
Building the Future of Eye Care January 2026 Vivian, an XDEMVY patient.

2 | © Tarsus Pharmaceuticals | For Investor Purposes Only Forward-looking Statements This presentation contains forward-looking statements that involve risks and uncertainties. These statements and the most recent Form 10-Q quarterly filing filed with the SEC include statements regarding the potential commercial success and growth of XDEMVY in Demodex blepharitis, including market size, acceptance, demand, adoption rate, and peak sales potential for XDEMVY; our ability maintain distribution and patient access for XDEMVY and timing and breadth of payer coverage; our ability to expand the clinical applications of XDEMVY in eye care; our ability to successfully maintain our sales force execution and the impact of our direct-to-consumer campaign including network television; our ability to continue to educate the market about Demodex blepharitis, the timing, objectives, and results of the clinical trials including planned initiation of a Phase 2 trial for the potential prevention of Lyme disease and the timing of clinical results for the Phase 2 trial for the potential treatment of ocular rosacea, the potential market size, opportunity, and ECP education for ocular rosacea and our other pipeline indications, anticipated regulatory and development milestones including the clarity of the regulatory path forward for TP-04 and TP-05 in the US, and potential Europe, Japan, and China regulatory pathways and approval for XDEMVY, our ability to continue investing in our business, add new programs, create value, and become an eye care leader, and the potential XDEMVY net sales, prescription demand, gross-to-net discount, and operating expense outlook for Q4 2025 and beyond. The words, without limitation, “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these or similar identifying words. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Further, there are other risks and uncertainties that could cause actual results to differ from those set forth in the forward-looking statements and they are detailed from time to time in the reports Tarsus files with the Securities and Exchange Commission, including Tarsus’ Form 10-K for the year December 31, 2024 filed on February 25, 2025 and Tarsus’ Form 10-Q for the quarter ended September 30, 2025 filed on November 4, 2025, which Tarsus incorporates by reference into this presentation, copies of which are or will be posted on its website and are available from Tarsus without charge. However, new risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Any forward-looking statements contained in this press release are based on the current expectations of Tarsus’ management team and speak only as of the date hereof, and Tarsus specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.

3 | © Tarsus Pharmaceuticals | For Investor Purposes Only Bobby Azamian, MD, PhD CEO & Chairman Jeff Farrow Chief Financial & Strategy Officer Aziz Mottiwala Chief Commercial Officer Dianne Whitfield Chief Human Resources Officer Elizabeth Yeu, MD Chief Medical Officer Bryan Wahl, MD, JD General Counsel Sesha Neervannan, PhD Chief Operating Officer World-Class Leadership Team With Proven Track Record of Success
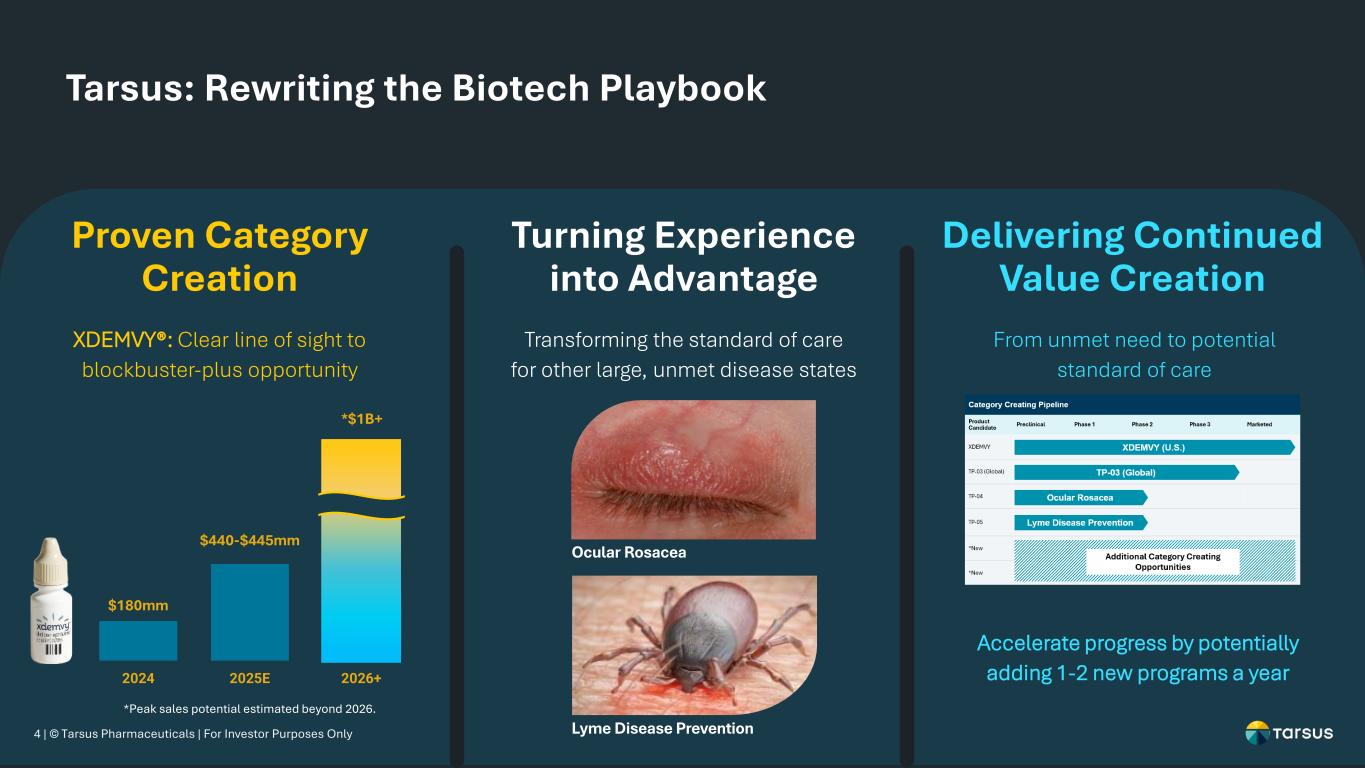
4 | © Tarsus Pharmaceuticals | For Investor Purposes Only Tarsus: Rewriting the Biotech Playbook XDEMVY®: Clear line of sight to blockbuster-plus opportunity Proven Category Creation Transforming the standard of care for other large, unmet disease states Turning Experience into Advantage From unmet need to potential standard of care Delivering Continued Value Creation $180mm $440-$445mm 2024 2025E 2026+ *$1B+ Accelerate progress by potentially adding 1-2 new programs a year Ocular Rosacea Lyme Disease Prevention *Peak sales potential estimated beyond 2026.

5 | © Tarsus Pharmaceuticals | For Investor Purposes Only World-class leadership team with track record of value creation and turning experience into a strategic advantage Poised to Lead the Next Era in Eye Care and Beyond Exceptional XDEMVY launch with blockbuster-plus potential driven by high-performing commercial engine Unique ability to identify untapped opportunities driven by patient need and the potential to transform the standard of care Financial strength and discipline driving expected sustained and scalable growth Well-positioned to potentially add 1-2 new programs each year

6 | © Tarsus Pharmaceuticals | For Investor Purposes Only The ONLY FDA-approved medicine and definitive standard of care for Demodex blepharitis Targets the root cause of disease Delivers strong, clinically meaningful results within 6 weeks Patent protection expected through 2038 (9 Orange Book-listed patents to date and counting) XDEMVY®
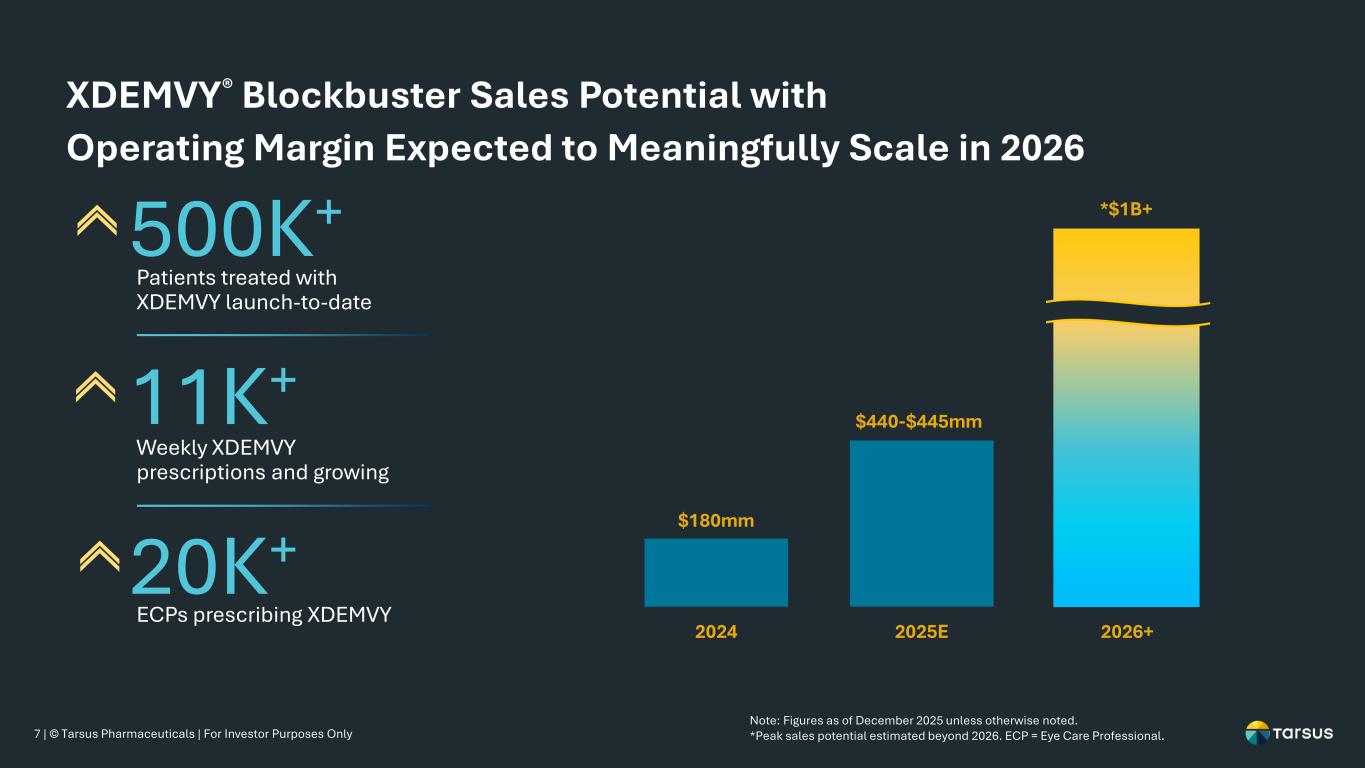
7 | © Tarsus Pharmaceuticals | For Investor Purposes Only XDEMVY® Blockbuster Sales Potential with Operating Margin Expected to Meaningfully Scale in 2026 $180mm $440-$445mm 2024 2025E 2026+ *$1B+ 20K+ ECPs prescribing XDEMVY 11K+ Weekly XDEMVY prescriptions and growing 500K+ Patients treated with XDEMVY launch-to-date Note: Figures as of December 2025 unless otherwise noted. *Peak sales potential estimated beyond 2026. ECP = Eye Care Professional.
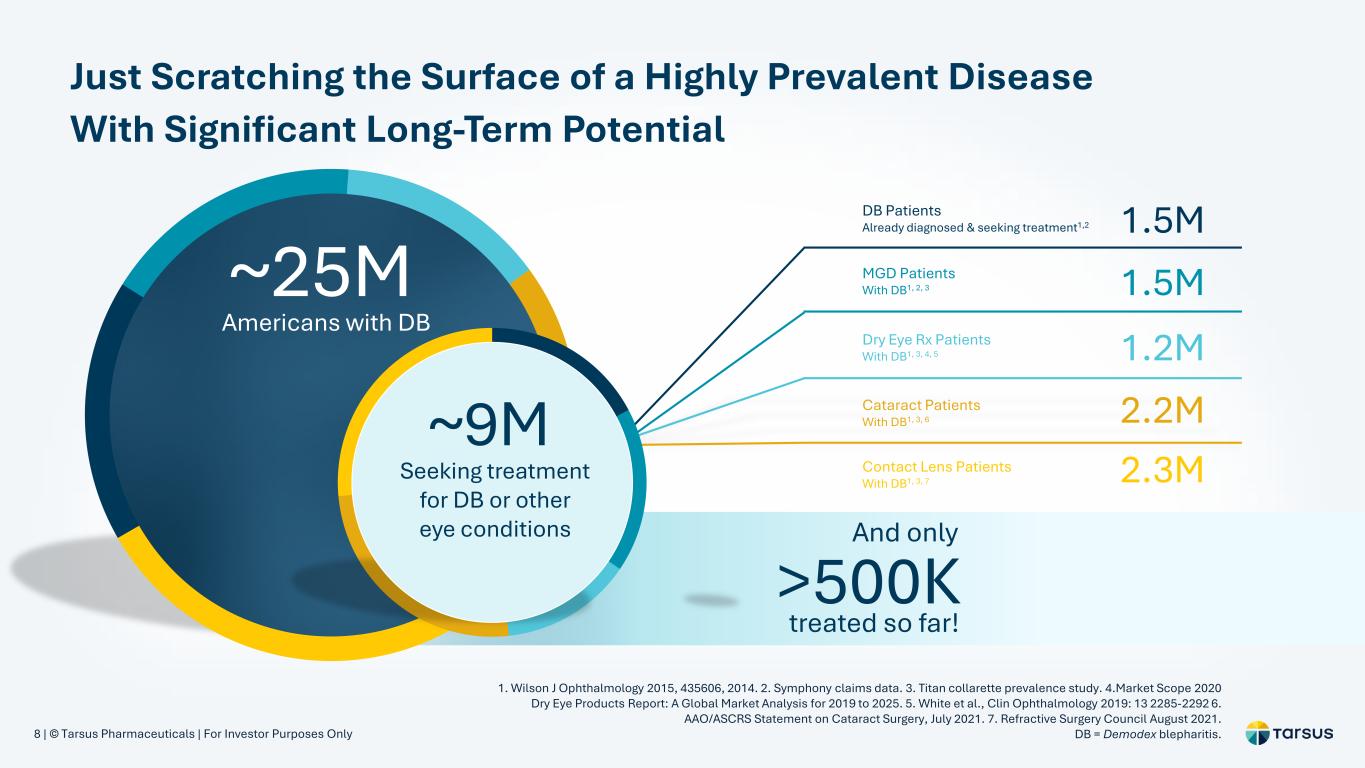
8 | © Tarsus Pharmaceuticals | For Investor Purposes Only 2.3M 1.5MDB Patients Already diagnosed & seeking treatment1,2 1.5M 1.2M 2.2MCataract Patients With DB1, 3, 6 Contact Lens Patients With DB1, 3, 7 MGD Patients With DB1, 2, 3 Dry Eye Rx Patients With DB1, 3, 4, 5 ~25M Americans with DB ~9M Seeking treatment for DB or other eye conditions And only >500K treated so far! Just Scratching the Surface of a Highly Prevalent Disease With Significant Long-Term Potential 1. Wilson J Ophthalmology 2015, 435606, 2014. 2. Symphony claims data. 3. Titan collarette prevalence study. 4.Market Scope 2020 Dry Eye Products Report: A Global Market Analysis for 2019 to 2025. 5. White et al., Clin Ophthalmology 2019: 13 2285-2292 6. AAO/ASCRS Statement on Cataract Surgery, July 2021. 7. Refractive Surgery Council August 2021. DB = Demodex blepharitis.

9 | © Tarsus Pharmaceuticals | For Investor Purposes Only Blockbuster-Plus Potential XDEMVY® Growth Engine Driving Access Broad, high-quality coverage with >90% of commercial, Medicare and Medicaid lives covered Execution Sales force transforming ECPs to weekly and daily prescribing Education Scaling the impact of our proven levers focused on increasing ECP utilization and enhancing DTC ROI Evidence Impactful evidence giving ECPs more reasons to look and treat $1B+ Peak Sales Potential DTC = Direct-To-Consumer. ROI = Return on Investment.
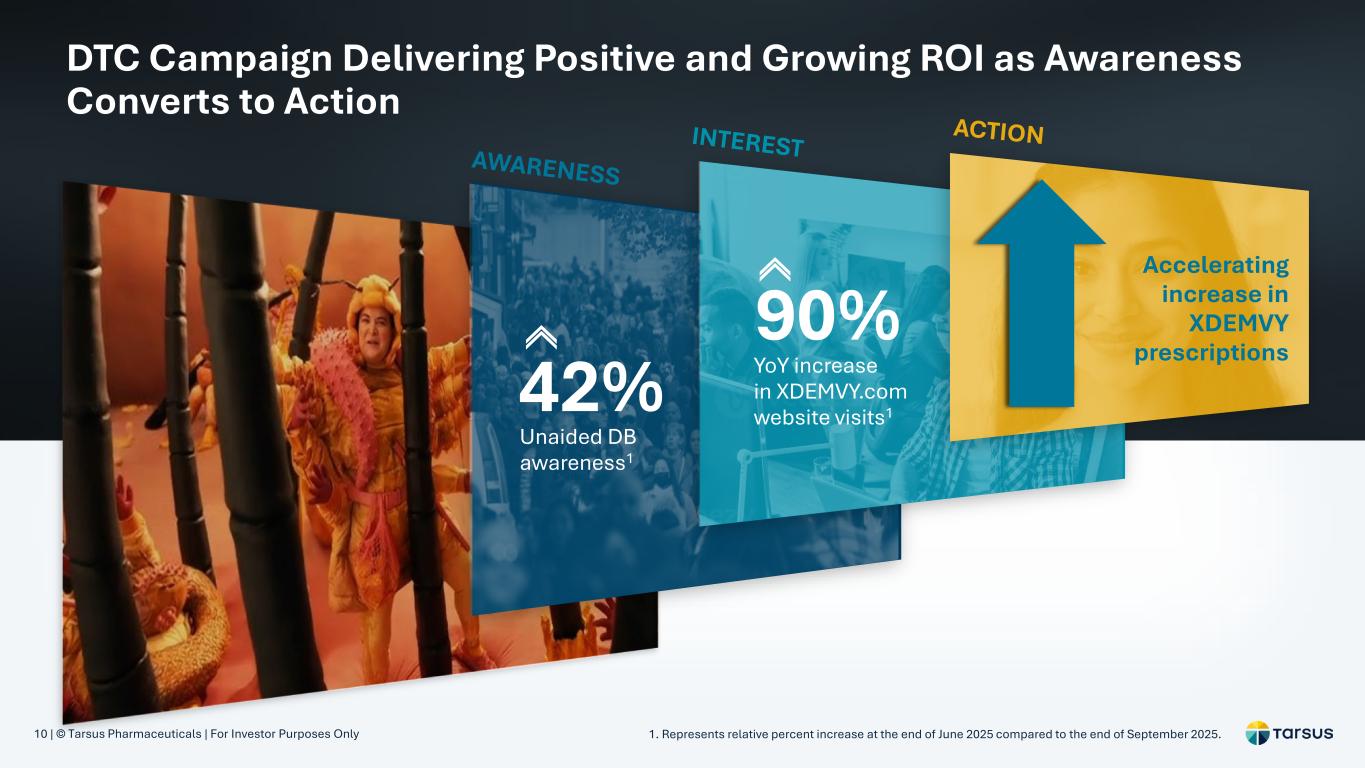
10 | © Tarsus Pharmaceuticals | For Investor Purposes Only DTC Campaign Delivering Positive and Growing ROI as Awareness Converts to Action 1. Represents relative percent increase at the end of June 2025 compared to the end of September 2025. Unaided DB awareness1 42% YoY increase in XDEMVY.com website visits1 90% Accelerating increase in XDEMVY prescriptions
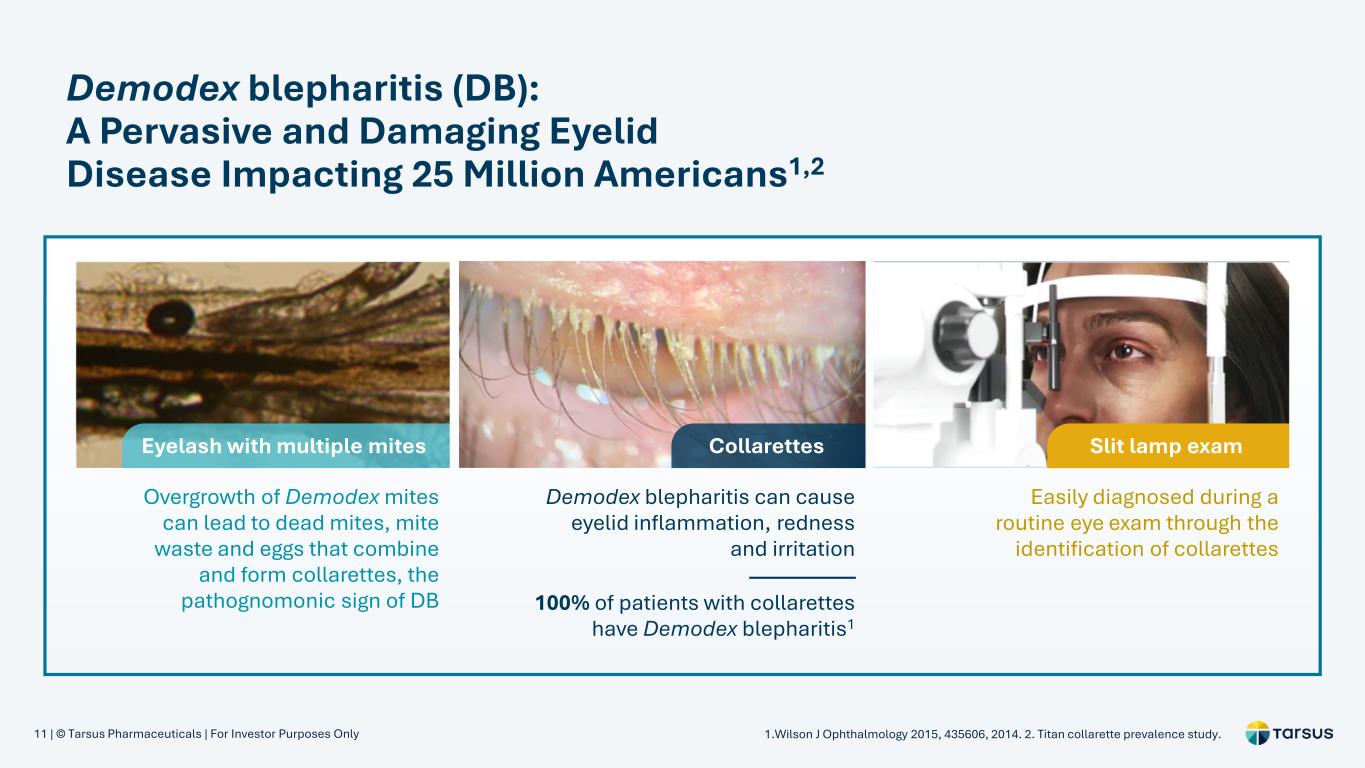
11 | © Tarsus Pharmaceuticals | For Investor Purposes Only Demodex blepharitis (DB): A Pervasive and Damaging Eyelid Disease Impacting 25 Million Americans1,2 Overgrowth of Demodex mites can lead to dead mites, mite waste and eggs that combine and form collarettes, the pathognomonic sign of DB Demodex blepharitis can cause eyelid inflammation, redness and irritation Easily diagnosed during a routine eye exam through the identification of collarettes 100% of patients with collarettes have Demodex blepharitis1 Eyelash with multiple mites Collarettes Slit lamp exam 1.Wilson J Ophthalmology 2015, 435606, 2014. 2. Titan collarette prevalence study.

12 | © Tarsus Pharmaceuticals | For Investor Purposes Only 1.Wilson J Ophthalmology 2015, 435606, 2014. 2. Titan collarette prevalence study. 3. Liu J et al. Curr Opin Allergy Clin Immunol. 2010;10(5):505-510. 4. Cheng AM et al. Curr Opin Ophthalmol. 2015;26(4):295-300.

13 | © Tarsus Pharmaceuticals | For Investor Purposes Only Record Third Quarter 2025 Results $118.7M XDEMVY Net Sales ($ in millions) >103K XDEMVY Bottles Delivered to Patients +147% YoY Net Sales Growth 44.7% Gross-to-Net Discount
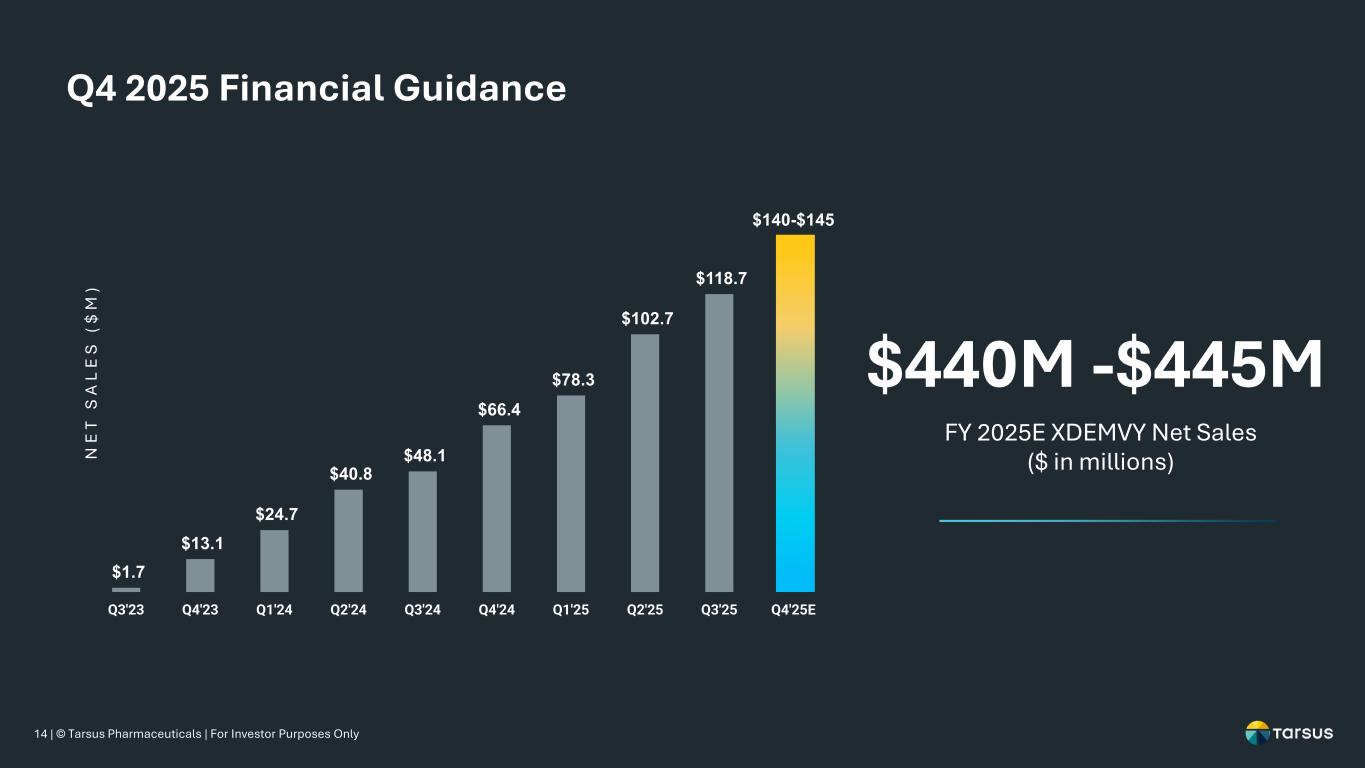
14 | © Tarsus Pharmaceuticals | For Investor Purposes Only Q4 2025 Financial Guidance $1.7 $13.1 $24.7 $40.8 $48.1 $66.4 $78.3 $102.7 $118.7 $140-$145 Q3'23 Q4'23 Q1'24 Q2'24 Q3'24 Q4'24 Q1'25 Q2'25 Q3'25 Q4'25E N E T S A L E S ( $ M ) $440M -$445M FY 2025E XDEMVY Net Sales ($ in millions)

15 | © Tarsus Pharmaceuticals | For Investor Purposes Only Extending Our Category-Creating Leadership with Potential to Add 1-2 Pipeline Programs Per Year Initiated Phase 2 study in December 2025 Plan to initiate Phase 2 study in 2026 On track for global expansion in 2026+ Lyme Prevention (TP-05)2 Global OpportunityOcular Rosacea (TP-04)1 1.TP-04 is an investigational therapy. 2. TP-05 is an investigational therapy.
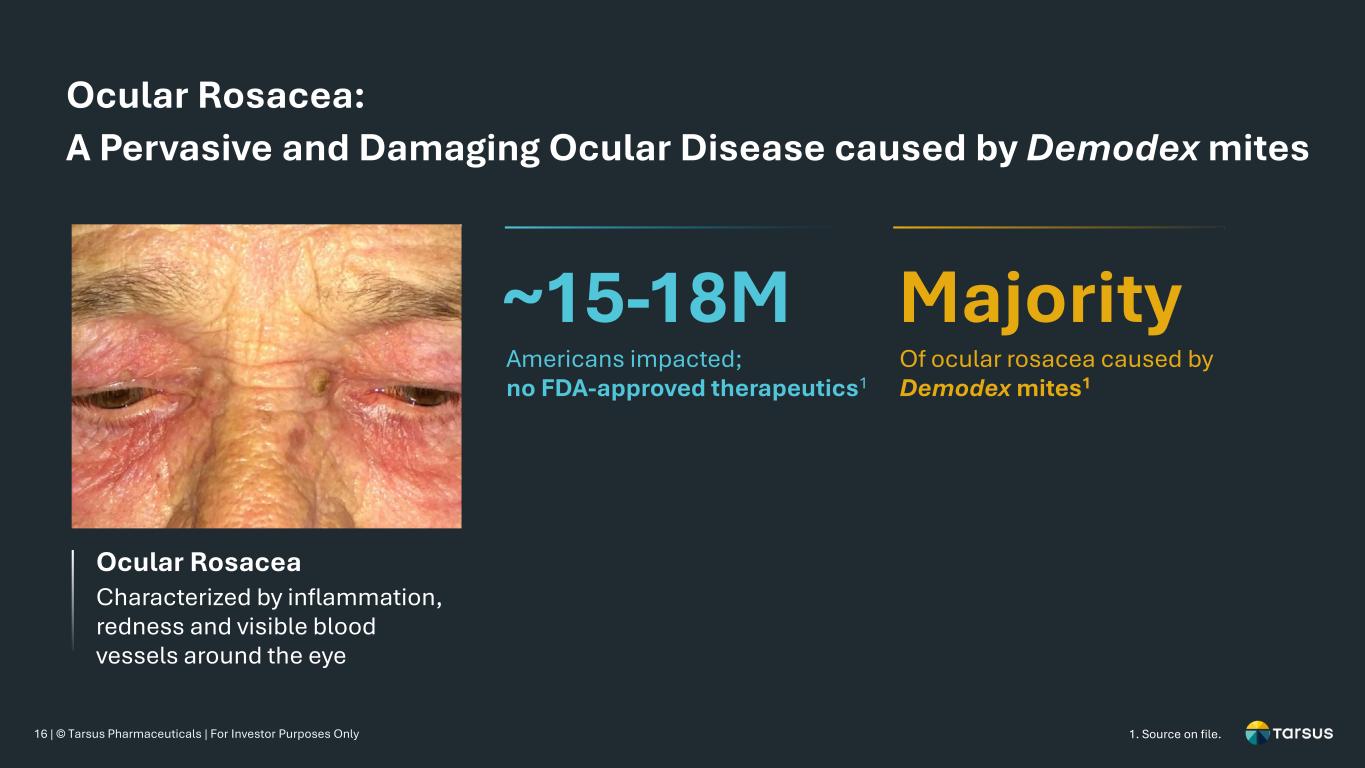
16 | © Tarsus Pharmaceuticals | For Investor Purposes Only Ocular Rosacea: A Pervasive and Damaging Ocular Disease caused by Demodex mites Ocular Rosacea Characterized by inflammation, redness and visible blood vessels around the eye ~15-18M Majority Americans impacted; no FDA-approved therapeutics1 Of ocular rosacea caused by Demodex mites1 1. Source on file.
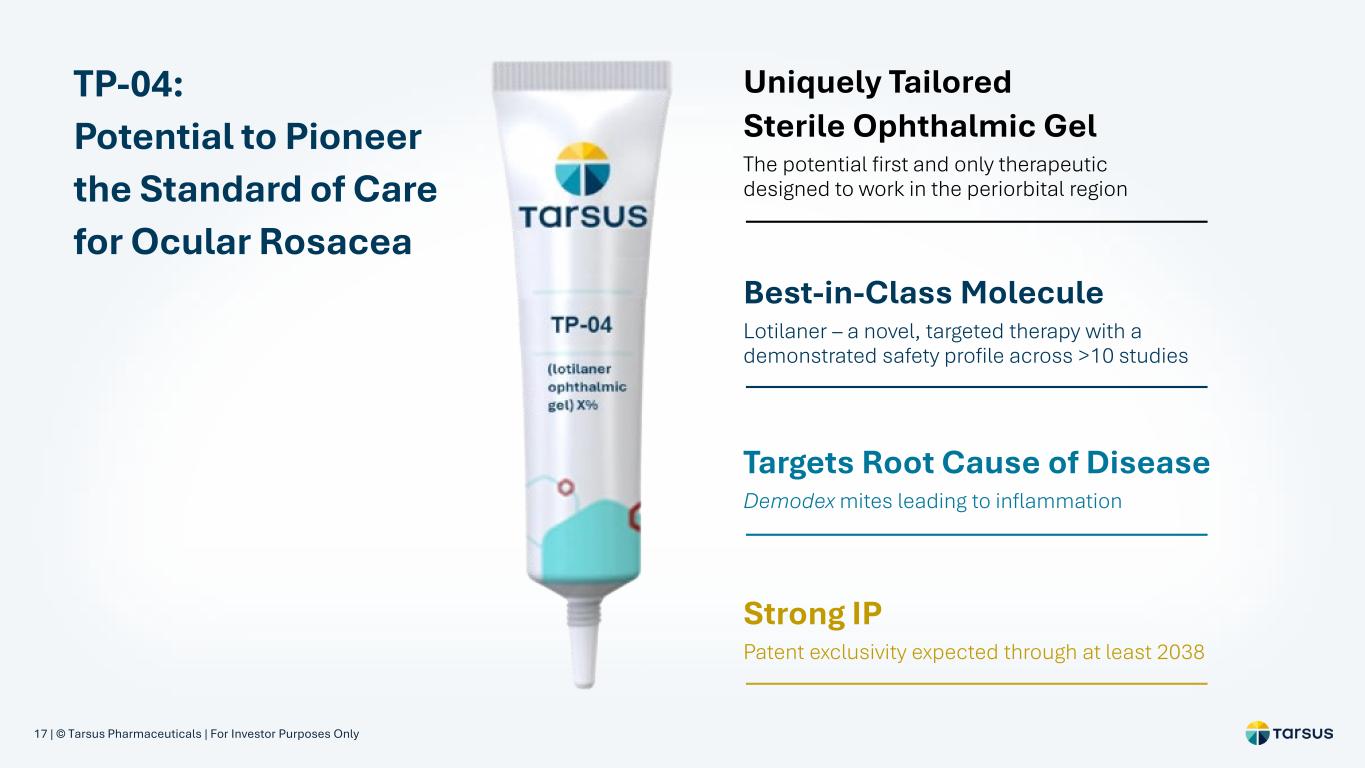
17 | © Tarsus Pharmaceuticals | For Investor Purposes Only TP-04: Potential to Pioneer the Standard of Care for Ocular Rosacea Uniquely Tailored Sterile Ophthalmic Gel The potential first and only therapeutic designed to work in the periorbital region Best-in-Class Molecule Lotilaner – a novel, targeted therapy with a demonstrated safety profile across >10 studies Strong IP Patent exclusivity expected through at least 2038 Targets Root Cause of Disease Demodex mites leading to inflammation

18 | © Tarsus Pharmaceuticals | For Investor Purposes Only Initiated First-Ever Phase 2 Study for the Treatment of Ocular Rosacea in December 2025 FDA-Endorsed Objective Endpoints Improvement in visible blood vessels (telangiectasia) & redness (erythema) of the periorbital region Clear Mechanism of Action Demonstrated statistically significant improvements in inflammatory lesions and reduction in redness compared to placebo in PPR Phase 2a trial1 • Vehicle-controlled 16-week study • ~100 patients with ocular rosacea • Newly developed endpoints with grading scales • Phase 2 data expected in 1H 2027 TP-04 Phase 2 Study Erythema Telangiectasia 1. Galatea Phase 2a trial for the treatment of papulopustular rosacea – February 2024. PPR = papulopustular rosacea.

19 | © Tarsus Pharmaceuticals | For Investor Purposes Only Lyme Disease Prevention: A Growing Public Health Crisis with Limited Treatment Options and No FDA-Approved Prophylaxis Lyme Disease A tick-borne infection caused by the transmission of Borrelia burgdorferi ~27M $1.3B Americans at high-to- moderate infection risk1 Impact to U.S. healthcare system2 1. CDC Estimate and Corsica Market Research. 2. Adrion E, et al, PLoS One, Feb 2015, Vol. 10(2):e0116767.

20 | © Tarsus Pharmaceuticals | For Investor Purposes Only TP-05: Potential to be First & Only On-Demand Oral Prophylaxis for Lyme Disease Designed to be Convenient, Fast-Acting and Durable Monthly oral tablet that gets to potentially therapeutic levels in hours Strong IP Patent exclusivity expected through at least 2040 Targets Root Cause of Disease Kills 99% of ticks before Borrelia transmission in animal studies Best-in-Class Molecule Lotilaner – a novel, targeted therapy with a demonstrated safety profile across >10 studies
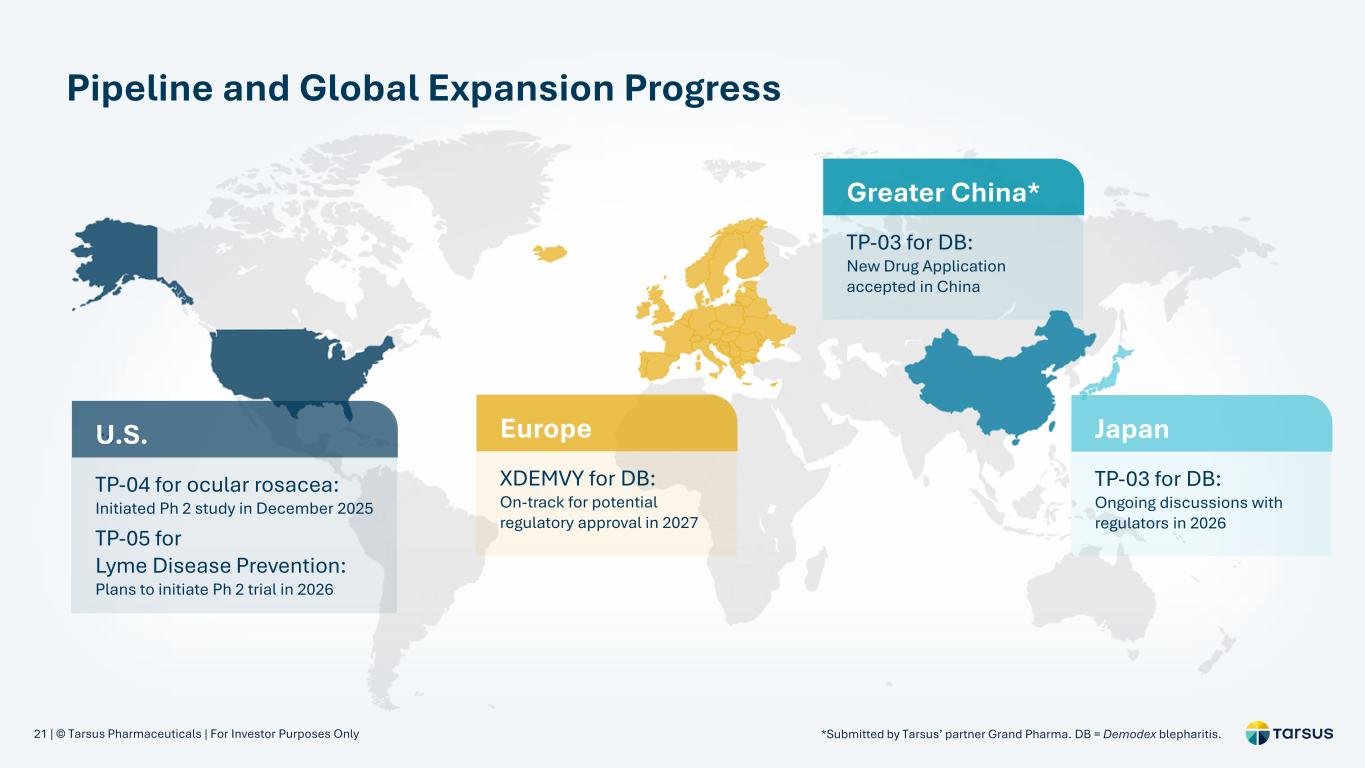
21 | © Tarsus Pharmaceuticals | For Investor Purposes Only Pipeline and Global Expansion Progress TP-04 for ocular rosacea: Initiated Ph 2 study in December 2025 U.S. XDEMVY for DB: On-track for potential regulatory approval in 2027 Europe TP-03 for DB: New Drug Application accepted in China Greater China* TP-03 for DB: Ongoing discussions with regulators in 2026 Japan TP-05 for Lyme Disease Prevention: Plans to initiate Ph 2 trial in 2026 *Submitted by Tarsus’ partner Grand Pharma. DB = Demodex blepharitis.

Thank You! 22 | © Tarsus Pharmaceuticals | For Investor Purposes Only Diamond, an XDEMVY patient.