
Unlocking the Value of our Pipeline for T1D January 2026

2 Forward - looking statements This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this presentation, other than statements of historical facts or statements that relate to present fa cts or current conditions, including but not limited to, statements regarding our clinical development plans and timelines and the initial safety and efficacy profiles of our product ca ndidates, and statements regarding our preclinical development programs, including initial preclinical data and development plans and timelines, and statements regarding cash r unw ay and expected use of potential offering proceeds are forward - looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by the forw ard - looking statements. In some cases, you can identify forward - looking statements by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “co uld,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions. The fo rward - looking statements in this presentation are only predictions. We have based these forward - looking statements largely on our current expectations and projections about future eve nts and financial trends that we believe may affect our business, financial condition, and results of operations. These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, incl udi ng, among others: our ability to successfully advance our current and future product candidates through development activities, preclinical studies, and clinical trials; o ur ability to progress our product candidates through clinical development; our ability to meet development milestones on anticipated timelines; uncertainties inherent in the results of pr eli minary data, pre - clinical studies and earlier - stage clinical trials, which may not be predictive of final results or the results of later - stage clinical trials; our ability to obtain cleara nce of our future IND or CTA submissions and commence and complete clinical trials on expected timelines, or at all; our reliance on the maintenance of certain key collaborative relat ion ships for the manufacturing and development of our product candidates; the timing, scope and likelihood of regulatory filings and approvals, including final regulatory approval of our pro duct candidates; the impact of geopolitical issues, trade disputes and tariffs, banking instability and inflation on our business and operations, supply chain and labor force; the per for mance of third parties in connection with the development of our product candidates, including third parties conducting our clinical trials as well as third - party suppliers and manufactu rers; our ability to successfully commercialize our product candidates and develop sales and marketing capabilities, if our product candidates are approved; our ability to recruit and m ain tain key members of management and our ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are desc rib ed more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward - lo oking statements as predictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge fro m t ime to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publi cly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
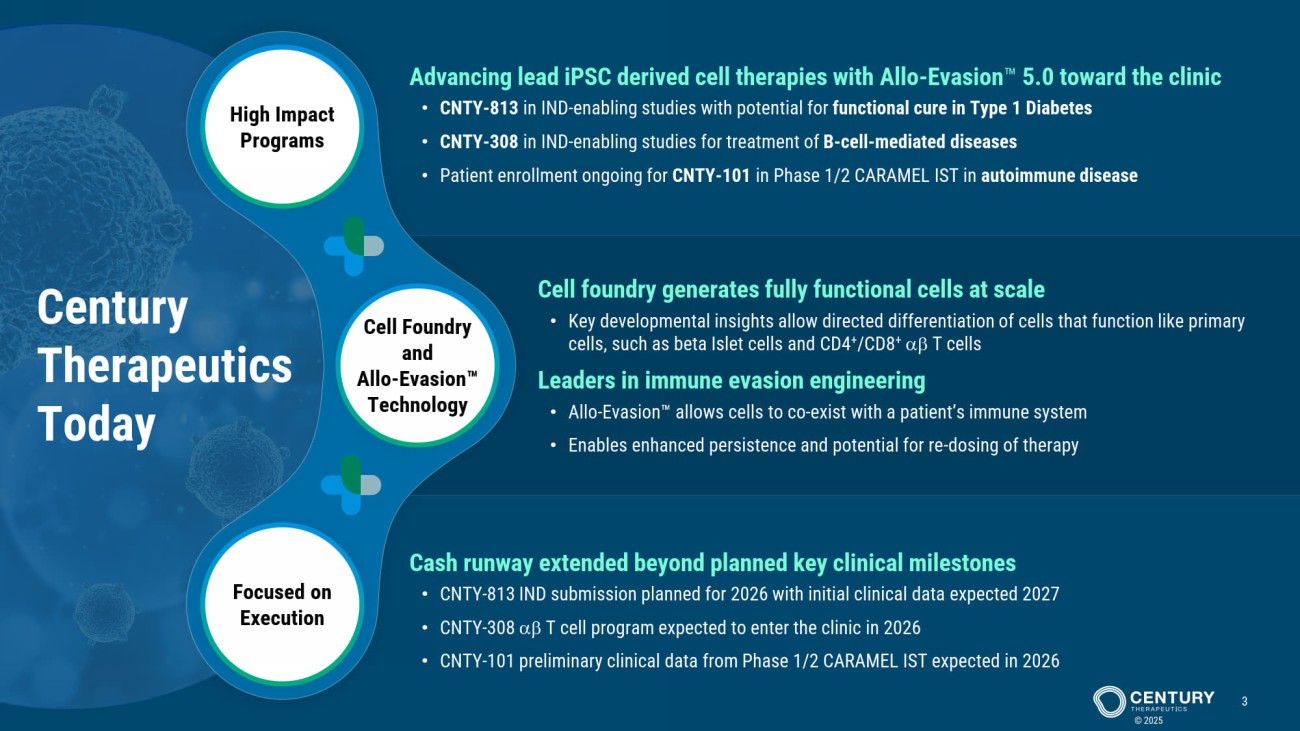
Cell Foundry and Allo - Evasion Technology High Impact Programs Focused on Execution Century Therapeutics Today © 2025 3 Cell foundry generates fully functional cells at scale • Key developmental insights allow directed differentiation of cells that function like primary cells, such as beta Islet cells and CD4 + / CD8 + T cells Leaders in immune evasion engineering • Allo - Evasion allows cells to co - exist with a patient’s immune system • Enables enhanced persistence and potential for re - dosing of therapy Advancing lead iPSC derived cell therapies with Allo - Evasion 5.0 toward the clinic • CNTY - 813 in IND - enabling studies with potential for functional cure in Type 1 Diabetes • CNTY - 308 in IND - enabling studies for treatment of B - cell - mediated diseases • Patient enrollment ongoing for CNTY - 101 in Phase 1/2 CARAMEL IST in autoimmune disease Cash runway extended beyond planned key clinical milestones • CNTY - 813 IND submission planned for 2026 with initial clinical data expected 2027 • CNTY - 308 T cell program expected to enter the clinic in 2026 • CNTY - 101 preliminary clinical data from Phase 1/2 CARAMEL IST expected in 2026
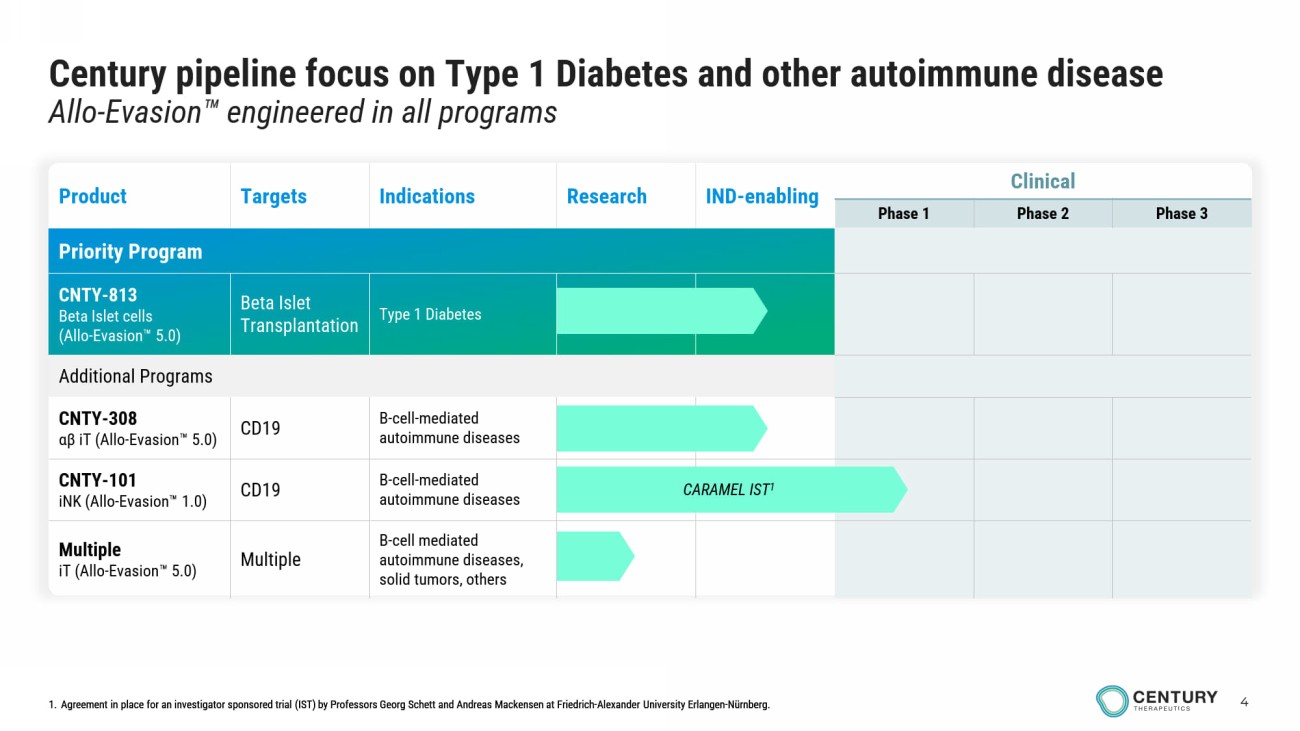
4 Century pipeline focus on Type 1 Diabetes and other autoimmune disease Allo - Evasion engineered in all programs infusions in outpatient setting 1. Agreement in place for an investigator sponsored trial (IST) by Professors Georg Schett and Andreas Mackensen at Friedrich - Alexander University Erlangen - Nürnberg. Clinical IND - enabling Research Indications Targets Product Phase 3 Phase 2 Phase 1 Priority Program Type 1 Diabetes Beta Islet Transplantation CNTY - 813 Beta Islet cells (Allo - Evasion 5.0) Additional Programs B - cell - mediated autoimmune diseases CD19 CNTY - 308 αβ iT ( Allo - Evasion 5.0) B - cell - mediated autoimmune diseases CD19 CNTY - 101 iNK ( Allo - Evasion 1.0) B - cell mediated autoimmune diseases, solid tumors, others Multiple Multiple iT ( Allo - Evasion 5.0) CARAMEL IST 1 1. Agreement in place for an investigator sponsored trial (IST) by Professors Georg Schett and Andreas Mackensen at Friedrich - Alexander University Erlangen - Nürnberg.
5 Century Executive Team Experienced leadership with a track record of driving innovation and success in cell therapy Chad Cowan, PhD Chief Scientific Officer Brent Pfeiffenberger, PharmD, MBA Chairman and Chief Executive Officer Greg Russotti, PhD Chief Technology and Manufacturing Officer Megan Bilson Chief People Officer Douglas Carr, CPA Head of Finance Principal Financial Officer Elizabeth Devlin Head of Development

Allo - Evasion
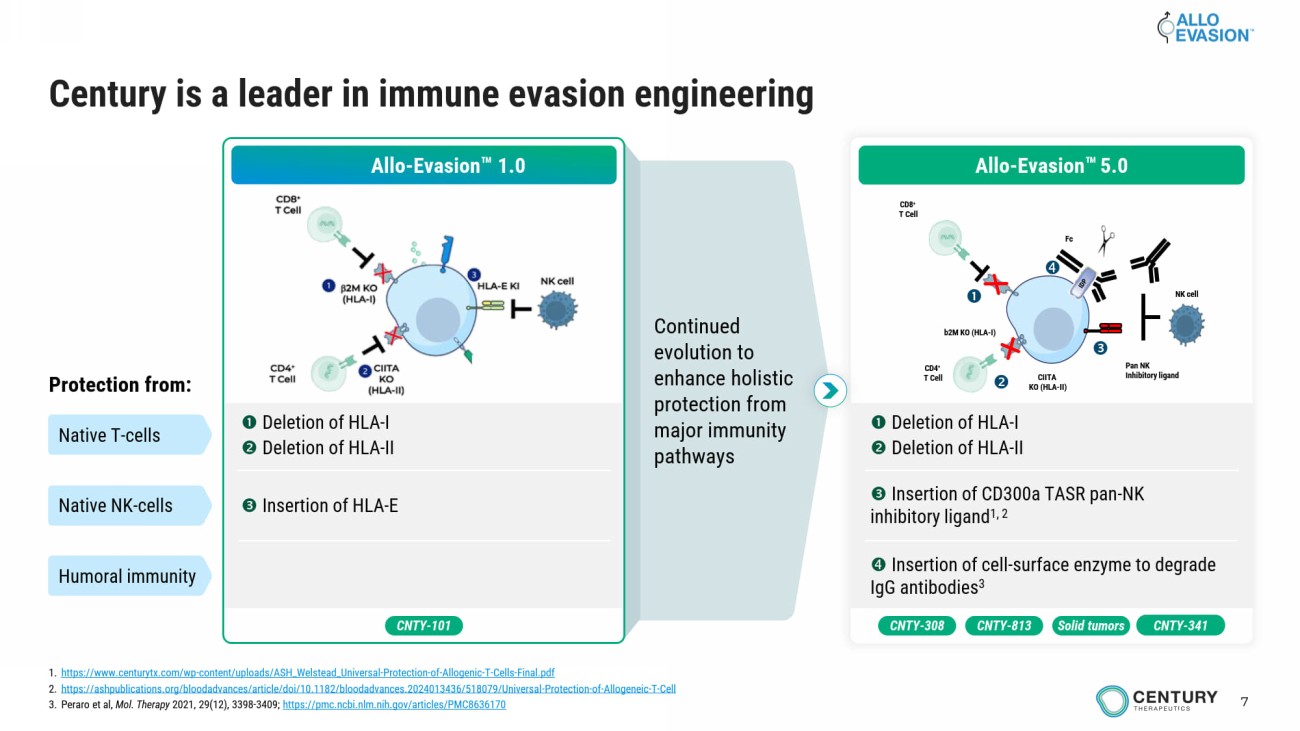
7 Continued evolution to enhance holistic protection from major immunity pathways Century is a leader in immune evasion engineering Allo - Evasion 1.0 Deletion of HLA - I Deletion of HLA - II Insertion of HLA - E Protection from: Native T - cells Native NK - cells Humoral immunity CNTY - 101 Deletion of HLA - I Deletion of HLA - II Insertion of CD300a TASR pan - NK inhibitory ligand 1, 2 Insertion of cell - surface enzyme to degrade IgG antibodies 3 b2M KO (HLA - I) CIITA KO (HLA - II) CD8 + T Cell CD4 + T Cell Pan NK Inhibitory ligand Fc NK cell Allo - Evasion 5.0 CNTY - 308 Solid tumors CNTY - 813 CNTY - 341 1. https://www.centurytx.com/wp - content/uploads/ASH_Welstead_Universal - Protection - of - Allogenic - T - Cells - Final.pdf 2. https://ashpublications.org/bloodadvances/article/doi/10.1182/bloodadvances.2024013436/518079/Universal - Protection - of - Allogeneic - T - Cell 3. Peraro et al, Mol. Therapy 2021, 29(12), 3398 - 3409; https://pmc.ncbi.nlm.nih.gov/articles/PMC8636170
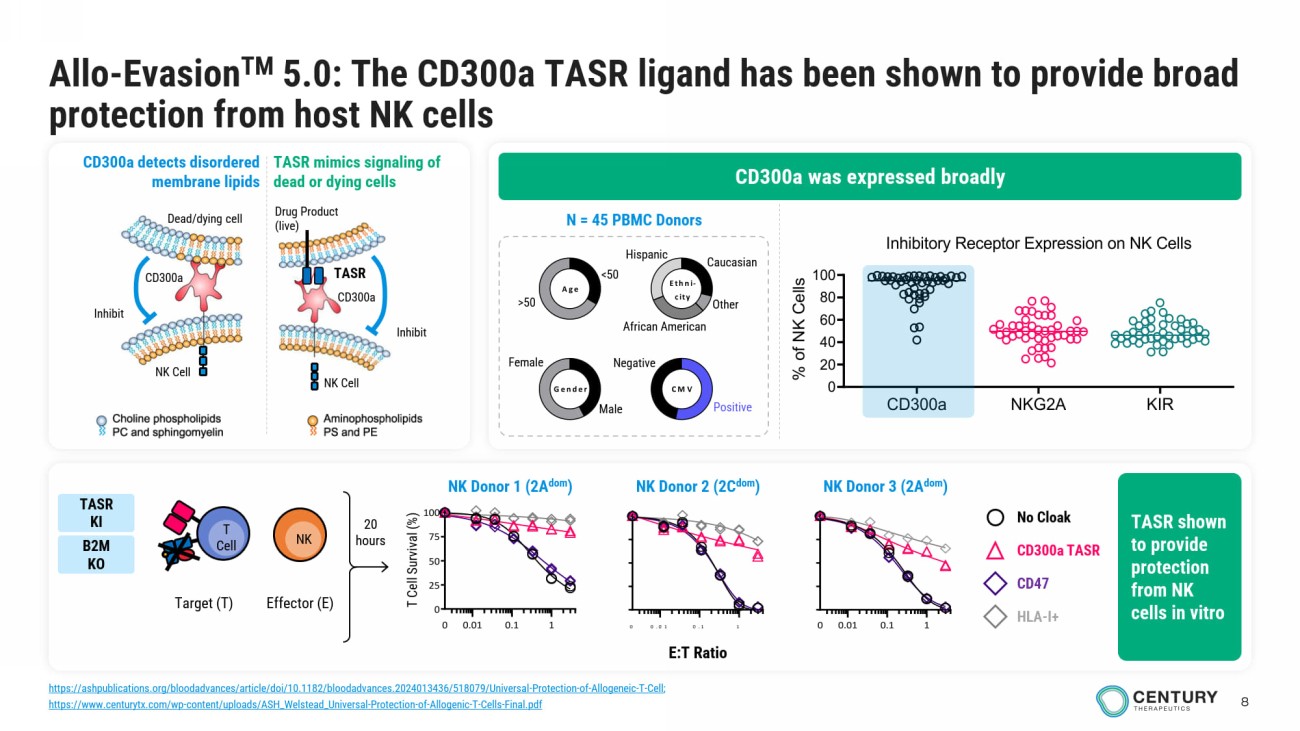
8 Allo - Evasion TM 5.0 : The CD300a TASR ligand has been shown to provide broad protection from host NK cells Male Female <50 >50 Gender Age Ethni- city CMV Caucasian Other African American Hispanic Negative Positive N = 45 PBMC Donors 0.01 0.1 10 0.01 0.1 1 0 25 50 75 100 0 E:T Ratio T Cell Survival (%) 0.01 0.1 10 No Cloak CD300a TASR CD47 HLA - I+ 20 hours Target (T) T Cell NK Effector (E) CD300a was expressed broadly CD300a detects disordered membrane lipids TASR mimics signaling of dead or dying cells Drug Product (live) Inhibit NK Cell TASR CD300a Dead/dying c ell NK Cell Inhibit CD300a TASR shown to provide protection from NK cells in vitro NK Donor 2 (2C dom ) NK Donor 1 (2A dom ) NK Donor 3 (2A dom ) TASR KI B2M KO CD300a NKG2A KIR 0 20 40 60 80 100 Inhibitory Receptor Expression on NK Cells (n = 46 donors) % o f N K C e l l s https://ashpublications.org/bloodadvances/article/doi/10.1182/bloodadvances.2024013436/518079/Universal - Protection - of - Allogeneic - T - Cell ; https://www.centurytx.com/wp - content/uploads/ASH_Welstead_Universal - Protection - of - Allogenic - T - Cells - Final.pdf
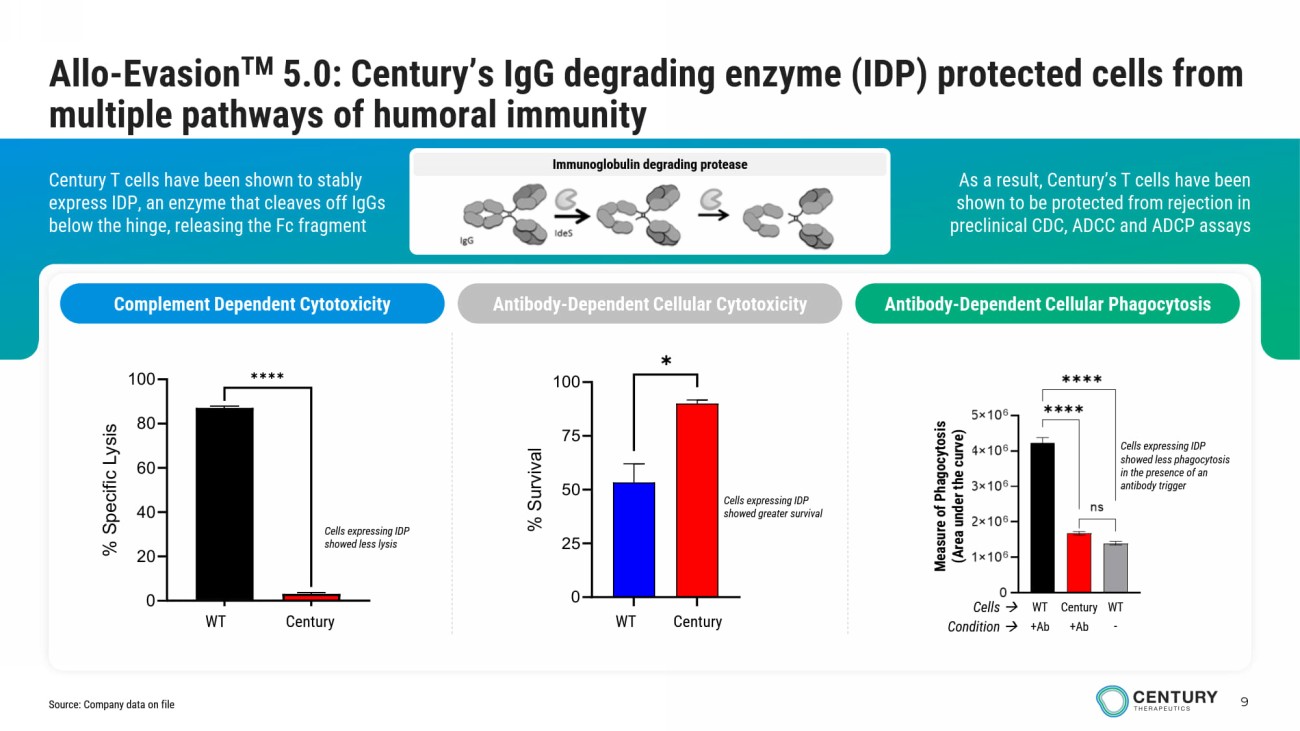
9 Immunoglobulin degrading protease As a result, Century’s T cells have been shown to be protected from rejection in preclinical CDC, ADCC and ADCP assays Century T cells have been shown to stably express IDP, an enzyme that cleaves off IgGs below the hinge, releasing the Fc fragment Allo - Evasion TM 5.0: Century’s IgG degrading enzyme (IDP) protected cells from multiple pathways of humoral immunity Complement Dependent Cytotoxicity Antibody - Dependent Cellular Cytotoxicity Antibody - Dependent Cellular Phagocytosis Measure of Phagocytosis (Area under the curve) Cells expressing IDP showed less phagocytosis in the presence of an antibody trigger Century WT WT Cells Condition +Ab +Ab - Cells expressing IDP showed greater survival GFP IdeStm 0 20 40 60 80 100 % S p e c i f i c L y s i s ✱✱✱✱ WT Century Cells expressing IDP showed less lysis WT Gen 2.3 0 25 50 75 100 % S u r v i v a l ✱ WT Century Source: Company data on file

Type 1 Diabetes Program
11 Century is uniquely positioned to deliver a successful T1D cell replacement therapy • T1D is a significant global market (9M pts WW) with high unmet medical need 1 • Beta islet cell replacement for T1D has clear, validated clinical Proof - of - Concept (POC) over 20+ years 2 • Transformational outcomes for patients BUT adoption is very limited due to 1) Cell source and 2) Chronic immunosuppression • Recent clinical data with stem cells demonstrate similar outcomes and potential solution to cell source/scale 3 1. Diabetes Res Clin Pract.2025 Jul: 225:112277.doi: 10.1016/j.diabres.2025.112277.Epub 2025 May 22 2. Approximately 1500 patients reported in https://www.citregistry.org/system/files/CITR%2012th%20Allograft%20Report_2025_Final.pdf 3. https://www.nejm.org/doi/10.1056/NEJMoa2506549?url_ver=Z39.88 - 2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed CNTY - 813 is an iPSC derived beta islet cell replacement therapy engineered with Allo - Evasion TM 5.0 CNTY - 813 has comprehensive pre - clinical data demonstrating unique potential for functional cure • Highly potent/pure iPSC derived beta islet cells functional glucose control • Allo - Evasion TM 5.0 engineering reduce/eliminate need for chronic immunosuppression • Highly scalable manufacturing (bioreactors) ensure broad patient access and supply Unique Company know - how and experience with iPSC development and supply (research, clinical, regulatory, manufacturing) CNTY - 813 Scalable Generation of Beta Islets with Allo - Evasion 5.0
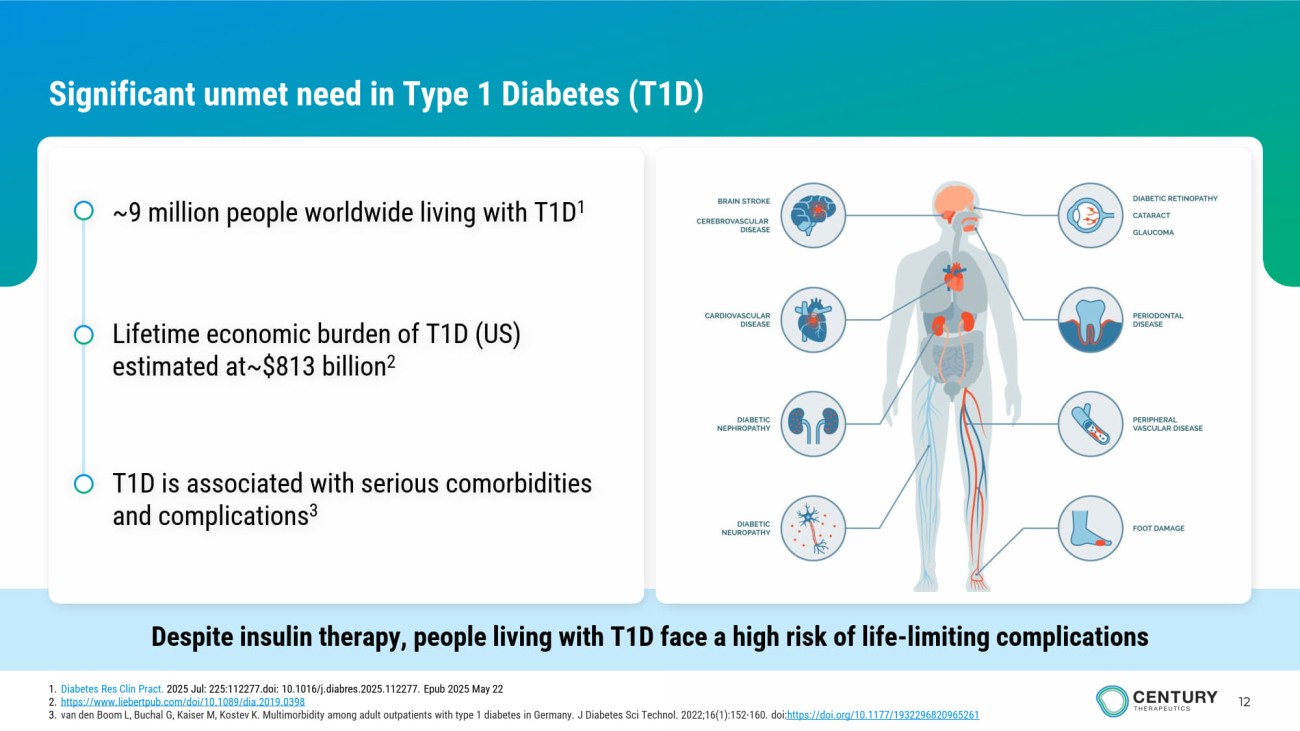
12 Significant unmet need in Type 1 Diabetes (T1D) Despite insulin therapy, people living with T1D face a high risk of life - limiting complications ~9 million people worldwide living with T1D 1 Lifetime economic burden of T1D (US) estimated at~$813 billion 2 T1D is associated with serious comorbidities and complications 3 1. Diabetes Res Clin Pract . 2025 Jul: 225:112277.doi: 10.1016/j.diabres.2025.112277. Epub 2025 May 22 2. https://www.liebertpub.com/doi/10.1089/dia.2019.0398 3. van den Boom L, Buchal G, Kaiser M, Kostev K. Multimorbidity among adult outpatients with type 1 diabetes in Germany. J Diabetes Sci Technol. 2022;16(1):152 - 160. doi: https ://doi.org/10.1177/1932296820965261
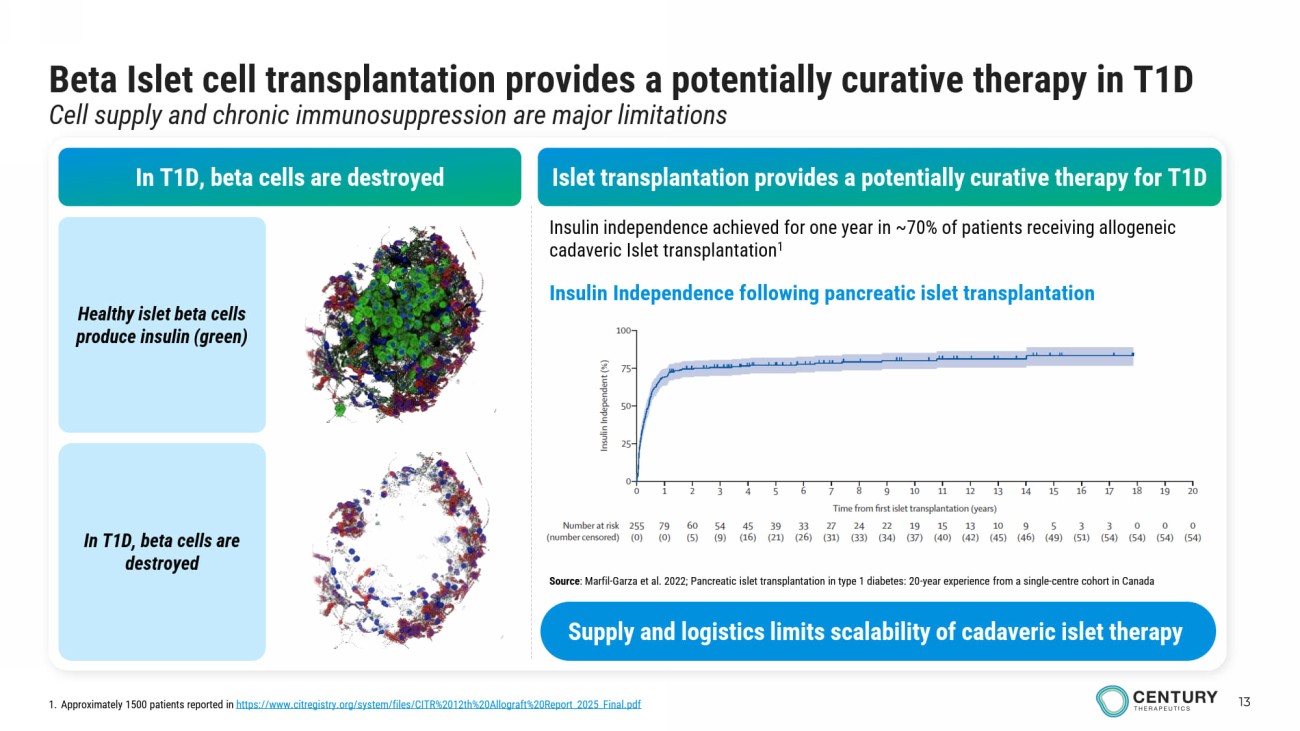
13 Beta Islet cell transplantation provides a potentially curative therapy in T1D Cell supply and chronic immunosuppression are major limitations In T1D, beta cells are destroyed Healthy islet beta cells produce insulin (green) In T1D, beta cells are destroyed Insulin independence achieved for one year in ~70% of patients receiving allogeneic cadaveric Islet transplantation 1 Islet transplantation provides a potentially curative therapy for T1D Insulin Independence following pancreatic islet transplantation 1. Approximately 1500 patients reported in https://www.citregistry.org/system/files/CITR%2012th%20Allograft%20Report_2025_Final.pdf Source : Marfil - Garza et al. 2022; Pancreatic islet transplantation in type 1 diabetes: 20 - year experience from a single - centre cohort in Canada Supply and logistics limits scalability of cadaveric islet therapy
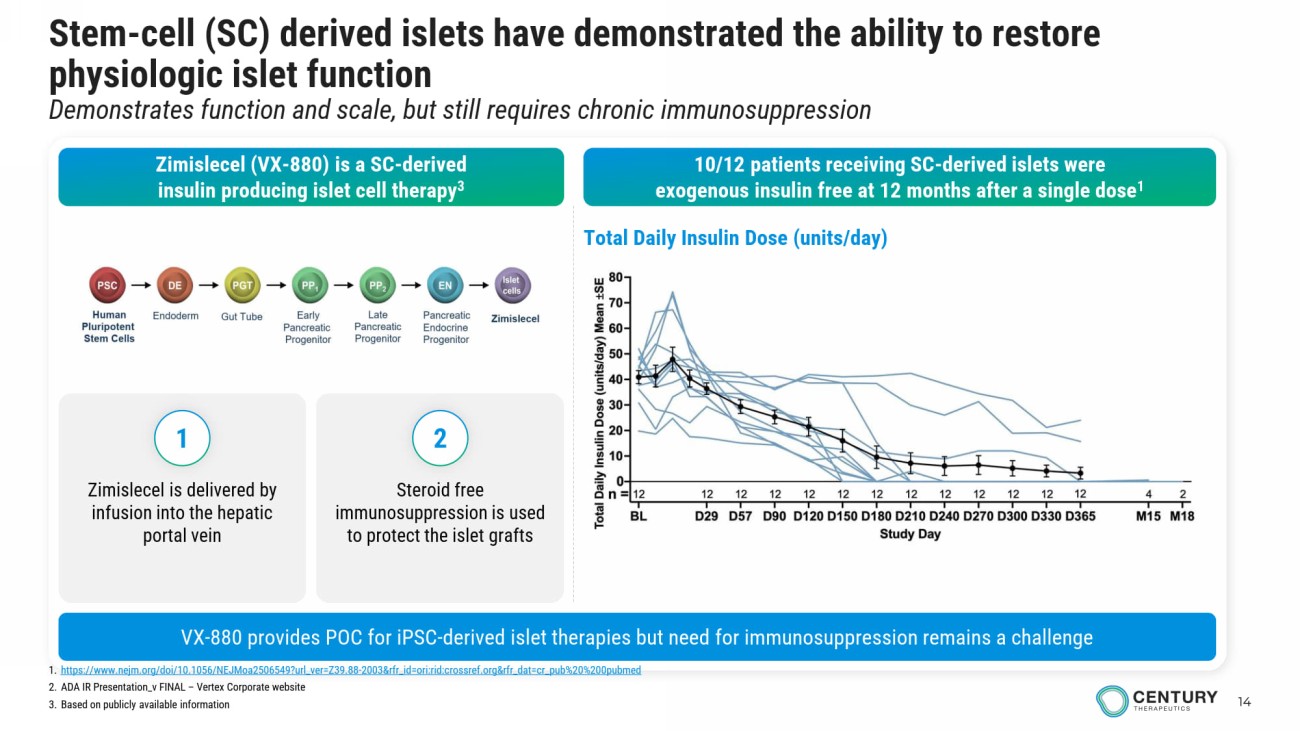
14 Stem - cell (SC) derived islets have demonstrated the ability to restore physiologic islet function Demonstrates function and scale, but still requires chronic immunosuppression Zimislecel (VX - 880) is a SC - derived insulin producing islet cell therapy 3 10/12 patients receiving SC - derived islets were exogenous insulin free at 12 months after a single dose 1 1. https://www.nejm.org/doi/10.1056/NEJMoa2506549?url_ver=Z39.88 - 2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed 2. ADA IR Presentation_v FINAL – Vertex Corporate website 3. Based on publicly available information VX - 880 provides POC for iPSC - derived islet therapies but need for immunosuppression remains a challenge Total Daily Insulin Dose (units/day) Zimislecel is delivered by infusion into the hepatic portal vein Steroid free immunosuppression is used to protect the islet grafts 1 2
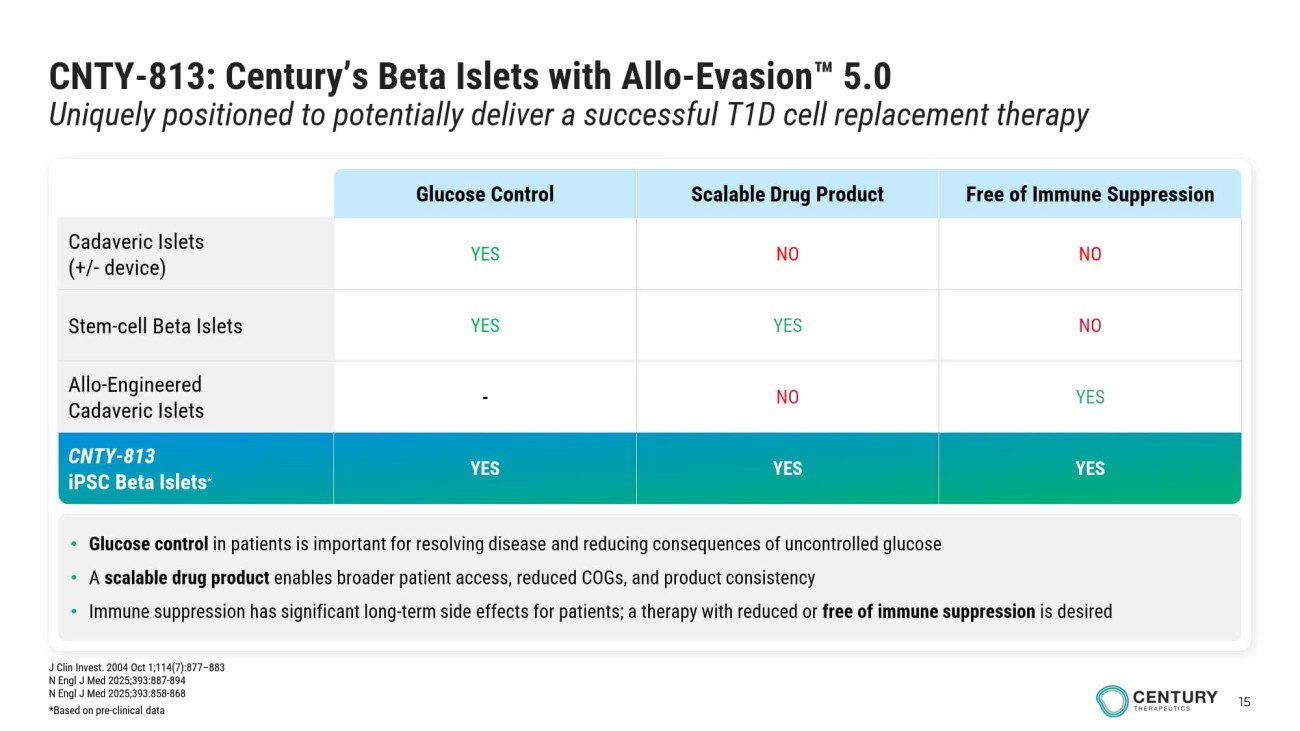
15 CNTY - 813: Century’s Beta Islets with Allo - Evasion 5.0 Uniquely positioned to potentially deliver a successful T1D cell replacement therapy • Glucose control in patients is important for resolving disease and reducing consequences of uncontrolled glucose • A scalable drug product enables broader patient access, reduced COGs, and product consistency • Immune suppression has significant long - term side effects for patients; a therapy with reduced or free of immune suppression is desired Free of Immune Suppression Scalable Drug Product Glucose Control NO NO YES Cadaveric Islets (+/ - device) NO YES YES Stem - cell Beta Islets YES NO - Allo - Engineered Cadaveric Islets YES YES YES CNTY - 813 iPSC Beta Islets * J Clin Invest. 2004 Oct 1;114(7):877 – 883 N Engl J Med 2025;393:887 - 894 N Engl J Med 2025;393:858 - 868 *Based on pre - clinical data
16 A fully scalable, bioreactor - enabled differentiation process yields mature, functional beta Islets from engineered iPSCs Clinical candidate selected with Century’s Allo - Evasion 5.0 to protect cells from immune rejection In vitro and in vivo data support potential to provide functional cure without systemic immunosuppression CNTY - 813 Scalable Generation of Beta Islets with Allo - Evasion 5.0
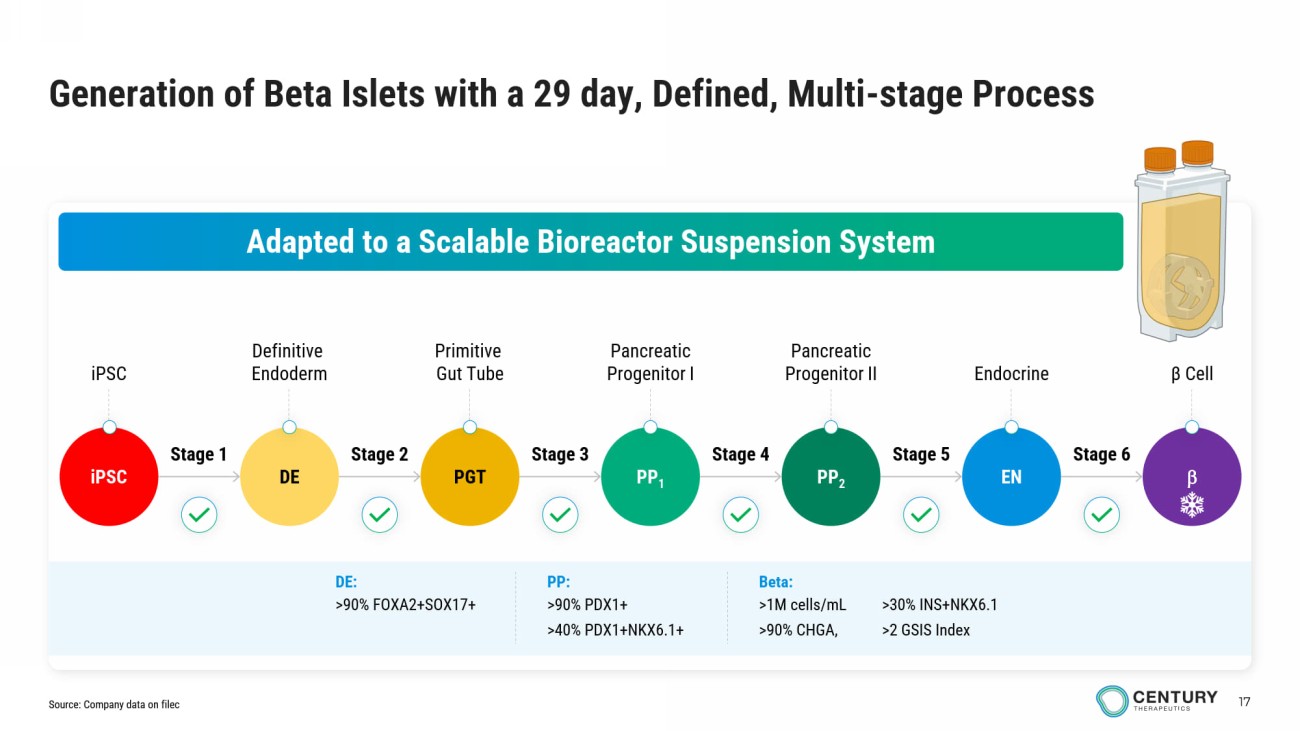
17 iPSC Definitive Endoderm Primitive Gut Tube Pancreatic Progenitor I Pancreatic Progenitor II β Cell Endocrine Generation of Beta Islets with a 29 day, Defined, Multi - stage Process Adapted to a Scalable Bioreactor Suspension System iPSC DE PGT PP 1 PP 2 EN Stage 1 Stage 2 Stage 4 Stage 5 Stage 6 Stage 3 Beta: >1M cells / mL >90% CHGA, >30% INS+NKX6.1 >2 GSIS Index PP: >90% PDX1+ >40% PDX1+NKX6.1+ DE: >90% FOXA2+SOX17+ Source: Company data on filec
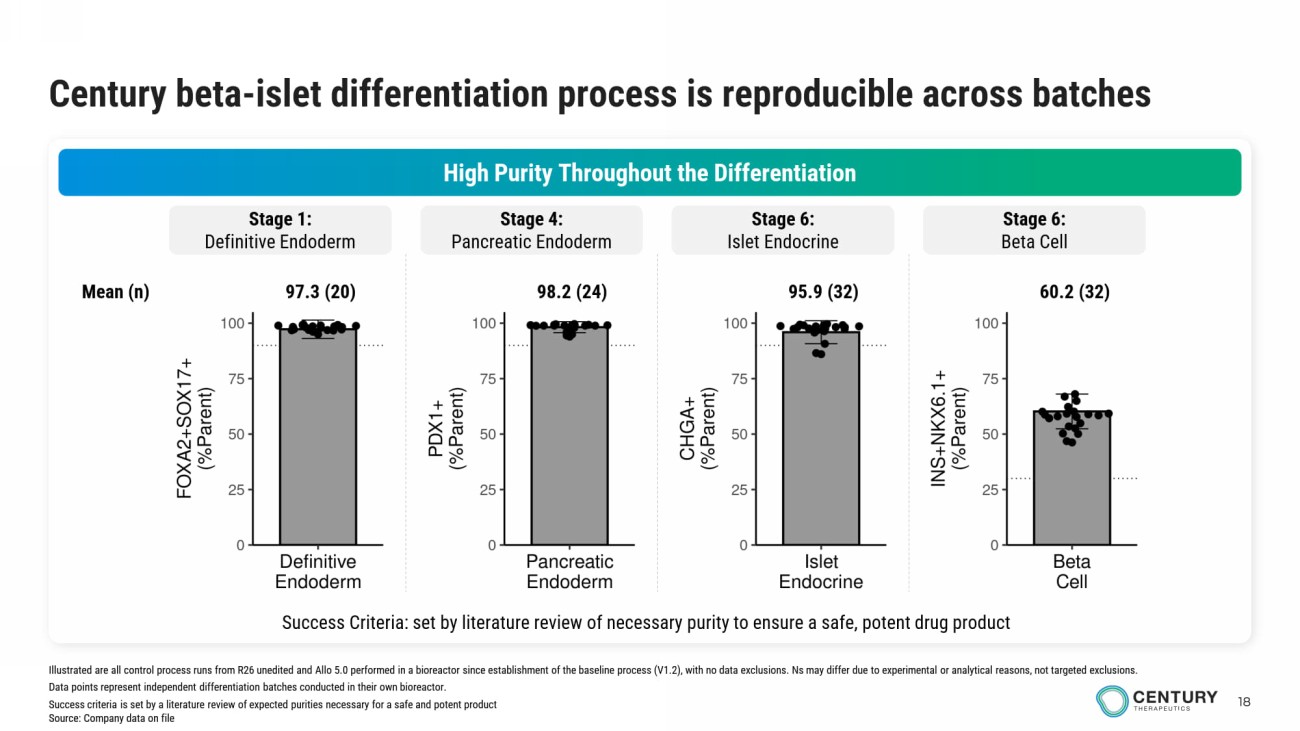
18 Century beta - islet differentiation process is reproducible across batches High Purity Throughout the Differentiation Stage 1: Definitive Endoderm Stage 4: Pancreatic Endoderm Stage 6: Islet Endocrine Stage 6: Beta Cell Success Criteria: set by literature review of necessary purity to ensure a safe, potent drug product 97.3 (20) 98.2 (24) 95.9 (32) 60.2 (32) Mean (n) Illustrated are all control process runs from R26 unedited and Allo 5.0 performed in a bioreactor since establishment of the baseline process (V1.2), with no data exclusions. Ns may differ due to experimental or analytical reasons, not targeted exclusions. Data points represent independent differentiation batches conducted in their own bioreactor. Success criteria is set by a literature review of expected purities necessary for a safe and potent product Source: Company data on file
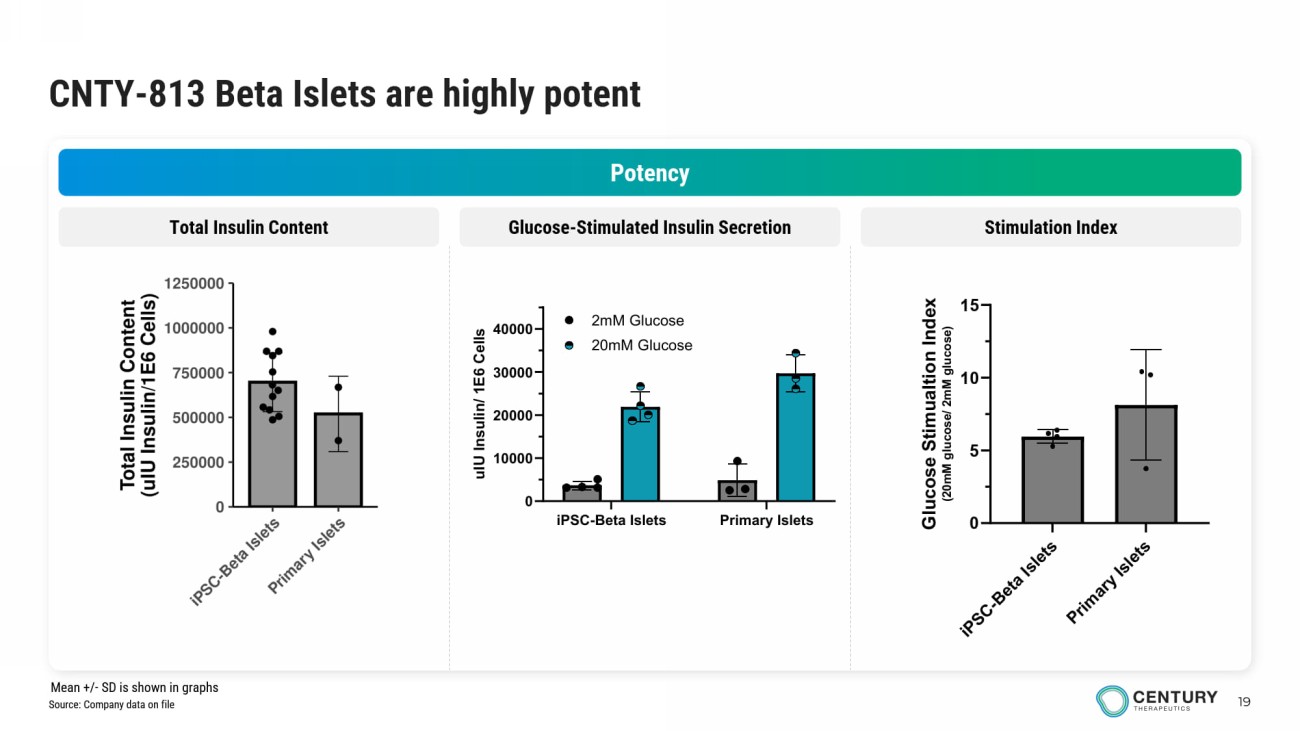
19 CNTY - 813 Beta Islets are highly potent Potency Glucose - Stimulated Insulin Secretion Stimulation Index Total Insulin Content iPSC-Beta Islets Primary Islets 0 10000 20000 30000 40000 u I U I n s u l i n / 1 E 6 C e l l s 2mM Glucose 20mM Glucose Source: Company data on file Mean +/ - SD is shown in graphs
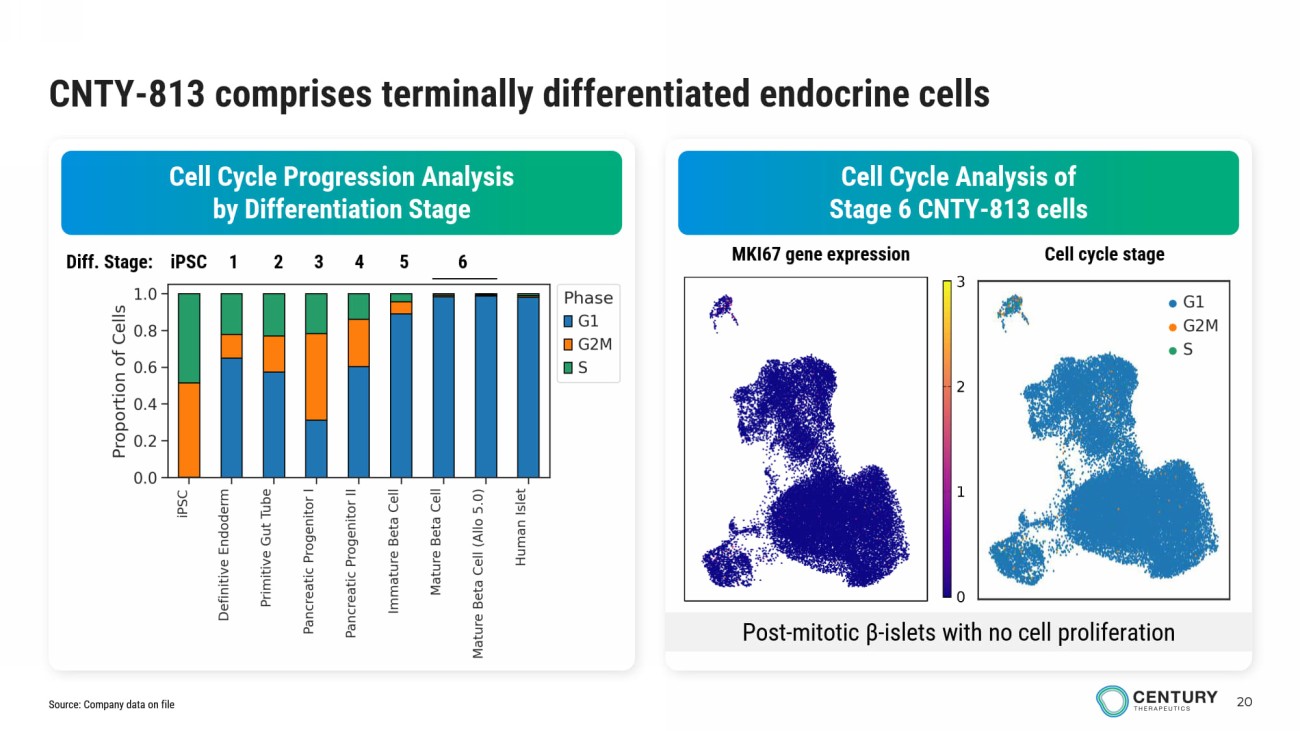
20 CNTY - 813 comprises terminally differentiated endocrine cells Cell Cycle Analysis of Stage 6 CNTY - 813 cells Cell Cycle Progression Analysis by Differentiation Stage Diff. Stage: iPSC 1 2 3 4 5 6 Post - mitotic β - islets with no cell proliferation MKI67 gene expression Cell cycle stage Source: Company data on file
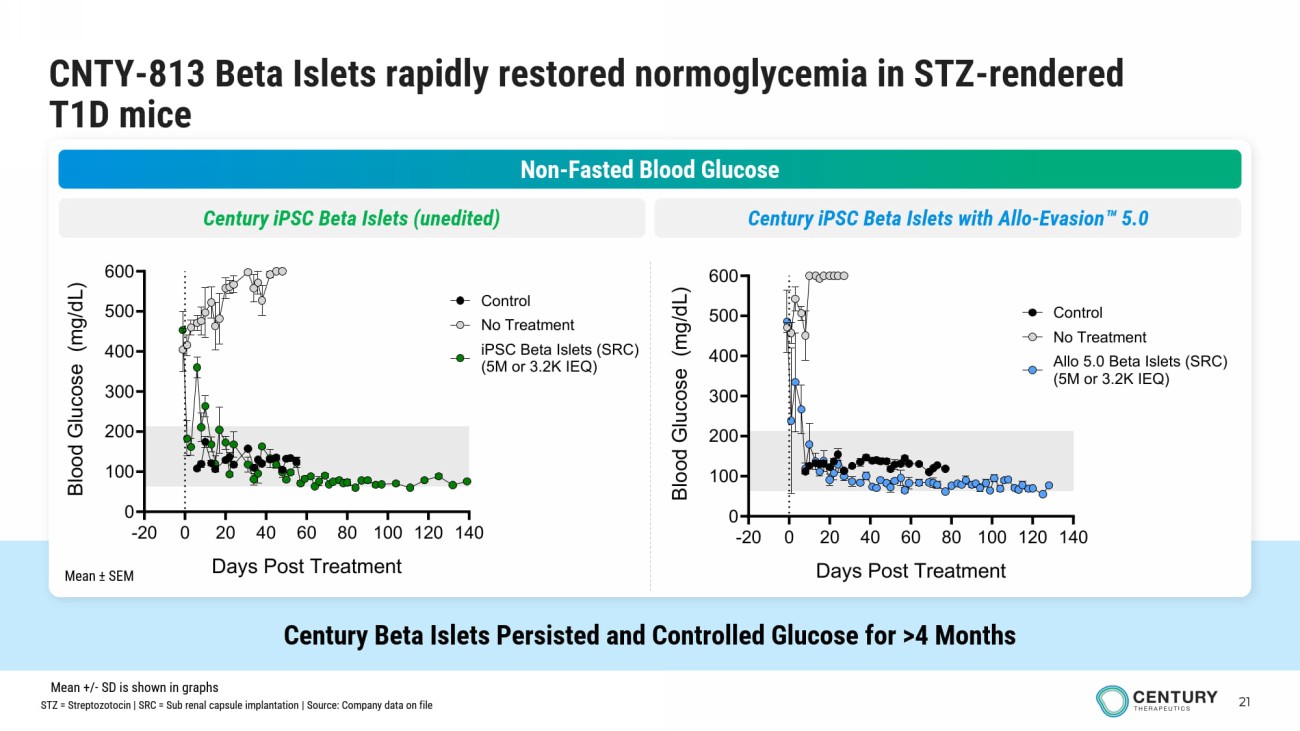
21 CNTY - 813 Beta Islets rapidly restored normoglycemia in STZ - rendered T1D mice Century Beta Islets Persisted and Controlled Glucose for >4 Months Non - Fasted Blood Glucose Century iPSC Beta Islets (unedited) Century iPSC Beta Islets with Allo - Evasion 5.0 Mean ± SEM -20 0 20 40 60 80 100 120 140 0 100 200 300 400 500 600 Days Post Treatment B l o o d G l u c o s e ( m g / d L ) No Treatment iPSC Beta Islets (SRC) (5M or 3.2K IEQ) Control -20 0 20 40 60 80 100 120 140 0 100 200 300 400 500 600 Days Post Treatment B l o o d G l u c o s e ( m g / d L ) No Treatment Allo 5.0 Beta Islets (SRC) (5M or 3.2K IEQ) Control STZ = Streptozotocin | SRC = Sub renal capsule implantation | Source: Company data on file Mean +/ - SD is shown in graphs
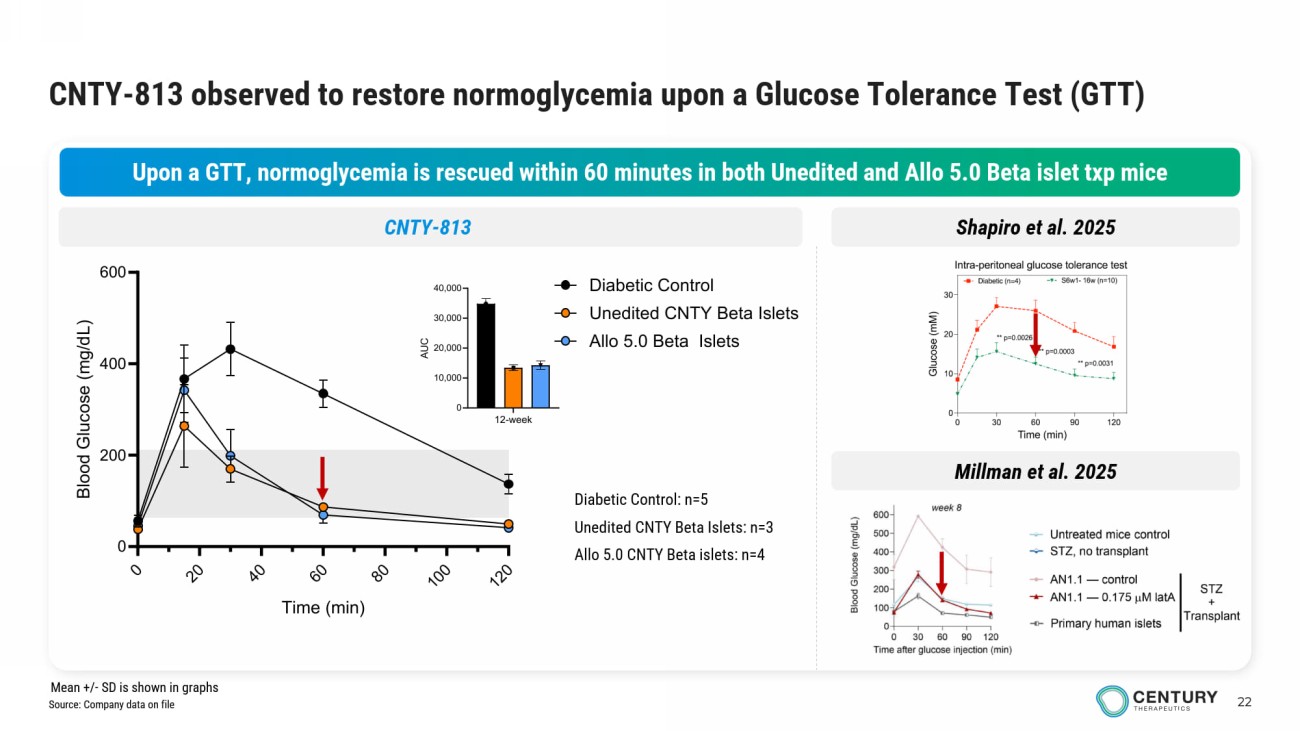
22 CNTY - 813 observed to restore normoglycemia upon a Glucose Tolerance Test (GTT) Upon a GTT, normoglycemia is rescued within 60 minutes in both Unedited and Allo 5.0 Beta islet txp mice 12-week 0 10,000 20,000 30,000 40,000 A U C Diabetic Control: n=5 Unedited CNTY Beta Islets: n=3 Allo 5.0 CNTY Beta islets: n=4 Shapiro et al. 2025 CNTY - 813 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 0 200 400 600 Time (min) B l o o d G l u c o s e ( m g / d L ) Diabetic Control Unedited CNTY Beta Islets Allo 5.0 Beta Islets Millman et al. 2025 Source: Company data on file Mean +/ - SD is shown in graphs
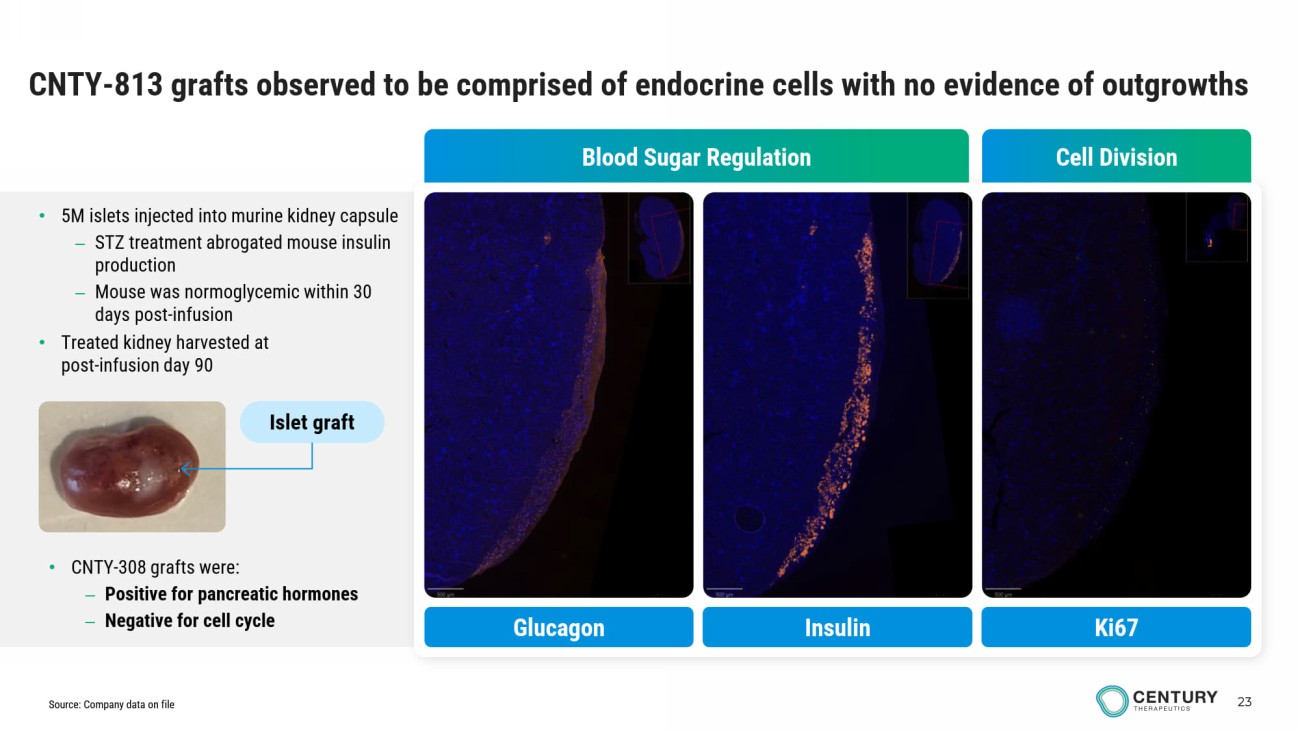
23 CNTY - 813 grafts observed to be comprised of endocrine cells with no evidence of outgrowths Cell Division Blood Sugar Regulation Insulin Glucagon Ki67 • 5M islets injected into murine kidney capsule – STZ treatment abrogated mouse insulin production – Mouse was normoglycemic within 30 days post - infusion • Treated kidney harvested at post - infusion day 90 • CNTY - 308 grafts were: – Positive for pancreatic hormones – Negative for cell cycle Islet graft Source: Company data on file
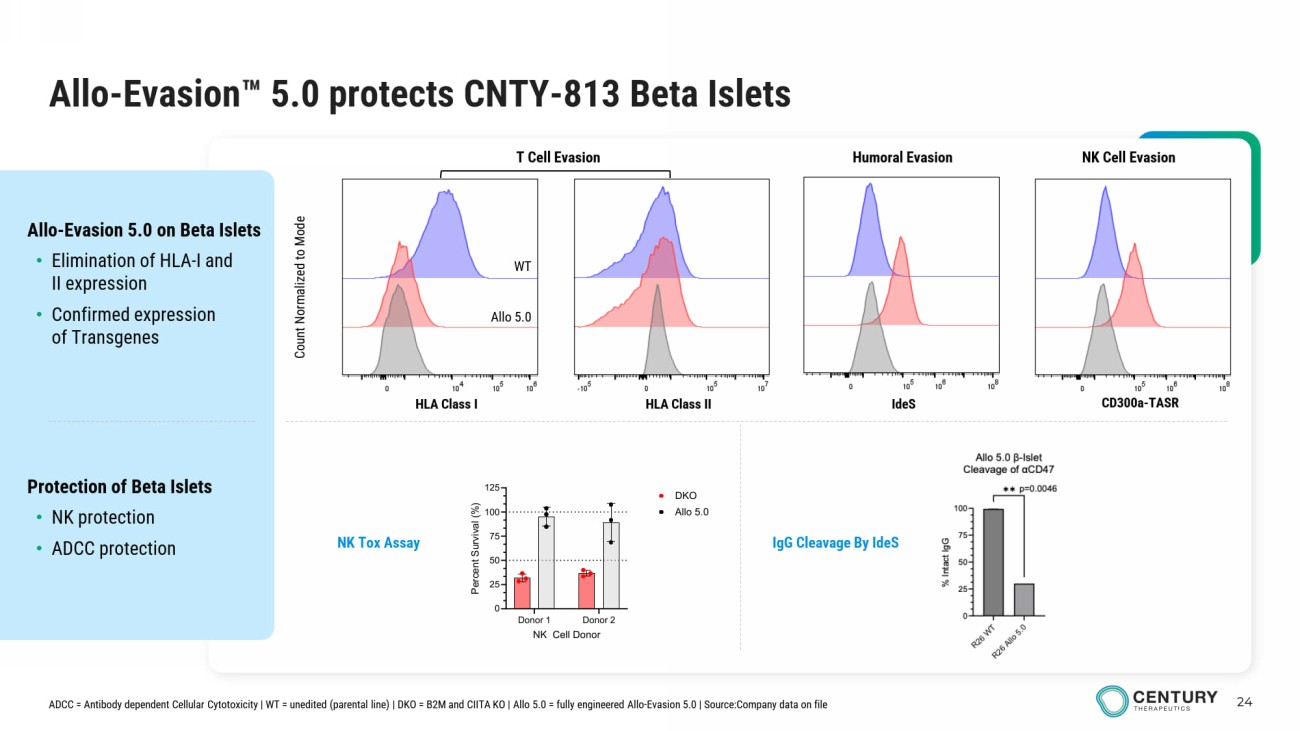
24 Allo - Evasion 5.0 protects CNTY - 813 Beta Islets Protection of Beta Islets • NK protection • ADCC protection Allo - Evasion 5.0 on Beta Islets • Elimination of HLA - I and II expression • Confirmed expression of Transgenes Count Normalized to Mode HLA Class I HLA Class II IdeS CD300a - TASR T Cell Evasion Humoral Evasion NK Cell Evasion NK Tox Assay WT Allo 5.0 ADCC = Antibody dependent Cellular Cytotoxicity | WT = unedited (parental line) | DKO = B2M and CIITA KO | Allo 5.0 = fully engineered Allo - Evasion 5.0 | Source:Company data on file IgG Cleavage By IdeS Donor 1 Donor 2 0 25 50 75 100 125 NK Cell Donor P e r c e n t S u r v i v a l ( % ) DKO Allo 5.0
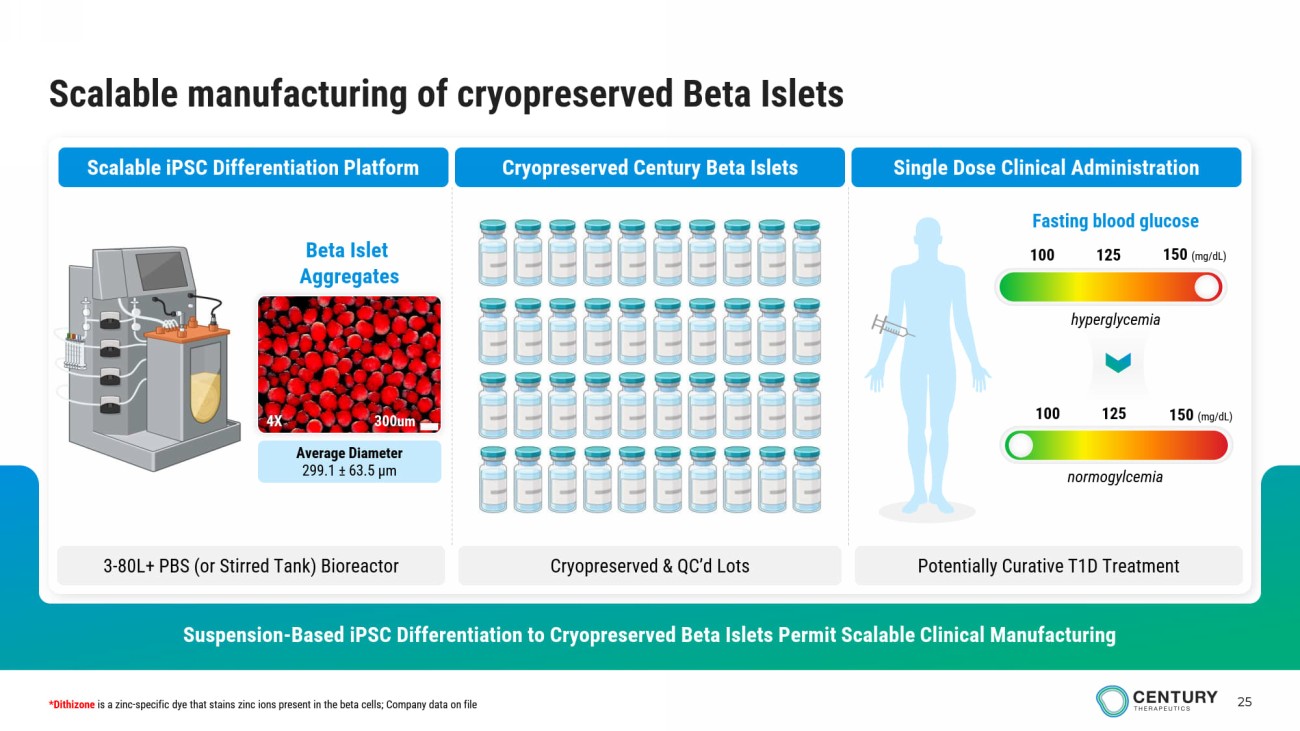
25 Scalable manufacturing of cryopreserved Beta Islets Suspension - Based iPSC Differentiation to Cryopreserved Beta Islets Permit Scalable Clinical Manufacturing Scalable iPSC Differentiation Platform Cryopreserved Century Beta Islets Single Dose Clinical Administration 3 - 80L+ PBS (or Stirred Tank ) Bioreactor Cryopreserved & QC’d Lots Potentially Curative T1D Treatment Average Diameter 299.1 ± 63.5 μm Beta Islet Aggregates 300um 4X 100 125 150 (mg/dL) Fasting blood glucose 100 125 150 (mg/dL) *Dithizone is a zinc - specific dye that stains zinc ions present in the beta cells; Company data on file hyperglycemia normogylcemia

Autoimmune Disease Programs
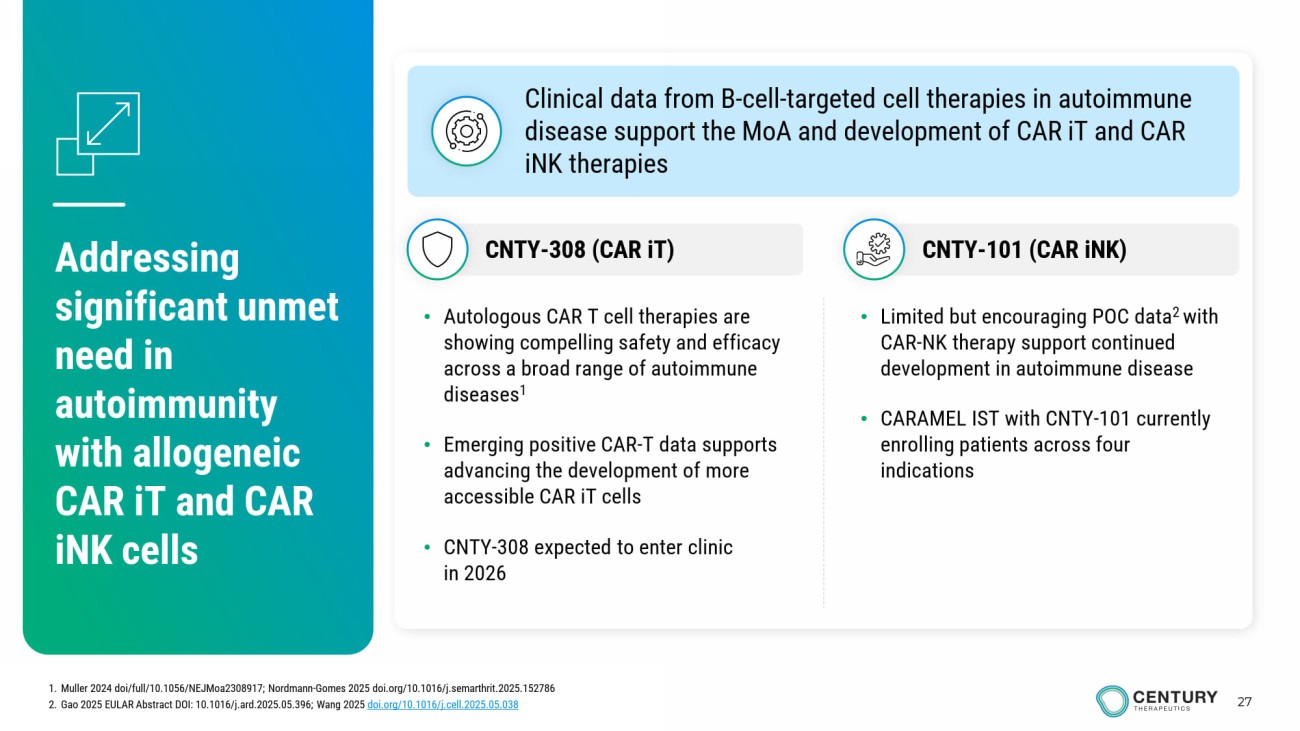
27 Addressing significant unmet need in autoimmunity with allogeneic CAR iT and CAR iNK cells • Limited but encouraging POC data 2 with CAR - NK therapy support continued development in autoimmune disease • CARAMEL IST with CNTY - 101 currently enrolling patients across four indications • Autologous CAR T cell therapies are showing compelling safety and efficacy across a broad range of autoimmune diseases 1 • Emerging positive CAR - T data supports advancing the development of more accessible CAR iT cells • CNTY - 308 expected to enter clinic in 2026 Clinical data from B - cell - targeted cell therapies in autoimmune disease support the MoA and development of CAR iT and CAR iNK therapies CNTY - 101 (CAR iNK ) CNTY - 308 (CAR iT ) 1. Muller 2024 doi /full/10.1056/NEJMoa2308917; Nordmann - Gomes 2025 doi.org/10.1016/j.semarthrit.2025.152786 2. Gao 2025 EULAR Abstract DOI: 10.1016/j.ard.2025.05.396; Wang 2025 doi.org/10.1016/j.cell.2025.05.038

CNTY - 308 CD4+/CD8+ αβ iT - cell with Allo - Evasion 5.0
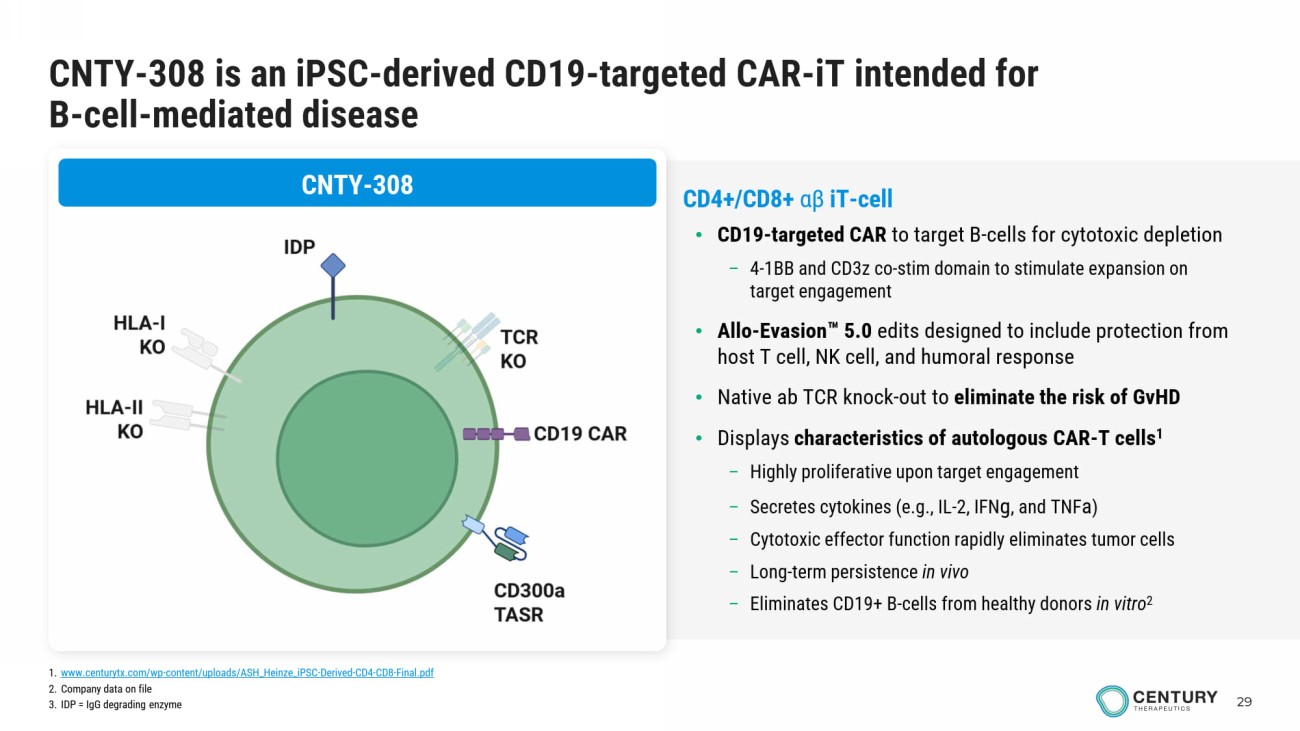
29 CNTY - 308 is an iPSC - derived CD19 - targeted CAR - iT intended for B - cell - mediated disease CNTY - 308 CD4+/CD8+ αβ iT - cell • CD19 - targeted CAR to target B - cells for cytotoxic depletion – 4 - 1BB and CD3z co - stim domain to stimulate expansion on target engagement • Allo - Evasion 5.0 edits designed to include protection from host T cell, NK cell, and humoral response • Native ab TCR knock - out to eliminate the risk of GvHD • Displays characteristics of autologous CAR - T cells 1 – Highly proliferative upon target engagement – Secretes cytokines (e.g., IL - 2, IFN g , and TNF a ) – Cytotoxic effector function rapidly eliminates tumor cells – Long - term persistence in vivo – Eliminates CD19+ B - cells from healthy donors in vitro 2 1. www.centurytx.com/wp - content/uploads/ASH_Heinze_iPSC - Derived - CD4 - CD8 - Final.pdf 2. Company data on file 3. IDP = IgG degrading enzyme
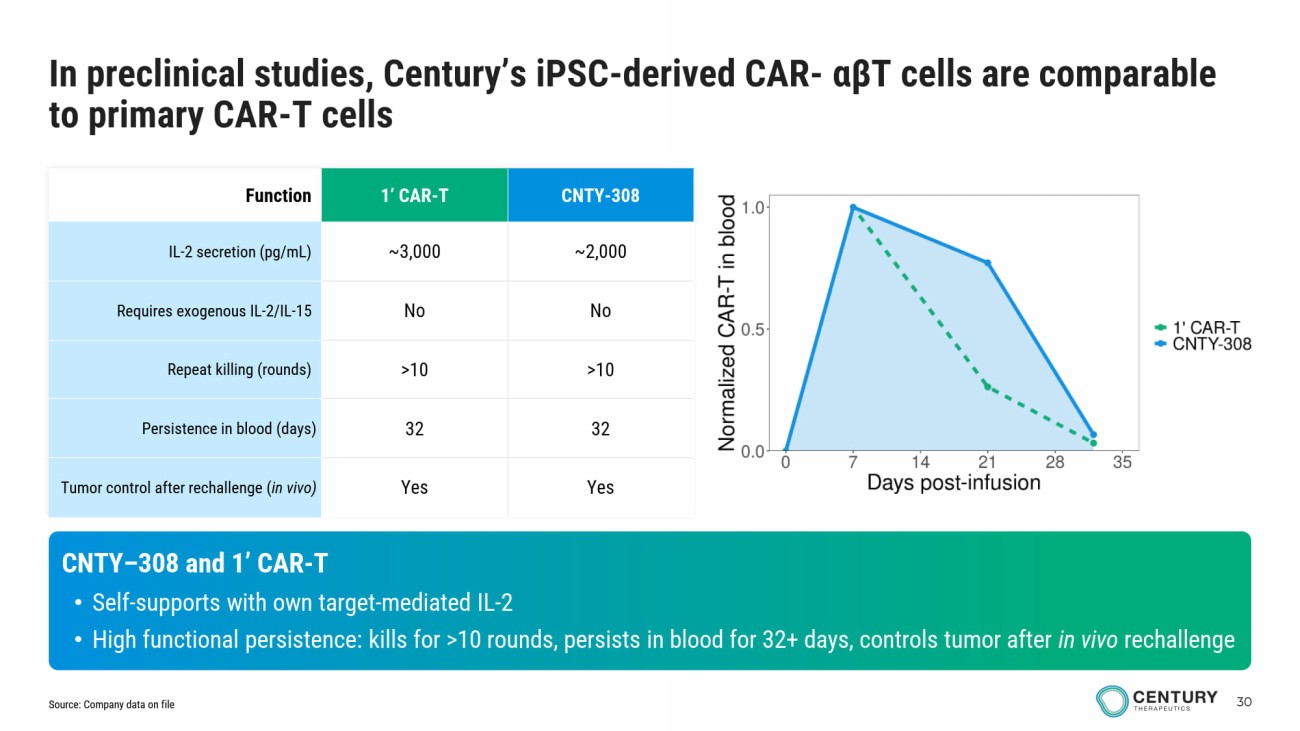
30 CNTY - 308 1’ CAR - T Function ~2,000 ~3,000 IL - 2 secretion (pg/mL) No No Requires exogenous IL - 2/IL - 15 >10 >10 Repeat killing (rounds) 32 32 Persistence in blood (days) Yes Yes Tumor control after rechallenge ( in vivo) CNTY – 308 and 1’ CAR - T • Self - supports with own target - mediated IL - 2 • High functional persistence: kills for >10 rounds, persists in blood for 32+ days, controls tumor after in vivo rechallenge In preclinical studies, Century’s iPSC - derived CAR - αβ T cells are comparable to primary CAR - T cells Source: Company data on file
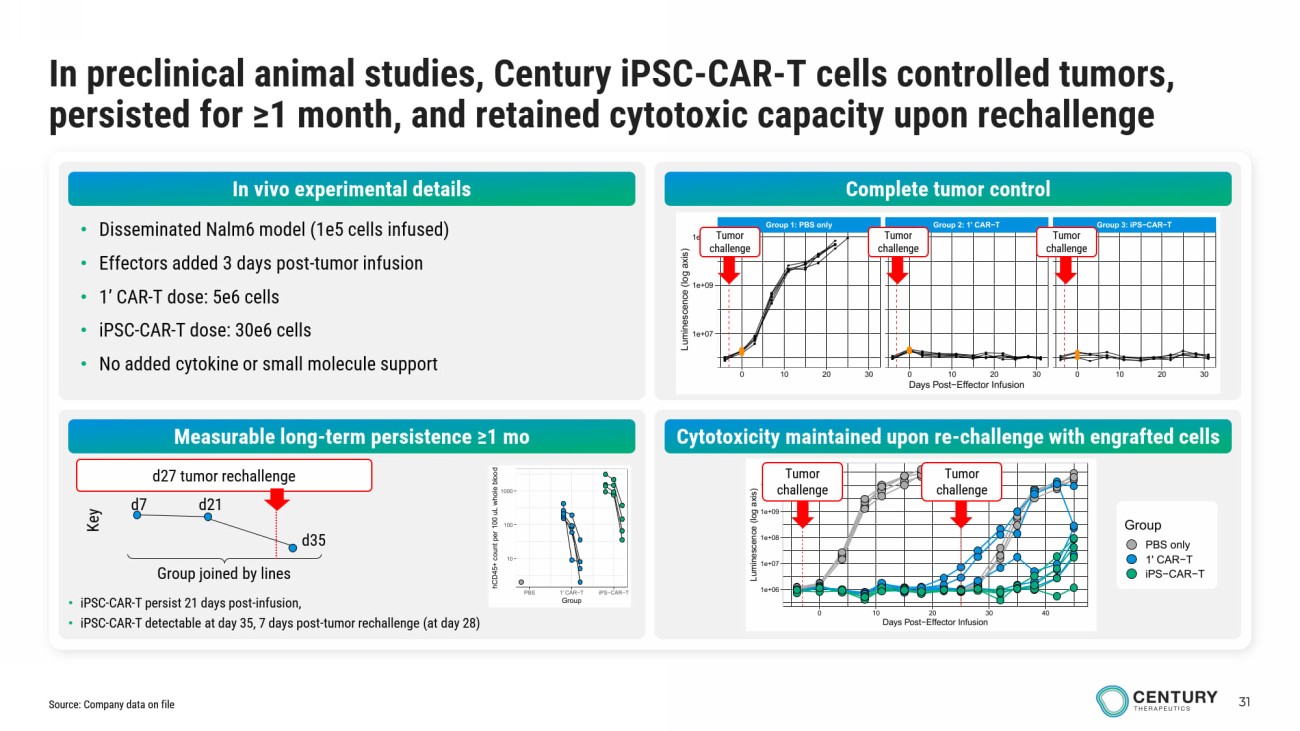
31 In preclinical animal studies, Century iPSC - CAR - T cells controlled tumors, persisted for ≥1 month, and retained cytotoxic capacity upon rechallenge • Disseminated Nalm6 model (1e5 cells infused) • Effectors added 3 days post - tumor infusion • 1’ CAR - T dose: 5e6 cells • iPSC - CAR - T dose: 30e6 cells • No added cytokine or small molecule support Complete tumor control Measurable long - term persistence ≥1 mo Cytotoxicity maintained upon re - challenge with engrafted cells Group 1: PBS only Group 2: 1' CAR−T Group 3: iPS−CAR−T 0 10 20 30 0 10 20 30 0 10 20 30 1e+07 1e+09 1e+11 Days Post−Effector Infusion L u m i n e s c e n c e ( l o g a x i s ) 1e+06 1e+07 1e+08 1e+09 1e+10 0 10 20 30 40 Days Post−Effector Infusion L u m i n e s c e n c e ( l o g a x i s ) Group PBS only 1' CAR−T iPS−CAR−T Tumor challenge Tumor challenge Tumor challenge Tumor challenge Tumor challenge 10 100 1000 PBS 1' CAR−T iPS−CAR−T Group h C D 4 5 + c o u n t p e r 1 0 0 u L w h o l e b l o o d • iPSC - CAR - T persist 21 days post - infusion, • iPSC - CAR - T detectable at day 35, 7 days post - tumor rechallenge (at day 28) Group joined by lines d7 d21 d35 Key d27 tumor rechallenge Source: Company data on file In vivo experimental details

CNTY - 101 CAR - iNK cell therapy with Allo - Evasion
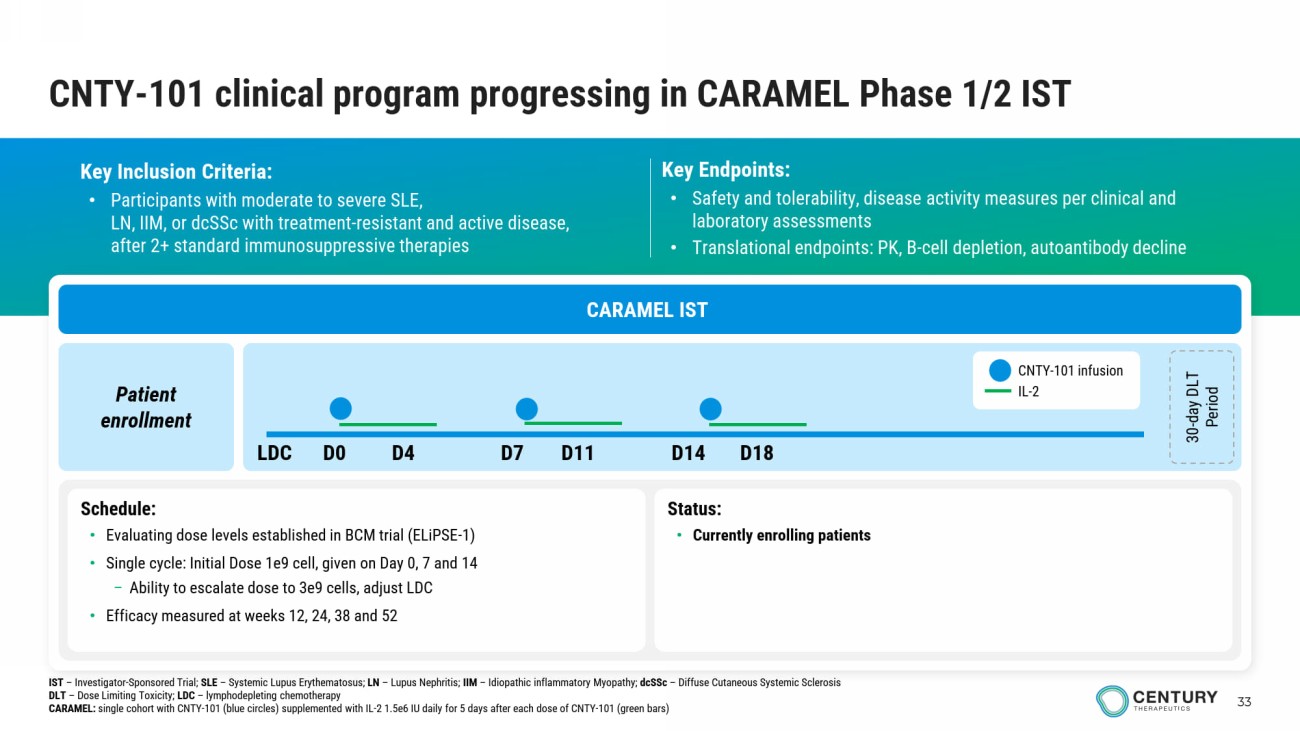
33 CNTY - 101 clinical program progressing in CARAMEL Phase 1/2 IST Key Inclusion Criteria: • Participants with moderate to severe SLE , LN, IIM, or dcSSc with treatment - resistant and active disease, after 2+ standard immunosuppressive therapies Key Endpoints: • Safety and tolerability, disease activity measures per clinical and laboratory assessments • Translational endpoints: PK, B - cell depletion, autoantibody decline CARAMEL IST Patient enrollment 30 - day DLT Period Schedule: • Evaluating dose levels established in BCM trial (ELiPSE - 1) • Single cycle: Initial Dose 1e9 cell , given on Day 0, 7 and 14 – Ability to escalate dose to 3e9 cells, adjust LDC • Efficacy measured at weeks 12, 24, 38 and 52 Status : • Currently enrolling patients CNTY - 101 infusion IL - 2 LDC D0 D4 D7 D11 D14 D18 IST – Investigator - Sponsored Trial; SLE – Systemic Lupus Erythematosus; LN – Lupus Nephritis; IIM – Idiopathic inflammatory Myopathy; dcSSc – Diffuse Cutaneous Systemic Sclerosis DLT – Dose Limiting Toxicity; LDC – lymphodepleting chemotherapy CARAMEL: single cohort with CNTY - 101 (blue circles) supplemented with IL - 2 1.5e6 IU daily for 5 days after each dose of CNTY - 101 (green b ars)
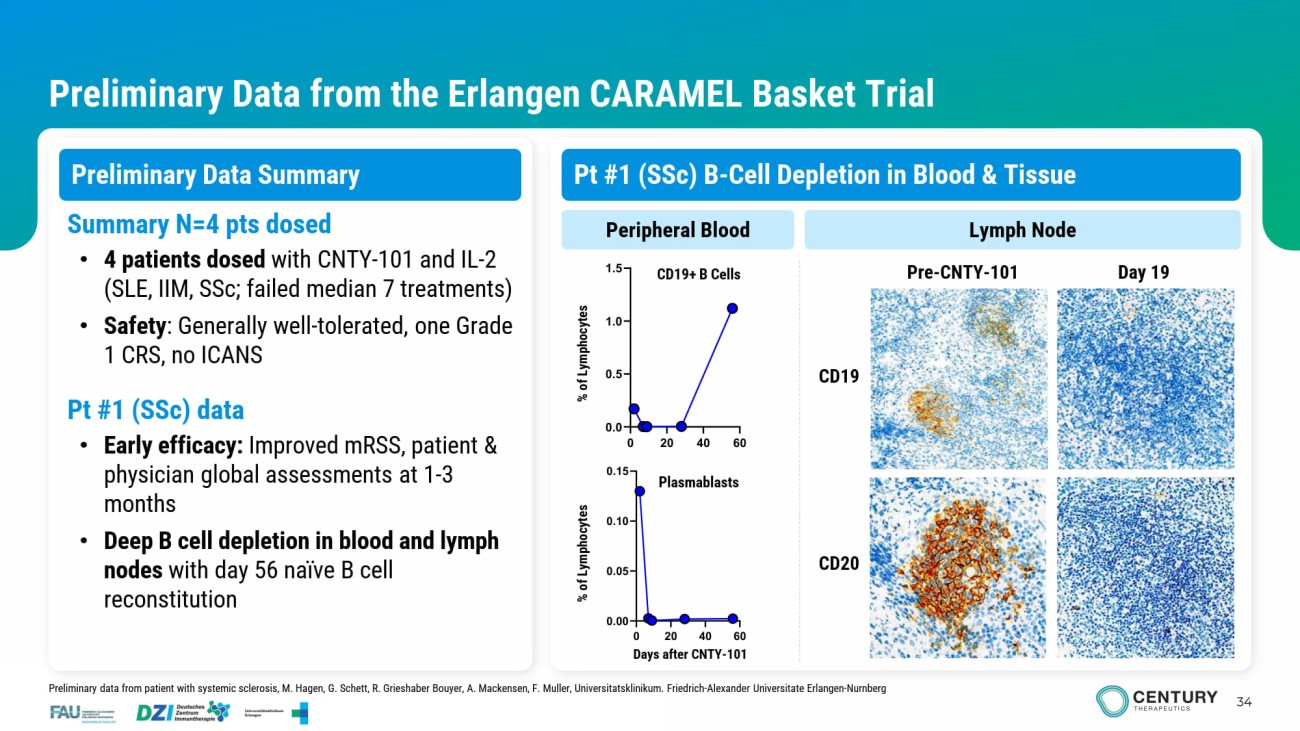
34 Preliminary Data from the Erlangen CARAMEL Basket Trial Preliminary Data Summary CD19 Pre - CNTY - 101 Day 19 Peripheral Blood Lymph Node Summary N=4 pts dosed • 4 patients dosed with CNTY - 101 and IL - 2 (SLE, IIM, SSc ; failed median 7 treatments) • Safety : Generally well - tolerated, one Grade 1 CRS, no ICANS Pt #1 ( SSc ) data • Early efficacy: Improved mRSS , patient & physician global assessments at 1 - 3 months • Deep B cell depletion in blood and lymph nodes with day 56 naïve B cell reconstitution Plasmablasts CD19+ B Cells CD20 Days after CNTY - 101 % of Lymphocytes % of Lymphocytes Preliminary data from patient with systemic sclerosis, M. Hagen, G. Schett , R. Grieshaber Bouyer, A. Mackensen, F. Muller, Universitatsklinikum . Friedrich - Alexander Universitate Erlangen - Nurnberg Pt #1 ( SSc ) B - Cell Depletion in Blood & Tissue

Corporate Summary

36 Established in - house manufacturing from development to launch Quality product at disruptive scale and cost of goods • Built - for - purpose 53,000 ft 2 cGMP facility • Produced and released clinical product for US and EU • Key leaders each with 1 – 2 decades of cell therapy manufacturing expertise, from leading commercial cell therapies • In - house team facilitates aligned priorities, learnings, faster product iteration for efficiency, speed, and product quality • Builds and protects proprietary know - how • Optionality with redundant sites (in - house, active CDMO) • Consistency: Control of manufacturing and single - donor master - cell - bank over product lifetime for batch - to - batch reproducibility • Increased cell fitness: Differentiated immune cells do not undergo excessive expansion cycles which often result in cell exhaustion • Product homogeneity: Clonal origin enables a well - characterized product • Potential to manufacture at antibody - like scale: Scalable platforms and optimized processes to maximize yield, reduce COGs, and meet demand Century platform and in - house manufacturing: Pathway to scalable, profitable cell therapy
Cell Foundry and Allo - Evasion Technology High Impact Programs Focused on Execution Century Therapeutics Today © 2025 37 Cell foundry generates fully functional cells at scale • Key developmental insights allow directed differentiation of cells that function like primary cells, such as beta Islet cells and CD4 + /CD8 + T cells Leaders in immune evasion engineering • Allo - Evasion allows cells to co - exist with a patient’s immune system • Enables enhanced persistence and potential for re - dosing of therapy Advancing lead iPSC derived cell therapies with Allo - Evasion 5.0 toward the clinic • CNTY - 813 in IND - enabling studies with potential for functional cure in Type 1 Diabetes • CNTY - 308 in IND - enabling studies for treatment of B - cell - mediated diseases • Patient enrollment ongoing for CNTY - 101 in Phase 1/2 CARAMEL IST in autoimmune disease Cash runway extended beyond planned key clinical milestones • CNTY - 813 IND submission planned for 2026 with initial clinical data expected 2027 • CNTY - 308 T cell program expected to enter the clinic in 2026 • CNTY - 101 preliminary clinical data from Phase 1/2 CARAMEL IST expected in 2026

www.centurytx.com