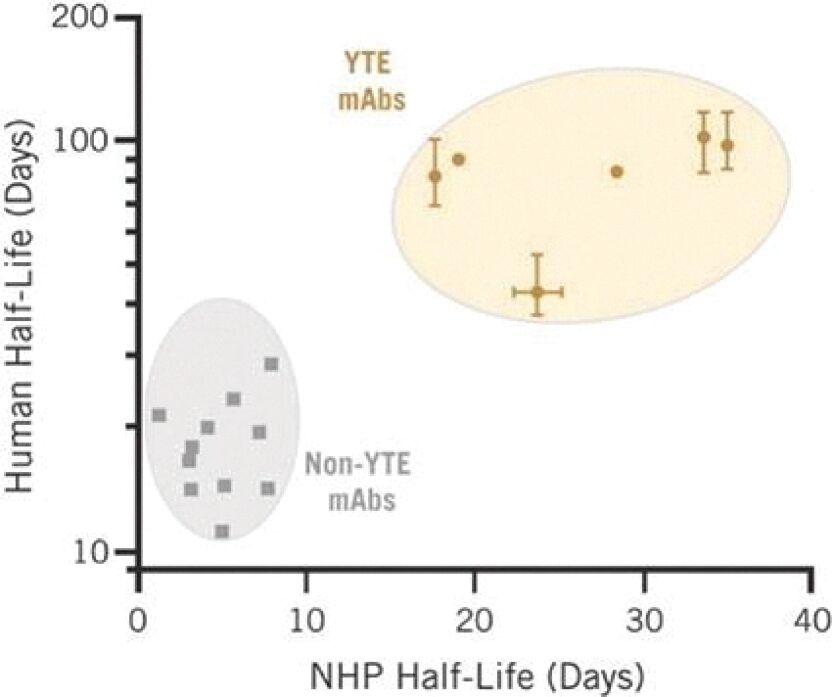
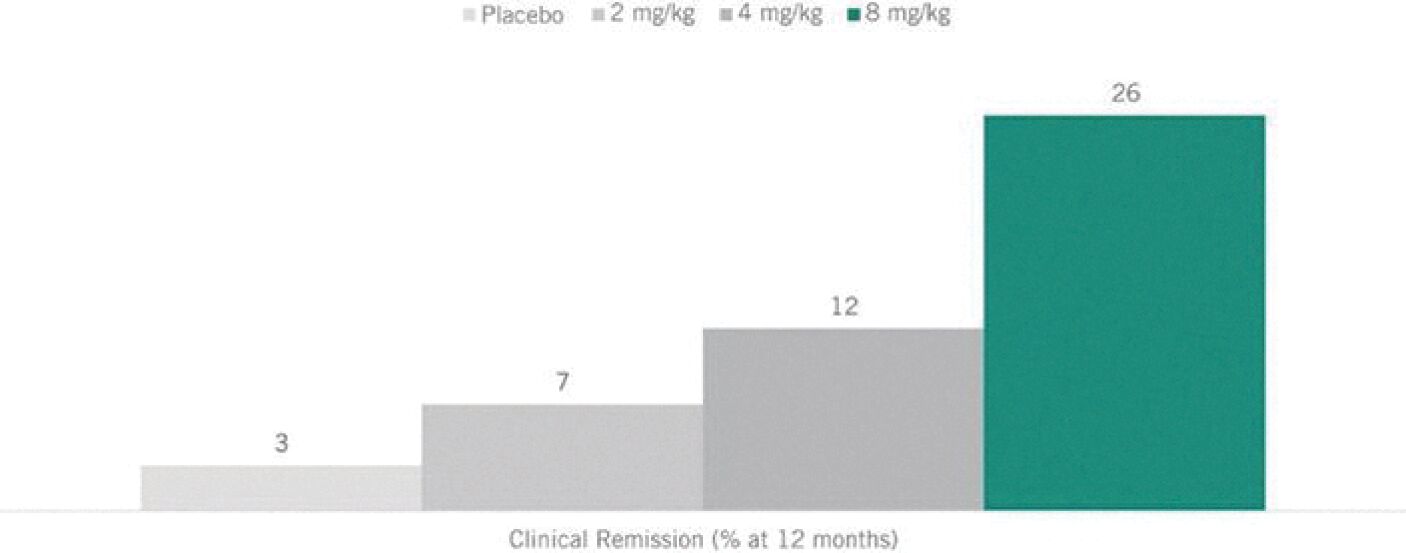
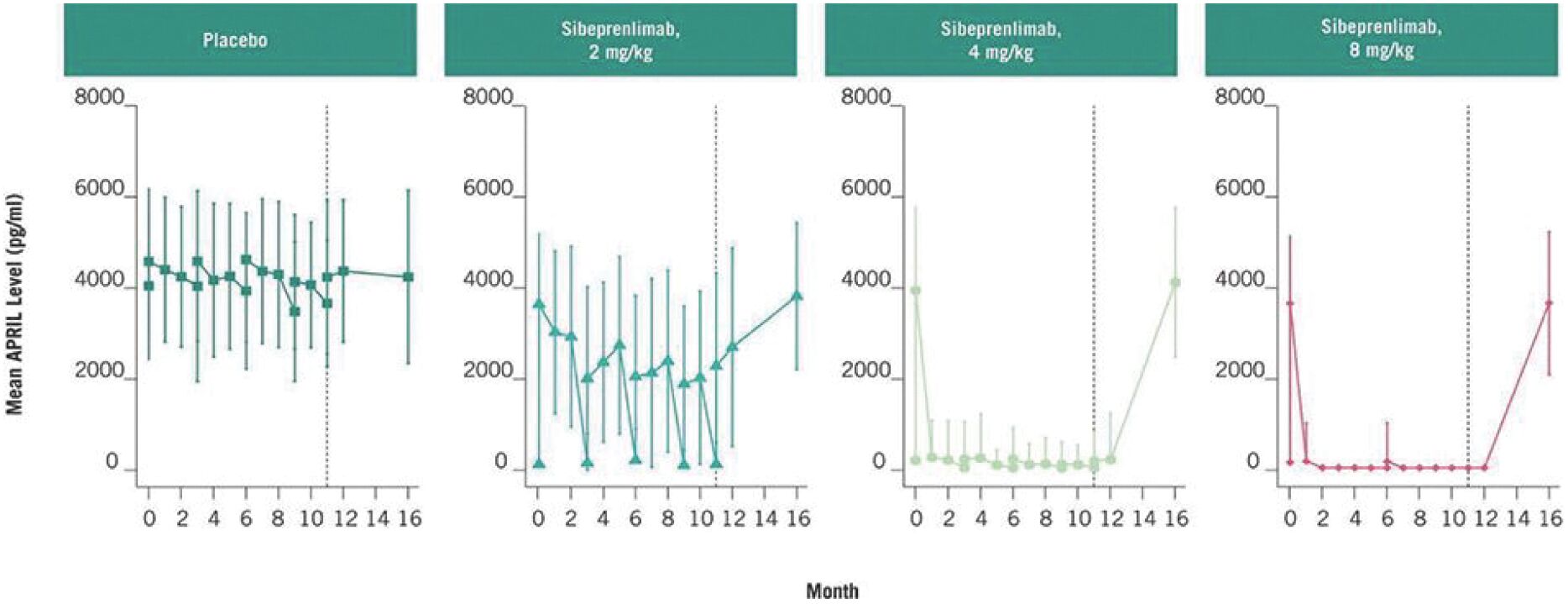
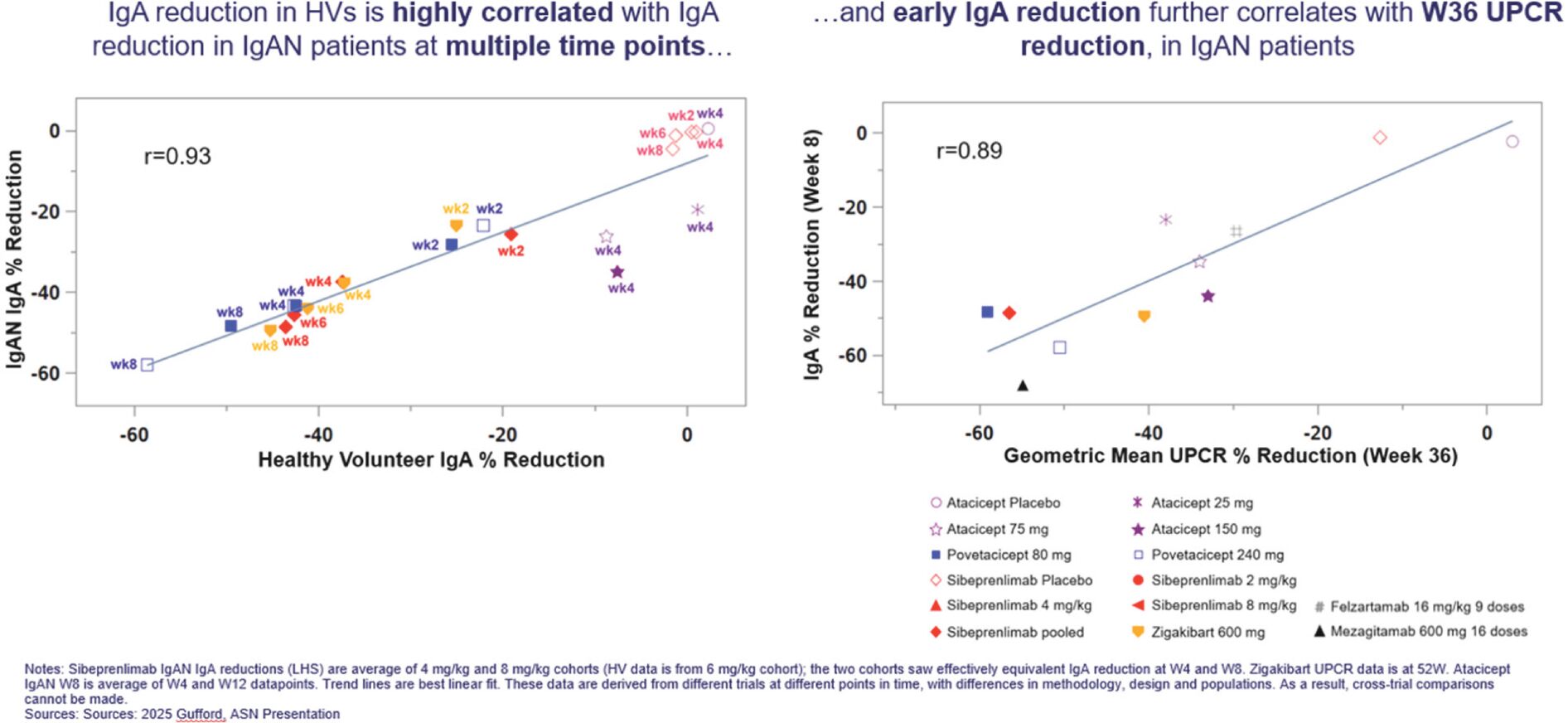
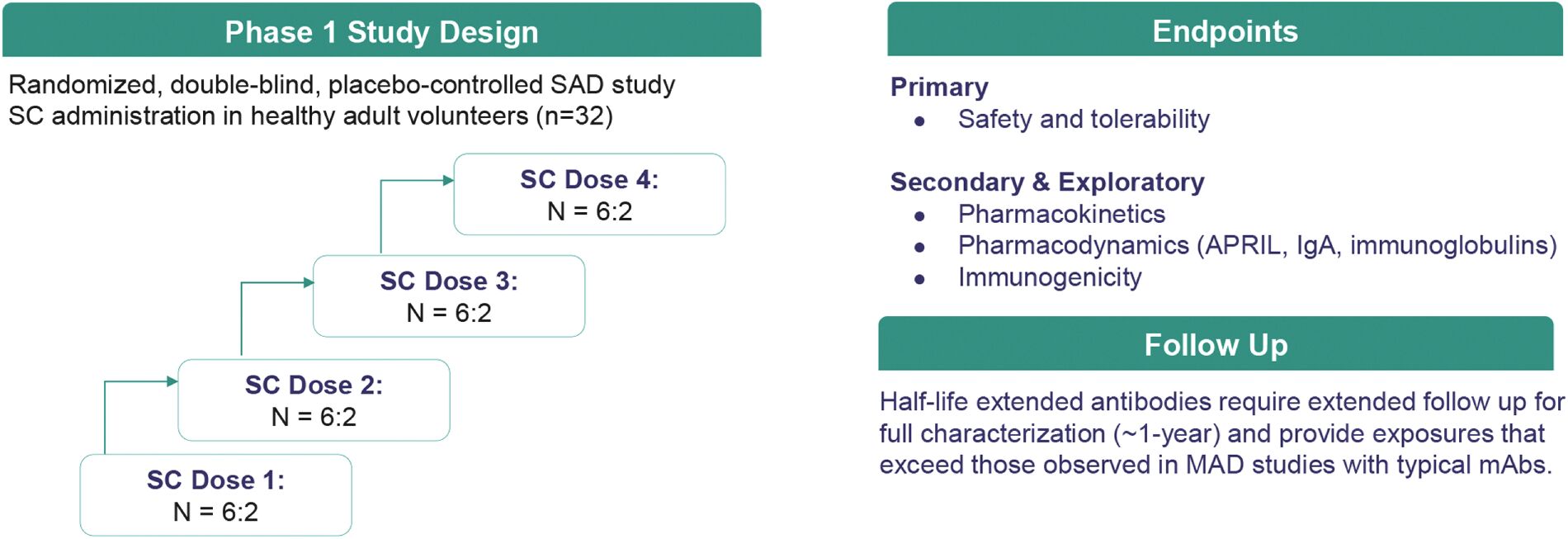
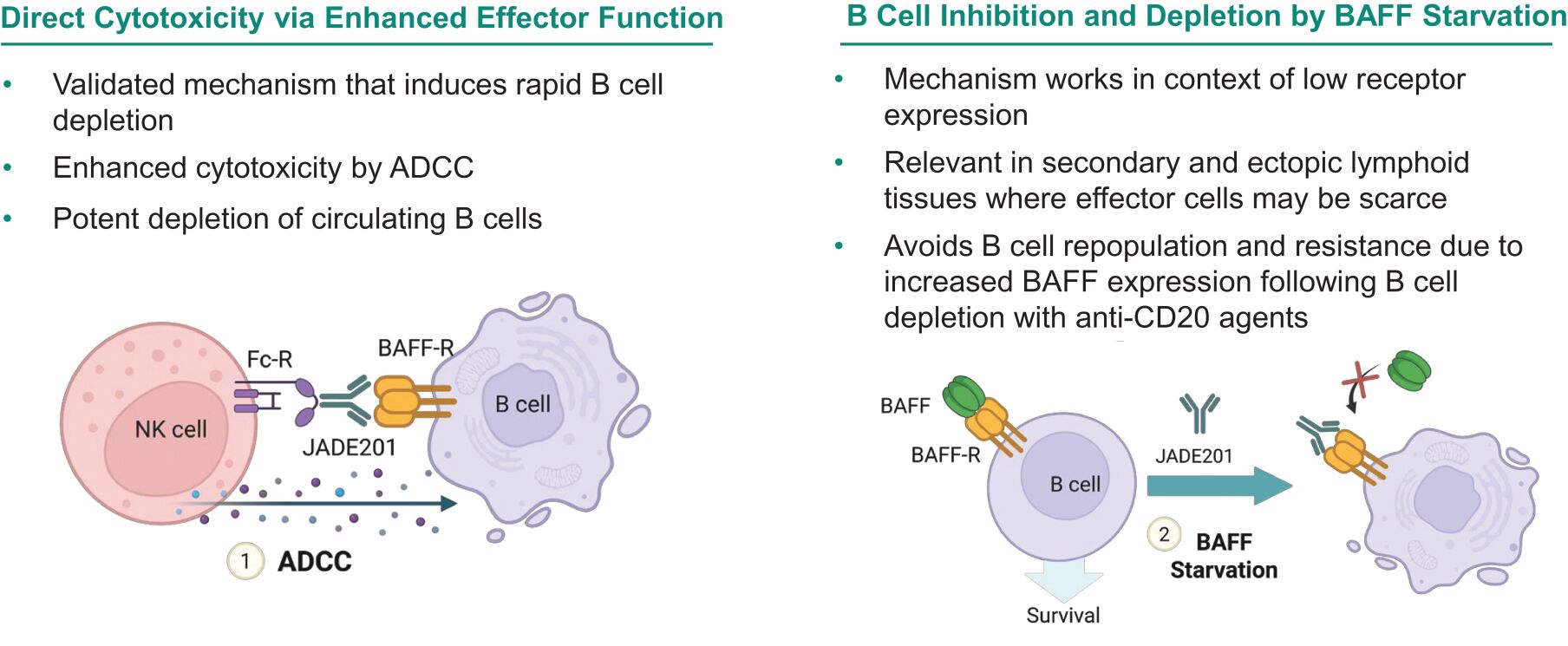
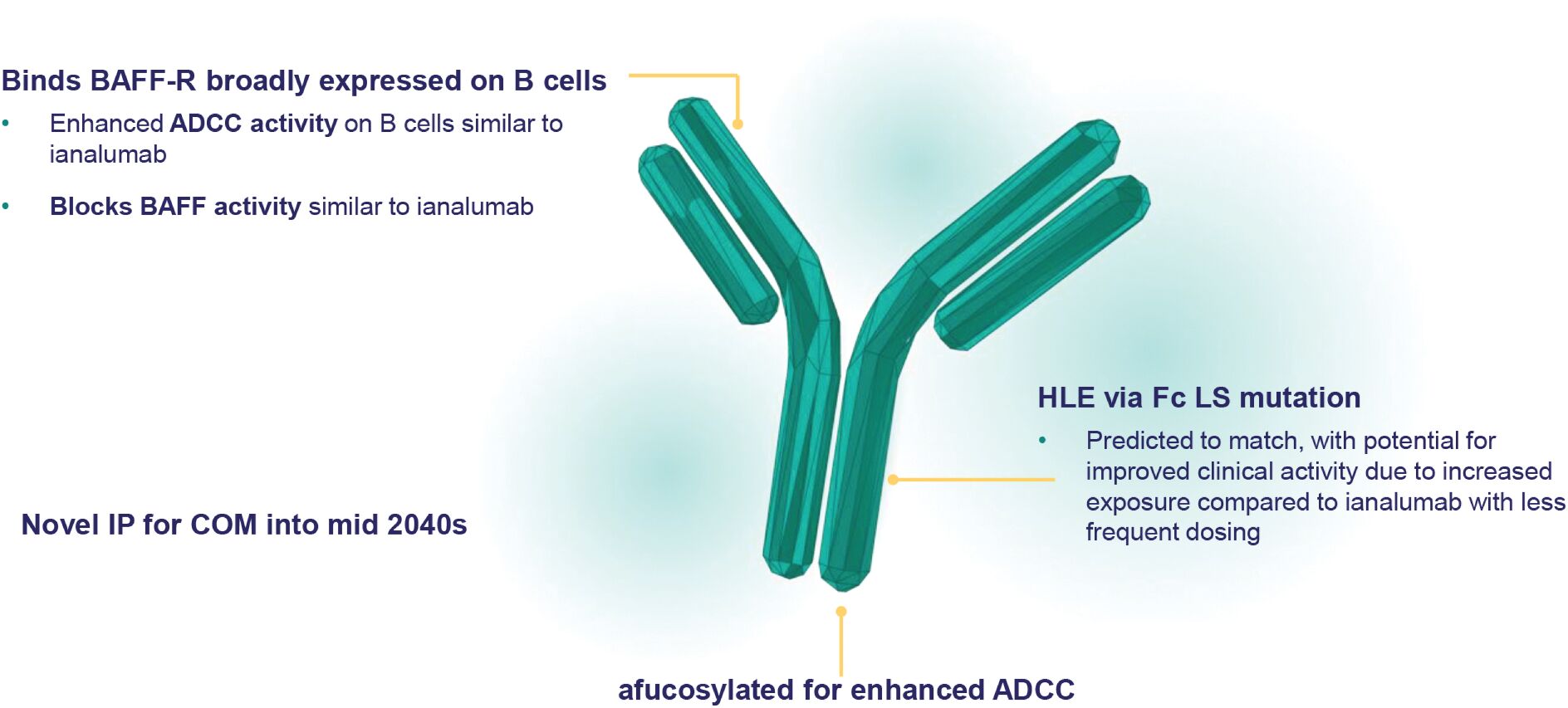
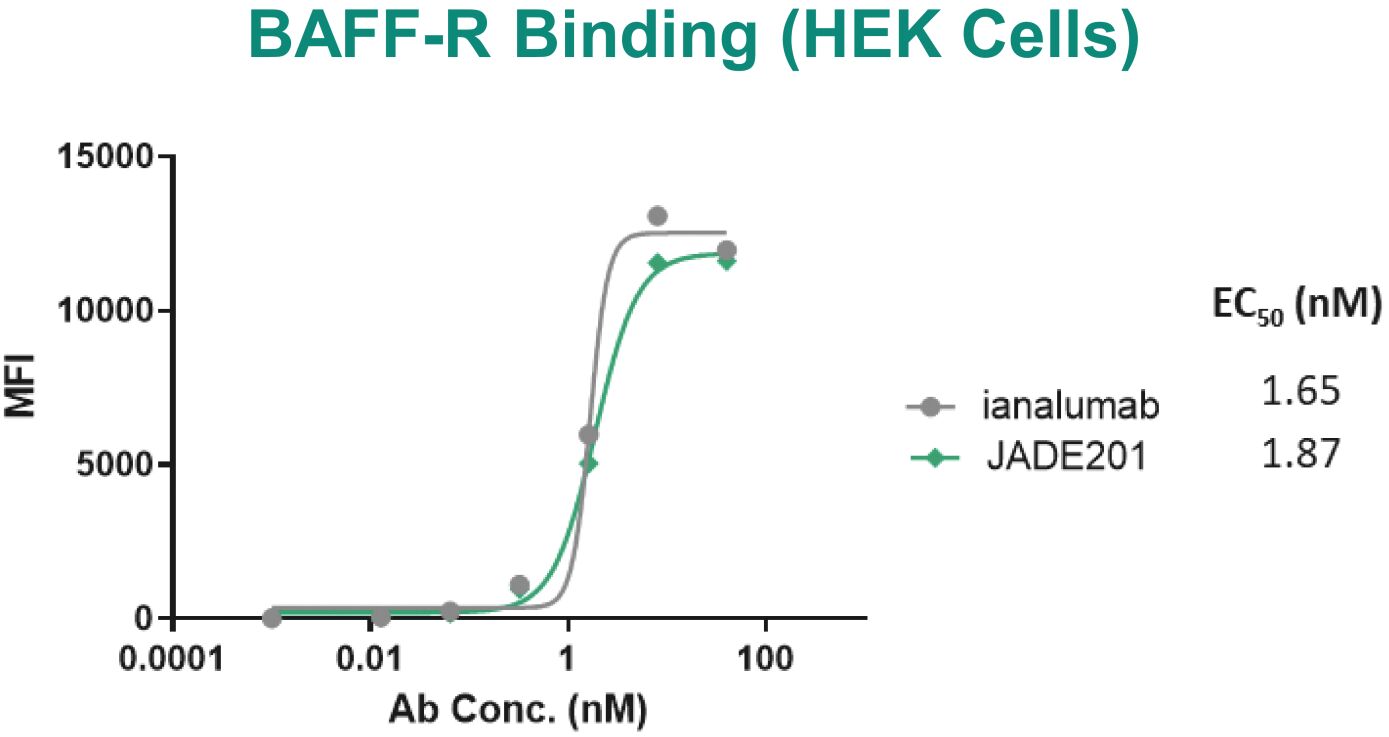
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-291541
PROSPECTUS

Jade Biosciences, Inc.
16,125,269 Shares
Common Stock
Offered by the Selling Stockholders
This prospectus relates to the proposed resale or other disposition by the selling stockholders identified herein (the “Selling Stockholders”) of up to (i) 13,368,164 shares (the “PIPE Shares”) of our common stock, par value $0.0001 per share (“Common Stock”), (ii) 1,402,092 shares of Common Stock issuable upon the exercise of pre-funded warrants (the “PIPE Pre-Funded Warrants”) and (iii) 1,355,013 shares of Common Stock (the “Existing Shares”) previously issued to a Selling Stockholder. The shares of Common Stock registered by this prospectus are referred to herein as the “Resale Shares.”
The PIPE Shares and the PIPE Pre-Funded Warrants were issued and sold to accredited investors in a private placement (the “October 2025 PIPE”), which closed on October 8, 2025. The Existing Shares consist of (i) 292,687 shares of Common Stock previously held by and (ii) 1,062,326 shares of Common Stock issued in the Pre-Closing Financing (as defined herein) to RA Capital Healthcare Fund, L.P. We are not selling any Resale Shares under this prospectus and will not receive any of the proceeds from the sale or other disposition of Resale Shares by the Selling Stockholders. Upon any exercise of the PIPE Pre-Funded Warrants by payment of cash, however, we will receive the nominal cash exercise price paid by the holders of the PIPE Pre-Funded Warrants. We intend to use those proceeds, if any, for general corporate purposes.
The Selling Stockholders may sell the Resale Shares on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, on the over-the-counter market, in one or more transactions otherwise than on these exchanges or systems, such as privately negotiated transactions, or using a combination of these methods, and at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. See the disclosure under the heading “Plan of Distribution” elsewhere in this prospectus for more information about how the Selling Stockholders may sell or otherwise dispose of their Resale Shares hereunder.
The Selling Stockholders may sell any, all or none of the securities offered by this prospectus and we do not know when or in what amount the Selling Stockholders may sell their Resale Shares hereunder following the effective date of the registration statement of which this prospectus forms a part. Discounts, concessions, commissions and similar selling expenses attributable to the sale of the Resale Shares will be borne by the Selling Stockholder. We will pay certain fees and expenses (other than discounts, concessions, commissions and similar selling expenses) incident to the registration of the Resale Shares with the Securities and Exchange Commission (“SEC”).
You should carefully read this prospectus and any applicable prospectus supplement before you invest in any of the securities being offered.
Our Common Stock is traded on The Nasdaq Capital Market under the symbol “JBIO.” On November 12, 2025, the last reported sale price for our Common Stock was $10.00 per share.
An investment in our securities involves a high degree of risk. You should carefully consider the information under the heading “Risk Factors” beginning on page 8 of this prospectus and any applicable prospectus supplement.
We are a “smaller reporting company” and an “emerging growth company” under applicable federal securities laws and are subject to reduced public company reporting requirements.
Neither the SEC nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is December 19, 2025