

January 2026 Redefining Neuroscience Drug Development

Important Disclosures This presentation contains forward-looking statements about Neumora Therapeutics, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws, including statements related to: Neumora’s intention to redefine neuroscience drug development by bringing forward the next generation of novel therapies that offer improved treatment outcomes and quality of life for patients; the timing, progress and plans for its therapeutic development programs, including the timing of clinical trial initiation and data readouts and upcoming milestones and catalysts; expectations and projections regarding future operating results and financial performance, including the sufficiency of its cash resources, intellectual property protection, and expectation of the timing of its cash runway; and other statements identified by words such as “could,” “expects,” “intends,” “may,” “plans,” “potential,” “should,” “will,” “would,” or similar expressions and the negatives of those terms. Other than statements of historical facts, all statements contained in this presentation are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. These statements are subject to risks and uncertainties that could cause the actual results to be materially different from the information expressed or implied by these forward-looking statements, including, among others: the risks related to the inherent uncertainty of clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals; risks related to the timely initiation and enrollment in our clinical trials; risks related to our reliance on third parties, including contract research organizations; risks related to serious or undesirable side effects of our therapeutic candidates; risks related to our ability to utilize and protect our intellectual property rights; and other matters that could affect sufficiency of capital resources to fund operations. For a detailed discussion of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Neumora’s business in general, please refer to the risk factors identified in the Company’s filings with the Securities and Exchange Commission (SEC), including but not limited to its Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 which was filed with the SEC on November 6, 2025. Forward-looking statements speak only as of the date hereof, and, except as required by law, Neumora undertakes no obligation to update or revise these forward-looking statements. Our results for the quarter ended September 30, 2025 are also not necessarily indicative of our operating results for any future periods.

Our Mission We are focused on bringing forward the next generation of novel therapies with brain-penetrant chemistry that offer improved treatment outcomes and quality of life for patients

Led by experienced company builders and leading neuroscience drug developers Leadership Paul L. Berns Co-Founder, Chief Executive Officer & Chairman of Board of Directors Bill Aurora, Pharm.D. Chief Operating & Development Officer Joshua Pinto, Ph.D. President Board of Directors Carol Suh Chief Strategy Officer & Co-Founder Nick Brandon, Ph.D. Chief Scientific Officer Jason Duncan Chief Legal & Administrative Officer Lori Houle Chief Technical Operations & Quality Officer Michael Milligan Chief Financial Officer Amy Sullivan Chief Human Resources Officer Pablo Gersberg Chief Information Officer Paul L. Berns Co-Founder, Chief Executive Officer, Chairman Kristina Burow Managing Director, ARCH Venture Partners Matthew K. Fust Biotechnology Advisor Alaa Halawa Executive Director, Mubadala Capital Maykin Ho, Ph.D. Retired Partner, Goldman Sachs David Piacquad Biotechnology Advisor
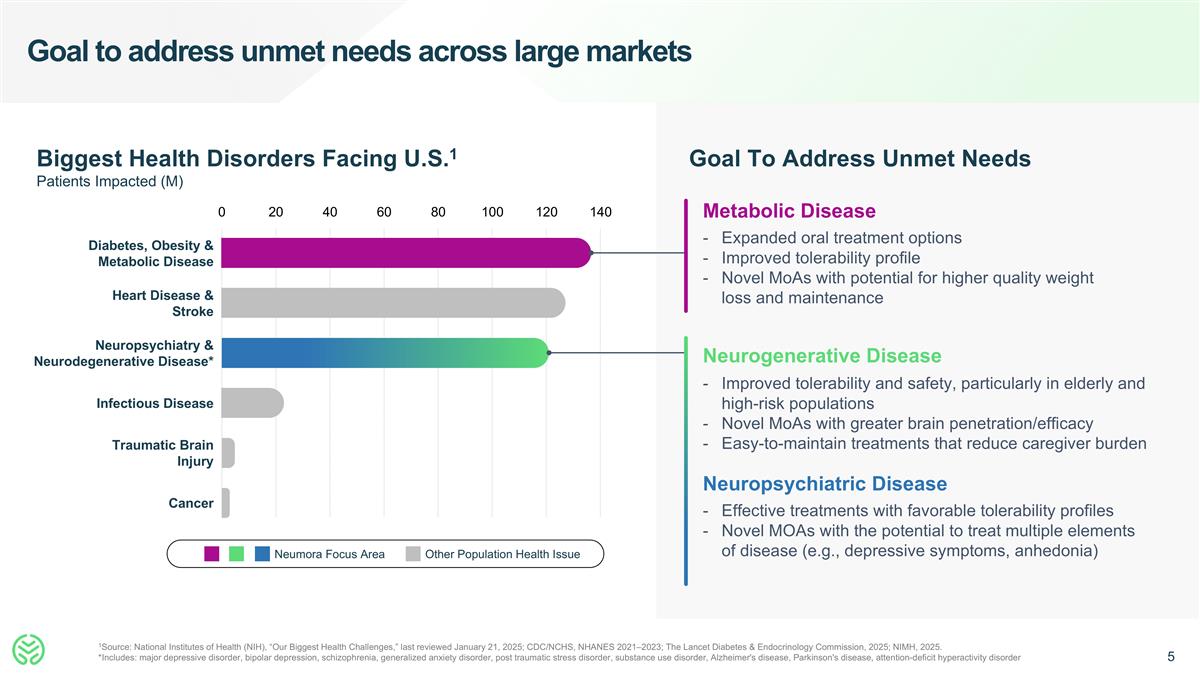
Goal to address unmet needs across large markets 1Source: National Institutes of Health (NIH), “Our Biggest Health Challenges,” last reviewed January 21, 2025; CDC/NCHS, NHANES 2021–2023; The Lancet Diabetes & Endocrinology Commission, 2025; NIMH, 2025. *Includes: major depressive disorder, bipolar depression, schizophrenia, generalized anxiety disorder, post traumatic stress disorder, substance use disorder, Alzheimer's disease, Parkinson's disease, attention-deficit hyperactivity disorder Neumora Focus Area Other Population Health Issue Biggest Health Disorders Facing U.S.1 Patients Impacted (M) Diabetes, Obesity & Metabolic Disease Neuropsychiatry & Neurodegenerative Disease* Infectious Disease Heart Disease & Stroke Traumatic Brain Injury Cancer 0 20 40 60 80 100 120 140 Goal To Address Unmet Needs Metabolic Disease Expanded oral treatment options Improved tolerability profile Novel MoAs with potential for higher quality weight loss and maintenance Neurogenerative Disease Improved tolerability and safety, particularly in elderly and high-risk populations Novel MoAs with greater brain penetration/efficacy Easy-to-maintain treatments that reduce caregiver burden Neuropsychiatric Disease Effective treatments with favorable tolerability profiles Novel MOAs with the potential to treat multiple elements of disease (e.g., depressive symptoms, anhedonia)
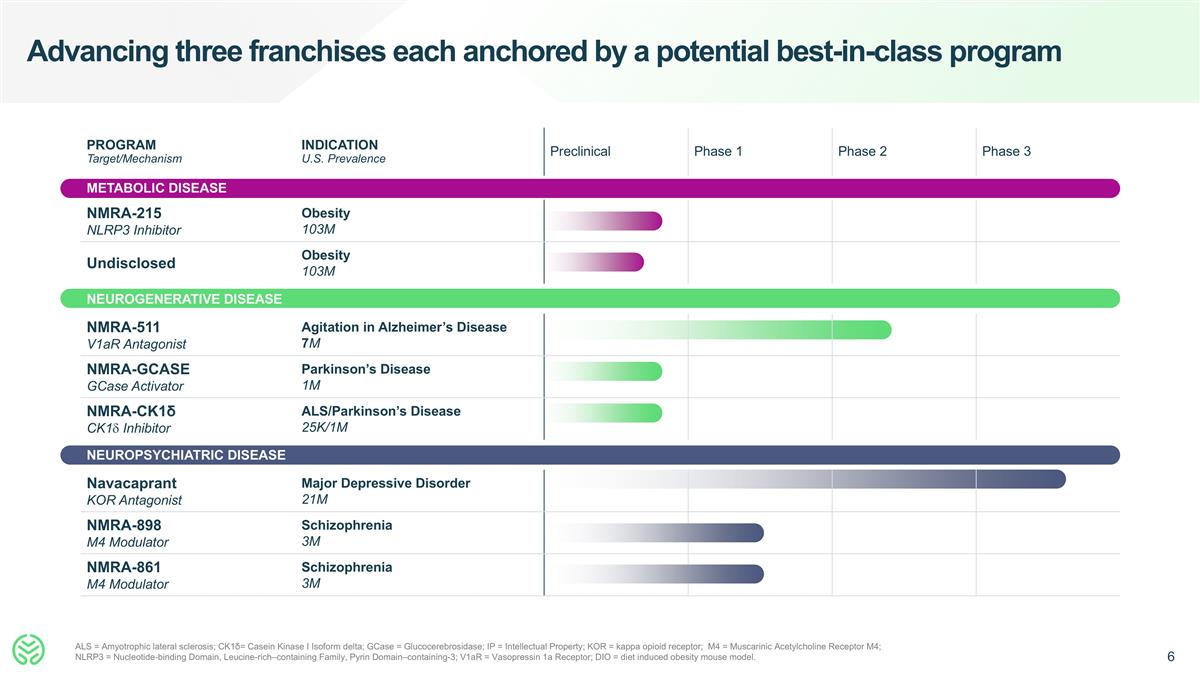
PROGRAM Target/Mechanism INDICATION U.S. Prevalence Preclinical Phase 1 Phase 2 Phase 3 METABOLIC DISEASE NMRA-215 NLRP3 Inhibitor Obesity 103M Undisclosed Obesity 103M NEUROGENERATIVE DISEASE NMRA-511 V1aR Antagonist Agitation in Alzheimer’s Disease 7M NMRA-GCASE GCase Activator Parkinson’s Disease 1M NMRA-CK1δ CK1d Inhibitor ALS/Parkinson’s Disease 25K/1M NEUROPSYCHIATRIC DISEASE Navacaprant KOR Antagonist Major Depressive Disorder 21M NMRA-898 M4 Modulator Schizophrenia 3M NMRA-861 M4 Modulator Schizophrenia 3M Advancing three franchises each anchored by a potential best-in-class program ALS = Amyotrophic lateral sclerosis; CK1δ= Casein Kinase I Isoform delta; GCase = Glucocerebrosidase; IP = Intellectual Property; KOR = kappa opioid receptor; M4 = Muscarinic Acetylcholine Receptor M4; NLRP3 = Nucleotide-binding Domain, Leucine-rich–containing Family, Pyrin Domain–containing-3; V1aR = Vasopressin 1a Receptor; DIO = diet induced obesity mouse model.

ANTICIPATED KEY MILESTONES Metabolic Disease NMRA-215 biomarker data NMRA-215 human weight loss data Neurogenerative Disease NMRA-511 MAD extension data Neuropsychiatric Disease KOASTAL-2 and -3 topline data (joint readout) Provide M4 franchise update Multiple catalysts expected in 2026 across three core franchises Well capitalized with a cash runway to support operations into 3Q 2027 3Q 2026 Year end ‘26 Year end ‘26 2Q 2026 Mid-2026
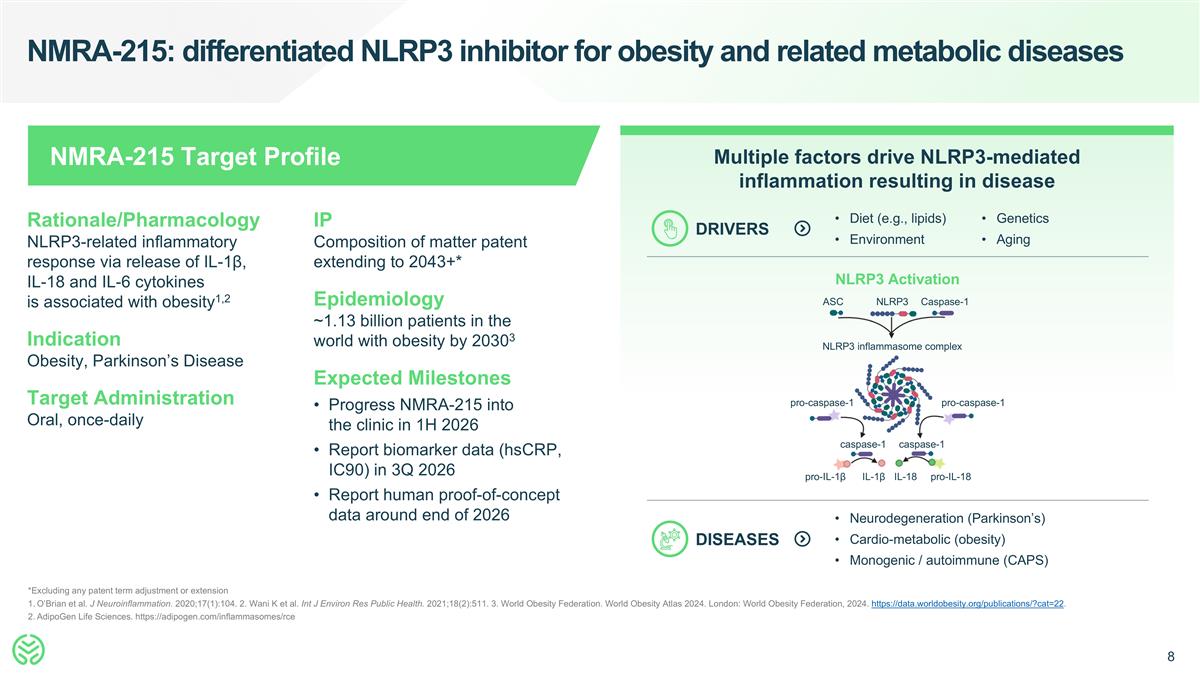
NMRA-215: differentiated NLRP3 inhibitor for obesity and related metabolic diseases *Excluding any patent term adjustment or extension O’Brian et al. J Neuroinflammation. 2020;17(1):104. 2. Wani K et al. Int J Environ Res Public Health. 2021;18(2):511. 3. World Obesity Federation. World Obesity Atlas 2024. London: World Obesity Federation, 2024. https://data.worldobesity.org/publications/?cat=22. AdipoGen Life Sciences. https://adipogen.com/inflammasomes/rce Rationale/Pharmacology NLRP3-related inflammatory response via release of IL-1β, IL-18 and IL-6 cytokines is associated with obesity1,2 Indication Obesity, Parkinson’s Disease Target Administration Oral, once-daily IP Composition of matter patent extending to 2043+* Epidemiology ~1.13 billion patients in the world with obesity by 20303 Expected Milestones Progress NMRA-215 into the clinic in 1H 2026 Report biomarker data (hsCRP, IC90) in 3Q 2026 Report human proof-of-concept data around end of 2026 NMRA-215 Target Profile Multiple factors drive NLRP3-mediated inflammation resulting in disease DRIVERS Diet (e.g., lipids) Environment Genetics Aging NLRP3 Activation DISEASES Neurodegeneration (Parkinson’s) Cardio-metabolic (obesity) Monogenic / autoimmune (CAPS) ASC NLRP3 Caspase-1 NLRP3 inflammasome complex pro-caspase-1 pro-caspase-1 caspase-1 caspase-1 pro-IL-1β IL-1β IL-18 pro-IL-18

Obesity represents one of the greatest public health challenges 1World Obesity Federation. World Obesity Atlas 2025. London: World Obesity Federation, 2025. https://data.worldobesity.org/publications/world-obesity-atlas-2025-v7.pdf 1.13 BILLION people worldwide will be living with obesity1 By 2030, $130 - $170 BILLION estimated obesity market size in 2030 Driving a significant market for obesity treatments Significant opportunity remains And yet, NLRP3 inhibition NLRP3 inhibition may offer benefit across monotherapy, combination therapy and maintenance paradigms: Incretin-like weight loss Increased response rates Better tolerability Convenience with no cold chain storage Lower COGS with oral small molecule Approved incretin therapies offer weight loss, but come with challenges: Significant AEs, such as nausea, vomiting, constipation and diarrhea High discontinuation rates Weight regain following discontinuation Cold chain storage required Emerging oral treatments produce less weight loss and are burdened by the same intolerable side effects May address unmet needs
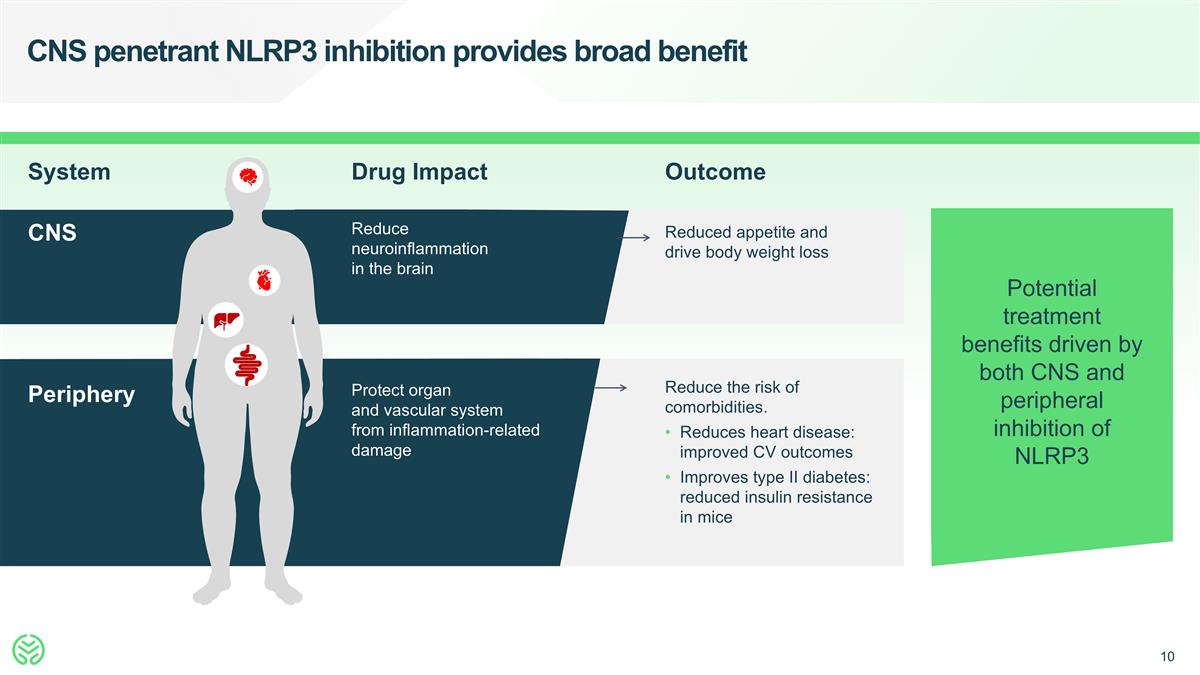
CNS penetrant NLRP3 inhibition provides broad benefit System Drug Impact Outcome Periphery Protect organ and vascular system from inflammation-related damage CNS Reduce neuroinflammation in the brain Reduced appetite and drive body weight loss Reduce the risk of comorbidities. Reduces heart disease: improved CV outcomes Improves type II diabetes: reduced insulin resistance in mice Potential treatment benefits driven by both CNS and peripheral inhibition of NLRP3
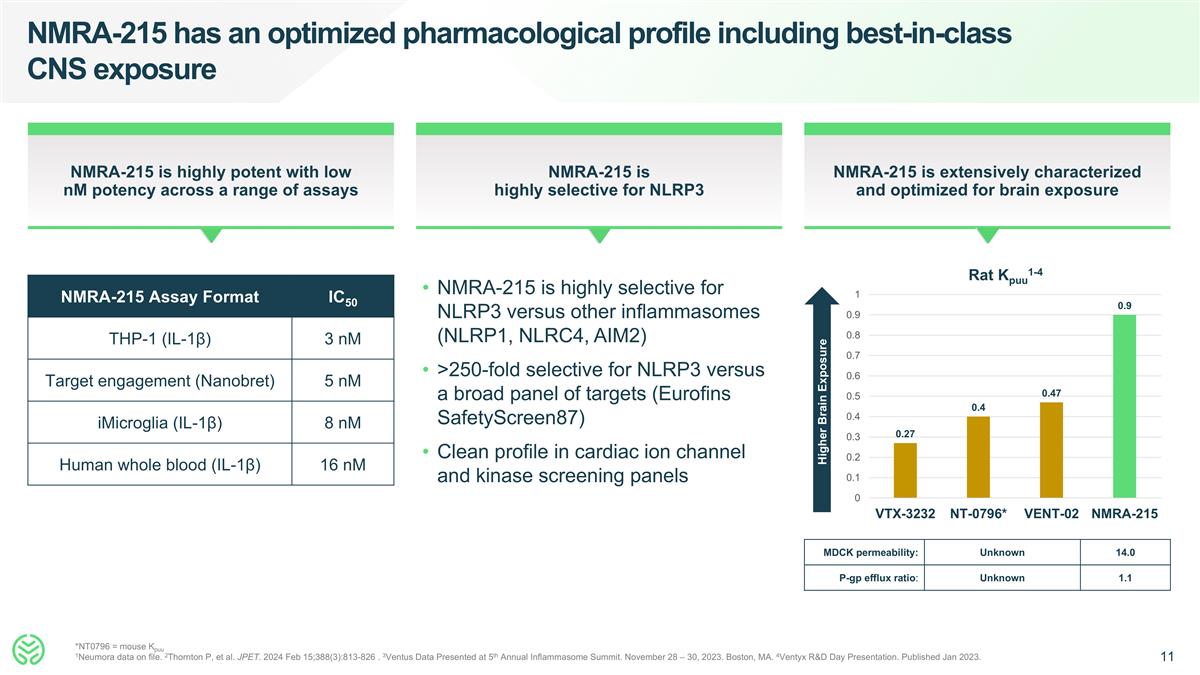
MDCK permeability: Unknown 14.0 P-gp efflux ratio: Unknown 1.1 NMRA-215 has an optimized pharmacological profile including best-in-class CNS exposure *NT0796 = mouse Kpuu 1Neumora data on file. 2Thornton P, et al. JPET. 2024 Feb 15;388(3):813-826 . 3Ventus Data Presented at 5th Annual Inflammasome Summit. November 28 – 30, 2023. Boston, MA. 4Ventyx R&D Day Presentation. Published Jan 2023. NMRA-215 is extensively characterized and optimized for brain exposure NMRA-215 is highly selective for NLRP3 NMRA-215 is highly potent with low nM potency across a range of assays NMRA-215 is highly selective for NLRP3 versus other inflammasomes (NLRP1, NLRC4, AIM2) >250-fold selective for NLRP3 versus a broad panel of targets (Eurofins SafetyScreen87) Clean profile in cardiac ion channel and kinase screening panels Higher Brain Exposure NMRA-215 Assay Format IC50 THP-1 (IL-1β) 3 nM Target engagement (Nanobret) 5 nM iMicroglia (IL-1β) 8 nM Human whole blood (IL-1β) 16 nM
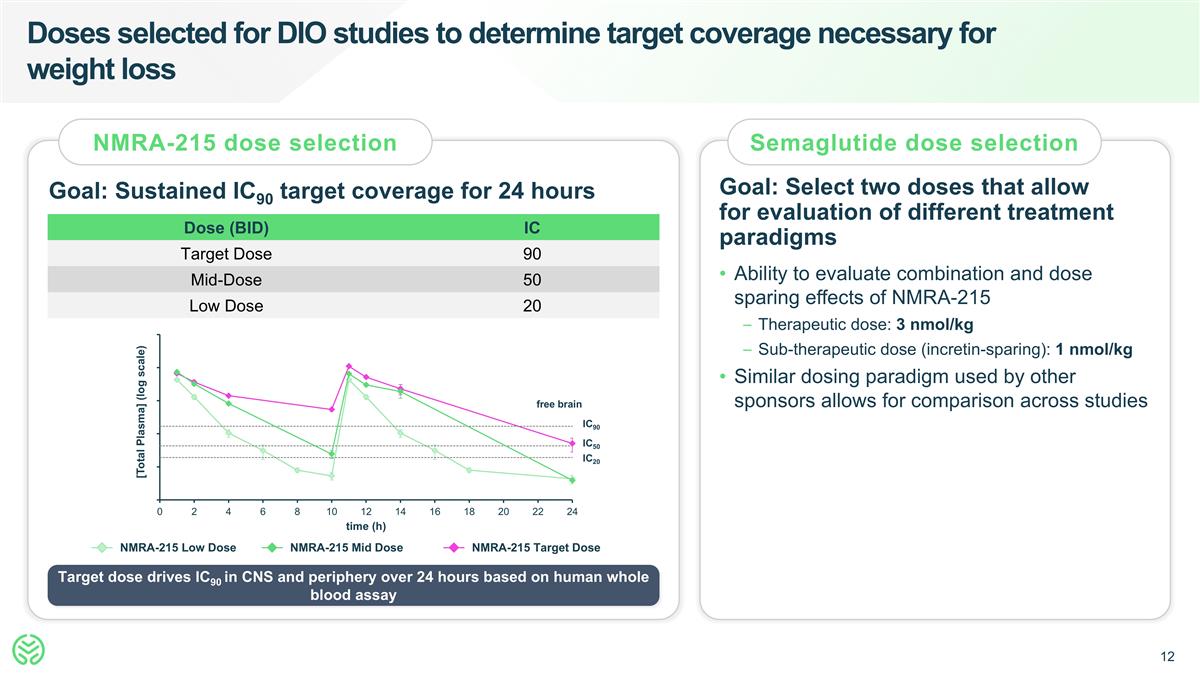
Doses selected for DIO studies to determine target coverage necessary for weight loss NMRA-215 dose selection Semaglutide dose selection Goal: Sustained IC90 target coverage for 24 hours Goal: Select two doses that allow for evaluation of different treatment paradigms Dose (BID) IC Target Dose 90 Mid-Dose 50 Low Dose 20 Ability to evaluate combination and dose sparing effects of NMRA-215 Therapeutic dose: 3 nmol/kg Sub-therapeutic dose (incretin-sparing): 1 nmol/kg Similar dosing paradigm used by other sponsors allows for comparison across studies NMRA-215 Low Dose NMRA-215 Mid Dose NMRA-215 Target Dose free brain IC90 IC50 IC20 [Total Plasma] (log scale) Target dose drives IC90 in CNS and periphery over 24 hours based on human whole blood assay
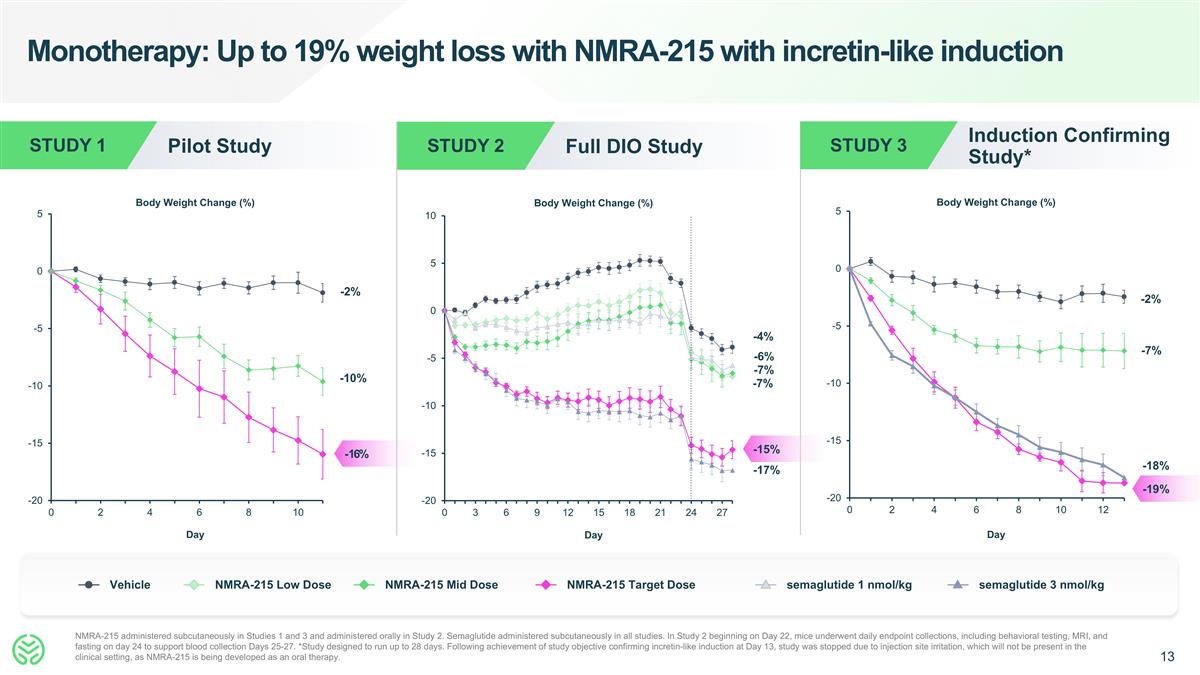
Monotherapy: Up to 19% weight loss with NMRA-215 with incretin-like induction NMRA-215 administered subcutaneously in Studies 1 and 3 and administered orally in Study 2. Semaglutide administered subcutaneously in all studies. In Study 2 beginning on Day 22, mice underwent daily endpoint collections, including behavioral testing, MRI, and fasting on day 24 to support blood collection Days 25-27. *Study designed to run up to 28 days. Following achievement of study objective confirming incretin-like induction at Day 13, study was stopped due to injection site irritation, which will not be present in the clinical setting, as NMRA-215 is being developed as an oral therapy. Day Body Weight Change (%) Pilot Study STUDY 1 -2% -10% -16% Day Body Weight Change (%) Induction Confirming Study* STUDY 3 -18% -2% -7% Day Body Weight Change (%) Full DIO Study STUDY 2 -4% -6% -7% -7% -15% -17% Vehicle NMRA-215 Low Dose NMRA-215 Mid Dose NMRA-215 Target Dose semaglutide 1 nmol/kg semaglutide 3 nmol/kg -19%
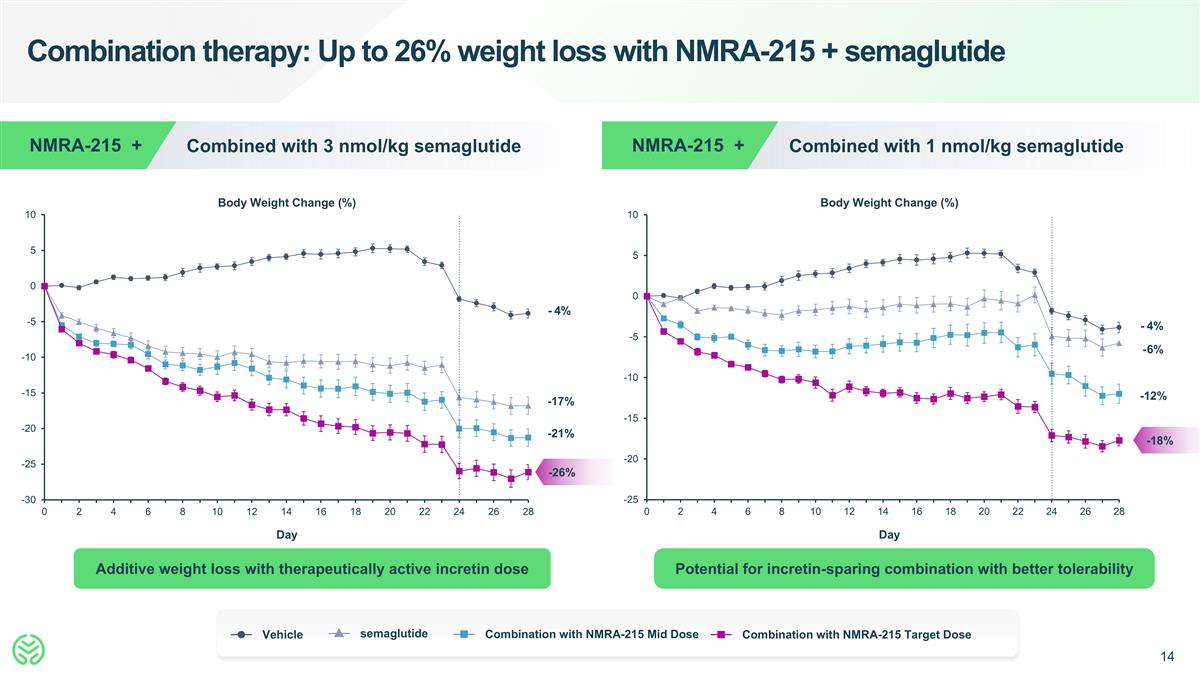
Combination therapy: Up to 26% weight loss with NMRA-215 + semaglutide Day Body Weight Change (%) Combined with 3 nmol/kg semaglutide NMRA-215 + Day Body Weight Change (%) Combined with 1 nmol/kg semaglutide NMRA-215 + -4% -17% -26% -21% -4% -6% -18% -12% Additive weight loss with therapeutically active incretin dose Vehicle Combination with NMRA-215 Mid Dose semaglutide Combination with NMRA-215 Target Dose Potential for incretin-sparing combination with better tolerability
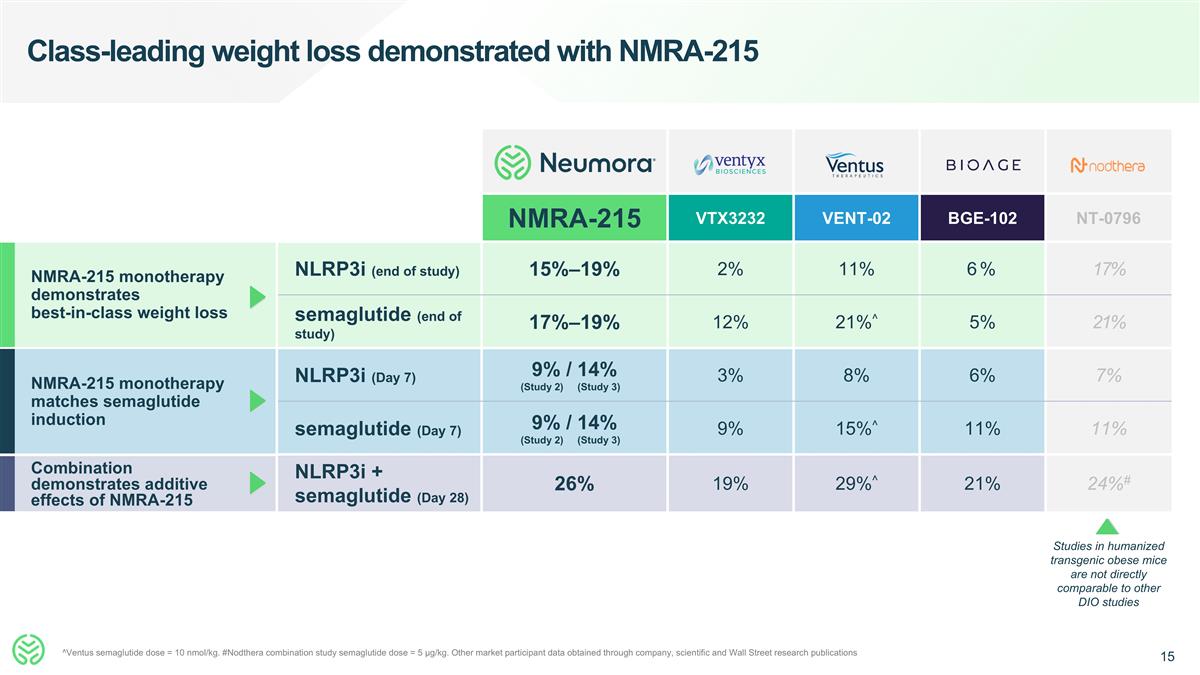
Combination demonstrates additive effects of NMRA-215 Class-leading weight loss demonstrated with NMRA-215 NMRA-215 VTX3232 VENT-02 BGE-102 NT-0796 NLRP3i (end of study) 15%–19% 2% 11% 6% 17% semaglutide (end of study) 17%–19% 12% 21%^ 5% 21% NLRP3i (Day 7) 9% / 14% (Study 2) (Study 3) 3% 8% 6% 7% semaglutide (Day 7) 9% / 14% (Study 2) (Study 3) 9% 15%^ 11% 11% NLRP3i + semaglutide (Day 28) 26% 19% 29%^ 21% 24%# NMRA-215 monotherapy demonstrates best-in-class weight loss NMRA-215 monotherapy matches semaglutide induction Studies in humanized transgenic obese mice are not directly comparable to other DIO studies ^Ventus semaglutide dose = 10 nmol/kg. #Nodthera combination study semaglutide dose = 5 μg/kg. Other market participant data obtained through company, scientific and Wall Street research publications
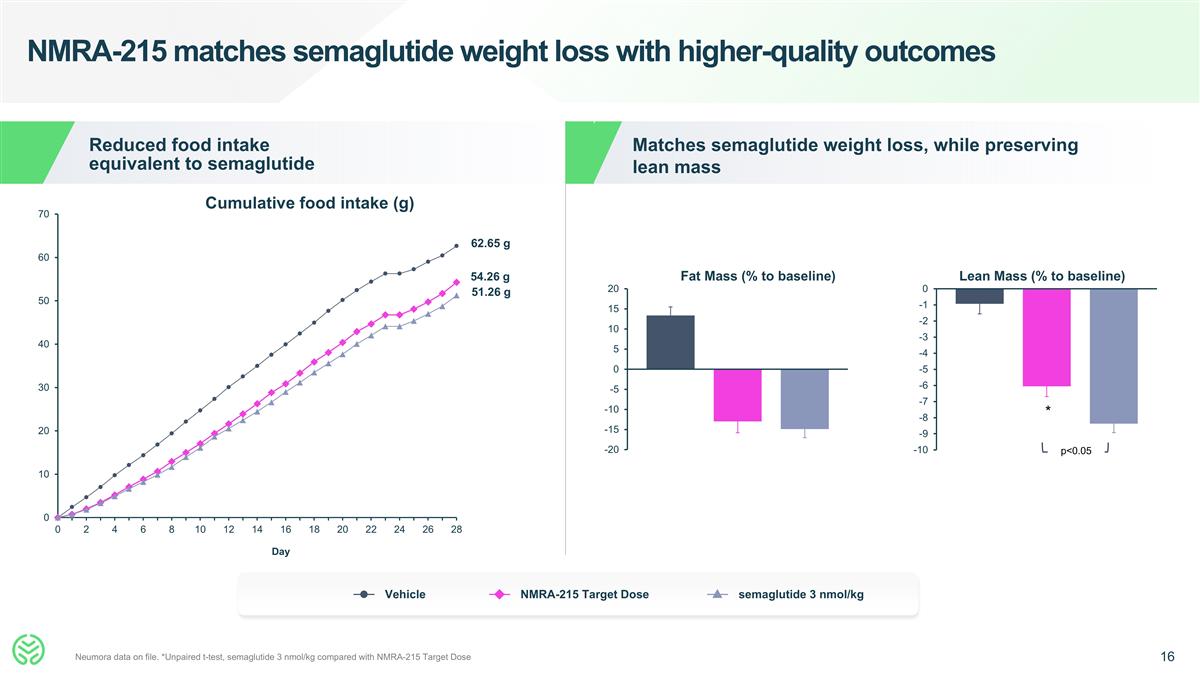
NMRA-215 matches semaglutide weight loss with higher-quality outcomes Neumora data on file. *Unpaired t-test, semaglutide 3 nmol/kg compared with NMRA-215 Target Dose Day Cumulative food intake (g) Reduced food intake equivalent to semaglutide Fat Mass (% to baseline) Vehicle NMRA-215 Target Dose semaglutide 3 nmol/kg Matches semaglutide weight loss, while preserving lean mass Lean Mass (% to baseline) 62.65 g 54.26 g 51.26 g * p<0.05
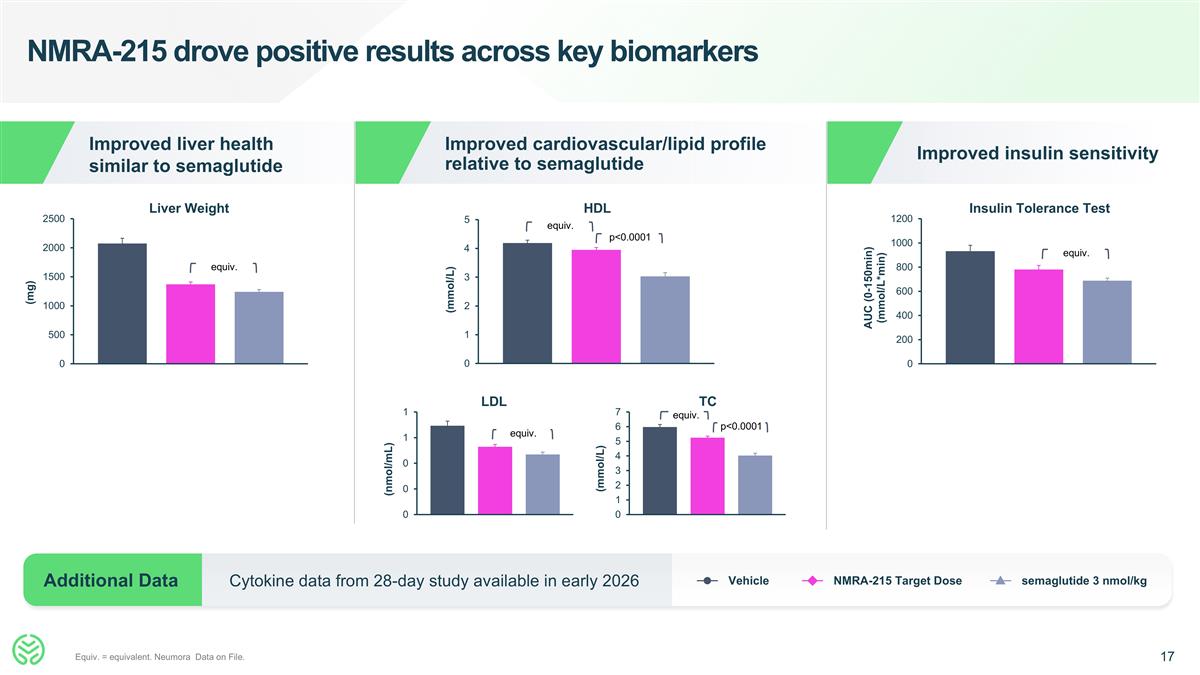
NMRA-215 drove positive results across key biomarkers Equiv. = equivalent. Neumora Data on File. Improved liver health similar to semaglutide Improved cardiovascular/lipid profile relative to semaglutide Vehicle NMRA-215 Target Dose semaglutide 3 nmol/kg Cytokine data from 28-day study available in early 2026 Additional Data Improved insulin sensitivity LDL TC Liver Weight HDL Insulin Tolerance Test equiv. p<0.0001 equiv. equiv. equiv. equiv. p<0.0001
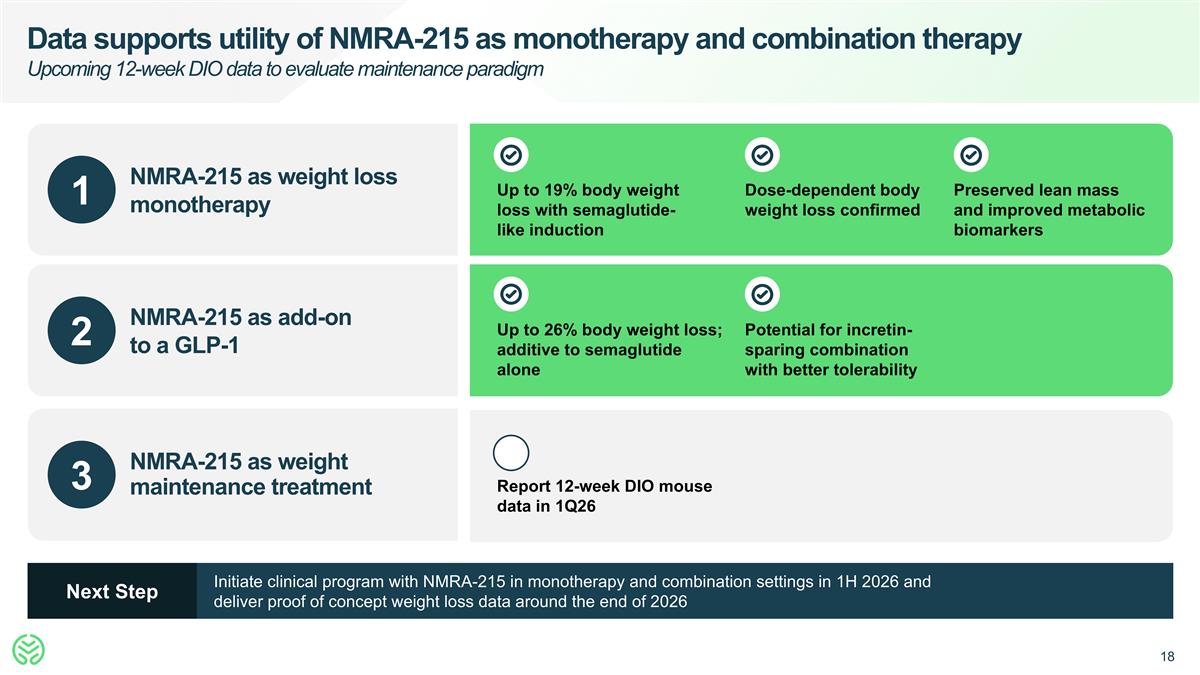
Data supports utility of NMRA-215 as monotherapy and combination therapy Upcoming 12-week DIO data to evaluate maintenance paradigm 1 2 3 NMRA-215 as weight loss monotherapy NMRA-215 as add-on to a GLP-1 NMRA-215 as weight maintenance treatment Up to 19% body weight loss with semaglutide-like induction Dose-dependent body weight loss confirmed Preserved lean mass and improved metabolic biomarkers Up to 26% body weight loss; additive to semaglutide alone Potential for incretin-sparing combination with better tolerability Report 12-week DIO mouse data in 1Q26 Initiate clinical program with NMRA-215 in monotherapy and combination settings in 1H 2026 and deliver proof of concept weight loss data around the end of 2026 Next Step
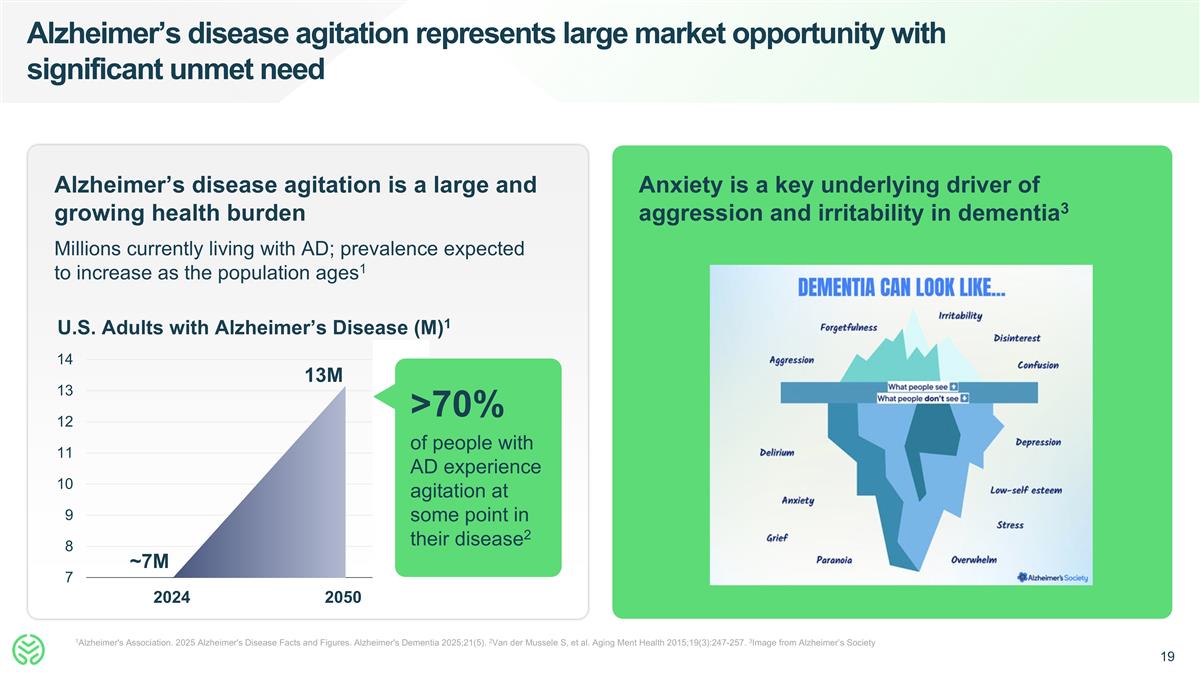
Alzheimer’s disease agitation represents large market opportunity with significant unmet need 1Alzheimer's Association. 2025 Alzheimer's Disease Facts and Figures. Alzheimer's Dementia 2025;21(5). 2Van der Mussele S, et al. Aging Ment Health 2015;19(3):247-257. 3Image from Alzheimer’s Society Alzheimer’s disease agitation is a large and growing health burden Millions currently living with AD; prevalence expected to increase as the population ages1 ~7M 13M U.S. Adults with Alzheimer’s Disease (M)1 >70% of people with AD experience agitation at some point in their disease2 Anxiety is a key underlying driver of aggression and irritability in dementia3
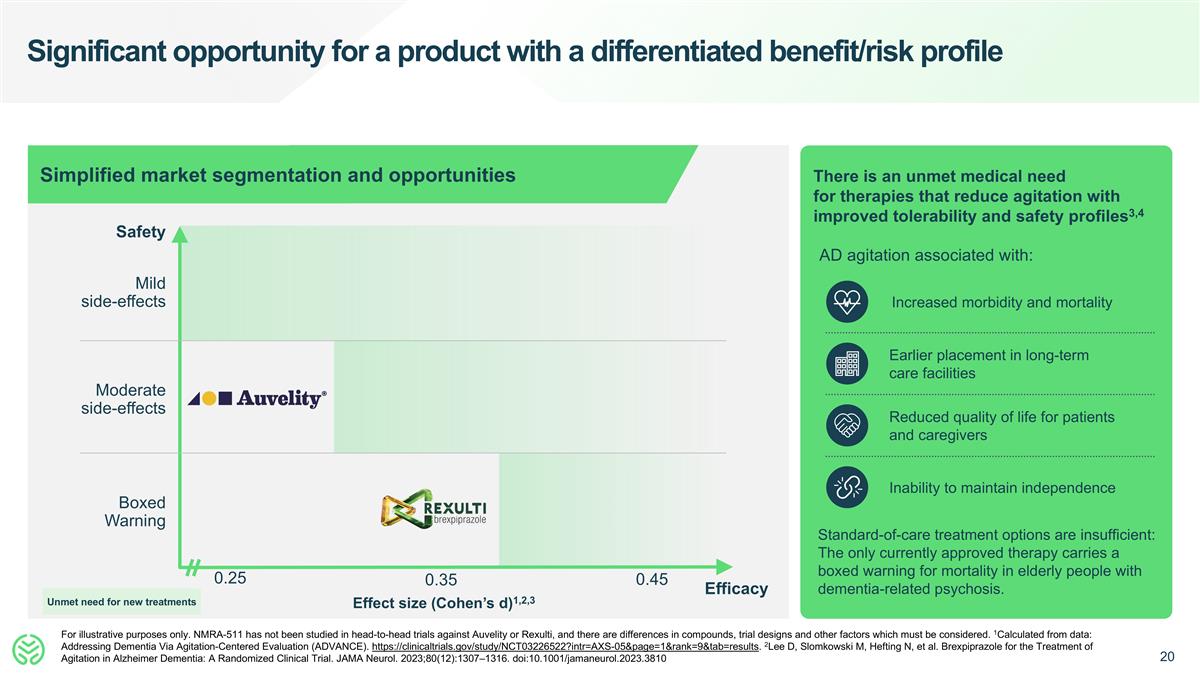
Significant opportunity for a product with a differentiated benefit/risk profile For illustrative purposes only. NMRA-511 has not been studied in head-to-head trials against Auvelity or Rexulti, and there are differences in compounds, trial designs and other factors which must be considered. 1Calculated from data: Addressing Dementia Via Agitation-Centered Evaluation (ADVANCE). https://clinicaltrials.gov/study/NCT03226522?intr=AXS-05&page=1&rank=9&tab=results. 2Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023;80(12):1307–1316. doi:10.1001/jamaneurol.2023.3810 0.25 Safety Effect size (Cohen’s d)1,2,3 Boxed Warning Moderate side-effects Mild side-effects Simplified market segmentation and opportunities Increased morbidity and mortality Earlier placement in long-term care facilities Reduced quality of life for patients and caregivers Inability to maintain independence There is an unmet medical need for therapies that reduce agitation with improved tolerability and safety profiles3,4 AD agitation associated with: Standard-of-care treatment options are insufficient: The only currently approved therapy carries a boxed warning for mortality in elderly people with dementia-related psychosis. 0.35 0.45 Efficacy Unmet need for new treatments
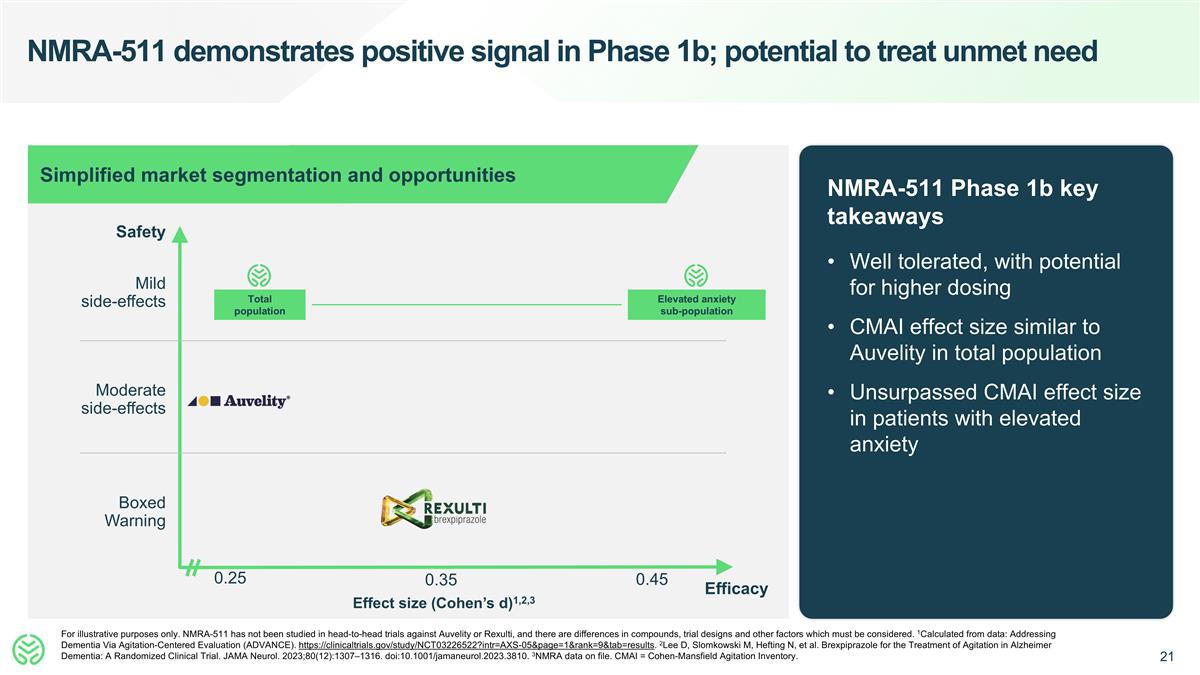
NMRA-511 demonstrates positive signal in Phase 1b; potential to treat unmet need NMRA-511 Phase 1b key takeaways Well tolerated, with potential for higher dosing CMAI effect size similar to Auvelity in total population Unsurpassed CMAI effect size in patients with elevated anxiety For illustrative purposes only. NMRA-511 has not been studied in head-to-head trials against Auvelity or Rexulti, and there are differences in compounds, trial designs and other factors which must be considered. 1Calculated from data: Addressing Dementia Via Agitation-Centered Evaluation (ADVANCE). https://clinicaltrials.gov/study/NCT03226522?intr=AXS-05&page=1&rank=9&tab=results. 2Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023;80(12):1307–1316. doi:10.1001/jamaneurol.2023.3810. 3NMRA data on file. CMAI = Cohen-Mansfield Agitation Inventory. 0.25 Safety Effect size (Cohen’s d)1,2,3 Boxed Warning Moderate side-effects Mild side-effects Simplified market segmentation and opportunities 0.35 0.45 Efficacy Total population Elevated anxiety sub-population
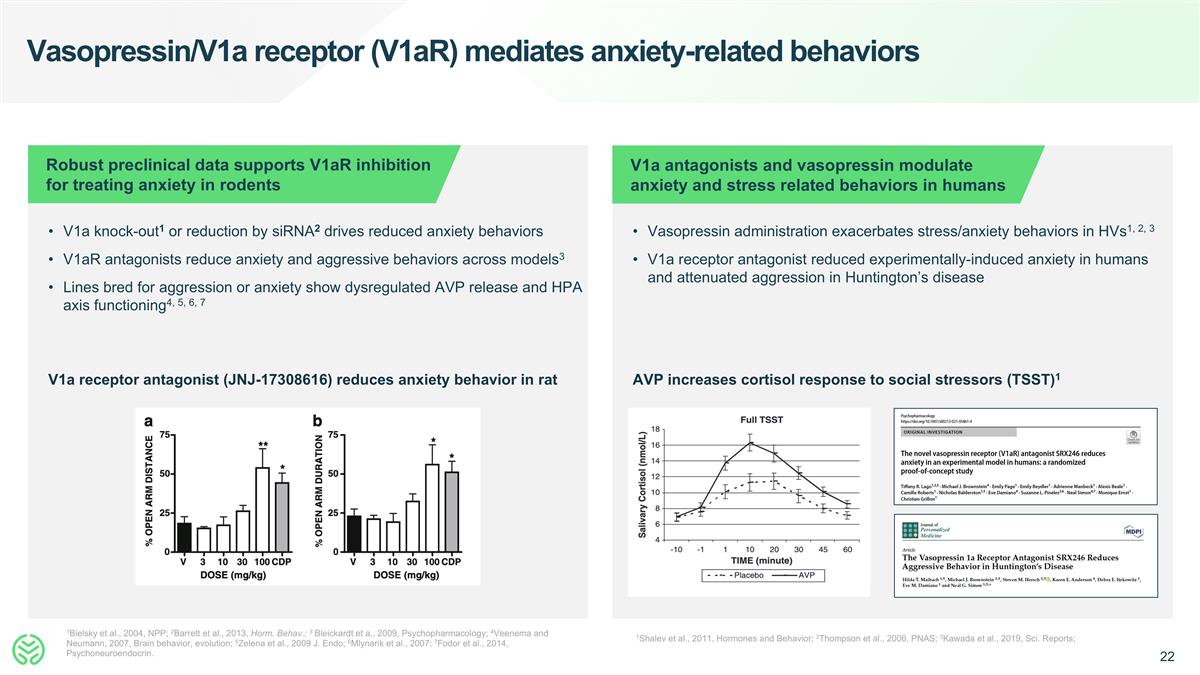
Vasopressin/V1a receptor (V1aR) mediates anxiety-related behaviors 1Bielsky et al., 2004, NPP; 2Barrett et al., 2013, Horm. Behav.; 3 Bleickardt et a., 2009, Psychopharmacology; 4Veenema and Neumann, 2007, Brain behavior, evolution; 5Zelena et al., 2009 J. Endo; 6Mlynarik et al., 2007; 7Fodor et al., 2014, Psychoneuroendocrin. 1Shalev et al., 2011, Hormones and Behavior; 2Thompson et al., 2006, PNAS; 3Kawada et al., 2019, Sci. Reports; Robust preclinical data supports V1aR inhibition for treating anxiety in rodents V1a antagonists and vasopressin modulate anxiety and stress related behaviors in humans V1a knock-out1 or reduction by siRNA2 drives reduced anxiety behaviors V1aR antagonists reduce anxiety and aggressive behaviors across models3 Lines bred for aggression or anxiety show dysregulated AVP release and HPA axis functioning4, 5, 6, 7 Vasopressin administration exacerbates stress/anxiety behaviors in HVs1, 2, 3 V1a receptor antagonist reduced experimentally-induced anxiety in humans and attenuated aggression in Huntington’s disease V1a receptor antagonist (JNJ-17308616) reduces anxiety behavior in rat AVP increases cortisol response to social stressors (TSST)1
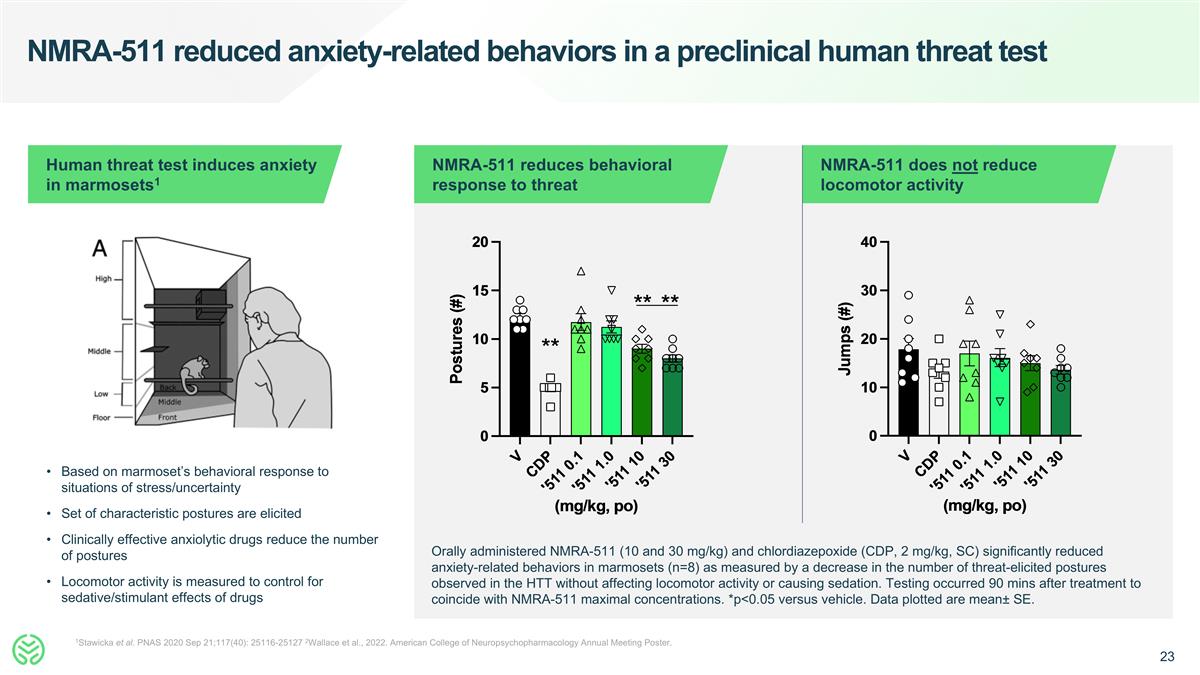
NMRA-511 reduced anxiety-related behaviors in a preclinical human threat test 1Stawicka et al. PNAS 2020 Sep 21;117(40): 25116-25127 2Wallace et al., 2022. American College of Neuropsychopharmacology Annual Meeting Poster. Based on marmoset’s behavioral response to situations of stress/uncertainty Set of characteristic postures are elicited Clinically effective anxiolytic drugs reduce the number of postures Locomotor activity is measured to control for sedative/stimulant effects of drugs Human threat test induces anxiety in marmosets1 NMRA-511 reduces behavioral response to threat NMRA-511 does not reduce locomotor activity Orally administered NMRA-511 (10 and 30 mg/kg) and chlordiazepoxide (CDP, 2 mg/kg, SC) significantly reduced anxiety-related behaviors in marmosets (n=8) as measured by a decrease in the number of threat-elicited postures observed in the HTT without affecting locomotor activity or causing sedation. Testing occurred 90 mins after treatment to coincide with NMRA-511 maximal concentrations. *p<0.05 versus vehicle. Data plotted are mean± SE.
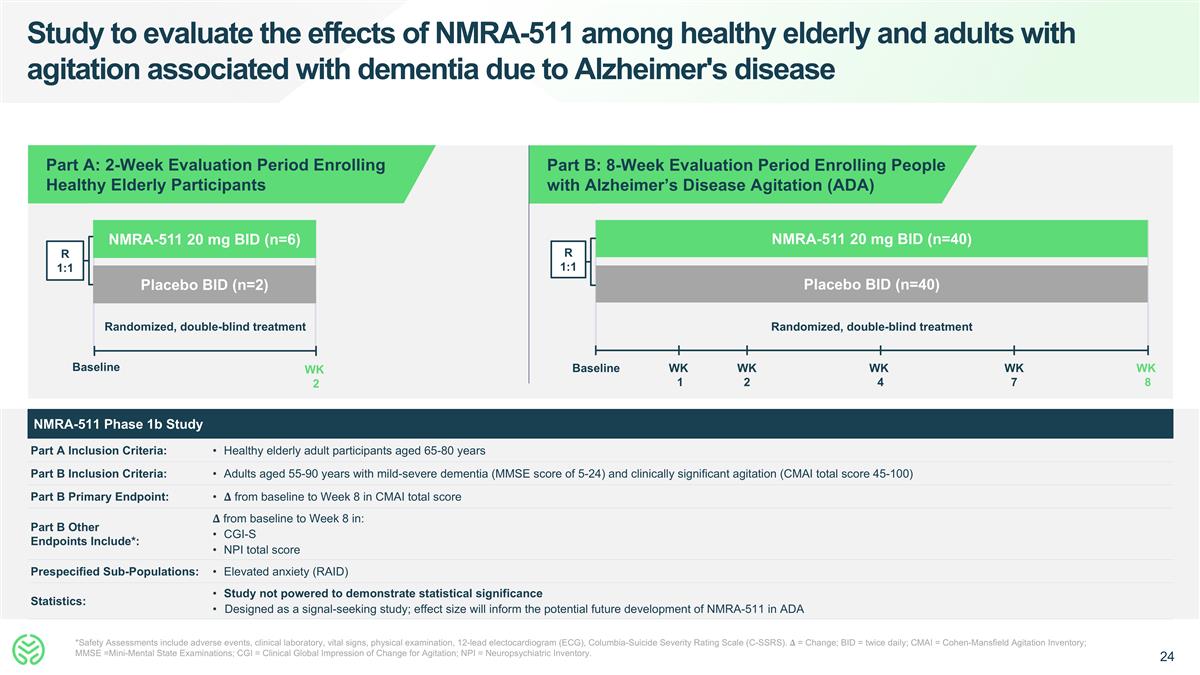
Study to evaluate the effects of NMRA-511 among healthy elderly and adults with agitation associated with dementia due to Alzheimer's disease *Safety Assessments include adverse events, clinical laboratory, vital signs, physical examination, 12-lead electocardiogram (ECG), Columbia-Suicide Severity Rating Scale (C-SSRS). �� = Change; BID = twice daily; CMAI = Cohen-Mansfield Agitation Inventory; MMSE =Mini-Mental State Examinations; CGI = Clinical Global Impression of Change for Agitation; NPI = Neuropsychiatric Inventory. R 1:1 Randomized, double-blind treatment NMRA-511 20 mg BID (n=6) Placebo BID (n=2) Baseline WK 2 Randomized, double-blind treatment NMRA-511 20 mg BID (n=40) Placebo BID (n=40) R 1:1 Baseline WK 8 WK 4 WK 2 WK 7 WK 1 Part B: 8-Week Evaluation Period Enrolling People with Alzheimer’s Disease Agitation (ADA) Part A: 2-Week Evaluation Period Enrolling Healthy Elderly Participants NMRA-511 Phase 1b Study Part A Inclusion Criteria: Healthy elderly adult participants aged 65-80 years Part B Inclusion Criteria: Adults aged 55-90 years with mild-severe dementia (MMSE score of 5-24) and clinically significant agitation (CMAI total score 45-100) Part B Primary Endpoint: �� from baseline to Week 8 in CMAI total score Part B Other Endpoints Include*: �� from baseline to Week 8 in: CGI-S NPI total score Prespecified Sub-Populations: Elevated anxiety (RAID) Statistics: Study not powered to demonstrate statistical significance Designed as a signal-seeking study; effect size will inform the potential future development of NMRA-511 in ADA
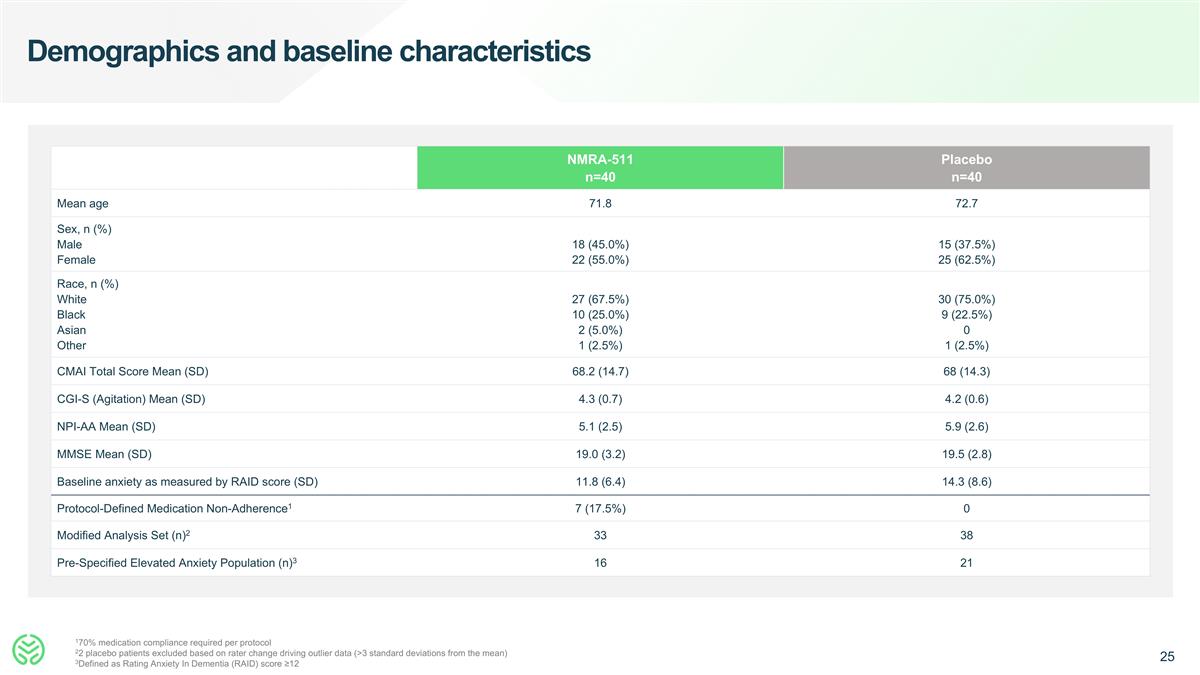
Demographics and baseline characteristics 170% medication compliance required per protocol 22 placebo patients excluded based on rater change driving outlier data (>3 standard deviations from the mean) 3Defined as Rating Anxiety In Dementia (RAID) score ≥12 NMRA-511 n=40 Placebo n=40 Mean age 71.8 72.7 Sex, n (%) Male Female 18 (45.0%) 22 (55.0%) 15 (37.5%) 25 (62.5%) Race, n (%) White Black Asian Other 27 (67.5%) 10 (25.0%) 2 (5.0%) 1 (2.5%) 30 (75.0%) 9 (22.5%) 0 1 (2.5%) CMAI Total Score Mean (SD) 68.2 (14.7) 68 (14.3) CGI-S (Agitation) Mean (SD) 4.3 (0.7) 4.2 (0.6) NPI-AA Mean (SD) 5.1 (2.5) 5.9 (2.6) MMSE Mean (SD) 19.0 (3.2) 19.5 (2.8) Baseline anxiety as measured by RAID score (SD) 11.8 (6.4) 14.3 (8.6) Protocol-Defined Medication Non-Adherence1 7 (17.5%) 0 Modified Analysis Set (n)2 33 38 Pre-Specified Elevated Anxiety Population (n)3 16 21
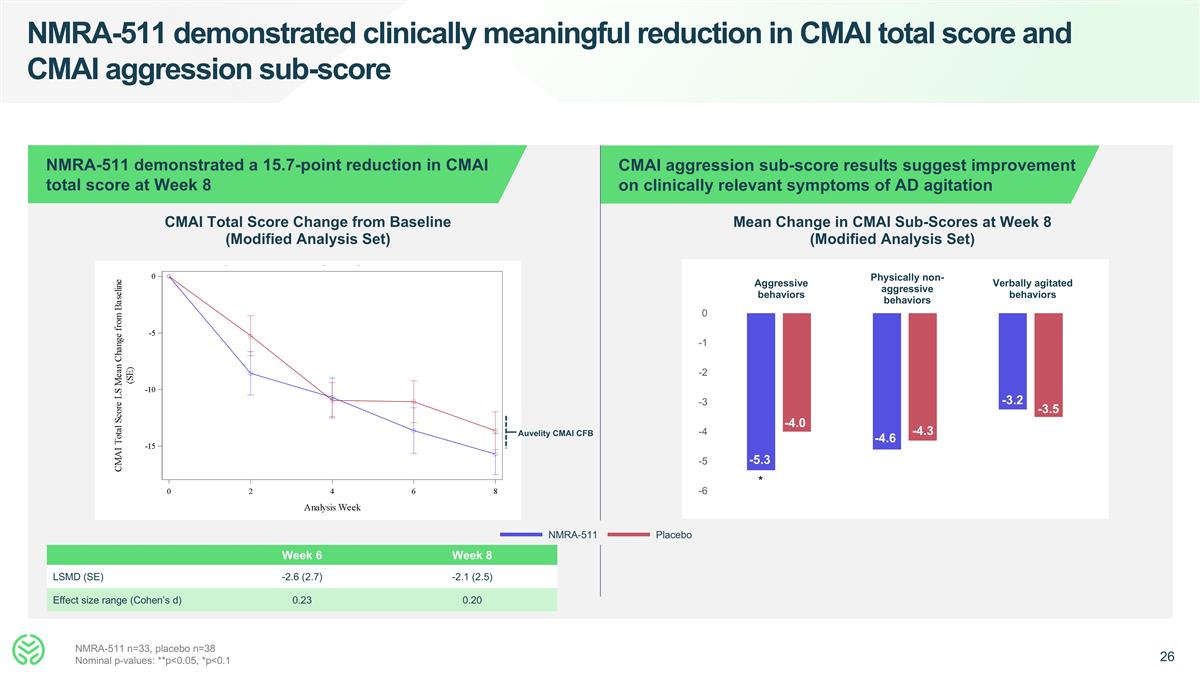
NMRA-511 demonstrated clinically meaningful reduction in CMAI total score and CMAI aggression sub-score NMRA-511 demonstrated a 15.7-point reduction in CMAI total score at Week 8 CMAI aggression sub-score results suggest improvement on clinically relevant symptoms of AD agitation CMAI Total Score Change from Baseline (Modified Analysis Set) Mean Change in CMAI Sub-Scores at Week 8 (Modified Analysis Set) Week 6 Week 8 LSMD (SE) -2.6 (2.7) -2.1 (2.5) Effect size range (Cohen’s d) 0.23 0.20 NMRA-511 Placebo Aggressive behaviors Physically non-aggressive behaviors Verbally agitated behaviors -5.3 -4.0 NMRA-511 n=33, placebo n=38 Nominal p-values: **p<0.05, *p<0.1 -4.6 -4.3 * -3.2 -3.5 Auvelity CMAI CFB
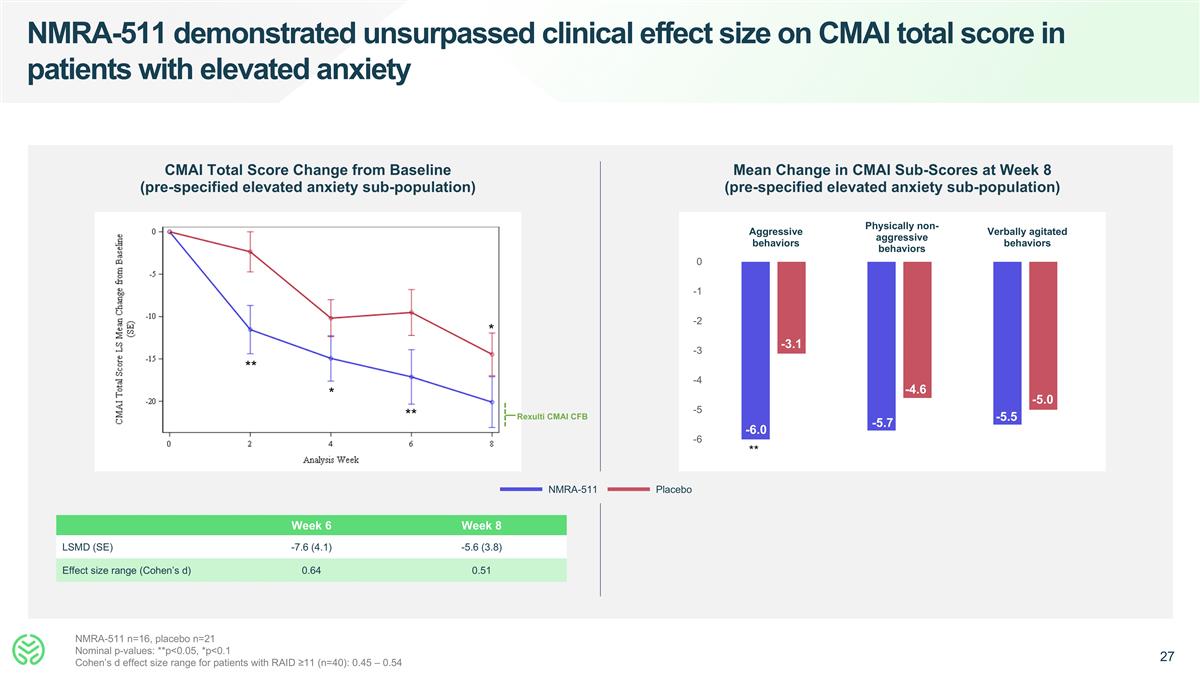
NMRA-511 demonstrated unsurpassed clinical effect size on CMAI total score in patients with elevated anxiety CMAI Total Score Change from Baseline (pre-specified elevated anxiety sub-population) Mean Change in CMAI Sub-Scores at Week 8 (pre-specified elevated anxiety sub-population) NMRA-511 Placebo Aggressive behaviors Physically non-aggressive behaviors Verbally agitated behaviors -6.0 -3.1 NMRA-511 n=16, placebo n=21 Nominal p-values: **p<0.05, *p<0.1 Cohen’s d effect size range for patients with RAID ≥11 (n=40): 0.45 – 0.54 Week 6 Week 8 LSMD (SE) -7.6 (4.1) -5.6 (3.8) Effect size range (Cohen’s d) 0.64 0.51 -5.7 -4.6 ** -5.5 -5.0 Rexulti CMAI CFB ** * ** *
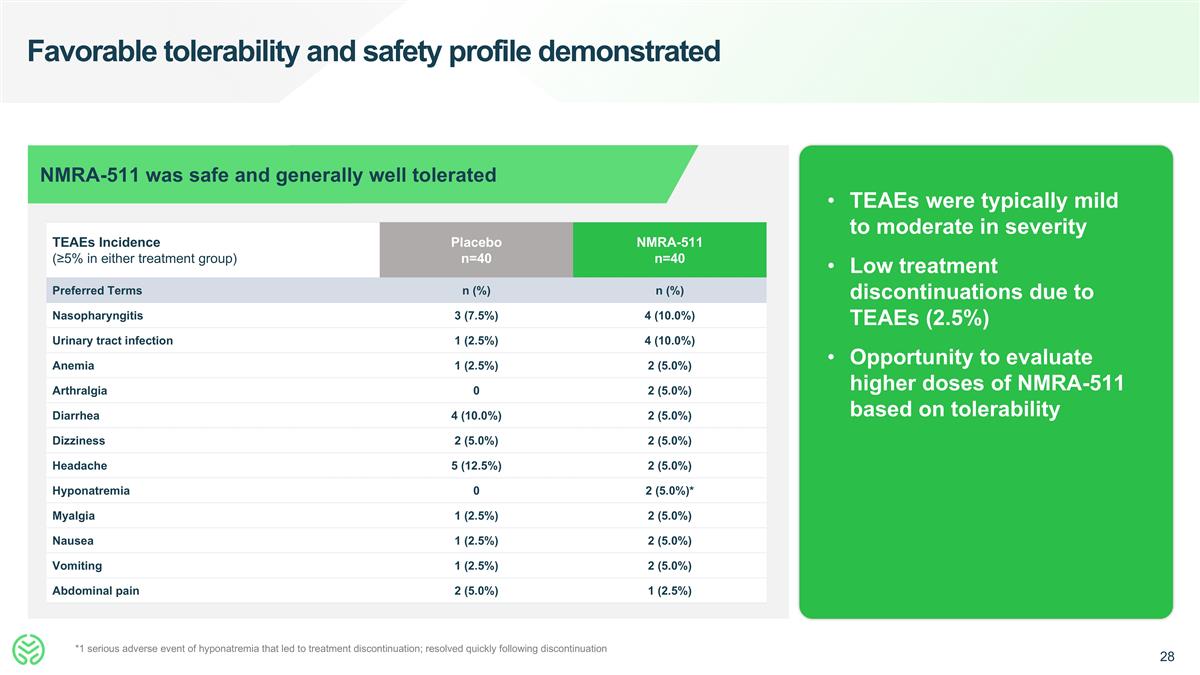
Favorable tolerability and safety profile demonstrated TEAEs were typically mild to moderate in severity Low treatment discontinuations due to TEAEs (2.5%) Opportunity to evaluate higher doses of NMRA-511 based on tolerability TEAEs Incidence (≥5% in either treatment group) Placebo n=40 NMRA-511 n=40 Preferred Terms n (%) n (%) Nasopharyngitis 3 (7.5%) 4 (10.0%) Urinary tract infection 1 (2.5%) 4 (10.0%) Anemia 1 (2.5%) 2 (5.0%) Arthralgia 0 2 (5.0%) Diarrhea 4 (10.0%) 2 (5.0%) Dizziness 2 (5.0%) 2 (5.0%) Headache 5 (12.5%) 2 (5.0%) Hyponatremia 0 2 (5.0%)* Myalgia 1 (2.5%) 2 (5.0%) Nausea 1 (2.5%) 2 (5.0%) Vomiting 1 (2.5%) 2 (5.0%) Abdominal pain 2 (5.0%) 1 (2.5%) NMRA-511 was safe and generally well tolerated *1 serious adverse event of hyponatremia that led to treatment discontinuation; resolved quickly following discontinuation
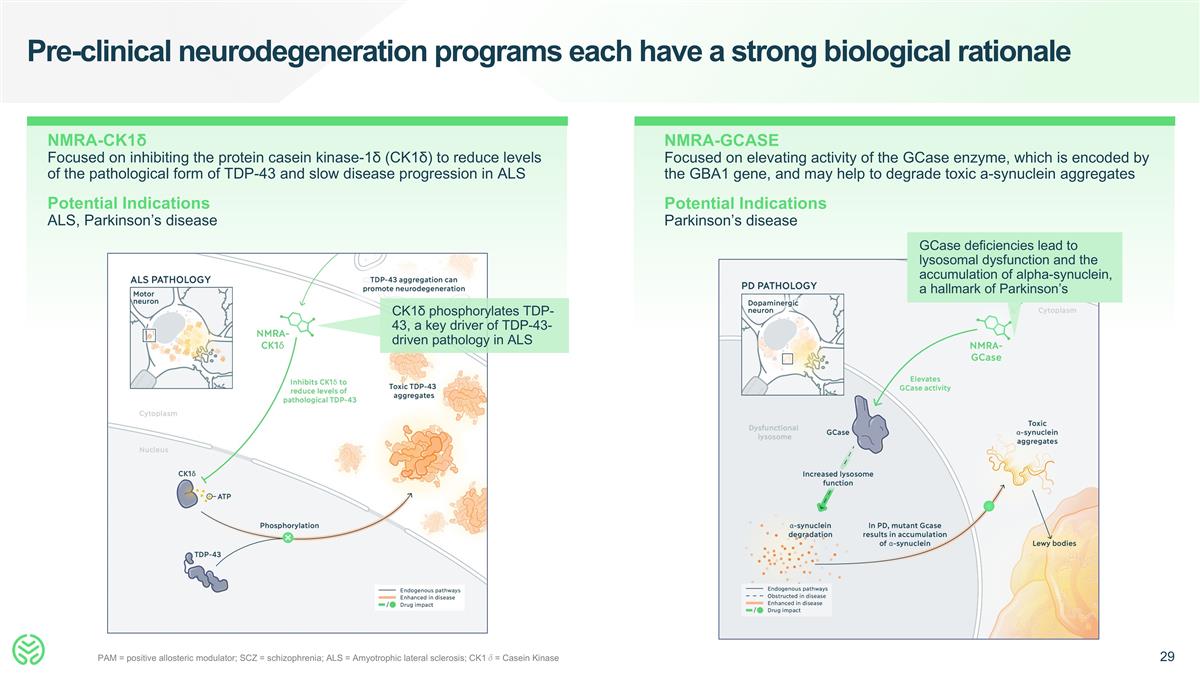
Pre-clinical neurodegeneration programs each have a strong biological rationale NMRA-GCASE Focused on elevating activity of the GCase enzyme, which is encoded by the GBA1 gene, and may help to degrade toxic a-synuclein aggregates Potential Indications Parkinson’s disease NMRA-CK1δ Focused on inhibiting the protein casein kinase-1δ (CK1δ) to reduce levels of the pathological form of TDP-43 and slow disease progression in ALS Potential Indications ALS, Parkinson’s disease PAM = positive allosteric modulator; SCZ = schizophrenia; ALS = Amyotrophic lateral sclerosis; CK1δ= Casein Kinase CK1δ phosphorylates TDP-43, a key driver of TDP-43-driven pathology in ALS GCase deficiencies lead to lysosomal dysfunction and the accumulation of alpha-synuclein, a hallmark of Parkinson’s
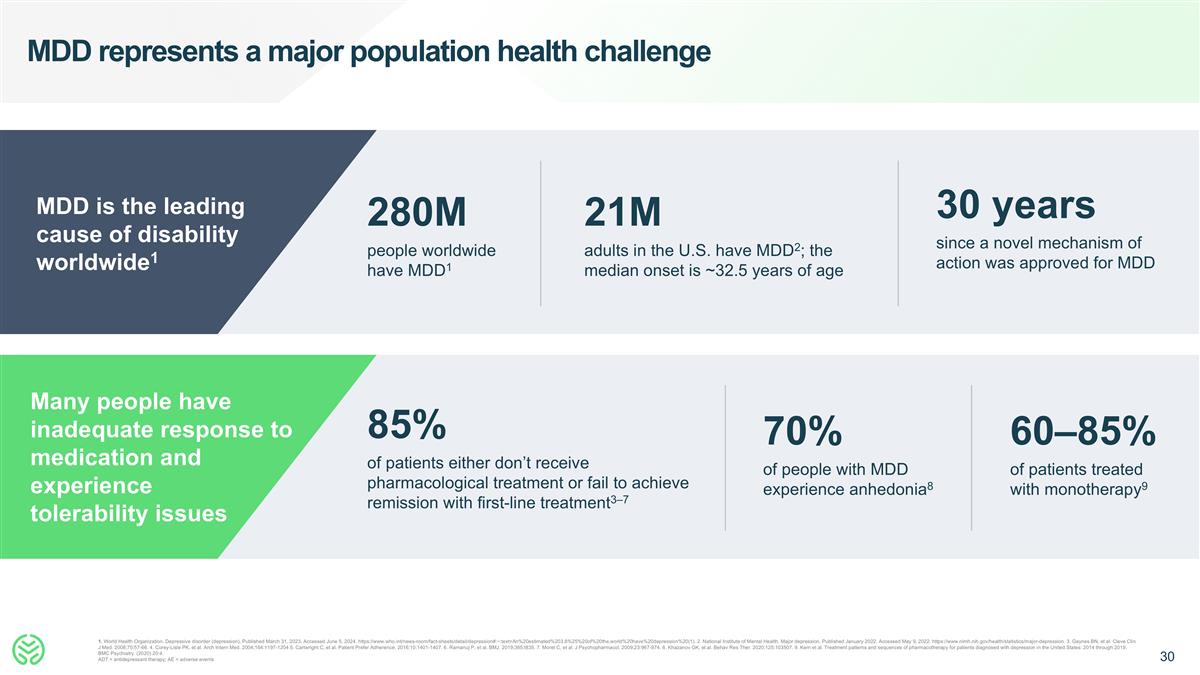
Many people have inadequate response to medication and experience tolerability issues MDD represents a major population health challenge 70% of people with MDD experience anhedonia8 60–85% of patients treated with monotherapy9 21M adults in the U.S. have MDD2; the median onset is ~32.5 years of age 1. World Health Organization. Depressive disorder (depression), Published March 31, 2023. Accessed June 5, 2024. https://www.who.int/news-room/fact-sheets/detail/depression#:~:text=An%20estimated%203.8%25%20of%20the,world%20have%20depression%20(1). 2. National Institute of Mental Health. Major depression. Published January 2022. Accessed May 9, 2022. https://www.nimh.nih.gov/health/statistics/major-depression. 3. Gaynes BN, et al. Cleve Clin J Med. 2008;75:57-66. 4. Corey-Lisle PK, et al. Arch Intern Med. 2004;164:1197-1204 5. Cartwright C, et al. Patient Prefer Adherence. 2016;10:1401-1407. 6. Ramanuj P, et al. BMJ. 2019;365:I835. 7. Moret C, et al. J Psychopharmacol. 2009;23:967-974. 8. Khazanov GK, et al. Behav Res Ther. 2020;125:103507. 9. Kern et al. Treatment patterns and sequences of pharmacotherapy for patients diagnosed with depression in the United States: 2014 through 2019. BMC Psychiatry. (2020) 20:4. ADT = antidepressant therapy; AE = adverse events 30 years since a novel mechanism of action was approved for MDD MDD is the leading cause of disability worldwide1 280M people worldwide have MDD1 Many people have inadequate response to medication and experience tolerability issues 85% of patients either don’t receive pharmacological treatment or fail to achieve remission with first-line treatment3–7
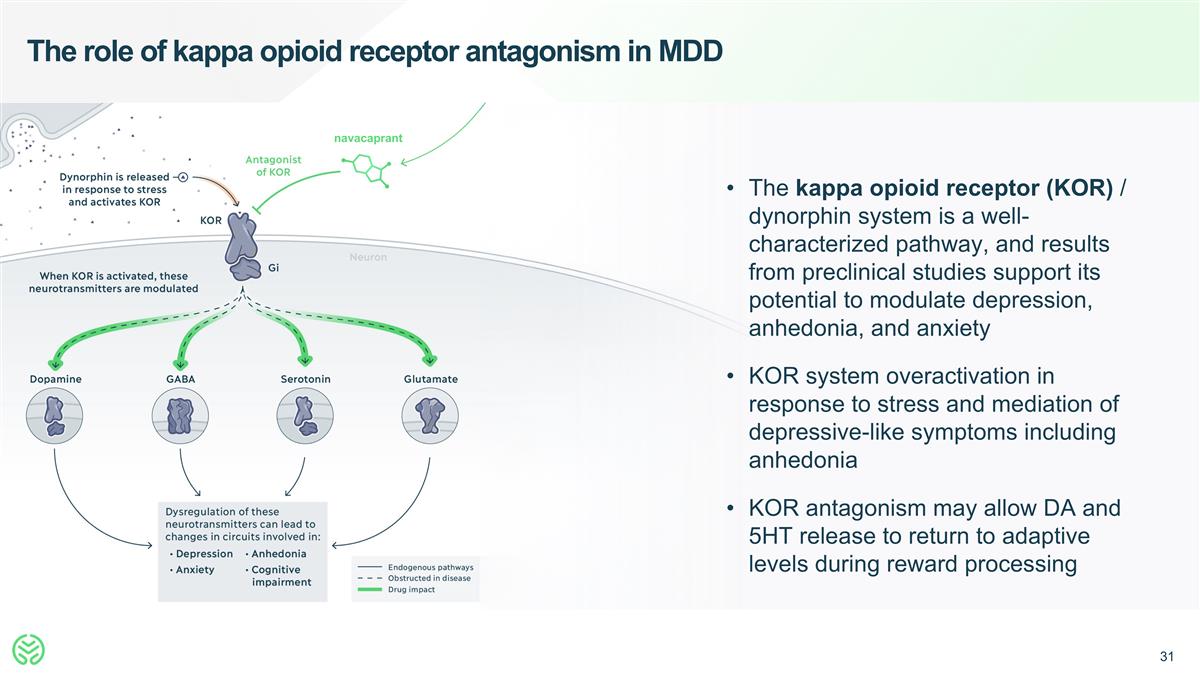
The role of kappa opioid receptor antagonism in MDD The kappa opioid receptor (KOR) / dynorphin system is a well-characterized pathway, and results from preclinical studies support its potential to modulate depression, anhedonia, and anxiety KOR system overactivation in response to stress and mediation of depressive-like symptoms including anhedonia KOR antagonism may allow DA and 5HT release to return to adaptive levels during reward processing navacaprant
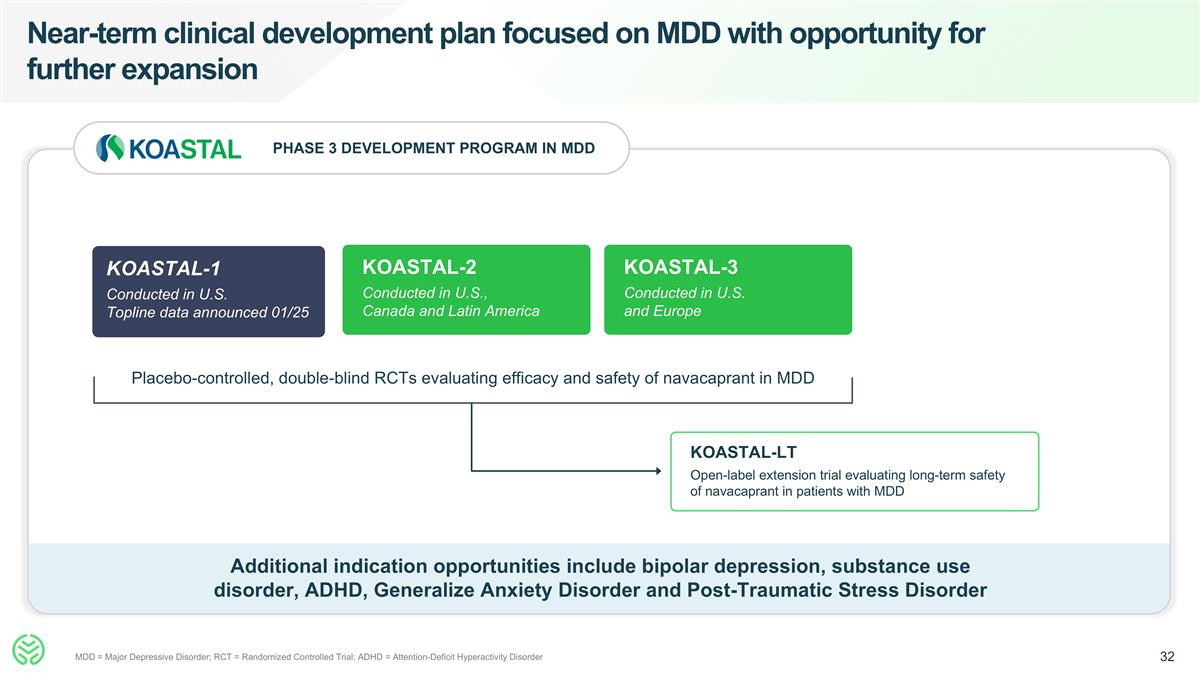
Near-term clinical development plan focused on MDD with opportunity for further expansion MDD = Major Depressive Disorder; RCT = Randomized Controlled Trial; ADHD = Attention-Deficit Hyperactivity Disorder KOASTAL-2 Conducted in U.S., Canada and Latin America KOASTAL-3 Conducted in U.S. and Europe KOASTAL-LT Open-label extension trial evaluating long-term safety of navacaprant in patients with MDD Additional indication opportunities include bipolar depression, substance use disorder, ADHD, Generalize Anxiety Disorder and Post-Traumatic Stress Disorder PHASE 3 DEVELOPMENT PROGRAM IN MDD KOASTAL-1 Conducted in U.S. Topline data announced 01/25 Placebo-controlled, double-blind RCTs evaluating efficacy and safety of navacaprant in MDD
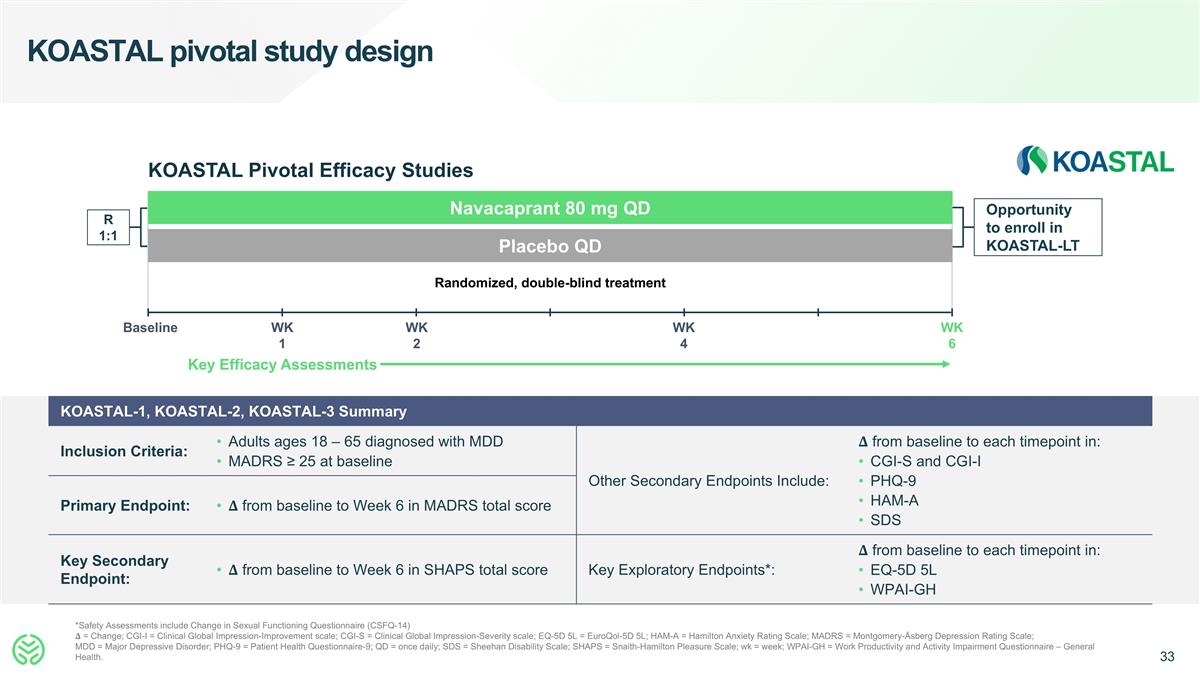
KOASTAL pivotal study design *Safety Assessments include Change in Sexual Functioning Questionnaire (CSFQ-14) �� = Change; CGI-I = Clinical Global Impression-Improvement scale; CGI-S = Clinical Global Impression-Severity scale; EQ-5D 5L = EuroQol-5D 5L; HAM-A = Hamilton Anxiety Rating Scale; MADRS = Montgomery-Åsberg Depression Rating Scale; MDD = Major Depressive Disorder; PHQ-9 = Patient Health Questionnaire-9; QD = once daily; SDS = Sheehan Disability Scale; SHAPS = Snaith-Hamilton Pleasure Scale; wk = week; WPAI-GH = Work Productivity and Activity Impairment Questionnaire – General Health. Randomized, double-blind treatment Baseline WK 6 Navacaprant 80 mg QD Placebo QD R 1:1 Opportunity to enroll in KOASTAL-LT WK 1 WK 2 KOASTAL Pivotal Efficacy Studies KOASTAL-1, KOASTAL-2, KOASTAL-3 Summary Inclusion Criteria: Adults ages 18 – 65 diagnosed with MDD MADRS ≥ 25 at baseline Other Secondary Endpoints Include: �� from baseline to each timepoint in: CGI-S and CGI-I PHQ-9 HAM-A SDS Primary Endpoint: �� from baseline to Week 6 in MADRS total score Key Secondary Endpoint: �� from baseline to Week 6 in SHAPS total score Key Exploratory Endpoints*: �� from baseline to each timepoint in: EQ-5D 5L WPAI-GH Key Efficacy Assessments WK 4
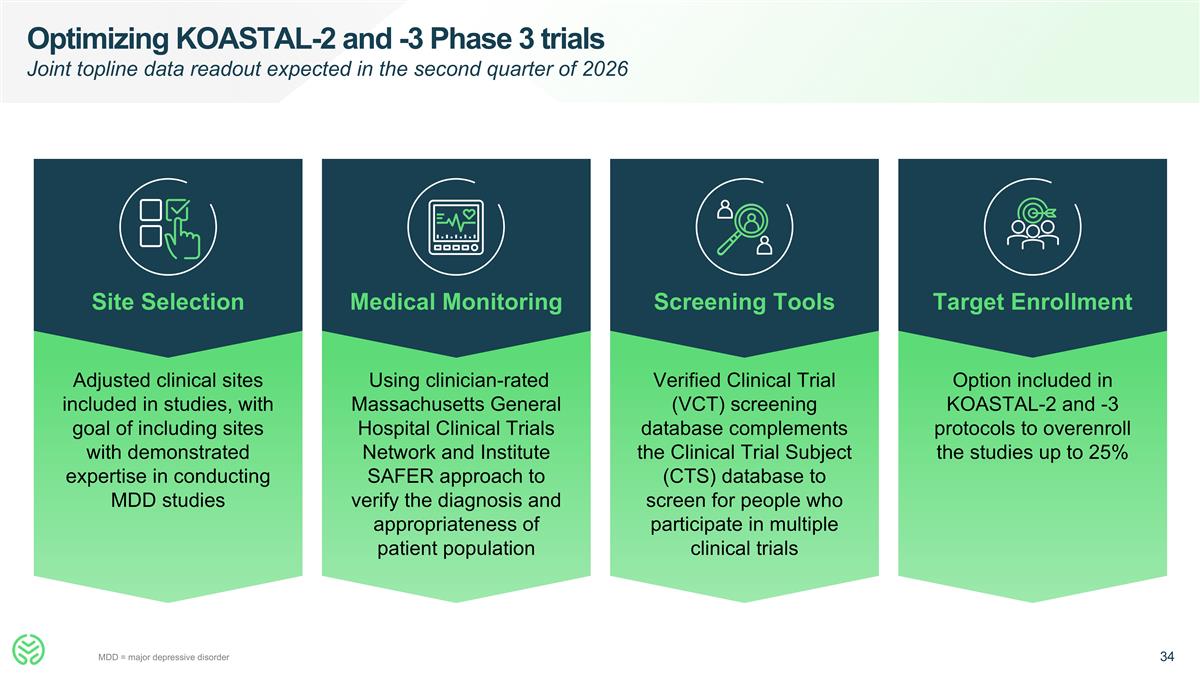
Optimizing KOASTAL-2 and -3 Phase 3 trials Joint topline data readout expected in the second quarter of 2026 Site Selection Adjusted clinical sites included in studies, with goal of including sites with demonstrated expertise in conducting MDD studies Medical Monitoring Using clinician-rated Massachusetts General Hospital Clinical Trials Network and Institute SAFER approach to verify the diagnosis and appropriateness of patient population Screening Tools Verified Clinical Trial (VCT) screening database complements the Clinical Trial Subject (CTS) database to screen for people who participate in multiple clinical trials Target Enrollment Option included in KOASTAL-2 and -3 protocols to overenroll the studies up to 25% MDD = major depressive disorder
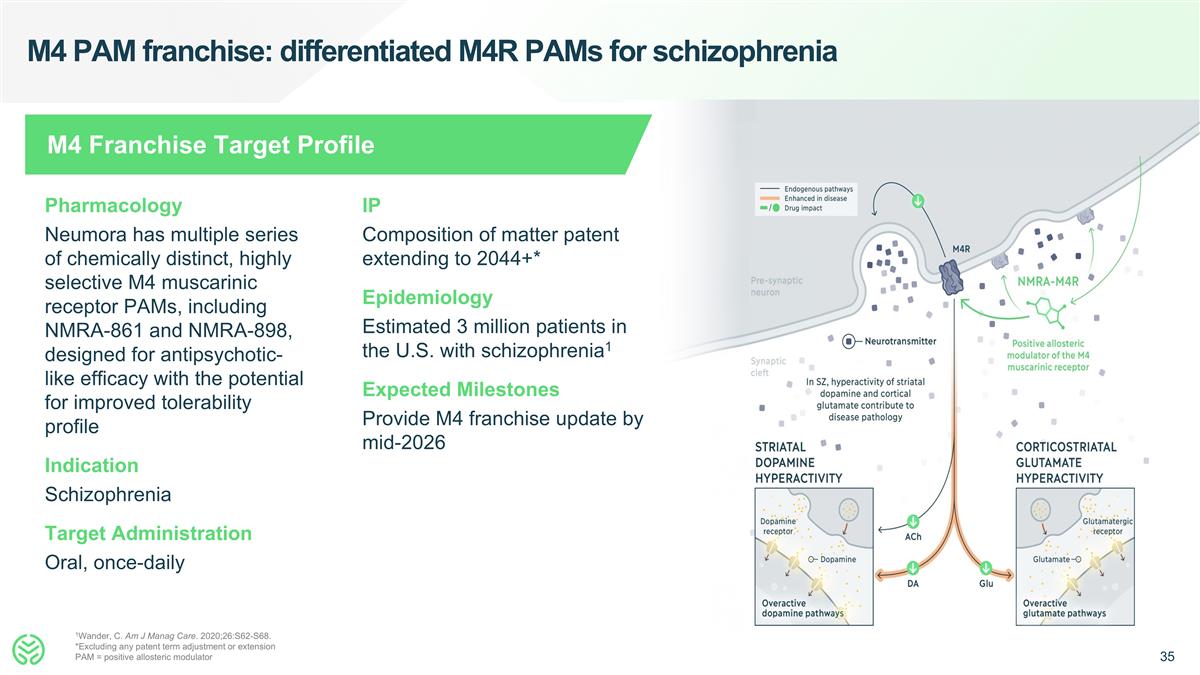
M4 PAM franchise: differentiated M4R PAMs for schizophrenia 1Wander, C. Am J Manag Care. 2020;26:S62-S68. *Excluding any patent term adjustment or extension PAM = positive allosteric modulator Pharmacology Neumora has multiple series of chemically distinct, highly selective M4 muscarinic receptor PAMs, including NMRA-861 and NMRA-898, designed for antipsychotic-like efficacy with the potential for improved tolerability profile Indication Schizophrenia Target Administration Oral, once-daily IP Composition of matter patent extending to 2044+* Epidemiology Estimated 3 million patients in the U.S. with schizophrenia1 Expected Milestones Provide M4 franchise update by mid-2026 M4 Franchise Target Profile
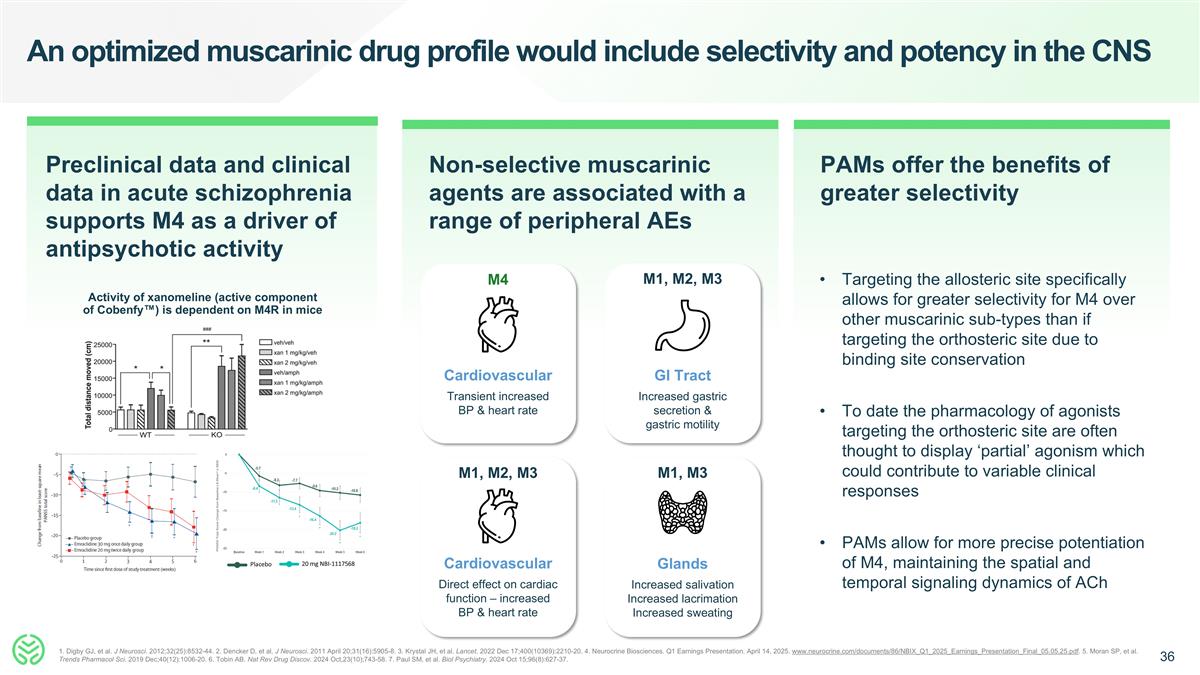
An optimized muscarinic drug profile would include selectivity and potency in the CNS Activity of xanomeline (active component of Cobenfy™) is dependent on M4R in mice Preclinical data and clinical data in acute schizophrenia supports M4 as a driver of antipsychotic activity Non-selective muscarinic agents are associated with a range of peripheral AEs Glands Increased salivation Increased lacrimation Increased sweating M1, M3 GI Tract Increased gastric secretion & gastric motility M1, M2, M3 M1, M2, M3 Cardiovascular Direct effect on cardiac function – increased BP & heart rate M4 Cardiovascular Transient increased BP & heart rate PAMs offer the benefits of greater selectivity Placebo 20 mg NBI-1117568 Targeting the allosteric site specifically allows for greater selectivity for M4 over other muscarinic sub-types than if targeting the orthosteric site due to binding site conservation To date the pharmacology of agonists targeting the orthosteric site are often thought to display ‘partial’ agonism which could contribute to variable clinical responses PAMs allow for more precise potentiation of M4, maintaining the spatial and temporal signaling dynamics of ACh 1. Digby GJ, et al. J Neurosci. 2012;32(25):8532-44. 2. Dencker D, et al. J Neurosci. 2011 April 20;31(16):5905-8. 3. Krystal JH, et al. Lancet. 2022 Dec 17;400(10369):2210-20. 4. Neurocrine Biosciences. Q1 Earnings Presentation. April 14, 2025. www.neurocrine.com/documents/86/NBIX_Q1_2025_Earnings_Presentation_Final_05.05.25.pdf. 5. Moran SP, et al. Trends Pharmacol Sci. 2019 Dec;40(12):1006-20. 6. Tobin AB. Nat Rev Drug Discov. 2024 Oct,23(10);743-58. 7. Paul SM, et al. Biol Psychiatry. 2024 Oct 15;96(8):627-37.
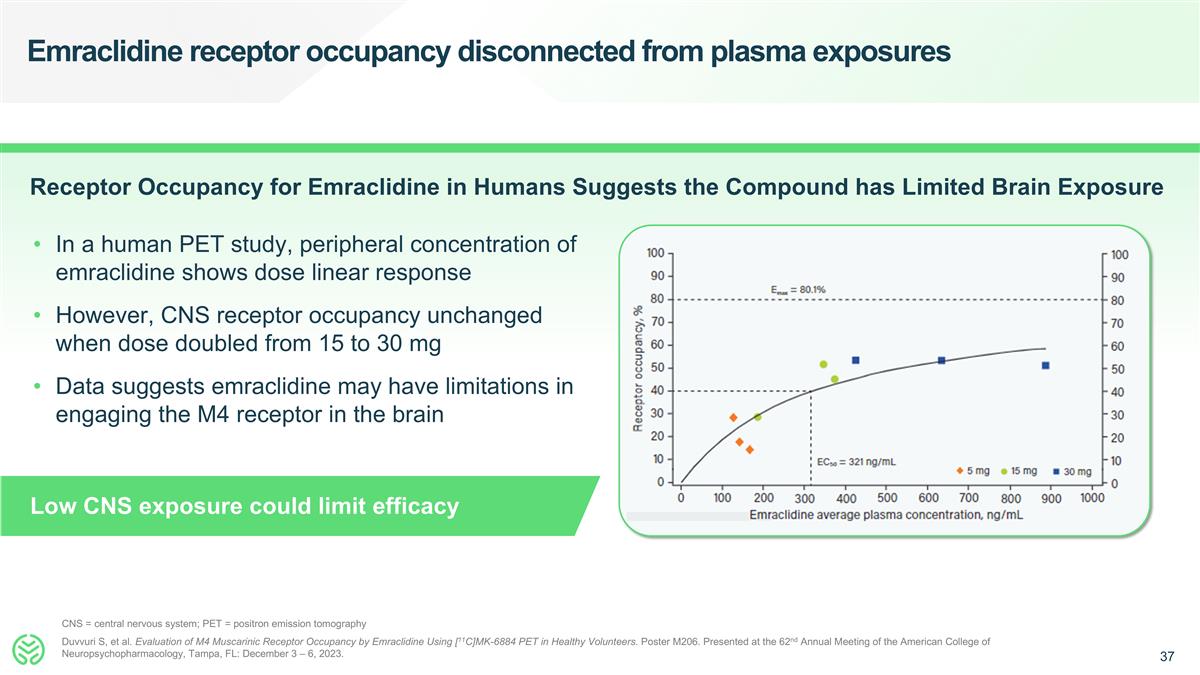
Emraclidine receptor occupancy disconnected from plasma exposures Duvvuri S, et al. Evaluation of M4 Muscarinic Receptor Occupancy by Emraclidine Using [11C]MK-6884 PET in Healthy Volunteers. Poster M206. Presented at the 62nd Annual Meeting of the American College of Neuropsychopharmacology, Tampa, FL: December 3 – 6, 2023. CNS = central nervous system; PET = positron emission tomography In a human PET study, peripheral concentration of emraclidine shows dose linear response However, CNS receptor occupancy unchanged when dose doubled from 15 to 30 mg Data suggests emraclidine may have limitations in engaging the M4 receptor in the brain Low CNS exposure could limit efficacy Receptor Occupancy for Emraclidine in Humans Suggests the Compound has Limited Brain Exposure
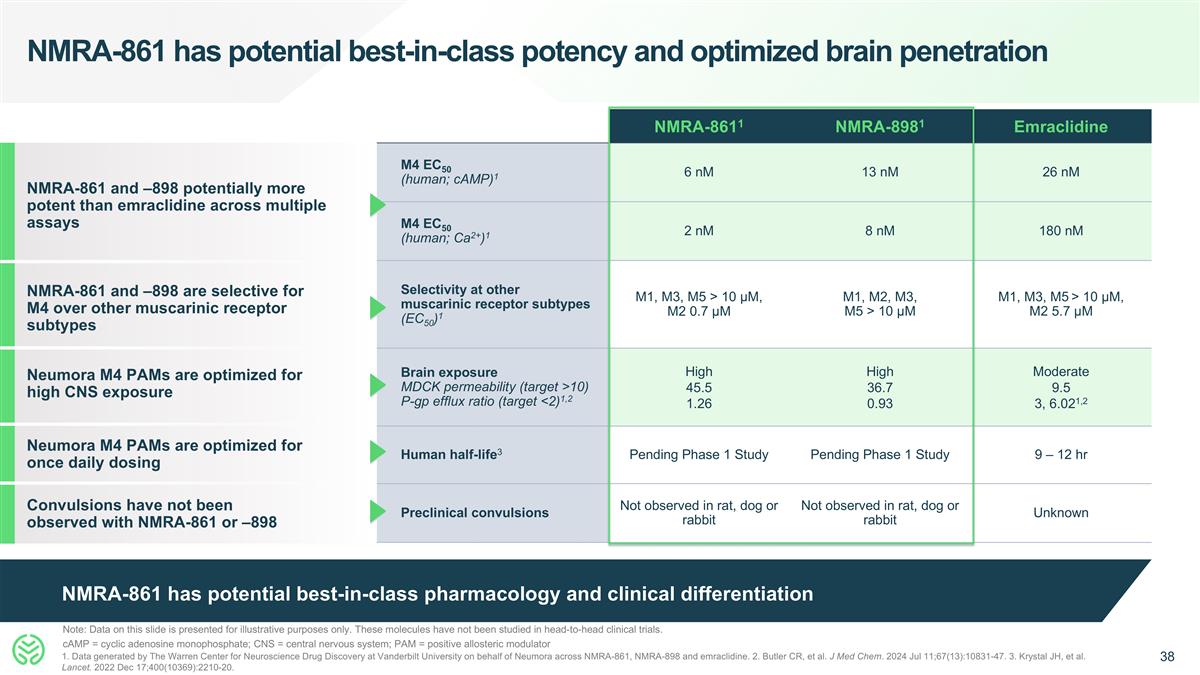
NMRA-861 has potential best-in-class pharmacology and clinical differentiation NMRA-861 has potential best-in-class potency and optimized brain penetration NMRA-8611 NMRA-8981 Emraclidine M4 EC50 (human; cAMP)1 6 nM 13 nM 26 nM M4 EC50 (human; Ca2+)1 2 nM 8 nM 180 nM Selectivity at other muscarinic receptor subtypes (EC50)1 M1, M3, M5 > 10 µM, M2 0.7 µM M1, M2, M3, M5 > 10 µM M1, M3, M5 > 10 µM, M2 5.7 µM Brain exposure MDCK permeability (target >10) P-gp efflux ratio (target <2)1,2 High 45.5 1.26 High 36.7 0.93 Moderate 9.5 3, 6.021,2 Human half-life3 Pending Phase 1 Study Pending Phase 1 Study 9 – 12 hr Preclinical convulsions Not observed in rat, dog or rabbit Not observed in rat, dog or rabbit Unknown NMRA-861 and –898 potentially more potent than emraclidine across multiple assays Convulsions have not been observed with NMRA-861 or –898 NMRA-861 and –898 are selective for M4 over other muscarinic receptor subtypes Neumora M4 PAMs are optimized for once daily dosing Note: Data on this slide is presented for illustrative purposes only. These molecules have not been studied in head-to-head clinical trials. cAMP = cyclic adenosine monophosphate; CNS = central nervous system; PAM = positive allosteric modulator 1. Data generated by The Warren Center for Neuroscience Drug Discovery at Vanderbilt University on behalf of Neumora across NMRA-861, NMRA-898 and emraclidine. 2. Butler CR, et al. J Med Chem. 2024 Jul 11;67(13):10831-47. 3. Krystal JH, et al. Lancet. 2022 Dec 17;400(10369):2210-20. Neumora M4 PAMs are optimized for high CNS exposure
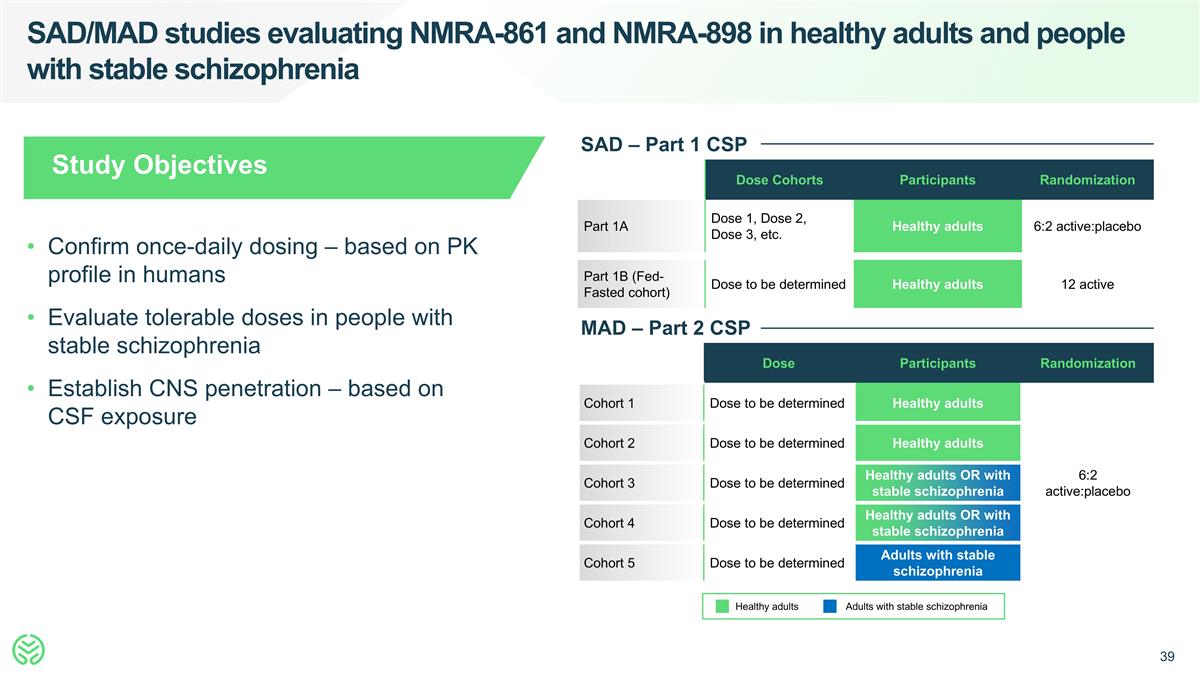
SAD/MAD studies evaluating NMRA-861 and NMRA-898 in healthy adults and people with stable schizophrenia Dose Cohorts Participants Randomization Part 1A Dose 1, Dose 2, Dose 3, etc. Healthy adults 6:2 active:placebo Part 1B (Fed-Fasted cohort) Dose to be determined Healthy adults 12 active SAD – Part 1 CSP Dose Participants Randomization Cohort 1 Dose to be determined Healthy adults 6:2 active:placebo Cohort 2 Dose to be determined Healthy adults Cohort 3 Dose to be determined Healthy adults OR with stable schizophrenia Cohort 4 Dose to be determined Healthy adults OR with stable schizophrenia Cohort 5 Dose to be determined Adults with stable schizophrenia MAD – Part 2 CSP Adults with stable schizophrenia Healthy adults Study Objectives Confirm once-daily dosing – based on PK profile in humans Evaluate tolerable doses in people with stable schizophrenia Establish CNS penetration – based on CSF exposure
