

.2 Positive Topline Results from Phase 1/2 DELIVER Trial of Zeleciment Rostudirsen (DYNE-251) in DMD to Support Potential U.S. Accelerated Approval Ravi, living with DMD DECEMBER 8, 2025

Forward-Looking Statements & Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform and its conjugates, the therapeutic potential of zeleciment basivarsen (z-basivarsen, also known as DYNE-101), zeleciment rostudirsen (z- rostudirsen, also known as DYNE-251), DYNE-302 and DYNE-401, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of accelerated approval pathways for z-basivarsen and z-rostudirsen, and expectations regarding the timing of submission of applications for U.S. Accelerated Approval and the timing of commercial launches, and the sufficiency of Dyne’s cash resources for the period anticipated, constitute forward- looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” “will,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward- looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and initial data from clinical trials will be predictive of the final results of the clinical trials or future trials; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne's clinical trials and acceptance of Dyne's clinical programs and the regulatory approval process; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses, debt service obligations, and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-Q and in subsequent filings Dyne may make with the SEC. In addition, the forward- looking statements included in this presentation represent Dyne’s views as of the date of this presentation. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other statistical data made by independent parties and by the Company relating to market size and growth and other data about the Company’s industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The Company has not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of the Company’s future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. 2

Program Opening Remarks John Cox, President & CEO Positive Topline Results from DELIVER Trial of Z-Rostudirsen Supporting Potential for U.S. Accelerated Approval Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO Available for Q&A Erick Lucera, Chief Financial Officer 3
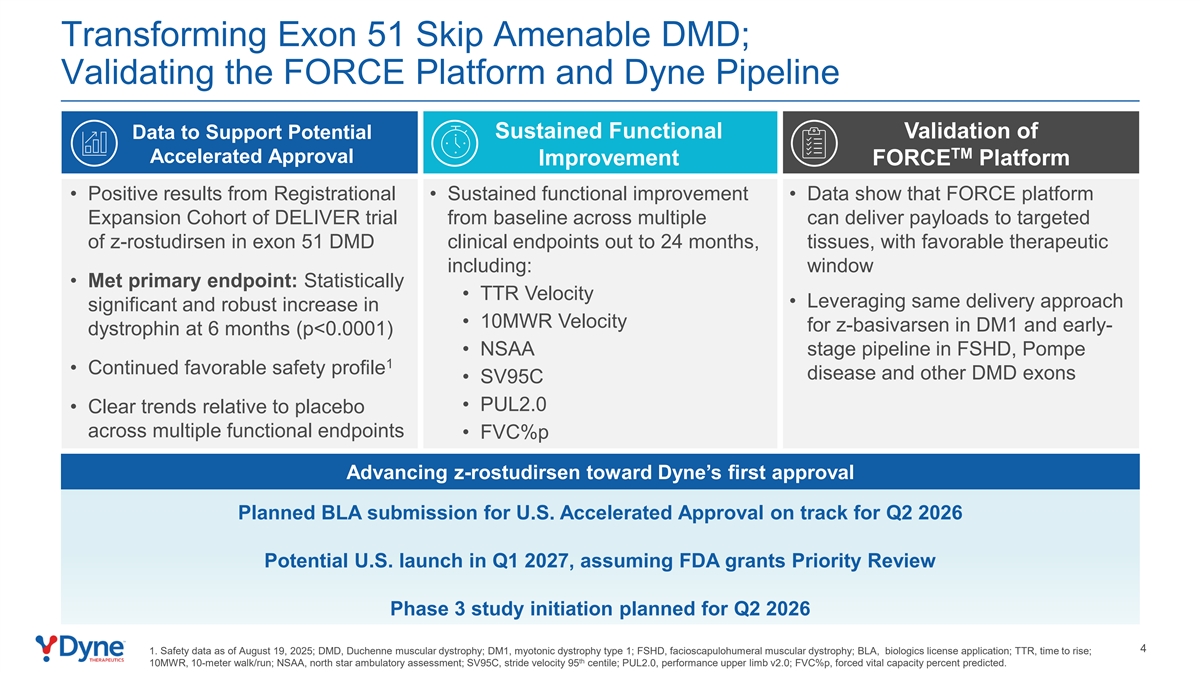
Transforming Exon 51 Skip Amenable DMD; Validating the FORCE Platform and Dyne Pipeline Data to Support Potential Sustained Functional Validation of TM Accelerated Approval Improvement FORCE Platform • Positive results from Registrational • Sustained functional improvement • Data show that FORCE platform Expansion Cohort of DELIVER trial from baseline across multiple can deliver payloads to targeted of z-rostudirsen in exon 51 DMD clinical endpoints out to 24 months, tissues, with favorable therapeutic including: window • Met primary endpoint: Statistically • TTR Velocity • Leveraging same delivery approach significant and robust increase in • 10MWR Velocity for z-basivarsen in DM1 and early- dystrophin at 6 months (p<0.0001) • NSAA stage pipeline in FSHD, Pompe 1 • Continued favorable safety profile disease and other DMD exons • SV95C • PUL2.0 • Clear trends relative to placebo across multiple functional endpoints • FVC%p Advancing z-rostudirsen toward Dyne’s first approval Planned BLA submission for U.S. Accelerated Approval on track for Q2 2026 Potential U.S. launch in Q1 2027, assuming FDA grants Priority Review Phase 3 study initiation planned for Q2 2026 4 1. Safety data as of August 19, 2025; DMD, Duchenne muscular dystrophy; DM1, myotonic dystrophy type 1; FSHD, facioscapulohumeral muscular dystrophy; BLA, biologics license application; TTR, time to rise; th 10MWR, 10-meter walk/run; NSAA, north star ambulatory assessment; SV95C, stride velocity 95 centile; PUL2.0, performance upper limb v2.0; FVC%p, forced vital capacity percent predicted.

Program Opening Remarks John Cox, President & CEO Positive Topline Results from DELIVER Trial of Z-Rostudirsen Supporting Potential for U.S. Accelerated Approval Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO Available for Q&A Erick Lucera, Chief Financial Officer 5

DELIVER Clinical Update Agenda Unmet Need in DMD and Potential for Z-Rostudirsen Positive Topline Results from Registrational Expansion Cohort (REC) of DELIVER Trial to Support Planned Submission for U.S. Accelerated Approval New Positive Long-Term Results from DELIVER Trial Showed Sustained Functional Improvement Across Multiple Functional Measures TM Results Validate the Potential of FORCE Platform 6

Exon 51 Skip Amenable DMD: A More Severe Population with Significant Unmet Need, Despite Approved Therapies Progressive Clinical Exon 51 Skip Amenable DMD – Limitations with Presentation Challenging Form of Duchenne Current Therapies • Muscle weakness and gait • Greatly reduced or absent dystrophin • Limited delivery to skeletal abnormalities protein expression muscle, heart, and CNS • Low baseline dystrophin levels compared • Progressive loss of upper and • High patient and caregiver 2-8 with most other DMD mutations lower limb strength and function burden due to frequent IV dosing 2 9-11 (e.g., Q1W) • Faster functional decline • Cognitive function impairment and neuropsychiatric disorders • <1% dystrophin production with • Earlier loss of ambulation (average ~11.5 10 currently approved exon 51 years) • Respiratory/cardiac failure 2 skipping therapy (leading cause of death) 12 • ~13% of DMD patient population • Microdystrophin lacks domains 1 • Life expectancy ~30 years 13 key for optimal functionality • Unknown durability and inability to redose with gene therapy • Safety considerations 1. Broomfield et al. Neurology. 2021;97:e2304-e2314; 2. Exondys 51 Prescribing Information; 3. McMillan et al. 2025 WMS Annual Meeting, 19O; 4. Vyondys53 Prescribing Information; 5. Amondys45 Prescribing Information; 6. Viltepso Prescribing Information; 7. Veerapandiyan et al. 2025 MDA Clinical and Scientific Conference O72; 8. Aoki et al. 2025 ASGCT Annual Meeting, Abstract 1351; 9. Muntoni 7 et al. Neurology. 2023;100:e1540–e1554; 10. Bello et al. Neurology. 2016;87:401-409. 11. Zygmunt et al. Muscle Nerve. 2024 Nov;70(5):1053-1061. 12. Aartsma-Rus et al. Hum Mutat. 2009;30:293-299; 13. Chamberlain et al. Hum Gene Ther 2023;34:404–15. DMD, Duchenne muscular dystrophy, CNS, central nervous system; Q1W, every 1 week.
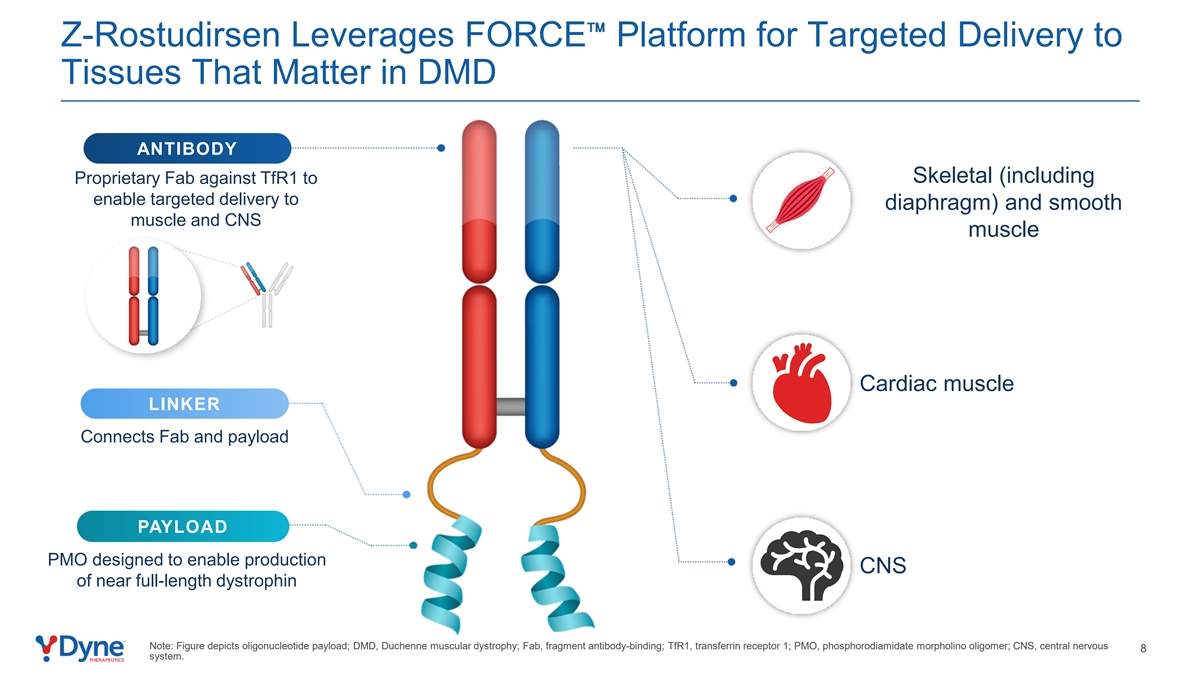
Z-Rostudirsen Leverages FORCE Platform for Targeted Delivery to Tissues That Matter in DMD ANTIBODY Skeletal (including Proprietary Fab against TfR1 to enable targeted delivery to diaphragm) and smooth muscle and CNS muscle Cardiac muscle LINKER Connects Fab and payload PAYLOAD PMO designed to enable production CNS of near full-length dystrophin Note: Figure depicts oligonucleotide payload; DMD, Duchenne muscular dystrophy; Fab, fragment antibody-binding; TfR1, transferrin receptor 1; PMO, phosphorodiamidate morpholino oligomer; CNS, central nervous 8 system.

DELIVER Clinical Update Agenda Unmet Need in DMD and Potential for Z-Rostudirsen Positive Topline Results from Registrational Expansion Cohort (REC) of DELIVER Trial to Support Planned Submission for U.S. Accelerated Approval New Positive Long-Term Results from DELIVER Trial Showed Sustained Functional Improvement Across Multiple Functional Measures TM Results Validate the Potential of FORCE Platform 9
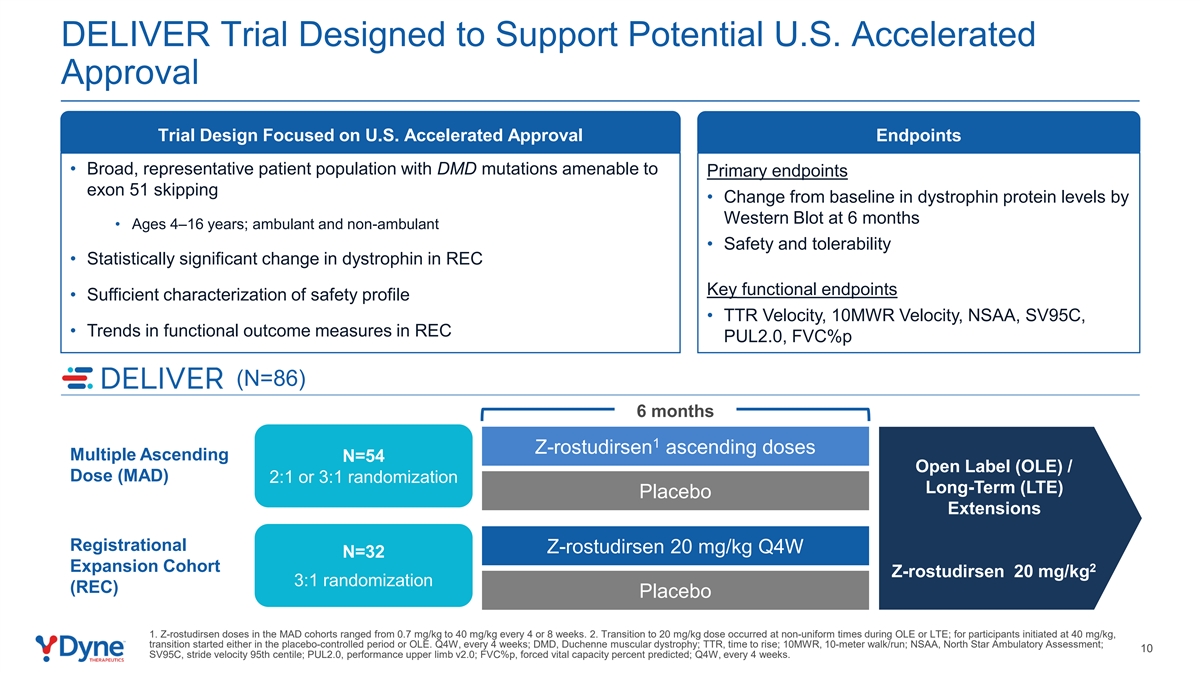
DELIVER Trial Designed to Support Potential U.S. Accelerated Approval Trial Design Focused on U.S. Accelerated Approval Endpoints • Broad, representative patient population with DMD mutations amenable to Primary endpoints exon 51 skipping • Change from baseline in dystrophin protein levels by Western Blot at 6 months • Ages 4–16 years; ambulant and non-ambulant • Safety and tolerability • Statistically significant change in dystrophin in REC Key functional endpoints • Sufficient characterization of safety profile • TTR Velocity, 10MWR Velocity, NSAA, SV95C, • Trends in functional outcome measures in REC PUL2.0, FVC%p (N=86) 6 months 1 Z-rostudirsen ascending doses Multiple Ascending N=54 Open Label (OLE) / Dose (MAD) 2:1 or 3:1 randomization Long-Term (LTE) Placebo Extensions Registrational Z-rostudirsen 20 mg/kg Q4W 6 months N=32 Expansion Cohort 2 Z-rostudirsen 20 mg/kg 3:1 randomization (REC) Placebo 1. Z-rostudirsen doses in the MAD cohorts ranged from 0.7 mg/kg to 40 mg/kg every 4 or 8 weeks. 2. Transition to 20 mg/kg dose occurred at non-uniform times during OLE or LTE; for participants initiated at 40 mg/kg, transition started either in the placebo-controlled period or OLE. Q4W, every 4 weeks; DMD, Duchenne muscular dystrophy; TTR, time to rise; 10MWR, 10-meter walk/run; NSAA, North Star Ambulatory Assessment; 10 SV95C, stride velocity 95th centile; PUL2.0, performance upper limb v2.0; FVC%p, forced vital capacity percent predicted; Q4W, every 4 weeks.
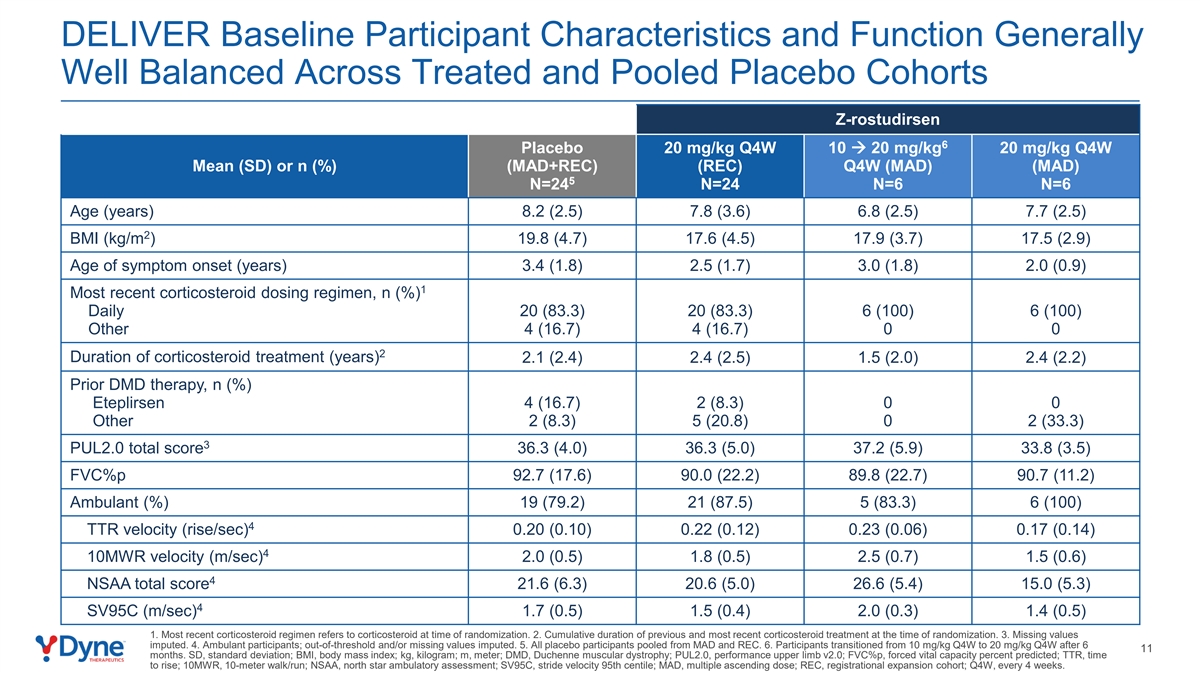
DELIVER Baseline Participant Characteristics and Function Generally Well Balanced Across Treated and Pooled Placebo Cohorts Z-rostudirsen 6 Placebo 20 mg/kg Q4W 10 à 20 mg/kg 20 mg/kg Q4W Mean (SD) or n (%) (MAD+REC) (REC) Q4W (MAD) (MAD) 5 N=24 N=24 N=6 N=6 Age (years) 8.2 (2.5) 7.8 (3.6) 6.8 (2.5) 7.7 (2.5) 2 BMI (kg/m ) 19.8 (4.7) 17.6 (4.5) 17.9 (3.7) 17.5 (2.9) Age of symptom onset (years) 3.4 (1.8) 2.5 (1.7) 3.0 (1.8) 2.0 (0.9) 1 Most recent corticosteroid dosing regimen, n (%) Daily 20 (83.3) 20 (83.3) 6 (100) 6 (100) Other 4 (16.7) 4 (16.7) 0 0 2 Duration of corticosteroid treatment (years) 2.1 (2.4) 2.4 (2.5) 1.5 (2.0) 2.4 (2.2) Prior DMD therapy, n (%) Eteplirsen 4 (16.7) 2 (8.3) 0 0 Other 2 (8.3) 5 (20.8) 0 2 (33.3) 3 PUL2.0 total score 36.3 (4.0) 36.3 (5.0) 37.2 (5.9) 33.8 (3.5) FVC%p 92.7 (17.6) 90.0 (22.2) 89.8 (22.7) 90.7 (11.2) Ambulant (%) 19 (79.2) 21 (87.5) 5 (83.3) 6 (100) 4 TTR velocity (rise/sec) 0.20 (0.10) 0.22 (0.12) 0.23 (0.06) 0.17 (0.14) 4 10MWR velocity (m/sec) 2.0 (0.5) 1.8 (0.5) 2.5 (0.7) 1.5 (0.6) 4 NSAA total score 21.6 (6.3) 20.6 (5.0) 26.6 (5.4) 15.0 (5.3) 4 SV95C (m/sec) 1.7 (0.5) 1.5 (0.4) 2.0 (0.3) 1.4 (0.5) 1. Most recent corticosteroid regimen refers to corticosteroid at time of randomization. 2. Cumulative duration of previous and most recent corticosteroid treatment at the time of randomization. 3. Missing values imputed. 4. Ambulant participants; out-of-threshold and/or missing values imputed. 5. All placebo participants pooled from MAD and REC. 6. Participants transitioned from 10 mg/kg Q4W to 20 mg/kg Q4W after 6 11 months. SD, standard deviation; BMI, body mass index; kg, kilogram; m, meter; DMD, Duchenne muscular dystrophy; PUL2.0, performance upper limb v2.0; FVC%p, forced vital capacity percent predicted; TTR, time to rise; 10MWR, 10-meter walk/run; NSAA, north star ambulatory assessment; SV95C, stride velocity 95th centile; MAD, multiple ascending dose; REC, registrational expansion cohort; Q4W, every 4 weeks.
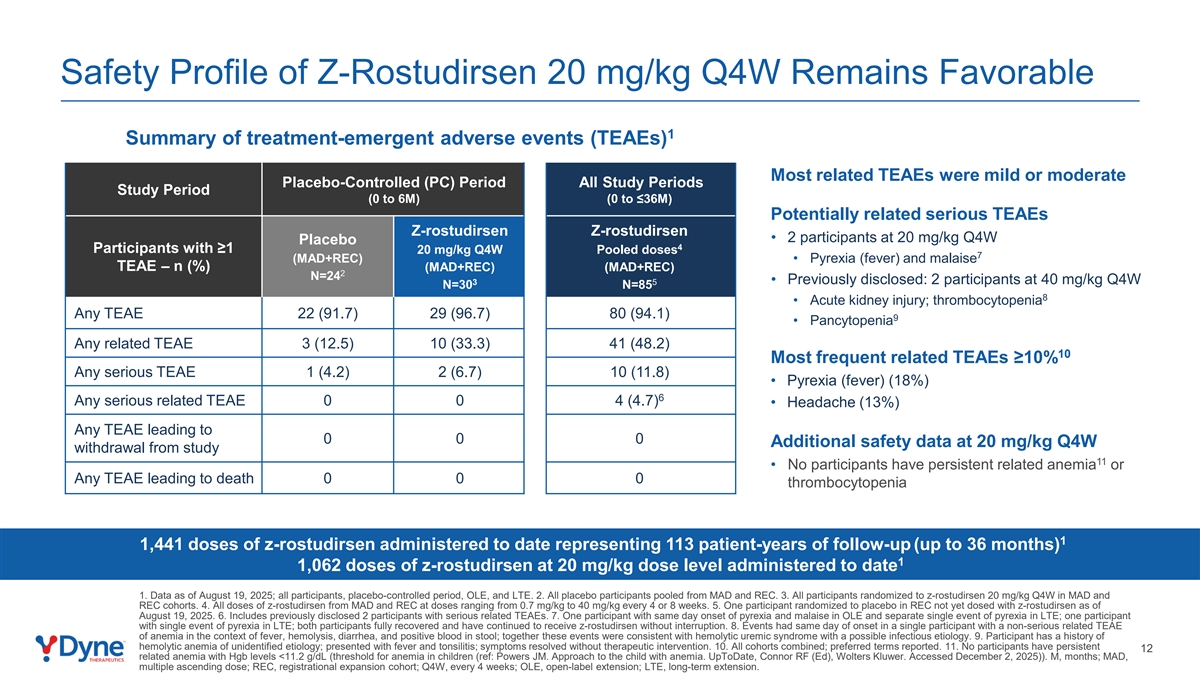
Safety Profile of Z-Rostudirsen 20 mg/kg Q4W Remains Favorable 1 Summary of treatment-emergent adverse events (TEAEs) Most related TEAEs were mild or moderate Placebo-Controlled (PC) Period All Study Periods Study Period (0 to 6M) (0 to ≤36M) Potentially related serious TEAEs Z-rostudirsen Z-rostudirsen • 2 participants at 20 mg/kg Q4W Placebo 4 Participants with ≥1 20 mg/kg Q4W Pooled doses 7 (MAD+REC) • Pyrexia (fever) and malaise TEAE – n (%) (MAD+REC) (MAD+REC) 2 N=24 3 5 • Previously disclosed: 2 participants at 40 mg/kg Q4W N=30 N=85 8 • Acute kidney injury; thrombocytopenia Any TEAE 22 (91.7) 29 (96.7) 80 (94.1) 9 • Pancytopenia Any related TEAE 3 (12.5) 10 (33.3) 41 (48.2) 10 Most frequent related TEAEs ≥10% Any serious TEAE 1 (4.2) 2 (6.7) 10 (11.8) • Pyrexia (fever) (18%) 6 Any serious related TEAE 0 0 4 (4.7) • Headache (13%) Any TEAE leading to 0 0 0 Additional safety data at 20 mg/kg Q4W withdrawal from study 11 • No participants have persistent related anemia or Any TEAE leading to death 0 0 0 thrombocytopenia 1 1,441 doses of z-rostudirsen administered to date representing 113 patient-years of follow-up (up to 36 months) 1 1,062 doses of z-rostudirsen at 20 mg/kg dose level administered to date 1. Data as of August 19, 2025; all participants, placebo-controlled period, OLE, and LTE. 2. All placebo participants pooled from MAD and REC. 3. All participants randomized to z-rostudirsen 20 mg/kg Q4W in MAD and REC cohorts. 4. All doses of z-rostudirsen from MAD and REC at doses ranging from 0.7 mg/kg to 40 mg/kg every 4 or 8 weeks. 5. One participant randomized to placebo in REC not yet dosed with z-rostudirsen as of August 19, 2025. 6. Includes previously disclosed 2 participants with serious related TEAEs. 7. One participant with same day onset of pyrexia and malaise in OLE and separate single event of pyrexia in LTE; one participant with single event of pyrexia in LTE; both participants fully recovered and have continued to receive z-rostudirsen without interruption. 8. Events had same day of onset in a single participant with a non-serious related TEAE of anemia in the context of fever, hemolysis, diarrhea, and positive blood in stool; together these events were consistent with hemolytic uremic syndrome with a possible infectious etiology. 9. Participant has a history of hemolytic anemia of unidentified etiology; presented with fever and tonsilitis; symptoms resolved without therapeutic intervention. 10. All cohorts combined; preferred terms reported. 11. No participants have persistent 12 related anemia with Hgb levels <11.2 g/dL (threshold for anemia in children (ref: Powers JM. Approach to the child with anemia. UpToDate, Connor RF (Ed), Wolters Kluwer. Accessed December 2, 2025)). M, months; MAD, multiple ascending dose; REC, registrational expansion cohort; Q4W, every 4 weeks; OLE, open-label extension; LTE, long-term extension.
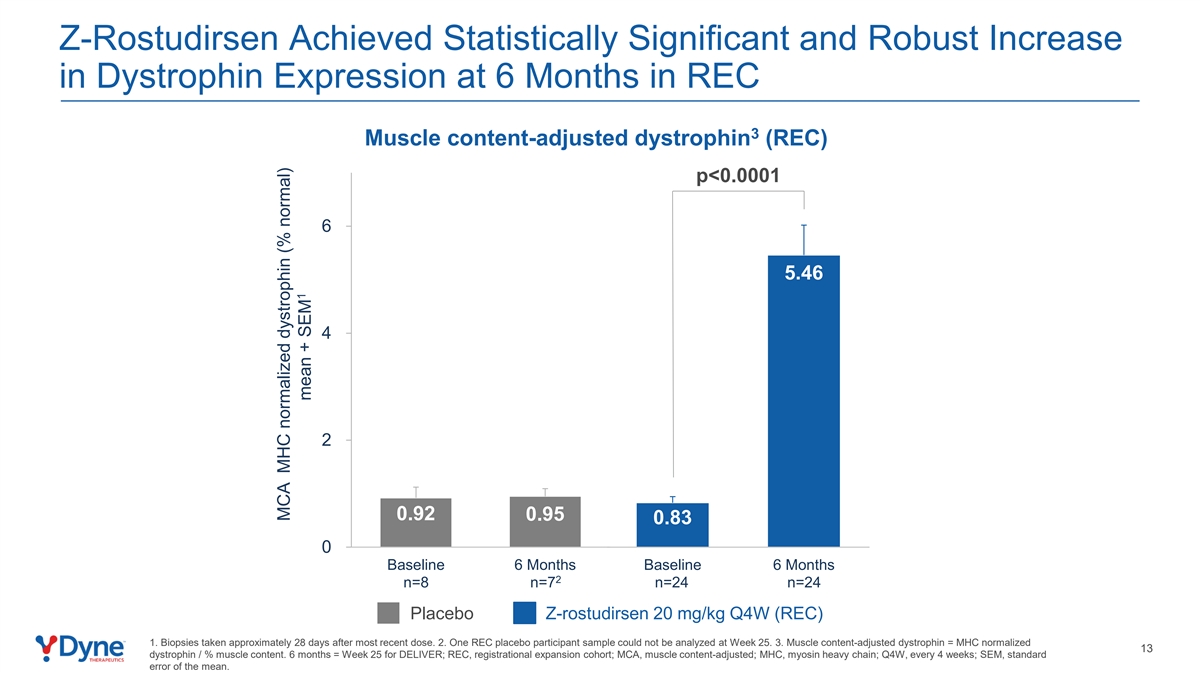
Z-Rostudirsen Achieved Statistically Significant and Robust Increase in Dystrophin Expression at 6 Months in REC 3 Muscle content-adjusted dystrophin (REC) p<0.0001 6 5.46 4 2 0.92 0.95 0.83 0 Baseline 6 Months Baseline 6 Months 2 n=8 n=7 n=24 n=24 Placebo Z-rostudirsen 20 mg/kg Q4W (REC) 1. Biopsies taken approximately 28 days after most recent dose. 2. One REC placebo participant sample could not be analyzed at Week 25. 3. Muscle content-adjusted dystrophin = MHC normalized 13 dystrophin / % muscle content. 6 months = Week 25 for DELIVER; REC, registrational expansion cohort; MCA, muscle content-adjusted; MHC, myosin heavy chain; Q4W, every 4 weeks; SEM, standard error of the mean. MCA MHC normalized dystrophin (% normal) 1 mean + SEM
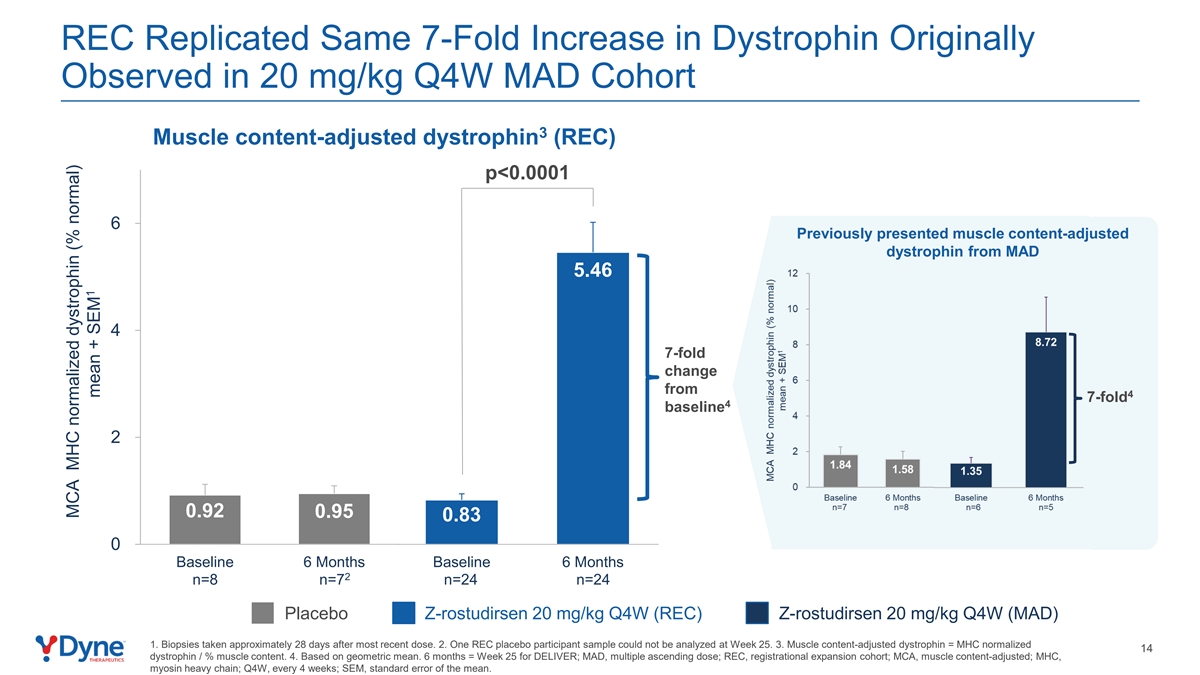
REC Replicated Same 7-Fold Increase in Dystrophin Originally Observed in 20 mg/kg Q4W MAD Cohort 3 Muscle content-adjusted dystrophin (REC) p<0.0001 6 Previously presented muscle content-adjusted dystrophin from MAD 5.46 4 7-fold change from 4 7-fold 4 baseline 2 0.92 0.95 0.83 0 Baseline 6 Months Baseline 6 Months 2 n=8 n=7 n=24 n=24 Placebo Z-rostudirsen 20 mg/kg Q4W (REC) Z-rostudirsen 20 mg/kg Q4W (MAD) 1. Biopsies taken approximately 28 days after most recent dose. 2. One REC placebo participant sample could not be analyzed at Week 25. 3. Muscle content-adjusted dystrophin = MHC normalized 14 dystrophin / % muscle content. 4. Based on geometric mean. 6 months = Week 25 for DELIVER; MAD, multiple ascending dose; REC, registrational expansion cohort; MCA, muscle content-adjusted; MHC, myosin heavy chain; Q4W, every 4 weeks; SEM, standard error of the mean. MCA MHC normalized dystrophin (% normal) 1 mean + SEM
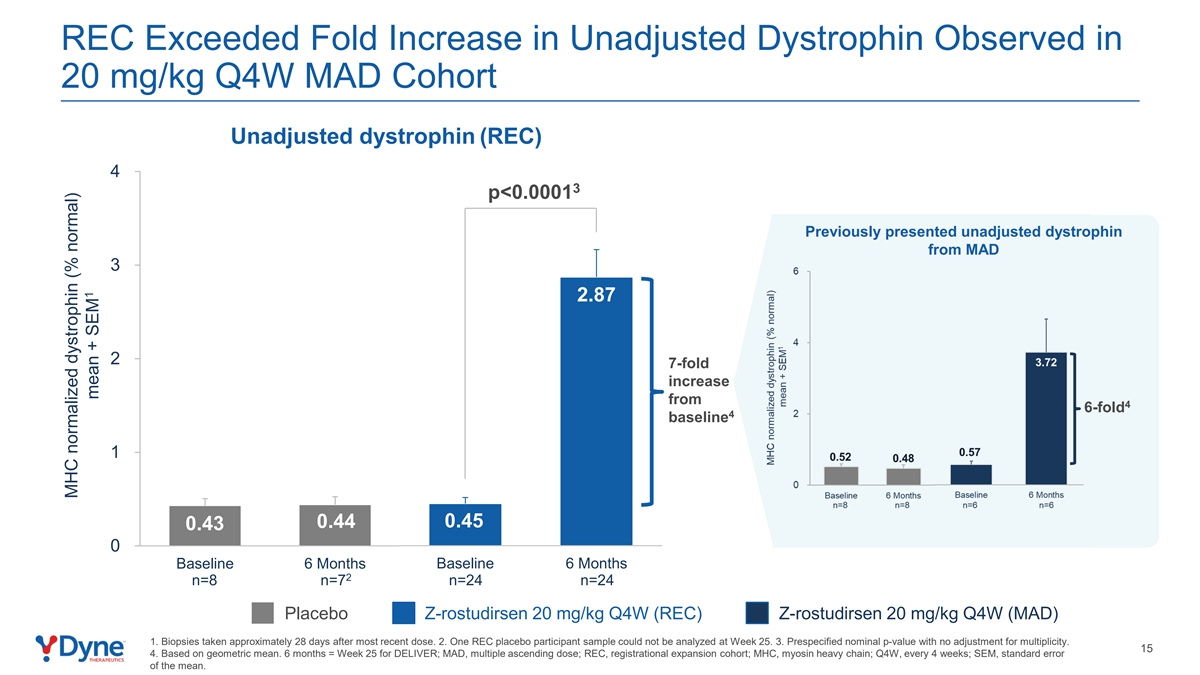
REC Exceeded Fold Increase in Unadjusted Dystrophin Observed in 20 mg/kg Q4W MAD Cohort Unadjusted dystrophin (REC) 4 3 p<0.0001 Previously presented unadjusted dystrophin from MAD 3 2.87 2 7-fold increase from 4 6-fold 4 baseline 1 0.45 0.44 0.43 0 Baseline 6 Months Baseline 6 Months 2 n=8 n=7 n=24 n=24 Placebo Z-rostudirsen 20 mg/kg Q4W (REC) Z-rostudirsen 20 mg/kg Q4W (MAD) 1. Biopsies taken approximately 28 days after most recent dose. 2. One REC placebo participant sample could not be analyzed at Week 25. 3. Prespecified nominal p-value with no adjustment for multiplicity. 15 4. Based on geometric mean. 6 months = Week 25 for DELIVER; MAD, multiple ascending dose; REC, registrational expansion cohort; MHC, myosin heavy chain; Q4W, every 4 weeks; SEM, standard error of the mean. MHC normalized dystrophin (% normal) 1 mean + SEM
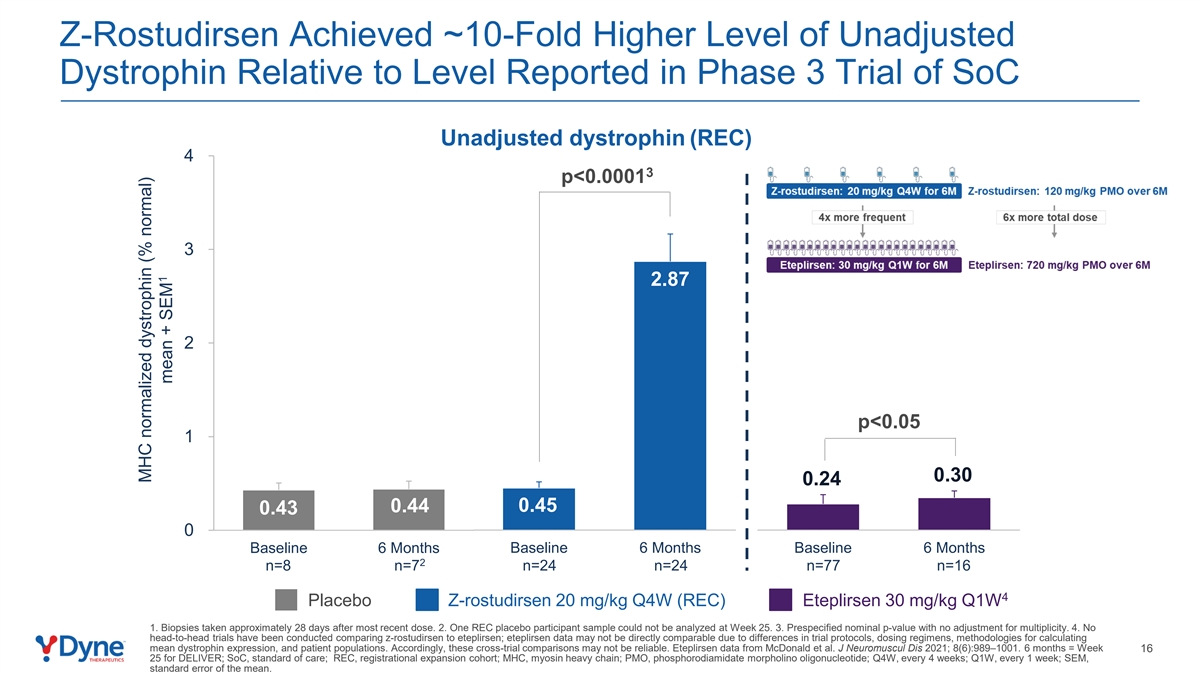
Z-Rostudirsen Achieved ~10-Fold Higher Level of Unadjusted Dystrophin Relative to Level Reported in Phase 3 Trial of SoC Unadjusted dystrophin (REC) 4 3 p<0.0001 3 2.87 2 p<0.05 1 0.30 0.24 0.44 0.45 0.43 0 Baseline 6 Months Baseline 6 Months Baseline 6 Months 2 n=8 n=7 n=24 n=24 n=77 n=16 4 Placebo Z-rostudirsen 20 mg/kg Q4W (REC) Eteplirsen 30 mg/kg Q1W 1. Biopsies taken approximately 28 days after most recent dose. 2. One REC placebo participant sample could not be analyzed at Week 25. 3. Prespecified nominal p-value with no adjustment for multiplicity. 4. No head-to-head trials have been conducted comparing z-rostudirsen to eteplirsen; eteplirsen data may not be directly comparable due to differences in trial protocols, dosing regimens, methodologies for calculating mean dystrophin expression, and patient populations. Accordingly, these cross-trial comparisons may not be reliable. Eteplirsen data from McDonald et al. J Neuromuscul Dis 2021; 8(6):989–1001. 6 months = Week 16 25 for DELIVER; SoC, standard of care; REC, registrational expansion cohort; MHC, myosin heavy chain; PMO, phosphorodiamidate morpholino oligonucleotide; Q4W, every 4 weeks; Q1W, every 1 week; SEM, standard error of the mean. MHC normalized dystrophin (% normal) 1 mean + SEM
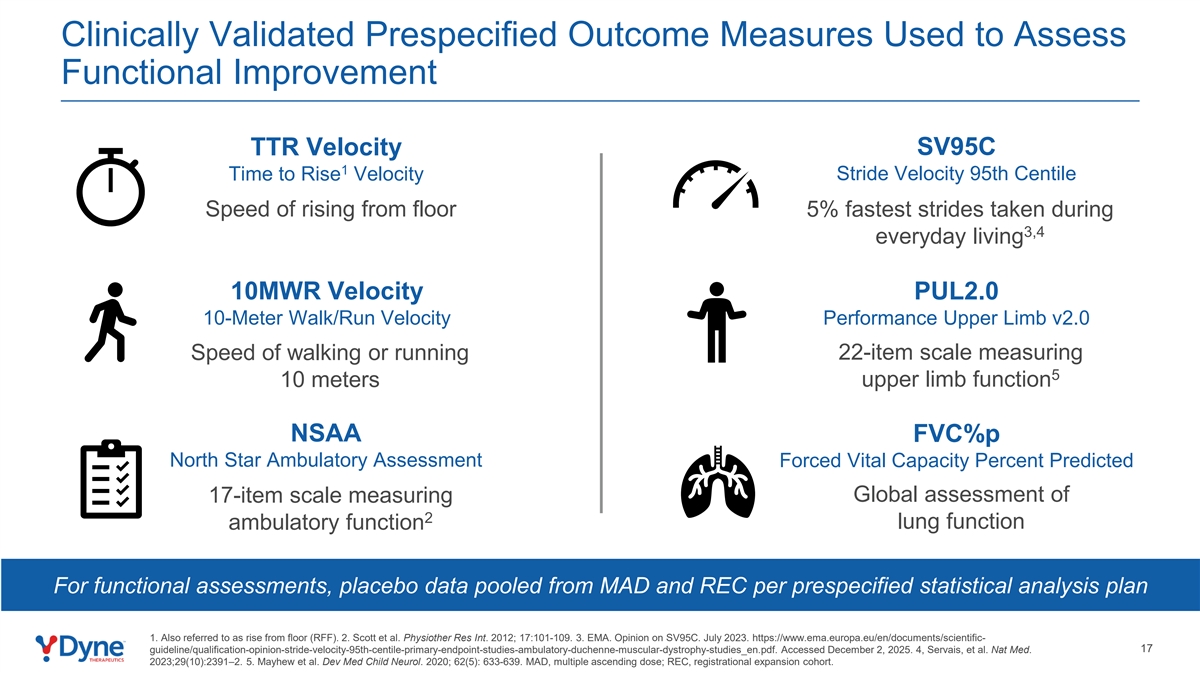
Clinically Validated Prespecified Outcome Measures Used to Assess Functional Improvement TTR Velocity SV95C 1 Stride Velocity 95th Centile Time to Rise Velocity Speed of rising from floor 5% fastest strides taken during 3,4 everyday living 10MWR Velocity PUL2.0 10-Meter Walk/Run Velocity Performance Upper Limb v2.0 Speed of walking or running 22-item scale measuring 5 10 meters upper limb function NSAA FVC%p North Star Ambulatory Assessment Forced Vital Capacity Percent Predicted 17-item scale measuring Global assessment of 2 lung function ambulatory function For functional assessments, placebo data pooled from MAD and REC per prespecified statistical analysis plan 1. Also referred to as rise from floor (RFF). 2. Scott et al. Physiother Res Int. 2012; 17:101-109. 3. EMA. Opinion on SV95C. July 2023. https://www.ema.europa.eu/en/documents/scientific- 17 guideline/qualification-opinion-stride-velocity-95th-centile-primary-endpoint-studies-ambulatory-duchenne-muscular-dystrophy-studies_en.pdf. Accessed December 2, 2025. 4, Servais, et al. Nat Med. 2023;29(10):2391–2. 5. Mayhew et al. Dev Med Child Neurol. 2020; 62(5): 633-639. MAD, multiple ascending dose; REC, registrational expansion cohort.
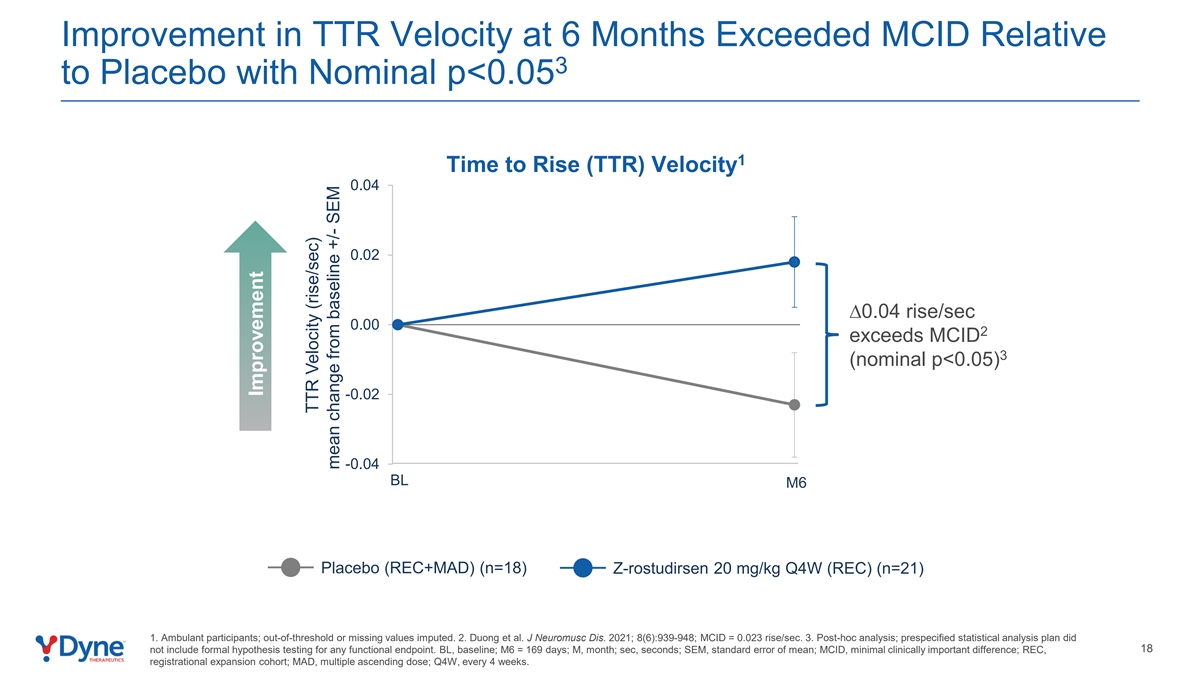
Improvement in TTR Velocity at 6 Months Exceeded MCID Relative 3 to Placebo with Nominal p<0.05 1 Time to Rise (TTR) Velocity 0.04 0.02 ∆0.04 rise/sec 0.00 2 exceeds MCID 3 (nominal p<0.05) -0.02 -0.04 BL M6 Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) 1. Ambulant participants; out-of-threshold or missing values imputed. 2. Duong et al. J Neuromusc Dis. 2021; 8(6):939-948; MCID = 0.023 rise/sec. 3. Post-hoc analysis; prespecified statistical analysis plan did 18 not include formal hypothesis testing for any functional endpoint. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; MCID, minimal clinically important difference; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Improvement TTR Velocity (rise/sec) mean change from baseline +/- SEM
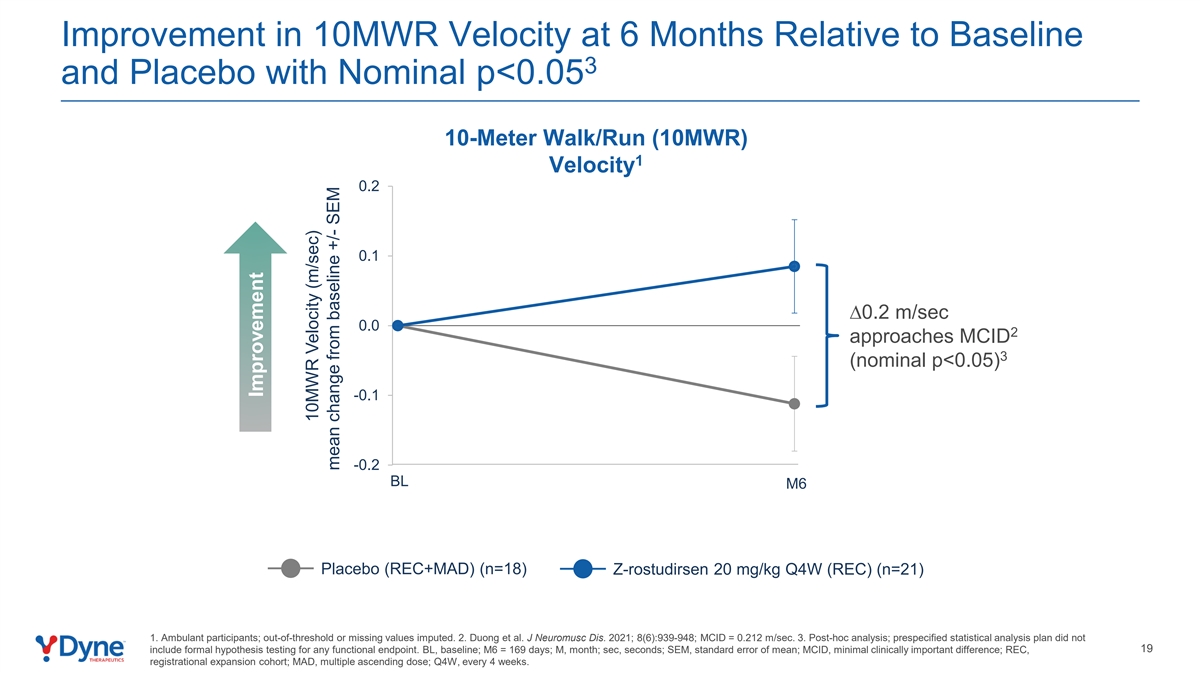
Improvement in 10MWR Velocity at 6 Months Relative to Baseline 3 and Placebo with Nominal p<0.05 10-Meter Walk/Run (10MWR) 1 Velocity 0.2 0.1 ∆0.2 m/sec 0.0 2 approaches MCID 3 (nominal p<0.05) -0.1 -0.2 BL M6 Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) 1. Ambulant participants; out-of-threshold or missing values imputed. 2. Duong et al. J Neuromusc Dis. 2021; 8(6):939-948; MCID = 0.212 m/sec. 3. Post-hoc analysis; prespecified statistical analysis plan did not 19 include formal hypothesis testing for any functional endpoint. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; MCID, minimal clinically important difference; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Improvement 10MWR Velocity (m/sec) mean change from baseline +/- SEM
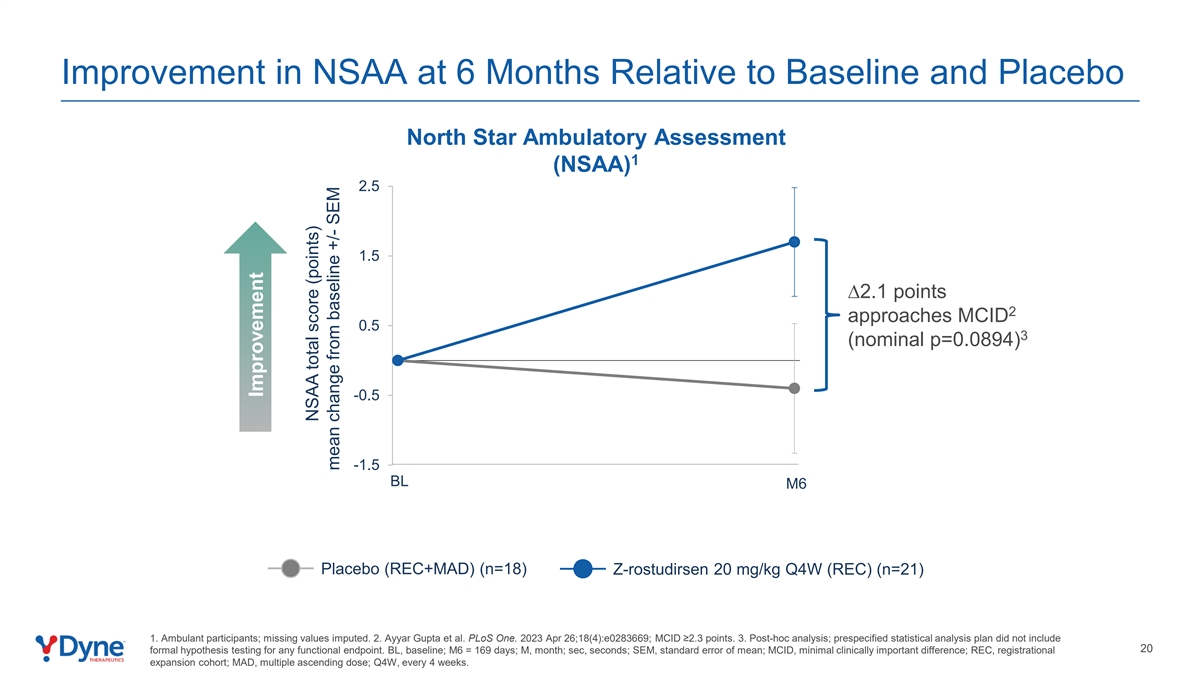
Improvement in NSAA at 6 Months Relative to Baseline and Placebo North Star Ambulatory Assessment 1 (NSAA) 2.5 1.5 ∆2.1 points 2 approaches MCID 0.5 3 (nominal p=0.0894) -0.5 -1.5 BL M6 Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) 1. Ambulant participants; missing values imputed. 2. Ayyar Gupta et al. PLoS One. 2023 Apr 26;18(4):e0283669; MCID ≥2.3 points. 3. Post-hoc analysis; prespecified statistical analysis plan did not include 20 formal hypothesis testing for any functional endpoint. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; MCID, minimal clinically important difference; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Improvement NSAA total score (points) mean change from baseline +/- SEM
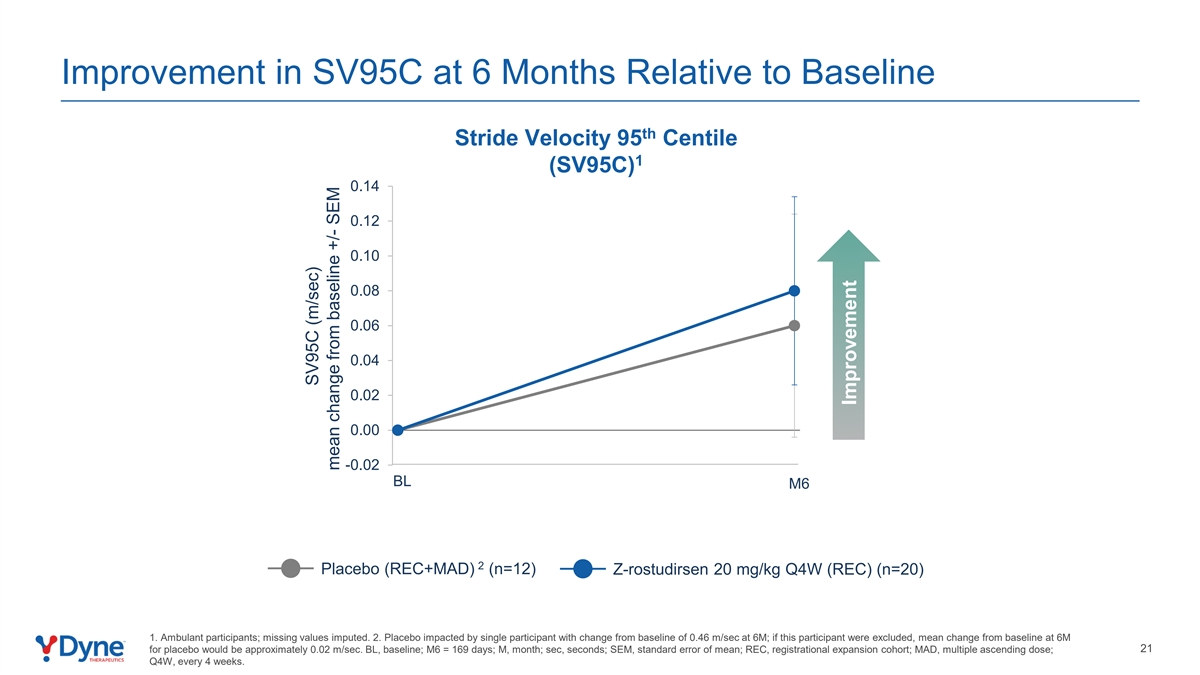
Improvement in SV95C at 6 Months Relative to Baseline th Stride Velocity 95 Centile 1 (SV95C) 0.14 0.12 0.10 0.08 0.06 0.04 0.02 0.00 -0.02 BL M6 2 Placebo (REC+MAD) (n=12) Z-rostudirsen 20 mg/kg Q4W (REC) (n=20) 1. Ambulant participants; missing values imputed. 2. Placebo impacted by single participant with change from baseline of 0.46 m/sec at 6M; if this participant were excluded, mean change from baseline at 6M 21 for placebo would be approximately 0.02 m/sec. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. SV95C (m/sec) mean change from baseline +/- SEM Improvement
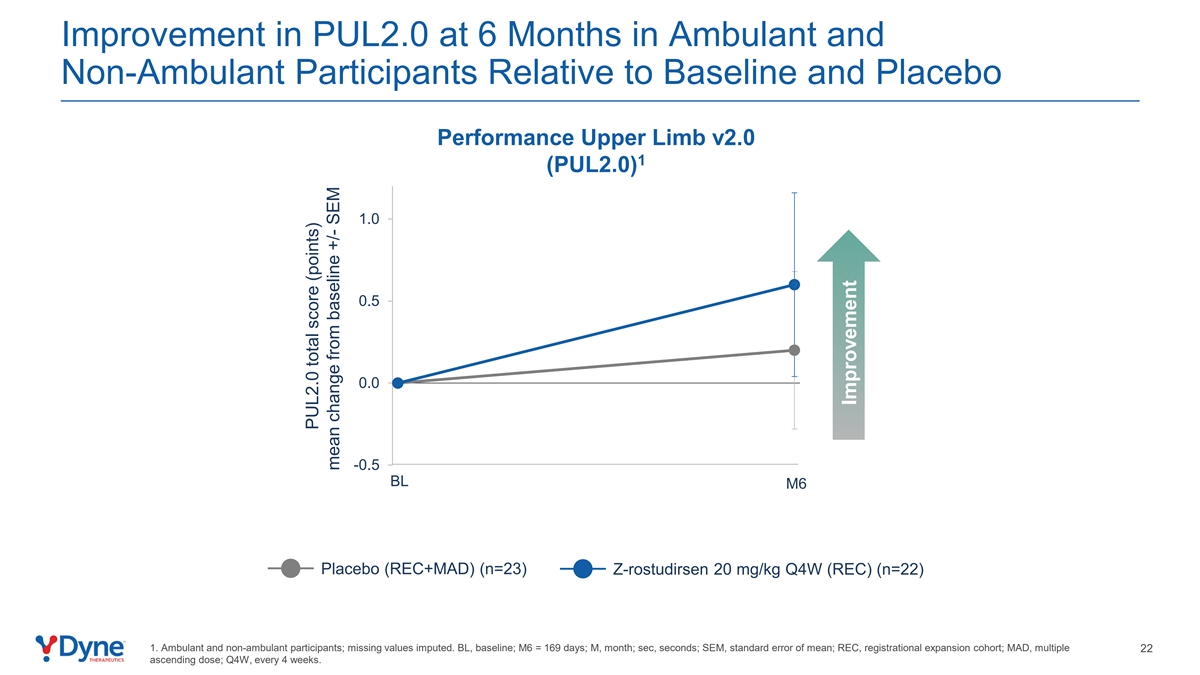
Improvement in PUL2.0 at 6 Months in Ambulant and Non-Ambulant Participants Relative to Baseline and Placebo Performance Upper Limb v2.0 1 (PUL2.0) 1.0 0.5 0.0 -0.5 BL M6 Placebo (REC+MAD) (n=23) Z-rostudirsen 20 mg/kg Q4W (REC) (n=22) 1. Ambulant and non-ambulant participants; missing values imputed. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; REC, registrational expansion cohort; MAD, multiple 22 ascending dose; Q4W, every 4 weeks. PUL2.0 total score (points) mean change from baseline +/- SEM Improvement
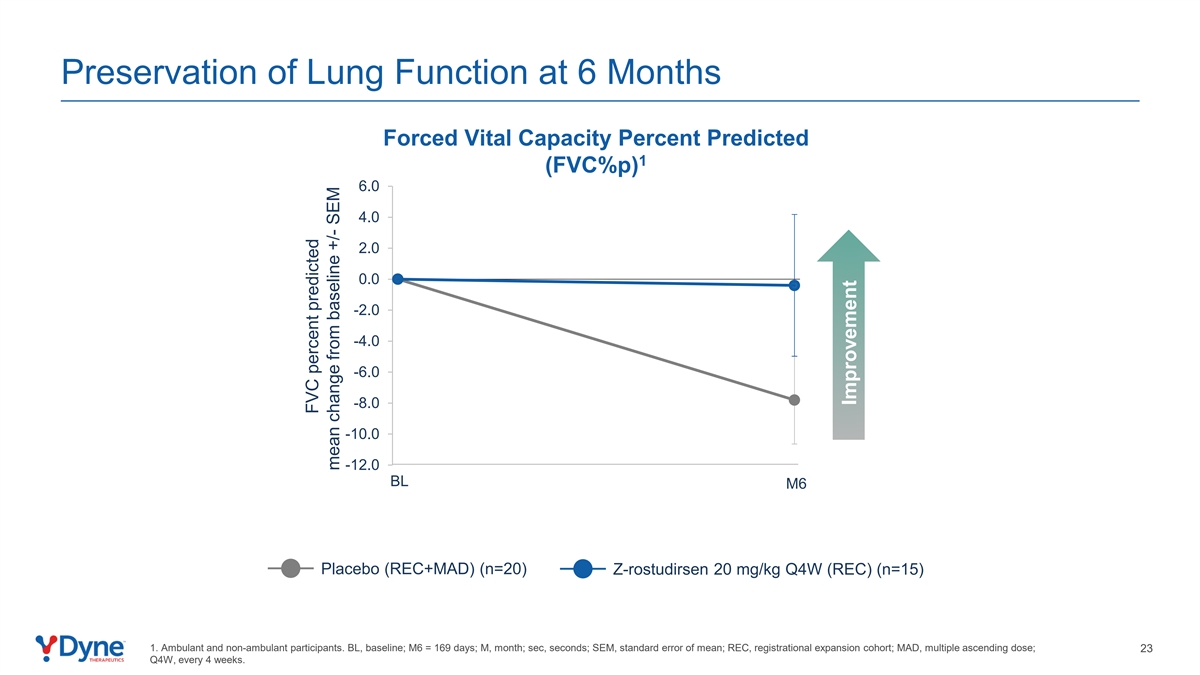
Preservation of Lung Function at 6 Months Forced Vital Capacity Percent Predicted 1 (FVC%p) 6.0 4.0 2.0 0.0 -2.0 -4.0 -6.0 -8.0 -10.0 -12.0 BL M6 Placebo (REC+MAD) (n=20) Z-rostudirsen 20 mg/kg Q4W (REC) (n=15) 1. Ambulant and non-ambulant participants. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; REC, registrational expansion cohort; MAD, multiple ascending dose; 23 Q4W, every 4 weeks. FVC percent predicted mean change from baseline +/- SEM Improvement
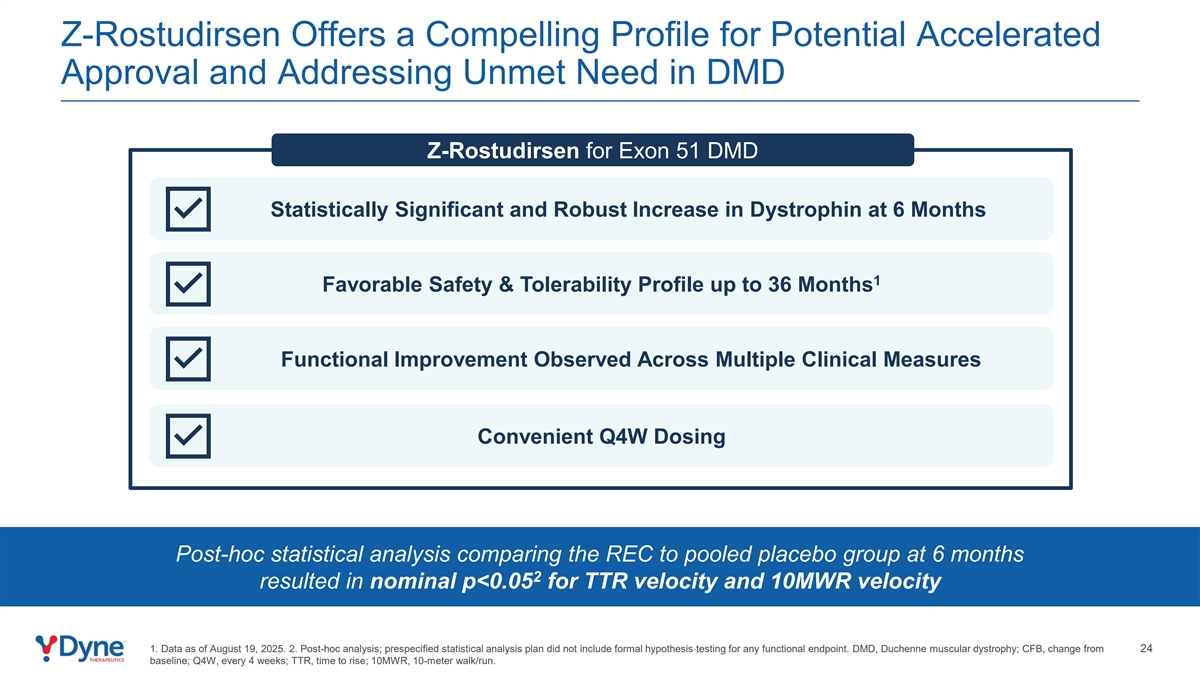
Z-Rostudirsen Offers a Compelling Profile for Potential Accelerated Approval and Addressing Unmet Need in DMD Z-Rostudirsen for Exon 51 DMD Statistically Significant and Robust Increase in Dystrophin at 6 Months 1 Favorable Safety & Tolerability Profile up to 36 Months Functional Improvement Observed Across Multiple Clinical Measures Convenient Q4W Dosing Post-hoc statistical analysis comparing the REC to pooled placebo group at 6 months 2 resulted in nominal p<0.05 for TTR velocity and 10MWR velocity 1. Data as of August 19, 2025. 2. Post-hoc analysis; prespecified statistical analysis plan did not include formal hypothesis testing for any functional endpoint. DMD, Duchenne muscular dystrophy; CFB, change from 24 baseline; Q4W, every 4 weeks; TTR, time to rise; 10MWR, 10-meter walk/run.

DELIVER Clinical Update Agenda Unmet Need in DMD and Potential for Z-Rostudirsen Positive Topline Results from Registrational Expansion Cohort (REC) of DELIVER Trial to Support Planned Submission for U.S. Accelerated Approval New Positive Long-Term Results from DELIVER Trial Showed Sustained Functional Improvement Across Multiple Functional Measures TM Results Validate the Potential of FORCE Platform 25

Sustained Functional Improvement Compared to Baseline Across All 6 Measures up to 24 Months in a Broad Participant Population 1 1 1 18-Month CFB 18-Month CFB 24-Month CFB 2 2 20 mg/kg Q4W Pooled 10 à 20 mg/kg Q4W 10 à 20 mg/kg Q4W MAD Cohort and 20 mg/kg Q4W MAD Cohorts MAD Cohort Improvement Improvement Improvement 1. Mean change from baseline +/- SEM; TTR velocity, 10MWR velocity, NSAA, and SV95C analyzed from ambulant participants; PUL2.0 and FVC%p analyzed from ambulant and non-ambulant participants; Out-of- threshold and/or missing values imputed except for FVC%p. 2. Participants transitioned from 10 mg/kg Q4W to 20 mg/kg Q4W after 6M; all participants treated with 20 mg/kg Q4W for at least 12M in the 24M th assessment. Q4W, every 4 weeks; CFB, change from baseline; MAD, multiple ascending dose; TTR, time to rise; 10MWR, 10-meter walk/run; NSAA, north star ambulatory assessment; SV95C, stride velocity 95 26 centile; PUL2.0, performance upper limb v2.0; FVC%p, forced vital capacity percent predicted.

DELIVER Clinical Update Agenda Unmet Need in DMD and Potential for Z-Rostudirsen Positive Topline Results from Registrational Expansion Cohort (REC) of DELIVER Trial to Support Planned Submission for U.S. Accelerated Approval New Positive Long-Term Results from DELIVER Trial Showed Sustained Functional Improvement Across Multiple Functional Measures TM Results Validate the Potential of FORCE Platform 27

Z-Rostudirsen Data Validate Potential of the FORCE Platform FORCE Design Principles of the FORCE Platform Validation with Z-Rostudirsen DELIVER Data TfR1-mediated delivery to muscle, heart and CNS with Statistically significant and robust increase in rationally selected payload to match disease biology dystrophin TfR1-binding Fab to enable robust and widespread Early and sustained functional improvement across tissue distribution multiple clinical endpoints 1 Designed not to interfere with TfR1 function in iron Favorable safety and tolerability with no persistent 2 homeostasis related anemia or thrombocytopenia at 20 mg/kg Achievement of target profile with infrequent dosing Convenient Q4W dosing 28 1. Data as of August 19, 2025. 2. No participants have demonstrated persistent related anemia with Hgb levels <11.2 g/dL (threshold for anemia in children (ref: Powers JM. Approach to the child with anemia. UpToDate, Connor RF (Ed), Wolters Kluwer. Accessed December 2, 2025)). TfR1, transferrin receptor 1; CNS, central nervous system; Fab, fragment antibody-binding; Q4W, every 4 weeks.
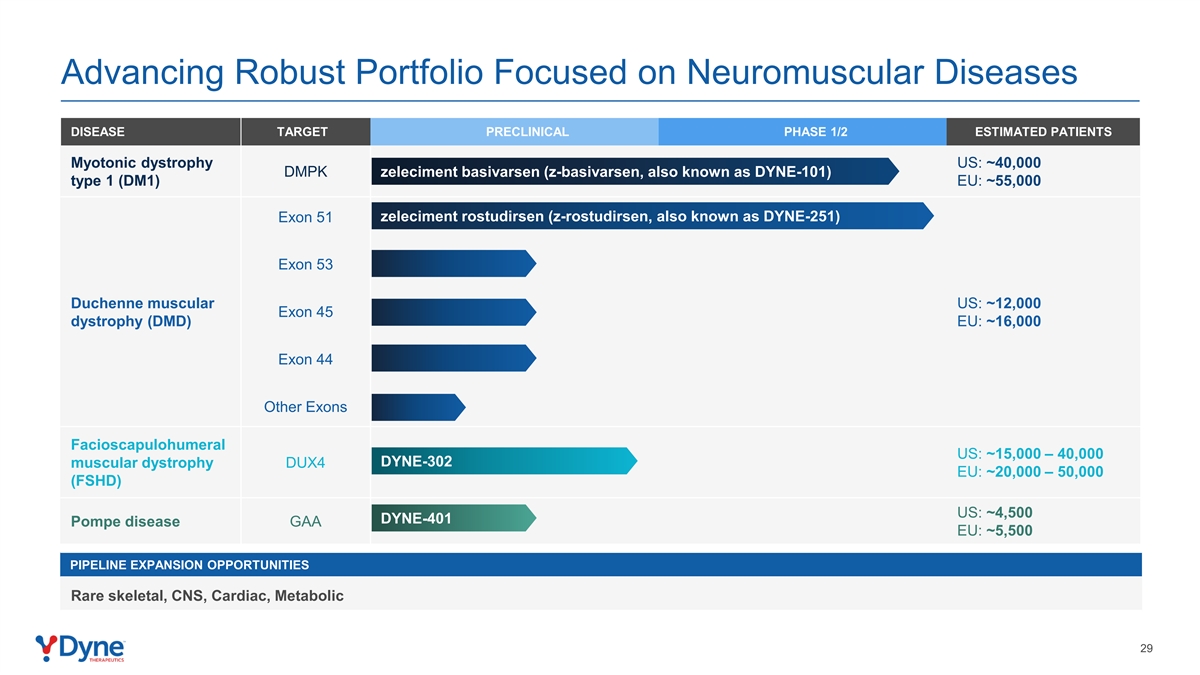
Advancing Robust Portfolio Focused on Neuromuscular Diseases DISEASE TARGET PRECLINICAL PHASE 1/2 ESTIMATED PATIENTS Myotonic dystrophy US: ~40,000 DMPK zeleciment basivarsen (z-basivarsen, also known as DYNE-101) type 1 (DM1) EU: ~55,000 zeleciment rostudirsen (z-rostudirsen, also known as DYNE-251) Exon 51 Exon 53 Duchenne muscular US: ~12,000 Exon 45 dystrophy (DMD) EU: ~16,000 Exon 44 Other Exons Facioscapulohumeral US: ~15,000 – 40,000 DYNE-302 muscular dystrophy DUX4 EU: ~20,000 – 50,000 (FSHD) US: ~4,500 DYNE-401 Pompe disease GAA EU: ~5,500 PIPELINE EXPANSION OPPORTUNITIES Rare skeletal, CNS, Cardiac, Metabolic 29

Program Opening Remarks John Cox, President & CEO Positive Topline Results from DELIVER Trial of Z-Rostudirsen Supporting Potential for U.S. Accelerated Approval Doug Kerr, M.D., Ph.D., Chief Medical Officer Closing Remarks John Cox, President & CEO Available for Q&A Erick Lucera, Chief Financial Officer 30
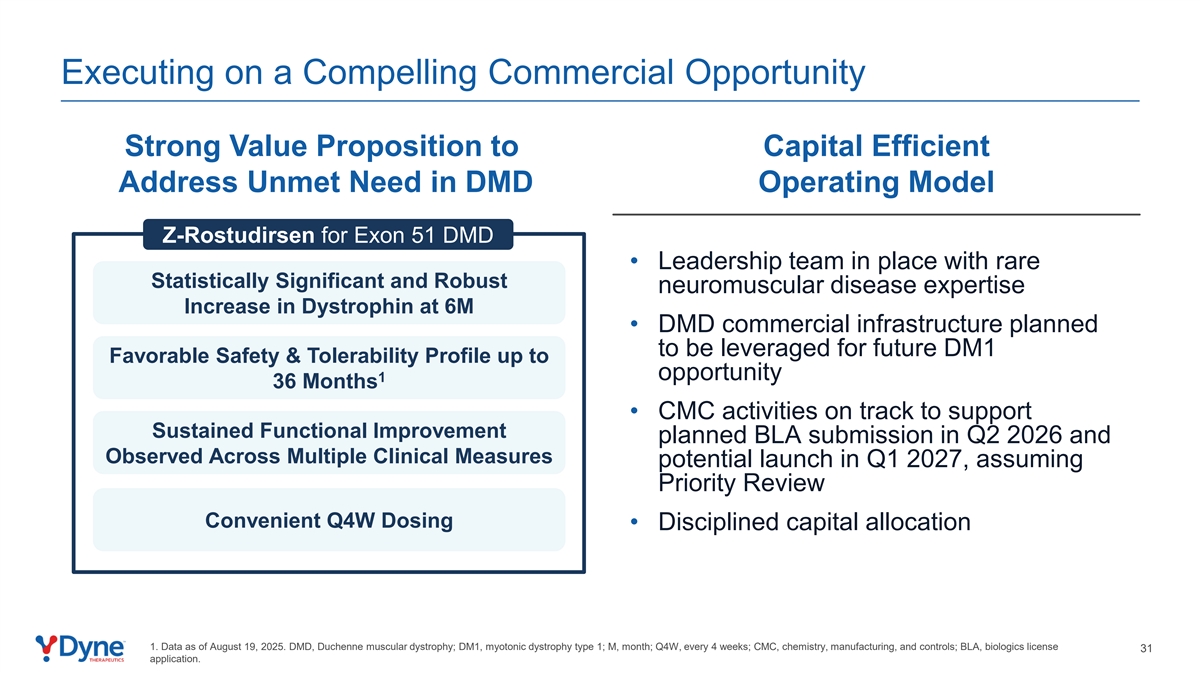
Executing on a Compelling Commercial Opportunity Strong Value Proposition to Capital Efficient Address Unmet Need in DMD Operating Model Z-Rostudirsen for Exon 51 DMD • Leadership team in place with rare Statistically Significant and Robust neuromuscular disease expertise Increase in Dystrophin at 6M • DMD commercial infrastructure planned to be leveraged for future DM1 Favorable Safety & Tolerability Profile up to opportunity 1 36 Months • CMC activities on track to support Sustained Functional Improvement planned BLA submission in Q2 2026 and Observed Across Multiple Clinical Measures potential launch in Q1 2027, assuming Priority Review Convenient Q4W Dosing • Disciplined capital allocation 1. Data as of August 19, 2025. DMD, Duchenne muscular dystrophy; DM1, myotonic dystrophy type 1; M, month; Q4W, every 4 weeks; CMC, chemistry, manufacturing, and controls; BLA, biologics license 31 application.
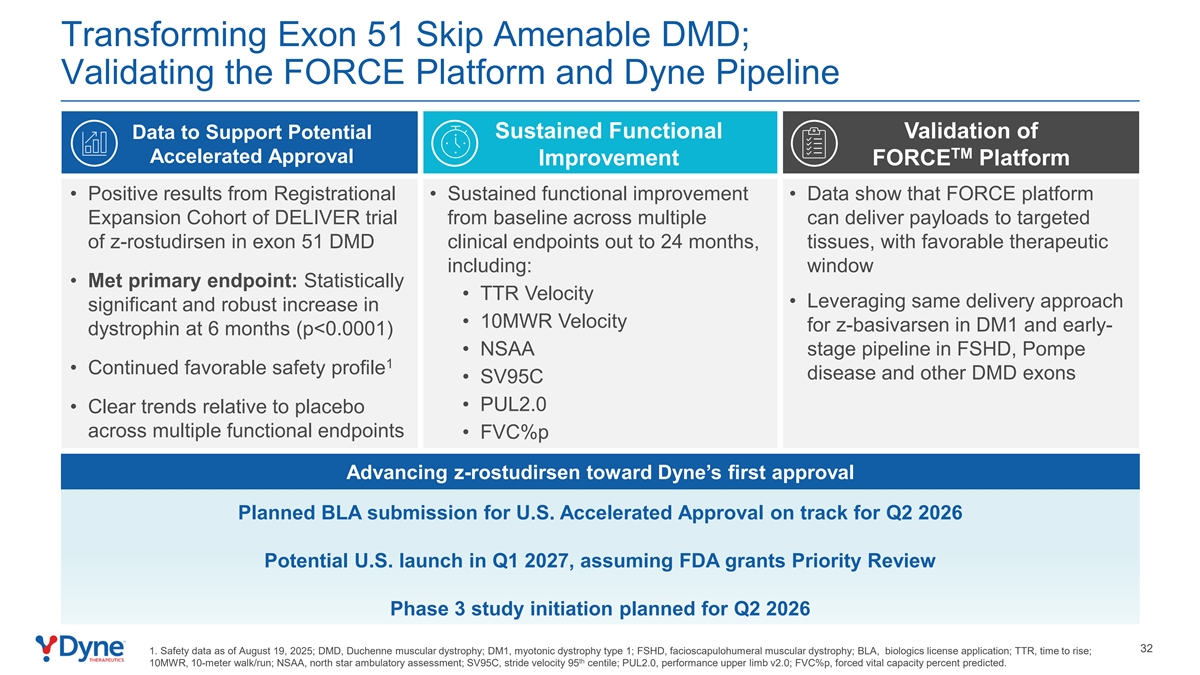
Transforming Exon 51 Skip Amenable DMD; Validating the FORCE Platform and Dyne Pipeline Data to Support Potential Sustained Functional Validation of TM Accelerated Approval Improvement FORCE Platform • Positive results from Registrational • Sustained functional improvement • Data show that FORCE platform Expansion Cohort of DELIVER trial from baseline across multiple can deliver payloads to targeted of z-rostudirsen in exon 51 DMD clinical endpoints out to 24 months, tissues, with favorable therapeutic including: window • Met primary endpoint: Statistically • TTR Velocity • Leveraging same delivery approach significant and robust increase in • 10MWR Velocity for z-basivarsen in DM1 and early- dystrophin at 6 months (p<0.0001) • NSAA stage pipeline in FSHD, Pompe 1 • Continued favorable safety profile disease and other DMD exons • SV95C • PUL2.0 • Clear trends relative to placebo across multiple functional endpoints • FVC%p Advancing z-rostudirsen toward Dyne’s first approval Planned BLA submission for U.S. Accelerated Approval on track for Q2 2026 Potential U.S. launch in Q1 2027, assuming FDA grants Priority Review Phase 3 study initiation planned for Q2 2026 32 1. Safety data as of August 19, 2025; DMD, Duchenne muscular dystrophy; DM1, myotonic dystrophy type 1; FSHD, facioscapulohumeral muscular dystrophy; BLA, biologics license application; TTR, time to rise; th 10MWR, 10-meter walk/run; NSAA, north star ambulatory assessment; SV95C, stride velocity 95 centile; PUL2.0, performance upper limb v2.0; FVC%p, forced vital capacity percent predicted.

Transforming Dyne into a Commercial Organization as Early as 2027 Z-rostudirsen for Exon 51 DMD Z-basivarsen for DM1 Completed enrollment of Early Complete enrollment planned for Q1 2025 Q2 2026 Registrational Expansion Cohort Registrational Expansion Cohort Positive topline results from Data planned for Registrational December Q1 2027 2025 Registrational Expansion Cohort Expansion Cohort Planned submission for U.S. Potential submission for U.S. Early Q2 2026 Q3 2027 Accelerated Approval Accelerated Approval st Potential U.S. launch, Potential U.S. launch, 1 potential Q1 2027 Q1 2028 launch for Dyne assuming Priority Review assuming Priority Review DMD = Duchenne muscular dystrophy; DM1 = myotonic dystrophy type 1. 33

THANK YOU to all who participated