
© 2025 Biohaven, Ltd. All rights reserved. JENNIFER Living with SCA3 Participant in the Troriluzole Clinical Study Corporate Presentation June 2025

Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Biohaven Ltd. (the “Company”) and our planned and ongoing trials for our troriluzole, taldefgrobep alfa, BHV-7000, BHV-2100, BHV-8000, BHV-1300, BHV-1400, BHV-1510, and BHV-1600 development programs, the timing of and the availability of data from our clinical trials, the timing and our decisions to proceed with our planned regulatory filings, the timing of and our ability to obtain regulatory approvals for our product candidates, the clinical potential utility of our product candidates, alone and as compared to other existing potential treatment options, and the potential advancement of our early phase programs including BHV-1310, BHV-1530, and BHV-1500. The use of certain words, including “continue”, “plan”, “will”, “believe”, “may”, “expect”, “anticipate” and similar expressions, is intended to identi fy forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of our development candidates, including the potential FDA approval and commercialization of troriluzole for SCA, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the Food and Drug Administration, including those regarding the potential FDA approval of troriluzole for SCA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; the potential for Biohaven’s product candidates to be first-in-class, best-in-class, best-in-clinic or best-in-category therapies; and the effectiveness and safety of Biohaven's product candidates, including open label clinical data in ongoing studies. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company’s filings with the Securities and Exchange Commission, including within the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. This presentation also contains market data and other information based on industry publications, reports by market research firms or published independent sources. Some market data and information are also based on the Company’s good faith estimates, which are derived from management’s knowledge of its industry and such independent sources referred to above. June 2025 Biohaven Investor Presentation2
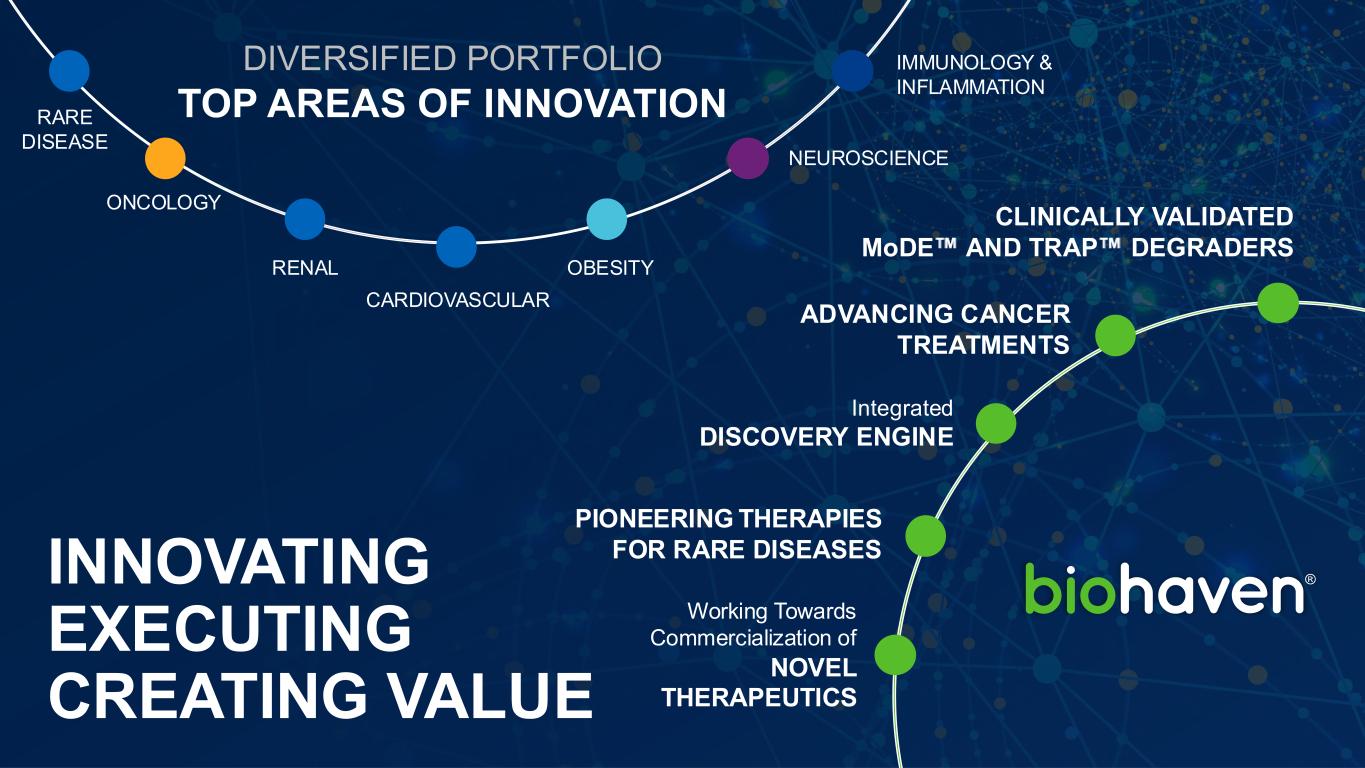
Working Towards Commercialization of NOVEL THERAPEUTICS INNOVATING EXECUTING CREATING VALUE PIONEERING THERAPIES FOR RARE DISEASES Integrated DISCOVERY ENGINE CLINICALLY VALIDATED MoDE AND TRAP DEGRADERS ADVANCING CANCER TREATMENTS DIVERSIFIED PORTFOLIO TOP AREAS OF INNOVATION IMMUNOLOGY & INFLAMMATION NEUROSCIENCE OBESITY ONCOLOGY CARDIOVASCULAR RENAL RARE DISEASE
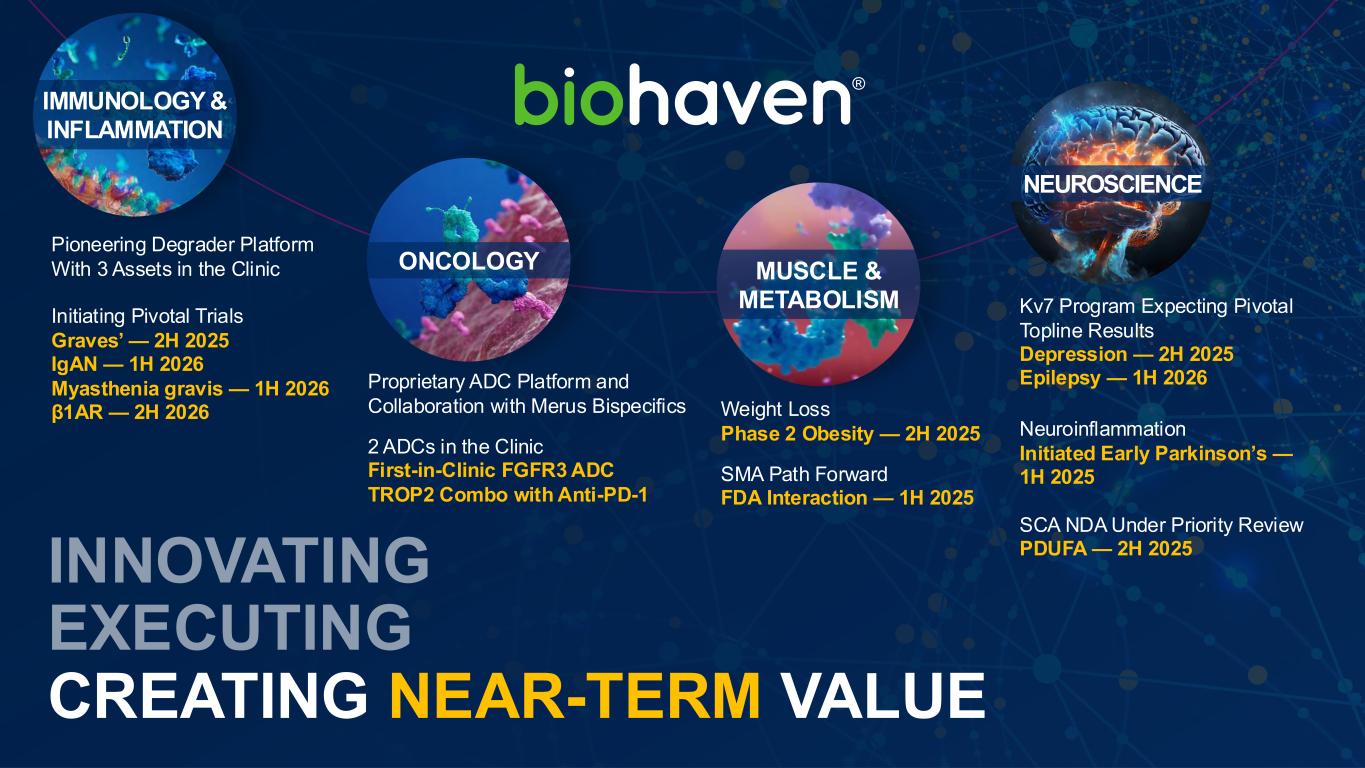
CREATING NEAR-TERM VALUE Kv7 Program Expecting Pivotal Topline Results Depression — 2H 2025 Epilepsy — 1H 2026 Neuroinflammation Initiated Early Parkinson’s — 1H 2025 SCA NDA Under Priority Review PDUFA — 2H 2025 Pioneering Degrader Platform With 3 Assets in the Clinic Initiating Pivotal Trials Graves’ — 2H 2025 IgAN — 1H 2026 Myasthenia gravis — 1H 2026 β1AR — 2H 2026 Proprietary ADC Platform and Collaboration with Merus Bispecifics 2 ADCs in the Clinic First-in-Clinic FGFR3 ADC TROP2 Combo with Anti-PD-1 Weight Loss Phase 2 Obesity — 2H 2025 SMA Path Forward FDA Interaction — 1H 2025 MUSCLE & METABOLISM IMMUNOLOGY & INFLAMMATION ONCOLOGY NEUROSCIENCE
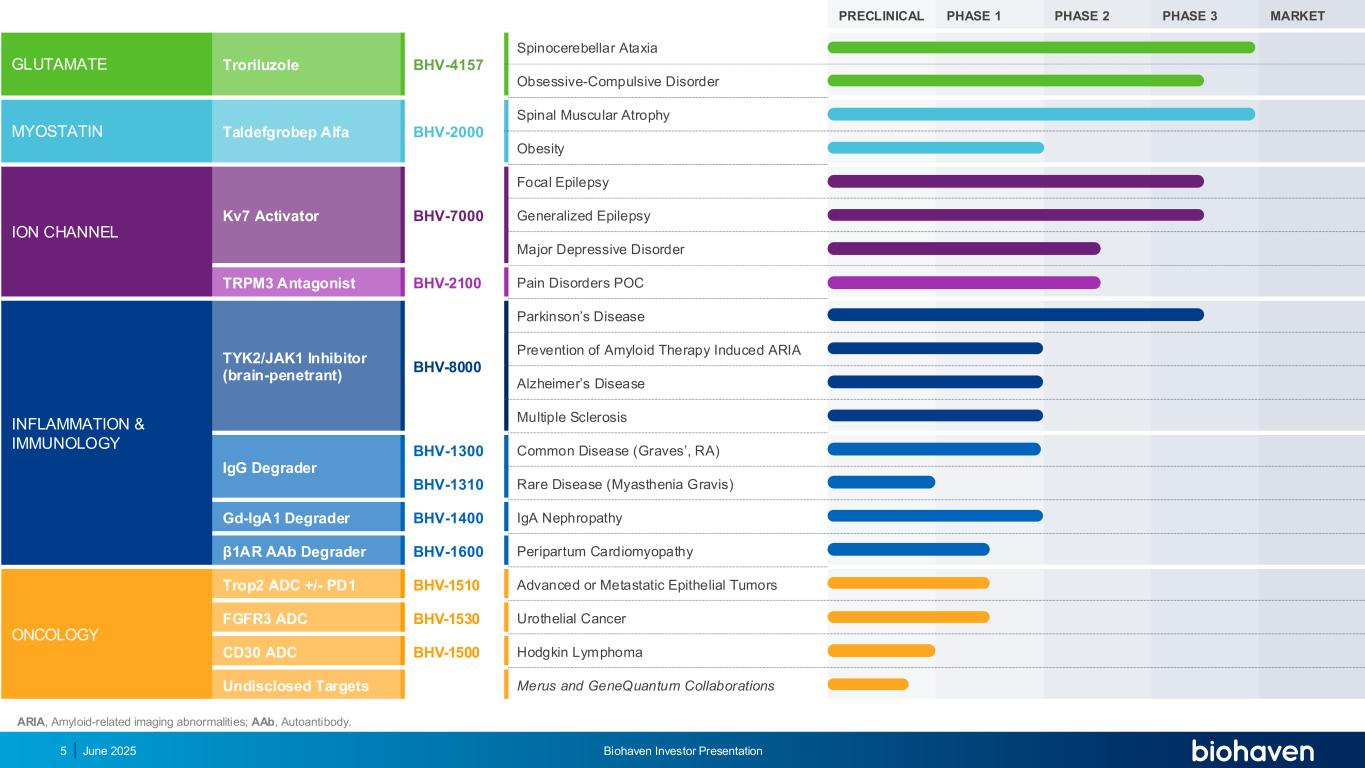
ARIA, Amyloid-related imaging abnormalities; AAb, Autoantibody. PRECLINICAL PHASE 1 PHASE 2 PHASE 3 MARKET GLUTAMATE Troriluzole BHV-4157 Spinocerebellar Ataxia Obsessive-Compulsive Disorder MYOSTATIN Taldefgrobep Alfa BHV-2000 Spinal Muscular Atrophy Obesity ION CHANNEL Kv7 Activator BHV-7000 Focal Epilepsy Generalized Epilepsy Major Depressive Disorder TRPM3 Antagonist BHV-2100 Pain Disorders POC INFLAMMATION & IMMUNOLOGY TYK2/JAK1 Inhibitor (brain-penetrant) BHV-8000 Parkinson’s Disease Prevention of Amyloid Therapy Induced ARIA Alzheimer’s Disease Multiple Sclerosis IgG Degrader BHV-1300 Common Disease (Graves’, RA) BHV-1310 Rare Disease (Myasthenia Gravis) Gd-IgA1 Degrader BHV-1400 IgA Nephropathy β1AR AAb Degrader BHV-1600 Peripartum Cardiomyopathy ONCOLOGY Trop2 ADC +/- PD1 BHV-1510 Advanced or Metastatic Epithelial Tumors FGFR3 ADC BHV-1530 Urothelial Cancer CD30 ADC BHV-1500 Hodgkin Lymphoma Undisclosed Targets Merus and GeneQuantum Collaborations June 2025 Biohaven Investor Presentation5
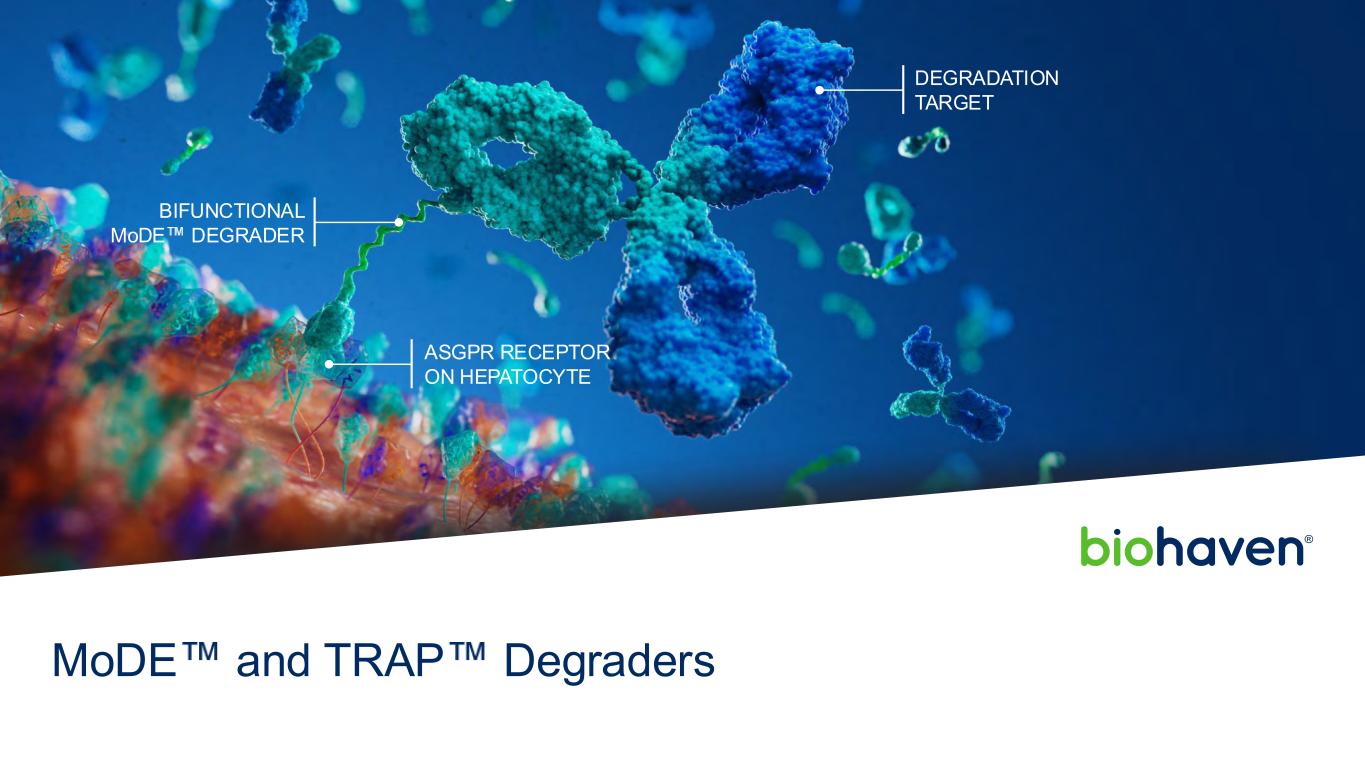
DEGRADATION TARGET BIFUNCTIONAL MoDE DEGRADER ASGPR RECEPTOR ON HEPATOCYTE MoDE and TRAP Degraders
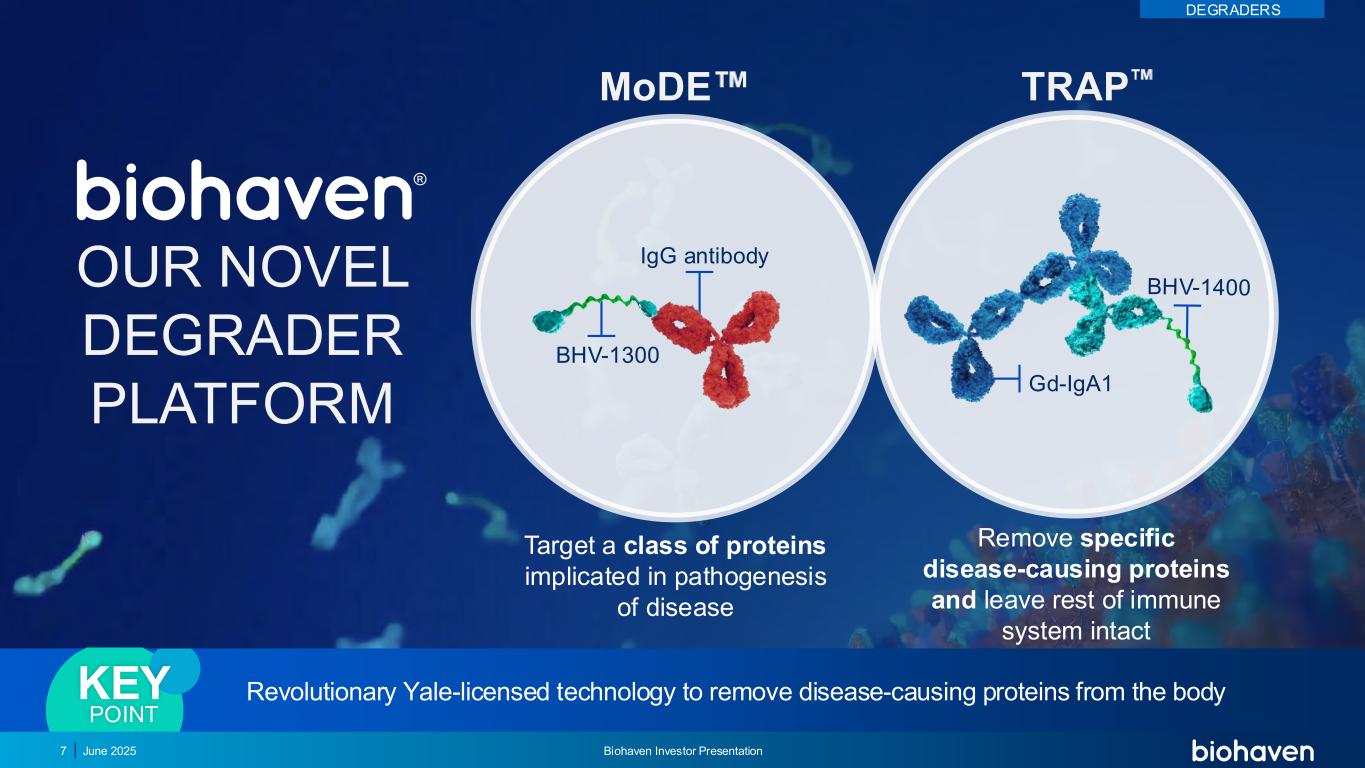
Gd-IgA1 IgG antibody BHV-1300 TRAPMoDE OUR NOVEL DEGRADER PLATFORM Revolutionary Yale-licensed technology to remove disease-causing proteins from the bodyKEY POINT Target a class of proteins implicated in pathogenesis of disease Remove specific disease-causing proteins and leave rest of immune system intact 7 June 2025 Biohaven Investor Presentation DEGRADERS
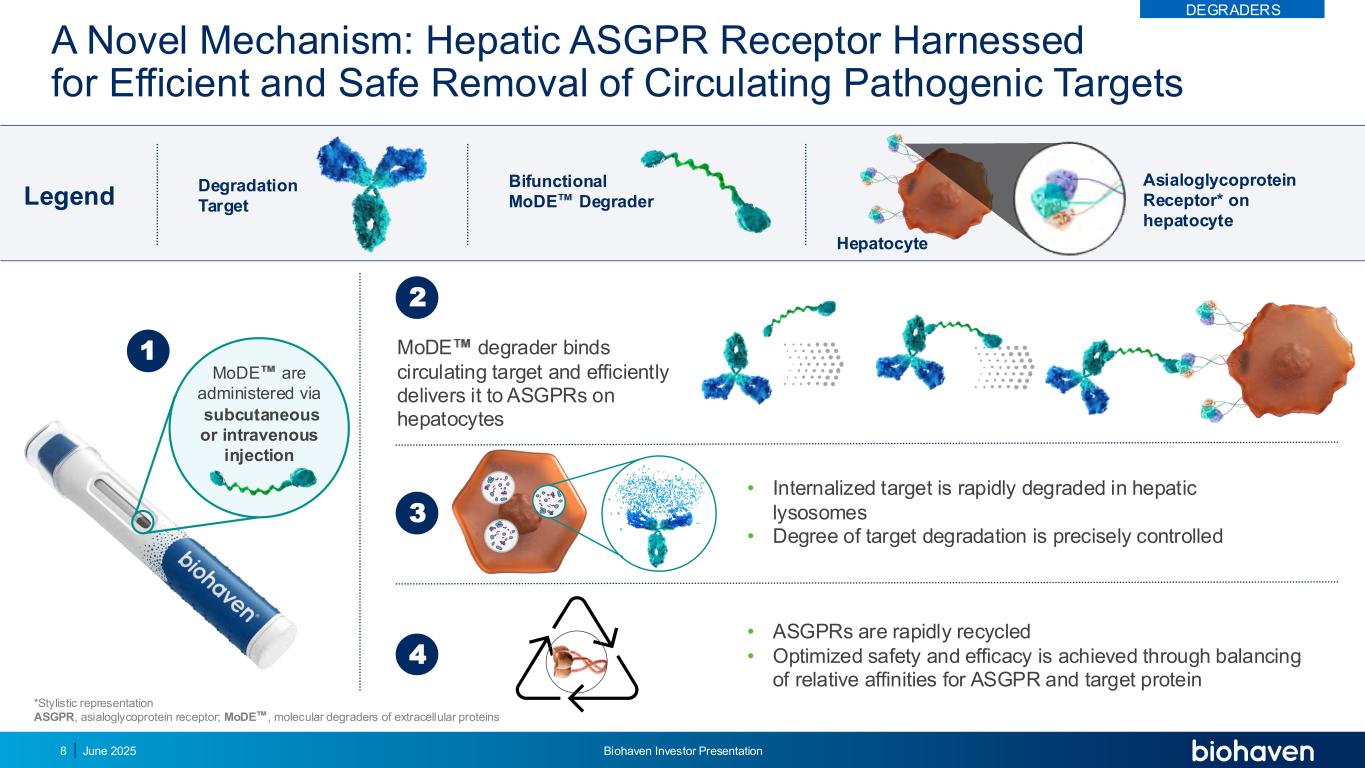
A Novel Mechanism: Hepatic ASGPR Receptor Harnessed for Efficient and Safe Removal of Circulating Pathogenic Targets subcutaneous or intravenous injection MoDE are administered via *Stylistic representation ASGPR, asialoglycoprotein receptor; MoDE , molecular degraders of extracellular proteins • Internalized target is rapidly degraded in hepatic lysosomes • Degree of target degradation is precisely controlled 3 1 Legend Degradation Target Bifunctional MoDE Degrader Asialoglycoprotein Receptor* on hepatocyte • ASGPRs are rapidly recycled • Optimized safety and efficacy is achieved through balancing of relative affinities for ASGPR and target protein 4 MoDE degrader binds circulating target and efficiently delivers it to ASGPRs on hepatocytes 2 DEGRADERS Hepatocyte June 2025 Biohaven Investor Presentation8
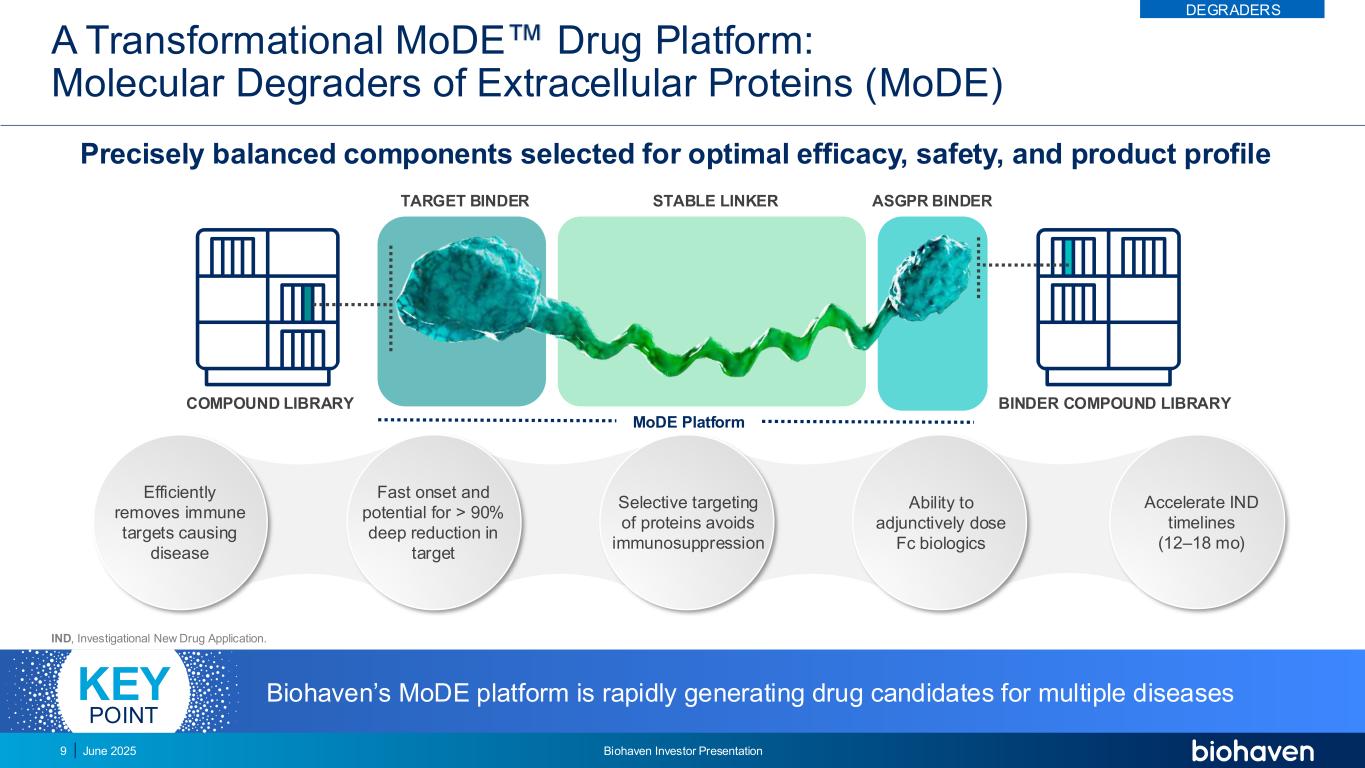
A Transformational MoDE Drug Platform: Molecular Degraders of Extracellular Proteins (MoDE) Precisely balanced components selected for optimal efficacy, safety, and product profile TARGET BINDER STABLE LINKER ASGPR BINDER COMPOUND LIBRARY BINDER COMPOUND LIBRARY MoDE Platform IND, Investigational New Drug Application. Accelerate IND timelines (12–18 mo) Efficiently removes immune targets causing disease Fast onset and potential for > 90% deep reduction in target Selective targeting of proteins avoids immunosuppression Ability to adjunctively dose Fc biologics Biohaven’s MoDE platform is rapidly generating drug candidates for multiple diseasesKEY POINT DEGRADERS June 2025 Biohaven Investor Presentation9
Catalyzing Innovation for Patients and Early Demonstration of Removal of Disease-Causing Protein June 2025 Biohaven Investor Presentation10 C R E A T I N G V A L U EE X E C U T I N GI N N O V A T I N G Follow the Science Understand the Need PRECISION IMMUNOLOGY TARGET THE DISEASE, NOT THE PATIENT KEEP THE PATIENT AT THE CENTER Establish Early Target Efficacy Create Value NEAR- AND LONG-TERM DEGRADERS PHASE 1 PD ENDPOINT N Y S E : B H V N
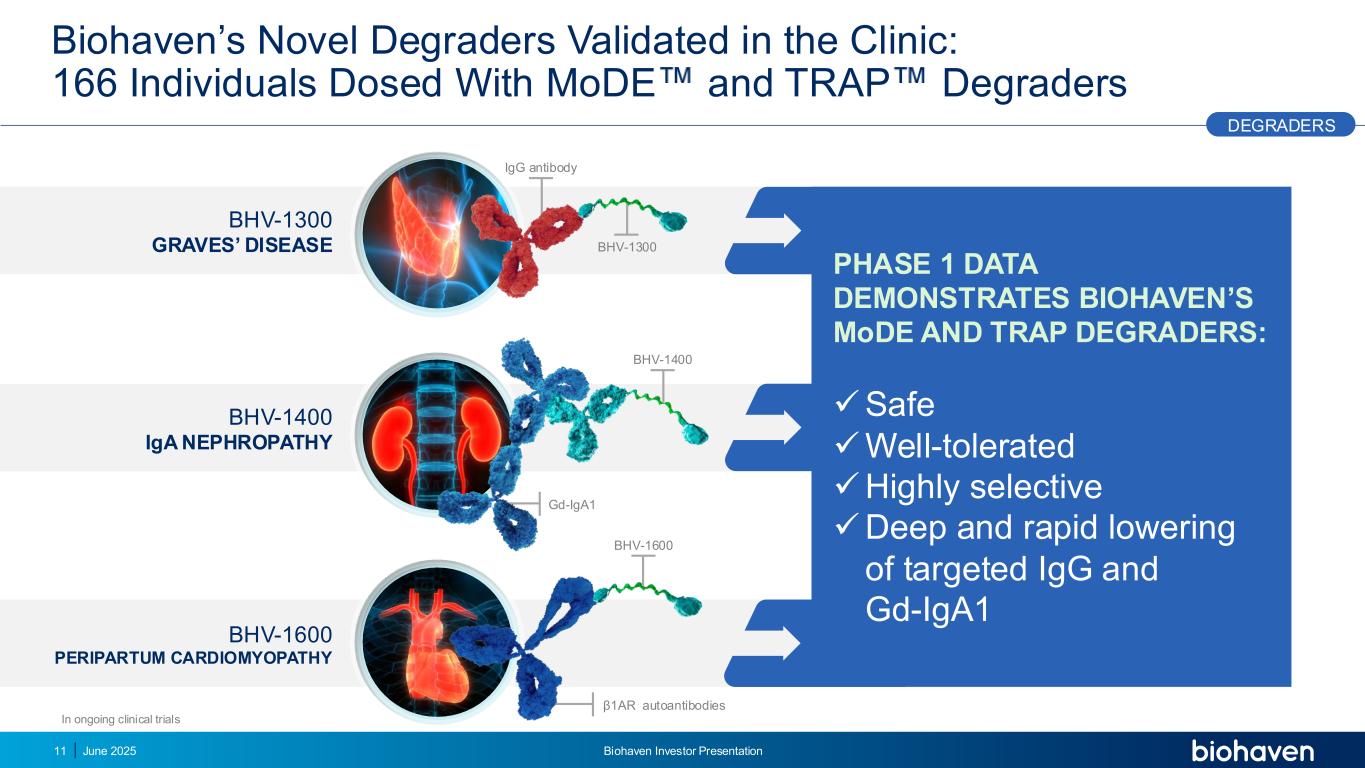
BHV-1600 PERIPARTUM CARDIOMYOPATHY Biohaven’s Novel Degraders Validated in the Clinic: 166 Individuals Dosed With MoDE and TRAP Degraders BHV-1400 IgA NEPHROPATHY BHV-1300 GRAVES’ DISEASE PHASE 1 DATA DEMONSTRATES BIOHAVEN’S MoDE AND TRAP DEGRADERS: ✓Safe ✓Well-tolerated ✓Highly selective ✓Deep and rapid lowering of targeted IgG and Gd-IgA1 Gd-IgA1 BHV-1400 IgG antibody BHV-1300 β1AR autoantibodies BHV-1600 In ongoing clinical trials DEGRADERS 11 June 2025 Biohaven Investor Presentation
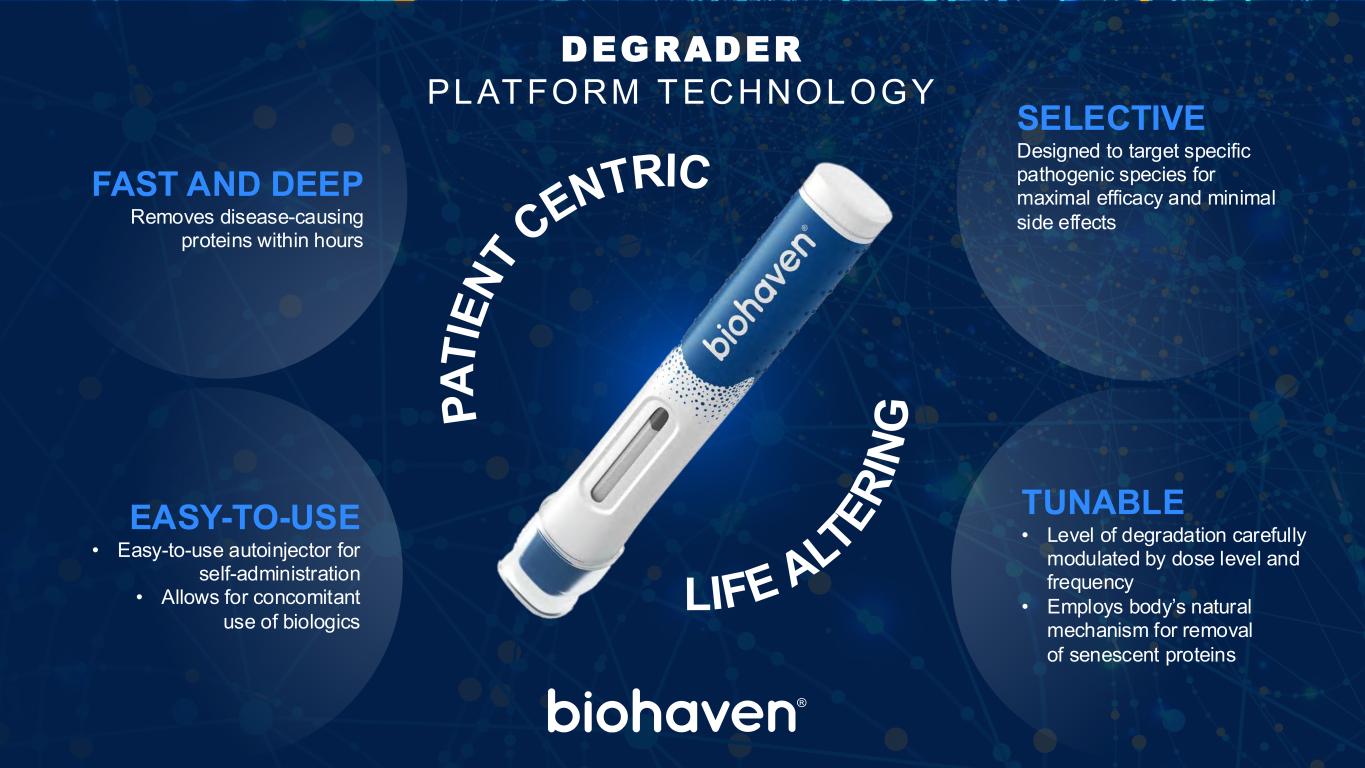
P A T IE N T CENTRIC LIFE ALTE R IN G FAST AND DEEP Removes disease-causing proteins within hours EASY-TO-USE • Easy-to-use autoinjector for self-administration • Allows for concomitant use of biologics SELECTIVE Designed to target specific pathogenic species for maximal efficacy and minimal side effects TUNABLE • Level of degradation carefully modulated by dose level and frequency • Employs body’s natural mechanism for removal of senescent proteins DEGRADER PLATFORM TECHNOLOGY
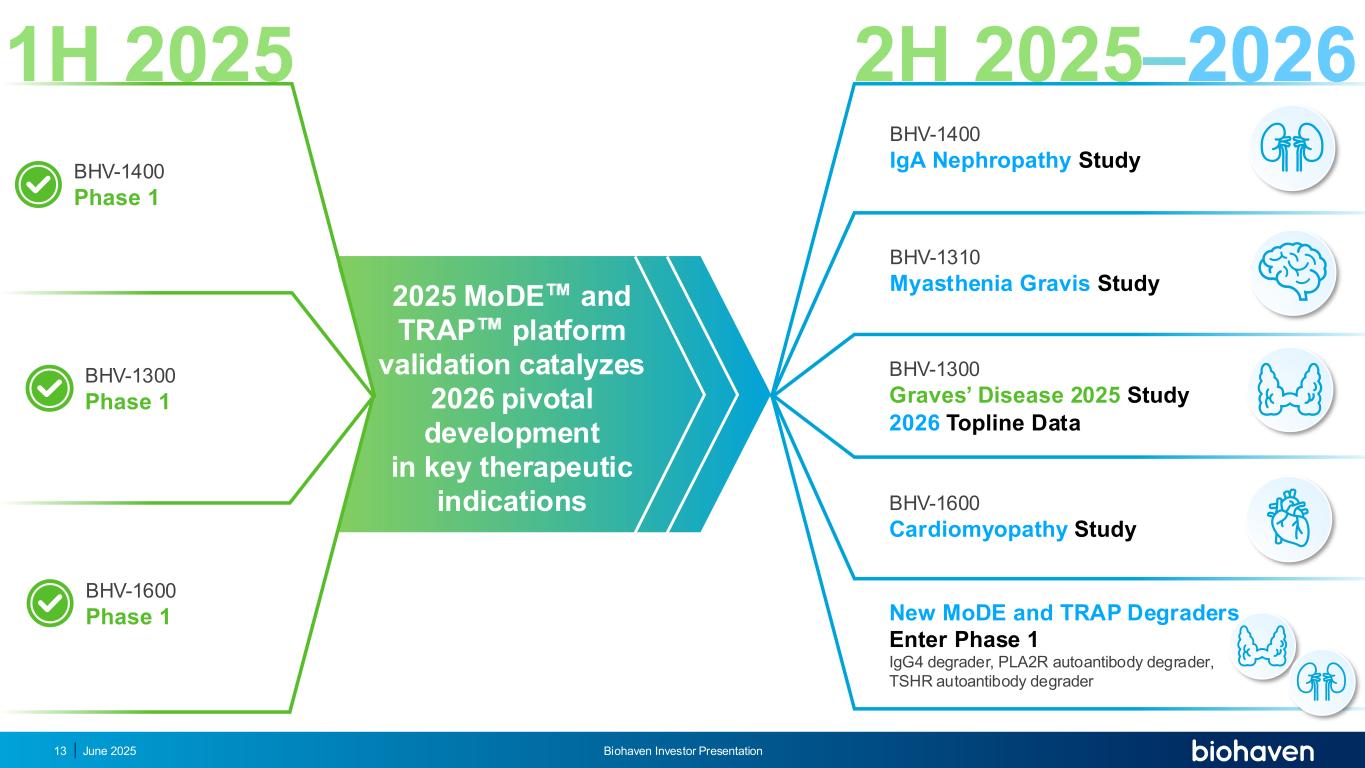
1H 2025 BHV-1300 Graves’ Disease 2025 Study 2026 Topline Data BHV-1310 Myasthenia Gravis Study BHV-1600 Cardiomyopathy Study New MoDE and TRAP Degraders Enter Phase 1 IgG4 degrader, PLA2R autoantibody degrader, TSHR autoantibody degrader 2H 2025–2026 BHV-1400 IgA Nephropathy Study BHV-1300 Phase 1 BHV-1400 Phase 1 BHV-1600 Phase 1 2025 MoDE and TRAP platform validation catalyzes 2026 pivotal development in key therapeutic indications June 2025 Biohaven Investor Presentation13
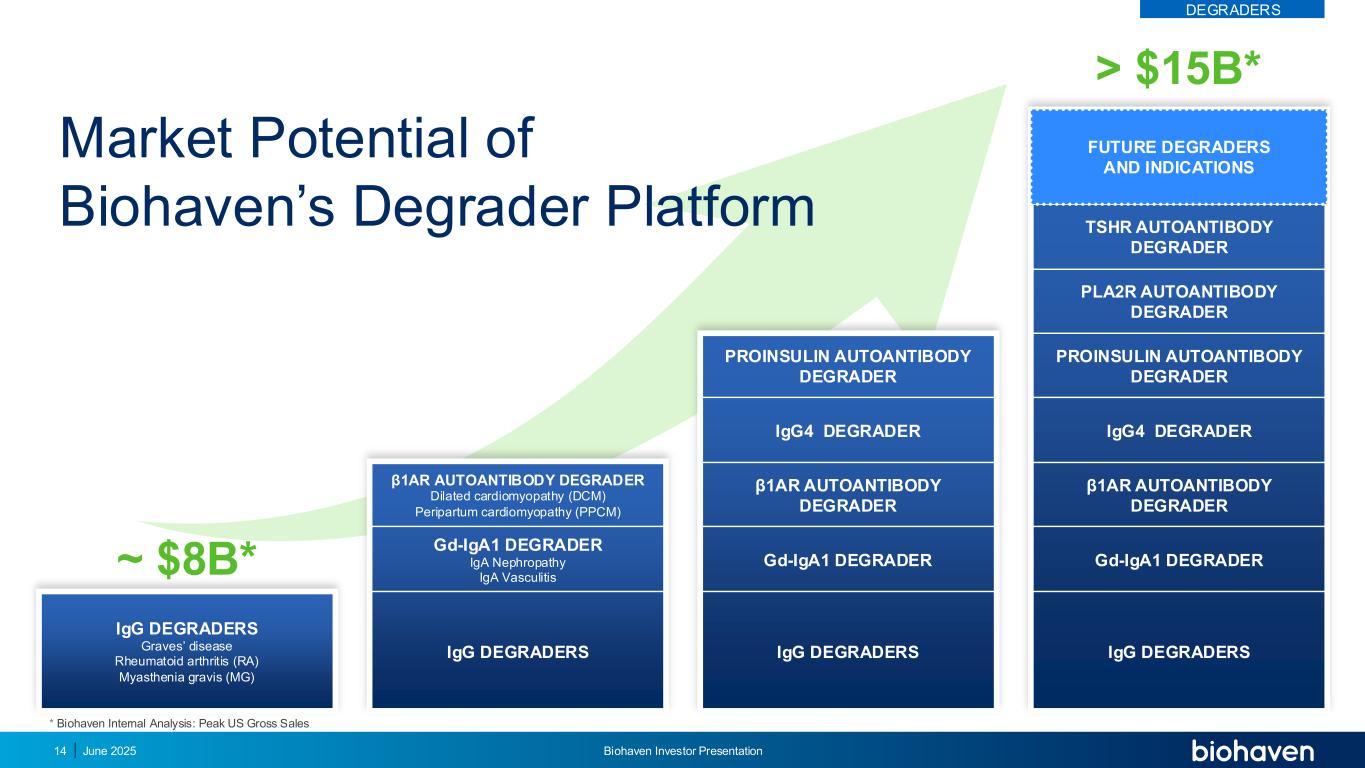
IgG DEGRADERS Graves’ disease Rheumatoid arthritis (RA) Myasthenia gravis (MG) IgG DEGRADERS Gd-IgA1 DEGRADER β1AR AUTOANTIBODY DEGRADER IgG4 DEGRADER PROINSULIN AUTOANTIBODY DEGRADER PLA2R AUTOANTIBODY DEGRADER TSHR AUTOANTIBODY DEGRADER ~ $8B* > $15B* Market Potential of Biohaven’s Degrader Platform FUTURE DEGRADERS AND INDICATIONS IgG DEGRADERS Gd-IgA1 DEGRADER β1AR AUTOANTIBODY DEGRADER IgG4 DEGRADER PROINSULIN AUTOANTIBODY DEGRADER IgG DEGRADERS Gd-IgA1 DEGRADER IgA Nephropathy IgA Vasculitis β1AR AUTOANTIBODY DEGRADER Dilated cardiomyopathy (DCM) Peripartum cardiomyopathy (PPCM) * Biohaven Internal Analysis: Peak US Gross Sales DEGRADERS June 2025 Biohaven Investor Presentation14

Gd-IgA1 TRAP Degrader
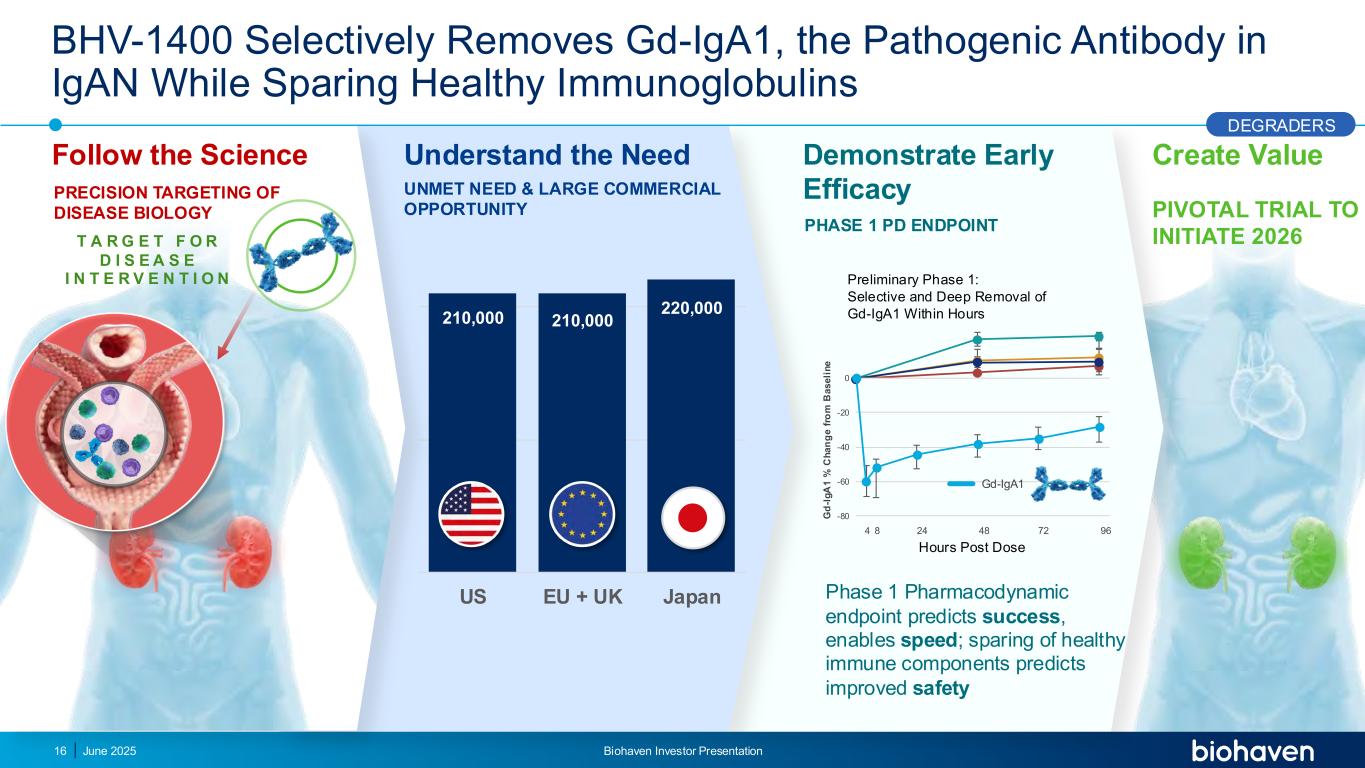
BHV-1400 Selectively Removes Gd-IgA1, the Pathogenic Antibody in IgAN While Sparing Healthy Immunoglobulins Phase 1 Pharmacodynamic endpoint predicts success, enables speed; sparing of healthy immune components predicts improved safety Demonstrate Early Efficacy Create Value PIVOTAL TRIAL TO INITIATE 2026 PHASE 1 PD ENDPOINT June 2025 Biohaven Investor Presentation16 Understand the Need UNMET NEED & LARGE COMMERCIAL OPPORTUNITY 210,000 210,000 220,000 US EU + UK Japan PRECISION TARGETING OF DISEASE BIOLOGY T A R G E T F O R D I S E A S E I N T E R V E N T I O N Follow the Science Preliminary Phase 1: Selective and Deep Removal of Gd-IgA1 Within Hours -80 -60 -40 -20 0 4 8 24 48 72 96 G d -I g A 1 % C h a n g e f ro m B a s e li n e Hours Post Dose DEGRADERS Gd-IgA1
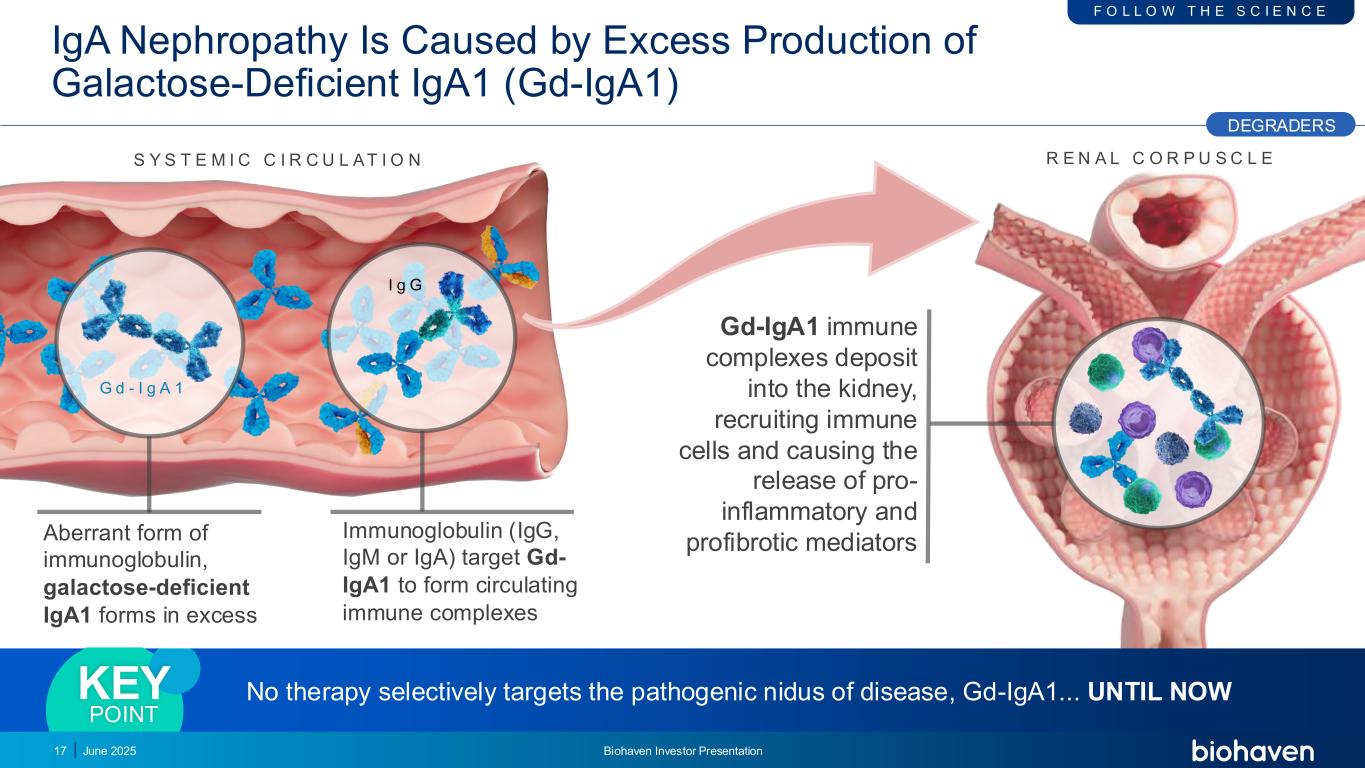
Gd-IgA1 immune complexes deposit into the kidney, recruiting immune cells and causing the release of pro- inflammatory and profibrotic mediators S Y S T E M I C C I R C U L A T I O N Aberrant form of immunoglobulin, galactose-deficient IgA1 forms in excess Immunoglobulin (IgG, IgM or IgA) target Gd- IgA1 to form circulating immune complexes G d - I g A 1 I g G R E N A L C O R P U S C L E IgA Nephropathy Is Caused by Excess Production of Galactose-Deficient IgA1 (Gd-IgA1) No therapy selectively targets the pathogenic nidus of disease, Gd-IgA1... UNTIL NOWKEY POINT DEGRADERS F O L L O W T H E S C I E N C E 17 June 2025 Biohaven Investor Presentation
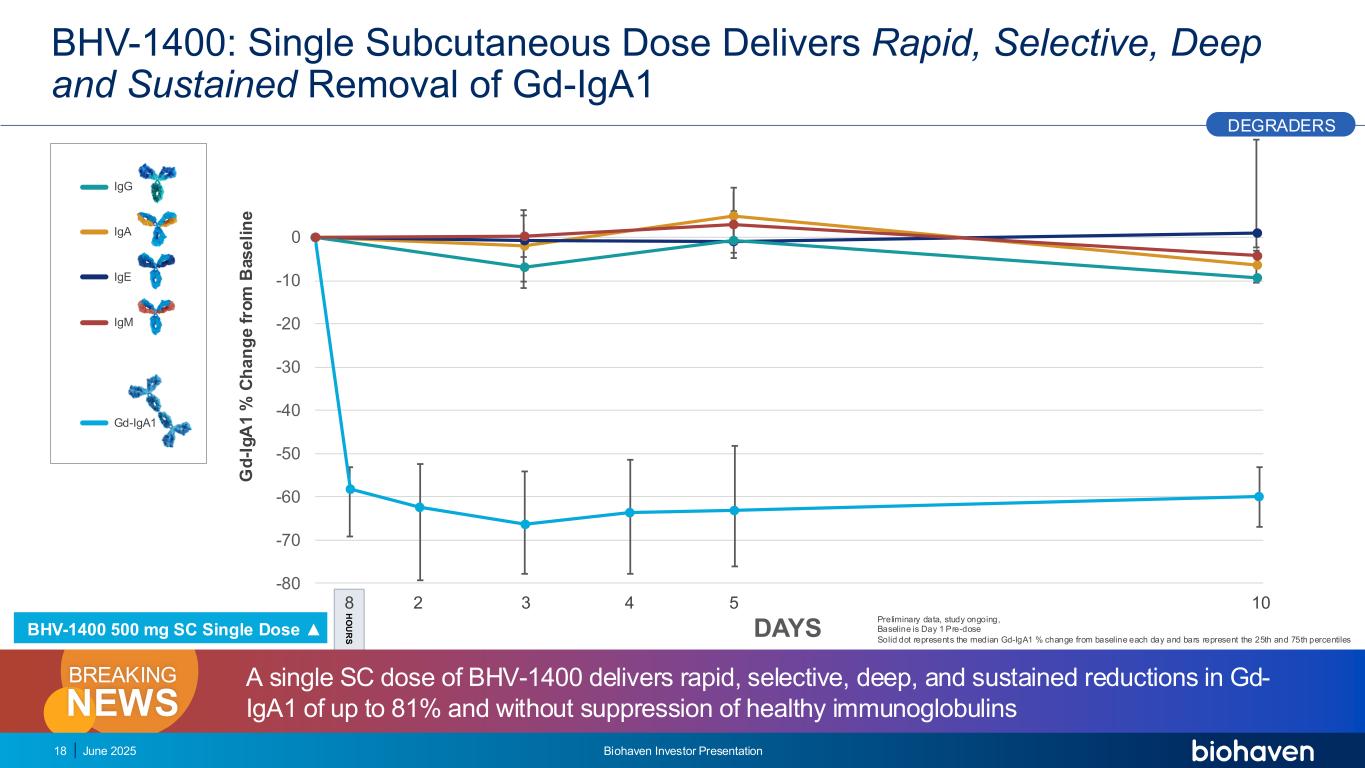
-80 -70 -60 -50 -40 -30 -20 -10 0 BHV-1400: Single Subcutaneous Dose Delivers Rapid, Selective, Deep and Sustained Removal of Gd-IgA1 G d -I g A 1 % C h a n g e f ro m B a s e li n e IgG IgA IgE IgM Gd-IgA1 18 DAYS 2 3 4 5 108 H O U R S DEGRADERS June 2025 Biohaven Investor Presentation A single SC dose of BHV-1400 delivers rapid, selective, deep, and sustained reductions in Gd- IgA1 of up to 81% and without suppression of healthy immunoglobulinsNEWS BREAKING Preliminary data, study ongoing, Baseline is Day 1 Pre-dose Solid dot represents the median Gd-IgA1 % change from baseline each day and bars represent the 25th and 75th percentiles BHV-1400 500 mg SC Single Dose ▲
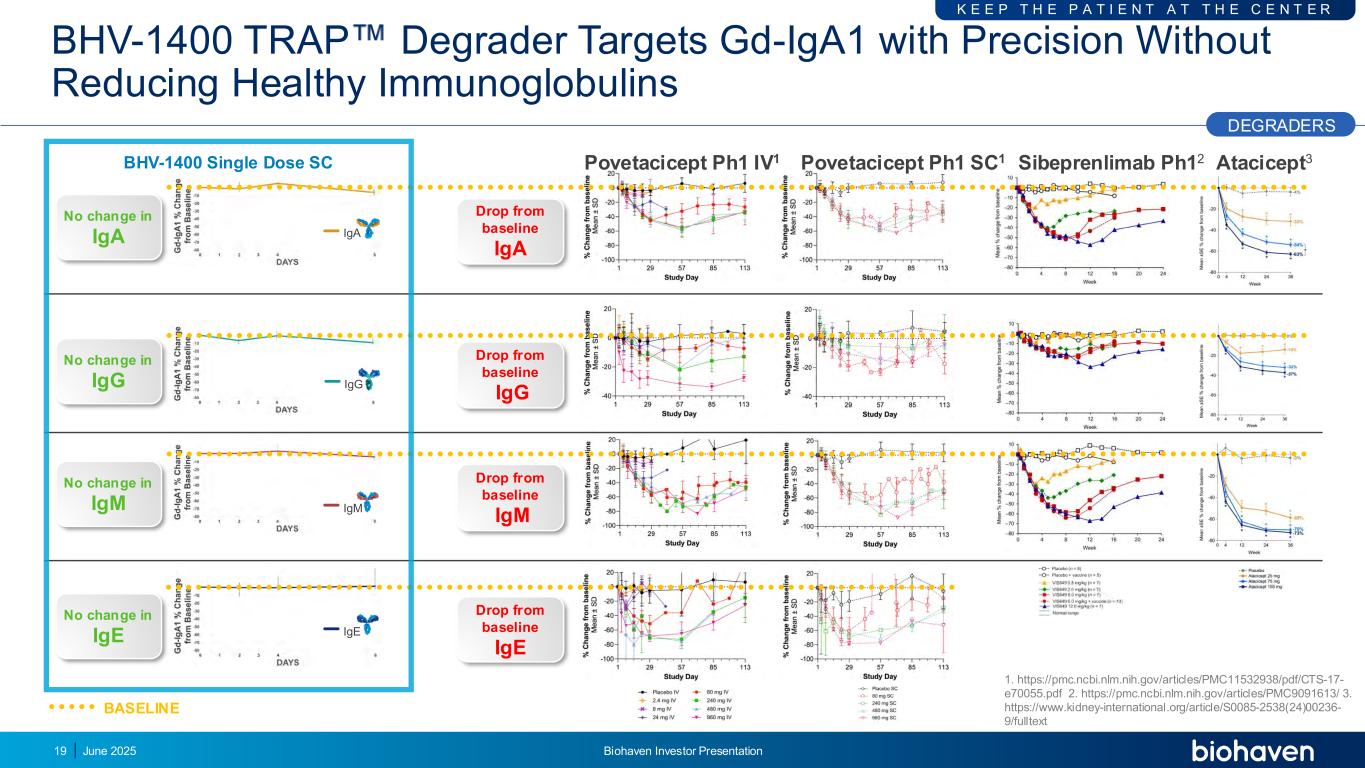
BHV-1400 TRAP Degrader Targets Gd-IgA1 with Precision Without Reducing Healthy Immunoglobulins June 2025 Biohaven Investor Presentation19 IgG IgA IgE IgM BHV-1400 Single Dose SC Sibeprenlimab Ph12Povetacicept Ph1 IV1 Povetacicept Ph1 SC1 Atacicept3 DEGRADERS K E E P T H E P A T I E N T A T T H E C E N T E R No change in IgA No change in IgG No change in IgM No change in IgE Drop from baseline IgA Drop from baseline IgG Drop from baseline IgM Drop from baseline IgE BASELINE 1. https://pmc.ncbi.nlm.nih.gov/articles/PMC11532938/pdf/CTS-17- e70055.pdf 2. https://pmc.ncbi.nlm.nih.gov/articles/PMC9091613/ 3. https://www.kidney-international.org/article/S0085-2538(24)00236- 9/fulltext
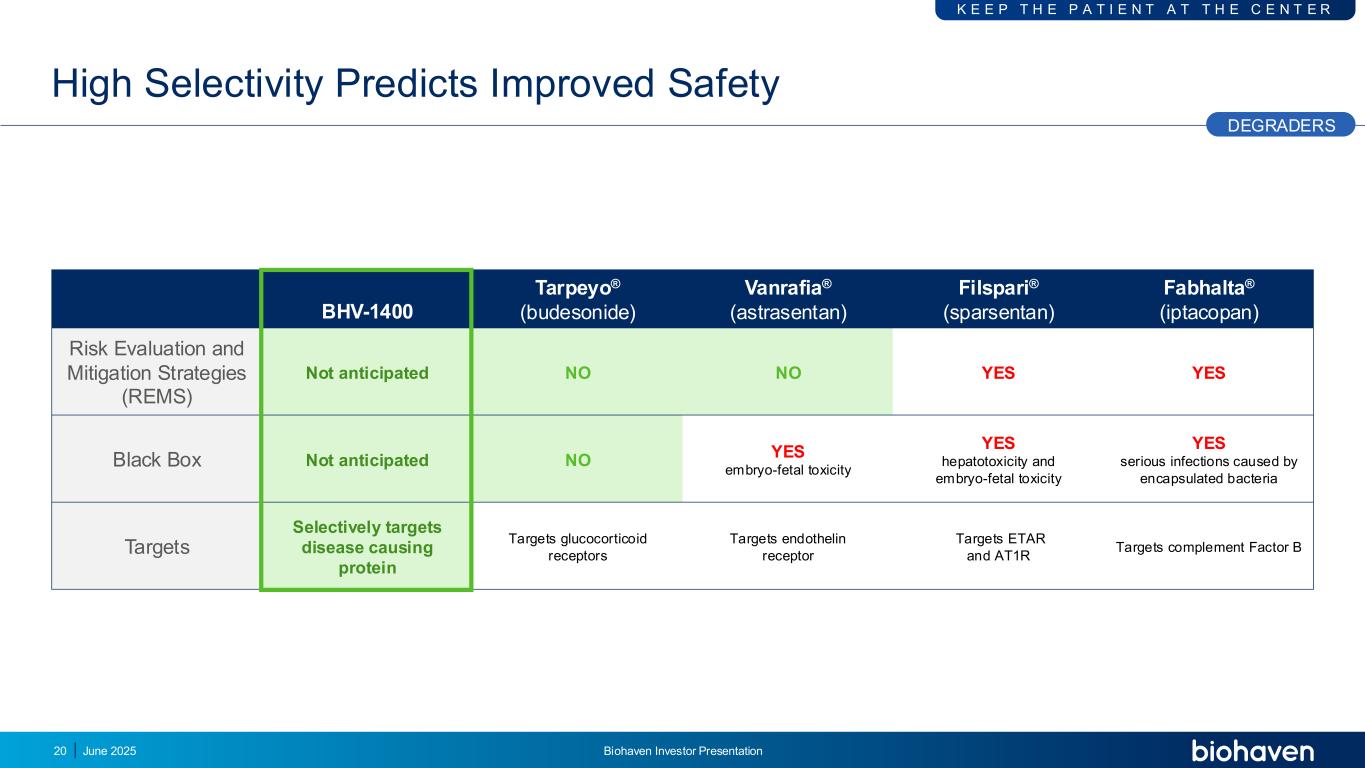
High Selectivity Predicts Improved Safety DEGRADERS BHV-1400 Tarpeyo® (budesonide) Vanrafia® (astrasentan) Filspari® (sparsentan) Fabhalta® (iptacopan) Risk Evaluation and Mitigation Strategies (REMS) Not anticipated NO NO YES YES Black Box Not anticipated NO YES embryo-fetal toxicity YES hepatotoxicity and embryo-fetal toxicity YES serious infections caused by encapsulated bacteria Targets Selectively targets disease causing protein Targets glucocorticoid receptors Targets endothelin receptor Targets ETAR and AT1R Targets complement Factor B 20 K E E P T H E P A T I E N T A T T H E C E N T E R June 2025 Biohaven Investor Presentation
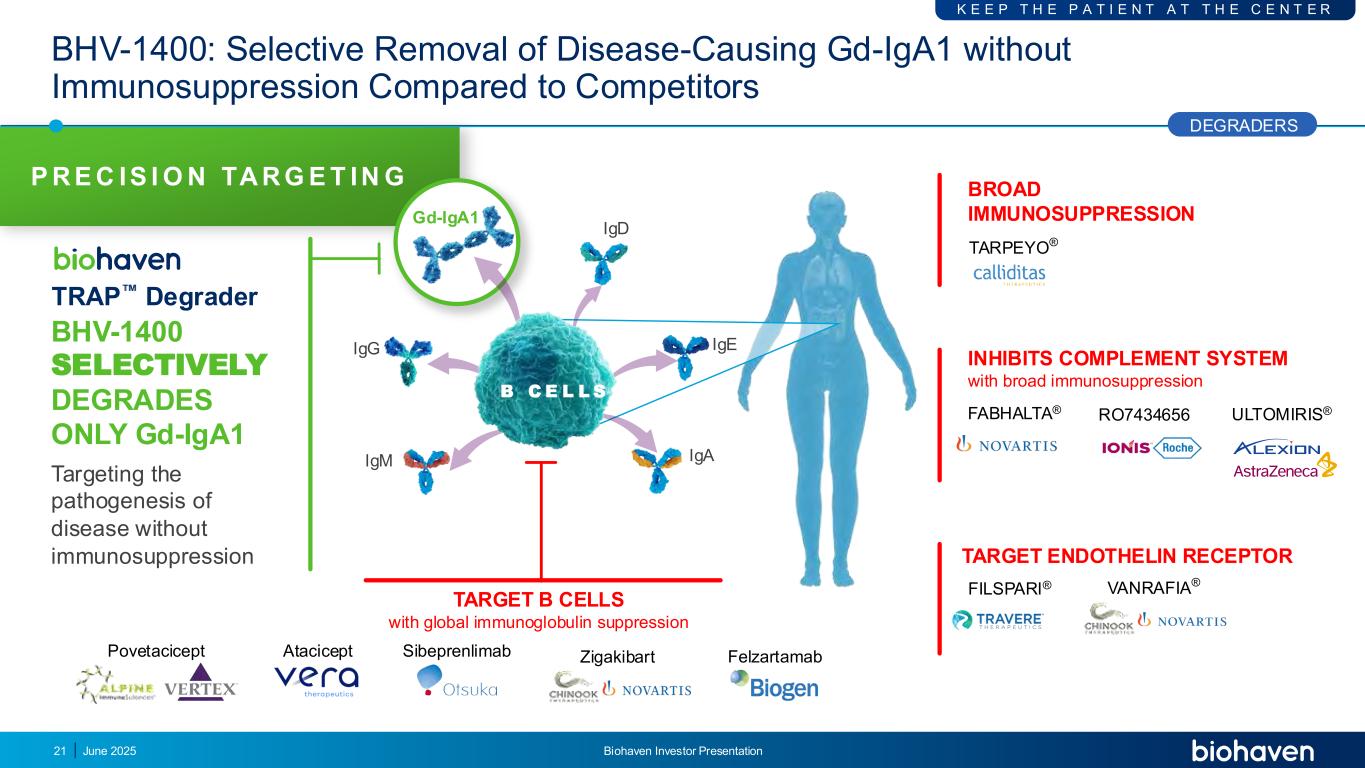
P R E C IS I O N TA R G E T IN G BHV-1400 SELECTIVELY DEGRADES ONLY Gd-IgA1 Targeting the pathogenesis of disease without immunosuppression TARGET B CELLS with global immunoglobulin suppression Povetacicept Atacicept Sibeprenlimab Zigakibart Felzartamab BHV-1400: Selective Removal of Disease-Causing Gd-IgA1 without Immunosuppression Compared to Competitors TRAP Degrader IgD IgM IgE IgA IgG Gd-IgA1 B C E L L S DEGRADERS K E E P T H E P A T I E N T A T T H E C E N T E R 21 INHIBITS COMPLEMENT SYSTEM with broad immunosuppression FABHALTA® RO7434656 VANRAFIA® FILSPARI® BROAD IMMUNOSUPPRESSION TARPEYO® ULTOMIRIS® TARGET ENDOTHELIN RECEPTOR June 2025 Biohaven Investor Presentation
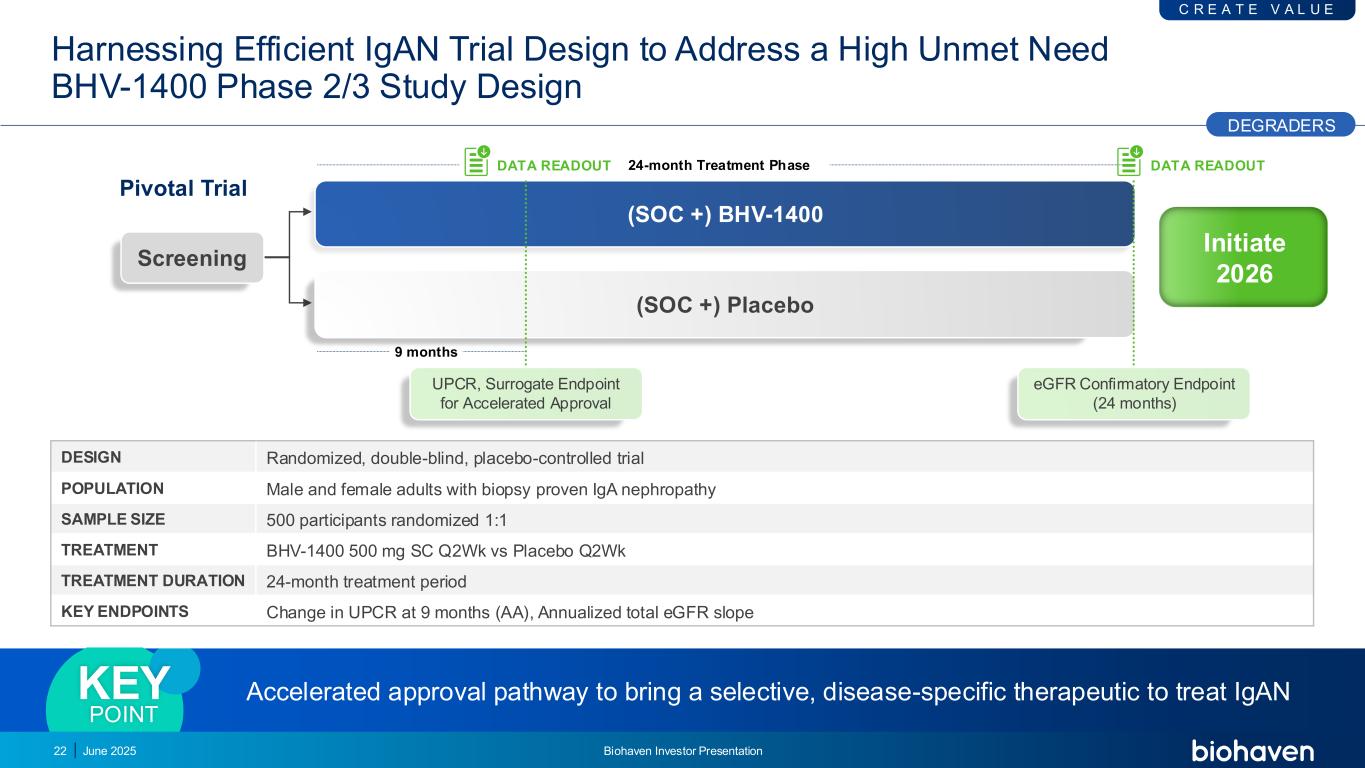
Harnessing Efficient IgAN Trial Design to Address a High Unmet Need BHV-1400 Phase 2/3 Study Design 22 9 months 24-month Treatment Phase (SOC +) BHV-1400 (SOC +) Placebo Screening Pivotal Trial UPCR, Surrogate Endpoint for Accelerated Approval DATA READOUT DATA READOUT eGFR Confirmatory Endpoint (24 months) DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Male and female adults with biopsy proven IgA nephropathy SAMPLE SIZE 500 participants randomized 1:1 TREATMENT BHV-1400 500 mg SC Q2Wk vs Placebo Q2Wk TREATMENT DURATION 24-month treatment period KEY ENDPOINTS Change in UPCR at 9 months (AA), Annualized total eGFR slope Accelerated approval pathway to bring a selective, disease-specific therapeutic to treat IgANKEY POINT DEGRADERS C R E A T E V A L U E June 2025 Biohaven Investor Presentation Initiate 2026
IgG MoDE Degrader
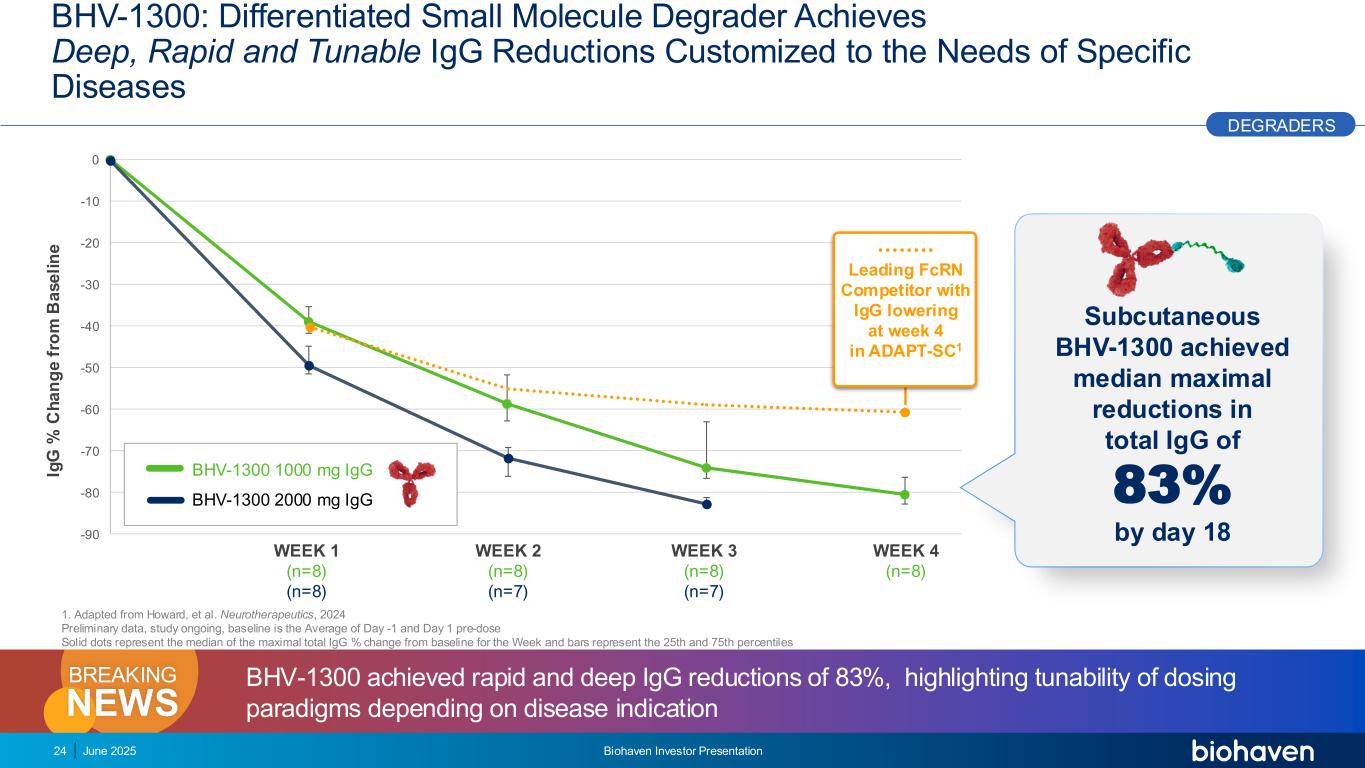
-90 -80 -70 -60 -50 -40 -30 -20 -10 0 BHV-1300: Differentiated Small Molecule Degrader Achieves Deep, Rapid and Tunable IgG Reductions Customized to the Needs of Specific Diseases 24 June 2025 Biohaven Investor Presentation Ig G % C h a n g e f ro m B a s e li n e WEEK 1 (n=8) (n=8) WEEK 2 (n=8) (n=7) WEEK 3 (n=8) (n=7) WEEK 4 (n=8) BHV-1300 1000 mg IgG BHV-1300 2000 mg IgG Subcutaneous BHV-1300 achieved median maximal reductions in total IgG of 83% by day 18 BHV-1300 achieved rapid and deep IgG reductions of 83%, highlighting tunability of dosing paradigms depending on disease indicationNEWS BREAKING 1. Adapted from Howard, et al. Neurotherapeutics, 2024 Preliminary data, study ongoing, baseline is the Average of Day -1 and Day 1 pre-dose Solid dots represent the median of the maximal total IgG % change from baseline for the Week and bars represent the 25th and 75th percentiles Leading FcRN Competitor with IgG lowering at week 4 in ADAPT-SC1 DEGRADERS
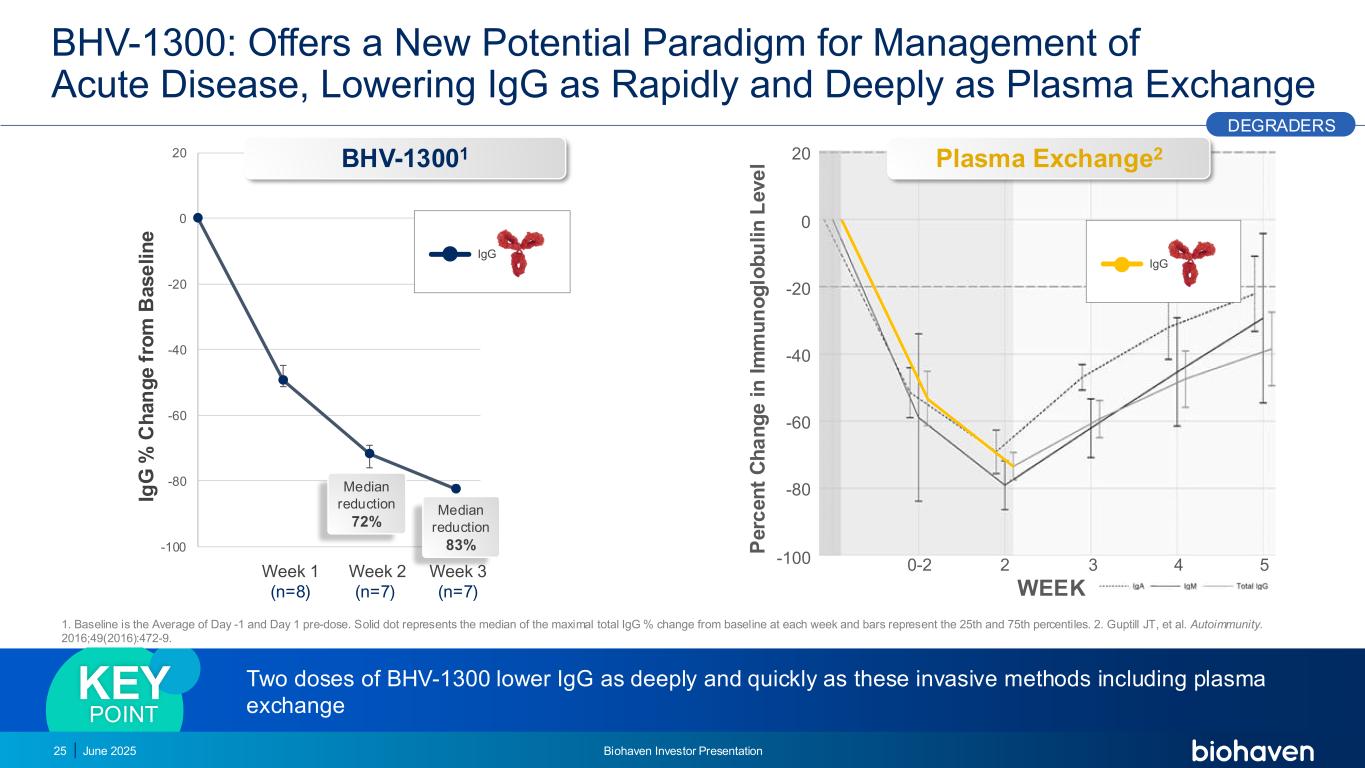
-100 -80 -60 -40 -20 0 20 IgG BHV-1300: Offers a New Potential Paradigm for Management of Acute Disease, Lowering IgG as Rapidly and Deeply as Plasma Exchange June 2025 Biohaven Investor Presentation25 Two doses of BHV-1300 lower IgG as deeply and quickly as these invasive methods including plasma exchange KEY POINT 1. Baseline is the Average of Day -1 and Day 1 pre-dose. Solid dot represents the median of the maximal total IgG % change from baseline at each week and bars represent the 25th and 75th percentiles. 2. Guptill JT, et al. Autoimmunity. 2016;49(2016):472-9. 0-2 2 3 4 5-100 -80 -60 -40 -20 0 20 IgG P e rc e n t C h a n g e i n I m m u n o g lo b u li n L e v e l WEEK BHV-13001 Plasma Exchange2 DEGRADERS Median reduction 72% Median reduction 83% Week 1 (n=8) Week 2 (n=7) Week 3 (n=7) Ig G % C h a n g e f ro m B a s e li n e
BHV-1300 Plasma Exchange BHV-1300 Could Represent Replacement Plasma Exchange (PLEX) in an Auto-Injector DEGRADERS K E E P T H E P A T I E N T A T T H E C E N T E R VS 26 June 2025 Biohaven Investor Presentation
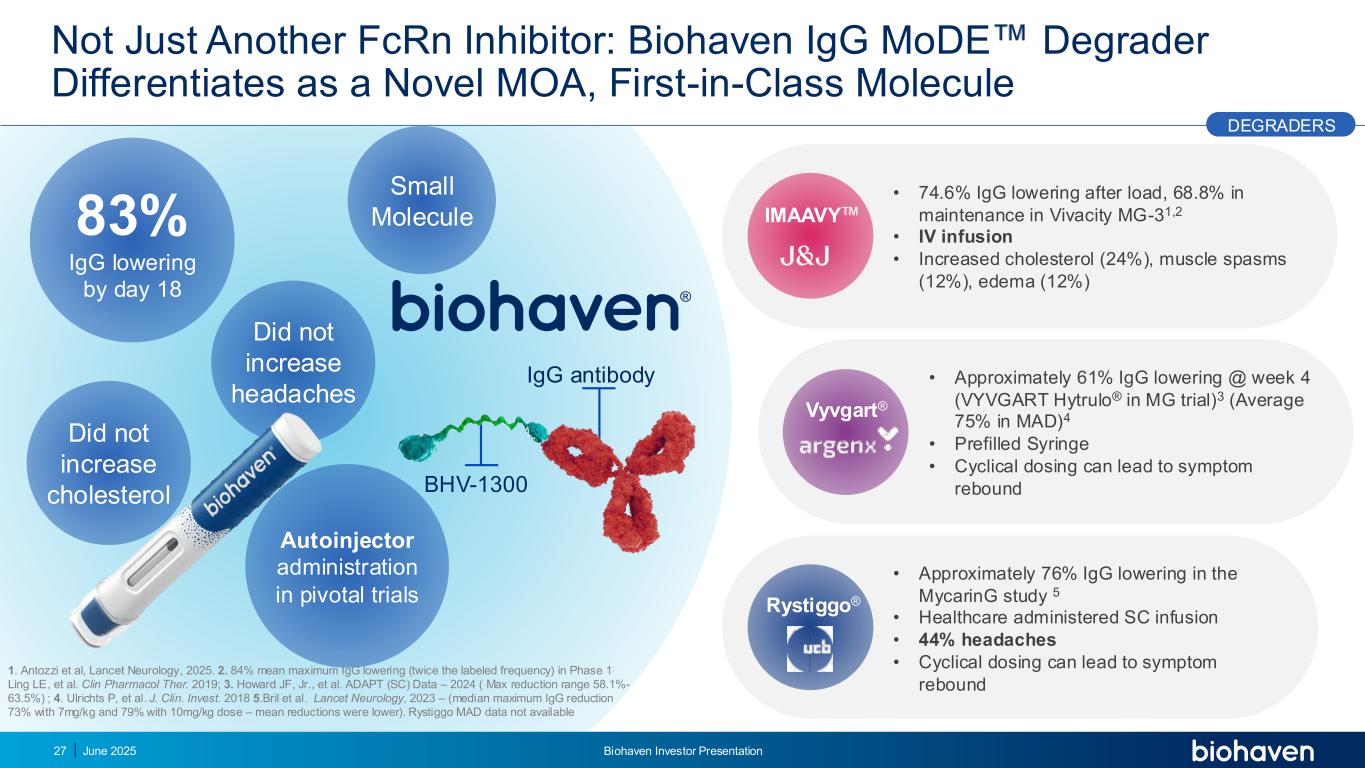
• 74.6% IgG lowering after load, 68.8% in maintenance in Vivacity MG-31,2 • IV infusion • Increased cholesterol (24%), muscle spasms (12%), edema (12%) • Approximately 61% IgG lowering @ week 4 (VYVGART Hytrulo® in MG trial)3 (Average 75% in MAD)4 • Prefilled Syringe • Cyclical dosing can lead to symptom rebound Not Just Another FcRn Inhibitor: Biohaven IgG MoDE Degrader Differentiates as a Novel MOA, First-in-Class Molecule IgG antibody BHV-1300 • Approximately 76% IgG lowering in the MycarinG study 5 • Healthcare administered SC infusion • 44% headaches • Cyclical dosing can lead to symptom rebound 83% IgG lowering by day 18 Did not increase cholesterol Did not increase headaches Autoinjector administration in pivotal trials Vyvgart® IMAAVY Rystiggo® DEGRADERS 27 Small Molecule 1. Antozzi et al, Lancet Neurology, 2025. 2. 84% mean maximum IgG lowering (twice the labeled frequency) in Phase 1: Ling LE, et al. Clin Pharmacol Ther. 2019; 3. Howard JF, Jr., et al. ADAPT (SC) Data – 2024 ( Max reduction range 58.1%- 63.5%) ; 4. Ulrichts P, et al. J. Clin. Invest. 2018 5.Bril et al. Lancet Neurology, 2023 – (median maximum IgG reduction 73% with 7mg/kg and 79% with 10mg/kg dose – mean reductions were lower). Rystiggo MAD data not available June 2025 Biohaven Investor Presentation
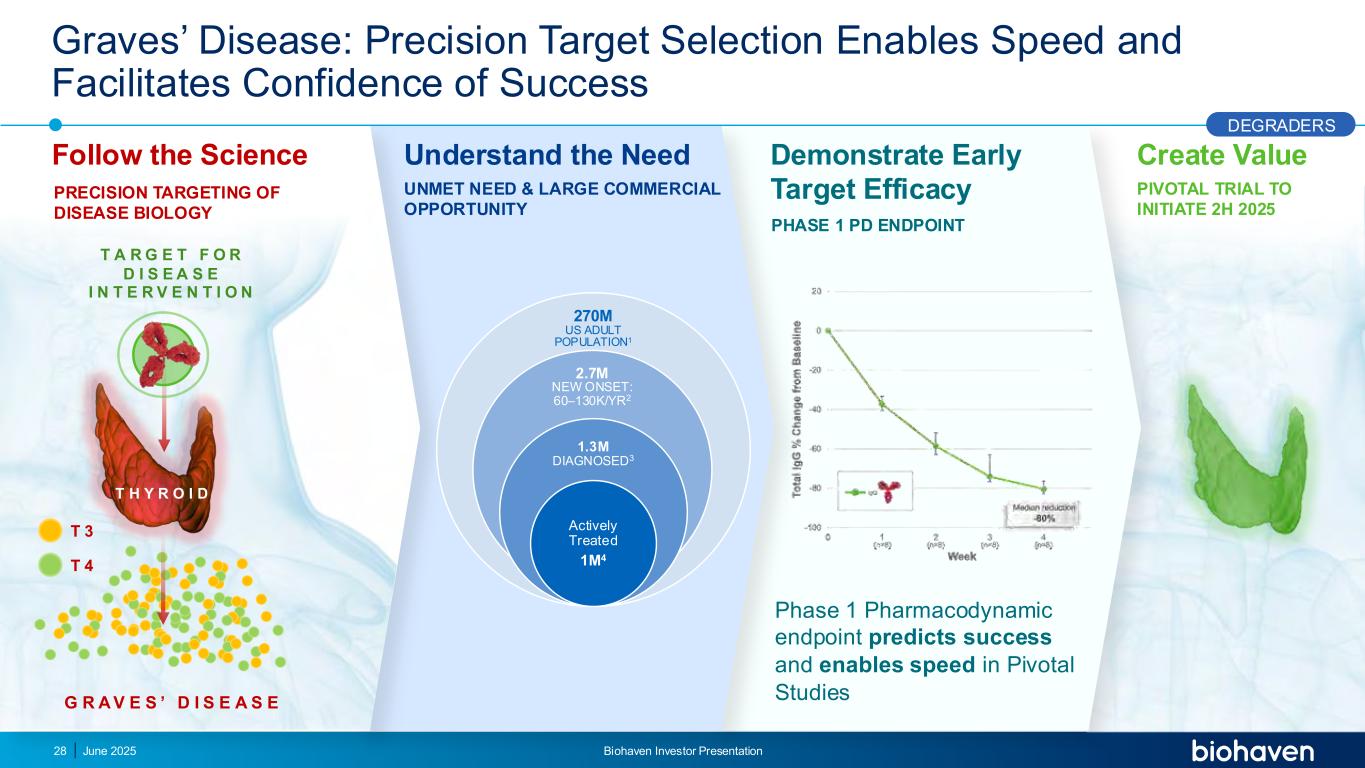
Graves’ Disease: Precision Target Selection Enables Speed and Facilitates Confidence of Success Follow the Science Understand the Need Phase 1 Pharmacodynamic endpoint predicts success and enables speed in Pivotal Studies PRECISION TARGETING OF DISEASE BIOLOGY 270M US ADULT POPULATION1 2.7M NEW ONSET: 60–130K/YR2 1.3M DIAGNOSED3 Actively Treated 1M4 UNMET NEED & LARGE COMMERCIAL OPPORTUNITY T H Y R O I D G R A V E S ’ D I S E A S E T 3 T 4 Demonstrate Early Target Efficacy T A R G E T F O R D I S E A S E I N T E R V E N T I O N Create Value PIVOTAL TRIAL TO INITIATE 2H 2025 PHASE 1 PD ENDPOINT DEGRADERS 28 June 2025 Biohaven Investor Presentation
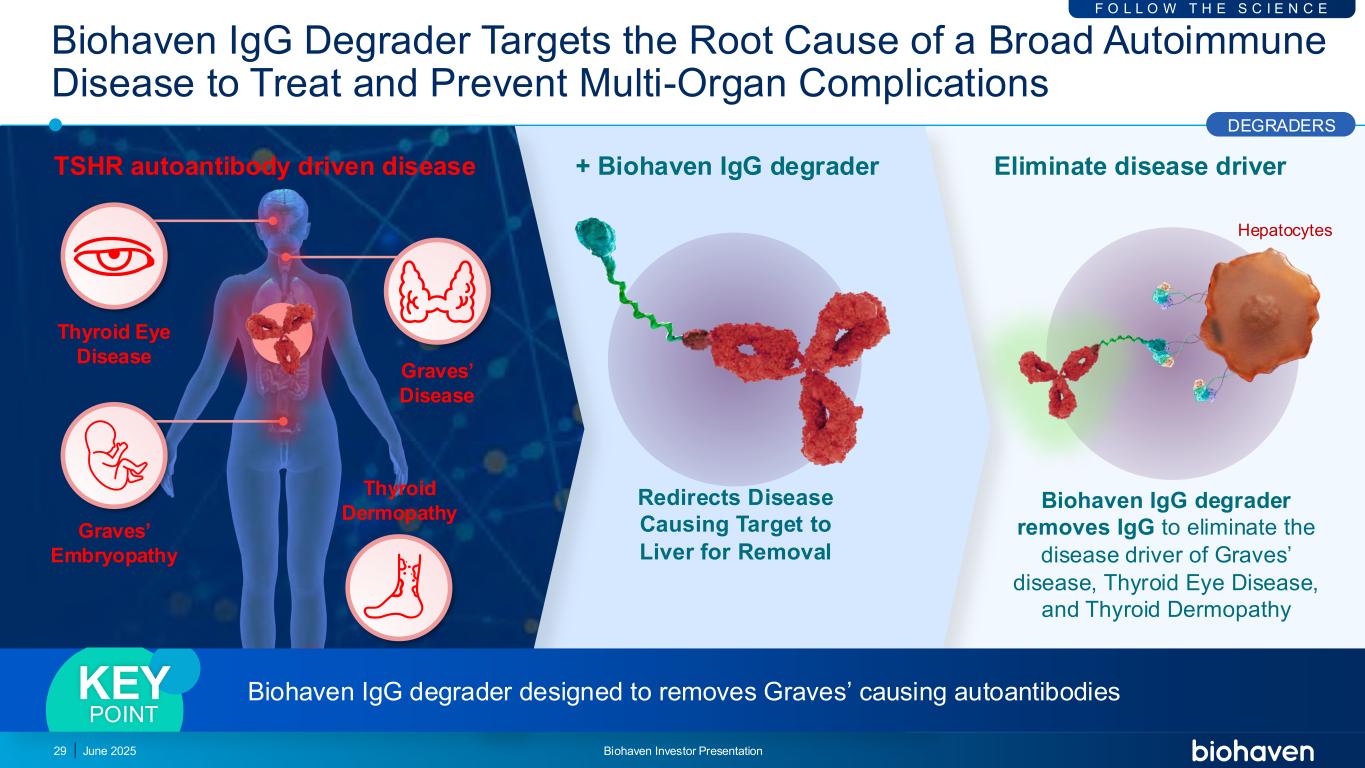
Hepatocytes Biohaven IgG Degrader Targets the Root Cause of a Broad Autoimmune Disease to Treat and Prevent Multi-Organ Complications June 2025 Biohaven Investor Presentation29 Redirects Disease Causing Target to Liver for Removal + Biohaven IgG degrader Biohaven IgG degrader removes IgG to eliminate the disease driver of Graves’ disease, Thyroid Eye Disease, and Thyroid Dermopathy Eliminate disease driver F O L L O W T H E S C I E N C E Thyroid Eye Disease Thyroid Dermopathy Graves’ Disease Graves’ Embryopathy TSHR autoantibody driven disease DEGRADERS Biohaven IgG degrader designed to removes Graves’ causing autoantibodiesKEY POINT
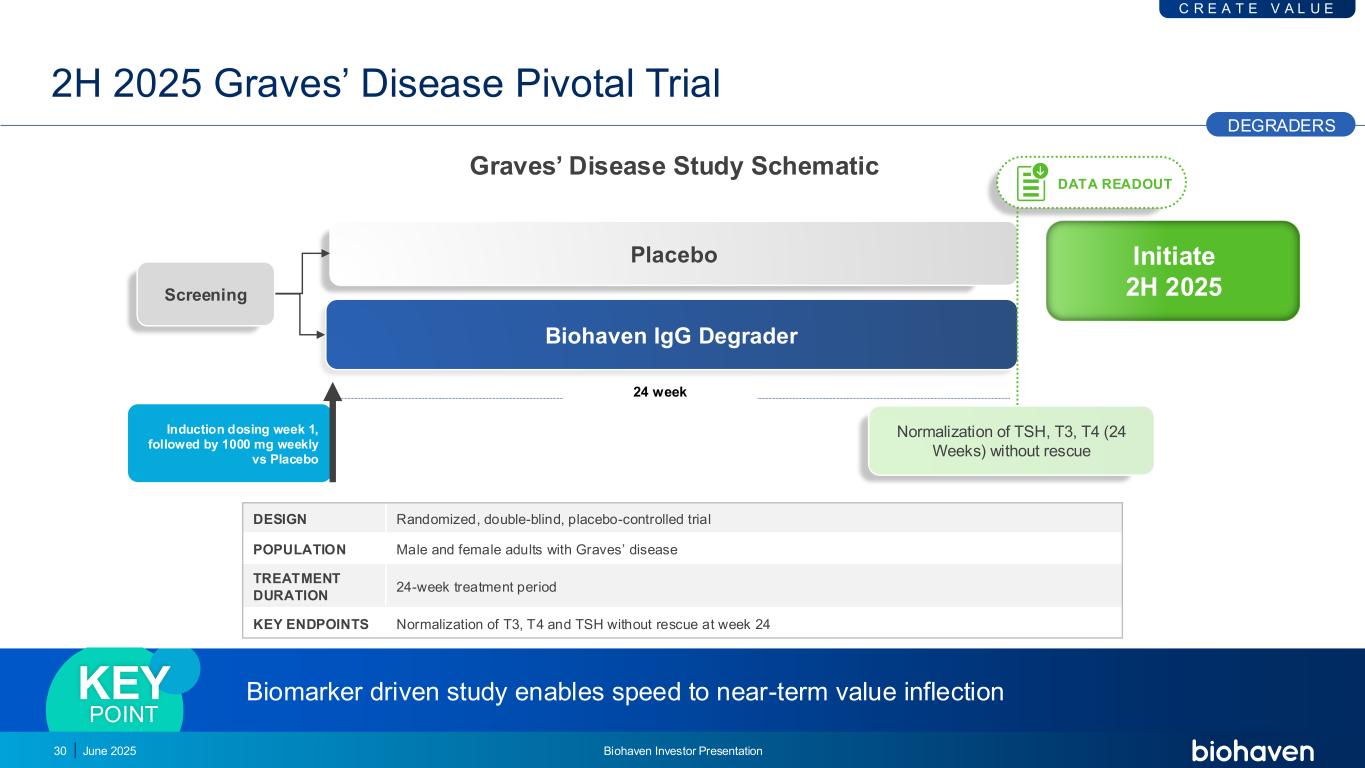
Initiate 2H 2025 2H 2025 Graves’ Disease Pivotal Trial DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Male and female adults with Graves’ disease TREATMENT DURATION 24-week treatment period KEY ENDPOINTS Normalization of T3, T4 and TSH without rescue at week 24 Biomarker driven study enables speed to near-term value inflectionKEY POINT 24 week Biohaven IgG Degrader Placebo Screening Induction dosing week 1, followed by 1000 mg weekly vs Placebo C R E A T E V A L U E DEGRADERS 30 DATA READOUT Normalization of TSH, T3, T4 (24 Weeks) without rescue June 2025 Biohaven Investor Presentation Graves’ Disease Study Schematic
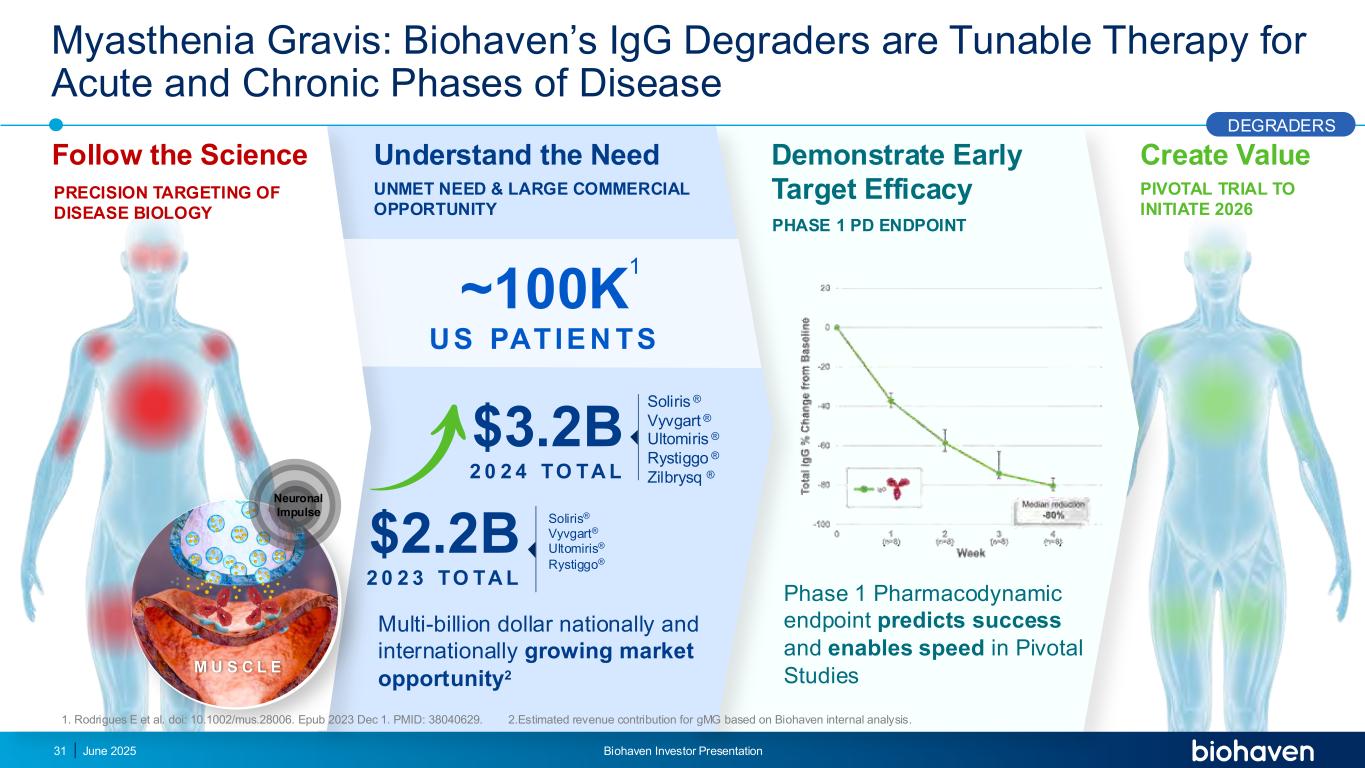
Create Value PIVOTAL TRIAL TO INITIATE 2026 Myasthenia Gravis: Biohaven’s IgG Degraders are Tunable Therapy for Acute and Chronic Phases of Disease Phase 1 Pharmacodynamic endpoint predicts success and enables speed in Pivotal Studies Multi-billion dollar nationally and internationally growing market opportunity2 ~100K US PATIE N TS $3.2B 2 0 2 4 TO TA L Soliris ® Vyvgart ® Ultomiris ® Rystiggo ® Zilbrysq ® $2.2B 2 0 2 3 TO TA L Soliris® Vyvgart® Ultomiris® Rystiggo® Understand the Need UNMET NEED & LARGE COMMERCIAL OPPORTUNITY Demonstrate Early Target Efficacy PHASE 1 PD ENDPOINT Neuronal Impulse M U S C L E Follow the Science PRECISION TARGETING OF DISEASE BIOLOGY DEGRADERS June 2025 1. Rodrigues E et al. doi: 10.1002/mus.28006. Epub 2023 Dec 1. PMID: 38040629. 2.Estimated revenue contribution for gMG based on Biohaven internal analysis. 1 Biohaven Investor Presentation31
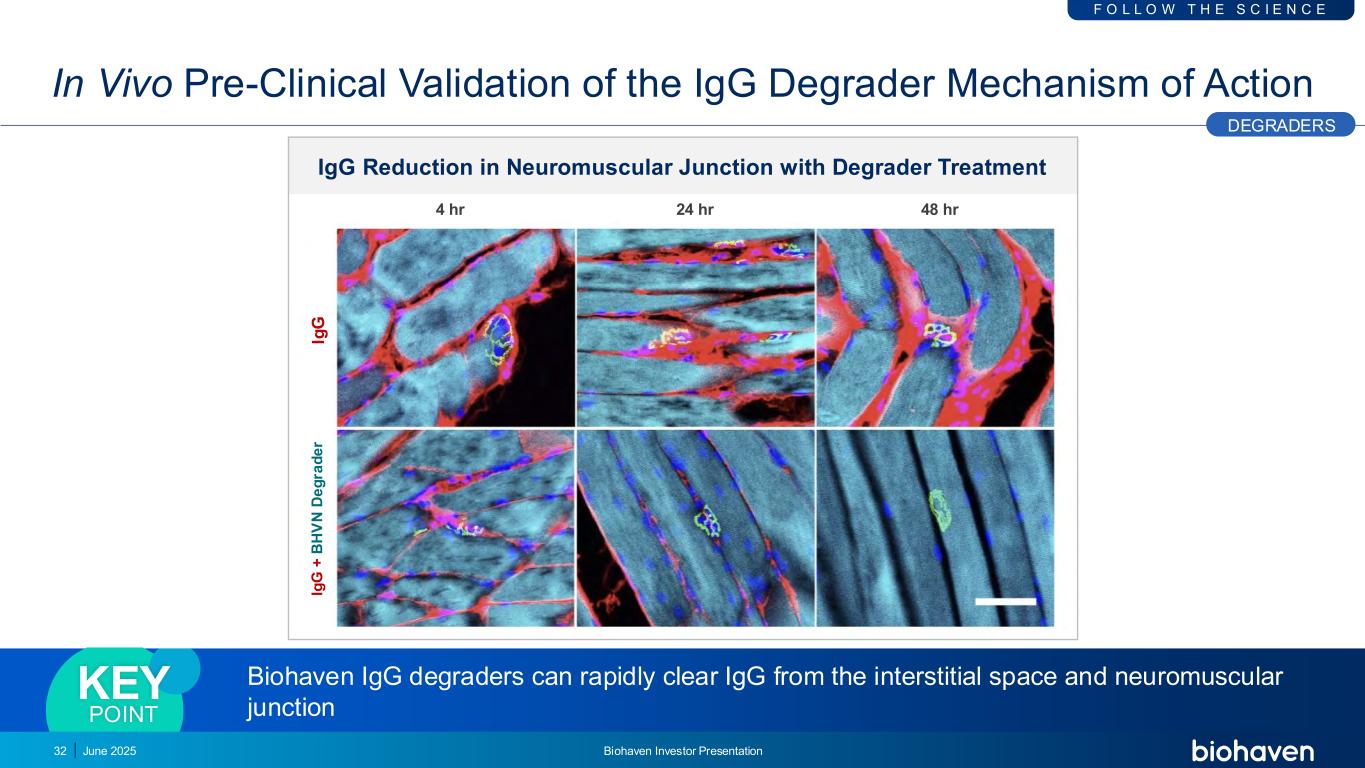
In Vivo Pre-Clinical Validation of the IgG Degrader Mechanism of Action Biohaven IgG degraders can rapidly clear IgG from the interstitial space and neuromuscular junction KEY POINT Ig G Ig G + B H V N D e g ra d e r IgG Reduction in Neuromuscular Junction with Degrader Treatment 4 hr 24 hr 48 hr DEGRADERS F O L L O W T H E S C I E N C E June 2025 Biohaven Investor Presentation32
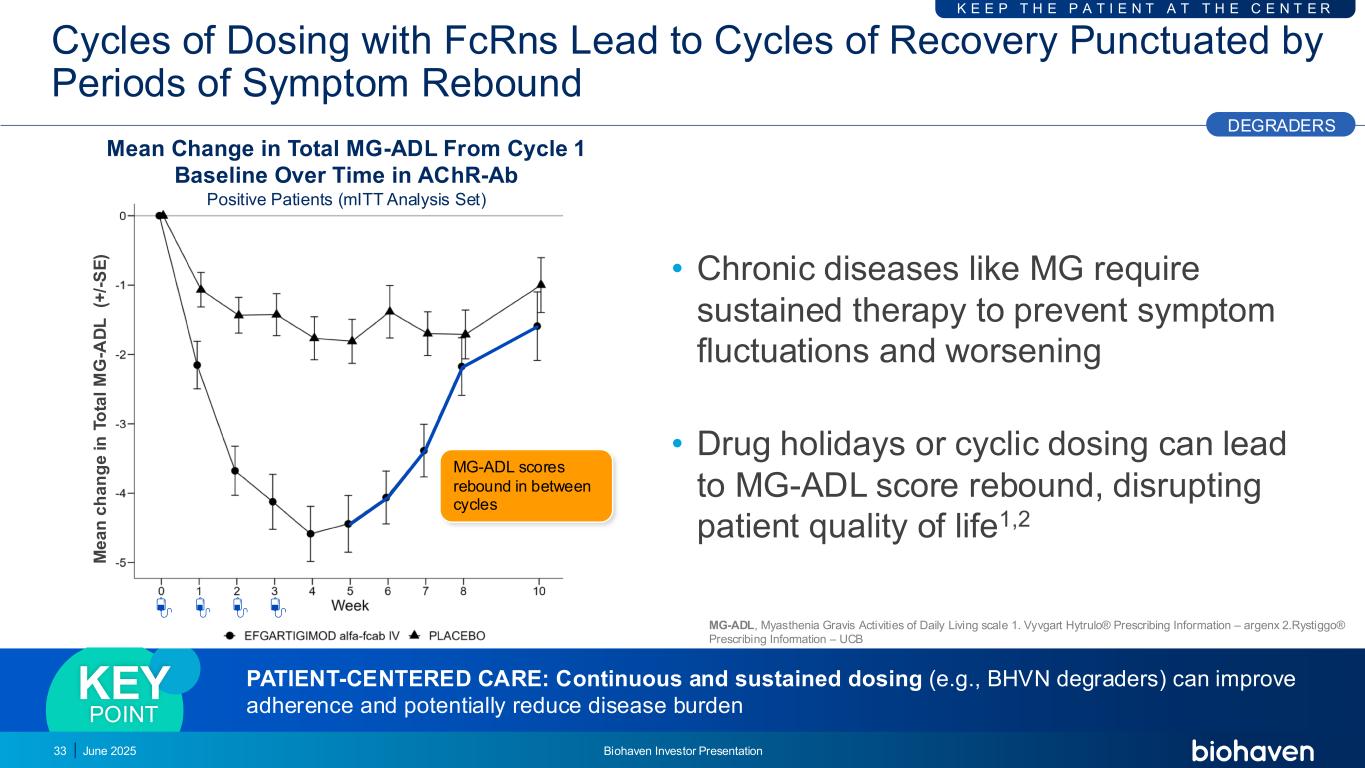
MG-ADL scores rebound in between cycles Cycles of Dosing with FcRns Lead to Cycles of Recovery Punctuated by Periods of Symptom Rebound • Chronic diseases like MG require sustained therapy to prevent symptom fluctuations and worsening • Drug holidays or cyclic dosing can lead to MG-ADL score rebound, disrupting patient quality of life1,2 June 2025 Biohaven Investor Presentation33 K E E P T H E P A T I E N T A T T H E C E N T E R Mean Change in Total MG-ADL From Cycle 1 Baseline Over Time in AChR-Ab Positive Patients (mITT Analysis Set) DEGRADERS M e a n c h a n g e i n T o ta l M G -A D L (+ /- S E ) PATIENT-CENTERED CARE: Continuous and sustained dosing (e.g., BHVN degraders) can improve adherence and potentially reduce disease burden KEY POINT MG-ADL, Myasthenia Gravis Activities of Daily Living scale 1. Vyvgart Hytrulo® Prescribing Information – argenx 2.Rystiggo® Prescribing Information – UCB
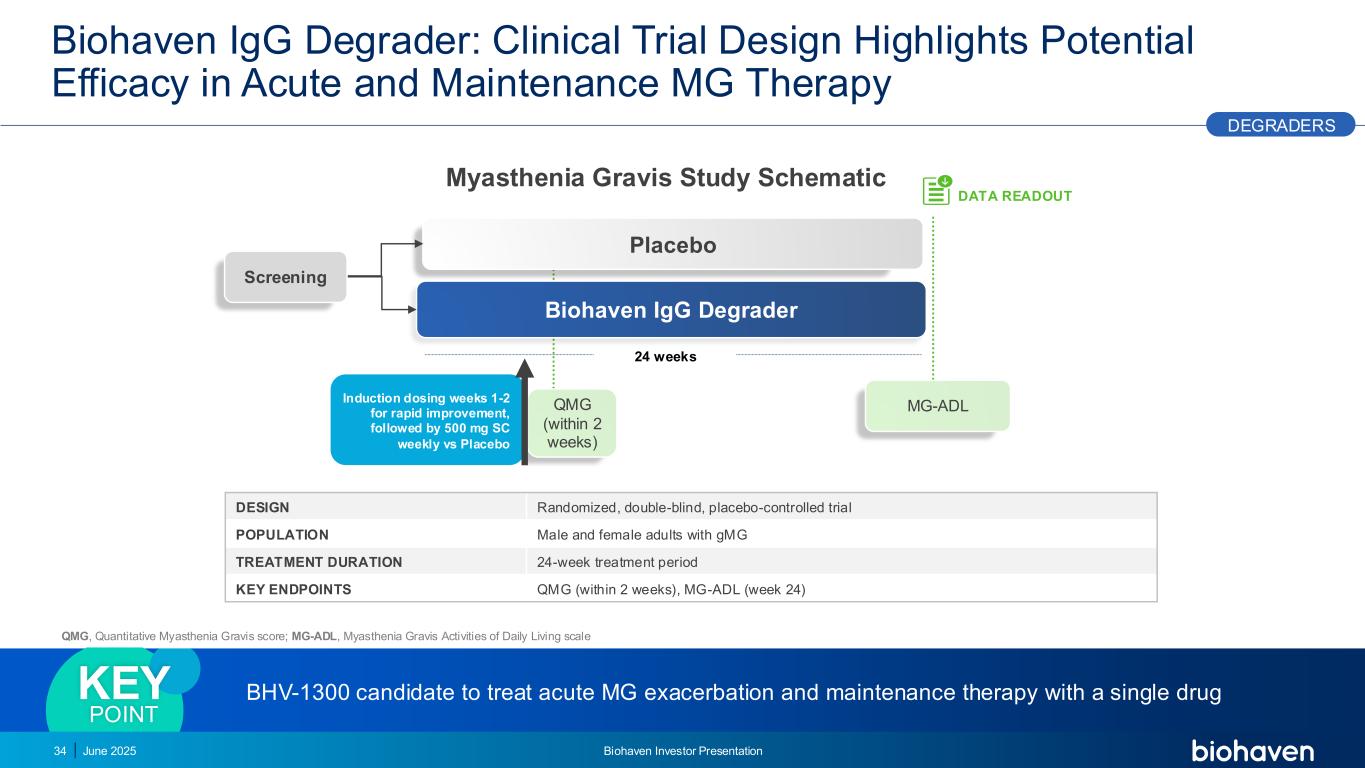
Biohaven IgG Degrader: Clinical Trial Design Highlights Potential Efficacy in Acute and Maintenance MG Therapy QMG (within 2 weeks) DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Male and female adults with gMG TREATMENT DURATION 24-week treatment period KEY ENDPOINTS QMG (within 2 weeks), MG-ADL (week 24) BHV-1300 candidate to treat acute MG exacerbation and maintenance therapy with a single drugKEY POINT 24 weeks DATA READOUT Biohaven IgG Degrader Placebo Screening MG-ADL Induction dosing weeks 1-2 for rapid improvement, followed by 500 mg SC weekly vs Placebo DEGRADERS 34 June 2025 Biohaven Investor Presentation Myasthenia Gravis Study Schematic QMG, Quantitative Myasthenia Gravis score; MG-ADL, Myasthenia Gravis Activities of Daily Living scale
Targeting the Role of Autoimmunity in PPCM Current treatment for PPCM • Guideline-Directed Medical Therapy: No disease specific therapy Treatment goals in PPCM • Intervene early • Treat acute heart failure • Prevent further myocardial injury and restore baseline normal cardiac function β1AR AAbs present in PPCM • Increased titers correlate with more severe disease • β1AR autoantibodies are a potential target to provide PPCM patients with their first disease specific therapy 35 DEGRADERS June 2025 Biohaven Investor Presentation
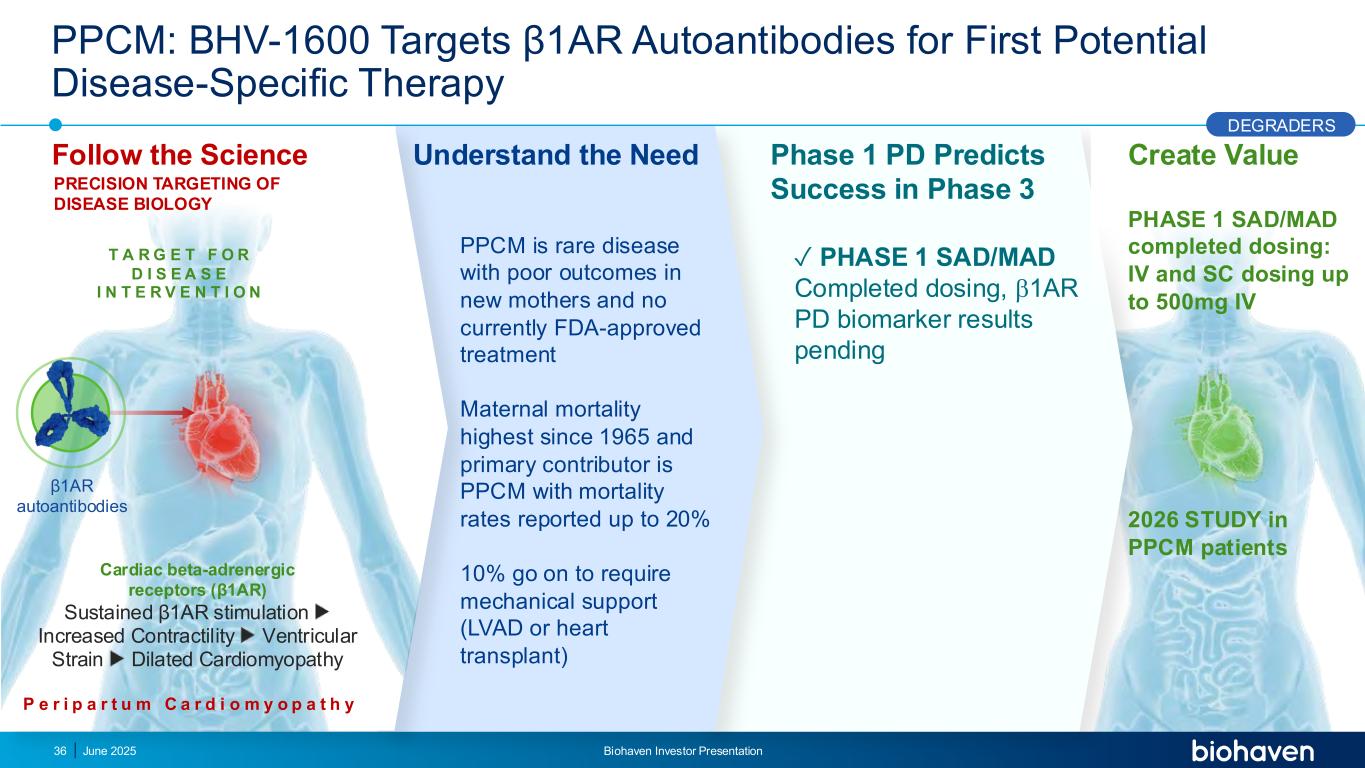
Create Value PHASE 1 SAD/MAD completed dosing: IV and SC dosing up to 500mg IV 2026 STUDY in PPCM patients PPCM: BHV-1600 Targets β1AR Autoantibodies for First Potential Disease-Specific Therapy Follow the Science Understand the Need ✓ PHASE 1 SAD/MAD Completed dosing, 1AR PD biomarker results pending PRECISION TARGETING OF DISEASE BIOLOGY Phase 1 PD Predicts Success in Phase 3 DEGRADERS P e r i p a r t u m C a r d i o m y o p a t h y Cardiac beta-adrenergic receptors (β1AR) Sustained β1AR stimulation Increased Contractility Ventricular Strain Dilated Cardiomyopathy β1AR autoantibodies T A R G E T F O R D I S E A S E I N T E R V E N T I O N June 2025 Biohaven Investor Presentation36 PPCM is rare disease with poor outcomes in new mothers and no currently FDA-approved treatment Maternal mortality highest since 1965 and primary contributor is PPCM with mortality rates reported up to 20% 10% go on to require mechanical support (LVAD or heart transplant)
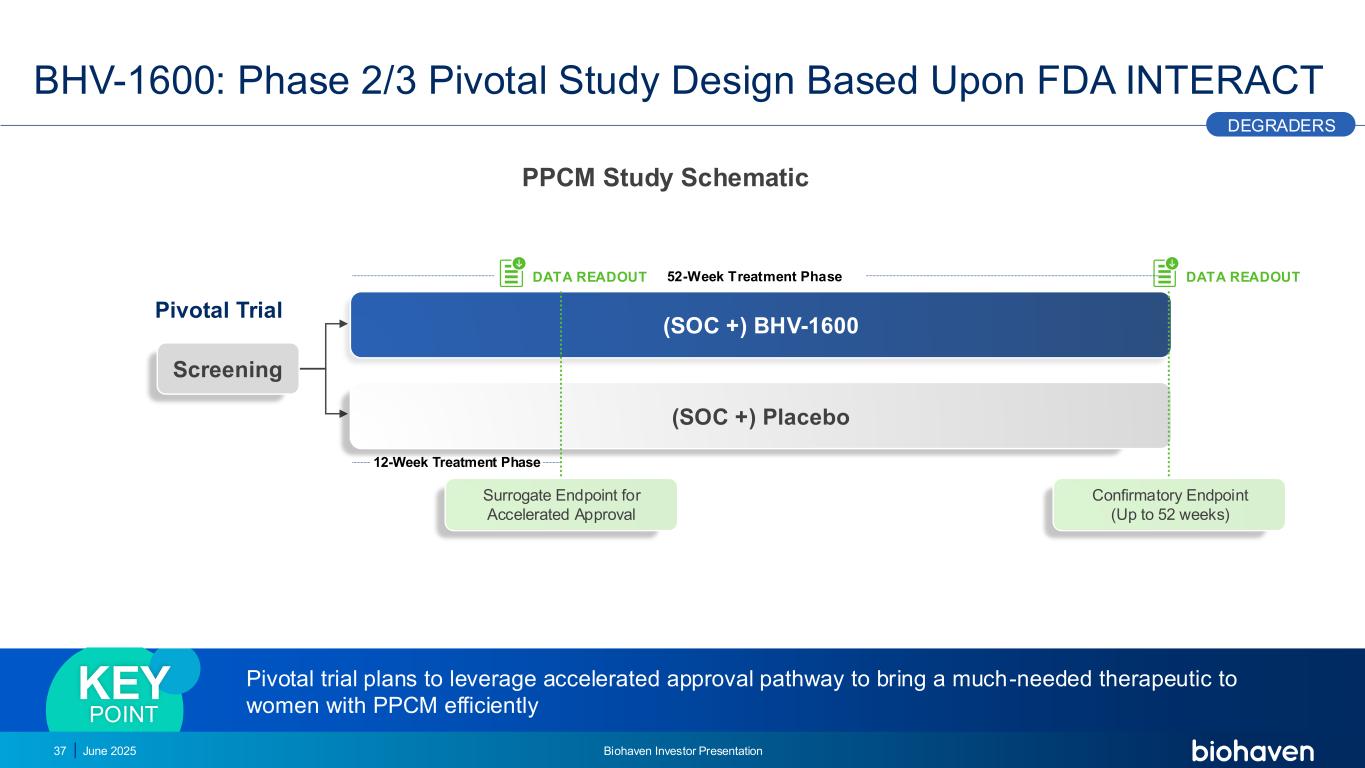
12-Week Treatment Phase 52-Week Treatment Phase (SOC +) BHV-1600 (SOC +) Placebo Screening BHV-1600: Phase 2/3 Pivotal Study Design Based Upon FDA INTERACT 37 Pivotal Trial Surrogate Endpoint for Accelerated Approval DATA READOUT DATA READOUT Confirmatory Endpoint (Up to 52 weeks) DEGRADERS Pivotal trial plans to leverage accelerated approval pathway to bring a much-needed therapeutic to women with PPCM efficiently KEY POINT June 2025 Biohaven Investor Presentation PPCM Study Schematic

Next-Gen Degraders
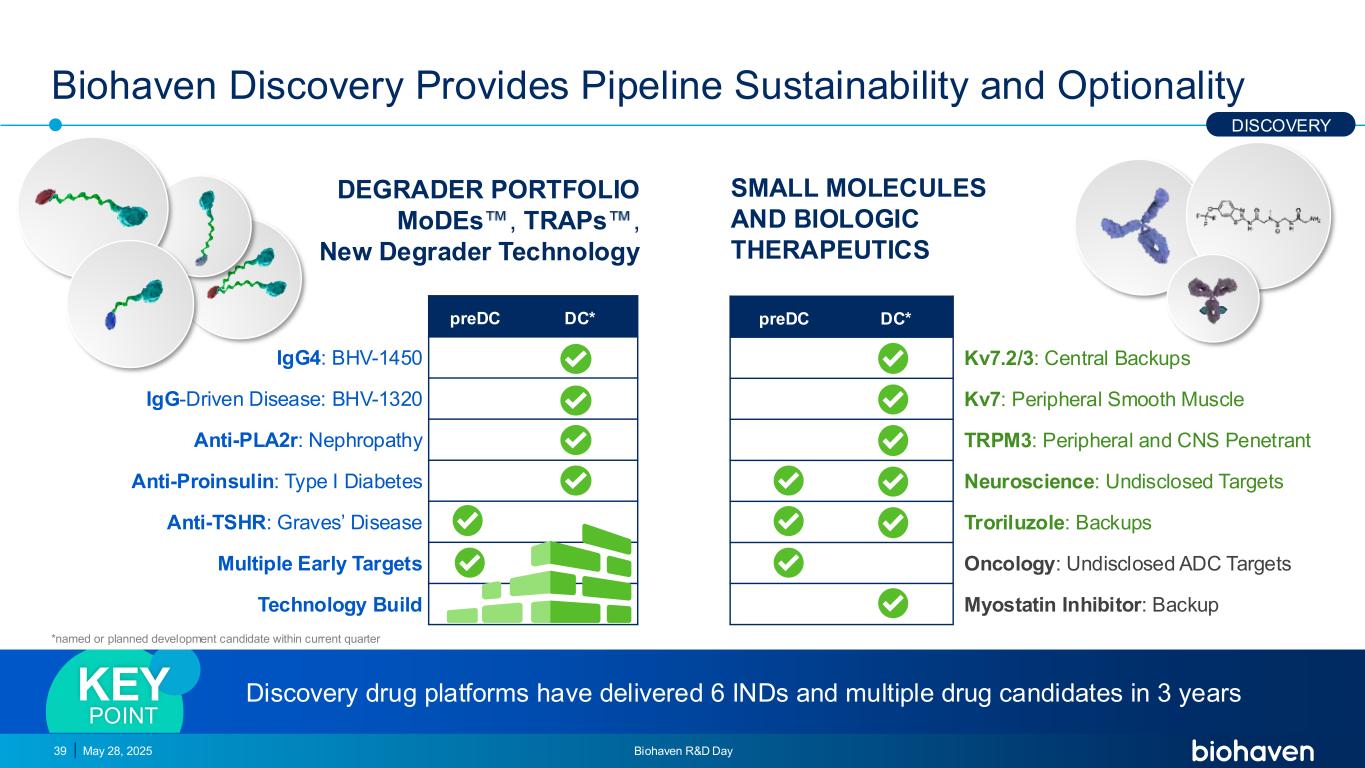
preDC DC* IgG4: BHV-1450 IgG-Driven Disease: BHV-1320 Anti-PLA2r: Nephropathy Anti-Proinsulin: Type I Diabetes Anti-TSHR: Graves’ Disease Multiple Early Targets Technology Build Biohaven Discovery Provides Pipeline Sustainability and Optionality May 28, 2025 Biohaven R&D Day39 DEGRADER PORTFOLIO MoDEs , TRAPs , New Degrader Technology SMALL MOLECULES AND BIOLOGIC THERAPEUTICS *DC named or anticipated within current quarter Discovery drug platforms have delivered 6 INDs and multiple drug candidates in 3 yearsKEY POINT preDC DC* Kv7.2/3: Central Backups Kv7: Peripheral Smooth Muscle TRPM3: Peripheral and CNS Penetrant Neuroscience: Undisclosed Targets Troriluzole: Backups Oncology: Undisclosed ADC Targets Myostatin Inhibitor: Backup DISCOVERY *named or planned development candidate within current quarter
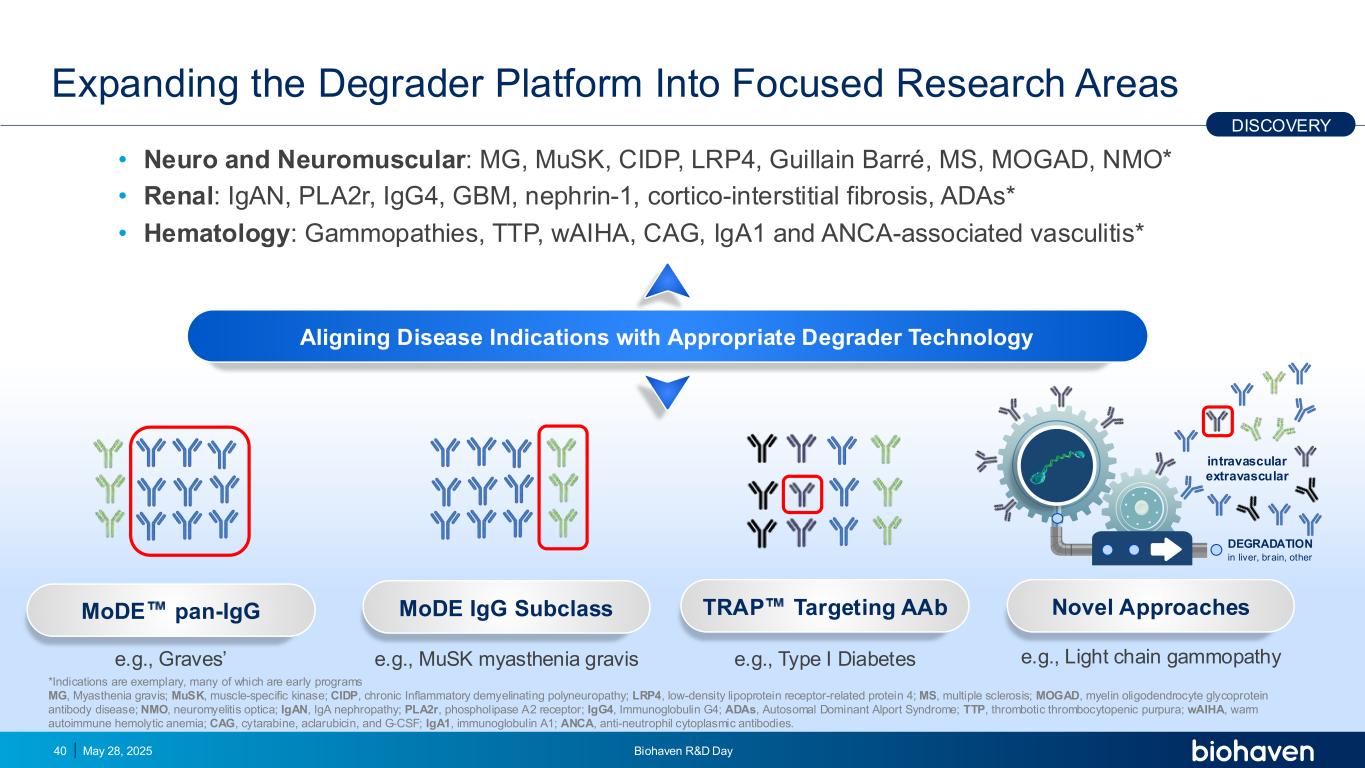
Expanding the Degrader Platform Into Focused Research Areas • Neuro and Neuromuscular: MG, MuSK, CIDP, LRP4, Guillain Barré, MS, MOGAD, NMO* • Renal: IgAN, PLA2r, IgG4, GBM, nephrin-1, cortico-interstitial fibrosis, ADAs* • Hematology: Gammopathies, TTP, wAIHA, CAG, IgA1 and ANCA-associated vasculitis* May 28, 2025 Biohaven R&D Day40 DISCOVERY MoDE pan-IgG MoDE IgG Subclass TRAP Targeting AAb Novel Approaches DEGRADATION in liver, brain, other intravascular extravascular Aligning Disease Indications with Appropriate Degrader Technology e.g., Graves’ e.g., MuSK myasthenia gravis e.g., Type I Diabetes e.g., Light chain gammopathy *Indications are exemplary, many of which are early programs MG, Myasthenia gravis; MuSK, muscle-specific kinase; CIDP, chronic Inflammatory demyelinating polyneuropathy; LRP4, low-density lipoprotein receptor-related protein 4; MS, multiple sclerosis; MOGAD, myelin oligodendrocyte glycoprotein antibody disease; NMO, neuromyelitis optica; IgAN, IgA nephropathy; PLA2r, phospholipase A2 receptor; IgG4, Immunoglobulin G4; ADAs, Autosomal Dominant Alport Syndrome; TTP, thrombotic thrombocytopenic purpura; wAIHA, warm autoimmune hemolytic anemia; CAG, cytarabine, aclarubicin, and G‐CSF; IgA1, immunoglobulin A1; ANCA, anti-neutrophil cytoplasmic antibodies.
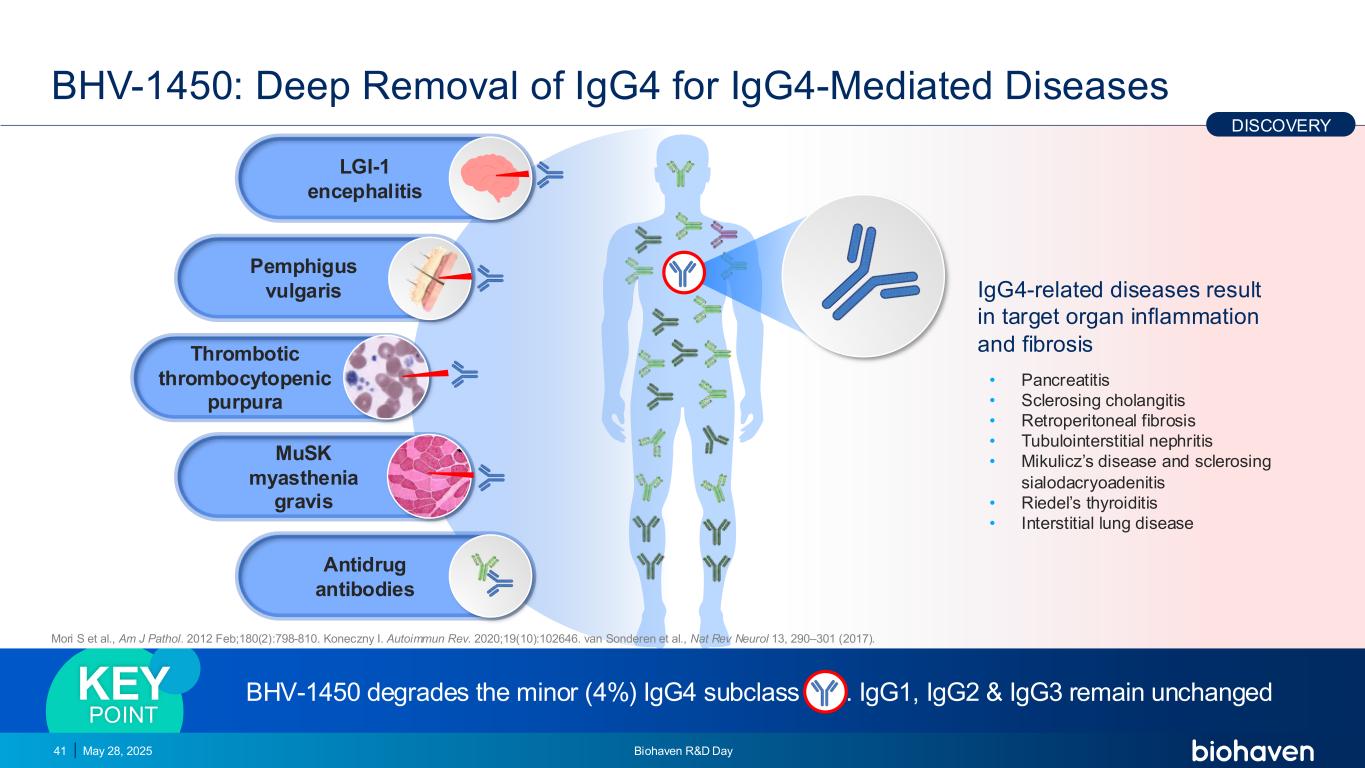
BHV-1450: Deep Removal of IgG4 for IgG4-Mediated Diseases May 28, 2025 Biohaven R&D Day41 IgG4-related diseases result in target organ inflammation and fibrosis • Pancreatitis • Sclerosing cholangitis • Retroperitoneal fibrosis • Tubulointerstitial nephritis • Mikulicz’s disease and sclerosing sialodacryoadenitis • Riedel’s thyroiditis • Interstitial lung disease BHV-1450 degrades the minor (4%) IgG4 subclass . IgG1, IgG2 & IgG3 remain unchangedKEY POINT Thrombotic thrombocytopenic purpura LGI-1 encephalitis Pemphigus vulgaris MuSK myasthenia gravis Antidrug antibodies DISCOVERY Mori S et al., Am J Pathol. 2012 Feb;180(2):798-810. Koneczny I. Autoimmun Rev. 2020;19(10):102646. van Sonderen et al., Nat Rev Neurol 13, 290–301 (2017).
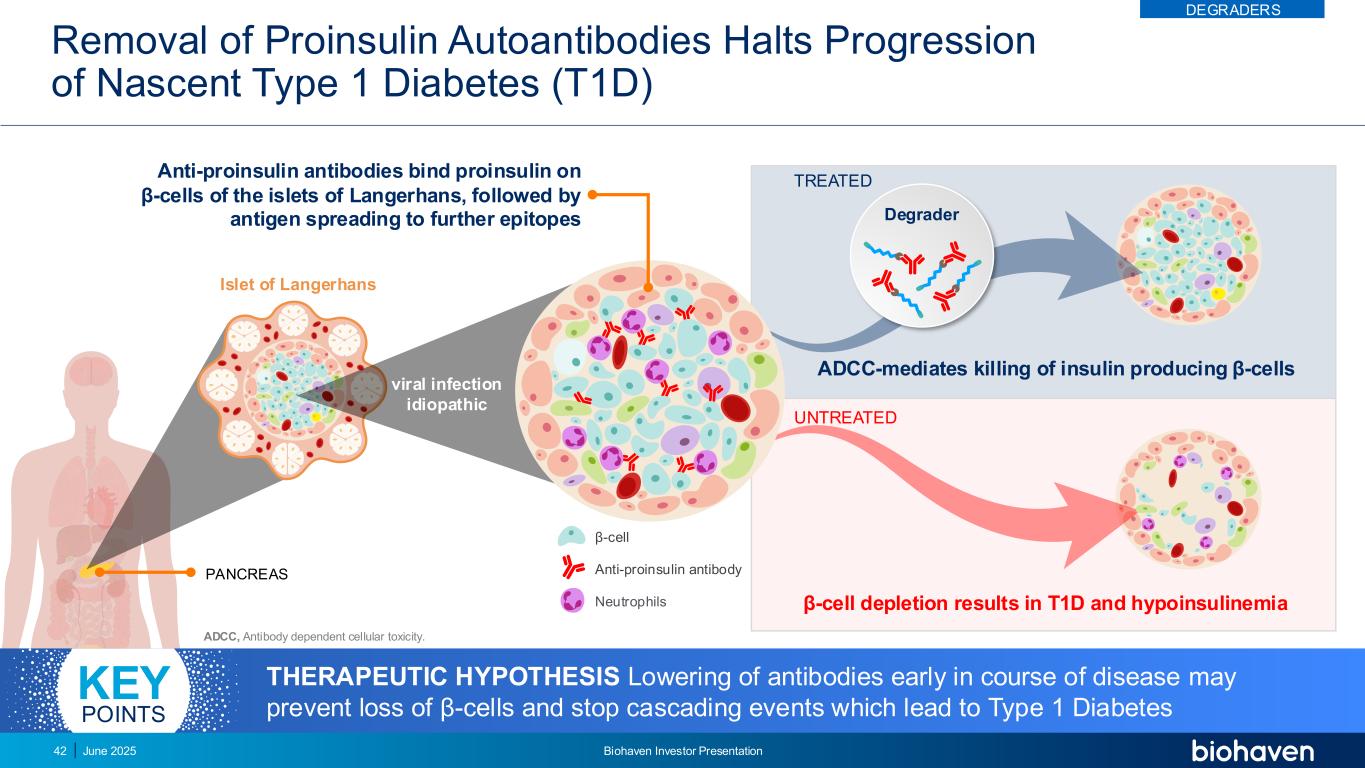
Removal of Proinsulin Autoantibodies Halts Progression of Nascent Type 1 Diabetes (T1D) June 2025 Biohaven Investor Presentation42 Islet of Langerhans PANCREAS β-cell depletion results in T1D and hypoinsulinemia ADCC-mediates killing of insulin producing β-cells UNTREATED viral infection idiopathic TREATED β-cell Anti-proinsulin antibody Neutrophils Degrader ADCC, Antibody dependent cellular toxicity. THERAPEUTIC HYPOTHESIS Lowering of antibodies early in course of disease may prevent loss of β-cells and stop cascading events which lead to Type 1 Diabetes KEY POINTS Anti-proinsulin antibodies bind proinsulin on β-cells of the islets of Langerhans, followed by antigen spreading to further epitopes DEGRADERS
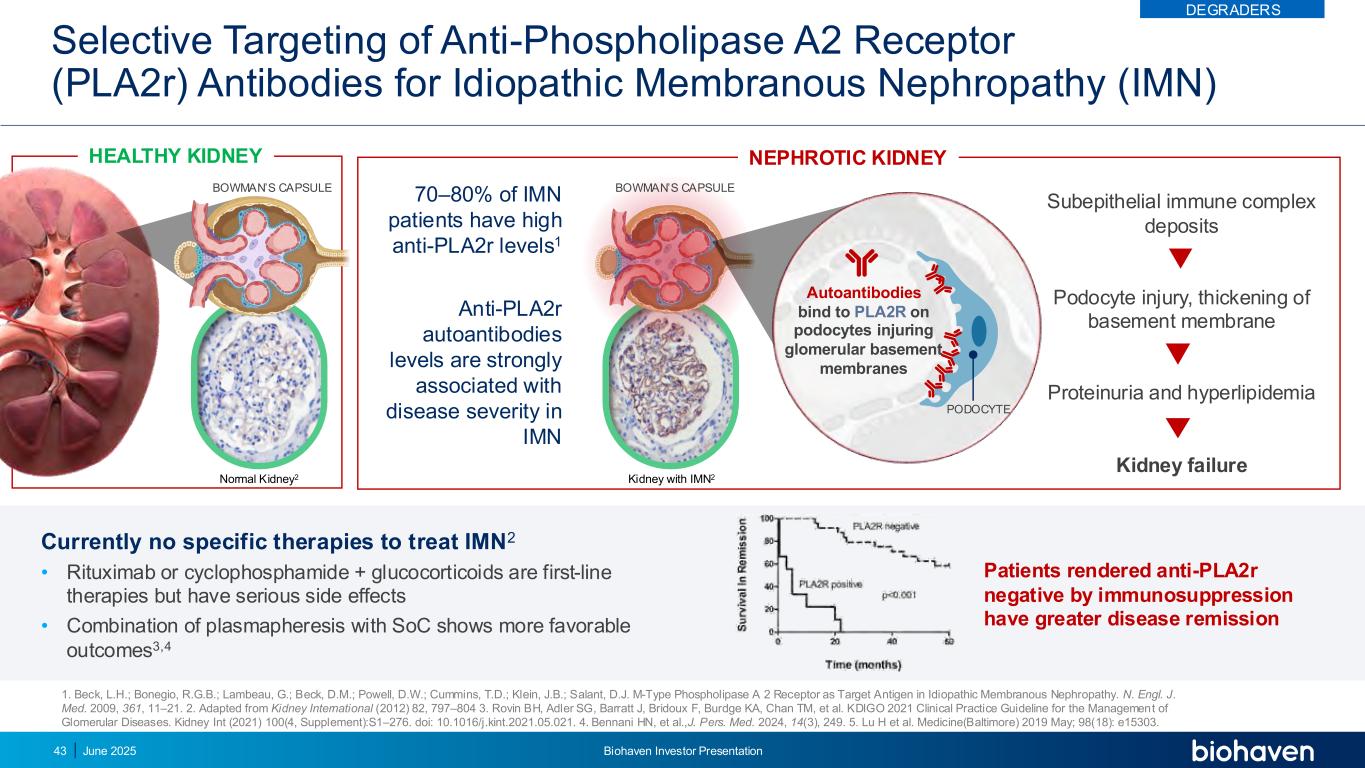
Selective Targeting of Anti-Phospholipase A2 Receptor (PLA2r) Antibodies for Idiopathic Membranous Nephropathy (IMN) HEALTHY KIDNEY Currently no specific therapies to treat IMN2 • Rituximab or cyclophosphamide + glucocorticoids are first-line therapies but have serious side effects • Combination of plasmapheresis with SoC shows more favorable outcomes3,4 Patients rendered anti-PLA2r negative by immunosuppression have greater disease remission 1. Beck, L.H.; Bonegio, R.G.B.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-Type Phospholipase A 2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2009, 361, 11–21. 2. Adapted from Kidney International (2012) 82, 797–804 3. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int (2021) 100(4, Supplement):S1–276. doi: 10.1016/j.kint.2021.05.021. 4. Bennani HN, et al.,J. Pers. Med. 2024, 14(3), 249. 5. Lu H et al. Medicine(Baltimore) 2019 May; 98(18): e15303. BOWMAN’S CAPSULE70–80% of IMN patients have high anti-PLA2r levels1 Anti-PLA2r autoantibodies levels are strongly associated with disease severity in IMN Kidney with IMN2 Autoantibodies bind to PLA2R on podocytes injuring glomerular basement membranes PODOCYTE Subepithelial immune complex deposits Podocyte injury, thickening of basement membrane Proteinuria and hyperlipidemia Kidney failure NEPHROTIC KIDNEY BOWMAN’S CAPSULE Normal Kidney2 DEGRADERS June 2025 Biohaven Investor Presentation43
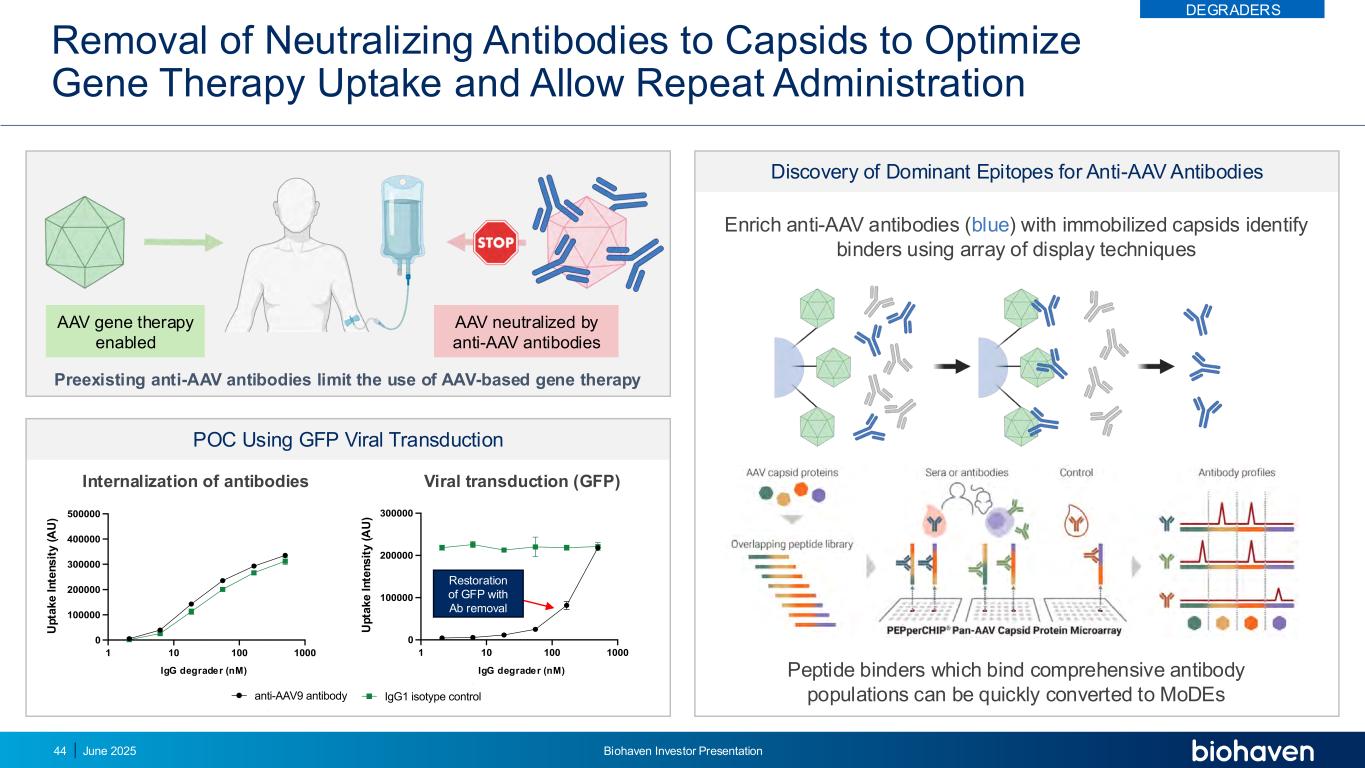
Removal of Neutralizing Antibodies to Capsids to Optimize Gene Therapy Uptake and Allow Repeat Administration Discovery of Dominant Epitopes for Anti-AAV Antibodies 1 10 100 1000 0 100000 200000 300000 400000 500000 BH3746 nM 6 4 7 a re a ( u m 2 /i m a g e ) Internalization anti-AAV9 antibody IgG1 isotype control Internalization of antibodies 1 10 100 1000 0 100000 200000 300000 BH3746 nM 4 8 8 a re a ( u m 2 /i m a g e ) Neutralization anti-AAV9 antibody IgG1 isotype control Viral transduction (GFP) IgG degrader (nM)IgG degrader (nM) Restoration of GFP with Ab removal Enrich anti-AAV antibodies (blue) with immobilized capsids identify binders using array of display techniques Peptide binders which bind comprehensive antibody populations can be quickly converted to MoDEs Preexisting anti-AAV antibodies limit the use of AAV-based gene therapy AAV gene therapy enabled AAV neutralized by anti-AAV antibodies POC Using GFP Viral Transduction 1 10 100 1000 0 100000 200000 300000 400000 500000 BH3746 nM 6 4 7 a re a ( u m 2 /i m a g e ) Internalization anti-AAV9 antibody IgG1 isotype control 1 10 100 1000 0 100000 200000 300000 400000 500000 BH3746 nM 6 4 7 a re a ( u m 2 /i m a g e ) Internalization anti-AAV9 antibody IgG1 isotype control U p ta k e I n te n s it y ( A U ) U p ta k e I n te n s it y ( A U ) DEGRADERS June 2025 Biohaven Investor Presentation44
BHV-7000 Kv7.2/7.3 Activator Potassium (K+) Ion Channel Ion Channel Platform

BHV-7000: Kv7 Activator for Epilepsy and Depression

June 2025 Biohaven Investor Presentation47 Kv7 Is a Transformational Target in Neurology and Neuropsychiatry • Selective Kv7 activation avoids unwanted CNS side effects • Clinically validated in epilepsy, depression and pain BHV-7000 Is Potential Best-in-Class Selective Kv7 Activator with Blockbuster Potential • Rationally designed to eliminate GABAA receptor activation • No dose-limiting CNS side effects observed in Phase 1 • CNS target engagement at predicted therapeutic concentrations confirmed in Phase 1 EEG study Late-stage Clinical Development 4 pivotal trials ongoing in epilepsy and MDD BHV-7000 SELECTIVE Kv7 ACTIVATOR • Pivotal MDD topline results expected 2H 2025 • 1st focal epilepsy study topline results expected 1H 2026 KEY POINT GABA, γ-aminobutyric acid.

BHV-7000: Potential Best-in-Clinic Selective Kv7 Activator Nears Completion of Pivotal Trials with Blockbuster Potential Epilepsy 3.5M Patients • Clinically validated MOA for epilepsy • Global Phase 2/3 program ongoing in focal epilepsy (2 trials) and idiopathic generalized epilepsy (1 trial) Major Depressive Disorder 21M Patients • Clinically validated MOA for MDD • Differentiated profile vs. SSRIs Topline results expected in 2H 2025 1st focal epilepsy study topline results expected in 1H 2026 ION CHANNELS June 2025 Biohaven Investor Presentation48
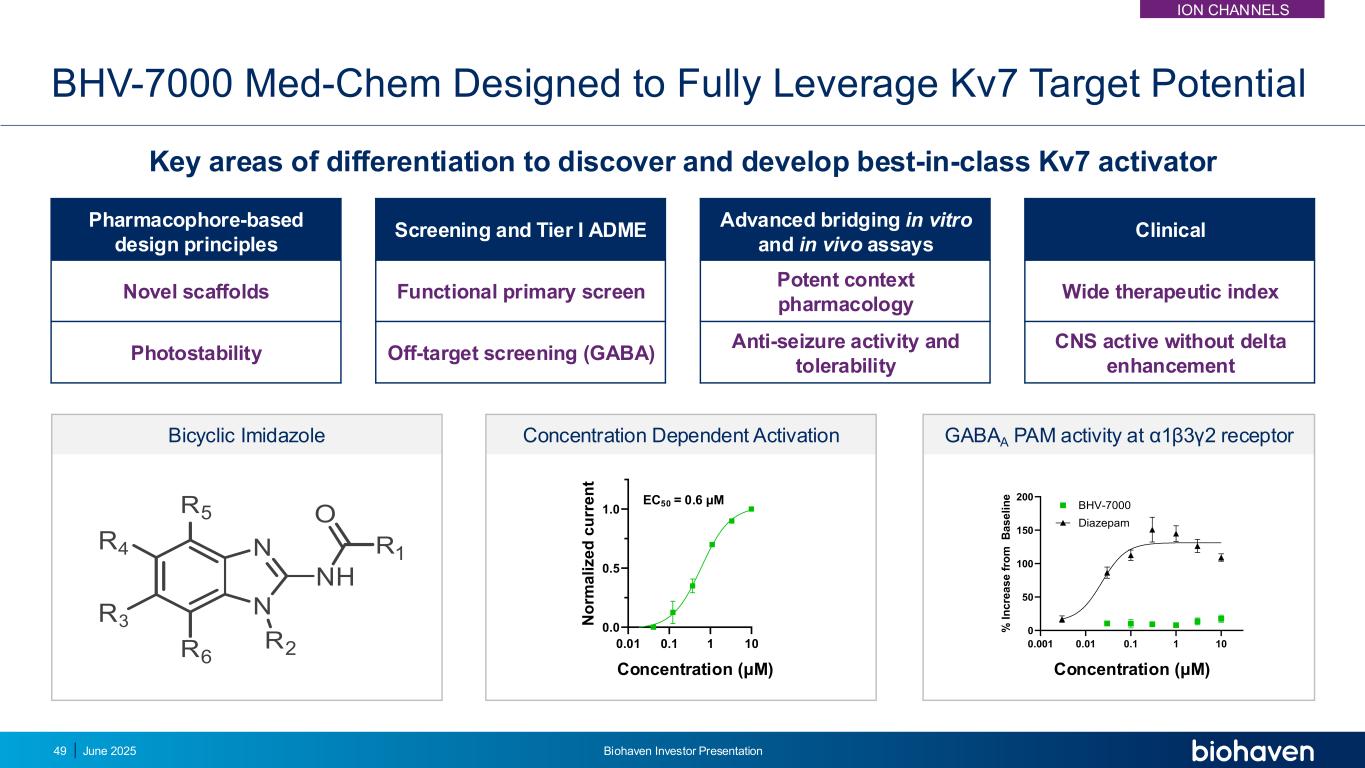
BHV-7000 Med-Chem Designed to Fully Leverage Kv7 Target Potential Key areas of differentiation to discover and develop best-in-class Kv7 activator 49 Pharmacophore-based design principles Screening and Tier I ADME Advanced bridging in vitro and in vivo assays Clinical Novel scaffolds Functional primary screen Potent context pharmacology Wide therapeutic index Photostability Off-target screening (GABA) Anti-seizure activity and tolerability CNS active without delta enhancement 0.001 0.01 0.1 1 10 0 50 100 150 200 Concentration (M) % I n c re a s e f ro m B a s e li n e BHV-7000 Diazepam 0.001 0.01 0.1 1 10 0 50 100 150 200 Concentration (M) % I n c re a s e f ro m B a s e li n e BHV-7000 Diazepam GABAA PAM activity at α1β3γ2 receptor 0.01 0.1 1 10 0.0 0.5 1.0 Concentration (M) N o rm a li z e d c u rr e n t EC50 = 0.6 µM Concentration Dependent Activation oncentration (μM) Concentration (μM) Bicyclic Imidazole June 2025 Biohaven Investor Presentation ION CHANNELS
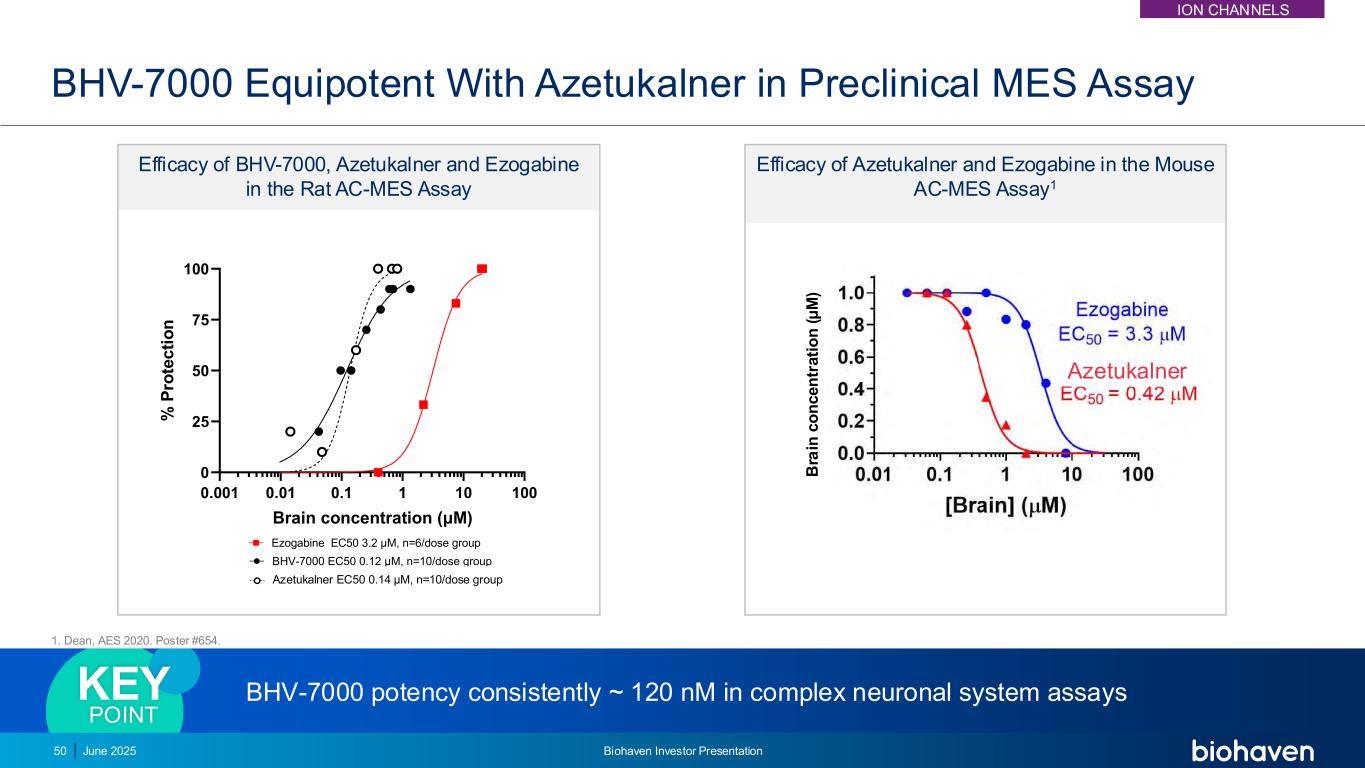
BHV-7000 potency consistently ~ 120 nM in complex neuronal system assaysKEY POINT BHV-7000 Equipotent With Azetukalner in Preclinical MES Assay Efficacy of BHV-7000, Azetukalner and Ezogabine in the Rat AC-MES Assay 0.001 0.01 0.1 1 10 100 0 25 50 75 100 Maximal Electroshock Model (rat) Brain concentration (μM) % P ro te c ti o n BHV-7000 EC50 0.12 μM, n=10/dose group XEN1101 EC50 0.14 μM, n=10/dose group Ezogabine EC50 3.2 μM, n=6/dose group 0.001 0.01 0.1 1 10 100 0 25 50 75 100 Maximal Electroshock Model (rat) Brain concentration (μM) % P ro te c ti o n BHV-7000 EC50 0.12 , n=10/dose group XEN1101 EC50 0.14 μM, n=10/dose group Ezogabine EC50 3.2 μM, n=6/dose group Efficacy of Azetukalner and Ezogabine in the Mouse AC-MES Assay1 1. Dean, AES 2020. Poster #654. Azetukalner EC50 0.14 μM, n=10/dose group Azetukalner 50 B ra in c o n c e n tr a ti o n ( μ M ) June 2025 Biohaven Investor Presentation ION CHANNELS
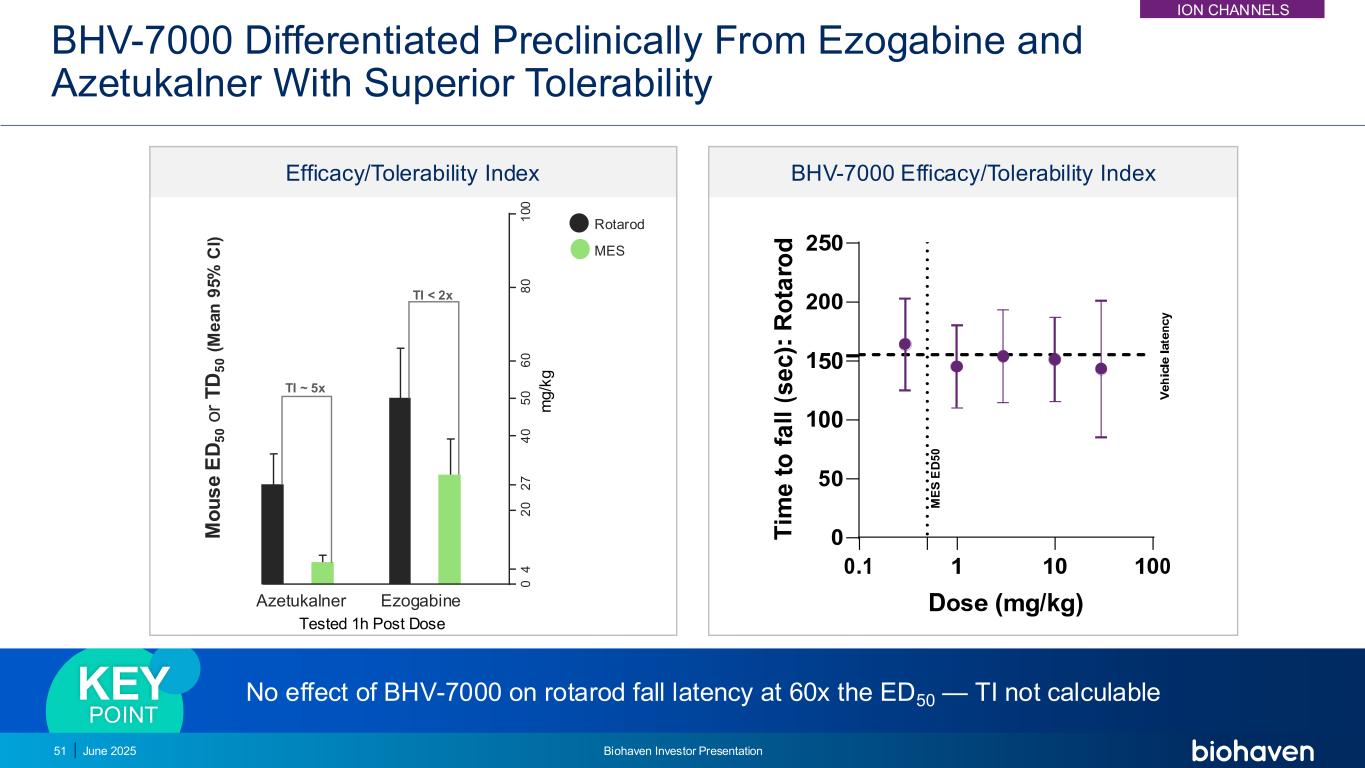
No effect of BHV-7000 on rotarod fall latency at 60x the ED50 — TI not calculable KEY POINT BHV-7000 Differentiated Preclinically From Ezogabine and Azetukalner With Superior Tolerability 51 BHV-7000 Efficacy/Tolerability Index Rotarod MES m g /k g 1 0 0 8 0 6 0 5 0 4 0 2 7 2 0 4 0 Tested 1h Post Dose M o u s e E D 5 0 o r T D 5 0 ( M e a n 9 5 % C I) Azetukalner Ezogabine TI ~ 5x TI < 2x Efficacy/Tolerability Index June 2025 Biohaven Investor Presentation ION CHANNELS
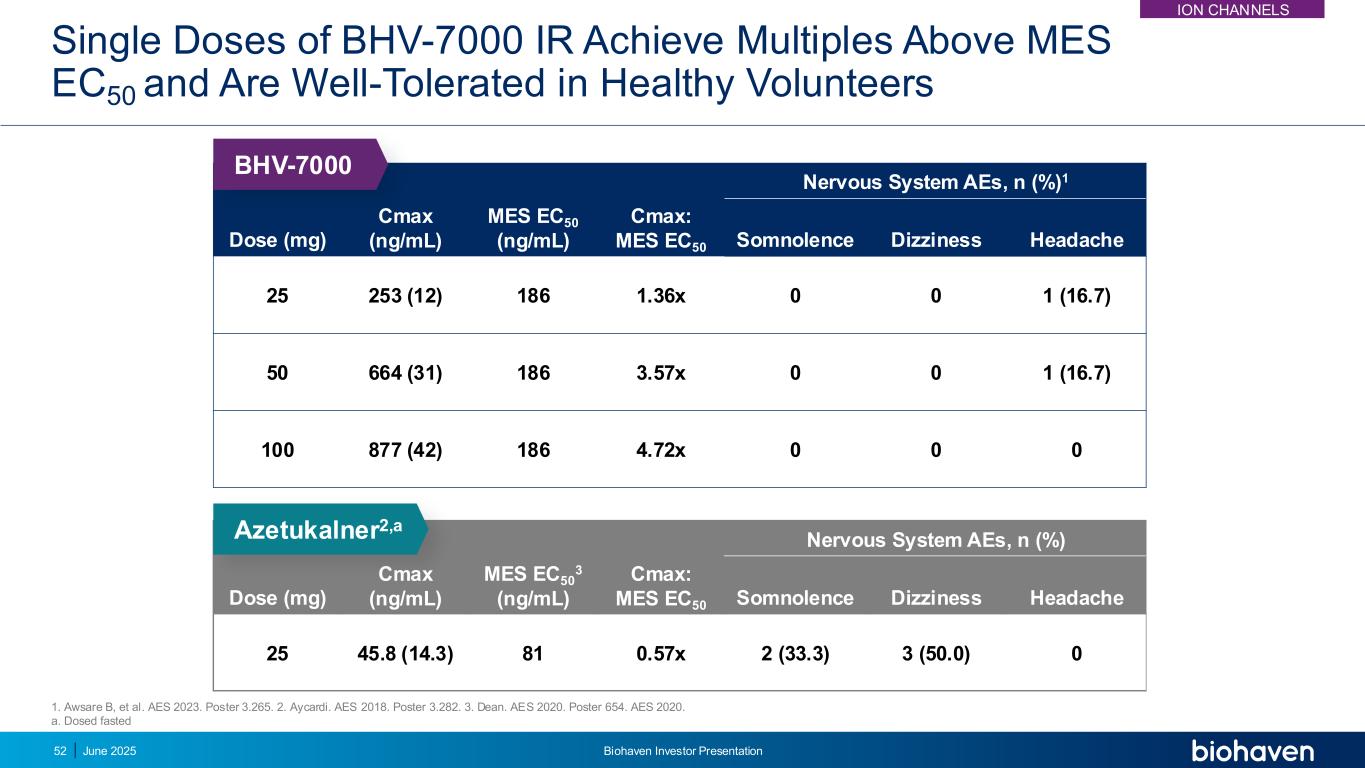
Single Doses of BHV-7000 IR Achieve Multiples Above MES EC50 and Are Well-Tolerated in Healthy Volunteers Dose (mg) Cmax (ng/mL) MES EC50 3 (ng/mL) Cmax: MES EC50 Nervous System AEs, n (%) Somnolence Dizziness Headache 25 45.8 (14.3) 81 0.57x 2 (33.3) 3 (50.0) 0 Azetukalner2,a Dose (mg) Cmax (ng/mL) MES EC50 (ng/mL) Cmax: MES EC50 Nervous System AEs, n (%)1 Somnolence Dizziness Headache 25 253 (12) 186 1.36x 0 0 1 (16.7) 50 664 (31) 186 3.57x 0 0 1 (16.7) 100 877 (42) 186 4.72x 0 0 0 BHV-7000 1. Awsare B, et al. AES 2023. Poster 3.265. 2. Aycardi. AES 2018. Poster 3.282. 3. Dean. AES 2020. Poster 654. AES 2020. a. Dosed fasted 52 June 2025 Biohaven Investor Presentation ION CHANNELS
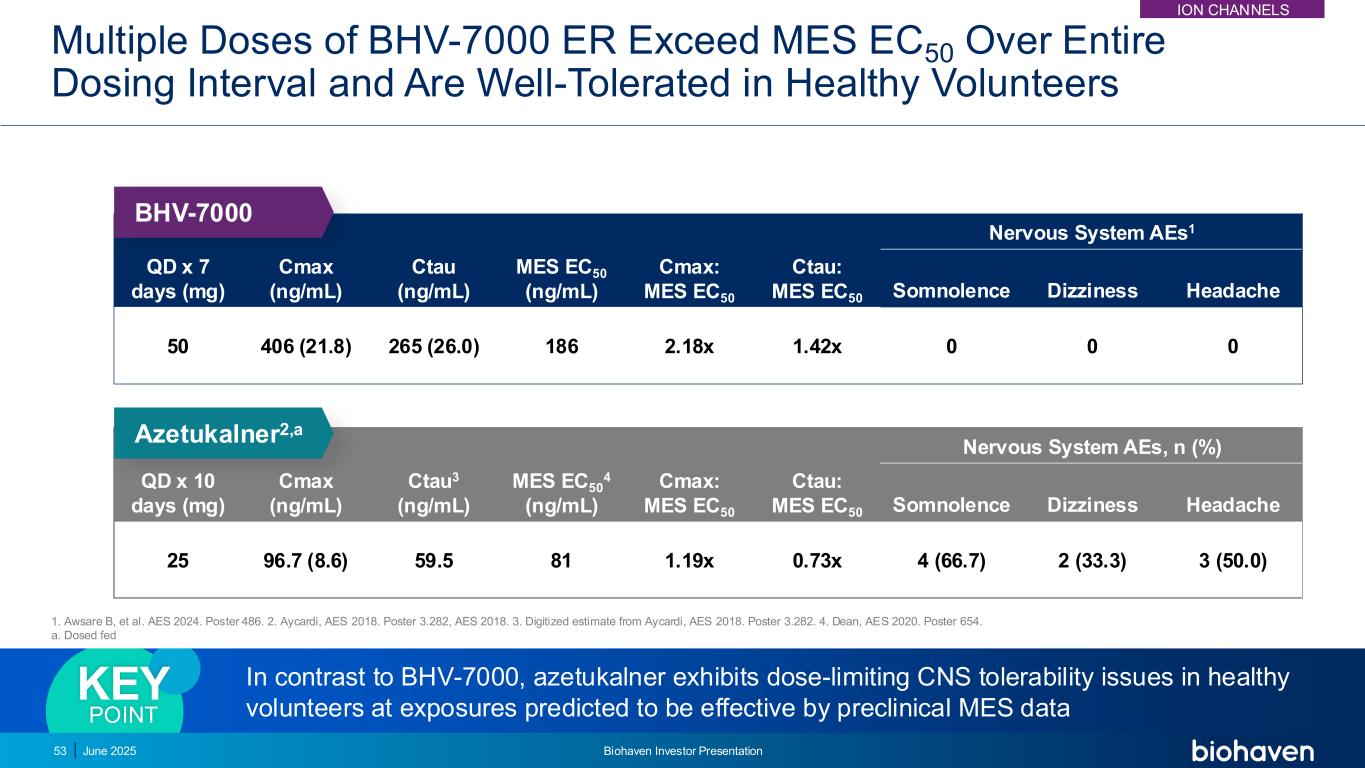
Multiple Doses of BHV-7000 ER Exceed MES EC50 Over Entire Dosing Interval and Are Well-Tolerated in Healthy Volunteers QD x 7 days (mg) Cmax (ng/mL) Ctau (ng/mL) MES EC50 (ng/mL) Cmax: MES EC50 Ctau: MES EC50 Nervous System AEs1 Somnolence Dizziness Headache 50 406 (21.8) 265 (26.0) 186 2.18x 1.42x 0 0 0 QD x 10 days (mg) Cmax (ng/mL) Ctau3 (ng/mL) MES EC50 4 (ng/mL) Cmax: MES EC50 Ctau: MES EC50 Nervous System AEs, n (%) Somnolence Dizziness Headache 25 96.7 (8.6) 59.5 81 1.19x 0.73x 4 (66.7) 2 (33.3) 3 (50.0) In contrast to BHV-7000, azetukalner exhibits dose-limiting CNS tolerability issues in healthy volunteers at exposures predicted to be effective by preclinical MES data KEY POINT Azetukalner2,a BHV-7000 53 June 2025 Biohaven Investor Presentation 1. Awsare B, et al. AES 2024. Poster 486. 2. Aycardi, AES 2018. Poster 3.282, AES 2018. 3. Digitized estimate from Aycardi, AES 2018. Poster 3.282. 4. Dean, AES 2020. Poster 654. a. Dosed fed ION CHANNELS
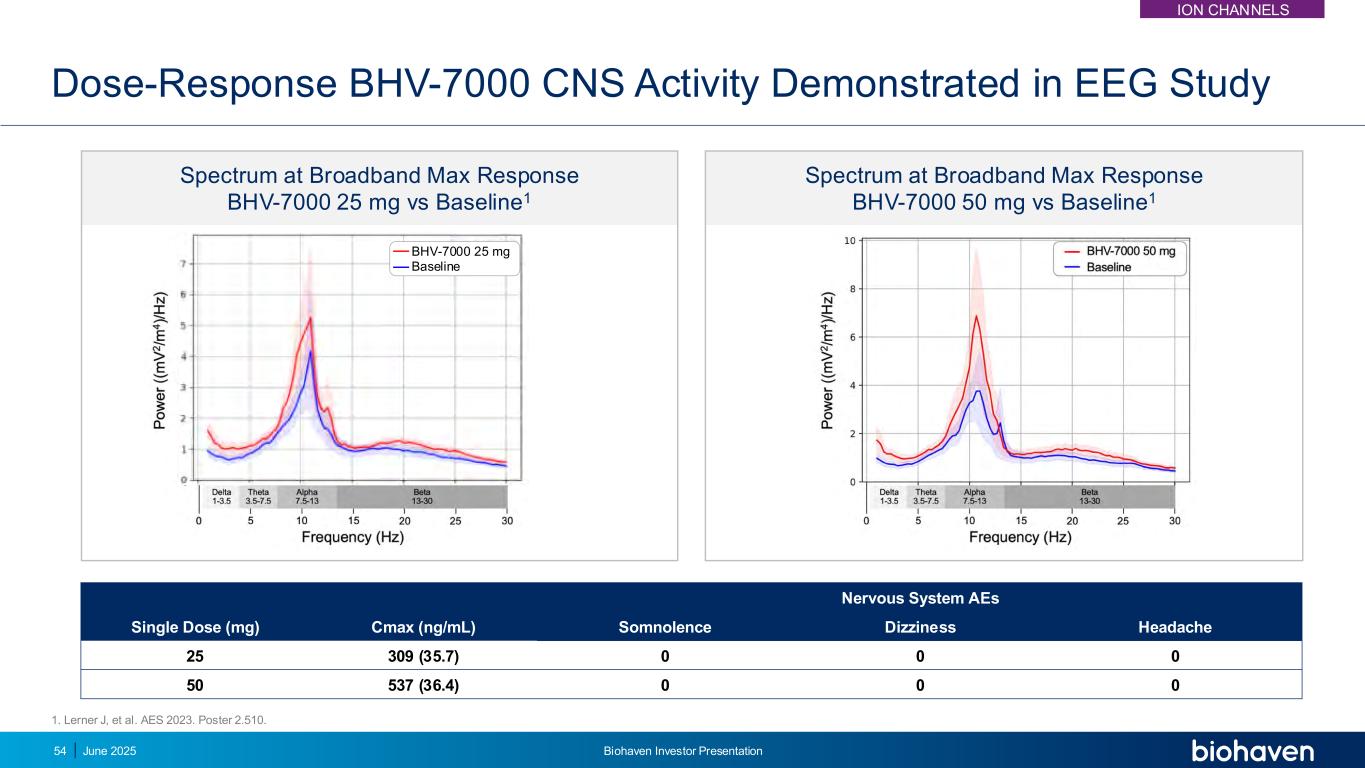
Spectrum at Broadband Max Response BHV-7000 50 mg vs Baseline1 Spectrum at Broadband Max Response BHV-7000 25 mg vs Baseline1 Dose-Response BHV-7000 CNS Activity Demonstrated in EEG Study Single Dose (mg) Cmax (ng/mL) Nervous System AEs Somnolence Dizziness Headache 25 309 (35.7) 0 0 0 50 537 (36.4) 0 0 0 BHV-7000 25 mg Baseline 54 June 2025 Biohaven Investor Presentation 1. Lerner J, et al. AES 2023. Poster 2.510. ION CHANNELS
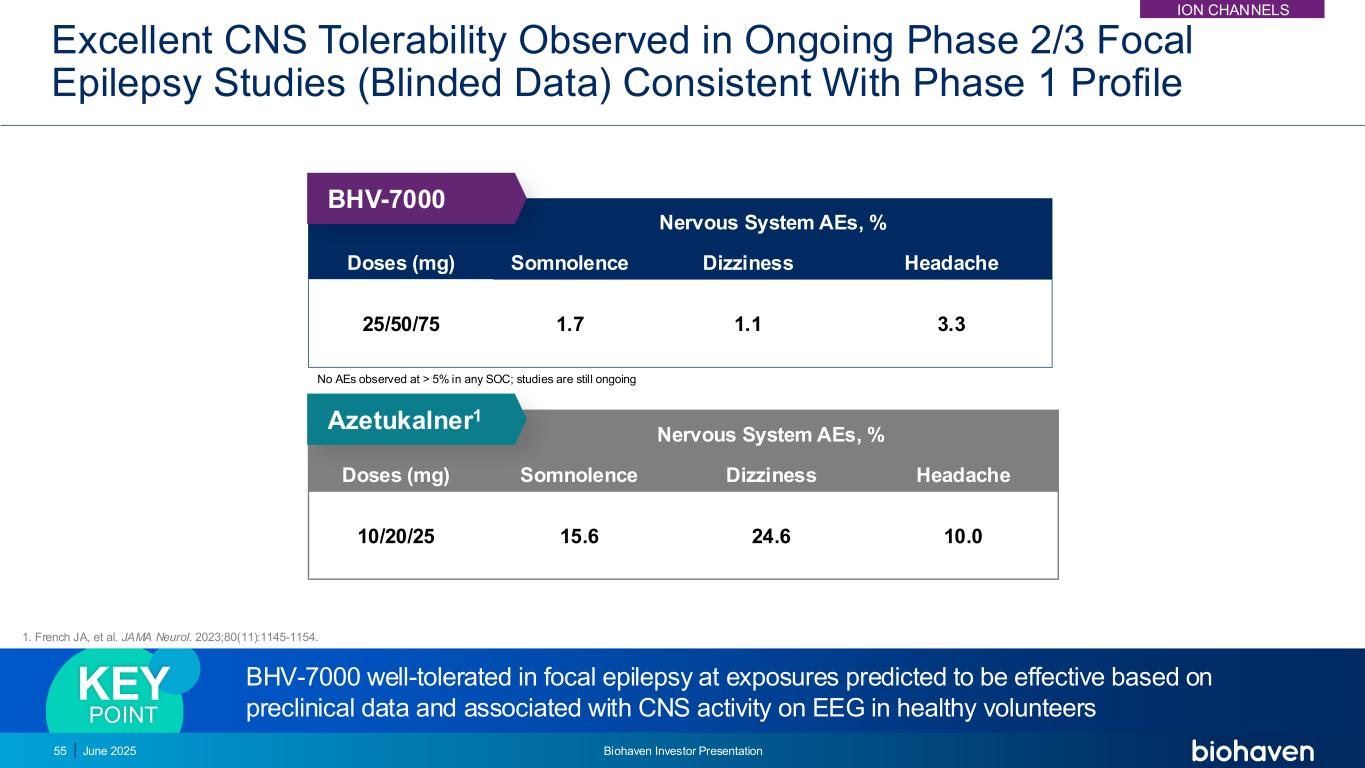
Excellent CNS Tolerability Observed in Ongoing Phase 2/3 Focal Epilepsy Studies (Blinded Data) Consistent With Phase 1 Profile BHV-7000 well-tolerated in focal epilepsy at exposures predicted to be effective based on preclinical data and associated with CNS activity on EEG in healthy volunteers KEY POINT Doses (mg) Nervous System AEs, % Somnolence Dizziness Headache 25/50/75 1.7 1.1 3.3 Doses (mg) Nervous System AEs, % Somnolence Dizziness Headache 10/20/25 15.6 24.6 10.0 Azetukalner1 BHV-7000 No AEs observed at > 5% in any SOC; studies are still ongoing 55 June 2025 Biohaven Investor Presentation 1. French JA, et al. JAMA Neurol. 2023;80(11):1145-1154. ION CHANNELS
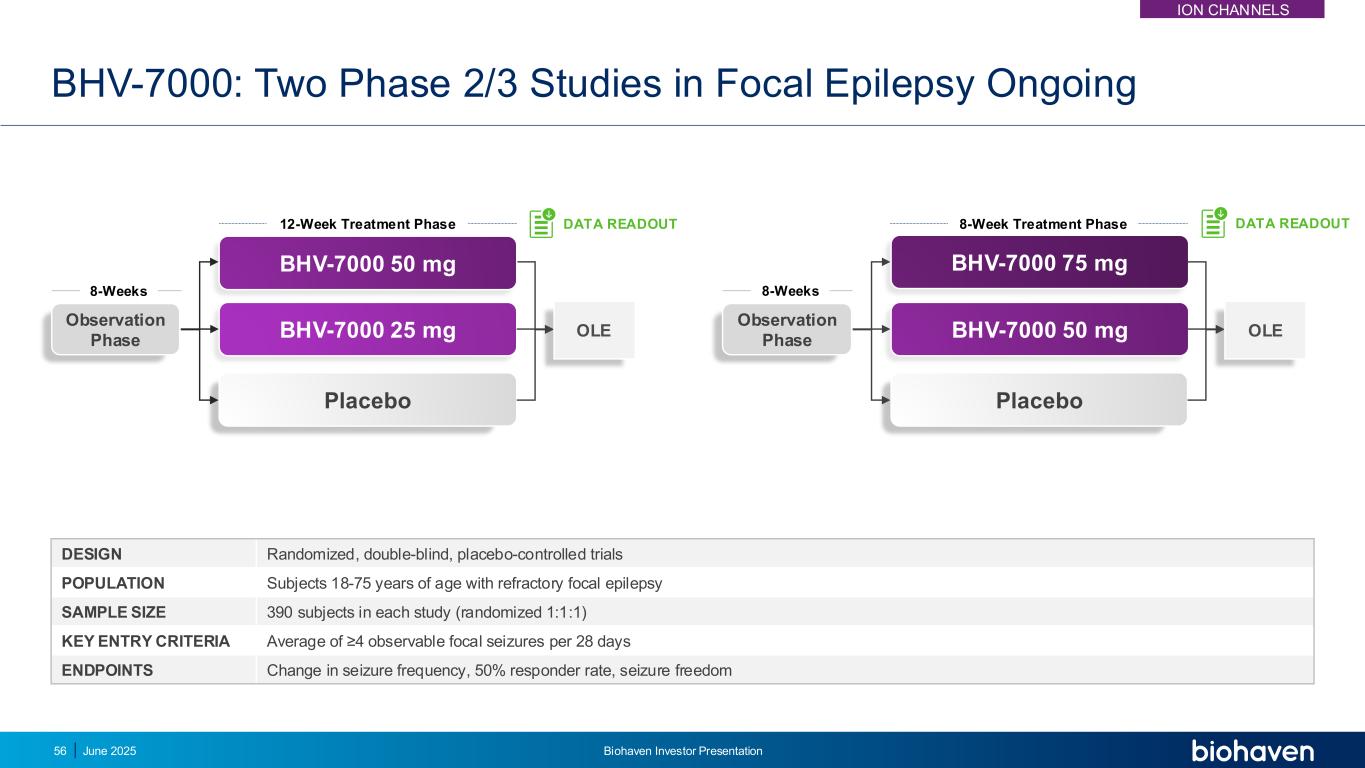
BHV-7000: Two Phase 2/3 Studies in Focal Epilepsy Ongoing DESIGN Randomized, double-blind, placebo-controlled trials POPULATION Subjects 18-75 years of age with refractory focal epilepsy SAMPLE SIZE 390 subjects in each study (randomized 1:1:1) KEY ENTRY CRITERIA Average of ≥4 observable focal seizures per 28 days ENDPOINTS Change in seizure frequency, 50% responder rate, seizure freedom Observation Phase OLEBHV-7000 50 mg BHV-7000 75 mg Placebo 8-Weeks 8-Week Treatment Phase DATA READOUT OLE Observation Phase BHV-7000 25 mg Placebo 12-Week Treatment Phase 8-Weeks DATA READOUT BHV-7000 50 mg ION CHANNELS June 2025 Biohaven Investor Presentation56
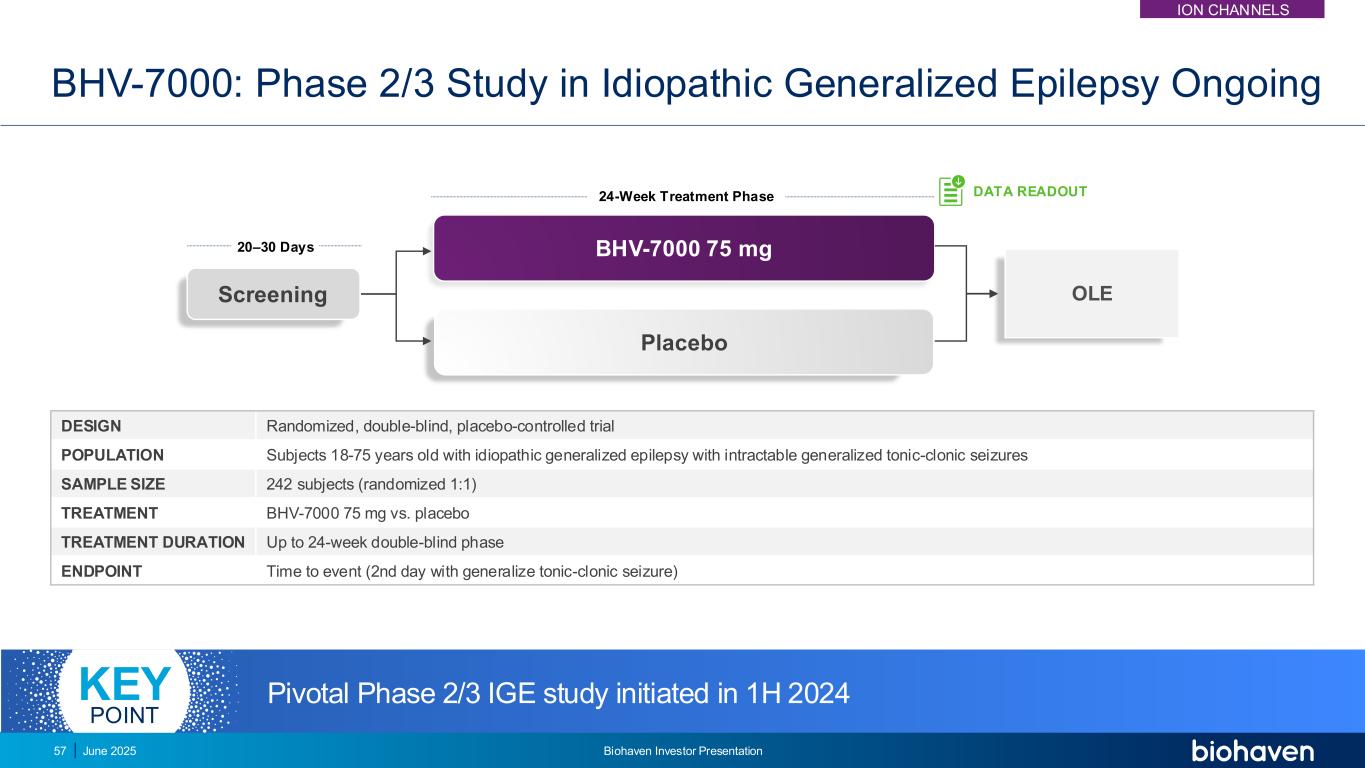
OLEScreening BHV-7000 75 mg Placebo BHV-7000: Phase 2/3 Study in Idiopathic Generalized Epilepsy Ongoing DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects 18-75 years old with idiopathic generalized epilepsy with intractable generalized tonic-clonic seizures SAMPLE SIZE 242 subjects (randomized 1:1) TREATMENT BHV-7000 75 mg vs. placebo TREATMENT DURATION Up to 24-week double-blind phase ENDPOINT Time to event (2nd day with generalize tonic-clonic seizure) 20–30 Days 24-Week Treatment Phase DATA READOUT Pivotal Phase 2/3 IGE study initiated in 1H 2024KEY POINT ION CHANNELS June 2025 Biohaven Investor Presentation57
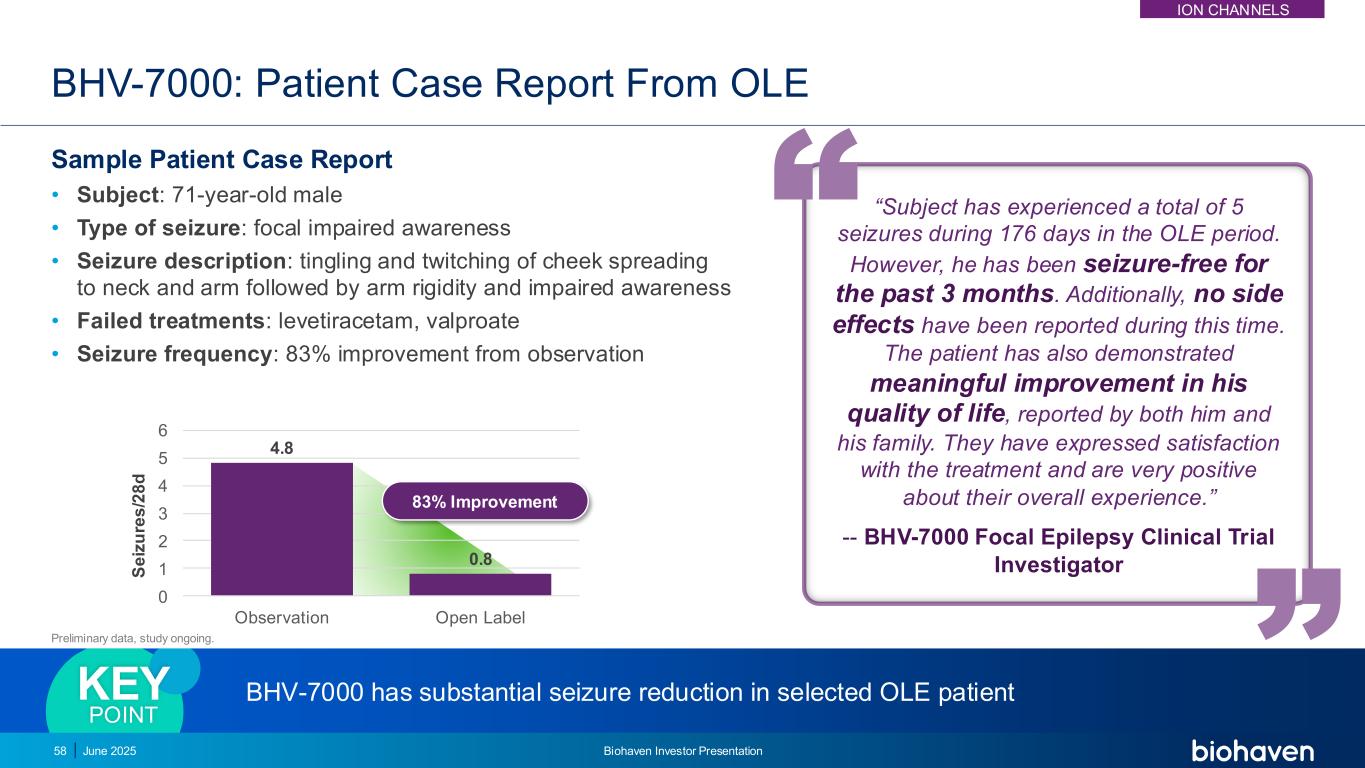
4.8 0.8 0 1 2 3 4 5 6 Observation Open Label BHV-7000: Patient Case Report From OLE Sample Patient Case Report • Subject: 71-year-old male • Type of seizure: focal impaired awareness • Seizure description: tingling and twitching of cheek spreading to neck and arm followed by arm rigidity and impaired awareness • Failed treatments: levetiracetam, valproate • Seizure frequency: 83% improvement from observation “Subject has experienced a total of 5 seizures during 176 days in the OLE period. However, he has been seizure-free for the past 3 months. Additionally, no side effects have been reported during this time. The patient has also demonstrated meaningful improvement in his quality of life, reported by both him and his family. They have expressed satisfaction with the treatment and are very positive about their overall experience.” -- BHV-7000 Focal Epilepsy Clinical Trial Investigator BHV-7000 has substantial seizure reduction in selected OLE patientKEY POINT S e iz u re s /2 8 d 83% Improvement 58 June 2025 Biohaven Investor Presentation Preliminary data, study ongoing. ION CHANNELS
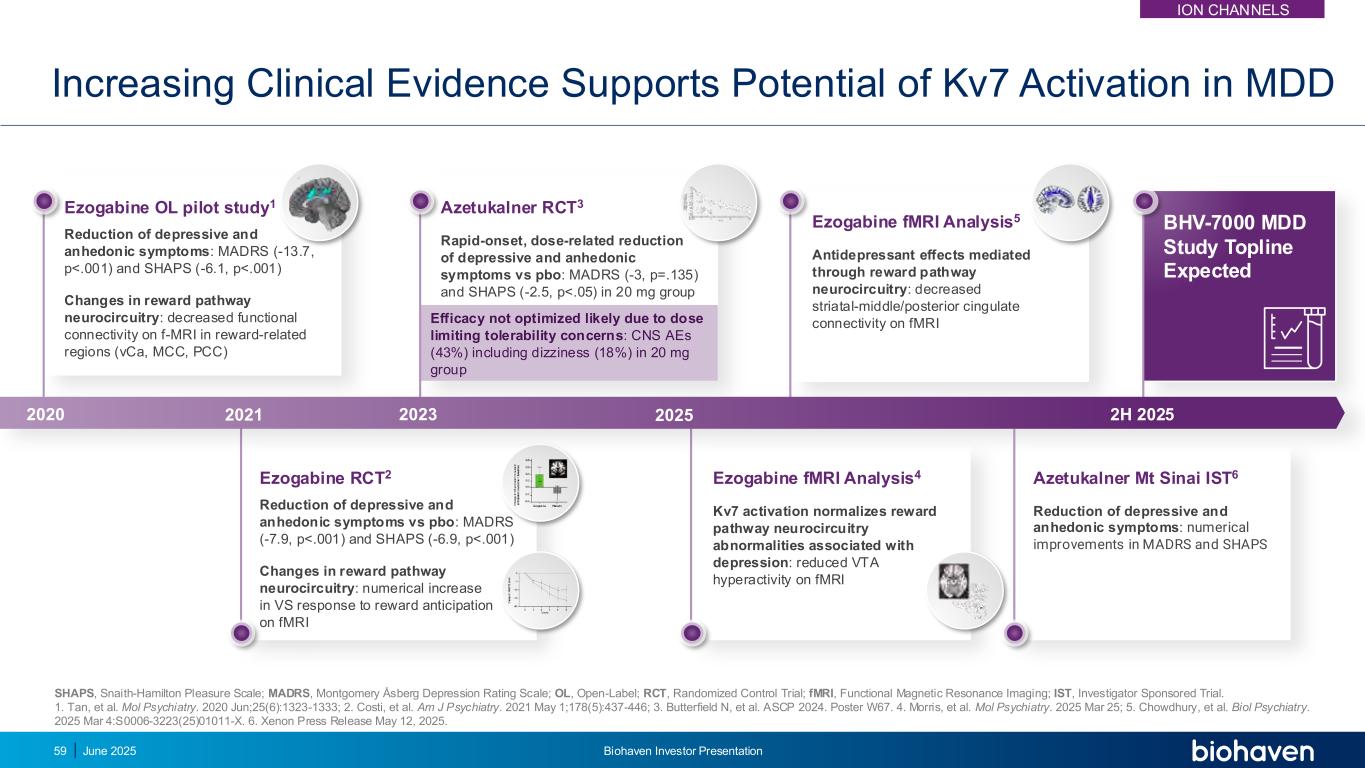
Azetukalner RCT3 Rapid-onset, dose-related reduction of depressive and anhedonic symptoms vs pbo: MADRS (-3, p=.135) and SHAPS (-2.5, p<.05) in 20 mg group Efficacy not optimized likely due to dose limiting tolerability concerns: CNS AEs (43%) including dizziness (18%) in 20 mg group Azetukalner Mt Sinai IST6 Reduction of depressive and anhedonic symptoms: numerical improvements in MADRS and SHAPS Increasing Clinical Evidence Supports Potential of Kv7 Activation in MDD Ezogabine RCT2 Reduction of depressive and anhedonic symptoms vs pbo: MADRS (-7.9, p<.001) and SHAPS (-6.9, p<.001) Changes in reward pathway neurocircuitry: numerical increase in VS response to reward anticipation on fMRI Ezogabine OL pilot study1 Reduction of depressive and anhedonic symptoms: MADRS (-13.7, p<.001) and SHAPS (-6.1, p<.001) Changes in reward pathway neurocircuitry: decreased functional connectivity on f-MRI in reward-related regions (vCa, MCC, PCC) SHAPS, Snaith-Hamilton Pleasure Scale; MADRS, Montgomery Åsberg Depression Rating Scale; OL, Open-Label; RCT, Randomized Control Trial; fMRI, Functional Magnetic Resonance Imaging; IST, Investigator Sponsored Trial. 1. Tan, et al. Mol Psychiatry. 2020 Jun;25(6):1323-1333; 2. Costi, et al. Am J Psychiatry. 2021 May 1;178(5):437-446; 3. Butterfield N, et al. ASCP 2024. Poster W67. 4. Morris, et al. Mol Psychiatry. 2025 Mar 25; 5. Chowdhury, et al. Biol Psychiatry. 2025 Mar 4:S0006-3223(25)01011-X. 6. Xenon Press Release May 12, 2025. BHV-7000 MDD Study Topline Expected Ezogabine fMRI Analysis4 Kv7 activation normalizes reward pathway neurocircuitry abnormalities associated with depression: reduced VTA hyperactivity on fMRI Ezogabine fMRI Analysis5 Antidepressant effects mediated through reward pathway neurocircuitry: decreased striatal-middle/posterior cingulate connectivity on fMRI 2020 2021 2023 2025 2H 2025 59 June 2025 Biohaven Investor Presentation ION CHANNELS
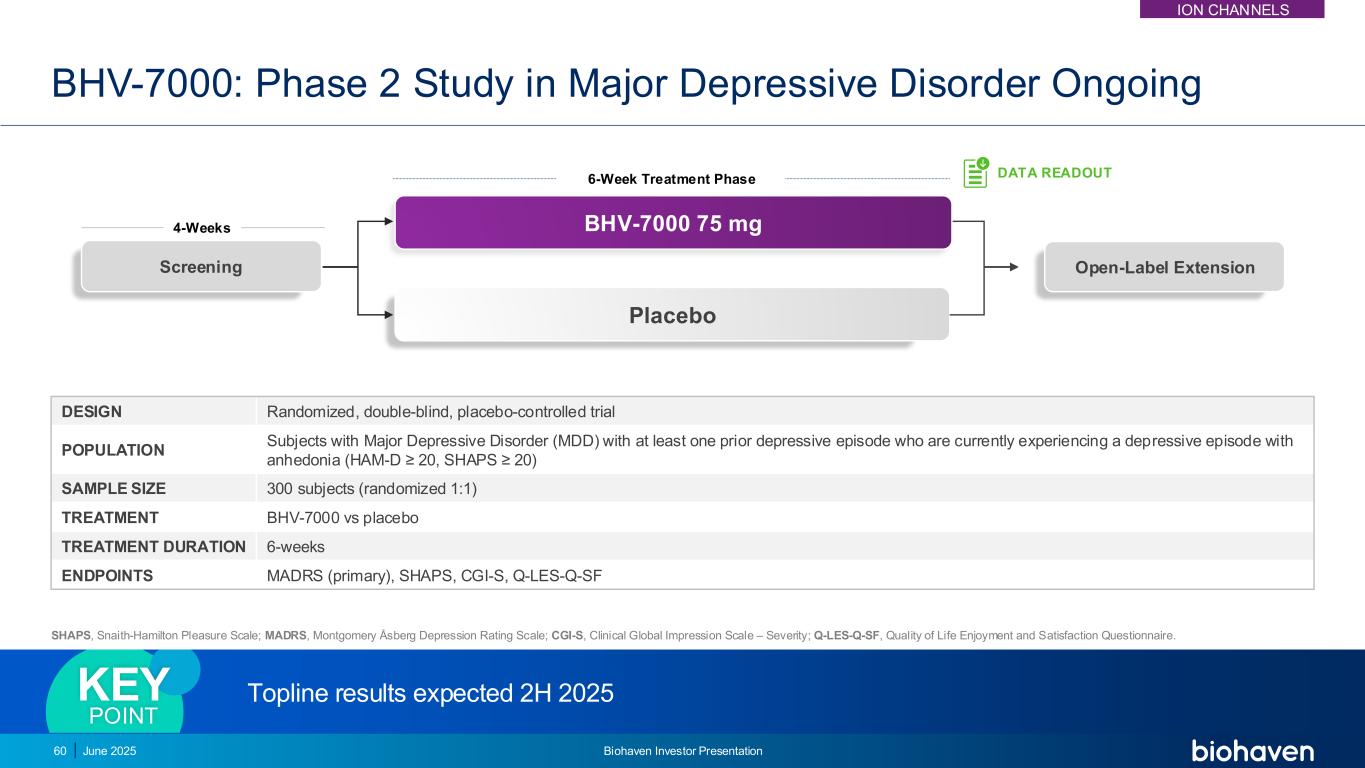
BHV-7000: Phase 2 Study in Major Depressive Disorder Ongoing DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Subjects with Major Depressive Disorder (MDD) with at least one prior depressive episode who are currently experiencing a depressive episode with anhedonia (HAM-D ≥ 20, SHAPS ≥ 20) SAMPLE SIZE 300 subjects (randomized 1:1) TREATMENT BHV-7000 vs placebo TREATMENT DURATION 6-weeks ENDPOINTS MADRS (primary), SHAPS, CGI-S, Q-LES-Q-SF SHAPS, Snaith-Hamilton Pleasure Scale; MADRS, Montgomery Åsberg Depression Rating Scale; CGI-S, Clinical Global Impression Scale – Severity; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire. DATA READOUT Screening Placebo 6-Week Treatment Phase 4-Weeks BHV-7000 75 mg Open-Label Extension Topline results expected 2H 2025 KEY POINT 60 June 2025 Biohaven Investor Presentation ION CHANNELS
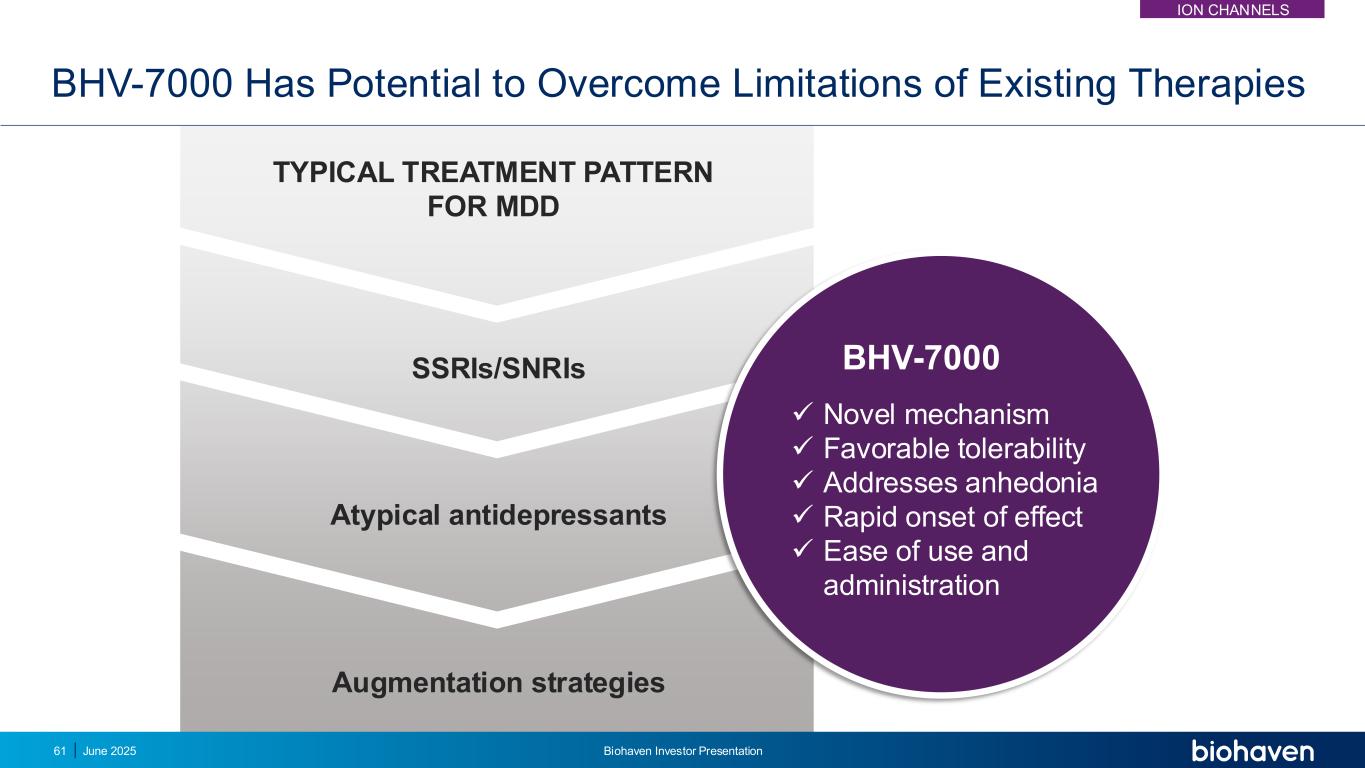
BHV-7000 Has Potential to Overcome Limitations of Existing Therapies SSRIs/SNRIs TYPICAL TREATMENT PATTERN FOR MDD Atypical antidepressants Augmentation strategies ✓ Novel mechanism ✓ Favorable tolerability ✓ Addresses anhedonia ✓ Rapid onset of effect ✓ Ease of use and administration BHV-7000 61 June 2025 Biohaven Investor Presentation ION CHANNELS

BHV-2100: TRPM3 Antagonist for Pain

June 2025 Biohaven Investor Presentation63 First-in-Clinic TRPM3 Antagonist — Novel Mechanism for the Treatment of Pain • BHV-2100 is an orally administered, peripherally-restricted and selective TRPM3 antagonist • Preclinical data shows robust efficacy in pain models • Highly differentiated from programs that target TRPV1 and TRPA1, avoids TRP family liabilities Phase 1 Study Data Supports Evaluation in Pain • BHV-2100 demonstrated excellent safety/tolerability and favorable PK • Proof of concept demonstrated for pain The Therapeutic Landscape of TRPM3-Targeted Therapies Is Evolving • Emerging data support the potential for targeting TRPM3 in nociceptive pain conditions • Non-clinical data support brain-penetrant TRPM3 program in epilepsy and pain Milestones Achieved • Early signal of efficacy observed in laser-evoked pain trial • Proof-of-concept trial in acute treatment of migraine completed with no efficacy signal detected BHV-2100 TRPM3 ANTAGONIST Proof-of-concept for pain demonstrated; program advancing to later stage pain trialsKEY POINT
BHV-2100: Targeting the Unmet Medical Need in Pain ACUTE PAIN & NEUROPATHIC PAIN Emerging role of novel mechanisms: ion channels in the periphery BHV-2100 is a selective, peripherally-restricted TRPM3 antagonist that is a potentially highly-effective, non-sedating, non-opioid treatment for pain KEY POINT ION CHANNELS June 2025 Biohaven Investor Presentation64
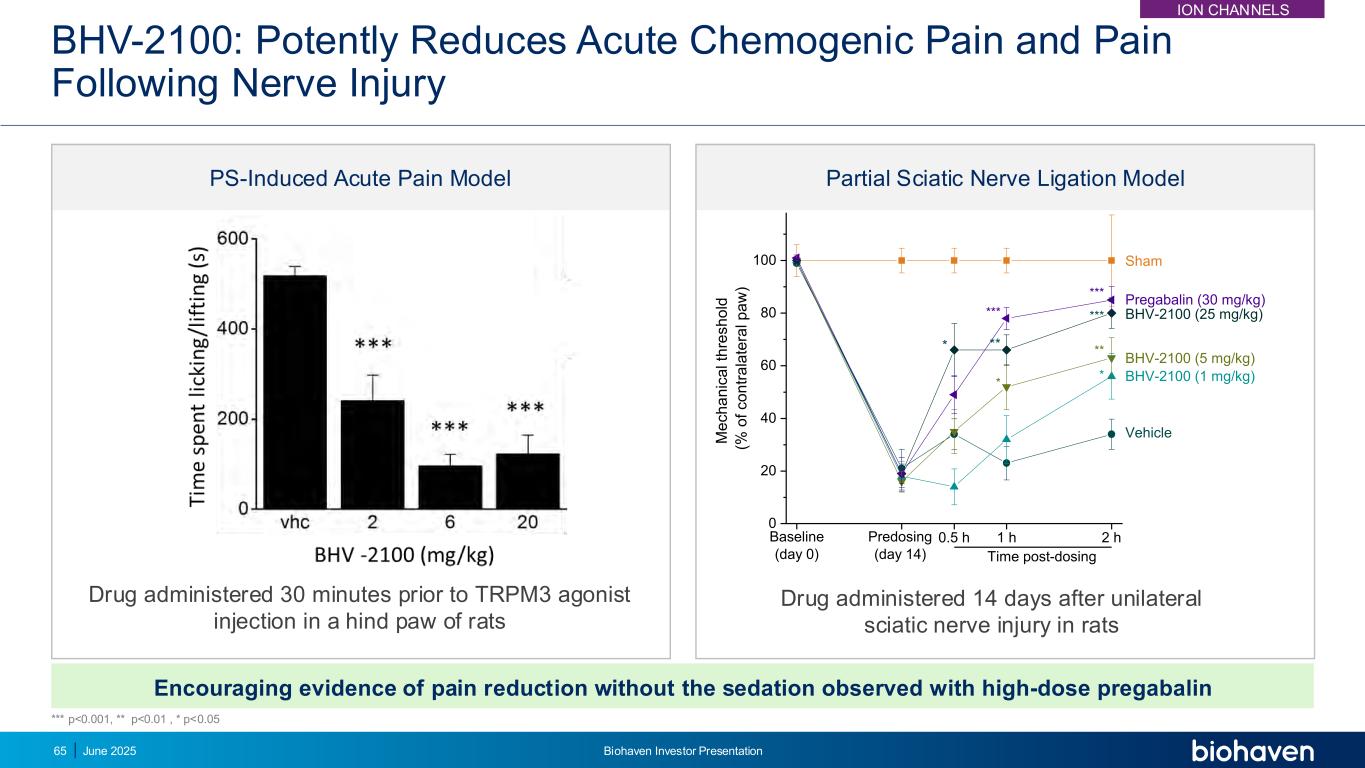
BHV-2100: Potently Reduces Acute Chemogenic Pain and Pain Following Nerve Injury Encouraging evidence of pain reduction without the sedation observed with high-dose pregabalin Drug administered 30 minutes prior to TRPM3 agonist injection in a hind paw of rats Drug administered 14 days after unilateral sciatic nerve injury in rats 0 20 40 60 80 100 * * ** * *** ** *** Vehicle BHV-2100 (1 mg/kg) BHV-2100 (5 mg/kg) BHV-2100 (25 mg/kg) Pregabalin (30 mg/kg) 2 h1 h0.5 hPredosing (day 14) M e c h a n ic a l th re s h o ld (% o f c o n tr a la te ra l p a w ) Baseline (day 0) Time post-dosing Sham *** PS-Induced Acute Pain Model Partial Sciatic Nerve Ligation Model *** p<0.001, ** p<0.01 , * p<0.05 ION CHANNELS June 2025 Biohaven Investor Presentation65
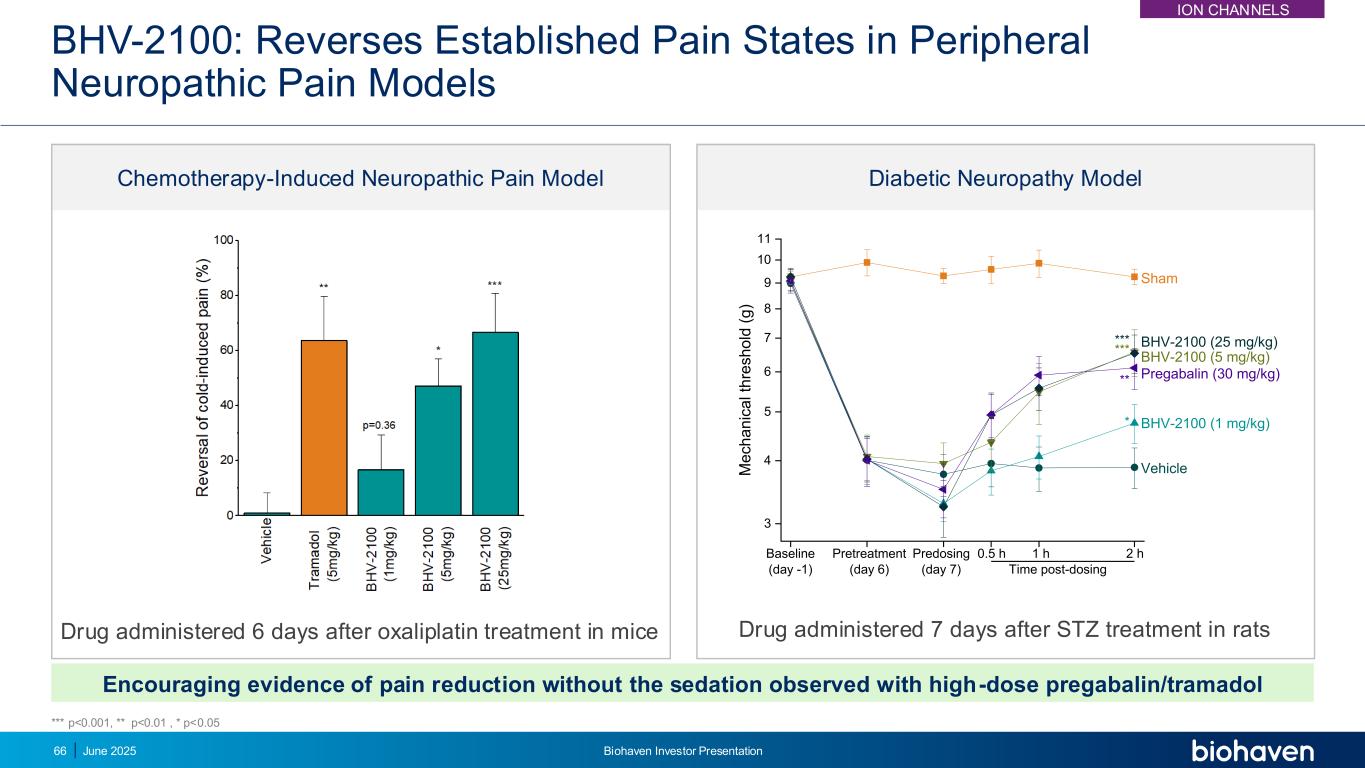
BHV-2100: Reverses Established Pain States in Peripheral Neuropathic Pain Models Drug administered 6 days after oxaliplatin treatment in mice Chemotherapy-Induced Neuropathic Pain Model Diabetic Neuropathy Model Drug administered 7 days after STZ treatment in rats 3 4 5 6 7 8 9 10 11 * ** *** 2 h1 hPredosing (day 7) Pretreatment (day 6) M e c h a n ic a l th re s h o ld ( g ) Baseline (day -1) 0.5 h Time post-dosing Vehicle BHV-2100 (1 mg/kg) BHV-2100 (5 mg/kg) BHV-2100 (25 mg/kg) Pregabalin (30 mg/kg) Sham *** Encouraging evidence of pain reduction without the sedation observed with high-dose pregabalin/tramadol *** p<0.001, ** p<0.01 , * p<0.05 ION CHANNELS June 2025 Biohaven Investor Presentation66
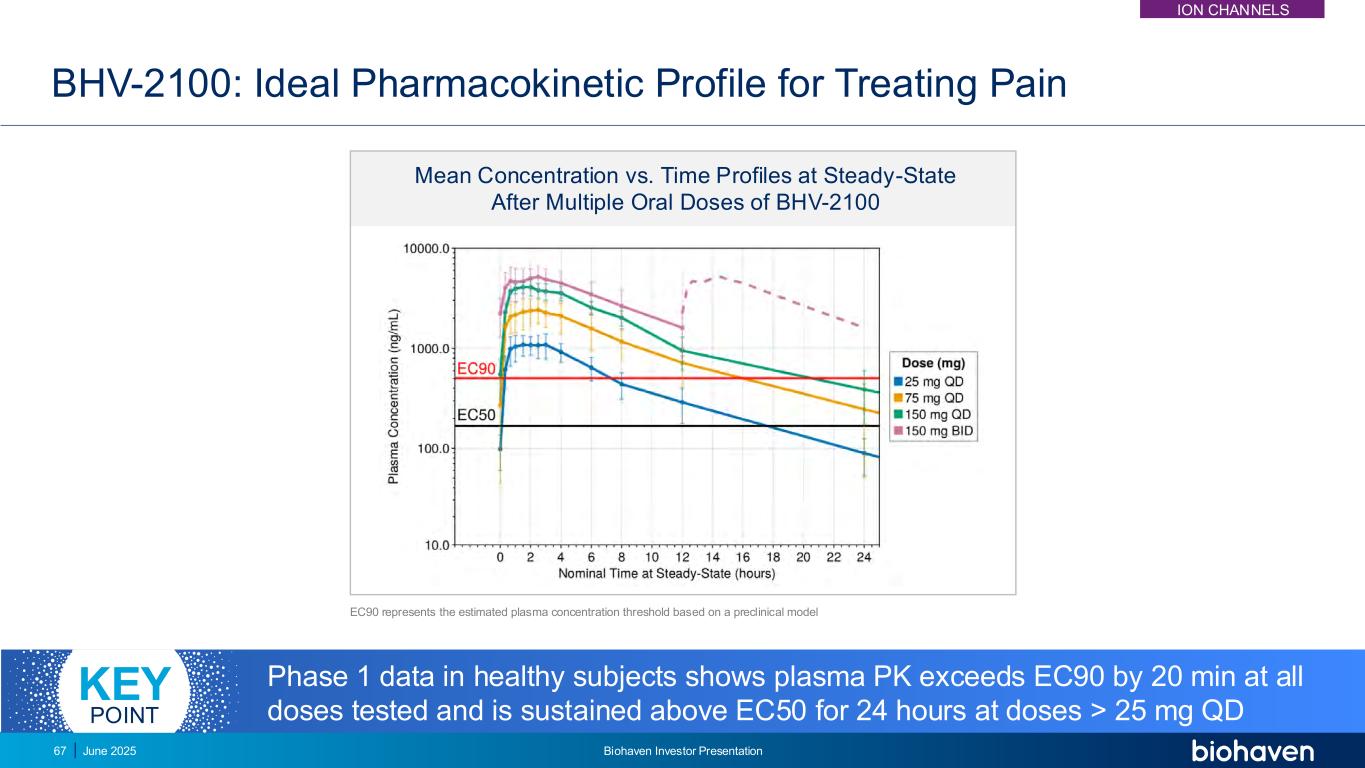
BHV-2100: Ideal Pharmacokinetic Profile for Treating Pain EC90 represents the estimated plasma concentration threshold based on a preclinical model Phase 1 data in healthy subjects shows plasma PK exceeds EC90 by 20 min at all doses tested and is sustained above EC50 for 24 hours at doses > 25 mg QD KEY POINT Mean Concentration vs. Time Profiles at Steady-State After Multiple Oral Doses of BHV-2100 ION CHANNELS June 2025 Biohaven Investor Presentation67
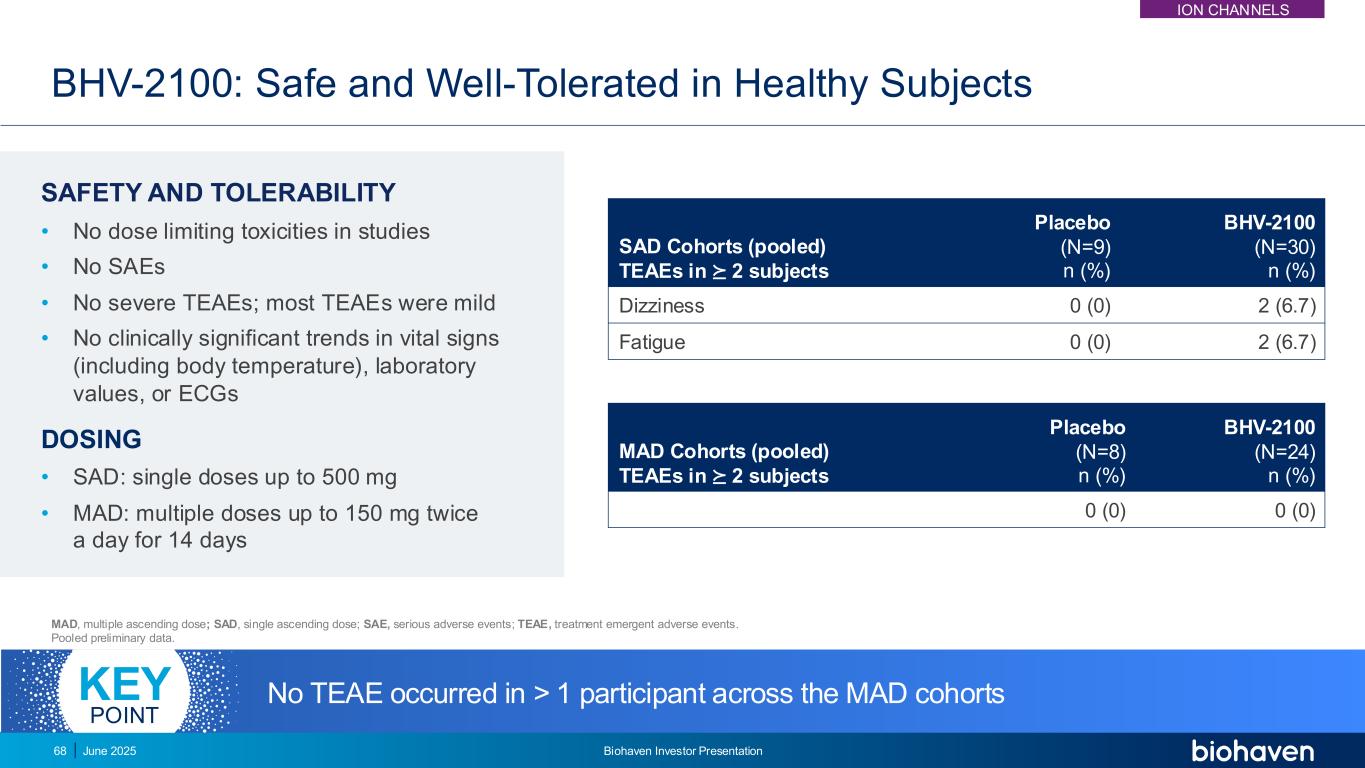
BHV-2100: Safe and Well-Tolerated in Healthy Subjects SAFETY AND TOLERABILITY • No dose limiting toxicities in studies • No SAEs • No severe TEAEs; most TEAEs were mild • No clinically significant trends in vital signs (including body temperature), laboratory values, or ECGs DOSING • SAD: single doses up to 500 mg • MAD: multiple doses up to 150 mg twice a day for 14 days MAD, multiple ascending dose; SAD, single ascending dose; SAE, serious adverse events; TEAE, treatment emergent adverse events. Pooled preliminary data. SAD Cohorts (pooled) TEAEs in ⪰ 2 subjects Placebo (N=9) n (%) BHV-2100 (N=30) n (%) Dizziness 0 (0) 2 (6.7) Fatigue 0 (0) 2 (6.7) MAD Cohorts (pooled) TEAEs in ⪰ 2 subjects Placebo (N=8) n (%) BHV-2100 (N=24) n (%) 0 (0) 0 (0) No TEAE occurred in > 1 participant across the MAD cohortsKEY POINT ION CHANNELS June 2025 Biohaven Investor Presentation68
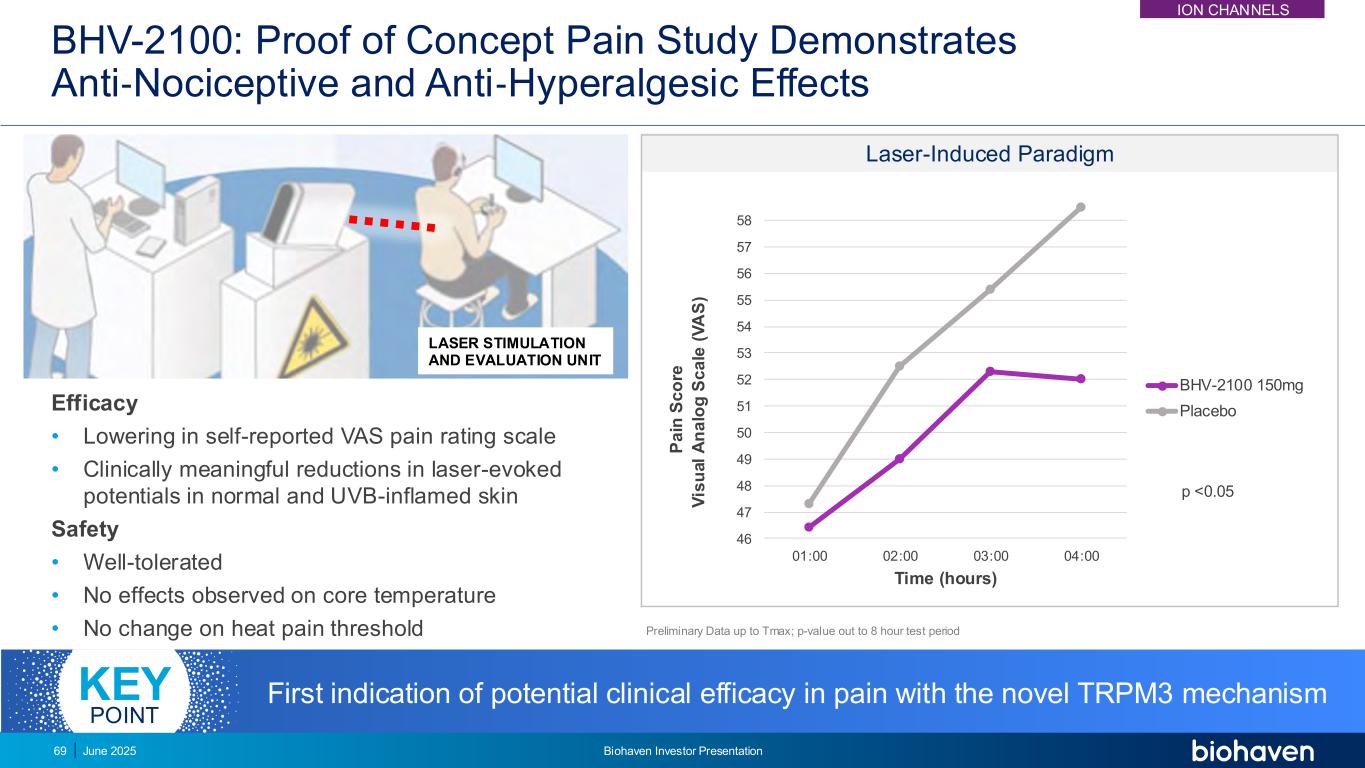
BHV-2100: Proof of Concept Pain Study Demonstrates Anti‐Nociceptive and Anti‐Hyperalgesic Effects Efficacy • Lowering in self-reported VAS pain rating scale • Clinically meaningful reductions in laser-evoked potentials in normal and UVB-inflamed skin Safety • Well-tolerated • No effects observed on core temperature • No change on heat pain threshold 46 47 48 49 50 51 52 53 54 55 56 57 58 01:00 02:00 03:00 04:00 V is u a l A n a lo g S c a le ( V A S ) Time (hours) BHV-2100 150mg Placebo Laser-Induced Paradigm p <0.05 Preliminary Data up to Tmax; p-value out to 8 hour test period First indication of potential clinical efficacy in pain with the novel TRPM3 mechanismKEY POINT LASER STIMULATION AND EVALUATION UNIT P a in S c o re ION CHANNELS June 2025 Biohaven Investor Presentation69
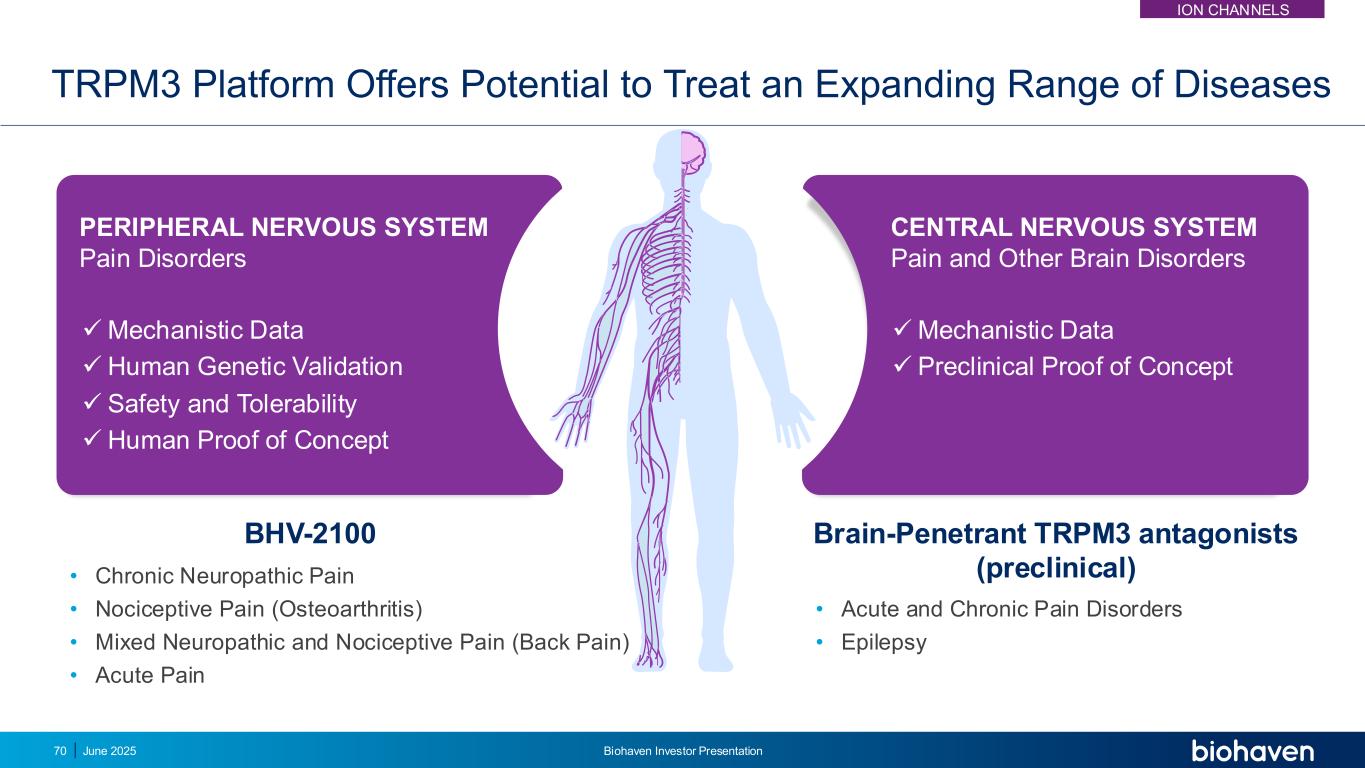
BHV-2100 TRPM3 Platform Offers Potential to Treat an Expanding Range of Diseases • Chronic Neuropathic Pain • Nociceptive Pain (Osteoarthritis) • Mixed Neuropathic and Nociceptive Pain (Back Pain) • Acute Pain ✓Mechanistic Data ✓Human Genetic Validation ✓ Safety and Tolerability ✓Human Proof of Concept PERIPHERAL NERVOUS SYSTEM Pain Disorders CENTRAL NERVOUS SYSTEM Pain and Other Brain Disorders ✓Mechanistic Data ✓ Preclinical Proof of Concept Brain-Penetrant TRPM3 antagonists (preclinical) • Acute and Chronic Pain Disorders • Epilepsy June 2025 Biohaven Investor Presentation70 ION CHANNELS

BHV-8000: Brain-Penetrant TYK2/JAK1 Inhibitor for Parkinson’s Disease

June 2025 Biohaven Investor Presentation72 First-in-Clinic, Brain-Penetrant, Selective TYK2/JAK1 Inhibitor for the Treatment of Neuroinflammatory and Neurodegenerative Diseases • TYK2 and JAK1 inhibition target key inflammatory signaling pathways • Selectivity profile avoids safety liabilities of JAK2/3 inhibition Breaks Cycle of Central and Peripheral Inflammation • Autoimmunity plays key role in Parkinson’s and is novel therapeutic target • TYK2/JAK1 inhibition reduces inflammatory impact of activated microglia and astrocytes in the CNS, and infiltrating T lymphocytes Encouraging Results from Phase 1 • Well-tolerated • Achieves target engagement • Robust brain penetration Phase 2/3 Parkinson’s Disease Study Ongoing Innovative study design optimized for efficiency and sensitivity in detecting clinically meaningful change BHV-8000 TYK2/JAK1 INHIBITOR (BRAIN-PENETRANT) Phase 2/3 Parkinson’s study initiated May 2025NEWS BREAKING
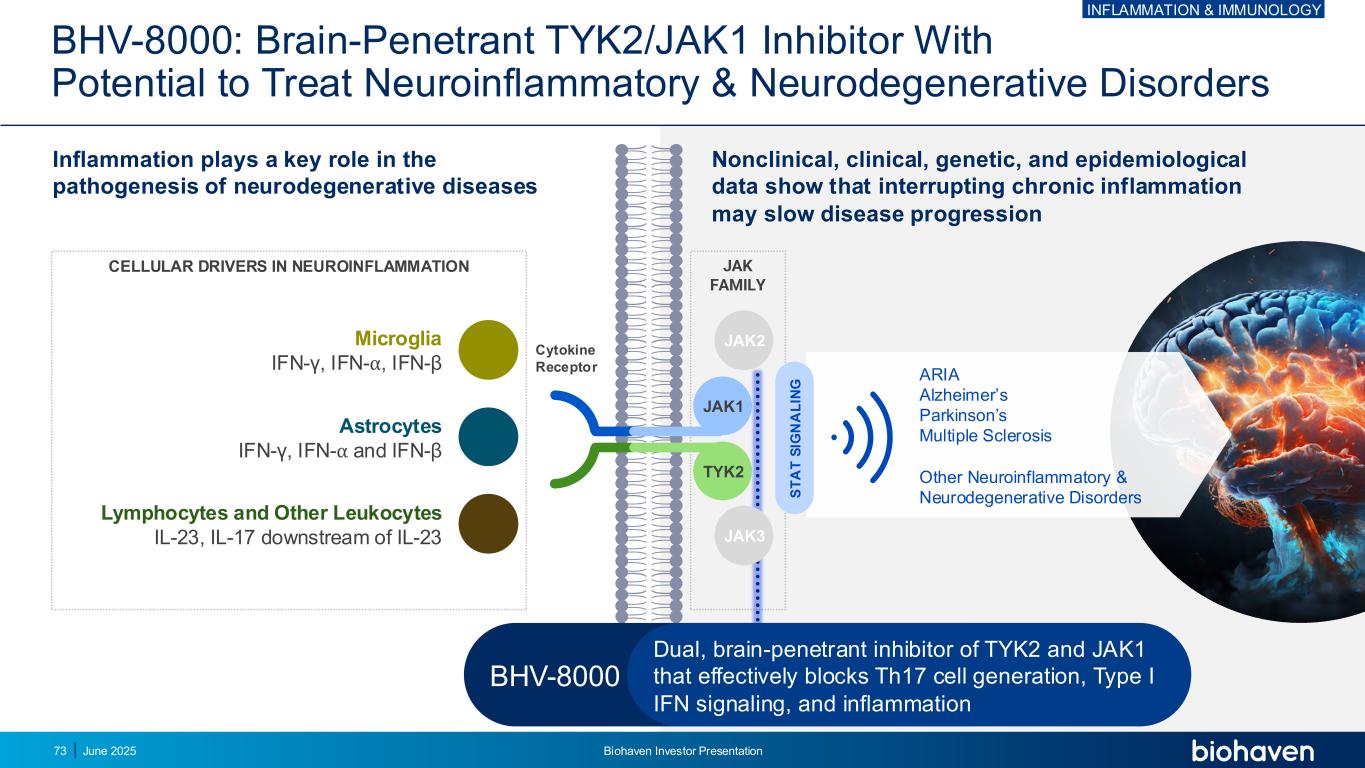
BHV-8000: Brain-Penetrant TYK2/JAK1 Inhibitor With Potential to Treat Neuroinflammatory & Neurodegenerative Disorders Cytokine Receptor JAK FAMILY Microglia IFN-γ, IFN-⍺, IFN-β Astrocytes IFN-γ, IFN-⍺ and IFN-β Lymphocytes and Other Leukocytes IL-23, IL-17 downstream of IL-23 CELLULAR DRIVERS IN NEUROINFLAMMATION Inflammation plays a key role in the pathogenesis of neurodegenerative diseases Nonclinical, clinical, genetic, and epidemiological data show that interrupting chronic inflammation may slow disease progression ARIA Alzheimer’s Parkinson’s Multiple Sclerosis Other Neuroinflammatory & Neurodegenerative Disorders JAK2 JAK1 TYK2 S T A T S IG N A L IN G JAK3 BHV-8000 Dual, brain-penetrant inhibitor of TYK2 and JAK1 that effectively blocks Th17 cell generation, Type I IFN signaling, and inflammation INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation73
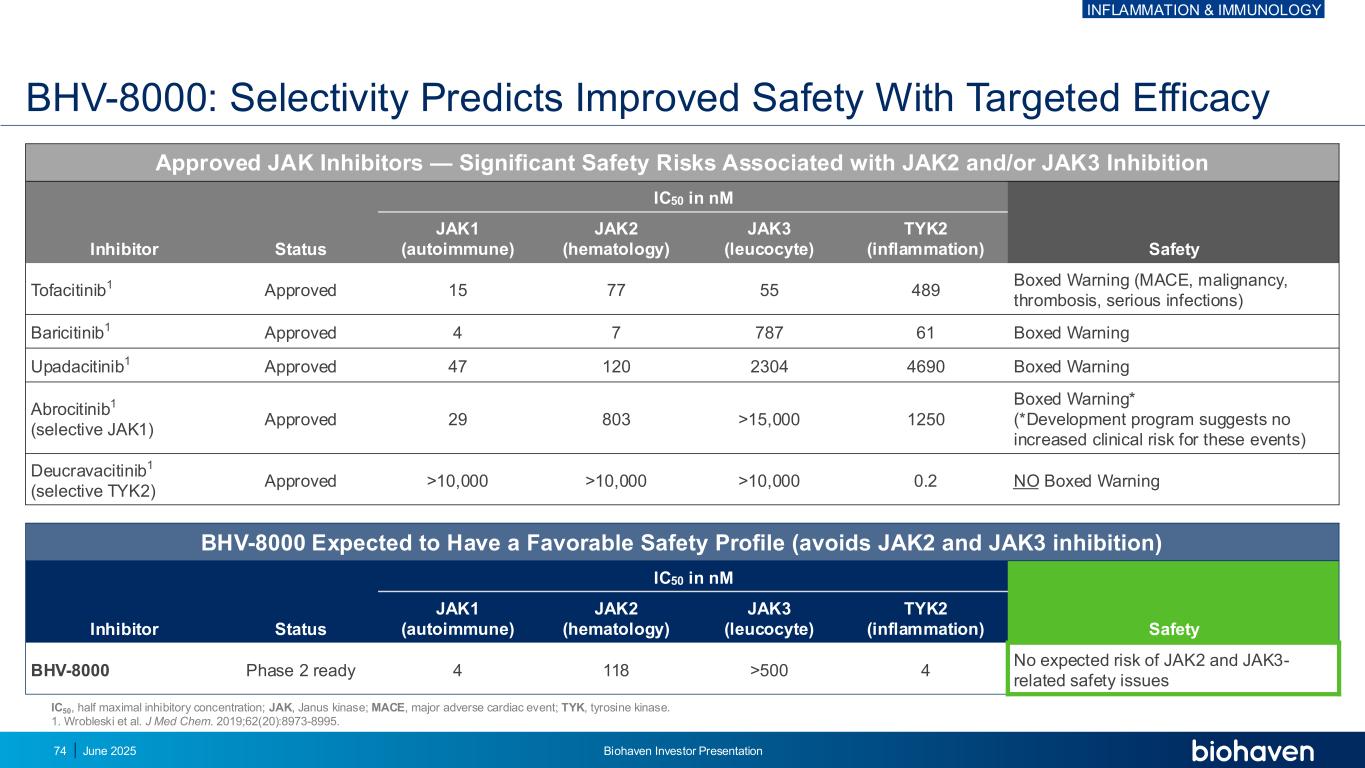
Approved JAK Inhibitors — Significant Safety Risks Associated with JAK2 and/or JAK3 Inhibition Inhibitor Status IC50 in nM Safety JAK1 (autoimmune) JAK2 (hematology) JAK3 (leucocyte) TYK2 (inflammation) Tofacitinib1 Approved 15 77 55 489 Boxed Warning (MACE, malignancy, thrombosis, serious infections) Baricitinib1 Approved 4 7 787 61 Boxed Warning Upadacitinib1 Approved 47 120 2304 4690 Boxed Warning Abrocitinib1 (selective JAK1) Approved 29 803 >15,000 1250 Boxed Warning* (*Development program suggests no increased clinical risk for these events) Deucravacitinib1 (selective TYK2) Approved >10,000 >10,000 >10,000 0.2 NO Boxed Warning BHV-8000 Expected to Have a Favorable Safety Profile (avoids JAK2 and JAK3 inhibition) Inhibitor Status IC50 in nM Safety JAK1 (autoimmune) JAK2 (hematology) JAK3 (leucocyte) TYK2 (inflammation) BHV-8000 Phase 2 ready 4 118 >500 4 No expected risk of JAK2 and JAK3- related safety issues IC50, half maximal inhibitory concentration; JAK, Janus kinase; MACE, major adverse cardiac event; TYK, tyrosine kinase. 1. Wrobleski et al. J Med Chem. 2019;62(20):8973-8995. BHV-8000: Selectivity Predicts Improved Safety With Targeted Efficacy INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation74
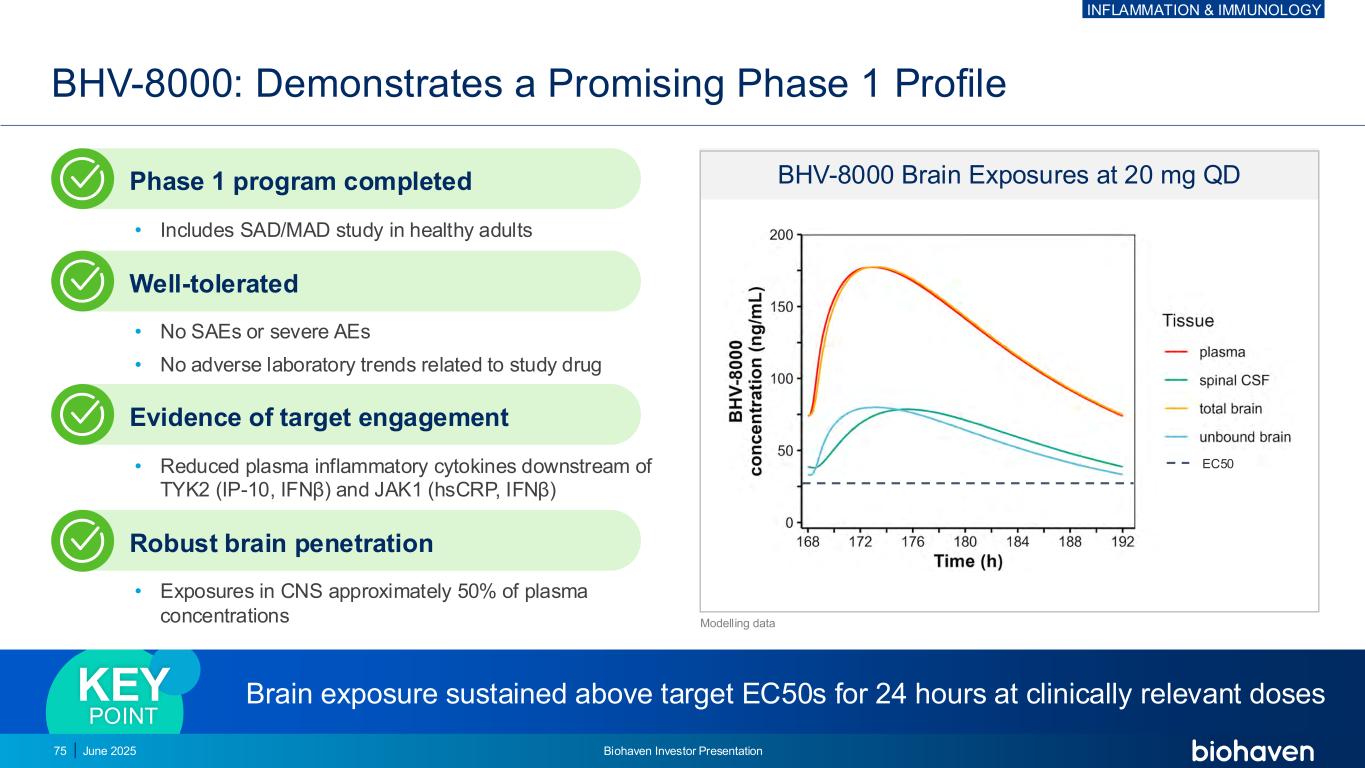
BHV-8000: Demonstrates a Promising Phase 1 Profile Brain exposure sustained above target EC50s for 24 hours at clinically relevant dosesKEY POINT BHV-8000 Brain Exposures at 20 mg QD EC50 Modelling data Phase 1 program completed • Includes SAD/MAD study in healthy adults Well-tolerated • No SAEs or severe AEs • No adverse laboratory trends related to study drug Evidence of target engagement • Reduced plasma inflammatory cytokines downstream of TYK2 (IP-10, IFNβ) and JAK1 (hsCRP, IFNβ) Robust brain penetration • Exposures in CNS approximately 50% of plasma concentrations 75 June 2025 Biohaven Investor Presentation INFLAMMATION & IMMUNOLOGY
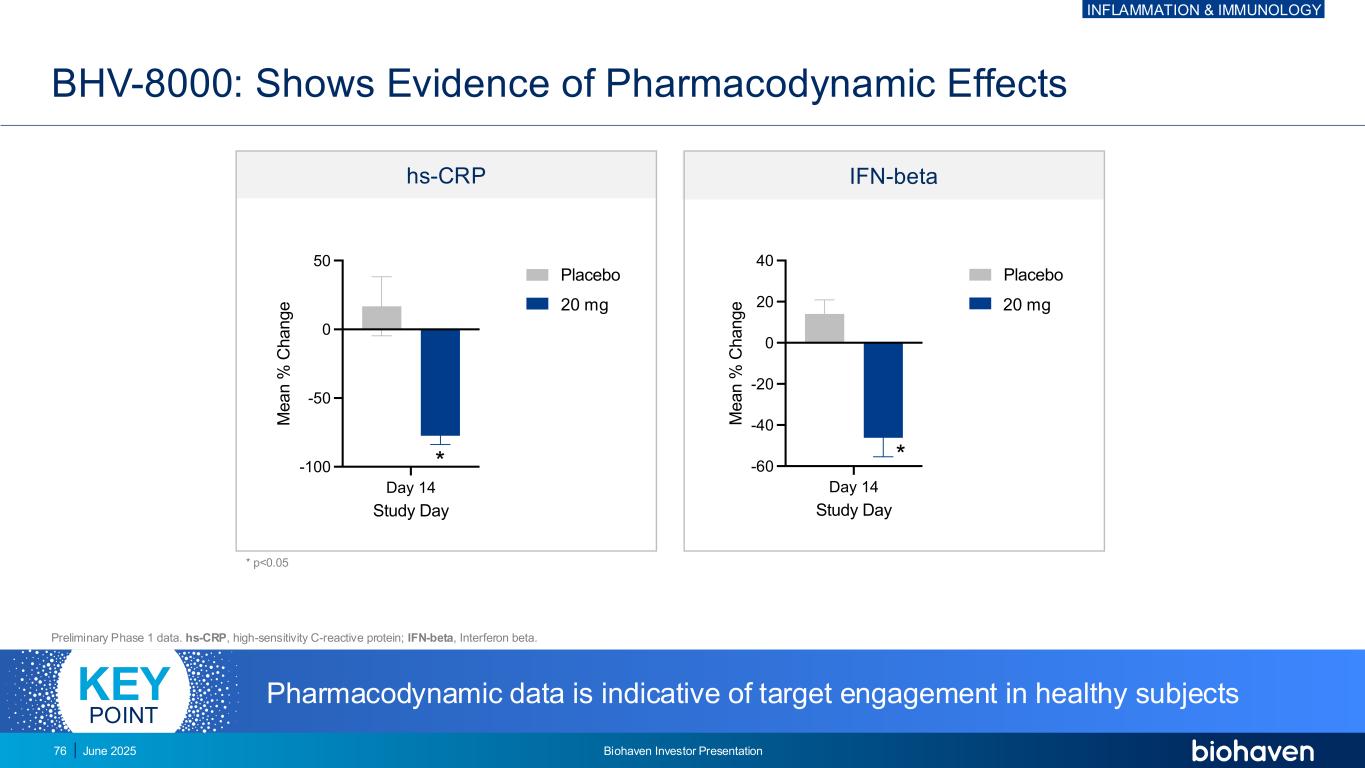
BHV-8000: Shows Evidence of Pharmacodynamic Effects Preliminary Phase 1 data. hs-CRP, high-sensitivity C-reactive protein; IFN-beta, Interferon beta. * p<0.05 IFN-betahs-CRP Day 14 -60 -40 -20 0 20 40 Study Day M e a n % C h a n g e IFNb Placebo 20mg Day 14 -100 -50 0 50 Study Day M e a n % C h a n g e hsCRP Placebo 20mg * * mg g Pharmacodynamic data is indicative of target engagement in healthy subjectsKEY POINT INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation76
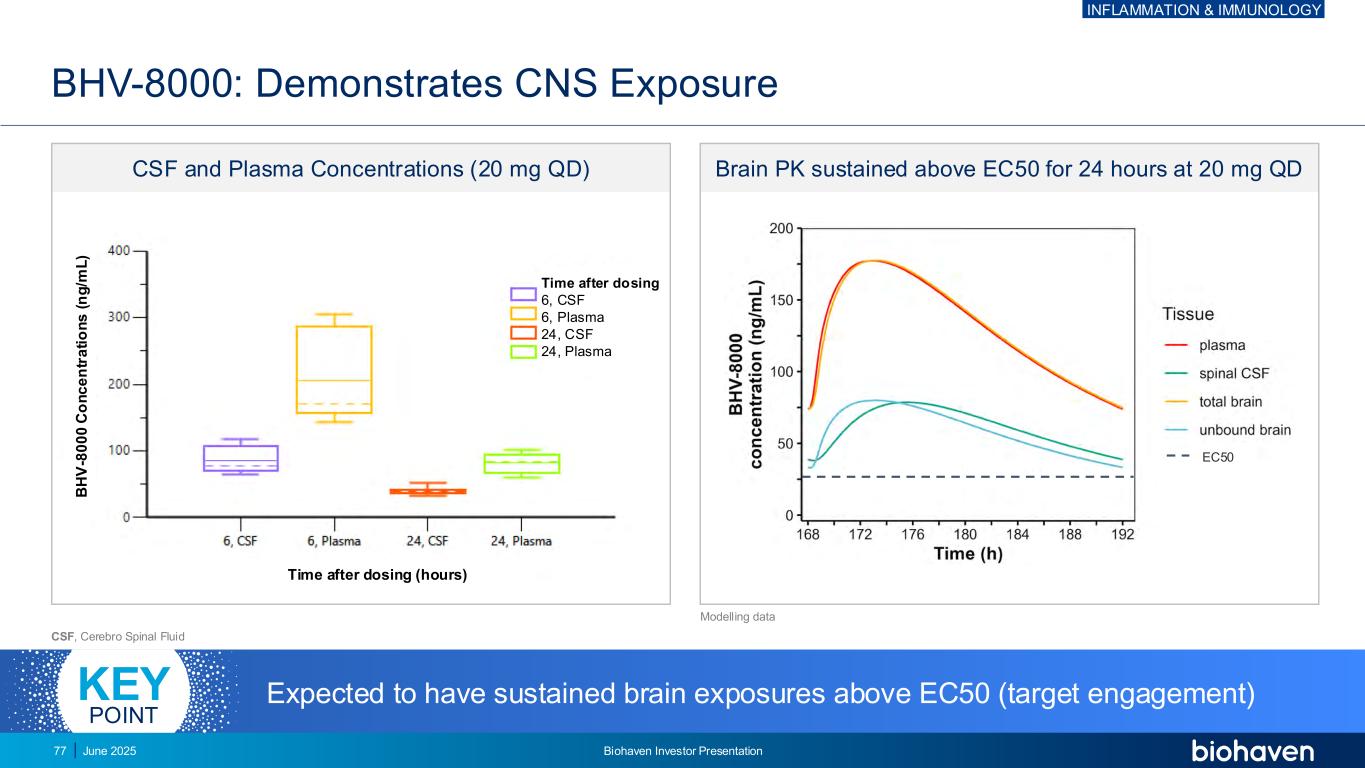
Time after dosing 6, CSF 6, Plasma 24, CSF 24, Plasma BHV-8000: Demonstrates CNS Exposure CSF, Cerebro Spinal Fluid Expected to have sustained brain exposures above EC50 (target engagement)KEY POINT CSF and Plasma Concentrations (20 mg QD) Brain PK sustained above EC50 for 24 hours at 20 mg QD B H V -8 0 0 0 C o n c e n tr a ti o n s ( n g /m L ) Time after dosing (hours) EC50 Modelling data INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation77
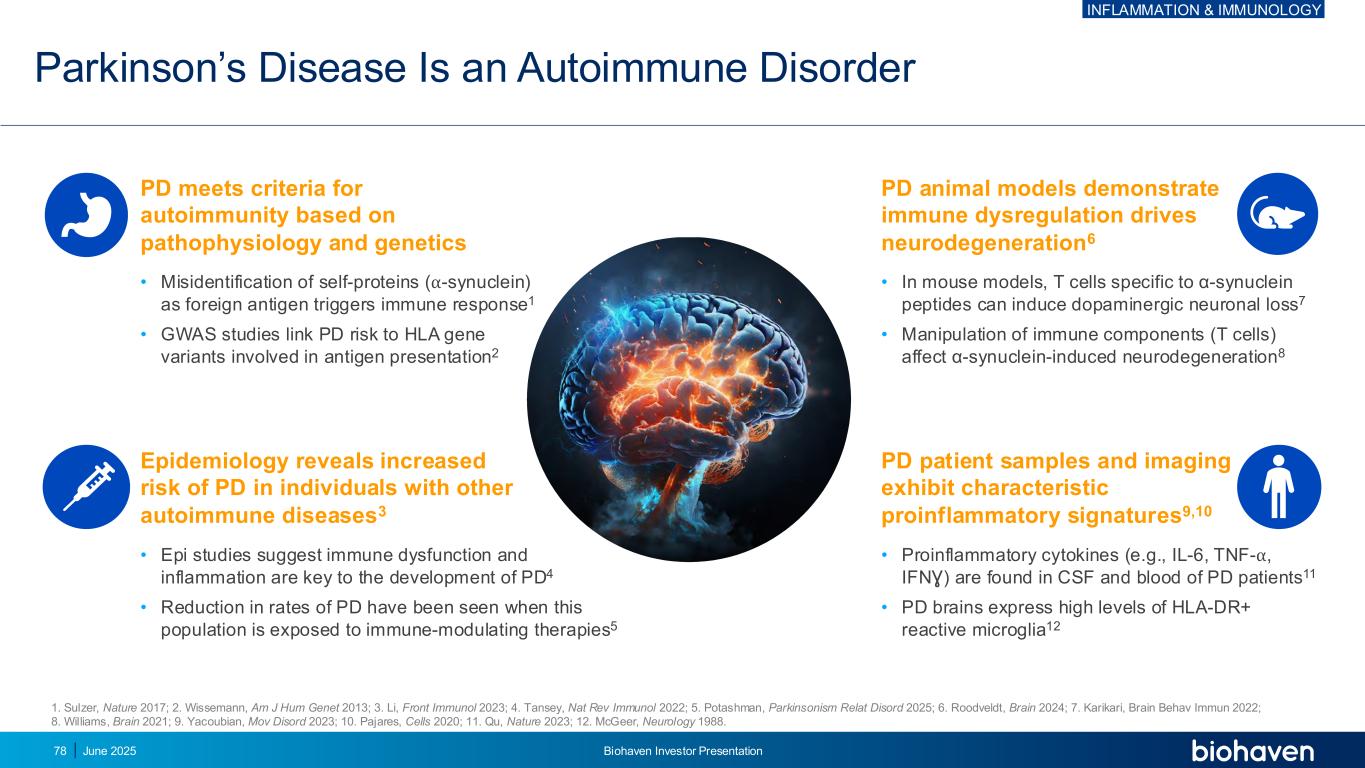
Parkinson’s Disease Is an Autoimmune Disorder 1. Sulzer, Nature 2017; 2. Wissemann, Am J Hum Genet 2013; 3. Li, Front Immunol 2023; 4. Tansey, Nat Rev Immunol 2022; 5. Potashman, Parkinsonism Relat Disord 2025; 6. Roodveldt, Brain 2024; 7. Karikari, Brain Behav Immun 2022; 8. Williams, Brain 2021; 9. Yacoubian, Mov Disord 2023; 10. Pajares, Cells 2020; 11. Qu, Nature 2023; 12. McGeer, Neurology 1988. • Misidentification of self-proteins (⍺-synuclein) as foreign antigen triggers immune response1 • GWAS studies link PD risk to HLA gene variants involved in antigen presentation2 • In mouse models, T cells specific to α-synuclein peptides can induce dopaminergic neuronal loss7 • Manipulation of immune components (T cells) affect α-synuclein-induced neurodegeneration8 • Epi studies suggest immune dysfunction and inflammation are key to the development of PD4 • Reduction in rates of PD have been seen when this population is exposed to immune-modulating therapies5 PD patient samples and imaging exhibit characteristic proinflammatory signatures9,10 PD meets criteria for autoimmunity based on pathophysiology and genetics Epidemiology reveals increased risk of PD in individuals with other autoimmune diseases3 PD animal models demonstrate immune dysregulation drives neurodegeneration6 • Proinflammatory cytokines (e.g., IL-6, TNF-⍺, IFNƔ) are found in CSF and blood of PD patients11 • PD brains express high levels of HLA-DR+ reactive microglia12 78 June 2025 Biohaven Investor Presentation INFLAMMATION & IMMUNOLOGY
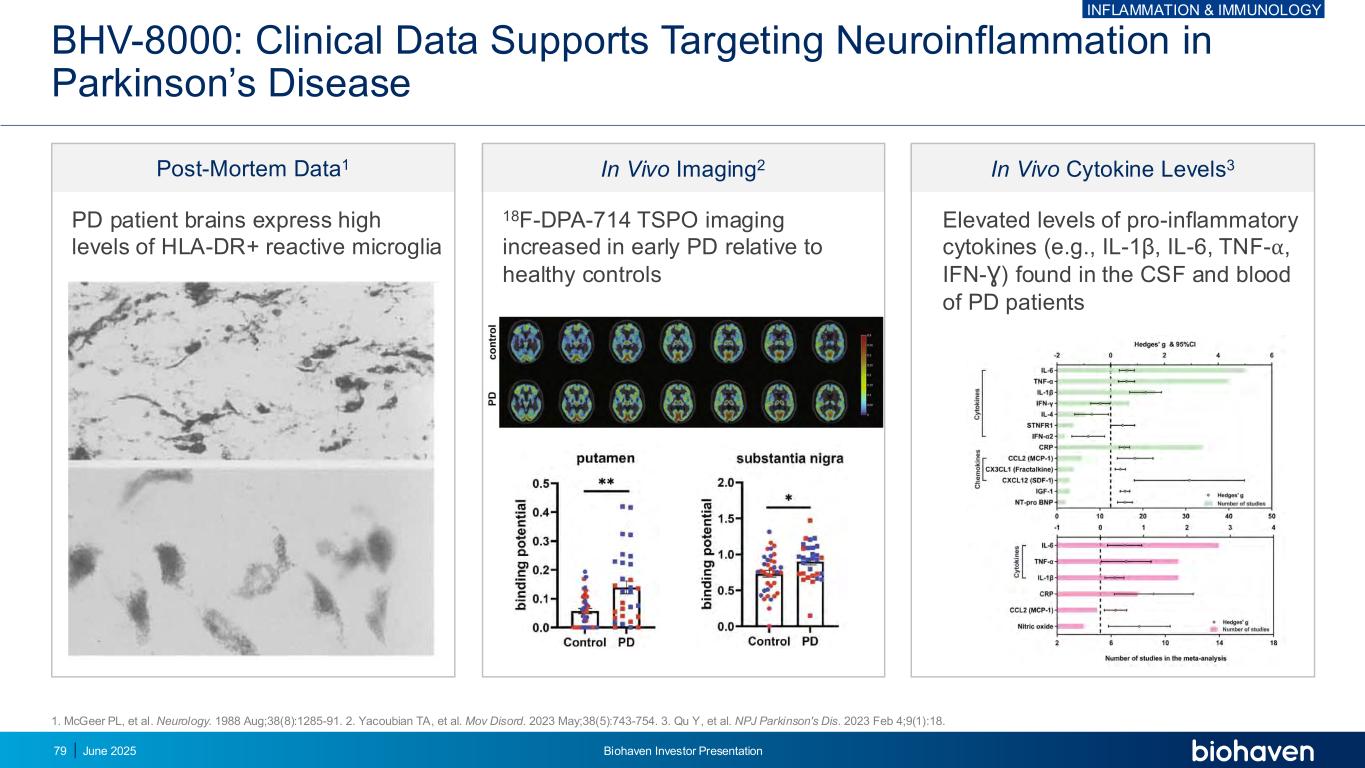
` BHV-8000: Clinical Data Supports Targeting Neuroinflammation in Parkinson’s Disease In Vivo Cytokine Levels3 Elevated levels of pro-inflammatory cytokines (e.g., IL-1β, IL-6, TNF-⍺, IFN-Ɣ) found in the CSF and blood of PD patients In Vivo Imaging2 18F-DPA-714 TSPO imaging increased in early PD relative to healthy controls Post-Mortem Data1 PD patient brains express high levels of HLA-DR+ reactive microglia 1. McGeer PL, et al. Neurology. 1988 Aug;38(8):1285-91. 2. Yacoubian TA, et al. Mov Disord. 2023 May;38(5):743-754. 3. Qu Y, et al. NPJ Parkinson's Dis. 2023 Feb 4;9(1):18. INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation79
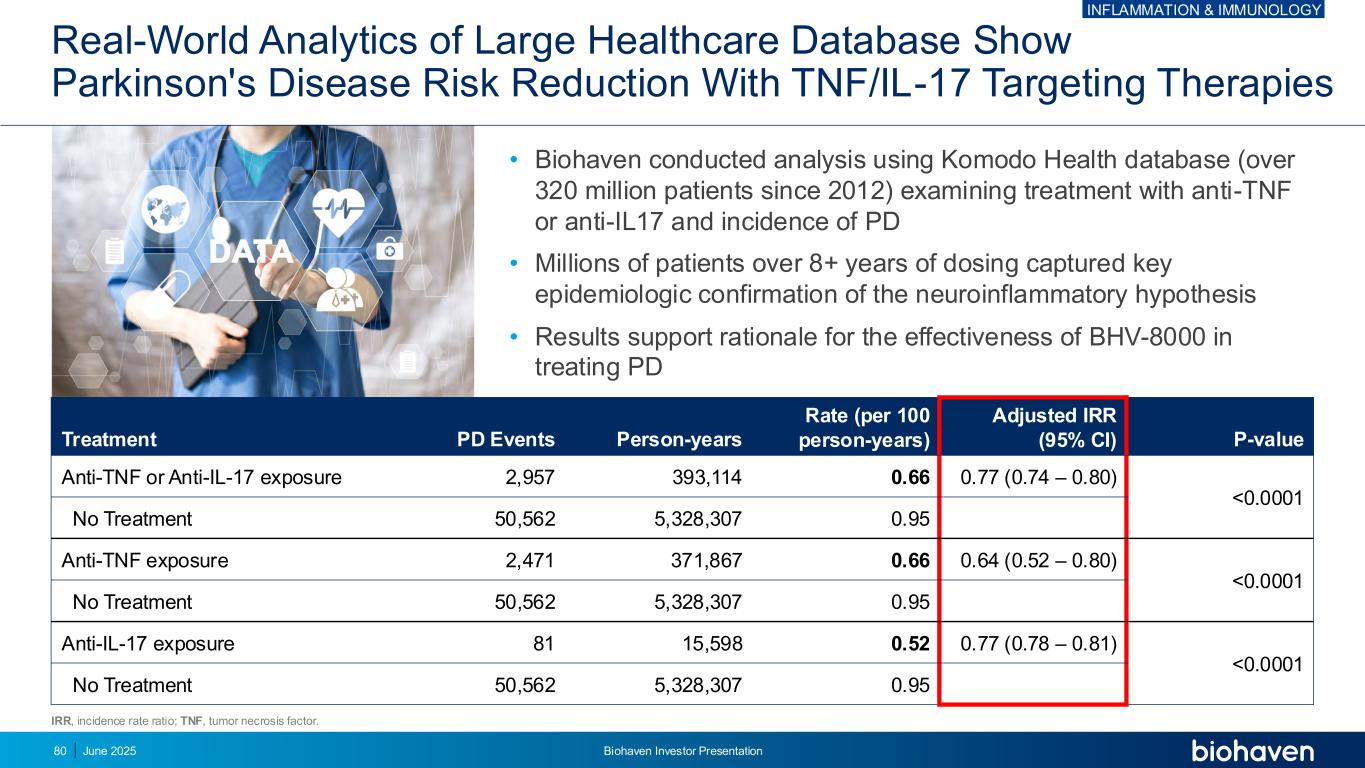
Real-World Analytics of Large Healthcare Database Show Parkinson's Disease Risk Reduction With TNF/IL-17 Targeting Therapies • Biohaven conducted analysis using Komodo Health database (over 320 million patients since 2012) examining treatment with anti-TNF or anti-IL17 and incidence of PD • Millions of patients over 8+ years of dosing captured key epidemiologic confirmation of the neuroinflammatory hypothesis • Results support rationale for the effectiveness of BHV-8000 in treating PD Treatment PD Events Person-years Rate (per 100 person-years) Adjusted IRR (95% CI) P-value Anti-TNF or Anti-IL-17 exposure 2,957 393,114 0.66 0.77 (0.74 – 0.80) <0.0001 No Treatment 50,562 5,328,307 0.95 Anti-TNF exposure 2,471 371,867 0.66 0.64 (0.52 – 0.80) <0.0001 No Treatment 50,562 5,328,307 0.95 Anti-IL-17 exposure 81 15,598 0.52 0.77 (0.78 – 0.81) <0.0001 No Treatment 50,562 5,328,307 0.95 IRR, incidence rate ratio; TNF, tumor necrosis factor. INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation80
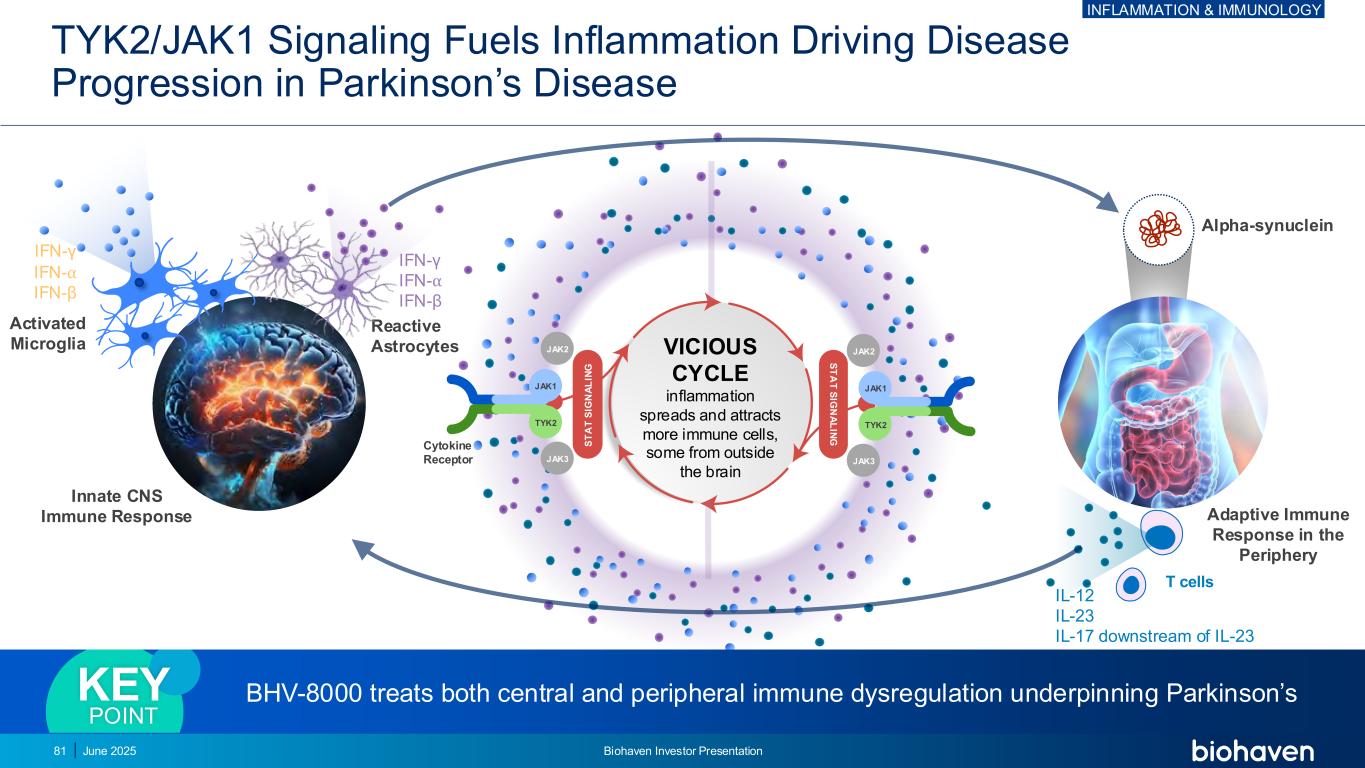
TYK2/JAK1 Signaling Fuels Inflammation Driving Disease Progression in Parkinson’s Disease Alpha-synuclein T cells IFN-γ IFN-⍺ IFN-β IFN-γ IFN-⍺ IFN-β Reactive Astrocytes Activated Microglia Cytokine Receptor JAK2 JAK1 TYK2 JAK3 S T A T S IG N A L IN G JAK2 JAK1 TYK2 JAK3 S T A T S IG N A L IN G VICIOUS CYCLE inflammation spreads and attracts more immune cells, some from outside the brain IL-12 IL-23 IL-17 downstream of IL-23 Adaptive Immune Response in the Periphery Innate CNS Immune Response BHV-8000 treats both central and peripheral immune dysregulation underpinning Parkinson’sKEY POINT 81 June 2025 Biohaven Investor Presentation INFLAMMATION & IMMUNOLOGY
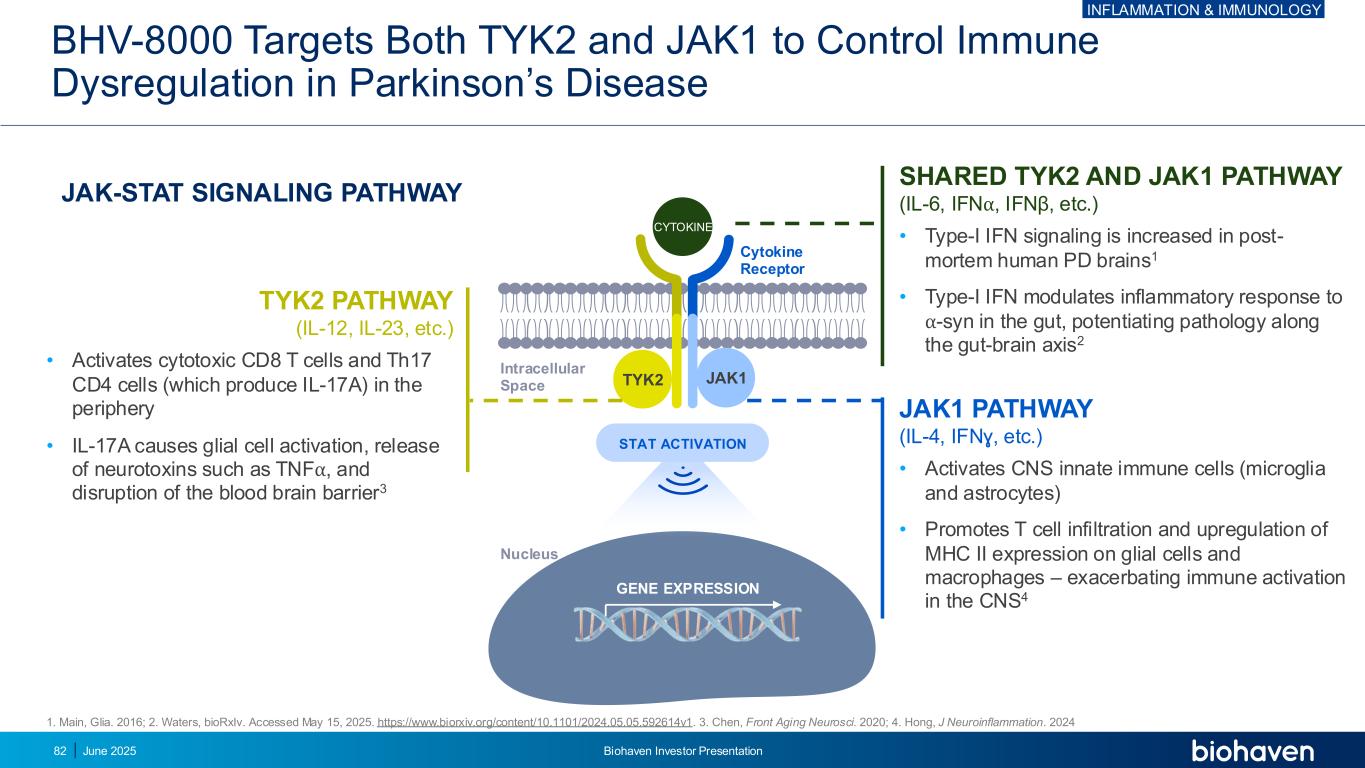
BHV-8000 Targets Both TYK2 and JAK1 to Control Immune Dysregulation in Parkinson’s Disease 82 1. Main, Glia. 2016; 2. Waters, bioRxIv. Accessed May 15, 2025. https://www.biorxiv.org/content/10.1101/2024.05.05.592614v1. 3. Chen, Front Aging Neurosci. 2020; 4. Hong, J Neuroinflammation. 2024 STAT ACTIVATION GENE EXPRESSION Cytokine Receptor JAK1TYK2 CYTOKINE JAK-STAT SIGNALING PATHWAY SHARED TYK2 AND JAK1 PATHWAY (IL-6, IFN⍺, IFNβ, etc.) • Type-I IFN signaling is increased in post- mortem human PD brains1 • Type-I IFN modulates inflammatory response to ⍺-syn in the gut, potentiating pathology along the gut-brain axis2 JAK1 PATHWAY (IL-4, IFNɣ, etc.) • Activates CNS innate immune cells (microglia and astrocytes) • Promotes T cell infiltration and upregulation of MHC II expression on glial cells and macrophages – exacerbating immune activation in the CNS4 TYK2 PATHWAY (IL-12, IL-23, etc.) • Activates cytotoxic CD8 T cells and Th17 CD4 cells (which produce IL-17A) in the periphery • IL-17A causes glial cell activation, release of neurotoxins such as TNF⍺, and disruption of the blood brain barrier3 Intracellular Space Nucleus June 2025 Biohaven Investor Presentation INFLAMMATION & IMMUNOLOGY
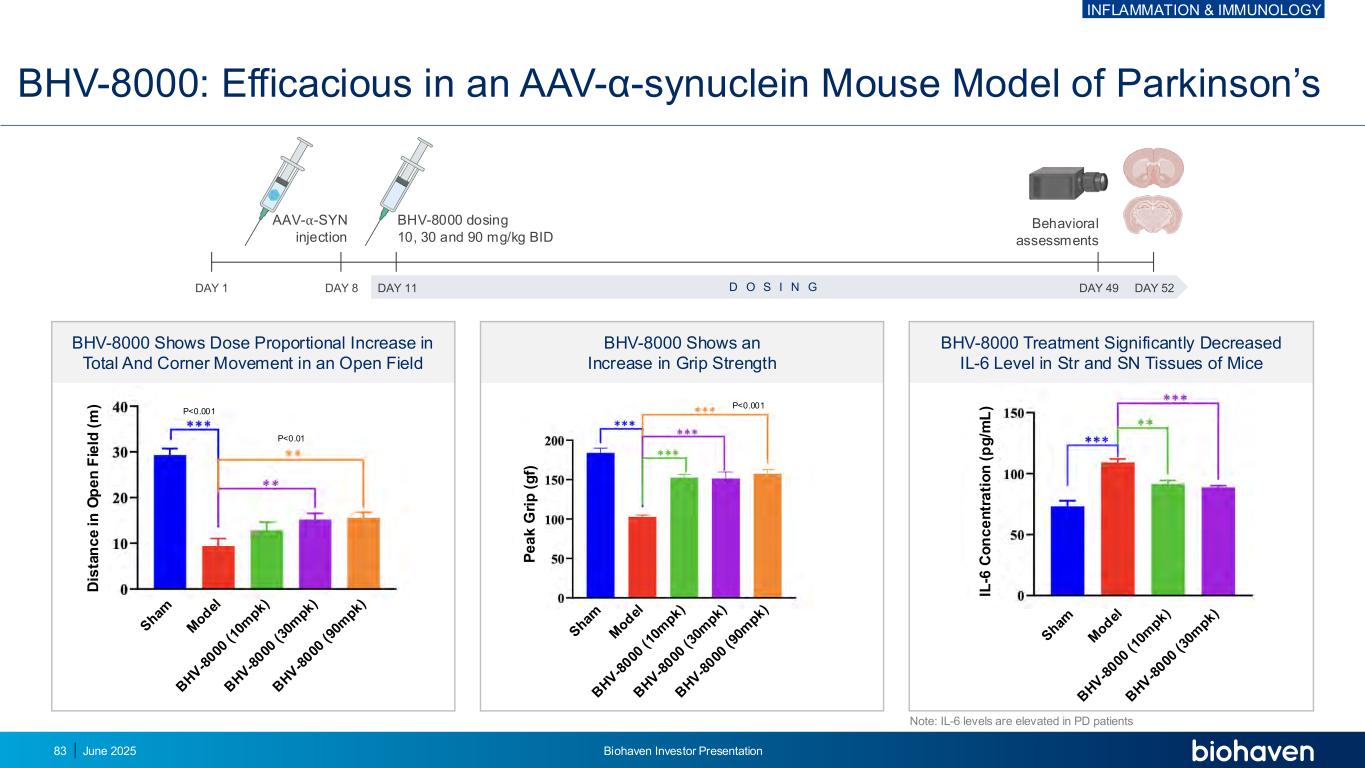
BHV-8000: Efficacious in an AAV-α-synuclein Mouse Model of Parkinson’s BHV-8000 Shows Dose Proportional Increase in Total And Corner Movement in an Open Field BHV-8000 Shows an Increase in Grip Strength P<0.001 P<0.01 P<0.001 BHV-8000 Treatment Significantly Decreased IL-6 Level in Str and SN Tissues of Mice Note: IL-6 levels are elevated in PD patients D is ta n c e i n O p e n F ie ld ( m ) P e a k G ri p ( g f) IL -6 C o n c e n tr a ti o n ( p g /m L ) AAV-⍺-SYN injection BHV-8000 dosing 10, 30 and 90 mg/kg BID DAY 1 DAY 8 DAY 11 DAY 49 DAY 52D O S I N G Behavioral assessments INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation83
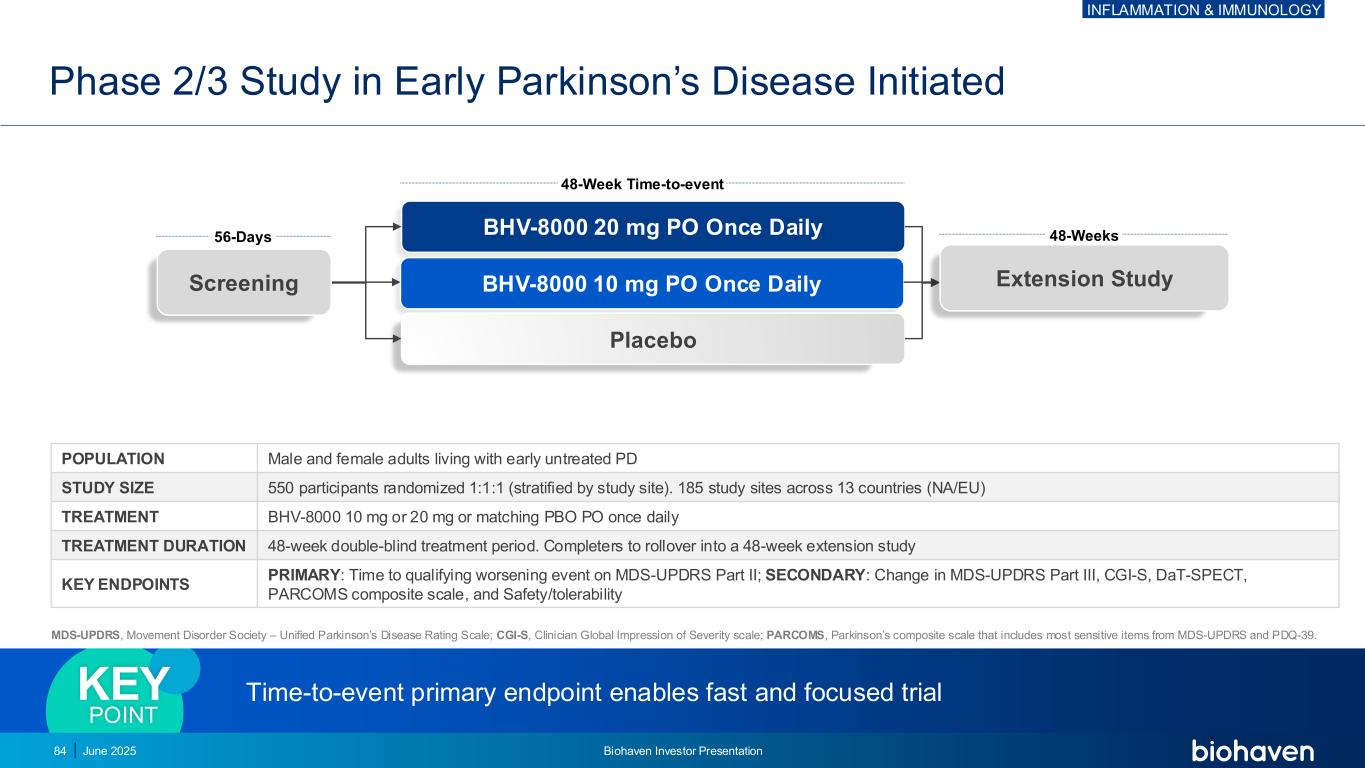
MDS-UPDRS, Movement Disorder Society – Unified Parkinson’s Disease Rating Scale; CGI-S, Clinician Global Impression of Severity scale; PARCOMS, Parkinson’s composite scale that includes most sensitive items from MDS-UPDRS and PDQ-39. 48-Week Time-to-event BHV-8000 20 mg PO Once Daily BHV-8000 10 mg PO Once Daily Placebo 56-Days 48-Weeks Screening POPULATION Male and female adults living with early untreated PD STUDY SIZE 550 participants randomized 1:1:1 (stratified by study site). 185 study sites across 13 countries (NA/EU) TREATMENT BHV-8000 10 mg or 20 mg or matching PBO PO once daily TREATMENT DURATION 48-week double-blind treatment period. Completers to rollover into a 48-week extension study KEY ENDPOINTS PRIMARY: Time to qualifying worsening event on MDS-UPDRS Part II; SECONDARY: Change in MDS-UPDRS Part III, CGI-S, DaT-SPECT, PARCOMS composite scale, and Safety/tolerability Extension Study Phase 2/3 Study in Early Parkinson’s Disease Initiated Time-to-event primary endpoint enables fast and focused trial KEY POINT 84 June 2025 Biohaven Investor Presentation INFLAMMATION & IMMUNOLOGY
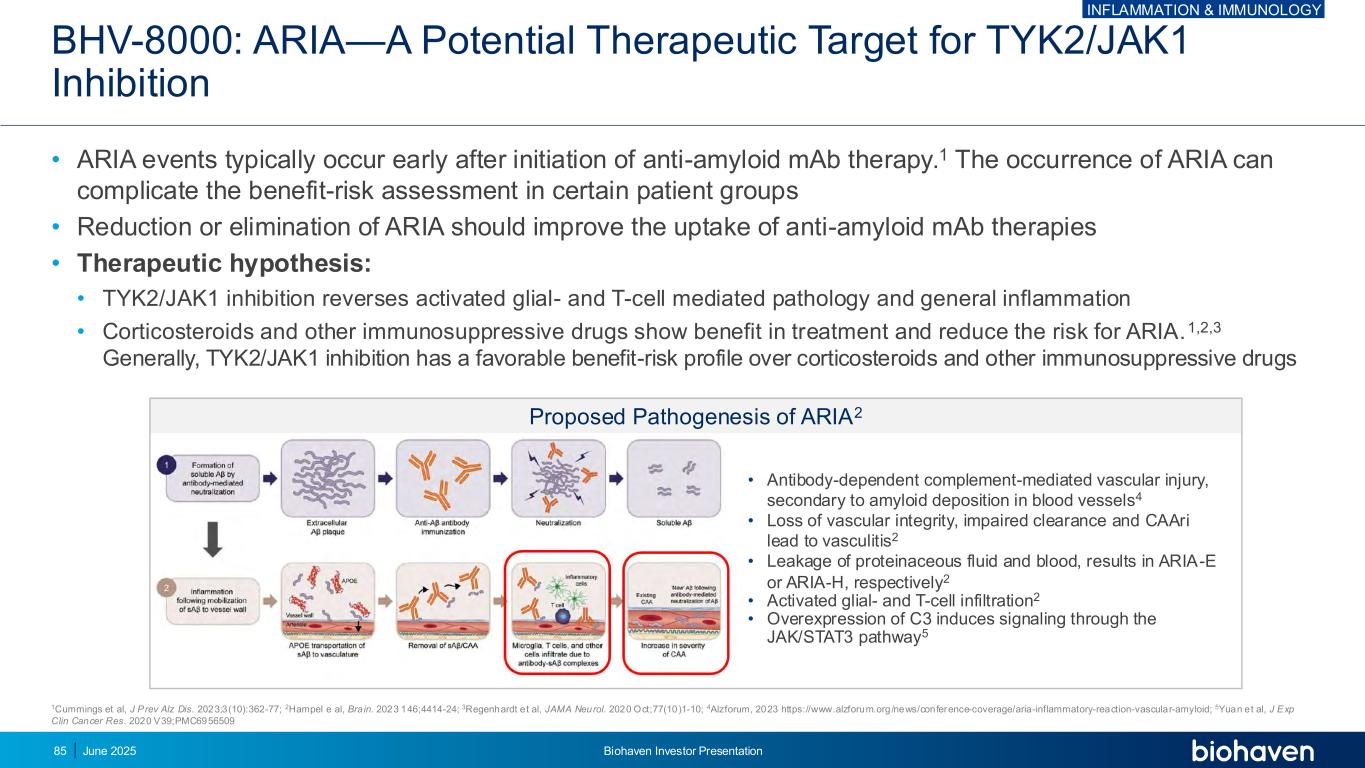
BHV-8000: ARIA—A Potential Therapeutic Target for TYK2/JAK1 Inhibition • ARIA events typically occur early after initiation of anti-amyloid mAb therapy.1 The occurrence of ARIA can complicate the benefit-risk assessment in certain patient groups • Reduction or elimination of ARIA should improve the uptake of anti-amyloid mAb therapies • Therapeutic hypothesis: • TYK2/JAK1 inhibition reverses activated glial- and T-cell mediated pathology and general inflammation • Corticosteroids and other immunosuppressive drugs show benefit in treatment and reduce the risk for ARIA.1,2,3 Generally, TYK2/JAK1 inhibition has a favorable benefit-risk profile over corticosteroids and other immunosuppressive drugs 1Cummings et al, J Prev Alz Dis. 2023;3(10):362-77; 2Hampel e al, Brain. 2023 146;4414-24; 3Regenhardt et al, JAMA Neurol. 2020 Oct;77(10)1-10; 4Alzforum, 2023 https://www.alzforum.org/news/conference-coverage/aria-inflammatory-reaction-vascular-amyloid; 5Yuan et al, J Exp Clin Cancer Res. 2020 V39;PMC6956509 Proposed Pathogenesis of ARIA2 • Antibody-dependent complement-mediated vascular injury, secondary to amyloid deposition in blood vessels4 • Loss of vascular integrity, impaired clearance and CAAri lead to vasculitis2 • Leakage of proteinaceous fluid and blood, results in ARIA-E or ARIA-H, respectively2 • Activated glial- and T-cell infiltration2 • Overexpression of C3 induces signaling through the JAK/STAT3 pathway5 INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation85
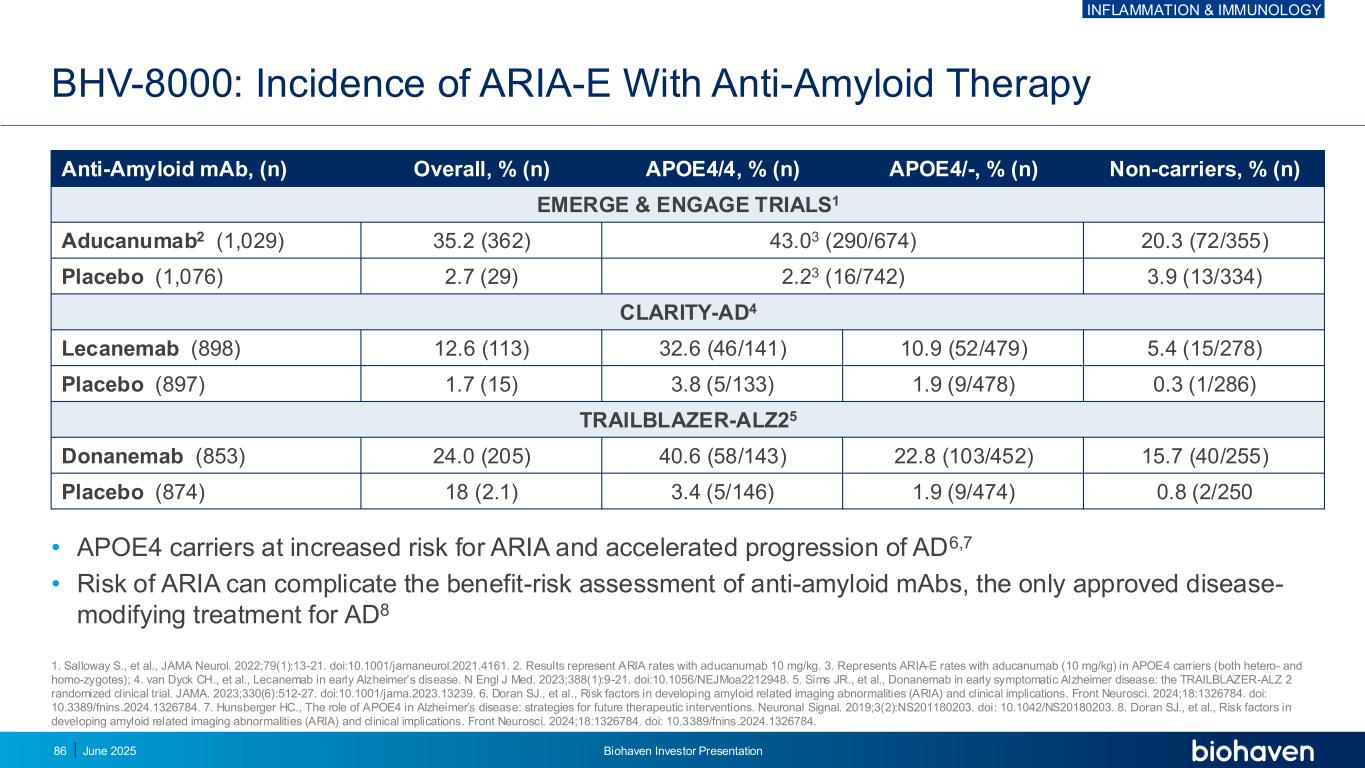
• APOE4 carriers at increased risk for ARIA and accelerated progression of AD6,7 • Risk of ARIA can complicate the benefit-risk assessment of anti-amyloid mAbs, the only approved disease- modifying treatment for AD8 BHV-8000: Incidence of ARIA-E With Anti-Amyloid Therapy Anti-Amyloid mAb, (n) Overall, % (n) APOE4/4, % (n) APOE4/-, % (n) Non-carriers, % (n) EMERGE & ENGAGE TRIALS1 Aducanumab2 (1,029) 35.2 (362) 43.03 (290/674) 20.3 (72/355) Placebo (1,076) 2.7 (29) 2.23 (16/742) 3.9 (13/334) CLARITY-AD4 Lecanemab (898) 12.6 (113) 32.6 (46/141) 10.9 (52/479) 5.4 (15/278) Placebo (897) 1.7 (15) 3.8 (5/133) 1.9 (9/478) 0.3 (1/286) TRAILBLAZER-ALZ25 Donanemab (853) 24.0 (205) 40.6 (58/143) 22.8 (103/452) 15.7 (40/255) Placebo (874) 18 (2.1) 3.4 (5/146) 1.9 (9/474) 0.8 (2/250 1. Salloway S., et al., JAMA Neurol. 2022;79(1):13-21. doi:10.1001/jamaneurol.2021.4161. 2. Results represent ARIA rates with aducanumab 10 mg/kg. 3. Represents ARIA-E rates with aducanumab (10 mg/kg) in APOE4 carriers (both hetero- and homo-zygotes); 4. van Dyck CH., et al., Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948. 5. Sims JR., et al., Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-27. doi:10.1001/jama.2023.13239. 6. Doran SJ., et al., Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications. Front Neurosci. 2024;18:1326784. doi: 10.3389/fnins.2024.1326784. 7. Hunsberger HC., The role of APOE4 in Alzheimer’s disease: strategies for future therapeutic interventions. Neuronal Signal. 2019;3(2):NS201180203. doi: 10.1042/NS20180203. 8. Doran SJ., et al., Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications. Front Neurosci. 2024;18:1326784. doi: 10.3389/fnins.2024.1326784. INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation86
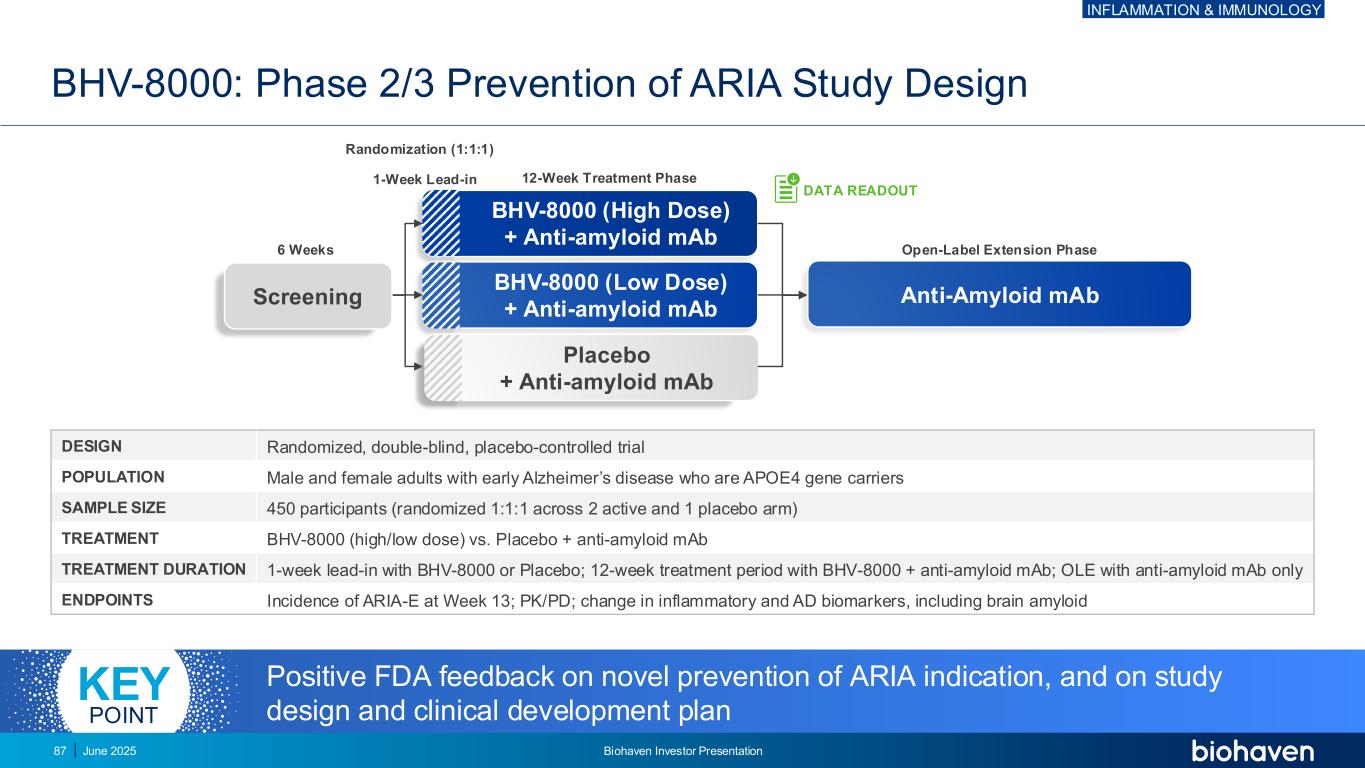
Anti-Amyloid mAb BHV-8000 (High Dose) + Anti-amyloid mAb BHV-8000 (Low Dose) + Anti-amyloid mAb Placebo + Anti-amyloid mAb BHV-8000: Phase 2/3 Prevention of ARIA Study Design DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Male and female adults with early Alzheimer’s disease who are APOE4 gene carriers SAMPLE SIZE 450 participants (randomized 1:1:1 across 2 active and 1 placebo arm) TREATMENT BHV-8000 (high/low dose) vs. Placebo + anti-amyloid mAb TREATMENT DURATION 1-week lead-in with BHV-8000 or Placebo; 12-week treatment period with BHV-8000 + anti-amyloid mAb; OLE with anti-amyloid mAb only ENDPOINTS Incidence of ARIA-E at Week 13; PK/PD; change in inflammatory and AD biomarkers, including brain amyloid 12-Week Treatment Phase1-Week Lead-in Randomization (1:1:1) Open-Label Extension Phase6 Weeks DATA READOUT Positive FDA feedback on novel prevention of ARIA indication, and on study design and clinical development plan KEY POINT Screening INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation87
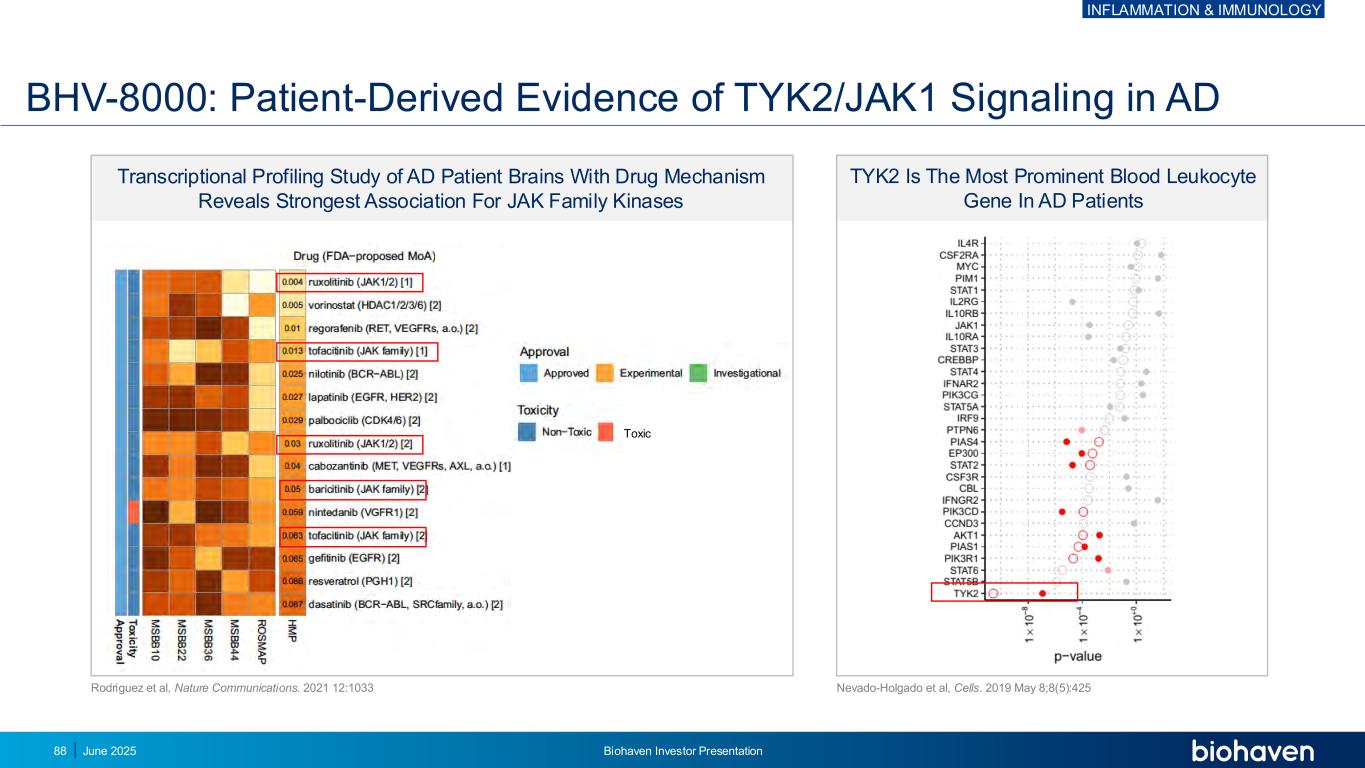
Transcriptional Profiling Study of AD Patient Brains With Drug Mechanism Reveals Strongest Association For JAK Family Kinases BHV-8000: Patient-Derived Evidence of TYK2/JAK1 Signaling in AD TYK2 Is The Most Prominent Blood Leukocyte Gene In AD Patients g e n e Nevado-Holgado et al, Cells. 2019 May 8;8(5):425 Rodriguez et al, Nature Communications. 2021 12:1033 Toxic INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation88
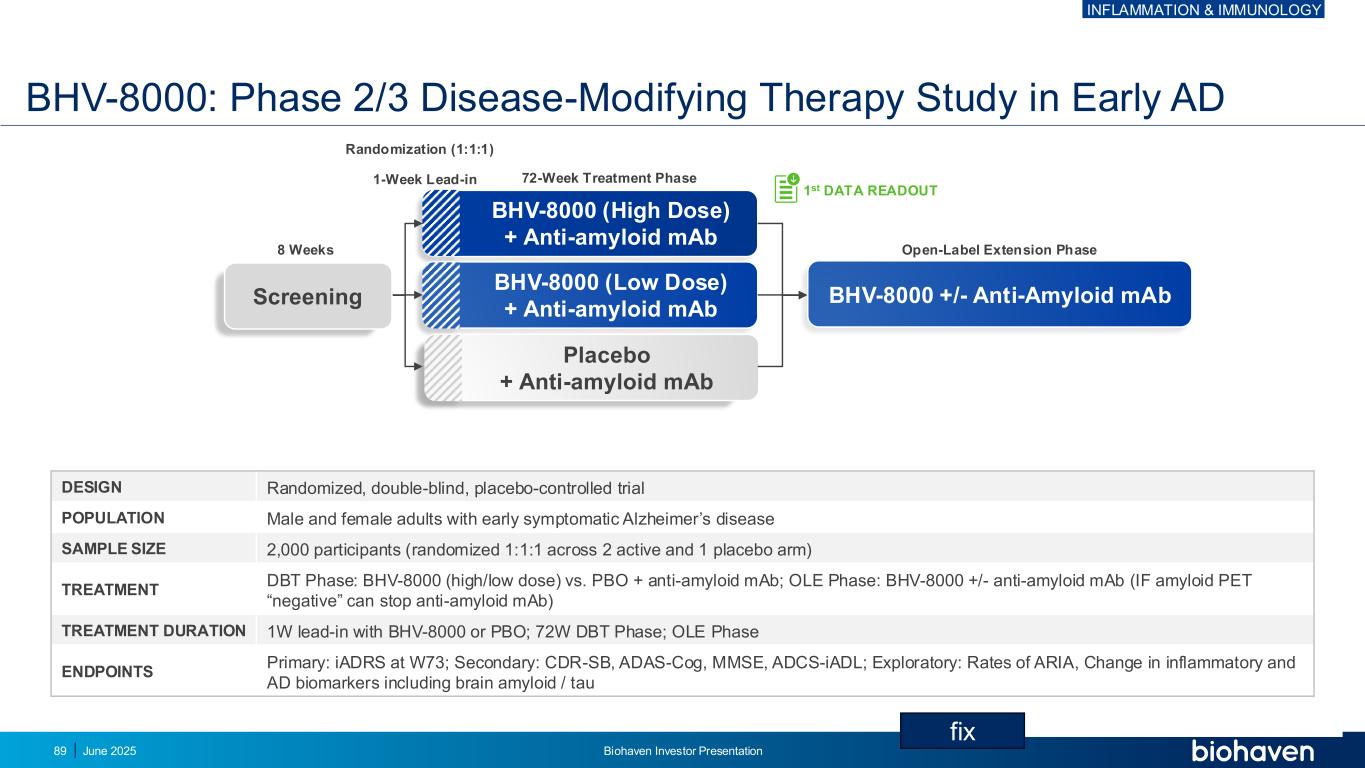
BHV-8000: Phase 2/3 Disease-Modifying Therapy Study in Early AD DESIGN Randomized, double-blind, placebo-controlled trial POPULATION Male and female adults with early symptomatic Alzheimer’s disease SAMPLE SIZE 2,000 participants (randomized 1:1:1 across 2 active and 1 placebo arm) TREATMENT DBT Phase: BHV-8000 (high/low dose) vs. PBO + anti-amyloid mAb; OLE Phase: BHV-8000 +/- anti-amyloid mAb (IF amyloid PET “negative” can stop anti-amyloid mAb) TREATMENT DURATION 1W lead-in with BHV-8000 or PBO; 72W DBT Phase; OLE Phase ENDPOINTS Primary: iADRS at W73; Secondary: CDR-SB, ADAS-Cog, MMSE, ADCS-iADL; Exploratory: Rates of ARIA, Change in inflammatory and AD biomarkers including brain amyloid / tau BHV-8000 +/- Anti-Amyloid mAb BHV-8000 (High Dose) + Anti-amyloid mAb BHV-8000 (Low Dose) + Anti-amyloid mAb Placebo + Anti-amyloid mAb 72-Week Treatment Phase1-Week Lead-in Randomization (1:1:1) Open-Label Extension Phase8 Weeks 1st DATA READOUT Screening INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation89 fix
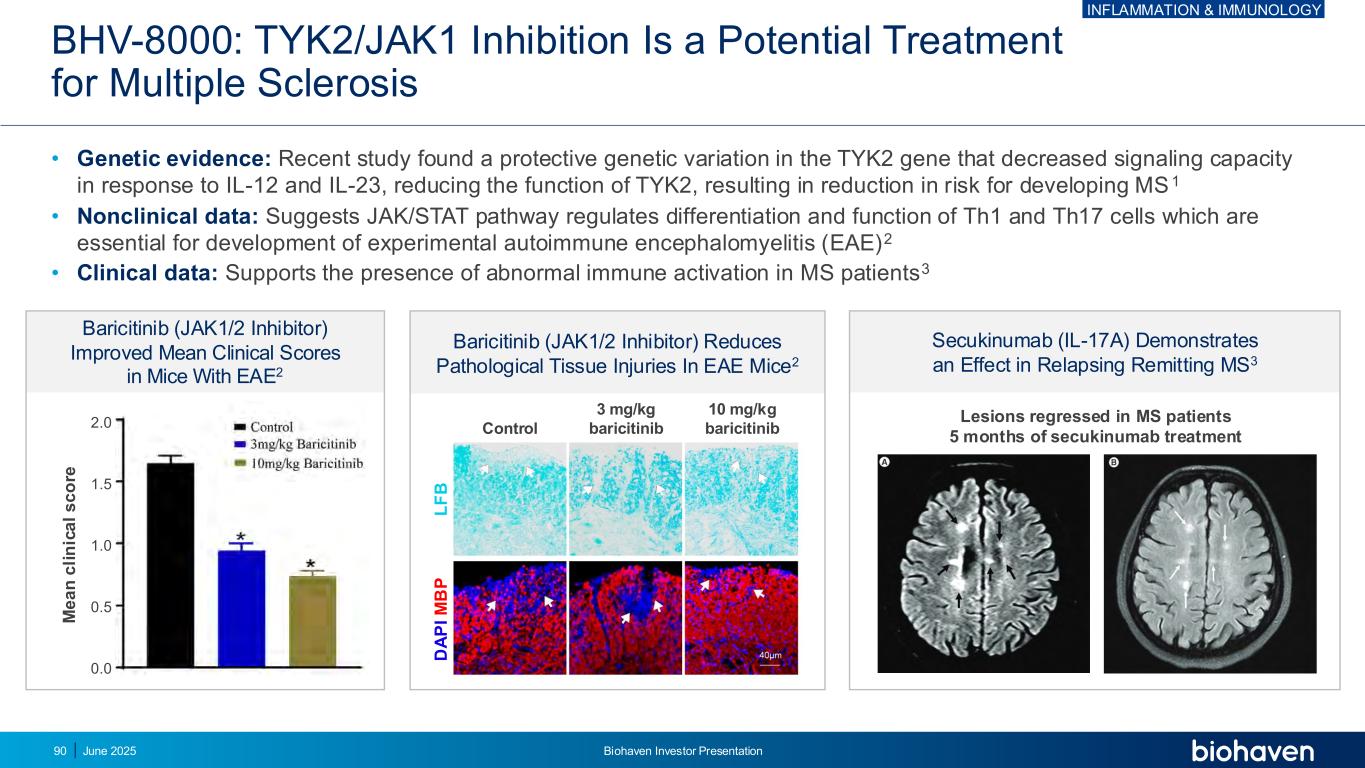
BHV-8000: TYK2/JAK1 Inhibition Is a Potential Treatment for Multiple Sclerosis • Genetic evidence: Recent study found a protective genetic variation in the TYK2 gene that decreased signaling capacity in response to IL-12 and IL-23, reducing the function of TYK2, resulting in reduction in risk for developing MS 1 • Nonclinical data: Suggests JAK/STAT pathway regulates differentiation and function of Th1 and Th17 cells which are essential for development of experimental autoimmune encephalomyelitis (EAE)2 • Clinical data: Supports the presence of abnormal immune activation in MS patients3 Secukinumab (IL-17A) Demonstrates an Effect in Relapsing Remitting MS3 Baricitinib (JAK1/2 Inhibitor) Reduces Pathological Tissue Injuries In EAE Mice2 Baricitinib (JAK1/2 Inhibitor) Improved Mean Clinical Scores in Mice With EAE2 Lesions regressed in MS patients 5 months of secukinumab treatment M e a n c li n ic a l s c o re 2.0 1.5 1.0 0.5 0.0 Control 3 mg/kg baricitinib 10 mg/kg baricitinib L F B D A P I M B P INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation90
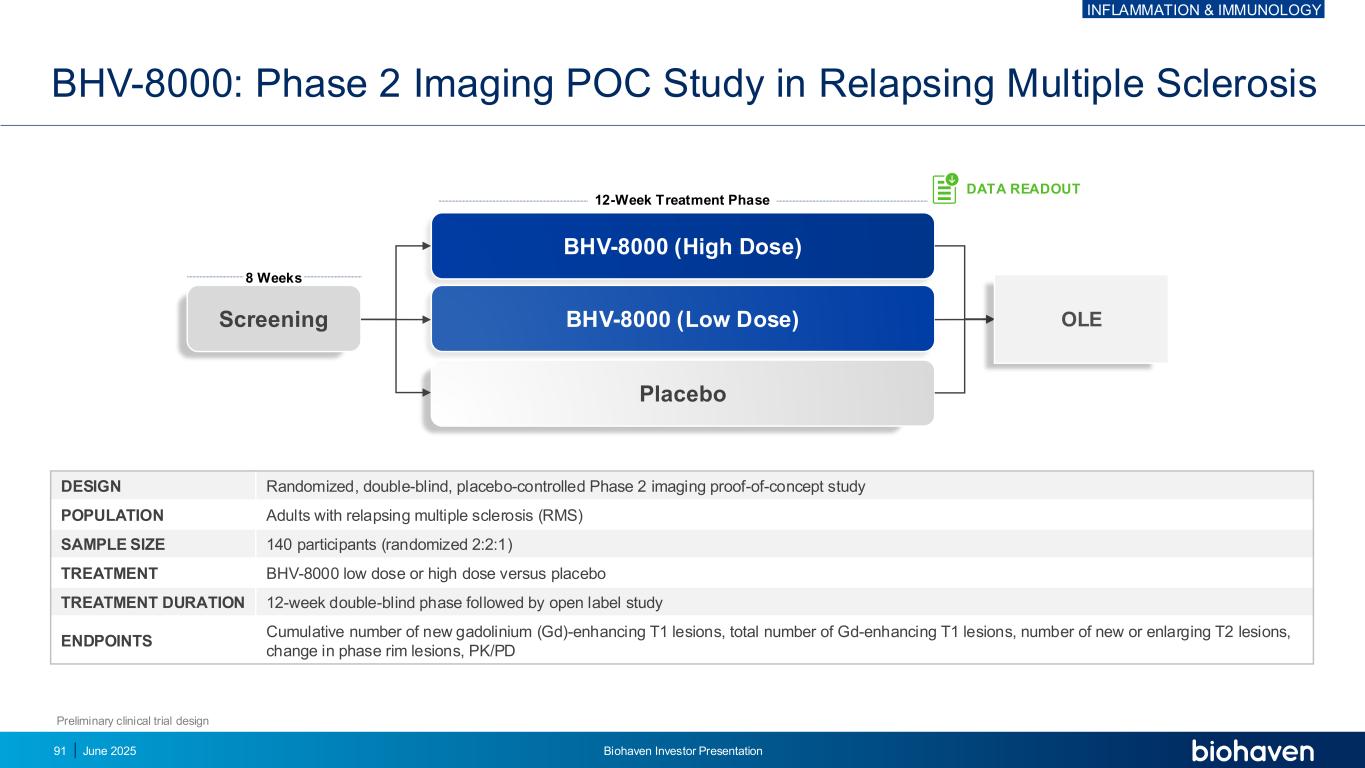
BHV-8000 (High Dose) BHV-8000 (Low Dose) Placebo Screening BHV-8000: Phase 2 Imaging POC Study in Relapsing Multiple Sclerosis Preliminary clinical trial design DESIGN Randomized, double-blind, placebo-controlled Phase 2 imaging proof-of-concept study POPULATION Adults with relapsing multiple sclerosis (RMS) SAMPLE SIZE 140 participants (randomized 2:2:1) TREATMENT BHV-8000 low dose or high dose versus placebo TREATMENT DURATION 12-week double-blind phase followed by open label study ENDPOINTS Cumulative number of new gadolinium (Gd)-enhancing T1 lesions, total number of Gd-enhancing T1 lesions, number of new or enlarging T2 lesions, change in phase rim lesions, PK/PD 8 Weeks 12-Week Treatment Phase DATA READOUT OLE INFLAMMATION & IMMUNOLOGY June 2025 Biohaven Investor Presentation91
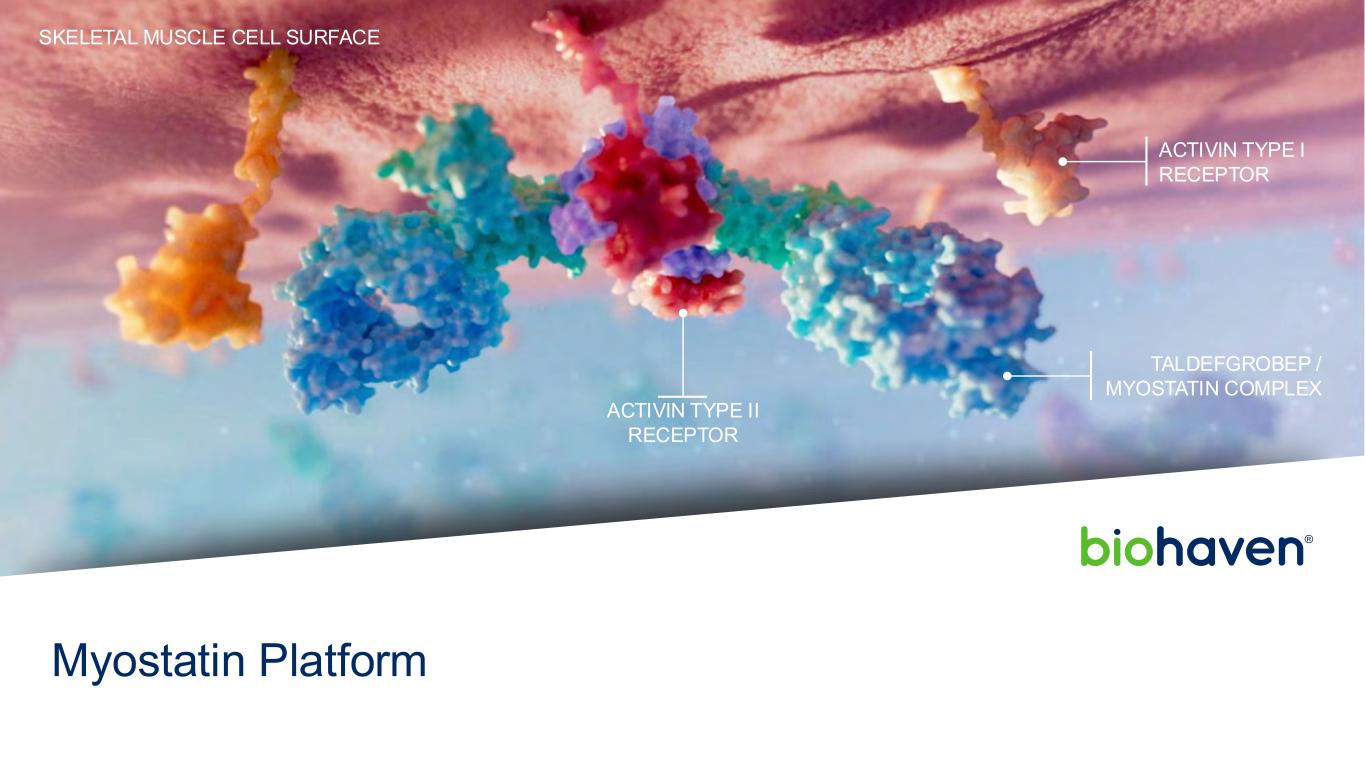
TALDEFGROBEP / MYOSTATIN COMPLEX ACTIVIN TYPE II RECEPTOR ACTIVIN TYPE I RECEPTOR SKELETAL MUSCLE CELL SURFACE Myostatin Platform

Taldefgrobep Alfa for Obesity

June 2025 Biohaven Investor Presentation94 Differentiated Pharmacology Key to Optimizing Benefit in Overweight and Obesity • Taldefgrobep-myostatin complex competitively inhibits multiple key ligands from signaling through Activin II receptors (ActRII) • Unique MOA leads to direct beneficial effects on both muscle and adipose tissues • Safety profile established in diverse clinical populations (N >700) well-suited to overweight and obesity indication Paradigm Shift to Improving Quality of Weight Loss • Ability to lower total body weight by reducing fat mass while preserving lean muscle mass • Use as monotherapy or in combination with Nutrient-Stimulated Hormone (NuSH)-based therapies, i.e., GLP-1 receptor agonists • Weekly SC administration via off-the-shelf autoinjector, with potential for extended dosing intervals TALDEFGROBEP ALFA (ANTI-MYOSTATIN) Phase 2 study in obesity planned for 2H 2025KEY UPDATE

Optimal Management of Obesity Remains a Critical Unmet Medical Need • By 2030, 1 billion people worldwide will be living with obesity, including 50% of American adults1 • Obesity is a disease of excess and/or abnormal adipose tissue, not excess mass • Incretin mimetics have revolutionized management of obesity, but present liabilities • Up to 40% of total body weight loss is lean mass2 • Gastrointestinal side effects3 • Reduced bone mass4 • Two-thirds stop GLP-1 therapy within 1 year5 • Two-thirds of lost body weight returns within 1 year of stopping GLP-1 therapy5,6 Endocrine • Diabetes mellitus • Hypothyroidism • Subfertility GI • Hiatus hernia • Gallbladder disease • Inguinal hernia Carcinoma • Breast • Colorectal • Endometrial Musculoskeletal • Osteoarthritis • Back pain Cardiovascular • Sudden death • Cardiomyopathy • Hypertension • Ischemic heart disease • Peripheral vascular disease • Deep vein thrombosis • Pulmonary embolism Respiratory • Restrictive lung disease • Obstructive sleep apnea • Obesity hypoventilation syndrome • Difficult intubation Genitourinary • Menstrual problems • Female incontinence • Renal calculi Complications of Obesity7 1 https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022; Accessed 9-JAN-2025. 2. Wilding JPH et al, N Engl J Med. 2021;384(11):989-1002. 3. Wilding, et. al., Diabetes Obes Metab. 2022; 24(8):1553-64. doi: 10.1111/dom.14725 4. Hansen MS, et al., eClinicalMedicine. 2024;72:102624 5. Scientific American. What happens when you quit Ozempic or Wegovy? APR 2024. https://www.scientificamerican.com/article/you-quit-ozempic-or-wegovy-what-happens-next/ Accessed 9-JAN-2025. 6. Sikirica MV. Et al., Diabetes Metab Syndr Obes. 2017;10:403-12. 7. UpToDate. Overweight and obesity in adults: health consequences. https://www.uptodate.com/contents/overweight-and-obesity-in-adults-health-consequences. Accessed 9-JAN-2025. T-ALFA June 2025 Biohaven Investor Presentation95
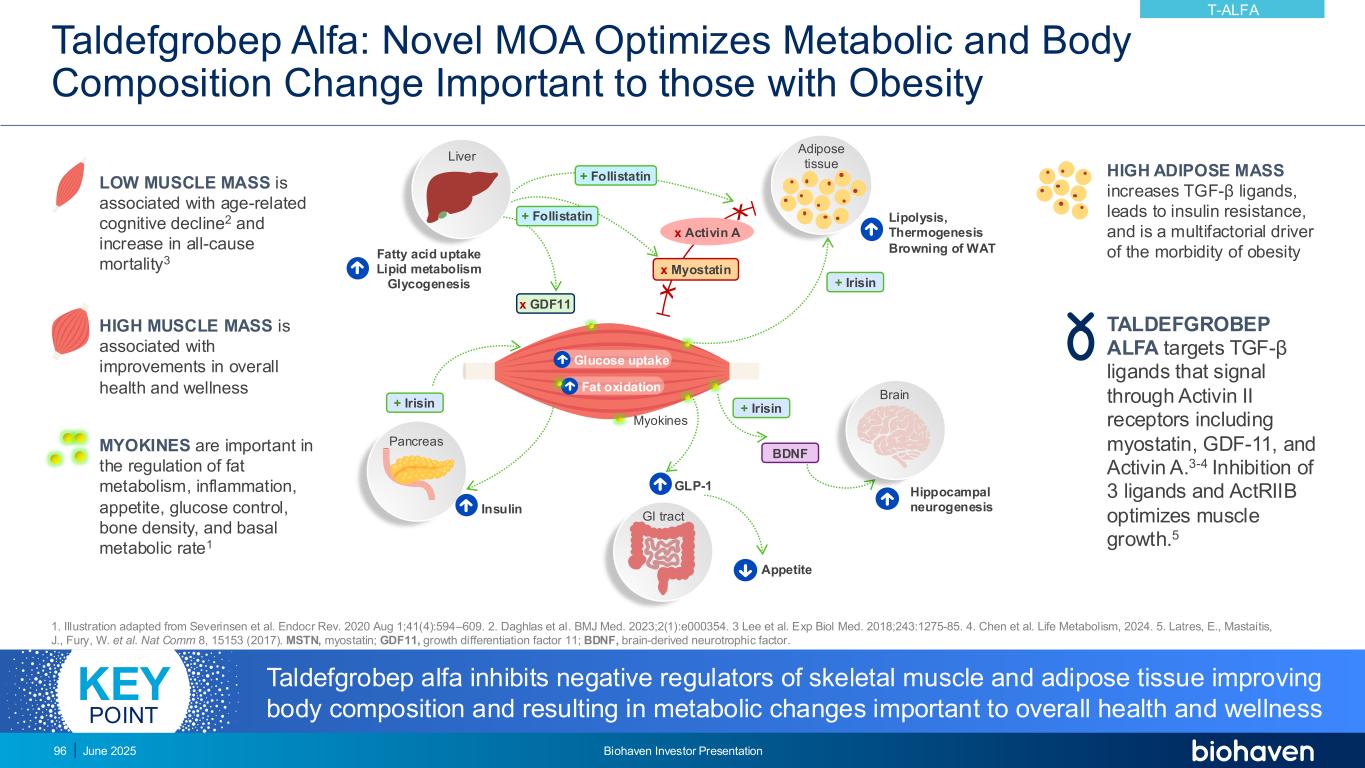
Taldefgrobep Alfa: Novel MOA Optimizes Metabolic and Body Composition Change Important to those with Obesity 1. Illustration adapted from Severinsen et al. Endocr Rev. 2020 Aug 1;41(4):594–609. 2. Daghlas et al. BMJ Med. 2023;2(1):e000354. 3 Lee et al. Exp Biol Med. 2018;243:1275-85. 4. Chen et al. Life Metabolism, 2024. 5. Latres, E., Mastaitis, J., Fury, W. et al. Nat Comm 8, 15153 (2017). MSTN, myostatin; GDF11, growth differentiation factor 11; BDNF, brain-derived neurotrophic factor. Lipolysis, Thermogenesis Browning of WAT Appetite GLP-1 Fatty acid uptake Lipid metabolism Glycogenesis Insulin Glucose uptake Fat oxidation Brain Myokines GI tract + Irisin+ Irisin BDNF Pancreas Liver x GDF11 Adipose tissue + Irisin x Myostatin x Activin A + Follistatin Hippocampal neurogenesis + Follistatin LOW MUSCLE MASS is associated with age-related cognitive decline2 and increase in all-cause mortality3 HIGH MUSCLE MASS is associated with improvements in overall health and wellness MYOKINES are important in the regulation of fat metabolism, inflammation, appetite, glucose control, bone density, and basal metabolic rate1 HIGH ADIPOSE MASS increases TGF-β ligands, leads to insulin resistance, and is a multifactorial driver of the morbidity of obesity TALDEFGROBEP ALFA targets TGF-β ligands that signal through Activin II receptors including myostatin, GDF-11, and Activin A.3-4 Inhibition of 3 ligands and ActRIIB optimizes muscle growth.5 T-ALFA June 2025 Biohaven Investor Presentation96 Taldefgrobep alfa inhibits negative regulators of skeletal muscle and adipose tissue improving body composition and resulting in metabolic changes important to overall health and wellness KEY POINT
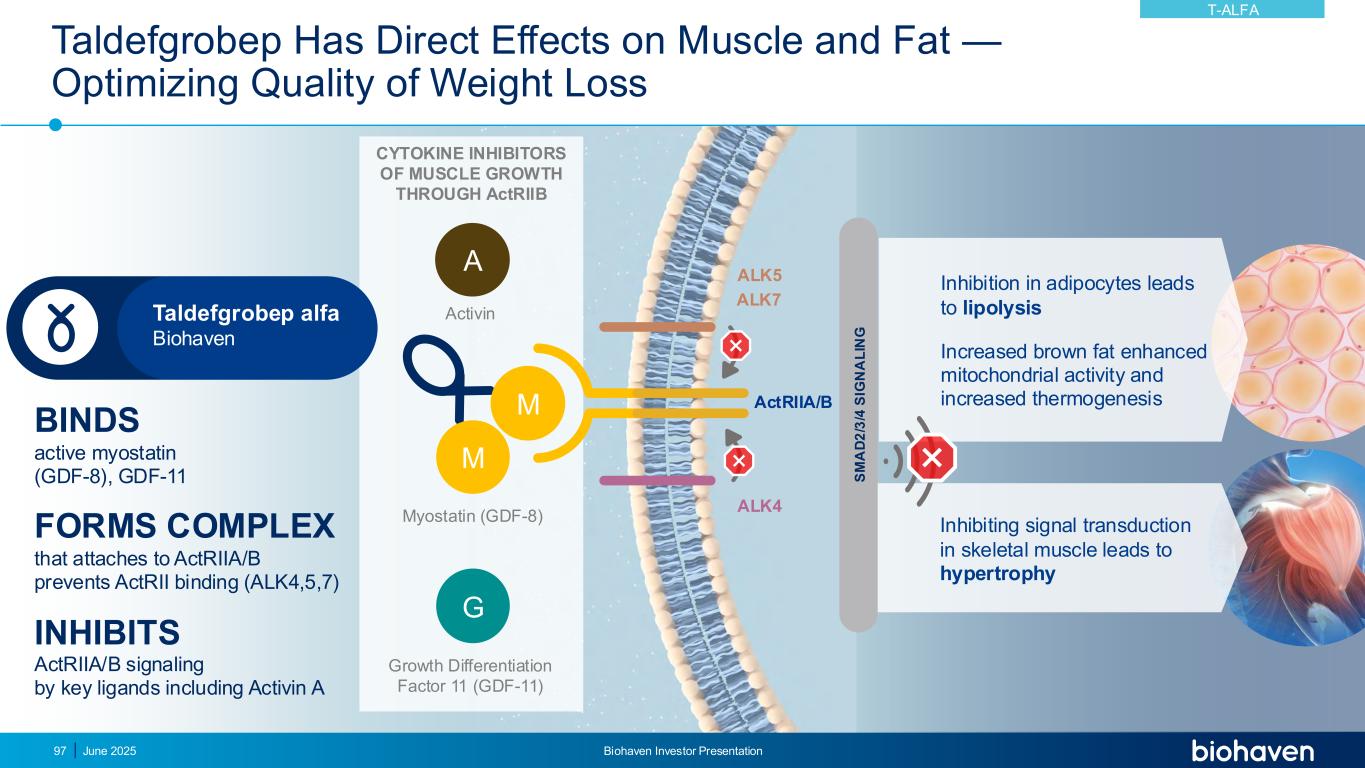
Taldefgrobep Has Direct Effects on Muscle and Fat — Optimizing Quality of Weight Loss June 2025 Biohaven Investor Presentation97 BINDS active myostatin (GDF-8), GDF-11 FORMS COMPLEX that attaches to ActRIIA/B prevents ActRII binding (ALK4,5,7) INHIBITS ActRIIA/B signaling by key ligands including Activin A S M A D 2 /3 /4 S IG N A L IN G ALK4 ALK5 ActRIIA/BM M G ALK7 A Myostatin (GDF-8) Growth Differentiation Factor 11 (GDF-11) Activin CYTOKINE INHIBITORS OF MUSCLE GROWTH THROUGH ActRIIB Inhibiting signal transduction in skeletal muscle leads to hypertrophy Inhibition in adipocytes leads to lipolysis Increased brown fat enhanced mitochondrial activity and increased thermogenesis Taldefgrobep alfa Biohaven T-ALFA
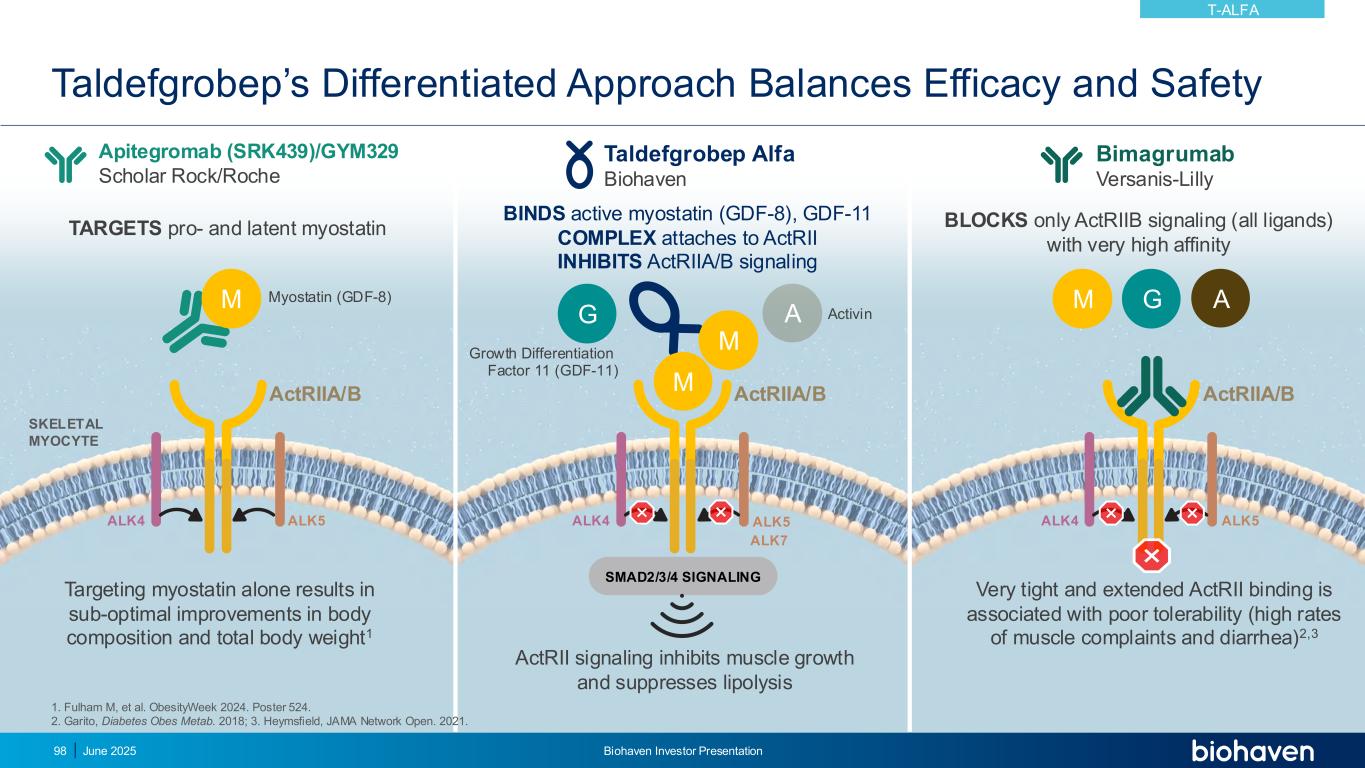
Taldefgrobep’s Differentiated Approach Balances Efficacy and Safety June 2025 Biohaven Investor Presentation98 ALK4 ALK5 ActRIIA/B SKELETAL MYOCYTE SMAD2/3/4 SIGNALING ActRII signaling inhibits muscle growth and suppresses lipolysis ALK4 ALK5 ActRIIA/B ALK4 ALK5 ActRIIA/B M M G A A M M TARGETS pro- and latent myostatin Apitegromab (SRK439)/GYM329 Scholar Rock/Roche Taldefgrobep Alfa Biohaven BINDS active myostatin (GDF-8), GDF-11 COMPLEX attaches to ActRII INHIBITS ActRIIA/B signaling Bimagrumab Versanis-Lilly BLOCKS only ActRIIB signaling (all ligands) with very high affinity G ALK7 Targeting myostatin alone results in sub-optimal improvements in body composition and total body weight1 Very tight and extended ActRII binding is associated with poor tolerability (high rates of muscle complaints and diarrhea)2,3 1. Fulham M, et al. ObesityWeek 2024. Poster 524. 2. Garito, Diabetes Obes Metab. 2018; 3. Heymsfield, JAMA Network Open. 2021. Myostatin (GDF-8) Growth Differentiation Factor 11 (GDF-11) Activin T-ALFA
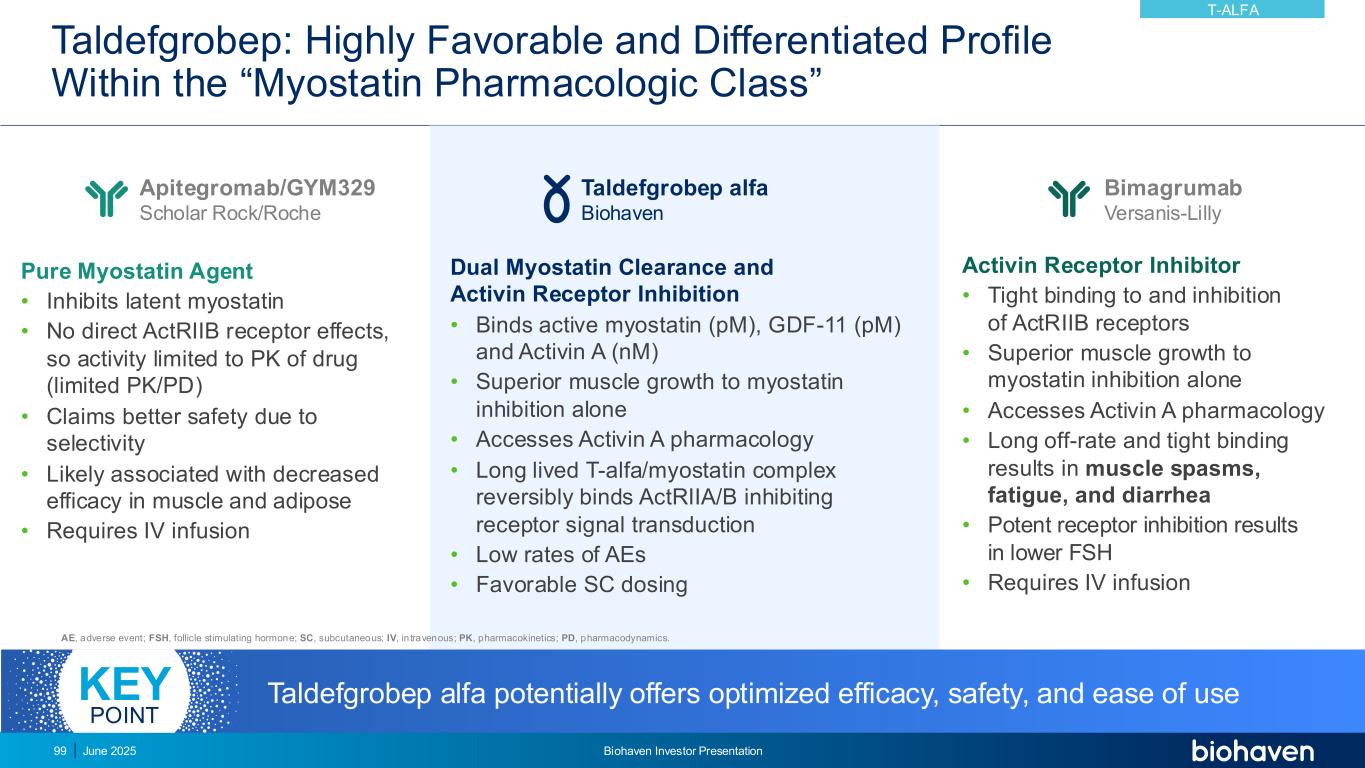
Taldefgrobep: Highly Favorable and Differentiated Profile Within the “Myostatin Pharmacologic Class” Pure Myostatin Agent • Inhibits latent myostatin • No direct ActRIIB receptor effects, so activity limited to PK of drug (limited PK/PD) • Claims better safety due to selectivity • Likely associated with decreased efficacy in muscle and adipose • Requires IV infusion Activin Receptor Inhibitor • Tight binding to and inhibition of ActRIIB receptors • Superior muscle growth to myostatin inhibition alone • Accesses Activin A pharmacology • Long off-rate and tight binding results in muscle spasms, fatigue, and diarrhea • Potent receptor inhibition results in lower FSH • Requires IV infusion Dual Myostatin Clearance and Activin Receptor Inhibition • Binds active myostatin (pM), GDF-11 (pM) and Activin A (nM) • Superior muscle growth to myostatin inhibition alone • Accesses Activin A pharmacology • Long lived T-alfa/myostatin complex reversibly binds ActRIIA/B inhibiting receptor signal transduction • Low rates of AEs • Favorable SC dosing Apitegromab/GYM329 Scholar Rock/Roche Taldefgrobep alfa Biohaven Bimagrumab Versanis-Lilly AE, adverse event; FSH, follicle stimulating hormone; SC, subcutaneous; IV, in travenous; PK, pharmacokinetics; PD, pharmacodynamics. Taldefgrobep alfa potentially offers optimized efficacy, safety, and ease of useKEY POINT T-ALFA June 2025 Biohaven Investor Presentation99
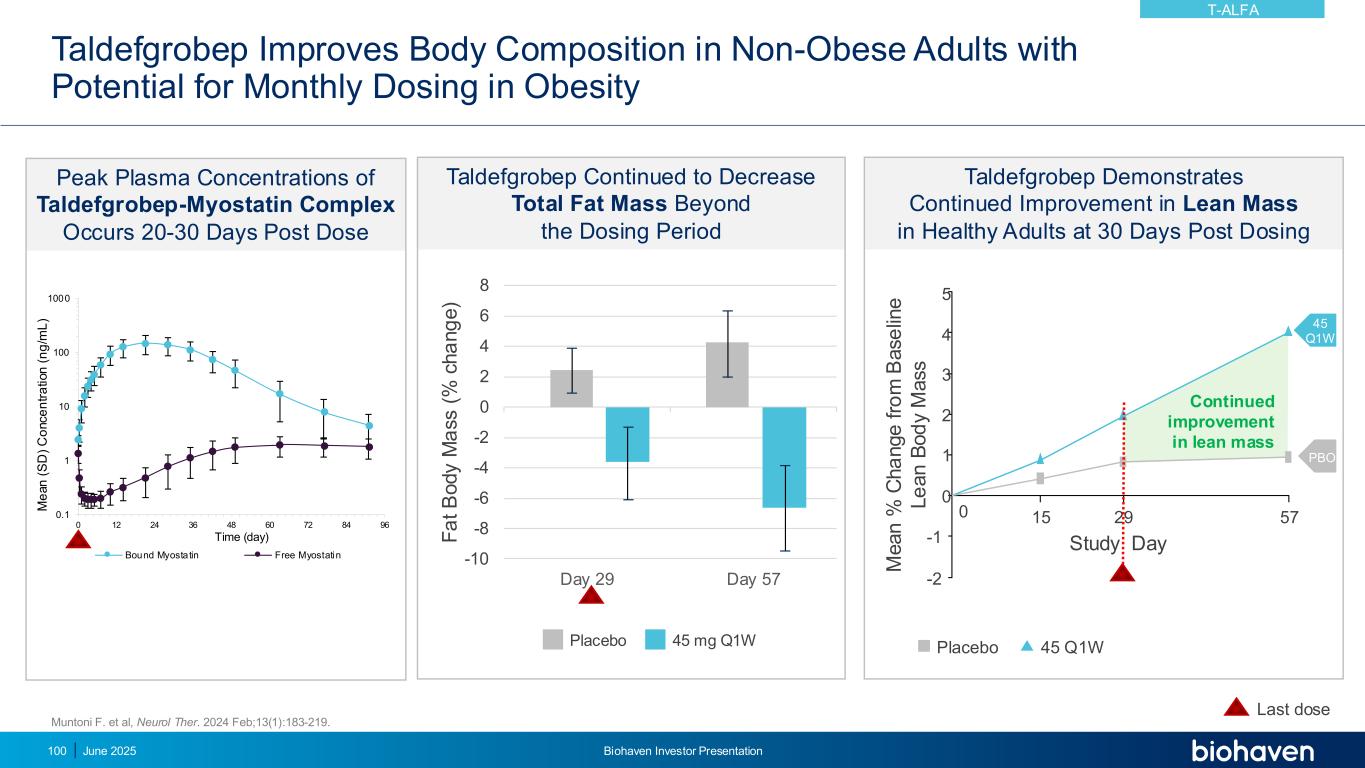
Taldefgrobep Improves Body Composition in Non-Obese Adults with Potential for Monthly Dosing in Obesity June 2025 Biohaven Investor Presentation100 Muntoni F. et al, Neurol Ther. 2024 Feb;13(1):183-219. Taldefgrobep Continued to Decrease Total Fat Mass Beyond the Dosing Period Placebo 45 mg Q1W Last dose -10 -8 -6 -4 -2 0 2 4 6 8 Day 29 Day 57 F a t B o d y M a s s ( % c h a n g e ) Peak Plasma Concentrations of Taldefgrobep-Myostatin Complex Occurs 20-30 Days Post Dose Taldefgrobep Demonstrates Continued Improvement in Lean Mass in Healthy Adults at 30 Days Post Dosing 45 Q1W 5 4 3 2 1 0 -1 -2 0 15 29 57 M e a n % C h a n g e f ro m B a s e lin e L e a n B o d y M a s s Study Day Continued improvement in lean mass PBO Placebo 45 Q1W 0.1 1 10 100 1000 0 12 24 36 48 60 72 84 96 M e a n ( S D ) C o n c e n tr a ti o n ( n g /m L ) Time (day) Bound Myostatin Free Myostatin T-ALFA
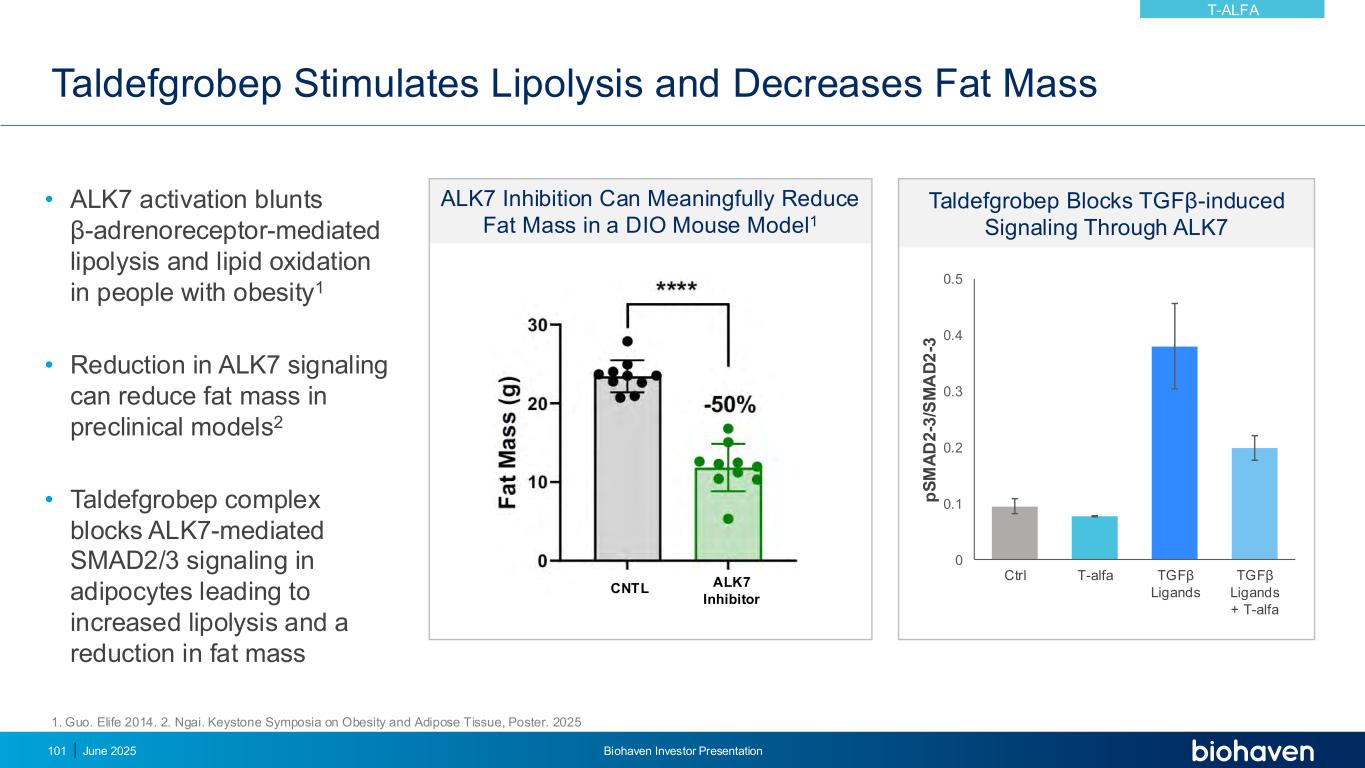
ALK7 Inhibition Can Meaningfully Reduce Fat Mass in a DIO Mouse Model1 Taldefgrobep Stimulates Lipolysis and Decreases Fat Mass June 2025 Biohaven Investor Presentation101 • ALK7 activation blunts β-adrenoreceptor-mediated lipolysis and lipid oxidation in people with obesity1 • Reduction in ALK7 signaling can reduce fat mass in preclinical models2 • Taldefgrobep complex blocks ALK7-mediated SMAD2/3 signaling in adipocytes leading to increased lipolysis and a reduction in fat mass ALK7 Inhibitor CNTL 1. Guo. Elife 2014. 2. Ngai. Keystone Symposia on Obesity and Adipose Tissue, Poster. 2025 Taldefgrobep Blocks TGFβ-induced Signaling Through ALK7 0 0.1 0.2 0.3 0.4 0.5 1 2 3 4 p S M A D 2 -3 /S M A D 2 -3 Ctrl T-alfa TGFβ Ligands TGFβ Ligands + T-alfa T-ALFA
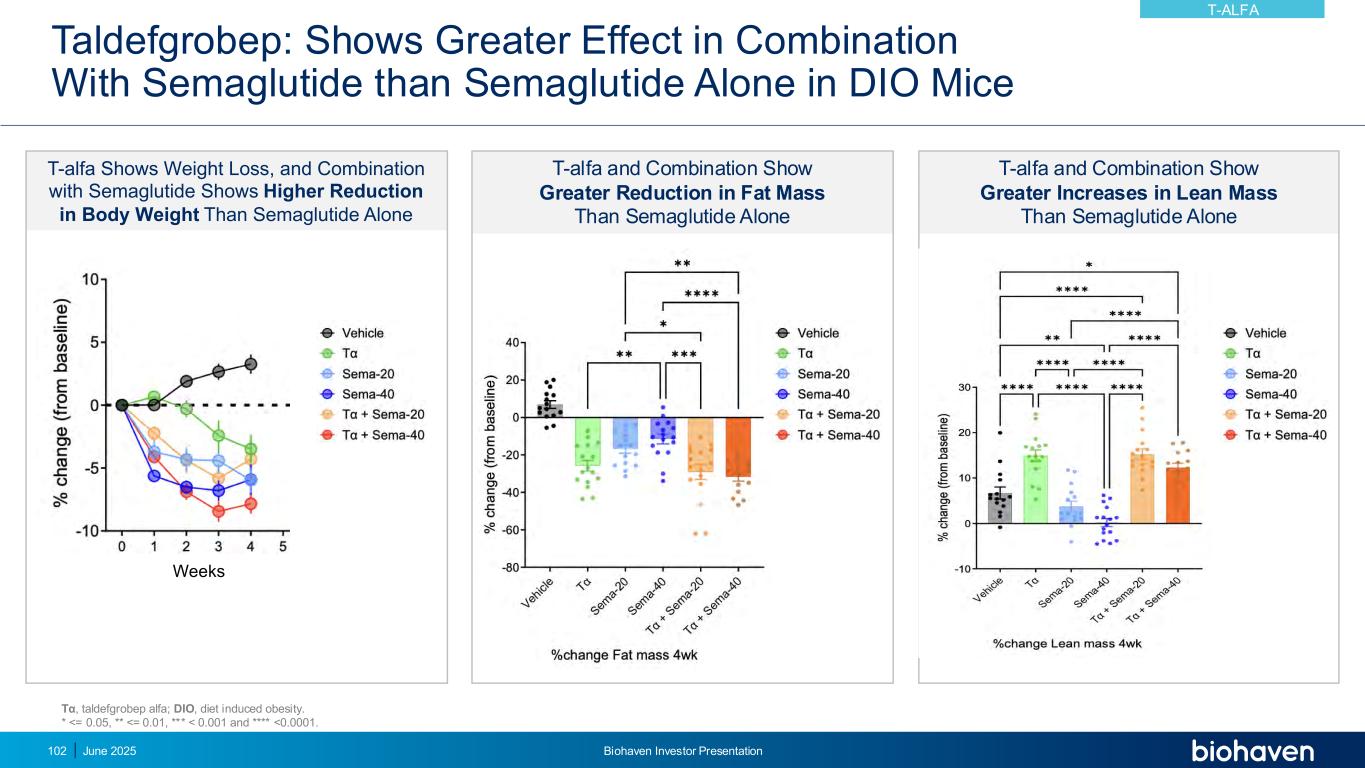
Taldefgrobep: Shows Greater Effect in Combination With Semaglutide than Semaglutide Alone in DIO Mice T-alfa Shows Weight Loss, and Combination with Semaglutide Shows Higher Reduction in Body Weight Than Semaglutide Alone Weeks T-alfa and Combination Show Greater Reduction in Fat Mass Than Semaglutide Alone T-alfa and Combination Show Greater Increases in Lean Mass Than Semaglutide Alone Tα, taldefgrobep alfa; DIO, diet induced obesity. * <= 0.05, ** <= 0.01, *** < 0.001 and **** <0.0001. T-ALFA June 2025 Biohaven Investor Presentation102

Taldefgrobep Monotherapy Offers Attractive Differentiation; but Also Potential for Combination With NuSH Therapies to Improve Quality of Weight Loss June 2025 Biohaven Investor Presentation103 MITIGATE AGAINST ✓ Excess loss of lean mass ✓ Excess loss of bone mass ✓ Rapid weight rebound following interruption of dosing with NuSH therapies ✓ Hepatic fat ✓ Intramuscular fat ✓ HbA1c ✓ BP Augment beneficial effects of NuSH therapies Address limitations of NuSH therapies FURTHER REDUCE ✓ Total body weight ✓ Total body fat ✓ Visceral adipose tissue ✓ Subcutaneous adipose tissue NuSH Therapies, Nutrient-stimulating hormone therapies. T-ALFA
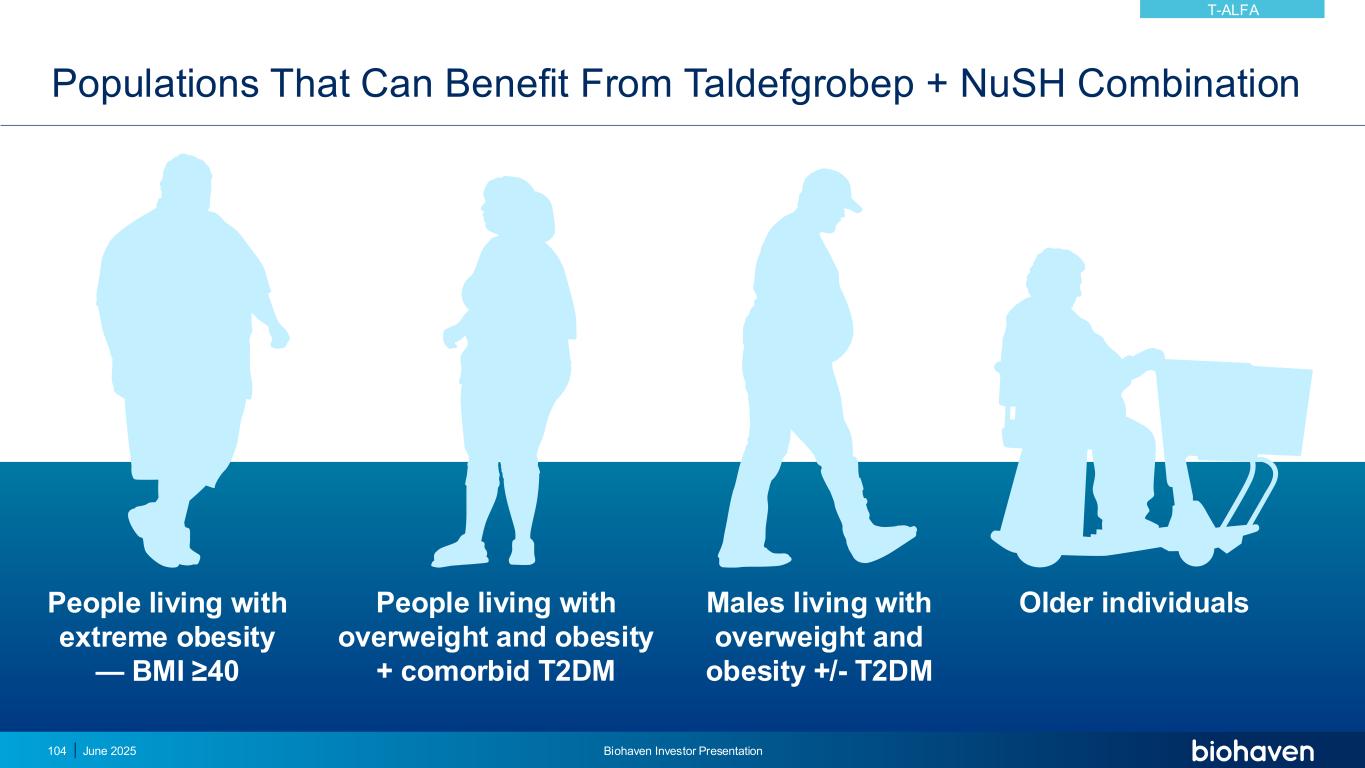
Populations That Can Benefit From Taldefgrobep + NuSH Combination June 2025 Biohaven Investor Presentation104 People living with overweight and obesity + comorbid T2DM Males living with overweight and obesity +/- T2DM Older individualsPeople living with extreme obesity — BMI ≥40 T-ALFA
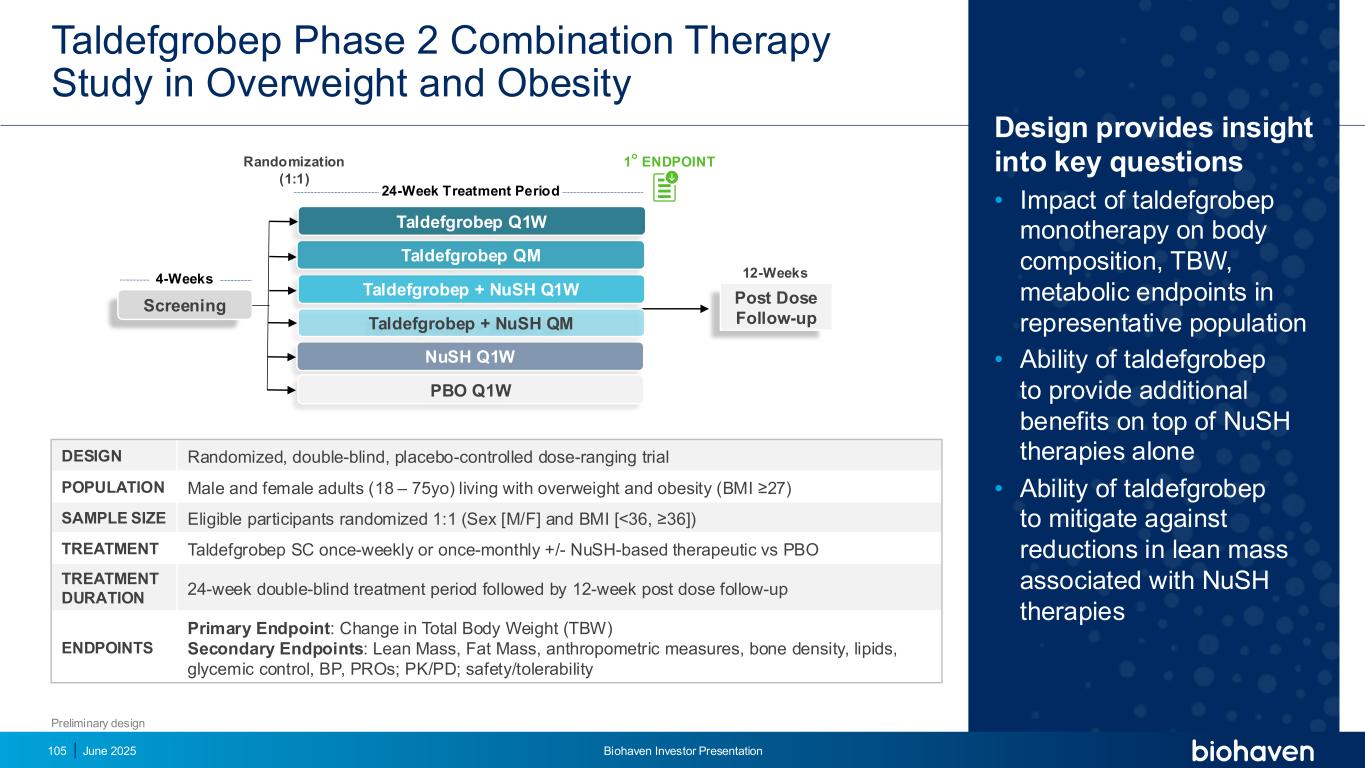
Design provides insight into key questions • Impact of taldefgrobep monotherapy on body composition, TBW, metabolic endpoints in representative population • Ability of taldefgrobep to provide additional benefits on top of NuSH therapies alone • Ability of taldefgrobep to mitigate against reductions in lean mass associated with NuSH therapies June 2025 Biohaven Investor Presentation105 Taldefgrobep Phase 2 Combination Therapy Study in Overweight and Obesity DESIGN Randomized, double-blind, placebo-controlled dose-ranging trial POPULATION Male and female adults (18 – 75yo) living with overweight and obesity (BMI ≥27) SAMPLE SIZE Eligible participants randomized 1:1 (Sex [M/F] and BMI [<36, ≥36]) TREATMENT Taldefgrobep SC once-weekly or once-monthly +/- NuSH-based therapeutic vs PBO TREATMENT DURATION 24-week double-blind treatment period followed by 12-week post dose follow-up ENDPOINTS Primary Endpoint: Change in Total Body Weight (TBW) Secondary Endpoints: Lean Mass, Fat Mass, anthropometric measures, bone density, lipids, glycemic control, BP, PROs; PK/PD; safety/tolerability 12-Weeks4-Weeks 24-Week Treatment Period Screening Randomization (1:1) Post Dose Follow-up Taldefgrobep + NuSH Q1W NuSH Q1W 1⚬ ENDPOINT Taldefgrobep + NuSH QM Taldefgrobep QM Taldefgrobep Q1W PBO Q1W Preliminary design

Taldefgrobep Alfa for Spinal Muscular Atrophy (SMA)
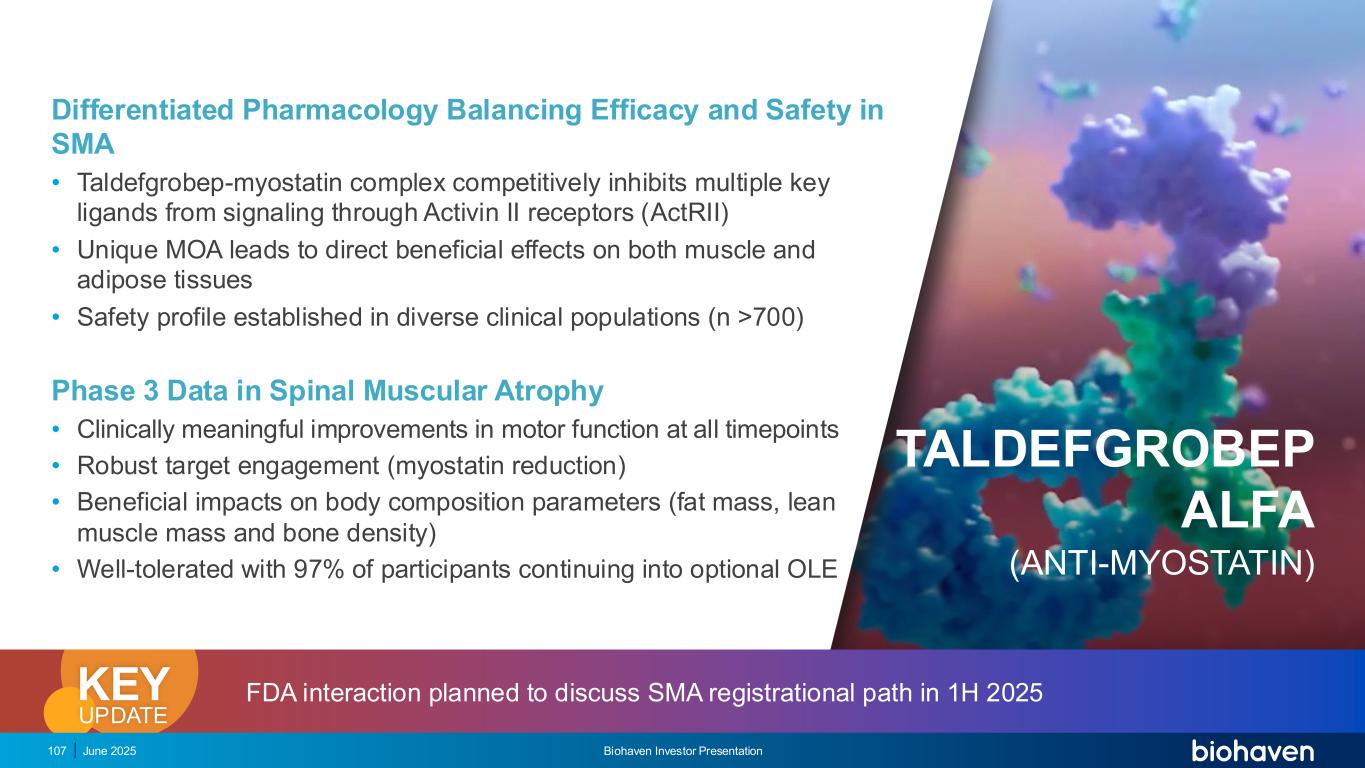
June 2025 Biohaven Investor Presentation107 Differentiated Pharmacology Balancing Efficacy and Safety in SMA • Taldefgrobep-myostatin complex competitively inhibits multiple key ligands from signaling through Activin II receptors (ActRII) • Unique MOA leads to direct beneficial effects on both muscle and adipose tissues • Safety profile established in diverse clinical populations (n >700) Phase 3 Data in Spinal Muscular Atrophy • Clinically meaningful improvements in motor function at all timepoints • Robust target engagement (myostatin reduction) • Beneficial impacts on body composition parameters (fat mass, lean muscle mass and bone density) • Well-tolerated with 97% of participants continuing into optional OLE TALDEFGROBEP ALFA (ANTI-MYOSTATIN) FDA interaction planned to discuss SMA registrational path in 1H 2025KEY UPDATE
High Unmet Need Still Remains For SMA 1. Mercuri E, et al. Nat Rev Dis Primers. 2022 Aug 4;8(1):52 . 2. Day JW, et al. BMC Pediatr. 2022;22(1):632. 3. Darras BT, et al. N Engl J Med. 2021;385(5):427-435. 4. Finkel RS, et al. N Engl J Med. 2017;377(18):1723-1732 5. Cure SMA. Accessed November 2024. https://www.curesma.org/wp-content/uploads/2018/01/SMA-VoP-for- publication-1-22-2018.pdf. 6. Darras BT, et al. N Engl J Med. 2021;385(5):427-435. 7. Finkel RS, et al. N Engl J Med. 2017;377(18):1723-1732. SOC treatments target motor neurons not muscle Patients on SOC treatment continue to experience significant muscle weakness, reduced functioning and decreased quality of life1–7 CALEB Living with SMA Clinical trial participant on open label treatment June 2025 Biohaven Investor Presentation108
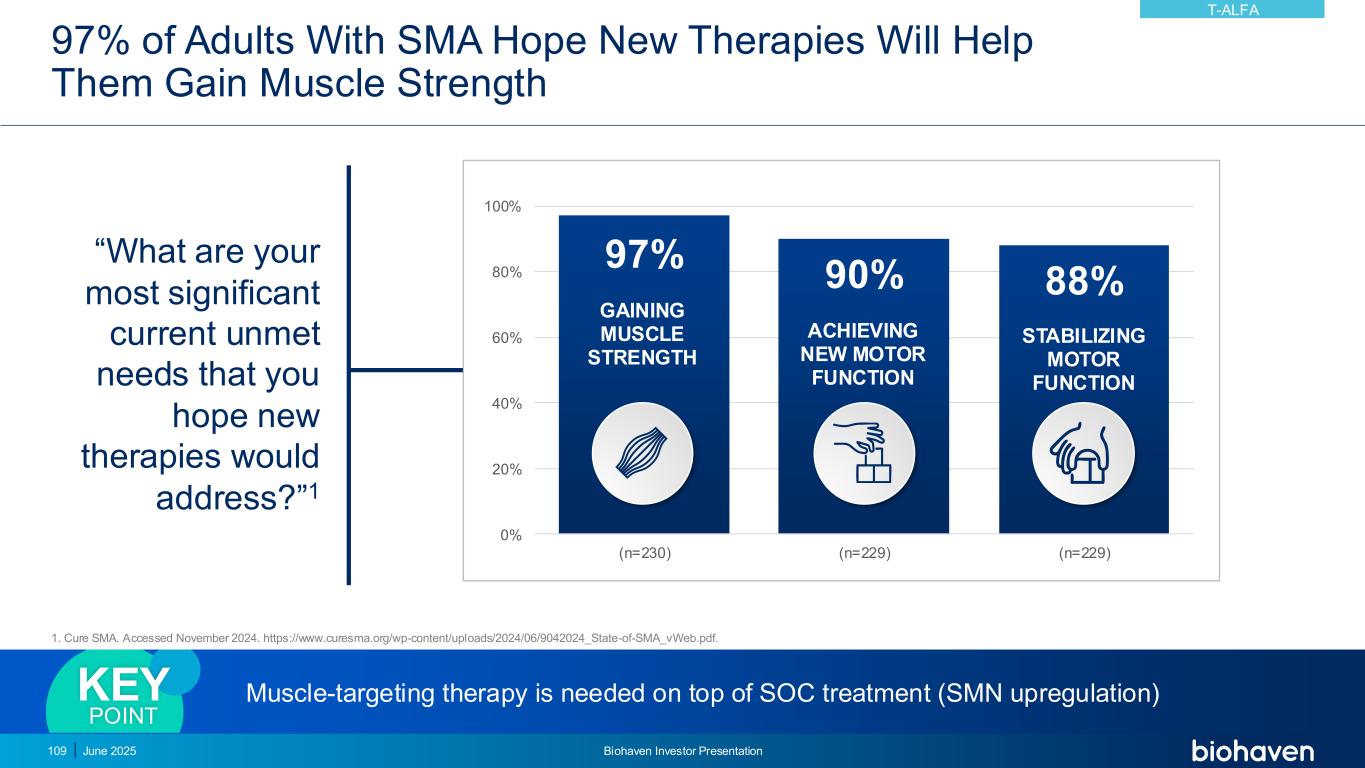
“What are your most significant current unmet needs that you hope new therapies would address?”1 97% of Adults With SMA Hope New Therapies Will Help Them Gain Muscle Strength 1. Cure SMA. Accessed November 2024. https://www.curesma.org/wp-content/uploads/2024/06/9042024_State-of-SMA_vWeb.pdf. 97% 90% 88% 0% 20% 40% 60% 80% 100% (n=230) (n=229) (n=229) GAINING MUSCLE STRENGTH ACHIEVING NEW MOTOR FUNCTION STABILIZING MOTOR FUNCTION Muscle-targeting therapy is needed on top of SOC treatment (SMN upregulation)KEY POINT June 2025 Biohaven Investor Presentation109 T-ALFA
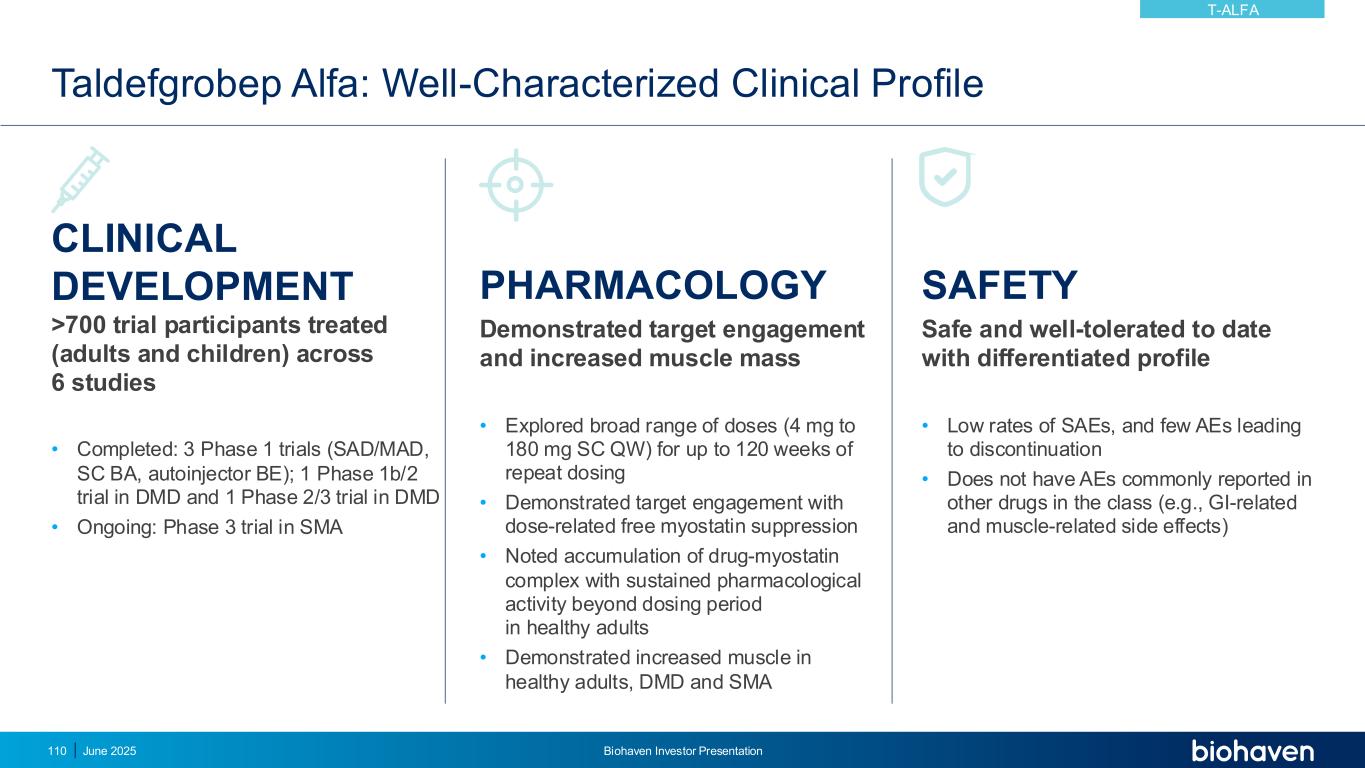
Taldefgrobep Alfa: Well-Characterized Clinical Profile CLINICAL DEVELOPMENT >700 trial participants treated (adults and children) across 6 studies • Completed: 3 Phase 1 trials (SAD/MAD, SC BA, autoinjector BE); 1 Phase 1b/2 trial in DMD and 1 Phase 2/3 trial in DMD • Ongoing: Phase 3 trial in SMA PHARMACOLOGY Demonstrated target engagement and increased muscle mass • Explored broad range of doses (4 mg to 180 mg SC QW) for up to 120 weeks of repeat dosing • Demonstrated target engagement with dose-related free myostatin suppression • Noted accumulation of drug-myostatin complex with sustained pharmacological activity beyond dosing period in healthy adults • Demonstrated increased muscle in healthy adults, DMD and SMA SAFETY Safe and well-tolerated to date with differentiated profile • Low rates of SAEs, and few AEs leading to discontinuation • Does not have AEs commonly reported in other drugs in the class (e.g., GI-related and muscle-related side effects) June 2025 Biohaven Investor Presentation110 T-ALFA
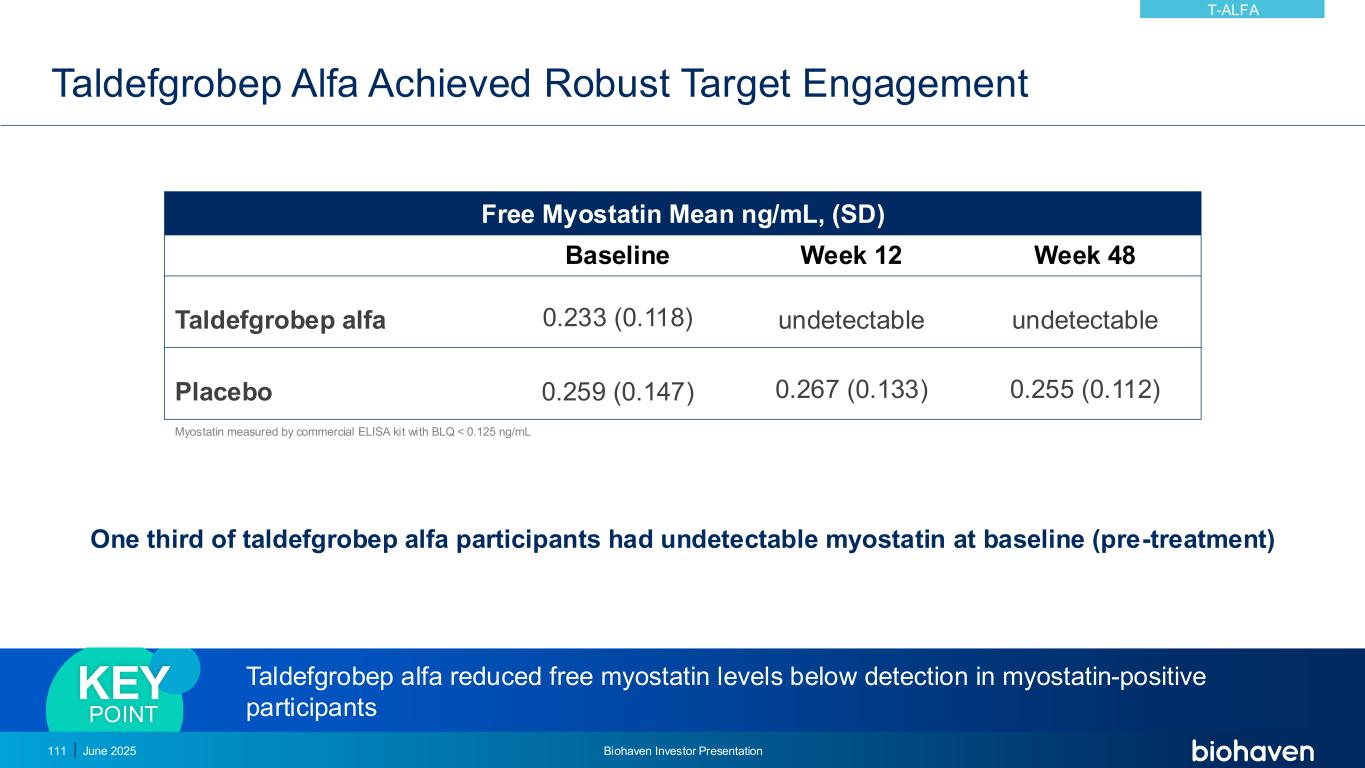
Taldefgrobep Alfa Achieved Robust Target Engagement One third of taldefgrobep alfa participants had undetectable myostatin at baseline (pre-treatment) Free Myostatin Mean ng/mL, (SD) Baseline Week 12 Week 48 Taldefgrobep alfa 0.233 (0.118) undetectable undetectable Placebo 0.259 (0.147) 0.267 (0.133) 0.255 (0.112) Myostatin measured by commercial ELISA kit with BLQ < 0.125 ng/mL Taldefgrobep alfa reduced free myostatin levels below detection in myostatin-positive participants KEY POINT June 2025 Biohaven Investor Presentation111 T-ALFA
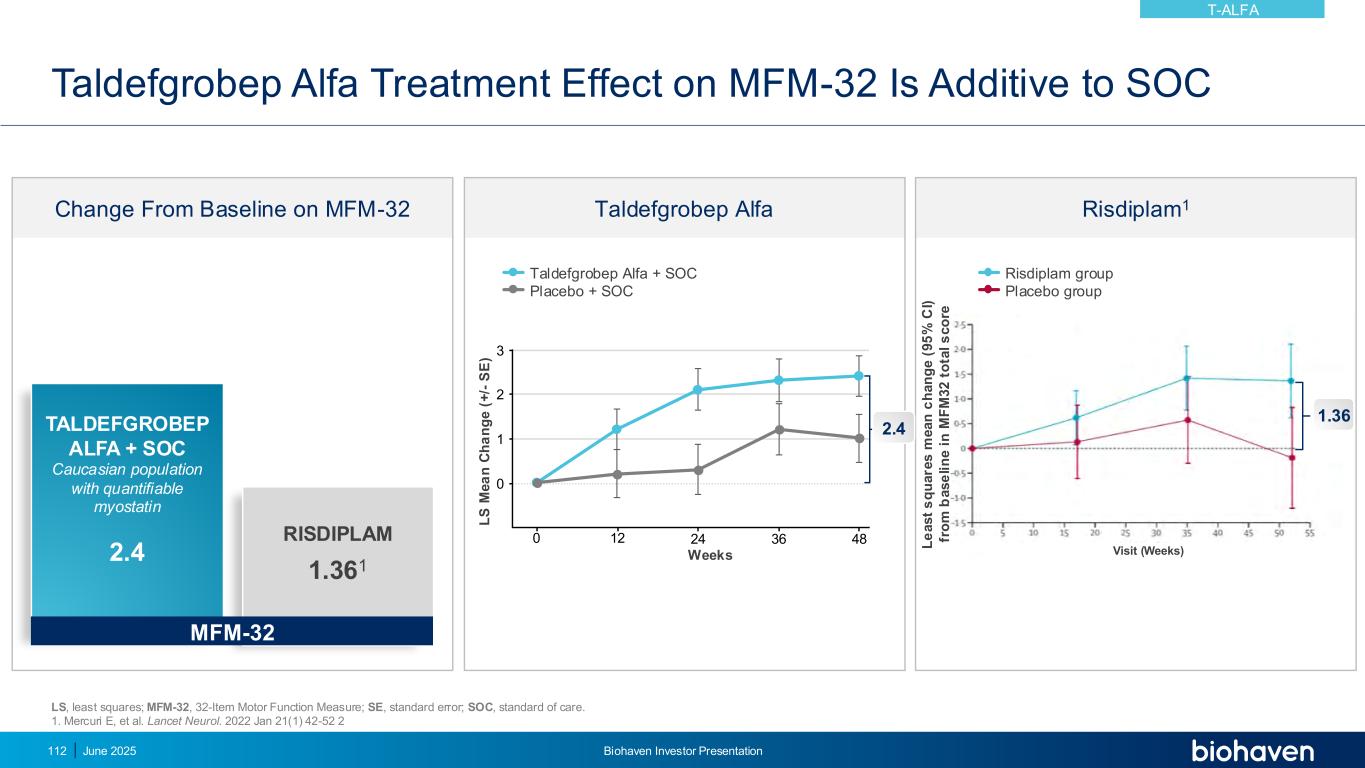
Taldefgrobep Alfa Treatment Effect on MFM-32 Is Additive to SOC MFM-32 TALDEFGROBEP ALFA + SOC Caucasian population with quantifiable myostatin 1.361 2.4 RISDIPLAM L e a s t s q u a re s m e a n c h a n g e ( 9 5 % C I) fr o m b a s e li n e i n M F M 3 2 t o ta l s c o re Risdiplam group Placebo group Change From Baseline on MFM-32 Risdiplam1Taldefgrobep Alfa 1.36 Visit (Weeks) LS, least squares; MFM-32, 32-Item Motor Function Measure; SE, standard error; SOC, standard of care. 1. Mercuri E, et al. Lancet Neurol. 2022 Jan 21(1) 42-52 2 0 12 24 36 48 0 1 2 3 L S M e a n C h a n g e ( + /- S E ) Weeks Taldefgrobep Alfa + SOC Placebo + SOC 2.4 June 2025 Biohaven Investor Presentation112 T-ALFA
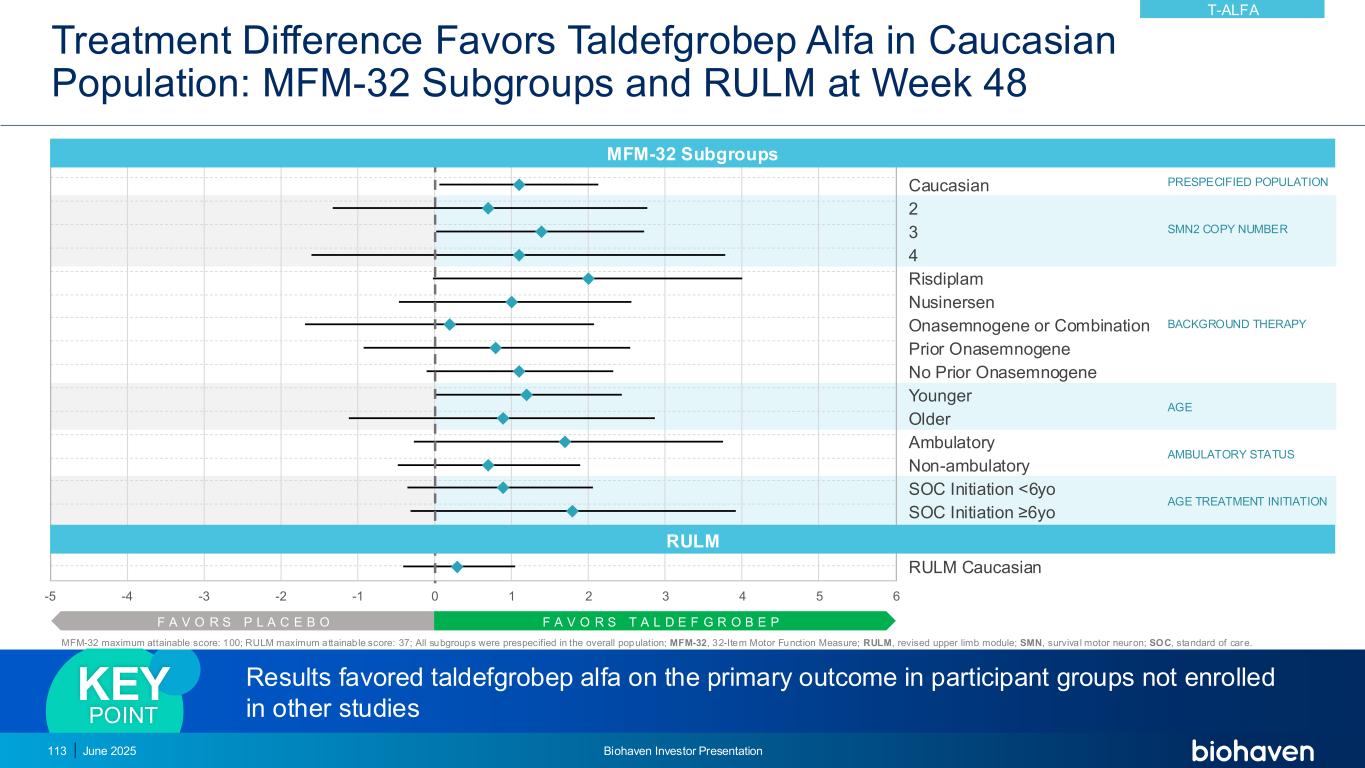
Treatment Difference Favors Taldefgrobep Alfa in Caucasian Population: MFM-32 Subgroups and RULM at Week 48 Caucasian 2 3 4 Risdiplam Nusinersen Onasemnogene or Combination Prior Onasemnogene No Prior Onasemnogene Younger Older Ambulatory Non-ambulatory SOC Initiation <6yo SOC Initiation ≥6yo F A V O R S T A L D E F G R O B E PF A V O R S P L A C E B O Results favored taldefgrobep alfa on the primary outcome in participant groups not enrolled in other studies KEY POINT PRESPECIFIED POPULATION SMN2 COPY NUMBER BACKGROUND THERAPY AGE AMBULATORY STATUS AGE TREATMENT INITIATION RULM Caucasian -5 -4 -3 -2 -1 0 1 2 3 4 5 6 MFM-32 Subgroups RULM MFM-32 maximum attainable score: 100; RULM maximum attainable score: 37; All subgroups were prespecified in the overall population; MFM-32, 32-Item Motor Function Measure; RULM, revised upper limb module; SMN, surviva l motor neuron; SOC, standard of care. June 2025 Biohaven Investor Presentation113 T-ALFA
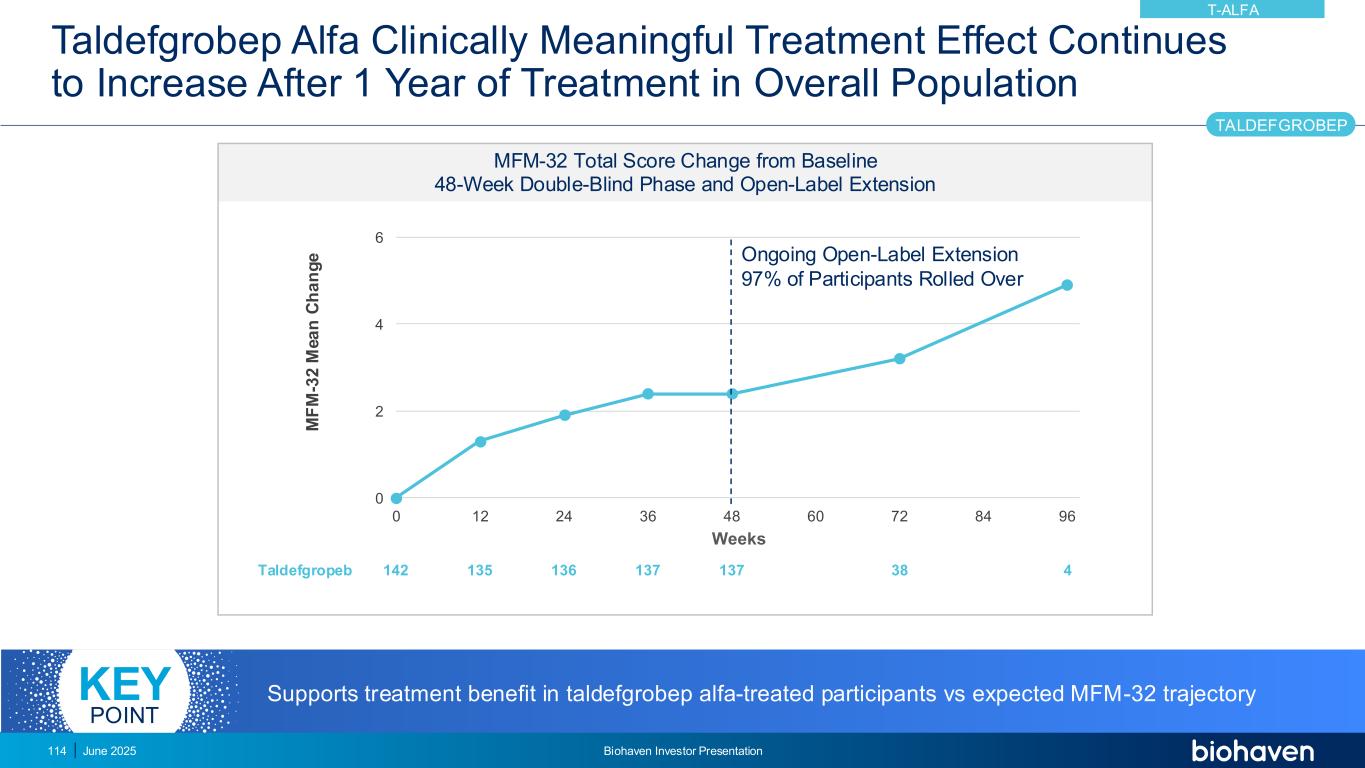
Taldefgrobep Alfa Clinically Meaningful Treatment Effect Continues to Increase After 1 Year of Treatment in Overall Population MFM-32 Total Score Change from Baseline 48-Week Double-Blind Phase and Open-Label Extension 0 2 4 6 0 12 24 36 48 60 72 84 96 M F M -3 2 M e a n C h a n g e Weeks Taldefgropeb 142 137 4135 136 137 38 Supports treatment benefit in taldefgrobep alfa-treated participants vs expected MFM-32 trajectoryKEY POINT June 2025 Biohaven Investor Presentation114 Ongoing Open-Label Extension 97% of Participants Rolled Over TALDEFGROBEP T-ALFA
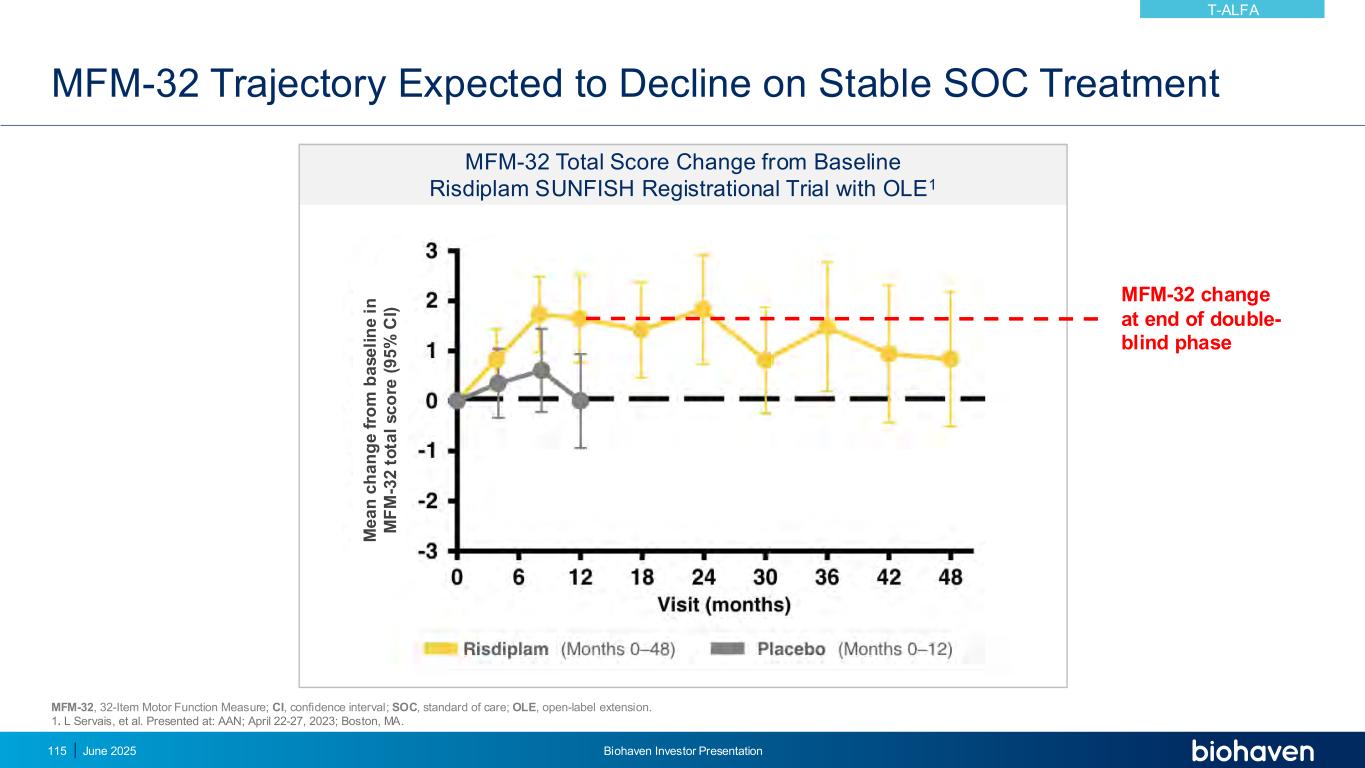
MFM-32 Trajectory Expected to Decline on Stable SOC Treatment MFM-32 Total Score Change from Baseline Risdiplam SUNFISH Registrational Trial with OLE1 MFM-32 change at end of double- blind phase M e a n c h a n g e f ro m b a s e li n e i n M F M -3 2 t o ta l s c o re ( 9 5 % C I) MFM-32, 32-Item Motor Function Measure; CI, confidence interval; SOC, standard of care; OLE, open-label extension. 1. L Servais, et al. Presented at: AAN; April 22-27, 2023; Boston, MA. June 2025 Biohaven Investor Presentation115 T-ALFA
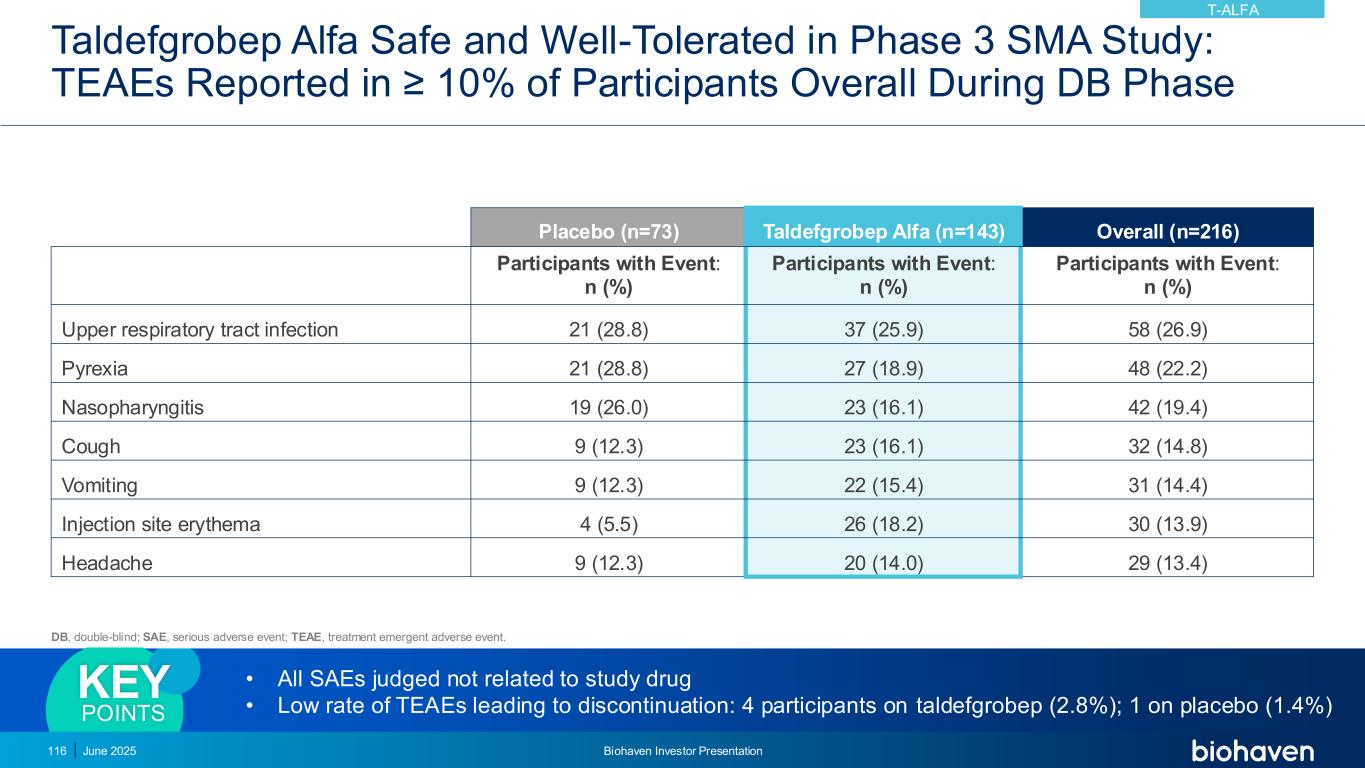
Taldefgrobep Alfa Safe and Well-Tolerated in Phase 3 SMA Study: TEAEs Reported in ≥ 10% of Participants Overall During DB Phase • All SAEs judged not related to study drug • Low rate of TEAEs leading to discontinuation: 4 participants on taldefgrobep (2.8%); 1 on placebo (1.4%) KEY POINTS Placebo (n=73) Taldefgrobep Alfa (n=143) Overall (n=216) Participants with Event: n (%) Participants with Event: n (%) Participants with Event: n (%) Upper respiratory tract infection 21 (28.8) 37 (25.9) 58 (26.9) Pyrexia 21 (28.8) 27 (18.9) 48 (22.2) Nasopharyngitis 19 (26.0) 23 (16.1) 42 (19.4) Cough 9 (12.3) 23 (16.1) 32 (14.8) Vomiting 9 (12.3) 22 (15.4) 31 (14.4) Injection site erythema 4 (5.5) 26 (18.2) 30 (13.9) Headache 9 (12.3) 20 (14.0) 29 (13.4) DB, double-blind; SAE, serious adverse event; TEAE, treatment emergent adverse event. June 2025 Biohaven Investor Presentation116 T-ALFA
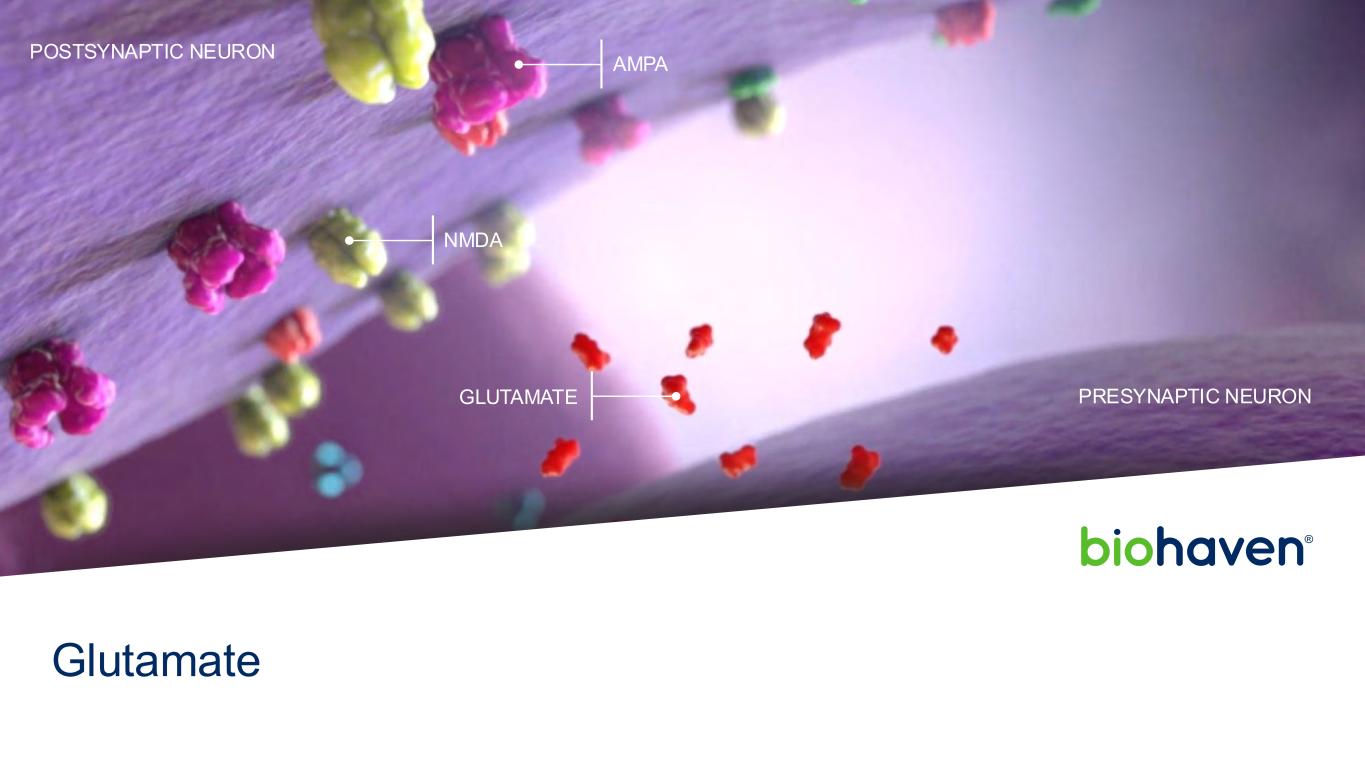
PRESYNAPTIC NEURON POSTSYNAPTIC NEURON AMPA NMDA GLUTAMATE Glutamate

June 2025 Biohaven Investor Presentation118 Significant Unmet Need in SCA With No Available Treatment • Rare, relentlessly progressive, inherited neurodegenerative disease • ~15,000 patients in US US Priority Review of NDA With FDA Is Underway • Completed mid-cycle review and inspections of HQ and clinical trial sites • Preparing for Advisory Committee and late-cycle meetings • PDUFA date 2H 2025 Efficacy and Safety Demonstrated Over 8 Years • Study 206-RWE met prespecified primary endpoint (f-SARA at Year 3) in all SCA genotypes • Additional confirmatory evidence from Study 206-RWE, Study 206 and Study 201 Biohaven Will Be Ready to Serve Patients Upon Anticipated Approval • EAP currently expanding across multiple sites in the US due to patient demand • Preparing for US commercial launch in 2H 2025 in event of approval TRORILUZOLE GLUTAMATE MODULATOR Troriluzole US NDA currently under priority review for SCA — 2H 2025NEWS BREAKING
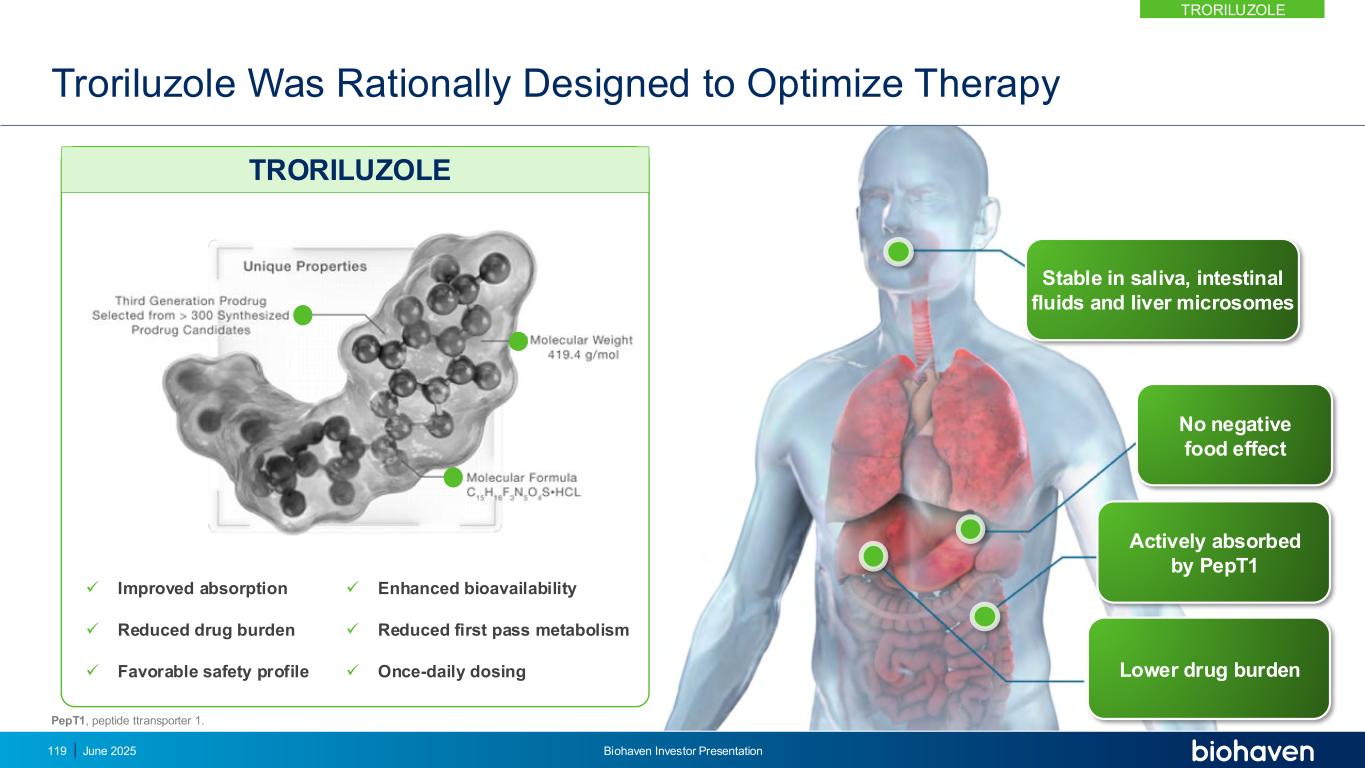
Troriluzole Was Rationally Designed to Optimize Therapy ✓ Improved absorption ✓ Enhanced bioavailability ✓ Reduced drug burden ✓ Reduced first pass metabolism ✓ Favorable safety profile ✓ Once-daily dosing Stable in saliva, intestinal fluids and liver microsomes No negative food effect Actively absorbed by PepT1 Lower drug burden PepT1, peptide ttransporter 1. TRORILUZOLE 119 June 2025 Biohaven Investor Presentation TRORILUZOLE
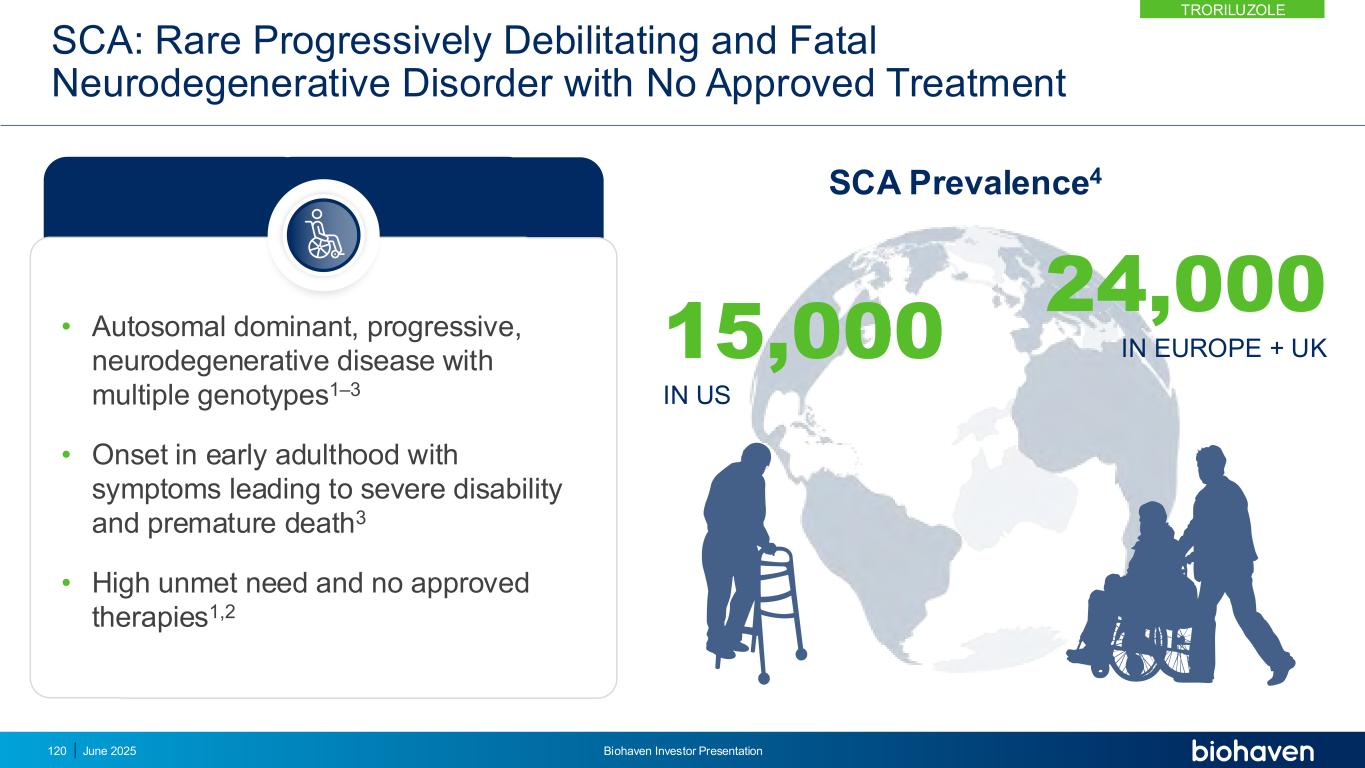
• Autosomal dominant, progressive, neurodegenerative disease with multiple genotypes1–3 • Onset in early adulthood with symptoms leading to severe disability and premature death3 • High unmet need and no approved therapies1,2 SCA: Rare Progressively Debilitating and Fatal Neurodegenerative Disorder with No Approved Treatment SCA Prevalence4 24,000 IN EUROPE + UK15,000 IN US TRORILUZOLE June 2025 Biohaven Investor Presentation120
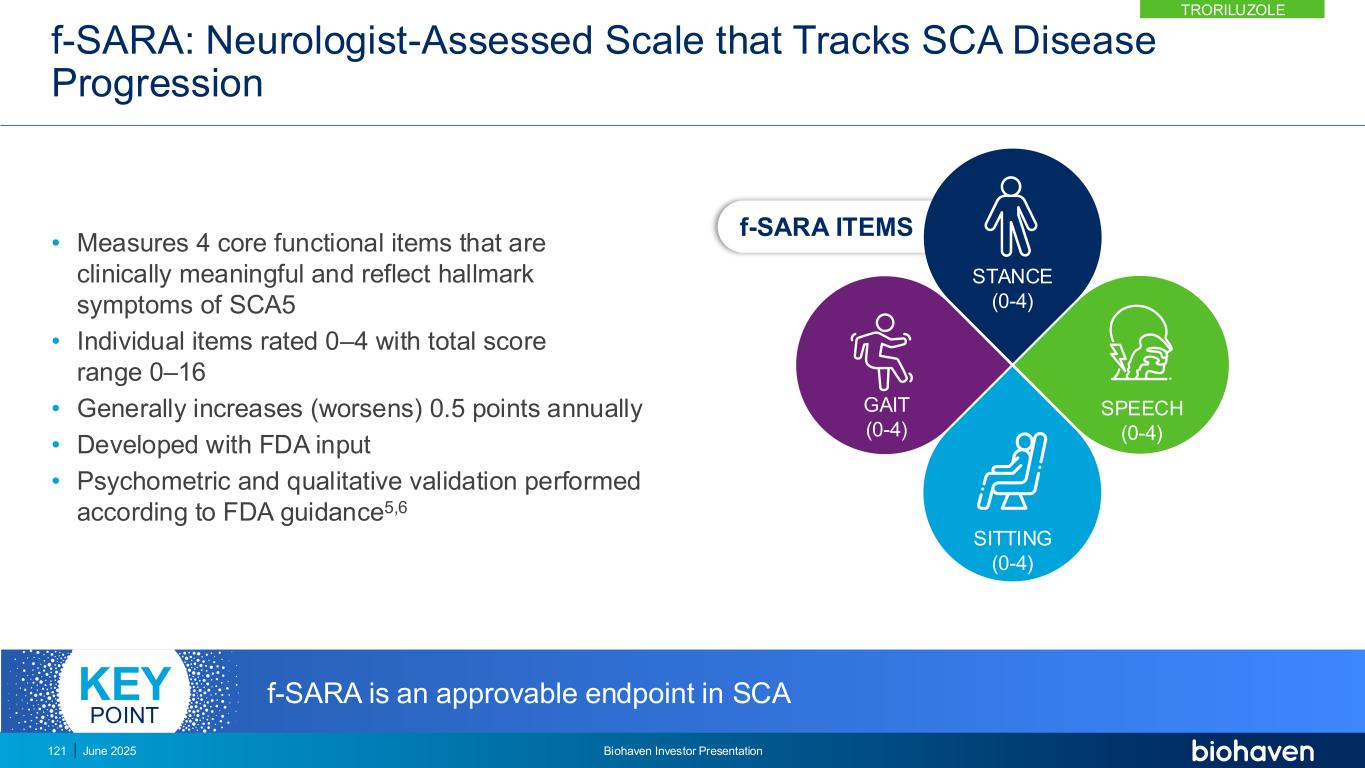
f-SARA: Neurologist-Assessed Scale that Tracks SCA Disease Progression • Measures 4 core functional items that are clinically meaningful and reflect hallmark symptoms of SCA5 • Individual items rated 0–4 with total score range 0–16 • Generally increases (worsens) 0.5 points annually • Developed with FDA input • Psychometric and qualitative validation performed according to FDA guidance5,6 f-SARA ITEMS GAIT (0-4) SITTING (0-4) SPEECH (0-4) STANCE (0-4) f-SARA is an approvable endpoint in SCAKEY POINT TRORILUZOLE June 2025 Biohaven Investor Presentation121

Troriluzole Restores Glutamate Homeostasis and Delays Disease Progression Across SCA Genotypes Clinically Meaningful Effect on f-SARA Strong Mechanistic Rationale Preclinical and clinical evidence for role of glutamate in SCA Reduction in Falls Falling is cardinal feature of SCAs leading to injury and reduced QoL Impact on Disease Progression Observed across the 8- year SCA development program Clear evidence for troriluzole benefit in SCA 122 June 2025 Biohaven Investor Presentation TRORILUZOLE
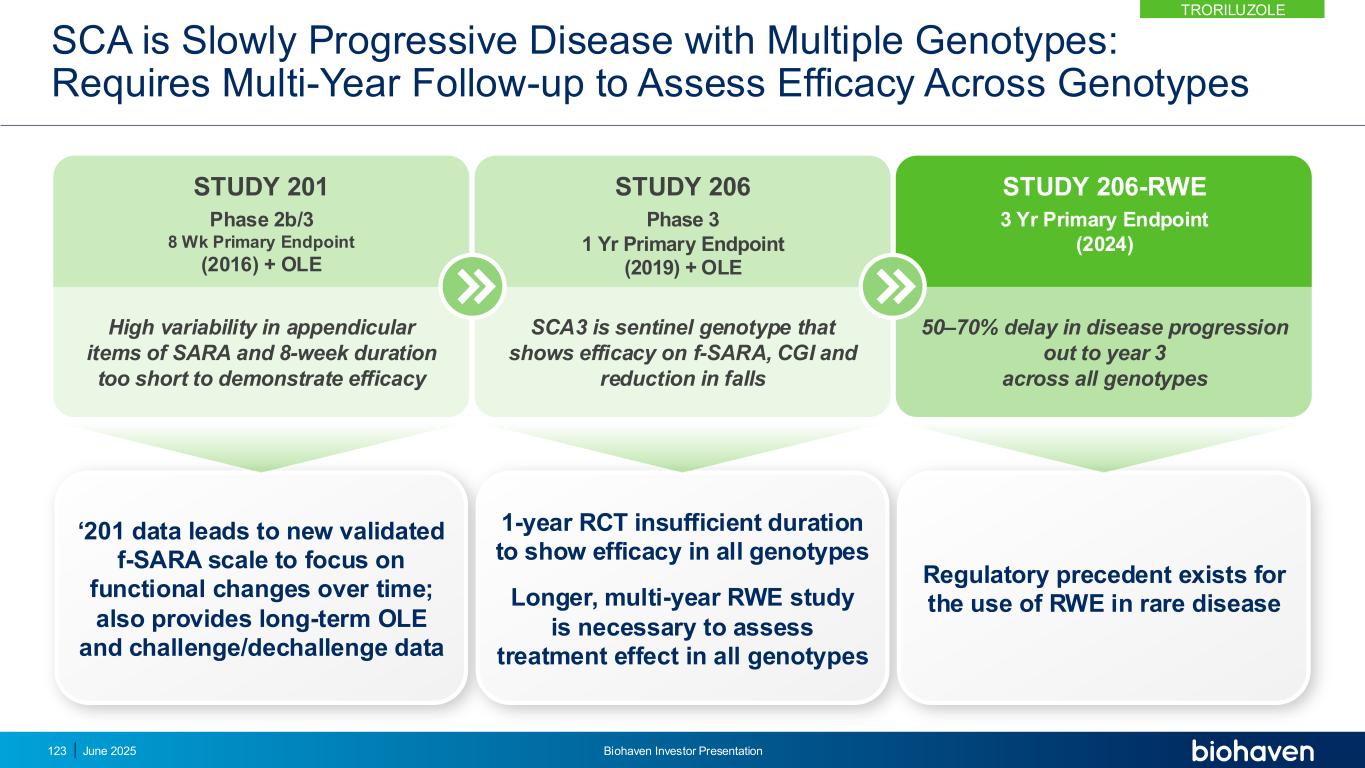
‘201 data leads to new validated f-SARA scale to focus on functional changes over time; also provides long-term OLE and challenge/dechallenge data 1-year RCT insufficient duration to show efficacy in all genotypes Longer, multi-year RWE study is necessary to assess treatment effect in all genotypes Regulatory precedent exists for the use of RWE in rare disease STUDY 201 Phase 2b/3 8 Wk Primary Endpoint (2016) + OLE STUDY 206 Phase 3 1 Yr Primary Endpoint (2019) + OLE STUDY 206-RWE 3 Yr Primary Endpoint (2024) High variability in appendicular items of SARA and 8-week duration too short to demonstrate efficacy SCA3 is sentinel genotype that shows efficacy on f-SARA, CGI and reduction in falls 50–70% delay in disease progression out to year 3 across all genotypes SCA is Slowly Progressive Disease with Multiple Genotypes: Requires Multi-Year Follow-up to Assess Efficacy Across Genotypes 123 June 2025 Biohaven Investor Presentation TRORILUZOLE
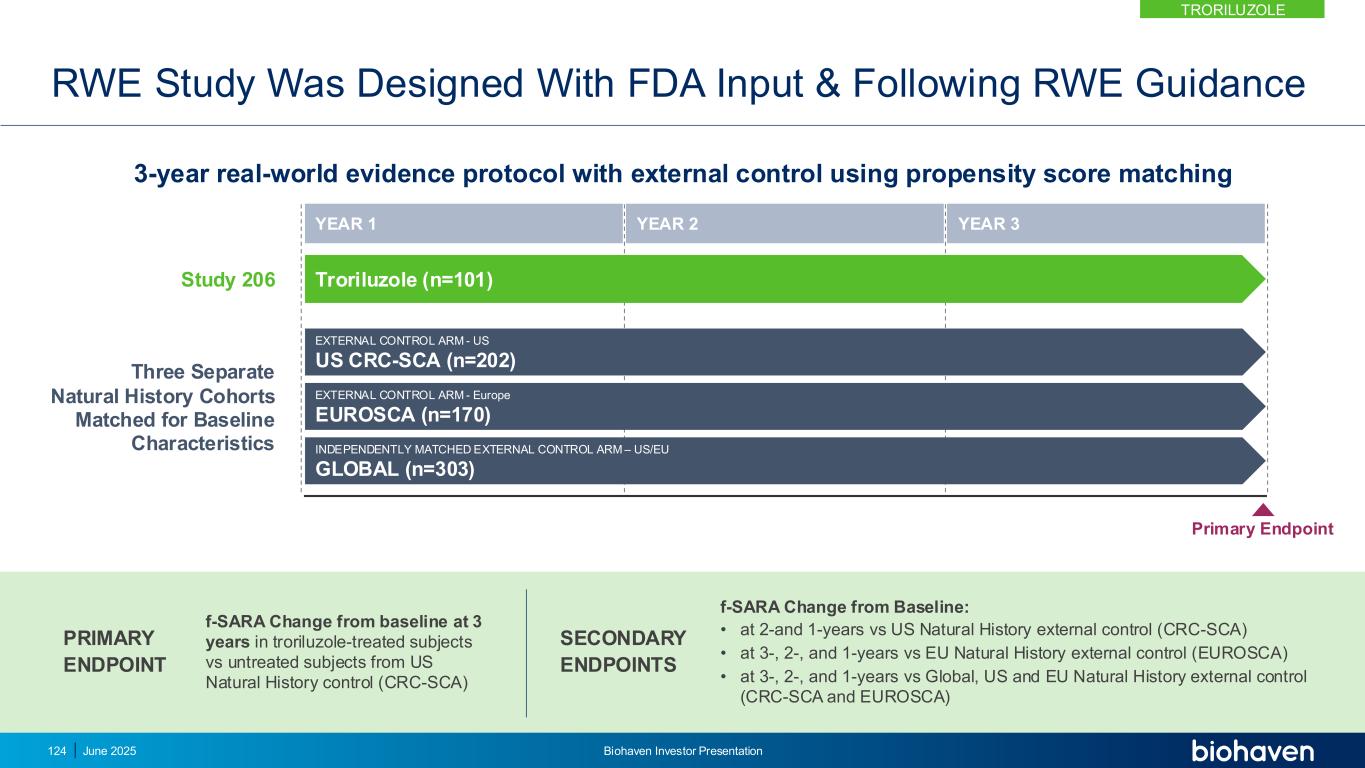
RWE Study Was Designed With FDA Input & Following RWE Guidance 3-year real-world evidence protocol with external control using propensity score matching YEAR 3YEAR 1 YEAR 2 Troriluzole (n=101)Study 206 EXTERNAL CONTROL ARM - US US CRC-SCA (n=202) EXTERNAL CONTROL ARM - Europe EUROSCA (n=170) INDEPENDENTLY MATCHED EXTERNAL CONTROL ARM – US/EU GLOBAL (n=303) Three Separate Natural History Cohorts Matched for Baseline Characteristics Primary Endpoint f-SARA Change from baseline at 3 years in troriluzole-treated subjects vs untreated subjects from US Natural History control (CRC-SCA) PRIMARY ENDPOINT f-SARA Change from Baseline: • at 2-and 1-years vs US Natural History external control (CRC-SCA) • at 3-, 2-, and 1-years vs EU Natural History external control (EUROSCA) • at 3-, 2-, and 1-years vs Global, US and EU Natural History external control (CRC-SCA and EUROSCA) SECONDARY ENDPOINTS 124 June 2025 Biohaven Investor Presentation TRORILUZOLE

RWE Study Designed With FDA Feedback to Reduce Bias Biohaven Proposal FDA Feedback BHV4157-206-RWE Protocol Analyze new data from subjects completing 3-years of treatment Follow Industry Guidance for RWE* Regulatory precedent for NDA approval based on RWE and external control for analysis of 3-year endpoint. Inclusion of new 3-year completers nearly doubles the sample size and thus increases power and precision as these data were not previously available Submit SAP for FDA review Submit both Protocol and SAP for FDA review prior to database lock Submitted Protocol and SAP to FDA with prespecified endpoints and analysis plan based on FDA input ahead of database lock; Biohaven adopted all feedback proposed by the FDA Use composite scale as primary outcome measure Use f-SARA as primary outcome measure f-SARA used as primary outcome measure; a reliable and validated scale to measure clinically meaningful change in function in SCA; designed with FDA input that is objective and minimizes effort dependence Use global SCA Natural History cohorts as external control Use US SCA Natural History cohort as external control for primary analysis Minimizes potential for bias by ensuring the Biohaven trial & US SCA Natural History study conducted by same sites/investigators, evaluating similar scales, over similar time period, with same population, on same standard of care treatment Use MAIC analysis as primary Use Propensity Score Matching (PSM) for primary analysis Minimizes potential for confounding bias by balancing baseline characteristics between treatment group and external control; Used in other NDAs leveraging RWE** Genetic risk factors not included in matching Match populations based on trinucleotide repeat length for primary analysis Minimizes potential for bias by further matching treatment group and external control based on additional genetic factors associated with disease burden *Guidance for Industry Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision Making for Drug and Biological Products https://www.fda.gov/media/171667/download **Lynch DR, et. al. Propensity matched comparison of omaveloxolone treatment to Friedreich ataxia natural history data. Ann Clin Transl Neurol. 2024 Jan;11(1):4-16 TRORILUZOLE June 2025 Biohaven Investor Presentation125
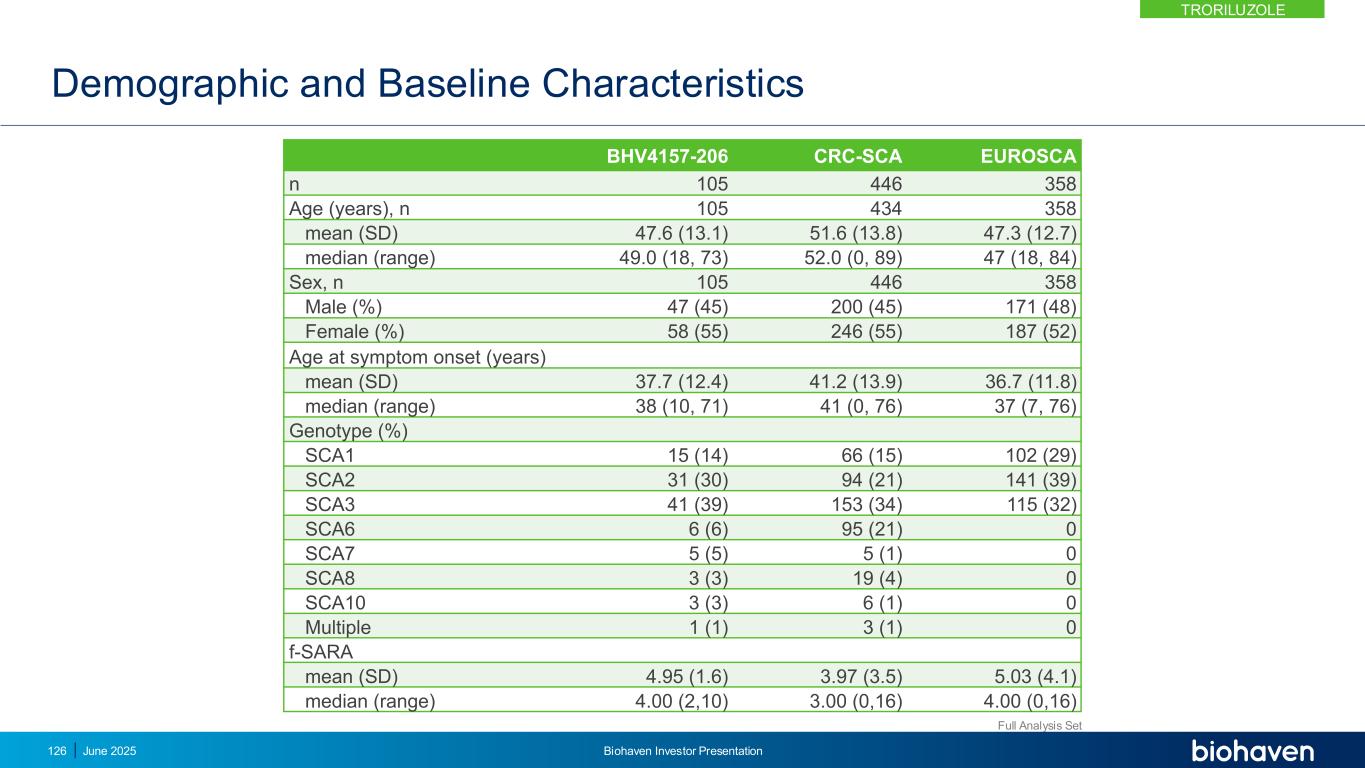
Demographic and Baseline Characteristics BHV4157-206 CRC-SCA EUROSCA n 105 446 358 Age (years), n 105 434 358 mean (SD) 47.6 (13.1) 51.6 (13.8) 47.3 (12.7) median (range) 49.0 (18, 73) 52.0 (0, 89) 47 (18, 84) Sex, n 105 446 358 Male (%) 47 (45) 200 (45) 171 (48) Female (%) 58 (55) 246 (55) 187 (52) Age at symptom onset (years) mean (SD) 37.7 (12.4) 41.2 (13.9) 36.7 (11.8) median (range) 38 (10, 71) 41 (0, 76) 37 (7, 76) Genotype (%) SCA1 15 (14) 66 (15) 102 (29) SCA2 31 (30) 94 (21) 141 (39) SCA3 41 (39) 153 (34) 115 (32) SCA6 6 (6) 95 (21) 0 SCA7 5 (5) 5 (1) 0 SCA8 3 (3) 19 (4) 0 SCA10 3 (3) 6 (1) 0 Multiple 1 (1) 3 (1) 0 f-SARA mean (SD) 4.95 (1.6) 3.97 (3.5) 5.03 (4.1) median (range) 4.00 (2,10) 3.00 (0,16) 4.00 (0,16) Full Analysis Set TRORILUZOLE June 2025 Biohaven Investor Presentation126
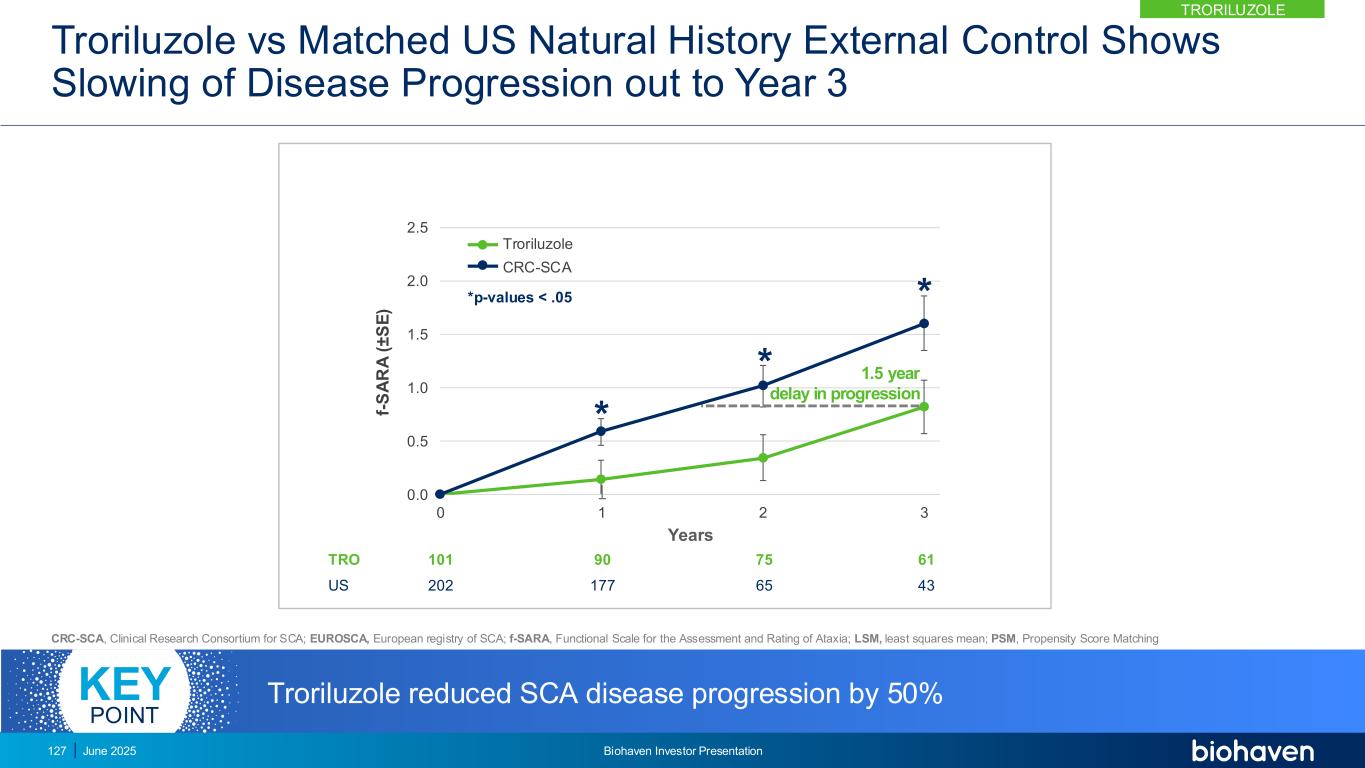
Troriluzole vs Matched US Natural History External Control Shows Slowing of Disease Progression out to Year 3 CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia; LSM, least squares mean; PSM, Propensity Score Matching 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years * **p-values < .05 Troriluzole CRC-SCA TRO US 101 202 90 177 75 65 61 43 * 1.5 year delay in progression Troriluzole reduced SCA disease progression by 50%KEY POINT TRORILUZOLE June 2025 Biohaven Investor Presentation127
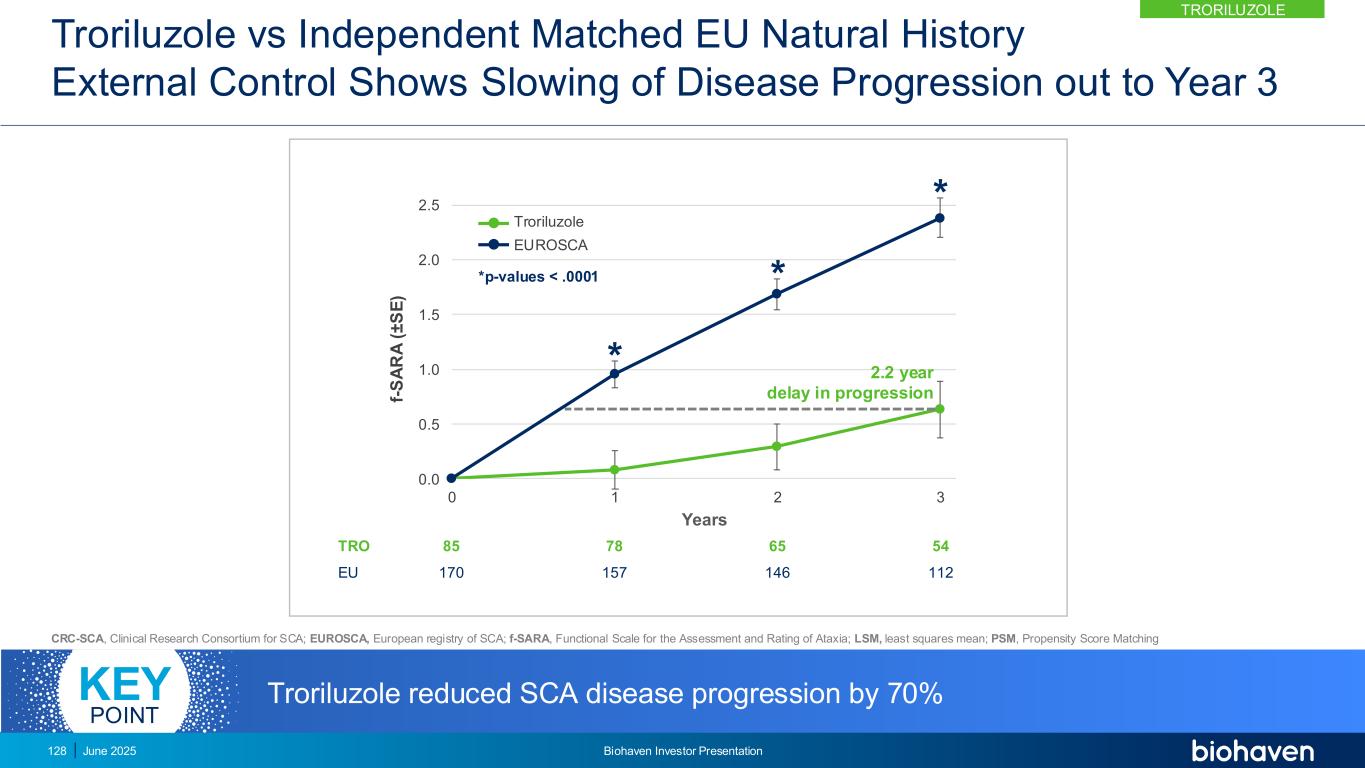
Troriluzole vs Independent Matched EU Natural History External Control Shows Slowing of Disease Progression out to Year 3 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years * * * TRO EU 85 170 78 157 65 146 54 112 *p-values < .0001 Troriluzole EUROSCA 2.2 year delay in progression Troriluzole reduced SCA disease progression by 70%KEY POINT TRORILUZOLE June 2025 Biohaven Investor Presentation128 CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia; LSM, least squares mean; PSM, Propensity Score Matching
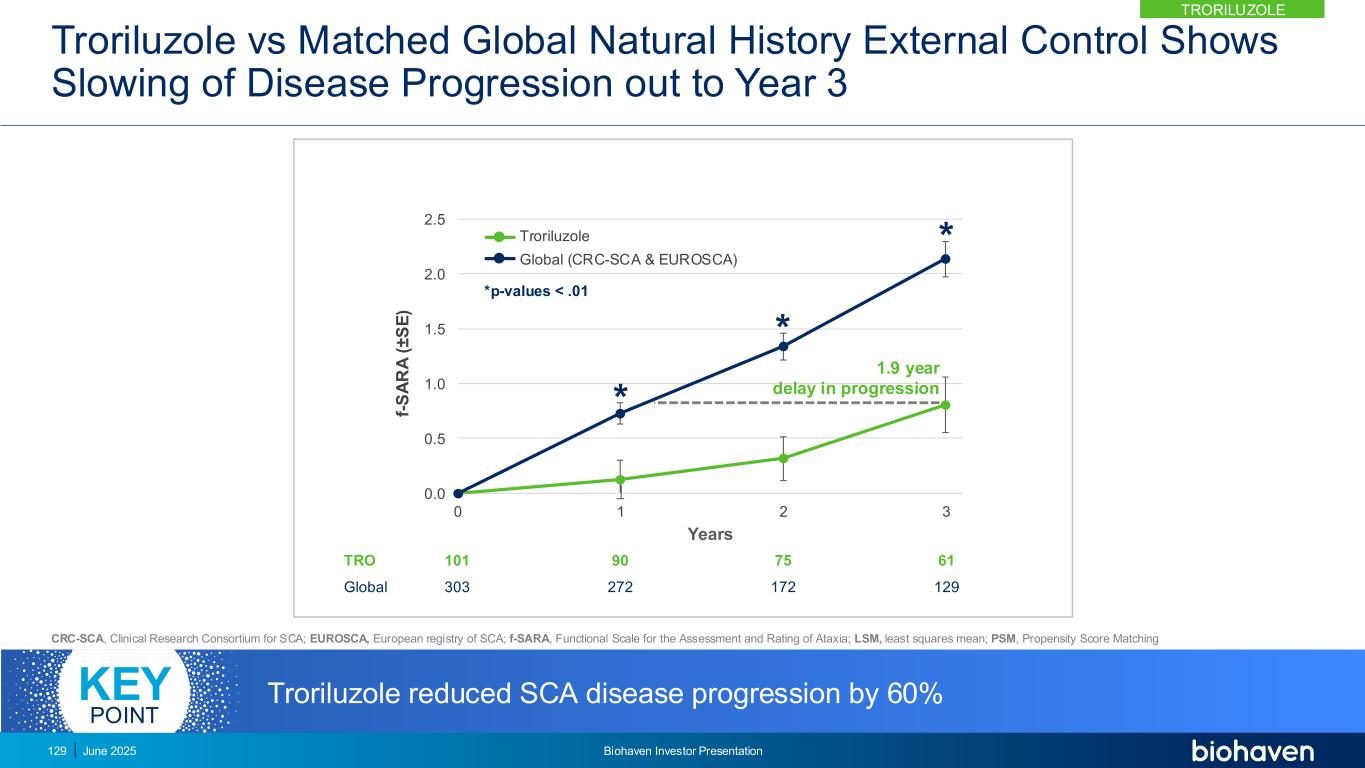
0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years * * * TRO Global 101 303 90 272 75 172 61 129 *p-values < .01 Troriluzole Global (CRC-SCA & EUROSCA) 1.9 year delay in progression Troriluzole reduced SCA disease progression by 60%KEY POINT TRORILUZOLE June 2025 Biohaven Investor Presentation129 Troriluzole vs Matched Global Natural History External Control Shows Slowing of Disease Progression out to Year 3 CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia; LSM, least squares mean; PSM, Propensity Score Matching
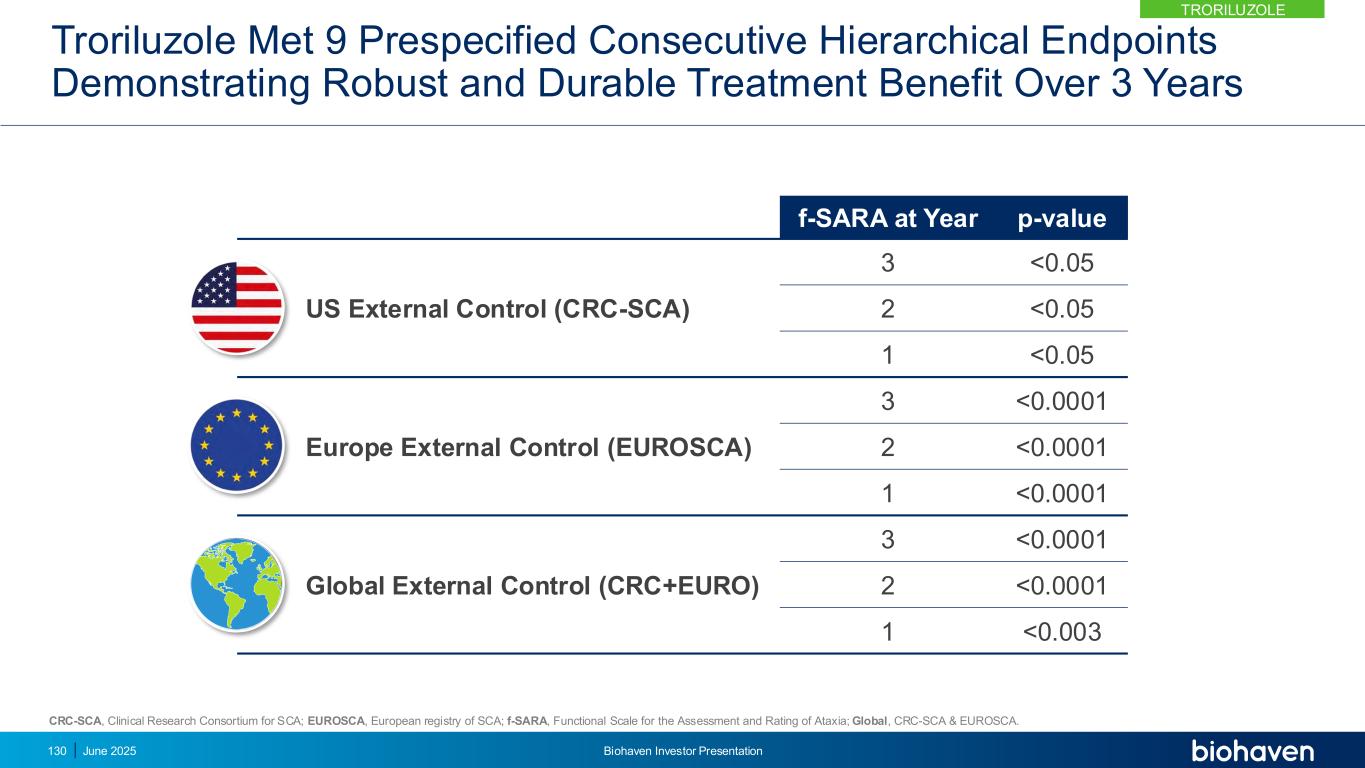
Troriluzole Met 9 Prespecified Consecutive Hierarchical Endpoints Demonstrating Robust and Durable Treatment Benefit Over 3 Years f-SARA at Year p-value US External Control (CRC-SCA) 3 <0.05 2 <0.05 1 <0.05 Europe External Control (EUROSCA) 3 <0.0001 2 <0.0001 1 <0.0001 Global External Control (CRC+EURO) 3 <0.0001 2 <0.0001 1 <0.003 CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia; Global, CRC-SCA & EUROSCA. 130 June 2025 Biohaven Investor Presentation TRORILUZOLE
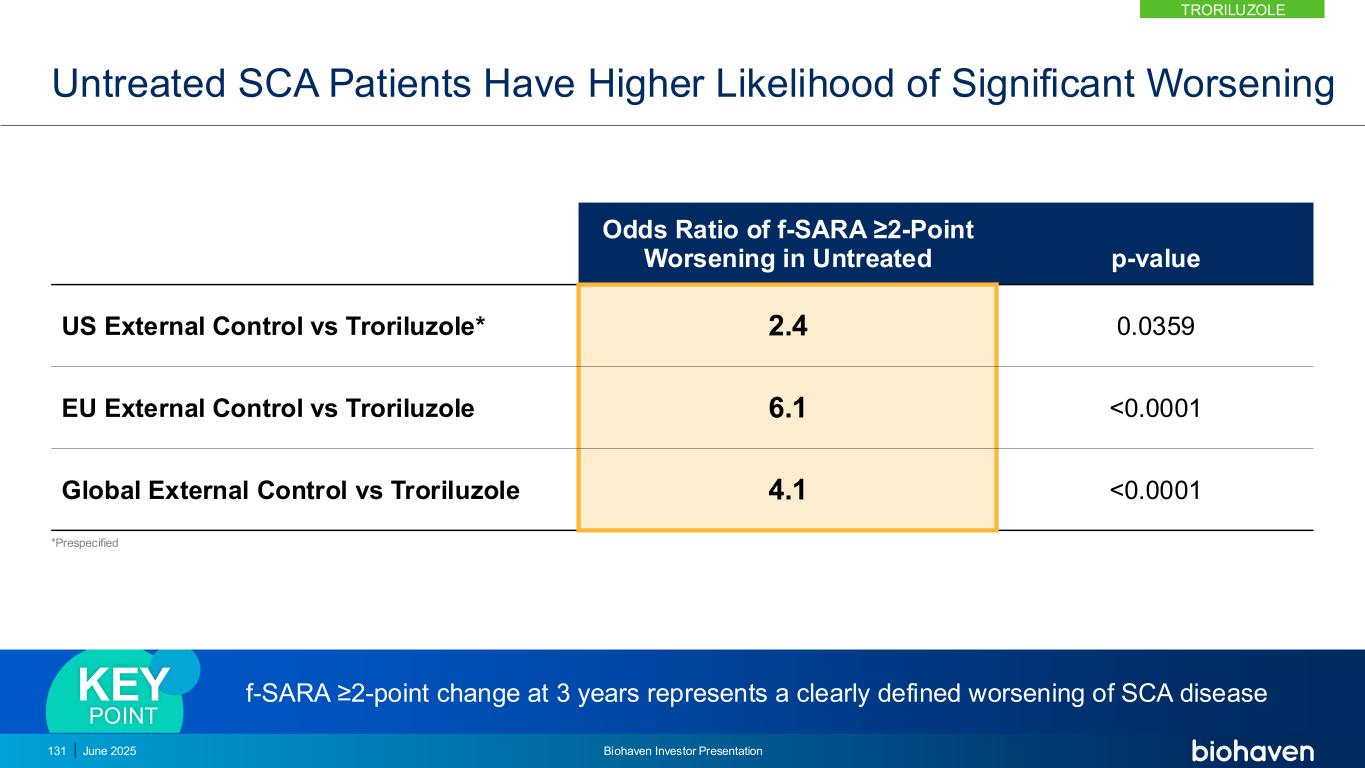
Untreated SCA Patients Have Higher Likelihood of Significant Worsening *Prespecified Odds Ratio of f-SARA ≥2-Point Worsening in Untreated p-value US External Control vs Troriluzole* 2.4 0.0359 EU External Control vs Troriluzole 6.1 <0.0001 Global External Control vs Troriluzole 4.1 <0.0001 f-SARA ≥2-point change at 3 years represents a clearly defined worsening of SCA diseaseKEY POINT 131 June 2025 Biohaven Investor Presentation TRORILUZOLE
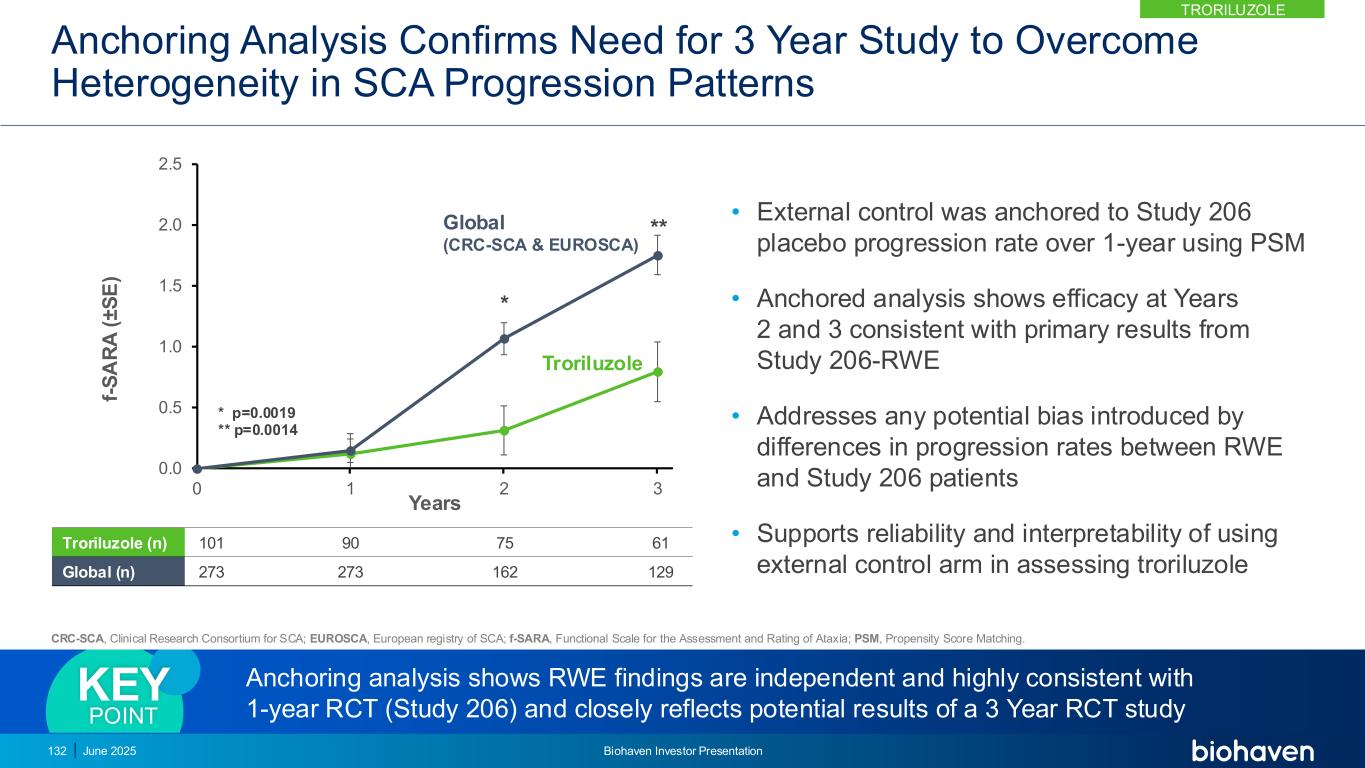
Anchoring Analysis Confirms Need for 3 Year Study to Overcome Heterogeneity in SCA Progression Patterns • External control was anchored to Study 206 placebo progression rate over 1-year using PSM • Anchored analysis shows efficacy at Years 2 and 3 consistent with primary results from Study 206-RWE • Addresses any potential bias introduced by differences in progression rates between RWE and Study 206 patients • Supports reliability and interpretability of using external control arm in assessing troriluzole 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years ** * Troriluzole (n) 101 90 75 61 Global (n) 273 273 162 129 * p=0.0019 ** p=0.0014 Global (CRC-SCA & EUROSCA) Troriluzole CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia; PSM, Propensity Score Matching. Anchoring analysis shows RWE findings are independent and highly consistent with 1-year RCT (Study 206) and closely reflects potential results of a 3 Year RCT study KEY POINT 132 June 2025 Biohaven Investor Presentation TRORILUZOLE
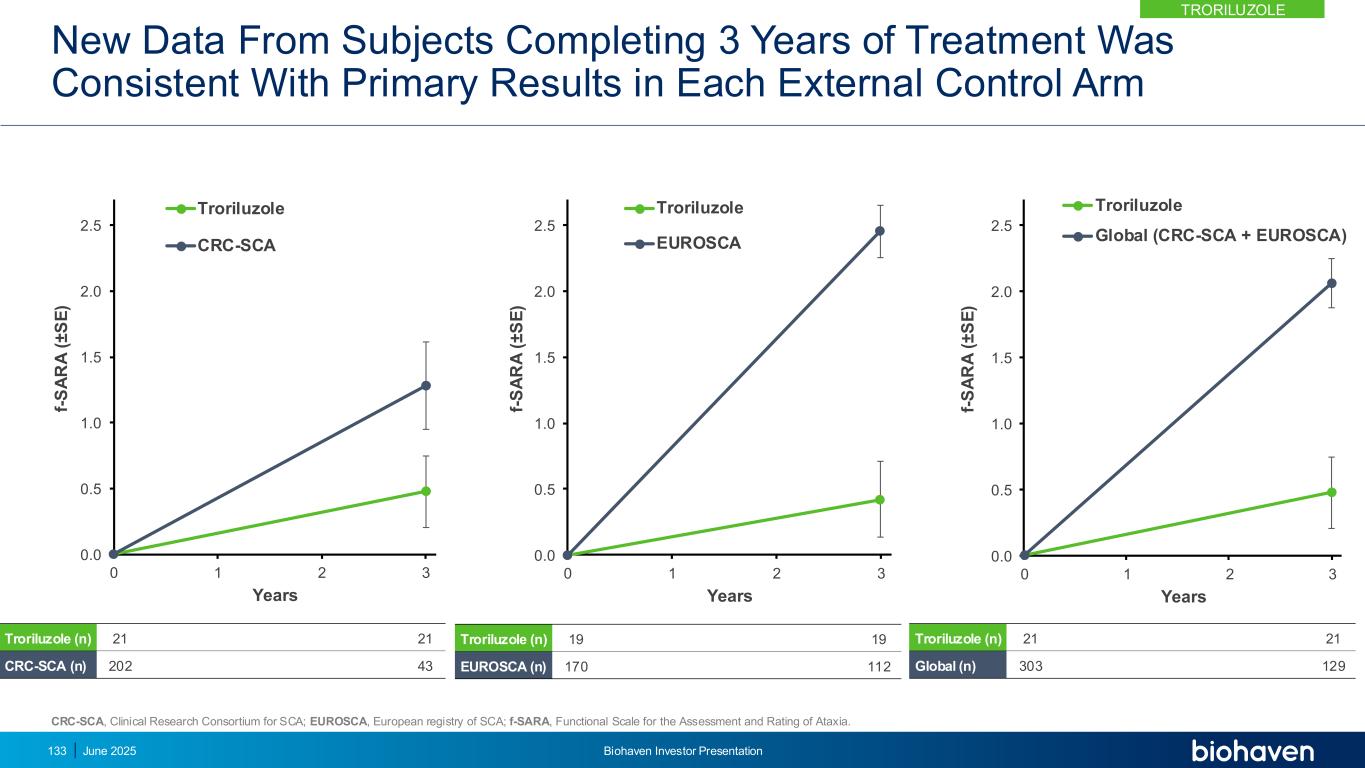
New Data From Subjects Completing 3 Years of Treatment Was Consistent With Primary Results in Each External Control Arm 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years Troriluzole CRC-SCA 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years Troriluzole EUROSCA 0.0 0.5 1.0 1.5 2.0 2.5 0 1 2 3 f- S A R A ( ± S E ) Years Troriluzole Global (CRC-SCA + EUROSCA) CRC-SCA, Clinical Research Consortium for SCA; EUROSCA, European registry of SCA; f-SARA, Functional Scale for the Assessment and Rating of Ataxia. 133 June 2025 Biohaven Investor Presentation Troriluzole (n) 21 21 CRC-SCA (n) 202 43 Troriluzole (n) 19 19 EUROSCA (n) 170 112 Troriluzole (n) 21 21 Global (n) 303 129 TRORILUZOLE
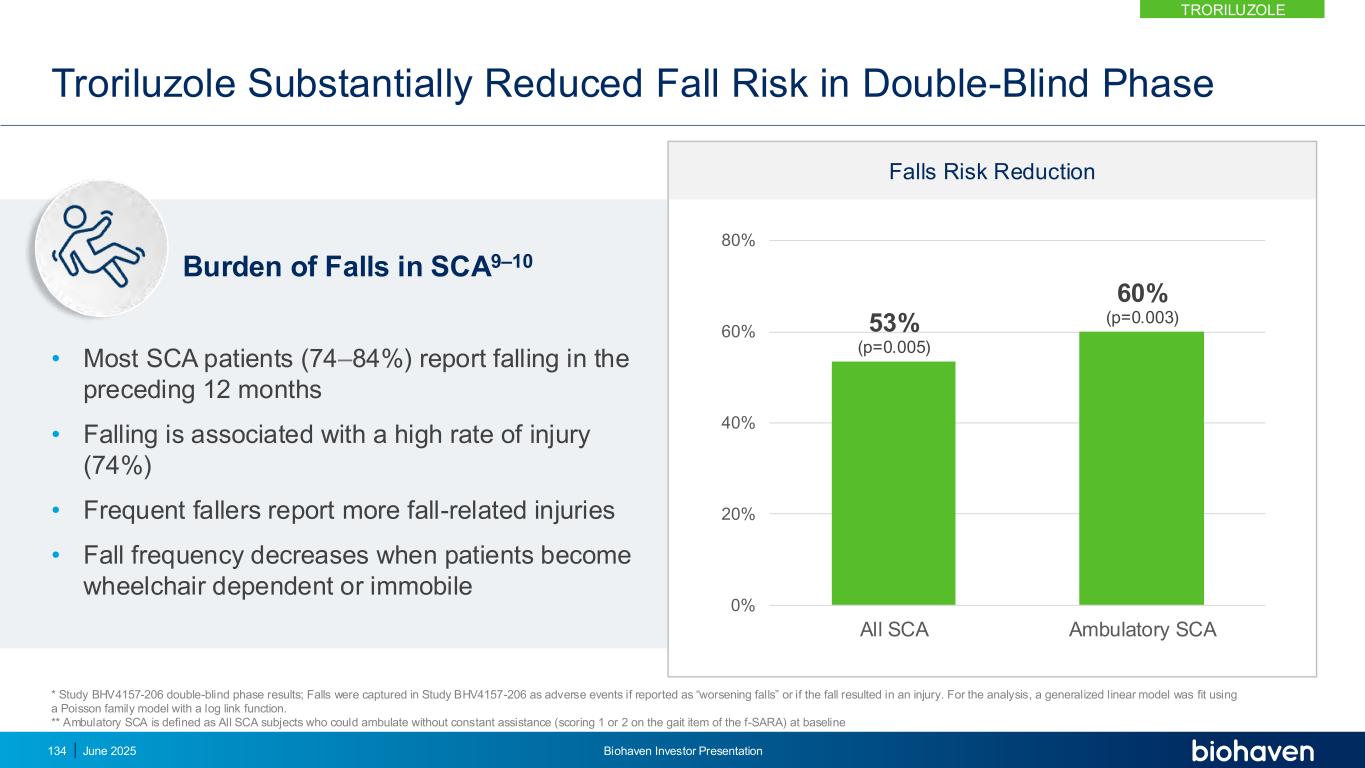
Burden of Falls in SCA9–10 • Most SCA patients (74–84%) report falling in the preceding 12 months • Falling is associated with a high rate of injury (74%) • Frequent fallers report more fall-related injuries • Fall frequency decreases when patients become wheelchair dependent or immobile Falls Risk Reduction Troriluzole Substantially Reduced Fall Risk in Double-Blind Phase * Study BHV4157-206 double-blind phase results; Falls were captured in Study BHV4157-206 as adverse events if reported as “worsening falls” or if the fall resulted in an injury. For the analysis, a generalized linear model was fit using a Poisson family model with a log link function. ** Ambulatory SCA is defined as All SCA subjects who could ambulate without constant assistance (scoring 1 or 2 on the gait item of the f-SARA) at baseline 53% (p=0.005) 60% (p=0.003) 0% 20% 40% 60% 80% All SCA Ambulatory SCA TRORILUZOLE June 2025 Biohaven Investor Presentation134
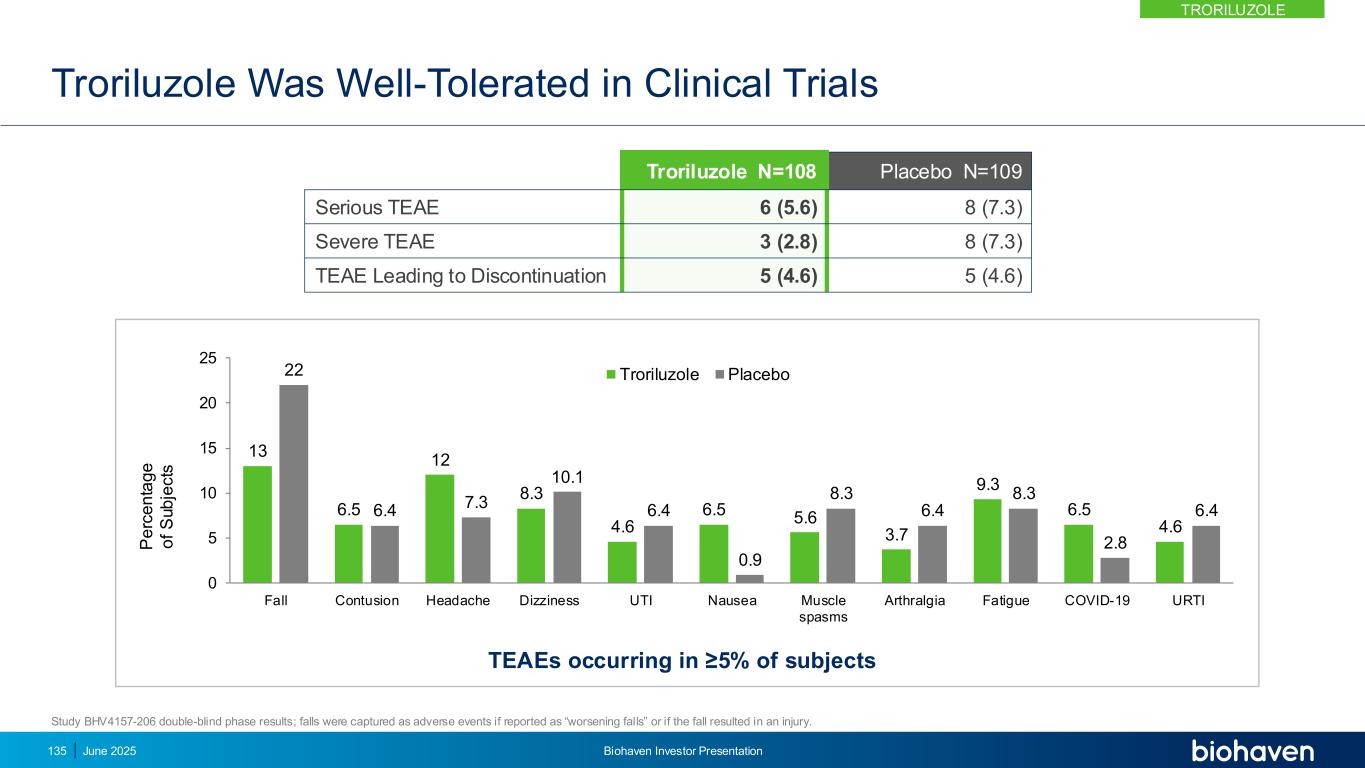
Troriluzole Was Well-Tolerated in Clinical Trials 13 6.5 12 8.3 4.6 6.5 5.6 3.7 9.3 6.5 4.6 22 6.4 7.3 10.1 6.4 0.9 8.3 6.4 8.3 2.8 6.4 0 5 10 15 20 25 Fall Contusion Headache Dizziness UTI Nausea Muscle spasms Arthralgia Fatigue COVID-19 URTI Troriluzole Placebo Troriluzole N=108 Placebo N=109 Serious TEAE 6 (5.6) 8 (7.3) Severe TEAE 3 (2.8) 8 (7.3) TEAE Leading to Discontinuation 5 (4.6) 5 (4.6) P e rc e n ta g e o f S u b je c ts TEAEs occurring in ≥5% of subjects Study BHV4157-206 double-blind phase results; falls were captured as adverse events if reported as “worsening falls” or if the fall resulted in an injury. TRORILUZOLE June 2025 Biohaven Investor Presentation135
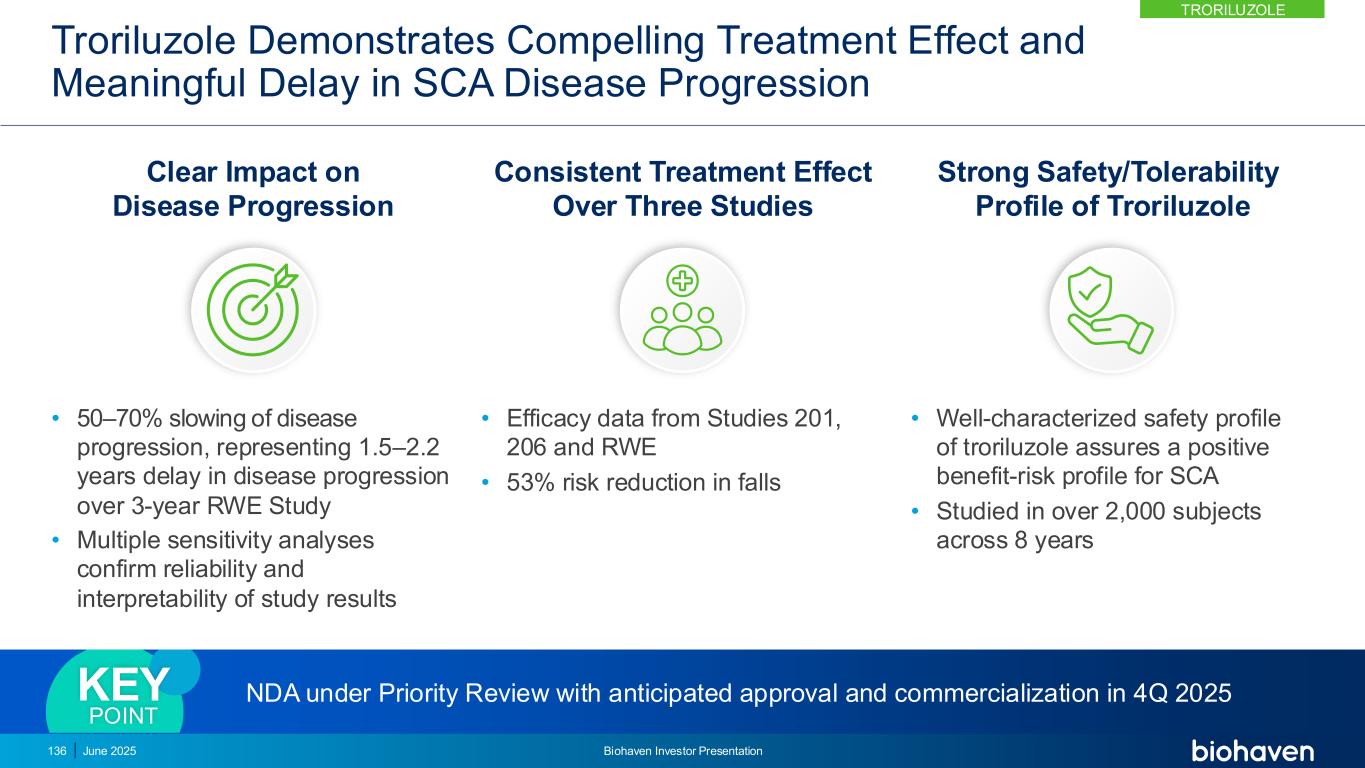
Troriluzole Demonstrates Compelling Treatment Effect and Meaningful Delay in SCA Disease Progression • Efficacy data from Studies 201, 206 and RWE • 53% risk reduction in falls • 50–70% slowing of disease progression, representing 1.5–2.2 years delay in disease progression over 3-year RWE Study • Multiple sensitivity analyses confirm reliability and interpretability of study results Consistent Treatment Effect Over Three Studies Clear Impact on Disease Progression • Well-characterized safety profile of troriluzole assures a positive benefit-risk profile for SCA • Studied in over 2,000 subjects across 8 years Strong Safety/Tolerability Profile of Troriluzole NDA under Priority Review with anticipated approval and commercialization in 4Q 2025KEY POINT 136 June 2025 Biohaven Investor Presentation TRORILUZOLE
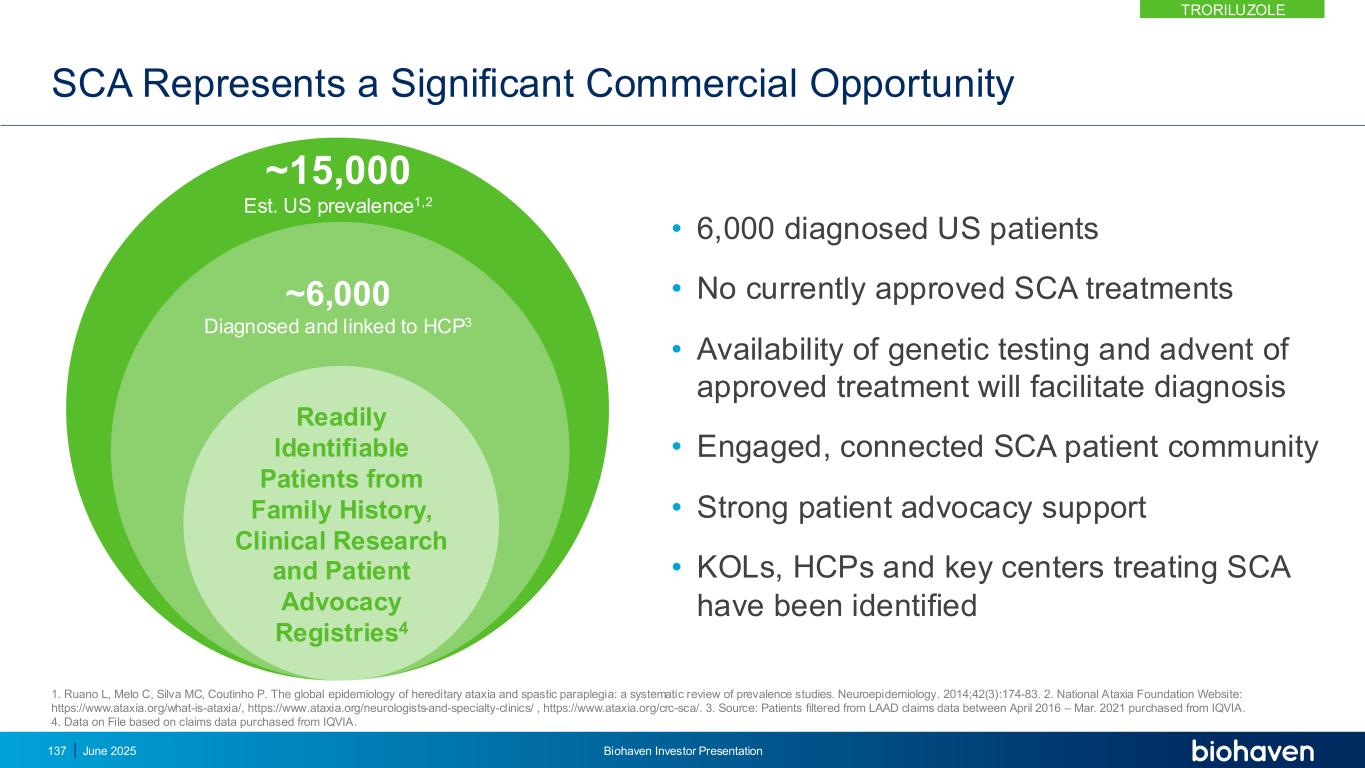
SCA Represents a Significant Commercial Opportunity • 6,000 diagnosed US patients • No currently approved SCA treatments • Availability of genetic testing and advent of approved treatment will facilitate diagnosis • Engaged, connected SCA patient community • Strong patient advocacy support • KOLs, HCPs and key centers treating SCA have been identified Readily Identifiable Patients from Family History, Clinical Research and Patient Advocacy Registries4 ~6,000 Diagnosed and linked to HCP3 ~15,000 Est. US prevalence1,2 1. Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174-83. 2. National Ataxia Foundation Website: https://www.ataxia.org/what-is-ataxia/, https://www.ataxia.org/neurologists-and-specialty-clinics/ , https://www.ataxia.org/crc-sca/. 3. Source: Patients filtered from LAAD claims data between April 2016 – Mar. 2021 purchased from IQVIA. 4. Data on File based on claims data purchased from IQVIA. TRORILUZOLE June 2025 Biohaven Investor Presentation137
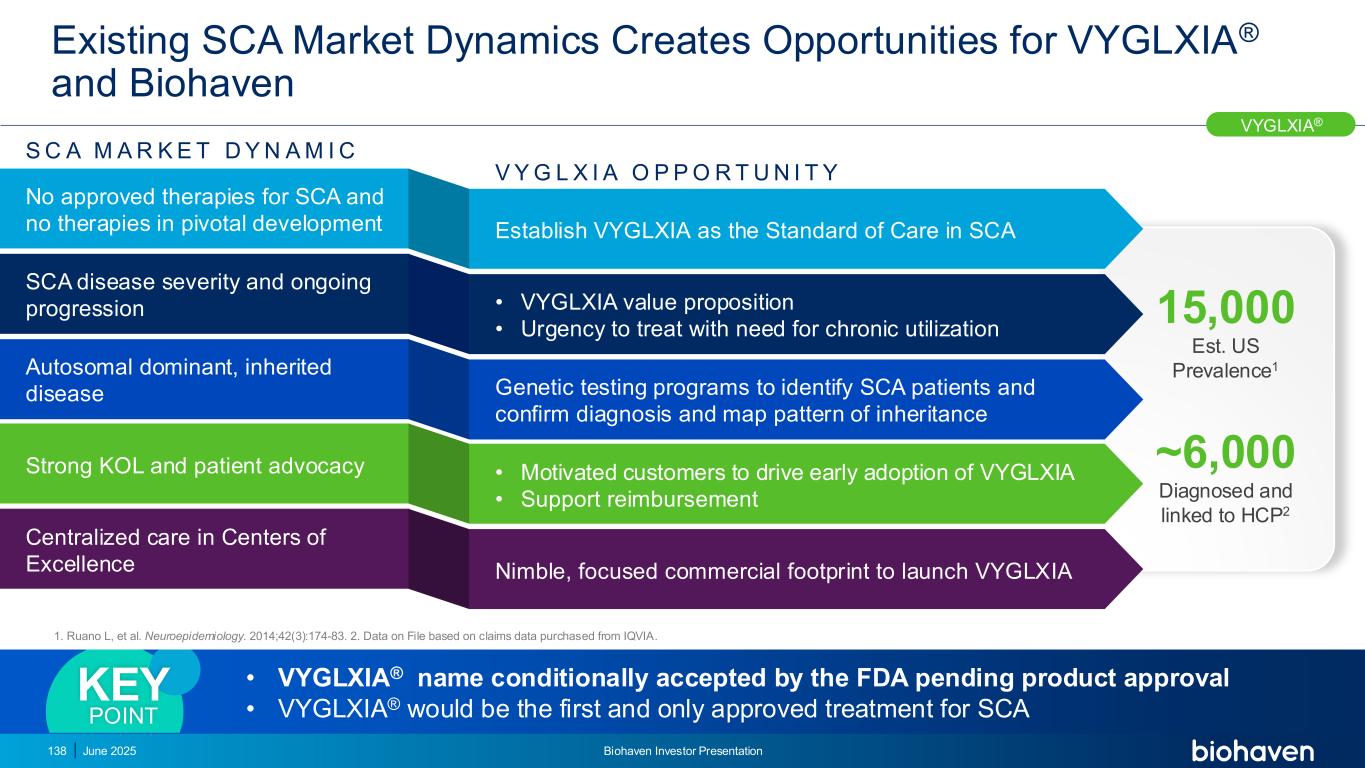
Existing SCA Market Dynamics Creates Opportunities for VYGLXIA® and Biohaven • VYGLXIA® name conditionally accepted by the FDA pending product approval • VYGLXIA® would be the first and only approved treatment for SCA KEY POINT No approved therapies for SCA and no therapies in pivotal development SCA disease severity and ongoing progression Autosomal dominant, inherited disease Strong KOL and patient advocacy Centralized care in Centers of Excellence Establish VYGLXIA as the Standard of Care in SCA • VYGLXIA value proposition • Urgency to treat with need for chronic utilization Genetic testing programs to identify SCA patients and confirm diagnosis and map pattern of inheritance • Motivated customers to drive early adoption of VYGLXIA • Support reimbursement Nimble, focused commercial footprint to launch VYGLXIA S C A M A R K E T D Y N A M I C V Y G L X I A O P P O R T U N I T Y VYGLXIA® ~6,000 Diagnosed and linked to HCP2 15,000 Est. US Prevalence1 1. Ruano L, et al. Neuroepidemiology. 2014;42(3):174-83. 2. Data on File based on claims data purchased from IQVIA. 138 June 2025 Biohaven Investor Presentation

Strategic Imperatives to Drive Successful Launch in SCA Identify patients, drive early diagnosis Establish VYGLXIA® as SOC in SCA Create access and reimbursement Ensure ongoing treatment 01 02 03 04 SCA Launch Priorities SCA launch relies on identifying patients and driving diagnosisKEY POINT VYGLXIA® 139 June 2025 Biohaven Investor Presentation
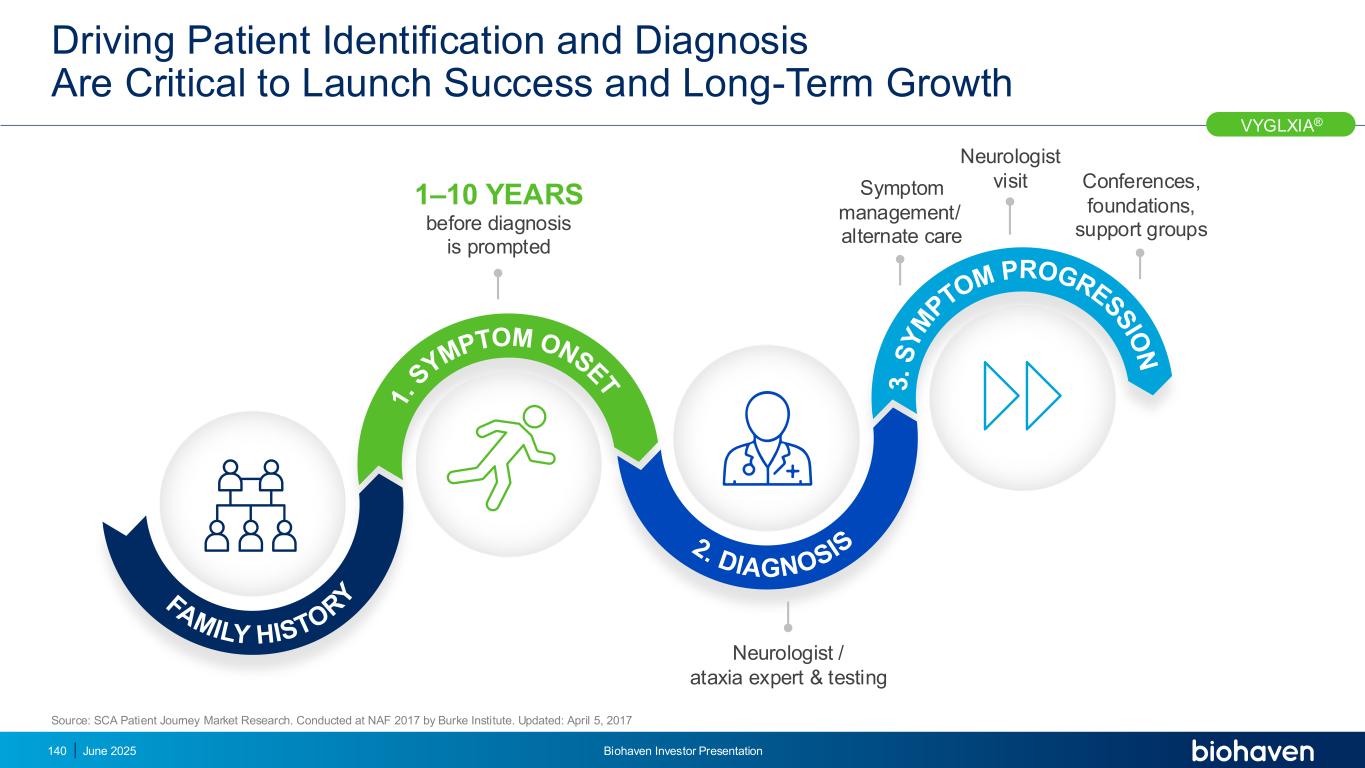
Driving Patient Identification and Diagnosis Are Critical to Launch Success and Long-Term Growth FAMILY HISTOR Y 2. DIAGNOSIS 3 . S Y M PTOM PROGRESS IO N 1. SYMPTOM ONSET 1–10 YEARS before diagnosis is prompted Neurologist / ataxia expert & testing Symptom management/ alternate care Neurologist visit Conferences, foundations, support groups Source: SCA Patient Journey Market Research. Conducted at NAF 2017 by Burke Institute. Updated: April 5, 2017 VYGLXIA® 140 June 2025 Biohaven Investor Presentation
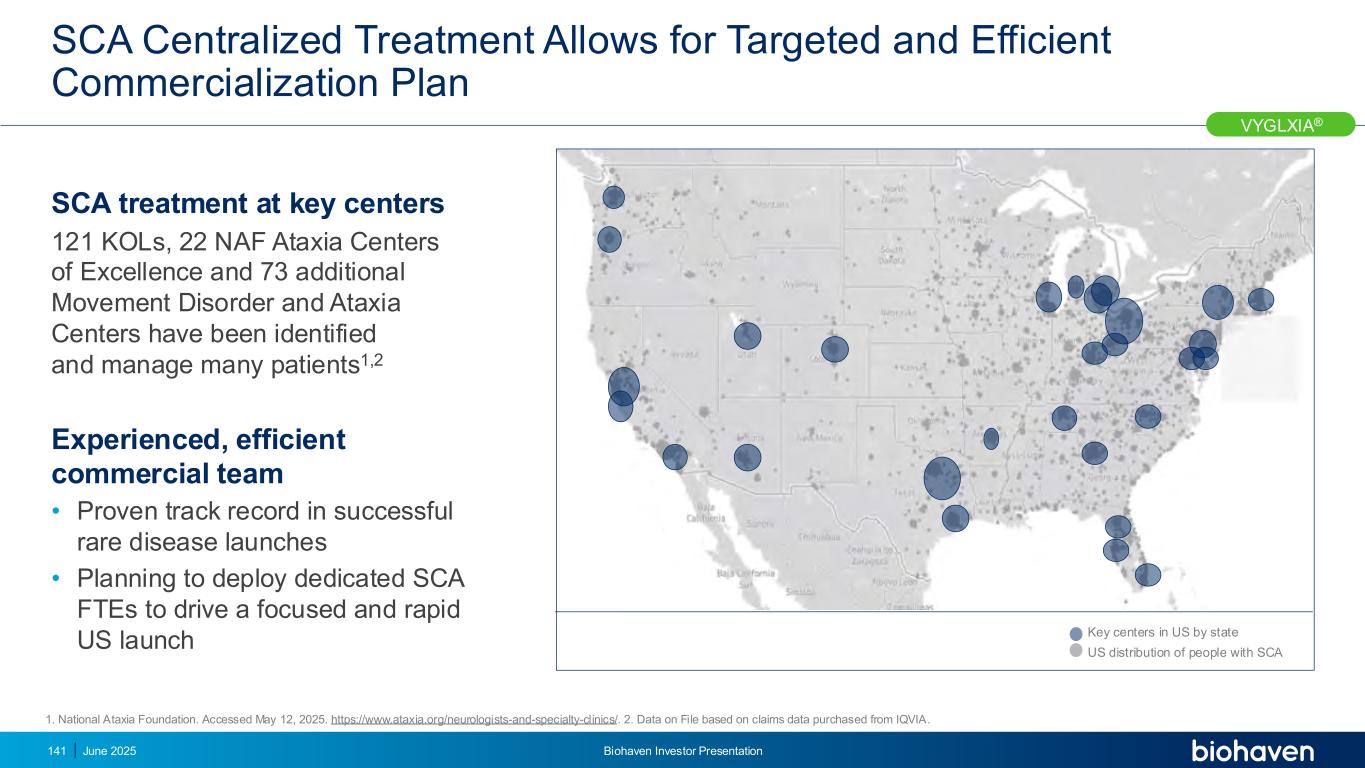
SCA Centralized Treatment Allows for Targeted and Efficient Commercialization Plan June 2025 Biohaven Investor Presentation141 Key centers in US by state US distribution of people with SCA 1. National Ataxia Foundation. Accessed May 12, 2025. https://www.ataxia.org/neurologists-and-specialty-clinics/. 2. Data on File based on claims data purchased from IQVIA. VYGLXIA® SCA treatment at key centers 121 KOLs, 22 NAF Ataxia Centers of Excellence and 73 additional Movement Disorder and Ataxia Centers have been identified and manage many patients1,2 Experienced, efficient commercial team • Proven track record in successful rare disease launches • Planning to deploy dedicated SCA FTEs to drive a focused and rapid US launch
VYGLXIA® in SCA Represents a Significant Commercial Opportunity • HUB will be a central point of contact for the SCA community • Strive to ensure all SCA patients will have access to VYGLXIA® • High-volume ataxia experts and people with SCA interested or involved in clinical research programs/registries • People already diagnosed with SCA and their families Biohaven's HUB will facilitate access to VYGLXIA® Readily identifiable patients will drive initial uptake • Focused initiatives that drive patient ID and early diagnosis strategy are critical • Targeted commercialization plan includes experienced rare disease leaders Patient ID and early diagnosis fuel long-term growth If approved, the Biohaven team will be prepared to serve people living with SCAKEY POINT 142 June 2025 Biohaven Investor Presentation
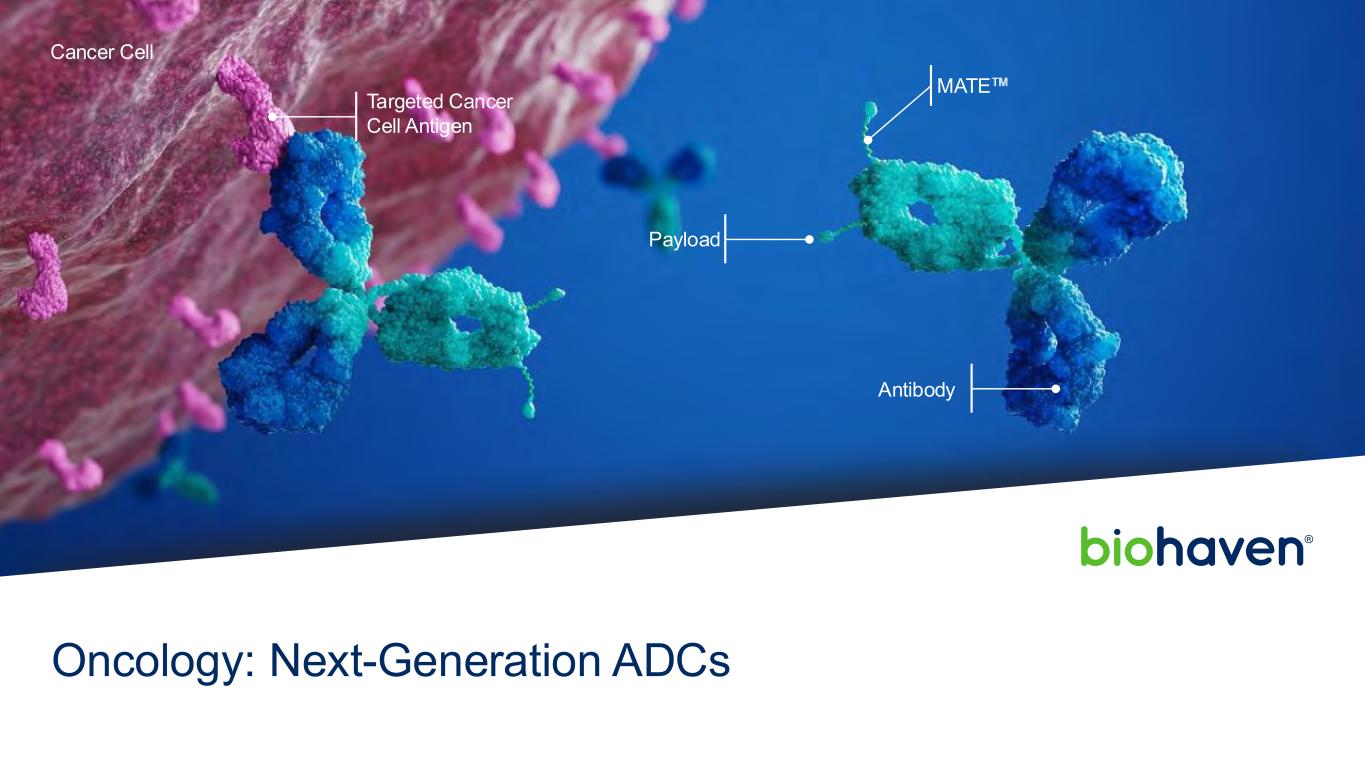
Antibody Cancer Cell MATE Payload Targeted Cancer Cell Antigen Oncology: Next-Generation ADCs
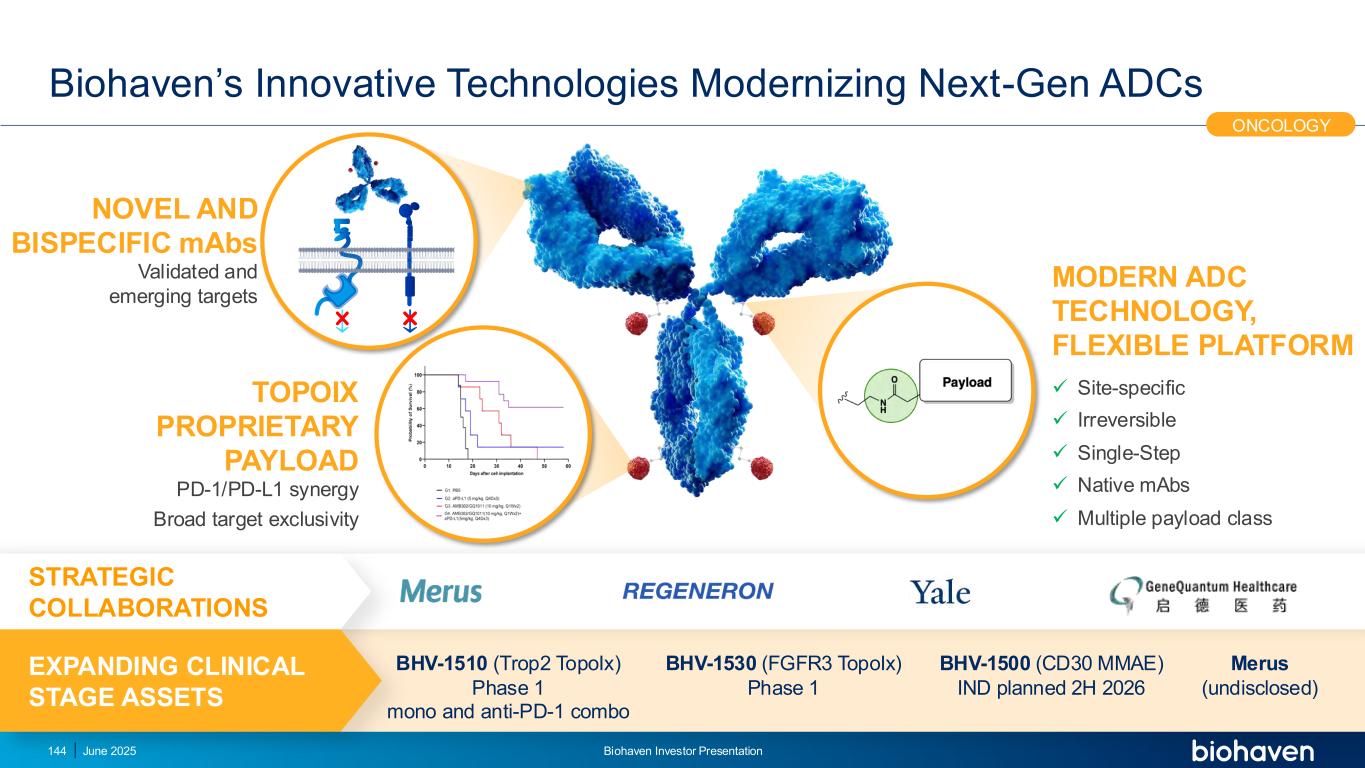
Merus (undisclosed) STRATEGIC COLLABORATIONS Biohaven’s Innovative Technologies Modernizing Next-Gen ADCs June 2025 Biohaven Investor Presentation144 BHV-1510 (Trop2 TopoIx) Phase 1 mono and anti-PD-1 combo BHV-1500 (CD30 MMAE) IND planned 2H 2026 BHV-1530 (FGFR3 TopoIx) Phase 1 EXPANDING CLINICAL STAGE ASSETS MODERN ADC TECHNOLOGY, FLEXIBLE PLATFORM ✓ Site-specific ✓ Irreversible ✓ Single-Step ✓ Native mAbs ✓ Multiple payload class NOVEL AND BISPECIFIC mAbs Validated and emerging targets TOPOIX PROPRIETARY PAYLOAD PD-1/PD-L1 synergy Broad target exclusivity ONCOLOGY

BHV-1510 Is a Highly Differentiated Trop2 ADC • Site-specific, highly stable conjugation-linker • TopoIx payload ideally positions for fast-to-market strategy with anti-PD-1 combo • Remains competitive as fast-follower Emerging Clinical Data Shows Predicted Profile and Potential of Proprietary TopoIx Payload • Clinical activity demonstrated as monotherapy and in combination with anti-PD-1 cemiplimab • No interstitial lung disease (ILD) with TopoIx observed in early cohorts • Target exclusivity of TopoIx payload for up to 18 ADCs June 2025 Biohaven Investor Presentation145 BHV-1510 TROP2ADC
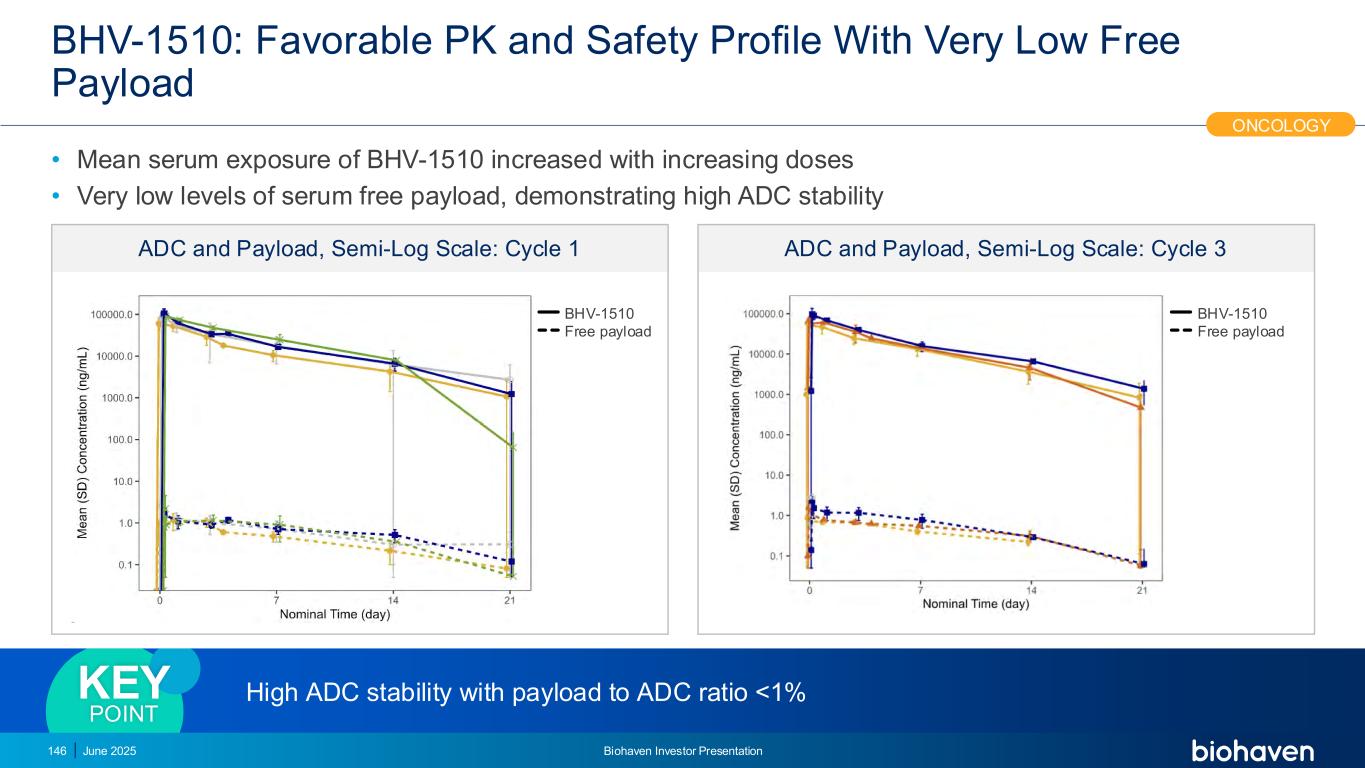
BHV-1510: Favorable PK and Safety Profile With Very Low Free Payload • Mean serum exposure of BHV-1510 increased with increasing doses • Very low levels of serum free payload, demonstrating high ADC stability June 2025 Biohaven Investor Presentation146 Prelim data from ongoing study ADC and Payload, Semi-Log Scale: Cycle 1 ADC and Payload, Semi-Log Scale: Cycle 3 ONCOLOGY BHV-1510 Free payload BHV-1510 Free payload High ADC stability with payload to ADC ratio <1%KEY POINT
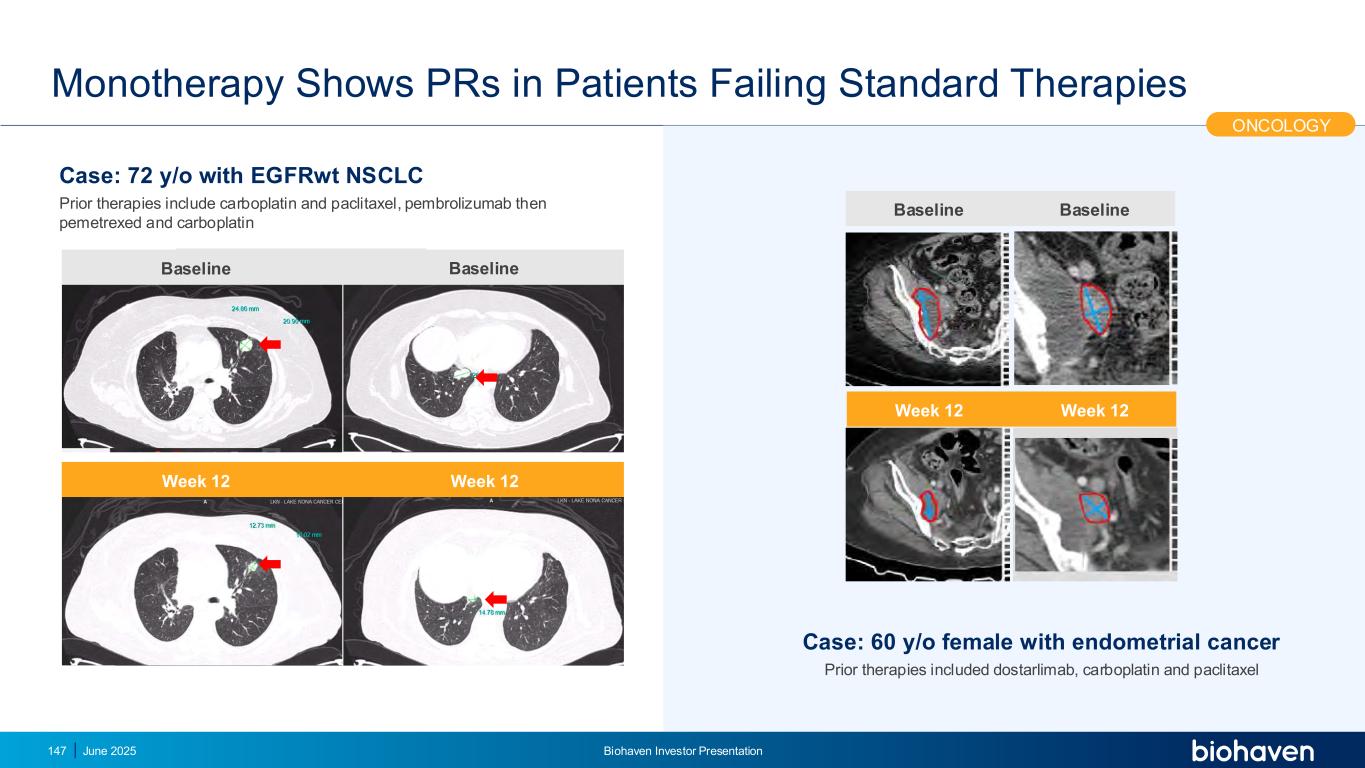
- Monotherapy Shows PRs in Patients Failing Standard Therapies June 2025 Biohaven Investor Presentation147 Case: 60 y/o female with endometrial cancer Prior therapies included dostarlimab, carboplatin and paclitaxel ONCOLOGY Case: 72 y/o with EGFRwt NSCLC Prior therapies include carboplatin and paclitaxel, pembrolizumab then pemetrexed and carboplatin Week 12 Baseline Week 12 Baseline BaselineBaseline Week 12Week 12

BHV-1510 Combination With Anti-PD-1
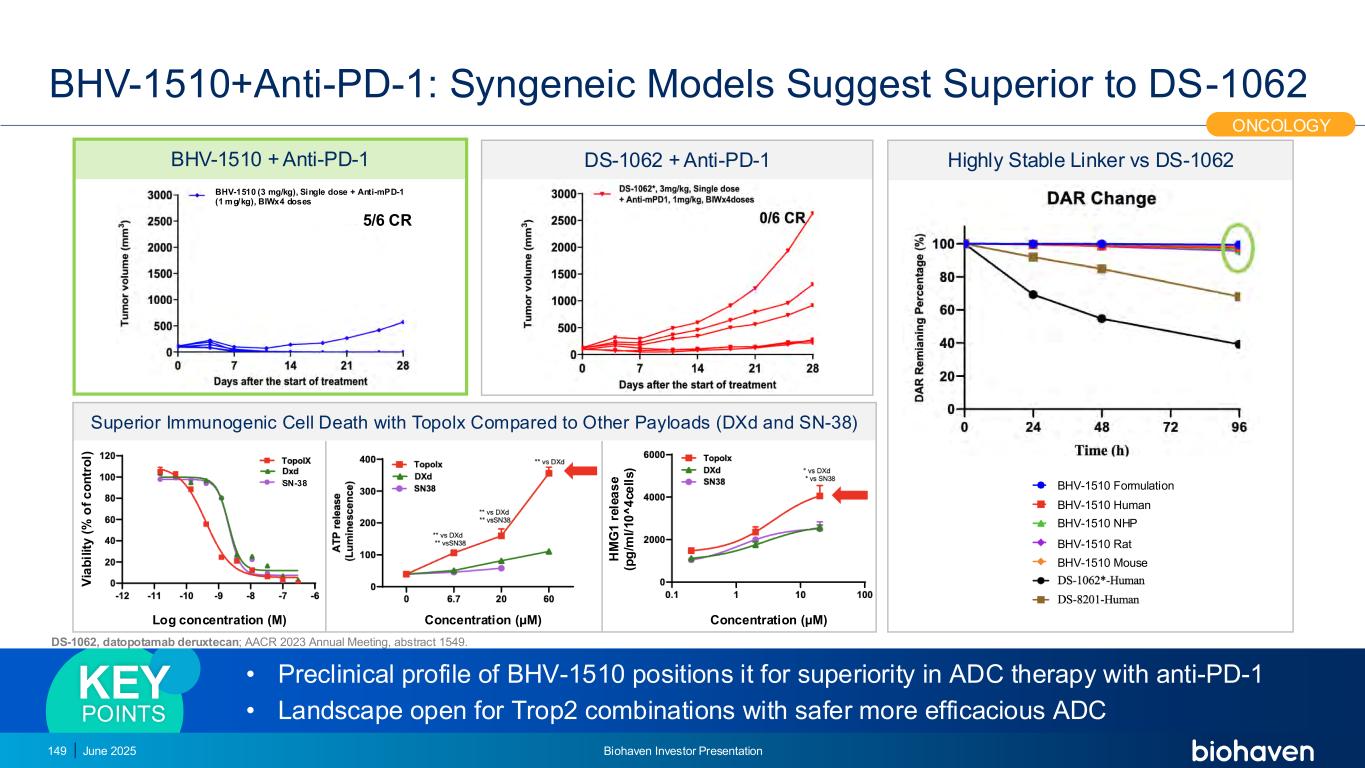
SN-38 ** vs DXd ** vs SN-38 Concentration (µM) H M G 1 r e le a s e (p g /m l/ 1 0 ^ 4 c e ll s ) Concentration (µM) A T P r e le a s e (L u m in e s c e n c e ) SN-38 ** vs DXd ** vs SN-38 ** vs DXd ** vs SN-38 ** vs DXd BHV-1510+Anti-PD-1: Syngeneic Models Suggest Superior to DS-1062 June 2025 Biohaven Investor Presentation149 DS-1062, datopotamab deruxtecan; AACR 2023 Annual Meeting, abstract 1549. V ia b il it y ( % o f c o n tr o l) Log concentration (M) SN-38 BHV-1510 + Anti-PD-1 DS-1062 + Anti-PD-1 BHV-1510 (3 mg/kg), Single dose + Anti-mPD-1 (1 mg/kg), BIWx4 doses • Preclinical profile of BHV-1510 positions it for superiority in ADC therapy with anti-PD-1 • Landscape open for Trop2 combinations with safer more efficacious ADC KEY POINTS ONCOLOGY Highly Stable Linker vs DS-1062 Superior Immunogenic Cell Death with Topolx Compared to Other Payloads (DXd and SN-38) 5/6 CR BHV-1510 Formulation BHV-1510 Human BHV-1510 NHP BHV-1510 Rat BHV-1510 Mouse
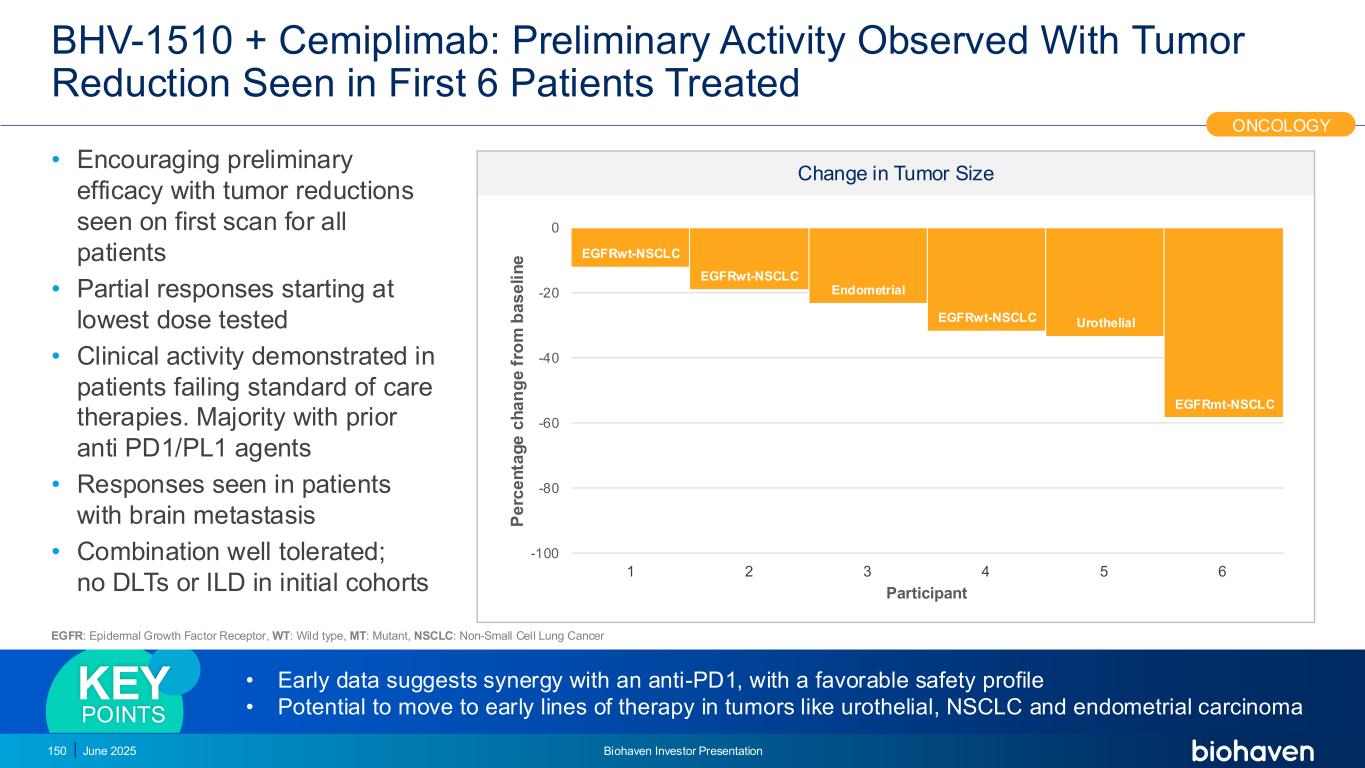
Change in Tumor Size BHV-1510 + Cemiplimab: Preliminary Activity Observed With Tumor Reduction Seen in First 6 Patients Treated • Encouraging preliminary efficacy with tumor reductions seen on first scan for all patients • Partial responses starting at lowest dose tested • Clinical activity demonstrated in patients failing standard of care therapies. Majority with prior anti PD1/PL1 agents • Responses seen in patients with brain metastasis • Combination well tolerated; no DLTs or ILD in initial cohorts Biohaven Investor Presentation150 ONCOLOGY EGFRwt-NSCLC EGFRwt-NSCLC Endometrial EGFRwt-NSCLC Urothelial EGFRmt-NSCLC -100 -80 -60 -40 -20 0 P e rc e n ta g e c h a n g e f ro m b a s e li n e EGFR: Epidermal Growth Factor Receptor, WT: Wild type, MT: Mutant, NSCLC: Non-Small Cell Lung Cancer • Early data suggests synergy with an anti-PD1, with a favorable safety profile • Potential to move to early lines of therapy in tumors like urothelial, NSCLC and endometrial carcinoma KEY POINTS June 2025 Participant 1 2 3 4 5 6
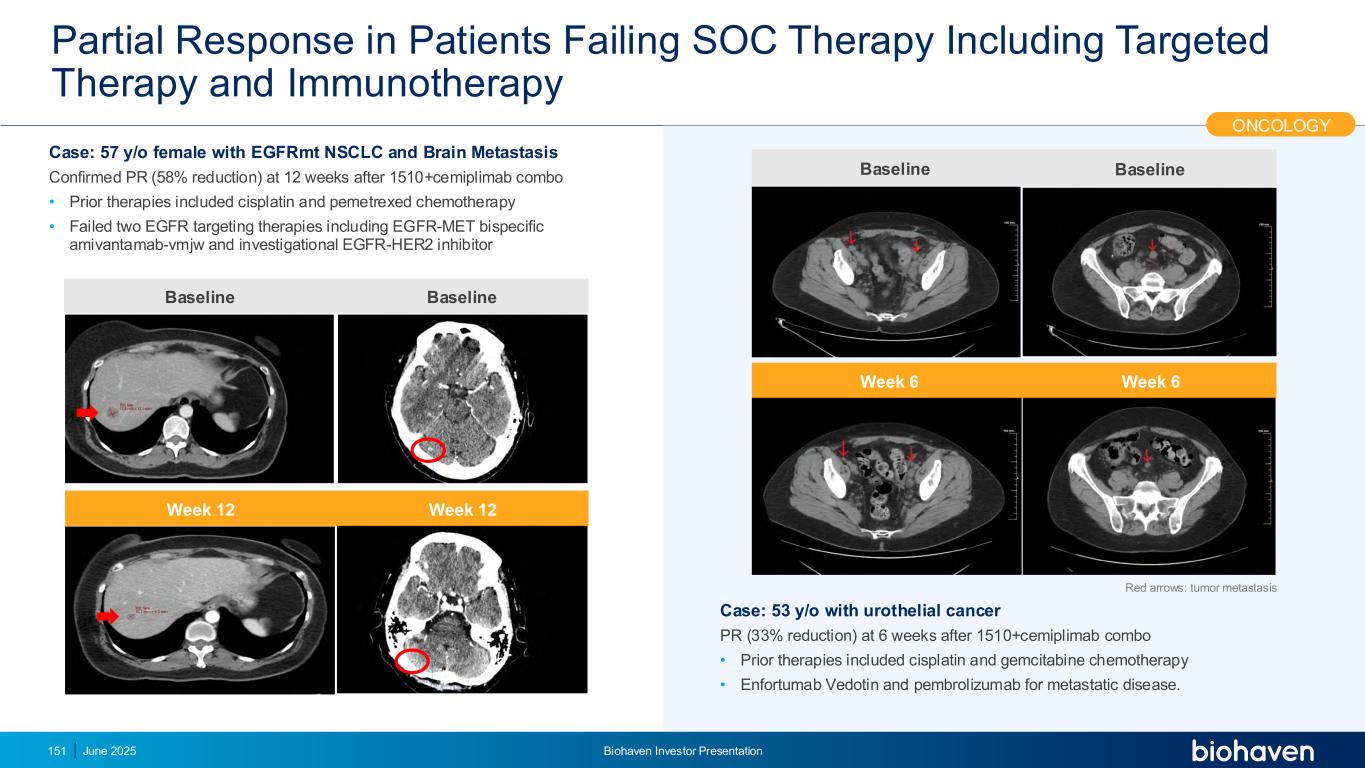
Partial Response in Patients Failing SOC Therapy Including Targeted Therapy and Immunotherapy June 2025 Biohaven Investor Presentation151 Red arrows: tumor metastasis Week 6 Baseline Week 6 Baseline Case: 53 y/o with urothelial cancer PR (33% reduction) at 6 weeks after 1510+cemiplimab combo • Prior therapies included cisplatin and gemcitabine chemotherapy • Enfortumab Vedotin and pembrolizumab for metastatic disease. ONCOLOGY Case: 57 y/o female with EGFRmt NSCLC and Brain Metastasis Confirmed PR (58% reduction) at 12 weeks after 1510+cemiplimab combo • Prior therapies included cisplatin and pemetrexed chemotherapy • Failed two EGFR targeting therapies including EGFR-MET bispecific amivantamab-vmjw and investigational EGFR-HER2 inhibitor Week 12 Baseline Week 12 Baseline
BHV-1510: Tumor Reduction Seen in Difficult to Treat Tumors, in Patients Progressing on Standard and Approved Therapies • Clinical activity seen across doses starting at the lowest dose • As monotherapy partial response seen in tumor types (SCLC, Endometrial, NSCLC) in patients that are heavily pretreated and have progressed on standard of care therapies • Patients on treatment with durable clinical benefit, several at 6 months and beyond • Tumor reductions seen in first 6/6 evaluable patients treated with cemiplimab combination; PRs in urothelial and NSCLC patients • Favorable preliminary safety profile • No payload-associated ILD, low GI toxicity like diarrhea, and low hematological toxicity • Main toxicity observed is on-target Trop2 ADC class mucositis; an expected and manageable effect • Combination with cemiplimab well tolerated with no DLTs to date in initial cohorts June 2025 Biohaven Investor Presentation152 ONCOLOGY Preliminary efficacy and tolerability of BHV-1510, incorporating the novel TopoIx payload and highly stable linker, indicate potential for use in earlier treatment settings across various cancers KEY POINTS Prelim data from ongoing study. Data cut off May 2, 2025

BHV-1530

Novel FGFR3 mAb With Proprietary TopoIx Payload • Only FGFR3 directed ADC in clinic • Site-specific conjugation with favorable nonclinical tox profile FGFR3 Is a Validated Target With Limited Competition • No current FGFR3 ADCs approved or in advanced development Core opportunity in FGFR3-altered metastatic urothelial cancer • Only 1 Tyrosine Kinase Inhibitor approved • TKI toxicity due to Pan FGFR inhibition • Potential extension into other FGFR3-driven solid tumors Synergistic Efficacy With Checkpoint Inhibitors In Vivo Anti-PD-L1 combination showed synergy similar to BHV-1510 June 2025 Biohaven Investor Presentation154 BHV-1530 CLINIC-READY FGFR3 ADC
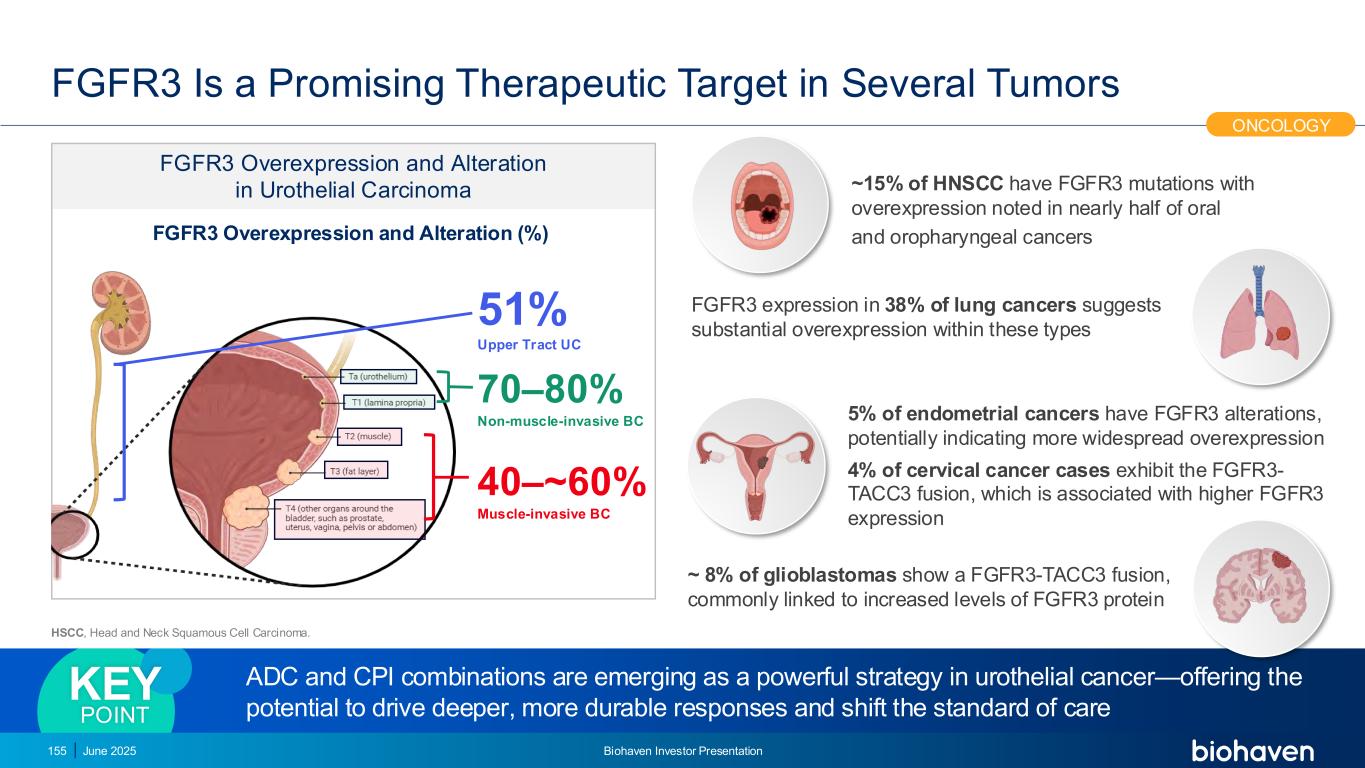
FGFR3 Is a Promising Therapeutic Target in Several Tumors June 2025 Biohaven Investor Presentation155 ONCOLOGY 5% of endometrial cancers have FGFR3 alterations, potentially indicating more widespread overexpression 4% of cervical cancer cases exhibit the FGFR3- TACC3 fusion, which is associated with higher FGFR3 expression FGFR3 expression in 38% of lung cancers suggests substantial overexpression within these types FGFR3 Overexpression and Alteration in Urothelial Carcinoma FGFR3 Overexpression and Alteration (%) 51% Upper Tract UC 40–~60% Muscle-invasive BC 70–80% Non-muscle-invasive BC ~15% of HNSCC have FGFR3 mutations with overexpression noted in nearly half of oral and oropharyngeal cancers ADC and CPI combinations are emerging as a powerful strategy in urothelial cancer—offering the potential to drive deeper, more durable responses and shift the standard of care KEY POINT ~ 8% of glioblastomas show a FGFR3-TACC3 fusion, commonly linked to increased levels of FGFR3 protein HSCC, Head and Neck Squamous Cell Carcinoma.
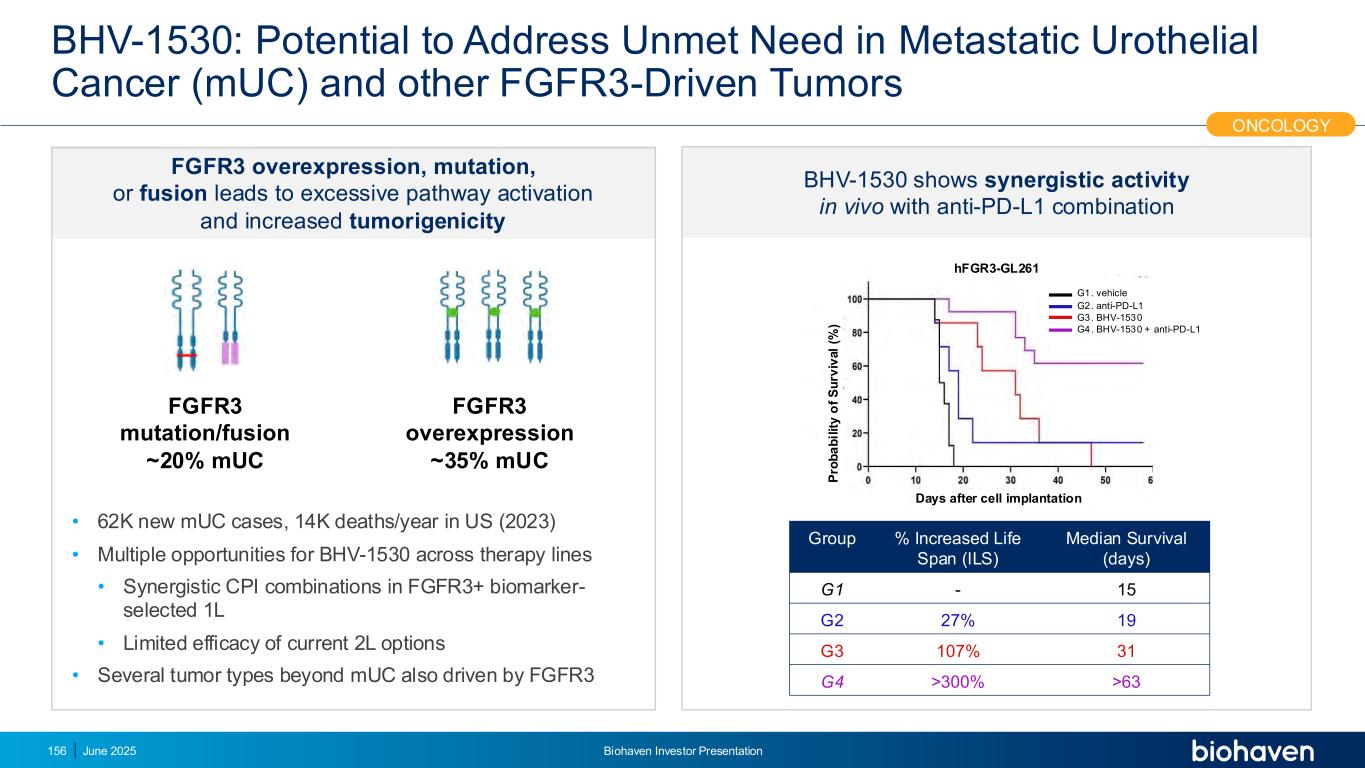
BHV-1530: Potential to Address Unmet Need in Metastatic Urothelial Cancer (mUC) and other FGFR3-Driven Tumors June 2025 Biohaven Investor Presentation156 P ro b a b il it y o f S u rv iv a l (% ) Days after cell implantation BHV-1530 shows synergistic activity in vivo with anti-PD-L1 combination G1. vehicle G2. anti-PD-L1 G3. BHV-1530 G4. BHV-1530 + anti-PD-L1 hFGR3-GL261 Group % Increased Life Span (ILS) Median Survival (days) G1 - 15 G2 27% 19 G3 107% 31 G4 >300% >63 • 62K new mUC cases, 14K deaths/year in US (2023) • Multiple opportunities for BHV-1530 across therapy lines • Synergistic CPI combinations in FGFR3+ biomarker- selected 1L • Limited efficacy of current 2L options • Several tumor types beyond mUC also driven by FGFR3 FGFR3 mutation/fusion ~20% mUC FGFR3 overexpression ~35% mUC FGFR3 overexpression, mutation, or fusion leads to excessive pathway activation and increased tumorigenicity ONCOLOGY
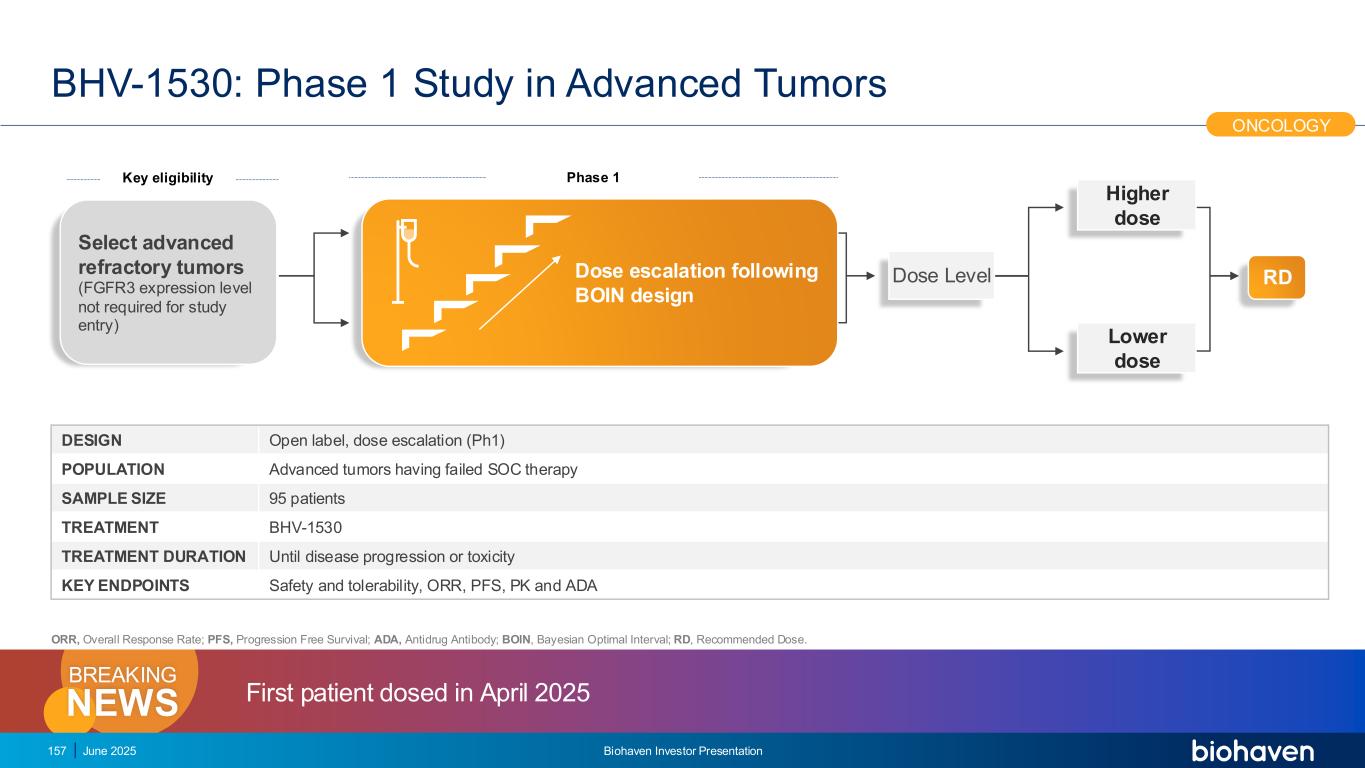
BHV-1530: Phase 1 Study in Advanced Tumors June 2025 Biohaven Investor Presentation157 DESIGN Open label, dose escalation (Ph1) POPULATION Advanced tumors having failed SOC therapy SAMPLE SIZE 95 patients TREATMENT BHV-1530 TREATMENT DURATION Until disease progression or toxicity KEY ENDPOINTS Safety and tolerability, ORR, PFS, PK and ADA ORR, Overall Response Rate; PFS, Progression Free Survival; ADA, Antidrug Antibody; BOIN, Bayesian Optimal Interval; RD, Recommended Dose. RD Lower dose Key eligibility Phase 1 Select advanced refractory tumors (FGFR3 expression level not required for study entry) Dose escalation following BOIN design Higher dose First patient dosed in April 2025NEWS BREAKING Dose Level ONCOLOGY
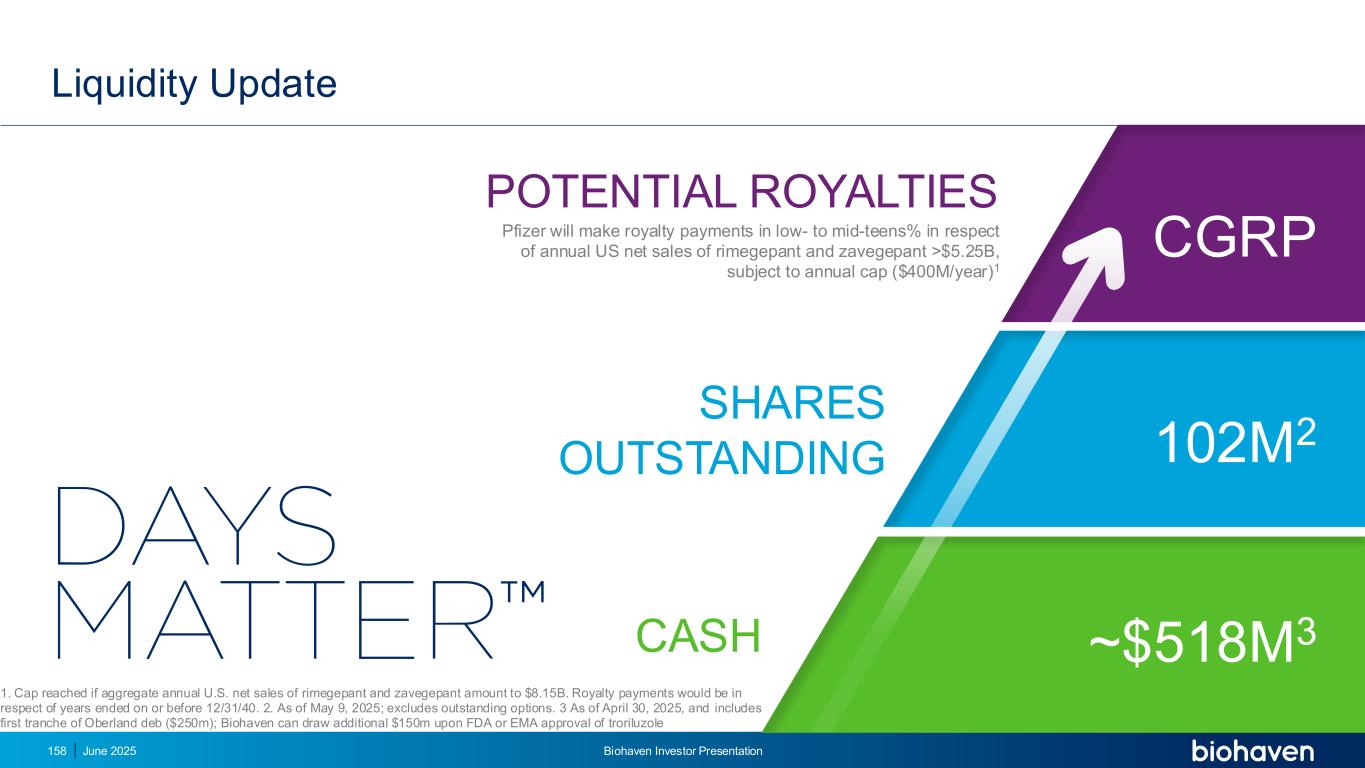
Liquidity Update CASH SHARES OUTSTANDING POTENTIAL ROYALTIES Pfizer will make royalty payments in low- to mid-teens% in respect of annual US net sales of rimegepant and zavegepant >$5.25B, subject to annual cap ($400M/year)1 ~$518M3 102M2 CGRP June 2025 Biohaven Investor Presentation158 1. Cap reached if aggregate annual U.S. net sales of rimegepant and zavegepant amount to $8.15B. Royalty payments would be in respect of years ended on or before 12/31/40. 2. As of May 9, 2025; excludes outstanding options. 3 As of April 30, 2025, and includes first tranche of Oberland deb ($250m); Biohaven can draw additional $150m upon FDA or EMA approval of troriluzole
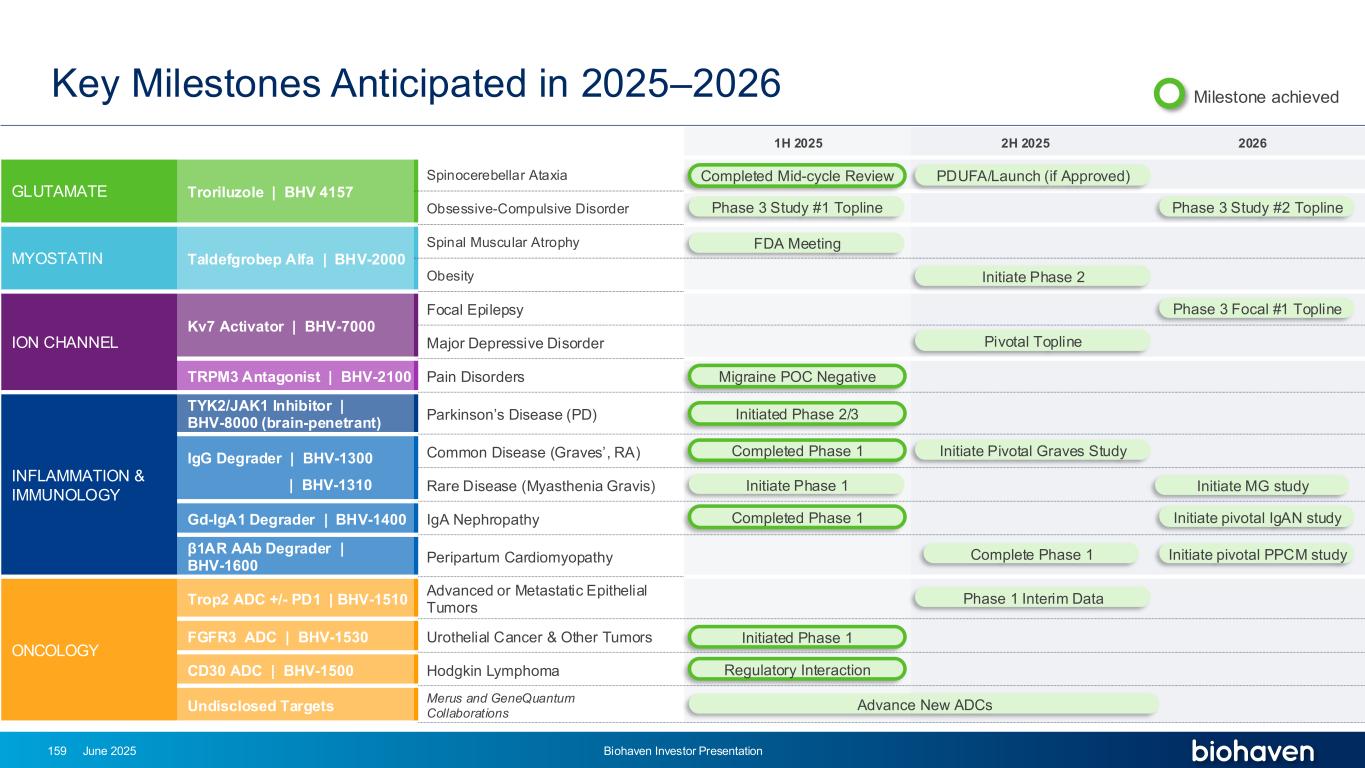
1H 2025 2H 2025 2026 GLUTAMATE Troriluzole | BHV 4157 Spinocerebellar Ataxia Obsessive-Compulsive Disorder MYOSTATIN Taldefgrobep Alfa | BHV-2000 Spinal Muscular Atrophy Obesity ION CHANNEL Kv7 Activator | BHV-7000 Focal Epilepsy Major Depressive Disorder TRPM3 Antagonist | BHV-2100 Pain Disorders INFLAMMATION & IMMUNOLOGY TYK2/JAK1 Inhibitor | BHV-8000 (brain-penetrant) Parkinson’s Disease (PD) IgG Degrader | BHV-1300 | BHV-1310 Common Disease (Graves’, RA) Rare Disease (Myasthenia Gravis) Gd-IgA1 Degrader | BHV-1400 IgA Nephropathy β1AR AAb Degrader | BHV-1600 Peripartum Cardiomyopathy ONCOLOGY Trop2 ADC +/- PD1 | BHV-1510 Advanced or Metastatic Epithelial Tumors FGFR3 ADC | BHV-1530 Urothelial Cancer & Other Tumors CD30 ADC | BHV-1500 Hodgkin Lymphoma Undisclosed Targets Merus and GeneQuantum Collaborations Phase 3 Study #1 Topline FDA Meeting Pivotal Topline Completed Phase 1 Complete Phase 1 Initiate Phase 1 Completed Phase 1 Phase 1 Interim Data Regulatory Interaction Key Milestones Anticipated in 2025–2026 PDUFA/Launch (if Approved)Completed Mid-cycle Review Initiated Phase 2/3 Initiate Phase 2 Phase 3 Study #2 Topline Initiate Pivotal Graves Study Initiate pivotal IgAN study Initiated Phase 1 Phase 3 Focal #1 Topline Initiate pivotal PPCM study Initiate MG study Advance New ADCs June 2025 Migraine POC Negative Biohaven Investor Presentation159 Milestone achieved
