| Corporate Overview January 12, 2026 Nasdaq Ticker: ZURA |
| ©2026 Zura Bio Ltd. 2 Forward Looking Statements Disclaimer This presentation contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,” “strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook,” “goal,” “mission,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations, estimates, and assumptions and, as a result, are subject to risks and uncertainties that could cause actual results to differ materially from the expected results. These statements are based on various assumptions, whether or not identified in this presentation. These forward-looking statements may include, without limitation: Zura Bio’s clinical development plans; the design, initiation, conduct, enrollment and timing of its clinical trials; expectations regarding the timing of key milestones and anticipated data readouts; expectations regarding Zura Bio’s clinical programs, including the safety, efficacy, and therapeutic potential of Zura Bio’s product candidates; expectations regarding the commercial potential of Zura Bio’s product candidates; expectations regarding data readouts from third parties; expectations regarding market opportunities, competitive landscape, addressable patient populations, or potential clinical differentiation; Zura Bio’s cash resources and projected cash runway; Zura Bio’s business strategies and objectives; and any other statements that are not historical facts. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on, by an investor as a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events are difficult or impossible to predict and could differ materially from those expressed or implied in such forward-looking statements, as a result of these risks and uncertainties, which include, but are not limited to: Zura Bio’s expectations regarding its product candidates and their related benefits, and Zura Bio’s beliefs regarding competing product candidates and products both in development and approved, may not be achieved; Zura Bio's vision and strategy may not be successful; the timing of key events and initiation of Zura Bio's studies, regulatory matters and release of clinical data may take longer than anticipated or may not be achieved at all; the potential general acceptability and maintenance of Zura Bio's product candidates by regulatory authorities, payors, physicians, and patients may not be achieved; Zura Bio's ability to attract and retain key personnel; Zura Bio's expectations with respect to its future operating expenses, capital requirements and needs for additional financing may not be achieved; Zura Bio has not completed any clinical trials, and has no products approved for commercial sale; Zura Bio has incurred significant losses since inception, and expects to incur significant losses for the foreseeable future and may not be able to achieve or sustain profitability in the future; Zura Bio requires substantial additional capital to finance its operations, and if it is unable to raise such capital when needed or on acceptable terms, Zura Bio may be forced to delay, reduce, and/or eliminate one or more of its development programs or future commercialization efforts; Zura Bio may be unable to renew existing contracts or enter into new contracts; Zura Bio relies on third-party contract development manufacturing organizations for the manufacture of clinical materials; Zura Bio relies on contract research organizations, clinical trial sites, and other third parties to conduct its preclinical studies and clinical trials; Zura Bio may be unable to obtain regulatory approval for its product candidates, and there may be related restrictions or limitations of any approved products; Zura Bio may be unable to successfully respond to general economic and geopolitical conditions; Zura Bio may be unable to effectively manage growth; Zura Bio faces competitive pressures from other companies worldwide; Zura Bio may be unable to adequately protect its intellectual property rights; and other factors set forth in documents filed, or to be filed by Zura Bio, with the Securities andExchange Commission (SEC), including the risks and uncertainties described in the “Risk Factors” section of Zura Bio’s Annual Report on Form 10-K for the year ended December 31, 2024, as supplemented by its Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2025, June 30, 2025 and September 30, 2025, and other filings with the SEC. These risks and uncertainties may be amplified by health epidemics or other unanticipated global disruption events, which may continue to cause economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking statements, which speak only as of the date made. Zura Bio gives no assurance that it will achieve its expectations. Zura Bio does not undertake or accept any obligation to update any forward-looking statements, whether as a result of new information, future developments, or otherwise, except as required by law.No assurance can be given that the expectations expressed herein will be achieved or that deviations from such expectations will not be material. This presentation discusses product candidates that are under clinical investigation and have not been approved for marketing by the U.S. Food and Drug Administration or any other regulatory authority. No representation is made as to the safety, efficacy, or likelihood of regulatory approval of these product candidates for the uses under investigation. Comparisons across clinical trials or product candidates should be interpreted with caution, as differences in study design, inclusion/exclusion criteria, patient populations, endpoints, dosing regimens, and other variables may limit interpretability. Statements in this presentation regarding clinical trials involving product candidates originating from third parties (including Eli Lilly, Pfizer and Novartis) have not been reviewed, verified, or endorsed by such parties. |
| ©2026 Zura Bio Ltd. Zura Bio: Advancing a Differentiated Approach for Complex Immune Disorders Our lead program, tibulizumab, is the first and currently only in-class bispecific antibody inhibiting both the IL-17 and BAFF pathways Fusion of components from tabalumab, a BAFF-binding antibody, and an IL-17–binding single-chain variable fragment derived from ixekizumab (marketed as Taltz®, ~$3B in global 2024 sales, as reported) Potent engagement of targets and low immunogenicity observed in completed Phase 1 studies Tibulizumab has been rationally designed to address complex autoimmune diseases not fully addressed by single-pathway therapies Dual-pathway inhibition offers the potential to overcome efficacy ceilings observed with single-pathway inhibition Potential to modulate both B-cell– and T-cell–driven pathobiology Initial clinical focus on hidradenitis suppurativa (HS) and systemic sclerosis (SSc), each characterized by complex immune pathobiology involving both B- and T-cell activation HS: potential to be the best-in-class treatment by inhibiting two pathways already independently validated SSc: potential to be first-in-disease treatment able to treat both skin and lung manifestations, where both IL-17 and BAFF have been implicated Zura Bio is financed beyond key near-term value inflection points Topline data expected from HS and SSc phase 2 studies in Q4 2026 and 1H 2027, respectively $139M in cash (as of Sept 30, 2025) expected to fund planned operations through 2027 ~101M shares outstanding (as-converted)* 3 Nasdaq: ZURA (*) Shares outstanding as of December 31, 2025; includes ~27.5 million shares issuable upon conversion of outstanding pre-funded warrants to purchase Class A ordinary shares. This figure does not reflect potential dilution from outstanding stock options or unvested RSUs. Sources: Zura Bio Ltd., public filings and disclosures; Evaluate Pharma; publicly available information on ixekizumab (Taltz®) and tabalumab. Acronyms: BAFF, B-cell activating factor; HS, hidradenitis suppurativa; IL-17, interleukin-17; scFv, single-chain variable fragment; SSc, systemic sclerosis. |
| ©2026 Zura Bio Ltd. Leadership Team and Board of Directors 4 Amit Munshi Chairman Sandeep Kulkarni, MD Founder & Director Someit Sidhu, MD Founder & Director Dan Becker, MD, PhD Director Neil Graham, MBBS, MD, MPH Director Jennifer Jarrett Director Steve Schoch Director Robert Lisicki CEO and Director Board of Directors Executive Team Kim Davis, JD Interim Chief Executive Officer and Chief Operating Officer Eric Hyllengren Chief Financial Officer Gary Whale, PhD Chief Technology Officer Kiran Nistala, MBBS, PhD Chief Medical Officer and Head of Development Nasdaq: ZURA |
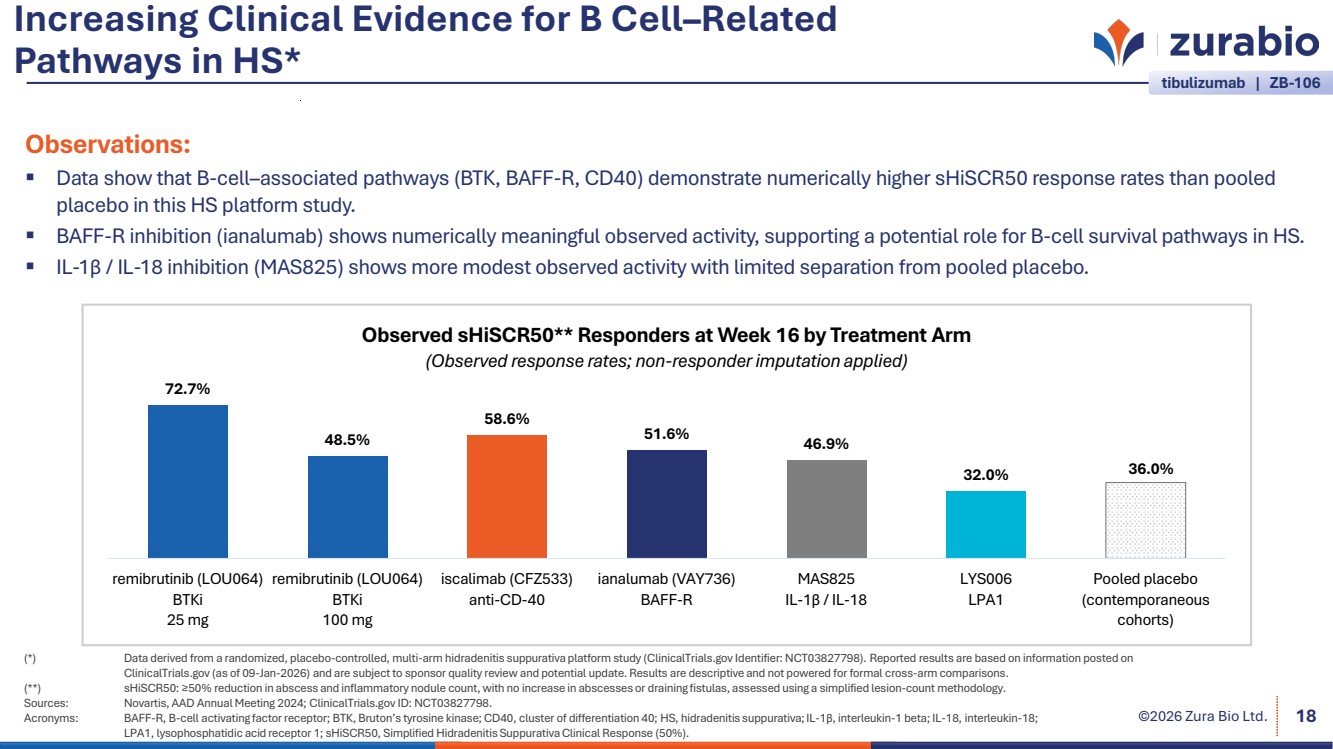
| ©2026 Zura Bio Ltd. Increasing Clinical Evidence for B Cell–Related Pathways in HS* 18 tibulizumab | ZB-106 (*) Data derived from a randomized, placebo-controlled, multi-arm hidradenitis suppurativa platform study (ClinicalTrials.gov Identifier: NCT03827798). Reported results are based on information posted on ClinicalTrials.gov (as of 09-Jan-2026) and are subject to sponsor quality review and potential update. Results are descriptive and not powered for formal cross-arm comparisons. (**) sHiSCR50: ≥50% reduction in abscess and inflammatory nodule count, with no increase in abscesses or draining fistulas, assessed using a simplified lesion-count methodology. Sources: Novartis, AAD Annual Meeting 2024; ClinicalTrials.gov ID: NCT03827798. Acronyms: BAFF-R, B-cell activating factor receptor; BTK, Bruton’s tyrosine kinase; CD40, cluster of differentiation 40; HS, hidradenitis suppurativa; IL-1 interleukin-1 beta; IL-18, interleukin-18; LPA1, lysophosphatidic acid receptor 1; sHiSCR50, Simplified Hidradenitis Suppurativa Clinical Response (50%). β, 72.7% 48.5% 58.6% 51.6% 46.9% 32.0% 36.0% remibrutinib (LOU064) BTKi 25 mg remibrutinib (LOU064) BTKi 100 mg iscalimab (CFZ533) anti-CD-40 ianalumab (VAY736) BAFF-R MAS825 IL-1β / IL-18 LYS006 LPA1 Pooled placebo (contemporaneous cohorts) Observed sHiSCR50** Responders at Week 16 by Treatment Arm (Observed response rates; non-responder imputation applied) Observations: Data show that B-cell–associated pathways (BTK, BAFF-R, CD40) demonstrate numerically higher sHiSCR50 response rates than pooled placebo in this HS platform study. BAFF-R inhibition (ianalumab) shows numerically meaningful observed activity, supporting a potential role for B-cell survival pathways in HS. IL-1β / IL-18 inhibition (MAS825) shows more modest observed activity with limited separation from pooled placebo. |
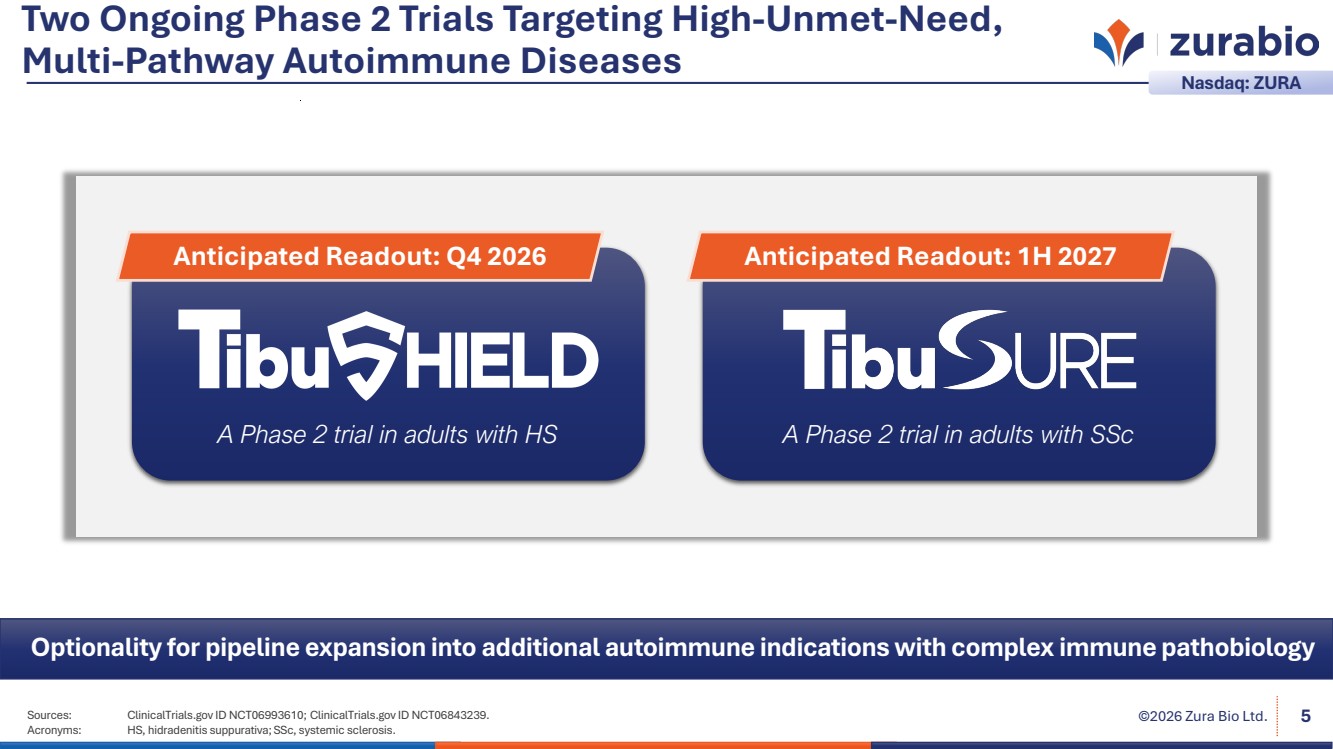
| Sources: ClinicalTrials.gov ID NCT06993610; ClinicalTrials.gov ID NCT06843239. ©2026 Zura Bio Ltd. Acronyms: HS, hidradenitis suppurativa; SSc, systemic sclerosis. 5 Two Ongoing Phase 2 Trials Targeting High-Unmet-Need, Multi-Pathway Autoimmune Diseases Nasdaq: ZURA A Phase 2 trial in adults with HS Anticipated Readout: Q4 2026 A Phase 2 trial in adults with SSc Anticipated Readout: 1H 2027 Optionality for pipeline expansion into additional autoimmune indications with complex immune pathobiology |
| ©2026 Zura Bio Ltd. Limits of Monotherapy in Multi-Pathway Autoimmune Disease Acronyms: HS, hidradenitis suppurativa; SSc, systemic sclerosis. 6 Nasdaq: ZURA Implication: These limitations support therapeutic approaches designed to address more than one disease-driving pathway with a single agent Autoimmune diseases, including HS and SSc, are driven by multiple, intersecting immune pathways, resulting in biological heterogeneity across patients Single-pathway biologics have delivered meaningful benefit, but durable disease control is not achieved in all patients Pathway redundancy and compensatory signaling can limit the depth and durability of response with monotherapy |
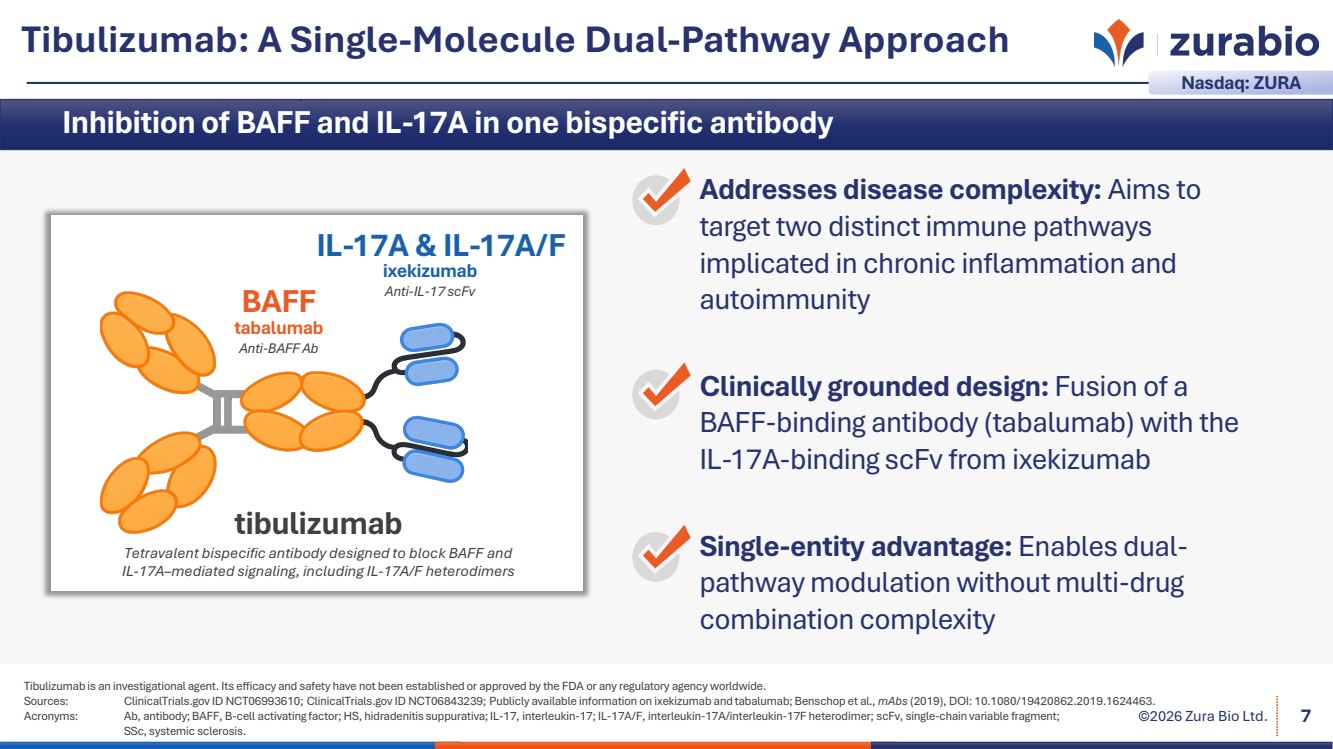
| ©2026 Zura Bio Ltd. Tibulizumab: A Single-Molecule Dual-Pathway Approach Tibulizumab is an investigational agent. Its efficacy and safety have not been established or approved by the FDA or any regulatory agency worldwide. Sources: ClinicalTrials.gov ID NCT06993610; ClinicalTrials.gov ID NCT06843239; Publicly available information on ixekizumab and tabalumab; Benschop et al., mAbs (2019), DOI: 10.1080/19420862.2019.1624463. Acronyms: Ab, antibody; BAFF, B-cell activating factor; HS, hidradenitis suppurativa; IL-17, interleukin-17; IL-17A/F, interleukin-17A/interleukin-17F heterodimer; scFv, single-chain variable fragment; SSc, systemic sclerosis. 7 Nasdaq: ZURA Addresses disease complexity: Aims to target two distinct immune pathways implicated in chronic inflammation and autoimmunity Clinically grounded design: Fusion of a BAFF-binding antibody (tabalumab) with the IL-17A-binding scFv from ixekizumab Single-entity advantage: Enables dual-pathway modulation without multi-drug combination complexity ixekizumab Anti-IL-17 scFv IL-17A & IL-17A/F tabalumab Anti-BAFF Ab BAFF tibulizumab Tetravalent bispecific antibody designed to block BAFF and IL-17A–mediated signaling, including IL-17A/F heterodimers Inhibition of BAFF and IL-17A in one bispecific antibody |
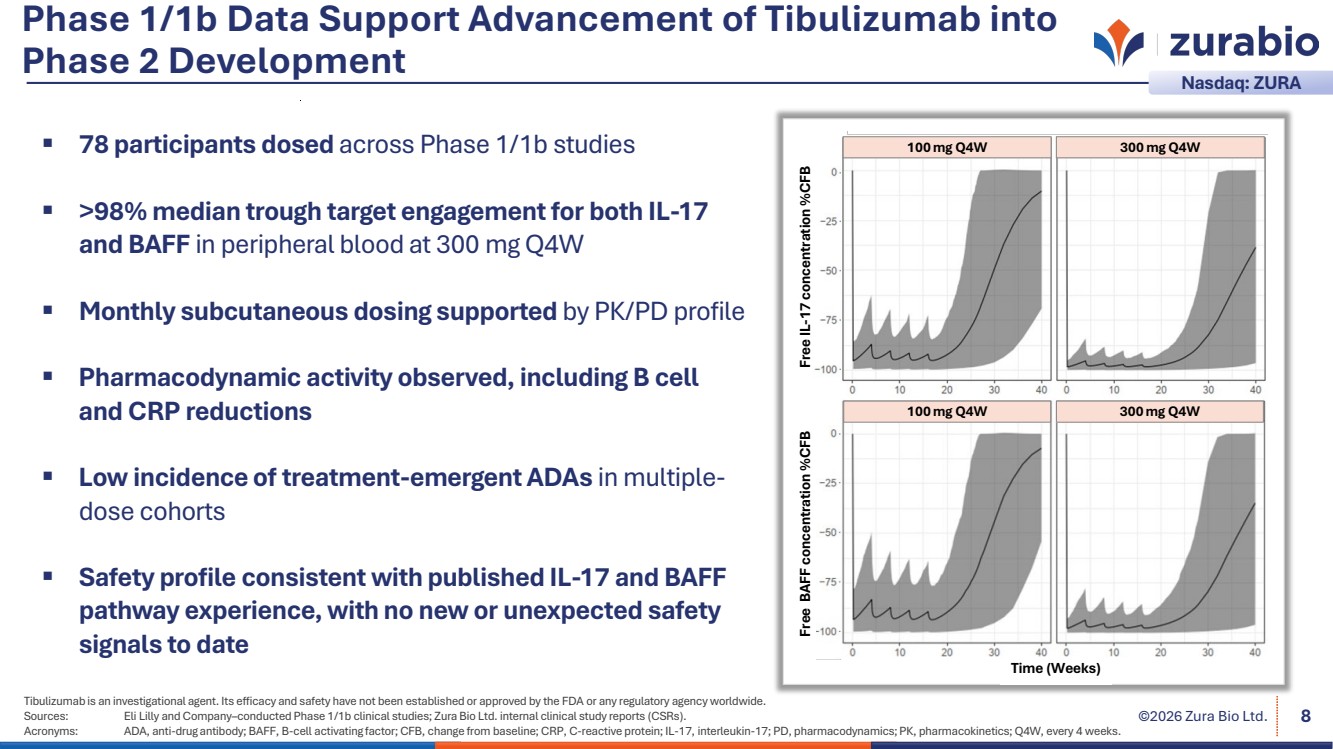
| ©2026 Zura Bio Ltd. Phase 1/1b Data Support Advancement of Tibulizumab into Phase 2 Development 8 78 participants dosed across Phase 1/1b studies >98% median trough target engagement for both IL-17 and BAFF in peripheral blood at 300 mg Q4W Monthly subcutaneous dosing supported by PK/PD profile Pharmacodynamic activity observed, including B cell and CRP reductions Low incidence of treatment-emergent ADAs in multiple-dose cohorts Safety profile consistent with published IL-17 and BAFF pathway experience, with no new or unexpected safety signals to date Time (Weeks) Free BAFF concentration %CFB Free IL-17 concentration %CFB 100 mg Q4W 300 mg Q4W 100 mg Q4W 300 mg Q4W Tibulizumab is an investigational agent. Its efficacy and safety have not been established or approved by the FDA or any regulatory agency worldwide. Sources: Eli Lilly and Company–conducted Phase 1/1b clinical studies; Zura Bio Ltd. internal clinical study reports (CSRs). Acronyms: ADA, anti-drug antibody; BAFF, B-cell activating factor; CFB, change from baseline; CRP, C-reactive protein; IL-17, interleukin-17; PD, pharmacodynamics; PK, pharmacokinetics; Q4W, every 4 weeks. Nasdaq: ZURA |
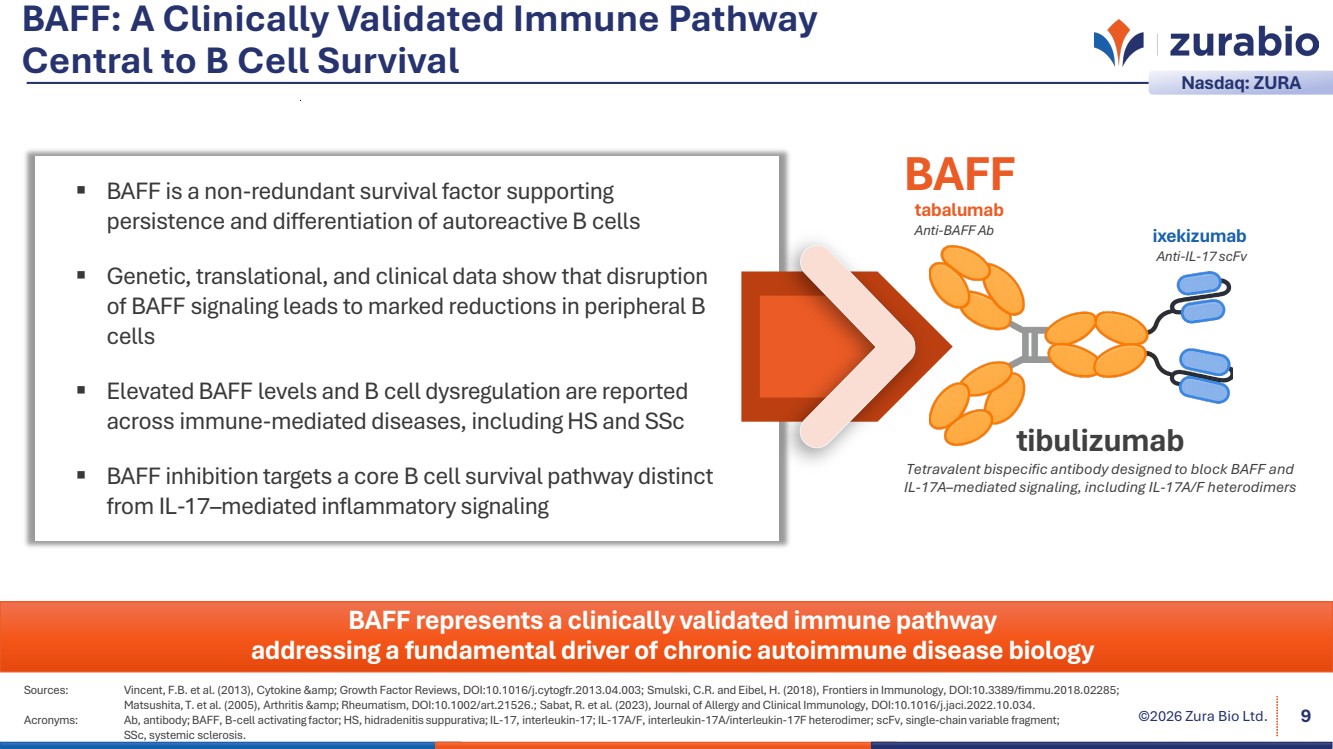
| ©2026 Zura Bio Ltd. BAFF: A Clinically Validated Immune Pathway Central to B Cell Survival 9 Sources: Vincent, F.B. et al. (2013), Cytokine & Growth Factor Reviews, DOI:10.1016/j.cytogfr.2013.04.003; Smulski, C.R. and Eibel, H. (2018), Frontiers in Immunology, DOI:10.3389/fimmu.2018.02285; Matsushita, T. et al. (2005), Arthritis & Rheumatism, DOI:10.1002/art.21526.; Sabat, R. et al. (2023), Journal of Allergy and Clinical Immunology, DOI:10.1016/j.jaci.2022.10.034. Acronyms: Ab, antibody; BAFF, B-cell activating factor; HS, hidradenitis suppurativa; IL-17, interleukin-17; IL-17A/F, interleukin-17A/interleukin-17F heterodimer; scFv, single-chain variable fragment; SSc, systemic sclerosis. Nasdaq: ZURA BAFF represents a clinically validated immune pathway addressing a fundamental driver of chronic autoimmune disease biology BAFF is a non-redundant survival factor supporting persistence and differentiation of autoreactive B cells Genetic, translational, and clinical data show that disruption of BAFF signaling leads to marked reductions in peripheral B cells Elevated BAFF levels and B cell dysregulation are reported across immune-mediated diseases, including HS and SSc BAFF inhibition targets a core B cell survival pathway distinct from IL-17–mediated inflammatory signaling ixekizumab Anti-IL-17 scFv tabalumab Anti-BAFF Ab BAFF tibulizumab Tetravalent bispecific antibody designed to block BAFF and IL-17A–mediated signaling, including IL-17A/F heterodimers |
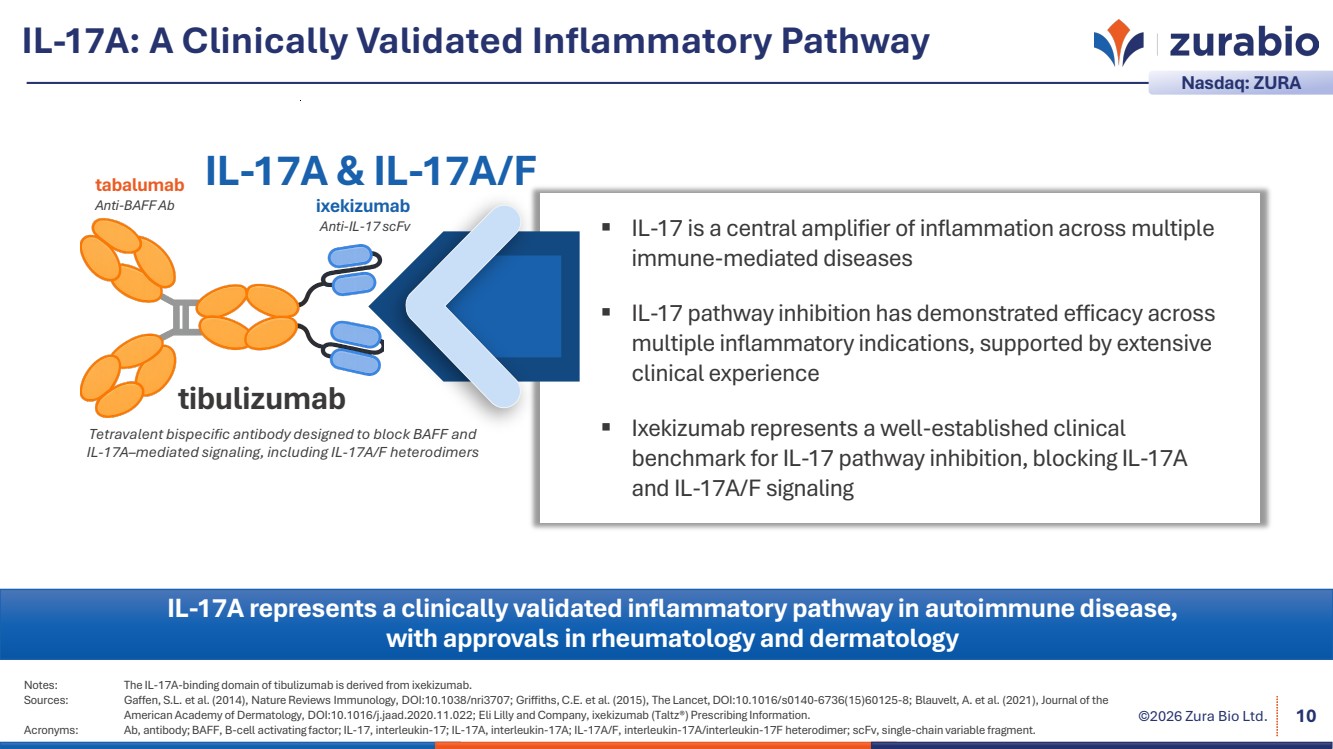
| ©2026 Zura Bio Ltd. IL-17A: A Clinically Validated Inflammatory Pathway 10 Notes: The IL-17A-binding domain of tibulizumab is derived from ixekizumab. Sources: Gaffen, S.L. et al. (2014), Nature Reviews Immunology, DOI:10.1038/nri3707; Griffiths, C.E. et al. (2015), The Lancet, DOI:10.1016/s0140-6736(15)60125-8; Blauvelt, A. et al. (2021), Journal of the American Academy of Dermatology, DOI:10.1016/j.jaad.2020.11.022; Eli Lilly and Company, ixekizumab (Taltz®) Prescribing Information. Acronyms: Ab, antibody; BAFF, B-cell activating factor; IL-17, interleukin-17; IL-17A, interleukin-17A; IL-17A/F, interleukin-17A/interleukin-17F heterodimer; scFv, single-chain variable fragment. IL-17A represents a clinically validated inflammatory pathway in autoimmune disease, with approvals in rheumatology and dermatology Nasdaq: ZURA IL-17 is a central amplifier of inflammation across multiple immune-mediated diseases IL-17 pathway inhibition has demonstrated efficacy across multiple inflammatory indications, supported by extensive clinical experience Ixekizumab represents a well-established clinical benchmark for IL-17 pathway inhibition, blocking IL-17A and IL-17A/F signaling ixekizumab Anti-IL-17 scFv tabalumab Anti-BAFF Ab tibulizumab IL-17A & IL-17A/F Tetravalent bispecific antibody designed to block BAFF and IL-17A–mediated signaling, including IL-17A/F heterodimers |
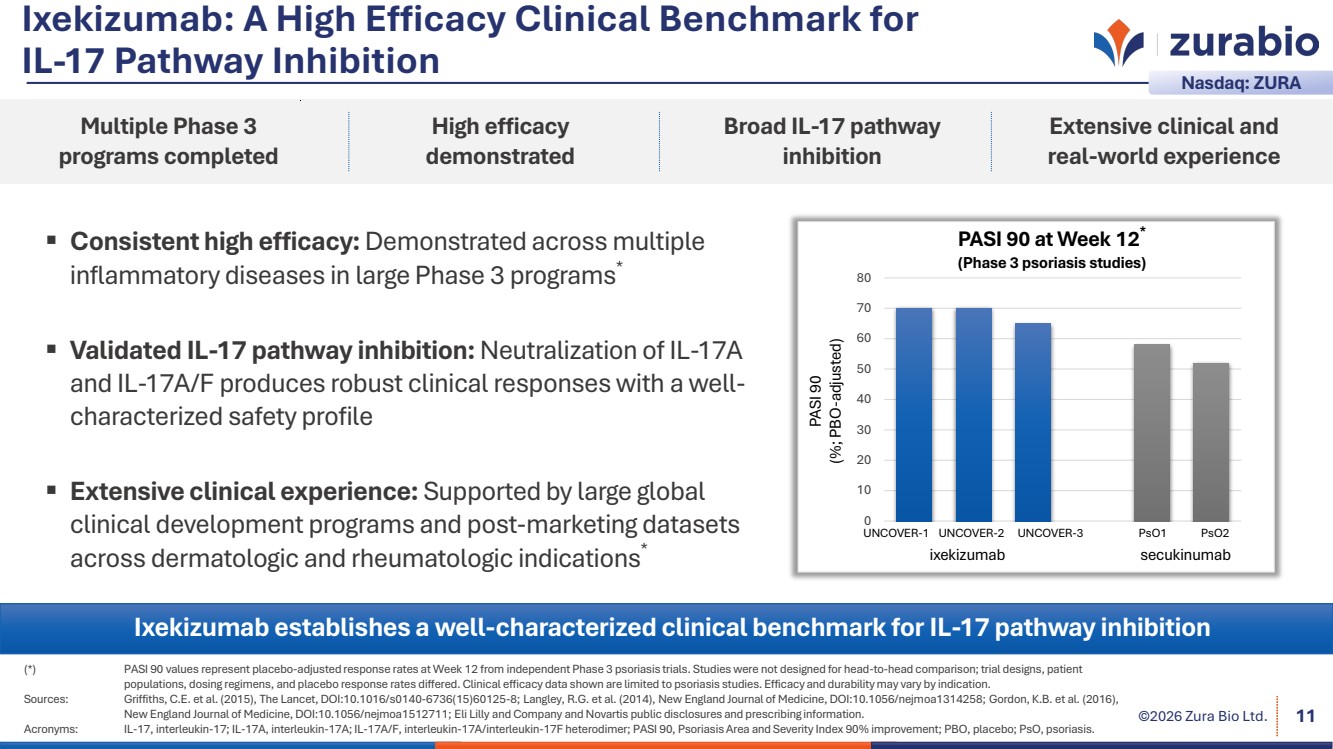
| ©2026 Zura Bio Ltd. Ixekizumab: A High Efficacy Clinical Benchmark for IL-17 Pathway Inhibition Consistent high efficacy: Demonstrated across multiple inflammatory diseases in large Phase 3 programs* Validated IL-17 pathway inhibition: Neutralization of IL-17A and IL-17A/F produces robust clinical responses with a well-characterized safety profile Extensive clinical experience: Supported by large global clinical development programs and post-marketing datasets across dermatologic and rheumatologic indications* (*) PASI 90 values represent placebo-adjusted response rates at Week 12 from independent Phase 3 psoriasis trials. Studies were not designed for head-to-head comparison; trial designs, patient populations, dosing regimens, and placebo response rates differed. Clinical efficacy data shown are limited to psoriasis studies. Efficacy and durability may vary by indication. Sources: Griffiths, C.E. et al. (2015), The Lancet, DOI:10.1016/s0140-6736(15)60125-8; Langley, R.G. et al. (2014), New England Journal of Medicine, DOI:10.1056/nejmoa1314258; Gordon, K.B. et al. (2016), New England Journal of Medicine, DOI:10.1056/nejmoa1512711; Eli Lilly and Company and Novartis public disclosures and prescribing information. Acronyms: IL-17, interleukin-17; IL-17A, interleukin-17A; IL-17A/F, interleukin-17A/interleukin-17F heterodimer; PASI 90, Psoriasis Area and Severity Index 90% improvement; PBO, placebo; PsO, psoriasis. 11 Ixekizumab establishes a well-characterized clinical benchmark for IL-17 pathway inhibition Multiple Phase 3 programs completed High efficacy demonstrated Broad IL-17 pathway inhibition Extensive clinical and real-world experience Nasdaq: ZURA secukinumab PASI 90 at Week 12* (Phase 3 psoriasis studies) PASI 90 (%; PBO-adjusted) 0 10 20 30 40 50 60 70 80 ixekizumab UNCOVER-1 UNCOVER-2 UNCOVER-3 PsO1 PsO2 |
| ©2026 Zura Bio Ltd. Tibulizumab Targets Two Potentially High-Value Autoimmune Market Opportunities 12 Nasdaq: ZURA Note: Market estimates based on third-party analyses and published literature, including DRG/Clarivate, Evaluate Pharma, GlobalData, peer-reviewed epidemiology studies, and company analyses. Acronyms: TAM, total addressable market. Potential Significant Multi-Billion Dollar Market Opportunities Growing biologics market supported by increasing diagnosis and utilization Chronic inflammatory disease with durable treatment demand and high unmet need Heterogeneous disease biology creates opportunity beyond single-pathway approaches Large and Expanding Chronic Disease HIDRADENITIS SUPPURATIVA ~$8B projected by the mid-2030’s ESTIMATED TAM Rare autoimmune disease with significant unmet need and limited effective treatment options Specialist-managed market with concentrated prescribing and premium pricing dynamics Multisystem inflammatory and fibrotic manifestations contribute to substantial disease burden High-Value Orphan Autoimmune Opportunity SYSTEMIC SCLEROSIS ~$4B projected by mid-2030’s ESTIMATED TAM |
| ©2026 Zura Bio Ltd. A Dual-Pathway Approach in HS tibulizumab ZB-106 Anti-BAFF + IL-17 First and currently only in-class bispecific antibody designed to target BAFF and IL-17 signaling, including IL-17A and IL-17A/F Phase 2 clinical trial (TibuSHIELD) ongoing; top line data anticipated in Q4 2026 Tibulizumab is an investigational agent. Its efficacy and safety have not been established or approved by the FDA or any other regulatory agency worldwide. |
| Nasdaq: ZURA | www.zurabio.com HS Is a Complex and Relapsing Disease ©2026 Zura Bio Ltd. The interplay of acute lesions, chronic inflammation, and complex immune drivers makes HS uniquely challenging, and highlights the opportunity for biology-driven solutions. Patient Experience Painful nodules and abscesses form in sensitive areas Lesions may progress to tunnels and scarring Acute flares coexist with chronic inflammation HS patients may experience delayed diagnosis and variable treatment results Underlying Biology HS lesions are driven by a diverse, dynamic immune microenvironment Prominent immune signatures: Th1/Th17, neutrophils, B cells Chronic immune activity underlies persistent, relapsing disease course Sources: Moran, B. et al. (2017), Journal of Investigative Dermatology, DOI:10.1016/j.jid.2017.05.033; Banerjee, A. et al. (2017), Immunological Investigations, doi:10.1080/08820139.2016.1230867; Sabat, R. et al. (2023), Journal of Allergy and Clinical Immunology, DOI:10.1016/j.jaci.2022.10.034; Garg, A. et al. (2017), JAMA Dermatology, DOI:10.1001/jamadermatol.2017.0201; Ingram, J.R. (2020), British Journal of Dermatology, DOI:10.1111/bjd.19435; Midgette, B. et al. (2022), British Journal of Dermatology, doi:10.1111/bjd.21798; MEDACorp key opinion leader (KOL) discussions. Acronyms: HS, hidradenitis suppurativa; Th1, T helper 1 cell; Th17, T helper 17 cell. tibulizumab | ZB-106 14 |
| ©2026 Zura Bio Ltd. A Dual-Pathway Approach to Hidradenitis Suppurativa 15 Targeting immune drivers of chronic inflammation and tissue damage Disease Chronic, inflammatory skin disease with recurrent painful lesions Significant impact on quality of life and long-term morbidity Biology Heterogeneous disease driven by activation of multiple immune pathways BAFF elevation and associated B cell dysregulation observed in HS lesions IL-17A–driven inflammation implicated in dysfunction of neutrophils, macrophages, and keratinocytes Program Tibulizumab (BAFF + IL-17A bispecific antibody) Phase 2 TibuSHIELD clinical study in adults with HS; Topline data expected Q4 2026 Th17 cell IL-17A IL-17A B cell tibulizumab | ZB-106 Acronyms: BAFF, B cell activating factor; HS, hidradenitis suppurativa; IL-17A, interleukin-17A; Th17, T helper 17 cells. |
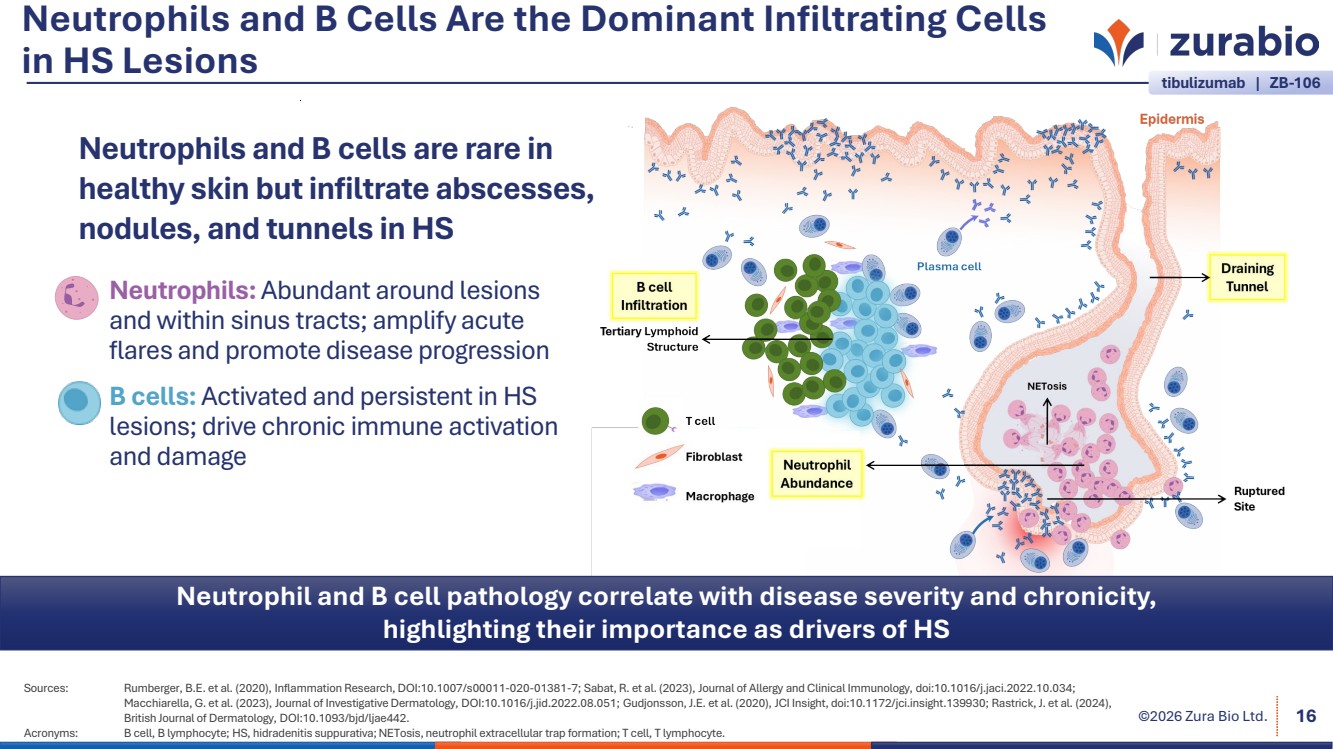
| ©2026 Zura Bio Ltd. Neutrophils and B Cells Are the Dominant Infiltrating Cells in HS Lesions Sources: Rumberger, B.E. et al. (2020), Inflammation Research, DOI:10.1007/s00011-020-01381-7; Sabat, R. et al. (2023), Journal of Allergy and Clinical Immunology, doi:10.1016/j.jaci.2022.10.034; Macchiarella, G. et al. (2023), Journal of Investigative Dermatology, DOI:10.1016/j.jid.2022.08.051; Gudjonsson, J.E. et al. (2020), JCI Insight, doi:10.1172/jci.insight.139930; Rastrick, J. et al. (2024), British Journal of Dermatology, DOI:10.1093/bjd/ljae442. Acronyms: B cell, B lymphocyte; HS, hidradenitis suppurativa; NETosis, neutrophil extracellular trap formation; T cell, T lymphocyte. 16 Neutrophil and B cell pathology correlate with disease severity and chronicity, highlighting their importance as drivers of HS Neutrophils: Abundant around lesions and within sinus tracts; amplify acute flares and promote disease progression B cells: Activated and persistent in HS lesions; drive chronic immune activation and damage tibulizumab | ZB-106 Neutrophils and B cells are rare in healthy skin but infiltrate abscesses, nodules, and tunnels in HS |
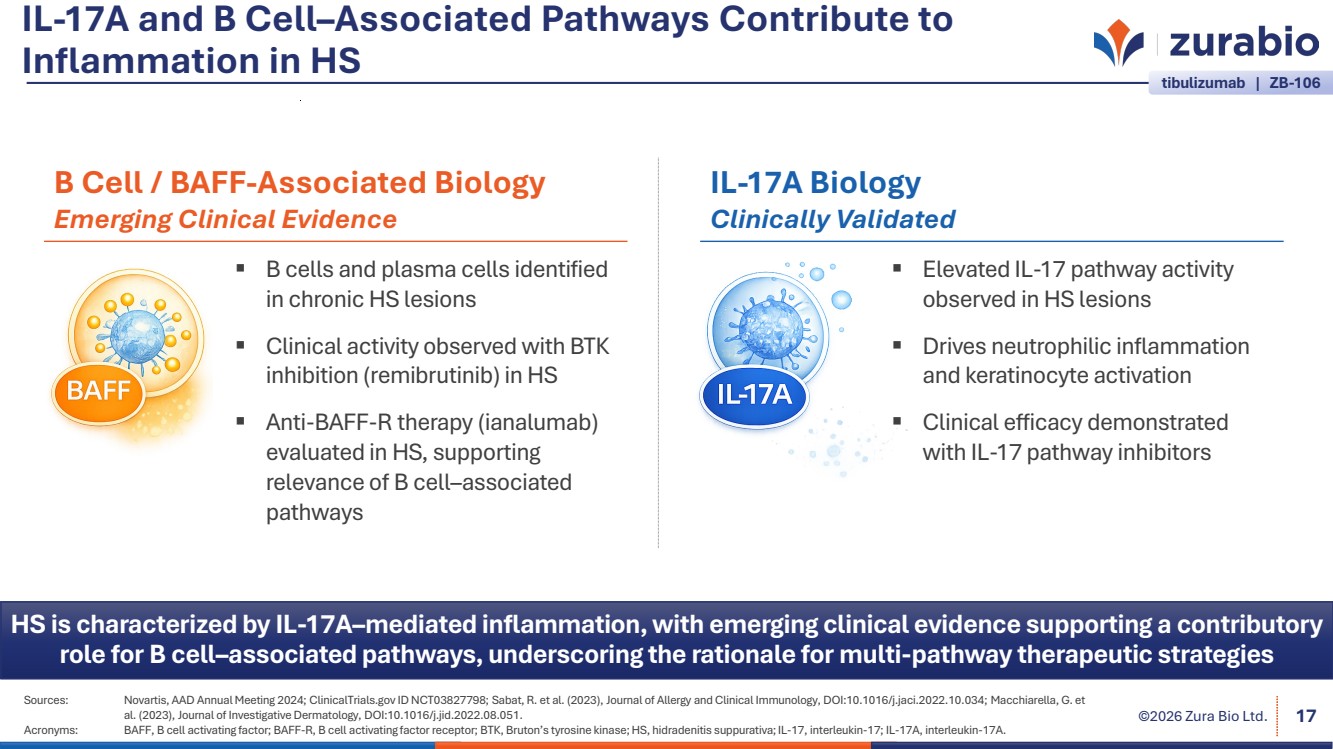
| ©2026 Zura Bio Ltd. IL-17A and B Cell–Associated Pathways Contribute to Inflammation in HS Sources: Novartis, AAD Annual Meeting 2024; ClinicalTrials.gov ID NCT03827798; Sabat, R. et al. (2023), Journal of Allergy and Clinical Immunology, DOI:10.1016/j.jaci.2022.10.034; Macchiarella, G. et al. (2023), Journal of Investigative Dermatology, DOI:10.1016/j.jid.2022.08.051. Acronyms: BAFF, B cell activating factor; BAFF-R, B cell activating factor receptor; BTK, Bruton’s tyrosine kinase; HS, hidradenitis suppurativa; IL-17, interleukin-17; IL-17A, interleukin-17A. 17 HS is characterized by IL-17A–mediated inflammation, with emerging clinical evidence supporting a contributory role for B cell–associated pathways, underscoring the rationale for multi-pathway therapeutic strategies tibulizumab | ZB-106 B cells and plasma cells identified in chronic HS lesions Clinical activity observed with BTK inhibition (remibrutinib) in HS Anti-BAFF-R therapy (ianalumab) evaluated in HS, supporting relevance of B cell–associated pathways B Cell / BAFF-Associated Biology Emerging Clinical Evidence Elevated IL-17 pathway activity observed in HS lesions Drives neutrophilic inflammation and keratinocyte activation Clinical efficacy demonstrated with IL-17 pathway inhibitors IL-17A Biology Clinically Validated |
| ©2026 Zura Bio Ltd. Increasing Clinical Evidence for B Cell–Related Pathways in HS* 18 tibulizumab | ZB-106 (*) Data derived from a randomized, placebo-controlled, multi-arm hidradenitis suppurativa platform study (ClinicalTrials.gov Identifier: NCT03827798). Reported results are based on information posted on ClinicalTrials.gov (as of 09-Jan-2026) and are subject to sponsor quality review and potential update. Results are descriptive and not powered for formal cross-arm comparisons. (**) sHiSCR50: ≥50% reduction in abscess and inflammatory nodule count, with no increase in abscesses or draining fistulas, assessed using a simplified lesion-count methodology. Sources: Novartis, AAD Annual Meeting 2024; ClinicalTrials.gov ID: NCT03827798. Acronyms: BAFF-R, B-cell activating factor receptor; BTK, Bruton’s tyrosine kinase; CD40, cluster of differentiation 40; HS, hidradenitis suppurativa; IL-1 interleukin-1 beta; IL-18, interleukin-18; LPA1, lysophosphatidic acid receptor 1; sHiSCR50, Simplified Hidradenitis Suppurativa Clinical Response (50%). β, 72.7% 48.5% 58.6% 51.6% 46.9% 32.0% 36.0% remibrutinib (LOU064) BTKi 25 mg remibrutinib (LOU064) BTKi 100 mg iscalimab (CFZ533) anti-CD-40 ianalumab (VAY736) BAFF-R MAS825 IL-1β / IL-18 LYS006 LPA1 Pooled placebo (contemporaneous cohorts) Observed sHiSCR50** Responders at Week 16 by Treatment Arm (Observed response rates; non-responder imputation applied) Observations:  Data show that B-cell–associated pathways (BTK, BAFF-R, CD40) demonstrate numerically higher sHiSCR50 response rates than pooled placebo in this HS platform study.  BAFF-R inhibition (ianalumab) shows numerically meaningful observed activity, supporting a potential role for B-cell survival pathways in HS.  IL-1β / IL-18 inhibition (MAS825) shows more modest observed activity with limited separation from pooled placebo. |
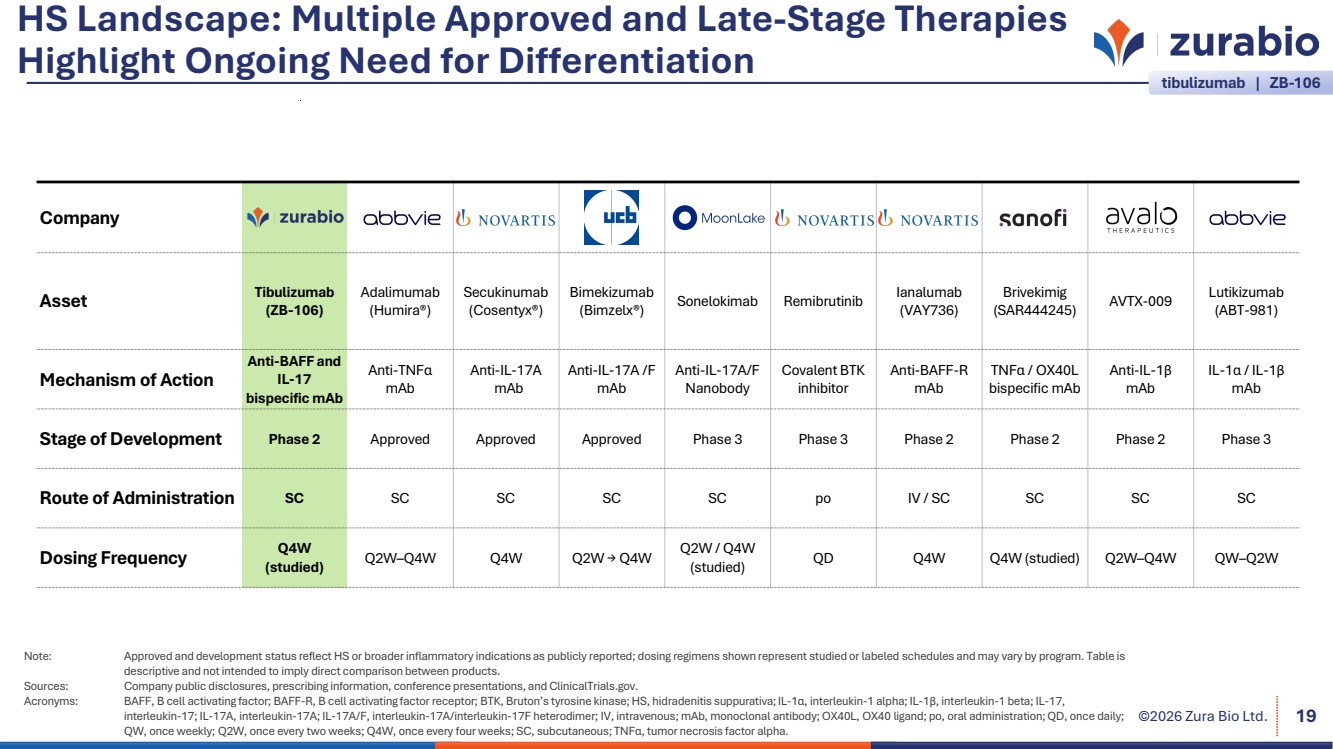
| ©2026 Zura Bio Ltd. HS Landscape: Multiple Approved and Late-Stage Therapies Highlight Ongoing Need for Differentiation 19 tibulizumab | ZB-106 Company Asset Tibulizumab (ZB-106) Adalimumab (Humira®) Secukinumab (Cosentyx®) Bimekizumab (Bimzelx®) Sonelokimab Remibrutinib Ianalumab (VAY736) Brivekimig (SAR444245) AVTX-009 Lutikizumab (ABT-981) Mechanism of Action Anti-BAFF and IL-17 bispecific mAb Anti-TNFα mAb Anti-IL-17A mAb Anti-IL-17A /F mAb Anti-IL-17A/F Nanobody Covalent BTK inhibitor Anti-BAFF-R mAb TNFα / OX40L bispecific mAb Anti-IL-1β mAb IL-1α / IL-1β mAb Stage of Development Phase 2 Approved Approved Approved Phase 3 Phase 3 Phase 2 Phase 2 Phase 2 Phase 3 Route of Administration SC SC SC SC SC po IV / SC SC SC SC Dosing Frequency Q4W (studied) Q2W–Q4W Q4W Q2W → Q4W Q2W / Q4W (studied) QD Q4W Q4W (studied) Q2W–Q4W QW–Q2W Note: Approved and development status reflect HS or broader inflammatory indications as publicly reported; dosing regimens shown represent studied or labeled schedules and may vary by program. Table is descriptive and not intended to imply direct comparison between products. Sources: Company public disclosures, prescribing information, conference presentations, and ClinicalTrials.gov. Acronyms: BAFF, B cell activating factor; BAFF-R, B cell activating factor receptor; BTK, Bruton’s tyrosine kinase; HS, hidradenitis suppurativa; IL-1α, interleukin-1 alpha; IL-1β, interleukin-1 beta; IL-17, interleukin-17; IL-17A, interleukin-17A; IL-17A/F, interleukin-17A/interleukin-17F heterodimer; IV, intravenous; mAb, monoclonal antibody; OX40L, OX40 ligand; po, oral administration; QD, once daily; QW, once weekly; Q2W, once every two weeks; Q4W, once every four weeks; SC, subcutaneous; TNFα, tumor necrosis factor alpha. |
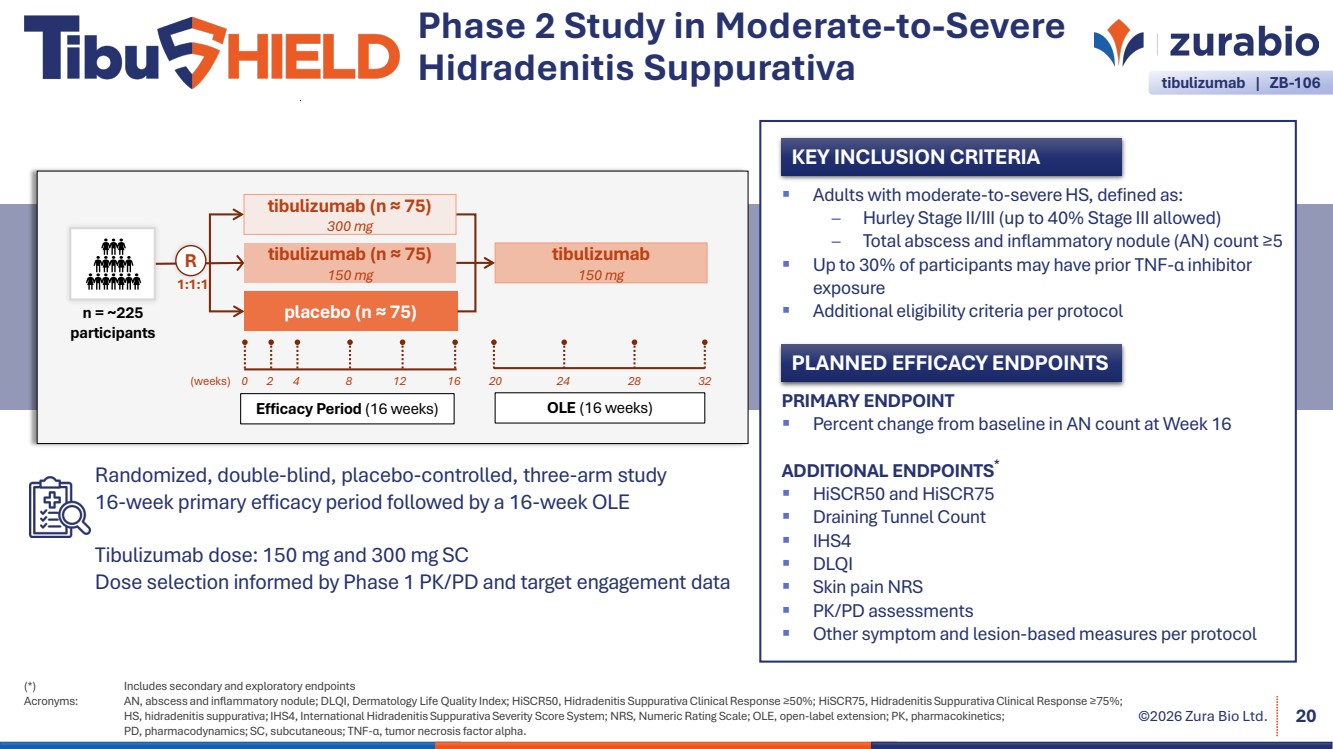
| ©2026 Zura Bio Ltd. 20 (*) Includes secondary and exploratory endpoints Acronyms: AN, abscess and inflammatory nodule; DLQI, Dermatology Life Quality Index; HiSCR50, Hidradenitis Suppurativa Clinical Response ≥50%; HiSCR75, Hidradenitis Suppurativa Clinical Response ≥75%; HS, hidradenitis suppurativa; IHS4, International Hidradenitis Suppurativa Severity Score System; NRS, Numeric Rating Scale; OLE, open-label extension; PK, pharmacokinetics; PD, pharmacodynamics; SC, subcutaneous; TNF-α, tumor necrosis factor alpha. KEY INCLUSION CRITERIA PLANNED EFFICACY ENDPOINTS PRIMARY ENDPOINT Percent change from baseline in AN count at Week 16 ADDITIONAL ENDPOINTS* HiSCR50 and HiSCR75 Draining Tunnel Count IHS4 DLQI Skin pain NRS PK/PD assessments Other symptom and lesion-based measures per protocol Adults with moderate-to-severe HS, defined as: – Hurley Stage II/III (up to 40% Stage III allowed) – Total abscess and inflammatory nodule (AN) count ≥5 Up to 30% of participants may have prior TNF-α inhibitor exposure Additional eligibility criteria per protocol Efficacy Period (16 weeks) OLE (16 weeks) (weeks) 1:1:1 R tibulizumab (n ≈ 75) 150 mg n = ~225 placebo (n ≈ 75) participants tibulizumab (n ≈ 75) 300 mg tibulizumab 150 mg 0 2 4 8 12 16 20 24 28 32 Randomized, double-blind, placebo-controlled, three-arm study 16-week primary efficacy period followed by a 16-week OLE Tibulizumab dose: 150 mg and 300 mg SC Dose selection informed by Phase 1 PK/PD and target engagement data Phase 2 Study in Moderate-to-Severe Hidradenitis Suppurativa tibulizumab | ZB-106 |
| ©2026 Zura Bio Ltd. A Dual-Pathway Approach in SSc tibulizumab ZB-106 Anti-BAFF + IL-17 First and currently only in-class bispecific antibody designed to target BAFF and IL-17 signaling, including IL-17A and IL-17A/F Phase 2 clinical trial (TibuSURE) ongoing; topline data anticipated in 1H 2027 Tibulizumab is an investigational agent. Its efficacy and safety have not been established or approved by the FDA or any other regulatory agency worldwide. |
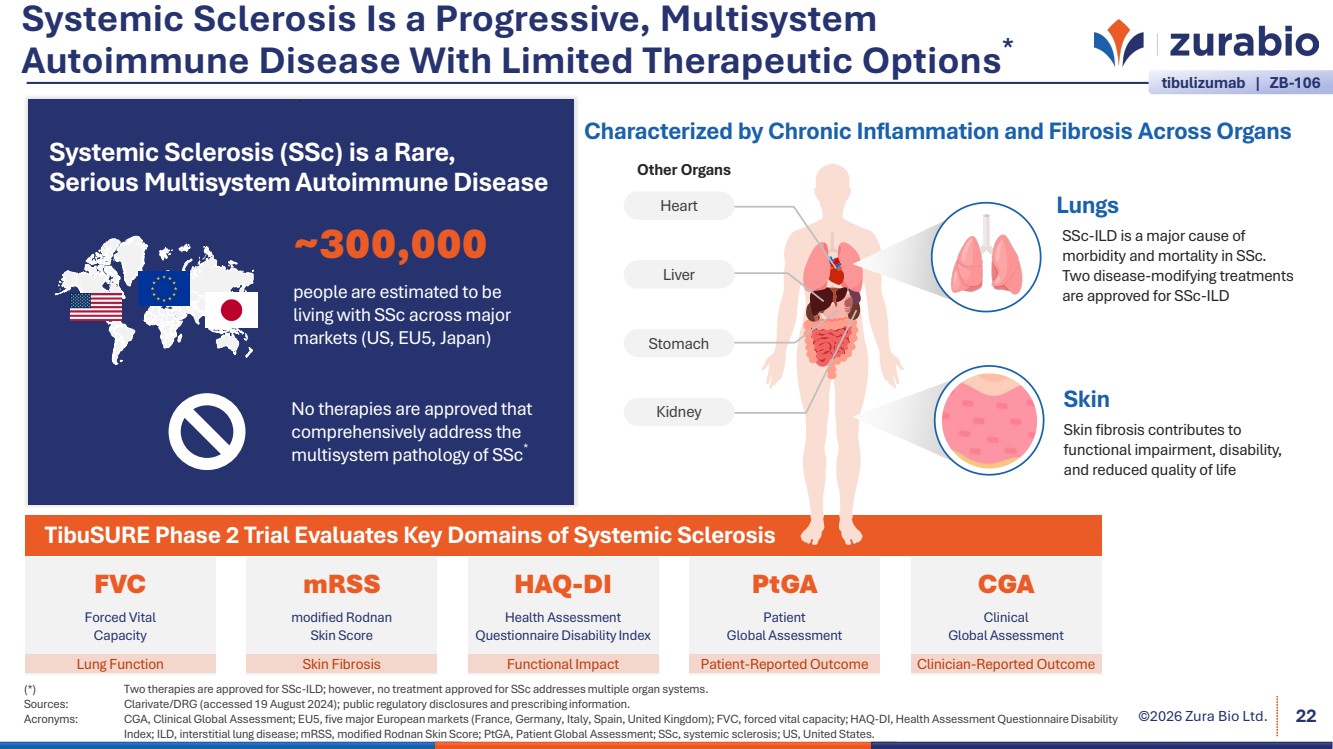
| ©2026 Zura Bio Ltd. Systemic Sclerosis Is a Progressive, Multisystem Autoimmune Disease With Limited Therapeutic Options* (*) Two therapies are approved for SSc-ILD; however, no treatment approved for SSc addresses multiple organ systems. Sources: Clarivate/DRG (accessed 19 August 2024); public regulatory disclosures and prescribing information. Acronyms: CGA, Clinical Global Assessment; EU5, five major European markets (France, Germany, Italy, Spain, United Kingdom); FVC, forced vital capacity; HAQ-DI, Health Assessment Questionnaire Disability Index; ILD, interstitial lung disease; mRSS, modified Rodnan Skin Score; PtGA, Patient Global Assessment; SSc, systemic sclerosis; US, United States. 22 Systemic Sclerosis (SSc) is a Rare, Serious Multisystem Autoimmune Disease No therapies are approved that comprehensively address the multisystem pathology of SSc* ~300,000 people are estimated to be living with SSc across major markets (US, EU5, Japan) TibuSURE Phase 2 Trial Evaluates Key Domains of Systemic Sclerosis Other Organs Heart Kidney Liver Stomach SSc-ILD is a major cause of morbidity and mortality in SSc. Two disease-modifying treatments are approved for SSc-ILD Lungs Skin Characterized by Chronic Inflammation and Fibrosis Across Organs Skin fibrosis contributes to functional impairment, disability, and reduced quality of life FVC Forced Vital Capacity mRSS modified Rodnan Skin Score HAQ-DI Health Assessment Questionnaire Disability Index PtGA Patient Global Assessment CGA Clinical Global Assessment Lung Function Skin Fibrosis Functional Impact Patient-Reported Outcome Clinician-Reported Outcome tibulizumab | ZB-106 |
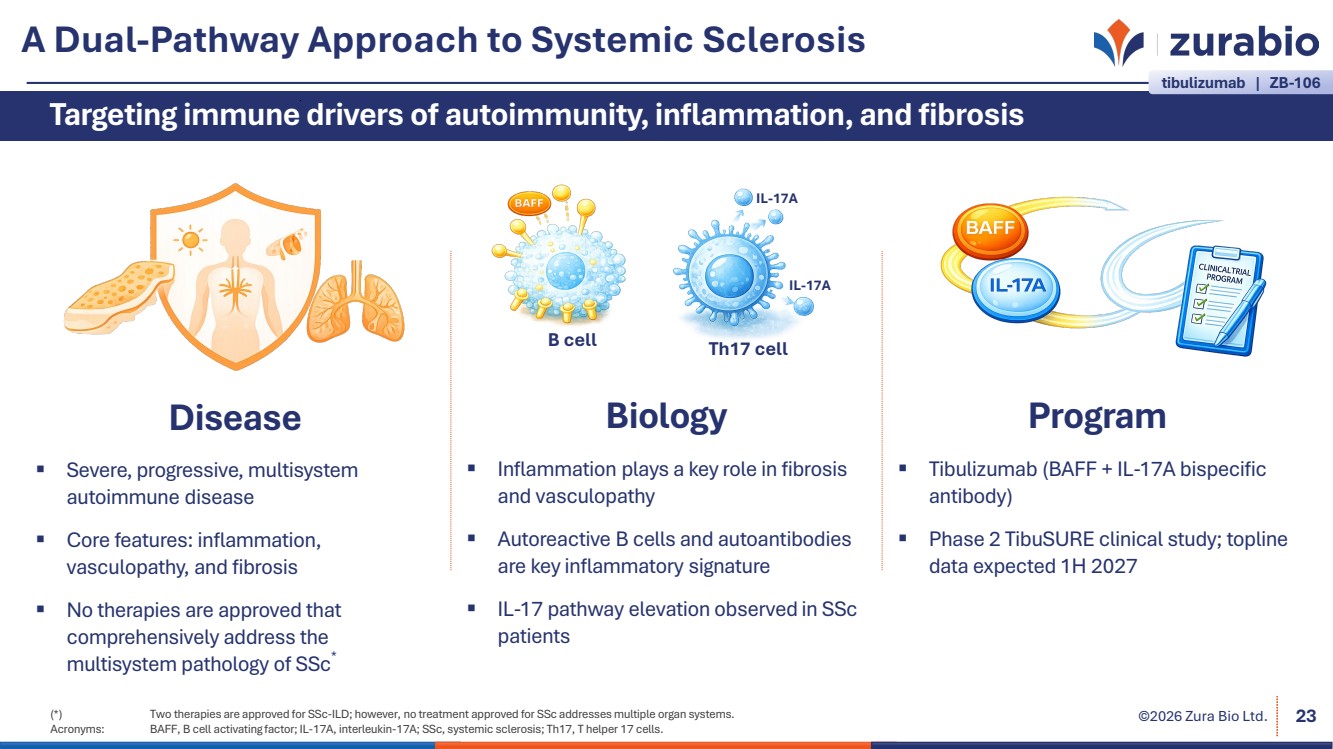
| ©2026 Zura Bio Ltd. A Dual-Pathway Approach to Systemic Sclerosis 23 Targeting immune drivers of autoimmunity, inflammation, and fibrosis Disease Severe, progressive, multisystem autoimmune disease Core features: inflammation, vasculopathy, and fibrosis No therapies are approved that comprehensively address the multisystem pathology of SSc* Program Tibulizumab (BAFF + IL-17A bispecific antibody) Phase 2 TibuSURE clinical study; topline data expected 1H 2027 Biology Inflammation plays a key role in fibrosis and vasculopathy Autoreactive B cells and autoantibodies are key inflammatory signature IL-17 pathway elevation observed in SSc patients Th17 cell IL-17A IL-17A B cell (*) Two therapies are approved for SSc-ILD; however, no treatment approved for SSc addresses multiple organ systems. Acronyms: BAFF, B cell activating factor; IL-17A, interleukin-17A; SSc, systemic sclerosis; Th17, T helper 17 cells. tibulizumab | ZB-106 |
| ©2026 Zura Bio Ltd. IL-17A and BAFF/B Cells Contribute to Inflammation, Vasculopathy, and Fibrosis in SSc Core Disease Features SSc is characterized by chronic inflammation, vasculopathy, and progressive fibrosis Infiltration of activated immune cells (macrophages, T cells, B cells) creates a persistent inflammatory tissue environment BAFF / B Cell Biology BAFF is elevated in SSc and supports survival and activation of autoreactive B cells Activated B cells produce autoantibodies and inflammatory cytokines (e.g., IL-6) associated with fibrosis and vascular dysfunction IL-17A Biology IL-17A has been reported to be elevated in subsets of patients with SSc Preclinical studies suggest IL-17A can activate fibroblasts and endothelial cells, promoting inflammation, immune cell recruitment, and tissue remodeling 24 tibulizumab | ZB-106 Sources: Seki, N. et al. (2024), Cytokine, DOI:10.1016/j.cyto.2024.156534; Ono, Y. et al. (2024), Scientific Reports, DOI:10.1038/s41598-024-76987-6; Lonati, P.A. et al. (2014), PLoS ONE, DOI:10.1371/journal.pone.0105008; Deng, C.-C. et al. (2025), Frontiers in Immunology, DOI:10.3389/fimmu.2024.1522076; Kurasawa, K. et al. (2000), Arthritis & Rheumatism, 43(11), pp. 2455– 2463. doi:10.1002/1529-0131(200011)43:11<2455::aid-anr12>3.0.co;2-k. Acronyms: BAFF, B cell activating factor; IL-6, interleukin-6; IL-17A, interleukin-17A; SSc, systemic sclerosis. |
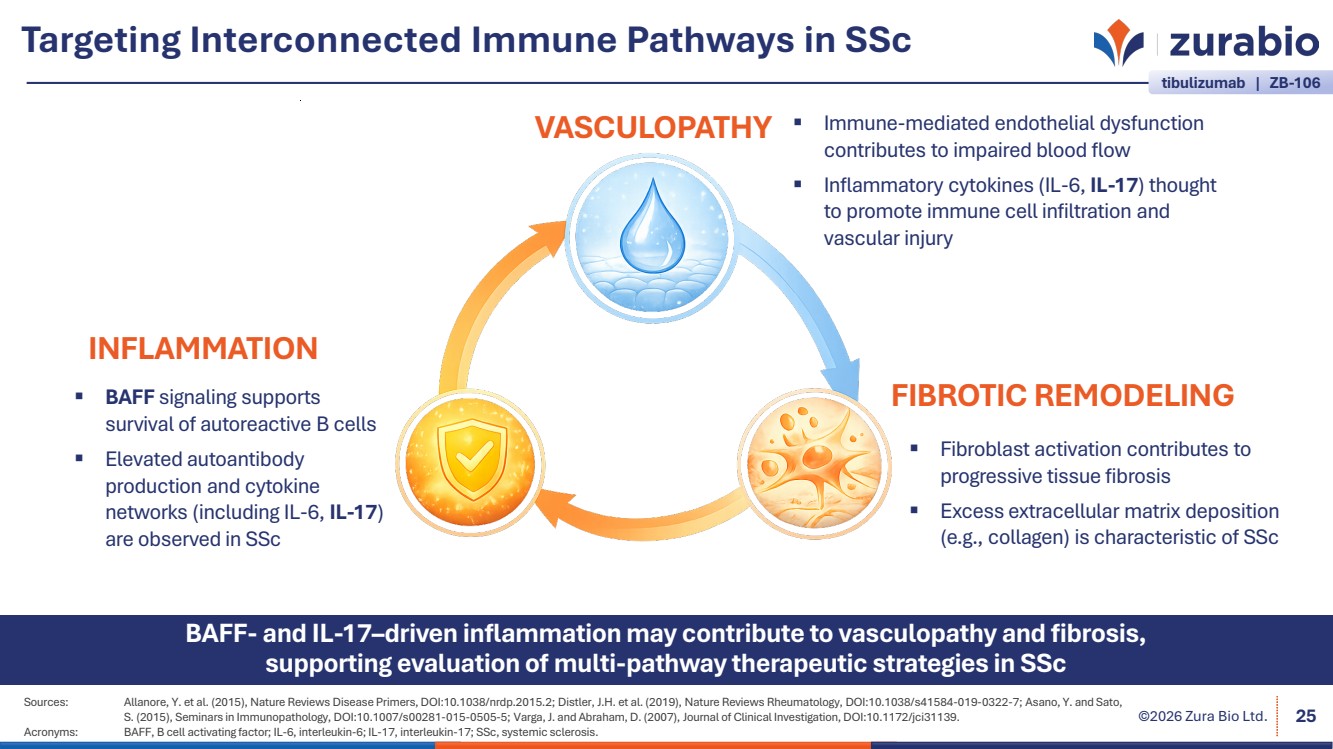
| ©2026 Zura Bio Ltd. Targeting Interconnected Immune Pathways in SSc 25 BAFF- and IL-17–driven inflammation may contribute to vasculopathy and fibrosis, supporting evaluation of multi-pathway therapeutic strategies in SSc BAFF signaling supports survival of autoreactive B cells Elevated autoantibody production and cytokine networks (including IL-6, IL-17) are observed in SSc Immune-mediated endothelial dysfunction contributes to impaired blood flow Inflammatory cytokines (IL-6, IL-17) thought to promote immune cell infiltration and vascular injury Fibroblast activation contributes to progressive tissue fibrosis Excess extracellular matrix deposition (e.g., collagen) is characteristic of SSc INFLAMMATION VASCULOPATHY FIBROTIC REMODELING Sources: Allanore, Y. et al. (2015), Nature Reviews Disease Primers, DOI:10.1038/nrdp.2015.2; Distler, J.H. et al. (2019), Nature Reviews Rheumatology, DOI:10.1038/s41584-019-0322-7; Asano, Y. and Sato, S. (2015), Seminars in Immunopathology, DOI:10.1007/s00281-015-0505-5; Varga, J. and Abraham, D. (2007), Journal of Clinical Investigation, DOI:10.1172/jci31139. Acronyms: BAFF, B cell activating factor; IL-6, interleukin-6; IL-17, interleukin-17; SSc, systemic sclerosis. tibulizumab | ZB-106 |
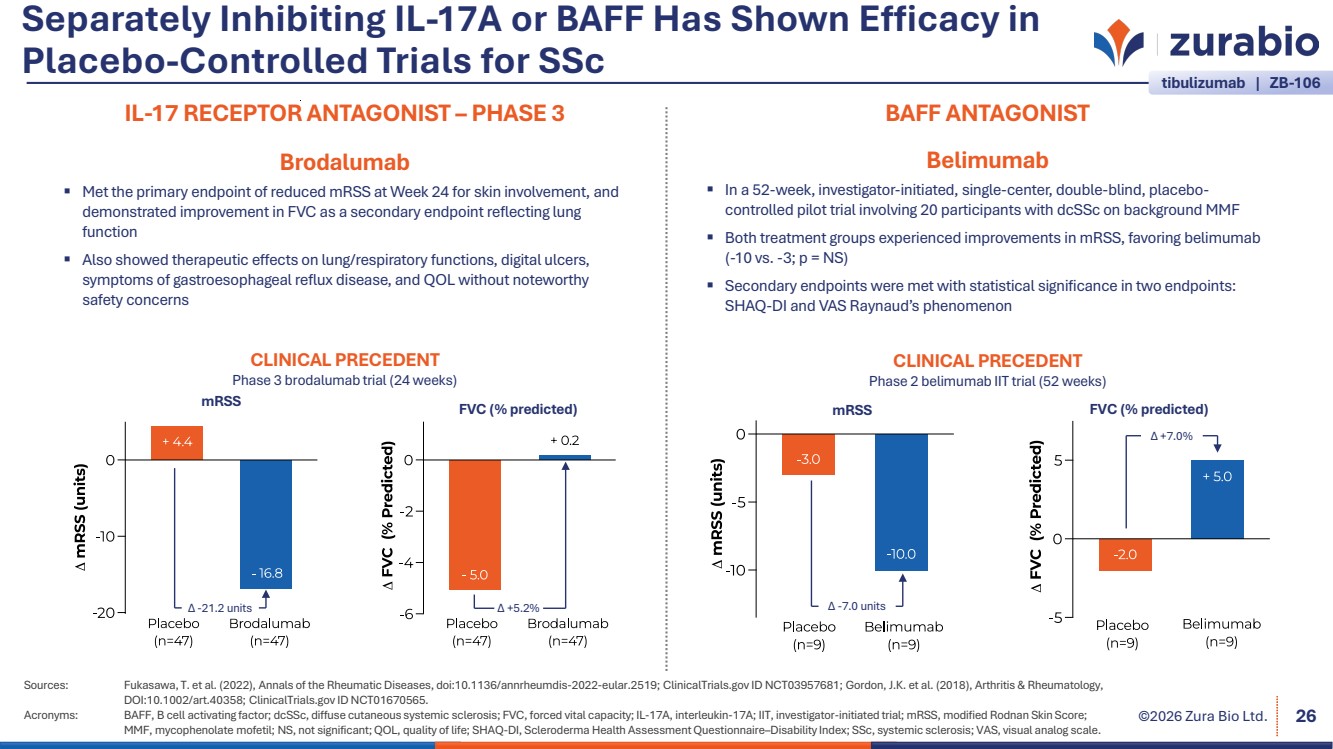
| ©2026 Zura Bio Ltd. Separately Inhibiting IL-17A or BAFF Has Shown Efficacy in Placebo-Controlled Trials for SSc Sources: Fukasawa, T. et al. (2022), Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2022-eular.2519; ClinicalTrials.gov ID NCT03957681; Gordon, J.K. et al. (2018), Arthritis & Rheumatology, DOI:10.1002/art.40358; ClinicalTrials.gov ID NCT01670565. Acronyms: BAFF, B cell activating factor; dcSSc, diffuse cutaneous systemic sclerosis; FVC, forced vital capacity; IL-17A, interleukin-17A; IIT, investigator-initiated trial; mRSS, modified Rodnan Skin Score; MMF, mycophenolate mofetil; NS, not significant; QOL, quality of life; SHAQ-DI, Scleroderma Health Assessment Questionnaire–Disability Index; SSc, systemic sclerosis; VAS, visual analog scale. 26 IL-17 RECEPTOR ANTAGONIST – PHASE 3 Brodalumab Met the primary endpoint of reduced mRSS at Week 24 for skin involvement, and demonstrated improvement in FVC as a secondary endpoint reflecting lung function Also showed therapeutic effects on lung/respiratory functions, digital ulcers, symptoms of gastroesophageal reflux disease, and QOL without noteworthy safety concerns Δ -21.2 units Δ +5.2% CLINICAL PRECEDENT Phase 3 brodalumab trial (24 weeks) mRSS FVC (% predicted) BAFF ANTAGONIST Belimumab In a 52-week, investigator-initiated, single-center, double-blind, placebo-controlled pilot trial involving 20 participants with dcSSc on background MMF Both treatment groups experienced improvements in mRSS, favoring belimumab (-10 vs. -3; p = NS) Secondary endpoints were met with statistical significance in two endpoints: SHAQ-DI and VAS Raynaud’s phenomenon CLINICAL PRECEDENT Phase 2 belimumab IIT trial (52 weeks) mRSS FVC (% predicted) Δ -7.0 units Δ +7.0% tibulizumab | ZB-106 |
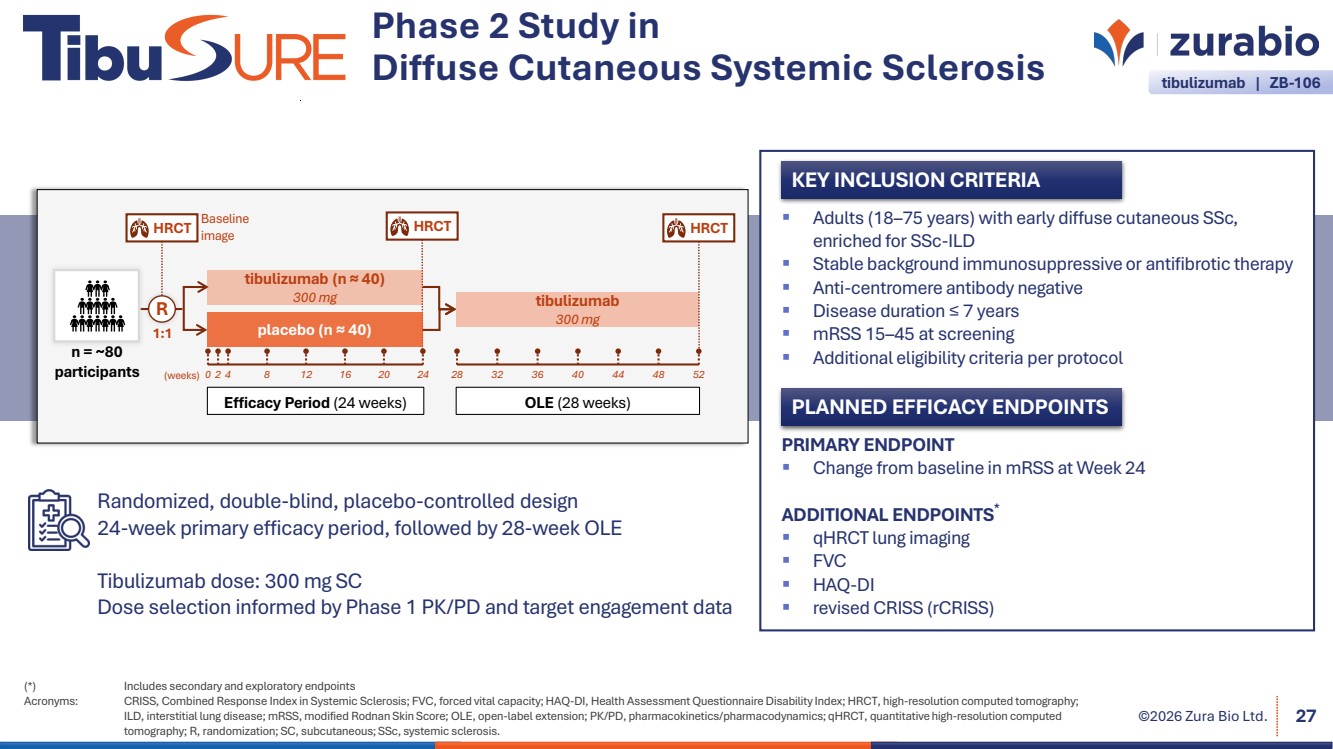
| ©2026 Zura Bio Ltd. 27 (*) Includes secondary and exploratory endpoints Acronyms: CRISS, Combined Response Index in Systemic Sclerosis; FVC, forced vital capacity; HAQ-DI, Health Assessment Questionnaire Disability Index; HRCT, high-resolution computed tomography; ILD, interstitial lung disease; mRSS, modified Rodnan Skin Score; OLE, open-label extension; PK/PD, pharmacokinetics/pharmacodynamics; qHRCT, quantitative high-resolution computed tomography; R, randomization; SC, subcutaneous; SSc, systemic sclerosis. Phase 2 Study in Diffuse Cutaneous Systemic Sclerosis KEY INCLUSION CRITERIA PLANNED EFFICACY ENDPOINTS PRIMARY ENDPOINT Change from baseline in mRSS at Week 24 ADDITIONAL ENDPOINTS* qHRCT lung imaging FVC HAQ-DI revised CRISS (rCRISS) Adults (18–75 years) with early diffuse cutaneous SSc, enriched for SSc-ILD Stable background immunosuppressive or antifibrotic therapy Anti-centromere antibody negative Disease duration ≤ 7 years mRSS 15–45 at screening Additional eligibility criteria per protocol Randomized, double-blind, placebo-controlled design 24-week primary efficacy period, followed by 28-week OLE Tibulizumab dose: 300 mg SC Dose selection informed by Phase 1 PK/PD and target engagement data n = ~80 participants Efficacy Period (24 weeks) Baseline image HRCT 1:1 R tibulizumab (n ≈ 40) 300 mg placebo (n ≈ 40) (weeks) 0 2 4 8 12 16 20 24 OLE (28 weeks) tibulizumab 300 mg 28 32 36 40 44 48 52 HRCT HRCT tibulizumab | ZB-106 |
| ©2026 Zura Bio Ltd. Key Takeaways Clear differentiation Tibulizumab is the first and currently only in-class bispecific antibody designed to simultaneously target BAFF and IL-17– mediated signaling, addressing immune complexity beyond single-pathway approaches 28 Acronyms: BAFF, B cell activating factor; IL-17, interleukin-17. Defined, anticipated near-term clinical catalysts Two independent Phase 2 trials (TibuSHIELD and TibuSURE) evaluating a dual-pathway strategy in diseases with significant unmet need and potential multi-billion dollar market opportunities Platform optionality Multi-pathway immune biology provides potential for expansion into additional autoimmune indications, subject to clinical validation Nasdaq: ZURA |