| Copyright © 2026 Harmony Biosciences. All rights reserved. 44th Annual J.P. Morgan Healthcare Conference JEFFREY M. DAYNO, MD January 13,2026 | San Francisco |
| Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding our full year 2026 net product revenue for WAKIX, expectations for the growth and value of WAKIX, plans to submit an NDA for pitolisant GR; plans to conduct trials, collect or receive data, or continue investigating any of our product candidates or potential indications; our plans to extend the pitolisant franchise into the 2040s; our future results of operations and financial position, business strategy, products, prospective products, product approvals, the plans and objectives of management for future operations and future results of anticipated products. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: our commercialization efforts and strategy for WAKIX and, if approved, our other product candidates; the rate and degree of market acceptance and clinical utility of pitolisant in additional indications, if approved, and any other product candidates we may develop or acquire, if approved; our research and development plans, including our efforts to explore the therapeutic potential of pitolisant in additional indications, including pitolisant GR and pitolisant HD; our ongoing and planned clinical trials; our ability to expand the scope of our license agreements with Bioprojet Société Civile de Recherche (“Bioprojet”); the availability of favorable insurance coverage and reimbursement for WAKIX and, if approved, our other product candidates; the timing of, and our ability to obtain, regulatory approvals for pitolisant for other indications, including pitolisant GR and pitolisant HD, as well as any other product candidates; our estimates regarding expenses, future revenue, capital requirements and additional financing needs; our ability to identify, acquire and integrate additional products or product candidates with significant commercial potential that are consistent with our commercial objectives; our commercialization, marketing and manufacturing capabilities and strategy; significant competition in our industry; our intellectual property position; loss or retirement of key members of management; failure to successfully execute our growth strategy, including any delays in our planned future growth; our failure to maintain effective internal controls; the impact of government laws and regulations; volatility and fluctuations in the price of our common stock; the significant costs and required management time as a result of operating as a public company; the fact that the price of Harmony's common stock may be volatile and fluctuate substantially; statements related to our intended share repurchases and repurchase timeframe and the significant costs and required management time as a result of operating as a public company. These and other important factors discussed under the caption "Risk Factors" in our Annual Report on Form 10-K filed with the Securities and Exchange Commission (the "SEC") on February 25, 2025, and our other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management's estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. 2 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
| 5 STRONG FINANCIAL PROFILE PHASE 3 PROGRAMS ON TRACK FOR BLOCKBUSTER STATUS |
| PITOLISANT FRANCHISE STRATEGY |
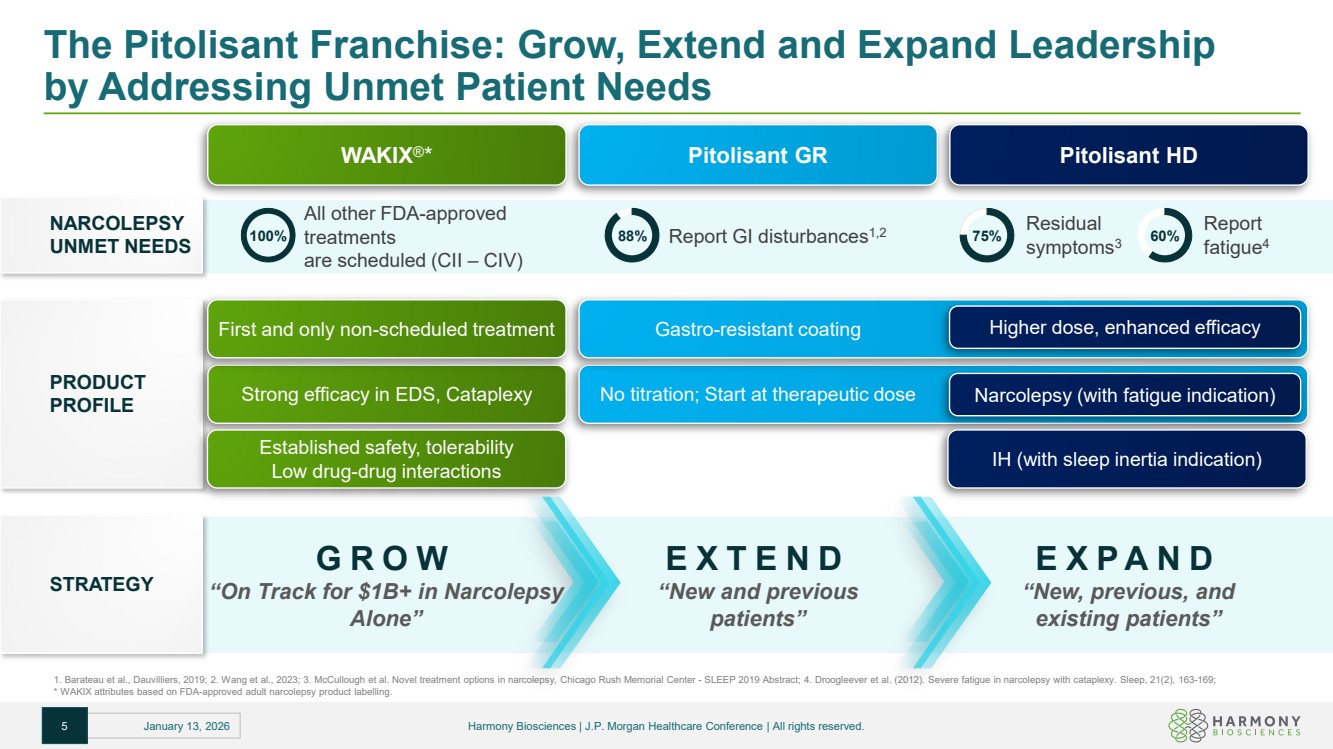
| The Pitolisant Franchise: Grow, Extend and Expand Leadership by Addressing Unmet Patient Needs 1. Barateau et al., Dauvilliers, 2019; 2. Wang et al., 2023; 3. McCullough et al. Novel treatment options in narcolepsy, Chicago Rush Memorial Center - SLEEP 2019 Abstract; 4. Droogleever et al. (2012). Severe fatigue in narcolepsy with cataplexy. Sleep, 21(2), 163-169; * WAKIX attributes based on FDA-approved adult narcolepsy product labelling. Pitolisant GR Pitolisant HD NARCOLEPSY UNMET NEEDS WAKIX®* All other FDA-approved treatments are scheduled (CII – CIV) 100% Report GI disturbances 88% 1,2 Residual symptoms3 75% Report fatigue4 60% PRODUCT PROFILE 5 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. Strong efficacy in EDS, Cataplexy No titration; Start at therapeutic dose First and only non-scheduled treatment Gastro-resistant coating Higher dose, enhanced efficacy Established safety, tolerability Low drug-drug interactions Narcolepsy (with fatigue indication) IH (with sleep inertia indication) STRATEGY GROW “On Track for $1B+ in Narcolepsy Alone” EXTEND “New and previous patients” EXPAND “New, previous, and existing patients” |
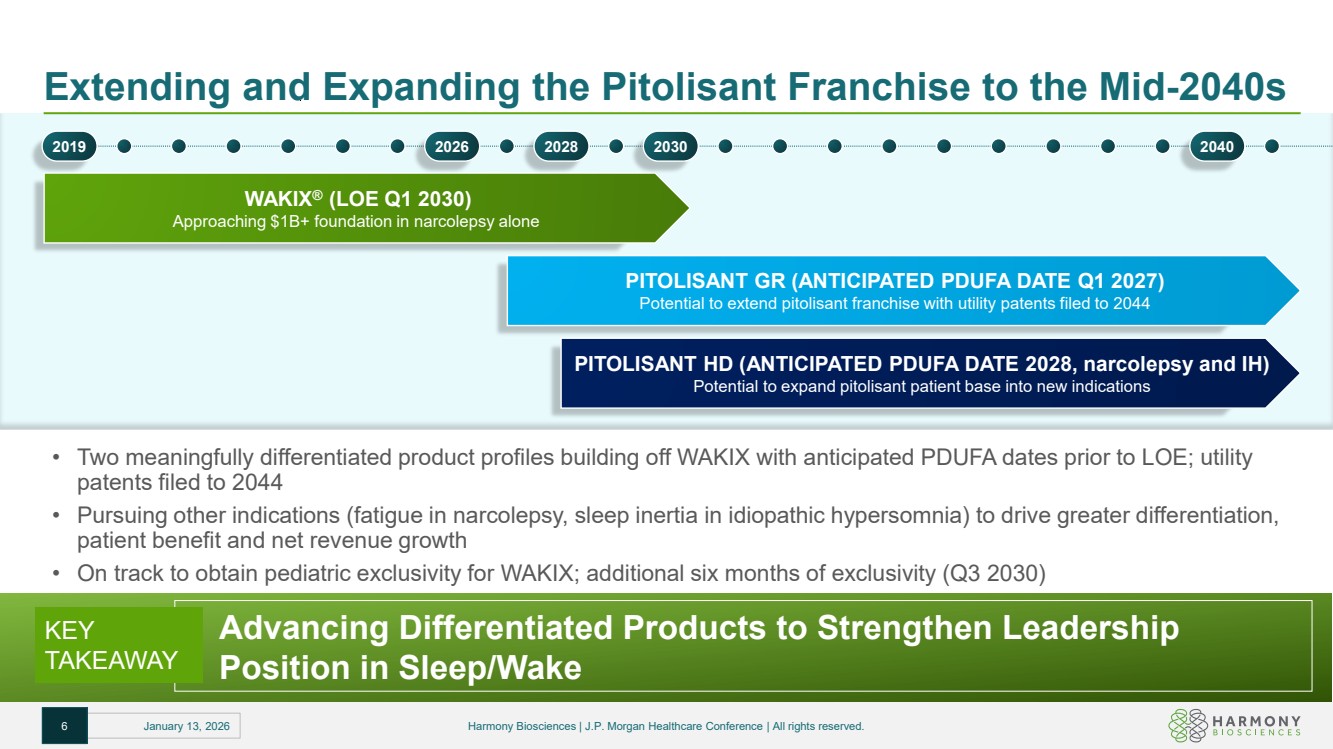
| Extending and Expanding the Pitolisant Franchise to the Mid-2040s • Two meaningfully differentiated product profiles building off WAKIX with anticipated PDUFA dates prior to LOE; utility patents filed to 2044 • Pursuing other indications (fatigue in narcolepsy, sleep inertia in idiopathic hypersomnia) to drive greater differentiation, patient benefit and net revenue growth • On track to obtain pediatric exclusivity for WAKIX; additional six months of exclusivity (Q3 2030) WAKIX® (LOE Q1 2030) Approaching $1B+ foundation in narcolepsy alone PITOLISANT GR (ANTICIPATED PDUFA DATE Q1 2027) Potential to extend pitolisant franchise with utility patents filed to 2044 PITOLISANT HD (ANTICIPATED PDUFA DATE 2028, narcolepsy and IH) Potential to expand pitolisant patient base into new indications KEY TAKEAWAY Advancing Differentiated Products to Strengthen Leadership Position in Sleep/Wake 2019 2026 2028 2030 2040 6 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
| GROW EXTEND EXPAND PITOLISANT FRANCHISE STRATEGY |
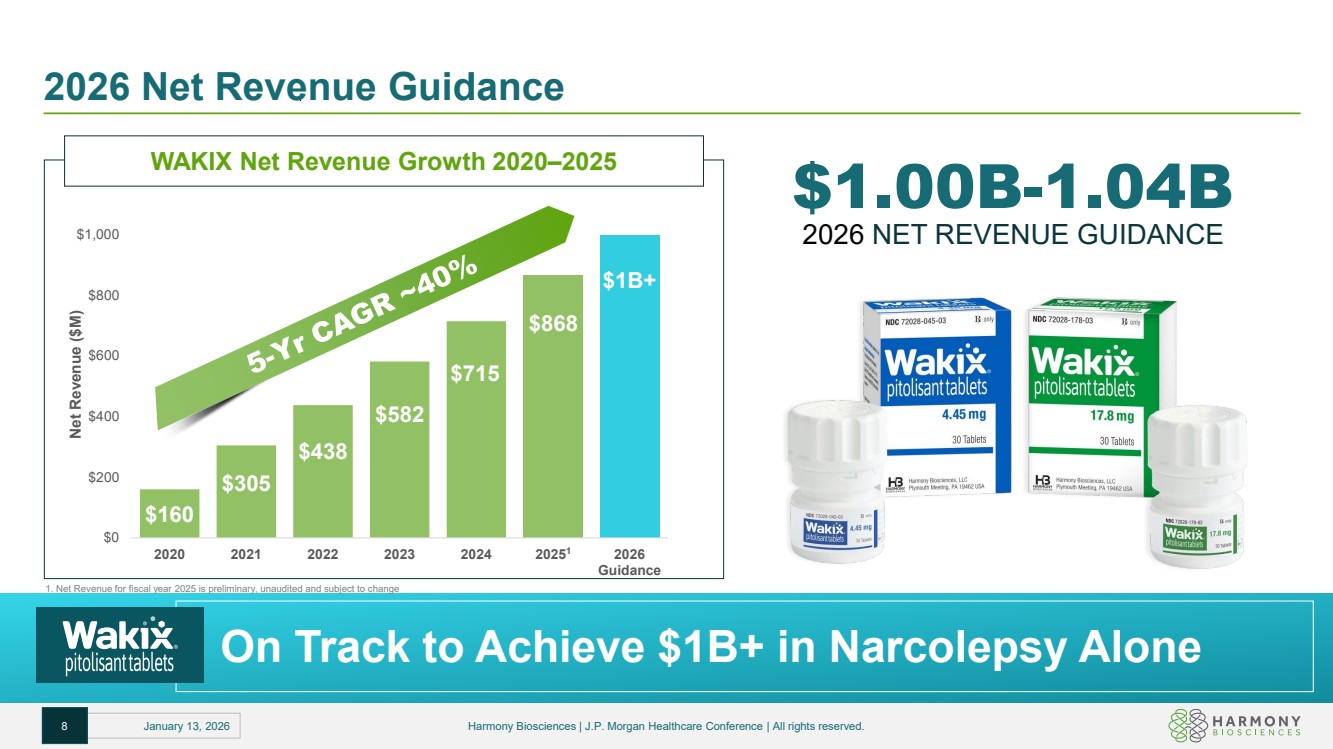
| 2026 Net Revenue Guidance $1.00B-1.04B 2026 NET REVENUE GUIDANCE WAKIX Net Revenue Growth 2020–2025 $160 $305 $438 $582 $715 $1B+ $0 $200 $400 $600 $800 $1,000 2020 2021 2022 2023 2024 2026 Guidance Net Revenue ($M) 20251 $868 On Track to Achieve $1B+ in Narcolepsy Alone 8 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. 1. Net Revenue for fiscal year 2025 is preliminary, unaudited and subject to change |
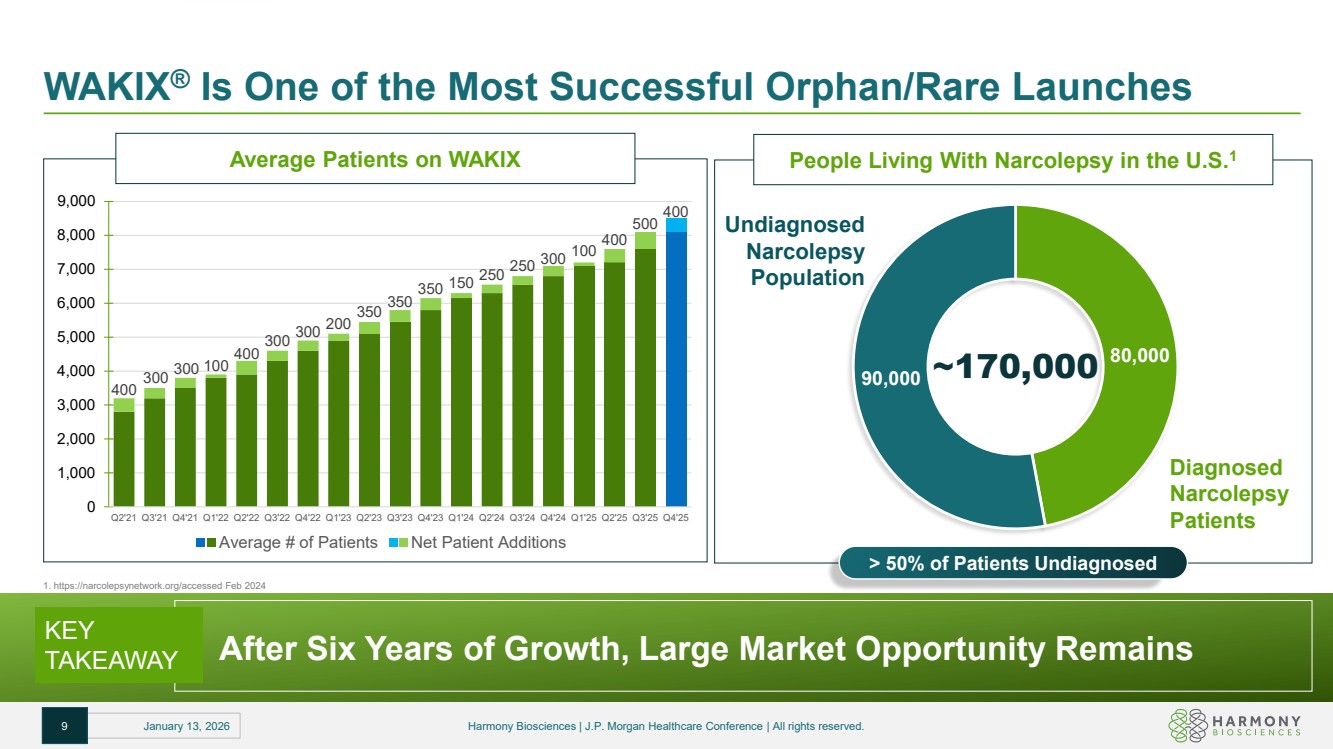
| WAKIX® Is One of the Most Successful Orphan/Rare Launches KEY TAKEAWAY After Six Years of Growth, Large Market Opportunity Remains 1. https://narcolepsynetwork.org/accessed Feb 2024 80,000 90,000 ~170,000 Diagnosed Narcolepsy Patients Undiagnosed Narcolepsy Population People Living With Narcolepsy in the U.S.1 > 50% of Patients Undiagnosed 9 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. Average Patients on WAKIX 400 300 300 100 400 300 300 200 350 350 350 150 250 250 300 100 400 500 400 0 1,000 2,000 3,000 4,000 5,000 6,000 7,000 8,000 9,000 Q2'21 Q3'21 Q4'21 Q1'22 Q2'22 Q3'22 Q4'22 Q1'23 Q2'23 Q3'23 Q4'23 Q1'24 Q2'24 Q3'24 Q4'24 Q1'25 Q2'25 Q3'25 Q4'25 Average # of Patients Net Patient Additions |
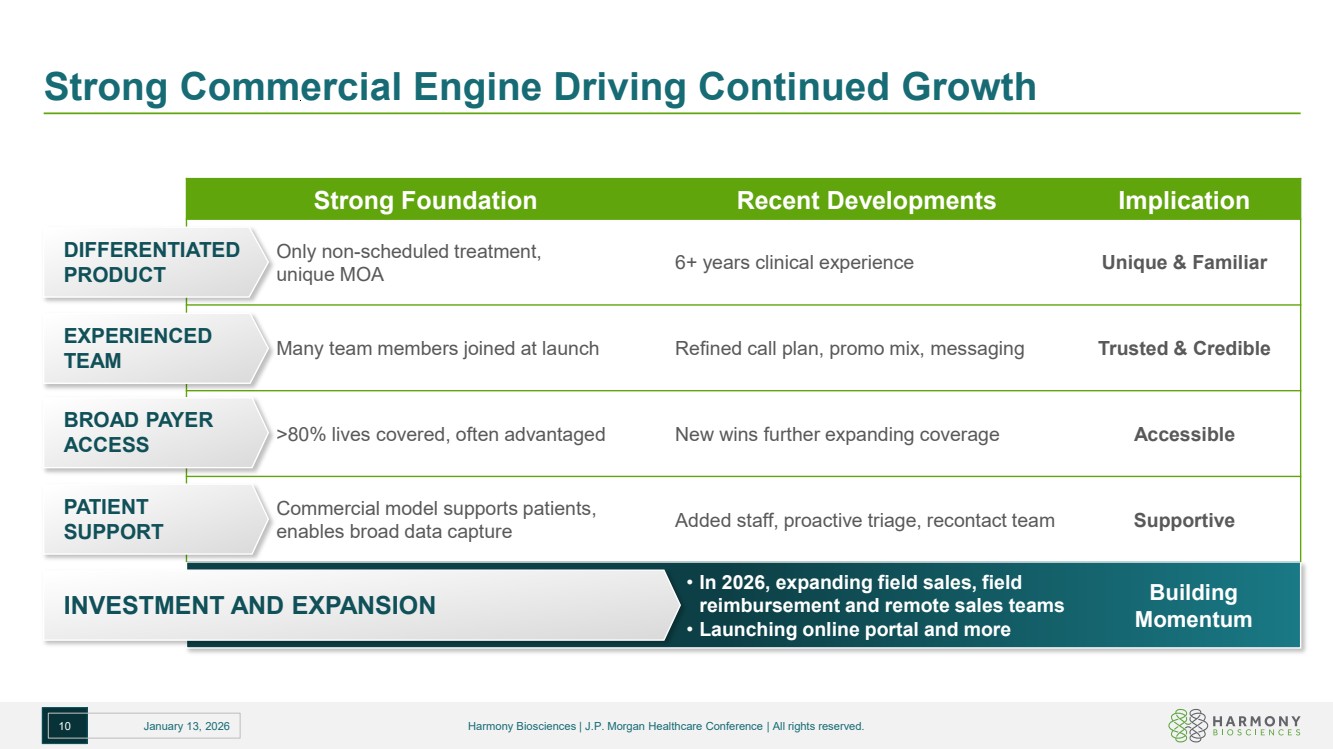
| Strong Commercial Engine Driving Continued Growth 10 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. Strong Foundation Recent Developments Implication Only non-scheduled treatment, unique MOA 6+ years clinical experience Unique & Familiar Many team members joined at launch Refined call plan, promo mix, messaging Trusted & Credible >80% lives covered, often advantaged New wins further expanding coverage Accessible Commercial model supports patients, enables broad data capture Added staff, proactive triage, recontact team Supportive DIFFERENTIATED PRODUCT EXPERIENCED TEAM BROAD PAYER ACCESS PATIENT SUPPORT INVESTMENT AND EXPANSION • In 2026, expanding field sales, field reimbursement and remote sales teams • Launching online portal and more Building Momentum |
| GROW EXTEND EXPAND PITOLISANT FRANCHISE STRATEGY |
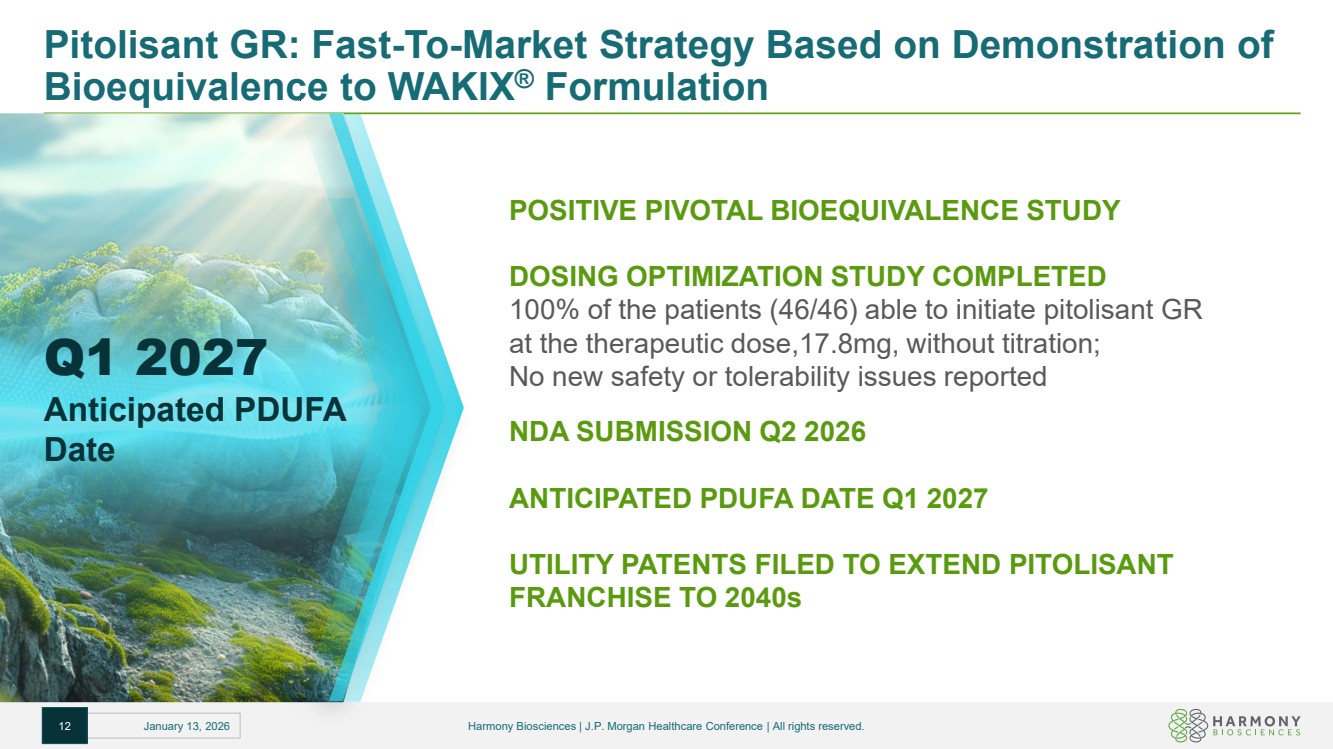
| Pitolisant GR: Fast-To-Market Strategy Based on Demonstration of Bioequivalence to WAKIX® Formulation Q1 2027 Anticipated PDUFA Date POSITIVE PIVOTAL BIOEQUIVALENCE STUDY DOSING OPTIMIZATION STUDY COMPLETED 100% of the patients (46/46) able to initiate pitolisant GR at the therapeutic dose,17.8mg, without titration; No new safety or tolerability issues reported NDA SUBMISSION Q2 2026 ANTICIPATED PDUFA DATE Q1 2027 UTILITY PATENTS FILED TO EXTEND PITOLISANT FRANCHISE TO 2040s 12 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
| GROW EXTEND EXPAND PITOLISANT FRANCHISE STRATEGY |
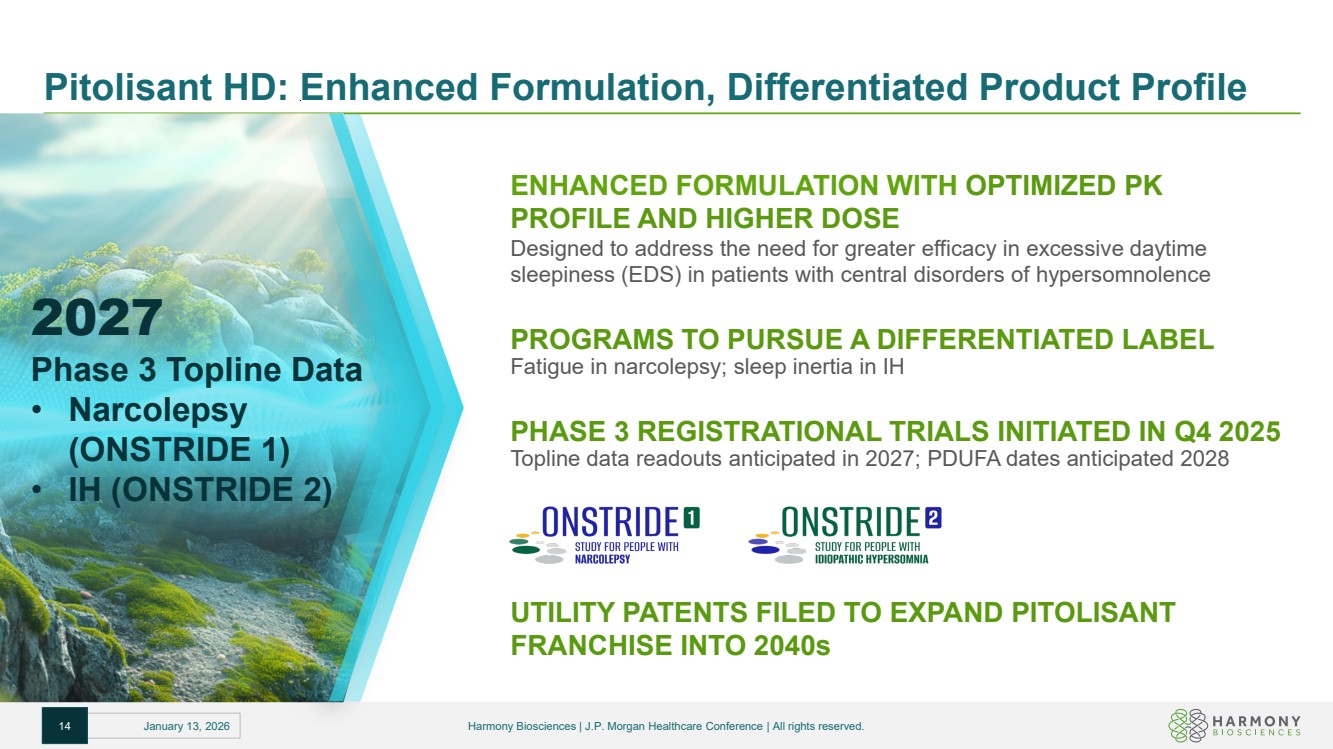
| Pitolisant HD: Enhanced Formulation, Differentiated Product Profile ENHANCED FORMULATION WITH OPTIMIZED PK PROFILE AND HIGHER DOSE Designed to address the need for greater efficacy in excessive daytime sleepiness (EDS) in patients with central disorders of hypersomnolence PROGRAMS TO PURSUE A DIFFERENTIATED LABEL Fatigue in narcolepsy; sleep inertia in IH PHASE 3 REGISTRATIONAL TRIALS INITIATED IN Q4 2025 Topline data readouts anticipated in 2027; PDUFA dates anticipated 2028 UTILITY PATENTS FILED TO EXPAND PITOLISANT FRANCHISE INTO 2040s 2027 Phase 3 Topline Data • Narcolepsy (ONSTRIDE 1) • IH (ONSTRIDE 2) 14 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
|
| ROBUST PIPELINE |
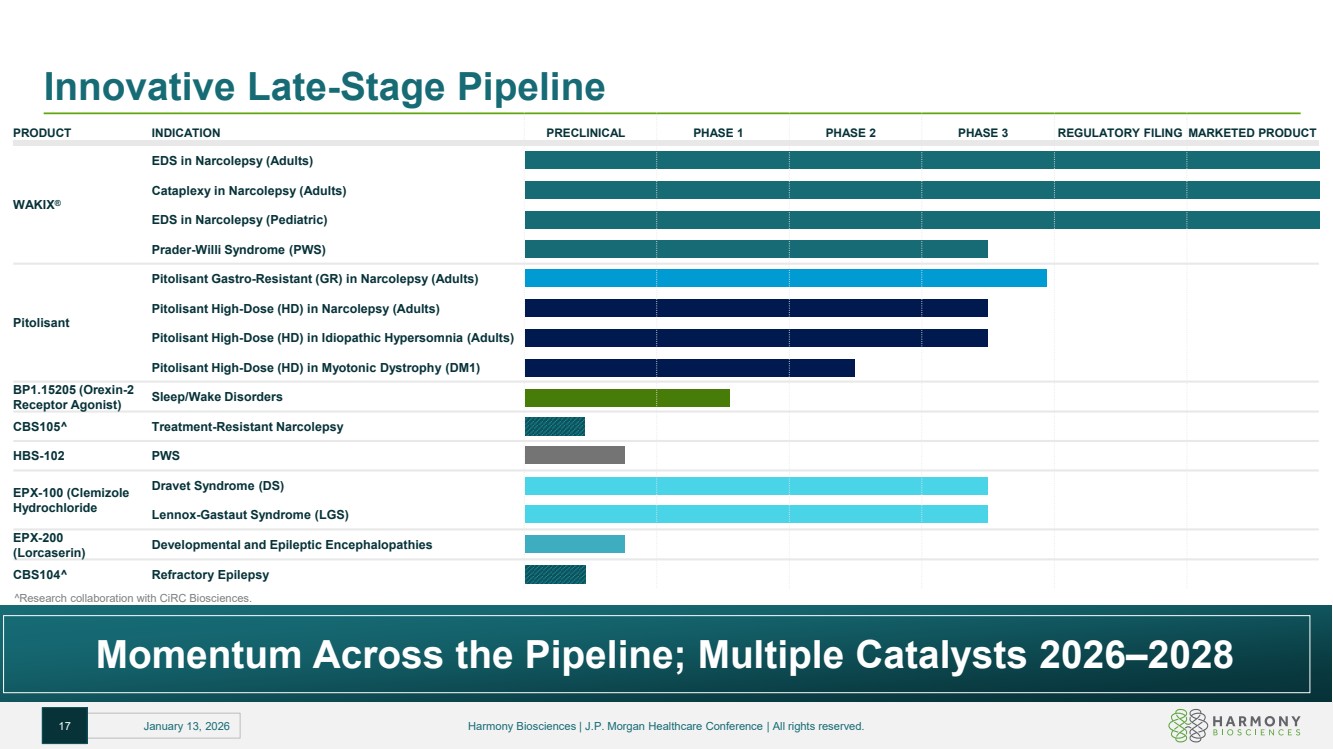
| Innovative Late-Stage Pipeline PRODUCT INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 REGULATORY FILING MARKETED PRODUCT WAKIX® EDS in Narcolepsy (Adults) Cataplexy in Narcolepsy (Adults) EDS in Narcolepsy (Pediatric) Prader-Willi Syndrome (PWS) Pitolisant Pitolisant Gastro-Resistant (GR) in Narcolepsy (Adults) Pitolisant High-Dose (HD) in Narcolepsy (Adults) Pitolisant High-Dose (HD) in Idiopathic Hypersomnia (Adults) Pitolisant High-Dose (HD) in Myotonic Dystrophy (DM1) BP1.15205 (Orexin-2 Receptor Agonist) Sleep/Wake Disorders CBS105 Treatment-Resistant Narcolepsy HBS-102 PWS EPX-100 (Clemizole Hydrochloride Dravet Syndrome (DS) Lennox-Gastaut Syndrome (LGS) EPX-200 (Lorcaserin) Developmental and Epileptic Encephalopathies CBS104 Refractory Epilepsy Momentum Across the Pipeline; Multiple Catalysts 2026–2028 Research collaboration with CiRC Biosciences. 17 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
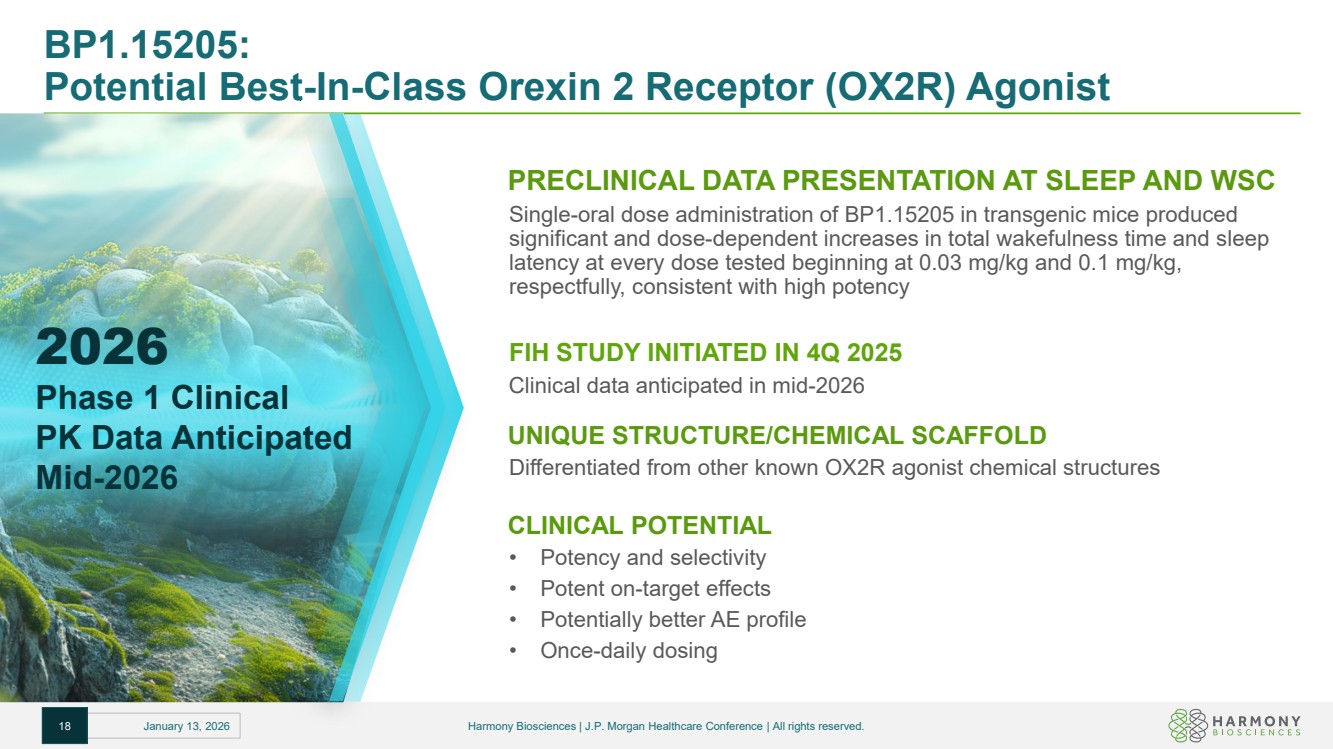
| BP1.15205: Potential Best-In-Class Orexin 2 Receptor (OX2R) Agonist PRECLINICAL DATA PRESENTATION AT SLEEP AND WSC Single-oral dose administration of BP1.15205 in transgenic mice produced significant and dose-dependent increases in total wakefulness time and sleep latency at every dose tested beginning at 0.03 mg/kg and 0.1 mg/kg, respectfully, consistent with high potency FIH STUDY INITIATED IN 4Q 2025 Clinical data anticipated in mid-2026 UNIQUE STRUCTURE/CHEMICAL SCAFFOLD Differentiated from other known OX2R agonist chemical structures CLINICAL POTENTIAL • Potency and selectivity • Potent on-target effects • Potentially better AE profile • Once-daily dosing 18 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. 2026 Phase 1 Clinical PK Data Anticipated Mid-2026 |
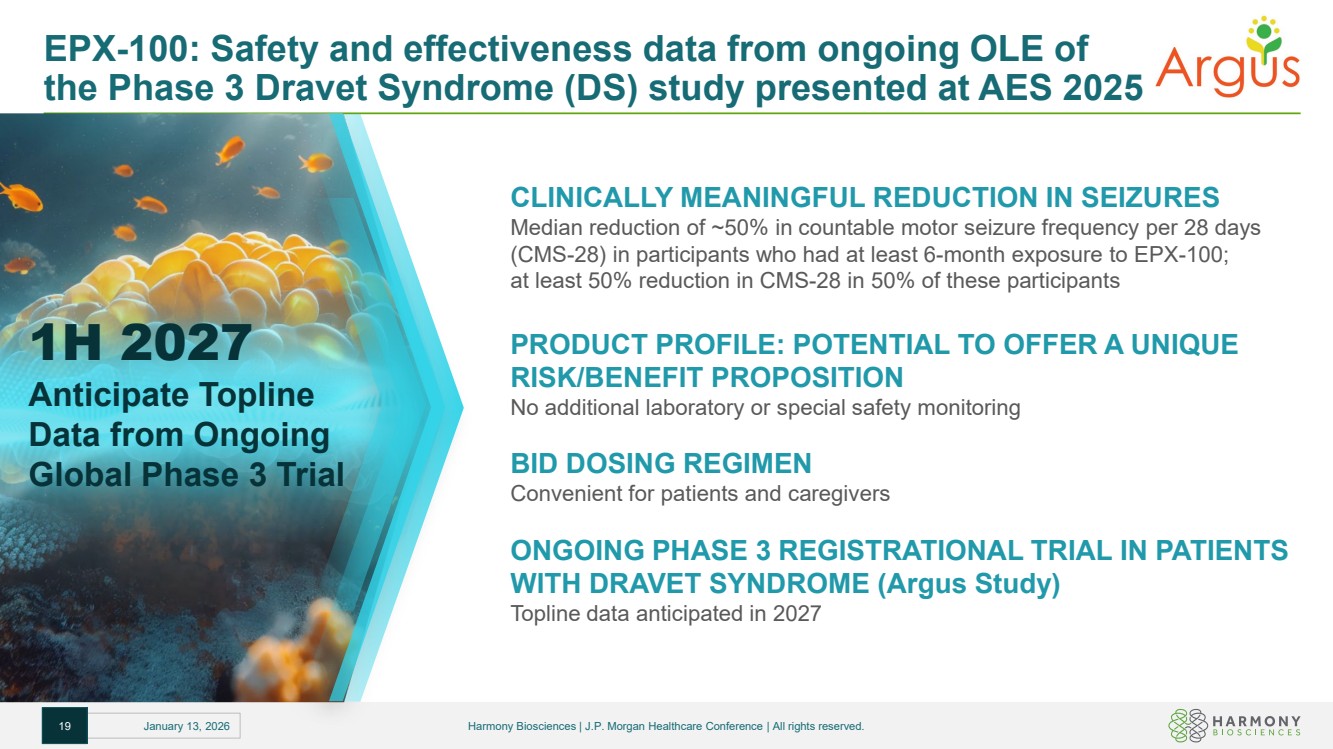
| EPX-100: Safety and effectiveness data from ongoing OLE of the Phase 3 Dravet Syndrome (DS) study presented at AES 2025 CLINICALLY MEANINGFUL REDUCTION IN SEIZURES Median reduction of ~50% in countable motor seizure frequency per 28 days (CMS-28) in participants who had at least 6-month exposure to EPX-100; at least 50% reduction in CMS-28 in 50% of these participants PRODUCT PROFILE: POTENTIAL TO OFFER A UNIQUE RISK/BENEFIT PROPOSITION No additional laboratory or special safety monitoring BID DOSING REGIMEN Convenient for patients and caregivers ONGOING PHASE 3 REGISTRATIONAL TRIAL IN PATIENTS WITH DRAVET SYNDROME (Argus Study) Topline data anticipated in 2027 1H 2027 Anticipate Topline Data from Ongoing Global Phase 3 Trial 19 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
| EPX-100: One of Most Advanced 5-HT2 (Serotonin) Agonist Programs in DEEs 20 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. ESTABLISHED 5-HT2 (SEROTONIN) AGONIST MECHANISM OF ACTION MoA validated via the zebrafish model ONGOING PHASE 3 TRIAL IN LENNOX-GASTAUT SYNDROME (LGS) (LIGHTHOUSE Study) Topline data anticipated 1H 2027 SAFETY: POTENTIAL TO OFFER A UNIQUE RISK/BENEFIT PROPOSITION No additional laboratory or special safety monitoring BID DOSING REGIMEN Convenient for patients and caregivers 1H 2027 Anticipate Topline Data from Ongoing Global Phase 3 Trial |
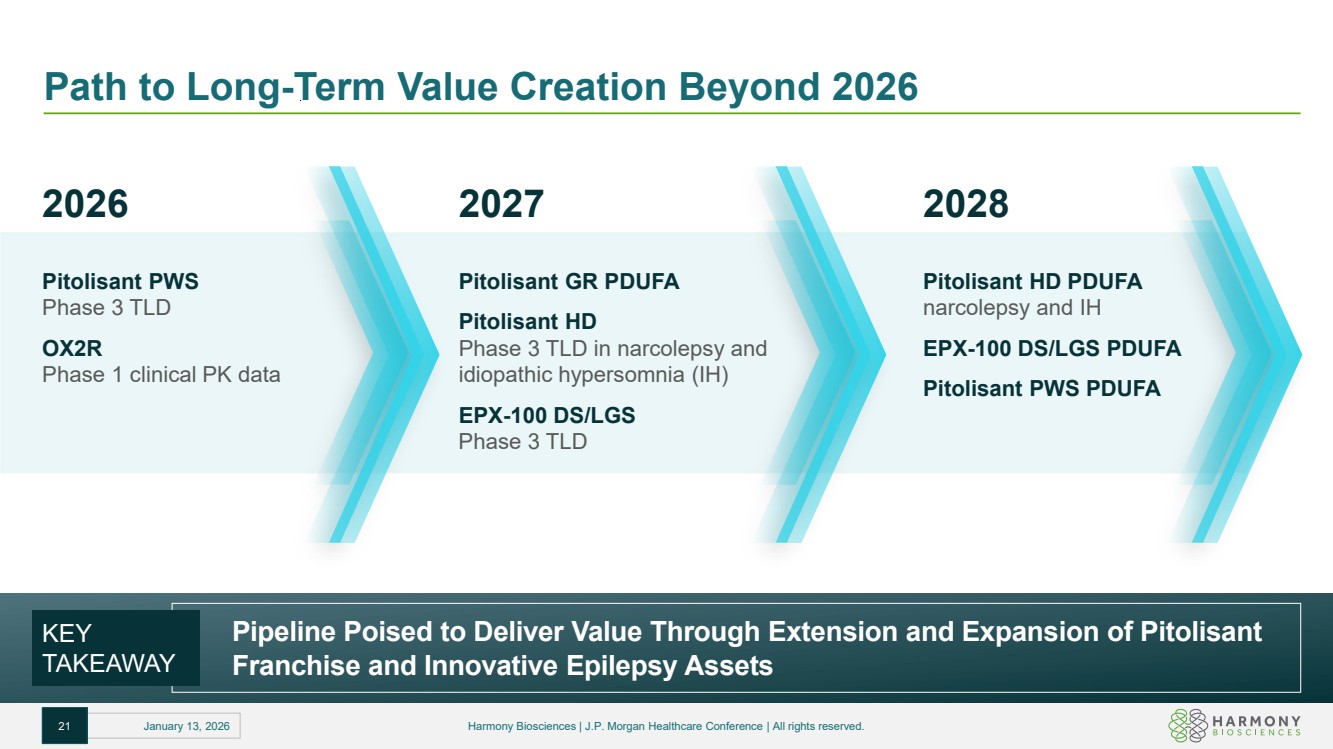
| Path to Long-Term Value Creation Beyond 2026 Pipeline Poised to Deliver Value Through Extension and Expansion of Pitolisant Franchise and Innovative Epilepsy Assets KEY TAKEAWAY 2027 Pitolisant GR PDUFA Pitolisant HD Phase 3 TLD in narcolepsy and idiopathic hypersomnia (IH) EPX-100 DS/LGS Phase 3 TLD 2028 Pitolisant HD PDUFA narcolepsy and IH EPX-100 DS/LGS PDUFA Pitolisant PWS PDUFA 2026 Pitolisant PWS Phase 3 TLD OX2R Phase 1 clinical PK data 21 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. |
| STRONG FINANCIAL PROFILE |
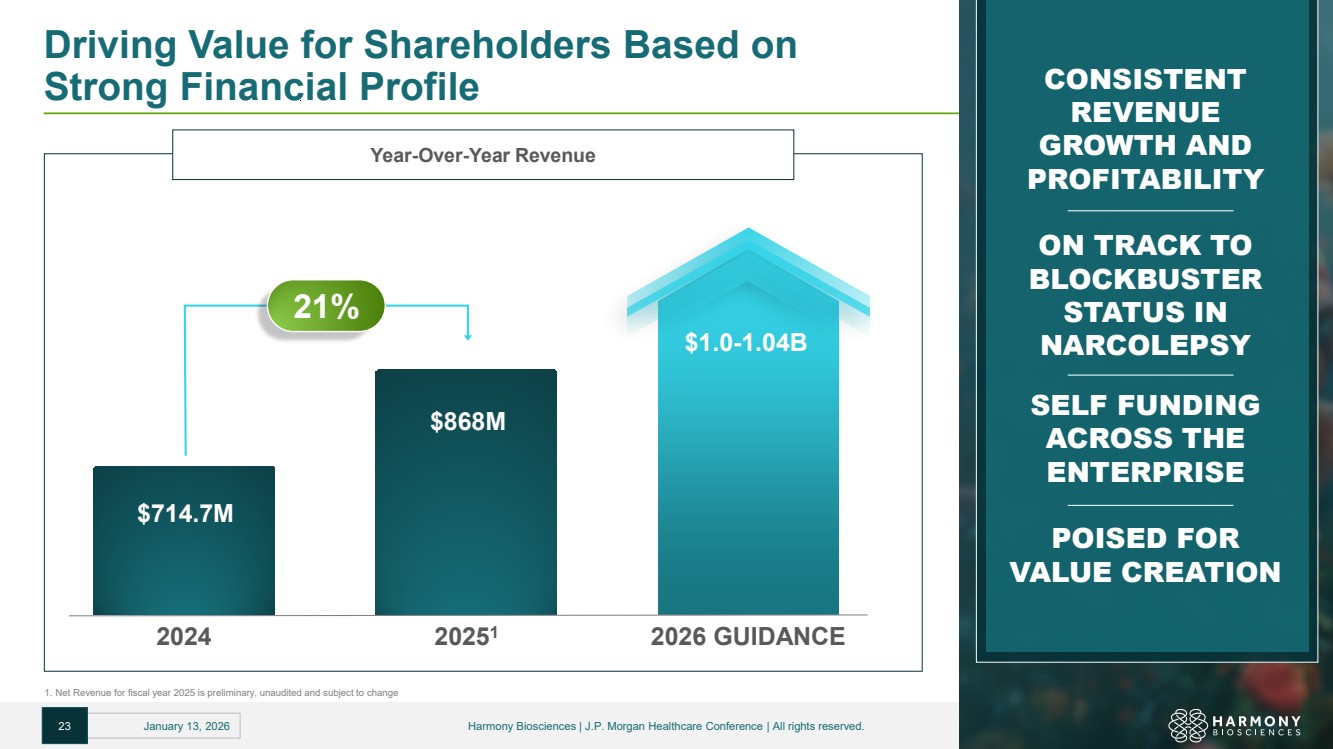
| Driving Value for Shareholders Based on Strong Financial Profile CONSISTENT REVENUE GROWTH AND PROFITABILITY ON TRACK TO BLOCKBUSTER STATUS IN NARCOLEPSY SELF FUNDING ACROSS THE ENTERPRISE POISED FOR VALUE CREATION Year-Over-Year Revenue 21% 2024 20251 2026 GUIDANCE $714.7M $868M $1.0-1.04B 23 January 13, 2026 Harmony Biosciences | J.P. Morgan Healthcare Conference | All rights reserved. 1. Net Revenue for fiscal year 2025 is preliminary, unaudited and subject to change |
| 5 STRONG FINANCIAL PROFILE PHASE 3 PROGRAMS ON TRACK FOR BLOCKBUSTER STATUS |
| To deliver on our promise to patients and create value for shareholders |
| company/harmonybiosciences/ @harmonybio harmony_biosciences www.harmonybiosciences.com |