

Transforming the Future of Chronic Kidney Disease Treatment Corporate Presentation January 2026 Preserving Kidney Function in Patients at High Risk of Kidney Failure

This presentation includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. ProKidney’s actual results may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s beliefs that its Phase 3 REGEN-006 (PROACT 1) trial could be sufficient to support a potential BLA submission and full regulatory approval, eGFR slope can be used as a surrogate endpoint on an accelerated approval pathway for rilparencel, expectations with respect to financial results and expected cash runway, including the Company’s expectation that current cash will support operating plans into mid-2027, future performance, development and commercialization of products, if approved, the potential benefits and impact of the Company’s products, if approved, potential regulatory approvals, the size and potential growth of current or future markets for the Company’s products, if approved, the advancement of the Company’s development programs into and through the clinic and the expected timing for reporting data, the making of regulatory filings or achieving other milestones related to the Company’s product candidates, and the advancement and funding of the Company’s developmental programs, generally. Most of these factors are outside of the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: disruptions to our business or that may otherwise materially harm our results of operations or financial condition as a result of our recent domestication to the United States; the inability to maintain the listing of the Company’s Class A common stock on Nasdaq; the inability of the Company’s Class A common stock to remain included in various indices and the potential negative impact on the trading price of the Class A common stock if excluded from such indices; the inability to implement business plans, forecasts, and other expectations or identify and realize additional opportunities, which may be affected by, among other things, competition and the ability of the Company to grow and manage growth profitably and retain its key employees; the risk of downturns and a changing regulatory landscape in the highly competitive biotechnology industry; the risk that results of the Company’s clinical trials may not support approval; the risk that the FDA could require additional studies before approving the Company’s drug candidates; the inability of the Company to raise financing in the future; the inability of the Company to obtain and maintain regulatory clearance or approval for its products, and any related restrictions and limitations of any cleared or approved product; the inability of the Company to identify, in-license or acquire additional technology; the inability of Company to compete with other companies currently marketing or engaged in the biologics market and in the area of treatment of kidney diseases; the size and growth potential of the markets for the Company’s products, if approved, and its ability to serve those markets, either alone or in partnership with others; the Company’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; the Company’s financial performance; the Company’s intellectual property rights; uncertainties inherent in cell therapy research and development, including the actual time it takes to initiate and complete clinical studies and the timing and content of decisions made by regulatory authorities; the fact that interim results from our clinical programs may not be indicative of future results; the impact of geo-political conflict on the Company’s business; and other risks and uncertainties included under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. The Company cautions readers that the foregoing list of factors is not exclusive and cautions readers not to place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based. Forward-looking Statements

Advanced CKD Patients Want More Time More time before dialysis More time for life’s moments More time and flexibility with the people who matter most Time for Hope

Rilparencel: Buying Meaningful Time NOVEL autologous cell therapy made from a patient’s own kidney cells CLINICAL DATA shows kidney function stabilization in multiple Phase 2 studies WELL-TOLERATED with no preconditioning or immunosuppression required PHASE 3 STUDY is ongoing with pivotal topline results expected in Q2 2027 For CKD Patients

ProKidney 2025 was a Pivotal Year for ProKidney Aligned with FDA on an accelerated approval pathway for rilparencel using eGFR slope as the surrogate endpoint Presented positive Phase 2 REGEN-007 data as a late-breaking clinical trial at American Society of Nephrology (ASN) Kidney Week 2025 Generated significant enrollment momentum in the Phase 3 PROACT 1 study; pivotal Phase 3 readout of surrogate endpoint anticipated in Q2 2027 Initiated expansion ProKidney’s in-house manufacturing footprint in two adjacent, company-owned manufacturing facilities totaling 180,000 SF in Winston-Salem, NC

ProKidney 2026 will be A Year of Highly Focused Execution Complete enrollment for the accelerated approval efficacy analysis by mid-2026 Present results from ongoing mechanism of action studies at key medical & scientific conferences Maintain dialogue with FDA under RMAT designation to prepare for BLA submission in 2H 2027 Ongoing expansion of in-house manufacturing facility capacity and prep for commercial launch
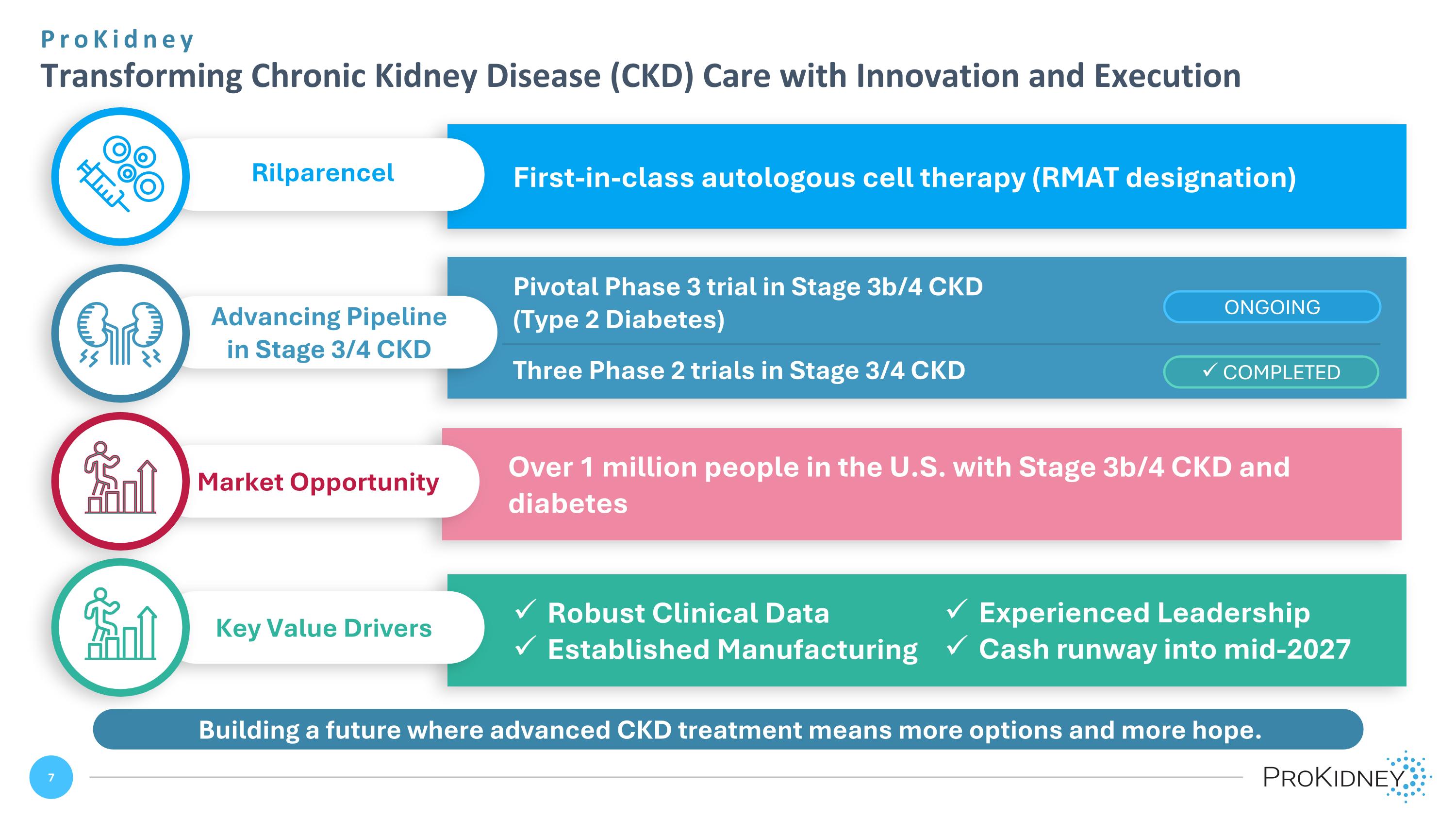
ProKidney Transforming Chronic Kidney Disease (CKD) Care with Innovation and Execution Building a future where advanced CKD treatment means more options and more hope. First-in-class autologous cell therapy (RMAT designation) Rilparencel Pivotal Phase 3 trial in Stage 3b/4 CKD (Type 2 Diabetes) Three Phase 2 trials in Stage 3/4 CKD Advancing Pipeline in Stage 3/4 CKD Robust Clinical Data Established Manufacturing Key Value Drivers Experienced Leadership Cash runway into mid-2027 COMPLETED ONGOING Over 1 million people in the U.S. with Stage 3b/4 CKD and diabetes Market Opportunity
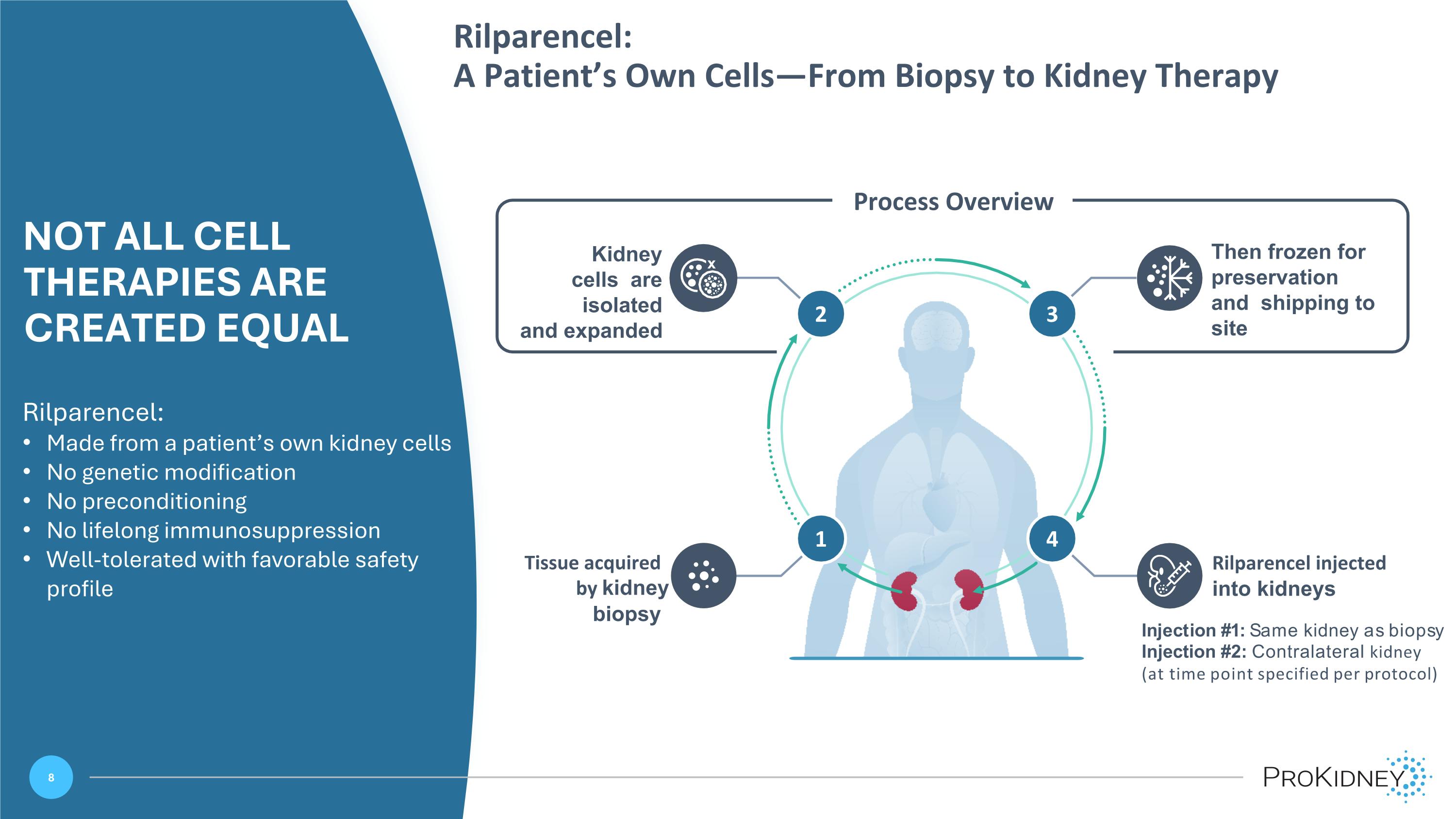
Rilparencel: A Patient’s Own Cells—From Biopsy to Kidney Therapy NOT ALL CELL THERAPIES ARE CREATED EQUAL Rilparencel: Made from a patient’s own kidney cells No genetic modification No preconditioning No lifelong immunosuppression Well-tolerated with favorable safety profile Then frozen for preservation and shipping to site Kidney cells are isolated and expanded Tissue acquired by kidney biopsy Rilparencel injected into kidneys Injection #1: Same kidney as biopsy Injection #2: Contralateral kidney (at time point specified per protocol) Process Overview 4 2 3 1 2 3 4 x

Purchased two adjacent buildings in Winston-Salem, NC in November 2024, totaling approximately 180,000 square feet Currently supports Phase 3 PROACT 1 clinical manufacturing, with capacity to accommodate future commercial supply Ongoing capital investment in manufacturing infrastructure and systems to support process readiness for BLA submission and commercial launch Facilities support office, research, and cGMP manufacturing operations for ProKidney’s autologous cell therapy platform Continued Expansion of In-House Manufacturing Facilities Purpose-built, scalable manufacturing infrastructure supporting Phase 3 study execution and longer-term commercialization

CHRONIC KIDNEY DISEASE Significant Unmet Need and Limitations with Standard-of-Care
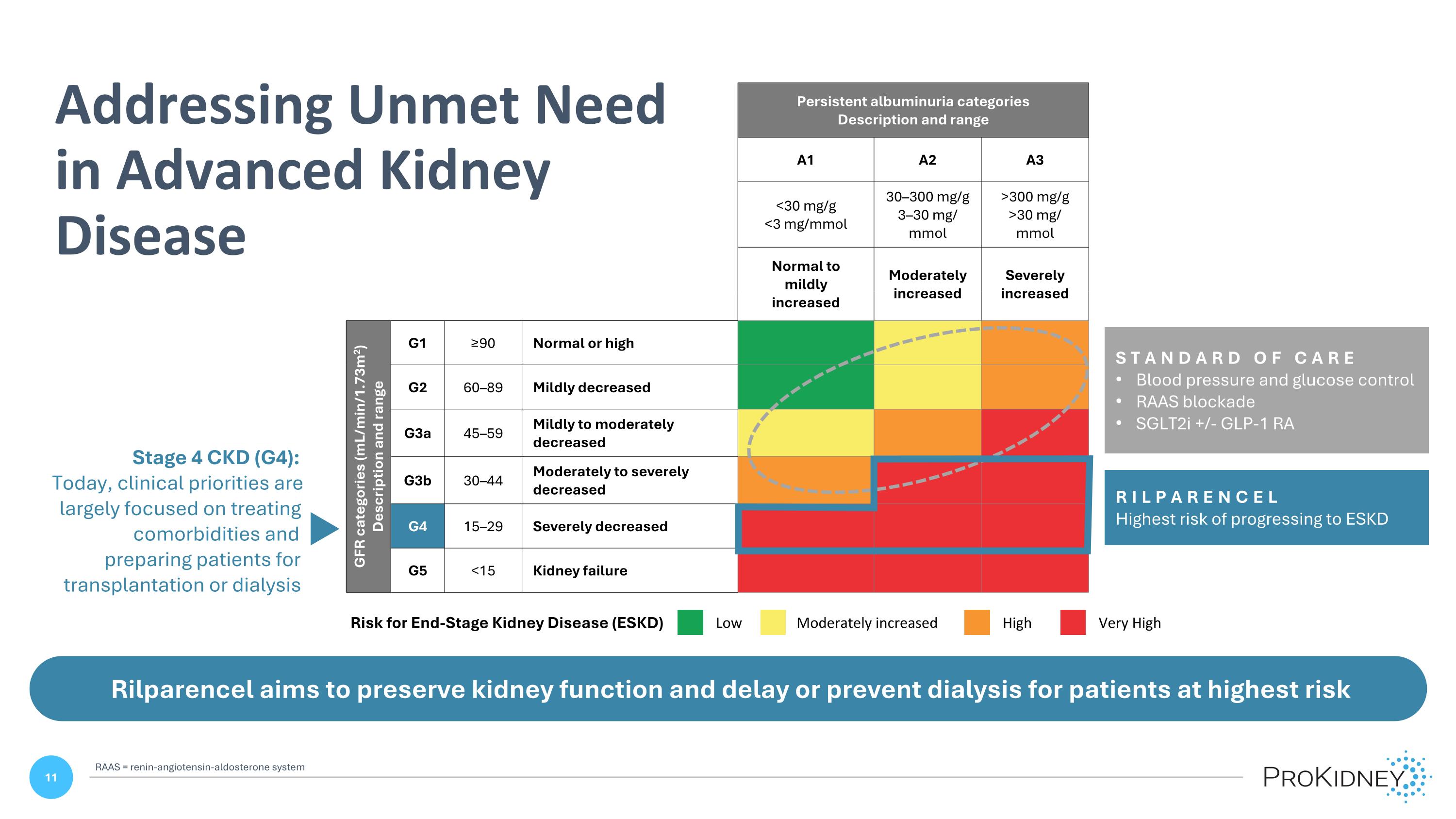
Persistent albuminuria categories Description and range A1 A2 A3 <30 mg/g <3 mg/mmol 30–300 mg/g 3–30 mg/ mmol >300 mg/g >30 mg/ mmol Normal to mildly increased Moderately increased Severely increased GFR categories (mL/min/1.73m2) Description and range G1 ≥90 Normal or high G2 60–89 Mildly decreased G3a 45–59 Mildly to moderately decreased G3b 30–44 Moderately to severely decreased G4 15–29 Severely decreased G5 <15 Kidney failure STANDARD OF CARE Blood pressure and glucose control RAAS blockade SGLT2i +/- GLP-1 RA RILPARENCEL Highest risk of progressing to ESKD Low Moderately increased High Very High Risk for End-Stage Kidney Disease (ESKD) Rilparencel aims to preserve kidney function and delay or prevent dialysis for patients at highest risk Stage 4 CKD (G4): Today, clinical priorities are largely focused on treating comorbidities and preparing patients for transplantation or dialysis Addressing Unmet Need in Advanced Kidney Disease RAAS = renin-angiotensin-aldosterone system
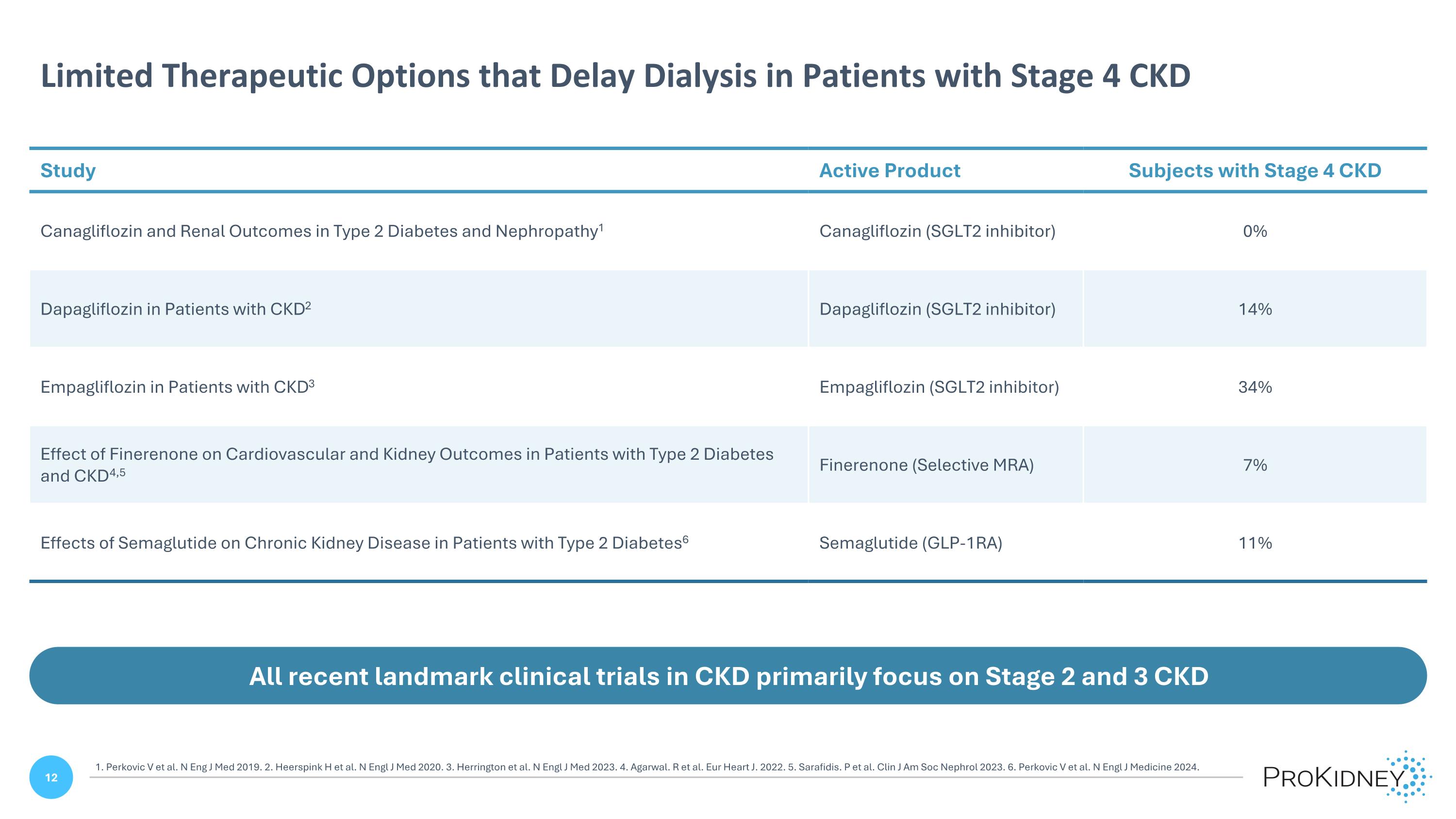
Limited Therapeutic Options that Delay Dialysis in Patients with Stage 4 CKD All recent landmark clinical trials in CKD primarily focus on Stage 2 and 3 CKD Study Active Product Subjects with Stage 4 CKD Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy1 Canagliflozin (SGLT2 inhibitor) 0% Dapagliflozin in Patients with CKD2 Dapagliflozin (SGLT2 inhibitor) 14% Empagliflozin in Patients with CKD3 Empagliflozin (SGLT2 inhibitor) 34% Effect of Finerenone on Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes and CKD4,5 Finerenone (Selective MRA) 7% Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes6 Semaglutide (GLP-1RA) 11% 1. Perkovic V et al. N Eng J Med 2019. 2. Heerspink H et al. N Engl J Med 2020. 3. Herrington et al. N Engl J Med 2023. 4. Agarwal. R et al. Eur Heart J. 2022. 5. Sarafidis. P et al. Clin J Am Soc Nephrol 2023. 6. Perkovic V et al. N Engl J Medicine 2024.
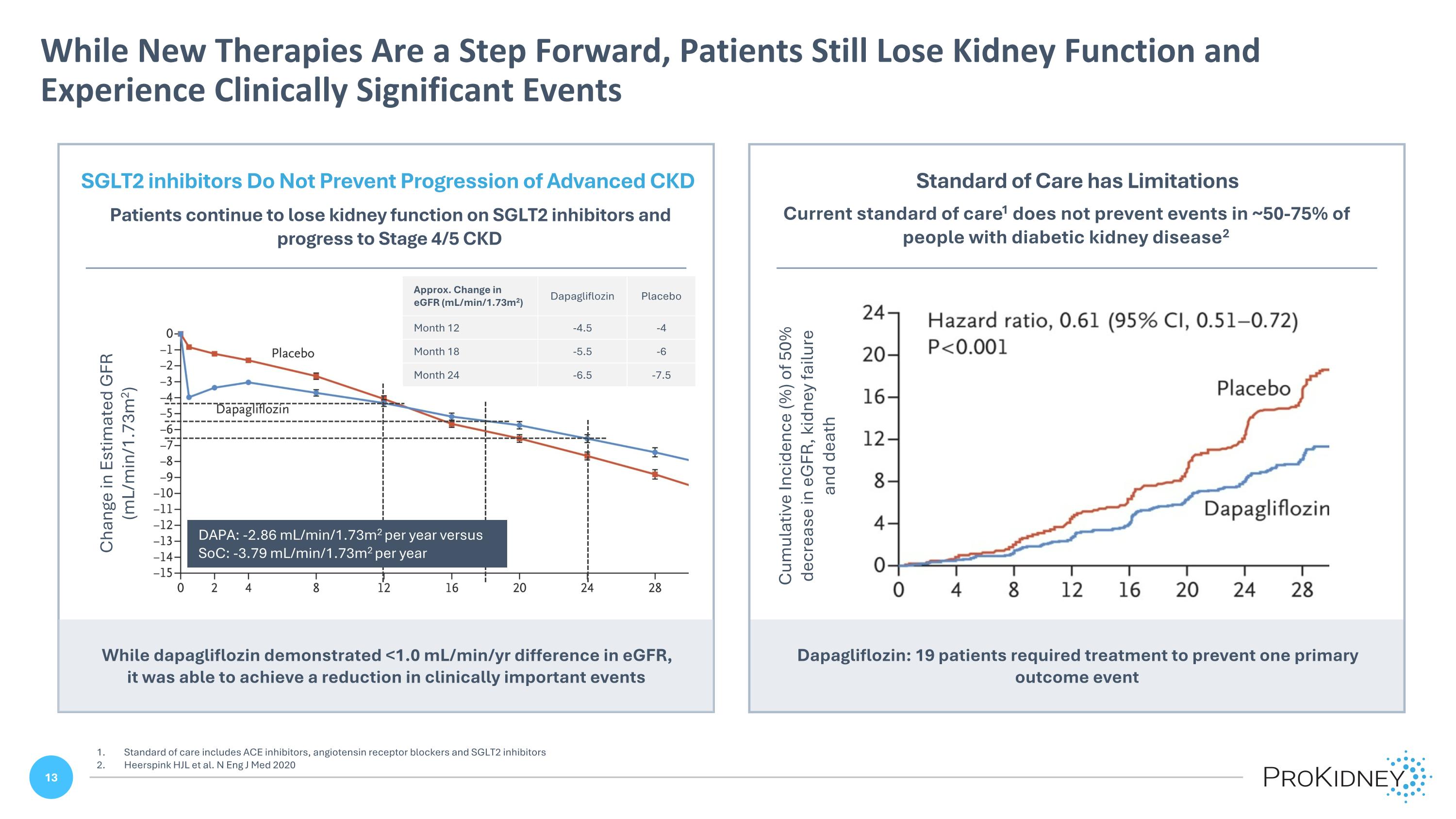
Dapagliflozin: 19 patients required treatment to prevent one primary outcome event While New Therapies Are a Step Forward, Patients Still Lose Kidney Function and Experience Clinically Significant Events Standard of care includes ACE inhibitors, angiotensin receptor blockers and SGLT2 inhibitors Heerspink HJL et al. N Eng J Med 2020 Current standard of care1 does not prevent events in ~50-75% of people with diabetic kidney disease2 Standard of Care has Limitations Cumulative Incidence (%) of 50% decrease in eGFR, kidney failure and death SGLT2 inhibitors Do Not Prevent Progression of Advanced CKD Patients continue to lose kidney function on SGLT2 inhibitors and progress to Stage 4/5 CKD Change in Estimated GFR (mL/min/1.73m2) While dapagliflozin demonstrated <1.0 mL/min/yr difference in eGFR, it was able to achieve a reduction in clinically important events Approx. Change in eGFR (mL/min/1.73m2) Dapagliflozin Placebo Month 12 -4.5 -4 Month 18 -5.5 -6 Month 24 -6.5 -7.5 DAPA: -2.86 mL/min/1.73m2 per year versus SoC: -3.79 mL/min/1.73m2 per year SGLT2 inhibitors Do Not Prevent Progression of Advanced CKD Patients continue to lose kidney function on SGLT2 inhibitors and progress to Stage 4/5 CKD Patients continue to lose kidney function on SGLT2 inhibitors and progress to Stage 4/5 CKD Approx. Change in eGFR (mL/min/1.73m2) Month 12 Month 18 Dapagliflozin -4.5 -5.5 -6.5 Placebo -4 -6 -7.5 Placebo dapagliflozin DAPA: -2.86 mL/min/1.73m2 per year versus SoC: -3.79 mL/min/1.73m2 per year Change in Estimated GFR (mL/min/1.73m2) 0 -1 -2 -3 -4 -5 -6 -7 -8 -9 -10 -11 -12 -13 -14 0 2 4 8 12 16 20 24 28 While dapagliflozin demonstrated <1.0 mL/min/yr difference in eGFR, it was able to achieve a reduction in clinically important events Standard of Care has Limitations Current standard of care1 does not prevent events in ~50-75% of people with diabetic kidney disease2 Cumulative Incidence (%) of 50% decrease in eGFR, kidney failure and death Dapagliflozin: 19 patients required treatment to prevent one primary outcome event 24 20 16 12 8 4 0 0 4 8 12 16 20 24 28 Hazard ratio, 0.61 (95% C1-0.72) P<0.001 Placebo Dapagliflozin
RILPARENCEL RENAL AUTOLOGOUS CELL THERAPY Transforming the Chronic Kidney Disease Treatment Landscape
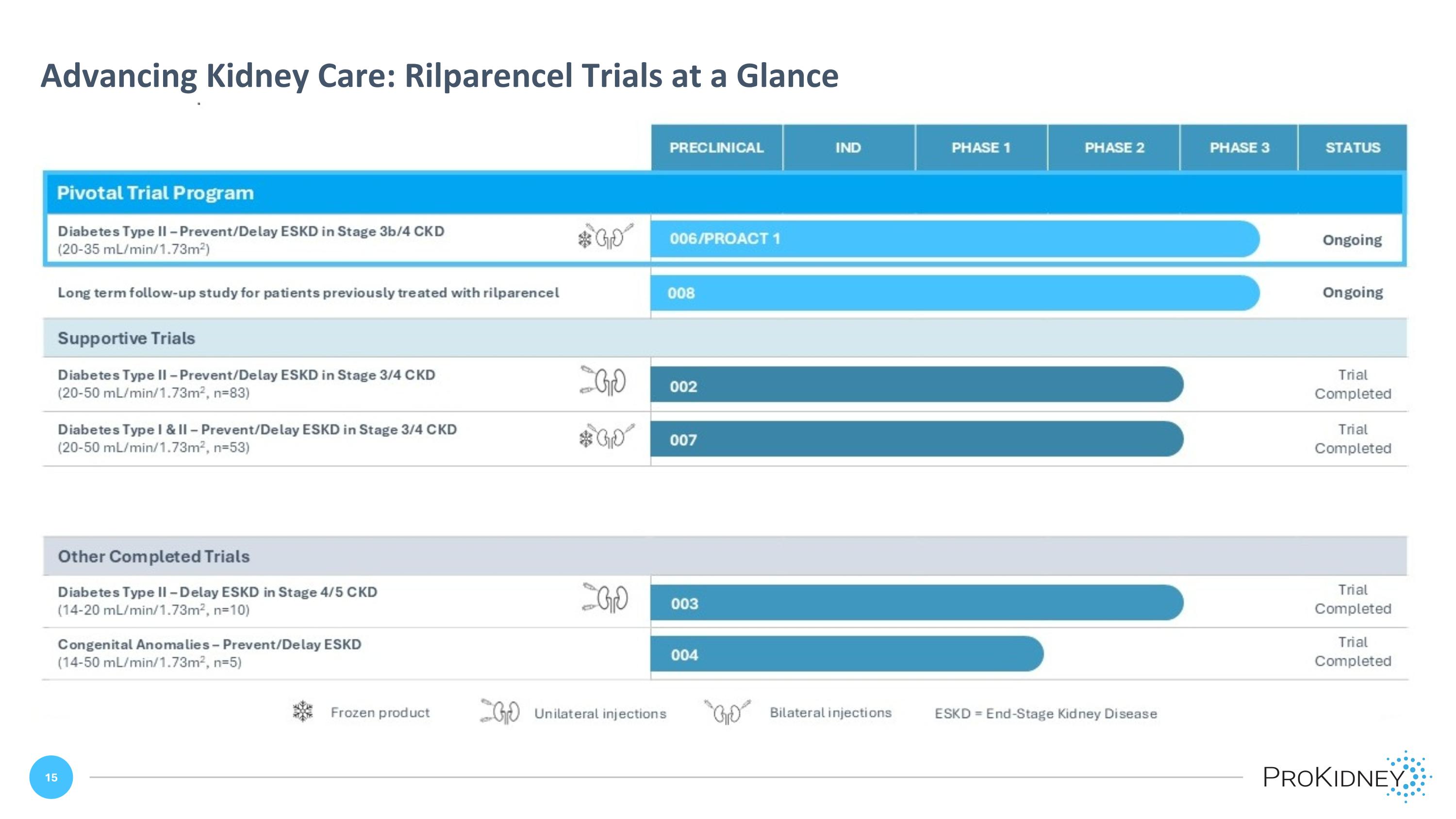
Advancing Kidney Care: Rilparencel Trials at a Glance 006/PROACT 1 PRECLINICAL IND PHASE 1 PHASE 2 PHASE 3 STATUS Pivotal Trial Program Diabetes Type II – Prevent/Delay ESKD in Stage 3b/4 CKD (20-35 mL/min/1.73m2) 008 002 007 003 004 Ongoing Long term follow-up study for patients previously treated with rilparencel Ongoing Supportive Trials Diabetes Type II – Prevent/Delay ESKD in Stage 3/4 CKD (20-50 mL/min/1.73m2, n=83) Trial Completed Diabetes Type I & II – Prevent/Delay ESKD in Stage 3/4 CKD (20-50 mL/min/1.73m2, n=53) Trial Completed Other Completed Trials Diabetes Type II – Delay ESKD in Stage 4/5 CKD (14-20 mL/min/1.73m2, n=10) Trial Completed Congenital Anomalies – Prevent/Delay ESKD (14-50 mL/min/1.73m2, n=5) Trial Completed Frozen product Unilateral injections Bilateral injections ESKD = End-Stage Kidney Disease
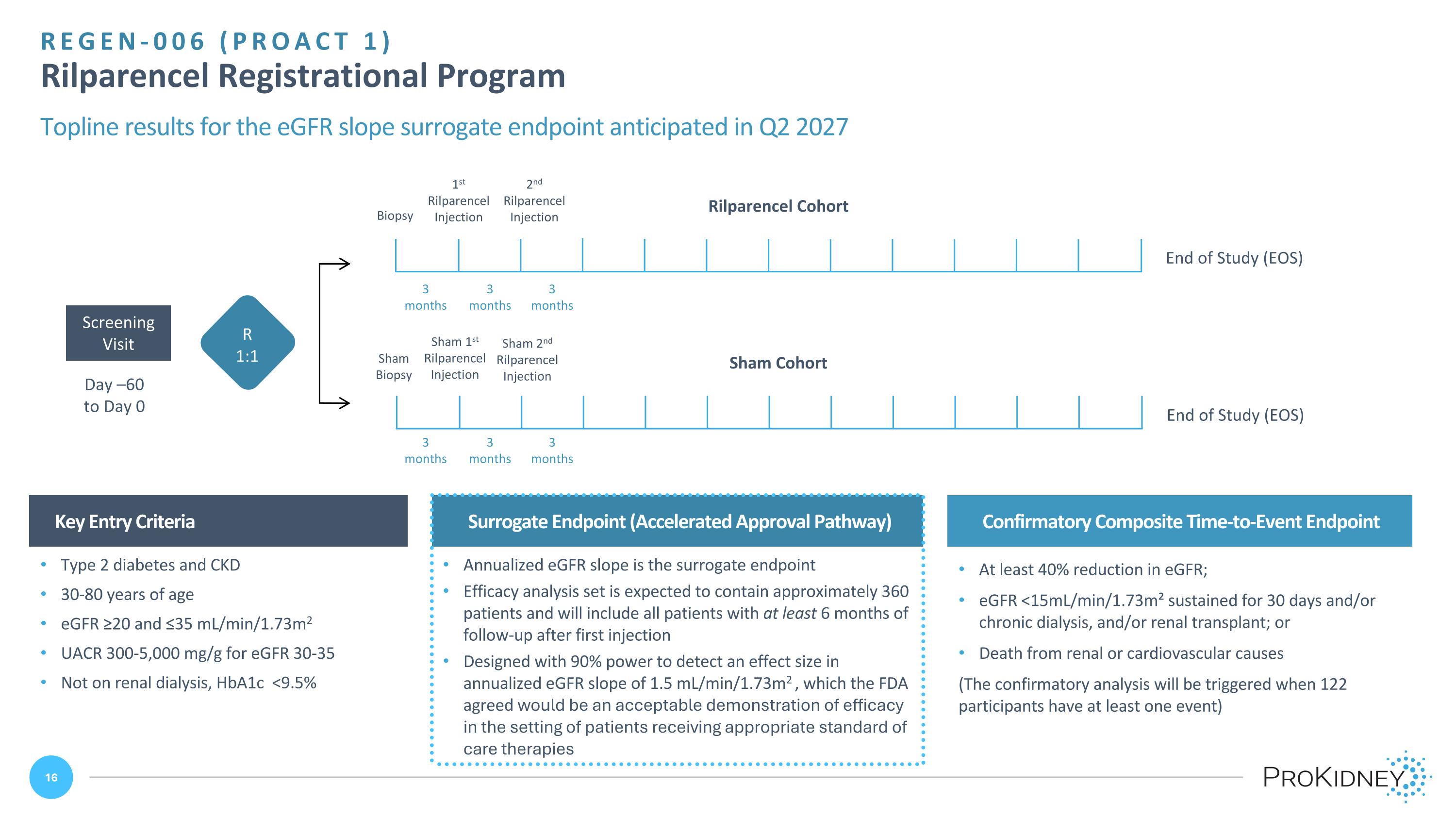
Day –60 to Day 0 Screening Visit Sham Cohort Sham 1st Rilparencel Injection Sham 2nd Rilparencel Injection Sham Biopsy End of Study (EOS) 1st Rilparencel Injection 2nd Rilparencel Injection Biopsy End of Study (EOS) REGEN-006 (PROACT 1) Rilparencel Registrational Program Topline results for the eGFR slope surrogate endpoint anticipated in Q2 2027 Key Entry Criteria Confirmatory Composite Time-to-Event Endpoint Type 2 diabetes and CKD 30-80 years of age eGFR ≥20 and ≤35 mL/min/1.73m2 UACR 300-5,000 mg/g for eGFR 30-35 Not on renal dialysis, HbA1c <9.5% 3 months 3 months 3 months 3 months R 1:1 Surrogate Endpoint (Accelerated Approval Pathway) Annualized eGFR slope is the surrogate endpoint Efficacy analysis set is expected to contain approximately 360 patients and will include all patients with at least 6 months of follow-up after first injection Designed with 90% power to detect an effect size in annualized eGFR slope of 1.5 mL/min/1.73m2 , which the FDA agreed would be an acceptable demonstration of efficacy in the setting of patients receiving appropriate standard of care therapies 3 months 3 months At least 40% reduction in eGFR; eGFR <15mL/min/1.73m² sustained for 30 days and/or chronic dialysis, and/or renal transplant; or Death from renal or cardiovascular causes (The confirmatory analysis will be triggered when 122 participants have at least one event) Rilparencel Cohort
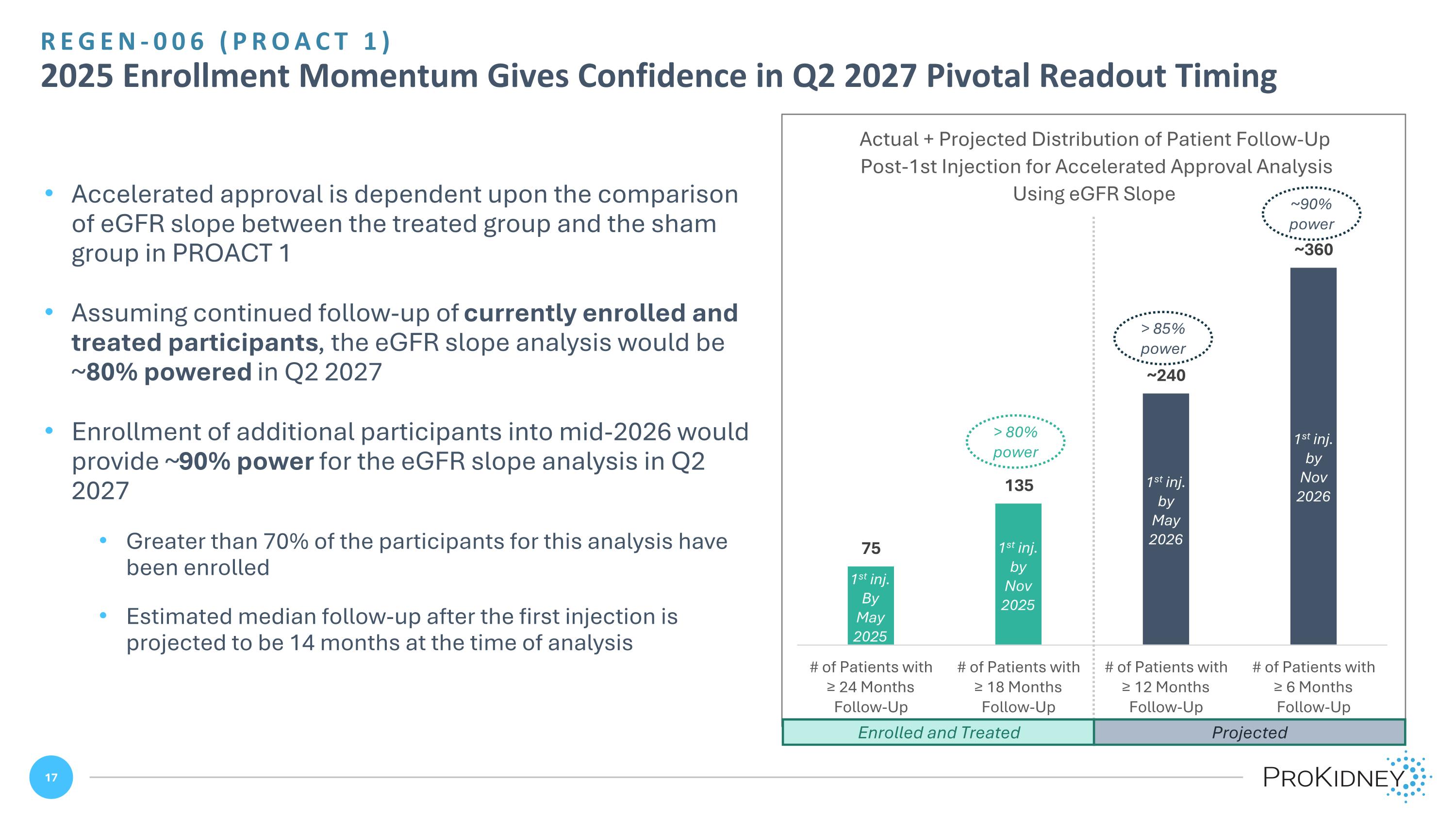
REGEN-006 (PROACT 1) 2025 Enrollment Momentum Gives Confidence in Q2 2027 Pivotal Readout Timing > 80% power > 85% power ~90% power Enrolled and Treated Projected Accelerated approval is dependent upon the comparison of eGFR slope between the treated group and the sham group in PROACT 1 Assuming continued follow-up of currently enrolled and treated participants, the eGFR slope analysis would be ~80% powered in Q2 2027 Enrollment of additional participants into mid-2026 would provide ~90% power for the eGFR slope analysis in Q2 2027 Greater than 70% of the participants for this analysis have been enrolled Estimated median follow-up after the first injection is projected to be 14 months at the time of analysis 1st inj. by Nov 2025 1st inj. By May 2025 1st inj. by May 2026 1st inj. by Nov 2026
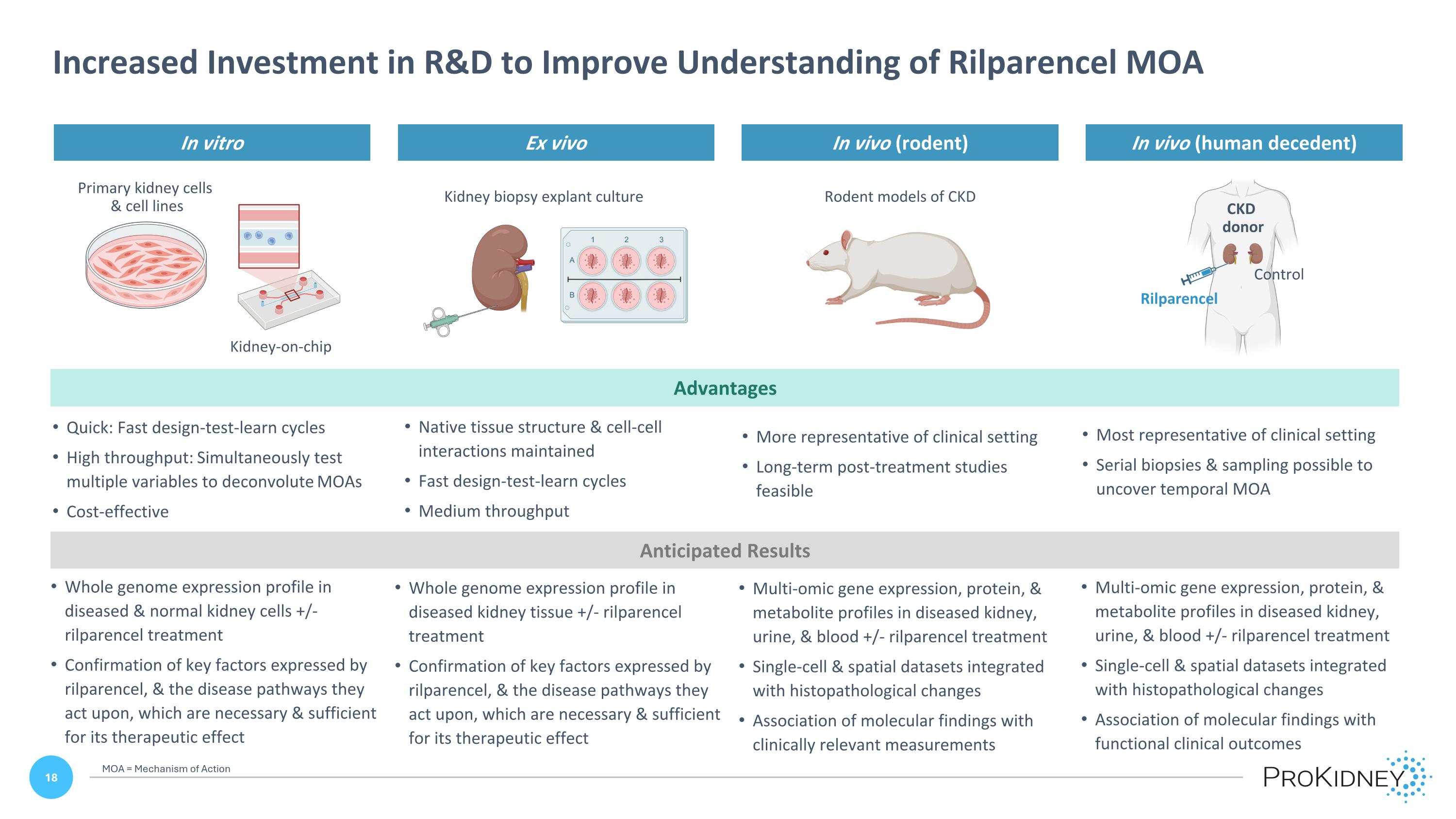
Anticipated Results Whole genome expression profile in diseased & normal kidney cells +/- rilparencel treatment Confirmation of key factors expressed by rilparencel, & the disease pathways they act upon, which are necessary & sufficient for its therapeutic effect Whole genome expression profile in diseased kidney tissue +/- rilparencel treatment Confirmation of key factors expressed by rilparencel, & the disease pathways they act upon, which are necessary & sufficient for its therapeutic effect Multi-omic gene expression, protein, & metabolite profiles in diseased kidney, urine, & blood +/- rilparencel treatment Single-cell & spatial datasets integrated with histopathological changes Association of molecular findings with clinically relevant measurements Multi-omic gene expression, protein, & metabolite profiles in diseased kidney, urine, & blood +/- rilparencel treatment Single-cell & spatial datasets integrated with histopathological changes Association of molecular findings with functional clinical outcomes In vitro Ex vivo In vivo (rodent) In vivo (human decedent) Quick: Fast design-test-learn cycles High throughput: Simultaneously test multiple variables to deconvolute MOAs Cost-effective Native tissue structure & cell-cell interactions maintained Fast design-test-learn cycles Medium throughput More representative of clinical setting Long-term post-treatment studies feasible Most representative of clinical setting Serial biopsies & sampling possible to uncover temporal MOA Advantages Kidney biopsy explant culture Primary kidney cells & cell lines Kidney-on-chip Rodent models of CKD Rilparencel Control CKD donor Increased Investment in R&D to Improve Understanding of Rilparencel MOA MOA = Mechanism of Action

RILPARENCEL CLINICAL RESULTS Advancing Cell Therapy For Chronic Kidney Disease
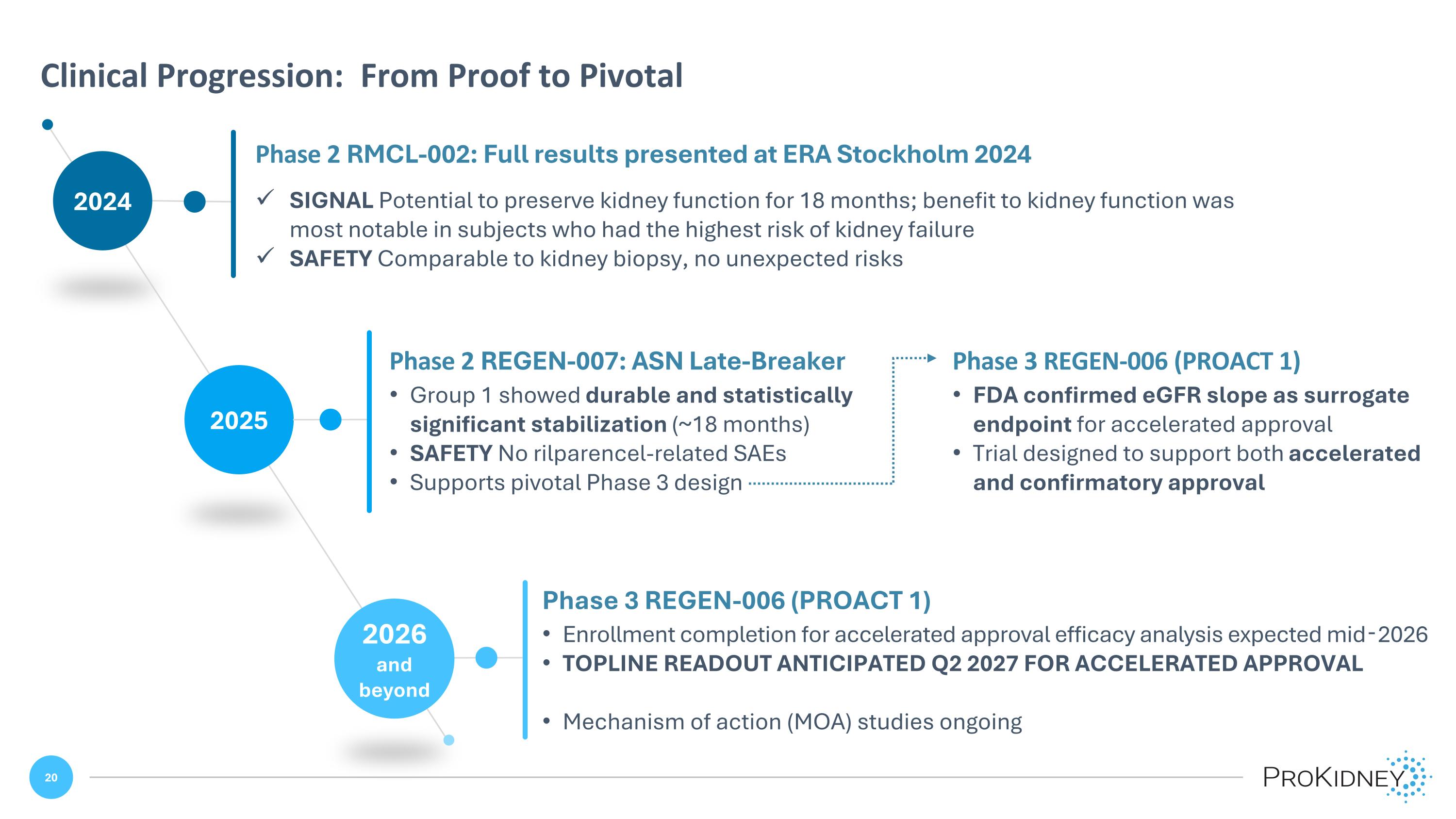
2024 Clinical Progression: From Proof to Pivotal Phase 2 RMCL-002: Full results presented at ERA Stockholm 2024 SIGNAL Potential to preserve kidney function for 18 months; benefit to kidney function was most notable in subjects who had the highest risk of kidney failure SAFETY Comparable to kidney biopsy, no unexpected risks 2026 and beyond 2025 Phase 2 REGEN-007: ASN Late-Breaker Group 1 showed durable and statistically significant stabilization (~18 months) SAFETY No rilparencel-related SAEs Supports pivotal Phase 3 design Phase 3 REGEN-006 (PROACT 1) FDA confirmed eGFR slope as surrogate endpoint for accelerated approval Trial designed to support both accelerated and confirmatory approval Phase 3 REGEN-006 (PROACT 1) Enrollment completion for accelerated approval efficacy analysis expected mid‑2026 TOPLINE READOUT ANTICIPATED Q2 2027 FOR ACCELERATED APPROVAL Mechanism of action (MOA) studies ongoing
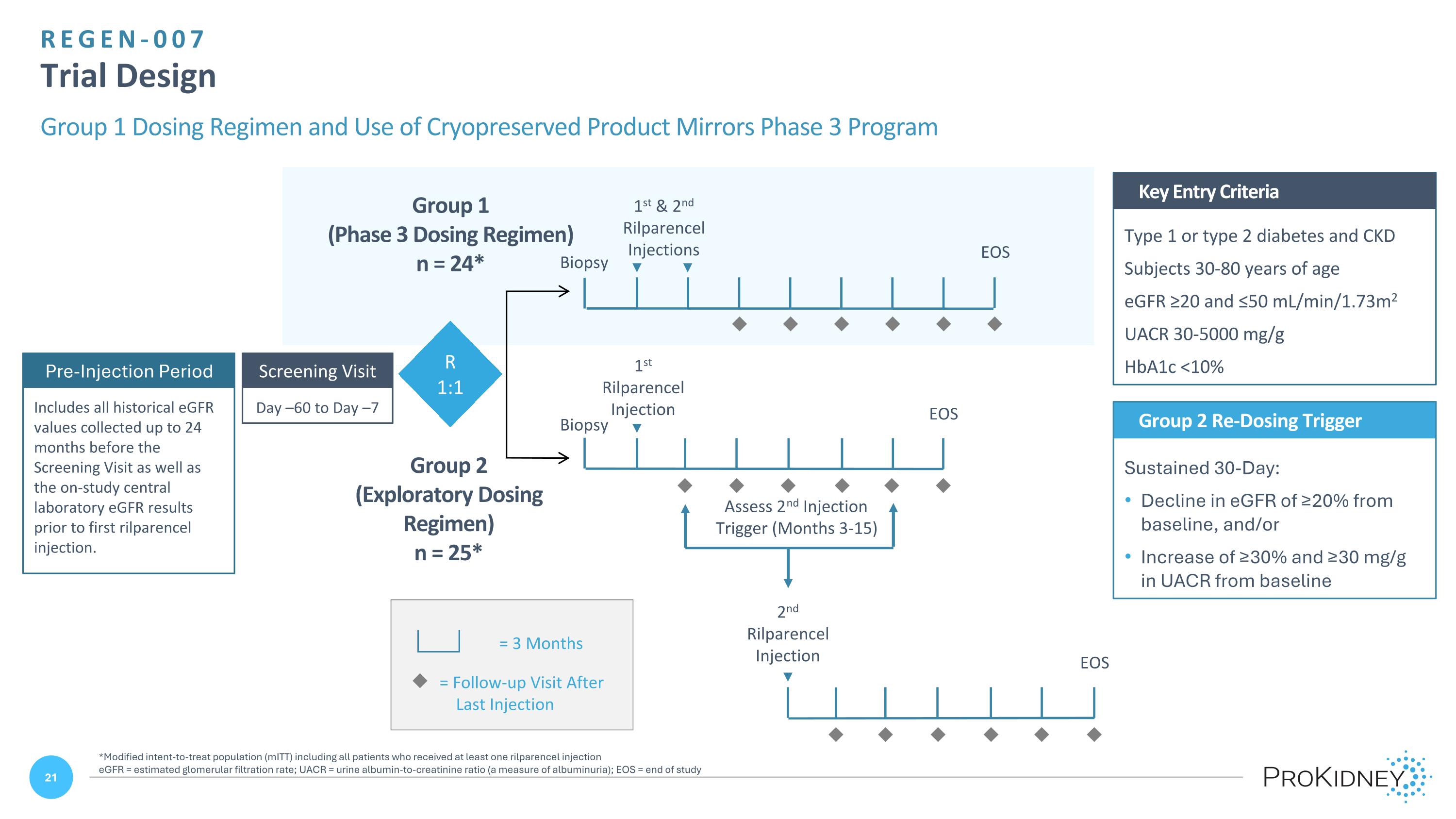
REGEN-007 Trial Design Group 1 Dosing Regimen and Use of Cryopreserved Product Mirrors Phase 3 Program Type 1 or type 2 diabetes and CKD Subjects 30-80 years of age eGFR ≥20 and ≤50 mL/min/1.73m2 UACR 30-5000 mg/g HbA1c <10% Key Entry Criteria Day –60 to Day –7 Screening Visit u = Follow-up Visit After Last Injection = 3 Months Group 2 Re-Dosing Trigger Sustained 30-Day: Decline in eGFR of ≥20% from baseline, and/or Increase of ≥30% and ≥30 mg/g in UACR from baseline 2nd Rilparencel Injection Assess 2nd Injection Trigger (Months 3-15) Group 2 (Exploratory Dosing Regimen) n = 25* R 1:1 1st & 2nd Rilparencel Injections EOS Group 1 (Phase 3 Dosing Regimen) n = 24* EOS 1st Rilparencel Injection u u u u u u u u u u u u u u u u u u EOS Biopsy Biopsy Pre-Injection Period Includes all historical eGFR values collected up to 24 months before the Screening Visit as well as the on-study central laboratory eGFR results prior to first rilparencel injection. *Modified intent-to-treat population (mITT) including all patients who received at least one rilparencel injection eGFR = estimated glomerular filtration rate; UACR = urine albumin-to-creatinine ratio (a measure of albuminuria); EOS = end of study
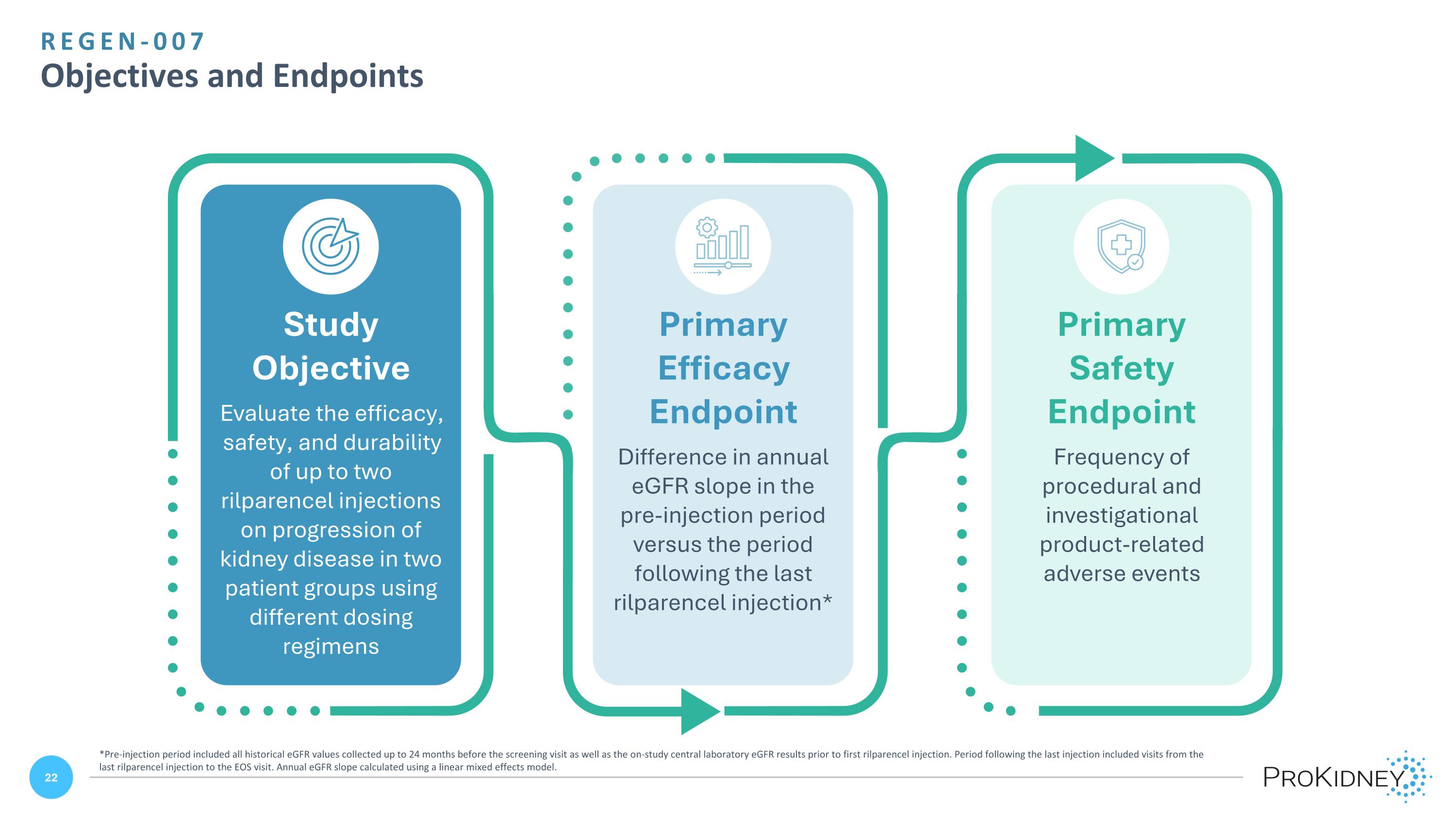
REGEN-007 Objectives and Endpoints Study Objective Evaluate the efficacy, safety, and durability of up to two rilparencel injections on progression of kidney disease in two patient groups using different dosing regimens Primary Efficacy Endpoint Difference in annual eGFR slope in the pre-injection period versus the period following the last rilparencel injection* Primary Safety Endpoint Frequency of procedural and investigational product-related adverse events *Pre-injection period included all historical eGFR values collected up to 24 months before the screening visit as well as the on-study central laboratory eGFR results prior to first rilparencel injection. Period following the last injection included visits from the last rilparencel injection to the EOS visit. Annual eGFR slope calculated using a linear mixed effects model.
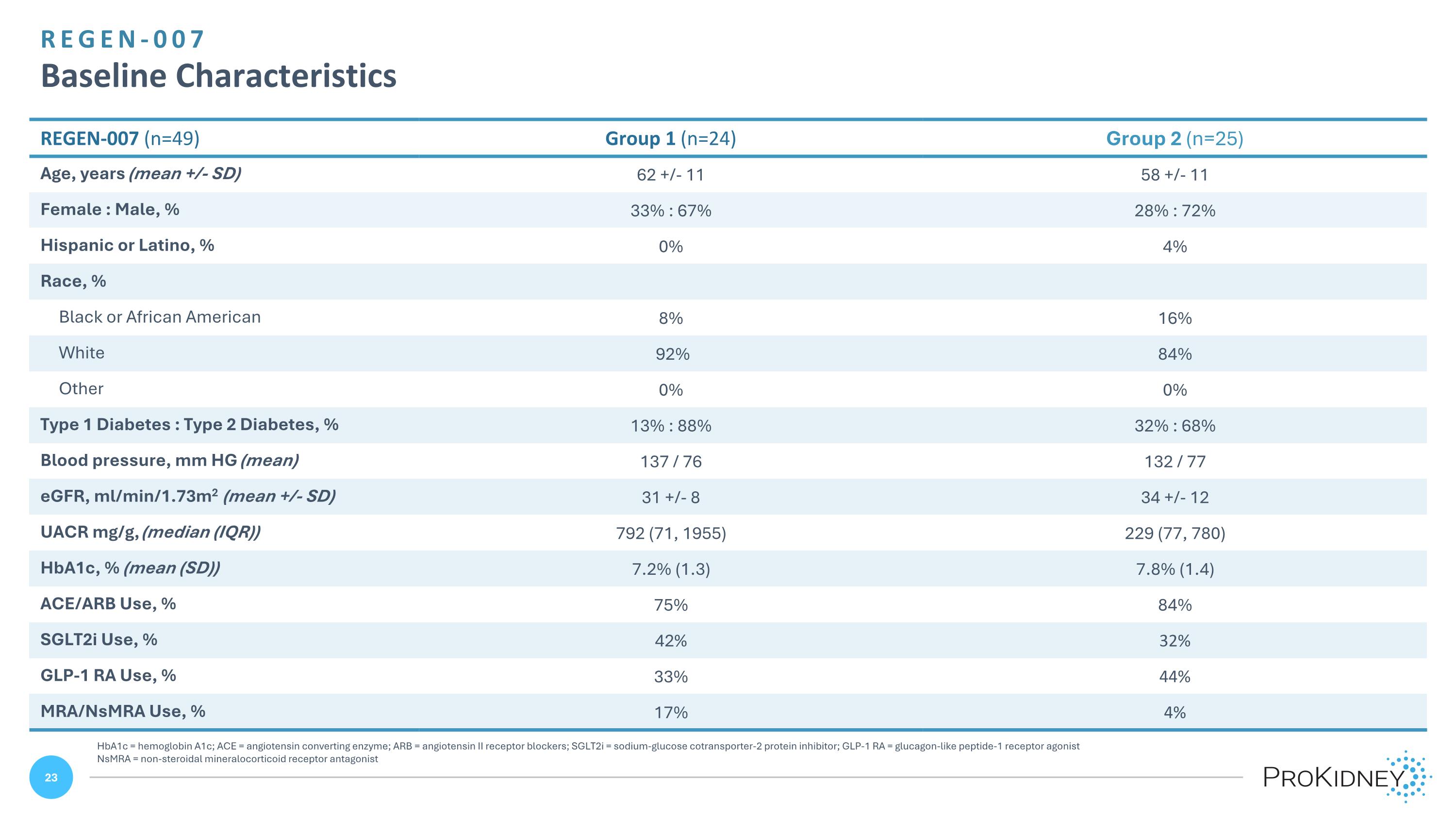
REGEN-007 Baseline Characteristics REGEN-007 (n=49) Group 1 (n=24) Group 2 (n=25) Age, years (mean +/- SD) 62 +/- 11 58 +/- 11 Female : Male, % 33% : 67% 28% : 72% Hispanic or Latino, % 0% 4% Race, % Black or African American 8% 16% White 92% 84% Other 0% 0% Type 1 Diabetes : Type 2 Diabetes, % 13% : 88% 32% : 68% Blood pressure, mm HG (mean) 137 / 76 132 / 77 eGFR, ml/min/1.73m2 (mean +/- SD) 31 +/- 8 34 +/- 12 UACR mg/g, (median (IQR)) 792 (71, 1955) 229 (77, 780) HbA1c, % (mean (SD)) 7.2% (1.3) 7.8% (1.4) ACE/ARB Use, % 75% 84% SGLT2i Use, % 42% 32% GLP-1 RA Use, % 33% 44% MRA/NsMRA Use, % 17% 4% HbA1c = hemoglobin A1c; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blockers; SGLT2i = sodium-glucose cotransporter-2 protein inhibitor; GLP-1 RA = glucagon-like peptide-1 receptor agonist NsMRA = non-steroidal mineralocorticoid receptor antagonist
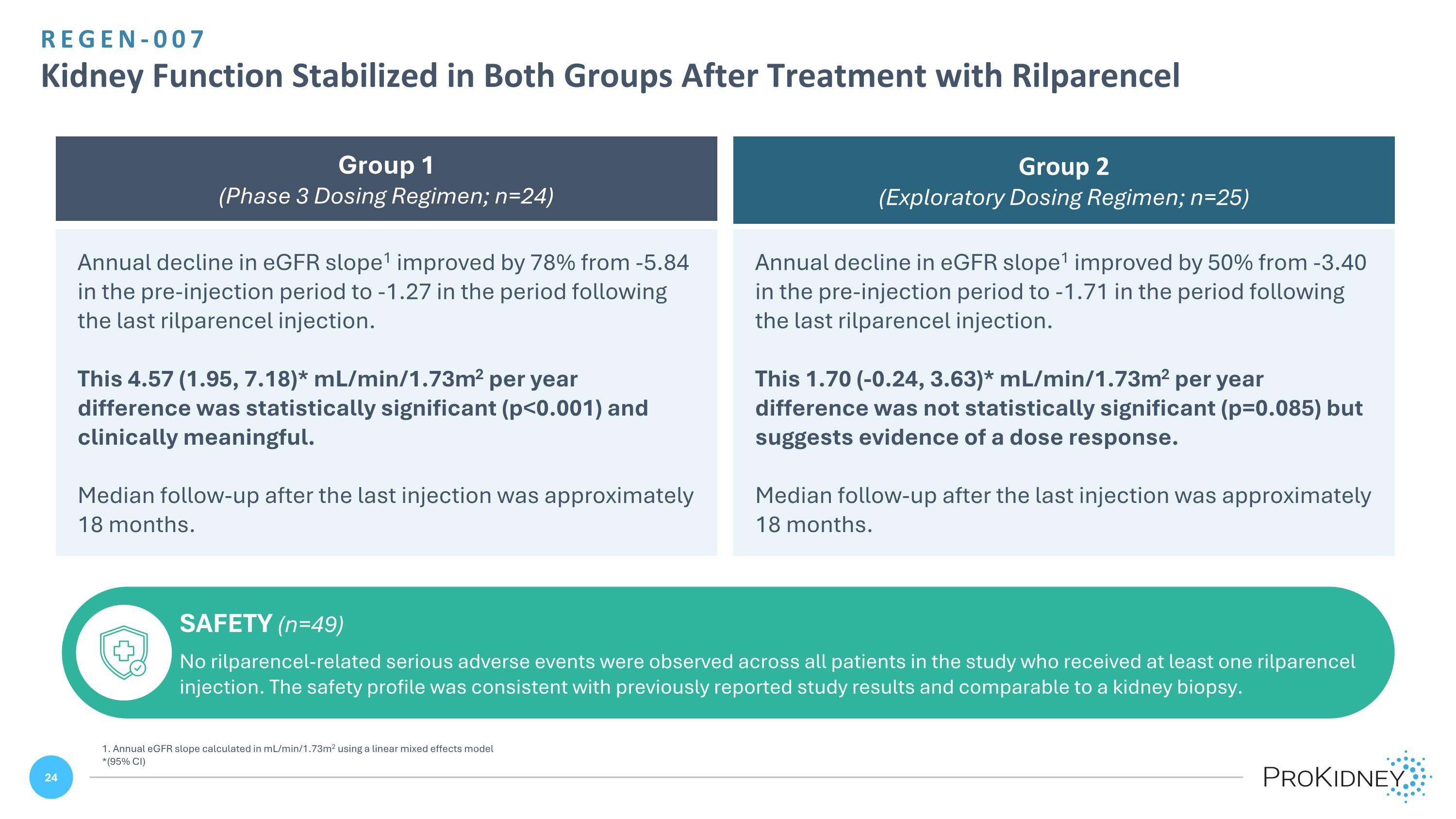
REGEN-007 Kidney Function Stabilized in Both Groups After Treatment with Rilparencel Annual decline in eGFR slope1 improved by 78% from -5.84 in the pre-injection period to -1.27 in the period following the last rilparencel injection. This 4.57 (1.95, 7.18)* mL/min/1.73m2 per year difference was statistically significant (p<0.001) and clinically meaningful. Median follow-up after the last injection was approximately 18 months. Annual decline in eGFR slope1 improved by 50% from -3.40 in the pre-injection period to -1.71 in the period following the last rilparencel injection. This 1.70 (-0.24, 3.63)* mL/min/1.73m2 per year difference was not statistically significant (p=0.085) but suggests evidence of a dose response. Median follow-up after the last injection was approximately 18 months. Group 1 (Phase 3 Dosing Regimen; n=24) Group 2 (Exploratory Dosing Regimen; n=25) SAFETY (n=49) No rilparencel-related serious adverse events were observed across all patients in the study who received at least one rilparencel injection. The safety profile was consistent with previously reported study results and comparable to a kidney biopsy. 1. Annual eGFR slope calculated in mL/min/1.73m2 using a linear mixed effects model *(95% CI)
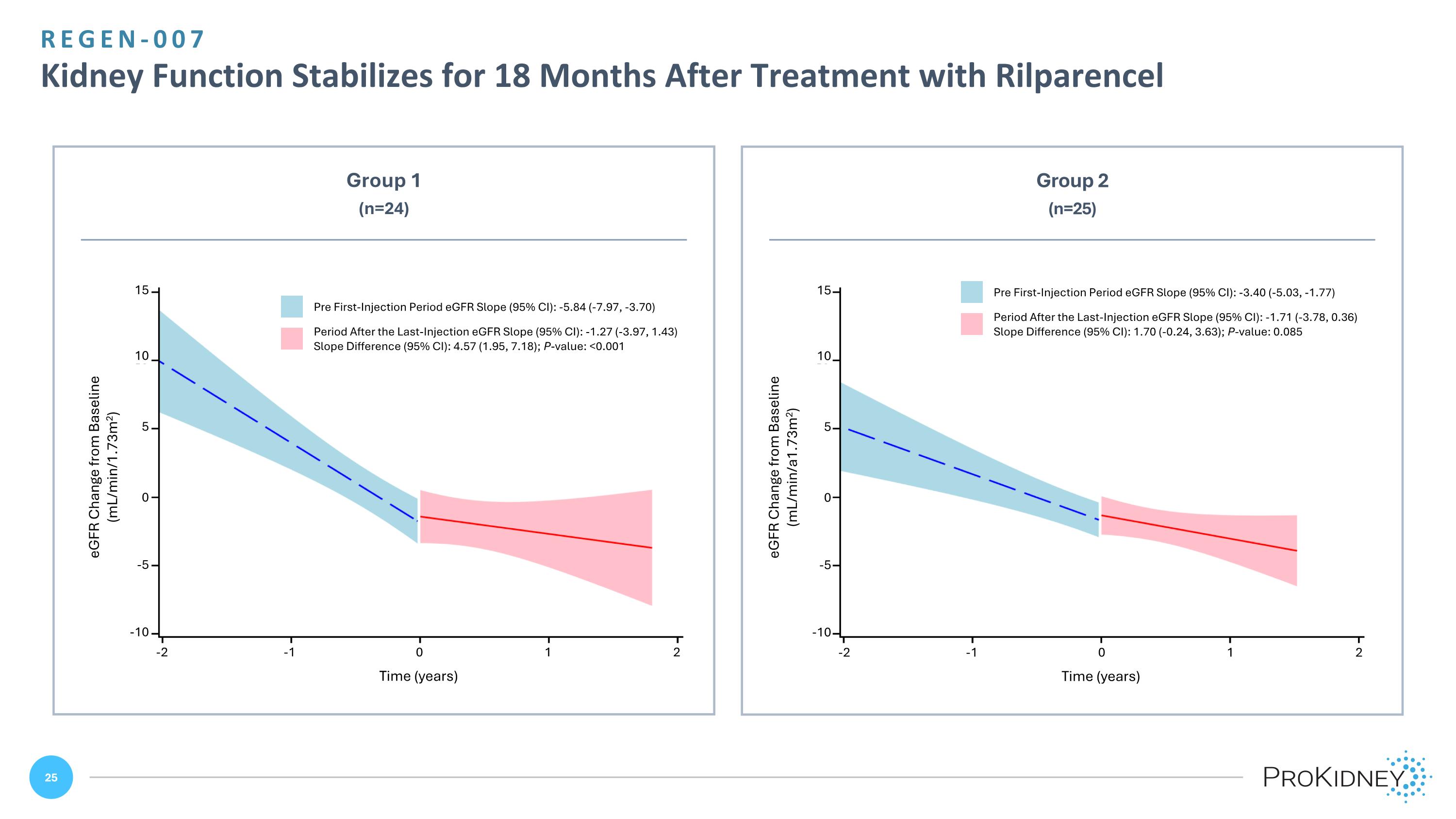
REGEN-007 Kidney Function Stabilizes for 18 Months After Treatment with Rilparencel 15 10 5 0 -5 -10 -2 -1 0 1 2 Time (years) eGFR Change from Baseline (mL/min/1.73m2) Group 1 (n=24) Group 2 (n=25) 15 10 5 0 -5 -10 -2 -1 0 1 2 Time (years) eGFR Change from Baseline (mL/min/a1.73m2) Pre First-Injection Period eGFR Slope (95% CI): -5.84 (-7.97, -3.70) Period After the Last-Injection eGFR Slope (95% CI): -1.27 (-3.97, 1.43) Slope Difference (95% CI): 4.57 (1.95, 7.18); P-value: <0.001 Pre First-Injection Period eGFR Slope (95% CI): -3.40 (-5.03, -1.77) Period After the Last-Injection eGFR Slope (95% CI): -1.71 (-3.78, 0.36) Slope Difference (95% CI): 1.70 (-0.24, 3.63); P-value: 0.085
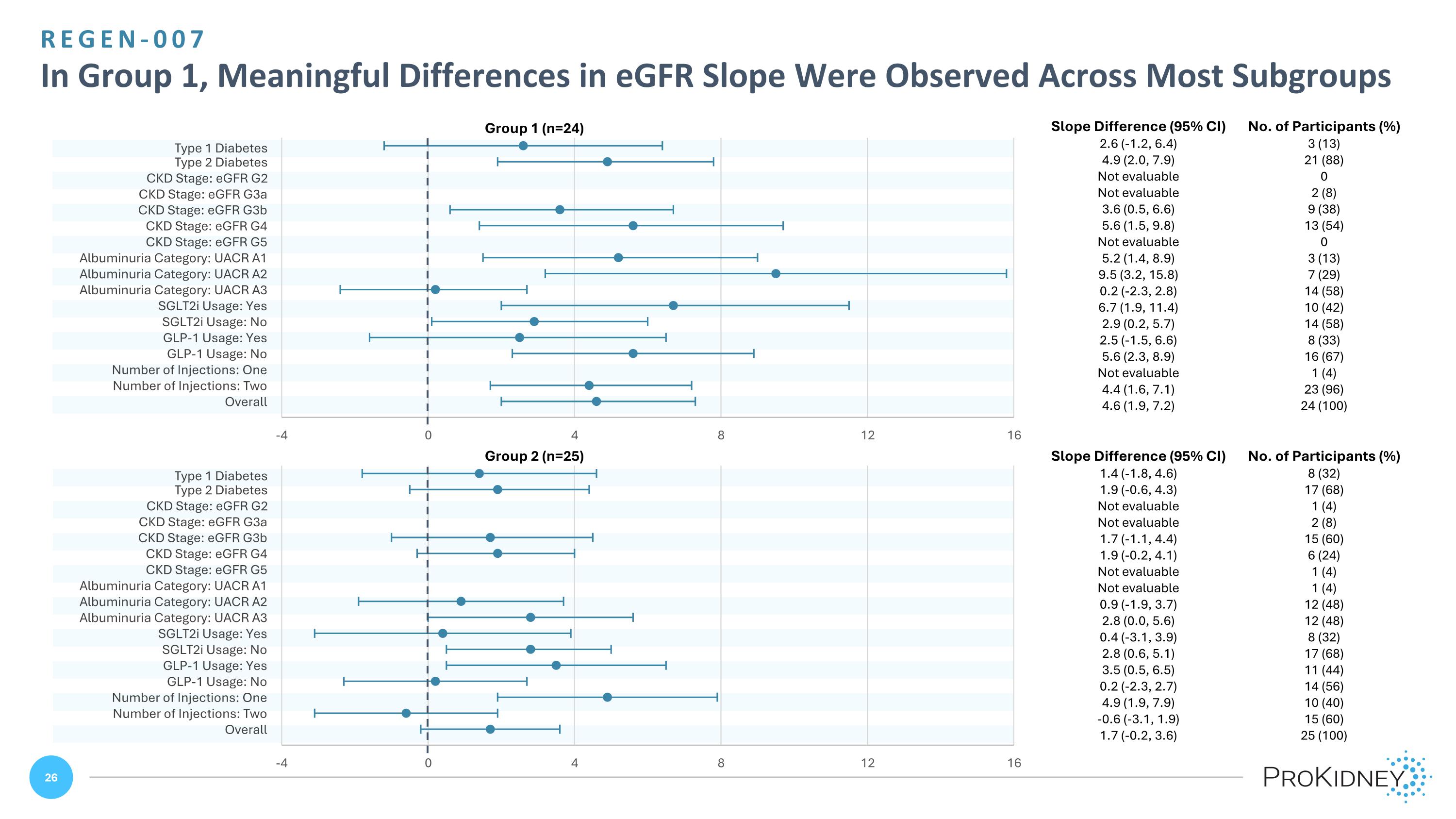
Group 1 (n=24) Group 2 (n=25) Slope Difference (95% CI) No. of Participants (%) 2.6 (-1.2, 6.4) 3 (13) 4.9 (2.0, 7.9) 21 (88) Not evaluable 0 Not evaluable 2 (8) 3.6 (0.5, 6.6) 9 (38) 5.6 (1.5, 9.8) 13 (54) Not evaluable 0 5.2 (1.4, 8.9) 3 (13) 9.5 (3.2, 15.8) 7 (29) 0.2 (-2.3, 2.8) 14 (58) 6.7 (1.9, 11.4) 10 (42) 2.9 (0.2, 5.7) 14 (58) 2.5 (-1.5, 6.6) 8 (33) 5.6 (2.3, 8.9) 16 (67) Not evaluable 1 (4) 4.4 (1.6, 7.1) 23 (96) 4.6 (1.9, 7.2) 24 (100) Slope Difference (95% CI) No. of Participants (%) 1.4 (-1.8, 4.6) 8 (32) 1.9 (-0.6, 4.3) 17 (68) Not evaluable 1 (4) Not evaluable 2 (8) 1.7 (-1.1, 4.4) 15 (60) 1.9 (-0.2, 4.1) 6 (24) Not evaluable 1 (4) Not evaluable 1 (4) 0.9 (-1.9, 3.7) 12 (48) 2.8 (0.0, 5.6) 12 (48) 0.4 (-3.1, 3.9) 8 (32) 2.8 (0.6, 5.1) 17 (68) 3.5 (0.5, 6.5) 11 (44) 0.2 (-2.3, 2.7) 14 (56) 4.9 (1.9, 7.9) 10 (40) -0.6 (-3.1, 1.9) 15 (60) 1.7 (-0.2, 3.6) 25 (100) REGEN-007 In Group 1, Meaningful Differences in eGFR Slope Were Observed Across Most Subgroups
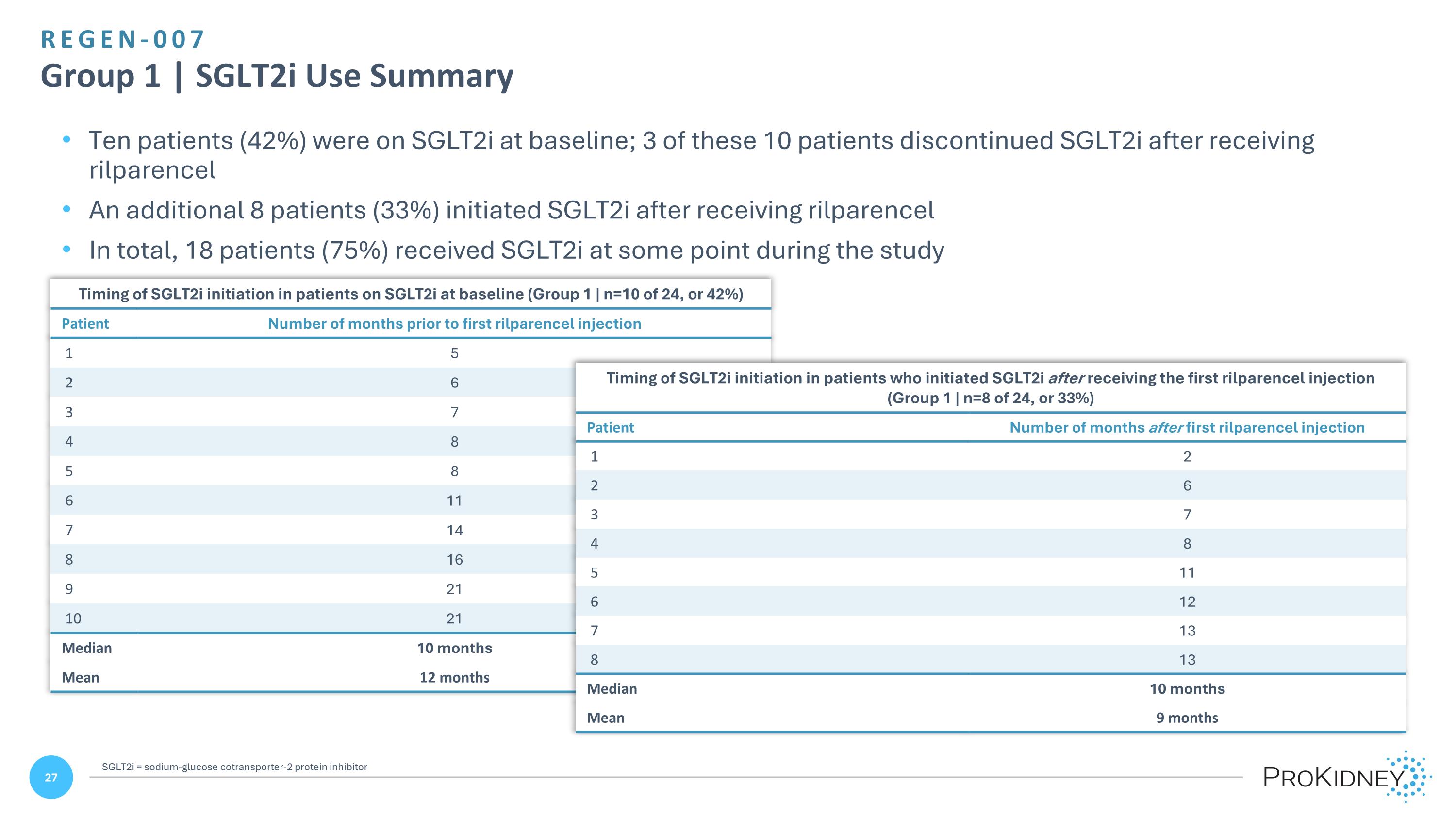
REGEN-007 Group 1 | SGLT2i Use Summary Ten patients (42%) were on SGLT2i at baseline; 3 of these 10 patients discontinued SGLT2i after receiving rilparencel An additional 8 patients (33%) initiated SGLT2i after receiving rilparencel In total, 18 patients (75%) received SGLT2i at some point during the study Timing of SGLT2i initiation in patients on SGLT2i at baseline (Group 1 | n=10 of 24, or 42%) Patient Number of months prior to first rilparencel injection 1 5 2 6 3 7 4 8 5 8 6 11 7 14 8 16 9 21 10 21 Median 10 months Mean 12 months Timing of SGLT2i initiation in patients who initiated SGLT2i after receiving the first rilparencel injection (Group 1 | n=8 of 24, or 33%) Patient Number of months after first rilparencel injection 1 2 2 6 3 7 4 8 5 11 6 12 7 13 8 13 Median 10 months Mean 9 months SGLT2i = sodium-glucose cotransporter-2 protein inhibitor
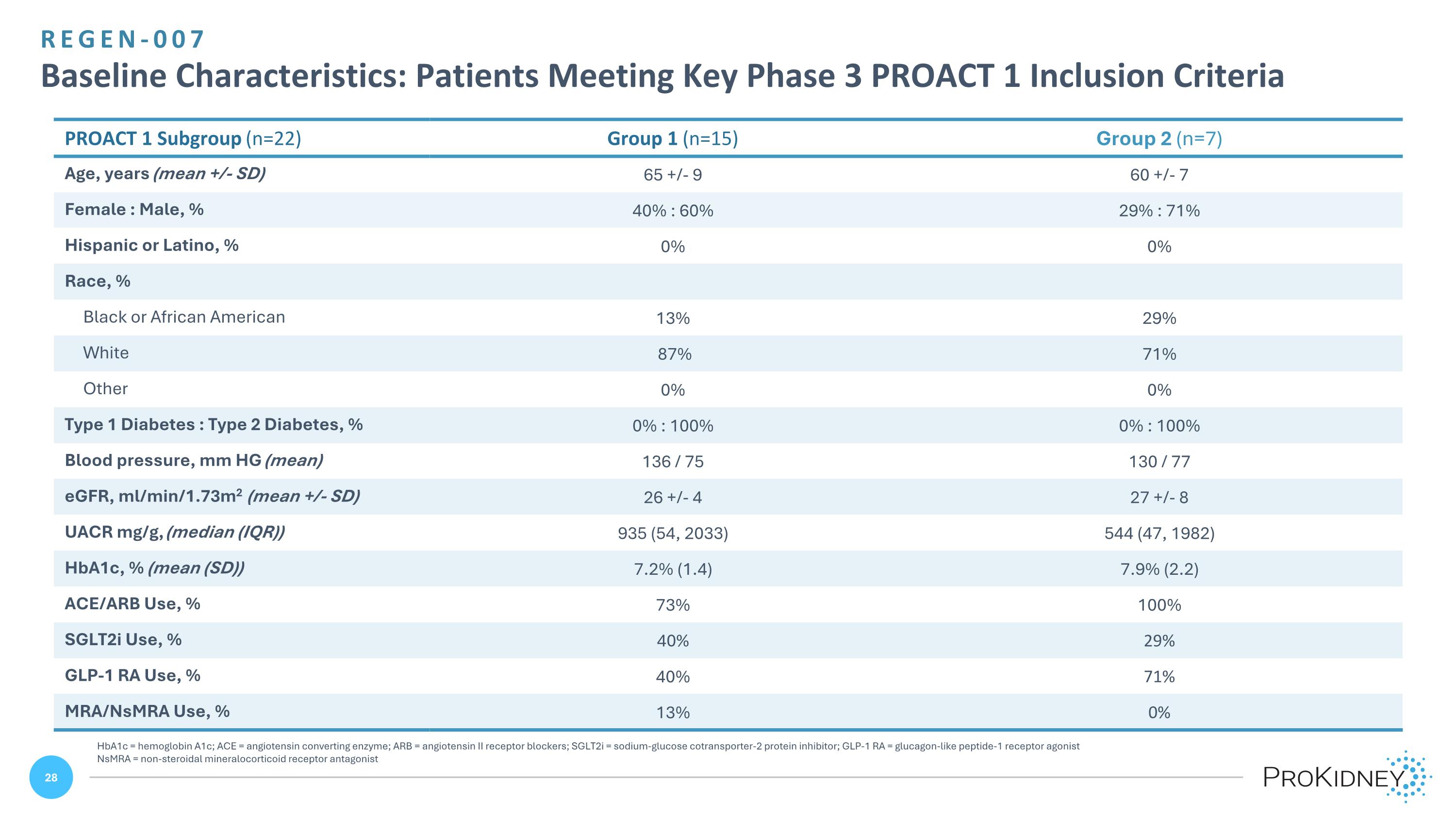
REGEN-007 Baseline Characteristics: Patients Meeting Key Phase 3 PROACT 1 Inclusion Criteria HbA1c = hemoglobin A1c; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blockers; SGLT2i = sodium-glucose cotransporter-2 protein inhibitor; GLP-1 RA = glucagon-like peptide-1 receptor agonist NsMRA = non-steroidal mineralocorticoid receptor antagonist PROACT 1 Subgroup (n=22) Group 1 (n=15) Group 2 (n=7) Age, years (mean +/- SD) 65 +/- 9 60 +/- 7 Female : Male, % 40% : 60% 29% : 71% Hispanic or Latino, % 0% 0% Race, % Black or African American 13% 29% White 87% 71% Other 0% 0% Type 1 Diabetes : Type 2 Diabetes, % 0% : 100% 0% : 100% Blood pressure, mm HG (mean) 136 / 75 130 / 77 eGFR, ml/min/1.73m2 (mean +/- SD) 26 +/- 4 27 +/- 8 UACR mg/g, (median (IQR)) 935 (54, 2033) 544 (47, 1982) HbA1c, % (mean (SD)) 7.2% (1.4) 7.9% (2.2) ACE/ARB Use, % 73% 100% SGLT2i Use, % 40% 29% GLP-1 RA Use, % 40% 71% MRA/NsMRA Use, % 13% 0%
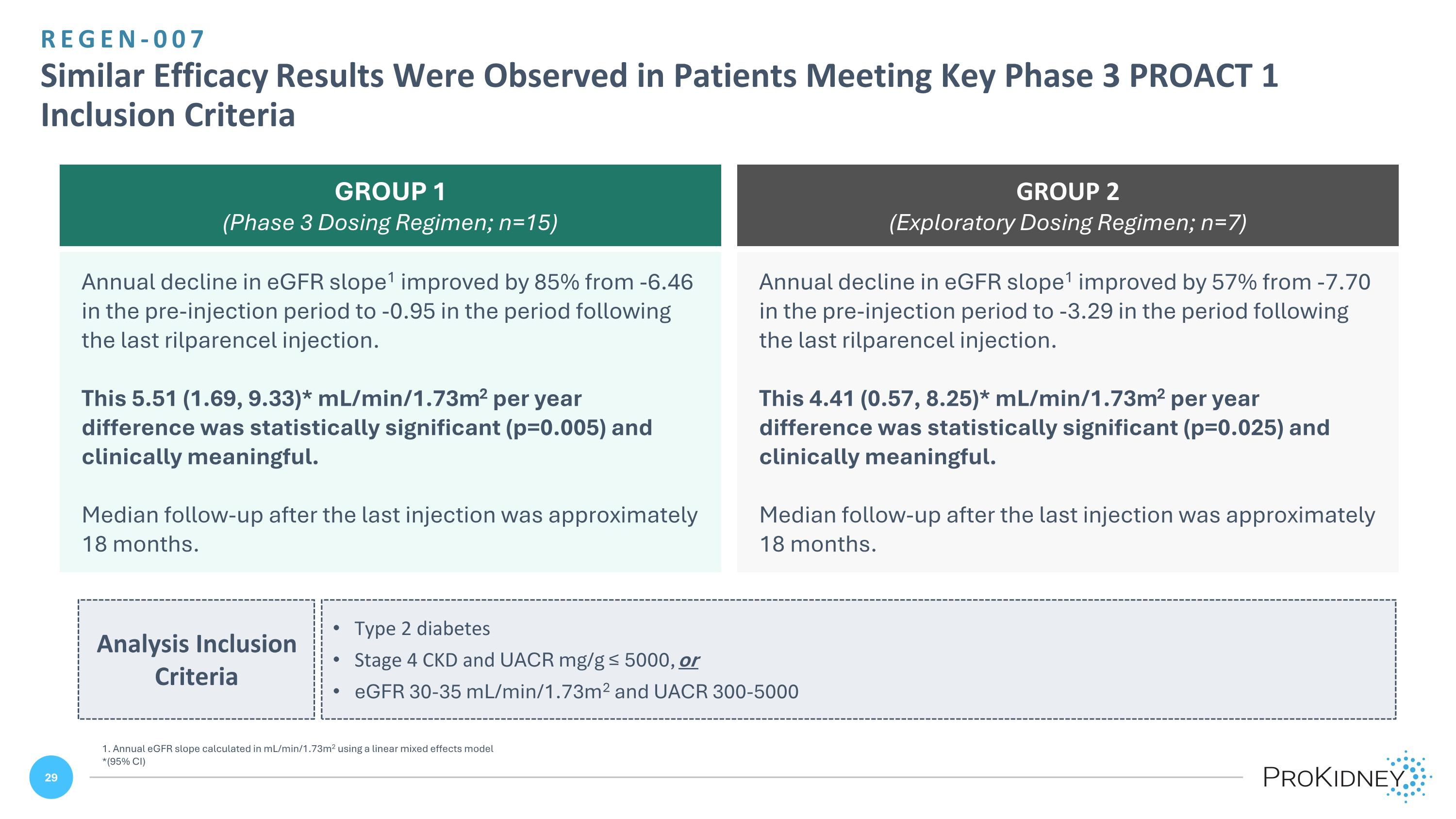
REGEN-007 Similar Efficacy Results Were Observed in Patients Meeting Key Phase 3 PROACT 1 Inclusion Criteria 1. Annual eGFR slope calculated in mL/min/1.73m2 using a linear mixed effects model *(95% CI) Annual decline in eGFR slope1 improved by 85% from -6.46 in the pre-injection period to -0.95 in the period following the last rilparencel injection. This 5.51 (1.69, 9.33)* mL/min/1.73m2 per year difference was statistically significant (p=0.005) and clinically meaningful. Median follow-up after the last injection was approximately 18 months. Annual decline in eGFR slope1 improved by 57% from -7.70 in the pre-injection period to -3.29 in the period following the last rilparencel injection. This 4.41 (0.57, 8.25)* mL/min/1.73m2 per year difference was statistically significant (p=0.025) and clinically meaningful. Median follow-up after the last injection was approximately 18 months. GROUP 1 (Phase 3 Dosing Regimen; n=15) GROUP 2 (Exploratory Dosing Regimen; n=7) Analysis Inclusion Criteria Type 2 diabetes Stage 4 CKD and UACR mg/g ≤ 5000, or eGFR 30-35 mL/min/1.73m2 and UACR 300-5000
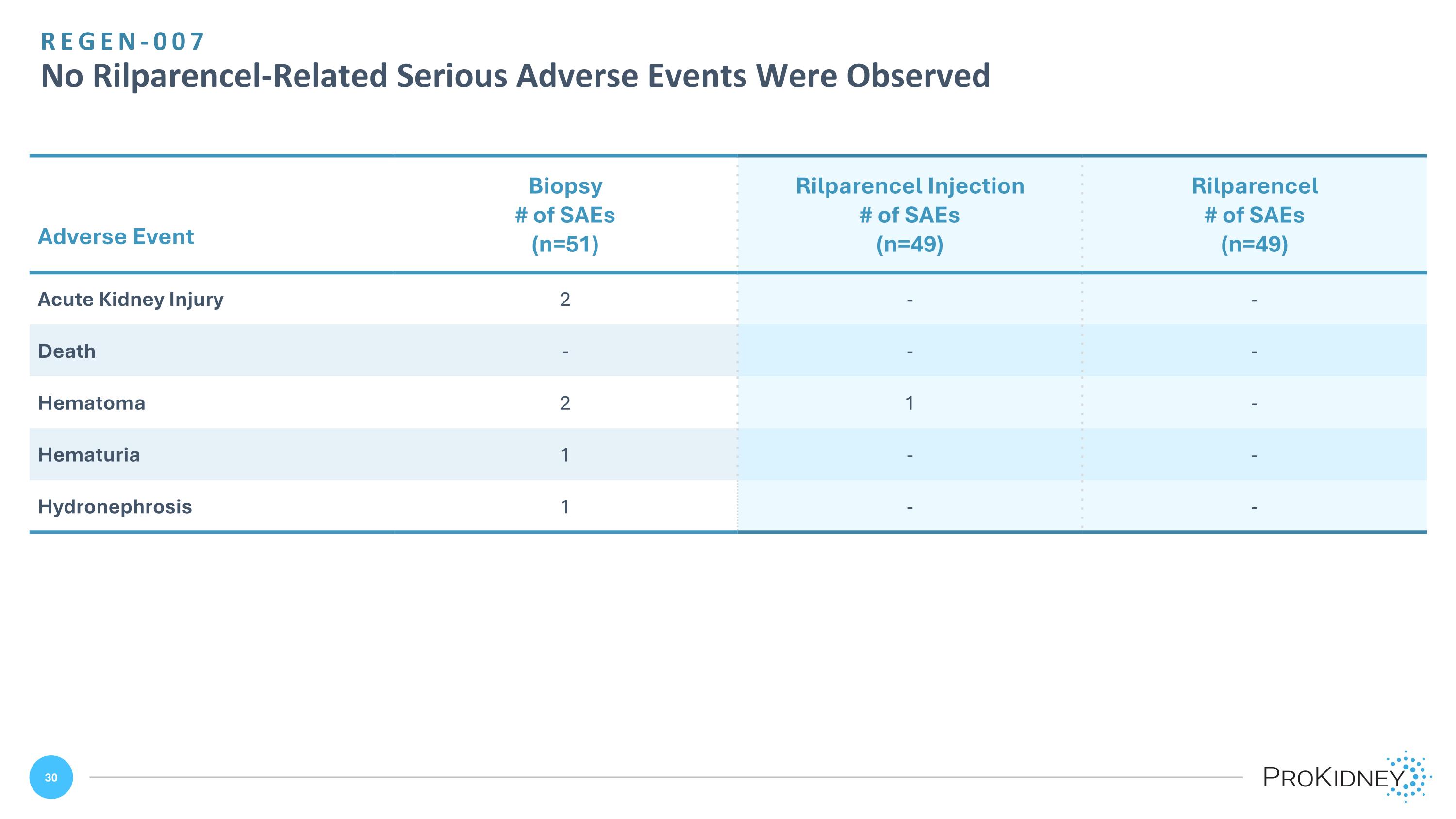
Adverse Event Biopsy # of SAEs (n=51) Rilparencel Injection # of SAEs (n=49) Rilparencel # of SAEs (n=49) Acute Kidney Injury 2 - - Death - - - Hematoma 2 1 - Hematuria 1 - - Hydronephrosis 1 - - REGEN-007 No Rilparencel-Related Serious Adverse Events Were Observed
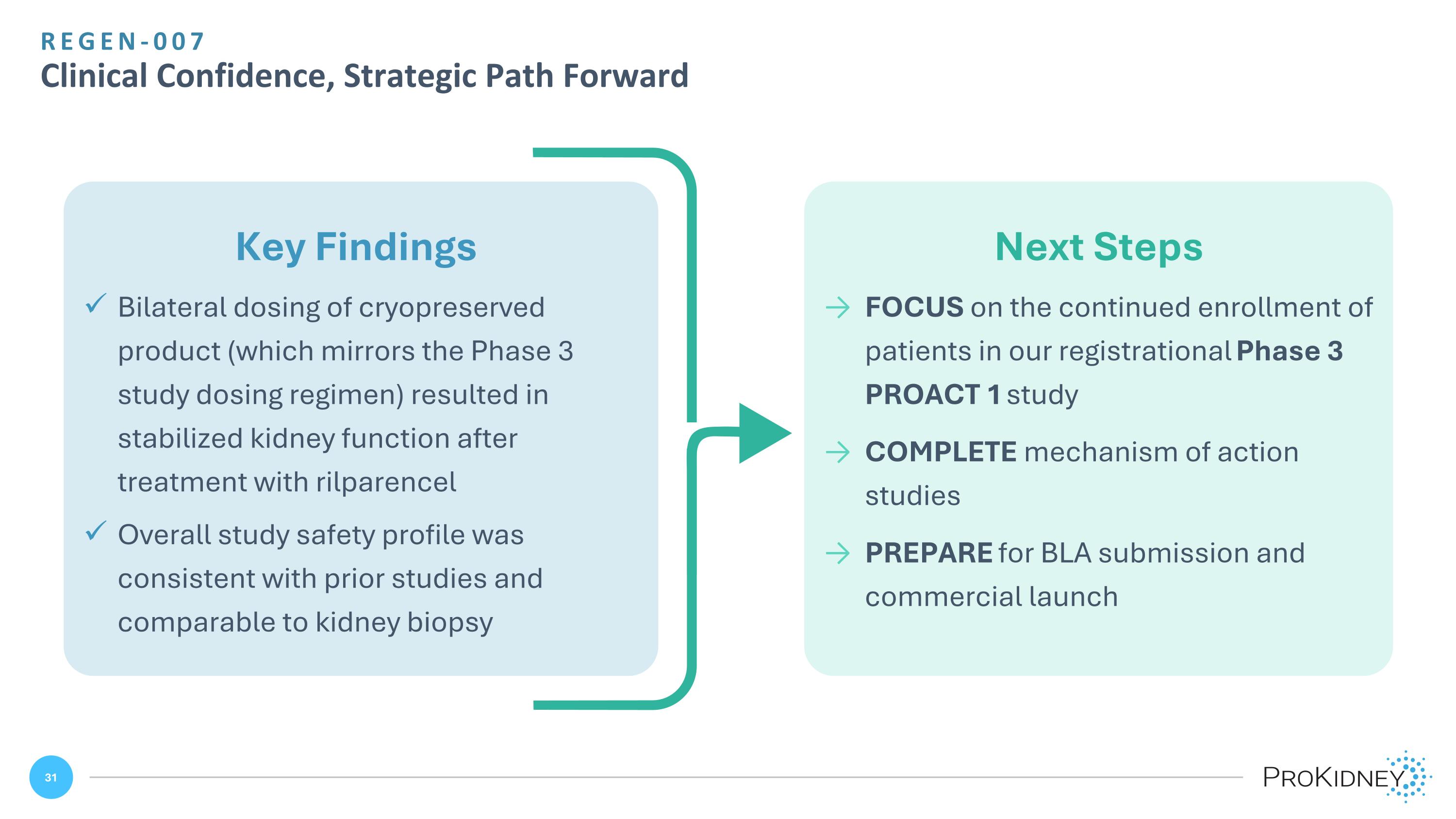
Next Steps FOCUS on the continued enrollment of patients in our registrational Phase 3 PROACT 1 study COMPLETE mechanism of action studies PREPARE for BLA submission and commercial launch Key Findings Bilateral dosing of cryopreserved product (which mirrors the Phase 3 study dosing regimen) resulted in stabilized kidney function after treatment with rilparencel Overall study safety profile was consistent with prior studies and comparable to kidney biopsy REGEN-007 Clinical Confidence, Strategic Path Forward

EXECUTING WITH STRENGTH Financial Snapshot
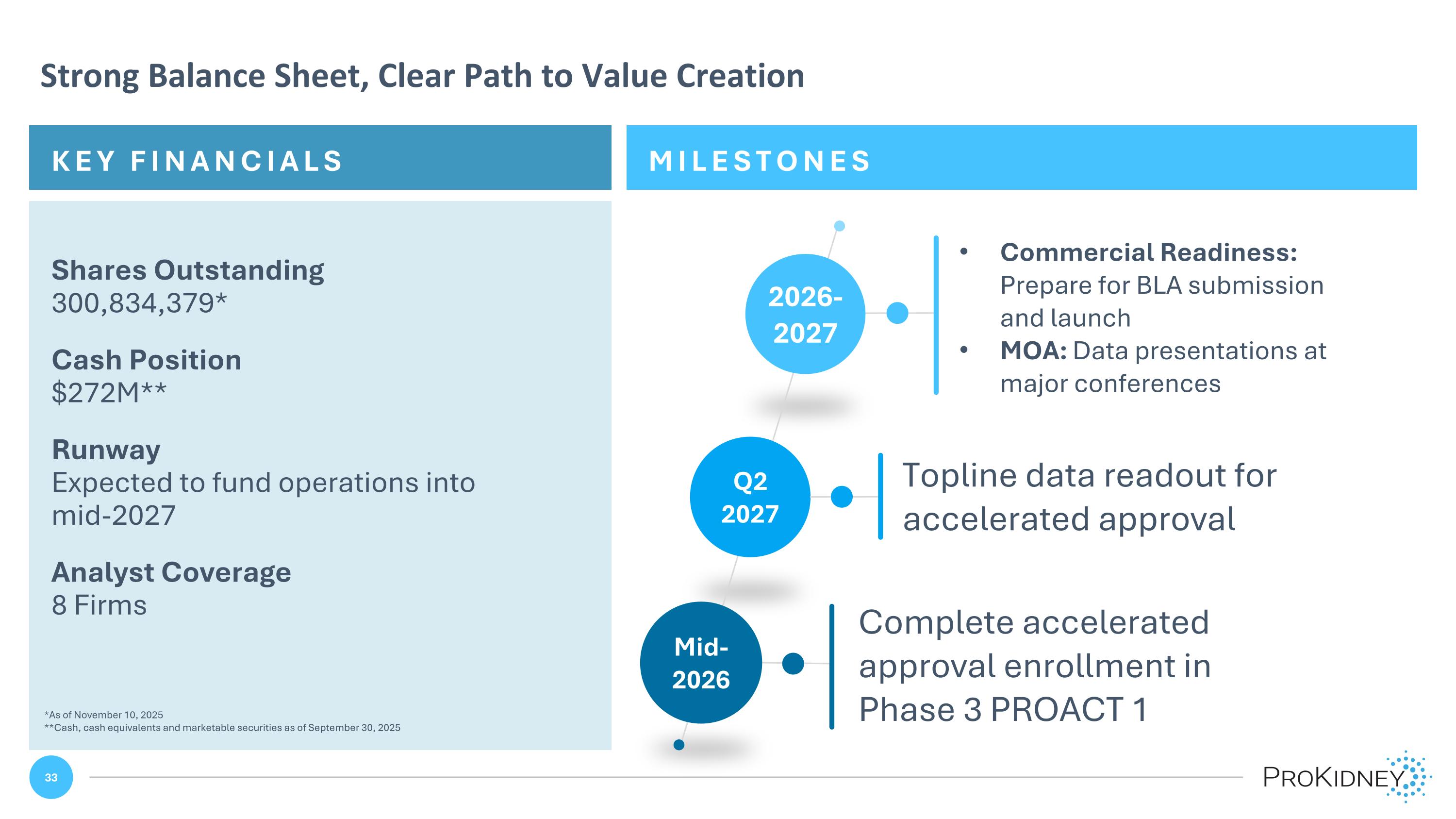
MILESTONES KEY FINANCIALS Strong Balance Sheet, Clear Path to Value Creation Shares Outstanding 300,834,379* Cash Position $272M** Runway Expected to fund operations into mid-2027 Analyst Coverage 8 Firms Mid-2026 2026-2027 Q2 2027 Complete accelerated approval enrollment in Phase 3 PROACT 1 Topline data readout for accelerated approval Commercial Readiness: Prepare for BLA submission and launch MOA: Data presentations at major conferences *As of November 10, 2025 **Cash, cash equivalents and marketable securities as of September 30, 2025

Patients Want More Time We are building a future where advanced CKD treatment means more options and more hope