

Global Science. One Purpose. 48-week Phase 3 Clinical Trial Data from China for Telitacicept in Primary Sjögren’s Disease October 28, 2025

Forward Looking Statement This presentation (the “Presentation”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 about Vor Biopharma Inc. (“Vor,” “Vor Bio” or the “Company”). The words “aim,” “anticipate,” “believe,” “can,” “could,” “design,” “enable” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “project,” “should,” “target,” “towards,” “will,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements in this presentation include those regarding Vor Bio's plans for development and commercialization of telitacicept; the potential therapeutic benefits of telitacicept in various indications including generalized myasthenia gravis (gMG) and primary Sjögren's disease; the potential of telitacicept to be a best-in-disease and a disease modification in gMG and Sjogren’s; the potential benefits of the dual BAFF/APRIL mechanism; the expected safety profile of telitacicept; the market opportunities for telitacicept; the addressable patient populations in the indications Vor Bio intends to treat; and other statements that are not historical fact. Vor Bio may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including: uncertainties inherent in the initiation, completion of, and availability and timing of results from, preclinical studies and clinical trials; whether preclinical data or interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; the uncertainty of regulatory approvals to conduct trials or to market products; Vor Bio's reliance on third parties over which it may not always have full control; and the availability of funding sufficient for Vor Bio's foreseeable and unforeseeable operating expenses and capital expenditure requirements. These and other risks are described in greater detail under the caption “Risk Factors” included in Vor Bio's most recent annual or quarterly report and in other reports it has filed or may file with the Securities and Exchange Commission. Any forward-looking statements contained in this Presentation speak only as of the date of this Presentation, and Vor Bio expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise, except as may be required by law. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and Vor Bio's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, the Company has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third party sources. In addition, there can be no guarantee as to the accuracy or reliability of any assumptions or limitations that may be included in such third-party information. While the Company believes its own internal research is reliable, such research has not been verified by any independent source. All brand names or trademarks appearing in this Presentation are the property of their respective owners.

Agenda Opening Remarks & Data Highlights Jean-Paul Kress, M.D., Chairman & Chief Executive Officer Sjögren’s Disease – A Major Unmet Need Ronald van Vollenhoven, M.D., Ph.D., Professor of Rheumatology at Amsterdam University Medical Center 1. 2. Primary Sjögren’s Disease China Phase 3 Results Qing Zuraw, M.D., M.P.H., M.B.A., Chief Development Officer 3. Competitive Landscape & Commercial Opportunity Dallan Murray, Chief Commercial Officer 4. Closing Remarks 5. Jean-Paul Kress, M.D., Chairman & Chief Executive Officer
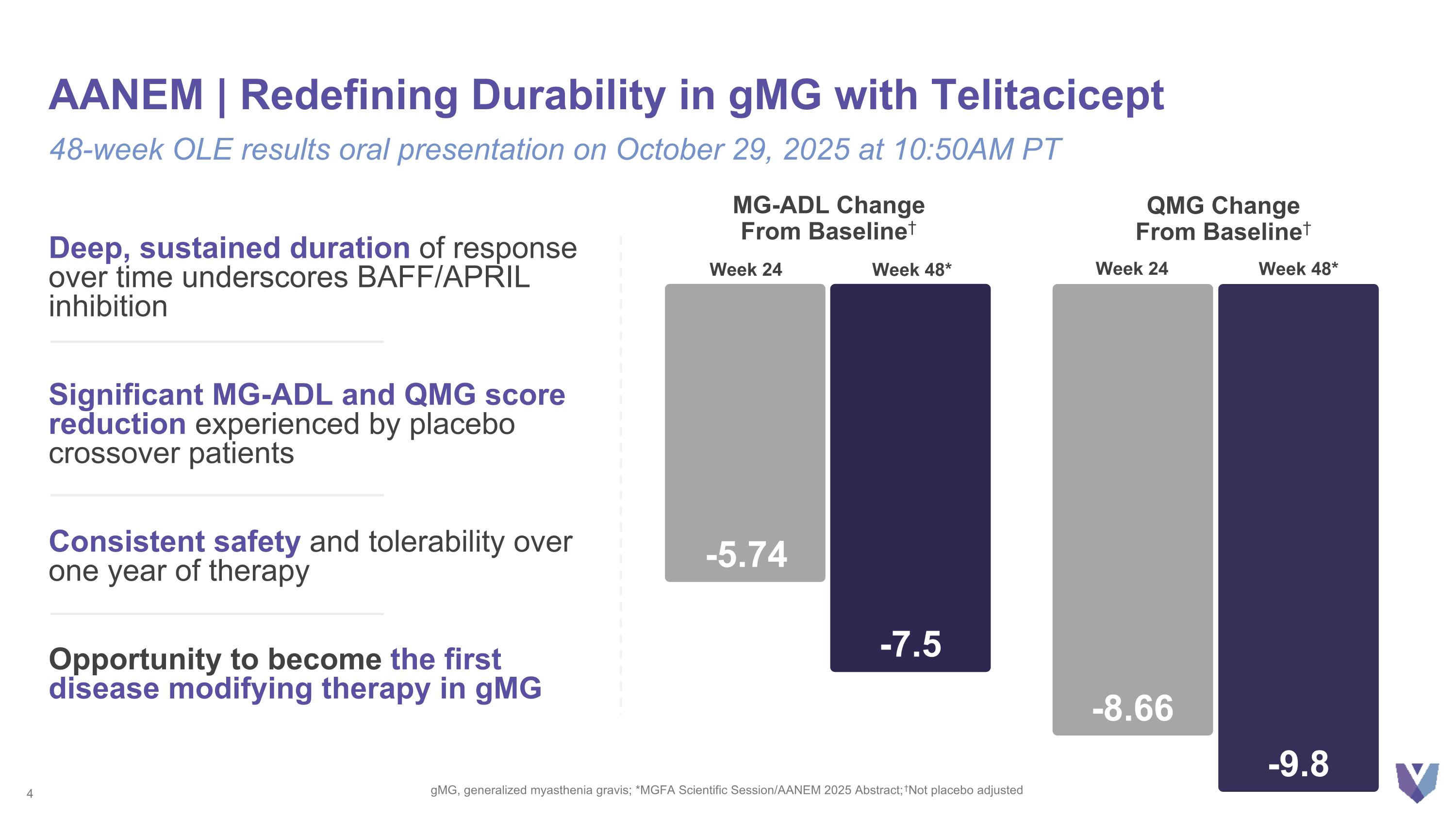
AANEM | Redefining Durability in gMG with Telitacicept gMG, generalized myasthenia gravis; *MGFA Scientific Session/AANEM 2025 Abstract; †Not placebo adjusted 48-week OLE results oral presentation on October 29, 2025 at 10:50AM PT MG-ADL Change From Baseline† -5.74 Week 24 -7.5 Week 48* -8.66 Week 24 -9.8 Week 48* QMG Change From Baseline† Deep, sustained duration of response over time underscores BAFF/APRIL inhibition Significant MG-ADL and QMG score reduction experienced by placebo crossover patients Consistent safety and tolerability over one year of therapy Opportunity to become the first disease modifying therapy in gMG
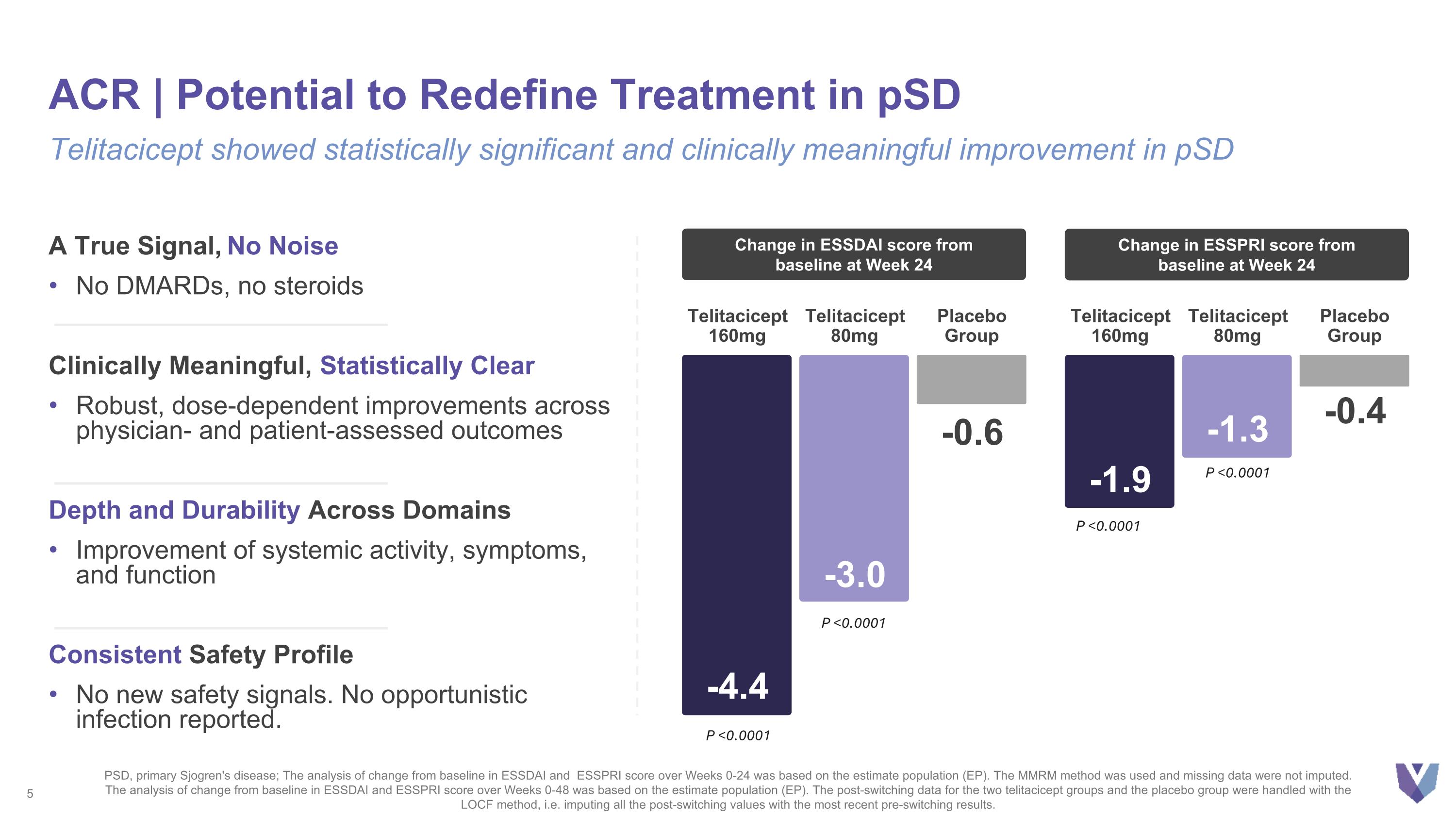
A True Signal, No Noise No DMARDs, no steroids Clinically Meaningful, Statistically Clear Robust, dose-dependent improvements across physician- and patient-assessed outcomes Depth and Durability Across Domains Improvement of systemic activity, symptoms, and function Consistent Safety Profile No new safety signals. No opportunistic infection reported. ACR | Potential to Redefine Treatment in pSD Telitacicept showed statistically significant and clinically meaningful improvement in pSD -4.4 Telitacicept 160mg -3.0 Telitacicept 80mg -0.6 Placebo Group Change in ESSDAI score from baseline at Week 24 -1.9 Telitacicept 160mg -1.3 Telitacicept 80mg -0.4 Placebo Group Change in ESSPRI score from baseline at Week 24 P <0.0001 P <0.0001 PSD, primary Sjogren's disease; The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. P <0.0001 P <0.0001
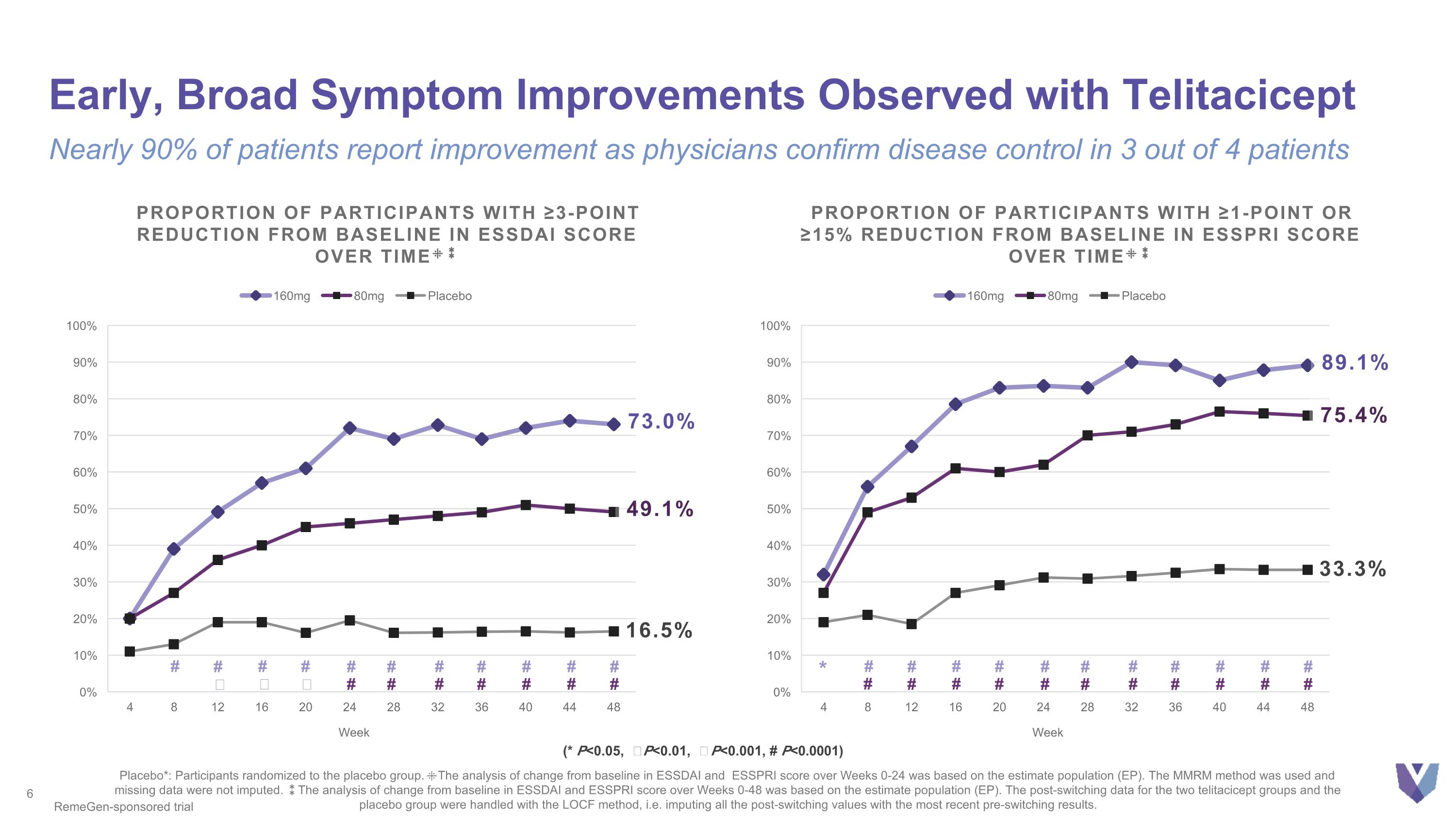
Early, Broad Symptom Improvements Observed with Telitacicept Nearly 90% of patients report improvement as physicians confirm disease control in 3 out of 4 patients Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. RemeGen-sponsored trial 73.0% 16.5% Proportion of Participants with ≥3-point Reduction from Baseline in ESSDAI Score Over Time⁜⁑ 49.1% # # Ϯ # Ϯ # ǂ # # # # # # # # # # # # # # (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001) 33.3% Proportion of Participants with ≥1-point or ≥15% Reduction from Baseline in ESSPRI Score Over Time⁜⁑ 89.1% 75.4% # # # # # # # # # # # # # # # # # # # # # * #
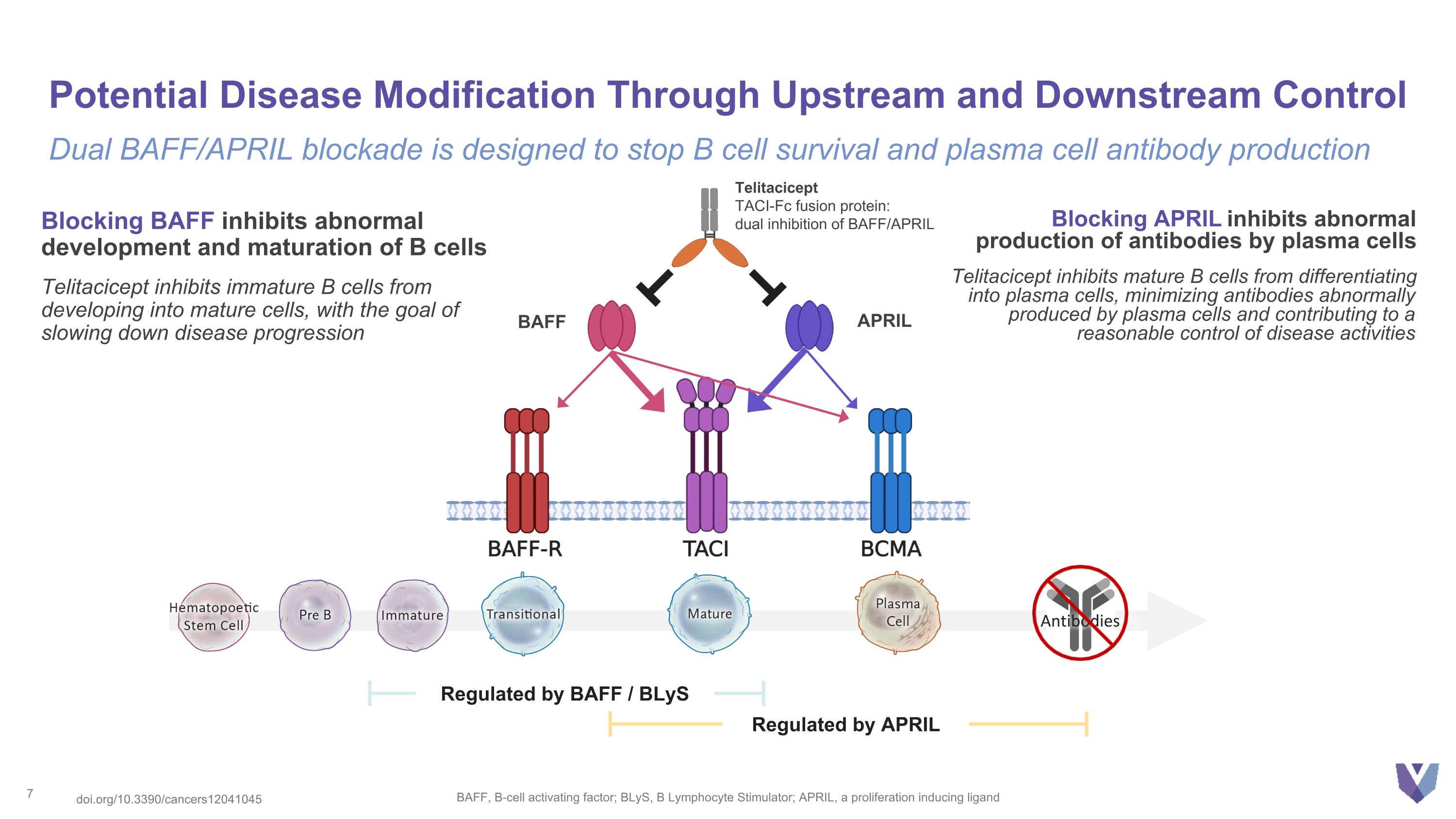
Potential Disease Modification Through Upstream and Downstream Control BAFF, B-cell activating factor; BLyS, B Lymphocyte Stimulator; APRIL, a proliferation inducing ligand Dual BAFF/APRIL blockade is designed to stop B cell survival and plasma cell antibody production Regulated by APRIL Regulated by BAFF / BLyS Antibodies Telitacicept TACI-Fc fusion protein: dual inhibition of BAFF/APRIL Blocking BAFF inhibits abnormal development and maturation of B cells Telitacicept inhibits immature B cells from developing into mature cells, with the goal of slowing down disease progression Blocking APRIL inhibits abnormal production of antibodies by plasma cells Telitacicept inhibits mature B cells from differentiating into plasma cells, minimizing antibodies abnormally produced by plasma cells and contributing to a reasonable control of disease activities BAFF APRIL doi.org/10.3390/cancers12041045
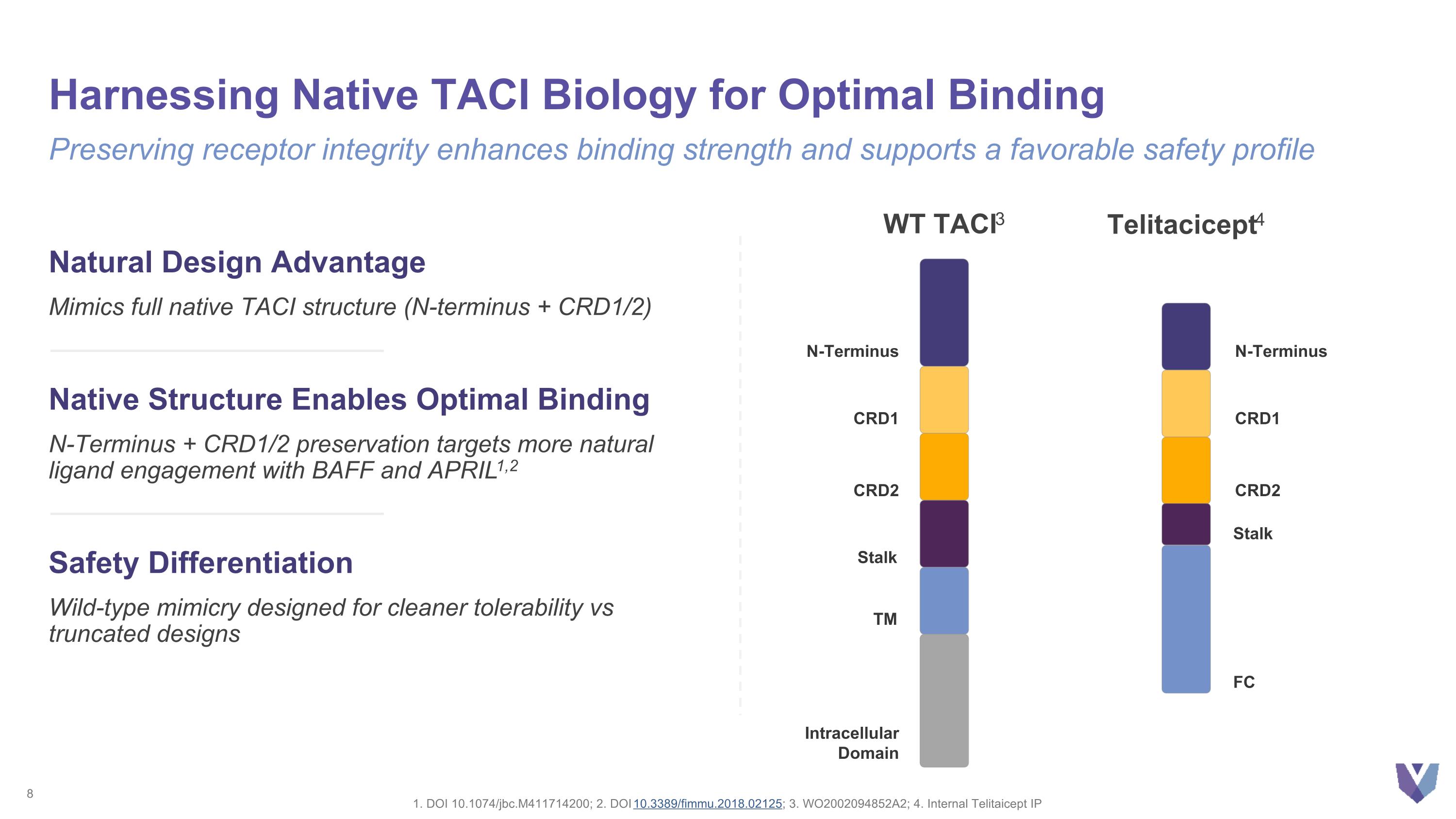
Harnessing Native TACI Biology for Optimal Binding 1. DOI 10.1074/jbc.M411714200; 2. DOI 10.3389/fimmu.2018.02125; 3. WO2002094852A2; 4. Internal Telitaicept IP Preserving receptor integrity enhances binding strength and supports a favorable safety profile Natural Design Advantage Mimics full native TACI structure (N-terminus + CRD1/2) Native Structure Enables Optimal Binding N-Terminus + CRD1/2 preservation targets more natural ligand engagement with BAFF and APRIL1,2 Safety Differentiation Wild-type mimicry designed for cleaner tolerability vs truncated designs N-Terminus CRD1 CRD2 Stalk TM Intracellular Domain N-Terminus CRD1 CRD2 Stalk FC Telitacicept4 WT TACI3

Telitacicept: Redefining What Success Looks Like In Autoimmune Disease 2021 - Systemic Lupus Erythematosus (SLE)† 2024 - Rheumatoid Arthritis (RA) 2025 – Generalized Myasthenia Gravis (gMG) †Conditional Approval, Full Approval in 2023; *Accelerated Approval in China Clinically meaningful results in every late-stage program with consistent efficacy and safety 1 2 3 4 5 6 3 Commercial Approvals in China Primary Sjogren’s Disease (pSD, Filed 2025) IgA Nephropathy (IgAN, Filed 2025)* 2 BLA Submissions in China Unique Dual BAFF/APRIL Inhibition Drives Superior Clinical Benefit in SLE, pSD, gMG in China Best-In-Disease Leadership Proven real-world impact at commercial scale 10s of Thousands of Patients Treated in China No burdensome vaccination No B-cell depletion-related SAEs Mild to moderate AEs Favorable Safety Profile Safety profile confirmed in ~1,800 patients across 6+ indications in China AE rates comparable to placebo Consistent Tolerability

Primary Sjögren’s Disease China Phase 3 Results Qing Zuraw, M.D., M.P.H., M.B.A., Chief Development Officer
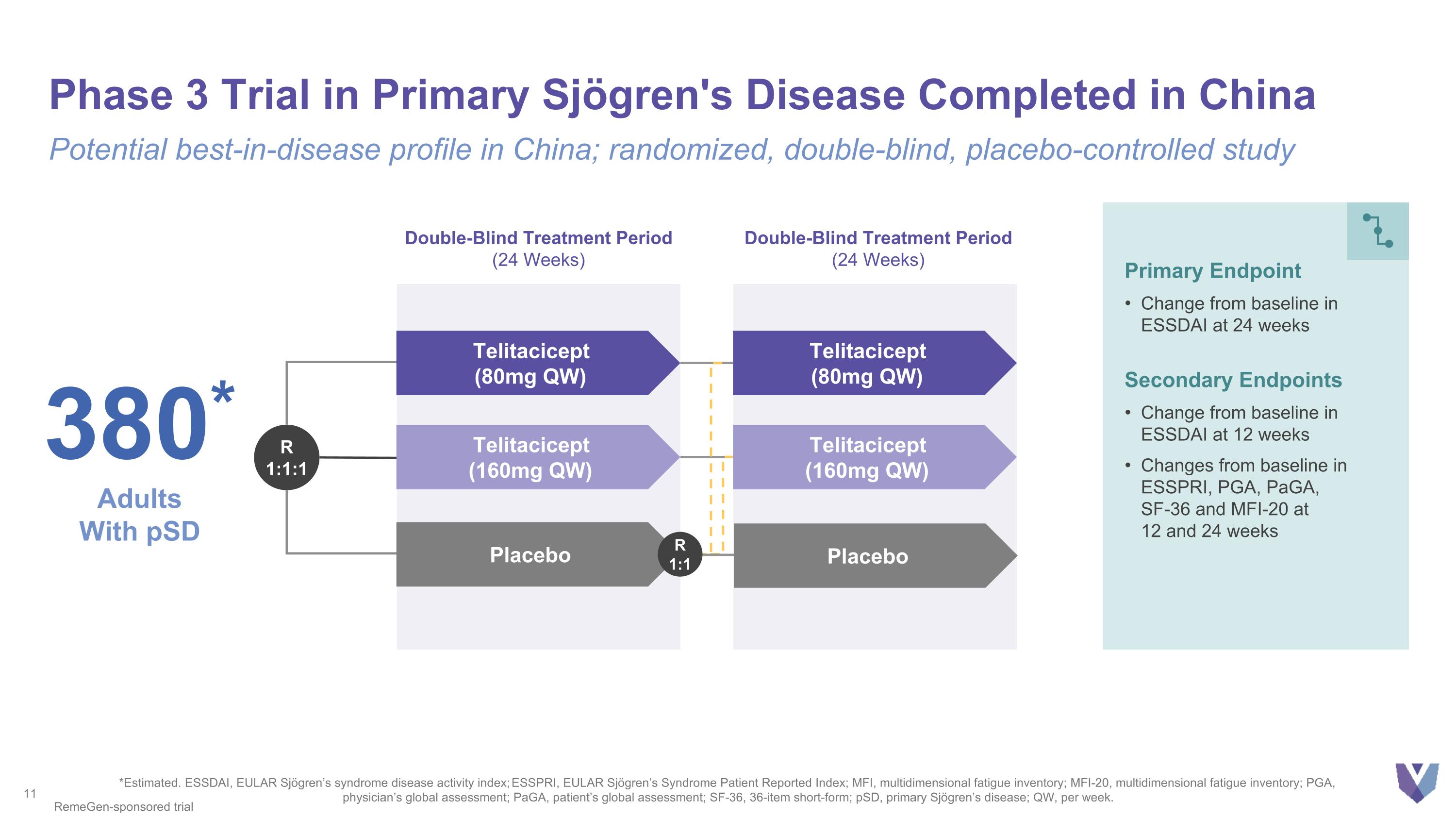
Phase 3 Trial in Primary Sjögren's Disease Completed in China Potential best-in-disease profile in China; randomized, double-blind, placebo-controlled study *Estimated. ESSDAI, EULAR Sjögren’s syndrome disease activity index; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; MFI, multidimensional fatigue inventory; MFI-20, multidimensional fatigue inventory; PGA, physician’s global assessment; PaGA, patient’s global assessment; SF-36, 36-item short-form; pSD, primary Sjögren’s disease; QW, per week. 380* Adults With pSD Primary Endpoint Change from baseline in ESSDAI at 24 weeks Secondary Endpoints Change from baseline in ESSDAI at 12 weeks Changes from baseline in ESSPRI, PGA, PaGA, SF-36 and MFI-20 at 12 and 24 weeks Double-Blind Treatment Period (24 Weeks) R 1:1:1 Telitacicept (80mg QW) Telitacicept (160mg QW) Double-Blind Treatment Period (24 Weeks) Telitacicept (80mg QW) Telitacicept (160mg QW) Placebo RemeGen-sponsored trial Placebo R 1:1
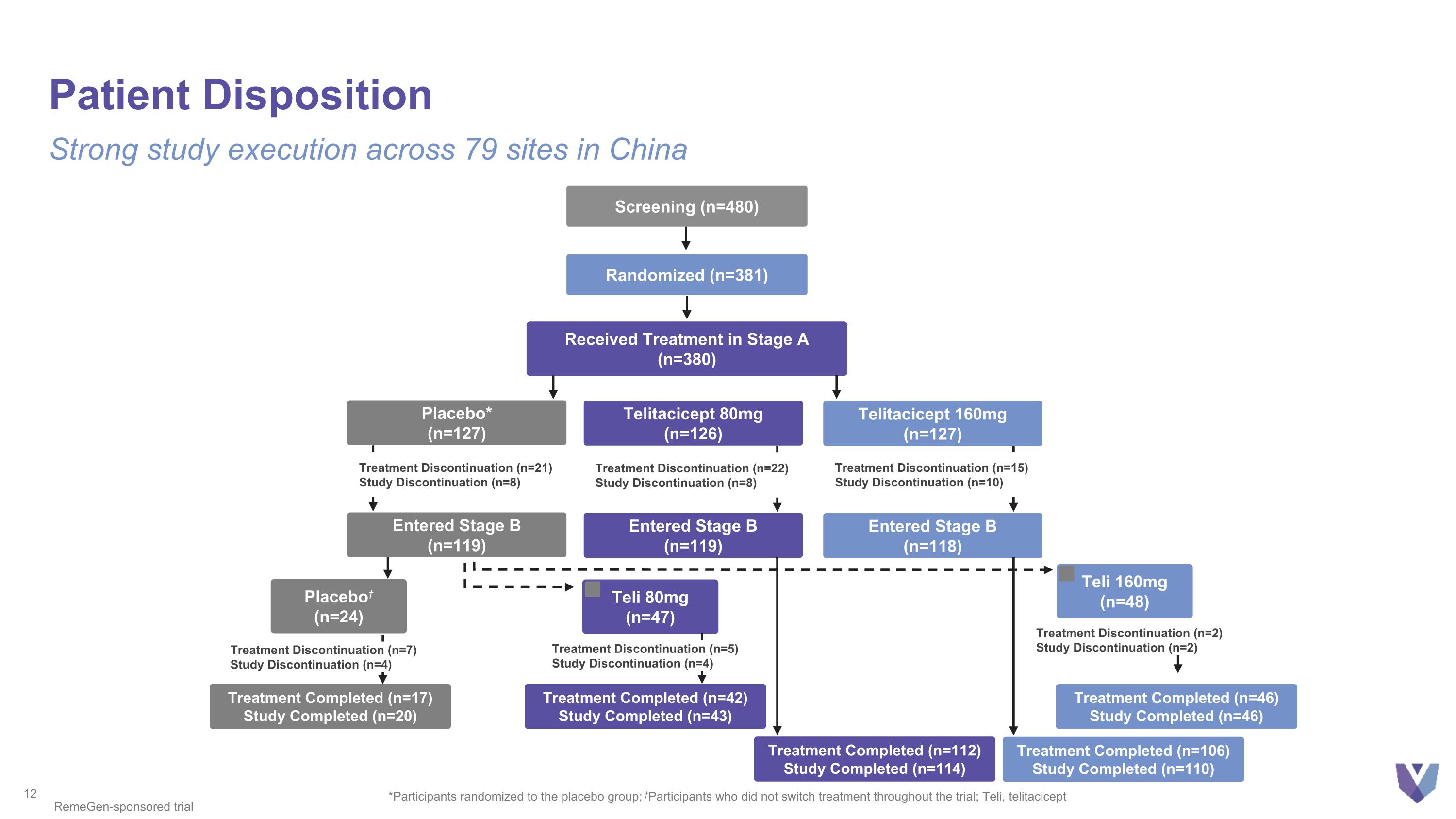
Patient Disposition Strong study execution across 79 sites in China *Participants randomized to the placebo group; †Participants who did not switch treatment throughout the trial; Teli, telitacicept RemeGen-sponsored trial Screening (n=480) Randomized (n=381) Received Treatment in Stage A (n=380) Placebo* (n=127) Telitacicept 160mg (n=127) Telitacicept 80mg (n=126) Treatment Discontinuation (n=21) Study Discontinuation (n=8) Treatment Discontinuation (n=15) Study Discontinuation (n=10) Treatment Discontinuation (n=22) Study Discontinuation (n=8) Entered Stage B (n=118) Entered Stage B (n=119) Placebo† (n=24) Teli 160mg (n=48) Teli 80mg (n=47) Treatment Completed (n=17) Study Completed (n=20) Treatment Completed (n=46) Study Completed (n=46) Treatment Completed (n=42) Study Completed (n=43) Treatment Completed (n=106) Study Completed (n=110) Treatment Completed (n=112) Study Completed (n=114) Entered Stage B (n=119) Treatment Discontinuation (n=7) Study Discontinuation (n=4) Treatment Discontinuation (n=5) Study Discontinuation (n=4) Treatment Discontinuation (n=2) Study Discontinuation (n=2)
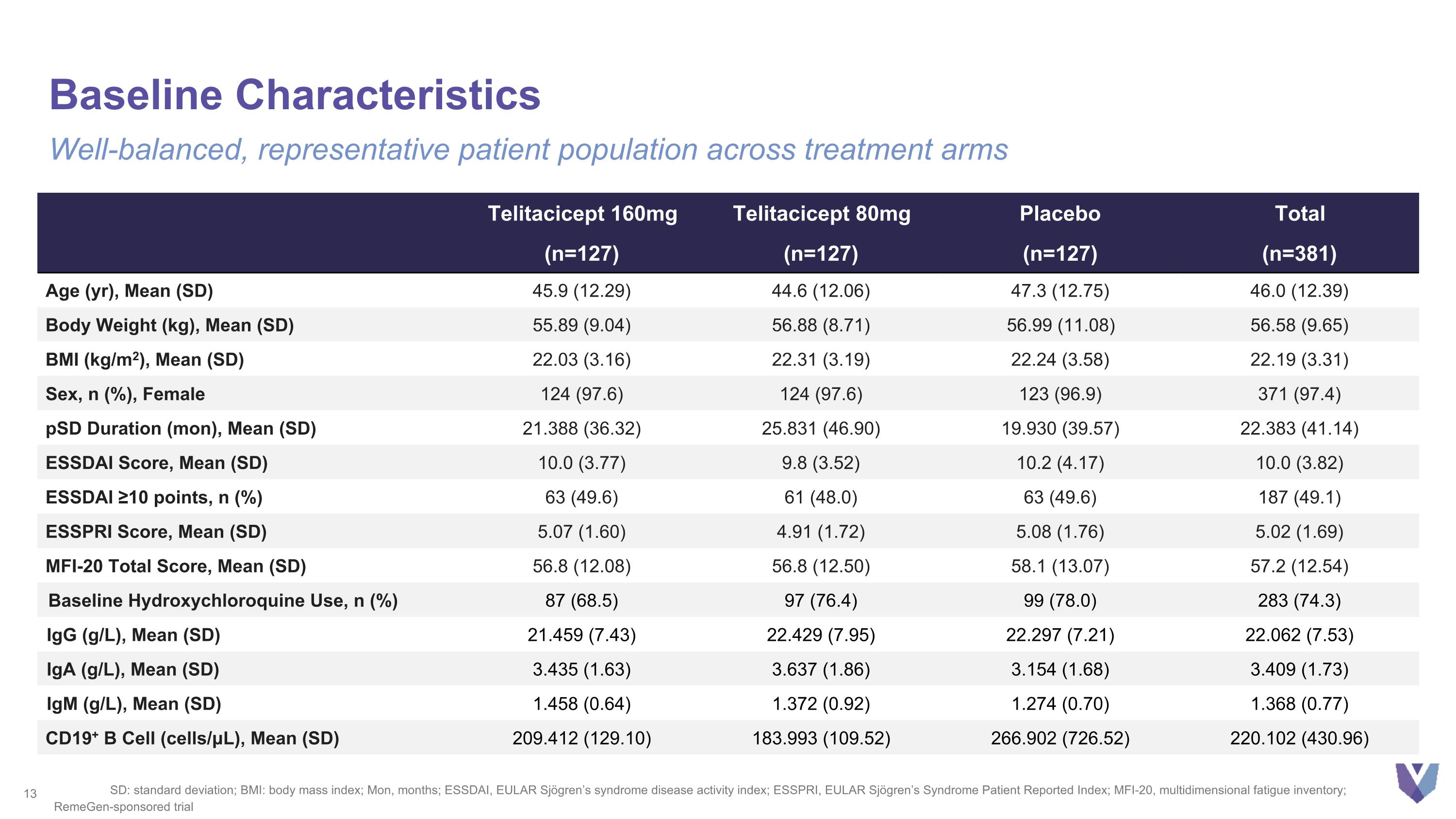
Baseline Characteristics Well-balanced, representative patient population across treatment arms SD: standard deviation; BMI: body mass index; Mon, months; ESSDAI, EULAR Sjögren’s syndrome disease activity index; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; MFI-20, multidimensional fatigue inventory; Telitacicept 160mg Telitacicept 80mg Placebo Total (n=127) (n=127) (n=127) (n=381) Age (yr), Mean (SD) 45.9 (12.29) 44.6 (12.06) 47.3 (12.75) 46.0 (12.39) Body Weight (kg), Mean (SD) 55.89 (9.04) 56.88 (8.71) 56.99 (11.08) 56.58 (9.65) BMI (kg/m2), Mean (SD) 22.03 (3.16) 22.31 (3.19) 22.24 (3.58) 22.19 (3.31) Sex, n (%), Female 124 (97.6) 124 (97.6) 123 (96.9) 371 (97.4) pSD Duration (mon), Mean (SD) 21.388 (36.32) 25.831 (46.90) 19.930 (39.57) 22.383 (41.14) ESSDAI Score, Mean (SD) 10.0 (3.77) 9.8 (3.52) 10.2 (4.17) 10.0 (3.82) ESSDAI ≥10 points, n (%) 63 (49.6) 61 (48.0) 63 (49.6) 187 (49.1) ESSPRI Score, Mean (SD) 5.07 (1.60) 4.91 (1.72) 5.08 (1.76) 5.02 (1.69) MFI-20 Total Score, Mean (SD) 56.8 (12.08) 56.8 (12.50) 58.1 (13.07) 57.2 (12.54) Baseline Hydroxychloroquine Use, n (%) 87 (68.5) 97 (76.4) 99 (78.0) 283 (74.3) IgG (g/L), Mean (SD) 21.459 (7.43) 22.429 (7.95) 22.297 (7.21) 22.062 (7.53) IgA (g/L), Mean (SD) 3.435 (1.63) 3.637 (1.86) 3.154 (1.68) 3.409 (1.73) IgM (g/L), Mean (SD) 1.458 (0.64) 1.372 (0.92) 1.274 (0.70) 1.368 (0.77) CD19+ B Cell (cells/μL), Mean (SD) 209.412 (129.10) 183.993 (109.52) 266.902 (726.52) 220.102 (430.96) RemeGen-sponsored trial
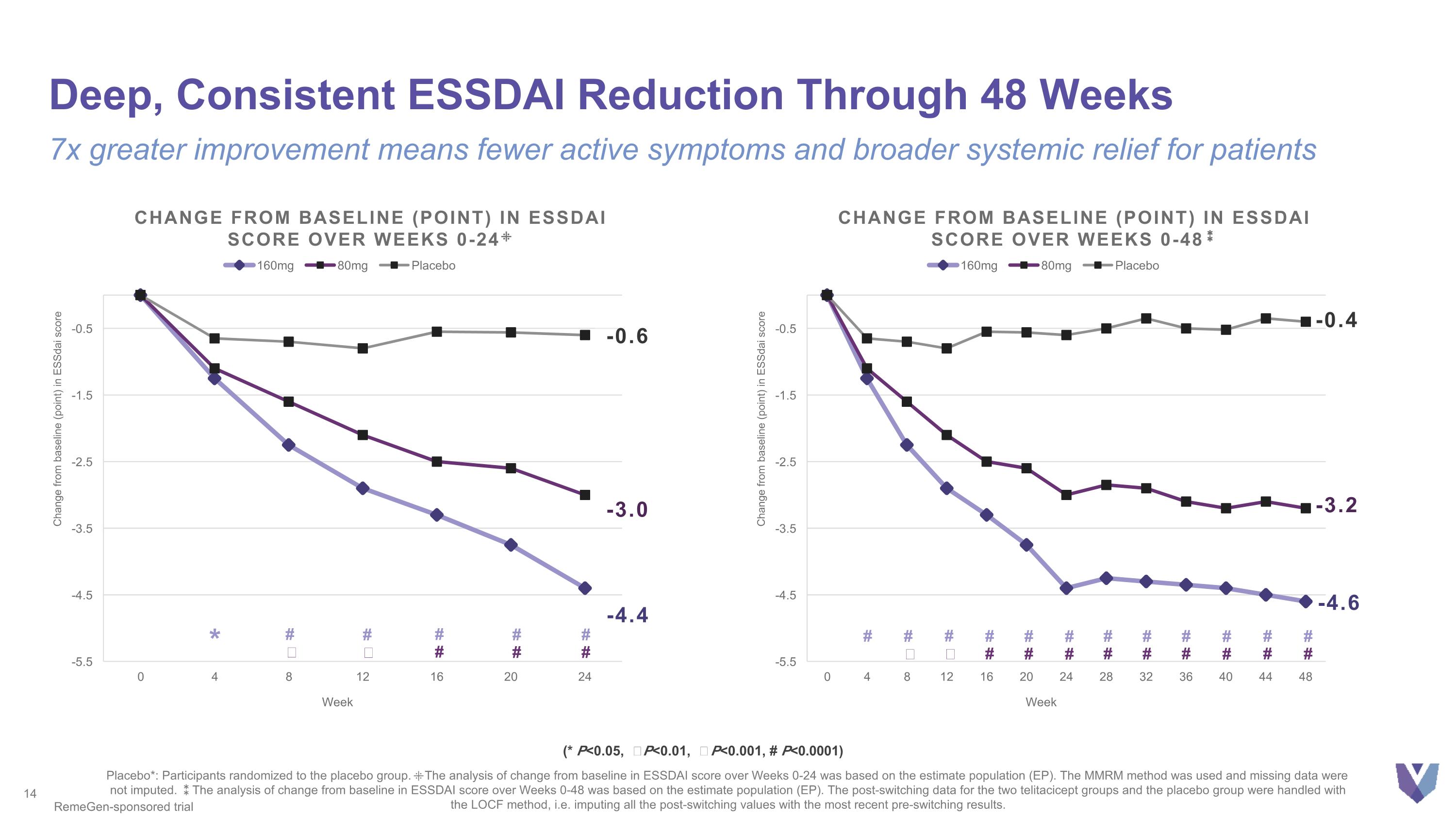
Deep, Consistent ESSDAI Reduction Through 48 Weeks 7x greater improvement means fewer active symptoms and broader systemic relief for patients Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ESSDAI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ESSDAI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. -4.4 Change from baseline (point) in essdai score over weeks 0-24⁜ -0.6 -3.0 * # # # # # Ϯ ǂ # # # -3.2 -0.4 -4.6 Change from baseline (point) in essdai score over weeks 0-48⁑ # # Ϯ # ǂ # # # # # # # # # # # # # # # # # # RemeGen-sponsored trial (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001)
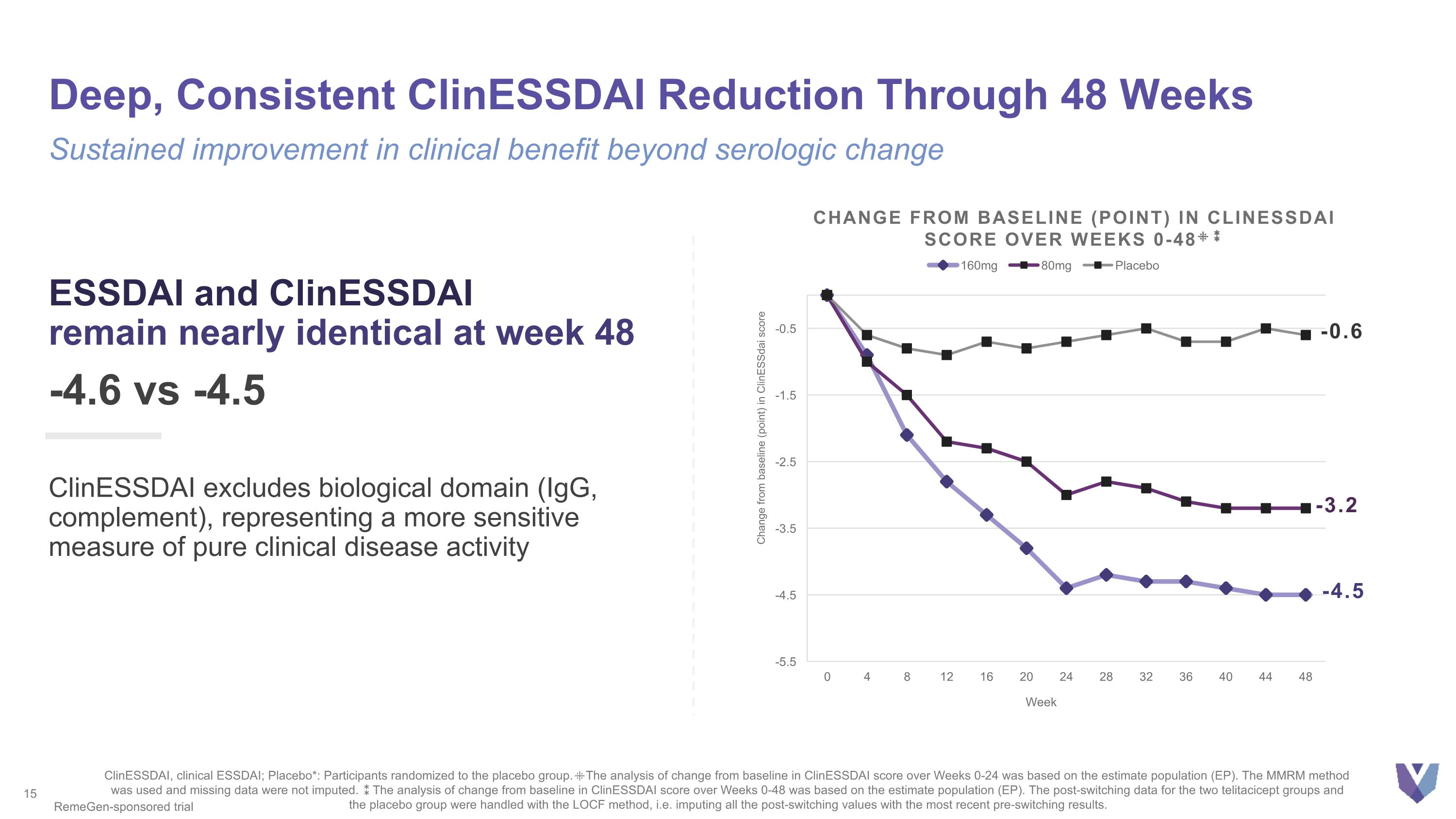
Deep, Consistent ClinESSDAI Reduction Through 48 Weeks ESSDAI and ClinESSDAI remain nearly identical at week 48 -4.6 vs -4.5 ClinESSDAI excludes biological domain (IgG, complement), representing a more sensitive measure of pure clinical disease activity Sustained improvement in clinical benefit beyond serologic change ClinESSDAI, clinical ESSDAI; Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ClinESSDAI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ClinESSDAI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. RemeGen-sponsored trial -3.2 -0.6 Change from baseline (point) in Clinessdai score over weeks 0-48⁜⁑ -4.5
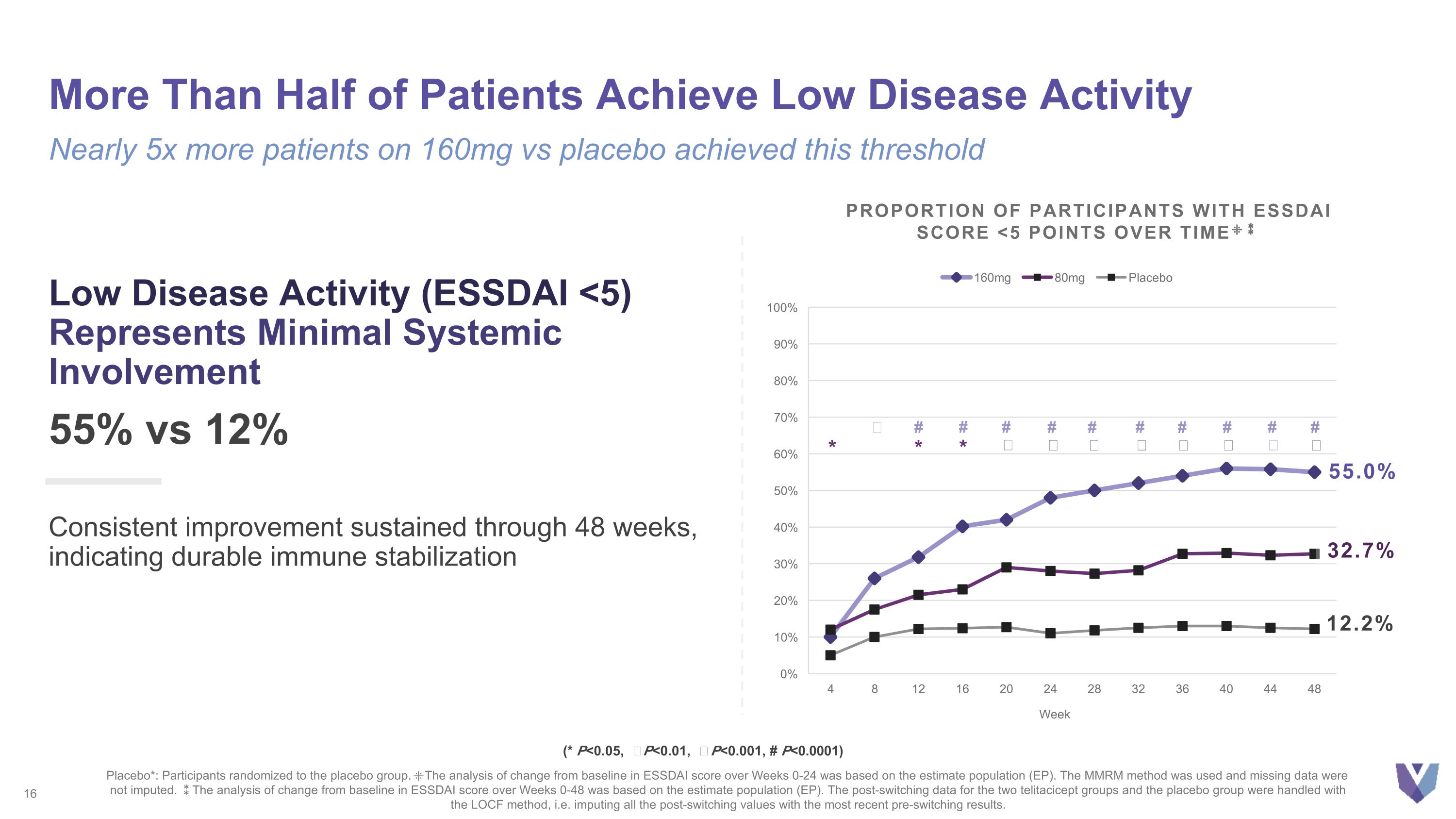
More Than Half of Patients Achieve Low Disease Activity Nearly 5x more patients on 160mg vs placebo achieved this threshold Low Disease Activity (ESSDAI <5) Represents Minimal Systemic Involvement 55% vs 12% Consistent improvement sustained through 48 weeks, indicating durable immune stabilization Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ESSDAI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ESSDAI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001) 55.0% 12.2% Proportion of Participants with ESSDAI Score <5 Points Over Time⁜⁑ 32.7% ǂ # * # * # Ϯ # ǂ # Ϯ # Ϯ # ǂ # ǂ # ǂ # ǂ *
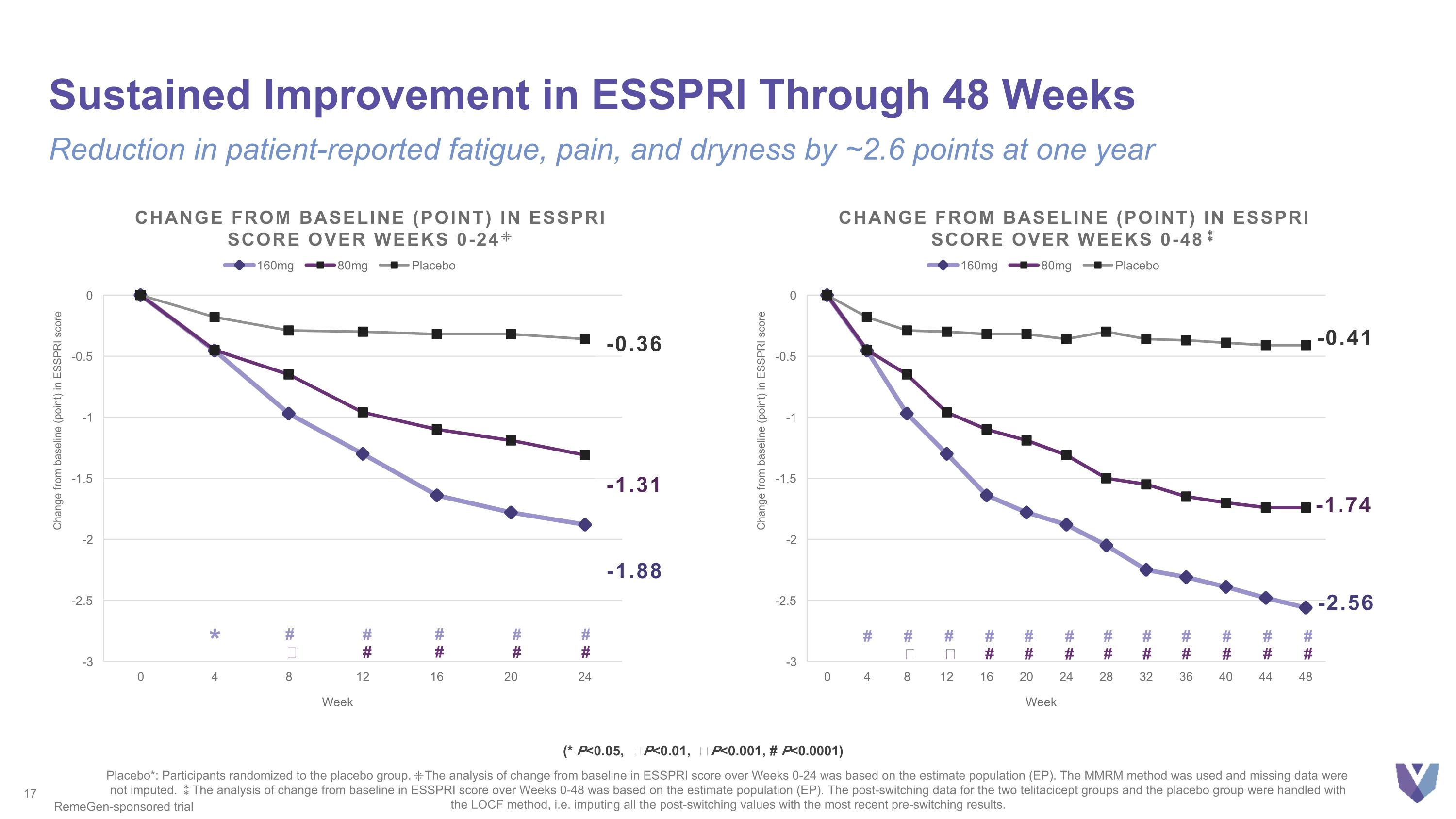
Sustained Improvement in ESSPRI Through 48 Weeks Reduction in patient-reported fatigue, pain, and dryness by ~2.6 points at one year Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ESSPRI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ESSPRI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. RemeGen-sponsored trial (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001) -1.88 Change from baseline (point) in ESSPRI score over weeks 0-24⁜ -0.36 -1.31 * # # # # # Ϯ # # # # -1.74 -0.41 -2.56 Change from baseline (point) in ESSPRI score over weeks 0-48⁑ # # Ϯ # ǂ # # # # # # # # # # # # # # # # # #
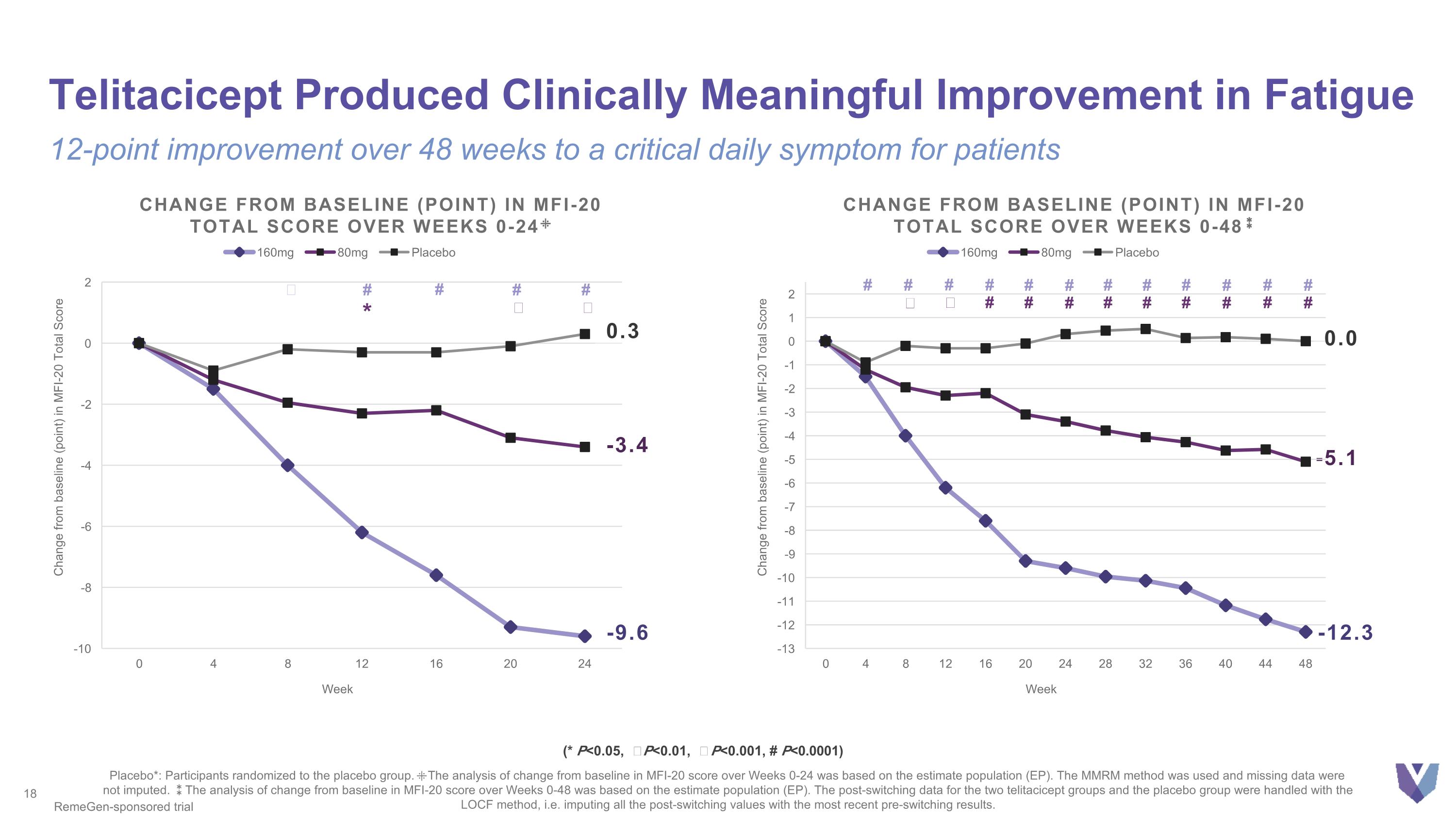
Telitacicept Produced Clinically Meaningful Improvement in Fatigue 12-point improvement over 48 weeks to a critical daily symptom for patients Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in MFI-20 score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in MFI-20 score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. -9.6 Change from baseline (point) in MFI-20 Total Score over weeks 0-24⁜ 0.3 -3.4 ǂ # # # # * Ϯ Ϯ -5.1 -12.3 Change from baseline (point) in MFI-20 Total Score over weeks 0-48⁑ 0.0 # # Ϯ # ǂ # # # # # # # # # # # # # # # # # # RemeGen-sponsored trial (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001)
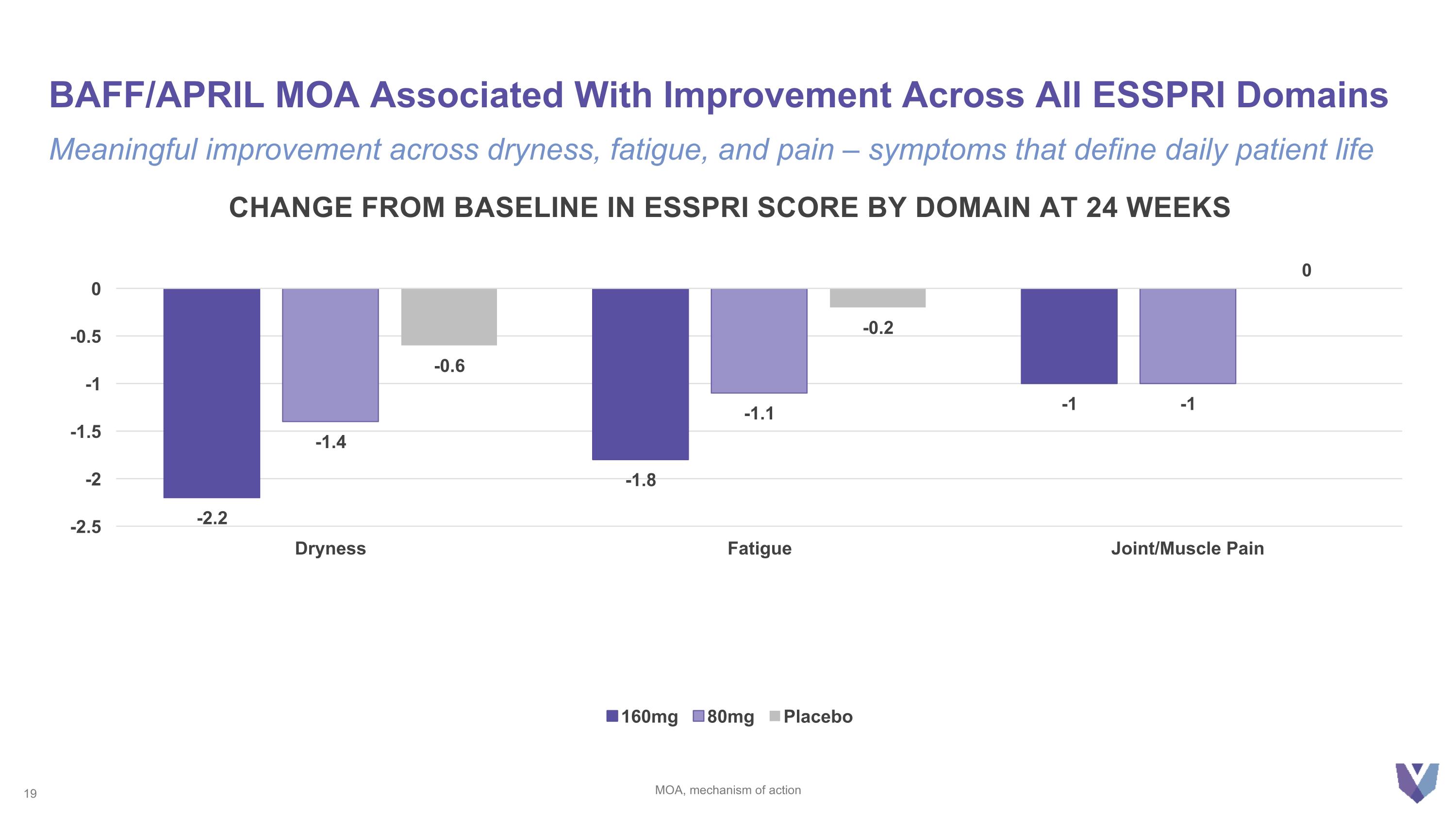
BAFF/APRIL MOA Associated With Improvement Across All ESSPRI Domains MOA, mechanism of action Meaningful improvement across dryness, fatigue, and pain – symptoms that define daily patient life
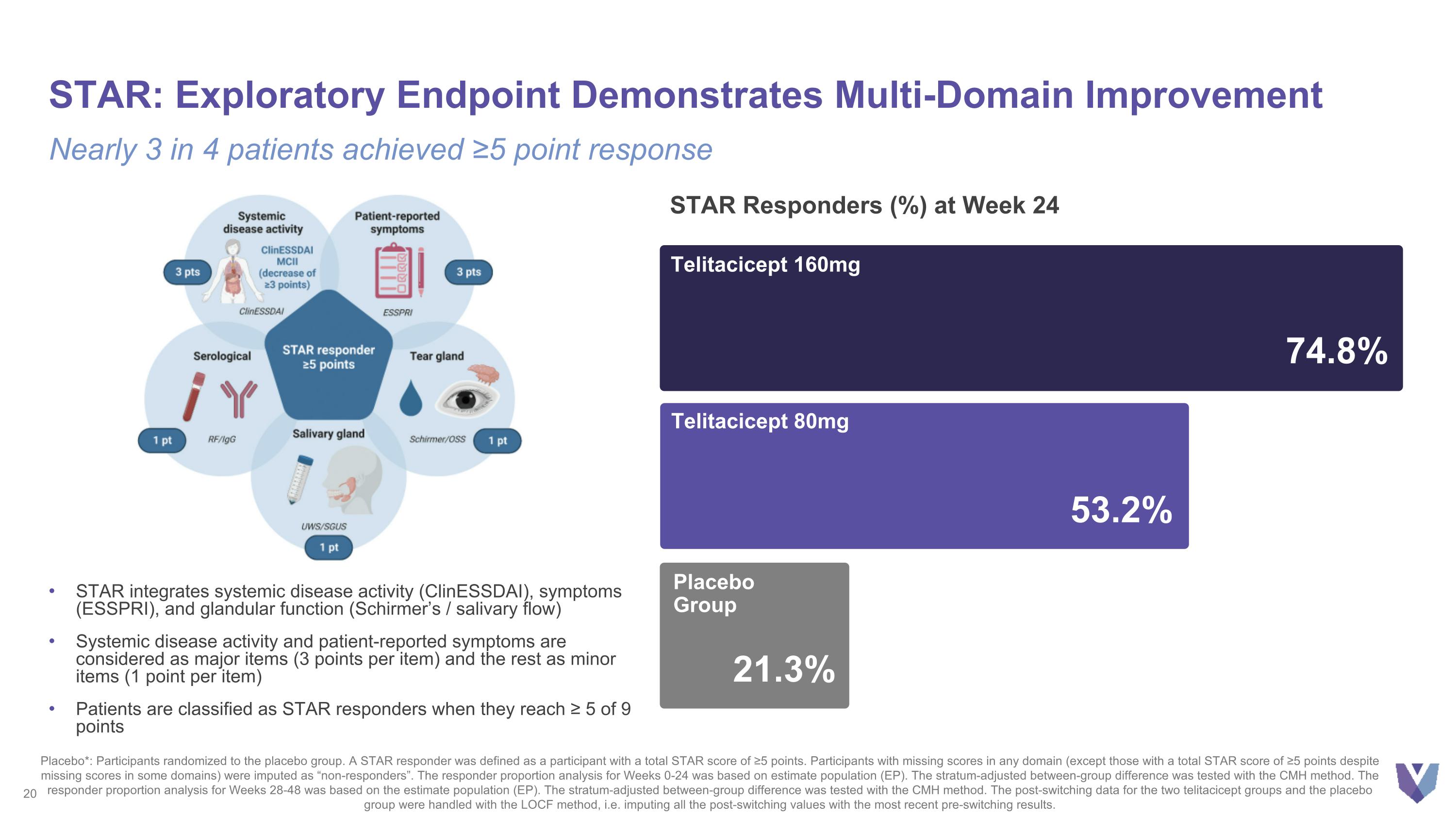
STAR: Exploratory Endpoint Demonstrates Multi-Domain Improvement Nearly 3 in 4 patients achieved ≥5 point response Placebo*: Participants randomized to the placebo group. A STAR responder was defined as a participant with a total STAR score of ≥5 points. Participants with missing scores in any domain (except those with a total STAR score of ≥5 points despite missing scores in some domains) were imputed as “non-responders”. The responder proportion analysis for Weeks 0-24 was based on estimate population (EP). The stratum-adjusted between-group difference was tested with the CMH method. The responder proportion analysis for Weeks 28-48 was based on the estimate population (EP). The stratum-adjusted between-group difference was tested with the CMH method. The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. STAR integrates systemic disease activity (ClinESSDAI), symptoms (ESSPRI), and glandular function (Schirmer’s / salivary flow) Systemic disease activity and patient-reported symptoms are considered as major items (3 points per item) and the rest as minor items (1 point per item) Patients are classified as STAR responders when they reach ≥ 5 of 9 points 74.8% Telitacicept 160mg 53.2% Telitacicept 80mg 21.3% Placebo Group STAR Responders (%) at Week 24
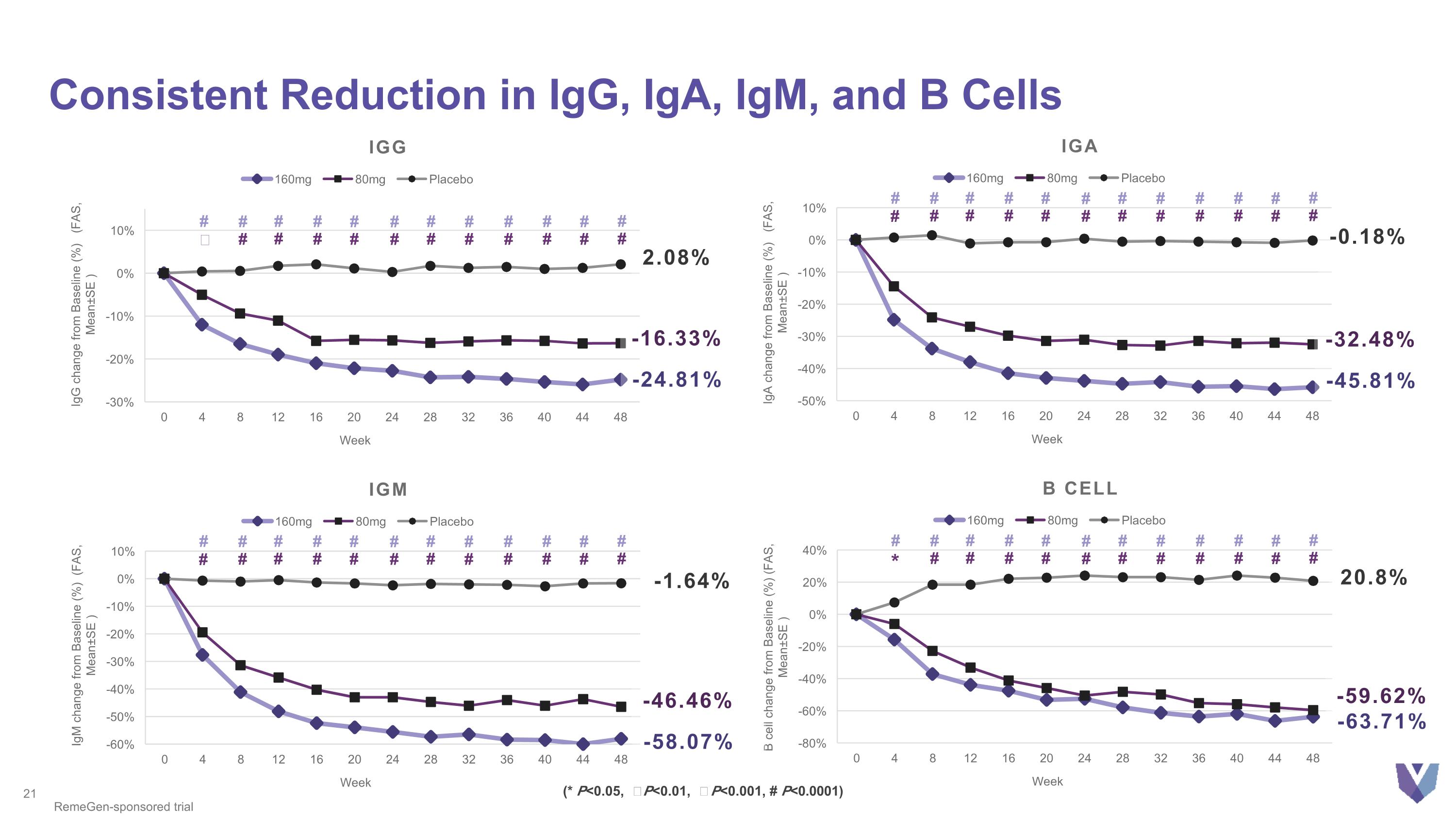
Consistent Reduction in IgG, IgA, IgM, and B Cells IgG IgA IGM B cell RemeGen-sponsored trial -24.81% 2.08% -16.33% -45.81% -0.18% -32.48% -58.07% -1.64% -46.46% -63.71% 20.8% -59.62% # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # * # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # # ǂ # # (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001)
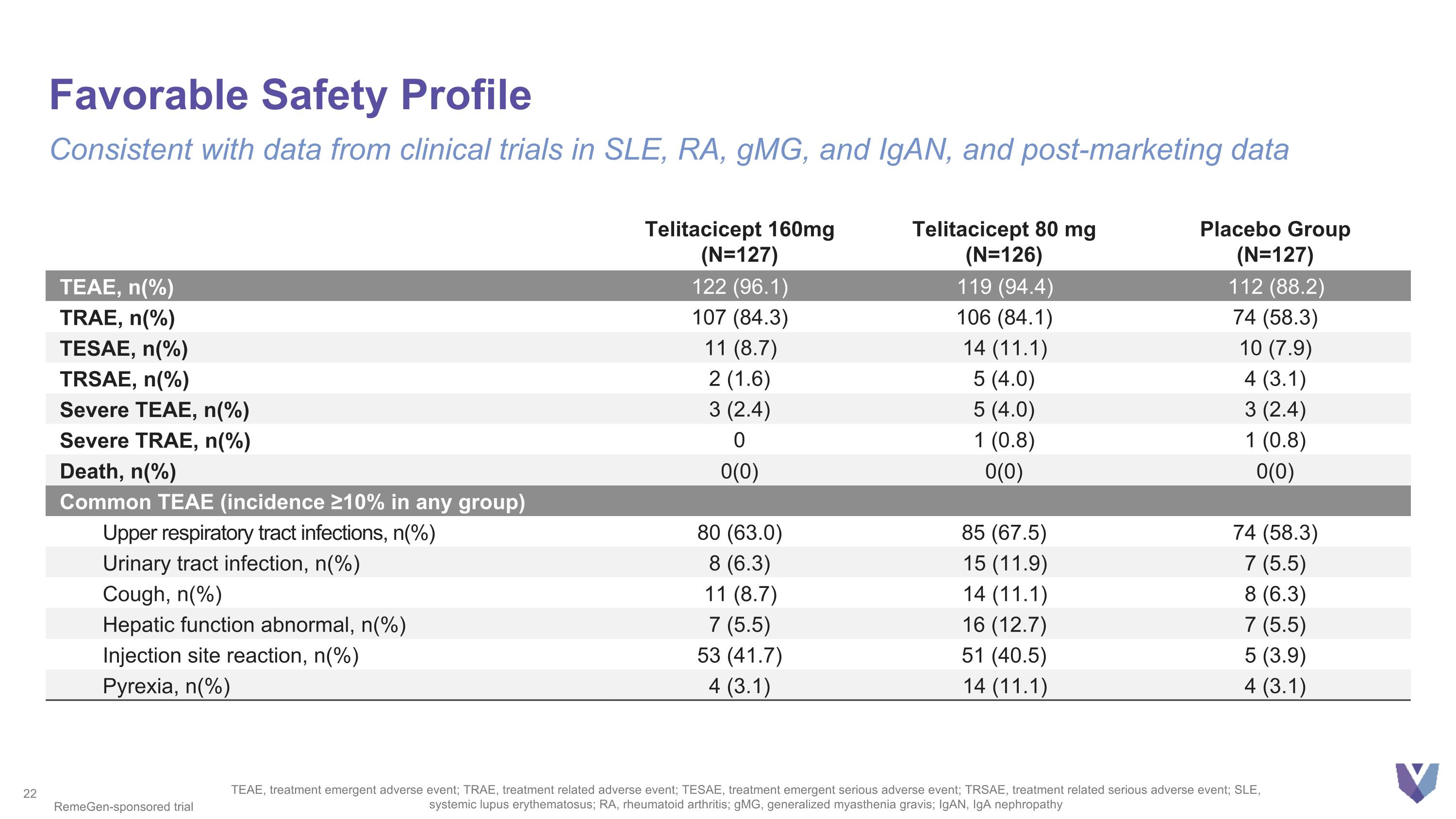
Favorable Safety Profile Consistent with data from clinical trials in SLE, RA, gMG, and IgAN, and post-marketing data TEAE, treatment emergent adverse event; TRAE, treatment related adverse event; TESAE, treatment emergent serious adverse event; TRSAE, treatment related serious adverse event; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; gMG, generalized myasthenia gravis; IgAN, IgA nephropathy RemeGen-sponsored trial Telitacicept 160mg (N=127) Telitacicept 80 mg (N=126) Placebo Group (N=127) TEAE, n(%) 122 (96.1) 119 (94.4) 112 (88.2) TRAE, n(%) 107 (84.3) 106 (84.1) 74 (58.3) TESAE, n(%) 11 (8.7) 14 (11.1) 10 (7.9) TRSAE, n(%) 2 (1.6) 5 (4.0) 4 (3.1) Severe TEAE, n(%) 3 (2.4) 5 (4.0) 3 (2.4) Severe TRAE, n(%) 0 1 (0.8) 1 (0.8) Death, n(%) 0(0) 0(0) 0(0) Common TEAE (incidence ≥10% in any group) Upper respiratory tract infections, n(%) 80 (63.0) 85 (67.5) 74 (58.3) Urinary tract infection, n(%) 8 (6.3) 15 (11.9) 7 (5.5) Cough, n(%) 11 (8.7) 14 (11.1) 8 (6.3) Hepatic function abnormal, n(%) 7 (5.5) 16 (12.7) 7 (5.5) Injection site reaction, n(%) 53 (41.7) 51 (40.5) 5 (3.9) Pyrexia, n(%) 4 (3.1) 14 (11.1) 4 (3.1)

pSD Commercial Opportunity Dallan Murray, Chief Commercial Officer
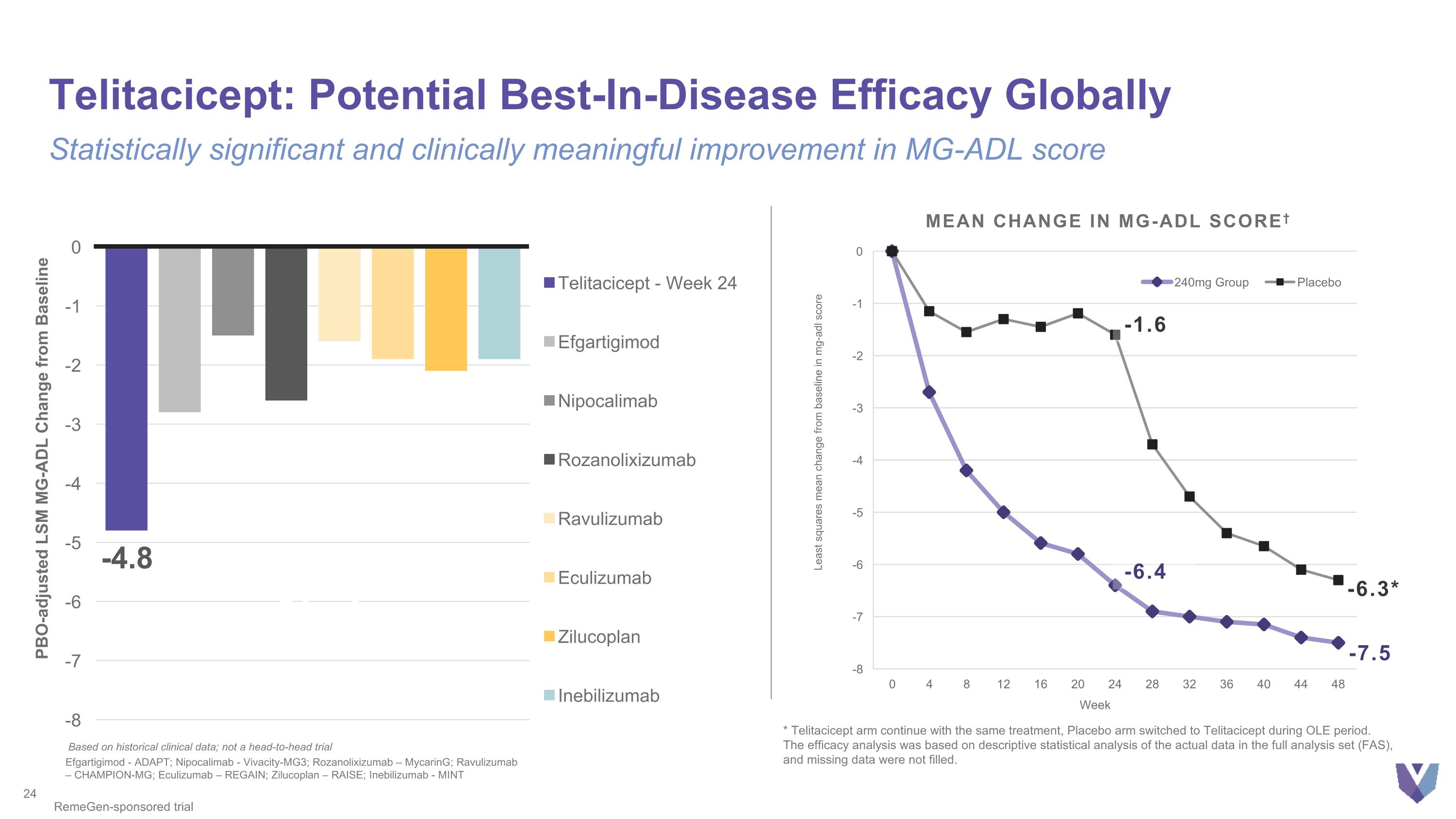
Telitacicept: Potential Best-In-Disease Efficacy Globally Statistically significant and clinically meaningful improvement in MG-ADL score PBO-adjusted LSM MG-ADL Change from Baseline Based on historical clinical data; not a head-to-head trial Efgartigimod - ADAPT; Nipocalimab - Vivacity-MG3; Rozanolixizumab – MycarinG; Ravulizumab – CHAMPION-MG; Eculizumab – REGAIN; Zilucoplan – RAISE; Inebilizumab - MINT RemeGen-sponsored trial -7.5 -6.3* -7.5 Mean Change in MG-ADL Score† -6.4 -1.6 * Telitacicept arm continue with the same treatment, Placebo arm switched to Telitacicept during OLE period. The efficacy analysis was based on descriptive statistical analysis of the actual data in the full analysis set (FAS), and missing data were not filled.
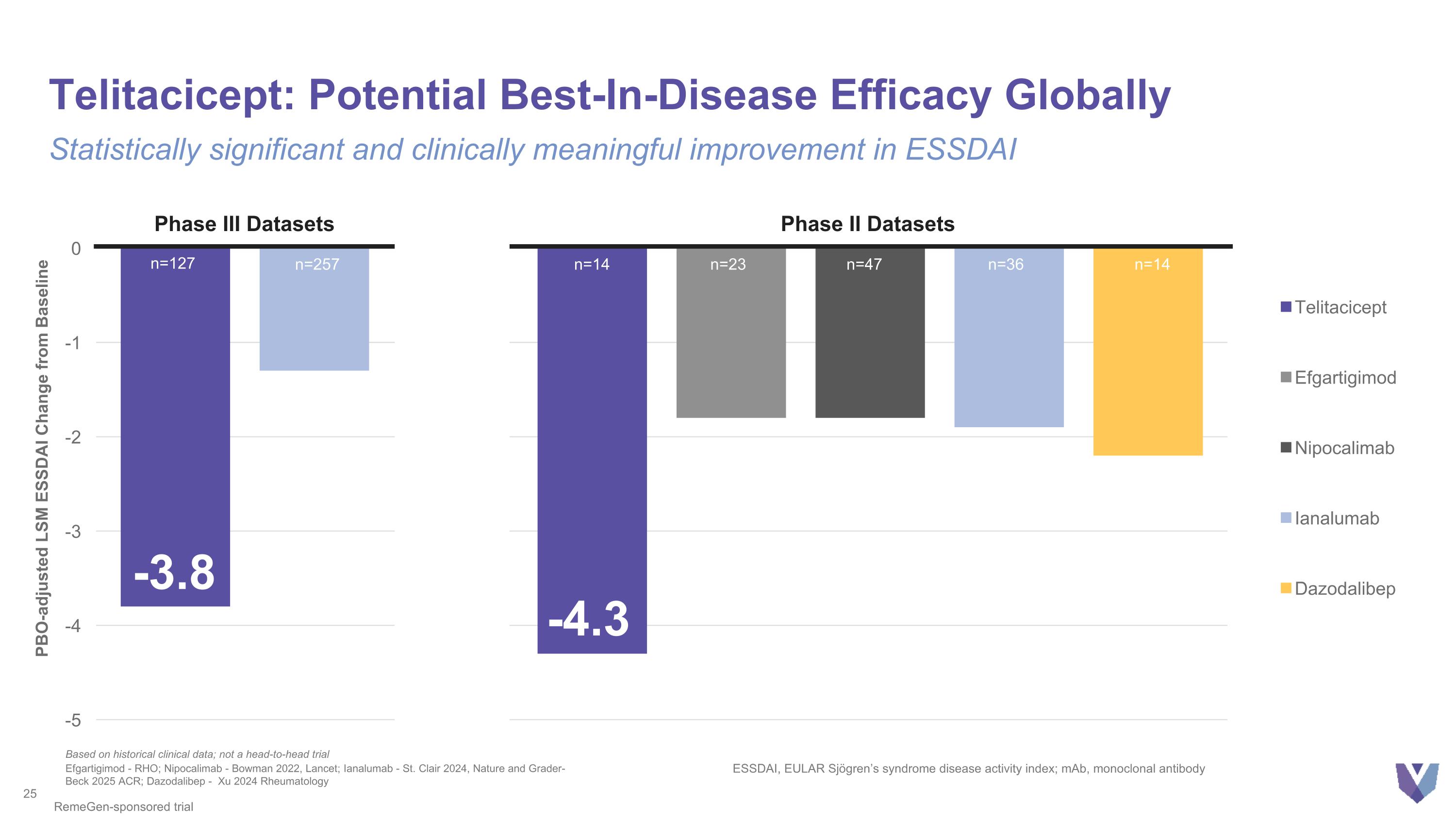
Telitacicept: Potential Best-In-Disease Efficacy Globally Statistically significant and clinically meaningful improvement in ESSDAI -4.3 PBO-adjusted LSM ESSDAI Change from Baseline Efgartigimod - RHO; Nipocalimab - Bowman 2022, Lancet; Ianalumab - St. Clair 2024, Nature and Grader-Beck 2025 ACR; Dazodalibep - Xu 2024 Rheumatology ESSDAI, EULAR Sjögren’s syndrome disease activity index; mAb, monoclonal antibody n=14 n=23 n=47 n=36 n=14 Based on historical clinical data; not a head-to-head trial RemeGen-sponsored trial n=257 n=127 Phase III Datasets Phase II Datasets ? -3.8
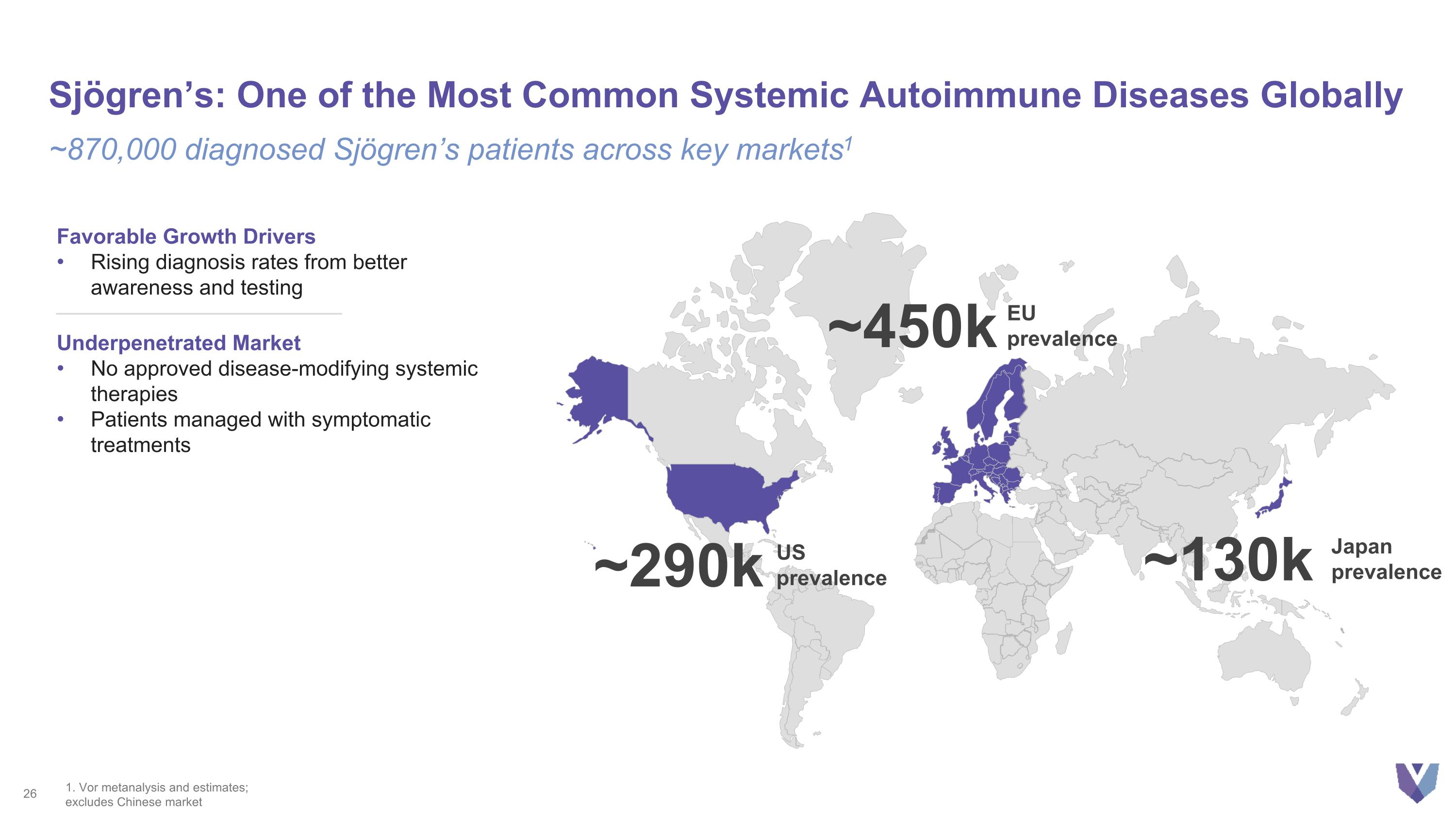
Sjögren’s: One of the Most Common Systemic Autoimmune Diseases Globally ~870,000 diagnosed Sjögren’s patients across key markets1 Favorable Growth Drivers Rising diagnosis rates from better awareness and testing Underpenetrated Market No approved disease-modifying systemic therapies Patients managed with symptomatic treatments ~290k US prevalence ~450k EU prevalence ~130k Japan prevalence 1. Vor metanalysis and estimates; excludes Chinese market
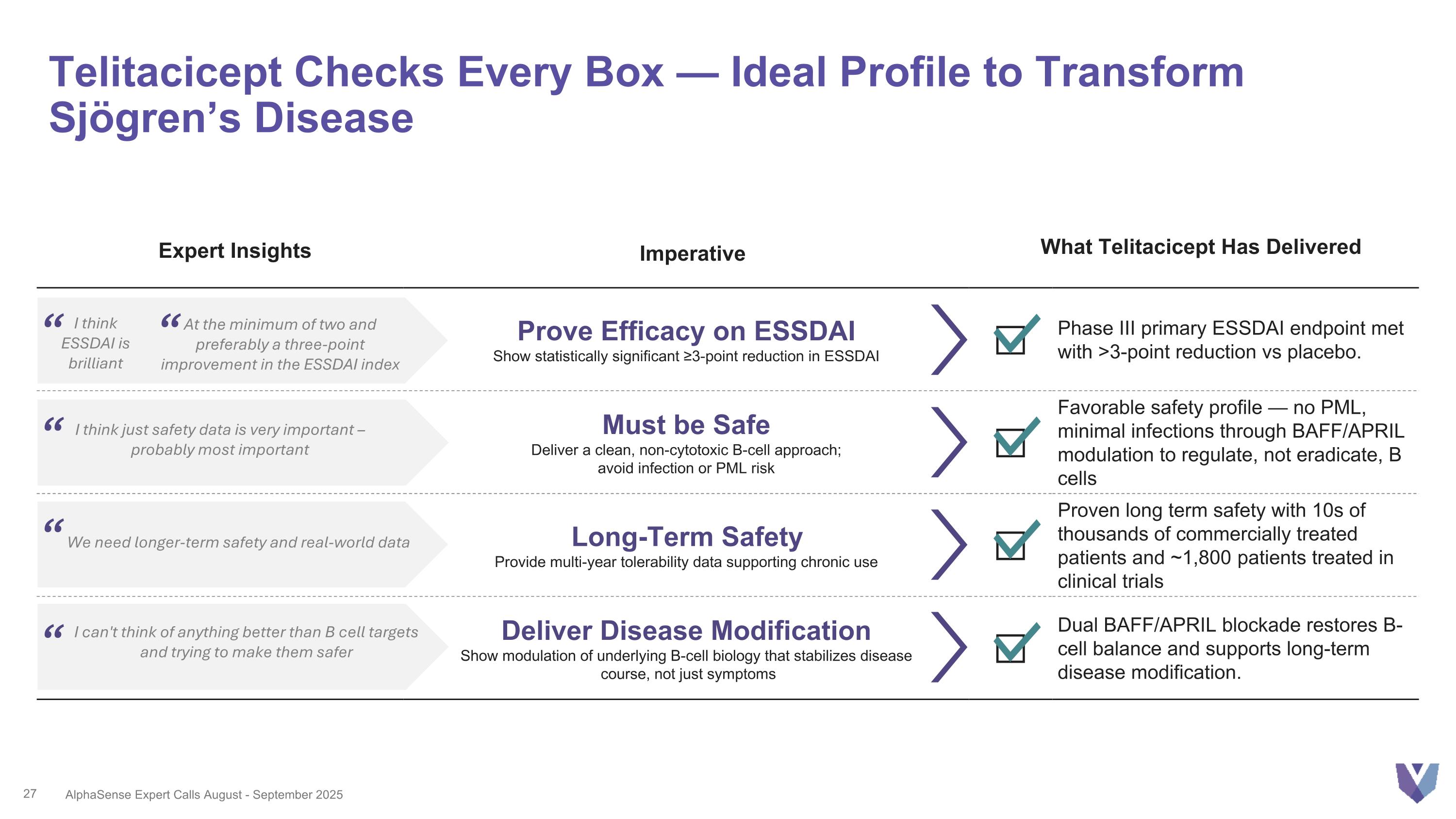
Prove Efficacy on ESSDAI Show statistically significant ≥3-point reduction in ESSDAI Phase III primary ESSDAI endpoint met with >3-point reduction vs placebo. Must be Safe Deliver a clean, non-cytotoxic B-cell approach; avoid infection or PML risk Favorable safety profile — no PML, minimal infections through BAFF/APRIL modulation to regulate, not eradicate, B cells Long-Term Safety Provide multi-year tolerability data supporting chronic use Proven long term safety with 10s of thousands of commercially treated patients and ~1,800 patients treated in clinical trials Deliver Disease Modification Show modulation of underlying B-cell biology that stabilizes disease course, not just symptoms Dual BAFF/APRIL blockade restores B-cell balance and supports long-term disease modification. Telitacicept Checks Every Box — Ideal Profile to Transform Sjögren’s Disease I think just safety data is very important – probably most important We need longer-term safety and real-world data I can't think of anything better than B cell targets and trying to make them safer I think ESSDAI is brilliant “ AlphaSense Expert Calls August - September 2025 At the minimum of two and preferably a three-point improvement in the ESSDAI index Expert Insights Imperative What Telitacicept Has Delivered “ “ “ “
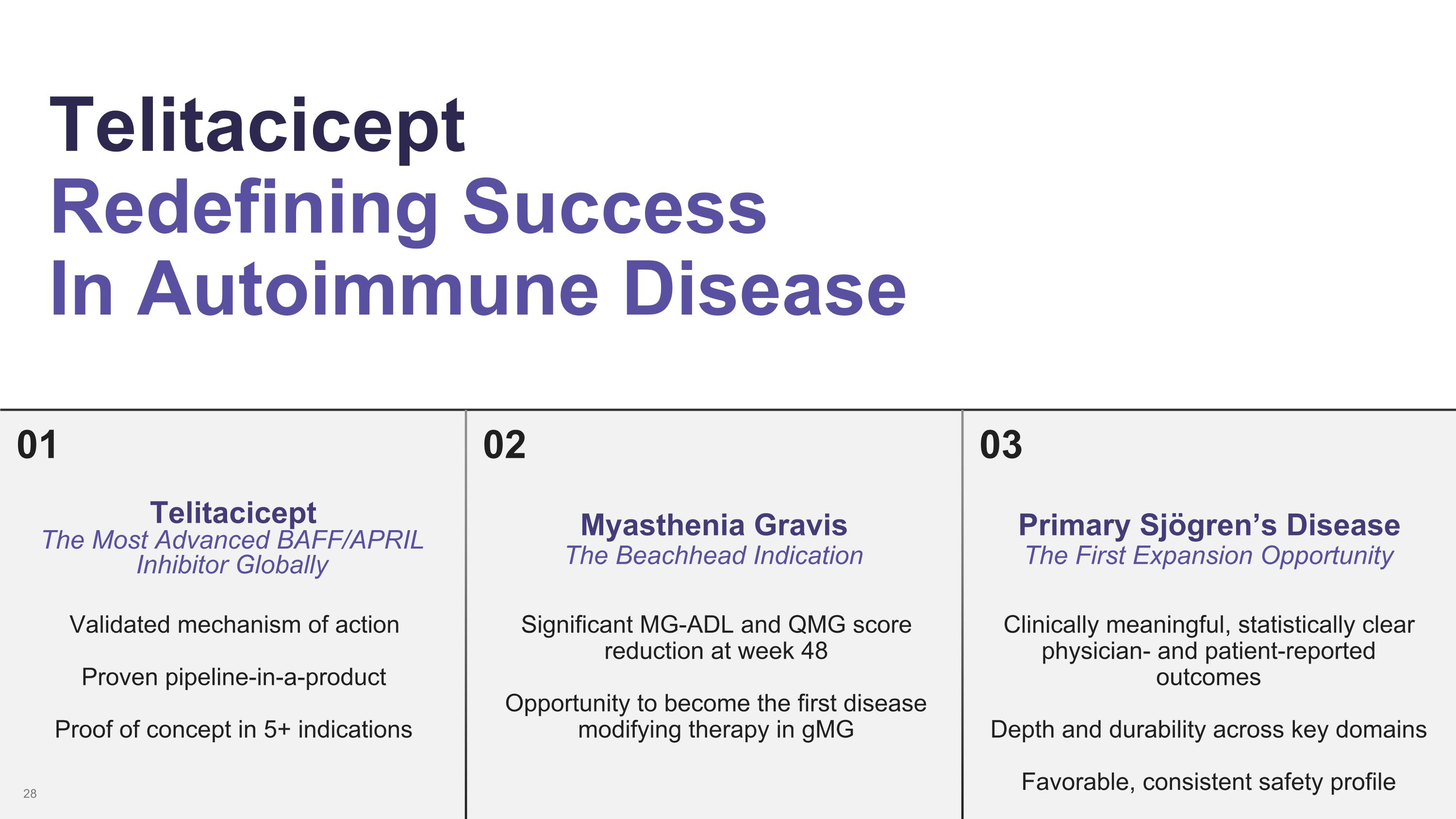
01 Telitacicept Redefining Success In Autoimmune Disease 02 03 Telitacicept The Most Advanced BAFF/APRIL Inhibitor Globally Validated mechanism of action Proven pipeline-in-a-product Proof of concept in 5+ indications Myasthenia Gravis The Beachhead Indication Significant MG-ADL and QMG score reduction at week 48 Opportunity to become the first disease modifying therapy in gMG Primary Sjögren’s Disease The First Expansion Opportunity Clinically meaningful, statistically clear physician- and patient-reported outcomes Depth and durability across key domains Favorable, consistent safety profile
Thank You. Investors@vorbio.com