

2025 ASH Management Call December 7, 2025 Confidential

Disclaimer and FLS This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Disc’s expectations with respect to: (i) the registrational pathway for bitopertin, including the potential for accelerated approval, benefits of the Commissioner’s National Priority Voucher (CNPV) pilot program, and the anticipated review period and action date; (ii) the potential commercial launch of bitopertin, including commercial readiness activities and the timeline for availability of drug supply; (iii) the timing, progress and results of preclinical studies and clinical trials for bitopertin, DISC-0974, DISC-3405 and other product candidates Disc may develop, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work and the period during which results will become available; (iv) Disc’s research and development plans, including plans to explore the therapeutic potential of DISC-0974 in other anemias of inflammation; (v) Disc’s analysis of the market potential for its product candidates; and (vi) Disc’s future cash position. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” or the negative of these terms and other similar words or expressions that are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Disc’s current beliefs, expectations and assumptions regarding the future of Disc’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Disc may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and investors should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements as a result of a number of material risks and uncertainties including but not limited to: the adequacy of Disc’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; the nature, strategy and focus of Disc; the difficulty in predicting the time and cost of development of Disc’s product candidates; Disc’s plans to research, develop and commercialize its current and future product candidates; the timing of initiation of Disc’s planned preclinical studies and clinical trials; the timing of the availability of data from Disc’s clinical trials; Disc’s ability to identify additional product candidates with significant commercial potential and to expand its pipeline in hematological diseases; the timing and anticipated results of Disc’s preclinical studies and clinical trials and the risk that the results of Disc’s preclinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; and the other risks and uncertainties described in Disc’s filings with the Securities and Exchange Commission, including in the “Risk Factors” section of Disc’s Annual Report on Form 10-K for the year ended December 31, 2024, and in subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement speaks only as of the date on which it was made. None of Disc, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.

Bitopertin, DISC-0974, and DISC-3405 are investigational agents and are not approved for use as therapies in any jurisdiction worldwide CONFIDENTIAL
Agenda 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 06 02 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer
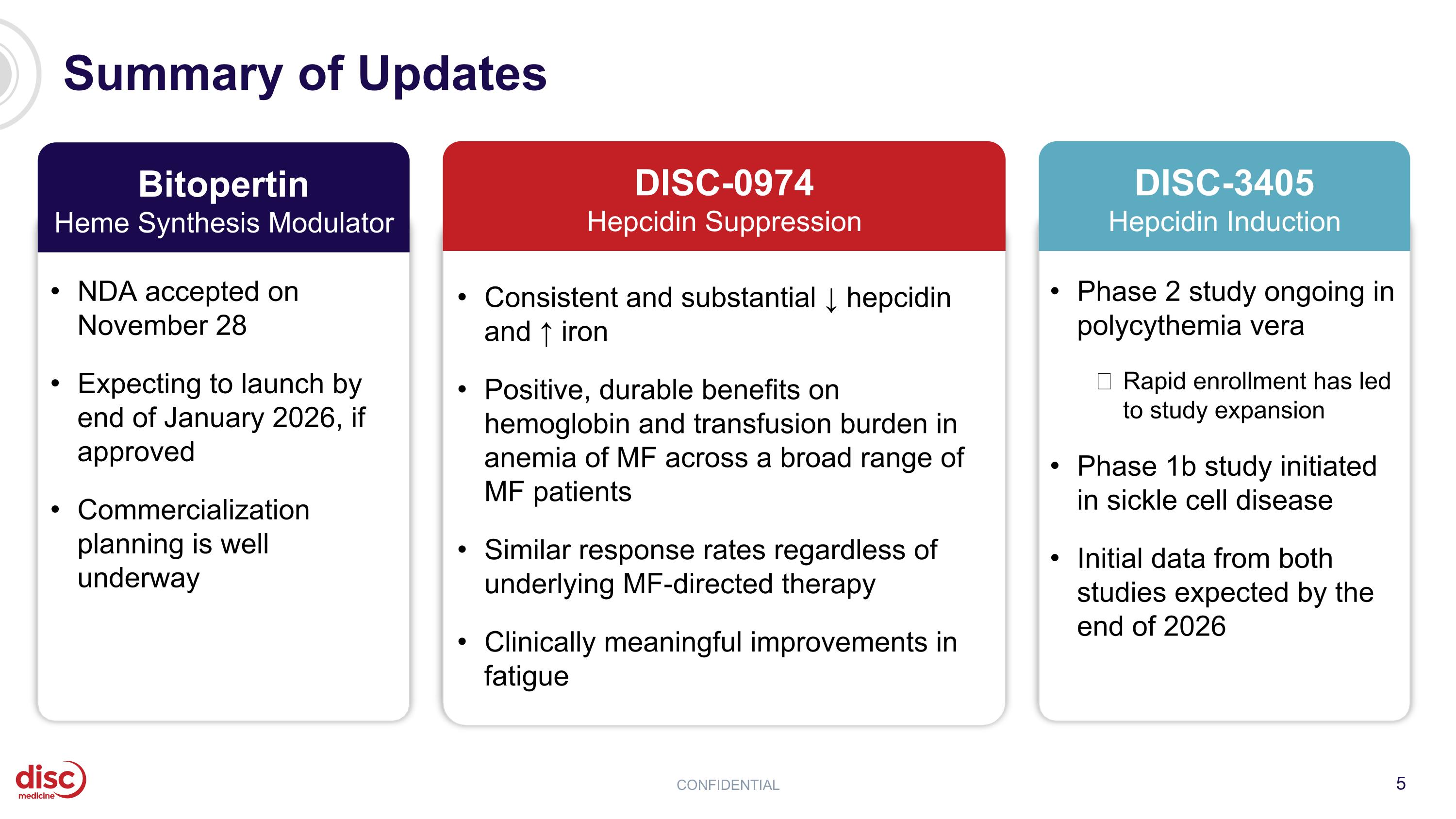
Summary of Updates CONFIDENTIAL Consistent and substantial ↓ hepcidin and ↑ iron Positive, durable benefits on hemoglobin and transfusion burden in anemia of MF across a broad range of MF patients Similar response rates regardless of underlying MF-directed therapy Clinically meaningful improvements in fatigue DISC-0974 Hepcidin Suppression NDA accepted on November 28 Expecting to launch by end of January 2026, if approved Commercialization planning is well underway Bitopertin Heme Synthesis Modulator Phase 2 study ongoing in polycythemia vera Rapid enrollment has led to study expansion Phase 1b study initiated in sickle cell disease Initial data from both studies expected by the end of 2026 DISC-3405 Hepcidin Induction

Agenda 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 06 02 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer
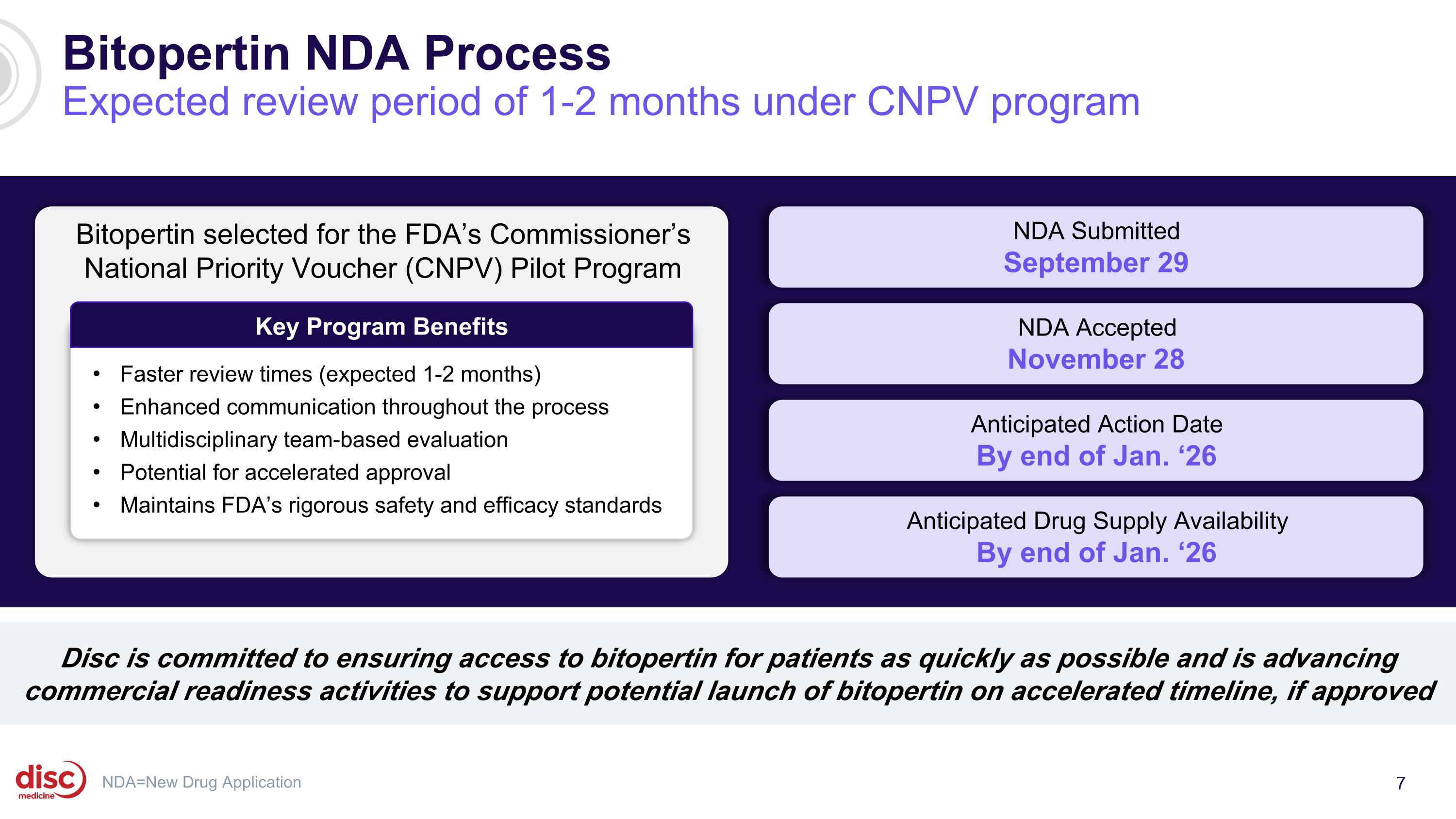
Disc is committed to ensuring access to bitopertin for patients as quickly as possible and is advancing commercial readiness activities to support potential launch of bitopertin on accelerated timeline, if approved NDA=New Drug Application Bitopertin selected for the FDA’s Commissioner’s National Priority Voucher (CNPV) Pilot Program Key Program Benefits Faster review times (expected 1-2 months) Enhanced communication throughout the process Multidisciplinary team-based evaluation Potential for accelerated approval Maintains FDA’s rigorous safety and efficacy standards Bitopertin NDA Process Expected review period of 1-2 months under CNPV program NDA Submitted September 29 Anticipated Action Date By end of Jan. ‘26 NDA Accepted November 28 Anticipated Drug Supply Availability By end of Jan. ‘26

Bitopertin Next Steps Collaborate with FDA throughout their ongoing review Continue accelerated activities to support a potential US approval and launch by end of January 2026 Drive enrollment of ongoing APOLLO confirmatory trial in US, UK, Canada, Australia and Europe Further details on commercialization and launch planning to be shared at the JP Morgan Healthcare Conference in January 2026
Agenda 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 06 02 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer
DISC-0974: Novel Anti-HJV mAb to Suppress Hepcidin Designed to enhance iron availability to address a wide range of hematologic disorders Releases iron stores Enables GI absorption Increases Iron Reduces Hepcidin Inhibits endogenous production of hepcidin Potential to treat wide range of anemias Enables RBC Production HJV = hemojuvelin
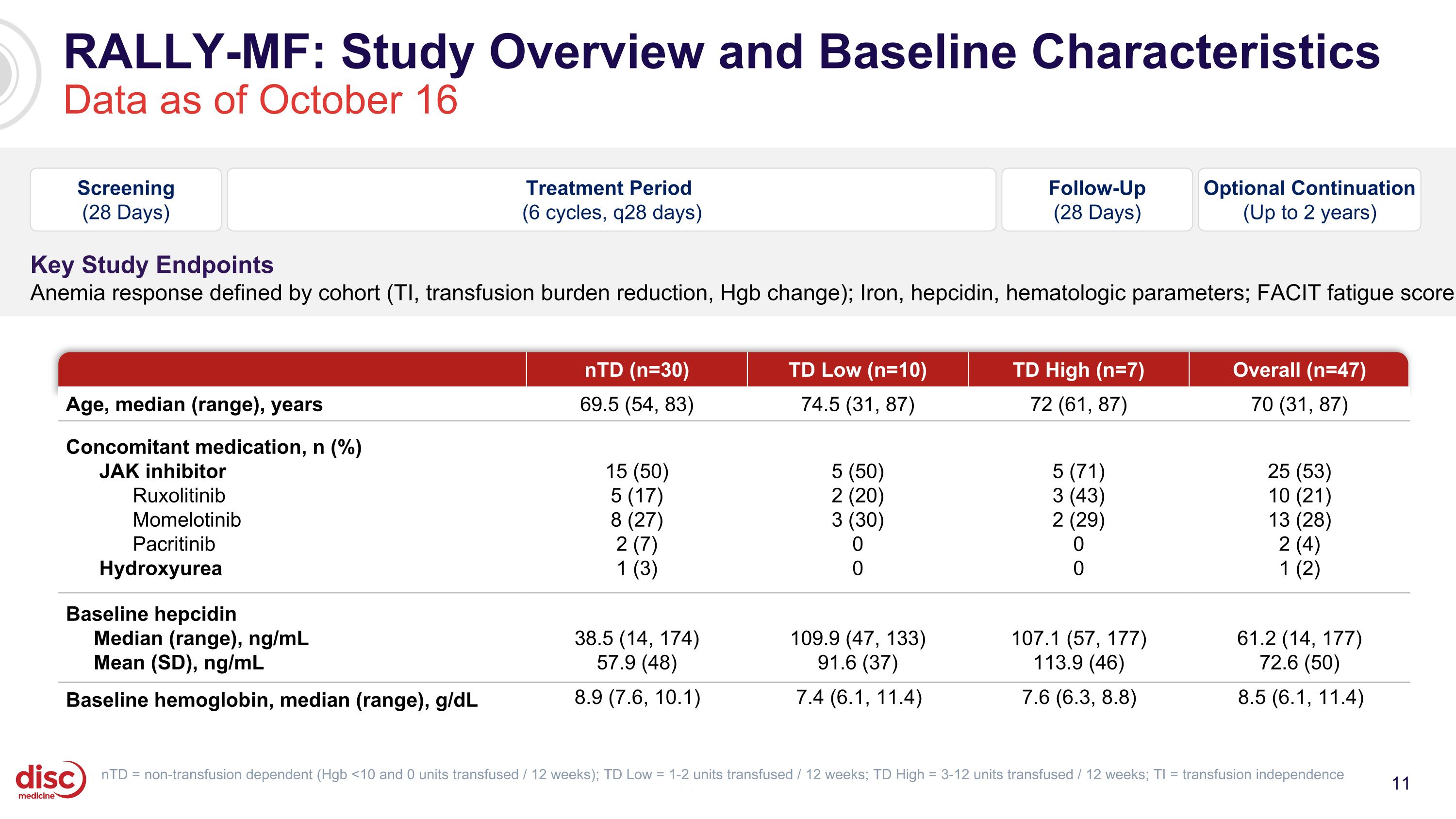
RALLY-MF: Study Overview and Baseline Characteristics Data as of October 16 CONFIDENTIAL nTD (n=30) TD Low (n=10) TD High (n=7) Overall (n=47) Age, median (range), years 69.5 (54, 83) 74.5 (31, 87) 72 (61, 87) 70 (31, 87) Concomitant medication, n (%) JAK inhibitor Ruxolitinib Momelotinib Pacritinib Hydroxyurea 15 (50) 5 (17) 8 (27) 2 (7) 1 (3) 5 (50) 2 (20) 3 (30) 0 0 5 (71) 3 (43) 2 (29) 0 0 25 (53) 10 (21) 13 (28) 2 (4) 1 (2) Baseline hepcidin Median (range), ng/mL Mean (SD), ng/mL 38.5 (14, 174) 57.9 (48) 109.9 (47, 133) 91.6 (37) 107.1 (57, 177) 113.9 (46) 61.2 (14, 177) 72.6 (50) Baseline hemoglobin, median (range), g/dL 8.9 (7.6, 10.1) 7.4 (6.1, 11.4) 7.6 (6.3, 8.8) 8.5 (6.1, 11.4) Screening (28 Days) Treatment Period (6 cycles, q28 days) Optional Continuation (Up to 2 years) Follow-Up (28 Days) Key Study Endpoints Anemia response defined by cohort (TI, transfusion burden reduction, Hgb change); Iron, hepcidin, hematologic parameters; FACIT fatigue score nTD = non-transfusion dependent (Hgb <10 and 0 units transfused / 12 weeks); TD Low = 1-2 units transfused / 12 weeks; TD High = 3-12 units transfused / 12 weeks; TI = transfusion independence
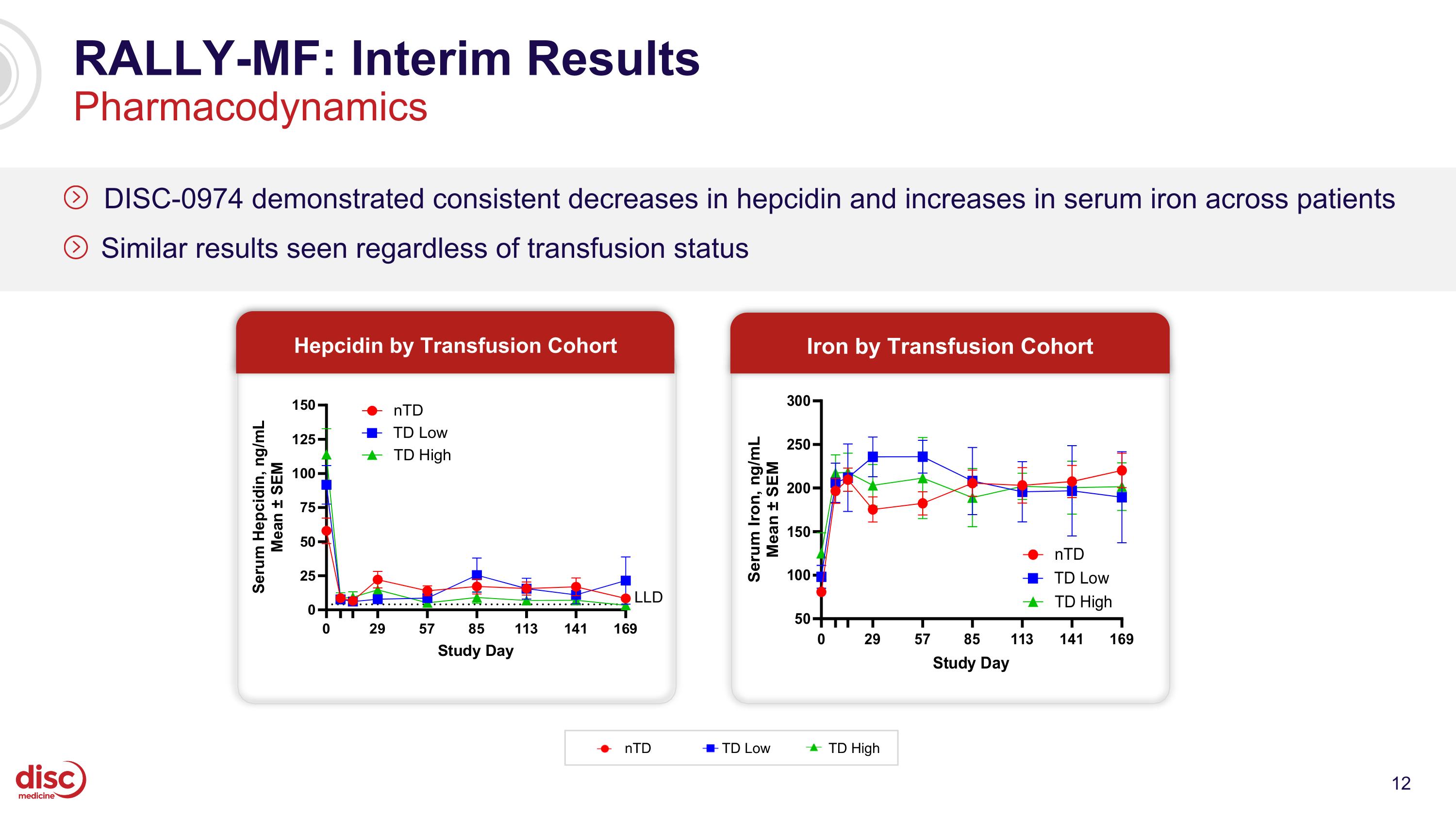
RALLY-MF: Interim Results Pharmacodynamics DISC-0974 demonstrated consistent decreases in hepcidin and increases in serum iron across patients Similar results seen regardless of transfusion status nTD TD Low TD High Hepcidin by Transfusion Cohort Iron by Transfusion Cohort
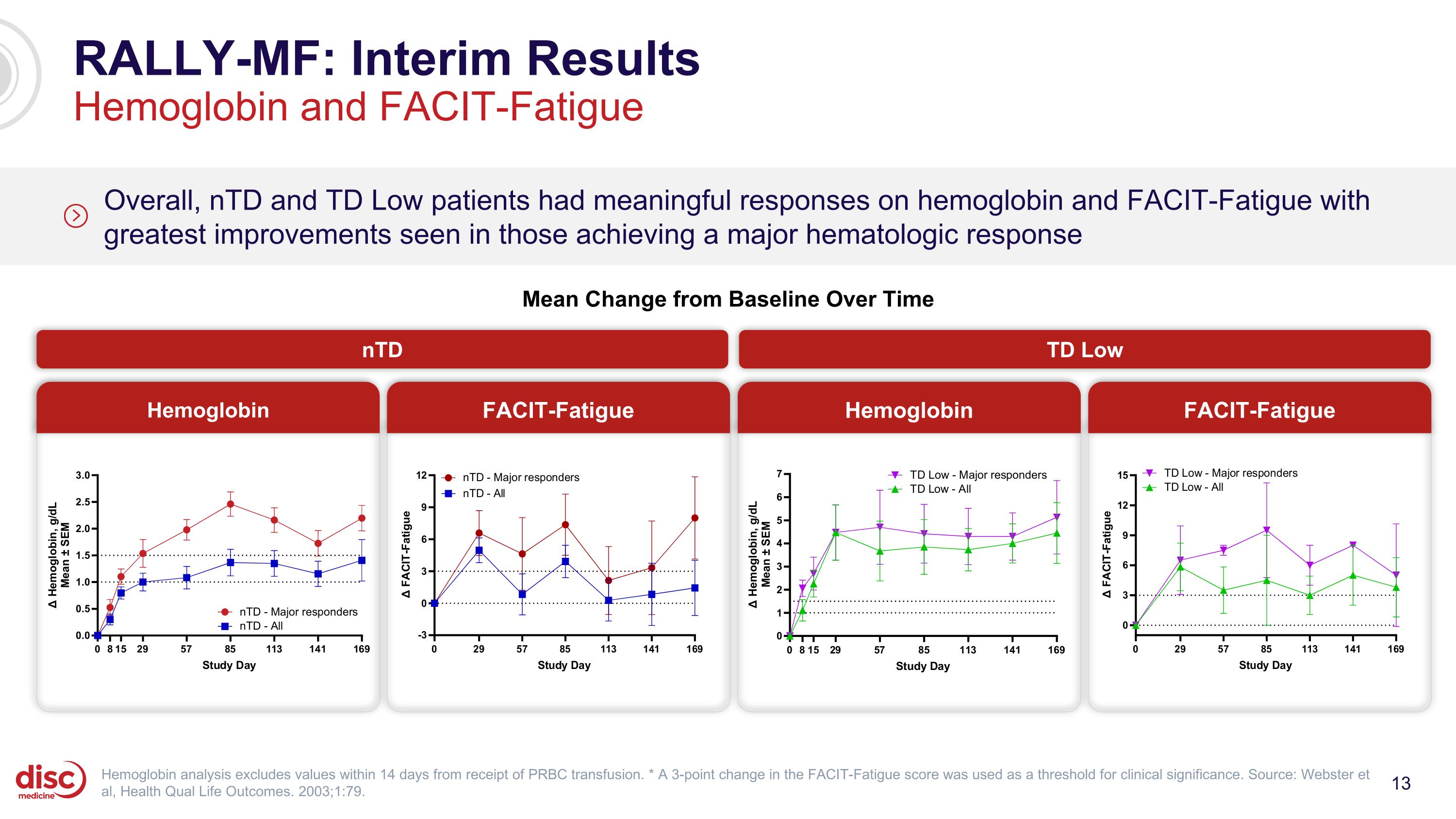
RALLY-MF: Interim Results Hemoglobin and FACIT-Fatigue Overall, nTD and TD Low patients had meaningful responses on hemoglobin and FACIT-Fatigue with greatest improvements seen in those achieving a major hematologic response Hemoglobin FACIT-Fatigue Hemoglobin FACIT-Fatigue Hemoglobin analysis excludes values within 14 days from receipt of PRBC transfusion. * A 3-point change in the FACIT-Fatigue score was used as a threshold for clinical significance. Source: Webster et al, Health Qual Life Outcomes. 2003;1:79. Mean Change from Baseline Over Time nTD TD Low
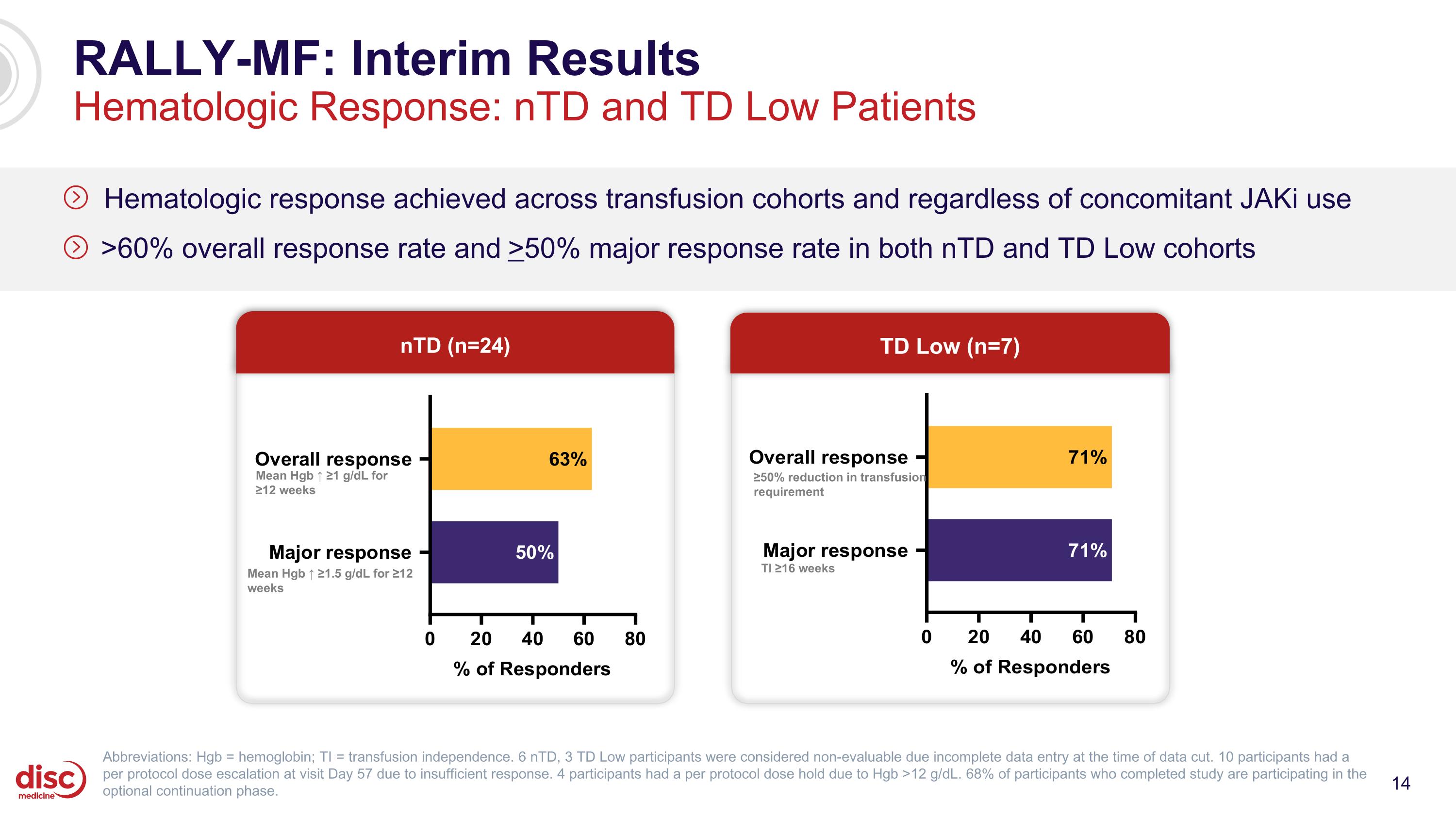
RALLY-MF: Interim Results Hematologic Response: nTD and TD Low Patients Hematologic response achieved across transfusion cohorts and regardless of concomitant JAKi use >60% overall response rate and >50% major response rate in both nTD and TD Low cohorts nTD (n=24) TD Low (n=7) Mean Hgb ↑ ≥1.5 g/dL for ≥12 weeks Mean Hgb ↑ ≥1 g/dL for ≥12 weeks ≥50% reduction in transfusion requirement TI ≥16 weeks Abbreviations: Hgb = hemoglobin; TI = transfusion independence. 6 nTD, 3 TD Low participants were considered non-evaluable due incomplete data entry at the time of data cut. 10 participants had a per protocol dose escalation at visit Day 57 due to insufficient response. 4 participants had a per protocol dose hold due to Hgb >12 g/dL. 68% of participants who completed study are participating in the optional continuation phase.
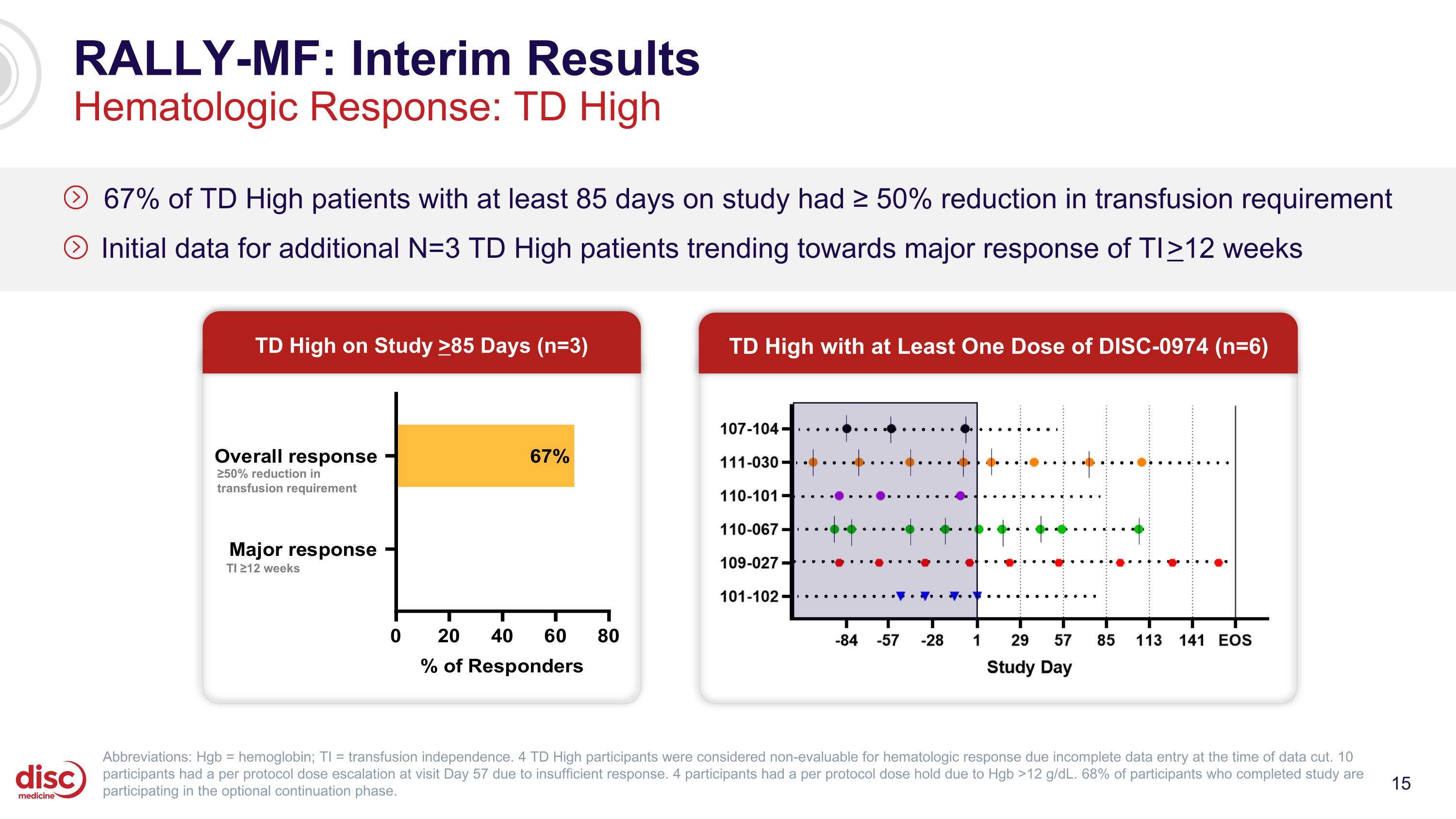
RALLY-MF: Interim Results Hematologic Response: TD High 67% of TD High patients with at least 85 days on study had ≥ 50% reduction in transfusion requirement Initial data for additional N=3 TD High patients trending towards major response of TI >12 weeks Abbreviations: Hgb = hemoglobin; TI = transfusion independence. 4 TD High participants were considered non-evaluable for hematologic response due incomplete data entry at the time of data cut. 10 participants had a per protocol dose escalation at visit Day 57 due to insufficient response. 4 participants had a per protocol dose hold due to Hgb >12 g/dL. 68% of participants who completed study are participating in the optional continuation phase. TD High on Study >85 Days (n=3) TD High with at Least One Dose of DISC-0974 (n=6) ≥50% reduction in transfusion requirement TI ≥12 weeks
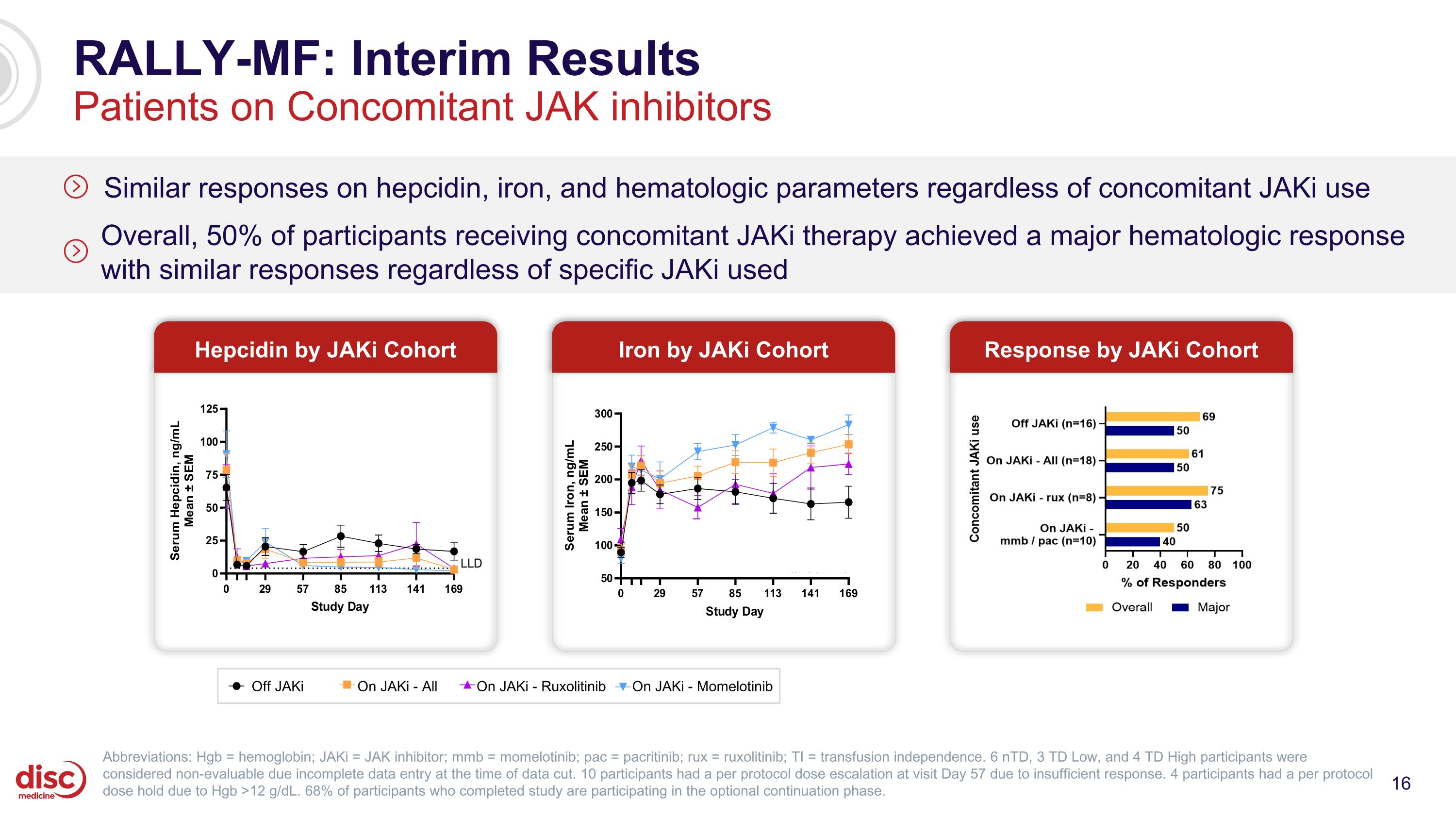
RALLY-MF: Interim Results Patients on Concomitant JAK inhibitors Similar responses on hepcidin, iron, and hematologic parameters regardless of concomitant JAKi use Overall, 50% of participants receiving concomitant JAKi therapy achieved a major hematologic response with similar responses regardless of specific JAKi used Response by JAKi Cohort Abbreviations: Hgb = hemoglobin; JAKi = JAK inhibitor; mmb = momelotinib; pac = pacritinib; rux = ruxolitinib; TI = transfusion independence. 6 nTD, 3 TD Low, and 4 TD High participants were considered non-evaluable due incomplete data entry at the time of data cut. 10 participants had a per protocol dose escalation at visit Day 57 due to insufficient response. 4 participants had a per protocol dose hold due to Hgb >12 g/dL. 68% of participants who completed study are participating in the optional continuation phase. Hepcidin by JAKi Cohort Iron by JAKi Cohort Off JAKi On JAKi - All On JAKi - Ruxolitinib On JAKi - Momelotinib
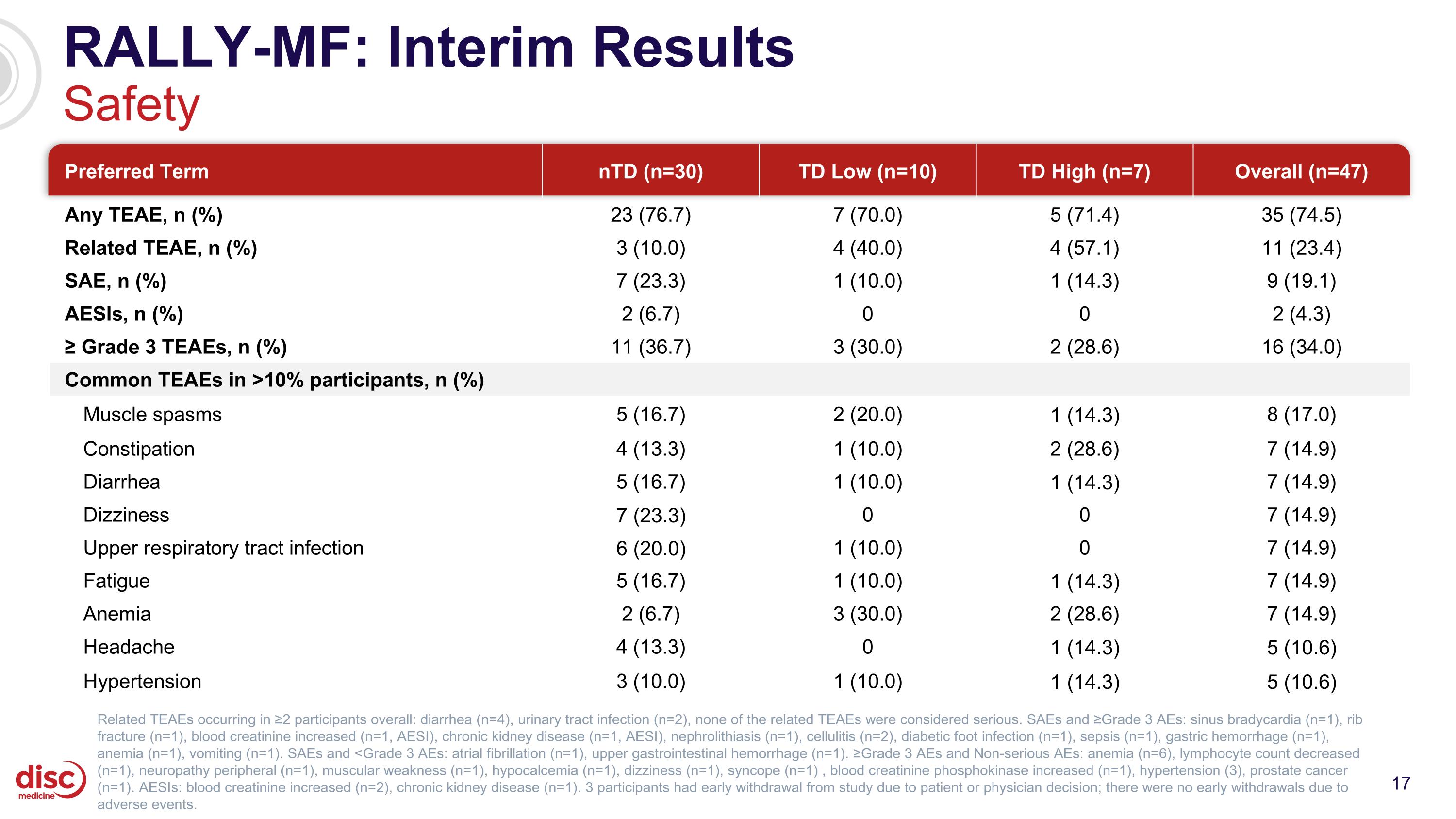
RALLY-MF: Interim Results Safety Preferred Term nTD (n=30) TD Low (n=10) TD High (n=7) Overall (n=47) Any TEAE, n (%) 23 (76.7) 7 (70.0) 5 (71.4) 35 (74.5) Related TEAE, n (%) 3 (10.0) 4 (40.0) 4 (57.1) 11 (23.4) SAE, n (%) 7 (23.3) 1 (10.0) 1 (14.3) 9 (19.1) AESIs, n (%) 2 (6.7) 0 0 2 (4.3) ≥ Grade 3 TEAEs, n (%) 11 (36.7) 3 (30.0) 2 (28.6) 16 (34.0) Common TEAEs in >10% participants, n (%) Muscle spasms 5 (16.7) 2 (20.0) 1 (14.3) 8 (17.0) Constipation 4 (13.3) 1 (10.0) 2 (28.6) 7 (14.9) Diarrhea 5 (16.7) 1 (10.0) 1 (14.3) 7 (14.9) Dizziness 7 (23.3) 0 0 7 (14.9) Upper respiratory tract infection 6 (20.0) 1 (10.0) 0 7 (14.9) Fatigue 5 (16.7) 1 (10.0) 1 (14.3) 7 (14.9) Anemia 2 (6.7) 3 (30.0) 2 (28.6) 7 (14.9) Headache 4 (13.3) 0 1 (14.3) 5 (10.6) Hypertension 3 (10.0) 1 (10.0) 1 (14.3) 5 (10.6) Related TEAEs occurring in ≥2 participants overall: diarrhea (n=4), urinary tract infection (n=2), none of the related TEAEs were considered serious. SAEs and ≥Grade 3 AEs: sinus bradycardia (n=1), rib fracture (n=1), blood creatinine increased (n=1, AESI), chronic kidney disease (n=1, AESI), nephrolithiasis (n=1), cellulitis (n=2), diabetic foot infection (n=1), sepsis (n=1), gastric hemorrhage (n=1), anemia (n=1), vomiting (n=1). SAEs and <Grade 3 AEs: atrial fibrillation (n=1), upper gastrointestinal hemorrhage (n=1). ≥Grade 3 AEs and Non-serious AEs: anemia (n=6), lymphocyte count decreased (n=1), neuropathy peripheral (n=1), muscular weakness (n=1), hypocalcemia (n=1), dizziness (n=1), syncope (n=1) , blood creatinine phosphokinase increased (n=1), hypertension (3), prostate cancer (n=1). AESIs: blood creatinine increased (n=2), chronic kidney disease (n=1). 3 participants had early withdrawal from study due to patient or physician decision; there were no early withdrawals due to adverse events.
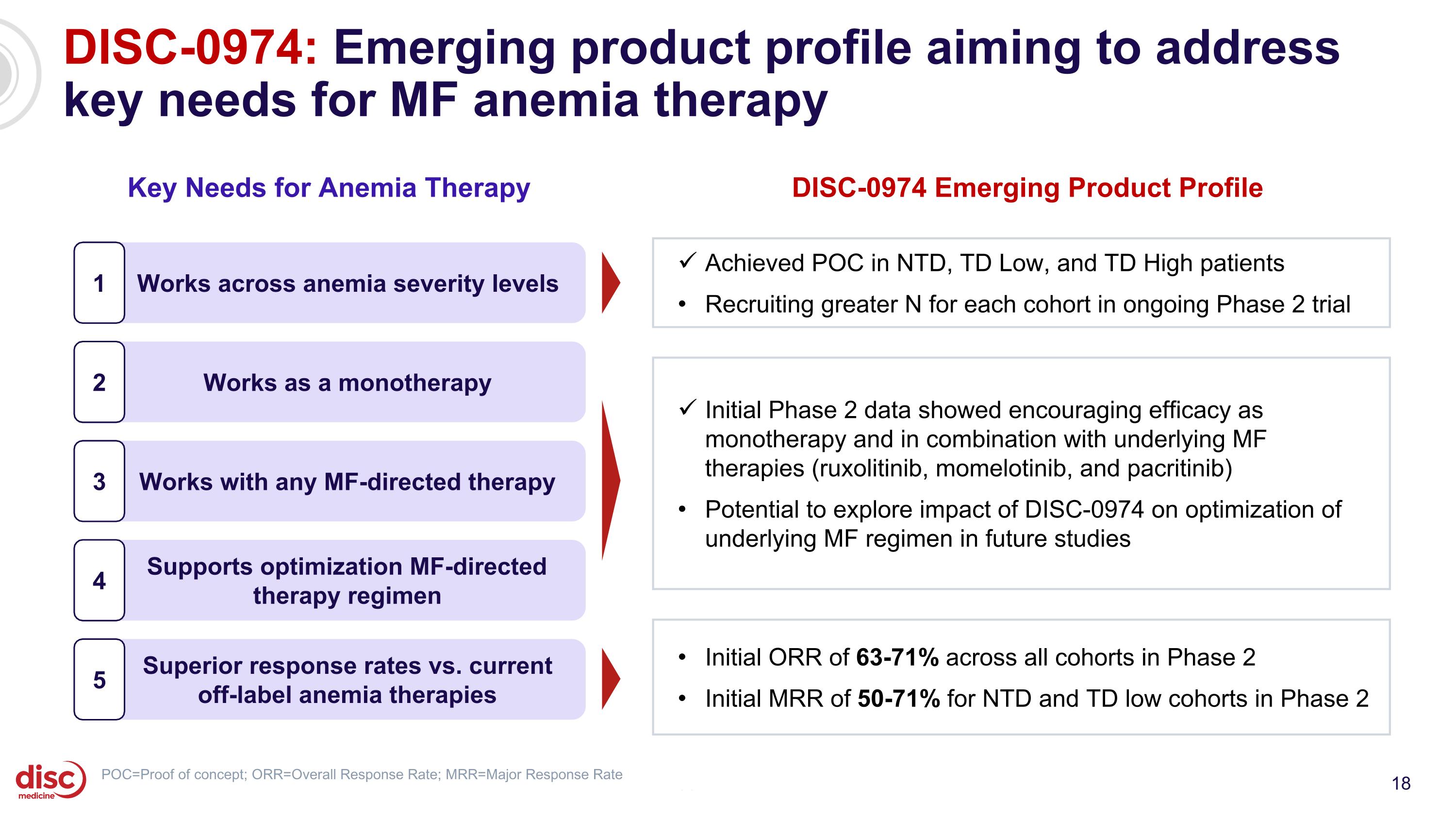
DISC-0974: Emerging product profile aiming to address key needs for MF anemia therapy CONFIDENTIAL Key Needs for Anemia Therapy Works across anemia severity levels 1 Works as a monotherapy 2 Works with any MF-directed therapy 3 Supports optimization MF-directed therapy regimen 4 Superior response rates vs. current off-label anemia therapies 5 DISC-0974 Emerging Product Profile Initial ORR of 63-71% across all cohorts in Phase 2 Initial MRR of 50-71% for NTD and TD low cohorts in Phase 2 Achieved POC in NTD, TD Low, and TD High patients Recruiting greater N for each cohort in ongoing Phase 2 trial Initial Phase 2 data showed encouraging efficacy as monotherapy and in combination with underlying MF therapies (ruxolitinib, momelotinib, and pacritinib) Potential to explore impact of DISC-0974 on optimization of underlying MF regimen in future studies POC=Proof of concept; ORR=Overall Response Rate; MRR=Major Response Rate
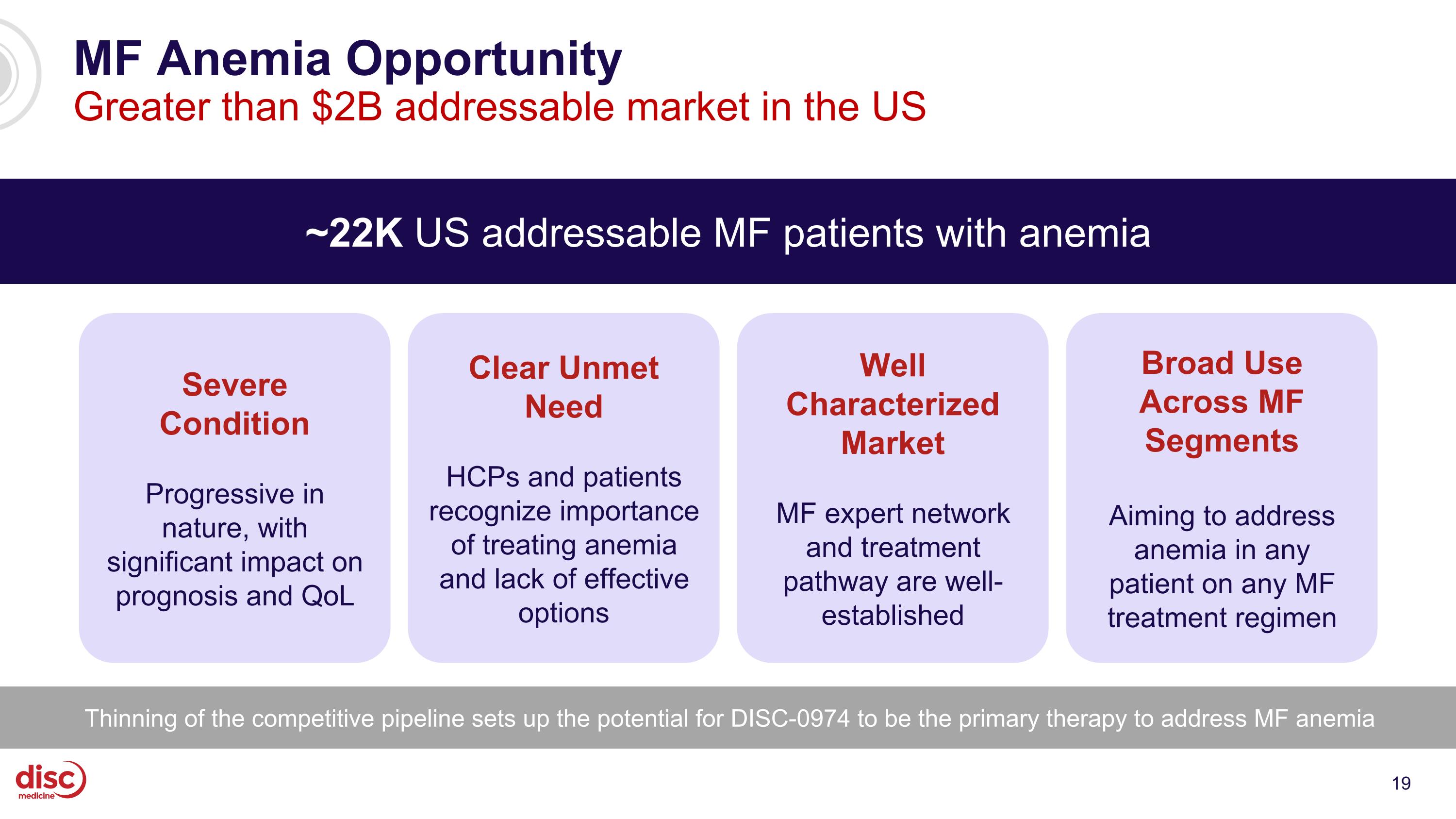
MF Anemia Opportunity Greater than $2B addressable market in the US ~22K US addressable MF patients with anemia Severe Condition Progressive in nature, with significant impact on prognosis and QoL Well Characterized Market MF expert network and treatment pathway are well-established Clear Unmet Need HCPs and patients recognize importance of treating anemia and lack of effective options Broad Use Across MF Segments Aiming to address anemia in any patient on any MF treatment regimen Thinning of the competitive pipeline sets up the potential for DISC-0974 to be the primary therapy to address MF anemia
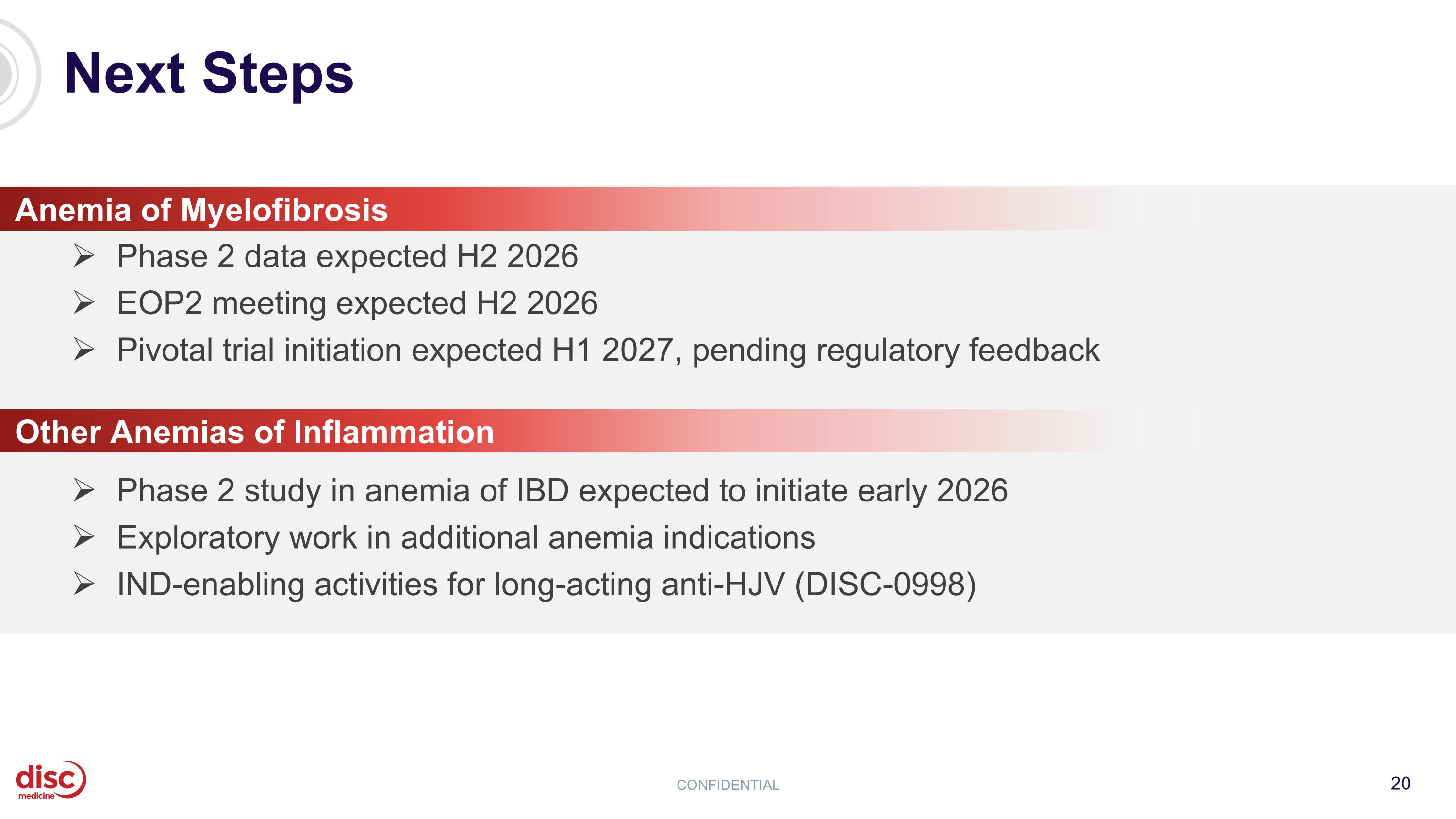
Next Steps CONFIDENTIAL Other Anemias of Inflammation Phase 2 study in anemia of IBD expected to initiate early 2026 Exploratory work in additional anemia indications IND-enabling activities for long-acting anti-HJV (DISC-0998) Phase 2 data expected H2 2026 EOP2 meeting expected H2 2026 Pivotal trial initiation expected H1 2027, pending regulatory feedback Anemia of Myelofibrosis
Agenda 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 06 02 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer
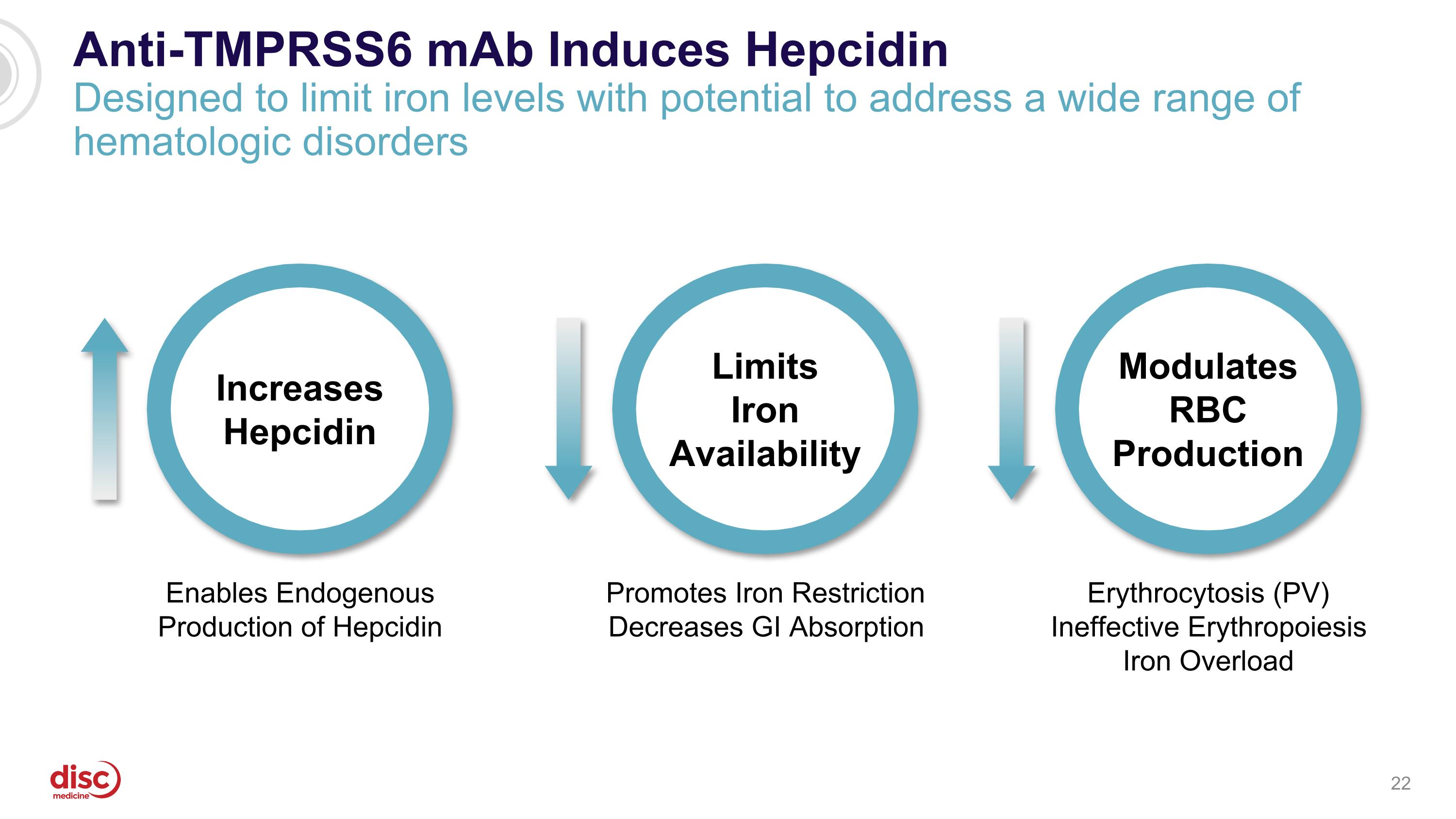
Anti-TMPRSS6 mAb Induces Hepcidin Designed to limit iron levels with potential to address a wide range of hematologic disorders Promotes Iron Restriction Decreases GI Absorption Limits Iron Availability Increases Hepcidin Enables Endogenous Production of Hepcidin Erythrocytosis (PV) Ineffective Erythropoiesis Iron Overload Modulates RBC Production
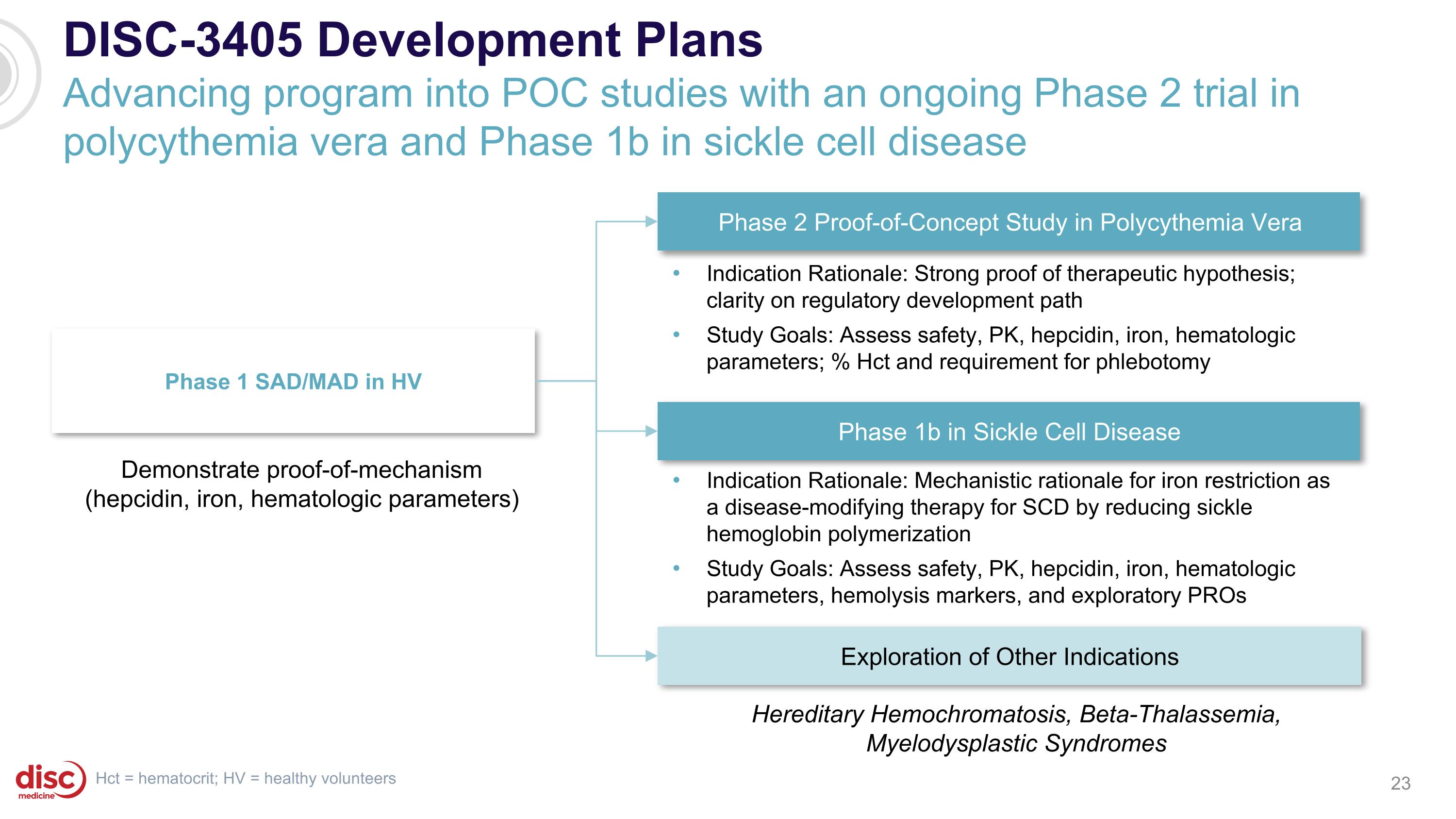
DISC-3405 Development Plans Advancing program into POC studies with an ongoing Phase 2 trial in polycythemia vera and Phase 1b in sickle cell disease Phase 1 SAD/MAD in HV Phase 2 Proof-of-Concept Study in Polycythemia Vera Exploration of Other Indications Demonstrate proof-of-mechanism (hepcidin, iron, hematologic parameters) Indication Rationale: Strong proof of therapeutic hypothesis; clarity on regulatory development path Study Goals: Assess safety, PK, hepcidin, iron, hematologic parameters; % Hct and requirement for phlebotomy Hereditary Hemochromatosis, Beta-Thalassemia, Myelodysplastic Syndromes Hct = hematocrit; HV = healthy volunteers Phase 1b in Sickle Cell Disease Indication Rationale: Mechanistic rationale for iron restriction as a disease-modifying therapy for SCD by reducing sickle hemoglobin polymerization Study Goals: Assess safety, PK, hepcidin, iron, hematologic parameters, hemolysis markers, and exploratory PROs
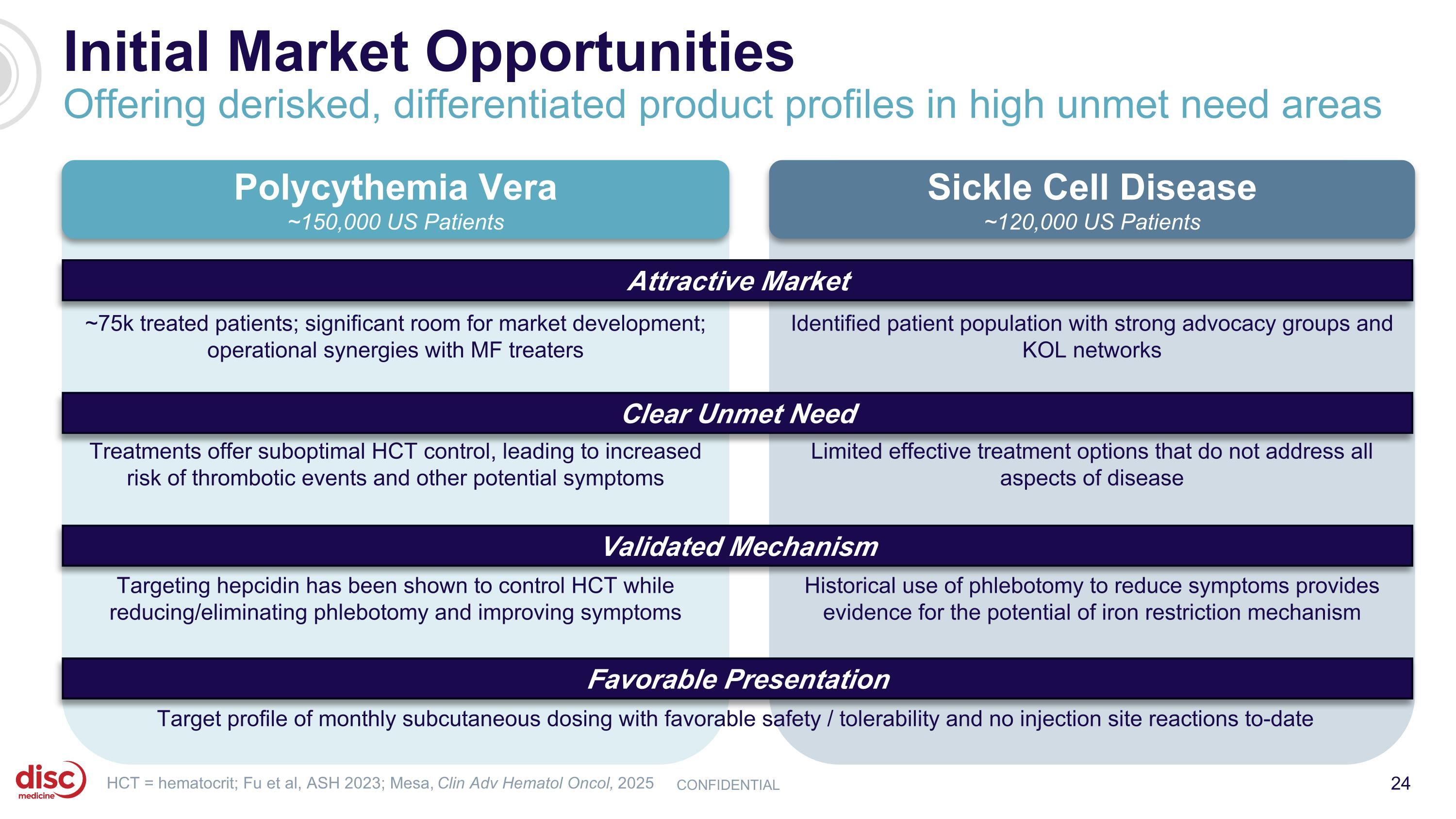
Initial Market Opportunities Offering derisked, differentiated product profiles in high unmet need areas CONFIDENTIAL Polycythemia Vera ~150,000 US Patients ~75k treated patients; significant room for market development; operational synergies with MF treaters Treatments offer suboptimal HCT control, leading to increased risk of thrombotic events and other potential symptoms Targeting hepcidin has been shown to control HCT while reducing/eliminating phlebotomy and improving symptoms Target profile of monthly subcutaneous dosing with favorable safety / tolerability and no injection site reactions to-date HCT = hematocrit; Fu et al, ASH 2023; Mesa, Clin Adv Hematol Oncol, 2025 Limited effective treatment options that do not address all aspects of disease Sickle Cell Disease ~120,000 US Patients Historical use of phlebotomy to reduce symptoms provides evidence for the potential of iron restriction mechanism Identified patient population with strong advocacy groups and KOL networks Attractive Market Clear Unmet Need Validated Mechanism Favorable Presentation
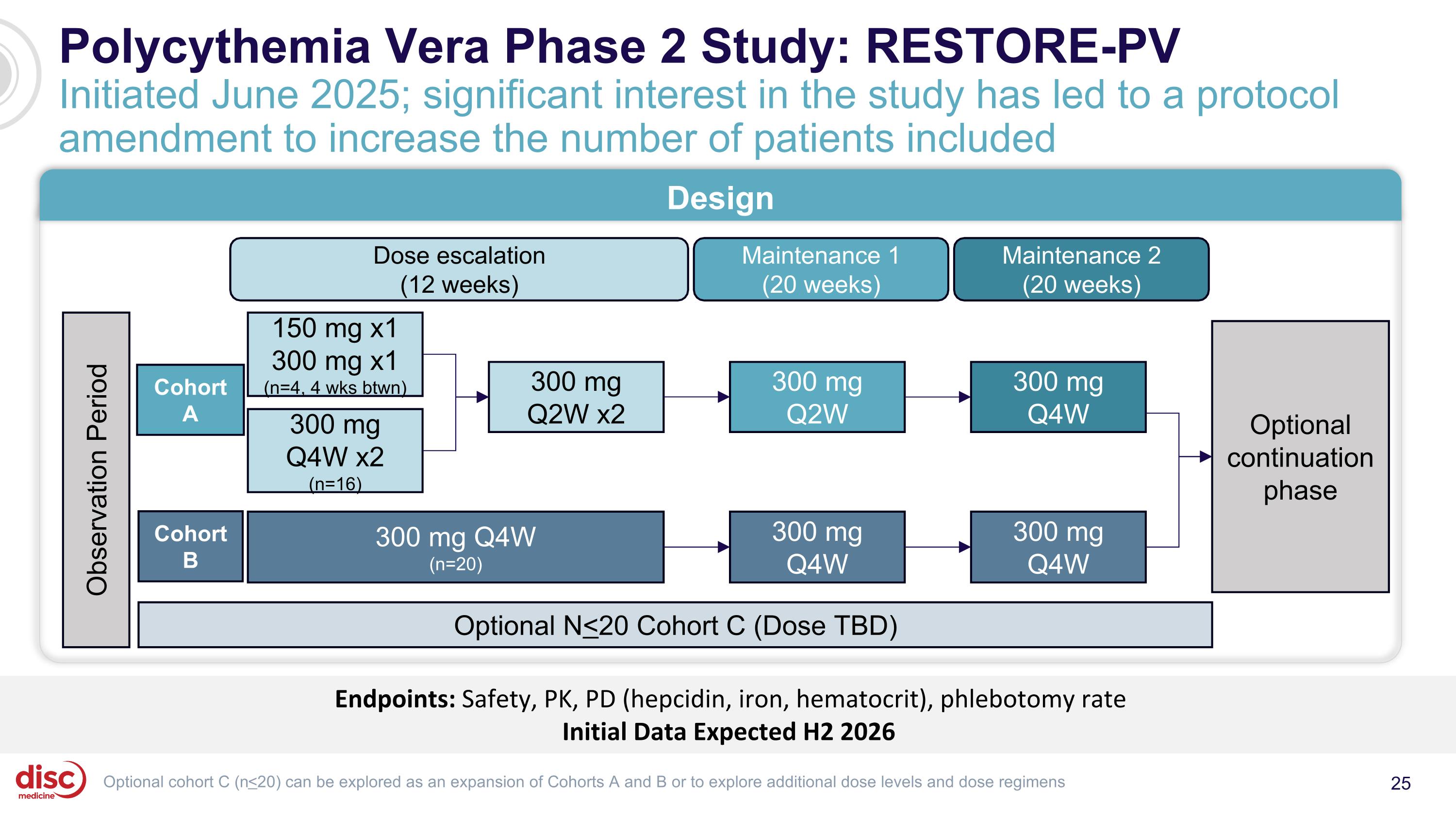
Polycythemia Vera Phase 2 Study: RESTORE-PV Initiated June 2025; significant interest in the study has led to a protocol amendment to increase the number of patients included Endpoints: Safety, PK, PD (hepcidin, iron, hematocrit), phlebotomy rate Initial Data Expected H2 2026 Design Maintenance 2 (20 weeks) Maintenance 1 (20 weeks) Dose escalation (12 weeks) 300 mg Q2W 300 mg Q4W 150 mg x1 300 mg x1 (n=4, 4 wks btwn) 300 mg Q4W x2 (n=16) 300 mg Q2W x2 Optional continuation phase Observation Period Optional cohort C (n<20) can be explored as an expansion of Cohorts A and B or to explore additional dose levels and dose regimens 300 mg Q4W (n=20) Optional N<20 Cohort C (Dose TBD) 300 mg Q4W 300 mg Q4W Cohort A Cohort B
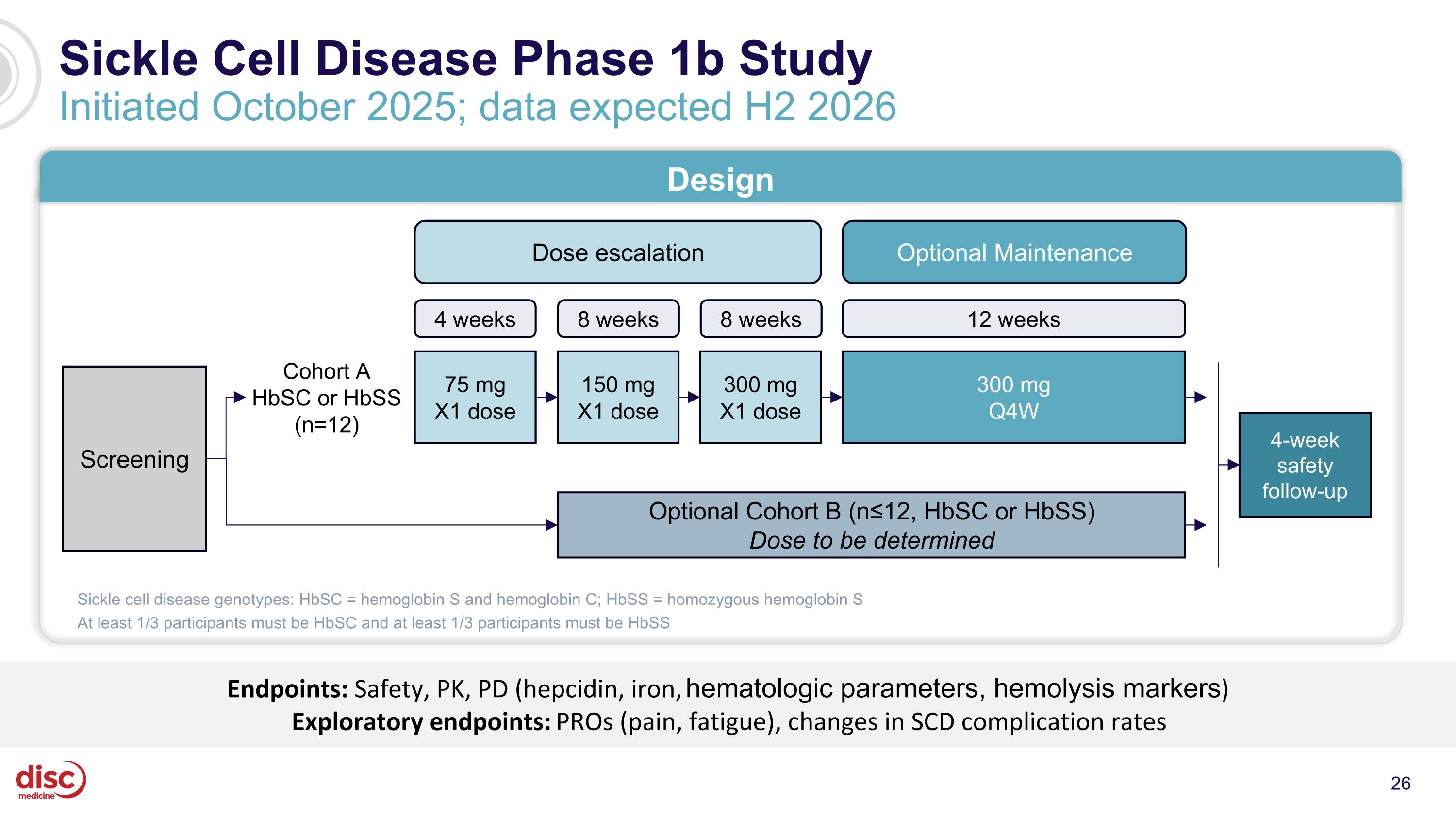
Sickle Cell Disease Phase 1b Study Initiated October 2025; data expected H2 2026 Endpoints: Safety, PK, PD (hepcidin, iron, hematologic parameters, hemolysis markers) Exploratory endpoints: PROs (pain, fatigue), changes in SCD complication rates Design Optional Maintenance Dose escalation Screening Cohort A HbSC or HbSS (n=12) Optional Cohort B (n≤12, HbSC or HbSS) Dose to be determined 4 weeks 8 weeks 8 weeks 12 weeks 75 mg X1 dose 150 mg X1 dose 300 mg X1 dose 300 mg Q4W 4-week safety follow-up Sickle cell disease genotypes: HbSC = hemoglobin S and hemoglobin C; HbSS = homozygous hemoglobin S At least 1/3 participants must be HbSC and at least 1/3 participants must be HbSS
Agenda 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 06 02
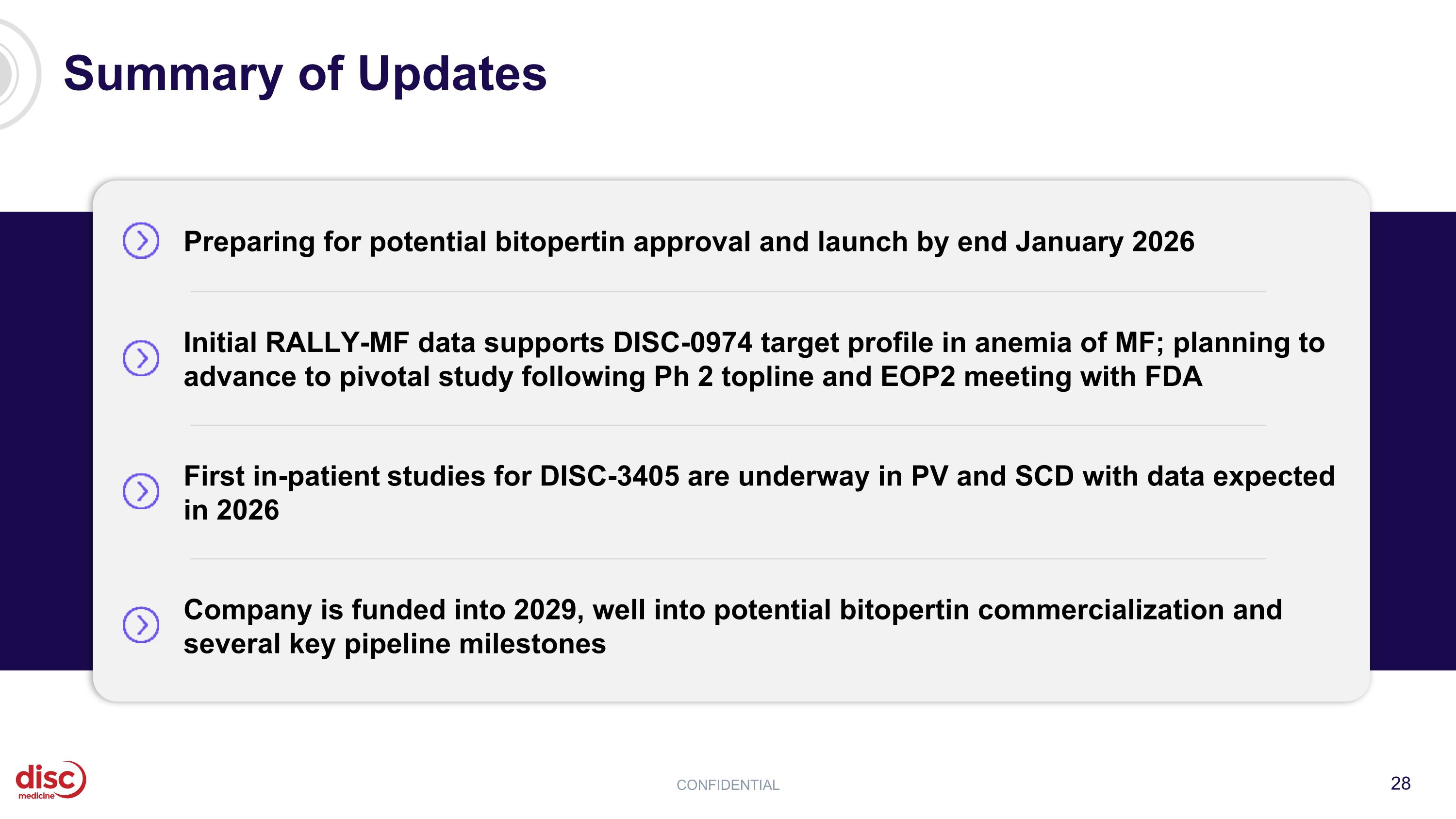
Summary of Updates CONFIDENTIAL Preparing for potential bitopertin approval and launch by end January 2026 Initial RALLY-MF data supports DISC-0974 target profile in anemia of MF; planning to advance to pivotal study following Ph 2 topline and EOP2 meeting with FDA First in-patient studies for DISC-3405 are underway in PV and SCD with data expected in 2026 Company is funded into 2029, well into potential bitopertin commercialization and several key pipeline milestones
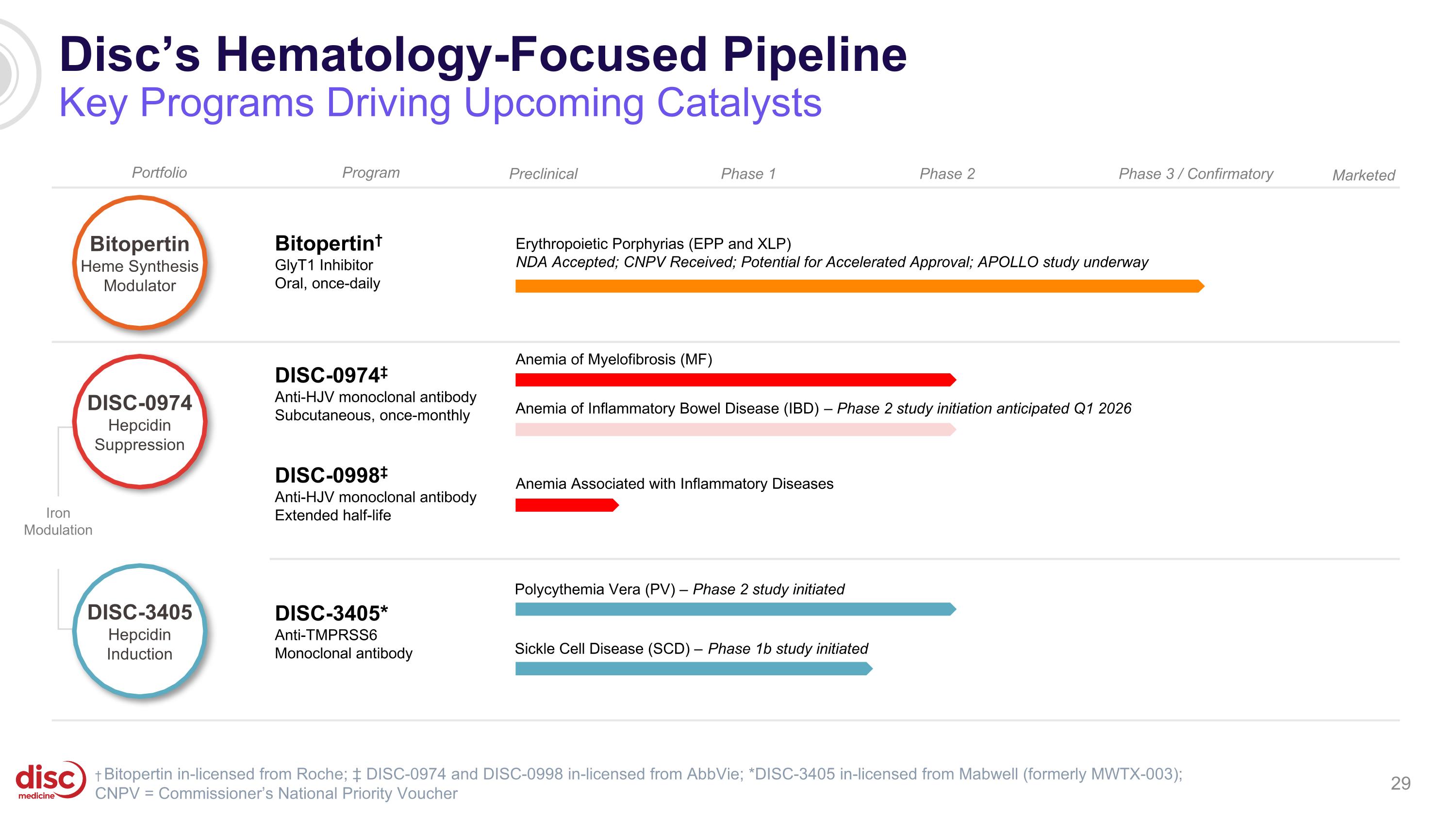
Disc’s Hematology-Focused Pipeline Key Programs Driving Upcoming Catalysts Preclinical Phase 1 Bitopertin† GlyT1 Inhibitor Oral, once-daily Phase 2 Phase 3 / Confirmatory DISC-0974‡ Anti-HJV monoclonal antibody Subcutaneous, once-monthly DISC-0998‡ Anti-HJV monoclonal antibody Extended half-life Erythropoietic Porphyrias (EPP and XLP) NDA Accepted; CNPV Received; Potential for Accelerated Approval; APOLLO study underway Anemia Associated with Inflammatory Diseases Iron Modulation Portfolio Program † Bitopertin in-licensed from Roche; ‡ DISC-0974 and DISC-0998 in-licensed from AbbVie; *DISC-3405 in-licensed from Mabwell (formerly MWTX-003); CNPV = Commissioner’s National Priority Voucher DISC-3405* Anti-TMPRSS6 Monoclonal antibody Polycythemia Vera (PV) – Phase 2 study initiated Sickle Cell Disease (SCD) – Phase 1b study initiated Anemia of Myelofibrosis (MF) Anemia of Inflammatory Bowel Disease (IBD) – Phase 2 study initiation anticipated Q1 2026 DISC-3405 Hepcidin Induction Bitopertin Heme Synthesis Modulator DISC-0974 Hepcidin Suppression Marketed
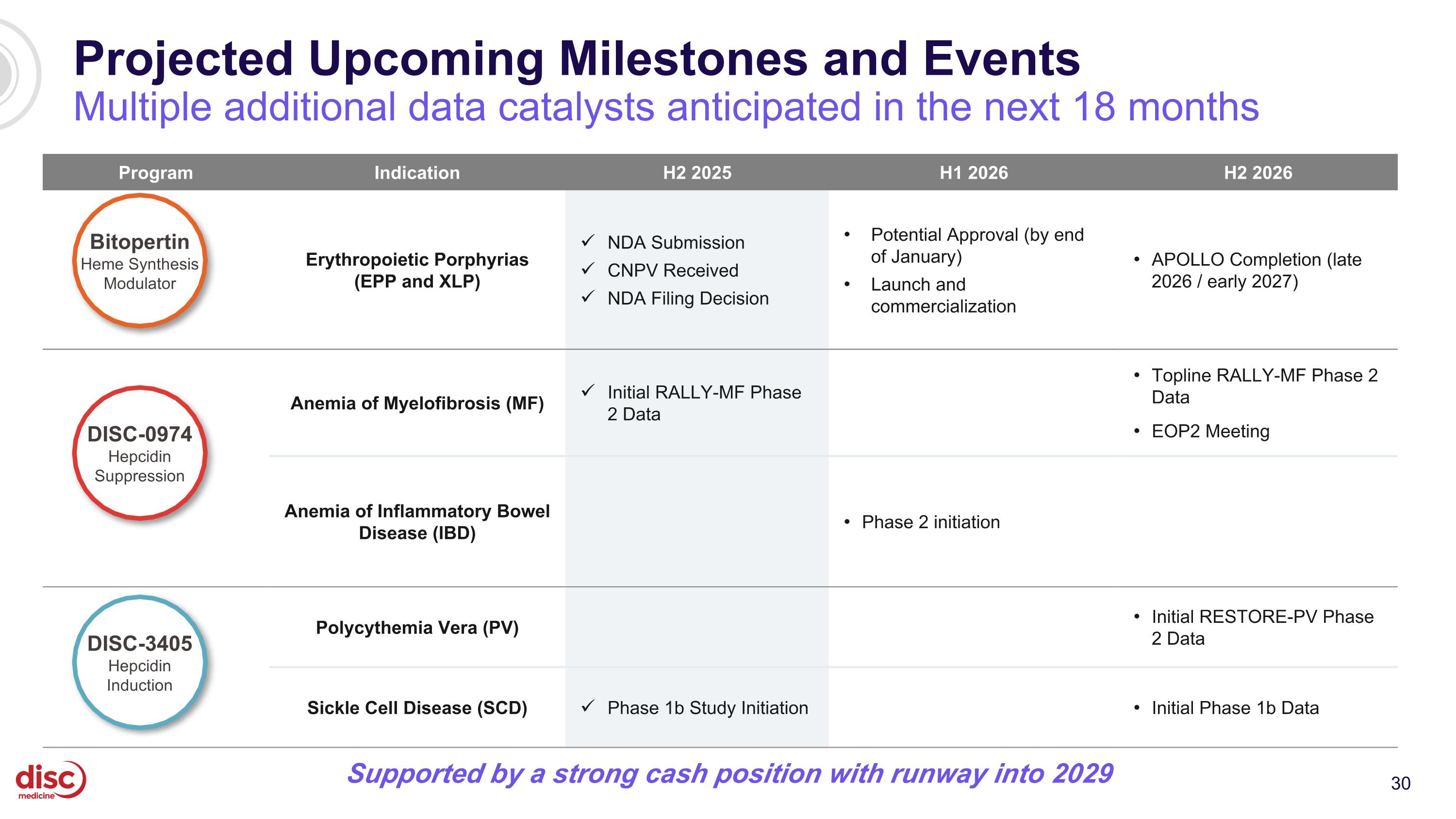
Projected Upcoming Milestones and Events Multiple additional data catalysts anticipated in the next 18 months Program Indication H2 2025 H1 2026 H2 2026 Erythropoietic Porphyrias (EPP and XLP) NDA Submission CNPV Received NDA Filing Decision Potential Approval (by end of January) Launch and commercialization APOLLO Completion (late 2026 / early 2027) Anemia of Myelofibrosis (MF) Initial RALLY-MF Phase 2 Data Topline RALLY-MF Phase 2 Data EOP2 Meeting Anemia of Inflammatory Bowel Disease (IBD) Phase 2 initiation Polycythemia Vera (PV) Initial RESTORE-PV Phase 2 Data Sickle Cell Disease (SCD) Phase 1b Study Initiation Initial Phase 1b Data DISC-3405 Hepcidin Induction Bitopertin Heme Synthesis Modulator DISC-0974 Hepcidin Suppression Supported by a strong cash position with runway into 2029
Agenda 01 Introduction and Summary John Quisel, JD, PhD, Chief Executive Officer 03 DISC-0974 Updated Data in Anemia of MF Will Savage, MD, PhD, Chief Medical Officer MF Anemia Market Opportunity Jonathan Yu, Chief Operating Officer 04 Bitopertin Updates John Quisel, JD, PhD, Chief Executive Officer DISC-3405 Updates Jonathan Yu, Chief Operating Officer Will Savage, MD, PhD, Chief Medical Officer 02 Closing Remarks John Quisel, JD, PhD, Chief Executive Officer 05 Q&A Session 06