
FOCUSED On advancing the next wave of medicine for inflammatory diseases MA R C H 2 0 2 5 ©2025 THIRD HARMONIC BIO

FOCUSED On advancing the next wave of medicine for inflammatory diseases MA R C H 2 0 2 5 ©2025 THIRD HARMONIC BIO

Forward Looking Statements This presentation contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995. Any statements made in this presentation that are not statements of historical fact, including statements about our beliefs and expectations, are forward-looking statements and should be evaluated as such. Forward-looking statements include information concerning the anticipated profile, efficacy and target indications of THB335, the expected timing of clinical trials of THB335, the expected development and timeline for clinical of THB335 and statements regarding Third Harmonic Bio’s plans to explore opportunities to maximize stockholder value, the company’s ability to enter into any agreements or transactions in connection with the exploration of potential strategic transactions, or if entered into, that any such agreements or transactions will be successful or on attractive terms. These statements often include words such as “anticipate,” “expect,” “suggests,” “plan,” “believe,” “intend,” “estimates,” “targets,” “projects,” “should,” “could,” “would,” “may,” “will,” “forecast” and other similar expressions. These forward-looking statements are contained throughout this presentation. We base these forward-looking statements on our current expectations, plans and assumptions that we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate under the circumstances at such time. As you read and consider this presentation, you should understand that these statements are not guarantees of future performance or results. The forward-looking statements are subject to and involve risks, uncertainties and assumptions, and you should not place undue reliance on these forward-looking statements. Although we believe that these forward-looking statements are based on reasonable assumptions at the time they are made, you should be aware that many factors could affect our actual results or results of operations and could cause actual results to differ materially from those expressed in the forward-looking statements. Factors that may materially affect such forward-looking statements include: our limited operating history and that we have not completed any clinical trials beyond Phase 1 and have not had any product candidates approved for commercial sale; our significant net losses incurred since inception and the likelihood of incurring additional losses for the foreseeable future; our need for substantial additional funding; the early stage of development of our programs and the possibility they may fail in development; our future performance is substantially dependent on our ability to identify and develop future product candidates; legal and regulatory risks; and intellectual property-related risks, among others. Additional risks and uncertainties that could affect our financial results and business are more fully described under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the three months ended September 30, 2024, filed with the SEC on November 7, 2024, and our other SEC filings, which are available on the Investor & Media page of our website at https://ir.thirdharmonicbio.com/ and on the SEC’s website at www.sec.gov. These cautionary statements should not be construed by you to be exhaustive and are made only as of the date of this presentation. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation also contains preliminary estimates and projections regarding cash and cash equivalents as of December 31, 2024 and June 30, 2025. These estimates are preliminary, unaudited and may change, were prepared by management and are based on the most current information available to management. Further, these amounts are subject to completion by management of the financial statements as of and for the year ended December 31, 2024 and three months ended June 30, 2025, including completion of the review procedures, final adjustments and other developments that may arise between now and the time the financial results for this period are finalized, completion of the audit or review (as applicable) of such financial statements, and in the case of the June 30, 2025 estimates, subject to completion of the relevant period. 2
THRD: Focused on KIT to Treat Mast Cell-Mediated Inflammatory Diseases LARGE MARKETS ESTABLISHED WITH HIGH CLINICALLY KIT: A NOVEL, VALIDATED “PIPELINE-IN-A-TARGET” UNMET NEED TARGET CLINICAL THB335 CANDIDATE: POTENTIAL Millions of patients living Clinical validation of KIT Highly selective, oral small Potential treatment options for a with severe mast cell-mediated as potentially transformative target for molecule with potential to optimize range of dermal, airway, and GI diseases; high residual need despite mast cell-mediated diseases therapeutic index and offer patient inflammatory diseases multiple approved products convenience over injectables 3

High Disease Burden in Chronic Spontaneous Urticaria A severe, yet undertreated dermal inflammatory condition “Out there, it’s a horrible world for urticaria patients“1 Prevalence: More than 1.5 million patients or 0.5-1% point prevalence; ~70-80% female, mean age ~46 years Disease impact: CSU severely impairs quality of life, causes significant physical discomfort and emotional distress, including anxiety, depression, insomnia and social isolation Limited treatment options: Oral anti-histamines effective in only ~50% of patients; single biologic therapy approved for second-line use New treatment options are imperative to driving disease awareness, diagnosis and treatment 1Marcus Maurer MD, LifeSci Immunology and Inflammation Day, May 10, 2022 4
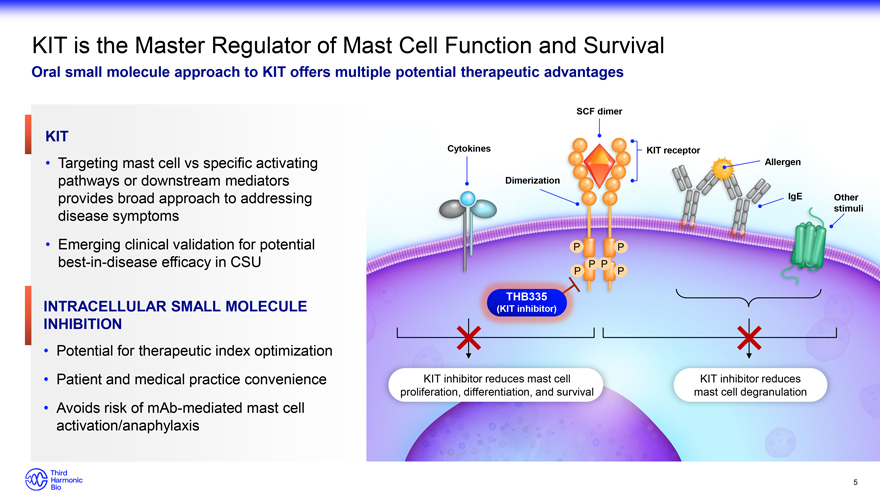
KIT is the Master Regulator of Mast Cell Function and Survival Oral small molecule approach to KIT offers multiple potential therapeutic advantages KIT Targeting mast cell vs specific activating pathways or downstream mediators provides broad approach to addressing disease symptoms Emerging clinical validation for potential best-in-disease efficacy in CSU INTRACELLULAR SMALL MOLECULE INHIBITION Potential for therapeutic index optimization Patient and medical practice convenience Avoids risk of mAb-mediated mast cell activation/anaphylaxis SCF dimer Cytokines KIT receptor Allergen Dimerization IgE Other stimuli P P P P P P THB335 (KIT inhibitor) KIT inhibitor reduces mast cell KIT inhibitor reduces proliferation, differentiation, and survival mast cell degranulation 5
THB335: A Next-Generation, Potent, and Highly Selective Wild-Type KIT Inhibitor THB335 Product Profile Low nanomolar KIT potency with excellent kinome selectivity in biochemical and cell-based assays Peripherally restricted biodistribution Structural and metabolic improvements vs THB001 to address DILI risk Favorable nonclinical pharmacokinetic profile, including high oral bioavailability, metabolic stability, and long circulating half-life No off-target toxicology findings in IND-enabling studies, consistent with THB001 experience through chronic studies in rodent and non-rodent species New composition of matter IP; base patent term through 2043 DILI = drug-induced liver injury 6
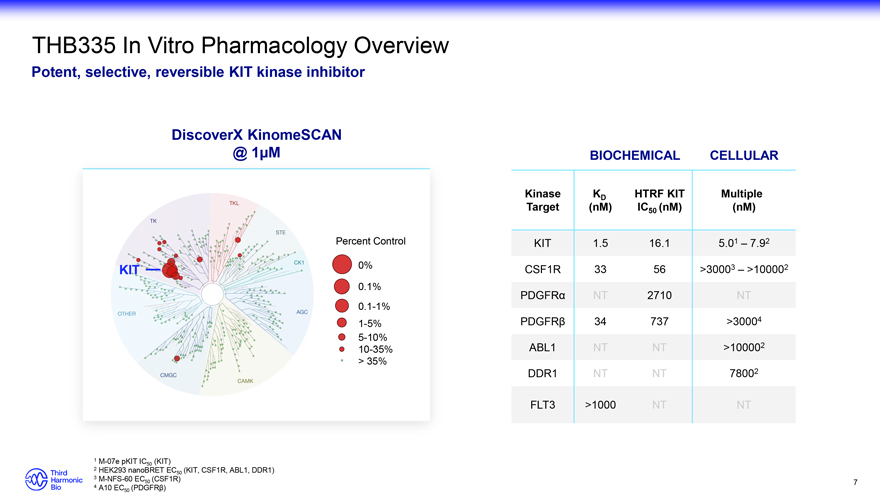
THB335 In Vitro Pharmacology Overview Potent, selective, reversible KIT kinase inhibitor DiscoverX KinomeSCAN @ 1 M 0% 0.1% 0.1-1% 1-5% 5-10% 10-35% > 35% Percent Control BIOCHEMICAL CELLULAR Kinase KD HTRF KIT Multiple Target (nM) IC50 (nM) (nM) KIT 1.5 16.1 5.01 – 7.92 CSF1R 33 56 >30003 – >100002 PDGFR NT 2710 NT PDGFR 34 737 >30004 ABL1 NT NT >100002 DDR1 NT NT 78002 FLT3 >1000 NT NT 1 M-07e pKIT IC (KIT) 2 50 HEK293 nanoBRET EC50 (KIT, CSF1R, ABL1, DDR1) 3 M-NFS-60 EC (CSF1R) 4 50 A10 EC50 (PDGFR?) 7
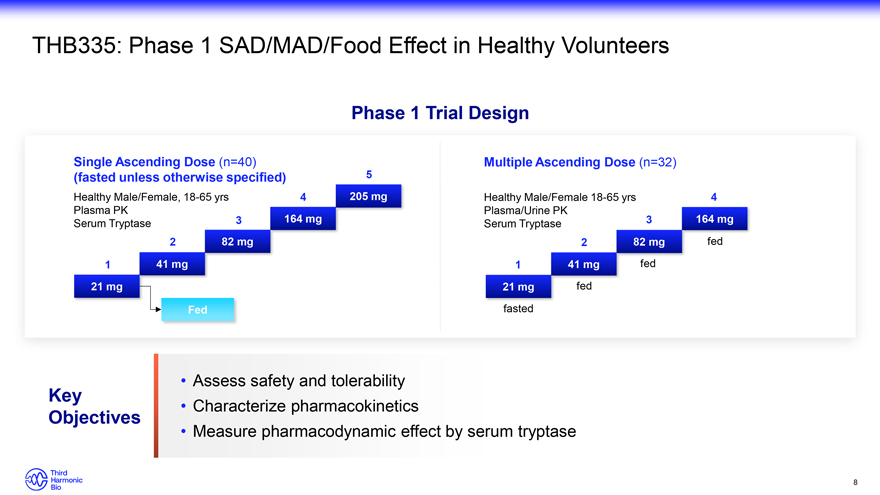
THB335: Phase 1 SAD/MAD/Food Effect in Healthy Volunteers Phase 1 Trial Design Single Ascending Dose (n=40) Multiple Ascending Dose (n=32) (fasted unless otherwise specified) 5 Healthy Male/Female, 18-65 yrs 4 205 mg Healthy Male/Female 18-65 yrs 4 Plasma PK Plasma/Urine PK Serum Tryptase 3 164 mg Serum Tryptase 3 164 mg 2 82 mg 2 82 mg fed 1 41 mg 1 41 mg fed 21 mg 21 mg fed Fed fasted Assess safety and tolerability Key Characterize pharmacokinetics Objectives Measure pharmacodynamic effect by serum tryptase 8
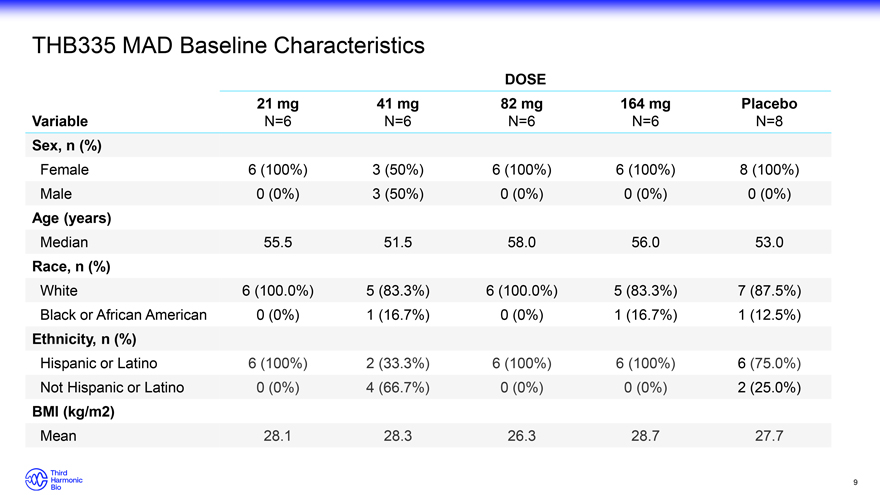
THB335 MAD Baseline Characteristics DOSE 21 mg 41 mg 82 mg 164 mg Placebo Variable N=6 N=6 N=6 N=6 N=8 Sex, n (%) Female 6 (100%) 3 (50%) 6 (100%) 6 (100%) 8 (100%) Male 0 (0%) 3 (50%) 0 (0%) 0 (0%) 0 (0%) Age (years) Median 55.5 51.5 58.0 56.0 53.0 Race, n (%) White 6 (100.0%) 5 (83.3%) 6 (100.0%) 5 (83.3%) 7 (87.5%) Black or African American 0 (0%) 1 (16.7%) 0 (0%) 1 (16.7%) 1 (12.5%) Ethnicity, n (%) Hispanic or Latino 6 (100%) 2 (33.3%) 6 (100%) 6 (100%) 6 (75.0%) Not Hispanic or Latino 0 (0%) 4 (66.7%) 0 (0%) 0 (0%) 2 (25.0%) BMI (kg/m2) Mean 28.1 28.3 26.3 28.7 27.7 9
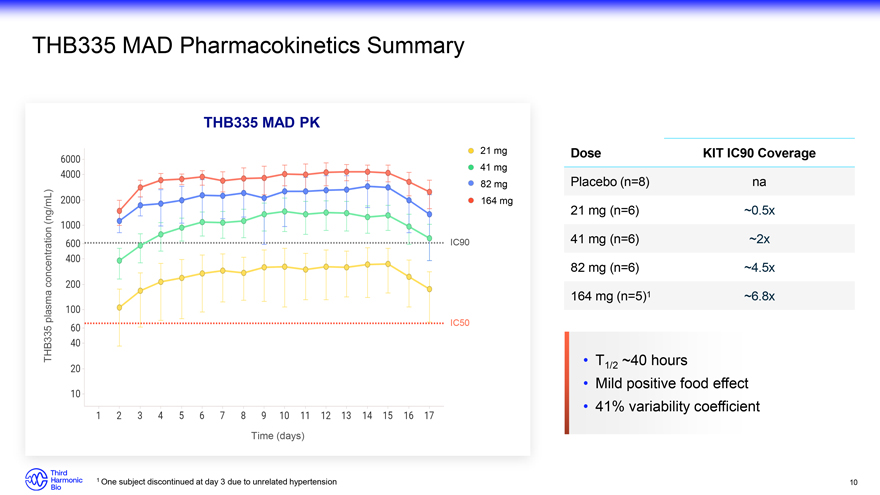
THB335 MAD Pharmacokinetics Summary THB335 MAD PK 21 mg Dose KIT IC90 Coverage 41 mg 82 mg Placebo (n=8) na 164 mg (ng/mL) 21 mg (n=6) ~0.5x IC90 41 mg (n=6) ~2x concentration 82 mg (n=6) ~4.5x plasma 164 mg (n=5)1 ~6.8x THB335 IC50 T1/2 ~40 hours Mild positive food effect 41% variability coefficient Time (days) 1 One subject discontinued at day 3 due to unrelated hypertension 10
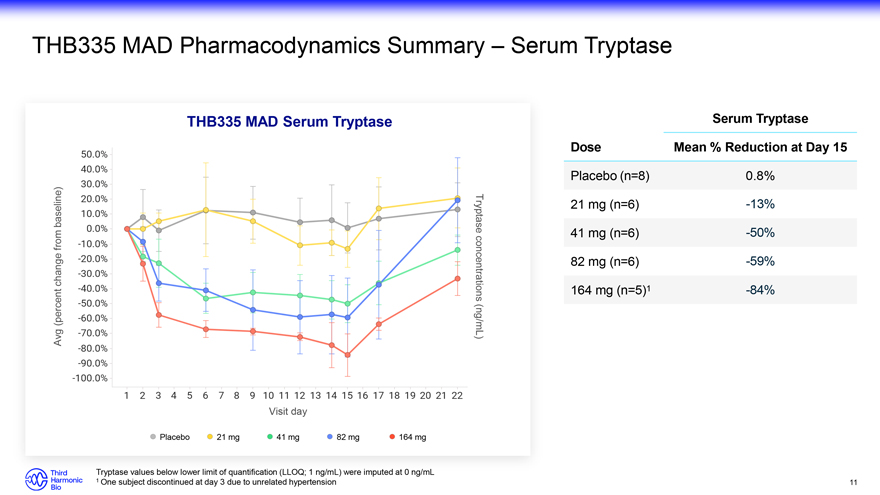
THB335 MAD Pharmacodynamics Summary – Serum Tryptase THB335 MAD Serum Tryptase Serum Tryptase Dose Mean % Reduction at Day 15 Placebo (n=8) 0.8% baseline) 21 mg (n=6) -13% Tryptase from 41 mg (n=6) -50% change 82 mg (n=6) -59% concentrations 164 mg (n=5)1 -84% (percent Avg (ng/mL) Visit day Placebo 21 mg 41 mg 82 mg 164 mg Tryptase values below lower limit of quantification (LLOQ; 1 ng/mL) were imputed at 0 ng/mL 1 One subject discontinued at day 3 due to unrelated hypertension 11
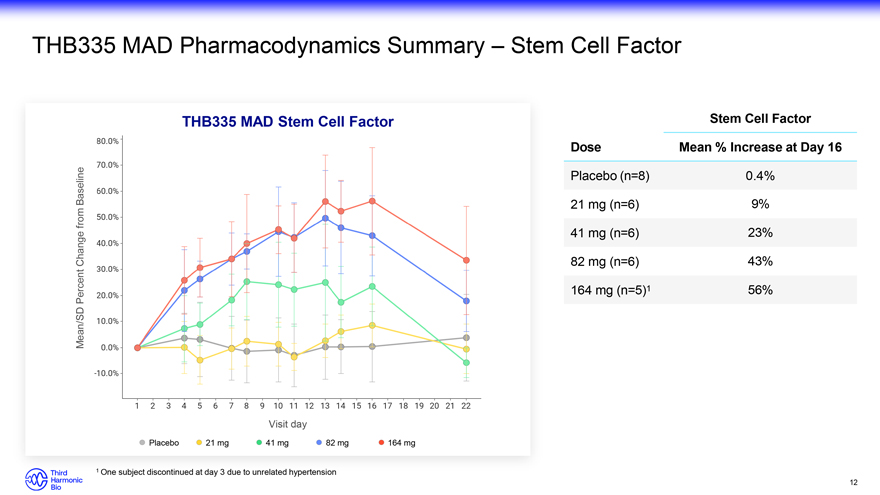
THB335 MAD Pharmacodynamics Summary – Stem Cell Factor THB335 MAD Stem Cell Factor Stem Cell Factor Dose Mean % Increase at Day 16 Baseline Placebo (n=8) 0.4% from 21 mg (n=6) 9% 41 mg (n=6) 23% Change 82 mg (n=6) 43% Percent 164 mg (n=5)1 56% Mean/SD Visit day Placebo 21 mg 41 mg 82 mg 164 mg 1 One subject discontinued at day 3 due to unrelated hypertension 12
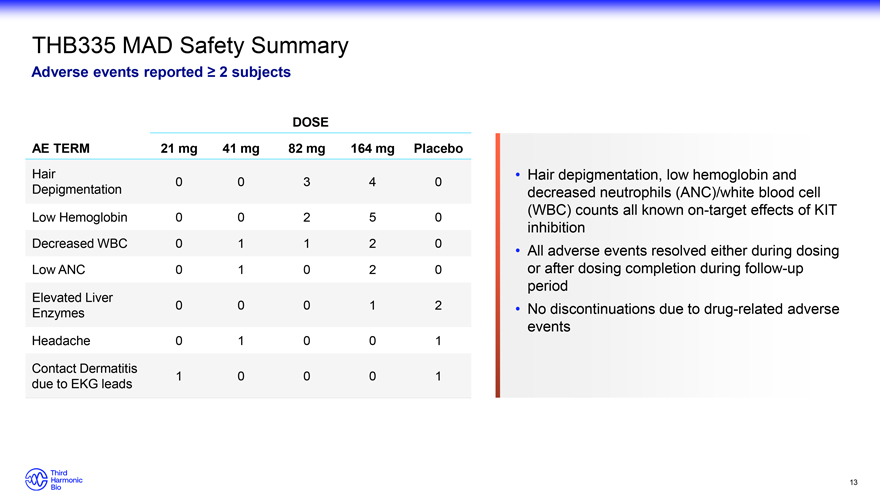
THB335 MAD Safety Summary Adverse events reported ? 2 subjects DOSE AE TERM 21 mg 41 mg 82 mg 164 mg Placebo Hair 0 0 3 4 0 Depigmentation Low Hemoglobin 0 0 2 5 0 Decreased WBC 0 1 1 2 0 Low ANC 0 1 0 2 0 Elevated Liver 0 0 0 1 2 Enzymes Headache 0 1 0 0 1 Contact Dermatitis 1 0 0 0 1 due to EKG leads Hair depigmentation, low hemoglobin and decreased neutrophils (ANC)/white blood cell (WBC) counts all known on-target effects of KIT inhibition All adverse events resolved either during dosing or after dosing completion during follow-up period No discontinuations due to drug-related adverse events 13
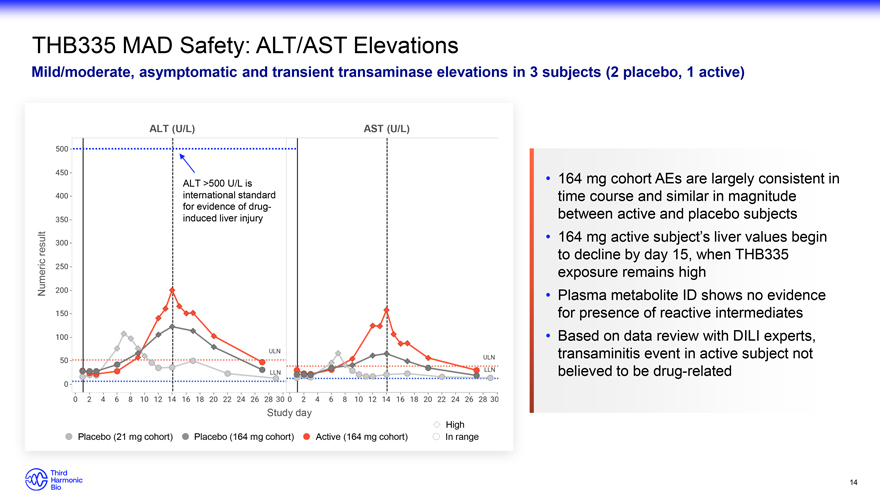
THB335 MAD Safety: ALT/AST Elevations Mild/moderate, asymptomatic and transient transaminase elevations in 3 subjects (2 placebo, 1 active) ALT (U/L) AST (U/L) ALT >500 U/L is 164 mg cohort AEs are largely consistent in international standard time course and similar in magnitude for evidence of drug- between active and placebo subjects induced liver injury result 164 mg active subject’s liver values begin to decline by day 15, when THB335 Numeric exposure remains high Plasma metabolite ID shows no evidence for presence of reactive intermediates Based on data review with DILI experts, transaminitis event in active subject not believed to be drug-related Study day High Placebo (21 mg cohort) Placebo (164 mg cohort) Active (164 mg cohort) In range 14
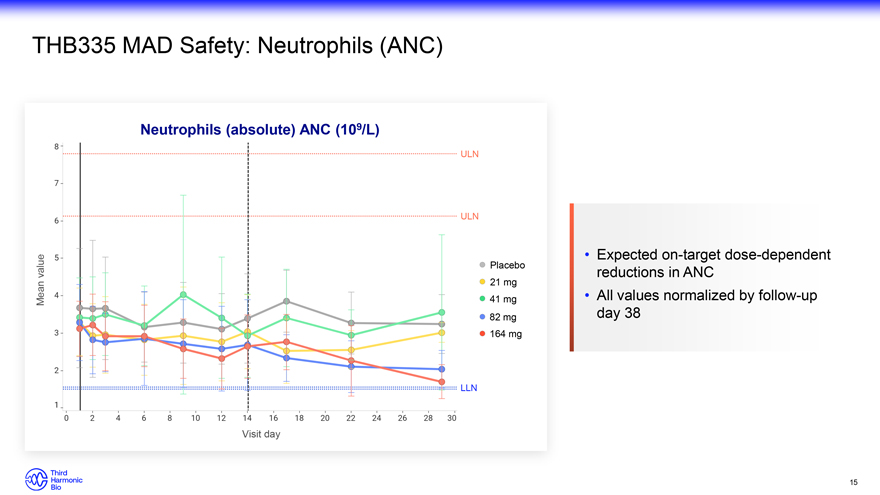
THB335 MAD Safety: Neutrophils (ANC) Neutrophils (absolute) ANC (109/L) ULN ULN Expected on-target dose-dependent value Placebo reductions in ANC 21 mg Mean 41 mg All values normalized by follow-up 82 mg day 38 164 mg LLN Visit day 15
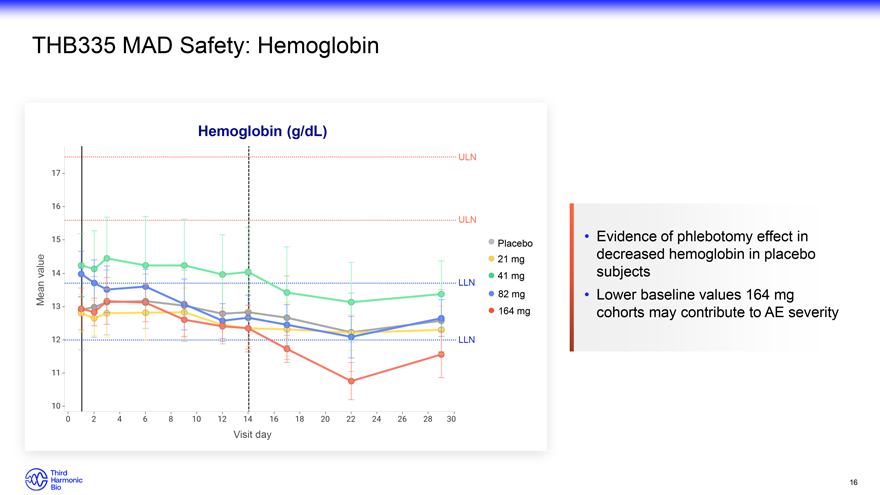
THB335 MAD Safety: Hemoglobin Hemoglobin (g/dL) ULN ULN Evidence of phlebotomy effect in Placebo 21 mg decreased hemoglobin in placebo value 41 mg subjects LLN Mean 82 mg Lower baseline values 164 mg 164 mg cohorts may contribute to AE severity LLN Visit day 16
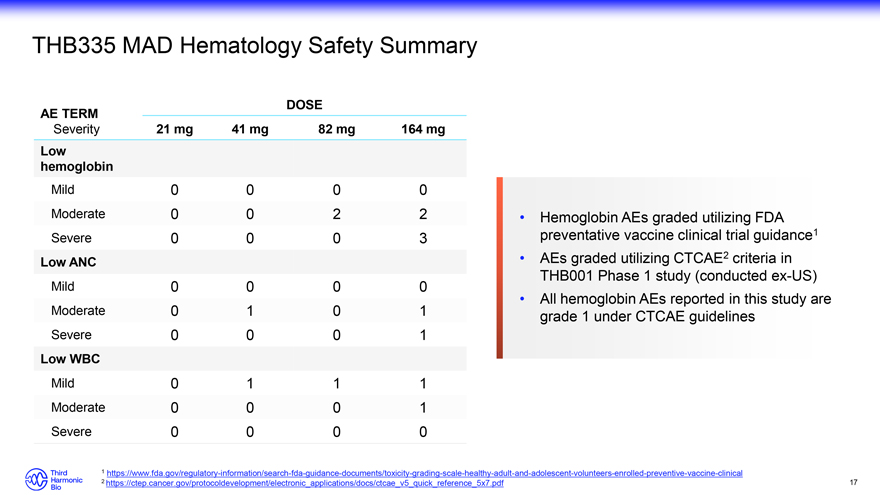
THB335 MAD Hematology Safety Summary DOSE AE TERM Severity 21 mg 41 mg 82 mg 164 mg Low hemoglobin Mild 0 0 0 0 Moderate 0 0 2 2 Severe 0 0 0 3 Low ANC Mild 0 0 0 0 Moderate 0 1 0 1 Severe 0 0 0 1 Low WBC Mild 0 1 1 1 Moderate 0 0 0 1 Severe 0 0 0 0 Hemoglobin AEs graded utilizing FDA preventative vaccine clinical trial guidance1 AEs graded utilizing CTCAE2 criteria in THB001 Phase 1 study (conducted ex-US) All hemoglobin AEs reported in this study are grade 1 under CTCAE guidelines 1 https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical 2 https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf 17
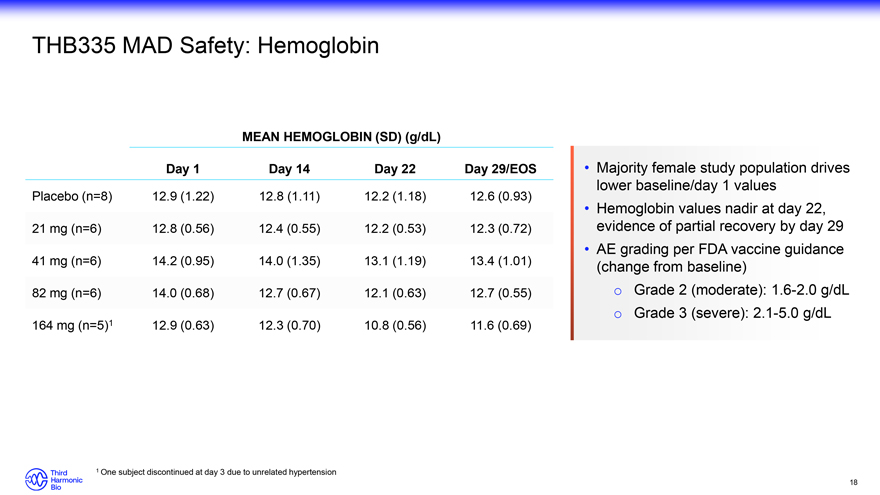
Majority female study population drives lower baseline/day 1 values Hemoglobin values nadir at day 22, evidence of partial recovery by day 29 AE grading per FDA vaccine guidance (change from baseline) o Grade 2 (moderate): 1.6-2.0 g/dL o Grade 3 (severe): 2.1-5.0 g/dL 1 One subject discontinued at day 3 due to unrelated hypertension 18
THB335 Phase 1 Clinical Trial Results Summary Once daily dosing with dose-dependent increases in exposure exceeding the KIT IC90 at doses > 41mg Sustained, dose-dependent decreases in serum tryptase Safety profile which supports evaluation of moderate KIT inhibition therapeutic index optimization hypothesis Provides basis for a Phase 2 clinical trial in CSU 19
“Pipeline-in-a-Target” Potential with KIT Inhibition Select Mast Cell-Mediated Diseases In addition to CSU, opportunity to pursue additional mast-cell mediated inflammatory disorders – Meaningful opportunity in severe asthma, where mast cells play a central role in pathophysiology and clear need exists for new oral therapies Other companies developing KIT inhibitors are pursuing proof-of-concept trials in atopic dermatitis, prurigo nodularis, asthma, eosinophilic esophagitis, allergic rhinitis/conjunctivitis, and mast cell activation syndrome – Clinical validation may enable fast-follow strategy 20

Summary and Next Steps Advancing THB335 toward Phase 2 trial in CSU and strategic review process underway Advancing THB335 toward Phase 2 readiness by mid-year 2025 Launched strategic review process to identify opportunities to maximize shareholder value Implemented restructuring to halt all non-THB335 R&D activities and reduce workforce by approximately 50% Cash and cash equivalents of $285 million as of December 31, 2024 (unaudited); projected cash and cash equivalents of approximately $262 to $267 million as of June 30, 2025 21

APPENDIX Previously Presented Data 22
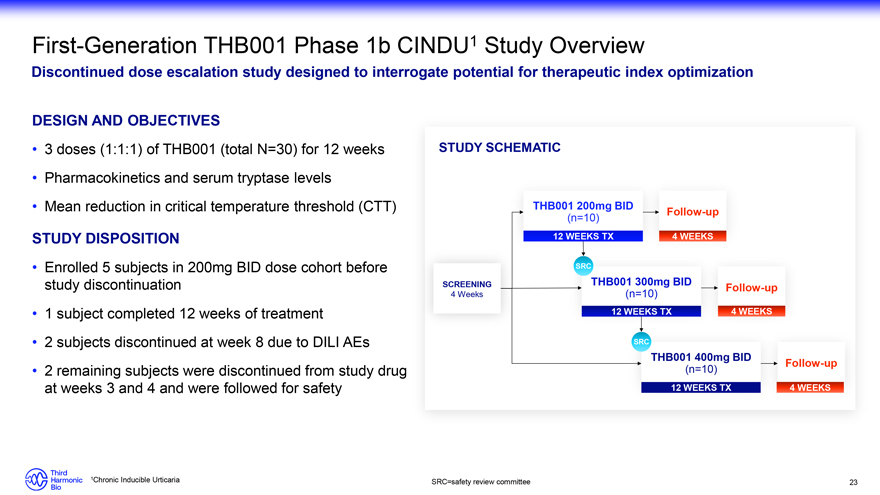
First-Generation THB001 Phase 1b CINDU1 Study Overview Discontinued dose escalation study designed to interrogate potential for therapeutic index optimization DESIGN AND OBJECTIVES 3 doses (1:1:1) of THB001 (total N=30) for 12 weeks Pharmacokinetics and serum tryptase levels Mean reduction in critical temperature threshold (CTT) STUDY DISPOSITION Enrolled 5 subjects in 200mg BID dose cohort before study discontinuation 1 subject completed 12 weeks of treatment• 2 subjects discontinued at week 8 due to DILI AEs 2 remaining subjects were discontinued from study drug at weeks 3 and 4 and were followed for safety STUDY SCHEMATIC THB001 200mg BID Follow-up (n=10) 12 WEEKS TX 4 WEEKS SRC SCREENING THB001 300mg BID Follow-up 4 Weeks (n=10) 12 WEEKS TX 4 WEEKS SRC THB001 400mg BID Follow-up (n=10) 12 WEEKS TX 4 WEEKS 1Chronic Inducible Urticaria SRC=safety review committee 23
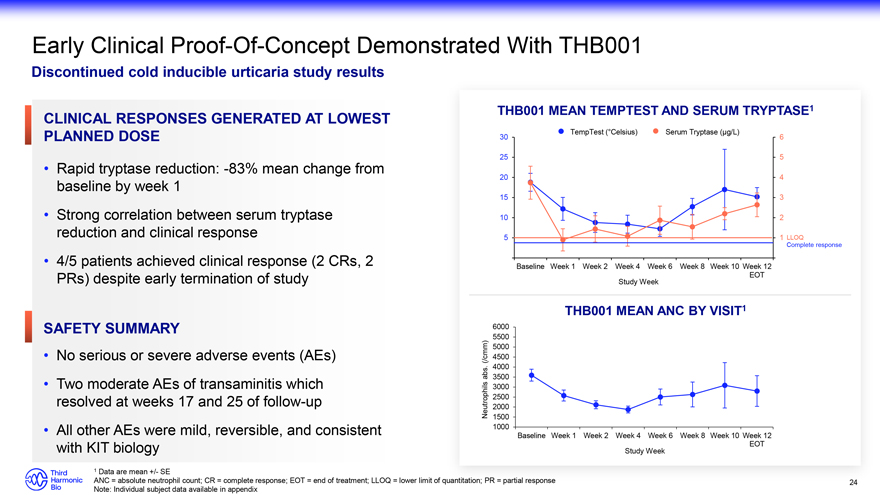
Early Clinical Proof-Of-Concept Demonstrated With THB001 Discontinued cold inducible urticaria study results THB001 MEAN TEMPTEST AND SERUM TRYPTASE1 TempTest (°Celsius) Serum Tryptase (µg/L) 30 6 25 5 20 4 15 3 10 2 5 1 Complete LLOQ response Baseline Week 1 Week 2 Week 4 Week 6 Week 8 Week 10 Week 12 EOT Study Week THB001 MEAN ANC BY VISIT1 6000 5500 5000 (/cmm) 4500 . 4000 abs 3500 3000 2500 Neutrophils 2000 1500 1000 Baseline Week 1 Week 2 Week 4 Week 6 Week 8 Week 10 Week 12 EOT Study Week 1 Data are mean +/- SE ANC = absolute neutrophil count; CR = complete response; EOT = end of treatment; LLOQ = lower limit of Note: Individual subject data available in appendix
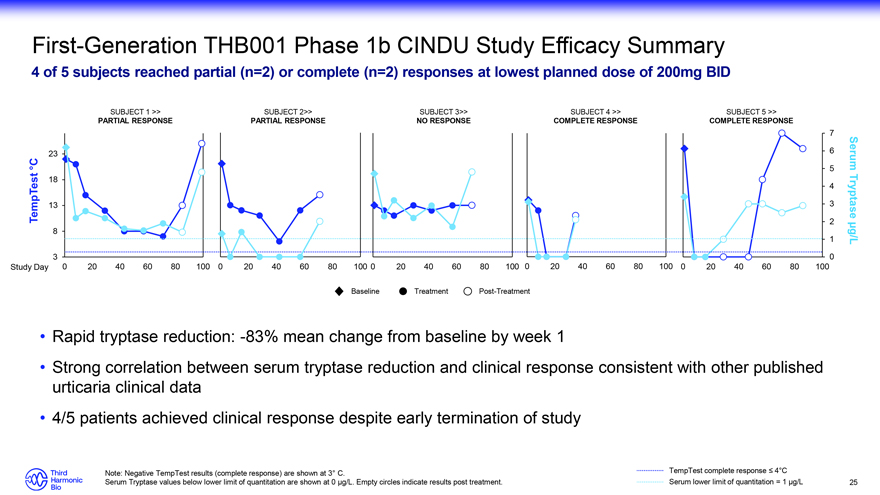
First-Generation THB001 Phase 1b CINDU Study Efficacy Summary 4 of 5 subjects reached partial (n=2) or complete (n=2) responses at lowest planned dose of 200mg BID SUBJECT 1 >> SUBJECT 2>> SUBJECT 3>> SUBJECT 4 >> SUBJECT 5 >> PARTIAL RESPONSE PARTIAL RESPONSE NO RESPONSE COMPLETE RESPONSE COMPLETE RESPONSE 7 23 6 °C 5 Serum 18 4 13 3 Tryptase TempTest 2 8 1 µg/L 3 0 Study Day 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 0 20 40 60 80 100 Baseline Treatment Post-Treatment Rapid tryptase reduction: -83% mean change from baseline by week 1 Strong correlation between serum tryptase reduction and clinical response consistent with other published urticaria clinical data 4/5 patients achieved clinical response despite early termination of study Note: Negative TempTest results (complete response) are shown at 3° C. TempTest complete response ? 4°C Serum Tryptase values below lower limit of quantitation are shown at 0 µg/L. Empty circles indicate results post treatment. Serum lower limit of quantitation = 1 µg/L 25
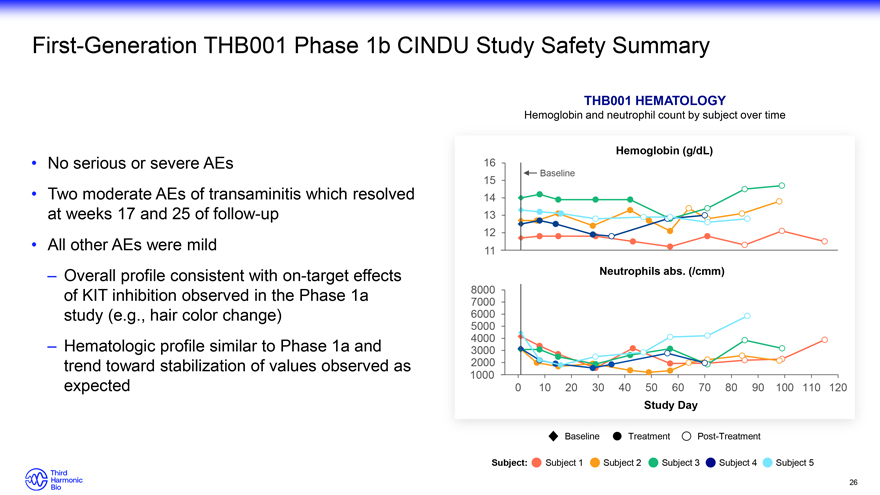
First-Generation THB001 Phase 1b CINDU Study Safety Summary No serious or severe AEs Two moderate AEs of transaminitis which resolved at weeks 17 and 25 of follow-up All other AEs were mild – Overall profile consistent with on-target effects of KIT inhibition observed in the Phase 1a study (e.g., hair color change) – Hematologic profile similar to Phase 1a and trend toward stabilization of values observed as expected THB001 HEMATOLOGY Hemoglobin and neutrophil count by subject over time Hemoglobin (g/dL) 16 Baseline 15 14 13 12 11 Neutrophils abs. (/cmm) 8000 7000 6000 5000 4000 3000 2000 1000 0 10 20 30 40 50 60 70 80 90 100 110 120 Study Day Baseline Treatment Post-Treatment Subject: Subject 1 Subject 2 Subject 3 Subject 4 Subject 5 26
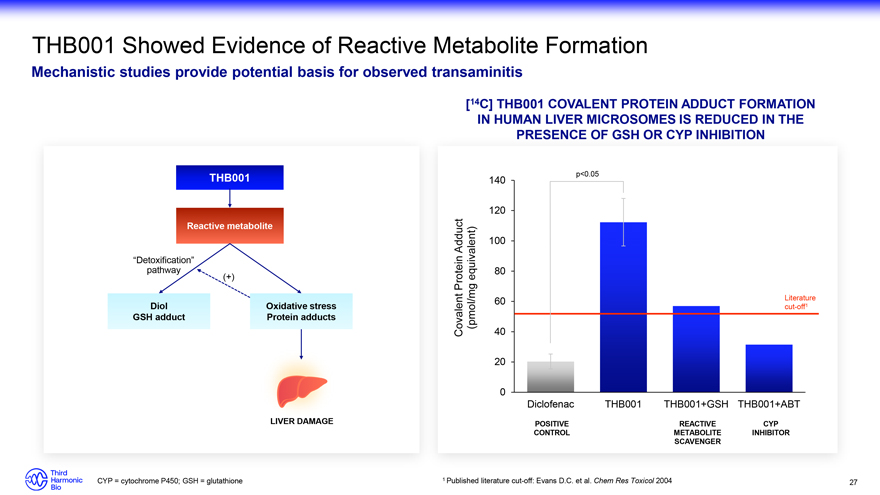
THB001 Showed Evidence of Reactive Metabolite Formation Mechanistic studies provide potential basis for observed transaminitis [14C] THB001 COVALENT PROTEIN ADDUCT FORMATION IN HUMAN LIVER MICROSOMES IS REDUCED IN THE PRESENCE OF GSH OR CYP INHIBITION THB001 Reactive metabolite “Detoxification” pathway (+) Diol Oxidative stress GSH adduct Protein adducts LIVER DAMAGE p<0.05 140 120 Adduct 100 equivalent) 80 Protein 60 Literature (pmol/mg cut-off1 Covalent 40 20 0 Diclofenac THB001 THB001+GSH THB001+ABT POSITIVE REACTIVE CYP CONTROL METABOLITE INHIBITOR SCAVENGER CYP = cytochrome P450; GSH = glutathione 1 Published literature cut-off: Evans D.C. et al. Chem Res Toxicol 2004 27
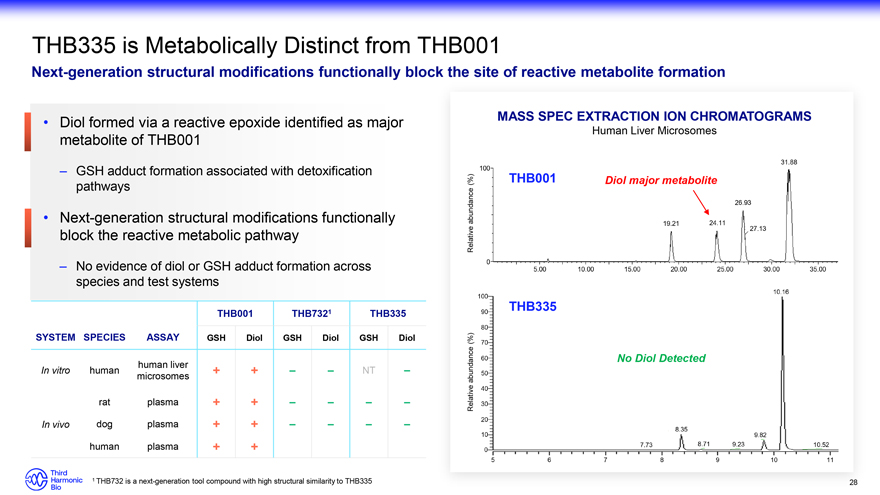
THB335 is Metabolically Distinct from THB001 Next-generation structural modifications functionally block the site of reactive metabolite formation Diol formed via a reactive epoxide identified as major metabolite of THB001 – GSH adduct formation associated with detoxification pathways Next-generation structural modifications functionally block the reactive metabolic pathway – No evidence of diol or GSH adduct formation across species and test systems THB001 THB7321 THB335 SYSTEM SPECIES ASSAY GSH Diol GSH Diol GSH Diol human liver In vitro human + + NT microsomes rat plasma + + In vivo dog plasma + + human plasma + + MASS SPEC EXTRACTION ION CHROMATOGRAMS Human Liver Microsomes (%) THB001 Diol major metabolite abundance Relative THB335 (%) abundance No Diol Detected Relative 1 THB732 is a next-generation tool compound with high structural similarity to THB335
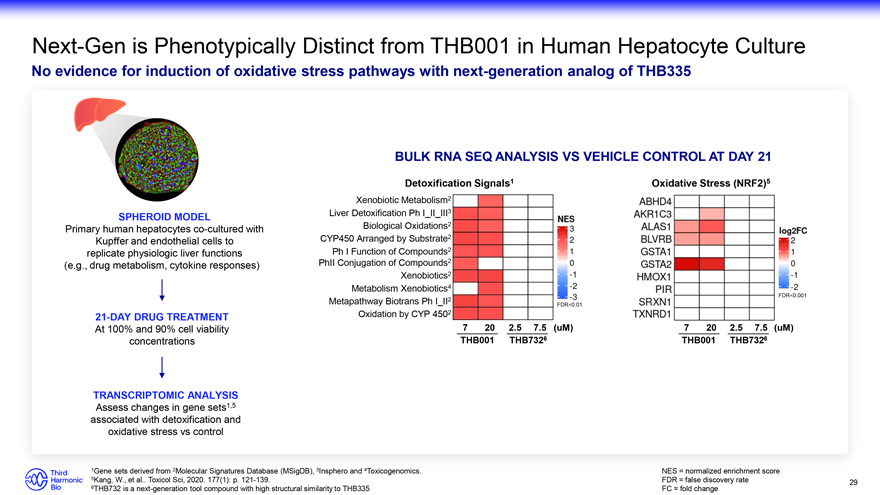
Next-Gen is Phenotypically Distinct from THB001 in Human Hepatocyte Culture No evidence for induction of oxidative stress pathways with next-generation analog of THB335 SPHEROID MODEL Primary human hepatocytes co-cultured with Kupffer and endothelial cells to replicate physiologic liver functions (e.g., drug metabolism, cytokine responses) 21-DAY DRUG TREATMENT At 100% and 90% cell viability concentrations TRANSCRIPTOMIC ANALYSIS Assess changes in gene sets1,5 associated with detoxification and oxidative stress vs control BULK RNA SEQ ANALYSIS VS VEHICLE CONTROL AT DAY 21 Detoxification Signals1 Oxidative Stress (NRF2)5 Xenobiotic Metabolism2 Liver Detoxification Ph I_II_III3 Biological Oxidations2 CYP450 Arranged by Substrate2 Ph I Function of Compounds2 PhII Conjugation of Compounds2 Xenobiotics2 Metabolism Xenobiotics4 FDR<0.001 Metapathway Biotrans Ph I_II2 FDR<0.01 Oxidation by CYP 4502 7 20 2.5 7.5 (uM) 7 20 2.5 7.5 (uM) THB001 THB7326 THB001 THB7326 NES = normalized enrichment score 5Kang, W., et al. Toxicol Sci, 2020. 177(1): p. 121-139. FDR = false discovery rate 29 6THB732 is a next-generation tool compound with high structural similarity to THB335 FC = fold change

Third Harmonic Bio 30