

2026 J.P. Morgan Healthcare Conference Mick Hitchcock, CEO Biomea Fusion January 14, 2026

Legal Disclaimer & Forward-looking Statements Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future business and financial performance of Biomea Fusion, Inc. (the “Company”) and involve known and unknown risks, uncertainties, and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any projections of financial information or profitability, the initiation, timing and results of pending or future preclinical studies and clinical trials, the actual or potential actions of the Food and Drug Administration (FDA), the status and timing of ongoing research, development and corporate partnering activities, any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for future operations and any statements of expectation or belief regarding future events, potential markets or market size, or technology developments. The Company has based these forward-looking statements on its current expectations, assumptions, estimates, and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission (the SEC), as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the SEC. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
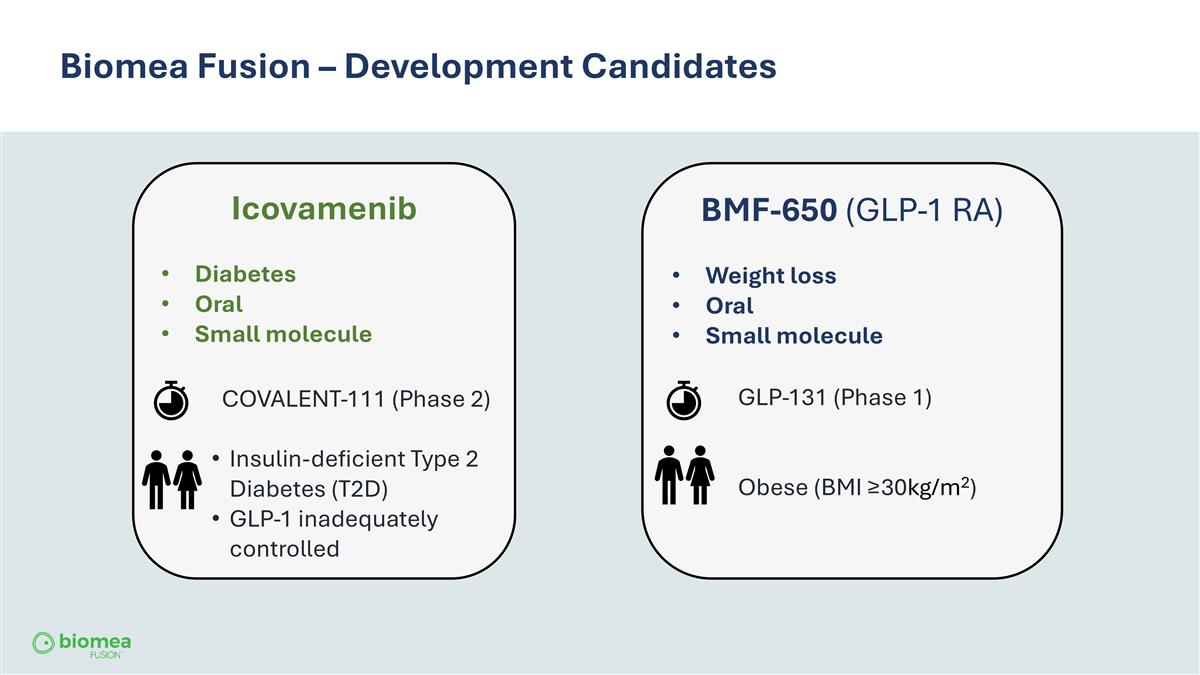
Biomea Fusion – Development Candidates Icovamenib Diabetes Oral Small molecule COVALENT-111 (Phase 2) Insulin-deficient Type 2 Diabetes (T2D) GLP-1 inadequately controlled BMF-650 (GLP-1 RA) Weight loss Oral Small molecule GLP-131 (Phase 1) Obese (BMI ≥30kg/m2)
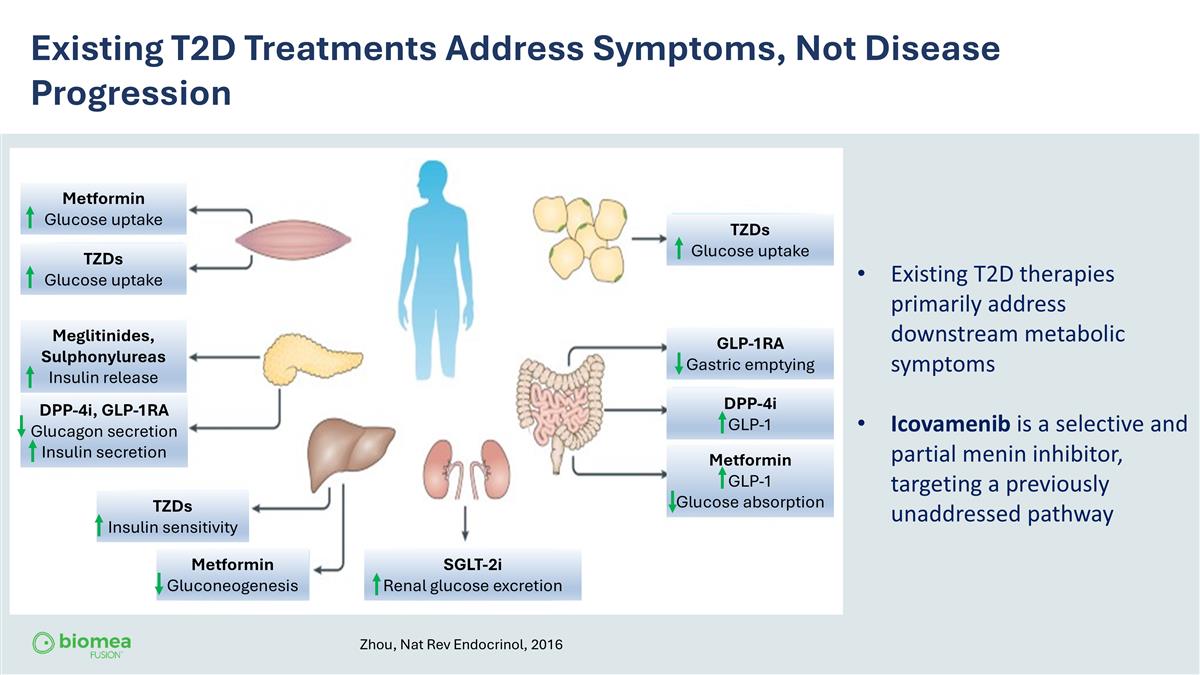
Existing T2D Treatments Address Symptoms, Not Disease Progression Existing T2D therapies primarily address downstream metabolic symptoms Icovamenib is a selective and partial menin inhibitor, targeting a previously unaddressed pathway Zhou, Nat Rev Endocrinol, 2016 Metformin Glucose uptake TZDs Glucose uptake TZDs Glucose uptake TZDs Insulin sensitivity Metformin Gluconeogenesis GLP-1RA Gastric emptying DPP-4i GLP-1 Metformin GLP-1 Glucose absorption Meglitinides, Sulphonylureas Insulin release DPP-4i, GLP-1RA Glucagon secretion Insulin secretion SGLT-2i Renal glucose excretion
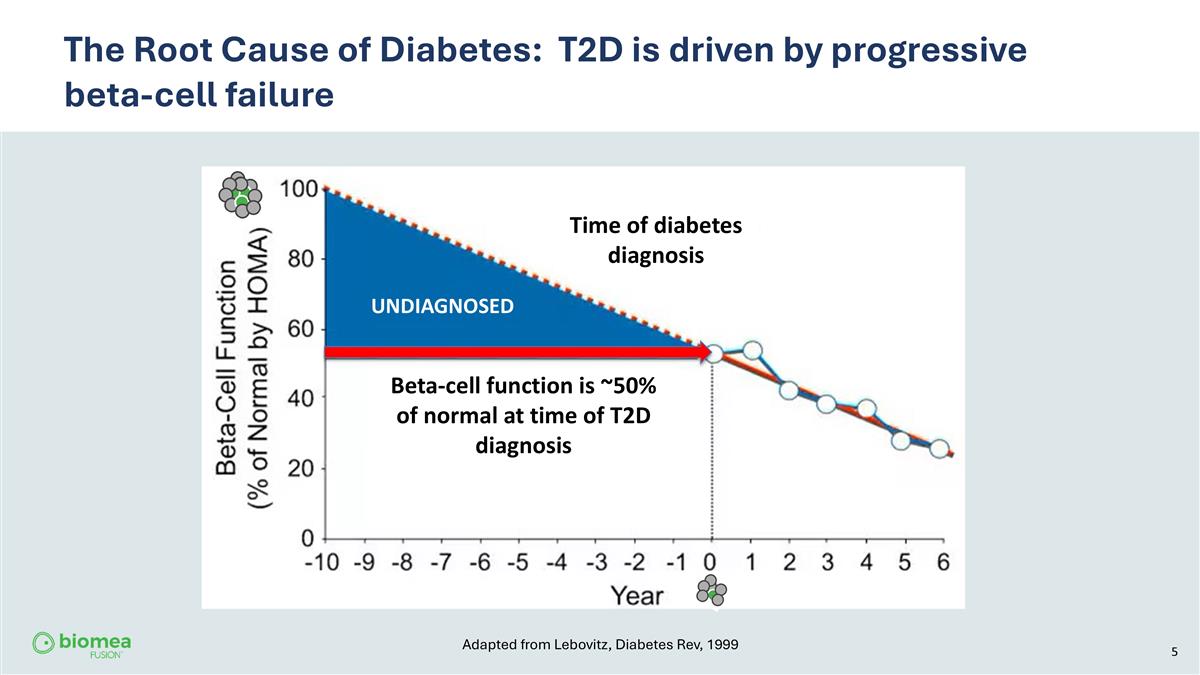
The Root Cause of Diabetes: T2D is driven by progressive beta-cell failure UNDIAGNOSED Time of diabetes diagnosis Beta-cell function is ~50% of normal at time of T2D diagnosis Adapted from Lebovitz, Diabetes Rev, 1999
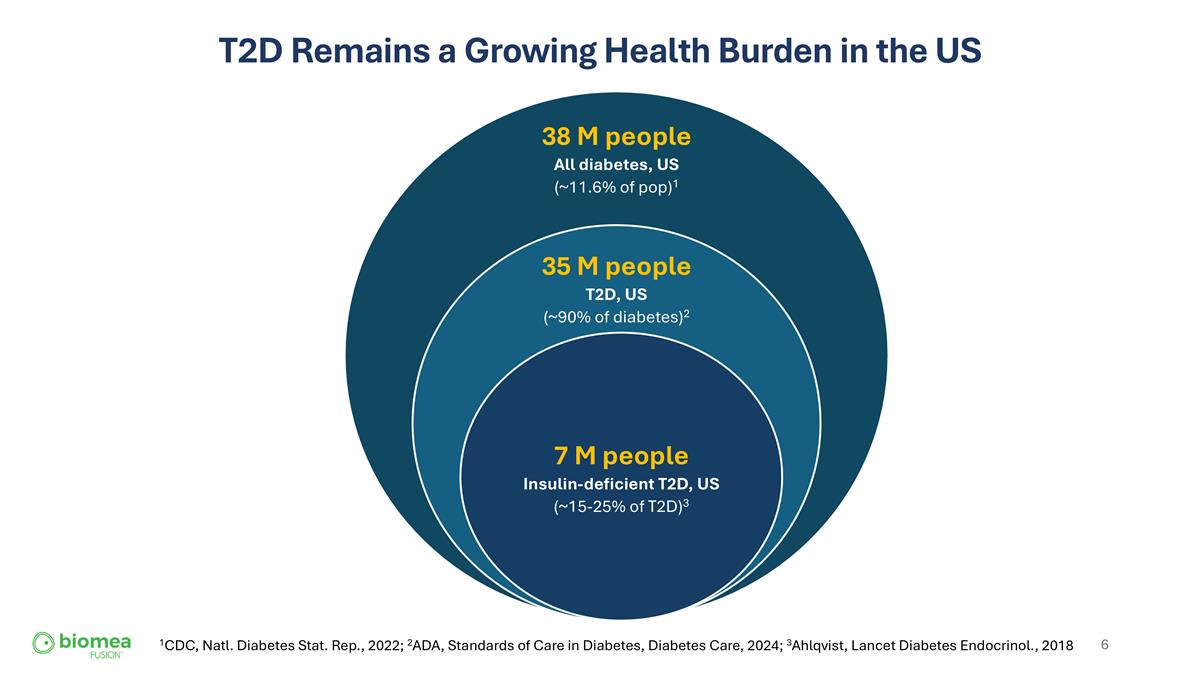
T2D Remains a Growing Health Burden in the US 1CDC, Natl. Diabetes Stat. Rep., 2022; 2ADA, Standards of Care in Diabetes, Diabetes Care, 2024; 3Ahlqvist, Lancet Diabetes Endocrinol., 2018
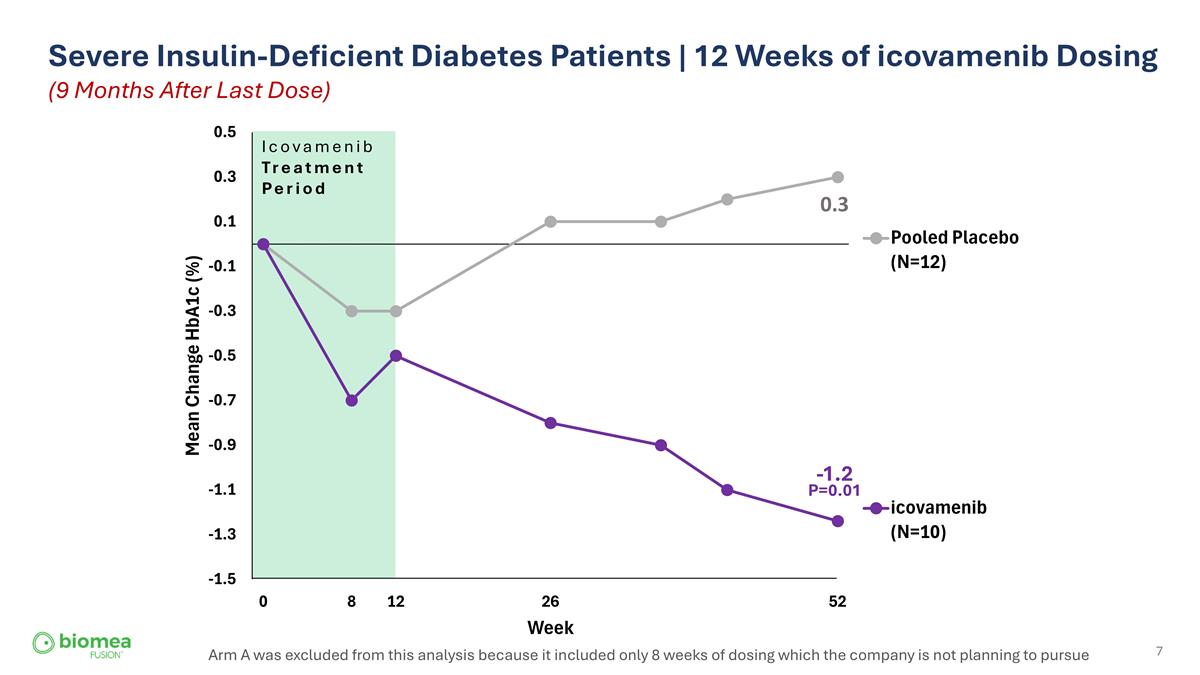
Severe Insulin-Deficient Diabetes Patients | 12 Weeks of icovamenib Dosing (9 Months After Last Dose) Arm A was excluded from this analysis because it included only 8 weeks of dosing which the company is not planning to pursue Icovamenib Treatment Period 0.3 -1.2 P=0.01
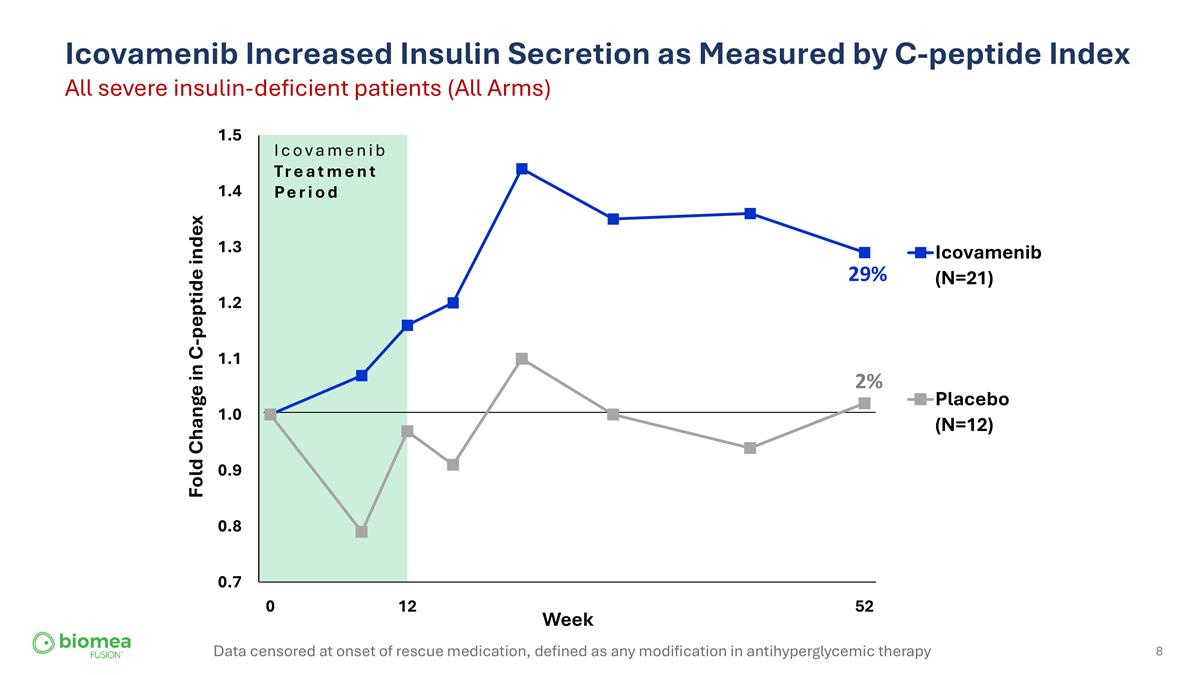
Icovamenib Increased Insulin Secretion as Measured by C-peptide Index All severe insulin-deficient patients (All Arms) 29% 2% Data censored at onset of rescue medication, defined as any modification in antihyperglycemic therapy Icovamenib Treatment Period
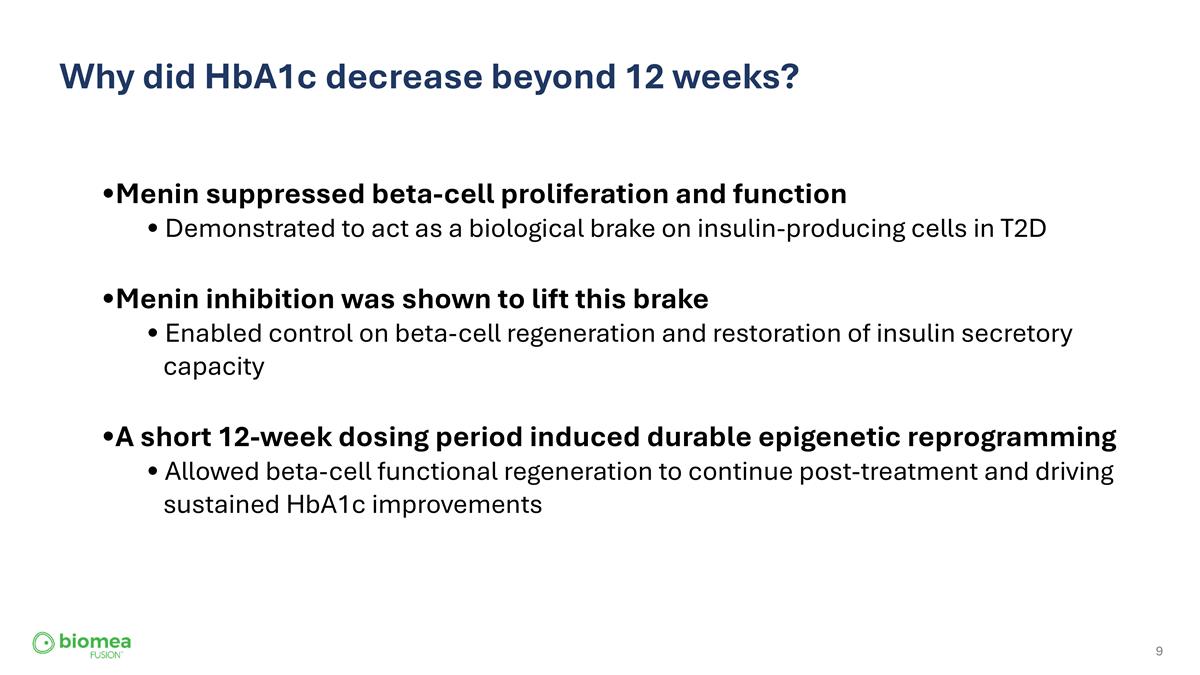
Why did HbA1c decrease beyond 12 weeks? Menin suppressed beta-cell proliferation and function Demonstrated to act as a biological brake on insulin-producing cells in T2D Menin inhibition was shown to lift this brake Enabled control on beta-cell regeneration and restoration of insulin secretory capacity A short 12-week dosing period induced durable epigenetic reprogramming Allowed beta-cell functional regeneration to continue post-treatment and driving sustained HbA1c improvements

10 Physiologic Suppression of Menin Can Expand Beta-Cell Mass Physiologic states such as pregnancy and lactation suppress menin, enabling beta-cell expansion and increased insulin output Preclinical and human data consistently link reduced menin signaling to improved beta-cell mass and function Icovamenib has been shown to directly inhibit menin, aiming to pharmacologically replicate a naturally occurring, validated biologic process Karnik, Science, 2007
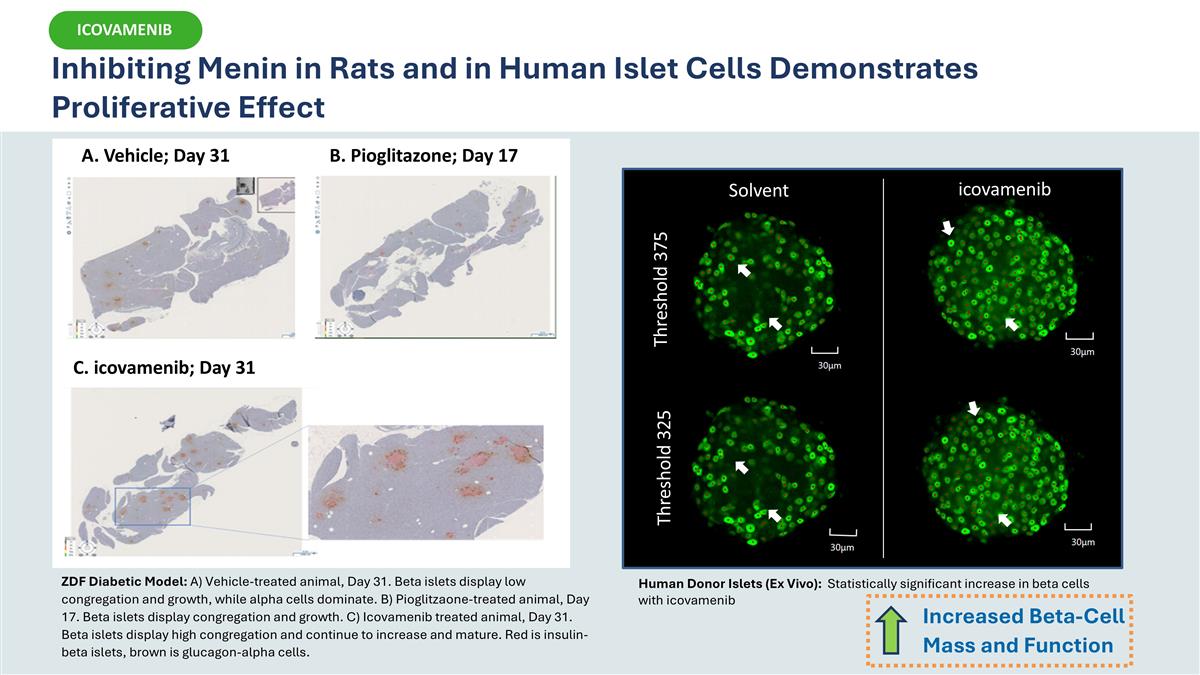
A. Vehicle; Day 31 B. Pioglitazone; Day 17 C. icovamenib; Day 31 ZDF Diabetic Model: A) Vehicle-treated animal, Day 31. Beta islets display low congregation and growth, while alpha cells dominate. B) Pioglitzaone-treated animal, Day 17. Beta islets display congregation and growth. C) Icovamenib treated animal, Day 31. Beta islets display high congregation and continue to increase and mature. Red is insulin-beta islets, brown is glucagon-alpha cells. Solvent icovamenib Threshold 375 Threshold 325 Human Donor Islets (Ex Vivo): Statistically significant increase in beta cells with icovamenib Inhibiting Menin in Rats and in Human Islet Cells Demonstrates Proliferative Effect Increased Beta-Cell Mass and Function ICOVAMENIB
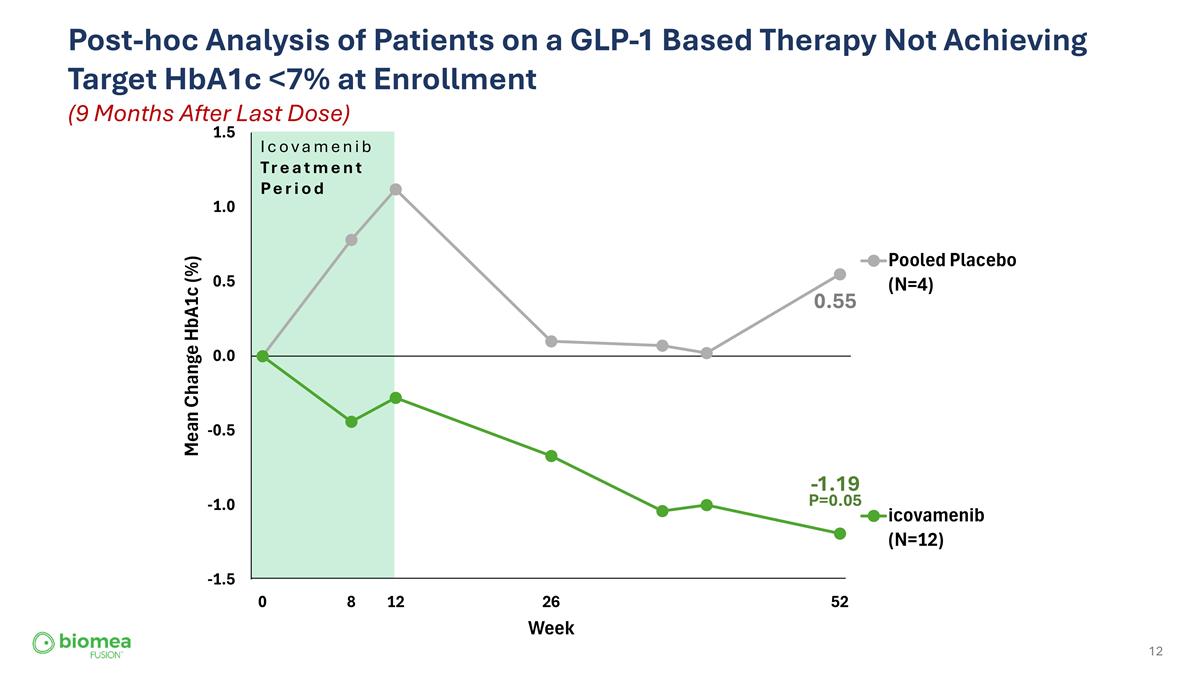
Post-hoc Analysis of Patients on a GLP-1 Based Therapy Not Achieving Target HbA1c <7% at Enrollment (9 Months After Last Dose) Icovamenib Treatment Period 0.55 -1.19 P=0.05
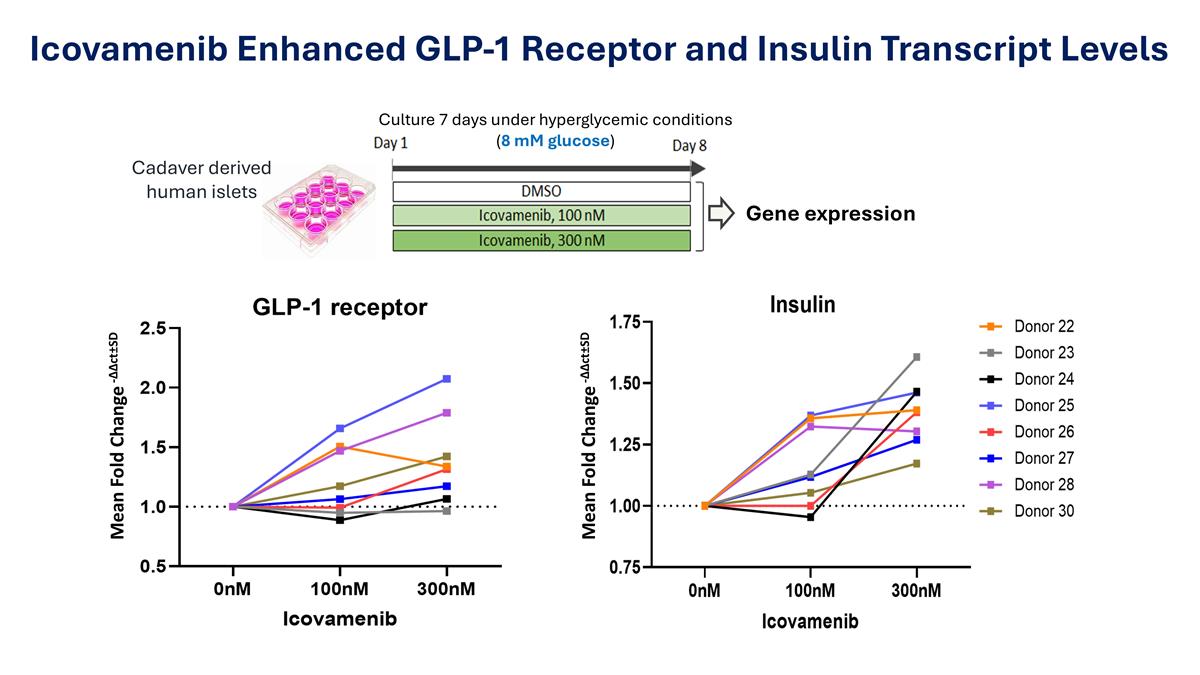
Icovamenib Enhanced GLP-1 Receptor and Insulin Transcript Levels Culture 7 days under hyperglycemic conditions (8 mM glucose) Cadaver derived human islets Gene expression Mean Fold Change -∆∆ct±SD Mean Fold Change -∆∆ct±SD
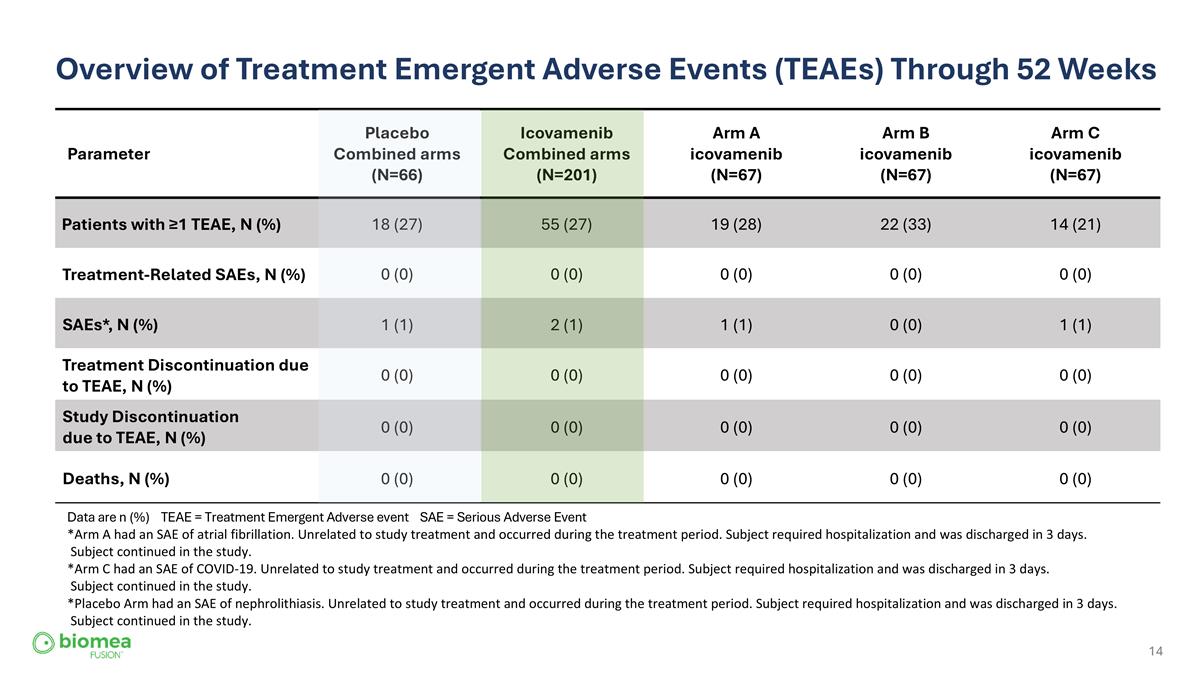
Overview of Treatment Emergent Adverse Events (TEAEs) Through 52 Weeks Parameter Placebo Combined arms (N=66) Icovamenib Combined arms (N=201) Arm A icovamenib (N=67) Arm B icovamenib (N=67) Arm C icovamenib (N=67) Patients with ≥1 TEAE, N (%) 18 (27) 55 (27) 19 (28) 22 (33) 14 (21) Treatment-Related SAEs, N (%) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) SAEs*, N (%) 1 (1) 2 (1) 1 (1) 0 (0) 1 (1) Treatment Discontinuation due to TEAE, N (%) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Study Discontinuation due to TEAE, N (%) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Deaths, N (%) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) Data are n (%) TEAE = Treatment Emergent Adverse event SAE = Serious Adverse Event *Arm A had an SAE of atrial fibrillation. Unrelated to study treatment and occurred during the treatment period. Subject required hospitalization and was discharged in 3 days. Subject continued in the study. *Arm C had an SAE of COVID-19. Unrelated to study treatment and occurred during the treatment period. Subject required hospitalization and was discharged in 3 days. Subject continued in the study. *Placebo Arm had an SAE of nephrolithiasis. Unrelated to study treatment and occurred during the treatment period. Subject required hospitalization and was discharged in 3 days. Subject continued in the study.
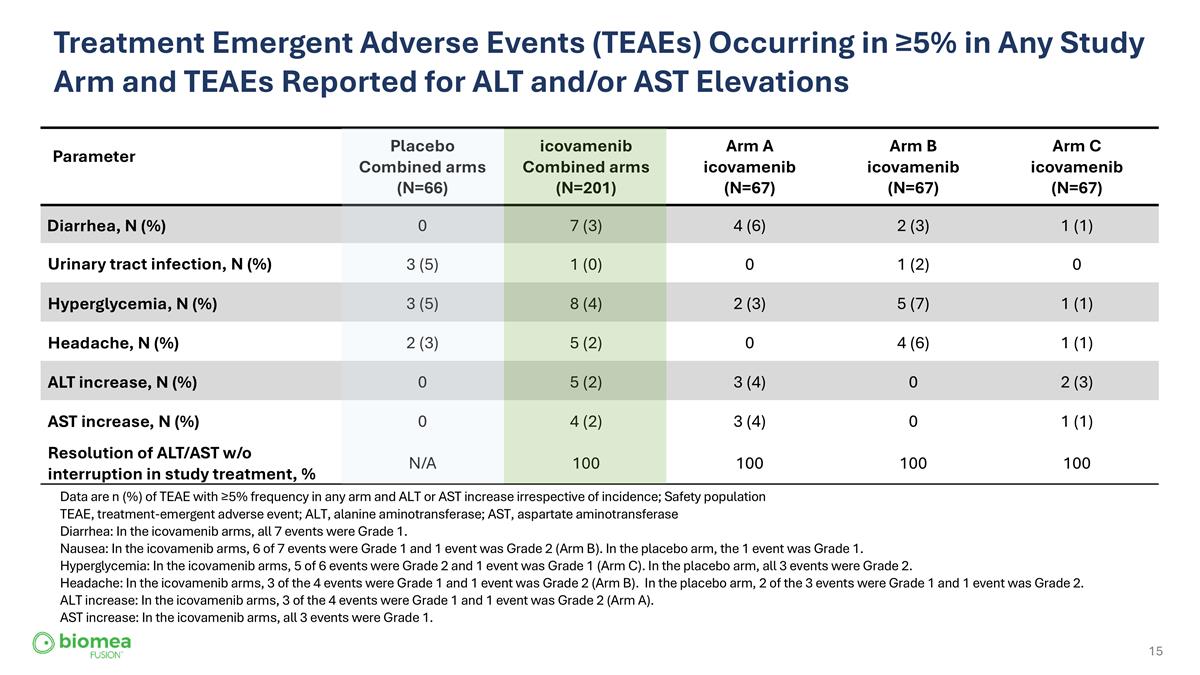
Treatment Emergent Adverse Events (TEAEs) Occurring in ≥5% in Any Study Arm and TEAEs Reported for ALT and/or AST Elevations Parameter Placebo Combined arms (N=66) icovamenib Combined arms (N=201) Arm A icovamenib (N=67) Arm B icovamenib (N=67) Arm C icovamenib (N=67) Diarrhea, N (%) 0 7 (3) 4 (6) 2 (3) 1 (1) Urinary tract infection, N (%) 3 (5) 1 (0) 0 1 (2) 0 Hyperglycemia, N (%) 3 (5) 8 (4) 2 (3) 5 (7) 1 (1) Headache, N (%) 2 (3) 5 (2) 0 4 (6) 1 (1) ALT increase, N (%) 0 5 (2) 3 (4) 0 2 (3) AST increase, N (%) 0 4 (2) 3 (4) 0 1 (1) Resolution of ALT/AST w/o interruption in study treatment, % N/A 100 100 100 100 Data are n (%) of TEAE with ≥5% frequency in any arm and ALT or AST increase irrespective of incidence; Safety population TEAE, treatment-emergent adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase Diarrhea: In the icovamenib arms, all 7 events were Grade 1. Nausea: In the icovamenib arms, 6 of 7 events were Grade 1 and 1 event was Grade 2 (Arm B). In the placebo arm, the 1 event was Grade 1. Hyperglycemia: In the icovamenib arms, 5 of 6 events were Grade 2 and 1 event was Grade 1 (Arm C). In the placebo arm, all 3 events were Grade 2. Headache: In the icovamenib arms, 3 of the 4 events were Grade 1 and 1 event was Grade 2 (Arm B). In the placebo arm, 2 of the 3 events were Grade 1 and 1 event was Grade 2. ALT increase: In the icovamenib arms, 3 of the 4 events were Grade 1 and 1 event was Grade 2 (Arm A). AST increase: In the icovamenib arms, all 3 events were Grade 1.
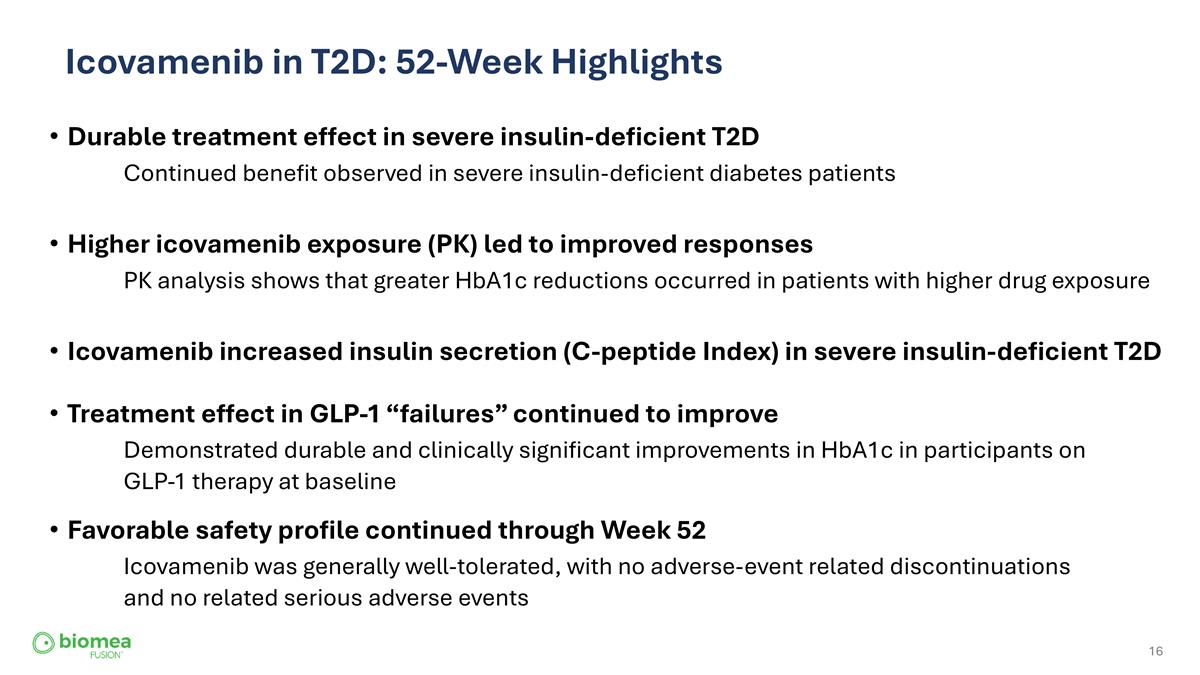
Icovamenib in T2D: 52-Week Highlights Durable treatment effect in severe insulin-deficient T2D Continued benefit observed in severe insulin-deficient diabetes patients Higher icovamenib exposure (PK) led to improved responses PK analysis shows that greater HbA1c reductions occurred in patients with higher drug exposure Treatment effect in GLP-1 “failures” continued to improve Demonstrated durable and clinically significant improvements in HbA1c in participants on GLP-1 therapy at baseline Favorable safety profile continued through Week 52 Icovamenib was generally well-tolerated, with no adverse-event related discontinuations and no related serious adverse events Icovamenib increased insulin secretion (C-peptide Index) in severe insulin-deficient T2D

COVALENT-111: Potential to Alter the Insulin Treatment Trajectory COVALENT-111 highlights the potential to restore endogenous insulin production capacity in patients who would otherwise progress to chronic insulin therapy—offering the possibility of short-term oral treatment rather than lifelong injectable management.
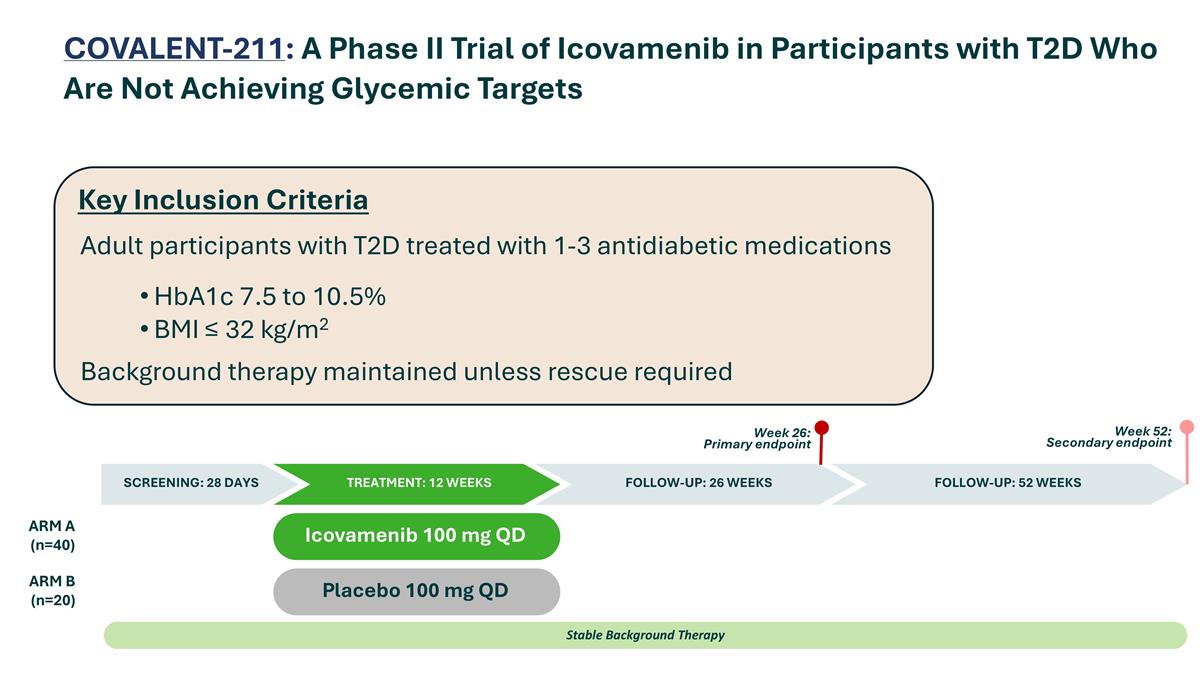
COVALENT-211: A Phase II Trial of Icovamenib in Participants with T2D Who Are Not Achieving Glycemic Targets Placebo 100 mg QD Icovamenib 100 mg QD ARM A (n=40) ARM B (n=20) Stable Background Therapy SCREENING: 28 DAYS TREATMENT: 12 WEEKS FOLLOW-UP: 52 WEEKS Week 26: Primary endpoint Week 52: Secondary endpoint FOLLOW-UP: 26 WEEKS Adult participants with T2D treated with 1-3 antidiabetic medications HbA1c 7.5 to 10.5% BMI ≤ 32 kg/m2 Background therapy maintained unless rescue required Key Inclusion Criteria
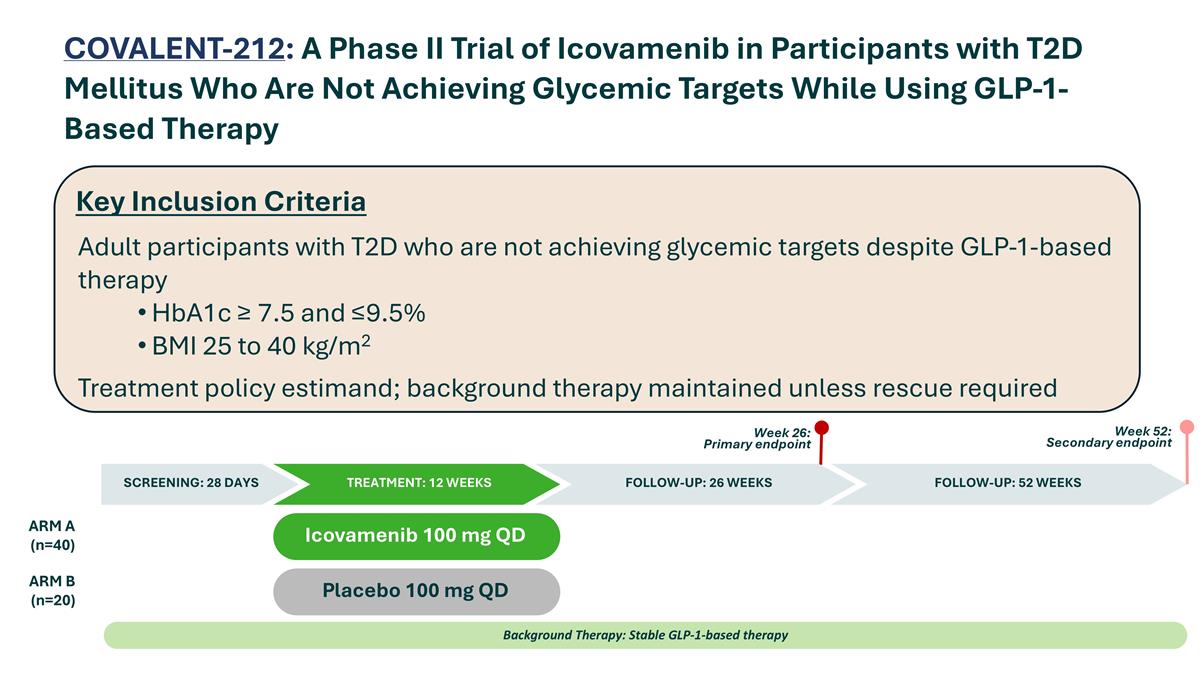
Placebo 100 mg QD Icovamenib 100 mg QD ARM A (n=40) ARM B (n=20) Background Therapy: Stable GLP-1-based therapy SCREENING: 28 DAYS TREATMENT: 12 WEEKS FOLLOW-UP: 52 WEEKS Week 26: Primary endpoint Week 52: Secondary endpoint FOLLOW-UP: 26 WEEKS Key Inclusion Criteria Adult participants with T2D who are not achieving glycemic targets despite GLP-1-based therapy HbA1c ≥ 7.5 and ≤9.5% BMI 25 to 40 kg/m2 Treatment policy estimand; background therapy maintained unless rescue required COVALENT-212: A Phase II Trial of Icovamenib in Participants with T2D Mellitus Who Are Not Achieving Glycemic Targets While Using GLP-1-Based Therapy
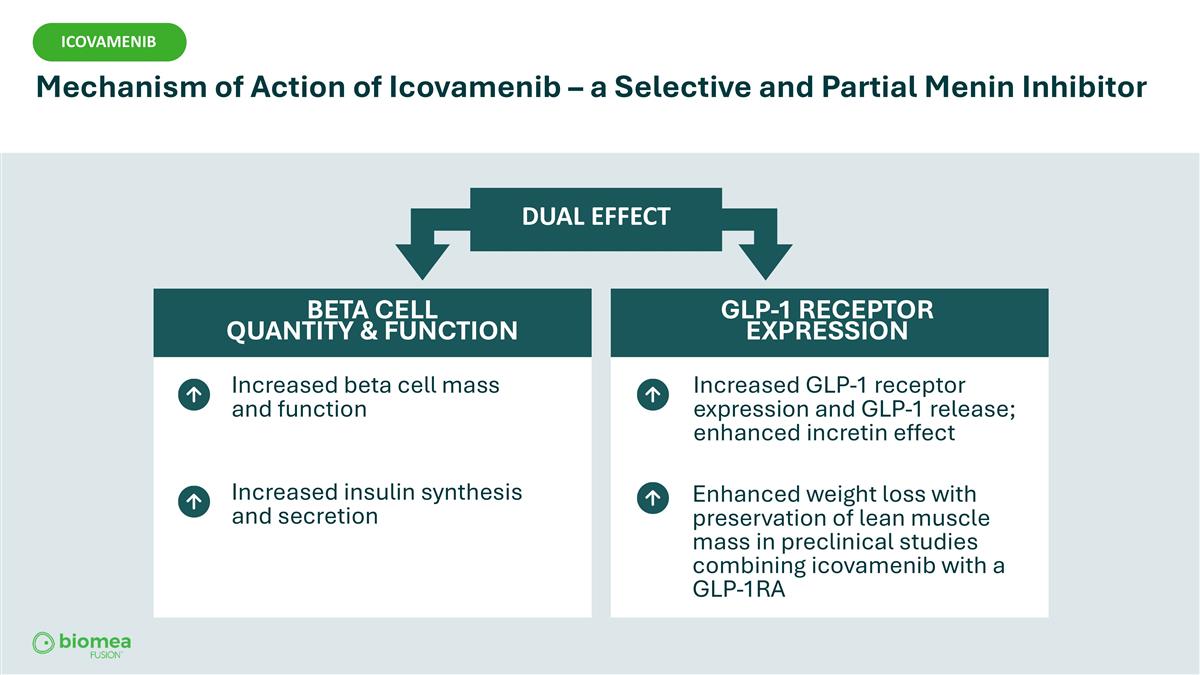
Increased beta cell mass and function Increased insulin synthesis and secretion DUAL EFFECT BETA CELL QUANTITY & FUNCTION GLP-1 RECEPTOR EXPRESSION Increased GLP-1 receptor expression and GLP-1 release; enhanced incretin effect Enhanced weight loss with preservation of lean muscle mass in preclinical studies combining icovamenib with a GLP-1RA ICOVAMENIB Mechanism of Action of Icovamenib – a Selective and Partial Menin Inhibitor ICOVAMENIB
Biomea Fusion – BMF-650 BMF-650 (GLP-1 RA) Weight loss Oral Small molecule GLP-131 (Phase 1) Obese (BMI ≥30kg/m2) Designed for better bioavailability >>> More consistent efficacy
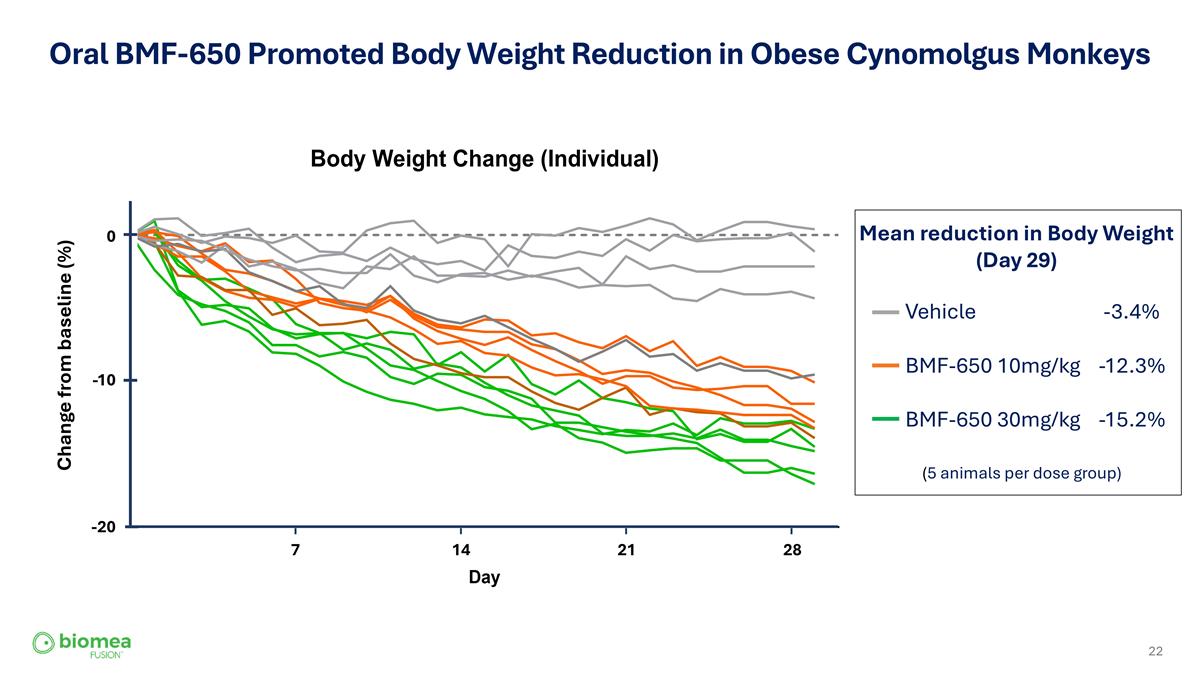
Oral BMF-650 Promoted Body Weight Reduction in Obese Cynomolgus Monkeys (5 animals per dose group) Vehicle -3.4% BMF-650 10mg/kg -12.3% BMF-650 30mg/kg -15.2% 0 -10 -20 Change from baseline (%) 7 14 21 28 Mean reduction in Body Weight (Day 29)
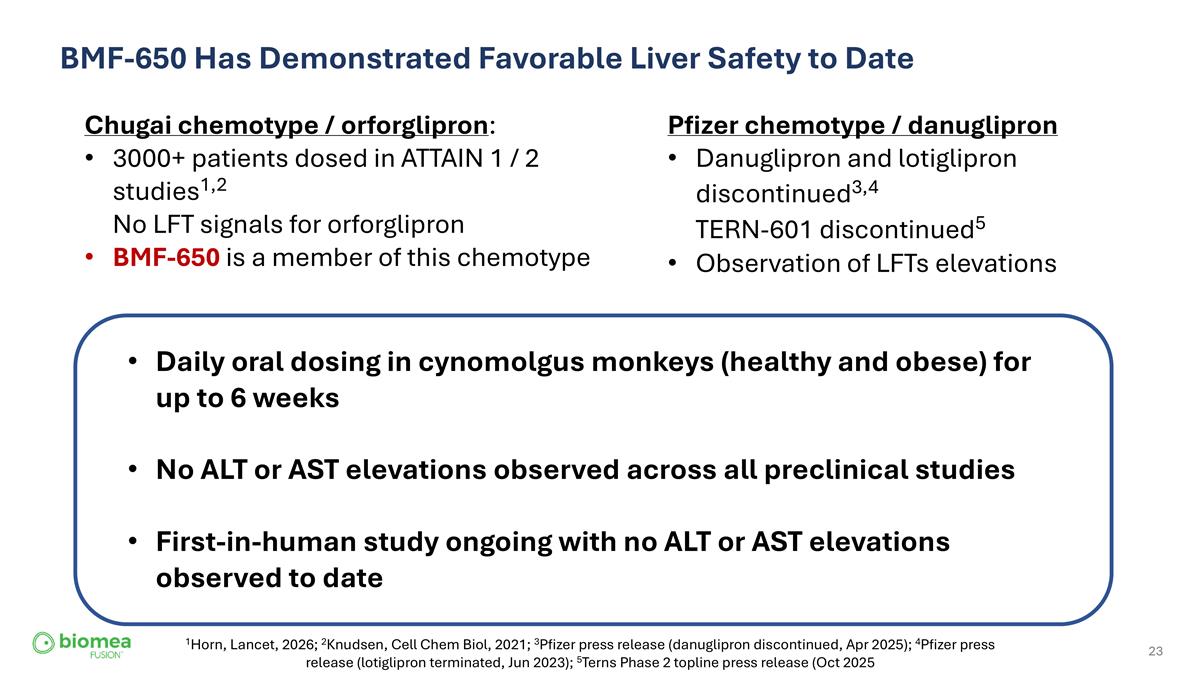
BMF-650 Has Demonstrated Favorable Liver Safety to Date Pfizer chemotype / danuglipron Danuglipron and lotiglipron discontinued3,4 TERN-601 discontinued5 Observation of LFTs elevations Chugai chemotype / orforglipron: 3000+ patients dosed in ATTAIN 1 / 2 studies1,2 No LFT signals for orforglipron BMF-650 is a member of this chemotype Daily oral dosing in cynomolgus monkeys (healthy and obese) for up to 6 weeks No ALT or AST elevations observed across all preclinical studies First-in-human study ongoing with no ALT or AST elevations observed to date 1Horn, Lancet, 2026; 2Knudsen, Cell Chem Biol, 2021; 3Pfizer press release (danuglipron discontinued, Apr 2025); 4Pfizer press release (lotiglipron terminated, Jun 2023); 5Terns Phase 2 topline press release (Oct 2025
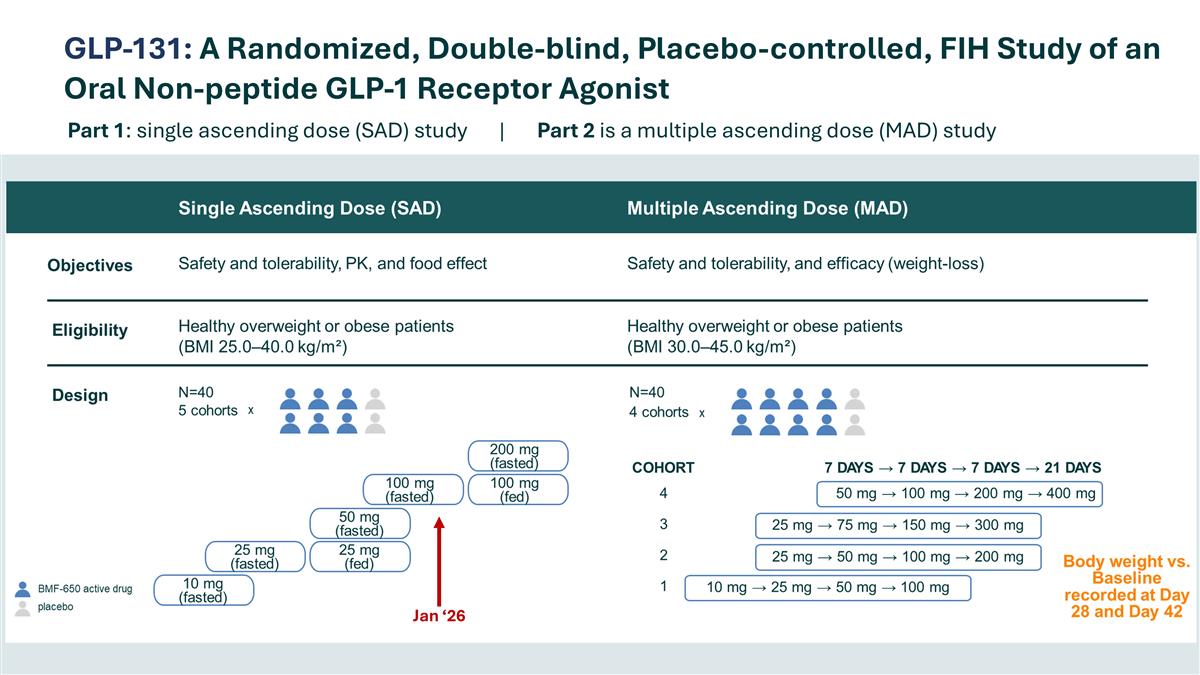
GLP-131: A Randomized, Double-blind, Placebo-controlled, FIH Study of an Oral Non-peptide GLP-1 Receptor Agonist Part 1: single ascending dose (SAD) study | Part 2 is a multiple ascending dose (MAD) study Jan ‘26
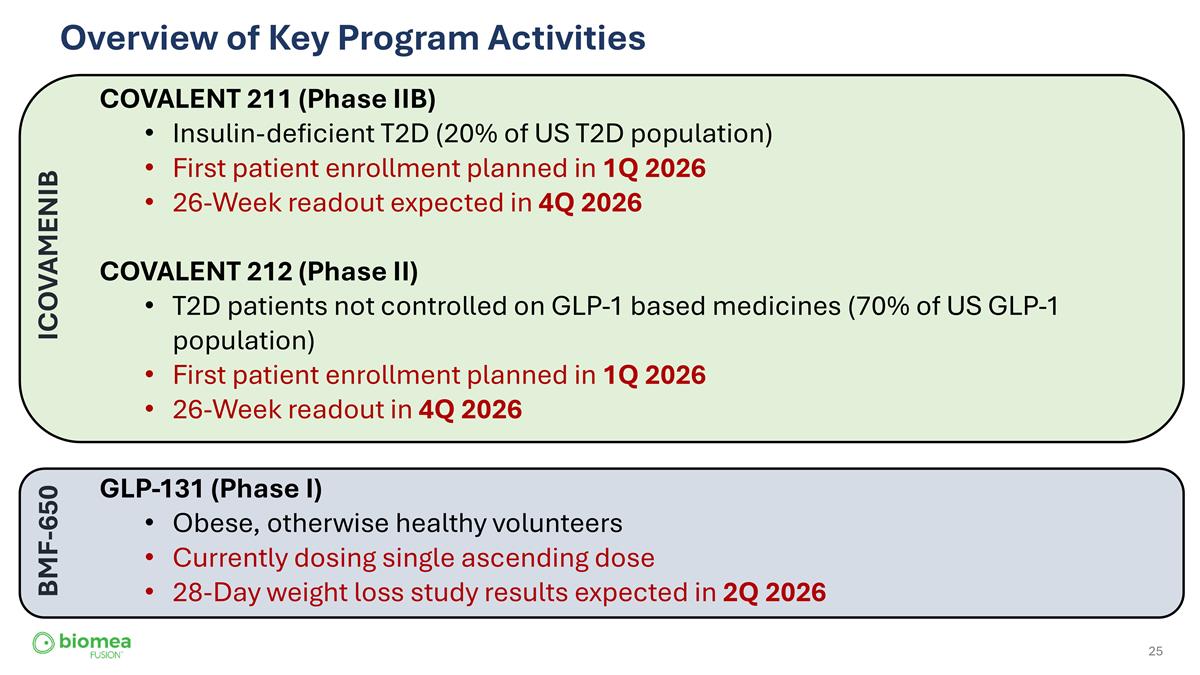
Overview of Key Program Activities COVALENT 211 (Phase IIB) Insulin-deficient T2D (20% of US T2D population) First patient enrollment planned in 1Q 2026 26-Week readout expected in 4Q 2026 COVALENT 212 (Phase II) T2D patients not controlled on GLP-1 based medicines (70% of US GLP-1 population) First patient enrollment planned in 1Q 2026 26-Week readout in 4Q 2026 GLP-131 (Phase I) Obese, otherwise healthy volunteers Currently dosing single ascending dose 28-Day weight loss study results expected in 2Q 2026 ICOVAMENIB BMF-650

Q&A

THANK YOU For questions or inquiries, please reach out to Meichiel Weiss at ir@biomeafusion.com www.biomeafusion.com