

Advancing Clinically Validated Platform Toward Commercialization 44TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE | JANUARY 2026

Forward-Looking Statements & Disclaimer This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this presentation, including statements regarding Dyne’s strategy, future operations, prospects and plans, objectives of management, the potential of the FORCE platform and its conjugates, the therapeutic potential of zeleciment basivarsen (z-basivarsen, also known as DYNE-101), zeleciment rostudirsen (z-rostudirsen, also known as DYNE-251), DYNE-302 and DYNE-401, the anticipated timelines for reporting additional data from the ACHIEVE clinical trial, enrolling registrational cohorts and initiating additional clinical trials, expectations regarding the timing and outcome of interactions with global regulatory authorities and the availability of expedited approval pathways for z-basivarsen and z-rostudirsen, expectations regarding the timing of submitting applications for U.S. Accelerated Approval, plans to provide future updates on pipeline programs, expectations regarding the commercialization of any of Dyne’s product candidates, and the sufficiency of Dyne's cash resources for the period anticipated, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” “will” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Dyne may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; the timing of and Dyne’s ability to enroll patients in clinical trials; whether results from preclinical studies and data from clinical trials will be predictive of the final results of the clinical trials or other trials; whether data from clinical trials will support submission for regulatory approvals; uncertainties as to the FDA’s and other regulatory authorities’ interpretation of the data from Dyne's clinical trials and acceptance of Dyne's clinical programs and as to the regulatory approval process for Dyne's product candidates; whether Dyne’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in Dyne’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-K and in subsequent filings Dyne may make with the SEC. In addition, the forward-looking statements included in this presentation represent Dyne’s views as of the date of this presentation. Dyne anticipates that subsequent events and developments will cause its views to change. However, while Dyne may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Dyne’s views as of any date subsequent to the date of this presentation. This presentation also contains estimates, projections and other statistical data made by independent parties and by Dyne relating to market size and growth and other data about Dyne’s industry and business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Dyne has not independently verified the accuracy and completeness of the information obtained from third parties included in this presentation. In addition, projections, assumptions and estimates of Dyne’s future performance and the future performance of the markets in which Dyne operates are necessarily subject to a high degree of uncertainty and risk.
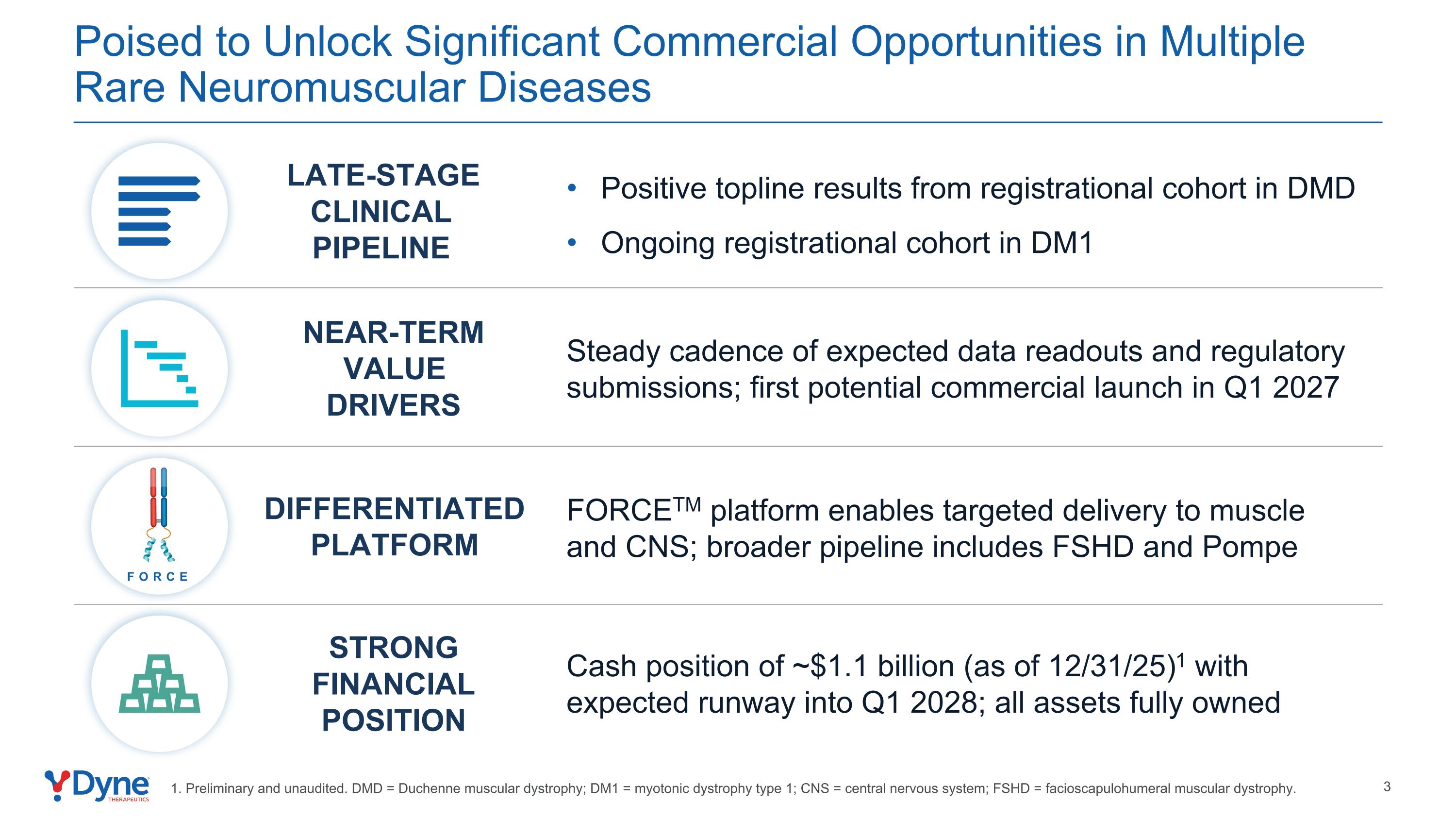
Poised to Unlock Significant Commercial Opportunities in Multiple Rare Neuromuscular Diseases Positive topline results from registrational cohort in DMD Ongoing registrational cohort in DM1 NEAR-TERM VALUE DRIVERS STRONG FINANCIAL POSITION LATE-STAGE CLINICAL PIPELINE Steady cadence of expected data readouts and regulatory submissions; first potential commercial launch in Q1 2027 Cash position of ~$1.1 billion (as of 12/31/25)1 with expected runway into Q1 2028; all assets fully owned FORCE DIFFERENTIATED PLATFORM FORCETM platform enables targeted delivery to muscle and CNS; broader pipeline includes FSHD and Pompe 1. Preliminary and unaudited. DMD = Duchenne muscular dystrophy; DM1 = myotonic dystrophy type 1; CNS = central nervous system; FSHD = facioscapulohumeral muscular dystrophy.
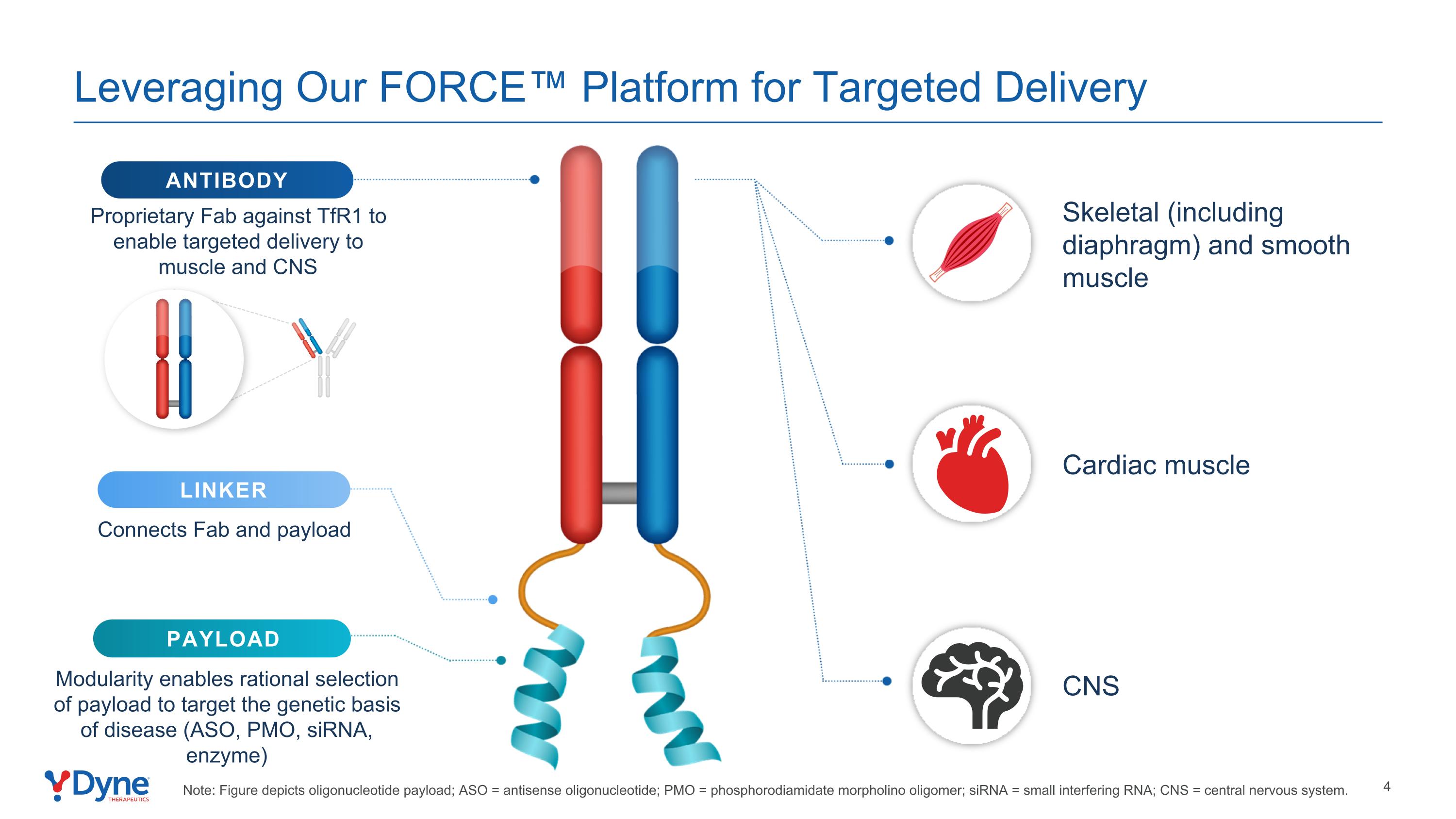
Leveraging Our FORCE™ Platform for Targeted Delivery LINKER Connects Fab and payload PAYLOAD Modularity enables rational selection of payload to target the genetic basis of disease (ASO, PMO, siRNA, enzyme) ANTIBODY Proprietary Fab against TfR1 to enable targeted delivery to muscle and CNS Skeletal (including diaphragm) and smooth muscle Cardiac muscle CNS Note: Figure depicts oligonucleotide payload; ASO = antisense oligonucleotide; PMO = phosphorodiamidate morpholino oligomer; siRNA = small interfering RNA; CNS = central nervous system.
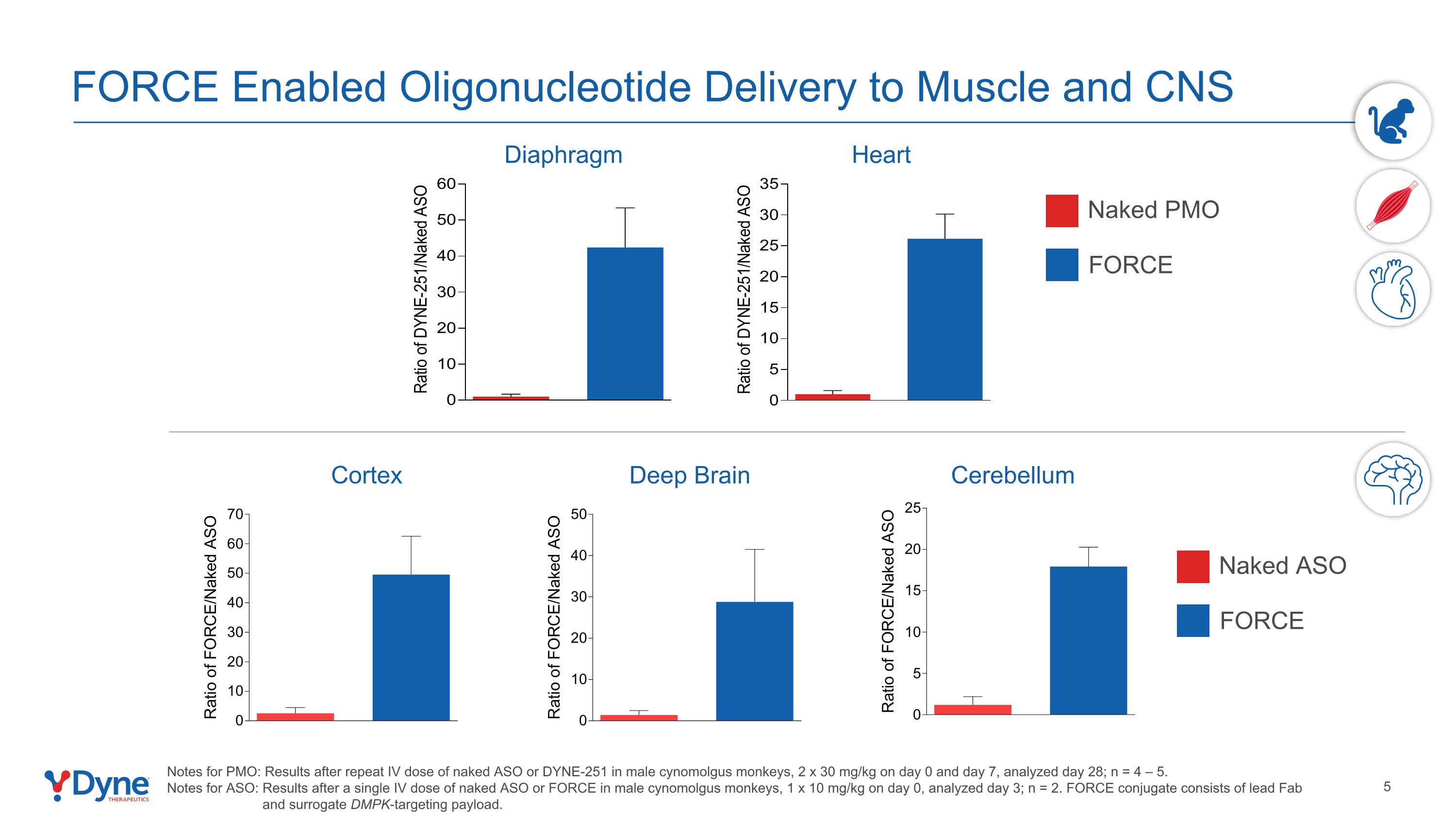
FORCE Enabled Oligonucleotide Delivery to Muscle and CNS Notes for PMO: Results after repeat IV dose of naked ASO or DYNE-251 in male cynomolgus monkeys, 2 x 30 mg/kg on day 0 and day 7, analyzed day 28; n = 4 – 5. Notes for ASO: Results after a single IV dose of naked ASO or FORCE in male cynomolgus monkeys, 1 x 10 mg/kg on day 0, analyzed day 3; n = 2. FORCE conjugate consists of lead Fab and surrogate DMPK-targeting payload. Cortex Cerebellum Deep Brain Diaphragm Heart Naked PMO FORCE Naked ASO FORCE
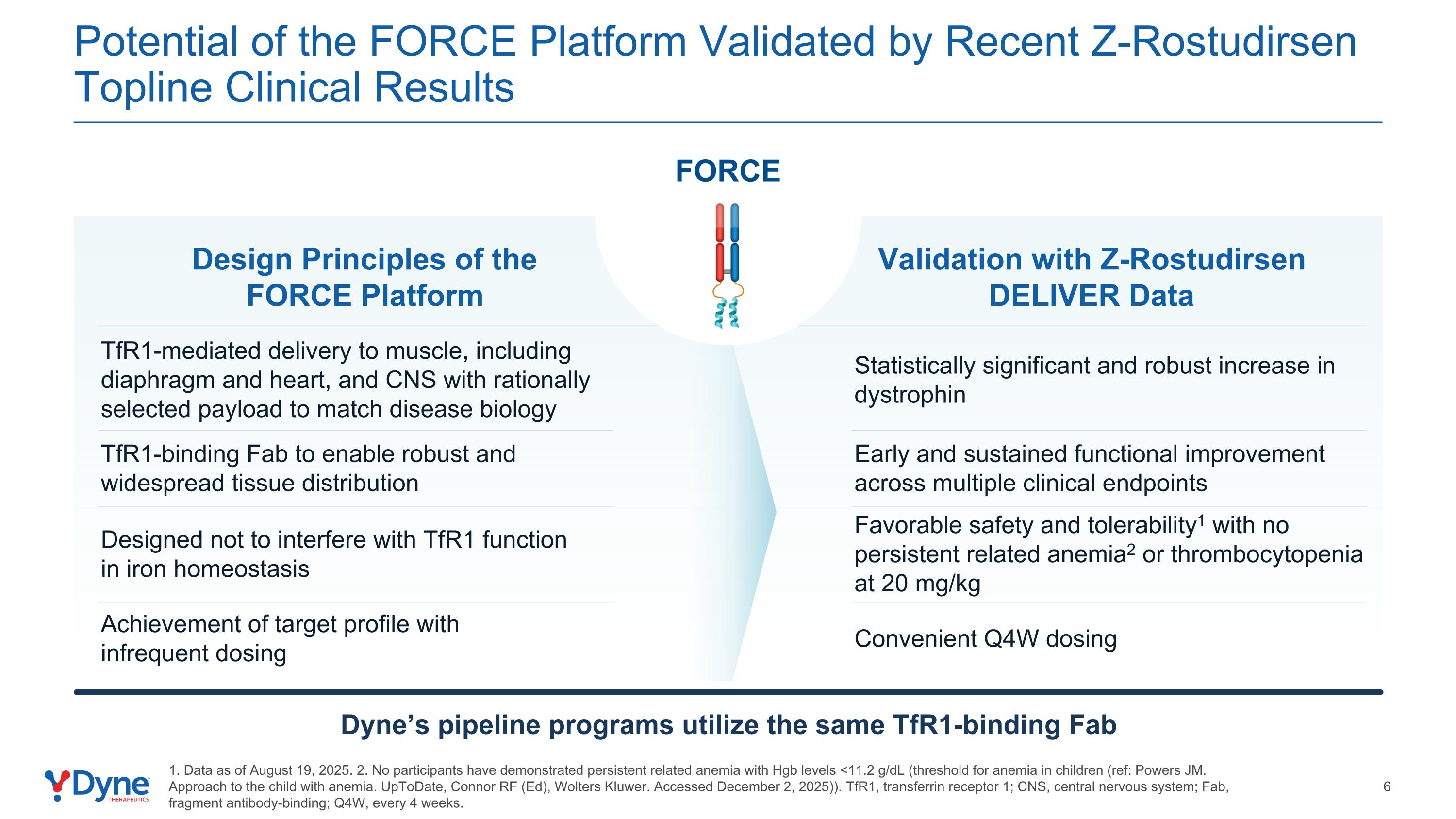
Potential of the FORCE Platform Validated by Recent Z-Rostudirsen Topline Clinical Results Validation with Z-Rostudirsen DELIVER Data 1. Data as of August 19, 2025. 2. No participants have demonstrated persistent related anemia with Hgb levels <11.2 g/dL (threshold for anemia in children (ref: Powers JM. Approach to the child with anemia. UpToDate, Connor RF (Ed), Wolters Kluwer. Accessed December 2, 2025)). TfR1, transferrin receptor 1; CNS, central nervous system; Fab, fragment antibody-binding; Q4W, every 4 weeks. Design Principles of the FORCE Platform Favorable safety and tolerability1 with no persistent related anemia2 or thrombocytopenia at 20 mg/kg Designed not to interfere with TfR1 function in iron homeostasis Convenient Q4W dosing Achievement of target profile with infrequent dosing TfR1-binding Fab to enable robust and widespread tissue distribution Early and sustained functional improvement across multiple clinical endpoints Statistically significant and robust increase in dystrophin TfR1-mediated delivery to muscle, including diaphragm and heart, and CNS with rationally selected payload to match disease biology Dyne’s pipeline programs utilize the same TfR1-binding Fab FORCE
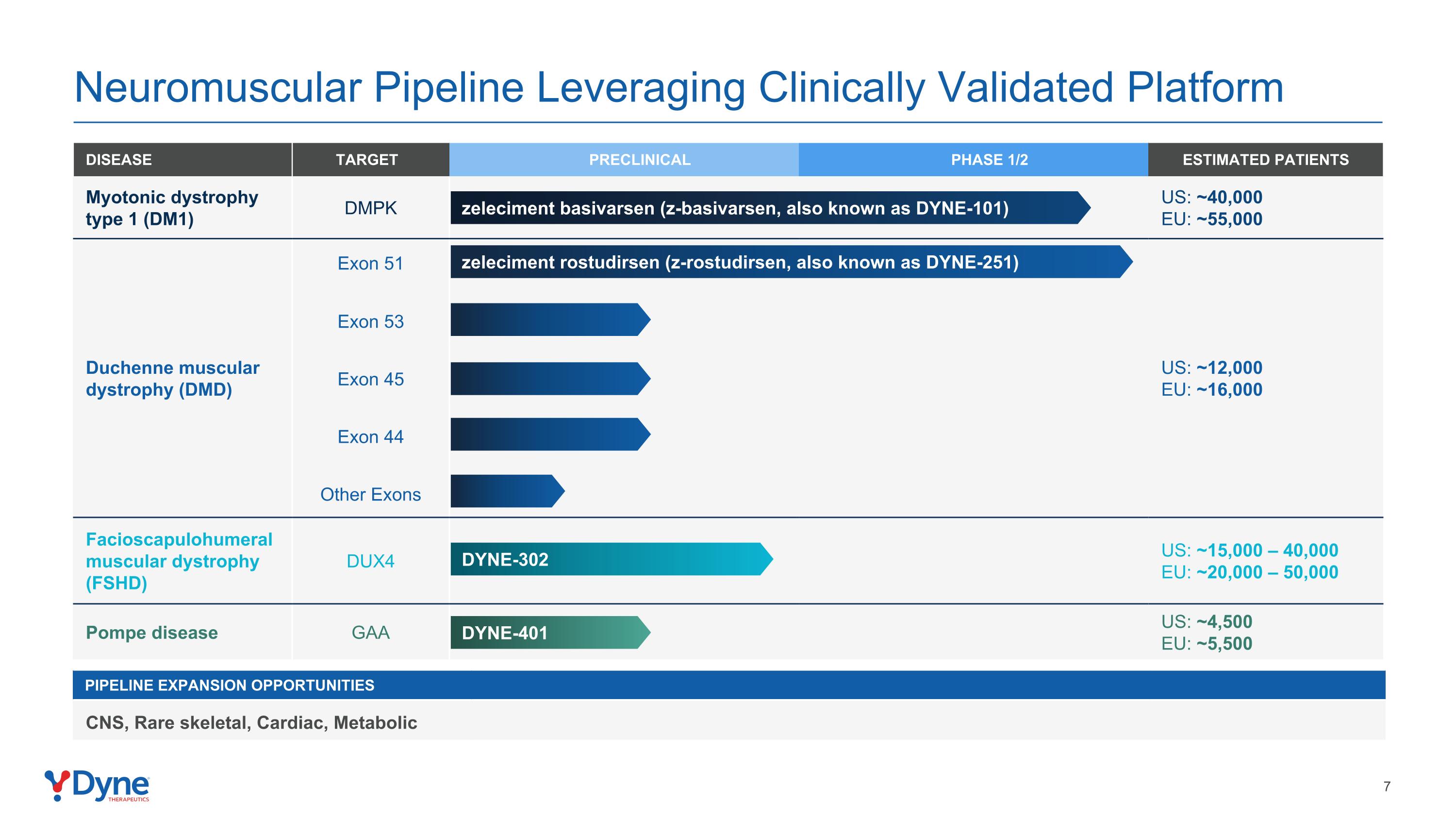
Neuromuscular Pipeline Leveraging Clinically Validated Platform Pipeline Expansion Opportunities CNS, Rare skeletal, Cardiac, Metabolic DISEASE TARGET PRECLINICAL PHASE 1/2 ESTIMATED PATIENTS Myotonic dystrophy type 1 (DM1) DMPK US: ~40,000 EU: ~55,000 Duchenne muscular dystrophy (DMD) Exon 51 US: ~12,000 EU: ~16,000 Exon 53 Exon 45 Exon 44 Other Exons Facioscapulohumeral muscular dystrophy (FSHD) DUX4 US: ~15,000 – 40,000 EU: ~20,000 – 50,000 Pompe disease GAA US: ~4,500 EU: ~5,500 zeleciment basivarsen (z-basivarsen, also known as DYNE-101) zeleciment rostudirsen (z-rostudirsen, also known as DYNE-251) DYNE-302 DYNE-401
Broad and Durable Functional Improvement Observed with Dyne's First Clinically Validated Program: Z-Rostudirsen in DMD
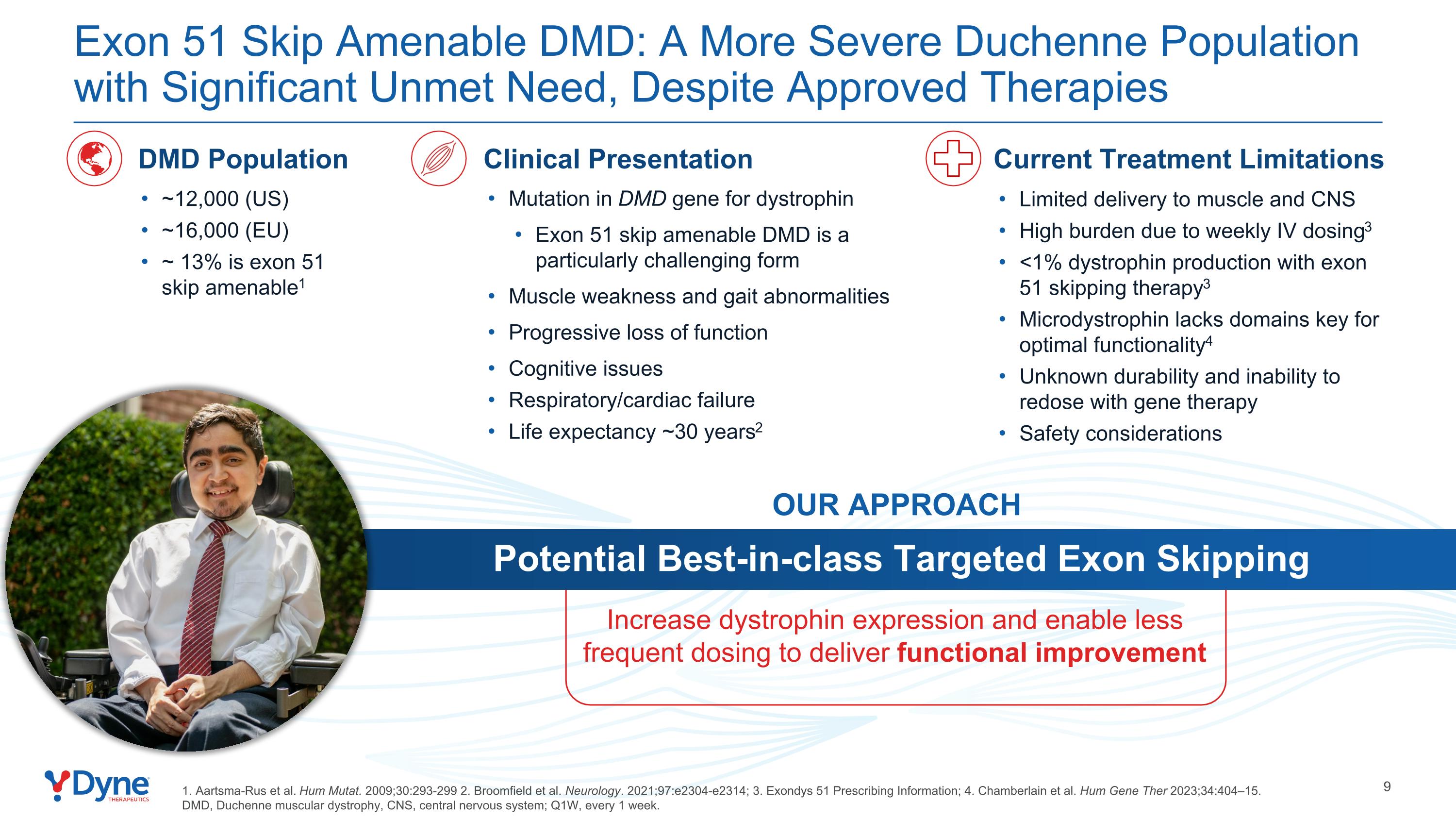
Potential Best-in-class Targeted Exon Skipping Increase dystrophin expression and enable less frequent dosing to deliver functional improvement OUR APPROACH ~12,000 (US) ~16,000 (EU) ~ 13% is exon 51 skip amenable1 DMD Population Mutation in DMD gene for dystrophin Exon 51 skip amenable DMD is a particularly challenging form Muscle weakness and gait abnormalities Progressive loss of function Cognitive issues Respiratory/cardiac failure Life expectancy ~30 years2 Clinical Presentation Exon 51 Skip Amenable DMD: A More Severe Duchenne Population with Significant Unmet Need, Despite Approved Therapies Current Treatment Limitations Limited delivery to muscle and CNS High burden due to weekly IV dosing3 <1% dystrophin production with exon 51 skipping therapy3 Microdystrophin lacks domains key for optimal functionality4 Unknown durability and inability to redose with gene therapy Safety considerations 1. Aartsma-Rus et al. Hum Mutat. 2009;30:293-299 2. Broomfield et al. Neurology. 2021;97:e2304-e2314; 3. Exondys 51 Prescribing Information; 4. Chamberlain et al. Hum Gene Ther 2023;34:404–15. DMD, Duchenne muscular dystrophy, CNS, central nervous system; Q1W, every 1 week.
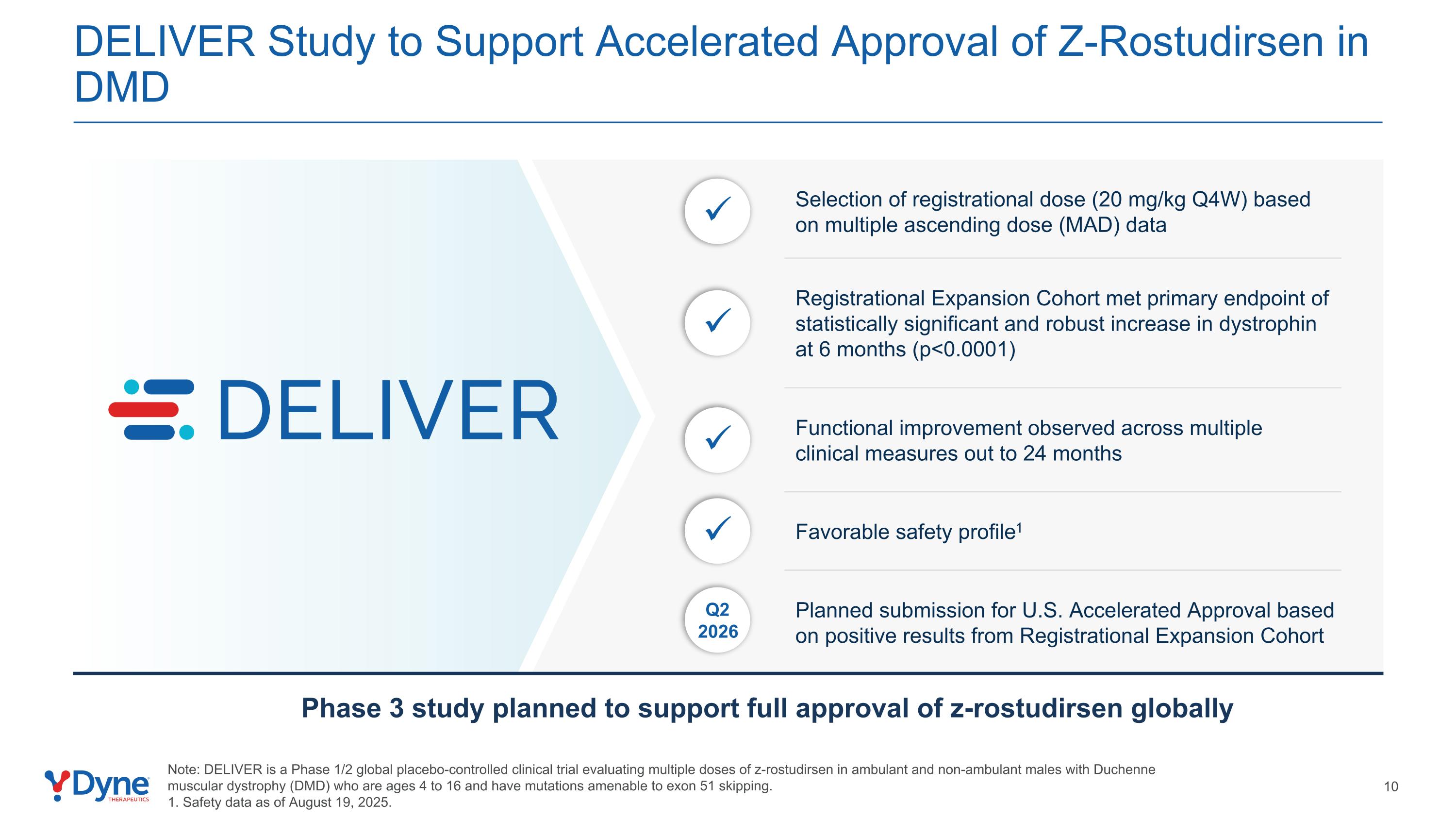
DELIVER Study to Support Accelerated Approval of Z-Rostudirsen in DMD Selection of registrational dose (20 mg/kg Q4W) based on multiple ascending dose (MAD) data Q2 2026 Planned submission for U.S. Accelerated Approval based on positive results from Registrational Expansion Cohort Functional improvement observed across multiple clinical measures out to 24 months Note: DELIVER is a Phase 1/2 global placebo-controlled clinical trial evaluating multiple doses of z-rostudirsen in ambulant and non-ambulant males with Duchenne muscular dystrophy (DMD) who are ages 4 to 16 and have mutations amenable to exon 51 skipping. 1. Safety data as of August 19, 2025. Registrational Expansion Cohort met primary endpoint of statistically significant and robust increase in dystrophin at 6 months (p<0.0001) Favorable safety profile1 Phase 3 study planned to support full approval of z-rostudirsen globally
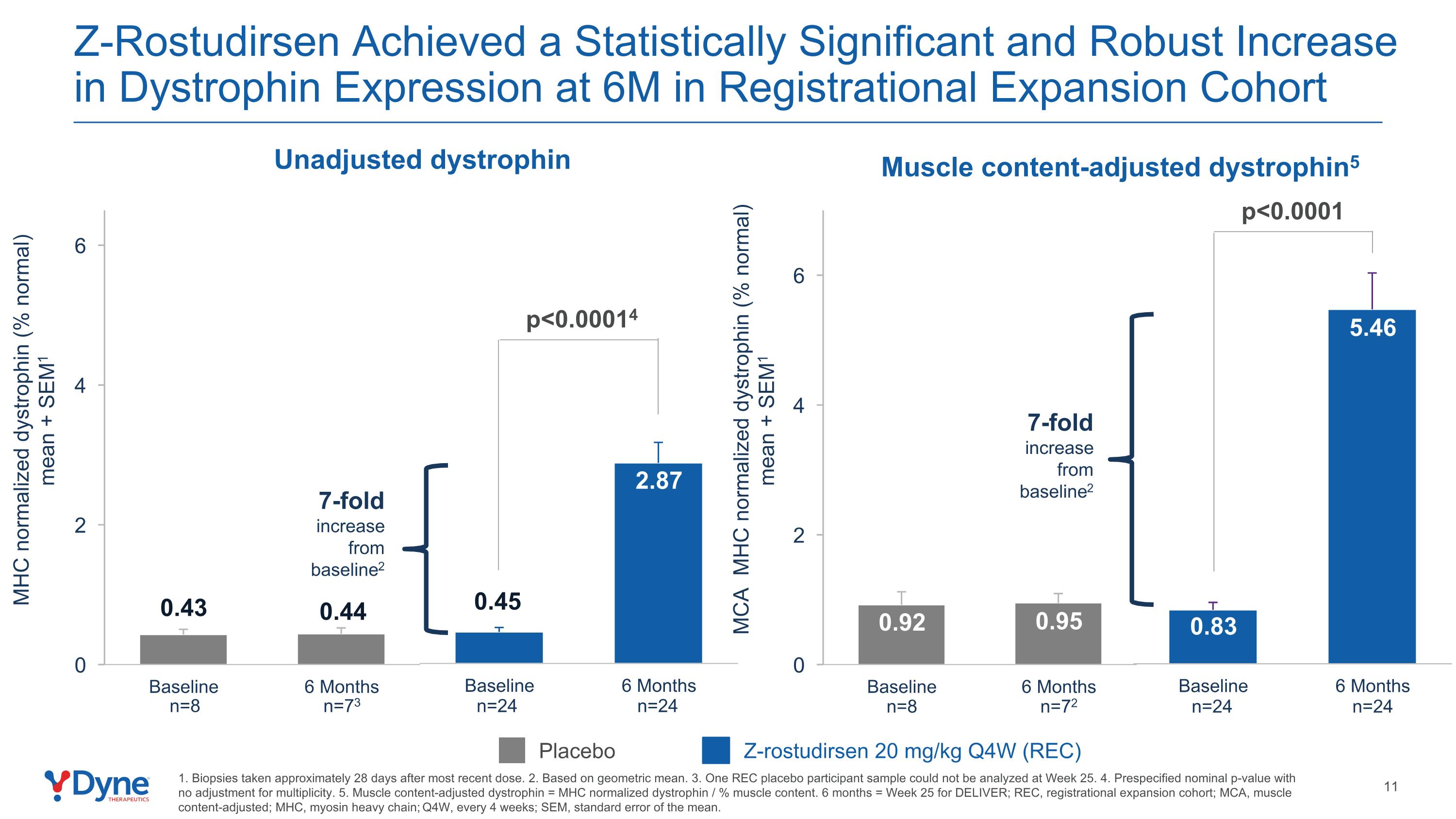
Z-Rostudirsen Achieved a Statistically Significant and Robust Increase in Dystrophin Expression at 6M in Registrational Expansion Cohort 1. Biopsies taken approximately 28 days after most recent dose. 2. Based on geometric mean. 3. One REC placebo participant sample could not be analyzed at Week 25. 4. Prespecified nominal p-value with no adjustment for multiplicity. 5. Muscle content-adjusted dystrophin = MHC normalized dystrophin / % muscle content. 6 months = Week 25 for DELIVER; REC, registrational expansion cohort; MCA, muscle content-adjusted; MHC, myosin heavy chain; Q4W, every 4 weeks; SEM, standard error of the mean. Unadjusted dystrophin Placebo Z-rostudirsen 20 mg/kg Q4W (REC) n=24 n=24 n=72 n=8 Muscle content-adjusted dystrophin5 p<0.0001 MHC normalized dystrophin (% normal) mean + SEM1 p<0.00014 MCA MHC normalized dystrophin (% normal) mean + SEM1 n=24 n=24 n=73 n=8 7-fold increase from baseline2 7-fold increase from baseline2
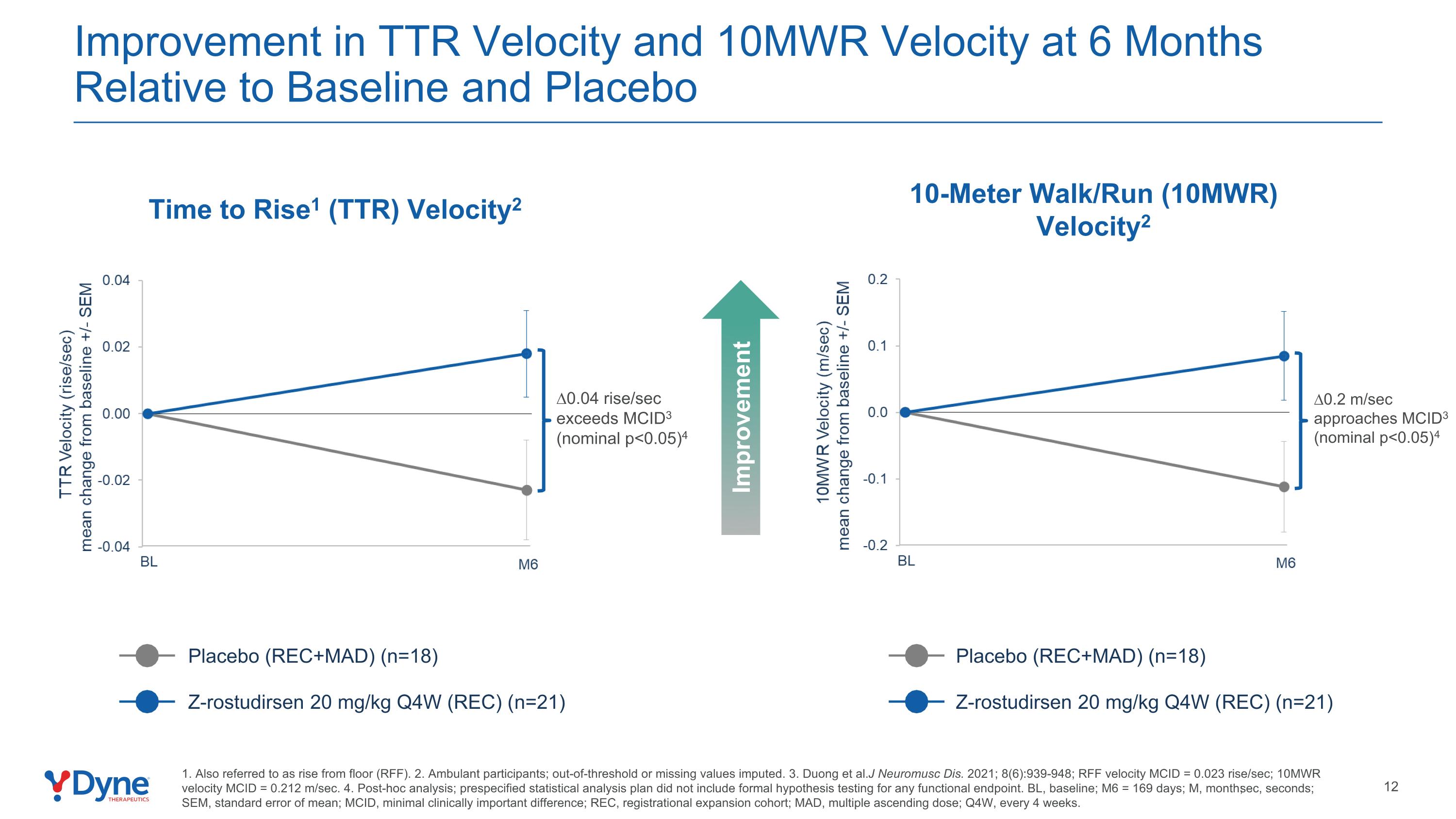
Improvement in TTR Velocity and 10MWR Velocity at 6 Months Relative to Baseline and Placebo Time to Rise1 (TTR) Velocity2 10-Meter Walk/Run (10MWR) Velocity2 Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) ∆0.2 m/sec approaches MCID3 (nominal p<0.05)4 ∆0.04 rise/sec exceeds MCID3 (nominal p<0.05)4 1. Also referred to as rise from floor (RFF). 2. Ambulant participants; out-of-threshold or missing values imputed. 3. Duong et al. J Neuromusc Dis. 2021; 8(6):939-948; RFF velocity MCID = 0.023 rise/sec; 10MWR velocity MCID = 0.212 m/sec. 4. Post-hoc analysis; prespecified statistical analysis plan did not include formal hypothesis testing for any functional endpoint. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; MCID, minimal clinically important difference; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Improvement
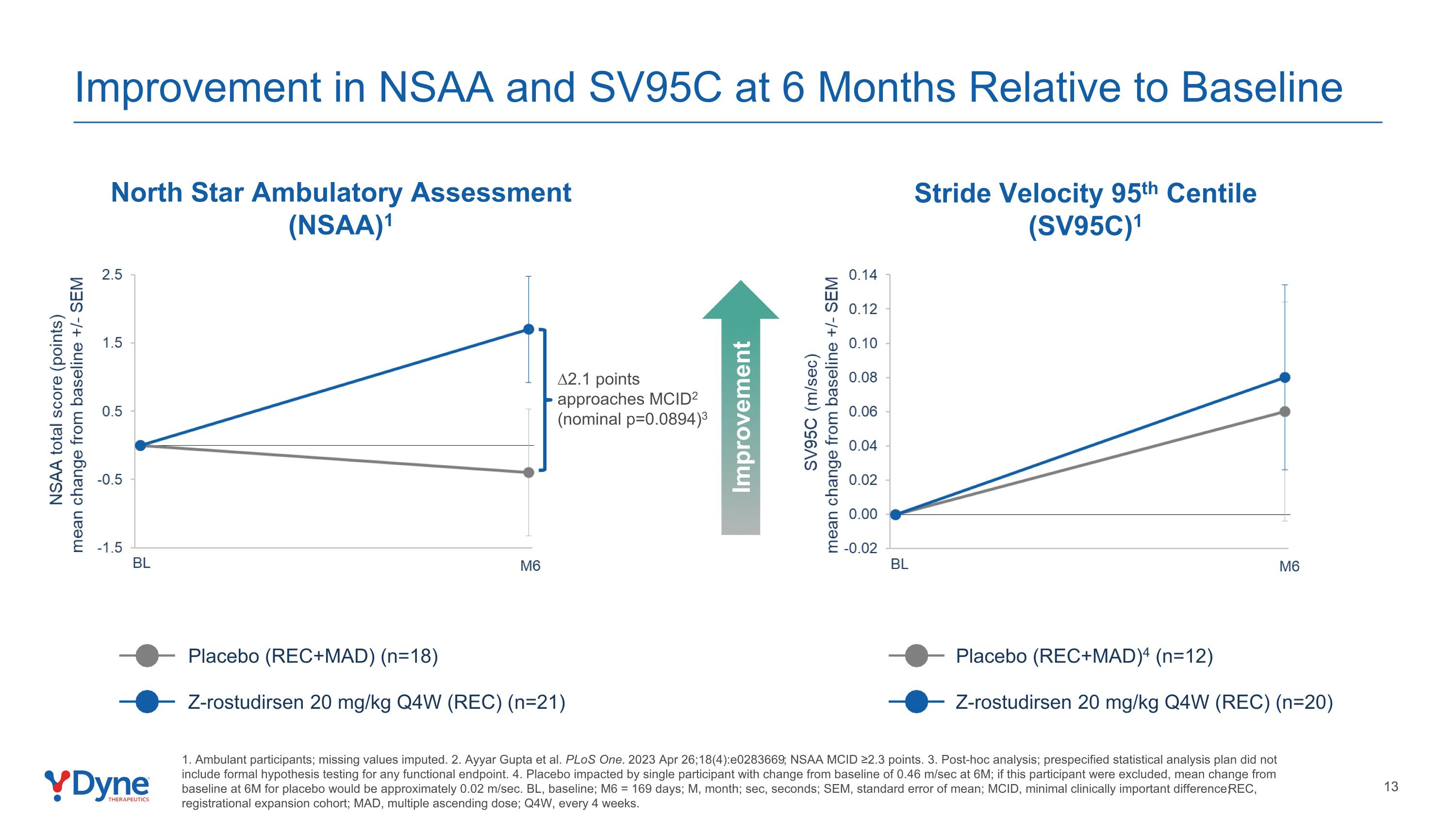
Improvement in NSAA and SV95C at 6 Months Relative to Baseline North Star Ambulatory Assessment (NSAA)1 Stride Velocity 95th Centile (SV95C)1 Improvement ∆2.1 points approaches MCID2 (nominal p=0.0894)3 1. Ambulant participants; missing values imputed. 2. Ayyar Gupta et al. PLoS One. 2023 Apr 26;18(4):e0283669; NSAA MCID ≥2.3 points. 3. Post-hoc analysis; prespecified statistical analysis plan did not include formal hypothesis testing for any functional endpoint. 4. Placebo impacted by single participant with change from baseline of 0.46 m/sec at 6M; if this participant were excluded, mean change from baseline at 6M for placebo would be approximately 0.02 m/sec. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; MCID, minimal clinically important difference; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Placebo (REC+MAD) (n=18) Z-rostudirsen 20 mg/kg Q4W (REC) (n=21) Placebo (REC+MAD)4 (n=12) Z-rostudirsen 20 mg/kg Q4W (REC) (n=20)
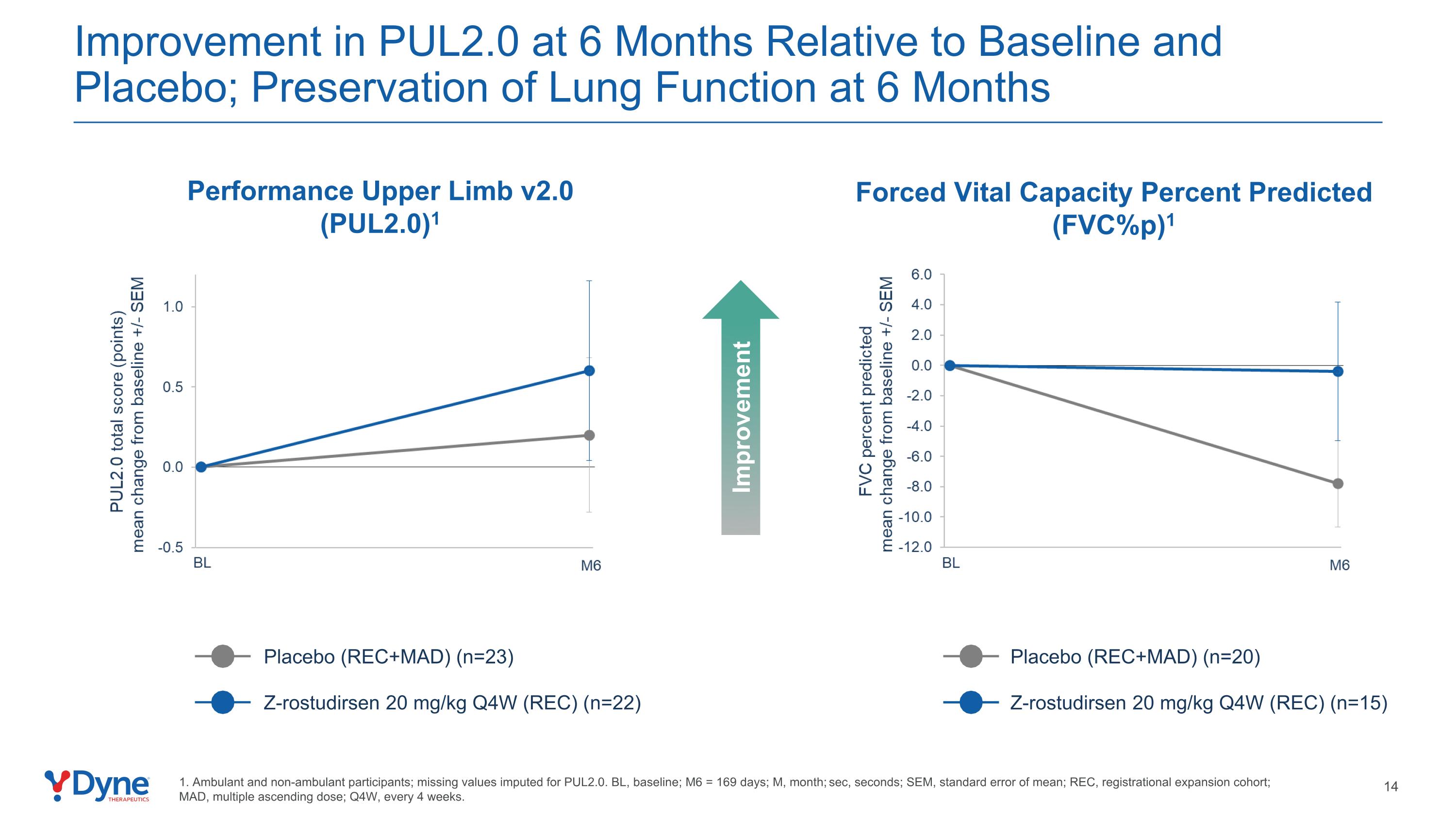
Improvement in PUL2.0 at 6 Months Relative to Baseline and Placebo; Preservation of Lung Function at 6 Months Forced Vital Capacity Percent Predicted (FVC%p)1 Placebo (REC+MAD) (n=23) Z-rostudirsen 20 mg/kg Q4W (REC) (n=22) Placebo (REC+MAD) (n=20) Z-rostudirsen 20 mg/kg Q4W (REC) (n=15) 1. Ambulant and non-ambulant participants; missing values imputed for PUL2.0. BL, baseline; M6 = 169 days; M, month; sec, seconds; SEM, standard error of mean; REC, registrational expansion cohort; MAD, multiple ascending dose; Q4W, every 4 weeks. Improvement Performance Upper Limb v2.0 (PUL2.0)1
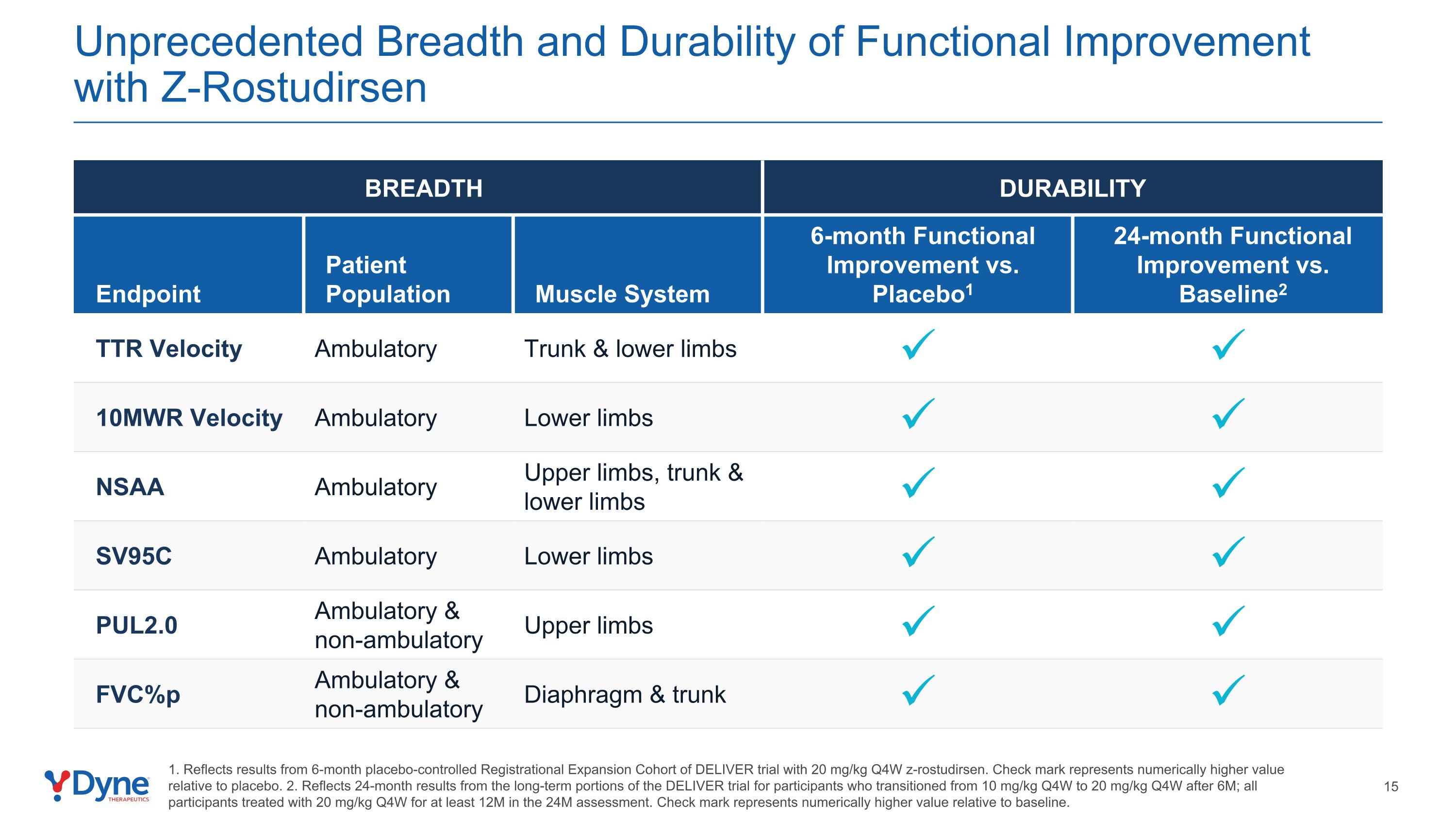
Unprecedented Breadth and Durability of Functional Improvement with Z-Rostudirsen BREADTH DURABILITY Endpoint Patient Population Muscle System 6-month Functional Improvement vs. Placebo1 24-month Functional Improvement vs. Baseline2 TTR Velocity Ambulatory Trunk & lower limbs 10MWR Velocity Ambulatory Lower limbs NSAA Ambulatory Upper limbs, trunk & lower limbs SV95C Ambulatory Lower limbs PUL2.0 Ambulatory & non-ambulatory Upper limbs FVC%p Ambulatory & non-ambulatory Diaphragm & trunk 1. Reflects results from 6-month placebo-controlled Registrational Expansion Cohort of DELIVER trial with 20 mg/kg Q4W z-rostudirsen. Check mark represents numerically higher value relative to placebo. 2. Reflects 24-month results from the long-term portions of the DELIVER trial for participants who transitioned from 10 mg/kg Q4W to 20 mg/kg Q4W after 6M; all participants treated with 20 mg/kg Q4W for at least 12M in the 24M assessment. Check mark represents numerically higher value relative to baseline.
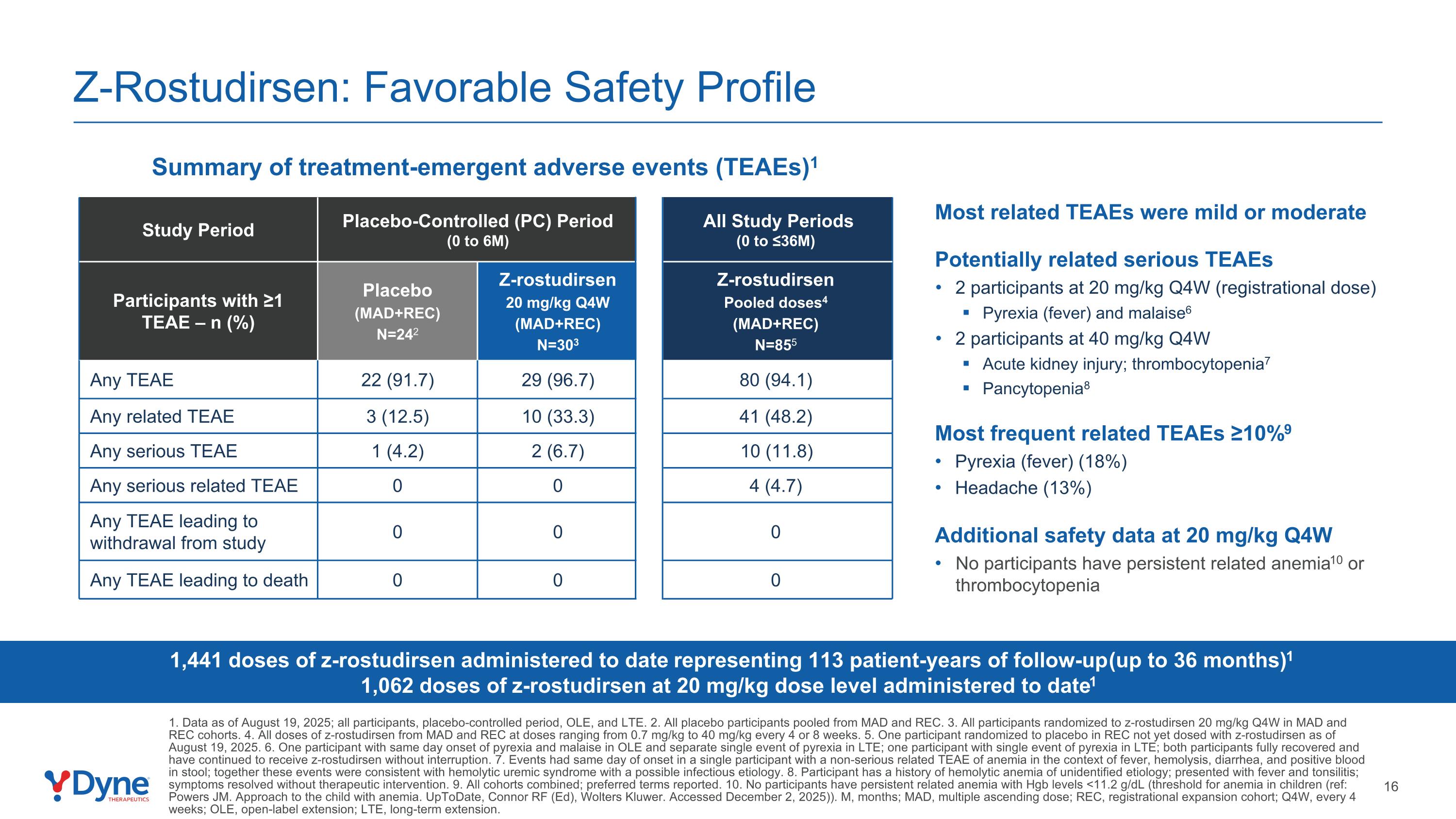
Z-Rostudirsen: Favorable Safety Profile Summary of treatment-emergent adverse events (TEAEs)1 1,441 doses of z-rostudirsen administered to date representing 113 patient-years of follow-up (up to 36 months)1 1,062 doses of z-rostudirsen at 20 mg/kg dose level administered to date1 Most related TEAEs were mild or moderate Potentially related serious TEAEs 2 participants at 20 mg/kg Q4W (registrational dose) Pyrexia (fever) and malaise6 2 participants at 40 mg/kg Q4W Acute kidney injury; thrombocytopenia7 Pancytopenia8 Most frequent related TEAEs ≥10%9 Pyrexia (fever) (18%) Headache (13%) Additional safety data at 20 mg/kg Q4W No participants have persistent related anemia10 or thrombocytopenia Study Period Placebo-Controlled (PC) Period (0 to 6M) All Study Periods (0 to ≤36M) Participants with ≥1 TEAE – n (%) Placebo (MAD+REC) N=242 Z-rostudirsen 20 mg/kg Q4W (MAD+REC) N=303 Z-rostudirsen Pooled doses4 (MAD+REC) N=855 Any TEAE 22 (91.7) 29 (96.7) 80 (94.1) Any related TEAE 3 (12.5) 10 (33.3) 41 (48.2) Any serious TEAE 1 (4.2) 2 (6.7) 10 (11.8) Any serious related TEAE 0 0 4 (4.7) Any TEAE leading to withdrawal from study 0 0 0 Any TEAE leading to death 0 0 0 1. Data as of August 19, 2025; all participants, placebo-controlled period, OLE, and LTE. 2. All placebo participants pooled from MAD and REC. 3. All participants randomized to z-rostudirsen 20 mg/kg Q4W in MAD and REC cohorts. 4. All doses of z-rostudirsen from MAD and REC at doses ranging from 0.7 mg/kg to 40 mg/kg every 4 or 8 weeks. 5. One participant randomized to placebo in REC not yet dosed with z-rostudirsen as of August 19, 2025. 6. One participant with same day onset of pyrexia and malaise in OLE and separate single event of pyrexia in LTE; one participant with single event of pyrexia in LTE; both participants fully recovered and have continued to receive z-rostudirsen without interruption. 7. Events had same day of onset in a single participant with a non-serious related TEAE of anemia in the context of fever, hemolysis, diarrhea, and positive blood in stool; together these events were consistent with hemolytic uremic syndrome with a possible infectious etiology. 8. Participant has a history of hemolytic anemia of unidentified etiology; presented with fever and tonsilitis; symptoms resolved without therapeutic intervention. 9. All cohorts combined; preferred terms reported. 10. No participants have persistent related anemia with Hgb levels <11.2 g/dL (threshold for anemia in children (ref: Powers JM. Approach to the child with anemia. UpToDate, Connor RF (Ed), Wolters Kluwer. Accessed December 2, 2025)). M, months; MAD, multiple ascending dose; REC, registrational expansion cohort; Q4W, every 4 weeks; OLE, open-label extension; LTE, long-term extension.

Z-Rostudirsen Offers a Compelling Profile for Potential Accelerated Approval and Addressing Unmet Need in DMD Z-Rostudirsen: Potential Best-in-class Profile for Exon 51 DMD Statistically Significant and Robust Increase in Dystrophin at 6 Months Favorable Safety & Tolerability Profile up to 36 Months1 Functional Improvement Observed Across Multiple Clinical Measures Convenient Q4W Dosing 1. Data as of August 19, 2025. 2. Post-hoc analysis; prespecified statistical analysis plan did not include formal hypothesis testing for any functional endpoint. DMD, Duchenne muscular dystrophy; CFB, change from baseline; Q4W, every 4 weeks; TTR, time to rise; 10MWR, 10-meter walk/run. Dr. Shieh is a professor of neurology and pediatrics at the David Geffen School of Medicine at UCLA, a neurologist at the Ronald Reagan UCLA Medical Center in Los Angeles, and a principal investigator for the DELIVER trial. “I am highly encouraged by these new results from the placebo-controlled Registrational Expansion Cohort and the longer-term portions of DELIVER, and I look forward to being able to offer z-rostudirsen to eligible DMD patients, if approved.” - Perry Shieh, M.D, Ph.D.
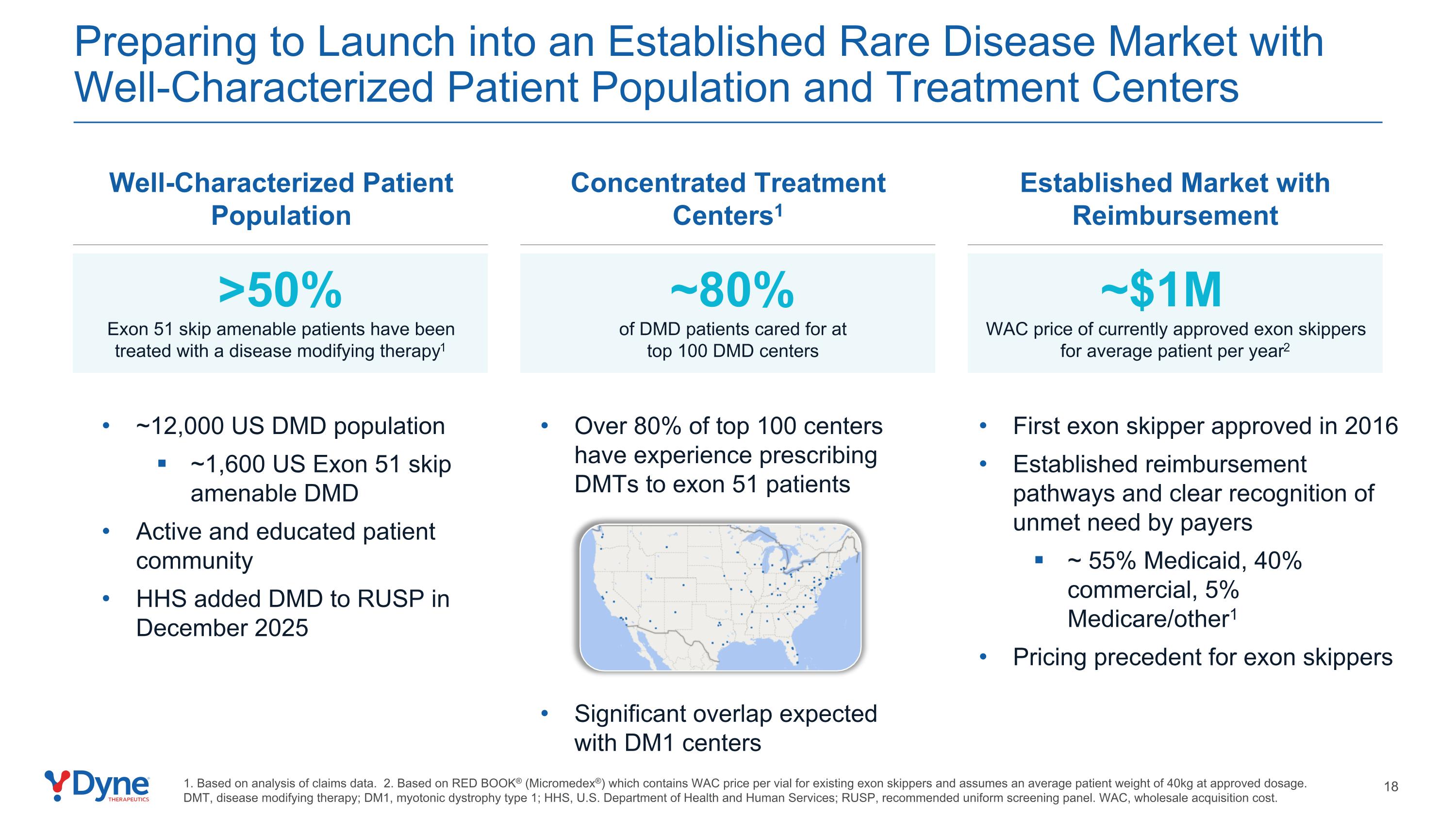
Preparing to Launch into an Established Rare Disease Market with Well-Characterized Patient Population and Treatment Centers Significant overlap expected with DM1 centers First exon skipper approved in 2016 Established reimbursement pathways and clear recognition of unmet need by payers ~ 55% Medicaid, 40% commercial, 5% Medicare/other1 Pricing precedent for exon skippers ~12,000 US DMD population ~1,600 US Exon 51 skip amenable DMD Active and educated patient community HHS added DMD to RUSP in December 2025 Over 80% of top 100 centers have experience prescribing DMTs to exon 51 patients 1. Based on analysis of claims data. 2. Based on RED BOOK® (Micromedex®) which contains WAC price per vial for existing exon skippers and assumes an average patient weight of 40kg at approved dosage. DMT, disease modifying therapy; DM1, myotonic dystrophy type 1; HHS, U.S. Department of Health and Human Services; RUSP, recommended uniform screening panel. WAC, wholesale acquisition cost. Well-Characterized Patient Population Concentrated Treatment Centers1 Established Market with Reimbursement ~$1M WAC price of currently approved exon skippers for average patient per year2 >50% Exon 51 skip amenable patients have been treated with a disease modifying therapy1 ~80% of DMD patients cared for at top 100 DMD centers
Capital-Efficient Commercial Infrastructure Designed to Deliver Potentially Transformative Therapies and Optimize Value Strong expertise in rare neurological and neuromuscular diseases Broad US launch experience including SPINRAZA, ELEVIDYS, VYONDYS, AMONDYS, and DAYBUE Deep expertise across Medical, Commercial, Value & Access, Manufacturing, Program Management, Patient Advocacy and Services, Government Affairs, Digital, Data & Analytics Experienced launch team assembled for targeted execution Ongoing education & awareness building Manufacturing capabilities and team ready to supply DMD and DM1 potential launches Site of care visits underway across Medical and Commercial field teams Ongoing engagement with payer community > 5 years of sustained patient community and advocacy organization engagement Proven track record of providing drug supply across global clinical trials since 2022 Leveraging same Fab and linker across DMD and DM1 programs Commercial-scale manufacturing partnerships in place with sufficient capacity and redundant, geographically-diverse suppliers Note: Fab = fragment antibody-binding; DMD = Duchenne muscular dystrophy; DM1 = myotonic dystrophy type 1
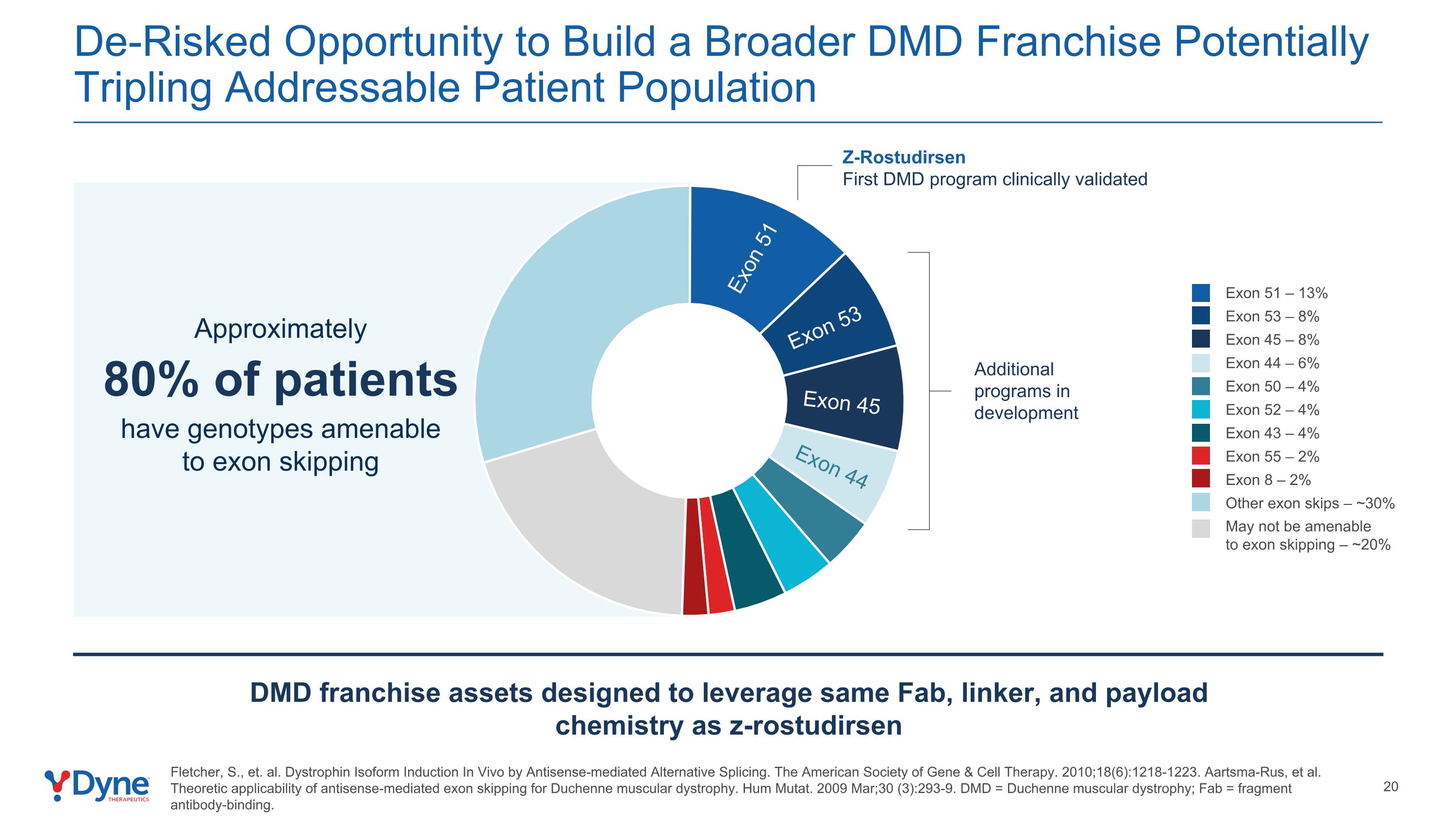
De-Risked Opportunity to Build a Broader DMD Franchise Potentially Tripling Addressable Patient Population May not be amenable to exon skipping – ~20% Exon 51 – 13% Exon 53 – 8% Exon 45 – 8% Exon 44 – 6% Exon 50 – 4% Other exon skips – ~30% Exon 52 – 4% Exon 43 – 4% Exon 55 – 2% Exon 8 – 2% Approximately 80% of patients have genotypes amenable to exon skipping Exon 51 Exon 53 Exon 45 Exon 44 DMD franchise assets designed to leverage same Fab, linker, and payload chemistry as z-rostudirsen Additional programs in development Z-Rostudirsen First DMD program clinically validated Fletcher, S., et. al. Dystrophin Isoform Induction In Vivo by Antisense-mediated Alternative Splicing. The American Society of Gene & Cell Therapy. 2010;18(6):1218-1223. Aartsma-Rus, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy. Hum Mutat. 2009 Mar;30 (3):293-9. DMD = Duchenne muscular dystrophy; Fab = fragment antibody-binding.
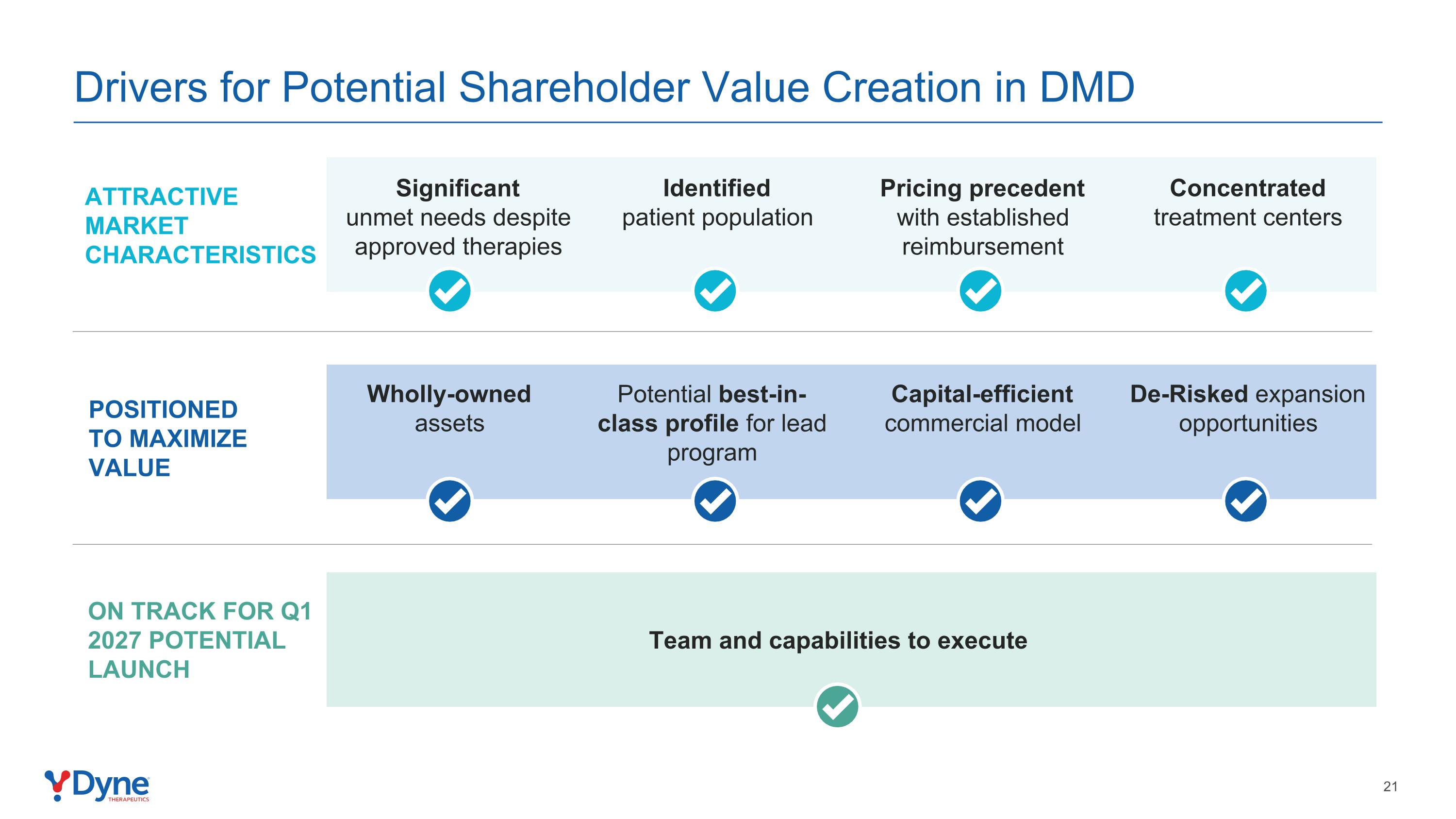
Drivers for Potential Shareholder Value Creation in DMD Significant unmet needs despite approved therapies Identified patient population Pricing precedent with established reimbursement Concentrated treatment centers attractive market characteristics Wholly-owned assets Potential best-in-class profile for lead program Capital-efficient commercial model De-Risked expansion opportunities Positioned to maximize value Team and capabilities to execute On track for Q1 2027 potential launch

Second Lead Program Leveraging Clinically Validated Platform for Larger Adjacent Indication: Z-Basivarsen in DM1
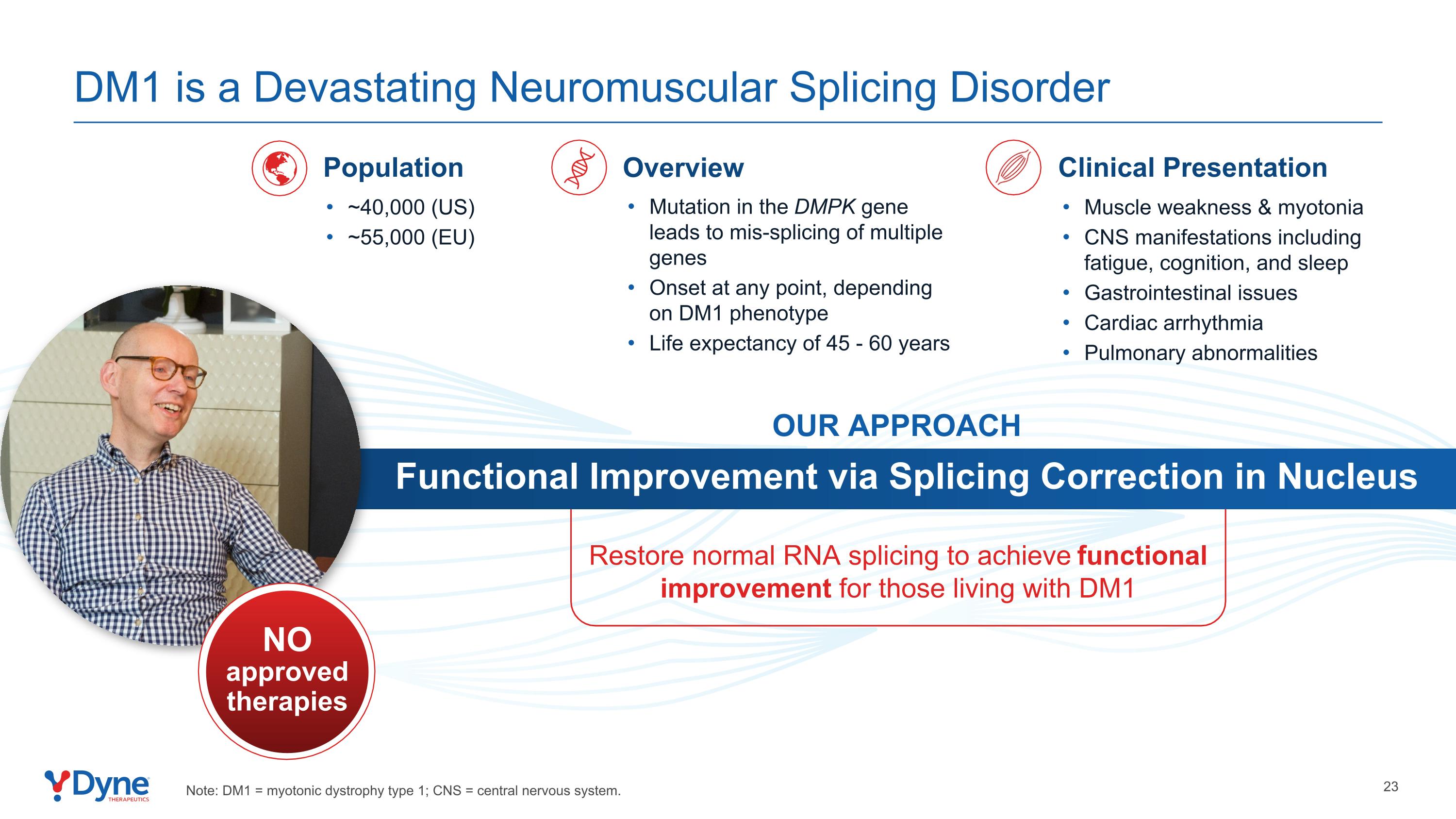
Restore normal RNA splicing to achieve functional improvement for those living with DM1 Functional Improvement via Splicing Correction in Nucleus OUR APPROACH Mutation in the DMPK gene leads to mis-splicing of multiple genes Onset at any point, depending on DM1 phenotype Life expectancy of 45 - 60 years Overview ~40,000 (US) ~55,000 (EU) Population Muscle weakness & myotonia CNS manifestations including fatigue, cognition, and sleep Gastrointestinal issues Cardiac arrhythmia Pulmonary abnormalities Clinical Presentation NO approved therapies DM1 is a Devastating Neuromuscular Splicing Disorder Note: DM1 = myotonic dystrophy type 1; CNS = central nervous system.
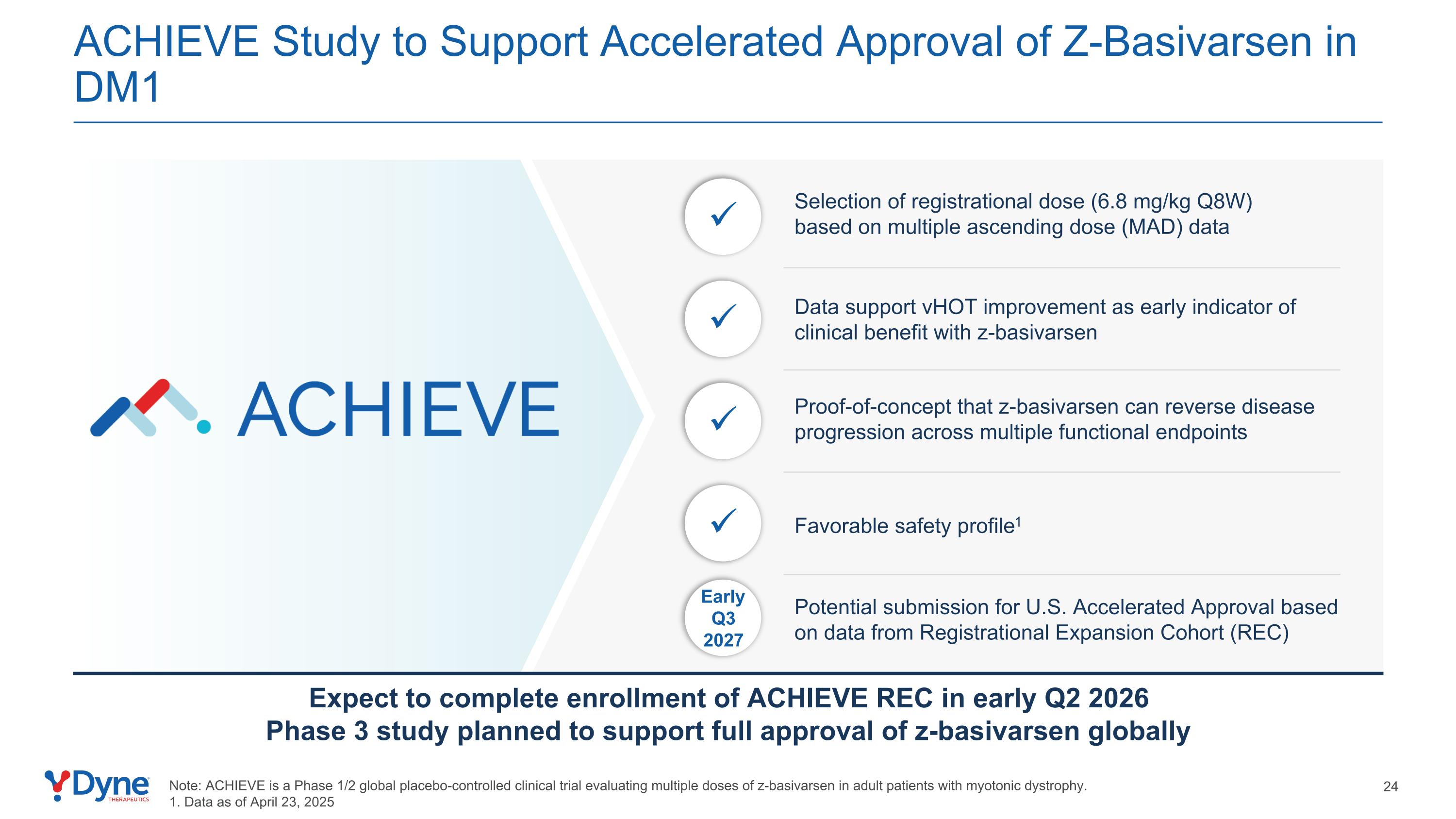
EarlyQ3 2027 ACHIEVE Study to Support Accelerated Approval of Z-Basivarsen in DM1 Selection of registrational dose (6.8 mg/kg Q8W) based on multiple ascending dose (MAD) data Potential submission for U.S. Accelerated Approval based on data from Registrational Expansion Cohort (REC) Proof-of-concept that z-basivarsen can reverse disease progression across multiple functional endpoints Note: ACHIEVE is a Phase 1/2 global placebo-controlled clinical trial evaluating multiple doses of z-basivarsen in adult patients with myotonic dystrophy. 1. Data as of April 23, 2025 Data support vHOT improvement as early indicator of clinical benefit with z-basivarsen Favorable safety profile1 Expect to complete enrollment of ACHIEVE REC in early Q2 2026 Phase 3 study planned to support full approval of z-basivarsen globally
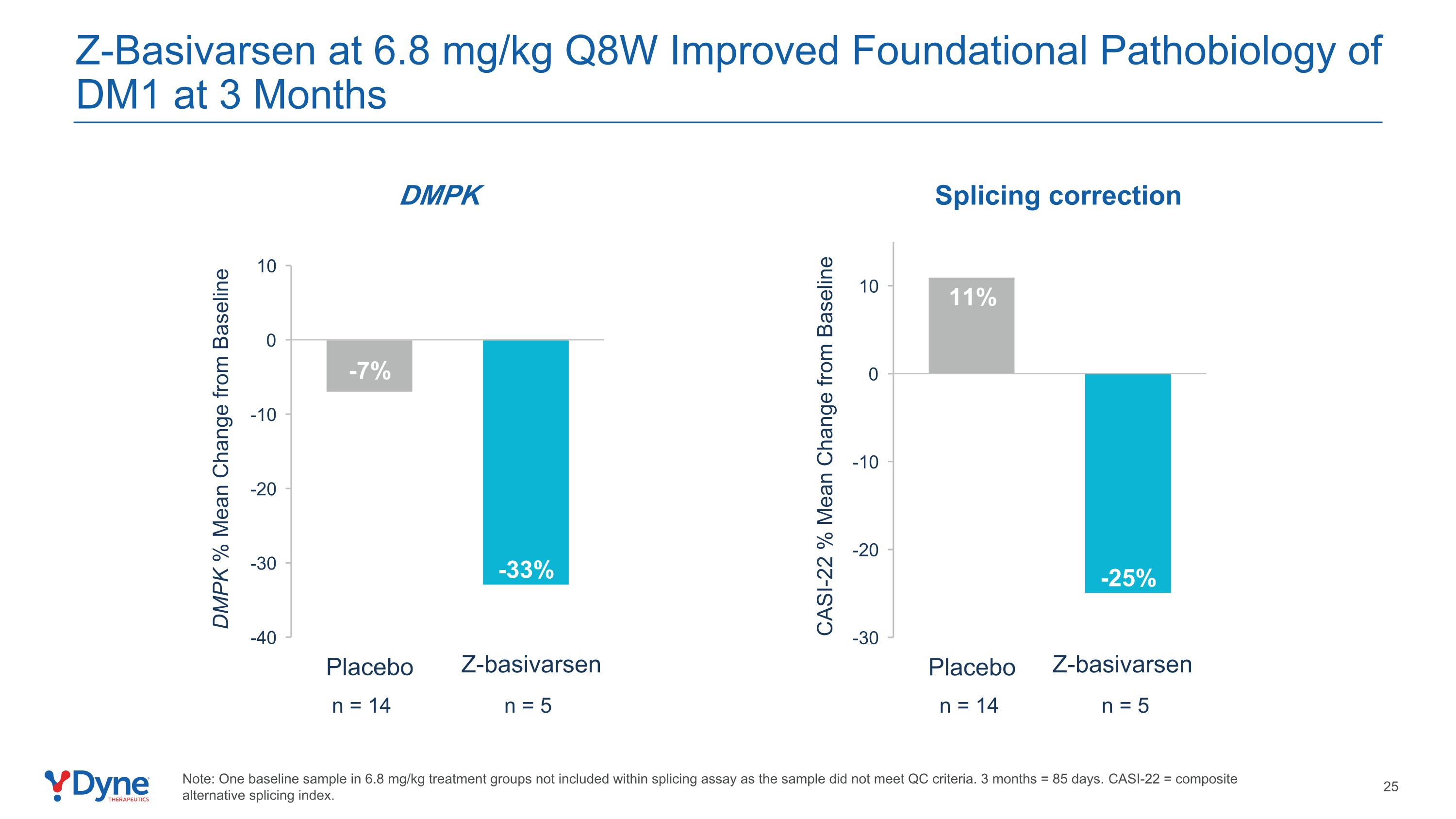
Z-Basivarsen at 6.8 mg/kg Q8W Improved Foundational Pathobiology of DM1 at 3 Months Note: One baseline sample in 6.8 mg/kg treatment groups not included within splicing assay as the sample did not meet QC criteria. 3 months = 85 days. CASI-22 = composite alternative splicing index. n = 5 DMPK % Mean Change from Baseline DMPK n = 14 CASI-22 % Mean Change from Baseline n = 5 n = 14 Splicing correction Z-basivarsen Z-basivarsen
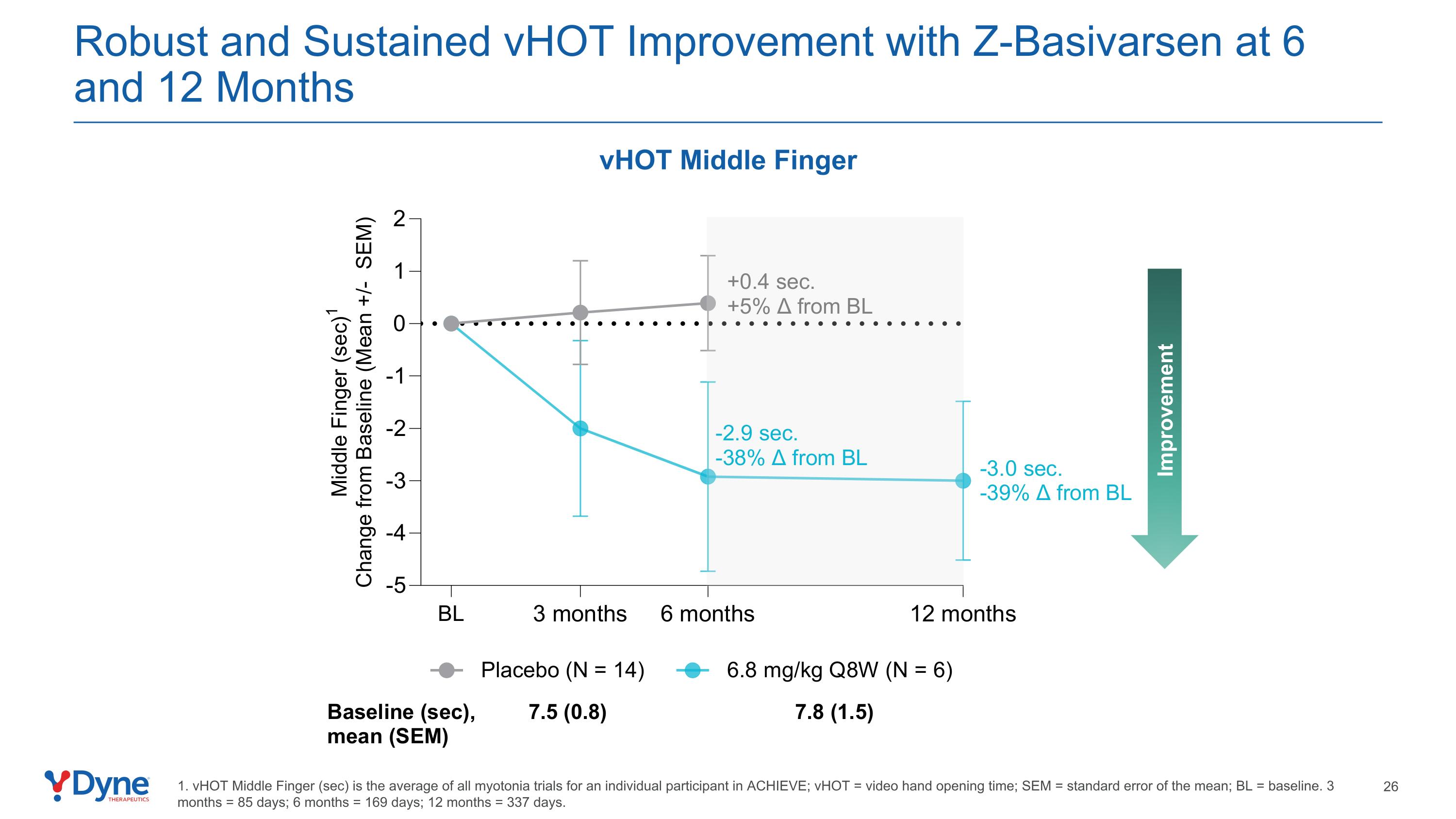
Robust and Sustained vHOT Improvement with Z-Basivarsen at 6 and 12 Months vHOT Middle Finger Improvement 1. vHOT Middle Finger (sec) is the average of all myotonia trials for an individual participant in ACHIEVE; vHOT = video hand opening time; SEM = standard error of the mean; BL = baseline. 3 months = 85 days; 6 months = 169 days; 12 months = 337 days.
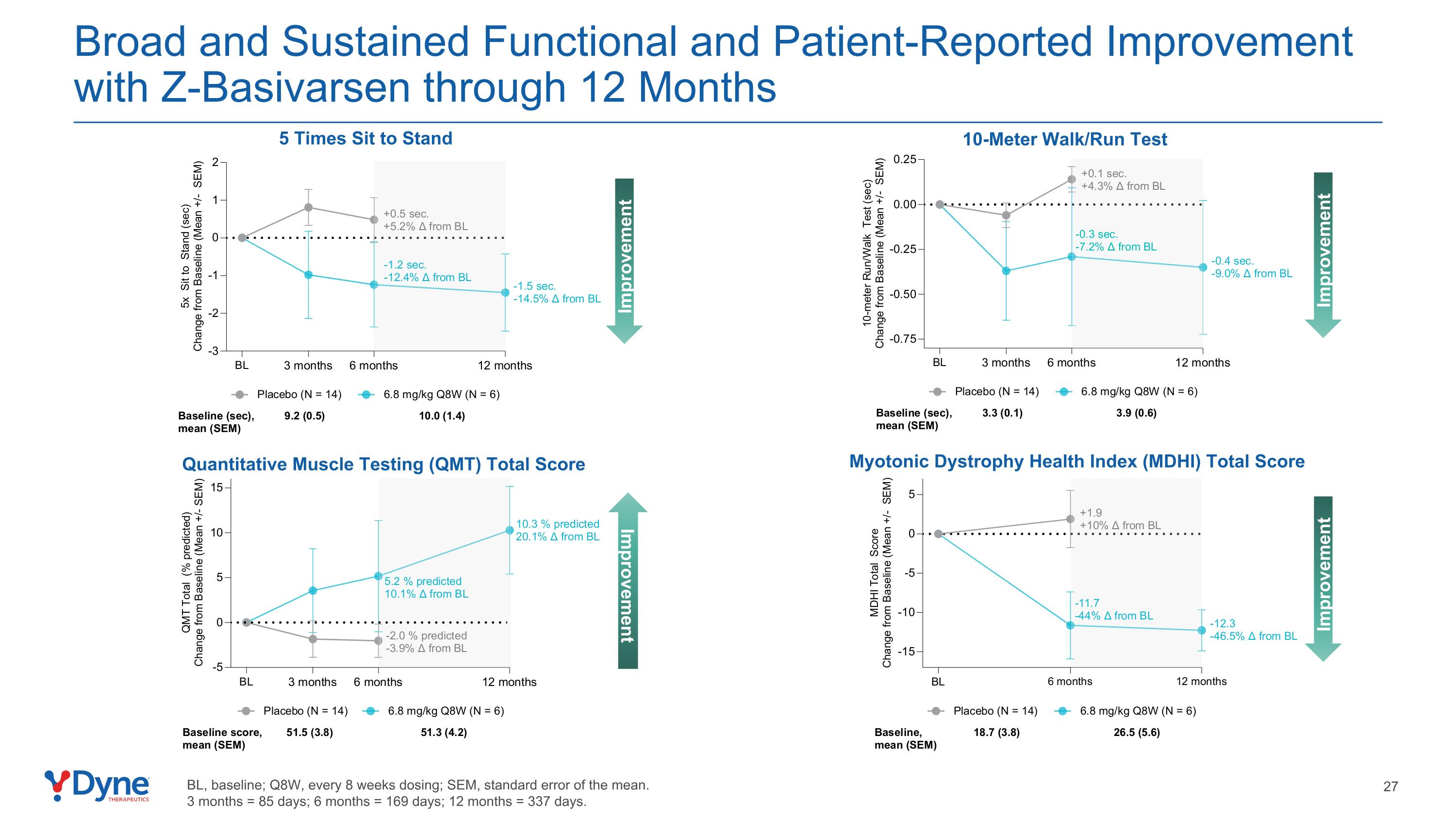
Broad and Sustained Functional and Patient-Reported Improvement with Z-Basivarsen through 12 Months Quantitative Muscle Testing (QMT) Total Score Improvement 10-Meter Walk/Run Test 5 Times Sit to Stand Improvement Improvement Improvement Myotonic Dystrophy Health Index (MDHI) Total Score BL, baseline; Q8W, every 8 weeks dosing; SEM, standard error of the mean. 3 months = 85 days; 6 months = 169 days; 12 months = 337 days.
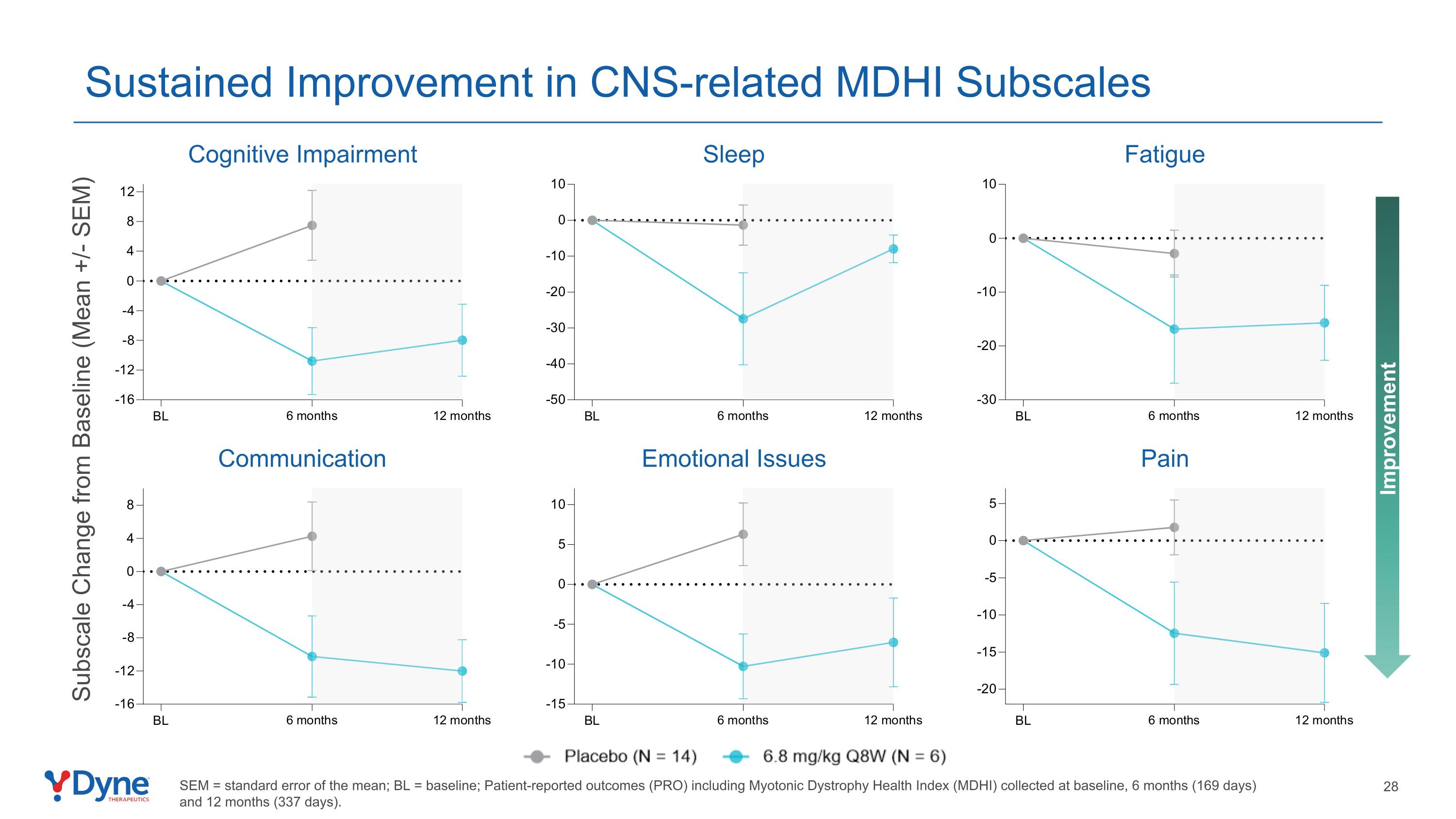
Cognitive Impairment Communication Emotional Issues Sleep Pain Fatigue Improvement Subscale Change from Baseline (Mean +/- SEM) SEM = standard error of the mean; BL = baseline; Patient-reported outcomes (PRO) including Myotonic Dystrophy Health Index (MDHI) collected at baseline, 6 months (169 days) and 12 months (337 days). Sustained Improvement in CNS-related MDHI Subscales
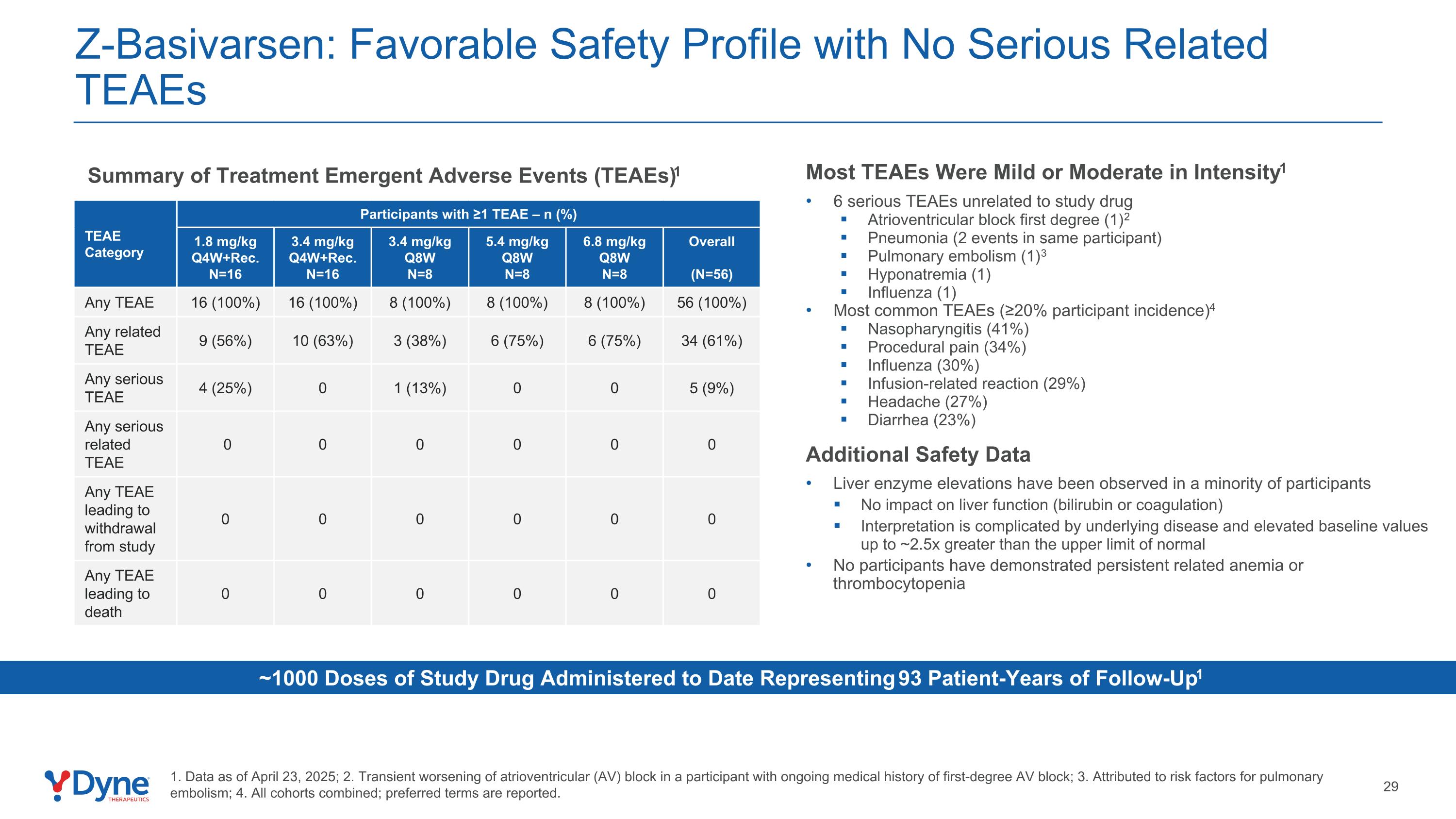
TEAE Category Participants with ≥1 TEAE – n (%) 1.8 mg/kg Q4W+Rec. N=16 3.4 mg/kg Q4W+Rec. N=16 3.4 mg/kg Q8W N=8 5.4 mg/kg Q8W N=8 6.8 mg/kg Q8W N=8 Overall (N=56) Any TEAE 16 (100%) 16 (100%) 8 (100%) 8 (100%) 8 (100%) 56 (100%) Any related TEAE 9 (56%) 10 (63%) 3 (38%) 6 (75%) 6 (75%) 34 (61%) Any serious TEAE 4 (25%) 0 1 (13%) 0 0 5 (9%) Any serious related TEAE 0 0 0 0 0 0 Any TEAE leading to withdrawal from study 0 0 0 0 0 0 Any TEAE leading to death 0 0 0 0 0 0 Most TEAEs Were Mild or Moderate in Intensity1 6 serious TEAEs unrelated to study drug Atrioventricular block first degree (1)2 Pneumonia (2 events in same participant) Pulmonary embolism (1)3 Hyponatremia (1) Influenza (1) Most common TEAEs (≥20% participant incidence)4 Nasopharyngitis (41%) Procedural pain (34%) Influenza (30%) Infusion-related reaction (29%) Headache (27%) Diarrhea (23%) Additional Safety Data Liver enzyme elevations have been observed in a minority of participants No impact on liver function (bilirubin or coagulation) Interpretation is complicated by underlying disease and elevated baseline values up to ~2.5x greater than the upper limit of normal No participants have demonstrated persistent related anemia or thrombocytopenia Z-Basivarsen: Favorable Safety Profile with No Serious Related TEAEs Summary of Treatment Emergent Adverse Events (TEAEs)1 ~1000 Doses of Study Drug Administered to Date Representing 93 Patient-Years of Follow-Up1 1. Data as of April 23, 2025; 2. Transient worsening of atrioventricular (AV) block in a participant with ongoing medical history of first-degree AV block; 3. Attributed to risk factors for pulmonary embolism; 4. All cohorts combined; preferred terms are reported.

Transforming Dyne into a Commercial Organization as Early as 2027 Q1 2025 Completed enrollment of Registrational Expansion Cohort Early Q2 2026 Complete enrollment planned for Registrational Expansion Cohort December 2025 Positive topline results from Registrational Expansion Cohort Q1 2027 Data planned for Registrational Expansion Cohort Q2 2026 Planned submission for U.S. Accelerated Approval Early Q3 2027 Potential submission for U.S. Accelerated Approval Q1 2027 Potential U.S. launch, assuming Priority Review Q1 2028 Potential U.S. launch, assuming Priority Review 1st potential launch for Dyne DMD = Duchenne muscular dystrophy; DM1 = myotonic dystrophy type 1. One capital efficient operating model to support multiple potential commercial launches Z-Rostudirsen for Exon 51 DMD Z-Basivarsen for DM1
Q&A