

February 2026 Guggenheim Emerging Outlook: Biotech Summit

Important Disclosures This presentation contains forward-looking statements about Neumora Therapeutics, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws, including statements related to: Neumora’s intention to redefine neuroscience drug development by bringing forward the next generation of novel therapies that offer improved treatment outcomes and quality of life for patients; the timing, progress and plans for its therapeutic development programs, including the timing of clinical trial initiation and data readouts and upcoming milestones and catalysts; expectations and projections regarding future operating results and financial performance, including the sufficiency of its cash resources, intellectual property protection, and expectation of the timing of its cash runway; and other statements identified by words such as “could,” “expects,” “intends,” “may,” “plans,” “potential,” “should,” “will,” “would,” or similar expressions and the negatives of those terms. Other than statements of historical facts, all statements contained in this presentation are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. These statements are subject to risks and uncertainties that could cause the actual results to be materially different from the information expressed or implied by these forward-looking statements, including, among others: the risks related to the inherent uncertainty of clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals; risks related to the timely initiation and enrollment in our clinical trials; risks related to our reliance on third parties, including contract research organizations; risks related to serious or undesirable side effects of our therapeutic candidates; risks related to our ability to utilize and protect our intellectual property rights; and other matters that could affect sufficiency of capital resources to fund operations. For a detailed discussion of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Neumora’s business in general, please refer to the risk factors identified in the Company’s filings with the Securities and Exchange Commission (SEC), including but not limited to its Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 which was filed with the SEC on November 6, 2025. Forward-looking statements speak only as of the date hereof, and, except as required by law, Neumora undertakes no obligation to update or revise these forward-looking statements. Our results for the quarter ended September 30, 2025 are also not necessarily indicative of our operating results for any future periods.
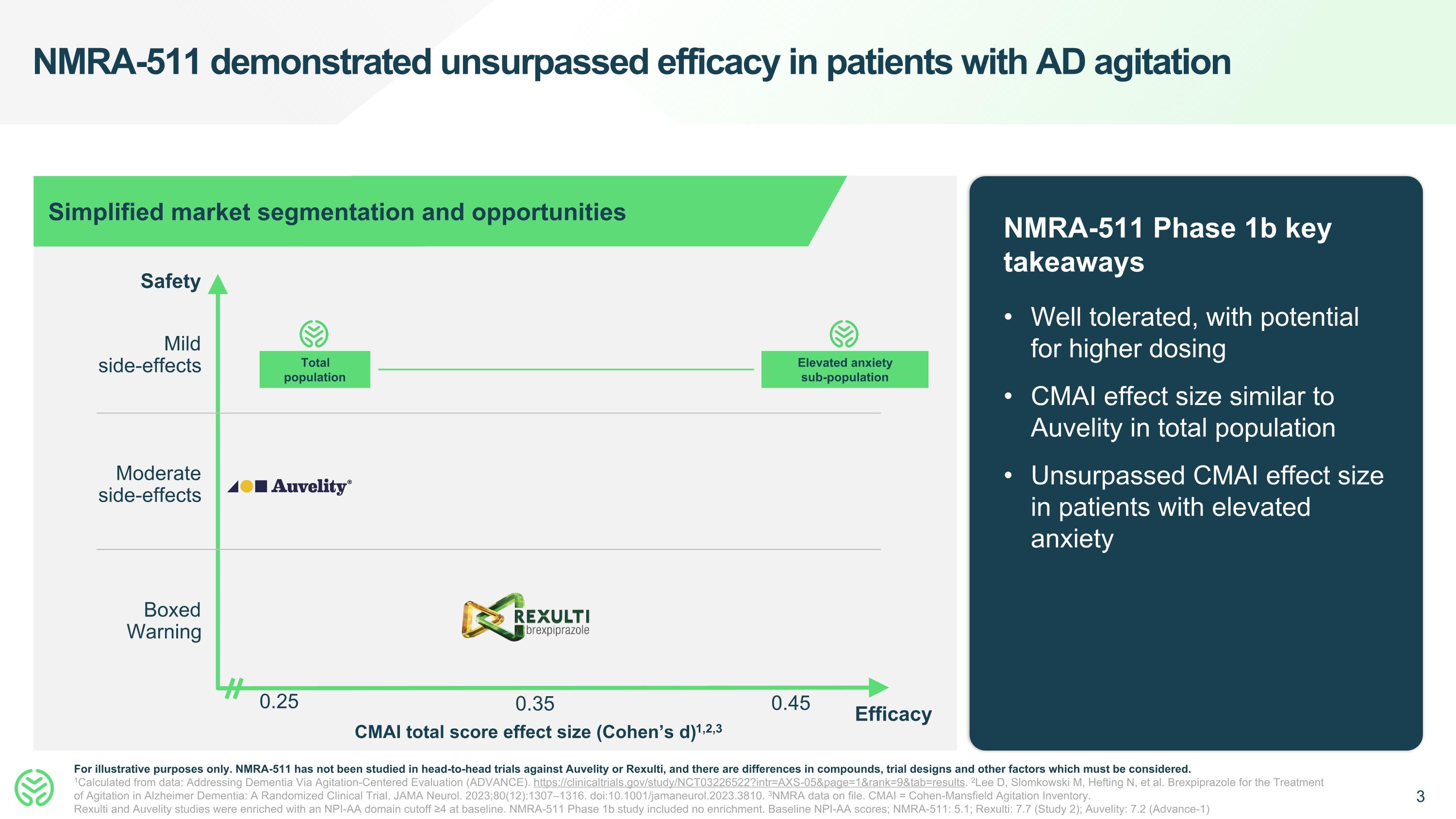
NMRA-511 demonstrated unsurpassed efficacy in patients with AD agitation NMRA-511 Phase 1b key takeaways Well tolerated, with potential for higher dosing CMAI effect size similar to Auvelity in total population Unsurpassed CMAI effect size in patients with elevated anxiety For illustrative purposes only. NMRA-511 has not been studied in head-to-head trials against Auvelity or Rexulti, and there are differences in compounds, trial designs and other factors which must be considered. 1Calculated from data: Addressing Dementia Via Agitation-Centered Evaluation (ADVANCE). https://clinicaltrials.gov/study/NCT03226522?intr=AXS-05&page=1&rank=9&tab=results. 2Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023;80(12):1307–1316. doi:10.1001/jamaneurol.2023.3810. 3NMRA data on file. CMAI = Cohen-Mansfield Agitation Inventory. Rexulti and Auvelity studies were enriched with an NPI-AA domain cutoff ≥4 at baseline. NMRA-511 Phase 1b study included no enrichment. Baseline NPI-AA scores; NMRA-511: 5.1; Rexulti: 7.7 (Study 2); Auvelity: 7.2 (Advance-1) 0.25 Safety CMAI total score effect size (Cohen’s d)1,2,3 Boxed Warning Moderate side-effects Mild side-effects Simplified market segmentation and opportunities 0.35 0.45 Efficacy Total population Elevated anxiety sub-population
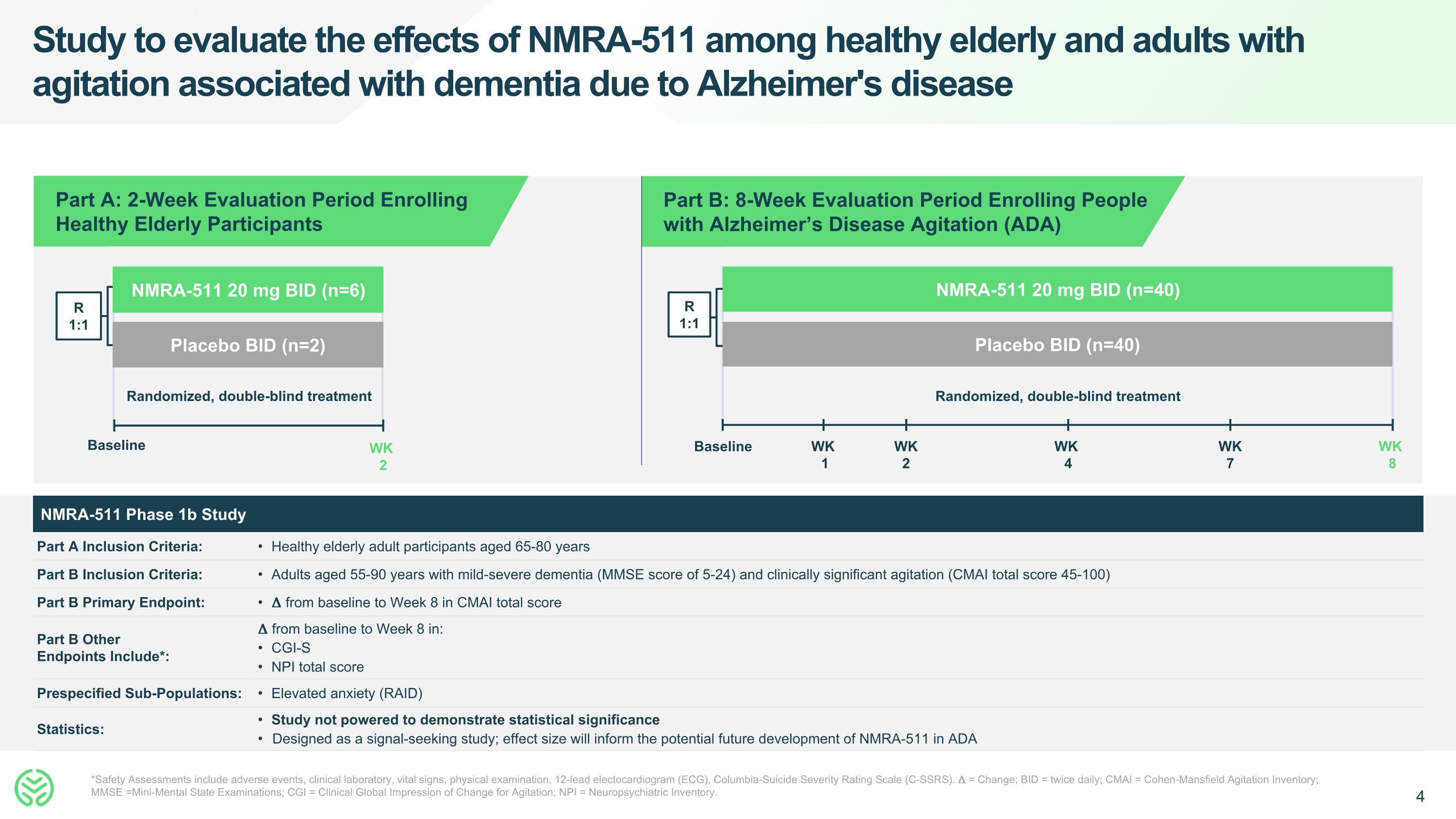
Study to evaluate the effects of NMRA-511 among healthy elderly and adults with agitation associated with dementia due to Alzheimer's disease *Safety Assessments include adverse events, clinical laboratory, vital signs, physical examination, 12-lead electocardiogram (ECG), Columbia-Suicide Severity Rating Scale (C-SSRS). 𝚫 = Change; BID = twice daily; CMAI = Cohen-Mansfield Agitation Inventory; MMSE =Mini-Mental State Examinations; CGI = Clinical Global Impression of Change for Agitation; NPI = Neuropsychiatric Inventory. R 1:1 Randomized, double-blind treatment NMRA-511 20 mg BID (n=6) Placebo BID (n=2) Baseline WK 2 Randomized, double-blind treatment NMRA-511 20 mg BID (n=40) Placebo BID (n=40) R 1:1 Baseline WK 8 WK 4 WK 2 WK 7 WK 1 Part B: 8-Week Evaluation Period Enrolling People with Alzheimer’s Disease Agitation (ADA) Part A: 2-Week Evaluation Period Enrolling Healthy Elderly Participants NMRA-511 Phase 1b Study Part A Inclusion Criteria: Healthy elderly adult participants aged 65-80 years Part B Inclusion Criteria: Adults aged 55-90 years with mild-severe dementia (MMSE score of 5-24) and clinically significant agitation (CMAI total score 45-100) Part B Primary Endpoint: 𝚫 from baseline to Week 8 in CMAI total score Part B Other Endpoints Include*: 𝚫 from baseline to Week 8 in: CGI-S NPI total score Prespecified Sub-Populations: Elevated anxiety (RAID) Statistics: Study not powered to demonstrate statistical significance Designed as a signal-seeking study; effect size will inform the potential future development of NMRA-511 in ADA
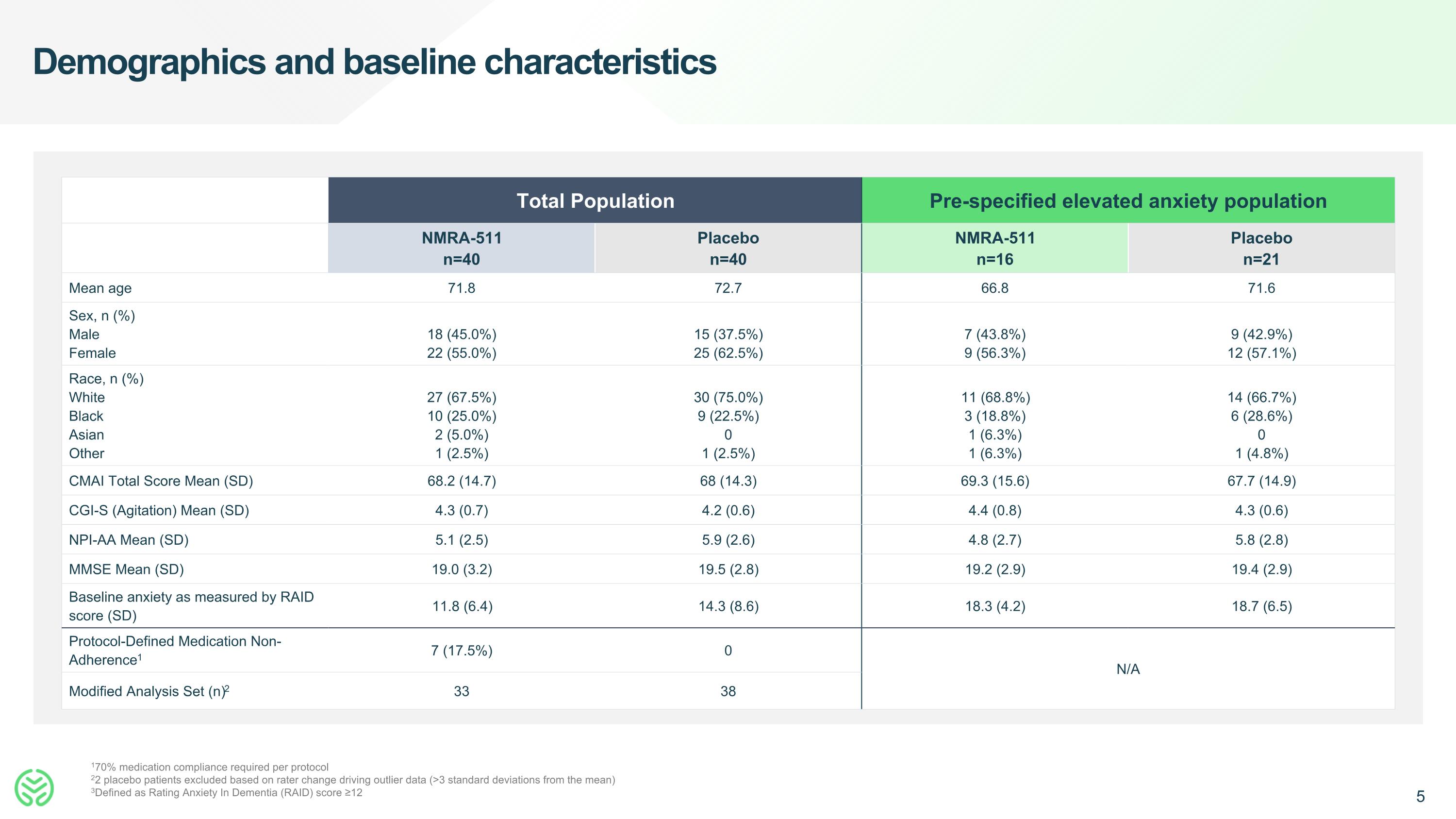
Demographics and baseline characteristics 170% medication compliance required per protocol 22 placebo patients excluded based on rater change driving outlier data (>3 standard deviations from the mean) 3Defined as Rating Anxiety In Dementia (RAID) score ≥12 Total Population Pre-specified elevated anxiety population NMRA-511 n=40 Placebo n=40 NMRA-511 n=16 Placebo n=21 Mean age 71.8 72.7 66.8 71.6 Sex, n (%) Male Female 18 (45.0%) 22 (55.0%) 15 (37.5%) 25 (62.5%) 7 (43.8%) 9 (56.3%) 9 (42.9%) 12 (57.1%) Race, n (%) White Black Asian Other 27 (67.5%) 10 (25.0%) 2 (5.0%) 1 (2.5%) 30 (75.0%) 9 (22.5%) 0 1 (2.5%) 11 (68.8%) 3 (18.8%) 1 (6.3%) 1 (6.3%) 14 (66.7%) 6 (28.6%) 0 1 (4.8%) CMAI Total Score Mean (SD) 68.2 (14.7) 68 (14.3) 69.3 (15.6) 67.7 (14.9) CGI-S (Agitation) Mean (SD) 4.3 (0.7) 4.2 (0.6) 4.4 (0.8) 4.3 (0.6) NPI-AA Mean (SD) 5.1 (2.5) 5.9 (2.6) 4.8 (2.7) 5.8 (2.8) MMSE Mean (SD) 19.0 (3.2) 19.5 (2.8) 19.2 (2.9) 19.4 (2.9) Baseline anxiety as measured by RAID score (SD) 11.8 (6.4) 14.3 (8.6) 18.3 (4.2) 18.7 (6.5) Protocol-Defined Medication Non-Adherence1 7 (17.5%) 0 N/A Modified Analysis Set (n)2 33 38
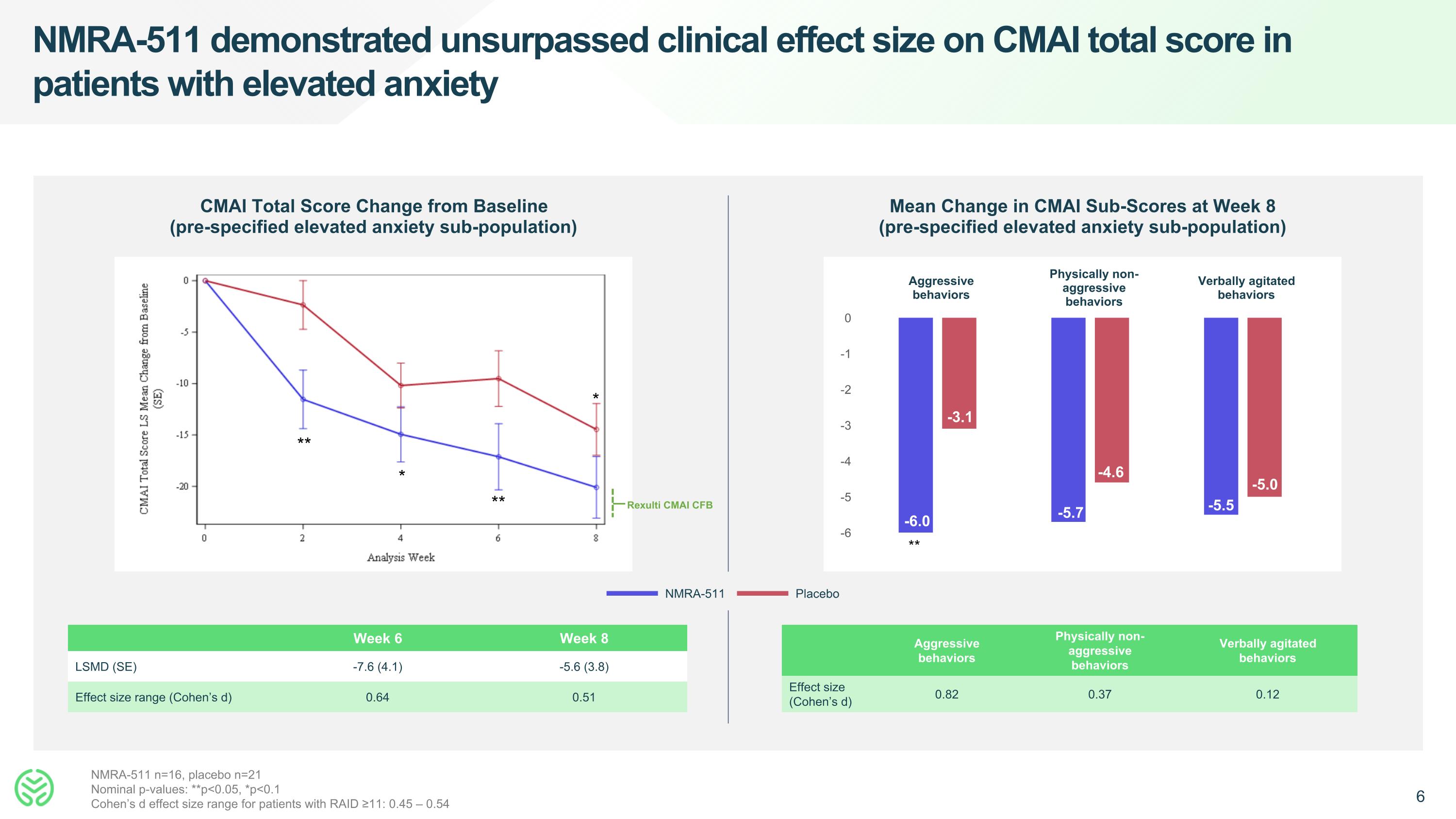
NMRA-511 demonstrated unsurpassed clinical effect size on CMAI total score in patients with elevated anxiety CMAI Total Score Change from Baseline (pre-specified elevated anxiety sub-population) Mean Change in CMAI Sub-Scores at Week 8 (pre-specified elevated anxiety sub-population) NMRA-511 Placebo Aggressive behaviors Physically non-aggressive behaviors Verbally agitated behaviors -6.0 -3.1 NMRA-511 n=16, placebo n=21 Nominal p-values: **p<0.05, *p<0.1 Cohen’s d effect size range for patients with RAID ≥11: 0.45 – 0.54 Week 6 Week 8 LSMD (SE) -7.6 (4.1) -5.6 (3.8) Effect size range (Cohen’s d) 0.64 0.51 -5.7 -4.6 ** -5.5 -5.0 Rexulti CMAI CFB ** * ** * Aggressive behaviors Physically non-aggressive behaviors Verbally agitated behaviors Effect size (Cohen’s d) 0.82 0.37 0.12
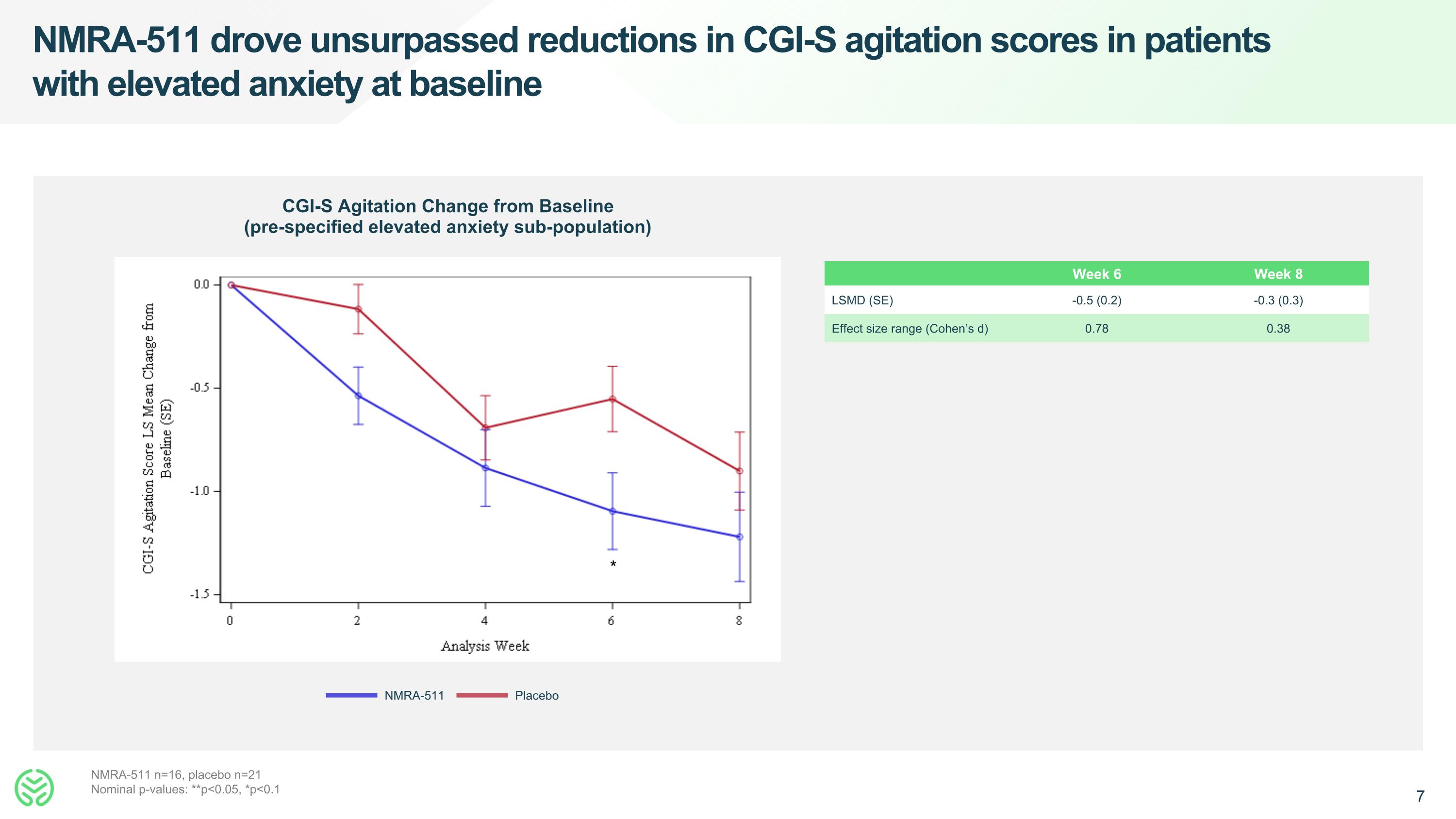
NMRA-511 drove unsurpassed reductions in CGI-S agitation scores in patients with elevated anxiety at baseline CGI-S Agitation Change from Baseline (pre-specified elevated anxiety sub-population) NMRA-511 Placebo NMRA-511 n=16, placebo n=21 Nominal p-values: **p<0.05, *p<0.1 Week 6 Week 8 LSMD (SE) -0.5 (0.2) -0.3 (0.3) Effect size range (Cohen’s d) 0.78 0.38 *
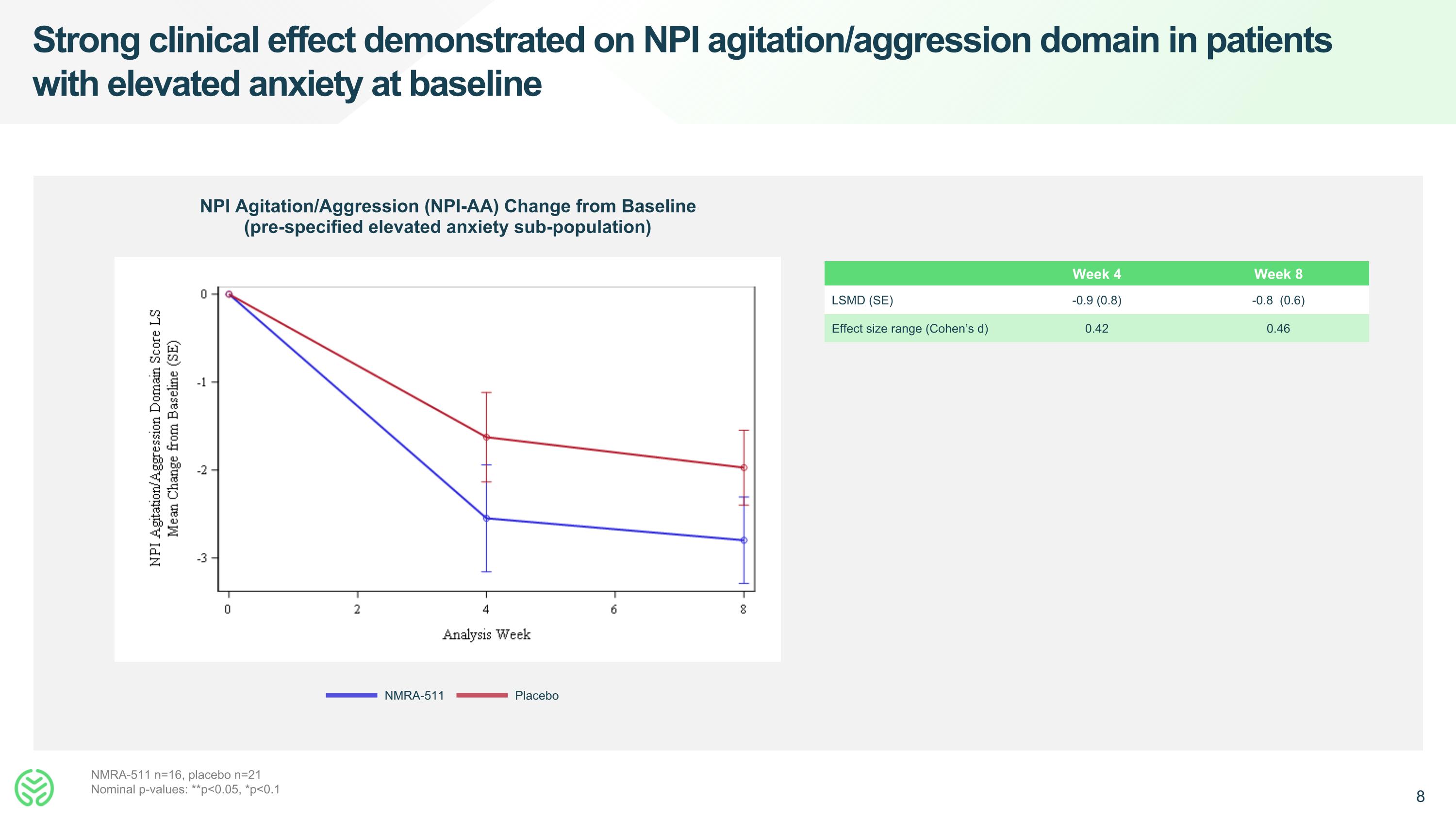
Strong clinical effect demonstrated on NPI agitation/aggression domain in patients with elevated anxiety at baseline NPI Agitation/Aggression (NPI-AA) Change from Baseline (pre-specified elevated anxiety sub-population) NMRA-511 Placebo NMRA-511 n=16, placebo n=21 Nominal p-values: **p<0.05, *p<0.1 Week 4 Week 8 LSMD (SE) -0.9 (0.8) -0.8 (0.6) Effect size range (Cohen’s d) 0.42 0.46
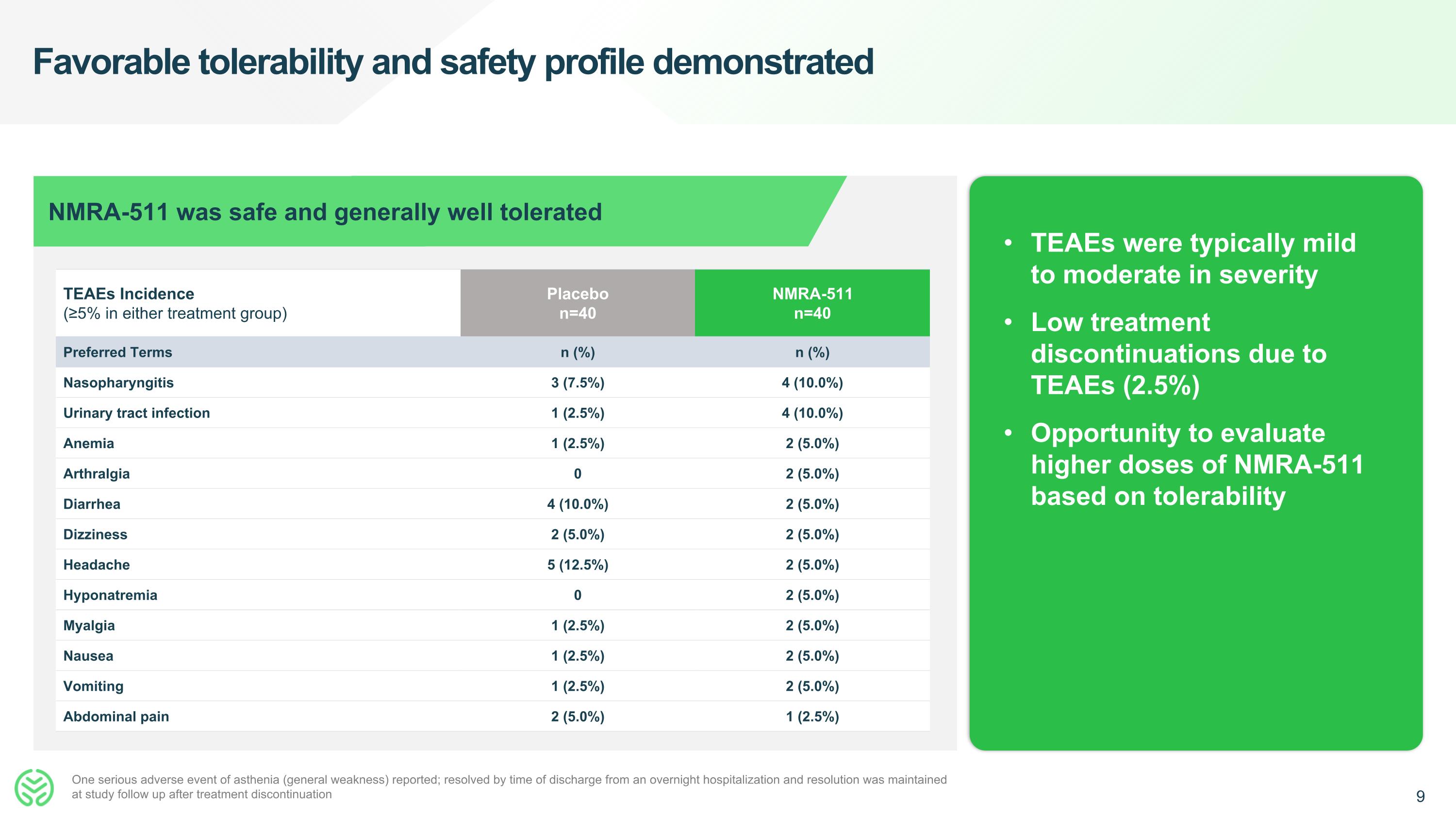
Favorable tolerability and safety profile demonstrated TEAEs were typically mild to moderate in severity Low treatment discontinuations due to TEAEs (2.5%) Opportunity to evaluate higher doses of NMRA-511 based on tolerability TEAEs Incidence (≥5% in either treatment group) Placebo n=40 NMRA-511 n=40 Preferred Terms n (%) n (%) Nasopharyngitis 3 (7.5%) 4 (10.0%) Urinary tract infection 1 (2.5%) 4 (10.0%) Anemia 1 (2.5%) 2 (5.0%) Arthralgia 0 2 (5.0%) Diarrhea 4 (10.0%) 2 (5.0%) Dizziness 2 (5.0%) 2 (5.0%) Headache 5 (12.5%) 2 (5.0%) Hyponatremia 0 2 (5.0%) Myalgia 1 (2.5%) 2 (5.0%) Nausea 1 (2.5%) 2 (5.0%) Vomiting 1 (2.5%) 2 (5.0%) Abdominal pain 2 (5.0%) 1 (2.5%) NMRA-511 was safe and generally well tolerated One serious adverse event of asthenia (general weakness) reported; resolved by time of discharge from an overnight hospitalization and resolution was maintained at study follow up after treatment discontinuation
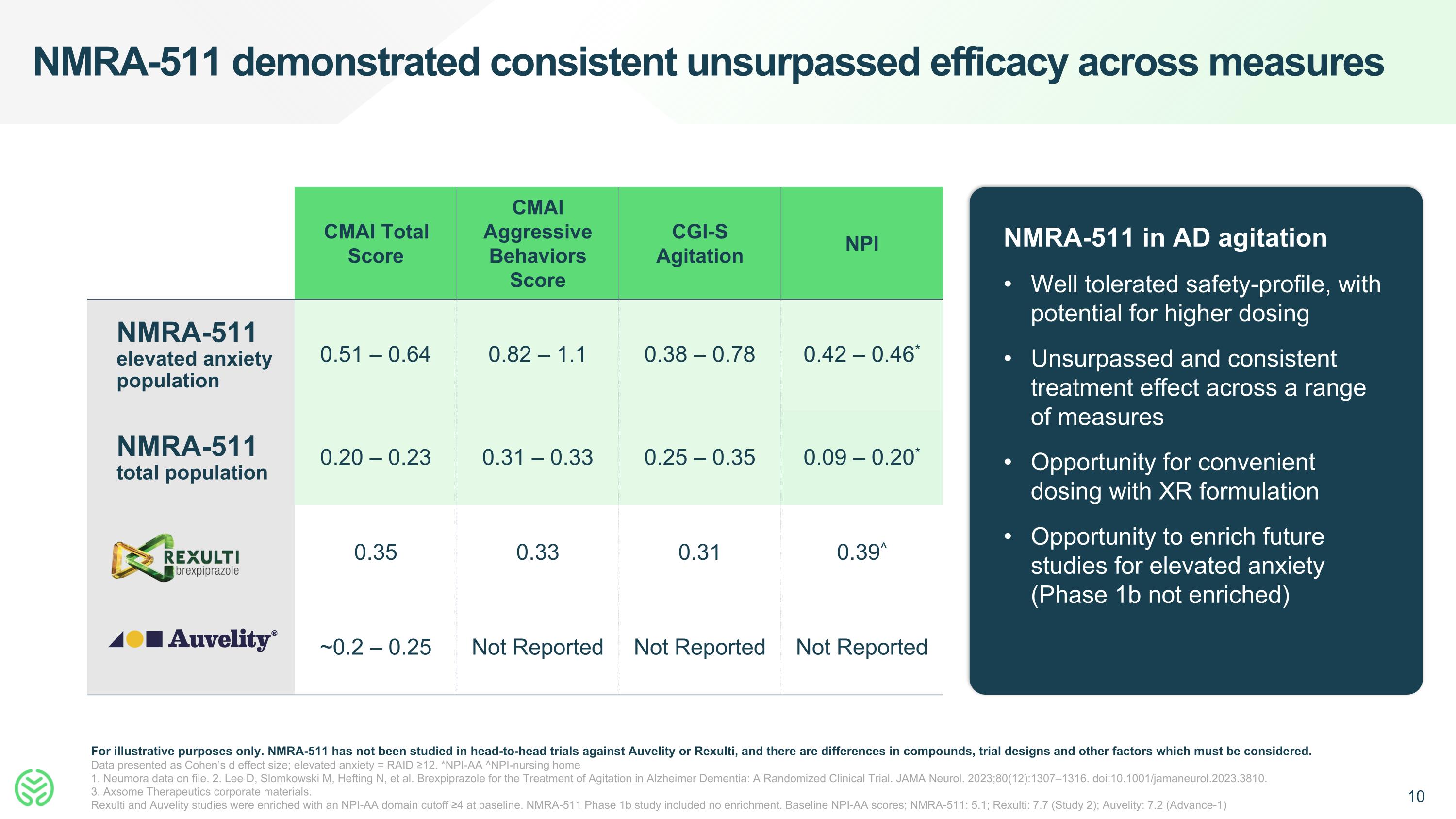
NMRA-511 demonstrated consistent unsurpassed efficacy across measures For illustrative purposes only. NMRA-511 has not been studied in head-to-head trials against Auvelity or Rexulti, and there are differences in compounds, trial designs and other factors which must be considered. Data presented as Cohen’s d effect size; elevated anxiety = RAID ≥12. *NPI-AA ^NPI-nursing home 1. Neumora data on file. 2. Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023;80(12):1307–1316. doi:10.1001/jamaneurol.2023.3810. 3. Axsome Therapeutics corporate materials. Rexulti and Auvelity studies were enriched with an NPI-AA domain cutoff ≥4 at baseline. NMRA-511 Phase 1b study included no enrichment. Baseline NPI-AA scores; NMRA-511: 5.1; Rexulti: 7.7 (Study 2); Auvelity: 7.2 (Advance-1) CMAI Total Score CMAI Aggressive Behaviors Score CGI-S Agitation NPI NMRA-511 elevated anxiety population 0.51 – 0.64 0.82 – 1.1 0.38 – 0.78 0.42 – 0.46* NMRA-511 total population 0.20 – 0.23 0.31 – 0.33 0.25 – 0.35 0.09 – 0.20* 0.35 0.33 0.31 0.39^ ~0.2 – 0.25 Not Reported Not Reported Not Reported NMRA-511 in AD agitation Well tolerated safety-profile, with potential for higher dosing Unsurpassed and consistent treatment effect across a range of measures Opportunity for convenient dosing with XR formulation Opportunity to enrich future studies for elevated anxiety (Phase 1b not enriched)

Appendix
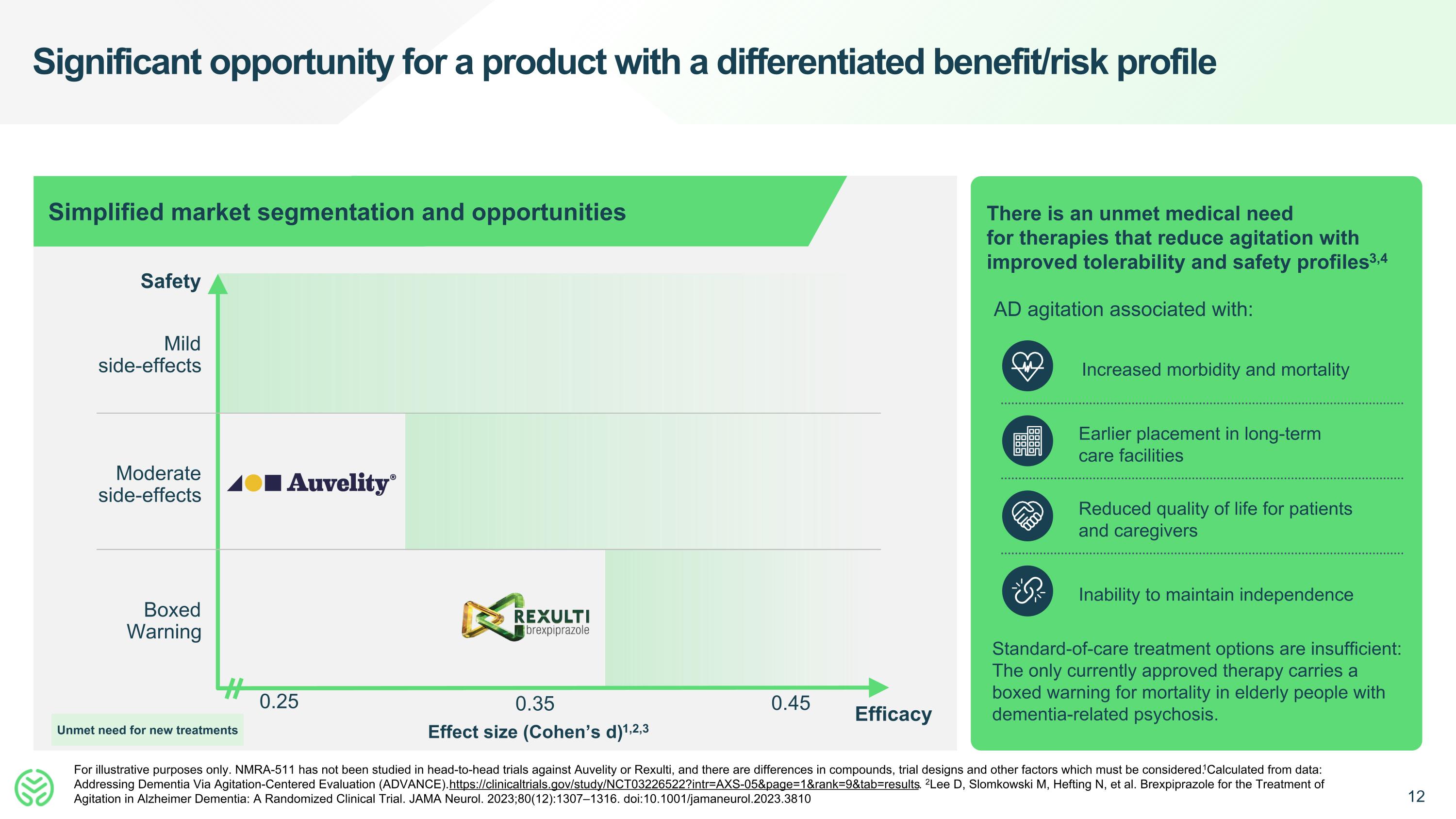
Significant opportunity for a product with a differentiated benefit/risk profile For illustrative purposes only. NMRA-511 has not been studied in head-to-head trials against Auvelity or Rexulti, and there are differences in compounds, trial designs and other factors which must be considered. 1Calculated from data: Addressing Dementia Via Agitation-Centered Evaluation (ADVANCE). https://clinicaltrials.gov/study/NCT03226522?intr=AXS-05&page=1&rank=9&tab=results. 2Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia: A Randomized Clinical Trial. JAMA Neurol. 2023;80(12):1307–1316. doi:10.1001/jamaneurol.2023.3810 0.25 Safety Effect size (Cohen’s d)1,2,3 Boxed Warning Moderate side-effects Mild side-effects Simplified market segmentation and opportunities Increased morbidity and mortality Earlier placement in long-term care facilities Reduced quality of life for patients and caregivers Inability to maintain independence There is an unmet medical need for therapies that reduce agitation with improved tolerability and safety profiles3,4 AD agitation associated with: Standard-of-care treatment options are insufficient: The only currently approved therapy carries a boxed warning for mortality in elderly people with dementia-related psychosis. 0.35 0.45 Efficacy Unmet need for new treatments
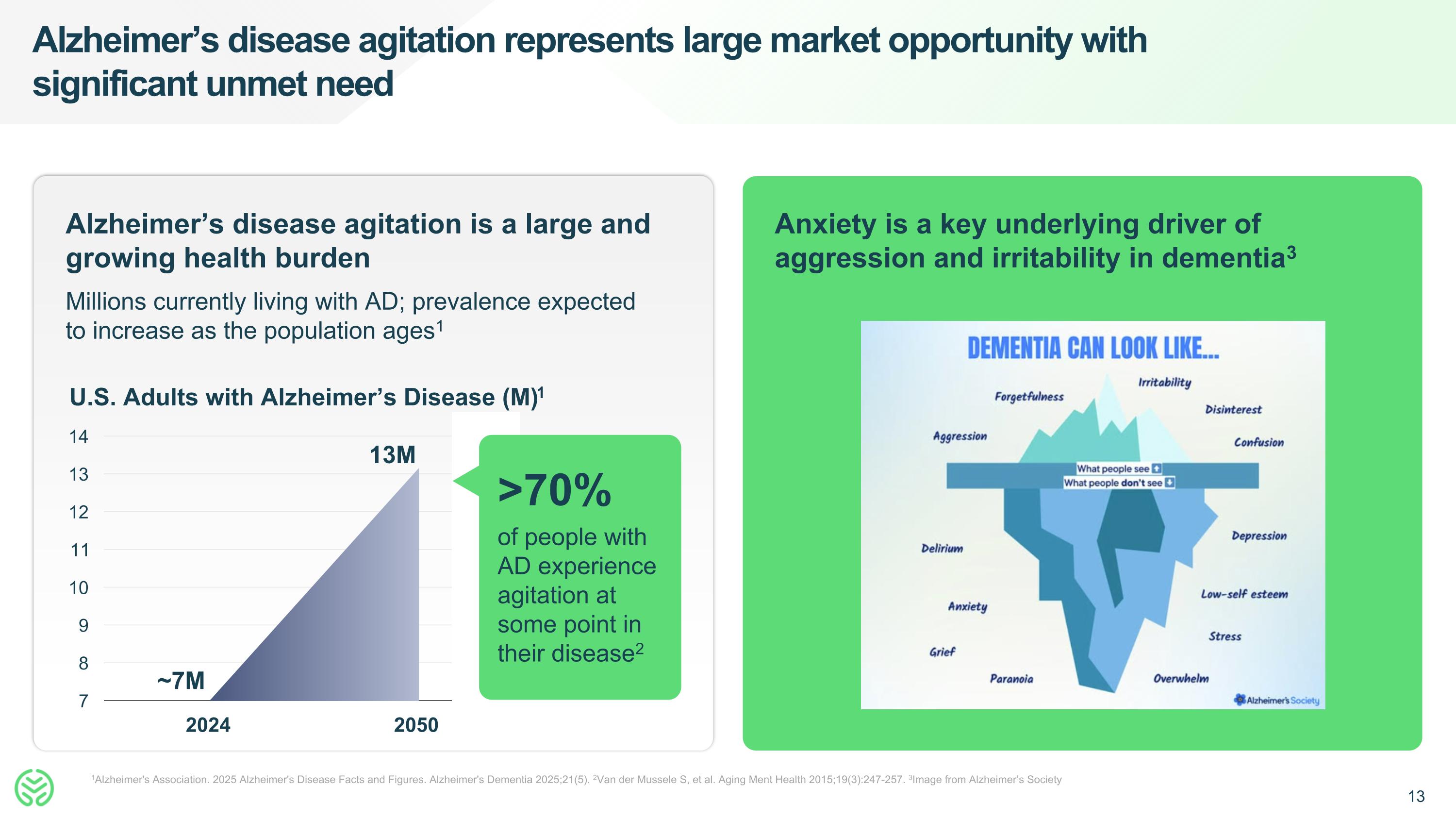
Alzheimer’s disease agitation represents large market opportunity with significant unmet need 1Alzheimer's Association. 2025 Alzheimer's Disease Facts and Figures. Alzheimer's Dementia 2025;21(5). 2Van der Mussele S, et al. Aging Ment Health 2015;19(3):247-257. 3Image from Alzheimer’s Society Alzheimer’s disease agitation is a large and growing health burden Millions currently living with AD; prevalence expected to increase as the population ages1 ~7M 13M U.S. Adults with Alzheimer’s Disease (M)1 >70% of people with AD experience agitation at some point in their disease2 Anxiety is a key underlying driver of aggression and irritability in dementia3
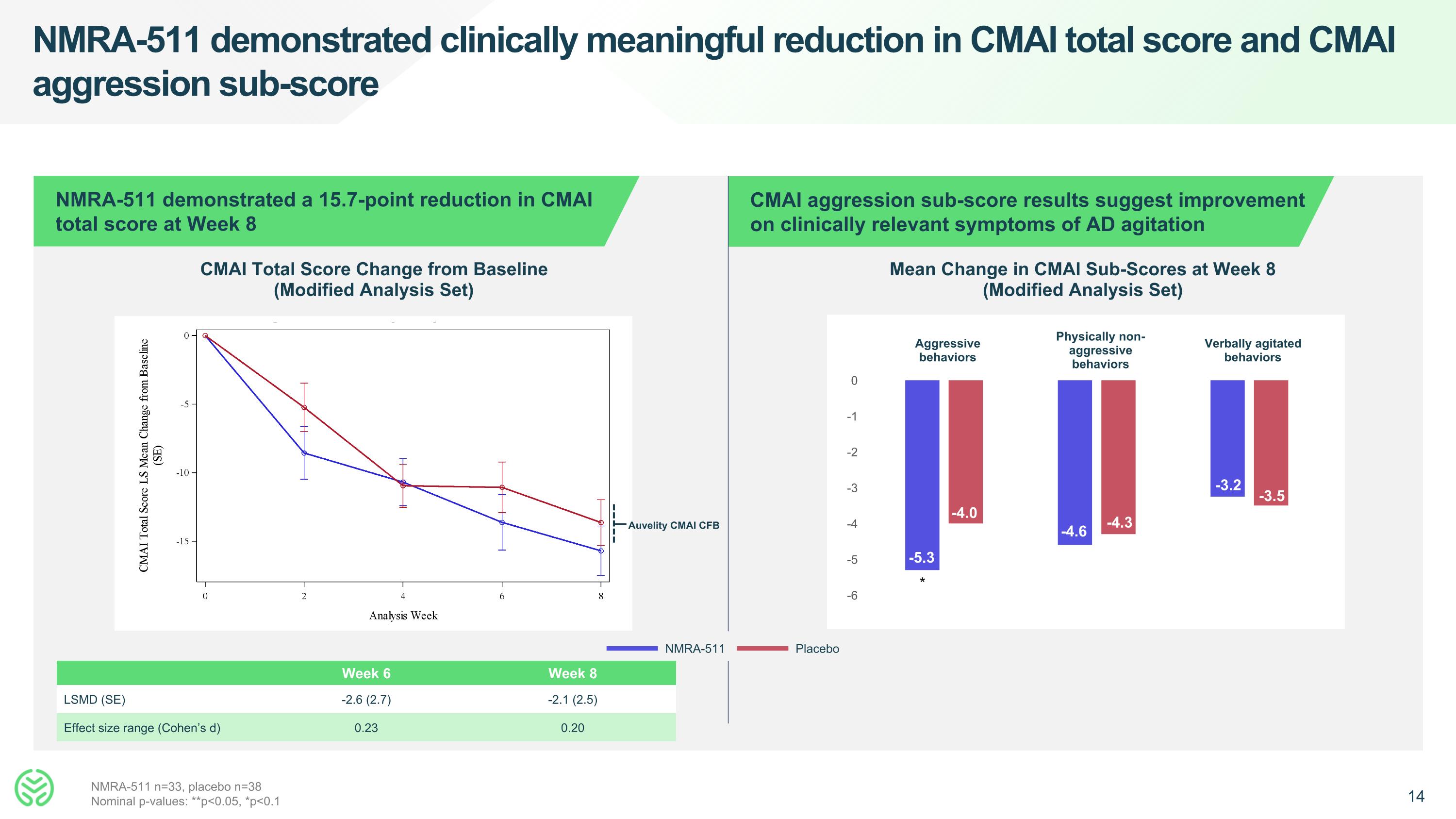
NMRA-511 demonstrated clinically meaningful reduction in CMAI total score and CMAI aggression sub-score NMRA-511 demonstrated a 15.7-point reduction in CMAI total score at Week 8 CMAI aggression sub-score results suggest improvement on clinically relevant symptoms of AD agitation CMAI Total Score Change from Baseline (Modified Analysis Set) Mean Change in CMAI Sub-Scores at Week 8 (Modified Analysis Set) Week 6 Week 8 LSMD (SE) -2.6 (2.7) -2.1 (2.5) Effect size range (Cohen’s d) 0.23 0.20 NMRA-511 Placebo Aggressive behaviors Physically non-aggressive behaviors Verbally agitated behaviors -5.3 -4.0 NMRA-511 n=33, placebo n=38 Nominal p-values: **p<0.05, *p<0.1 -4.6 -4.3 * -3.2 -3.5 Auvelity CMAI CFB

15