.2

The next wave of targeted therapies in oncology JP Morgan Healthcare Conference January 12, 2026
.2

The next wave of targeted therapies in oncology JP Morgan Healthcare Conference January 12, 2026

Disclaimer and safe harbor statement Certain statements in this presentation may be considered forward-looking statements. Forward-looking statements generally relate to future events, Tango’s future financial and operating performance, goals, expectations, beliefs, development plans, as well as development and clinical trial objectives for Tango’s product pipeline (as individual therapies and combination therapies with other party’s drugs). In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “path”, “achievable”, “milestones”, “goal”, “forecast”, “estimate”, “potential”, “anticipate”, “believe”, “predict”, or “continue”, or the negatives of these terms or variations of them or similar terminology. For example, express or implied statements concerning the following include or constitute forward-looking statements: the potential for the Company to have best-in-class oral PRMT5 inhibitors; the Company’s belief that it has a significant opportunity to treat multiple common cancers; the Company’s expected cash runway into 2028; the potential for vopimetostat to have best-in-class tolerability; the Company’s expectations regarding its PRMT5 inhibitors as compared to competitor molecules, including in terms of safety and tolerability; the anticipated milestones and timing for the Company’s drug programs (including registrational studies), including the timing for clinical trial initiation, enrollment, patient dosing, dose escalation, dose expansion, and clinical updates; of initial, interim, and final safety and efficacy or clinical activity data and results from clinical trial(s); the Company’s expectations regarding a registrational trial for vopimetostat; the Company’s plans for and timing of combination trials for vopimetostat and TNG456, including with RAS(ON) inhibitors for vopimetostat and with abemaciclib for TNG456; the Company’s belief that vopimetostat has the potential to transform care in front line pancreatic cancer; the Company’s expectations regarding the combination study with RAS(ON) inhibitors, including future enrollment, study design, and the ability of early signs of activity and tolerability to translate to positive results; the Company’s expectations regarding TNG456’s predicted brain exposure may not be realized; the expected benefits of the Company’s development candidates and other product candidates (including for combination studies); the Company’s expectations around the size and value of the potential patient population for PRMT5 inhibitors (including for lung and pancreatic cancers); potential combination strategies and uses for PRMT5 inhibitors, including vopimetostat and TNG456; the development plans for the PRMT5 franchise (including future single agent and combination clinical trials); future clinical trial designs; TNG260 future clinical trials strategy and implementation; expectations regarding the benefits and success of collaborations and combination clinical trials; and the anticipated benefits of its current and future product candidates; expectations around TNG456’s clinical efficacy, including its potential to treat glioblastoma and expectations around the brain exposure required for clinical efficacy; the development and regulatory pathway for vopimetostat, TNG456, or TNG961; the Company’s belief that TN961 may be a first-in-class HBS1L degrader; and the Company’s belief that there is potential for single agent and vopimetostat combination activity for TNG961. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Tango and its management at the time of this presentation, are inherently uncertain. Drug development, clinical trials and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: Tango has a limited operating history and has not generated any revenue to date from drug sales, and may never become profitable; future clinical trial data releases may differ materially from initial or interim data from our current and future clinical trials; Tango has limited experience with conducting clinical trials (and does and will rely on third parties to operate its clinical trials) and may not be able to commence any clinical trial, enroll and dose patients when expected and may not generate results in the anticipated timeframe (or at all); dosing (including dose expansion) in clinical trials may need be delayed or may be stopped for various reasons, including due to any potential issues at the site, safety issues or supply disruptions; any significant changes required to be made to an applicable IND application or protocol could significantly delay on-going clinical trials); the benefits of Tango pipeline products (stand-alone and as potential combination therapies) that are seen in preclinical experiments may not be present in clinical trials or in use commercially or may not be safe and/or effective in humans (and Tango or a third-party may not be able to obtain approval or commercial sales of any stand-alone or combination therapies); Tango has incurred significant operating losses and anticipates continued losses for the foreseeable future; Tango will need to raise capital in the future and if it is unable to raise capital when needed or on attractive terms, the Company would be forced to delay, reduce, or eliminate or discontinue some development programs or future commercialization efforts; Tango may be unable to advance its preclinical development programs into and through the clinic for safety or efficacy reasons or experience significant delays in doing so as a result of factors beyond Tango’s control; the expected benefits of our product candidates in patients as single agents and/or in combination may not be realized; the Company may experience delays or difficulties in the initiation, enrollment, or dosing of patients in clinical trials or the announcement of initial, interim, or final clinical trial results; Tango’s approach to the discovery and development of product candidates is novel and unproven, which makes it difficult to predict the time, cost of development, and likelihood of successfully developing any products; Tango may not identify or discover development candidates (including next generation products) or may expend a portion of its limited resources to pursue a particular product candidate or indications and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success; delays or difficulties in the initiation, enrollment or dosing of patients in clinical trials could delay or prevent receipt of regulatory approvals or reporting trial results; our product candidates may cause adverse or other undesirable side effects that could, among other things, delay or prevent regulatory approval; our dependence on third parties for conducting clinical trials and producing drug product and drug substance (including the potential impact of the BIOSECURE Act on our suppliers); the impact of trade restrictions such as sanctions, tariffs, reciprocal and retaliatory tariffs, legal actions or enforcement and inflation rates on our business, financial condition, and results of operations; inadequate funding for or disruptions at the U.S. Food and Drug Administration or other government agencies may slow the time necessary for new drugs to be reviewed and/or approved or prevent these agencies from performing business functions on which the operation of our business may rely (which could negatively impact our business), and uncertainty around the U.S. presidential administration’s approach to governmental agencies and/or product candidate approvals may present challenges for our business or create a more costly environment in which to pursue the development of new therapeutic candidates; our ability to obtain and maintain patent and other intellectual property protection for our technology and product candidates or the scope of intellectual property protection obtained is not sufficiently broad; and delays and other impacts on product development and clinical trials from public health events. Additional information concerning risks, uncertainties and assumptions can be found in Tango’s filings with the SEC, including the risk factors referenced in Tango’s Annual Report on Form 10-K for the year ended December 31, 2024, as may be supplemented and/or modified by its most recent Quarterly Report on Form 10-Q. You should not place undue reliance on forward-looking statements in this presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Tango specifically disclaims any duty to update these forward-looking statements. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Tango’s own internal estimates and research. In addition, market data included in this presentation involve assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Tango believes its internal research is reliable, such research has not been verified by any independent source.

Malte Peters, MD President and CEO to lead our next phase of growth Accomplished leader with deep clinical development expertise • Member of Tango Board of Directors since 2018 • Previous roles - Chief Research and Development Officer, MorphoSys AG and member of the Management Board - Global Head of Clinical Development, Biopharmaceuticals Business Unit, Sandoz International - Global Clinical Head, Oncology Translational Medicine, Novartis Oncology • Track record of innovative global regulatory approvals in particular of combination regimens
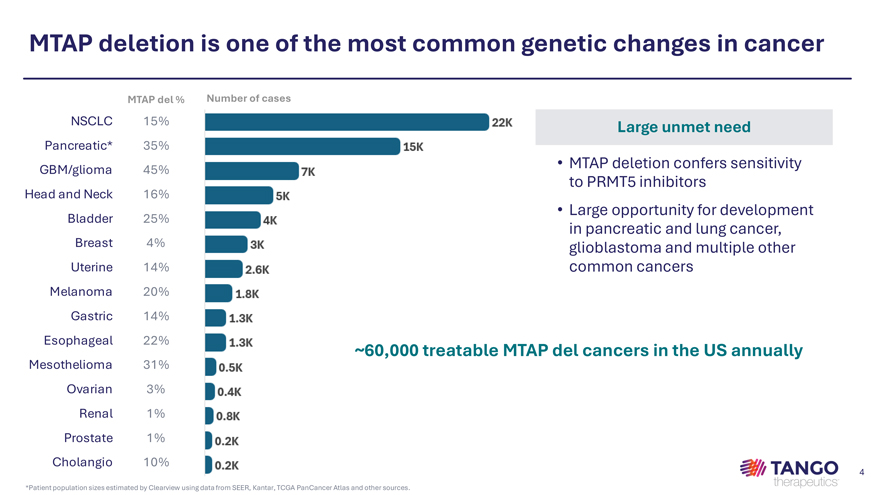
MTAP deletion is one of the most common genetic changes in cancer MTAP del % Number of cases NSCLC 15% Large unmet need Pancreatic* 35% GBM/glioma 45% • MTAP deletion confers sensitivity to PRMT5 inhibitors Head and Neck 16% • Large opportunity for development Bladder 25% in pancreatic and lung cancer, Breast 4% glioblastoma and multiple other Uterine 14% common cancers Melanoma 20% Gastric 14% Esophageal 22% Mesothelioma 31% ~60,000 treatable MTAP del cancers in the US annually Ovarian 3% Renal 1% Prostate 1%
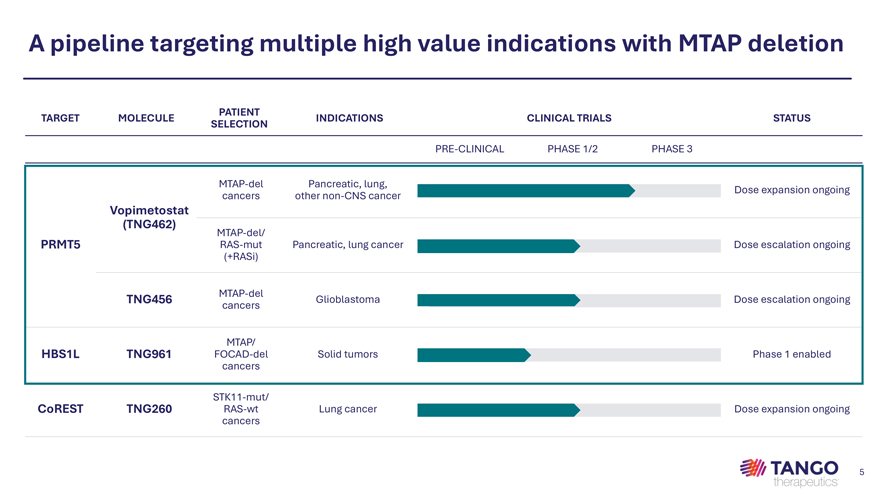
A pipeline targeting multiple high value indications with MTAP deletion PATIENT TARGET MOLECULE INDICATIONS CLINICAL TRIALS STATUS SELECTION PRE-CLINICAL PHASE 1/2 PHASE 3 MTAP-del Pancreatic, lung, Dose expansion ongoing cancers other non-CNS cancer Vopimetostat (TNG462) MTAP-del/ PRMT5 RAS-mut Pancreatic, lung cancer Dose escalation ongoing (+RASi) MTAP-del TNG456 Glioblastoma Dose escalation ongoing cancers MTAP/ HBS1L TNG961 FOCAD-del Solid tumors Phase 1 enabled cancers STK11-mut/ CoREST TNG260 RAS-wt Lung cancer Dose expansion ongoing cancers
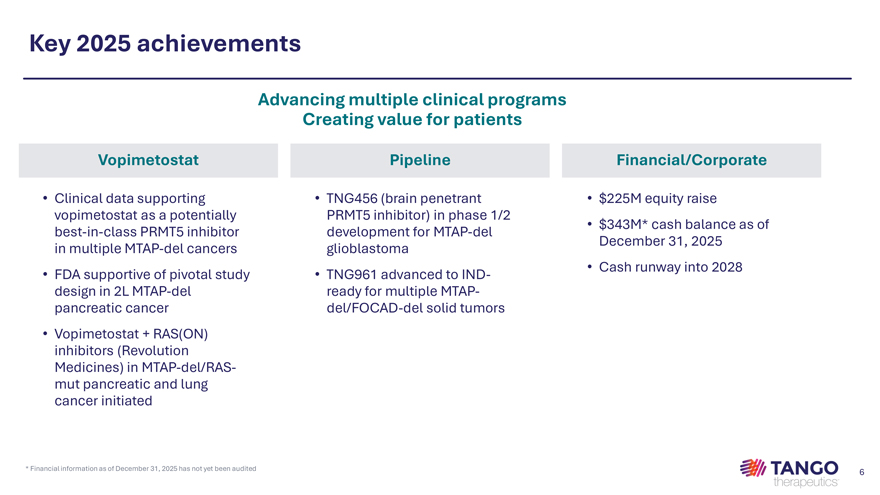
Key 2025 achievements Advancing multiple clinical programs Creating value for patients Vopimetostat Pipeline Financial/Corporate • Clinical data supporting • TNG456 (brain penetrant • $225M equity raise vopimetostat as a potentially PRMT5 inhibitor) in phase 1/2 • $343M* cash balance as of best-in-class PRMT5 inhibitor development for MTAP-del December 31, 2025 in multiple MTAP-del cancers glioblastoma • Cash runway into 2028 • FDA supportive of pivotal study • TNG961 advanced to IND-design in 2L MTAP-del ready for multiple MTAP-pancreatic cancer del/FOCAD-del solid tumors • Vopimetostat + RAS(ON) inhibitors (Revolution Medicines) in MTAP-del/RAS-mut pancreatic and lung cancer initiated
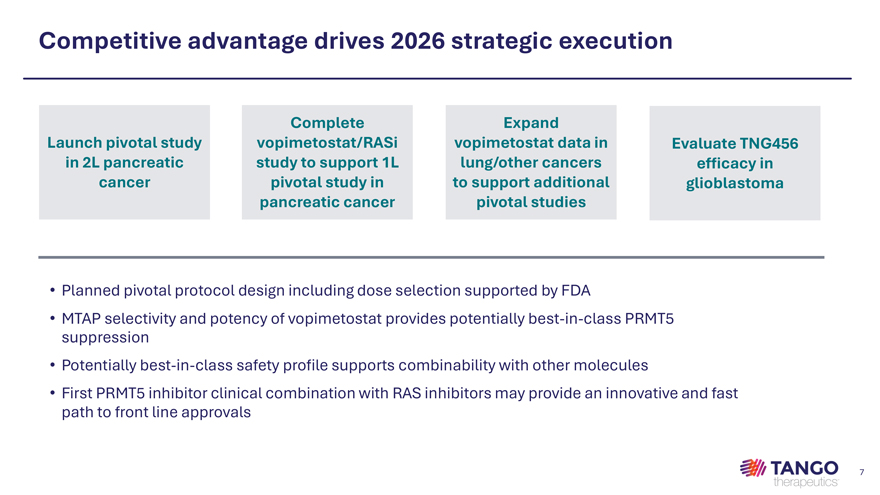
Competitive advantage drives 2026 strategic execution Complete Expand Launch pivotal study vopimetostat/RASi vopimetostat data in Evaluate TNG456 in 2L pancreatic study to support 1L lung/other cancers efficacy in cancer pivotal study in to support additional glioblastoma pancreatic cancer pivotal studies • Planned pivotal protocol design including dose selection supported by FDA • MTAP selectivity and potency of vopimetostat provides potentially best-in-class PRMT5 suppression • Potentially best-in-class safety profile supports combinability with other molecules • First PRMT5 inhibitor clinical combination with RAS inhibitors may provide an innovative and fast path to front line approvals
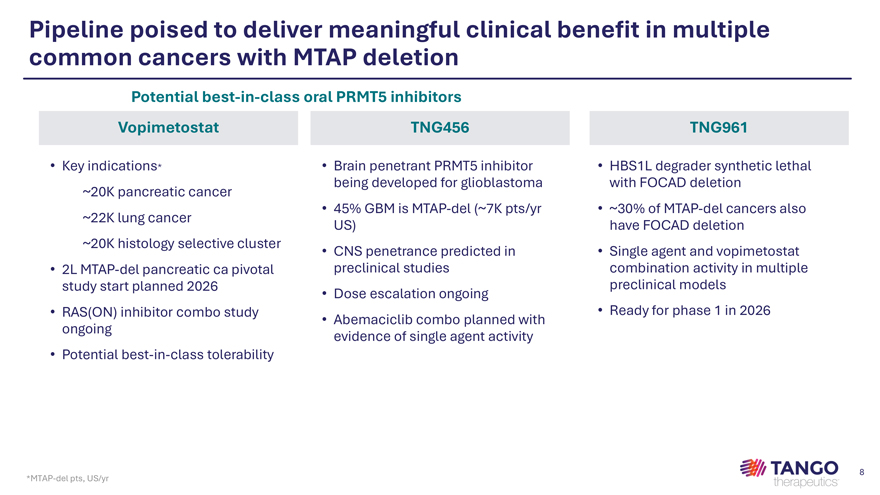
Pipeline poised to deliver meaningful clinical benefit in multiple common cancers with MTAP deletion Potential best-in-class oral PRMT5 inhibitors Vopimetostat TNG456 TNG961 • Key indications* • Brain penetrant PRMT5 inhibitor • HBS1L degrader synthetic lethal being developed for glioblastoma with FOCAD deletion ~20K pancreatic cancer • 45% GBM is MTAP-del (~7K pts/yr • ~30% of MTAP-del cancers also ~22K lung cancer US) have FOCAD deletion ~20K histology selective cluster • CNS penetrance predicted in • Single agent and vopimetostat • 2L MTAP-del pancreatic ca pivotal preclinical studies combination activity in multiple study start planned 2026 preclinical models • Dose escalation ongoing • RAS(ON) inhibitor combo study • Ready for phase 1 in 2026 • Abemaciclib combo planned with ongoing evidence of single agent activity • Potential best-in-class tolerability
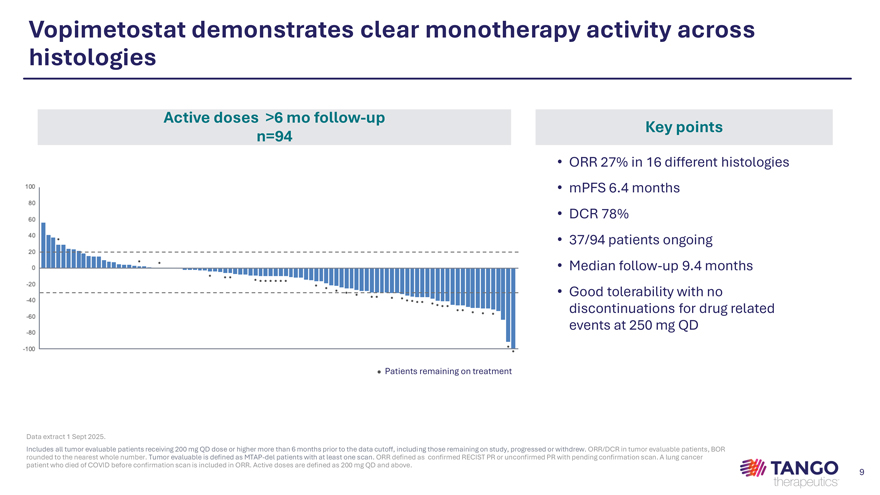
Vopimetostat demonstrates clear monotherapy activity across histologies Active doses >6 mo follow-up Key points n=94 • ORR 27% in 16 different histologies • mPFS 6.4 months • DCR 78% • 37/94 patients ongoing • Median follow-up 9.4 months • Good tolerability with no discontinuations for drug related events at 250 mg QD • Patients remaining on treatment
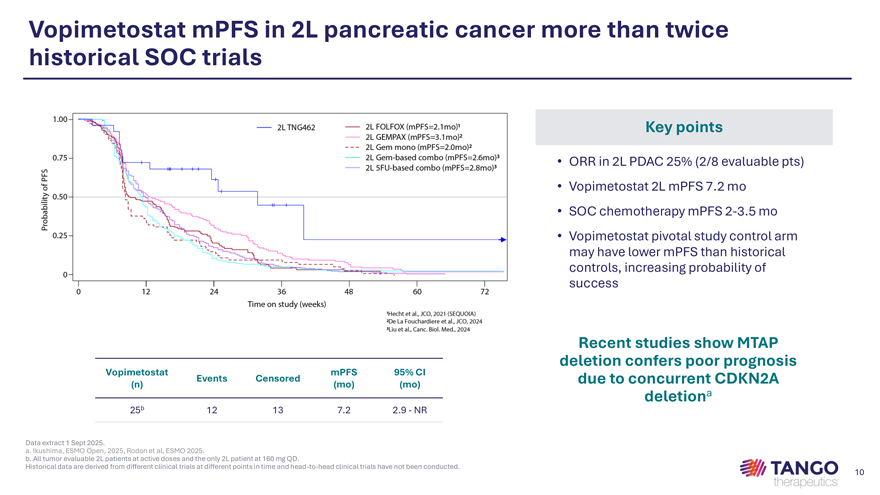
Vopimetostat mPFS in 2L pancreatic cancer more than twice historical SOC trials Key points • ORR in 2L PDAC 25% (2/8 evaluable pts) • Vopimetostat 2L mPFS 7.2 mo • SOC chemotherapy mPFS 2-3.5 mo • Vopimetostat pivotal study control arm may have lower mPFS than historical controls, increasing probability of success Recent studies show MTAP Vopimetostat mPFS 95% CI deletion confers poor prognosis Events Censored due to concurrent CDKN2A (n) (mo) (mo) deletiona 25b 12 13 7.2 2.9—NR Data extract 1 Sept 2025.
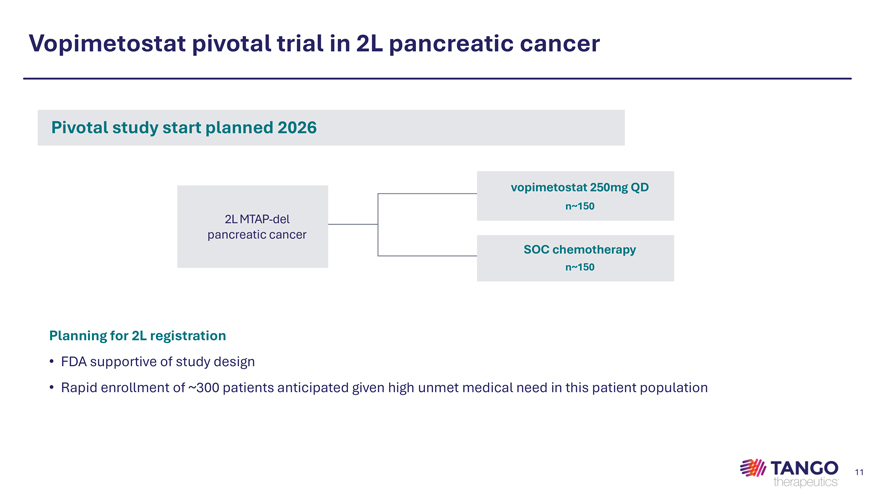
Vopimetostat pivotal trial in 2L pancreatic cancer Pivotal study start planned 2026 vopimetostat 250mg QD n~150 2L MTAP-del pancreatic cancer SOC chemotherapy n~150 Planning for 2L registration • FDA supportive of study design • Rapid enrollment of ~300 patients anticipated given high unmet medical need in this patient population
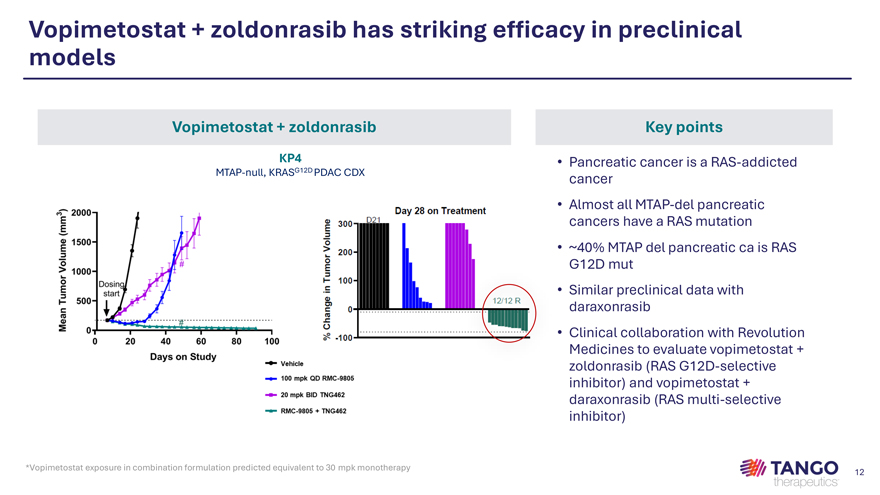
Vopimetostat + zoldonrasib has striking efficacy in preclinical models Vopimetostat + zoldonrasib Key points KP4 • Pancreatic cancer is a RAS-addicted MTAP-null, KRASG12D PDAC CDX cancer • Almost all MTAP-del pancreatic cancers have a RAS mutation • ~40% MTAP del pancreatic ca is RAS G12D mut • Similar preclinical data with daraxonrasib • Clinical collaboration with Revolution Medicines to evaluate vopimetostat + zoldonrasib (RAS G12D-selective inhibitor) and vopimetostat + daraxonrasib (RAS multi-selective inhibitor)
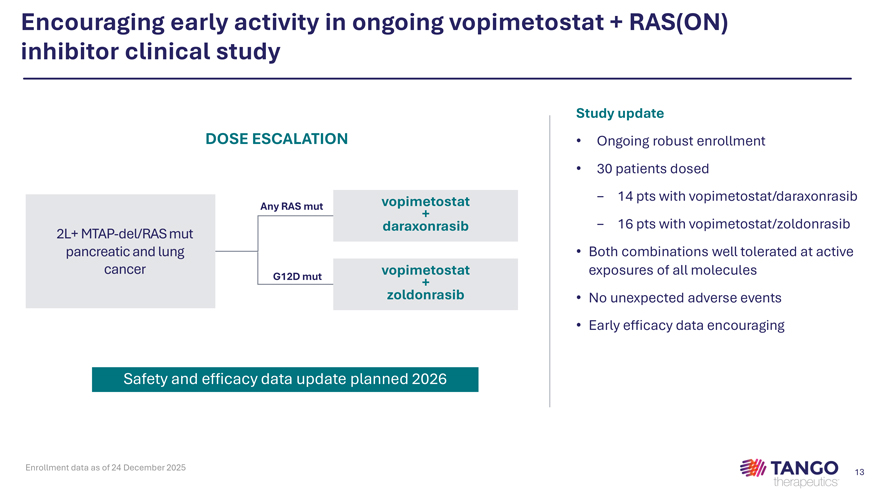
Encouraging early activity in ongoing vopimetostat + RAS(ON) inhibitor clinical study Study update DOSE ESCALATION • Ongoing robust enrollment • 30 patients dosed vopimetostat—14 pts with vopimetostat/daraxonrasib Any RAS mut + daraxonrasib—16 pts with vopimetostat/zoldonrasib 2L+ MTAP-del/RAS mut pancreatic and lung • Both combinations well tolerated at active cancer vopimetostat exposures of all molecules G12D mut + zoldonrasib • No unexpected adverse events • Early efficacy data encouraging Safety and efficacy data update planned 2026
Vopimetostat + RASi strategy for 1L pancreatic cancer RAS inhibitors likely to replace chemotherapy as SOC in 1L pancreatic cancer • More than 90% of pancreatic cancers are RAS-driven1 • Almost all MTAP-del pancreatic cancers have a RAS mutation • ~18k pts with MTAP-del and RAS-mut pancreatic cancer annually in the U.S. Potential path to vopimetostat 1L pivotal study with chemo-sparing RASi combinations • Encouraging early activity and robust enrollment in ongoing study in 2L+ pancreatic and lung cancer • Addition of 1L pancreatic cancer cohort to ongoing study planned 2026 • Phase 1/2 combo data in 2026 could support rapid move into 1L pancreatic cancer pivotal study - Single agent daraxonrasib 2L pancreatic ca ORR 29%, 1L pancreatic ca 47%, 2L+ lung ca 39%2 - Single agent zoldonrasib 2L+ pancreatic ca ORR 30%2 • First PRMT5 inhibitor being clinically evaluated in combination with RAS inhibitors
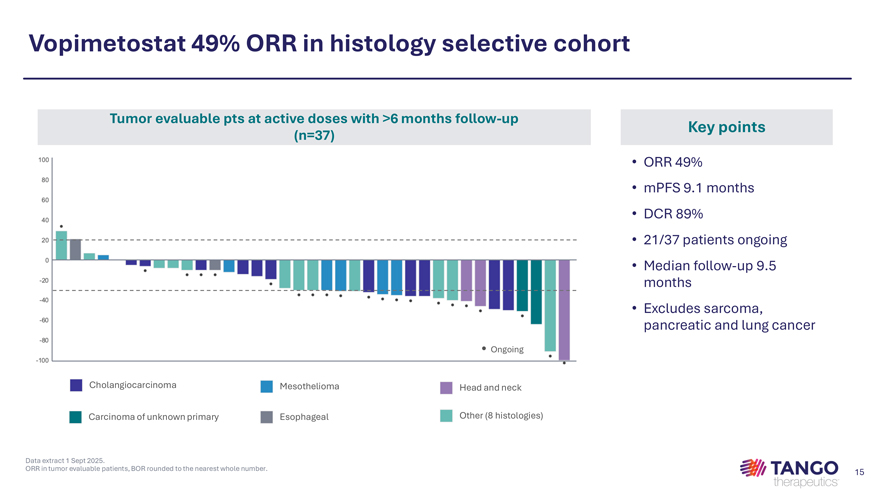
Vopimetostat 49% ORR in histology selective cohort Tumor evaluable pts at active doses with >6 months follow-up Key points (n=37) • ORR 49% • mPFS 9.1 months • DCR 89% • 21/37 patients ongoing • Median follow-up 9.5 months • Excludes sarcoma, pancreatic and lung cancer • Ongoing Cholangiocarcinoma Mesothelioma Head and neck Carcinoma of unknown primary Esophageal Other (8 histologies)
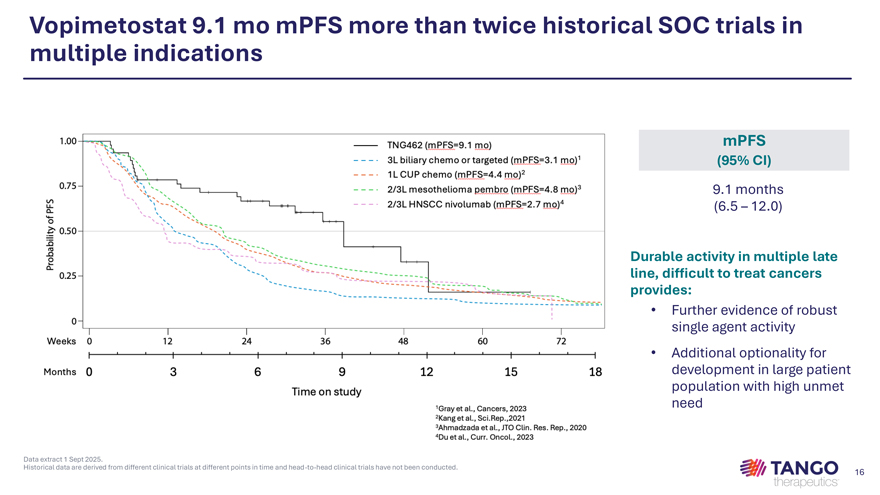
Vopimetostat 9.1 mo mPFS more than twice historical SOC trials in multiple indications mPFS (95% CI) 9.1 months (6.5 – 12.0) Durable activity in multiple late line, difficult to treat cancers provides: • Further evidence of robust single agent activity • Additional optionality for development in large patient population with high unmet need

TNG456 PRMT5 inhibition being developed for MTAP-deleted glioblastoma
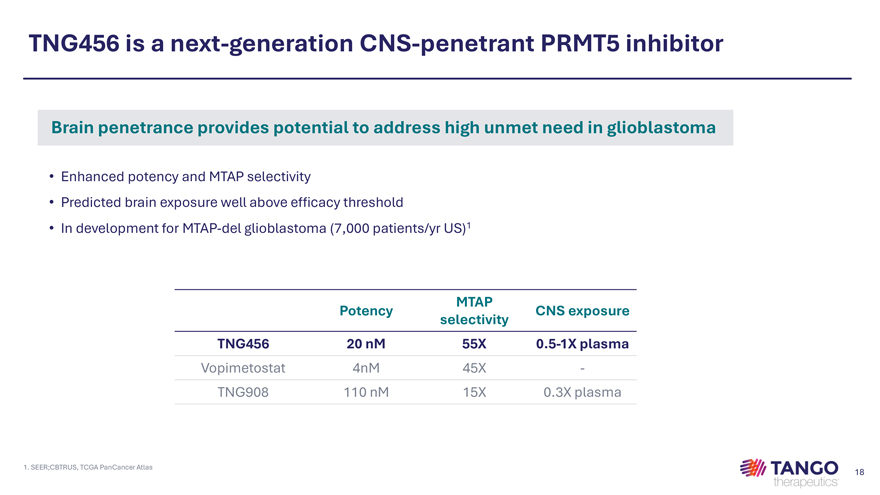
TNG456 is a next-generation CNS-penetrant PRMT5 inhibitor Brain penetrance provides potential to address high unmet need in glioblastoma • Enhanced potency and MTAP selectivity • Predicted brain exposure well above efficacy threshold • In development for MTAP-del glioblastoma (7,000 patients/yr US)1 MTAP Potency CNS exposure selectivity TNG456 20 nM 55X 0.5-1X plasma Vopimetostat 4nM 45X -TNG908 110 nM 15X 0.3X plasma
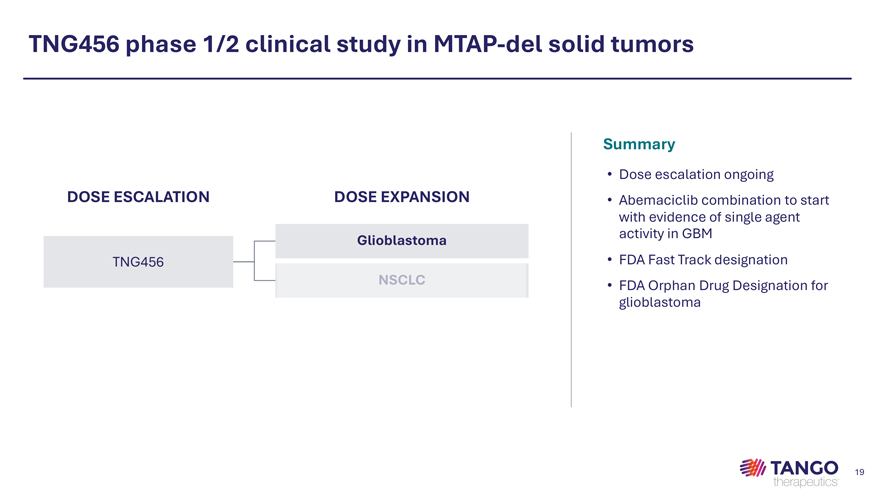
TNG456 phase 1/2 clinical study in MTAP-del solid tumors Summary • Dose escalation ongoing DOSE ESCALATION DOSE EXPANSION • Abemaciclib combination to start with evidence of single agent Glioblastoma activity in GBM TNG456 • FDA Fast Track designation • FDA Orphan Drug Designation for glioblastoma

TNG961 HBS1L degrader for FOCAD-del/MTAP-del cancers
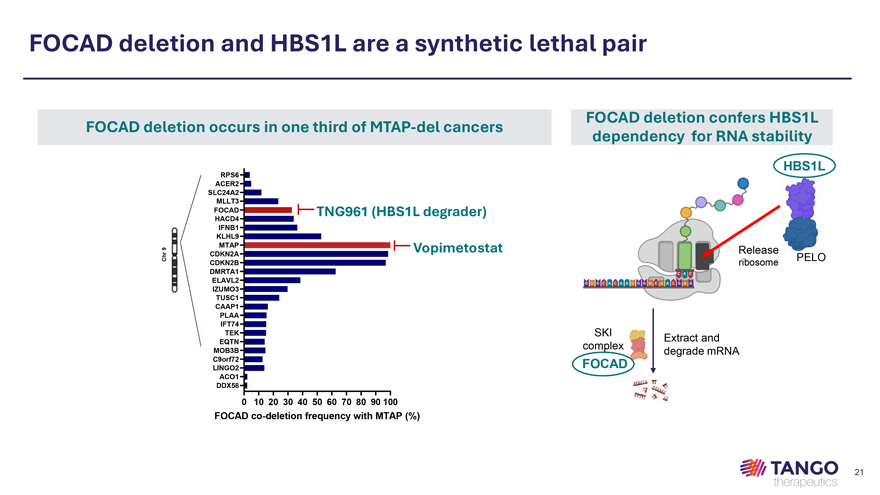
FOCAD deletion and HBS1L are a synthetic lethal pair FOCAD deletion confers HBS1L FOCAD deletion occurs in one third of MTAP-del cancers dependency for RNA stability HBS1L RPS6 ACER2 SLC24A2 MLLT3 FOCAD TNG961 (HBS1L degrader) HACD4 IFNB1 KLHL9 MTAP Vopimetostat CDKN2A Release PELO CDKN2B ribosome DMRTA1 ELAVL2 IZUMO3 TUSC1 CAAP1 PLAA IFT74 TEK SKI Extract and EQTN complex MOB3B degrade mRNA C9orf72 FOCAD LINGO2 ACO1 DDX58 0 10 20 30 40 50 60 70 80 90 100 FOCAD co-deletion frequency with MTAP (%)
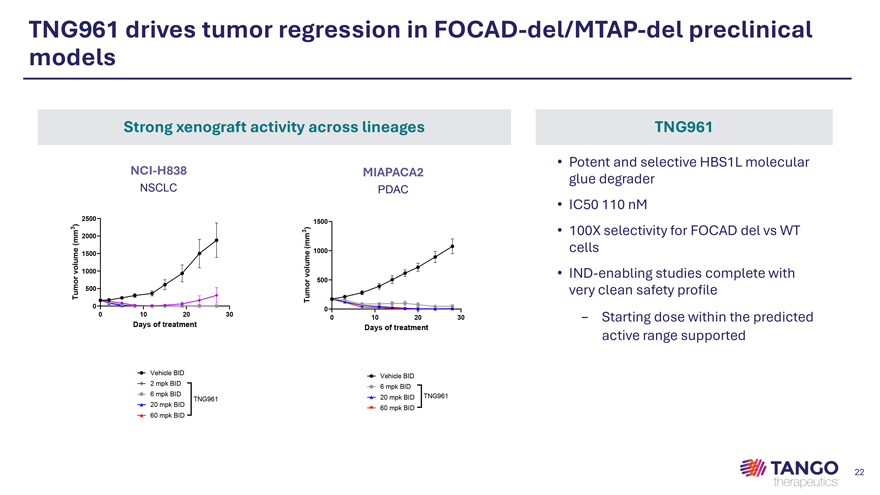
TNG961 drives tumor regression in FOCAD-del/MTAP-del preclinical models Strong xenograft activity across lineages TNG961 • Potent and selective HBS1L molecular NCI-H838 MIAPACA2 glue degrader NSCLC PDAC • IC50 110 nM 2500 ) 1500 3 3 ) • 100X selectivity for FOCAD del vs WT m m 2000 m ( ( m e e 1000 cells m 1500 u m l u vo 1000 r vol • IND-enabling studies complete with o r 500 m Tu 500 mo very clean safety profile 0 Tu 0 0 10 20 30 0 10 20 30—Starting dose within the predicted Days of treatment Days of treatment active range supported Vehicle BID Vehicle BID 2 mpk BID 6 mpk BID 6 mpk BID TNG961 TNG961 20 mpk BID 20 mpk BID 60 mpk BID 60 mpk BID
Multiple projected key milestones and strong balance sheet Clinical milestones Cash balance Vopimetostat lung ca data 2026 • $343M cash, cash equivalents and marketable Vopimetostat + daraxonrasib/zoldonrasib data securities as of December 31, 2025* 2026 • Cash runway into 2028 2L PDAC pivotal study start 2026 TNG456 data 2026

TANGO therapeutics