

Global Science. One Purpose. 44th Annual J.P. Morgan Healthcare Conference January 2026

Forward Looking Statement This presentation (the “Presentation”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 about Vor Biopharma Inc. (“Vor,” “Vor Bio” or the “Company”). The words “aim,” “anticipate,” “believe,” “can,” “could,” “design,” “enable” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “project,” “should,” “target,” “towards,” “will,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements in this Presentation include those regarding Vor Bio's plans for development and commercialization of telitacicept, including expected milestones such as trial initiation and data readout; the potential of telitacicept in various indications including generalized myasthenia gravis (gMG) and primary Sjögren's disease; the Company’s ambition to transform the approach to B cell-driven autoimmune disease; the potential of telitacicept to be a best- and first-in-class BAFF/APRIL inhibitor; the expected safety profile of telitacicept; the market opportunities for telitacicept; the addressable patient populations in the indications Vor Bio intends to treat; Vor Bio's cash runway; and other statements that are not historical fact. Vor Bio may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including: uncertainties inherent in the initiation, completion of, and availability and timing of results from, clinical trials; whether preclinical data or interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; the uncertainty of regulatory approvals to conduct trials or to market products; Vor Bio's reliance on third parties over which it may not always have full control; and the availability of funding sufficient for Vor Bio's foreseeable and unforeseeable operating expenses and capital expenditure requirements. These and other risks are described in greater detail under the caption “Risk Factors” included in Vor Bio's most recent annual or quarterly report and in other reports it has filed or may file with the Securities and Exchange Commission. Any forward-looking statements contained in this Presentation speak only as of the date of this Presentation, and Vor Bio expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise, except as may be required by law. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and Vor Bio's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, the Company has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third party sources. In addition, there can be no guarantee as to the accuracy or reliability of any assumptions or limitations that may be included in such third-party information. While the Company believes its own internal research is reliable, such research has not been verified by any independent source. All brand names or trademarks appearing in this Presentation are the property of their respective owners.
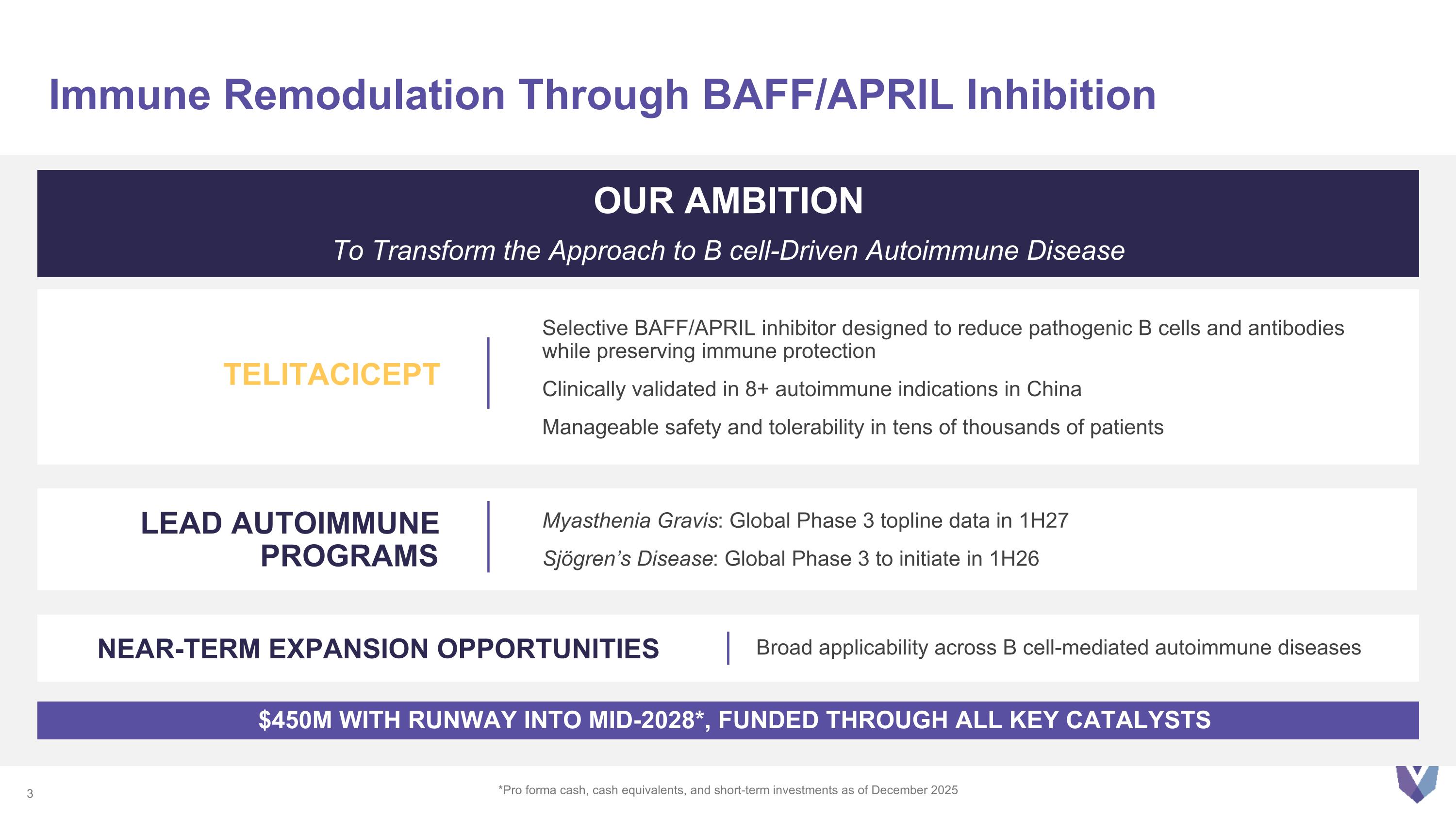
Immune Remodulation Through BAFF/APRIL Inhibition *Pro forma cash, cash equivalents, and short-term investments as of December 2025 OUR AMBITION To Transform the Approach to B cell-Driven Autoimmune Disease TELITACICEPT Selective BAFF/APRIL inhibitor designed to reduce pathogenic B cells and antibodies while preserving immune protection Clinically validated in 8+ autoimmune indications in China Manageable safety and tolerability in tens of thousands of patients $450M WITH RUNWAY INTO MID-2028*, FUNDED THROUGH ALL KEY CATALYSTS LEAD AUTOIMMUNE PROGRAMS Myasthenia Gravis: Global Phase 3 topline data in 1H27 Sjögren’s Disease: Global Phase 3 to initiate in 1H26 NEAR-TERM EXPANSION OPPORTUNITIES Broad applicability across B cell-mediated autoimmune diseases
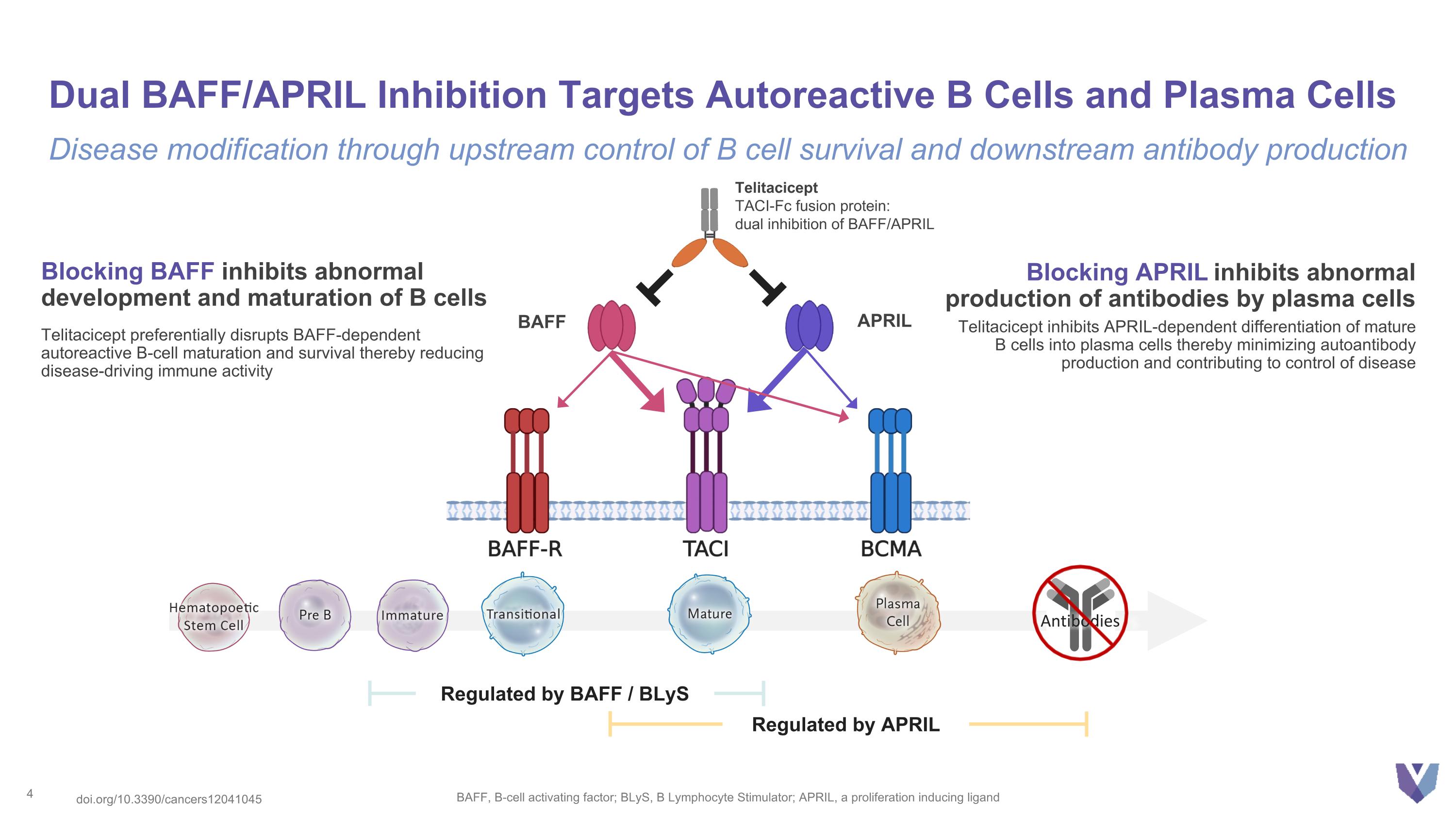
Dual BAFF/APRIL Inhibition Targets Autoreactive B Cells and Plasma Cells BAFF, B-cell activating factor; BLyS, B Lymphocyte Stimulator; APRIL, a proliferation inducing ligand Disease modification through upstream control of B cell survival and downstream antibody production Regulated by APRIL Regulated by BAFF / BLyS Antibodies Telitacicept TACI-Fc fusion protein: dual inhibition of BAFF/APRIL Blocking BAFF inhibits abnormal development and maturation of B cells Telitacicept preferentially disrupts BAFF-dependent autoreactive B-cell maturation and survival thereby reducing disease-driving immune activity Blocking APRIL inhibits abnormal production of antibodies by plasma cells Telitacicept inhibits APRIL-dependent differentiation of mature B cells into plasma cells thereby minimizing autoantibody production and contributing to control of disease BAFF APRIL doi.org/10.3390/cancers12041045

Commercial Approvals †Conditional Approval, Full Approval in 2023; *Accelerated Approval in China Established Efficacy in China Across Autoimmune Diseases BLA Submissions Best-In-Disease 3 2 3 2021 - Systemic Lupus Erythematosus (SLE)† 2024 - Rheumatoid Arthritis (RA) 2025 - Myasthenia Gravis (MG) Filed 2025 – Sjögren’s Disease (SD) Filed 2025 – IgA Nephropathy (IgAN)* Validated Commercial Therapy in China Across Diverse Autoimmune Diseases Poised to Further Expand Telitacicept Footprint in Large, Underserved Diseases in China Unique Dual BAFF/APRIL Inhibition Drives Superior Clinical Benefit Systemic Lupus Erythematosus Myasthenia Gravis Sjögren’s Disease

*From pooled safety analysis across all telitacicept trials. AEs, adverse events; SAEs, serious adverse events. Favorable Safety At Scale Favorable and Predictable Safety Profile Observed Among ~1,800* Patients Studied in Clinical Trials No Burdensome Vaccination Requirements No Signature B Cell Depletion Associated SAEs Mild to Moderate AEs Placebo (n=527) Telitacicept (n=1211) Cough 3 5 Diarrhea 5 5 Urinary tract infection 9 10 Injection site reaction 2 17 Upper respiratory tract infection 30 35 10s of Thousands Patients Treated Commercially in China Frequency (%) of safety events reported in clinical trials
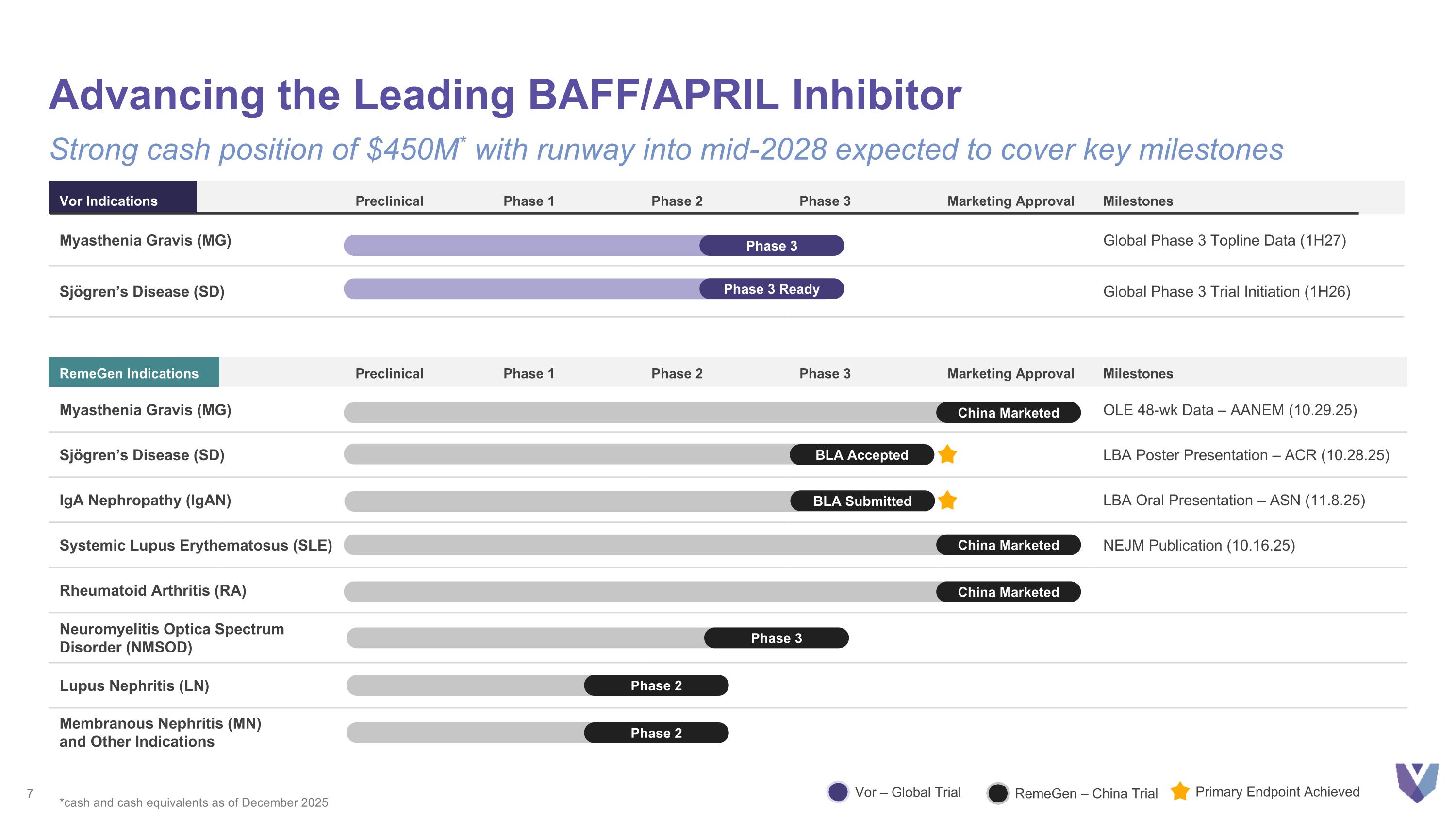
RemeGen Indications Preclinical Phase 1 Phase 2 Phase 3 Marketing Approval Milestones Myasthenia Gravis (MG) OLE 48-wk Data – AANEM (10.29.25) Sjögren’s Disease (SD) LBA Poster Presentation – ACR (10.28.25) IgA Nephropathy (IgAN) LBA Oral Presentation – ASN (11.8.25) Systemic Lupus Erythematosus (SLE) NEJM Publication (10.16.25) Rheumatoid Arthritis (RA) Neuromyelitis Optica Spectrum Disorder (NMSOD) Lupus Nephritis (LN) Membranous Nephritis (MN) and Other Indications Advancing the Leading BAFF/APRIL Inhibitor Strong cash position of $450M* with runway into mid-2028 expected to cover key milestones Vor Indications Preclinical Phase 1 Phase 2 Phase 3 Marketing Approval Milestones Myasthenia Gravis (MG) Global Phase 3 Topline Data (1H27) Sjögren’s Disease (SD) Global Phase 3 Trial Initiation (1H26) Phase 3 Phase 2 Phase 3 Phase 2 Vor – Global Trial RemeGen – China Trial BLA Submitted Primary Endpoint Achieved *cash and cash equivalents as of December 2025 China Marketed China Marketed Phase 3 Ready BLA Accepted China Marketed

Myasthenia Gravis Moving Beyond IgG Therapies
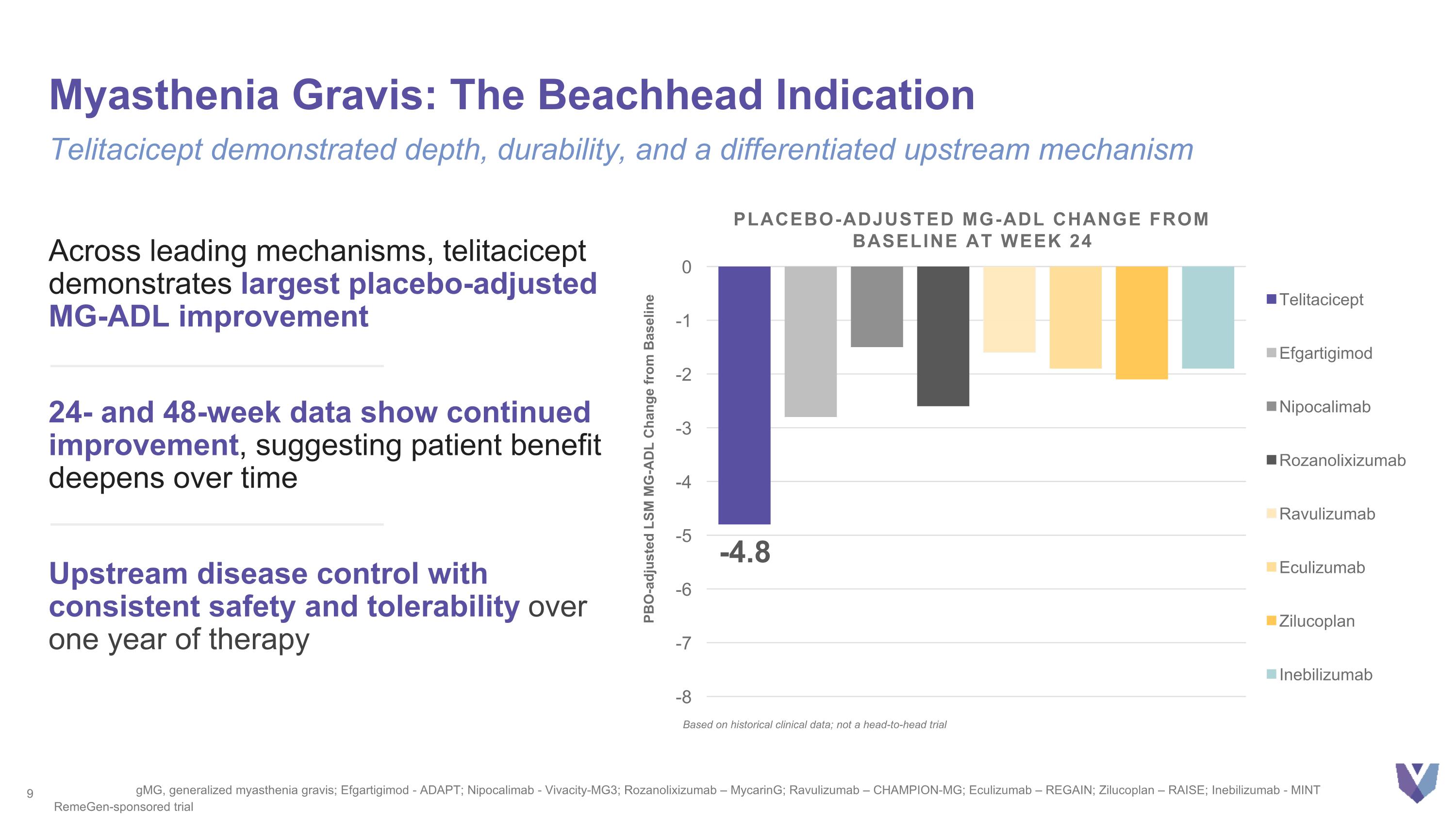
Myasthenia Gravis: The Beachhead Indication Telitacicept demonstrated depth, durability, and a differentiated upstream mechanism Across leading mechanisms, telitacicept demonstrates largest placebo-adjusted MG-ADL improvement 24- and 48-week data show continued improvement, suggesting patient benefit deepens over time Upstream disease control with consistent safety and tolerability over one year of therapy gMG, generalized myasthenia gravis; Efgartigimod - ADAPT; Nipocalimab - Vivacity-MG3; Rozanolixizumab – MycarinG; Ravulizumab – CHAMPION-MG; Eculizumab – REGAIN; Zilucoplan – RAISE; Inebilizumab - MINT RemeGen-sponsored trial Based on historical clinical data; not a head-to-head trial PBO-adjusted LSM MG-ADL Change from Baseline PLACEBO-ADJUSTED MG-ADL CHANGE FROM BASELINE At Week 24
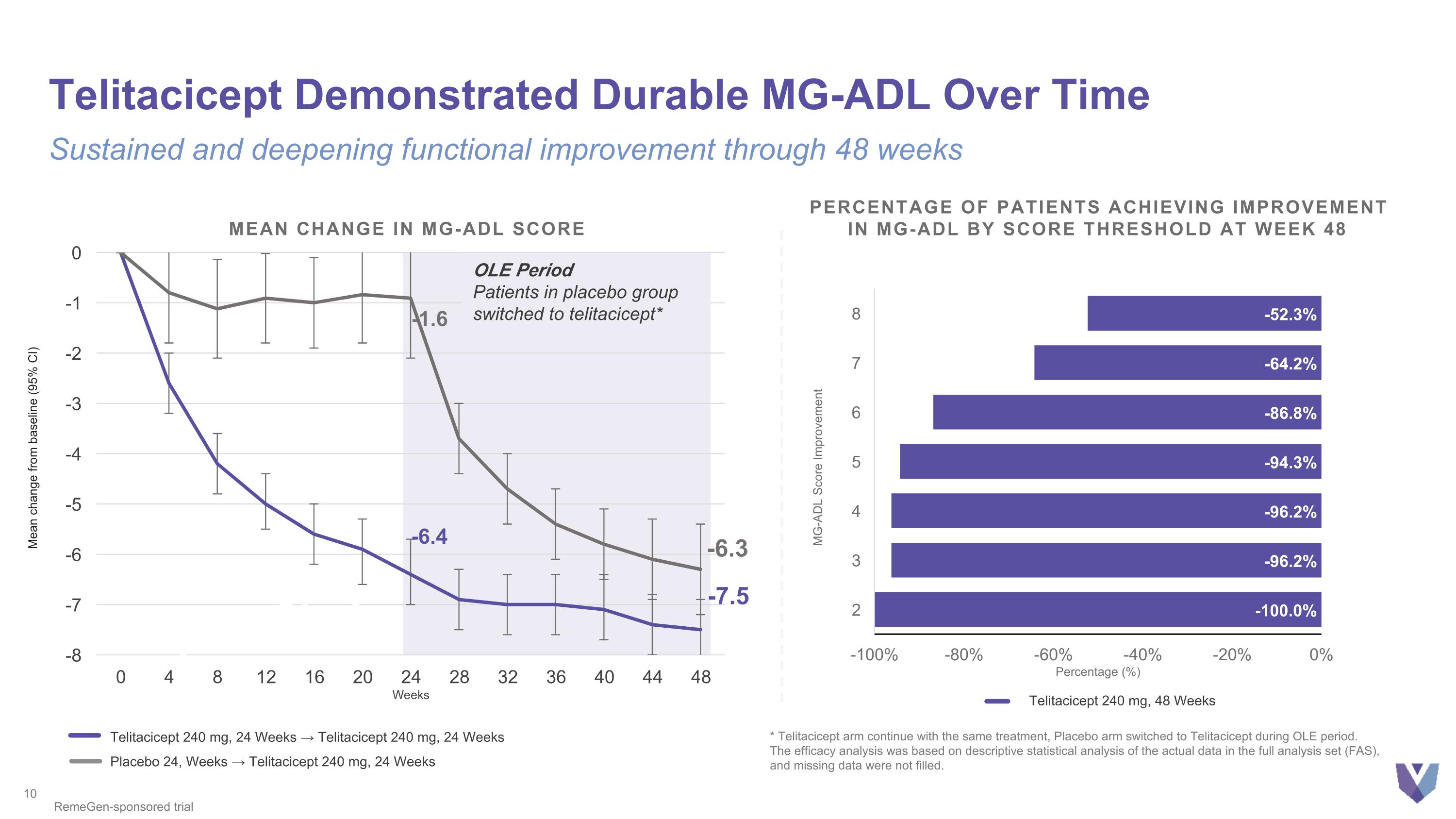
-7.5 -6.3 -7.5 -1.6 -6.4 Mean change from baseline (95% CI) MEAN CHANGE IN MG-ADL SCORE OLE Period Patients in placebo group switched to telitacicept* Telitacicept Demonstrated Durable MG-ADL Over Time Sustained and deepening functional improvement through 48 weeks RemeGen-sponsored trial -7.5 * Telitacicept arm continue with the same treatment, Placebo arm switched to Telitacicept during OLE period. The efficacy analysis was based on descriptive statistical analysis of the actual data in the full analysis set (FAS), and missing data were not filled. Percentage of Patients Achieving Improvement in MG-ADL by Score Threshold at Week 48 Telitacicept 240 mg, 48 Weeks Telitacicept 240 mg, 24 Weeks → Telitacicept 240 mg, 24 Weeks Placebo 24, Weeks → Telitacicept 240 mg, 24 Weeks
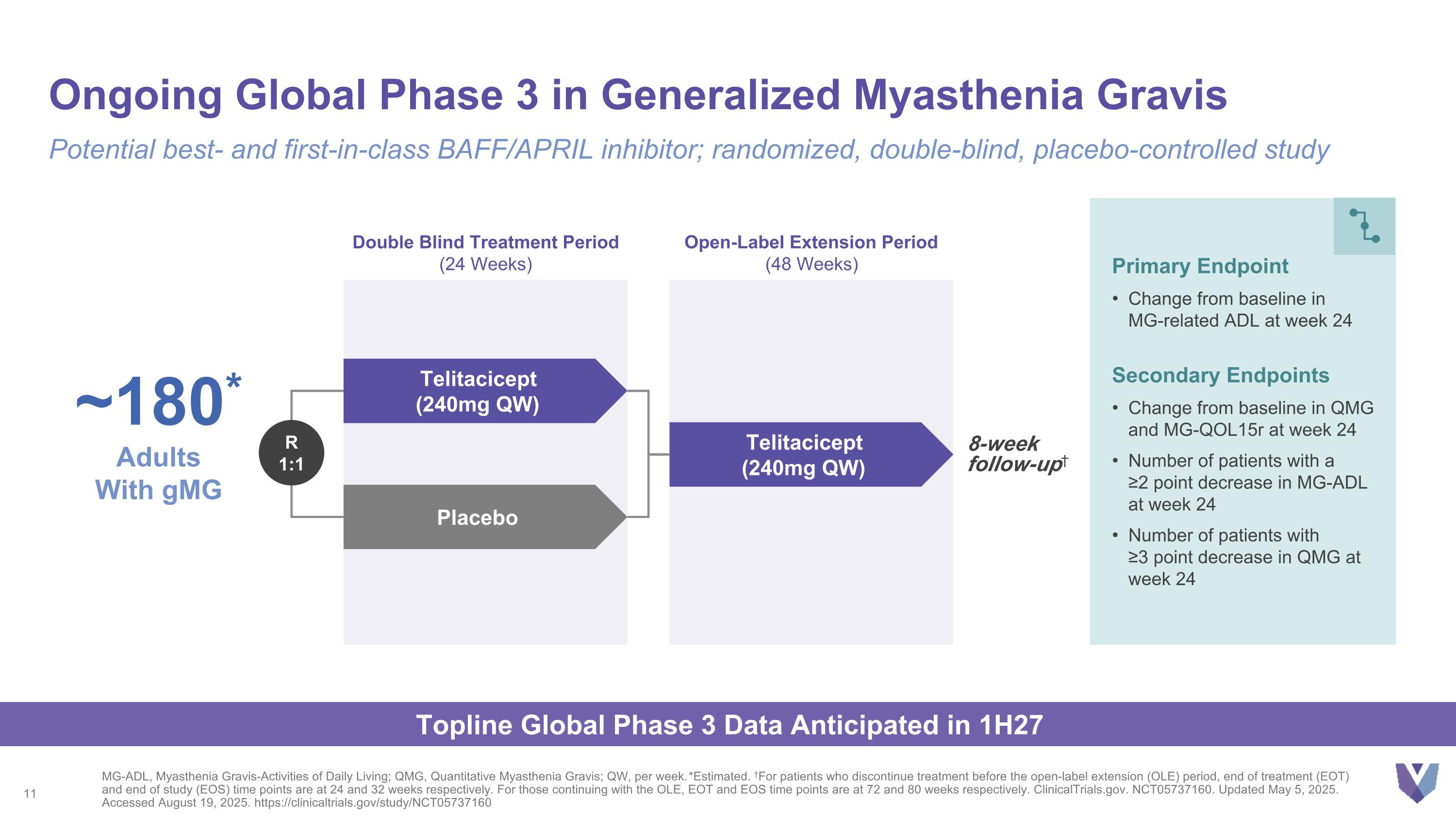
Ongoing Global Phase 3 in Generalized Myasthenia Gravis MG-ADL, Myasthenia Gravis-Activities of Daily Living; QMG, Quantitative Myasthenia Gravis; QW, per week. *Estimated. †For patients who discontinue treatment before the open-label extension (OLE) period, end of treatment (EOT) and end of study (EOS) time points are at 24 and 32 weeks respectively. For those continuing with the OLE, EOT and EOS time points are at 72 and 80 weeks respectively. ClinicalTrials.gov. NCT05737160. Updated May 5, 2025. Accessed August 19, 2025. https://clinicaltrials.gov/study/NCT05737160 Potential best- and first-in-class BAFF/APRIL inhibitor; randomized, double-blind, placebo-controlled study Double Blind Treatment Period (24 Weeks) Open-Label Extension Period (48 Weeks) Telitacicept (240mg QW) Placebo Telitacicept (240mg QW) R 1:1 Primary Endpoint Change from baseline in MG-related ADL at week 24 Secondary Endpoints Change from baseline in QMG and MG-QOL15r at week 24 Number of patients with a ≥2 point decrease in MG-ADL at week 24 Number of patients with ≥3 point decrease in QMG at week 24 8-week follow-up† ~180* Adults With gMG Topline Global Phase 3 Data Anticipated in 1H27

Sjögren's Disease Moving Beyond Symptom Management
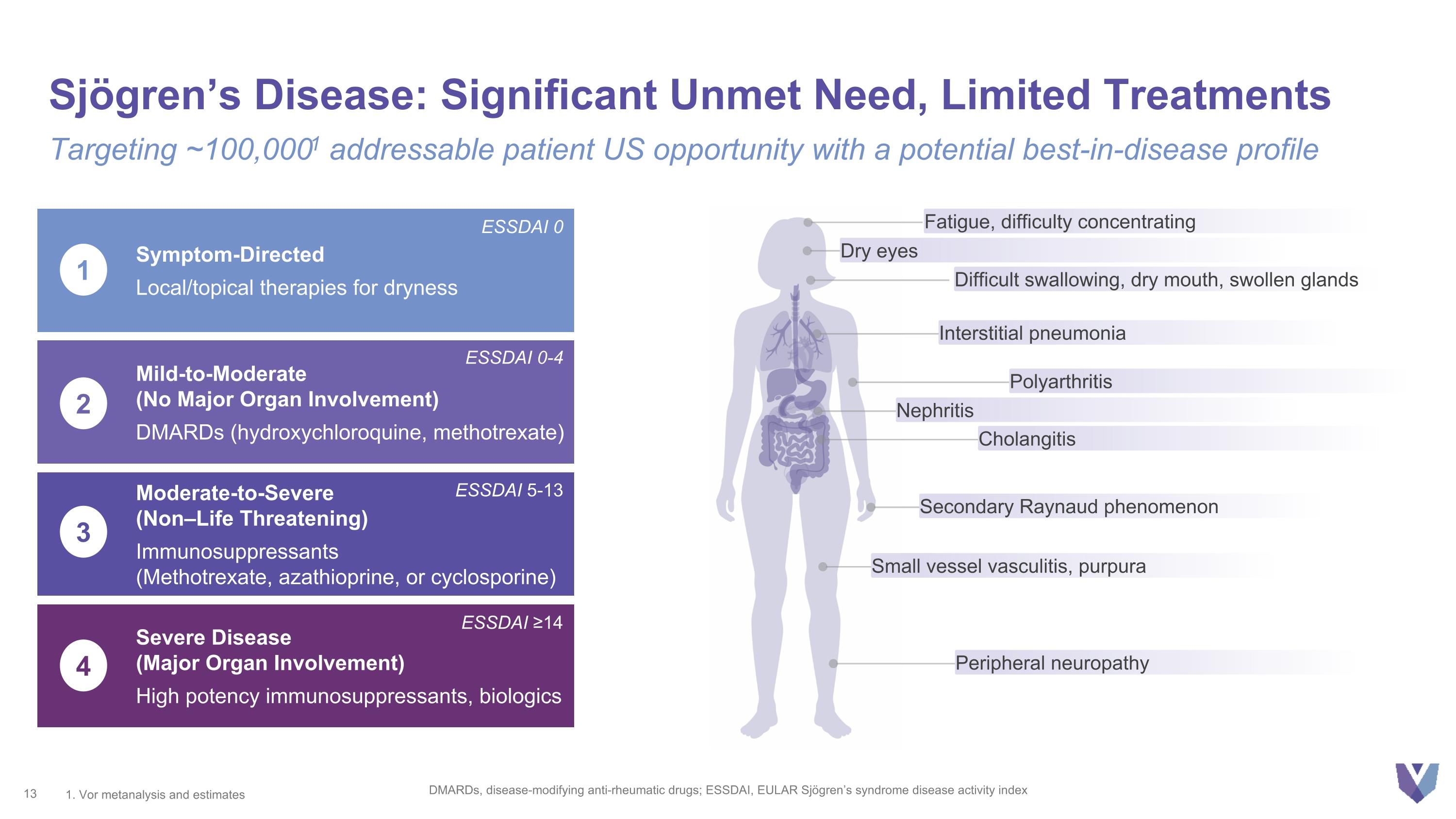
Sjögren’s Disease: Significant Unmet Need, Limited Treatments DMARDs, disease-modifying anti-rheumatic drugs; ESSDAI, EULAR Sjögren’s syndrome disease activity index Targeting ~100,0001 addressable patient US opportunity with a potential best-in-disease profile Interstitial pneumonia Secondary Raynaud phenomenon Fatigue, difficulty concentrating Difficult swallowing, dry mouth, swollen glands Peripheral neuropathy Nephritis Cholangitis Small vessel vasculitis, purpura Polyarthritis Dry eyes 1. Vor metanalysis and estimates Symptom-Directed Local/topical therapies for dryness Mild-to-Moderate (No Major Organ Involvement) DMARDs (hydroxychloroquine, methotrexate) Moderate-to-Severe (Non–Life Threatening) Immunosuppressants (Methotrexate, azathioprine, or cyclosporine) Severe Disease (Major Organ Involvement) High potency immunosuppressants, biologics 1 2 3 4 ESSDAI 0 ESSDAI 0-4 ESSDAI 5-13 ESSDAI ≥14
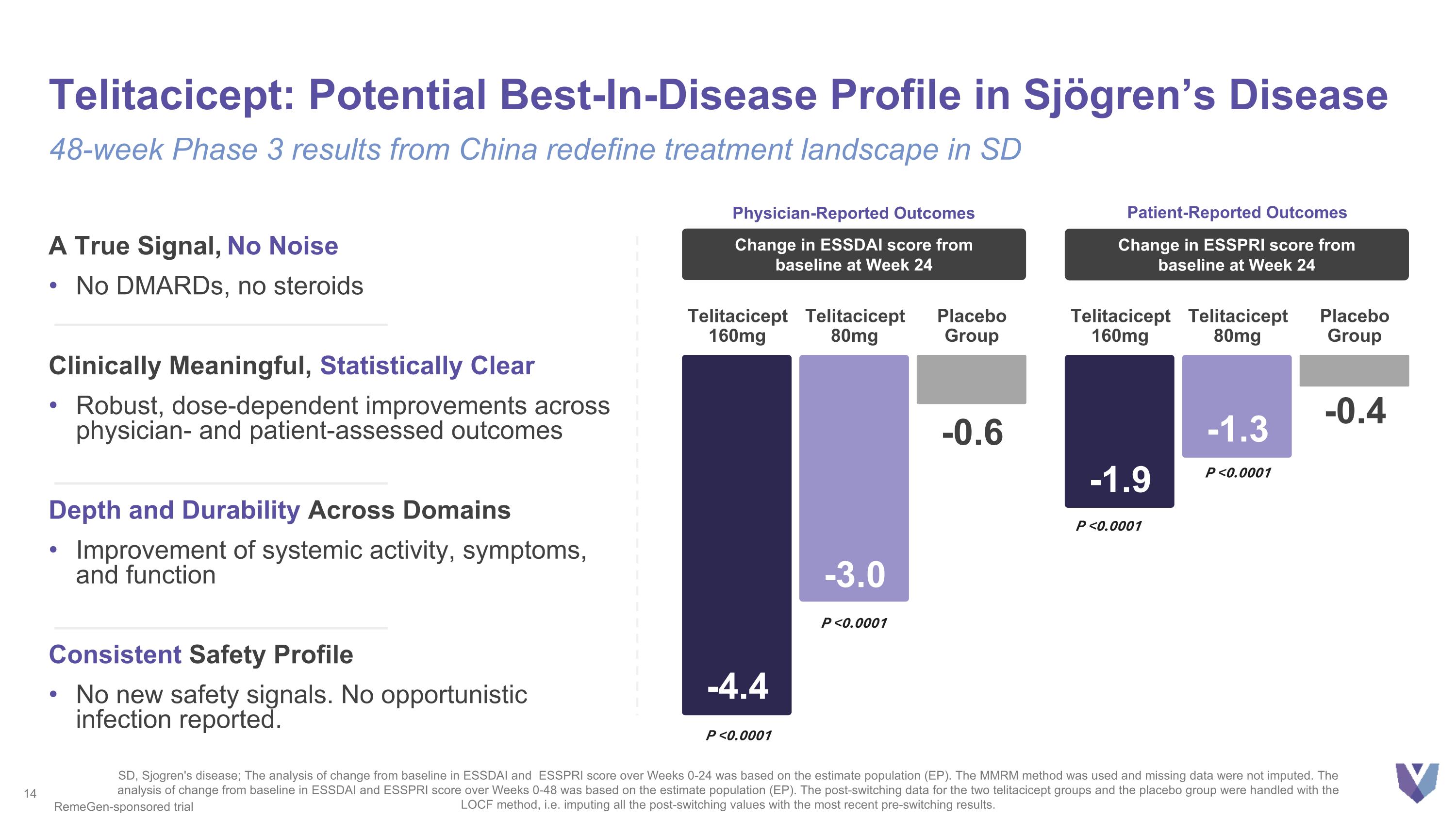
A True Signal, No Noise No DMARDs, no steroids Clinically Meaningful, Statistically Clear Robust, dose-dependent improvements across physician- and patient-assessed outcomes Depth and Durability Across Domains Improvement of systemic activity, symptoms, and function Consistent Safety Profile No new safety signals. No opportunistic infection reported. Telitacicept: Potential Best-In-Disease Profile in Sjögren’s Disease 48-week Phase 3 results from China redefine treatment landscape in SD -4.4 Telitacicept 160mg -3.0 Telitacicept 80mg -0.6 Placebo Group Change in ESSDAI score from baseline at Week 24 -1.9 Telitacicept 160mg -1.3 Telitacicept 80mg -0.4 Placebo Group Change in ESSPRI score from baseline at Week 24 P <0.0001 P <0.0001 SD, Sjogren's disease; The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. The analysis of change from baseline in ESSDAI and ESSPRI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. P <0.0001 P <0.0001 RemeGen-sponsored trial Physician-Reported Outcomes Patient-Reported Outcomes
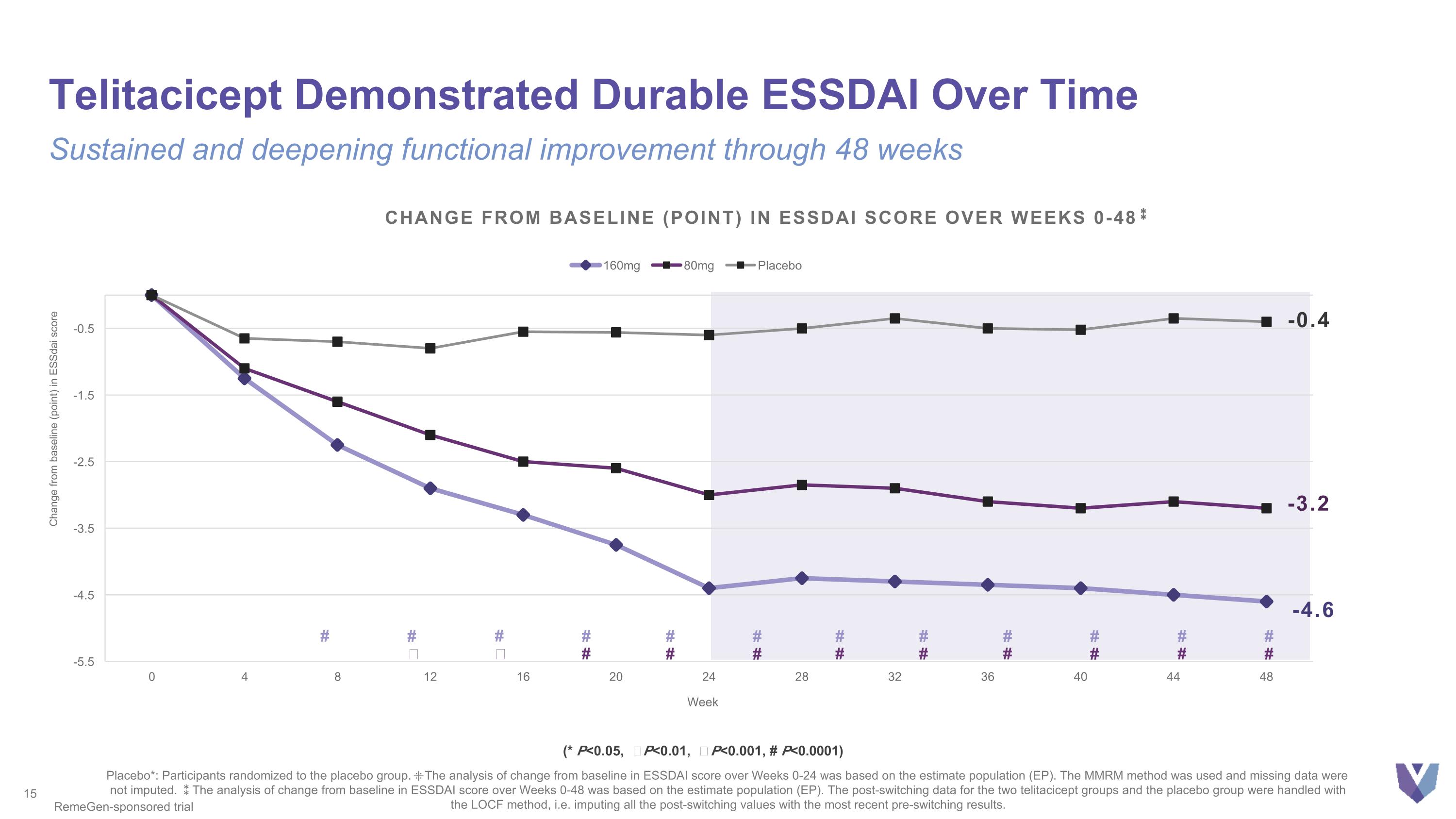
-3.2 -4.6 Change from baseline (point) in essdai score over weeks 0-48⁑ -0.4 # # Ϯ # ǂ # # # # # # # # # # # # # # # # # # Telitacicept Demonstrated Durable ESSDAI Over Time Placebo*: Participants randomized to the placebo group. ⁜The analysis of change from baseline in ESSDAI score over Weeks 0-24 was based on the estimate population (EP). The MMRM method was used and missing data were not imputed. ⁑The analysis of change from baseline in ESSDAI score over Weeks 0-48 was based on the estimate population (EP). The post-switching data for the two telitacicept groups and the placebo group were handled with the LOCF method, i.e. imputing all the post-switching values with the most recent pre-switching results. RemeGen-sponsored trial (* P<0.05, Ϯ P<0.01, ǂ P<0.001, # P<0.0001) Sustained and deepening functional improvement through 48 weeks
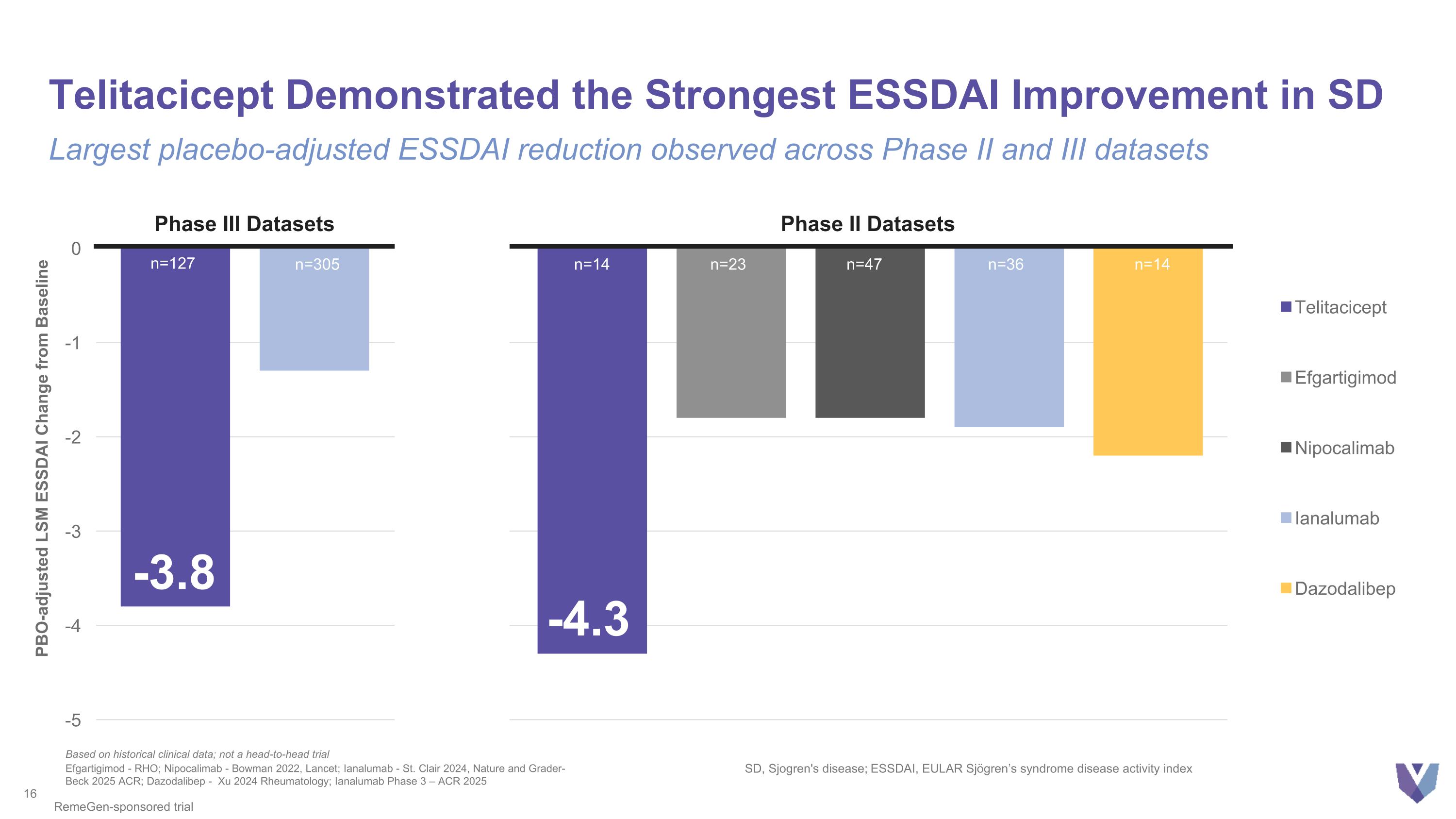
Telitacicept Demonstrated the Strongest ESSDAI Improvement in SD Largest placebo-adjusted ESSDAI reduction observed across Phase II and III datasets -4.3 PBO-adjusted LSM ESSDAI Change from Baseline Efgartigimod - RHO; Nipocalimab - Bowman 2022, Lancet; Ianalumab - St. Clair 2024, Nature and Grader-Beck 2025 ACR; Dazodalibep - Xu 2024 Rheumatology; Ianalumab Phase 3 – ACR 2025 SD, Sjogren's disease; ESSDAI, EULAR Sjögren’s syndrome disease activity index n=14 n=23 n=47 n=36 n=14 Based on historical clinical data; not a head-to-head trial RemeGen-sponsored trial n=305 n=127 Phase III Datasets Phase II Datasets ? -3.8
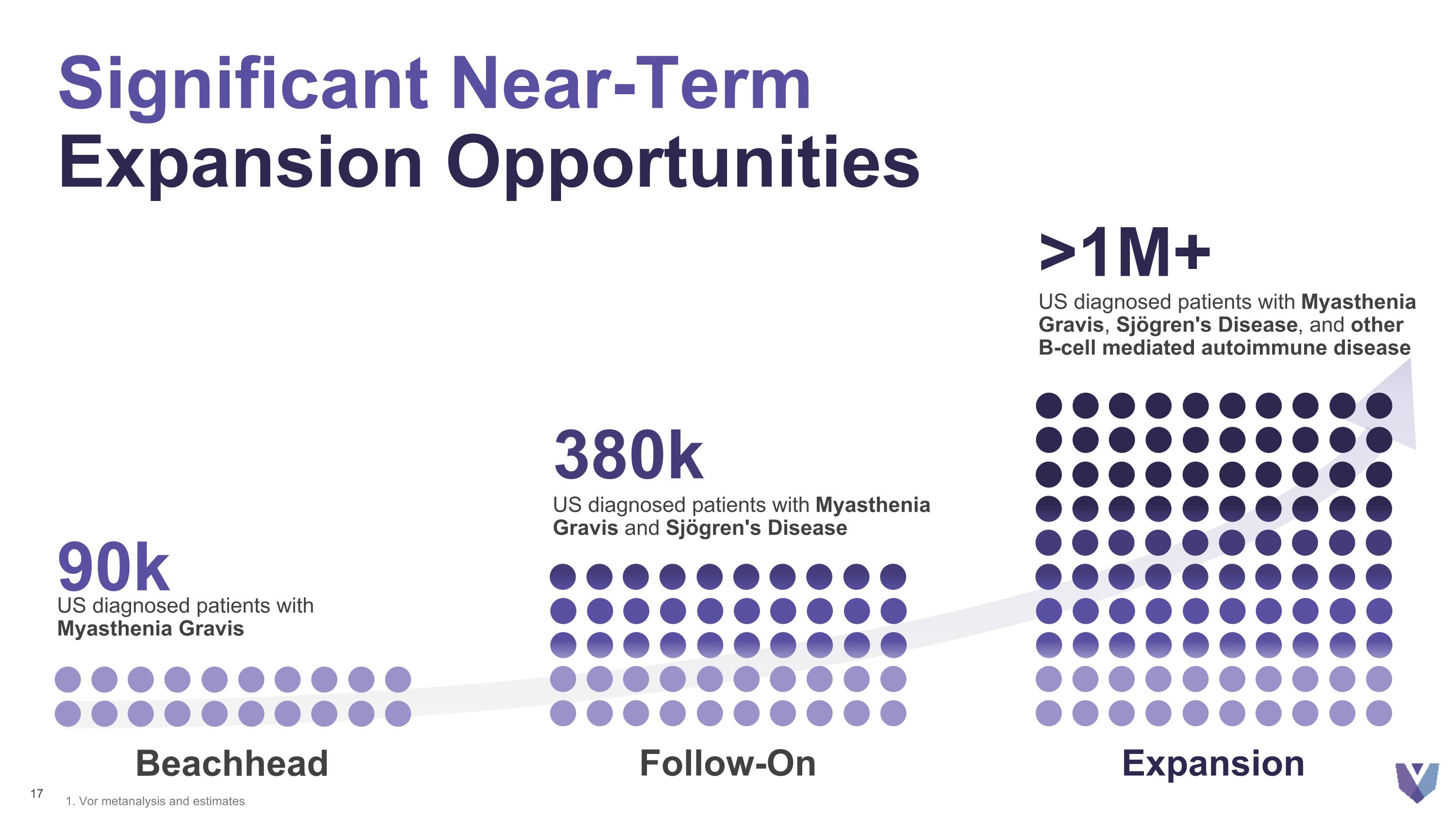
90k US diagnosed patients with Myasthenia Gravis 1. Vor metanalysis and estimates Beachhead 380k US diagnosed patients with Myasthenia Gravis and Sjögren's Disease Follow-On >1M+ US diagnosed patients with Myasthenia Gravis, Sjögren's Disease, and other B-cell mediated autoimmune disease Expansion Significant Near-Term Expansion Opportunities

2026: Advancing The Leading BAFF/APRIL Inhibitor *Pro forma cash, cash equivalents, and short-term investments as of December 2025 ~$450M Runway into Mid-2028* MYASTHENIA GRAVIS 01 02 SJÖGREN’S DISEASE Global Phase 3 Topline Data in 1H27 Best-in-Disease, Commercially Approved in China Global Phase 3 to Initiate in 1H26 Best-in-Disease, BLA Submission Accepted in China 03 EXPANSION OPPORTUNITIES Broad Potential Across B Cell-Driven Immune Diseases Indication Focus on High Unmet Need And Clinical Value
Thank You. Investors@vorbio.com