| Corporate Presentation September 2024 GAIN THERAPEUTICS Corporate Deck February 2026 NASDAQ: GANX |
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 22 Certain statements set forth in this presentation are forward-looking and reflect the Company’s plans, beliefs, expectations and current views with respect to, among other things, future events and financial performance (collectively referred to herein as “forward-looking statements”). Forward-looking statements can be identified by the fact that they do not relate strictly to historical or current facts and are often characterized by the use of words such as “believe,” “can,” “could,” “potential,” “plan,” “predict,” “goals,” “seek,” “should,” “may,” “may have,” “would,” “estimate,” “continue,” “anticipate,” “intend,” “expect” or by discussions of strategy, plans or intentions. Such forward-looking statements involve known and unknown risks, uncertainties, assumptions and other important factors that could cause our actual results, performance or achievements or industry results to differ materially from historical results or any future results, performance or achievements expressed, suggested or implied by such forward-looking statements. These include, but are not limited to, statements about the Company’s ability to develop, obtain regulatory approval for and commercialize its product candidates; the timing of future IND submissions, initiation of preclinical studies and clinical trials, and timing of expected clinical results for our product candidates; the Company’s success in early preclinical studies, which may not be indicative of results obtained in later studies or clinical trials; the outbreak of the novel strain of coronavirus disease, COVID- 19, which could adversely impact our business, including our preclinical studies and any future clinical trials; the potential benefits of our product candidates; the Company’s ability to obtain regulatory approval to commercialize our existing or any future product candidates; the Company’s ability to identify patients with the diseases treated by our product candidates, and to enroll patients in clinical trials; the success of our efforts to expand our pipeline of product candidates and develop marketable products through the use of our Magellan platform; the Company’s expectations regarding collaborations and other agreements with third parties and their potential benefits; the Company’s ability to obtain, maintain and protect our intellectual property; the Company’s reliance upon intellectual property licensed from third parties, including the license to use the Company’s Magellan platform; the Company’s ability to identify, recruit and retain key personnel; the Company’s financial performance; developments or projections relating to the Company’s competitors or industry; the impact of laws and regulations; the Company’s expectations regarding the time during which it will be an emerging growth company under the JOBS Act; and other factors and assumptions described in the Company’s public filings. These statements are based on the Company’s historical performance and on its current plans, estimates and projections in light of information currently available to the Company, and therefore, you should not place undue reliance on them. The inclusion of forward-looking information should not be regarded as a representation by the Company or any other personthat the future plans, estimates or expectations contemplated by us will be achieved. Forward-looking statements made in this presentation speak only asof the date of this presentation, and the Company undertakes no obligation to update them in light of new information or future events, except as required by law. You should carefully consider the above factors, as well as the factors discussed elsewhere in this presentation and our public filings, before deciding to invest in our common stock. The factors identified above should not be construed as an exhaustive list of factors that could affect the Company’s future results and should be read in conjunction with the other cautionary statements that are included in this presentation and our public filings. New risks and uncertainties arise from time to time, and it is impossible for the Company to predict those events or how they may affect the Company. If any of these trends, risks or uncertainties actually occurs or continues, the Company’s business, revenue and financial results could be harmed, the trading prices of its securities could decline, and you could lose all or part of your investment. All forward-looking statements attributable to the Company or persons actingon its behalf are expressly qualified in their entirety by this cautionary statement. Trademarks, Service Marks, and Trade Names This presentation includes our trademarks, and trade names, which are protected under applicable intellectual property laws. This presentation also may contain trademarks, service marks, trade names, and copyrights of other companies, which are the property of their respective owners. Solely for convenience, the trademarks, service marks, trade names, and copyrights referred to in this presentation are listed without the TM, SM, ©, and ® symbols, but we will assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors, if any, to these trademarks, service marks, trade names, and copyrights. Industry Information Market data and industry information used throughout this presentation are based on management’s knowledge of the industry and the good faith estimates of management. We also relied, to the extent available, upon management’s review of independent industry surveys and publications and other publicly available information prepared by a number of third-party sources. All of the market data and industry information used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Although we believe that these sources are reliable, we cannot guarantee the accuracy or completeness of this information, and we have not independently verified this information. While we believe the estimated market position, market opportunity and market size information included in this presentation are generally reliable, such information, which is derived in part from management’s estimates and beliefs, is inherently uncertain and imprecise. No representations or warranties are made by the Company or any of its affiliates as to the accuracy of any such statements or projections. Projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described above. These and other factors could cause results to differ materially from those expressed in our estimatesand beliefs and in the estimates prepared by independent parties. Forward-LookingStatements |
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 33 GANXCorporateHighlights Lead Program GT-02287 Moving to Phase 2 Clinical Evaluation in Parkinson’s Disease Multiple Assets in Discovery andPreclinical Development StrongIntellectual Property Estate Upcoming Milestones • Complete FDA IND review – 1H 2026 • GT-02287 Phase 1b study extension analysis – 2H 2026 • Commencement of Phase 2 in people with Parkinson’s disease – 2H 2026 • Assets discovered and developed with our proprietary Magellan AI platform • Initial disease targets include neurodegenerative diseases, lysosomal storage disorders including Gaucher disease as well as metabolic disease and solid tumors • Gain retains full WW rites to GT-02287 with composition of matter patent protection through 2038 not including Hatch Waxman extension for R&D • Patent applications for 5 NCEfamilies under review • Studies completed in both Phase 1a healthy volunteers and Phase1b open-label trial in PD (90-day) with 9-month open-label extension ongoing • Biomarker evidence from Phase 1b supports disease modifying hypothesis for GT-02287 |
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 44 Leadership: ExtensiveBiotechandPharma Experience Gene Mack, MBA Chief Executive Officer Jonas Hannestad, MD, PhD Chief Medical Officer Gianluca Fuggetta Senior Vice President, Finance Joanne Taylor, PhD SVPResearch Terenzio Ignoni, PharmD SVPTechnical Operations |
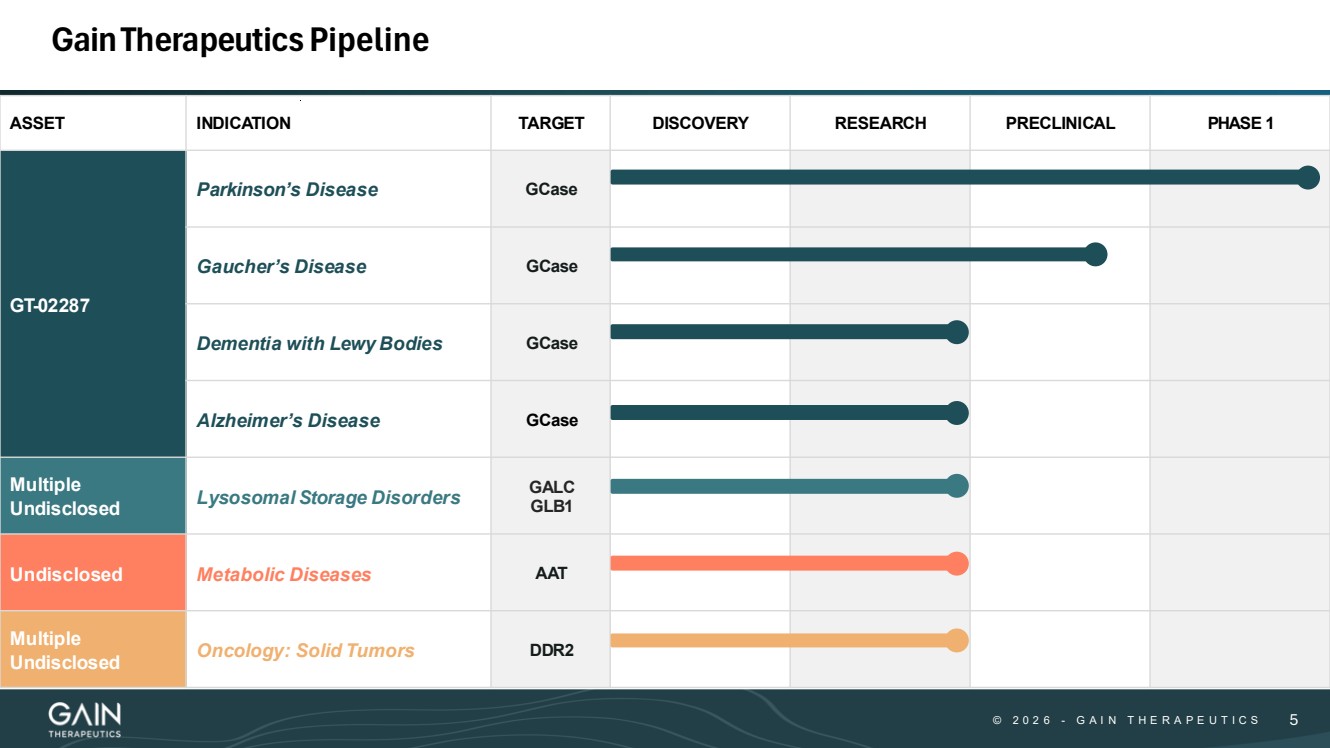
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 55 GainTherapeuticsPipeline ASSET INDICATION TARGET DISCOVERY RESEARCH PRECLINICAL PHASE 1 GT-02287 Parkinson’s Disease GCase Gaucher’s Disease GCase Dementia with Lewy Bodies GCase Alzheimer’s Disease GCase Multiple Undisclosed Lysosomal Storage Disorders GALC GLB1 Undisclosed Metabolic Diseases AAT Multiple Undisclosed Oncology: Solid Tumors DDR2 |
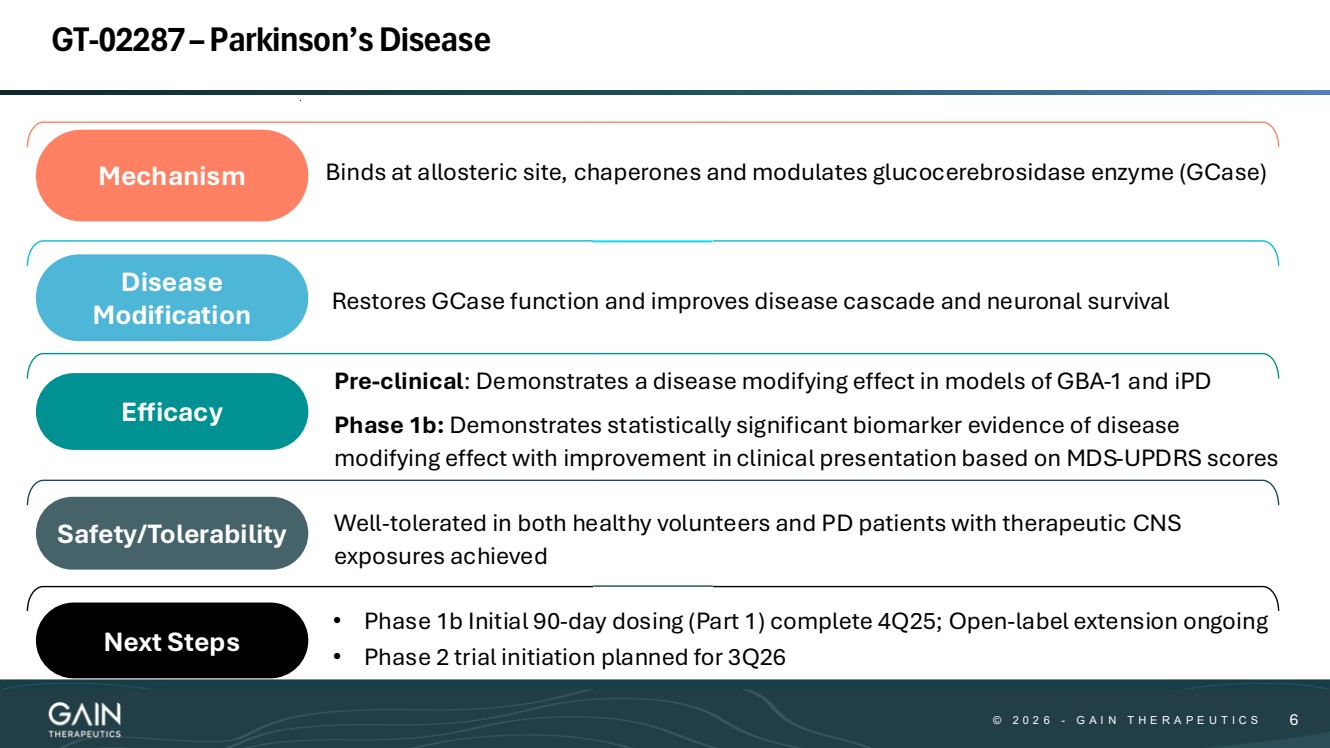
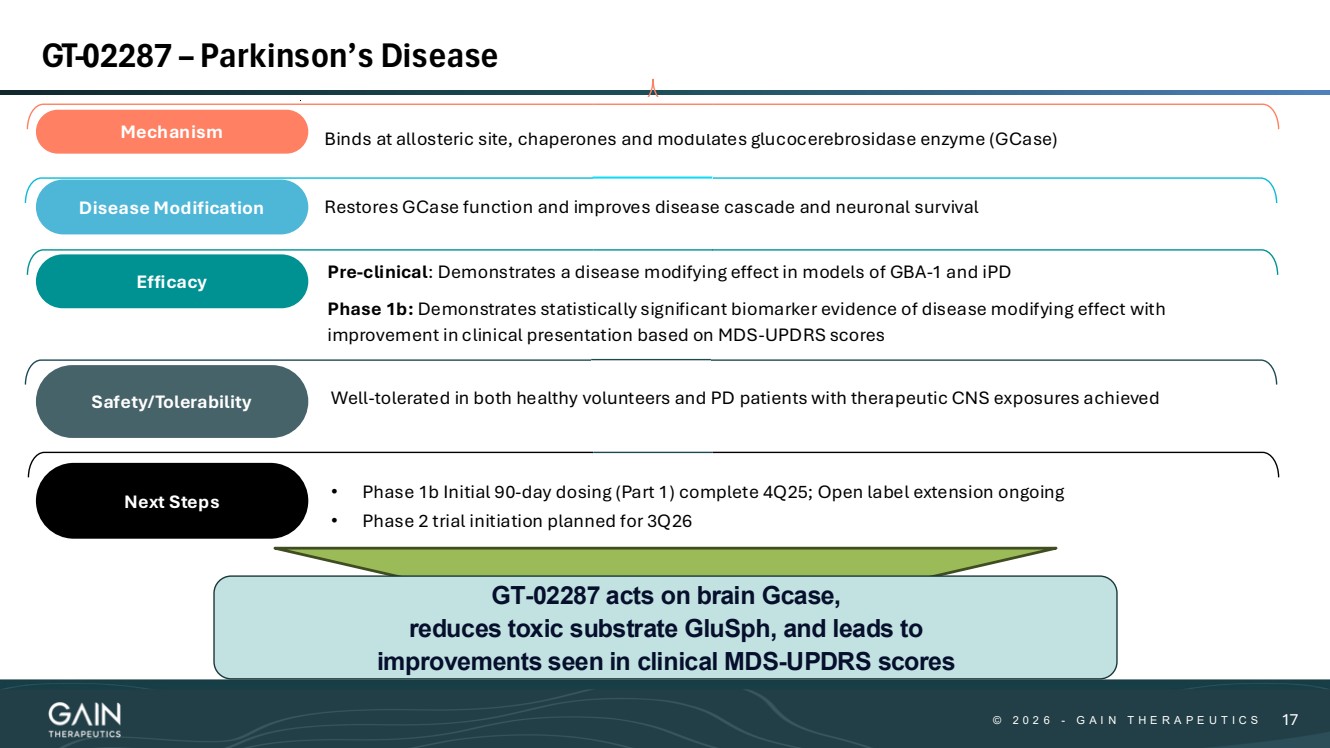
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 66 Well-tolerated in both healthy volunteers and PD patients with therapeutic CNS exposures achieved Mechanism Binds at allosteric site, chaperones and modulates glucocerebrosidase enzyme (GCase) Disease Modification Restores GCase function and improves disease cascade and neuronal survival Efficacy Safety/Tolerability • Phase 1b Initial 90-day dosing (Part 1) complete 4Q25; Open-label extension ongoing • Phase 2 trial initiation planned for 3Q26 Next Steps Pre-clinical: Demonstrates a disease modifying effect in models of GBA-1 and iPD Phase 1b: Demonstrates statistically significant biomarker evidence of disease modifying effect with improvement in clinical presentation based on MDS-UPDRS scores GT-02287 –Parkinson’s Disease |
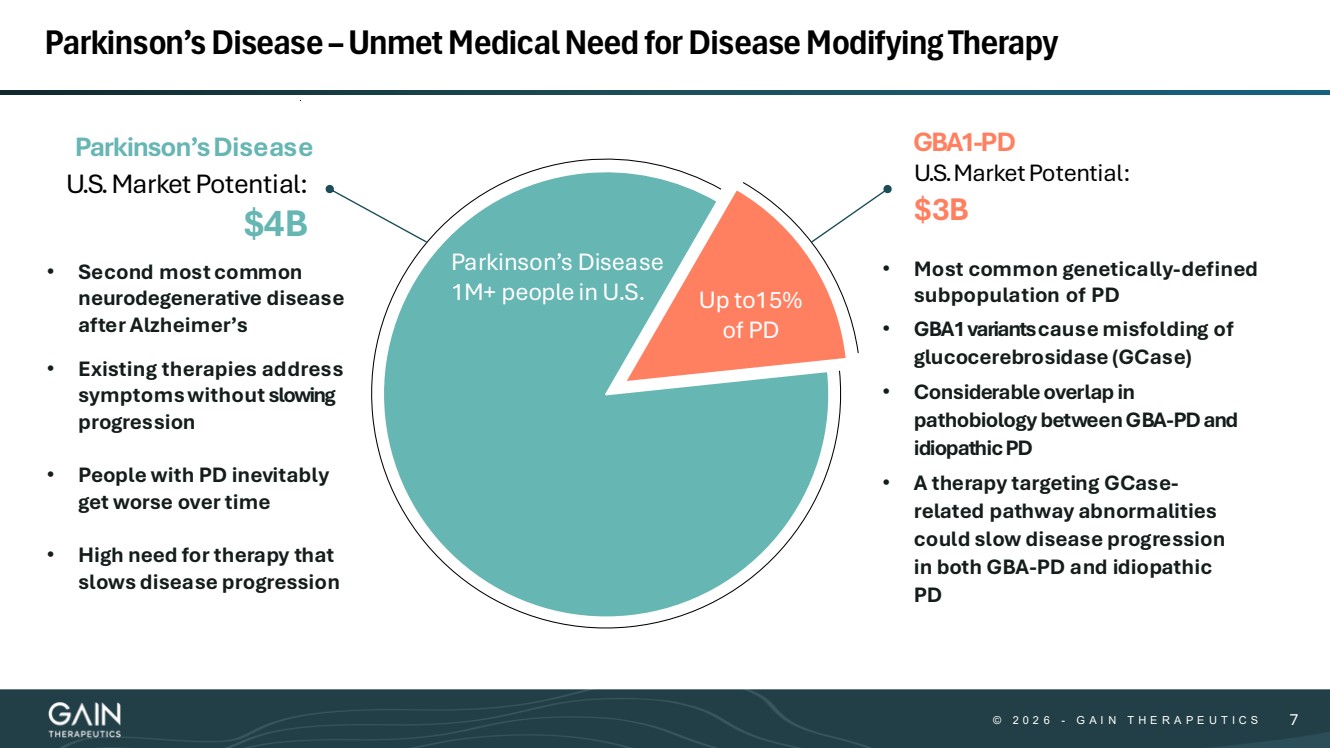
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 77 Parkinson’s Disease –Unmet Medical Need for Disease Modifying Therapy 7 Parkinson’sDisease GBA1-PD • Most common genetically-defined subpopulation of PD • GBA1variantscause misfolding of glucocerebrosidase (GCase) • Considerable overlap in pathobiology between GBA-PD and idiopathic PD • A therapy targeting GCase-related pathway abnormalities could slow disease progression in both GBA-PD and idiopathic PD Parkinson’s Disease 1M+ people in U.S. Up to15% of PD • Second most common neurodegenerative disease after Alzheimer’s • Existing therapies address symptoms without slowing progression • People with PD inevitably get worse over time • High need for therapy that slows disease progression U.S.MarketPotential: $4B U.S.MarketPotential: $3B |
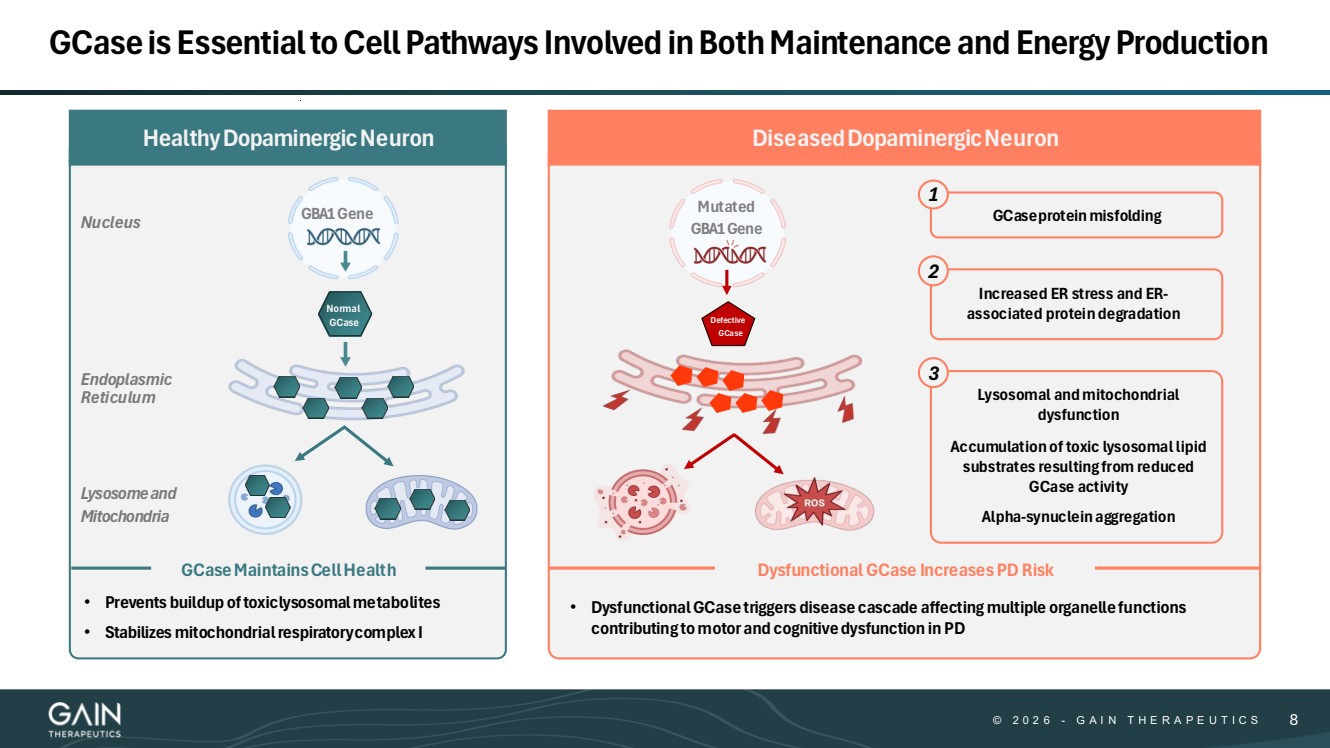
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 88 GCase is Essential to Cell Pathways Involved in Both Maintenance and Energy Production Nucleus Endoplasmic Reticulum Lysosomeand Mitochondria GBA1Gene Normal GCase GCaseMaintainsCellHealth • Prevents buildup oftoxiclysosomalmetabolites • Stabilizes mitochondrial respiratorycomplex I HealthyDopaminergicNeuron DiseasedDopaminergicNeuron Mutated GBA1Gene Dysfunctional GCase Increases PD Risk Defective GCase GCaseprotein misfolding Increased ER stress and ER-associated protein degradation Lysosomal and mitochondrial dysfunction Accumulation of toxic lysosomal lipid substrates resulting from reduced GCase activity Alpha-synuclein aggregation 1 2 3 • Dysfunctional GCase triggers disease cascade affecting multiple organelle functions contributing to motor and cognitive dysfunction in PD |
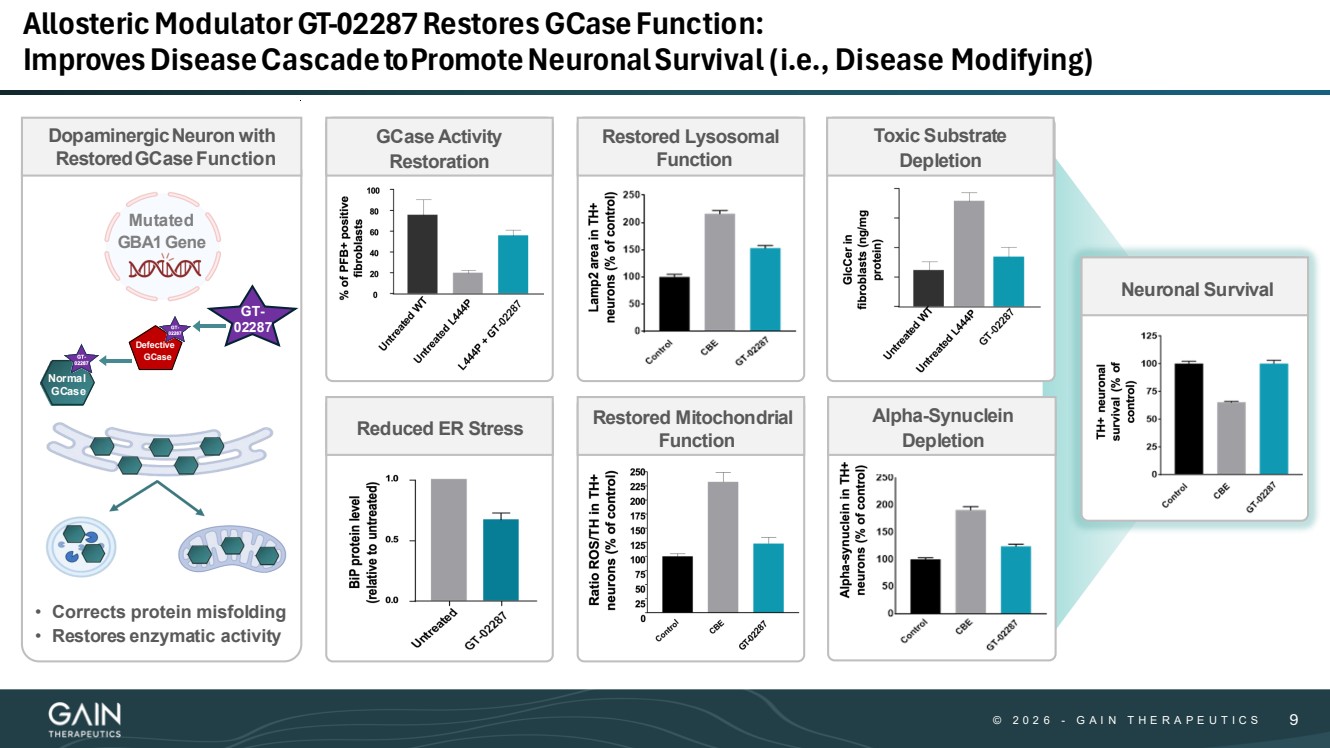
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 99 AllostericModulatorGT-02287RestoresGCaseFunction: ImprovesDiseaseCascadeto PromoteNeuronalSurvival (i.e., Disease Modifying) DopaminergicNeuron with RestoredGCaseFunction Mutated GBA1 Gene Defective GCase GT-02287 GCase Activity Restoration Reduced ER Stress Restored Lysosomal Function Restored Mitochondrial Function Toxic Substrate Depletion Alpha-Synuclein Depletion Neuronal Survival Normal GCase • Corrects protein misfolding • Restores enzymatic activity |
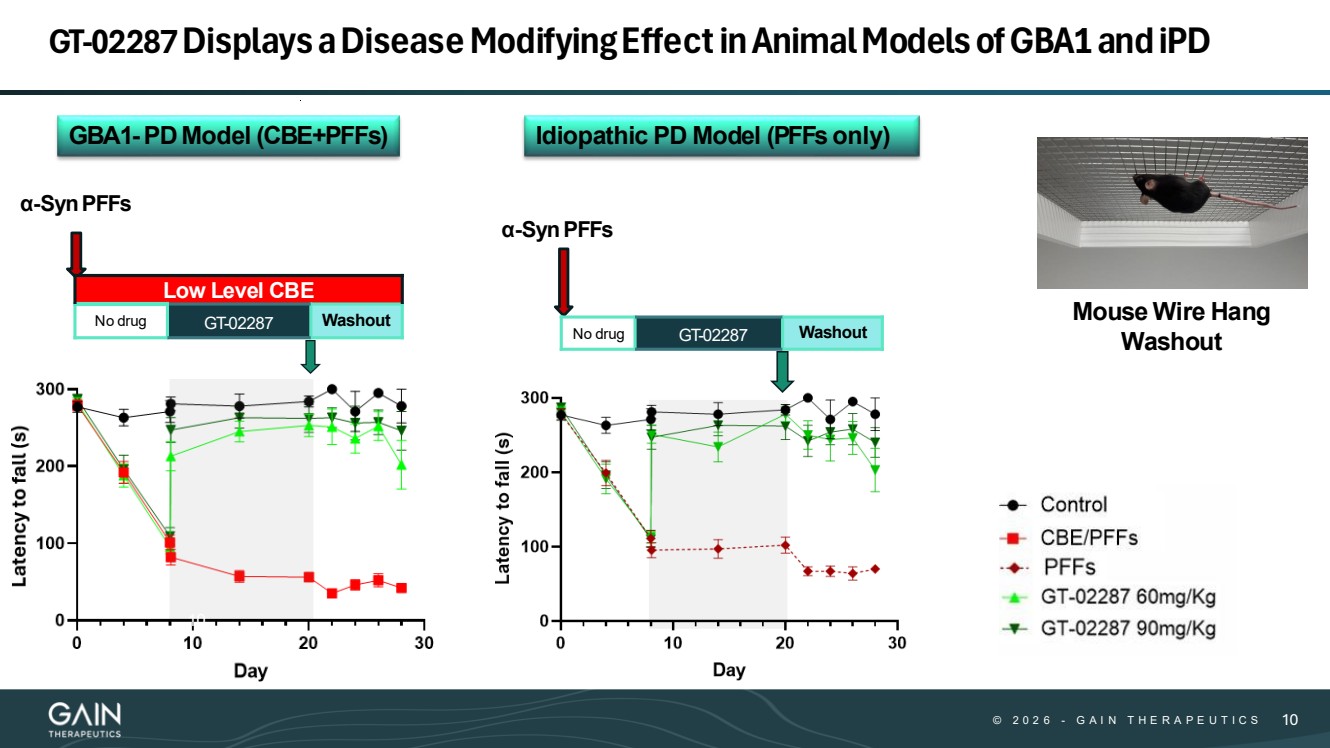
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1010 GT-02287Displaysa Disease ModifyingEffectin Animal Models of GBA1 and iPD α-SynPFFs α-SynPFFs No drug GT-02287 Washout Low Level CBE No drug GT-02287 Washout GBA1-PDModel (CBE+PFFs) Idiopathic PD Model (PFFs only) Mouse Wire Hang Washout 10 |
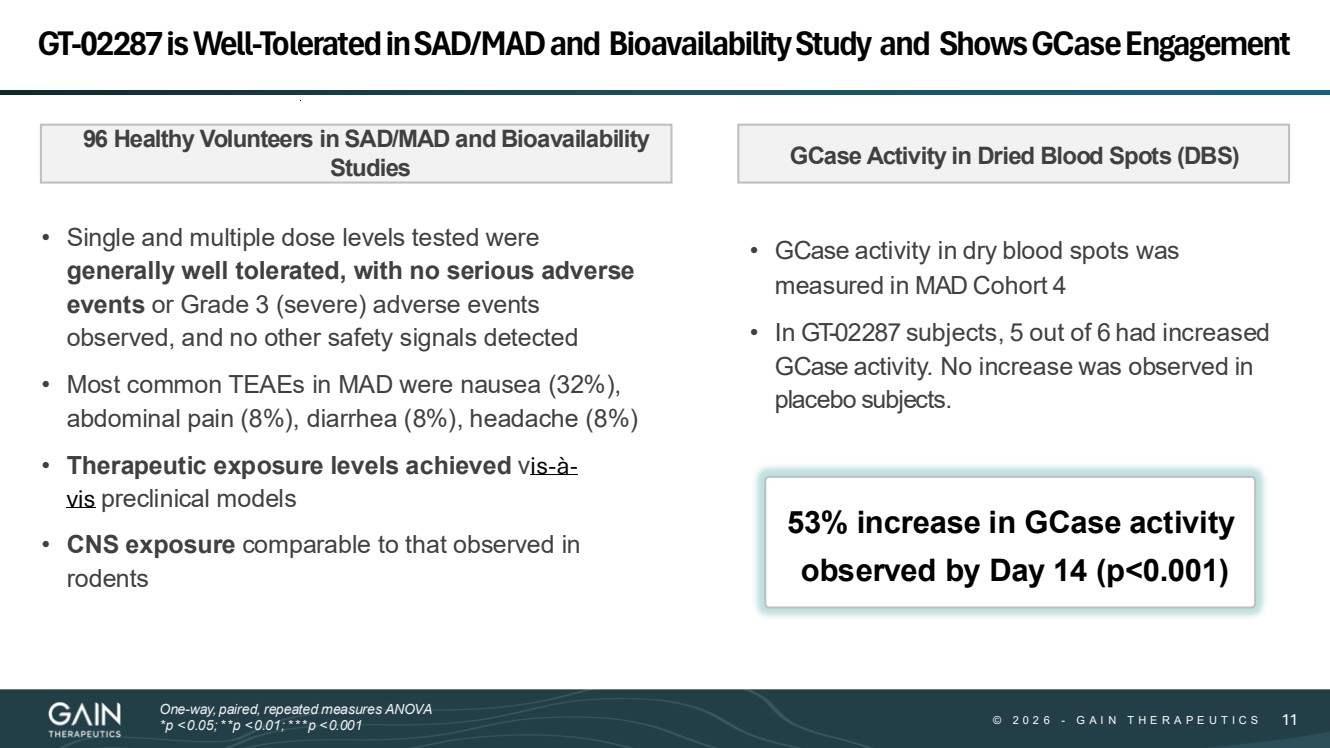
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1111 GT-02287 is Well-Tolerated in SAD/MAD and Bioavailability Study and Shows GCaseEngagement GCase Activity in Dried Blood Spots (DBS) • Single and multiple dose levels tested were generally well tolerated, with no serious adverse events or Grade 3 (severe) adverse events observed, and no other safety signals detected • Most common TEAEs in MAD were nausea (32%), abdominal pain (8%), diarrhea (8%), headache (8%) • Therapeutic exposure levels achieved vis-à-vis preclinical models • CNS exposure comparable to that observed in rodents 96 Healthy Volunteers in SAD/MAD and Bioavailability Studies 53% increase in GCase activity observed by Day 14 (p<0.001) One-way, paired, repeated measures ANOVA *p <0.05;**p <0.01;***p <0.001 • GCase activity in dry blood spots was measured in MAD Cohort 4 • In GT-02287 subjects, 5 out of 6 had increased GCase activity. No increase was observed in placebo subjects. |
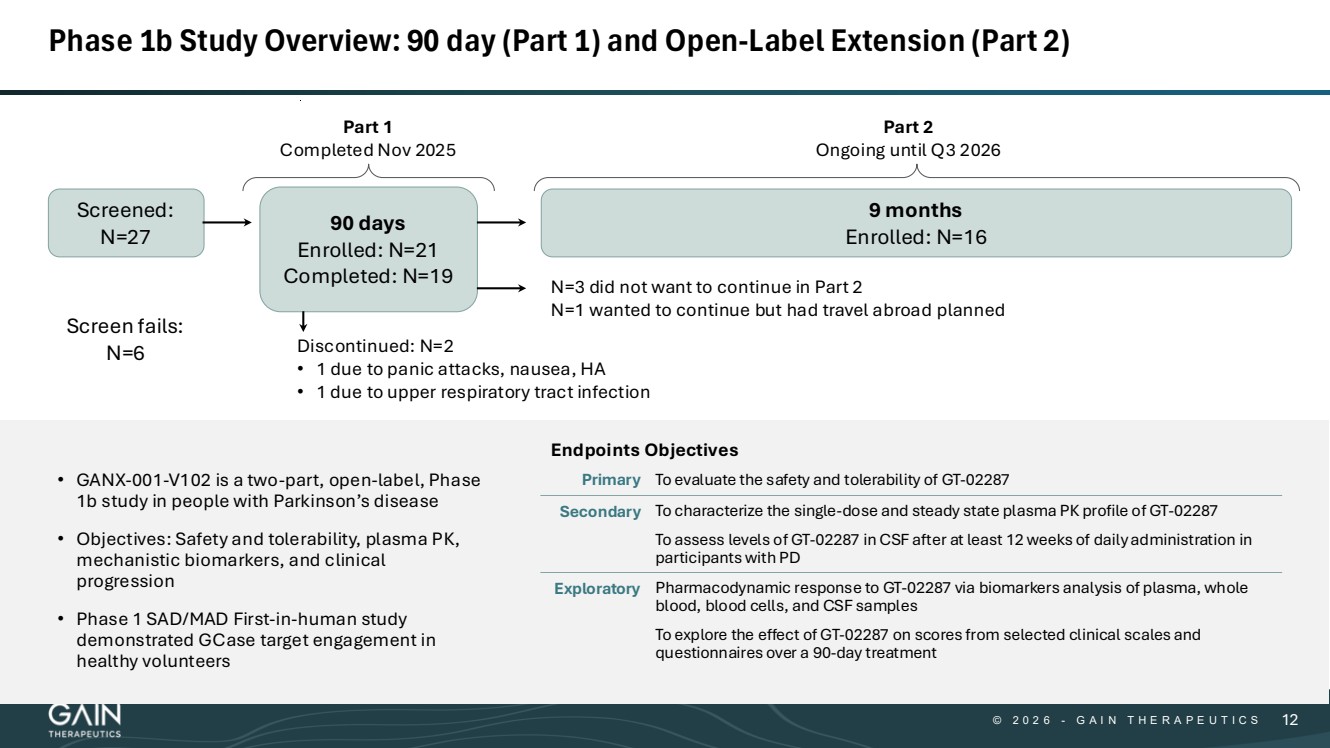
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1212 Phase 1b Study Overview: 90 day (Part 1) and Open-Label Extension (Part 2) • GANX-001-V102 is a two-part, open-label, Phase 1b study in people with Parkinson’s disease • Objectives: Safety and tolerability, plasma PK, mechanistic biomarkers, and clinical progression • Phase 1 SAD/MAD First-in-human study demonstrated GCase target engagement in healthy volunteers 90 days Enrolled: N=21 Completed: N=19 9 months Enrolled: N=16 Screened: N=27 Discontinued: N=2 • 1 due to panic attacks, nausea, HA • 1 due to upper respiratory tract infection Screen fails: N=6 Part 1 Completed Nov 2025 Part 2 Ongoing until Q3 2026 N=3 did not want to continue in Part 2 N=1 wanted to continue but had travel abroad planned Endpoints Objectives Primary To evaluate the safety and tolerability of GT-02287 Secondary To characterize the single-dose and steady state plasma PK profile of GT-02287 To assess levels of GT-02287 in CSF after at least 12 weeks of daily administration in participants with PD Exploratory Pharmacodynamic response to GT-02287 via biomarkers analysis of plasma, whole blood, blood cells, and CSF samples To explore the effect of GT-02287 on scores from selected clinical scales and questionnaires over a 90-day treatment |
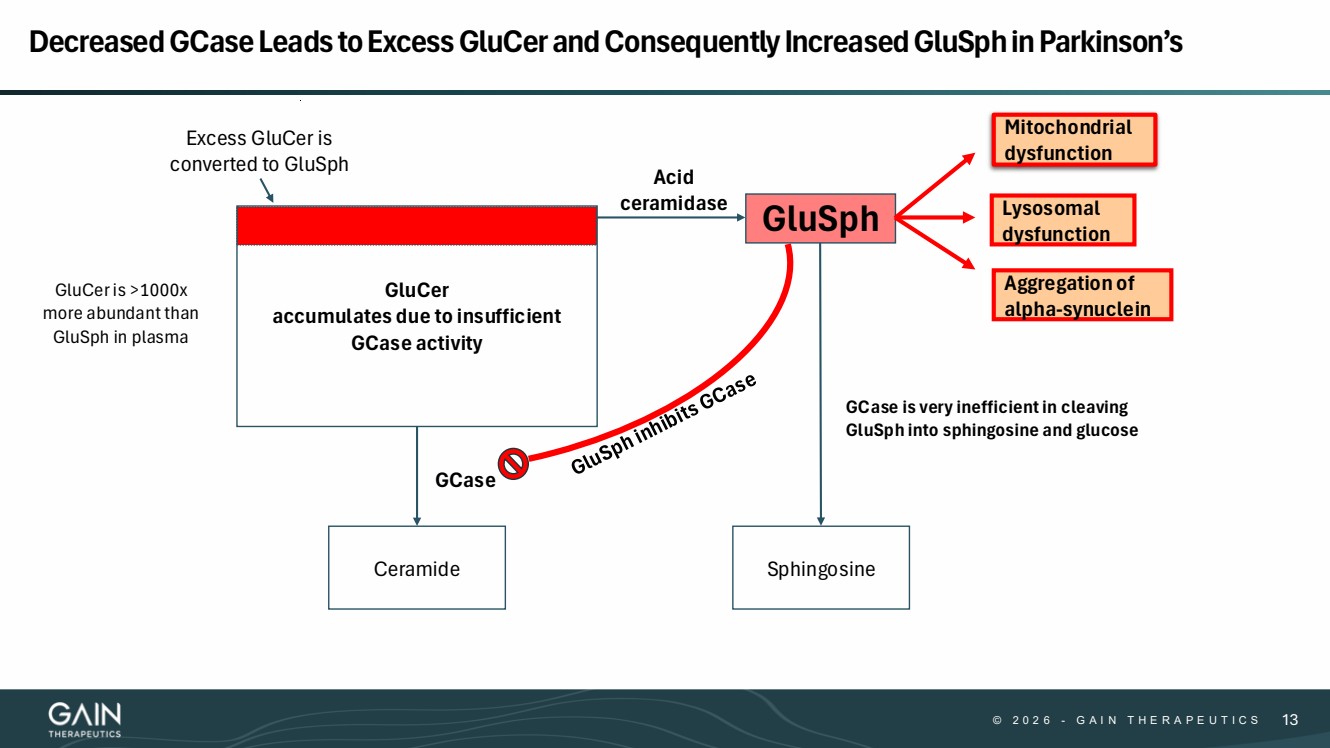
| © 2 0 2 6 - G A I N T H E R A P E U T I C S 13 Decreased GCaseLeads to Excess GluCerand Consequently Increased GluSphin Parkinson’s Sphingosine GCase GluSph Ceramide GCase is very inefficient in cleaving GluSph into sphingosine and glucose Excess GluCer is converted to GluSph Acid ceramidase GluCer accumulates due to insufficient GCase activity Aggregation of alpha-synuclein Mitochondrial dysfunction Lysosomal dysfunction GluCer is >1000x more abundant than GluSph in plasma |
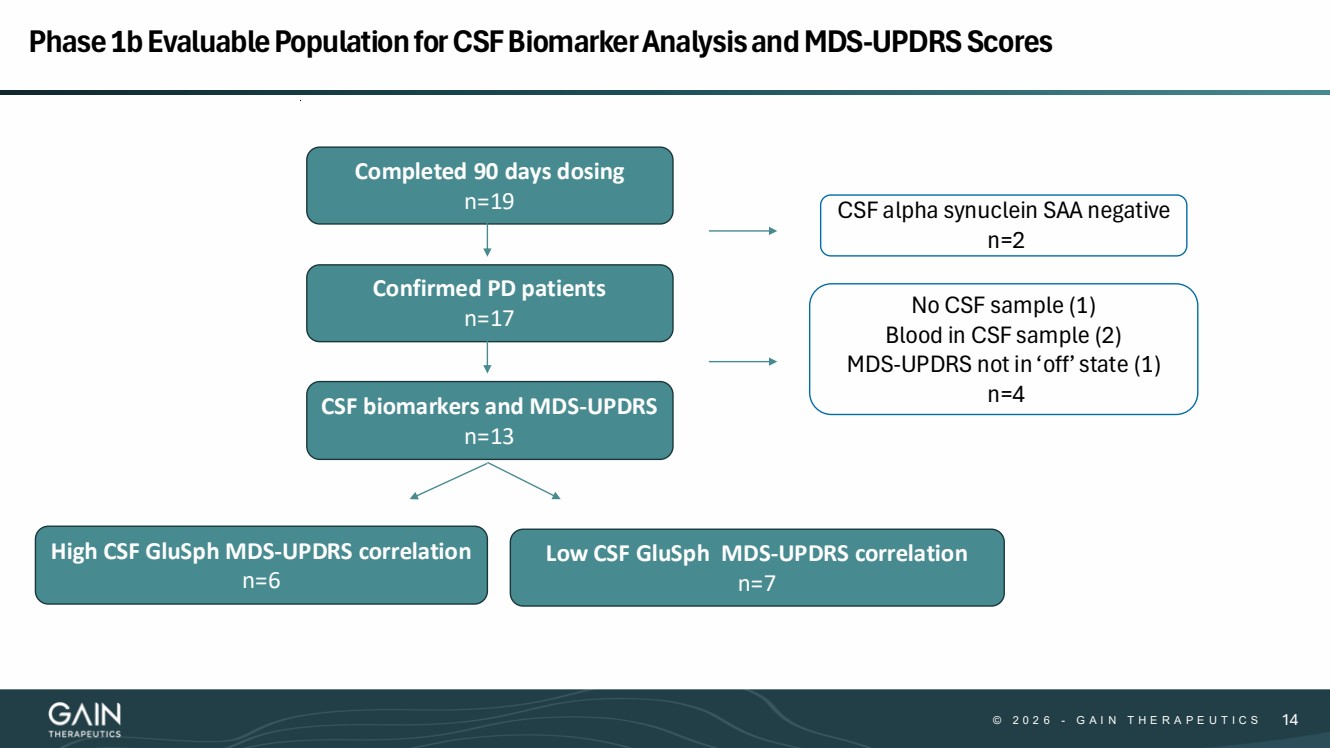
| © 2 0 2 6 - G A I N T H E R A P E U T I C S 14 CSF biomarkers and MDS-UPDRS n=13 High CSF GluSph MDS-UPDRS correlation n=6 Low CSF GluSph MDS-UPDRS correlation n=7 CSF alpha synuclein SAA negative n=2 Confirmed PD patients n=17 No CSF sample (1) Blood in CSF sample (2) MDS-UPDRS not in ‘off’ state (1) n=4 Completed 90 days dosing n=19 Phase 1b Evaluable Population for CSF Biomarker Analysis and MDS-UPDRS Scores |
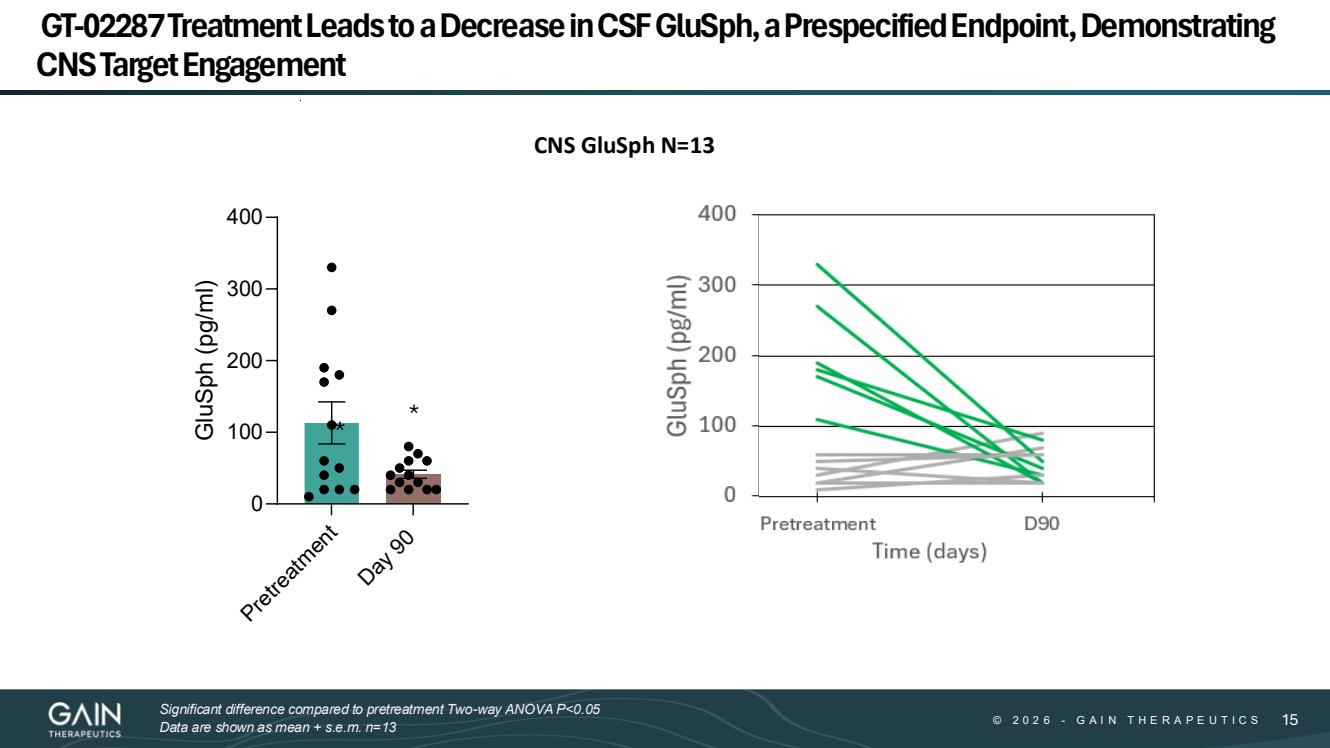
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1515 GT-02287 Treatment Leads to a Decrease in CSF GluSph, a Prespecified Endpoint, Demonstrating CNS Target Engagement Pretreatment Day 90 0 100 200 300 400 GluSph (pg/ml) CNS GluSph N=13 * * Significant difference compared to pretreatment Two-way ANOVA P<0.05 Data are shown as mean + s.e.m. n=13 |
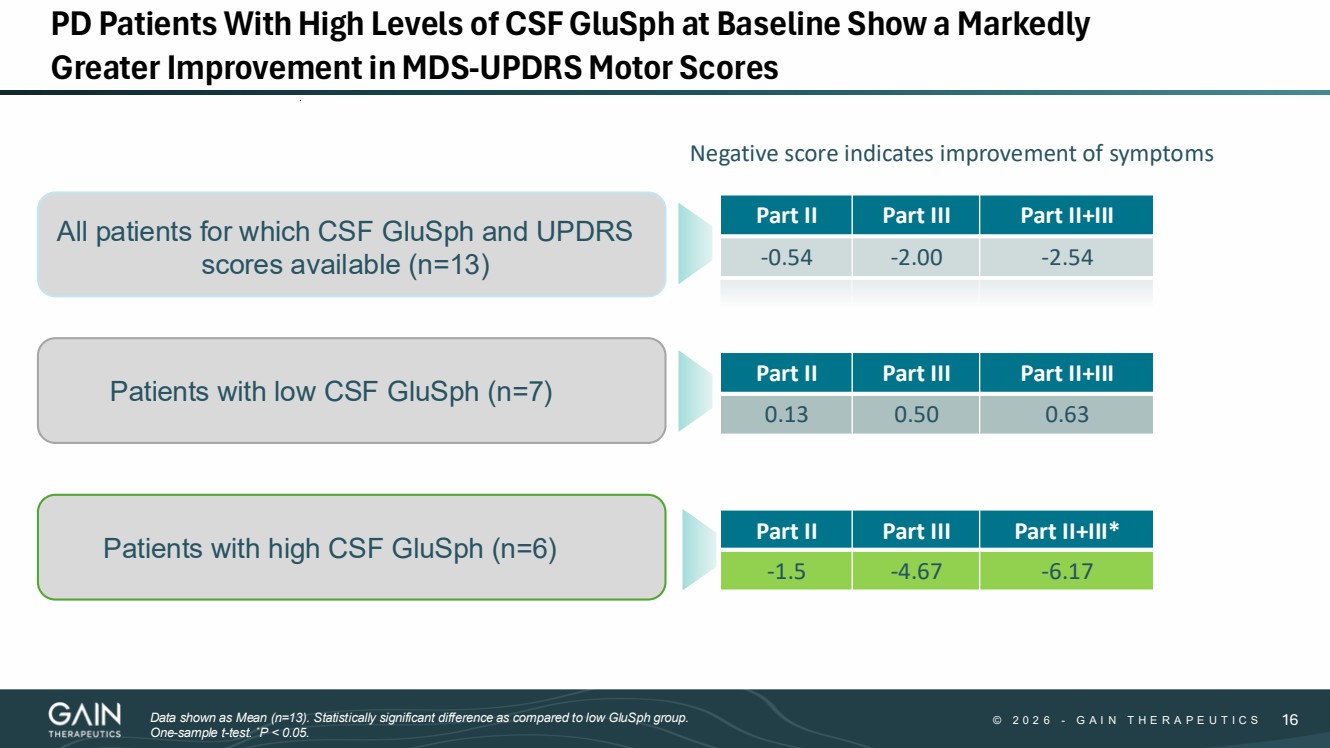
| 2/3/2026 © 2 0 2 6 - G A I N T H E R A P E U T I C S 16 16 PD Patients With High Levels of CSF GluSph at Baseline Show a Markedly Greater Improvement in MDS-UPDRS Motor Scores Part II Part III Part II+III -0.54 -2.00 -2.54 All patients for which CSF GluSph and UPDRS scores available (n=13) Part II Part III Part II+III* -1.5 -4.67 -6.17 Part II Part III Part II+III 0.13 0.50 0.63 Patients with high CSF GluSph (n=6) Patients with low CSF GluSph (n=7) Negative score indicates improvement of symptoms Data shown as Mean (n=13). Statistically significant difference as compared to low GluSph group. One-sample t-test. *P < 0.05. |
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1717 GT-02287 – Parkinson’s Disease Well-tolerated in both healthy volunteers and PD patients with therapeutic CNS exposures achieved Mechanism Binds at allosteric site, chaperones and modulates glucocerebrosidase enzyme (GCase) Disease Modification Restores GCase function and improves disease cascade and neuronal survival Efficacy Safety/Tolerability • Phase 1b Initial 90-day dosing (Part 1) complete 4Q25; Open label extension ongoing • Phase 2 trial initiation planned for 3Q26 Next Steps Pre-clinical: Demonstrates a disease modifying effect in models of GBA-1 and iPD Phase 1b: Demonstrates statistically significant biomarker evidence of disease modifying effect with improvement in clinical presentation based on MDS-UPDRS scores GT-02287 acts on brain Gcase, reduces toxic substrate GluSph, and leads to improvements seen in clinical MDS-UPDRS scores |
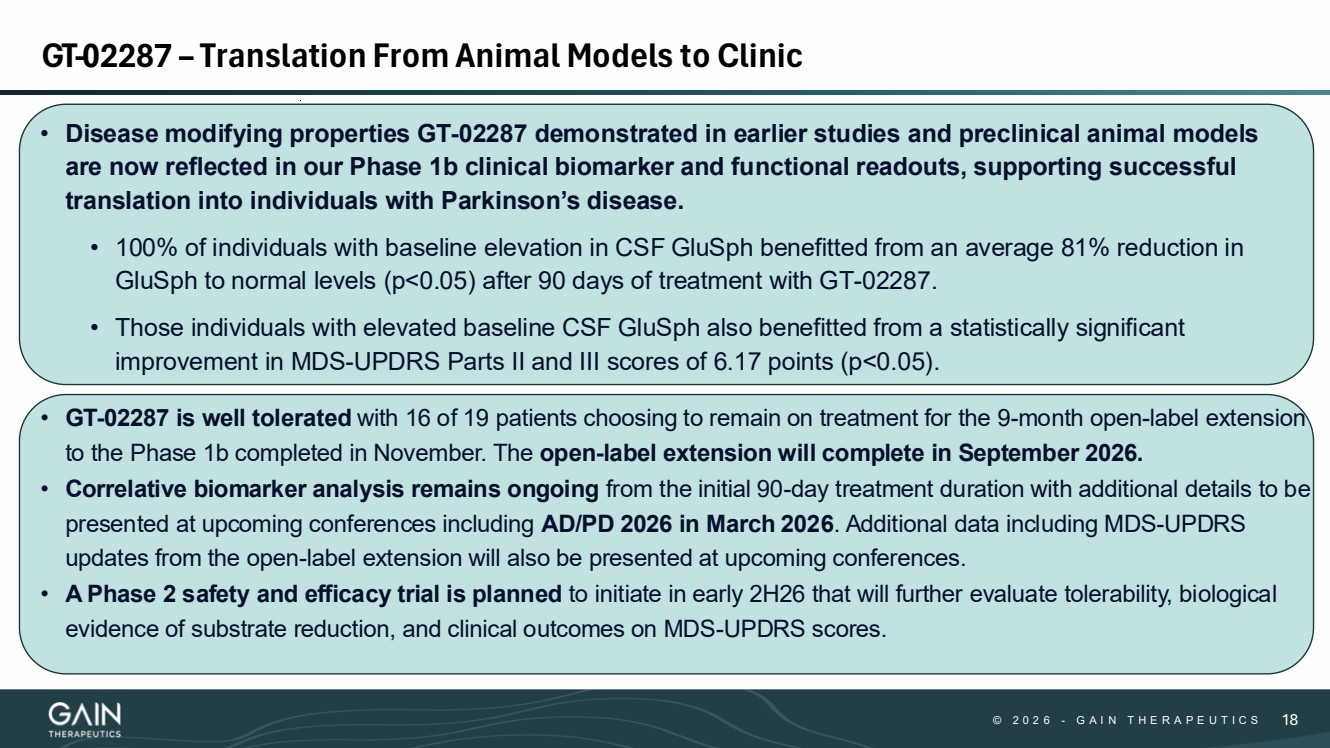
| © 2 0 2 6 - G A I N T H E R A P E U T I C S 18 • Disease modifying properties GT-02287 demonstrated in earlier studies and preclinical animal models are now reflected in our Phase 1b clinical biomarker and functional readouts, supporting successful translation into individuals with Parkinson’s disease. • 100% of individuals with baseline elevation in CSF GluSph benefitted from an average 81% reduction in GluSph to normal levels (p<0.05) after 90 days of treatment with GT-02287. • Those individuals with elevated baseline CSF GluSph also benefitted from a statistically significant improvement in MDS-UPDRS Parts II and III scores of 6.17 points (p<0.05). • GT-02287 is well tolerated with 16 of 19 patients choosing to remain on treatment for the 9-month open-label extension to the Phase 1b completed in November. The open-label extension will complete in September 2026. • Correlative biomarker analysis remains ongoing from the initial 90-day treatment duration with additional details to be presented at upcoming conferences including AD/PD 2026 in March 2026. Additional data including MDS-UPDRS updates from the open-label extension will also be presented at upcoming conferences. • A Phase 2 safety and efficacy trial is planned to initiate in early 2H26 that will further evaluate tolerability, biological evidence of substrate reduction, and clinical outcomes on MDS-UPDRS scores. GT-02287 – Translation From Animal Models to Clinic |
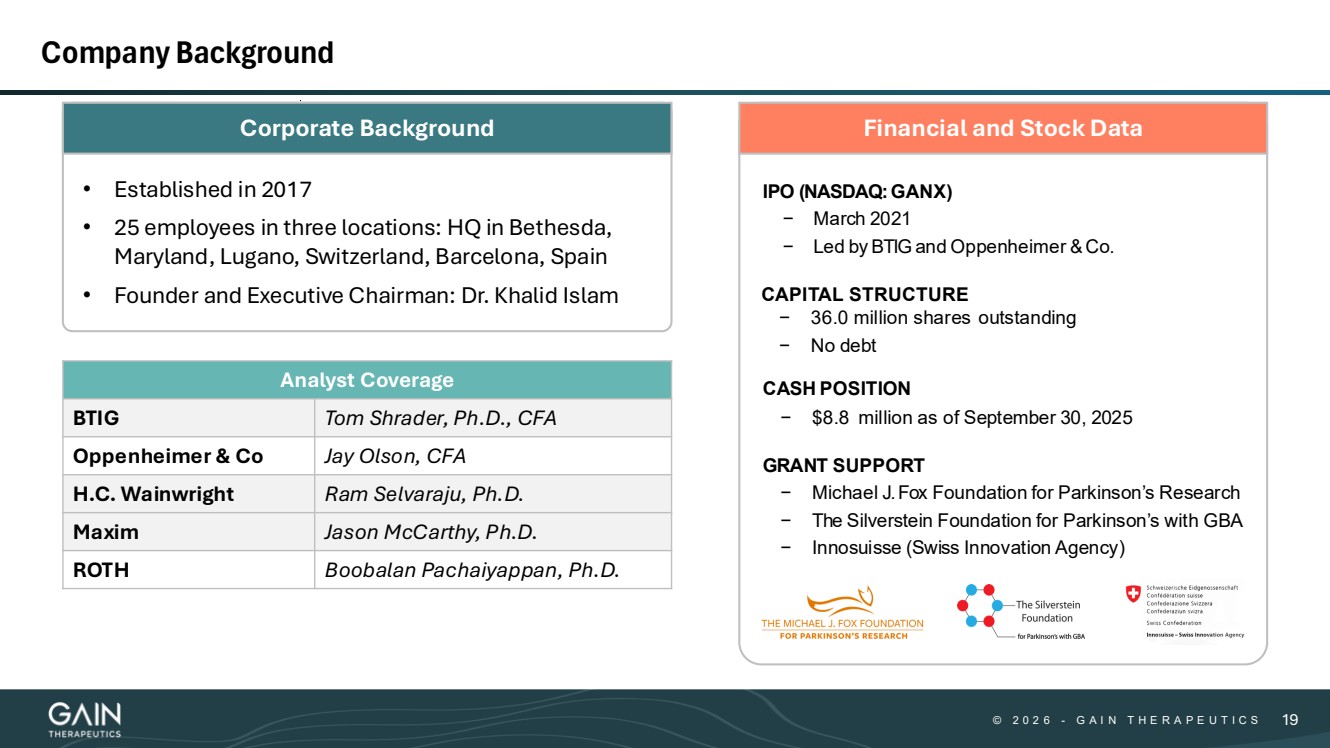
| © © 2 0 2 6 2 0 2 5 - -G AG A I N T H E R A P E U T I C S I N T H E R A P E U T I C S 1919 Company Background Analyst Coverage BTIG Tom Shrader, Ph.D., CFA Oppenheimer & Co Jay Olson, CFA H.C. Wainwright Ram Selvaraju, Ph.D. Maxim Jason McCarthy, Ph.D. ROTH Boobalan Pachaiyappan, Ph.D. Financial and Stock Data IPO (NASDAQ:GANX) − March 2021 − Led by BTIG and Oppenheimer & Co. CAPITAL STRUCTURE − 36.0 million shares outstanding − No debt CASH POSITION − $8.8 million as of September 30, 2025 GRANT SUPPORT − Michael J.Fox Foundation for Parkinson’s Research − The Silverstein Foundation for Parkinson’s with GBA − Innosuisse (Swiss Innovation Agency) Corporate Background • Established in 2017 • 25 employees in three locations: HQ in Bethesda, Maryland, Lugano, Switzerland, Barcelona, Spain • Founder and Executive Chairman: Dr. Khalid Islam |