

Interim Clinical Data from the Phase 1/2 HEROIC-PKP2 Trial of LX2020 for Arrhythmogenic Cardiomyopathy Caused by Mutations in the PKP2 Gene January 12, 2026 .2

Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, statements regarding Lexeo’s expectations and plans regarding its current product candidates and programs, including statements regarding the timing, progress and results of preclinical and clinical trials of Lexeo’s gene therapy product candidates and the anticipated benefits of its current product candidates. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the SEC), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward looking statements as a result of many factors, including but not limited to: risks and uncertainties related to expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025, filed with the SEC on November 5, 2025, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law.
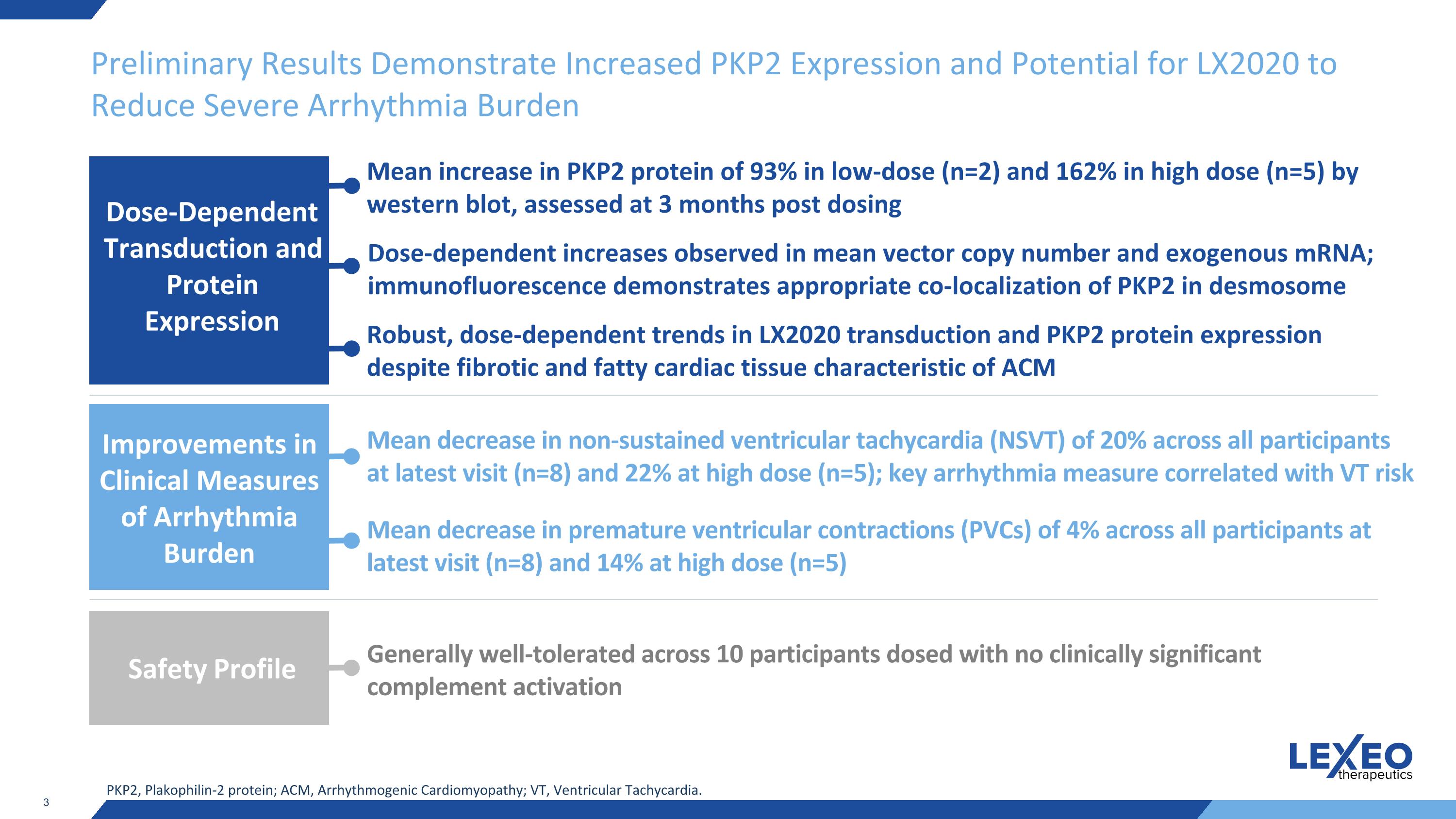
Preliminary Results Demonstrate Increased PKP2 Expression and Potential for LX2020 to Reduce Severe Arrhythmia Burden Mean decrease in non-sustained ventricular tachycardia (NSVT) of 20% across all participants at latest visit (n=8) and 22% at high dose (n=5); key arrhythmia measure correlated with VT risk Mean decrease in premature ventricular contractions (PVCs) of 4% across all participants at latest visit (n=8) and 14% at high dose (n=5) Improvements in Clinical Measures of Arrhythmia Burden PKP2, Plakophilin-2 protein; ACM, Arrhythmogenic Cardiomyopathy; VT, Ventricular Tachycardia. Generally well-tolerated across 10 participants dosed with no clinically significant complement activation Safety Profile Robust, dose-dependent trends in LX2020 transduction and PKP2 protein expression despite fibrotic and fatty cardiac tissue characteristic of ACM Mean increase in PKP2 protein of 93% in low-dose (n=2) and 162% in high dose (n=5) by western blot, assessed at 3 months post dosing Dose-dependent increases observed in mean vector copy number and exogenous mRNA; immunofluorescence demonstrates appropriate co-localization of PKP2 in desmosome Dose-Dependent Transduction and Protein Expression
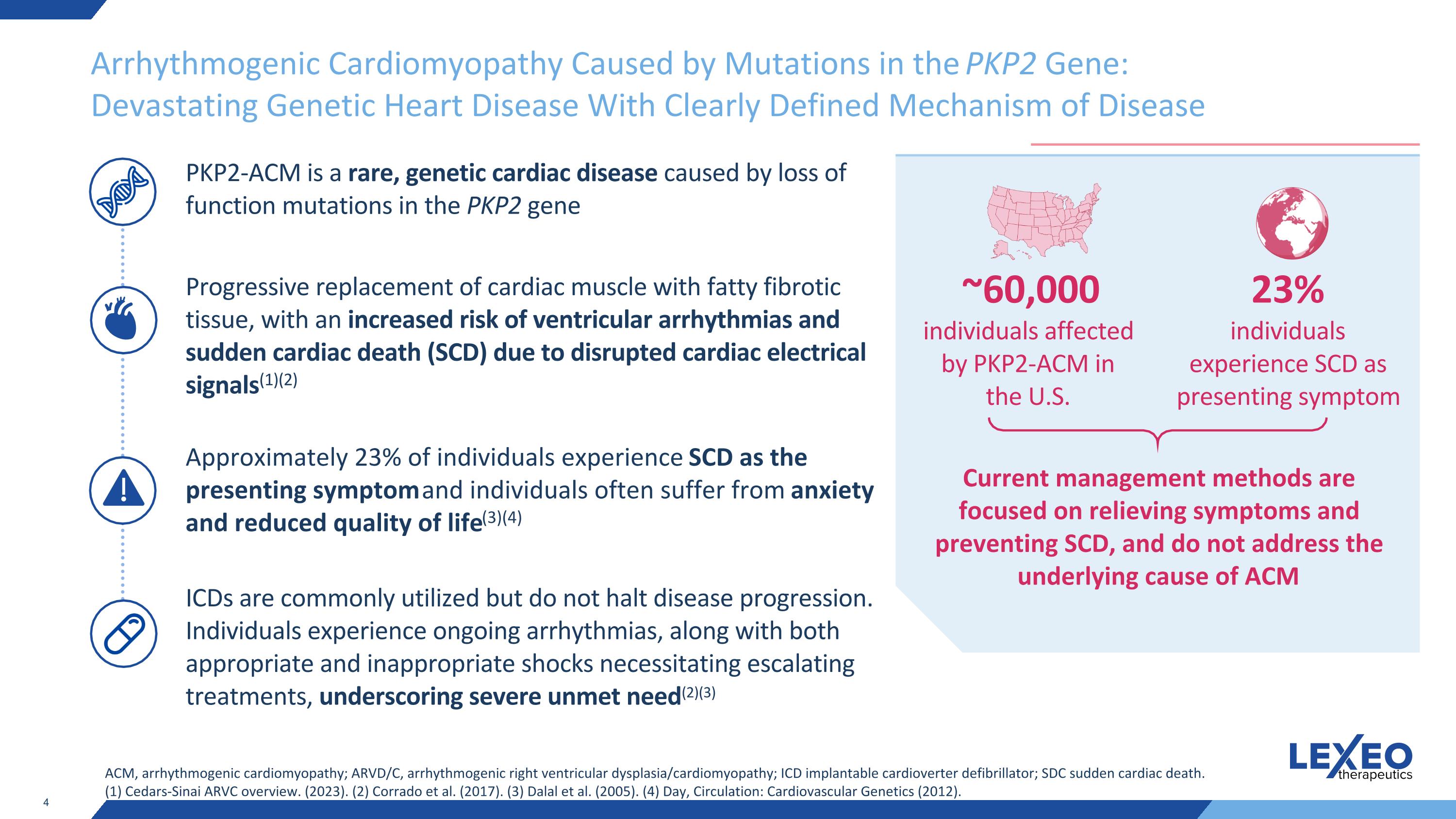
Arrhythmogenic Cardiomyopathy Caused by Mutations in the PKP2 Gene: Devastating Genetic Heart Disease With Clearly Defined Mechanism of Disease ACM, arrhythmogenic cardiomyopathy; ARVD/C, arrhythmogenic right ventricular dysplasia/cardiomyopathy; ICD implantable cardioverter defibrillator; SDC sudden cardiac death. (1) Cedars-Sinai ARVC overview. (2023). (2) Corrado et al. (2017). (3) Dalal et al. (2005). (4) Day, Circulation: Cardiovascular Genetics (2012). PKP2-ACM is a rare, genetic cardiac disease caused by loss of function mutations in the PKP2 gene Progressive replacement of cardiac muscle with fatty fibrotic tissue, with an increased risk of ventricular arrhythmias and sudden cardiac death (SCD) due to disrupted cardiac electrical signals(1)(2) Approximately 23% of individuals experience SCD as the presenting symptom and individuals often suffer from anxiety and reduced quality of life(3)(4) ICDs are commonly utilized but do not halt disease progression. Individuals experience ongoing arrhythmias, along with both appropriate and inappropriate shocks necessitating escalating treatments, underscoring severe unmet need(2)(3) Current management methods are focused on relieving symptoms and preventing SCD, and do not address the underlying cause of ACM 23% individuals experience SCD as presenting symptom ~60,000 individuals affected by PKP2-ACM in the U.S.
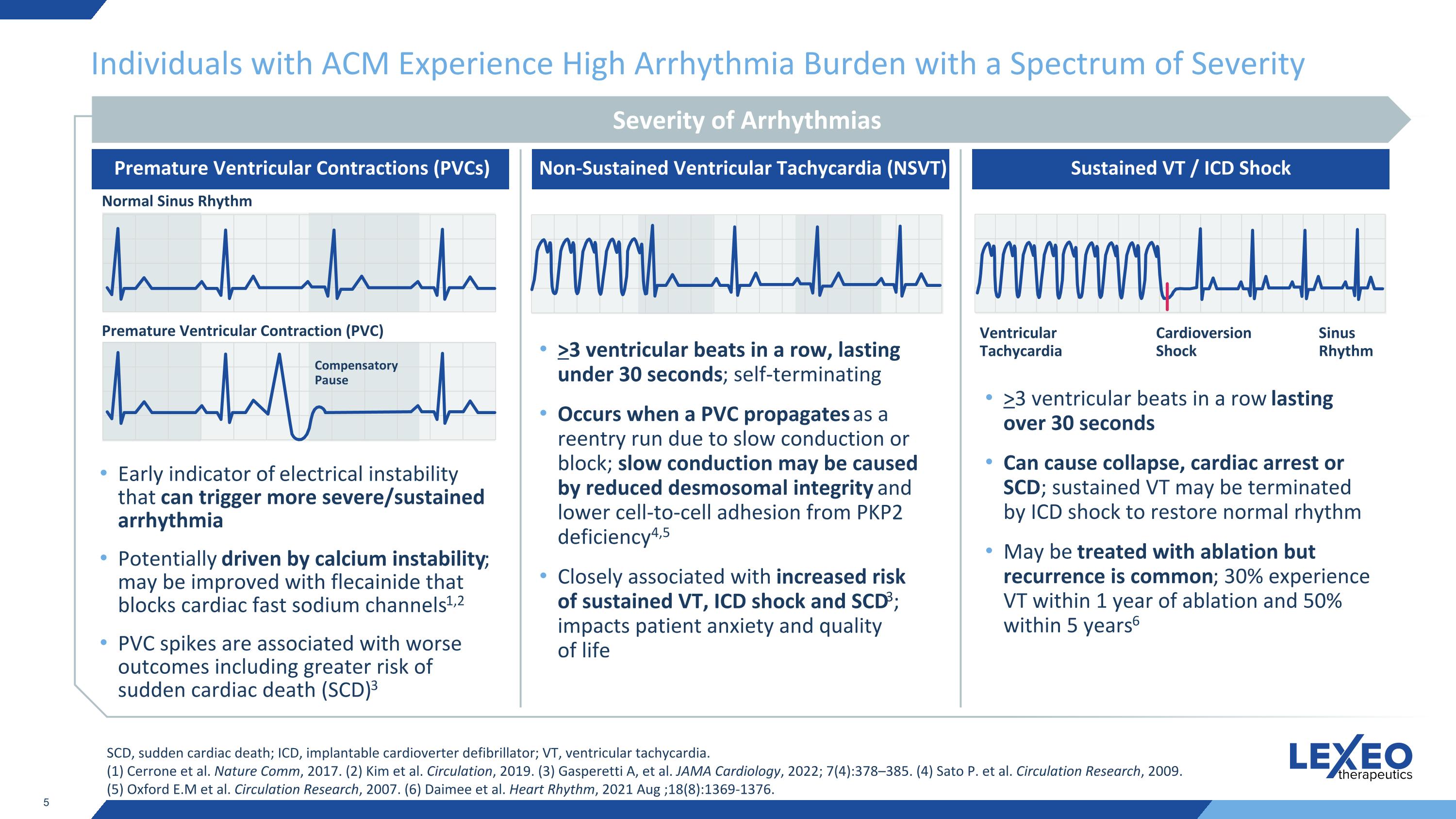
Individuals with ACM Experience High Arrhythmia Burden with a Spectrum of Severity Early indicator of electrical instability that can trigger more severe/sustained arrhythmia Potentially driven by calcium instability; may be improved with flecainide that blocks cardiac fast sodium channels1,2 PVC spikes are associated with worse outcomes including greater risk of sudden cardiac death (SCD)3 >3 ventricular beats in a row, lasting under 30 seconds; self-terminating Occurs when a PVC propagates as a reentry run due to slow conduction or block; slow conduction may be caused by reduced desmosomal integrity and lower cell-to-cell adhesion from PKP2 deficiency4,5 Closely associated with increased risk of sustained VT, ICD shock and SCD3; impacts patient anxiety and quality of life >3 ventricular beats in a row lasting over 30 seconds Can cause collapse, cardiac arrest or SCD; sustained VT may be terminated by ICD shock to restore normal rhythm May be treated with ablation but recurrence is common; 30% experience VT within 1 year of ablation and 50% within 5 years6 SCD, sudden cardiac death; ICD, implantable cardioverter defibrillator; VT, ventricular tachycardia. (1) Cerrone et al. Nature Comm, 2017. (2) Kim et al. Circulation, 2019. (3) Gasperetti A, et al. JAMA Cardiology, 2022; 7(4):378–385. (4) Sato P. et al. Circulation Research, 2009. (5) Oxford E.M et al. Circulation Research, 2007. (6) Daimee et al. Heart Rhythm, 2021 Aug ;18(8):1369-1376. Severity of Arrhythmias Premature Ventricular Contractions (PVCs) Non-Sustained Ventricular Tachycardia (NSVT) Sustained VT / ICD Shock Normal Sinus Rhythm Premature Ventricular Contraction (PVC) Compensatory Pause Ventricular Tachycardia Cardioversion Shock Sinus Rhythm
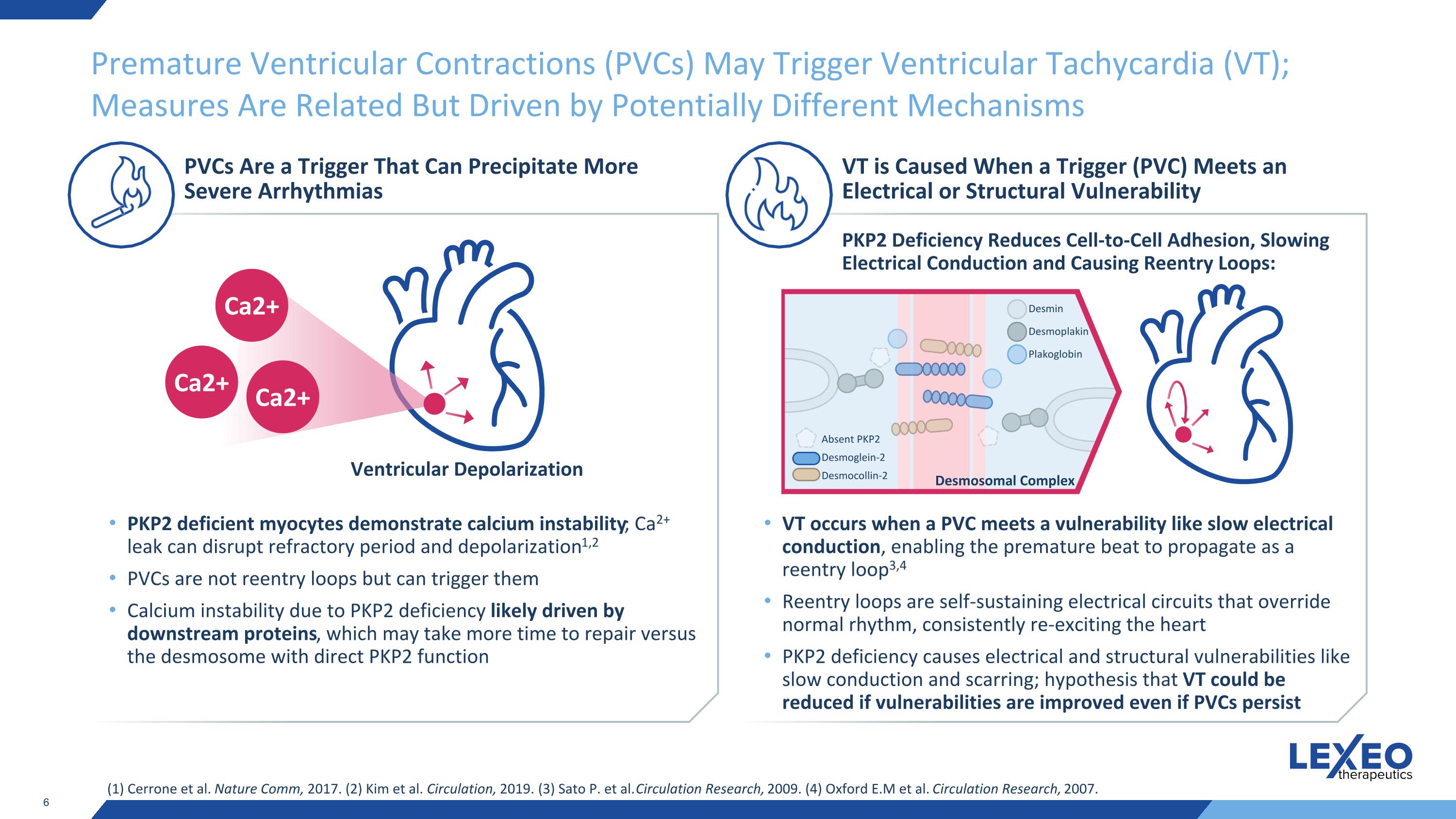
Premature Ventricular Contractions (PVCs) May Trigger Ventricular Tachycardia (VT); Measures Are Related But Driven by Potentially Different Mechanisms (1) Cerrone et al. Nature Comm, 2017. (2) Kim et al. Circulation, 2019. (3) Sato P. et al. Circulation Research, 2009. (4) Oxford E.M et al. Circulation Research, 2007. PVCs Are a Trigger That Can Precipitate More Severe Arrhythmias VT is Caused When a Trigger (PVC) Meets an Electrical or Structural Vulnerability PKP2 deficient myocytes demonstrate calcium instability; Ca2+ leak can disrupt refractory period and depolarization1,2 PVCs are not reentry loops but can trigger them Calcium instability due to PKP2 deficiency likely driven by downstream proteins, which may take more time to repair versus the desmosome with direct PKP2 function VT occurs when a PVC meets a vulnerability like slow electrical conduction, enabling the premature beat to propagate as a reentry loop3,4 Reentry loops are self-sustaining electrical circuits that override normal rhythm, consistently re-exciting the heart PKP2 deficiency causes electrical and structural vulnerabilities like slow conduction and scarring; hypothesis that VT could be reduced if vulnerabilities are improved even if PVCs persist Ventricular Depolarization Ca2+ Ca2+ Ca2+ PKP2 Deficiency Reduces Cell-to-Cell Adhesion, Slowing Electrical Conduction and Causing Reentry Loops: Absent PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin Desmosomal Complex
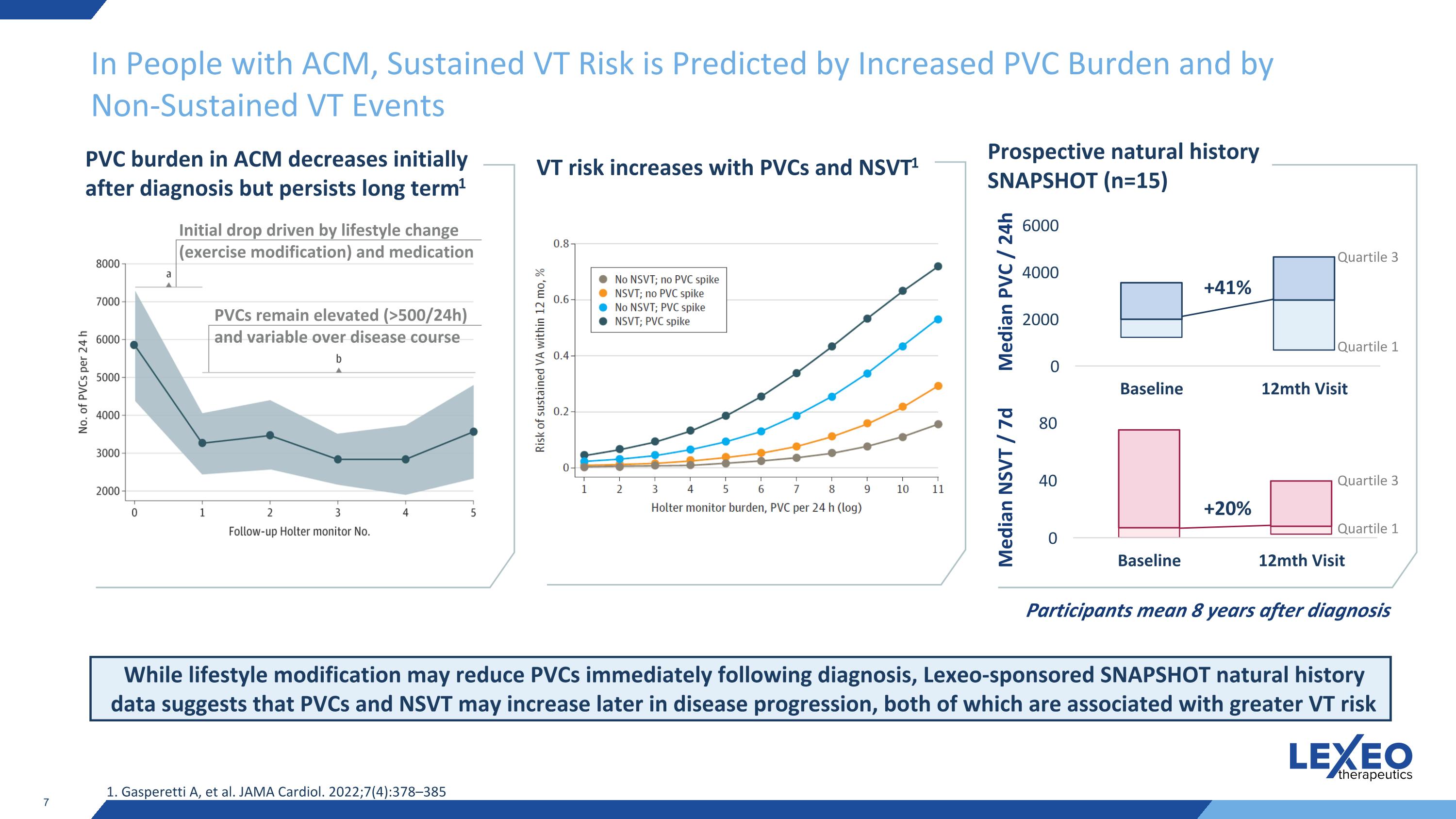
In People with ACM, Sustained VT Risk is Predicted by Increased PVC Burden and by Non-Sustained VT Events PVC burden in ACM decreases initially after diagnosis but persists long term1 Initial drop driven by lifestyle change (exercise modification) and medication PVCs remain elevated (>500/24h) and variable over disease course While lifestyle modification may reduce PVCs immediately following diagnosis, Lexeo-sponsored SNAPSHOT natural history data suggests that PVCs and NSVT may increase later in disease progression, both of which are associated with greater VT risk 1. Gasperetti A, et al. JAMA Cardiol. 2022;7(4):378–385 VT risk increases with PVCs and NSVT1 Prospective natural history SNAPSHOT (n=15) Participants mean 8 years after diagnosis Median PVC / 24h Median NSVT / 7d Quartile 1 +41% Quartile 3 +20% Quartile 1 Quartile 3
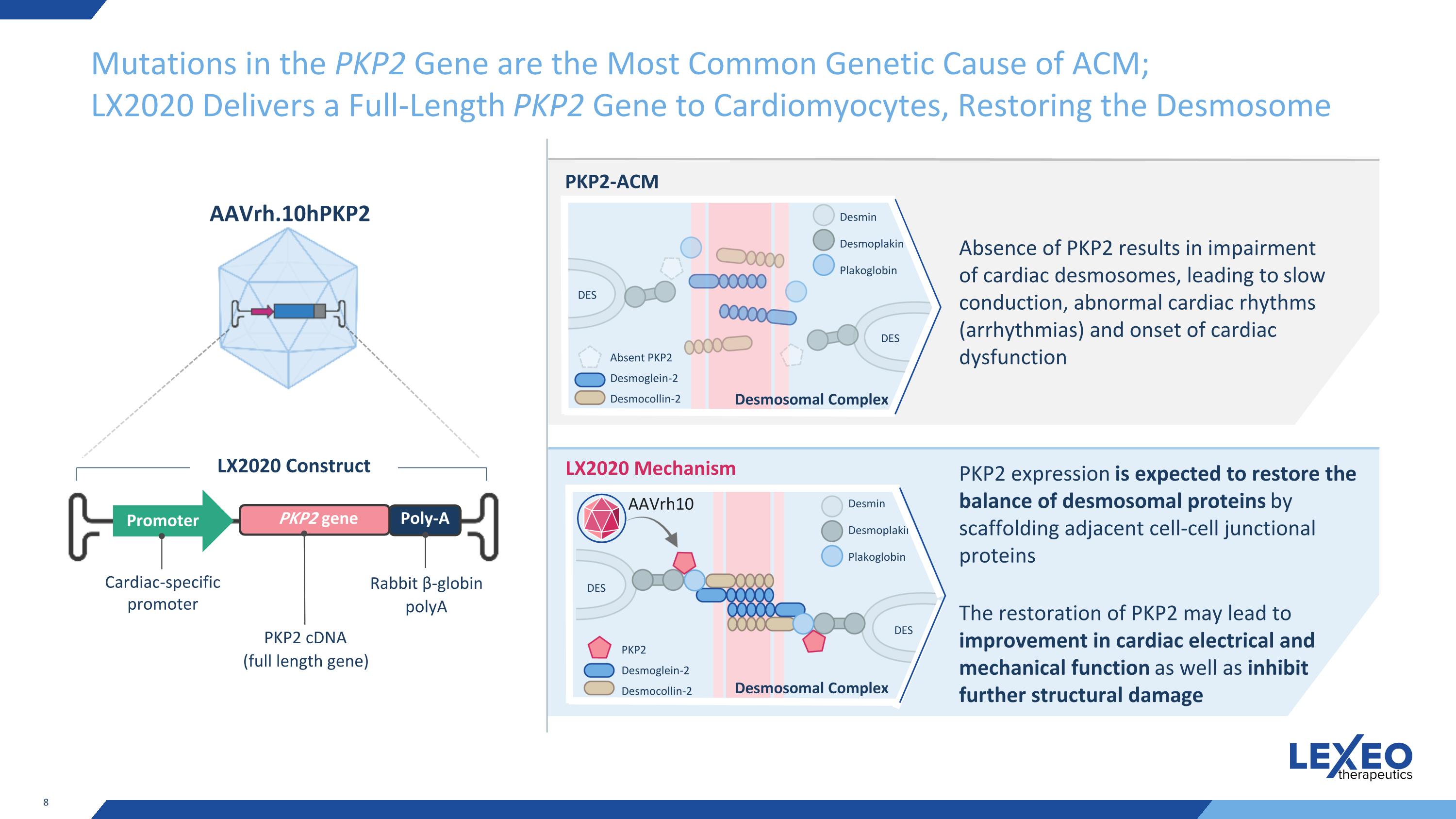
Mutations in the PKP2 Gene are the Most Common Genetic Cause of ACM; LX2020 Delivers a Full-Length PKP2 Gene to Cardiomyocytes, Restoring the Desmosome Cardiac-specific promoter Promoter PKP2 gene Poly-A Rabbit β-globin polyA LX2020 Construct AAVrh.10hPKP2 PKP2-ACM LX2020 Mechanism Absence of PKP2 results in impairment of cardiac desmosomes, leading to slow conduction, abnormal cardiac rhythms (arrhythmias) and onset of cardiac dysfunction DES DES Absent PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin DES DES PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin PKP2 expression is expected to restore the balance of desmosomal proteins by scaffolding adjacent cell-cell junctional proteins The restoration of PKP2 may lead to improvement in cardiac electrical and mechanical function as well as inhibit further structural damage AAVrh10 PKP2 cDNA (full length gene) Desmosomal Complex Desmosomal Complex
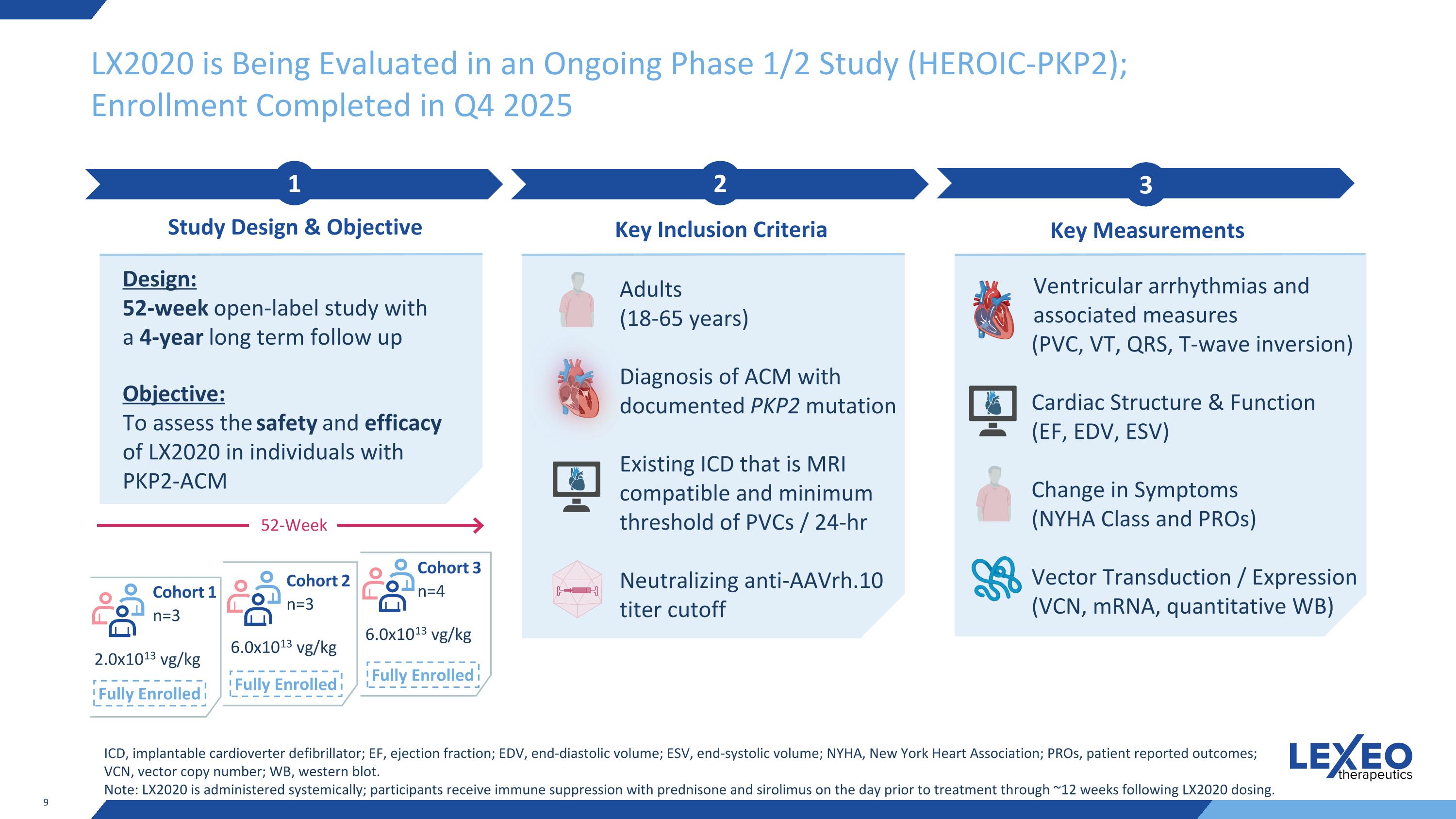
LX2020 is Being Evaluated in an Ongoing Phase 1/2 Study (HEROIC-PKP2); Enrollment Completed in Q4 2025 2 Key Inclusion Criteria 3 Key Measurements 1 Study Design & Objective Design: 52-week open-label study with a 4-year long term follow up Objective: To assess the safety and efficacy of LX2020 in individuals with PKP2-ACM Adults (18-65 years) Diagnosis of ACM with documented PKP2 mutation Existing ICD that is MRI compatible and minimum threshold of PVCs / 24-hr Neutralizing anti-AAVrh.10 titer cutoff Ventricular arrhythmias and associated measures (PVC, VT, QRS, T-wave inversion) Cardiac Structure & Function (EF, EDV, ESV) Change in Symptoms (NYHA Class and PROs) Vector Transduction / Expression (VCN, mRNA, quantitative WB) ICD, implantable cardioverter defibrillator; EF, ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume; NYHA, New York Heart Association; PROs, patient reported outcomes; VCN, vector copy number; WB, western blot. Note: LX2020 is administered systemically; participants receive immune suppression with prednisone and sirolimus on the day prior to treatment through ~12 weeks following LX2020 dosing. 2.0x1013 vg/kg 6.0x1013 vg/kg 6.0x1013 vg/kg Cohort 1 n=3 Cohort 2 n=3 Cohort 3 n=4 52-Week Fully Enrolled Fully Enrolled Fully Enrolled
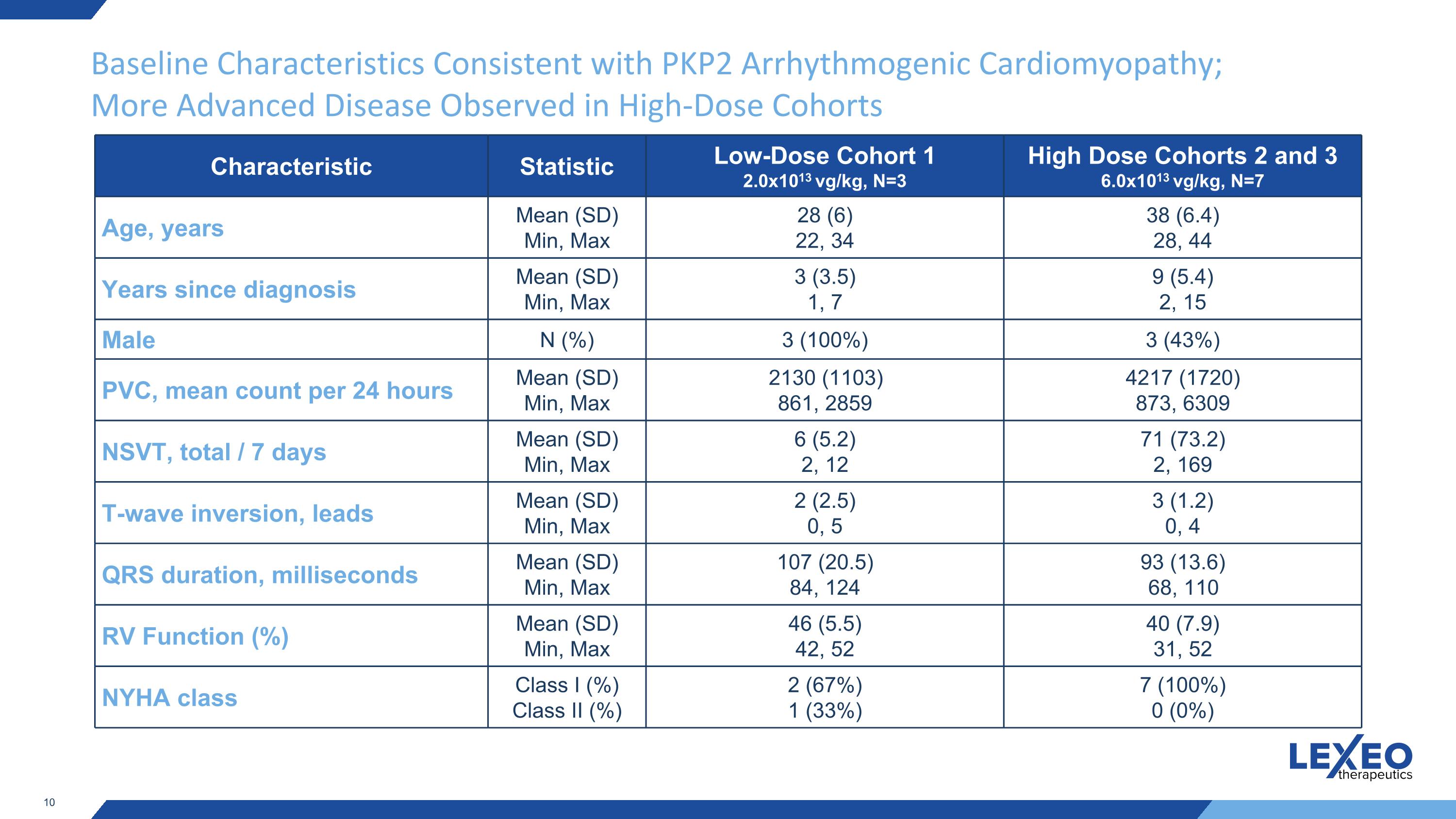
Baseline Characteristics Consistent with PKP2 Arrhythmogenic Cardiomyopathy; More Advanced Disease Observed in High-Dose Cohorts Characteristic Statistic Low-Dose Cohort 1 2.0x1013 vg/kg, N=3 High Dose Cohorts 2 and 3 6.0x1013 vg/kg, N=7 Age, years Mean (SD) Min, Max 28 (6) 22, 34 38 (6.4) 28, 44 Years since diagnosis Mean (SD) Min, Max 3 (3.5) 1, 7 9 (5.4) 2, 15 Male N (%) 3 (100%) 3 (43%) PVC, mean count per 24 hours Mean (SD) Min, Max 2130 (1103) 861, 2859 4217 (1720) 873, 6309 NSVT, total / 7 days Mean (SD) Min, Max 6 (5.2) 2, 12 71 (73.2) 2, 169 T-wave inversion, leads Mean (SD) Min, Max 2 (2.5) 0, 5 3 (1.2) 0, 4 QRS duration, milliseconds Mean (SD) Min, Max 107 (20.5) 84, 124 93 (13.6) 68, 110 RV Function (%) Mean (SD) Min, Max 46 (5.5) 42, 52 40 (7.9) 31, 52 NYHA class Class I (%) Class II (%) 2 (67%) 1 (33%) 7 (100%) 0 (0%)
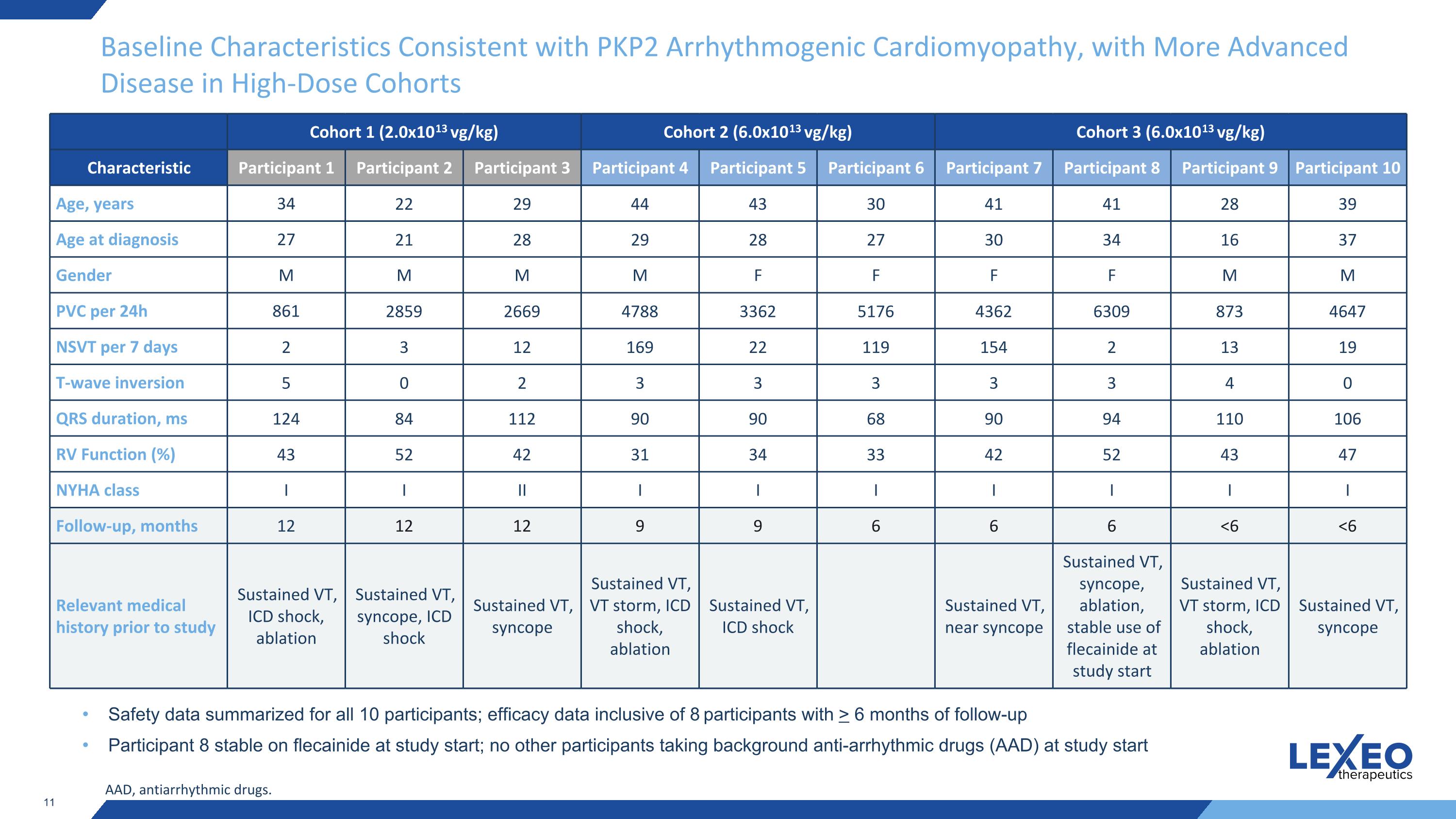
Baseline Characteristics Consistent with PKP2 Arrhythmogenic Cardiomyopathy, with More Advanced Disease in High-Dose Cohorts Cohort 1 (2.0x1013 vg/kg) Cohort 2 (6.0x1013 vg/kg) Cohort 3 (6.0x1013 vg/kg) Characteristic Participant 1 Participant 2 Participant 3 Participant 4 Participant 5 Participant 6 Participant 7 Participant 8 Participant 9 Participant 10 Age, years 34 22 29 44 43 30 41 41 28 39 Age at diagnosis 27 21 28 29 28 27 30 34 16 37 Gender M M M M F F F F M M PVC per 24h 861 2859 2669 4788 3362 5176 4362 6309 873 4647 NSVT per 7 days 2 3 12 169 22 119 154 2 13 19 T-wave inversion 5 0 2 3 3 3 3 3 4 0 QRS duration, ms 124 84 112 90 90 68 90 94 110 106 RV Function (%) 43 52 42 31 34 33 42 52 43 47 NYHA class I I II I I I I I I I Follow-up, months 12 12 12 9 9 6 6 6 <6 <6 Relevant medical history prior to study Sustained VT, ICD shock, ablation Sustained VT, syncope, ICD shock Sustained VT, syncope Sustained VT, VT storm, ICD shock, ablation Sustained VT, ICD shock Sustained VT, near syncope Sustained VT, syncope, ablation, stable use of flecainide at study start Sustained VT, VT storm, ICD shock, ablation Sustained VT, syncope Safety data summarized for all 10 participants; efficacy data inclusive of 8 participants with > 6 months of follow-up Participant 8 stable on flecainide at study start; no other participants taking background anti-arrhythmic drugs (AAD) at study start AAD, antiarrhythmic drugs.

Treatment with LX2020 Has Been Well Tolerated to Date LX2020 generally well tolerated across ten participants dosed No clinically significant complement activation Elevations in liver function tests (LFT) observed in five participants at the high-dose, treated successfully with modified immunosuppression per the trial protocol: Three participants’ elevations occurred following steroid tapering and resolved with re-introduction of low-dose prednisone Two participants’ elevations occurred prior to steroid tapering and resolved with increased prednisone and sirolimus treatment All elevations have since resolved without other complications or hospitalization, and no other medications were required for resolution No participants discontinued from study One previously disclosed Grade 3 serious adverse event of sustained ventricular tachycardia (VT) was observed three months after dosing in a single participant in the high dose cohort and assessed as possibly treatment related. The participant was successfully treated with anti-arrhythmic medication and discharged with no additional intervention required Interim Safety Update: 10 Participants Dosed
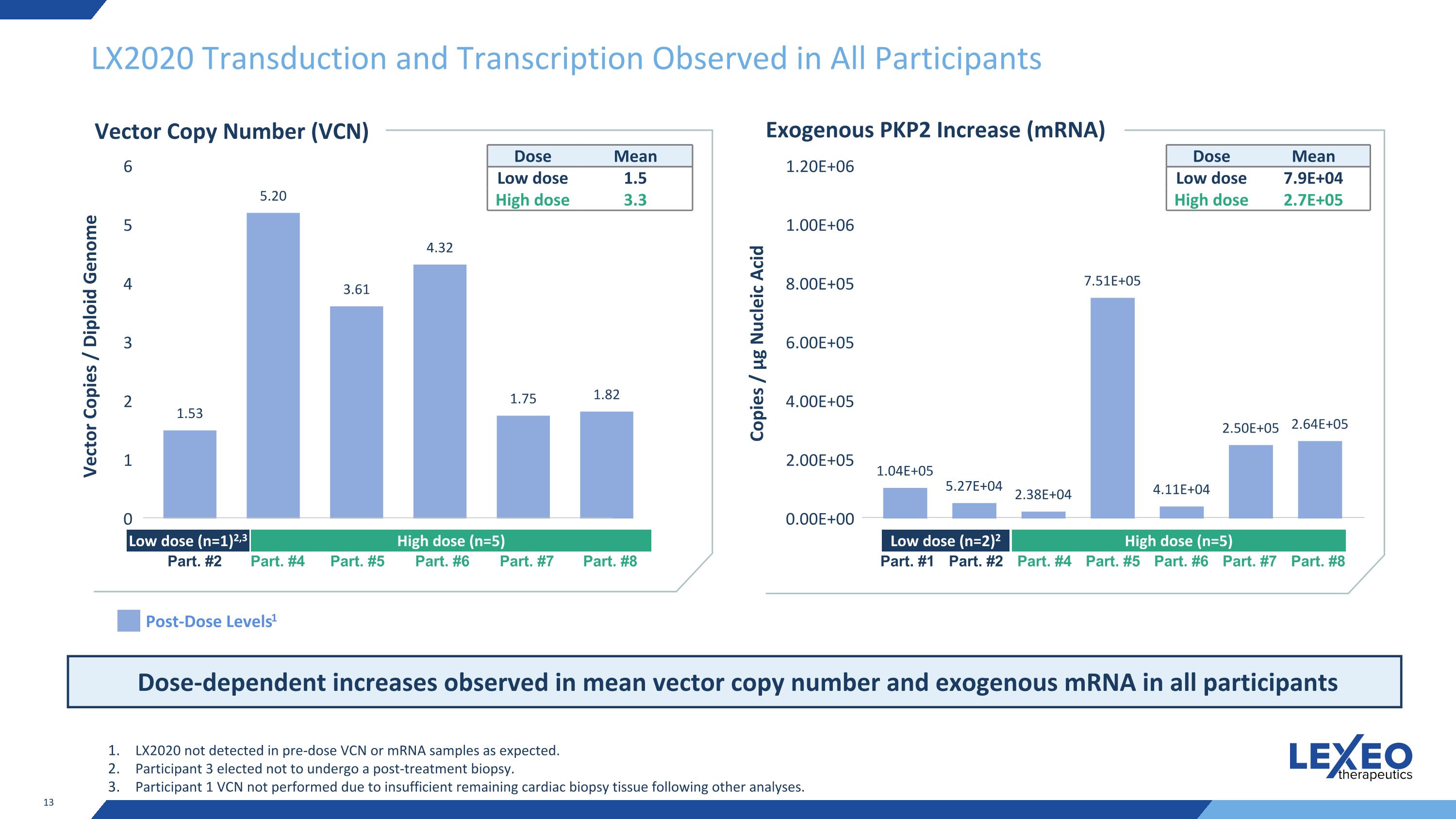
LX2020 Transduction and Transcription Observed in All Participants Post-Dose Levels1 Vector Copies / Diploid Genome LX2020 not detected in pre-dose VCN or mRNA samples as expected. Participant 3 elected not to undergo a post-treatment biopsy. Participant 1 VCN not performed due to insufficient remaining cardiac biopsy tissue following other analyses. Vector Copy Number (VCN) Low dose (n=1)2,3 High dose (n=5) Part. #2 Part. #4 Part. #5 Part. #6 Part. #7 Part. #8 Dose Mean Low dose 1.5 High dose 3.3 Dose-dependent increases observed in mean vector copy number and exogenous mRNA in all participants Exogenous PKP2 Increase (mRNA) Copies / μg Nucleic Acid Low dose (n=2)2 High dose (n=5) Part. #1 Part. #2 Part. #4 Part. #5 Part. #6 Part. #7 Part. #8 Dose Mean Low dose 7.9E+04 High dose 2.7E+05
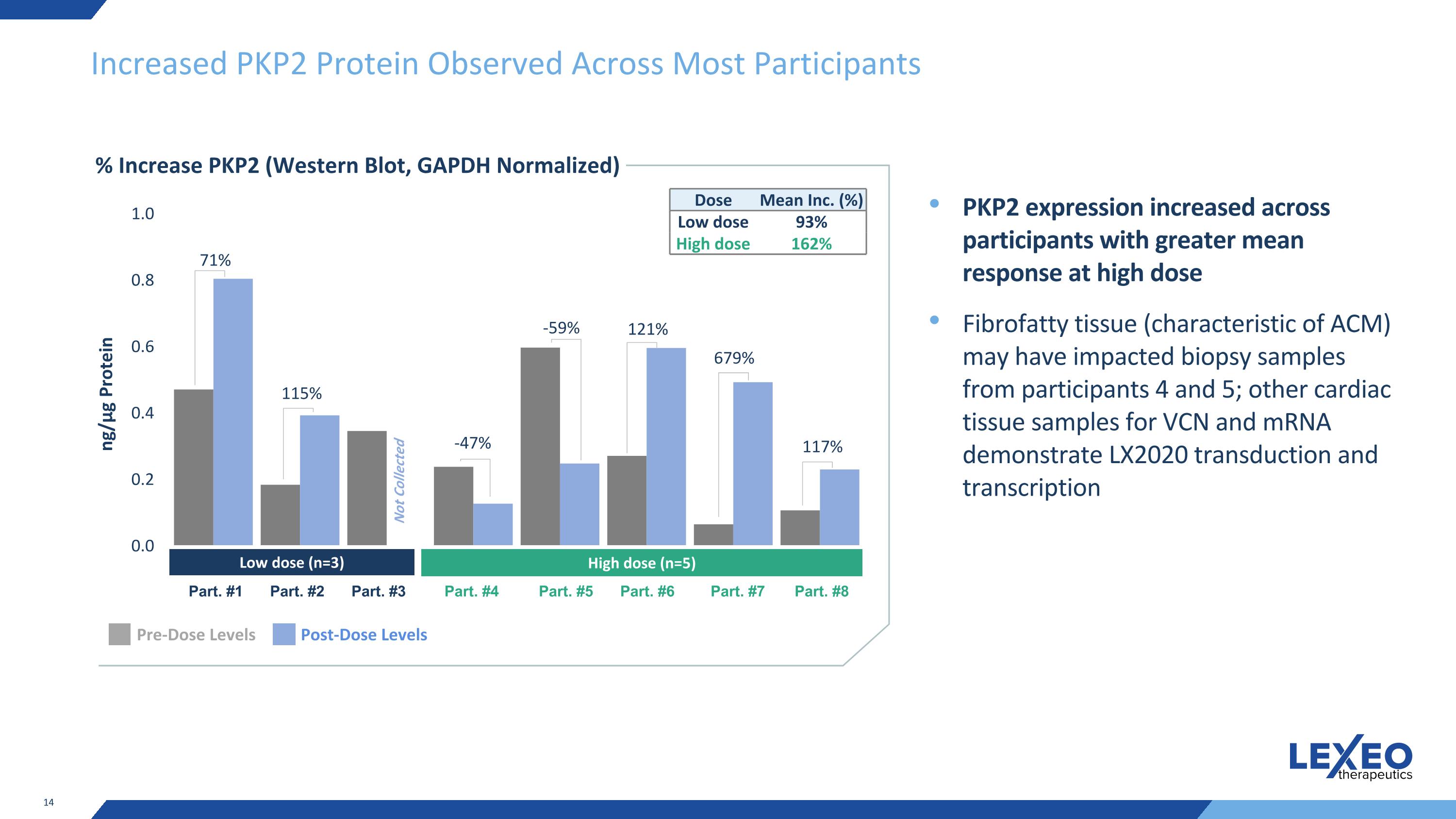
Increased PKP2 Protein Observed Across Most Participants % Increase PKP2 (Western Blot, GAPDH Normalized) PKP2 expression increased across participants with greater mean response at high dose Fibrofatty tissue (characteristic of ACM) may have impacted biopsy samples from participants 4 and 5; other cardiac tissue samples for VCN and mRNA demonstrate LX2020 transduction and transcription ng/μg Protein Dose Mean Inc. (%) Low dose 93% High dose 162% Pre-Dose Levels Post-Dose Levels Low dose (n=3) High dose (n=5) Part. #1 Part. #2 Part. #3 Part. #4 Part. #5 Part. #6 Part. #7 Part. #8 Not Collected 71% 115% -47% -59% 121% 679% 117%
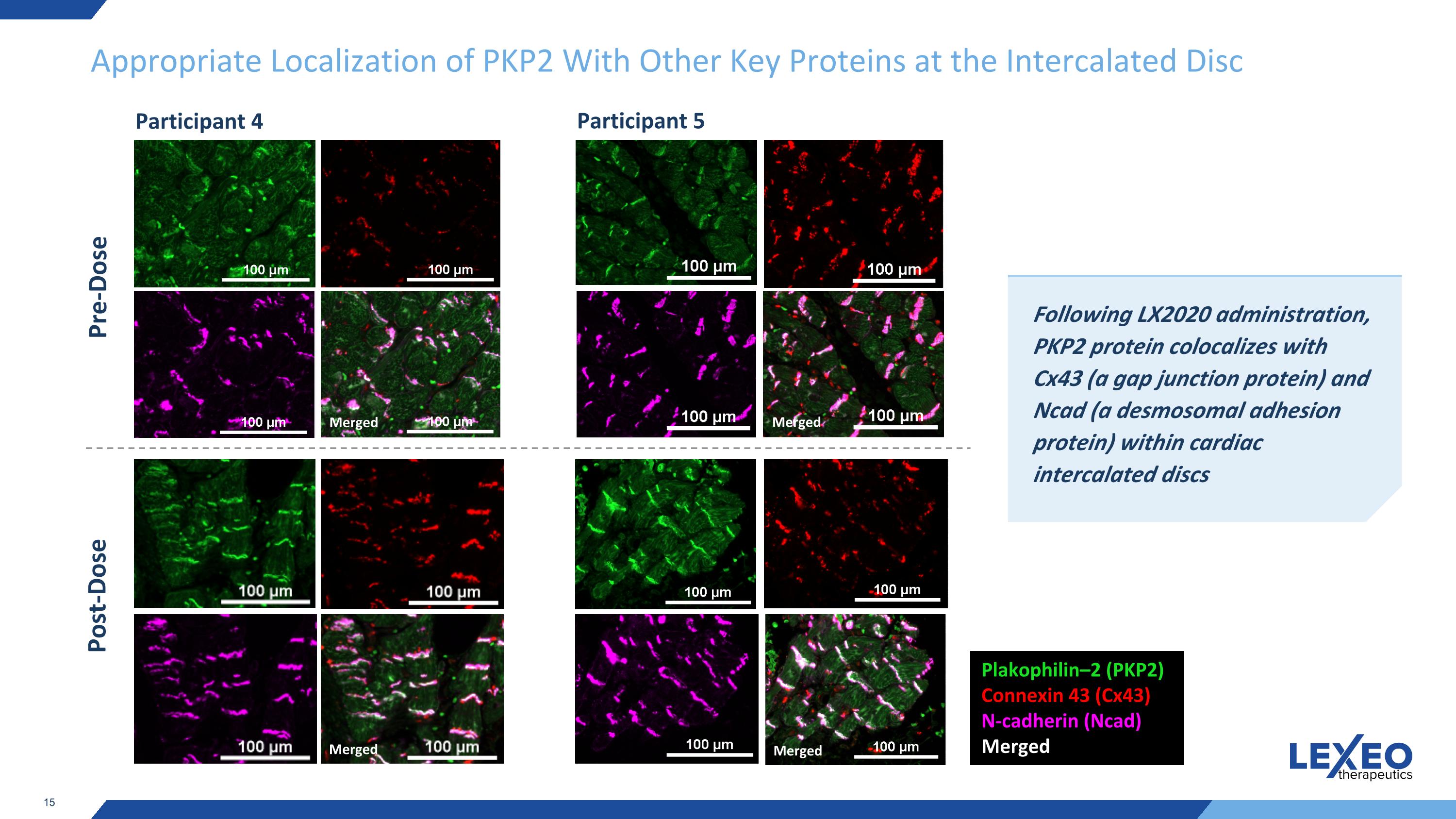
Appropriate Localization of PKP2 With Other Key Proteins at the Intercalated Disc Pre-Dose Following LX2020 administration, PKP2 protein colocalizes with Cx43 (a gap junction protein) and Ncad (a desmosomal adhesion protein) within cardiac intercalated discs Plakophilin–2 (PKP2) Connexin 43 (Cx43) N-cadherin (Ncad) Merged Merged Post-Dose Merged Merged Merged Participant 4 Participant 5
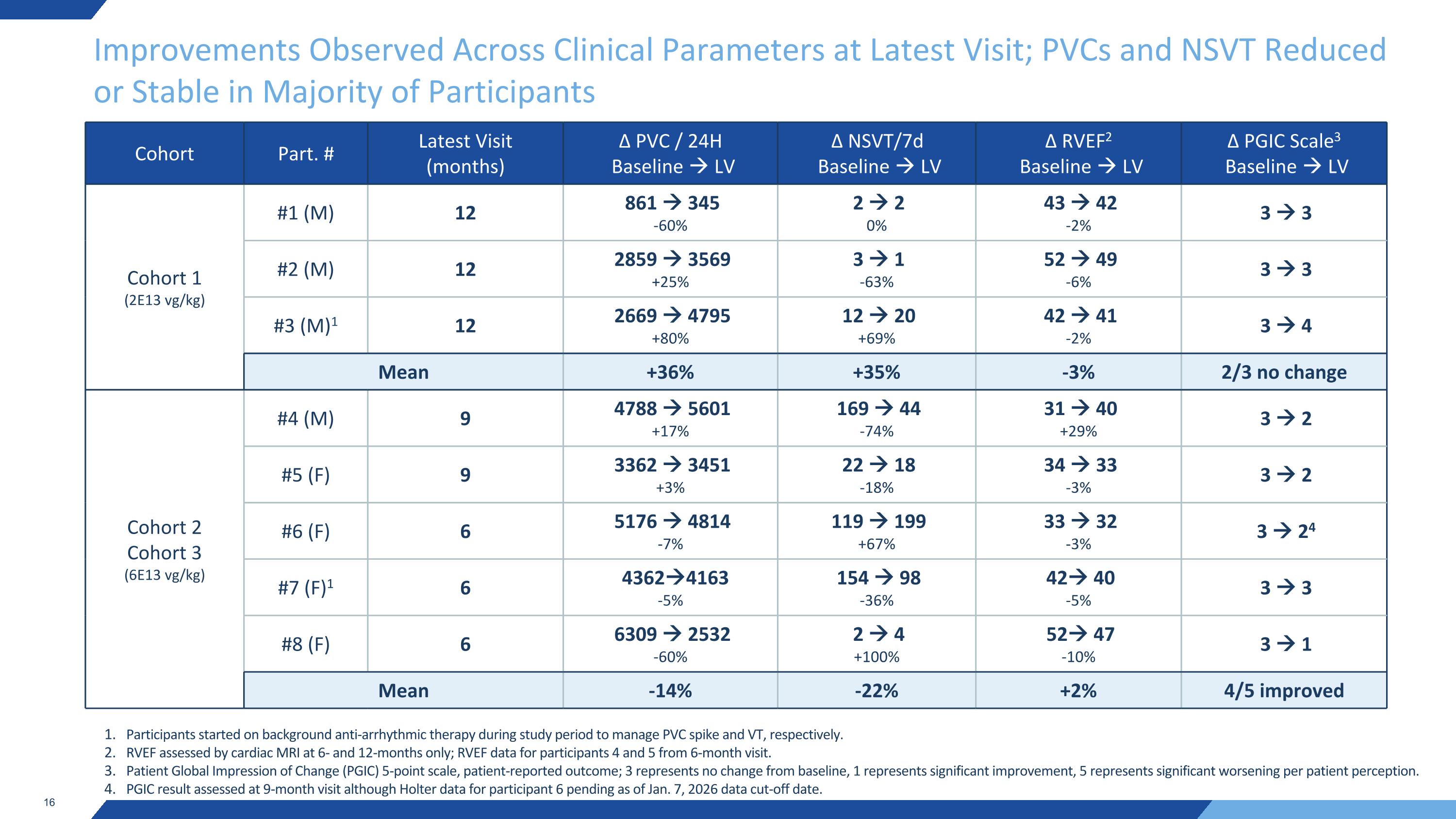
Improvements Observed Across Clinical Parameters at Latest Visit; PVCs and NSVT Reduced or Stable in Majority of Participants Cohort Part. # Latest Visit (months) ∆ PVC / 24H Baseline LV ∆ NSVT/7d Baseline LV ∆ RVEF2 Baseline LV ∆ PGIC Scale3 Baseline LV Cohort 1 (2E13 vg/kg) #1 (M) 12 861 345 -60% 2 2 0% 43 42 -2% 3 3 #2 (M) 12 2859 3569 +25% 3 1 -63% 52 49 -6% 3 3 #3 (M)1 12 2669 4795 +80% 12 20 +69% 42 41 -2% 3 4 Mean +36% +35% -3% 2/3 no change Cohort 2 Cohort 3 (6E13 vg/kg) #4 (M) 9 4788 5601 +17% 169 44 -74% 31 40 +29% 3 2 #5 (F) 9 3362 3451 +3% 22 18 -18% 34 33 -3% 3 2 #6 (F) 6 5176 4814 -7% 119 199 +67% 33 32 -3% 3 24 #7 (F)1 6 43624163 -5% 154 98 -36% 42 40 -5% 3 3 #8 (F) 6 6309 2532 -60% 2 4 +100% 52 47 -10% 3 1 Mean -14% -22% +2% 4/5 improved Participants started on background anti-arrhythmic therapy during study period to manage PVC spike and VT, respectively. RVEF assessed by cardiac MRI at 6- and 12-months only; RVEF data for participants 4 and 5 from 6-month visit. Patient Global Impression of Change (PGIC) 5-point scale, patient-reported outcome; 3 represents no change from baseline, 1 represents significant improvement, 5 represents significant worsening per patient perception. PGIC result assessed at 9-month visit although Holter data for participant 6 pending as of Jan. 7, 2026 data cut-off date.
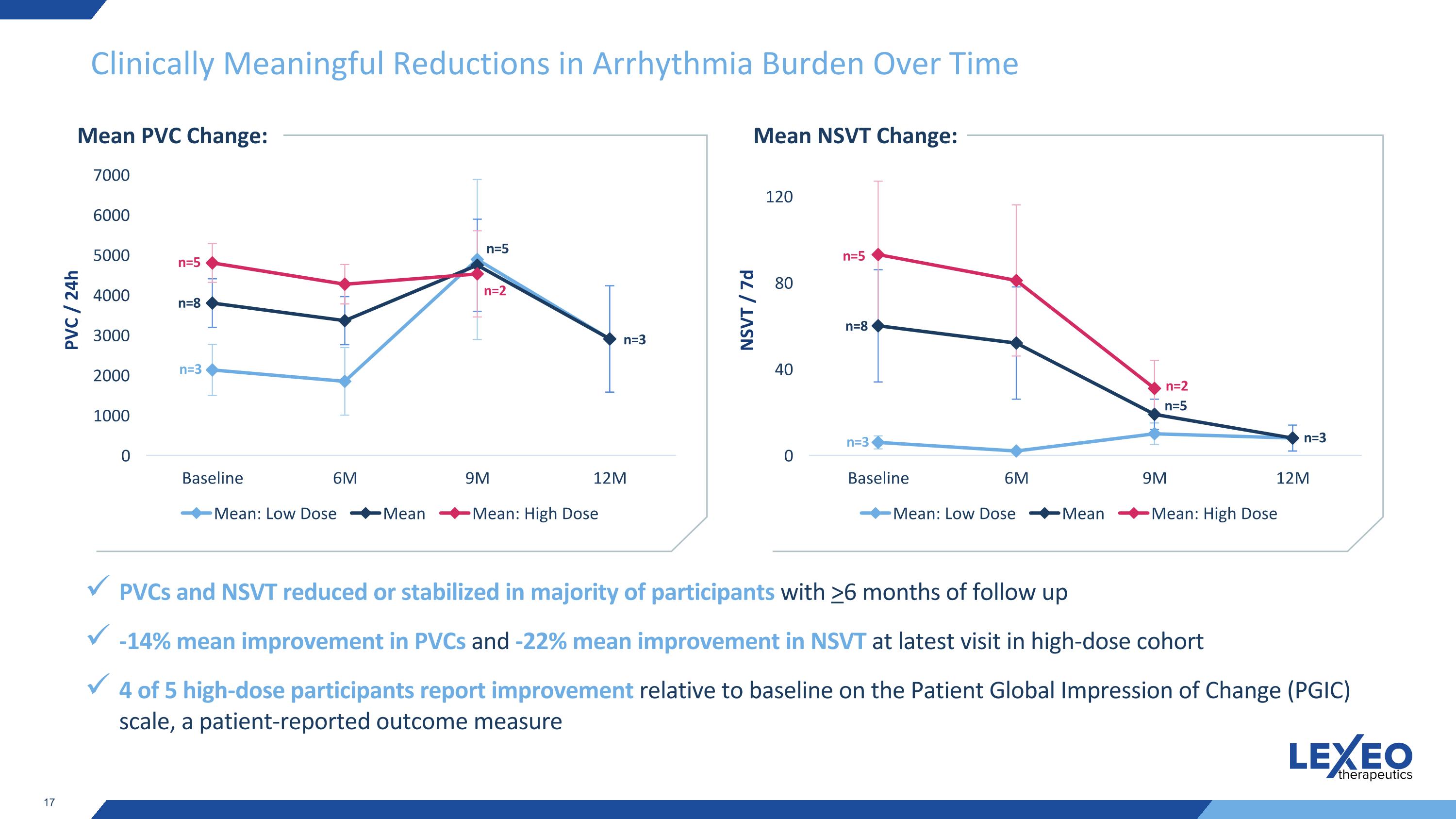
Clinically Meaningful Reductions in Arrhythmia Burden Over Time Mean PVC Change: PVCs and NSVT reduced or stabilized in majority of participants with >6 months of follow up -14% mean improvement in PVCs and -22% mean improvement in NSVT at latest visit in high-dose cohort 4 of 5 high-dose participants report improvement relative to baseline on the Patient Global Impression of Change (PGIC) scale, a patient-reported outcome measure n=3 n=8 n=5 n=5 n=2 n=3 Mean NSVT Change: n=3 n=8 n=5 n=5 n=2 n=3 PVC / 24h NSVT / 7d
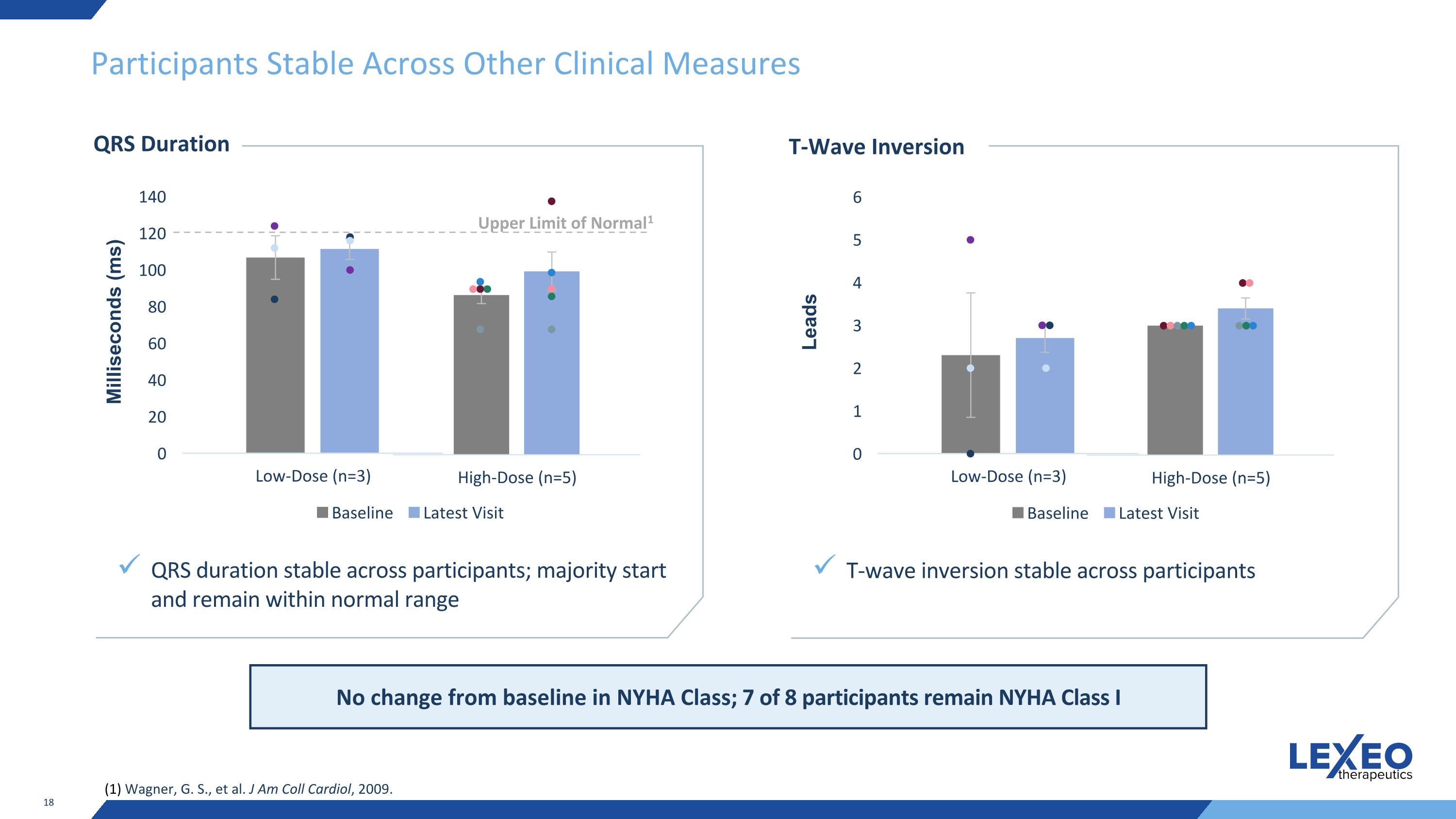
Participants Stable Across Other Clinical Measures QRS Duration QRS duration stable across participants; majority start and remain within normal range Milliseconds (ms) Upper Limit of Normal1 (1) Wagner, G. S., et al. J Am Coll Cardiol, 2009. T-Wave Inversion T-wave inversion stable across participants Leads No change from baseline in NYHA Class; 7 of 8 participants remain NYHA Class I

Summary of Results and Next Steps for LX2020 LX2020 generally well tolerated across 10 participants dosed Biopsies demonstrate robust LX2020 transduction and increased PKP2 expression at 3 months post treatment, with dose-dependent increases: Mean vector copy number of 1.5 at low dose (n=1) and 3.3 at high dose (n=5) Mean exogenous mRNA of 7.9E+04 at low dose (n=2) and 2.7E+05 at high dose (n=5) Mean increase in PKP2 protein expression of 93% at low dose (n=2) and 162% at high dose (n=5) Appropriate PKP2 colocalization observed at cardiac intercalated disc via immunofluorescence staining Improvements in key clinical parameters observed at latest visit, particularly in high-dose cohort: PVCs reduced or stable in majority of participants; 14% mean improvement in high-dose at latest visit NSVT reduced or stable in majority of participants; 22% mean improvement in high-dose at latest visit 4 of 5 high-dose participants report improvement relative to baseline on the Patient Global Impression of Change (PGIC) scale Participants stable across other clinical measures Enrollment completed in Q4 2025; further clinical updates expected in 2026 Biopsy results pending for last two participants in Cohort 3 12-month data available for all high-dose participants in Q4 2026

Lexeo Thanks the Arrhythmogenic Cardiomyopathy Community Individuals and families impacted by genetically mediated cardiovascular diseases are at the center of our mission Lexeo is grateful to the study participants, caregivers, investigators and other members of the ACM community who have helped us reach this exciting milestone We will continue to collaborate with those impacted by PKP2-ACM to increase screening and diagnosis and advance critical research

Thank you