
GeneDx (Nasdaq: WGS) 3Q 2025 Earnings Presentation October 28, 2025 .2

2 Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and the U.S. Private Securities Litigation Reform Act of 1995, including statements regarding our future performance and our market opportunity, including our expected full year 2025 reported revenue, growth in exome and genome revenue and volume, adjusted gross margin and adjusted net income guidance. These forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this press release, including but not limited to: (i) our ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (ii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iii) the size and growth of the market in which we operate, and (iv) our ability to pursue our new strategic direction. The foregoing list of factors is not exhaustive. A further list and description of risks, uncertainties and other matters can be found in the “Risk Factors” section of our Annual Report on Form 10-K for the fiscal year ended December 31, 2024 and our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2025, June 30, 2025 and September 30, 2025, and other documents filed by us from time to time with the SEC. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and we assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. We do not give any assurance that we will achieve our expectations. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our periodic reports and other filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us are available www.sec.gov. Requests for copies of such documents should be directed to our Investor Relations department at GeneDx Holdings Corp. 333 Ludlow Street, North Tower 7th Floor, Stamford, Connecticut, 06902. Our telephone number is 888-729-1206.

3 Grew revenues to $116.7 million, an increase of 52%2 year-over-year Delivered adjusted gross margin of 74%, up from 64% in the third quarter of 2024 Grew exome and genome test revenue to $98.9 million, an increase of 65% year-over-year Generated adjusted net income of $14.7 million in the third quarter of 2025 1. Adjusted gross margin and adjusted net income are non-GAAP financial measures. See appendix for a reconciliation of GAAP to non-GAAP figures presented. 2. When compared to 2024 revenue from continuing operations, excluding the exited Legacy Sema4 business. Third Quarter 2025 Results1

4 Granted FDA Breakthrough Device Designation for the GeneDx ExomeDx and GenomeDx Introduced GeneDx InfinityTM and exhibited at the American Academy of Pediatrics (AAP) Annual Meeting, educating pediatricians about the updated AAP guidance recommending exome and genome sequencing Third Quarter Business Highlights Announced participation in two flagship genomic newborn screening initiatives – the NIH's BEACONS Initiative and Florida’s Sunshine Genetics Network Announced the Autism Partnership Program in partnership with Jaguar Gene Therapy, expanding access to testing for patients with SHANK3-related autism spectrum disorder (ASD) and Phelan-McDermid syndrome Appointed Lisa Gurry as Chief Business Officer and Dr. Mimi Lee as Chief Precision Medicine Officer to accelerate precision medicine and help more families with the power of data, AI, and clinical expertise, and added Dr. Thomas Fuchs, Chief AI Officer at Lilly to Board of Directors
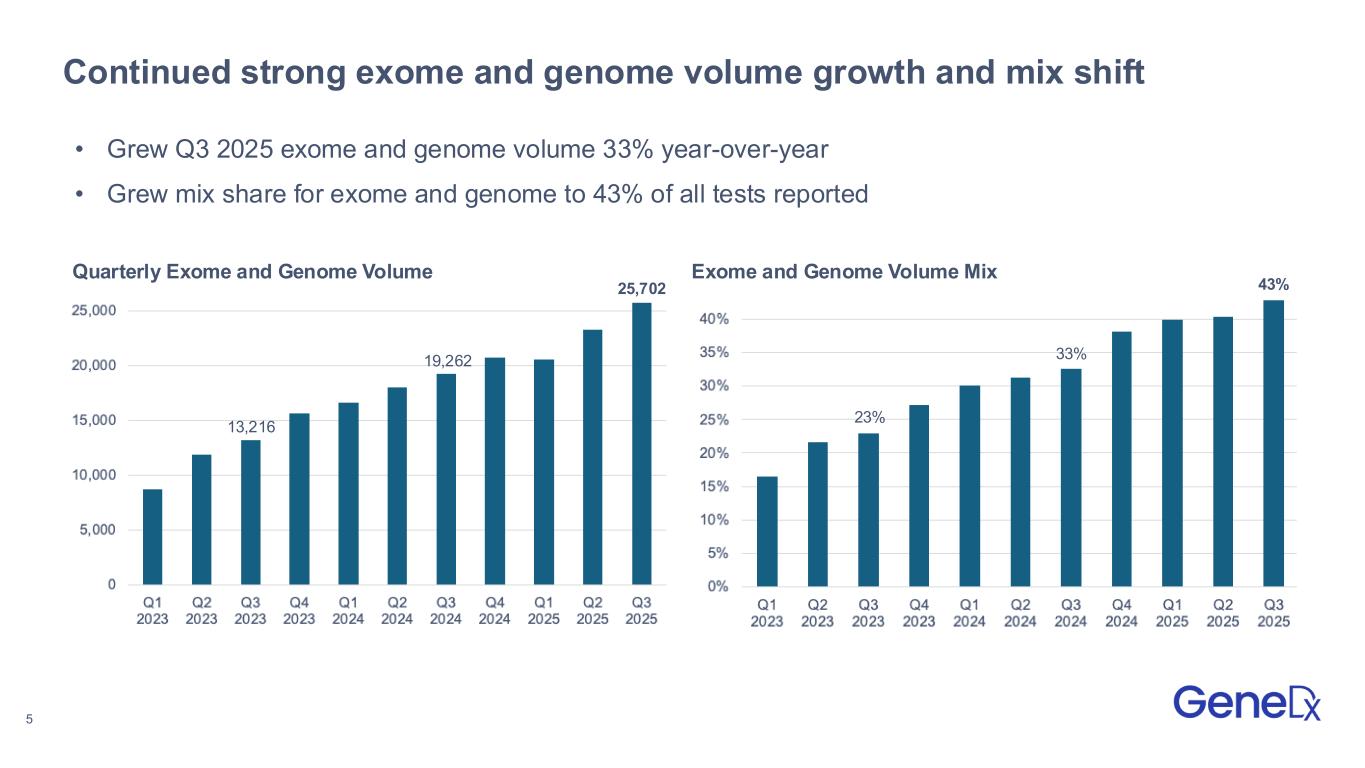
5 43% • Grew Q3 2025 exome and genome volume 33% year-over-year • Grew mix share for exome and genome to 43% of all tests reported Continued strong exome and genome volume growth and mix shift 23% 33% 25,702 19,262 13,216 Quarterly Exome and Genome Volume Exome and Genome Volume Mix
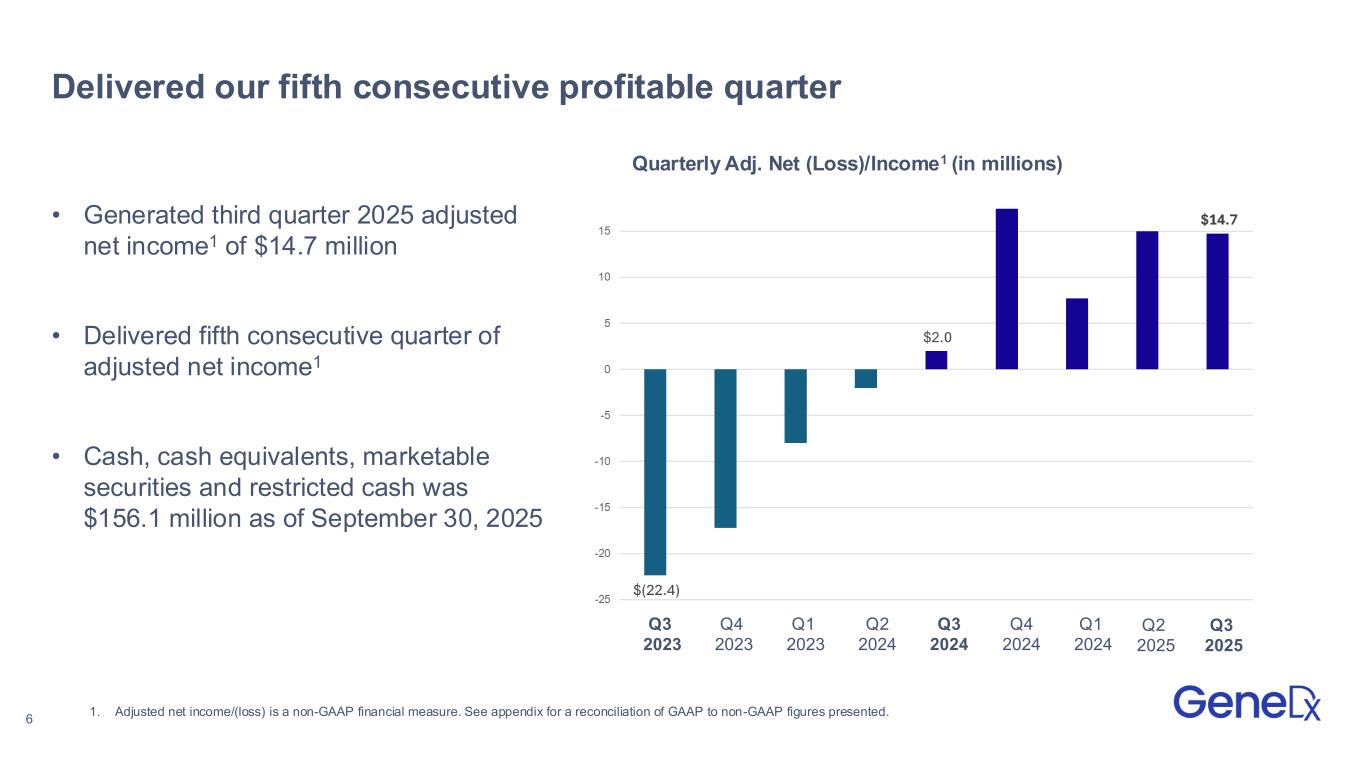
6 Q1 2023 Q4 2024 Q1 2024 Q3 2024 Q2 2024 Q4 2023 Q3 2023 Delivered our fifth consecutive profitable quarter • Generated third quarter 2025 adjusted net income1 of $14.7 million • Delivered fifth consecutive quarter of adjusted net income1 • Cash, cash equivalents, marketable securities and restricted cash was $156.1 million as of September 30, 2025 1. Adjusted net income/(loss) is a non-GAAP financial measure. See appendix for a reconciliation of GAAP to non-GAAP figures presented. Q2 2025 Quarterly Adj. Net (Loss)/Income1 (in millions) Q3 2025
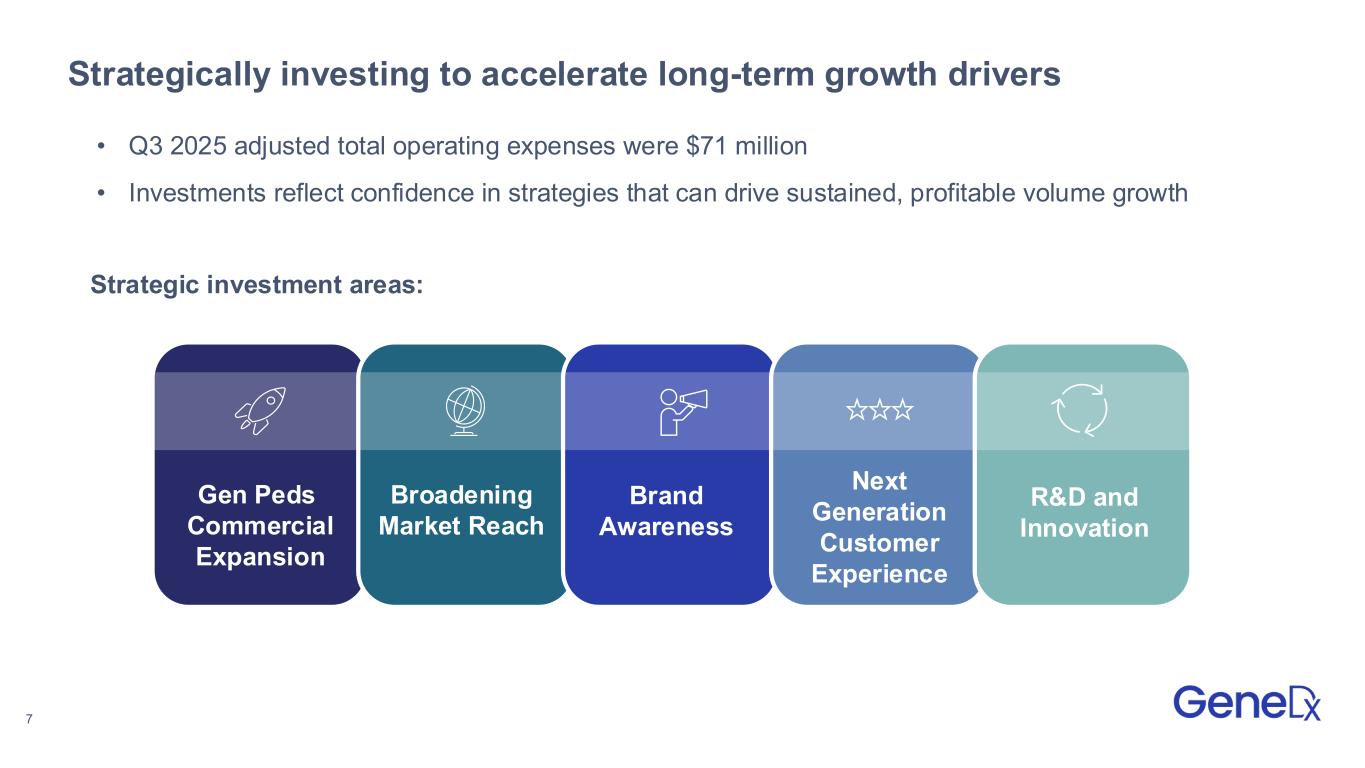
7 Strategically investing to accelerate long-term growth drivers Gen Peds Commercial Expansion Broadening Market Reach Brand Awareness Next Generation Customer Experience R&D and Innovation • Q3 2025 adjusted total operating expenses were $71 million • Investments reflect confidence in strategies that can drive sustained, profitable volume growth Strategic investment areas:
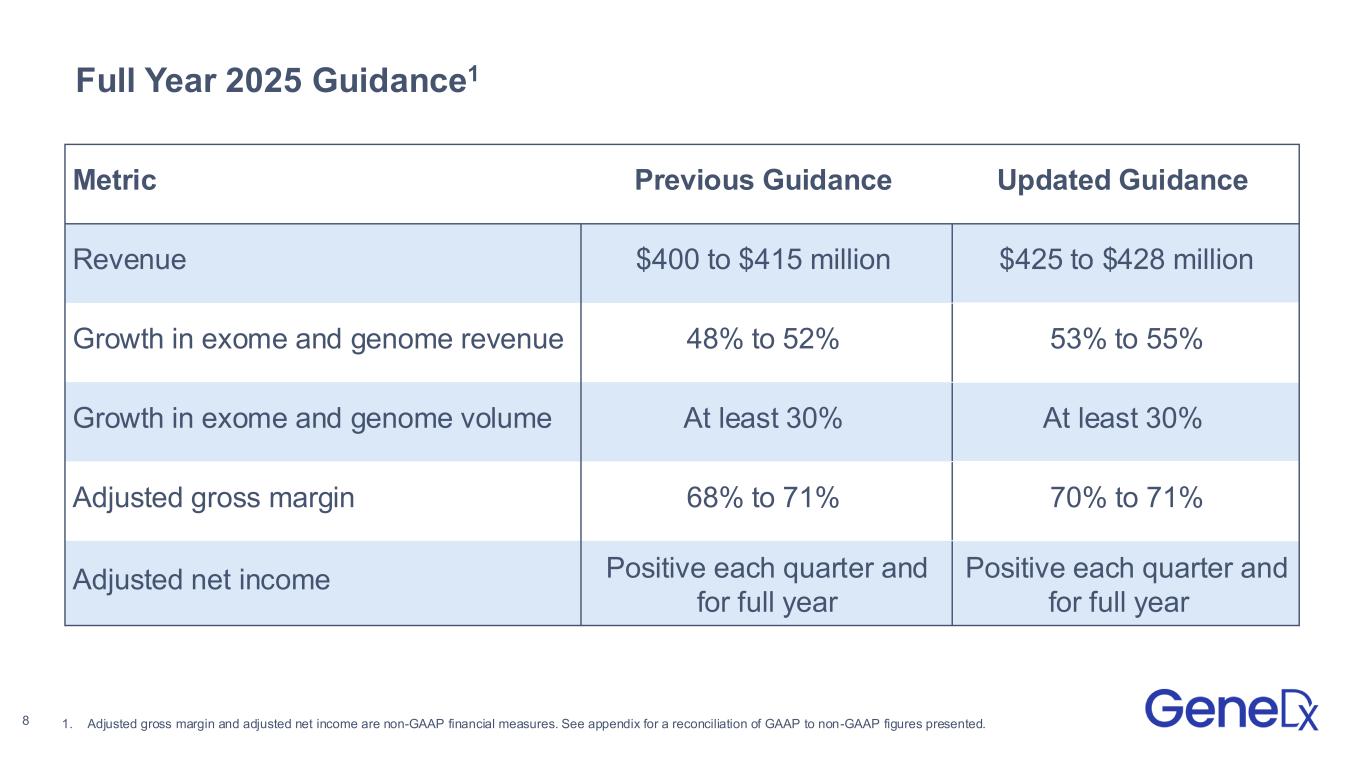
Full Year 2025 Guidance1 8 Metric Previous Guidance Updated Guidance Revenue $400 to $415 million $425 to $428 million Growth in exome and genome revenue 48% to 52% 53% to 55% Growth in exome and genome volume At least 30% At least 30% Adjusted gross margin 68% to 71% 70% to 71% Adjusted net income Positive each quarter and for full year Positive each quarter and for full year 1. Adjusted gross margin and adjusted net income are non-GAAP financial measures. See appendix for a reconciliation of GAAP to non-GAAP figures presented.

9 We envision a world where any genetic disorder is diagnosed quickly to prevent disease progression and ensure long and healthy lives for all.
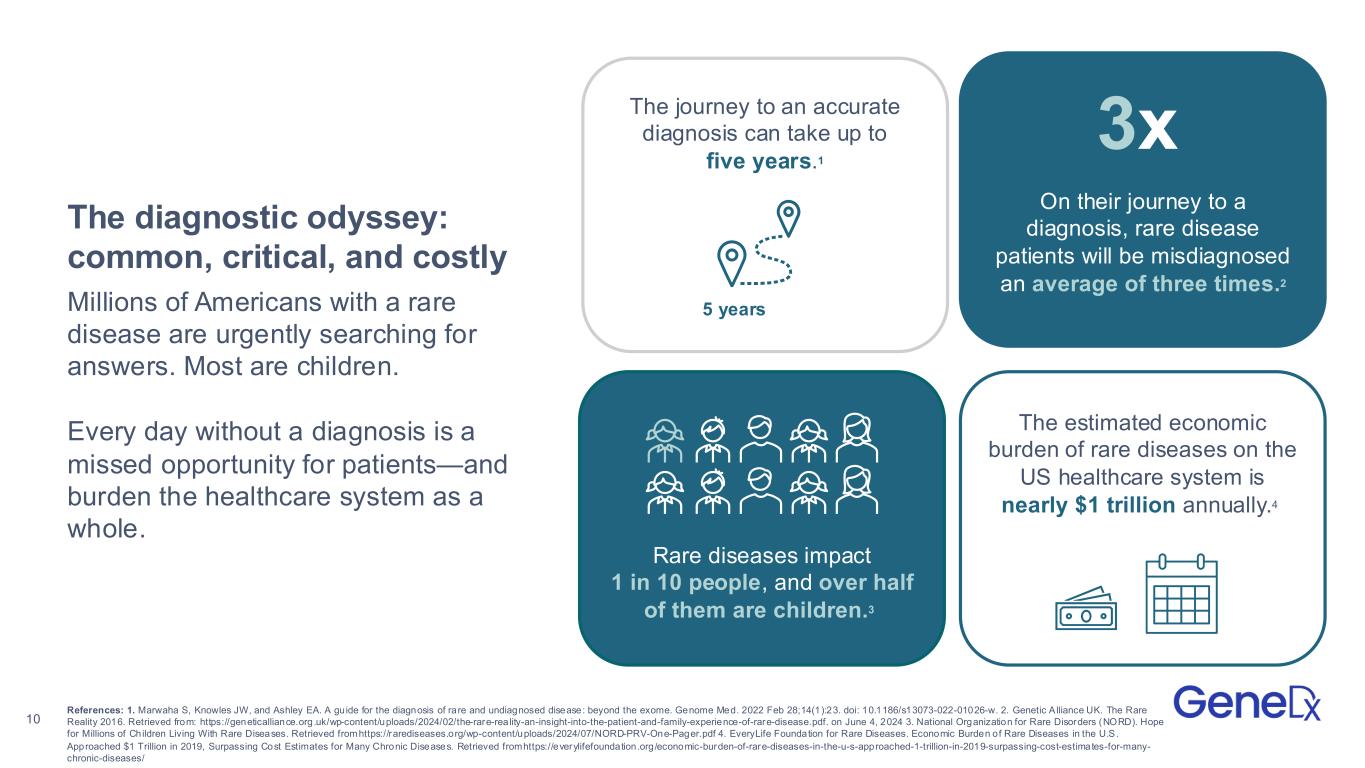
10 Rare diseases impact 1 in 10 people, and over half of them are children.3 The estimated economic burden of rare diseases on the US healthcare system is nearly $1 trillion annually.4 On their journey to a diagnosis, rare disease patients will be misdiagnosed an average of three times.2 3x The journey to an accurate diagnosis can take up to five years.1 5 years The diagnostic odyssey: common, critical, and costly Millions of Americans with a rare disease are urgently searching for answers. Most are children. Every day without a diagnosis is a missed opportunity for patients—and burden the healthcare system as a whole. References: 1. Marwaha S, Knowles JW, and Ashley EA. A guide for the diagnosis of rare and undiagnosed disease: beyond the exome. Genome Med. 2022 Feb 28;14(1):23. doi: 10.1186/s13073-022-01026-w. 2. Genetic A lliance UK. The Rare Reality 2016. Retrieved from: https://geneticalliance.org.uk/wp-content/uploads/2024/02/the-rare-rea lity-an-insight-into-the-patient-and-family-experience-of-rare-disease.pdf. on June 4, 2024 3. National Organization for Rare Disorders (NORD). Hope for Millions of Children Living With Rare Diseases. Retrieved fromhttps://rarediseases.org/wp-content/uploads/2024/07/NORD-PRV-One-Pager.pdf 4. EveryLife Foundation for Rare Diseases. Economic Burden of Rare Diseases in the U.S. Approached $1 Trillion in 2019, Surpassing Cost Estimates for Many Chronic Diseases. Retrieved fromhttps://everylifefoundation.org/economic-burden-of-rare-diseases-in-the-u-s-approached-1-trillion-in-2019-surpassing-cost-estimates-for-many- chronic-diseases/
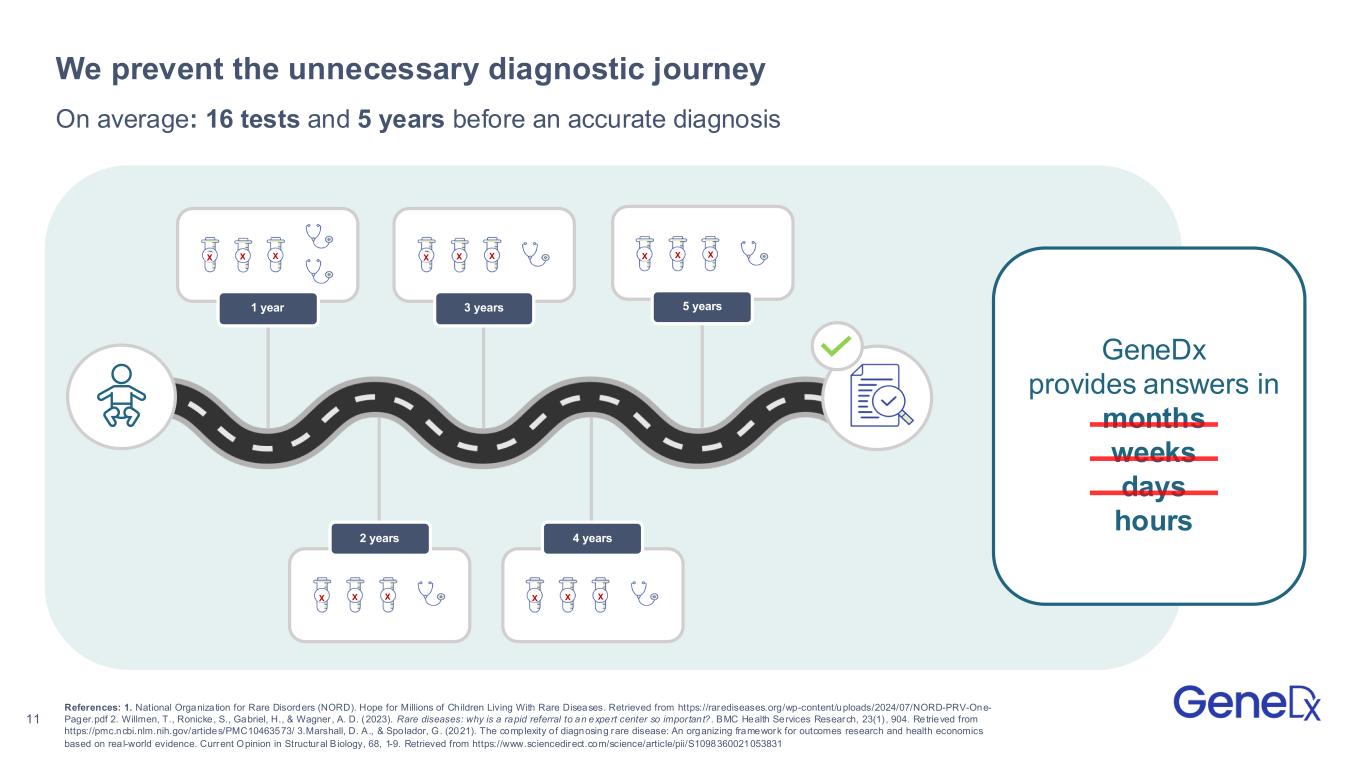
11 X X X 1 year X X X 2 years X X X 3 years X X X 4 years X X X 5 years On average: 16 tests and 5 years before an accurate diagnosis We prevent the unnecessary diagnostic journey GeneDx provides answers in months weeks days hours References: 1. National Organization for Rare Disorders (NORD). Hope for Millions of Children Living With Rare Diseases. Retrieved from https://rarediseases.org/wp-content/uploads/2024/07/NORD-PRV-One- Pager.pdf 2. Willmen, T., Ronicke, S., Gabriel, H., & Wagner , A. D. (2023). Rare diseases: why is a rapid referral to an expert center so impor tant? . BMC Health Services Research, 23(1) , 904. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10463573/ 3.Marshall, D. A., & Spolador, G. (2021). The complexity of diagnosing rare disease: An organizing framework for outcomes research and health economics based on rea l-world evidence. Current Opinion in Structural B iology, 68, 1-9. Retrieved from https://www.sciencedirect.com/science/article/pii/S1098360021053831
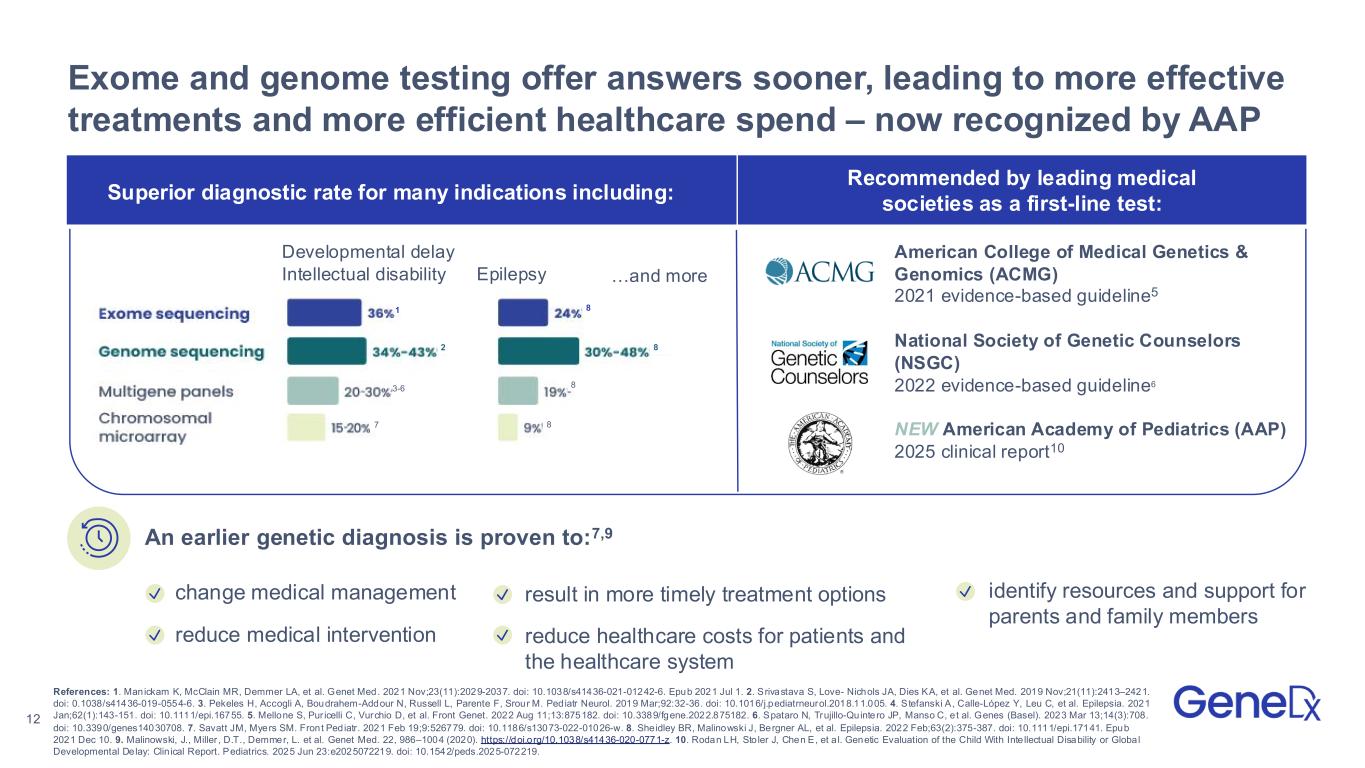
12 Exome and genome testing offer answers sooner, leading to more effective treatments and more efficient healthcare spend – now recognized by AAP change medical management References: 1. Manickam K, McClain MR, Demmer LA, et al. Genet Med. 2021 Nov;23(11):2029-2037. doi: 10.1038/s41436-021-01242-6. Epub 2021 Jul 1. 2. Srivastava S, Love- Nichols JA, Dies KA, et al. Genet Med. 2019 Nov;21(11):2413–2421. doi: 0.1038/s41436-019-0554-6. 3. Pekeles H, Accogli A, Boudrahem-Addour N, Russell L, Parente F, Srour M. Pediatr Neurol. 2019 Mar;92:32-36. doi: 10.1016/j.pediatrneurol.2018.11.005. 4. Stefanski A, Calle-López Y, Leu C, et al. Epilepsia. 2021 Jan;62(1):143-151. doi: 10.1111/epi.16755. 5. Mellone S, Pur icelli C, Vurchio D, et al. Front Genet. 2022 Aug 11;13:875182. doi: 10.3389/fgene.2022.875182. 6. Spataro N, Trujillo-Quintero JP, Manso C, e t al. Genes (Basel). 2023 Mar 13;14(3):708. doi: 10.3390/genes14030708. 7. Savatt JM, Myers SM. Front Pediatr. 2021 Feb 19;9:526779. doi: 10.1186/s13073-022-01026-w. 8. Sheidley BR, Malinowski J, Bergner AL, et al. Epilepsia. 2022 Feb;63(2):375-387. doi: 10.1111/epi.17141. Epub 2021 Dec 10. 9. Malinowski, J., Miller , D.T., Demmer, L. et al. Genet Med. 22, 986–1004 (2020). https://doi.org/10.1038/s41436-020-0771-z. 10. Rodan LH, Sto ler J, Chen E, et a l. Genetic Evaluation of the Child With Inte llectual Disability or Global Developmental Delay: Clin ical Report. Pediatrics. 2025 Jun 23:e2025072219. doi: 10.1542/peds.2025-072219. identify resources and support for parents and family members An earlier genetic diagnosis is proven to:7,9 reduce medical intervention American College of Medical Genetics & Genomics (ACMG) 2021 evidence-based guideline5 National Society of Genetic Counselors (NSGC) 2022 evidence-based guideline6 NEW American Academy of Pediatrics (AAP) 2025 clinical report10 result in more timely treatment options reduce healthcare costs for patients and the healthcare system Recommended by leading medical societies as a first-line test: Superior diagnostic rate for many indications including: …and moreEpilepsy Developmental delay Intellectual disability 1 2 3-6 7 8 8 8 8
13 Reference: Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Only 43% of epilepsy genes are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures. 20

14 Reference: Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Exome and genome sequencing checks all 768 genes Only 43% of epilepsy genes are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures. 21
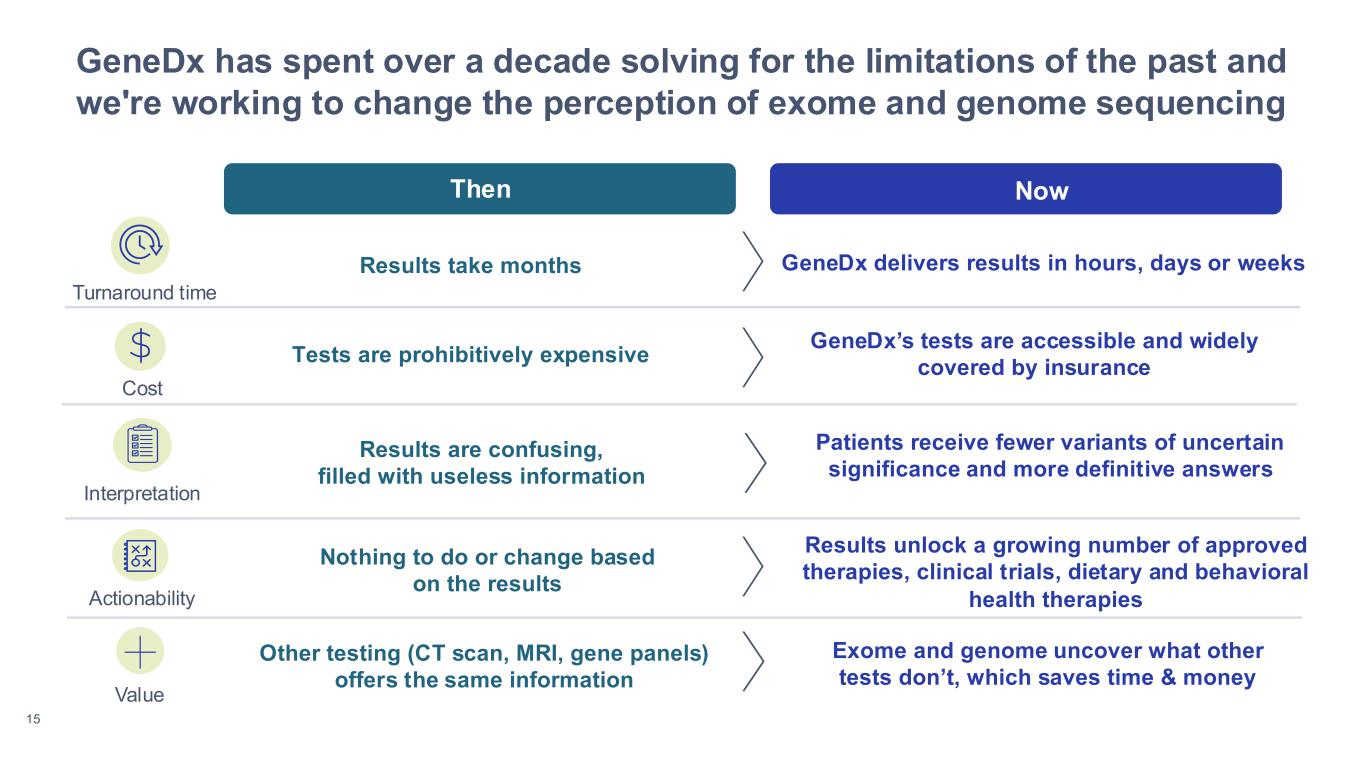
15 GeneDx has spent over a decade solving for the limitations of the past and we're working to change the perception of exome and genome sequencing Then Now Results take months Tests are prohibitively expensive GeneDx’s tests are accessible and widely covered by insurance Results are confusing, filled with useless information Patients receive fewer variants of uncertain significance and more definitive answers Turnaround time Cost Interpretation Nothing to do or change based on the results Results unlock a growing number of approved therapies, clinical trials, dietary and behavioral health therapies GeneDx delivers results in hours, days or weeks Other testing (CT scan, MRI, gene panels) offers the same information Exome and genome uncover what other tests don’t, which saves time & money Actionability Value
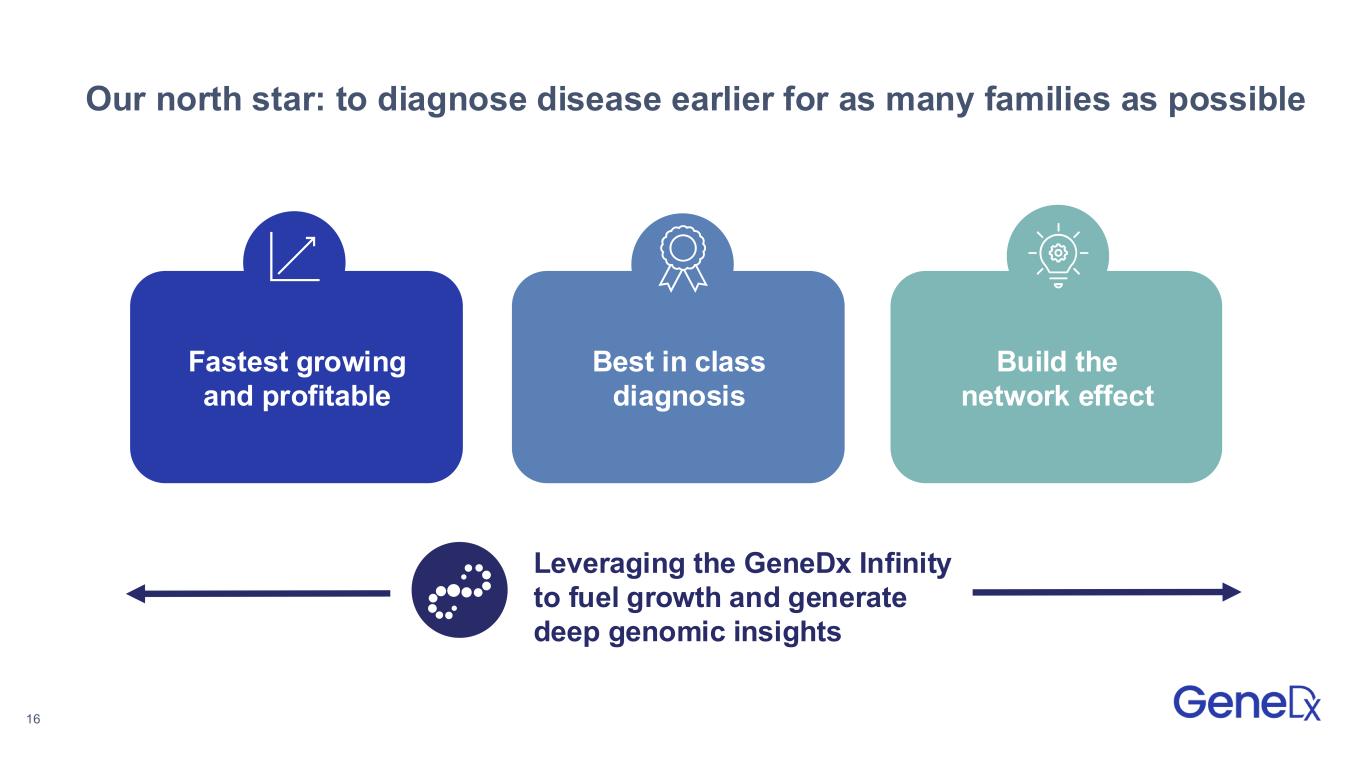
16 Fastest growing and profitable Best in class diagnosis Our north star: to diagnose disease earlier for as many families as possible Build the network effect Leveraging the GeneDx Infinity to fuel growth and generate deep genomic insights
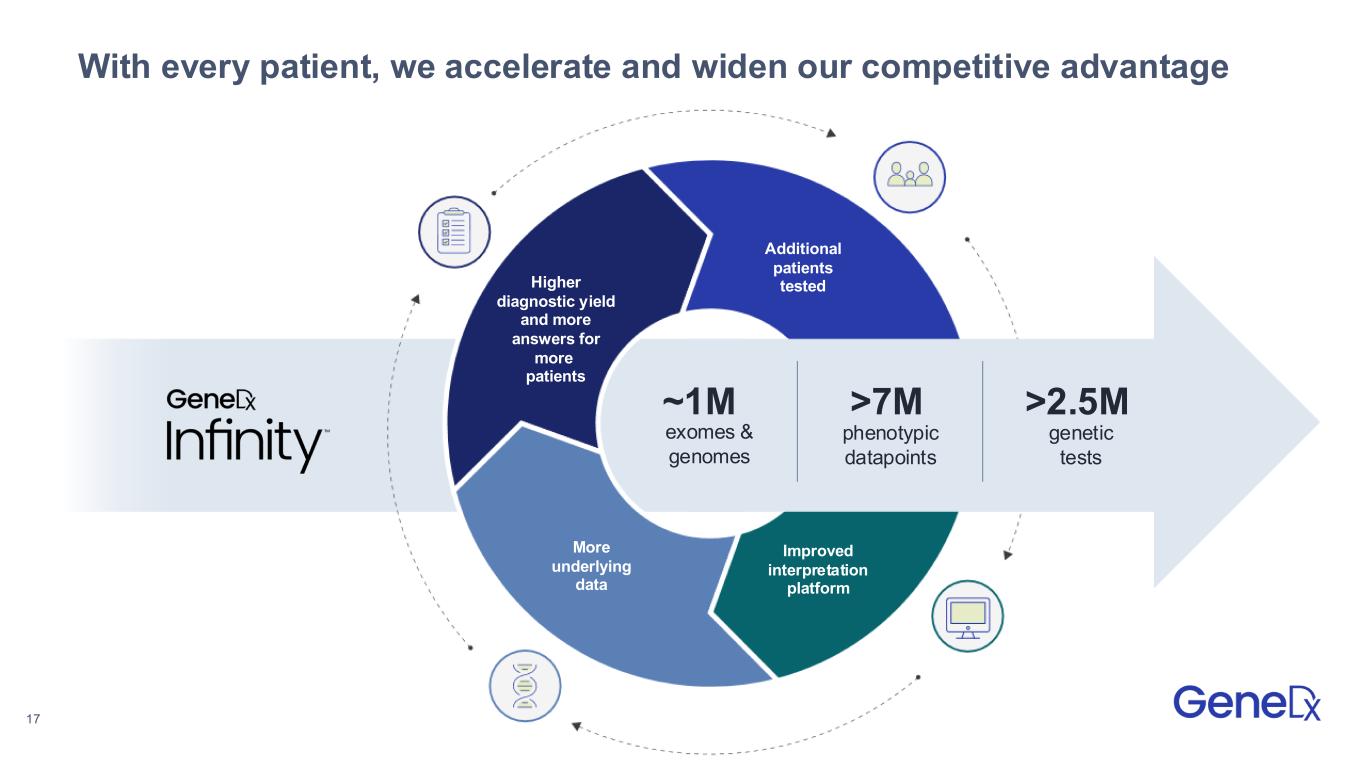
17 With every patient, we accelerate and widen our competitive advantage ~1M exomes & genomes >7M phenotypic datapoints Additional patients tested Improved interpretation platform More underlying data Higher diagnostic yield and more answers for more patients >2.5M genetic tests
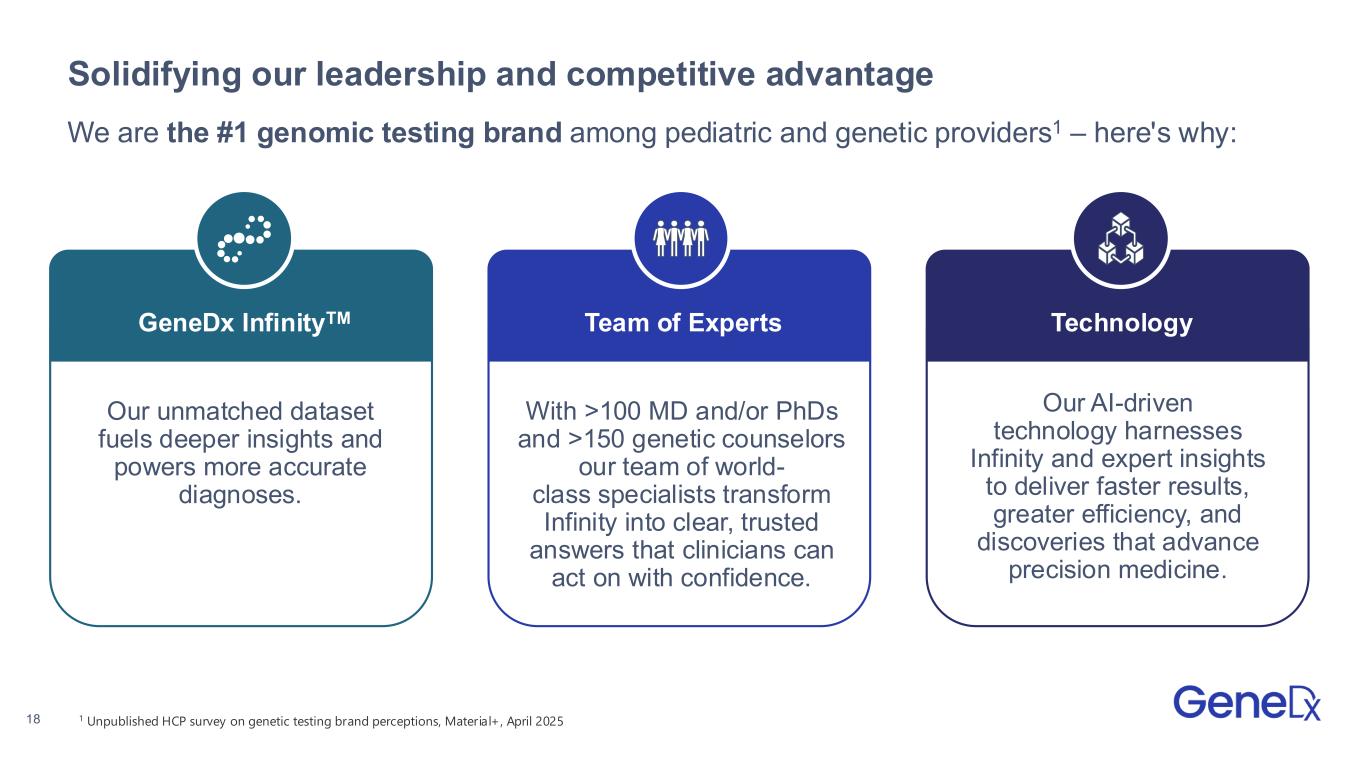
18 Solidifying our leadership and competitive advantage Our unmatched dataset fuels deeper insights and powers more accurate diagnoses. GeneDx InfinityTM With >100 MD and/or PhDs and >150 genetic counselors our team of world- class specialists transform Infinity into clear, trusted answers that clinicians can act on with confidence. Team of Experts Our AI-driven technology harnesses Infinity and expert insights to deliver faster results, greater efficiency, and discoveries that advance precision medicine. Technology We are the #1 genomic testing brand among pediatric and genetic providers1 – here's why: 1 Unpublished HCP survey on genetic testing brand perceptions, Material+, April 2025
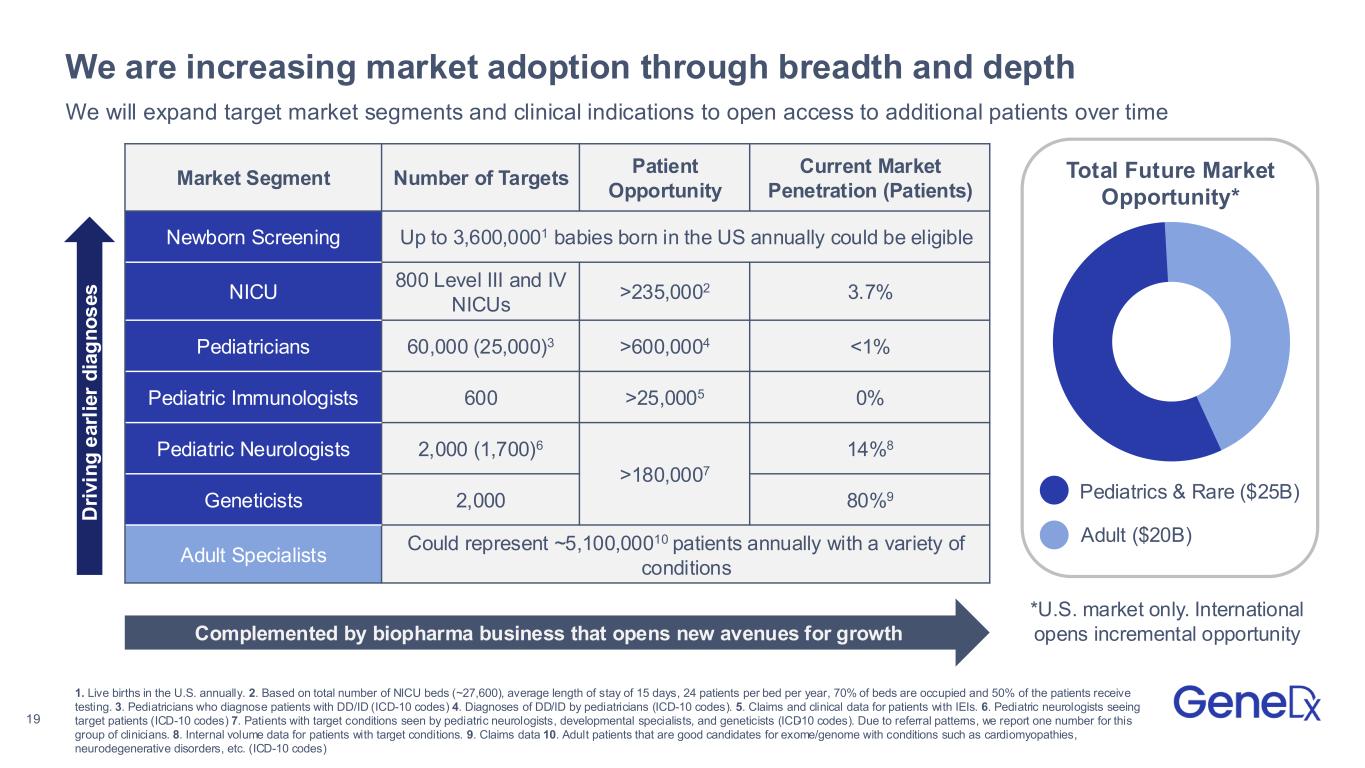
19 We are increasing market adoption through breadth and depth Market Segment Number of Targets Patient Opportunity Current Market Penetration (Patients) Newborn Screening Up to 3,600,0001 babies born in the US annually could be eligible NICU 800 Level III and IV NICUs >235,0002 3.7% Pediatricians 60,000 (25,000)3 >600,0004 <1% Pediatric Immunologists 600 >25,0005 0% Pediatric Neurologists 2,000 (1,700)6 >180,0007 14%8 Geneticists 2,000 80%9 Adult Specialists Could represent ~5,100,00010 patients annually with a variety of conditions We will expand target market segments and clinical indications to open access to additional patients over time Complemented by biopharma business that opens new avenues for growth Total Future Market Opportunity* Pediatrics & Rare ($25B) Adult ($20B) 1. Live births in the U.S. annually. 2. Based on total number of NICU beds (~27,600), average length of stay of 15 days, 24 patients per bed per year, 70% of beds are occupied and 50% of the patients receive testing. 3. Pediatricians who diagnose patients with DD/ID (ICD-10 codes) 4. Diagnoses of DD/ID by pediatricians (ICD-10 codes). 5. Claims and clinical data for patients with IEIs. 6. Pediatric neurologists seeing target patients (ICD-10 codes) 7. Patients with target conditions seen by pediatric neurologists, developmental specialists, and geneticists (ICD-10 codes). Due to referral patterns, we report one number for this group of clinicians. 8. Internal volume data for patients with target conditions. 9. Claims data 10. Adult patients that are good candidates for exome/genome with conditions such as cardiomyopathies, neurodegenerative disorders, etc. (ICD-10 codes) D ri v in g e a rl ie r d ia g n o s e s *U.S. market only. International opens incremental opportunity

20 Fueling growth and better outcomes in the outpatient setting Growing from the core: • Scaling utilization by geneticists and pediatric neurologists diagnosing epilepsy, autism and intellectual disability/developmental delay, and rare disease Expanding to new indications: • Started with cerebral palsy and immunological disorders • Taking a disciplined approach to entering markets with clinical utility and favorable reimbursement Looking ahead to general pediatricians: • American Academy of Pediatrics recommends exome or genome testing as first-line for patients with intellectual disability/developmental delay • Expect 18-24 months before meaningful volume contributions

21 Delivering answers to improve care in the NICU References: 1.SeqFirst 2. Kingsmore SF, Nofsinger R. & Ellswor th K. NPJ Genom. Med. 2024;9(17). doi:10.1038/s41525-024-00404-0. 3. Lavelle TA, Maron JL, Kingsmore SF, et a l. Rapid Genome Sequencing Compared to a Gene Panel in Critica lly Ill Infants with a Suspected Genetic Disorder: An Economic Eva luation. medRxiv [Prepr int]. 2025 Apr 10:2024.10.18.24315740. doi: 10.1101/2024.10.18.24315740 Demonstrating clinical and economic utility: • SeqFirst: 60% of infants in Level IV NICUs should receive a rapid genome test1 • Genome testing is severely underutilized, currently ordered for <5% of children who could benefit2 • A genetic diagnosis in the NICU can result in total cost savings of $150K/patient over a single year3 Delivering the leading product and experience: • Epic Aura integrations streamline workflows and improve experience • UltraRapid genome sequencing deliver results in as soon as 48 hours • Genome product updates (buccal, 5-day TAT, etc.) deliver value Leveraging our relationships and reputation: • 200 of the 800 Level IV and V NICUs are current GeneDx clients
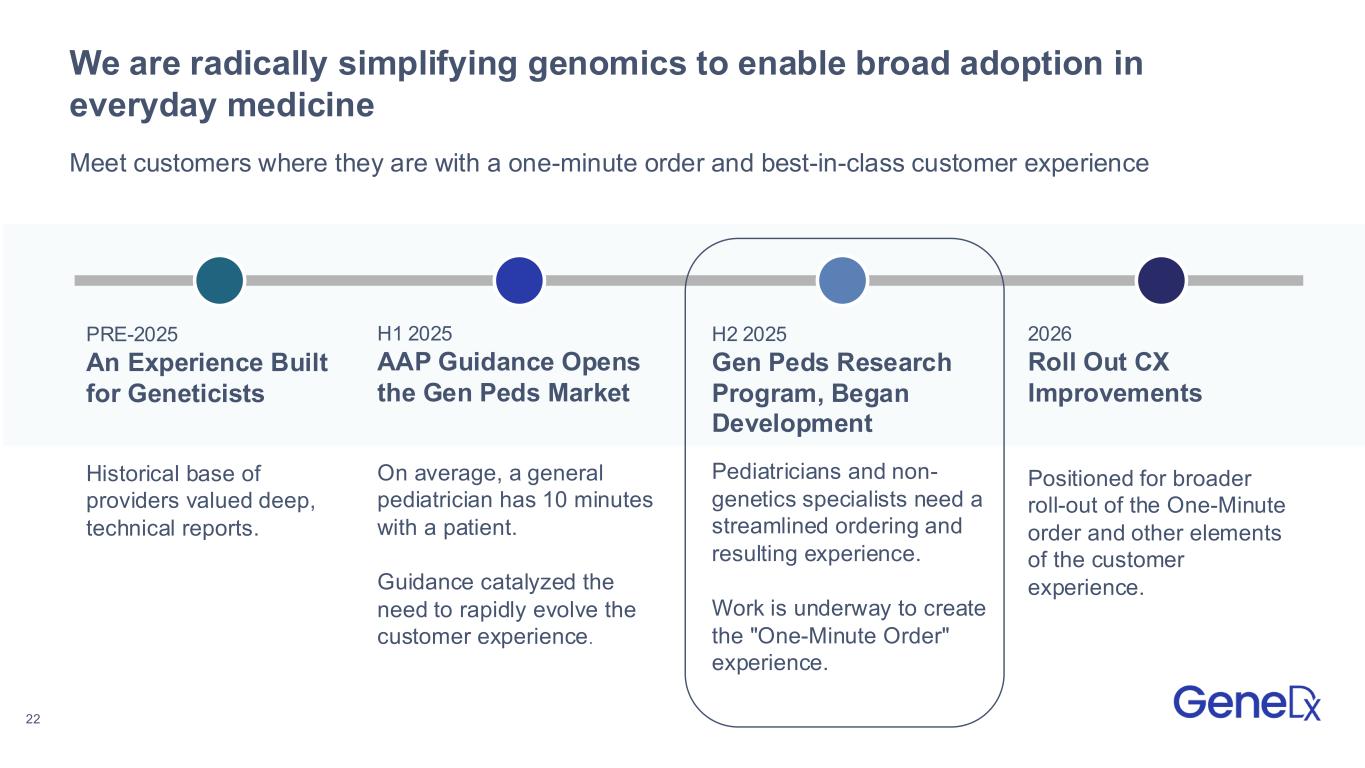
22 We are radically simplifying genomics to enable broad adoption in everyday medicine PRE-2025 An Experience Built for Geneticists Historical base of providers valued deep, technical reports. H1 2025 AAP Guidance Opens the Gen Peds Market On average, a general pediatrician has 10 minutes with a patient. Guidance catalyzed the need to rapidly evolve the customer experience. H2 2025 Gen Peds Research Program, Began Development Pediatricians and non- genetics specialists need a streamlined ordering and resulting experience. Work is underway to create the "One-Minute Order" experience. 2026 Roll Out CX Improvements Positioned for broader roll-out of the One-Minute order and other elements of the customer experience. Meet customers where they are with a one-minute order and best-in-class customer experience
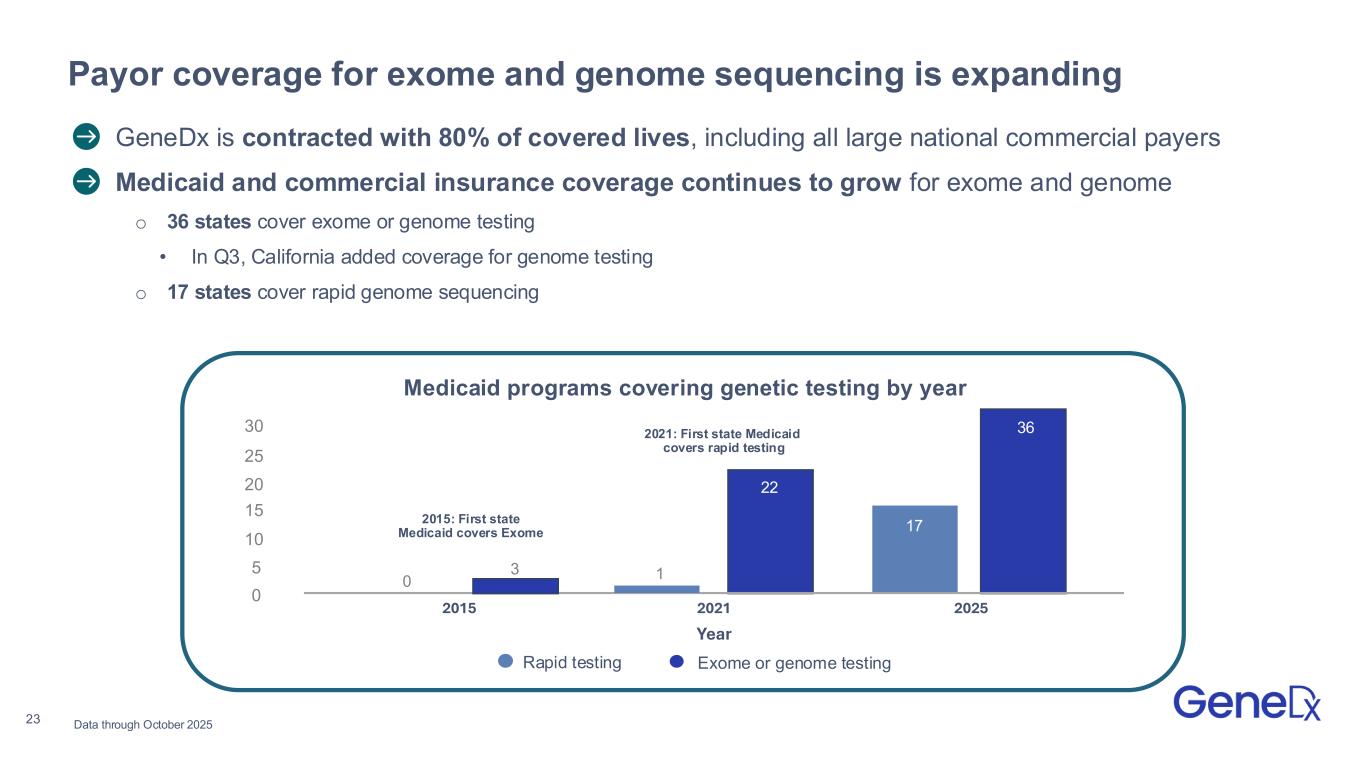
23 〉 GeneDx is contracted with 80% of covered lives, including all large national commercial payers 〉Medicaid and commercial insurance coverage continues to grow for exome and genome o 36 states cover exome or genome testing • In Q3, California added coverage for genome testing o 17 states cover rapid genome sequencing Payor coverage for exome and genome sequencing is expanding Data through October 2025 Medicaid programs covering genetic testing by year 2015: First state Medicaid covers Exome 2021: First state Medicaid covers rapid testing 30 25 20 15 10 5 0 0 3 1 36 2015 2021 2025 Year Rapid testing Exome or genome testing 22 17
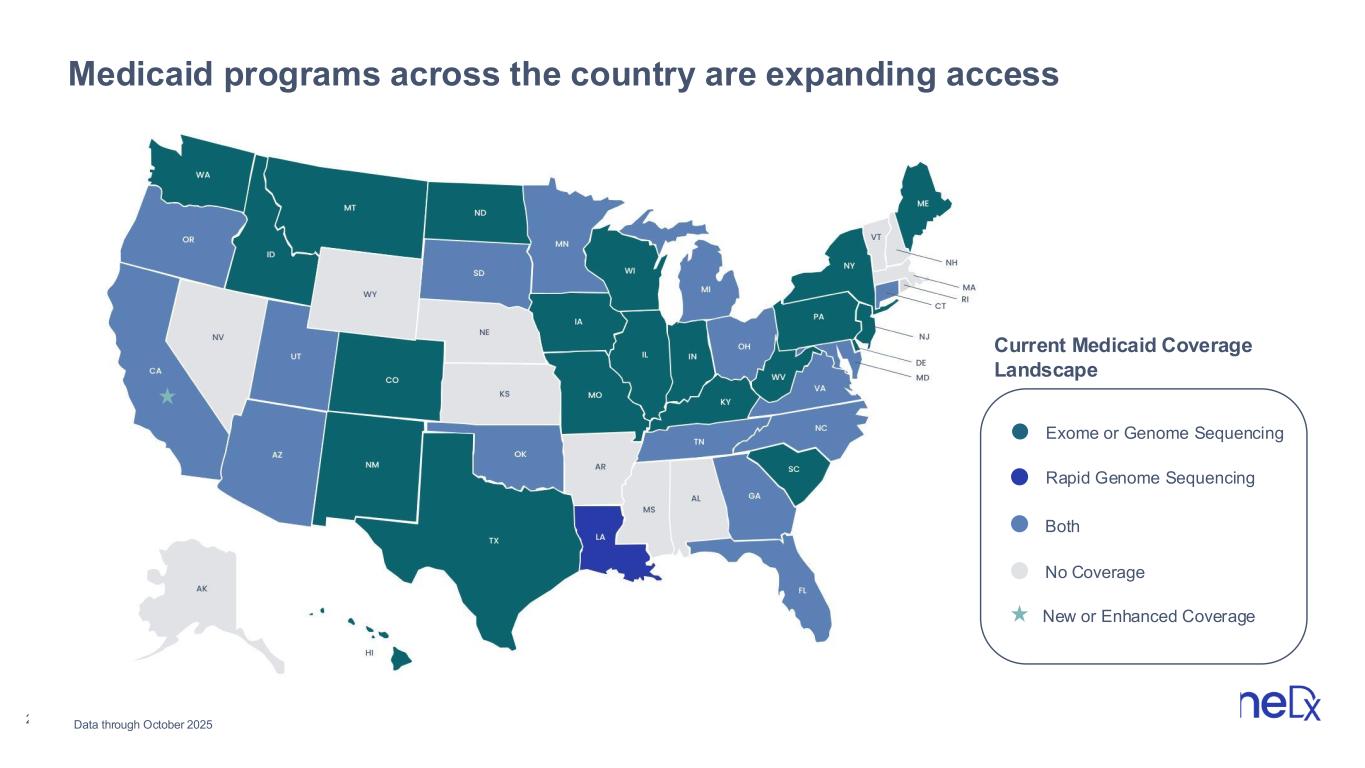
24 Medicaid programs across the country are expanding access Exome or Genome Sequencing Rapid Genome Sequencing Both No Coverage Current Medicaid Coverage Landscape Data through October 2025 New or Enhanced Coverage
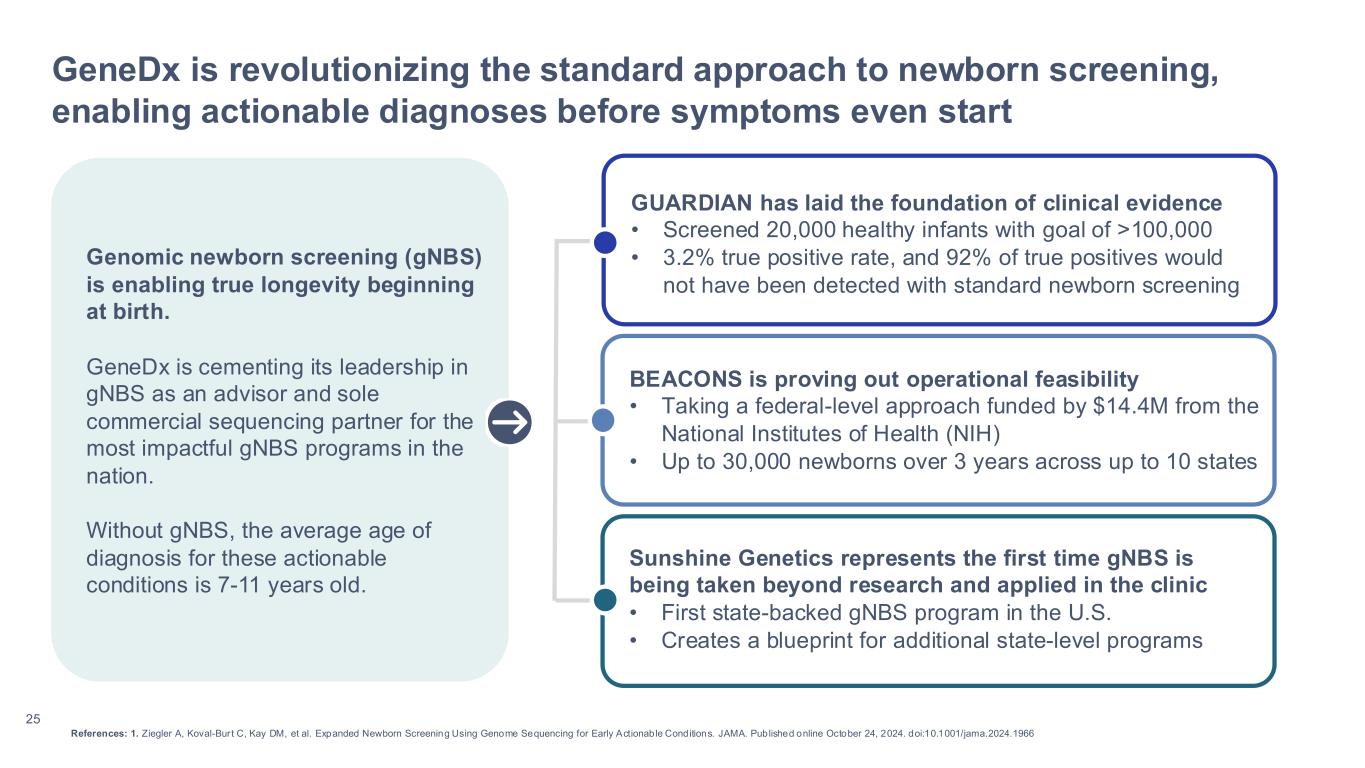
25 GeneDx is revolutionizing the standard approach to newborn screening, enabling actionable diagnoses before symptoms even start GUARDIAN has laid the foundation of clinical evidence • Screened 20,000 healthy infants with goal of >100,000 • 3.2% true positive rate, and 92% of true positives would not have been detected with standard newborn screening References: 1. Ziegler A, Koval-Bur t C, Kay DM, et al. Expanded Newborn Screening Using Genome Sequencing for Early Actionable Conditions. JAMA. Published online October 24, 2024. doi:10.1001/jama.2024.1966 BEACONS is proving out operational feasibility • Taking a federal-level approach funded by $14.4M from the National Institutes of Health (NIH) • Up to 30,000 newborns over 3 years across up to 10 states Sunshine Genetics represents the first time gNBS is being taken beyond research and applied in the clinic • First state-backed gNBS program in the U.S. • Creates a blueprint for additional state-level programs Genomic newborn screening (gNBS) is enabling true longevity beginning at birth. GeneDx is cementing its leadership in gNBS as an advisor and sole commercial sequencing partner for the most impactful gNBS programs in the nation. Without gNBS, the average age of diagnosis for these actionable conditions is 7-11 years old.
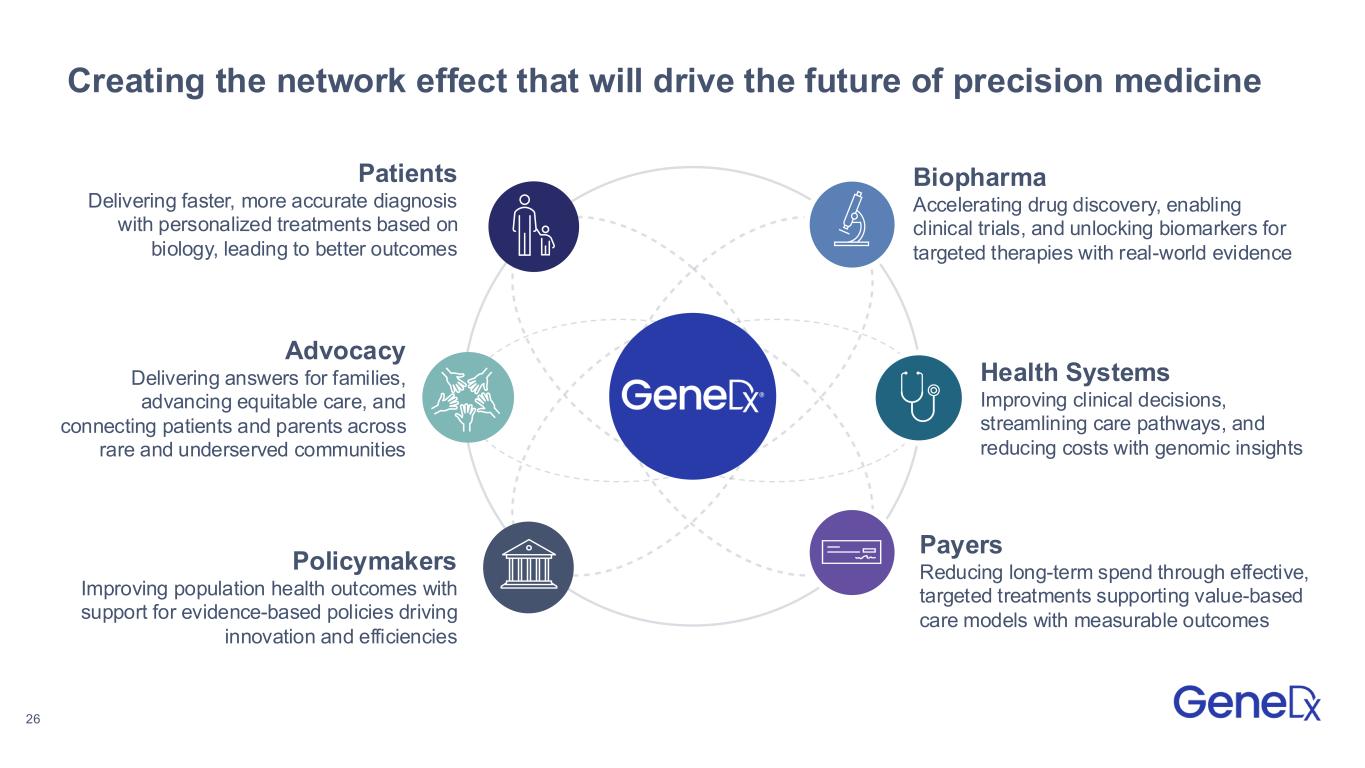
26 Creating the network effect that will drive the future of precision medicine Biopharma Accelerating drug discovery, enabling clinical trials, and unlocking biomarkers for targeted therapies with real-world evidence Policymakers Improving population health outcomes with support for evidence-based policies driving innovation and efficiencies Patients Delivering faster, more accurate diagnosis with personalized treatments based on biology, leading to better outcomes Health Systems Improving clinical decisions, streamlining care pathways, and reducing costs with genomic insights Payers Reducing long-term spend through effective, targeted treatments supporting value-based care models with measurable outcomes Advocacy Delivering answers for families, advancing equitable care, and connecting patients and parents across rare and underserved communities
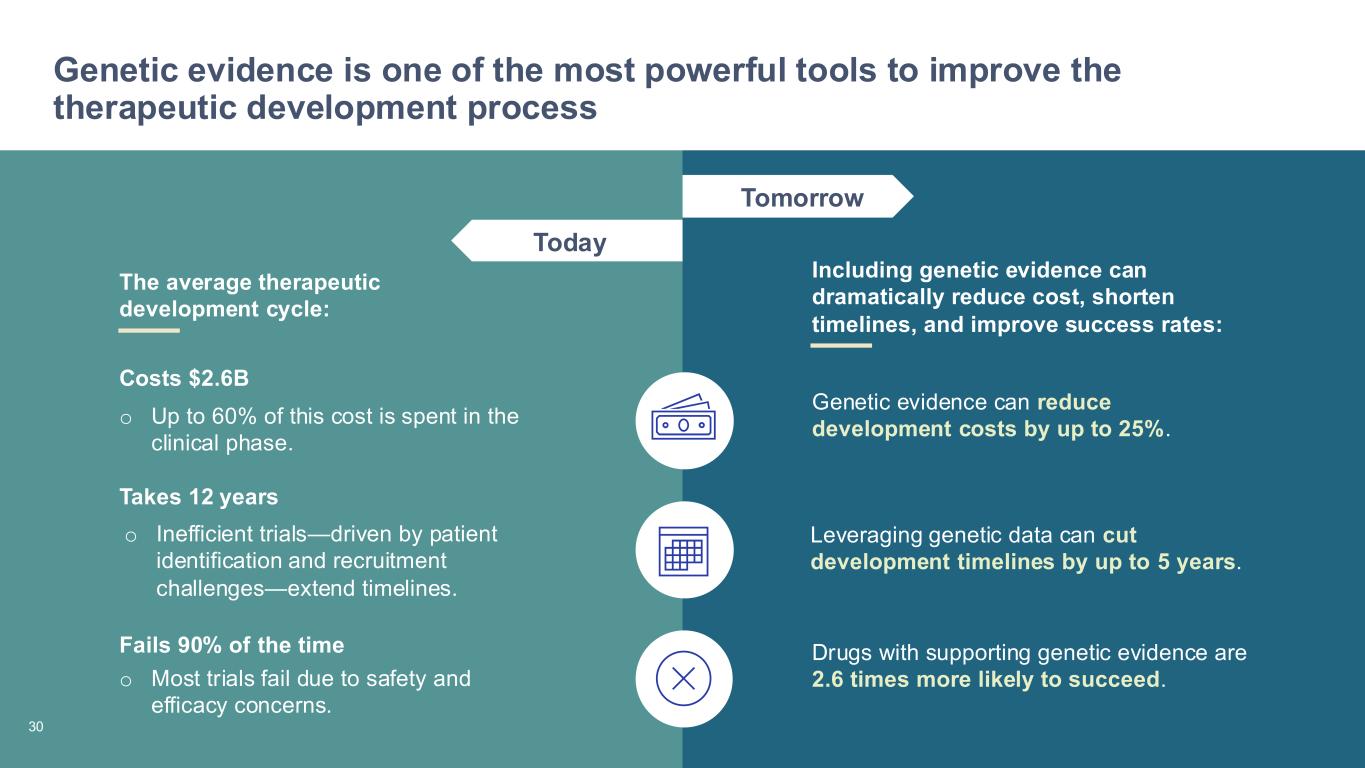
27 Genetic evidence is one of the most powerful tools to improve the therapeutic development process Today Tomorrow The average therapeutic development cycle: Including genetic evidence can dramatically reduce cost, shorten timelines, and improve success rates: o Up to 60% of this cost is spent in the clinical phase. Costs $2.6B o Inefficient trials—driven by patient identification and recruitment challenges—extend timelines. Takes 12 years o Most trials fail due to safety and efficacy concerns. Fails 90% of the time Genetic evidence can reduce development costs by up to 25%. Leveraging genetic data can cut development timelines by up to 5 years. Drugs with supporting genetic evidence are 2.6 times more likely to succeed. 30
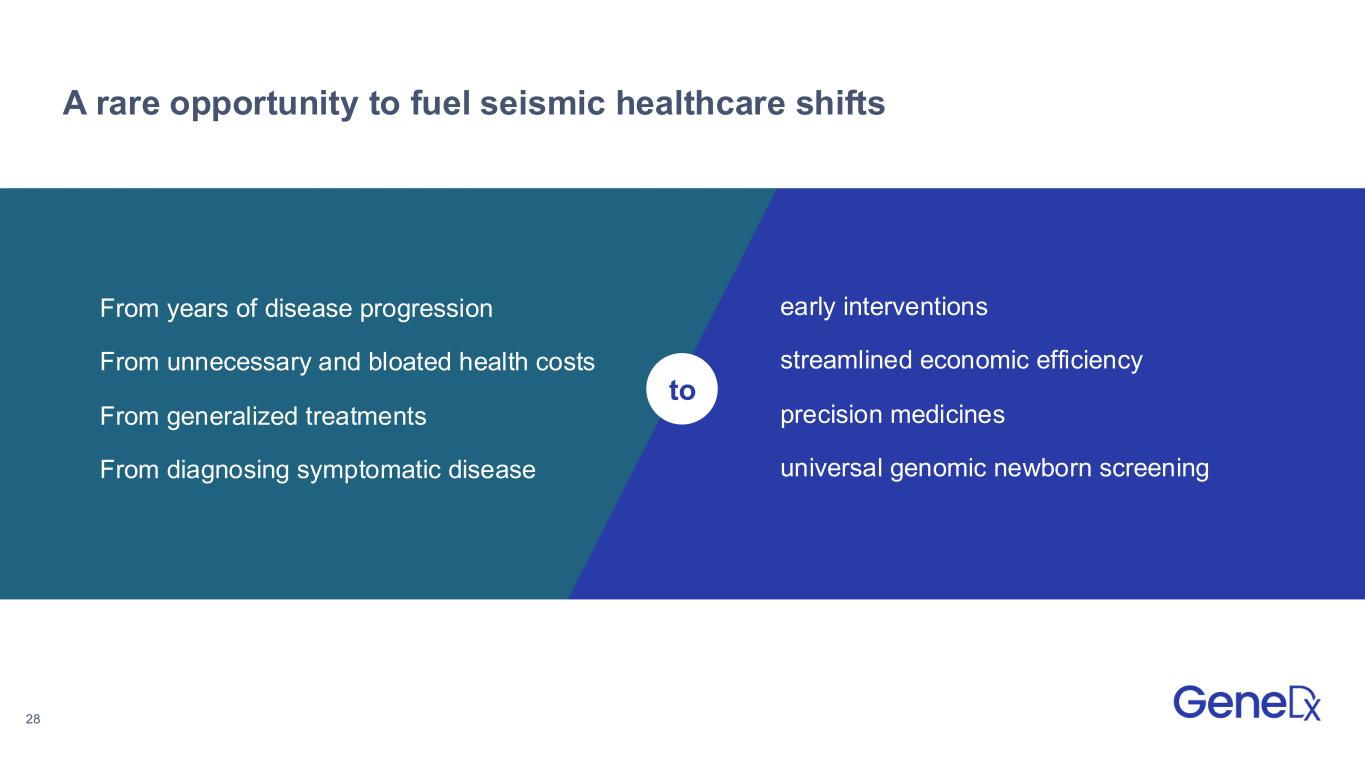
28 A rare opportunity to fuel seismic healthcare shifts to From years of disease progression From unnecessary and bloated health costs From generalized treatments From diagnosing symptomatic disease early interventions streamlined economic efficiency precision medicines universal genomic newborn screening

29 The future of healthcare is about proactive, personalized care. GeneDx is leading the way.

30 Confidential & Proprietary. Do Not Distribute. Appendix
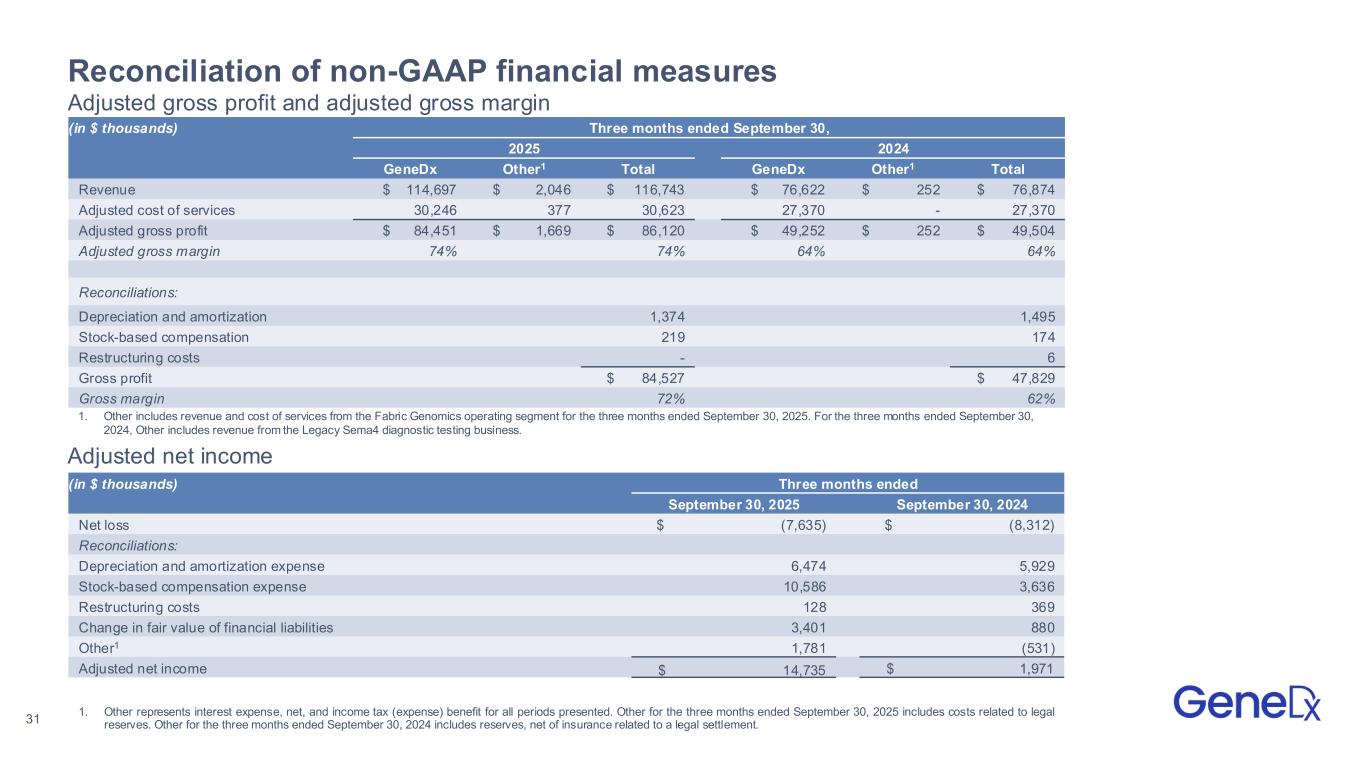
31 Reconciliation of non-GAAP financial measures Adjusted gross profit and adjusted gross margin (in $ thousands) Three months ended September 30, 2025 2024 GeneDx Other1 Total GeneDx Other1 Total Revenue $ 114,697 $ 2,046 $ 116,743 $ 76,622 $ 252 $ 76,874 Adjusted cost of services 30,246 377 30,623 27,370 - 27,370 Adjusted gross profit $ 84,451 $ 1,669 $ 86,120 $ 49,252 $ 252 $ 49,504 Adjusted gross margin 74% 74% 64% 64% Reconciliations: Depreciation and amortization 1,374 1,495 Stock-based compensation 219 174 Restructuring costs - 6 Gross profit $ 84,527 $ 47,829 Gross margin 72% 62% (in $ thousands) Three months ended September 30, 2025 September 30, 2024 Net loss $ (7,635) $ (8,312) Reconciliations: Depreciation and amortization expense 6,474 5,929 Stock-based compensation expense 10,586 3,636 Restructuring costs 128 369 Change in fair value of financial liabilities 3,401 880 Other1 1,781 (531) Adjusted net income $ 14,735 $ 1,971 Adjusted net income 1. Other represents interest expense, net, and income tax (expense) benefit for all periods presented. Other for the three months ended September 30, 2025 includes costs related to legal reserves. Other for the three months ended September 30, 2024 includes reserves, net of insurance related to a legal settlement. 1. Other includes revenue and cost of services from the Fabric Genomics operating segment for the three months ended September 30, 2025. For the three months ended September 30, 2024, Other includes revenue from the Legacy Sema4 diagnostic testing business.