
Investor and Analyst Day June 3, 2025 1

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Welcome and Agenda Allison Wey SVP, Investor Relations and Corporate Communications 2

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Important Disclosures Forward-Looking Statements and Other Information Any statements in presentation other than statements of historical fact are forward-looking statements. Forward-looking statements include, but are not limited to, statements about future expectations, plans and prospects for Xeris Biopharma Holdings, Inc., including statements regarding financial guidance for 2025, including its expected total revenue and commitment to remaining adjusted EBITDA positive, the outlook for 2030 and outlook for 2035 and beyond, including statements regarding total revenue, product growth, annual net revenue expected for Recorlev® and XP-8121, the market and therapeutic potential of its products and product candidates, including Recorlev and XP-8121, the ability to continue to demonstrate rapid revenue growth, sustained momentum across the portfolio and maintain disciplined execution of the Company's growth strategy, the beneficial impact on the lives of patients, including XP-8121’s potential to transform the treatment landscape for millions living with hypothyroidism, capital management enabling the self-funding of both near and long-term growth, and other statements containing the words “will,” “would,” “continue,” “expect,” “should,” “anticipate,” “new,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on numerous assumptions and assessments made in light of Xeris’ experience and perception of historical trends, current conditions, business strategies, operating environment, future developments, geopolitical factors and other factors it believes appropriate. By their nature, forward-looking statements involve known and unknown risks and uncertainties because they relate to events and depend on circumstances that will occur in the future. The various factors that could cause Xeris’ actual results, performance or achievements, industry results and developments to differ materially from those expressed in or implied by such forward-looking statements, include, but are not limited to, its financial position and need for financing, including to fund its product development programs or commercialization efforts, whether its products will achieve and maintain market acceptance in a competitive business environment, its reliance on third-party suppliers, including single-source suppliers, its reliance on third parties to conduct clinical trials, the ability of its product candidates to compete successfully with existing and new drugs, and its collaborators’ ability to protect its intellectual property and proprietary technology, and general macroeconomic and geopolitical conditions, including the possibility of an economic downturn, changes in governmental priorities and resources, announced or implemented tariffs, and market volatility. No assurance can be given that such expectations will be realized and persons reading this communication are, therefore, cautioned not to place undue reliance on these forward-looking statements. Additional risks and information about potential impacts of financial, operational, economic, competitive, regulatory, governmental, technological, and other factors that may affect Xeris can be found in Xeris’ filings, including its most recent Annual Report on Form 10-K and subsequent Quarterly Reports on Form 10-Q, as well as subsequent filings with the U.S. Securities and Exchange Commission, the contents of which are not incorporated by reference into, nor do they form part of, this communication. Forward-looking statements in this communication are based on information available to management, as of the date of this communication and, while the Company believes its assumptions are reasonable, actual results may differ materially. Subject to any obligations under applicable law, the Company does not undertake any obligation to update any forward-looking statement whether as a result of new information, future developments or otherwise, or to conform any forward-looking statement to actual results, future events, or to changes in expectations. The trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. Non-GAAP Financial Measures In addition to U.S. GAAP financial measures, this presentation includes references to Adjusted EBITDA, which is a non-GAAP financial measure. The Company defines Adjusted EBITDA as GAAP net income (loss) before income tax (benefit) expense, plus interest and other income, less depreciation and amortization, interest expenses, share based compensation and debt refinancing fees. The Company believes this non-GAAP financial measure helps indicate underlying trends in the Company’s business and is important in comparing current results with prior period results and understanding expected operating performance. Also, management uses this non-GAAP financial measure to establish budgets and operational goals, and to manage the Company’s business and evaluate its performance. In addition, management believes that Adjusted EBIDTA is important in evaluating the administrative costs of operating the Company’s business. The Company is unable to reconcile its forward-looking guidance for Adjusted EBITDA to its most directly comparable GAAP measure. This is because the Company cannot predict with reasonable certainty and without unreasonable efforts the ultimate outcome of certain components of such GAAP metric and reconciliations due to market-related assumptions such as information regarding future compensation charges, future changes in the market price of its common stock or other costs which may arise. 3

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Today’s Agenda 4 Topic Speaker - Welcome & Introductions Allison Wey, SVP, IR & Corporate Communications - Unlocking the Future of Xeris John Shannon, Chief Executive Officer - Recorlev®: Tremendous Growth Opportunities Mary Beth McNerney, Chief Commercial Officer Break - XP-8121, Building a Blockbuster in Hypothyroidism Dr. Anh Nguyen Chief Medical Officer - Leveraging Our Strengths to Build Our Future Kevin McCulloch, President and Chief Operating Officer - Positioned for Long-Term Value Creation Steven Pieper, Chief Financial Officer Q&A - Closing Remarks John Shannon, Chief Executive Officer Joshua Bennett VP, Strategic Initiatives Guest Speakers Today Eliza B. Geer, MD (Memorial Sloan Kettering Cancer Center) Antonio C. Bianco, MD, PhD (University of Texas Medical Branch) Francesco S. Celi, MD, MHSc (UConn Health)

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Key Opinion Leaders (KOLs) Joining Us Today Eliza B. Geer, MD (Memorial Sloan Kettering Cancer Center), is the Medical Director of Memorial Sloan Kettering’s Multidisciplinary Pituitary & Skull Base Tumor Center and Professor of Medicine and Neurosurgery. She is an endocrinologist who specializes in caring for people with pituitary and neuroendocrine diseases. She is involved in clinical trials investigating new medical therapies for patients with Cushing’s, acromegaly, and prolactinoma. Her current research interests focus on characterizing patient reported outcomes in patients with Cushing’s and acromegaly, and treatment of aggressive pituitary tumors. Antonio C. Bianco, MD, PhD (University of Texas Medical Branch), is the Nelda C. and H.J. Lutcher Professor in Internal Medicine, Senior Vice President and Dean of the John Sealy School of Medicine, and Chief Research Officer. Dr. Bianco has published extensively in the area of thyroid hormone metabolism and action. He has been recognized with numerous awards from national and international professional associations, and membership in the American Society for Clinical Investigation and the Association of American Physicians. He served as a regular member of NIH study sections for almost 10 years, and the Board of Scientific Counselors of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Bianco was elected and served as president of the American Thyroid Association (ATA) in 2016. Francesco S. Celi, MD, MHSc (UConn Health), is the Professor of Medicine and James E.C. Walker Chair of the Department of Medicine at UConn Health. Dr. Celi conducts clinical and translational research, and his scientific interest is focused on the physiology and pathophysiology of thyroid hormone action as it relates to energy metabolism. Dr. Celi served as Principal Investigator of several clinical trials on innovative treatments for hypothyroidism. Dr. Celi is a practicing endocrinologist, and his clinical interests are diabetes and thyroid disease, with a specific focus on thyroid hormone replacement therapy, imaging, fine needle aspiration biopsy, and multidisciplinary management of thyroid cancer. 5 * The KOLs for today's event are being compensated by Xeris for their consulting and time. ** Eliza Geer’s presentation is not an endorsement of the product.

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Unlocking the Future of Xeris John Shannon Chief Executive Officer 6

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Xeris’ Innovative Therapies Address Serious, Unmet Medical Needs and Make a Difference in Patient Lives Built on a strong foundation of proprietary formulation technologies and deep expertise in clinical development and commercialization 7

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Proven, Experienced Team Confidence to Execute Our Strategic Vision A seasoned team with a track record of operational excellence that is well-positioned to take Xeris to new heights 8 Presenting Today Other Members of Leadership Team Beth Hecht / Chief Legal Officer Brian Conner / Chief Compliance & Risk Kendal Korte / SVP, Human Resources John Shannon Chief Executive Officer Allison Wey SVP, IR and Corporate Comms Steven Pieper Chief Financial Officer Kevin McCulloch President & Chief Operating Officer Mary Beth McNerney Chief Commercial Officer Anh Nguyen Chief Medical Officer Josh Bennett VP, Strategic Initiatives Extensive experience across pharmaceutical, medical device, and healthcare sectors — from large global enterprises to emerging companies — leading the development, launch, and commercialization of products, including several with over $1 billion in revenue
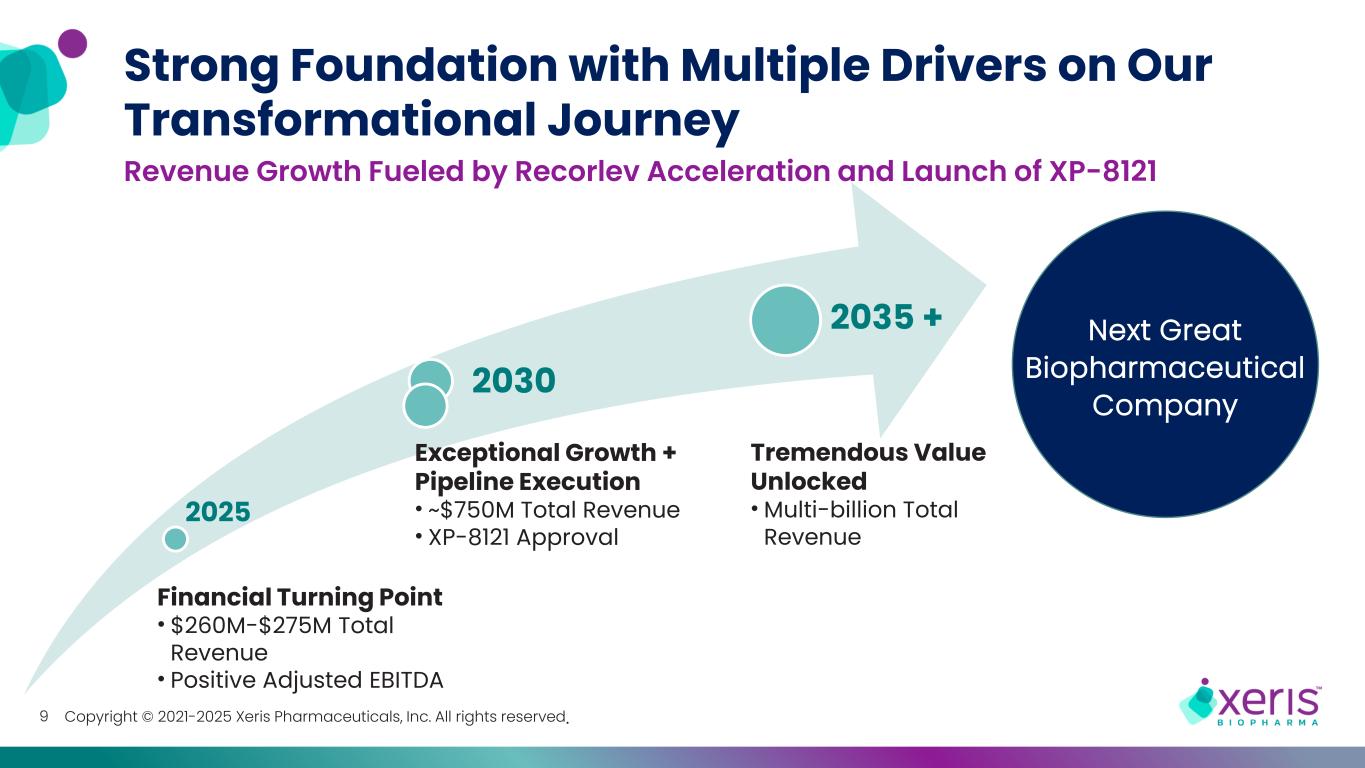
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. 2025 2030 2035 + 9 Revenue Growth Fueled by Recorlev Acceleration and Launch of XP-8121 Strong Foundation with Multiple Drivers on Our Transformational Journey Tremendous Value Unlocked • Multi-billion Total Revenue Exceptional Growth + Pipeline Execution • ~$750M Total Revenue • XP-8121 Approval Financial Turning Point • $260M-$275M Total Revenue • Positive Adjusted EBITDA Next Great Biopharmaceutical Company
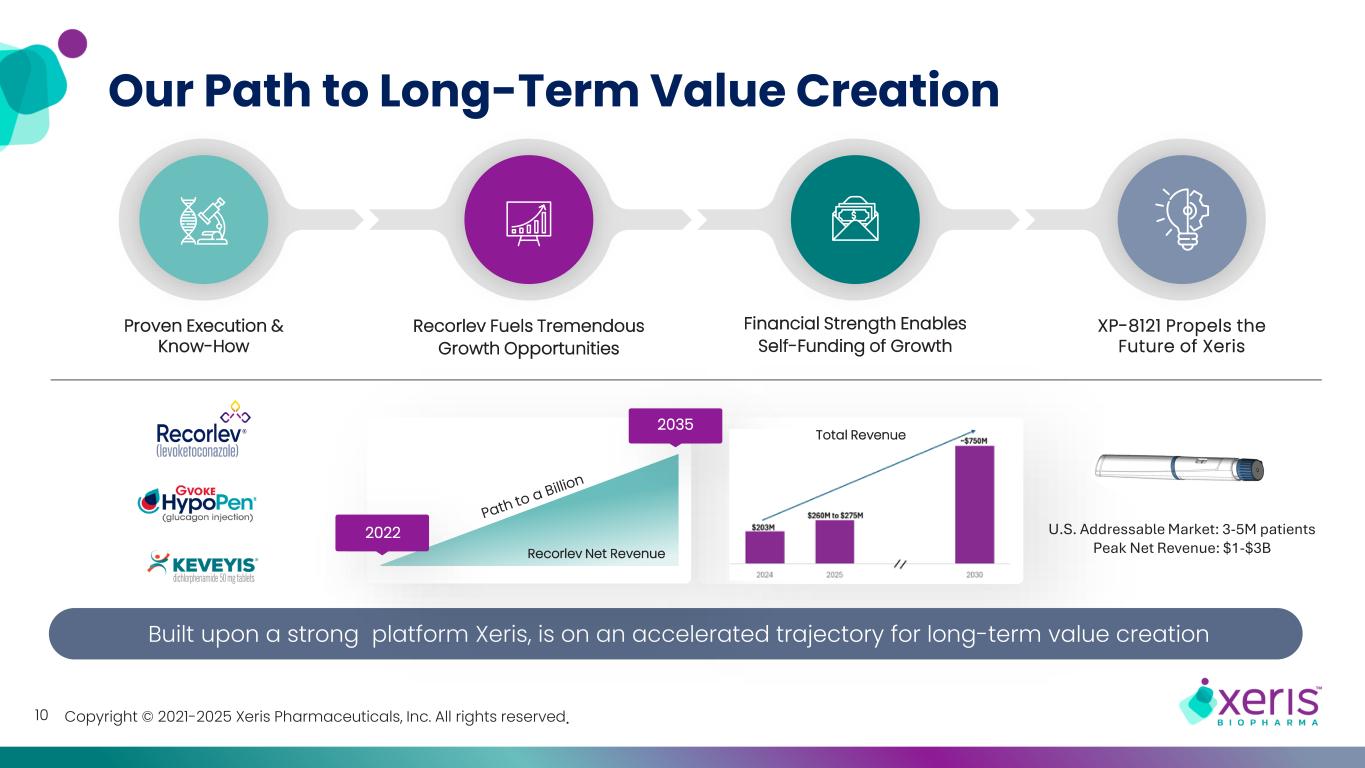
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.10 Our Path to Long-Term Value Creation Built upon a strong platform Xeris, is on an accelerated trajectory for long-term value creation Recorlev Fuels Tremendous Growth Opportunities 2035 2022 Recorlev Net Revenue Financial Strength Enables Self-Funding of Growth Total Revenue Proven Execution & Know-How U.S. Addressable Market: 3-5M patients Peak Net Revenue: $1-$3B XP-8121 Propels the Future of Xeris

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Recorlev®: Tremendous Growth Opportunities 11 Mary Beth McNerney Chief Commercial Officer

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Recorlev is Our Growth Engine in an Expanding Hypercortisolism Market Our most significant growth driver, expected to approach $1 billion in annual net revenue Long expected runway of cash generation with highly favorable economic value Highest commercial priority for the company where further investment is expected to drive tremendous shareholder value Recorlev treats the root cause of hypercortisolism in adult patients with Cushing’s Syndrome1 12
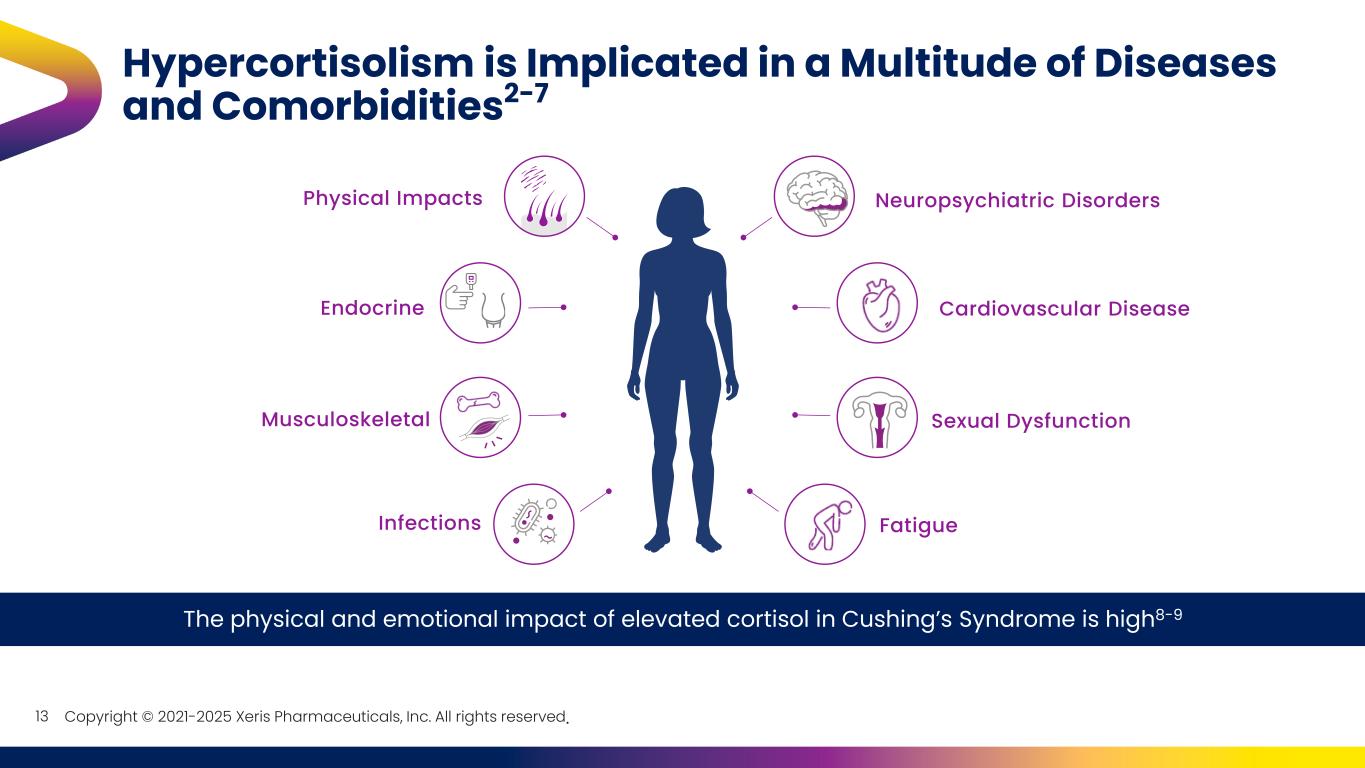
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Hypercortisolism is Implicated in a Multitude of Diseases and Comorbidities2-7 The physical and emotional impact of elevated cortisol in Cushing’s Syndrome is high8-9 Infections Musculoskeletal Neuropsychiatric Disorders Fatigue Sexual Dysfunction Cardiovascular Disease Physical Impacts Endocrine 13
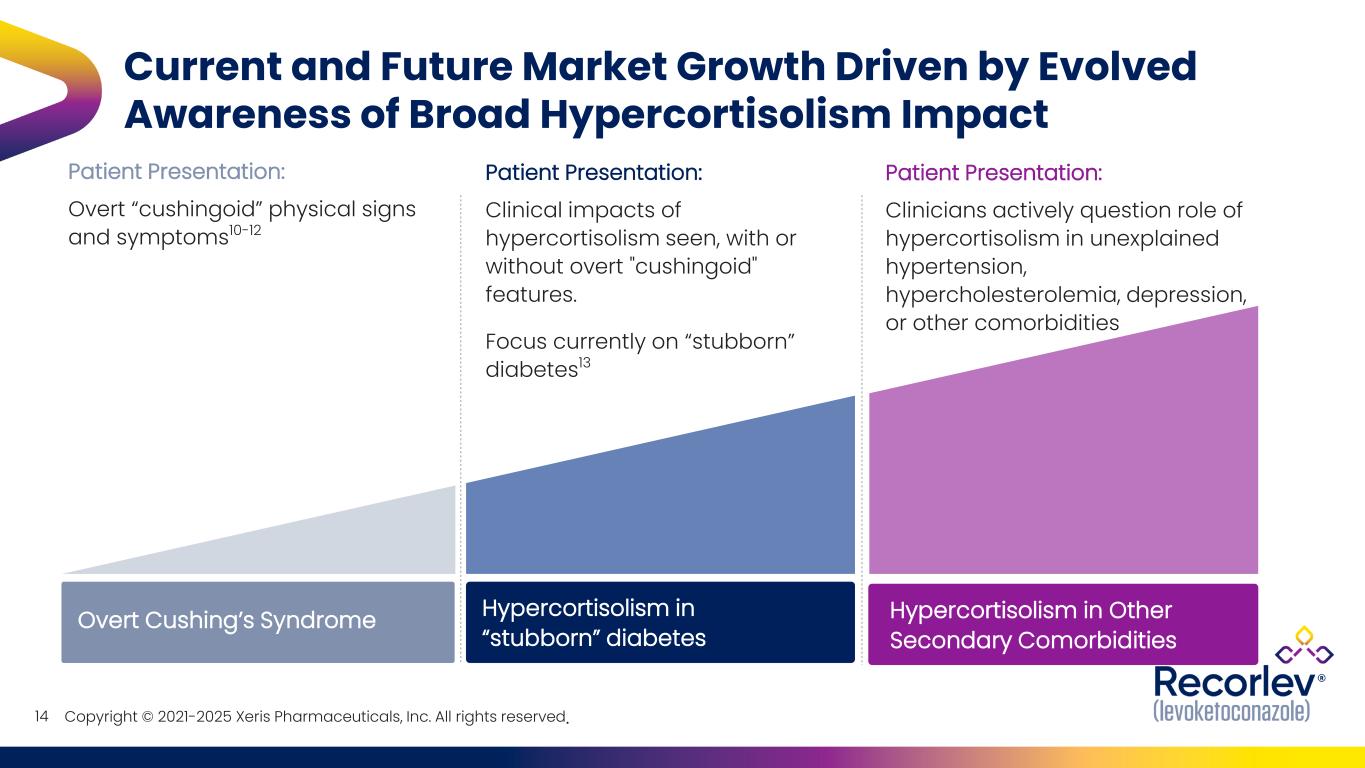
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Patient Presentation: Overt “cushingoid” physical signs and symptoms10-12 Patient Presentation: Clinical impacts of hypercortisolism seen, with or without overt "cushingoid" features. Focus currently on “stubborn” diabetes13 Current and Future Market Growth Driven by Evolved Awareness of Broad Hypercortisolism Impact Overt Cushing’s Syndrome Hypercortisolism in “stubborn” diabetes Hypercortisolism in Other Secondary Comorbidities Patient Presentation: Clinicians actively question role of hypercortisolism in unexplained hypertension, hypercholesterolemia, depression, or other comorbidities 14
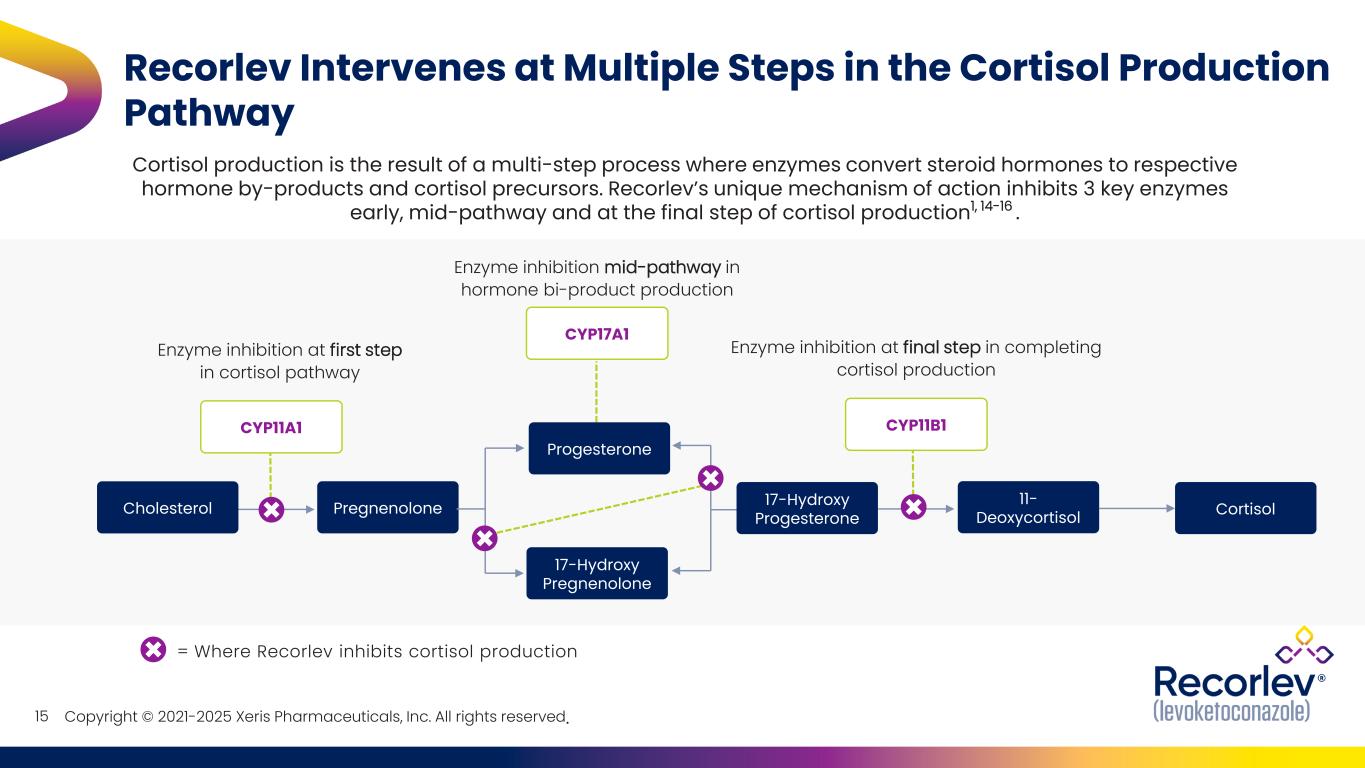
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Recorlev Intervenes at Multiple Steps in the Cortisol Production Pathway 15 Cortisol production is the result of a multi-step process where enzymes convert steroid hormones to respective hormone by-products and cortisol precursors. Recorlev’s unique mechanism of action inhibits 3 key enzymes early, mid-pathway and at the final step of cortisol production1, 14-16 . Enzyme inhibition at first step in cortisol pathway Cholesterol CYP11A1 Pregnenolone Progesterone 17-Hydroxy Pregnenolone 17-Hydroxy Progesterone CYP17A1 11- Deoxycortisol Cortisol Enzyme inhibition mid-pathway in hormone bi-product production Enzyme inhibition at final step in completing cortisol production CYP11B1 = Where Recorlev inhibits cortisol production
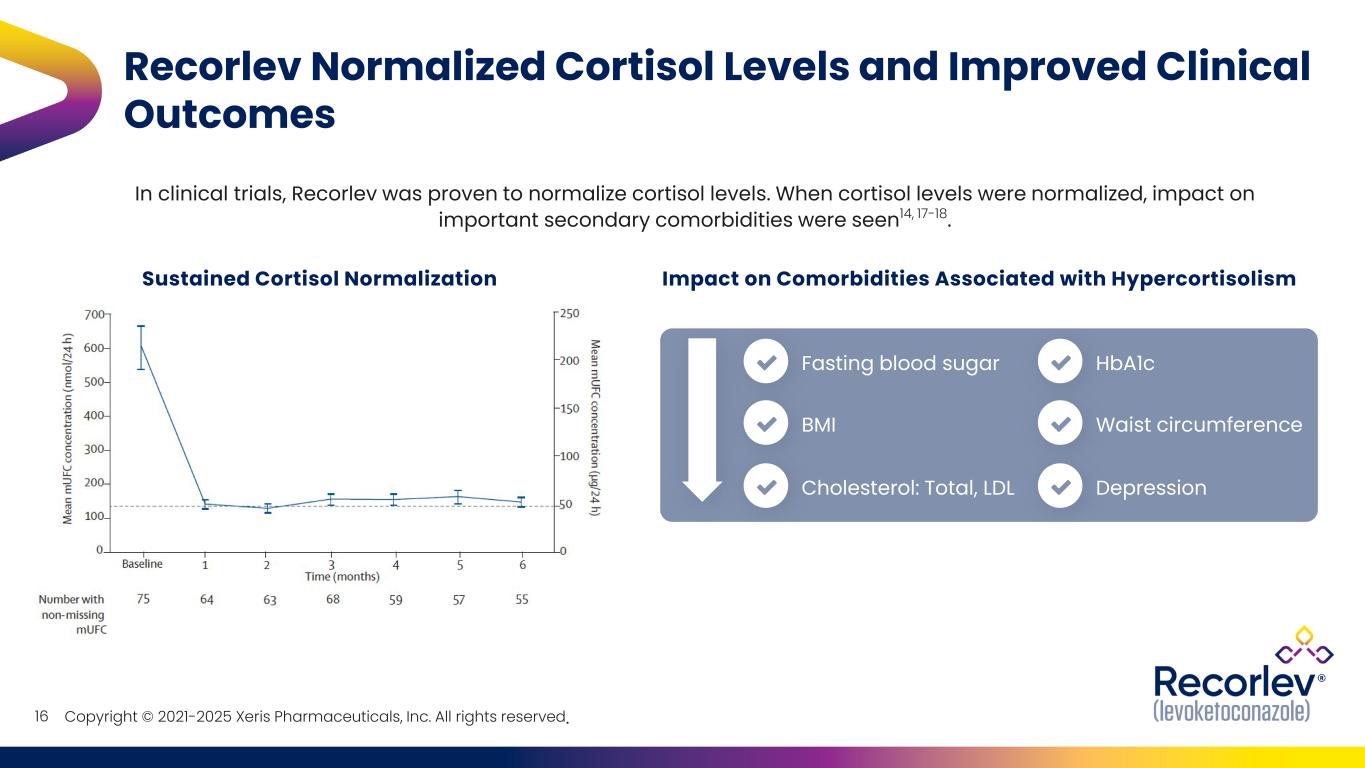
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Recorlev Normalized Cortisol Levels and Improved Clinical Outcomes Sustained Cortisol Normalization Impact on Comorbidities Associated with Hypercortisolism Fasting blood sugar BMI Cholesterol: Total, LDL HbA1c Waist circumference Depression 16 In clinical trials, Recorlev was proven to normalize cortisol levels. When cortisol levels were normalized, impact on important secondary comorbidities were seen14, 17-18.

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. 20352022 Re co rle v N et R ev en ue 17 Recorlev is the right product at the right time. Recorlev is on its Way to $1 Billion in Revenue Stepwise investment in Recorlev leadership position by leveraging existing capabilities and expertise: • Seize current market opportunity • Expand clinical role of Recorlev in broader hypercortisolism market • Sustained long-term value $1 Billion
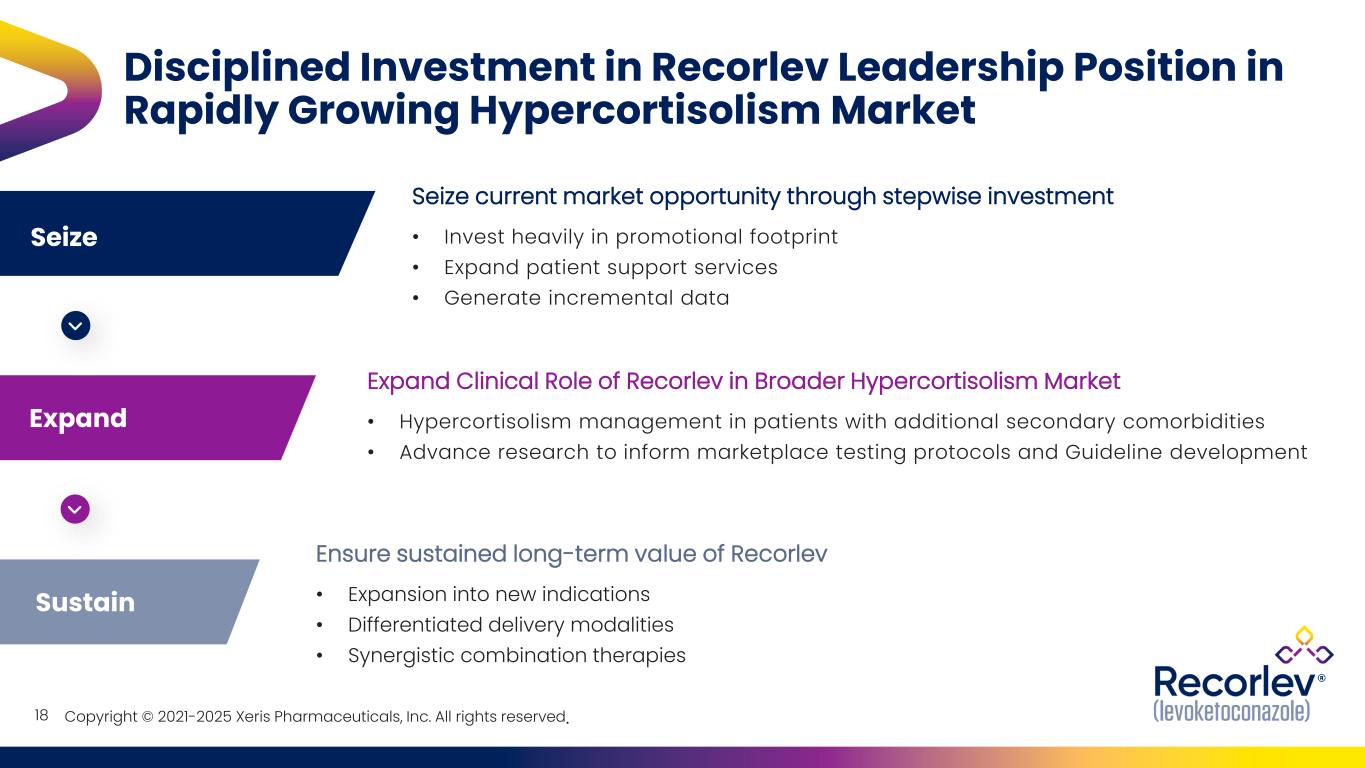
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Seize Expand 18 Disciplined Investment in Recorlev Leadership Position in Rapidly Growing Hypercortisolism Market Seize current market opportunity through stepwise investment • Invest heavily in promotional footprint • Expand patient support services • Generate incremental data Sustain Expand Clinical Role of Recorlev in Broader Hypercortisolism Market • Hypercortisolism management in patients with additional secondary comorbidities • Advance research to inform marketplace testing protocols and Guideline development Ensure sustained long-term value of Recorlev • Expansion into new indications • Differentiated delivery modalities • Synergistic combination therapies

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Recorlev Investment Summary 19 01 Hypercortisolemia market is evolving and growing rapidly as physicians shift to screening patients with less “cushingoid” presentations 02 Mechanism of Action inhibiting cortisol production at multiple points is unique and allows Recorlev to address the root cause of Cushing’s Syndrome and impact important secondary comorbidities 03 Proven commercial execution allows us to deliver on growth expectations

20 Evolving Understanding of Cortisol, Cortisol Regulation, and Cushing's Syndrome Eliza B. Geer, MD
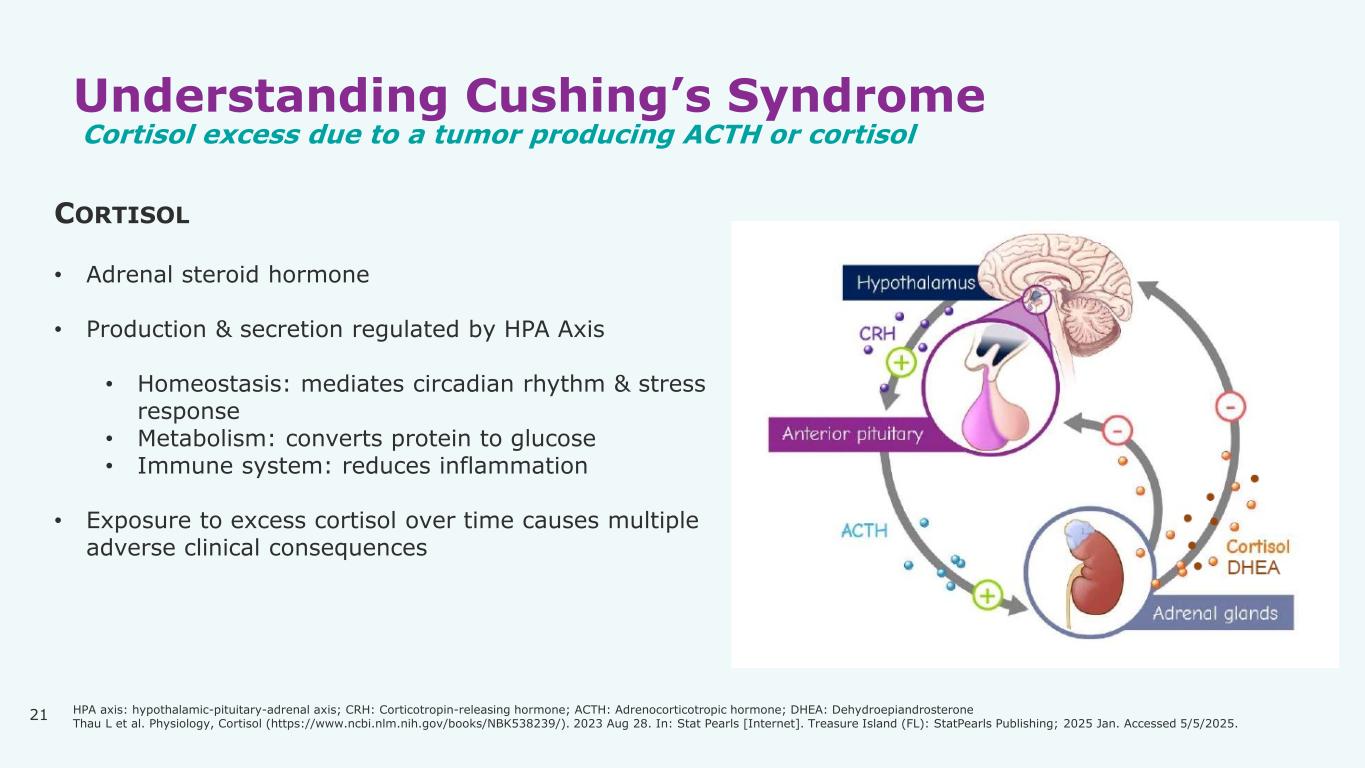
21 Understanding Cushing’s Syndrome Cortisol excess due to a tumor producing ACTH or cortisol CORTISOL • Adrenal steroid hormone • Production & secretion regulated by HPA Axis • Homeostasis: mediates circadian rhythm & stress response • Metabolism: converts protein to glucose • Immune system: reduces inflammation • Exposure to excess cortisol over time causes multiple adverse clinical consequences HPA axis: hypothalamic-pituitary-adrenal axis; CRH: Corticotropin-releasing hormone; ACTH: Adrenocorticotropic hormone; DHEA: Dehydroepiandrosterone Thau L et al. Physiology, Cortisol (https://www.ncbi.nlm.nih.gov/books/NBK538239/). 2023 Aug 28. In: Stat Pearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Accessed 5/5/2025.

22 Unique Presentations of Cushing’s Syndrome • ADRENAL CUSHING’S SYNDROME • 13-YEAR DIAGNOSTIC JOURNEY • ADRENAL CUSHING’S SYNDROME • 2-YEAR DIAGNOSTIC JOURNEY; FOLLOWING IDENTIFICATION OF TUMORS ON KIDNEY SCAN Actual Patient Actual Patient
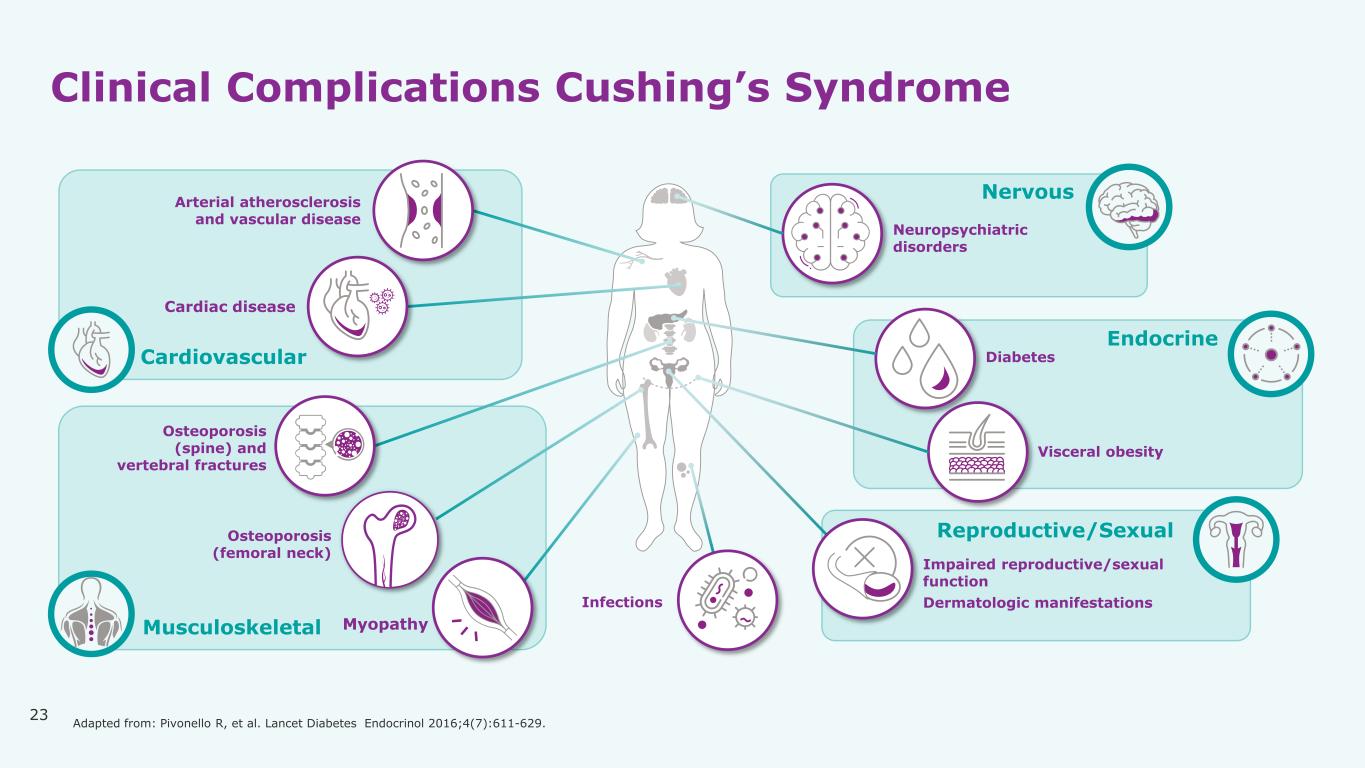
23 Clinical Complications Cushing’s Syndrome Reproductive/Sexual Nervous Endocrine Cardiovascular Musculoskeletal Neuropsychiatric disorders Arterial atherosclerosis and vascular disease Diabetes Osteoporosis (femoral neck) Myopathy Cardiac disease Impaired reproductive/sexual function Dermatologic manifestations Visceral obesity Infections Osteoporosis (spine) and vertebral fractures Adapted from: Pivonello R, et al. Lancet Diabetes Endocrinol 2016;4(7):611-629.
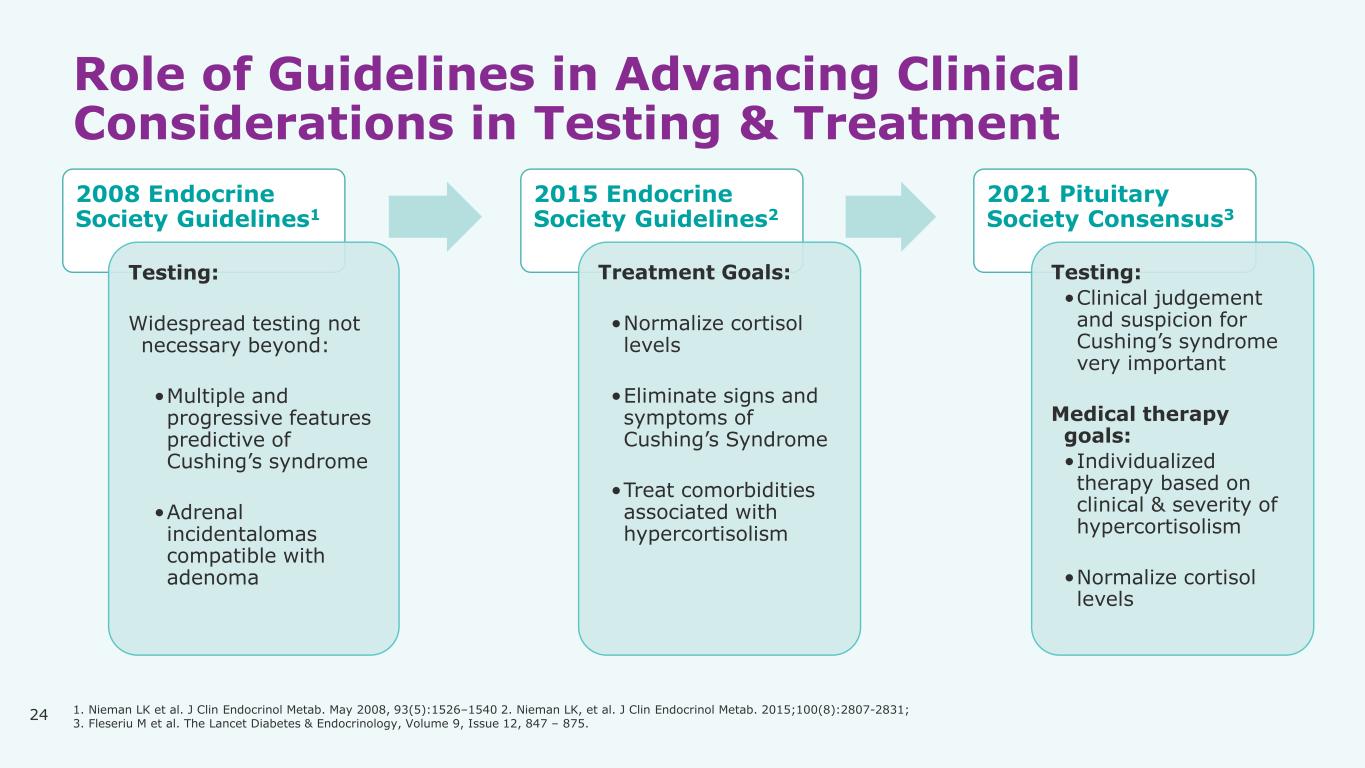
24 Role of Guidelines in Advancing Clinical Considerations in Testing & Treatment 2008 Endocrine Society Guidelines1 Testing: Widespread testing not necessary beyond: •Multiple and progressive features predictive of Cushing’s syndrome •Adrenal incidentalomas compatible with adenoma 2015 Endocrine Society Guidelines2 Treatment Goals: •Normalize cortisol levels •Eliminate signs and symptoms of Cushing’s Syndrome •Treat comorbidities associated with hypercortisolism 2021 Pituitary Society Consensus3 Testing: •Clinical judgement and suspicion for Cushing’s syndrome very important Medical therapy goals: •Individualized therapy based on clinical & severity of hypercortisolism •Normalize cortisol levels 1. Nieman LK et al. J Clin Endocrinol Metab. May 2008, 93(5):1526–1540 2. Nieman LK, et al. J Clin Endocrinol Metab. 2015;100(8):2807-2831; 3. Fleseriu M et al. The Lancet Diabetes & Endocrinology, Volume 9, Issue 12, 847 – 875.
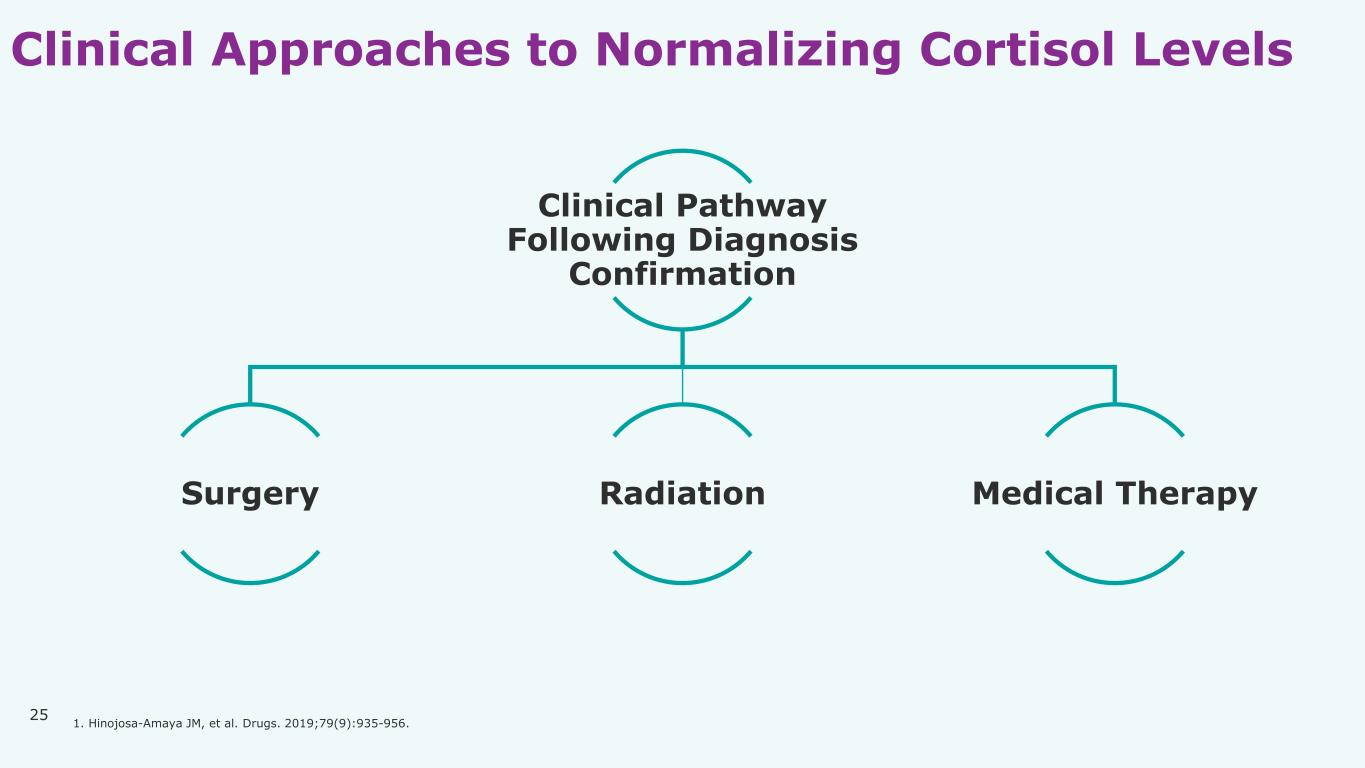
25 Clinical Approaches to Normalizing Cortisol Levels Clinical Pathway Following Diagnosis Confirmation Surgery Radiation Medical Therapy 1. Hinojosa-Amaya JM, et al. Drugs. 2019;79(9):935-956.
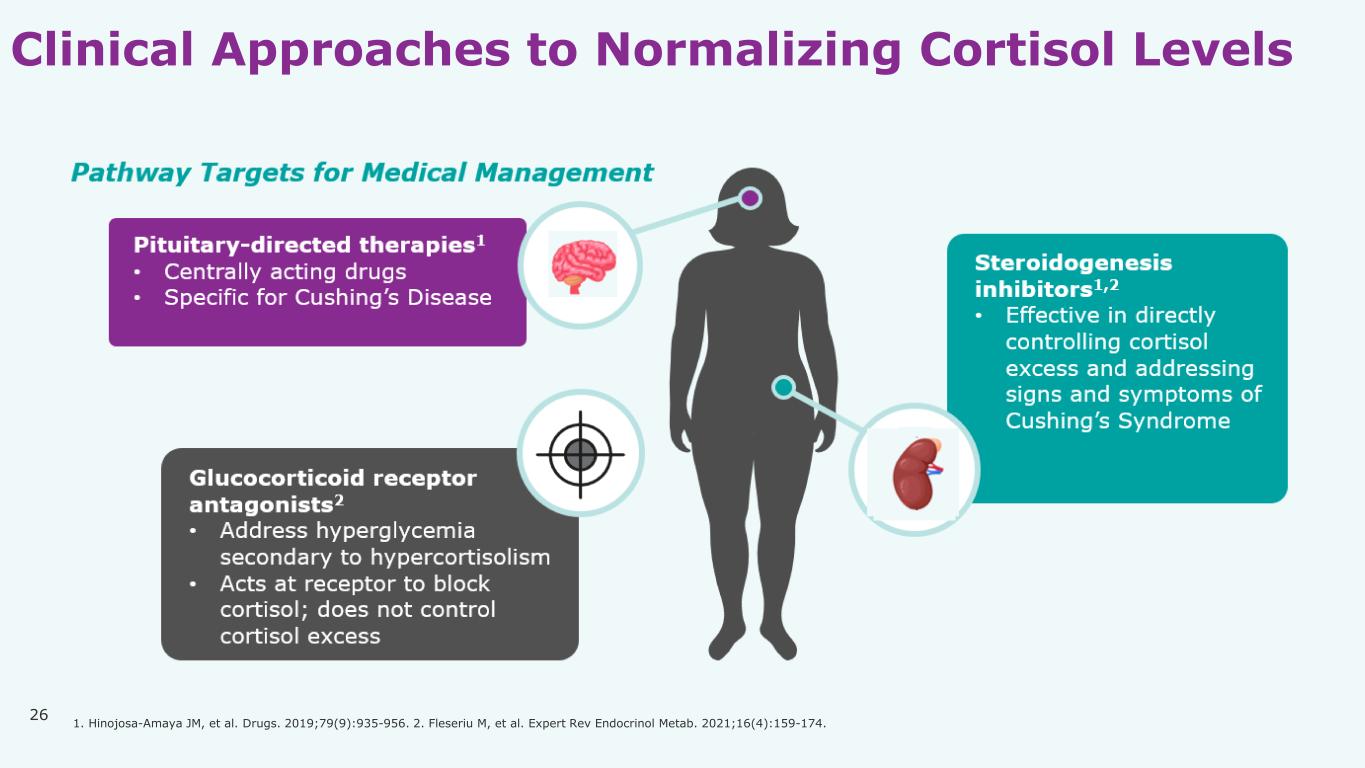
26 Clinical Approaches to Normalizing Cortisol Levels 1. Hinojosa-Amaya JM, et al. Drugs. 2019;79(9):935-956. 2. Fleseriu M, et al. Expert Rev Endocrinol Metab. 2021;16(4):159-174.
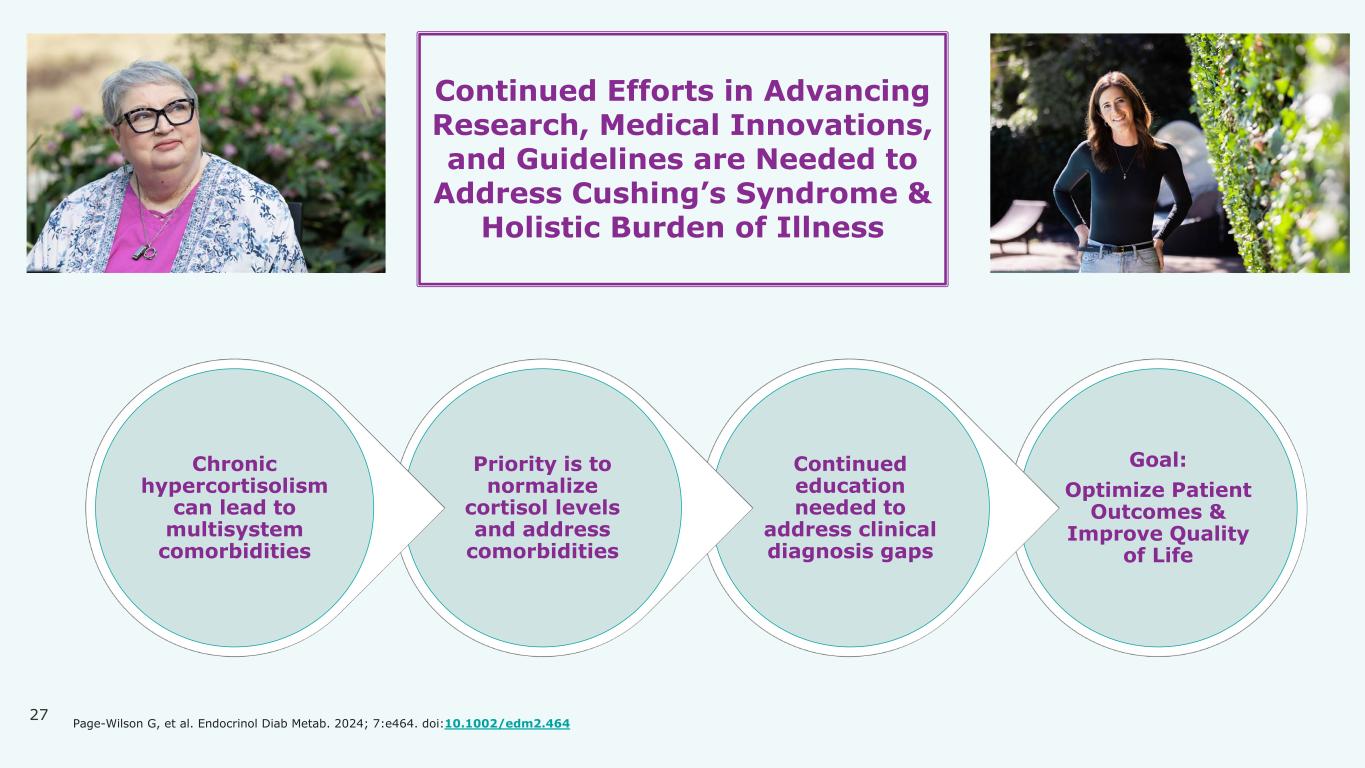
27 Goal: Optimize Patient Outcomes & Improve Quality of Life Continued education needed to address clinical diagnosis gaps Priority is to normalize cortisol levels and address comorbidities Chronic hypercortisolism can lead to multisystem comorbidities Continued Efforts in Advancing Research, Medical Innovations, and Guidelines are Needed to Address Cushing’s Syndrome & Holistic Burden of Illness Page-Wilson G, et al. Endocrinol Diab Metab. 2024; 7:e464. doi:10.1002/edm2.464

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Break Please be back in 10-minutes 28

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. XP-8121 Building a Blockbuster in Hypothyroidism Clinical Overview Anh Nguyen, MD, MBA Chief Medical Officer Expert Panel on Hypothyroidism Anh Nguyen, MD, MBA Antonio C. Bianco, MD, PhD University of Texas Medical Branch Francesco S. Celi, MD, MHSc UConn Health Opportunity and Path to Launch Joshua Bennett VP, Strategic Initiatives 29
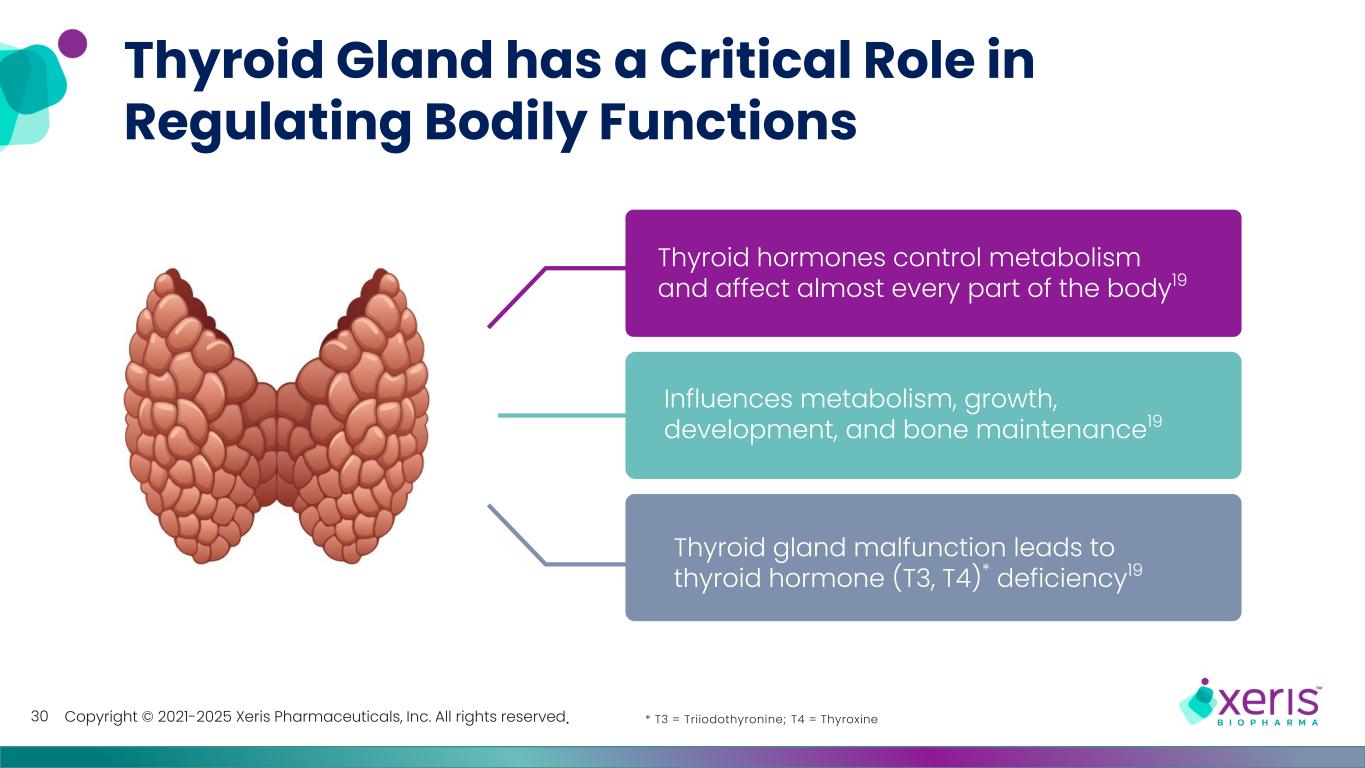
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Thyroid Gland has a Critical Role in Regulating Bodily Functions 30 Thyroid gland malfunction leads to thyroid hormone (T3, T4)* deficiency19 Influences metabolism, growth, development, and bone maintenance19 Thyroid hormones control metabolism and affect almost every part of the body19 * T3 = Triiodothyronine; T4 = Thyroxine
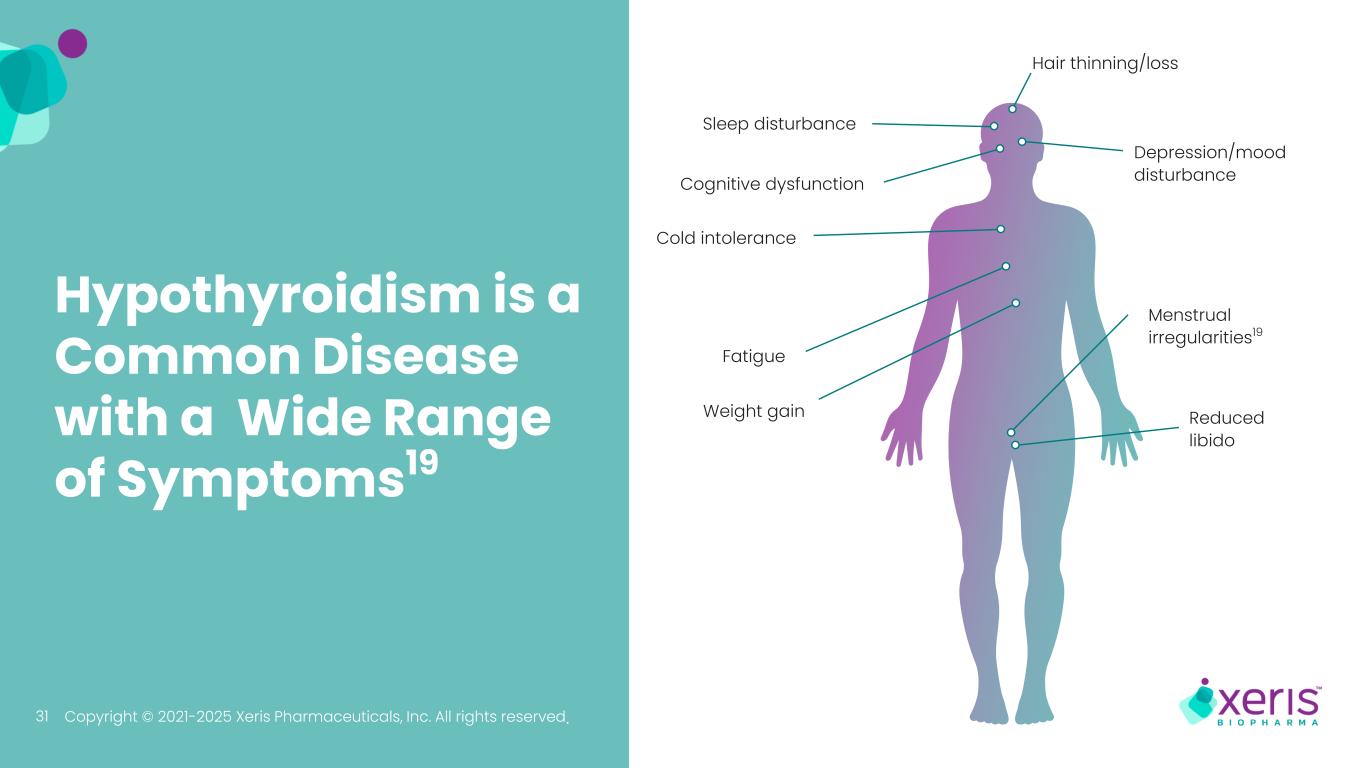
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Sleep disturbance Menstrual irregularities19 Reduced libido Hair thinning/loss Cold intolerance Depression/mood disturbance Weight gain Cognitive dysfunction Fatigue Hypothyroidism is a Common Disease with a Wide Range of Symptoms19 31
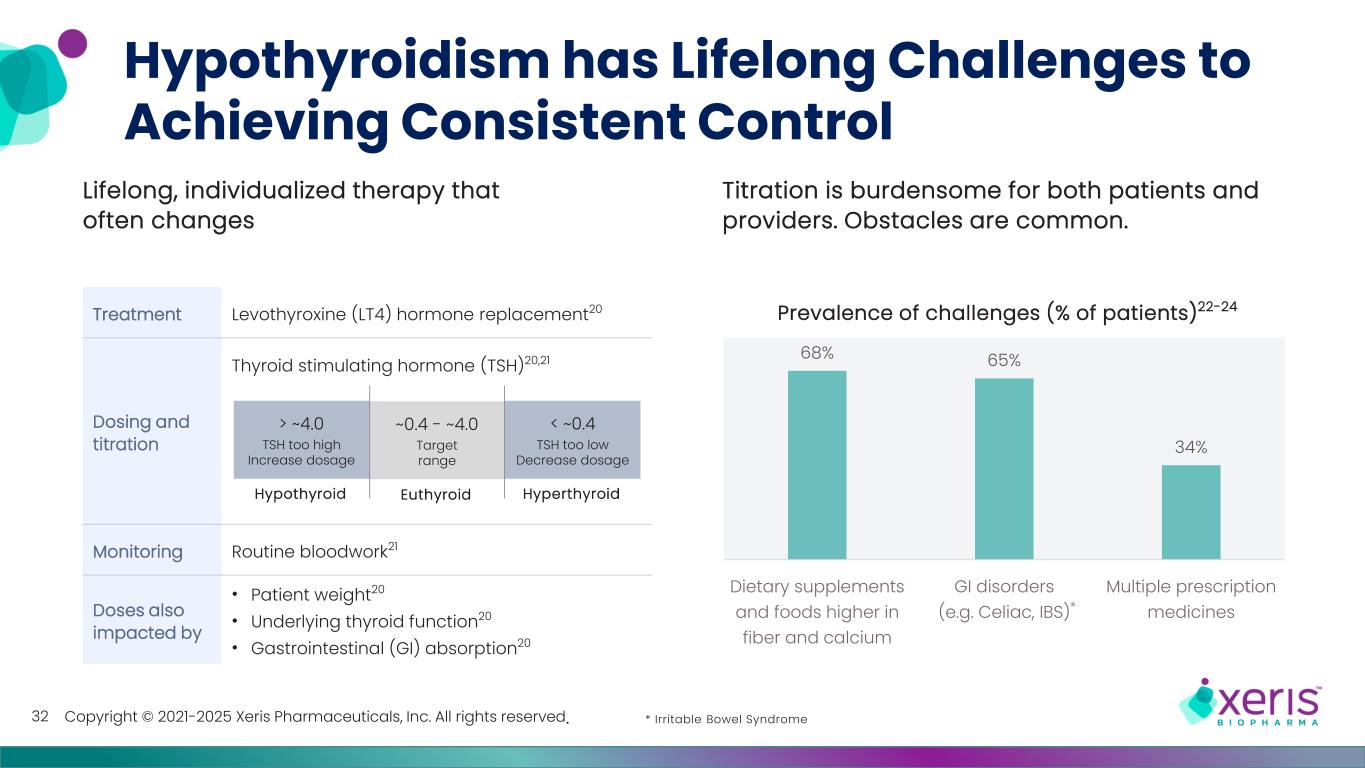
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Hypothyroidism has Lifelong Challenges to Achieving Consistent Control 32 Lifelong, individualized therapy that often changes Titration is burdensome for both patients and providers. Obstacles are common. 68% 65% 34% Dietary supplements and foods higher in fiber and calcium GI disorders (e.g. Celiac, IBS) Multiple prescription medicines Prevalence of challenges (% of patients)1-3Treatment Levothyroxine (LT4) hormone replacement20 Dosing and titration Thyroid stimulating hormone (TSH)20,21 Monitoring Routine bloodwork21 Doses also impacted by • Patient weight20 • Underlying thyroid function20 • Gastrointestinal (GI) absorption20 Hypothyroid > ~4.0 TSH too high Increase dosage Hyperthyroid < ~0.4 TSH too low Decrease dosage ~0.4 - ~4.0 Target range Euthyroid Prevalence of challenges ( ts)22-24 * * Irritable Bowel Syndrome
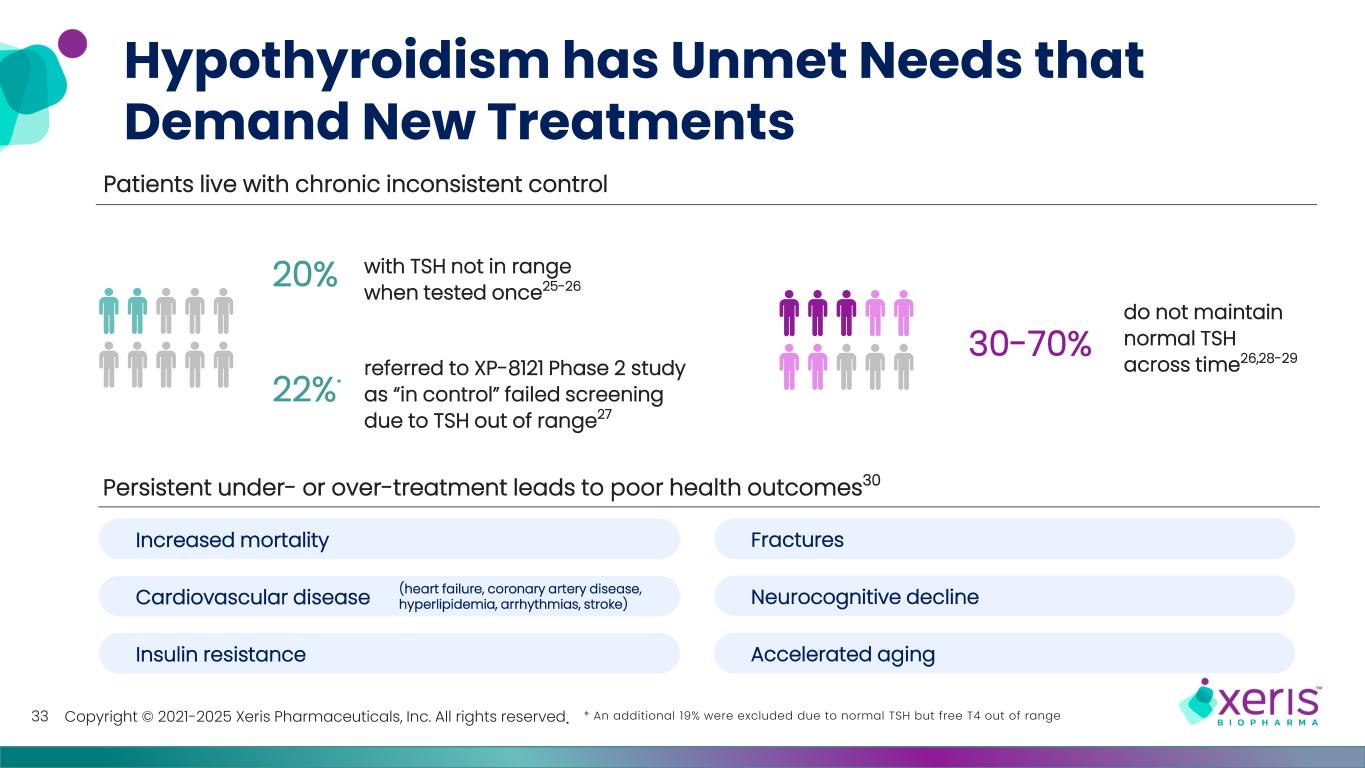
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Hypothyroidism has Unmet Needs that Demand New Treatments 33 Persistent under- or over-treatment leads to poor health outcomes30 * An additional 19% were excluded due to normal TSH but free T4 out of range Patients live with chronic inconsistent control 20% with TSH not in range when tested once25-26 22%* referred to XP-8121 Phase 2 study as “in control” failed screening due to TSH out of range27 30-70% do not maintain normal TSH across time26,28-29 Increased mortality Cardiovascular disease Insulin resistance Fractures Neurocognitive decline Accelerated aging (heart failure, coronary artery disease, hyperlipidemia, arrhythmias, stroke)
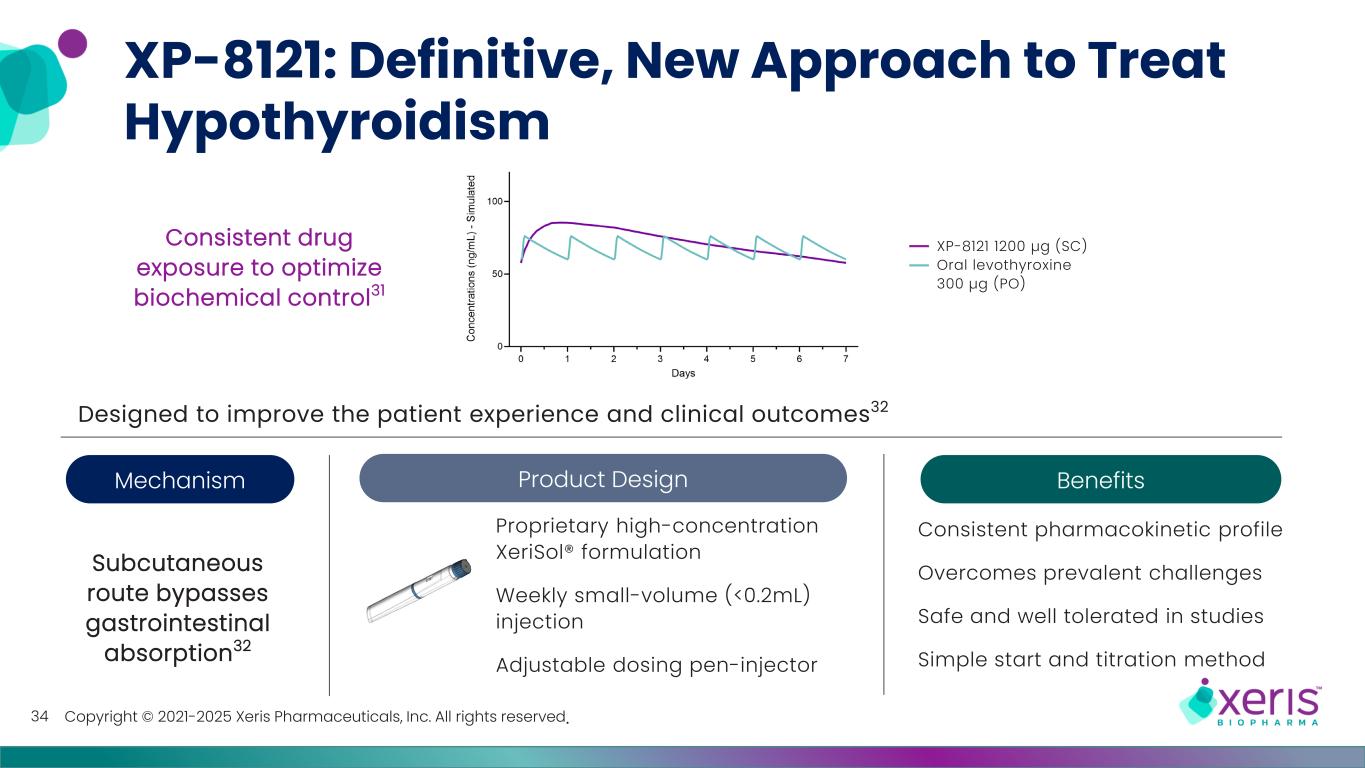
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. XP-8121: Definitive, New Approach to Treat Hypothyroidism 34 Proprietary high-concentration XeriSol® formulation Weekly small-volume (<0.2mL) injection Adjustable dosing pen-injector Consistent pharmacokinetic profile Overcomes prevalent challenges Safe and well tolerated in studies Simple start and titration method Designed to improve the patient experience and clinical outcomes32 Mechanism XP-8121 1200 µg (SC) Oral levothyroxine 300 µg (PO) Consistent drug exposure to optimize biochemical control31 Subcutaneous route bypasses gastrointestinal absorption32 Product Design Benefits

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. XP-8121 Building a Blockbuster in Hypothyroidism Clinical Overview Anh Nguyen, MD, MBA Chief Medical Officer Expert Panel on Hypothyroidism Anh Nguyen, MD, MBA Antonio C. Bianco, MD, PhD University of Texas Medical Branch Francesco S. Celi, MD, MHSc UConn Health Opportunity and Path to Launch Joshua Bennett VP, Strategic Initiatives 35

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. XP-8121 Building a Blockbuster in Hypothyroidism Clinical Overview Anh Nguyen, MD, MBA Chief Medical Officer Expert Panel on Hypothyroidism Anh Nguyen, MD, MBA Antonio C. Bianco, MD, PhD University of Texas Medical Branch Francesco S. Celi, MD, MHSc UConn Health Opportunity and Path to Launch Joshua Bennett VP, Strategic Initiatives 36
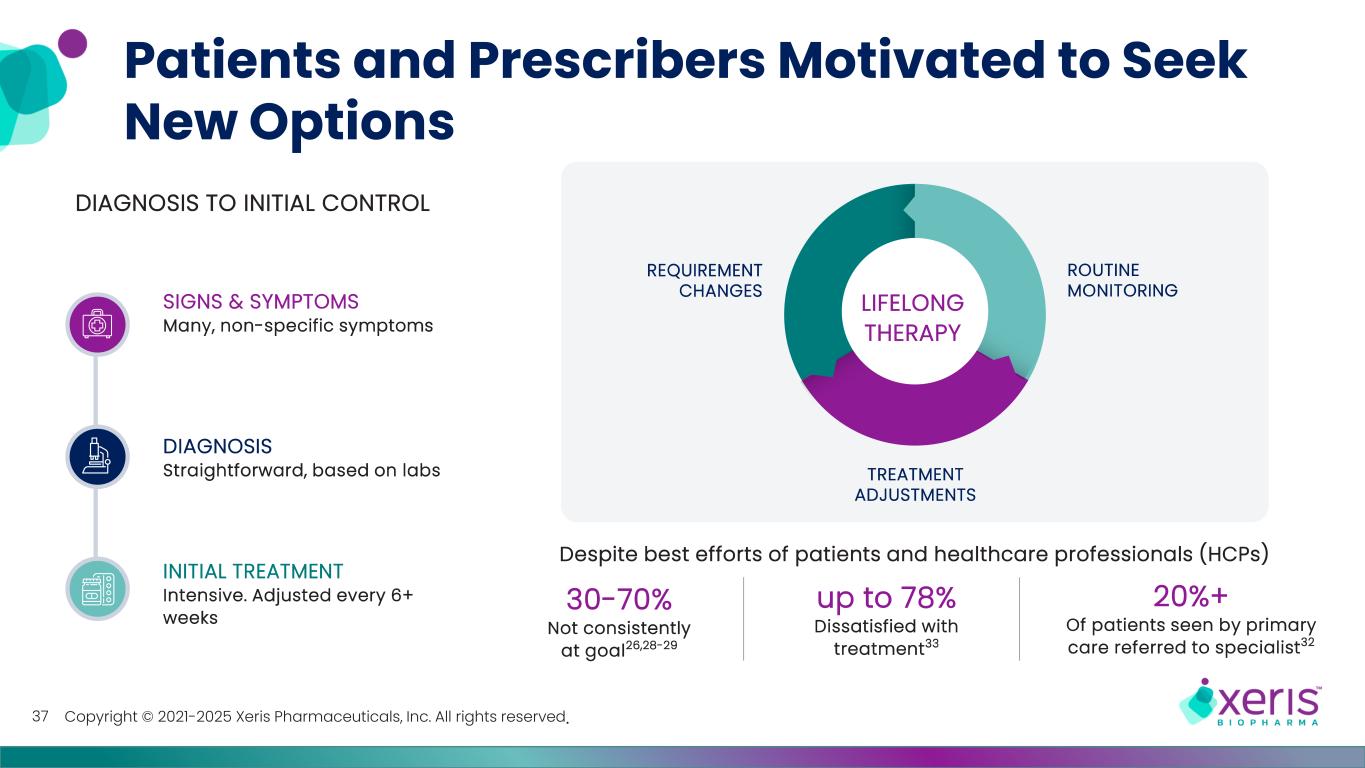
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Patients and Prescribers Motivated to Seek New Options 37 Despite best efforts of patients and healthcare professionals (HCPs) 30-70% Not consistently at goal26,28-29 up to 78% Dissatisfied with treatment33 20%+ Of patients seen by primary care referred to specialist32 REQUIREMENT CHANGES ROUTINE MONITORING TREATMENT ADJUSTMENTS LIFELONG THERAPY SIGNS & SYMPTOMS Many, non-specific symptoms DIAGNOSIS Straightforward, based on labs INITIAL TREATMENT Intensive. Adjusted every 6+ weeks DIAGNOSIS TO INITIAL CONTROL
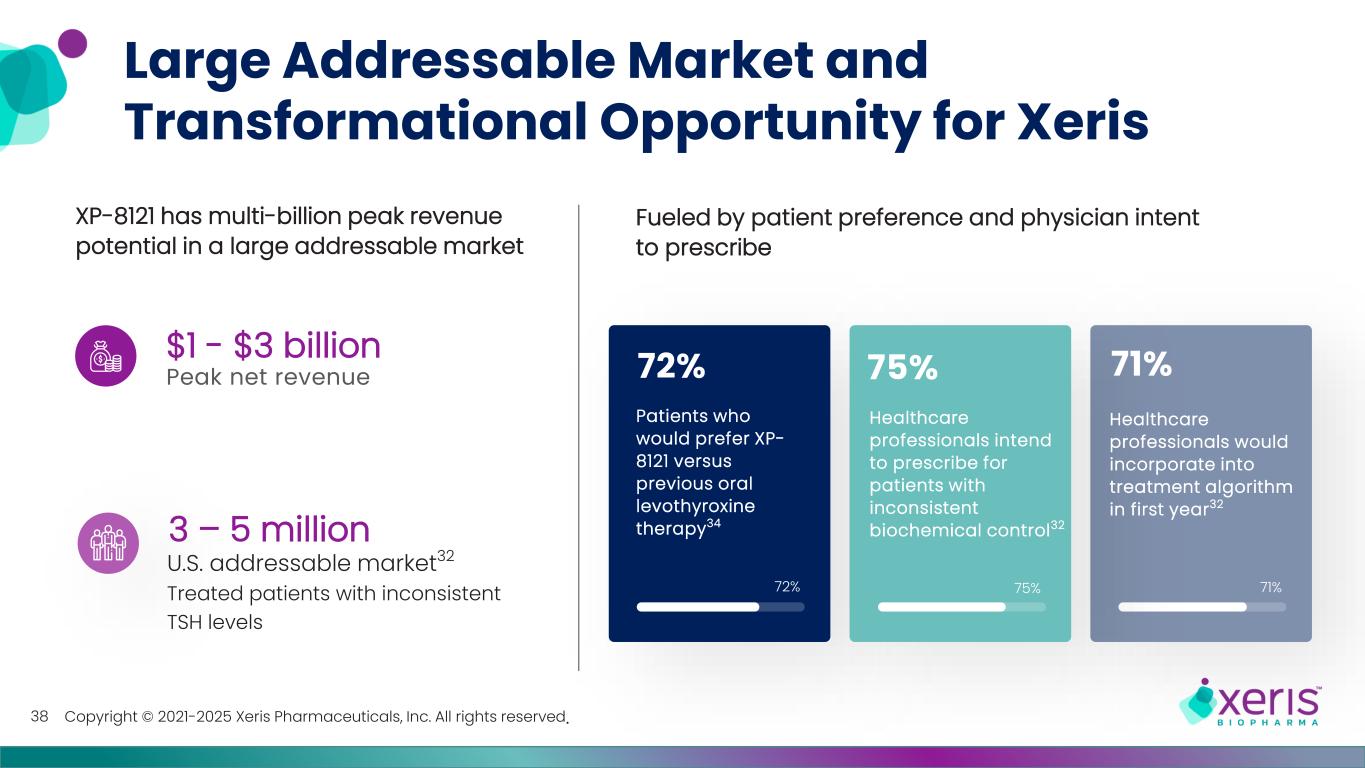
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. 72% 71%75% Large Addressable Market and Transformational Opportunity for Xeris 75% Healthcare professionals intend to prescribe for patients with inconsistent biochemical control32 71% Healthcare professionals would incorporate into treatment algorithm in first year32 72% Patients who would prefer XP- 8121 versus previous oral levothyroxine therapy34 Fueled by patient preference and physician intent to prescribe XP-8121 has multi-billion peak revenue potential in a large addressable market Peak net revenue $1 - $3 billion U.S. addressable market32 Treated patients with inconsistent TSH levels 3 – 5 million 38
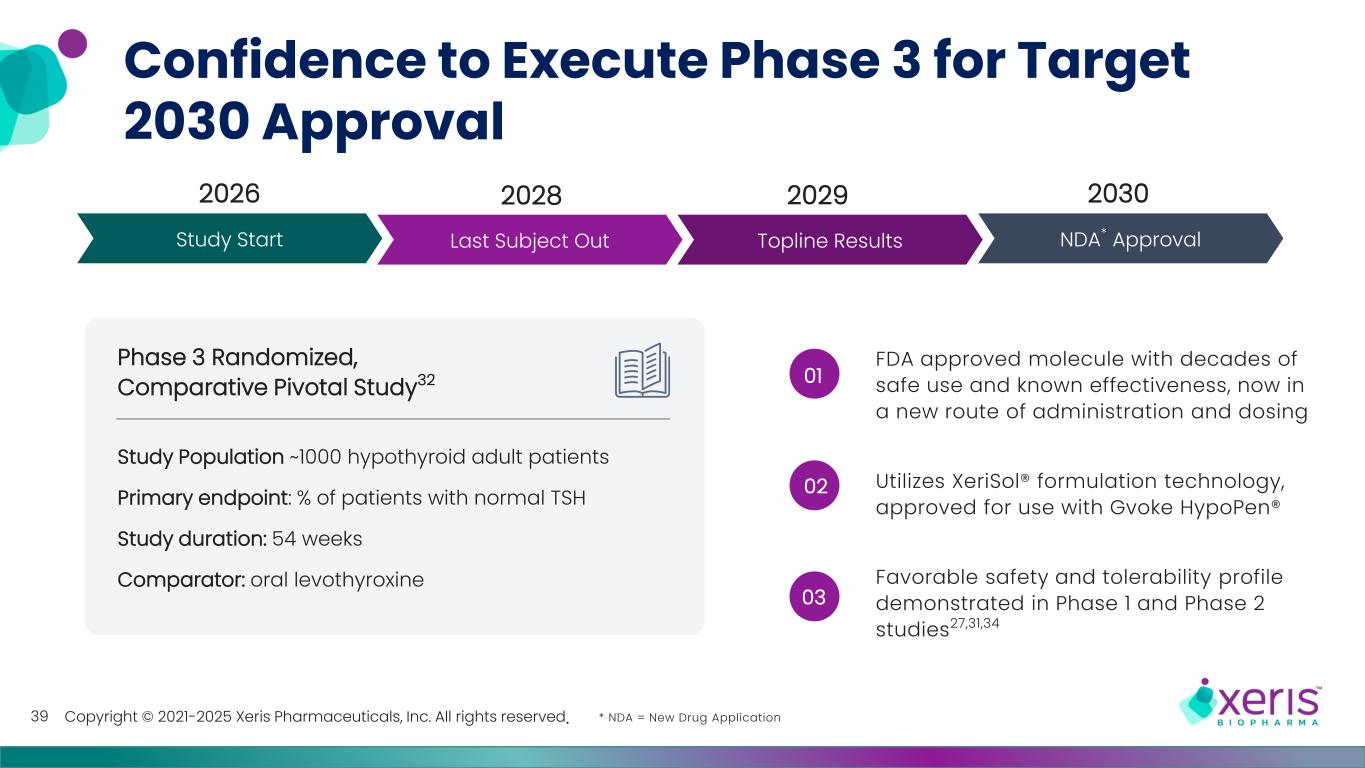
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Confidence to Execute Phase 3 for Target 2030 Approval 39 2026 2029 20302028 Study Start Last Subject Out Topline Results NDA* Approval FDA approved molecule with decades of safe use and known effectiveness, now in a new route of administration and dosing Utilizes XeriSol® formulation technology, approved for use with Gvoke HypoPen® Favorable safety and tolerability profile demonstrated in Phase 1 and Phase 2 studies27,31,34 01 02 03 Phase 3 Randomized, Comparative Pivotal Study32 Study Population ~1000 hypothyroid adult patients Primary endpoint: % of patients with normal TSH Study duration: 54 weeks Comparator: oral levothyroxine * NDA = New Drug Application

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. XP-8121 Investment Summary Significant Unmet Need | Seminal Program | Transformational Opportunity 40 Unmet need not resolved after 50+ years of oral levothyroxine availability Long-term health risks, symptom burden, healthcare practice burden First pivotal Phase 3 study evaluating clinical efficacy of levothyroxine for NDA submission Established molecule, innovative product, defined development program $1 - $3 billion peak net revenue Treated patients, identifiable via regular labs. Leverages Xeris capabilities

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Leveraging Our Strengths to Deliver Our Future Kevin McCulloch President and Chief Operating Officer 41
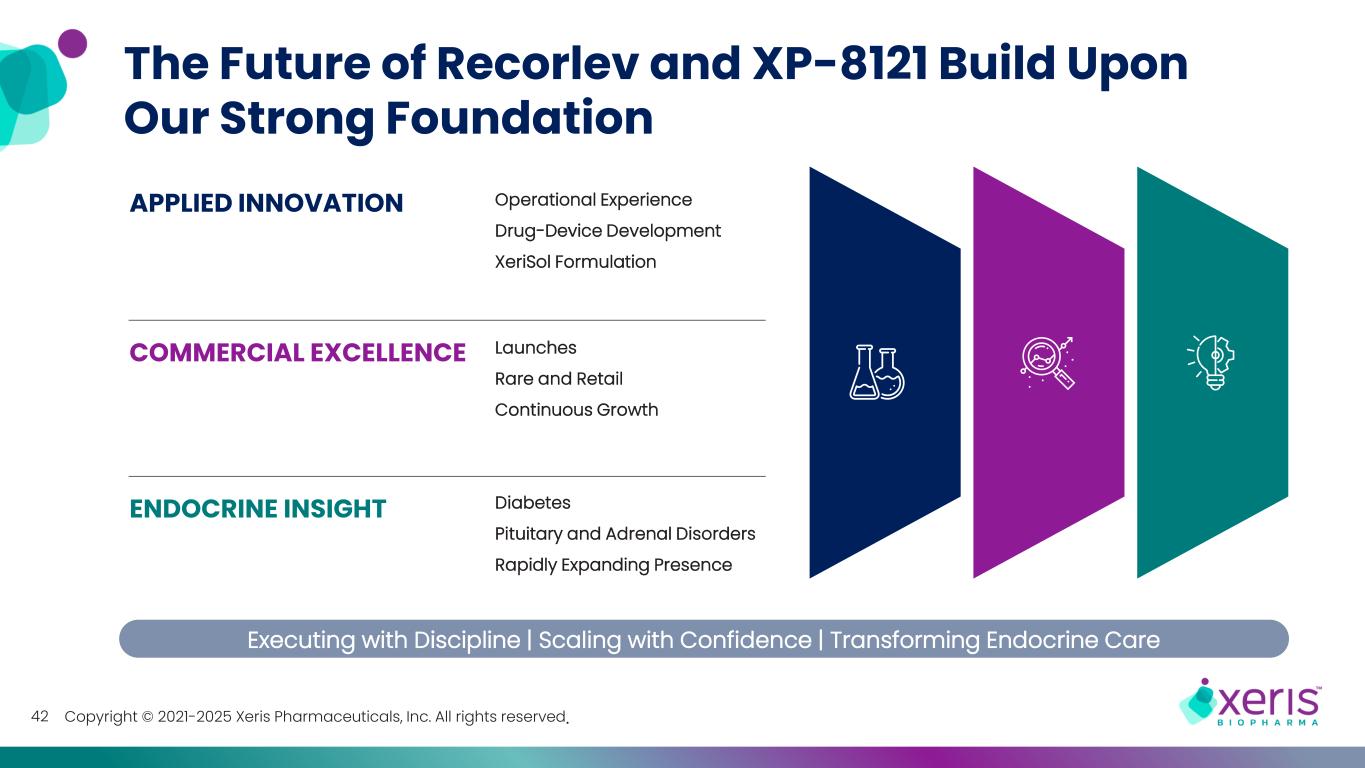
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.42 The Future of Recorlev and XP-8121 Build Upon Our Strong Foundation Operational Experience Drug-Device Development XeriSol Formulation APPLIED INNOVATION COMMERCIAL EXCELLENCE Launches Rare and Retail Continuous Growth ENDOCRINE INSIGHT Diabetes Pituitary and Adrenal Disorders Rapidly Expanding Presence Executing with Discipline | Scaling with Confidence | Transforming Endocrine Care
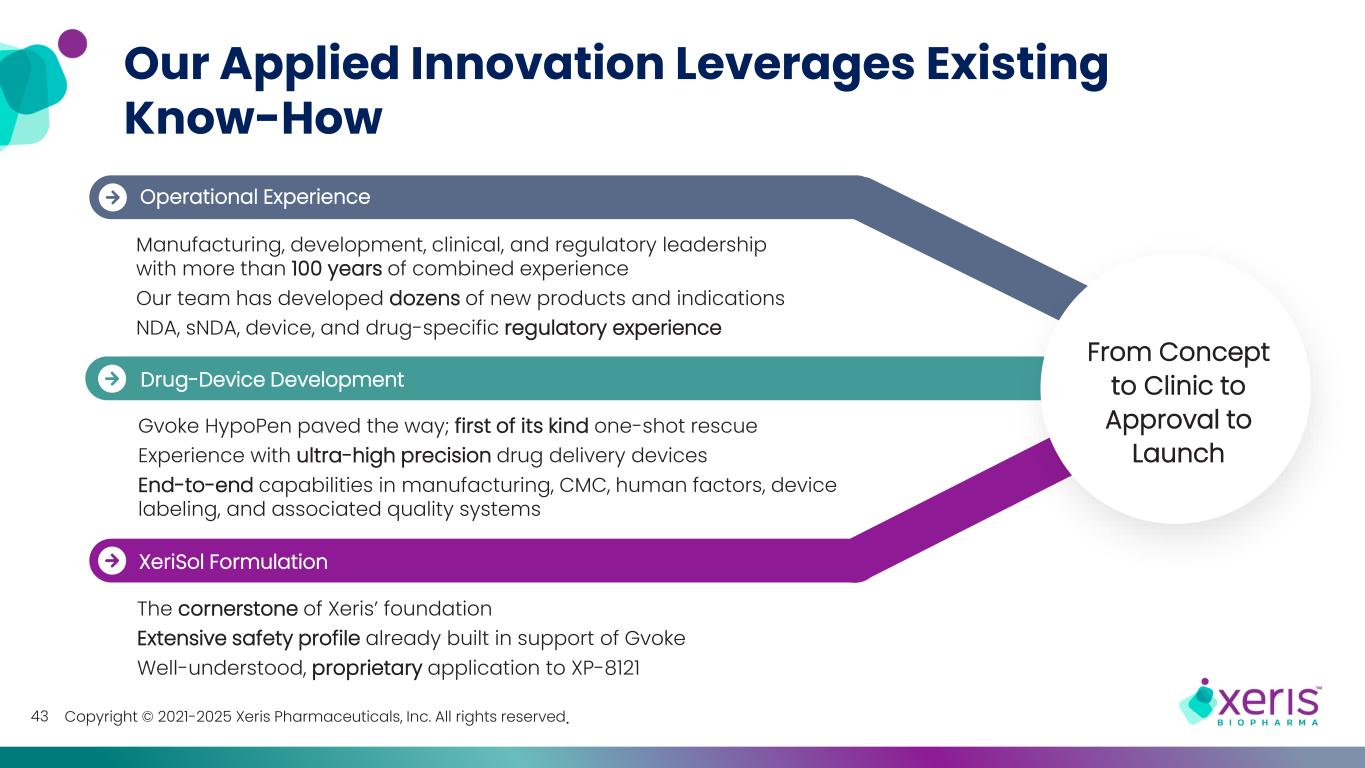
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.43 Our Applied Innovation Leverages Existing Know-How Operational Experience Manufacturing, development, clinical, and regulatory leadership with more than 100 years of combined experience Our team has developed dozens of new products and indications NDA, sNDA, device, and drug-specific regulatory experience Drug-Device Development Gvoke HypoPen paved the way; first of its kind one-shot rescue Experience with ultra-high precision drug delivery devices End-to-end capabilities in manufacturing, CMC, human factors, device labeling, and associated quality systems XeriSol Formulation The cornerstone of Xeris’ foundation Extensive safety profile already built in support of Gvoke Well-understood, proprietary application to XP-8121 From Concept to Clinic to Approval to Launch

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.44 Commercial Excellence is Our Standard Management team with deep experience in the promotion of pharmaceutical products Market preparedness expertise in research, medical affairs, payer management and business analytics Dedicated sales, patient support, medical education, and patient advocacy across all related disease categories Established specialty pharmacy and distribution networks Our brands expand disease state understanding We build promotional strategies that navigate the competitive landscape Xeris’ commercial model is purpose-built to drive continuous rapid growth Market Expansion and Lifecyle Management In-house Capability Across Rare, Ultra-Rare, and Retail Highly Successful Launches of Gvoke and Recorlev

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.45 FIRST in Cushing’s Syndrome Committed to supporting those who suffer from either pituitary or adrenal-based diseases Laser-focused on delivering the insight needed to address the root causes of hypercortisolemia Delivering a Best-in-Class Therapy to Normalize Cortisol Clinical development partnerships with the endocrinology academic community started more than 10 YEARS ago Captured groundbreaking insight across two separate therapeutic areas Our studies in hypothyroidism will leverage our well-established relationships 20-year history that created the ONLY ready-to-use subcutaneous form of glucagon Deep partnering with advocacy organizations and patient groups Leading voice for the unprotected Protecting People with Diabetes from Harm Aligned to Serve the Endocrinology Community Building upon our history, commitment, and expertise Investing in Research and Disease Awareness

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Positioned for Long-Term Value Creation Steven Pieper Chief Financial Officer 46
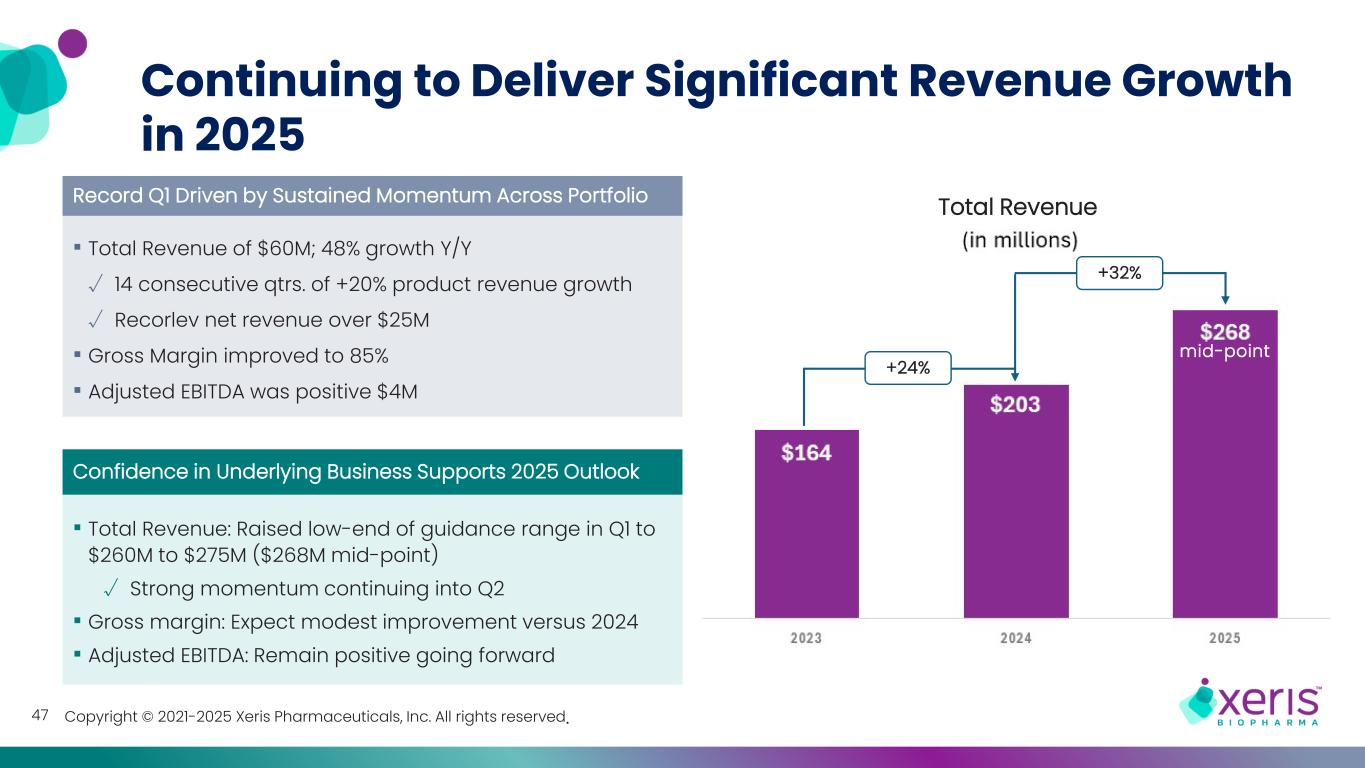
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Continuing to Deliver Significant Revenue Growth in 2025 Total Revenue of $60M; 48% growth Y/Y √ 14 consecutive qtrs. of +20% product revenue growth √ Recorlev net revenue over $25M Gross Margin improved to 85% Adjusted EBITDA was positive $4M Record Q1 Driven by Sustained Momentum Across Portfolio Confidence in Underlying Business Supports 2025 Outlook Total Revenue: Raised low-end of guidance range in Q1 to $260M to $275M ($268M mid-point) √ Strong momentum continuing into Q2 Gross margin: Expect modest improvement versus 2024 Adjusted EBITDA: Remain positive going forward +24% +32% mid-point 47 Total Revenue
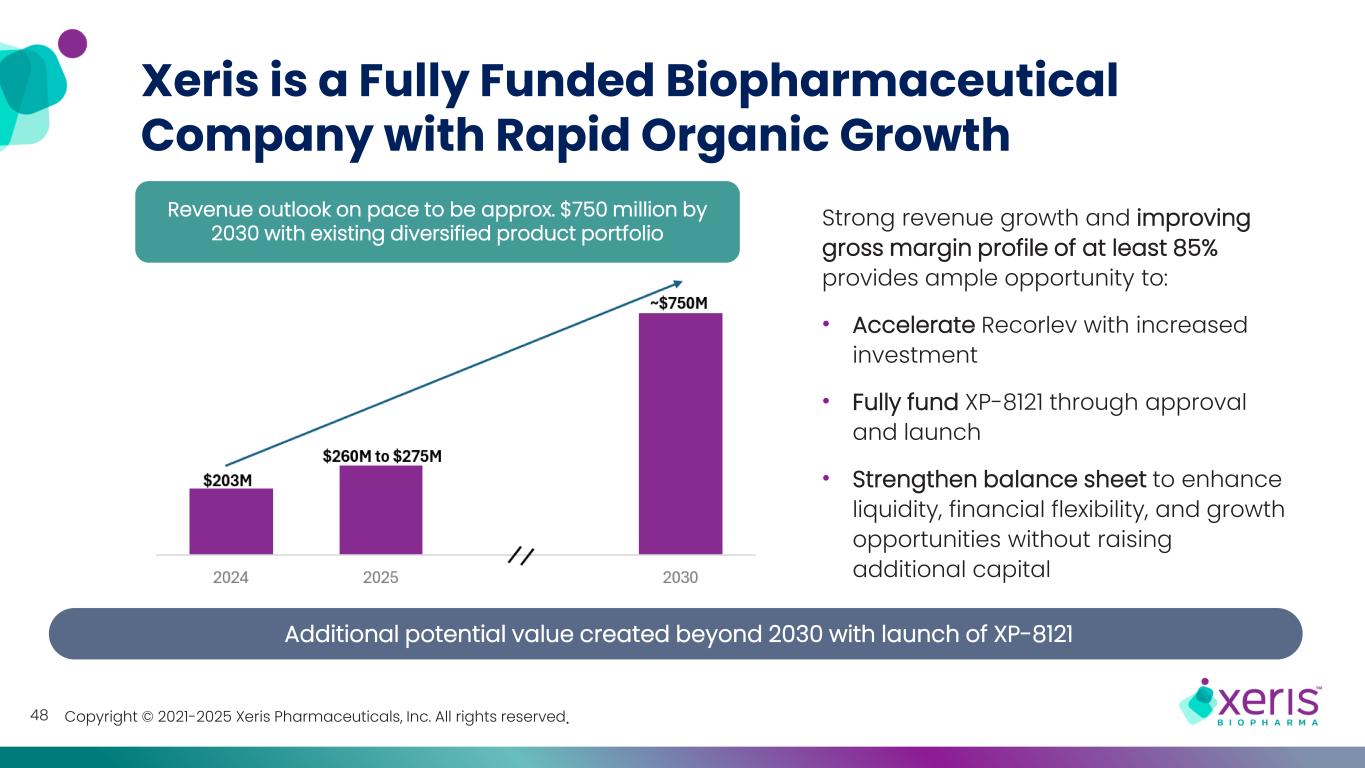
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Strong revenue growth and improving gross margin profile of at least 85% provides ample opportunity to: • Accelerate Recorlev with increased investment • Fully fund XP-8121 through approval and launch • Strengthen balance sheet to enhance liquidity, financial flexibility, and growth opportunities without raising additional capital Revenue outlook on pace to be approx. $750 million by 2030 with existing diversified product portfolio Xeris is a Fully Funded Biopharmaceutical Company with Rapid Organic Growth 48 Additional potential value created beyond 2030 with launch of XP-8121
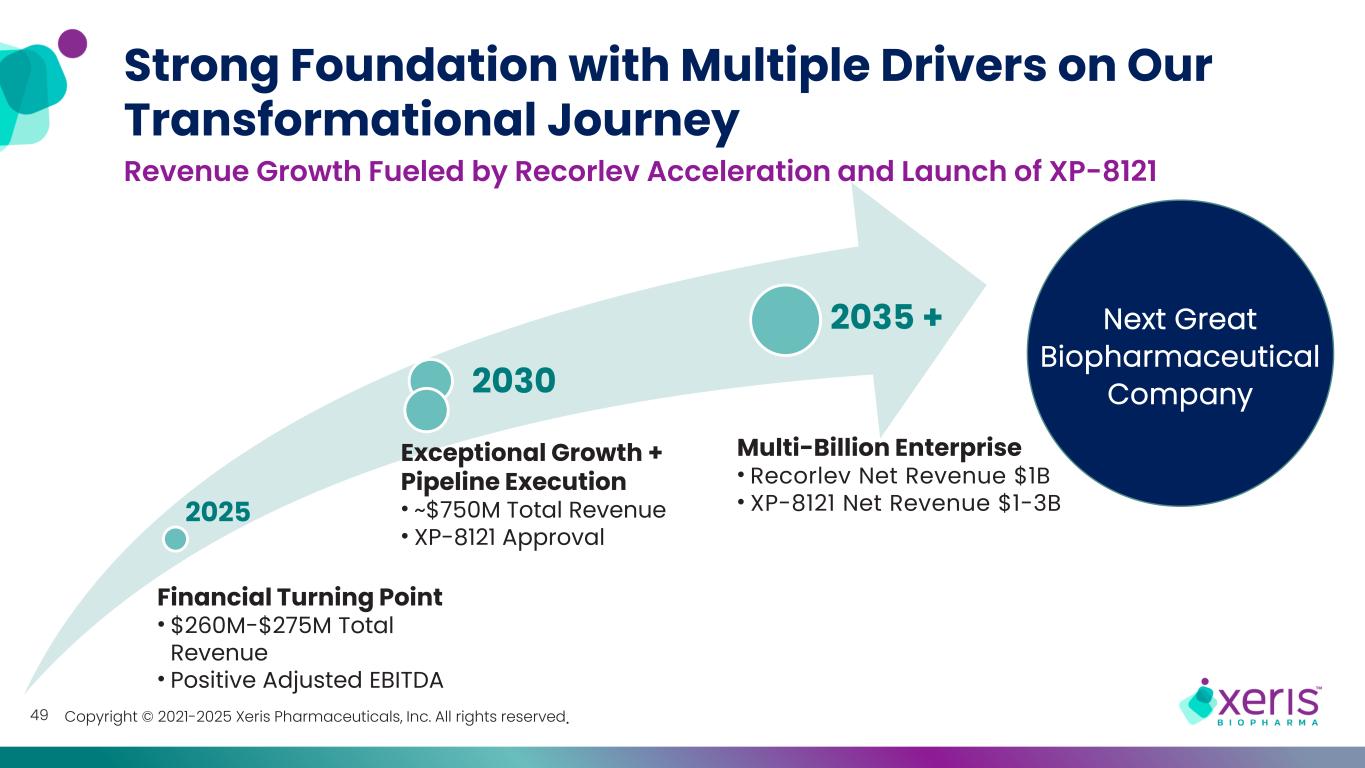
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. 2025 2030 2035 + Revenue Growth Fueled by Recorlev Acceleration and Launch of XP-8121 Strong Foundation with Multiple Drivers on Our Transformational Journey Multi-Billion Enterprise • Recorlev Net Revenue $1B • XP-8121 Net Revenue $1-3B Exceptional Growth + Pipeline Execution • ~$750M Total Revenue • XP-8121 Approval Financial Turning Point • $260M-$275M Total Revenue • Positive Adjusted EBITDA Next Great Biopharmaceutical Company 49

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved.50 Q&A Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. 50

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Closing Remarks John Shannon Chief Executive Officer 51

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Thank You XERIS INVESTOR RELATIONS Allison Wey awey@xerispharma.com 52

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. IMPORTANT SAFETY INFORMATION about Recorlev Indication RECORLEV (levoketoconazole) is a cortisol synthesis inhibitor indicated for the treatment of endogenous hypercortisolemia in adult patients with Cushing’s syndrome for whom surgery is not an option or has not been curative. Limitations of use: RECORLEV is not approved for the treatment of fungal infections. 53

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. IMPORTANT SAFETY INFORMATION about Recorlev (continued) • RECORLEV is contraindicated in patients: – With cirrhosis, acute liver disease or poorly controlled chronic liver disease, baseline AST or ALT >3 times the upper limit of normal, recurrent symptomatic cholelithiasis, a prior history of drug-induced liver injury due to ketoconazole or any azole antifungal therapy that required discontinuation of treatment, or extensive metastatic liver disease – Taking drugs that cause QT prolongation associated with ventricular arrhythmias, including torsades de pointes – With prolonged QTcF interval >470 msec at baseline, history of torsades de pointes, ventricular tachycardia, ventricular fibrillation, or long QT syndrome – With hypersensitivity to levoketoconazole, ketoconazole, or any excipient in RECORLEV – Taking certain drugs that are sensitive substrates of CYP3A4 or CYP3A4 and P-gp • RECORLEV may lead to hypocortisolism with a potential for life-threatening adrenal insufficiency. Dosage reduction or interruption may be necessary • Hypersensitivity to RECORLEV has been reported. Anaphylaxis has been reported with oral ketoconazole • RECORLEV may lower serum testosterone in men and women. Inform patients to report associated symptoms • Most common adverse reactions are nausea/vomiting, hypokalemia, hemorrhage/contusion, systemic hypertension, headache, hepatic injury, abnormal uterine bleeding, erythema, fatigue, abdominal pain/dyspepsia, arthritis, upper respiratory infection, myalgia, arrhythmia, back pain, insomnia/sleep disturbances, and peripheral edema • Avoid use of strong CYP3A4 inhibitors and inducers 2 weeks before and during RECORLEV treatment. Consult approved product labeling for drugs that are substrates of CYP3A4, P-gp, OCT2, and MATE prior to initiating RECORLEV. For atorvastatin, metformin, and gastric acid modulators, see full Prescribing Information for recommendations regarding concomitant use with RECORLEV • Breastfeeding is not recommended during treatment and for one day after final dose Please see Medication Guide and full Prescribing Information, including Boxed Warning, for RECORLEV. 54

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Citations 55 1. Fleseriu M, et al. Expert Rev Endocrinol Metab. 2021;16(4):159-174. 2. Dekkers OM, et al. J Clin Endocrinol Metab. 2013;98(6):2277-2284. 3. Limumpornpetch P, et al. J Clin Endocrinol Metab. 2022;107(8):2377-2388 4. Mancini T, et al. Clin Endocrinol. 2004;61(6):768-777. 6. 5. Nieman LK, et al. J Clin Endocrinol Metab. 2015;100(8):2807-2831. 6. Nieman LK, et al. J Clin Endocrinol Metab. 2008;93(5):1526-1540. 7. Pivonello R, et al. Lancet Diabetes Endocrinol. 2016;4(7):611-629. 8. Page-Wilson G, et al. Pituitary. 2023;26(4):364-374. 9. Ragnarsson O, et al. J Clin Endocrinol Metab. 2019;104(6):2375-2384. 10. Gadelha M, et al. Lancet. 2023 Dec 9;402(10418):2237-2252. 11. Wengander S et al. Clin Endocrinol 2019; 91: 263–70. 12. Hakami OA, et al. Best Pract Res Clin Endocrinol Metab 2021; 35: 101521 13. Buse JB, et al. Diabetes Care 2025; dc242841. https://doi.org/10.2337/dc24-2841 14. Recorlev [prescribing information]. Chicago, IL: Xeris Pharmaceuticals, Inc. 15. Creemers SG, et al. J Clin Endocrinol Metab. 2021;106(4):e1618-e1630. 16. Auchus RJ, et al. Poster presented at: the Endocrine Society 100th Annual Meeting; March 17-20, 2018; Chicago, IL. 17. Fleseriu M, et al. Lancet Diabetes Endocrinol. 2019;7(11):1-12. 18. Fleseriu M, et al. Eur J Endocrinol. 2022;187(6):859-871. doi:10.1530/EJE-22-0506 8

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Citations (continued) 19. Patil N, Rehman A, Anastasopoulou C, Jialal I. Hypothyroidism. 2024 Feb 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–2024 Feb 18. 20. Esfandiari NH, Papaleontiou M. Levothyroxine prescribing: why simple is so complex. J Clin Endocrinol Metab. 2024; 109(5):e1406-e1407. 21. Jonklaas J, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014 Dec;24(12):1670-751. 22. Wiesner A, Gajewska D, Paśko P. Levothyroxine Interactions with Food and Dietary Supplements-A Systematic Review. Pharmaceuticals (Basel). 2021 Mar 2;14(3):206. 23. McMillan M, Rotenberg KS, Vora K, Sterman AB, Thevathasan L, Ryan MF, Mehra M, Sandulli W. Comorbidities, Concomitant Medications, and Diet as Factors Affecting Levothyroxine Therapy: Results of the CONTROL Surveillance Project. Drugs R D. 2016 Mar;16(1):53-68. 24. Henderson BB, Smith SP, Mengelkamp ME, Rhymer EK, Gray KN, Jackson AG, Henry SF, Chuang S, Stavrakas EH, Blair OM, Heaps M. Liquid Thyroxine Improves Outcomes in Hypothyroid Patients With Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome. Endocr Pract. 2024 Jun;30(6):505-512. 25. Bianco AC, Bao Y, Antunez Flores O, et al. Levothyroxine Treatment Adequacy and Formulation Changes in Patients with Hypothyroidism: A Retrospective Study of Real-World Data from the United States. Thyroid. 2023;33(8):940-949. 26. Kuye R, Riggs C, King J, Heilmann R, Kurz D, Milchak J. Thyroid Stimulating Hormone Stability in Patients Prescribed Synthetic or Desiccated Thyroid Products: A Retrospective Study. Ann Fam Med. 2020;18(5):452-454. 27. Conoscenti V, Meyer J, Huang R, Harper D. Screening Failures in a Phase 2, Multicenter Non-Randomized, Open-Label, Single Arm, Self-Controlled Study of Once-Weekly Subcutaneous Levothyroxine (XP-8121) [abstract and poster]. Presented at the American Thyroid Association Annaul Meeting (ATA); October 30-Nopvember 3, 2024. 28. Lindgård Nielsen J, Karmisholt J, Bülow Pedersen I, Carlé A. Prevalence and predictors of adequate treatment of overt hypothyroidism - a population-based study. EXCLI J. 2022;21:104-116; Xeris analysis. 29. Ettleson MD, Penna GCE, Wan W, Benseñor IM, Laiteerapong N, Bianco AC. TSH Trajectories During Levothyroxine Treatment in the Brazilian Longitudinal Study of Adult Health (ELSABrasil) Cohort. J Clin Endocrinol Metab. Published online May 23, 2024. 30. Feldt-Rasmussen U, Effraimidis G, Bliddal S, Klose M. Risks of suboptimal and excessive thyroid hormone replacement across ages. J Endocrinol Invest. 2024 May;47(5):1083-1090. 31. Fitch R, Mould DR, Conoscenti V, Huang R, Harper D. Phase 1 Study Evaluating the Pharmacokinetics, Dose Proportionality, Bioavailability, and Tolerability of Subcutaneous Levothyroxine Sodium (XP- 8121). Clin Transl Sci. 2025 May;18(5):e70244. 32. Data on File. Xeris Pharmaceuticals, LLC. Chicago, IL 2025. 33. Mitchell A, Hegedüs L, Žarković M, Hickey J, Perros P. Patient Satisfaction and Quality of Life in Hypothyroidism: An Online Survey by the British Thyroid Foundation. Clin Endocrinol. 2021 Mar;94(3):513-520. 34. Xeris Biopharma Announces Positive Topline Phase 2 Clinical Data of Its Investigational XeriSol -Formulated Once-Weekly Subcutaneous (SC) Levothyroxine (XP-8121). Press Release. May 30, 2024. Accessed November 13, 2024. https://xerispharma.com/news-releases/news-release-details/xeris-biopharma-announces-positive-topline-phase-2-clinical-data 56

Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Appendix 57
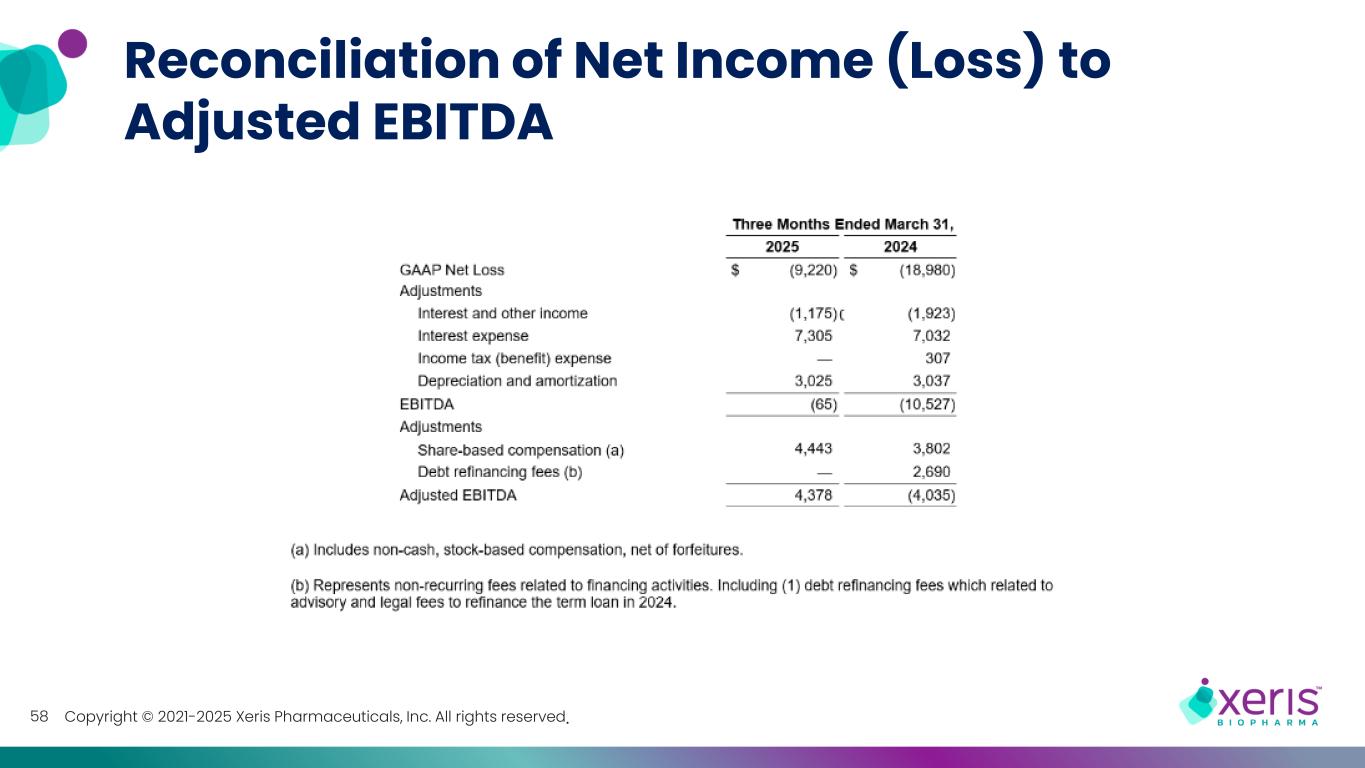
Copyright © 2021-2025 Xeris Pharmaceuticals, Inc. All rights reserved. Reconciliation of Net Income (Loss) to Adjusted EBITDA 58