

Corporate Presentation November 6, 2025
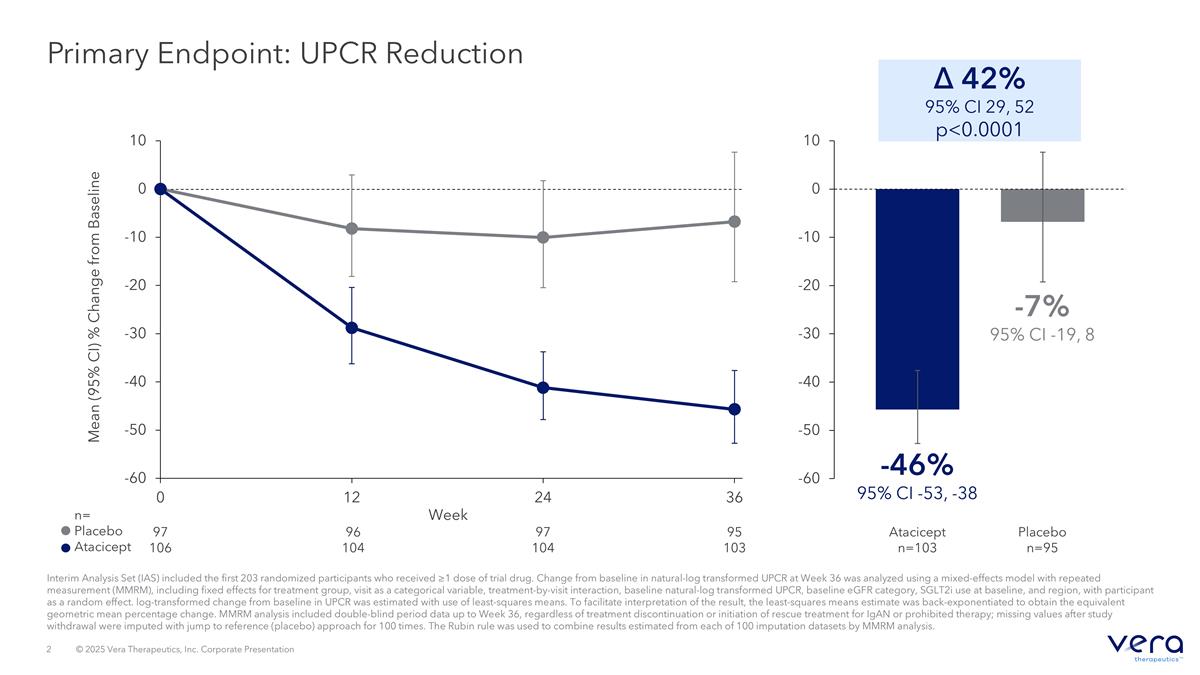
Primary Endpoint: UPCR Reduction Interim Analysis Set (IAS) included the first 203 randomized participants who received ≥1 dose of trial drug. Change from baseline in natural-log transformed UPCR at Week 36 was analyzed using a mixed-effects model with repeated measurement (MMRM), including fixed effects for treatment group, visit as a categorical variable, treatment-by-visit interaction, baseline natural-log transformed UPCR, baseline eGFR category, SGLT2i use at baseline, and region, with participant as a random effect. log-transformed change from baseline in UPCR was estimated with use of least-squares means. To facilitate interpretation of the result, the least-squares means estimate was back-exponentiated to obtain the equivalent geometric mean percentage change. MMRM analysis included double-blind period data up to Week 36, regardless of treatment discontinuation or initiation of rescue treatment for IgAN or prohibited therapy; missing values after study withdrawal were imputed with jump to reference (placebo) approach for 100 times. The Rubin rule was used to combine results estimated from each of 100 imputation datasets by MMRM analysis. Mean (95% CI) % Change from Baseline n= Placebo Atacicept Week 97 106 96 104 97 104 95 103 Δ 42% 95% CI 29, 52 p<0.0001 -46% 95% CI -53, -38 -7% 95% CI -19, 8 Placebo n=95 Atacicept n=103
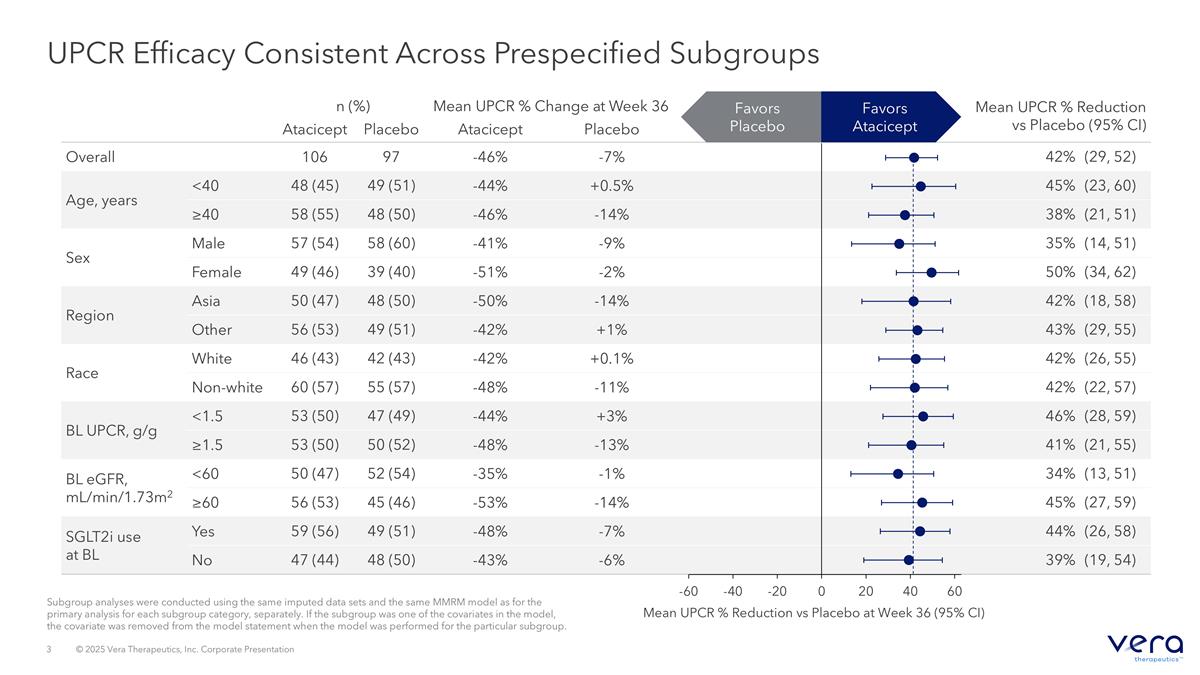
Subgroup analyses were conducted using the same imputed data sets and the same MMRM model as for the primary analysis for each subgroup category, separately. If the subgroup was one of the covariates in the model, the covariate was removed from the model statement when the model was performed for the particular subgroup. UPCR Efficacy Consistent Across Prespecified Subgroups n (%) Mean UPCR % Change at Week 36 Mean UPCR % Reduction vs Placebo (95% CI) Atacicept Placebo Atacicept Placebo Overall 106 97 -46% -7% 42% (29, 52) Age, years <40 48 (45) 49 (51) -44% +0.5% 45% (23, 60) ≥40 58 (55) 48 (50) -46% -14% 38% (21, 51) Sex Male 57 (54) 58 (60) -41% -9% 35% (14, 51) Female 49 (46) 39 (40) -51% -2% 50% (34, 62) Region Asia 50 (47) 48 (50) -50% -14% 42% (18, 58) Other 56 (53) 49 (51) -42% +1% 43% (29, 55) Race White 46 (43) 42 (43) -42% +0.1% 42% (26, 55) Non-white 60 (57) 55 (57) -48% -11% 42% (22, 57) BL UPCR, g/g <1.5 53 (50) 47 (49) -44% +3% 46% (28, 59) ≥1.5 53 (50) 50 (52) -48% -13% 41% (21, 55) BL eGFR, mL/min/1.73m2 <60 50 (47) 52 (54) -35% -1% 34% (13, 51) ≥60 56 (53) 45 (46) -53% -14% 45% (27, 59) SGLT2i use at BL Yes 59 (56) 49 (51) -48% -7% 44% (26, 58) No 47 (44) 48 (50) -43% -6% 39% (19, 54) Mean UPCR % Reduction vs Placebo at Week 36 (95% CI) Favors Atacicept Favors Placebo
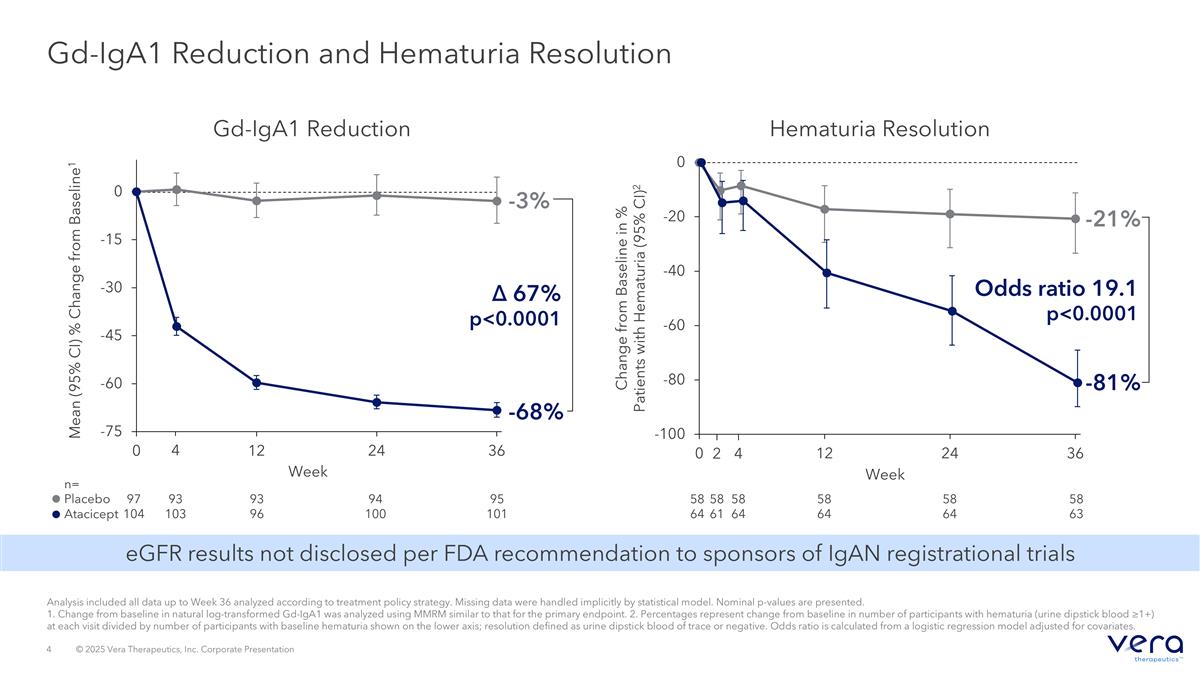
Gd-IgA1 Reduction and Hematuria Resolution Mean (95% CI) % Change from Baseline1 97 104 95 101 n= Placebo Atacicept Week 94 100 93 96 93 103 -3% 4 Gd-IgA1 Reduction -68% Δ 67% p<0.0001 Change from Baseline in % Patients with Hematuria (95% CI)2 Week 58 64 58 63 58 64 58 64 -81% -21% Odds ratio 19.1 p<0.0001 Hematuria Resolution 58 64 4 58 61 2 Analysis included all data up to Week 36 analyzed according to treatment policy strategy. Missing data were handled implicitly by statistical model. Nominal p-values are presented. 1. Change from baseline in natural log-transformed Gd-IgA1 was analyzed using MMRM similar to that for the primary endpoint. 2. Percentages represent change from baseline in number of participants with hematuria (urine dipstick blood ≥1+) at each visit divided by number of participants with baseline hematuria shown on the lower axis; resolution defined as urine dipstick blood of trace or negative. Odds ratio is calculated from a logistic regression model adjusted for covariates. eGFR results not disclosed per FDA recommendation to sponsors of IgAN registrational trials
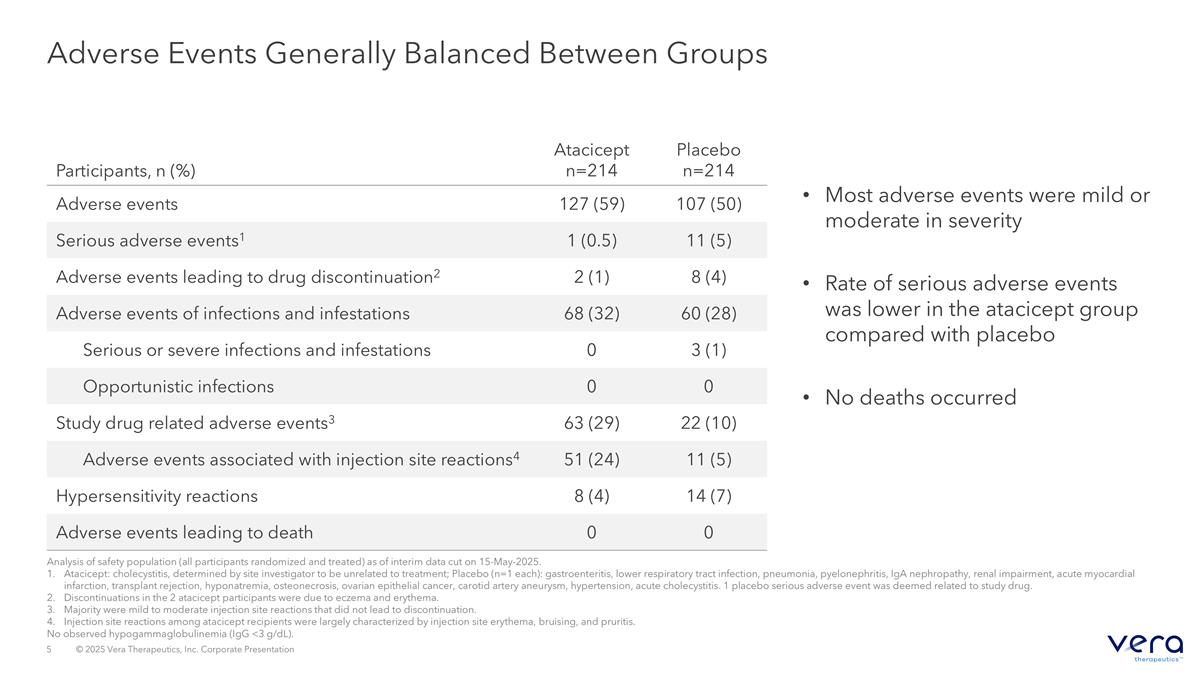
Adverse Events Generally Balanced Between Groups Most adverse events were mild or moderate in severity Rate of serious adverse events was lower in the atacicept group compared with placebo No deaths occurred Participants, n (%) Atacicept n=214 Placebo n=214 Adverse events 127 (59) 107 (50) Serious adverse events1 1 (0.5) 11 (5) Adverse events leading to drug discontinuation2 2 (1) 8 (4) Adverse events of infections and infestations 68 (32) 60 (28) Serious or severe infections and infestations 0 3 (1) Opportunistic infections 0 0 Study drug related adverse events3 63 (29) 22 (10) Adverse events associated with injection site reactions4 51 (24) 11 (5) Hypersensitivity reactions 8 (4) 14 (7) Adverse events leading to death 0 0 Analysis of safety population (all participants randomized and treated) as of interim data cut on 15-May-2025. Atacicept: cholecystitis, determined by site investigator to be unrelated to treatment; Placebo (n=1 each): gastroenteritis, lower respiratory tract infection, pneumonia, pyelonephritis, IgA nephropathy, renal impairment, acute myocardial infarction, transplant rejection, hyponatremia, osteonecrosis, ovarian epithelial cancer, carotid artery aneurysm, hypertension, acute cholecystitis. 1 placebo serious adverse event was deemed related to study drug. Discontinuations in the 2 atacicept participants were due to eczema and erythema. Majority were mild to moderate injection site reactions that did not lead to discontinuation. Injection site reactions among atacicept recipients were largely characterized by injection site erythema, bruising, and pruritis. No observed hypogammaglobulinemia (IgG <3 g/dL).
