
© 2026 Biohaven, Ltd. All rights reserved. Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Corporate Presentation January 2026 CAMERON Living with Graves’ Disease PODIUM

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Biohaven Ltd. (the “Company”) and our planned and ongoing trials (including those for our taldefgrobep alfa, opakalim, BHV- 2100, BHV-8000, BHV-1300, BHV-1400, BHV-1510 and BHV-1600 development programs), the timing of and the availability of data from our clinical trials, the timing and our decisions to proceed with our planned regulatory filings, the timing of and our ability to obtain regulatory approvals for our product candidates, the clinical potential utility of our product candidates, alone and as compared to other existing potential treatment options, and the potential advancement of our early phase programs including BHV-1310, BHV-1530 and BHV-1500. The use of certain words, including “continue”, “plan”, “will”, “believe”, “may”, “expect”, “anticipate” and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of our development candidates are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the Food and Drug Administration, including those regarding the resubmission of our new drug application for troriluzole for SCA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; the potential for Biohaven’s product candidates to be first-in- class, best-in-class, best-in-clinic or best-in-category therapies; and the effectiveness and safety of Biohaven's product candidates, including open label clinical data in ongoing studies. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Additional important factors to be considered in connection with forward-looking statements are described in the Company’s filings with the Securities and Exchange Commission, including within the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. This presentation also contains market data and other information based on industry publications, reports by market research firms or published independent sources. Some market data and information are also based on the Company’s good faith estimates, which are derived from management’s knowledge of its indust ry and such independent sources referred to above. All images are actors unless otherwise noted. Biohaven is a registered trademark, and MoDE, TRAP and Days Matter are trademarks of Biohaven Therapeutics Ltd. January 12, 2026 Biohaven Corporate Presentation2 PODIUM

Innovating Tomorrow’s Medicines Today PODIUM
Created a durable engine, built to scale, for near-term catalysts and long-term value Diversified platforms delivering multiple innovative therapies because DAYS MATTER DEGRADERS Selectively removing what harms while preserving what heals ION CHANNEL A modern Kv7 activator that offers efficacy without burdensome CNS side effects MYOSTATIN ACTIVIN Targeting high quality weight loss, preserving muscle mass and reducing fat January 12, 2026 Biohaven Corporate Presentation4

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Targeting the Root Cause of Autoimmune Disease DEGRADERS: MoDE AND TRAP
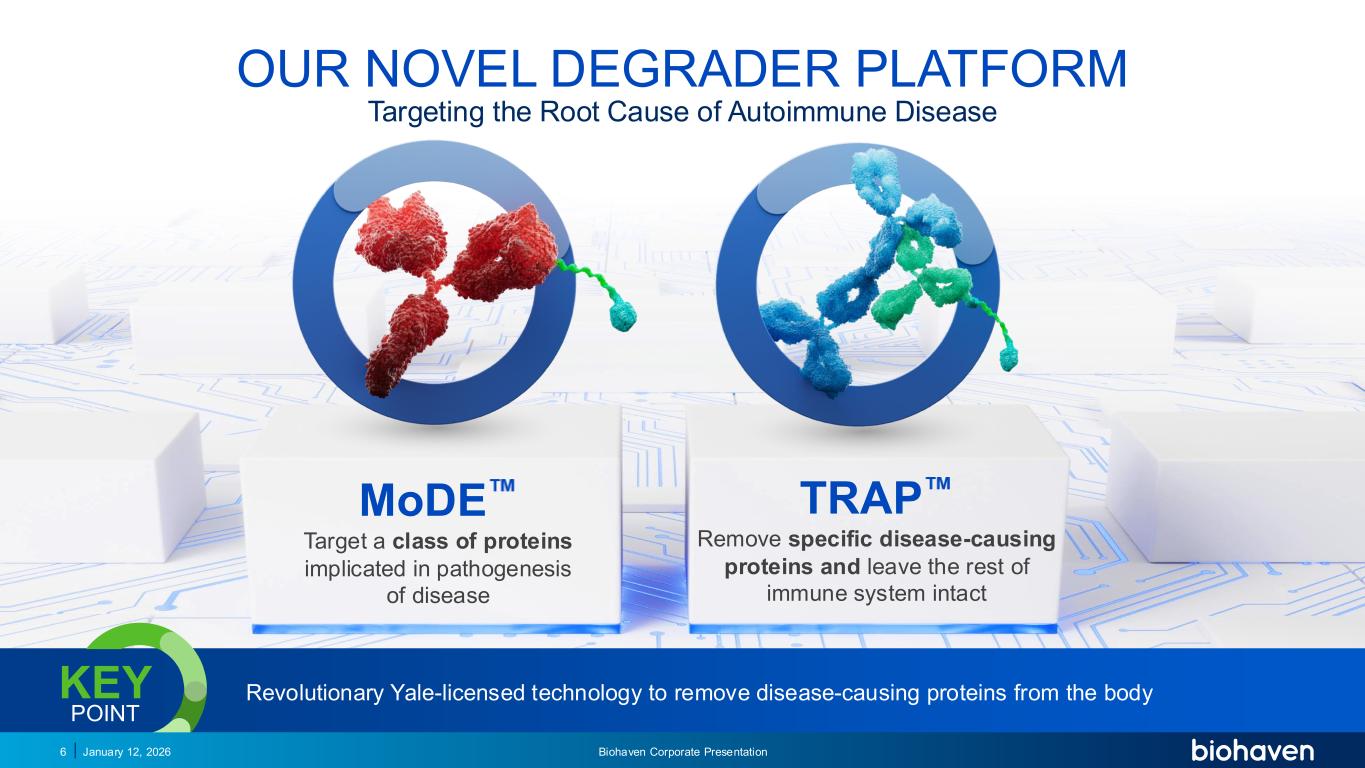
OUR NOVEL DEGRADER PLATFORM Biohaven Corporate Presentation6 January 12, 2026 MoDE Target a class of proteins implicated in pathogenesis of disease TRAP Remove specific disease-causing proteins and leave the rest of immune system intact Revolutionary Yale-licensed technology to remove disease-causing proteins from the bodyKEY POINT PODIUM Targeting the Root Cause of Autoimmune Disease
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYMoDE and TRAP Degraders: Pioneering the First and Only Extracellular Degraders in the Clinic January 12, 2026 Biohaven Corporate Presentation7 DEGRADERS PODIUM PATIENT FRIENDLY ADMINISTRATION Easy-to-use autoinjector; rapid, selective, tunable for patient needs POSITIONED TO INITIATE PIVOTAL TRIALS 2026 IgAN lead indication for BHV-1400 and Graves’ for BHV-1300 SCALABLE TO MULTIPLE TARGETS AND STRONG IP Biohaven leads in technology and IP position with this modality HIGHLY SELECTIVE TARGETING OF DISEASE-DRIVING PROTEINS Validated in the clinic: Safe and well-tolerated
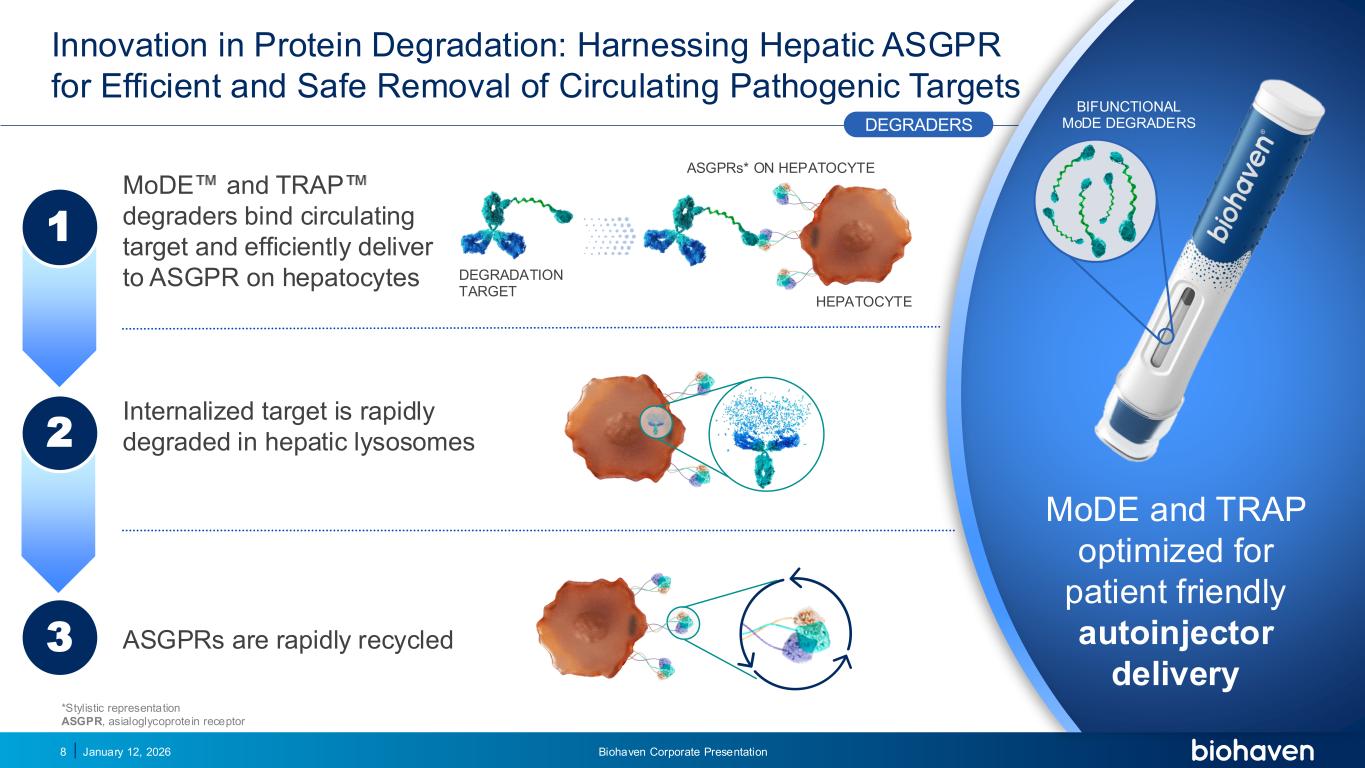
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Innovation in Protein Degradation: Harnessing Hepatic ASGPR for Efficient and Safe Removal of Circulating Pathogenic Targets January 12, 2026 Biohaven Corporate Presentation8 MoDE and TRAP degraders bind circulating target and efficiently deliver to ASGPR on hepatocytes DEGRADATION TARGET HEPATOCYTE ASGPRs* ON HEPATOCYTE Internalized target is rapidly degraded in hepatic lysosomes ASGPRs are rapidly recycled 1 3 2 MoDE and TRAP optimized for patient friendly autoinjector delivery BIFUNCTIONAL MoDE DEGRADERSDEGRADERS *Stylistic representation ASGPR, asialoglycoprotein receptor

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-1400 Gd-IgA1 IgA Nephropathy (IgAN) DEGRADERS: BHV-1400 TRAP PODIUM
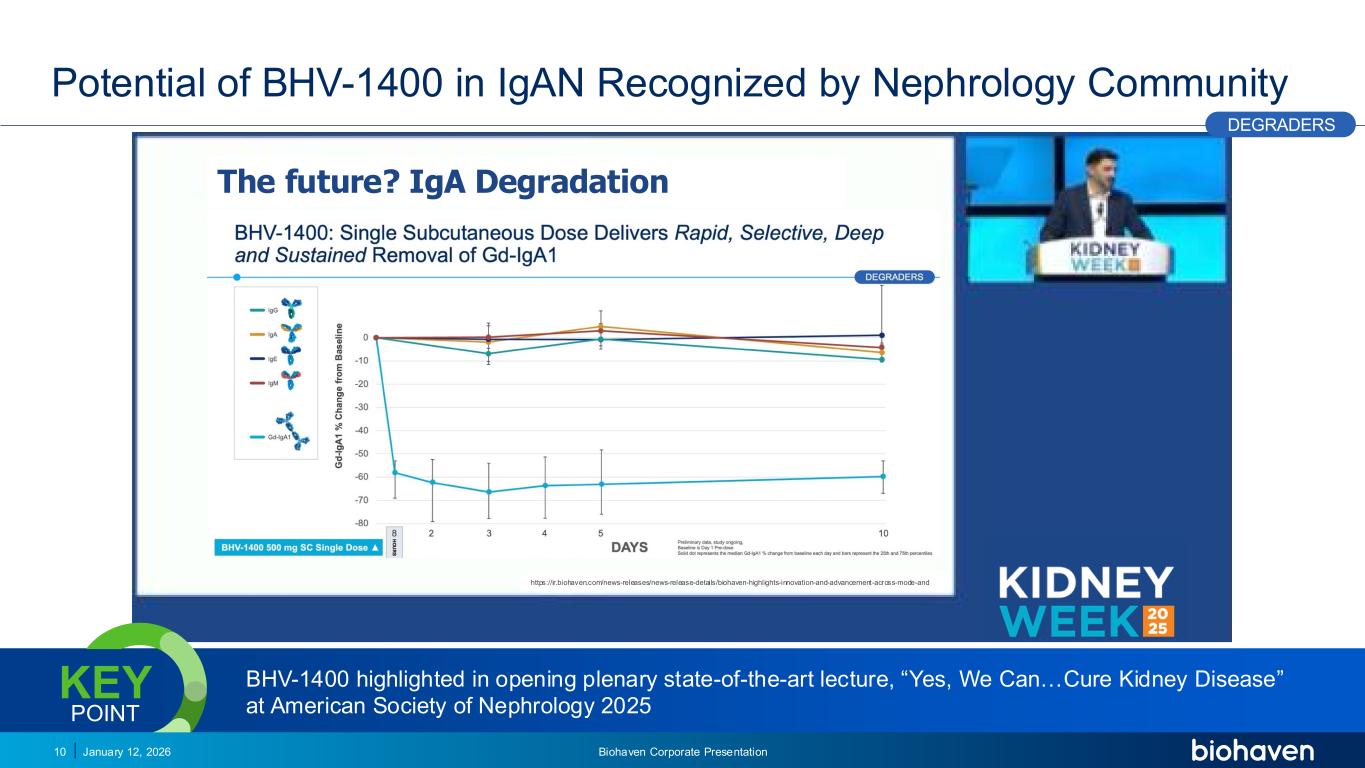
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY The future? IgA Degradation https://ir.biohaven.com/news-releases/news-release-details/biohaven-highlights-innovation-and-advancement-across-mode-and Potential of BHV-1400 in IgAN Recognized by Nephrology Community January 12, 2026 Biohaven Corporate Presentation10 Source: https://www.asn-online.org/education/kidneyweek/2025 BHV-1400 highlighted in opening plenary state-of-the-art lecture, “Yes, We Can…Cure Kidney Disease” at American Society of Nephrology 2025 KEY POINT PODIUM DEGRADERS
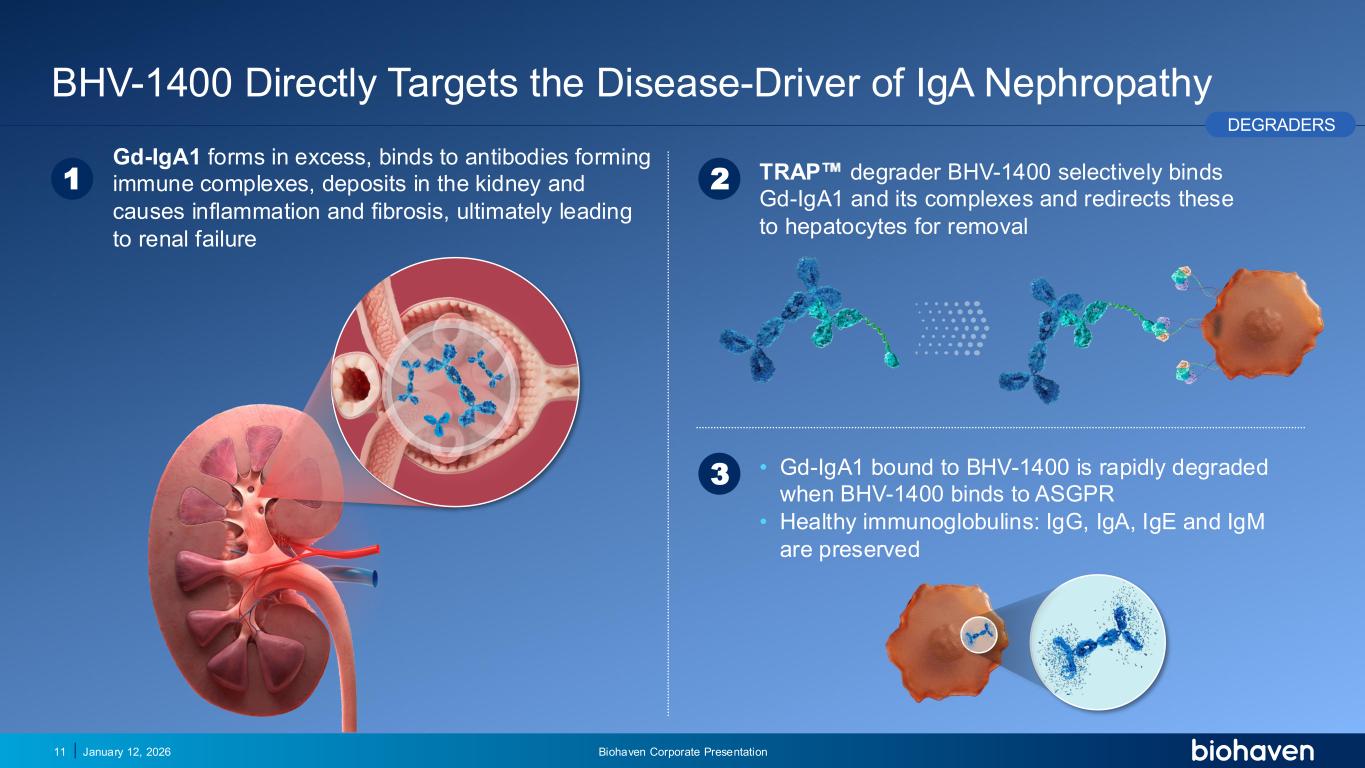
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-1400 Directly Targets the Disease-Driver of IgA Nephropathy January 12, 2026 Biohaven Corporate Presentation11 PODIUM 2 TRAP degrader BHV-1400 selectively binds Gd-IgA1 and its complexes and redirects these to hepatocytes for removal 1 Gd-IgA1 forms in excess, binds to antibodies forming immune complexes, deposits in the kidney and causes inflammation and fibrosis, ultimately leading to renal failure 3 • Gd-IgA1 bound to BHV-1400 is rapidly degraded when BHV-1400 binds to ASGPR • Healthy immunoglobulins: IgG, IgA, IgE and IgM are preserved DEGRADERS PR

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Updated KDIGO Guidelines: Treat Earlier and Target Pathogenic Gd-IgA1 January 12, 2026 Biohaven Corporate Presentation12 KDIGO: Kidney Disease Improving Global Outcomes; https://kdigo.org/wp-content/uploads/2024/08/KDIGO-2025-IgAN-IgAV-Guideline.pdf IgAN Treatment Is Now Defined by Targeting of Gd-IgA1 New KDIGO 2025 Guidance BHV-1400 First-line intervention now targets pathogenic Gd-IgA1 for disease modification Designed to selectively remove pathogenic Gd-IgA1, the root cause of IgAN Proteinuria threshold lowered to >0.5 g/g Meaningfully expands the first- line addressable population Early, disease-specific therapy recommended Positions BHV-1400 as a potential foundational therapy BHV-1400 is the only therapy in clinical development that directly and selectively removes Gd-IgA1 KEY POINT DEGRADERS PODIUM
BHV-1400 Across the Spectrum ADVANCING A THERAPY FOR ALL STAGES OF DISEASE ADVANCEDEARLY PODIUM
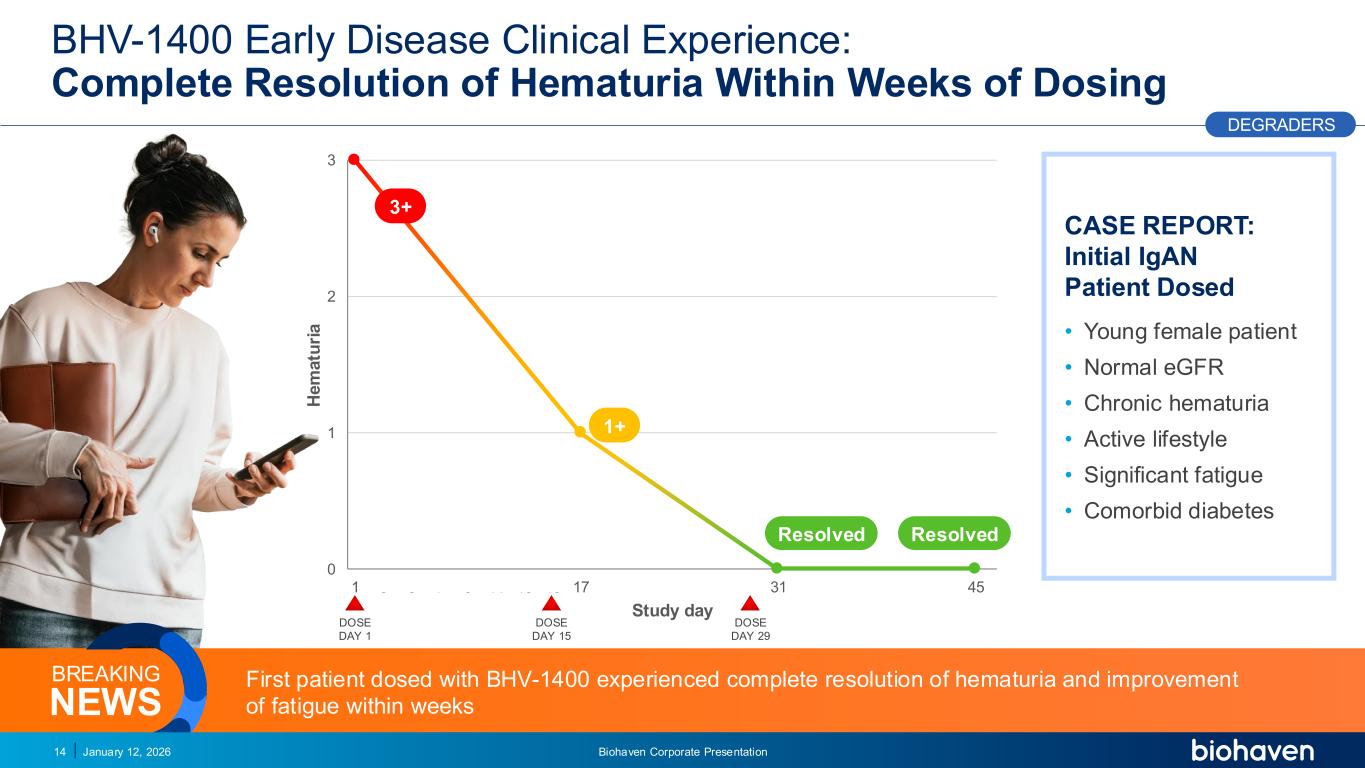
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1400 Early Disease Clinical Experience: Complete Resolution of Hematuria Within Weeks of Dosing January 12, 2026 Biohaven Corporate Presentation14 DEGRADERS CASE REPORT: Initial IgAN Patient Dosed • Young female patient • Normal eGFR • Chronic hematuria • Active lifestyle • Significant fatigue • Comorbid diabetes First patient dosed with BHV-1400 experienced complete resolution of hematuria and improvement of fatigue within weeksNEWS BREAKING 0 1 2 3 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 H e m a tu ri a Study day 3+ 1+ DOSE DAY 1 Resolved Resolved PODIUM DOSE DAY 15 DOSE DAY 29 PR
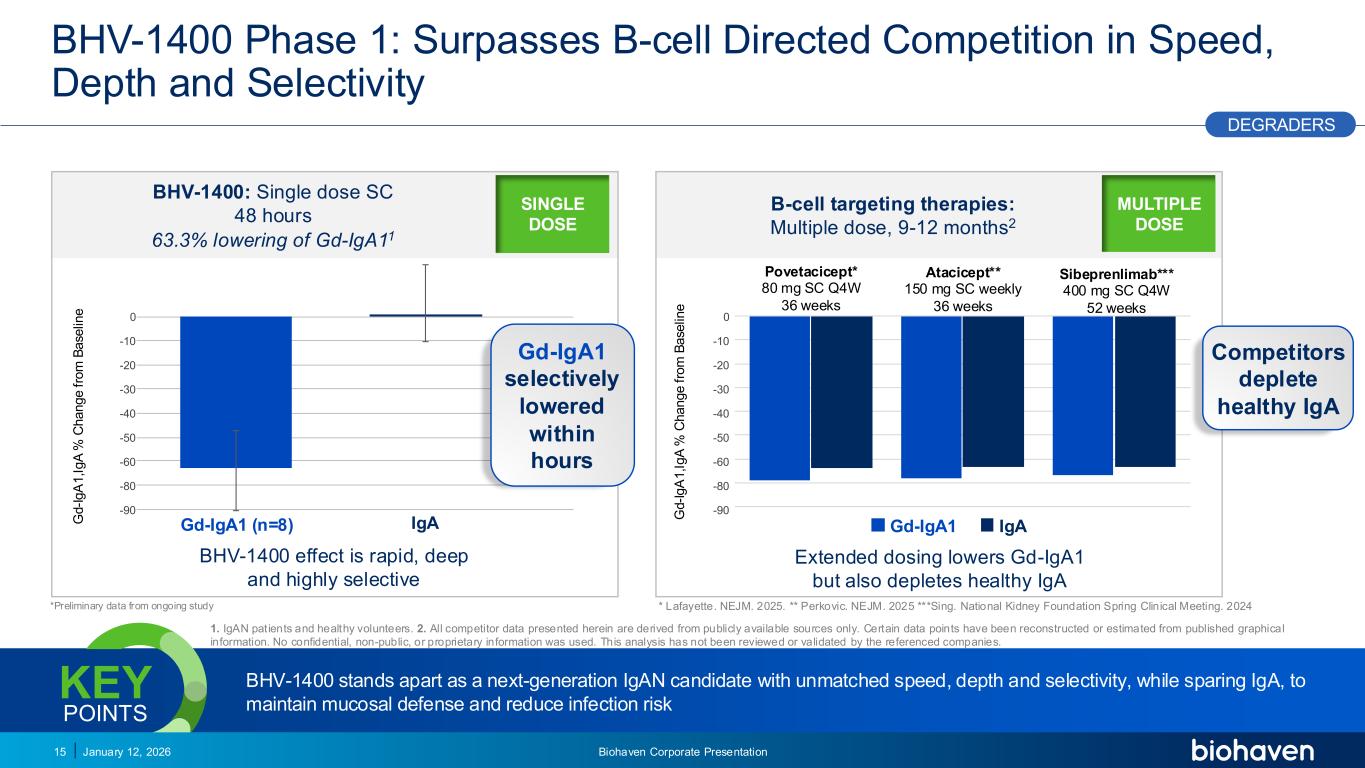
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1400 Phase 1: Surpasses B-cell Directed Competition in Speed, Depth and Selectivity January 12, 2026 Biohaven Corporate Presentation15 BHV-1400 stands apart as a next-generation IgAN candidate with unmatched speed, depth and selectivity, while sparing IgA, to maintain mucosal defense and reduce infection risk KEY POINTS B-cell targeting therapies: Multiple dose, 9-12 months2 Extended dosing lowers Gd-IgA1 but also depletes healthy IgA G d -I g A 1 ,I g A % C h a n g e f ro m B a se lin e Gd-IgA1 (n=8) BHV-1400: Single dose SC 48 hours 63.3% lowering of Gd-IgA11 G d -I g A 1 ,I g A % C h a n g e f ro m B a se lin e BHV-1400 effect is rapid, deep and highly selective Gd-IgA1 selectively lowered within hours *Preliminary data from ongoing study DEGRADERS IgA Competitors deplete healthy IgA PODIUM 1. IgAN patients and healthy volunteers. 2. All competitor data presented herein are derived from publicly available sources only. Certain data points have been reconstructed or estimated from published graphical information. No confidential, non-public, or proprietary information was used. This analysis has not been reviewed or validated by the referenced companies. Povetacicept* 80 mg SC Q4W 36 weeks Atacicept** 150 mg SC weekly 36 weeks Sibeprenlimab*** 400 mg SC Q4W 52 weeks -IgA1 - - Atacicept -68.30% -63.50% Atacicept 150 mg SC once weekly 36 weeks Sibeprenlimab -67.10% -63.50% Sibeprenlimab 400 mg SC Q4W 52 weeks 0 -10 -20 -30 -40 -50 -60 -80 -90 0 -10 -20 -30 -40 -50 -60 -80 -90 Gd-IgA1 IgA MULTIPLE DOSE SINGLE DOSE * Lafayette. NEJM. 2025. ** Perkovic. NEJM. 2025 ***Sing. National Kidney Foundation Spring Clinical Meeting. 2024
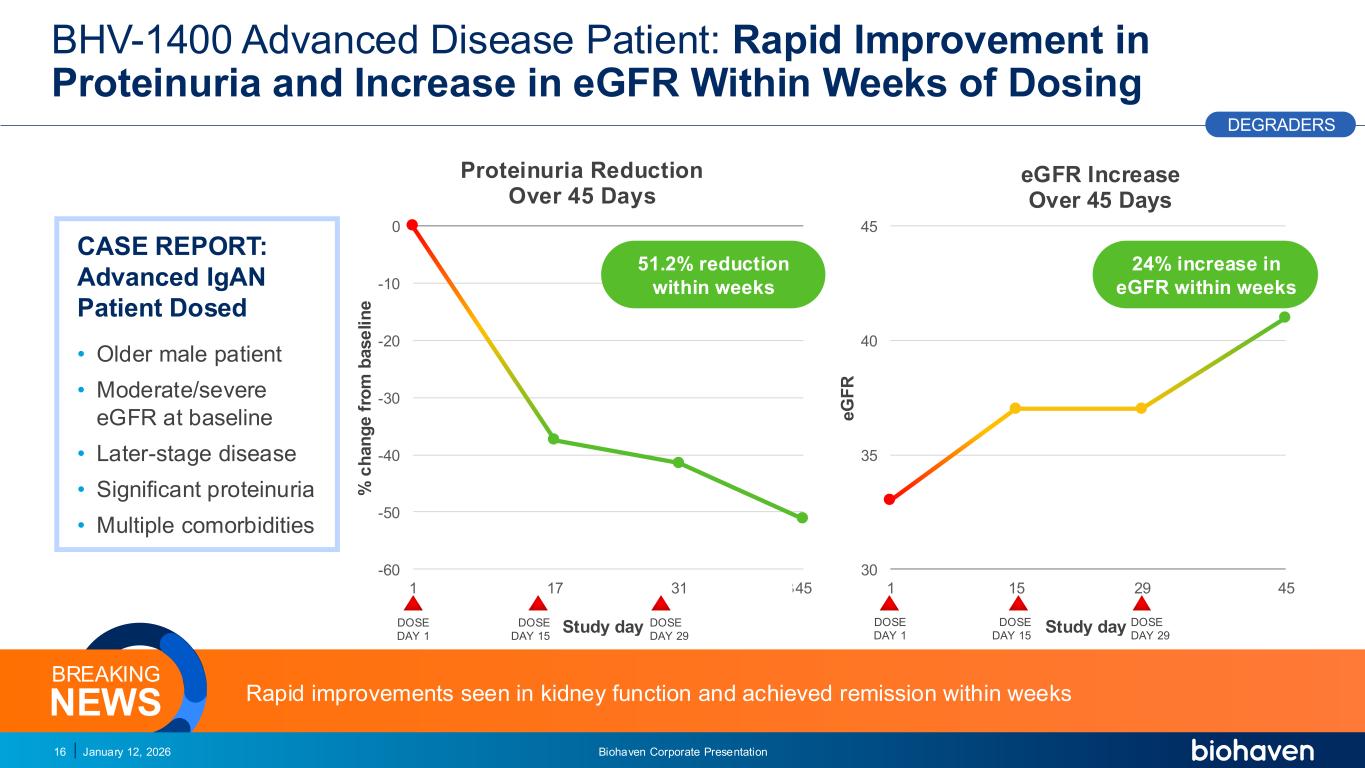
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1400 Advanced Disease Patient: Rapid Improvement in Proteinuria and Increase in eGFR Within Weeks of Dosing January 12, 2026 Biohaven Corporate Presentation16 DEGRADERS Rapid improvements seen in kidney function and achieved remission within weeks NEWS BREAKING CASE REPORT: Advanced IgAN Patient Dosed • Older male patient • Moderate/severe eGFR at baseline • Later-stage disease • Significant proteinuria • Multiple comorbidities PODIUM 30 35 40 45 1 3 5 7 9 111315171921232527293133353739414345 e G F R Study day eGFR Increase Over 45 Days 24% increase in eGFR within weeks -60 -50 -40 -30 -20 -10 0 1 3 5 7 9 111315171921232527293133353739414345 % c h a n g e f ro m b a s e li n e Study day Proteinuria Reduction Over 45 Days 51.2% reduction within weeks DOSE DAY 1 DOSE DAY 15 DOSE DAY 29 DOSE DAY 1 DOSE DAY 15 DOSE DAY 29 PR
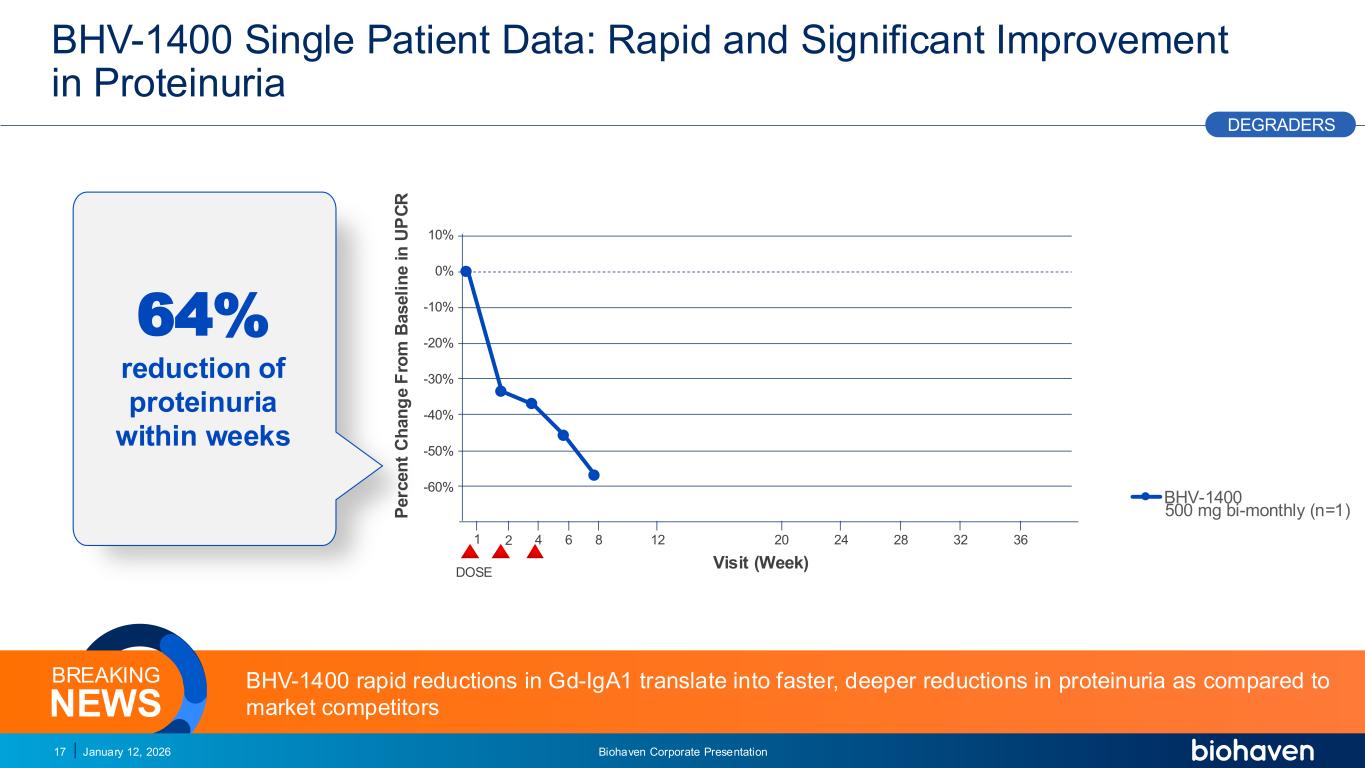
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY 0% -10% -20% -30% -40% -50% -60% 10% BHV-1400 Single Patient Data: Rapid and Significant Improvement in Proteinuria January 12, 2026 Biohaven Corporate Presentation17 Visit (Week) P e rc e n t C h a n g e F ro m B a s e li n e i n U P C R BHV-1400 rapid reductions in Gd-IgA1 translate into faster, deeper reductions in proteinuria as compared to market competitorsNEWS BREAKING PODIUM DEGRADERS 4 8 12 20 24 28 32 36621 64% reduction of proteinuria within weeks atacicept OLE atacicept, ORIGIN3 atrasentan nefecon mezagitamab povetacicept sibeprenlimab zigakibart BHV-1400 DOSE 500 mg bi-monthly (n=1)
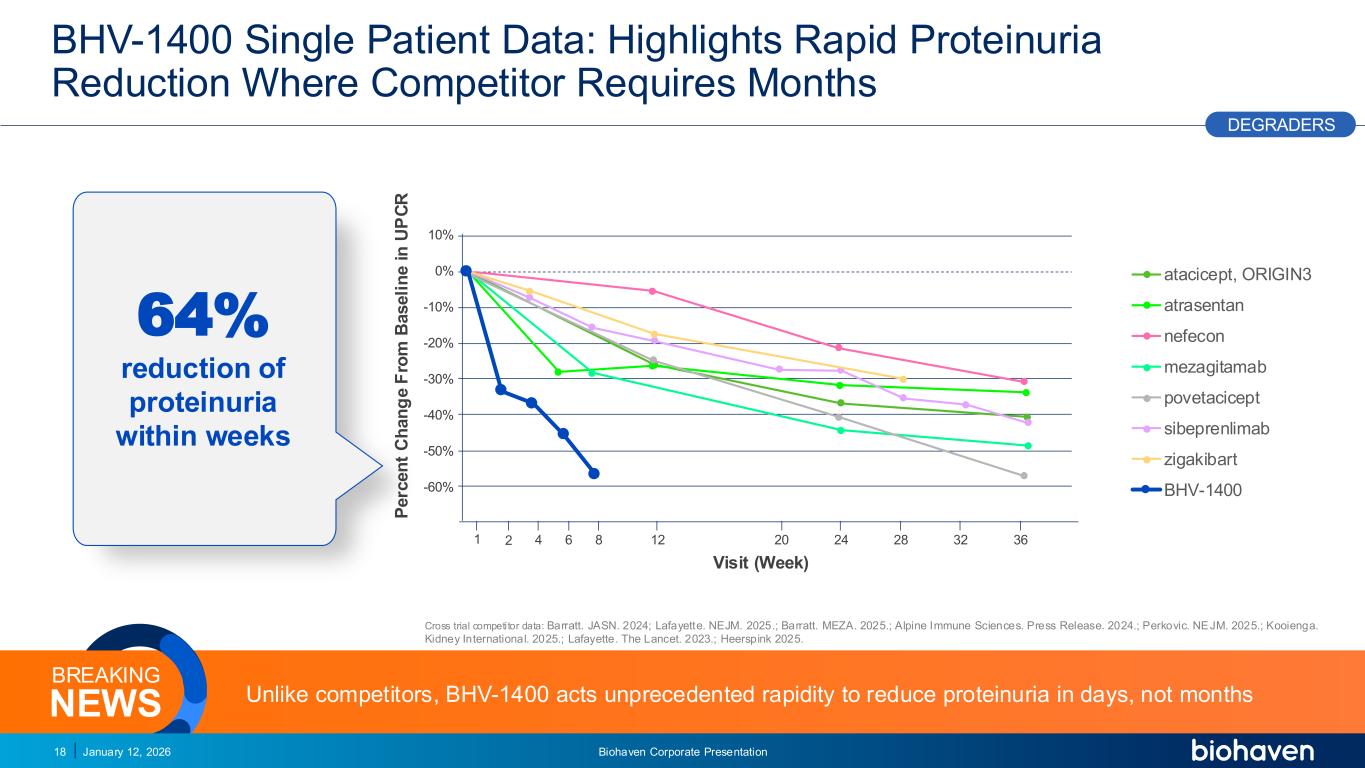
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY 0% -10% -20% -30% -40% -50% -60% 10% BHV-1400 Single Patient Data: Highlights Rapid Proteinuria Reduction Where Competitor Requires Months January 12, 2026 Biohaven Corporate Presentation18 Visit (Week) P e rc e n t C h a n g e F ro m B a s e li n e i n U P C R Unlike competitors, BHV-1400 acts unprecedented rapidity to reduce proteinuria in days, not monthsNEWS BREAKING PODIUM DEGRADERS 4 8 12 20 24 28 32 36621 64% reduction of proteinuria within weeks Cross trial competitor data: Barratt. JASN. 2024; Lafayette. NEJM. 2025.; Barratt. MEZA. 2025.; Alpine Immune Sciences. Press Release. 2024.; Perkovic. NEJM. 2025.; Kooienga. Kidney International. 2025.; Lafayette. The Lancet. 2023.; Heerspink 2025. atacicept, ORIGIN3 atrasentan nefecon mezagitamab povetacicept sibeprenlimab zigakibart BHV-1400 PR
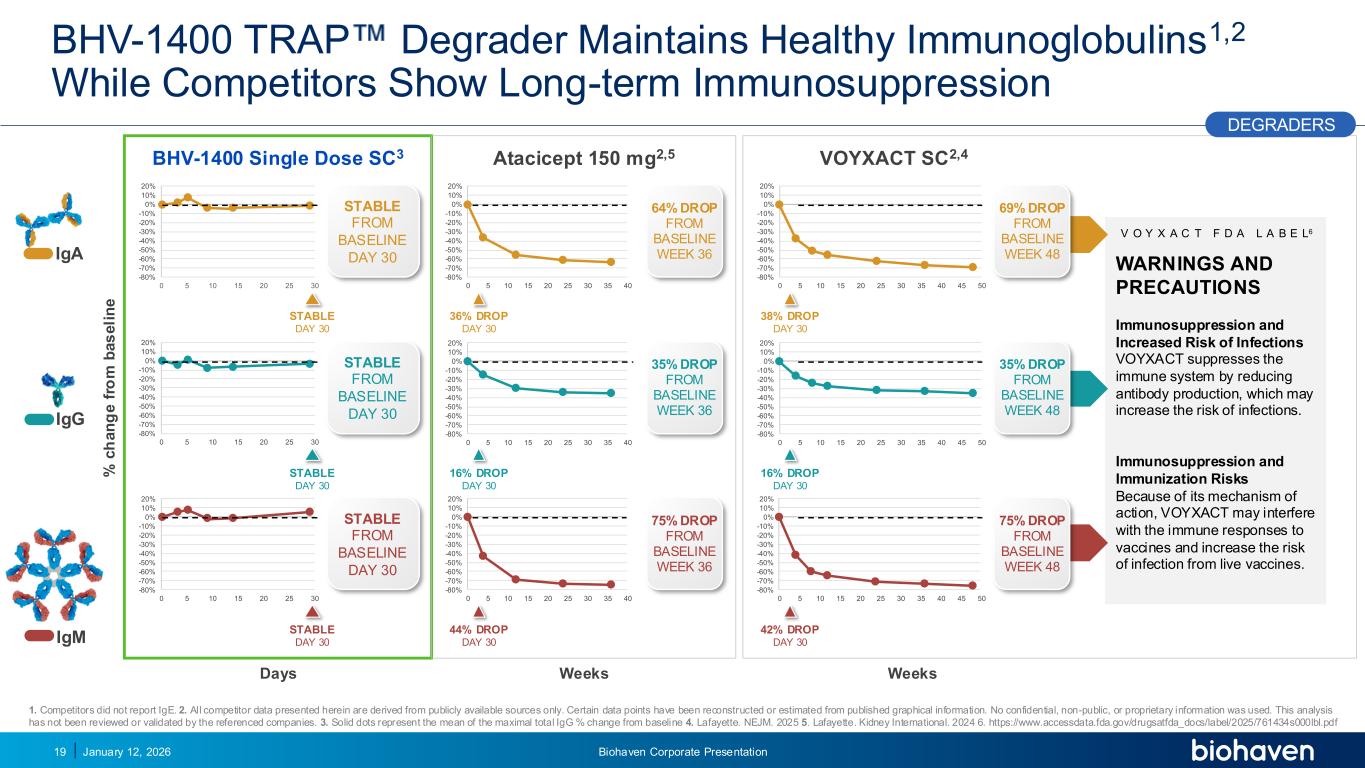
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY WARNINGS AND PRECAUTIONS Immunosuppression and Increased Risk of Infections VOYXACT suppresses the immune system by reducing antibody production, which may increase the risk of infections. Immunosuppression and Immunization Risks Because of its mechanism of action, VOYXACT may interfere with the immune responses to vaccines and increase the risk of infection from live vaccines. -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 BHV-1400 TRAP Degrader Maintains Healthy Immunoglobulins1,2 While Competitors Show Long-term Immunosuppression IgG IgA 1. Competitors did not report IgE. 2. All competitor data presented herein are derived from publicly available sources only. Certain data points have been reconstructed or estimated from published graphical information. No confidential, non-public, or proprietary information was used. This analysis has not been reviewed or validated by the referenced companies. 3. Solid dots represent the mean of the maximal total IgG % change from baseline 4. Lafayette. NEJM. 2025 5. Lafayette. Kidney International. 2024 6. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761434s000lbl.pdf % c h a n g e f ro m b a s e li n e BHV-1400 Single Dose SC3 Days -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 STABLE DAY 30 STABLE DAY 30 STABLE DAY 30 January 12, 2026 Biohaven Corporate Presentation19 STABLE FROM BASELINE DAY 30 STABLE FROM BASELINE DAY 30 STABLE FROM BASELINE DAY 30 VOYXACT SC2,4 Weeks -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 45 50 -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 45 50 -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 45 50 35% DROP FROM BASELINE WEEK 48 75% DROP FROM BASELINE WEEK 48 69% DROP FROM BASELINE WEEK 48 38% DROP DAY 30 16% DROP DAY 30 42% DROP DAY 30 Atacicept 150 mg2,5 Weeks -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 -80% -70% -60% -50% -40% -30% -20% -10% 0% 10% 20% 0 5 10 15 20 25 30 35 40 35% DROP FROM BASELINE WEEK 36 75% DROP FROM BASELINE WEEK 36 64% DROP FROM BASELINE WEEK 36 36% DROP DAY 30 16% DROP DAY 30 44% DROP DAY 30 PODIUM IgM DEGRADERS V O Y X A C T F D A L A B E L6
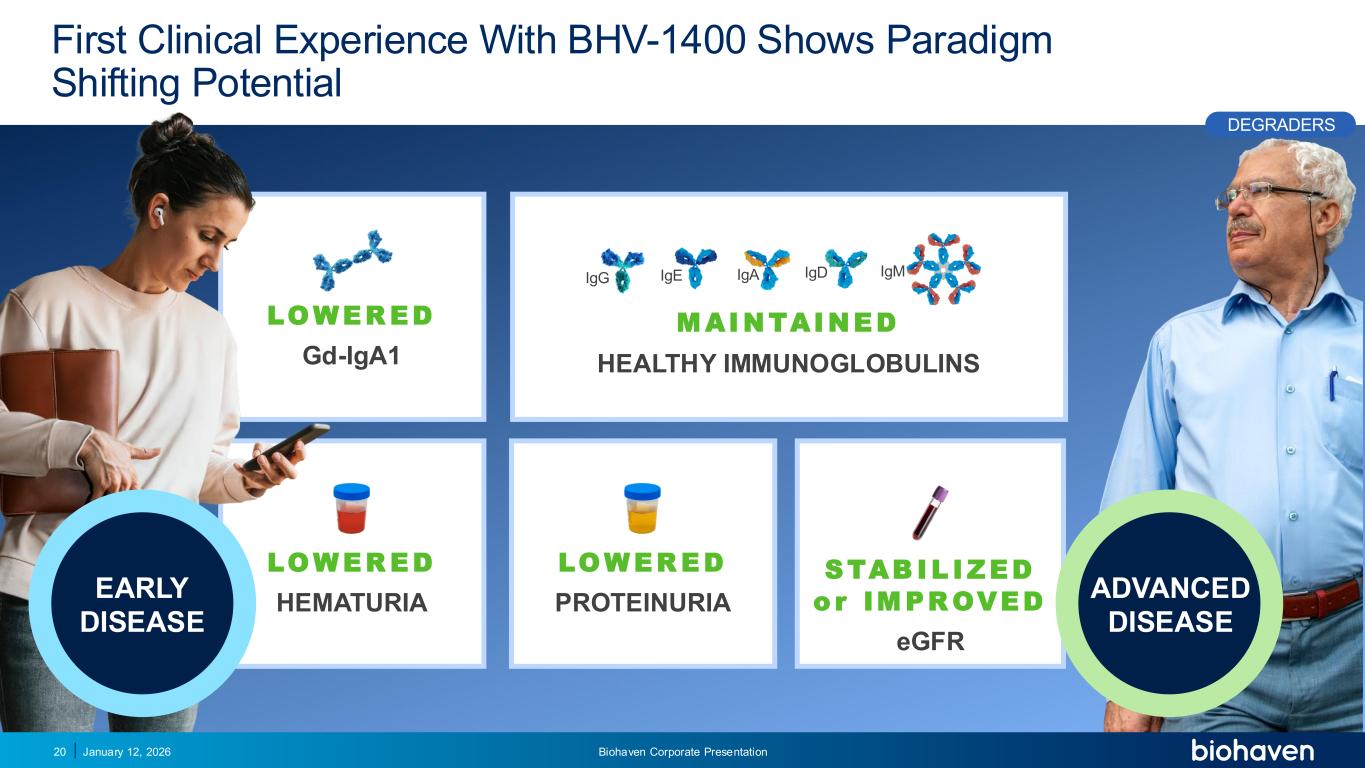
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY LOWER ED Gd-IgA1 LOWER ED PROTEINURIA LOWER ED HEMATURIA STAB I L I ZED o r IM PR OVED eGFR M AI NTAI NED HEALTHY IMMUNOGLOBULINS First Clinical Experience With BHV-1400 Shows Paradigm Shifting Potential January 12, 2026 Biohaven Corporate Presentation20 IgD IgMIgE IgAIgG PODIUM ADVANCED DISEASE EARLY DISEASE DEGRADERS
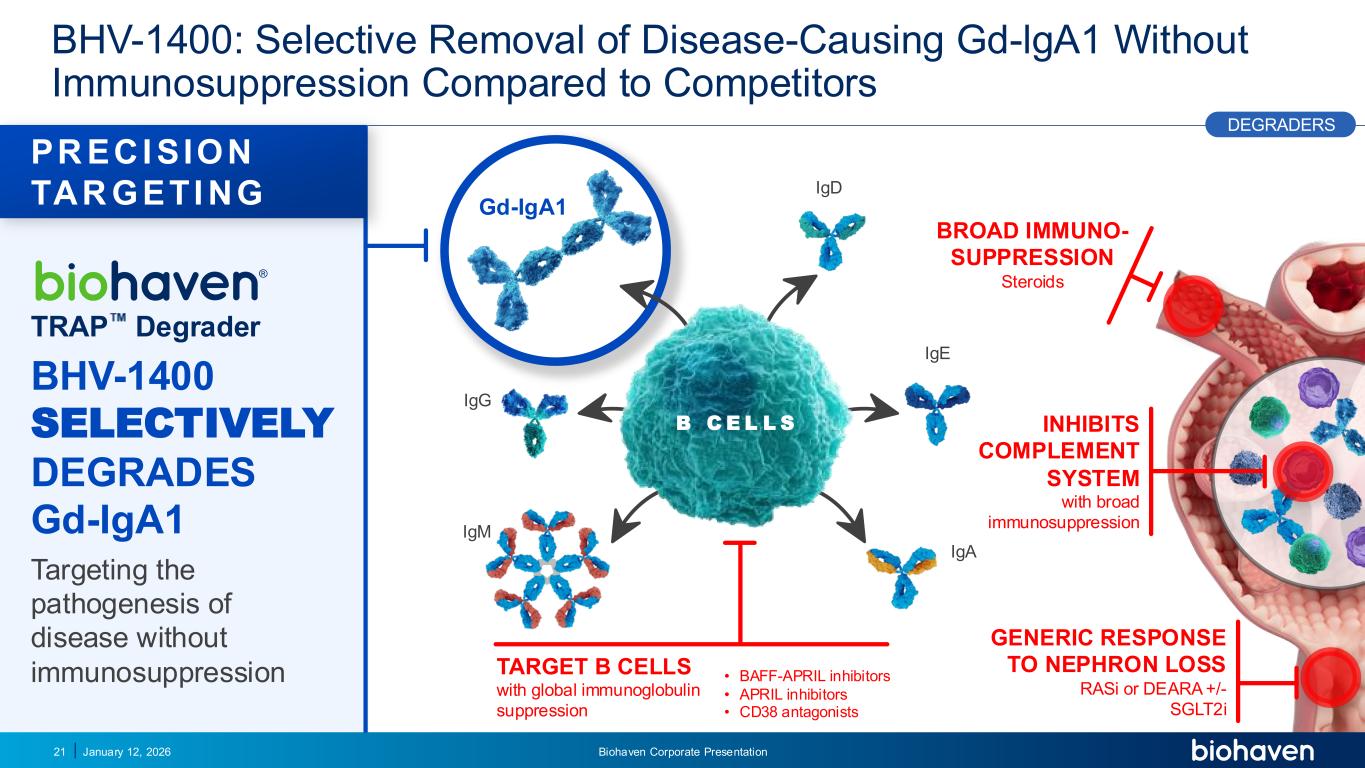
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Gd-IgA1 TRAP Degrader BHV-1400 SELECTIVELY DEGRADES Gd-IgA1 Targeting the pathogenesis of disease without immunosuppression BHV-1400: Selective Removal of Disease-Causing Gd-lgA1 Without Immunosuppression Compared to Competitors January 12, 2026 Biohaven Corporate Presentation21 BROAD IMMUNO- SUPPRESSION Steroids GENERIC RESPONSE TO NEPHRON LOSS RASi or DEARA +/- SGLT2i PR ECI SION TAR GETI NG DEGRADERS TARGET B CELLS with global immunoglobulin suppression IgD IgM IgE IgA IgG B C E L L S • BAFF-APRIL inhibitors • APRIL inhibitors • CD38 antagonists INHIBITS COMPLEMENT SYSTEM with broad immunosuppression
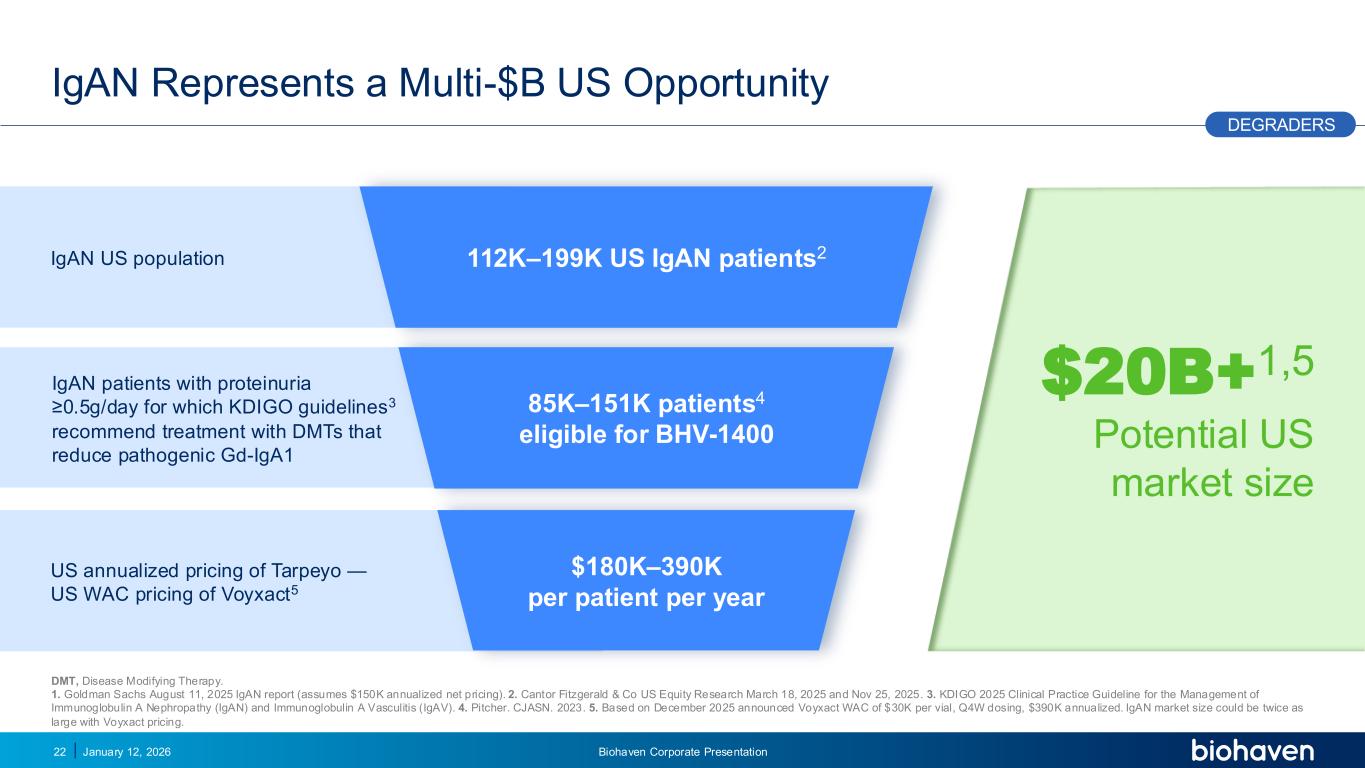
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY $20B+1,5 Potential US market size IgAN Represents a Multi-$B US Opportunity January 12, 2026 Biohaven Corporate Presentation22 IgAN patients with proteinuria ≥0.5g/day for which KDIGO guidelines3 recommend treatment with DMTs that reduce pathogenic Gd-IgA1 IgAN US population US annualized pricing of Tarpeyo — US WAC pricing of Voyxact5 112K–199K US IgAN patients2 85K–151K patients4 eligible for BHV-1400 $180K–390K per patient per year DEGRADERS DMT, Disease Modifying Therapy. 1. Goldman Sachs August 11, 2025 IgAN report (assumes $150K annualized net pricing). 2. Cantor Fitzgerald & Co US Equity Research March 18, 2025 and Nov 25, 2025. 3. KDIGO 2025 Clinical Practice Guideline for the Management of Immunoglobulin A Nephropathy (IgAN) and Immunoglobulin A Vasculitis (IgAV). 4. Pitcher. CJASN. 2023. 5. Based on December 2025 announced Voyxact WAC of $30K per vial, Q4W dosing, $390K annualized. IgAN market size could be twice as large with Voyxact pricing. PODIUM
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1400: A Singular Asset Combining a First-in-Class Mechanism, Compelling Human Data and a Clear Commercial Path January 12, 2026 Biohaven Corporate Presentation23 DIFFERENTIATED SCIENCE The only therapy that directly targets and eliminates the root pathogenic driver of IgAN A true paradigm shift from production inhibition or downstream damage control to precision antigen removal DE-RISKED BIOLOGY Human proof-of-concept achieved with rapid (>70%), deep and durable target engagement The fundamental mechanism is validated, shifting focus to clinical execution MASSIVE OPPORTUNITY Positioned to become a foundational therapy by addressing the core unmet need in a multibillion-dollar market A clear, regulator-endorsed clinical path to approval and commercialization NO BROAD IMMUNOSUPPRESSION Leverages a natural clearance pathway rather than inhibiting immune cell function DEGRADERS

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-1300 IgG antibody Graves’ Disease DEGRADERS: BHV-1300 MoDE PODIUM
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1300: Potential to Transform Care Across Multiple IgG Mediated Diseases January 12, 2026 Biohaven Corporate Presentation25 DERMATOLOGY Pemphigus vulgaris, bullous pemphigoid, pemphigus foliaceus RENAL Lupus nephritis, antibody- mediated rejection, membranous nephropathy IMMUNOLOGY AND INFLAMMATION Graves’ diseases, thyroid eye disease, anti-drug antibodies for gene therapies and biologics DEGRADERS NEUROLOGY AND NEUROMUSCULAR Myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, neuromyelitis optica spectrum disorder, autoimmune encephalitis, myelin oligodendrocyte glycoprotein antibody disease RHEUMATOLOGY Sjogren’s, systemic sclerosis, rheumatoid arthritis, cutaneous lupus erythematosus, myositis, fibromyalgia HEMATOLOGY Immune thrombocytopenia, autoimmune hemolytic anemia
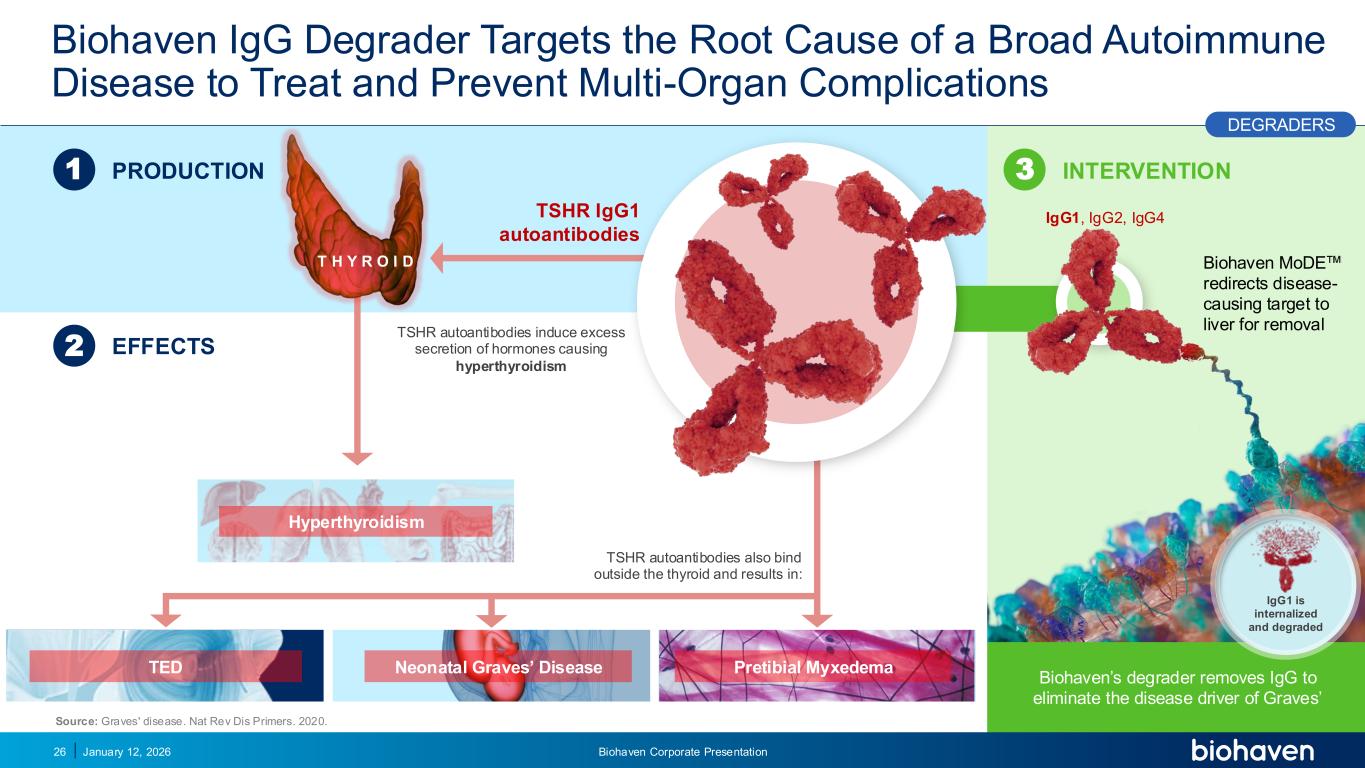
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBiohaven IgG Degrader Targets the Root Cause of a Broad Autoimmune Disease to Treat and Prevent Multi-Organ Complications January 12, 2026 Biohaven Corporate Presentation26 TSHR IgG1 autoantibodies 1 2 PRODUCTION EFFECTS INTERVENTION3 Biohaven’s degrader removes IgG to eliminate the disease driver of Graves’ IgG1, IgG2, IgG4 TSHR autoantibodies also bind outside the thyroid and results in: Biohaven MoDE redirects disease- causing target to liver for removal IgG1 is internalized and degraded TSHR autoantibodies induce excess secretion of hormones causing hyperthyroidism TED Neonatal Graves’ Disease Pretibial Myxedema Source: Graves' disease. Nat Rev Dis Primers. 2020. DEGRADERS T H Y R O I D Hyperthyroidism PODIUM PR
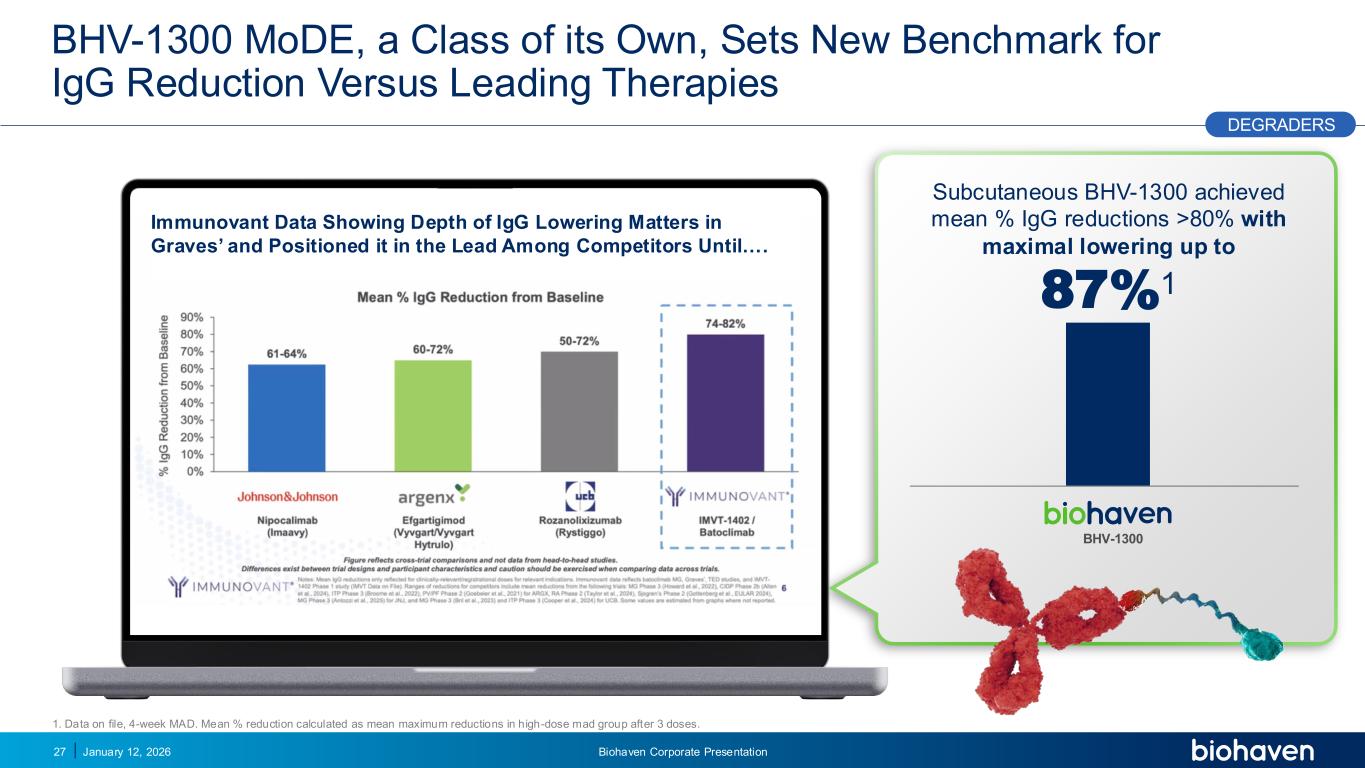
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Immunovant Data Showing Depth of IgG Lowering Matters in Graves’ and Positioned it in the Lead Among Competitors Until…. BHV-1300 MoDE, a Class of its Own, Sets New Benchmark for IgG Reduction Versus Leading Therapies January 12, 2026 Biohaven Corporate Presentation27 BHV-1300 Subcutaneous BHV-1300 achieved mean % IgG reductions >80% with maximal lowering up to 87%1 DEGRADERS PODIUM 1. Data on file, 4-week MAD. Mean % reduction calculated as mean maximum reductions in high-dose mad group after 3 doses.
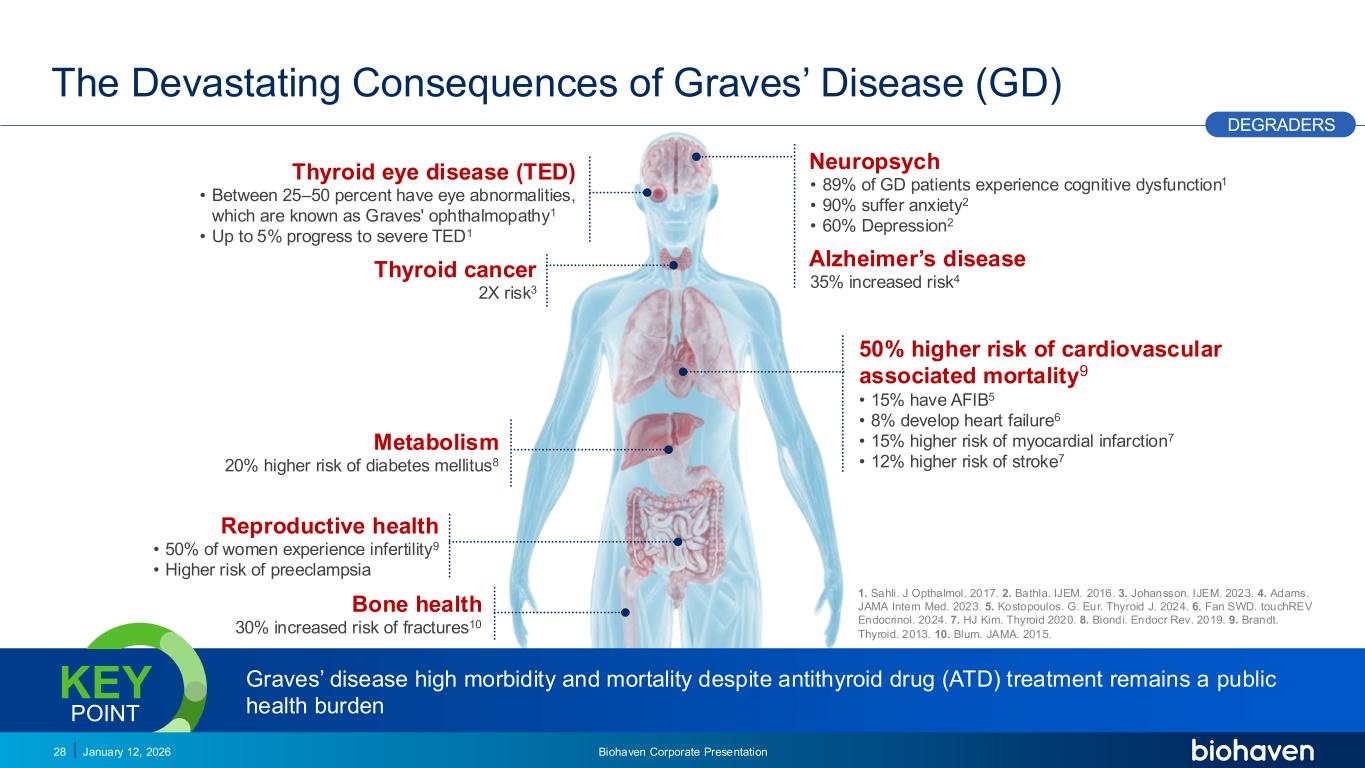
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Graves’ disease high morbidity and mortality despite antithyroid drug (ATD) treatment remains a public health burden KEY POINT The Devastating Consequences of Graves’ Disease (GD) January 12, 2026 Biohaven Corporate Presentation28 Thyroid eye disease (TED) • Between 25–50 percent have eye abnormalities, which are known as Graves' ophthalmopathy1 • Up to 5% progress to severe TED1 Thyroid cancer 2X risk3 Neuropsych • 89% of GD patients experience cognitive dysfunction1 • 90% suffer anxiety2 • 60% Depression2 Alzheimer’s disease 35% increased risk4 50% higher risk of cardiovascular associated mortality9 • 15% have AFIB5 • 8% develop heart failure6 • 15% higher risk of myocardial infarction7 • 12% higher risk of stroke7 Bone health 30% increased risk of fractures10 Metabolism 20% higher risk of diabetes mellitus8 Reproductive health • 50% of women experience infertility9 • Higher risk of preeclampsia 1. Sahli. J Opthalmol. 2017. 2. Bathla. IJEM. 2016. 3. Johansson. IJEM. 2023. 4. Adams. JAMA Intern Med. 2023. 5. Kostopoulos. G. Eur. Thyroid J. 2024. 6. Fan SWD. touchREV Endocrinol. 2024. 7. HJ Kim. Thyroid 2020. 8. Biondi. Endocr Rev. 2019. 9. Brandt. Thyroid. 2013. 10. Blum. JAMA. 2015. DEGRADERS
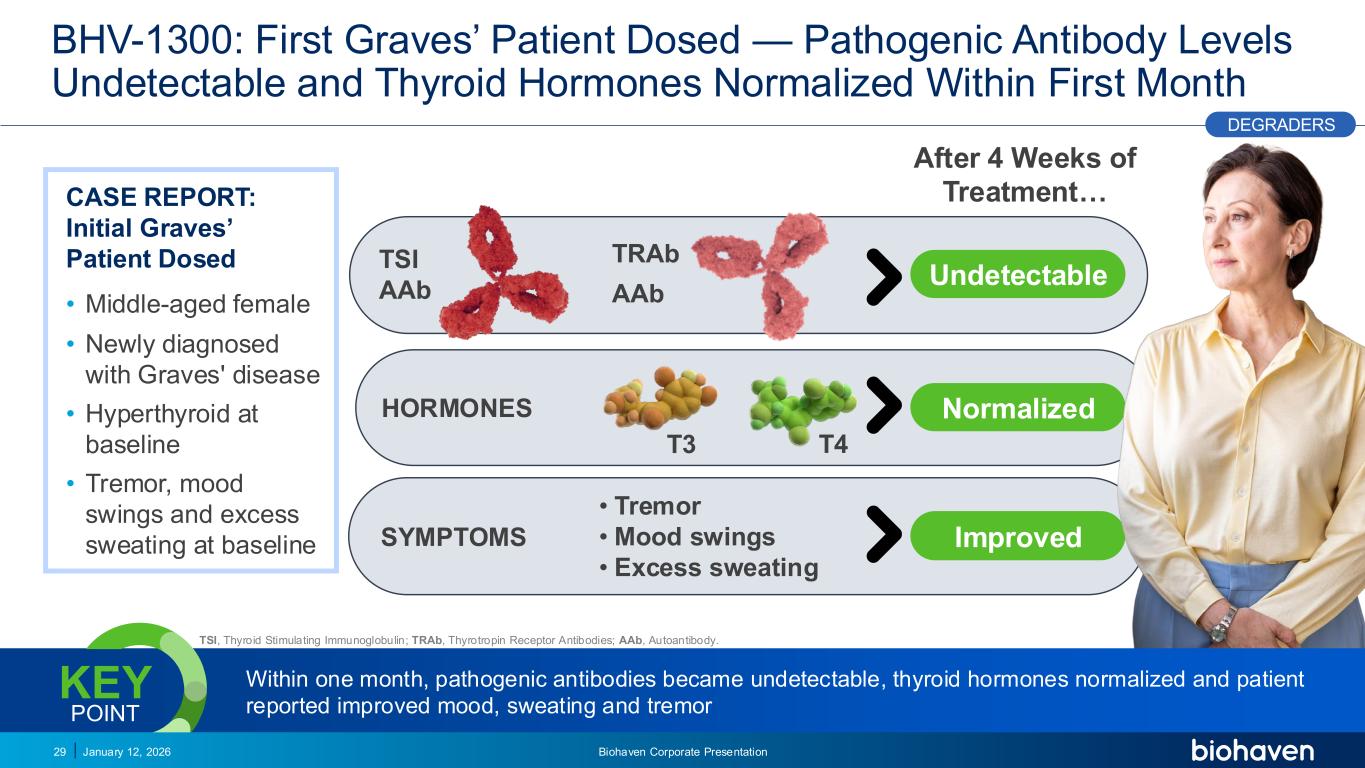
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY After 4 Weeks of Treatment… T3 T4 TSI AAb TRAb AAb HORMONES Undetectable Normalized BHV-1300: First Graves’ Patient Dosed — Pathogenic Antibody Levels Undetectable and Thyroid Hormones Normalized Within First Month January 12, 2026 Biohaven Corporate Presentation29 Within one month, pathogenic antibodies became undetectable, thyroid hormones normalized and patient reported improved mood, sweating and tremor KEY POINT PODIUM DEGRADERS CASE REPORT: Initial Graves’ Patient Dosed • Middle-aged female • Newly diagnosed with Graves' disease • Hyperthyroid at baseline • Tremor, mood swings and excess sweating at baseline SYMPTOMS Improved • Tremor •Mood swings • Excess sweating TSI, Thyroid Stimulating Immunoglobulin; TRAb, Thyrotropin Receptor Antibodies; AAb, Autoantibody. PR
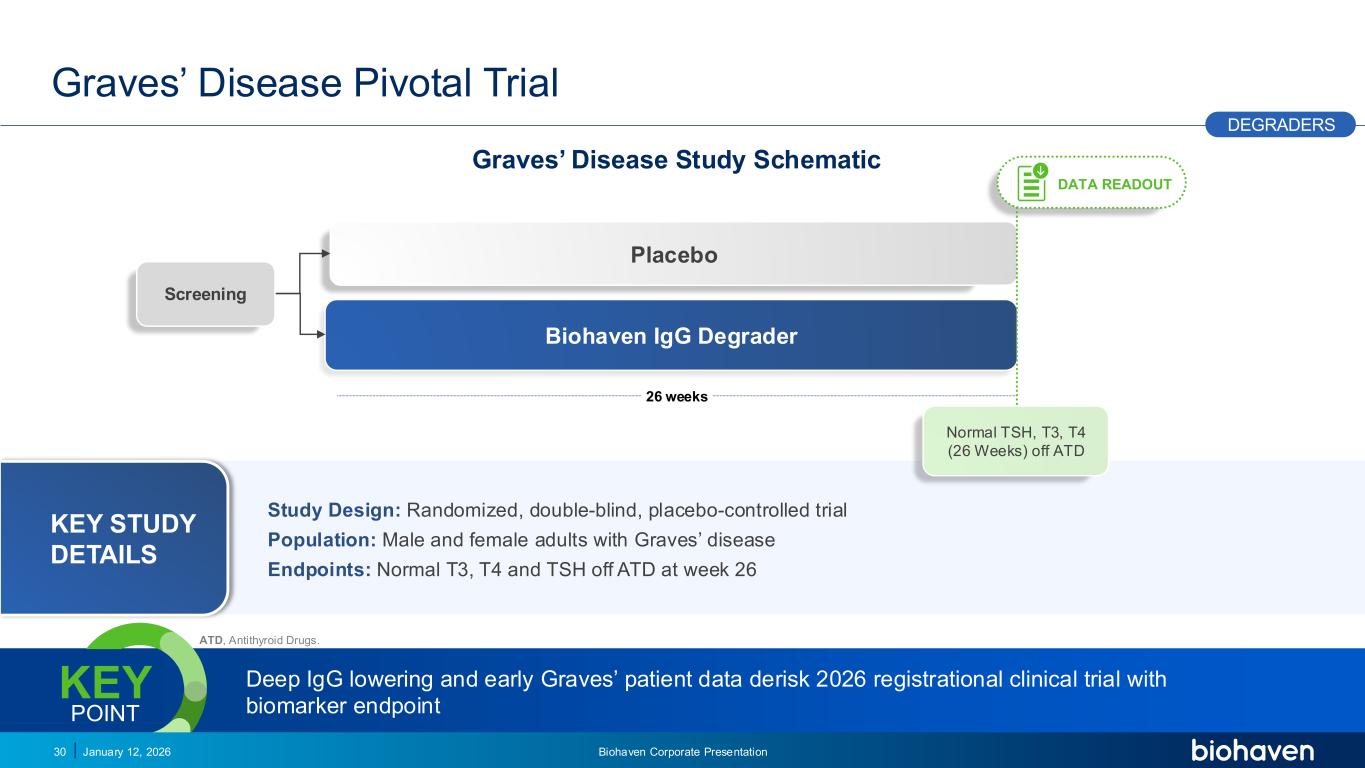
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Graves’ Disease Pivotal Trial January 12, 2026 Biohaven Corporate Presentation30 26 weeks Biohaven IgG Degrader Placebo Screening DEGRADERS DATA READOUT Graves’ Disease Study Schematic Deep IgG lowering and early Graves’ patient data derisk 2026 registrational clinical trial with biomarker endpoint KEY POINT Study Design: Randomized, double-blind, placebo-controlled trial Population: Male and female adults with Graves’ disease Endpoints: Normal T3, T4 and TSH off ATD at week 26 KEY STUDY DETAILS Normal TSH, T3, T4 (26 Weeks) off ATD PODIUM ATD, Antithyroid Drugs.
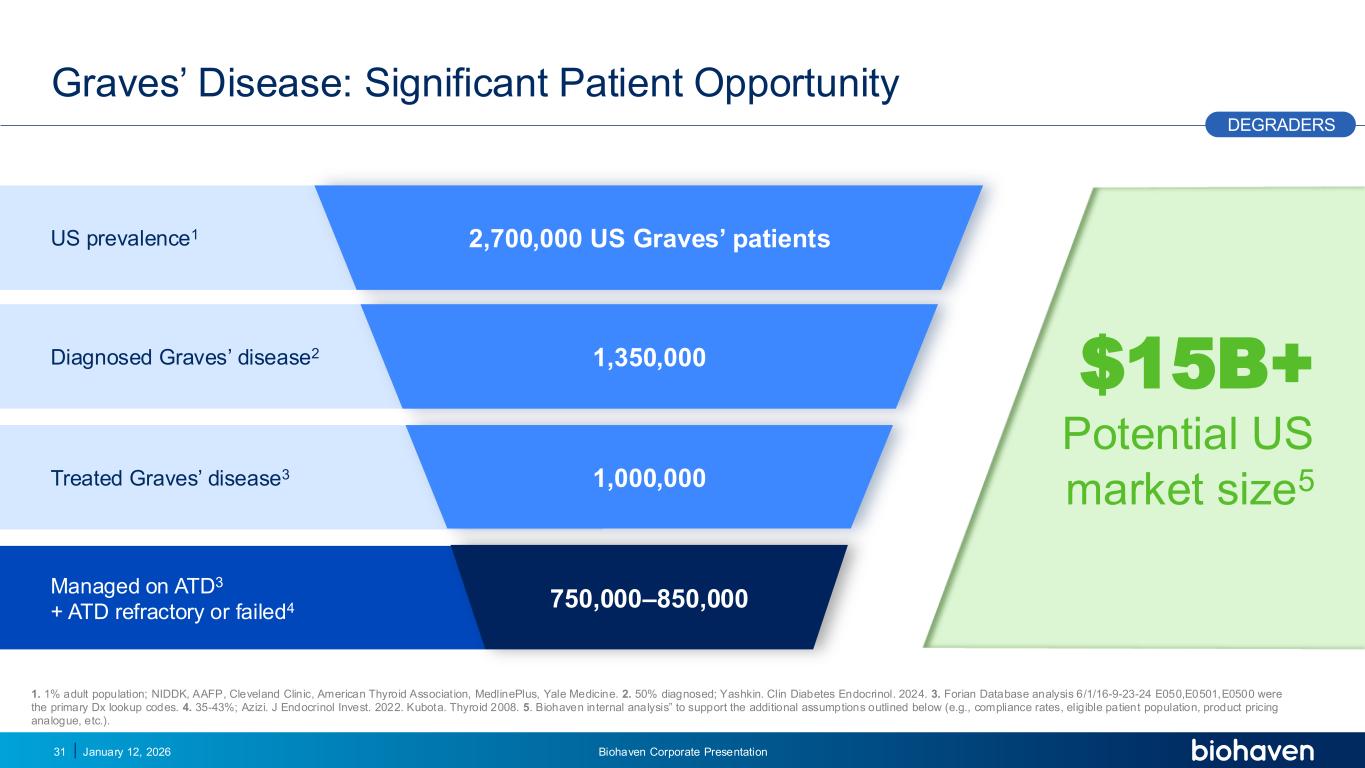
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY January 12, 2026 Biohaven Corporate Presentation31 Graves’ Disease: Significant Patient Opportunity DEGRADERS Diagnosed Graves’ disease2 US prevalence1 Treated Graves’ disease3 Managed on ATD3 + ATD refractory or failed4 2,700,000 US Graves’ patients 1,350,000 1,000,000 750,000–850,000 1. 1% adult population; NIDDK, AAFP, Cleveland Clinic, American Thyroid Association, MedlinePlus, Yale Medicine. 2. 50% diagnosed; Yashkin. Clin Diabetes Endocrinol. 2024. 3. Forian Database analysis 6/1/16-9-23-24 E050,E0501,E0500 were the primary Dx lookup codes. 4. 35-43%; Azizi. J Endocrinol Invest. 2022. Kubota. Thyroid 2008. 5. Biohaven internal analysis” to support the additional assumptions outlined below (e.g., compliance rates, eligible patient population, product pricing analogue, etc.). PODIUM $15B+ Potential US market size5

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY OPAKALIM Kv7 ACTIVATOR ION CHANNEL Revolutionizing Epilepsy Treatment With a Modern Kv7 Activator ION CHANNEL: OPAKALIM SELECTIVE Kv7 ACTIVATOR PODIUM
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYKv7 Activation Is a Clinically Validated Mechanism of Action for the Treatment of Epilepsy January 12, 2026 Biohaven Corporate Presentation33 Opakalim, a selective Kv7 activator, offers paradigm-shifting potential to control seizures without the burdensome side effects frequently reported with approved antiseizure medicines and those in development Kv7 DESIGNED TO SELECTIVELY ACTIVATE Kv7.2/7.3 CHANNELS WITHOUT IMPACTING GABA RECEPTORS DEMONSTRATES EXCEPTIONAL SAFETY PROFILE WITH LOW RATES OF CNS ADVERSE EVENTS ACROSS STUDIES DELIVERING FOCAL EPILEPSY TOPLINE DATA IN 1H 2026 Efficacy signals observed in open-label epilepsy data, comparable to competitor data, with favorable tolerabilityNEWS BREAKING

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYHigh Unmet Need for Novel, Well-tolerated and Effective Antiseizure Medicines January 12, 2026 Biohaven Corporate Presentation34 P R E V A L E N C E 1 3.5 MILLION people with epilepsy (PWE) in the US Kv7 H E A L T H C A R E S P E N D I N G 3 $24.5 BILLION annual spending in the US (direct costs) 1. www.cdc.gov/mmwr/volumes/66/wr/mm6631a1.htm. 2. French. Epilepsia. 2007. 3. www.cdc.gov/epilepsy/data-research/facts-stats/index.html#:~:text= Health%20care%20spending,3. R E F R A C T O R Y 2 UP TO 40% of PWE continue to have seizures despite treatment EMILY, Living with Epilepsy
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Q U A L I T Y O F L I F E 4 50% of PWE report lower QoL A D H E R E N C E 2 UP TO 60% of PWE report non-adherence to ASMs High Unmet Need for Novel, Well-tolerated and Effective Antiseizure Medicines January 12, 2026 Biohaven Corporate Presentation35 C O M O R B I D I T I E S 3 50% of PWE report at least 1 comorbidity A D V E R S E E V E N T S 1 80% of PWE report AEs after starting an ASM Kv7 1. Baker. Epilepsia. 1997. 2. Donahue. Neurol Clin Pract. 2025. 3. Bosak. Epilepsy & Behavior. 2025. 4. Strzelczyk. Epilepsy & Behavior. 2023. VENIKA, Living with Epilepsy
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Opakalim Overcomes the Challenges of Approved Epilepsy Therapies January 12, 2026 Biohaven Corporate Presentation36 Kv7 OPAKALIMAPPROVED THERAPIES Easy-to-use • Once-daily tablet • No need to titrate Minimal CNS side effects Very low rates of CNS AEs Does not make comorbidities worse Improved seizure control Clinically validated MoA complementary to existing ASMs allowing rational combinations Burdensome • Dosing multiple times per day • Months long titration schedules Poor CNS tolerability • High rates of CNS AEs • Somnolence, dizziness, cognitive problems Make comorbidities worse 50% of patients report comorbidities i.e., mood Limited seizure control Up to 40% of patients are refractory to treatment ✕ ✕ ✕ ✕ Opakalim offers compelling ASM profile for patients with potential to drive enhanced clinical outcomes and superior quality of life KEY POINT PODIUM ASMs, Antiseizure Medications.
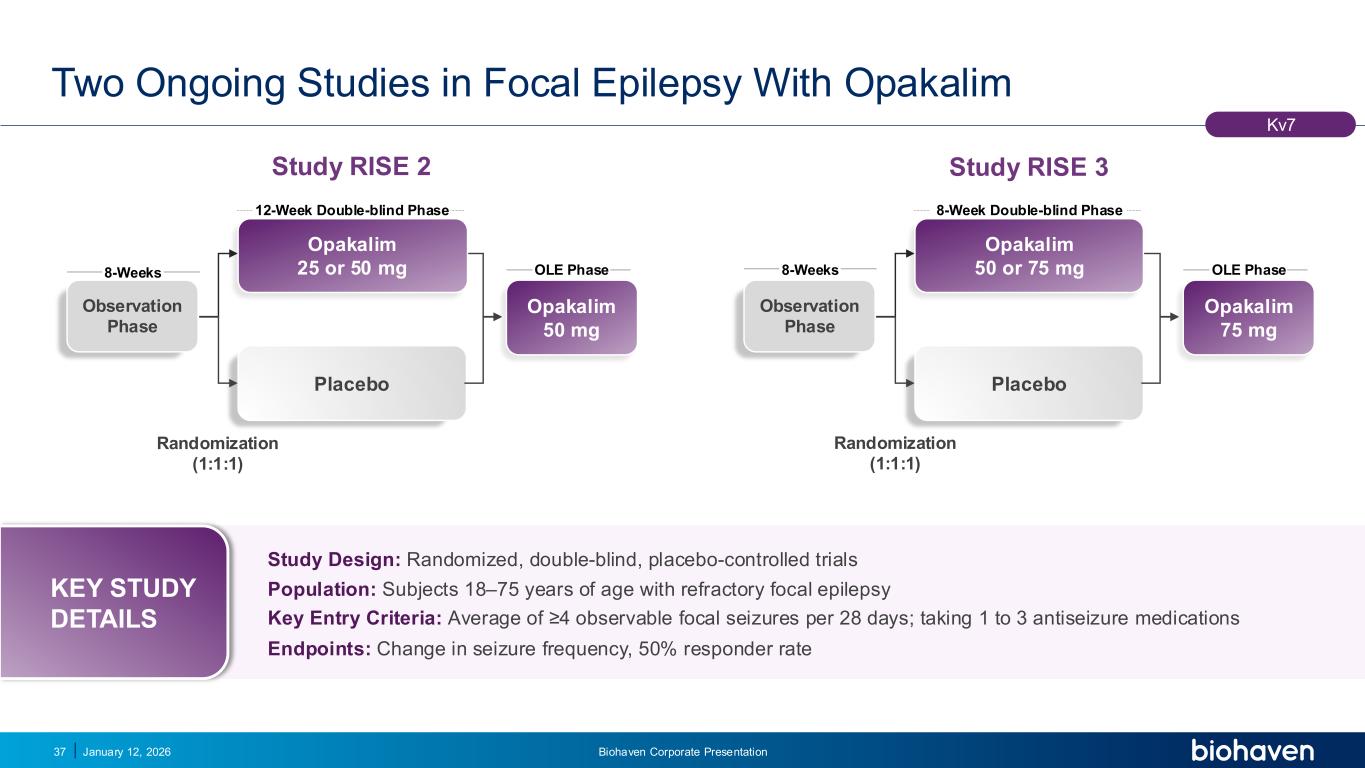
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Study Design: Randomized, double-blind, placebo-controlled trials Population: Subjects 18–75 years of age with refractory focal epilepsy Key Entry Criteria: Average of ≥4 observable focal seizures per 28 days; taking 1 to 3 antiseizure medications Endpoints: Change in seizure frequency, 50% responder rate KEY STUDY DETAILS Two Ongoing Studies in Focal Epilepsy With Opakalim January 12, 2026 Biohaven Corporate Presentation37 Observation Phase Placebo 12-Week Double-blind Phase 8-Weeks OLE Phase Opakalim 25 or 50 mg Opakalim 50 mg Observation Phase Placebo 8-Week Double-blind Phase 8-Weeks OLE Phase Opakalim 50 or 75 mg Opakalim 75 mg Randomization (1:1:1) Randomization (1:1:1) Kv7 Study RISE 2 Study RISE 3
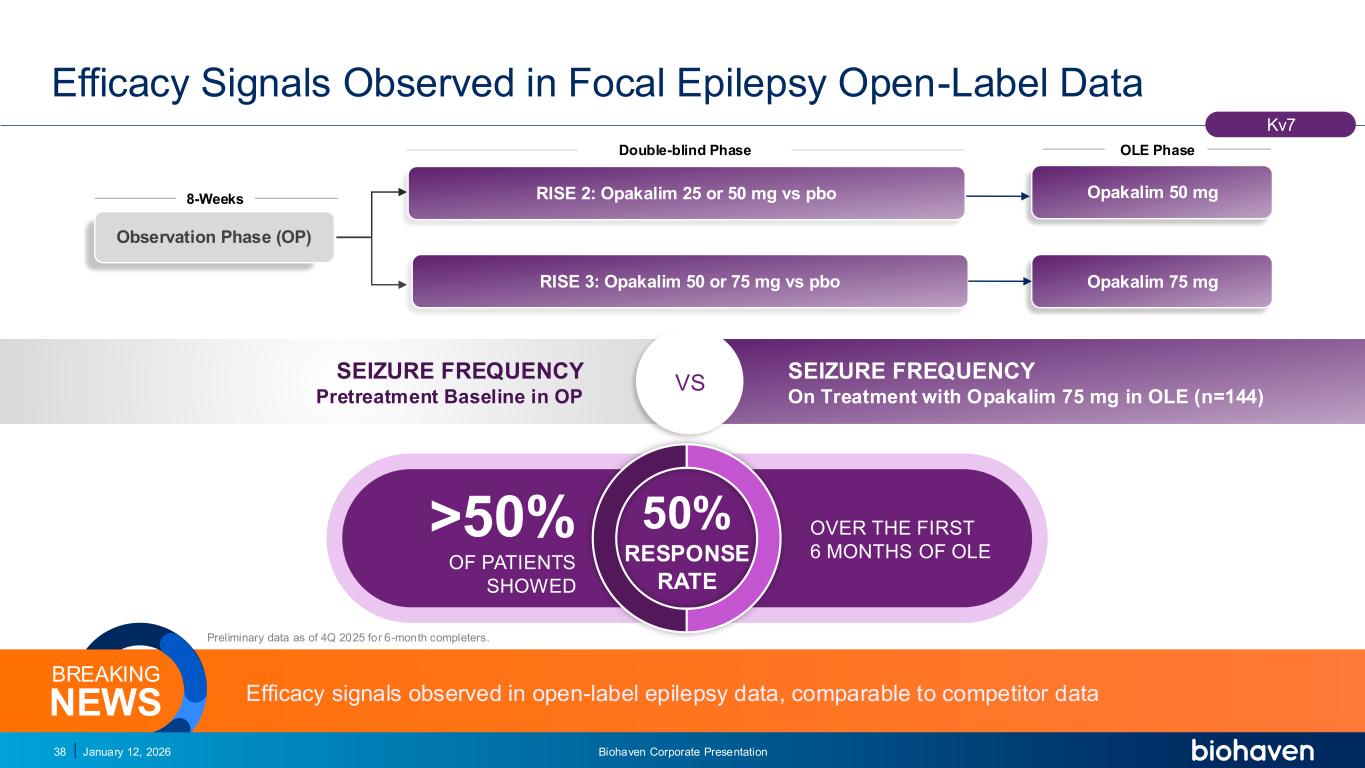
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY RISE 3: Opakalim 50 or 75 mg vs pbo RISE 2: Opakalim 25 or 50 mg vs pbo Double-blind Phase 8-Weeks OLE Phase Efficacy Signals Observed in Focal Epilepsy Open-Label Data January 12, 2026 Biohaven Corporate Presentation38 Kv7 Preliminary data as of 4Q 2025 for 6-month completers. Observation Phase (OP) Opakalim 75 mg SEIZURE FREQUENCY Pretreatment Baseline in OP SEIZURE FREQUENCY On Treatment with Opakalim 75 mg in OLE (n=144) VS Efficacy signals observed in open-label epilepsy data, comparable to competitor dataNEWS BREAKING Opakalim 50 mg PODIUM >50% OF PATIENTS SHOWED OVER THE FIRST 6 MONTHS OF OLE 50% RESPONSE RATE
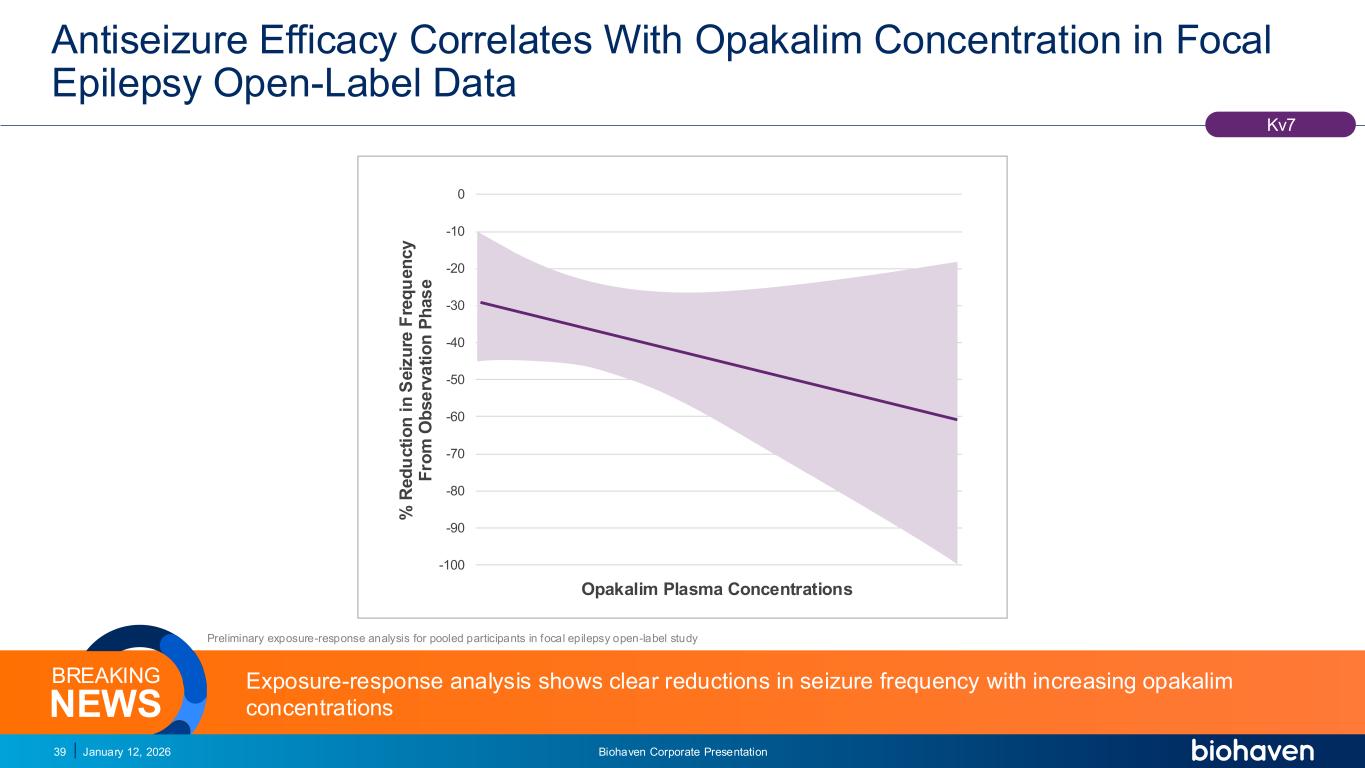
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 % R e d u c ti o n i n S e iz u re F re q u e n c y F ro m O b s e rv a ti o n P h a s e Opakalim Plasma Concentrations Antiseizure Efficacy Correlates With Opakalim Concentration in Focal Epilepsy Open-Label Data January 12, 2026 Biohaven Corporate Presentation39 Kv7 Preliminary exposure-response analysis for pooled participants in focal epilepsy open-label study Exposure-response analysis shows clear reductions in seizure frequency with increasing opakalim concentrationsNEWS BREAKING PODIUM
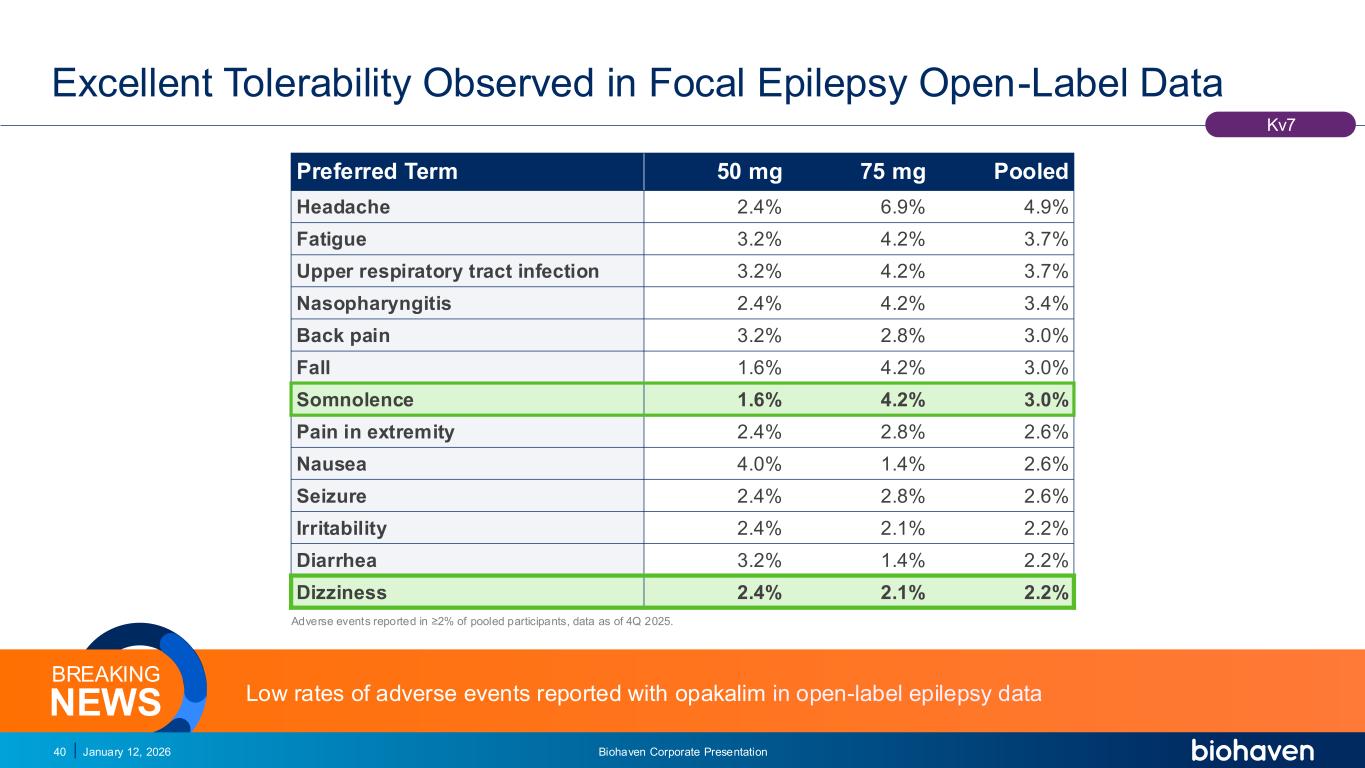
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Excellent Tolerability Observed in Focal Epilepsy Open-Label Data January 12, 2026 Biohaven Corporate Presentation40 Kv7 Preferred Term 50 mg 75 mg Pooled Headache 2.4% 6.9% 4.9% Fatigue 3.2% 4.2% 3.7% Upper respiratory tract infection 3.2% 4.2% 3.7% Nasopharyngitis 2.4% 4.2% 3.4% Back pain 3.2% 2.8% 3.0% Fall 1.6% 4.2% 3.0% Somnolence 1.6% 4.2% 3.0% Pain in extremity 2.4% 2.8% 2.6% Nausea 4.0% 1.4% 2.6% Seizure 2.4% 2.8% 2.6% Irritability 2.4% 2.1% 2.2% Diarrhea 3.2% 1.4% 2.2% Dizziness 2.4% 2.1% 2.2% Adverse events reported in ≥2% of pooled participants, data as of 4Q 2025. Low rates of adverse events reported with opakalim in open-label epilepsy dataNEWS BREAKING
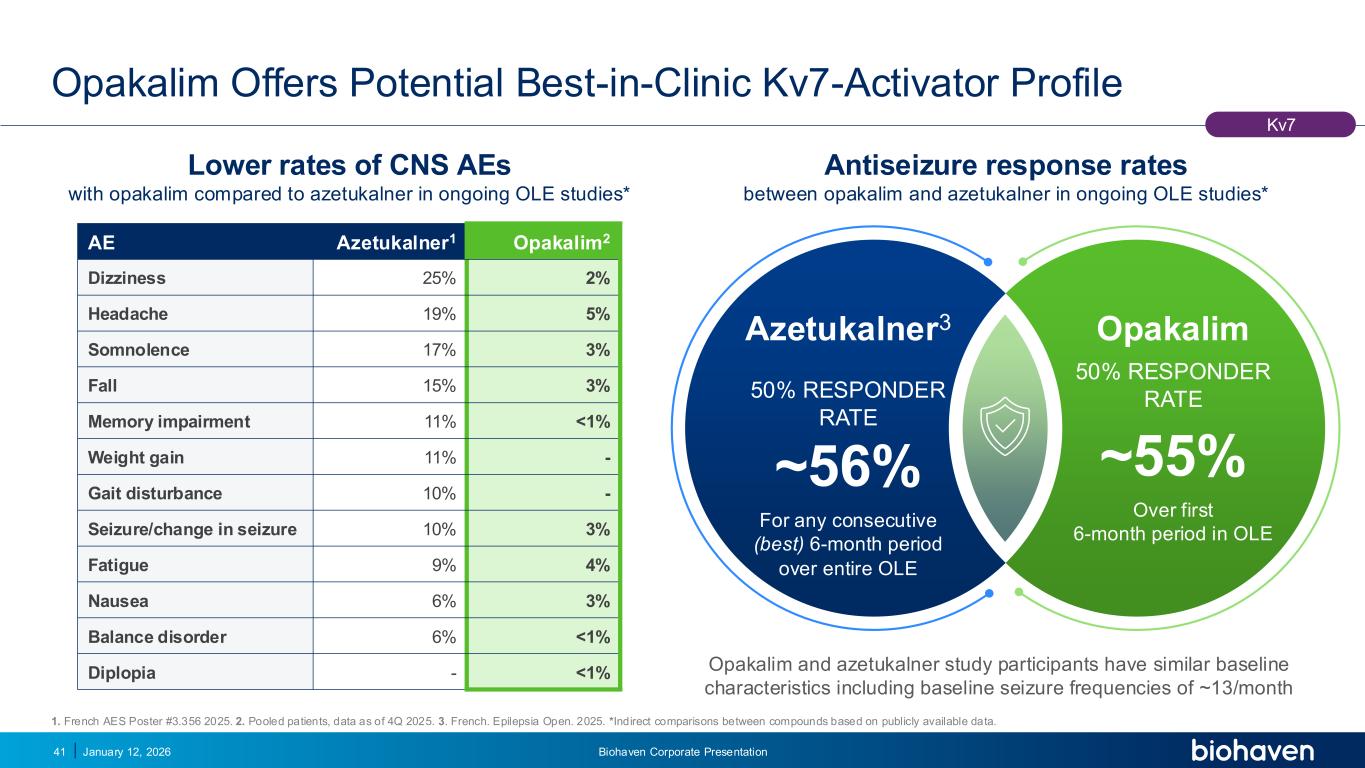
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Opakalim Offers Potential Best-in-Clinic Kv7-Activator Profile January 12, 2026 Biohaven Corporate Presentation41 1. French AES Poster #3.356 2025. 2. Pooled patients, data as of 4Q 2025. 3. French. Epilepsia Open. 2025. *Indirect comparisons between compounds based on publicly available data. Kv7 Opakalim and azetukalner study participants have similar baseline characteristics including baseline seizure frequencies of ~13/month AE Azetukalner1 Opakalim2 Dizziness 25% 2% Headache 19% 5% Somnolence 17% 3% Fall 15% 3% Memory impairment 11% <1% Weight gain 11% - Gait disturbance 10% - Seizure/change in seizure 10% 3% Fatigue 9% 4% Nausea 6% 3% Balance disorder 6% <1% Diplopia - <1% Azetukalner3 50% RESPONDER RATE ~56% For any consecutive (best) 6-month period over entire OLE Lower rates of CNS AEs with opakalim compared to azetukalner in ongoing OLE studies* Antiseizure response rates between opakalim and azetukalner in ongoing OLE studies* Opakalim 50% RESPONDER RATE ~55% Over first 6-month period in OLE PODIUM PR
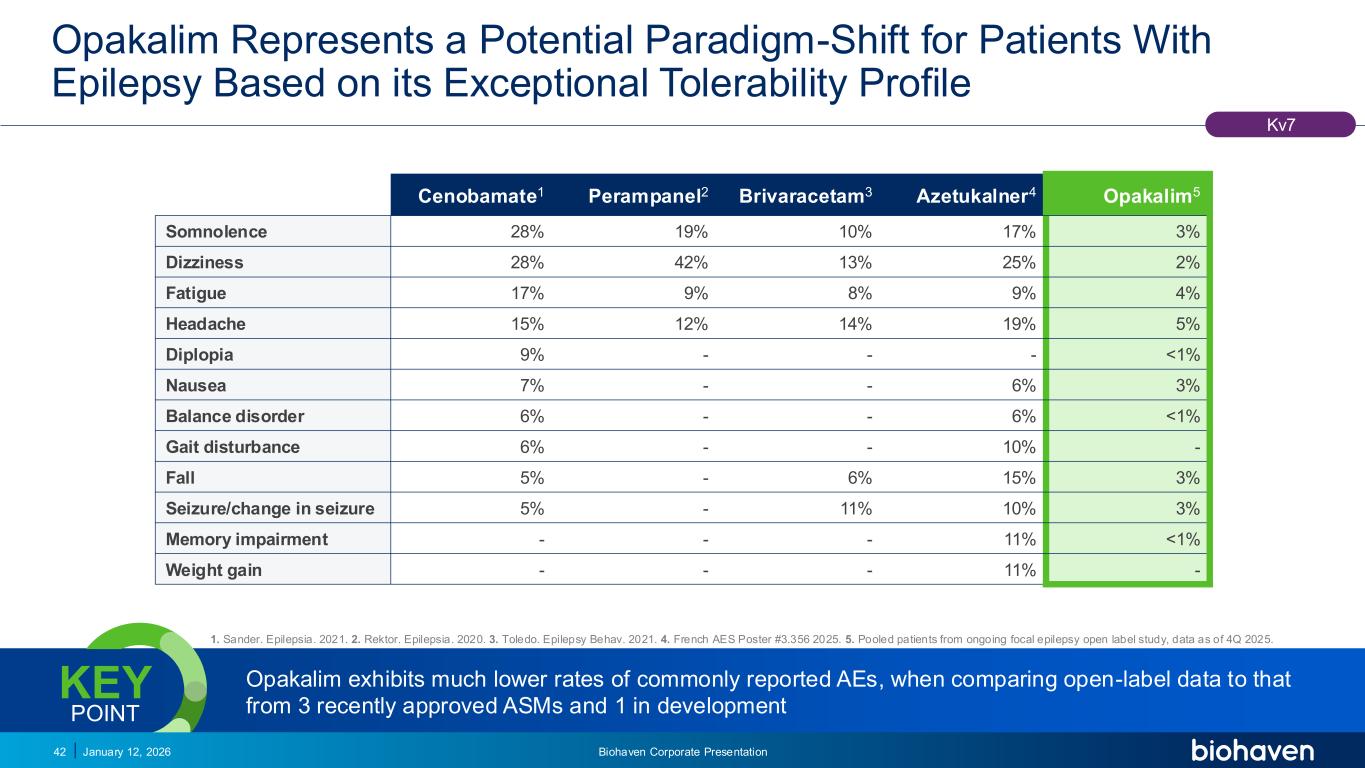
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYOpakalim Represents a Potential Paradigm-Shift for Patients With Epilepsy Based on its Exceptional Tolerability Profile January 12, 2026 Biohaven Corporate Presentation42 1. Sander. Epilepsia. 2021. 2. Rektor. Epilepsia. 2020. 3. Toledo. Epilepsy Behav. 2021. 4. French AES Poster #3.356 2025. 5. Pooled patients from ongoing focal epilepsy open label study, data as of 4Q 2025. Opakalim exhibits much lower rates of commonly reported AEs, when comparing open-label data to that from 3 recently approved ASMs and 1 in development KEY POINT Kv7 Cenobamate1 Perampanel2 Brivaracetam3 Azetukalner4 Opakalim5 Somnolence 28% 19% 10% 17% 3% Dizziness 28% 42% 13% 25% 2% Fatigue 17% 9% 8% 9% 4% Headache 15% 12% 14% 19% 5% Diplopia 9% - - - <1% Nausea 7% - - 6% 3% Balance disorder 6% - - 6% <1% Gait disturbance 6% - - 10% - Fall 5% - 6% 15% 3% Seizure/change in seizure 5% - 11% 10% 3% Memory impairment - - - 11% <1% Weight gain - - - 11% -
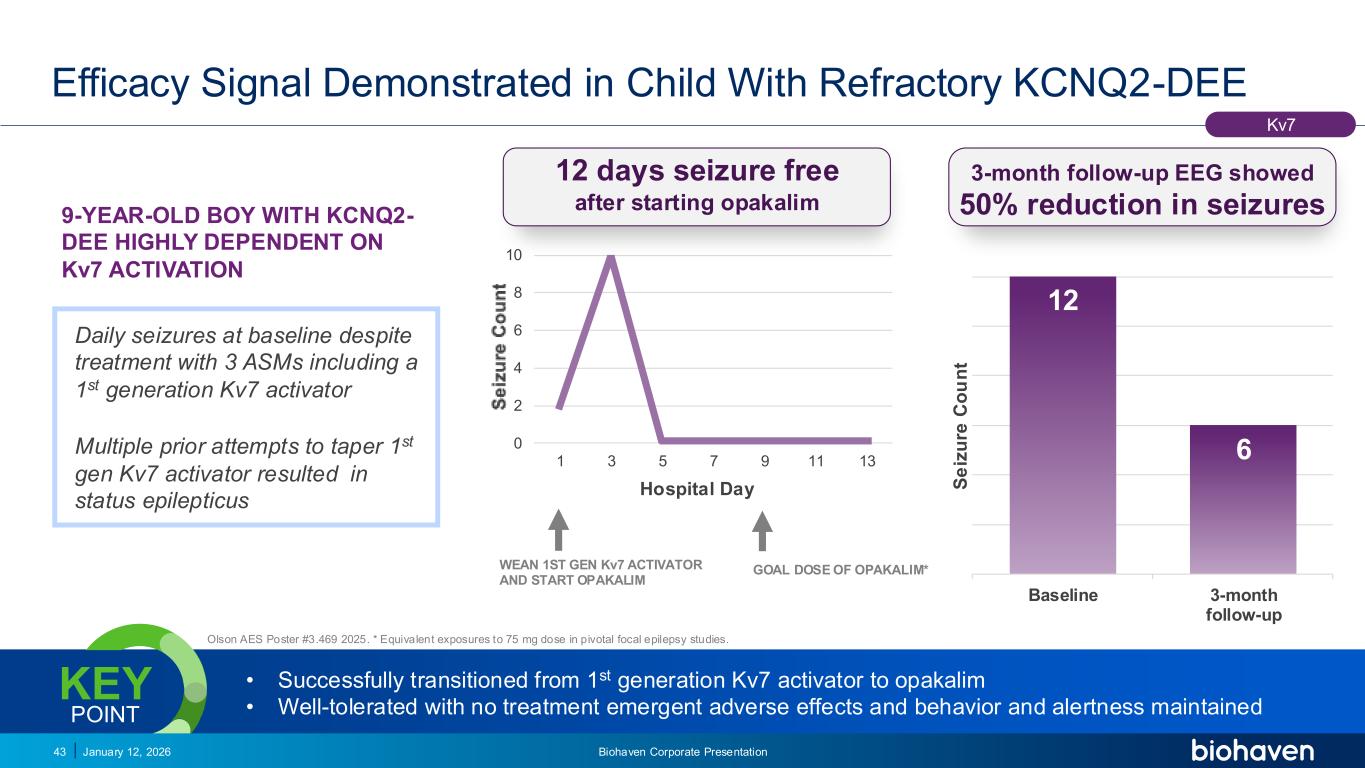
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Daily seizures at baseline despite treatment with 3 ASMs including a 1st generation Kv7 activator Multiple prior attempts to taper 1st gen Kv7 activator resulted in status epilepticus Efficacy Signal Demonstrated in Child With Refractory KCNQ2-DEE January 12, 2026 Biohaven Corporate Presentation43 Olson AES Poster #3.469 2025. * Equivalent exposures to 75 mg dose in pivotal focal epilepsy studies. • Successfully transitioned from 1st generation Kv7 activator to opakalim • Well-tolerated with no treatment emergent adverse effects and behavior and alertness maintained KEY POINT 12 6 Baseline 3-month follow-up S e iz u re C o u n t 3-month follow-up EEG showed 50% reduction in seizures Kv7 9-YEAR-OLD BOY WITH KCNQ2- DEE HIGHLY DEPENDENT ON Kv7 ACTIVATION 0 2 4 6 8 10 1 3 5 7 9 11 13 Hospital Day 12 days seizure free after starting opakalim WEAN 1ST GEN Kv7 ACTIVATOR AND START OPAKALIM GOAL DOSE OF OPAKALIM*
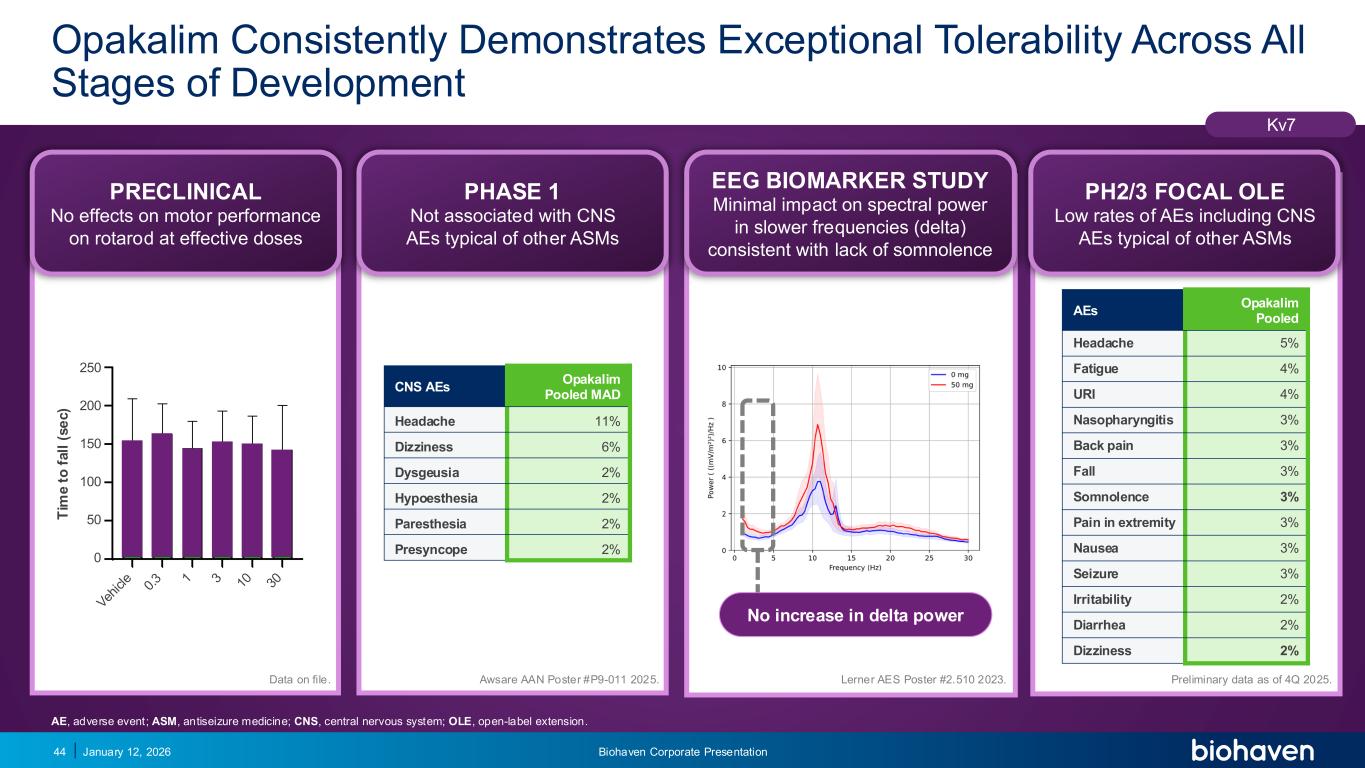
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYOpakalim Consistently Demonstrates Exceptional Tolerability Across All Stages of Development January 12, 2026 Biohaven Corporate Presentation44 V eh ic le 0. 3 1 3 10 30 0 50 100 150 200 250 BHV-7000 (mg/kg) T im e t o f a ll ( s e c ): R o ta ro d T im e t o f a ll ( s e c ) Kv7 PRECLINICAL No effects on motor performance on rotarod at effective doses PHASE 1 Not associated with CNS AEs typical of other ASMs EEG BIOMARKER STUDY Minimal impact on spectral power in slower frequencies (delta) consistent with lack of somnolence PH2/3 FOCAL OLE Low rates of AEs including CNS AEs typical of other ASMs AEs Opakalim Pooled Headache 5% Fatigue 4% URI 4% Nasopharyngitis 3% Back pain 3% Fall 3% Somnolence 3% Pain in extremity 3% Nausea 3% Seizure 3% Irritability 2% Diarrhea 2% Dizziness 2% No increase in delta power Preliminary data as of 4Q 2025.Data on file. Awsare AAN Poster #P9-011 2025. CNS AEs Opakalim Pooled MAD Headache 11% Dizziness 6% Dysgeusia 2% Hypoesthesia 2% Paresthesia 2% Presyncope 2% Lerner AES Poster #2.510 2023. AE, adverse event; ASM, antiseizure medicine; CNS, central nervous system; OLE, open-label extension.
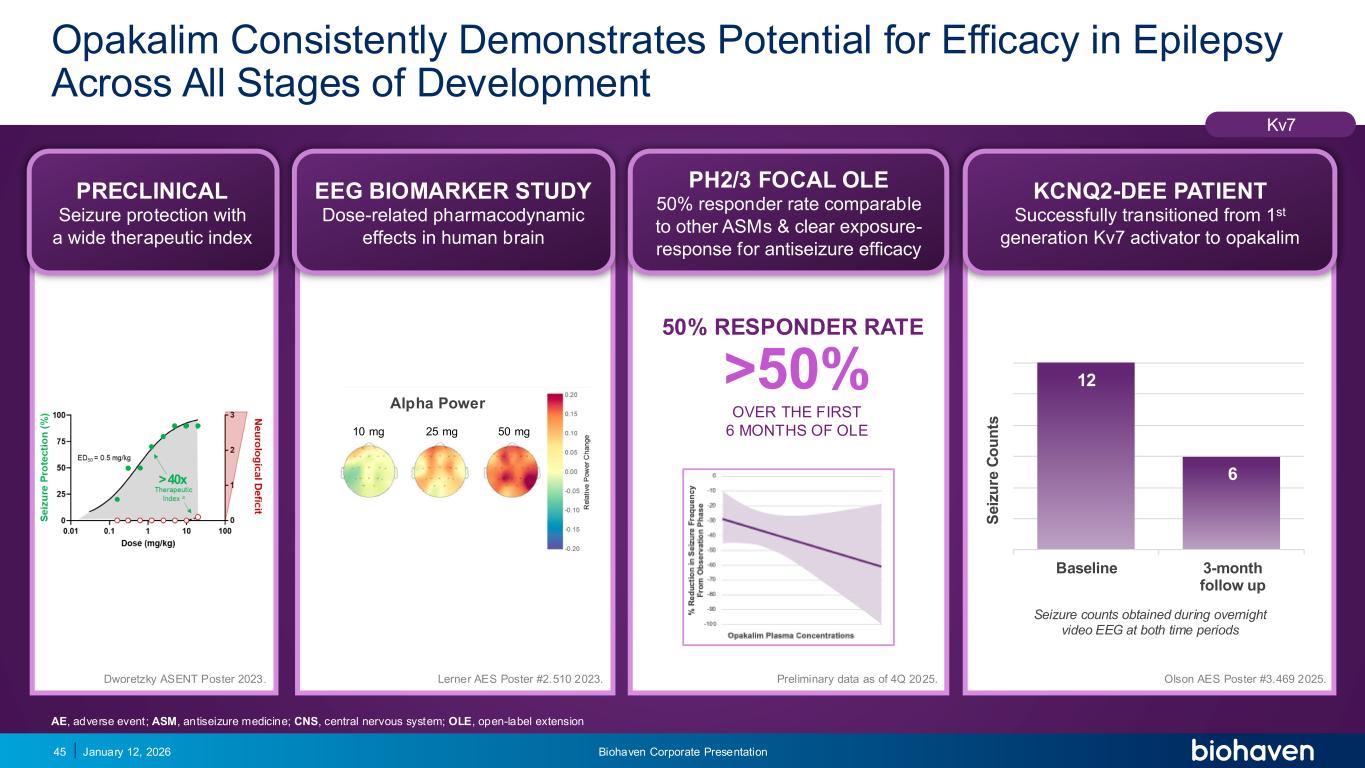
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYOpakalim Consistently Demonstrates Potential for Efficacy in Epilepsy Across All Stages of Development January 12, 2026 Biohaven Corporate Presentation45 PRECLINICAL Seizure protection with a wide therapeutic index Kv7 12 6 Baseline 3-month follow up S e iz u re C o u n ts PRECLINICAL Seizure protection with a wide therapeutic index EEG BIOMARKER STUDY Dose-related pharmacodynamic effects in human brain PH2/3 FOCAL OLE 50% responder rate comparable to other ASMs & clear exposure- response for antiseizure efficacy KCNQ2-DEE PATIENT Successfully transitioned from 1st generation Kv7 activator to opakalim Preliminary data as of 4Q 2025. Seizure counts obtained during overnight video EEG at both time periods Olson AES Poster #3.469 2025.Dworetzky ASENT Poster 2023. Alpha Power 10 mg 25 mg 50 mg Lerner AES Poster #2.510 2023. 50% RESPONDER RATE >50% OVER THE FIRST 6 MONTHS OF OLE AE, adverse event; ASM, antiseizure medicine; CNS, central nervous system; OLE, open-label extension
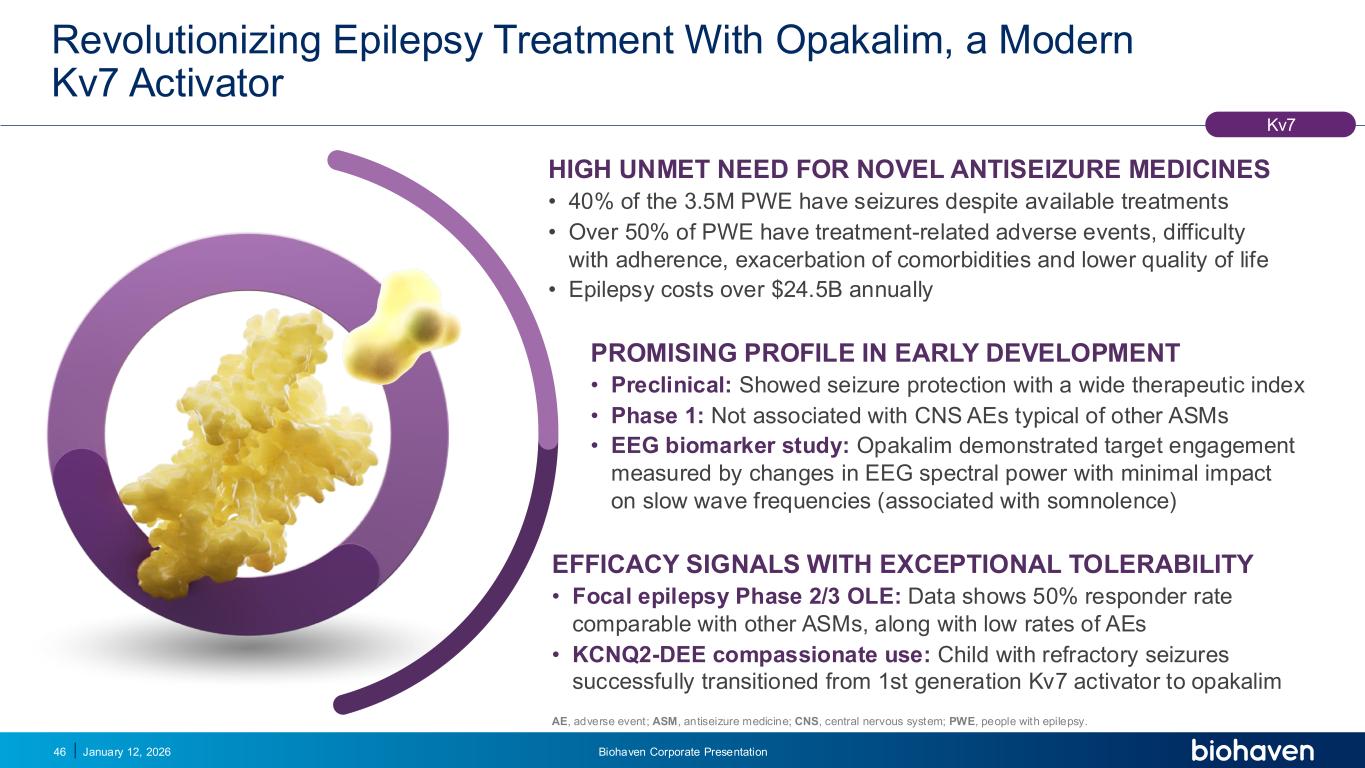
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYRevolutionizing Epilepsy Treatment With Opakalim, a Modern Kv7 Activator January 12, 2026 Biohaven Corporate Presentation46 HIGH UNMET NEED FOR NOVEL ANTISEIZURE MEDICINES • 40% of the 3.5M PWE have seizures despite available treatments • Over 50% of PWE have treatment-related adverse events, difficulty with adherence, exacerbation of comorbidities and lower quality of life • Epilepsy costs over $24.5B annually PROMISING PROFILE IN EARLY DEVELOPMENT • Preclinical: Showed seizure protection with a wide therapeutic index • Phase 1: Not associated with CNS AEs typical of other ASMs • EEG biomarker study: Opakalim demonstrated target engagement measured by changes in EEG spectral power with minimal impact on slow wave frequencies (associated with somnolence) EFFICACY SIGNALS WITH EXCEPTIONAL TOLERABILITY • Focal epilepsy Phase 2/3 OLE: Data shows 50% responder rate comparable with other ASMs, along with low rates of AEs • KCNQ2-DEE compassionate use: Child with refractory seizures successfully transitioned from 1st generation Kv7 activator to opakalim Kv7 AE, adverse event; ASM, antiseizure medicine; CNS, central nervous system; PWE, people with epilepsy.

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY TALDEFGROBEP / MYOSTATIN COMPLEX Targeting High-Quality Weight Loss MYOSTATIN ACTIVIN INHIBITOR: TALDEFGROBEP ALFA PODIUM

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY MYOSTATIN ACTIVIN MOA FOR HEALTHY WEIGHT LOSS Targeting myostatin, activin A and activin E/ALK7 signaling SAFETY DATABASE IN >700 TREATED TO DATE Differentiated safety profile CONVENIENT DOSING Potential for once monthly self-administration Targeting High-Quality Weight Loss With Myostatin Activin Mechanism January 12, 2026 Biohaven Corporate Presentation48 Taldefgrobep directly targets fat, builds muscle and increases bone density while avoiding intolerable adverse effects TALDEFGROBEP Initiated Phase 2 monotherapy study with topline expected 2026NEWS BREAKING PODIUM
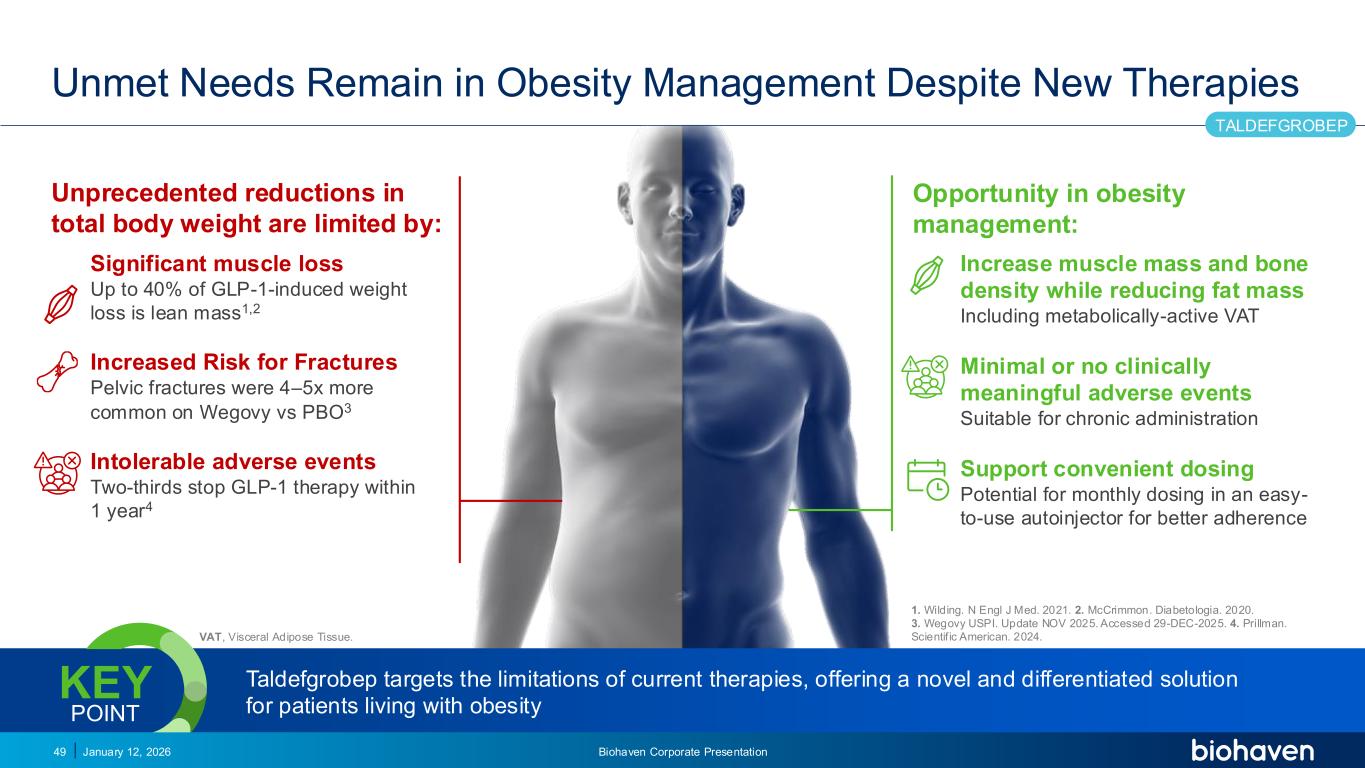
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Unmet Needs Remain in Obesity Management Despite New Therapies January 12, 2026 Biohaven Corporate Presentation49 Taldefgrobep targets the limitations of current therapies, offering a novel and differentiated solution for patients living with obesity KEY POINT Increase muscle mass and bone density while reducing fat mass Including metabolically-active VAT Minimal or no clinically meaningful adverse events Suitable for chronic administration Support convenient dosing Potential for monthly dosing in an easy- to-use autoinjector for better adherence Opportunity in obesity management: TALDEFGROBEP Unprecedented reductions in total body weight are limited by: Significant muscle loss Up to 40% of GLP-1-induced weight loss is lean mass1,2 Increased Risk for Fractures Pelvic fractures were 4–5x more common on Wegovy vs PBO3 Intolerable adverse events Two-thirds stop GLP-1 therapy within 1 year4 1. Wilding. N Engl J Med. 2021. 2. McCrimmon. Diabetologia. 2020. 3. Wegovy USPI. Update NOV 2025. Accessed 29-DEC-2025. 4. Prillman. Scientif ic American. 2024. VAT, Visceral Adipose Tissue.
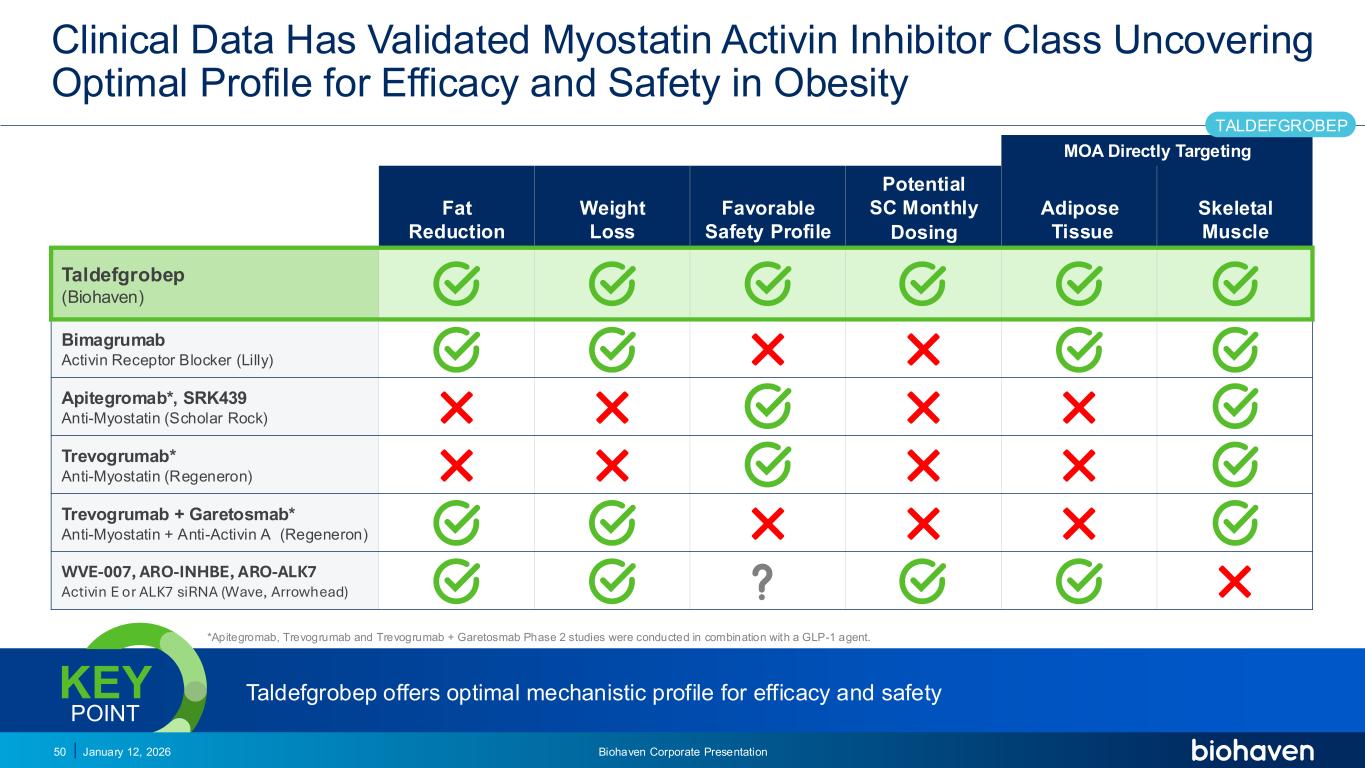
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYClinical Data Has Validated Myostatin Activin Inhibitor Class Uncovering Optimal Profile for Efficacy and Safety in Obesity MOA Directly Targeting Fat Reduction Weight Loss Favorable Safety Profile Potential SC Monthly Dosing Adipose Tissue Skeletal Muscle Taldefgrobep (Biohaven) Bimagrumab Activin Receptor Blocker (Lilly) ✕ ✕ Apitegromab*, SRK439 Anti-Myostatin (Scholar Rock) ✕ ✕ ✕ ✕ Trevogrumab* Anti-Myostatin (Regeneron) ✕ ✕ ✕ ✕ Trevogrumab + Garetosmab* Anti-Myostatin + Anti-Activin A (Regeneron) ✕ ✕ ✕ WVE-007, ARO-INHBE, ARO-ALK7 Activin E or ALK7 siRNA (Wave, Arrowhead) ✕ January 12, 2026 Biohaven Corporate Presentation50 *Apitegromab, Trevogrumab and Trevogrumab + Garetosmab Phase 2 studies were conducted in combination with a GLP-1 agent. Taldefgrobep offers optimal mechanistic profile for efficacy and safety KEY POINT TALDEFGROBEP
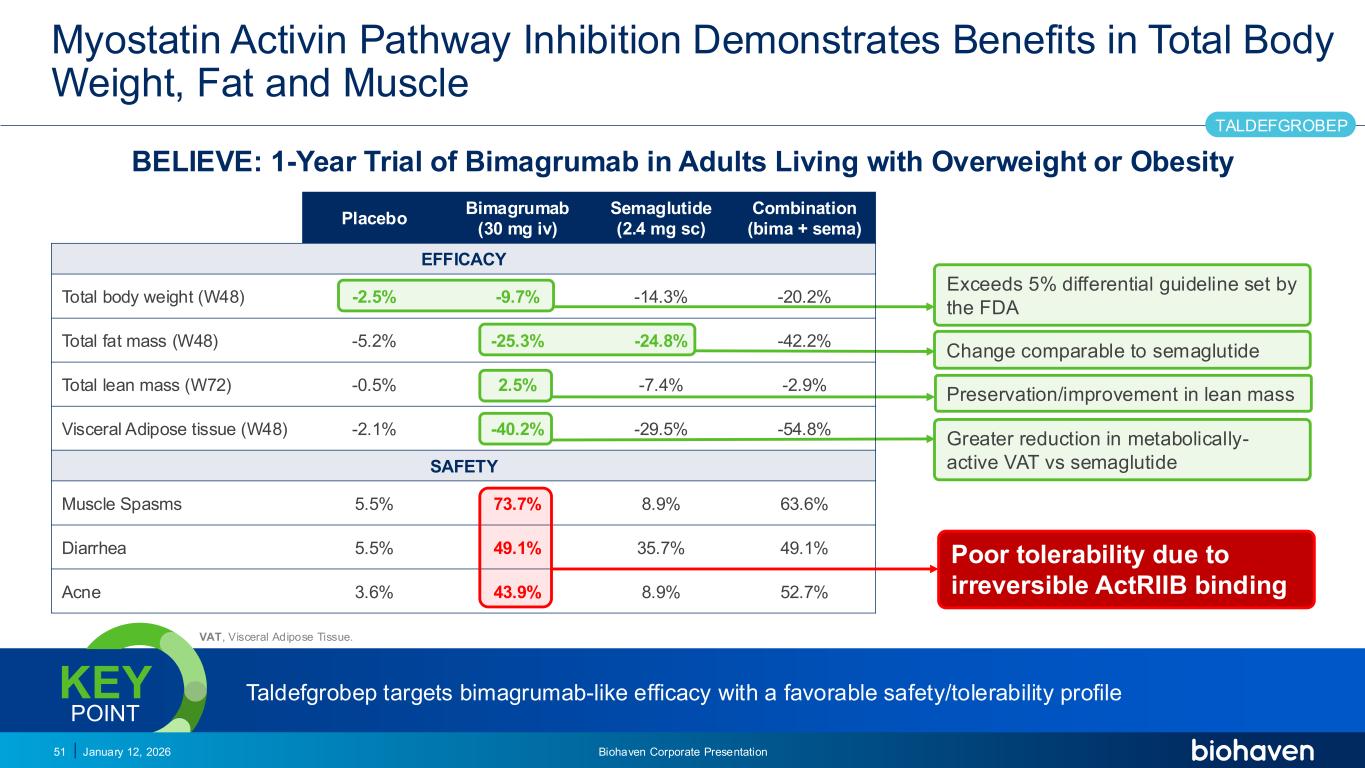
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYMyostatin Activin Pathway Inhibition Demonstrates Benefits in Total Body Weight, Fat and Muscle BELIEVE: 1-Year Trial of Bimagrumab in Adults Living with Overweight or Obesity January 12, 2026 Biohaven Corporate Presentation51 Exceeds 5% differential guideline set by the FDA Change comparable to semaglutide Preservation/improvement in lean mass Poor tolerability due to irreversible ActRIIB binding Placebo Bimagrumab (30 mg iv) Semaglutide (2.4 mg sc) Combination (bima + sema) EFFICACY Total body weight (W48) -2.5% -9.7% -14.3% -20.2% Total fat mass (W48) -5.2% -25.3% -24.8% -42.2% Total lean mass (W72) -0.5% 2.5% -7.4% -2.9% Visceral Adipose tissue (W48) -2.1% -40.2% -29.5% -54.8% SAFETY Muscle Spasms 5.5% 73.7% 8.9% 63.6% Diarrhea 5.5% 49.1% 35.7% 49.1% Acne 3.6% 43.9% 8.9% 52.7% TALDEFGROBEP Greater reduction in metabolically- active VAT vs semaglutide Taldefgrobep targets bimagrumab-like efficacy with a favorable safety/tolerability profileKEY POINT PODIUM VAT, Visceral Adipose Tissue.
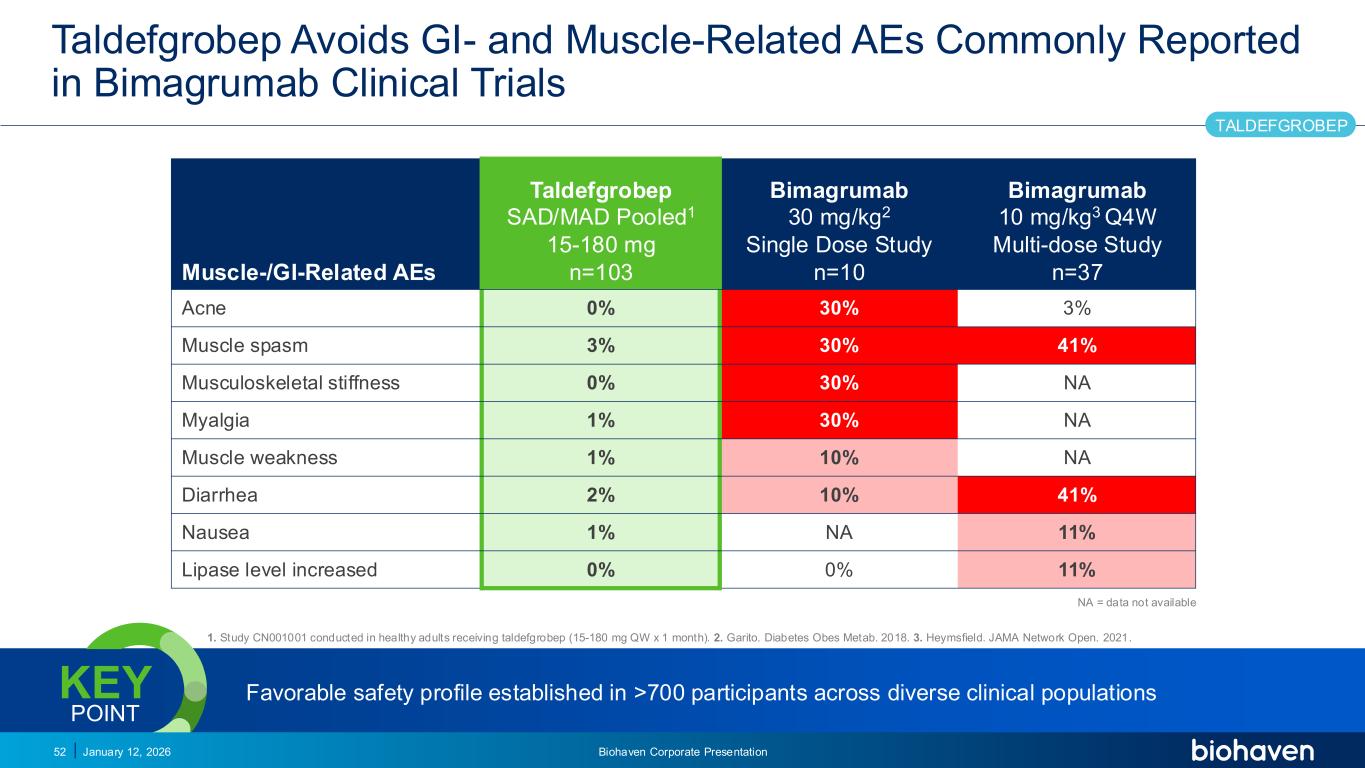
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYTaldefgrobep Avoids GI- and Muscle-Related AEs Commonly Reported in Bimagrumab Clinical Trials January 12, 2026 Biohaven Corporate Presentation52 Muscle-/GI-Related AEs Taldefgrobep SAD/MAD Pooled1 15-180 mg n=103 Bimagrumab 30 mg/kg2 Single Dose Study n=10 Bimagrumab 10 mg/kg3 Q4W Multi-dose Study n=37 Acne 0% 30% 3% Muscle spasm 3% 30% 41% Musculoskeletal stiffness 0% 30% NA Myalgia 1% 30% NA Muscle weakness 1% 10% NA Diarrhea 2% 10% 41% Nausea 1% NA 11% Lipase level increased 0% 0% 11% NA = data not available 1. Study CN001001 conducted in healthy adults receiving taldefgrobep (15-180 mg QW x 1 month). 2. Garito. Diabetes Obes Metab. 2018. 3. Heymsfield. JAMA Network Open. 2021. TALDEFGROBEP Favorable safety profile established in >700 participants across diverse clinical populationsKEY POINT PODIUM
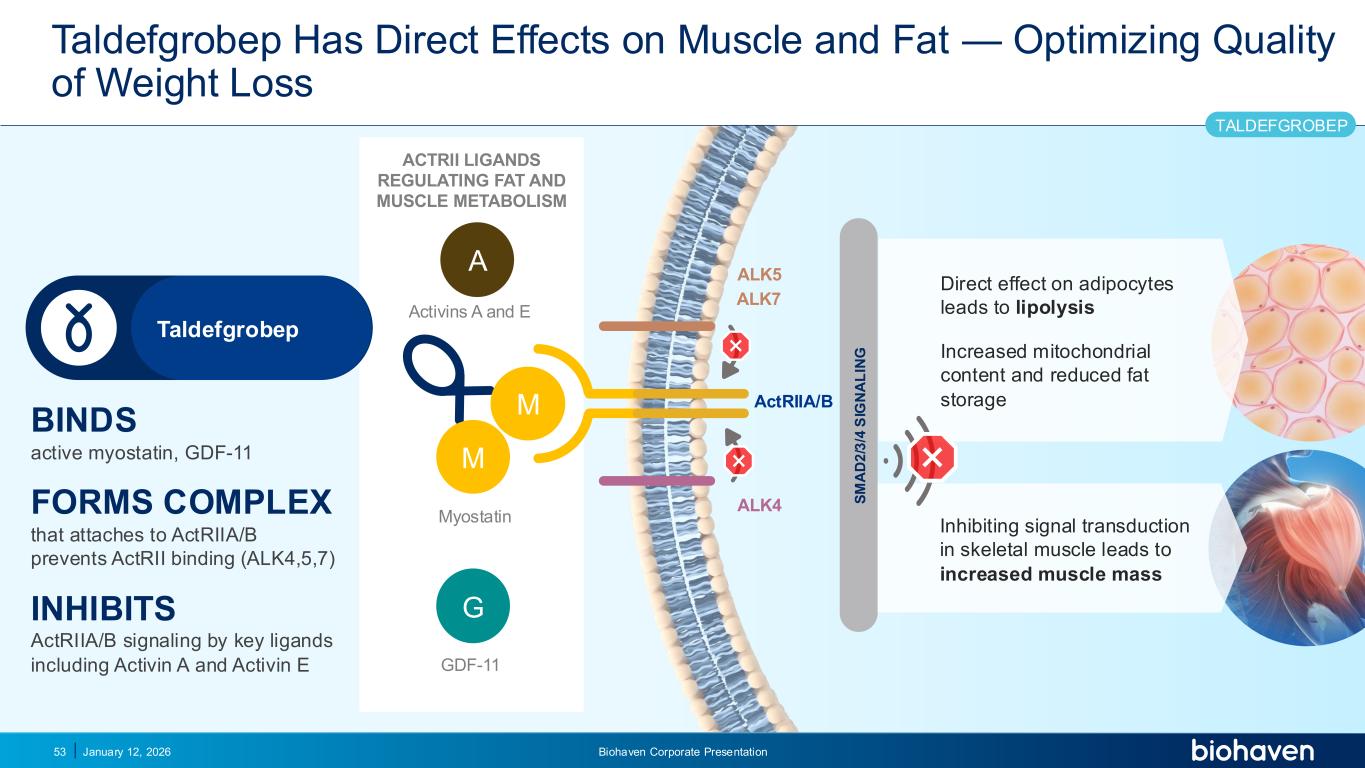
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYTaldefgrobep Has Direct Effects on Muscle and Fat — Optimizing Quality of Weight Loss January 12, 2026 Biohaven Corporate Presentation53 BINDS active myostatin, GDF-11 FORMS COMPLEX that attaches to ActRIIA/B prevents ActRII binding (ALK4,5,7) INHIBITS ActRIIA/B signaling by key ligands including Activin A and Activin E S M A D 2 /3 /4 S IG N A L IN G ALK4 ALK5 ActRIIA/BM M G ALK7 A Myostatin GDF-11 ACTRII LIGANDS REGULATING FAT AND MUSCLE METABOLISM Activins A and E Inhibiting signal transduction in skeletal muscle leads to increased muscle mass Direct effect on adipocytes leads to lipolysis Increased mitochondrial content and reduced fat storage Taldefgrobep TALDEFGROBEP
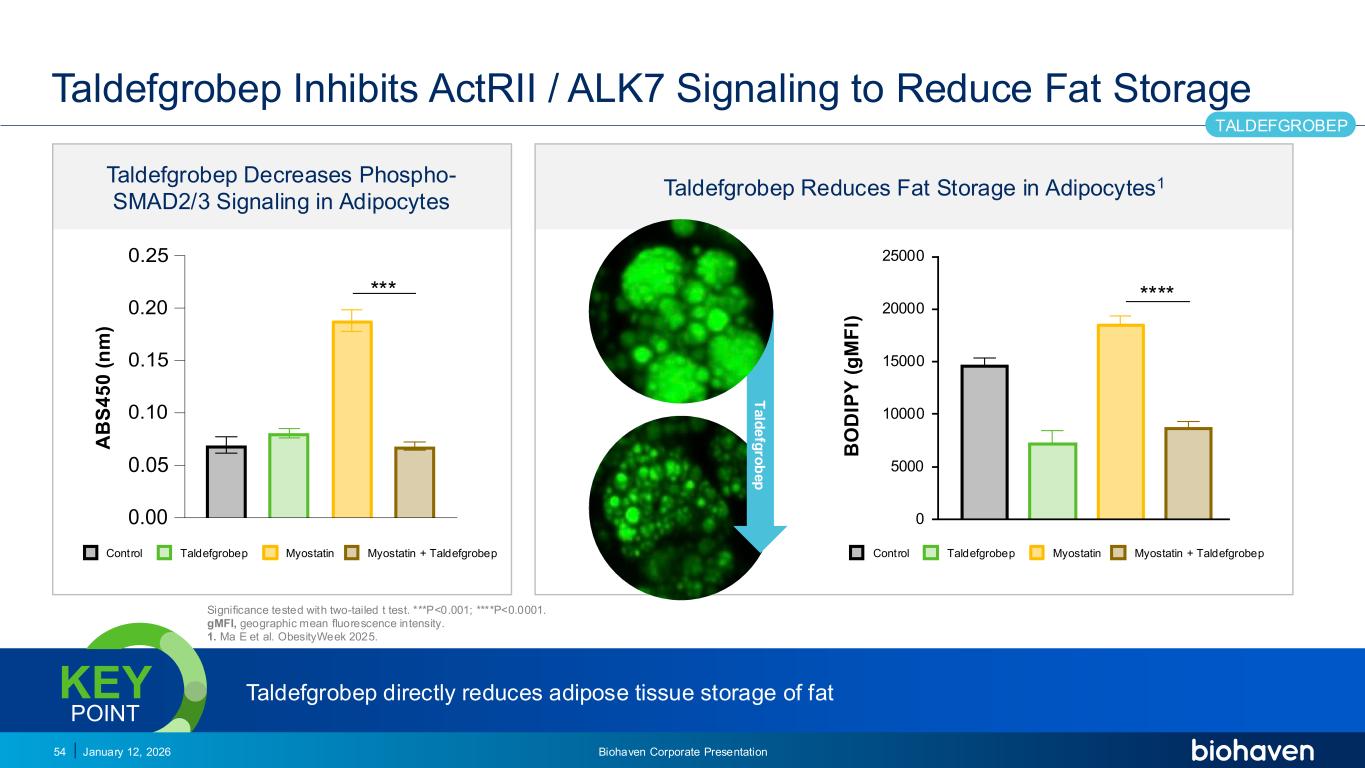
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Taldefgrobep Reduces Fat Storage in Adipocytes1 Taldefgrobep Inhibits ActRII / ALK7 Signaling to Reduce Fat Storage January 12, 2026 Biohaven Corporate Presentation54 TALDEFGROBEP 0 5000 10000 15000 20000 25000 B O D IP Y ( g M F I) Significance tested with two-tailed t test. ***P<0.001; ****P<0.0001. gMFI, geographic mean fluorescence intensity. 1. Ma E et al. ObesityWeek 2025. T a ld e fg ro b e p Taldefgrobep directly reduces adipose tissue storage of fatKEY POINT **** Taldefgrobep Decreases Phospho- SMAD2/3 Signaling in Adipocytes *** Control Taldefgrobep Myostatin Myostatin + Taldefgrobep A B S 4 5 0 ( n m ) Control Taldefgrobep Myostatin Myostatin + Taldefgrobep
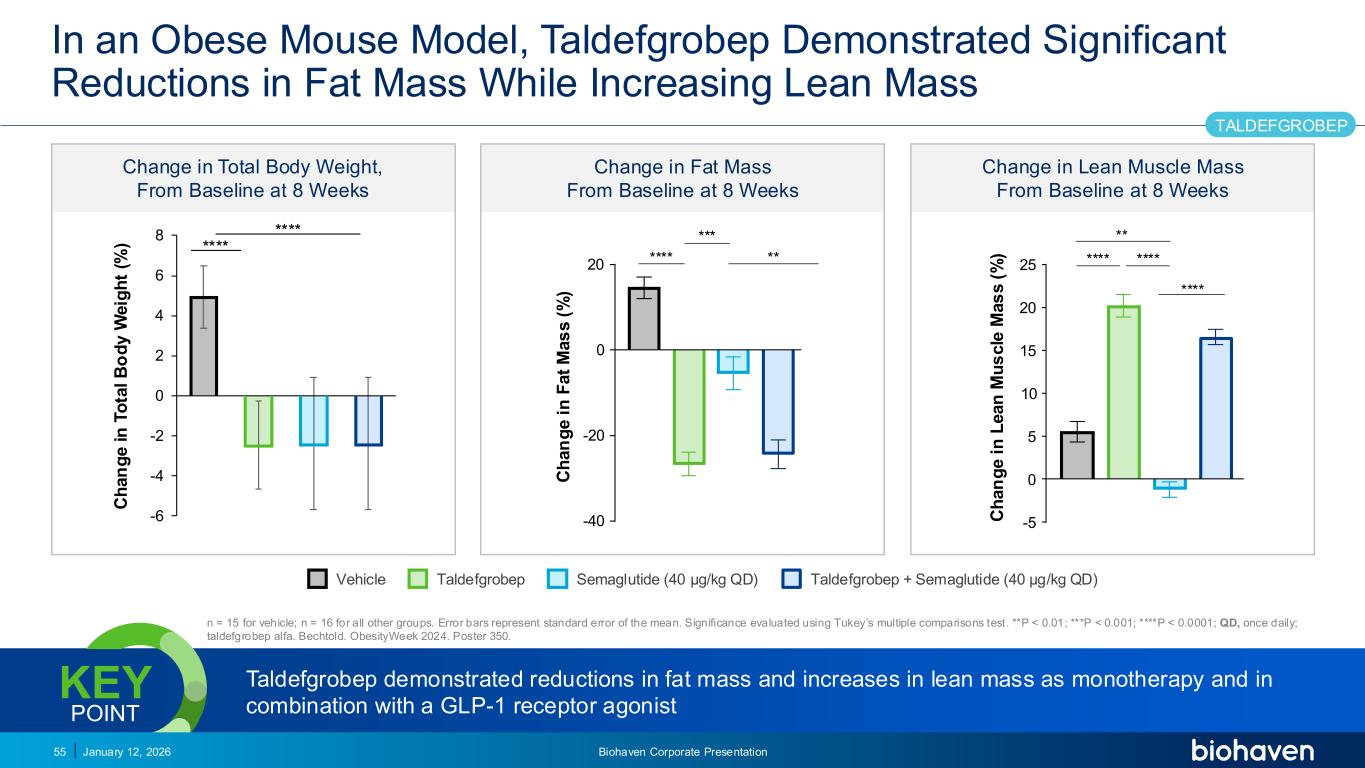
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Change in Total Body Weight, From Baseline at 8 Weeks Change in Fat Mass From Baseline at 8 Weeks Change in Lean Muscle Mass From Baseline at 8 Weeks In an Obese Mouse Model, Taldefgrobep Demonstrated Significant Reductions in Fat Mass While Increasing Lean Mass January 12, 2026 Biohaven Corporate Presentation55 n = 15 for vehicle; n = 16 for all other groups. Error bars represent standard error of the mean. Significance evaluated using Tukey’s multiple comparisons test. **P < 0.01; ***P < 0.001; ****P < 0.0001; QD, once daily; taldefgrobep alfa. Bechtold. ObesityWeek 2024. Poster 350. Taldefgrobep demonstrated reductions in fat mass and increases in lean mass as monotherapy and in combination with a GLP-1 receptor agonist KEY POINT TALDEFGROBEP -6 -4 -2 0 2 4 6 8 C h a n g e i n T o ta l B o d y W e ig h t (% ) **** **** C h a n g e i n L e a n M u s c le M a s s ( % ) -5 0 5 10 15 20 25 **** ** **** **** C h a n g e i n F a t M a s s ( % ) 20 -40 -20 0 **** *** ** Vehicle Taldefgrobep Semaglutide (40 µg/kg QD) Taldefgrobep + Semaglutide (40 µg/kg QD)
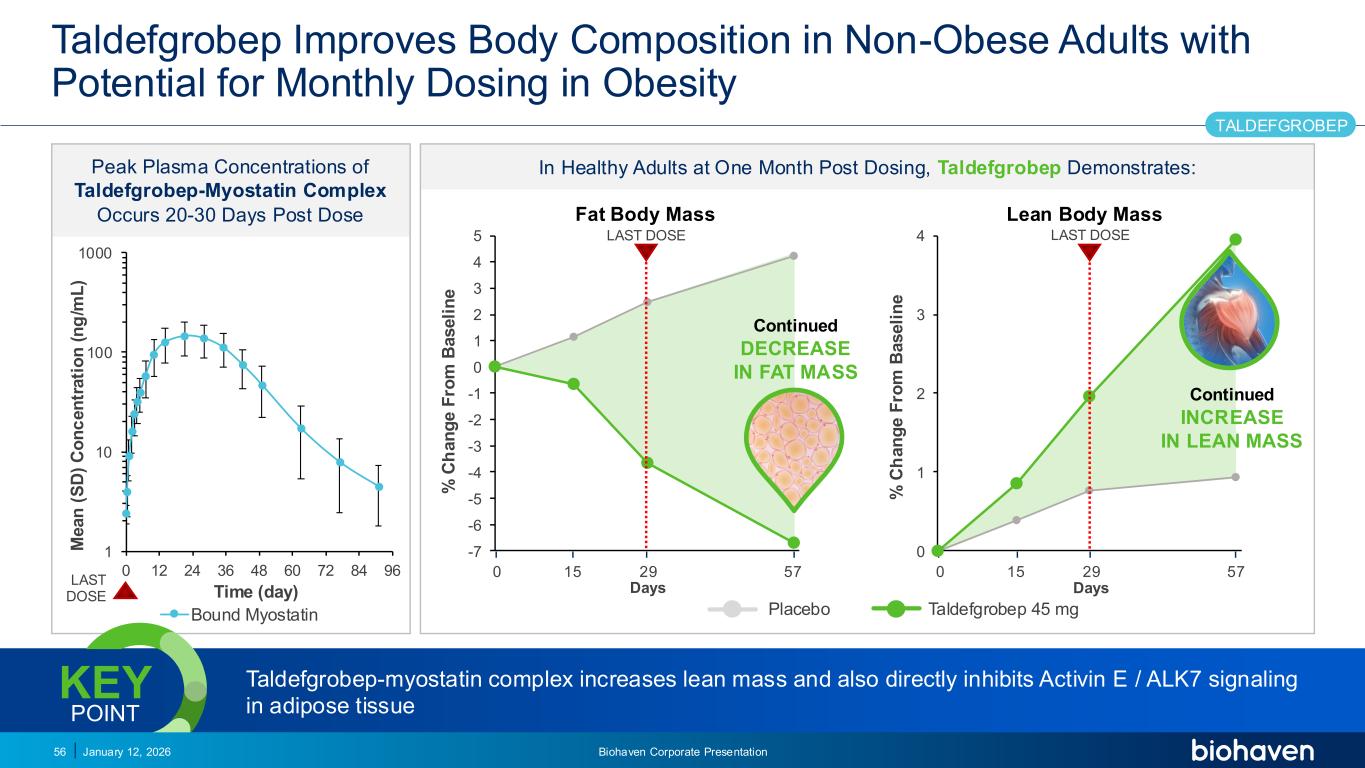
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYTaldefgrobep Improves Body Composition in Non-Obese Adults with Potential for Monthly Dosing in Obesity January 12, 2026 Biohaven Corporate Presentation56 TALDEFGROBEP Peak Plasma Concentrations of Taldefgrobep-Myostatin Complex Occurs 20-30 Days Post Dose 1 10 100 1000 0 12 24 36 48 60 72 84 96 M e a n ( S D ) C o n c e n tr a ti o n ( n g /m L ) Time (day) Bound Myostatin In Healthy Adults at One Month Post Dosing, Taldefgrobep Demonstrates: 0 1 2 3 4 0 5 10 15 20 25 30 35 40 45 50 55 LAST DOSE % C h a n g e F ro m B a s e li n e 29 7 Days Lean Body Mass Continued INCREASE IN LEAN MASS -7 -6 -5 -4 -3 -2 -1 0 1 2 3 4 5 0 5 10 15 20 25 30 35 40 45 50 55 LAST DOSE % C h a n g e F ro m B a s e li n e Fat Body Mass Continued DECREASE IN FAT MASS Placebo Taldefgrobep 45 mg LAST DOSE Taldefgrobep-myostatin complex increases lean mass and also directly inhibits Activin E / ALK7 signaling in adipose tissue KEY POINT 29 7 Days
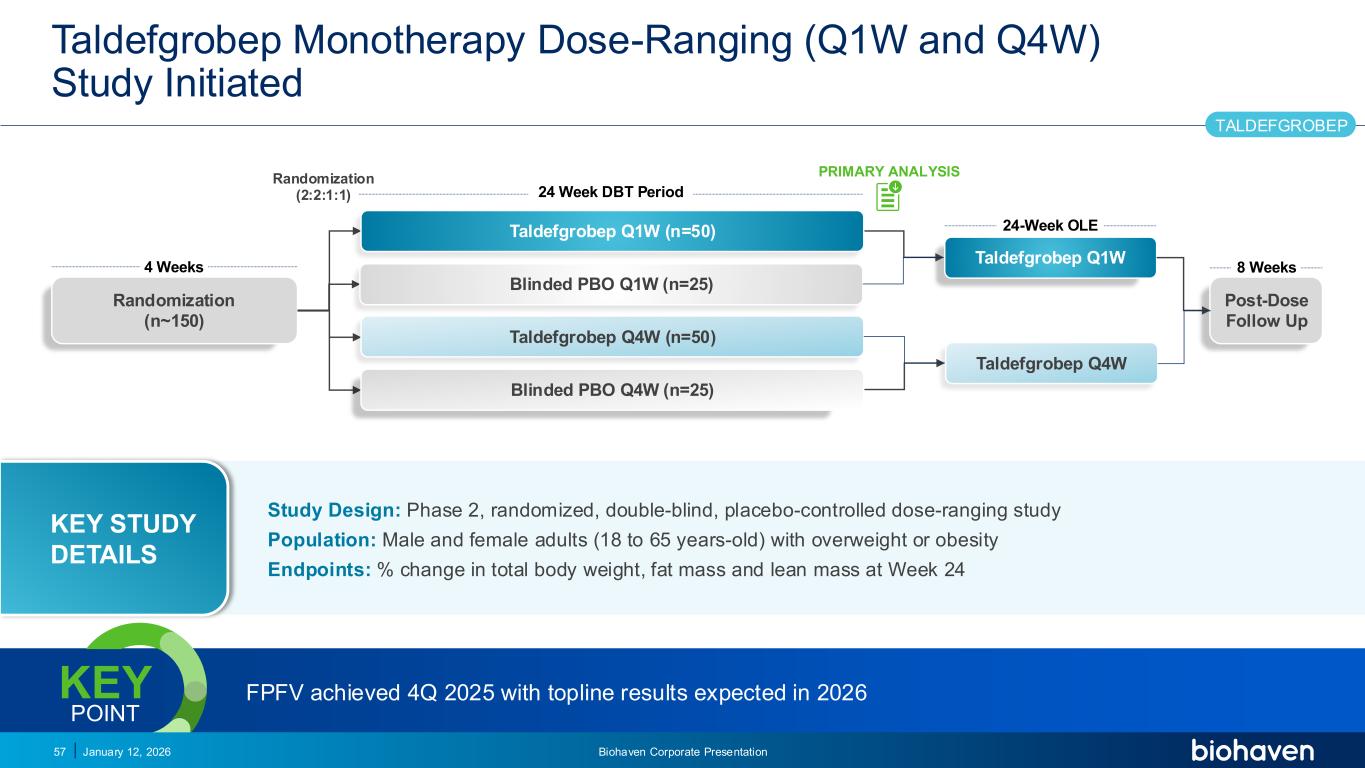
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYTaldefgrobep Monotherapy Dose-Ranging (Q1W and Q4W) Study Initiated January 12, 2026 Biohaven Corporate Presentation57 24 Week DBT Period 4 Weeks Randomization (n~150) Randomization (2:2:1:1) 8 Weeks PRIMARY ANALYSIS Post-Dose Follow Up TALDEFGROBEP Taldefgrobep Q1W Taldefgrobep Q4W 24-Week OLETaldefgrobep Q1W (n=50) Blinded PBO Q1W (n=25) Taldefgrobep Q4W (n=50) Blinded PBO Q4W (n=25) FPFV achieved 4Q 2025 with topline results expected in 2026KEY POINT Study Design: Phase 2, randomized, double-blind, placebo-controlled dose-ranging study Population: Male and female adults (18 to 65 years-old) with overweight or obesity Endpoints: % change in total body weight, fat mass and lean mass at Week 24 KEY STUDY DETAILS PODIUM PR
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Fat mass loss comparable to GLP-1 therapies Total body weight loss meeting current regulatory standards Increase in bone density Convenient subcutaneous autoinjector with potential for monthly dosing Visceral adipose tissue loss favorable to GLP-1 therapies Favorable safety and tolerability Increase in lean muscle mass Benefit as monotherapy and in combination with GLP-1 therapies Taldefgrobep Offers a Novel Approach to Address the Needs for People Living With Obesity Q1M TALDEFGROBEP January 12, 2026 Biohaven Corporate Presentation58

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Next-Generation ADC Technologies: Built to Deliver Improved Efficacy, Safety and Scalability ONCOLOGY PODIUM

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Developing Highly Differentiated Next-Generation ADCs January 12, 2026 Biohaven Corporate Presentation60 ONCOLOGY Proprietary platform solving for superior efficacy, safety, stability, and scalability FIRST CLINICAL DEMONSTRATION WITH BHV-1510 TROP2 ADC • Compelling activity with checkpoint combination • Highly stable, differentiated PK and safety FIRST-IN-HUMAN FGFR3 ADC (BHV-1530) • Early dose escalation with no DLTs • First-in-class potential in FGFR3 driven tumors CLINICALLY DIFFERENTIATED TOPOIX PAYLOAD Expanding high-value targets including bispecifics First patients dosed with novel subcutaneous ADC (BHV-1510)NEWS BREAKING

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Trop2 ADC BHV-1510 PODIUM
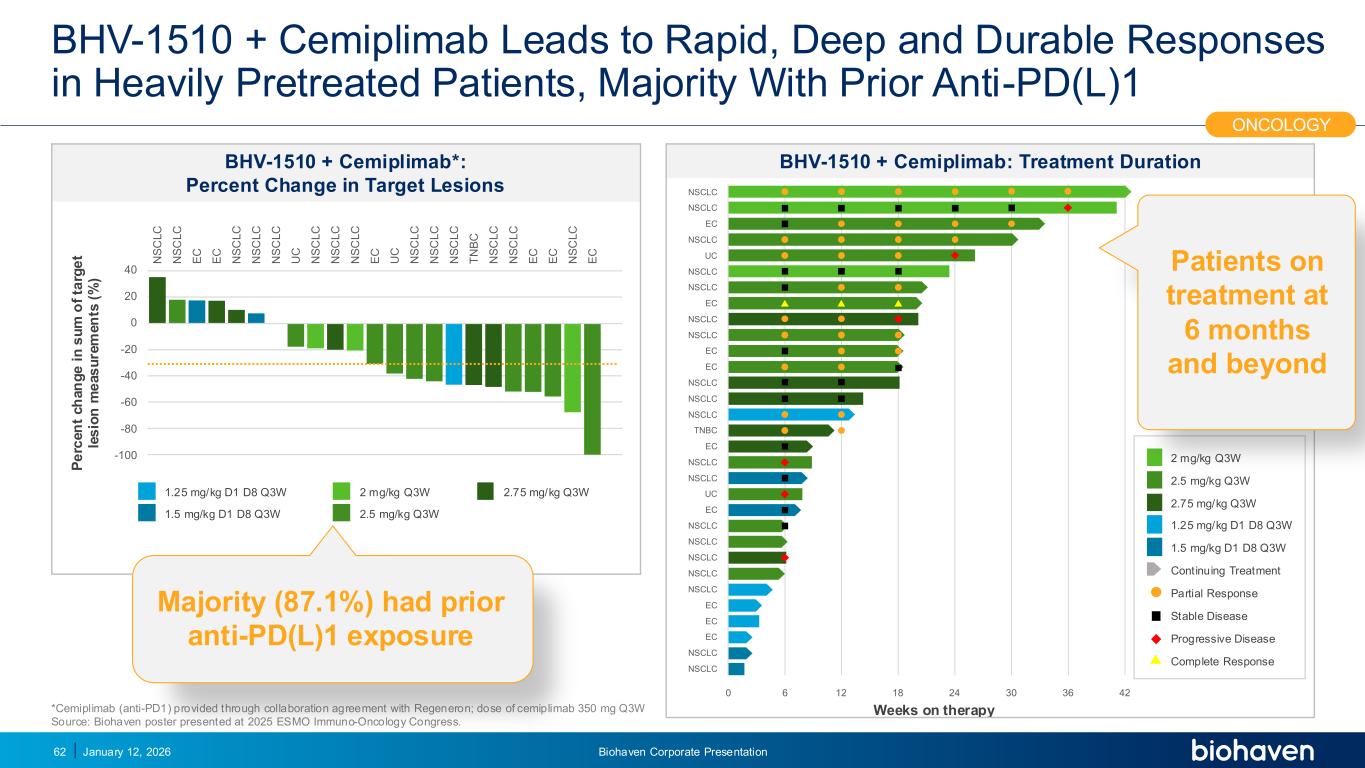
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1510 + Cemiplimab Leads to Rapid, Deep and Durable Responses in Heavily Pretreated Patients, Majority With Prior Anti-PD(L)1 January 12, 202662 P e rc e n t c h a n g e i n s u m o f ta rg e t le s io n m e a s u re m e n ts ( % ) 2 mg/kg Q3W 2.5 mg/kg Q3W 2.75 mg/kg Q3W1.25 mg/kg D1 D8 Q3W 1.5 mg/kg D1 D8 Q3W 100 80 60 40 20 0 20 40 S C L C S C L C E C E C S C L C S C L C S C L C C S C L C S C L C S C L C E C C S C L C S C L C S C L C T B C S C L C S C L C E C E C S C L C E C Weeks on therapy 0 6 12 18 24 30 36 42 SCLC SCLC EC SCLC C SCLC SCLC EC SCLC SCLC EC EC SCLC SCLC SCLC T BC EC SCLC SCLC SCLC C EC SCLC SCLC SCLC SCLC EC EC EC SCLC SCLC 2 mg/kg Q3W 2.5 mg/kg Q3W 2.75 mg/kg Q3W 1.25 mg/kg D1 D8 Q3W 1.5 mg/kg D1 D8 Q3W Partial Response Stable Disease Progressive Disease Complete Response Continuing Treatment BHV-1510 + Cemiplimab*: Percent Change in Target Lesions BHV-1510 + Cemiplimab: Treatment Duration *Cemiplimab (anti-PD1) provided through collaboration agreement with Regeneron; dose of cemiplimab 350 mg Q3W Source: Biohaven poster presented at 2025 ESMO Immuno-Oncology Congress. ONCOLOGY Patients on treatment at 6 months and beyond PODIUM Majority (87.1%) had prior anti-PD(L)1 exposure Biohaven Corporate Presentation
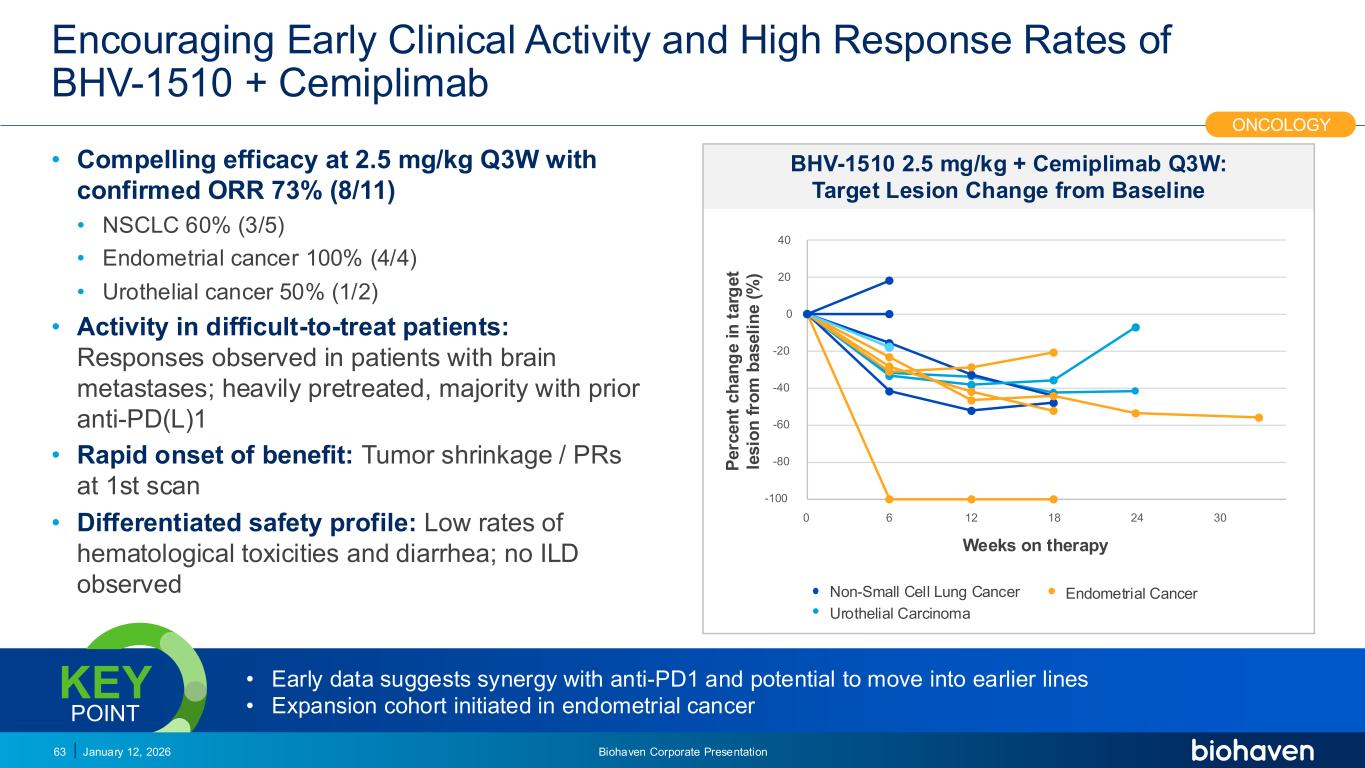
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYEncouraging Early Clinical Activity and High Response Rates of BHV-1510 + Cemiplimab • Compelling efficacy at 2.5 mg/kg Q3W with confirmed ORR 73% (8/11) • SCLC 60% (3/5) • Endometrial cancer 100% (4/4) • Urothelial cancer 50% (1/2) • Activity in difficult-to-treat patients: Responses observed in patients with brain metastases; heavily pretreated, majority with prior anti-PD(L)1 • Rapid onset of benefit: Tumor shrinkage / PRs at 1st scan • Differentiated safety profile: Low rates of hematological toxicities and diarrhea; no ILD observed January 12, 2026 Biohaven Corporate Presentation63 ONCOLOGY 100 80 60 40 20 0 20 40 0 6 12 18 24 30 P e rc e n t c h a n g e i n t a rg e t le s io n f ro m b a s e li n e ( % ) Non-Small Cell Lung Cancer Endometrial Cancer Urothelial Carcinoma Weeks on therapy BHV-1510 2.5 mg/kg + Cemiplimab Q3W: Target Lesion Change from Baseline • Early data suggests synergy with anti-PD1 and potential to move into earlier lines • Expansion cohort initiated in endometrial cancer KEY POINT PODIUM PR
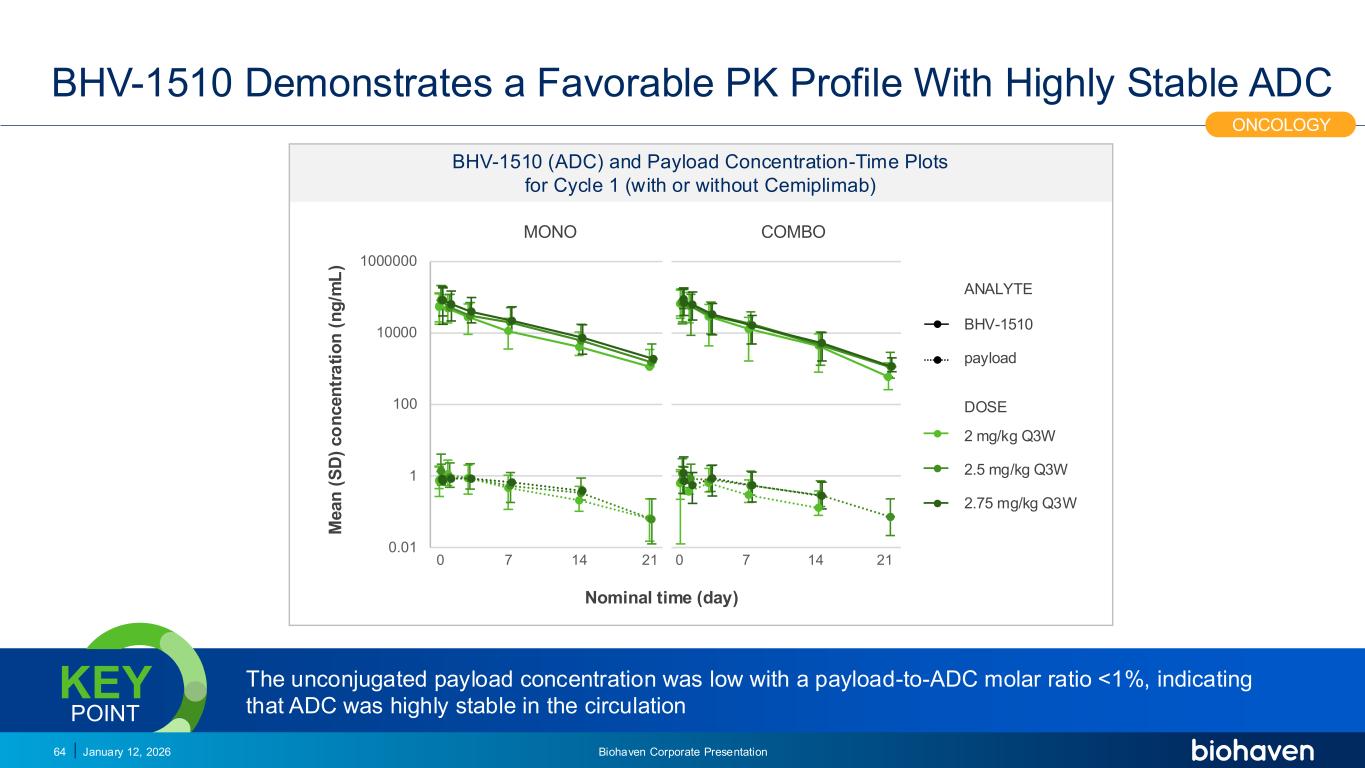
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-1510 Demonstrates a Favorable PK Profile With Highly Stable ADC January 12, 2026 Biohaven Corporate Presentation64 ONCOLOGY 2 mg/kg Q3W 2.5 mg/kg Q3W 2.75 mg/kg Q3W BHV-1510 payload ANALYTE DOSE M e a n ( S D ) c o n c e n tr a ti o n ( n g /m L ) Nominal time (day) MONO COMBO 0 7 14 21 0 7 14 21 0.01 1 100 10000 1000000 BHV-1510 (ADC) and Payload Concentration-Time Plots for Cycle 1 (with or without Cemiplimab) The unconjugated payload concentration was low with a payload-to-ADC molar ratio <1%, indicating that ADC was highly stable in the circulation KEY POINT
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY First and Only Trop2 ADC in Clinic With Subcutaneous Delivery • Target PK profile achieved • Early data shows optimized potential with no dose limiting toxicities, no stomatitis in cycle 1 • First patient dosed with 25% reduction in target lesions after single SC administration • Patient friendly delivery: No IV access needed; minutes versus hours with competitors • Reduced site-of-care burden: Less infusion- suite demand; supports community clinics • Optimized PK: Potential for improved tolerability and efficacy Initial patients dosed with SC BHV-1510 SC delivery offers several key advantages January 12, 2026 Biohaven Corporate Presentation65 ONCOLOGY Advancing SC to optimize safety and efficacy; potentially differentiating Biohaven’s ADC platform from competitors KEY POINT PODIUM??

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY FGFR3 ADC BHV-1530 PODIUM
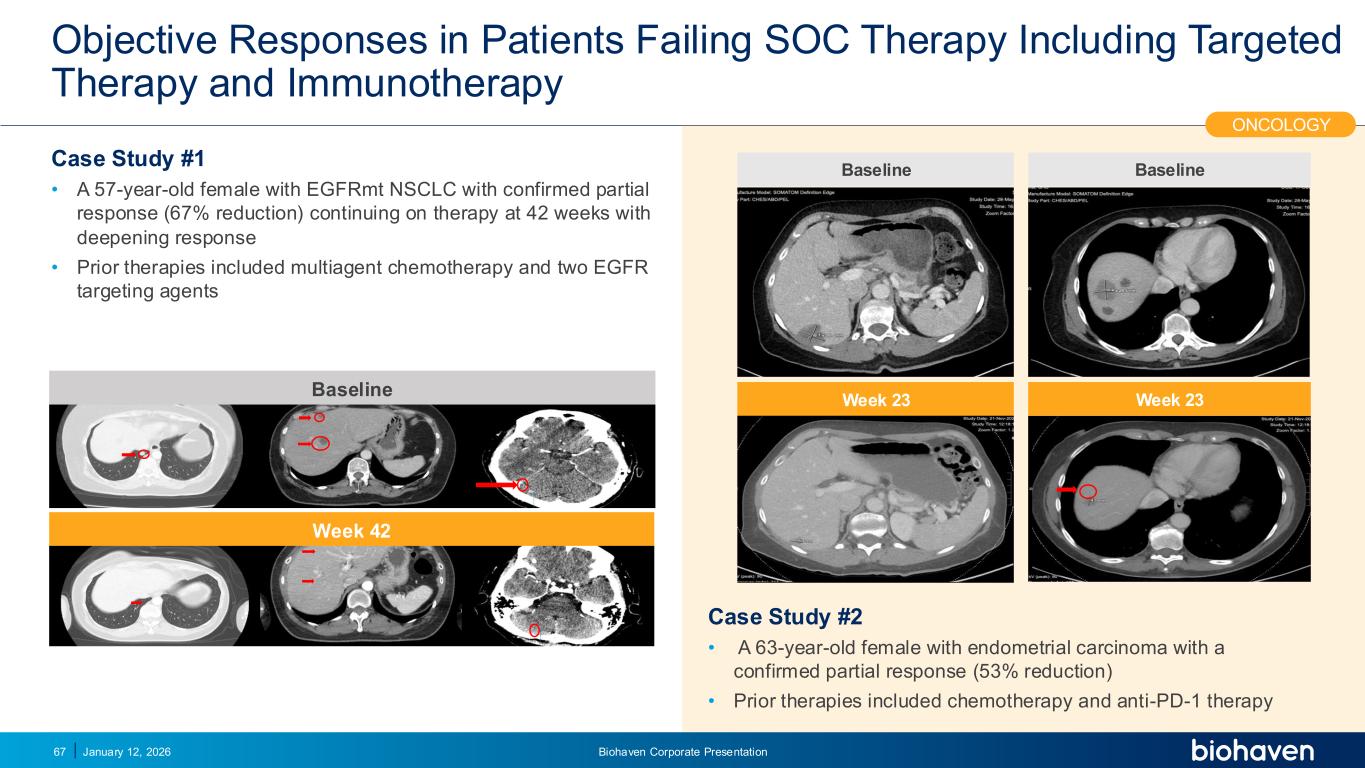
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYObjective Responses in Patients Failing SOC Therapy Including Targeted Therapy and Immunotherapy Case Study #1 • A 57-year-old female with EGFRmt NSCLC with confirmed partial response (67% reduction) continuing on therapy at 42 weeks with deepening response • Prior therapies included multiagent chemotherapy and two EGFR targeting agents Case Study #2 • A 63-year-old female with endometrial carcinoma with a confirmed partial response (53% reduction) • Prior therapies included chemotherapy and anti-PD-1 therapy January 12, 2026 Biohaven Corporate Presentation67 ONCOLOGY Baseline Week 42 Baseline Week 23 Baseline Week 23
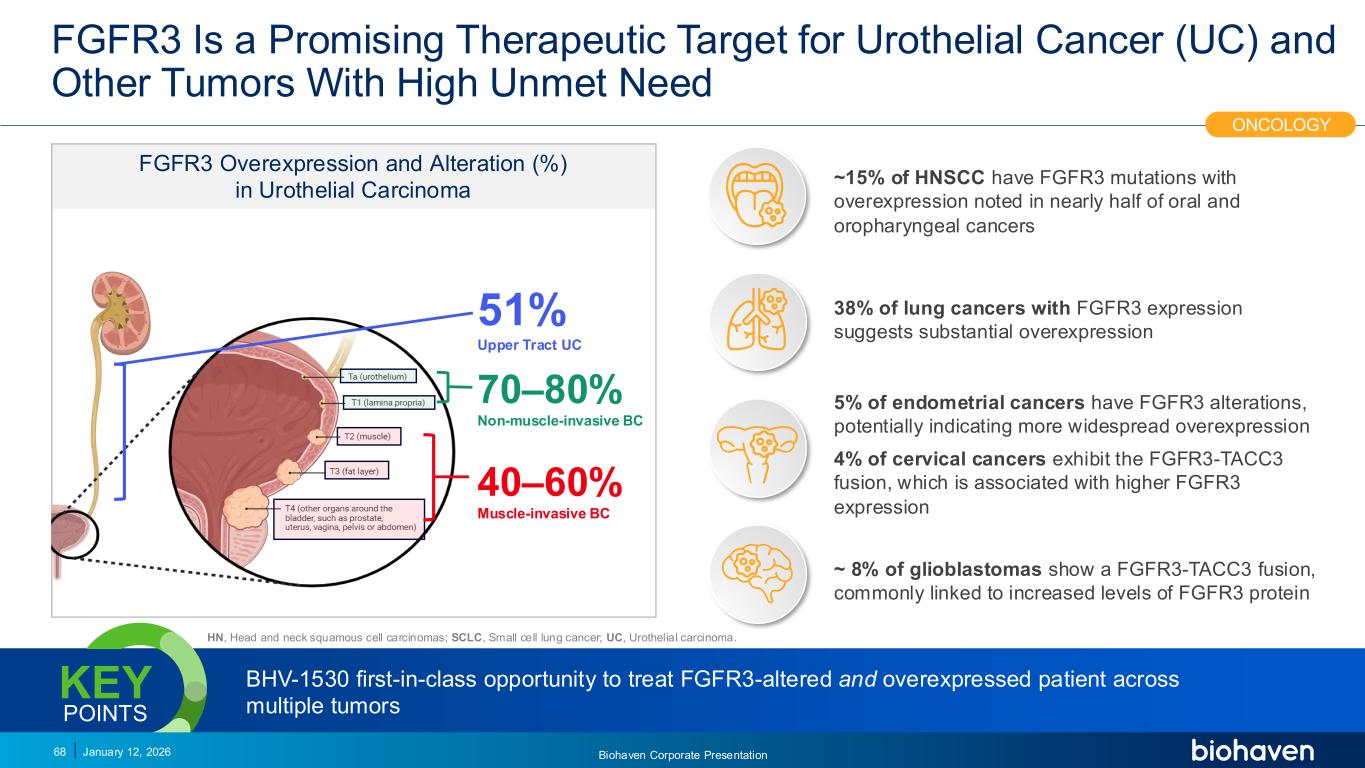
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYFGFR3 Is a Promising Therapeutic Target for Urothelial Cancer (UC) and Other Tumors With High Unmet Need January 12, 202668 FGFR3 Overexpression and Alteration (%) in Urothelial Carcinoma 51% Upper Tract UC 40–60% Muscle-invasive BC 70–80% Non-muscle-invasive BC Biohaven Corporate Presentation ONCOLOGY BHV-1530 first-in-class opportunity to treat FGFR3-altered and overexpressed patient across multiple tumors KEY POINTS 5% of endometrial cancers have FGFR3 alterations, potentially indicating more widespread overexpression 4% of cervical cancers exhibit the FGFR3-TACC3 fusion, which is associated with higher FGFR3 expression ~15% of HNSCC have FGFR3 mutations with overexpression noted in nearly half of oral and oropharyngeal cancers 38% of lung cancers with FGFR3 expression suggests substantial overexpression ~ 8% of glioblastomas show a FGFR3-TACC3 fusion, commonly linked to increased levels of FGFR3 protein PODIUM HN, Head and neck squamous cell carcinomas; SCLC, Small cell lung cancer; UC, Urothelial carcinoma. Head and neck squamous cell carcinoma - PMC
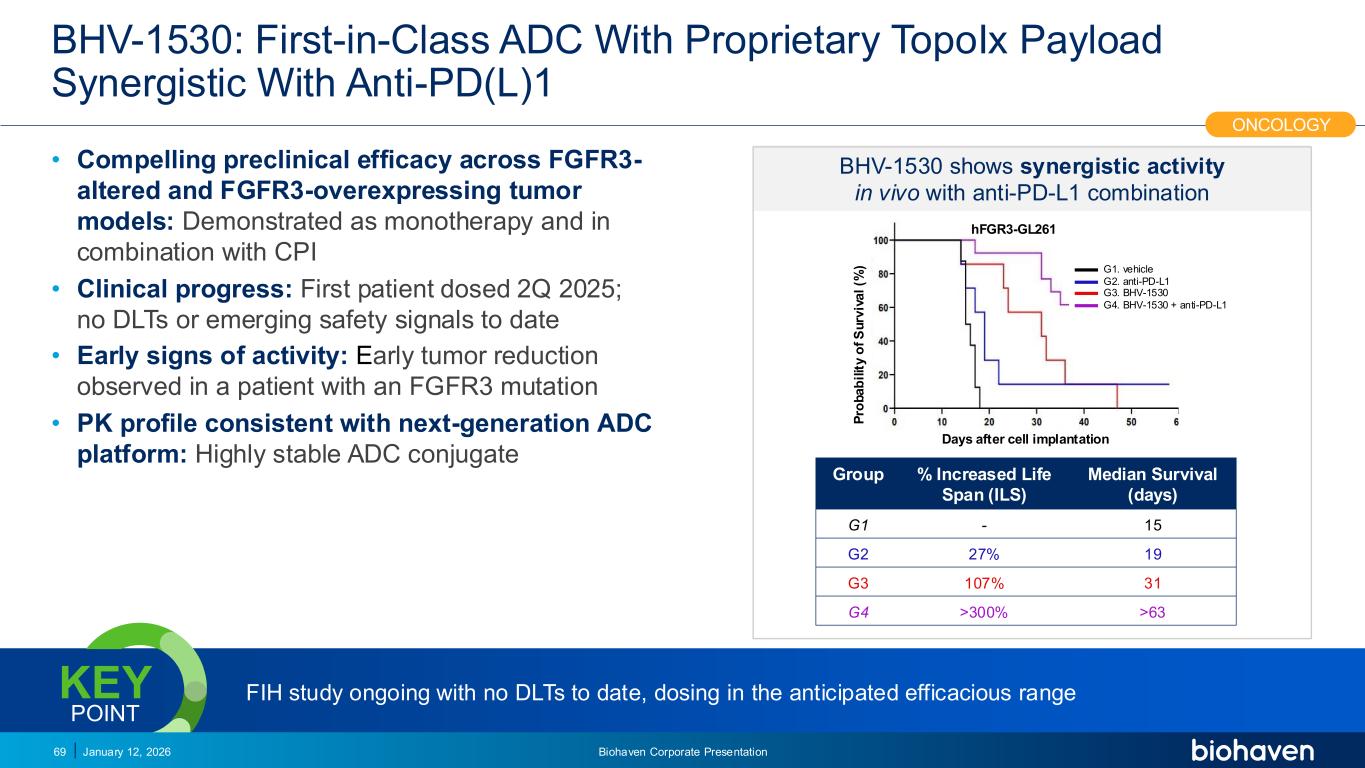
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-1530: First-in-Class ADC With Proprietary TopoIx Payload Synergistic With Anti-PD(L)1 • Compelling preclinical efficacy across FGFR3- altered and FGFR3-overexpressing tumor models: Demonstrated as monotherapy and in combination with CPI • Clinical progress: First patient dosed 2Q 2025; no DLTs or emerging safety signals to date • Early signs of activity: Early tumor reduction observed in a patient with an FGFR3 mutation • PK profile consistent with next-generation ADC platform: Highly stable ADC conjugate January 12, 2026 Biohaven Corporate Presentation69 P ro b a b il it y o f S u rv iv a l (% ) Days after cell implantation BHV-1530 shows synergistic activity in vivo with anti-PD-L1 combination G1. vehicle G2. anti-PD-L1 G3. BHV-1530 G4. BHV-1530 + anti-PD-L1 hFGR3-GL261 Group % Increased Life Span (ILS) Median Survival (days) G1 - 15 G2 27% 19 G3 107% 31 G4 >300% >63 ONCOLOGY FIH study ongoing with no DLTs to date, dosing in the anticipated efficacious rangeKEY POINT PODIUM

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY TRPM3 Targeting Pain at its Source ION CHANNEL: BHV-2100 TRPM3 ANTAGONIST

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-2100: Phase 2 First-in-Clinic TRPM3 Antagonist for Pain January 12, 2026 Biohaven Corporate Presentation71 Oral, peripherally acting small molecule that targets pain at its source—peripheral nociceptors—and avoids the liabilities of other TRP channel modulators TRPM3 NOVEL MOA WITH HUMAN GENETIC VALIDATION ROBUST EFFICACY IN NONCLINICAL PAIN MODELS FAVORABLE CLINICAL PHARMACOLOGY LOW POTENTIAL FOR CNS SIDE EFFECTS NO TEMPERATURE-RELATED LIABILITY IN HUMANS
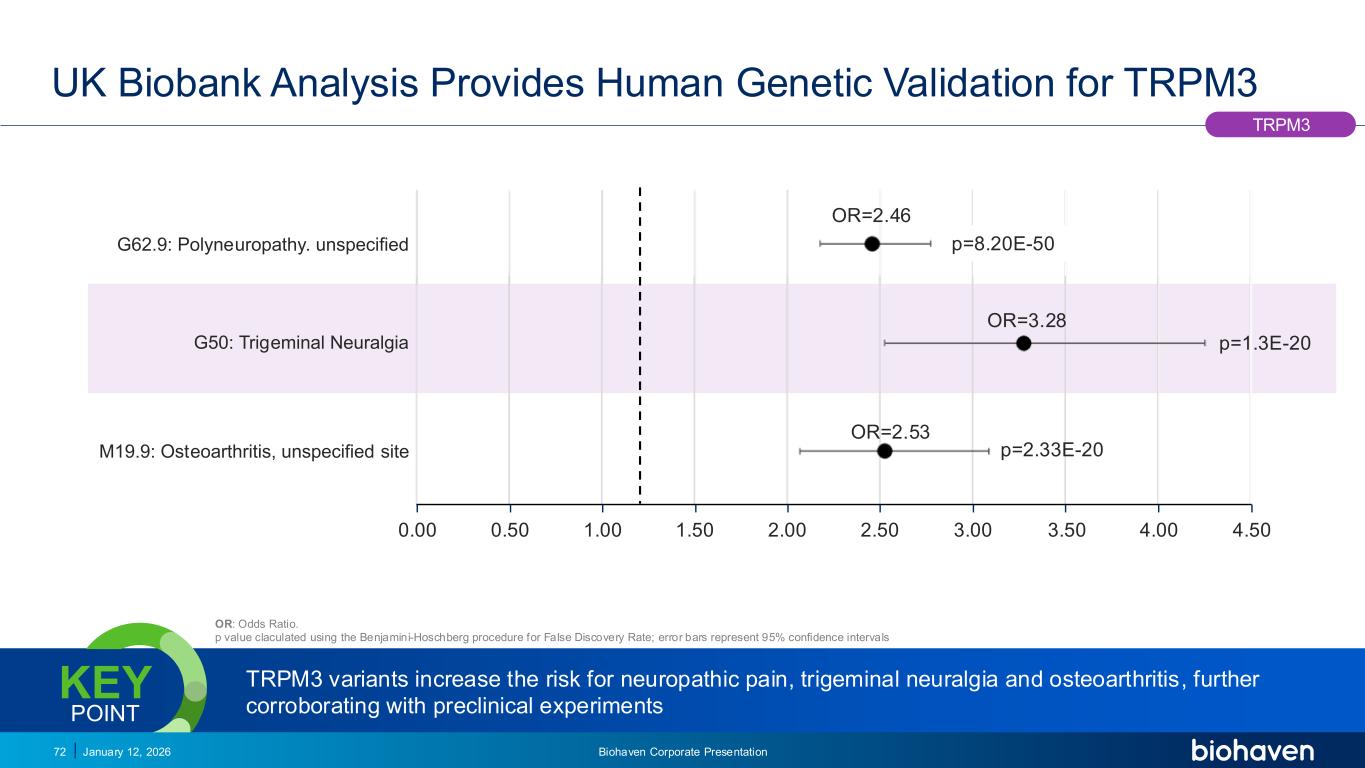
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY UK Biobank Analysis Provides Human Genetic Validation for TRPM3 OR: Odds Ratio. p value claculated using the Benjamini-Hoschberg procedure for False Discovery Rate; error bars represent 95% confidence intervals TRPM3 variants increase the risk for neuropathic pain, trigeminal neuralgia and osteoarthritis, further corroborating with preclinical experiments KEY POINT January 12, 2026 Biohaven Corporate Presentation72 OR=2.53 OR=3.28 OR=2.46 p=8.20E-50 p=1.3E-20 p=2.33E-20M19.9: Osteoarthritis, unspecified site G50: Trigeminal Neuralgia G62.9: Polyneuropathy. unspecified 0.00 0.50 1.00 1.50 2.00 2.50 3.00 3.50 4.00 4.50 TRPM3
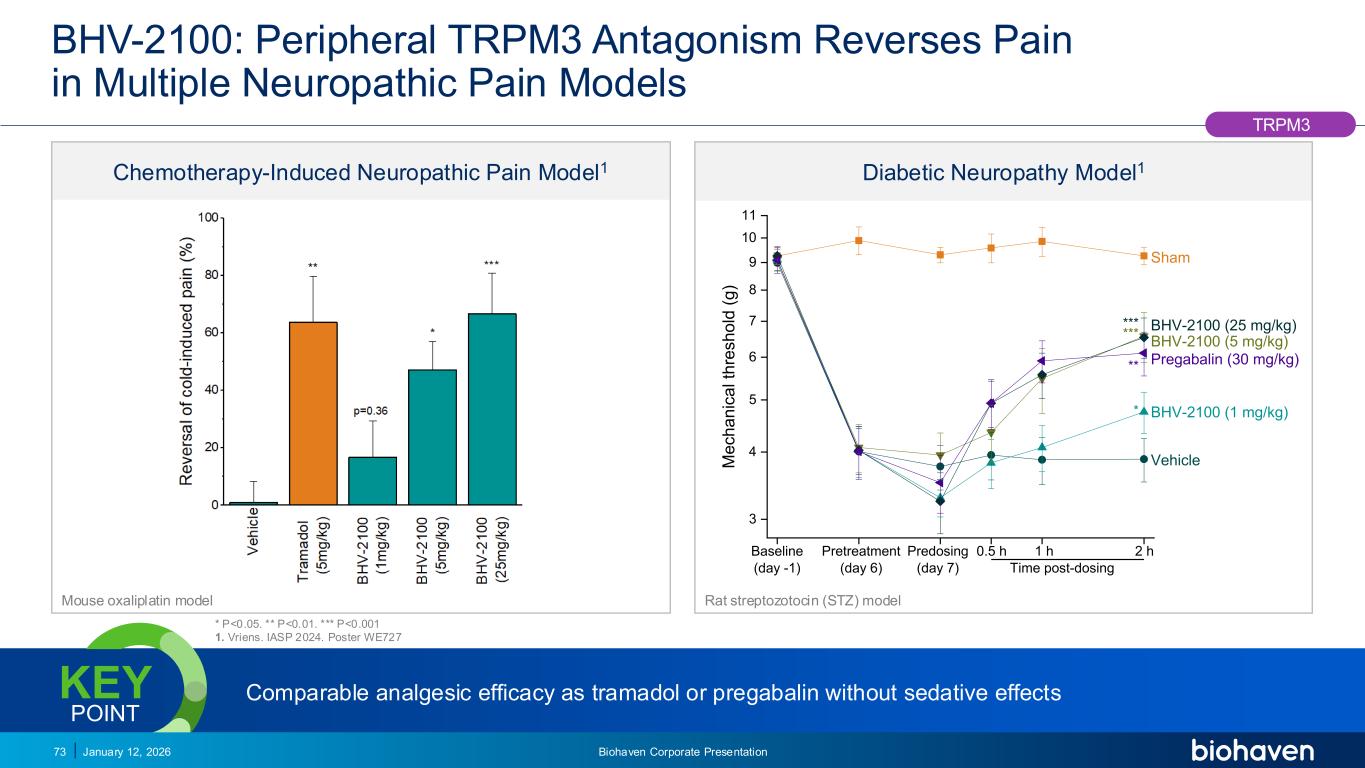
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY 3 4 5 6 7 8 9 10 11 * ** *** 2 h1 hPredosing (day 7) Pretreatment (day 6) M e c h a n ic a l th re s h o ld ( g ) Baseline (day -1) 0.5 h Time post-dosing Vehicle BHV-2100 (1 mg/kg) BHV-2100 (5 mg/kg) BHV-2100 (25 mg/kg) Pregabalin (30 mg/kg) Sham *** Mouse oxaliplatin model Rat streptozotocin (STZ) model BHV-2100: Peripheral TRPM3 Antagonism Reverses Pain in Multiple Neuropathic Pain Models TRPM3 Chemotherapy-Induced Neuropathic Pain Model1 Diabetic Neuropathy Model1 Comparable analgesic efficacy as tramadol or pregabalin without sedative effectsKEY POINT * P<0.05. ** P<0.01. *** P<0.001 1. Vriens. IASP 2024. Poster WE727 January 12, 2026 Biohaven Corporate Presentation73
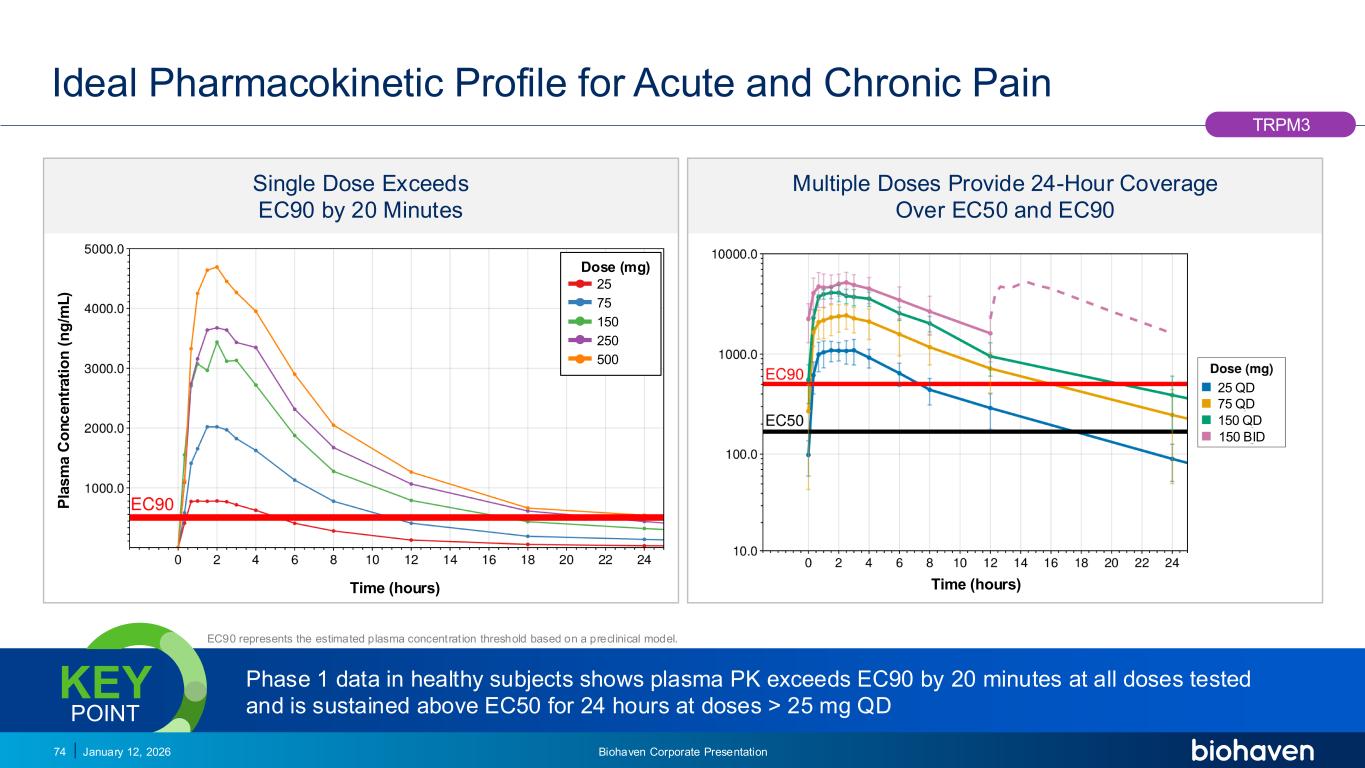
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Ideal Pharmacokinetic Profile for Acute and Chronic Pain January 12, 2026 Biohaven Corporate Presentation74 EC90 represents the estimated plasma concentration threshold based on a preclinical model. Single Dose Exceeds EC90 by 20 Minutes Dose (mg) P la s m a C o n c e n tr a ti o n ( n g /m L ) Time (hours) Multiple Doses Provide 24-Hour Coverage Over EC50 and EC90 Phase 1 data in healthy subjects shows plasma PK exceeds EC90 by 20 minutes at all doses tested and is sustained above EC50 for 24 hours at doses > 25 mg QD KEY POINT TRPM3 Time (hours) 25 QD 75 QD 150 QD 150 BID 25 75 150 250 500
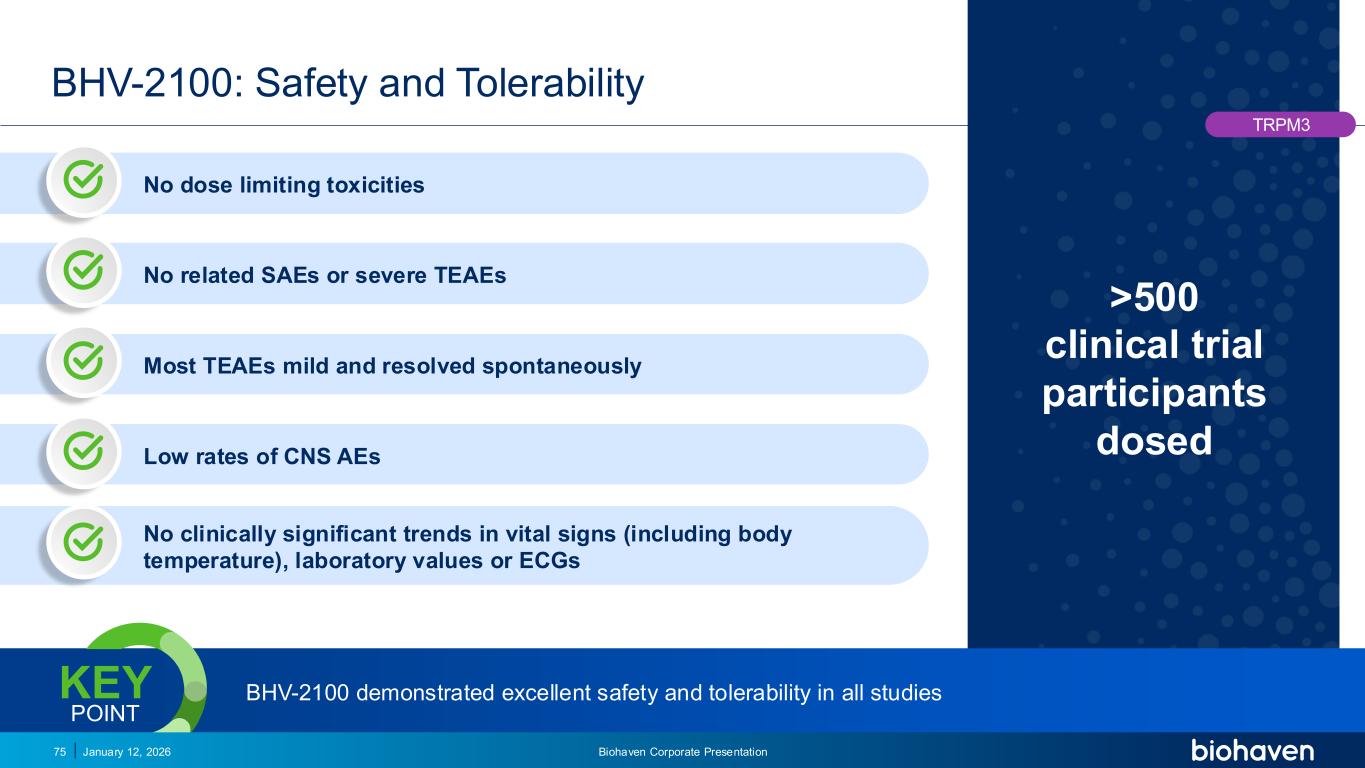
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY >500 clinical trial participants dosed January 12, 2026 Biohaven Corporate Presentation75 BHV-2100: Safety and Tolerability TRPM3 BHV-2100 demonstrated excellent safety and tolerability in all studiesKEY POINT No dose limiting toxicities No clinically significant trends in vital signs (including body temperature), laboratory values or ECGs Low rates of CNS AEs Most TEAEs mild and resolved spontaneously No related SAEs or severe TEAEs
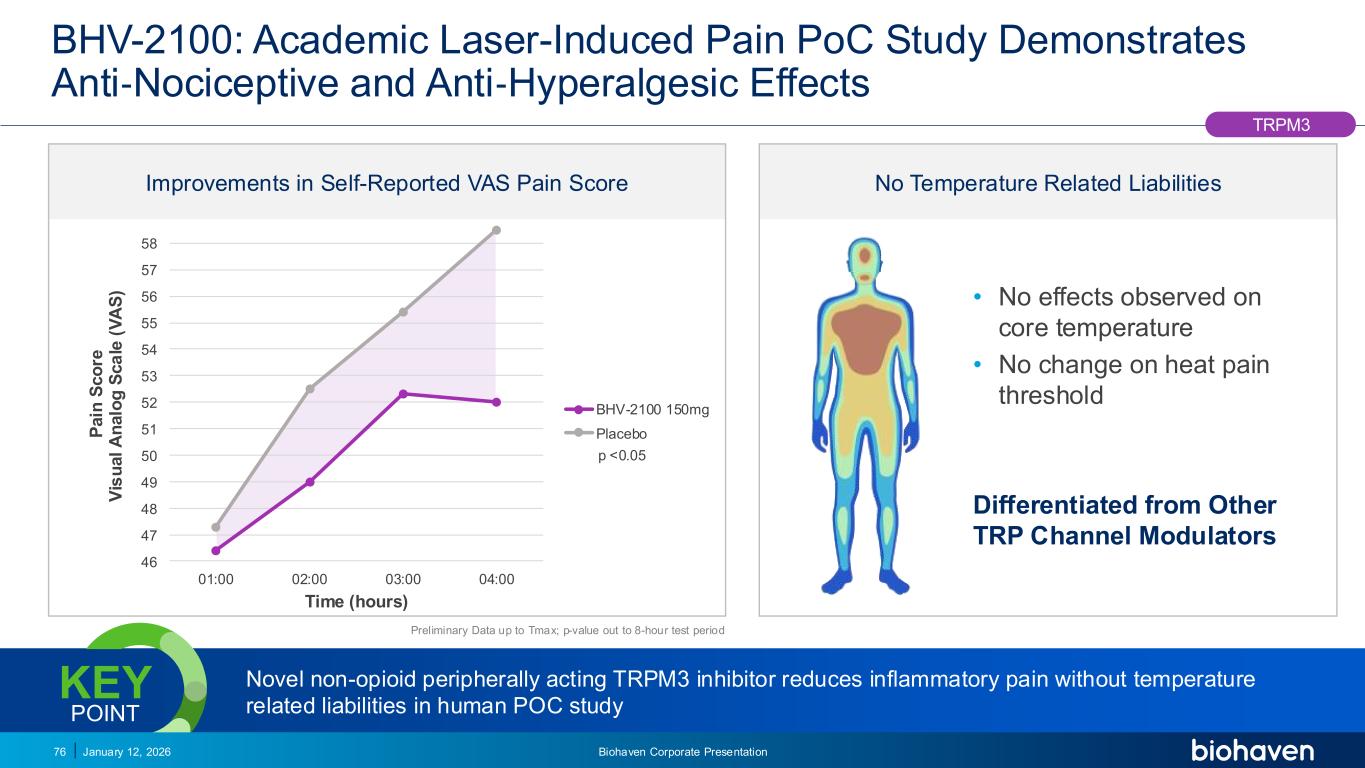
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Improvements in Self-Reported VAS Pain Score BHV-2100: Academic Laser-Induced Pain PoC Study Demonstrates Anti‐Nociceptive and Anti‐Hyperalgesic Effects January 12, 2026 Biohaven Corporate Presentation76 46 47 48 49 50 51 52 53 54 55 56 57 58 01:00 02:00 03:00 04:00 P a in S c o re V is u a l A n a lo g S c a le ( V A S ) Time (hours) BHV-2100 150mg Placebo p <0.05 Preliminary Data up to Tmax; p-value out to 8-hour test period TRPM3 Novel non-opioid peripherally acting TRPM3 inhibitor reduces inflammatory pain without temperature related liabilities in human POC study KEY POINT • No effects observed on core temperature • No change on heat pain threshold Differentiated from Other TRP Channel Modulators No Temperature Related Liabilities

Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY BHV-8000 Leveraging Validated Immune Targets To Stop Neuroinflammation I&I: BHV-8000 BRAIN-PENETRANT TYK2/JAK1 INHIBITOR
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Brain-Penetrant TYK2/JAK1 Inhibitor To Treat Parkinson’s Disease January 12, 202678 TYK2/JAK1 BHV-8000 addresses the widespread immune dysregulation that drives the onset and progression of neurodegenerative disorders SUPPRESSES INNATE AND ADAPTIVE IMMUNE CELL ACTIVATION AND INFILTRATION HIGH SELECTIVITY FOR TYK2/JAK1 AVOIDS JAK2/3 SAFETY LIABILITIES DEMONSTRATED TARGET ENGAGEMENT, ROBUST BRAIN PENETRATION AND A PROMISING SAFETY PROFILE PHASE 2/3 TRIAL IN EARLY PD ONGOING Biohaven Corporate Presentation
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Widespread Immune Dysregulation Is a Hallmark of PD January 12, 202679 1. Sulzer. Nature. 2017. 2. Wissemann. Am J Hum Genet. 2013. 3. Li. Front Immunol. 2023. 4. Tansey. Nat Rev Immunol. 2022. 5. Potashman. Parkinsonism Relat Disord. 2025. 6. Roodveldt. Brain. 2024. 7. Karikari. Brain Behav Immun. 2022. 8. Williams. Brain. 2021. 9. Yacoubian. Mov Disord. 2023. 10. Pajares. Cells. 2020. 11. Qu. Nature. 2023. 12. McGeer. Neurology. 1988. • Misidentification of self-proteins (⍺-synuclein) as foreign antigen triggers immune response1 • GWAS studies link PD risk to HLA gene variants involved in antigen presentation2 PD meets criteria for autoimmunity based on pathophysiology and genetics • Epidemiological studies suggest immune dysfunction and inflammation are key to the development of PD4 • Reduction in rates of PD have been seen when this population is exposed to immune-modulating therapies5 Epidemiology reveals increased risk of PD in individuals with other autoimmune diseases3 • In mouse models, T cells specific to α-synuclein peptides can induce dopaminergic neuronal loss7 • Manipulation of immune components (T cells) affect α-synuclein-induced neurodegeneration8 PD animal models demonstrate immune dysregulation drives neurodegeneration6 PD patient samples and imaging exhibit characteristic proinflammatory signatures9,10 • Proinflammatory cytokines (e.g., IL-6, TNF-⍺, IF Ɣ) are found in CSF and blood of PD patients11 • PD brains express high levels of HLA-DR+ reactive microglia12 TYK2/JAK1 Biohaven Corporate Presentation
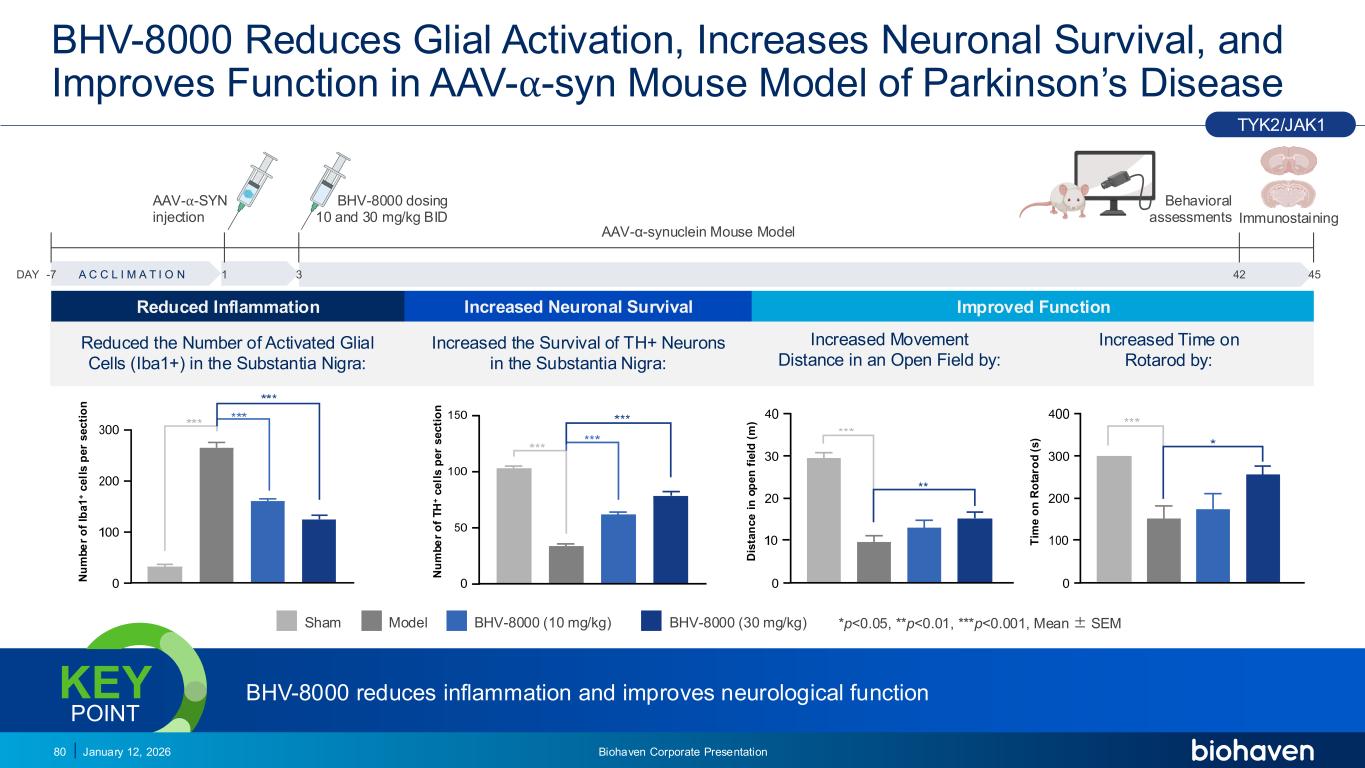
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-8000 Reduces Glial Activation, Increases Neuronal Survival, and Improves Function in AAV-⍺-syn Mouse Model of Parkinson’s Disease 80 Increased Time on Rotarod by: Increased Movement Distance in an Open Field by: Sham Model BHV-8000 (10 mg/kg) BHV-8000 (30 mg/kg) *p<0.05, **p<0.01, ***p<0.001, Mean ± SEM A C C L I M A T I O N Immunostaining Behavioral assessments AAV-⍺-SYN injection BHV-8000 dosing 10 and 30 mg/kg BID -7 1 3 45DAY 42 AAV-α-synuclein Mouse Model Increased the Survival of TH+ Neurons in the Substantia Nigra: Reduced the Number of Activated Glial Cells (Iba1+) in the Substantia Nigra: TYK2/JAK1 BHV-8000 reduces inflammation and improves neurological functionKEY POINT January 12, 2026 Biohaven Corporate Presentation Reduced Inflammation Increased Neuronal Survival Improved Function N u m b e r o f Ib a 1 + c e ll s p e r s e c ti o n N u m b e r o f T H + c e ll s p e r s e c ti o n D is ta n c e i n o p e n f ie ld ( m ) T im e o n R o ta ro d ( s )
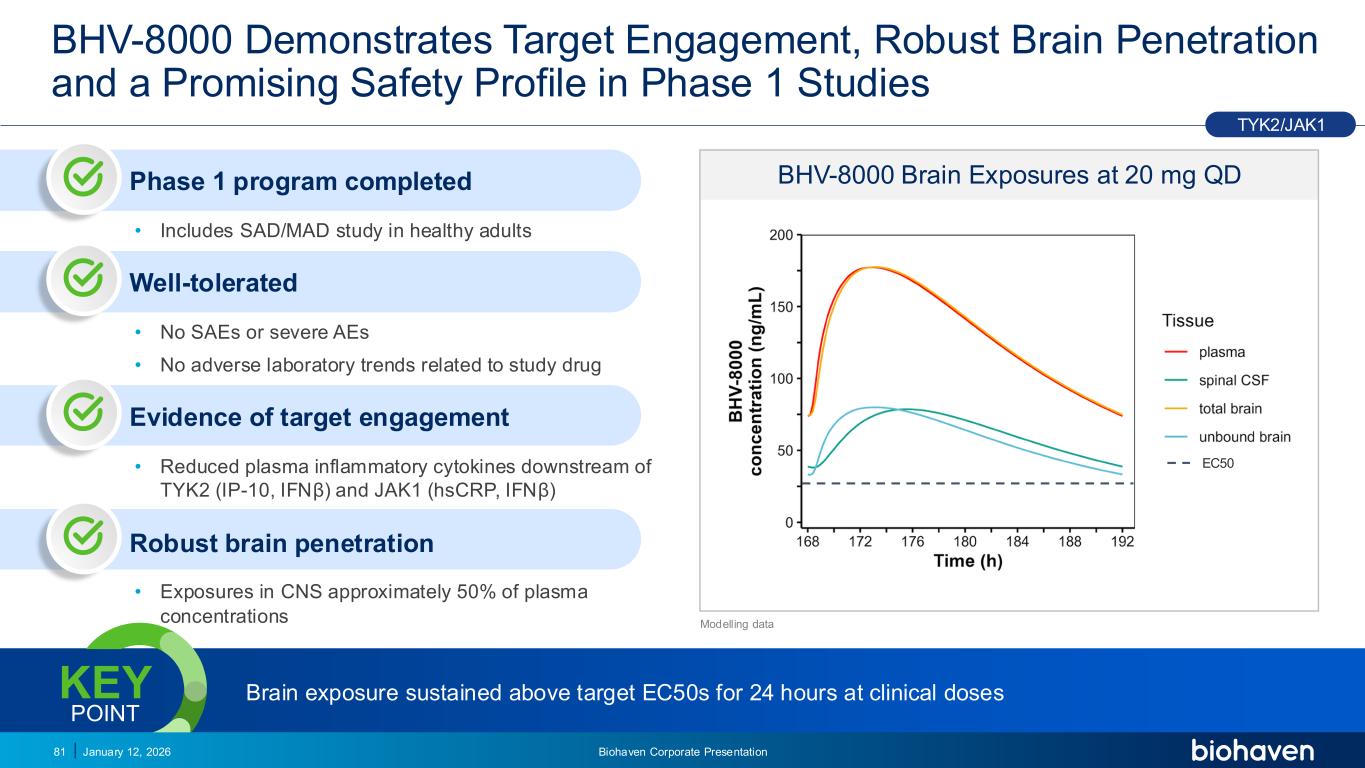
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORYBHV-8000 Demonstrates Target Engagement, Robust Brain Penetration and a Promising Safety Profile in Phase 1 Studies 81 BHV-8000 Brain Exposures at 20 mg QD EC50 Modelling data Phase 1 program completed • Includes SAD/MAD study in healthy adults Well-tolerated • No SAEs or severe AEs • No adverse laboratory trends related to study drug Evidence of target engagement • Reduced plasma inflammatory cytokines downstream of TYK2 (IP-10, IF β) and JAK1 (hsCRP, IF β) Robust brain penetration • Exposures in CNS approximately 50% of plasma concentrations TYK2/JAK1 Brain exposure sustained above target EC50s for 24 hours at clinical dosesKEY POINT January 12, 2026 Biohaven Corporate Presentation
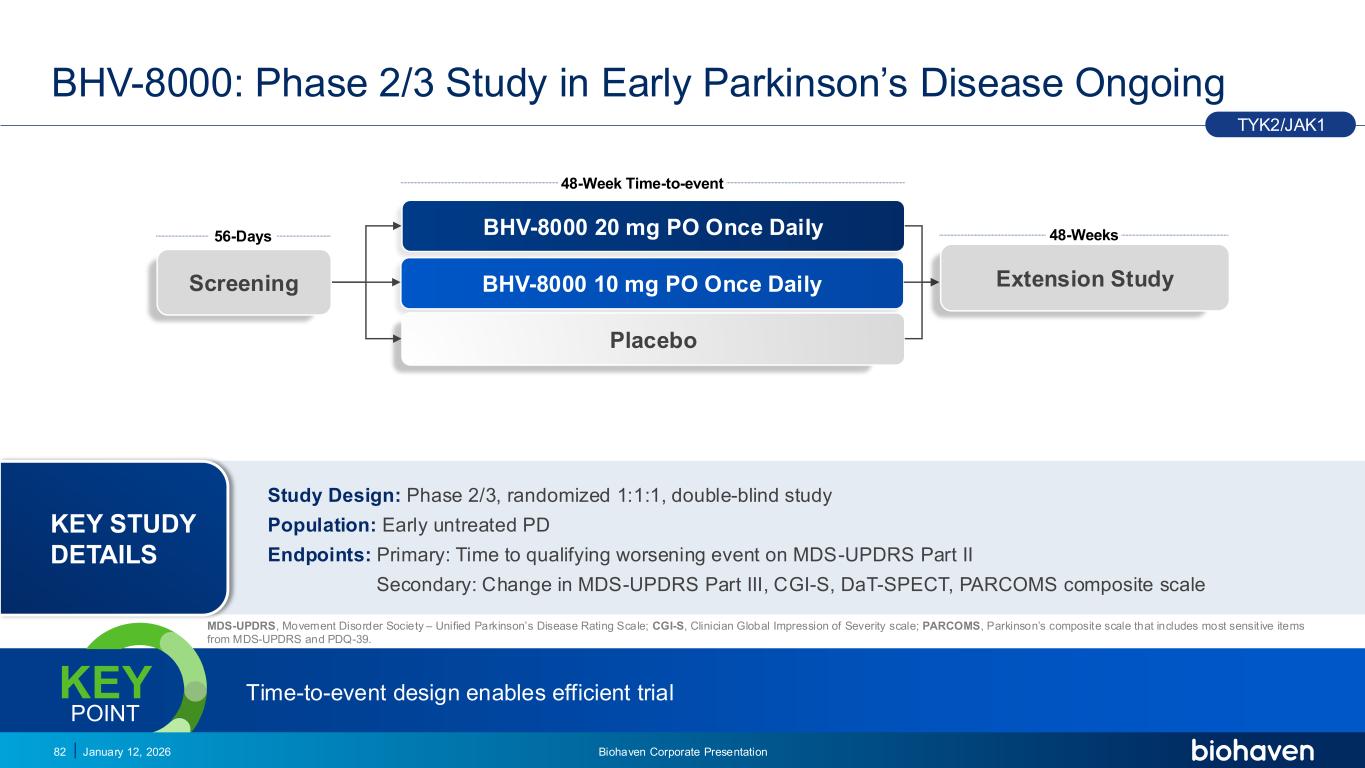
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY MDS-UPDRS, Movement Disorder Society – nified Parkinson’s Disease Rating Scale; CGI-S, Clinician Global Impression of Severity scale; PARCOMS, Parkinson’s composite scale that includes most sensit ive items from MDS-UPDRS and PDQ-39. 48-Week Time-to-event BHV-8000 20 mg PO Once Daily BHV-8000 10 mg PO Once Daily Placebo 56-Days 48-Weeks Screening Extension Study BHV-8000: Phase 2/3 Study in Early Parkinson’s Disease Ongoing January 12, 202682 TYK2/JAK1 Time-to-event design enables efficient trial KEY POINT Biohaven Corporate Presentation Study Design: Phase 2/3, randomized 1:1:1, double-blind study Population: Early untreated PD Endpoints: Primary: Time to qualifying worsening event on MDS-UPDRS Part II Secondary: Change in MDS-UPDRS Part III, CGI-S, DaT-SPECT, PARCOMS composite scale KEY STUDY DETAILS
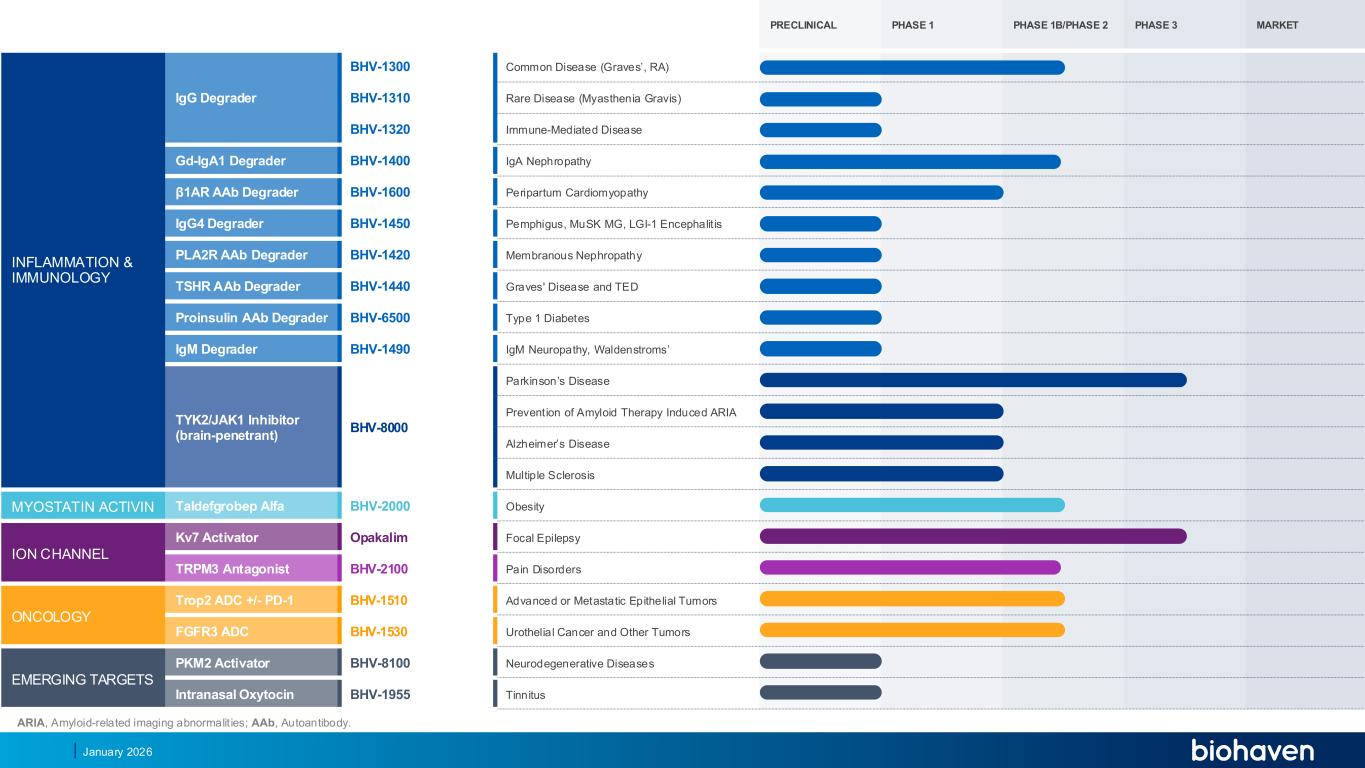
ARIA, Amyloid-related imaging abnormalit ies; AAb, Autoantibody. January 2026 PRECLINICAL PHASE 1 PHASE 1B/PHASE 2 PHASE 3 MARKET INFLAMMATION & IMMUNOLOGY IgG Degrader BHV-1300 Common Disease (Graves’, RA) BHV-1310 Rare Disease (Myasthenia Gravis) BHV-1320 Immune-Mediated Disease Gd-IgA1 Degrader BHV-1400 IgA Nephropathy β1AR AAb Degrader BHV-1600 Peripartum Cardiomyopathy IgG4 Degrader BHV-1450 Pemphigus, MuSK MG, LGI-1 Encephalit is PLA2R AAb Degrader BHV-1420 Membranous Nephropathy TSHR AAb Degrader BHV-1440 Graves' Disease and TED Proinsulin AAb Degrader BHV-6500 Type 1 Diabetes IgM Degrader BHV-1490 IgM europathy, Waldenstroms’ TYK2/JAK1 Inhibitor (brain-penetrant) BHV-8000 Parkinson’s Disease Prevention of Amyloid Therapy Induced ARIA Alzheimer’s Disease Multiple Sclerosis MYOSTATIN ACTIVIN Taldefgrobep Alfa BHV-2000 Obesity ION CHANNEL Kv7 Activator Opakalim Focal Epilepsy TRPM3 Antagonist BHV-2100 Pain Disorders ONCOLOGY Trop2 ADC +/- PD-1 BHV-1510 Advanced or Metastatic Epithelial Tumors FGFR3 ADC BHV-1530 Urothelial Cancer and Other Tumors EMERGING TARGETS PKM2 Activator BHV-8100 Neurodegenerative Diseases Intranasal Oxytocin BHV-1955 Tinnitus PR
PODIUM Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY Next-Generation Pipeline…
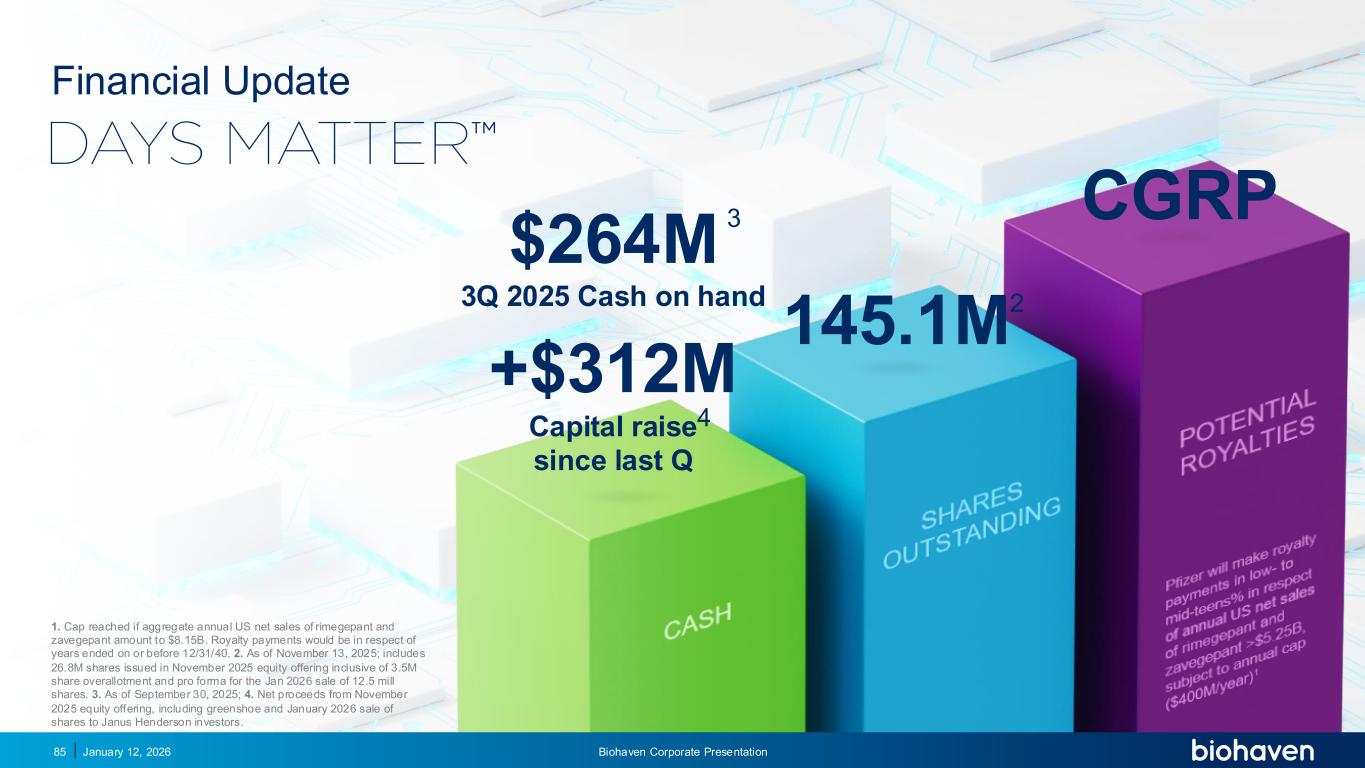
January 12, 2026 Biohaven Corporate Presentation85 CGRP 1. Cap reached if aggregate annual US net sales of rimegepant and zavegepant amount to $8.15B. Royalty payments would be in respect of years ended on or before 12/31/40. 2. As of November 13, 2025; includes 26.8M shares issued in November 2025 equity offering inclusive of 3.5M share overallotment and pro forma for the Jan 2026 sale of 12.5 mill shares. 3. As of September 30, 2025; 4. Net proceeds from November 2025 equity offering, including greenshoe and January 2026 sale of shares to Janus Henderson investors. Financial Update $264M 3Q 2025 Cash on hand +$312M Capital raise since last Q 3 4 145.1M2
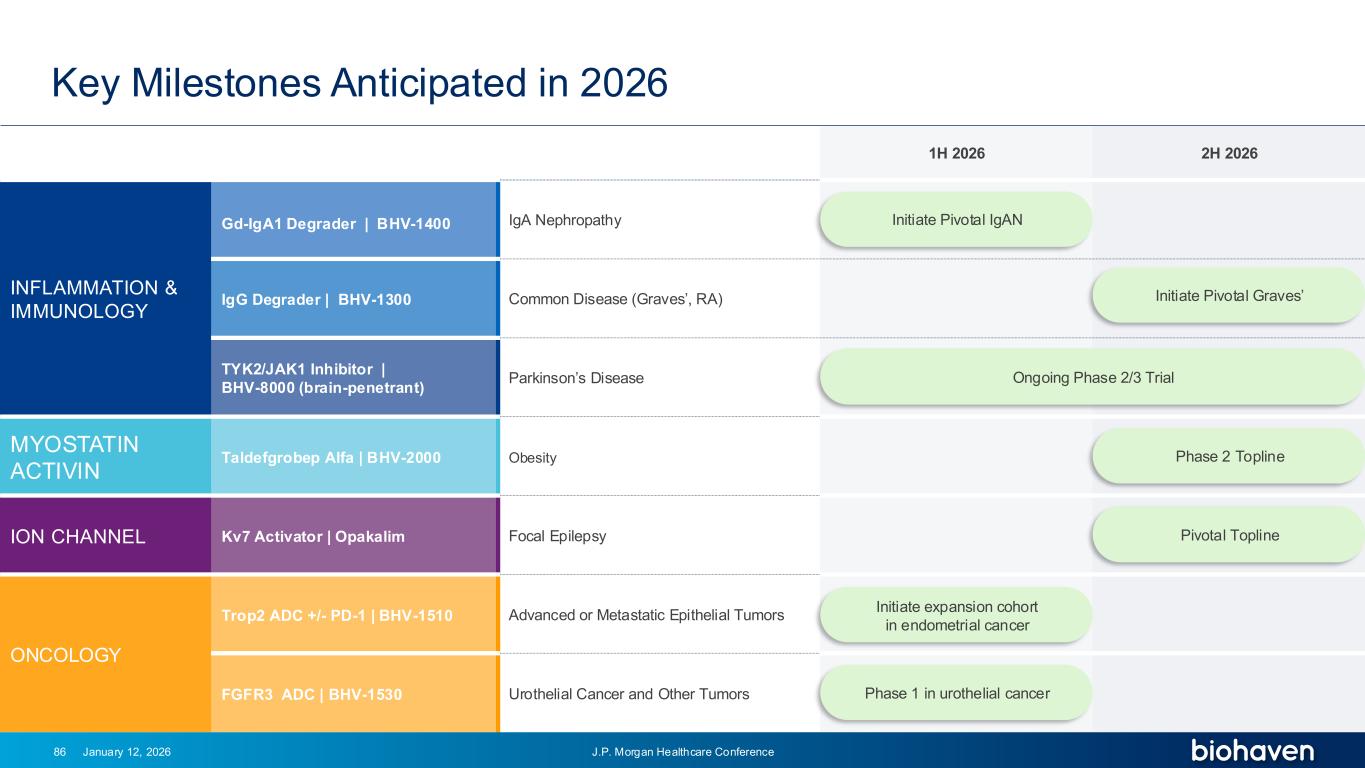
Trop2 ADC FGFR3 ADC CD30 ADC ONCOLOGY DEGRADERS TYK2/JAK1 INHIBITOR INFLAMMATION & IMMUNOLOGY TRPM3 ANTAGONIST KV7 ACTIVATORION CHANNEL T-ALFAMYOSTATIN TRORILUZOLEGLUTAMATE PROGRAMSCATEGORY 1H 2026 2H 2026 INFLAMMATION & IMMUNOLOGY Gd-IgA1 Degrader | BHV-1400 IgA Nephropathy IgG Degrader | BHV-1300 Common Disease (Graves’, RA) TYK2/JAK1 Inhibitor | BHV-8000 (brain-penetrant) Parkinson’s Disease MYOSTATIN ACTIVIN Taldefgrobep Alfa | BHV-2000 Obesity ION CHANNEL Kv7 Activator | Opakalim Focal Epilepsy ONCOLOGY Trop2 ADC +/- PD-1 | BHV-1510 Advanced or Metastatic Epithelial Tumors FGFR3 ADC | BHV-1530 Urothelial Cancer and Other Tumors Key Milestones Anticipated in 2026 January 12, 2026 J.P. Morgan Healthcare Conference86 Phase 2 Topline Pivotal Topline Ongoing Phase 2/3 Trial Initiate Pivotal IgAN Initiate Pivotal Graves’ Initiate expansion cohort in endometrial cancer Phase 1 in urothelial cancer

PODIUM P A I G E D A V I D K I M J E N N I F E R L A U R E NE M M A V E N I K A E M I L Y M I C H E L E E L L I E