Please wait
As filed with the Securities and Exchange Commission on January 28, 2026.
Registration No. 333-292657
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
To
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Veradermics, Incorporated
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | 2834 | 84-3304423 |
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
470 James St.
New Haven, CT 06513
(228) 372-3376
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Reid Waldman, M.D.
Chief Executive Officer
Veradermics, Incorporated
470 James St.
New Haven, CT 06513
(228) 372-3376
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| | | | | | | | |
Zachary Blume Nicholas Roper Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199-3600 (617) 951-7000 | Michael Greco General Counsel Veradermics, Incorporated 470 James St. New Haven, CT 06513 (228) 372-3376 | Divakar S. Gupta Ryan Sansom Madison A. Jones Cooley LLP 55 Hudson Yards New York, NY 10001 (212) 479-6000 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | | | | | | | | | | |
Large accelerated filer | ☐ | Accelerated filer | ☐ | Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | | | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 28, 2026
PRELIMINARY PROSPECTUS
13,350,000 Shares
Common Stock
We are offering 13,350,000 shares of our common stock. This is our initial public offering, and no public market currently exists for our common stock. We expect that the initial public offering price will be between $14.00 and $16.00 per share of our common stock. We have applied to list our common stock on the New York Stock Exchange under the symbol “MANE.” We believe that upon the consummation of this offering, we will meet the standards for listing on the New York Stock Exchange, and the consummation of this offering is contingent upon such listing.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 14 of this prospectus. Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| | | | | | | | | | | |
| PER SHARE | | TOTAL |
Initial Public Offering Price | $ | | $ |
Underwriting Discounts and Commissions(1) | | | |
Proceeds, Before Expenses, to Veradermics, Incorporated | | | |
| | | |
(1)See the section titled “Underwriting” for additional disclosure regarding underwriting compensation. Delivery of the shares of common stock is expected to be made on or about , 2026.
We have granted the underwriters an option for a period of 30 days to purchase up to an additional 2,002,500 shares of common stock. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
Wellington Management (the “cornerstone investor”) has indicated an interest in purchasing up to $30.0 million in shares of common stock in this offering at the initial public offering price. The shares of common stock to be purchased by the cornerstone investor will not be subject to a lock-up agreement with the underwriters. Because this indication of interest is not a binding agreement or commitment to purchase, the cornerstone investor may determine to purchase more, less or no shares in this offering or the underwriters may determine to sell more, less or no shares to the cornerstone investor. The underwriters will receive the same underwriting discounts and commissions on any of our shares of common stock purchased by the cornerstone investor as they will from any other shares of common stock sold to the public in this offering. The number of shares of common stock available for sale to the public will be reduced to the extent that the cornerstone investor purchases shares of common stock in the offering.
| | | | | | | | | | | |
Jefferies | Leerink Partners | Citigroup | Cantor |
Prospectus dated , 2026.
TABLE OF CONTENTS
Neither we nor the underwriters have authorized anyone to provide any information other than that contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside of the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside of the United States.
TRADEMARKS
This prospectus contains references to our trademarks and those trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other entities’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other entity.
MARKET AND INDUSTRY DATA
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations, market position and market opportunity, is based on our management’s estimates and research, as well as industry and general publications and research and studies conducted by third parties. We believe that the information from these third-party publications, research and studies included in this prospectus is reliable. However, we have not separately verified this data. Management’s estimates are derived from publicly available information, their knowledge of our industry and their assumptions based on such information and knowledge, which we believe to be reasonable; however, such research has not been verified by any third party. This data involves a number of assumptions and limitations which are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and “Special Note Regarding Forward-Looking Statements.” These and other factors could cause our future performance to differ materially from our assumptions and estimates.
PROSPECTUS SUMMARY
This summary highlights information included elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in our common stock. You should read and consider this entire prospectus carefully, including the sections titled “Risk Factors,” “Special Note Regarding Forward-Looking Statements” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in the prospectus, before making any investment decision. Except where the context otherwise requires or where otherwise indicated, the terms “Veradermics,” “we,” “us,” “our,” “the Company,” and “our business” refer to Veradermics, Incorporated and its consolidated subsidiaries. Overview
We are a dermatologist-founded, late clinical-stage biopharmaceutical company focused on developing innovative therapeutics to address pervasive treatment challenges in highly prevalent aesthetic and dermatological conditions. Our initial focus is developing better treatments for pattern hair loss, or PHL, a condition affecting approximately 50 million men and 30 million women in the United States. Current PHL treatment options are limited and therefore are consistently plagued with high rates of treatment failure, patient dissatisfaction and treatment discontinuation. Patients and healthcare providers routinely identify the following shortcomings with currently available treatment options:
nSlow onset of hair growth
nInconsistent results
nInsufficient density of hair growth for patient satisfaction
nTolerability issues related to hormonal, mood and cardiac side effects
nInconvenient administration
nLimited U.S. Food and Drug Administration, or FDA, approved treatment options, and no FDA-approved oral options for women
We are developing VDPHL01 as an oral, non-hormonal treatment for men and women with PHL to reduce the barriers to wide adoption of chronic hair loss therapy and potentially transform PHL treatment. We believe that a marketing application could initially seek approval in male patients, followed by a supplemental new drug application, or sNDA, for female patients, or could alternatively pursue approval in both male and female patients simultaneously depending on the timing of the completion of our clinical trials.
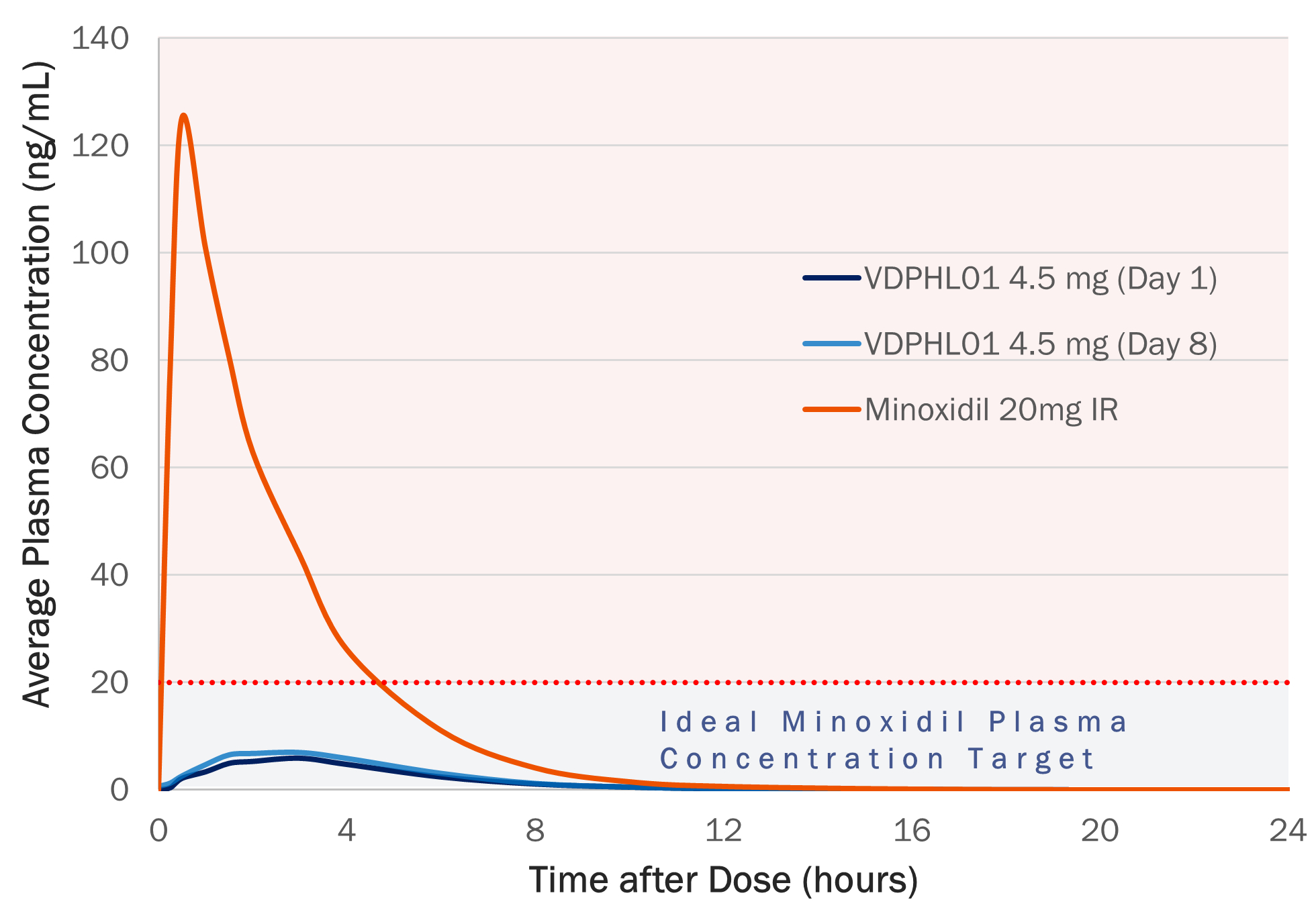
VDPHL01 is an oral, extended-release, or ER, formulation of minoxidil, a proven hair growth agent, designed to maximize minoxidil’s impact on hair restoration while minimizing the risk of cardiac activity. Though immediate-release, or IR, oral minoxidil was originally designed to treat resistant hypertension, it has been used off label as a treatment for PHL after hair growth was observed as a side effect. However, IR oral minoxidil’s release profile was not designed for hair growth as its short duration of circulation allows less time for follicular saturation and must be used at lower doses to reduce the likelihood of reaching off target cardiac stimulative levels. VDPHL01 builds on minoxidil’s validated hair growth biology via a novel and proprietary ER formulation designed to maximize the total plasma concentrations of minoxidil known to grow hair without inducing changes in cardiac activity. We believe that our efforts mark the first attempt to bring an ER formulation of minoxidil to patients with these optimized pharmacokinetic, or PK, and pharmacodynamic, or PD, qualities that raise the ceiling of hair growth.
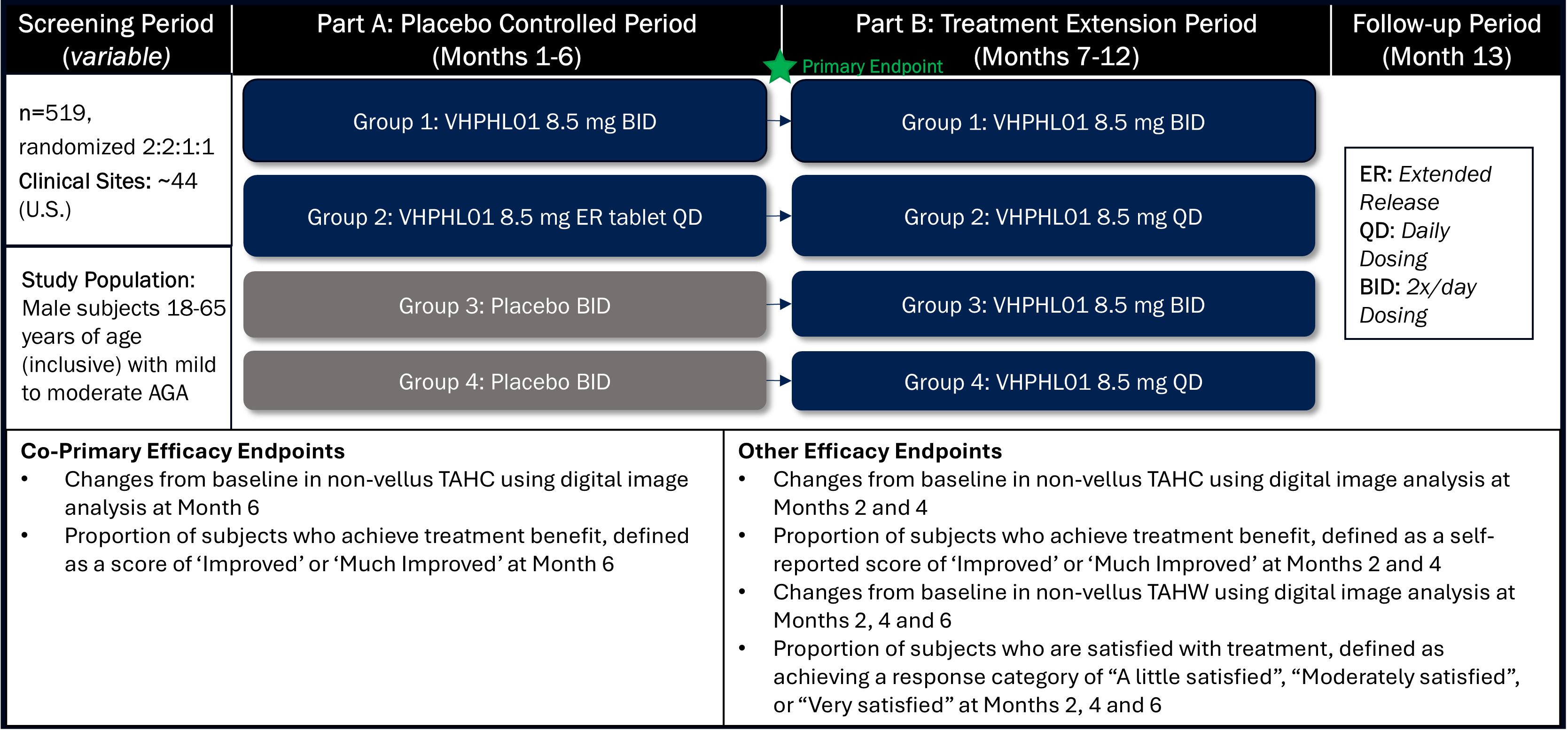
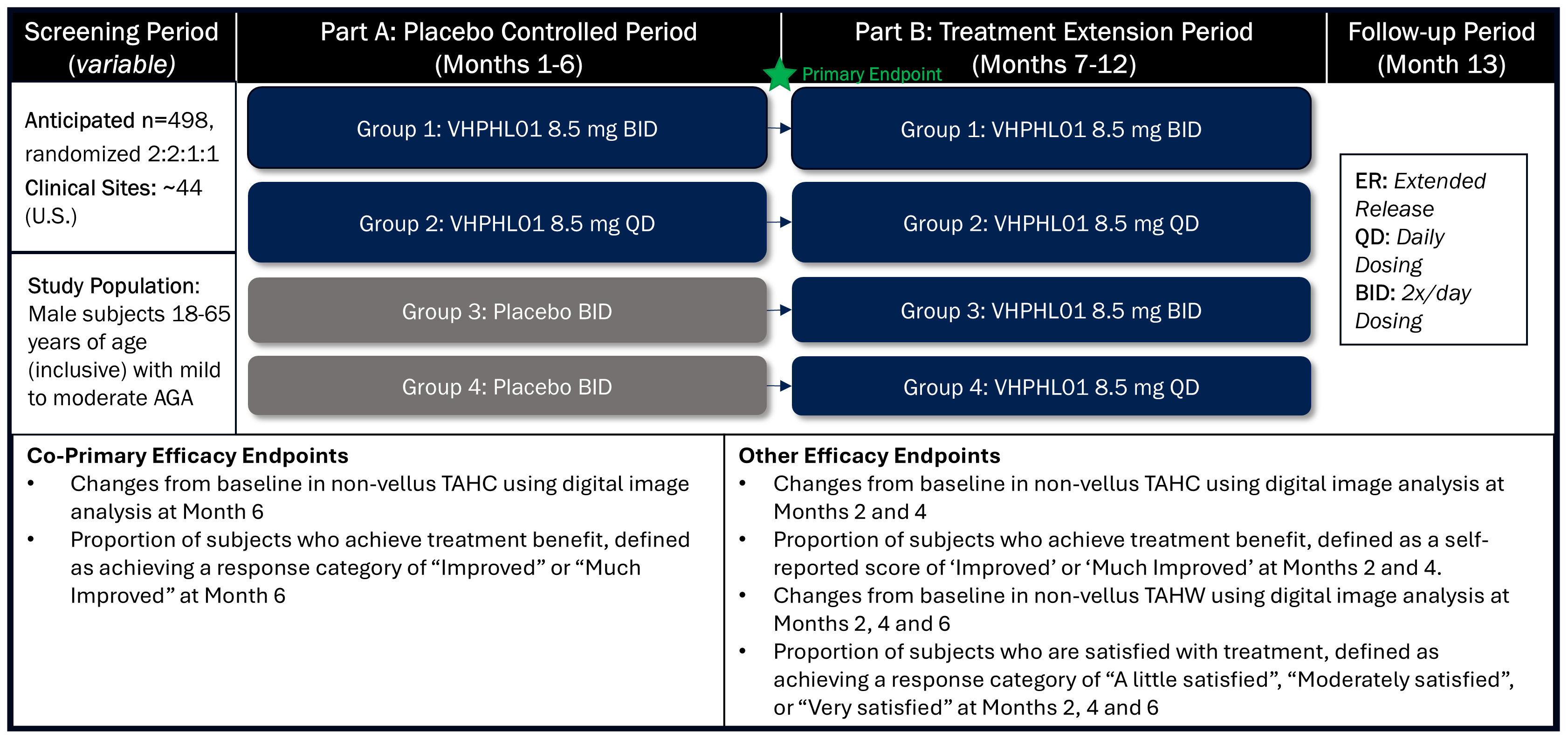
We are currently dosing patients with VDPHL01 in our registration-directed clinical program consisting of three pivotal, multi-center, randomized, double-blind, placebo-controlled clinical trials: two in male patients and one in female patients with PHL. These trials are designed to support our planned submissions to the FDA for regulatory approval across both male and female patient populations through a 505(b)(2) New Drug Application, or NDA. We have fully enrolled the first of these registration-directed clinical trials, a Phase 2/3 trial evaluating VDPHL01 in 519 male patients with mild-to-moderate PHL. This first trial assesses two dose regimens of VDPHL01 over 52 weeks of treatment. The co-primary endpoints are change in non-vellus hair count per square
centimeter and patient self-assessment of hair coverage benefit after 24 weeks from treatment initiation. We anticipate topline data from this clinical trial in the first half of 2026.
We have also initiated our confirmatory registration-directed Phase 3 trial targeting the enrollment of 498 male patients with mild-to-moderate PHL and our registrational-directed Phase 2/3 trial targeting the enrollment of 552 female patients with mild-to-moderate PHL. We expect to report data from this male Phase 3 trial in the second half of 2026. Enrollment in the female Phase 2/3 trial is ongoing and our projected time to topline data will be determined as the trial progresses. The clinical trials designated as Phase 2/3 are designed and conducted as registration-directed Phase 3 trials to support the planned marketing application, or NDA, for VDPHL01 for the treatment of PHL. The Phase 2/3 trials include a parallel Phase 2 component intended to further assess proprietary patient-reported outcome, or PRO, measures used as endpoints in all three registration-directed trials.
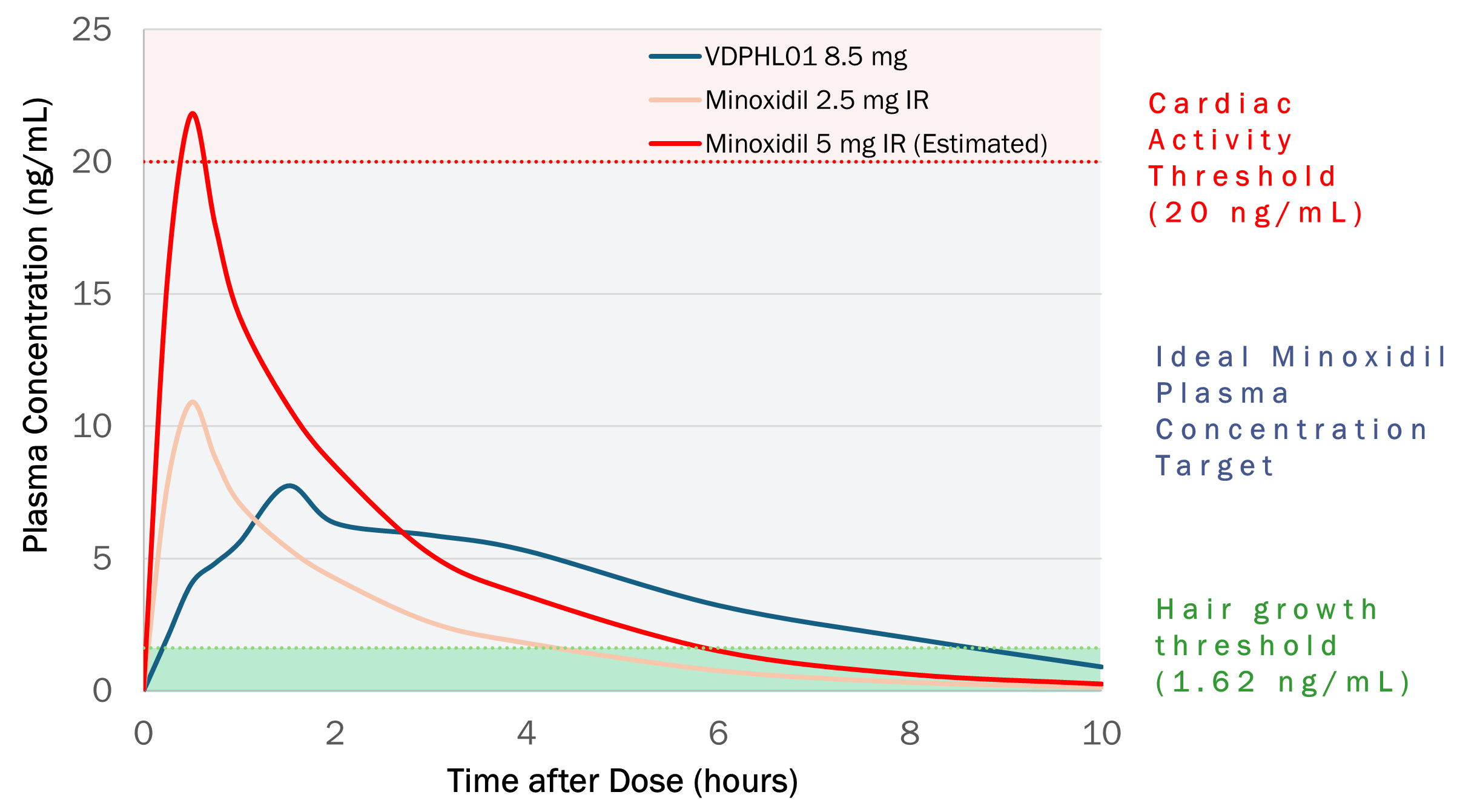
In a Phase 1 clinical trial, which we refer to as Study QSC300720, we studied VDPHL01 prototypes in doses up to 10 mg against a single reference dose of commercially available 2.5 mg IR oral minoxidil. When comparing the results of VDPHL01 8.5 mg with 2.5 mg IR oral minoxidil in male patients who were administered both dosage forms in this study, we found that VDPHL01 8.5 mg:
ndelivered nearly twice the total amount of minoxidil in plasma over 12 hours than the total amount delivered by the 2.5 mg IR oral minoxidil tablet;
nsustained plasma concentrations above minoxidil's hair growth threshold two times longer than a 2.5 mg IR oral minoxidil tablet;
nmaintained peak concentrations below the threshold at which signs of cardiac activity are typically observed; and
nwas generally well tolerated with no serious adverse events, or SAEs, observed.
As this study was exploratory, no formal sample size calculation was made related to statistical power. The data presented above are reflective of the study’s per-protocol primary objective, namely, to evaluate the PK profile and determine the relative bioavailability of VDPHL01 following single oral dosing of VDPHL01 versus the reference IR oral minoxidil formulation in healthy subjects.
We are currently conducting a Phase 2 clinical trial evaluating VDPHL01 in male patients and female patients with mild-to-moderate PHL. As this trial is exploratory, no formal sample size calculation was made related to statistical power. Therefore, all trial endpoints, including objective hair count measures and subjective patient and investigator assessments, are exploratory and are planned to be reported as descriptive statistics. In October 2025, we announced preliminary data from the male cohort for those who had completed four months of treatment in this trial (n=21; the female cohort, currently with 22 subjects dosing, initiated enrollment in this trial later than males, and similar data is not yet available).
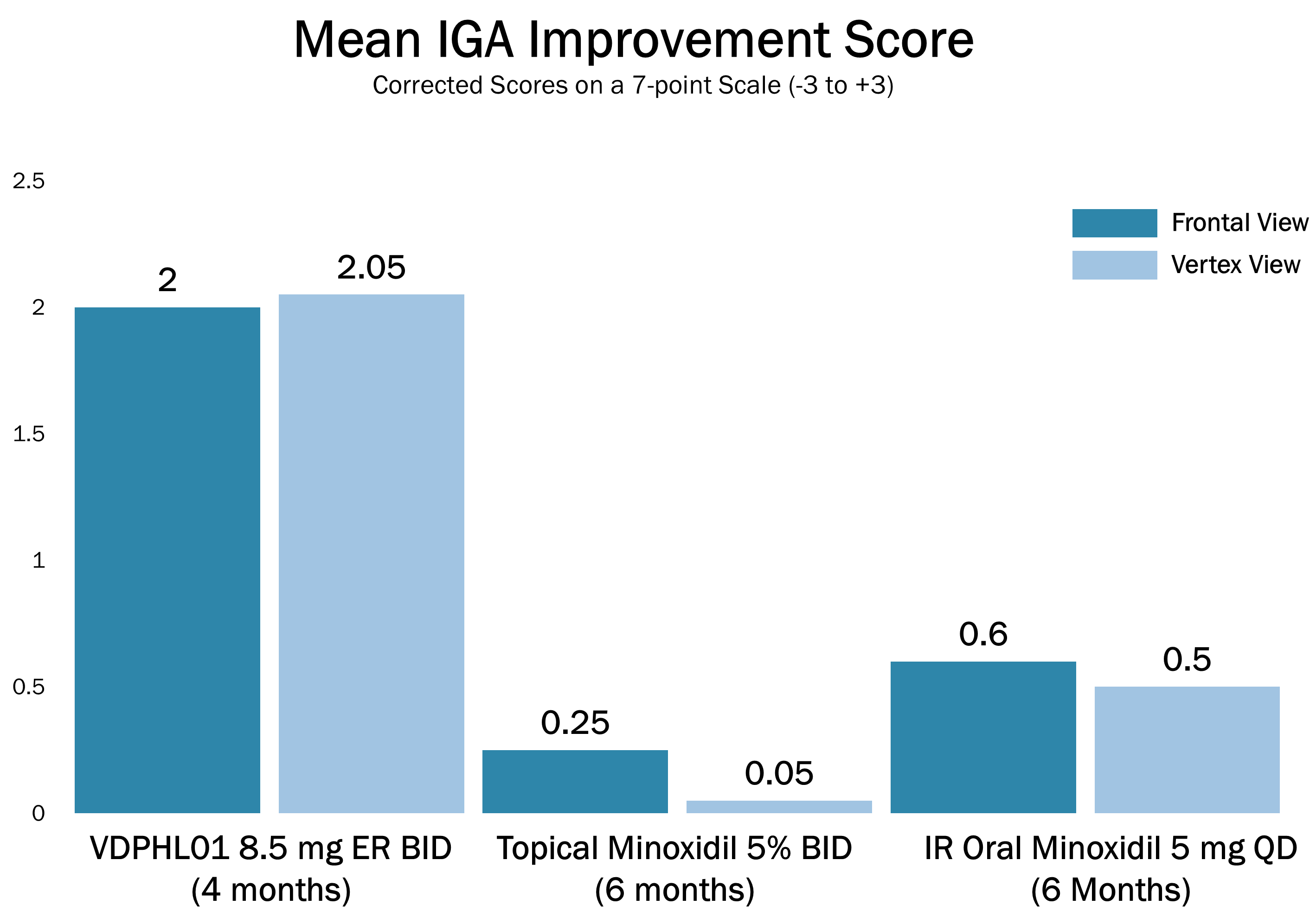
The preliminary data show that VDPHL01 drove favorable outcomes, which we believe underscores its potential to deliver a convenient, oral treatment that provides visible hair regrowth to the majority of users as early as two months, while maintaining a favorable tolerability profile. We believe that the results of this trial support the emerging product profile for VDPHL01 with the following key attributes:
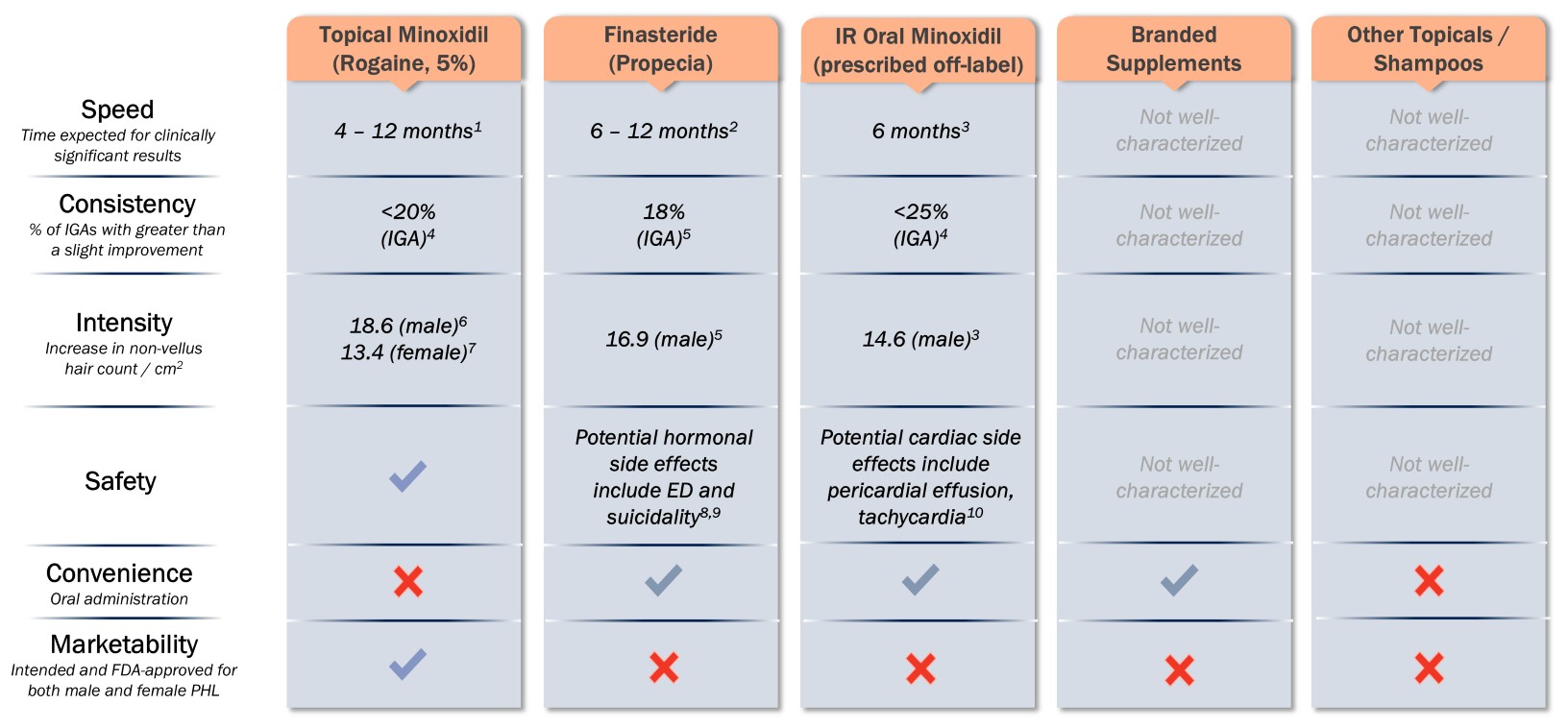
nSpeed: Visibly noticeable hair growth as early as two months after treatment in a majority of patients based on PRO, and an Investigator Global Assessment, or an IGA.
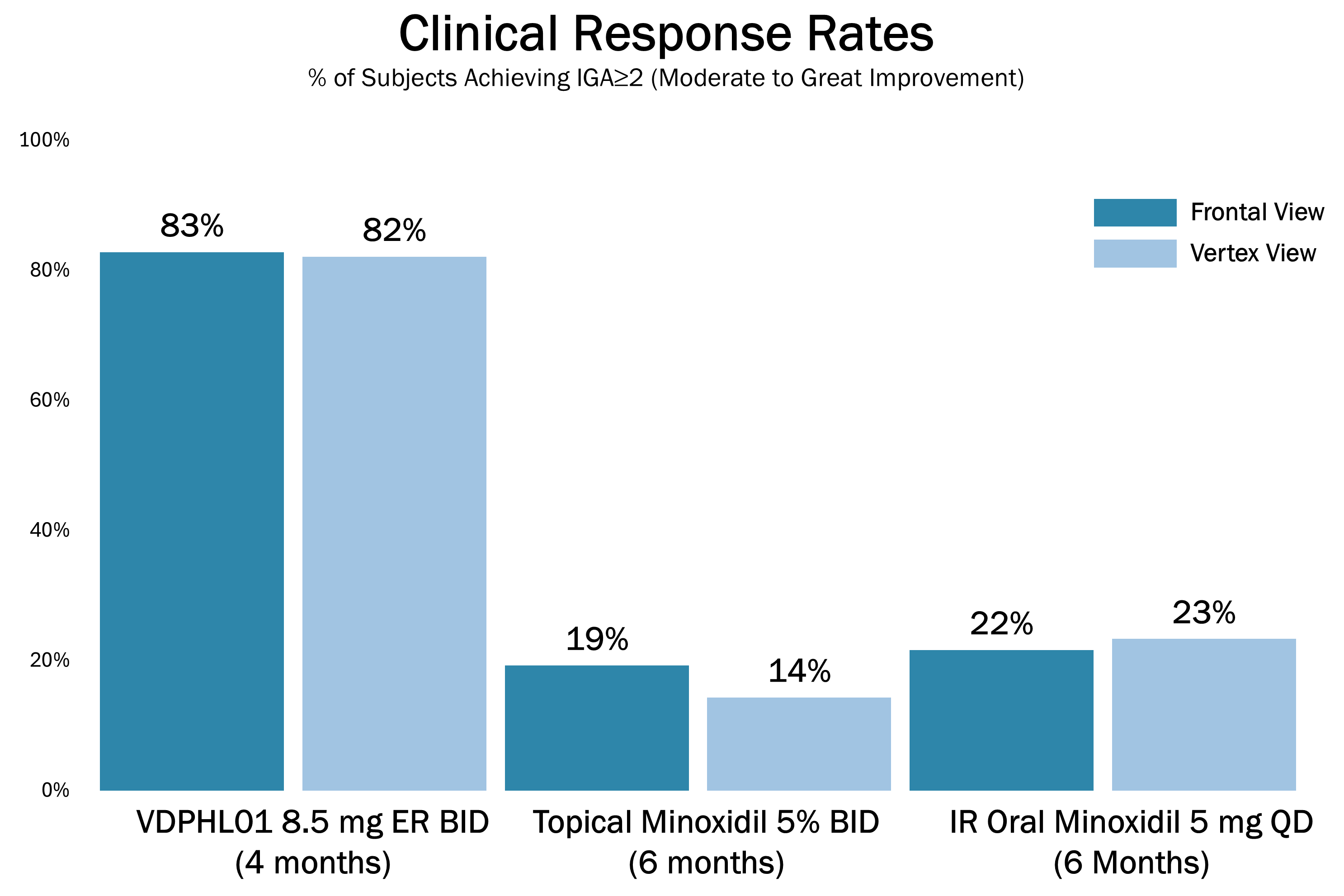
nConsistency: 90.5% treatment response at four months based on PRO of participants reporting “improved” or “much improved” hair coverage.
nIntensity: Average non-vellus (greater than 30 microns) hair count change of 47.3 hairs per cm2, with double digit absolute non-vellus hair count changes in greater than 90% of patients completing four months of treatment.
nSafety: Generally well tolerated, with no treatment-related SAEs, including no cardiac or hormonal-related issues to date.
nConvenience: Preference for oral over topical administration supported by third-party research.
nMarketability: Potential to be the first oral, non-hormonal FDA-approved therapy for PHL.
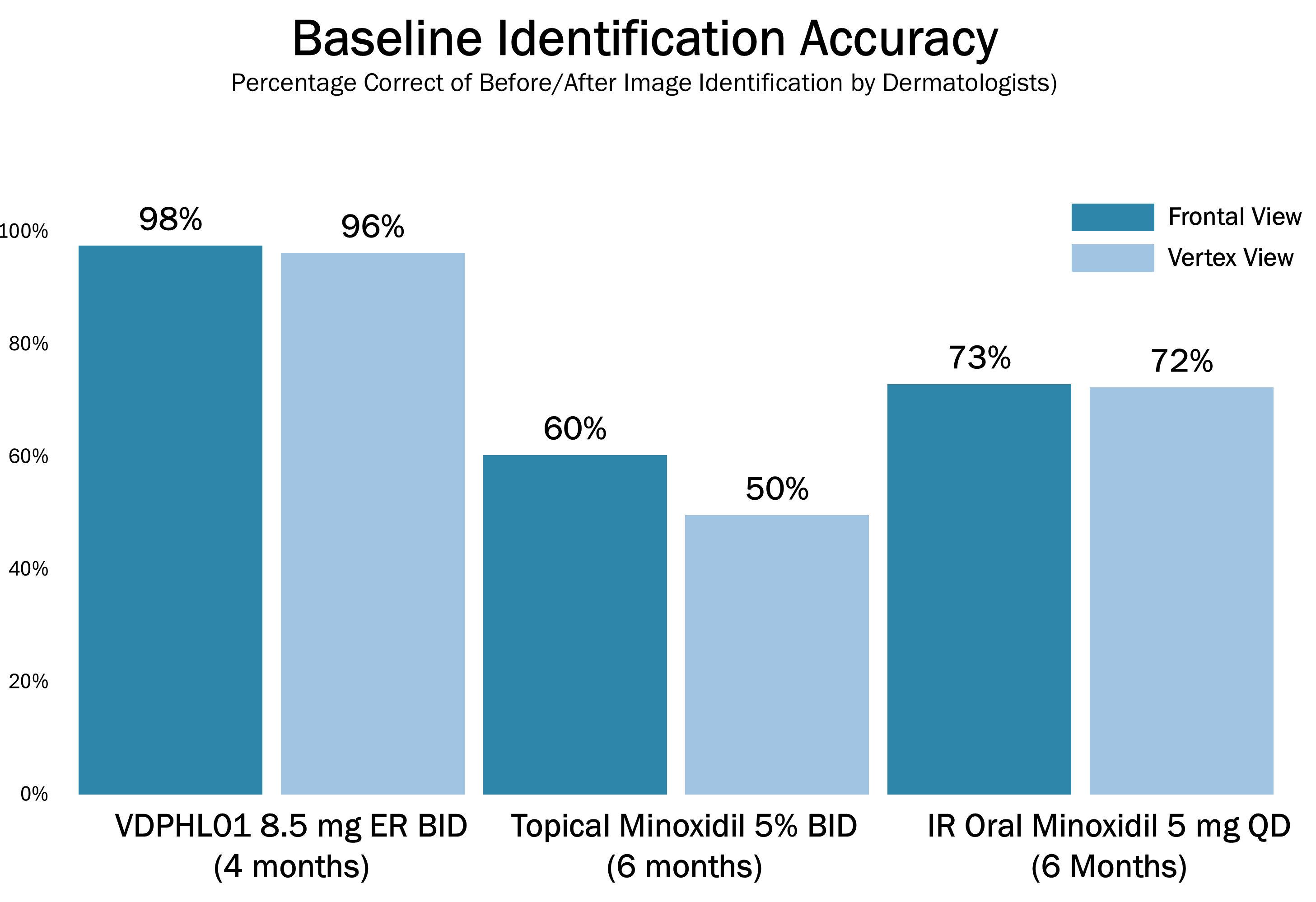
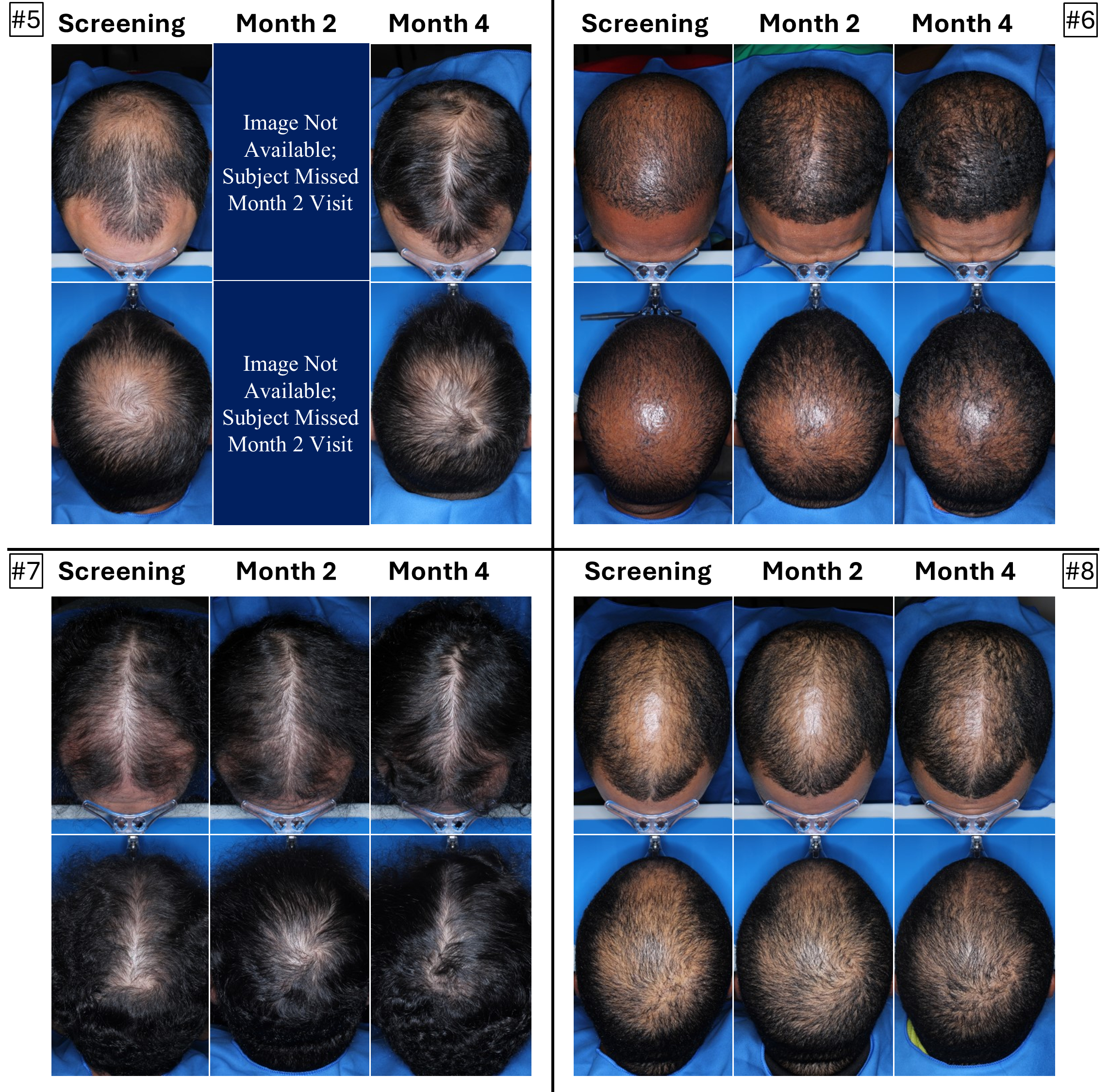
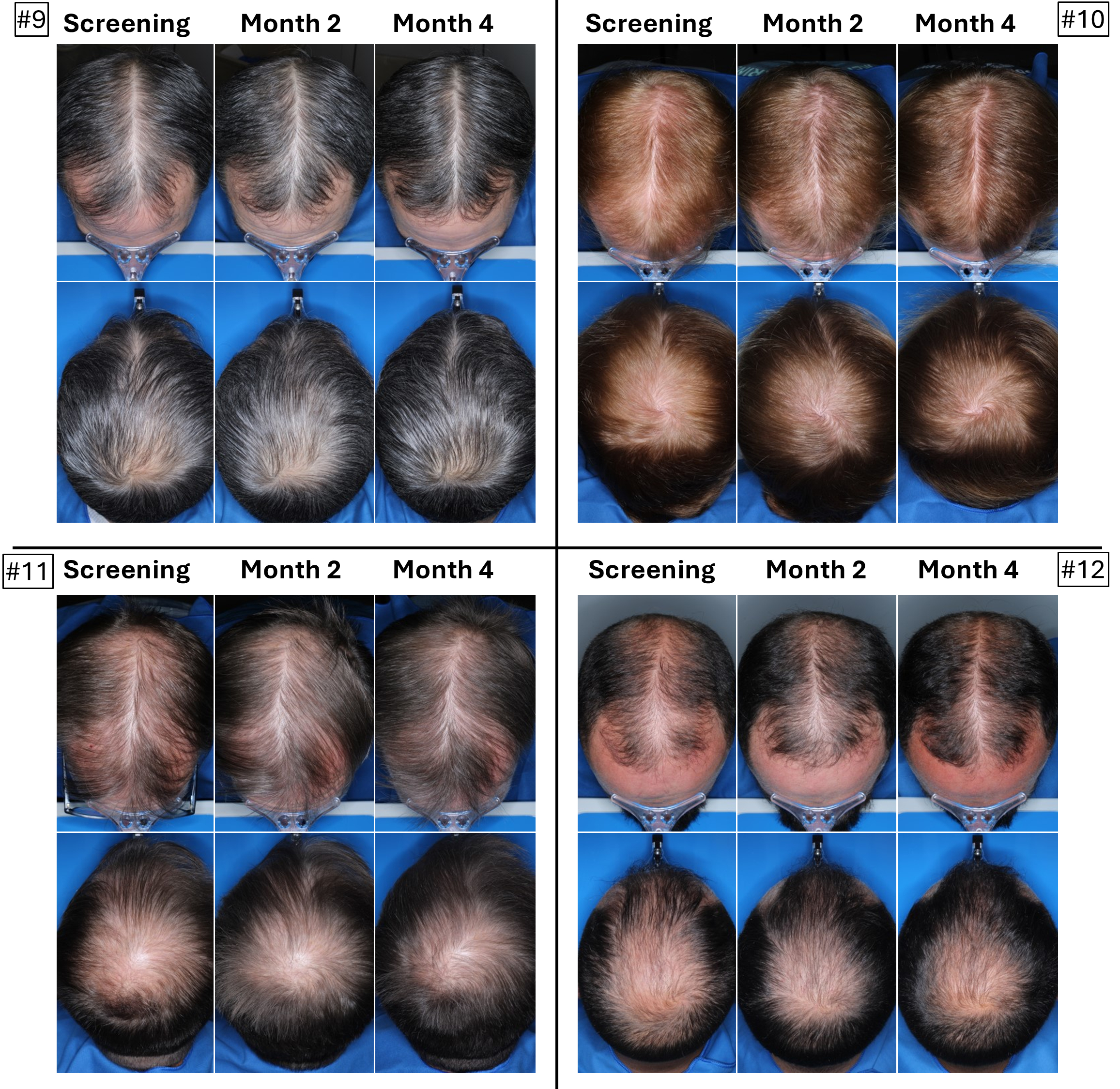
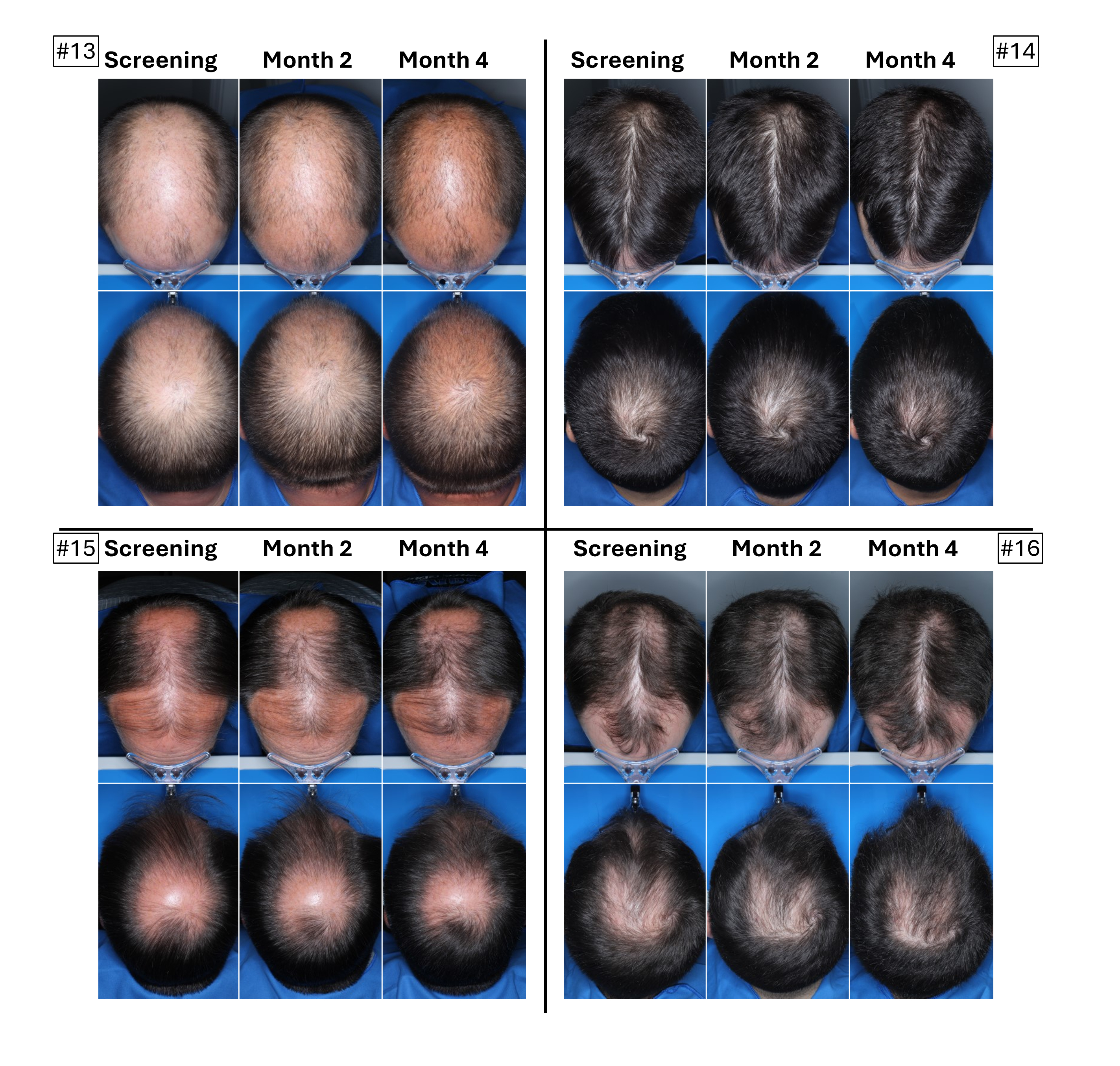
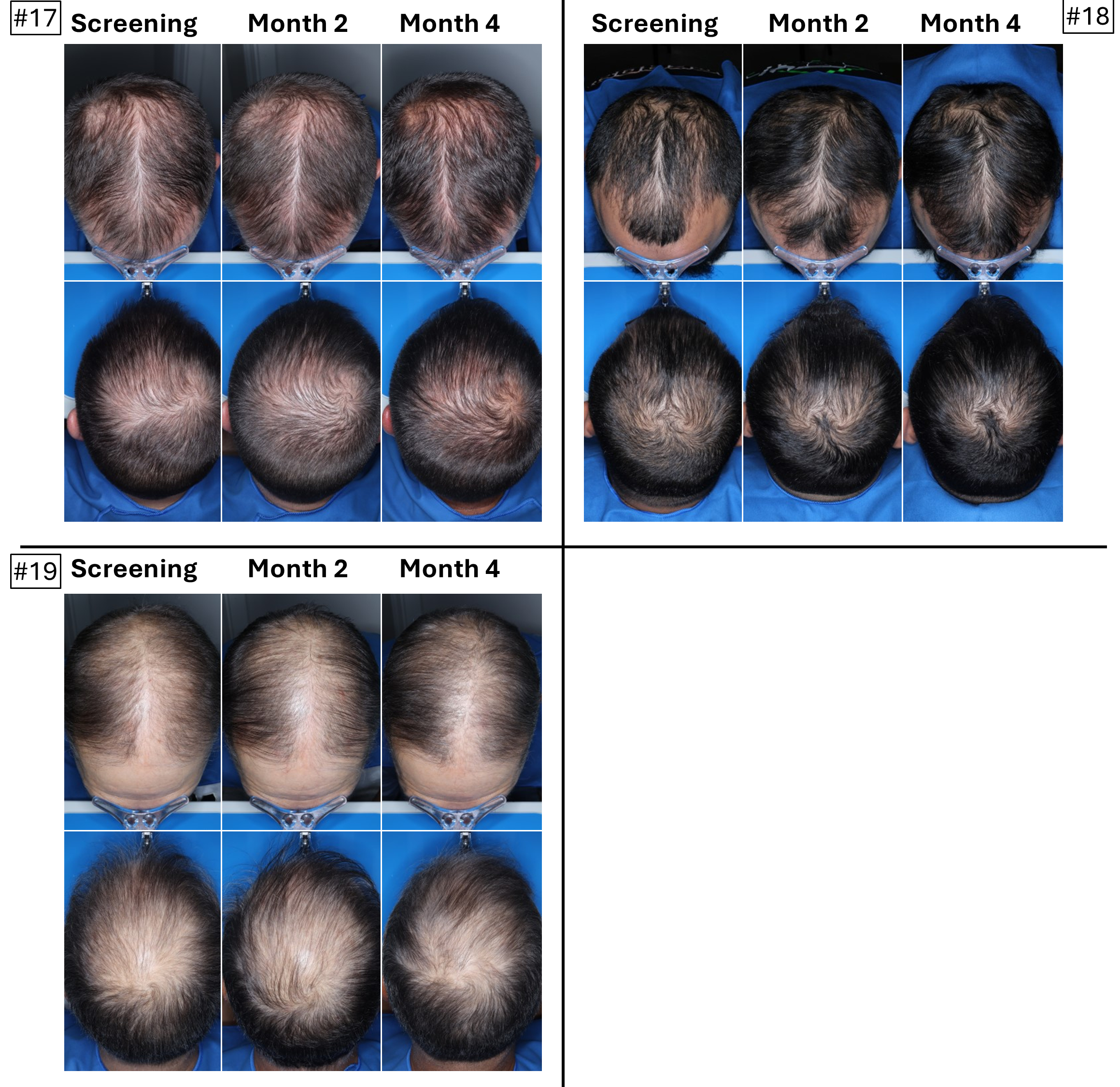
The below images are of the four patients, of 20 patients in the cohort as of August 2025, the time at which a blinded image analysis study was conducted. These patients were observed to have the highest average hair growth improvement scores as determined by expert graders using images from our Phase 2 clinical trial in male patients at month four. This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale, a commonly used tool for assessing change in hair growth ranging from greatly decreased (-3) to greatly increased (+3) with intermediate categories of no change (0), slightly decreased/increased (-1/+1) and moderately decreased/increased (-2/+2). Images for patients from screening, month two and month four are presented in order (highest to lowest) of the average improvement scores at month four. Average IGA improvement scores shown represent average improvement scores as assessed by the blinded expert graders ranging from +2.7 (patient #4) to +3.0 (patient #1). See “—Our Solution for PHL: VDPHL01—Ongoing Studies—Study 207: Our Phase 2 Open Label Proof of Concept Trial” for the full set of images from the study (images are excluded for patients (n=1) with improvement scores less than or equal to the 5th percentile or greater than or equal to the 95th percentile in both image views at month four).
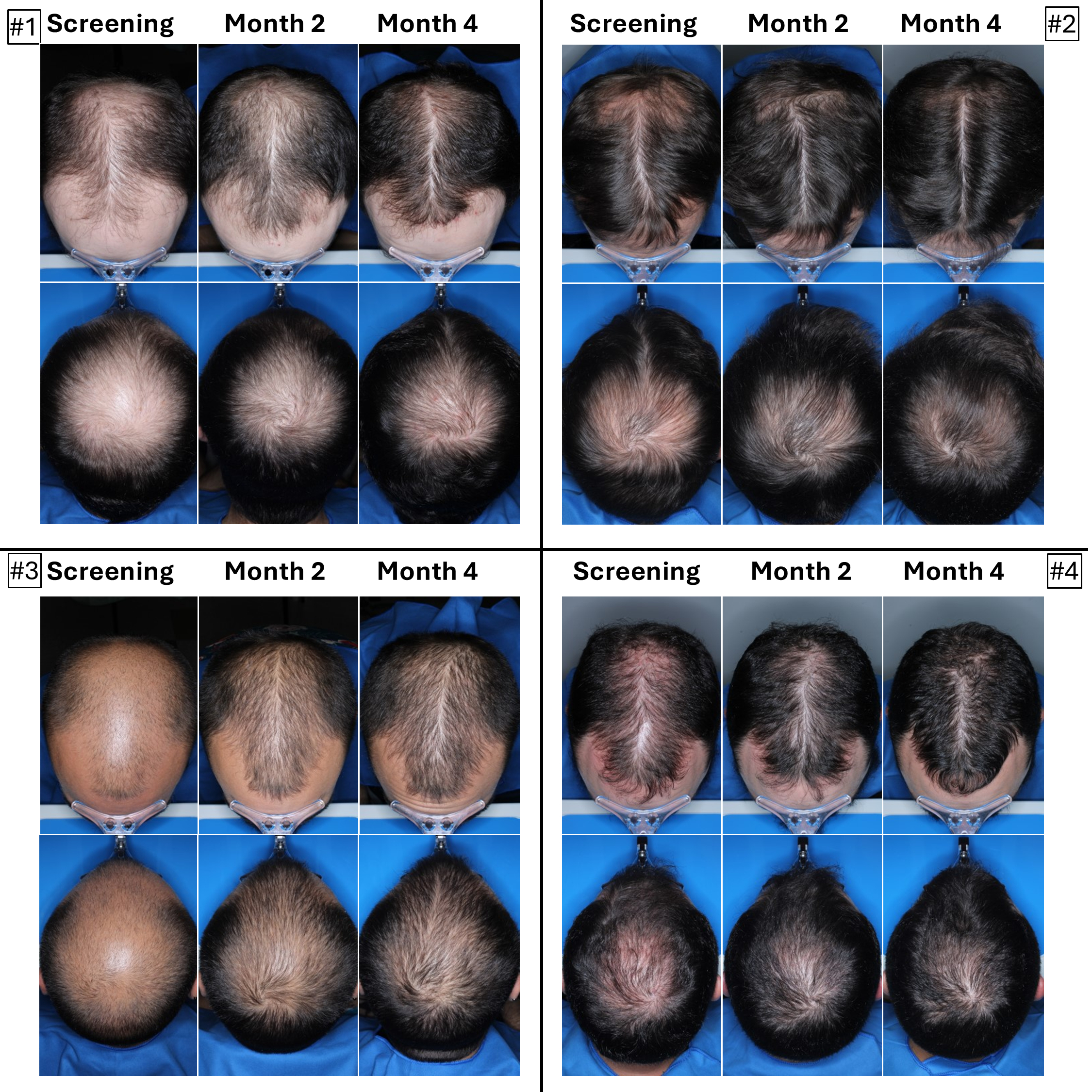
This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs of each treatment from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale (–3 to +3), a commonly used tool for assessing hair growth, measuring whether scalp hair growth has “slightly,” “moderately,” or “greatly” increased or decreased or no change has occurred.
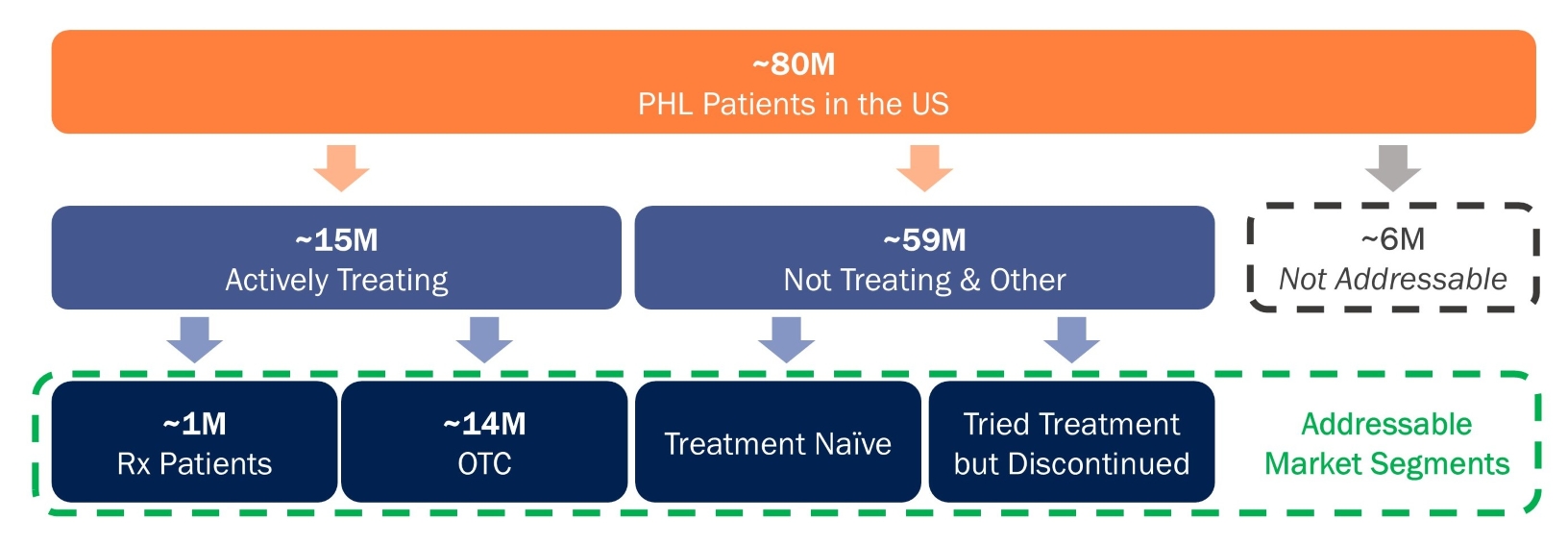
Our Phase 2 trial evaluating VDPHL01 in male patients and female patients with PHL is ongoing, and we have initiated three registration-directed trials evaluating VDPHL01, two in male patients with PHL and one in female patients with PHL. If approved, we believe VDPHL01’s commercial potential would be substantial, as we estimate that the current U.S. commercial opportunity for PHL treatments for men and women is valued at
approximately $9 billion annually, despite low patient engagement and high levels of dissatisfaction with current options reported. We believe that VDPHL01 could gain a meaningful share of this existing opportunity while also catalyzing substantial growth by offering a previously unavailable, transformative product profile to patients in an area of high unmet need. Our proprietary research indicates that 93% of patients would like to address their PHL yet only 9% are satisfied with their current treatment. Based on the initial product profile we have established for VDPHL01, we believe it could drive significant adoption in this large, motivated but underserved PHL patient population.
In anticipation of a potential NDA submission for VDPHL01 leveraging data from our registration-directed Phase 3 trials, we are developing a comprehensive multi-channel commercialization plan that will integrate patient identification, physician education, direct-to-consumer, or DTC, advertising, social media and telehealth engagement and customer care. This commercialization strategy will be designed to drive patient awareness of VDPHL01’s differentiated profile and demand for hair loss treatment. We believe that the success of analog therapies highlights both the unmet need and motivation among patients and reinforces the potential for VDPHL01 to create demand in a cash-pay market, convert over-the-counter, or OTC, patients to prescribed therapies and activate a population of untreated patients. We plan to focus commercial efforts at launch on establishing a dermatology-focused field force and conducting DTC advertising. We believe these commercial pillars will drive significant VDPHL01 adoption, if approved.
We maintain a broad library of patents and patent applications related to the key innovations of VDPHL01, including its method of utilizing an oral route of administration, ER formulation to stimulate hair growth as well as its optimized PK and PD qualities and profile. The earliest expiring patent term is 2043.
Our Pipeline
Our goal is to develop a focused portfolio of aesthetic dermatology product candidates targeting high-prevalence dermatologic conditions, with potential selective development of medical dermatology product candidates. We are advancing our lead product candidate, VDPHL01, in clinical trials as depicted below.
Our Team and Investors
Our team comprises a seasoned management team with deep connections in the dermatology physician and patient communities and expertise spanning dermatology, clinical development, regulatory affairs, operations, manufacturing and commercialization. The company is led by dermatologists who are well-regarded within the community, which we believe provides important credibility among physicians and insight into patient needs. Our corporate management team includes industry veterans with significant experience in both entrepreneurial
biotechnology and global pharmaceutical companies, and collectively, have authored more than 100 peer-reviewed publications. Our team has extensive late-stage clinical trial experience, having been involved in the successful development and approval of more than forty products. We are further supported by a board of directors comprised of thought-leading dermatologists, biotechnology industry leaders and corporate executives with collective decades of experience.
Since inception, we have raised $263.0 million from a broad syndicate of investors, including several leading investment advisers and life sciences funds, such as Longitude Capital, SR One, Surveyor Capital (a Citadel company), Suvretta Capital and Viking Global. Potential investors should not consider investments made by our existing investors when making a decision to purchase shares in this offering since our existing investors may have had different risk tolerances and paid less per share than the price at which the shares are being offered in this offering.
Our Competitive Strengths
We believe the following strengths will enable us to achieve our goal of becoming the leader in hair loss treatment:
nPotentially transformative late-stage lead product candidate, VDPHL01, innovating on validated science to address the millions of men and women suffering from PHL in the United States.
nClinical data to date supports a potentially differentiated profile for VDPHL01, with topline data from our initial registration-directed trial anticipated to read out in the first half of 2026.
nDermatology-rooted leadership with deep understanding of needs of PHL patients and physicians.
nComprehensive commercialization strategy to educate and engage physicians and patients.
nRobust intellectual property portfolio designed to protect the key innovations that drive VDPHL01’s therapeutic differentiation.
Our Strategy
Our goal is to develop a leading aesthetics- and dermatology-focused biopharmaceutical company that advances therapies to address significant unmet needs for large, underserved patient populations. In order to achieve this goal, we are currently advancing our lead candidate, VDPHL01, for the treatment of PHL. Our strategy involves the following key elements:
nEfficiently develop VDPHL01 through registration-directed trials to be the leading treatment of PHL for both men and women.
nBuild a sales and marketing organization focused on prescribers and dedicated to increasing patient awareness and activation.
nPosition for early and wide adoption through the cash-pay model.
nDeliver and commercialize VDPHL01, if approved, as the first FDA-approved oral treatment for female PHL.
nEvaluate strategic collaboration to maximize the value of our product candidates and deliver meaningful benefits to patients.
nIdentify additional solutions to pervasive unmet needs in dermatology.
Summary of Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks could materially and adversely impact our business, financial condition and results of operations, which could cause the trading price of our common stock to decline and could result in a loss of all or part of your investment. Additional risks, beyond those summarized below or discussed elsewhere in this prospectus, may apply to our business, activities or operations as currently
conducted or as we may conduct them in the future or in the markets in which we operate or may in the future operate. These risks include the following:
nWe are a clinical-stage biopharmaceutical company with a limited operating history and no products approved for commercial sale. We have incurred substantial losses since our inception, and we anticipate incurring substantial and increasing losses for the foreseeable future;
nEven if this offering is successful, we will require substantial additional financing to achieve our goals, and failure to obtain additional capital when needed, or on acceptable terms, would cause us to delay, limit, reduce or terminate our product development or commercialization efforts;
nWe currently anticipate that our success will depend on the approval and successful commercialization of VDPHL01, which is our lead product candidate. If we are unable to obtain regulatory approval for, and successfully commercialize, VDPHL01, or any of our other current or future product candidates, or experience significant delays in doing so, our business will be materially harmed;
nPreclinical and clinical development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future preclinical studies or clinical trial results. We may encounter substantial delays in preclinical and clinical trials, or may not be able to conduct or complete preclinical or clinical trials on the expected timelines, if at all. If our preclinical studies and clinical trials are not sufficient to support regulatory approval of any of our product candidates, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development of such product candidate;
nIf the FDA does not conclude that VDPHL01 satisfies the requirements for the Section 505(b)(2) regulatory approval pathway, or if the requirements for VDPHL01 under Section 505(b)(2) are not as we expect, the approval pathway for those product candidates will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not be successful;
nAny significant AEs or undesirable side effects caused by our product candidates may delay or prevent regulatory approval or market acceptance of our product candidates, or result in significant negative consequences following marketing approval, if any. Additionally, the clinical profile of VDPHL01 in female patients may differ from the clinical profile in male patients, and the outcomes observed to date in male patients may not be reflective or predictive of future outcomes for female patients;
nWe operate in highly competitive markets and face competition from large, well-established companies with significant resources as well as other entities, and, as a result, we may not be able to compete effectively;
nWe currently have limited marketing, sales or distribution infrastructure. If we are unable to fully develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we may not be successful in commercializing our product candidates;
nEven if we obtain regulatory approval for VDPHL01 or any other product candidates, such products may fail to achieve market acceptance which would adversely affect our efforts to commercialize any such product successfully;
nThe commercial opportunity for VDPHL01 and any of our other current or future product candidates we may develop may be smaller than we expect;
nOur strategy of focusing on the cash-pay healthcare market for VDPHL01 may limit our ability to increase sales or achieve profitability;
nIf we fail to effectively maintain, promote, and enhance our reputation and VDPHL01 brand recognition in a cost-effective manner, our business and competitive advantage may be harmed;
nWe are dependent on the services of our senior management and other key personnel, and if we are not able to retain these individuals or recruit additional management or key personnel, our business will suffer;
nWe will need to grow our organization, and we may experience difficulties in managing our growth and expanding our operations, which could adversely affect our business;
nOur commercial success depends on our ability to obtain and maintain sufficient intellectual property protection for VDPHL01 and our other current and any future product candidates and other proprietary technologies;
nIf the scope of any patent protection we obtain is not sufficiently broad, or if we lose any of our patent protection, our ability to prevent our competitors from commercializing similar or identical product candidates would be adversely affected;
nWe may not be able to protect our intellectual property rights throughout the world;
nThe regulatory approval process is highly uncertain, and we may be unable to obtain, or may be delayed in obtaining, U.S. regulatory approval and, as a result, unable to commercialize our product candidates or any future product candidates. Even if we believe our current, or planned clinical trials are successful, regulatory authorities may not agree that they provide adequate data on safety or efficacy;
nEven if we receive regulatory approval for any of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal. We may also be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates;
nWe currently rely on third parties for the manufacture of drug or biological substances for our preclinical studies and clinical trials and expect to continue to do so for commercialization of any product candidates that we may develop that are approved for marketing. Our reliance on third parties may increase the risk that we will not have sufficient quantities of such drug substance, product candidates, or any products that we may develop and commercialize, or that such supply will not be available to us at an acceptable cost, which could delay, prevent, or impair our development or commercialization efforts;
nWe have relied and expect to continue to rely on third parties to conduct our preclinical studies and clinical trials. If those third parties do not perform as contractually required, fail to satisfy legal or regulatory requirements, miss deadlines or terminate the relationship, our development programs could be delayed, more costly or unsuccessful, and we may never be able to seek or obtain regulatory approval for or commercialize our product candidates;
nAn active and liquid trading market for our common stock may not develop, and you may not be able to resell your shares of common stock at or above the public offering price, if at all; and
nThe market price of our common stock may be volatile, which could result in substantial losses for investors purchasing shares in this offering.
The foregoing is only a summary of some of our risks. For a more detailed discussion of these and other risks you should consider before making an investment in our common stock, see “Risk Factors.” Our Corporate Information
We were originally incorporated on October 5, 2019, as VeraDermics, Incorporated, a Texas corporation. On September 15, 2021, we converted to a Delaware corporation. Our principal executive offices are located at 470 James St., New Haven, Connecticut 06513, and our telephone number is (228) 372-3376. Our corporate website address is www.veradermics.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies, including reduced disclosure about our executive compensation arrangements, exemption from the requirements to hold non-binding advisory votes on executive compensation and golden parachute payments and exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting.
We may take advantage of these exemptions until the last day of the fiscal year following the fifth anniversary of this offering or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company earlier if we have more than $1.235 billion in annual revenue, we have more than $700.0 million in market value of our stock held by non-affiliates (and we have been a public company for at least 12 months and have filed one Annual Report on Form 10-K) or we issue more than $1.0 billion of non-convertible debt securities over a three year period. For so long as we remain an emerging growth company, we are permitted, and intend, to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. We may choose to take advantage of some, but not all, of the available exemptions.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. In particular, in this prospectus, we have provided only two years of audited financial statements and have not included all of the executive compensation-related information that would be required if we were not an emerging growth company. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected not to “opt out” of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we will adopt the new or revised standard at the time private companies adopt the new or revised standard and will do so until such time that we either (i) irrevocably elect to “opt out” of such extended transition period or (ii) no longer qualify as an emerging growth company. We may choose to early adopt any new or revised accounting standards whenever such early adoption is permitted for private companies. Therefore, the reported results of operations contained in our financial statements may not be directly comparable to those of other public companies.
We are also a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates plus the proposed aggregate amount of gross proceeds to us as a result of this offering is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company after this offering if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation. We may continue to be a smaller reporting company until the fiscal year following the determination that we no longer meet the requirements necessary to be considered a smaller reporting company.
THE OFFERING
| | | | | | | | |
| Common stock offered by us | | 13,350,000 shares. |
| | |
| Common stock to be outstanding after this offering | | 33,350,170 shares (35,352,670 shares if the underwriters exercise their option to purchase additional shares in full). |
| | |
| Underwriters’ option to purchase additional shares of common stock from us | | We have granted the underwriters an option to purchase up to an aggregate of 2,002,500 additional shares of common stock from us at the initial public offering price, less the estimated underwriting discounts and commissions, for a period of 30 days after the date of this prospectus. |
| | |
| Use of proceeds | | We estimate that our net proceeds from the sale of our common stock in this offering will be approximately $181.8 million, assuming an initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and marketable securities, to advance VDPHL01 through NDA approval and initial commercialization in the United States, if approved. These funds will support physician education, brand awareness initiatives, marketing, pre-commercial and commercial launch activities, including building our commercialization infrastructure and manufacture of commercial supply, with any remaining proceeds for business development activities and other general corporate purposes. See “Use of Proceeds.” |
Indication of interest | | The cornerstone investor has indicated an interest in purchasing up to $30.0 million in shares of common stock in this offering at the initial public offering price. The shares of common stock to be purchased by the cornerstone investor will not be subject to a lock-up agreement with the underwriters. Because this indication of interest is not a binding agreement or commitment to purchase, the cornerstone investor may determine to purchase more, less or no shares in this offering or the underwriters may determine to sell more, less or no shares to the cornerstone investor. The underwriters will receive the same underwriting discounts and commissions on any of our shares of common stock purchased by the cornerstone investor as they will from any other shares of common stock sold to the public in this offering. The number of shares of common stock available for sale to the public will be reduced to the extent that the cornerstone investor purchases shares of common stock in the offering. |
| | |
| Risk factors | | You should carefully read the “Risk Factors” section of this prospectus and the other information included in this prospectus for a discussion of factors that you should consider before deciding to invest in our common stock. |
| | |
| Proposed New York Stock Exchange symbol | | “MANE” |
The number of shares of our common stock to be outstanding immediately following the consummation of this offering is based on 19,999,437 shares outstanding as of September 30, 2025, after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Convertible Preferred Stock, or Series C Preferred Stock, issued after September 30, 2025
into an aggregate of 11,789,280 shares of our common stock, in each case immediately prior to the consummation of this offering. These amounts exclude:
n739,331 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2025, with a weighted average exercise price of $12.19 per share;
n2,974,637 shares of our common stock issuable upon the exercise of stock options granted after September 30, 2025, with a weighted average exercise price of $12.77 per share;
n685,080 shares of our common stock reserved for issuance under the VeraDermics, Incorporated 2021 Equity Incentive Plan, as amended, or the 2021 Plan, as of December 31, 2025, which will cease to be available for issuance at the time the Veradermics, Incorporated 2026 Incentive Plan, or the 2026 Plan, becomes effective and will be added to, and become available for issuance under, the 2026 Plan;
na number of shares of our common stock equal to 10.8% of shares issued and outstanding as of immediately following the consummation of this offering (not to exceed 4,518,426 shares) reserved for issuance under the 2026 Plan, which will become effective in connection with this offering (which includes up to 1,768,254 shares of common stock underlying stock option awards to be granted to certain of our directors, executive officers and other employees under the 2026 Plan upon the pricing of this offering with an exercise price per share equal to the initial public offering price per share), as well as any automatic increases in the number of shares of common stock reserved for future issuance thereunder; and
n316,668 shares of our common stock reserved for issuance under the Veradermics, Incorporated 2026 Employee Stock Purchase Plan, or the ESPP, which will become effective in connection with this offering.
Except as otherwise noted, all information in this prospectus assumes or gives effect to:
na 1-for-10.067 reverse stock split of our common stock, which was effected on January 27, 2026;
n(i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock, in each case immediately prior to the consummation of this offering;
nno exercise by the underwriters of their option to purchase up to an additional 2,002,500 shares of our common stock from us;
nthe filing and effectiveness of our restated certificate of incorporation and the adoption of our amended and restated bylaws immediately prior to the consummation of this offering; and
nno vesting or exercise of the outstanding stock options described above.
SUMMARY FINANCIAL DATA
You should read the following summary financial data together with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this prospectus and our financial statements and the related notes included elsewhere in this prospectus. The statements of operations for the years ended December 31, 2024 and 2023 have been derived from our audited financial statements included elsewhere in this prospectus. The statements of operations and comprehensive loss data for the nine months ended September 30, 2025 and 2024 and our balance sheet data as of September 30, 2025 have been derived from our unaudited financial statements included elsewhere in this prospectus. In the opinion of management, the unaudited financial data reflect all adjustments, consisting only of normal recurring adjustments, necessary for a fair statement of such financial information in those statements. Our historical results are not necessarily indicative of the results that may be expected in the future. The summary financial date in this section are not intended to replace the financial statements and the related notes thereto included elsewhere in this prospectus and are qualified in their entirety by those financial statements and the related notes. | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | Nine Months ended September 30, |
| (in thousands, except share and per share amounts) | 2024 | | 2023 | | 2025 | | 2024 |
| Operating Expenses: | | | | | | | |
Research and development | $ | 23,283 | | | $ | 14,971 | | | $ | 43,873 | | | $ | 18,333 | |
General and administrative | 3,495 | | | 2,353 | | | 5,463 | | | 2,474 | |
Total operating expenses | 26,778 | | | 17,324 | | | 49,336 | | | 20,807 | |
Loss from operations | (26,778) | | | (17,324) | | | (49,336) | | | (20,807) | |
| Other income (expenses): | | | | | | | |
Interest income | 481 | | | 870 | | | 656 | | | 272 | |
Other income | 394 | | | — | | | 532 | | | — | |
Interest expense | (585) | | | (36) | | | — | | | (293) | |
Total other income (expense), net | 290 | | | 834 | | | 1,188 | | | (21) | |
Loss before income taxes | (26,488) | | | (16,490) | | | (48,148) | | | (20,828) | |
Income tax benefit | — | | | — | | | — | | | — | |
Net loss | $ | (26,488) | | | $ | (16,490) | | | $ | (48,148) | | | $ | (20,828) | |
Net loss attributable to common stock | $ | (27,303) | | | $ | (16,490) | | | $ | (55,166) | | | $ | (20,828) | |
Net loss per share of common stock, basic and diluted(1) | $ | (37.05) | | | $ | (22.38) | | | $ | (74.21) | | | $ | (28.26) | |
Weighted average common stock outstanding, basic and diluted(1) | 736,933 | | | 736,933 | | | 743,401 | | 736,933 |
Pro forma net loss per share of common stock, basic and diluted(2) | $ | (1.85) | | | $ | (1.21) | | | $ | (2.42) | | | $ | (1.51) | |
Pro forma weighted average common stock outstanding, basic and diluted(2) | 14,351,418 | | | 13,648,780 | | | 19,906,853 | | | 13,804,188 | |
| | | | | | | |
(1)See Note 7 to our unaudited condensed consolidated financial statements and Note 7 to our audited consolidated financial statements included elsewhere in this prospectus for details on the calculation of basic and diluted net loss per share attributable to holders of our common stock and the weighted-average number of shares of common stock used in the computation of the per share amounts.
(2)Pro forma basic and diluted net loss per share of common stock has been prepared to give effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock, in each case as if such conversion had occurred at the beginning
of the period presented or the issuance dates of the respective preferred stock, if later, and (iii) the filing and effectiveness of our restated certificate of incorporation, which will be effective immediately prior to the consummation of this offering.
| | | | | | | | | | | |
| As of December 31, |
| (in thousands) | 2024 | | 2023 |
Consolidated Balance Sheet Data: | | | |
| Cash and cash equivalents | $ | 53,084 | | | $ | 16,296 | |
Working capital | 50,817 | | | 14,510 | |
Total assets | 55,535 | | | 17,562 | |
Total liabilities | 4,718 | | | 3,838 | |
Redeemable convertible preferred stock | 99,297 | | | 35,966 | |
Accumulated deficit | (49,231) | | | (22,743) | |
Total stockholders' deficit | $ | (48,480) | | | $ | (22,242) | |
| | | |
| | | | | | | | | | | | | | | | | |
| As of September 30, 2025 |
| (in thousands) | Actual | | Pro Forma(1) | | Pro Forma as Adjusted(2) |
Consolidated Balance Sheet Data: | | | | | |
Cash, cash equivalents and marketable securities | $ | 15,139 | | | $ | 165,702 | | | $ | 347,535 | |
Working capital(3) | 13,019 | | | 163,582 | | | 345,415 | |
Total assets | 18,797 | | | 169,360 | | | 351,193 | |
Total liabilities | 5,710 | | | 5,710 | | | 5,710 | |
Redeemable convertible preferred stock | 109,102 | | | — | | | — | |
Accumulated deficit | (97,379) | | | (97,379) | | | (97,379) | |
Total stockholders’ deficit | (96,015) | | | 163,649 | | | 345,482 | |
| | | | | |
(1)The pro forma balance sheet data gives effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock immediately prior to the consummation of this offering, (ii) the issuance and sale of 118,682,683 shares of Series C Preferred Stock, including the net proceeds from such sale, which occurred subsequent to September 30, 2025, and the automatic conversion of all outstanding shares of Series C Preferred Stock into an aggregate of 11,789,280 shares of our common stock immediately prior to the consummation of this offering, and (iii) the filing and effectiveness of our restated certificate of incorporation and amended and restated bylaws, which will be effective immediately prior to the consummation of this offering.
(2)The pro forma as adjusted balance sheet data give further effect to our issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
(3)We define working capital as current assets less current liabilities. See our unaudited condensed consolidated financial statements and the related notes included elsewhere in this prospectus for further details regarding our current assets and current liabilities.
The pro forma as adjusted information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing. A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash, cash equivalents and marketable securities, working capital, total assets and total stockholders’ (deficit) equity by $12.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash, cash equivalents and marketable securities, working capital, total assets and total stockholders’ (deficit) equity by $14.0 million, assuming no change in the assumed initial public offering price per share and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
RISK FACTORS
Investing in our common stock involves a high degree of risk. Before deciding to invest in shares of our common stock, you should carefully consider the risks described below, together with the other information contained in this prospectus, including in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in our audited financial statements and the related notes. The events discussed below may occur and adversely impact our business, financial condition, results of operations and prospects, which may cause the trading price of our common stock to decline, resulting in you losing all or part of your investment. Risks Related to Our Financial Position and Need for Capital
We are a clinical-stage biopharmaceutical company with a limited operating history and no products approved for commercial sale. We have incurred substantial losses since our inception, and we anticipate incurring substantial and increasing losses for the foreseeable future.
We are a clinical-stage biopharmaceutical company with a limited operating history on which to base your investment decision. We were formed in 2019 and our operations to date have been limited to organizing, staffing, and financing our company, conducting research and development activities, conducting clinical trials for our product candidates and establishing our intellectual property portfolio. We have never obtained regulatory approval for, or commercialized, a pharmaceutical product. We currently generate no revenue from sales of any products, and we may never be able to develop or commercialize a marketable product. We have financed our operations with $263.0 million in gross proceeds from equity financings to date. Biopharmaceutical product development is a highly speculative undertaking, involving substantial upfront capital expenditure and significant risk. Any product candidate may fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval or become commercially viable, despite substantial investment on development or commercialization.
We have incurred, and will continue to incur, significant expenses related to the clinical development of VDPHL01 and our other current and any future product candidates and ongoing operations. Our net losses for the nine months ended September 30, 2025 and 2024 were $48.1 million and $20.8 million, respectively. Our net losses for the years ended December 31, 2024 and 2023 were $26.5 million and $16.5 million, respectively. As of September 30, 2025, we had an accumulated deficit of $97.4 million. Substantially all of our operating losses have resulted from expenses incurred in connection with development of VDPHL01 and our other product candidates and from general and administrative costs associated with our operations. We expect to incur significant losses for the foreseeable future, and we expect these losses to increase as we advance VDPHL01 in our ongoing Phase 3 trials and, if results are positive, prepare for the commercialization of VDPHL01, if approved.
We anticipate that our expenses will increase substantially if, and as, we:
ncontinue development of VDPHL01 and our other current product candidates, including preclinical development and conducting clinical trials;
nseek marketing regulatory approvals for VDPHL01 and for any of our other current or any future product candidates that successfully complete clinical trials;
ntake steps toward supporting commercial activities, including establishing sales, marketing and distribution infrastructure;
nincrease marketing in connection with the potential commercialization of VDPHL01, if approved;
nadvance additional product candidates through preclinical development and clinical trials;
nidentify additional product candidates and acquire rights from third parties to those product candidates through licenses or acquisitions and conduct development activities, including preclinical studies and clinical trials;
nmake royalty, milestone or other payments under any current or future license or collaboration agreements;
nprocure the manufacturing of preclinical, clinical and commercial supply of our current or any future product candidates;
nestablish agreements with contract research organizations, or CROs, and contract manufacturing organizations, or CMOs;
nattract, hire and retain additional qualified clinical, scientific, operations and management personnel;
nseek to continue to develop, maintain and defend our intellectual property portfolio, including against third-party interference, infringement and other intellectual property claims, if any;
nadd and maintain operational, financial and information management systems;
nattempt to address any competing therapies and market developments;
nexperience delays in our preclinical studies, clinical trials or regulatory approval for our current or any future product candidates, including with respect to failed studies, inconclusive results, safety issues or other regulatory challenges; and
nincur additional costs associated with being a public company, including audit, legal, regulatory and tax-related services associated with maintaining compliance with an exchange listing and the Securities and Exchange Commission, or the SEC, requirements, director and officer insurance premiums and investor relations costs.
We may never succeed in these activities and, even if we do, may never generate any revenue or revenue that is significant enough to achieve profitability. Even if we succeed in commercializing VDPHL01 or one or more of our other product candidates, we will incur substantial expenditures to develop and market additional product candidates. We also may encounter unforeseen expenses, difficulties, complications, delays and other events that adversely affect our business. As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of public or private equity offerings and debt financings or other sources, such as potential collaboration agreements, strategic alliances and licensing arrangements. The size of our future net losses will depend, in part, on the rate that our expenses increase and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and our working capital.
Even if we achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the value of our company and could impair our ability to raise capital, expand our business, maintain our development efforts, obtain product approvals, diversify our offerings or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Even if this offering is successful, we will require substantial additional financing to achieve our goals, and failure to obtain additional capital when needed, or on acceptable terms, would cause us to delay, limit, reduce or terminate our product development or commercialization efforts.
The development of biopharmaceutical product candidates, including conducting preclinical studies and clinical trials, is a time-consuming, capital-intensive and uncertain process. We expect our expenses to substantially increase in connection with our ongoing and future activities, specifically as we advance development of VDPHL01 in our ongoing Phase 3 trials and prepare for potential commercialization of VDPHL01, if approved. In addition, because the outcome of any clinical trial or preclinical study is uncertain, we cannot reliably estimate the actual amount of capital necessary to successfully complete the development and commercialization of our other product candidates, and if we obtain regulatory approval for our other current or any future product candidates we also expect to incur significant commercialization expenses of such products. Furthermore, following the completion of this offering, we expect to incur additional costs associated with operating as a public company including significant legal, accounting, investor relations, and other expenses that we did not incur as a private company.
Subsequent to December 31, 2024, we completed the second funding of our Series B Preferred Stock financing of $8.4 million in the first quarter of 2025, followed by a Series C Preferred Stock financing in the fourth quarter of 2025, from which we received gross proceeds of $151.0 million. As of September 30, 2025, we had $15.1 million in cash, cash equivalents and marketable securities, which did not include the proceeds from the Series C Preferred Stock financing. We expect the net proceeds from this offering, together with our existing cash, cash equivalents and marketable securities, will be sufficient to fund our operating expenses and capital expenditure requirements for more than twelve months from the date of this prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect. We expect to attempt to raise additional cash in advance of exhausting our available capital resources.
We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for a product candidate, and we may never generate significant revenue or profits. If we obtain regulatory approval for VDPHL01 or any of our other current or any future product candidates, and do not enter into a third-party commercialization partnership, we expect to incur significant expenses related to developing our commercialization capability to support product sales, marketing, manufacturing and distribution activities. We also may require additional capital to pursue in-licenses or acquisitions of other product candidates. In addition, upon the completion of this offering, we expect to incur additional costs associated with operating as a public company, including significant legal, accounting, investor relations, and other expenses that we did not incur as a private company. As a result, we expect to incur substantial operating losses and negative operating cash flows for the foreseeable future.
Because of the numerous risks and uncertainties, length of time and scope of activities associated with research, development and commercialization of product candidates, we are unable to estimate the exact amount of our working capital requirements. Our future funding requirements, both near and long-term, will depend on, and could increase significantly as a result of, many factors, including, but not limited to:
nthe initiation, progress, timing, costs and results of our clinical trials through all phases of development, including our ongoing clinical trials for VDPHL01 and the development of our other current and any future product candidates;
nthe number and scope of preclinical and clinical programs we decide to pursue;
nthe identification, assessment, acquisition and/or development of additional research programs and additional product candidates;
nthe timing of and successful patient enrollment in, and the initiation and completion of, clinical trials;
nthe outcome, timing and costs of meeting regulatory requirements established by the FDA or any comparable foreign regulatory authority, including any additional clinical trials required by the FDA or any comparable foreign regulatory authority;
nthe willingness of the FDA or any comparable foreign regulatory authorities to accept our clinical trial designs, as well as data from our completed and planned preclinical studies and clinical trials, as the basis for review and approval of VDPHL01 and any other product candidates;
nthe progress, timing and costs of the development by us or third parties of companion diagnostics, if required, for VDPHL01 or any other product candidates, including design, manufacturing and regulatory approval;
nthe timing, receipt and terms of any marketing approvals from applicable regulatory authorities;
nour ability to establish new licensing or collaboration arrangements;
nthe performance of our future collaborators, if any;
ndevelopment and timely delivery of commercial-grade drug formulations that can be used in our planned clinical trials and for commercialization;
nthe cost of filing, prosecuting and enforcing our patent claims and other intellectual property rights;
nthe cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us;
nthe costs associated with potential clinical trial liability or product liability claims, including the costs associated with obtaining insurance against such claims and with defending against such claims;
nthe effect of competing technological and market developments;
nour ability to hire additional personnel and consultants as our business grows, including additional executive officers and clinical development, regulatory, chemistry, manufacturing and controls, quality and commercial personnel;
nour ability to develop and commercialize products that are considered by physicians, patients and payors as medically and/or financially differentiated as compared to competitive products;
nour ability to establish arrangements with third-party manufacturers for the commercial supply of products that receive marketing approval, if any;
nthe cost of making royalty, milestone or other payments under any future in-license agreements;
nthe extent to which we in-license or acquire additional product candidates or technologies;
nthe cost of establishing sales, marketing and distribution capabilities for our product candidates, if approved;
nthe initiation, progress and timing of our commercialization of VDPHL01, if approved, or any other product candidates;
nour ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors, if applicable, and adequate market share and revenue;
nmaintaining a continued acceptable safety profile of the product candidates following approval; and
nthe costs of operating as a public company.
We expect that our commercial revenue, if any, will initially be derived from sales of VDPHL01, which we do not expect to be commercially available in the near-term, if ever. Accordingly, we will need to rely on additional financing to achieve our business objectives until such time as we can generate sufficient commercial revenue. Adequate additional financing may not be available to us on acceptable terms, or at all, including as a result of financial and credit market deterioration or instability, including as a result of tariffs, fluctuations in inflation rates and concerns of a recession in the United States or other major markets, market-wide liquidity shortages, geopolitical events or otherwise. Weakness and volatility in the capital markets and the economy in general could also increase our costs of borrowing. If we are unable to raise sufficient additional capital, we would be forced to curtail our planned operations and the pursuit of our growth strategy.
Raising additional capital may cause dilution to our stockholders, including purchasers of common stock in this offering, imposing restrictions on our operations or require us to relinquish rights to our product candidates.
Until such time, if ever, that we generate substantial product revenue, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, including potential collaborations, licenses and other arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our common stock. Any future debt or preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, selling or licensing our assets, making capital expenditures, declaring dividends or encumbering our assets to secure future indebtedness. Such restrictions could adversely impact our ability to conduct our operations and execute our business plan.
If we raise additional funds through future collaborations, licenses and other arrangements, we likely would relinquish valuable rights to our potential future revenue streams or product candidates. We also may grant licenses on terms that may not be favorable to us or that reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, or on acceptable terms, we would be required to delay, limit, reduce or terminate our product development efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Further, we may not be able to access a portion of our existing cash due to market conditions. If banks and financial institutions with whom we hold accounts enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our existing cash may be threatened and could have a material adverse effect on our business and financial condition.
Risks Related to the Development of Our Product Candidates
We currently anticipate that our success will substantially depend on the approval and successful commercialization of VDPHL01, which is our lead product candidate. If we are unable to obtain regulatory approval for, and successfully commercialize, VDPHL01, or any of our other current or future product candidates, or experience significant delays in doing so, our business will be materially harmed.
We have never obtained regulatory approval for, or commercialized, a pharmaceutical product. With respect to VDPHL01 in PHL, we may pursue a marketing application to initially seek approval in male patients followed by an sNDA for female patients, or could alternatively pursue approval in both male and female patients simultaneously. The FDA may require us to conduct additional trials to support approval if it does not think our existing data are sufficient to support approval. We have invested a significant portion of our efforts and financial resources in the development of our most advanced product candidate, VDPHL01, for the treatment of PHL in male patients and female patients. Our business substantially depends on the successful development and commercialization of our product candidates, and in particular, VDPHL01, which may never occur. We currently generate no revenue from sales of any products, and we may never be able to develop or commercialize a marketable product. We do not expect to generate significant revenue, if any, unless and until we are able to obtain regulatory approval for, and successfully commercialize, VDPHL01, or for any of our other current or future product candidates. Successful commercialization of a product candidate requires achievement of many key milestones, including obtaining regulatory approval. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We are not permitted to market or promote any of our product candidates, including VDPHL01, before we receive regulatory approval from the FDA or comparable foreign regulatory authorities, and we may never receive such regulatory approval for any of our product candidates. Even if VDPHL01 or any of our other current or future product candidates is approved, they may be subject to limitations on the indicated uses for which they may be marketed, distribution restrictions, or to other conditions of approval, may contain significant safety warnings, including boxed warnings, contraindications, and precautions, may not be approved with label statements necessary or desirable for successful commercialization, or may contain requirements for costly post-market testing and surveillance, or other requirements, including the submission of a risk evaluation and mitigation strategy, or REMS, to monitor the safety or efficacy of the products.
We have not previously submitted an NDA to the FDA, or similar drug approval filings to comparable foreign authorities, for any product candidate, and we cannot be certain that VDPHL01 or any other of our current or future product candidates will be successful in clinical trials or receive regulatory approval. Our product candidates are susceptible to the risks of failure inherent at any stage of product development, including the appearance of unexpected AEs or failure to achieve its primary endpoints in subsequent clinical trials. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials.
The future regulatory and commercial success of VDPHL01 and any of our other current or future product candidates is subject to a number of risks, including the following:
neffective Investigational New Drug, or IND, applications from the FDA that allow commencement of our planned or future clinical trials for our product candidates;
ntimely and successful completion of preclinical studies and clinical trials;
nsuccessful patient enrollment in clinical trials;
nsuccessful data from our preclinical studies and clinical trials that support an acceptable risk-benefit profile of VDPHL01 and any of our other current or future product candidates in the intended populations and indications;
nsatisfaction of applicable regulatory requirements;
npotential unforeseen safety issues or adverse side effects, either in clinical trials or following approval;
nreceipt and maintenance of marketing approvals from applicable regulatory authorities;
nremaining in compliance with post-marketing regulatory requirements;
nobtaining and maintaining patent and trade secret protection and regulatory exclusivity for VDPHL01 and any of our other current or future product candidates;
nmaking arrangements or maintaining existing arrangements with third-party manufacturers, or establishing manufacturing capabilities, for both clinical and commercial supplies of VDPHL01 and any of our other current or future product candidates;
nentry into collaborations for the commercialization of VDPHL01 or to further the development of any of our other current or future product candidates;
nestablishing sales, marketing and distribution capabilities and launching commercial sales of any approved products, whether alone or in collaboration with others;
nsuccessfully launching commercial sales of VDPHL01 and any of our other current or future product candidates, if and when approved;
nsuccessfully creating market demand for VDPHL01 and any of our other current or future product candidates through our own marketing and sales activities, and any other arrangements to promote these product candidates that we may otherwise establish;
nmanufacturing VDPHL01 and any of our other current or future product candidates in sufficient quantities and at acceptable quality and manufacturing cost to meet commercial demand at launch, if approved, and thereafter;
nacceptance of VDPHL01 and any of our other current or future product candidates, if and when approved, by patients, the medical community and third-party payors;
nproducts, following approval, maintaining a continued acceptable safety profile;
neffectively competing with other therapies;
nensuring that we promote and distribute our products consistent with all applicable healthcare laws; and
nenforcing and defending intellectual property rights and claims.
If we are unable to develop, receive regulatory approval for, or successfully commercialize VDPHL01 for the indications we are developing it for, or if we experience delays as a result of any of these risks or otherwise, our business and financial condition will be materially harmed.
In addition, of the large number of products in development in the pharmaceutical industry, only a small percentage result in the submission of an NDA to the FDA, and even fewer are approved for marketing and commercialization. Furthermore, even if we receive regulatory approval to market VDPHL01 for any indication, any such approval may be subject to limitations on the indications or uses or the patient populations for which we may market the product. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development activities, we may not be able to successfully develop or commercialize VDPHL01 for any indication. If we or any of our current or licensing and collaboration partners are unable to develop, or obtain regulatory approval for, or, if approved, successfully commercialize VDPHL01 for its initial indication or potential additional indications, we may not be able to generate sufficient revenue to continue our business.
We also are preparing to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition, and we may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors that delay, impair or prevent us from achieving our business objectives. Our failure to become and remain profitable may depress the market price of our common stock and could impair our ability to raise capital, expand our business or continue our operations.
Preclinical and clinical development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future preclinical studies or clinical trial results. We may encounter substantial delays in preclinical and clinical trials, or may not be able to conduct or complete preclinical or clinical trials on the expected timelines, if at all. If our preclinical studies and clinical trials are not sufficient to support regulatory approval of any of our product candidates, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development of such product candidate.
It is impossible to predict when or if any of our product candidates will prove effective and safe in humans or will receive regulatory approval. Our ongoing, planned or future clinical trials may not ultimately be successful or support further clinical development or regulatory approval of VDPHL01 or any of our other current or future product candidates. Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical studies and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical development testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in later stage clinical trials even after achieving promising results in earlier stage clinical trials. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their product candidates. Our preclinical studies and future clinical trials may not be successful.
We may experience delays in initiating or completing preclinical studies or clinical trials, including as a result of delays in obtaining or failure to obtain the FDA’s clearance to initiate clinical trials under INDs. Additionally, we cannot be certain that preclinical studies or clinical trials for our product candidates will not require redesign, enroll an adequate number of subjects on time, or be completed on schedule, if at all. We may experience adverse or unforeseen events during, or as a result of, preclinical studies and clinical trials that could delay or terminate our trials, or delay or prevent our ability to receive marketing approval or commercialize our product candidates, including:
nwe may receive feedback from regulatory authorities that requires us to modify the design or implementation of our preclinical studies or clinical trials, including our ability to commence a clinical trial;
nwe may fail or be delayed in reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical trial sites;
nwe may be unable to or delayed in adding a sufficient number of clinical trial sites and obtaining Institutional Review Board, or IRB, or independent ethics committee approval at each clinical trial site;
npreclinical studies or clinical trials of our product candidates may fail to show safety or efficacy or otherwise produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials or abandon our research efforts for our other product candidates;
nthe number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of our clinical trials or fail to return for post-treatment follow-up at a higher rate than we anticipate;
nour third-party contractors may fail to comply with regulatory requirements, fail to maintain adequate quality controls or be unable to provide us with sufficient product supply to conduct and complete preclinical studies or clinical trials of our product candidates in a timely manner, or at all;
nwe or our investigators might have to suspend or terminate clinical trials of our product candidates for various reasons, including non-compliance with regulatory requirements, a finding that our product candidates have undesirable side effects or other unexpected characteristics or a finding that the participants are being exposed to unacceptable health risks;
nwe may experience difficulties in having subjects complete a clinical trial or return for post-treatment follow-up;
nclinical trial sites may deviate from clinical trial protocol or drop out of a clinical trial;
nwe may be unable or delayed in obtaining sufficient product supply of product candidate for use in preclinical studies or clinical trials from third-party suppliers;
nthe quality of our product candidates or other materials necessary to conduct preclinical studies or clinical trials of our product candidates may be insufficient or inadequate, and any transfer of manufacturing activities may require unforeseen manufacturing or formulation changes;
nreports from clinical testing of other therapies may raise safety or efficacy concerns about our product candidates;
nregulators may revise the requirements for approving our product candidates, or such requirements may not be as we anticipate;
nregulators may not consider the endpoints of our clinical trials to provide clinically meaningful results if, for example, we cannot establish that the PRO measures used in our registration-directed trials are fit-for-purpose; and
nfuture collaborators may conduct clinical trials in ways they view as advantageous to them but that are suboptimal for us.
If we are required to conduct additional preclinical studies or clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these studies, trials or tests are not positive or are only moderately positive or if there are safety concerns, our business and results of operations may be adversely affected and we may incur significant additional costs.
We would also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs, or ethics committees of the institutions in which such clinical trials are being conducted, by the Data Safety Monitoring Board, if any, for such clinical trial or by the FDA or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical trial protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from the product candidates, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Moreover, principal investigators for our current and future clinical trials who serve as scientific advisors or consultants to us from time to time receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA or comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authority may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable foreign regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA or comparable foreign regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of one or more of our product candidates.
If VDPHL01, or any of our other current or future product candidates, generally prove to be ineffective, unsafe or commercially unviable, our entire pipeline may have little, if any, value, which would have a material and adverse effect on our business, financial condition, results of operations and prospects.
If the FDA does not conclude that VDPHL01 satisfies the requirements for the Section 505(b)(2) regulatory approval pathway, or if the requirements for VDPHL01 under Section 505(b)(2) are not as we expect, the approval pathway for those product candidates will likely take significantly longer, cost significantly more and entail significantly greater complications and risks than anticipated, and in either case may not be successful.
While we believe that we will have the necessary supporting data to submit to the FDA a marketing application under the Federal Food, Drug and Cosmetic Act, or the FDCA, Section 505(b)(2) regulatory pathway for VDPHL01 for the treatment of patients with PHL upon completion of our ongoing registration-directed clinical trials of VDPHL01, the FDA may not agree that the Section 505(b)(2) pathway is appropriate and may not approve any such application or any future application for additional indication or future product candidates.
The Drug Price Competition and Patent Term Restoration Act of 1984, as amended, or the Hatch-Waxman Act, added Section 505(b)(2) to the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Section 505(b)(2), if available to us, would allow an NDA we submit to the FDA to rely in part on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of approved compounds, which could expedite the development program for our future product candidates by potentially decreasing the amount of preclinical and/or clinical data that we would need to generate in order to obtain FDA approval. This pathway does not, however, expedite the FDA review process timelines.
If the FDA does not allow us to pursue the Section 505(b)(2) regulatory pathway as anticipated, we may need to conduct additional preclinical studies and/or clinical trials, provide additional data and information, and meet additional standards for regulatory approval. If this were to occur, the time and financial resources required to obtain FDA approval for VDPHL01 or any of our other current or future product candidates, and complications and risks associated with such product candidates, would likely substantially increase. Moreover, inability to pursue the Section 505(b)(2) regulatory pathway could result in new competitive products reaching the market more quickly than any product candidates we develop, which could adversely impact our competitive position and prospects. Even if we are allowed to pursue the Section 505(b)(2) regulatory pathway, we cannot be certain that VDPHL01 or any of our other current or future product candidates we develop will receive the requisite approval for commercialization.
In addition, notwithstanding the approval of a number of products by the FDA under Section 505(b)(2), certain pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA’s interpretation of Section 505(b)(2) is successfully challenged, the FDA may change its Section 505(b)(2) policies and practices, which could delay or even prevent the FDA from approving any NDA that we submit under Section 505(b)(2). In addition, the pharmaceutical industry is highly competitive, and Section 505(b)(2) NDAs are subject to certain requirements designed to protect the patent rights of sponsors of previously approved drugs that are referenced in a Section 505(b)(2) NDA. These requirements may give rise to patent litigation and mandatory delays in approval of our NDAs for up to 30 months or longer depending on the outcome of any litigation. It is not uncommon for a manufacturer of an approved product to file a citizen petition with the FDA seeking to delay approval of, or impose additional approval requirements for, pending competing products.
Any significant AEs or undesirable side effects caused by our product candidates may delay or prevent regulatory approval or market acceptance of our product candidates, or result in significant negative consequences following marketing approval, if any. Additionally, the clinical profile of VDPHL01 in female patients may differ from the clinical profile in male patients, and the outcomes observed to date in male patients may not be reflective or predictive of future outcomes for female patients.
Unacceptable, undesirable or clinically unmanageable side effects, caused by any of our product candidates, could cause us or regulatory authorities to interrupt, delay or halt our clinical trials and could result in a more restrictive label or the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities.
AEs, SAEs or other side effects in clinical trials often make it difficult to recruit participants to clinical trials and results in participants dropping out of trials. While certain side effects may be reversible following discontinuation of the product candidate with sufficient recovery periods, we will need to monitor the severity and duration of side effects in our clinical trials. If such effects are more severe, less reversible than we expect or not reversible at all, we may decide, or be required, to perform additional studies or to halt or delay further clinical development of our product candidates.
We have observed certain AEs in our clinical trials of VDPHL01, including viral upper respiratory tract infection and headache. The occurrence of AEs, side effects or other safety or tolerability concerns, could limit our opportunity to disrupt the current standard of care, particularly if AEs and SAEs are deemed to be related to VDPHL01 or any of our other current or future product candidates. Such a determination may require longer and more extensive clinical development, or regulatory authorities may increase the amount of data and information required to approve, market or maintain approval of our product candidates. In addition, unwanted hair growth
on non-scalp parts of the body where hair is already naturally growing has been observed as a side effect of VDPHL01, which could deter adoption and continued use of VDPHL01 by those who find such additional hair growth to be undesirable. Additionally, although we are developing VDPHL01 in both male patients and female patients, we have less clinical experience to date with VDPHL01 in female patients and the tolerability profile of VDPHL01 in female patients may differ from the profile in male patients. The clinical profile of VDPHL01 in female patients may differ from the clinical profile in male patients, and the outcomes observed to date in male patients may not be reflective or predictive of future outcomes for female patients.
We, the FDA or other applicable regulatory authorities, or an IRB, may suspend clinical trials of a product candidate at any time for various reasons, including a belief that participants in such trials are being exposed to unacceptable health risks or adverse side effects. Many potential product candidates developed in the biotechnology industry that initially showed promise in early-stage trials have later been found to cause side effects that prevented their further development and approval. Even if side effects do not preclude the product candidate from obtaining or maintaining marketing approval, undesirable side effects may inhibit market acceptance.
Even if we successfully develop a product candidate and it receives marketing approval, the FDA could require additional precautions, including requiring us to adopt a REMS to ensure that the benefits of treatment outweigh the risks for each potential patient, which may include, among other things, a medication guide outlining the risks of the product for distribution to patients, a communication plan to healthcare practitioners, extensive patient monitoring or distribution systems and processes that are highly controlled, restrictive, and more costly than what is typical for the industry. Additionally, the FDA has required the inclusion of a boxed warning on the labeling for FDA-approved IR oral minoxidil for treatment of blood pressure and cardiovascular indications due to serious heart-related adverse effects. Although we have designed VDPHL01 to prevent the peak minoxidil concentrations from exceeding the drug’s known cardiac activity threshold, and to date we have not observed any cardiac AEs in our clinical trials of VDPHL01, we do not know at this stage whether FDA is likely to require inclusion of a boxed warning for VDPHL01, if approved. Additionally, concerns about cardiac risks associated with minoxidil may impact the perception of tolerability of VDPHL01.
Although the clinical trial process is designed to identify and assess potential side effects and AEs, clinical development does not always fully characterize the safety and efficacy profile of a new drug, and it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. AEs or side effects during clinical trials or after approval expose us to several potential negative consequences, including:
nregulatory authorities may limit, suspend or withdraw approvals of such product, or may refuse to approve supplemental applications for such product;
nregulatory authorities may require additional warnings on the label, such as a boxed warning, contraindications or precautions, or otherwise limit the approved use of such product;
nregulatory authorities may impose additional restrictions on the marketing of, or the manufacturing processes for, the particular product, including requiring a REMS;
nwe may be required to recall the product or change the way it is administered in patients;
nwe may be required to conduct additional clinical trials;
nwe may decide to remove such product from the market;
nwe could be sued and held liable for harm caused to patients; and
nour reputation may suffer.
Any of these events could prevent us from obtaining or maintaining regulatory approvals or achieving or maintaining market acceptance of our current and future product candidates or could substantially increase the costs and expenses of commercializing the affected product, which in turn could significantly impact our ability to successfully commercialize our product candidates and generate revenues.
We operate in highly competitive markets and face competition from large, well-established companies with significant resources as well as other entities, and, as a result, we may not be able to compete effectively.
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on intellectual property. While we believe our deep scientific knowledge of dermatology paired with our differentiated lead product candidate, VDPHL01, provides a strong competitive advantage, we face competition from many different sources, including pharmaceutical and biotechnology companies, generic drug companies, compounding pharmacies and consumer health companies as well as from academic institutions, governmental agencies and public and private research institutions. If our competitors develop technologies or product candidates more rapidly than we do, or if their technologies or product candidates are more effective or safer than ours, our ability to develop and successfully commercialize VDPHL01 or any of our future product candidates may be adversely affected.
Our competitors may have significantly greater financial resources, established presence in the market and expertise in research and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and reimbursement and marketing approved products than we do. Accordingly, our competitors may be more successful than we may be in obtaining regulatory approval for therapies and achieving widespread market acceptance. Our competitors’ products may be more effective, safer, or more effectively marketed and sold, than any product candidate we may commercialize and may render VDPHL01 or any future product candidates obsolete or non-competitive before we can recover development and commercialization expenses. In addition, our competitors may succeed in developing, acquiring or licensing technologies and drug products that are more effective or less costly than VDPHL01 or any future product candidates that we may develop, which could render such product candidates obsolete and noncompetitive. They may also compete in recruiting and retaining qualified scientific, sales, marketing and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for, our product candidates. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Our commercial opportunity can be reduced or eliminated if competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Competitors also may obtain FDA or other regulatory approval for their products more rapidly or earlier than us, which could result in our competitors establishing a strong market position before we are able to enter the market. Additionally, technologies developed by our competitors may render our potential product candidates uneconomical or obsolete and we may not be successful in marketing our product candidates against competitors.
Further, our current or potential competitors may be acquired by third-parties with greater available resources, which has recently occurred in our industry. As a result, our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards, or customer requirements and may have the ability to initiate or withstand substantial price competition. In addition, our competitors have established, and may in the future establish, cooperative relationships with vendors of complementary products, technologies or services to increase the availability of their solutions in the marketplace.
If approved, we anticipate that VDPHL01 will compete primarily against existing treatment options for PHL, including oral finasteride and topical minoxidil, off label IR oral minoxidil, low light laser therapy, or LLLT, and fractional laser non-ablative therapy as well as non-FDA approved non-prescription options such as “nutraceutical” supplements and shampoos. In particular, VDPHL01, if approved, will need to compete with an established OTC market and will require potential patients to consult with a doctor and receive a prescription, which may detract potential patients. Further, OTC products may be more competitively priced than VDPHL01, which may impact our ability to attract potential patients. We may also face competition from compounding pharmacies producing oral minoxidil formulations. Although FDA rules would not permit compounding pharmacies to prepare formulations that are essentially copies of VDPHL01 regularly or in inordinate amounts, FDA may not adequately regulate or enforce its requirements with respect to such compounding pharmacies, which could in turn lead to consumer confusion in the marketplace or result in potential VDPHL01 sales being diverted to compounding pharmacies.
There are several product candidates in clinical development which could potentially pose a direct competitive threat to VDPHL01 in the future for the treatment of PHL. To our knowledge, the only other late-stage product candidate that is currently under evaluation in a Phase 3 study in the U.S. for the treatment of PHL is Breezula, a topical androgen receptor antagonist by Cosmo Pharmaceuticals NV. We are also aware of other product candidates in clinical development, including: KX-826, a topical androgen receptor antagonist by Kintor Pharmaceutical Limited, PP405, a topical mitochondrial pyruvate carrier inhibitor by Pelage Pharmaceuticals, Inc., TDM-105795, topical thyromimetic by TechnoDerma Medicines Inc. and ET-02, a topical treatment of undisclosed mechanism by Eirion Therapeutics, Inc. We are also aware of other companies with programs for hair loss, including Hope Medicine Inc, Samson Clinical Pty Ltd, Amplifica Holdings Group Inc., Dermaliq Therapeutics, Inc. and Absci Corporation.
Our ability to compete effectively depends on our ability to distinguish our company and our offerings from our competitors and their products, and includes factors such as:
nefficacy, safety and tolerability of our products;
ntiming and scope of regulatory approvals for these products;
navailability and cost of manufacturing;
npatent position;
naccessibility, ease of use and convenience;
nprice and affordability;
nreimbursement coverage;
nbrand recognition;
nlong-term outcomes;
nbreadth and efficacy of offerings;
nmarket penetration;
nmarketing and sales capabilities;
npartnerships and alliances;
nrelationships with providers, suppliers and partners; and
nregulatory compliance recourses.
If we are unable to successfully compete with existing and potential competitors, our business, financial condition, and results of operations could be adversely affected.
Delays or difficulties in the enrollment and dosing of patients in clinical trials may delay or prevent receipt of necessary regulatory approvals.
The timing of our clinical trials depends on our ability to recruit patients to participate in our studies as well as the dosing of such patients and completion of required follow-up periods. Participant enrollment, a significant factor in the timing of clinical trials, is affected by many factors, including the size and nature of the patient population, the number and location of clinical sites, the proximity of participants to clinical sites, the eligibility and exclusion criteria for the trial, the design of the clinical trial, challenges in obtaining and maintaining participant consents, enrolled participants dropping out, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications being investigated by us.
In addition, clinical trials commonly compete with other clinical trials for product candidates that address the same diseases or conditions, and this competition reduces the number and types of participants available to us, because some participants who might have opted to enroll in our trials instead opt to enroll in a trial conducted by a competitor or elect to use a marketed therapy. We also could encounter delays if doctors face ethical challenges associated with enrolling participants in a clinical trial rather than prescribing an existing treatment with an established safety and efficacy profile.
If we or our collaborators, if any, are unable to enroll a sufficient number of eligible patients to participate in our clinical trials, we may not be able to initiate, continue or complete clinical trials for our product candidates.
Even if we are able to enroll a sufficient number of participants in our clinical trials, delays in enrollment may result in increased costs, delay completion or adversely impact the outcome of the trial.
Participants, including in any control groups, frequently withdraw from a clinical trial if they are not experiencing improvement in their underlying disease or condition or if they experience adverse side effects or other issues. Withdrawal of participants from our clinical trials may compromise the quality of our data.
Difficulties enrolling a sufficient number of patients to conduct our clinical trials as planned may require us to delay, limit or terminate clinical trials for our product candidates, or expand to additional jurisdictions, which could impose additional challenges on our company. Failure to successfully conduct our clinical trials as planned, would have an adverse effect on our business, financial condition, results of operations and prospects.
As an organization, we have only recently initiated registrational-directed clinical trials, and may be unable to complete such trials for any product candidates we may develop, including VDPHL01.
We will need to successfully complete our ongoing and planned clinical trials, including registrational clinical trials, in order to obtain FDA approval to market our product candidates. Carrying out later-stage clinical trials and the submission of a successful NDA is a complicated process. As an organization, we have initiated Phase 2 and Phase 3 clinical trials, but we have not previously completed such later stage or registrational clinical trials and have not previously submitted an NDA for any product candidate. Consequently, we may be unable to successfully and efficiently execute and complete necessary clinical trials in a way that leads to NDA submission and approval of any product candidate. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of product candidates that we develop. Failure to commence or complete, or delays in, our planned clinical trials, including trials of VDPHL01 for men or for women could prevent us from or delay us in commercializing our product candidates, including VDPHL01.
Interim, initial, “top-line” and preliminary data from our clinical trials that we announce or publish from time to time are subject to audit and verification procedures and may differ materially from final data as more patient data become available.
Preliminary or top-line data from our preclinical studies and clinical trials that we publish from time to time are based on preliminary analyses of then-available data, and the results, related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular preclinical study or clinical trial. We also make assumptions, estimations, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the top-line or preliminary results may differ from future results of the same studies or trials, or different conclusions or considerations may qualify such results once additional data have been received and fully evaluated. Top-line data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data. As a result, preliminary and top-line data should be viewed with caution until the final data are available.
From time to time, we also may disclose interim data from our preclinical studies and clinical trials. Interim data from clinical trials are subject to the risk that one or more of the clinical outcomes may materially change as participant enrollment continues and more participant data become available or as participants from our clinical trials continue other treatments for their disease. For example, in October 2025, we reported preliminary data in male patients from our Phase 2 trial of VDPHL01 in mild-to-moderate PHL; however, these results may not ultimately be reproducible or durable.
Furthermore, third parties, including regulatory authorities, may not accept or agree with our assumptions, estimates, calculations, conclusions or analyses or may interpret or weigh the importance of data differently, which could delay or prevent regulatory approval of, or limit commercial prospects for, the particular product candidate. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and you or others may not agree with what we determine to disclose.
If the interim, top-line or preliminary data that we report differ from final results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed, which could harm our business, financial condition, results of operations and prospects. Further, disclosure of interim, top-line or preliminary data by us or by our competitors could result in volatility in the price of our common stock after the completion of this offering.
We intend to expend our resources to pursue VDPHL01 and therefore may fail to capitalize on, or identify product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we are currently focusing our development efforts on our lead product candidate, VDPHL01, for the treatment of PHL. As a result, we may forgo or delay pursuit of opportunities with other existing or potential product candidates or other indications for our existing product candidates that later prove to have greater commercial potential. Our resource allocation decisions may result in our failure to capitalize on viable commercial products or profitable commercial opportunities. Our spending on VDPHL01 and other current and future development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
The increasing use of social media platforms presents new risks and challenges.
Social media is increasingly being used to communicate about our clinical development activities and PHL, which is the indication VDPHL01 is being developed to treat, and we intend to utilize appropriate social media in connection with our commercialization efforts of VDPHL01, if approved. For example, we have engaged in social media campaigns to increase awareness of VDPHL01 and to attract potential patients our clinical trials. Social media practices in the biotechnology and biopharmaceutical industries continue to evolve and regulations and regulatory guidance relating to such use are evolving and not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business, resulting in potential regulatory actions against us, along with the potential for litigation related to off-label marketing, promotion of an unapproved new drug, false or misleading advertising, or other prohibited activities and heightened scrutiny by the FDA, the Federal Trade Commission, or FTC, the SEC and other regulators. For example, patients may use social media channels to comment on their experience in an ongoing clinical trial or to report an alleged side effect or adverse event, or AE. If such disclosures occur, there is a risk that trial enrollment may be adversely impacted, that we may fail to monitor and comply with applicable AE reporting obligations or that we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our product candidates. There is also a risk of inappropriate disclosure of sensitive, personal or confidential information or negative or inaccurate posts or comments about us on any social networking website. In addition, we may encounter attacks on social media regarding us, our management, VDPHL01 or any of our other current or future product candidates. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions, suffers reputational harm or incur other harm to our business.
We may not be successful in our efforts to build a pipeline of additional product candidates.
Our goal is to develop a focused portfolio of aesthetic dermatology product candidates targeting high-prevalence dermatologic conditions, with potential selective development of medical dermatology product candidates. We may not be able to continue to identify and develop new product candidates in addition to the pipeline of product candidates that we have established. Even if we are successful in continuing to build our pipeline, the potential product candidates that we identify may not be suitable for clinical development. For example, they may be shown to have harmful side effects or other characteristics that indicate that they are unlikely to be drugs that will receive marketing approval and achieve market acceptance. If we do not successfully develop and commercialize product candidates based upon our approach, we will not be able to obtain product revenue in future periods, which likely would result in significant harm to our financial position and adversely affect our stock price.
If we do not achieve our projected development goals in the timeframes we announce and expect, the commercialization of our products may be delayed.
From time to time, we may estimate the timing of the accomplishment of various scientific, clinical, regulatory, manufacturing and other product development goals. These milestones may include the commencement or completion of preclinical studies and clinical trials and the submission of regulatory filings, including IND and NDA submissions. From time to time, we may publicly announce the expected timing of such milestones. The achievement of these milestones is, and will be, based on a variety of assumptions. The actual timing of these milestones can vary significantly compared to our estimates, in some cases for reasons beyond our control. We may experience numerous unforeseen events during, or as a result of, any future clinical trials that we conduct that could delay or prevent our ability to receive marketing approval or commercialize our product candidates.
Product liability lawsuits against us or any of our licensing and collaboration partners could divert our resources and attention, cause us to incur substantial liabilities and limit commercialization of VDPHL01 or any of our other current or future product candidates.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Currently, we have no products that have been approved for commercial sale; however, the use of VDPHL01 or any of our other current or future product candidates by us and any current and licensing and collaboration partners in clinical trials, and the sale of VDPHL01 or any of our other current or future product candidates, if approved, in the future, may expose us to liability claims. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, health care providers, pharmaceutical companies, our current and licensing and collaboration partners or others using, administering or selling any of our future approved products. If we cannot successfully defend ourself against any such claims, we may incur substantial liabilities or be required to cease or limit commercialization of VDPHL01 or any of our other current or future product candidates if approved. Regardless of the merits or eventual outcome, liability claims may result in:
ndecreased demand for any of our future approved products;
ninjury to our reputation;
nwithdrawal of clinical trial participants;
ntermination of clinical trial sites or entire trial programs;
nsignificant litigation costs, including with respect to potential class action lawsuits;
nsubstantial monetary awards to, or costly settlements with, patients or other claimants;
nproduct recalls or a change in the indications for which they may be used;
nloss of revenue;
ndiversion of management and scientific resources from our business operations; and
nthe inability to commercialize VDPHL01 or any of our other current or future product candidates.
Although we maintain product liability insurance coverage of up to $5 million in the aggregate, including clinical trial liability, this insurance may not fully cover potential liabilities that we may incur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. We will need to increase our insurance coverage if we commercialize VDPHL01 or any of our other current or future product candidates that receives regulatory approval. In addition, insurance coverage is becoming increasingly expensive. Failure to maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, could prevent or inhibit the development and commercial production and sale of VDPHL01 or any of our other current or future product candidates, which could harm our business, financial condition, results of operations and prospects. Furthermore, if any of our current or future product candidates, including VDPHL01, are approved for marketing and commercial sale, we will be highly dependent upon consumer perceptions of us and the safety and quality of our products. We could be adversely affected if we are subject to negative publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies.
Risks Related to Commercialization of Our Product Candidates
We currently have limited marketing, sales or distribution infrastructure. If we are unable to fully develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we may not be successful in commercializing our product candidates.
We are currently building our marketing, sales or distribution capabilities. As a company we have not commercialized or marketed any products to date. If VDPHL01 is approved for the treatment of PHL or if any of our other current or future product candidate is approved, we will need to expand our sales and marketing organization, on our own or in collaboration with third parties, and add further technical expertise and supporting distribution capabilities to commercialize the approved product in key territories, which will require substantial additional resources. We anticipate that a large portion of these costs will be incurred in advance of any approval of VDPHL01. There are risks involved with both establishing our own sales, marketing and distribution capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a commercial organization is expensive and time consuming and could delay any product launch. If the commercial launch of VDPHL01 or any other product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. Any failure or delay in the development of our or third parties’ internal sales, marketing and distribution capabilities would adversely impact the commercialization of VDPHL01 and any other product candidates.
Factors that may inhibit our efforts to commercialize VDPHL01 or any of our other current or future product candidates on its own include:
nour inability to recruit and retain adequate numbers of effective sales and marketing personnel;
nthe inability of sales personnel to obtain access to or our failure to educate adequate numbers of dermatologists or other physicians on the benefits of our products;
nthe lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and
nunforeseen costs and expenses associated with building out an independent sales and marketing organization.
We may enter into licensing and collaboration agreements in foreign territories for the commercialization of VDPHL01 or any of our other current or future product candidates, however, we may be unable to enter into such agreements on favorable terms, if at all. Our product revenue may be lower than if we directly marketed or sold our products, if approved. In addition, any revenue we receive will depend in whole or in part upon the efforts of these third parties, which may not be successful and are generally not within our control. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively.
We also will compete with many companies that currently have extensive, experienced and well-funded sales, distribution and marketing operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of VDPHL01 or any of our other current or future product candidates, if approved. Without an internal team or the support of a third-party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
If we do not expand our sales and marketing capabilities successfully, on our own or in collaboration with third parties, we will not be successful in commercializing VDPHL01, if approved, or any of our other current or future product candidates. If we are not successful in commercializing any approved products, our future product revenue will suffer and we may incur significant additional losses.
Furthermore, our efforts to educate patients, dermatologists and other physicians, on the benefits of VDPHL01 or any of our other or future product candidates may require more resources than we anticipate and may never be successful. Even if VDPHL01 or any of our other or future product candidates is approved, if we are unable to market our products successfully we will not be able to generate significant revenues from such products.
Even if we obtain regulatory approval for VDPHL01 or any other product candidates, such products may fail to achieve market acceptance which would adversely affect our efforts to commercialize any such product successfully.
Our current business strategy is highly dependent on the successful development and commercialization of VDPHL01, if approved, and achieving and maintaining market acceptance of VDPHL01. VDPHL01, if approved as a hair loss treatment, may not be covered by government health benefit programs or private insurance as such treatments are generally not considered medically necessary. Lack of insurance coverage may adversely affect our ability to successfully commercialize VDPHL01 if health care practitioners are unwilling to prescribe products that are not covered and consumers are unwilling to pay the full cost of such products.
If we are not successful in demonstrating to existing and potential customers the benefits of VDPHL01 or any of our other current or future product candidates, if approved, our business will be adversely affected. We have never commercialized a product, and even if VDPHL01, or any of our other current or future product candidates, is approved by the appropriate regulatory authorities for marketing and sale, it may nonetheless fail to gain sufficient market acceptance by physicians, patients and others in the medical community. The rate of adoption by health care practitioners of any products for which we may receive marketing approval and consumer demand for such products will likely depend on several factors, including:
nthe safety and effectiveness of the product;
nthe prevalence and severity of any side effects;
nthe convenience and ease of use of the product;
nlimitations or warnings, including distribution or use restrictions contained in the product’s approved labeling;
nchanges in the standard of care for the targeted indications for the product;
nour ability to offer the product for sale at competitive prices;
nthe cost, availability, and effectiveness of competitor therapies, including OTC drugs;
nchanges in pricing and promotional efforts by competitors;
nthe cost of the product for consumers;
nthe willingness of consumers to try the product;
nconsumer satisfaction with the results of the product;
nthe extent to which health care practitioners recommend the product to their patients;
nthe willingness of consumers to pay for products indicated for aesthetic purposes;
nthe success of efforts to educate potential prescribers and consumers regarding the benefits of our product as compared to those of other treatments;
nthe strength of sales, marketing, and distribution support; and
nthe success of any DTC marketing efforts for the product, which may depend on current and future restrictions on any DTC advertising.
If we fail to generate sufficient market acceptance of our product candidates, if approved, our ability to obtain an appropriate return on investment on such products will be impacted.
Additionally, in order to attract new customers and incentivize existing customers to purchase more of our offerings, we plan to use social media, emails, text messages, celebrity influencers, and other marketing strategies, including DTC marketing strategies, to reach new and existing customers. In September 2025, the FDA stated that it intends to more aggressively enforce requirements for DTC drug advertising, as well as expand its oversight of digital and social media advertising. In connection with this announcement, the FDA sent more than 100 warnings or untitled letters to companies for allegedly deceptive prescription drug advertising, which represents a dramatic increase in FDA actions as compared to prior years. The administration has also announced plans to propose a rulemaking that would call for drug companies to disclose additional safety information in DTC broadcast advertisements. The nature and extent of changes to FDA regulations and enforcement approach is unclear but may impact pharmaceutical marketing efforts industry-wide, which could in turn impact our potential future sales and operations.
The commercial opportunity for VDPHL01 and any of our other current or future product candidates we may develop may be smaller than we expect.
We have focused our development of VDPHL01 for the treatment of PHL. We base our commercial opportunity estimates on a variety of factors, including our estimates of the number of people who have experienced PHL and the number of those we expect would use VDPHL01, if approved, including those who currently do not treat their PHL, have discontinued use of past treatment for PHL or currently use other OTC products or off-label therapies. These estimates are based on many assumptions and may prove incorrect, and new studies or market research may reduce our estimated patient population and potential sales. The number of patients in the United States may turn out to be lower than expected or may not be otherwise amenable to treatment with VDPHL01. Further, while our regulatory strategy is to seek approval of VDPHL01 in male patients and female patients with PHL, we may only receive a more limited approval, which would impact our estimated commercial opportunity. If we are unable to advance VDPHL01, or any of our other current or future product candidates with attractive commercial opportunities or if our commercial opportunities are smaller than we expected, our future product revenues will be smaller than anticipated, which would adversely affect our business, financial condition, results of operations and prospects.
The total addressable commercial opportunity for VDPHL01 candidates will ultimately depend upon a number of factors, including the diagnosis and treatment criteria included in the final label, if approved for sale in specified indications, acceptance by the medical community, patient access and product pricing and, to the extent applicable, reimbursement. Incidence and prevalence estimates are frequently based on information and assumptions that are not exact and may not be appropriate, and the methodology is forward-looking and speculative. The process we have used in developing an estimated incidence and prevalence range for the indications we are targeting has involved collating limited data from multiple sources. Accordingly, the incidence and prevalence estimates included in this prospectus should be viewed with caution. Further, the data and statistical information used in this prospectus, including estimates derived from them, may differ from information and estimates made by our competitors or from current or future studies conducted by independent sources.
Our strategy of focusing on the cash-pay healthcare market for VDPHL01 may limit our ability to increase sales or achieve profitability
Our strategy is to focus on the cash-pay healthcare market for VDPHL01, if approved. This focus may limit our ability to increase sales or achieve profitability. For example, to maintain our business model, we will not offer products or services available in the broader healthcare market that are reimbursed by third-party payors such as Medicare, Medicaid or commercial insurance. We believe pursuing cash-pay non-reimbursed product strategy allows for meaningful strategic advantages in the United States, including pricing and marketing flexibility and which we believe makes the market less sensitive to changes in insurance coverage and reimbursement. However, our business strategy was developed based on a number of important assumptions about the cash-pay healthcare market for VDPHL01. For example, we believe that the use of cash-pay for VDPHL01 will be attractive to patients as compared to traditional insurance models. Our expectations could be incorrect and sales of VDPHL01 or any of our current or future product candidates could differ materially from our projections. In
addition, companies offering competitive products, whether they pursue a non-reimbursed product strategy or not, may nonetheless try to compete with VDPHL01 on price both directly through rebates, promotional programs and coupons and indirectly through attractive product bundling and customer loyalty programs. Further, changes in healthcare legislation and healthcare cost containment measures, as discussed in greater detail below, could impact the pricing of other products and procedures that compete with VDPHL01, which can indirectly impact our pricing strategy and profitability. If a competitor treatment is covered by third-party payors or has more favorable pricing for consumers, the pricing of VDPHL01 may be negatively impacted, which could have a material adverse effect on our ability to generate revenue and to attain profitability. Additionally, the out-of-pocket, cash-pay market for our patient population may be negatively impacted by other price increases and market conditions, including rising costs of other consumer goods, which patients may prioritize over any product candidates we may commercialize. Our business, financial results and future prospects will be materially harmed if we cannot generate sufficient consumer demand for VDPHL01, if approved.
If we fail to effectively maintain, promote, and enhance our reputation and VDPHL01 brand recognition in a cost-effective manner, our business and competitive advantage may be harmed.
We believe that maintaining and enhancing our reputation and brand recognition for VDPHL01 will be critical to our relationships with customers, physicians and other partners, and to our ability to attract new customers, physicians and other partners. The promotion of VDPHL01 will require us to make substantial investments. Brand promotion and marketing activities may not be successful or yield increased revenue, and to the extent that these activities yield increased revenue, the increased revenue may not offset the expenses we incur and our results of operations could be harmed. In addition, any factor that diminishes our reputation or that of our management, including failing to meet the expectations of our customers, physicians and other partners, would make it substantially more difficult for us to attract new customers, physicians and other partners. If we do not successfully maintain and enhance our reputation and brand recognition in a cost-effective manner, our business may not grow and we could lose our relationships with customers, physicians and other partners, which could harm our business, financial condition and results of operations.
Our products rely on consumer discretionary spending and the purchasing decisions of our customers, both of which are sensitive to difficult to predict global economic conditions, including the imposition of tariffs, or changes in consumer or customer sentiment.
We do not expect VDPHL01, if approved, to be reimbursed by any government or third-party payor and therefore VDPHL01 will continue to be paid for directly by the consumer. As a result, demand for VDPHL01 will be tied to the discretionary spending levels of our targeted consumer population. Sales of VDPHL01, if approved, may depend on short-term purchasing decisions made by our customers in response to consumer demand, aesthetics trends, our competitor’s sales tactics, inventory management, seasonality, and other factors affecting consumer and customer purchasing behavior. As a result, it will be difficult to forecast demand for VDPHL01, if approved, and our revenues in a given period may be subject to volatility based on any of these factors.
Recent macroeconomic events, including inflationary pressures and threatened and imposed tariffs have negatively impacted consumer sentiment, resulted in decreased procedural volume for cash-pay medical aesthetics treatments, especially in the United States, and have impacted consumer purchasing behaviors. If these or similar conditions persist or worsen, our business, financial condition, and results of operations could be materially harmed.
Coverage and reimbursement by third-party payors may not be available or may be difficult to obtain for any product candidates for which we receive marketing approval. Failure to obtain or maintain coverage and adequate reimbursement for any such approved products could limit our ability to market those products and would decrease our ability to generate revenue.
While our commercialization plan for VDPHL01 is currently focused solely on cash-pay sales to patients outside private health insurance and government healthcare programs, such as the Medicare and Medicaid programs, we may in the future seek coverage and reimbursement from third-party payors for VDPHL01 or other product candidates if approved for marketing.
We cannot be sure that coverage will be available for any product that we commercialize and, if coverage is available, that the level of reimbursement will be adequate. Specifically, prescription drug products used for cosmetic purposes will generally not be covered by third-party payors because they are not considered medically necessary. And the availability of coverage and the adequacy of the reimbursement of prescription drugs used for medical purposes is uncertain. Without adequate coverage and reimbursement, commercial success for any such product may be adversely affected.
Third-party payors may limit coverage or otherwise seek to control the utilization and cost of drug products. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the FDA-approved products for a particular indication. Some third-party payors may impose coverage restrictions through medical policies or manage utilization of a particular product by requiring pre-approval, known as “prior authorization,” for coverage of particular prescriptions, which allows the payor to assess medical necessity. A third-party payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain net price levels sufficient to realize an appropriate return on our investment in product development. Additionally, coverage and reimbursement for drug products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular drug product does not ensure that other payors will also provide coverage for the drug product or will provide coverage at an adequate reimbursement rate.
There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved products. Third-party payors are increasingly challenging the price and examining the cost-effectiveness of new products and services in addition to their safety and efficacy. Factors considered by these payors in determining whether to cover drug products and what reimbursement to provide include product efficacy, cost-effectiveness, and safety, as well as the availability of other treatments, including generic prescription drugs or OTC drugs. To obtain or maintain coverage and reimbursement for any approved drug product, we may need to collect real-world evidence and conduct pharmacoeconomic studies to demonstrate the medical necessity and cost-effectiveness of our product candidates, if approved. These studies will be in addition to the studies required to obtain or maintain regulatory approvals. If third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover the product or, if they do, the level of payment may not be sufficient to allow sale of a product at a profit. Thus, obtaining and maintaining coverage and adequate reimbursement status is complex, costly, and uncertain.
Federal and state initiatives to control healthcare costs, including measures to lower prescription drug pricing or reduce prescription reimbursement, may result in more restricted coverage and put additional downward pressure on pharmaceutical pricings, which could negatively impact coverage and reimbursement for our product candidates, if approved, our revenue, and our ability to compete with other marketed products and to recoup the costs of our research and development.
We may be required to provide discounts or rebates under government healthcare programs or to certain government and private purchasers in order to obtain coverage under federal healthcare programs such as Medicare and Medicaid or to sell products to government purchasers. More generally, we may need to offer price concessions to third-party payors to obtain favorable coverage or to purchasers to achieve sales.
We expect to experience coverage challenges and pricing pressures for any of our product candidates that may be approved and for which we seek coverage and reimbursement by third-party payors. Pricing pressures and healthcare reform efforts have become intense. As a result, increasingly high barriers are being erected to the entry of new products.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
We are dependent on the services of our senior management and other key personnel, and if we are not able to retain these individuals or recruit additional management or key personnel, our business will suffer.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management and other key personnel. We are highly dependent upon our co-founders, Chief Executive Officer, Reid Waldman, M.D., and President, Tim Durso, M.D., as well as our Chief Financial Officer, Dominic Carrano,
CPA and other members of our senior management and clinical development teams. The loss of services of any of these individuals could delay or prevent the successful development of our product pipeline, initiation or completion of our preclinical studies and clinical trials or the commercialization of our product candidates, if approved. Although we have executed employment agreements or offer letters with certain members of our management team, these agreements are terminable at will with or without notice and, therefore, we may not be able to retain their services. We do not currently maintain “key person” life insurance on the lives of our executives or any of our employees. This lack of insurance means that we may not have adequate compensation for the loss of the services of these individuals.
We must expand and effectively manage our managerial, operational, financial and other resources in order to successfully pursue our clinical development and commercialization efforts. We may not be successful in maintaining our company culture and continuing to attract or retain qualified management and scientific and clinical personnel in the future due to the intense competition for qualified personnel among biopharmaceutical, biotechnology and other businesses, particularly in the greater New England area. If we are not able to attract, integrate, retain and motivate personnel necessary to accomplish our business objectives, we may experience constraints that significantly impede the achievement of our commercial and development objectives, our ability to raise additional capital and our ability to implement our business strategy.
We will need to grow our organization, and we may experience difficulties in managing our growth and expanding our operations, which could adversely affect our business.
As of September 30, 2025, we had 19 full-time employees. We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of regulatory affairs and sales, marketing and distribution, as well as to support our public company operations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures, and we may not be able to implement improvements in an efficient or timely manner or may discover deficiencies in existing systems and controls. Our management may need to devote a significant amount of our attention to managing these growth activities. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion or relocation of our operations, retain key employees, or identify, recruit and train additional qualified personnel. Our inability to manage the expansion or relocation of our operations effectively may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth, in particular in connection with the potential commercial launch of VDPHL01, if approved, will also require significant capital expenditures and may divert financial resources from other product candidates or business initiatives. If we are unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate revenues could be reduced and we may not be able to implement our business strategy, including the successful commercialization of VDPHL01 or any other current or future product candidates, which could adversely affect our business, financial condition, results of operations and prospects.
Misconduct or other improper actions, including noncompliance with regulatory standards and requirements, by our employees, independent contractors, consultants, commercial partners and vendors exposes us to potential noncompliance with regulatory standards and requirements.
Employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners, CROs, CMOs and vendors exposes us to liability. Misconduct by these parties could be intentional, reckless and/or negligent conduct, including failure to comply with FDA or other regulations, provide true, complete and accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we may establish, comply with healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begin commercializing those products in the United States, our potential exposure under these laws will increase significantly, as will our costs associated with compliance. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct could also involve the
improper use of information obtained in the course of clinical trials or creation of fraudulent data in preclinical studies or clinical trials, which could result in regulatory sanctions and serious harm to our reputation. Additionally, a person could allege fraud or other misconduct even if none occurred. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling known or unknown risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Any such actions instituted against us could have a material and adverse effect on our business, financial condition, results of operations and prospects, including the imposition of significant civil, criminal or administrative penalties, damages, fines, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, integrity oversight and reporting obligations, or reputational harm.
If our information technology systems or data, or those of third parties with whom we work, are or were compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions, litigation, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits and other adverse consequences.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share, or, collectively, process, personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, data we collect about trial participants in connection with clinical trials, sensitive third-party data, business plans, transactions, and financial information, or, collectively, sensitive data.
We and the third parties with whom we work face a variety of evolving threats, including but not limited to ransomware attacks, which could cause security incidents. Cyber-attacks, malicious internet-based activity, online and offline fraud, and other similar activities threaten the confidentiality, integrity, and availability of our sensitive data and information technology systems, and those of the third parties with whom we work. Such threats are prevalent and continue to rise, are increasingly difficult to detect, and come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists,” organized criminal threat actors, rogue personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors.
Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we and the third parties with whom we work may be vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks, which could materially disrupt our systems and operations, supply chain, and ability to produce, sell and distribute our services.
We and the third parties with whom we work are subject to a variety of evolving threats, including but not limited to social-engineering attacks (including through deep fakes, which may be increasingly more difficult to identify as fake, and phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks credential stuffing attacks, credential harvesting, personnel misconduct or error, ransomware attacks, supply-chain attacks, attacks enhanced or facilitated by AI, software bugs, server malfunctions, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fires, floods, and other similar threats.
In particular, severe ransomware attacks are becoming increasingly prevalent and can lead to significant interruptions in our operations, ability to provide our products or services, loss of sensitive data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the negative impact of a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments.
Remote work has increased risks to our information technology systems and data, as our personnel utilize network connections, computers, and devices outside our premises or network, including working at home, while in transit and in public locations. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may discover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to integrate companies into our information technology environment and security program.
In addition, our reliance on third-party service providers could introduce new cybersecurity risks and vulnerabilities, including supply-chain attacks, and other threats to our business operations. We rely on third-party service providers and technologies to operate critical business systems to process sensitive data in a variety of contexts, including, without limitation, cloud-based infrastructure, information technology, data hosting, data center facilities, encryption and authentication technology, employee email, content delivery to customers, and other functions.
Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If third parties with whom we work experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages if these third parties fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties’ infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised.
Any of the previously identified or similar threats could cause a security incident or other interruption that could result in unauthorized, unlawful, or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to our sensitive data or our information technology systems, or those of the third parties with whom we work. A security incident or other interruption could disrupt our ability (and that of third parties with whom we work) to provide our services.
We may expend significant resources or modify our business activities (including clinical trials) to try to protect against security incidents. Additionally, certain data privacy and security obligations may require us to implement and maintain specific security measures or industry-standard or reasonable security measures to protect our information technology systems and sensitive data. It may be difficult and/or costly to detect, investigate, mitigate, contain, and remediate a security incident. Our efforts to do so may not be successful. Actions taken by us or the third parties with whom we work to detect, investigate, mitigate, contain, and remediate a security incident could result in outages, data losses, and disruptions of our business. Threat actors may also gain access to other networks and systems after a compromise of our networks and systems.
While we have implemented security measures designed to protect against security incidents, there can be no assurance that these measures will be effective. We take steps designed to detect, mitigate and remediate vulnerabilities in our information systems, but we may not be able to detect and remediate all vulnerabilities including in a timely manner. Further, we may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities. Vulnerabilities could be exploited and result in a security incident.
Applicable data privacy and security obligations may require us to notify data subjects, customers, partners, regulators and the media relevant stakeholders of security incidents. Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to adverse consequences.
If we (or a third party with whom we work) experience a security incident or are perceived to have experienced a security incident, we may experience adverse consequences, such as government enforcement actions (for example, investigations, fines, penalties, audits, and inspections); additional reporting requirements and/or oversight; restrictions on processing sensitive data (including personal data); litigation (including class claims); indemnification obligations; negative publicity; reputational harm; monetary fund diversions; interruptions in our operations (including availability of data); financial loss; and other similar harms. Security incidents and attendant consequences may prevent or cause customers to stop using our services, deter new customers from using our services, and negatively impact our ability to grow and operate our business.
Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or claims related to our data privacy and security obligations. We cannot be sure that our insurance coverages (if any) will be adequate or sufficient to protect us from or to mitigate liabilities arising out of our privacy and security practices, that such coverage will continue to be available on commercially reasonable terms or at all, or that such coverage will pay future claims.
In addition to experiencing a security incident, third parties may gather, collect, or infer sensitive information about us from public sources, data brokers, or other means that reveals competitively sensitive details about our organization and could be used to undermine our competitive advantage or market position. Additionally, sensitive information could be leaked, disclosed, or revealed as a result of or in connection with our employees’, personnels’, or vendors’ use of generative artificial intelligence, or AI, technologies.
Risks Related to Our Intellectual Property
Our commercial success depends on our ability to obtain and maintain sufficient intellectual property protection for VDPHL01 and our other current and any future product candidates and other proprietary technologies.
Our commercial success will depend, in part, on our ability to obtain and maintain patent protection in the United States and other countries with respect to VDPHL01 and our other current and any future product candidates. If we are unable to obtain or maintain patent protection with respect to VDPHL01 or any of our other current or any future product candidates and their uses, our business, financial condition, results of operations and prospects could be materially harmed.
We generally seek to protect our proprietary position by filing patents or patent applications in the United States and abroad related to VDPHL01 and our other current and any future product candidates that are important to our business, as appropriate. Our pending and future patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents issue from such applications, and then only to the extent the issued claims cover the technology. Our patent applications may not result in patents being issued and issued patents may not afford sufficient protection against competitors with similar technology. Furthermore any issued patents may be infringed, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. The failure to obtain meaningful protection from the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
The patent application process is subject to numerous risks and uncertainties, and we or any of our potential future collaborators may not be successful in protecting our product candidates by obtaining and defending patents. Obtaining and enforcing patents is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain and/or enforce patents that may issue based on our patent applications, at a reasonable cost or in a timely manner. We may fail to identify patentable aspects of our research and development results before it is too late to obtain patent protection.
Although we enter into non-disclosure and confidentiality agreements with parties who have access to patentable aspects of our research and development output, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, independent contractors, advisors and other third parties, any
of these parties may breach these agreements and disclose such results before a patent application is filed, thereby jeopardizing our ability to seek adequate patent protection.
If the scope of any patent protection we obtain is not sufficiently broad, or if we lose any of our patent protection, our ability to prevent our competitors from commercializing similar or identical product candidates would be adversely affected.
The patent position of pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation, resulting in court decisions, including U.S. Supreme Court decisions, which have increased uncertainties as to the ability to enforce patent rights in the future. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa.
Further, we may not be aware of all third-party intellectual property rights potentially relating to our research programs and product candidates, or their intended uses, and as a result the potential impact of such third-party intellectual property rights upon the patentability of our own patents and patent applications, as well as the potential impact of such third-party intellectual property upon our freedom to operate, is highly uncertain. Because patent applications are maintained as confidential for a certain period of time, until the relevant application is published, we may be unaware of third-party patents that may be infringed by commercialization of any of our product candidates, and we cannot be certain that we were the first to file a patent application related to any product candidates or technologies. Moreover, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. There may be prior art of which we are aware, but which we do not believe is relevant to our business, which may, nonetheless, ultimately be found to limit our ability to make, use, sell, offer for sale or import our products that may be approved in the future, or impair our competitive position. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain.
Our patents or pending patent applications, or the patents or pending patent applications that we license, may be challenged in the courts or patent offices in the United States and foreign jurisdictions. The legal threshold for initiating such proceedings may be low, so that even proceedings with a low probability of success might be initiated. An adverse determination in any such challenge may result in loss of exclusivity or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may not be able to protect our intellectual property rights throughout the world.
Patents are of national or regional effect. Filing, prosecuting and defending patents on all of our research programs and product candidates in all countries throughout the world would be prohibitively expensive, and our and our licensors’ intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States, even in jurisdictions where we do pursue patent protection. Consequently, we may not be able to prevent third parties from practicing our or our licensors’ inventions in all countries outside the United States, even in jurisdictions where we pursue patent protection, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These
competitor products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Various companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many countries do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights.
Our success depends on a market that is observant of intellectual property rights and regulatory requirements. Developments that undermine that landscape can significantly impact our business and reputation. For example, inadequately regulated sales of unapproved compounded versions of our product candidates or approved products could materially impact our business by exposing patients to significant risk, diverting sales of any approved products and harming our reputation. Our actions intended to stop or prevent illegal sales of such medicines also may be costly or ineffective.
Various countries outside the United States have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. As a result, a patent owner may have limited remedies in certain circumstances, which could materially diminish the value of such patent. If we or our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations and prospects may be adversely affected. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Further, the standards applied by the U.S. Patent and Trademark Office, or USPTO, and foreign patent offices in granting patents are not always applied uniformly or predictably. As such, we do not know the degree of future protection that we will have on our product candidates. While we will endeavor to try to protect our product candidates with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time-consuming, expensive and unpredictable.
In addition, geopolitical actions in the United States and in foreign countries could increase the uncertainties and costs surrounding the prosecution or maintenance of our patent applications or those of any future licensors and the maintenance, enforcement, or defense of our issued patents or those of any future licensors. For example, the United States and foreign government actions related to Russia’s invasion of Ukraine may limit or prevent filing, prosecution, and maintenance of patent applications in Russia. Government actions may also prevent maintenance of issued patents in Russia. These actions could result in abandonment or lapse of our patents or patent applications, resulting in partial or complete loss of patent rights in Russia. If such an event were to occur, it could have a material adverse effect on our business. In addition, a decree was adopted by the Russian government in March 2022, allowing Russian companies and individuals to exploit inventions owned by patentees from the United States without consent or compensation. Consequently, we would not be able to prevent third parties from practicing our inventions in Russia or from selling or importing products made using our inventions in and into Russia. As a result, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
Changes in patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining, defending, maintaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is therefore costly, time consuming and inherently uncertain. Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents, and may diminish our ability to protect our inventions, obtain, maintain, enforce and protect our intellectual property rights and, more generally, could affect the value of our intellectual property or
narrow the scope of our future owned and licensed patents, all of which could adversely affect our business, financial condition, results of operations and prospects. Patent reform legislation in the United States, including the Leahy-Smith America Invents Act, or the Leahy-Smith Act, signed into law on September 16, 2011, redefined prior art and provided more efficient and cost-effective avenues for competitors to challenge the validity of patents. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Further, because of a lower evidentiary standard in these USPTO post-grant proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our or our licensors’ patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
After March 2013, under the Leahy-Smith Act, the United States transitioned to a first inventor to file system in which, assuming that the other statutory requirements are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before we file an application covering the same invention, could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This requires us to be cognizant of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we were the first to either (i) file any patent application related to our product candidates and other proprietary technologies or (ii) invent any of the inventions claimed in our patents or patent applications. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing the claimed invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our future issued patents, all of which could adversely affect our business, financial condition, results of operations and prospects.
In addition, the patent positions of companies in the development and commercialization of pharmaceuticals are particularly uncertain. Changes in either the patent laws or interpretation of the patent laws in the United States or in other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. In the United States, numerous recent changes to the patent laws and proposed changes to the rules of the USPTO may have a significant impact on our ability to protect our technology, products and enforce our intellectual property rights. Subsequent rulings could adversely impact our patents or patent applications. In addition to increasing uncertainty regarding our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once granted. For example, the U.S. Supreme Court, in the case Amgen v. Sanofi, held that broad functional antibody claims are invalid for lack of enablement.
Depending on decisions by the U.S. Congress, the federal courts and the USPTO, and similar legislative and regulatory bodies in other countries in which we may pursue patent protection, the laws and regulations governing patents could change in unpredictable ways, particularly with respect to pharmaceutical patent protection, that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents. Any similar adverse change in the patent laws of other jurisdictions could also adversely affect our business, financial condition, results of operations and prospects.
In 2012, the European Union Patent Package, or the EU Patent Package, regulations were passed with the goal of providing a single pan-European Unitary Patent and a new European Unified Patent Court, or the UPC, for litigation involving European patents. The EU Patent Package was implemented on June 1, 2023. As a result, all European patents, including those issued prior to ratification of the EU Patent Package, now by default
automatically fall under the jurisdiction of the UPC, unless otherwise opted out. It is uncertain how the UPC will impact granted European patents in the biotechnology and pharmaceutical industries. Our European patent applications, if issued, could be challenged in the UPC. During the first seven years of the UPC’s existence, the UPC legislation allows a patent owner to opt its European patents out of the jurisdiction of the UPC. We may decide to opt out European patents from the UPC, but doing so may preclude us from realizing the benefits of the UPC. Moreover, if we do not meet all of the formalities and requirements for opt out under the UPC, said European patents could remain under the jurisdiction of the UPC. The UPC will provide our competitors with a new forum to centrally revoke European patents, and allow for the possibility of a competitor to obtain a pan-European injunction in UPC member states. Such a loss of patent protection could have a material adverse impact on our business and our ability to commercialize our technology and any future product candidates due to increased competition and, resultantly, on our business, financial condition, results of operations and prospects in Europe. The UPC and Unitary Patent are significant changes in European patent practice. As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty of any litigation in the UPC.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated as a result of noncompliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or applications will be due to be paid to the USPTO and various government patent agencies outside of the United States over the lifetime of our patents and patent applications. The USPTO and various non-U.S. government patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. We may, in some cases, be dependent on our licensors to take the necessary action to comply with these requirements with respect to licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could adversely affect our business, financial condition, results of operations and prospects.
Patent terms may be inadequate to protect our competitive position on products or product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional or international patent application filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our products or product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of products or new product candidates, patents protecting such products or candidates might expire before or shortly after such products or candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient and continuing rights to exclude others from commercializing products similar or identical to ours.
Patent rights relating to inventions described and claimed in our pending patent applications may not issue and patents based on our patent applications may be challenged and rendered invalid and/or unenforceable.
We own patents and patent applications in our portfolio relating to VDPHL01 that are pending at the patent offices in the United States, Europe, Japan, and other foreign jurisdictions, however, we cannot predict:
nif and when patents may issue based on our patent applications;
nthe scope of protection of any patent issuing based on our patent applications;
nwhether the claims of any patent issuing based on our patent applications will provide protection against competitors;
nwhether or not third parties will find ways to invalidate or circumvent our patent rights;
nwhether or not others will obtain patents claiming aspects similar to those covered by our patent applications;
nwhether we will need to initiate litigation or administrative proceedings to enforce and/or defend our patent rights which will be costly whether we win or lose;
nwhether our patent applications will result in issued patents with claims that cover VDPHL01 or any of our other current and any future product candidates or uses thereof; and/or
nwhether we may experience patent office interruption or delays to our ability to timely secure patent coverage to our product candidates.
We cannot be certain that the claims in our pending patent applications directed to VDPHL01 or any of our other current and any future product candidates will be considered patentable by the USPTO or by patent offices in foreign countries. One aspect of the determination of patentability of our inventions depends on the scope and content of the “prior art,” information that was or is deemed available to a person of skill in the relevant art prior to the priority date of the claimed invention. There may be prior art of which we are not aware that may affect the patentability of our patent claims or, if issued, affect the validity or enforceability of a patent claim relevant to our business, or prior art of which we are aware, but which we do not believe is relevant to our business, which may, nonetheless, ultimately be found to limit our ability to make, use, sell, offer for sale or import our products that may be approved in the future, or impair our competitive position. Minoxidil, the ingredient in VDPHL01, was previously known, and thus our patents and patent applications are not to this ingredient itself as a chemical compound, but rather, to other aspects of VDPHL01, including its formulation and dosage form. Even if the patents do issue based on the patent applications we own, co-own or exclusively license, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, patents in our portfolio may not adequately exclude third parties from practicing relevant technology or prevent others from designing around our claims. If the breadth or strength of our intellectual property position with respect to our product candidates is threatened, it could dissuade companies from collaborating with us to develop, and threaten our ability to commercialize, our product candidates. In the event of litigation or administrative proceedings, we cannot be certain that the claims in any of our issued patents will be considered valid by courts in the United States or foreign countries.
Issued patents covering our product candidates, or the method of use of our product candidates, could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
After initiation of legal proceedings against a third party to enforce a patent covering a our product candidates or other proprietary technologies, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement, insufficient written description, or failure to claim patent-eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. In addition to such counterclaims, third parties may raise claims challenging the validity or enforceability of a patent before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of, or amendment to our patent rights in such a way that they no longer cover our product candidates, therapeutic programs, and other proprietary technologies we may develop. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art of which we and the patent examiner were unaware during prosecution. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection provided to our product candidates, proprietary technologies, or other components of our therapeutic programs, as applicable. Even if a third party does not prevail on a legal assertion of invalidity or unenforceability, our patent claims may be construed in a manner that would limit our ability to enforce such claims against the defendant and others. Such a loss of patent protection could have a material adverse impact on our business, financial condition, results of operations, and prospects.
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent which might adversely affect our ability to develop and market VDPHL01 or any of our other current and any future product candidates.
As the biopharmaceutical industry expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties. Our operations may, or may in the future, infringe existing or future third-party patents. Identification of third-party patent rights that may be relevant to our operations is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. We cannot guarantee that any of our patent searches or analyses, including the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending application in the United States and abroad that is relevant to our operations or necessary for the commercialization of our product candidates in any jurisdiction.
Numerous U.S. and foreign patents and pending patent applications exist in our market that are owned by third parties. Our competitors in both the United States and abroad, many of which have substantially greater resources and have made substantial investments in patent portfolios and competing technologies, may have applied for or obtained or may in the future apply for and obtain, patents that will prevent, limit or otherwise interfere with our ability to make, use and sell our products. We do not always conduct independent reviews of pending patent applications and patents issued to third parties. Patent applications in the United States and elsewhere are typically published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Certain U.S. patent applications that will not be filed outside the United States can remain confidential until patents issue. In addition, patent applications in the United States and elsewhere can be pending for many years before issuance, or unintentionally abandoned patents or applications can be revived. Furthermore, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our technologies, our products or the use of our products. As such, there may be applications of others now pending or recently revived patents of which we are unaware. These patent applications may later result in issued patents, or the revival of previously abandoned patents that will prevent, limit or otherwise interfere with our ability to make, use or sell VDPHL01 or any of our other current and any future product candidates.
The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect, which may negatively impact our ability to market VDPHL01 or any of our other current and any future product candidates. We may incorrectly determine that our products are not covered by a third-party patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States or abroad that we consider relevant may be incorrect, which may negatively impact our ability to develop and market VDPHL01 or any of our other current and any future product candidates. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market VDPHL01 or any of our other current and any future product candidates.
Third-party patents may exist that might be enforced against our current technology, including our research programs, product candidates, their respective methods of use, and manufacture thereof, which could result in either an injunction prohibiting our manufacture or future sales, or, with respect to our future sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties, which could be significant.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing VDPHL01 or any of our other current and any future product candidates.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell VDPHL01 or any of our other current and any future product candidates without infringing the intellectual property and other proprietary rights of third parties. Third parties may allege that we have infringed or misappropriated their intellectual property. Litigation or other legal proceedings relating to intellectual property claims, with or without
merit, is unpredictable and generally expensive and time consuming and, even if resolved in our favor, is likely to divert significant resources from our core business, including distracting our technical and management personnel from their normal responsibilities. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
There is a substantial amount of intellectual property litigation in the biopharmaceutical industry, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to VDPHL01 or any of our other current and any future product candidates. Third parties may assert infringement claims against us based on existing or future intellectual property rights. The biopharmaceutical industry has produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that our product candidates, or of use either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity of third-party patents may be difficult and uncertain. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in defending our rights in these proceedings, which could have a material adverse effect on our business and operations. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe a third party’s intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product candidate or product. Alternatively, we may be required, or may choose, to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product candidate. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time-consuming and unsuccessful.
Competitors or other third parties may infringe our patents, trademarks or other intellectual property. To counter infringement or unauthorized use, we or one of our licensing partners may file infringement claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents, in addition to counterclaims asserting that our patents are invalid or unenforceable, or both. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement, insufficient written description or failure to claim patent-eligible subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. The outcome following legal assertions of invalidity and unenforceability is unpredictable. In any patent infringement proceeding, there is a risk that a court will decide that a patent of ours or our licensors is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of
such patents is upheld, the court will construe the patent’s claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patent claims do not cover the invention, or decide that the other party’s use of our patented technology falls under a safe harbor to patent infringement. An adverse outcome in a litigation or proceeding involving our patents could limit our ability to assert our patents against those parties or other competitors and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products. Any of these occurrences could adversely affect our competitive position, business, financial condition, results of operations or prospects. Similarly, if we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
Even if we establish infringement, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could adversely affect the price of shares of our common stock. Moreover, we may not have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
VDPHL01, or any of our other current or future product candidates, may face competition sooner than expected, and our patents may be challenged.
Our success will depend in part on our ability to obtain and maintain patent protection for VDPHL01 and our other current and any future product candidates and technologies and to prevent third parties from infringing upon our proprietary rights. We must also operate without infringing upon patents and proprietary rights of others, including by obtaining appropriate licenses to patents or other proprietary rights held by third parties, if necessary. However, the patent applications we have filed or may file in the future may never yield patents that protect our inventions and intellectual property assets. Failure to obtain patents that sufficiently cover our formulations and technologies would limit our protection against generic drug manufacturers, pharmaceutical companies and other parties who may seek to copy our products, produce substantially similar products or use technologies substantially similar to those we own, co-own, or exclusively license.
In the United States, when an NDA is approved under Section 505(b)(2), such NDA may be eligible for a period of non-patent exclusivity. When an NDA is approved, the product covered thereby becomes a “reference listed drug” in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations,” or the Orange Book. Manufacturers may seek approval of generic versions of reference listed drugs through submission of Abbreviated New Drug Applications, or ANDAs, in the United States. In support of an ANDA a generic manufacturer generally must show that its product has the same active ingredient(s), dosage form, strength, route of administration, and adequate labeling as the reference listed drug and that the generic version is bioequivalent to the reference listed drug, meaning, in part, that it is absorbed in the body at the same rate and to the same extent. Generic products may be significantly less costly to bring to market than the reference listed drug and companies that produce generic products are generally able to offer them at lower prices. Moreover, third-party insurers require, and many states allow or require, substitution of therapeutically equivalent generic drugs at the pharmacy level even if the branded drug is prescribed. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference listed drug may be lost to the generic product.
The FDA may not finally approve an ANDA for a generic product or a Section 505(b)(2) NDA of a competitor until any applicable period of non-patent exclusivity for the reference listed drug has expired. The FDCA provides a period of three years of non-patent exclusivity for a drug product that contains an active moiety that has been previously approved by FDA, when the application contains reports of new clinical investigations conducted or sponsored by the sponsor that were essential to the application. For example, the changes in an approved drug product that affect its active ingredient(s), strength, dosage form, route of administration or conditions of use
may be granted exclusivity if clinical investigations were essential to approval of the application containing those changes. In such an instance, FDA may not for a period of three years after the date of approval of the NDA approve another 505(b)(2) application or an ANDA for the conditions of approval of the NDA, or an ANDA submitted pursuant to an approved petition under that relies on the information supporting the conditions of approval of an original NDA. While VDPHL01 does not qualify for the five-year non-patent exclusivity provision under Section 505(b)(2), VDPHL01 may qualify for three years of non-patent exclusivity; if FDA does not grant VDPHL01 or other product candidates appropriate periods of non-patent exclusivity before approving generic versions of such products, the sales of such products could be adversely affected.
We may become subject to claims challenging the inventorship or ownership of our or our licensors’ patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our or our licensors’ patents or other intellectual property as an inventor or co-inventor. The failure to name the proper inventors on a patent application can result in the patents issuing thereon being unenforceable. Inventorship disputes may arise from conflicting views regarding the contributions of different individuals named as inventors, the effects of foreign laws where foreign nationals are involved in the development of the subject matter of the patent, conflicting obligations of third parties involved in developing our product candidates or as a result of questions regarding co-ownership of potential joint inventions. Litigation may be necessary to resolve these and other claims challenging inventorship or ownership. Alternatively, or additionally, we may enter into agreements to clarify the scope of our rights in such intellectual property. If we fail to defend any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could adversely affect our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could adversely affect our business, financial condition, results of operations, and prospects.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. During trademark registration proceedings, we may receive rejections of our applications by the USPTO or in other foreign jurisdictions. Although we are given an opportunity to respond to such rejections, we may be unable to overcome them. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, which may not survive such proceedings. Moreover, any name we have proposed to use with our product candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. Similar requirements exist in Europe. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA or an equivalent administrative body in a foreign jurisdiction objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. Furthermore, in many countries, owning and maintaining a trademark registration may not provide an adequate defense against a subsequent infringement claim asserted by the owner of a senior trademark.
We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, domain name or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. We may also rely on trade secret protection as temporary protection for concepts that may be included in a future patent filing. However, trade secret protection will not protect us from innovations that a competitor develops independently of our proprietary know how. If a competitor independently develops a technology that we protect as a trade secret and files a patent application on that technology, then we may not be able to patent that technology in the future, may require a license from the competitor to use our own know-how, and if the license is not available on commercially viable terms, then we may not be able to launch our product candidates. Additionally, trade secrets can be difficult to protect and some courts inside and outside the United States are less willing or unwilling to protect trade secrets. Although we require all of our employees to assign their inventions to us, and require all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, our trade secrets and other confidential proprietary information could be disclosed or competitors could otherwise gain access to our trade secrets. If our trade secrets are not adequately protected, our business, financial condition, results of operations and prospects could be adversely affected.
These risks are heightened due to our reliance on third parties, including third party CROs and CMOs, for certain aspects of our business. The activities conducted by our third party vendors require us to share our trade secrets with them, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
We may be subject to claims asserting that our employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants or advisors have in the past and may in the future be employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. An inability to incorporate such technologies or features would harm our business and may prevent us from successfully commercializing our product candidates. In addition, we may lose personnel as a result of such claims and any such litigation or the threat thereof may adversely affect our ability to hire employees or contract with independent contractors. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our product candidates, which could adversely affect our business, financial condition, results of operations and prospects. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
If we fail to comply with our obligations under our intellectual property licenses with third parties, we could lose license rights that are important to our business.
License agreements that we currently or in the future are party to impose and may impose various obligations on us. If we fail to comply with our obligations under our licenses, the licensors may have the right to terminate their respective license agreements, in which event we might not be able to market any product that is covered by the agreements. Termination of our license agreements or reduction or elimination of our licensed rights may result in our having to negotiate new or reinstated licenses with less favorable terms, which could adversely affect our business, financial condition, results of operations and prospects. Moreover, license agreements may be complex and subject to interpretation, and there may be disputes between us and our licensors about the scope or interpretation of the license agreement. Disputes related to license agreement could be distracting, time consuming and, if determined adversely, adversely affect our business, financial condition, results of operations and prospects.
We may not be successful in obtaining or maintaining necessary rights to third party patents for our product candidates through acquisitions and in-licenses.
The growth of our business may depend in part on our ability to acquire, in-license, or use third-party intellectual property and proprietary rights. Other pharmaceutical companies and academic institutions may own patents or may have filed, or be planning to file, patent applications potentially relevant to our business. In order to avoid infringing such patent rights, we may find it necessary or prudent to obtain licenses to such patent rights from such third parties. For example, we may be required by the FDA or comparable foreign regulatory authorities to provide a specific companion diagnostic test or tests with our product candidates, any of which could require us to obtain rights to use patents or know-how owned or controlled by third parties. In addition, with respect to any patent or other intellectual property rights we may co-own with third parties in the future, we may require licenses to such co-owners’ interest to such patent or other intellectual property rights. We may be unable to acquire or in-license any compositions, methods of use, processes, or other third-party intellectual property rights from third parties that we identify as necessary or important to our business operations. In addition, we may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. Were that to happen, we may need to cease use of the compositions or methods covered by those third-party intellectual property rights and may need to seek to develop alternative approaches that do not infringe, misappropriate, or otherwise violate those intellectual property rights, which may entail additional costs and development delays, even if we were able to develop such alternatives, which may not be feasible. Even if we are able to obtain a license, it may be non-exclusive, which means that our competitors may also receive access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology.
The licensing and acquisition of third-party intellectual property rights is a competitive area, and companies that may be more established or have greater resources than we do may also be pursuing strategies to license or acquire third-party intellectual property rights that we may consider necessary or attractive in order to commercialize our product candidates. More established companies may have a competitive advantage over us due to their size, resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. There can be no assurance that we will be able to successfully complete these types of negotiations and ultimately acquire the rights to the intellectual property related to the products or product candidates that we may seek to develop or market. If we are unable to successfully obtain rights to required third-party intellectual property or to maintain the existing intellectual property rights we have, we may have to abandon development of certain programs and our business, financial condition, results of operations, and prospects could suffer.
Certain intellectual property which we have in-licensed may have been discovered through government funded programs and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for U.S. industry. Compliance with such regulations may limit our exclusive rights, and limit our ability to contract with non-U.S. manufacturers.
Certain intellectual property rights we have or may license have been generated through the use of U.S. government funding and may therefore be subject to certain federal laws and regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future product candidates
pursuant to the Bayh-Dole Act of 1980, or the Bayh-Dole Act. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations, which are commonly referred to as “march-in rights.” The U.S. government also has the right to take title to these inventions if the applicable licensor, fails to disclose the invention to the government and fails to file an application to register the intellectual property within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require the applicable licensor to expend substantial resources. In addition, the U.S. government requires that any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property. To the extent any of our current or future intellectual property is generated through the use of U.S. government funding, the provisions of the Bayh-Dole Act may similarly apply. Any exercise by the government of certain of its rights could adversely affect our competitive position, business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
nothers may be able to make product candidates that are similar to ours but that are not covered by our patents;
nwe or our licensors or future collaborators might not have been the first to make the inventions covered by our patents or pending patent application;
nwe or our licensors or future collaborators might not have been the first to file patent applications covering certain inventions;
nothers may independently develop similar or alternative technologies or duplicate any of our technologies without infringing or otherwise violating our intellectual property rights;
nit is possible that noncompliance with the USPTO and foreign governmental patent agencies requirement for a number of procedural, documentary, fee payment and other provisions during the patent process can result in abandonment or lapse of a patent or patent application, and partial or complete loss of patent rights in the relevant jurisdiction;
nit is possible that our pending patent applications will not lead to issued patents;
nissued patents may be revoked, modified, or held invalid or unenforceable, as a result of legal challenges by our competitors;
nour competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
nwe may not develop additional proprietary technologies that are patentable;
nthere may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns;
ncountries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates;
nthe claims of any patent issuing based on our patent applications may not provide protection against competitors or any competitive advantages, or may be challenged by third parties;
nif enforced, a court may not hold that our patents are valid, enforceable and infringed;
nwe may need to initiate litigation or administrative proceedings to enforce and/or defend our patent rights which will be costly whether we win or lose;
nwe may choose not to file a patent application in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent application covering such intellectual property;
nwe may fail to adequately protect and police our trademarks and trade secrets; and
nthe patents of others may have an adverse effect on our business, including if others obtain patents claiming subject matter similar to or improving that covered by our patent applications.
Should any of these or similar events occur, they could significantly harm our business, financial condition, results of operations and prospects.
Risks Related to Government Regulation
The regulatory approval process is highly uncertain, and we may be unable to obtain, or may be delayed in obtaining, U.S. regulatory approval and, as a result, unable to commercialize our product candidates or any future product candidates. Even if we believe our current, or planned clinical trials are successful, regulatory authorities may not agree that they provide adequate data on safety or efficacy.
Our current product candidates are, and any future product candidates will be, subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, approval, recordkeeping, reporting, labeling, storage, packaging, advertising and promotion, pricing, post-approval monitoring, marketing and distribution of products. Rigorous preclinical studies and clinical trials and an extensive regulatory approval process are required to be completed successfully in the United States and in many foreign jurisdictions before a new product can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that none of our product candidates will obtain the regulatory approvals necessary for us to begin selling them.
We have no prior experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA. The time required to obtain FDA and other approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the product candidate. Any analysis we perform of data from preclinical studies and clinical trials is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. It is impossible to predict whether additional legislative changes will be enacted, or whether FDA regulations, guidance or interpretations will be changed, or the impact of such changes, if any. Any elongation or de-prioritization of preclinical studies or clinical trials or delay in regulatory review resulting from such disruptions could materially affect the development and study of VDPHL01 or any of our other product candidates or any future product candidates.
Further, the FDA may respond to any NDA that we may submit by requesting additional data or studies that we do not anticipate. Such responses could delay clinical development of our product candidates or any future product candidates. The FDA also may consider its approvals of competing products, which may alter the treatment landscape, including changes to requirements for clinical data or clinical trial design. Such changes could delay approval or necessitate withdrawal of our NDA submissions.
Any delay or failure in obtaining required approvals would adversely affect our ability to generate revenue from the particular product candidate for which we are seeking approval. Furthermore, any regulatory approval to market a product may be subject to limitations on the approved uses for which we may market the product or on the labeling or other restrictions.
We also may in the future become subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and may include all of the risks associated with the FDA approval process described above, as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. FDA approval does not ensure approval by regulatory authorities outside the United States and vice versa. Any delay or failure to obtain U.S. or foreign regulatory approval for a product candidate could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Even if we receive regulatory approval for any of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal. We may also be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any regulatory approvals that we or our existing or future collaborators obtain for our product candidates may also be subject to limitations on the approved indicated uses for which a product may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing and surveillance to monitor the safety and efficacy of the product candidate.
In addition, if the FDA or a comparable foreign regulatory authority approves any of our product candidates, the manufacturing processes, labeling, packaging, distribution, post-approval monitoring and AE reporting, storage, import, export, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. The FDA has significant post-market authority, including the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate safety risks related to the use of a product or to require withdrawal of the product from the market. The FDA also has the authority to require a REMS plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, or to require the inclusion of a boxed warning, which highlights a specific life-threatening safety risk. FDA has required the inclusion of a boxed warning on the labeling for FDA-approved IR oral minoxidil for treatment of blood pressure and cardiovascular indications due to serious heart-related adverse effects. It is unclear at this stage whether FDA is likely to require inclusion of a boxed warning for our VDPHL01, as an ER oral minoxidil product. The manufacturing facilities we use to make a future product, if any, will also be subject to periodic review and inspection by the FDA and other regulatory authorities, including for continued compliance with Current Good Manufacturing Practices, or cGMPs. The discovery of any new or previously unknown problems with our third-party manufacturers, manufacturing processes or facilities may result in restrictions on the product, manufacturer or facility, including withdrawal of the product from the market.
Any product promotion and advertising will also be subject to regulatory requirements and continuing regulatory review. The FDA imposes stringent restrictions on manufacturers’ communications regarding use of their products. Although clinicians may prescribe products for off-label uses as the FDA and other regulatory authorities do not regulate a physician’s choice of drug treatment made in the physician’s independent medical judgment, they do restrict promotional communications from companies or their sales force with respect to off-label uses of products. If we promote our products in a manner inconsistent with FDA-approved labeling or otherwise not in compliance with FDA regulations, we may be subject to enforcement action. The failure by us or our collaborators to comply with applicable regulatory requirements in the United States or foreign jurisdictions in which we seek to market our product candidates may result in, among other things, fines, warning or untitled letters, holds on clinical trials, delay of approval or refusal by the FDA or comparable foreign regulatory authorities to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, administrative detention of products, refusal to permit the import or export of products, operating restrictions, injunction, civil penalties and criminal prosecution. Even if it is later determined we were not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our actions and have to divert significant management resources from other matters.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. Changes in FDA staffing could result in delays in the FDA’s responsiveness or in its ability to review submissions or applications, issue regulations or guidance, or implement or enforce regulatory requirements in a timely fashion or at all.
Disruptions at the FDA, SEC or comparable foreign regulatory authorities caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel necessary for the review, approval and commercialization of new products in a timely manner or otherwise prevent those authorities from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA and comparable foreign regulatory authorities to review and approve new products is affected by a variety of factors, including government budget and funding levels, statutory, regulatory and policy changes, the ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the regulatory authority’s ability to perform routine functions. Average review times at the FDA and other regulatory authorities have fluctuated in recent years. In addition, government funding of other authorities and agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable. Disruptions at the FDA and other regulatory authorities may also slow the time necessary for new drugs or modifications to approved drugs to be reviewed and/or approved, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times, including in 2025, and certain regulatory authorities, such as the FDA and the SEC, have had to furlough critical employees and stop critical activities.
If any future prolonged government shutdown occurs, or if global health concerns prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
If we fail to comply with broad and complex healthcare and other laws, we could face substantial penalties and our business, operations, and financial condition could be adversely affected.
The marketing of pharmaceutical products and related arrangements with healthcare providers, third-party payors, patients, and other third parties in the healthcare industry are subject to a wide range of federal and state healthcare laws and regulations that may constrain our business and/or financial arrangements. Some of these laws apply to us now, while other laws may apply to us only if and when we have marketed products or have marketed products that are covered by government health benefit programs or private health care insurance. These laws include:
nthe federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid;
nfederal civil and criminal false claims laws, including the federal False Claims Act, which can be enforced through civil whistleblower, or qui tam actions, as well as civil monetary penalty laws can impose criminal and civil penalties, assessment, and exclusion from participation for various forms of fraud and abuse involving the federal healthcare programs, such as Medicare and Medicaid;
nthe federal Health Insurance Portability and Accountability Act of 1996, as amended, or HIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also establishes requirements related to the privacy, security, and transmission of individually identifiable health information which apply to many healthcare providers, physicians, and third-party payors with whom we interact;
nthe federal Food, Drug, and Cosmetic Act, or FDCA, which, among other things, strictly regulates drug product and medical device marketing, prohibits manufacturers from marketing such products for off-label use, and regulates the distribution of samples;
nfederal laws that require pharmaceutical manufacturers to calculate, report, and certify certain complex product prices and other data to the government or provide certain discounts or rebates to government authorities or private entities, often as a condition of reimbursement under government healthcare programs, which data may be used in the calculation of reimbursement and/or discounts on approved products;
nthe so-called federal “sunshine law” or Open Payments which requires manufacturers of drugs, devices, biologics, and medical supplies covered under certain government health benefit programs to report to the Centers for Medicare & Medicaid Services, or CMS, information related to payments and other transfers of value to teaching hospitals, physicians, and other healthcare practitioners, as well as ownership and investment interests held by physicians and their immediate family members;
nfederal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
nstate laws and regulations analogous to federal laws, including anti-kickback or related laws, some of which apply regardless of whether products or services are covered by government health benefit programs or private insurance, false claims laws, laws prohibiting consumer protection and unfair competition laws, and laws governing privacy, security, and breaches of health (and other personal) information in certain circumstances, many of which differ in significant ways from federal laws and across states and are often not preempted by federal law, thus complicating compliance efforts; and
nstate laws that require pharmaceutical companies to comply with specific compliance standards, restrict financial interactions between pharmaceutical companies and healthcare providers, report drug product pricing information, financial interactions with health care providers, or marketing expenditures, and/or require the registration of pharmaceutical sales representatives.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage, and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
To the extent that we implement a telehealth platform, activities undertaken and arrangements implemented in connection with such a platform may implicate other laws such as state physician, pharmacy and telehealth licensure laws, corporate practice of medicine, and fee-splitting laws.
Efforts to ensure that our activities comply with applicable healthcare laws and regulations will involve substantial costs. Given the breadth of the laws and regulations, limited guidance for certain laws and regulations, and evolving government interpretations of the laws and regulations, governmental authorities may possibly conclude that our business practices may not comply with such laws. For example, we have engaged physicians to serve as investigators and/or consultants, including service on advisory boards, and our commercialization plan may include significant physician outreach and education. Federal and state enforcement agencies scrutinize interactions between pharmaceutical companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions, and settlements in the healthcare industry. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal, and administrative penalties, damages, fines, exclusion from participation in federal health care programs such as Medicare and Medicaid, the curtailment or restructuring of our operations, and other actions. Further, defending against any such actions can be costly, time-consuming, and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
The U.S. Supreme Court’s June 2024 decision in Loper Bright Enterprises v. Raimondo overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statutes. The Loper decision could result in additional legal challenges to regulations and guidance issued by federal agencies, including FDA and CMS, on which we rely. Any such legal challenges, if successful, could have a material impact on our business. Additionally, the Loper decision may result in increased regulatory uncertainty, inconsistent judicial interpretations, and other impacts to the agency rulemaking process, any of which could adversely impact our business and operations. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action or as a result of legal challenges, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, our business could be materially harmed.
Legislative, regulatory, and executive healthcare and other reform initiatives aimed at reducing healthcare costs may have a material adverse effect on our business and results of operations.
Legislative, regulatory, and executive healthcare and other reform initiatives in the United States, including those aimed at containing or lowering the cost of healthcare, may adversely impact our business, operations, and financial condition.
A number of the healthcare reform initiatives have focused on pricing and payment for prescription drugs, including some recent initiatives that address pricing in DTC offerings. For example, the Inflation Reduction Act of 2022, or the IRA, which is having a significant and ongoing impact on the pharmaceutical industry, includes a number of changes intended to address rising prescription drug prices in Medicare Parts B and D, such as caps on Medicare Part D out-of-pocket costs and a drug price negotiation program for certain high-spend Medicare Part B and D drugs.
More recently, the Trump Administration has taken action intended to reduce the cost of prescription drugs, including drugs purchased directly by consumers. The administration issued two Executive Orders aimed at lowering drug prices through multiple directives, including directives to government agencies and officials to identify most-favored-nation pricing targets for prescription drugs (and looking to pharmaceutical manufacturers to make significant progress towards delivering target prices to patients), to facilitate DTC purchasing programs for pharmaceutical manufacturers to sell their products to patients at the most-favored-nation price, to enhance competition for high-cost prescription drugs by accelerating approval of generics and biosimilars, facilitating the process for re-classifying prescription drugs as OTC drugs, and increasing drug importation. In the wake of the Executive Orders and related executive initiatives, a number of pharmaceutical manufacturers have announced new or expanded DTC offerings with discounted prices and/or reached agreement with the federal government regarding discounted pricing for drugs, including prices for Medicaid drugs and newly launched products. TrumpRx, a website sponsored by the federal government that is anticipated to offer pharmaceutical DTC channels, has also been announced. Federal agencies are also developing and proposing new drug pricing and payment pilot programs based on international pricing metrics under Medicare Parts B and D as well as Medicaid.
Other healthcare reform efforts or actions under the Trump Administration may affect access to healthcare coverage or the funding of health care benefits, although the full impact of such efforts or actions cannot be predicted. For example, Congressional Budget Office has estimated that Medicaid provisions in the 2025 budget reconciliation legislation, including restrictions in eligibility and funding for Medicaid, as well as changes to the healthcare marketplace, will increase the number of uninsured.
Healthcare reform efforts have been and may continue to be subject to scrutiny and legal challenge, which increases uncertainty. For example, the IRA drug price negotiation program has been challenged in litigation filed by various pharmaceutical manufacturers and industry groups.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and, in some cases, encourage importation from other countries and bulk purchasing. This could reduce the ultimate demand for our product candidates, if approved, or put pressure on our product pricing, which could negatively affect our business, financial condition, results of operations, and prospects.
Other recent government actions also may affect prices or payments for prescription drugs. For example, the Trump Administration’s recently announced tariff on branded or patented drugs for manufacturers that do not invest in manufacturing plants in the United States or reach a drug pricing agreement with the Trump Administration may adversely impact our ability to realize an adequate return on the sale of any drug products imported from abroad or manufactured using products or materials imported from abroad. The timeline for implementation of this tariff has not yet been finalized. As another example, the Budget Control Act, as amended, resulted in the imposition of reductions in Medicare (but not Medicaid) payments to providers in 2013 and will remain in effect into 2032 unless additional Congressional action is taken.
The nature and extent of future healthcare or other reforms cannot be predicted. There is significant uncertainty regarding the nature or impact of any drug pricing or broader healthcare reform implemented by the current presidential administration through executive action or by Congress and the extent to which such action may be subject to litigation or other challenges. Reform at the federal or state level could affect demand for, or pricing of, any future products if approved for sale. We cannot, however, predict the ultimate content, timing, or effect of any federal and state reform efforts. There is no assurance that federal or state health care reform will not adversely affect our future business and financial results.
We are or may become subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, industry standards and policies related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; loss of customers or sales; and other adverse business consequences.
Our data processing activities subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations relating to data privacy and security. Various federal, state, local and foreign legislative and regulatory bodies, or self-regulatory organizations, may expand current laws, rules or regulations, enact new laws, rules or regulations or issue revised rules or guidance regarding data privacy and security. In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws). For example, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, imposes specific requirements relating to the privacy, security, and transmission of individually identifiable health information.
Numerous U.S. states have enacted comprehensive privacy laws that impose certain obligations on covered businesses, including providing specific disclosures in privacy notices and affording residents with certain rights concerning their personal data. As applicable, such rights may include the right to access, correct, or delete certain personal data, and to opt-out of certain data processing activities, such as targeted advertising, profiling, and automated decision-making. The exercise of these rights may impact our business and ability to provide our products and services. Certain states also impose stricter requirements for processing certain personal data, including sensitive information, such as conducting data privacy impact assessments. These state laws allow for statutory fines for noncompliance.
For example, the California Consumer Privacy Act, or CCPA, provides for civil penalties per violation as well as a private right of action with statutory damages for certain data breaches. The CCPA also exempts some data processed in the context of clinical trials. These laws may further complicate compliance efforts, and increase legal risk and compliance costs for us and the third parties upon whom we rely. In addition to government activity, privacy advocacy groups and technology and other industries are considering various new, additional or
different self-regulatory standards that may place additional burdens on us. Similar laws are being considered in several other states, as well as at the federal and local levels, and we expect more states to pass similar laws in the future.
There are many other federal and state-based data privacy and security laws and regulations, such as the Federal Communications Act, the Electronic Communications Privacy Act, the Telephone Consumer Protection Act, or the TCPA, the Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003, or the CAN-SPAM Act, and similar state consumer protection and communication privacy laws, such as California’s Invasion of Privacy Act, or the CIPA. For example, the CAN-SPAM Act and the TCPA impose specific requirements on communications with consumers. The TCPA and analogous state laws impose various consumer consent requirements and other requirements on certain communications with consumers by phone or text message. TCPA violations can result in significant financial penalties, including penalties or criminal fines imposed by the Federal Communications Commission, or the FCC, or statutory damages of up to $1,500 per violation imposed through private litigation or by state authorities. The TCPA provides for substantial penalties and statutory damages and has generated significant class action activity. The costs of litigating and/or settling a TCPA or similar legal claim could be significant. There has also been a noticeable uptick in class action litigation wherein plaintiffs have utilized a variety of laws, including state wiretapping laws such as the CIPA, in relation to companies’ use of certain tracking technologies, such as cookies and pixels. Actual or perceived failure to comply with these laws and regulations could subject us to legal proceedings (such as class action litigation and mass arbitration demands), which could result in adverse publicity, substantial monetary damages and legal defense costs, injunctive relief and fines or penalties.
We are or may become subject to laws governing the privacy of consumer health data, including reproductive, sexual orientation, and gender identity privacy rights. For example, Washington’s My Health My Data Act, or MHMD, as applicable to our operations, broadly defines consumer health data, places restrictions on the processing of consumer health data (including imposing stringent requirements for consents), provides consumers certain rights with respect to their health data, and creates a private right of action to allow individuals to sue for violations of the law. Other states have passed, are considering, and may adopt similar laws.
In addition to data privacy and security laws, we are also bound by other contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful.
Our employees and personnel use or may in the future use generative AI and/or automated decision-making technologies to perform their work, and the disclosure and use of personal data in AI technologies is subject to various privacy laws and other privacy obligations. Governments have passed and are likely to pass additional laws regulating AI and/or automated decision-making technologies. Our use of this technology could result in additional compliance costs, regulatory investigations and actions, and lawsuits. If we are unable to use AI and/or automated decision-making technologies, it could make our business less efficient and result in competitive disadvantages.
The U.S. Department of Justice, or the DOJ, issued a rule entitled Access to U.S. Sensitive Personal Data and Government-Related Data by Countries of Concern or Covered Persons, which places additional restriction on certain data transactions involving countries of concern (e.g., China, Russia, Iran) and covered individuals (i.e., individuals and entities located in or controlled by individuals or entities located in those jurisdictions) that may impact certain business activities such as vendor engagements, sale or sharing of data, employment of certain individuals, and investor agreements. Violations of the rule could lead to significant civil and criminal fines and penalties. The rule applies regardless of whether data is anonymized, key-coded, pseudonymized, de-identified or encrypted, which presents particular challenges for companies like ours and may impact our ability to transfer data in connection with certain transactions or agreements.
We also publicly post certain of our privacy policies and practices concerning our collection, use, disclosure and other processing of the personal information. Regulators are increasingly scrutinizing these statements, and if these policies, materials or statements are found to be deficient, lacking in transparency, deceptive, unfair, misleading, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators or other adverse consequences.
Obligations related to data privacy and security (and consumers’ data privacy expectations) are quickly changing, becoming increasingly stringent, and creating uncertainty. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires us to devote significant resources, which may necessitate changes to our services, information technologies, systems, and practices and to those of any third parties that process personal data on our behalf.
Each of these laws, rules, regulations and contractual obligations relating to data privacy and security, and any other such changes or new laws, rules, regulations or contractual obligations could impose significant limitations, require changes to our business, or restrict our collection, use, storage or processing of personal information, which may increase our compliance expenses and make our business more costly or less efficient to conduct. In addition, any such changes could compromise our ability to develop an adequate marketing strategy and pursue our growth strategy effectively or even prevent us from providing certain products in jurisdictions in the future or incur potential liability in an effort to comply with such legislation, which, in turn, could adversely affect our business, financial condition, results of operations and prospects. Complying with these numerous, complex and often changing regulations is expensive and difficult, and failure to comply with any data privacy or security laws, whether by us, one of our CROs, CMOs or another third party, could adversely affect our business, financial condition, results of operations and prospects, including but not limited to: investigation costs; material fines and penalties; compensatory, special, punitive and statutory damages; litigation; consent orders regarding our privacy and security practices; requirements that we provide notices, credit monitoring services and/or credit restoration services or other relevant services to impacted individuals; adverse actions against our licenses to do business; reputational damage; and injunctive relief.
We may at times fail (or be perceived to have failed) in our efforts to comply with our data privacy and security obligations. Moreover, despite our efforts, our personnel or third-parties with whom we work may fail to comply with such obligations. Any actual or perceived failure by us or the third parties with whom we work to comply with any federal, state or foreign laws, rules, regulations, industry self-regulatory principles, industry standards or codes of conduct, regulatory guidance, orders to which we may be subject or other legal obligations relating to privacy, data protection, data security or consumer protection could adversely affect our reputation, brand and business. We may also be contractually required to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, rules and regulations or other legal obligations relating to privacy or any inadvertent or unauthorized use or disclosure of data that we process as part of operating our business. Any of these events could adversely affect our reputation, business, or financial condition, including but not limited to: loss of customers; interruptions or stoppages in our business operations (including clinical trials); inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize our products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to our business model or operations.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations as well as U.S. and certain foreign export controls, trade sanctions, and import laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the United States Export Administration Regulations, United States Customs regulations, various economic and trade sanctions regulations administered by the United States Treasury Department’s Office of Foreign Assets Controls, the United States Foreign Corrupt Practices Act of 1977, as amended, or FCPA, the United States domestic bribery statute contained in 18 U.S.C. § 201, the United States Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties to sell our products outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We
can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences. Recently the SEC and the DOJ have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all of our employees, agents or contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or employees, disgorgement, and other sanctions and remedial measures, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer VDPHL01 or any future product candidates in one or more countries and could materially damage our reputation, brand, international activities, ability to attract and retain employees, and business, prospects, operating results and financial condition.
In addition, VDPHL01 or any of our other current or future product candidates and activities may be subject to U.S. and foreign export controls, trade sanctions and import laws and regulations. Governmental regulation of the import or export of VDPHL01 or any of our other current or future product candidates, or our failure to obtain any required import or export authorization for VDPHL01 or any of our other current or future product candidates, when applicable, could harm our international sales and adversely affect our revenue. Compliance with applicable regulatory requirements regarding the export of VDPHL01 or any of our other current or future product candidates may create delays in the introduction of our product candidates in international markets or, in some cases, prevent the export of our product candidates to some countries altogether. Furthermore, U.S. export control laws and economic sanctions prohibit the shipment of certain products and services to countries, governments, and persons targeted by U.S. sanctions. If we fail to comply with export and import regulations and such economic sanctions, penalties could be imposed, including fines and/or denial of certain export privileges. Moreover, any new export or import restrictions, new legislation or shifting approaches in the enforcement or scope of existing regulations, or in the countries, persons, or products targeted by such regulations, could result in decreased use of VDPHL01 or any of our other current or future product candidates by, or in our decreased ability to export VDPHL01 or any of our other current or future product candidates to existing or potential customers with international operations. Any decreased use of VDPHL01 or any of our other current or future product candidates or limitation on our ability to export or sell access to VDPHL01 or any of our other current or future product candidates would likely adversely affect our business.
Risks Related to Our Dependence on Third Parties
We currently rely on third parties for the manufacture of drug or biological substances for our preclinical studies and clinical trials and expect to continue to do so for commercialization of any product candidates that we may develop that are approved for marketing. Our reliance on third parties may increase the risk that we will not have sufficient quantities of such drug substance, product candidates, or any products that we may develop and commercialize, or that such supply will not be available to us at an acceptable cost, which could delay, prevent, or impair our development or commercialization efforts.
We have limited personnel with experience in manufacturing, and we do not own facilities for manufacturing VDPHL01 or any other product candidate. Instead, we rely on and expect to continue to rely on CMOs for the supply of cGMP-drug substance and drug product of VDPHL01 and any other product candidates we develop and, in the future, for commercial supply for any approved product candidates. Currently, we rely on one CMO for the supply of drug product of VDPHL01. Reliance on third parties may expose us to more risk than if we were to manufacture our product candidates ourselves. For example, should demand for our drug significantly exceed what is expected, we may experience supply shortages in trying to obtain or source material to meet such demand. Additionally, we do not have long-term supply contracts with any of our CMOs for preclinical or clinical supply, and they are not obligated to supply drug products to us for any period, in any specified quantity or at any certain price beyond the delivery contemplated by the relevant purchase orders. As a result, our suppliers could stop selling to us at commercially reasonable prices, or at all. We may be unsuccessful in negotiating and entering into long-term master supply agreements with certain of our current or future CMOs on favorable terms or at all, which would likely jeopardize our ability to provide any product candidates to participants in clinical trials and products to market, if approved.
We have entered into a commercial supply agreement with a third-party manufacturer for commercial supply of VDPHL01, if approved. We intend to rely on this manufacturer as the primary source for drug product manufacturing and final packaging for VDPHL01. Unless and until we can secure an alternative source for drug product manufacturing and final packaging, our dependence on one manufacturer will subject us to the possible risks of shortages, interruptions and price fluctuations if VDPHL01 is approved for commercialization. We may not be able to establish agreements with other third-party manufacturers if necessary for VDPHL01 or any other product candidate that receives marketing approval, on acceptable terms or at all.
Reliance on third-party manufacturers entails additional risks, including:
nthe failure of the third party to manufacture VDPHL01 or any other current or future product candidates according to our schedule, or at all, including if our third-party contractors give greater priority to the supply of other products over our products or product candidates or otherwise do not satisfactorily perform according to the terms of the agreements between us and them;
nthe failure of the third party to manufacture VDPHL01 or any other current or future product candidates according to our specifications;
nthe reduction or termination of production or deliveries by suppliers, or the raising of prices or renegotiation of terms;
nthe possible breach of the manufacturing agreement by the third-party;
nthe possible termination or nonrenewal of the agreement by the third-party at a time that is costly or inconvenient for us;
nthe mislabeling of clinical supplies, potentially resulting in the wrong dose amounts being supplied or study drug or placebo not being properly identified;
nthe failure of third-party contractors to comply with applicable regulatory requirements, whether related to VDPHL01 or any other current or future product candidates or products;
nclinical supplies not being delivered to clinical sites on time, leading to clinical trial interruptions, or of drug supplies not being distributed to commercial vendors in a timely manner, resulting in lost sales;
nthe misappropriation of our proprietary information, including our trade secrets and know-how;
nreliance on the third-party for regulatory compliance, quality assurance, safety, and pharmacovigilance and related reporting; and
nthe possible inability of third-party suppliers to supply and/or transport materials, components and products to us in a timely manner as a result of disruptions to the global supply chain.
The manufacturing process for our product candidates is subject to the FDA and comparable foreign regulatory authority review. We and our suppliers and manufacturers, some of which are currently our sole source of supply, must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as cGMPs. Any failure to follow cGMP or other regulatory requirements or delay, interruption or other issues that arise in the manufacture, fill-finish, packaging, or storage of our product candidates as a result of a failure of our facilities or the facilities or operations of third parties to comply with regulatory requirements or pass any regulatory authority inspection could significantly impair our ability to develop and commercialize our product candidates, including leading to significant delays in the availability of our product candidates for our clinical trials or the termination of or suspension of a clinical trial, or the delay or prevention of a filing or approval of marketing applications for our product candidates. Moreover, our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocations, seizures or recalls of product candidates or therapies, operating restrictions, and criminal prosecutions, any of which could significantly and adversely affect supplies of our therapies and harm our business, financial condition, results of operations, and prospects.
While we provide oversight of manufacturing activities, we have limited ability to control the execution of manufacturing activities by, and are or will be dependent on, our CMOs for compliance with cGMP requirements for the manufacture of our product candidates by our CMOs. As a result, we are subject to the risk that our product candidates may have manufacturing defects or fail to comply with regulatory requirements, which we
have limited ability to prevent. CMOs may also have competing obligations that prevent them from manufacturing our product candidates in a timely manner. If a CMO cannot successfully manufacture drug substance that conforms to our specifications and the regulatory requirements, we will not be able to secure or maintain regulatory approval for the use of our product candidates in clinical trials, or for commercial distribution of our product candidates, if approved. In addition, we have limited control over the ability of our CMOs to maintain adequate quality control, quality assurance, and qualified personnel, and we were not involved in developing our CMOs’ policies and procedures.
The facilities and processes used to manufacture our product candidates are subject to inspection by the FDA and other comparable foreign authorities. If the FDA or other comparable foreign regulatory authority finds deficiencies with or does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval or finds deficiencies in the future, we may need to find alternative manufacturing facilities or conduct additional studies, which would delay our development program and significantly impact our ability to develop, obtain regulatory approval for, or commercialize our product candidates, if approved. Furthermore, CMOs may breach existing agreements they have with us because of factors beyond our control. They may also terminate or refuse to renew their agreement at a time that is costly or otherwise inconvenient for us. Finding new CMOs or third-party suppliers involves additional cost and requires our management’s time and focus. In addition, there is typically a transition period when a new CMO commences work. Any significant delay in the supply of our product candidates or the raw materials needed to produce our product candidates, could considerably delay conducting our clinical trials and potential regulatory approval of our product candidates. If we were unable to find an adequate CMO or another acceptable solution in time, our clinical trials could be delayed, or our commercial activities could be harmed.
We rely on and will continue to rely on CMOs to purchase from third-party suppliers raw materials necessary to produce our product candidates. We have limited ability to control the process or timing of the acquisition of these raw materials by our CMOs. Supplies of raw materials could be interrupted from time to time and we cannot be certain that alternative supplies could be obtained within a reasonable time frame, at an acceptable cost, or at all. In addition, a disruption in the supply of raw materials could delay the commercial launch of our product candidates, if approved, or result in a shortage in supply, which would impair our ability to generate revenues from the sale of our product candidates. Growth in the costs and expenses of raw materials may also impair our ability to cost effectively manufacture our product candidates. There are a limited number of suppliers for the raw materials that we may use to manufacture our product candidates and we may need to assess alternative suppliers to prevent a possible disruption of the manufacture of our product candidates. Moreover, our product candidates utilize drug substances that are produced on a small scale, which could limit our ability to reach agreements with alternative suppliers.
As part of their manufacture of our product candidates, our CMOs and third-party suppliers are expected to comply with and respect the intellectual property and proprietary rights of others. If a CMO or third-party supplier fails to acquire the proper licenses or otherwise infringes, misappropriates or otherwise violates the intellectual property or the proprietary rights of others in the course of providing services to us, we may have to find alternative CMOs or third-party suppliers or defend against claims of infringement, either of which would significantly impact our ability to develop, obtain regulatory approval for, or commercialize our product candidates, if approved.
Furthermore, any of the sole source and limited source suppliers upon whom we rely could stop producing our supplies, cease operations or be acquired by, or enter into exclusive arrangements with, our competitors. Any interruption or delay in the supply of sole source or limited source components for our product candidates, including as a result of us needing to seek alternative sources, which may not be available at reasonable prices or at all, would adversely affect our ability to meet scheduled timelines and budget for the development and commercialization of our product candidates, could result in higher expenses and delayed revenue, if our product candidates are approved, and would harm our business. Although we have not experienced any significant disruption as a result of our reliance on limited or sole source suppliers, we have a limited operating history and cannot assure you that we will not experience disruptions in our supply chain in the future as a result of such reliance or otherwise.
We have relied and expect to continue to rely on third parties to conduct our preclinical studies and clinical trials. If those third parties do not perform as contractually required, fail to satisfy legal or regulatory requirements, miss deadlines or terminate the relationship, our development programs could be delayed, more costly or unsuccessful, and we may never be able to seek or obtain regulatory approval for or commercialize our product candidates.
We rely and intend to continue to rely on third-party clinical investigators, CROs and clinical data management organizations to conduct, supervise and monitor preclinical studies and clinical trials of our current and future product candidates. Currently, Therapeutics, Inc. is our exclusive provider of clinical trial management, regulatory affairs activities (including acting as our designated agent with the FDA), program support and other CRO services, and will continue to be our exclusive provider of such services through the completion of Phase 2 of VDMN (or, if later, the first Veradermics product candidate to reach completion of Phase 2).
Because of this reliance, we have less control over the timing, quality and other aspects of preclinical studies and clinical trials than if we conduct them ourselves. Third parties are not our employees and we have limited control over the amount of time and resources that they dedicate to our programs. Additionally, such parties have contractual relationships with other entities, some of which may be our competitors, which may divert time and resources from our programs.
Our reliance on third parties reduces our control over our development activities. Nevertheless, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable trial protocol and legal, regulatory and scientific standards. For example, we remain responsible for ensuring that each of our preclinical studies are conducted in accordance with GLPs and clinical trials are conducted in accordance with Good Clinical Practice, or GCPs. Moreover, the FDA and comparable foreign regulatory authorities require us to comply with GCP for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Regulatory authorities enforce these requirements through periodic inspections (including pre-approval inspections once an NDA is submitted to the FDA) of trial sponsors, clinical investigators, trial sites and certain third parties including CROs. If we, our CROs, clinical trial sites or other third parties fail to comply with applicable GCP or other regulatory requirements, we or they may be subject to enforcement or other legal actions, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials. Moreover, our business may be significantly impacted if our CROs, clinical investigators or other third parties violate federal or state healthcare fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
If our third party contractors do not successfully carry out their contractual duties, meet deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, our clinical trials may need to be repeated, extended, delayed or terminated, we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates, we will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates or we or they may be subject to regulatory enforcement actions. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
If any of our relationships with these third parties terminate, we may not be able to enter into alternative arrangements or do so on commercially reasonable terms. Switching or adding contractors involves cost, takes time and diverts management’s attention. In addition, there is a natural transition period when a new third party commences work. Delays could compromise our ability to meet our desired development timelines. In addition, if an agreement with any of our collaborators terminates, our access to technology and intellectual property licensed to us by that collaborator may be restricted or terminate entirely, which may delay our continued development of our product candidates utilizing the collaborator’s technology or intellectual property or require us to stop development of those product candidates completely.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. We may be required to report some of these relationships to the FDA, and the FDA may conclude that a financial relationship between us and/or a principal investigator has created a conflict of interest or otherwise affects interpretation of the study. The FDA
may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
If our third-party manufacturer of VDPHL01 is unable to increase the scale of its production or we are not able to qualify additional manufacturers, then commercialization may be delayed or interrupted.
In order to produce sufficient quantities to meet the commercial demand for VDPHL01, if approved, our third-party manufacturers may be required to increase their production and optimize their manufacturing processes while maintaining the quality of VDPHL01. The transition to larger scale production could prove difficult. In addition, if we are unable to qualify additional manufacturers or our third-party manufacturers are not able to produce increased amounts of our product candidates to meet demand while maintaining the same quality then we may not be able to meet market demand for VDPHL01, which could decrease our ability to generate profits and have a material adverse impact on our business and results of operations.
We depend on third-party suppliers for materials used in the manufacture of our product candidates, and the loss of these third-party suppliers or their inability to supply us with adequate materials could harm our business.
We rely on third-party suppliers for certain materials and components required for the production of our product candidates. Our dependence on these third-party suppliers and the challenges we may face in obtaining adequate supplies of materials involve several risks, including limited control over pricing, availability, and quality and delivery schedules. As a small company, our negotiation leverage is limited and we are likely to get lower priority than our competitors that are larger than we are. We cannot be certain that our suppliers will continue to provide us with the quantities of these raw materials that we require or satisfy our anticipated specifications and quality requirements. Any supply interruption in limited or sole sourced raw materials could materially harm our ability to manufacture our product candidates until a new source of supply, if any, could be identified and qualified. We may be unable to find a sufficient alternative supply channel in a reasonable time or on commercially reasonable terms. Any performance failure on the part of our suppliers could delay the development and potential commercialization of our product candidates, including limiting supplies necessary for clinical trials and regulatory approvals, which would have a material adverse effect on our business.
The operations of our supplier for minoxidil API for VDPHL01 are located outside of the United States and are subject to additional risks that are beyond our control and that could harm our business, financial condition, results of operations and prospects.
Currently, our supplier for minoxidil API for VDPHL01 primarily operates outside of the United States. As a result, we are subject to risks associated with doing business abroad, including:
ngeopolitical tensions, political unrest, terrorism, labor disputes and economic instability resulting in the disruption of trade from foreign countries in which our products are manufactured, particularly China;
nthe imposition of new laws and regulations, including those relating to labor conditions and safety standards, information and data transfer, imports, duties, taxes, and other charges on imports, as well as trade restrictions and restrictions on currency exchange or the transfer of funds, particularly new or increased tariffs imposed on imports from countries where our suppliers operate;
ngreater challenges and increased costs with enforcing and periodically auditing or reviewing our suppliers’ and manufacturers’ compliance with cGMPs or status acceptable to the FDA or comparable foreign regulatory authorities;
nreduced protection for intellectual property rights, including trade secret protection, in some countries, particularly China;
ndisruptions in operations due to global, regional, or local epidemics, pandemics and other public health crises, or other emergencies or natural disasters;
ndisruptions or delays in shipments; and
nchanges in local economic conditions in countries where our manufacturers or suppliers are located.
These and other factors beyond our control could interrupt our supplier’s production, influence the ability of our suppliers to export our clinical supplies cost-effectively or at all and inhibit our suppliers’ ability to procure certain materials, any of which could delay our clinical trials or otherwise harm our business, financial condition, results of operations and prospects.
Risks associated with the in-licensing or acquisition of product candidates could cause substantial delays in the preclinical and clinical development of our product candidates.
We may seek to enter into collaborations, licenses and other similar arrangements for VDPHL01 or any of our other current or future product candidates and may not be successful in doing so, and even if we are, we may relinquish valuable rights and may not realize the benefits of such relationships.
We may seek to enter into collaborations, licenses and other similar arrangements for the development or commercialization of VDPHL01 outside of the United States or of any of our other current or future product candidates, due to capital costs required to develop or commercialize our product candidates or manufacturing constraints. Such collaborative efforts may not be profitable. We may not be successful in our efforts to establish or maintain collaborations for our product candidates because our research and development pipeline may be insufficient, our product candidates may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy or significant commercial opportunity. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process is often time-consuming and complex. We likely would relinquish valuable rights to our future revenue streams, research programs or product candidates, or may grant licenses on terms that may not be favorable to us, as part of any such arrangement, and such arrangements restrict us from entering into additional agreements with other potential licensing and collaboration partners. We may not achieve an economic benefit that justifies any such transaction.
The success of any collaboration arrangements will depend heavily on the efforts and activities of our collaborators. Collaborations are subject to numerous risks, which may include that:
ncollaborators have significant discretion in determining the efforts and resources that they will apply to collaborations;
ncollaborators may not pursue development and commercialization of our products or may elect not to continue or renew development or commercialization programs based on trial or test results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities;
ncollaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates;
na collaborator with marketing, manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities;
nwe could grant exclusive rights to our collaborators that would prevent us from collaborating with others;
ncollaborators may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
ndisputes may arise between us and a collaborator that causes the delay or termination of the research, development or commercialization of our current or future products or that results in costly litigation or arbitration that diverts management attention and resources;
ncollaborations may be terminated, and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable current or future products;
ncollaborators may own or co-own intellectual property covering our products that results from our collaborating with them, and in such cases, we would not have the exclusive right to develop or commercialize such intellectual property; and
na collaborator’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings.
Even if we are successful in our efforts to establish such collaborations, the terms that we agree upon may not be favorable to us, and we may not be able to maintain such collaborations if, for example, the development or approval of VDPHL01 or any of our other current or future product candidates is delayed, the safety of VDPHL01 or any of our other current or future product candidates is questioned or the sales of an approved product candidate are unsatisfactory.
In addition, any potential future collaborations may be terminable by our strategic partners, and we may not be able to adequately protect our rights under these agreements. Furthermore, strategic partners often negotiate for certain rights to control decisions regarding the development and commercialization of product candidates and products, if approved, and may not conduct those activities in the same manner as we do. Any termination of collaborations we enter into in the future, or any delay in entering into collaborations related to VDPHL01 or any of our other current or future product candidates, would delay the development and commercialization of such product or product candidate and reduce their competitiveness if they reach the market, which could have a material adverse effect on our business, financial condition and results of operations.
Our reliance on third parties requires us to share our trade secrets, know-how and other proprietary information, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we currently rely on third parties to manufacture our product candidates and to perform other preclinical and clinical services, we must, at times, share our proprietary information, including trade secrets and know-how, with them. We seek to protect our proprietary information, in part, by entering into confidentiality agreements, and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our CROs, CMOs, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our proprietary information. Despite the contractual provisions employed when working with third parties, the need to share trade secrets, know-how and other proprietary information increases the risk that such proprietary information become known by our competitors, are intentionally or inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. We rely, in part, on trade secrets, know-how and other proprietary information to develop and maintain our competitive position and a competitor’s discovery of our proprietary information or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business, financial condition, results of operations and prospects.
Changes in methods of product candidate manufacturing or formulation may result in additional costs or delay.
As product candidates proceed through preclinical studies to late-stage clinical trials towards potential approval and commercialization, various aspects of the development program, such as manufacturing methods and formulation, may be altered along the way in an effort to optimize processes and product characteristics. Such changes carry the risk that they will not achieve our intended objectives. Any such changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the materials manufactured using altered processes. Such changes may also require additional testing, FDA notification or FDA approval, or comparable foreign regulatory requirements. This could delay completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidates and jeopardize our ability to commence sales and generate revenue. In addition, we may be required to make significant changes to our upstream and downstream processes across our pipeline, which could delay the development of our future product candidates.
Risks Related to this Offering and Ownership of Our Common Stock
An active and liquid trading market for our common stock may not develop, and you may not be able to resell your shares of common stock at or above the public offering price, if at all.
Prior to this offering, no market for shares of our common stock existed. We have applied to list our common stock on NYSE under the symbol “MANE,” and this offering is contingent upon obtaining such approval. Assuming that our common stock is listed, an active or liquid trading market for our common stock may never develop or be sustained following the consummation of this offering. Moreover, the initial public offering price for our common stock will be determined through negotiations with the underwriters, and may vary from the market price of our common stock following the consummation of this offering. As a result of these and other factors, you may be unable to resell your shares of our common stock at or above the initial public offering price or at a price that you consider reasonable. Furthermore, an inactive market may reduce the fair market value of your shares, impair our ability to raise capital by selling shares of our common stock in the future, and may impair our ability to enter into strategic collaborations or acquire companies or products by using our shares of common stock as consideration.
The market price of our common stock may be volatile, which could result in substantial losses for investors purchasing shares in this offering.
The initial public offering price for our common stock was determined through negotiations with the underwriters. This initial public offering price may vary from the market price of our common stock after the offering. As a result, you may not be able to sell your common stock at or above the initial public offering price. Some of the factors that may cause the market price of our common stock to fluctuate include:
nvolatility in our operating results or the failure of our operating results to meet the expectations of investors or securities analysts;
nthe success of existing or new competitive product candidates or technologies;
nthe timing and results of preclinical and clinical studies for any product candidates that we may develop or changes in the development status of any of these programs;
nany delay in our regulatory filings for VDPHL01 or our other current or any future product candidates;
nfailure or discontinuation of any of our product development and research programs;
nour failure to commercialize VDPHL01 or our other current or any future product candidates;
nresults of preclinical studies, clinical trials, or regulatory approvals of product candidates of our competitors, or announcements about new research programs or product candidates of our competitors;
ncommencement or termination of collaborations for our product development and research programs;
nregulatory or legal developments in the United States and other countries;
ndevelopments or disputes concerning patent applications, issued patents, or other proprietary rights;
nthe recruitment or departure of key personnel;
nthe level of expenses related to any of our research programs or product candidates that we may develop;
nthe results of our efforts to develop additional product candidates or products;
nactual or anticipated changes in estimates as to financial results, development timelines, or recommendations by securities analysts;
nannouncement or expectation of additional financing efforts;
nsales or perceived potential sales of our common stock by us, our insiders or other stockholders;
nexpiration of market stand-off or lock-up agreements;
nany changes to our relationship with manufacturers, suppliers, collaborators or other strategic partners;
nmanufacturing or supply shortages;
nvariations in our financial results or those of companies that are perceived to be similar to us;
nchanges in estimates or recommendations by securities analysts, if any, that cover our stock;
npress reports, whether or not true, about our business;
nour failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public;
nchanges in the structure of healthcare payment systems;
nfluctuations in the valuation of companies perceived by investors to be comparable to us;
nannouncement or expectation of additional financing efforts;
nthe inability to obtain additional funding;
nmarket conditions in the pharmaceutical and biotechnology sectors;
ngeneral global economic, industry, political and market conditions, such as military conflict or war, inflation and financial institution instability, or pandemic or epidemic disease outbreaks, many of which are beyond our control; and
nthe other factors described in this “Risk Factors” section and elsewhere in this prospectus, including those which are outside of our control. In recent years, the stock market in general, and the market for biopharmaceutical and biotechnology companies in particular, has experienced significant price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our stock shortly following the consummation of this offering. The market price of our common stock may decline below the initial public offering price, and you may lose some or all of your investment.
Our quarterly and annual operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts or any guidance we may publicly provide, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly and annual fluctuations which may, in turn, cause the price of our common stock to fluctuate substantially. Our net loss and other operating results will be affected by numerous factors, including:
nvariations in the level of expense related to the ongoing development of VDPHL01 or future development programs;
nresults and timing of preclinical studies and ongoing and future clinical trials, or the addition or termination of any such clinical trials;
nour execution of any strategic transactions, including acquisitions, collaborations, licenses, or similar arrangements, and the timing and amount of payments we may make or receive in connection with such transactions;
nany intellectual property infringement lawsuit or opposition, interference, or cancellation proceeding in which we may become involved;
nrecruitment and departures of key personnel;
nif our product candidate receives regulatory approval in the future, the terms of such approval, and market acceptance and demand for such products;
nregulatory developments affecting our product candidate or those of our competitors;
nglobal or regional public health emergencies, including any health epidemics and their residual effects, natural disasters, or major catastrophic events;
nadverse macroeconomic conditions or geopolitical events, including the conflict between Ukraine and Russia, the conflict between Israel and Hamas, high levels of inflation, heightened interest rates, and bank failures;
nthe impacts of inflation and rising interest rates on our business and operations; and
nchanges in general market and economic conditions.
If our quarterly or annual operating results fall below the expectations of investors or securities analysts or any forecasts or guidance we may provide to the market, the price of our common stock could decline substantially. Such a stock price decline could occur even when we have met any previously publicly stated guidance we may provide. We believe that quarterly or annual comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
You will incur immediate and substantial dilution as a result of the consummation of this offering.
If you purchase common stock in this offering, you will incur immediate and substantial dilution of $4.64 per share, representing the difference between the assumed initial public offering price of $15.00 per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, and our pro forma as adjusted net tangible book value per share as of September 30, 2025 after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock, (ii) the issuance and sale of 118,682,683 shares of Series C Preferred Stock, including the net proceeds from such sale, which occurred subsequent to September 30, 2025, and the automatic conversion of all outstanding shares of Series C Preferred Stock into an aggregate of 11,789,280 shares of our common stock and (iii) the filing and effectiveness of our restated certificate of incorporation and amended and restated bylaws. To the extent that shares are issued upon the exercise of options or the underwriters exercise their over-allotment option, you will incur further dilution. For a further description of the dilution you will experience immediately after the consummation of this offering, see the section titled “Dilution.” To the extent that stock options are exercised, new equity awards are issued under our equity incentive plans or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
A significant portion of our total outstanding shares is restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to decline significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales upon the expiration of the lock-up agreements (described in the “Underwriting” section of this prospectus), the early release of the lock-ups, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. After the consummation of this offering, we will have 33,350,170 shares of common stock outstanding, or 35,352,670 shares if the underwriters exercise their over-allotment option in full, in each case based on the shares of our common stock outstanding as of September 30, 2025. Of these shares, the 13,350,000 shares (or 15,352,500 shares if the underwriters exercise their over-allotment option in full) we are selling in this offering may be resold in the public market immediately, unless purchased by our affiliates. The remaining shares are currently restricted under securities laws or as a result of lock-up or other agreements, but will be able to be sold after the consummation of this offering as described in the “Shares Eligible for Future Sale” section of this prospectus. The representatives of the underwriters may release some or all of the shares of common stock subject to lock-up agreements at any time in their sole discretion and without notice, except for directors and officers, which would allow for earlier sales of shares in the public market. In addition, the cornerstone investor has indicated an interest in purchasing up to $30.0 million in shares of common stock in this offering at the initial public offering price. The shares of common stock to be purchased by the cornerstone investor will not be subject to a lock-up agreement with the underwriters. Because this indication of interest is not a binding agreement or commitment to purchase, the cornerstone investor may determine to purchase more, less or no shares in this offering or the underwriters may determine to sell more, less or no shares to the cornerstone investor. If the cornerstone investor is allocated all or a portion of the shares it has indicated an interest in purchasing in this offering or more, and purchases any such shares, such purchases could reduce the available public float for our shares of common stock if the cornerstone investor holds such shares long-term.
Moreover, after the consummation of this offering, holders of an aggregate of 19,250,410 shares of our common stock will have rights, subject to conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also plan to register all shares of common stock that we may issue under our equity compensation plans or that are issuable upon exercise of outstanding options. Once we register these shares, they can be freely sold in the
public market upon issuance and once vested, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus. If any of these additional shares are sold, or if it is perceived that they will be sold, in the public market, the market price of our common stock could decline. Insiders will continue to have substantial influence over us after the consummation of this offering, which could limit your ability to affect the outcome of key transactions, including a change of control.
After the consummation of this offering, our directors, executive officers and greater than 5% stockholders and their affiliates, in the aggregate, will beneficially own shares representing approximately 13,779,032 of our outstanding common stock (assuming no exercise of the underwriters’ over-allotment option and no exercise of outstanding options and excluding any purchases that may be made in this offering). As a result, these stockholders, if they act together, will be able to influence our management and affairs and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. The interests of these holders may not always coincide with our corporate interests or the interests of other stockholders, and they may act in a manner with which you may not agree or that may not be in the best interests of our other stockholders. This concentration of ownership may have the effect of delaying or preventing a change in control of our company and might affect the market price of our common stock.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. As a result, capital appreciation, if any, of our common stock will be your sole source of gain on an investment in our common stock in the foreseeable future. See the section titled “Dividend Policy” for additional information. We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to emerging growth and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act and we may remain an emerging growth company until December 31, 2031. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or SOX Section 404, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, the information we provide stockholders will be different than the information that is available with respect to other public companies. In this prospectus, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
Even after we no longer qualify as an emerging growth company, we could still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements and reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to
private companies. We have elected not to “opt out” of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we will adopt the new or revised standard at the time private companies adopt the new or revised standard and will do so until such time that we either (i) irrevocably elect to “opt out” of such extended transition period, or (ii) no longer qualify as an emerging growth company. Therefore, the reported results of operations contained in our financial statements may not be directly comparable to those of other public companies.
We are also a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and our common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
Provisions in our restated certificate of incorporation, our amended and restated bylaws and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and amended and restated bylaws, which will become effective prior to the consummation of this offering, and Delaware law contain provisions that may have the effect of discouraging, delaying or preventing a change in control of us or changes in our management that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Our restated certificate of incorporation and amended and restated bylaws, which will become effective prior to the consummation of this offering, include provisions that:
nauthorize “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend and other rights superior to our common stock;
ncreate a classified board of directors whose members serve staggered three-year terms;
nspecify that special meetings of our stockholders can be called only by our board of directors;
nprohibit stockholder action by written consent;
nestablish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors;
nprovide that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum;
nprovide that our directors may be removed only for cause;
nspecify that no stockholder is permitted to cumulate votes at any election of directors;
nexpressly authorize our board of directors to modify, alter or repeal our amended and restated bylaws; and
nrequire supermajority votes of the holders of our common stock to amend specified provisions of our restated certificate of incorporation and amended and restated bylaws.
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock.
In addition, because we are incorporated in the State of Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware, or the DGCL, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Any provision of our restated certificate of incorporation, amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive
a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our restated certificate of incorporation will designate specific courts as the sole and exclusive forum for certain claims or causes of action that may be brought by our stockholders, which could discourage lawsuits against us and our directors and officers.
Our restated certificate of incorporation will provide that, subject to limited exceptions, the Court of Chancery of the State of Delaware (or, if, and only if, the Court of Chancery of the State of Delaware dismisses a Covered Claim (as defined below) for lack of subject matter jurisdiction, any other state or federal court in the State of Delaware that does have subject matter jurisdiction) will, to the fullest extent permitted by applicable law, be the sole and exclusive forum for the following types of claims: (i) any derivative claim brought in the right of the Company, (ii) any claim asserting a breach of a fiduciary duty to the Company or the Company’s stockholders owed by any current or former director, officer or other employee or stockholder of the Company, (iii) any claim against the Company arising pursuant to any provision of the DGCL, our restated certificate of incorporation or amended and restated bylaws, (iv) any claim to interpret, apply, enforce or determine the validity of our restated certificate of incorporation or amended and restated bylaws, (v) any claim against the Company governed by the internal affairs doctrine, and (vi) any other claim, not subject to exclusive federal jurisdiction and not asserting a cause of action arising under the Securities Act of 1933, as amended, or the Securities Act, brought in any action asserting one or more of the claims specified in clauses (a)(i) through (v) herein above, or each a Covered Claim. This provision would not apply to claims brought to enforce a duty or liability created by the Exchange Act.
Our restated certificate of incorporation will further provide that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. In addition, our restated certificate of incorporation will provide that any person or entity purchasing or otherwise acquiring any interest in the shares of capital stock of the Company will be deemed to have notice of and consented to these choice-of-forum provisions and waived any argument relating to the inconvenience of the forums in connection with any Covered Claim.
The choice of forum provisions to be contained in our restated certificate of incorporation may make it more costly for a stockholder to bring a claim, and it may also limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, other employees or stockholders, which may discourage lawsuits with respect to such claims, although our stockholders will not be deemed to have waived our compliance with federal securities laws and the rules and regulations thereunder. While the Delaware courts have determined that such choice of forum provisions are facially valid, it is possible that a court of law in another jurisdiction could rule that the choice of forum provisions to be contained in our restated certificate of incorporation are inapplicable or unenforceable if they are challenged in a proceeding or otherwise, which could cause us to incur additional costs associated with resolving such action in other jurisdictions. The choice of forum provisions may also impose additional litigation costs on stockholders who assert that the provisions are not enforceable or invalid.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
We cannot specify with certainty the particular uses of the net proceeds we will receive from this offering. Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in the “Use of Proceeds” section of this prospectus. Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds, with only limited information concerning management’s specific intentions. Our management may spend a portion or all of the net proceeds from this offering in ways that our stockholders may not desire or that may not yield a favorable return. The failure by our management to apply these funds effectively could harm our business, financial condition, results of operations and prospects. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
Participation in this offering by our existing stockholders and/or their affiliated entities may reduce the public float for our common stock.
To the extent certain of our existing stockholders and their affiliated entities participate in this offering, such purchases would reduce the non-affiliate public float of our shares, meaning the number of shares of our common stock that are not held by officers, directors and controlling stockholders. A reduction in the public float could reduce the number of shares that are available to be traded at any given time, thereby adversely impacting the liquidity of our common stock and depressing the price at which you may be able to sell shares of common stock purchased in this offering.
If securities or industry analysts do not publish research or reports about our business, or if they publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will be influenced in part by the research and reports that securities or industry analysts publish about us or our business. We do not have any control over the industry or securities analysts, or the content and opinions included in their reports and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, or if analysts cease coverage of us, we could lose visibility in the financial markets, and the trading price for our common stock could be impacted negatively. If any of the analysts who cover us publish inaccurate or unfavorable research or opinions regarding us, our business model, our intellectual property or our stock performance, or if our preclinical studies and clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline.
General Risk Factors
Unstable economic and market conditions may have serious adverse consequences on our business, financial condition and stock price.
Global economic and business activities continue to face widespread uncertainties, and global credit and financial markets have experienced extreme volatility and disruptions in the past several years, including severely diminished liquidity and credit availability, fluctuating inflation and monetary supply shifts, rising interest rates, labor shortages, declines in consumer confidence, declines in economic growth, increases in unemployment rates, recession risks, tariffs and uncertainty about economic and geopolitical stability (for example, related to the ongoing Russia-Ukraine conflict). The extent of the impact of these conditions on our operational and financial performance, including our ability to execute our business strategies and initiatives in the expected timeframe, as well as that of third parties upon whom we rely, will depend on future developments which are uncertain and cannot be predicted. There can be no assurance that further deterioration in economic or market conditions will not occur, or how long these challenges will persist. If the current equity and credit markets further deteriorate, or do not improve, it may make any necessary debt or equity financing more difficult, more costly, and more dilutive. Furthermore, our stock price may decline due in part to the volatility of the stock market and the general economic downturn.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
After the completion of this offering, as a public company, and particularly after we are no longer an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Securities Act, the Exchange Act, Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the NYSE and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain sufficient coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also
make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. The increased costs may require us to reduce costs in other areas of our business. Moreover, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Failure to establish and maintain effective internal control over financial reporting could adversely affect our business and if investors lose confidence in the accuracy and completeness of our financial reports, the market price of our common stock could be negatively affected.
We are not currently required to comply with the rules of the SEC implementing SOX Section 404 and are therefore not required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. Upon becoming a public company, we will be required to comply with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley Act, which will require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of internal control over financial reporting. Although we will be required to disclose changes made in our internal control over financial reporting on a quarterly basis, we will not be required to make our first annual assessment of our internal control over financial reporting until our second annual report on Form 10-K. However, as an emerging growth company, our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the later of the year following our first annual report required to be filed with the SEC or the date we are no longer an emerging growth company. At such time, our independent registered public accounting firm would need to issue a report that is adverse in the event that there are material weaknesses in our internal control over financial reporting.
As a private company, we do not currently have any internal audit function. To comply with the requirements of being a public company, we have undertaken various actions, and will need to take additional actions, such as implementing numerous internal controls and procedures and hiring additional accounting or internal audit staff or consultants. Testing and maintaining internal controls can divert our management’s attention from other matters that are important to the operation of our business.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Upon the completion of this offering, we will become subject to the periodic reporting requirements of the Exchange Act. We must design our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. Any disclosure controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. For example, our directors or executive officers could inadvertently fail to disclose a new relationship or arrangement causing us to fail to make any related party transaction disclosures. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected. In addition, we do not have a formal risk management program for identifying and addressing risks to our business in other areas.
Our insurance policies are expensive and only protect us from some business risks, which will leave us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include workers’ compensation, clinical trials, and directors’ and officers’ liability insurance. We do not know, however, if we will be able to maintain insurance with adequate levels of coverage. Any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our
business, financial condition, results of operations and prospects. We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock is likely to be volatile. The stock market in general, and NYSE and biopharmaceutical companies in particular, have experienced significant price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies. In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation (including the cost to defend against, and any potential adverse outcome resulting from any such proceeding) can be expensive, time-consuming, damage our reputation and divert our management’s attention from other business concerns, which could seriously harm our business.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been instituted against companies following periods of volatility in the trading price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition. Additionally, the dramatic increase in the cost of directors’ and officers’ liability insurance may cause us to opt for lower overall policy limits or to forgo insurance that we may otherwise rely on to cover significant defense costs, settlements and damages awarded to plaintiffs.
Our operations or those of the third parties upon whom we depend might be affected by the occurrence of a natural disaster, pandemic or other catastrophic event.
We depend on our employees, consultants, vendors, service providers, and other contractors (including CMOs and CROs), as well as regulatory agencies and other third parties, for the continued operation of our business. Despite any precautions we take for natural disasters or other catastrophic events, these events, including terrorist attack, pandemics, hurricanes, fire, floods and ice and snowstorms, could result in significant disruptions to our research and development, preclinical studies, clinical trials, and, ultimately, commercialization of our products. Long-term disruptions in infrastructure caused by events, such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism or other “acts of God,” particularly involving those places in which we maintain office space or at our manufacturing or clinical trial sites, could adversely affect our businesses. Although we carry business interruption insurance policies and typically have provisions in our contracts that protect us in certain events, our coverage might not respond or be adequate to compensate us for all losses that may occur. Any natural disaster or catastrophic event affecting us, our consultants, vendors, service providers, and other contractors, regulatory agencies or other parties with which we are engaged could have a significant negative impact on our operations and financial performance.
We could be subject to changes in tax rates or new tax legislation or could otherwise have exposure to additional tax liabilities, which could harm our business.
Changes to tax laws or regulations, or to the interpretation of such laws or regulations, in the jurisdictions in which we operate could significantly increase our effective tax rate and materially affect our financial condition. In addition, other factors or events, including business combinations and investments, changes in our stock-based compensation, changes in the valuation of our deferred tax assets and liabilities, adjustments to our taxes upon finalization of any of our various tax returns or as a result of deficiencies asserted by taxing authorities against us, increases in any of our expenses that are not deductible for tax purposes, changes in our available tax credits, and changes in the apportionment of our income and our activities among tax jurisdictions, could also increase our effective tax rate. Our tax filings are subject to review or audit by the U.S. Internal Revenue Service, or the IRS, and state, local and foreign taxing authorities. We may also be liable for taxes in connection with businesses we acquire. Our determinations in respect of our tax liabilities are not binding on the IRS or any other taxing authorities, and accordingly the final determination in an audit or other proceeding may be materially different than the treatment reflected in our tax provisions, accruals and returns. An assessment of additional taxes because of an audit or other proceeding could harm our business.
Our ability to use certain net operating loss, or NOL, carryforwards and certain other tax attributes may be limited.
As of December 31, 2024, we had federal and state NOL carryforwards of approximately $29.3 million. Federal NOL carryforwards generated in taxable years beginning after December 31, 2017, may be carried forward indefinitely but are permitted to be used in any taxable year to offset only up to 80% of taxable income in such taxable year, if any. It is uncertain if and to what extent various states will conform to federal law. There also may be periods during which the use of state NOL carryforwards is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. Certain of the state NOL carryforwards will begin to expire in 2040.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change NOL carryforwards and other pre-change tax attributes to offset its post-change income and taxes may be limited. Similar rules may apply under state tax laws. In general, an “ownership change” occurs if there is a cumulative change in ownership of the corporation by “5% shareholders” that exceeds 50 percentage points over a rolling three-year period. We have initiated but not yet completed a study under Section 382 of the Code to determine whether we have previously experienced an ownership change. We may also experience an ownership change upon future issuances of our stock or due to secondary trading of our stock which may be outside of our control. Any of these ownership changes and their resulting limitations on our ability to use NOL carryforwards and other tax attributes could adversely impact our business, financial condition, results of operations and cash flows.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are based on management’s beliefs and assumptions and on information currently available to management. All statements other than statements of historical facts contained in this prospectus are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements include, but are not limited to, statements concerning:
nthe initiation, timing, enrollment, progress, results, and cost of our research and development programs, and our current and future preclinical and clinical studies, including statements regarding the timing of initiation or completion of our clinical trials for VDPHL01 and our other product candidates, and related preparatory work, and the period during which the results of the trials will become available;
nour regulatory strategy and the timing of our planned NDA submission for VDPHL01;
nthe success, cost and timing of our clinical development of VDPHL01 and our other product candidates;
nour ability to initiate, recruit and enroll patients in and conduct our clinical trials at the pace that we project;
nthe timing of and our ability to obtain and maintain regulatory approval of our product candidates, and any related restrictions, limitations or warnings in the label of any of our product candidates, if approved;
nour ability to compete with companies currently selling, marketing or engaged in the development of treatments for diseases that our product candidates are designed to target, including PHL;
nour reliance on third parties to conduct our clinical trials;
nour reliance on third parties to manufacture drug substance for use in our clinical trials;
nour estimates regarding the size and growth potential of the commercial opportunity for VDPHL01 and our current product candidates or other product candidates we may identify and pursue, and our ability to serve those markets;
nour ability to expand our pipeline through collaborations, partnerships and other transactions with third parties;
nour ability to identify and advance through clinical development any additional product candidates;
nthe commercialization of VDPHL01 and our other current product candidates and any other product candidates we may identify and pursue, if approved, including our ability to successfully build commercial infrastructure or enter into collaborations with third parties to market our current product candidates and any other product candidates we may identify and pursue;
nthe effectiveness of physician outreach and education, direct‑to‑consumer advertising, telehealth engagement and social media campaigns on physician and patient adoption rates;
nour ability to develop and commercialize products that are considered by physicians, patients and payors as medically and/or financially differentiated as compared to competitive products;
nour ability to retain and recruit key personnel;
nour ability to obtain. maintain and successfully enforce adequate intellectual property rights;
nour patent portfolio, including issued, allowed and pending applications, and plans for future applications;
nour expectations about patient willingness to pay, the effect of macroeconomic conditions on discretionary spending and implications of limited or no third-party payor coverage and reimbursement on VPHL01, if approved;
nour estimates of our expenses, ongoing losses, capital requirements and our needs for or ability to obtain additional financing;
nour expected uses of the net proceeds to us from this offering and our existing cash, cash equivalents and marketable securities and the sufficiency of such capital resources to fund our future operating expenses and capital expenditure requirements;
nour expectations regarding the time during which we will be an emerging growth company under the JOBS Act;
nour financial performance;
ndevelopments and projections relating to our competitors or our industry; and
nother risks and uncertainties, including those listed under the section titled “Risk Factors.” The forward-looking statements in this prospectus are only predictions and are based largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of known and unknown risks, uncertainties and assumptions, including those described under the sections in this prospectus titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this prospectus and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to unduly rely on these statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as guarantees of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual future results, levels of activity, performance and events and circumstances could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risks and uncertainties may emerge from time to time, and management cannot predict all risks and uncertainties. Except as required by applicable law, we are not obligated to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
USE OF PROCEEDS
We estimate that the net proceeds to us from the sale of the shares of our common stock in this offering will be approximately $181.8 million (or approximately $209.8 million if the underwriters exercise their option to purchase additional shares in full), assuming an initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $12.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, an increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $14.0 million, assuming no change in the assumed initial public offering price per share, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
As of September 30, 2025, we had cash, cash equivalents and marketable securities of $15.1 million. Subsequent to September 30, 2025, we completed our Series C Preferred Stock financing, from which we received gross proceeds of $151.0 million.
We intend to use the net proceeds from this offering, together with our existing cash, cash equivalents and marketable securities:
nto advance VDPHL01 through NDA approval and initial commercialization in the United States, if approved. These funds will support physician education, brand awareness initiatives, marketing, pre-commercial and commercial launch activities, including building our commercialization infrastructure and manufacture of commercial supply, with
nany remaining proceeds for business development activities and other general corporate purposes.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress of our preclinical development efforts, our operating costs and other factors described under “Risk Factors” in this prospectus. Based upon our current operating plan, we believe that the anticipated net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient to fund our operating expenses and capital expenditure requirements into the second half of 2028. This estimate is based on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. We do not anticipate that the expected net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient for us to fund VDPHL01, or any of our other product candidates, through regulatory approval and commercialization, and we will need to raise substantial additional capital in order to do so. We may satisfy our future cash needs through the sale of equity securities, debt financings, working capital lines of credit, corporate collaborations or license agreements, grant funding, interest income earned on invested cash balances or a combination of one or more of these sources. We may also use a portion of the net proceeds from this offering to in-license, acquire, or invest in complementary businesses, technologies, products, or assets. However, we have no current plans, commitments, or obligations to do so.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with complete certainty all of the particular uses for the net proceeds to be received upon the consummation of this offering or the actual amounts that we will spend on the uses set forth above.
Our management will have broad discretion in the application of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of those net proceeds.
The timing and amount of our actual expenditures will be based on many factors, including the anticipated growth of our business, and we may find it necessary or advisable to use the net proceeds for other purposes. Pending the uses described above, we plan to invest the net proceeds from this offering in short-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
DIVIDEND POLICY
We have never declared or paid any dividends on our capital stock. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors our board of directors deems relevant, and subject to the restrictions contained in any future financing instruments. Our ability to pay cash dividends on our capital stock in the future may also be limited by the terms of any preferred securities we may issue or agreements governing any indebtedness we may incur.
CAPITALIZATION
The following table sets forth our cash and capitalization as of September 30, 2025:
non an actual basis;
non a pro forma basis, to give effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock, (ii) the issuance and sale of 118,682,683 shares of Series C Preferred Stock, including the net proceeds from such sale, which occurred subsequent to September 30, 2025, and the automatic conversion of all outstanding shares of Series C Preferred Stock into an aggregate of 11,789,280 shares of our common stock, (iii) the filing and effectiveness of our restated certificate of incorporation and amended and restated bylaws immediately prior to the consummation of this offering and (iv) the reclassification of our subscription receivable, associated with our historical Preferred Stock transactions, from temporary equity to permanent stockholders' equity upon automatic conversion of all outstanding Preferred Stock; and
non a pro forma as adjusted basis, to give further effect to our issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The pro forma as adjusted information below is illustrative only and our capitalization following the consummation of this offering will change based on the initial public offering price and other terms of this offering determined at pricing. You should read the information in this table together with the financial
| | | | | | | | | | | | | | | | | |
| AS OF SEPTEMBER 30, 2025 |
| (in thousands, except share and per share amounts) | ACTUAL | | PRO FORMA | | PRO FORMA AS ADJUSTED |
Cash, cash equivalents and marketable securities | $ | 15,139 | | | $ | 165,702 | | | $ | 347,535 | |
Series A preferred stock, $0.00001 par value; 12,865,375 shares authorized, issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 36,860 | | | — | | | — | |
Series B preferred stock, $0.00001 par value; 62,245,806 shares authorized, 62,245,805 issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 74,708 | | | — | | | — | |
Series C preferred stock, $0.00001 par value; no shares authorized, issued or outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | — | | — | | | — | |
Subscription receivable | (2,466) | | — | | | — | |
Total stockholders’ (deficit) equity | | | | | |
Preferred stock, $0.00001 par value; no shares authorized, issued and outstanding, actual; 25,000,000 shares authorized and no shares issued or outstanding, pro forma and pro forma as adjusted | — | | — | | | — | |
Common stock, $0.00001 par value; 98,774,582 shares authorized, 749,027 shares issued and outstanding, actual; 26,568,670 shares authorized, 19,999,437 issued and outstanding, pro forma; 200,000,000 shares authorized, 33,349,437 shares issued and outstanding, pro forma as adjusted | 1 | | | 1 | | | 1 | |
Additional paid-in capital | 1,363 | | | 263,493 | | | 445,326 | |
Subscription receivable | — | | | (2,466) | | | (2,466) | |
Accumulated deficit | (97,379) | | | (97,379) | | | (97,379) | |
Total stockholders’ (deficit) equity | (96,015) | | | 163,649 | | | 345,482 | |
Total capitalization | $ | 13,087 | | | $ | 163,649 | | | $ | 345,482 | |
| | | | | |
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ deficit and total capitalization on a pro forma as adjusted basis by approximately $12.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ deficit and total capitalization on a pro forma as adjusted basis by approximately $14.0 million, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The outstanding share information in the table above as of September 30, 2025 excludes:
n739,331 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2025, with a weighted average exercise price of $12.19 per share;
n2,974,637 shares of our common stock issuable upon the exercise of stock options granted after September 30, 2025, with a weighted average exercise price of $12.77 per share;
n685,080 shares of our common stock reserved for issuance under the 2021 Plan, as of December 31, 2025, which will cease to be available for issuance at the time the 2026 Plan becomes effective and will be added to, and become available for issuance under, the 2026 Plan;
na number of shares of our common stock equal to 10.8% of shares issued and outstanding as of immediately following the consummation of this offering (not to exceed 4,518,426 shares) reserved for issuance under the 2026 Plan, which will become effective in connection with this offering (which includes up to 1,768,254 shares of common stock underlying stock option awards to be granted to our directors, executive officers and other employees under the 2026 Plan upon the pricing of this offering with an exercise price per share equal to the initial public offering price per share), as well as any automatic increases in the number of shares of common stock reserved for future issuance thereunder; and
n316,668 shares of our common stock reserved for issuance under the ESPP, which will become effective in connection with this offering, as well as any automatic increases in the number of shares of common stock reserved for future issuance thereunder.
DILUTION
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible book value (deficit) as of September 30, 2025 was $(96.0) million, or $(128.19) per share of common stock. Our historical net tangible book value (deficit) is the amount of our total tangible assets less our total liabilities and the carrying value of our redeemable convertible preferred stock, which is not included within stockholders (deficit) equity. Historical net tangible book value (deficit) per share represents historical net tangible book value (deficit) divided by the 749,027 shares of common stock outstanding as of September 30, 2025.
Our pro forma net tangible book value as of September 30, 2025 was $163.6 million, or $8.18 per share of common stock. Pro forma net tangible book value represents the amount of our total tangible assets less our total liabilities, after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock, (ii) the issuance and sale of 118,682,683 shares of Series C Preferred Stock, including the net proceeds from such sale, which occurred subsequent to September 30, 2025, and the automatic conversion of all outstanding shares of Series C Preferred Stock into an aggregate of 11,789,280 shares of our common stock and (iii) the filing and effectiveness of our restated certificate of incorporation and amended and restated bylaws. Pro forma net tangible book value per share represents pro forma net tangible book value divided by the total number of shares outstanding as of September 30, 2025 after giving effect to the pro forma adjustments described above.
After giving further effect to our issuance and sale of 13,350,000 shares of common stock in this offering at an assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of September 30, 2025 would have been $345.5 million, or $10.36 per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $2.18 per share to existing stockholders and an immediate dilution of $4.64 in pro forma as adjusted net tangible book value per share to new investors participating in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the assumed initial public offering price per share paid by new investors. The following table illustrates this dilution on a per share basis:
| | | | | | | | | | | |
Assumed initial public offering price per share | | | $ | 15.00 | |
Historical net tangible book value (deficit) per share as of September 30, 2025 | $ | (128.19) | | | |
Increase per share attributable to pro forma adjustments as described above | 136.37 | | |
Pro forma net tangible book value per share of common stock as of September 30, 2025 | 8.18 | | |
Increase in net tangible book value per share of common stock attributable to this offering | 2.18 | | |
Pro forma as adjusted net tangible book value per share of common stock after this offering | | | 10.36 |
Dilution per share of common stock to new investors participating in this offering | | | $ | 4.64 | |
| | | |
The dilution information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing. A $1.00 increase in the assumed initial price to the public of $15.00 per share, which is the midpoint of the price range set forth on the cover page of
this prospectus, would increase the pro forma as adjusted net tangible book value by $12.4 million, or $0.37 per share, and increase the dilution per share to investors participating in this offering by $0.63 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 1,000,000 in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase the pro forma as adjusted net tangible book value by $14.0 million, or $0.10 per share, and the dilution per share to new investors participating in this offering would be $4.54 per share, assuming that the assumed initial price to the public remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each $1.00 decrease in the assumed initial price to the public of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would decrease the pro forma as adjusted net tangible book value by $12.4 million, or $0.37 per share, and decrease the dilution per share to investors participating in this offering by $0.63 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease the pro forma as adjusted net tangible book value by $14.0 million, or $0.11 per share, and the dilution per share to investors participating in this offering would be $4.75 per share, assuming that the assumed initial price to the public remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise in full their option to purchase 2,002,500 additional shares of common stock from us in this offering, our pro forma as adjusted net tangible book value per share after the offering would be $10.56, representing an immediate increase in pro forma as adjusted net tangible book value per share of $2.38 to existing stockholders and immediate dilution in pro forma as adjusted net tangible book value per share of approximately $4.44 to new investors purchasing common stock in this offering, assuming an initial public offering price of approximately $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The following table summarizes, as of September 30, 2025, on the pro forma as adjusted basis described above, the total number of shares of common stock purchased from us, the total consideration and the average price per share (1) paid by existing stockholders and (2) to be paid by new investors participating in this offering at the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting the underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing common stock in this offering will pay an average price per share substantially higher than our existing stockholders paid.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| SHARES PURCHASED | | TOTAL CONSIDERATION | | AVERAGE PRICE PER SHARE |
| NUMBER | | PERCENT | | AMOUNT (in thousands) | | PERCENT | | |
Existing stockholders | 19,999,437 | | | 60.0 | % | | $ | 263,387 | | | 56.8 | % | | $ | 13.17 | |
New investors | 13,350,000 | | | 40.0 | % | | 200,250 | | | 43.2 | % | | 15.00 | |
Total | 33,349,437 | | | 100.0 | % | | $ | 463,637 | | | 100.0 | % | | |
| | | | | | | | | |
The table above assumes no exercise of the underwriters’ option to purchase additional shares in this offering. If the underwriters’ option to purchase 2,002,500 additional shares is exercised in full, the number of shares of common stock held by existing stockholders would be reduced to 56.6% of the total number of shares of common stock to be outstanding upon consummation of this offering, and the number of shares of common stock held by new investors participating in this offering will be increased to 43.4% of the total number of shares of our common stock to be outstanding upon consummation of the offering.
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the total
consideration paid by new investors by approximately $12.4 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. Similarly, an increase (decrease) of 1,000,000 in the number of shares offered by us would increase (decrease) total consideration paid by new investors by approximately $14.0 million, assuming no change in the assumed initial public offering price.
The share information presented in the tables and discussions above as of September 30, 2025 excludes:
n739,331 shares of our common stock issuable upon the exercise of stock options outstanding as of September 30, 2025, with a weighted average exercise price of $12.19 per share;
n2,974,637 shares of our common stock issuable upon the exercise of stock options granted after September 30, 2025, with a weighted average exercise price of $12.77 per share;
n685,080 shares of our common stock reserved for issuance under the 2021 Plan, as of December 31, 2025, which will cease to be available for issuance at the time the 2026 Plan becomes effective and will be added to, and become available for issuance under, the 2026 Plan;
na number of shares of our common stock equal to 10.8% of shares issued and outstanding as of immediately following the consummation of this offering (not to exceed 4,518,426 shares) reserved for issuance under the 2026 Plan, which will become effective in connection with this offering (which includes up to 1,768,254, shares of common stock underlying stock option awards to be granted to certain of our directors, executive officers and other employees under the 2026 Plan upon the pricing of this offering with an exercise price per share equal to the initial public offering price per share), as well as any automatic increases in the number of shares of common stock reserved for future issuance thereunder; and
n316,668 shares of our common stock reserved for issuance under the ESPP, which will become effective in connection with this offering, as well as any automatic increases in the number of shares of common stock reserved for future issuance thereunder.
New investors participating in this offering will experience further dilution when any new options are issued and exercised under our equity incentive plans or we issue additional shares of common stock, other equity securities or convertible debt securities for lower consideration per share than in this offering in the future. In addition, we may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the “Selected Financial Data” section of this prospectus and our financial statements and related notes included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. See “Special Note Regarding Forward-Looking Statements.” Overview
We are a dermatologist-founded, late clinical-stage biopharmaceutical company focused on developing innovative therapeutics to address pervasive treatment challenges in highly prevalent aesthetic and dermatological conditions. Our initial focus is developing better treatments for PHL, a condition affecting approximately 50 million men and 30 million women in the United States. Current PHL treatment options are limited and therefore are consistently plagued with high rates of treatment failure, patient dissatisfaction and treatment discontinuation. Patients and healthcare providers routinely identify the following shortcomings with currently available treatment options:
nSlow onset of hair growth
nInconsistent results
nInsufficient density of hair growth for patient satisfaction
nTolerability issues related to hormonal, mood and cardiac side effects
nInconvenient administration
nLimited FDA-approved treatment options, and no FDA-approved oral options for women
We are developing VDPHL01 as an oral, non-hormonal treatment for men and women with PHL to reduce the barriers to wide adoption of chronic hair loss therapy and potentially transform PHL treatment. We believe that a marketing application could initially seek approval in male patients, followed by an sNDA for female patients, or could alternatively pursue approval in both male and female patients simultaneously depending on the timing of the completion of our clinical trials.
VDPHL01 is an oral, ER formulation of minoxidil, a proven hair growth agent, designed to maximize minoxidil’s impact on hair restoration while minimizing the risk of cardiac activity. Though IR oral minoxidil was originally designed to treat resistant hypertension, it has been used off label as a treatment for PHL after hair growth was observed as a side effect. However, IR oral minoxidil’s release profile was not designed for hair growth as its short duration of circulation allows less time for follicular saturation and must be used at lower doses to reduce the likelihood of reaching off target cardiac stimulative levels. VDPHL01 builds on minoxidil’s validated hair growth biology via a novel and proprietary ER formulation designed to maximize the total plasma concentrations of minoxidil known to grow hair without inducing changes in cardiac activity. We believe that our efforts mark the first attempt to bring an ER formulation of minoxidil to patients with these optimized PK and PD qualities that raise the ceiling of hair growth.
In the first half of 2024 we completed several PK studies of VDPHL01 and then initiated our Phase 2 trial evaluating VDPHL01 in male patients and female patients with mild-to-moderate PHL. In the fourth quarter of 2024 we started dosing patients in our first Phase 2/3 trial evaluating VDPHL01 in male patients with mild-to-moderate PHL subsequent to the first closing of our Series B Convertible Preferred Stock, or Series B Preferred Stock, financing. The following milestones have been achieved to date through 2025:
nthe initiation of a confirmatory registration-directed Phase 3 trial in male patients with mild-to-moderate PHL and the initiation of a registration-directed Phase 2/3 trial in female patients with mild-to-moderate PHL;
ncompletion of enrollment in our lead Phase 2/3 trial in male patients with mild-to-moderate PHL initiated in the fourth quarter of 2024;
nannouncement of preliminary data from our Phase 2 trial in male patients with PHL who had completed four months of treatment, in which VDPHL01 drove favorable outcomes, which we believe underscores its potential to deliver a convenient, oral treatment that provides visible hair regrowth to the majority of users as early as two months, while maintaining a favorable tolerability profile; and
nthe completion of the second funding of Series B Preferred Stock of $8.4 million in the first quarter of 2025, followed by a Series C Preferred Stock financing in the fourth quarter of 2025, from which we received gross proceeds of $151.0 million.
Since our inception we have devoted substantially all of our time and efforts to performing research and development activities, raising capital and recruiting management and technical staff to support our operations. We have never obtained regulatory approval for, or commercialized, a pharmaceutical product. We currently generate no revenue from sales of any products, and we may never be able to develop or commercialize a marketable product. To date, we have financed our operations primarily with proceeds from the sales of our redeemable convertible preferred stock.
We have incurred recurring net losses since inception, including net losses of $48.1 million and $20.8 million for the nine months ended September 30, 2025 and 2024, respectively, and net losses of $26.5 million and $16.5 million for the years ended December 31, 2024 and 2023, respectively. We have incurred negative cash flows from operations of $48.0 million and $20.1 million for the nine months ended September 30, 2025 and 2024, respectively, and negative cash flows from operations of $23.7 million and $13.5 million for the years ended December 31, 2024 and 2023, respectively. In addition, as of September 30, 2025, the Company had an accumulated deficit of $97.4 million. Substantially all of our operating losses have resulted from expenses incurred in connection with development of VDPHL01 and our other product candidates and from general and administrative costs associated with our operations. We expect to incur significant losses for the foreseeable future, as we advance VDPHL01 or any of our other current and future product candidates through clinical development, seek regulatory approval for such product candidates, maintain and expand our intellectual property portfolio, hire additional research and development and business personnel, conduct pre-commercial launch activities and infrastructure building, and operate as a public company.
We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for our product candidates. In addition, if we obtain regulatory approval for our product candidates, we expect to incur significant expenses related to developing our commercialization capability to support product sales, marketing, manufacturing and distribution activities initially in the United States.
We anticipate that our expenses will increase substantially if, and as, we:
ncontinue development of VDPHL01 and our other current product candidates, including preclinical development and conducting clinical trials;
nseek marketing regulatory approvals for VDPHL01 and for any of our other current or any future product candidates that successfully complete clinical trials;
ntake steps toward supporting commercial activities, including establishing sales, marketing and distribution infrastructure;
nincrease marketing in connection with the potential commercialization of VDPHL01, if approved;
nadvance additional product candidates through preclinical development and clinical trials;
nidentify additional product candidates and acquire rights from third parties to those product candidates through licenses or acquisitions and conduct development activities, including preclinical studies and clinical trials;
nmake royalty, milestone or other payments under any current or future license or collaboration agreements;
nprocure the manufacturing of preclinical, clinical and commercial supply of our current or any future product candidates;
nestablish agreements with contract research organizations and CMOs;
nattract, hire and retain additional qualified clinical, scientific, operations and management personnel;
nseek to continue to develop, maintain and defend our intellectual property portfolio, including against third-party interference, infringement and other intellectual property claims, if any;
nadd and maintain operational, financial and information management systems;
nattempt to address any competing therapies and market developments;
nexperience delays in our preclinical studies, clinical trials or regulatory approval for our current or any future product candidates, including with respect to failed studies, inconclusive results, safety issues or other regulatory challenges; and
nincur additional costs associated with being a public company, including audit, legal, regulatory and tax-related services associated with maintaining compliance with an exchange listing and the SEC requirements, director and officer insurance premiums and investor relations costs.
We may never succeed in these activities and, even if we do, may never generate any revenue or revenue that is significant enough to achieve profitability. Even if we succeed in commercializing VDPHL01 or one or more of our other product candidates, we will incur substantial expenditures to develop and market additional product candidates. We also may encounter unforeseen expenses, difficulties, complications, delays and other events that adversely affect our business. As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of public or private equity offerings and debt financings or other sources, such as potential collaboration agreements, strategic alliances and licensing arrangements. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on acceptable terms, or at all. Our failure to raise capital or enter into such agreements as and when needed could have a material adverse effect on our business, results of operations and financial condition.
At this time, due to the inherently unpredictable nature of clinical development we cannot reasonably estimate the costs we will incur and the timelines that will be required to complete development, obtain marketing approval and commercialize our current product candidates or any future product candidates, if at all. For the same reasons, we are also unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we fail to become profitable or sustain profitability on a continuing basis, then we may be unable to raise additional capital, maintain our research and development efforts, expand our business or continue our operations at planned levels, and as a result we may be forced to substantially reduce or terminate our operations.
Components of Results of Operations
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with our research and development activities, these can include drug discovery efforts and the development of our product candidates. We expense research and development costs as incurred, which include:
nexternal research and development expenses incurred under agreements with third parties, such as CROs, as well as investigative sites and consultants that conduct our clinical trials and other scientific development services;
ncosts related to manufacturing material for our clinical trials, including fees paid to CMOs;
nmanufacturing scale-up expenses and the cost of acquiring and manufacturing clinical trial materials;
nemployee-related expenses, including salaries, bonuses, benefits, stock-based compensation and other related costs for those employees involved in research and development efforts;
ncosts of outside consultants supporting research and development;
nthe costs of acquiring and developing clinical trial materials;
nclinical trial recruitment costs;
nexpenses to acquire technologies, such as intellectual property, to be used in research and development including in-process research and development, that has no alternative future use at the time of asset acquisitions;
ncosts related to compliance with regulatory requirements; and
nother indirect costs.
Costs for certain activities are recognized based on an evaluation of the progress to completion of each specific contract using information and data provided to us by our vendors and analyzing the progress of our research studies or other services performed. Significant judgments and estimates are made in determining the expenses incurred balances at the end of any reporting period.
Our direct, external research and development expenses consist primarily of fees paid to outside consultants, CROs, CMOs and research laboratories in connection with our process development, manufacturing and clinical development activities. Our direct external research and development expenses also include fees incurred under license and intellectual property purchase agreements. We track these external research and development costs on a program-by-program basis.
We do not allocate employee costs, costs associated with our development efforts and facilities, including depreciation or other indirect costs, to specific programs because these costs are deployed across multiple programs and, as such, are not separately classified. We use internal resources and third-party consultants primarily to conduct our research and development activities as well as for managing our process development, manufacturing and clinical development activities.
The successful development of our product candidates is highly uncertain. We plan to substantially increase our research and development expenses in the foreseeable future as we continue the development of our product candidates and manufacturing processes and conduct discovery and research activities for our clinical programs. We cannot determine with certainty the timing of initiation, the duration or the completion costs of current or future clinical trials of our product candidates due to the inherently unpredictable nature of preclinical and clinical development. We will need to raise substantial additional capital in the future. Our clinical development costs are expected to increase significantly with our ongoing clinical trials. We anticipate that our expenses will increase substantially, particularly due to the numerous risks and uncertainties associated with developing product candidates, including the uncertainty of:
nthe scope, rate of progress and expenses of our ongoing research activities and clinical trials and other research and development activities;
nsuccessful enrollment in and completion of clinical trials;
nwhether our product candidates show safety and efficacy in our clinical trials;
nreceipt of marketing approvals from applicable regulatory authorities;
nestablishing commercial manufacturing capabilities or making arrangements with third-party manufacturers;
nobtaining and maintaining patent and trade secret protection and regulatory exclusivity for our product candidates;
ncommercializing product candidates, if and when approved, whether alone or in collaboration with others; and
ncontinued acceptable safety profile of the products following any regulatory approval.
Any changes in the outcome of any of these variables with respect to the development of our product candidates in clinical development could mean a significant change in the costs and timing associated with the development of these product candidates.
In addition, clinical development timelines, the probability of success and development costs can differ materially from expectations. We anticipate that we will make determinations as to which product candidates to pursue and how much funding to direct to each product candidate on an ongoing basis in response to the results of ongoing and future clinical trials, regulatory developments and our ongoing assessment as to each product candidate’s commercial potential. However, we may never succeed in achieving regulatory approval for any of
our product candidates and may obtain unexpected results from our clinical trials. As a result, we may elect to discontinue, delay or modify clinical trials of some product candidates or focus on others. For example, if the FDA or another regulatory authority were to delay our planned start of clinical trials or require us to conduct clinical trials or other testing beyond those that we currently expect or if we experience significant delays in enrollment in any of our planned clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development of that product candidate.
We anticipate that our research and development expenses will continue to increase as we continue our current research programs, initiate new research programs, continue our preclinical development of product candidates and conduct future clinical trials for any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and stock-based compensation for our personnel in executive, legal, finance and accounting, and other administrative functions. General and administrative expenses also include legal fees relating to intellectual property and corporate matters, professional fees paid for accounting, auditing, tax and consulting services, insurance costs, travel expenses and direct facility costs not otherwise included in research and development expenses.
We anticipate that our general and administrative expenses will increase as we increase headcount to provide additional administrative support to our research and development activities. We also anticipate that we will incur significantly increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs, as well as investor and public relations expenses associated with operating as a public company. Additionally, if and when we believe a regulatory approval of a product candidate appears likely, we anticipate an increase in payroll and other employee-related expenses as a result of our preparation for commercial operations, especially as it relates to the sales and marketing of that product candidate.
Total Other Income, Net
Total other income, net, includes interest income earned on cash and cash equivalents, interest expense incurred on convertible and promissory notes payable, tax credits and other income and expense items.
Results of Operations
Comparison of the Nine months ended September 30, 2025 and 2024
The following table summarizes our results of operations:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Nine Months Ended September 30, | | |
| (in thousands) | | 2025 | | 2024 | | CHANGE |
Operating Expenses: | | | | | | |
Research and development | | $ | 43,873 | | | $ | 18,333 | | | $ | 25,540 | |
General and administrative | | 5,463 | | | 2,474 | | | 2,989 | |
Total operating expenses | | 49,336 | | | 20,807 | | | 28,529 | |
Loss from operations | | (49,336) | | | (20,807) | | | (28,529) | |
Total other income (expense), net | | 1,188 | | | (21) | | | 1,209 | |
Income tax benefit | | — | | | — | | | — | |
Net loss | | $ | (48,148) | | | $ | (20,828) | | | $ | (27,320) | |
| | | | | | |
Operating Expenses
Research and Development Expenses
The following table summarizes our research and development costs for each of the periods presented:
| | | | | | | | | | | | | | | | | | | | |
| | Nine Months Ended September 30, | | |
(in thousands) | | 2025 | | 2024 | | CHANGE |
| VDPHL01 | | $ | 39,458 | | | $ | 11,395 | | | $ | 28,063 | |
| VDMN | | 978 | | | 3,100 | | | (2,122) | |
| VDMC | | 22 | | | 1,154 | | | (1,132) | |
| VDAA | | 9 | | | 439 | | | (430) | |
| Other program candidates and expenses | | 48 | | | 1,322 | | | (1,274) | |
Other unallocated research and development costs | | | | | | |
| Personnel expenses (including stock-based compensation) | | 3,012 | | | 830 | | | 2,182 | |
Other expenses | | 346 | | | 93 | | | 253 | |
Total research and development expenses | | $ | 43,873 | | | $ | 18,333 | | | $ | 25,540 | |
| | | | | | |
Research and development expenses were $43.9 million for the nine months ended September 30, 2025, compared to $18.3 million for the nine months ended September 30, 2024. The increase of $25.5 million was primarily due to:
na $28.1 million increase in costs related to VDPHL01 primarily due to a $22.6 million increase in clinical trial expenses, including investigator fees, pass-through costs, and program management fees resulting from expanded registrational and confirmatory study activity in 2025 compared to the prior period, as well as an additional $4.6 million increase in clinical trial recruitment costs. The remaining increase is related to chemistry, manufacturing and controls activities, including costs associated with GMP manufacturing and clinical materials to support ongoing and planned clinical studies as compared to the prior year; and
na $2.2 million increase in personnel-related costs, including stock-based compensation expense, primarily due to an increase in research and development related headcount as compared to the same period in the nine months ended September 30, 2024.
These increases were partially offset by:
na decrease in costs related to VDMN, VDMC, VDAA, and other program candidates of $2.1 million, $1.1 million, $0.4 million and $1.3 million respectively. These decreases were primarily related to decreases in chemistry, manufacturing and controls activities for VDMN and other program candidates, a decrease in clinical trial expenses for VDMN, and decreases in other research and development costs related across these programs.
General and Administrative Expenses
General and administrative expenses were $5.5 million for the nine months ended September 30, 2025, compared to $2.5 million for the nine months ended September 30, 2024. The increase of $3.0 million was primarily due to an increase in payroll and personnel-related costs, including stock-based compensation, primarily as a result of an increase in general and administrative related headcount and other professional fees associated with legal and intellectual property matters, operating activities, commercial readiness and preparations for becoming a public company.
Total Other Income, Net
Total other income, net, was $1.2 million for the nine months ended September 30, 2025, compared to net expense of less than $0.1 million for the nine months ended September 30, 2024. The increase of $1.2 million is primarily attributable to a $0.5 million increase in accretion income on investments, a $0.4 million increase in interest income reflecting higher yields on average cash and investment balances during the period, and a $0.3 million decrease in interest expense resulting from the repayment of convertible notes and promissory notes using proceeds from the Series B Preferred Stock financing in 2024.
Comparison of the Years Ended December 31, 2024 and 2023
The following table summarizes our results of operations:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | | Year Ended December 31, | | |
| (in thousands) | | 2024 | | 2023 | | CHANGE |
| Operating Expenses: | | | | | | |
| Research and development | | $ | 23,283 | | | $ | 14,971 | | | $ | 8,312 | |
| General and administrative | | 3,495 | | | 2,353 | | | 1,142 | |
| Total operating expenses | | 26,778 | | | 17,324 | | | 9,454 | |
| Loss from operations | | (26,778) | | | (17,324) | | | (9,454) | |
| Total other income, net | | 290 | | | 834 | | | (544) | |
| Income tax benefit | | — | | | — | | | — | |
| Net loss | | $ | (26,488) | | | $ | (16,490) | | | $ | (9,998) | |
| | | | | | |
Operating Expenses
Research and Development Expenses
The following table summarizes our research and development costs for each of the periods presented:
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, | | |
(in thousands) | | 2024 | | 2023 | | CHANGE |
| VDPHL01 | | $ | 14,641 | | | $ | 2,953 | | | $ | 11,688 | |
| VDMN | | 3,747 | | | 5,056 | | | (1,309) | |
| VDMC | | 1,329 | | | 3,648 | | | (2,319) | |
| VDAA | | 402 | | | 1,146 | | | (744) | |
| Other program candidates and expenses | | 1,370 | | | 1,036 | | | 334 | |
Other unallocated research and development costs | | | | | | |
| Personnel expenses (including stock-based compensation) | | 1,499 | | | 1,131 | | | 368 | |
Other expenses | | 295 | | | 1 | | | 294 | |
Total research and development expenses | | $ | 23,283 | | | $ | 14,971 | | | $ | 8,312 | |
| | | | | | |
Research and development expenses were $23.3 million for the year ended December 31, 2024, compared to $15.0 million for the year ended December 31, 2023. The increase of $8.3 million was primarily due to:
nan $11.7 million increase in costs related to VDPHL01 primarily due a $9.0 million increase in clinical trial expenses, including investigator fees and pass-through costs. Remaining increase related to chemistry,
manufacturing and controls activities, including costs associated with GMP manufacturing and clinical materials to support ongoing and planned clinical studies as compared to the prior year;
na $0.4 million increase in personnel-related costs, including stock-based compensation expense, primarily due to an increase in research and development related headcount as compared to the same period in the year ended December 31, 2023;
These increases were partially offset by:
na decrease in costs related to VDMN, VDMC and VDAA of $1.3 million, $2.3 million and $0.7 million respectively. These decreases were primarily related to decreases in chemistry, manufacturing and controls activities for VDMN and VDMC and a decrease in other research and development costs related to VDAA;
General and Administrative Expenses
General and administrative expenses were $3.5 million for the year ended December 31, 2024, compared to $2.4 million for the year ended December 31, 2023. The increase of $1.1 million was primarily due to an increase in payroll and personnel-related costs, including stock-based compensation, primarily as a result of an increase in general and administrative related headcount and other professional fees associated with operating activities and the preparations for becoming a public company.
Total Other Income, Net
Total other income, net, was $0.3 million for the year ended December 31, 2024, compared to $0.8 million for the year ended December 31, 2023. The decrease of $0.5 million is primarily attributable to $0.4 million decrease in interest income, which reflected lower yields on average cash balances during the period, as well as a $0.5 million increase in interest expense related to convertible notes and promissory notes paid off with proceeds from the Series B Preferred Stock financing. These items were partially offset by a $0.4 million increase in other income resulting from the recognition of certain tax credits that we elected to exchange for cash.
Liquidity and Capital Resources
Sources of Liquidity
Since our inception, we have funded our operations primarily through equity financings, and through September 30, 2025, had received proceeds of approximately $112.2 million, net of issuance costs of $0.7 million, from the sale of our Series A and B redeemable convertible preferred stock. As of September 30, 2025, we had $15.1 million of cash, cash equivalents and marketable securities.
Uses of Liquidity
We currently have no ongoing material financing commitments, such as lines of credit or guarantees, that are expected to affect our liquidity over the next five years, other than our manufacturing, licensing and lease obligations described further below.
Future Funding Requirements
Subsequent to September 30, 2025, we completed a Series C Preferred Stock financing in the fourth quarter of 2025, from which we received gross proceeds of $151.0 million. We expect the net proceeds from this offering, together with our existing cash, cash equivalents and marketable securities, will be sufficient to fund our operating expenses and capital expenditure requirements for more than twelve months from the date of this prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect.
We expect to incur significant expenses and operating losses in the foreseeable future as we advance VPHL01 or any of our other current or any future product candidates through clinical development, seek regulatory approval and pursue commercialization of any approved product candidates. Furthermore, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company.
Because of the numerous risks and uncertainties, length of time and scope of activities associated with research, development and commercialization of product candidates, we are unable to estimate the exact amount of our working capital requirements. Our future funding requirements, both near and long-term, will depend on, and could increase significantly as a result of, many factors, including, but not limited to:
nthe initiation, progress, timing, costs and results of our clinical trials through all phases of development, including our ongoing clinical trials for VDPHL01 and the development of our other current and any future product candidates;
nthe number and scope of preclinical and clinical programs we decide to pursue;
nthe identification, assessment, acquisition and/or development of additional research programs and additional product candidates;
nthe timing of and successful patient enrollment in, and the initiation and completion of, clinical trials;
nthe outcome, timing and costs of meeting regulatory requirements established by the FDA or any comparable foreign regulatory authority, including any additional clinical trials required by the FDA or any comparable foreign regulatory authority;
nthe willingness of the FDA or any comparable foreign regulatory authorities to accept our clinical trial designs, as well as data from our completed and planned preclinical studies and clinical trials, as the basis for review and approval of VDPHL01 and any other product candidates;
nthe progress, timing and costs of the development by us or third parties of companion diagnostics, if required, for VDPHL01 or any other product candidates, including design, manufacturing and regulatory approval;
nthe timing, receipt and terms of any marketing approvals from applicable regulatory authorities;
nour ability to establish new licensing or collaboration arrangements;
nthe performance of our future collaborators, if any;
ndevelopment and timely delivery of commercial-grade drug formulations that can be used in our planned clinical trials and for commercialization;
nthe cost of filing, prosecuting and enforcing our patent claims and other intellectual property rights;
nthe cost of defending potential intellectual property disputes, including patent infringement actions brought by third parties against us;
nthe costs associated with potential clinical trial liability or product liability claims, including the costs associated with obtaining insurance against such claims and with defending against such claims;
nthe effect of competing technological and market developments;
nour ability to hire additional personnel and consultants as our business grows, including additional executive officers and clinical development, regulatory, chemistry, manufacturing and controls, quality and commercial personnel;
nour ability to develop and commercialize products that are considered by physicians, patients and payors as medically and/or financially differentiated as compared to competitive products;
nour ability to establish arrangements with third-party manufacturers for the commercial supply of products that receive marketing approval, if any;
nthe cost of making royalty, milestone or other payments under any future in-license agreements;
nthe extent to which we in-license or acquire additional product candidates or technologies;
nthe cost of establishing sales, marketing and distribution capabilities for our product candidates, if approved;
nthe initiation, progress and timing of our commercialization of VDPHL01, if approved, or any other product candidates;
nour ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors, if applicable, and adequate market share and revenue;
nmaintaining a continued acceptable safety profile of the product candidates following approval; and
nthe costs of operating as a public company.
A change in the outcome of any of these, or other variables with respect to the development of any of our product candidates, could significantly change the costs and timing associated with the development of that product candidate. We will need to continue to rely on additional financing to achieve our business objectives.
In addition to the variables described above, if and when any of our product candidates successfully complete development, we will incur substantial additional costs associated with regulatory filings, marketing approval, post-marketing requirements, maintaining our intellectual property rights and regulatory protection, in addition to other commercial costs. We cannot reasonably estimate these costs at this time.
Until such time, if ever, as we generate significant revenue from product sales, we expect to finance our operations through the sale of equity, debt financings, marketing and distribution arrangements and collaborations, strategic alliances and licensing arrangements or other sources. We currently have no credit facility or committed sources of capital. If we raise additional funds through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, and we may need to dedicate a substantial additional portion of any operating cash flows to the payment of principal and interest on such indebtedness. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, intellectual property, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate product candidate development or future commercialization efforts.
Cash Flows
The following table summarizes our cash flows for each of the periods presented:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months Ended September 30, | | Year Ended December 31, |
| (in thousands) | | 2025 | | 2024 | | 2024 | | 2023 |
| Net cash used in operating activities | | $ | (47,974) | | | $ | (20,056) | | | $ | (23,688) | | | $ | (13,510) | |
Net cash used in investing activities | | (9,080) | | | — | | | — | | | — | |
| Net cash provided by financing activities | | 9,630 | | | 10,170 | | | 60,476 | | | 13,895 | |
| Net increase in cash and cash equivalents | | $ | (47,424) | | | $ | (9,886) | | | $ | 36,788 | | | $ | 385 | |
| | | | | | | | |
Operating Activities
Net cash used in operating activities was $48.0 million for the nine months ended September 30, 2025 as compared to $20.1 million for the nine months ended September 30, 2024. The increase in cash used in operations was primarily due to the increase of $25.5 million in research and development expense for the advancement of our lead asset VDPHL01 and $3.0 million in general and administrative expenses as compared to the same period the prior year. The increase in research and development spending reflects greater clinical trial activity, manufacturing scale-up, and personnel costs related to support the advancement of VDPHL01. The increase in general and administrative expenses was driven by higher external professional fees, including legal and intellectual property matters, accounting, and consulting costs to support our corporate activities including commercial readiness and preparations for becoming a public company.
Net cash used in operating activities was $23.7 million for the year ended December 31, 2024 as compared to $13.5 million for the year ended December 31, 2023. The increase in cash used in operations was primarily due to the increase of $8.3 million in research and development expense for the advancement of our lead asset VDPHL01 and $1.1 million in general and administrative expenses as compared to the same period the prior year. The increase in research and development spending reflects greater clinical trial activity, manufacturing scale-up, and personnel-related costs to support the advancement of VDPHL01. The increase in general and administrative expenses was driven by higher external professional fees, including legal, accounting, and consulting costs to support our corporate activities.
Investing Activities
Net cash used in investing activities was $9.1 million for the nine months ended September 30, 2025. The increase was primarily driven by the purchase of investment securities partially offset by maturities of short-term investments during the period.
Financing Activities
Net cash provided by financing activities was $9.6 million for the nine months ended September 30, 2025 as compared to $10.2 million for the nine months ended September 30, 2024. The decrease of $0.6 million was primarily attributable to lower proceeds from financing transactions driven primarily from the issuance of $8.4 million of preferred stock in connection with the Series B financing in 2025, compared to the issuance of $10.2 million of convertible notes in the prior period, partially offset by an increase of $1.1 million in pre-funded equity financing as compared to the prior period.
Net cash provided by financing activities was $60.5 million for the year ended December 31, 2024 as compared to $13.9 million for the year ended December 31, 2023. The increase was primarily attributable to higher proceeds from financing transactions, reflecting a net increase of $46.6 million from the issuance of preferred stock and convertible notes in connection with the Series B financing in 2024, compared to the proceeds from the issuance of preferred stock related to the Series A financing in the prior year.
Contractual Obligations
In February 2025, we entered into an office lease agreement in New Haven, Connecticut. We currently lease a total of 1,202 square feet, and the term of the lease extends to February 2027. We have the option to extend the lease for an additional two years after initial expiration. We believe our existing facilities are sufficient for our current needs. To meet the future needs of our business, we expect to lease additional or alternate office space, and we believe suitable additional or alternative space will be available in the future on commercially reasonable terms. Remaining lease payments from September 30, 2025 through the end of the lease term are less than $0.1 million.
Purchase and Other Obligations
We enter contracts in the normal course of business with CROs and other third-party vendors for clinical trials and testing and manufacturing services. Most contracts do not contain minimum purchase commitments and are cancellable by us upon written notice. Payments that may be due upon cancellation consist of payments for services provided or expenses incurred. As of September 30, 2025 and 2024 there were no amounts accrued related to termination charges.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Changes in estimates and assumptions are reflected in reported results in the period in which they become known. Actual results could differ from those estimates. Management considers many factors in selecting appropriate financial accounting policies and controls, and in developing the estimates and assumptions that are used in the preparation of these financial statements. Management must apply significant judgment in this process. In addition, other factors may affect estimates, including expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates, and whether historical trends are expected to be representative of future trends. The estimation process often may yield a range of potentially reasonable estimates of the ultimate future outcomes, and management must select an amount that falls within that range of reasonable estimates. Estimates are used in the following areas, among others: valuation of prepaid and accrued expenses related to certain research and development contracts, and stock-based compensation expense which includes estimating the fair value of its common stock.
Estimates related to research and development contract accruals and prepayments are reasonably likely to change in future periods as project scopes, timelines, and vendor billing patterns evolve. Similarly, estimates of stock-based compensation expense are sensitive to changes in assumptions such as the fair value of common stock, expected volatility, and the estimated term of awards. Management will continue to evaluate these estimates each reporting period and adjust them as additional information becomes available.
While our significant accounting policies are described in more detail in Note 2 “Summary of Significant Accounting Policies” to our audited consolidated financial statements included elsewhere in this prospectus, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our consolidated financial statements. Research and Development Expenses
We estimate and expense research and development costs as incurred, which involves determining the expenses for personnel, stock-based compensation, external services, clinical trials, and other contracted activities. This estimation process requires reviewing open contracts, purchase orders, and communicating with personnel and vendors to assess the level of services performed, particularly when an invoice hasn't been received. We base our estimates on the progress to completion of each contract, using data from vendors and clinical sites. Depending on the timing of payments, we recognize either prepaid or accrued expenses, which are management's estimates based on work performed, milestones achieved, and experience with similar contracts. We monitor these factors and adjust the prepaid or accrual balances if the actual timing or effort differs from our initial estimate, including for non-refundable advance payments. While we expect our estimates to be materially accurate, any difference between the estimated and actual status and timing of services performed could cause research and development expenses to be overstated or understated in a given reporting period.
Stock-Based Compensation Expense
We measure stock-based compensation using the grant date fair value of the awards and recognize the expense on a straight-line basis over the requisite service period, which is typically the vesting period, classifying the expense according to the function to which the services relate. Forfeitures are recognized as they occur. Given the absence of a public market for our common stock, the fair value was determined by the Board of Directors, considering independent third-party valuation reports. These reports primarily used an Option Pricing Method (OPM), which treats common and preferred stock as call options on the company's total equity value and applies a discount for lack of marketability to value the common stock. Key inputs for this valuation, particularly the Black-Scholes model, involved significant estimates: expected volatility was based on historical volatility of publicly-traded peer companies; the expected term was estimated at 10 years (the full grant term) due to a lack of sufficient company history; and the risk-free interest rate was based on the U.S. Treasury yield curve. Because we don't expect to pay dividends, the expected dividend yield is zero. These valuations incorporate management's best estimates and significant judgments regarding future operating performance, product development, and the timing of a potential liquidity event; consequently, if different assumptions were used, the resulting expense could be materially different. Following a public offering, the fair value of our common stock will be determined by its quoted market price. Additional information regarding stock-based compensation expense is provided in Note 2 “Summary of Significant Accounting Policies” to our audited consolidated financial statements included elsewhere in this prospectus. Common Stock Valuations
As there has been no public market for our common stock, the estimated fair value of our common stock has been determined by our Board of Directors after considering valuation reports provided by an independent third-party valuation firm and exercising reasonable judgment and considering numerous objective and subjective factors to determine the best estimate of the fair value of our common stock at each stock option grant date. In accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, a third-party valuation firm prepared valuations of our common stock using an OPM. The total equity value can be implied from the OPM, using the preferred stock financing price if transacted around each valuation date.
Given the absence of a public trading market, our Board of Directors, with input from management considered numerous objective and subjective factors to determine the fair value of our common stock. The factors included, but were not limited to:
nthe prices at which we sold preferred stock and preferences of the preferred stock relative to our common stock at the time of each grant;
nthe progress of our research and development efforts, including the status of clinical development for our product candidates;
nthe lack of liquidity of our equity as a private company;
nour stage of development and business strategy and the material risks related to our business and industry;
nthe achievement of enterprise milestones; and
nthe likelihood of achieving a liquidity event for the holders of our preferred stock and holders of our common stock, such as an initial public offering, or a sale of our company, given prevailing market conditions.
The assumptions underlying these valuations were highly complex and subjective and represented management’s best estimates, which involved inherent uncertainties and the application of management’s judgment. As a result, if we had used significantly different assumptions or estimates, the fair value of our common stock and our stock-based compensation expense could have been materially different.
Once a public trading market for our common stock has been established in connection with the completion of this offering, it will no longer be necessary for our Board of Directors to estimate the fair value of our common stock in connection with our accounting for granted stock options and other such awards we may grant, as the fair value of our common stock will be determined based on the quoted market price of our common stock.
Emerging Growth Company and Smaller Reporting Company
As an emerging growth company under the JOBS Act we may delay the adoption of certain accounting standards until such time as those standards apply to private companies. Other exemptions and reduced reporting requirements under the JOBS Act for emerging growth companies include presentation of only two years of audited financial statements in a registration statement for an IPO, an exemption from the requirement to provide an auditor’s report on internal controls over financial reporting pursuant to SOX Section 404, an exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation, and less extensive disclosure about our executive compensation arrangements. Additionally, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected not to “opt out” of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we will adopt the new or revised standard at the time private companies adopt the new or revised standard and will do so until such time that we either (i) irrevocably elect to “opt out” of such extended transition period or (ii) no longer qualify as an emerging growth company. We may choose to early adopt any new or revised accounting standards whenever such early adoption is permitted for private companies. Therefore, the reported results of operations contained in our consolidated financial statements may not be directly comparable to those of other public companies.
We may remain classified as an emerging growth company until the end of the fiscal year in which the fifth anniversary of this offering occurs, although if the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have annual gross revenues of $1.235 billion or more in any fiscal year, we will cease to be an emerging growth company as of December 31 of the applicable year. We also will cease to be an emerging growth company if we issue more than $1.0 billion of non-convertible debt over a three-year period. We intend to rely on certain of the other exemptions and reduced reporting requirements provided by the JOBS Act.
We are also a “smaller reporting company” meaning that the market value of our stock held by non-affiliates plus the proposed aggregate amount of gross proceeds to us as a result of this offering is less than $700.0 million and our annual revenue was less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company after this offering if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue was less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 “Summary of Significant Accounting Policies” to our audited consolidated financial statements included elsewhere in this prospectus.
BUSINESS
Overview
We are a dermatologist-founded, late clinical-stage biopharmaceutical company focused on developing innovative therapeutics to address pervasive treatment challenges in highly prevalent aesthetic and dermatological conditions. Our initial focus is developing better treatments for PHL, a condition affecting approximately 50 million men and 30 million women in the United States. Current PHL treatment options are limited and therefore are consistently plagued with high rates of treatment failure, patient dissatisfaction and treatment discontinuation. Patients and healthcare providers routinely identify the following shortcomings with currently available treatment options:
nSlow onset of hair growth
nInconsistent results
nInsufficient density of hair growth for patient satisfaction
nTolerability issues related to hormonal, mood and cardiac side effects
nInconvenient administration
nLimited FDA-approved treatment options and no FDA-approved oral options for women
We are developing VDPHL01 as an oral, non-hormonal treatment for men and women with PHL to reduce the barriers to wide adoption of chronic hair loss therapy and potentially transform PHL treatment. We believe that a marketing application could initially seek approval in male patients, followed by an sNDA for female patients, or could alternatively pursue approval in both male and female patients simultaneously depending on the timing of the completion of our clinical trials.
VDPHL01 is an oral, ER formulation of minoxidil, a proven hair growth agent, designed to maximize minoxidil’s impact on hair restoration while minimizing the risk of cardiac activity. Though IR oral minoxidil was originally designed to treat resistant hypertension, it has been used off label as a treatment for PHL after hair growth was observed as a side effect. However, IR oral minoxidil’s release profile was not designed for hair growth as its short duration of circulation allows less time for follicular saturation and must be used at lower doses to reduce the likelihood of reaching off target cardiac stimulative levels. VDPHL01 builds on minoxidil’s validated hair growth biology via a novel and proprietary ER formulation designed to maximize the total plasma concentrations of minoxidil known to grow hair without inducing changes in cardiac activity. We believe that our efforts mark the first attempt to bring an ER formulation of minoxidil to patients with these optimized PK and PD qualities that raise the ceiling of hair growth.
We are currently dosing patients with VDPHL01 in our registration-directed clinical program consisting of three pivotal, multi-center, randomized, double-blind, placebo-controlled clinical trials: two in male patients and one in female patients with PHL. These trials are designed to support our planned submissions to the FDA for regulatory approval across both male and female patient populations through a 505(b)(2) NDA. We have fully enrolled the first of these registration-directed clinical trials, a Phase 2/3 trial evaluating VDPHL01 in 519 male patients with mild-to-moderate PHL. This first trial assesses two dose regimens of VDPHL01 over 52 weeks of treatment. The co-primary endpoints are change in non-vellus hair count per square centimeter and patient self-assessment of hair coverage benefit after 24 weeks from treatment initiation. We anticipate topline data from this clinical trial in the first half of 2026.
We have also initiated our confirmatory registration-directed Phase 3 trial targeting the enrollment of 498 male patients with mild-to-moderate PHL and our registrational-directed Phase 2/3 trial targeting the enrollment of 552 female patients with mild-to-moderate PHL. We expect to report data from this male Phase 3 trial in the second half of 2026. Enrollment in the female Phase 2/3 trial is ongoing and projected time to topline data will be determined as the trial progresses. The clinical trials designated as Phase 2/3 are designed and conducted as registration-directed Phase 3 trials to support the planned marketing application, or NDA, for VDPHL01 for the treatment of PHL. The Phase 2/3 trials include a parallel Phase 2 component intended to further assess proprietary patient-reported outcome, or PRO, measures used as endpoints in all three registration-directed trials.
In a Phase 1 clinical trial, which we refer to as Study QSC300720, we studied VDPHL01 prototypes in doses up to 10 mg against a single reference dose of commercially available 2.5 mg IR oral minoxidil. When comparing the results of VDPHL01 8.5 mg with 2.5 mg IR oral minoxidil in male patients who were administered both dosage forms in this study, we found that VDPHL01 8.5 mg:
ndelivered nearly twice the total amount of minoxidil in plasma over 12 hours than the total amount delivered by the 2.5 mg IR oral minoxidil tablet;
nsustained plasma concentrations above minoxidil's hair growth threshold two times longer than a 2.5 mg IR oral minoxidil tablet;
nmaintained peak concentrations below the threshold at which signs of cardiac activity are typically observed; and
nwas generally well tolerated with no SAEs observed.
As this study was exploratory, no formal sample size calculation was made related to statistical power. The data presented above are reflective of the study’s per-protocol primary objective, namely, to evaluate the PK profile and determine the relative bioavailability of VDPHL01 following single oral dosing of VDPHL01 versus the reference IR oral minoxidil formulation in healthy subjects.
We are currently conducting a Phase 2 clinical trial evaluating VDPHL01 in male patients and female patients with mild-to-moderate PHL. As this trial is exploratory, no formal sample size calculation was made related to statistical power. Therefore, all trial endpoints, including objective hair count measures and subjective patient and investigator assessments, are exploratory and are planned to be reported as descriptive statistics. In October 2025, we announced preliminary data from the male cohort for those who had completed four months of treatment in this trial (n=21; the female cohort, currently with 22 subjects dosing, initiated enrollment in this trial later than males, and similar data is not yet available).
The preliminary data show that VDPHL01 drove favorable outcomes, which we believe underscores its potential to deliver a convenient, oral treatment that provides visible hair regrowth to the majority of users as early as two months, while maintaining a favorable tolerability profile. We believe that the results of this trial support the emerging product profile for VDPHL01 with the following key attributes:
nSpeed: Visibly noticeable hair growth as early as two months after treatment in a majority of patients based on PRO and an IGA.
nConsistency: 90.5% treatment response at four months based on PRO of participants reporting “improved” or “much improved” hair coverage.
nIntensity: Average non-vellus (greater than 30 microns) hair count change of 47.3 hairs per cm2, with double digit absolute non-vellus hair count changes in greater than 90% of patients completing four months of treatment.
nSafety: Generally well tolerated, with no treatment-related SAEs, including no cardiac or hormonal-related issues to date.
nConvenience: Preference for oral over topical administration supported by third-party research.
nMarketability: Potential to be the first oral, non-hormonal FDA-approved therapy for PHL.
The below images are of the four patients, of 20 patients in the cohort as of August 2025, the time at which a blinded image analysis study was conducted. These patients were observed to have the highest average hair growth improvement scores as determined by expert graders using images from our Phase 2 clinical trial in male patients at month four. This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale, a commonly used tool for assessing change in hair growth ranging from greatly decreased (-3) to greatly increased (+3) with intermediate categories of no change (0), slightly decreased/increased (-1/+1) and moderately decreased/increased (-2/+2). Images for patients from screening, month two and month four are presented in order (highest to lowest) of the average improvement scores at month four. Average IGA improvement scores shown represent average improvement scores as assessed by the blinded expert graders
ranging from +2.7 (patient #4) to +3.0 (patient #1). See “—Our Solution for PHL: VDPHL01—Ongoing Studies—Study 207: Our Phase 2 Open Label Proof of Concept Trial” for the full set of images from the study (images are excluded for patients (n=1) with improvement scores less than or equal to the 5th percentile or greater than or equal to the 95th percentile in both image views at month four).

This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs of each treatment from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale (–3 to +3), a commonly used tool for assessing hair growth, measuring whether scalp hair growth has “slightly,” “moderately,” or “greatly” increased or decreased or no change has occurred.
Our Phase 2 trial evaluating VDPHL01 in male patients and female patients with PHL is ongoing, and we have initiated three registration-directed trials evaluating VDPHL01, two in male patients with PHL and one in female patients with PHL. If approved, we believe VDPHL01’s commercial potential would be substantial, as we estimate that the current U.S. commercial opportunity for PHL treatments for men and women is valued at approximately $9 billion annually, despite low patient engagement and high levels of dissatisfaction with current options reported. We believe that VDPHL01 could gain a meaningful share of this existing opportunity while also catalyzing substantial growth by offering a previously unavailable, transformative product profile to patients in an area of high unmet need. Our proprietary research indicates that 93% of patients would like to address their PHL yet only 9% are satisfied with their current treatment. Based on the initial product profile we have established for VDPHL01, we believe it could drive significant adoption in this large, motivated but underserved PHL patient population.
In anticipation of a potential NDA submission for VDPHL01 leveraging data from our registration-directed Phase 3 trials, we are developing a comprehensive multi-channel commercialization plan that will integrate patient
identification, physician education, DTC, advertising, social media and telehealth engagement and customer care. This commercialization strategy will be designed to drive patient awareness of VDPHL01’s differentiated profile and demand for hair loss treatment. We believe that the success of analog therapies highlights both the unmet need and motivation among patients and reinforces the potential for VDPHL01 to create demand in a cash-pay market, convert OTC patients to prescribed therapies and activate a population of untreated patients. We plan to focus commercial efforts at launch on establishing a dermatology-focused field force and conducting DTC advertising. We believe these commercial pillars will drive significant VDPHL01 adoption, if approved.
We maintain a broad library of patents and patent applications related to the key innovations of VDPHL01, including its method of utilizing an oral route of administration, ER formulation to stimulate hair growth as well as its optimized PK and PD qualities and profile. The earliest expiring patent term is 2043.
Our Pipeline
Our goal is to develop a focused portfolio of aesthetic dermatology product candidates targeting high-prevalence dermatologic conditions, with potential selective development of medical dermatology product candidates. We are advancing our lead product candidate, VDPHL01, in clinical trials as depicted below.
Our Team and Investors
Our team comprises a seasoned management team with deep connections in the dermatology physician and patient communities and expertise spanning dermatology, clinical development, regulatory affairs, operations, manufacturing and commercialization. The company is led by dermatologists who are well-regarded within the community, which we believe provides important credibility among physicians and insight into patient needs. Our corporate management team includes industry veterans with significant experience in both entrepreneurial biotechnology and global pharmaceutical companies, and collectively, have authored more than 100 peer-reviewed publications. Our team has extensive late-stage clinical trial experience, having been involved in the successful development and approval of more than forty products. We are further supported by a board of directors comprised of thought-leading dermatologists, biotechnology industry leaders and corporate executives with collective decades of experience.
Since inception, we have raised $263.0 million from a broad syndicate of investors, including several leading investment advisers and life sciences funds, such as Longitude Capital, SR One, Surveyor Capital (a Citadel company), Suvretta Capital and Viking Global. Potential investors should not consider investments made by our existing investors when making a decision to purchase shares in this offering since our existing investors may
have had different risk tolerances and paid less per share than the price at which the shares are being offered in this offering.
Our Competitive Strengths
We believe the following strengths will enable us to achieve our goal of becoming the leader in hair loss treatment:
nPotentially transformative late-stage lead product candidate, VDPHL01, innovating on validated science to address the millions of men and women suffering from PHL in the United States. PHL represents an attractive commercial opportunity. Existing treatments provide insufficient hair growth and have potential tolerability issues and/or inconvenient administration. To our knowledge, currently there is only one FDA-approved oral treatment (finasteride 1 mg tablet) in male patients and none in female patients. Topical minoxidil is FDA-approved for use in both male patients and female patients, but delivers only modest efficacy, variable response rates and a burdensome application to the scalp that results in 86% of patients discontinuing within a year. We designed VDPHL01 to build upon minoxidil’s validated biology for stimulating hair growth to maximize the total amount of minoxidil that can be delivered to follicles over a 12- or 24-hour period without inciting spikes in peak plasma concentrations, or Cmax, of minoxidil known to induce changes in cardiac activity. We are currently in registration-directed trials and we believe VDPHL01 has the potential to be the first oral, non-hormonal FDA-approved therapy for PHL.
nClinical data to date supports a potentially differentiated profile for VDPHL01, with topline data from our initial registration-directed trial anticipated to read out in the first half of 2026. We believe the preliminary data reported in October 2025 from our Phase 2 clinical trial in males support VDPHL01’s differentiated clinical profile with the following key attributes:
nSpeed: Visibly noticeable hair growth as early as two months after treatment in a majority of patients based on PRO and an IGA.
nConsistency: 90.5% treatment response at four months based on PRO of participants reporting “improved” or “much improved” hair coverage.
nIntensity: Average non-vellus (greater than 30 microns) hair count change of 47.3 hairs per cm2, with double digit absolute non-vellus hair count changes in greater than 90% of patients completing four months of treatment.
nSafety: Generally well tolerated, with no treatment-related SAEs, including cardiac or hormonal-related issues to date.
nConvenience: Preference for oral over topical administration supported by third-party research.
nMarketability: Potential to be the first oral, non-hormonal FDA-approved therapy for PHL.
nDermatology-rooted leadership with deep understanding of needs of PHL patients and physicians. Our founding dermatologists have direct experience with PHL patients, the impact of the disease and the lack of suitable treatment options, all of which inform our efforts to bring a novel solution to patients. With this base of first-hand knowledge, we have strategically built our network and connectivity with the major PHL stakeholders and opinion leaders, that has enhanced our deep understanding of approaches and challenges to treatment today and areas of unmet patient needs. These insights coupled with our own experiences as dermatologists benefit both our product development and commercialization planning as we strive to bring a potential solution to the millions of men and women suffering from PHL.
nComprehensive commercialization strategy to educate and engage physicians and patients. Our commercial strategy will be designed to establish VDPHL01, if approved, as the product of choice for the treatment of PHL. We are developing a comprehensive multi-channel commercialization plan that will integrate patient identification, physician education, DTC, advertising, social media and telehealth engagement and customer care. This commercialization strategy will be designed to drive patient awareness of VDPHL01’s differentiated profile and demand for hair loss treatment. We believe that success of analog therapies highlights both the unmet need and motivation among patients and reinforces the potential for VDPHL01 to create demand in a cash-pay market, convert OTC patients to prescribed therapies and activate a population of untreated patients. We plan to focus commercial efforts at launch on establishing a dermatology-focused field force and conducting DTC advertising. We believe these commercial pillars will drive significant VDPHL01 adoption, if approved.
nRobust intellectual property portfolio designed to protect the key innovations that drive VDPHL01’s therapeutic differentiation. We maintain a broad library of patents and patent applications related to the key innovations of VDPHL01, including its method of utilizing an oral route of administration, ER formulation to stimulate hair growth as well as its optimized PK and PD qualities and profile. We believe each of these elements contributes to a potentially differentiated therapeutic profile that patients may find compelling, if VDPHL01 is approved. This strategy has elements that have been successfully utilized to protect other pharmaceutical products approved under the 505(b)2 NDA pathway.
Our Strategy
Our goal is to develop a leading aesthetics- and dermatology-focused biopharmaceutical company that advances therapies to address significant unmet needs for large, underserved patient populations. In order to achieve this goal, we are currently advancing our lead candidate, VDPHL01, for the treatment of PHL. Our strategy involves the following key elements:
nEfficiently develop VDPHL01 through registration-directed trials to be the leading treatment of PHL for both men and women. We believe that VDPHL01 has the potential to transform the treatment of PHL because of its encouraging preliminary Phase 2 clinical results with respect to fast, consistent and intense hair growth and its favorable tolerability profile as observed in our clinical trials to date. We are currently advancing VDPHL01 through late-stage clinical development in our registration-directed Phase 3 program consisting of three clinical trials: two in male patients and one in female patients with PHL. If approved, VDPHL01 would be the first FDA-approved oral minoxidil product for the treatment of PHL, the first FDA-approved oral product for the treatment of PHL in female patients, and we believe VDPHL01 would be the only FDA-approved branded product for the treatment of PHL that would be actively marketed in the United States at the anticipated time of product launch. We believe an FDA-approved ER oral minoxidil product for hair loss will drive broader prescriber adoption, particularly among those who currently prescribe infrequently or have never prescribed IR oral minoxidil for PHL. Furthermore, we believe that the ability to actively promote an approved product has the potential to enable widespread physician and patient awareness and adoption of VDPHL01.
nBuild a sales and marketing organization focused on prescribers and dedicated to increasing patient awareness and activation. We are developing a targeted commercial infrastructure designed to educate dermatologists and patients about VDPHL01’s differentiated clinical profile to maximize adoption upon potential approval. Our plan is to target based on detailed patient and prescriber segmentation to focus on those most likely to adopt VDPHL01. We will seek to raise awareness of VDPHL01 via expert voice and advertising and generate excitement and drive activation of patients to proactively seek treatment.
nPosition for early and wide adoption through the cash-pay model. We plan to sell VDPHL01 directly to patients through the cash-pay model, which bypasses the pharmacy benefit managers and eliminates the inconvenience of dealing with insurance companies. Multiple analogs in PHL and other indications have demonstrated patient willingness to pay. We believe this cash pay model will facilitate product access and enable VDPHL01, if approved, to become widely available to patients.
nDeliver and commercialize VDPHL01, if approved, as the first FDA-approved oral treatment for female PHL. Women represent nearly 40% of PHL patients in the United States and experience a disproportionately higher psychological burden from the condition. Women represent approximately 85% of the U.S. aesthetics market and account for the majority of hair supplement purchases. If VDPHL01 is approved as the first oral therapy for women, we believe it could be especially well-received among the approximately 30 million female PHL patients in the United States.
nEvaluate strategic collaboration to maximize the value of our product candidates and deliver meaningful benefits to patients. Our goal is to be a premier aesthetic dermatology company and to maximize the number of patients who benefit from our product candidates. We retain exclusive worldwide rights to all of our product candidates, including VDPHL01. We aim to establish VDPHL01, if approved, as the product of choice for men and women experiencing PHL. We plan to establish partnerships with physicians and tele-dermatologists, helping them identify potential patients for VDPHL01, if approved, to accelerate consultation and prescription. We may selectively consider strategic collaboration opportunities to expand the reach and maximize the value of VDPHL01.
nIdentify additional solutions to pervasive unmet needs in dermatology. We aim to utilize our deep experience in treating patients as practicing dermatologists to identify additional opportunities that could potentially
create paradigm shifts in aesthetic or potentially medical dermatology. We intend to focus on identifying innovative therapeutics that target highly prevalent and bothersome skin conditions. We also plan to continue to explore opportunities to internally develop or in-license assets to address unmet medical needs in dermatology.
PHL: The Largest Unmet Need in Aesthetic Dermatology
PHL is the largest unmet need in aesthetic dermatology. A genetically predetermined, progressive disorder, PHL impacts approximately 50 million men and 30 million women in the United States alone. For men, approximately two-thirds will experience some degree of noticeable hair loss by age 35, and by age 50, 85% will have experienced significant thinning. Approximately 40% of women will experience PHL by age 50. PHL demonstrates a lifetime prevalence of nearly 50%, significantly higher than psoriasis (2–3% of individuals) or atopic dermatitis (approximately 10% of adults). Approximately two times as many people in the United States actively treat their PHL each year than suffer from psoriasis. As demonstrated in the graphic below, PHL impacts more Americans than any other chronic dermatological condition, according to the American Academy of Dermatology:
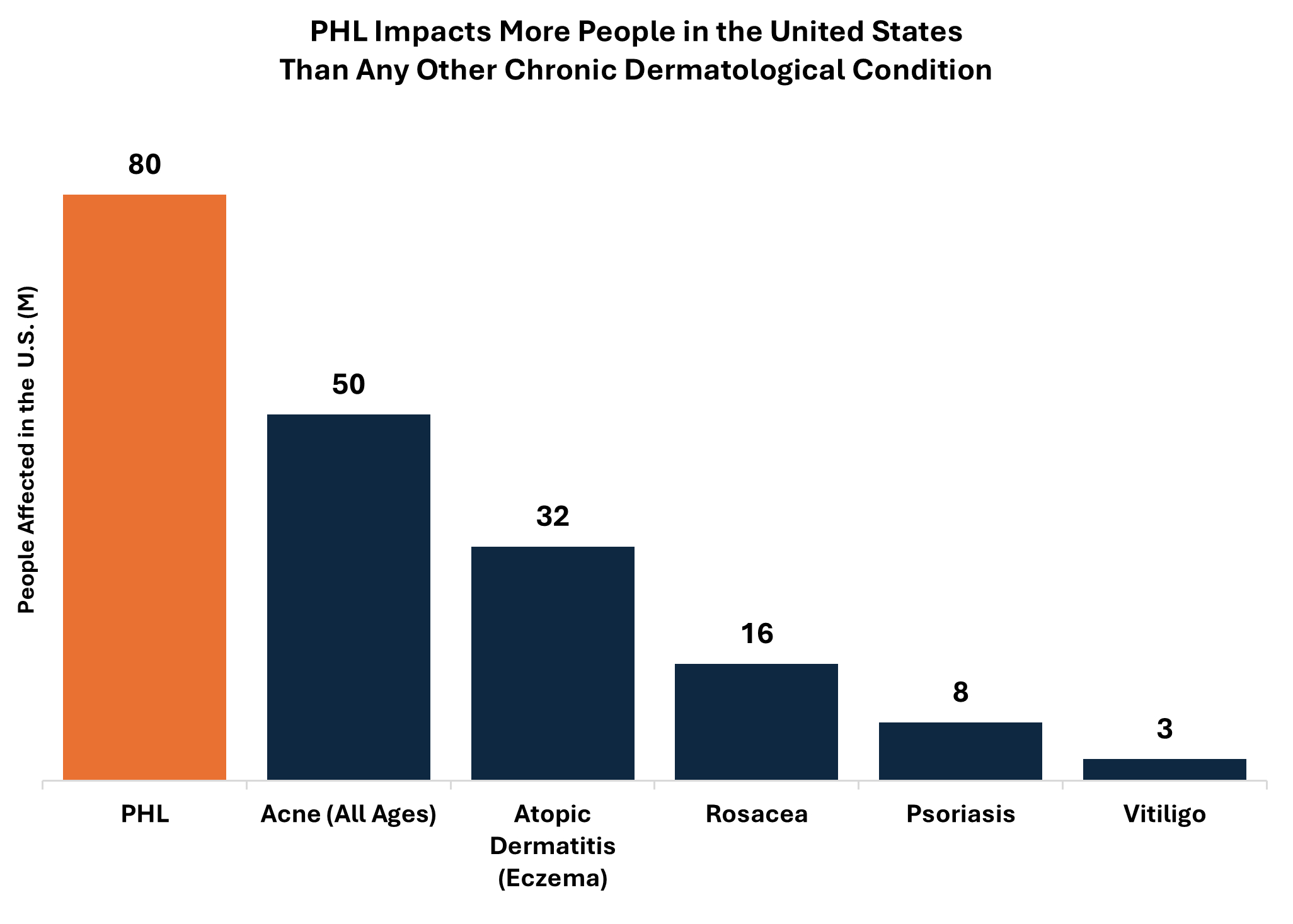
The detrimental effect of PHL on quality of life rivals that of other skin diseases with obvious presentation, such as alopecia areata, or AA, or acne vulgaris. Hair loss negatively impacts the self-image and self-confidence of the affected individual and alters social behavior, causing health-related impairments in quality of life. A study investigating the detrimental psychosocial effects of PHL found that hair loss can increase negative socioemotional events, such as looking older or feeling less attractive, having lower self-esteem and decreased life satisfaction compared with the general population. Although PHL is more commonly associated with men, psychological studies of male and female PHL patients consistently reported that the negative impacts of the condition are felt more acutely by women, who remain an underserved community with no FDA–approved oral treatment options. The impact on the female PHL population is further demonstrated by women’s willingness to
treat with topical minoxidil at higher rates than men, despite the inconvenience of topical application. Despite its large prevalence and associated quality of life impairments, the treatment landscape for PHL has experienced a lack of innovation and has remained fragmented, mostly dominated by products that have either limited efficacy, safety concerns, undesired topical administration or are costly and invasive surgical options.
Limitations of the Current PHL Treatment Landscape
While there are therapeutic as well as procedural and supplement-based treatments, outcomes for PHL patients continue to be very poor. Collectively, these options, whether used as a monotherapy or in combination, offer limited benefit, can be costly or burdensome and, in some cases, expose patients to serious, potentially life-threatening side effects. These incomplete therapeutic profiles are the primary reason that, despite the expressed desire of the vast majority of patients to address their PHL, only about 20% of PHL patients are actively engaged in treatment and only about 9% of those engaged are satisfied with their treatment outcomes, based on a proprietary commercial survey of 410 hair loss patients. Despite the low overall satisfaction with the outcomes offered by these treatments, patient willingness to pay remains high, often including significant upfront or recurring costs ranging from $700 up to $12,000 depending on the treatment. Current treatments for PHL can be categorized as follows:
nTherapeutics
nFDA-approved: oral finasteride (prescription, male patients only) and topical minoxidil (OTC).
nOff-label: drugs that were FDA-approved for other indications and are used “off-label” for hair growth, such as IR oral minoxidil.
nProcedures and Other
nFDA-cleared laser-based procedures: LLLT and fractional laser non-ablative therapy.
nSurgical procedures: platelet rich plasma therapy, or PRP, and hair transplant surgery.
nVarious non-FDA-approved, non-prescription options: such as “nutraceutical” supplements and shampoos.
Finasteride
Finasteride, sold under the brand name Propecia, is one of only two FDA-approved products for the treatment of PHL in men. The drug is an oral 5 alpha reductase inhibitor anti-androgenic that addresses the hormonally driven elements of PHL by decreasing the production of DHT and reducing the conversion of other forms of male hormones into DHT. We believe finasteride has several key limitations that have impacted adoption:
nTolerability issues associated with hormonal AEs: Due to its anti-androgenic impact, finasteride is associated with multiple reproductive, psychological and hormonal side effects. Finasteride’s original warning label includes references to adverse reactions of decreased libido, erectile dysfunction and ejaculation disorder. In a 2012 label update, a warning for potential increased risk of high-grade prostate cancer was added as were notations of incidences of depression and gynecomastia. In 2022, the FDA updated the finasteride label to include reports of suicidality and suicidal behavior, and, in 2025, the European Medicine Agency confirmed that suicidal ideation is a potential side effect of treatment with finasteride. In 2025, the FDA also provided guidance recognizing potential irreversibility of certain finasteride-related AEs.
nLack of female approval: Finasteride is not approved for the treatment of female PHL and studies of its efficacy in treating female PHL have been limited. Elevated DHT levels — a primary driver of PHL in men — play a less significant role in women, limiting the effectiveness of hormonal treatments such as finasteride.
nModest hair growth benefit: In two pivotal trials of finasteride in male PHL, patients treated with finasteride achieved the following hair growth results following twelve months of treatment:
nNon-vellus hair count: Growth of 16.9 hairs per cm2 (86 hairs) at twelve months from baseline.
nPRO: Only 39% of finasteride-treated patients were satisfied with the overall appearance of their hair vs. 22% treated with placebo.
nExpert Grading: Trial investigators rated 65% of men treated with finasteride as having increased hair growth compared with 37% in the placebo group. Amongst the treatment group, however, only 18% were judged to have achieved more than slight improvement.
OTC Supplements
OTC supplements for PHL are widely available and utilized by many patients. As dietary supplements, these products are subject to far fewer requirements for transparency regarding safety, efficacy and composition as compared to FDA-approved treatments. Consequently, advertised results for supplements typically have not been validated through large, independent, peer-reviewed clinical trials.
The most widely used of these non-FDA-approved supplements, Nutrafol, is an oral nutraceutical supplement composed of phytoactive ingredients, vitamins and minerals that is used by 1.5 million people at an estimated average annual cost of $700 to $1,000. Nutrafol’s small, manufacturer-sponsored studies are not required to be conducted pursuant to FDA review and oversight and have not, to date, been independently confirmed or reproduced.
Procedures
Procedural interventions for PHL include LLLT, fractional non-ablative laser therapy, PRP, injections and hair transplant surgery. While these approaches have expanded the treatment landscape for PHL patients, they remain constrained by limited efficacy, considerable invasiveness, high cost and high patient burden.
LLLT devices, which are FDA-cleared, use low-intensity light to stimulate hair follicles and promote regrowth. Although non-invasive, LLLT typically demonstrates modest clinical benefit and requires prolonged, time-intensive treatment regimens that impedes adherence. These devices also carry high upfront costs and are rarely reimbursed by insurance, limiting their practical utility.
Fractional non ablative laser therapy aims to induce controlled microthermal injury to the scalp to stimulate new hair growth. While also FDA-cleared, the clinical evidence supporting its benefit remains limited to small, often non controlled studies. The procedure can cause discomfort, erythema and post treatment irritation, further restricting adoption outside of cosmetic clinics.
PRP therapy, in which autologous plasma enriched with platelets and growth factors is injected into the scalp, has shown outcomes inferior to topical minoxidil and oral finasteride as monotherapy with mean hair count increase of approximately 10.4 per cm2 after 6 months of treatment. However, PRP is not FDA-approved for hair loss, requires multiple treatment sessions and can cost several thousand dollars per year, with no insurance coverage. Clinical response is also highly variable, dependent on preparation technique and operator experience.
Hair transplantation, such as follicular unit extraction or follicular unit transplantation, is a procedure that offers permanent redistribution of hair follicles from a donor site at the back of the head to recipient sites at the front or vertex of the scalp. Despite technological advances, these surgeries remain invasive, costly (typically $8,000–$12,000 per procedure) and associated with post operative discomfort, potential scarring and downtime. Importantly, transplanted follicles and native hair both require adjunctive pharmacologic therapy before and after surgery to optimize graft survival and minimize ongoing hair loss. Based on utilization of topical minoxidil-based products in the pre- and post-transplant settings, we believe that, if approved, VDPHL01 could potentially capture a portion of both the pre- and post-transplant adjunctive populations.
Collectively, these procedural modalities offer limited or temporary benefit while often imposing high economic, time and recovery burdens
Minoxidil-based PHL Treatments
Minoxidil is a drug that, following conversion to its active metabolite minoxidil sulfate by the sulfotransferase enzymes, SULT1A1 or SULT2A1, acts as a peripheral vasodilator by opening potassium channels on smooth muscle, causing membrane hyperpolarization to ultimately reduce vascular resistance. This potent vasodilation
drives significant reductions in both systolic and diastolic blood pressure, which led to minoxidil’s initial approval in 1979 for the treatment of resistant hypertension under the brand name Loniten. During its use for this indication in the 1980s, reports of hair growth among hypertensive patients emerged, sparking interest in its potential as a treatment for hair loss, consequently supporting eventual FDA approval as a topical solution. Oral formulations for hair loss, however, were not pursued due to concerns regarding potential adverse effects.
Minoxidil Topical Solution
Topical minoxidil, marketed as Rogaine, was first approved by the FDA in 1988 for men as a 2% prescription solution and in 1991 for women at the same concentration. These approvals were followed by subsequent clearance for nonprescription OTC use for both genders at either 2% or 5% concentrations. Despite its widespread availability and long-standing use, topical minoxidil has several limitations:
nModest efficacy: Rogaine 5% Foam was cleared for female patients based on results showing hair count increase at week 24 of 13.4 hairs per cm2 and achieved hair growth totals in male patients of 18.6 hairs per cm2 at 48 weeks in clinical studies. Across multiple clinical trials, topical minoxidil’s profile has remained consistent in both magnitude and speed of hair growth simulation, with 5% topical formulation consistently generating superior results to 2% topical formulation in head-to-head studies.
nVariable response: Self-administered and applied directly to the scalp, minoxidil is absorbed into hair follicles and converted to its active metabolite by SULT1A1. Clinical studies show that hair growth response to topical minoxidil correlates with follicular SULT1A1 expression, with up to 60% of patients potentially responding poorly to topical minoxidil therapy due to low baseline levels of SULT1A1 activity.
nBurdensome method of administration: For optimal results, minoxidil must be applied at least once daily to a dry scalp, with showering avoided for four hours post-application. Application can leave residue on the hair that can impact cosmetics and styling. This regimen is time-consuming, often leading to poor adherence and inconsistent drug exposure, which can diminish treatment results.
We believe there are approximately five million users of topical minoxidil in the United States annually, yet 86% discontinue within one year for the aforementioned reasons.
IR Oral Minoxidil Has Potential as a Hair Loss Treatment; However, Inherent Limitations of IR Oral Minoxidil Have Limited Adoption
IR oral minoxidil, an FDA-approved treatment for resistant hypertension at doses starting at 5 mg, is the most commonly prescribed hair loss treatment in the United States. Despite its label explicitly stating that it is not an approved treatment to promote hair growth, IR oral minoxidil has been prescribed approximately 3 million times in 2024, with its prescriptions surging approximately ten-fold following the 2022 publication of a New York Times article extolling its potential utility for PHL. This growth in IR oral minoxidil use points to strong underlying demand for a convenient non hormonal oral hair loss treatment, yet adoption remains limited to approximately 0.6% of the eligible population, with nearly 30% of all prescriptions written by fewer than 1,000 healthcare providers. While mechanistically promising, off-label IR oral minoxidil faces several widespread adoption barriers:
nHair growth ceiling: We believe IR oral minoxidil has a mismatch between its PK profile and what hair follicles require for hair growth. IR oral minoxidil has a half-life of approximately 90 minutes, creating peaks and troughs in plasma concentration. During the peaks, IR oral minoxidil delivers a rapid antihypertensive effect, reaching Cmax within one hour and materially clearing within four hours. While this drug release profile is intentionally suited for blood pressure control, it is ill-suited for hair growth as the short exposure hinders follicular conversion of minoxidil to active metabolite minoxidil sulfate via local sulfotransferase enzymes, as this enzymatic conversion is capacity-limited and time-dependent. IR oral minoxidil’s oscillating exposure pattern increases cardiac risk without added hair growth potential, as the majority of minoxidil exposure occurs within two hours of administration during a brief spike, rather than remaining below the threshold at which signs of cardiac activity are typically observed. In investigator-sponsored studies, off-label use of IR oral minoxidil has shown an efficacy ceiling demonstrating non-vellus hair count change on par with topical minoxidil.
nDose-dependent cardiac risk: Doses producing the strongest hair growth also generate plasma concentrations that trigger adverse cardiac activity. The greatest hair regrowth for oral minoxidil occurs as the dose level
increases. However, for 5 mg IR oral minoxidil, which is the consensus maximum dose of IR oral minoxidil for hair growth treatment, peak plasma levels reach 37 ng/mL, which exceed the FDA threshold of 20 ng/mL for cardiac and vascular changes. Despite peak regrowth potential, these peak plasma levels exceed the FDA threshold of about 20 ng/mL for cardiac and vascular changes, as described further below. These spikes in plasma concentration are not required for hair growth and they may drive off-target effects. Trials have reported patients experiencing symptomatic hypotension, dizziness, tachycardia, palpitations, blood pressure and EKG changes after 5 mg of IR oral minoxidil, as well as rare, potentially life-threatening complications such as pericardial effusion. We believe that prescribers recognize the potential for AEs at the 5 mg IR level, as physicians currently prescribe the 2.5 mg IR dosage form for approximately 90% of patients, despite its inferior stimulation of hair growth. The below graph depicts the plasma concentration levels of minoxidil after oral dosing at both IR 2.5 mg and IR 5 mg, relative to the cardiac threshold and the threshold required for hair growth.
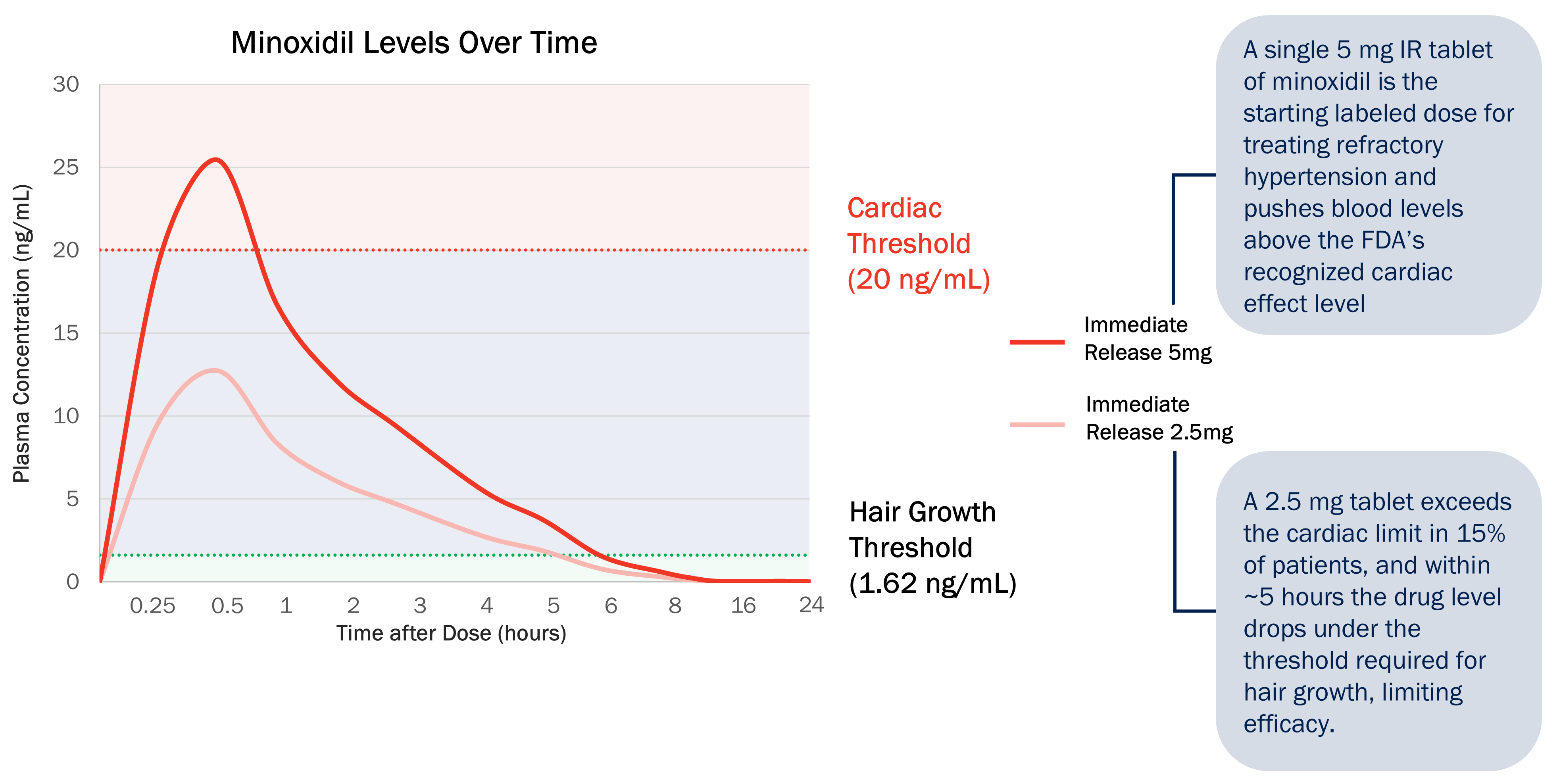
Minoxidil 2.5 mg IR data represent average plasma concentrations for all patients (n=15) from the relevant dosing period in Study QSC300720.
Minoxidil 5 mg IR data represent average plasma concentrations estimates using dose linear PK of minoxidil 2.5 mg IR data for all patients (n=15) from the relevant dosing period in Study QSC300720.
nLack of FDA Approval: We believe that lack of FDA approval for the treatment of PHL and other forms of hair loss limits prescriber willingness and patient awareness to adopt oral minoxidil. The absence of large-scale randomized trials and clinical data contributes to prescriber and patient hesitancy to adopt use and limited awareness of oral minoxidil for treating PHL and may contribute to variability in dosing of minoxidil that may further increase prescriber hesitancy and result in suboptimal outcomes. Furthermore, as IR oral minoxidil is not FDA-approved for any hair loss indication, it cannot be actively promoted for the treatment of hair loss, limiting awareness. In a proprietary commercial survey of 410 hair loss patients, the second most common reason why individuals using over the counter treatments for treating their hair loss do not opt for a prescription treatment is because they are unaware of prescription treatments for hair loss.
Our Solution for PHL: VDPHL01
VDPHL01’s key innovation is its release profile, which is designed to maximize the total amount of drug that can be delivered to follicles over a 12- or 24-hour period without inciting spikes in Cmax of minoxidil known to induce changes in cardiac activity. VDPHL01 aims to provide long lasting concentrations of minoxidil above the drug’s ‘hair growth threshold’ (i.e., 1.62 ng/mL, the minimally effective concentration needed to grow hair) while maintaining peak concentrations well below the drug’s ‘cardiac activity threshold’ (i.e., about 20 ng/mL, the
threshold concentration level at which cardiac effects occur). The key innovation that enables VDPHL01’s differentiated profile is the performance of the proprietary hydrogel tablet formulation, which is designed to:
nprevent Cmax from exceeding the ‘cardiac activity threshold’ to preserve the cardiac safety of minoxidil;
nmaximize total drug exposure and duration of drug exposure above the ‘hair growth threshold’ to increase hair growth potential; and
nincrease the metabolism of minoxidil to its active metabolite that is responsible for hair growth, minoxidil sulfate, by increasing amount and duration of drug exposure at the follicular level where the activating SULT1A1 enzyme is present.
The below graphic depicts the plasma concentration levels of minoxidil representing the cardiac activity threshold as compared to the hair growth threshold and the proposed target concentration range for hair growth.
We designed VDPHL01’s PK profile on the cardiac activity threshold established during the topical minoxidil OTC-switch process in the 1990s. This threshold was discovered via IV steady-state studies that identified the plasma concentration at which heart rate changes greater than 5 beats per minute can be detected. The cardiac activity threshold has been repeatedly recognized by the FDA in multiple subsequent approvals of topical minoxidil products. The hair growth threshold is the minimal Cmax concentration that must be reached to achieve hair growth, which was established based on the following data points:
nProspective PK data tying systemic administration to hair growth — hair growth was observed at Cmax as low as 1.62 ng/mL.
nIR oral minoxidil data demonstrating hair growth at 0.25 mg daily — based on dose proportionality of minoxidil PKs, a 0.25 mg dose would be anticipated to have a Cmax of 1.4 ng/mL-1.6 ng/mL.
nRogaine 5% data demonstrating distant site hair growth — the Cmax of Rogaine 5% foam is less than 2 ng/mL.
Furthermore, VDPHL01’s ER formulation of minoxidil is designed to overcome high inter-individual variability in sulfation capacity by enabling sustained drug exposure and consistent local formation in the hair follicle of the active metabolite, minoxidil sulfate. The prolonged exposure from VDPHL01’s ER tablet is designed to facilitate increased minoxidil sulfate formation, improving the likelihood of transitioning follicles into the anagen growth phase. This prolonged exposure is intended to avoid spiking systemic concentrations of minoxidil that otherwise could lead to enzymatic saturation (i.e., capacity-limited). We believe this key catalyzing interaction is enabled by VDPHL01, which increases the total amount of minoxidil available for conversion and extends the duration of engagement with the SULT1A1 sulfotransferase enzyme responsible for producing minoxidil sulfate (i.e., time dependent).
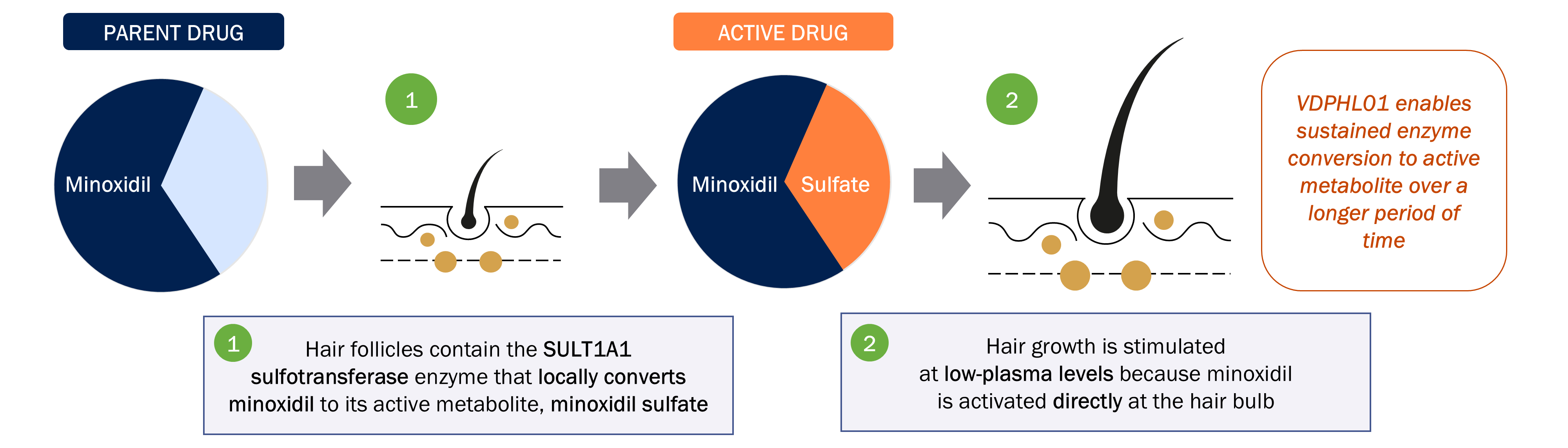
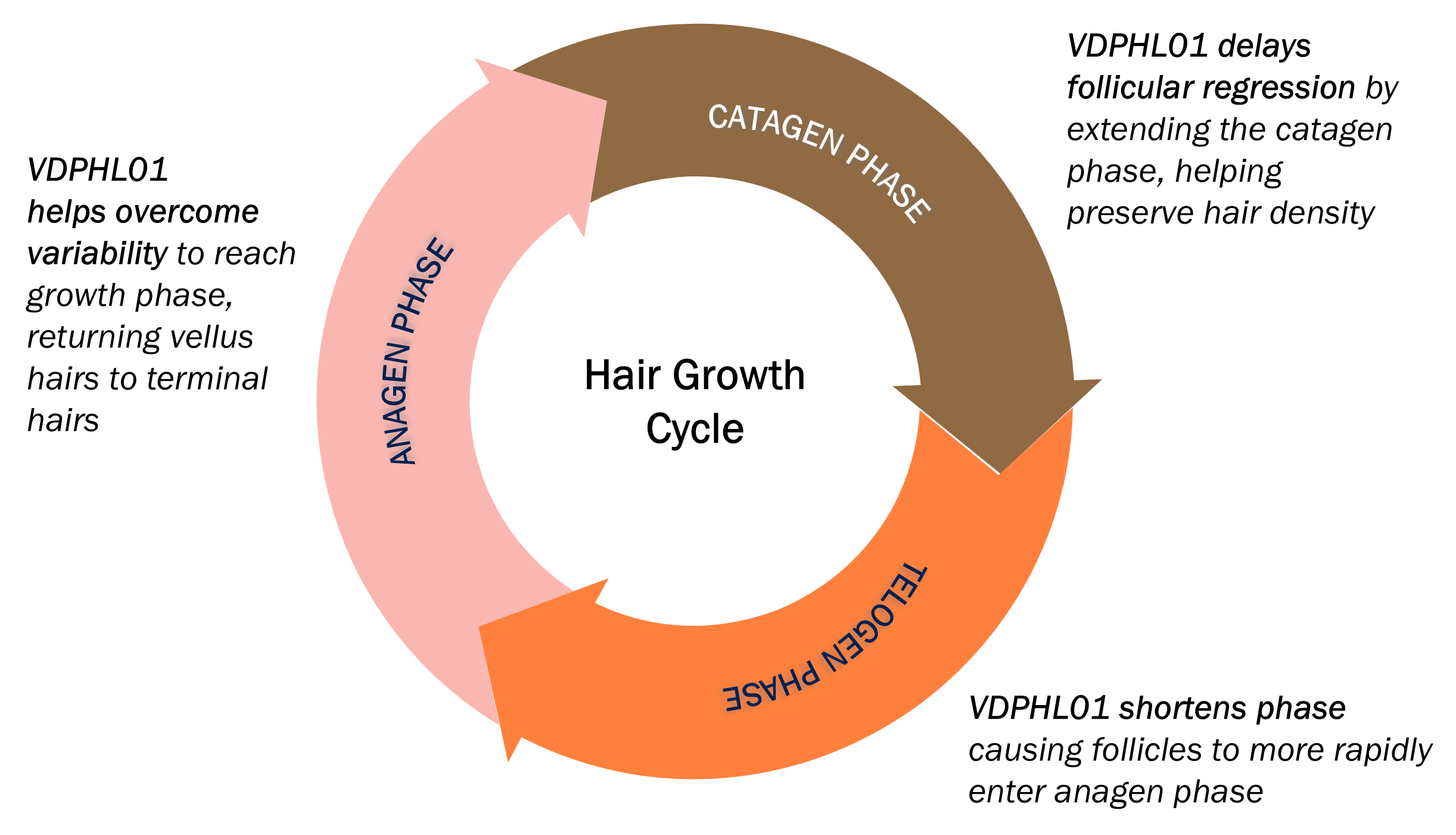
The ability of VDPHL01 to drive a differentiated metabolite profile is demonstrated by Phase 1 data in healthy volunteers, which showed a higher than anticipated ratio of minoxidil glucuronide to minoxidil, demonstrating an ability to increase metabolism of minoxidil to its most common metabolite. The ability to potentially increase sulfation of minoxidil is important since approximately 40% of individuals exhibit low SULT1A1 enzyme activity that has been shown to have a greater than 90% negative predictive value related to topical minoxidil response. We believe VDPHL01 has the potential to improve positive outcomes among individuals that exhibit low SULT1A1 enzyme activity due to prolonged exposure to minoxidil at concentrations above the hair growth threshold.
VDPHL01 has the potential to address inherent limitations in the current PHL treatment landscape
We believe that VDPHL01 could transform the PHL treatment paradigm and reduce the high levels of dissatisfaction and frustration associated with the current patient journey. VDPHL01 may deliver improved outcomes versus existing treatment options across the product attributes most desired by patients. In particular, we believe VDPHL01 will raise the hair growth ceiling with improved speed, consistency and intensity of effect by enabling greater sustained exposures to minoxidil throughout the day, without driving peak concentrations of systemic minoxidil associated with known cardiac effects. Many experts believe that variability in the response to
minoxidil as a treatment for hair loss is due to variability in hair follicle sulfation of minoxidil to active minoxidil sulfate. We believe that increasing the duration of exposure of minoxidil above levels that have been shown to grow hair may increase the percentage of individuals that respond and the intensity of response to a minoxidil treatment by driving capacity-limited and time-dependent conversion of minoxidil to active minoxidil sulfate. Supported by the preliminary data reported in October 2025 from our Phase 2 clinical trial data in male patients with PHL, VDPHL01’s emerging profile has following potential benefits:
nSpeed. In our proprietary market surveys of Americans with PHL, survey respondents cited more rapid onset of growth as a primary desire in a treatment for PHL. In our Phase 2 open-label trial, the majority of male patients receiving VDPHL01 8.5 mg twice daily reported that their hair coverage was “improved” or “much improved” after only two months of treatment.
nConsistency. Survey respondents seek hair growth responses consistent in magnitude and rapidity. Studies on the efficacy of finasteride in male patients showed slight improvement at month 12 according to expert grader review. In our Phase 2 open-label trial, 90.5% of male patients receiving VDPHL01 8.5 mg twice daily for four months of treatment reported that their hair coverage was “improved” or “much improved.”
nIntensity. Survey respondents identified more robust density and total hair growth as a critical attribute for a hair growth product. In our Phase 2 open-label trial, males receiving VDPHL01 8.5 mg BID for four months of treatment had an average non-vellus (greater than 30 microns) hair count change of 47.3 hairs per cm2, with double digit absolute non-vellus hair count changes in greater than 90% of patients.
nSafety. Both patients and physicians prioritize a safety profile devoid of hormonal and mood or psychological side effects, with minimized cardiac side effects associated with currently available treatment.
nCardiac. IV minoxidil steady-state work has supported that cardiac effects are driven by a Cmax threshold of about 20 ng/mL. VDPHL01 is designed to enable dosing of higher amounts of minoxidil than would be otherwise achievable with maintained cardiac tolerability by reducing peak-to-trough variability of minoxidil exposure and overall reducing peak levels of minoxidil.
nHormonal and Psychological. Minoxidil is non-hormonal and minoxidil treatments are not typically associated with hormonal and psychological side effects.
nConvenience. Patient respondents to surveys regarding their preferred route of administration for any medicine consistently cite an oral tablet as their top choice given its ease of use.
nMarketability. VDPHL01 has the potential to be the first oral, non-hormonal FDA-approved therapy for PHL. We believe an FDA-approved oral minoxidil product for hair loss will drive broader prescriber adoption, particularly among those who currently prescribe infrequently or have never prescribed oral minoxidil for PHL. Furthermore, we believe that the ability to actively promote an approved product has the potential to enable widespread physician and patient awareness and adoption of VDPHL01. Our proprietary market research identified that the second most common reason that current OTC users are not using a prescription product is lack of awareness of prescription offerings.
If approved, VDPHL01 could provide an attractive treatment option by combining these attributes in a way not currently available. We believe VDPHL01’s commercial potential could be substantial, as we estimate that the current commercial opportunity for PHL treatments for men and women in the United States is approximately $9 billion, based on publicly available industry data and patient surveys to determine the number of PHL patients using prescription and OTC therapies in the United States in 2024, our proprietary third-party market research of estimates of duration of therapy and our analysis of pricing for prescription and OTC treatments. We believe that VDPHL01 could not only gain a meaningful share of this existing commercial opportunity, but also capture patients previously deterred by inadequate treatments and expand the addressable commercial opportunity.
Summary of our clinical trials and data generated to date
Our belief in VDPHL01’s ability to deliver a differentiated, target PK profile relative to other forms of minoxidil is bolstered by data from our Phase 1 PK studies. The first was a dose ranging Phase 1 clinical trial of VDPHL01 prototypes in doses up to 10 mg against a single reference dose of commercially available 2.5 mg IR oral minoxidil administered to the same cohort of healthy male patients and female patients in sequential dosing periods. As this study was exploratory, no formal sample size calculation was made related to statistical power. The data presented below are reflective of the study’s per-protocol primary objective, namely, to evaluate the PK profile and determine the relative bioavailability of VDPHL01 following single oral dosing of VDPHL01 versus the
reference IR oral minoxidil formulation in healthy subjects. The study demonstrated the substantially different Cmaxs and total drug exposure levels achieved by the multiple prototype ER doses and 2.5 mg IR, the most prescribed off-label dose form. In the study, fasted administration of all dose levels from 5 mg up to 10 mg ER achieved both higher total exposures and lower Cmaxs than 2.5 mg IR. The 8.5 mg dose, which we expect would be VDPHL01’s commercially available dose for male patients and is the dose we are evaluating in Study 302, our first registration-directed clinical trial in male patients, specifically achieved an average total drug exposure, or AUC, of 42.5 ng/mL and average peak Cmax, of 8.32 ng/mL versus 25.3 ng/mL and 13.90 ng/mL respective levels for 2.5 mg IR. In addition, no patients dosed with VDPHL01 8.5 mg ER Tablet achieved a Cmax that exceeded the reported threshold for cardiac effects, which are seen at concentrations exceeding 20 ng/mL, whereas 13.3% of patients dosed with 2.5 mg IR oral minoxidil achieved a Cmax greater than 20 ng/mL. Additional details related to this PK study are outlined below in “— Completed Study QSC300720: A Phase 1, Single-Part, Open-Label, Six Period Sequential Crossover Study Designed to Evaluate the Pharmacokinetic Profile of VDPHL01 in Male and Female Subjects.”
In our second PK study, a Phase 1 clinical trial in healthy male patients, we assessed the potential for twice daily dosing to further boost overall minoxidil exposure. In this study, VDPHL01 8.5 mg was given either once or twice daily to twelve healthy male volunteers, and results showed consistent profiles in blunting Cmax irrespective of dosage frequency. The results of this PK study are summarized below in “— Study 250-13951-105: A Two-Period Randomized Crossover Design Study to Evaluate the Pharmacokinetic Profile of VDPHL01 In Healthy Male Subjects.”
Based on these results, VDPHL01 8.5 mg maintained plasma levels of minoxidil that fell between the thresholds for promoting hair growth and inducing cardiac activity for more than 7.5 to 12 hours if given once daily or 15 to 24 hours if given twice daily. In this same study, VDPHL01 8.5 mg was generally well tolerated in both arms, and we saw no signs of cardiac activity or drug-related severe AEs and had no patient withdrawals for drug-related AEs.
Completed Study QSC300720: A Phase 1, Single-Part, Open-Label, Six Period Sequential Crossover Study Designed to Evaluate the Pharmacokinetic Profile of VDPHL01 in Male and Female Subjects
Study QSC300720 was a single center, open-label, six-period sequential crossover single dose Phase 1 clinical trial in 16 healthy male and female patients to establish and assess the PK and safety of VDPHL01 prototype formulations at a range of potential doses and release rates in comparison to a reference 2.5 mg IR oral minoxidil tablet formulation. A summary of relevant PK parameters from this study are as outlined above.
The VDPHL01 prototype formulations were generally well tolerated at all doses and release rates. We observed no SAEs, and no patients were withdrawn for treatment-related AEs. Overall, seven patients reported a total of eight mild AEs across the six treatment periods. The only AE reported by more than one patient was viral upper respiratory tract infection, reported by two patients. Importantly, there were no clinically significant observations or changes from baseline on vital signs, ECG, physical examinations or clinical laboratory safety tests.
We reviewed the results of Study QSC300720 to compare the head-to-head PK profile of VDPHL01 8.5 mg with the PK profile of 2.5 mg IR oral minoxidil for male patients who were administered both dosage forms, as well as the estimated PK profile of 5 mg IR oral minoxidil using published dose linear PK in the same male subjects. Based on this comparison, we observed that the PK profile of VDPHL01 8.5 mg demonstrated a differentiated systemic delivery profile as compared to both 2.5 mg observed and 5 mg estimated IR oral minoxidil. The below graphic illustrates representative average plasma concentration levels of minoxidil for VDPHL01 8.5 mg compared to those for IR oral minoxidil in the same male subjects relative to the cardiac activity threshold, the hair growth threshold and the proposed target concentration range for hair growth.
nVDPHL01 8.5 mg curve represents average plasma concentrations for male patients (n=10) from Study QSC300720.
nMinoxidil 2.5 mg IR data represent average plasma concentrations for male patients (n=10) from Study QSC300720.
nMinoxidil 5 mg IR data represent average plasma concentrations estimates using dose linear PK of minoxidil 2.5 mg IR data for male patients (n=10) from Study QSC300720.
Representative average plasma concentration levels for VDPHL01 8.5 mg demonstrated blunting of Cmax below the cardiac threshold and lengthening of time above the hair growth threshold as compared to IR oral minoxidil (2.5 mg observed head-to-head, 5 mg estimated) of male subjects from Study QSC300720.
Study 250-13951-105: A Two-Period Randomized Crossover Design Study to Evaluate the Pharmacokinetic Profile of VDPHL01 In Healthy Male Subjects
Study 250-13951-105, or Study 105, was a multi-dose PK Phase 1 study in 12 healthy male volunteers to evaluate the safety and PK profile of 8.5 mg of VDPHL01 either once (QPM) or twice (BID) daily, to determine the PK profile of expected registrational dosing.
Both dosing regimens, 8.5 mg once daily and 8.5 mg twice daily, were generally well tolerated over an 8-day dosing period. No SAEs and/or cardiac-related AEs were reported, and no patients were withdrawn for treatment-related AEs. One patient reported AEs (intermittent headache and nasal congestion), all of which resolved.
Consistent with previous results, no individual Cmax value exceeded the cardiac threshold concentration of 20 ng/mL following single and multiple dosing of VDPHL01 once or twice daily. Consistent with the short half-life of minoxidil, minimal accumulation of minoxidil was observed following either once or twice daily administration of
minoxidil. The below graphic illustrates our Cmax, AUC24 and AUC12 findings for both dosing regimens on Day 8 in this trial:
| | | | | | | | | | | |
| Dosing Regimen | Cmax (ng/mL) | AUC12 (h*ng/mL) | AUC24 (h*ng/mL) |
| VDPHL01 8.5 mg QPM | 8.219 | NA | 40.81 |
| VDPHL01 8.5 mg BID | 8.014 | 37.27 | 74.54* |
Select respective PK parameters (Cmax, AUC24 and AUC12) are presented from Study 105 after the last dose of VDPHL01 8.5 mg on Day 8 of dosing following preceding administration either once daily or twice daily. Cmax = Maximum observed concentration; AUC12 = Area under the curve from time 0 to 12 hours; AUC24 = Area under the curve from time 0 to 24 hours *estimated based on anticipated doubling of AUC upon twice daily administration.
Study 250-13951--109: PK Study of VDPHL01 4.5 mg of ER Oral Minoxidil and 20 mg of IR Oral Minoxidil in Female Patients
To establish VDPHL01 4.5 mg (ER oral minoxidil) as an appropriate dose for female PHL patients and establish a PK bridge to the reference to IR oral tablet, we conducted Study 250-13951-109, or Study 109, a Phase 1 study of 24 healthy female patients who were administered VDPHL01 4.5 mg as a single dose on day one, a twice daily dose on days two through seven and a single morning dose on day eight. After a washout period, the same patients were administered 20 mg of IR oral minoxidil on the morning of day eleven. The below graphic illustrates our geometric mean AUC, geometric mean Cmax and maximum observed Cmax findings for VDPHL01 4.5 mg and 20 mg IR oral minoxidil in this trial:
| | | | | | | | | | | |
| Dosing Regimen | Cmax
(ng/mL) (n=24) | Maximum Observed Cmax (h*ng/mL) (n=1) | AUC24
(h*ng/mL) (n=24) |
| VDPHL01 4.5 mg - Day 1 | 6.417 | 12.3 | 27.84 |
| VDPHL01 4.5 mg - Day 8 | 7.735 | 13.4 | 36.94 |
| IR Oral Minoxidil 20 mg - Day 11 | 135.1 | 250 | 315.3 |
Select respective PK parameters (geometric mean AUC24, geometric mean Cmax and peak Cmax) are presented from Study 109 for VDPHL01 4.5 mg after the initial dose on Day 1 and after the last dose on Day 8 following preceding administration twice daily. These same parameters are presented for 20 mg IR oral minoxidil administered on Day 11 after washout from VDPHL01.Cmax = Maximum observed concentration; AUC24 = Area under the curve from time 0 to 24 hours
We observed the comparative bioavailability of PK profile of VDPHL01 4.5 mg to that of IR oral minoxidil 20 mg in the Study 109. The below graphic illustrates average plasma concentration levels of minoxidil for VDPHL01 4.5 mg at Day 1 and Day 8 compared to those for IR oral minoxidil 20 mg at Day 11 in Study 109 relative to
the cardiac activity threshold, the hair growth threshold and the proposed target concentration range for hair growth.
Average plasma concentration levels for VDPHL01 4.5 mg from Study 109 demonstrated significant blunting of Cmax below the cardiac threshold as compared to IR oral minoxidil while preserving time above the hair growth threshold.
Adverse Events (AEs)
The AE summary presented in the graphic below from Study 109 illustrates the AE profile of VDPHL01 4.5 mg as compared to that of IR oral minoxidil. After multiple-day dosing of VDPHL01 4.5 mg, only 5 of the 23 total reported AEs were classified as “possibly related” or “probably related” to VDPHL01, and no cardiac AEs were reported. After single dose administration of IR oral minoxidil in these same patients, 42 of the 43 total reported
AEs were classified as “possibly related” or “probably related” to the drug, and cardiac effects (palpitations, heart rate increase) were reported. No SAEs were reported in any dosing cohort in this study.
| | | | | | | | | | | | | | |
Dosing Period 1: VDPHL01 4.5 mg (Day 1-8, n=24 subjects) | Dosing Break
Day 9-10 | Dosing Period 2: Minoxidil 20 mg IR
(Day 11, n=24 subjects) |
23 total Adverse Events | | 43 total Adverse Events |
10 most frequent adverse events | | 10 most frequent adverse events |
Headache | 4 | | Headache | 8 |
Myalgia | 2 | | Heart Rate Increased | 5 |
Bruising Due to Venipuncture Site | 1 | | Palpitations | 4 |
Constipation | 1 | | Orthostatic Tachycardia | 2 |
Sore Throat | 1 | | Dizziness, Postural | 2 |
Abdominal Discomfort | 1 | | Fatigue | 2 |
Burning with Urination | 1 | | Nausea | 2 |
Dry Lips | 1 | | Vomiting | 2 |
Labia Irritation | 1 | | Hyperhidrosis | 2 |
White Discharge, Vaginal | 1 | | Blurred Vision | 1 |
| | | | | | | | | | | | | | |
| AEs and relation to VDPHL01 | | AEs and relation to Minoxidl IR |
| Not Related | 11 | | Not Related | 0 |
| Unlikely Related | 7 | | Unlikely Related | 1 |
| Possibly Related | 3 | | Possibly Related | 18 |
| Probably Related | 2 | | Probably Related | 24 |
AEs from Study 109 are summarized by dosing regimen (VDPHL01 4.5 mg and 20 mg IR oral minoxidil) with listing of the most common AEs reported for each group. Relatedness of all AEs is also summarized by group. Overall, the AE summary demonstrates that VDPHL01 was generally well tolerated.
Study 108: PK Study of VDPHL01 8.5 mg (ER Oral Minoxidil) and 20 mg of IR Oral Minoxidil in Male Patients
A similar study has been conducted for VDPHL01 8.5 mg, the male dose form, with a nearly identical study design that is intended to establish a PK bridge to the reference IR oral tablet. In September 2025, 24 men were administered VDPHL01 8.5 mg as a single dose on day one, a twice daily dose on days two through seven and a single morning dose on day eight. After a three-day washout period, the same patients were administered 20 mg of IR oral minoxidil. Data from this study is anticipated in the first half of 2026.
Preliminary Phase 2 Data Demonstrated VDPHL01’s Differentiated Impact on Hair Growth at Four Months
We are currently conducting an ongoing Phase 2 open-label trial of VDPHL01 in 21 adult male patients and 22 adult female patients with PHL. Preliminary data from 21 male patients dosed with VDPHL01, 8.5 mg twice daily, in the Phase 2 clinical trial demonstrated consistent hair growth responses at 2 and 4 months, improved PRO and that VDPHL01 was generally well tolerated. Preliminary data showed male patients receiving VDPHL01 achieved restoration of 37.5 hairs per cm2 and 47.3 hairs per cm2 from baseline after two and four months of therapy, respectively. We believe these preliminary data results support our belief that VDPHL01 possesses a differentiated profile.
The degree of visible aesthetic improvement resulting from both this magnitude and speed of change in hair count was identified by both patients and trial investigators. 50% and 90.5% of male patients indicated an improvement in hair coverage at two months and four months, respectively. 80% and 90.5% of physicians indicated an improvement in hair coverage on the IGA, at 8 weeks and 16 weeks, respectively. Importantly,
these outcomes were captured at or before the 16-week mark from initial treatment, with the initial observation of visibly noticeable improvements as early as 8 weeks.
As this trial is exploratory, no formal sample size calculation will be made related to statistical power. Therefore, all trial endpoints, including objective hair count measures and subjective patient and investigator assessments, are exploratory and are planned to be reported as descriptive statistics.
Blinded Review Presented at 2025 Congress of the European Academy of Dermatology and Venerology Indicated VDPHL01’s Encouraging Results and Rapid Onset of Benefit
We sponsored a blinded, retrospective grader imaging analysis that was presented at the 2025 Congress of the European Academy of Dermatology and Venerology. This study compared outcomes via IGA, from the 20 male patients who completed four months of treatment with VDPHL01 8.5 mg twice daily in our Phase 2 clinical trial with completed and published trial dataset from a double-blind, randomized controlled study of 33 male patients receiving 5 mg IR oral minoxidil once daily over 6 months and 34 male patients receiving 5% minoxidil solution twice daily over 6 months. Subjects across both our trial and the published trial data set demonstrated similar baseline hair loss characteristics as measured by Norwood Hamilton subtypes. The physical assessment of a person with PHL involves measuring the pattern and extent of hair loss. The Norwood-Hamilton scale is commonly used to classify male-pattern baldness. On this scale, the stages of hair loss are described with a number from 1 (least severe) to 7 (most severe).
This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs of each treatment from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale, a commonly used tool for assessing change in hair growth ranging from greatly decreased (-3) to greatly increased (+3) with intermediate categories of no change (0), slightly decreased/increased (-1/+1) and moderately increased/decreased (-2/+2).
Mean IGA Improvement
In cases where the grader incorrectly selected the baseline photo (i.e., they selected the follow-up image as being the baseline image and assigned an IGA improvement score based on that perception), they would in reality have perceived a worsening in scalp hair growth. Therefore, after completion of blinded grading, scores were corrected for baseline misidentification by reversing score direction. After correction for mis-sequencing, mean IGA improvement scores (mean ± standard deviation) for the frontal and vertex views, respectively, were: 2.02 ± 0.75 and 2.05 ± 0.79 for VDPHL01, 0.29 ± 1.32 and 0.02 ± 1.03 for topical minoxidil, and 0.59 ± 1.28 and 0.53 ± 1.15 for IR oral minoxidil. As illustrated in the graphic below, the mean improvement for VDPHL01 was superior and as compared to both topical minoxidil and IR oral minoxidil. Additionally, there was
no meaningful difference in mean improvement when comparing the frontal view for topical minoxidil and IR oral minoxidil.
After completion of blinded grading, the accuracy of correct baseline identification (i.e., cases where the grader correctly selected the baseline image from the two images per subject in randomized order) was determined for each treatment group. As illustrated in the graphic below, the frequency of correct baseline identification (percentage of correct over total) for frontal and vertex views, respectively, was: 97.5% and 96.2% for VDPHL01, 60.3% and 49.6% for topical minoxidil, and 72.9% and 72.3% for IR oral minoxidil. The frequency of correct baseline identification for VDPHL01 was superior as compared to both topical minoxidil and IR oral minoxidil. Additionally, there was no meaningful difference in the frequency of correct baseline identification when comparing either the frontal or vertex view for topical minoxidil and IR oral minoxidil.
After completion of blinded grading and correction for mis-sequencing, the magnitude of individual clinical responses was assessed by calculating the proportion of subjects within each treatment group who were assessed as having achieved corrected IGA scores ≥ +2 (indicating moderate-to-great improvement). The proportion of meeting this threshold for frontal and vertex views, respectively, were: 82.8% and 82.1% for VDPHL01, 19.2% and 14.3% for topical minoxidil, and 21.6% and 23.3% for IR oral minoxidil. The results for VDPHL01 were superior as compared to both topical minoxidil and IR oral minoxidil. Additionally, there was no meaningful difference when comparing either the frontal or vertex view for topical minoxidil and IR oral minoxidil.
After blinded IGA grading including correction for mis-sequencing errors, VDPHL01 demonstrated superior proportion of subjects achieving corrected IGA scores ≥ +2 (indicating moderate-to-great improvement) as compared to topical minoxidil and IR oral minoxidil, both of which were not meaningfully different when compared to each other for both views.
This analysis demonstrated that four months of treatment with VDPHL01 resulted in superior IGA scores, as assessed by expert graders, compared to six months of treatment with topical and IR oral minoxidil and that there was no meaningful difference when comparing topical and IR oral minoxidil to each other.
Ongoing Studies
Study 207: Our Phase 2 Open Label Proof of Concept Trial
Study 207 is a multicenter Phase 2 open label study in 21 adult male and 22 adult female PHL patients initiated in June 2024 to obtain proof of concept for the safety and efficacy of twelve months of treatment with VDPHL01 8.5 mg twice daily in male patients and VDPHL01 4.5 mg either once or twice in female patients with PHL. Both cohorts of male and female patients are fully enrolled. Additional topline data from this study is anticipated to read out in the first half of 2026.
Select efficacy endpoints include:
nNon-vellus Target Area Hair Count, or TAHC: Change from baseline using digital image analysis at Months 1, 2, 4, 6, 8, 10 and 12.
nPRO: Proportion of patients with each score of a proprietary questionnaire, summarized individually by question at Months 1, 2, 4, 6, 8, 10 and 12.
As this trial is exploratory, no formal sample size calculation will be made related to statistical power. Therefore, all trial endpoints, including objective hair count measures and subjective patient and investigator assessments, are exploratory and are planned to be reported as descriptive statistics.
Images from Patients: Preliminary Data Announced in October 2025
The below images are of male patients from our Phase 2 clinical trial who had completed their month 4 visit as of August 2025 (n=20), the time at which a blinded image analysis study was conducted. This blinded, retrospective image assessment study used image files containing before-and-after (in randomized order) scalp photographs from both the vertex and frontal view. These image files were reviewed independently by three U.S. board certified dermatologists who were blinded to treatment group and asked to identify the baseline image before assigning an IGA score for improvement using a 7-point Likert scale, a commonly used tool for assessing change in hair growth ranging from greatly decreased (-3) to greatly increased (+3) with intermediate categories of no change (0), slightly decreased/increased (-1/+1) and moderately increased/decreased (-2/+2). Images for patients from screening, month two and month four are presented in order (highest to lowest) of the average improvement scores at month four. Average IGA improvement scores shown represent average improvement scores as assessed by the blinded expert graders ranging from +1.2 (patient #19) to +3.0 (patient #1). Images have been excluded for patients (n=1) with improvement scores less than or equal to the 5th percentile or greater than or equal to the 95th percentile in both image views at month four.

Treatment Emergent Adverse Events: Preliminary Data Announced in October 2025
The treatment emergent adverse event, or TEAE, summary presented in the graphic below from Study 207 covers TEAEs through four months of treatment with VDPHL01 8.5 mg, administered twice daily. No cardiac AEs or SAEs were reported. Overall, the complete list of TEAEs, which includes all AEs observed in study subjects independent of relatedness, suggests that VDPHL01 is generally well tolerated. No SAEs have been observed to date.
| | | | | |
Category | VDPHL018.5 mg BID N*2S, n (% of subjects) |
| Total TEAEs | 14 |
Subjects with any TEAE | 7(28.0%) |
| Subjects with any TEAE Leading to Treatment Discontinuation | 1 (4.0%) |
Subjects with any TEAE Leading to Study Discontinuation | 0 |
| |
| Severity | VDPHL01 8.5 mg BID N=14; n (% of Total AES) |
| Mild | 6 (42.9%) |
| Moderate | 8 (57.1%) |
| Severe | 0 |
| | | | | |
AEs by Type | VDPHL01 8.5 mg BID N=25; n {% of subjects) |
| Headache | 4 (16.0%) |
Upper Respiratory Tract Infection | 2 (4.0%) |
| Sinus Congestion | 1 (4.0%) |
| Dehydration | 1 (4.0%) |
| Heat Exhaustion | 1 (4.0%) |
| Asymptomatic Orthostatic Hypertension | 1 (4.0%) |
| Pain | 1 (4.0%) |
| Oedema Peripheral | 1 (4.0%) |
Libido Decreased | 1 (4.0%) |
| Malignant Melanoma | 1 (4.0%) |
TEAEs from Study 207 through four months of treatment are listed by type and severity.
Study 302: Our Phase 2/3 Registration-Directed Trial in Male Patients
We are currently conducting Study 302, the first of three registration-directed trials clinical trials of VDPHL01 in patients with PHL. Study 302 is a four-arm multicenter, randomized, double-blinded, placebo-controlled, Phase 2/3 clinical trial investigating the safety and efficacy of VDPHL01 8.5 mg in male patients. We are evaluating the effects of VDPHL01 8.5 mg once daily or VDPHL01 8.5 mg twice daily versus placebo and are randomizing patients in a 2:2:1:1 ratio across the four arms of the trial. Patients randomized to placebo at baseline will cross over into dosing with VDPHL01 8.5 mg once daily or VDPHL01 8.5 mg twice daily after six months on trial). This study is fully enrolled with a final enrollment of 519 subjects.
The co-primary endpoints of the trial are changes from baseline in non-vellus TAHC using digital image analysis and proportion of patients who achieve treatment benefit, defined as a score of “Improved” or “Much improved” on Item 1 of a proprietary questionnaire, at six months. The secondary endpoints include changes from baseline in non-vellus TAHC at Months 2 and 4, proportion of patients who achieve treatment benefit at Months 2 and 4, changes from baseline in non-vellus Target Area Hair Width, or TAHW, at Months 2, 4 and 6, and the proportion of patients who are satisfied with treatment, defined as achieving a response category of “A little satisfied,” “Moderately satisfied” or “Very satisfied” at Months 2, 4 and 6. Study 302 also includes a parallel Phase 2 component involving a psychometric analysis intended to further assess that the proprietary questionnaire used to measure the co-primary endpoint and one of the secondary endpoints is an appropriate, fit-for-purpose
instrument. We began enrolling Study 302 in November 2024 and expect to report topline data in the first half of 2026. The trial design for Study 302 is depicted below.
Study 304: Our Confirmatory Phase 3 Trial in Male Patients
We are also conducting Study 304, our confirmatory registration-directed Phase 3 clinical trial of VDPHL01 in male patients with PHL. Study 304 is a four-arm, multicenter, randomized, double-blinded, placebo-controlled, Phase 3 clinical trial investigating the safety and efficacy of VDPHL01 in male PHL patients. This trial also evaluates the effects of VDPHL01 8.5 mg once daily or VDPHL01 8.5 mg twice daily versus placebo and randomizes patients in a 2:2:1:1 ratio across the four arms of the trial (with the same placebo crossover design as in Study 302). We expect to enroll a total of approximately 498 subjects in this study. The primary endpoints of the trial are the same as in Study 302. Secondary endpoints include the same as those listed for Study 302. We began enrolling Study 304 in March 2025 and expect to report topline data in the second half of 2026. The trial design for Study 304 is depicted below.
Study 306: Our Registration-Directed Trial in Female Patients
Study 306 is our registration-directed clinical trial of VDPHL01 in women with PHL. The four-arm, multicenter, randomized, double-blinded, placebo-controlled, Phase 2/3 clinical trial is investigating the safety and efficacy of VDPHL01 in female PHL patients. We are evaluating the effects of VDPHL01 4.5 mg once daily or VDPHL01 4.5 mg twice daily versus placebo and are randomizing patients in a 2:2:1:1 ratio across the four arms of the trial (with the same placebo crossover design as in Study 302). We expect to enroll a total of approximately 552 subjects in this study. The primary endpoints of the trial are the same as those listed for Study 302. Other efficacy endpoints include the proportion of patients who are satisfied with treatment at Month 6, the proportion of patients by response category for every other question of a proprietary questionnaire at Month 6, and changes from baseline in non-vellus TAHW Month 6. Study 306 also includes a parallel Phase 2 component involving a psychometric analysis intended to further assess that the proprietary questionnaire used to measure the co-primary endpoint and one of the secondary endpoints is an appropriate, fit-for-purpose instrument. We began enrolling Study 306 in July 2025. The trial design for Study 306 is depicted below.
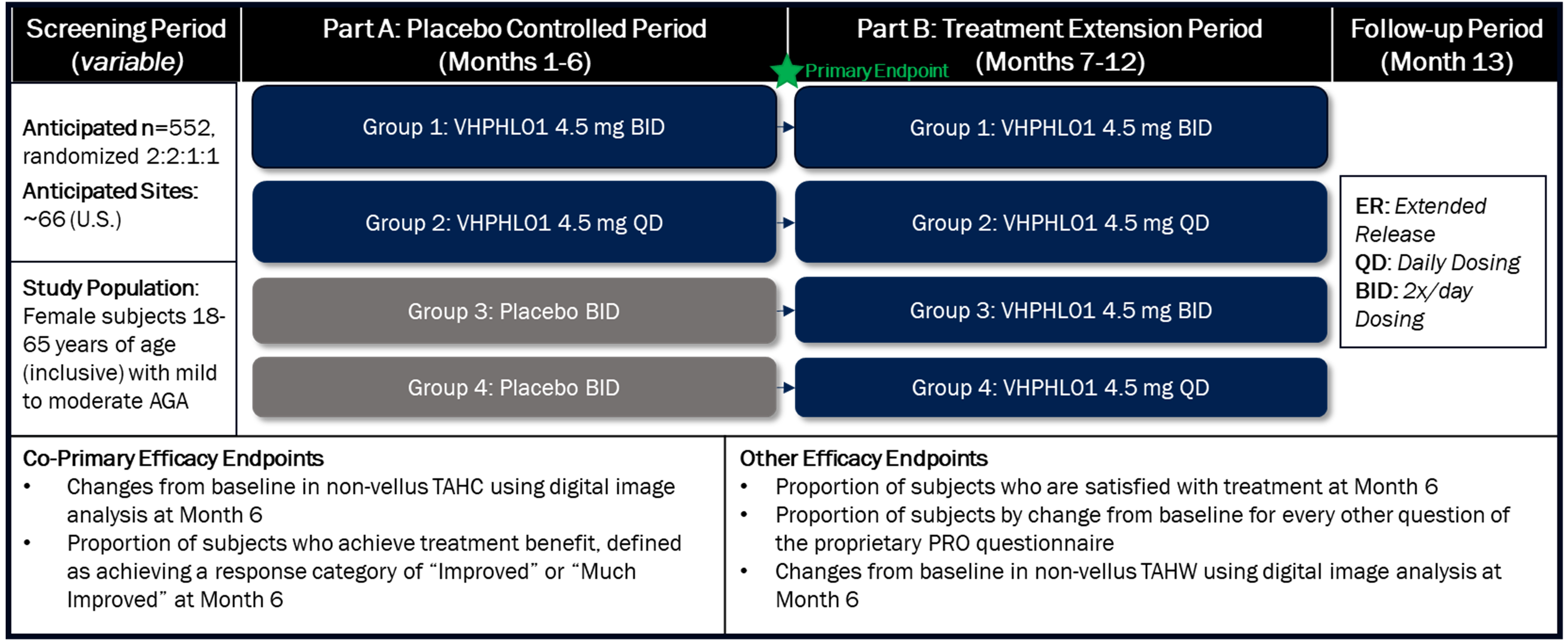
505(b)(2) Approval Pathway
Following the conclusion of our pivotal studies, we anticipate submitting VDPHL01 for approval under the 505 (b)(2) approval pathway. The benefit of this pathway is that it eliminates costly, time consuming and potentially duplicate studies on the already established elements of minoxidil but allows a period of regulatory exclusivity for VDPHL01, if approved. The typical regulatory market exclusivity period for a new product approved under the 505(b)(2) pathway that contains an active moiety that has been previously approved is 3 years. In addition to the 505(b)(2) regulatory exclusivity period, we are executing an intellectual property strategy that is intended to protect the novel innovations underlying VDPHL01 into at least 2043 (see “—Intellectual Property—VDPHL01 is protected by issued IP”).
There is an Attractive Potential Commercial Opportunity for an Approved Oral Therapeutic for PHL
Prevalence: A Widespread, Underserved Condition
PHL represents one of the most prevalent chronic dermatological conditions in the United States, affecting approximately 80 million men and women. PHL demonstrates a lifetime prevalence of nearly 50%, significantly higher than psoriasis (2–3% of individuals) or atopic dermatitis (approximately 10% of adults). As demonstrated
in the graphic below, PHL impacts more Americans than any other chronic dermatological condition, according to the American Academy of Dermatology:
Prevalence increases with age: approximately 50% of men and 40% of women show clinically significant PHL by age 50 in the United States. The condition can manifest as early as the late teens or early twenties and progressively worsens over time. It is estimated that the active treatment-seeking population for PHL was approximately double the size the size of the entire psoriasis population in the United States in 2024. The interplay of biological, genetic and hormonal drivers has contributed to PHL's widespread nature, yet effective treatment options have remained limited, creating substantial unmet medical need across a large patient population.
Quality of Life Impact: A Highly-Motivated Patient Population
PHL has significant, multidimensional effects on patients’ quality of life, including mental and emotional well-being. This substantial burden makes the target population heavily motivated to seek treatment, but many remain untreated given lack of attractive options. Timely intervention can profoundly and positively influence psychosocial functioning from an early stage, yet the absence of effective solutions underscores a critical gap in care.
Public and clinical interest in hair loss has accelerated, driven by heightened aesthetic awareness, increased media exposure and changing social norms. Since the beginning of 2024, over 100,000 news articles in the United States have addressed PHL. Social media engagement is substantial: hashtags including #hairloss, #hairthinning, #hairgrowth, #hairlosstreatment and #hairlosssolutions have been used over 13 million times on Instagram and TikTok. The PHL-focused sub-Reddit r/tressless has over 400,000 weekly visitors — four times the combined weekly visitors of r/Ozempic and r/Wegovy, two of the largest Glucagon-Like Peptide-1, or GLP-1, sub-Reddit communities. Proprietary social listening research conducted among approximately 700,000
conversations across X, Reddit and other digital forums from March 2024 to April 2025 revealed that only 6.5% of social posts expressed satisfaction with treatment — underscoring widespread dissatisfaction with current options.
Current Commercial Opportunity: Substantial Spending Despite Limited Efficacy
We estimate that the current commercial opportunity for PHL treatments for men and women in the United States is approximately $9 billion, based on publicly available industry data and patient surveys to determine the number of PHL patients using prescription and OTC therapies in the United States in 2024, our proprietary third-party market research of estimates of duration of therapy and our analysis of pricing for prescription and OTC treatments. The PHL commercial opportunity was estimated to be approximately $21 billion annually worldwide in 2021, and is projected to reach approximately $30 billion by 2028. Despite limited efficacy, the market exhibits strong growth across prescription and OTC categories.
Patient spending patterns demonstrate significant willingness to pay for treatment. According to a proprietary third-party market assessment in November 2024, which surveyed 410 PHL patients in the United States, over 50% of patients currently spend more than $40 per month on hair loss treatments, with 21% spending more than $80 per month, consistent with commercially available third-party sources.
Patients today cycle through existing products in a predominantly cash-pay market. It is estimated that approximately 3.4 million individuals in the United States have undergone hair transplant procedures in recent years, a market growing year over year, demonstrating willingness to pursue costly, intensive elective surgical procedures.
Unmet Need: High Dissatisfaction Creates Opportunity
Despite widespread usage and spending, existing treatments demonstrate poor patient satisfaction. A 2024 third-party survey found that only 9% of current patients were satisfied with available treatments, while 46% were actively seeking new options. We believe that due to lack of satisfactory options, the majority of interested and motivated patients are not currently treated.
We believe this dissatisfaction persists because PHL markets have historically been dominated by suboptimal treatments that remain largely stagnant, and patients seek safer, more effective solutions that current options fail to provide.
Precedent: Adjacent Markets Transformed by Innovation
Multiple analogous therapeutic categories demonstrate how innovation can transform markets dominated by inadequate legacy treatments. The PHL market today has similarities to the atopic dermatitis and psoriasis markets before the introduction of transformative therapies. Prior to the approval of Dupixent in 2017, atopic dermatitis patients cycled through topical corticosteroids and immunosuppressants with concerning safety profiles, resulting in sub-optimal treatment satisfaction despite AD affecting millions of patients. Similarly, before biologic therapies emerged in the early 2000s, psoriasis patients had few options beyond topical treatments and systemic agents with significant toxicity concerns. In both cases, large patient populations actively sought treatment suffered from chronic, quality-of-life-impairing conditions while existing treatments failed to meet patient needs, creating substantial latent demand that materialized rapidly once effective, tolerable therapies became available. We believe the PHL market exhibits these same characteristics: high prevalence, significant unmet need, widespread utilization despite poor satisfaction with current options, and demonstrated willingness to pay for treatment, suggesting similar potential for market transformation with an innovative therapeutic approach.
In recent years, large therapeutic categories within personal care, including treatments for wrinkles, erectile dysfunction and obesity, have been created or substantially expanded through rapid patient adoption of treatment options offering attractive value propositions. For example, the global weight loss pharmaceuticals market has grown over ten-fold from approximately $3 billion in 2020 to greater than $30 billion in 2024, while the U.S. erectile dysfunction market grew seven-fold within one month of Viagra’s launch. We believe that the U.S. facial injectables market, which was estimated to be approximately $9.5 billion in 2024, further supports the potential for rapid adoption and favorable adherence in a chronically managed aesthetic condition.
We believe these examples show that consumers in the United States consistently demonstrate high willingness to pay out-of-pocket for differentiated products addressing aesthetic and quality-of-life concerns. Importantly, PHL shares a critical characteristic with these successful categories: it is a chronic condition requiring long-term management, which drives durable commercial opportunities as evidenced by persistence and adherence trends in categories such as Botox for cosmetic use.
Our Potential Commercial Opportunity
We believe VDPHL01 could gain meaningful share of the existing $9 billion commercial opportunity for PHL treatments for men and women in the United States while catalyzing substantial expansion. Third-party studies have shown that a large percentage of PHL patients have not found existing treatment options sufficiently compelling to engage in treatment, representing significant latent demand.
We believe that multiple structural and behavioral factors support favorable market dynamics for an FDA-approved prescription therapy for PHL. These include: a large and expanding prevalence of the condition; the growing proportion of single adults within the general population, a demographic segment we believe is particularly motivated to pursue appearance-related treatments; increased social media engagement and accelerating public discourse surrounding hair loss; rapid expansion of DTC and telehealth-based marketing channels; growing consumer awareness and concern regarding hair health; and the increasing normalization and acceptance of prescription therapies as part of aesthetic self-care.
As the aesthetics market continues to grow, individuals remain active, career-oriented and aesthetically conscious for longer periods over their lifespans, we believe that effective pharmacologic options for PHL will become increasingly important to address both the cosmetic and psychosocial impact of the condition.
We believe demand for a differentiated pharmaceutical option is driven by the convergence of high prevalence, significant quality of life impairment, patient population motivated to seek treatment, lack of receptivity to existing treatments and substantial dissatisfaction among current patients — factors that together define a compelling commercial opportunity. In a proprietary commercial survey of 410 PHL patients in the United States:
n60% of patients reported viewing VDPHL01 as highly positively differentiated given safety and efficacy data to date;
nover 90% of patients reported willingness to seek out a health care provider for VDPHL01, if approved; and
nover 60% of patients indicated they are very likely to both ask a health care provider about and switch to or add VDPHL01 as a treatment, if approved.
Patient Segmentation and Addressable Commercial Opportunity
We estimate that of the approximately 80 million total PHL patient population in the United States, the total addressable patient population of this group is approximately 74 million patients, segmented into two primary categories: patients currently engaged in treatment (approximately 15 million) and patients not currently treating or other patients (approximately 59 million) based on proprietary third-party market research and publicly
available industry and market data. The below graphic depicts what we believe to be the total addressable commercial opportunities of the approximately 80 million total PHL patient population in the United States.
Patients Currently Treating: 15 Million
We estimate that the current U.S. commercial opportunity for PHL patients for men and women is valued at approximately $9 billion annually. However, in both third-party research and our proprietary research, many patients within this segment expressed dissatisfaction with their current options and were actively seeking new treatments. According to patient interviews and clinical experience, most patients cycle through multiple treatments with unsatisfactory results.
Current Prescription IR Oral Minoxidil and Finasteride Users
The number of PHL patients who are current prescription IR oral minoxidil and finasteride users in the United States was one million in 2025, and we assume that these patient refill those prescriptions six times per year. We believe patients currently treating their PHL under physician care with oral therapies represent the highest-probability early adopters of VDPHL01. These patients may find an oral, non-hormonal FDA-approved treatment with potentially superior efficacy attractive relative to existing prescription options. Therefore, adoption could be straightforward: patients can receive a new prescription at routine physician visits and fill it at their local pharmacy. In a proprietary commercial survey of 410 hair loss patients in the United States, 76% of current prescription medicine users indicated they would be very likely to ask their doctor about VDPHL01, if approved.
Patients currently using finasteride have expressed concerns regarding the hormonal mechanism that does not lend to intense hair growth and associated side effect profile, while those using off-label IR oral minoxidil face an efficacy ceiling, dose-limiting tolerability issues and uncertainty about using medications approved for other indications without formal PHL-specific efficacy and safety data.
Current OTC Topical Minoxidil Users
We estimate the number of PHL patients who are current OTC topical minoxidil users in the United States was approximately 4 million in 2025. We believe patients comfortable with minoxidil-based treatment will find VDPHL01’s potential for enhanced efficacy, faster onset of action and convenient oral dosing attractive relative to topical minoxidil's profile. The most common reason for topical minoxidil discontinuation is burdensome topical application, a concern directly addressed by VDPHL01’s oral administration. In a proprietary commercial survey of 410 PHL patients in the United States, 65% of OTC users indicated they would ask their doctor about VDPHL01, if approved.
Current Users of Other OTC Products
We estimate the number of PHL patients who are current users of other OTC products, including branded supplements, in the United States was 10 million in 2025. We believe an FDA-approved product with a robust clinical data package validating efficacy and safety claims could appeal to patients whose current OTC treatments are not backed by the same type of data to verify hair growth claims and product composition. DTC advertising aims to activate this population by creating awareness. However, our assumption for conversion to
VDPHL01 among this cohort is lower than other segments, as these patients are neither treating under physician care nor using minoxidil-based regimens and may be unaware of FDA-approved treatment options for PHL.
Patients Not Currently Treating and Other Patients: 59 Million
We believe a large portion of the remaining current PHL patients in the US are not currently receiving or taking treatment. This substantial patient population comprises of two distinct segments with different engagement patterns and motivations. In a proprietary commercial survey of 410 PHL patients in the United States, 37% of those not currently treating reported they would inquire about VDPHL01, if approved, and greater than 60% of those not currently treating indicated they may be open to new therapeutic options. Even low-single-digit percentage uptake within these segments would provide significant revenue opportunity due to segment size. Our estimates of this segment also include other individuals who may be taking treatments prescribed by telehealth platforms or who are using other surgical or LLLT devices that we do not have publicly available industry or market data for. We believe the differentiated VDPHL01 product profile would be attractive to this other segment due to the limitations of the current treatment landscape.
Treatment-Experienced Patients Currently Not Treating
These patients have previously engaged with PHL treatments but exited the treatment paradigm. Treatment discontinuation most commonly results from lack of effectiveness with available options. We believe VDPHL01's differentiated therapeutic profile could activate patient populations previously disengaged from treatment paradigms due to inadequate existing options.
This segment represents a particularly compelling opportunity, as these patients have demonstrated treatment-seeking behavior and willingness to pay, but found existing options insufficient to sustain engagement. Historical precedent from other therapeutic categories, like obesity, erectile dysfunction, smoking cessation and psoriasis, suggests that differentiated products can successfully reactivate previously disappointed patient populations. It is estimated that 19% of PHL patients have tried treatment but have discontinued and up to 86% of topical minoxidil treaters are believed to have tried but discontinued treatment.
Treatment-Naïve Patients
Treatment-naïve patients are not currently engaged in any therapy and may be refraining from treatment for various reasons beyond dissatisfaction with available options, including lack of awareness or the assumption that effective treatments do not exist as identified by our proprietary market research. These patients could be engaged through DTC advertising, social media engagement and physician education initiatives, though we believe meaningful penetration of this segment could develop over extended time periods as awareness builds and VDPHL01, if approved, establishes clinical reputation.
Prescription Demand and Growth Potential
We believe this segmentation analysis demonstrates both immediate commercial opportunity among currently treating patients and substantial long-term growth potential through market expansion. The 15 million patients currently treating, despite widespread dissatisfaction with available options, validate baseline demand and willingness to pay. Patients not currently treating represent significant latent demand that could be activated by a differentiated therapeutic profile, as demonstrated in analogous categories where innovation expanded markets substantially beyond pre-existing treatment populations.
VDPHL01, ER Oral Minoxidil Optimized for Hair Growth
VDPHL01 is our novel oral, ER formulation of minoxidil that is designed to maximize minoxidil’s impact on hair restoration. VDPHL01’s key innovation is its release profile that is designed to maximize the total amount of drug that can be delivered to follicles over either a 12 or 24-hour period without inciting spikes in Cmax of minoxidil known to induce changes in cardiac activity. We believe our efforts mark the first attempt to bring an
ER formulation of minoxidil to patients with these optimized PK and PD qualities that raise the ceiling of hair growth.
Characteristics of Existing PHL Solutions
1 Koperski JA, Orenberg EK, Wilkinson DI. Topical minoxidil therapy for androgenetic alopecia. A 30-month study. Arch Dermatol. 1987 Nov;123(11):1483-7.
2 Van Neste D et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br J Dermatol. 2000 Oct;143(4):804-10.
3 Penha MA et al. Oral Minoxidil vs Topical Minoxidil for Male Androgenetic Alopecia: A Randomized Clinical Trial. JAMA Dermatol. 2024 Jun 1;160(6):600-605.
4 Shapiro J et al. Comparative Efficacy of an Investigational Oral Minoxidil Extended-Release Tablet (VDPHL01) Versus Existing Minoxidil Formulations in Androgenetic Alopecia: A Blinded Retrospective IGA Analysis. Abstract presented at: European Academy of Dermatology and Venereology (EADV) Congress; Sep 19, 2025; Paris, France.
5 Kaufman KD et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998 Oct;39(4 Pt 1):578-89.
6 Olsen EA et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002 Sep;47(3):377-85.
7 Bergfeld W et al. A Phase III, Multicenter, Parallel-Design Clinical Trial to Compare the Efficacy and Safety of 5% Minoxidil Foam Versus Vehicle in Women With Female Pattern Hair Loss. J Drugs Dermatol. 2016 Jul 1;15(7):874-81.
8 Medicines and Healthcare products Regulatory Agency. Drug Safety Update volume 17, issue 9: April 2024: 1
9 European Medicines Agency. Measures to minimise risk of suicidal thoughts with finasteride and dutasteride medicines. EMA/202053/2025. 22 August 2025.
10 Loniten Minoxidil Tablets, USP. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/018154s026lbl.pdf
VDPHL01 Commercialization Strategy
To unlock the full commercial potential of VDPHL01, if approved, we are preparing a multi-channel commercial strategy designed to maximize reach and patient access. Our approach is informed by substantial market research indicating that only 9% of U.S. PHL patients in 2024 were satisfied with current therapies and that a majority of healthcare providers in third-party studies cite consistency, intensity and speed of effect, safety, tolerability and convenience as key areas of dissatisfaction. This model will combine a dedicated field force, peer-to-peer education and omnichannel outreach to establish clinical confidence in VDPHL01’s differentiated profile. The key elements to our commercial strategy will include:
nConcentrated Healthcare Provider Targeting Including Physician Outreach and Education
nPatient Identification and Segmentation
nDTC Advertising and Social Media Engagement
nTelehealth Integration
nProduct Distribution and Customer Support
nWillingness to Pay in a Cash Pay Market
Concentrated Healthcare Provider Targeting Including Physician Outreach and Education
The PHL prescription market is highly concentrated, with approximately 7,000 healthcare providers writing 70% of all prescriptions to PHL patients. We intend to focus our initial direct physician outreach and education efforts initially on these high-volume dermatology and primary care physician, or PCP, practices. These physicians constitute the leading source of information for PHL patients and counsel them in making treatment decisions and therefore it will be important to educate them about VDPHL01’s differentiated profile. As the group of physicians most familiar with PHL treatment, they will likely be the most comfortable acting as early adopters of VDPHL01, if approved. In a proprietary commercial survey of 150 health care providers:
napproximately 70% viewed VDPHL01 as highly positively differentiated, given safety and efficacy data to date;
ngreater than 70% indicated they would be “very likely” likely to prescribe VDPHL01 to male and female patients, if approved; and
ngreater than 40% indicated willingness switch or add VDPHL01 as a treatment for PHL, if approved.
For prescribers that fall into the category of moderate-volume PCPs and the broader prescriber universe, we will plan to target these categories of physicians via less frequent field sales force in-person education, virtual sales representative engagement, digital engagement and marketing, non-personal promotion, medical education programs and omnichannel engagement strategies. The number of unique prescribers of IR oral minoxidil has increased from approximately 18,000 in 2022 to approximately 32,000 in 2023 and approximately 46,000 in 2024. We believe that a 100+ person field force can effectively cover approximately 11,000 unique IR oral minoxidil prescribers, which we believe would cover over 80% of existing IR oral minoxidil prescriptions.
Patient Identification and Segmentation
Patient identification and segmentation will be foundational to our commercial strategy for VDPHL01. PHL affects approximately 50 million men and approximately 30 million women in the United States, encompassing a wide range of treatment experiences, motivations and barriers to care. To effectively engage this diverse population, we will develop a data-driven segmentation framework to classify potential patients based on distinct behavioral drivers and varying levels of awareness and willingness to pursue prescription treatment. Across these groups, patients are primarily motivated by the desire for greater efficacy, faster and more visible results, improved safety profiles — particularly the absence of cardiac, hormonal, or sexual side effects — and the credibility of a product backed by clinical data. These motivations are often shaped by dissatisfaction with prior therapies, emotional distress related to hair loss and a strong interest in convenient, trustworthy solutions. We plan to tailor messaging and engagement efforts to these drivers, with the goal of identifying and activating patients most likely to be long-term beneficiaries of VDPHL01’s differentiated profile.
DTC Advertising and Social Media Engagement
To drive broad awareness and accelerate adoption of VDPHL01, if approved, as potentially the only FDA-approved oral minoxidil treatment for PHL in male patients and female patients, we will plan to deploy a comprehensive, cost-efficient DTC, advertising strategy including advertising across social media platforms, search engines and programmatic display advertising targeting demographics most affected by PHL. These methods of advertising have emerged as key sources of treatment information among patients affected by PHL. In a proprietary commercial survey of 410 hair loss patients identified that lack of awareness of prescription treatments as the second most common reason for not seeking prescription hair loss treatment among current OTC users, further supporting importance of patient activation via DTC marketing. We believe this approach is supported by the demonstrated success of our digital outreach during clinical trial recruitment, where approximately 50% of male patients were enrolled through a targeted social media strategy. The 302 Study was enrolled approximately two times faster than the most recent non-Veradermics Phase 3 clinical trial in male PHL. Female engagement with trial recruitment advertising has been even greater than male engagement with a higher average number of daily website submissions generated by the female campaign than by the male campaign. By integrating social media into a broader multi-channel infrastructure, we may be able to reach
patients at scale, particularly those who are engaged with digital platforms, treatment-experienced and actively seeking alternatives to current options. The cash-pay nature of our plans would further enable direct engagement without reimbursement friction, which we believe positions our DTC strategy to convert awareness into action across a broad spectrum of patient segments.
Telehealth Integration
The rapid expansion of telehealth platforms has transformed how patients access both prescription and OTC treatments for aesthetic, dermatologic and lifestyle conditions, including hair loss. We estimate that there are approximately 5.2 million subscribers across existing telehealth platforms across all indications. These platforms offer a seamless experience by integrating virtual consultations with streamlined prescribing and fulfillment, making them particularly well-suited for conditions where convenience, privacy and accessibility are paramount. We expect that VDPHL01, if approved, could align well with this model as a user-friendly component of our patient access strategy given its targeted profile of favorable tolerability and efficacy, oral administration and appeal to patients seeking effective, non-invasive solutions. Hair loss diagnosis and treatment is already an established telehealth vertical for many platforms, which we believe would make integration of VDPHL01 less cumbersome. We believe VDPHL01, if approved, could entice established telehealth players to include it on formulary as its profile may drive additional retention should its target profile be achieved, and that VDPHL01 could prolong customer retention, which is a primary measure for telehealth companies seeking to have product sales recover customer acquisition costs. While we have not established any formal partnerships, we are evaluating the potential of telehealth as a key channel for expanding patient access and driving adoption of VDPHL01, if approved. By enabling patients to initiate treatment without the friction of traditional in-office and pharmacy visits, telehealth may be especially effective in reaching the large population of untreated individuals and those accustomed to managing aesthetic conditions through digital-first experiences. We believe VDPHL01 could serve as a high-value offering within telehealth ecosystems, attracting new users and supporting longer-term engagement through its chronic use profile and differentiated therapeutic benefits.
Product Distribution and Customer Support
To maximize patient access and convenience, we plan to maintain inventories of VDPHL01 sufficient to ensure broad product availability, if approved, and work to establish distribution channels that encompass traditional and specialty pharmacy, including major retail chains, telehealth channels, direct mail and provider dispensing. Additionally, we plan to provide 24/7 customer service to respond to patient medical inquiries and provide general assistance. We believe this multi-channel approach will ensure that patients can access VDPHL01 through their preferred channel, whether that's their existing dermatologist, a new telehealth consultation, or their local pharmacy.
Willingness to Pay
Given the aesthetic and quality-of-life nature of PHL, we expect VDPHL01, if approved, to be offered to patients as a cash-pay product, consistent with other cash-pay markets such as botulinum toxins for wrinkle reduction. Cash-pay offers key advantages over traditional insurance-based reimbursement, including the ability to bypass payer negotiations, prior authorization requirements and reimbursement delays. Additionally, the cash-pay model potentially provides insulation from potential pricing pressures associated with evolving healthcare policy, such as the IRA that authorize CMS to negotiate drug prices with pharmaceutical companies for some drugs covered under Medicare Part D and Part B or potential future “most favored nation” frameworks. This approach supports a more direct and durable commercial relationship with patients and aligns with established consumer behavior in the aesthetic treatment market.
Intellectual Property
We strive to protect and enhance proprietary technology, inventions, improvements and other rights that are commercially important to our business, including seeking, maintaining and defending patent rights, trademark rights and trade secrets. With respect to patents, our policy is to seek to protect our proprietary position by, among other methods, filing patent applications in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions, improvements and product candidates that are important to the development and implementation of our business. We additionally rely on data exclusivity and market exclusivity. Our commercial success will depend in part on our ability to obtain and maintain patents and other proprietary protection for our technology, inventions and improvements; to preserve the confidentiality of
our trade secrets; to maintain our licenses to use intellectual property owned by third parties; to defend and enforce our proprietary rights, including our patents; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third parties.
VDPHL01 is protected by issued IP
In addition to the regulatory exclusivity period afforded by the 505(b)(2) pathway, we are also constructing an extensive intellectual property portfolio designed to protect the innovations of VDPHL01. As of December 2025, the VDPHL01 intellectual property portfolio includes 2 U.S. issued patents, 1 U.S. allowed patent and more than 60 U.S. pending applications. The issued and allowed patents are method of use patents that will expire in 2043. In jurisdictions outside of the U.S., we have 14 VDPHL01 pending patent applications in Australia, Brazil, Canada, China, European Patent Organization, Hong Kong, Israel, Japan, Malaysia, Mexico, New Zealand, Republic of Korea, Singapore, and Taiwan. The foreign patents, if issued, are expected to expire between 2043 and 2045. Neither we, nor any other company, owns or has rights to composition of matter claims covering the active ingredient minoxidil. Current VDPHL01 patent applications include claims directed to three inventive concepts: 1) composition of the tablet and related manufacturing, 2) methods of use and clinical data and 3) PKs. The construction of our intellectual property portfolio is based on successful strategies utilized by other biotechnology companies to protect similar novel innovations that have been generated from established compounds. We intend to continue to execute our intellectual property strategy, which emphasizes building a robust portfolio of Orange Book-listable patents to protect our inventive concepts.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on intellectual property. While we believe our deep scientific knowledge of dermatology paired with our differentiated lead product candidate, VDPHL01, provides a strong competitive advantage, we face competition from many different sources, including pharmaceutical and biotechnology companies, generic drug companies, compounding pharmacies and consumer health companies as well as from academic institutions, governmental agencies and public and private research institutions.
Our competitors may have significantly greater financial resources, established presence in the market and expertise in research and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and reimbursement and marketing approved products than we do. They may also compete in recruiting and retaining qualified scientific, sales, marketing and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for, our product candidates. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Our commercial opportunity can be reduced or eliminated if competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Competitors also may obtain FDA or other regulatory approval for their products more rapidly or earlier than us, which could result in our competitors establishing a strong market position before we are able to enter the market. Additionally, technologies developed by our competitors may render our potential product candidates uneconomical or obsolete and we may not be successful in marketing our product candidates against competitors.
We are not aware of any competitors developing ER oral minoxidil for the treatment of PHL. Existing treatment options for PHL include oral finasteride and topical minoxidil, off label IR oral minoxidil, low light laser therapy and fractional laser non-ablative therapy as well as non-FDA approved non-prescription options such as “nutraceutical” supplements and shampoos. There are several product candidates in clinical development which could potentially pose a direct competitive threat to VDPHL01 in the future for the treatment of PHL. To our knowledge, the only other late-stage product candidate that is currently under evaluation in a Phase 3 study in the U.S. for the treatment of PHL is Breezula, a topical androgen receptor antagonist by Cosmo Pharmaceuticals NV. We are also aware of other product candidates in clinical development, including: KX-826, a topical androgen receptor antagonist by Kintor Pharmaceutical Limited, PP405, a topical mitochondrial pyruvate carrier inhibitor by Pelage Pharmaceuticals, Inc., TDM-105795, topical thyromimetic by TechnoDerma Medicines Inc. and ET-02, a topical treatment of undisclosed mechanism by Eirion Therapeutics, Inc. We are also aware of
other companies with programs for hair loss, including Hope Medicine Inc, Samson Clinical Pty Ltd, Amplifica Holdings Group Inc., Dermaliq Therapeutics, Inc. and Absci Corporation.
Our Pipeline
Our goal is to develop a focused portfolio of aesthetic dermatology product candidates targeting high-prevalence dermatologic conditions, with potential selective development of medical dermatology product candidates. We are advancing our lead product candidate, VDPHL01, in clinical trials as depicted below.
Additional Pipeline Assets
Beyond VDPHL01, we have created a portfolio utilizing our real-world experience as dermatologists to generate compelling pipeline assets. We are not currently advancing these other assets but we maintain worldwide rights and may choose to advance them in the future, subject to the availability of additional capital and strategic priorities or we may evaluate strategic collaborations to maximize the value of these other assets.
nVDMN: VDMN is a novel, injection-free product candidate for the treatment of common warts in the form of a dissolvable microarray patch that incorporates candida antigen extract. The patch contains an FDA-approved immunologic diagnostic (skin test) that has also been used off-label address treatment-resistant warts for decades and we believe is a patient-friendly approach.
nVDAA: VDAA is our once-monthly proprietary topical formulation of squaric acid dibutyl ester, a locally acting treatment for AA, a chronic autoimmune disease characterized by an attack on hair follicle by immune T-Cells that leads to the cessation of hair production. VDAA is a single-use applicator designed to deliver a topical treatment for AA and has the potential to become the first FDA-approved topical therapy, positioning it as a potential first-line option for patients with mild-to-moderate disease.
nVDMC: VDMC is a novel, direct acting topical antiviral gel for the treatment of molluscum contagiosum, or MC, a viral skin infection. VDMC is designed to directly target a key, virus-specific protein that mediates viral replication.
Manufacturing and Supply
We do not own or operate, and have no plans to establish, any internal manufacturing facilities. We currently rely and expect to continue to rely on third-party CMOs for the manufacture of our clinical supply requirements, including drug substances and drug products, and label and packaging for our clinical trials, as well as for commercial production of any product candidates that are approved. We employ internal resources to manage our CMO relationships and have entered into an agreement with a CMO for the commercial production of VDPHL01, if approved.
We believe that we will be able to contract with other CMOs to manufacture drug substances if our existing sources of drug substances were no longer available to us or with sufficient capacity, but drug substance capacity may not be available from other CMOs on acceptable terms, on the timeframe that our business would require, or at all.
Any products to be used in clinical trials and any approved product that we may commercialize will need to be manufactured in facilities, and by processes, that comply with the FDA’s cGMP requirements and comparable requirements of the regulatory agencies of other jurisdictions in which we are seeking approval.
We believe that VDPHL01 can be manufactured in reliable and reproducible chemical processes from readily available starting materials. We believe that our manufacturing processes are amenable to scale-up and will not require unusual or expensive equipment.
Agreements with Therapeutics, Inc.
Master Services Agreement
In September 2020, we entered into a Master Services Agreement, or the MSA, with Therapeutics, Inc, or TI. In accordance with the MSA and related work orders, TI provides clinical trial management, regulatory affairs activities, program support and other CRO services for the Company. TI provides services supporting the Company’s product candidates, including VDPHL01, for which the Company has agreed to pay TI an aggregate of approximately $95.0 million as of September 30, 2025 through the completion of our Phase 3 clinical trials of VDPHL01 and subsequent NDA filing, of which we have paid approximately $55.0 million as of September 30, 2025.
The MSA had an initial term of three years and automatically renews for additional one-year terms. The Company may terminate the MSA with 90 days’ prior written notice. Either party may terminate a work order for material breach (subject to a cure period) and the Company may terminate any work order for any reason on 90 days’ written notice, subject to payment of fees earned and expenses payable, in addition to a termination fee equal to a low-double-digit percentage of the remaining work order budget. The MSA contains customary provisions regarding confidentiality, intellectual property ownership and non-solicitation of personnel.
Collaboration Agreement
In September 2020, we entered into a Collaboration Agreement, or the Collaboration Agreement, with TI. Pursuant to the Collaboration Agreement, we issued TI an unsecured convertible promissory note with a principal amount of approximately $476,100 to evidence our obligation to pay TI certain program management fees pursuant to Work Order Number One of the MSA. On September 22, 2021, the unsecured convertible promissory note was converted into 238,008 shares of our Series A‑2 Preferred Stock. As consideration for the unsecured convertible promissory note, the Company agreed to make TI the exclusive provider of clinical trial management, regulatory affairs activities (including acting as the Company’s designated agent with the FDA), program support and other CRO services for the Company through the completion of Phase 2 of VDMN (or, if later, the first Veradermics product to reach completion of Phase 2). We have made no further payments to TI in connection with the Collaboration Agreement. We also granted TI with certain governance, including board observer rights, and information rights for so long as TI holds any of our securities.
The Collaboration Agreement requires that TI provide services at commercially reasonable rates consistent with its standard fee schedule, with a rolling low single-digit percent annual increases and on other commercially reasonable terms and conditions.
We may terminate TI’s exclusive rights to provide clinical trial management, regulatory affairs activities (including acting as the Company’ designated agent with the FDA), program support, and other CRO services for the Company at any time by paying mid-six figure liquidated damage amount to TI, subject to reduction for each in-human clinical study under an IND that TI performs and completes.
Government Regulation
The research, development, testing, manufacture, quality control, packaging, labeling, storage, record-keeping, distribution, import, export, promotion, advertising, marketing, sale, pricing and reimbursement of drug products
are extensively regulated by governmental authorities in the United States and other countries. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory requirements, both pre-approval and post-approval, require the expenditure of substantial time and financial resources. The regulatory requirements applicable to drug product development, approval and marketing are subject to change, and regulations and administrative guidance often are revised or reinterpreted by the agencies in ways that may have a significant impact on our business.
FDA Regulation
The FDA and other U.S. regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs such as those we are developing. We, along with our vendors, collaboration partners, clinical research organizations, or CROs, clinical trial investigators, and CMOs, will be required to navigate the various preclinical, clinical, manufacturing and commercial approval requirements of the FDA and of any governing regulatory agencies of the countries in which we wish to conduct studies or seek approval of our product candidates. The process of obtaining regulatory approvals of drugs and ensuring subsequent compliance with appropriate United States federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable regulatory requirements at any time during the product development process or post-approval may subject an applicant to delays in development or approval, as well as administrative and judicial sanctions.
In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. Drugs products are also subject to other federal, state and local statutes and regulations.
Our product candidates must be approved for therapeutic indications by the FDA before they may be marketed in the United States. For drug product candidates regulated under the FDCA, FDA must approve an NDA. The process generally involves the following:
ncompletion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with Good Laboratory Practice, or GLP, requirements;
nsubmission to the FDA of an IND application which must become effective before clinical trials may begin and must be updated annually and when certain changes are made;
napproval by an IRB or independent ethics committee at each clinical trial site before each trial may be initiated;
nperformance of adequate and well-controlled clinical trials in accordance with GCP requirements and other clinical trial-related regulations to establish the safety and efficacy of the investigational product for each proposed indication;
npreparation and submission to the FDA of an NDA;
npayment of user fees for FDA review of the NDA;
na determination by the FDA within 60 days of its receipt of an NDA to file the application for review;
nsatisfactory completion of one or more FDA pre-approval inspections of the manufacturing facility or facilities where the product will be produced to assess compliance with cGMPs to assure that the facilities, methods and controls are adequate to ensure and preserve the drug product’s identity, strength, quality and purity;
nsatisfactory completion of any FDA audits of the clinical trial sites that generated the data in support of the NDA; and
nFDA review and approval of the NDA, including, where applicable, consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the drug in the United States.
Preclinical and Clinical Trials
Before testing any drug in humans, the product candidate must undergo rigorous preclinical testing. Preclinical studies include laboratory evaluations of chemistry, formulation and stability, as well as in vitro and animal
studies to assess safety and in some cases to establish the rationale for therapeutic use. The conduct of preclinical studies is subject to federal and state regulations and requirements, including GLP requirements for safety and toxicology studies. In the United States, the results of the preclinical studies, together with manufacturing information and analytical data must be submitted to the FDA as part of an IND.
An IND is a request for authorization from the FDA to administer an investigational product to humans, and must become effective before clinical trials may begin. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, PKs, pharmacology, and PD characteristics of the product; chemistry, manufacturing and controls information; and any available human data or literature to support the use of the investigational product. In the United States, the IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks, and imposes a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Some long-term preclinical testing may continue after the IND is submitted. Accordingly, submission of an IND may or may not result in FDA authorization to begin a trial.
The clinical stage of development involves the administration of the product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCP requirements, which include the requirements that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters and criteria to be used in monitoring safety and evaluating effectiveness. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRB, either centrally or at each institution at which the clinical trial will be conducted, to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable related to the anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative, and must monitor the clinical trial until completed.
The FDA may, at any time during the initial 30-day IND review period or while clinical trials are ongoing under the IND, impose a partial or complete clinical hold based on concerns for patient safety and/or noncompliance with regulatory requirements. This order issued by the FDA would delay a proposed clinical study or cause suspension of an ongoing study until all outstanding concerns have been adequately addressed, and the FDA has notified the company that investigations may proceed. Imposition of a clinical hold could cause significant delays or difficulties in completing planned clinical studies in a timely manner. In addition, the IRB, or the sponsor may suspend or discontinue a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trials to public registries. In the United States, information about applicable clinical trials, including clinical trials results, must be submitted within specific timeframes for publication on the www.clinicaltrials.gov website.
A sponsor who wishes to conduct a clinical trial outside of the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. The FDA will accept a well-designed and well-conducted foreign clinical study not conducted under an IND if the study was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection if deemed necessary.
Clinical trials to evaluate therapeutic indications to support NDAs for marketing approval are typically conducted in three sequential phases, which may overlap. For example, a clinical trial designated as a Phase 2/3 clinical trial may be a registration directed Phase 3 trial that includes a Phase 2 component.
nPhase 1—Phase 1 clinical trials involve initial introduction of the investigational product in a limited population of healthy human volunteers or patients with the target disease or condition. These studies are typically designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, evaluate the side effects associated with increasing doses, and, if possible, to gain early evidence of effectiveness.
nPhase 2—Phase 2 clinical trials typically involve administration of the investigational product to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
nPhase 3—Phase 3 clinical trials typically involve administration of the investigational product to an expanded patient population to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA.
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and are commonly intended to generate additional safety data regarding use of the product in a clinical setting. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in withdrawal of approval for products.
Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators 15 days after the trial sponsor determines the information qualifies for reporting for serious and unexpected suspected AEs, findings from other studies or animal or in vitro testing that suggest a significant risk for human participants exposed to the drug and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must also notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction as soon as possible but in no case later than seven calendar days after the sponsor’s initial receipt of the information.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the drug characteristics of the product candidate and finalize a process for manufacturing the drug product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and manufacturers must develop, among other things, methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life and to identify appropriate storage conditions for the product candidate.
FDA Marketing Application Review Process
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the United States FDA as part of an NDA requesting approval to market the product for one or more indications. An NDA is a request for approval to market a new drug for one or more specified indications. The NDA pathway includes both a traditional pathway, referred to as the 505(b)(1) pathway, and a streamlined pathway, referred to as the 505(b)(2) pathway. The 505(b)(1) pathway applies for new drugs with novel active ingredients, and requires sponsors to submit a complete set of preclinical and clinical data to demonstrate the safety and efficacy of the drug. The 505(b)(2) pathway, however, is designed to expedite approvals for product candidates that are modifications or new formulations of
previously approved drugs that allows sponsors to rely upon FDA’s prior conclusions of safety or efficacy to establish certain baseline characteristics of a product candidate while requiring a sponsor to “bridge” to trials for approval similar to a standard NDA. Any NDA must include all relevant data available from pertinent pre-clinical studies and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational drug, to the satisfaction of the FDA. FDA approval of an NDA must be obtained before a drug may be marketed in the United States.
In addition, under the Pediatric Research Equity Act, or PREA, certain NDAs and certain supplements to an NDA must contain data to assess the safety and effectiveness of the drug product candidate for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. An initial Pediatric Study Plan must be submitted within 60 days after an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA.
In the United States, the FDA reviews all submitted NDAs to ensure they are sufficiently complete to permit substantive review before it accepts them for filing, and may request additional information rather than accepting the NDA for filing. The FDA makes a decision on accepting an NDA for filing within 60 days of receipt, and such decision could include a refusal to file by the FDA. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the application. The FDA reviews an NDA to determine, among other things, whether the product is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards, including cGMP requirements, designed to assure and preserve the product’s identity, strength, quality and purity. Under the goals and polices agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA targets ten months, from the filing date, in which to complete its initial review of an original NDA and respond to the applicant, and six months from the filing date of an original NDA filed for priority review. The FDA does not always meet its PDUFA goal dates for standard or priority NDAs, and the review process is often extended by FDA requests for additional information or clarification.
Further, under PDUFA, as amended, each NDA must be accompanied by a user fee, and the sponsor of an approved NDA is also subject to an annual program fee. FDA adjusts the PDUFA user fees on an annual basis. Fee waivers or reductions may be available in certain circumstances, including a waiver of the application fee for the first application filed by a small business.
The FDA may refer an application for a drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, which reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP and other requirements and the integrity of the clinical data submitted to the FDA.
After evaluating the application and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a Complete Response Letter. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. A Complete Response Letter will usually describe all of the deficiencies that the FDA has identified in the NDA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the Complete Response Letter without first conducting required inspections, testing submitted product lots, and/
or reviewing proposed labeling. In issuing the Complete Response Letter, the FDA may recommend actions that the applicant might take to place the NDA in condition for approval, including requests for additional information or clarification. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications.
Even if the FDA approves a product, depending on the specific risk(s) to be addressed, the FDA may limit the approved indications for use of the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a product’s safety or efficacy after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patent registries. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Requirements for Drugs in the United States
In the United States, drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, reporting of adverse experiences with the product, complying with promotion and advertising requirements, which include restrictions on promoting products for unapproved uses or patient populations (known as “off-label use”) and limitations on industry-sponsored scientific and educational activities. Although physicians may prescribe approved products for off-label uses, manufacturers may not market or promote such uses. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, including not only by Company employees but also by agents of the Company or those speaking on the Company’s behalf, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties, including liabilities under the False Claims Act where products carry reimbursement under federal health care programs. Promotional materials for approved drugs must be submitted to the FDA in conjunction with their first use or first publication. Further, if there are any modifications to the product, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new or supplemental NDA, which may require the development of additional data or preclinical studies and clinical trials.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-market testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and their contract manufacturers of approved products are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP, which impose certain procedural and documentation requirements upon us and our CMOs. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance Failure to comply with statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, product seizures,
injunctions, civil penalties or criminal prosecution. There is also a continuing, annual program fee for any marketed product.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, requirements for post-market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a REMS. Other potential consequences include, among other things:
nrestrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
nsafety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings or other safety information about the product;
nmandated modification of promotional materials and labeling and issuance of corrective information;
nfines, warning letters, or untitled letters;
nholds on clinical trials;
nrefusal of the FDA to approve applications or supplements to approved applications, or suspension or revocation of product approvals;
nproduct recall, seizure or detention, or refusal to permit the import or export of products;
ninjunctions or the imposition of civil or criminal penalties; and
nconsent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs.
United States Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval, some of our United States patents may be eligible for limited patent term extension under the Hatch-Waxman Act. The Hatch-Waxman Act permits restoration of the patent term of up to five years as compensation for patent term lost during the FDA regulatory review process. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended. The patent-term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA plus the time between the submission date of an NDA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Regulatory exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement.
The FDCA also provides three years of exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent for other conditions of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to
conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
In addition, drugs can also obtain pediatric exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study.
Other U.S. Regulatory Matters
Manufacturing, sales, promotion, and other activities involving product candidates following product approval, where applicable, or commercialization are also subject to regulation by numerous regulatory authorities in the U.S. in addition to the FDA, which may include CMS other divisions of the Department of Health and Human Services, or HHS, the DOJ, the Drug Enforcement Administration, the Consumer Product Safety Commission, the FTC, the Occupational Safety & Health Administration, the Environmental Protection Agency, and state and local governments and governmental agencies.
Other U.S. Healthcare Laws
Pharmaceutical manufacturers and their products are subject to extensive regulation that constrain their business operations and/or financial or other interactions with third parties. These laws include the following, some of which apply to pharmaceutical companies only after the companies have marketed products or have marketed products that are covered by government health benefit programs or private health care insurance.
nthe federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid;
nfederal civil and criminal false claims laws, including the federal False Claims Act, which can be enforced through civil whistleblower, or qui tam actions, as well as civil monetary penalty laws can impose criminal and civil penalties, assessment, and exclusion from participation for various forms of fraud and abuse involving the federal healthcare programs, such as Medicare and Medicaid;
nHIPAA, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also establishes requirements related to the privacy, security, and transmission of individually identifiable health information which apply to many healthcare providers, physicians, and third-party payers with whom we interact;
nthe federal FDCA, which, among other things, strictly regulates drug product and medical device marketing, prohibits manufacturers from marketing such products for off-label use, and regulates the distribution of samples;
nfederal laws that require pharmaceutical manufacturers to calculate, report, and certify certain complex product prices and other data to the government or provide certain discounts or rebates to government authorities or private entities, often as a condition of reimbursement under government healthcare programs, which data may be used in the calculation of reimbursement and/or discounts on approved products;
nthe so-called federal “sunshine law,” or Open Payments, which requires manufacturers of drugs, devices, biologics, and medical supplies covered under certain government health benefit programs to report to CMS information related to payments and other transfers of value to teaching hospitals, physicians, and other healthcare practitioners, as well as ownership and investment interests held by physicians and their immediate family members;
nfederal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
nstate laws and regulations analogous to federal laws, including anti-kickback or related laws, some of which apply regardless of whether products or services are covered by government health benefit programs or private insurance, false claims laws, laws prohibiting consumer protection and unfair competition laws, and laws governing privacy, security, and breaches of health (and other personal) information in certain
circumstances, many of which differ in significant ways from federal laws and across states and are often not preempted by federal law, thus complicating compliance efforts; and
nstate laws that require pharmaceutical companies to comply with specific compliance standards, restrict financial interactions between pharmaceutical companies and healthcare providers, report drug product pricing information, financial interactions with health care providers, or marketing expenditures, and/or require the registration of pharmaceutical sales representatives.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage, and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
Violations of these laws may result in significant penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, or the imposition of restrictions on operation.
U.S. Market Acceptance
Within the U.S., commercial success for a prescription drug product often depends in significant part on adequate coverage and reimbursement from third-party payors, including government healthcare programs, such as Medicare and Medicaid programs, and private entities, such as managed care organizations and private health insurers. Prescription drug products used for cosmetic purposes will generally not be covered by third-party payors because they are not considered medically necessary. As a result, commercial success for such products depends instead on market acceptance by health care practitioners who may prescribe such products and patients who may use and pay for such products. Such patients must be willing to pay for the products entirely “out-of-pocket.” Various factors may affect the market acceptance by health care practitioners and patients, including the cost of the product, characteristics and effectiveness of the product, ease of access to such product, and the cost, availability and effectiveness of competitor therapies, including OTC drug products. Prescription drugs used for medical purposes may also encounter similar challenges in achieving market acceptance if there is limited or no coverage by third-party payors or significant reliance upon cash-pay offerings to patients.
For prescription drugs used for medical purposes, no uniform policy of coverage and reimbursement for drug products exists among third-party payors and coverage and reimbursement for drug products can differ significantly from payor to payor. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved.
Third-party payors are increasingly challenging the prices charged, examining the medical necessity, reviewing the cost-effectiveness of medical products and services, and imposing controls to manage costs. To obtain or maintain coverage and reimbursement for any approved drug product, a pharmaceutical manufacturer may need to conduct expensive pharmacoeconomic studies or otherwise provide evidence to demonstrate to demonstrate the medical necessity and cost-effectiveness of our product candidates, if approved. These studies will be in addition to the studies required to obtain or maintain regulatory approvals. If third-party payors do not consider a product to be cost-effective compared to other available therapies, the payors may not cover the product or, if they do, the level of payment may not be sufficient to allow sale of a product at a profit.
Even if third-party payors provide some coverage, the third-party payors may impose limits on the coverage or controls to manage utilization of products. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication and can exclude drugs from their formularies in favor of competitor drugs or alternative treatments. Payors may also impose step edits that require patients to try alternative, including generic, treatments before authorizing payment for our product candidates, limit the types of diagnoses for which coverage will be provided, require pre-approval (known as “prior authorization”) for coverage of a prescription for each patient (to allow the payor to assess medical necessity), or impose a moratorium on coverage for products while the payor makes a coverage decision.
Moreover, a third-party payor’s decision to provide coverage for a product does not mean that an adequate reimbursement rate will be approved. We may be required to provide mandatory discounts or rebates to certain purchasers to obtain coverage under federal healthcare programs or to sell products to government purchasers. A pharmaceutical manufacturer may have to offer discounts or rebates to private third-party payors to obtain favorable coverage. Adequate third-party reimbursement may not be available to enable us to maintain net price levels sufficient to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of federal and state governments, and the prices of products have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement, and requirements for substitution of generic products. Adoption or enhancement of price controls and cost-containment measures could further limit a company’s revenue generated from the sale of any approved products. Coverage policies and third-party payor reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company receives regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Healthcare Reform
In the U.S., there have been, and likely will continue to be, a number of legislative, regulatory, and executive reform initiatives regarding the healthcare system directed at broadening the availability of healthcare, improving the quality of healthcare, and containing or lowering the cost of healthcare. These initiatives are wide-ranging and a number have focused on pricing and payment for prescription drugs, including some recent initiatives that address pricing in DTC offerings. For example, the IRA, which is having a significant and ongoing impact on the pharmaceutical industry, includes a number of changes intended to address rising prescription drug prices in Medicare Parts B and D, such as caps on Medicare Part D out-of-pocket costs and a drug price negotiation program for certain high-spend Medicare Part B and D drugs.
More recently, the Trump Administration has taken action intended to reduce the cost of prescription drugs, including drugs purchased directly by consumers. Two Executive Orders are aimed at lowering drug prices through multiple directives, including directives to government agencies and officials to identify most-favored-nation pricing targets for prescription drugs (and looking to pharmaceutical manufacturers to make significant progress towards delivering target prices to patients); to facilitate DTC purchasing programs for pharmaceutical manufacturers to sell their products to patients at the most-favored-nation price; to enhance competition for high-cost prescription drugs by accelerating approval of generics and biosimilars, facilitating the process for re-classifying prescription drugs as OTC drugs, and increasing drug importation. In the wake of the Executive Orders and related executive initiatives, a number of pharmaceutical manufacturers have announced new or expanded DTC offerings with discounted prices and/or reached agreement with the federal government regarding discounted pricing for drugs, including prices for Medicaid drugs and newly launched products. TrumpRx, a website sponsored by the federal government that is anticipated to offer pharmaceutical DTC channels, has also been announced. Federal agencies are also developing and proposing new drug pricing and payment pilot programs based on international pricing metrics under Medicare Parts B and D as well as Medicaid.
Other healthcare reform efforts or actions under the Trump Administration may affect access to healthcare coverage or the funding of health care benefits, although the full impact of such efforts or actions cannot be predicted. For example, Congressional Budget Office has estimated that Medicaid provisions in the 2025 budget reconciliation legislation, including restrictions in eligibility and funding for Medicaid, as well as changes to the healthcare marketplace, will increase the number of uninsured.
At the state level, new state laws and ongoing state legislative efforts seek to address drug costs, including by increasing transparency around drug costs or limiting drug prices.
Healthcare reform efforts have been and may continue to be subject to scrutiny and legal challenge, which increases uncertainty. For example, the IRA drug price negotiation program has been challenged in litigation filed by various pharmaceutical manufacturers and industry groups.
Other government, including general budget control measures, actions may affect prices or payments for prescription drugs. For example, new or increased tariffs implemented under the Trump Administration,
including the implementation of a recently announced tariff on branded or patented drugs for manufacturers that do not invest in manufacturing plants in the United States or reach a drug pricing agreement with the Trump Administration, could adversely affect the ability of pharmaceutical companies to realize an adequate return on sales of products imported from abroad or manufactured using materials or products imported from abroad. The timeline for implementing such tariff on branded or patented drugs has not been finalized. As another example, the Budget Control Act, as amended, resulted in the imposition of reductions in Medicare (but not Medicaid) payments to providers in 2013 and will remain in effect into 2032 unless additional Congressional action is taken.
The nature and extent of future healthcare or related reforms cannot be predicted. There is significant uncertainty regarding the nature or impact of any drug pricing or broader healthcare reform implemented by the current presidential administration through executive action or by Congress and regulatory agencies as well as the extent to which such action may be subject to litigation or other challenges.
Data Privacy Regulation
U.S. Privacy Laws
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, process or processing) personal data, data we collect about trial participants in connection with clinical trials, and other sensitive third-party data (collectively, sensitive data). Our data processing activities subject us to numerous data privacy and security obligations, such as various laws and regulations relating to data privacy and security. There are numerous U.S. federal and state laws and regulations addressing the privacy and security of personal information, including laws requiring the safeguarding of personal information and laws requiring notification to governmental authorities and data subjects as well as remediation in the event of a data breach.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws). Numerous U.S. states have enacted comprehensive privacy laws that impose certain obligations on covered businesses, including providing specific disclosures in privacy notices and affording residents with certain rights concerning their personal data. As applicable, such rights may include the right to access, correct, or delete certain personal data, and to opt-out of certain data processing activities, such as targeted advertising, profiling, and automated decision-making. Certain states also impose stricter requirements for processing certain personal data, including sensitive information, such as conducting data privacy impact assessments. These state laws allow for statutory fines for noncompliance. For example, California passed into law the CCPA, which took effect on January 1, 2020 and has been amended since, imposed requirements on certain businesses that process the personal information of California residents. The CCPA provides for fines and allows private litigants affected by certain data breaches to recover significant statutory damages. Compliance with these laws is a rigorous and time-intensive process that may increase the cost of doing business or require companies to change their business practices to ensure full compliance, to the extent the laws are applicable. Additionally, a number of states have passed laws that protect biometric information and a smaller number of states have passed or are considering laws that are specifically focused upon the privacy of consumer health data, including reproductive, sexual orientation, and gender identity privacy rights, such as Washington’s MHMD. MHMD broadly defines consumer health data, places restrictions on processing consumer health data (including imposing stringent requirements for consents), provides consumers certain rights with respect to their health data, and creates a private right of action.
There are many other federal and state-based data privacy and security laws and regulations, such as the Electronic Communications Privacy Act, the TCPA, the CAN-SPAM Act, and similar state consumer protection and communication privacy laws, such as CIPA. For example, the CAN-SPAM Act and the TCPA impose specific requirements on communications with consumers. The TCPA and analogous state laws impose various consumer consent requirements and other requirements on certain communications with consumers by phone or text message. TCPA violations can result in significant financial penalties, including penalties or criminal fines imposed by the FCC or statutory damages per violation imposed through private litigation or by state authorities. The TCPA provides for substantial penalties and statutory damages and has generated significant class action activity. The costs of litigating and/or settling a TCPA or similar legal claim could be significant. There has also
been a noticeable uptick in class action litigation wherein plaintiffs have utilized a variety of laws, including state wiretapping laws such as CIPA, in relation to companies’ use of certain tracking technologies, such as cookies and pixels. Actual or perceived failure to comply with applicable privacy requirements could result in adverse publicity, substantial monetary damages and legal defense costs, injunctive relief and fines or penalties.
Facilities
Our corporate headquarters is located at 470 James St, New Haven, CT 06513, where we lease and occupy approximately 1,200 square feet of office space. The term of our New Haven lease commenced in February 2025 and expires in February 2027 and we have the option to extent the lease for an additional two years after initial expiration. We believe our existing facilities are sufficient for our current needs. To meet the future needs of our business, we expect to lease additional or alternate office space, and we believe suitable additional or alternative space will be available in the future on commercially reasonable terms.
Employees and Human Capital Resources
Our employees are driven by our mission to utilize collective front-line clinical experience to identify and develop innovative solutions to solve pervasive treatment challenges in dermatology practice. We believe that our deep commitment to high ethical and professional standards is fundamental to our mission, and we are determined to build a culture that empowers a skilled and experienced workforce to perform at the highest levels. We commit our resources and make investments, including through recruiting, training and collaboration, to promote the culture that we desire, and we expect our employees to embrace the company’s values and culture in all that we do.
As of September 30, 2025, we employed 19 full-time employees. Of our full-time employees, 13 employees are engaged in business development, research, manufacturing, product development and clinical development, and 6 are engaged in finance, human resources, legal and other administrative functions. Five of our employees hold doctoral (Ph.D., M.D. or Pharm.D.) degrees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based incentive awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives. We offer a benefits program that provides resources to help employees manage their health, finances and life outside of work.
Legal Proceedings
From time to time, we may become involved in litigation or other legal proceedings. We are not a party to any litigation or legal proceedings that, in the opinion of our management, are probable to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on our business, financial condition, results of operations and prospects because of defense and settlement costs, diversion of management resources, negative publicity and reputational harm and other factors.
MANAGEMENT
Executive Officers and Directors
Our executive officers and directors, their ages as of December 31, 2025, and their positions, are as set forth below:
| | | | | | | | | | | | | | |
NAME | | AGE | | POSITION(S) |
Executive Officers | | | | |
Reid Waldman, M.D. | | 31 | | Chief Executive Officer and Director |
Tim Durso, M.D. | | 36 | | President |
Dominic Carrano, CPA | | 40 | | Chief Financial Officer |
Mark Neumann | | 62 | | Chief Commercial and Strategy Officer |
Michael V. Greco, J.D. | | 54 | | General Counsel |
Non-Management Directors | | | | |
John W. Childs | | 84 | | Director |
Vlad Coric, M.D. | | 55 | | Director |
Patrick Enright | | 63 | | Director |
David Friedman, M.D. | | 50 | | Director |
Jane Grant-Kels, M.D. | | 75 | | Director |
| Katarina Pance, Ph.D. | | 29 | | Director |
| | | | |
Executive Officers
Reid Waldman, M.D. is our co-founder and has served as our Chief Executive Officer since December 2022. Prior to his role as Chief Executive Officer, Dr. Waldman served as our Chief Operating Officer from March 2020 to December 2022. Dr. Waldman has served as a member of our board of directors since October 2019. From March 2019 to June 2021, Dr. Waldman served as a Clinical Trials Resident at The University of Connecticut Health Center, where he also served as a Dermatology Resident from June 2018 to June 2021. Prior to his residency, Dr. Waldman completed his internship at the Cedars-Sinai Medical Center. Dr. Waldman is a board-certified dermatologist and received his B.A. and M.D. from the University of Missouri-Kansas City 6 Year B.A./M.D. Program. We believe Dr. Waldman is qualified to serve on our board of directors because of his extensive experience as a dermatologist and based on his role as our Chief Executive Officer.
Tim Durso, M.D. is our co-founder and has served as our President since August 2020. Dr. Durso has also served as a Dermatologist at Edward Hines VA Hospital. From July 2016 to June 2022, Dr. Durso served as the Chief of Dermatology at the Malcolm Grow Medical Clinics and Surgery Center. Dr. Durso completed his internship and dermatology residency at the San Antonio Uniformed Services Health Education Consortium, and previously served on active duty in the U.S. Air Force. Dr. Durso is a board-certified dermatologist and received his B.S. in Pre-Medical Studies and Greek and Roman Civilizations from the University of Notre Dame and his M.D. from Loyola University Chicago Stritch School of Medicine.
Dominic Carrano, CPA has served as our Chief Financial Officer since February 2025. Mr. Carrano served as our Vice President, Finance from October 2023 to February 2025. Previously, Mr. Carrano served as Vice President, Finance and Controller from October 2022 to October 2023 and Controller from September 2020 to October 2022 at Rallybio Corporation where he oversaw financial and accounting operations including Rallybio Corporation’s transition to a public company and its follow on offering. Prior to Rallybio Corporation, Mr. Carrano held positions of increasing responsibility at Alexion Pharmaceuticals, Inc within the SEC reporting and technical accounting functions. Mr. Carrano began his career at Deloitte & Touche, LLP. Mr. Carrano received his B.S. and M.S. in Accounting from the University of Connecticut.
Mark Neumann has served as our Chief Commercial and Strategy Officer since December 2025. Previously, Mr. Neumann served as EVP and Chief Commercial Officer at Intra-Cellular Therapies, Inc. from October 2018 to April 2025. Before that, he worked at Amgen Inc. as Vice President and Global Therapeutic Area Marketing Head for Bone Health, Inflammation, and Nephrology from September 2016 to September 2018 and Head of Marketing for the U.S. Cardiology & Bone Health Business Unit from June 2014 to August 2016. Prior, Mr. Neumann spent over 25 years at Bristol-Myers Squibb Co in various roles of increasing responsibility across sales, marketing, and finance from June 1988 to May 2014. He started his career as an accountant at Arthur Andersen & Co. Mr. Neumann received his B.A. in Economics and Business Administration from Lafayette College.
Michael V. Greco, J.D. has served as our General Counsel since July 2025. From April 2020 until July 2025, he served as General Counsel and Secretary of Rallybio Corporation. From June 2018 until October 2019, he served as General Counsel and Secretary of SpringWorks Therapeutics, Inc. From February 2007 until June 2018, Mr. Greco held positions of increasing responsibility in the legal department at Alexion Pharmaceuticals, Inc., most recently as Senior Vice President of Law and Corporate Secretary from August 2015 to June 2018. Prior to Alexion, he was a corporate and transactional attorney in private practice at Bingham McCutchen LLP (now Morgan, Lewis & Bockius LLP) and Wiggin and Dana LLP. Prior to attending law school, Mr. Greco served in the U.S. Army Corps of Engineers. He received a J.D. from Suffolk University Law School and a B.S. from the United States Military Academy, West Point.
Non-employee Directors
John W. Childs has served as a member of our board of directors since September 2021. Since 1995, Mr. Childs has served as Chairman of J.W. Childs Associates, L.P. Previously, Mr. Childs was Senior Managing Director of the Thomas H. Lee Company and held various executive positions in the investment area at the Prudential Insurance Company of America, ultimately serving as Senior Managing Director in charge of the Capital Markets Group. Mr. Childs is currently a member of the board of directors of Biohaven Ltd., or Biohaven. Mr. Childs received his B.A. in History and French from Yale University and his M.B.A. from Columbia Business School at Columbia University. We believe Mr. Childs is qualified to serve on our board of directors because of his extensive experience in private equity, venture capital and life sciences.
Vlad Coric, M.D. has served as a member of our board of directors since September 2021. Dr. Coric has served as Chief Executive Officer of Biohaven since October 2022. Dr. Coric has also served as a Clinical Professor at Yale School of Medicine since July 2021. From October 2015 to October 2022, Dr. Coric served as Chief Executive Officer of Biohaven Pharmaceutical Holding Company Limited, until it was sold to Pfizer Inc. Prior to that, Dr. Coric served a Group Director, Global Clinical Research at Bristol-Myers Squibb Company. Dr. Coric is currently a member of the board of directors of Biohaven and Royalty Pharma plc. Dr. Coric also served on the board of directors of Revance Therapeutics, Inc. from March 2023 to February 2025, Biohaven Pharmaceutical Holding Company Ltd., from October 2015 to October 2022, and Social Capital Suvretta Holdings Corp. I, from June 2021 through July 2022. Dr. Coric received his B.S. in Neurobiology and Physiology from the University of Connecticut and his M.D. from Wake Forest University School of Medicine. He completed his internship at Yale-New Haven Hospital and residency training at the Yale Psychiatry Residency Training Program. We believe Dr. Coric is qualified to serve on our board of directors because of his extensive experience in the biopharmaceutical industry.
Patrick Enright has served as a member of our board of directors since December 2024. Mr. Enright has served as a Managing Director of Longitude Capital Management Co., LLC, a healthcare venture capital firm co-founded by Mr. Enright, since 2006. Previously, Mr. Enright was a Managing Director of Pequot Ventures, a venture capital firm, where he co-led the life sciences investment practice, and served in various senior executive positions at Valentis Inc., Boehringer Mannheim Pharmaceuticals Corp. and Sandoz Inc. Mr. Enright is currently a member of the board of directors of BioAge Labs, Inc., Jazz Pharmaceuticals PLC, Vera Therapeutics, Inc. and Zenas BioPharma, Inc., and has served on such boards since February 2024, January 2022, October 2020 and November 2022, respectively. Mr. Enright also served on the board of directors of Aptinyx Inc. from March 2016 to December 2022. Mr. Enright received his B.S. in Biological Sciences from Stanford University and his M.B.A. from the Wharton School of the University of Pennsylvania. We believe Mr. Enright is qualified to serve on our board of directors because of his experience serving on the board of directors of numerous biotechnology companies and his investment experience in the life sciences industry.
David Friedman, M.D., has served as a member of our board of directors since December 2025. Since January 2020, Dr. Friedman has served as a Managing Director and Senior Analyst at Suvretta Capital Management, LLC, or Suvretta Capital. Prior to Suvretta Capital, Dr. Friedman served as a healthcare analyst at Scopia Capital Management LP and worked in biotechnology equity research at Morgan Stanley & Co. LLC. Dr. Friedman received his B.S. in Biology from Duke University, his M.D. from the University of Pittsburgh School of Medicine and his M.B.A. from Harvard Business School. We believe Dr. Friedman is qualified to serve on our board of directors because of his experience investing in life sciences companies.
Jane M. Grant-Kels, M.D. has served as a member of our board of directors since September 2021. Dr. Grant-Kels currently serves the Vice Chair of the Department of Dermatology, the Founding Director of the Cutaneous Oncology Center and Melanoma Programs and Professor of Dermatology, Pathology and Pediatrics at the University of Connecticut Health Center, where she has held numerous positions since 1979 including Founding Chair of the Dermatology Department at UCONN Health. Dr. Grant-Kels received her B.A. in 1971 from Smith College and her M.D. from Cornell University Medical College in 1974. She completed her internship at the New York Hospital — Cornell Medical Center and residency training at the New York Hospital — Cornell Medical Center Residency Program. We believe that Dr. Grant-Kel is qualified to serve on our board of directors because of her extensive experience as a dermatologist.
Katarina Pance, Ph.D. has served as a member of our board of directors since October 2025. Dr. Pance has served as a Senior Associate at SR One Capital Management since May 2023. From February 2022 to May 2023, Dr. Pance served as Senior Scientist, Head of Discovery Biology at EpiBiologics, Inc., which she co-founded in July 2021. Dr. Pance also worked as a Venture Fellow at MPM Capital from July 2021 to January 2022. Dr. Pance received her B.A. in Biochemistry from the University of Pennsylvania and her Ph.D. from the University of California, San Francisco. We believe Dr. Pance is qualified to serve on our board of directors because of her experience serving on the board of directors of several biotechnology companies and her investment experience in the life sciences industry.
Family Relationships
There are no family relationships among any of our executive officers or directors.
Composition of Our Board of Directors
Our business and affairs are organized under the direction of our board of directors, which currently consists of seven members with one vacancy. The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required.
Our directors were each elected as directors pursuant to the board composition provisions of our Third Amended and Restated Voting Agreement, or the Voting Agreement, among us and our stockholders. The Voting Agreement will terminate upon the consummation of this offering, at which point no stockholder will have any special rights regarding the election or designation of the members of our board of directors. Our current directors elected to our board of directors pursuant to the Voting Agreement will continue to serve as directors until a successor is duly elected and qualified, or until his or her earlier resignation or removal.
Our board of directors may establish the authorized number of directors from time to time by resolution. In accordance with our restated certificate of incorporation that will become effective immediately prior to the consummation of this offering, our board of directors will be divided into three classes with staggered three-year terms. At each annual meeting of stockholders, a class of directors will be elected for a three-year term to succeed the class whose terms are then expiring, to serve from the time of election and qualification until the third annual meeting following their election or until their earlier death, resignation or removal. Upon the consummation of this offering, our directors will be divided among the three classes as follows:
nThe Class I directors will be Dr. Waldman and Mr. Childs, and their terms will expire at our 2027 annual meeting of stockholders.
nThe Class II directors will be Drs. Friedman, Grant-Kels and Pance, and their terms will expire at our 2028 annual meeting of stockholders.
nThe Class III directors will be Dr. Coric and Mr. Enright, and their terms will expire at our 2029 annual meeting of stockholders.
Our restated certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of our board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control. See the section of this prospectus captioned “Description of Capital Stock—Anti-takeover Effects of Our Restated Certificate of Incorporation and Amended and Restated Bylaws” for a discussion of these and other anti-takeover provisions found in our restated certificate of incorporation and amended and restated bylaws, which will be in effect prior to the consummation of this offering. Director Independence
Under the rules of the NYSE, independent directors must comprise a majority of a listed company’s board of directors within one year of the consummation of its initial public offering. In addition, the rules of the NYSE require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating/corporate governance committees be independent and that director nominees be selected or recommended for the board of directors’ selection by independent directors constituting a majority of the independent directors or by a nominating and corporate governance committee comprised solely of independent directors. Under the rules of the NYSE, a director will only qualify as “independent” if our board of directors affirmatively determines that he or she has no material relationship with us, either directly or as a partner, stockholder or officer of an organization that has a relationship with us. Ownership of a significant amount of our stock, by itself, does not constitute a material relationship.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors or any other board committee: (1) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries or (2) be an affiliated person of the listed company or any of its subsidiaries.
Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Dr. Waldman, is an “independent director” as defined under applicable rules of the NYSE, including, in the case of John W. Childs and Katarina Pance, the independence criteria set forth in Rule 10A-3 under the Exchange Act, and in the case of all the members of our compensation committee, the independence criteria set forth in Rule 10C-1 under the Exchange Act and are “non-employee directors” as defined in Section 16b-3 of the Exchange Act. Our board of directors has determined that Dr. Waldman, by virtue of his position as our chief executive officer, is not independent under applicable rules and regulations of the SEC and the rules of the NYSE. In making such determinations, our board of directors considered the relationships that each such non-employee director has with the Company and all other facts and circumstances that our board of directors deemed relevant in determining his or her independence, including the beneficial ownership of our capital stock by each non-employee director.
Committees of Our Board of Directors
Our board of directors will establish an audit committee, a compensation committee and a nominating and corporate governance committee prior to the listing of our common stock on the NYSE. The composition and responsibilities of each of the committees of our board of directors are described below. Members serve on these committees until their resignation or until otherwise determined by our board of directors. Each committee will operate pursuant to a written charter that satisfies the application rules and regulation of the SEC and the rules of the NYSE, which we will post to our website at www.veradermics.com prior to the listing of our common stock on the NYSE upon the consummation of this offering. Our board of directors may establish other committees as it deems necessary or appropriate from time to time. Information contained on, or accessible through, our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is only an inactive textual reference.
Audit Committee
Our audit committee will operate under a written charter, to be effective upon the effectiveness of the registration statement of which this prospectus forms a part, that satisfies the applicable rules of the NYSE.
The audit committee’s responsibilities upon consummation of this offering will include:
nappointing, approving the compensation of, and evaluating the qualifications, performance, procedures and independence of, our independent registered public accounting firm;
noverseeing the work of our independent registered public accounting firm, including through the receipt and consideration of written periodic reports from such firm;
npre-approving all audit and permitted non-audit services to be performed by our independent registered public accounting firm;
nreviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures, including earnings releases;
noverseeing and periodically reviewing with our independent registered public accounting firm our compliance with all applicable requirements of the Public Company Accounting Oversight Board;
nreviewing and discussing with management and our independent registered public accounting firm any material issues regarding accounting principles and financial statement presentations and the steps taken to deal with such issues;
nreviewing disclosures about any significant deficiencies or material weaknesses in our internal control structures and procedures, including disclosures in our annual and quarterly reports;
ncoordinating our board of directors’ oversight of our internal control over financial reporting, disclosure controls and procedures, code of business conduct and ethics, procedures for complaints and legal and regulatory matters;
nreviewing and discussing with management and our independent registered public accounting firm any material issues regarding cybersecurity risks and processes for assessing, identifying and managing material risks from cybersecurity threats;
ndiscussing our risk management policies with management;
nestablishing policies regarding hiring employees from our independent registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns;
nmeeting independently with our independent registered public accounting firm and management;
nreviewing and approving any related person transactions;
noverseeing our guidelines and policies governing risk assessment and risk management;
noverseeing and periodically reviewing the integrity of our information technology systems, process and data;
npreparing the audit committee report required by SEC rules;
nreviewing and assessing, at least annually, the adequacy of the audit committee’s charter; and
nperforming, at least annually, an evaluation of the performance of the audit committee.
All audit services and all non-audit services, other than de minimis non-audit services, to be provided to us by our independent registered public accounting firm must be approved in advance by our audit committee.
The members of our audit committee are Messrs. Enright and Childs and Dr. Pance. Mr. Enright chairs the audit committee. Our board of directors has determined that each member of our audit committee has sufficient knowledge in financial and auditing matters to serve on the audit committee. Our board of directors has also determined that Mr. Enright is an “audit committee financial expert,” as defined under Item 407 of Regulation S-K. We expect to satisfy the member independence requirements for the audit committee prior to the end of the transition period provided under the rules of the NYSE and SEC rules and regulations for companies completing their initial public offering.
Compensation Committee
Our compensation committee will operate under a written charter, to be effective upon the effectiveness of the registration statement of which this prospectus forms a part, that satisfies the applicable rules of the NYSE.
Our compensation committee’s responsibilities upon consummation of this offering will include:
nreviewing and establishing our overall management compensation strategy and benefits philosophy and policies, including base salary, incentive compensation and equity-based grants;
nreviewing and approving performance goals and objectives relevant to compensation of our chief executive officer and other executive officers;
nevaluating the performance of the chief executive officer and executive officers in light of their performance goals and objectives, including during executive sessions of non-employee directors, and recommending to our board of directors the compensation of our chief executive officer and other executive officers;
nreviewing and making recommendations to the board of directors with respect to non-employee director compensation;
nreviewing, overseeing and administering our equity incentive plans, granting awards under such plan and making recommendations to the board of directors about the adoption of any new or modifying existing equity-based, cash-based, management incentive and deferred compensation plans;
nestablishing and reviewing “clawback” policies that allow the recouping of incentive compensation;
nreviewing, considering and selecting, to the extent determined to be advisable, a peer group of appropriate companies for purposing of benchmarking and analysis of compensation for our executive officers and non-employee directors;
nrecommending to our board of directors any stock ownership guidelines for our executive officers and non-employee directors, periodically assessing these guidelines and recommending revisions as appropriate, and monitoring individual compliance with these guidelines;
nretaining, appointing or obtaining advice of a compensation consultant, legal counsel or other advisor and determining the compensation and independence of such consultant or advisor;
npreparing, if required, the compensation committee report on executive compensation for inclusion in our annual report on Form 10-K and our annual proxy statement in accordance with SEC proxy and disclosure rules;
nmonitoring our compliance with the requirements of Sarbanes-Oxley relating to loans to directors and officers;
nreviewing and approving all employment contract and other compensation, severance and change-in-control arrangements for our executive officers;
nestablishing and periodically reviewing policies and procedures with respect to perquisites as they relate to our executive officers;
nreviewing the risks associated with our compensation policies and practices;
nperiodically reviewing all equity compensation plans that are not subject to stockholder approval under the listing standards of the NYSE;
noverseeing the maintenance and presentation to our board of directors of management’s plans for succession to senior management positions based on guidelines developed and recommended to the compensation committee to the full board of directors;
nreviewing our strategies, initiatives and programs with respect to our culture, talent recruitment, development, and retention, employee engagement and diversity and inclusion;
nmaintaining minutes of the compensation committee and reporting its actions and any recommendations to the board of directors on a periodic basis;
nreviewing and assessing, at least annually, the adequacy of the compensation committee’s charter; and
nperforming, on an annual basis, an evaluation of the performance of the compensation committee.
The members of our compensation committee are Mr. Childs, Drs. Coric and Grant-Kels. Dr. Coric chairs the compensation committee. Our board of directors has determined that each member of the compensation committee satisfies the independence standards of the applicable rules of the NYSE.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee will operate under a written charter, to be effective upon the effectiveness of the registration statement of which this prospectus forms a part, that satisfies the applicable rules of the NYSE.
Our nominating and corporate governance committee’s responsibilities upon consummation of this offering will include:
nactively seeking and identifying individuals qualified to become members of our board of directors consistent with criteria approved by the board of directors and receiving nominations for such qualified individuals;
nrecommending to our board of directors the persons to be nominated for election as directors and to each committee of the board of directors;
ndeveloping and recommending to our board of directors a set of corporate governance guidelines applicable to the Company;
nestablishing a policy under which our stockholders may recommend a candidate to the nominating and corporate governance committee for consideration for nomination as a director;
nreviewing and recommending committee slates on an annual basis;
nrecommending to our board of directors qualified candidates to fill vacancies on our board of directors;
ndeveloping and recommending to our board of directors a set of corporate governance principles applicable to us and reviewing the principles on at least an annual basis;
nreviewing and making recommendations to our board of directors with respect to our board size, composition, leadership structure and board of directors committee structure;
nreviewing, in concert with our board of directors, our policies with respect to significant issues of corporate public responsibility, including but not limited to sustainability, diversity and inclusion and environmental, social and governance initiatives;
nmaking recommendations to our board of directors of processes for annual evaluations of the performance of our board of directors and committees of our board of directors;
noverseeing the process for annual evaluations of our board of directors and committees of our board of directors;
nconsidering and reporting to our board of directors any questions of possible conflicts of interest of members of our board of directors;
nreviewing with management the Company’s social corporate responsibility activities, policies, and program;
nproviding new director orientation and continuing education for existing directors on a periodic basis;
noverseeing the maintenance and presentation to our board of directors of management’s plans for succession to senior management positions in the Company;
nreviewing and assessing, at least annually, the adequacy of the nominating and corporate governance committee’s charter; and
nperforming, on an annual basis, an evaluation of the performance of the nominating and corporate governance committee.
The members of our nominating and corporate governance committee are Mr. Friedman and Drs. Grant-Kels and Pance. Dr. Grant-Kels chairs the nominating and corporate governance committee. Our board of directors has determined that each member of the nominating and corporate governance committee satisfies the independence standards of the applicable rules of the NYSE Stock Market.
Our board of directors may establish other committees from time to time.
Role of the Board of Directors in Risk Oversight
Our board of directors has, and, upon the consummation of this offering, its committees will also have, an active role in overseeing the management of our risks. Our board of directors is responsible for general oversight of risks and regular review of information regarding our risks, including credit risks, liquidity risks and operational risks. The compensation committee will be responsible for overseeing the management of risks relating to our executive compensation plans and arrangements. The audit committee will be responsible for overseeing the management of risks relating to accounting matters and financial reporting. The nominating and governance committee will be responsible for overseeing the management of risks associated with the independence of our board of directors and potential conflicts of interest. Although each committee will be responsible for evaluating
certain risks and overseeing the management of such risks, the entire board of directors will be regularly informed through discussions from committee members about such risks.
Code of Business Conduct and Ethics
We have adopted a written Code of Business Conduct and Ethics that applies to all our employees, officers and directors. This includes our principal executive officer, principal financial officer and principal accounting officer or controller, or persons performing similar functions. The full text of our Code of Business Conduct and Ethics will be posted on our website at www.veradermics.com prior to the consummation of this offering. We intend to disclose on our website any future amendments of our Code of Business Conduct and Ethics or waivers that exempt any principal executive officer, principal financial officer, principal accounting officer or controller, persons performing similar functions or our directors from provisions in the Code of Business Conduct and Ethics. Information contained on, or accessible through, our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is only an inactive textual reference.
Compensation Committee Interlocks and Insider Participation
None of the members of the compensation committee is currently, or has been at any time, one of our officers or employees. None of our executive officers currently serves, or has served during the last completed fiscal year, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
EXECUTIVE AND DIRECTOR COMPENSATION
The following discussion and analysis of compensation arrangements should be read with the compensation tables and related disclosures set forth below. This discussion contains forward looking statements that are based on our current plans and expectations regarding future compensation programs. The compensation programs that we adopt in the future may differ materially from the programs summarized in this discussion.
Introduction
This section provides an overview of the compensation awarded to, earned by, or paid to our principal executive officer and our next two most highly compensated executive officers listed below in respect of their service to us for the fiscal year ended December 31, 2025. We refer to these individuals as our named executive officers. Our named executive officers are:
nReid Waldman M.D., Chief Executive Officer and Director;
nTim Durso, M.D.*, President; and
nDominic Carrano, CPA, Chief Financial Officer.
*In connection with this offering, Dr. Durso will be appointed to the position of Chief Technical Officer.
Prior to this offering, our board of directors was responsible for determining the compensation of our executive officers, based on recommendations from our compensation committee. In addition, our Chief Executive Officer made recommendations to our board of directors regarding the compensation of his direct reports. Following this offering, our compensation committee will be responsible for making such determinations.
Summary compensation table
The following table sets forth the compensation awarded to, earned by, or paid to our named executive officers in respect of their service to us for the fiscal year ended December 31, 2024, and the fiscal year ended December 31, 2025:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
NAME AND PRINCIPAL POSITION | | YEAR | | SALARY ($) | | BONUS ($)(1) | | OPTION AWARDS ($) (2) | | ALL OTHER COMPENSATION ($) (3) | | TOTAL ($) |
Reid Waldman, M.D. | | 2025 | | 450,000 | | | 449,700 | | | 7,538,090 | | | 17,717 | | | 8,455,507 | |
| Chief Executive Officer and Director | | 2024 | | 371,280 | | | 329,070 | | | 94,800 | | | 17,217 | | | 812,367 | |
Tim Durso, M.D. | | 2025 | | 392,000 | | | 248,800 | | | 2,649,971 | | | 17,717 | | | 3,308,488 | |
| President | | 2024 | | 346,280 | | | 202,100 | | | 94,800 | | | 17,197 | | | 660,377 | |
Dominic Carrano, CPA | | 2025 | | 373,900 | | | 242,900 | | | 1,578,204 | | | 16,464 | | | 2,211,468 | |
| Chief Financial Officer | | 2024 | | 322,400 | | | 175,080 | | | 1,586 | | | 12,673 | | | 511,739 | |
| | | | | | | | | | | | |
(1)The amounts shown in this column represent discretionary annual bonuses paid to our named executive officers in respect of the applicable year, and, for Mr. Carrano the amount in respect of fiscal year 2024 also includes the second portion of a sign-on bonus paid to him ($30,000), described below under “Agreements with our named executive officers.”
(2)The amounts shown in this column for fiscal year 2025 include (i) the grant date fair value of time-based stock option awards granted to Dr. Waldman ($7,485,568), Dr. Durso ($2,597,449) and Mr. Carrano ($1,571,044) in fiscal year 2025 and (ii) the incremental fair value associated with the modification to outstanding stock options held by Dr. Waldman ($52,522), Dr. Durso ($52,522) and Mr. Carrano ($7,160) in fiscal year 2025, as described in more detail under “Equity Compensation” below, in each case, computed in accordance with FASB ASC Topic 718, excluding the effect of estimated forfeitures. No amounts have been included with respect to performance-based stock options granted in fiscal year 2025 because the performance conditions associated with such grants were deemed to not be probable of being satisfied at the time of grant and therefore no compensation cost was recognized for 2025. The grant date
fair value of such options granted during 2025, assuming that such performance conditions are satisfied in full and calculated using a Monte Carlo simulation model, are: Dr. Waldman ($1,252,660), Dr. Durso ($626,330) and Mr. Carrano ($220,184). These amounts do not necessarily correspond to the actual value that may be recognized by the named executive officers. The amounts shown in this column for fiscal year 2024 represent the grant date fair value of stock option awards granted to Drs. Waldman and Durso and Mr. Carrano in fiscal year 2024 computed in accordance with FASB ASC Topic 718, excluding the effect of estimated forfeitures. The assumptions used to value the time-based stock options for this purpose are set forth in Note 8 to our audited consolidated financial statements included elsewhere in this prospectus.
(3)The amounts shown in the “All Other Compensation” column for fiscal year 2025 reflect company contributions under the SIMPLE IRA matching program ($16,500 for each of Drs. Waldman and Durso and $15,247 for Mr. Carrano), company paid life insurance premiums ($317 for each of our named executive officers), and mobile phone reimbursement ($900 for each of our named executive officers).
Narrative disclosure to summary compensation table
Annual base salary
The employment agreement with each named executive officer (as amended and restated, in the case of Drs. Waldman and Durso), described below, establishes an annual base salary for the executive officer, which is subject to periodic review. Effective January 1, 2025, the annual base salaries of Drs. Waldman and Durso and Mr. Carrano were increased to $450,000, $392,000, and $373,900, respectively. Effective January 1, 2026, the annual base salaries of Drs. Waldman and Durso and Mr. Carrano were increased to $553,100, $436,400, and $431,800, respectively. Effective as of the date on which the registration statement of which this prospectus is a part is declared effective by the SEC, the annual base salaries of Drs. Waldman and Durso and Mr. Carrano will be increased to $680,200, $508,700, and $502,200, respectively.
Annual bonuses
With respect to fiscal year 2025, each of Drs. Waldman and Durso and Mr. Carrano was eligible to receive a discretionary annual bonus. After reviewing Company performance, our board of directors approved bonuses for Drs. Waldman and Durso and Mr. Carrano for fiscal year 2025 in the amounts of $449,700, $248,800, and $242,900, respectively. Effective as of the date on which the registration statement of which this prospectus is a part is declared effective by the SEC, the target bonus for each of Drs. Waldman and Durso and Mr. Carrano, expressed as a percentage of base salary, will be 55%, 40%, and 40%, respectively.
Agreements with our named executive officers
In connection with this offering, and effective as of the date on which the registration statement of which this prospectus is a part is declared effective by the SEC, we entered into an amended and restated employment agreement with each of Drs. Waldman and Durso, and an employment agreement with Mr. Carrano, setting forth the terms and conditions of such named executive officer’s continued employment with us. The material terms of the agreements are described below. Unless otherwise noted below, the terms “cause,” “good reason,” and “change of control” referred to below are defined in the respective named executive officer’s employment agreement.
Drs. Waldman and Durso. The amended and restated employment agreement with each of Drs. Waldman and Durso provides for an initial base salary (described above), which is subject to periodic review, and a target annual bonus equal to a percentage of the named executive officer’s annual base salary (described above).
Mr. Carrano. The employment agreement with Mr. Carrano provides for an initial base salary (described above), which is subject to periodic review, and a target annual bonus equal to 40% of Mr. Carrano’s base salary. Prior to entering into the employment agreement, Mr. Carrano was party to an offer letter, which provided for a sign-on bonus in the amount of $60,000, of which $30,000 was paid in 2023 and $30,000 was paid in 2024.
Severance upon termination of employment; change of control; restrictive covenants.
Severance Upon Termination of Employment; Change of Control. Each of Drs. Waldman and Durso and Mr. Carrano is entitled to severance payments and benefits in connection with certain qualifying terminations of employment under his respective employment agreement, as amended and restated in the case of Drs. Waldman and Durso. If the named executive officer’s employment is terminated by us without cause or by him for good
reason, in each case, not within the period beginning three (3) months before and ending twelve (12) months following a change of control, or the change of control period, he will be entitled to receive (i) an amount equal to the sum of the named executive officer’s annual base salary and target annual bonus, payable over twelve (12) months following termination, (ii) subject to his timely election of COBRA coverage, payment of a monthly amount equal to the monthly health premiums paid by us on behalf of the named executive officer and his eligible dependents for a period of twelve (12) months following termination (or, if earlier, until the named executive officer ceases to be eligible for COBRA coverage or obtains health coverage from another employer), and (iii) for each of Drs. Waldman and Durso, any unpaid prior year annual bonus. If Dr. Waldman’s or Dr. Durso’s employment is terminated by reason of his death or disability, he will be entitled to receive any unpaid prior year annual bonus.
If the named executive officer’s employment is terminated by us without cause or by him for good reason, in each case, within the change of control period, the named executive officer will instead be entitled to receive (i) a lump sum payment in an amount equal to 1.5 times the sum of (A) the named executive officer’s annual base salary and (B) the named executive officer’s target annual bonus, (ii) subject to his timely election of COBRA coverage, payment of a monthly amount equal to the monthly health premiums paid by us on behalf of the named executive officer and his eligible dependents for a period of eighteen (18) months following termination (or, if earlier, until the named executive officer ceases to be eligible for COBRA coverage or obtains health coverage from another employer), (iii) full acceleration of all of the named executive officer’s outstanding and unvested time-based equity awards, and (iv) for each of Drs. Waldman and Durso, any unpaid prior year annual bonus.
In addition, pursuant to the terms of the applicable option award agreements, certain options held by our named executive officers vest in full immediately prior to the consummation of a change of control (as defined in the 2021 Plan), subject to the named executive officer’s continued service through such change of control.
Each named executive officer’s respective employment agreement , as amended, provides for a Section 280G "better-of provision" such that payments or benefits that each or our named executive officers receives in connection with a change of control will be reduced to the extent necessary to avoid the imposition of any excise tax under Sections 280G and 4999 of the Code if such reduction would result in greater after-tax payment amount for such named executive officer.
Severance Subject to Release of Claims. Our obligation to provide a named executive officer with severance payments and other benefits under his respective employment agreement is conditioned on the executive officer signing a release of claims in favor of us.
Restrictive Covenants. Under their respective employment agreements, each of Drs. Waldman and Durso and Mr. Carrano has agreed to a perpetual non-disparagement covenant. Each of our named executive officers is also party to a Confidentiality, Assignment of Inventions, and Restrictive Covenant Agreement under which each named executive officer has agreed to a perpetual confidentiality covenant, an assignment of intellectual property covenant, and covenants not to compete with us or solicit our service providers or customers during his employment and for two years (or one (1) year in the event of a termination other than for cause or by the executive for good reason) following his termination of employment.
Equity compensation
Drs. Waldman and Durso and Mr. Carrano received incentive equity grants in fiscal year 2025 under the 2021 Plan.
On February 27, 2025, each of Drs. Waldman and Durso and Mr. Carrano was granted an option to purchase 224,247, 89,699, and 53,819 shares of our common stock, respectively, under the 2021 Plan, which vests in 48 equal monthly installments following November 25, 2024, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
On February 27, 2025, our board of directors determined to reduce the exercise price applicable to outstanding stock options, including those held by our named executive officers, to an amount not less than the then fair market value of a share of our common stock as of such date. The incremental fair value associated with the
modification to these options is quantified and described in footnote (2) to the Summary Compensation Table above.
On November 14, 2025, each of Drs. Waldman and Durso and Mr. Carrano was granted an option to purchase 1,039,490, 356,689, and 215,887 shares of our common stock, respectively, under the 2021 Plan, which vests in 48 equal monthly installments following October 14, 2025, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
On November 14, 2025, each of Drs. Waldman and Durso and Mr. Carrano was also granted a performance-based option to purchase 243,984, 121,992, and42,886 shares of our common stock, respectively, under the 2021 Plan, which is eligible to vest as to 100% of the underlying shares upon our achievement of specified market capitalization hurdles prior to specified dates following our initial public offering, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
Employee and retirement benefits
We currently provide broad-based health and welfare benefits that are available to all of our employees, including our named executive officers, as well as a SIMPLE IRA retirement plan for our full-time employees, under which we make matching employer contributions (subject to tax code limits). Other than the SIMPLE IRA plan, we do not provide any qualified or non-qualified retirement or deferred compensation benefits to our employees, including our named executive officers. Each of our named executive officers received company paid life insurance premiums and reimbursement for cell phone expenses in 2025 in the amounts reported in the Summary Compensation Table.
Clawback policy
In accordance with the requirements of the Dodd-Frank Act, final SEC rules, and applicable NYSE listing standards, our board of directors has adopted a compensation recovery policy, which will become effective upon the date on which the registration statement of which this prospectus is a part is declared effective by the SEC. The compensation recovery policy will provide that in the event we are required to prepare a restatement of financial statements due to material noncompliance with any financial reporting requirement under securities laws, certain incentive-based compensation paid or awarded to covered executives will be subject to reduction and/or repayment if the amount of such compensation was calculated based on the achievement of financial results that were the subject of the restatement and the amount of such compensation that would have been received by the covered executives had the financial results been properly reported would have been lower than the amount actually awarded.
Outstanding awards at fiscal year-end 2025
The following table sets forth information concerning outstanding equity awards held by each of our named executive officers as of December 31, 2025:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Option awards |
| Name | | Grant date | | Number of securities underlying unexercised options exercisable (#) | | Number of securities underlying unexercised options unexercisable (#) | | Equity Incentive Plan Awards: Number of securities underlying unexercised unearned options (#) | | Option exercise price ($/share)(18) | | Option expiration date |
| Reid Waldman, M.D. | | 12/13/2021(1) | | 3,452 | | | — | | | — | | | 12.19 | | | 12/12/2026 |
| | 12/13/2021(2) | | 84,504 | | | — | | | — | | | 12.19 | | | 12/12/2031 |
| | 12/22/2022(3) | | 3,452 | | | — | | | — | | | 12.19 | | | 12/21/2027 |
| | 12/22/2022(4) | | 5,343 | | | — | | | — | | | 12.19 | | | 12/21/2032 |
| | 3/19/2024(5) | | 6,300 | | | 3,564 | | | — | | | 12.19 | | | 3/18/2029 |
| | 3/19/2024(6) | | 3,216 | | | 1,819 | | | — | | | 12.19 | | | 3/18/2034 |
| | 2/27/2025(7) | | 60,733 | | | 163,513 | | | — | | | 12.19 | | | 2/26/2035 |
| | 11/14/2025(8) | | 43,312 | | | 996,178 | | | — | | | 12.79 | | | 11/13/2035 |
| | 11/14/2025(9) | | — | | | 243,984 | | | — | | | 12.79 | | | 11/13/2035 |
| | | | | | | | | | | | |
| Tim Durso, M.D. | | 12/13/2021(1) | | 3,452 | | | — | | | — | | | 12.19 | | | 12/12/2026 |
| | 12/13/2021(2) | | 84,504 | | | — | | | — | | | 12.19 | | | 12/12/2031 |
| | 12/22/2022(3) | | 3,452 | | | — | | | — | | | 12.19 | | | 12/21/2027 |
| | 12/22/2022(4) | | 5,343 | | | — | | | — | | | 12.19 | | | 12/21/2032 |
| | 3/19/2024(5) | | 6,300 | | | 3,564 | | | — | | | 12.19 | | | 3/18/2029 |
| | 3/19/2024(6) | | 3,216 | | | 1,819 | | | — | | | 12.19 | | | 3/18/2034 |
| | 2/27/2025(10) | | 24,293 | | | 65,405 | | | — | | | 12.19 | | | 2/26/2035 |
| | 11/14/2025(11) | | 14,862 | | | 341,827 | | | — | | | 12.79 | | | 11/13/2035 |
| | 11/14/2025(12) | | — | | | 121,992 | | | — | | | 12.79 | | | 11/13/2035 |
| | | | | | | | | | | | |
| Dominic Carrano, CPA | | 10/6/2023(13) | | 8,640 | | | 7,311 | | | — | | | 12.19 | | | 10/5/2033 |
| | 3/19/2024(14) | | 119 | | | 129 | | | — | | | 12.19 | | | 3/18/2034 |
| | 2/27/2025(15) | | 14,576 | | | 39,243 | | | — | | | 12.19 | | | 2/26/2035 |
| | 11/14/2025(16) | | 8,995 | | | 206,891 | | | — | | | 12.79 | | | 11/13/2035 |
| | 11/14/2025(17) | | — | | | 42,886 | | | — | | | 12.79 | | | 11/13/2035 |
| | | | | | | | | | | | |
(1)Represents an option to purchase 3,452 shares of our common stock, which was fully vested as of September 22, 2024.
(2)Represents an option to purchase 84,504 shares of our common stock, which was fully vested as of September 22, 2024.
(3)Represents an option to purchase 3,452 shares of our common stock, which was fully vested as of December 22, 2025.
(4)Represents an option to purchase 5,343 shares of our common stock, which was fully vested as of December 22, 2025.
(5)Represents an option to purchase 9,864 shares of our common stock, which vested as to 33.3% of the underlying shares on January 1, 2025 and vests in 24 equal monthly installments thereafter, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(6)Represents an option to purchase 5,035 shares of our common stock, which vested as to 33.3% of the underlying shares on January 1, 2025 and vests in 24 equal monthly installments thereafter, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(7)Represents an option to purchase 224,247 shares of our common stock, which vests in 48 equal monthly installments following November 25, 2024, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(8)Represents an option to purchase 1,039,490 shares of our common stock, which vests in 48 equal monthly installments following October 14, 2025, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(9)Represents an option to purchase 243,984 shares of our common stock, which is eligible to vest as to 100% of the underlying shares upon our achievement of specified market capitalization hurdles prior to specified dates following our initial public offering, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(10)Represents an option to purchase 89,699 shares of our common stock, which vests in 48 equal monthly installments following November 25, 2024, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(11)Represents an option to purchase 356,689 shares of our common stock, which vests in 48 equal monthly installments following October 14, 2025, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(12)Represents an option to purchase 121,992 shares of our common stock, which is eligible to vest as to 100% of the underlying shares upon our achievement of specified market capitalization hurdles prior to specified dates following our initial public offering, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(13)Represents an option to purchase 15,951 shares of our common stock, which vested as to 25% of the underlying shares on October 6, 2024 and vests in 36 equal monthly installments thereafter, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(14)Represents an option to purchase 249 shares of our common stock, which vested as to 25% of the underlying shares on January 1, 2025 and vests in 36 equal monthly installments thereafter, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(15)Represents an option to purchase 53,819 shares of our common stock, which vests in 48 equal monthly installments following November 25, 2024, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(16)Represents an option to purchase 215,887 shares of our common stock, which vests in 48 equal monthly installments following October 14, 2025, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(17)Represents an option to purchase 42,886 shares of our common stock, which is eligible to vest as to 100% of the underlying shares upon our achievement of specified market capitalization hurdles prior to specified dates following our initial public offering, generally subject to the named executive officer’s continued employment with us through the applicable vesting date.
(18)The amounts reported in the “Option exercise price” column reflect the modification to outstanding stock options held by Drs. Waldman and Durso and Mr. Carrano in fiscal year 2025, as applicable, as described in more detail under “Equity Compensation” above.
Director compensation
The following table sets forth the compensation received by our non-employee directors during the fiscal year ended December 31, 2025. None of our non-employee directors received compensation for their service on our board of directors during the fiscal year ended December 31, 2025. The amount for Dr. Coric reflects the incremental fair value associated with the modification to his stock options in fiscal year 2025. Dr. Waldman
does not receive compensation for his service as a director. His compensation for 2025 is included with that of our other named executive officers above.
| | | | | | | | | | | | | | |
NAME(1) | | Option Awards ($)(6) | | Total ($) |
Amanda Birdsey-Benson, Ph.D.(2) | | $ | — | | $ | — |
John W. Childs | | $ | — | | $ | — |
Vlad Coric, M.D. | | $ | 7,886 | | $ | 7,886 |
Christopher Eklund(3) | | $ | — | | $ | — |
Patrick Enright | | $ | — | | $ | — |
Jane Grant-Kels, M.D. | | $ | — | | $ | — |
Katarina Pance, Ph.D.(4) | | $ | — | | $ | — |
David Friedman, M.D.(5) | | $ | — | | $ | — |
| | | | |
(1)As of December 31, 2025, Dr. Coric held an option to purchase 4,764 shares of our common stock and none of our other non-employee directors held any outstanding equity awards.
(2)Dr. Birdsey-Benson ceased service on our board of directors in December 2025.
(3)Mr. Eklund ceased service on our board of directors in October 2025.
(4)Dr. Pance joined our board of directors in October 2025.
(5)Dr. Friedman joined our board of directors in December 2025.
(6)The amount reported in the “Option Awards” column for fiscal year 2025 represents the incremental fair value associated with the modification to outstanding stock options held by Dr. Coric in fiscal year 2025, computed in accordance with FASB ASC Topic 718. On February 27, 2025, our board of directors determined to reduce the exercise price applicable to outstanding stock options, including those held by Dr. Coric, to an amount not less than the then fair market value of a share of our common stock as of such date.
Director compensation policy
In connection with this offering, our board of directors has adopted and our stockholders have approved a formal non-employee director compensation policy for members of our board of directors, which will become effective upon the date on which the registration statement of which this prospectus is a part is declared effective by the SEC. Under the non-employee director compensation policy, following this offering our non-employee directors will be compensated as follows:
neach non-employee director will receive an annual cash fee of $40,000 (and the chair of our board of directors will receive an additional annual cash fee of $32,500);
neach non-employee director who is a member of the audit committee will receive an additional annual cash fee of $10,000 ($20,000 for the audit committee chair);
neach non-employee director who is a member of our compensation committee will receive an additional annual cash fee of $7,500 ($15,000 for our compensation committee chair);
neach non-employee director who is a member of the nominating and corporate governance committee will receive an additional annual cash fee of $5,000 ($10,000 for the nominating and corporate governance committee chair);
neach non-employee director who is first elected or appointed to our board of directors will be granted, upon his or her initial election to our board of directors, an option under our 2026 Plan to purchase 43,000 shares of our common stock, or the initial option grants; and
neach non-employee director will annually be granted, on the date of the first meeting of our board of directors held after the annual meeting of our stockholders (other than a director who was first elected or appointed to our board of directors during the calendar year of such meeting), an option under our 2026 Plan to purchase 21,500 shares of our common stock, or the annual option grants.
The stock options granted to our non-employee directors will have a per share exercise price equal to the fair market value of a share of our common stock on the date of grant, will expire not later than ten years after the
date of grant, and will be subject to the terms and conditions of our 2026 Plan. The initial option grants will vest in 36 equal monthly installments over a period of three years commencing from the date of grant, subject to the director’s continued service on our board of directors through each applicable vesting date. The annual option grants will vest in full on the earlier of the first anniversary of the date of grant or the next annual meeting of our stockholders, subject to the director’s continued service on our board of directors through the applicable vesting date. Upon a change of control (as defined in the applicable stock option award agreements), each initial option grant and each annual option grant that is then outstanding will vest in full, subject to the director’s continued service on our board of directors through such change of control.
All cash fees will be paid quarterly, in arrears, or upon the earlier resignation or removal of the non-employee director. The amount of each payment will be prorated for any portion of a calendar quarter that a non-employee director is not serving on our board of directors, based on the number of calendar days served by such non-employee director. For the calendar quarter in which this offering occurs, the amount of each payment will be prorated based on the number of calendar days such non-employee director was a member of our board of directors following this offering.
Each non-employee director is entitled to reimbursement for reasonable travel and other expenses incurred in connection with attending meetings of our board of directors and any committee on which he or she serves.
Equity incentive plans
2021 Plan
In 2021, our board of directors approved the 2021 Plan. The 2021 Plan has been amended from time to time to increase the aggregate number of shares of our common stock reserved for issuance under the 2021 Plan, and was most recently amended on December 21, 2025. The 2021 Plan permits the grant of stock options and restricted and unrestricted shares of common stock. Subject to adjustment, the maximum number of shares of common stock that may be granted under the 2021 Plan is 4,411,143. As of September 30, 2025, options to purchase 739,331 shares of common stock were outstanding under the 2021 Plan and 20,407 shares of common stock remained available for future issuance. Shares underlying awards that expire or become unexercisable without having been exercised, or that are forfeited to or repurchased by us will become available for subsequent awards under the 2021 Plan. It is anticipated that no further awards will be made under the 2021 Plan following the completion of this offering. In connection with this offering, we intend to adopt a new omnibus equity plan under which we will grant equity-based awards in connection with or following this offering. This summary is not a complete description of all provisions of the 2021 Plan and is qualified in its entirety by reference to the 2021 Plan, which is filed as an exhibit to the registration statement of which this prospectus is part.
Plan administration
Our board of directors administers the 2021 Plan. Our board of directors has the discretionary authority to grant awards, to construe award agreements and the 2021 Plan, to prescribe, amend and rescind rules and regulations relating to the 2021 Plan, to determine the terms and provisions of the award agreements and to make all other determinations in the judgment of our board of directors necessary or desirable for the administration of the 2021 Plan. Our board of directors may delegate any or all of its powers to a committee. As used in this summary, the term “Administrator” refers to our board of directors and its authorized delegates, as applicable.
Eligibility
Our and our affiliates’ employees, officers, directors, advisors and consultants are eligible to participate in the 2021 Plan. Eligibility for incentive stock options is limited to our employees or employees of certain affiliates.
Vesting; terms of awards
The Administrator determines the terms and conditions of all awards granted under the 2021 Plan, including the time or times an award vests or becomes exercisable, and the terms on which an award remains exercisable. No option granted to a director or executive officer may be exercisable during the first six months of the date of grant. Subject to the immediately preceding sentence, the Administrator may at any time accelerate the vesting or exercisability of an award.
Transferability of awards
Except as the Administrator may otherwise determine, options may not be transferred other than by will or by the laws of descent and distribution or pursuant to a qualified domestic relations order. Shares of common stock may not be transferred unless the transfer complies with all provisions of the 2021 Plan.
Effect of certain transactions
In the event of change of control, as defined in the 2021 Plan, the Administrator may, with respect to outstanding awards, provide for the acceleration of exercisability or lapse of restrictions in respect of any award, in full or in part and/or the cash-out of awards. If not accelerated or cashed out, the awards will be assumed or substituted by the successor company, and to the extent not assumed or substituted, any outstanding option will terminate and shares of restricted common stock will be repurchased.
Adjustment provisions
If, through or as a result of any recapitalization, reclassification, stock dividend, stock split, reverse stock split, liquidation, exchange of shares, spin-off, combination, consolidation or other similar transaction, (i) the outstanding shares of common stock are increased, decreased or exchanged for a different number or kind of shares or other securities or (ii) additional shares or new or different shares or other non-cash assets are distributed with respect to such shares or other securities, an appropriate and proportionate adjustment will be made to (x) the maximum number and kind of shares reserved for issuance under the 2021 Plan, (y) the number and kind of securities underlying awards then outstanding and (z) the exercise price for each option, without changing the aggregate purchase price as to which such options remain exercisable.
Amendments and termination
The Administrator may at any time amend the 2021 Plan and may at any time terminate the 2021 Plan as to future grants. However, except as expressly provided in the 2021 Plan, the Administrator may not alter the terms of the plan so as to adversely affect a participant’s rights under an award without the participant’s consent. Any amendments to the 2021 Plan will be conditioned on stockholder approval to the extent required.
2026 compensation plans
2026 Equity Incentive Plan
In connection with this offering, our board of directors has adopted and our stockholders have approved the 2026 Plan and, in connection with and following this offering, all equity-based awards to our employees, directors and consultants will be granted under the 2026 Plan. The following summary describes what we expect to be the material terms of the 2026 Plan. This summary is not a complete description of all provisions of the 2026 Plan and is qualified in its entirety by reference to the 2026 Plan, which will be filed as an exhibit to the registration statement of which this prospectus is a part.
Grants in connection with this Offering
In connection with this offering, effective upon pricing of this offering, our board of directors has granted an option to purchase 51,525 shares of our common stock to each of John W. Childs, Vladimir Coric and Jane Grant-Kels, an option to purchase 45,131 shares of our common stock to Patrick Enright, and an option to purchase 43,000 shares of our common stock to each of David Friedman and Katarina Pance. These options will vest in full on the first anniversary of the date of grant, generally subject to the director’s continued service through such date, and will have an exercise price equal to the price per share of our common stock in this offering.
In addition, our board of directors has granted an option to purchase shares of our common stock to our named executive officers and certain of our other employees, in each case effective upon the pricing of this offering. These options will vest as to 25% of the underlying shares on the first anniversary of the date of grant, and as to the remaining 75% of the underlying shares in 36 equal monthly installments thereafter, generally subject to the grantee’s continued employment through each applicable vesting date, and will have an exercise price equal to the price per share of our common stock in this offering. Dr. Waldman will receive an option to purchase 556,399 shares of our common stock. Dr. Durso will receive an option to purchase 213,352 shares of our common stock, Mr. Carrano will receive an option to purchase 148,011 shares of our common stock, and other employees will receive options to purchase an aggregate of 564,786 shares of our common stock.
Purpose. The purpose of the 2026 Plan is to advance our interests by providing for the grant of stock and stock-based awards to our employees, directors and consultants.
Plan Administration. The 2026 Plan will generally be administered by the compensation committee. The compensation committee (or our board of directors, as applicable) will have the discretionary authority to interpret the 2026 Plan and any awards granted under it, determine eligibility for and grant awards, determine the exercise price, base value from which appreciation is measured, or purchase price, if any, applicable to any award, determine, modify, accelerate and waive the terms and conditions of any award, determine the form of settlement of awards, prescribe forms, rules and procedures relating to the 2026 Plan and awards and otherwise do all things necessary or desirable to carry out the purposes of the 2026 Plan or any award. The compensation committee may delegate such of its duties, powers and responsibilities as it may determine to one or more of its members, members of our board of directors and, to the extent permitted by law, our officers, and may delegate to employees and other persons such ministerial tasks as it deems appropriate. As used in this summary, the term “Administrator” refers to the compensation committee and its authorized delegates, as applicable.
Eligibility. Our employees, directors and consultants are eligible to participate in the 2026 Plan. Eligibility for stock options intended to be incentive stock options, or ISOs, is limited to our employees or employees of certain affiliates. Eligibility for stock options, other than ISOs, and stock appreciation rights, or SARs, is limited to individuals who are providing direct services to us or certain affiliates on the date of grant of the award.
Authorized Shares. Subject to adjustment as described below, the maximum number of shares of our common stock that may be delivered in satisfaction of awards under the 2026 Plan is (i) the number of shares equal to 10.8% of the shares of our common stock issued and outstanding as of immediately following the consummation of this offering (not to exceed 4,518,426 shares), or the share pool, plus (ii) the number of shares of common stock underlying awards under the 2021 Plan that on or after the date the 2026 Plan is adopted are reacquired by us or are forfeited, expire, terminate or are cancelled for any reason without having been exercised or become vested in full, or otherwise become available again for grant under the 2021 Plan, in each case in accordance with its terms (up to an aggregate of 685,080 shares). The share pool will automatically increase on January 1st of each year beginning in 2027 and continuing through and including 2036 by the lesser of (i) five percent of the number of shares of our common stock outstanding as of the close of business on the immediately preceding December 31st and (ii) the number of shares determined by our board of directors on or prior to such date for such year. The share pool will automatically increase on January 1st of each year beginning in 2027 and continuing through and including 2036 by the lesser of (i) five percent of the number of shares of our common stock outstanding as of the close of business on the immediately preceding December 31st and (ii) the number of shares determined by our board of directors on or prior to such date for such year. Up to 24,289,722 shares may be delivered in satisfaction of ISOs. The number of shares of our common stock delivered in satisfaction of awards under the 2026 Plan is determined (i) by excluding shares withheld by us in payment of the exercise price or purchase price of the award or in satisfaction of tax withholding requirements with respect to the award, (ii) by including only the number of shares delivered in settlement of a SAR that is settled in shares of our common stock, and (iii) by excluding any shares underlying awards settled in cash or that expire, become unexercisable, terminate or are forfeited to or repurchased by us, in each case, without the delivery of shares of our common stock (or retention, in the case of restricted stock or unrestricted stock). The number of shares available for delivery under the 2026 Plan will not be increased by any shares that have been delivered under the 2026 Plan and are subsequently repurchased using proceeds directly attributable to stock option exercises. Shares that may be delivered under the 2026 Plan may be authorized but unissued shares, treasury shares or previously issued shares acquired by us.
Types of Awards. The 2026 Plan provides for the grant of stock options, SARs, restricted and unrestricted stock and stock units, performance awards and other awards that are convertible into or otherwise based on our common stock. Dividend equivalents may also be provided in connection with awards under the 2026 Plan.
•Stock Options and SARs. The Administrator may grant stock options, including ISOs, and SARs. A stock option is a right entitling the holder to acquire shares of our common stock upon payment of the applicable exercise price. A SAR is a right entitling the holder upon exercise to receive an amount (payable in cash or shares of equivalent value) equal to the excess of the closing price of the shares subject to the right over the base value from which appreciation is measured. The exercise price per share of each stock option, and the
base value of each SAR, granted under the 2026 Plan will not be less than 100% of the closing price of a share on the date of grant (or, if no closing price is reported on that date, the closing price on the immediately preceding date on which a closing price was reported) (110% in the case of certain ISOs). Other than in connection with certain corporate transactions or changes to our capital structure, stock options and SARs granted under the 2026 Plan may not be repriced, amended, or substituted for with new stock options or SARs having a lower exercise price or base value, nor may any consideration be paid upon the cancellation of any stock options or SARs that have a per share exercise or base price greater than the closing price of a share on the date of such cancellation (or, if no closing price is reported on that date, the closing price on the immediately preceding date on which a closing price was reported), in each case, without stockholder approval. Each stock option and SAR will have a maximum term of not more than ten years from the date of grant (or five years, in the case of certain ISOs).
•Restricted and Unrestricted Stock and Stock Units. The Administrator may grant awards of stock, stock units, restricted stock and restricted stock units. A stock unit is an unfunded and unsecured promise, denominated in shares, to deliver shares or cash measured by the value of shares in the future, and a restricted stock unit is a stock unit that is subject to the satisfaction of specified performance or other vesting conditions. Restricted stock are shares subject to restrictions requiring that they be forfeited, redelivered or offered for sale to us if specified performance or other vesting conditions are not satisfied.
•Performance Awards. The Administrator may grant performance awards, which are awards that vest subject to the achievement of performance criteria.
•Other Share-Based Awards. The Administrator may grant other awards that are convertible into or otherwise based on shares of our common stock, subject to such terms and conditions as it determines.
•Substitute Awards. The Administrator may grant substitute awards in connection with certain corporate transactions, which may have terms and conditions that are different from the terms and conditions of the 2026 Plan.
Non-Employee Director Limits. The aggregate value of all compensation granted or paid to any director with respect to any calendar year, including the grant date fair value of awards granted under the 2026 Plan and cash fees or other compensation paid by us to such director outside of the 2026 Plan for services as a director during such calendar year (which, for the avoidance of doubt, will not include compensation granted or paid to a director for services other than as a director, including without limitation, for services as a consultant or advisor to the Company), is subject to a limit of $750,000 in the aggregate ($1,000,000 in the aggregate with respect to a director’s first year of service on our board of directors).
Vesting; Terms of Awards. The Administrator determines the terms and conditions of all awards granted under the 2026 Plan, including the time or times an award vests or becomes exercisable, the terms and conditions on which an award remains exercisable, and the effect of termination of a participant’s employment or service on an award. The Administrator may at any time accelerate the vesting or exercisability of an award.
Non transferability of Awards. Except as the Administrator may otherwise determine, awards may not be transferred other than by will or by the laws of descent and distribution.
Adjustments upon Certain Covered Transactions. In the event of certain covered transactions (including the consummation of a consolidation, merger or similar transaction, the sale of all or substantially all of our assets or shares of our common stock, or our dissolution or liquidation), the Administrator may, with respect to outstanding awards, provide for (in each case, on such terms and subject to such conditions as it deems appropriate):
•The assumption, substitution or continuation of some or all awards (or any portion thereof) by the acquiror or surviving entity;
•The acceleration of exercisability or delivery of shares in respect of any award, in full or in part; and/or
•The cash payment in respect of some or all awards (or any portion thereof) equal to the difference between the fair market value of the shares subject to the award and its exercise or base price, if any.
Except as the Administrator may otherwise determine, each award will automatically terminate or be forfeited immediately upon the consummation of the covered transaction, other than awards that are substituted for, assumed, or that continue following the covered transaction.
Adjustments upon Changes in Capitalization. In the event of certain corporate transactions, including a recapitalization, reclassification, stock dividend, stock split, reverse stock split, liquidation, exchange of shares, spin-off, combination, consolidation, or other similar change in our capital structure, the Administrator will make appropriate adjustments to the maximum number of shares that may be delivered under the 2026 Plan, the number and kind of securities subject to, and, if applicable, the exercise or purchase prices (or base values) of outstanding awards, and any other provisions affected by such event.
Recovery of Compensation. The Administrator may provide that any outstanding award, the proceeds of any award or shares acquired thereunder and any other amounts received in respect of any award or shares acquired thereunder will be subject to forfeiture and disgorgement to us, with interest and other related earnings, if the participant to whom the award was granted is not in compliance with any provision of the 2026 Plan or any award, or violates any non-competition, non-solicitation, no-hire, non-disparagement, confidentiality, invention assignment or other restrictive covenant in favor of the Company or any of its affiliates, or any Company policy that relates to trading on non-public information and permitted transactions with respect to shares of our common stock or provides for forfeiture, disgorgement or clawback, or as otherwise required by law or applicable stock exchange listing standards.
Amendment and Termination. The Administrator may at any time amend the 2026 Plan or any outstanding award and may at any time terminate the 2026 Plan as to future awards. However, except as expressly provided in the 2026 Plan, the Administrator may not alter the terms of an award so as to materially and adversely affect a participant’s rights without the participant’s consent (unless the Administrator expressly reserved the right to do so in the 2026 Plan or at the time the award was granted). Any amendments to the 2026 Plan will be conditioned on stockholder approval to the extent required by applicable law or stock exchange requirements.
2026 Employee Stock Purchase Plan
In connection with this offering, our board of directors has adopted and the stockholders have approved the Veradermics, Incorporated 2026 Employee Stock Purchase Plan, or the ESPP. The following summary describes what we expect to be the material terms of the ESPP. This summary is not a complete description of all provisions of the ESPP and is qualified in its entirety by reference to the ESPP, which will be filed as an exhibit to the registration statement of which this prospectus is a part.
Purpose. The purpose of the ESPP is to enable eligible employees of us and our participating subsidiaries to use payroll deductions to purchase shares of our common stock, and thereby acquire an interest in us. The ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Code.
Administration. The ESPP will be administered by the compensation committee, which will have the discretionary authority to administer and interpret the ESPP, determine eligibility under the ESPP, prescribe forms, rules and procedures relating to the ESPP, and otherwise do all things necessary or desirable to carry out the purposes of the ESPP. The compensation committee may delegate such of its duties, powers and responsibilities as it may determine to one or more of its members, members of our board of directors and our officers and employees, in each case, to the extent permitted by law. As used in this summary, the term “Administrator” refers to the compensation committee and its authorized delegates, as applicable.
Shares Subject to the ESPP. Subject to adjustment as described below, the aggregate number of shares of our common stock available for purchase pursuant to the exercise of options under the ESPP is 316,668 shares, plus an automatic annual increase, as of January 1st of each year beginning in 2027 and continuing through and including 2036, equal to the lesser of (i) one percent of the number of shares of our common stock outstanding as of the close of business on the immediately preceding December 31st and (ii) the number of shares determined by our board of directors on or prior to such date for such year. Shares to be delivered upon exercise of options under the ESPP may be authorized but unissued shares, treasury shares, or previously issued shares acquired by us. If any option granted under the ESPP expires or terminates for any reason without having
been exercised in full or ceases for any reason to be exercisable in whole or in part, the unpurchased shares subject to such option will remain available for purchase under the ESPP.
Eligibility. Participation in the ESPP will generally be limited to our employees and employees of our subsidiaries who are employed by us or one of our subsidiaries, as applicable, as of the first day of an applicable offering period and who satisfy the requirements set forth in the ESPP. The Administrator may establish additional or other eligibility requirements, or change the requirements set forth in the ESPP, to the extent consistent with Section 423 of the Code. Any employee who owns (or is deemed under statutory attribution rules to own) shares possessing five percent or more of the total combined voting power or value of all classes of shares of us or our parent or subsidiaries, if any, will not be eligible to participate in the ESPP.
General Terms of Participation. The ESPP allows eligible employees to purchase shares of our common stock during specified offering periods. On the first day of each offering period, eligible employees will be granted an option to purchase shares of our common stock on the last business day of the offering period. A participant may purchase a maximum of 5,000 shares with respect to any offering period (or such lesser number as the Administrator may prescribe). No participant will be granted an option under the ESPP that permits the participant’s right to purchase shares of our common stock under the ESPP and under all other employee stock purchase plans of us or our parent or subsidiaries, if any, to accrue at a rate that exceeds $25,000 in fair market value (or such other maximum as may be prescribed by the Code) for each calendar year during which any option granted to the participant is outstanding at any time, determined in accordance with Section 423 of the Code.
The purchase price of each share issued pursuant to the exercise of an option under the ESPP on an exercise date will be 85% (or such greater percentage as specified by the Administrator) of the lesser of: (a) the closing price of a share of our common stock on the date the option is granted (or, if no closing price is reported on that date, the closing price on the immediately preceding date on which a closing price was reported), which will be the first day of the offering period, and (b) the closing price of a share of our common stock on the exercise date (or, if no closing price is reported on that date, the closing price on the immediately preceding date on which a closing price was reported), which will be the last business day of the offering period.
The Administrator has the discretion to change the commencement and exercise dates of offering periods, the purchase price, the maximum number of shares that may be purchased with respect to any offering period, the duration of any offering periods and other terms of the ESPP, in each case, without stockholder approval, except as required by law.
Participants in the ESPP will pay for shares purchased under the ESPP through payroll deductions. Participants may elect to authorize payroll deductions in an amount equal to a pre-established percentage of the participant’s eligible compensation each payroll period.
Transfer Restrictions. For participants who have purchased shares under the ESPP, the Administrator may impose restrictions prohibiting the transfer, sale, pledge or alienation of such shares, other than by will or by the laws of descent and distribution, for such period as may be determined by the Administrator.
Adjustments upon Changes in Capitalization. In the event of a recapitalization, reclassification, stock dividend, stock split, reverse stock split, liquidation, exchange of shares, spin-off, combination, consolidation or other similar change in our capital structure that constitutes an equity restructuring, the Administrator will make appropriate adjustments to the maximum number and type of shares available for purchase under the ESPP, the number and type of shares granted under any outstanding options, the maximum number and type of shares purchasable under any outstanding option and/or the purchase price per share under any outstanding option.
Covered Transactions. In the event of (i) a consolidation, merger or similar transaction or series of related transactions, including a sale or other disposition of stock, in which we are not the surviving entity or which results in the acquisition of all or substantially all of our then outstanding common stock by another person, (ii) a sale or transfer of all or substantially all of our assets, or (iii) a dissolution or liquidation, the Administrator may provide that each outstanding option will be assumed or substituted for or will be cancelled and the
balances of participants’ accounts returned, or that the option period will end before the date of the proposed covered transaction.
Amendment and Termination. The Administrator has discretion to amend the ESPP to any extent and in any manner it may deem advisable, provided that any amendment that would be treated as the adoption of a new plan for purposes of Section 423 of the Code will require stockholder approval. The Administrator may suspend or terminate the ESPP at any time.
Cash Incentive Plan
In connection with this offering, our board of directors has adopted the Veradermics, Incorporated 2026 Cash Incentive Plan, or the Cash Incentive Plan, to be effective upon the effectiveness of the registration statement of which this prospectus forms a part. Following its adoption, the Cash Incentive Plan will provide for the grant of cash-based incentive awards to our executive officers and other key employees. The following summary describes what we expect to be the material terms of the Cash Incentive Plan. This summary is not a complete description of all provisions of the Cash Incentive Plan and is qualified in its entirety by reference to the Cash Incentive Plan, which will be filed as an exhibit to the registration statement of which this prospectus is a part.
Purpose. The purpose of the Cash Incentive Plan is to advance our interests by providing for the grant of cash-based incentive awards to our executive officers and other key employees that will attract, retain, and reward such persons and incentivize them to attain key Company performance criteria and metrics.
Plan Administration. The Cash Incentive Plan will be administered by the compensation committee and its delegates. As used in this summary, the term “Administrator” refers to the compensation committee and its authorized delegates, as applicable. The Administrator will have the discretionary authority to administer and interpret the Cash Incentive Plan and any awards; determine eligibility for and grant awards; adjust the performance criterion or criteria applicable to awards; determine, modify or waive the terms and conditions of any award; prescribe forms, rules and procedures relating to the Cash Incentive Plan and awards, and otherwise do all things necessary or desirable to carry out the purposes of the Cash Incentive Plan.
Eligibility and Participation. Executive officers and other key employees of us and our subsidiaries will be eligible to participate in the Cash Incentive Plan and will be selected from time to time by the Administrator to participate in the Cash Incentive Plan.
Awards; Performance Criteria. Awards under the Cash Incentive Plan will be made based on, and subject to achieving, specified criteria established by the Administrator. For each award granted under the Cash Incentive Plan, the Administrator will establish the performance criteria applicable to the award, the amount or amounts payable if the performance criteria are achieved and such other terms and conditions as the Administrator deems appropriate.
Payments under an Award. A participant will be entitled to payment under an award only if all conditions to payment have been satisfied in accordance with the Cash Incentive Plan and the terms of the award. Following the end of a performance period, the Administrator will determine whether and to what extent the applicable performance criteria have been satisfied and will determine the amount payable under each award. The Administrator has the discretionary authority to increase or decrease the amount actually paid under any award.
Recovery of Compensation. Payments in respect of an award will be subject to forfeiture and disgorgement to us if the participant violates a non-competition, non-solicitation, confidentiality or other restrictive covenant or to the extent provided in any applicable Company policy that provides for forfeiture or disgorgement, or as otherwise required by law or applicable stock exchange listing standards.
Amendment and Termination. The Administrator may amend the Cash Incentive Plan or any outstanding award for any purpose, and may at any time terminate the Cash Incentive Plan as to any future grant of awards.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a summary of transactions since January 1, 2022, to which we have been a party in which the amount involved exceeded the lesser of (i) $120,000 or (ii) one percent of the average of our total assets at year end for the last two completed fiscal years, and in which any of our executive officers, directors, promoters or beneficial holders of more than 5% of our capital stock had or will have a direct or indirect material interest, other than compensation arrangements which are described under the section of this prospectus captioned “Executive and Director Compensation.” We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions with unrelated third parties. Private Placements
Series A-4 Preferred Stock
In April 2023, we entered into a Series A‑4 Preferred Stock Purchase Agreement pursuant to which we issued an aggregate of 4,965,572 shares of our Series A-4 Preferred Stock at a purchase price of $3.0208 per share. Each share of our Series A-4 Preferred Stock will convert into shares of our common stock immediately prior to the consummation of this offering, including adjustments in connection with the 1-for-10.067 reverse stock split of our common stock, which was effected on January 27, 2026. The following table summarizes the Series A-4 Preferred Stock issued to our directors, executive officers and beneficial holders of more than 5% of our capital stock:
| | | | | | | | | | | | | | |
| Purchaser | | Shares of Series A-4 Preferred Stock | | Aggregate Purchase Price of Shares Purchased ($) |
Entities affiliated with J.W. Childs Associates(1) | | 1,655,191 | | | 5,000,001 | |
| Entities Affiliated with ChemWerth, Inc. | | 827,595 | | | 2,499,999 | |
| Connecticut Innovations, Incorporated | | 794,491 | | | 2,399,998 | |
Therapeutics, Inc. | | 354,212 | | | 1,070,004 | |
| Mediqventures Limited | | 331,038 | | | 1,000,000 | |
Trusts affiliated with Vladimir Coric(2) | | 331,038 | | | 1,000,000 | |
| Christopher S. Eklund Trust U/A/D 8/31/2000 | | 281,382 | | | 849,999 | |
| | | | |
(1)Mr. Childs, a member of our board of directors, serves as Chairman of J.W. Childs Associates, L.P. Entities related to J.W. Childs Associates beneficially own more than 5% of our capital stock.
(2)Dr. Coric, a member of our board of directors, is a beneficiary of the Vladimir Coric Family Trust 2013 and the Vladimir Coric Marital Trust 2013, each of which is administered by Elizabeth Ann Coric as trustee. Trusts affiliated with Vladimir Coric beneficially own more than 5% of our capital stock.
Series B Preferred Stock
In November 2024, we entered into a Series B Preferred Stock Purchase Agreement, or the Series B Preferred Stock Purchase Agreement, pursuant to which we issued an aggregate of 62,245,805 shares of our Series B Preferred Stock at a purchase price of $1.2049 per share. Where applicable, the payment of the purchase price consisted of or included the automatic conversion of certain convertible promissory notes, representing an aggregate outstanding principal amount and accrued interest of $10.4 million, into 8,661,917 shares of Series B Preferred Stock. Each share of our Series B Preferred Stock will convert into shares of our common stock immediately prior to the consummation of this offering, including adjustments in connection with the 1-for-10.067 reverse stock split of our common stock, which was effected on January 27, 2026. The following
table summarizes the Series B Preferred Stock issued to our directors, executive officers and beneficial holders of more than 5% of our capital stock:
| | | | | | | | | | | | | | | | | | | | |
| Purchaser | | Shares of Series B Preferred Stock | | Aggregate Purchase Price of Shares Purchased ($) | | Aggregate Principal Amount of Converted Convertible Promissory Notes ($) |
Longitude Venture Partners V, L.P.(1) | | 12,449,165 | | | 14,999,999 | | | — | |
Entities affiliated with J.W. Childs Associates(2) | | 9,129,388 | | | 6,151,611 | | | 4,848,389 | |
Entities affiliated with Suvretta Capital (3) | | 8,299,443 | | | 9,999,999 | | | — | |
| Citadel Multi-Strategy Equities (Surveyor) | | 6,224,582 | | | 7,499,999 | | | — | |
Therapeutics, Inc. | | 3,533,209 | | | 3,032,917 | | | 1,224,246 | |
| Connecticut Innovations, Incorporated | | 7,413,807 | | | 8,932,896 | | | — | |
Trusts affiliated with Vladimir Coric(4) | | 2,995,836 | | | 2,748,006 | | | 861,677 | |
| Christopher S. Eklund Trust U/A/D 8/31/2000 | | 1,872,561 | | | 1,117,551 | | | 1,138,698 | |
| Entities affiliated with ChemWerth, Inc. | | 873,308 | | | — | | | 1,052,250 | |
| | | | | | |
(1)Mr. Enright, a member of our board of directors, is co-founder and serves as a Managing Director of Longitude Capital Management Co., LLC, an entity affiliated with Longitude Venture Partners V, L.P, or LVPV. LVPV beneficially owns more than 5% of our capital stock.
(2)Mr. Childs, a member of our board of directors, serves as Chairman of J.W. Childs Associates, L.P. Entities related to J.W. Childs Associates beneficially own more than 5% of our capital stock.
(3)Dr. Friedman, a member of our board of directors, serves as a Managing Director of Suvretta Capital. Dr. Birdsey-Benson, a former member of our board of directors, serves as a Managing Director of Suvretta Capital. Entities related to Suvretta Capital beneficially own more than 5% of our capital stock.
(4)Dr. Coric, a member of our board of directors, is a beneficiary of the Vladimir Coric Family Trust 2013 and the Vladimir Coric Marital Trust 2013, each of which is administered by Elizabeth Ann Coric as trustee. Trusts affiliated with Vladimir Coric beneficially own more than 5% of our capital stock.
Series C Preferred Stock
In October 2025, we entered into a Series C Preferred Stock Purchase Agreement pursuant to which we issued an aggregate of 118,682,683 shares of our Series C Preferred Stock at a purchase price of $1.2723 per share. Each share of our Series C Preferred Stock will convert into shares of our common stock immediately prior to the consummation of this offering, including adjustments in connection with the 1-for-10.067 reverse stock split of our common stock, which was effected on January 27, 2026. The following table summarizes the Series C Preferred Stock issued to our directors, executive officers and beneficial holders of more than 5% of our capital stock:
| | | | | | | | | | | | | | |
| Purchaser | | Shares of Series C Preferred Stock | | Aggregate Purchase Price of Shares Purchased ($) |
Entities affiliated with Longitude Capital(1) | | 23,579,344 | | | 30,000,000 | |
Entities affiliated with SR One(2) | | 19,649,453 | | | 24,999,999 | |
| Entities affiliated with Viking Global | | 15,719,562 | | | 19,999,999 | |
Entities affiliated with Suvretta Capital(3) | | 9,431,736 | | | 11,999,998 | |
| Citadel Multi-Strategy Equities (Surveyor) | | 7,859,781 | | | 9,999,999 | |
Entities affiliated with J.W. Childs Associates(4) | | 5,242,474 | | | 6,670,000 | |
Vladimir Coric(5) | | 2,750,923 | | | 3,499,999 | |
| | | | |
(1)Mr. Enright, a member of our board of directors, is co-founder and serves as a Managing Director of Longitude Capital Management Co., LLC, an entity affiliated with LVPV and L103. LVPV and L103 collectively beneficially own more than 5% of our capital stock.
(2)Dr. Pance, a member of our board of directors, serves as a Senior Associate at SR One Capital Management. Entities related to SR One beneficially own more than 5% of our capital stock.
(3)Dr. Friedman, a member of our board of directors, serves as a Managing Director of Suvretta Capital. Dr. Birdsey-Benson, a former member of our board of directors, serves as a Managing Director of Suvretta Capital. Entities related to Survetta Capital beneficially own more than 5% of our capital stock.
(4)Mr. Childs, a member of our board of directors, serves as Chairman of J.W. Childs Associates, L.P. Entities related to J.W. Childs Associates beneficially own more than 5% of our capital stock.
(5)Dr. Coric, a member of our board of directors, is a beneficiary of the Vladimir Coric Family Trust 2013 and the Vladimir Coric Marital Trust 2013, each of which is administered by Elizabeth Ann Coric as trustee. Trusts affiliated with Vladimir Coric beneficially own more than 5% of our capital stock.
Investors’ Rights Agreement
We are party to a Third Amended and Restated Investors’ Rights Agreement, or the Investors’ Rights Agreement, with our current stockholders. Pursuant to the terms of the Investors’ Rights Agreement, we granted these stockholders certain information rights and the right to participate in future stock issuances, which rights terminate upon this offering. The Investors’ Rights Agreement also grants these stockholders certain registration rights. See the section titled “Description of Capital Stock—Registration Rights” for additional information regarding these registration rights. Other provisions of the Investors’ Rights Agreement will terminate upon completion of this offering. Director Affiliations
Some of our directors are affiliated with and, prior to the closing of this offering, have served on our board of directors as representatives of entities which beneficially own or owned 5% or more of our voting securities, as indicated in the table below:
| | | | | | | | |
| Director | | Affiliated Stockholder |
David Friedman, M.D. | | Entities affiliated with Suvretta Capital |
| John W. Childs | | Entities affiliated with J.W. Childs Associates |
Patrick Enright | | Entities affiliated with Longitude Capital |
| Katarina Pance, Ph.D | | Entities affiliated with SR One |
| | |
Agreements with Therapeutics, Inc.
In September 2020, we entered into the MSA and the Collaboration Agreement with TI. The MSA and Collaboration Agreement are described in more detail under “Business—Agreements with Therapeutics, Inc.” Pursuant to the Collaboration Agreement, we issued TI a convertible promissory note with a principal amount of $0.5 million. On September 22, 2021, the convertible promissory note was converted into 238,008 shares of our Series A‑2 Preferred Stock. In August 2024, we issued to TI a convertible promissory note with a principal amount of $1.2 million for committed research and development services. In November 2024, the convertible promissory note was converted into 1,016,056 shares of our Series B Preferred Stock pursuant to the Series B Preferred Stock Purchase Agreement. Prior to the conversion, the aggregate principal amount and unpaid interest of the convertible promissory note was $1.2 million. During the years ended December 31, 2024, 2023 and 2022, and the nine months ended September 30, 2025, we paid TI $14.4 million, $5.9 million, $1.4 million and $31.9 million, respectively, in connection with the MSA and work orders thereunder. TI beneficially owned more than 5% of our capital stock during the years ended December 31, 2024, 2023 and 2022.
Agreement with ChemWerth, Inc.
In July 2022, we entered into a Development and Manufacturing Agreement, or the Development and Manufacturing Agreement with ChemWerth, Inc., or ChemWerth. In accordance with the Development and Manufacturing Agreement, ChemWerth provides development and manufacturing services for the production of active pharmaceutical ingredients or drug substances identified by the Company. In May 2023, we issued to ChemWerth a promissory note with principal amount of $0.8 million, and in August 2024, we issued to ChemWerth a convertible note with principal amount of $0.3 million, each in connection with the Development and Manufacturing Agreement. In November 2024, we repaid the promissory note, including accrued interest, in full with proceeds from our Series B Preferred Stock financing, and the convertible note, including accrued interest, was converted into 873,3088 shares of our Series B Preferred Stock pursuant to the Series B Preferred Stock Purchase Agreement. Prior to the repayment of the promissory note and the conversion of the convertible note, the aggregate principal amount and unpaid interest of the promissory note and the convertible note was $0.8 million and $0.3 million, respectively. During the years ended December 31, 2024, 2023 and 2022, and the nine months ended September 30, 2025, we paid ChemWerth $1.1 million, $2.7 million, $0.5 million and $0.5 million, respectively, in connection with the Development and Manufacturing Agreement. ChemWerth and its affiliates together beneficially owned more than 5% of our capital stock during the years ended December 31, 2024, 2023 and 2022, respectively.
Agreements with Green Line Talent Group LLC
From December 2024 to July 2025, we entered into certain talent search agreements with Green Line Talent Group LLC, or Green Line, pursuant to which we retained Green Line on an exclusive basis to provide end-to-end recruitment services for various roles at the Company. During the nine months ended September 30, 2025, we paid Green Line $0.1 million pursuant to the talent search agreements. The Green Line Agreements may be considered a related party transactions because Kristen Nielsen, a Partner at Green Line, is the spouse of Mr. Carrano, our Chief Financial Officer. The Green Line agreements were negotiated on an arm’s-length basis and are market rate transaction on terms that we believe are no less favorable than would have been reached with an unrelated third party.
Director and Officer Indemnification and Insurance
We have agreed to indemnify each of our directors and executive officers against certain liabilities, costs and expenses, and have purchased directors’ and officers’ liability insurance. We also maintain a general liability insurance policy which covers certain liabilities of directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers.
Related Person Transactions Policy
Our board of directors has adopted a written related person transaction policy, to be effective upon the effectiveness of the registration statement of which this prospectus forms a part, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved exceeds the lesser of (i) $120,000 or (ii) one percent of the average of our total assets at year end for the last two completed fiscal years, in any fiscal year and a related person had, has or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked with considering all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of September 30, 2025, as adjusted to reflect the conversion of all preferred stock into common stock prior to the consummation of this offering and the sale of common stock offered by us in this offering, for:
neach person who we know beneficially owns more than 5% of our common stock;
neach of our directors;
neach of our named executive officers; and
nall of our directors and executive officers as a group.
The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC. Under these rules, a person is deemed to be a “beneficial” owner of a security if that person has or shares voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. Except as indicated in the footnotes below, we believe, based on the information furnished to us, that the individuals and entities named in the table below have sole voting and investment power with respect to all shares of common stock beneficially owned by them, subject to any applicable community property laws.
Percentage ownership of our common stock before this offering is based on 20,000,170 shares of our common stock outstanding as of December 31, 2025, prior to the consummation of this offering, and after giving effect to the automatic conversion of all outstanding shares of our convertible preferred stock as of December 31, 2025 into an aggregate of 19,250,410 shares of our common stock immediately prior to the consummation of this offering. Percentage ownership of our common stock after this offering is based on shares of our common stock outstanding as of December 31, 2025, after giving effect to the transactions as described above and our issuance of shares of our common stock in this offering. The table below excludes any purchases that may be made in this offering. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to options or other rights held by such person that are currently exercisable or that will become exercisable within 60 days of December 31, 2025 are considered outstanding, although these shares are not considered outstanding for purposes of computing the
percentage ownership of any other person. Unless noted otherwise, the address of all listed stockholders is 470 James St. New Haven, CT, 06513.
| | | | | | | | | | | | | | | | | | | | |
| NAME OF BENEFICIAL OWNER | | NUMBER OF SHARES BENEFICIALLY OWNED | | PERCENTAGE OF SHARES BENEFICIALLY OWNED |
| | BEFORE OFFERING | | AFTER OFFERING |
5% or greater stockholders: | | | |
| |
|
Entities affiliated with Longitude Capital(1) | | 3,578,873 | | | 17.9 | % | | 10.7 | % |
Entities affiliated with SR One(2) | | 1,951,868 | | | 9.8 | % | | 5.9 | % |
Entities affiliated with J.W. Childs Associates(3) | | 1,907,889 | | | 9.5 | % | | 5.7 | % |
Entities affiliated with Suvretta Capital(4) | | 1,761,317 | | | 8.8 | % | | 5.3 | % |
Entities affiliated with Viking Global(5) | | 1,561,494 | | | 7.8 | % | | 4.7 | % |
Citadel Multi-Strategy Equities (Surveyor)(6) | | 1,399,062 | | | 7.0 | % | | 4.2 | % |
Directors and Named Executive Officers: | | | |
| |
|
Reid Waldman, M.D.(7) | | 499,121 | | | 2.5 | % | | 1.5 | % |
Timothy Durso, M.D.(8) | | 400,175 | | | 2.0 | % | | 1.2 | % |
Dominic Carrano, CPA(9) | | 44,249 | | | * | | * |
David Friedman, M.D. | | — | | | — | % | | — | % |
John W. Childs(3) | | 1,907,889 | | | 9.5 | % | | 5.7 | % |
Vlad Coric, M.D.(10) | | 652,554 | | | 3.3 | % | | 2.0 | % |
Katarina Pance, Ph.D. | | — | | | — | % | | — | % |
Patrick Enright(1) | | 3,578,872 | | | 17.9 | % | | 10.7 | % |
Jane Grant-Kels, M.D.(11) | | 22,431 | | | * | | * |
All executive officers and directors as a group (11 persons)(12) | | 7,105,291 | | | 35.5 | % | | 21.3 | % |
| | | | | | |
* Represents beneficial ownership of less than one percent.
(1)Consists of (i) 2,407,752 shares of common stock held by Longitude Venture Partners V, L.P, or LVPV and (ii) 1,171,121 shares of common stock held by Longitude 103.8 East, L.P., or L103. Longitude Capital Partners V, LLC, or LCPV, is the general partner of LVPV and may be deemed to have voting, investment and dispositive power with respect to these securities. Longitude 103.8 East Partners, LLC, or L103P is the general partner of L103 and may be deemed to have voting, investment and dispositive power with respect to these securities. Patrick G. Enright, a member of our Board, and Juliet Tammenoms Bakker are the managing members of each of LCPV and L103P, and may each be deemed to share voting, investment and dispositive power with respect to these securities. Each of LVPV, LCPV, L103, L103P, Mr. Enright and Ms. Tammenoms Bakker disclaims beneficial ownership of such securities except to the extent of their respective pecuniary interests therein. The address for these individuals and entities is 2740 Sand Hill Road, 2nd Floor, Menlo Park, CA 94025.
(2)Consists of (i) 1,171,121 shares of common stock held by SR One Capital Fund II Aggregator, LP, or SR One Capital Fund II Aggregator and (ii) 780,747 shares of common stock held by AMZL, LP, or AMZL. SR One Capital Fund II Aggregator is directly controlled by its general partner, SR One Capital Partners II, LP, or SR One Capital Partners. AMZL is directly controlled by its general partner, SR One Capital SMA Partners, LP, or SMA Partners. SMA Partners and SR One Capital Partners are directly controlled by their general partners, SR One Capital Management, LLC, or SR One Capital Management, and Simeon George, M.D. controls SR One Capital Management. Accordingly, each of SR One Capital Management and Simeon George, M.D. may be deemed to have voting and dispositive power with respect to the 1,951,868 shares of common stock held of record by SR One Fund II Aggregator and AMZL. Katarina Pance, Ph.D. is a Senior Associate at SR One Capital Management, LP, an entity affiliated with SR One Fund II Aggregator and AMZL, and a member of our board of directors, and has no voting or dispositive power with respect to any of the above referenced shares and disclaims beneficial ownership of such shares except to the extent of her
actual pecuniary interest therein. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their pecuniary interest therein. The address for these entities is 929 Main Street, Suite 200, Redwood City, California, 94063.
(3)Consists of 1,907,889 shares of common stock held by J.W. Childs Associates (FL), L.P. John W. Childs 2013 Revocable Trust is the sole owner of J.W. Childs Associates (FL), L.P. John W. Childs, a member of our Board, is Trustee of John W. Childs 2013 Revocable Trust and may be deemed to hold voting and dispositive power with respect to these securities. The principal business address of J.W. Childs Associates (FL), L.P. is 180 Lakeview Avenue, Suite 2500, West Palm Beach, FL 33401.
(4)Consists of (i) 1,701,918 shares of common stock held by Averill Master Fund, Ltd., or Averill Master Fund, and (ii) 59,399 shares of common stock held by Averill Madison Master Fund, Ltd., or Averill Madison Fund and, together with Averill Master Fund, the Averill Funds. Suvretta Capital Management, LLC is the investment manager of the Averill Funds. Aaron Cowen is a control person of Suvretta Capital Management, LLC and as such may be deemed to beneficially own these shares. The principal business address of Suvretta Capital Management, LLC is 540 Madison Avenue, 7th Floor, New York, NY 10022.
(5)Consists of (i) 936,896 shares of common stock held by Viking Global Opportunities Illiquid Investments Sub-Master LP, or the Opportunities Fund, and (ii) 624,598 shares of common stock held by Viking Global Opportunities Drawdown (Aggregator) LP, or the Drawdown Fund. The Opportunities Fund has the authority to dispose of and vote the shares directly owned by it, which power may be exercised by its general partner, Viking Global Opportunities Portfolio GP LLC, or the Opportunities GP, and by Viking Global Investors LP, or VGI, which provides managerial services to the Opportunities Fund. The Drawdown Fund has the authority to dispose of and vote the shares directly owned by it, which power may be exercised by its general partner, Viking Global Opportunities Drawdown Portfolio GP LLC, or the Drawdown GP, and by VGI, which provides managerial services to the Drawdown Fund. Andreas Halvorsen, David C. Ott and Rose S. Shabet, as Executive Committee members of Viking Global Partners LLC (the general partner of VGI) and Viking Global Opportunities Parent GP LLC (the ultimate parent of the Opportunities GP and the Drawdown GP), have shared authority to direct the voting and disposition of investments beneficially owned by VGI, the Opportunities Fund, the Opportunities GP, the Drawdown Fund and the Drawdown GP. The business address of each of the entities is c/o Viking Global Investors LP, 600, Washington Blvd. Floor 11, Stamford, CT 06901.
(6)Consists of 1,399,062 shares of common stock held by Citadel Multi-Strategy Equities Master Fund Ltd. (Surveyor). The principal business address of Citadel Multi-Strategy Equities Master Fund Ltd. is c/o Citadel Enterprises Americas LLC, 830 Brickell Plaza, Miami FL 33131.
(7)Consists of (i) 234,908 shares of common stock held directly and (ii) 264,213 shares of common stock underlying outstanding stock options exercisable within 60 days of December 31, 2025.
(8)Consists of (i) 234,908 shares of common stock held directly and (ii) 165,267 shares of common stock underlying outstanding stock options exercisable within 60 days of December 31, 2025.
(9)Consists of 44,249 shares of common stock underlying outstanding stock options exercisable within 60 days of December 31, 2025.
(10)Consists of (i) 129,939 shares of common stock held directly, (ii) 521,150 shares of common stock held in trust and (iii) 1,465 shares of common stock underlying outstanding stock options exercisable within 60 days of December 31, 2025.
(11)Consists of 2,455 shares of common stock held directly and (ii) 19,976 shares of common stock held in trust.
(12)Consists of (i) 6,088,971 shares of common stock held directly, (ii) 541,126 shares of common stock held in trust and (iii) 475,194 shares of common stock underlying outstanding stock options exercisable within 60 days of December 31, 2025.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock and provisions of our restated certificate of incorporation and amended and restated bylaws as they will be in effect prior to the consummation of this offering are summaries and are qualified in their entirety by reference to our restated certificate of incorporation and amended and restated bylaws that will be in effect prior to the consummation of this offering. Copies of these documents are filed as exhibits to the registration statement of which this prospectus is a part.
General
Upon the consummation of this offering, our authorized capital stock will consist of 200,000,000 shares of common stock, with a par value of $0.00001 per share, and 25,000,000 shares of preferred stock, with a par value of $0.00001 per share, all of which preferred stock will be undesignated. Our board of directors may establish the rights and preferences of the preferred stock from time to time.
As of September 30, 2025, after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of 118,682,683 shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock, there would have been 19,999,437 shares of our common stock outstanding, held by 76 stockholders of record, and no shares of preferred stock outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of any series of preferred stock that we may designate and issue in the future.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately our net assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. Our outstanding shares of common stock are, and the shares offered by us in this offering will be, when issued and paid for, validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our restated certificate of incorporation that will be in effect prior to the consummation of this offering, our board of directors is authorized to direct us to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third-party to acquire, or could discourage a third-party from seeking to acquire, a majority of our outstanding voting stock. Upon the consummation of this offering, there will be no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock.
Options
Registration Rights
On October 14, 2025, we entered into a Third Amended and Restated Investors’ Right Agreement, or the Investors’ Rights Agreement, with certain holders of our capital stock, or the Investors, including the purchasers of our Series C Preferred Stock and other specified stockholders.
The Investors’ Rights Agreement grants the parties thereto certain registration rights in respect of the “registrable securities” held by them, which securities include (i) shares of our common stock issued or issuable upon conversion of our preferred stock (ii) shares of our common stock issued or issuable (directly or indirectly) upon conversion and/or exercise of any other securities of the Company, acquired by the Investors following the date of the Investors’ Rights Agreement and prior to the consummation of this offering, and (iii) any common stock issued as (or issuable upon the conversion or exercise of any warrant, right, or other security that is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, the shares referenced in clauses (i), (ii) and (iii). The registration of shares of our common stock pursuant to the exercise of these registration rights will enable the holders thereof to sell such shares without restriction under the Securities Act when the applicable registration statement is declared effective. Under the Investors’ Rights Agreement, we will pay all expenses relating to such registrations, including the reasonable fees of one counsel for the participating holders, and the holders will pay all underwriting discounts, selling commissions and stock transfer taxes relating to the sale of their shares. The Investors’ Rights Agreement also includes customary indemnification and procedural terms.
Holders of shares of our common stock (after giving effect to the conversion of preferred stock into shares of our common stock) are be entitled to such registration rights pursuant to the Investors’ Rights Agreement. These registration rights will expire on the earlier of (i) such time after this offering as Rule 144 or another similar exemption under the Securities Act is available for the sale of all of such holder’s shares without limitation during a three-month period without registration and (ii) the fifth anniversary of the consummation of this offering.
Demand Registration Rights
At any time beginning 180 days after the effectiveness of the registration statement of which this prospectus forms a part, the holders of at least 35% of the registrable securities then outstanding may request that we file a registration statement on Form S-1 with respect to a majority of the registrable securities then outstanding, if the aggregate offering price of the registrable securities requested to be registered would exceed $20 million.
Once we are eligible to use a registration statement on Form S-3, the holders of not less than 20% of the registrable shares then outstanding may request that we file a registration statement on Form S-3 with respect to such holders’ registrable securities then outstanding, if the aggregate offering price of the registrable securities requested to be registered would exceed $1 million.
Piggyback Registration Rights
In the event that we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the stockholders party to the Investors’ Rights Agreement will be entitled to certain “piggyback” registration rights allowing them to include their registrable securities in such registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act other than with respect to a demand registration or a registration statement on Form S-4 or S-8, these holders will be entitled to notice of the registration and will have the right to include their registrable securities in the registration subject to certain limitations.
Anti-takeover Effects of Our Restated Certificate of Incorporation and Our Amended and Restated Bylaws
Our restated certificate of incorporation and amended and restated bylaws, which will be in effect prior to the consummation of this offering, will contain certain provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors, but which may have the effect of delaying, deferring or preventing a future takeover or change in control of us unless such takeover or change in control is approved by our board of directors.
These provisions include:
Classified board. Our restated certificate of incorporation will provide that our board of directors will be divided into three classes of directors, with the classes as nearly equal in number as possible. As a result, approximately one-third of our board of directors will be elected each year. The classification of directors will have the effect of making it more difficult for stockholders to change the composition of our board of directors. Our restated certificate of incorporation will also provide that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances, the number of directors will be fixed exclusively pursuant to a resolution adopted by our board of directors. Upon consummation of this offering, we expect that our board of directors will have members.
Action by written consent; special meetings of stockholders. Our restated certificate of incorporation will provide that stockholder action can be taken only at an annual or special meeting of stockholders and cannot be taken by written consent in lieu of a meeting. Our restated certificate of incorporation and the bylaws will also provide that, except as otherwise required by law, special meetings of the stockholders can only be called pursuant to a resolution adopted by a majority of our board of directors. Except as described above, stockholders will not be permitted to call a special meeting or to require our board of directors to call a special meeting.
Removal of directors. Our restated certificate of incorporation will provide that our directors may be removed only for cause by the affirmative vote of at least 75% of the voting power of our outstanding shares of capital stock, voting together as a single class. This requirement of a supermajority vote to remove directors could enable a minority of our stockholders to prevent a change in the composition of our board of directors.
Advance notice procedures. Our bylaws will establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of our stockholders, including proposed nominations of persons for election to our board of directors. Stockholders at an annual meeting will only be able to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors or by a stockholder who was a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has given our Secretary timely written notice, in proper form, of the stockholder’s intention to bring that business before the meeting. Although the bylaws will not give our board of directors the power to approve or disapprove stockholder nominations of candidates or proposals regarding other business to be conducted at a special or annual meeting, the bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempting to obtain control of us.
Supermajority approval requirements. The DGCL generally provides that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless either a corporation’s certificate of incorporation or bylaws requires a greater percentage. Our restated certificate of incorporation and bylaws will provide that the affirmative vote of holders of at least 75% of the total votes eligible to be cast in the election of directors will be required to amend, alter, change or repeal specified provisions. This requirement of a supermajority vote to approve amendments to our restated certificate of incorporation and bylaws could enable a minority of our stockholders to exercise veto power over any such amendments.
Authorized but unissued shares. Our authorized but unissued shares of common stock and preferred stock will be available for future issuance without stockholder approval. These additional shares may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued shares of common stock and preferred
stock could render more difficult or discourage an attempt to obtain control of a majority of our common stock by means of a proxy contest, tender offer, merger or otherwise.
Exclusive forum.
Our restated certificate of incorporation will provide that, subject to limited exceptions, the Court of Chancery of the State of Delaware (or, if, and only if, the Court of Chancery of the State of Delaware dismisses a Covered Claim (as defined below) for lack of subject matter jurisdiction, any other state or federal court in the State of Delaware that does have subject matter jurisdiction) will, to the fullest extent permitted by applicable law, be the sole and exclusive forum for the following types of claims: (i) any derivative claim brought in the right of the Company, (ii) any claim asserting a breach of a fiduciary duty to the Company or the Company’s stockholders owed by any current or former director, officer or other employee or stockholder of the Company, (iii) any claim against the Company arising pursuant to any provision of the DGCL, our restated certificate of incorporation or amended and restated bylaws, (iv) any claim to interpret, apply, enforce or determine the validity of our restated certificate of incorporation or amended and restated bylaws, (v) any claim against the Company governed by the internal affairs doctrine, and (vi) any other claim, not subject to exclusive federal jurisdiction and not asserting a cause of action arising under the Securities Act, brought in any action asserting one or more of the claims specified in clauses (a)(i) through (v) herein above, each a Covered Claim. This provision would not apply to claims brought to enforce a duty or liability created by the Exchange Act.
Our restated certificate of incorporation will further provide that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. In addition, our restated certificate of incorporation will provide that any person or entity purchasing or otherwise acquiring any interest in the shares of capital stock of the Company will be deemed to have notice of and consented to these choice-of-forum provisions and waived any argument relating to the inconvenience of the forums in connection with any Covered Claim.
Section 203 of the DGCL
Upon consummation of this offering, we will be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock.
Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions: before the stockholder became interested, the corporation’s board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock
plans, in some instances; or at or after the time the stockholder became interested, the business combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Limitations of Liability and Indemnification Matters
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equiniti Trust Company, LLC.
Listing
We have applied for listing of our common stock on the NYSE under the symbol “MANE.”
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was no public market for our common stock, and no predictions can be made about the effect, if any, that market sales of our common stock or the availability of such shares for sale will have on the market price prevailing from time to time. Nevertheless, future sales of our common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock and could impair our ability to raise capital through future sales of our securities. See the section titled “Risk Factors—Risks Related to This Offering and Our Common Stock—A significant portion of our total outstanding shares is restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to decline significantly, even if our business is doing well.” Furthermore, although we have applied to have our common stock approved for listing on the NYSE, we cannot assure you that there will be an active public trading market for our common stock. Upon the consummation of this offering, based on the number of shares of our common stock outstanding as of September 30, 2025, after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock, in each case immediately prior to the consummation of this offering, we will have an aggregate of 33,350,170 shares of our common stock outstanding (or 35,352,670 shares of our common stock if the underwriters exercise in full their option to purchase additional shares). Of these shares of our common stock, all of the 13,350,000 shares sold in this offering (or 15,352,500 shares if the underwriters exercise in full their option to purchase additional shares) will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
All remaining shares of common stock held by existing stockholders immediately prior to the consummation of this offering will be “restricted securities” as such term is defined in Rule 144. These restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below. We expect that substantially all of these shares will be subject to the 180-day lock-up period under the lock-up agreements described below. Upon expiration of the lock-up period, we estimate that approximately 20,000,170 shares of our common stock will become available for sale in the public market, subject in some cases to applicable volume limitations under Rule 144.
Lock-Up Agreements
We and each of our directors and executive officers and holders of substantially all of our outstanding capital stock, who will collectively own approximately 19,878,828 shares of our common stock upon the consummation of this offering (based on our shares outstanding as of September 30, 2025 after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock), have agreed, among other things and subject to certain exceptions, not to sell or transfer any common stock or securities convertible into, exchangeable for, exercisable for, or repayable with common stock, for 180 days after the date of this prospectus without first obtaining the written consent of the representatives of the underwriters.
Upon the expiration of the lock-up period, substantially all of the shares subject to such lock-up restrictions will become eligible for sale, subject to the limitations discussed above. For a further description of these lock-up agreements, please see the section titled “Underwriters.” After the date of the initial public filing of the prospectus, certain of our employees, including our executive officers, and/or directors may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements relating to the offering described above.
Rule 144
Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our common stock for at least six months would be entitled to sell (subject to the lock-up agreement referred to above, if applicable) in “broker’s transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three-month period that does not exceed the greater of:
n1% of the number of shares of our common stock then outstanding, which will equal approximately 333,502 shares (or 353,527 shares if the underwriters exercise their option to purchase additional shares in full) of our common stock immediately after this offering; or
nthe average weekly trading volume in shares of our common stock on the NYSE during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale.
An “affiliate” is a person that directly, or indirectly through one or more intermediaries, controls or is controlled by, or is under common control with an issuer. Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the three months preceding a sale, and who has beneficially owned shares of our common stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us (as well as the lock-up agreement referred to above, if applicable). If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90 day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Rule 701
In general, under Rule 701, any of an issuer’s employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Act is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The SEC has indicated that Rule 701 will apply to typical options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Equity Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register all shares of our common stock subject to outstanding options and shares of our common stock issued or issuable under our incentive plans. We expect to file the registration statement covering shares offered pursuant to our incentive plans shortly after the date of this prospectus, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144.
Registration Rights
Upon the consummation of this offering, the holders of 19,250,410 shares of our common stock or their transferees, after giving effect to (i) the automatic conversion of all outstanding shares of our convertible preferred stock as of September 30, 2025 into an aggregate of 7,461,130 shares of our common stock and (ii) the automatic conversion of all outstanding shares of Series C Preferred Stock issued after September 30, 2025 into an aggregate of 11,789,280 shares of our common stock, in each case immediately prior to the consummation of this offering, will be entitled to various rights with respect to the registration of these shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates. See the section titled “Description of Capital Stock—Registration Rights” for additional information. Shares covered by a registration statement will be eligible for sale in the public market upon the expiration or release from the terms of the lock-up agreement.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material U.S. federal income tax considerations relating to the purchase, ownership and disposition of our common stock by Non-U.S. Holders (as defined below). This summary does not purport to be a complete analysis of all the potential tax considerations relevant to Non-U.S. Holders of our common stock. This summary is based upon the Code, the Treasury regulations promulgated or proposed thereunder and administrative and judicial interpretations thereof, all as of the date hereof and all of which are subject to change at any time, possibly on a retroactive basis.
This discussion is limited to Non-U.S. Holders that hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). For purposes of this summary, a “Non-U.S. Holder” means a beneficial owner of our common stock that for U.S. federal income tax purposes is not classified as a partnership and is not:
nan individual who is a citizen or resident of the United States;
na corporation or any other organization taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof or the District of Columbia;
nan estate, the income of which is included in gross income for U.S. federal income tax purposes regardless of its source; or
na trust if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more U.S. persons have the authority to control all of the trust’s substantial decisions or (2) the trust has a valid election in effect under applicable Treasury regulations to be treated as a U.S. person.
This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including any impact of any alternative minimum tax, any estate or gift tax, the special tax accounting rules under Section 451(b) of the Code or the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
nU.S. expatriates and former citizens or long-term residents of the United States;
npersons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment;
nbanks, insurance companies and other financial institutions;
nbrokers, dealers or traders in securities;
n“controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax;
npartnerships or other entities or arrangements treated as partnerships or other pass-through entities for U.S. federal income tax purposes (and investors therein);
ntax-exempt organizations or governmental organizations;
npersons deemed to sell our common stock under the constructive sale provisions of the Code;
npersons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation;
ntax-qualified retirement plans;
npersons who hold common stock that constitutes “qualified small business stock” under Section 1202 of the Code, or “Section 1244 stock” under Section 1244 of the Code;
npersons who acquired our common stock in a transaction subject to the gain rollover provisions of the Code (including Section 1045 of the Code);
npersons that acquired our common stock pursuant to the exercise of warrants or conversion rights under convertible instruments;
npersons who have elected to mark securities to market;
npersons that own, or have owned, actually or constructively, more than 5% of our common stock; and
n“qualified foreign pension funds” as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds.
If an entity that is classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of persons treated as its partners for U.S. federal income tax purposes will generally depend upon the status of the partner and the activities of the partnership. Partnerships and other entities that are classified as partnerships for U.S. federal income tax purposes and persons holding our common stock through a partnership or other entity classified as a partnership for U.S. federal income tax purposes are urged to consult their own tax advisors.
There can be no assurance that the IRS will not challenge one or more of the tax consequences described herein, and we have not obtained, nor do we intend to obtain, a ruling from the IRS with respect to the U.S. federal income tax consequences to a Non-U.S. Holder of the purchase, ownership or disposition of our common stock.
THIS SUMMARY IS FOR GENERAL INFORMATION ONLY AND IS NOT INTENDED TO BE TAX ADVICE. NON-U.S. HOLDERS ARE URGED TO CONSULT THEIR TAX ADVISORS CONCERNING THE U.S. FEDERAL INCOME, STATE, LOCAL, NON-U.S. AND OTHER TAX CONSEQUENCES TO THEM OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK.
Distributions on Our Common Stock
We do not currently expect to make distributions with respect to our common stock. If we make a distribution of cash or property with respect to our common stock, any such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent of our current and accumulated earnings and profits, if any, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will constitute a return of capital and will first reduce the holder’s adjusted tax basis in our common stock, but not below zero. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in “—Gain on Sale, Exchange or Other Taxable Disposition of Our Common Stock.” Any such distribution would also be subject to the discussions below regarding effectively connected income and under the sections titled “—Additional Withholding and Reporting Requirements” and “—Backup Withholding and Information Reporting.”
Dividends paid to a Non-U.S. Holder generally will be subject to a 30% U.S. federal withholding tax unless such Non-U.S. Holder provides us or our agent, as the case may be, with an appropriate IRS Form W-8 establishing a reduction or exemption, such as:
nIRS Form W-8BEN or W-8BEN-E (or successor form) certifying, under penalties of perjury, a reduction or exemption in withholding under an applicable income tax treaty, or
nIRS Form W-8ECI (or successor form) certifying that a dividend paid on our common stock is not subject to withholding tax because it is effectively connected with a trade or business in the United States of the Non-U.S. Holder (in which case such dividend generally will be subject to regular graduated U.S. tax rates as described below).
The certification requirement described above must be provided to us or our agent prior to the payment of dividends and must be updated periodically. The certification also may require a Non-U.S. Holder that provides an IRS form or that claims treaty benefits to provide its U.S. taxpayer identification number. Special certification and other requirements apply in the case of certain Non-U.S. Holders that hold shares of our common stock through intermediaries or are pass-through entities for U.S. federal income tax purposes.
Each Non-U.S. Holder is urged to consult its own tax advisor about the specific methods for satisfying these requirements. A claim for reduction or exemption will not be valid if the person receiving the applicable form has actual knowledge or reason to know that the statements on the form are false.
If dividends are effectively connected with a trade or business in the United States of a Non-U.S. Holder (and, if required by an applicable income tax treaty, are attributable to a permanent establishment maintained by such Non-U.S. Holder in the United States), the Non-U.S. Holder, although eligible for a reduction or exemption from
the withholding tax as described above (provided that the certification requirement described above is satisfied), generally will be subject to U.S. federal income tax on such dividends on a net income basis in the same manner as if it were a resident of the United States. In addition, if a Non-U.S. Holder is treated as a corporation for U.S. federal income tax purposes, the Non-U.S. Holder may be subject to an additional “branch profits tax” equal to 30% (unless reduced by an applicable income treaty) of its earnings and profits in respect of such effectively connected dividend income, as adjusted for certain items.
Non-U.S. Holders that do not timely provide us or our agent with the required certification, but which are eligible for a reduced rate of or exemption from U.S. federal withholding tax pursuant to an income tax treaty, may obtain a refund or credit of any excess amount withheld by timely filing an appropriate claim for refund with the IRS.
Gain on Sale, Exchange or Other Taxable Disposition of Our Common Stock
Subject to the discussions below under the sections titled “—Additional Withholding and Reporting Requirements” and “—Backup Withholding and Information Reporting,” in general, a Non-U.S. Holder will not be subject to U.S. federal income tax or withholding tax on gain realized upon such holder’s sale, exchange or other taxable disposition of shares of our common stock, unless (1) such Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of disposition, and certain other conditions are met, (2) we are or have been a “United States real property holding corporation,” as defined in the Code, or a USRPHC, at any time within the shorter of the five-year period preceding the disposition and the Non-U.S. Holder’s holding period in the shares of our common stock, and certain other requirements are met, or (3) such gain is effectively connected with the conduct by such Non-U.S. Holder of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment maintained by such Non-U.S. Holder in the United States).
If the first exception described above applies, the Non-U.S. Holder generally will be subject to U.S. federal income tax at a rate of 30% (or at a reduced rate under an applicable income tax treaty) on the amount by which such Non-U.S. Holder’s capital gains allocable to U.S. sources exceed capital losses allocable to U.S. sources during the taxable year of the disposition (provided that the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses). If the third exception described above applies, the Non-U.S. Holder generally will be subject to U.S. federal income tax with respect to such gain on a net income basis in the same manner as if it were a resident of the United States and a Non-U.S. Holder that is a corporation for U.S. federal income tax purposes may also be subject to a branch profits tax with respect to any earnings and profits attributable to such gain at a rate of 30% (or at a reduced rate under an applicable income tax treaty).
With respect to the second exception described above, generally, a corporation is a USRPHC only if the fair market value of its U.S. real property interests (as defined in the Code) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not, and do not anticipate becoming, a USRPHC. Even if we became a USRPHC, a Non-U.S. Holder would not be subject to U.S. federal income tax on a sale, exchange or other taxable disposition of our common stock by reason of our status as USRPHC so long as our common stock is regularly traded on an established securities market at any time during the calendar year in which the disposition occurs and the Non-U.S. Holder does not own and is not deemed to own (directly, indirectly or constructively) more than 5% of our common stock at any time during the shorter of the five year period ending on the date of disposition and the holder’s holding period.
Additional Withholding and Reporting Requirements
The Foreign Account Tax Compliance Act, Sections 1471 through 1474 of the Code, and related Treasury regulations, together with other U.S. Treasury Department and IRS guidance issued thereunder, or FATCA, impose a U.S. federal withholding tax of 30% on certain payments, including dividends paid on our common stock, paid to (1) a “foreign financial institution” (as defined under FATCA) unless such institution furnishes proper documentation (typically on IRS Form W-8BEN-E) evidencing either (i) an exemption from FATCA withholding, (ii) its compliance (or deemed compliance) with specified due diligence, reporting, withholding and certification obligations under FATCA or (iii) residence in a jurisdiction that has entered into an intergovernmental agreement with the United States relating to FATCA and compliance with the diligence and
reporting requirements of the intergovernmental agreement and local implementing rules; or (2) a “non-financial foreign entity” (as defined under FATCA) that does not furnish proper documentation, typically on IRS Form W-8BEN-E, evidencing either (i) an exemption from FATCA or (ii) adequate information regarding substantial U.S. beneficial owners of such entity (if any). An intergovernmental agreement between the United States and an applicable foreign country, and legislation, rules and other official guidance adopted pursuant to such intergovernmental agreement, may modify these requirements.
The IRS and the U.S. Treasury Department have issued proposed regulations on which taxpayers may rely providing that these withholding rules will not apply to the gross proceeds of a sale or other disposition of shares of our common stock. Prospective investors should consult their own tax advisors regarding the effect of FATCA on their ownership and disposition of our common stock.
Backup Withholding and Information Reporting
We must report annually to the IRS and to each Non-U.S. Holder the gross amount of the distributions on our common stock paid to the holder and the tax withheld, if any, with respect to the distributions. Non-U.S. Holders may have to comply with specific certification procedures (such as the provision of a properly completed IRS Form W-8BEN or IRS Form W-8BEN-E) to establish that the holder is not a United States person (as defined in the Code) in order to avoid backup withholding at the applicable rate, currently 24%, with respect to dividends on our common stock. Dividends paid to Non-U.S. Holders subject to the U.S. withholding tax, as described above under the section titled “—Distributions on Our Common Stock,” generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a Non-U.S. Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a Non-U.S. Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Prospective investors should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them, including the availability of and procedure for obtaining an exemption from backup withholding.
Copies of information returns may be made available to the tax authorities of the country in which the Non-U.S. Holder resides or in which the Non-U.S. Holder is incorporated, under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S. Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
UNDERWRITING
Subject to the terms and conditions set forth in the underwriting agreement, dated 2026, among us and Jefferies LLC, Leerink Partners LLC, Citigroup Global Markets Inc. and Cantor Fitzgerald & Co. as the representatives of the underwriters named below and the book-running managers of this offering, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the respective number of shares of common stock shown opposite its name below:
| | | | | | | | |
Underwriter | | Number of Shares |
Jefferies LLC | | |
Leerink Partners LLC | | |
Citigroup Global Markets Inc. | | |
Cantor Fitzgerald & Co. | | |
Total | | 13,350,000 | |
| | |
The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will purchase all of the shares of common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters have advised us that, following the consummation of this offering, they currently intend to make a market in the common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters are offering the shares of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
The cornerstone investor has indicated an interest in purchasing up to $30.0 million in shares of common stock in this offering at the initial public offering price. The shares of common stock to be purchased by the cornerstone investor will not be subject to a lock-up agreement with the underwriters. Because this indication of interest is not a binding agreement or commitment to purchase, the cornerstone investor may determine to purchase more, less or no shares in this offering or the underwriters may determine to sell more, less or no shares to the cornerstone investor. The underwriters will receive the same underwriting discounts and commissions on any of our shares of common stock purchased by the cornerstone investor as they will from any other shares of common stock sold to the public in this offering. The number of shares of common stock available for sale to the public will be reduced to the extent that the cornerstone investor purchases shares of common stock in the offering.
Commission and Expenses
The underwriters have advised us that they propose to offer the shares of common stock to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers, which may include the underwriters, at that price less a concession not in excess of $ per share of common stock. After the offering, the initial public offering price, concession and reallowance to dealers may be reduced by the
representatives. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| | | | | | | | | | | | | | | | | | | | | | | |
| Per Share | | Total |
| Without
Option to Purchase Additional Shares | | With
Option to Purchase Additional Shares | | Without
Option to Purchase Additional Shares | | With
Option to Purchase Additional Shares |
Public offering price | $ | | $ | | $ | | $ |
Underwriting discounts and commissions paid by us | $ | | $ | | $ | | $ |
Proceeds to us, before expenses | $ | | $ | | $ | | $ |
| | | | | | | |
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $4.4 million. We have agreed to reimburse the underwriters for certain of their expenses relating to clearance of this offering with the Financial Industry Regulatory Authority up to $40,000.
Determination of Offering Price
Prior to this offering, there has not been a public market for our common stock. Consequently, the initial public offering price for our common stock will be determined by negotiations between us and the representatives. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
We offer no assurances that the initial public offering price will correspond to the price at which the common stock will trade in the public market subsequent to the offering or that an active trading market for the common stock will develop and continue after the offering.
Listing
We have applied to have our common stock listed on the New York Stock Exchange under the trading symbol “MANE.”
Stamp Taxes
If you purchase shares of common stock offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus.
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of 2,002,500 shares from us at the public offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to specified conditions, to purchase a number of additional shares proportionate to that underwriter’s initial purchase commitment as indicated in the table above. This option may be exercised only if the underwriters sell more shares than the total number set forth on the cover page of this prospectus.
No Sales of Similar Securities
We, our officers, directors and holders of all or substantially all of our outstanding capital stock have agreed, subject to specified exceptions, not to directly or indirectly:
nsell, offer to sell, contract to sell or lend, effect any short sale or establish or increase any “put equivalent position” within the meaning of Rule 16a-l(h) under the Exchange Act, pledge, hypothecate or grant any security interest in, or in any other way transfer or dispose of any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially, or
nenter into any swap, hedge or similar arrangement that transfers, in whole or in part, the economic risk of ownership of shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock, regardless of whether any such transaction is to be settled in securities, in cash or otherwise, or
nmake any demand for, or exercise any right with respect to, the registration under the Securities Act of the offer and sale of any shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock, or cause to be filed a registration statement, prospectus or prospectus supplement (or an amendment or supplement thereto) with respect to any such registration, or
npublicly announce an intention to do any of the foregoing.
This restriction terminates after the close of trading of the common stock on and including the 180th day after the date of this prospectus.
The foregoing restrictions shall not apply to:
(i) transfers or dispositions of shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock as a bona fide gift or for estate planning purposes, or by will, other testamentary document or intestate succession to the legal representative, heir, beneficiary or any family member, to a trust for the direct or indirect benefit of the securityholder and/or a family member, or to a charitable organization or educational institution in each case under this clause (i) in a transfer not involving a disposition for value;
(ii) transfers or dispositions of shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock to any corporation, partnership, limited liability company or other entity, all of the beneficial ownership interests of which are held by the securityholder or any family member;
(iii) if the securityholder is an entity, transfers, dispositions or distributions of shares of common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock to another corporation, partnership, limited liability company, trust or other business entity that is an affiliate (within the meaning set forth in Rule 405 under the Securities Act, and including the subsidiaries of the securityholder) of the securityholder, to any investment fund or other entity controlling, controlled by, managing or managed by or under common control with the securityholder or affiliates of the securityholder (including, for the avoidance of doubt, where the securityholder is a partnership, to its general partner or a successor partnership or fund, or any other funds managed by such partnership) or to its stockholders, limited partners, general partners, limited liability company members or other equityholders or to the estate of any such stockholders, limited partners, general partners, limited liability company members or equityholders;
(iv) the exercise of an option to purchase shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock granted under any equity incentive plan or stock purchase plan described in the registration statement of which this prospectus is a part, provided that the underlying shares of common stock shall continue to be subject to the lock-up agreement;
(v) the establishment or amendment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of shares of common stock; provided that such plan does not provide for any transfers of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock during the lock-up period and any required public disclosure, announcement or filing under the Exchange Act made by us or any person regarding the establishment or amendment of such plan during the lock-up period shall include a statement that the undersigned is not permitted to transfer, sell or otherwise dispose of securities under such plan during the lock-up period in contravention of this agreement, and no public announcement, report or filing under the Exchange Act, or any other public filing, report or announcement, shall be voluntarily made regarding the establishment or amendment of such plan during the lock-up period;
(vi) transfers or dispositions of shares of common stock acquired in this offering or on the open market following this offering, provided that no public disclosure or filing under the Exchange Act (other than any required filing on Schedule 13G, Schedule 13G/A or Form 13F) shall be made;
(vii) the transfer of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock to a nominee or custodian of a person or entity to whom a disposition or transfer would be permissible under clauses (i)-(iii) above;
(viii) transfers or dispositions of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock as forfeitures to satisfy tax withholding obligations of the undersigned in connection with the vesting or exercise of equity awards by the undersigned pursuant to our equity incentive, stock option, stock bonus or other stock plan or arrangement described in the registration statement of which this prospectus is a part;
(ix) transfers or dispositions of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock pursuant to a net exercise or cashless exercise by the undersigned of outstanding equity awards pursuant to our equity incentive, stock option, stock bonus or other stock plan or arrangement described in the registration statement of which this prospectus is a part;
(x) transfers or dispositions of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock pursuant to a bona fide third-party tender offer for our shares, merger, consolidation or other similar transaction made to all holders of our securities involving a change of control (including, without limitation, the entering into any lock-up, voting or similar agreement pursuant to which the undersigned may agree to transfer, sell, tender or otherwise dispose of common stock or other such securities in connection with such transaction, or vote any common stock or other such securities in favor of any such transaction), provided that in the event the tender offer, merger, consolidation or other such transaction is not completed, the securityholder’s securities shall remain subject to the restrictions set forth in such lock-up agreement;
(xi) transfers or dispositions of shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock by operation of law, including pursuant to a domestic order or negotiated divorce settlement, or pursuant to a court order; or
(xii) transfers or dispositions of securities that may be deemed to have occurred as a result of the conversion of any of our securities held by the securityholder in connection with or prior to the consummation of this offering;
provided that, (A) in the case of a transfer pursuant to clause (viii) or clause (ix) above, if the securityholder is required to make or voluntarily makes a filing under the Exchange Act reporting a reduction in beneficial ownership of shares of common stock during the lock-up period, the securityholder shall include a statement in such report to the effect that the filing relates to the circumstances described in such clause, (B) any shares of common stock acquired upon conversion or exercise described in clause (ix) and clause (xii) shall be subject to the restrictions set forth in the lock-up agreement, and (C) in the case of clauses (i)-(iii) and (vii) above it shall be a condition to such transfer or disposition that:
neach transferee executes and delivers a similar lock-up agreement and such shares of common stock shall remain subject to the restrictions set forth above, and
nprior to the expiration of the lock-up period, no public disclosure or filing under the Exchange Act by any party to the transfer (donor, donee, transferor or transferee) shall be required, or made voluntarily, reporting a reduction in beneficial ownership of shares of common stock in connection with such transfer.
Jefferies LLC, Leerink Partners LLC, Citigroup Global Markets Inc. and Cantor Fitzgerald & Co. may, in their sole discretion and at any time or from time to time before the termination of the 180-day period, release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization
The underwriters have advised us that they, pursuant to Regulation M under the Exchange Act, certain persons participating in the offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either “covered” short sales or “naked” short sales.
“Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
“Naked” short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriter’s purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
None of we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the
prospectus in electronic format, the information on the underwriters’ web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certain of their respective affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and certain of their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The underwriters and certain of their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Disclaimers About Non-U.S. Jurisdictions
Canada
(A) Resale Restrictions
The distribution of the shares of common stock in Canada is being made only in the provinces of Ontario, Quebec, Alberta and British Columbia on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of these securities are made. Any resale of the shares of common stock in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the securities.
(B) Representations of Canadian Purchasers
By purchasing the shares of common stock in Canada and accepting delivery of a purchase confirmation, a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
nthe purchaser is entitled under applicable provincial securities laws to purchase the shares of common stock without the benefit of a prospectus qualified under those securities laws as it is an “accredited investor” as defined under National Instrument 45-106 – Prospectus Exemptions,
nthe purchaser is a “permitted client” as defined in National Instrument 31-103 - Registration Requirements, Exemptions and Ongoing Registrant Obligations,
nwhere required by law, the purchaser is purchasing as principal and not as agent, and
nthe purchaser has reviewed the text above under Resale Restrictions.
(C) Conflicts of Interest
Canadian purchasers are hereby notified that Jefferies LLC, Leerink Partners LLC, Citigroup Global Markets Inc. and Cantor Fitzgerald & Co. are relying on the exemption set out in section 3A.3 or 3A.4, if applicable, of National Instrument 33-105 – Underwriting Conflicts from having to provide certain conflict of interest disclosure in this document.
(D) Statutory Rights of Action
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if the prospectus (including any amendment thereto) such as this document contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser of these securities in Canada should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
(E) Enforcement of Legal Rights
All of our directors and officers as well as the experts named may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
(F) Taxation and Eligibility for Investment
Canadian purchasers of shares of our common stock should consult their own legal and tax advisors with respect to the tax consequences of an investment in the shares of common stock in their particular circumstances and about the eligibility of the shares of common stock for investment by the purchaser under relevant Canadian legislation.
(G) Language of Documents
The purchaser confirms its express wish and that it has requested that this document, all documents evidencing or relating to the sale of the securities described herein and all other related documents be drawn up exclusively in the English language. L’acquéreur confirme sa volonté expresse et qu’il a demandé que le présent document, tous les documents attestant de la vente des titres décrits dans le présent document ou s'y rapportant ainsi que tous les autres documents s’y rattachant soient rédigés exclusivement en langue anglaise.
Australia
This prospectus is not a disclosure document for the purposes of Australia’s Corporations Act 2001 (Cth) of Australia, or Corporations Act, has not been lodged with the Australian Securities & Investments Commission and is only directed to the categories of exempt persons set out below. Accordingly, if you receive this prospectus in Australia:
(A) You confirm and warrant that you are either:
na “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act;
na “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to the Company which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made;
na person associated with the Company under Section 708(12) of the Corporations Act; or
na “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act.
To the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under this prospectus is void and incapable of acceptance.
(B) You warrant and agree that you will not offer any of the securities issued to you pursuant to this prospectus for resale in Australia within 12 months of those securities being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
European Economic Area
In relation to each Member State of the European Economic Area (each, a “Relevant State”), no shares have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which have been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that the shares may be offered to the public in that Relevant State at any time:
(1)to any legal entity which is a “qualified investor” as defined under Article 2 of the Prospectus Regulation;
(2)to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the Prospectus Regulation), subject to obtaining the prior consent of representatives for any such offer; or
(3)in any other circumstances falling within Article 1(4) of the Prospectus Regulation,
provided that no such offer of the shares shall require us or any of the representatives to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression “offer to the public” in relation to the shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
Hong Kong
No shares of common stock have been offered or sold, and no shares of common stock may be offered or sold, in Hong Kong, by means of any document, other than to persons whose ordinary business is to buy or sell shares or debentures, whether as principal or agent; or to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong, or SFO, and any rules made under that Ordinance; or in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong, or CO, or which do not constitute an offer or invitation to the public for the purpose of the CO or the SFO. No document, invitation or advertisement relating to the shares of common stock has been issued or may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted under the securities laws of Hong Kong) other than with respect to shares of common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made under that Ordinance.
This prospectus has not been registered with the Registrar of Companies in Hong Kong. Accordingly, this prospectus may not be issued, circulated or distributed in Hong Kong, and the shares of common stock may not be offered for subscription to members of the public in Hong Kong. Each person acquiring the shares of common stock will be required, and is deemed by the acquisition of the securities, to confirm that he is aware of the restriction on offers of the shares of common stock described in this prospectus and the relevant offering documents and that he is not acquiring, and has not been offered any shares of common stock in circumstances that contravene any such restrictions.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel Securities Authority. In Israel, this prospectus is being distributed only to, and is directed only at, and any offer of the shares of common stock is directed only at, (i) a limited number of persons in accordance with the Israeli Securities Law and (ii) investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals,” each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case, purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors are required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Japan
The offering has not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948 of Japan, as amended), or FIEL, and the Initial Purchaser will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the FIEL and any other applicable laws, regulations and ministerial guidelines of Japan.
Singapore
This prospectus has not been and will not be lodged or registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the notes may not be circulated or distributed, nor may the notes be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA,, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the common stock is subscribed or purchased under Section 275 of the SFA by a relevant person which is:
(1)a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
(2)a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the notes pursuant to an offer made under Section 275 of the SFA except:
(1)to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA;
(2)where no consideration is or will be given for the transfer;
(3)where the transfer is by operation of law;
(4)as specified in Section 276(7) of the SFA; or
(5)as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
Switzerland
The shares of common stock may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This prospectus has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this prospectus nor any other offering or marketing material relating to the offering, the Company or the shares of common stock have been or will be filed with or approved by any Swiss regulatory authority. In particular, this prospectus will not be filed with, and the offer of the shares of common stock will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of the shares of common stock has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares of common stock.
United Kingdom
No shares of common stock have been offered or will be offered pursuant to the offering to the public in the United Kingdom except that the shares of common stock may be offered to the public in the United Kingdom at any time:
(a)where (i) the offer is conditional on the admission of the shares of common stock to trading on the London Stock Exchange plc's main market (in reliance on the exception in paragraph 6(a) of Schedule 1 of the POATR) or (ii) the shares of common stock being offered are at the time of the offer already admitted to trading on London Stock Exchange plc's main market (in reliance on the exception in paragraph 6(b) of Schedule 1 of the POATR);
(b)to any “qualified investor” as defined in paragraph 15 of Schedule 1 of the POATR;
(c)to fewer than 150 persons (other than qualified investors as defined in paragraph 15 of Schedule 1 of the POATR), subject to obtaining the prior consent of the underwriters for any such offer; or
(d)in any other circumstances falling within Part 1 of Schedule 1 of the POATR.
For the purposes of this provision, the expression an “offer to the public” in relation to the shares of common stock in the United Kingdom means the communication to any person which presents sufficient information on: (a) the shares of common stock to be offered; and (b) the terms on which they are to be offered, to enable an investor to decide to buy or subscribe for the shares of common stock and the expression "POATR" means the Public Offers and Admissions to Trading Regulations 2024.
This prospectus is only being distributed to and is only directed at: (A) persons who are outside the United Kingdom, or (B) qualified investors who are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”), or (ii) high-net-worth companies, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons falling within (1) - (3) together being referred to as “relevant persons”). The shares of common stock are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire the shares of common stock will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
LEGAL MATTERS
The validity of the shares of common stock offered by this prospectus will be passed upon for us by Ropes & Gray LLP, Boston, Massachusetts. Certain legal matters in connection with this offering will be passed upon for the underwriters by Cooley LLP, New York, New York.
EXPERTS
The financial statements of Veradermics, Incorporated as of December 31, 2024 and 2023, and for each of the two years in the period ended December 31, 2024, included in this Prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are included in reliance upon the report of such firm given their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the shares of common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed thereto.
Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The SEC also maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that site is www.sec.gov.
Upon the effectiveness of the registration statement, we will be subject to the informational requirements of the Exchange Act and, in accordance with the Exchange Act, will file reports, proxy and information statements and other information with the SEC. Such annual, quarterly and special reports, proxy and information statements and other information can be accessed at the SEC’s website referenced above. We also intend to make this information available on the investor relations section of our website, which is located at www.veradermics.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | |
| PAGE |
Audited financial statements for the years ended December 31, 2024 and 2023: | |
| |
| |
| |
| |
| |
| |
| |
Unaudited financial statements for the nine months ended September 30, 2025 and 2024: | |
| |
| |
| |
| |
| |
| |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of Veradermics, Incorporated
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Veradermics, Incorporated and subsidiaries (the “Company”) as of December 31, 2024 and 2023, the related consolidated statements of operations, changes in redeemable convertible preferred stock and shareholders’ deficit, and cash flows, for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
Hartford, Connecticut
October 24, 2025 (January 28, 2026, as to the effects of the reverse stock split discussed in Note 13)
We have served as the Company’s auditor since 2023.
VERADERMICS, INCORPORATED
Consolidated Balance Sheets
| | | | | | | | | | | |
| As of December 31, |
| (in thousands, except share and per share amounts) | 2024 | | 2023 |
Assets | | | |
Current assets: | | | |
Cash and cash equivalents | $ | 53,084 | | | $ | 16,296 | |
Prepaid expenses and other current assets | 2,451 | | | 1,266 | |
Total current assets | 55,535 | | | 17,562 | |
Total assets | $ | 55,535 | | | $ | 17,562 | |
Liabilities and stockholders’ deficit | | | |
Current liabilities: | | | |
Accounts payable | $ | 2,248 | | | $ | 1,852 | |
Accrued expenses | 2,470 | | | 1,200 | |
Total current liabilities | 4,718 | | | 3,052 | |
Promissory note | — | | | 786 | |
Total liabilities | 4,718 | | | 3,838 | |
Commitments and contingencies (Note 10) | | | |
Redeemable convertible preferred stock: | | | |
Series A preferred stock, $0.00001 par value, 12,865,375 shares authorized, issued and outstanding with an aggregate liquidation preference of $37,238 net of issuance costs | 36,860 | | | 36,860 | |
Series B preferred stock, $0.00001 par value, 62,245,806 and 0 shares authorized; 55,246,971 and 0 issued and outstanding with an aggregate liquidation preference of $133,944 and $0 as of December 31, 2024 and 2023, respectively, net of issuance costs | 66,285 | | | — | |
Subscription receivable | (3,848) | | | (894) | |
Stockholders’ equity (deficit): | | | |
Common stock, $0.00001 par value, 98,774,582 and 25,500,000 authorized as of December 31, 2024 and 2023, respectively and 736,933 shares issued and outstanding | 1 | | | 1 | |
Additional paid-in capital | 750 | | | 500 | |
Accumulated deficit | (49,231) | | | (22,743) | |
Total stockholders’ deficit | (48,480) | | | (22,242) | |
Total liabilities, redeemable convertible preferred stock, and stockholders’ deficit | $ | 55,535 | | | $ | 17,562 | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Consolidated Statements of Operations
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands, except share and per share amounts) | 2024 | | 2023 |
Operating Expenses: | | | |
Research and development | $ | 23,283 | | | $ | 14,971 | |
General and administrative | 3,495 | | | 2,353 | |
Total operating expenses | 26,778 | | | 17,324 | |
Loss from operations | (26,778) | | | (17,324) | |
Other income (expenses): | | | |
Interest income | 481 | | | 870 | |
Other income | 394 | | | — | |
Interest expense | (585) | | | (36) | |
Total other income, net | 290 | | | 834 | |
Loss before income taxes | (26,488) | | | (16,490) | |
Income tax benefit | — | | | — | |
Net loss | $ | (26,488) | | | $ | (16,490) | |
Net loss attributable to common stock | $ | (27,303) | | | $ | (16,490) | |
Net loss per share of common stock, basic and diluted | $ | (37.05) | | | $ | (22.38) | |
Weighted average common stock outstanding, basic and diluted | 736,933 | | | 736,933 | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and Stockholders’ Deficit
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Redeemable Convertible Preferred Stock | | Stockholders' Deficit |
| Series A Redeemable Convertible Preferred Stock | | Series B Redeemable Convertible Preferred Stock | | Subscription Receivable | | Common Stock | | Additional Paid-In Capital | | Accumulated Deficit | | Total Stockholders’ Deficit |
| (in thousands, except share amounts) | Shares | | Amount | | Shares | | Amount | Shares | | Amount |
Balance, January 1, 2023 | 7,899,803 | | | $ | 21,896 | | | — | | | $ | — | | | $ | (892) | | | 736,933 | | | $ | 1 | | | $ | 294 | | | $ | (6,253) | | | $ | (5,958) | |
Issuance of Series A-4 Redeemable Convertible Preferred Stock, net of issuance costs of $35 | 4,965,572 | | | 14,964 | | | — | | | — | | | (1,070) | | | — | | | — | | | — | | | — | | | — | |
| Subscription receivable settled through research and development services received | — | | | — | | | — | | | — | | | 1,068 | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 206 | | | — | | | 206 | |
Net loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (16,490) | | | (16,490) | |
Balance, December 31, 2023 | 12,865,375 | | | $ | 36,860 | | | — | | | $ | — | | | $ | (894) | | | 736,933 | | | $ | 1 | | | 500 | | | $ | (22,743) | | | $ | (22,242) | |
Issuance of Series B Redeemable Convertible Preferred Stock, net of issuance costs of $283 | — | | | — | | | 55,246,971 | | | 66,285 | | | (3,965) | | | — | | | — | | | — | | | — | | | — | |
| Subscription receivable settled through for research and development services received | — | | | — | | | — | | | — | | | 1,011 | | | — | | | — | | | — | | | — | | | — | |
Stock-based compensation | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 250 | | | — | | | 250 | |
Net loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (26,488) | | | (26,488) | |
Balance, December 31, 2024 | 12,865,375 | | | $ | 36,860 | | | 55,246,971 | | | $ | 66,285 | | | $ | (3,848) | | | 736,933 | | | $ | 1 | | | 750 | | | $ | (49,231) | | | $ | (48,480) | |
| | | | | | | | | | | | | | | | | | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Consolidated Statements of Cash Flows
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
Cash Flows from Operating Activities | | | |
Net loss | $ | (26,488) | | | $ | (16,490) | |
Adjustments to reconcile net loss to net cash used in operating activities: | | | |
Stock-based compensation | 250 | | | 206 | |
Research and development expenses paid with convertible and promissory notes | 234 | | | 750 | |
Non-cash research and development services | 1,011 | | | 1,068 | |
Other | 573 | | | 36 | |
Changes in operating assets and liabilities: | | | |
Prepaid expenses and other current assets | (1,185) | | | (983) | |
Accounts payable | 646 | | | 1,354 | |
Accrued expenses | 1,271 | | | 549 | |
| Net cash used in operating activities | (23,688) | | | (13,510) | |
Cash Flows from Financing Activities: | | | |
Issuance of convertible notes | 8,507 | | | — | |
Payment of promissory note | (845) | | | — | |
Issuance of Series A-4 preferred stock | — | | | 13,930 | |
Issuance of Series B preferred stock | 53,097 | | | — | |
Preferred stock issuance costs | (283) | | | (35) | |
| Net cash provided by financing activities | 60,476 | | | 13,895 | |
| Net increase in cash and cash equivalents | 36,788 | | | 385 | |
Cash and cash equivalents—beginning of year | 16,296 | | | 15,911 | |
Cash and cash equivalents—end of year | $ | 53,084 | | | $ | 16,296 | |
Supplemental Disclosure of Cash Flow Information: | | | |
Cash paid during the year for interest | $ | 95 | | | $ | — | |
Cash paid during the year for income taxes | $ | — | | | $ | — | |
Supplemental Schedule of Noncash Financing Activities: | | | |
Issuance of Series A-4 preferred stock for contractual research and development services | $ | — | | | $ | 1,070 | |
Issuance of Series B preferred stock for contractual research and development services | $ | 3,033 | | | $ | — | |
Issuance of Series B preferred stock as payment for convertible notes | $ | 10,438 | | | $ | — | |
Issuance of convertible note for research and development services | $ | 1,166 | | | $ | — | |
Conversion of other receivable in exchange for research and development services to subscription receivable | $ | 932 | | | $ | — | |
Issuance of convertible note for accounts payable | $ | 250 | | | $ | — | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Notes to Financial Statements
1. Nature of the Business
Veradermics, Incorporated and its wholly owned subsidiary (collectively, the “Company” or “Veradermics”) is a dermatologist-founded, late clinical-stage biopharmaceutical company focused on developing innovative therapeutics to address pervasive treatment challenges in highly prevalent aesthetic and dermatological conditions. The Company’s initial focus is developing better treatments for pattern hair loss, or PHL, a condition affecting approximately 50 million men and approximately 30 million women in the United States. Beyond VDPHL01, the Company has created a portfolio utilizing its real-world experience as dermatologists to generate compelling pipeline assets, including VDMN for the treatment of common warts, VDAA for the treatment of alopecia areata, and VDMC for the treatment of molluscum contagiosum.
Veradermics began its operations in 2019 as a company incorporated under the laws of the State of Texas. Effective on September 14, 2021, the Company was converted into a company incorporated under the laws of the State of Delaware. The Company is headquartered in New Haven, CT.
Liquidity and Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business.
In accordance with Accounting Standards Update (“ASU”) 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (Subtopic 205-40), the Company has evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
The Company has incurred recurring losses since its inception, including net losses of $26.5 million and $16.5 million for the years ended December 31, 2024 and 2023, as well as incurred negative cash flows from operations of $23.7 million and $13.5 million, respectively. In addition, as of December 31, 2024, the Company had an accumulated deficit of $49.2 million. The Company expects to continue to generate operating losses for the foreseeable future. Through December 31, 2024, the Company has financed its operations primarily from the sale of equity securities. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital to fund its operations.
In November 2024, the Company completed the first closing of a $66.5 million Series B redeemable convertible preferred stock financing. Subsequent to December 31, 2024, the Company completed the second funding of Series B preferred stock of $8.4 million in the first quarter of 2025 and then completed a $150.0 million Series C preferred stock financing in the fourth quarter of 2025. As of the date of these financial statements $135.0 million of the Series C preferred stock financing had been received, with an additional $15.0 million that may be purchased at an additional closing for the same per share purchase price. The 2025 financings, along with cash on hand, will enable the Company to meet its obligations for at least the twelve-month period from the date the financial statements are available to be issued.
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the pharmaceutical industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations and those specific to the pharmaceutical industry such as the U.S. Food and Drug Administration, and the ability to secure additional capital to fund operations. Products currently under development will require significant additional research and development efforts, including preclinical and clinical testing and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel and infrastructure, and extensive compliance and reporting capabilities. The Company’s future clinical trials require significant compliance and monitoring by government agencies and there can be no assurances that such agencies will approve procedures followed in the Company’s trials. Another likely scenario is that such agencies would require additional procedures to be performed which would push out commercialization timing. Further, even if the Company's product development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
If the Company’s product development efforts are successful, they are subject to significant risks and uncertainties related to product commercialization and launch, including being unable to secure additional funding to make support to Company’s commercial launch efforts. Additionally, the Company’s potential product would compete in the market of medical dermatology. The industry is subject to technology advancements as well as being affected by political conditions which could impact the market’s reimbursement and regulatory policy, and by economic conditions surrounding availability and affordability of health insurance and access to health services. The pharmaceutical industry is heavily regulated by the need for approval in order to sell a product, to reimbursement policy for use of the product, and how companies can and cannot interact and sell to physicians or hospitals.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted (“U.S. GAAP”) in the United States. Any reference in these notes to applicable guidance is meant to refer to GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) promulgated by the Financial Accounting Standards Board (“FASB”).
Principles of Consolidation
The accompanying financial statements include the accounts of the Company and the Company’s subsidiary which the Company controls. Accordingly, all intercompany balances and transactions between these entities have been eliminated within the financial statements.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Changes in estimates and assumptions are reflected in reported results in the period in which they become known. Actual results could differ from those estimates. Management considers many factors in selecting appropriate financial accounting policies and controls, and in developing the estimates and assumptions that are used in the preparation of these financial statements. Management must apply significant judgment in this process. In addition, other factors may affect estimates, including expected business and operational changes, sensitivity and volatility associated with the assumptions used in developing estimates, and whether historical trends are expected to be representative of future trends. The estimation process often may yield a range of potentially reasonable estimates of the ultimate future outcomes, and management must select an amount that falls within that range of reasonable estimates. Estimates are used in the following areas, among others: valuation of prepaid and accrued expenses related to certain research and development contracts, and share-based compensation expense which includes estimating the fair value of its common stock.
Cash and Cash Equivalents
Cash and cash equivalents are short-term, highly liquid investments with original maturities of three months or less at the date of purchase.
Leases
The Company has made an accounting policy election not to recognize right-of-use assets and lease liabilities that arise from leases with a term of one year or less for any class of underlying asset. The Company did not have any leases with lease terms in excess of one-year for the years ended December 31, 2024 and 2023.
Fair Value of Financial Instruments
ASC Topic 820, Fair Value Measurement (“ASC 820”), establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances.
ASC 820 identifies fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tier fair value hierarchy that distinguishes among the following:
nLevel 1—Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access.
nLevel 2—Valuations based on quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active and models for which all significant inputs are observable, either directly or indirectly.
nLevel 3—Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Financial instruments consist of cash and cash equivalents, accounts payable, accrued expenses and promissory notes. These financial instruments are stated at their respective historical carrying amounts, which approximate fair value due to their short-term nature.
Subscription Receivable
Subscription receivable is recorded when preferred stock has been issued, and the Company has a legal right to receive payment or contractual services from the subscriber, but the contractual payment or services have not yet been received as of the balance sheet date. The Company records this amount as a subscription receivable in the mezzanine equity section along with the preferred shares on the consolidated balance sheets.
Accrued Expenses
As part of the process of preparing financial statements, the Company is required to estimate accrued expenses. This process involves identifying services that have been performed on the Company’s behalf and estimating the level of services performed and the associated costs incurred for such services where the Company has not yet been invoiced or otherwise notified of actual cost. The Company records these estimates in its consolidated financial statements as of the balance sheet date. Examples of estimated accrued expenses include:
nfees due to vendors in connection with the research and development; and
nprofessional service fees.
In accruing fees, the Company estimates the time period over which services will be provided and the level of effort in each period. If the actual timing of the provision of services or the level of effort varies from the estimate, the Company adjusts the accrual accordingly. In the event that the Company does not identify costs that have been incurred or it under or overestimates the level of services performed or the costs of such services, its actual expenses could differ from such estimates. The date on which some services commence, the level of services performed on or before a given date and the cost of such services can be subjective determinations. The Company prepares its estimates based on the facts and circumstances known to it at the time.
Research and Development Expenses
Research and development expenses are comprised of costs incurred in performing research and development activities, including personnel-related costs, stock-based compensation, external research and development expenses, clinical trial costs, contracted services, consultants license fees and other external costs. The accrual process involves reviewing open contracts and purchase orders, communicating with the Company’s personnel and with vendors to identify services that have been performed on behalf of the Company and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of the actual cost. Costs incurred in connection with research and development activities are expensed as incurred. Costs are considered incurred based on an evaluation of the progress to completion of each contract using information and data provided by the respective vendors, including the clinical sites. Depending upon the timing of payments to the service providers, the Company recognizes prepaid expenses or accrued expenses related to these costs. These accrued or prepaid expenses are based on management’s estimates of the work performed under service agreements, milestones achieved, and experience with similar contracts. The Company monitors each of these factors and adjusts estimates accordingly.
The Company bases its expenses related to research and development activities on the estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on behalf of the Company. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to the vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the estimate, the Company adjusts the accrual or prepaid balance accordingly. Non-refundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although the Company does not expect the estimates to be materially different from amounts incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in reporting amounts that are too high or too low in any particular period.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits, and stock-based compensation, for personnel in the Company’s executive, legal, finance and accounting, and other administrative functions. General and administrative expenses also include legal fees relating to intellectual property and corporate matters, professional fees paid for accounting, auditing, tax and consulting services, insurance costs, travel expenses, and direct facility costs not otherwise included in research and development expenses. General and administrative expenses are expensed as incurred.
Preferred Stock and Issuance Costs
The Company classifies convertible preferred stock as mezzanine equity in the accompanying consolidated balance sheets because, in certain deemed liquidation events, which are outside the Company’s control, such as a merger, acquisition or sale of all or substantially all of the Company’s assets, the preferred stock will become redeemable. Preferred stock issuance costs are legal fees incurred in obtaining funding. Preferred stock issuance costs are presented as a direct deduction of the carrying amount of the Series B preferred stock totaled $0.3 million for the year ended December 31, 2024 and the Series A-4 preferred stock was less than $0.1 million for the year ended December 31, 2023.
The Company has determined that the Series A and Series B preferred stock are contingently redeemable, based on the Company’s amended and restated certificate of incorporation that states upon the occurrence of certain events that equate to deemed liquidation events, the holders of preferred stock are entitled to receive cash or other assets. Additionally, the deemed liquidation events are not in the sole control of the Company and the preferred stock does not meet any limited exceptions under ASC 480, Distinguishing Liabilities From Equity. As such, the Company classified its redeemable preferred stock as mezzanine equity.
Stock-Based Compensation Expense
The Company measures stock-based compensation based on the grant date fair value of the stock-based awards and recognizes stock-based compensation expense on a straight-line basis over the requisite service period of the awards, which is generally the vesting period of the respective award. Stock-based compensation expense on grants to employees is classified in the consolidated statements of operations based on the function to which the related services are provided or in the same manner in which the grantee’s payroll costs are classified. Board of Directors expense is classified as general and administrative expenses. Forfeitures are accounted for as they occur.
As there has been no public market for the common stock, the estimated fair value of the common stock has been determined by the Company’s Board of Directors (“Board”) after considering valuation reports provided by an independent third-party valuation firm and exercising reasonable judgment and considering numerous objective and subjective factors to determine the best estimate of the fair value of the Company’s common stock at each stock option grant date. In accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, a third-party valuation firm prepared valuations of the common stock using an option pricing method (“OPM”), which is a Black-Scholes option pricing model. The total equity value can be implied from the OPM, using the recent preferred stock financing price if transacted by the Company around each valuation date. The OPM treats common stock and preferred stock as call options on the total equity value of a company, with exercise prices based on the value thresholds at which the allocation among the various holders of a company’s securities changes to value the common stock of the company. A discount for lack of marketability of the common stock is then applied to arrive at an indication of value for the common stock as of the valuation date.
The Company lacks sufficient history of company-specific historical and implied volatility information for its common stock. Therefore, under the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held Company Equity Securities Issued as Compensation, the Company estimates its expected stock price volatility based on the historical volatility of publicly traded peer companies. The Company did not meet the requirements for using the simplified method for determining the expected term and lacks sufficient history to determine an expected term of the options. The Company estimated a term of 10 years based on the details of the grants issued. This is the full term of the stock option grants which was the best estimate. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is based on the fact that the Company has never paid cash dividends on its common stock and does not expect to pay any cash dividends in the foreseeable future.
There are significant judgments related to volatility and time to liquidity as well as estimates inherent in these valuations. These judgments and estimates include assumptions regarding the Company’s future operating performance, the stage of development of the Company’s product candidates, the timing of a potential IPO or other liquidity events and the determination of the appropriate valuation methodology at each valuation date. The assumptions underlying these valuations represent management’s best estimates, which involve inherent uncertainties and the application of management judgment. As a result, if factors or expected outcomes change and the Company use significantly different assumptions or estimates, the stock-based compensation expense could be materially different. Following the completion of this offering, the fair value of the Company’s common stock will be determined based on the quoted market price of the common stock.
Income Taxes
Income taxes are accounted for under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the associated temporary differences become deductible. A valuation allowance is established for those jurisdictions in which the realization of deferred tax assets is not deemed to be more likely than not.
Accounting guidance prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This accounting guidance also provides guidance on derecognition, classification, interest and penalties, accounting in the interim periods, disclosure, and transition. Penalties and interest related to uncertain tax positions are recognized as a component of income tax expense. To the extent penalties and interest are not assessed with respect to uncertain tax positions, amounts accrued are reduced and reflected as a reduction of the overall income tax provision.
Concentration of Credit Risk and Other Risks
Financial instruments that subject the Company to credit risk consist primarily of cash. The Company places its cash deposits in accredited financial institutions and, therefore, the Company’s management believes these funds are subject to minimal credit risk. The Company has no off-balance sheet concentrations of credit risk such as foreign currency exchange contracts, option contracts or other hedging arrangements.
The Company’s three largest vendors, which provide services under the Development and Manufacturing Agreement and Master Services Agreement (see Note 11), accounted for approximately 72% and 60% of its research and development expenses for the years ended December 31, 2024 and 2023, respectively. As of December 31, 2024 and 2023 one and three vendors accounted for more than 10% of the Company’s accounts payable and accrued expenses.
Segment Information
Operating segments are defined as components of an enterprise for which discrete financial information is regularly reviewed by the chief operating decision maker (“CODM”) in deciding how to allocate resources and in assessing operating performance. The Company manages its operations as a single segment for the purposes of allocating resources, assessing performance, and making operating decisions.
Basic and Diluted Net Loss Per Common Share
The Company calculates basic net loss per common share by dividing the net loss, adjusted for the unpaid cumulative Series B preferred stock dividends, the weighted average number of shares of common stock outstanding during the period, without consideration of potential dilutive securities. Diluted net loss per share is computed by dividing the net loss, adjusted for the unpaid cumulative Series B preferred stock dividends, by the sum of the weighted average number of shares of common stock outstanding during the period plus the dilutive effects of potentially dilutive securities outstanding during the period. Potentially dilutive securities include outstanding stock options and redeemable convertible preferred stock. The dilutive effect of redeemable convertible preferred stock is calculated using the if-converted method. Under the if-converted method, it is assumed that all convertible preferred stock are converted into common stock at the beginning of the period, or at the time of issuance, if later. The Company has generated a net loss for all periods presented, therefore diluted net loss per common share is the same as basic net loss per common share since the inclusion of potentially dilutive securities would be anti-dilutive. Preferred stockholders do not have a contractual obligation to share in the Company’s losses and are not obligated to fund future losses.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2023-09 (“ASU 2023-09”), Income Taxes (Topic 740): Improvements to Income Tax Disclosures to enhance the transparency and usefulness of income tax disclosures. ASU 2023-09 is effective for annual periods beginning after December 15, 2025, on a prospective basis. Early adoption is permitted. As the amendments apply to income tax disclosures only, the Company does not expect adoption to have a material impact on its financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This update enhances the segment reporting requirements by increasing transparency and providing investors with additional insights into how an entity’s CODM evaluates segment performance and allocates resources.
The standard requires public entities to disclose significant segment expenses that are regularly reviewed by the CODM and included in the reported measure of segment profit or loss. Additionally, entities must provide a reconciliation of total segment amounts to financial statements, as well as enhanced qualitative disclosures regarding the methodology used to identify reportable segments and assess performance. The update applies to all public entities, including those with a single reportable segment, and expands segment disclosures in interim financial statements.
ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. The Company adopted ASU 2023-07 for the year ended December 31, 2024, and has updated its segment disclosures accordingly. The adoption of this standard did not impact the recognition or measurement of the Company’s financial statements but resulted in enhanced segment-related disclosures in the notes to its consolidated financial statements.
3. Prepaid Expenses, Other Current Assets, and Accrued Expenses
Prepaid expenses and other current asset as of December 31, 2024 and 2023, consisted of the following:
| | | | | | | | | | | |
| (in thousands) | 2024 | | 2023 |
Prepaid research and development | $ | 1,038 | | | $ | 1,220 | |
Tax credit receivable | 1,375 | | | — | |
| Other | $ | 38 | | | $ | 46 | |
| $ | 2,451 | | | $ | 1,266 | |
| | | |
Accrued expenses as of December 31, 2024 and 2023, consisted of the following:
| | | | | | | | | | | |
| (in thousands) | 2024 | | 2023 |
Research and development | $ | 1,064 | | | $ | 293 | |
Bonus | 1,033 | | | 764 | |
Professional fees | 371 | | | 138 | |
Other | 2 | | | 5 | |
| $ | 2,470 | | | $ | 1,200 | |
| | | |
4. Promissory Note
On May 3, 2023, the Company entered into a Promissory Note (the “Promissory Note”) that provided for up to $0.8 million in notes pursuant to the Development and Manufacturing Agreement dated July 26, 2022 (see Note 11). The Promissory Note was used to pay for services provided. The Promissory Note was originally scheduled to mature in October 2025 or on the date the Company consummates an equity financing with gross proceeds of greater than $20.0 million. The completion of the Series B preferred stock financing in November 2024 caused the Promissory Note to reach maturity.
The Promissory Note bears interest on the outstanding principal amount at the rate of 8.0% per annum, compounded annually. Interest commences on the date of issuance and continues until the outstanding principal amount is paid in full. Interest is computed on the basis of a year of 365 days for the actual number of days elapsed.
The Company incurred interest expense of approximately $0.1 million for the year ended December 31, 2024. In the fourth quarter of 2024, the Promissory Note, including accrued interest, was paid in full with the proceeds of the Series B preferred stock financing. Interest expense for the year ended December 31, 2023 was not material.
5. Convertible Notes
In August of 2024, the Company issued nine convertible promissory notes (the “Convertible Notes”) for a total of $9.9 million of which $8.5 million was for cash and $1.4 million was for committed research and development services. The Convertible Notes accrued simple interest at the rate of 1.5% per month (18% per annum) on commencement and continuing until the outstanding principal amount is fully paid or converted. The Convertible Notes automatically convert under a qualified equity financing event or change of control event.
On November 25, 2024, the Company entered into a Series B preferred stock purchase agreement which converted the Convertible Notes’ outstanding principal and accrued interest of $10.4 million through the issuance of 8,661,917 shares of Series B preferred stock at a purchase price of $1.2049 per share of which $1.5 million was a subscription receivable for committed research and development services. Interest expense related to the Convertible Notes for the year ended December 31, 2024 was $0.5 million.
6. Stockholders’ Equity – Common and Preferred Stock
Common Stock
In accordance with the Third Amended and Restated Certificate of Incorporation filed on November 22, 2024, the Company is authorized to issue 98.8 million shares of common stock, par value $0.00001 per share, of which 92.6 million are designated as voting common stock and 6.2 million are designated as non-voting common stock. Each share of non-voting common stock shall be convertible, at the option of the holder thereof, at any time, and without the payment of additional consideration by the holder thereof, into one fully paid and nonassessable share of voting common stock. The Company had no outstanding shares of non-voting common stock as of December 31, 2024 and 2023. The voting, dividend and liquidation rights of the holders of the common stock are subject to and qualified by the rights, power, and preferences of the preferred stockholders.
As of December 31, 2024 and 2023, a total of 736,933 shares of common stock were issued and an aggregate of 771,832 shares (subject to certain anti-dilution adjustments) of common stock were reserved for the granting of stock-based compensation. In addition, the Company has reserved sufficient shares of common stock for issuance upon conversion of convertible preferred stock.
The holders of the common stock are entitled to one vote for each share of common stock held at all meetings of stockholders (and written actions in lieu of meetings), and there are no cumulative voting rights. The number of authorized shares of common stock may be increased or decreased by the affirmative vote of the holders of common stock of the Company; however, the issuance of common stock may be subject to the vote of the holders of one or more series of preferred stock that may be required by terms of the Third Amended and Restated Certificate of Incorporation.
Preferred Stock
Preferred stock outstanding as of December 31, 2024 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Preferred Series Shares | | Date Sold | | Shares Sold | | Par Value | | Sales
Price/Share | | Total Proceeds
(in millions) | | Liquidation Preference as of December 31, 2024 (in millions) |
| Series A-1 | | September 2021 | | 6,515,849 | | | $ | 0.00001 | | | $ | 2.8770 | | | $ | 18.7 | | | $ | 18.7 | |
| Series A-2 | | September 2021 | | 680,479 | | | 0.00001 | | | 2.1578 | | | 1.5 | | | 1.5 | |
| Series A-3 | | September 2021 | | 703,475 | | | 0.00001 | | | 2.8770 | | | 2.0 | | | 2.0 | |
| Series A-4 | | April 2023 | | 4,965,572 | | | 0.00001 | | | 3.0208 | | | 15.0 | | | 15.0 | |
| Series B | | November 2024 | | 55,246,971 | | | 0.00001 | | | 1.2049 | | | 66.6 | | | 133.9 | |
| | | | | | | | | | | | |
In connection with the Series A4 Preferred Stock Purchase Agreement, the Company increased the number of shares of common stock authorized from 18,000,000 to 25,500,000 under the Second Amended and Restated Certificate of Incorporation dated as of April 13, 2023.
In connection with the Series B Preferred Stock Purchase Agreement, the Company increased the number of shares of common stock authorized from 25,500,000 to 98,774,582 under the Third Amended and Restated Certificate of Incorporation dated as of November 22, 2024.
Collectively, the shares of Series A1, A2, A3 and A4 preferred stock are referred to as “Series A preferred stock.” The following terms detailed below for the Series A and B preferred stock are documented in the Company’s Third Amended and Restated Certificate of Incorporation dated as of November 22, 2024 and other equity-related documents:
Conversion
Each share of Series A and Series B preferred stock, at the option of the holder, is convertible into a number of fully paid and non-assessable common shares as determined by multiplying the number of shares of Series A and Series B preferred stock being converted by the applicable conversion rate. The conversion rate in effect at any time is determined by dividing the preferred stock issue price by the conversion price in effect at that time. The conversion price applicable (all adjusted for the reverse stock split) to the Series A1 preferred stock is equal to $28.963 per share, the conversion price applicable to the Series A2 preferred stock is equal to $21.7226 per share, the conversion price applicable to the Series A3 preferred stock is equal to $28.963 per share, the conversion price applicable to the Series A4 preferred stock is equal to $30.4104 per share, and the conversion price applicable to the Series B preferred stock is equal to $12.1297 per share. Such initial conversion price, and the rate at which shares of preferred stock may be converted into common stock, is subject to adjustment. See Note 13 for information on the reverse stock split that adjusted the preferred stock conversion ratio.
The Series A and Series B preferred stock will automatically convert to common stock either upon the closing of a public offering of the Company's common stock at a price of at least five times the conversion price, noted above, resulting in aggregate proceeds of at least $50.0 million, or upon the written election of a majority of the holders of Series B preferred stock.
Dividends
The Series B preferred stock contains cumulative dividend rights at the annual rate of 12%, if and when declared by the Board, such that the Series B preferred stockholders, acting as one class, shall first receive, or simultaneously receive, preferential dividends as calculated under the terms of the Company’s Third Amended and Restated Certificate of Incorporation prior to Series A and common stockholders receiving any declared dividend. Since inception, no dividends have been declared or paid on the Series B preferred stock. As of December 31, 2024, cumulative undeclared dividends totaled approximately $0.8 million.
The Series A preferred stock contains non-cumulative dividend rights at the annual rate of 8%, if and when declared by the Board, such that the holders of the preferred stock, acting as one class, shall receive, or simultaneously receive, preferential dividends as calculated under the terms of the Company’s Third Amended and Restated Certificate of Incorporation prior to common stockholders receiving any declared dividend. As of December 31, 2024, no dividends have been declared by the Board.
Deemed Liquidation Event
Certain transactions are defined as Deemed Liquidation Events, unless waived by the holders of a majority of the outstanding preferred stock. These events generally include a merger or consolidation involving the Company, the sale or disposition of all or substantially all of the Company’s assets, a SPAC transaction, or a merger or other business combination with a public company (a “Public Listing Transaction”). Transactions in which existing stockholders retain a majority of the voting power of the surviving entity are not considered Deemed Liquidation Events.
Liquidation Preference
In the event of any liquidation, dissolution, or winding up of the Company, the Series B preferred stockholders are entitled to receive prior to, and in preference to, any distribution to the Series A preferred and common stockholders, an amount equal to the greater of the applicable of two times the Original Issue Price per share plus accrued but unpaid dividends whether or not declared, or such amount per share as would have been payable had all shares of Series B preferred stock been converted to shares of common stock immediately prior to such event of liquidation, dissolution or winding up. In the event that upon liquidation or dissolution, if the assets and funds of the Company are insufficient to permit the payment to preferred stockholders of the full preferential amounts, then the entire assets and funds of the Company legally available for distribution would be distributed ratably among the Series B preferred stockholders in proportion to the full preferential amount each is otherwise entitled to receive.
Following the completion of the distributions to the Series B Preferred stockholders, the Series A preferred stockholders are entitled to receive prior to, and in preference to, any distribution to the common stockholders, an amount equal to the greater of the applicable Original Issue Price per share plus accrued but unpaid dividends declared, or such amount per share as would have been payable had all shares of Series A preferred stock been converted to shares of common stock immediately prior to such event of liquidation, dissolution or winding up. In the event that upon liquidation or dissolution, if the assets and funds of the Company are insufficient to permit the payment to preferred stockholders of the full preferential amounts, then the entire assets and funds of the Company legally available for distribution are to be distributed ratably among the Series A preferred stockholders in proportion to the full preferential amount each is otherwise entitled to receive. After the distributions described above have been paid in full, the remaining assets of the Company available for distribution would be distributed pro-rata to the common stockholders.
Voting Rights
Each Series A and Series B preferred stockholder is entitled to the number of votes equal to the number of shares of voting common stock into which such holder's shares are convertible.
7. Net Loss Per Common Share
Basic and diluted net loss per common share was calculated as follows:
| | | | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 |
| (in thousands except for share and per share amounts) | | | |
Net loss | $ | (26,488) | | | $ | (16,490) | |
Less: Cumulative dividends on Series B preferred stock | 815 | | | — | |
Net loss attributable to common stock | (27,303) | | | (16,490) | |
Weighted-average number of shares of common stock outstanding, basic and diluted | 736,933 | | | 736,933 | |
Net loss per common share, basic and diluted | $ | (37.05) | | | $ | (22.38) | |
| | | |
Basic net loss per common share is calculated by dividing the net loss, adjusted for the unpaid cumulative Series B preferred stock dividends, by the weighted-average number of shares of common stock outstanding during the period. Diluted loss per share is computed by dividing net loss for the period by the weighted-average number of shares of common stock and common stock equivalents outstanding (unless their effect is anti-dilutive) for the period. Common share equivalents include shares issuable upon the exercise of stock options and the conversion of preferred stock. During the years ended December 31, 2024 and 2023, convertible preferred stock on an as if converted basis and unexercised options have been excluded as their effect is antidilutive. There were no other potentially dilutive, unissued shares of common stock for the years ended December 31, 2024 and 2023. The Company had no outstanding non-voting common stock as of December 31, 2024 and 2023.
The Company reported net losses for each of the years ended December 31, 2024 and 2023 and therefore excluded all options and convertible preferred stock from the computation of diluted net loss per common share as their inclusion would have had an antidilutive effect, as summarized below:
| | | | | | | | | |
| Year Ended December 31, |
| 2024 | | 2023 |
| Stock options | 279,208 | | | 244,551 | |
| Convertible preferred stock | 6,765,903 | | | 1,277,975 | |
| Total potentially dilutive shares | 7,045,111 | | | 1,522,526 | |
| | | |
8. Stock-Based Compensation
The Company adopted the 2021 Equity Incentive Plan (the “2021 Plan”) to grant option awards to its officers, directors and employees as compensation for their services to the Company. Under the 2021 Plan, up to 771,832 shares of voting common stock were made available for issuance.
Stock option awards under the 2021 Plan must be issued at an exercise price of not less than 100% of the fair market value of the voting common stock at the date of the grant. The 2021 Plan is administered by the Board, which has the authority to grant awards, interpret the plan and related agreements, establish rules and regulations, and make all other determinations necessary for its administration. The board may delegate these powers to a committee. Stock option awards granted under the 2021 Plan generally vest over 36 or 48 months, with 33.3% or 25% vesting one year after the grant date and the remainder vesting in equal monthly installments over the following 24 or 36 months, respectively.
To estimate the fair value of the Company’s stock options, the Company used the Black-Scholes OPM. The following key assumptions were used to estimate the fair value:
| | | | | | | | | | | |
| 2024 | | 2023 |
Expected volatility | 82.6 | % | | 81.2 | % |
Expected term (years) | 10.0 | | | 10.0 | |
Risk free interest rate | 4.2 | % | | 4.3 | % |
Expected dividend yield | 0.0 | % | | 0.0 | % |
| | | |
During the years ended December 31, 2024 and 2023, the Company recognized stock-based compensation expense of $0.3 million and $0.2 million, respectively. Stock-based compensation expense for the years ended December 31, 2024 and 2023 was allocated as follows:
| | | | | | | | | | | |
| (in thousands) | 2024 | | 2023 |
| Research and development | $ | 156 | | | $ | 138 | |
| General and administrative | 94 | | | 68 | |
| $ | 250 | | | $ | 206 | |
| | | |
As of December 31, 2024 and 2023, there was $0.3 million and $0.4 million of unrecognized stock-based compensation expense that is expected to be recognized over a weighted average period of approximately 2.3 and 2.7 years.
Total option activity for the years ended December 31, 2024 and 2023, is summarized as follows:
| | | | | | | | | | | | | | | | | |
| Number of Stock Options | | Weighted Average Exercise Price | | Weighted Average Contractual Term (in years) |
| Outstanding as of January 1, 2023 | 211,095 | | $ | 29.00 | | | 9.11 |
| Granted | 33,456 | | 30.41 | | | |
| Forfeited | — | | — | | | |
| Outstanding as of December 31,2023 | 244,551 | | $ | 29.20 | | | 8.34 |
| Granted | 34,657 | | 30.41 | | | |
| Forfeited | — | | — | | | |
| Outstanding as of December 31,2024 | 279,208 | | | $ | 29.30 | | | 7.55 |
| Stock options exercisable as of December 31, 2024 | 208,256 | | $ | 29.00 | | | 7.14 |
| | | | | |
Using the Black-Scholes OPM, the weighted average grant-date fair value per unit of options granted during the years ended December 31, 2024 and 2023, was $6.35 per share. The intrinsic value of options outstanding and exercisable as of December 31, 2024 and 2023 was $0, as the exercise price of all options exceeded the fair value of the Company’s common stock on that date.
9. Income Taxes
The Company did not record a provision for income taxes for the years ended December 31, 2024 and 2023, due to a full valuation allowance against its deferred tax assets.
The reconciliation of the Company’s effective tax rate to the U.S. federal statutory rate is as follows:
| | | | | | | | | | | |
| (in thousands) | 2024 | | 2023 |
Federal tax at statutory rate | 21.0 | % | | 21.0 | % |
State taxes, net of federal benefit | 5.9 | | | 5.9 | |
Permanent differences | 0.3 | | | — | |
Research and development credits | 5.3 | | | 4.1 | |
Change in valuation allowance | (32.5) | | | (31.0) | |
Effective tax rate | — | % | | — | % |
| | | |
The significant components of the Company’s net deferred tax assets as of December 31, 2024 and 2023 are shown below. In determining the realizability of the Company’s net deferred tax assets, the Company considered numerous factors, including historical profitability and estimated future taxable income. Based on this information and management’s assessment, the Company has provided a valuation allowance for the full amount of its net deferred tax assets because the Company has determined that it is more likely than not it will not be realized. Significant components of the Company’s deferred tax assets are as follows:
| | | | | | | | | | | |
| December 31, |
| (in thousands) | 2024 | | 2023 |
Deferred tax assets: | | | |
Federal and state net operating losses | $ | 3,942 | | | $ | 1,372 | |
Capitalized research and development | 8,795 | | | 4,450 | |
Federal and state research and development tax credits | 2,239 | | | 853 | |
Accrued expenses | 278 | | | — | |
Other | 137 | | | 92 | |
Valuation allowance | (15,391) | | | (6,767) | |
Total deferred tax assets: | — | | | — | |
| | | |
As of December 31, 2024 a full valuation allowance of $15.4 million was established against its deferred tax assets due to the uncertainty surrounding the realization of such assets. The valuation allowance increased $8.6 million compared to December 31, 2023 primarily due to increases in federal and state net operating losses, capitalized research and development and federal and state research and development tax credits.
As of December 31, 2024, the Company had federal and state net operating loss carryforwards of $29.3 million. Federal net operating losses carryforward indefinitely. State net operating loss carryforwards will begin to expire in 2040.
The Company had federal research and development credit carryforwards of $2.1 million as of December 31, 2024. The federal research tax credit carryforwards will begin to expire in 2041. The Company had state research and development credit carryforwards of $0.1 million as of December 31, 2024. The state research tax credit carryforwards will begin to expire in 2037.
Pursuant to Section 382 and 383 of the Internal Revenue Code (“IRC”), utilization of the Company’s federal net operating loss carryforwards and research and development credit carryforwards may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating loss and research and development credit carryforwards prior to utilization. The Company has not completed an IRC Section 382 and 383 analyses regarding the limitation of net operating loss and research and development credit carryforwards.
No liability is recorded on the financial statements related to uncertain tax positions. There are no unrecognized tax benefits as of December 31, 2024. The Company does not expect that uncertain tax benefits will materially change in the next 12 months.
The Company’s policy is to record estimated interest and penalties related to uncertain tax benefits as income tax expense. As of December 31, 2024, the Company had no accrued interest or penalties recorded related to uncertain tax positions.
10. Commitments and Contingencies
From time to time, in the ordinary course of business, the Company is subject to litigation and regulatory examinations as well as information gathering requests, inquiries and investigations. As of December 31, 2024 and 2023, there were no matters which would have a material impact on the Company’s financial results.
In July 2022, the Company entered into a license agreement with The Trustees of the University of Pennsylvania (“Penn”) to which the Company was granted intellectual property rights in certain technology, to develop, manufacture and commercialize such technology related to the advancement of its VDMC asset. The Company is required to make development and regulatory milestone payments up to $3.3 million and commercial and sales milestone up to $16 million. The Company is also required to pay annual single-digit royalties on net product sales over the term of the License Agreement. As of December 31, 2024, it is not practicable to estimate the future payments of any such milestone payments or royalties that may arise due to the clinical stage of development of VDMC.
11. Development and Manufacturing Agreement and Master Service Agreement
Development and Manufacturing Agreement
On July 26, 2022, the Company entered into a Development and Manufacturing Agreement (the “DMA”). The Company engaged the counterparty to perform development and manufacturing services for the production of API (active pharmaceutical ingredients or drug substances) identified by the Company. The specific terms for each API will be documented in a development plan. The counterparty provided $1.0 million worth of research and development services to the Company in exchange for 347,584 shares of Series A3 preferred stock. The Company issued a promissory note for $0.8 million to settle payables arising from the research and development services provided under the DMA as described in Note 4 herein. During the year ended December 31, 2023, the aggregate invoices exceeded $0.8 million and the Company paid all remaining invoices under the DMA in cash. The Company issued a convertible note for $0.3 million for committed research and development services. On November 25, 2024 the Company entered into a Series B preferred stock purchase agreement which converted the convertible note’s outstanding principal and accrued interest through the issuance of 217,860 shares of Series B preferred stock at a purchase price of $1.2049. As discussed in Note 4 the full principal and interest was paid during the year ended December 31, 2024.
The DMA commenced on July 26, 2022, and will expire after all development plans are complete or five years whichever date occurs first. Upon expiration, the DMA shall automatically renew for a total of ten one-year periods, unless the Company sends a notice of non-renewal. Either party can terminate the DMA upon written notice.
During the years ended December 31, 2024 and 2023, the Company incurred research and development expense of $2.4 million and $3.5 million, respectively, in connection with the DMA. As of December 31, 2023, the Company had deposits with the counterparty of $0.2 million which is included in prepaid expenses on the consolidated balance sheets. There were no deposits as of December 31, 2024.
Master Service Agreement
On September 21, 2020, the Company entered into a Master Service Agreement (the “MSA”). The MSA provides the terms and conditions upon which the Company may engage for the purpose of managing the preclinical and clinical development of its products in the field of dermatology, and other related services or projects, by executing work orders specifying the details of the service and the related terms and conditions. The MSA automatically renews for additional 1-year terms until the MSA is terminated upon written notice. Either party may terminate a work order for material breach (subject to a cure period) and the Company may terminate any work order for any reason on 90 days’ written notice, subject to payment of fees earned and expenses payable, in addition to a termination fee equal to a low-double-digit percentage of the remaining work order budget. As of December 31, 2024, there are no outstanding work order cancellation fees.
During the years ended December 31, 2024 and 2023, the Company recognized research and development expense of $13.4 million and $4.8 million, respectively, in connection with the MSA. As of December 31, 2024 and 2023, the Company has deposits with the counterparty of $0.3 million and $0.6 million, respectively, which is included in prepaid expenses on its consolidated balance sheets for each year.
Collaboration Agreement
In September 2020, the Company entered into a Collaboration Agreement, pursuant to which the counterparty serves as the exclusive provider of certain clinical trial management, regulatory, and related CRO services through completion of Work Order Number One of the MSA noted herein. In connection with the agreement, the Company issued the counterparty an unsecured convertible promissory note with a principal amount of approximately $0.5 million, which was converted into 238,008 shares of Series A-2 Preferred Stock in September 2021. The agreement may be terminated by the Company upon payment of a mid-six-figure liquidated damages amount, subject to reduction based on completed in-human clinical studies. As of December 31, 2024, there are no outstanding termination fees.
12. Segments
The Company defines its segments on the basis of the way in which internally reported financial information is regularly reviewed by the CODM to analyze financial performance, make decisions, and allocate resources. The Company’s CODM is the Chief Executive Officer. The Company manages its operations as a single operating and reportable segment and the measure of segment profit or loss is net loss. The CODM uses net loss in the budget and forecasting process and considers budget-to-actual variances on a quarterly basis when making decisions about the allocation of operating and capital resources. The following table summarizes the significant segment expenses presented on the Company's consolidated statements of operations:
| | | | | | | | | | | |
| Year Ended December 31, |
| (in thousands) | 2024 | | 2023 |
Direct research and development by program | | | |
VDPHL01 | 14,641 | | | 2,953 | |
| VDMN | 3,747 | | | 5,056 | |
| VDMC | 1,329 | | | 3,648 | |
| VDAA | 402 | | | 1,146 | |
Other program candidates and expenses | 1,370 | | | 1,036 | |
Other unallocated research and development costs | | | |
| Personnel expenses (including share-based compensation) | 1,499 | | | 1,131 | |
Other expenses | 295 | | | 1 | |
General and administrative, excluding personnel expenses | 2,319 | | | 1,559 | |
General and administrative, personnel expenses (including share-based compensation) | 1,176 | | | 794 | |
Other segment items(1) | (290) | | | (834) | |
Segment net loss | (26,488) | | | (16,490) | |
| | | |
(1)For the year ended December 31, 2024 other segment items include interest income of $0.5 million and research and development tax credit of $0.4 million, partially offset by interest expense of $0.6 million. For the year ended December 31, 2023, other segment items include interest income of $0.9 million, partially offset by less than $0.1 million of interest expense.
13. SUBSEQUENT EVENTS
The Company has evaluated all subsequent events through October 24, 2025, the date the consolidated financial statements were available to be issued, and through January 28, 2026, for the effects of the reverse stock split described below. The following events occurred subsequent to December 31, 2024:
In October 2025, the Company issued 106,107,033 shares of Series C Preferred Stock, $.0.00001 par value, at a purchase price of $1.2723 per share, for total cash consideration of $135.0 million, with an additional $15.0 million that may be purchased at an additional closing for the same per share purchase price. Additionally, in connection with the issuance of the Series C redeemable preferred stock, certain other terms of the Series A and B redeemable preferred stock terms were amended. The liquidation preference for Series B redeemable preferred stock was changed from two times to one times its original purchase price and is paid pari passu with the Series C redeemable preferred stock, and the Series C redeemable preferred stock is entitled to dividends prior to the Series B redeemable preferred stock.
In the first quarter of 2025 the Company issued an additional 6,998,834 shares of Series B redeemable convertible preferred stock, $0.00001 par value, at a purchase price of $1.2049 per share, for total cash proceeds of $8.4 million.
On January 27, 2026, the Company effected a 1-for-10.067 reverse stock split of the Company’s issued and outstanding common stock and adjusted the conversion ratio of the Company’s outstanding convertible preferred stock. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been retroactively adjusted, where applicable, to reflect the reverse stock split and the adjustment of the preferred stock conversion ratios.
VERADERMICS, INCORPORATED
Condensed Consolidated Balance Sheets
(Unaudited)
| | | | | | | | | | | |
| September 30, | | December 31, |
| (in thousands, except share and per share amounts) | 2025 | | 2024 |
Assets | | | |
Current assets: | | | |
Cash and cash equivalents | $ | 5,660 | | | $ | 53,084 | |
Marketable securities at fair value | 9,479 | | | — | |
Prepaid expenses and other current assets | 3,574 | | | 2,451 | |
Total current assets | 18,713 | | | 55,535 | |
Property, plant and equipment, net | 18 | | | — | |
Right-of-use asset and other long-term assets | 66 | | | — | |
Total assets | $ | 18,797 | | | $ | 55,535 | |
Liabilities and stockholders’ deficit | | | |
Current liabilities: | | | |
Accounts payable | 1,985 | | | 2,248 | |
Accrued expenses | 2,602 | | | 2,470 | |
Other current liabilities and lease liabilities | 1,107 | | | — | |
Total current liabilities | 5,694 | | | 4,718 | |
Lease liabilities - non-current | 16 | | | — | |
Total liabilities | 5,710 | | | 4,718 | |
Commitments and contingencies (Note 9) | | | |
Redeemable convertible preferred stock: | | | |
Series A preferred stock, $0.00001 par value, 12,865,375 shares authorized, issued and outstanding with an aggregate liquidation preference of $37,238 net of issuance costs as of September 30, 2025 and December 31, 2024, net of issuance costs | 36,860 | | | 36,860 | |
Series B preferred stock, $0.00001 par value, 62,245,806 shares authorized; 62,245,805 and 55,246,971 issued and outstanding with an aggregate liquidation preference of $157,833 and $133,944 as of September 30, 2025 and December 31, 2024, respectively, net of issuance costs | 74,708 | | | 66,285 | |
Subscription receivable | (2,466) | | | (3,848) | |
Stockholders’ equity (deficit): | | | |
Common stock, $0.00001 par value, 749,027 and 736,933 shares issued and outstanding as of September 30, 2025 and December 31, 2024, respectively, and 98,774,582 authorized as of September 30, 2025 and December 31, 2024 | 1 | | | 1 | |
Additional paid-in capital | 1,363 | | | 750 | |
Accumulated deficit | (97,379) | | | (49,231) | |
Total stockholders’ deficit | (96,015) | | | (48,480) | |
Total liabilities, redeemable convertible preferred stock, and stockholders’ deficit | $ | 18,797 | | | $ | 55,535 | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Condensed Consolidated Statements of Operations
(Unaudited)
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| (in thousands, except share and per share amounts) | 2025 | | 2024 |
| Operating Expenses: | | | |
| Research and development | $ | 43,873 | | | $ | 18,333 | |
| General and administrative | 5,463 | | | 2,474 | |
| Total operating expenses | 49,336 | | | 20,807 | |
| Loss from operations | (49,336) | | | (20,807) | |
| Other income (expense): | | | |
| Interest income | 656 | | | 272 | |
| Interest expense | — | | | (293) | |
| Other income | 532 | | | — | |
| Total other income (expense), net | 1,188 | | | (21) | |
| Loss before income taxes | (48,148) | | | (20,828) | |
| Income tax benefit | — | | | — | |
| Net loss | $ | (48,148) | | | $ | (20,828) | |
| Net loss attributable to common shares | $ | (55,166) | | | $ | (20,828) | |
| Net loss per common share, basic and diluted | $ | (74.21) | | | $ | (28.26) | |
| Weighted average common shares outstanding, basic and diluted | 743,401 | | | 736,933 | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Condensed Consolidated Statements of Changes in Redeemable Convertible Preferred Stock and Stockholders’ Deficit
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series A Redeemable Convertible Preferred Stock | | Series B Redeemable Convertible Preferred Stock | | Subscription Receivable | | Common Stock | | Additional Paid-In Capital | | Accumulated
Deficit | | Total Stockholders’ Deficit |
(in thousands, except share amounts) | Shares | | Amount | Shares | | Amount | Shares | | Amount | |
| Balance, January 1, 2024 | 12,865,375 | | | $ | 36,860 | | | — | | | $ | — | | | $ | (894) | | | 736,933 | | | $ | 1 | | | $ | 501 | | | $ | (22,743) | | | $ | (22,241) | |
| Release of subscription receivable | — | | | — | | | — | | | | | 680 | | | — | | | — | | | — | | | — | | | — | |
| Stock-based compensation | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 159 | | | — | | | 159 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (20,828) | | | (20,828) | |
Balance, September 30, 2024 | 12,865,375 | | | $ | 36,860 | | | — | | | $ | — | | | $ | (214) | | | 736,933 | | | $ | 1 | | | $ | 660 | | | $ | (43,571) | | | $ | (42,910) | |
| | | | | | | | | | | | | | | | | | | |
| Balance, January 1, 2025 | 12,865,375 | | | $ | 36,860 | | | 55,246,971 | | | $ | 66,285 | | | $ | (3,848) | | | 736,933 | | | $ | 1 | | | $ | 750 | | | $ | (49,231) | | | $ | (48,480) | |
| Issuance of Series B Redeemable Convertible Preferred stock, net of issuance costs of $9,863 | — | | | — | | | 6,998,834 | | | 8,423 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Subscription receivable settled through for research and development services received | — | | | — | | | — | | | — | | | 1,382 | | | — | | | — | | | — | | | — | | | — | |
| Stock-based compensation | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 466 | | | — | | | 466 | |
| Stock option exercises | — | | | — | | | — | | | — | | | — | | | 12,094 | | | — | | | 147 | | | — | | | 147 | |
| Net loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (48,148) | | | (48,148) | |
| Balance, September 30, 2025 | 12,865,375 | | | $ | 36,860 | | | 62,245,805 | | | $ | 74,708 | | | $ | (2,466) | | | 749,027 | | | $ | 1 | | | $ | 1,363 | | | $ | (97,379) | | | $ | (96,015) | |
| | | | | | | | | | | | | | | | | | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| (in thousands, except share amounts) | 2025 | | 2024 |
| Cash Flows from Operating Activities | | | |
| Net loss | $ | (48,148) | | | $ | (20,828) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Stock-based compensation | 466 | | | 159 | |
| Non-cash research and development services | 1,382 | | | 680 | |
| Net accretion of discount/premium on debt securities | (418) | | | — | |
| Other | 29 | | | 45 | |
| Changes in operating assets and liabilities: | | | |
| Prepaid expenses and other current assets | (1,126) | | | (1,139) | |
| Accounts payable | (263) | | | (397) | |
| Accrued expenses | 132 | | | 1,424 | |
| Other current liabilities and lease liabilities | (28) | | | — | |
| Net cash used in operating activities | (47,974) | | | (20,056) | |
| Cash Flows from Investing Activities: | | | |
| Purchase of property, plant and equipment | (19) | | | — | |
| Purchases of marketable securities | (24,061) | | | — | |
| Maturities of marketable securities | 15,000 | | | — | |
| Net cash used in investing activities | (9,080) | | | — | |
| Cash Flows from Financing Activities: | | | |
| Issuance of convertible notes | — | | | 10,170 | |
| Issuance of Series B preferred stock | 8,433 | | | — | |
| Preferred stock issuance costs | (10) | | | — | |
| Prefunded equity financing | 1,060 | | | — | |
| Exercise of stock options | 147 | | | — | |
| Net cash provided by financing activities | 9,630 | | | 10,170 | |
| Net decrease in cash and cash equivalents | (47,424) | | | (9,886) | |
| Cash and cash equivalents—beginning of period | 53,084 | | | 16,296 | |
| Cash and cash equivalents—end of period | $ | 5,660 | | | $ | 6,410 | |
| Supplemental Disclosure of Cash Flow Information: | | | |
| Cash paid during the period for interest | $ | — | | | $ | — | |
| Cash paid during the period for income taxes | $ | — | | | $ | — | |
| Supplemental Schedule of Noncash Financing Activities: | | | |
| Right-of-use asset exchanged for lease liabilities | $ | 90 | | | $ | — | |
| | | |
See accompanying notes of the financial statements
VERADERMICS, INCORPORATED
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Nature of Business
Veradermics, Incorporated and its wholly owned subsidiary (collectively, the “Company” or “Veradermics”) is a dermatologist-founded, late clinical-stage biopharmaceutical company focused on developing innovative therapeutics to address pervasive treatment challenges in highly prevalent aesthetic and dermatological conditions. The Company’s initial focus is developing better treatments for pattern hair loss, or PHL, a condition affecting approximately 50 million men and approximately 30 million women in the United States. Beyond VDPHL01, the Company has created a portfolio utilizing its real-world experience as dermatologists to generate compelling pipeline assets, including VDMN for the treatment of common warts, VDAA for the treatment of alopecia areata, and VDMC for the treatment of molluscum contagiosum.
Veradermics began its operations in 2019 as a company incorporated under the laws of the State of Texas. Effective on September 14, 2021, the Company was converted into a company incorporated under the laws of the State of Delaware. The Company is headquartered in New Haven, CT.
Liquidity and Ability to Continue as a Going Concern
The accompanying condensed consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business.
In accordance with Accounting Standards Update (“ASU”) 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (Subtopic 205-40), the Company has evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
The Company has incurred recurring losses since its inception, including net losses of $48.1 million and $20.8 million for the nine months ended September 30, 2025 and 2024, as well as incurred negative cash flows from operations of $48.0 million and $20.1 million, respectively. In addition, as of September 30, 2025, the Company had an accumulated deficit of $97.4 million. The Company expects to continue to generate operating losses for the foreseeable future. Through September 30, 2025, the Company has financed its operations primarily from the sale of equity securities. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital to fund its operations.
In November 2024, the Company completed the first closing of a $66.5 million Series B redeemable convertible preferred stock financing. In the first quarter of 2025, the Company completed the second funding of Series B preferred stock of $8.4 million and then completed a $151.0 million Series C preferred stock financing in the fourth quarter of 2025. The 2025 financings, along with cash on hand, will enable the Company to meet its obligations for at least the twelve-month period from the date the financial statements are available to be issued.
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the pharmaceutical industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations and those specific to the pharmaceutical industry such as the U.S. Food and Drug Administration, and the ability to secure additional capital to fund operations. Products currently under development will require significant additional research and development efforts, including preclinical and clinical testing and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel and infrastructure, and extensive compliance and reporting capabilities. The Company’s future clinical trials require significant compliance and monitoring by government agencies and there can be no assurances that such agencies will approve procedures followed in the Company’s trials. Another likely scenario is that such agencies would require additional procedures to be performed which would push out commercialization timing. Further, even if the Company's product development efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
If the Company’s product development efforts are successful, they are subject to significant risks and uncertainties related to product commercialization and launch, including being unable to secure additional funding to make support to Company’s commercial launch efforts. Additionally, the Company’s potential product would compete in the market of medical dermatology. The industry is subject to technology advancements as well as being affected by political conditions which could impact the market’s reimbursement and regulatory policy, and by economic conditions surrounding availability and affordability of health insurance and access to health services. The pharmaceutical industry is heavily regulated by the need for approval in order to sell a product, to reimbursement policy for use of the product, and how companies can and cannot interact and sell to physicians or hospitals.
2. Summary of Significant Accounting Policies
Basis of Presentation
Our financial statements included herein have been prepared in conformity with accounting principles generally accepted in the United States of America, or GAAP. In our opinion, the information furnished reflects all adjustments, all of which are of a normal and recurring nature, necessary for a fair presentation of the financial position and results of operations for the reported interim periods. We consider events or transactions that occur after the balance sheet date but before the financial statements are issued to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. The results of operations for interim periods are not necessarily indicative of results to be expected for the full year or any other interim period. These unaudited condensed consolidated interim financial statements should be read in conjunction with the audited consolidated financial statements, including the related notes thereto, for the year ended December 31, 2024.
There were no changes to the Company’s significant accounting policies during the nine months ended September 30, 2025 other than noted below.
Deferred Offering Costs
The Company capitalizes incremental legal, professional accounting and other third-party fees that are directly associated with the IPO as other current assets until the IPO is consummated. After consummation of the IPO, these costs will be recorded in stockholders’ deficit as a reduction of additional paid-in-capital generated as a result of the offering. As of September 30, 2025, there were no deferred offering costs included in prepaid expenses and other assets.
Prefunded Equity Financing
Proceeds received from prefunded equity financings are recorded as liabilities when no firm commitment for the issuance of equity instruments exists and therefore the criteria for equity classification are not met. During the nine months ended September 30, 2025, $1.1 million is included in other current liabilities in the condensed consolidated balance sheet. Upon authorization and issuance of the related shares, the liability is reclassified to stockholders’ equity.
Property, Plant and Equipment
Property and equipment are recorded at cost and consists of computer and other equipment, furniture and fixtures, leasehold improvements, and capitalized software. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, which range from three to six years. Maintenance and repairs which do not extend the lives of the assets are charged directly to expense as incurred. Upon retirement or disposal, cost and related accumulated depreciation are removed from the related accounts, and any resulting gain or loss is recognized as a component of income or loss in the consolidated statements of operations.
Marketable Securities
The Company invests excess cash balances in highly rated United States ("U.S.") government-backed debt securities and treasuries. We classify our marketable securities as available-for-sale and accordingly, record such securities at fair value. Debt securities with original maturities of greater than 90 days are classified as available-for-sale marketable securities and debt securities with original maturities of less than 90 days from the date of purchase are classified as cash equivalents.
Unrealized gains and losses on our marketable debt securities that are deemed temporary are included in accumulated other comprehensive gain (loss) as a separate component of stockholders’ equity. If any adjustment to fair value reflects a significant decline in the value of the security, we evaluate the extent to which the decline is determined to be other-than-temporary and would mark the security to market through a charge to our consolidated statements of operations. Credit losses are identified when we do not expect to receive cash flows sufficient to recover the amortized cost basis of a security. In the event of a credit loss, only the amount associated with the credit loss is recognized in operating results, with the amount of loss relating to other factors recorded in accumulated other comprehensive gain (loss).
Fair Value
ASC Topic 820, Fair Value Measurement (“ASC 820”), establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the assets or liability and are developed based on the best information available in the circumstances. ASC 820 identifies fair value as the price that would be received to sell an asset or paid to transfer a liability, in an orderly transaction between market participants at the measurement date. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tiered value hierarchy that distinguishes between the following:
Level 1—Quoted market prices in active markets for identical assets or liabilities.
Level 2—Inputs other than Level 1 inputs that are either directly or indirectly observable, such as quoted market prices, interest rates and yield curves.
Level 3—Unobservable inputs for the asset or liability (i.e. supported by little or no market activity). Level 3 inputs include management’s own assumptions about the assumptions that market participants would use in pricing the asset or liability (including assumptions about risk).
To the extent the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair values requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized as Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
In determining fair value, the Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, as well as considers counterparty credit risk in its assessment of fair value.
Leases
In the first quarter of 2025, the Company entered into an office lease agreement in New Haven, Connecticut. The Company leases approximately 1,202 square feet of office space under a lease that expires in February 2027, with an option to extend the lease for an additional two-year period.
The Company accounts for leases under ASC Topic 842, Leases (“ASC 842”). At the inception of an arrangement, the Company determines if an arrangement is, or contains, a lease based on the facts and circumstances present in that arrangement. Lease classification, recognition, and measurement are then determined at the lease commencement date. For arrangements that contain a lease the Company (i) identify lease and non-lease components, (ii) determine the consideration in the contract, (iii) determine whether the lease is an operating or financing lease; and (iv) recognize lease right-of-use ("ROU") assets and liabilities. Lease liabilities and their corresponding ROU assets are recorded based on the present value of fixed, or in substance fixed, lease payments over the expected lease term. When the interest rate implicit in lease contracts is not readily determinable the Company uses the incremental borrowing rate based on the information available at the lease commencement date, which represents an internally developed rate that would be incurred to borrow, on a collateralized basis, over a similar term, an amount equal to the lease payments in a similar economic environment.
The Company elected to combine lease components with non-lease components on the office real estate asset class. Fixed, or in substance fixed, lease payments on operating leases are recognized over the expected term of the lease on a straight-line basis. Variable lease expenses that are not considered fixed, or in substance fixed, are recognized as incurred. Fixed and variable lease expense on operating leases is recognized within operating expenses within the condensed consolidated statements of operations. Some leases include options to extend the lease and the Company includes these options in the recognition of the Company’s ROU assets and lease liabilities when it is reasonably certain that the Company will exercise such options. During the nine months ended September 30, 2025, $0.1 million is included in ROU assets, other current liabilities, and non-current lease liabilities in the condensed consolidated balance sheet.
Recent accounting pronouncements not yet adopted
In November 2024, the FASB issued ASU No. 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures, (“ASU 2024-03”). ASU 2024-3 requires disclosure of additional information about specific expense categories in the notes to the financial statements on an interim and annual basis. The standard is effective for fiscal years beginning after December 15, 2026, and for interim periods beginning after December 15, 2027, with prospective or retrospective application and early adoption permitted. The Company is currently evaluating the disclosure requirements related to this new standard.
3. Prepaid Expenses, Other Current Assets, and Accrued Expenses
Prepaid expenses and other current assets as of September 30, 2025 and December 31, 2024, consisted of the following:
| | | | | | | | | | | |
| (in thousands) | September 30, 2025 | | December 31, 2024 |
Prepaid research and development | $ | 2,067 | | | $ | 1,038 | |
Tax credit receivable | 1,234 | | | 1,375 | |
| Other | 273 | | | 38 | |
| $ | 3,574 | | | $ | 2,451 | |
| | | |
Accrued expenses as of September 30, 2025 and December 31, 2024, consisted of the following:
| | | | | | | | | | | |
| (in thousands) | September 30, 2025 | | December 31, 2024 |
| Bonus | $ | 1,032 | | | $ | 1,033 | |
| Research and development | 838 | | | 1,064 | |
| Professional fees | 640 | | | 371 | |
| Other | 92 | | | 2 | |
| $ | 2,602 | | | $ | 2,470 | |
| | | |
4. Marketable Securities
The amortized cost and fair value of our marketable securities by type of security as of September 30, 2025 was as follows:
| | | | | | | | | | | | | | | | | | | | |
| | Fair Value Hierarchy Level | | Amortized Cost | | Fair Value |
| Money market funds | | Level 1 | | $ | 1,795 | | | $ | 1,795 | |
| U.S. treasury securities | | Level 1 | | 7,480 | | | 7,481 | |
| U.S. government agency securities | | Level 2 | | 1,999 | | | 1,998 | |
| | | | | | |
Total unrealized gains and losses were not material as of September 30, 2025.
The fair values of marketable securities by classification in the condensed consolidated balance sheet as of September 30, 2025 was as follows:
| | | | | | | | |
| | September 30, 2025 |
| Cash and cash equivalents | | 1,795 | |
| Marketable securities | | $ | 9,479 | |
| | |
The contractual maturity of all the marketable securities as of September 30, 2025 was one year or less.
The Company had sales and maturities of marketable securities of $15.0 million and purchases of $24.1 million during the nine months ended September 30, 2025. The Company had an immaterial amount of realized gains or losses during the nine months ended September 30, 2025.
As of September 30, 2025, the Company believes that the cost basis of our marketable securities is recoverable and no allowance for credit losses was recorded.
5. Convertible Notes
In August of 2024, the Company issued nine convertible promissory notes (the “Convertible Notes”) for a total of $9.9 million of which $8.5 million was for cash and $1.4 million was for committed research and development services. The Convertible Notes accrued simple interest at the rate of 1.5% per month (18% per annum) on commencement and continuing until the outstanding principal amount is fully paid or converted. The Convertible Notes automatically convert under a qualified equity financing event or change of control event.
On November 25, 2024, the Company entered into a Series B preferred stock purchase agreement which converted the Convertible Notes’ outstanding principal and accrued interest of $10.4 million through the issuance of 8,661,917 shares of Series B preferred stock at a purchase price of $1.2049 per share of which $1.5 million was a subscription receivable for committed research and development services. Interest expense related to the Convertible Notes for the nine months ended September 30, 2024 was $0.2 million.
6. Stockholders’ Equity – Common and Preferred Stock
In accordance with the Third Amended and Restated Certificate of Incorporation filed on November 22, 2024, the Company is authorized to issue 98.8 million shares of common stock, par value $0.00001 per share, of which 92.6 million are designated as voting common stock and 6.2 million are designated as non-voting common stock. Each share of non-voting common stock shall be convertible, at the option of the holder thereof, at any time, and without the payment of additional consideration by the holder thereof, into one fully paid and nonassessable share of voting common stock. The Company had no outstanding shares of non-voting common stock as of September 30, 2025 and December 31, 2024. The voting, dividend and liquidation rights of the holders of the common stock are subject to and qualified by the rights, power, and preferences of the preferred stockholders.
As of September 30, 2025 and December 31, 2024 a total of 749,027 and 736,933 shares of common stock were issued and an aggregate of 771,832 shares (subject to certain anti-dilution adjustments) of common stock were reserved for the granting of stock-based compensation. In addition, the Company has reserved sufficient shares of common stock for issuance upon conversion of convertible preferred stock.
The holders of the common stock are entitled to one vote for each share of common stock held at all meetings of stockholders (and written actions in lieu of meetings), and there are no cumulative voting rights. The number of authorized shares of common stock may be increased or decreased by the affirmative vote of the holders of common stock of the Company; however, the issuance of common stock may be subject to the vote of the holders of one or more series of preferred stock that may be required by terms of the Third Amended and Restated Certificate of Incorporation.
Preferred Stock
Preferred stock outstanding as of September 30, 2025 consisted of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Preferred Series Shares | | Date Sold | | Shares Sold | | Par Value | | Sales
Price/Share | | Total Proceeds
(in millions) | | Liquidation Preference as of September 30, 2025 (in millions) |
| Series A-1 | | September 2021 | | 6,515,849 | | | $ | 0.00001 | | | $ | 2.8770 | | | $ | 18.7 | | | $ | 18.7 | |
| Series A-2 | | September 2021 | | 680,479 | | | 0.00001 | | | 2.1578 | | | 1.5 | | | 1.5 | |
| Series A-3 | | September 2021 | | 703,475 | | | 0.00001 | | | 2.8770 | | | 2.0 | | | 2.0 | |
| Series A-4 | | April 2023 | | 4,965,572 | | | 0.00001 | | | 3.0208 | | | 15.0 | | | 15.0 | |
| Series B | | November 2024 - February 2025 | | 62,245,806 | | | 0.00001 | | | 1.2049 | | | 75.0 | | | 157.8 | |
| | | | | | | | | | | | |
In connection with the Series A4 Preferred Stock Purchase Agreement, the Company increased the number of shares of common stock authorized from 18,000,000 to 25,500,000 under the Second Amended and Restated Certificate of Incorporation dated as of April 13, 2023.
In connection with the Series B Preferred Stock Purchase Agreement, the Company increased the number of shares of common stock authorized from 25,500,000 to 98,774,582 under the Third Amended and Restated Certificate of Incorporation dated as of November 22, 2024.
Collectively, the shares of Series A1, A2, A3 and A4 preferred stock are referred to as the “Series A preferred stock.” The following terms detailed below for the Series A and B preferred stock are documented in the Company’s Third Amended and Restated Certificate of Incorporation dated as of November 22, 2024 and other equity-related documents:
Conversion
Each share of Series A and Series B preferred stock, at the option of the holder, is convertible into a number of fully paid and non-assessable common shares as determined by multiplying the number of shares of Series A and Series B preferred stock being converted by the applicable conversion rate. The conversion rate in effect at any time is determined by dividing the preferred stock issue price by the conversion price in effect at that time. The conversion price applicable (all adjusted for the reverse stock split) to the Series A1 preferred stock is equal to $28.963 per share, the conversion price applicable to the Series A2 preferred stock is equal to $21.7226 per share, the conversion price applicable to the Series A3 preferred stock is equal to $28.963 per share, the conversion price applicable to the Series A4 preferred stock is equal to $30.4104 per share, and the conversion price applicable to the Series B preferred stock is equal to $12.1297 per share. Such initial conversion price, and the rate at which shares of preferred stock may be converted into common stock, is subject to adjustment. See Note 11 for information on the reverse stock split that adjusted the preferred stock conversion ratio.
The Series A and Series B preferred stock will automatically convert to common stock either upon the closing of a public offering of the Company's common stock at a price of at least five times the conversion price, noted above, resulting in aggregate proceeds of at least $50.0 million, or upon the written election of a majority of the holders of Series B preferred stock.
Dividends
The Series B preferred stock contains cumulative dividend rights at the annual rate of 12%, if and when declared by the Board, such that the Series B preferred stockholders, acting as one class, shall first receive, or simultaneously receive, preferential dividends as calculated under the terms of the Company’s Third Amended and Restated Certificate of Incorporation prior to Series A and common stockholders receiving any declared dividend. Since inception, no dividends have been declared or paid on the Series B preferred stock. As of September 30, 2025, cumulative undeclared dividends totaled approximately $7.8 million.
The Series A preferred stock contains non-cumulative dividend rights at the annual rate of 8%, if and when declared by the Board, such that the holders of the preferred stock, acting as one class, shall receive, or simultaneously receive, preferential dividends as calculated under the terms of the Company’s Third Amended and Restated Certificate of Incorporation prior to common stockholders receiving any declared dividend. As of September 30, 2025 and 2024, no dividends have been declared by the Board.
Deemed Liquidation Event
Certain transactions are defined as Deemed Liquidation Events, unless waived by the holders of a majority of the outstanding preferred stock. These events generally include a merger or consolidation involving the Company, the sale or disposition of all or substantially all of the Company’s assets, a SPAC transaction, or a merger or other business combination with a public company (a “Public Listing Transaction”). Transactions in which existing stockholders retain a majority of the voting power of the surviving entity are not considered Deemed Liquidation Events.
Liquidation Preference
In the event of any liquidation, dissolution, or winding up of the Company, the Series B preferred stockholders are entitled to receive prior to, and in preference to, any distribution to the Series A preferred and common stockholders, an amount equal to the greater of the applicable of two times the Original Issue Price per share plus accrued but unpaid dividends whether or not declared, or such amount per share as would have been payable had all shares of Series B preferred stock been converted to shares of common stock immediately prior to such event of liquidation, dissolution or winding up. In the event that upon liquidation or dissolution, if the assets and funds of the Company are insufficient to permit the payment to preferred stockholders of the full preferential amounts, then the entire assets and funds of the Company legally available for distribution would be distributed ratably among the Series B preferred stockholders in proportion to the full preferential amount each is otherwise entitled to receive.
Following the completion of the distributions to the Series B Preferred stockholders, the Series A preferred stockholders are entitled to receive prior to, and in preference to, any distribution to the common stockholders, an amount equal to the greater of the applicable Original Issue Price per share plus accrued but unpaid dividends declared, or such amount per share as would have been payable had all shares of Series A preferred stock been converted to shares of common stock immediately prior to such event of liquidation, dissolution or winding up. In the event that upon liquidation or dissolution, if the assets and funds of the Company are insufficient to permit the payment to preferred stockholders of the full preferential amounts, then the entire assets and funds of the Company legally available for distribution are to be distributed ratably among the Series A preferred stockholders in proportion to the full preferential amount each is otherwise entitled to receive. After the distributions described above have been paid in full, the remaining assets of the Company available for distribution would be distributed pro-rata to the common stockholders.
Voting Rights
Each Series A and Series B preferred stockholder is entitled to the number of votes equal to the number of shares of voting common stock into which such holder's shares are convertible.
7. Net Loss Per Common Share
Basic and diluted net loss per common share was calculated as follows:
| | | | | | | | | | | |
(in thousands except share and per share amounts) | Nine Months Ended September 30, |
| 2025 | | 2024 |
| Net loss | $ | (48,148) | | | $ | (20,828) | |
Less: Cumulative dividends on Series B preferred stock | 7,018 | | | — | |
Net loss attributable to common stock | (55,166) | | | (20,828) | |
Weighted-average number of shares of common stock outstanding, basic and diluted | 743,401 | | | 736,933 | |
Net loss per common share, basic and diluted | $ | (74.21) | | | $ | (28.26) | |
| | | |
Basic net loss per common share is calculated by dividing the net loss, adjusted for the unpaid cumulative Series B preferred stock dividends, by the weighted-average number of shares of common stock outstanding during the period. Diluted loss per share is computed by dividing net loss for the period by the weighted-average number of shares of common stock and common stock equivalents outstanding (unless their effect is anti-dilutive) for the period. Common share equivalents include shares issuable upon the exercise of stock options and the conversion of preferred stock. During the nine months ended September 30, 2025 and 2024, convertible preferred stock on an as if converted basis and unexercised options have been excluded as their effect is antidilutive. There were no other potentially dilutive, unissued shares of common stock for the nine months ended September 30, 2025 and 2024. The Company had no outstanding non-voting common stock as of nine months ended September 30, 2025 and 2024.
The Company reported net losses for each of the nine months ended September 30, 2025 and 2024 and therefore excluded all options and convertible preferred stock from the computation of diluted net loss per common share as their inclusion would have had an antidilutive effect, as summarized below:
| | | | | | | | | |
| Nine Months Ended September 30, |
| 2025 | | 2024 |
| Stock options | 739,331 | | 279,208 |
| Convertible preferred stock | 7,461,130 | | 1,277,975 |
| Total potentially dilutive shares | 8,200,461 | | 1,557,183 |
| | | |
8. Stock-Based Compensation
In September 2021, the Company adopted the 2021 Equity Incentive Plan (the “2021 Plan”) to grant stock option awards to its officers and employees as compensation for their services to the Company. Under the 2021 Plan, up to 771,832 shares of common stock were made available for issuance.
Stock option awards under the 2021 Plan must be issued at an exercise price of not less than 100% of the fair market value of the common stock at the date of the grant. The 2021 Plan is administered by the Board of Directors, which has the authority to determine to whom options may be granted, the period of exercise and what other restrictions, if any, should apply. Vesting for awards granted to date under the 2021 Plan is principally over four years from the date of an employee’s commencement of service, with awards vesting over a period of 36 or 48 months.
To estimate the fair value of the Company’s stock options, the Company used the Black-Scholes option pricing model (“OPM”). The following key assumptions were used to estimate the fair value:
| | | | | |
| September 30, |
| 2025 |
| Expected volatility | 77.0% - 82.7% |
| Expected term (years) | 10.0 | |
| Risk free interest rate | 3.6% - 4.5% |
| Expected dividend yield | — | |
| |
| |
During the nine months ended September 30, 2025 and 2024, the Company recognized stock-based compensation of $0.5 million and $0.2 million, respectively. Stock-based compensation expense for the nine months ended September 30, 2025 and 2024 was allocated as follows:
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| (in thousands) | 2025 | | 2024 |
| Research and development | $ | 266 | | | $ | 102 | |
| General and administrative | 200 | | | 58 | |
| $ | 466 | | | $ | 160 | |
| | | |
Total option activity for the nine months ended September 30, 2025 is summarized as follows:
| | | | | | | | | | | | | | | | | |
| Number of Stock Options | | Weighted Average Exercise Price | | Weighted Average Contractual Term (in years) |
| Outstanding at December 31, 2024 | 279,208 | | | $ | 29.30 | | | 7.55 |
| Granted | 472,217 | | | 12.19 | | | |
| Exercised | (12,094) | | | 12.19 | | | |
| Forfeited | — | | | — | | | |
| Cancelled | — | | | — | | | |
| Outstanding at September 30, 2025 | 739,331 | | | $ | 12.19 | | | 8.25 |
| Stock options exercisable as of September 30, 2025 | 314,150 | | | $ | 12.19 | | | 6.98 |
| | | | | |
Using the Black-Scholes OPM, the weighted average grant-date fair value per unit of stock options granted during the nine months ended September 30, 2025 was $2.82 per share. The intrinsic value of options outstanding and exercisable as of nine months ended September 30, 2025 and 2024 was $0, as the exercise price of all options exceeded the fair value of the Company’s common stock on that date.
In the first quarter of 2025, the Company repriced an aggregate of 279,208 previously granted stock options to employees and other individuals, to an exercise price of $12.19 per share. The modification resulted in an immaterial amount of incremental stock-based compensation expense recognized in the first quarter of 2025.
9. Commitments and Contingencies
From time to time, in the ordinary course of business, the Company is subject to litigation and regulatory examinations as well as information gathering requests, inquiries and investigations. As of September 30, 2025, there were no matters which would have a material impact on the Company’s financial results.
In July 2022, the Company entered into a license agreement with a third party to which the Company was granted intellectual property rights in certain technology, to develop, manufacture and commercialize such technology related to the advancement of its VDMC asset. The Company is required to make development and regulatory milestone payments up to $3.3 million and commercial and sales milestone up to $16 million. The Company is also required to pay annual single-digit royalties on net product sales over the term of the License Agreement. As of September 30, 2025, it is not practicable to estimate the future payments of any such milestone payments or royalties that may arise due to the clinical stage of development of VDMC.
10. Segments
The Company defines its segments on the basis of the way in which internally reported financial information is regularly reviewed by the CODM to analyze financial performance, make decisions, and allocate resources. The Company’s CODM is the Chief Executive Officer. The Company manages its operations as a single operating and reportable segment and the measure of segment profit or loss is net loss. The CODM uses net loss in the budget and forecasting process and considers budget-to-actual variances on a quarterly basis when making decisions about the allocation of operating and capital resources. The following table summarizes the significant segment expenses presented on the Company's condensed consolidated statements of operations:
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| (in thousands) | 2025 | | 2024 |
| | | |
Direct research and development costs by program | | | |
VDPHL01 | $ | 39,458 | | | $ | 11,395 | |
| VDMN | 978 | | | 3,100 | |
| VDMC | 22 | | | 1,154 | |
| VDAA | 9 | | | 439 | |
Other program candidates and expenses | 48 | | | 1,322 | |
Other unallocated research and development costs | | | |
| Personnel expenses (including stock-based compensation) | 3,012 | | | 830 | |
Other expenses | 346 | | | 93 | |
General and administrative, excluding personnel expenses | 3,975 | | | 1,626 | |
General and administrative, personnel expenses (including stock-based compensation) | 1,488 | | | 848 | |
Other segment items(1) | (1,188) | | | 21 | |
Segment net loss | $ | (48,148) | | | $ | (20,828) | |
| | | |
(1)For the nine months ended September 30, 2025 other segment items included $0.7 million of interest income and $0.5 million of accretion income on investments. For the nine months ended September 30, 2024, other segment items include interest expense of $0.3 million, partially offset by $0.3 million of interest income.
11. SUBSEQUENT EVENTS
The Company evaluated all material subsequent events through January 9, 2026 the date the financial statements were available to be issued, and through January 28, 2026, for the effects of the reverse stock split described below. The Company noted the following material events occurred subsequent to September 30, 2025.
In the fourth quarter of 2025, the Company issued 118,682,683 shares of Series C Preferred Stock, $0.00001 par value, at a purchase price of $1.2723 per share, for total cash consideration of $151.0 million. Additionally, in connection with the issuance of the Series C redeemable preferred stock, certain other terms of the Series A and B redeemable preferred stock terms were amended. The liquidation preference for Series B redeemable preferred stock was changed from two times to one times its original purchase price and is paid pari passu with the Series C redeemable preferred stock, and the Series C redeemable preferred stock is entitled to dividends prior to the Series B redeemable preferred stock.
In accordance with the Company’s Certificate of Amendment to the Fourth Amended and Restated Certificate of Incorporation filed in December 2025, the Company increased the number of authorized shares of common stock to 267,466,797 shares, par value $0.00001 per share, of which 253,382,434 are designated as voting shares and 14,084,363 are designated as non-voting shares.
In the fourth quarter of 2025, the Board of Directors approved increases to the aggregate shares of common stock available for issuance under the 2021 Plan from 7,770,032 (771,832 as adjusted for the reverse stock split) to 44,406,983 shares (4,411,143 shares as adjusted for the reverse stock split) .
In the fourth quarter of 2025, the Board of Directors granted performance-based stock option awards of 543,635 and non-performance based stock awards of 2,431,003 to certain employees and other individuals under the 2021 Plan. The exercise prices range between $12.19 and $12.79. The performance based stock option awards are earned based on achievement of a specified corporate milestone and will vest immediately upon Board certification of milestone achievement. The total estimated unrecognized stock-based compensation expense related to these fourth quarter grants was approximately $18.0 million.
On January 27, 2026, the Company effected a 1-for-10.067 reverse stock split of the Company’s issued and outstanding common stock and adjusted the conversion ratio of the Company’s outstanding convertible preferred stock. Accordingly, all share and per share amounts for all periods presented in the accompanying consolidated financial statements and notes thereto have been retroactively adjusted, where applicable, to reflect the reverse stock split and the adjustment of the preferred stock conversion ratios.
Shares
Veradermics, Incorporated
Common Stock
PRELIMINARY PROSPECTUS
| | | | | | | | | | | | | | | | | | | | |
| Jefferies | | Leerink Partners | | Citigroup | | Cantor |
, 2026
Through and including , 2026 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
Information Not Required in Prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of common stock being registered. All amounts are estimates except for the SEC registration fee, the FINRA filing fee and the NYSE listing fee:
| | | | | | | | |
| ITEM | | AMOUNT TO BE PAID |
SEC registration fee | | $ | 33,923 | |
FINRA filing fee | | 37,346 | |
NYSE listing fee | | 100,000 | |
Printing and engraving expenses | | 277,000 | |
Legal fees and expenses | | 3,000,000 | |
Accounting fees and expenses | | 875,000 | |
Transfer agent fees and expenses | | 4,000 | |
Miscellaneous expenses | | 72,731 | |
Total | | $ | 4,400,000 | |
| | |
Item 14. Indemnification of Directors and Officers.
As permitted by Section 102(b)(7) of the DGCL, we plan to include in our restated certificate of incorporation a provision to eliminate the personal liability of our directors and officers for monetary damages for breach of their fiduciary duties as directors or officers, subject to certain exceptions. In addition, our restated certificate of incorporation and bylaws will provide that we are required to indemnify our officers and directors under certain circumstances, including those circumstances in which indemnification would otherwise be discretionary, and we are required to advance expenses to our officers and directors as incurred in connection with proceedings against them for which they may be indemnified, in each case except to the extent that the DGCL prohibits the elimination or limitation of liability of directors or officers for breaches of fiduciary duty.
Section 145(a) of the DGCL provides that a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interest of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendere or its equivalent shall not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that his conduct was unlawful.
Section 145(b) of the DGCL provides that a corporation shall have the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise
against expenses (including attorneys’ fees) actually and reasonably incurred by him in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
We have entered into indemnification agreements with our directors and, prior to the consummation of this offering, intend to enter into indemnification agreements with certain of our officers. These indemnification agreements will provide broader indemnity rights than those provided under the DGCL and our restated certificate of incorporation. These indemnification agreements are not intended to deny or otherwise limit third-party or derivative suits against us or our directors or officers, but to the extent a director or officer were entitled to indemnity or contribution under the indemnification agreement, the financial burden of a third-party suit would be borne by us, and we would not benefit from derivative recoveries against the director or officer. Such recoveries would accrue to our benefit but would be offset by our obligations to the director or officer under the indemnification agreement.
The underwriting agreement will provide that the underwriters are obligated, under certain circumstances, to indemnify our directors, officers and controlling persons against certain liabilities, including liabilities under the Securities Act.
We maintain directors’ and officers’ liability insurance for the benefit of our directors and officers.
Item 15. Recent Sales of Unregistered Securities.
The following list sets forth information regarding all unregistered securities sold by us since September 30, 2022. No underwriters were involved in the sales and the certificates representing the securities sold and issued contain legends restricting transfer of the securities without registration under the Securities Act or an applicable exemption from registration. The issuances of the below securities were exempt either pursuant to Rule 701, as transactions pursuant to a compensatory benefit plan, or pursuant to Section 4(a)(2), as transactions by an issuer not involving a public offering.
(a) Grants of Stock Options
Equity Awards
In February 2025, the Company repriced and granted an aggregate of 279,208 stock options to employees and other individuals, with an exercise price of $12.19 per share, pursuant to the 2021 Plan.
In 2025, the Company granted an aggregate of 2,866,462 stock options to certain employees, with an exercise price of $12.79 per share, pursuant to the 2021 Plan.
In 2025, the Company granted an aggregate of 580,400 stock options to certain employees, with an exercise price of $12.19 per share, pursuant to the 2021 Plan.
In 2025, 12,827 shares of our Common Stock were issued upon the exercise of stock options at a weighted-average exercise price of $12.19 per share.
(b) Issuances of Convertible Preferred Stock
In November 2025, the Company issued 12,575,650 shares of Series C Preferred Stock at a purchase price of $1.2723 per share, for an aggregate purchase price of approximately $16.0 million.
In October 2025, the Company issued 106,107,033 shares of Series C Preferred Stock at a purchase price of $1.2723 per share, for an aggregate purchase price of approximately $135.0 million.
In February 2025, the Company issued 4,923,974 shares of Series B Preferred Stock at a purchase price of $1.2049 per share, for an aggregate purchase price of approximately $5.9 million.
In January 2025, the Company issued 2,074,860 shares of Series B Preferred Stock at a purchase price of $1.2049 per share, for an aggregate purchase price of approximately $2.5 million.
In November 2024, the Company issued and sold 55,246,971 shares of Series B Preferred Stock at a purchase price of $1.2049 per share, for an aggregate purchase price of approximately $66.2 million, including the conversion of certain convertible promissory notes, representing an aggregate outstanding principal amount and accrued interest of $10.4 million, into 8,661,917 shares of Series B Preferred Stock.
In April 2023, the Company issued and sold 4,965,572 shares of Series A-4 Preferred Stock at a purchase price of $3.0208 per share, for an aggregate purchase price of approximately $15.0 million.
(c) Issuances of Convertible Notes
In August 2024, the Company issued and sold an aggregate of $9.9 million of convertible promissory notes to several investors. In November 2024, concurrently with the issuance and sale of Series B Preferred Stock, such notes, representing an aggregate outstanding principal amount and accrued interest of $10.4 million, were automatically converted into an aggregate of 8,661,917 shares of Series B Preferred Stock.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
See the Exhibit Index attached to this Registration Statement, which is incorporated by reference herein.
(b) Financial Statement Schedules
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer, or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
1. For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
2. For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
EXHIBIT INDEX
| | | | | | | | |
| EXHIBIT NUMBER | | DESCRIPTION OF DOCUMENT |
| | |
1.1 | | |
| | |
3.1 | | |
| | |
3.2 | | |
| | |
4.1 | | |
| | |
4.2 | | |
| | |
5.1 | | |
| | |
10.1*+ | | |
| | |
10.2*+ | | |
| | |
| | | | | | | | |
10.3# | | |
| | |
10.4# | | |
| | |
10.5# | | |
| | |
10.6# | | |
| | |
10.7# | | |
| | |
10.8# | | |
| | |
10.9# | | |
| | |
10.10# | | |
| | |
10.11# | | |
| | |
10.12# | | |
| | |
10.13# | | |
| | |
10.14# | | |
| | |
10.15# | | |
| | |
21.1 | | |
| | |
| 23.1 | | |
| | |
23.2 | | |
| | |
24.1* | | |
| | |
107 | | |
| | |
*Previously filed.
# Indicates management contract or compensatory plan.
+ Portions of this exhibit (indicated by asterisks) have been redacted because they are both not material and the registrant customarily and actually treats such information as private or confidential.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New Haven, Connecticut, on the 28th day of January, 2026.
| | | | | | | | |
| VERADERMICS, INCORPORATED |
| | |
| By: | /s/ Reid Waldman |
| | Reid Waldman, M.D. Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| | | | | | | | | | | | | | |
| SIGNATURE | | TITLE | | DATE |
| | | | |
/s/ Reid Waldman, M.D. | | Chief Executive Officer and Director (Principal Executive Officer) | | January 28, 2026 |
Reid Waldman, M.D. | | |
| | | | |
/s/ Dominic Carrano, CPA | | Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) | | January 28, 2026 |
Dominic Carrano, CPA | | |
| | | | |
* | | Director | | January 28, 2026 |
David Friedman, M.D. | | |
| | | | |
* | | Director | | January 28, 2026 |
John W. Childs | | |
| | | | |
* | | Director | | January 28, 2026 |
Vlad Coric, M.D. | | |
| | | | |
* | | Director | | January 28, 2026 |
Katarina Pance, Ph.D. | | |
| | | | |
* | | Director | | January 28, 2026 |
Patrick Enright | | |
| | | | |
* | | Director | | January 28, 2026 |
Jane Grant-Kels, M.D. | | |
| | | | | |
*By: | /s/ Reid Waldman, M.D. |
| Reid Waldman, M.D. |
| Attorney-in-fact |