

Lexeo Therapeutics Corporate Overview January 2026 .3

Forward-looking statements This presentation contains “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, Lexeo’s expectations and plans regarding its current product candidates and programs and the timing for receipt and announcement of data from its clinical trials, the timing and likelihood of potential regulatory approval, and expectations regarding the time period over which Lexeo’s capital resources will be sufficient to fund its anticipated operations and estimates regarding Lexeo’s financial condition. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward-looking statements as a result of many factors, including but not limited to: risks and uncertainties related to global macroeconomic conditions and related volatility; expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025, filed with the SEC on November 5, 2025, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law.

Dedicated to reshaping heart health by applying pioneering science to fundamentally change how cardiovascular disease is treated Genetic medicine leader with rare cardiac disease focus Proven experience in the clinic Platform designed for safety and scalability Individuals and families impacted by Friedreich ataxia
Building a leading cardiac gene therapy platform Differentiated AAVrh10 capsid Genetic cardiac disease expertise Innovative AAV manufacturing Operating experience Deep cardiac genetic medicine know-how, anchored by two clinical and two preclinical programs Strong financial position Cash runway into 2028, supporting multiple value creating milestones Proven cardiac tropism allows for lower doses and improved therapeutic index Optimized Sf9 baculovirus manufacturing platform designed to support future commercial scale-up Leader in genetic medicine for inherited cardiac diseases
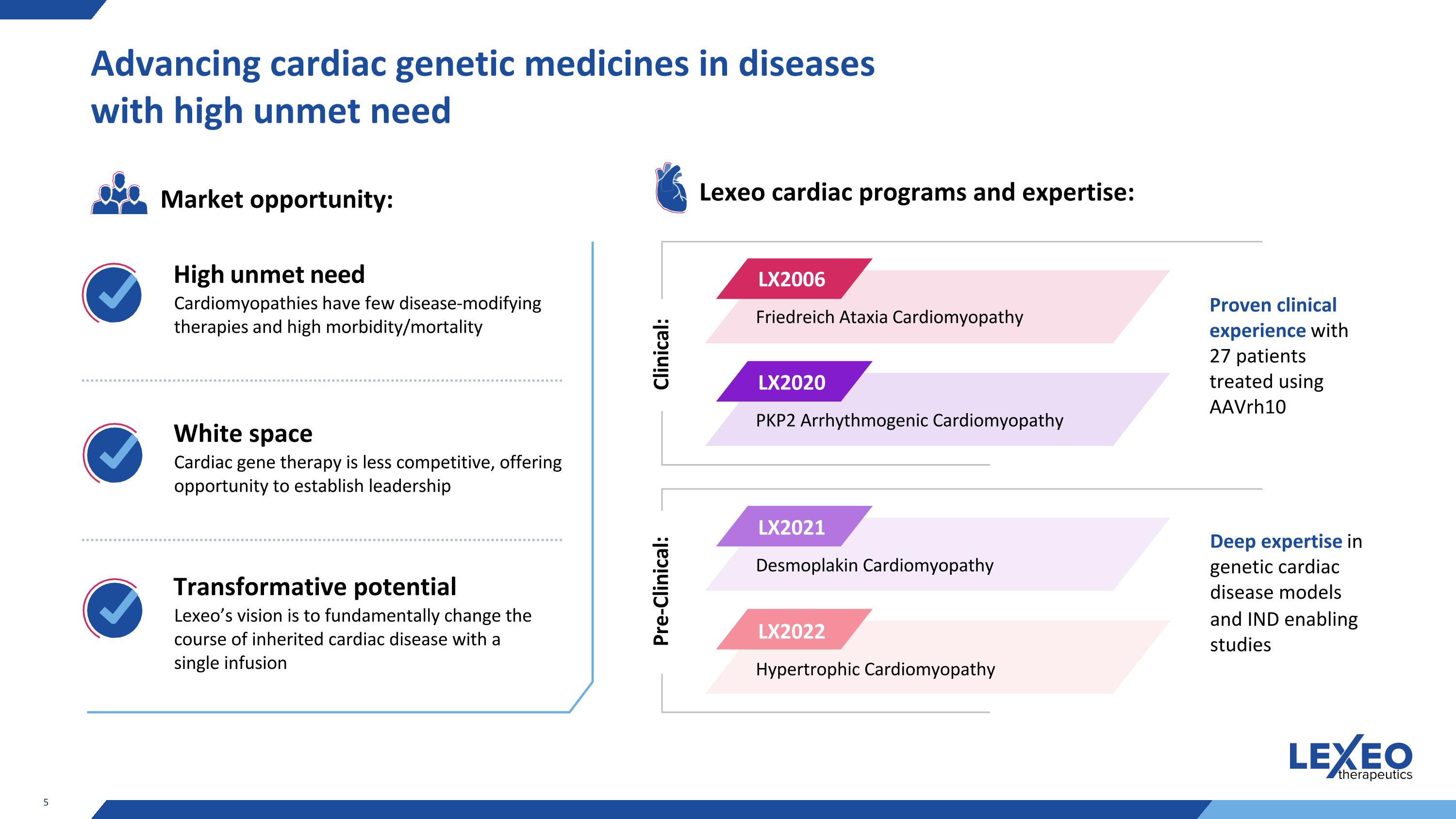
Advancing cardiac genetic medicines in diseases with high unmet need Lexeo cardiac programs and expertise: Clinical: LX2006 Friedreich Ataxia Cardiomyopathy LX2020 PKP2 Arrhythmogenic Cardiomyopathy LX2021 Desmoplakin Cardiomyopathy LX2022 Hypertrophic Cardiomyopathy Cardiomyopathies have few disease-modifying therapies and high morbidity/mortality High unmet need Cardiac gene therapy is less competitive, offering opportunity to establish leadership White space Lexeo’s vision is to fundamentally change the course of inherited cardiac disease with a single infusion Transformative potential Proven clinical experience with 27 patients treated using AAVrh10 Deep expertise in genetic cardiac disease models and IND enabling studies Market opportunity: Pre-Clinical:
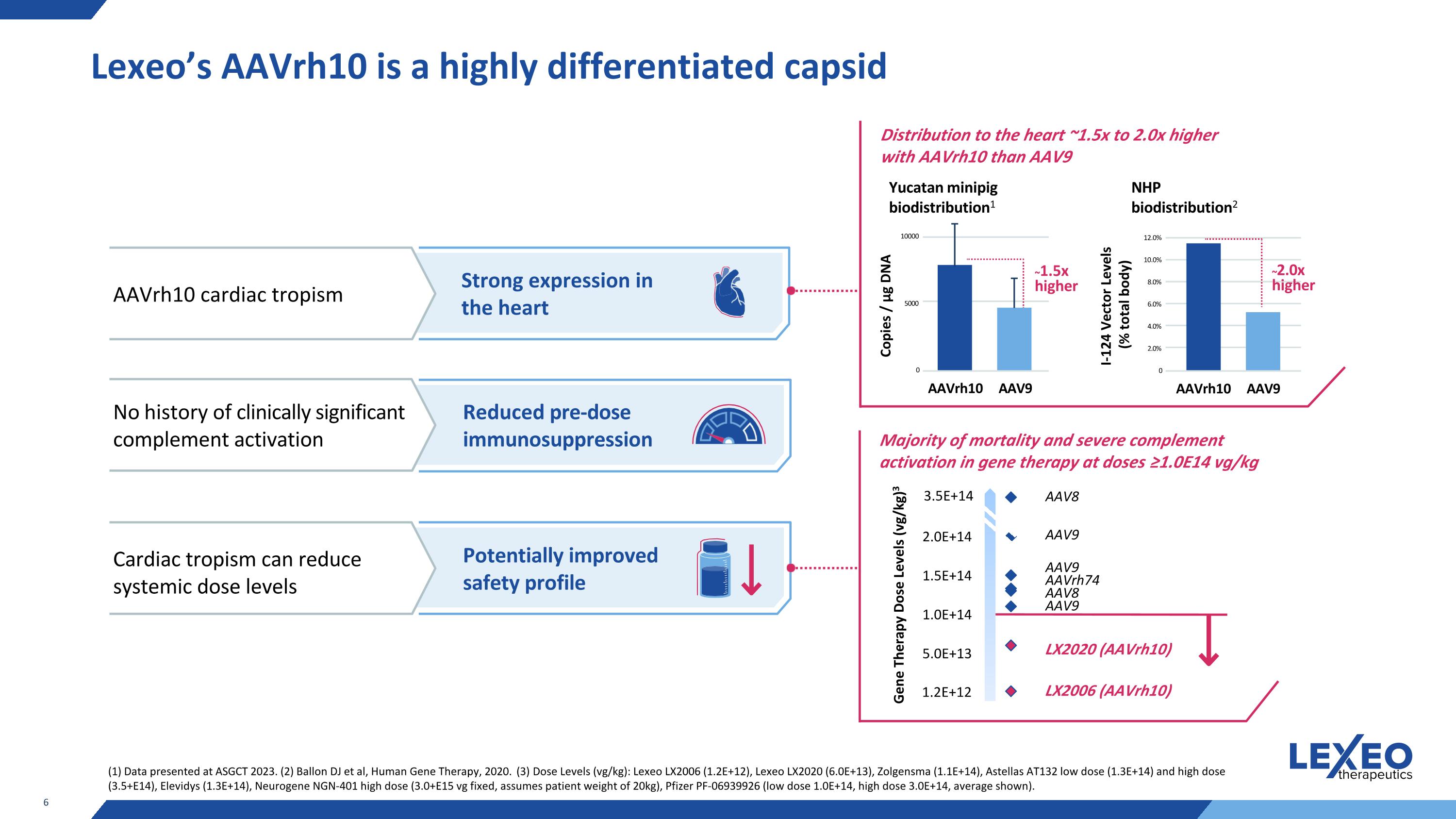
Majority of mortality and severe complement activation in gene therapy at doses ≥1.0E14 vg/kg Lexeo’s AAVrh10 is a highly differentiated capsid Strong expression in the heart AAVrh10 cardiac tropism No history of clinically significant complement activation Cardiac tropism can reduce systemic dose levels Reduced pre-dose immunosuppression Potentially improved safety profile Distribution to the heart ~1.5x to 2.0x higher with AAVrh10 than AAV9 Gene Therapy Dose Levels (vg/kg)3 3.5E+14 1.2E+12 LX2020 (AAVrh10) LX2006 (AAVrh10) AAV9 AAV8 AAVrh74 AAV9 AAV8 AAV9 Yucatan minipig biodistribution1 ~1.5x higher 5000 10000 0 AAVrh10 AAV9 NHP biodistribution2 ~2.0x higher 2.0% 6.0% 12.0% 10.0% 4.0% 8.0% 0 (1) Data presented at ASGCT 2023. (2) Ballon DJ et al, Human Gene Therapy, 2020. (3) Dose Levels (vg/kg): Lexeo LX2006 (1.2E+12), Lexeo LX2020 (6.0E+13), Zolgensma (1.1E+14), Astellas AT132 low dose (1.3E+14) and high dose (3.5+E14), Elevidys (1.3E+14), Neurogene NGN-401 high dose (3.0+E15 vg fixed, assumes patient weight of 20kg), Pfizer PF-06939926 (low dose 1.0E+14, high dose 3.0E+14, average shown). AAVrh10 AAV9 Copies / μg DNA I-124 Vector Levels (% total body)
Lexeo manufactures AAVrh10 utilizing an optimized Sf9 baculovirus process Innovative approach High yield, high quality Sf9 baculovirus manufacturing platform compared to conventional manufacturing (e.g. HEK based) Optimal potency Higher yields (1.0E15 vg/L) Greater downstream recovery (>55%) Fewer empty AAV capsids (<25%) Improved genomic purity owing to lack of plasmid transfections Scalable manufacturing Sustainable and defined starting materials, similar to therapeutic protein process (e.g. cell banks, virus banks) Low overall complexity Enables robust commercialization Poised to deliver an industry-leading and potentially transformational COGS profile LX2006 selected for FDA CDRP program, created to facilitate CMC registrational readiness and support faster patient access
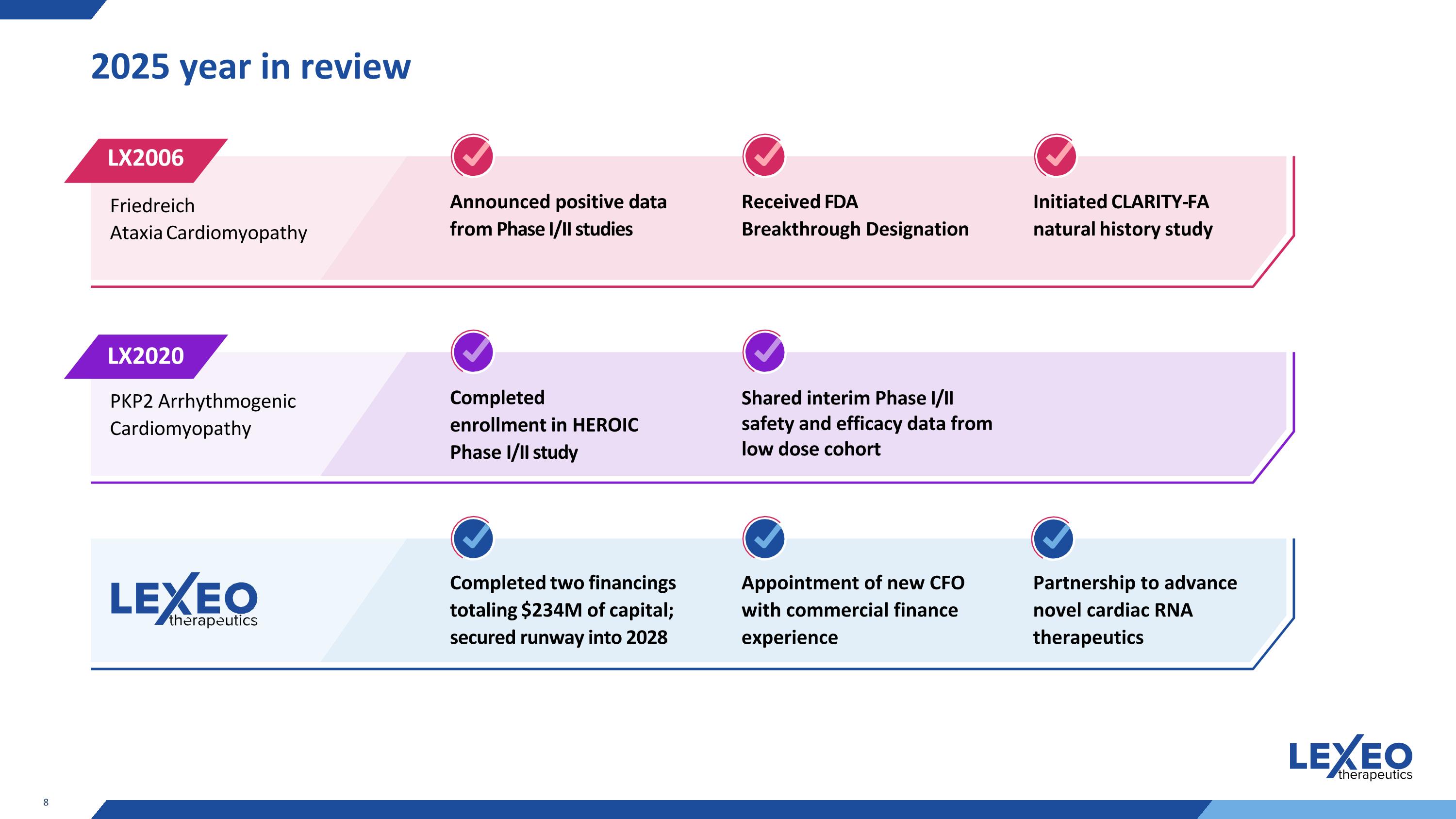
2025 year in review Announced positive data from Phase I/II studies Completed enrollment in HEROIC Phase I/II study Completed two financings totaling $234M of capital; secured runway into 2028 Received FDA Breakthrough Designation Shared interim Phase I/II safety and efficacy data from low dose cohort Initiated CLARITY-FA natural history study Friedreich Ataxia Cardiomyopathy PKP2 Arrhythmogenic Cardiomyopathy LX2006 LX2020 Appointment of new CFO with commercial finance experience Partnership to advance novel cardiac RNA therapeutics
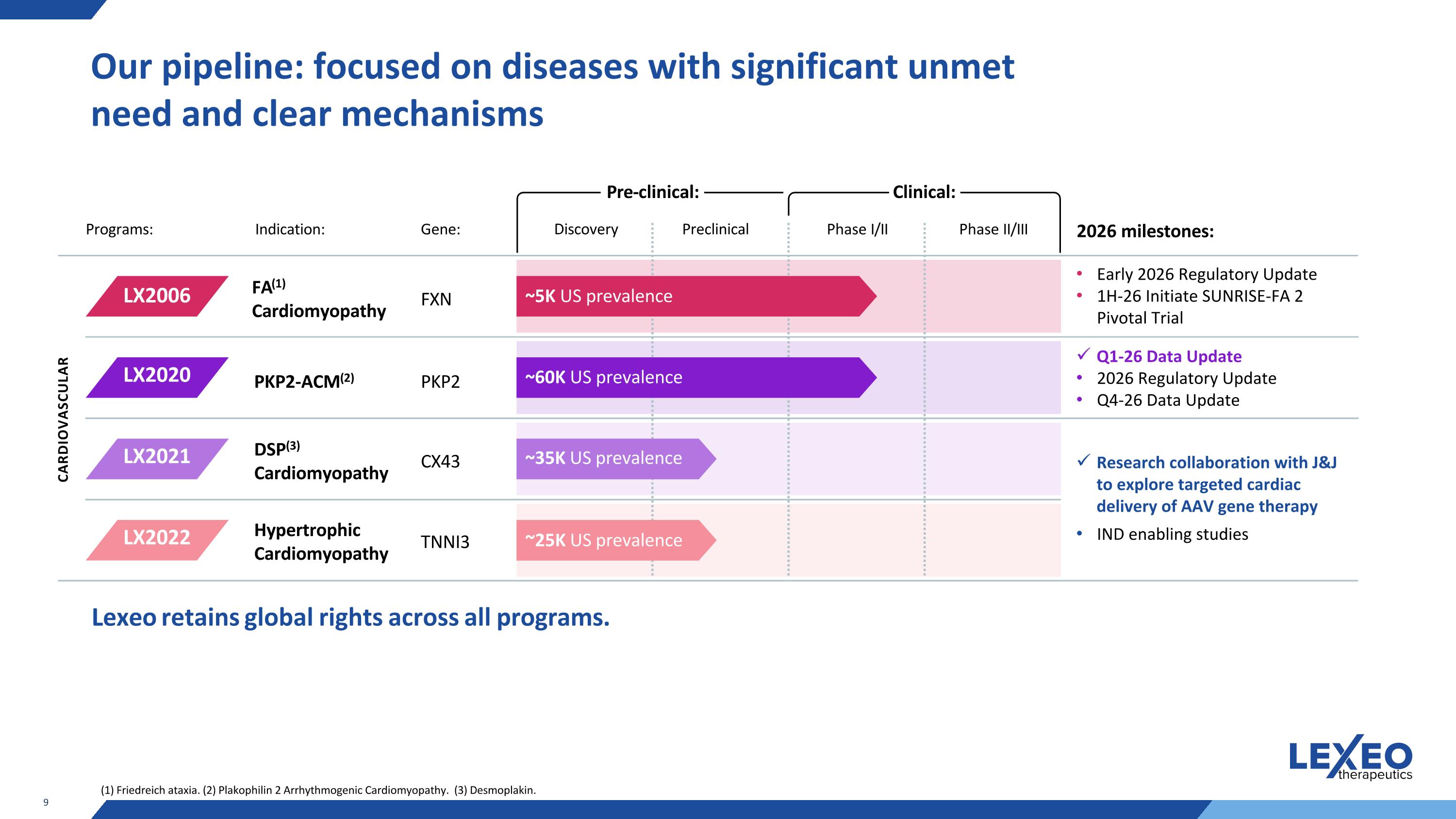
Our pipeline: focused on diseases with significant unmet need and clear mechanisms Programs: Indication: Gene: Phase I/II Phase II/III 2026 milestones: CARDIOVASCULAR FXN PKP2 CX43 TNNI3 Lexeo retains global rights across all programs. ~5K US prevalence ~60K US prevalence ~35K US prevalence ~25K US prevalence LX2006 LX2020 LX2021 LX2022 Clinical: Pre-clinical: Discovery Preclinical FA(1) Cardiomyopathy DSP(3) Cardiomyopathy PKP2-ACM(2) Hypertrophic Cardiomyopathy (1) Friedreich ataxia. (2) Plakophilin 2 Arrhythmogenic Cardiomyopathy. (3) Desmoplakin. Early 2026 Regulatory Update 1H-26 Initiate SUNRISE-FA 2 Pivotal Trial Q1-26 Data Update 2026 Regulatory Update Q4-26 Data Update Research collaboration with J&J to explore targeted cardiac delivery of AAV gene therapy IND enabling studies

Friedreich Ataxia Cardiomyopathy (FA-CM) LX2006

Cardiac complications are the leading cause of death in Friedreich Ataxia Ron and his son, Keith, who passed from FA cardiomyopathy at age 24. There are no approved treatments for the cardiomyopathy of FA. Time is of the essence.” - Ron Bartek, Co-founder of FARA Prevalence: Mortality: ~5,000 ~15,000 Global: US: Standard of care: (2) (2) Cardiac complications account for up to 80% of deaths in those with FA, with an average life expectancy of 35-40 years(1)(4) Omaveloxolone (SKYCLARYS®) is FDA-approved for FA based on neurologic endpoints; cardiac efficacy was not established(3) FA is a rare, progressive and devastating multisystem disease caused by a loss of function mutation in the FXN gene(1) With a typical age of onset between 5 and 15 years(2), individuals with FA experience cardiac and neurological manifestations Cardiac dysfunction in FA presents as cardiac hypertrophy and subsequent heart failure(1); with up to 40% of adults with FA having left ventricular hypertrophy as defined by abnormal LVMI(5)(6) The only approved disease-specific treatment for FA was not evaluated for the treatment of cardiac dysfunction in clinical trials, leaving significant unmet need within FA cardiomyopathy(3) FA, Friedreich Ataxia; FXN, Frataxin; LVMI, Left Ventricular Mass Index. (1) Payne R.M. JACC Basic Transl Sci, 2022;13;7(12):1267-1283. (2) Friedreich’s Ataxia Research Alliance, 2024. (3) Reetz, K., et al. Lancet Neurol, 2025;24(7):614-624. (4) Indelicato, E., et al. Mov Disord, 2024;39(3), 510–518. (5) Clinical Management Guidelines for Friedreich Ataxia. Chapter 4. The heart and cardiovascular system in Friedreich ataxia. 2022. (6) Lexeo Therapeutics, Data on File, 2025.
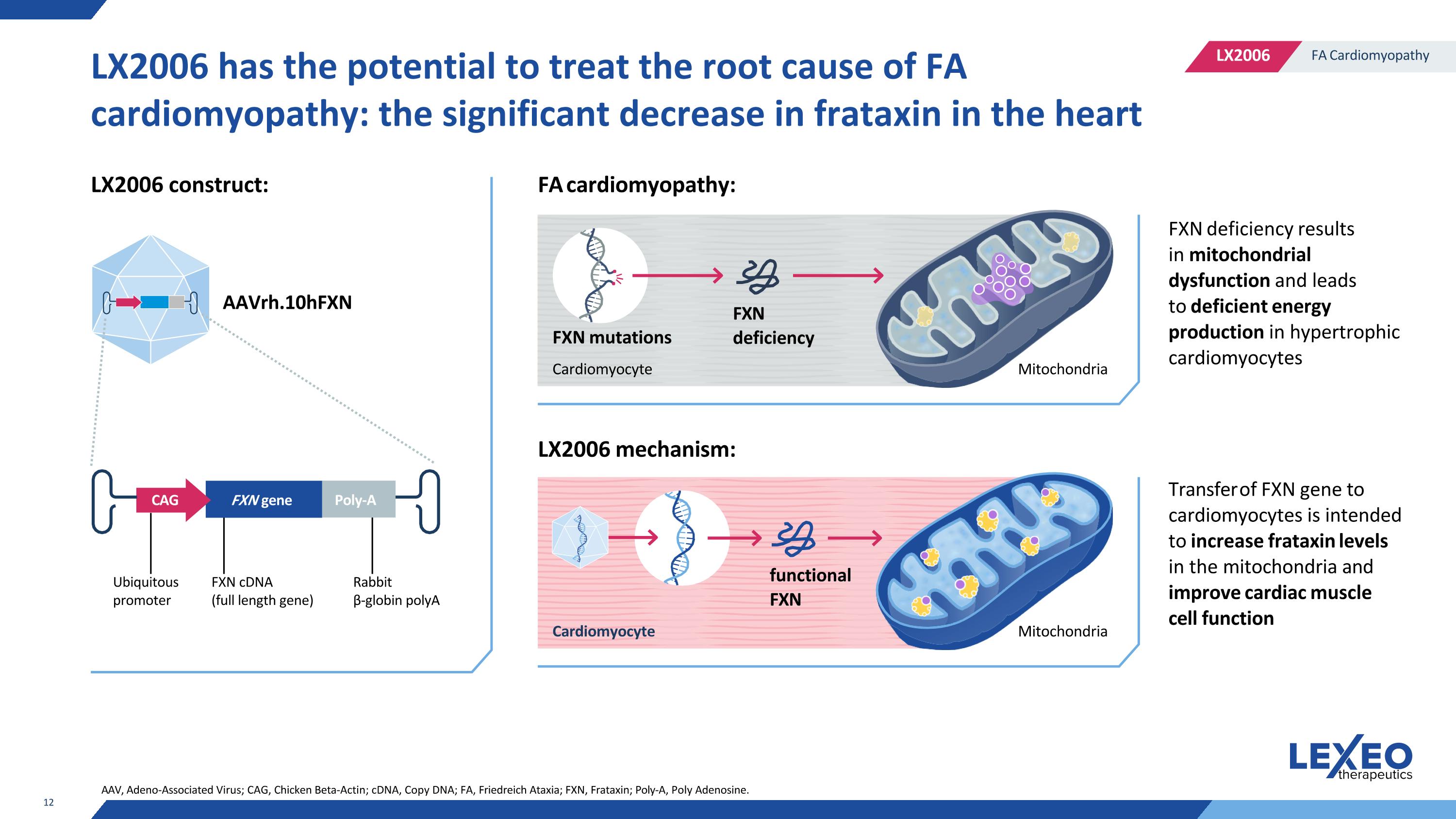
LX2006 has the potential to treat the root cause of FA cardiomyopathy: the significant decrease in frataxin in the heart AAVrh.10hFXN LX2006 construct: LX2006 mechanism: FXN deficiency results in mitochondrial dysfunction and leads to deficient energy production in hypertrophic cardiomyocytes Transfer of FXN gene to cardiomyocytes is intended to increase frataxin levels in the mitochondria and improve cardiac muscle cell function functional FXN Mitochondria Cardiomyocyte AAV, Adeno-Associated Virus; CAG, Chicken Beta-Actin; cDNA, Copy DNA; FA, Friedreich Ataxia; FXN, Frataxin; Poly-A, Poly Adenosine. Ubiquitous promoter FXN cDNA (full length gene) Rabbit β-globin polyA FA cardiomyopathy: Cardiomyocyte FXN mutations FXN deficiency Mitochondria CAG FXN gene Poly-A
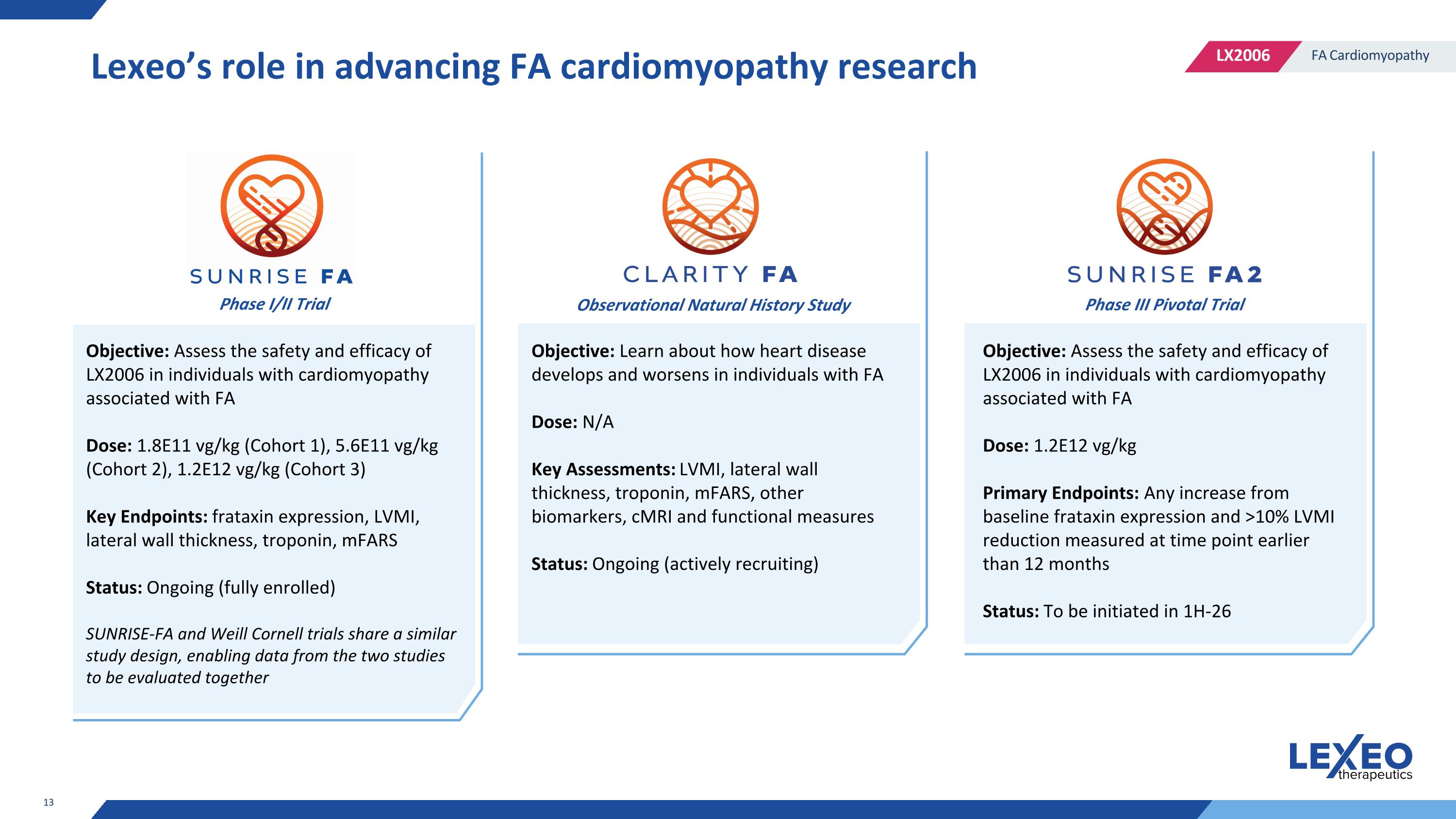
Lexeo’s role in advancing FA cardiomyopathy research Objective: Assess the safety and efficacy of LX2006 in individuals with cardiomyopathy associated with FA Dose: 1.8E11 vg/kg (Cohort 1), 5.6E11 vg/kg (Cohort 2), 1.2E12 vg/kg (Cohort 3) Key Endpoints: frataxin expression, LVMI, lateral wall thickness, troponin, mFARS Status: Ongoing (fully enrolled) SUNRISE-FA and Weill Cornell trials share a similar study design, enabling data from the two studies to be evaluated together Objective: Learn about how heart disease develops and worsens in individuals with FA Dose: N/A Key Assessments: LVMI, lateral wall thickness, troponin, mFARS, other biomarkers, cMRI and functional measures Status: Ongoing (actively recruiting) Objective: Assess the safety and efficacy of LX2006 in individuals with cardiomyopathy associated with FA Dose: 1.2E12 vg/kg Primary Endpoints: Any increase from baseline frataxin expression and >10% LVMI reduction measured at time point earlier than 12 months Status: To be initiated in 1H-26 Phase I/II Trial Observational Natural History Study Phase III Pivotal Trial
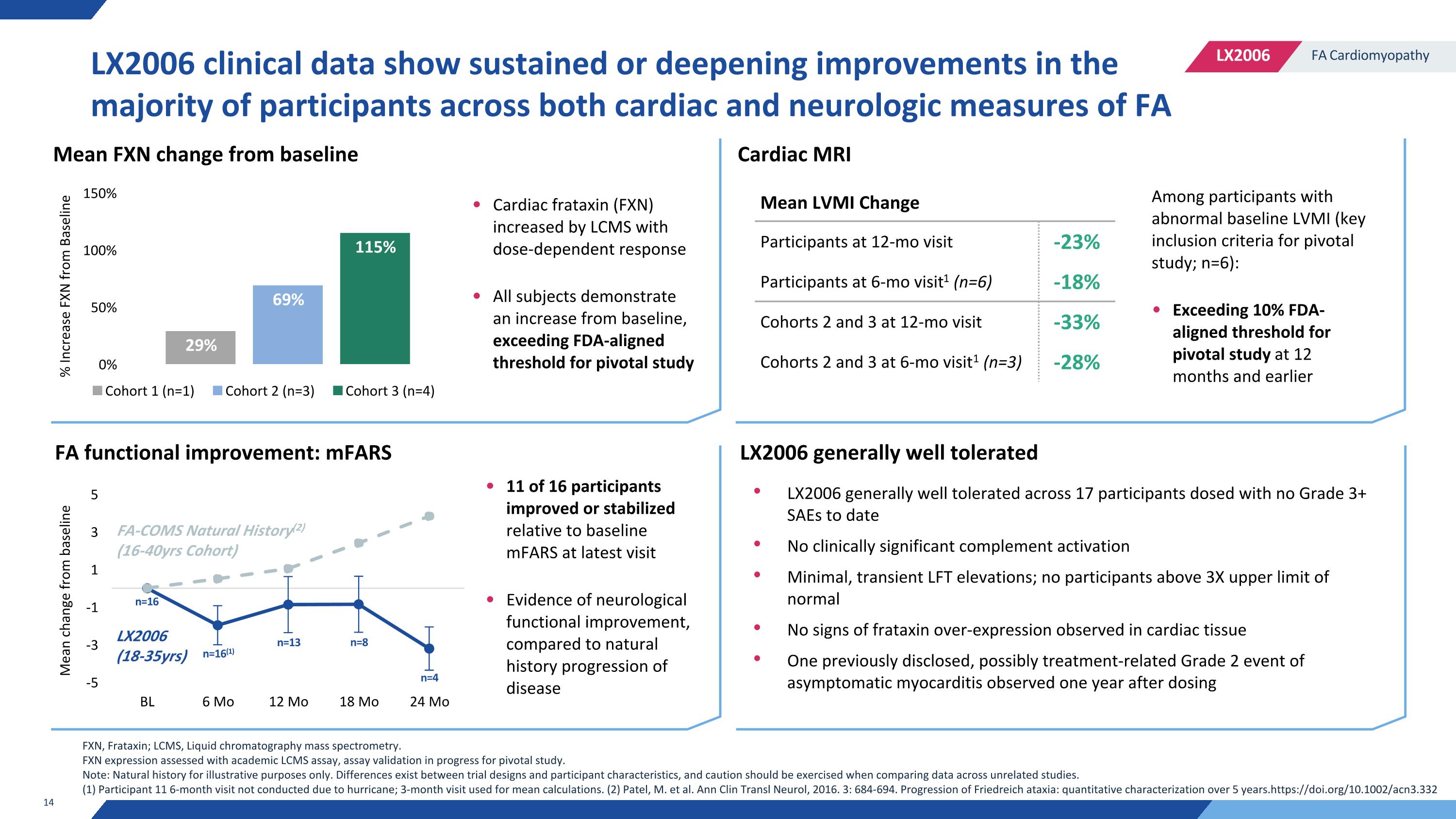
LX2006 clinical data show sustained or deepening improvements in the majority of participants across both cardiac and neurologic measures of FA LX2006 generally well tolerated across 17 participants dosed with no Grade 3+ SAEs to date No clinically significant complement activation Minimal, transient LFT elevations; no participants above 3X upper limit of normal No signs of frataxin over-expression observed in cardiac tissue One previously disclosed, possibly treatment-related Grade 2 event of asymptomatic myocarditis observed one year after dosing FA-COMS Natural History(2) (16-40yrs Cohort) LX2006 (18-35yrs) Mean LVMI Change Participants at 12-mo visit -23% Participants at 6-mo visit1 (n=6) -18% Cohorts 2 and 3 at 12-mo visit -33% Cohorts 2 and 3 at 6-mo visit1 (n=3) -28% Mean FXN change from baseline FA functional improvement: mFARS Cardiac frataxin (FXN) increased by LCMS with dose-dependent response All subjects demonstrate an increase from baseline, exceeding FDA-aligned threshold for pivotal study 11 of 16 participants improved or stabilized relative to baseline mFARS at latest visit Evidence of neurological functional improvement, compared to natural history progression of disease LX2006 generally well tolerated Cardiac MRI Among participants with abnormal baseline LVMI (key inclusion criteria for pivotal study; n=6): Exceeding 10% FDA-aligned threshold for pivotal study at 12 months and earlier FXN, Frataxin; LCMS, Liquid chromatography mass spectrometry. FXN expression assessed with academic LCMS assay, assay validation in progress for pivotal study. Note: Natural history for illustrative purposes only. Differences exist between trial designs and participant characteristics, and caution should be exercised when comparing data across unrelated studies. (1) Participant 11 6-month visit not conducted due to hurricane; 3-month visit used for mean calculations. (2) Patel, M. et al. Ann Clin Transl Neurol, 2016. 3: 684-694. Progression of Friedreich ataxia: quantitative characterization over 5 years.https://doi.org/10.1002/acn3.332 n=16 n=16(1) n=13 n=8 n=4 % Increase FXN from Baseline Mean change from baseline

Plakophilin-2 Arrhythmogenic Cardiomyopathy (PKP2-ACM) LX2020
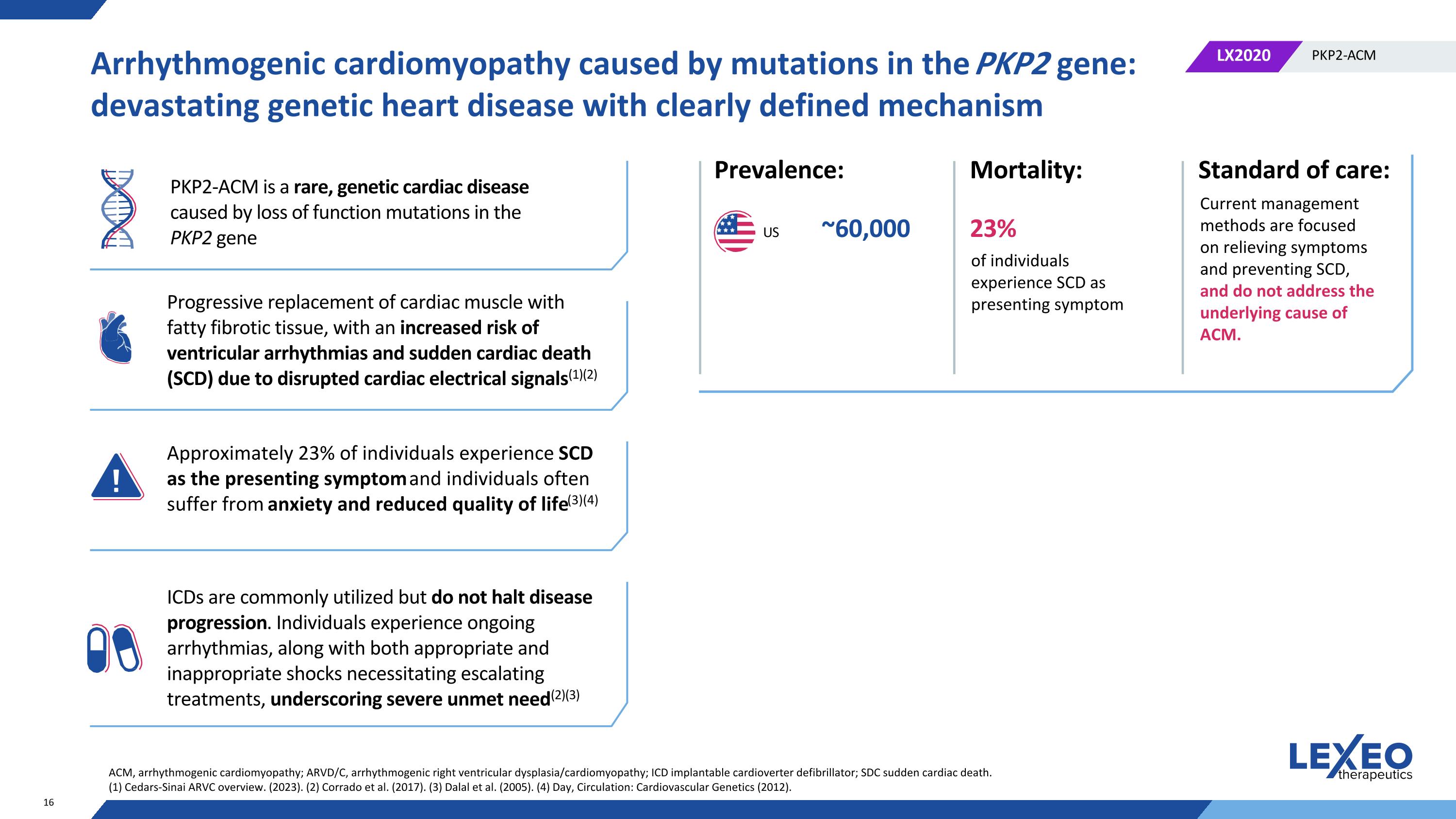
Arrhythmogenic cardiomyopathy caused by mutations in the PKP2 gene: devastating genetic heart disease with clearly defined mechanism PKP2-ACM is a rare, genetic cardiac disease caused by loss of function mutations in the PKP2 gene Progressive replacement of cardiac muscle with fatty fibrotic tissue, with an increased risk of ventricular arrhythmias and sudden cardiac death (SCD) due to disrupted cardiac electrical signals(1)(2) Approximately 23% of individuals experience SCD as the presenting symptom and individuals often suffer from anxiety and reduced quality of life(3)(4) ICDs are commonly utilized but do not halt disease progression. Individuals experience ongoing arrhythmias, along with both appropriate and inappropriate shocks necessitating escalating treatments, underscoring severe unmet need(2)(3) Prevalence: Mortality: ~60,000 US 23% Standard of care: of individuals experience SCD as presenting symptom Current management methods are focused on relieving symptoms and preventing SCD, and do not address the underlying cause of ACM. ACM, arrhythmogenic cardiomyopathy; ARVD/C, arrhythmogenic right ventricular dysplasia/cardiomyopathy; ICD implantable cardioverter defibrillator; SDC sudden cardiac death. (1) Cedars-Sinai ARVC overview. (2023). (2) Corrado et al. (2017). (3) Dalal et al. (2005). (4) Day, Circulation: Cardiovascular Genetics (2012).
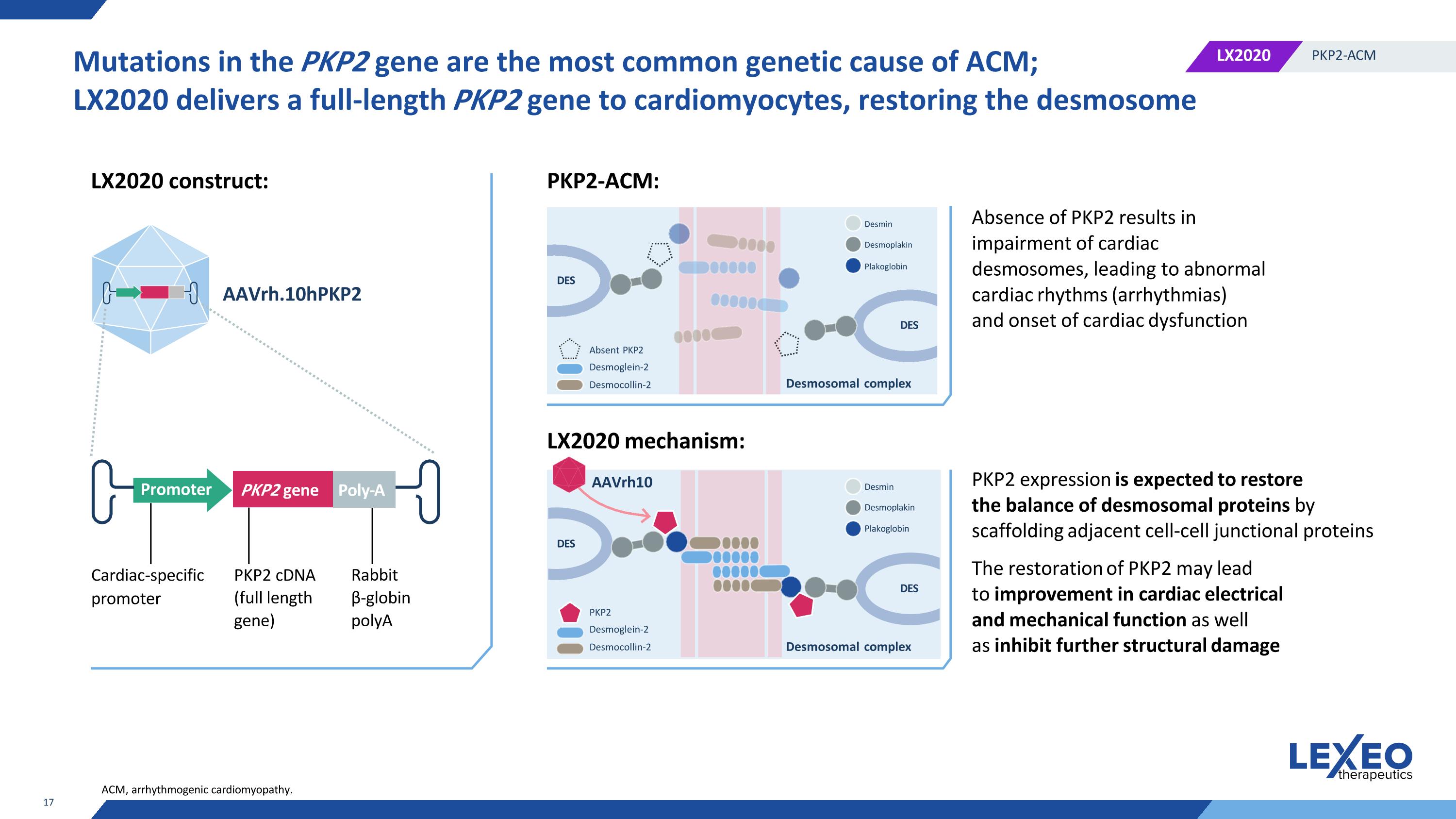
Mutations in the PKP2 gene are the most common genetic cause of ACM; LX2020 delivers a full-length PKP2 gene to cardiomyocytes, restoring the desmosome PKP2 cDNA (full length gene) Rabbit β-globin polyA AAVrh.10hPKP2 LX2020 construct: PKP2-ACM: LX2020 mechanism: Cardiac-specific promoter Absence of PKP2 results in impairment of cardiac desmosomes, leading to abnormal cardiac rhythms (arrhythmias) and onset of cardiac dysfunction PKP2 expression is expected to restore the balance of desmosomal proteins by scaffolding adjacent cell-cell junctional proteins The restoration of PKP2 may lead to improvement in cardiac electrical and mechanical function as well as inhibit further structural damage Desmosomal сomplex AAVrh10 ACM, arrhythmogenic cardiomyopathy. Desmin Desmoplakin DES DES Plakoglobin PKP2 Desmoglein-2 Desmocollin-2 DES DES Desmosomal сomplex Absent PKP2 Desmoglein-2 Desmocollin-2 Desmin Desmoplakin Plakoglobin Promoter PKP2 gene Poly-A
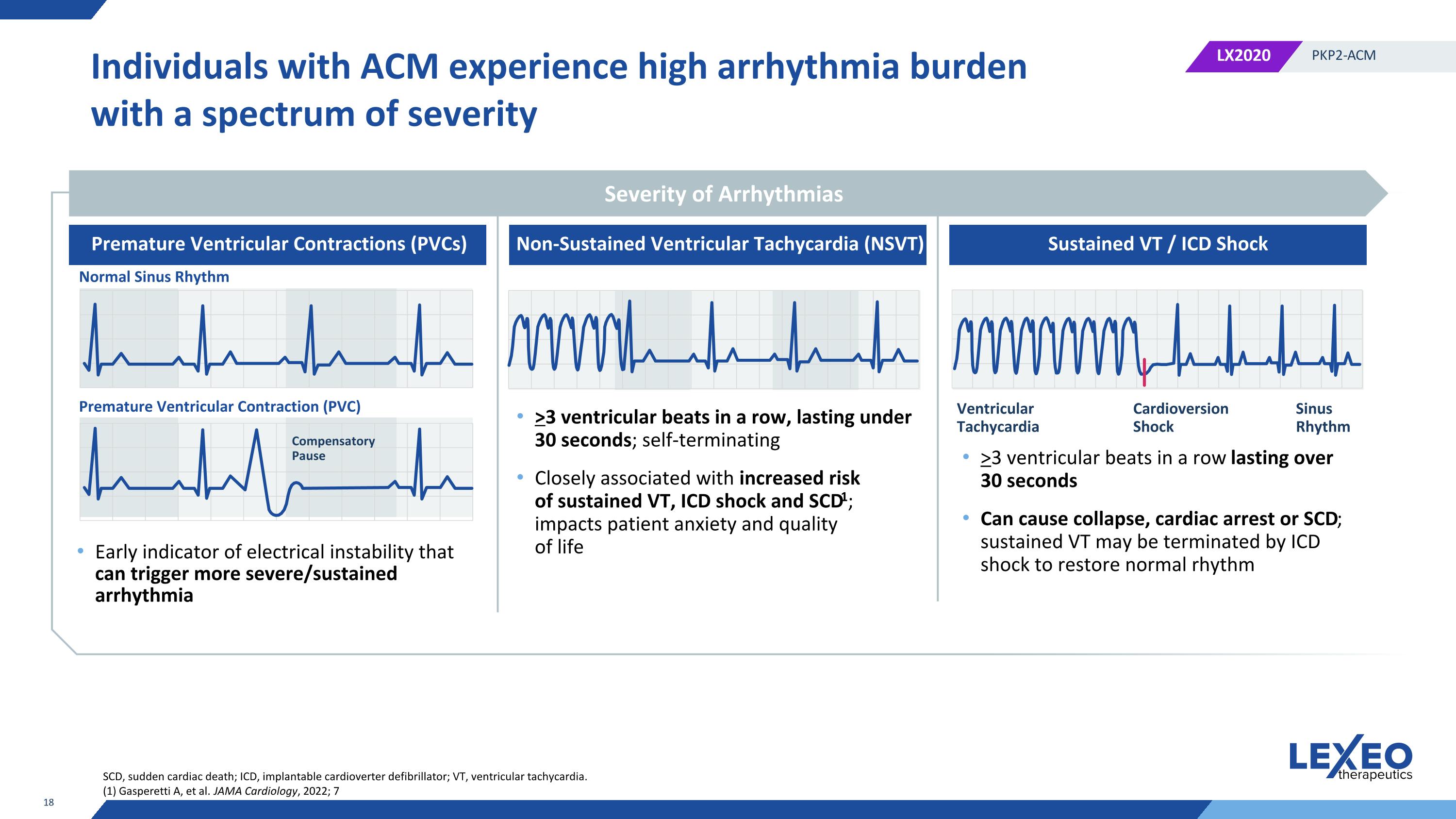
Individuals with ACM experience high arrhythmia burden with a spectrum of severity Early indicator of electrical instability that can trigger more severe/sustained arrhythmia >3 ventricular beats in a row, lasting under 30 seconds; self-terminating Closely associated with increased risk of sustained VT, ICD shock and SCD1; impacts patient anxiety and quality of life >3 ventricular beats in a row lasting over 30 seconds Can cause collapse, cardiac arrest or SCD; sustained VT may be terminated by ICD shock to restore normal rhythm SCD, sudden cardiac death; ICD, implantable cardioverter defibrillator; VT, ventricular tachycardia. (1) Gasperetti A, et al. JAMA Cardiology, 2022; 7 Severity of Arrhythmias Premature Ventricular Contractions (PVCs) Non-Sustained Ventricular Tachycardia (NSVT) Sustained VT / ICD Shock Normal Sinus Rhythm Premature Ventricular Contraction (PVC) Compensatory Pause Ventricular Tachycardia Cardioversion Shock Sinus Rhythm

Phase I/II Trial Lexeo’s role in advancing PKP2-ACM research Retrospective EMR Review and Prospective Observational Natural History Study Objective: Assess the safety and efficacy of LX2020 in individuals with PKP2-ACM Dose: 2.0E13 vg/kg (Cohort 1), 6.0E13 vg/kg (Cohorts 2, 3) Key Endpoints: PKP2 expression, VT, PVC, QRS, T-wave inversion, cardiac function, PROs Status: Ongoing (fully enrolled, n=10) Objective: Evaluate the clinical burden of illness for patients with PKP2-ACM, and prospectively evaluate changes in key cardiac parameters and patient-reported outcome measures (PROs) associated with PKP2-ACM progression Dose: N/A Key Assessments: VT, PVC, QRS, T-wave inversion, cardiac function, PROs Status: Ongoing (actively recruiting)
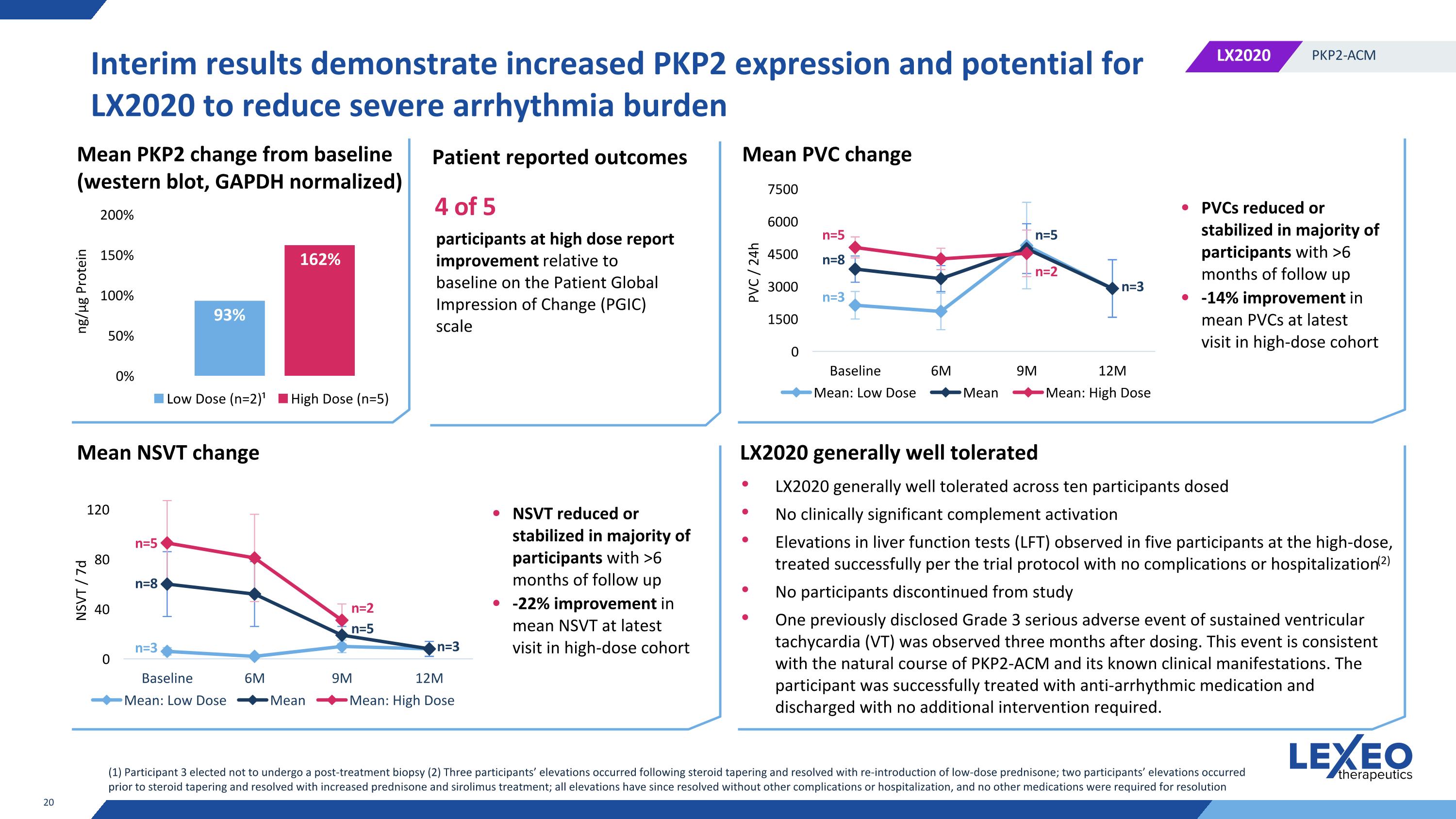
Interim results demonstrate increased PKP2 expression and potential for LX2020 to reduce severe arrhythmia burden n=3 n=8 n=5 n=5 n=2 n=3 n=3 n=8 n=5 n=5 n=2 n=3 Mean PKP2 change from baseline (western blot, GAPDH normalized) Mean PVC change PVCs reduced or stabilized in majority of participants with >6 months of follow up -14% improvement in mean PVCs at latest visit in high-dose cohort Mean NSVT change NSVT reduced or stabilized in majority of participants with >6 months of follow up -22% improvement in mean NSVT at latest visit in high-dose cohort LX2020 generally well tolerated LX2020 generally well tolerated across ten participants dosed No clinically significant complement activation Elevations in liver function tests (LFT) observed in five participants at the high-dose, treated successfully per the trial protocol with no complications or hospitalization(2) No participants discontinued from study One previously disclosed Grade 3 serious adverse event of sustained ventricular tachycardia (VT) was observed three months after dosing. This event is consistent with the natural course of PKP2-ACM and its known clinical manifestations. The participant was successfully treated with anti-arrhythmic medication and discharged with no additional intervention required. (1) Participant 3 elected not to undergo a post-treatment biopsy (2) Three participants’ elevations occurred following steroid tapering and resolved with re-introduction of low-dose prednisone; two participants’ elevations occurred prior to steroid tapering and resolved with increased prednisone and sirolimus treatment; all elevations have since resolved without other complications or hospitalization, and no other medications were required for resolution Patient reported outcomes 4 of 5 participants at high dose report improvement relative to baseline on the Patient Global Impression of Change (PGIC) scale NSVT / 7d ng/μg Protein PVC / 24h
Lexeo is also advancing two preclinical cardiac gene therapy programs LX2021 +2026 research collaboration with Johnson & Johnson exploring novel routes of administration for cardiac AAV gene therapy to maximize safety and efficacy LX2021 Desmoplakin Cardiomyopathy LX2022 Hypertrophic Cardiomyopathy High unmet need characterized by extensive fibrosis, high arrhythmic risk, and high heart failure burden 30-50% mortality within 5 years of diagnosis for dilated phenotype ~35K patients in U.S. IND-enabling studies and regulatory engagement expected in 2026 TNNI3 variants compose 3-5% of all HCM cases, causing cardiomyopathy, clinical heart failure and shortened lifespan Non-obstructive phenotype, often with preserved EF; myosin inhibitors not effective ~25K patients in U.S.
Lexeo – a leader in cardiac gene therapy Leader in cardiac genetic medicine addressing high unmet need and clear market opportunity Catalyst rich 2026 with multiple key milestones expected across two clinical stage programs Differentiated AAVrh10 capsid and innovative Sf9 baculovirus manufacturing platform Advancing towards pivotal stage; Phase III trial in FA-CM expected to initiate in 2026 with potential path to accelerated approval Strong financial position with cash runway into 2028 1 2 3 4 5

Thank You