
Life can only be understood backwards; but it must be lived forwards… KIERKEGAARD Invivyd 1 October 30, 2025 © 2025 Invivyd, Inc. All trademarks used in this presentation are the property of their respective owners.

Life can only be understood backwards; but it must be lived forwards… KIERKEGAARD Invivyd 1 October 30, 2025 © 2025 Invivyd, Inc. All trademarks used in this presentation are the property of their respective owners.

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This historical presentation fact are contains forward forward -looking- looking statements statements . Words within such as the “may,” meaning “will,” of the “should,” U.S. Private “expect,” Securities “plan,” Litigation “anticipate,” Reform “seek,” Act of 1995 “could,” . Statements “intend,” in “target,” this presentation “aim,” “project,” that are not “designed statements to,” of “estimate,” looking statements “believe,” contain “predict,” these “potential” identifying or words “continue” . Forward or the -looking negative statements of these terms include or statements other similar concerning, expressions among are intended other things, to identify expectations forward -about looking the statements, COVID landscape; though not beliefs all forward about—variants; limitations expectations of COVID regarding vaccines and durability the expected and stability advantages of the of company’s monoclonal antibodies; antibodies plans (mAbs), related including to the company’s the potential research to engineer and development mAbs for consistent activities, and high the activity timing against and potential SARS- CoV results -2 indication thereof; the and potential potential of VYD2311 administration as a mAb paradigm candidate; for VYD2311; expectations PEMGARDA® regarding the company’s (pemivibart) clinical as a mAb trial designs for pre- and exposure enrollment, prophylaxis regulatory (PrEP) pathway, of COVID product -19 profile, in certain target immunocompromised patient population, patients; commercialization estimates plans, regarding strategies, the size goals of target and expectations; patient populations the potential and the of the potential company’s market pipeline opportunity and discovery for the company’s efforts, including product for candidates, COVID, Long as well COVID, as its respiratory market position; syncytial the virus company’s (RSV) may and measles; not actually the achieve company’s the plans, business intentions strategies or and expectations objectives, disclosed and ability in the to company’s execute on forward them; the -looking company’s statements future and prospects; you should and not other place statements undue reliance that are on not the historical company’s fact forward . The company -looking forward statements -looking . These statements, forward-looking including, statements without involve limitation: risks the and timing, uncertainties progress that and could results cause of the the company’s company’s discovery, actual results preclinical to differ and materially clinical development from the results activities, described including in or the implied initiation by the of U the .S DECLARATION . Food & Drug Administration clinical trial, and (FDA); finalization clinical and trial initiation site activation of other aspects or enrollment of the REVOLUTION rates; unexpected clinical safety program, or efficacy such as data the observed LIBERTY during clinical preclinical trial, subject studies to final or alignment clinical trials; with the the predictability predictive of future of clinical results, success and interim of the company’s data are subject product to candidates further analysis; based on how neutralizing long the emergency activity in nonclinical use authorization studies; (EUA) the risk granted that results by the of FDA nonclinical for PEMGARDA studies or clinical for COVID trials- 19 may PrEP not be in certain engagement immunocompromised with regulators; uncertainties patients will related remain to in the effect regulatory and whether authorization such EUA or approval is revised process, or revoked and available by the FDA; development changes and in the regulatory regulatory pathways; environment; the company’s the outcome ability of to the generate company’s the product data needed candidate to support following a potential regulatory Biologics authorization License Application or approval; (BLA) the success submission of the for company’s VYD2311; in the -house ability sales to maintain force, and a continued company’s acceptable ability to safety, maintain tolerability and expand and efficacy sales, marketing profile of and any potential distribution variability capabilities in neutralizing to successfully activity commercialize of product candidates any authorized tested or in approved different assays, product such candidates; as pseudovirus changes assays in expected and authentic or existing assays; competition; variability of the results company’s in models reliance and on methods third parties; used to demonstrate predict activity and against sustain SARS neutralizing -CoV- 2 activity variants; against whether major the SARS epitopes -CoV that -2 variants, pemivibart particularly and VYD2311 in the face target of remain viral evolution; structurally the intact; complexities whether of the manufacturing company’s mAb product therapies, candidates and availability are able to of the quantities company of has commercial adequate funding launch product to meet in future the future, operating if authorized expenses or and approved; capital expenditure macroeconomic requirements and political . Other uncertainties; factors that the may company’s cause the ability company’s to continue actual as results a going to differ concern; materially and whether from ended those expressed December or 31, implied 2024 and in the its forward Quarterly -looking Report statements on Form 10 in- Q this for presentation the quarter are ended described June 30, under 2025, the each heading filed with “Risk the Factors” Securities in the and company’s Exchange Annual Commission Report (SEC), on Form and 10 in -the K for company’s the year other and Invivyd filings undertakes with the SEC, no duty and in to its update future such reports information to be filed whether with as the a SEC result and of new available information, at www future .sec.gov events . Forward or otherwise, -looking except statements as required contained under in this applicable press release law. are made as of this date, This presentation contains hyperlinks to information that is not deemed to be incorporated by reference in this presentation.

Life can only be understood backwards; but it must be lived forwards… KIERKEGAARD Invivyd 3 October 30, 2025. © 2025 Invivyd, Inc. All trademarks used in this presentation are the property of their respective owners.

Invivyd is committed to developing best-in-class antibody protection and treatment of viral threats COVID PEMGARDA® (pemivibart) VYD2311 LONG COVID SPEAR Study Group RSV Discovery-stage program MEASLES Discovery-stage program

agenda 01 The COVID Situation 02 Invivyd Antibodies 03 REVOLUTION Clinical Program 04 Future Commercial Landscape

Perceived as a “respiratory” virus because of transmission, but actually a vascular, prothrombotic, immunomodulatory novel virus Influenza Entry via sialic acid receptor Largely bronchoepithelial cells RSV Entry via CX3CR1 Largely bronchoepithelial cells SARS-CoV-2 Entry via ACE2 Epithelial and endothelial cells

COVID vaccines aren’t the problem: humans are. Humans don’t make long lasting antibodies against coronaviruses. Our findings raise concern that humoral immunity against SARS-CoV-2 may not be long lasting… the results call for caution regarding antibody-based “immunity passports,” herd immunity, and perhaps vaccine durability, especially in light of short-lived immunity against common human coronaviruses. Otto Yang, UCLA, July 2020 New England Journal of Medicine

Omicron made COVID even harder for vaccines. “There is no world, I think, where [the effectiveness] is the same level… we had with [the] Delta [variant],” Stéphane Bancel told the Financial Times Financial Times, November 30, 2021
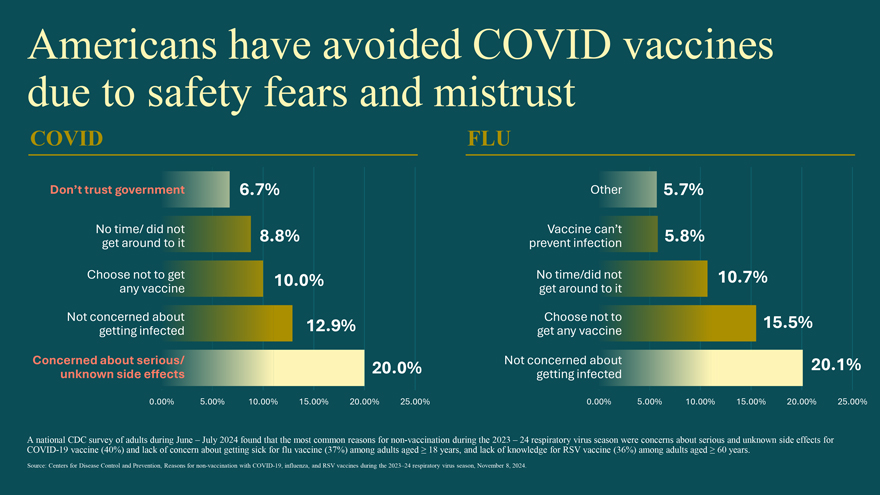
Americans have avoided COVID vaccines due to safety fears and mistrust COVID Don’t trust government 6.7% No time/ did not 8.8% get around to it Choose not to get 10.0% any vaccine Not concerned about 12.9% getting infected Concerned about serious/ 20.0% unknown side effects 0.00% 5.00% 10.00% 15.00% 20.00% 25.00% FLU Other 5.7% Vaccine can’t 5.8% prevent infection No time/did not 10.7% get around to it Choose not to 15.5% get any vaccine Not concerned about 20.1% getting infected 0.00% 5.00% 10.00% 15.00% 20.00% 25.00% A COVID national -19 CDC vaccine survey (40%) of adults and lack during of concern June – about July 2024 getting found sick that for the flu most vaccine common (37%) reasons among adults for non aged -vaccination ≥ 18 years, during and the lack 2023 of knowledge – 24 respiratory for RSV virus vaccine season (36%) were among concerns adults about aged serious ≥ 60 and years unknown . side effects for Source: Centers for Disease Control and Prevention, Reasons for non-vaccination with COVID-19, influenza, and RSV vaccines during the 2023–24 respiratory virus season, November 8, 2024.

We want to break the COVID stalemate COVID Conundrum Infection nobody wants Vaccines few trust Option 3

agenda 01 The COVID Situation 02 Invivyd Antibodies 03 REVOLUTION Clinical Program 04 Future Commercial Landscape

COVID today demands a monoclonal antibody In contrast to vaccines, monoclonal antibodies can be engineered for consistent high activity
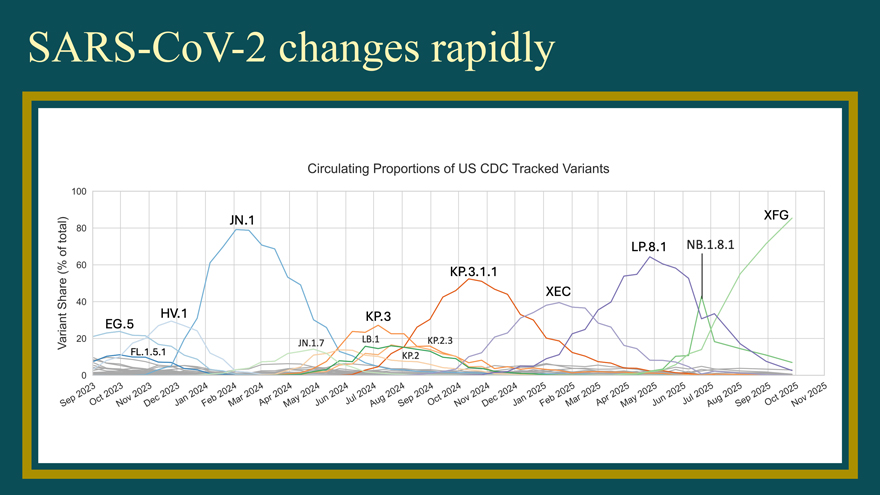
SARS-CoV-2 changes rapidly Circulating Proportions of US CDC Tracked Variants - ....... ro 0 ‘+ - 0— ~ - 0 Q) “— .ro r: (/) ....... c ro ·c ~
SARS-CoV-2 changes rapidly Circulating Proportions of US CDC Tracked Variants - ....... ro 0 ‘+ - 0— ~ - 0 Q) “— .ro r: (/) ....... c ro ·c ~
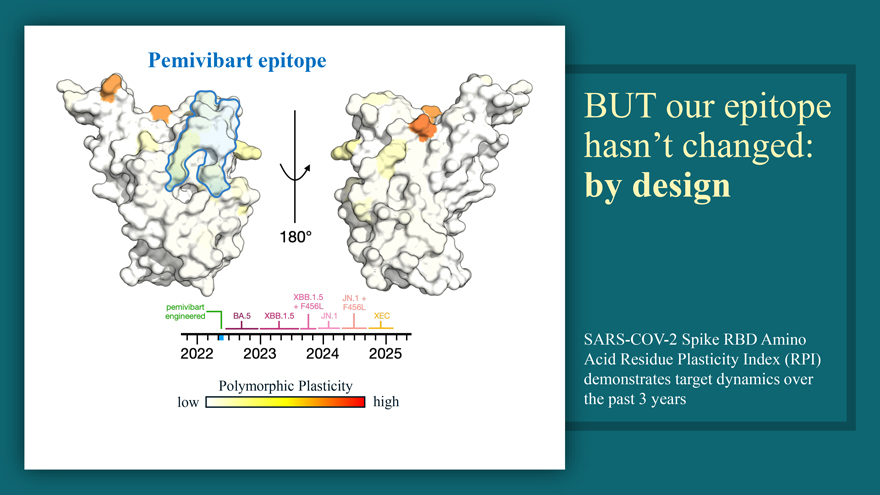
Pemivibart epitope Polymorphic Plasticity low high BUT our epitope hasn’t changed: by design SARS-COV-2 Spike RBD Amino Acid Residue Plasticity Index (RPI) demonstrates target dynamics over the past 3 years
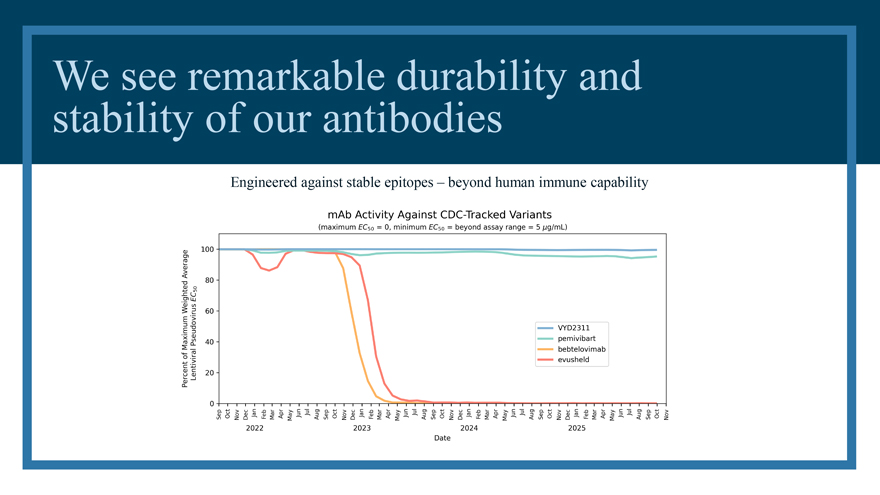
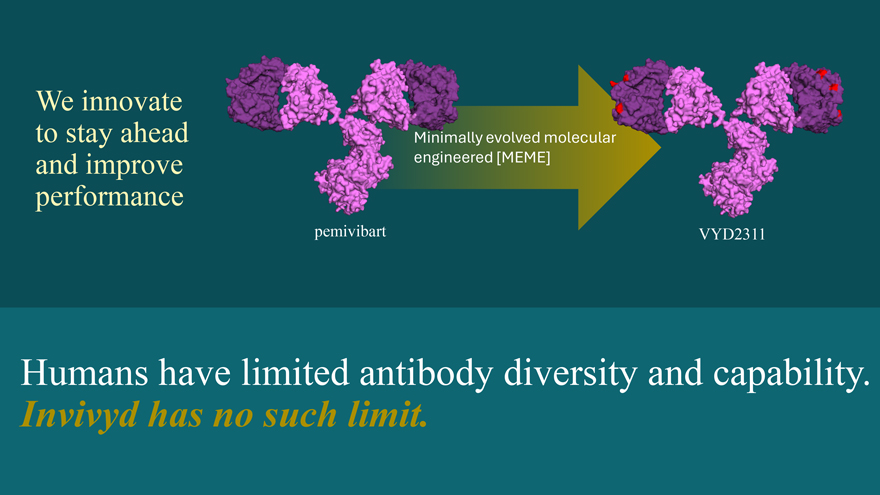
We innovate to stay ahead and improve performance pemivibart Minimally evolved molecular engineered [MEME] VYD2311 Humans have limited antibody diversity and capability. Invivyd has no such limit.

We see remarkable durability and stability of our antibodies Engineered against stable epitopes – beyond human immune capability

agenda The COVID Situation Invivyd Antibodies REVOLUTION Clinical Program Future Commercial Landscape
The covid situation Invivyd Antibodies 03 REvolutions Clinical Program
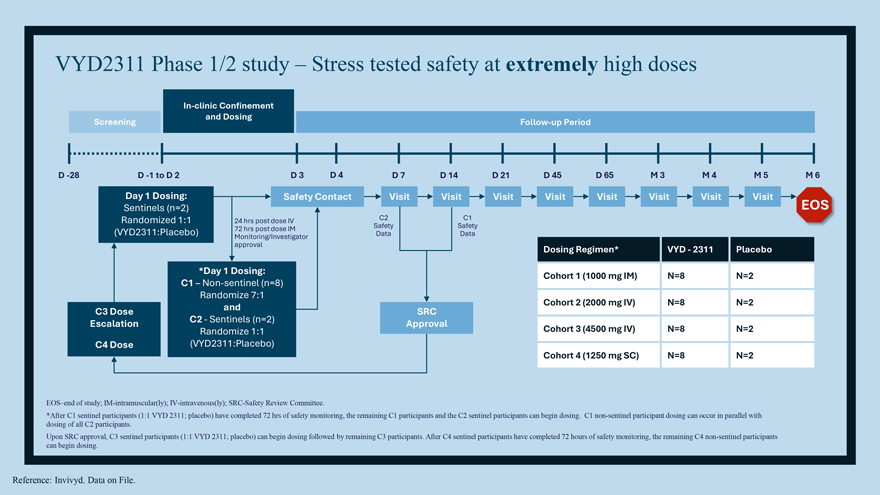
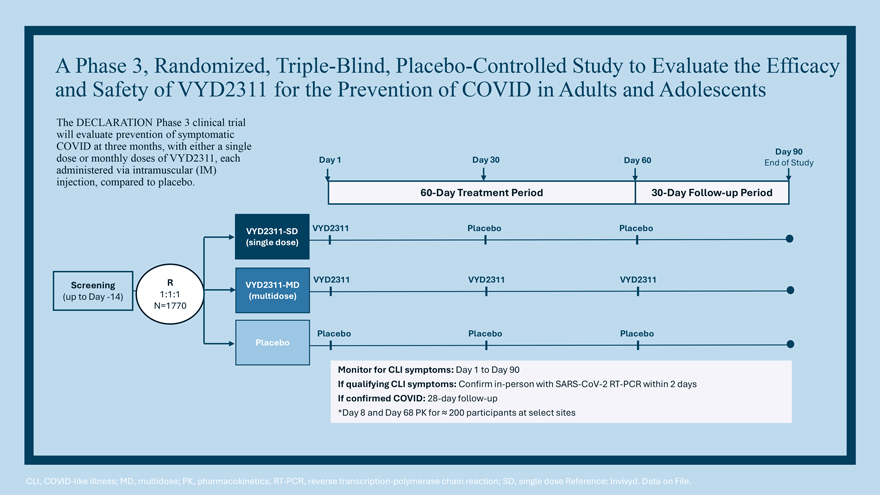
VYD2311/REVOLUTION Clinical Program Phase 1/2 Study Randomized, double-blind, placebo-controlled trial was conducted to evaluate safety, tolerability, pharmacokinetics, and immunogenicity in healthy participants Pivotal Efficacy Study ‘DECLARATION’ Phase 3, placebo-controlled efficacy trial in prevention of symptomatic COVID Vaccine Study ‘LIBERTY’ Safety/Tolerability comparison with COVID mRNA vaccines (and co-administration)

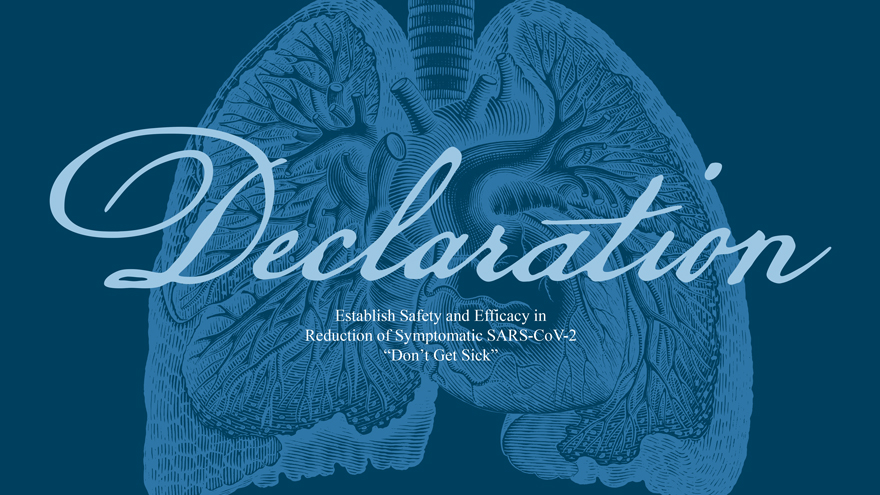
VYD2311 Phase 1/2 study – Stress tested safety at extremely high doses In-clinic Confinement and Dosing Screening Follow-up Period D -28 D -1 to D 2 D 3 D 4 D 7 D 14 D 21 D 45 D 65 M 3 M 4 M 5 M 6 Day 1 Dosing: Safety Contact Visit Visit Visit Visit Visit Visit Visit Visit Sentinels (n=2) EOS Randomized 1:1 24 hrs post dose IV C2 C1 72 hrs post dose IM Safety Safety (VYD2311:Placebo) Monitoring/Investigator Data Data approval Dosing Regimen* VYD—2311 Placebo *Day 1 Dosing: Cohort 1 (1000 mg IM) N=8 N=2 C1 – Non-sentinel (n=8) Randomize 7:1 and Cohort 2 (2000 mg IV) N=8 N=2 C3 Dose SRC Escalation C2—Sentinels (n=2) Approval Cohort 3 (4500 mg IV) N=8 N=2 Randomize 1:1 C4 Dose (VYD2311:Placebo) Cohort 4 (1250 mg SC) N=8 N=2 EOS–end of study; IM-intramuscular(ly); IV-intravenous(ly); SRC-Safety Review Committee. *After C1 sentinel participants (1:1 VYD 2311; placebo) have completed 72 hrs of safety monitoring, the remaining C1 participants and the C2 sentinel participants can begin dosing. C1 non-sentinel participant dosing can occur in parallel with dosing of all C2 participants. Upon SRC approval, C3 sentinel participants (1:1 VYD 2311; placebo) can begin dosing followed by remaining C3 participants. After C4 sentinel participants have completed 72 hours of safety monitoring, the remaining C4 non-sentinel participants can begin dosing. Reference: Invivyd. Data on File.
Initial Phase 1/2 extreme dose safety data -attractive and reflective of ordinary mAb safety VYD2311 administered IV at up to 4500 mg in Phase 1/2, which was as much as 18 years of antibody at the dose to be used in the Declaration study Injection site reactions • IM: 1 (12.5%) VYD2311 participant with mild pain, duration 1 hour, not requiring treatment • SC: 8 (100%) VYD2311 participants all mild, of short duration, not requiring treatment Infusion related reactions • 3 (37.5%) VYD2311 participants receiving 4500 mg infusion • 2 mild and 1 moderate, duration 3 to 20 minutes, the moderate reaction had study drug interruption and received paracetamol and loratadine, resumed full infusion without recurrence No deaths, SAEs, hypersensitivity reactions, severe TEAEs, or other significant TEAEs No trends in laboratory, vital signs, ECG values, or physical examination findings indicating a safety risk IM, intramuscular; SC, subcutaneous; SAEs, serious adverse events; TEAEs, treatment emergent adverse events

Declaration Establish safetyand Efficacy in
We aim to demonstrate a subsatantial reduction in yours risk of getting covid
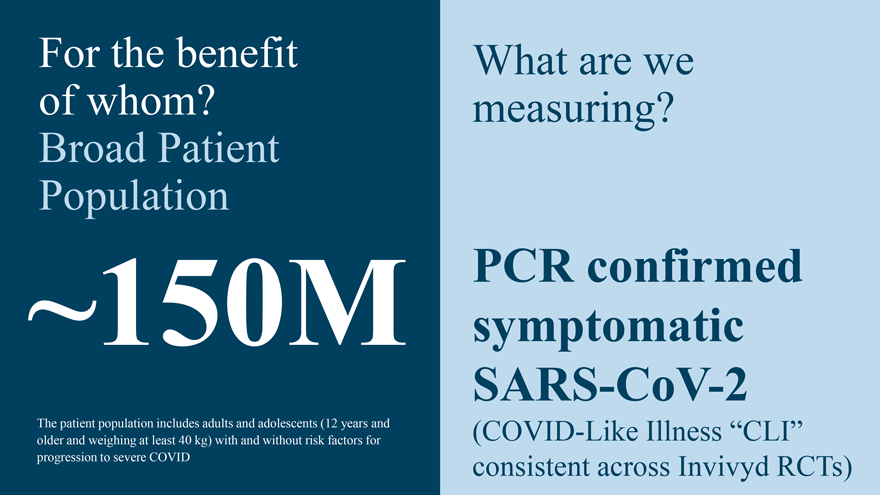
For the benefit of whom? Broad Patient ~150M Population The patient population includes adults and adolescents (12 years and older and weighing at least 40 kg) with and without risk factors for progression to severe COVID What are we measuring? PCR confirmed symptomatic SARS-CoV-2 (COVID-Like Illness “CLI” consistent across Invivyd RCTs)
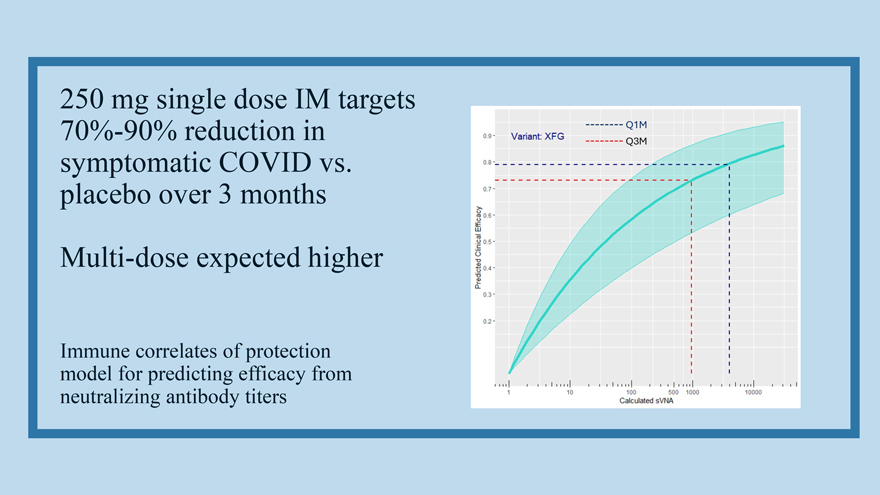
250 mg single dose IM targets 70%-90% reduction in symptomatic COVID vs. placebo over 3 months Multi-dose expected higher Immune correlates of protection model for predicting efficacy from neutralizing antibody titers

Long Half Life (76 days) and relatively flat titer: protection curve allows long-term protection following a single dose At one-year, single dose sVNA titer would still confer greater than 50% protection Every 90-day period requires only 1.2 product half-lives sVNA = serum virus neutralizing antibody
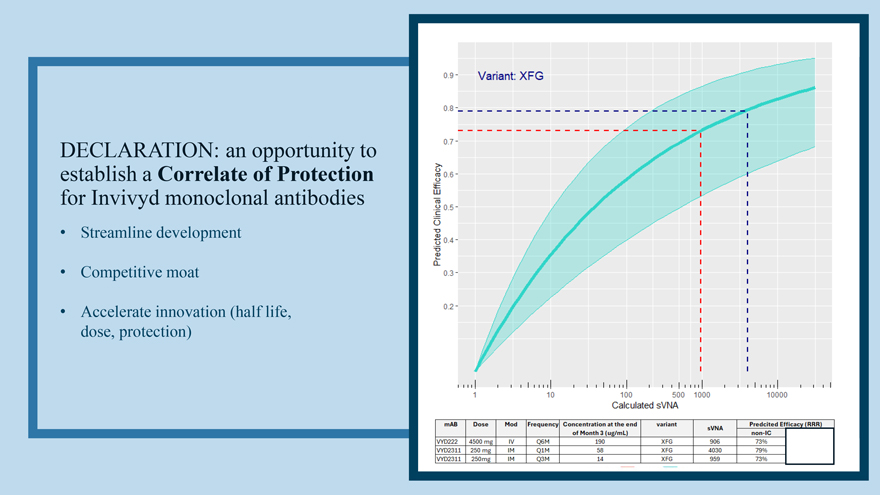
DECLARATION: an opportunity to establish a Correlate of Protection for Invivyd monoclonal antibodies • Streamline development • Competitive moat • Accelerate innovation (half life, dose, protection)

A Phase 3, Randomized, Pooled-Vaccine, Double-Blind Clinical Trial to Evaluate Head-to-Head Safety/Tolerability and Co-Administration Interaction of VYD2311 with Approved mRNA COVID Vaccines in Adults
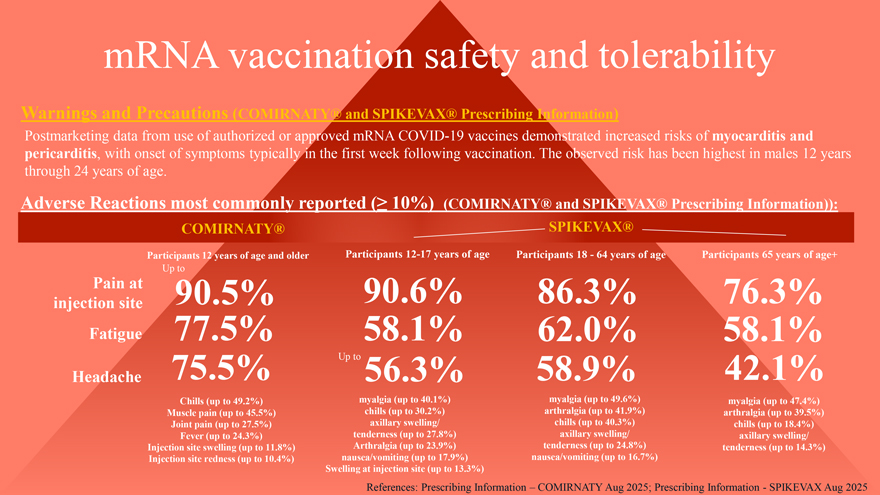
mRNA vaccination safety and tolerability Warnings and Precautions (COMIRNATY® and SPIKEVAX® Prescribing Information) Postmarketing data from use of authorized or approved mRNA COVID-19 vaccines demonstrated increased risks of myocarditis and pericarditis, with onset of symptoms typically in the first week following vaccination. The observed risk has been highest in males 12 years through 24 years of age. Adverse Reactions most commonly reported (≥ 10%) (COMIRNATY® and SPIKEVAX® Prescribing Information)): COMIRNATY® SPIKEVAX® Participants 12 years of age and older Participants 12-17 years of age Participants 18—64 years of age Participants 65 years of age+ Up to Pain at 90.5% 90.6% 86.3% 76.3% injection site Fatigue 77.5% 58.1% 62.0% 58.1% 75.5% Up to 56.3% 58.9% 42.1% Headache Chills (up to 49.2%) myalgia (up to 40.1%) myalgia (up to 49.6%) myalgia (up to 47.4%) Muscle pain (up to 45.5%) chills (up to 30.2%) arthralgia (up to 41.9%) arthralgia (up to 39.5%) Joint pain (up to 27.5%) axillary swelling/ chills (up to 40.3%) chills (up to 18.4%) Fever (up to 24.3%) tenderness (up to 27.8%) axillary swelling/ axillary swelling/ Injection site swelling (up to 11.8%) Arthralgia (up to 23.9%) tenderness (up to 24.8%) tenderness (up to 14.3%) Injection site redness (up to 10.4%) nausea/vomiting (up to 17.9%) nausea/vomiting (up to 16.7%) Swelling at injection site (up to 13.3%) References: Prescribing Information – COMIRNATY Aug 2025; Prescribing Information—SPIKEVAX Aug 2025
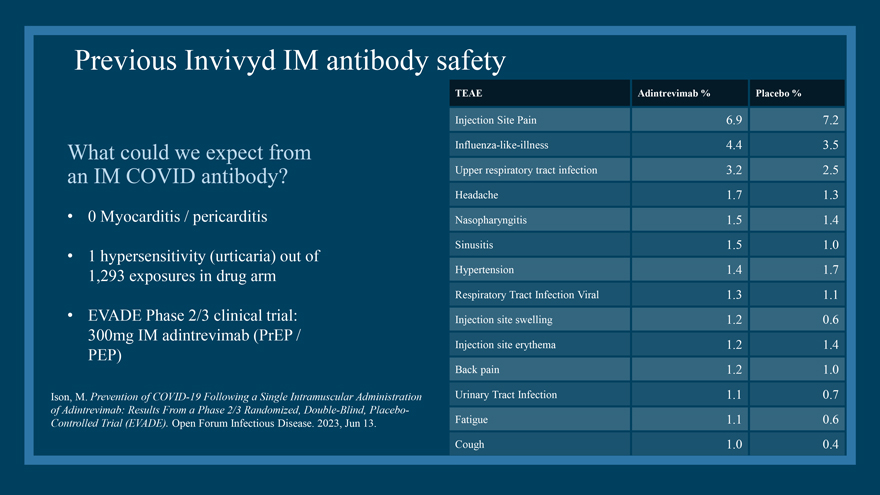
Previous Invivyd IM antibody safety What could we expect from an IM COVID antibody? • 0 Myocarditis / pericarditis • 1 hypersensitivity (urticaria) out of 1,293 exposures in drug arm • EVADE Phase 2/3 clinical trial: 300mg IM adintrevimab (PrEP / PEP) Ison, M. Prevention of COVID-19 Following a Single Intramuscular Administration of Adintrevimab: Results From a Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial (EVADE). Open Forum Infectious Disease. 2023, Jun 13. TEAE Adintrevimab % Placebo % Injection Site Pain 6.9 7.2 Influenza-like-illness 4.4 3.5 Upper respiratory tract infection 3.2 2.5 Headache 1.7 1.3 Nasopharyngitis 1.5 1.4 Sinusitis 1.5 1.0 Hypertension 1.4 1.7 Respiratory Tract Infection Viral 1.3 1.1 Injection site swelling 1.2 0.6 Injection site erythema 1.2 1.4 Back pain 1.2 1.0 Urinary Tract Infection 1.1 0.7 Fatigue 1.1 0.6 Cough 1.0 0.4
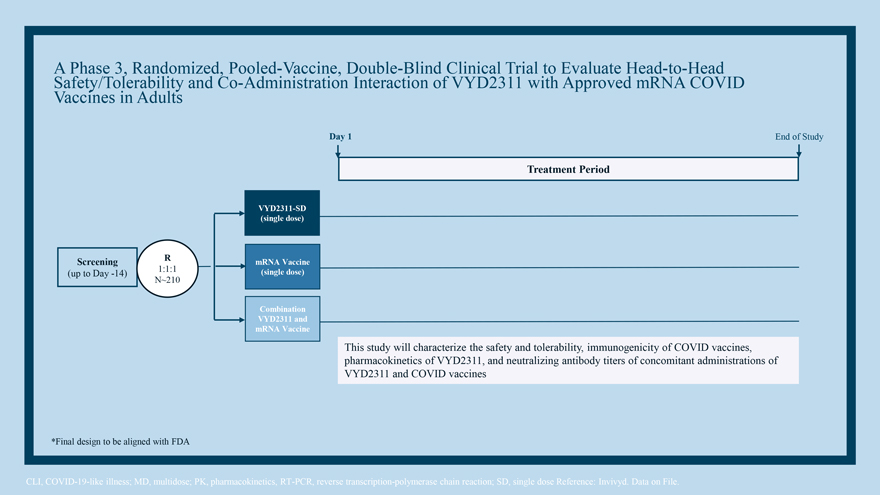
A Phase 3, Randomized, Pooled-Vaccine, Double-Blind Clinical Trial to Evaluate Head-to-Head Safety/Tolerability and Co-Administration Interaction of VYD2311 with Approved mRNA COVID Vaccines in Adults Day 1 End of Study Treatment Period VYD2311-SD (single dose) Screening R mRNA Vaccine (up to Day -14) 1:1:1 (single dose) N~210 Combination VYD2311 and mRNA Vaccine This study will characterize the safety and tolerability, immunogenicity of COVID vaccines, pharmacokinetics of VYD2311, and neutralizing antibody titers of concomitant administrations of VYD2311 and COVID vaccines *Final design to be aligned with FDA CLI, COVID-19-like illness; MD, multidose; PK, pharmacokinetics, RT-PCR, reverse transcription-polymerase chain reaction; SD, single dose Reference: Invivyd. Data on File.
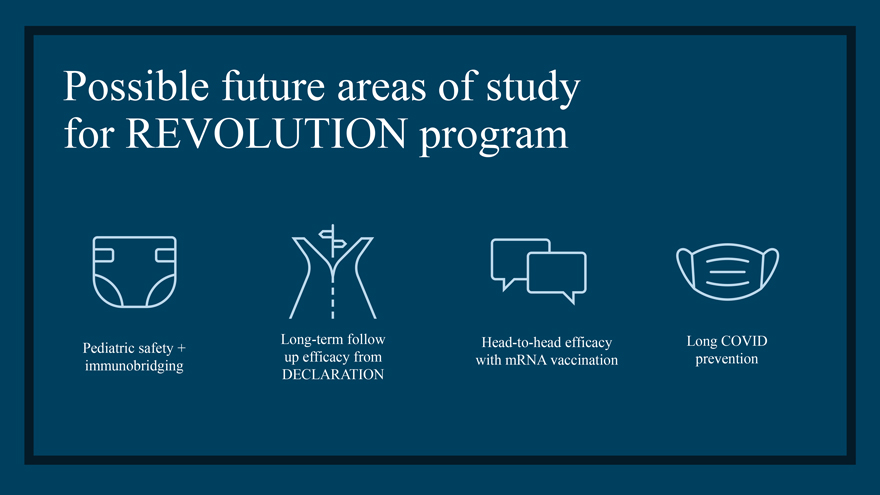
Possible future areas of study for REVOLUTION program Long-term follow Head-to-head efficacy Long COVID Pediatric safety + up efficacy from with mRNA vaccination prevention immunobridging DECLARATION

agenda 01 The COVID Situation 02 Invivyd Antibodies 03 REVOLUTION Clinical Program 04 Future Commercial Landscape

COVID Vaccines: Big Revenue, Small Protection 18 and over vaccine efficacy (VE) reduction in hospitalization estimate from CDC $3.8B 2023–2024 vaccine dose, ≥7 days 36% FY24 7–59 days earlier 51% (45–56) U.S. Revenue 60–119 days earlier 42% (35–48) 120–179 days earlier 15% (3–26)

293,000,000 U.S. Pop 12+ 147M Flu Vaccine doses in 24-25 flu season 34.2M ~100 -112M ~146 -158M COVID vaccinated last Received a flu shot, but not COVID last Did not receive COVID or flu shot in last respiratory season respiratory season respiratory season

Broad recognition from Societies and Guidelines for Antibodies in COVID SOCIETY / GUIDELINE PEMGARDA OR MAB TARGET AUDIENCE HIV.gov PEMGARDA Immunodeficiency IDSA Pemivibart Infectious disease NCCN – B-Cell Lymphomas Pemivibart Oncology NCCN – Infection Prevention Pemivibart Oncology Immune Deficiency Foundation (IDF) PEMGARDA Immunodeficiency Leukemia and Lymphoma Society (LLS) Pemivibart Oncology MS Society PEMGARDA Rheumatology National Kidney Foundation PEMGARDA Solid organ transplant American Cancer Society PEMGARDA Oncology American College of Rheumatology mAbs Rheumatology American Lung Association Pemivibart Oncology National Council on Aging (NCOA) PEMGARDA Elderly BreastCancer.org PEMGARDA Oncology CLL Society PEMGARDA Oncology American Academy of Allergy, Asthma, PEMGARDA Immunology and Immunology (AAAAI) Vasculitis Foundation PEMGARDA Rheumatology Invivyd poised to deliver on scalable form factor for broad access
P

Launch Preparation Underway *If approved