

January 7. 2026 CERTAINT - 1 Trial Update

CERO This presentation (this “Presentation”) is provided for informational purposes only and for no other purpose and has been prepared to assist interested parties in making their own evaluation with respect to a potential investment in CERo The information contained herein is preliminary in nature and is subject to change, and such changes may be material . CERo’s business is subject to a number of risks that are not described in this presentation, including those set forth in the statements below and in the Summary of Risk Factors at the end of this presentation . By reviewing or reading this Presentation, you will be deemed to have agreed to the obligations and restrictions set out below . This Presentation supersedes and written communications between the parties hereto relating to the subject matter hereof . No representations or warranties, express or implied are given in, or in respect of, this Presentation . To the fullest extent permitted by law, in no circumstances will CERo or any of their respective subsidiaries, stockholders, affiliates, representatives, officers, employees, advisers or agents be responsible or liable for any direct, indirect or consequential loss or loss of profit arising from the use of this Presentation, its contents (including internal economic models), its omissions, reliance on the it, or opinions communicated in relation thereto or otherwise arising in connection therewith . Industry and market data used in this Presentation have been obtained from third - party industry publications and sources as well as from research reports purposes . CERo has not independently verified the data obtained from these sources and cannot assure you of the data’s accuracy or completeness . This data is subject to change . Recipients of this Presentation are not to construe its contents, or communication from or with CERo or their respective representatives as investment, legal or tax advice . In addition, this Presentation does not purpose to be all - inclusive or contain all of the information that may be required to make a full analysis Presentation should each make their own evaluation of Cero and of the relevance and adequacy of the information and should make such other investigations as they deem necessary . No Offer or Solicitation This Presentation and any oral statements made in connection with this Presentation do not constitute an offer to sell, or a solicitation of an offer to buy, or a recommendation to purchase, any securities in any jurisdiction . This Presentation does not a recommendation regarding any securities . Any offer to sell securities will be made only pursuant to a definitive subscription agreement and will be made in reliance on an exemption from registration under the Securities Act of 1933 , as amended, securities that do not involve a public offering . CERo reserves the right to withdraw or amend for any reason any offering and to reject any subscription agreement for any reason . The communication of this Presentation is restricted by law ; it is not or use by any person in, any jurisdiction where such distribution or use would be contrary to local law or regulation . Investors should consult with their counsel as to the applicable requirements for a purchaser to avail itself of any exemption under the Securities Act . The transfer of the securities may also be subject to conditions set forth in an agreement under Investors should be aware that they might be required to bear the final risk of their investment for an indefinite period of time . Cero is not making an offer of the securities in any jurisdiction where the offer is not permitted . This presentation shall not “solicitation” as defined in Section 14 of the Securities Exchange Act of 1934 , as amended . Forward - Looking Statements Certain statements included in this Presentation are not historical facts but are forward - looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995 . Forward - looking accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” and similar expressions that predict or indicate future events that are not statements of historical matters . These forward - looking statements include, but are not limited to, statements regarding our financial performance ; the accuracy of our estimates regarding expenses, future revenue, capital financing ; the scope, progress, results and costs of developing CER - 1236 or any other product candidates we may develop, and conducting preclinical studies and clinical trials ; the timing and costs involved in obtaining and maintaining regulatory other product candidates we may develop, and the timing or likelihood of regulatory filings and approvals, including our expectation to seek special designations or accelerated approvals for our drug candidates for various indications ; our ability to into and successfully complete clinical trials ; the ability of our clinical trials to demonstrate the safety and efficacy of CER - 1236 and any other product candidates we may develop, and other positive results ; the size and growth potential of the candidates, and our ability to serve those markets ; our expectations regarding our ability to obtain, maintain, protect and enforce intellectual property protection for CER - 1236 and for any other product candidate ; our ability to realize the anticipated transactions ; and the ability to obtain or maintain the listing of our common stock, and our public warrants on Nasdaq ; and other matters . These statements are based on various assumptions, whether or not identified in this Presentation, and on the CERo and are not predictions of actual performance . These forward - looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by, any investor as, a guarantee, an assurance, a of fact or probability . Actual events and circumstances are difficult or impossible to predict and will differ from assumptions . Many actual events and circumstances are beyond the control of CERo . These forward - looking statements are subject to a number of risks and uncertainties including the risks and uncertainties described under the heading “Risk Factors” in CERo’s most recent Annual Report on Form 10 - K filed with the SEC and any may be additional risks that CERo does not presently know or that CERo currently believes are immaterial that could also cause actual results to differ from those contained in the forward - looking statements . In addition, forward - looking statements plans or forecasts of future events as of the date of this Presentation . CERo anticipates that subsequent events and developments will cause CERo’s assessments to change . However, while CERo may elect to update these forward - looking future, CERo specifically disclaims any obligation to do so . These forward - looking statements should not be relied upon as representing CERo assessments as of any date subsequent to the date of this Presentation . Accordingly, undue reliance forward - looking statements . This presentation discusses product candidates that are under preclinical or clinical study and which have not yet been approved for marketing by the U . S . Food and Drug Administration . No representation is made as to the safety or effectiveness for the uses for which they are being studied . Trademarks This Presentation contains trademarks, service marks, trade names, and copyrights of CERo and third parties, which are the property of their respective owners . The use or display of third parties’ trademarks, service marks, trade names or intended to, and does not imply, a relationship with CERo, or an endorsement or sponsorship by or of CERo . Solely for convenience, the trademarks, service marks, trade names and copyrights referred to in this Presentation may appear without the but such references are not intended to indicate, in any way, that CERo will not assert, to the fullest extent under applicable law, their rights or the rights of the applicable licensor to these trademarks, service marks, trade names and copyrights 2 Disclaimers and Other Important Information
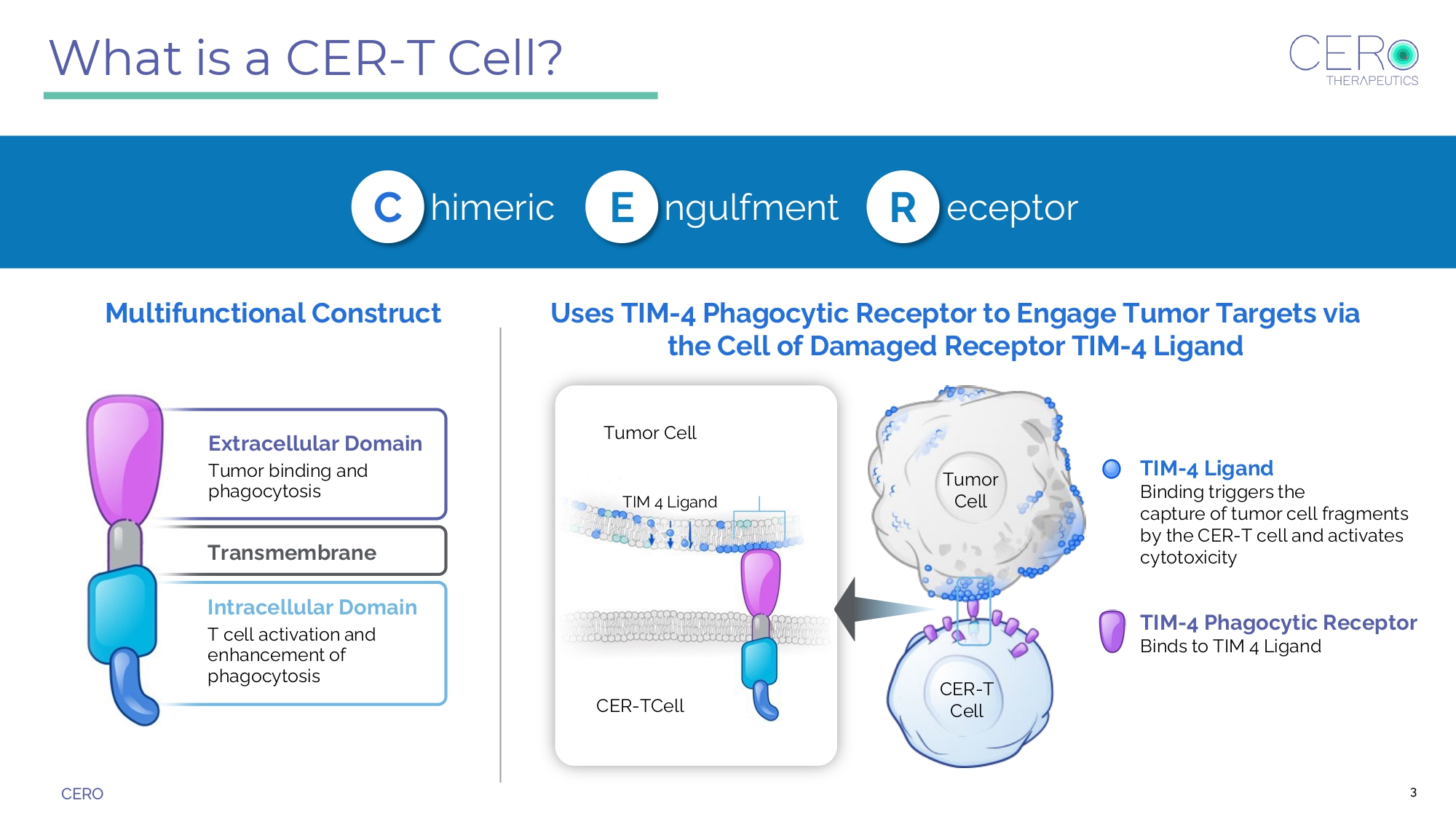
CERO 3 What is a CER - T Cell? himeric ngulfment eceptor C E R Uses TIM - 4 Phagocytic Receptor to Engage Tumor Targets via the Cell of Damaged Receptor TIM - 4 Ligand Tumor Cell TIM 4 Ligand T umor Cell CER - T Cell Multifunctional Construct Transmembrane Extracellular Domain Tumor binding and phagocytosis Intracellular Domain T cell activation and enhancement of phagocytosis TIM - 4 Ligand Binding triggers the capture of tumor cell fragments by the CER - T cell and activates cytotoxicity TIM - 4 Phagocytic Receptor Binds to TIM 4 Ligand CER - T Cell
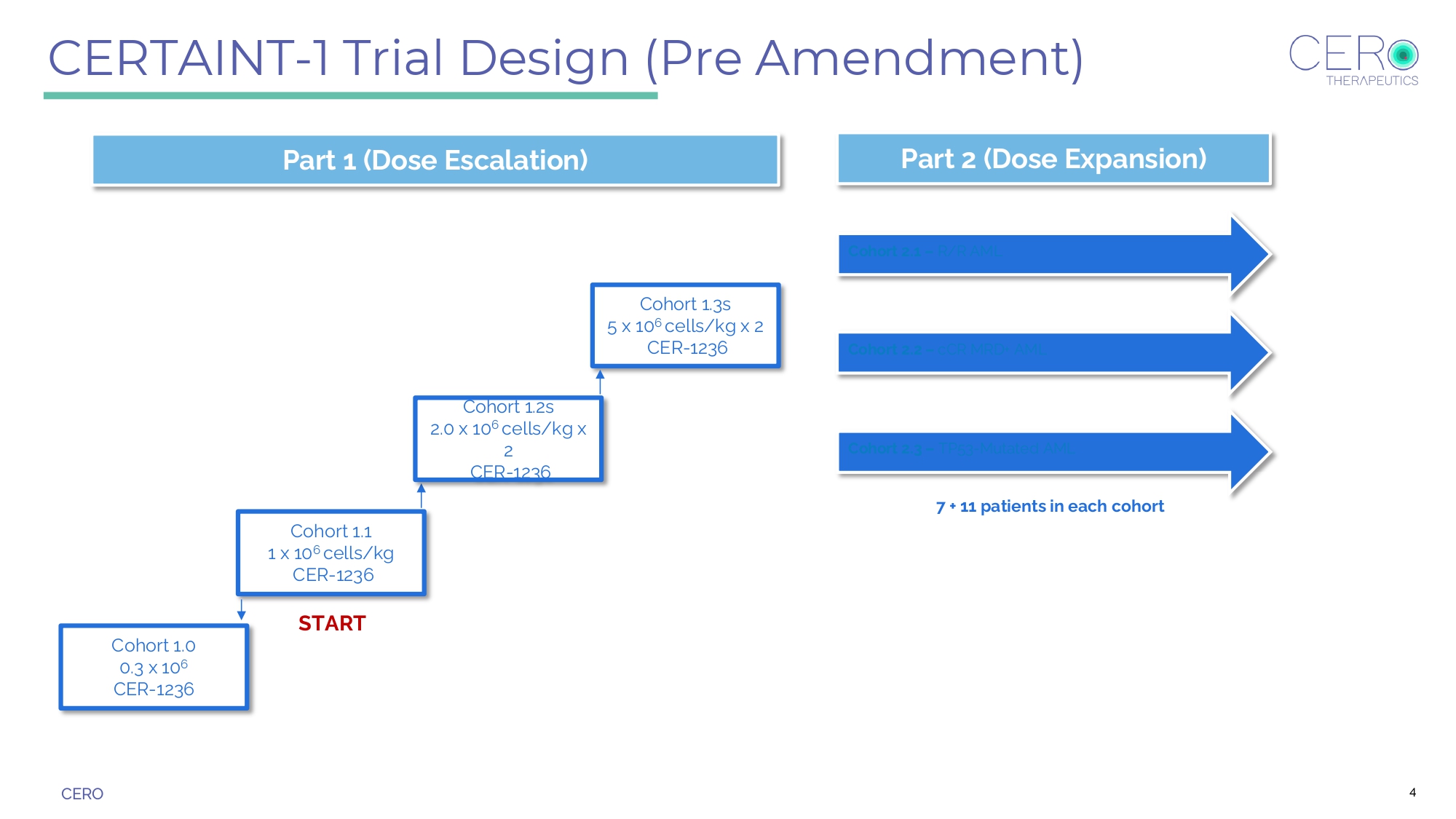
CERO Cohort 1.1 1 x 10 6 cells/kg CER - 1236 Cohort 1.3s 5 x 10 6 cells/kg x 2 CER - 1236 START Cohort 1.2s 2.0 x 10 6 cells/kg x 2 CER - 1236 Cohort 2.1 – R/R AML 7 + 11 patients in each cohort Cohort 1.0 0.3 x 10 6 CER - 1236 Part 1 (Dose Escalation) Part 2 (Dose Expansion) Cohort 2.2 – cCR MRD+ AML Cohort 2.3 – TP53 - Mutated AML CERTAINT - 1 Trial Design (Pre Amendment) 4

CERO Three Key Findings in Current Program 1. Robust cell expansion with no CRS, ICANS, or treatment - related adverse events reported to date. 2. Repeat dosing is feasible without LD. 3. Platelet recovery observed in patient 2 with MDS/AML. 5
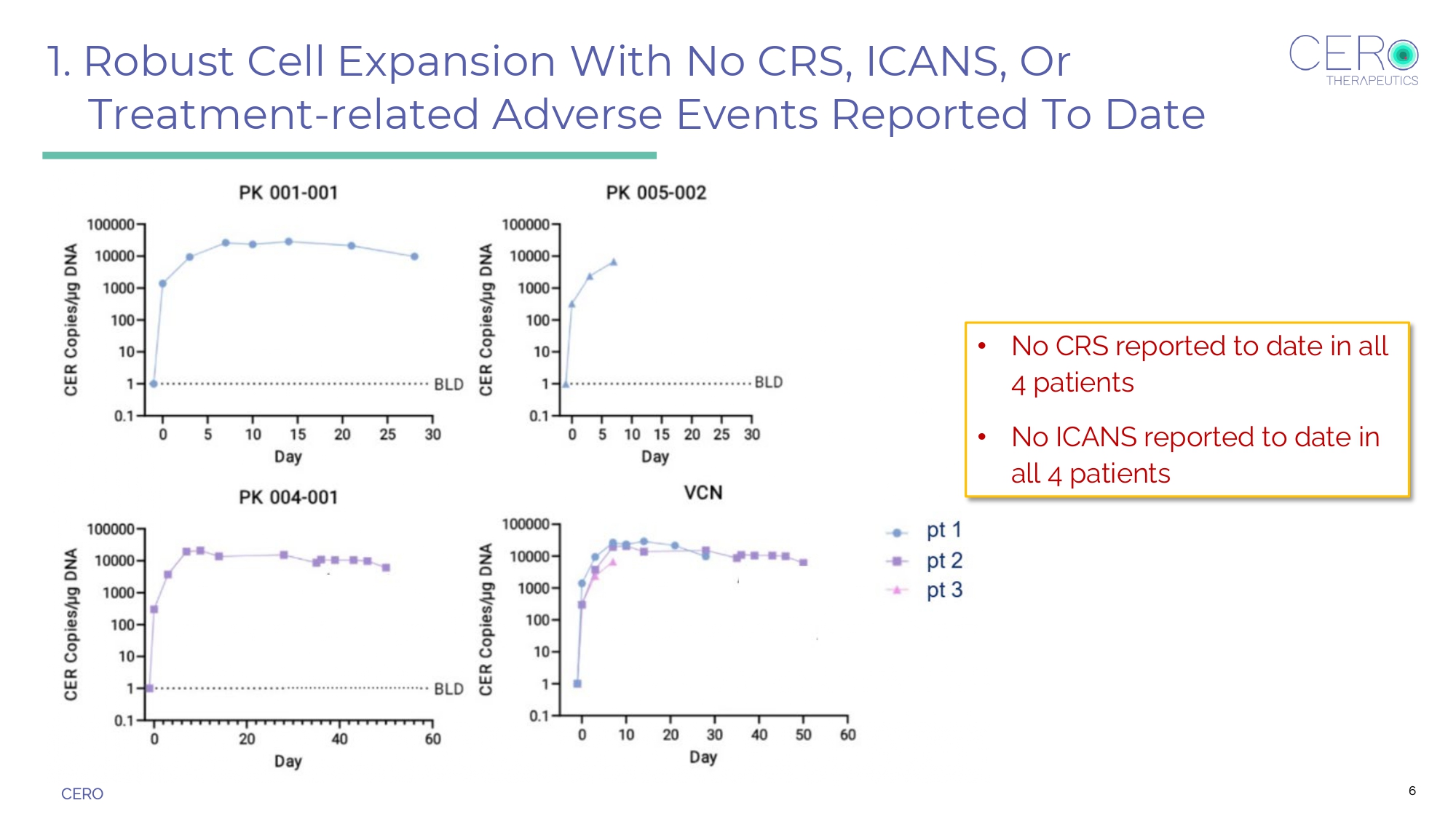
CERO 1. Robust Cell Expansion With No CRS, ICANS, Or Treatment - related Adverse Events Reported To Date • No CRS reported to date in all 4 patients • No ICANS reported to date in all 4 patients 6
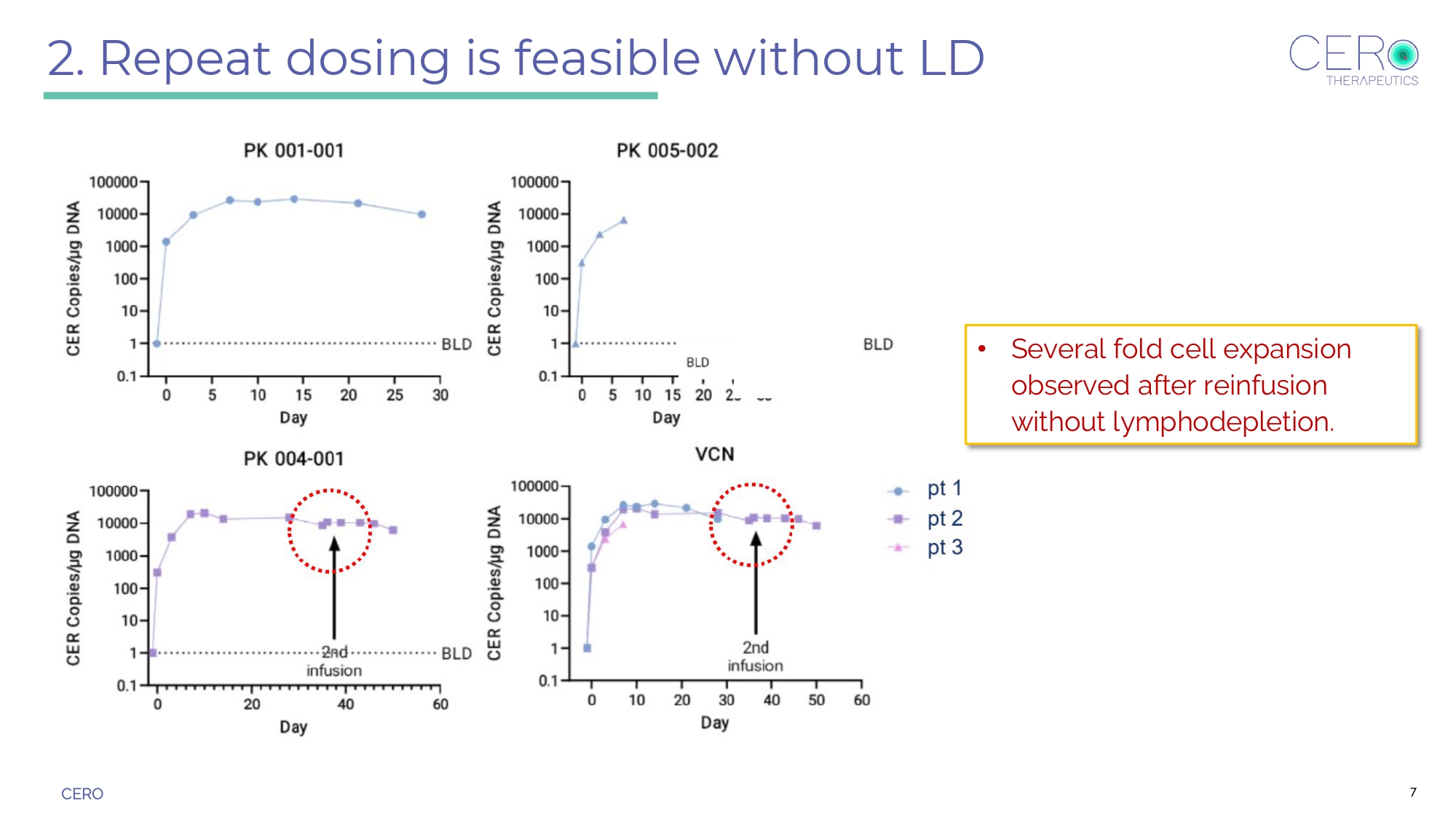
CERO 2. Repeat dosing is feasible without LD • Several fold cell expansion observed after reinfusion without lymphodepletion. 7
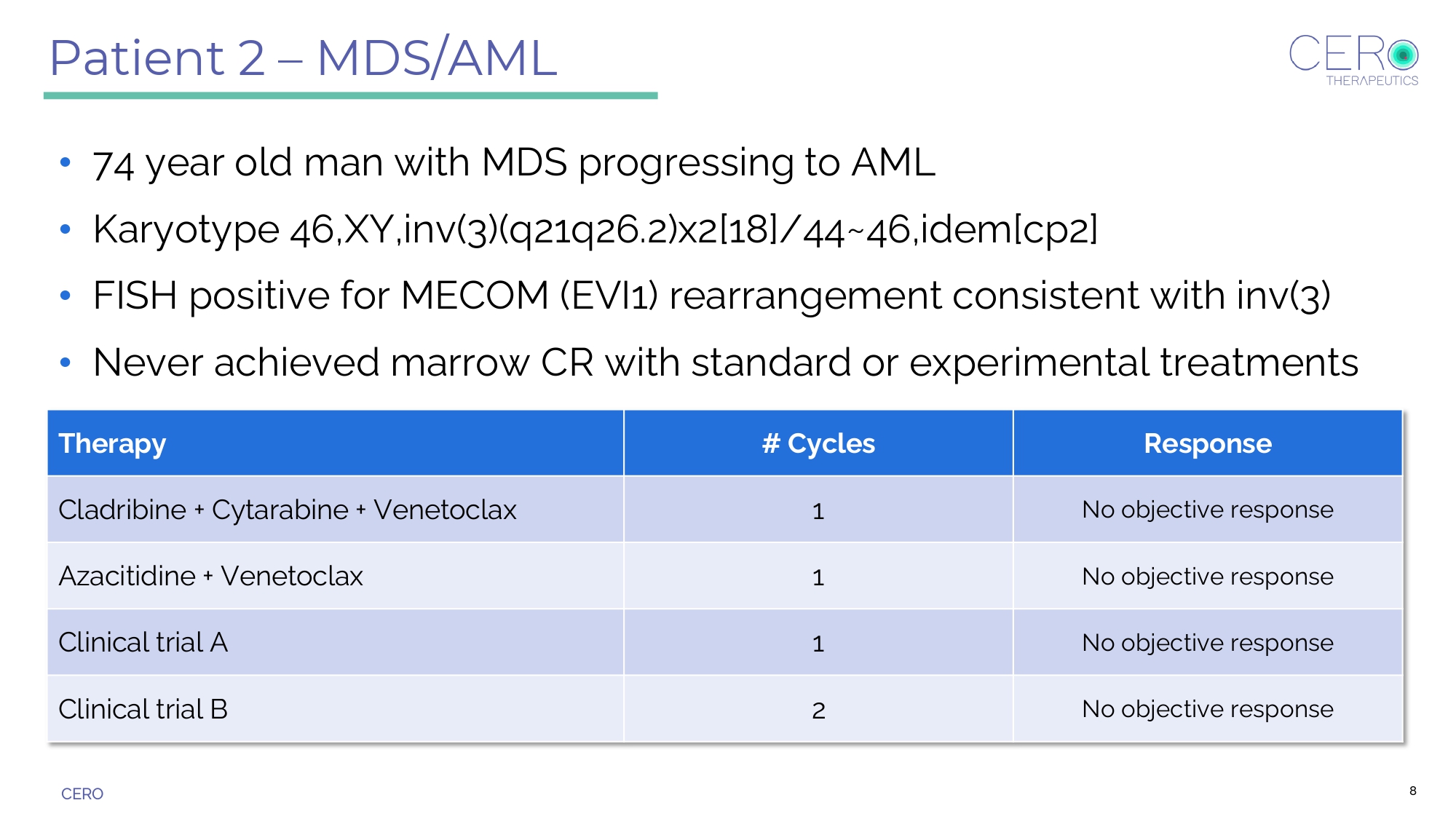
CERO Patient 2 – MDS/AML • 74 year old man with MDS progressing to AML • Karyotype 46,XY,inv(3)(q21q26.2)x2[18]/44~46,idem[cp2] • FISH positive for MECOM (EVI1) rearrangement consistent with inv(3) • Never achieved marrow CR with standard or experimental treatments Response # Cycles Therapy No objective response 1 Cladribine + Cytarabine + Venetoclax No objective response 1 Azacitidine + Venetoclax No objective response 1 Clinical trial A No objective response 2 Clinical trial B 8
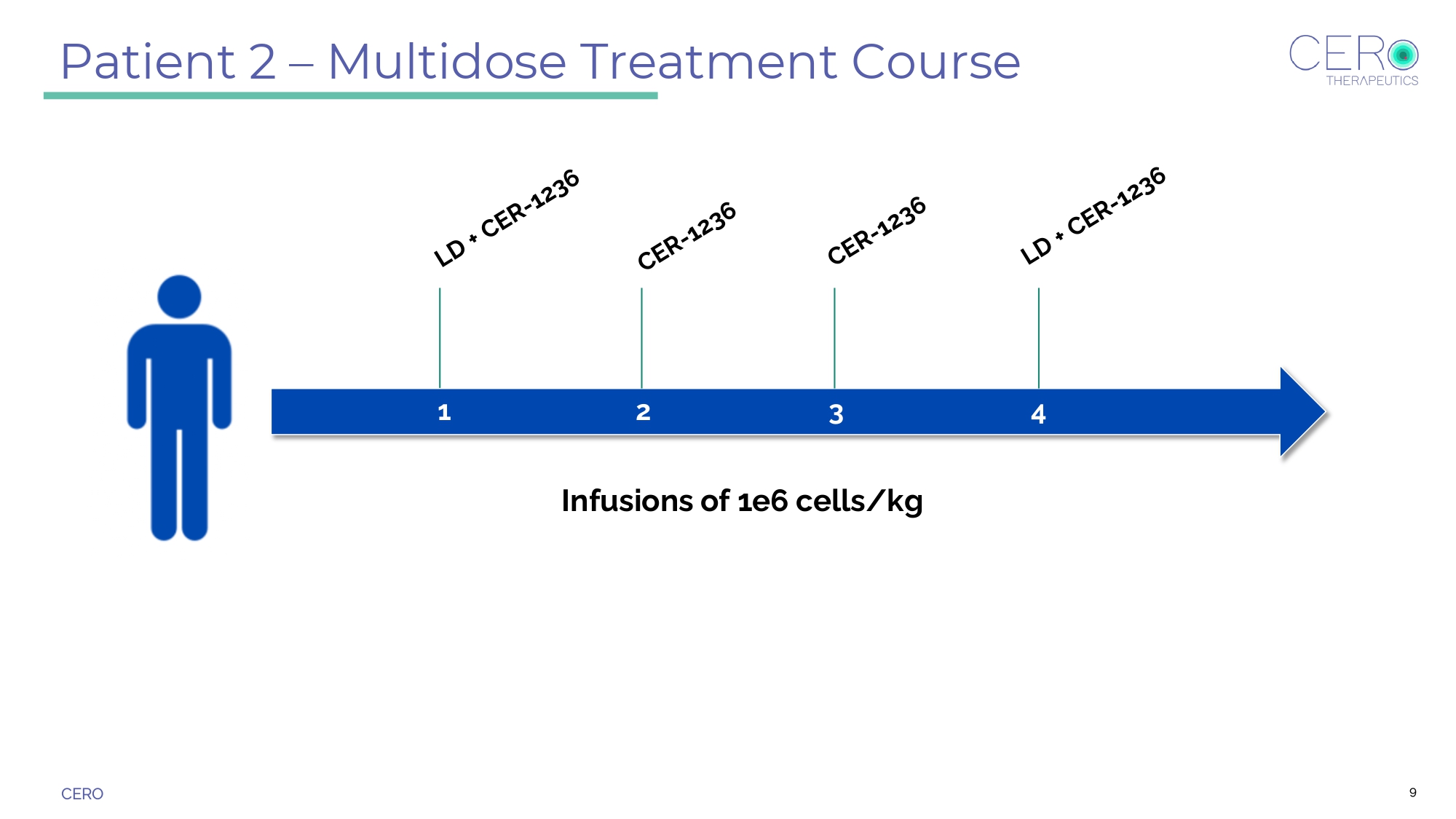
CERO Patient 2 – Multidose Treatment Course Infusions of 1e6 cells/kg 1 2 3 4 9
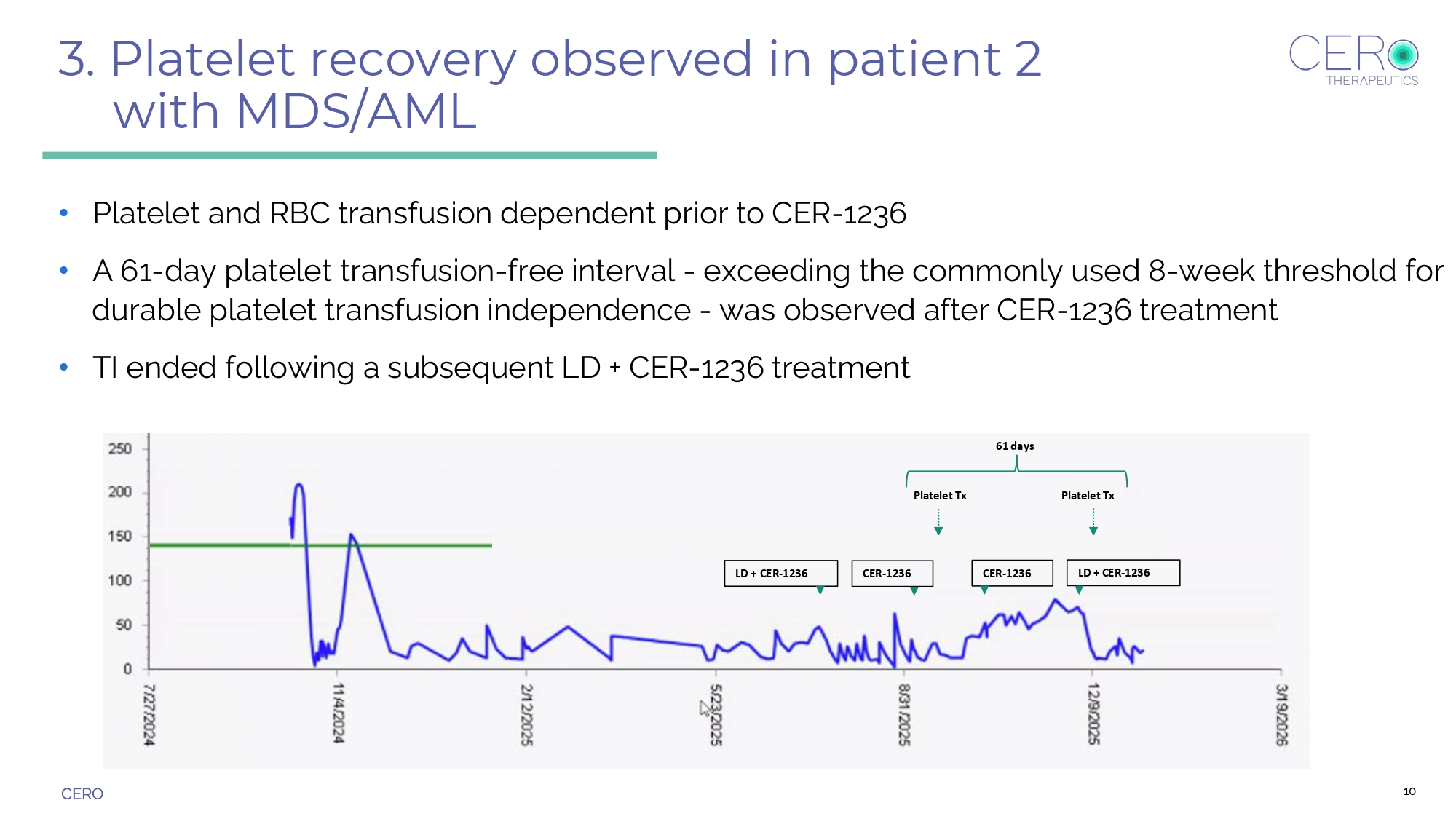
CERO LD + CER - 1236 CER - 1236 CER - 1236 Platelet Tx Platelet Tx LD + CER - 1236 61 days 3. Platelet recovery observed in patient 2 with MDS/AML • Platelet and RBC transfusion dependent prior to CER - 1236 • A 61 - day platelet transfusion - free interval - exceeding the commonly used 8 - week threshold for durable platelet transfusion independence - was observed after CER - 1236 treatment • TI ended following a subsequent LD + CER - 1236 treatment 10
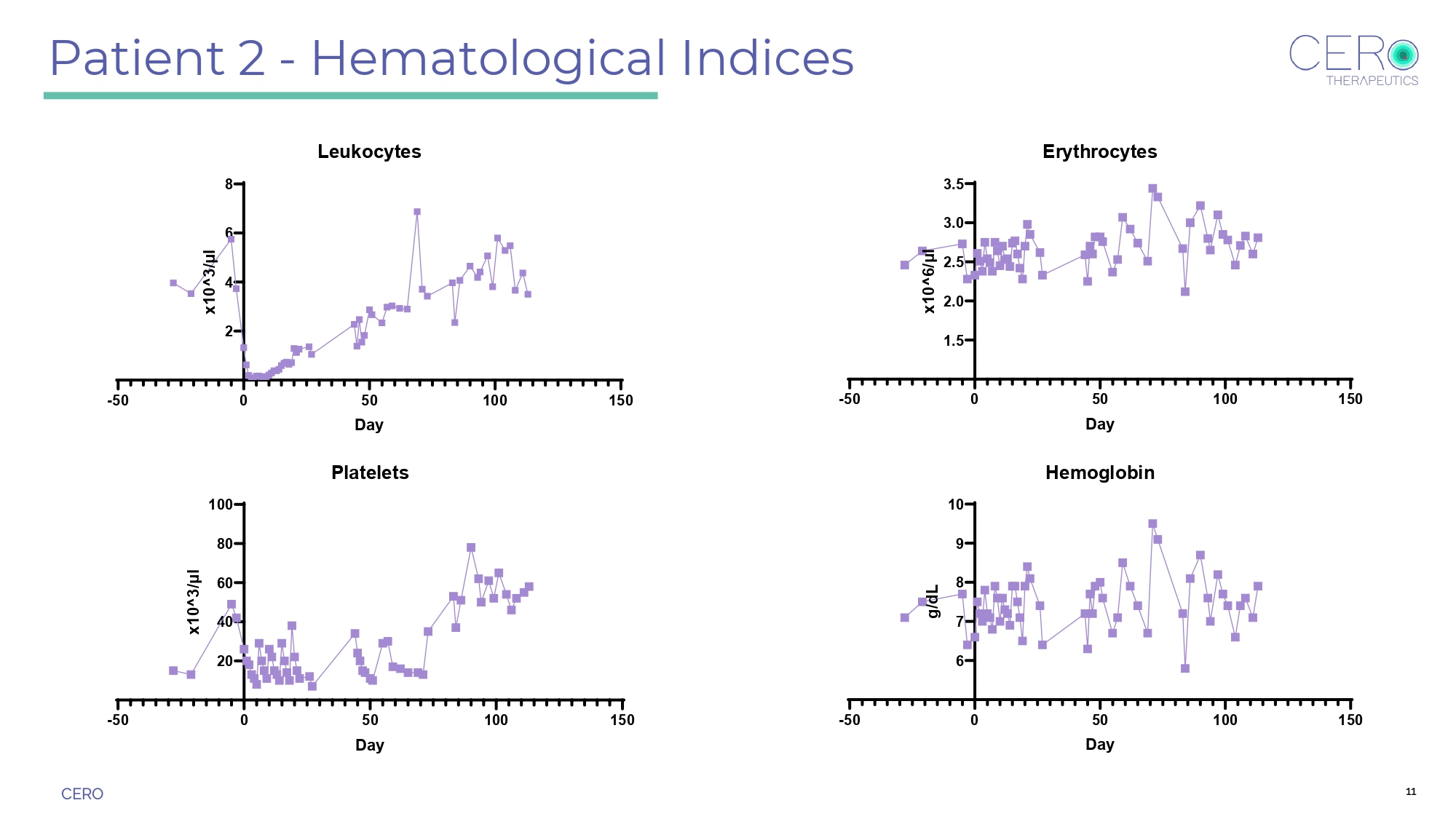
CERO Patient 2 - Hematological Indices -50 0 50 100 150 20 40 60 80 100 Platelets Day x 1 0 ^ 3 / μ l -50 0 50 100 150 6 7 8 9 10 Hemoglobin Day g / d L 11

CERO 12 Planned Next Steps x Protocol amended in December to include additional indications x Completed source data verification of foundational datasets x Engaged in exploratory discussions with potential partners at ASH □ Evaluating submission of data for scientific publication
CERO Opportunities in Myelodysplastic Syndrome (MDS) • HMAs (azacitidine/decitabine) remain the only approved disease - modifying therapies in higher - risk MDS • The VERONA trial did not demonstrate an overall survival benefit for venetoclax added to HMAs • Observed transfusion independence (e.g., patient #2) represents an early, clinically meaningful signal that supports further evaluation in MDS 13
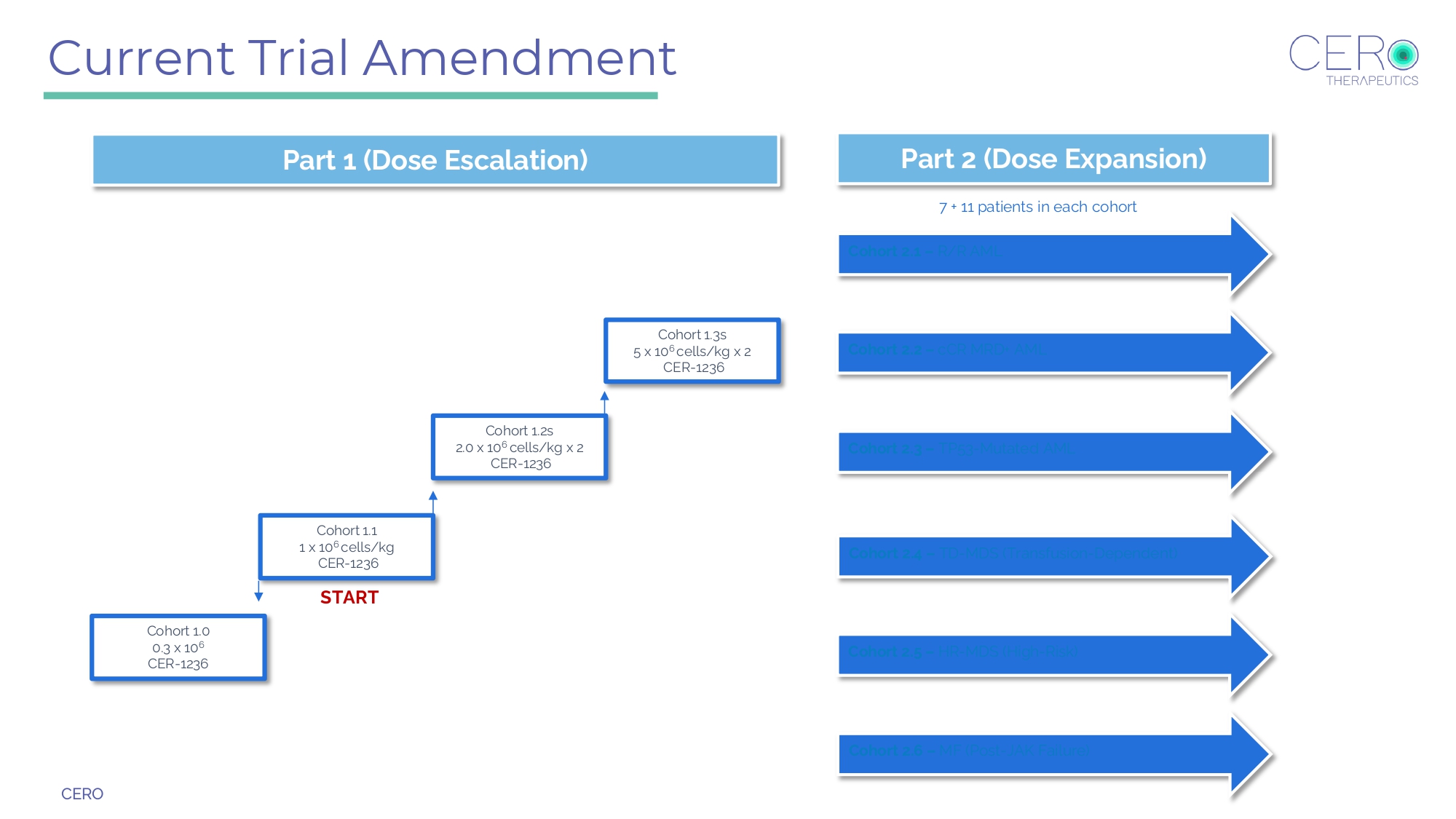
CERO Cohort 1.1 1 x 10 6 cells/kg CER - 1236 Cohort 1.3s 5 x 10 6 cells/kg x 2 CER - 1236 START Cohort 1.2s 2.0 x 10 6 cells/kg x 2 CER - 1236 Cohort 2.1 – R/R AML Cohort 1.0 0.3 x 10 6 CER - 1236 Part 1 (Dose Escalation) Part 2 (Dose Expansion) Cohort 2.2 – cCR MRD+ AML Cohort 2.3 – TP53 - Mutated AML Cohort 2.4 – TD - MDS (Transfusion - Dependent) Cohort 2.5 – HR - MDS (High - Risk) Cohort 2.6 – MF (Post - JAK Failure) Current Trial Amendment
