

Bitopertin Regulatory Update February 2026

Disclaimer and FLS This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, express or implied statements regarding Disc’s expectations with respect to its preclinical studies, clinical trials and research and development programs, in particular with respect to bitopertin, DISC-0974 and DISC-3405, and any developments or results in connection therewith; projected timelines for the initiation and completion of its clinical trials, anticipated timing of release of data, and other clinical activities; the registrational pathway for bitopertin, including the potential for traditional approval, the potential for the APOLLO clinical trial to serve as the basis for any such approval, and the timing of any such approval, if granted; anticipated discussions with and submissions to regulatory agencies; Disc’s expectations with respect to the potential of bitopertin as a treatment for EPP, if approved; and the time period over which Disc’s capital resources will be sufficient to fund its anticipated operations. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” or the negative of these terms and other similar words or expressions that are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Disc’s current beliefs, expectations and assumptions regarding the future of Disc’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Disc may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and investors should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements as a result of a number of material risks and uncertainties including but not limited to: the adequacy of Disc’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; the nature, strategy and focus of Disc; the difficulty in predicting the time and cost of development of Disc’s product candidates; Disc’s plans to research, develop and commercialize its current and future product candidates; the timing of initiation of Disc’s planned preclinical studies and clinical trials; the timing of the availability of data from Disc’s clinical trials; Disc’s ability to identify additional product candidates with significant commercial potential and to expand its pipeline in hematological diseases; the timing and anticipated results of Disc’s preclinical studies and clinical trials and the risk that the results of Disc’s preclinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; the content and timing of decisions made by the FDA and other regulatory authorities; and the other risks and uncertainties described in Disc’s filings with the Securities and Exchange Commission, including in the “Risk Factors” section of Disc’s Annual Report on Form 10-K for the year ended December 31, 2024, and in subsequent Quarterly Reports on Form 10-Q. Any forward-looking statement speaks only as of the date on which it was made. None of Disc, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.
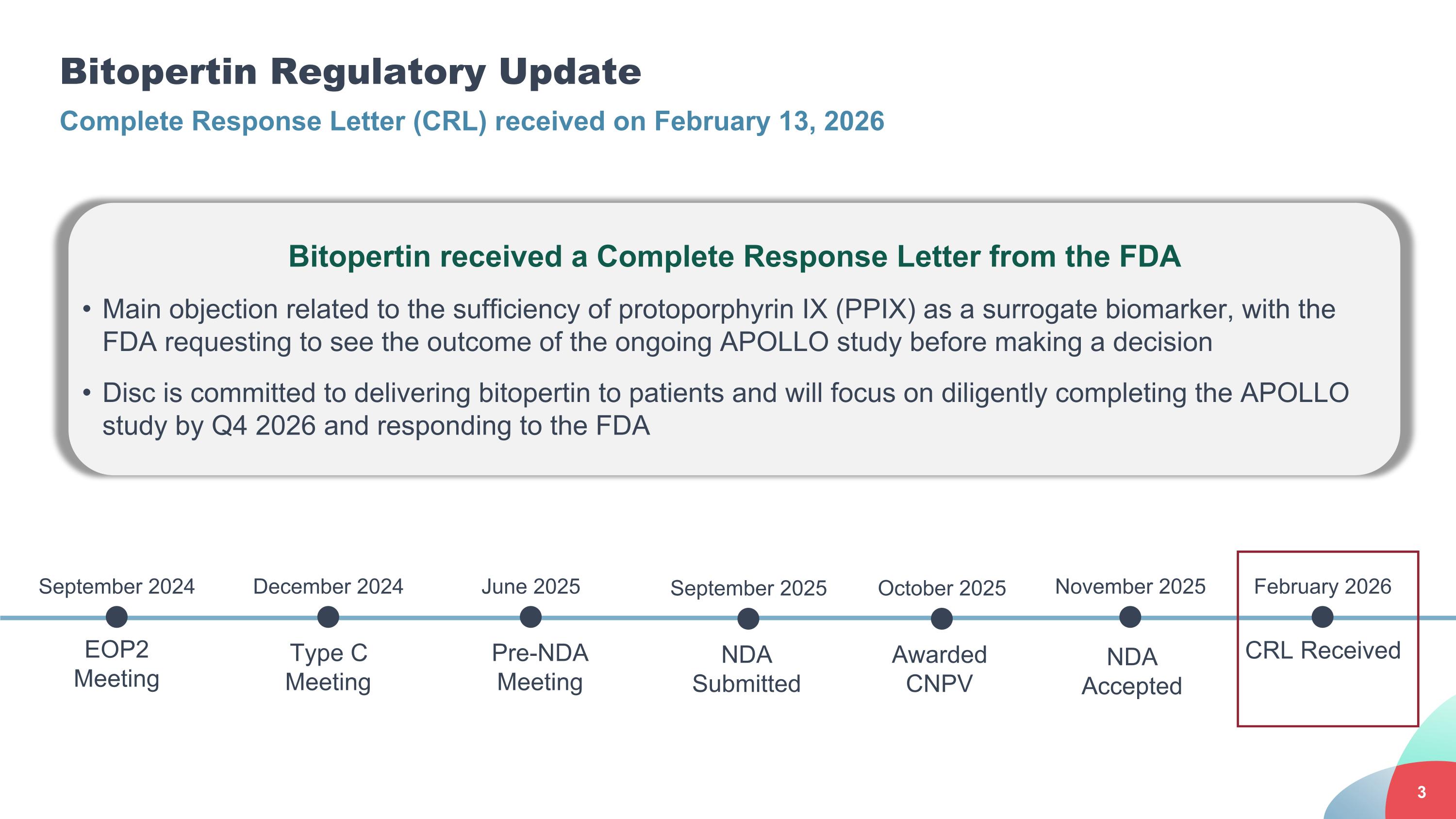
Bitopertin Regulatory Update Complete Response Letter (CRL) received on February 13, 2026 Type C Meeting EOP2 Meeting September 2024 December 2024 Pre-NDA Meeting June 2025 NDA Submitted September 2025 Awarded CNPV October 2025 NDA Accepted CRL Received November 2025 February 2026 Bitopertin received a Complete Response Letter from the FDA Main objection related to the sufficiency of protoporphyrin IX (PPIX) as a surrogate biomarker, with the FDA requesting to see the outcome of the ongoing APOLLO study before making a decision Disc is committed to delivering bitopertin to patients and will focus on diligently completing the APOLLO study by Q4 2026 and responding to the FDA
CRL Details Accelerated approval relies on (1) whether there is evidence of an effect on the proposed surrogate endpoint (% change in whole blood metal-free PPIX) and (2) whether the proposed surrogate endpoint, including the magnitude of change, is reasonably likely to predict a clinical benefit AURORA and BEACON provided sufficient evidence that bitopertin significantly lowers whole blood metal-free PPIX Based on review of AURORA and BEACON results, FDA concluded that the trials did not show evidence of association between percent change in PPIX and sunlight exposure-based endpoints, as measured in the trials, despite the strong mechanistic and biological plausibility supporting the use of the PPIX biomarker in protoporphyria FDA guided that for this application to be approved, Disc will need to provide additional evidence demonstrating the efficacy of bitopertin for the treatment of protoporphyria based on clinical endpoint(s) Stated willingness to discuss options to meet this requirement, including completion and submission of results from the ongoing APOLLO trial Other requests: FDA requested that Disc provide a standard safety update as part of the resubmission Disc will provide the new APOLLO safety data in the CRL response, which will be integrated with the prior safety database of over 4,000 subjects FDA indicated a need to see the results of the ongoing Phase 3 APOLLO study before making a decision
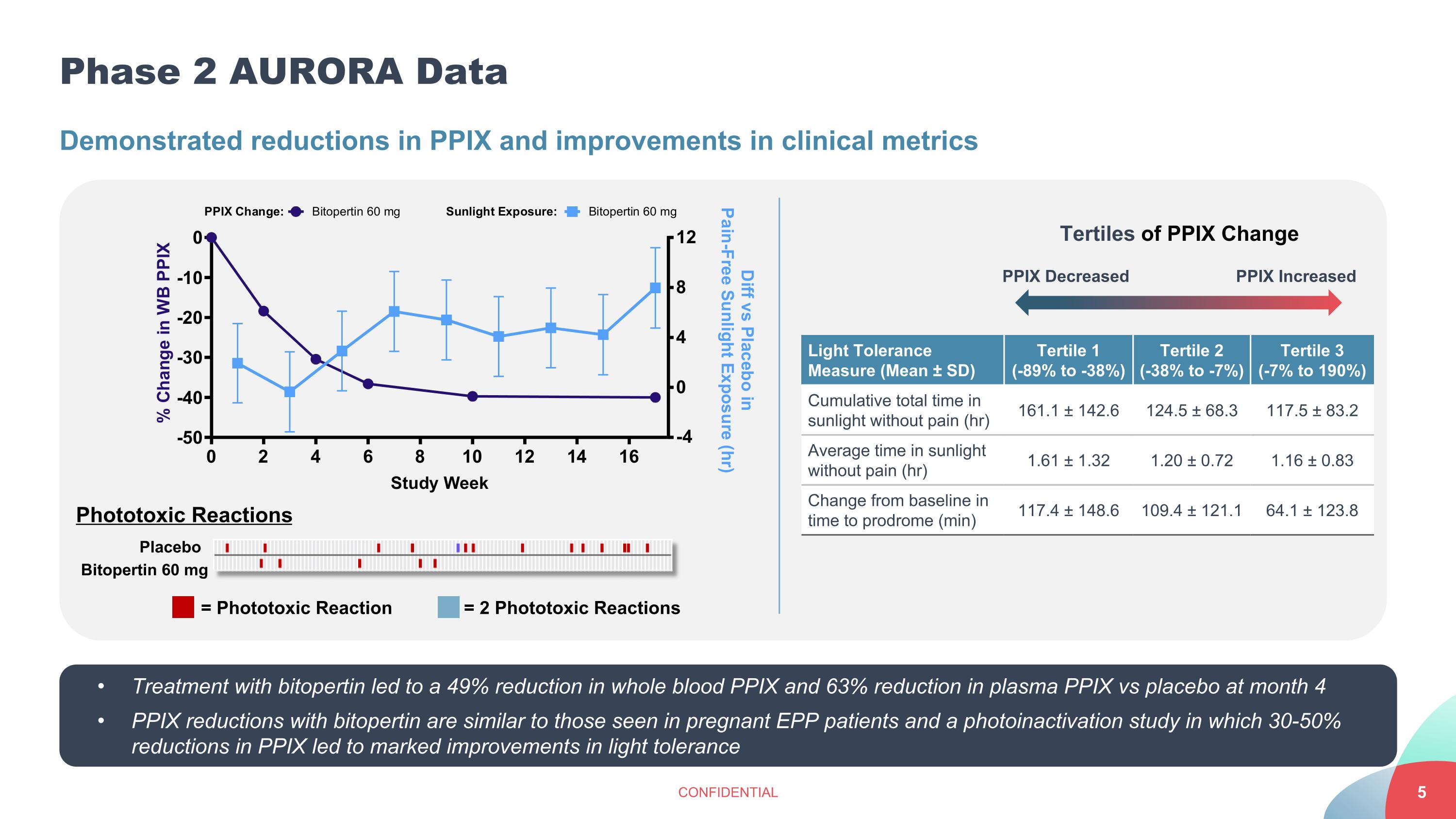
Phase 2 AURORA Data CONFIDENTIAL Demonstrated reductions in PPIX and improvements in clinical metrics Placebo Bitopertin 60 mg Phototoxic Reactions = Phototoxic Reaction = 2 Phototoxic Reactions Light Tolerance Measure (Mean ± SD) Tertile 1 (-89% to -38%) Tertile 2 (-38% to -7%) Tertile 3 (-7% to 190%) Cumulative total time in sunlight without pain (hr) 161.1 ± 142.6 124.5 ± 68.3 117.5 ± 83.2 Average time in sunlight without pain (hr) 1.61 ± 1.32 1.20 ± 0.72 1.16 ± 0.83 Change from baseline in time to prodrome (min) 117.4 ± 148.6 109.4 ± 121.1 64.1 ± 123.8 PPIX Increased PPIX Decreased Tertiles of PPIX Change Treatment with bitopertin led to a 49% reduction in whole blood PPIX and 63% reduction in plasma PPIX vs placebo at month 4 PPIX reductions with bitopertin are similar to those seen in pregnant EPP patients and a photoinactivation study in which 30-50% reductions in PPIX led to marked improvements in light tolerance
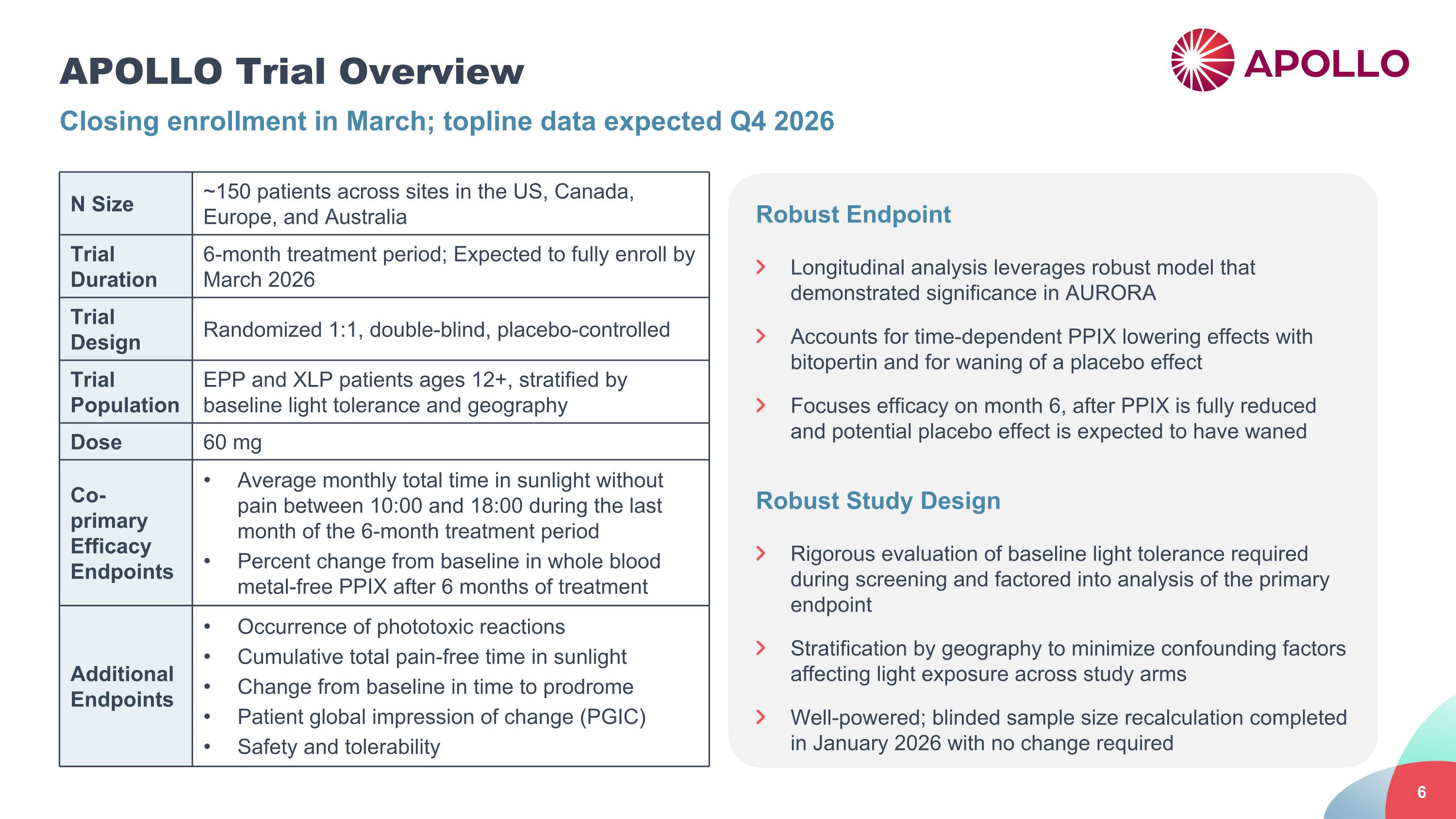
APOLLO Trial Overview Closing enrollment in March; topline data expected Q4 2026 N Size ~150 patients across sites in the US, Canada, Europe, and Australia Trial Duration 6-month treatment period; Expected to fully enroll by March 2026 Trial Design Randomized 1:1, double-blind, placebo-controlled Trial Population EPP and XLP patients ages 12+, stratified by baseline light tolerance and geography Dose 60 mg Co-primary Efficacy Endpoints Average monthly total time in sunlight without pain between 10:00 and 18:00 during the last month of the 6-month treatment period Percent change from baseline in whole blood metal-free PPIX after 6 months of treatment Additional Endpoints Occurrence of phototoxic reactions Cumulative total pain-free time in sunlight Change from baseline in time to prodrome Patient global impression of change (PGIC) Safety and tolerability Robust Endpoint Longitudinal analysis leverages robust model that demonstrated significance in AURORA Accounts for time-dependent PPIX lowering effects with bitopertin and for waning of a placebo effect Focuses efficacy on month 6, after PPIX is fully reduced and potential placebo effect is expected to have waned Robust Study Design Rigorous evaluation of baseline light tolerance required during screening and factored into analysis of the primary endpoint Stratification by geography to minimize confounding factors affecting light exposure across study arms Well-powered; blinded sample size recalculation completed in January 2026 with no change required
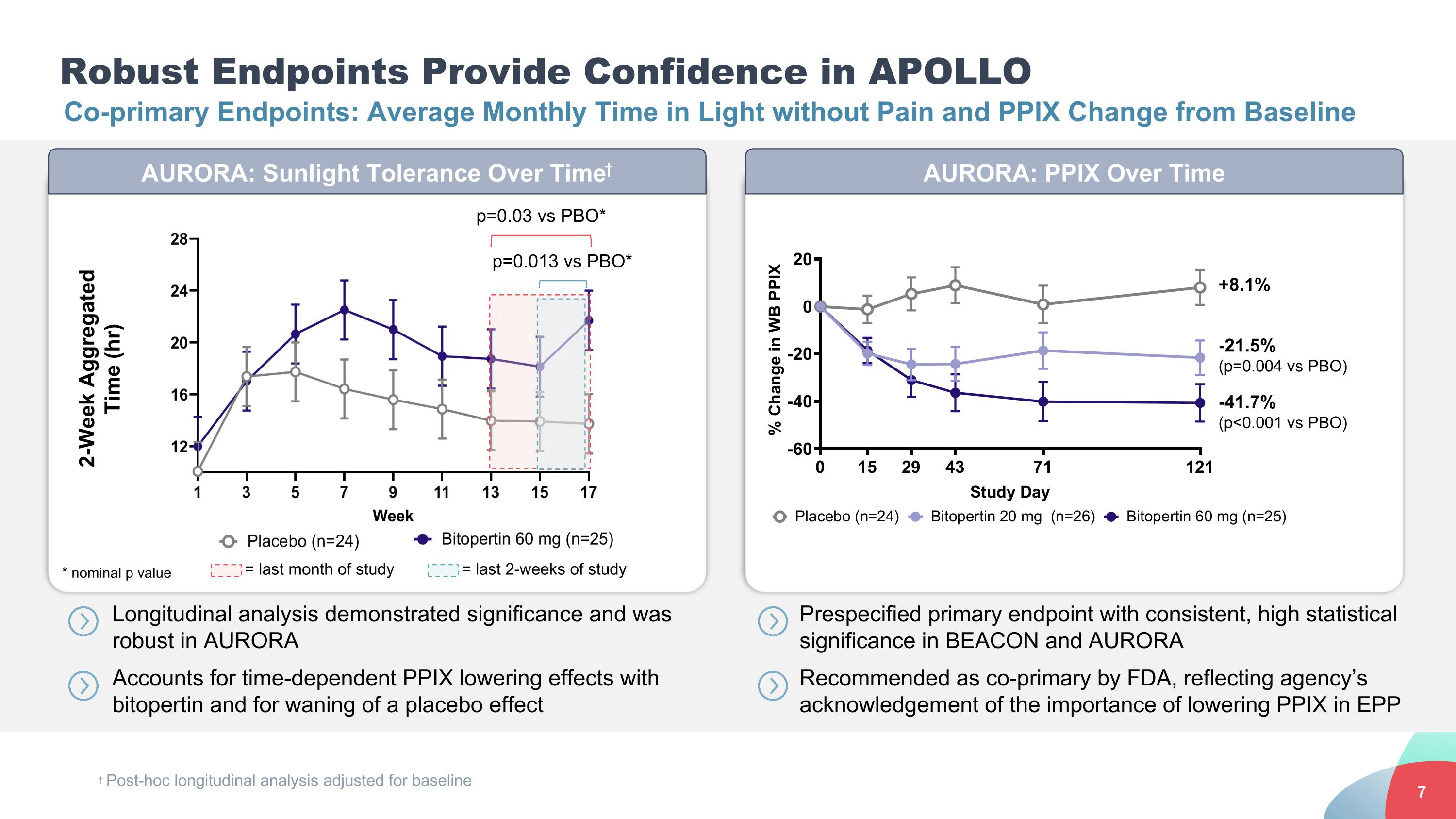
AURORA: Sunlight Tolerance Over Time† AURORA: PPIX Over Time Robust Endpoints Provide Confidence in APOLLO Co-primary Endpoints: Average Monthly Time in Light without Pain and PPIX Change from Baseline 2-Week Aggregated Time (hr) = last month of study = last 2-weeks of study p=0.013 vs PBO* p=0.03 vs PBO* † Post-hoc longitudinal analysis adjusted for baseline * nominal p value +8.1% -21.5% (p=0.004 vs PBO) -41.7% (p<0.001 vs PBO) Longitudinal analysis demonstrated significance and was robust in AURORA Accounts for time-dependent PPIX lowering effects with bitopertin and for waning of a placebo effect Prespecified primary endpoint with consistent, high statistical significance in BEACON and AURORA Recommended as co-primary by FDA, reflecting agency’s acknowledgement of the importance of lowering PPIX in EPP

Next Steps Disc plans to respond to the CRL with data from the ongoing APOLLO trial Expect to request Type A meeting with FDA to review our approach for resubmission Expect to complete enrollment in March 2026, several months ahead of guidance Expect to present topline data in Q4 2026 and use this data to submit a response to the CRL Typical FDA goal date for review of CRL response is ~6 months, implying potential for an updated decision by mid-2027

Disc’s EPP Community Commitment Disc’s belief in the importance of bringing a potential disease modifying therapy to the EPP community and the potential for bitopertin to set a new standard of care in EPP has not changed Disc has made every effort to bring bitopertin to the EPP community more quickly; while these efforts have not come to fruition, we remain committed to advancing the program and pursuing FDA approval of bitopertin During this process, Disc is committed to providing continued access to bitopertin for current clinical trial participants
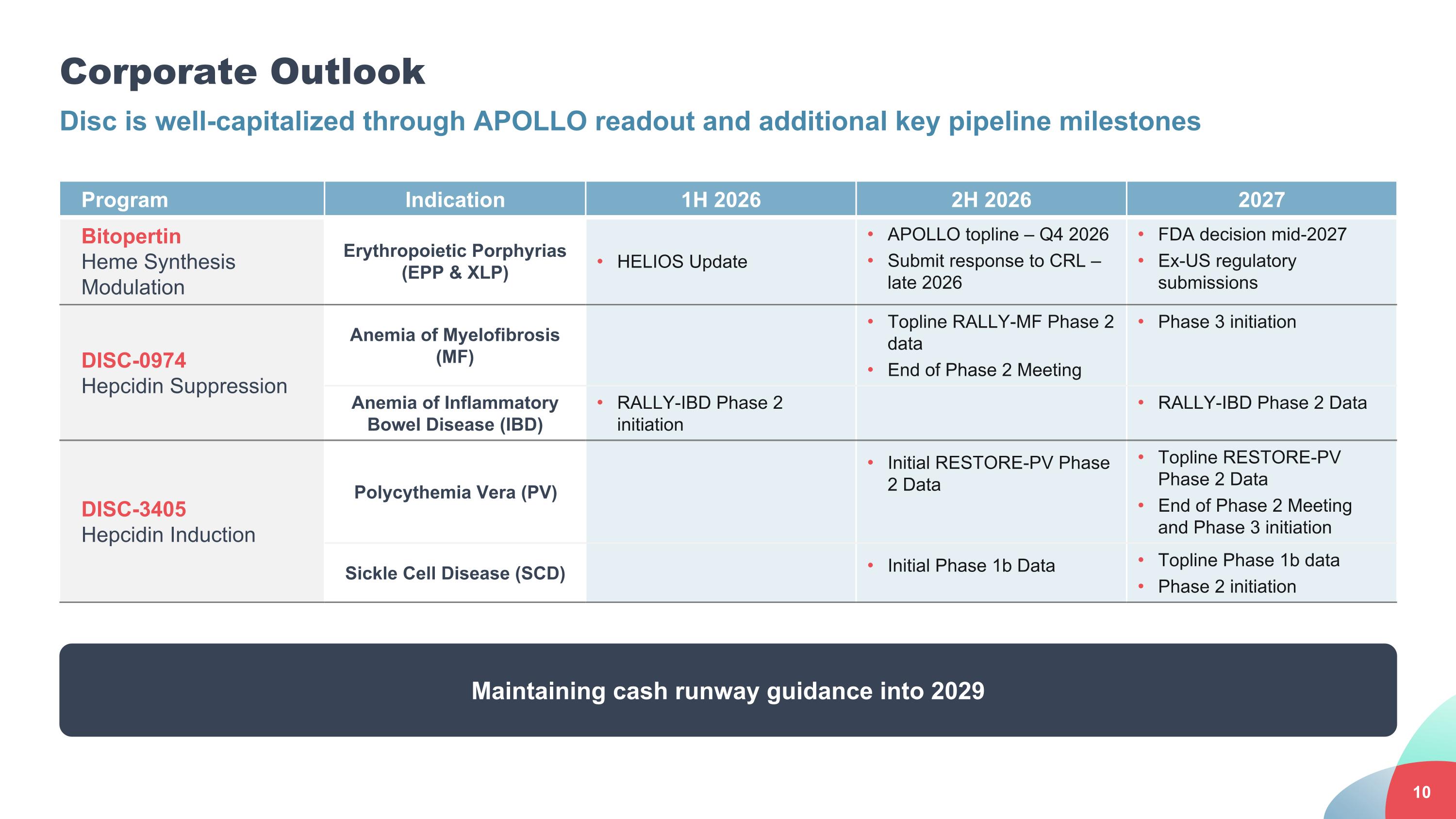
Corporate Outlook Disc is well-capitalized through APOLLO readout and additional key pipeline milestones Program Indication 1H 2026 2H 2026 2027 Bitopertin Heme Synthesis Modulation Erythropoietic Porphyrias (EPP & XLP) HELIOS Update APOLLO topline – Q4 2026 Submit response to CRL – late 2026 FDA decision mid-2027 Ex-US regulatory submissions DISC-0974 Hepcidin Suppression Anemia of Myelofibrosis (MF) Topline RALLY-MF Phase 2 data End of Phase 2 Meeting Phase 3 initiation Anemia of Inflammatory Bowel Disease (IBD) RALLY-IBD Phase 2 initiation RALLY-IBD Phase 2 Data DISC-3405 Hepcidin Induction Polycythemia Vera (PV) Initial RESTORE-PV Phase 2 Data Topline RESTORE-PV Phase 2 Data End of Phase 2 Meeting and Phase 3 initiation Sickle Cell Disease (SCD) Initial Phase 1b Data Topline Phase 1b data Phase 2 initiation Maintaining cash runway guidance into 2029

Q&A