
.2

Bridging Important Gaps in Anticoagulation Management for High - Risk Patients December 2025 Corporate Presentation NASDAQ: CVKD

2 Forward - looking Statements This document contains forward - looking statements . In addition, from time to time, we or our representatives may make forward - looking statements orally or in writing . We base these forward - looking statements on our expectations and projections about future events, which we derive from the information currently available to us . Such forward - looking statements relate to future events or our future performance, including : our financial performance and projections ; our growth in revenue and earnings ; and our business prospects and opportunities . You can identify forward - looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” or “hopes” or the negative of these or similar terms . In evaluating these forward - looking statements, you should consider various factors, including : our ability to successfully develop and commercialize product candidates, our ability to raise capital when needed, and the competitive environment of our business . These and other factors may cause our actual results to differ materially from any forward - looking statement, including those risk factors disclosed in our Annual Report on Form 10 - K for the year ended December 31 , 2024 , filed with the Securities and Exchange Commission on March 13 , 2025 , and our Quarterly Reports for the periods ended March 31 , 2025 , June 30 , 2025 , and September 30 , 2025 . Forward - looking statements are only predictions . The forward - looking events discussed in this document and other statements made from time to time by us or our representatives may not occur, and actual events and results may differ materially and are subject to risks, uncertainties, and assumptions about us . We are not obligated to publicly update or revise any forward - looking statement, whether as a result of uncertainties and assumptions, the forward - looking events discussed in this document, and other statements made from time to time by us or our representatives might not occur .

3 Our Mission Develop novel, differentiated products that bridge critical gaps in current acute and chronic anticoagulation management for rare and high - risk patient populations.
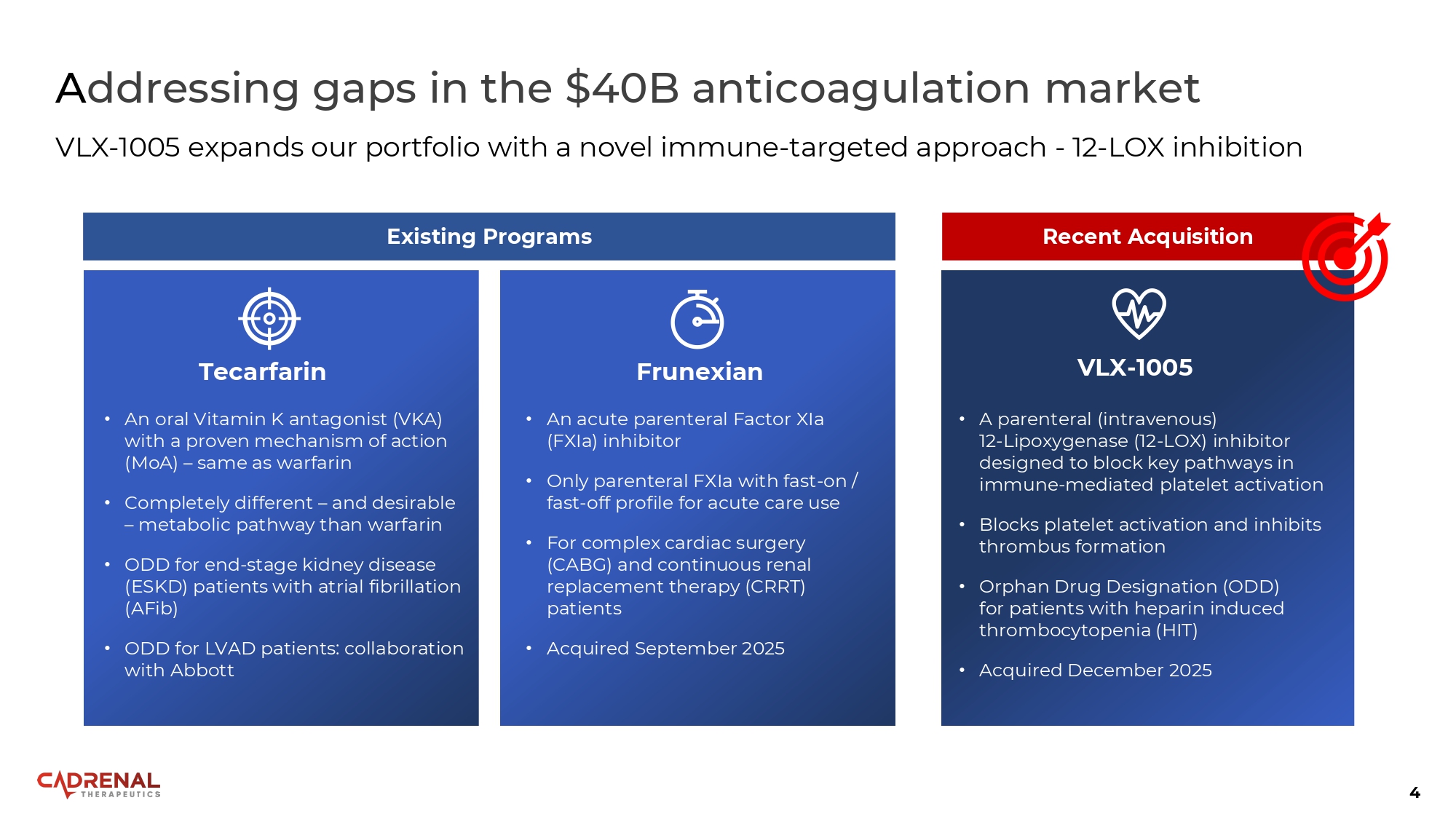
4 A ddressing gaps in the $40B anticoagulation market VLX - 1005 expands our portfolio with a novel immune - targeted approach - 12 - LOX inhibition • A parenteral (intravenous) 12 - Lipoxygenase (12 - LOX) inhibitor designed to block key pathways in immune - mediated platelet activation • Blocks platelet activation and inhibits thrombus formation • Orphan Drug Designation (ODD) for patients with heparin induced thrombocytopenia (HIT) • Acquired December 2025 Recent Acquisition VLX - 1005 Tecarfarin • An oral Vitamin K antagonist (VKA) with a proven mechanism of action (MoA) – same as warfarin • Completely different – and desirable – metabolic pathway than warfarin • ODD for end - stage kidney disease (ESKD) patients with atrial fibrillation (AFib) • ODD for LVAD patients : collaboration with Abbott Frunexian • An acute parenteral Factor XIa (FXIa) inhibitor • Only parenteral FXIa with fast - on / fast - off profile for acute care use • For complex cardiac surgery (CABG) and continuous renal replacement therapy (CRRT) patients • Acquired September 2025 Existing Programs
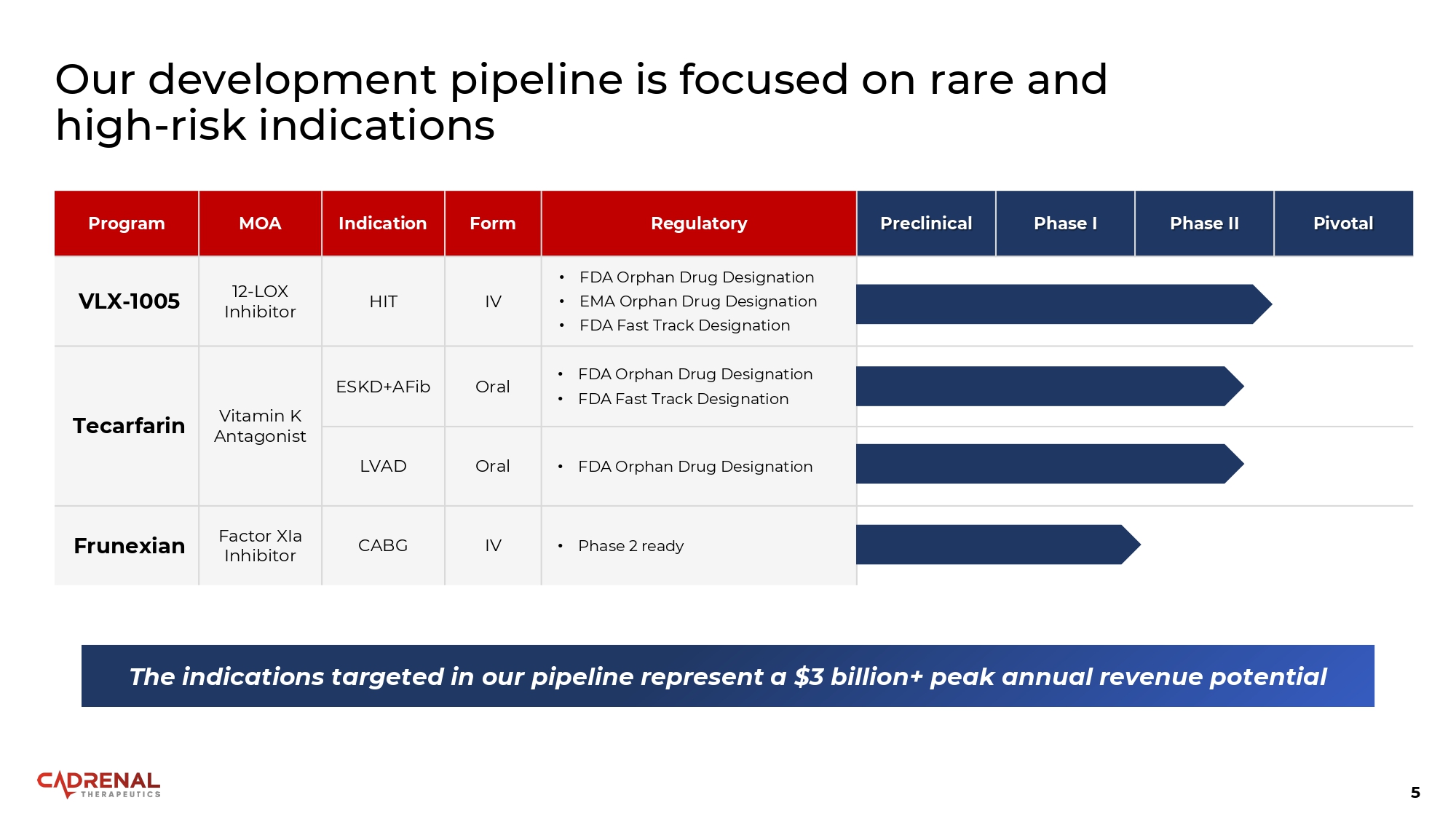
5 Our development pipeline is focused on rare and high - risk indications Pivotal Phase II Phase I Preclinical Regulatory Form Indication MOA Program • FDA Orphan Drug Designation • EMA Orphan Drug Designation • FDA Fast Track Designation IV HIT 12 - LOX Inhibitor VLX - 1005 • FDA Orphan Drug Designation • FDA Fast Track Designation Oral ESKD+AFib Vitamin K Antagonist Tecarfarin • FDA Orphan Drug Designation Oral LVAD • Phase 2 ready IV CABG Factor XIa Inhibitor Frunexian The indications targeted in our pipeline represent a $3 billion+ peak annual revenue potential

Target: Immune - mediated Platelet Activation VLX - 1005
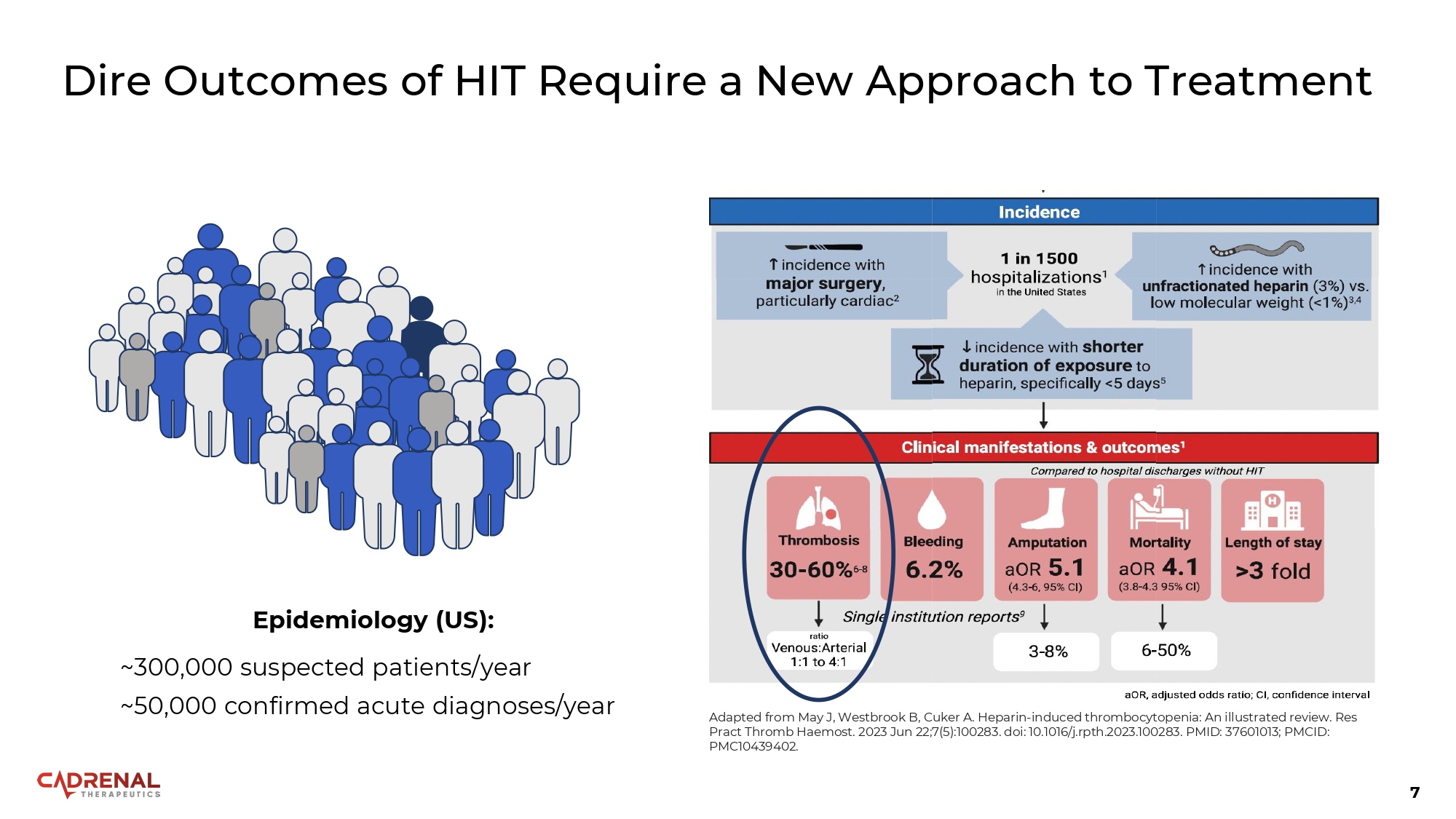
7 Dire Outcomes of HIT Require a New Approach to Treatment Epidemiology (US): ~300,000 suspected patients/year ~50,000 confirmed acute diagnoses/year Adapted from May J, Westbrook B, Cuker A. Heparin - induced thrombocytopenia: An illustrated review. Res Pract Thromb Haemost. 2023 Jun 22;7(5):100283. doi: 10.1016/j.rpth.2023.100283. PMID: 37601013; PMCID: PMC10439402.
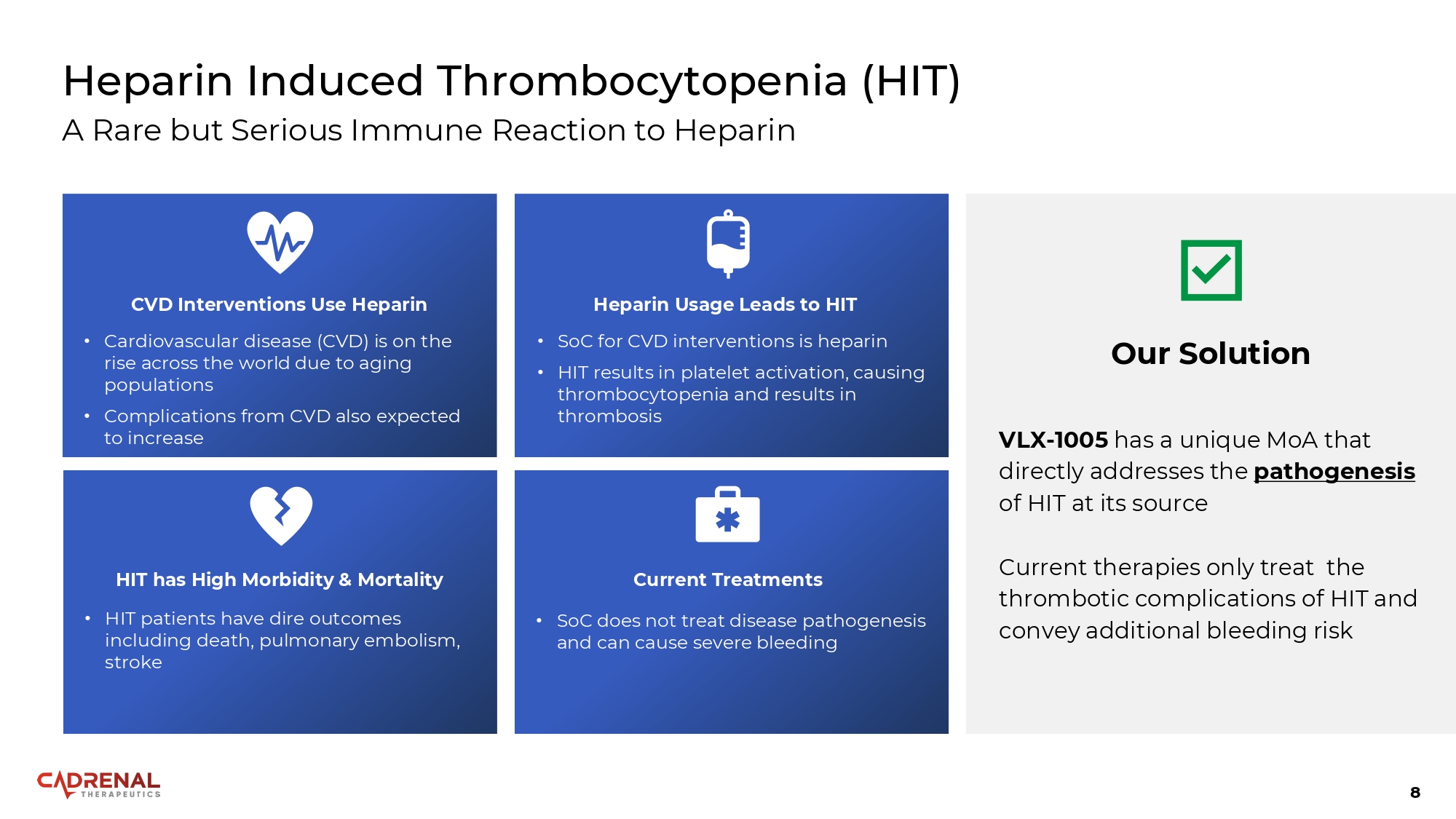
8 Heparin Induced Thrombocytopenia (HIT) A Rare but Serious Immune Reaction to Heparin CVD Interventions Use Heparin • Cardiovascular disease (CVD) is on the rise across the world due to aging populations • Complications from CVD also expected to increase Heparin Usage Leads to HIT • SoC for CVD interventions is heparin • HIT results in platelet activation, causing thrombocytopenia and results in thrombosis Current Treatments • SoC does not treat disease pathogenesis and can cause severe bleeding HIT has High Morbidity & Mortality • HIT patients have dire outcomes including death, pulmonary embolism, stroke Our Solution VLX - 1005 has a unique MoA that directly addresses the pathogenesis of HIT at its source Current therapies only treat the thrombotic complications of HIT and convey additional bleeding risk
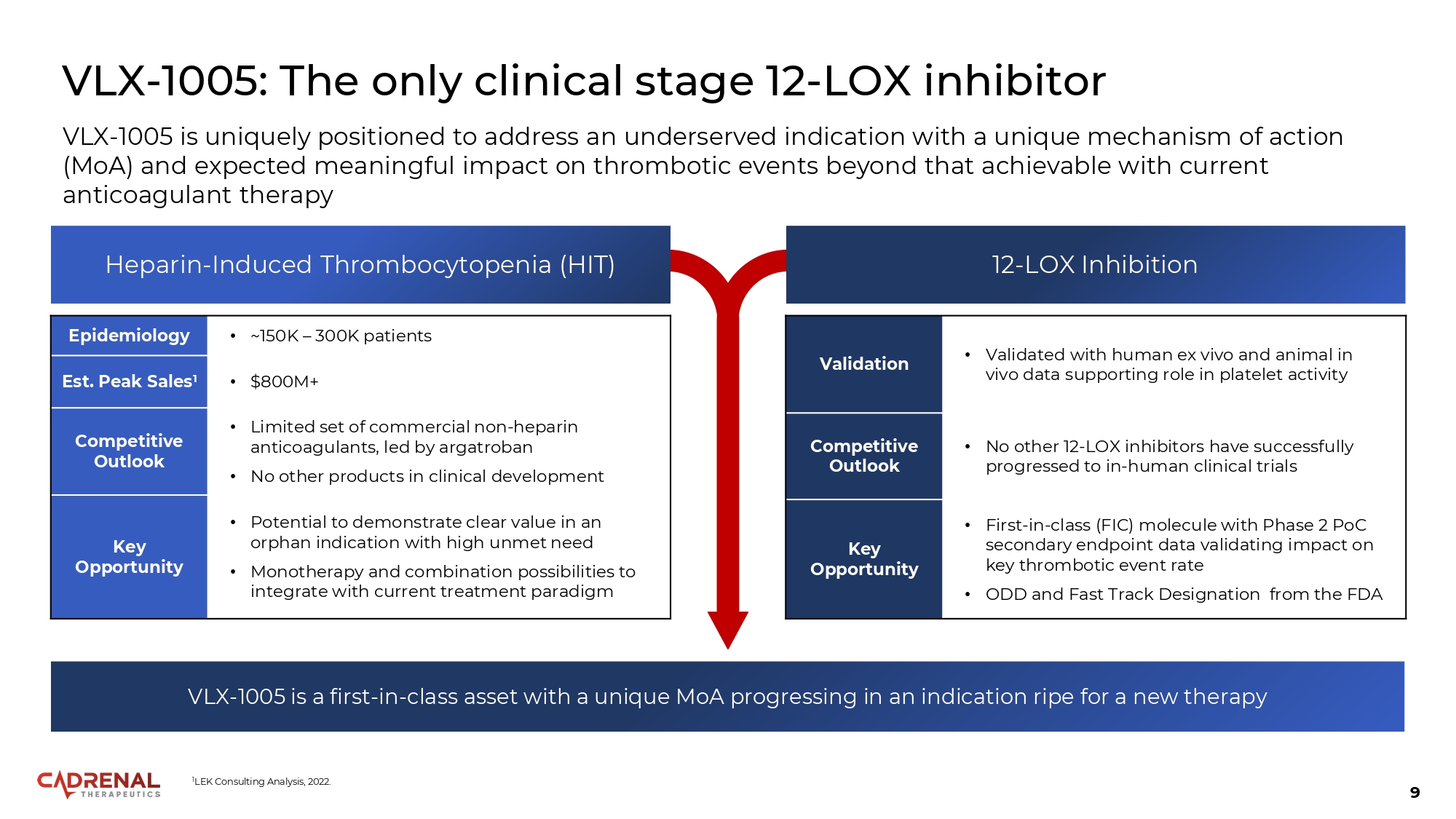
9 VLX - 1005: The only clinical stage 12 - LOX inhibitor • ~150K – 300K patients Epidemiology • $800M+ Est. Peak Sales 1 • Limited set of commercial non - heparin anticoagulants, led by argatroban • No other products in clinical development Competitive Outlook • Potential to demonstrate clear value in an orphan indication with high unmet need • Monotherapy and combination possibilities to integrate with current treatment paradigm Key Opportunity VLX - 1005 is uniquely positioned to address an underserved indication with a unique mechanism of action (MoA) and expected meaningful impact on thrombotic events beyond that achievable with current anticoagulant therapy Heparin - Induced Thrombocytopenia (HIT) 12 - LOX Inhibition • Validated with human ex vivo and animal in vivo data supporting role in platelet activity Validation • No other 12 - LOX inhibitors have successfully progressed to in - human clinical trials Competitive Outlook • First - in - class (FIC) molecule with Phase 2 PoC secondary endpoint data validating impact on key thrombotic event rate • ODD and Fast Track Designation from the FDA Key Opportunity VLX - 1005 is a first - in - class asset with a unique MoA progressing in an indication ripe for a new therapy 1 LEK Consulting Analysis, 2022.
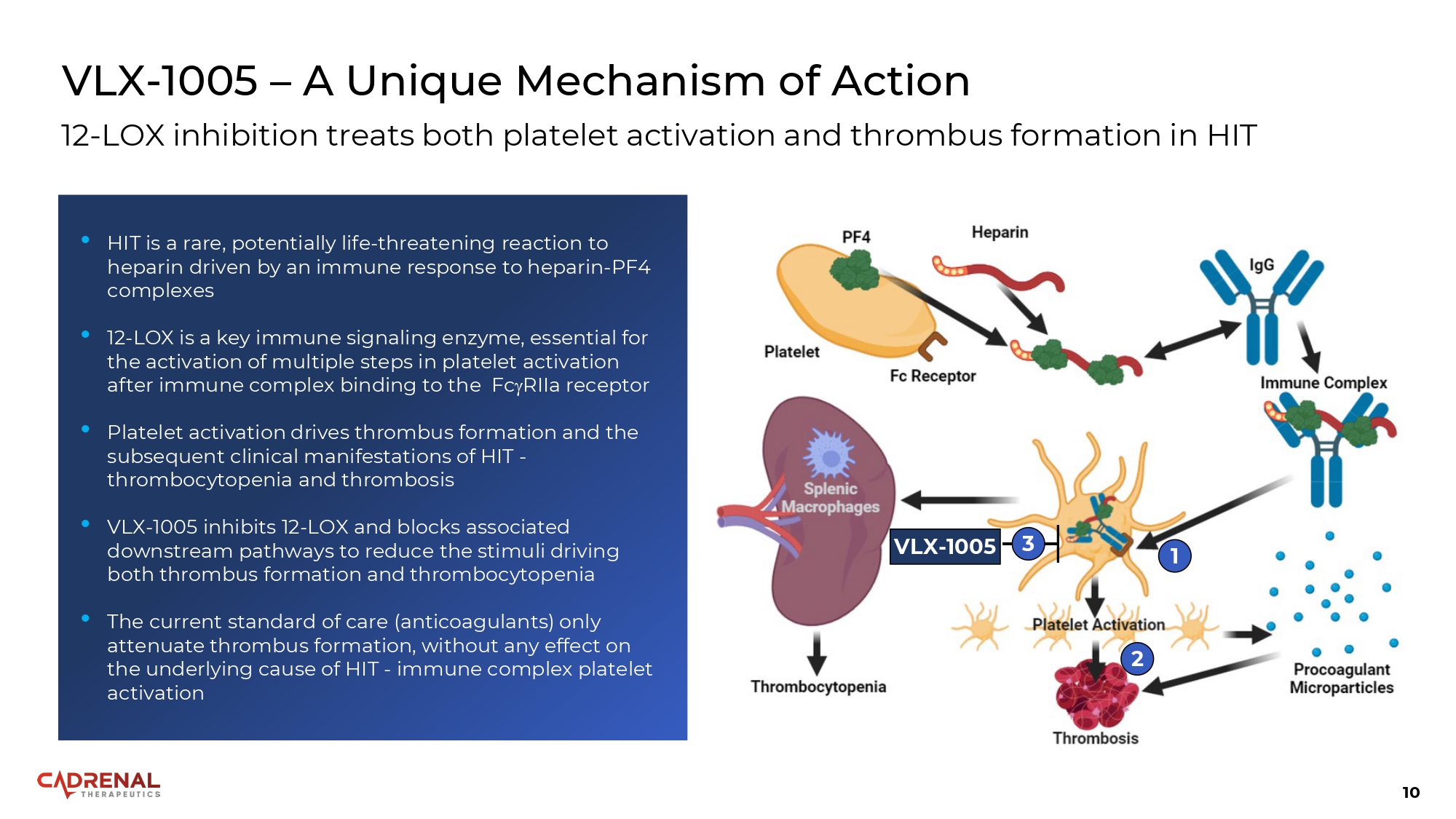
10 VLX - 1005 – A Unique Mechanism of Action 12 - LOX inhibition treats both platelet activation and thrombus formation in HIT VLX - 1005 1 2 3 • HIT is a rare, potentially life - threatening reaction to heparin driven by an immune response to heparin - PF4 complexes • 12 - LOX is a key immune signaling enzyme, essential for the activation of multiple steps in platelet activation after immune complex binding to the Fc RIIa receptor • Platelet activation drives thrombus formation and the subsequent clinical manifestations of HIT - thrombocytopenia and thrombosis • VLX - 1005 inhibits 12 - LOX and blocks associated downstream pathways to reduce the stimuli driving both thrombus formation and thrombocytopenia • The current standard of care (anticoagulants) only attenuate thrombus formation, without any effect on the underlying cause of HIT - immune complex platelet activation
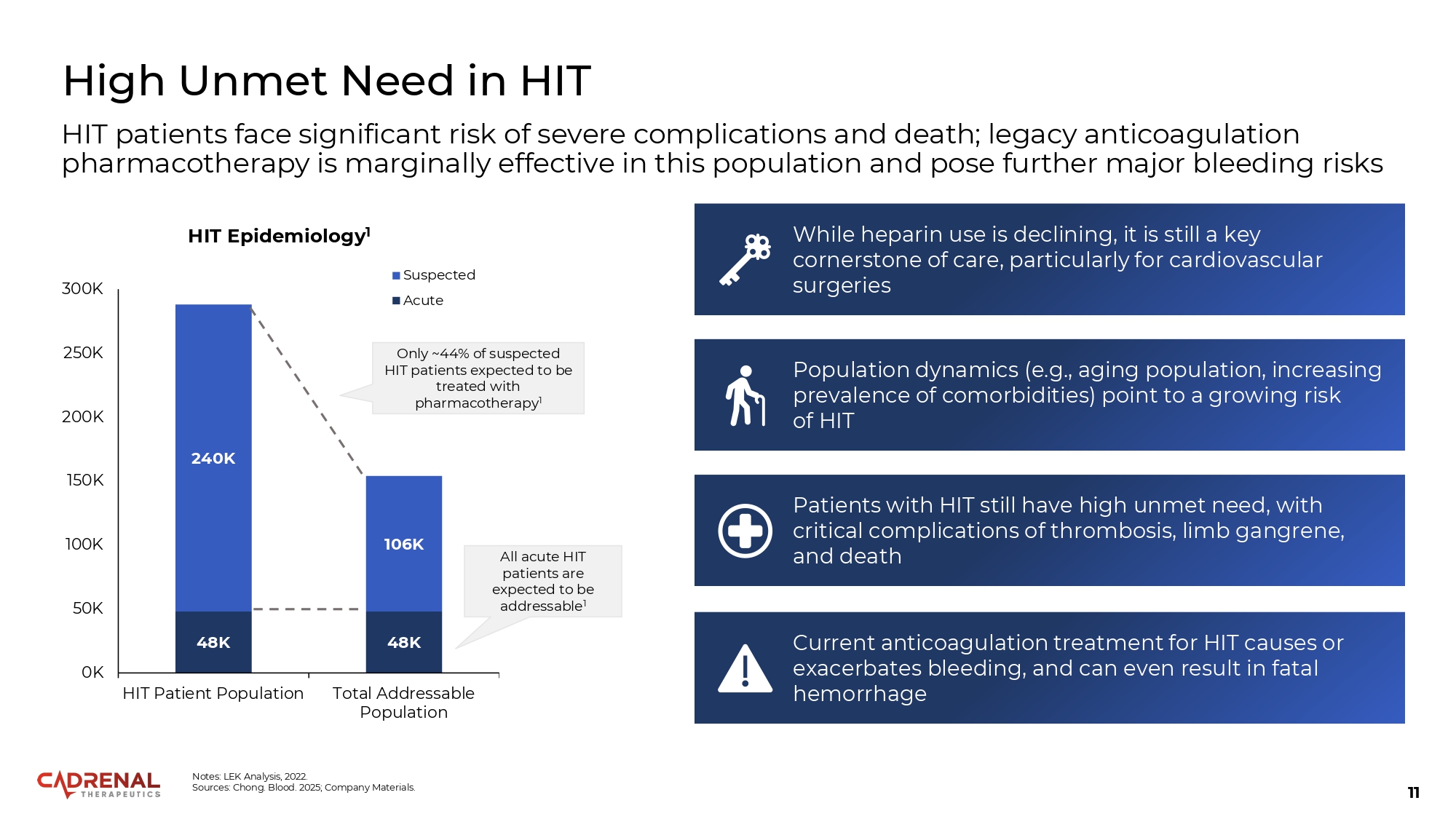
11 High Unmet Need in HIT HIT patients face significant risk of severe complications and death; legacy anticoagulation pharmacotherapy is marginally effective in this population and pose further major bleeding risks While heparin use is declining, it is still a key cornerstone of care, particularly for cardiovascular surgeries Patients with HIT still have high unmet need, with critical complications of thrombosis, limb gangrene, and death Current anticoagulation treatment for HIT causes or exacerbates bleeding, and can even result in fatal hemorrhage Population dynamics (e.g., aging population, increasing prevalence of comorbidities) point to a growing risk of HIT 48K 48K 240K 106K 0K 50K 100K 150K 200K 250K 300K HIT Patient Population Total Addressable Population HIT Epidemiology 1 Suspected Acute Only ~44% of suspected HIT patients expected to be treated with pharmacotherapy 1 All acute HIT patients are expected to be addressable 1 Notes: LEK Analysis, 2022. Sources: Chong. Blood. 2025; Company Materials.
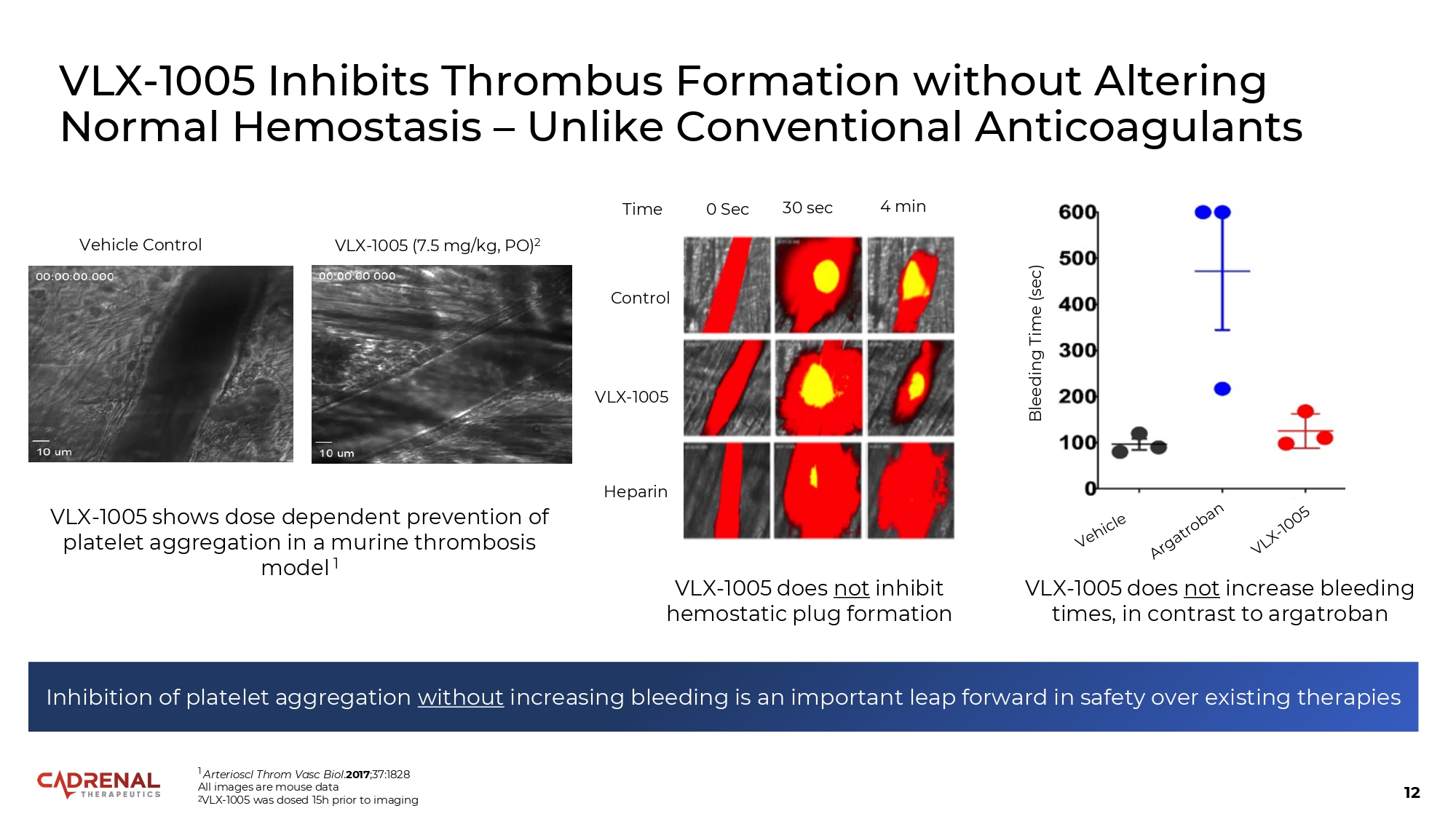
12 Vehicle Control VLX - 1005 (7.5 mg/kg, PO) 2 1 Arterioscl Throm Vasc Biol . 2017 ;37:1828 All images are mouse data 2 VLX - 1005 was dosed 15h prior to imaging Inhibition of platelet aggregation without increasing bleeding is an important leap forward in safety over existing therapies 0 Sec 30 sec 4 min Control VLX - 1005 Heparin VLX - 1005 shows dose dependent prevention of platelet aggregation in a murine thrombosis model 1 Bleeding Time (sec) Time VLX - 1005 Inhibits Thrombus Formation without Altering Normal Hemostasis – Unlike Conventional Anticoagulants VLX - 1005 does not inhibit hemostatic plug formation VLX - 1005 does not increase bleeding times, in contrast to argatroban
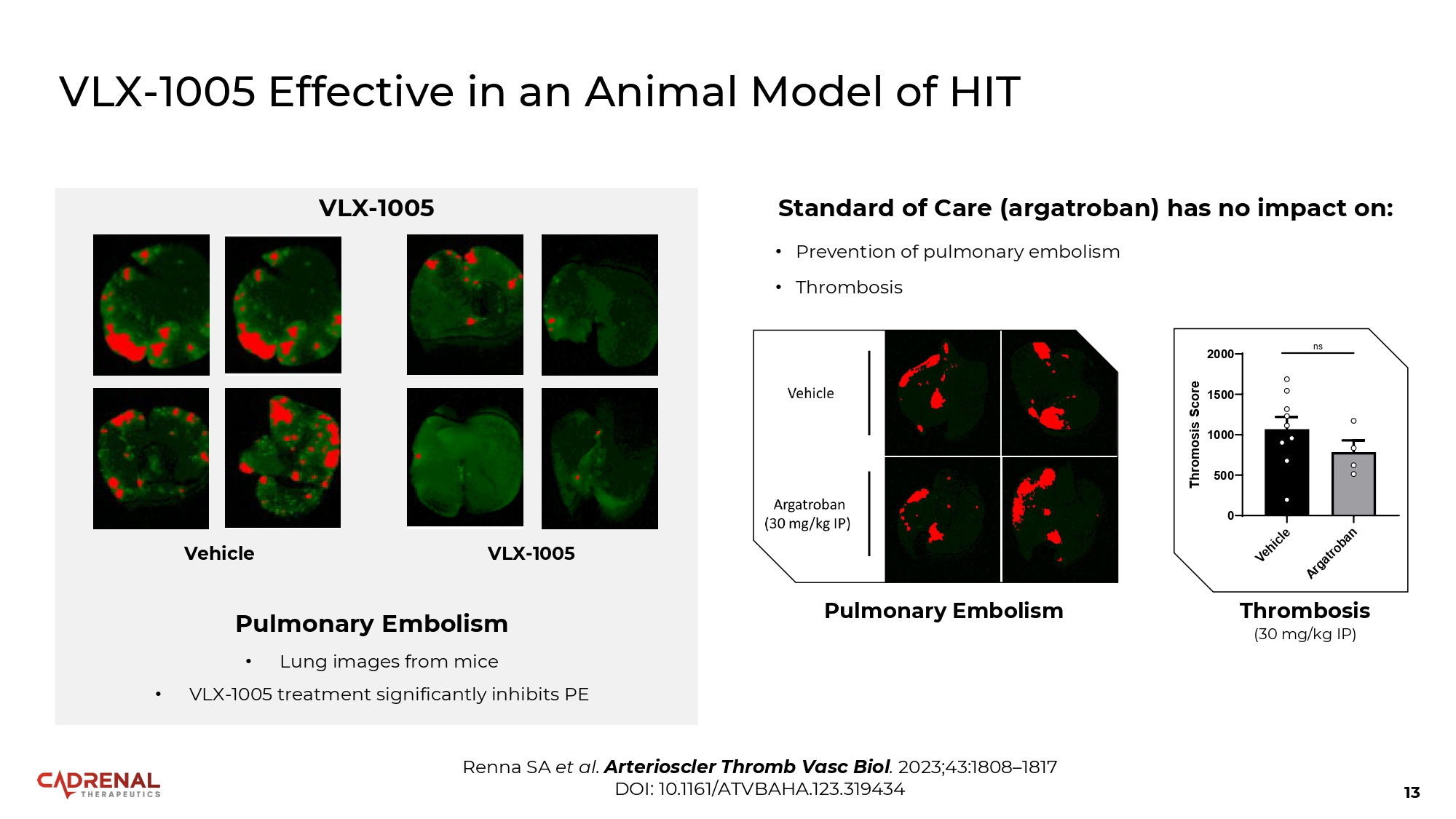
13 VLX - 1005 Effective in an Animal Model of HIT Renna SA et al. Arterioscler Thromb Vasc Biol . 2023;43:1808 – 1817 DOI: 10.1161/ATVBAHA.123.319434 1500 1000 500 0 2000 Thromosis Score ns Thrombosis (30 mg/kg IP) Pulmonary Embolism Standard of Care (argatroban) has no impact on: • Prevention of pulmonary embolism • Thrombosis VLX - 1005 Vehicle VLX - 1005 Pulmonary Embolism • Lung images from mice • VLX - 1005 treatment significantly inhibits PE

14 Phase 1 Studies Demonstrate VLX - 1005 Well - Tolerated VLX - 1005 well - tolerated in Phase 1 SAD/MAD and DDI studies • Over 100 subjects dosed • Adverse events were uncommon and mild in severity • Orphan Drug Designation in US and EMA • Fast Track Designation in US Composite SAD/MAD results: • No treatment - based discontinuations • 69 treatment emergent adverse events were observed - 30% in VLX - 1005 treated subjects vs. 36% in placebo • No SAEs reported, no dose limiting toxicities • PK well behaved & dose proportional • No drug - drug interaction between VLX - 1005 & argatroban
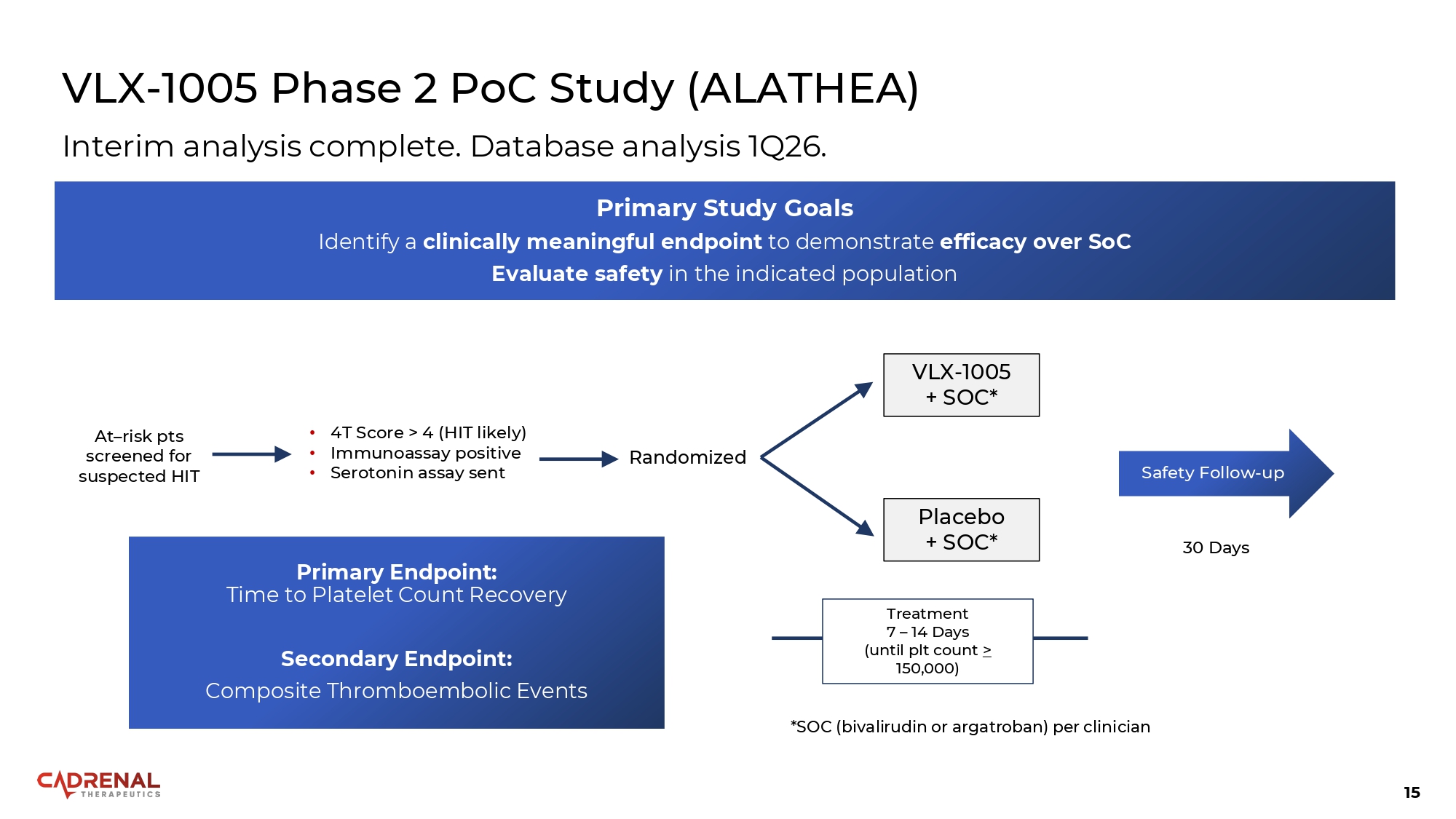
15 VLX - 1005 Phase 2 PoC Study (ALATHEA) Primary Endpoint: Time to Platelet Count Recovery Secondary Endpoint: Composite Thromboembolic Events At – risk pts screened for suspected HIT • 4T Score > 4 (HIT likely) • Immunoassay positive • Serotonin assay sent Randomized VLX - 1005 + SOC* Placebo + SOC* *SOC (bivalirudin or argatroban) per clinician Treatment 7 – 14 Days (until plt count > 150,000) 30 Days Safety Follow - up Interim analysis complete. Database analysis 1Q26. Primary Study Goals Identify a clinically meaningful endpoint to demonstrate efficacy over SoC Evaluate safety in the indicated population
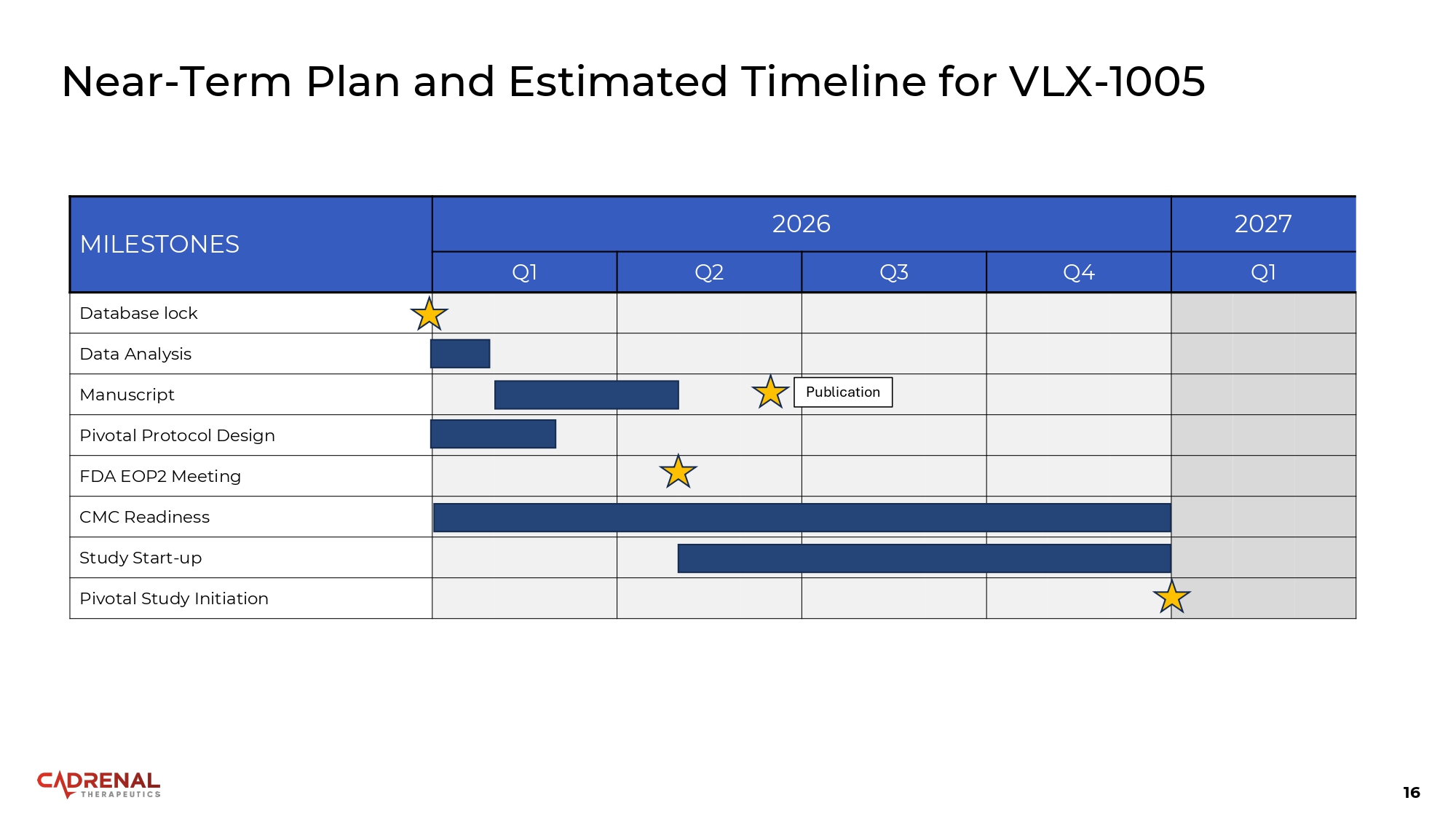
16 Near - Term Plan and Estimated Timeline for VLX - 1005 2027 2026 MILESTONES Q1 Q4 Q3 Q2 Q1 Database lock Data Analysis Publication Manuscript Pivotal Protocol Design FDA EOP2 Meeting CMC Readiness Study Start - up Pivotal Study Initiation

Target: Coagulation Tecarfarin and Frunexian
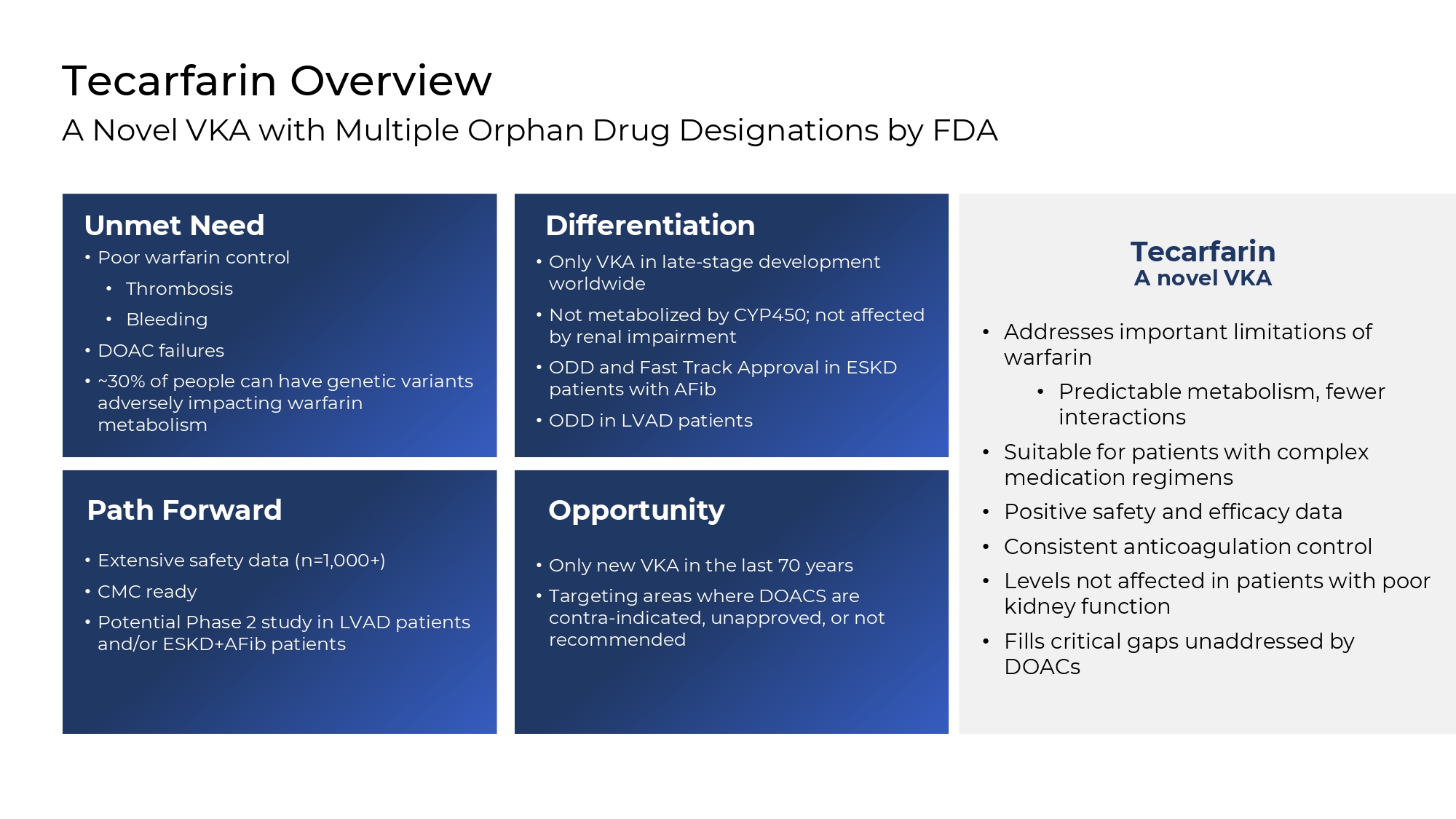
Opportunity • Only new VKA in the last 70 years • Targeting areas where DOACS are contra - indicated, unapproved, or not recommended Path Forward • Extensive safety data (n=1,000+) • CMC ready • Potential Phase 2 study in LVAD patients and/or ESKD+AFib patients Differentiation • Only VKA in late - stage development worldwide • Not metabolized by CYP450; not affected by renal impairment • ODD and Fast Track Approval in ESKD patients with AFib • ODD in LVAD patients Tecarfarin Overview A Novel VKA with Multiple Orphan Drug Designations by FDA Unmet Need • Poor warfarin control • Thrombosis • Bleeding • DOAC failures • ~30% of people can have genetic variants adversely impacting warfarin metabolism Tecarfarin A novel VKA • Addresses important limitations of warfarin • Predictable metabolism, fewer interactions • Suitable for patients with complex medication regimens • Positive safety and efficacy data • Consistent anticoagulation control • Levels not affected in patients with poor kidney function • Fills critical gaps unaddressed by DOACs

• Acute - care settings where there are opportunities to address treatment gaps with heparin Path Forward • Solidify CMC • Phase 2 dosing in preparation for potential Phase 3 program(s) • Complex cardiac surgery • Continuous Renal Replacement Therapy Differentiation • Fast - on / fast - off potent, dose - proportionate parenteral FXIa inhibition • Only acute - care parenteral XIa in development Frunexian Overview An Acute Parenteral Factor XIa inhibitor • Breakthrough Factor XIa asset(s) • Bridging opportunity (IV to Oral) Frunexian Acute parenteral FXIa inhibitor • Potent (> 95%) XIa inhibition • Effective blockade of contact activation • Predictable PK/PD • Parenteral administration • Rapid onset, short half - life • Phase 2 ready Unmet Need Opportunity

Leadership & Financial Summary

21 Experienced Leadership Across clinical to commercial drug development John R. Murphy Board Member Steven Zelenkofske, DO Board Member Glynn Wilson, PhD Board Member Quang X. Pham CEO & Founder, Chairman Matthew Szot, CPA Chief Financial Officer Jeff Cole Chief Operating Officer James Ferguson, MD, FACC, FAHA Chief Medical Officer Lee Golden, MD Board Member
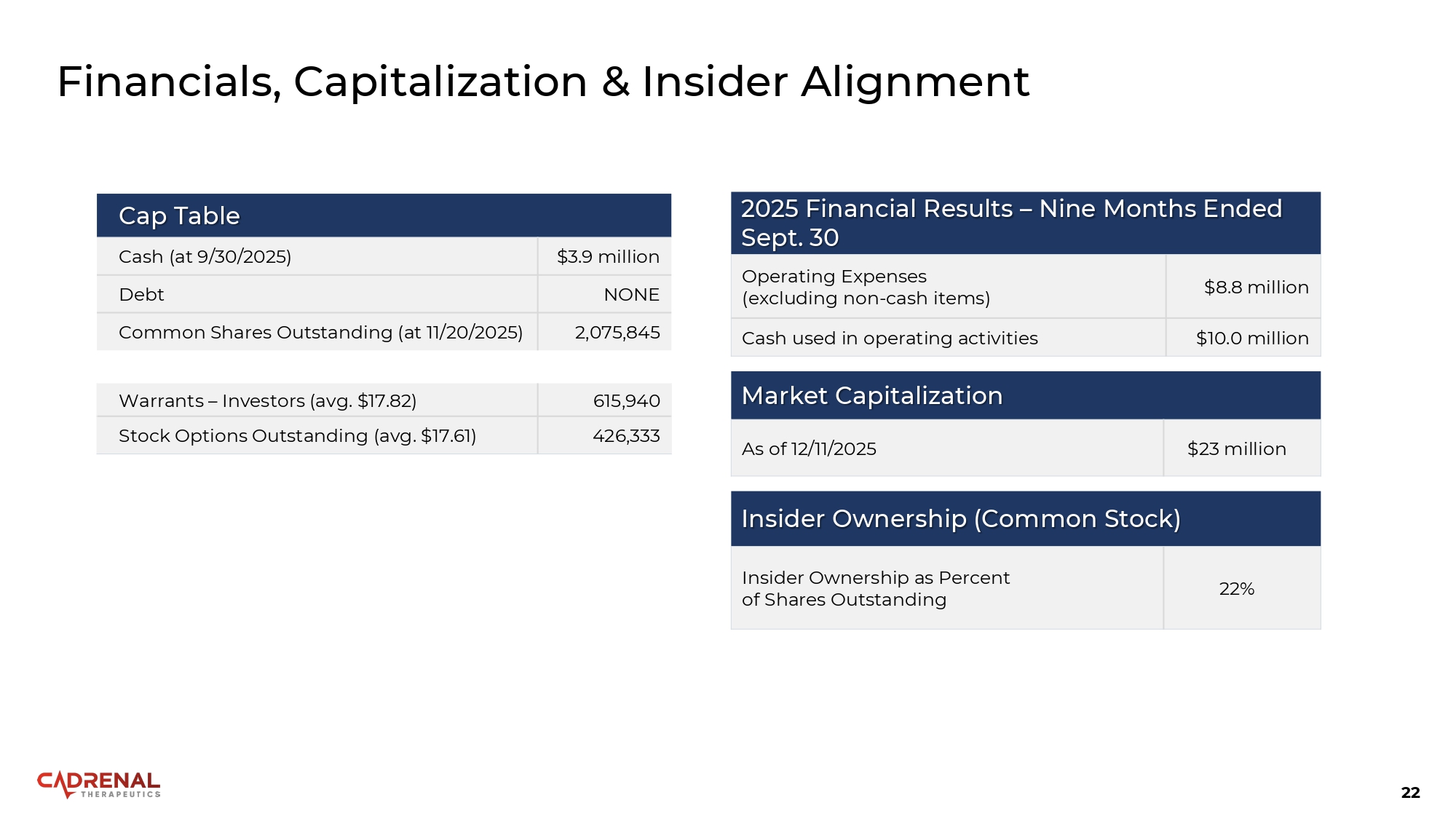
22 Financials, Capitalization & Insider Alignment Cap Table $3.9 million Cash (at 9/30/2025) NONE Debt 2,075,845 Common Shares Outstanding (at 11/20/2025) 615,940 Warrants – Investors (avg. $17.82) 426,333 Stock Options Outstanding (avg. $17.61) Market Capitalization $23 million As of 12/11/2025 Insider Ownership (Common Stock) 22% Insider Ownership as Percent of Shares Outstanding 2025 Financial Results – Nine Months Ended Sept. 30 $8.8 million Operating Expenses (excluding non - cash items) $10.0 million Cash used in operating activities

23 Quang X. Pham CEO & Founder quang.pham@cadrenal.com Matthew Szot CFO matthew.szot@cadrenal.com Contact Us: