
.2

Efficacy and Safety of Anzutresgene Autoleucel (IMA203), a PRAME - directed T - cell Receptor T - cell Therapy, in Patients with Previously Treated Advanced or Metastatic Uveal Melanoma from a Phase 1 Trial S. P. Patel 1 , A.M. Tsimberidou 2 , J.J. Luke 3 , W. Alsdorf 4 , A. Busse 5 , S. C. DeVane 6 , S. Hengler 7 , N. Hilf 7 , M.A. Sapna Patel, MD Presidential Symposium III Proffered Paper #1600O 20 October 2025 Kursunel 7 , A. Mayer - Mokler 7 , D. Pankov 6 , C.M. Britten 7 , M. Wermke 8 , L.F. Hernandez - Aya 1 University of Colorado, Aurora, CO; 2 University of Texas MD Anderson Center, Houston, TX; 3 University of Pittsburgh, Pittsburgh, PA; 4 University Medical Center Hamburg - Eppendorf, Hamburg, Germany; 5 Charité University Medicine Berlin, Berlin, Germany; 6 Immatics US Inc, Stafford, TX; 7 Immatics GmbH, Tuebingen, Germany; 8 University Hospital Dresden, Dresden, Germany; 9 University of Miami, Miller School of Medicine, Miami, FL. Copies of this presentation obtained through QR, AR and/or text key codes are for personal use only and may not be reproduced without written permission of the authors .

Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. DECLARATION OF INTERESTS Institutional clinical trial support: Linnaeus, Replimune Advisory board, steering committee, data safety monitoring board, consulting: Bristol - Myers Squibb, Daiichi Sankyo, Fortvita, Ideaya, Immatics, IO Biotech, MSD, Natera, Novartis, Obsidian, Pfizer, Replimune, Scancell, TriSalus Life Sciences, T3 Pharmaceuticals, Sun Pharmaceuticals, Veda Trials Commercial Medical Education providers: Clinical Education Alliance, Clinical Care Options, MJH Life Sciences, Melanoma Research Foundation, Vindico Medical The views and opinions expressed in this presentation are those of the presenter and do not necessarily represent the views of Immatics U.S. Inc. or its affiliates. The data discussed in this presentation are part of a study sponsored by Immatics U.S. Inc. Any statements about future development plans or anticipated results are solely those of the presenter and do not necessary reflect that of Immatics U.S. Inc. 2
In a cold tumour, does targeting a highly expressed antigen with TCR T - cell therapy lead to clinical benefit?
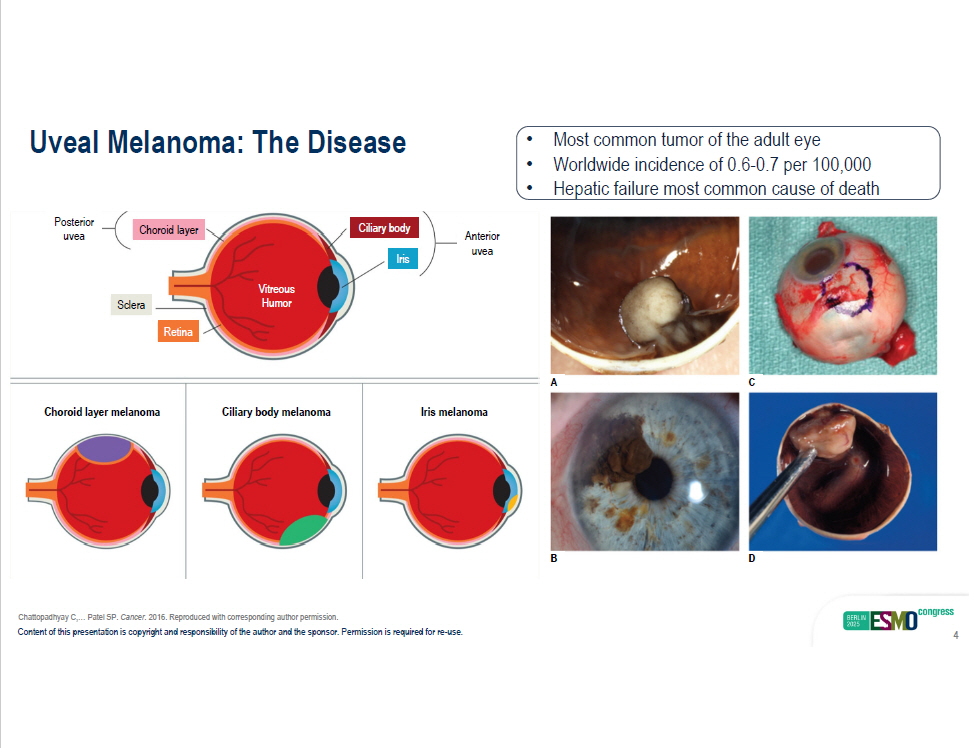
A C B D Chattopadhyay C,… Patel SP. Cancer . 2016. Reproduced with corresponding author permission. Uveal Melanoma: The Disease Posterior uvea Anterior uvea Ciliary body Choroid layer Iris Retina Sclera Vitreous Humor Choroid layer melanoma Ciliary body melanoma Iris melanoma • Most common tumor of the adult eye • Worldwide incidence of 0.6 - 0.7 per 100,000 Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 4 • Hepatic failure most common cause of death
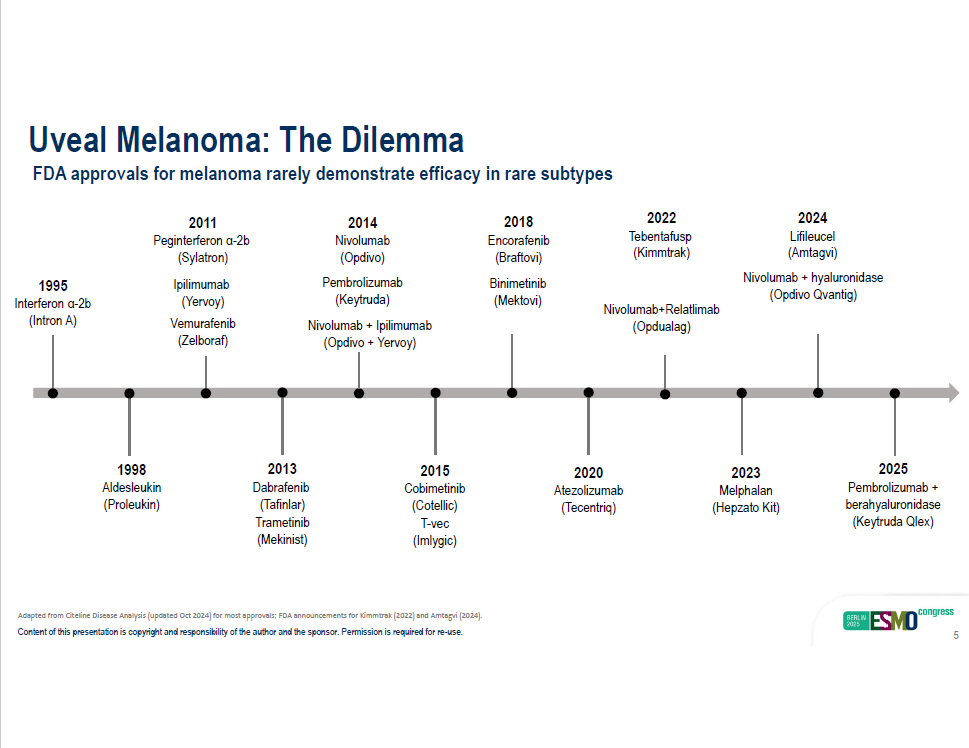
Uveal Melanoma: The Dilemma FDA approvals for melanoma rarely demonstrate efficacy in rare subtypes 1995 Interferon α - 2b (Intron A) 1998 Aldesleukin (Proleukin) 2015 Cobimetinib (Cotellic) T - vec (Imlygic) 2018 Encorafenib (Braftovi) Binimetin i b (Mektovi) 2020 Atezolizumab (Tecentriq) Nivolumab+Relatlimab (Opdualag) 2022 T ebentafusp (Kimmtrak) 2011 Peginterferon α - 2b (Sylatron) Ipilimumab (Yervoy) V e murafenib (Zelboraf) 2013 Dabrafenib (Tafinlar) T r ametinib (Mekinist) 2024 Lifileucel (Amtagvi) Nivolumab + hyaluronidase (Opdivo Qvantig) 2023 Melphalan (Hepzato Kit) 2025 Pembrolizumab + berahyaluronidase (Keytruda Qlex) 2014 Nivolumab (Opdivo) Pembrolizumab (Keytruda) Nivolumab + Ipilimumab (Opdivo + Yervoy) Adapted from Citeline Disease Analysis (updated Oct 2024) for most approvals; FDA announcements for Kimmtrak (2022) and Amtagvi (2024). Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 5
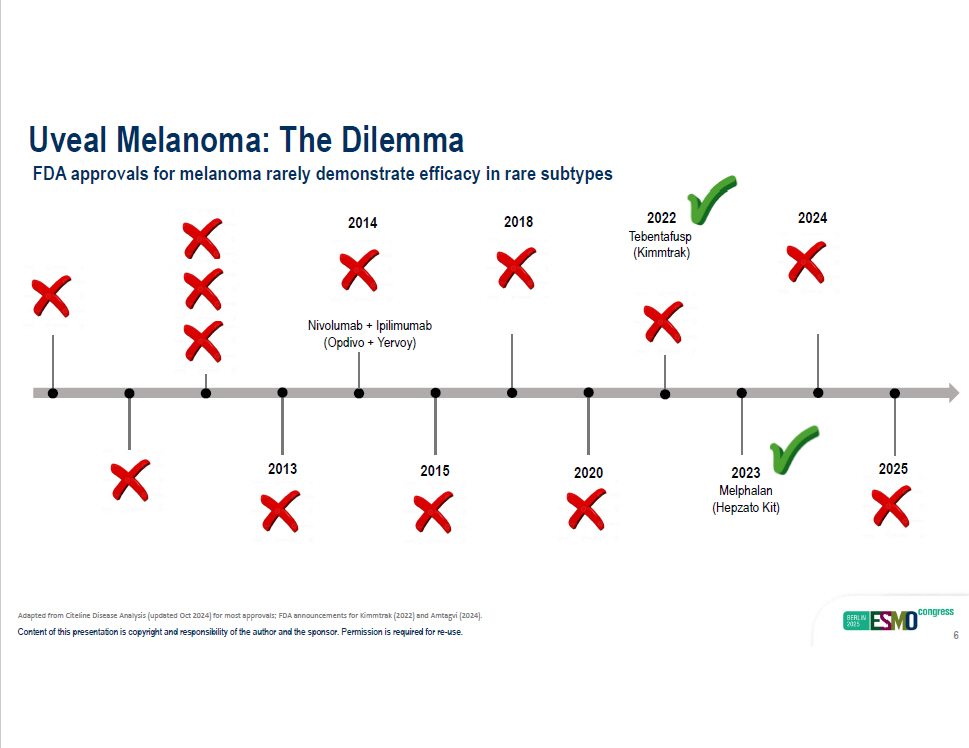
Uveal Melanoma: The Dilemma FDA approvals for melanoma rarely demonstrate efficacy in rare subtypes 2015 (Cotellic) 201 4 2018 2020 ( T ecentriq) 2022 T ebentafusp (Kimmtrak) 2013 2024 2023 Melphalan (Hepzato Kit) 2025 Nivolumab + Ipilimumab (Opdivo + Yervoy) Adapted from Citeline Disease Analysis (updated Oct 2024) for most approvals; FDA announcements for Kimmtrak (2022) and Amtagvi (2024). Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 6
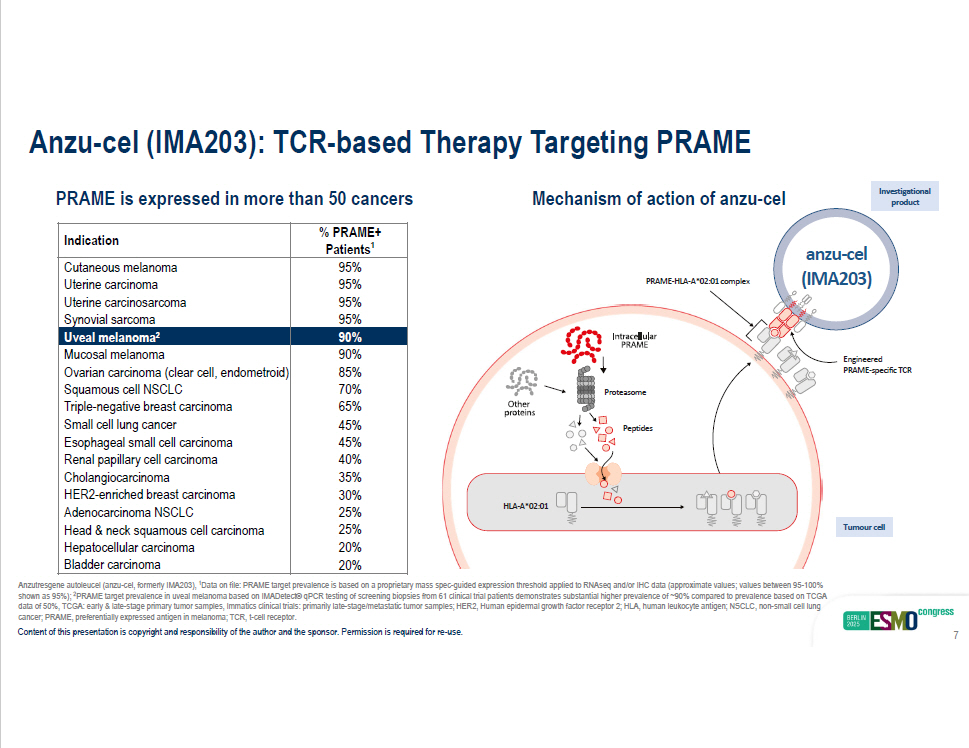
% PRAME+ Patients 1 Indication 95% Cutaneous melanoma 95% Uterine carcinoma 95% Uterine carcinosarcoma 95% Synovial sarcoma 90% Uveal melanoma 2 90% Mucosal melanoma 85% Ovarian carcinoma (clear cell, endometroid) 70% Squamous cell NSCLC 65% Triple - negative breast carcinoma 45% Small cell lung cancer 45% Esophageal small cell carcinoma 40% Renal papillary cell carcinoma 35% Cholangiocarcinoma 30% HER2 - enriched breast carcinoma 25% Adenocarcinoma NSCLC 25% Head & neck squamous cell carcinoma 20% Hepatocellular carcinoma 20% Bladder carcinoma PRAME is expressed in more than 50 cancers Mechanism of action of anzu - cel anzu - cel (IMA203) cancer; PRAME, preferentially expressed antigen in melanoma; TCR, t - cell receptor. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 7 PRAME - HLA - A*02:01 complex Proteasome Peptides HLA - A*02:01 Tumour cell Engineered PRAME - specific TCR Anzu - cel (IMA203): TCR - based Therapy Targeting PRAME Anzutresgene autoleucel (anzu - cel, formerly IMA203), 1 Data on file: PRAME target prevalence is based on a proprietary mass spec - guided expression threshold applied to RNAseq and/or IHC data (approximate values; values between 95 - 100% shown as 95%); 2 PRAME target prevalence in uveal melanoma based on IMADetect® qPCR testing of screening biopsies from 61 clinical trial patie nts demonstrates substantial higher prevalence of ~90% compared to prevalence based on TCGA data of 50%, TCGA: early & late - stage primary tumor samples, Immatics clinical trials: primarily late - stage/metastatic tumor sam ples; HER2, Human epidermal growth factor receptor 2; HLA, human leukocyte antigen; NSCLC, non - small cell lung I nv esti g ati ona l product
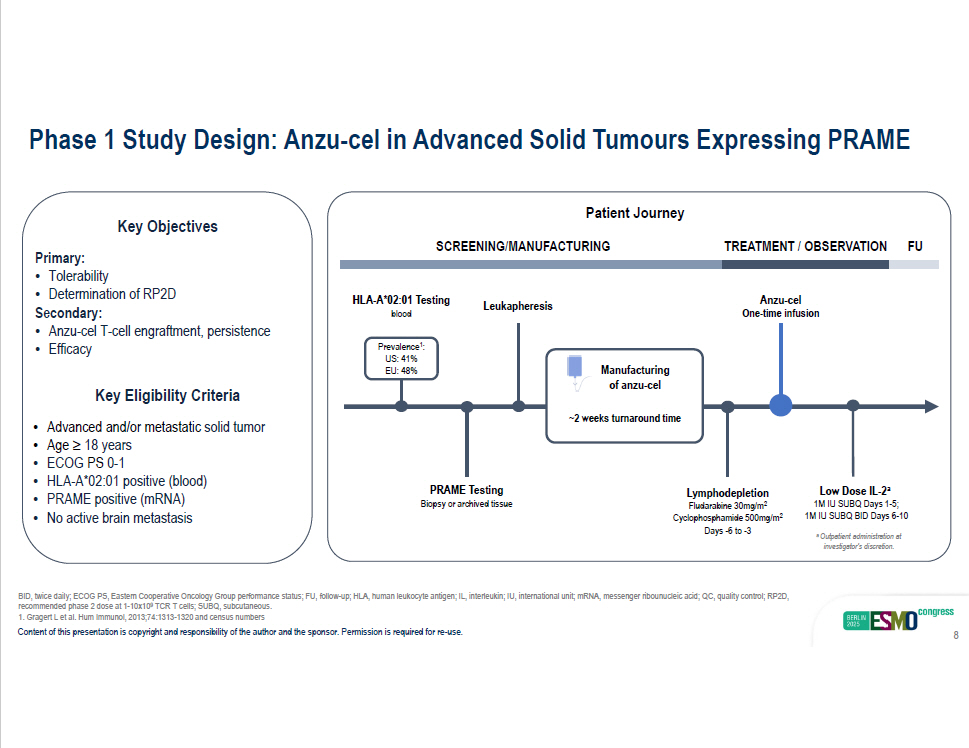
BID, twice daily; ECOG PS, Eastern Cooperative Oncology Group performance status; FU, follow - up; HLA, human leukocyte antigen; I L, interleukin; IU, international unit; mRNA, messenger ribounucleic acid; QC, quality control; RP2D, recommended phase 2 dose at 1 - 10x10 9 TCR T cells; SUBQ, subcutaneous. Phase 1 Study Design: Anzu - cel in Advanced Solid Tumours Expressing PRAME Key Objectives Primary: • Tolerability • Determination of RP2D Secondary: • Anzu - cel T - cell engraftment, persistence • Efficacy Key Eligibility Criteria • Advanced and/or metastatic solid tumor • Age ≥ 18 years • ECOG PS 0 - 1 • HLA - A*02:01 positive (blood) • PRAME positive (mRNA) • No active brain metastasis SCREENING/MANUFACTURING Leukapheresis Anzu - cel One - time infusion Lymphodepletion Fludarabine 30mg/m 2 Cyclophosphamide 500mg/m 2 Days - 6 to - 3 PRAME Testing Biopsy or archived tissue TRE A TME N T / OBSE R V A T ION FU HLA - A*02:01 Testing blood Prevale n c e 1 : US: 41% EU: 48% Manufacturing of anzu - cel ~2 weeks turnaround time Patient Journey Low Dose IL - 2 a 1M IU SUBQ Days 1 - 5; 1M IU SUBQ BID Days 6 - 10 a Outpatient administration at investigator’s discretion. 1. Gragert L et al. Hum Immunol, 2013;74:1313 - 1320 and census numbers Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 8
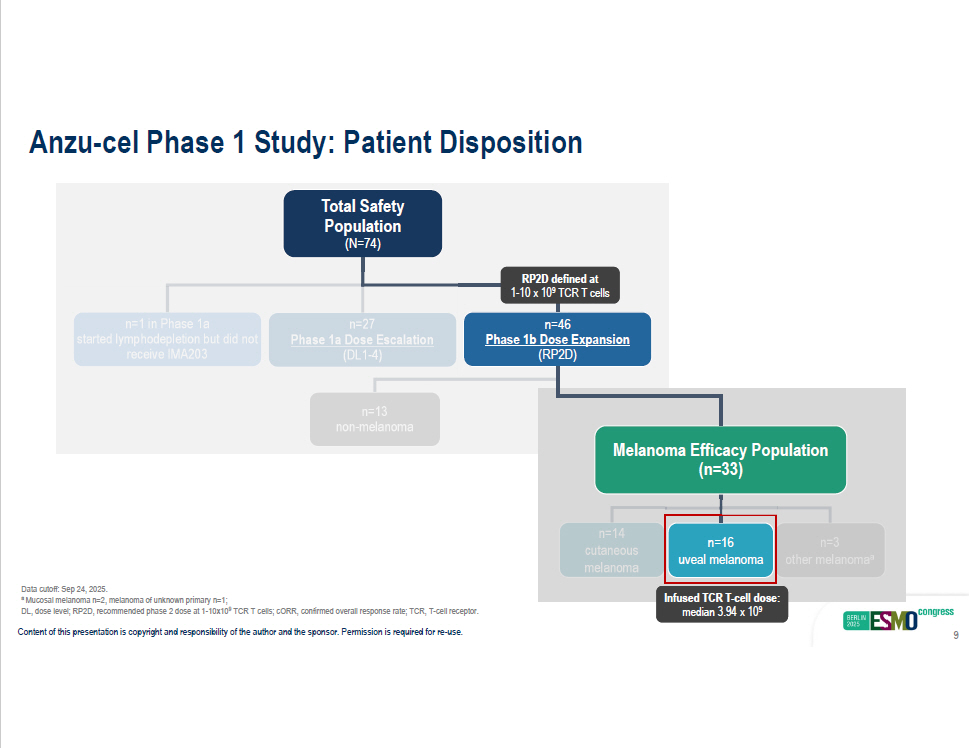
Data cutoff: Sep 24, 2025. a Mucosal melanoma n=2, melanoma of unknown primary n=1; DL, dose level; RP2D, recommended phase 2 dose at 1 - 10x10 9 TCR T cells; cORR, confirmed overall response rate; TCR, T - cell receptor. Total Safety Population (N=74) n=13 non - melanoma n=1 in Phase 1a n=27 started lymphodepletion but did not Phase 1a Dose Escalation receive IMA203 (DL1 - 4) n=3 other melanoma a n=14 cutaneous melanoma n=16 uveal melanoma Melanom a Efficacy Population (n=33) Infused TCR T - cell dose: median 3.94 x 10 9 RP2D defined at 1 - 10 x 10 9 TCR T cells n=46 Phase 1b Dose Expansion (RP2D) Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 9 Anzu - cel Phase 1 Study: Patient Disposition
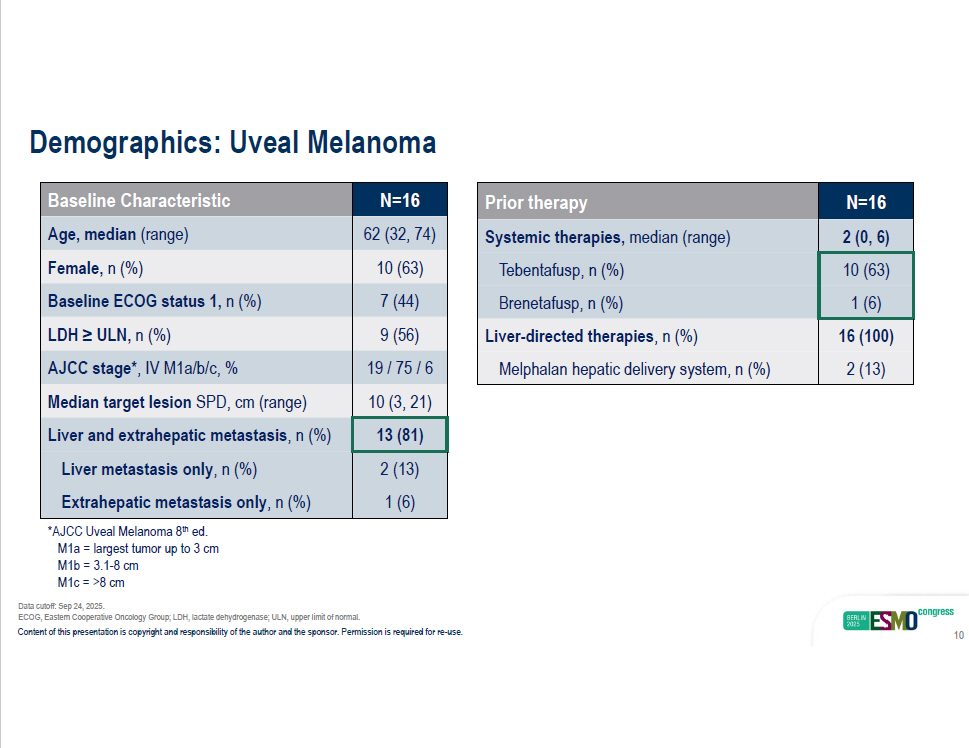
N=16 Baseline Characteristic 62 (32, 74) Age, median (range) 10 (63) Female, n (%) 7 (44) Baseline ECOG status 1, n (%) 9 (56) LDH ≥ ULN, n (%) 19 / 75 / 6 AJCC stage* , IV M1a/b/c, % 10 (3, 21) Median target lesion SPD, cm (range) 13 (81) Liver and extrahepatic metastasis , n (%) 2 (13) Liver metastasis only , n (%) 1 (6) Extrahepatic metastasis only , n (%) N=16 Prior therapy 2 (0, 6) Systemic therapies, median (range) 10 (63) Tebentafusp, n (%) 1 (6) Brenetafusp, n (%) 16 (100) Liver - directed therapies , n (%) 2 (13) Melphalan hepatic delivery system, n (%) ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 10 Demographics: Uveal Melanoma *AJCC Uveal Melanoma 8 th ed. M1a = largest tumor up to 3 cm M1b = 3.1 - 8 cm M1c = >8 cm Data cutoff: Sep 24, 2025.
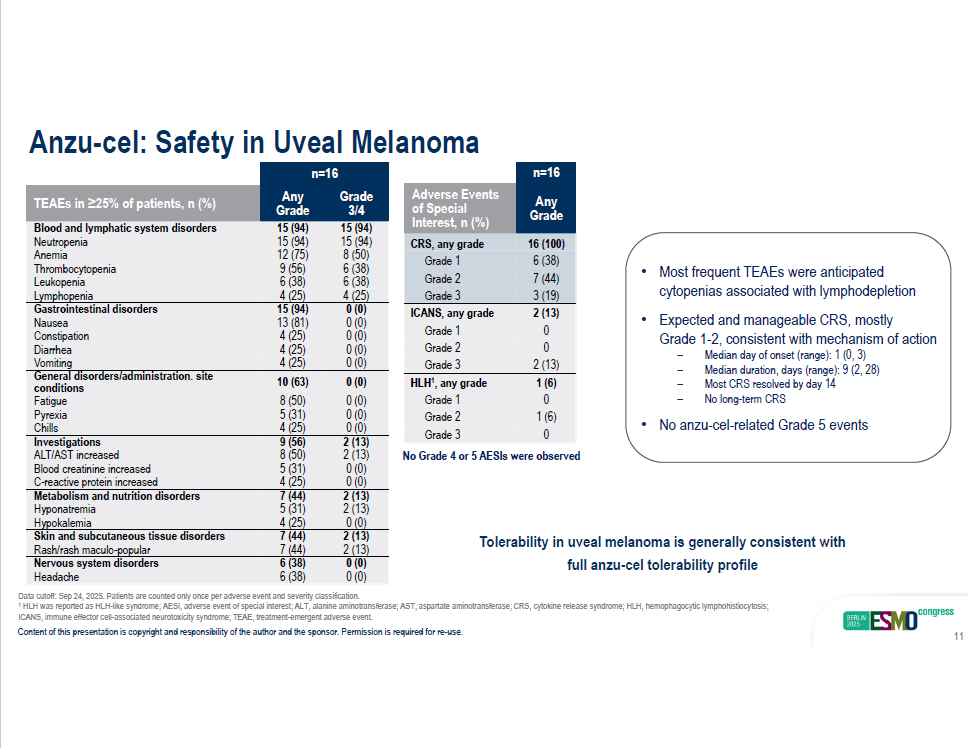
n=16 Grade Any 3/4 Grade TEAEs in ≥25% of patients, n (%) 15 (94) 15 (94) Blood and lymphatic system disorders 15 (94) 15 (94) Neutropenia 8 (50) 12 (75) Anemia 6 (38) 9 (56) Thrombocytopenia 6 (38) 6 (38) Leukopenia Lymphopenia 4 (25) 4 (25) 0 (0) 15 (94) Gastrointestinal disorders 0 (0) 13 (81) Nausea 0 (0) 4 (25) Constipation 0 (0) 4 (25) Diarrhea Chills 4 (25) 0 (0) 2 (13) 9 (56) Investigations 2 (13) 8 (50) ALT/AST increased 0 (0) 5 (31) Blood creatinine increased 0 (0) 4 (25) C - reactive protein increased 2 (13) 7 (44) Metabolism and nutrition disorders 2 (13) 5 (31) Hyponatremia 0 (0) 4 (25) Hypokalemia 2 (13) 7 (44) Skin and subcutaneous tissue disorders 2 (13) 7 (44) Rash/rash maculo - popular 0 (0) 6 (38) Nervous system disorders 0 (0) 6 (38) Headache Vomiting 4 (25) 0 (0) General disorders/administration. site 0 (0) 0 (0) 10 (63) 8 (50) conditions Fatigue Pyrexia 5 (31) 0 (0) Anzu - cel: Safety in Uveal Melanoma Data cutoff: Sep 24, 2025. Patients are counted only once per adverse event and severity classification. 1 HLH was reported as HLH - like syndrome; AESI, adverse event of special interest; ALT, alanine aminotransferase; AST, aspartate am inotransferase; CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistiocytosis; n=16 Any Grade Adverse Events of Special Interest, n (%) 16 (100) 6 (38) 7 (44) CRS, any grade Grade 1 Grade 2 Grade 3 3 (19) 2 (13) 0 0 ICANS, any grade Grade 1 Grade 2 Grade 3 2 (13) 1 (6) 0 1 (6) 0 HLH 1 , any grade Grade 1 Grade 2 Grade 3 • Most frequent TEAEs were anticipated cytopenias associated with lymphodepletion • Expected and manageable CRS, mostly Grade 1 - 2, consistent with mechanism of action ICANS, immune effector cell - associated neurotoxicity syndrome; TEAE, treatment - emergent adverse event. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 11 – Median day of onset (range): 1 (0, 3) – Median duration, days (range): 9 (2, 28) – Most CRS resolved by day 14 – No long - term CRS • No anzu - cel - related Grade 5 events Tolerability in uveal melanoma is generally consistent with full anzu - cel tolerability profile No Grade 4 or 5 AESIs were observed
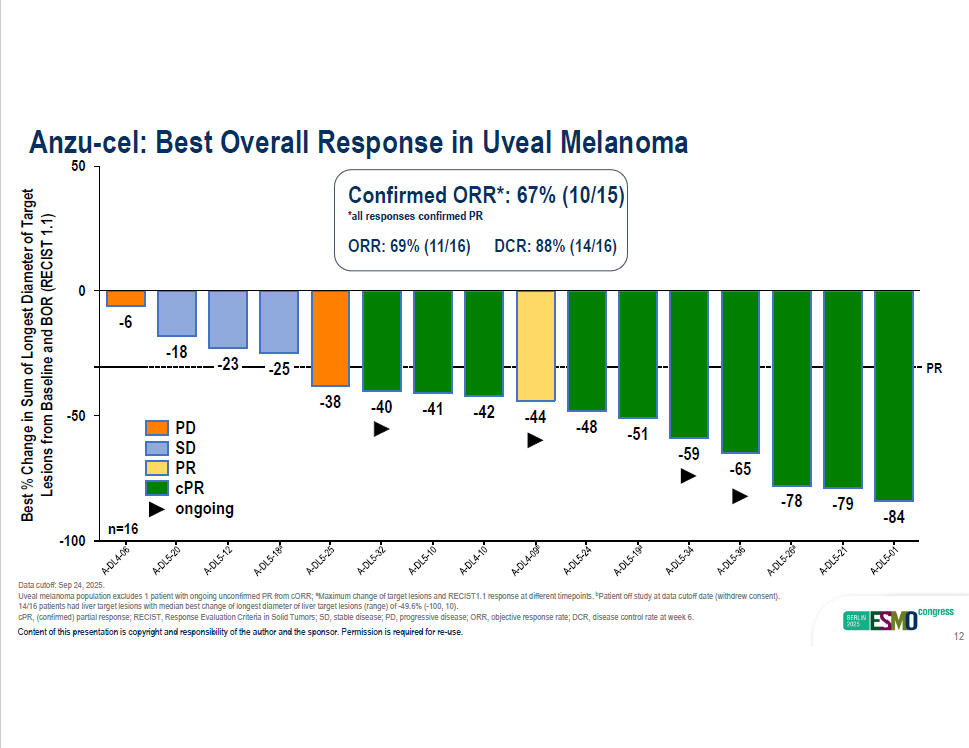
- 18 - 23 - 25 - 38 - 40 - 41 - 42 - 44 - 48 - 51 - 59 - 65 - 78 - 79 - 84 - 100 - 50 0 - 6 50 PD SD PR cPR ongoing Anzu - cel: Best Overall Response in Uveal Melanoma Best % Change in Sum of Longest Diameter of Target Lesions from Baseline and BOR (RECIST 1.1) Confirmed ORR*: 67% (10/15) *all responses confirmed PR ORR: 69% (11/16) DCR: 88% (14/16) PR n=16 Data cutoff: Sep 24, 2025. Uveal melanoma population excludes 1 patient with ongoing unconfirmed PR from cORR; a Maximum change of target lesions and RECIST1.1 response at different timepoints. b Patient off study at data cutoff date (withdrew consent). 14/16 patients had liver target lesions with median best change of longest diameter of liver target lesions (range) of - 49.6% ( - 100, 10). cPR, (confirmed) partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; PD, progressive dis ease; ORR, objective response rate; DCR, disease control rate at week 6. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 12
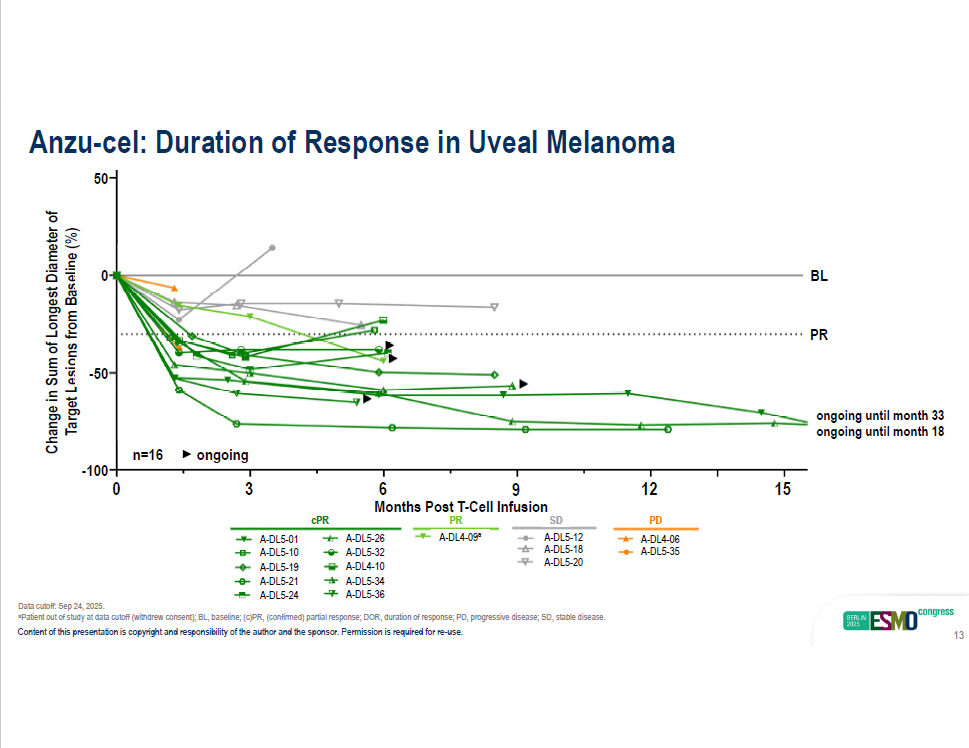
Months Post T - Cell Infusion Change in Sum of Longest Diameter of TargeLt esi on s fro m B as eli ne (%) BL PR n=16 Anzu - cel: Duration of Response in Uveal Melanoma cPR A - DL5 - 01 A - DL5 - 10 A - DL5 - 19 A - DL5 - 21 A - DL5 - 26 A - DL5 - 32 A - DL4 - 10 A - DL5 - 34 A - DL4 - 09 a A - DL5 - 12 A - DL5 - 18 A - DL5 - 20 A - DL4 - 06 A - DL5 - 35 PR SD PD ongoing - 50 0 50 0 3 12 6 9 15 - 100 A - DL5 - 24 A - DL5 - 36 Data cutoff: Sep 24, 2025. a Patient out of study at data cutoff (withdrew consent); BL, baseline; (c)PR, (confirmed) partial response; DOR, duration of response; PD, progressive disease; SD, stable disease. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 13 ongoing until month 33 ongoing until month 18
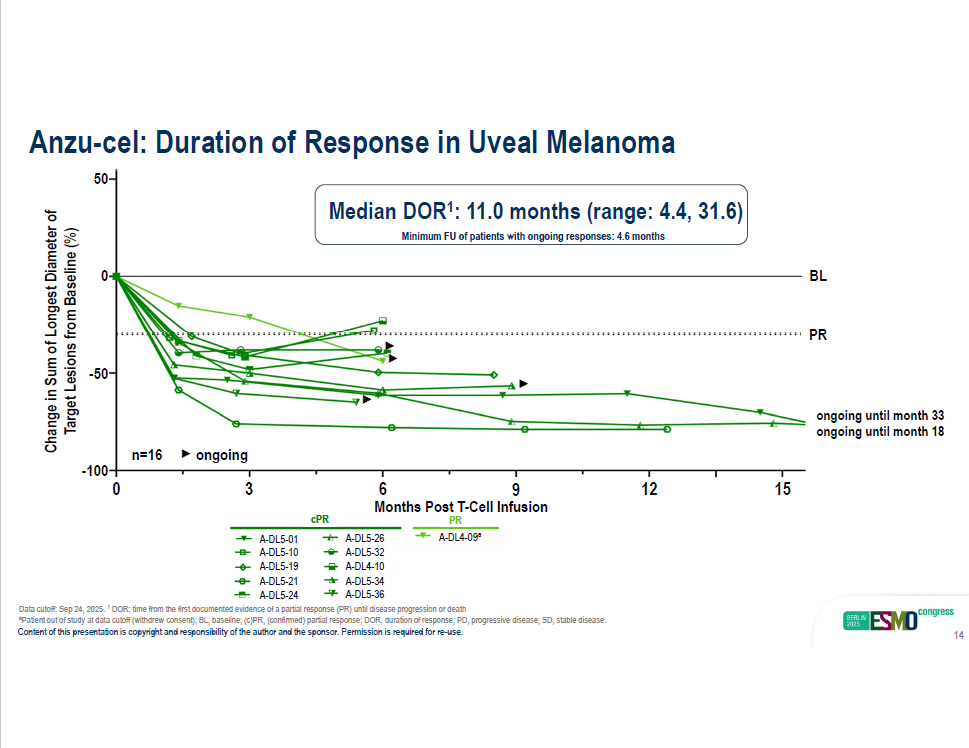
Months Post T - Cell Infusion - 50 0 50 Change in Sum of Longest Diameter of Target Lesions from Baseline (%) 0 3 12 6 9 15 BL PR Anzu - cel: Duration of Response in Uveal Melanoma - 100 cPR A - DL5 - 01 A - DL5 - 10 A - DL5 - 19 A - DL5 - 21 A - DL5 - 26 A - DL5 - 32 A - DL4 - 10 A - DL5 - 34 A - DL4 - 09 a PR n=16 ongoing ongoing until month 33 ongoing until month 18 Median DOR 1 : 11.0 months (range: 4.4, 31.6) Minimum FU of patients with ongoing responses: 4.6 months A - DL5 - 24 A - DL5 - 36 Data cutoff: Sep 24, 2025. 1 DOR: time from the first documented evidence of a partial response (PR) until disease progression or death a Patient out of study at data cutoff (withdrew consent); BL, baseline; (c)PR, (confirmed) partial response; DOR, duration of response; PD, progressive disease; SD, stable disease. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 14
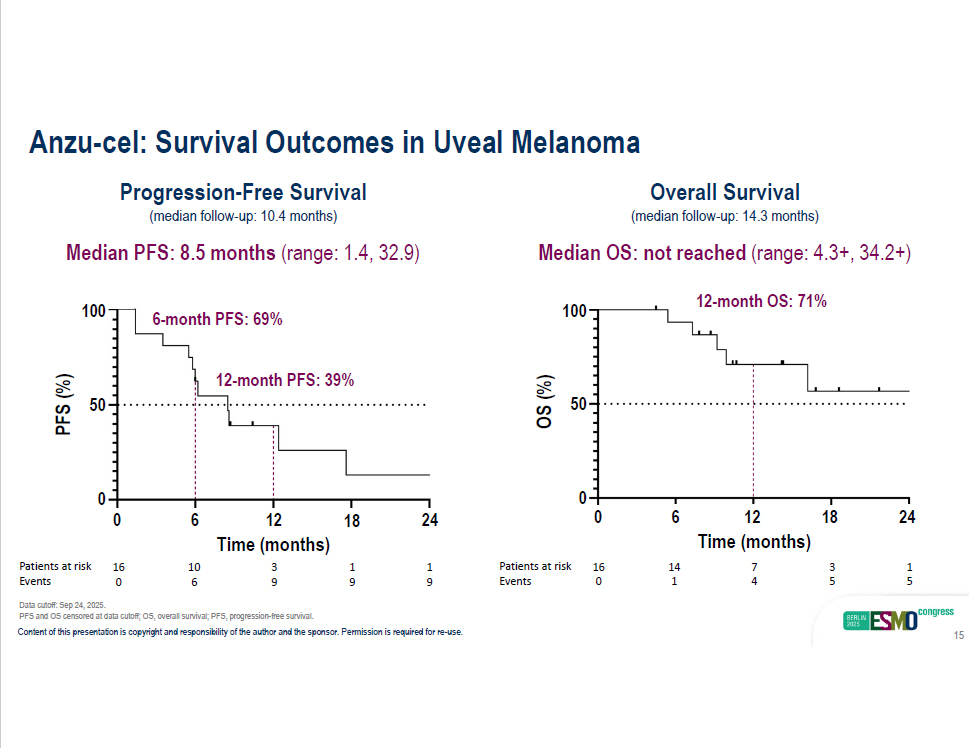
Anzu - cel: Survival Outcomes in Uveal Melanoma Progression - Free Survival (median follow - up: 10.4 months) Median PFS: 8.5 months (range: 1.4, 32.9) 12 - month PFS: 39% Overall Survival (median follow - up: 14.3 months) Median OS: not reached (range: 4.3+, 34.2+) 12 - month OS: 71% 100 50 PFS (%) 100 50 OS (%) 0 0 2 4 0 6 1 2 1 8 24 18 12 6 0 Time (months) Time (months) 1 3 7 14 16 Patients at risk 1 1 3 10 16 Patients at risk 5 5 4 1 0 Events 9 9 9 6 0 Events 6 - month PFS: 69% Data cutoff: Sep 24, 2025. PFS and OS censored at data cutoff; OS, overall survival; PFS, progression - free survival. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 15
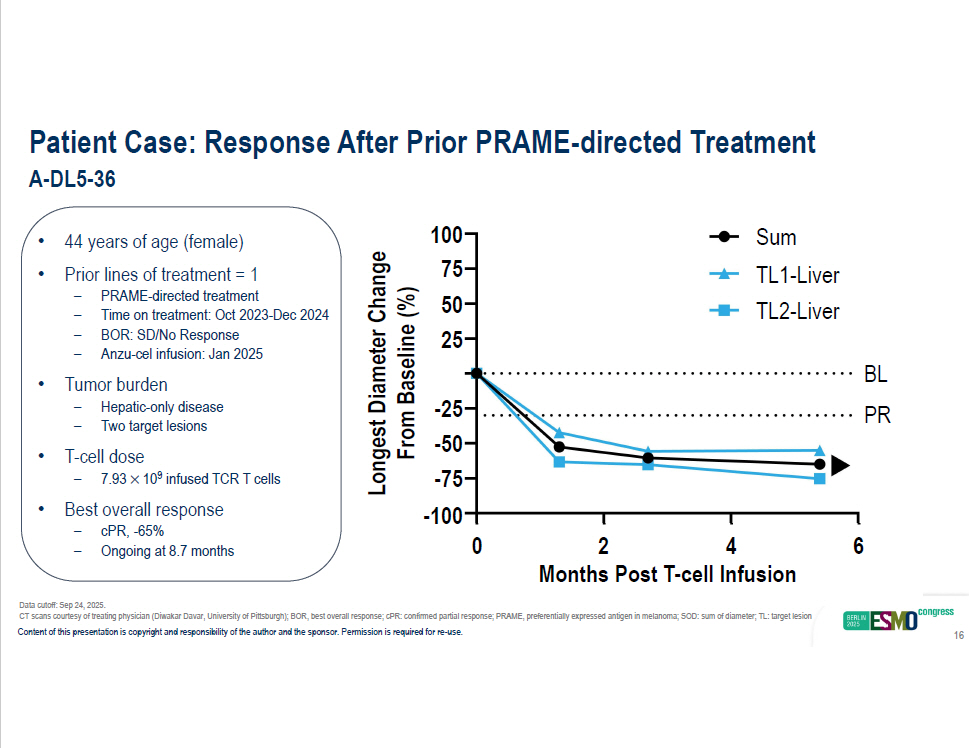
Patient Case: Response After Prior PRAME - directed Treatment A - DL5 - 36 • 44 years of age (female) • Prior lines of treatment = 1 – PRAME - directed treatment – Time on treatment: Oct 2023 - Dec 2024 – BOR: SD/No Response – Anzu - cel infusion: Jan 2025 • Tumor burden – Hepatic - only disease – Two target lesions • T - cell dose – 7.93 10 9 infused TCR T cells • Best overall response – cPR, - 65% – Ongoing at 8.7 months 0 6 100 75 50 25 - 25 - 50 - 75 - 100 2 4 Months Post T - cell Infusion Longest Diameter Change From Baseline (%) BL PR Sum TL1 - Liver TL2 - Liver Data cutoff: Sep 24, 2025. CT scans courtesy of treating physician (Diwakar Davar, University of Pittsburgh); BOR, best overall response; cPR: confirmed pa rti al response; PRAME, preferentially expressed antigen in melanoma; SOD: sum of diameter; TL: target lesion Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 16

• Anzu - cel tolerability profile: – Most frequent TEAEs: anticipated cytopaenias – CRS was mostly grade 1 - 2 • One - time infusion of anzu - cel in this cohort shows high response rates to immunotherapy for patients with metastatic uveal melanoma: – 67% cORR and 11.0 months mDOR – mPFS: 8.5 months (mFU: 10.4 months) and mOS not reached (mFU: 14.3 months) • Antitumor activity of anzu - cel was observed in patients with prior TCR - based or PRAME - targeted therapies mPFS, median progression - free survival; mOS, median overall survival. Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 17 Conclusions Data cutoff: Sep 24, 2025. cORR, confirmed objective response rate; CRS, cytokine release syndrome; ICANS, Immune effector cell - associated neurotoxicity syndrome; mDOR, median duration of response; mFU, median follow - up;
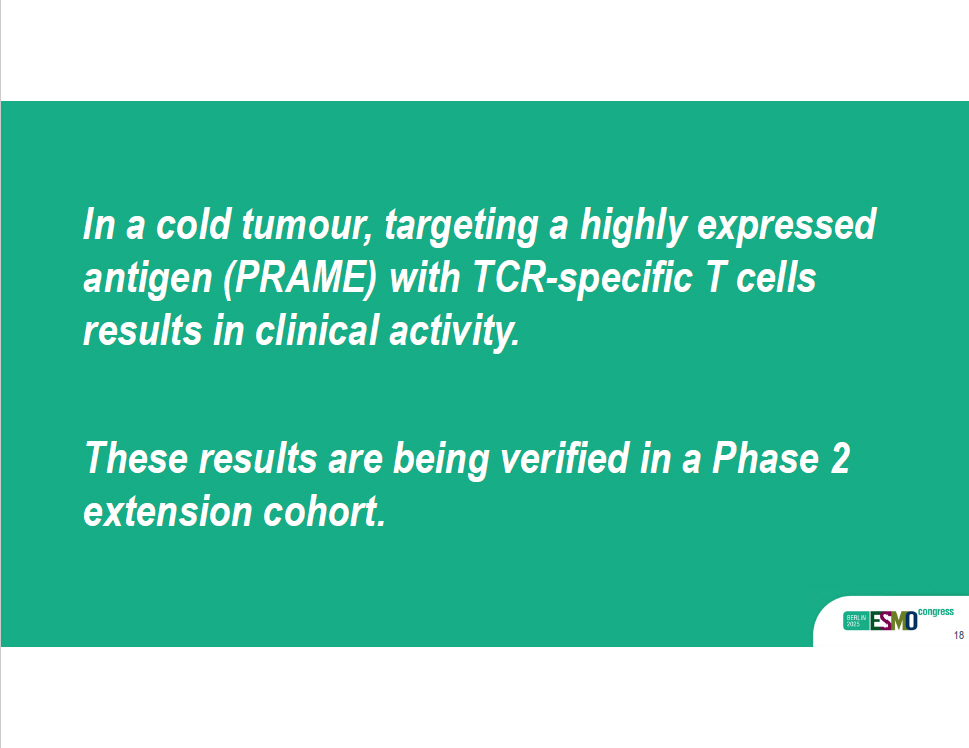
In a cold tumour, targeting a highly expressed antigen (PRAME) with TCR - specific T cells results in clinical activity. These results are being verified in a Phase 2 extension cohort. 18

Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. Thank You – Study Participants & Caregivers Anzu - cel Phase 1 Study Sponsor: Immatics University of Colorado Cancer Center Aurora, CO, USA (current) University of Texas MD Anderson Cancer Center Houston, TX, USA (during study conduct) Sapna Patel University of Texas MD Anderson Cancer Center Houston, TX, USA Apostolia Tsimberidou Strand Therapeutics (current) University of Pittsburgh Medical Center Pittsburgh, PA, USA (during study conduct) Jason Luke University Medical Center Hamburg – Eppendorf Hamburg, Germany Winfried Alsdorf Charite University Medicine Berlin, Germany Antonia Busse University Hospital Dresden Dresden, Germany Martin Wermke University of Miami, Miller School of Medicine Miami, FL, USA Leo Hernandez - Aya 19 Clinical Trial Sites Copies of this presentation obtained through QR, AR and/or text key codes are for personal use only and may not be reprod u c ed w i t h o ut w ritt e n permission of the authors.

Supplemental Data Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 20
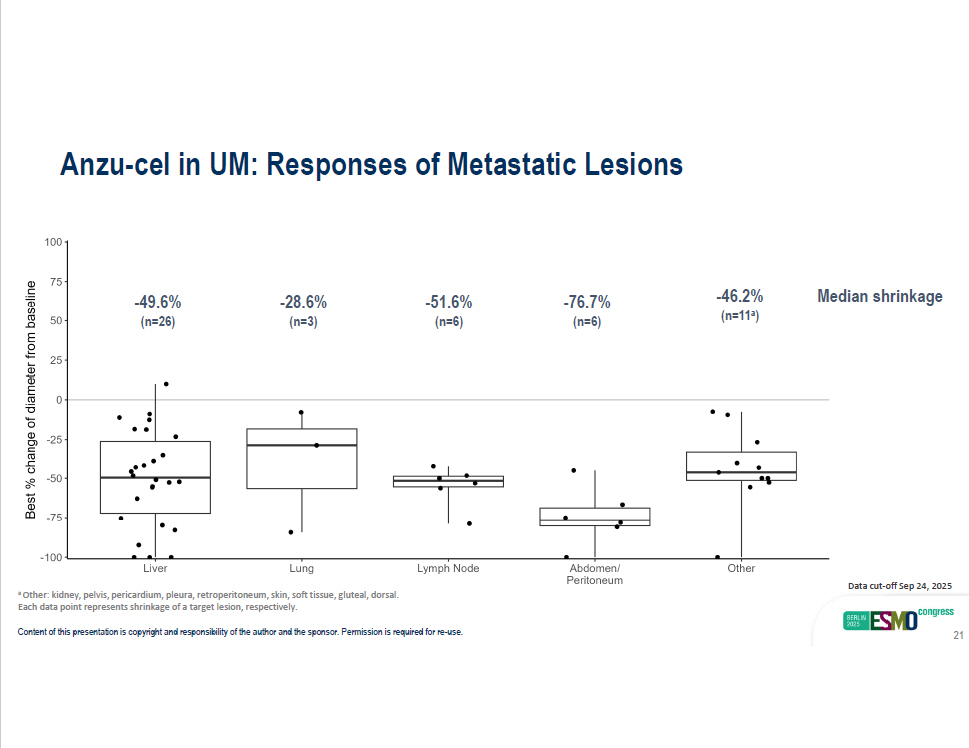
Anzu - cel in UM: Responses of Metastatic Lesions Median shrinkage Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. 21 - 49.6% (n=26) - 28.6% (n=3) - 51.6% (n=6) - 76.7% (n=6) - 46.2% (n=11 a ) Data cut - off Sep 24, 2025 a Other: kidney, pelvis, pericardium, pleura, retroperitoneum, skin, soft tissue, gluteal, dorsal. Each data point represents shrinkage of a target lesion, respectively.
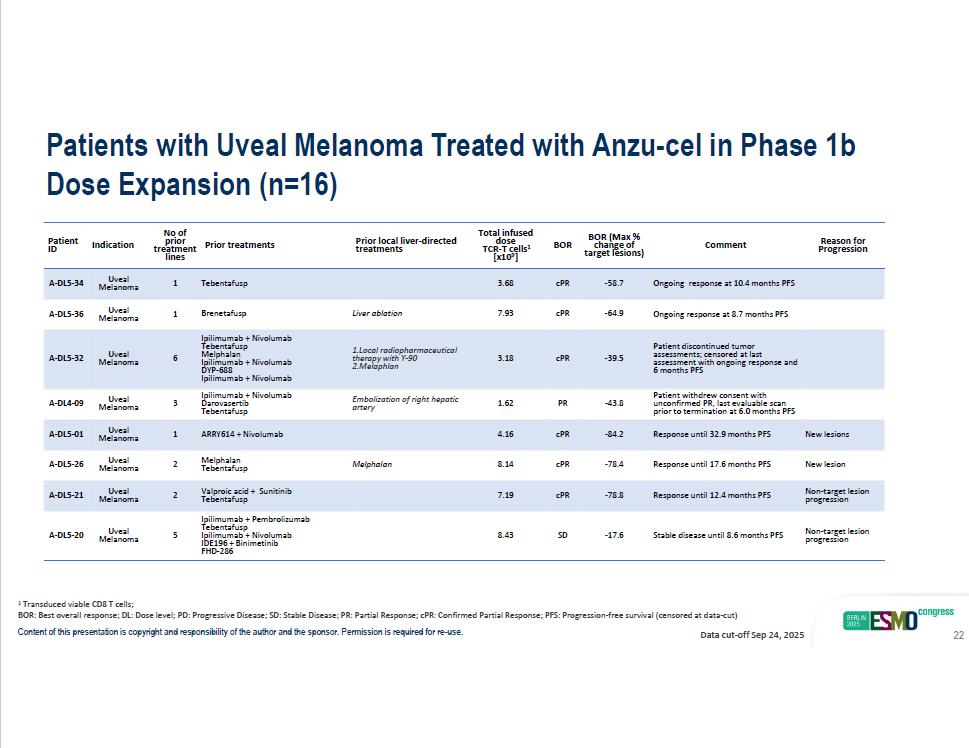
Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. Reason for Comment BOR (Max % change of BOR Total infused dose TCR - T cells 1 Prior local liver - directed treatments Prior treatments No of prior treatment rget lesions) Progression ta [x1 0 9 ] lines Ongoing response at 10.4 months PFS - 58.7 cPR 3.68 Tebentafusp A - DL5 - 34 Uveal 1 Ongoing response at 8.7 months PFS - 64.9 cPR 7.93 Liver ablation Brenetafusp Ipilimumab + Nivolumab A - DL5 - 36 Uveal 1 Indication Patient ID Melanoma Melanoma Patient discontinued tumor assessments; censored at last assessment with ongoing response and 6 months PFS - 39.5 cPR 3.18 1.Local radiopharmaceutical therapy with Y - 90 2.Melaphlan 6 Uveal Melanoma A - DL5 - 32 Patient withdrew consent with unconfirmed PR, last evaluable scan prior to termination at 6.0 months PFS - 43.8 PR 1.62 Embolization of right hepatic artery Tebentafu sp Melphalan Ipilimumab + Nivolumab DYP - 688 Ipilimumab + Nivolumab Ipilimumab + Nivolumab Darovasertib Tebentafusp 3 Uveal Melanoma A - DL4 - 09 New lesions Response until 32.9 months PFS - 84.2 cPR 4.16 ARRY614 + Nivolumab 1 Uveal Melanoma A - DL5 - 01 New lesion Response until 17.6 months PFS - 78.4 cPR 8.14 Me l pha l an Melphalan Tebentafu sp 2 Uveal Melanoma A - DL5 - 26 Non - target lesion progression Response until 12.4 months PFS - 78.8 cPR 7.19 Valproic acid + Sunitinib Tebentafusp 2 Uveal Melanoma A - DL5 - 21 Non - target lesion progression Stable disease until 8.6 months PFS - 17.6 SD 8.43 Ipilimumab + Pembrolizumab Tebentafusp Ipilimumab + Nivolumab IDE196 + Binimetinib FHD - 286 5 Uveal Melanoma A - DL5 - 20 1 Transduced viable CD8 T cells; BOR: Best overall response; DL: Dose level; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; PFS: Progression - free survival (censored at data - cut) Data cut - off Sep 24, 2025 Patients with Uveal Melanoma Treated with Anzu - cel in Phase 1b Dose Expansion (n=16) 22
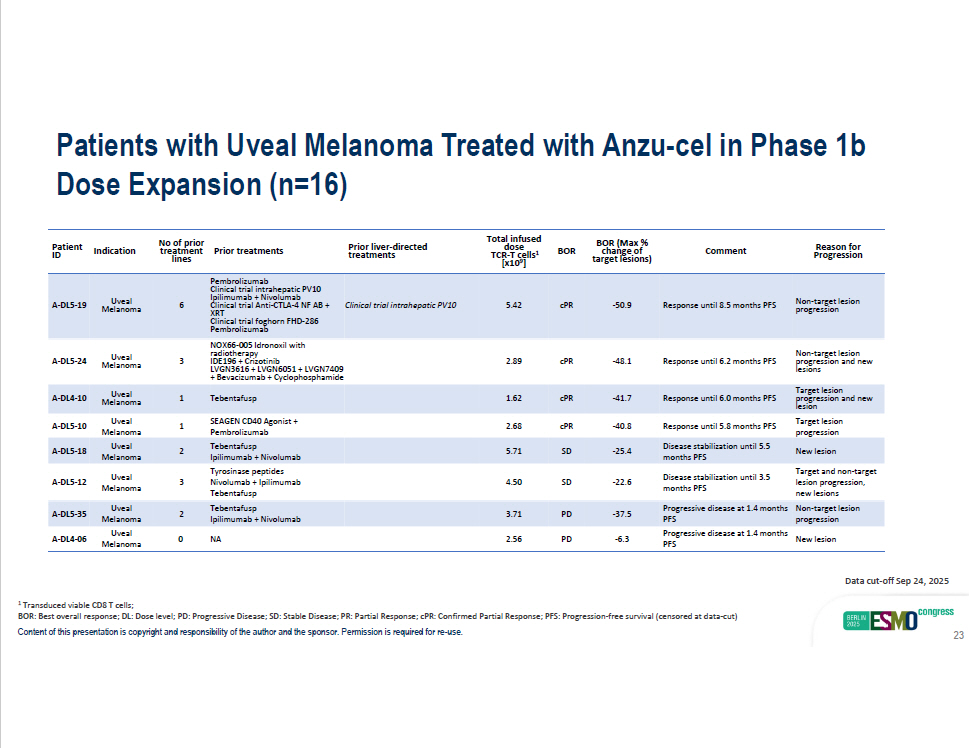
Patients with Uveal Melanoma Treated with Anzu - cel in Phase 1b Dose Expansion (n=16) - 37.5 Progressive disease at 1.4 months Non - target lesion PFS progression PD 3.71 Tebentafusp Ipilimumab + Nivolumab A - DL5 - 35 Uveal 2 Melanoma - 6.3 Progressive disease at 1.4 months New lesion PFS PD 2.56 NA A - DL4 - 06 Uveal 0 Melanoma Reason for Progression Comment BOR (Max % change of target lesions) BOR Total infused 1 dose TCR - T cells [x10 9 ] Prior liver - directed treatments Prior treatments No of prior treatment lines Indication Patient ID Non - target lesion progression Response until 8.5 months PFS - 50.9 cPR 5.42 Clinical trial intrahepatic PV10 6 Uveal Melanoma A - DL5 - 19 Non - target lesion progression and new lesions Response until 6.2 months PFS - 48.1 cPR 2.89 Pembrolizumab Clinical trial intrahepatic PV10 Ipilimumab + Nivolumab Clinical trial Anti - CTLA - 4 NF AB + XRT Clinical trial foghorn FHD - 286 Pembrolizumab NOX66 - 005 Idronoxil with radiotherapy IDE196 + Crizotinib LVGN3616 + LVGN6051 + LVGN7409 + Bevacizumab + Cyclophosphamide 3 Uveal Melanoma A - DL5 - 24 Response until 6.0 months PFS - 41.7 cPR 1.62 Tebentafusp 1 Uveal Melanoma A - DL4 - 10 Target lesion progression and new lesion Target lesion progression Response until 5.8 months PFS - 40.8 cPR 2.68 1 A - DL5 - 10 New lesion Disease stabilization until 5.5 months PFS - 25.4 SD 5.71 2 Uveal Melanoma Uveal Melanoma A - DL5 - 18 Target and non - target lesion progression, new lesions Disease stabilization until 3.5 months PFS - 22.6 SD 4.50 SEAGEN CD40 Agonist + Pembrolizumab Tebentafusp Ipilimumab + Nivolumab Tyrosinase peptides Nivolumab + Ipilimumab Tebentafusp 3 Uveal Melanoma A - DL5 - 12 1 Transduced viable CD8 T cells; BOR: Best overall response; DL: Dose level; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; PFS: Progression - free survival (censored at data - cut) Content of this presentation is copyright and responsibility of the author and the sponsor. Permission is required for re - use. Data cut - off Sep 24, 2025 23