
1 Corporate Presentation

2 Disclaimer and Other Information Forward-looking Statements This presentation includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. The actual results of the Company may differ from its expectations, estimates, and projections and, consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s expectations with respect to future performance and development and commercialization of products and services, its anticipated cash runway, anticipated data and product launches (including Proteus), investor confidence in Quantum-Si and our strategic roadmap, and any financial guidance. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from those discussed in the forward-looking statements. Most of these factors are outside the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: the inability to maintain the listing of the Company’s Class A common stock on The Nasdaq Stock Market; the ability of the Company to grow and manage growth and retain its key employees; the Company’s ongoing leadership transitions and succession planning; changes in applicable laws or regulations; the ability of the Company to raise financing in the future; the success, cost and timing of the Company’s product development and commercialization activities, including the use and benefit of artificial intelligence in these and other activities; the commercialization and adoption of the Company’s existing products and the success of any product the Company may offer in the future; the potential attributes and benefits of the Company’s commercialized Platinum protein sequencing instruments and kits and the Company’s other products (including Proteus) once commercialized; the Company’s ability to obtain and maintain regulatory approval for its products, and any related restrictions and limitations of any approved product; the Company’s ability to identify, in-license or acquire additional technology; the Company’s ability to maintain its existing lease, license, manufacture and supply agreements; the Company’s ability to compete with other companies currently marketing or engaged in the development or commercialization of products and services that serve customers engaged in proteomic analysis, many of which have greater financial and marketing resources than the Company; the size and growth potential of the markets for the Company’s products and services, and its ability to serve those markets once commercialized, either alone or in partnership with others; the Company’s estimates regarding future expenses, future revenue, capital requirements and needs for additional financing; the Company’s financial performance; the Company's defense and initiation of litigation matters; and other risks and uncertainties described under “Risk Factors” in the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q and in the Company’s other filings with the SEC. The Company cautions that the foregoing list of factors is not exclusive. The Company cautions readers not to place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions, or circumstances on which any such statement is based. Non-GAAP Financial Measures This presentation includes certain non-GAAP financial measures including “adjusted total operating expenses.” Please see .2 to the Company’s Current Report on Form 8-K filed with the SEC on November 5, 2025, for further discussion of the Company’s use of non-GAAP financial measures.

3 Quantum-Si Highlights Experienced team + board from leading life sciences companies Large and growing proteomics market opportunity valued at $75B+ Differentiated core technology, patent- protected, extensible to wide range of proteomics applications Partnerships in place spanning commercialization, product development, and manufacturing Well-capitalized with cash runway into Q2 2028 Proteus platform (est. YE26) designed to perform high-value applications not easily addressed with current technologies Global distribution network in place, covering all major markets Expanding user base and published data with first-to- market Platinum® Pro system

4 Proteins Are the Core of Biological Discoveries Across Many End Markets Academic Research Pharma + Biotech Industrial Defense Clinical Diagnostics Translational Research Agriculture
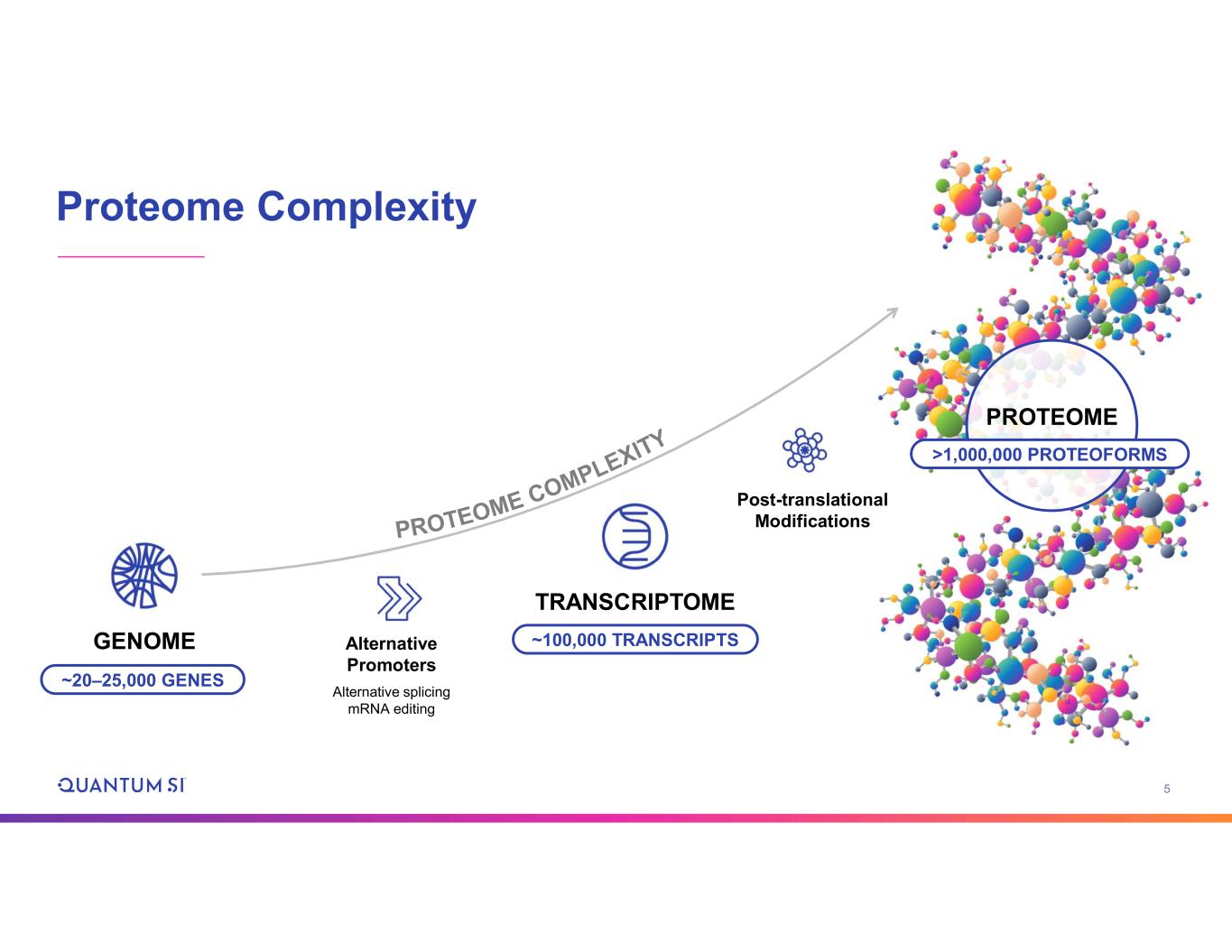
5 Proteome Complexity GENOME TRANSCRIPTOME Alternative Promoters Alternative splicing mRNA editing Post-translational Modifications ~20–25,000 GENES ~100,000 TRANSCRIPTS PROTEOME >1,000,000 PROTEOFORMS
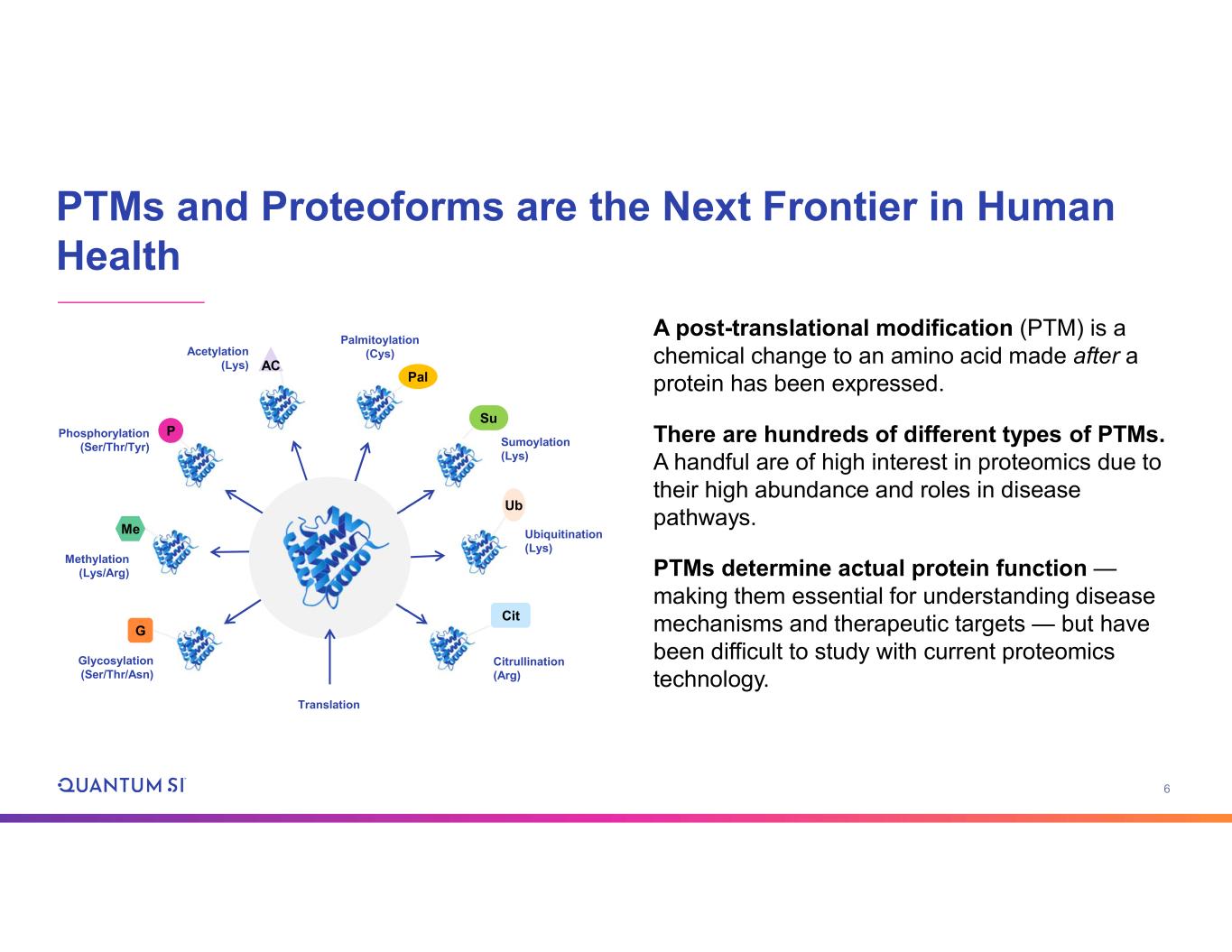
6 Su P G Me Methylation (Lys/Arg) Phosphorylation (Ser/Thr/Tyr) Palmitoylation (Cys) Sumoylation (Lys) Ubiquitination (Lys) Citrullination (Arg) AC Pal Ub Glycosylation (Ser/Thr/Asn) Acetylation (Lys) Translation Cit A post-translational modification (PTM) is a chemical change to an amino acid made after a protein has been expressed. There are hundreds of different types of PTMs. A handful are of high interest in proteomics due to their high abundance and roles in disease pathways. PTMs determine actual protein function — making them essential for understanding disease mechanisms and therapeutic targets — but have been difficult to study with current proteomics technology. PTMs and Proteoforms are the Next Frontier in Human Health
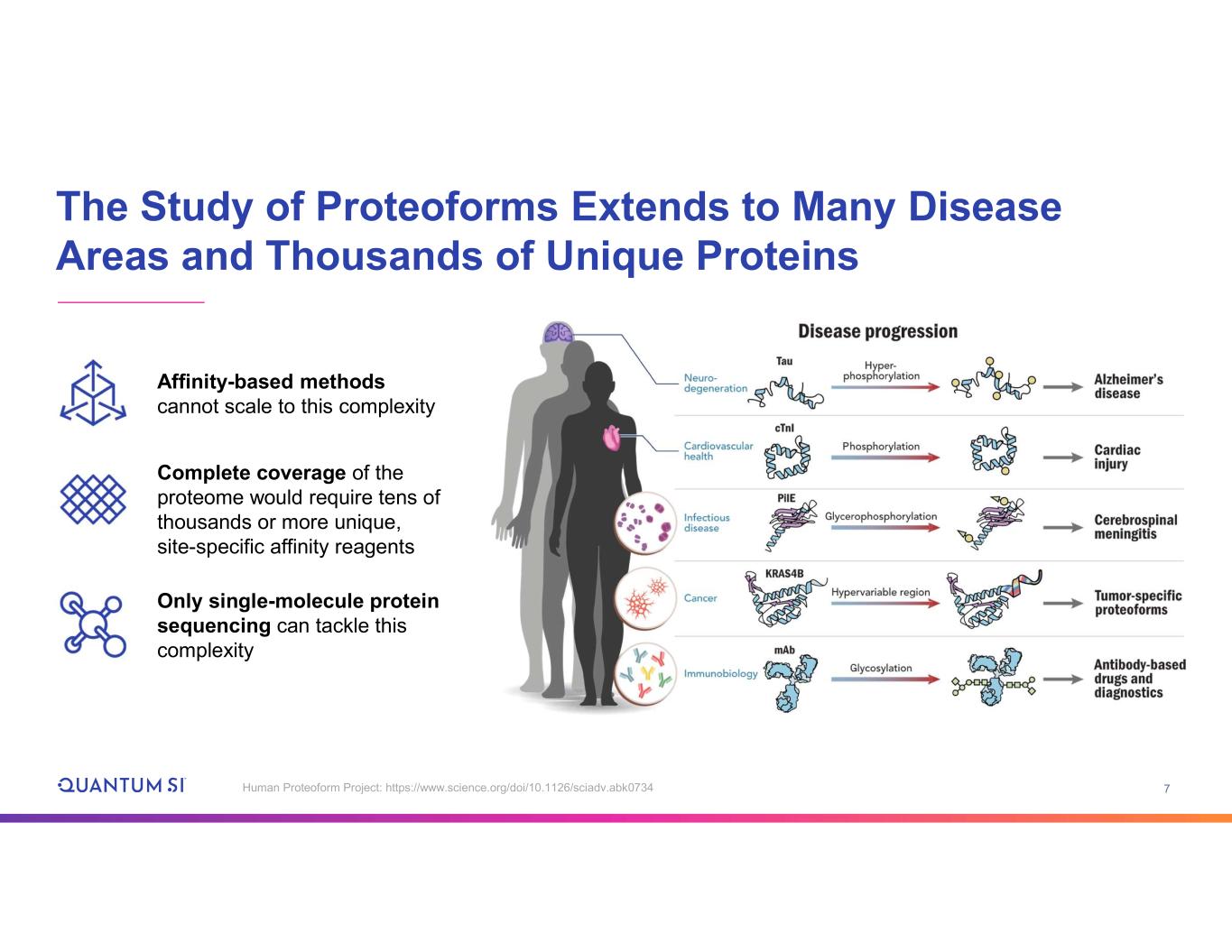
7 The Study of Proteoforms Extends to Many Disease Areas and Thousands of Unique Proteins Human Proteoform Project: https://www.science.org/doi/10.1126/sciadv.abk0734 Complete coverage of the proteome would require tens of thousands or more unique, site-specific affinity reagents Only single-molecule protein sequencing can tackle this complexity Affinity-based methods cannot scale to this complexity
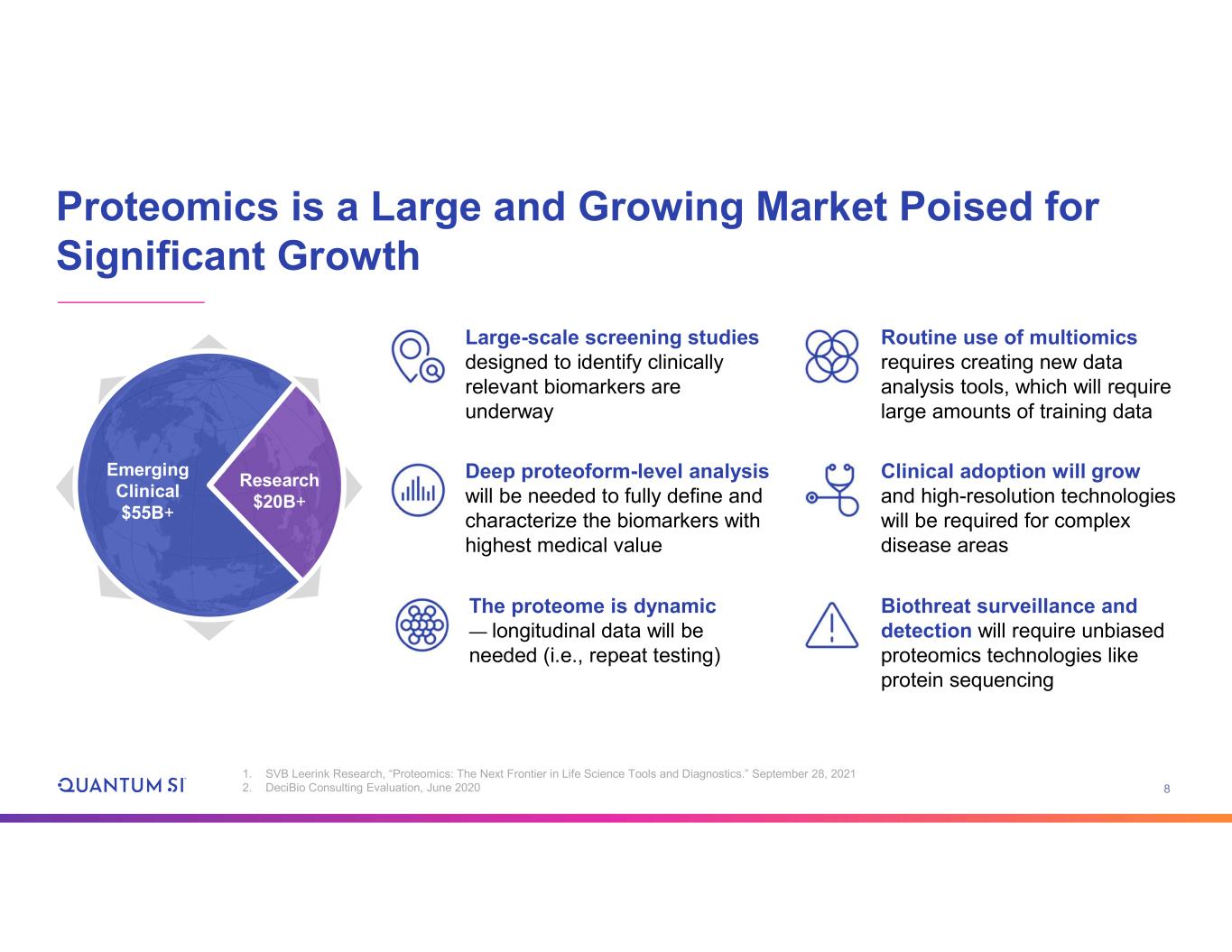
8 Proteomics is a Large and Growing Market Poised for Significant Growth Research $20B+ Emerging Clinical $55B+ Deep proteoform-level analysis will be needed to fully define and characterize the biomarkers with highest medical value Large-scale screening studies designed to identify clinically relevant biomarkers are underway Biothreat surveillance and detection will require unbiased proteomics technologies like protein sequencing Routine use of multiomics requires creating new data analysis tools, which will require large amounts of training data The proteome is dynamic — longitudinal data will be needed (i.e., repeat testing) Clinical adoption will grow and high-resolution technologies will be required for complex disease areas 1. SVB Leerink Research, “Proteomics: The Next Frontier in Life Science Tools and Diagnostics.” September 28, 2021 2. DeciBio Consulting Evaluation, June 2020

9 Specialized Platforms With High Capital Costs Limit Adoption Outside of Core Labs in Proteomics Many specialized platforms needed to fully interrogate the proteome Technical tradeoffs when selecting between the breadth of protein coverage and depth of insights High capital costs with the top-end instruments costing $1M or more each Manual laboratory and data analysis workflows limit the number of laboratories capable of performing proteomics
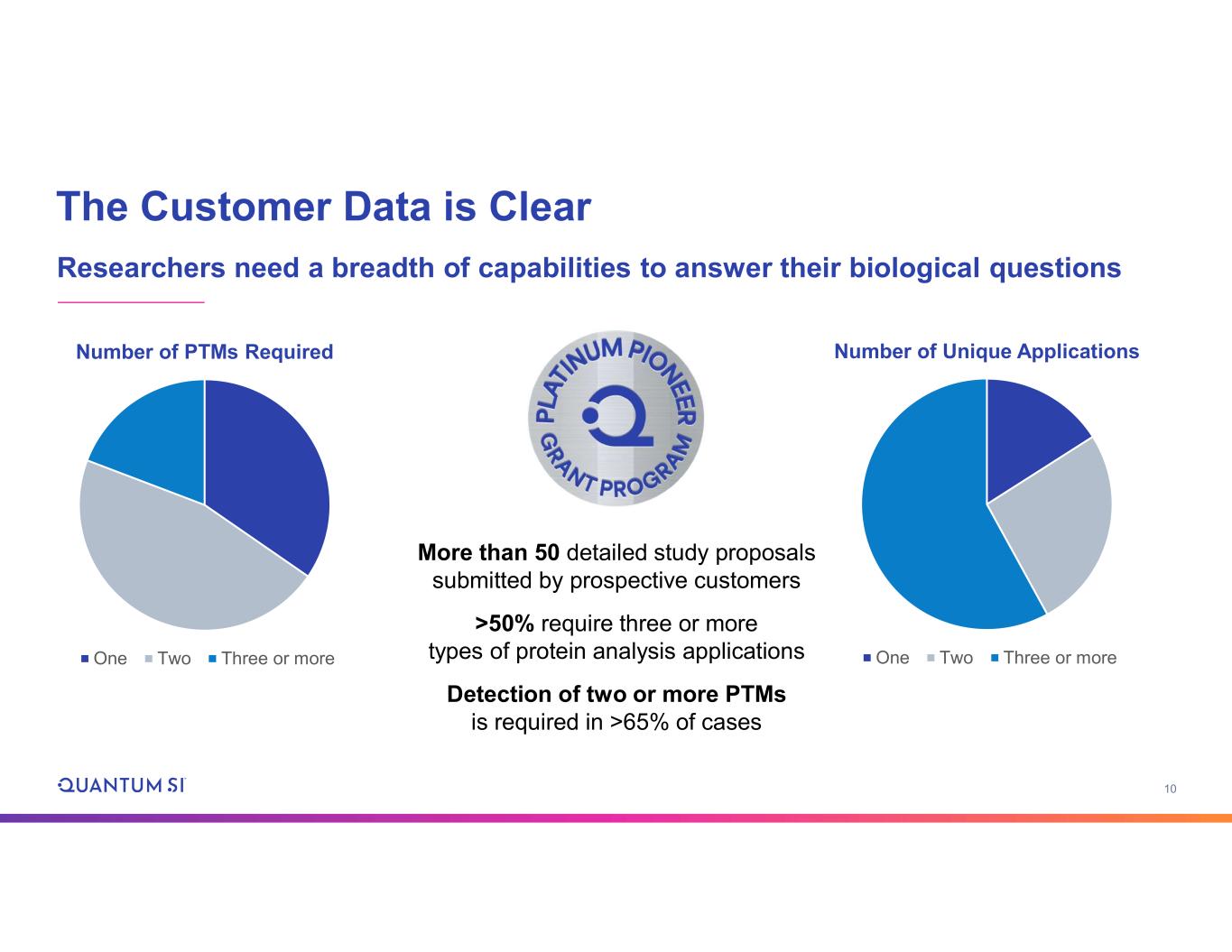
10 The Customer Data is Clear Researchers need a breadth of capabilities to answer their biological questions Number of Unique Applications One Two Three or more Number of PTMs Required One Two Three or more More than 50 detailed study proposals submitted by prospective customers >50% require three or more types of protein analysis applications Detection of two or more PTMs is required in >65% of cases

11 Moving Beyond the Core Lab: Proteus Will Enable Deep Protein Analysis and Accelerate the Field of Proteomics Automation simplifies laboratory workflow, increases throughput, and minimizes the need for specialized staff Affordable, allowing any lab — anywhere — to be a proteomics core lab Single-molecule protein sequencing will be the most versatile technology in proteomics — single amino acid variants, broad PTM coverage, and protein-agnostic
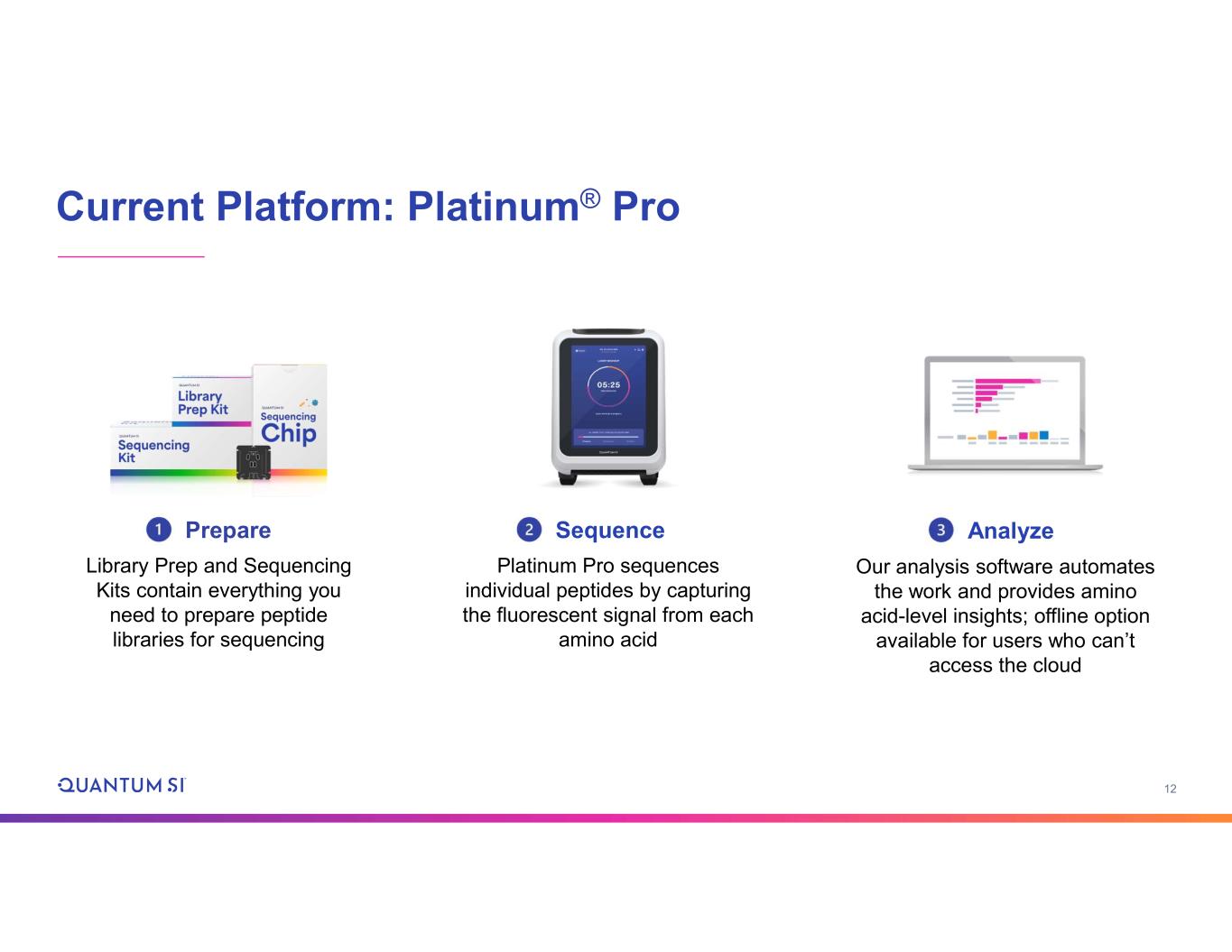
12 Current Platform: Platinum® Pro Platinum Pro sequences individual peptides by capturing the fluorescent signal from each amino acid Sequence Analyze Our analysis software automates the work and provides amino acid-level insights; offline option available for users who can’t access the cloud Library Prep and Sequencing Kits contain everything you need to prepare peptide libraries for sequencing Prepare

13 Leveraging Platinum Pro for Market Development and Commercial Infrastructure Build for Proteus Launch Global distribution network in place, covering all major markets Expanding user base across many market segments including academic, biopharma, defense, and agriculture Focusing on independent data generation to validate technology and deliver publications Collaborating with customers and corporate partners to develop and validate high-value applications
14 Publication Pipeline is Growing All publications Proteoforms relevant to biological interactions Peptide barcoding Single-molecule binding kinetics Single-amino acid variants associated with disease

15 Proteus On Track to Far Exceed Platinum Pro at Launch * Proteus is in development. Estimated launch specifications. Switch from semiconductor to optical architecture with patterned nanowell array Liquid handling automation dramatically simplifies workflow 40× more sequencing throughput per consumable relative to Platinum at initial launch* Run up to 4 samples in one sequencing run* Proteus development data is already superior to Platinum, with further improvements expected before launch

16 Proteus Next-generation Architecture Platinum® Pro + 2M Chip • Integrated semiconductor consumable with 2 million wells • Benchtop instrument with manual workflow Proteus + KinetIQ Array • Simple, passive consumable with estimated 80 million wells at launch • Benchtop instrument with imaging system • Superior sequencing performance • Workflow automation
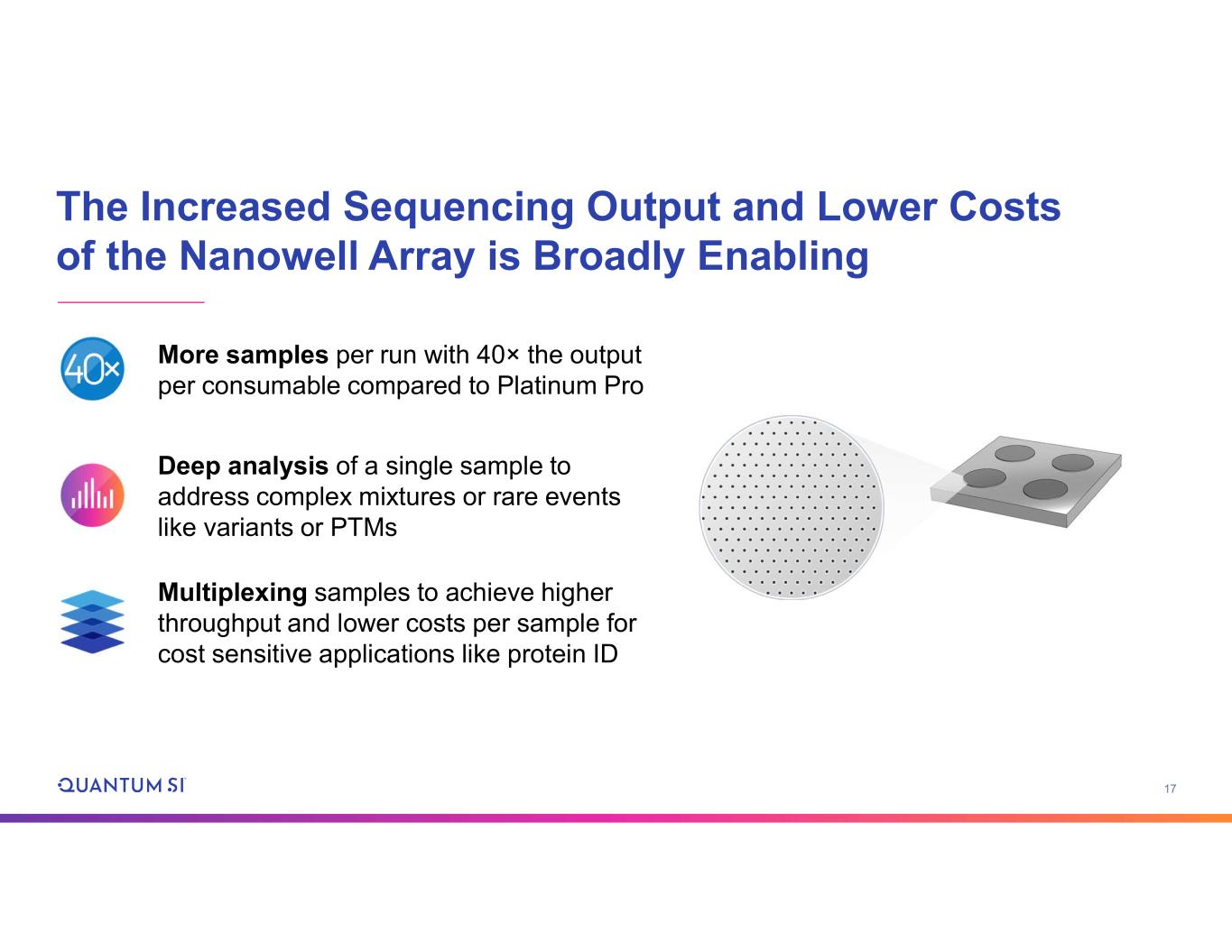
17 The Increased Sequencing Output and Lower Costs of the Nanowell Array is Broadly Enabling More samples per run with 40× the output per consumable compared to Platinum Pro Deep analysis of a single sample to address complex mixtures or rare events like variants or PTMs Multiplexing samples to achieve higher throughput and lower costs per sample for cost sensitive applications like protein ID
18 Accelerating the Core Sequencing Technology and Analytics to Unlock the Highest-value Applications Robust PTM detection and multi-PTM profiling Single-amino acid variant detection Sequencing of variable regions of antibodies Biothreat detection and surveillance
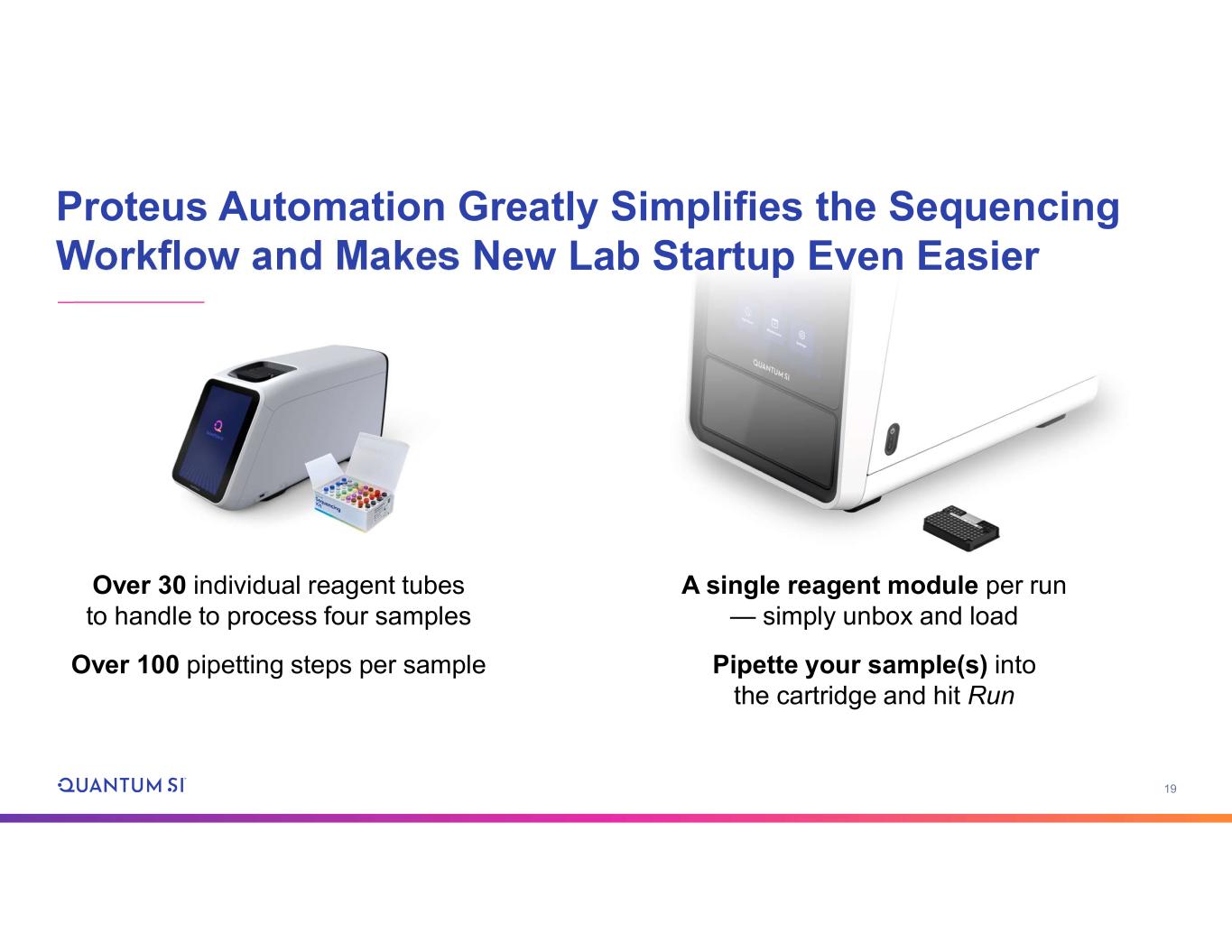
19 Proteus Automation Greatly Simplifies the Sequencing Workflow and Makes New Lab Startup Even Easier A single reagent module per run — simply unbox and load Pipette your sample(s) into the cartridge and hit Run Over 30 individual reagent tubes to handle to process four samples Over 100 pipetting steps per sample
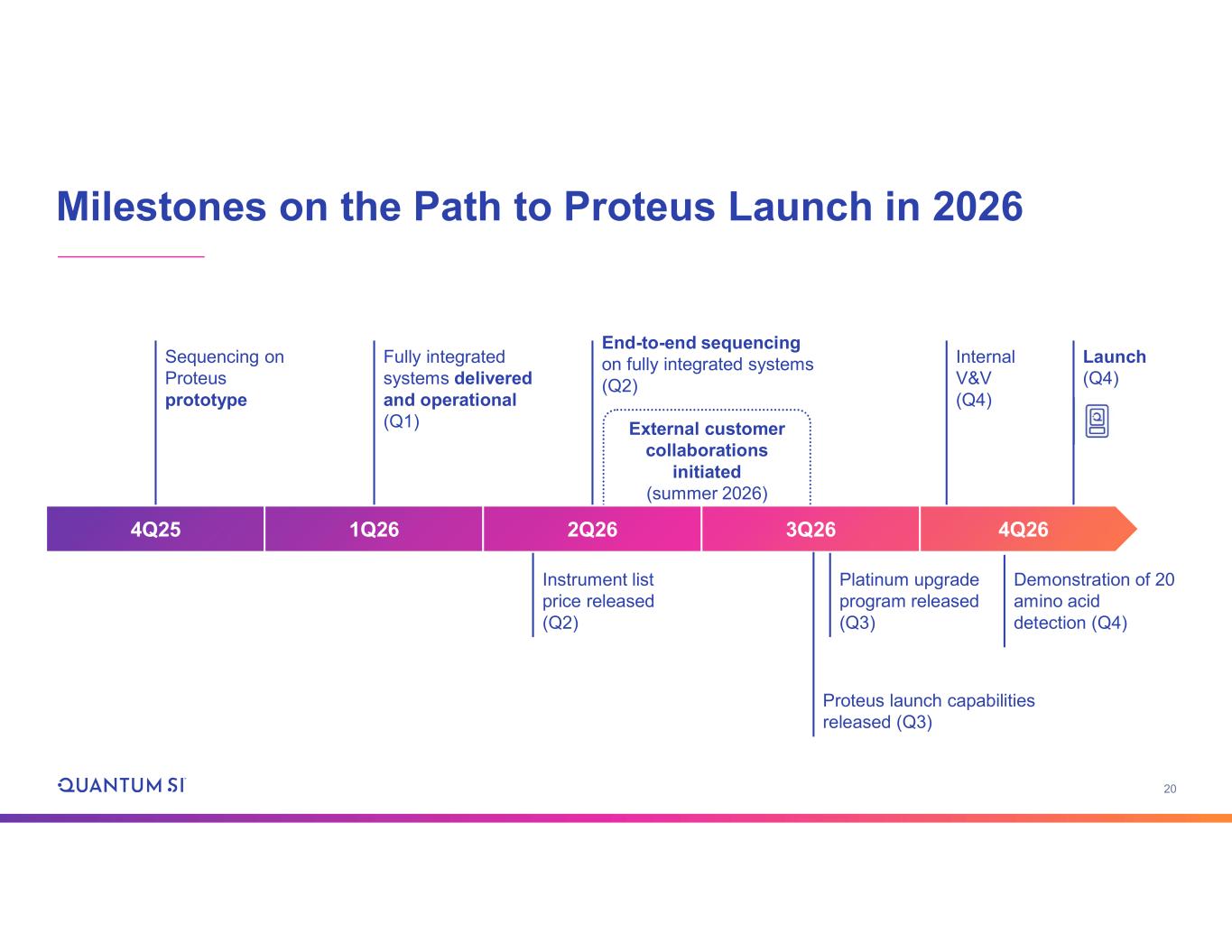
20 Milestones on the Path to Proteus Launch in 2026 External customer collaborations initiated (summer 2026) End-to-end sequencing on fully integrated systems (Q2) Platinum upgrade program released (Q3) Instrument list price released (Q2) Internal V&V (Q4) Sequencing on Proteus prototype Fully integrated systems delivered and operational (Q1) Launch (Q4) 4Q25 1Q26 2Q26 3Q26 4Q26 Demonstration of 20 amino acid detection (Q4) Proteus launch capabilities released (Q3)
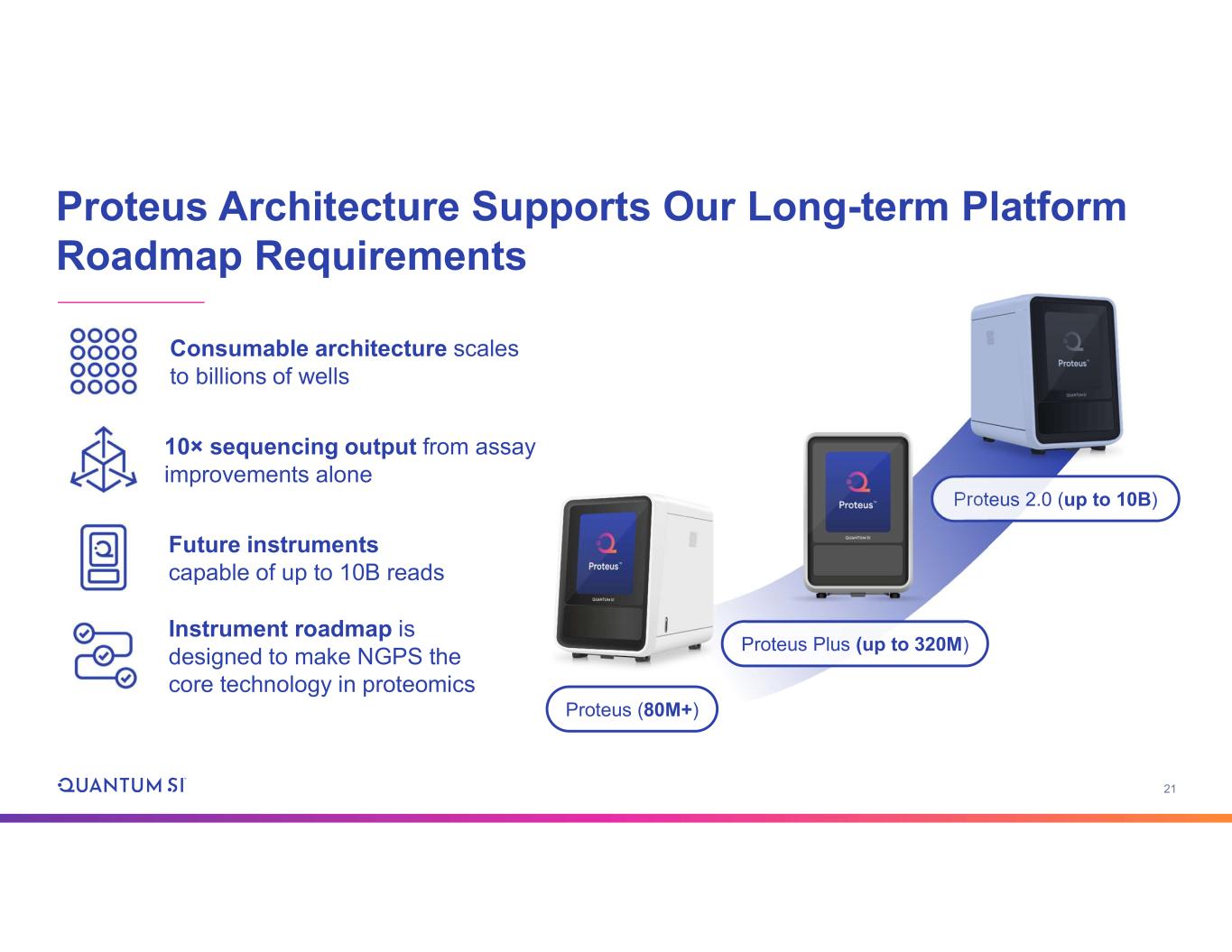
21 Proteus Architecture Supports Our Long-term Platform Roadmap Requirements Proteus 2.0 (up to 10B) Proteus (80M+) Proteus Plus (up to 320M) Consumable architecture scales to billions of wells 10× sequencing output from assay improvements alone Future instruments capable of up to 10B reads Instrument roadmap is designed to make NGPS the core technology in proteomics

22 QSI is Well-positioned to Build Upon Our First-to-market Advantage and Extend Our Leadership Into the Future Extend our technology lead through best-in- class R&D innovation and execution Execute on development roadmap to deliver a compelling Proteus launch Build a partner ecosystem to accelerate application development + deliver sample-to-report workflows Grow user base with Platinum Pro and develop the market for Proteus launch

23 Q&A