| Title text 2 January 6, 2026 Zumilokibart (APG777) Asthma Phase 1b Interim Results |
| © Apogee Therapeutics, Inc 2 This presentation contains certain "forward-looking statements" within the meaning of applicable securities laws. Other than statements of historical facts, all statements included in this presentation are forward-looking statements, including statements about the potential for zumilokibart (APG777) in asthma; Apogee's plans for its current and future product candidates and programs; the anticipated timing of its clinical trials, including the APEX 52-week Part A in AD, APEX 16-week Part B in AD, APG279 Phase 1b head-to-head readout against DUPIXENT in AD, the potential Phase 3 trial of zumilokibart and the potential launch of zumilokibart; its planned clinical trial designs; its plans for current and future clinical trials; the potential clinical benefit and half-life, PK profile and dosing regimen, and treatment outcomes of zumilokibart and APG279; the potential to expand zumilokibart for other indications; Apogee's other product candidates, including combination therapies, and any other potential programs; its planned business strategies; potential market sizes; and its expectations regarding the time period over which Apogee's capital resources will be sufficient to fund its anticipated operations.Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2025, filed with the SEC on August 11, 2025, Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025, filed with the SEC on November 10, 2025 . In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “can,” “could,” “design,” “estimate,” “expect,” “intend,” “likely,” “may,” “might,” “plan,” “potential,” “predict,” “suggest,” “target,” “will,” “would,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. The forward-looking statements are based on our beliefs, assumptions and expectations of future performance, taking into account the information currently available to us. These statements are only predictions based upon our current expectations and projections about future events. Forward-looking statements are subject to known and unknown risks, uncertainties and other factors that may cause our actual results, level of activity, performance or achievements to be materially different from those expressed or implied by such forward-looking statements, including those risks described in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the U.S. Securities and Exchange Commission (“SEC”) on March 3, 2025, our Quarterly Report on Form 10-Q for the three months ended March 31, 2025, to be filed with the SEC on May 12, 2025, Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2025, filed with the SEC on August 11, 2025, Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2025, filed with the SEC on November 10, 2025, and subsequent disclosure documents we may file with the SEC. Although we have attempted to identify important factors that could cause actual results to differ materially from those contained in forward-looking statements, there may be other factors that cause results not to be as anticipated, estimated or intended. This presentation concerns a drug candidate that is under clinical investigation, and which has not yet been approved by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. The assumptions used in the preparation of this presentation, although considered reasonable by us at the time of preparation, may prove to be incorrect. You are cautioned that the information is based on assumptions as to many factors and that actual results may vary from the results projected, and such variations may be material. Accordingly, you should not place undue reliance on any forward-looking statements contained herein or rely on them as predictions of future events. All forward-looking statements in this presentation apply only as of the date made and are expressly qualified by the cautionary statements included in this presentation. We do not undertake to update any forward-looking statements, except in accordance with applicable securities laws. The trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. Certain information contained in this presentation relate to or are based on studies, publications and other data obtained from third-party sources as well as our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Disclaimers and Forward-looking Statements |
| © Apogee Therapeutics, Inc 3 Agenda Introduction Michael Henderson, MD Chief Executive Officer Carl Dambkowski, MD Chief Medical Officer Invited KOL: Mario Castro, MD, MPH University of Kansas School of Medicine Michael Henderson, MD, CEO Carl Dambkowski, MD, CMO Jane Pritchett Henderson, CFO Jeff Hartness, CCO Invited KOL: Mario Castro, MD, MPH Zumilokibart (APG777) Asthma Phase 1b Results IL-13 inhibition in asthma Closing remarks Analyst Q&A Michael Henderson, MD Chief Executive Officer |
| Introduction Michael Henderson, MD Chief Executive Officer |
| © Apogee Therapeutics, Inc. Q1 NOTE: 1APG279 is a combination of APG777 and APG990. APG279 will be co-administered in the proof-of-concept Phase 1b trial; coformulation planned for future clinical studies and commercialization. FeNO = fractional exhaled nitric oxide. © Apogee Therapeutics, Inc. 5 2026 could be a transformational year for Apogee Zumilokibart (APG777) Asthma Phase 1b positive data UPDATE: Robust effect on FeNO comparable to DUPIXENT; sustained FeNO suppression after a single dose for 16 weeks, with continued FeNO suppression through 32 weeks for patients with available follow-up Zumilokibart AD Phase 2: Part A (52-week readout) Q2 Zumilokibart AD Phase 2: Part B (16-week readout) UPDATE: Part B enrollment complete and on track for Q2 2026 readout; overenrolled (N=347) due to strong interest from physicians and patients 2H APG279 AD Phase 1b POC readout (vs DUPIXENT)1 UPDATE: On track for 2H 2026 readout with expanded enrollment (N ~80) due to strong interest from physicians and patients Zumilokibart AD Phase 3 initiation Expected Key 2026 Milestones: |
| © Apogee Therapeutics, Inc 6 Zumilokibart (APG777) targets IL -13, the core driver of multiple I&I diseases Atopic dermatitis • Part A 16 -week data demonstrated potentially best -in -class profile • Patients in Part A with comorbid asthma or sinusitis saw improvement based on ACQ -5 and SNOT -22 Expansion indications • 8+ potential additional expansions (e.g., EoE, COPD, CRSwNP, PN) • Asthma Phase 1b: Depth and durability of response support continued expansion to asthma with potential every 3 - or 6 -month dosing UPDATE 6 |
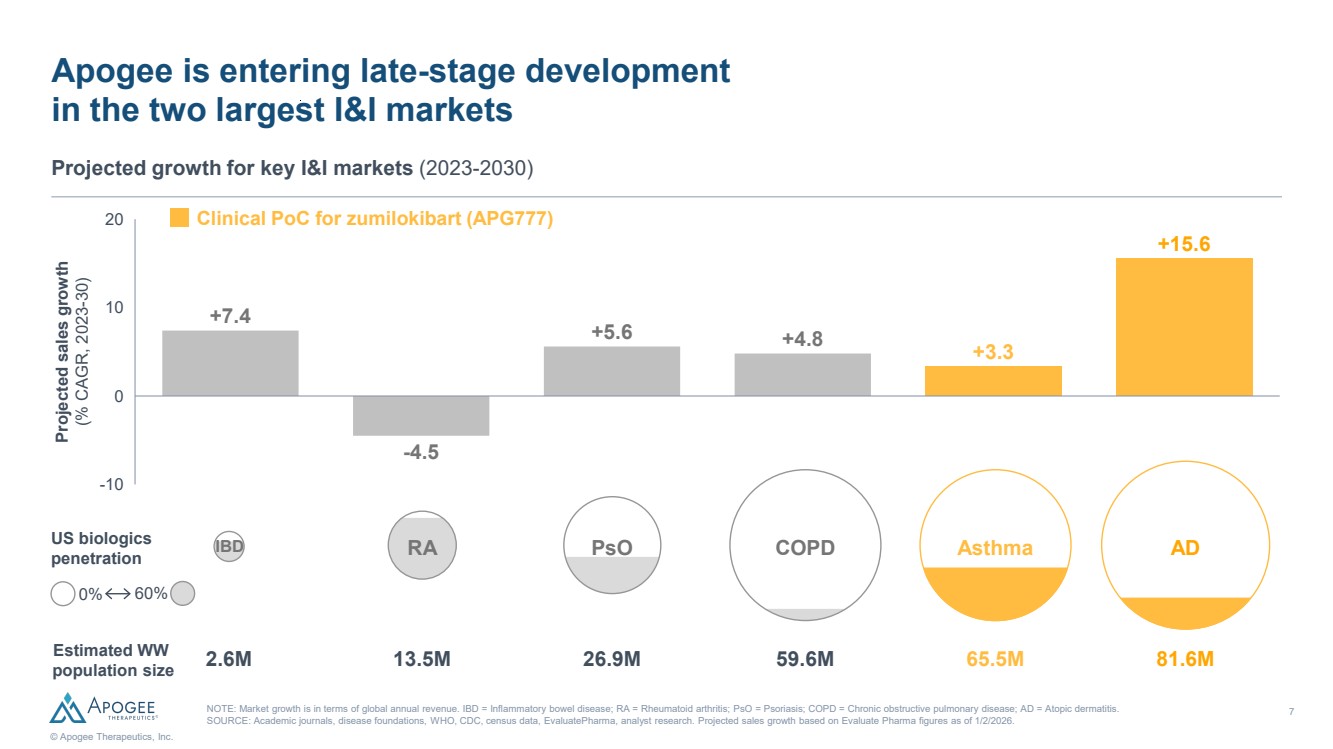
| 7 © Apogee Therapeutics, Inc. Apogee is entering late-stage development in the two largest I&I markets Projected growth for key I&I markets (2023-2030) NOTE: Market growth is in terms of global annual revenue. IBD = Inflammatory bowel disease; RA = Rheumatoid arthritis; PsO = Psoriasis; COPD = Chronic obstructive pulmonary disease; AD = Atopic dermatitis. SOURCE: Academic journals, disease foundations, WHO, CDC, census data, EvaluatePharma, analyst research. Projected sales growth based on Evaluate Pharma figures as of 1/2/2026. Projected sales growth (% CAGR, 2023-30) Estimated WW population size PsO COPD Asthma 2.6M 13.5M 26.9M 59.6M 65.5M 81.6M US biologics penetration AD 0% 60% Clinical PoC for zumilokibart (APG777) -4.5 -10 0 10 20 +7.4 +5.6 +4.8 +3.3 +15.6 IBD RA |
| Zumilokibart (APG777) Asthma Phase 1b Interim Results Carl Dambkowski, MD Chief Medical Officer |
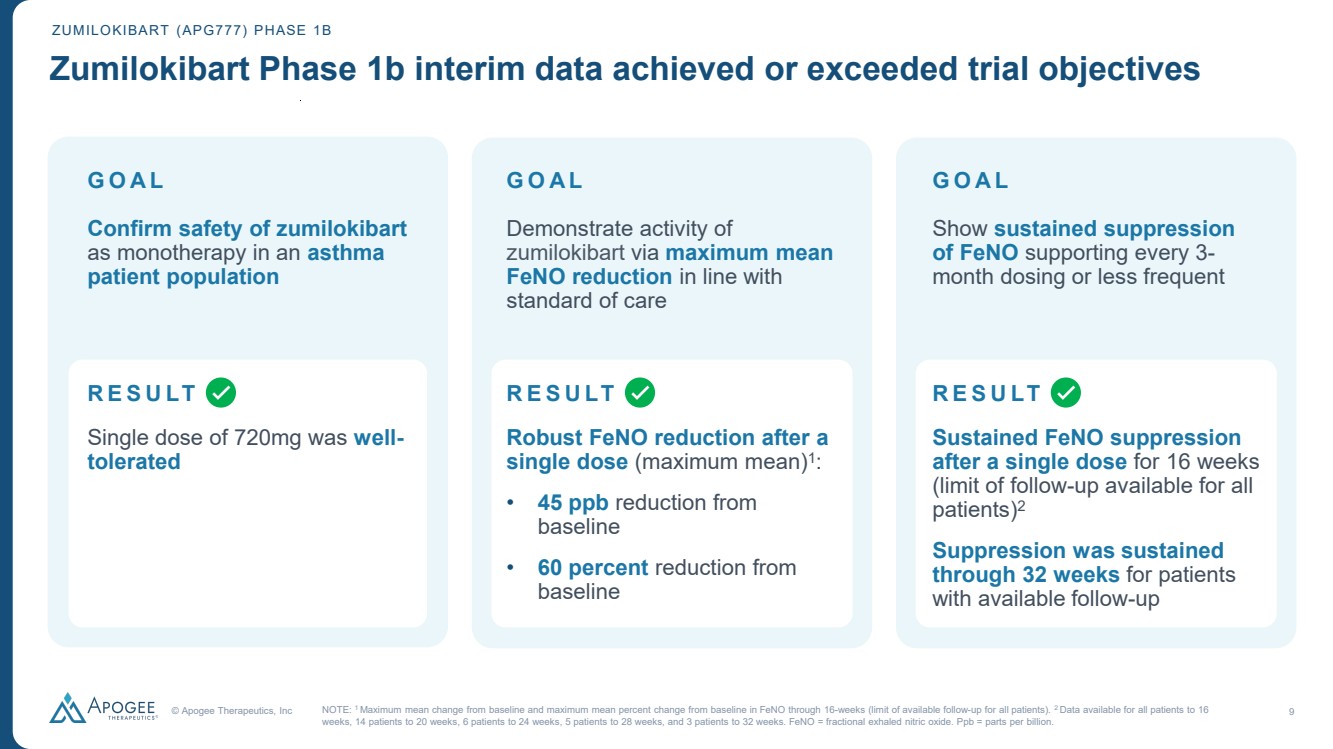
| © Apogee Therapeutics, Inc 9 GOAL Confirm safety of zumilokibart as monotherapy in an asthma patient population Single dose of 720mg was well-tolerated Demonstrate activity of zumilokibart via maximum mean FeNO reduction in line with standard of care GOAL Show sustained suppression of FeNO supporting every 3- month dosing or less frequent Sustained FeNO suppression after a single dose for 16 weeks (limit of follow-up available for all patients)2 Suppression was sustained through 32 weeks for patients with available follow-up GOAL ZUMILOKIBART (APG777) PHASE 1B Zumilokibart Phase 1b interim data achieved or exceeded trial objectives NOTE: 1 Maximum mean change from baseline and maximum mean percent change from baseline in FeNO through 16-weeks (limit of available follow-up for all patients). 2 Data available for all patients to 16 weeks, 14 patients to 20 weeks, 6 patients to 24 weeks, 5 patients to 28 weeks, and 3 patients to 32 weeks. FeNO = fractional exhaled nitric oxide. Ppb = parts per billion. Robust FeNO reduction after a single dose (maximum mean)1: • 45 ppb reduction from baseline • 60 percent reduction from baseline RESULT RESULT RESULT |
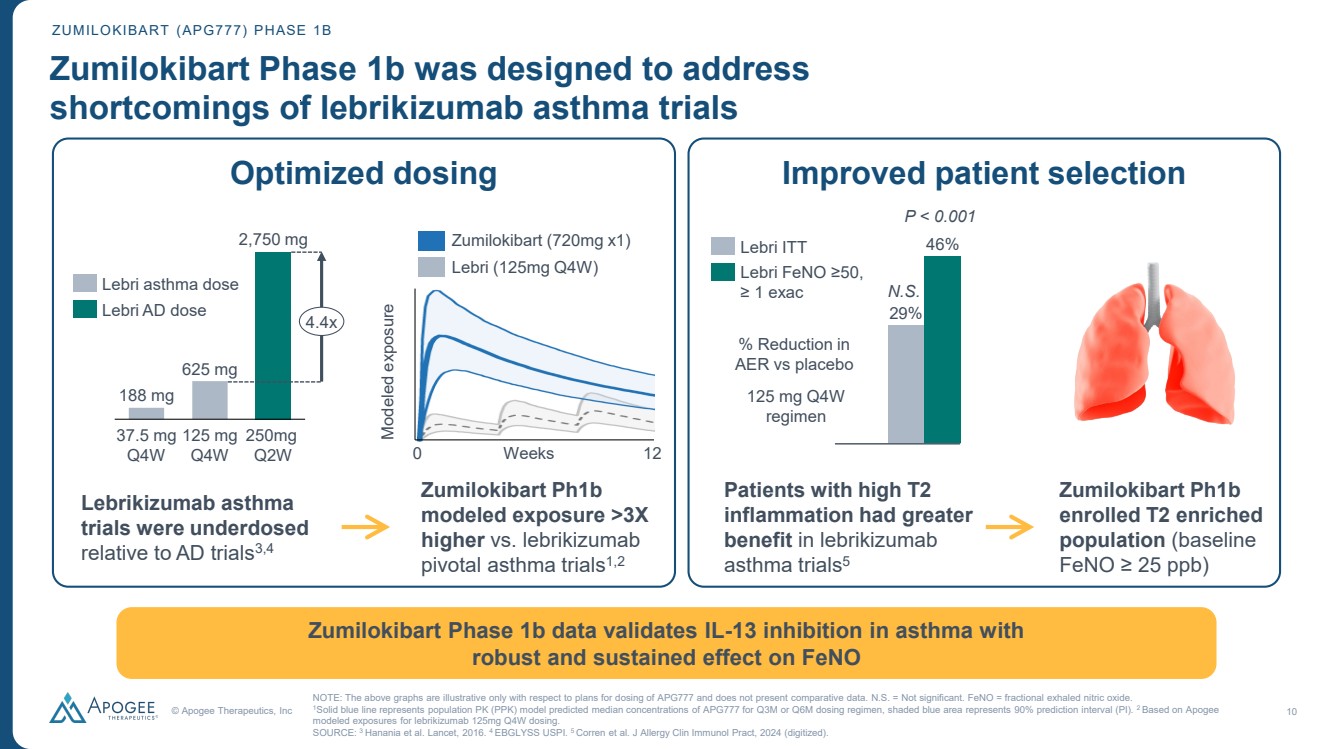
| © Apogee Therapeutics, Inc 10 ZUMILOKIBART (APG777) PHASE 1B Zumilokibart Phase 1b was designed to address shortcomings of lebrikizumab asthma trials Zumilokibart Phase 1b data validates IL-13 inhibition in asthma with robust and sustained effect on FeNO Lebrikizumab asthma trials were underdosed relative to AD trials3,4 Zumilokibart Ph1b modeled exposure >3X higher vs. lebrikizumab pivotal asthma trials1,2 37.5 mg Q4W 125 mg Q4W 250mg Q2W 188 mg 625 mg 2,750 mg 4.4x Lebri asthma dose Lebri AD dose Modeled exposure 0 Weeks 12 Zumilokibart (720mg x1) Lebri (125mg Q4W) Optimized dosing Improved patient selection 29% Lebri ITT 46% Lebri FeNO ≥50, ≥ 1 exac % Reduction in AER vs placebo 125 mg Q4W regimen Patients with high T2 inflammation had greater benefit in lebrikizumab asthma trials5 Zumilokibart Ph1b enrolled T2 enriched population (baseline FeNO ≥ 25 ppb) NOTE: The above graphs are illustrative only with respect to plans for dosing of APG777 and does not present comparative data. N.S. = Not significant. FeNO = fractional exhaled nitric oxide. 1Solid blue line represents population PK (PPK) model predicted median concentrations of APG777 for Q3M or Q6M dosing regimen, shaded blue area represents 90% prediction interval (PI). 2 Based on Apogee modeled exposures for lebrikizumab 125mg Q4W dosing. SOURCE: 3 Hanania et al. Lancet, 2016. 4 EBGLYSS USPI. 5 Corren et al. J Allergy Clin Immunol Pract, 2024 (digitized). P < 0.001 N.S. |
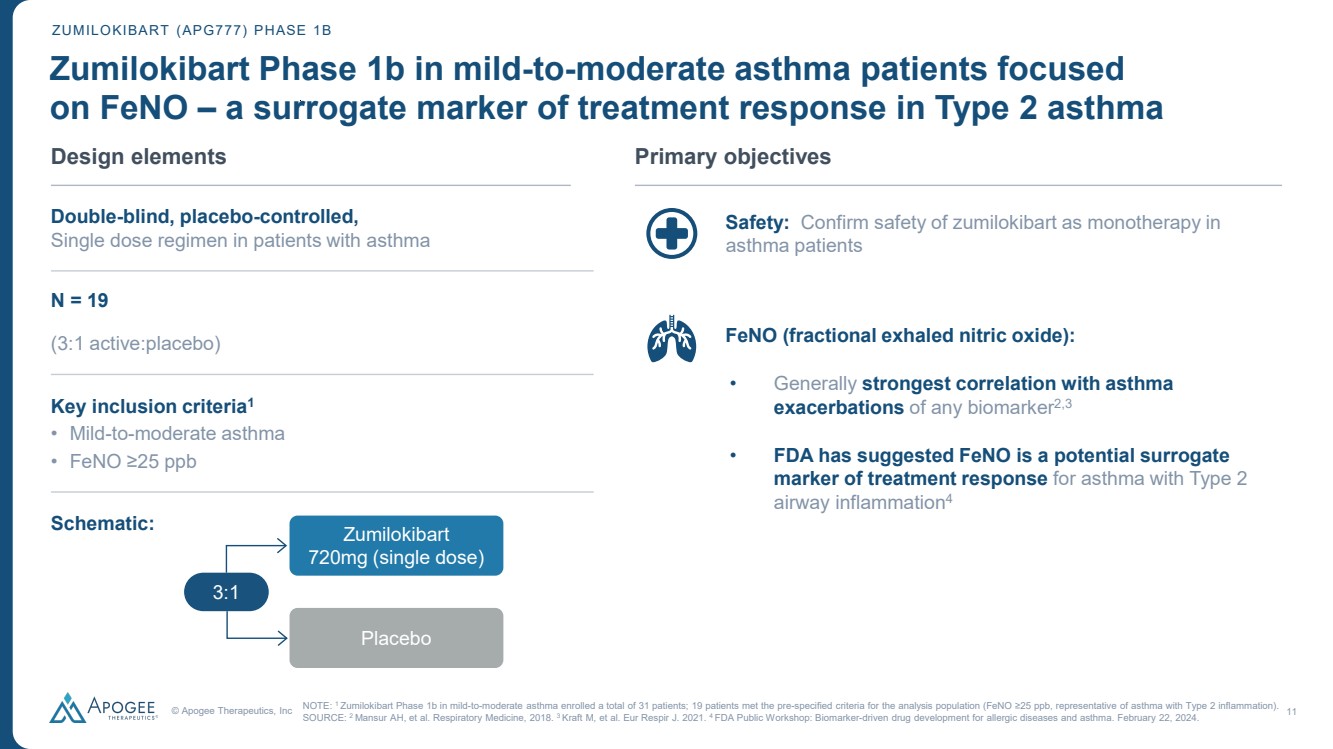
| © Apogee Therapeutics, Inc 11 • Safety: Confirm safety of zumilokibart as monotherapy in asthma patients • FeNO (fractional exhaled nitric oxide): • Generally strongest correlation with asthma exacerbations of any biomarker2,3 • FDA has suggested FeNO is a potential surrogate marker of treatment response for asthma with Type 2 airway inflammation4 ZUMILOKIBART (APG777) PHASE 1B Zumilokibart Phase 1b in mild-to-moderate asthma patients focused on FeNO – a surrogate marker of treatment response in Type 2 asthma Design elements Double-blind, placebo-controlled, Single dose regimen in patients with asthma N = 19 (3:1 active:placebo) Key inclusion criteria1 • Mild-to-moderate asthma • FeNO ≥25 ppb Schematic: Primary objectives NOTE: 1 Zumilokibart Phase 1b in mild-to-moderate asthma enrolled a total of 31 patients; 19 patients met the pre-specified criteria for the analysis population (FeNO ≥25 ppb, representative of asthma with Type 2 inflammation). SOURCE: 2 Mansur AH, et al. Respiratory Medicine, 2018. 3 Kraft M, et al. Eur Respir J. 2021. 4 FDA Public Workshop: Biomarker-driven drug development for allergic diseases and asthma. February 22, 2024. 3:1 Zumilokibart 720mg (single dose) Placebo |
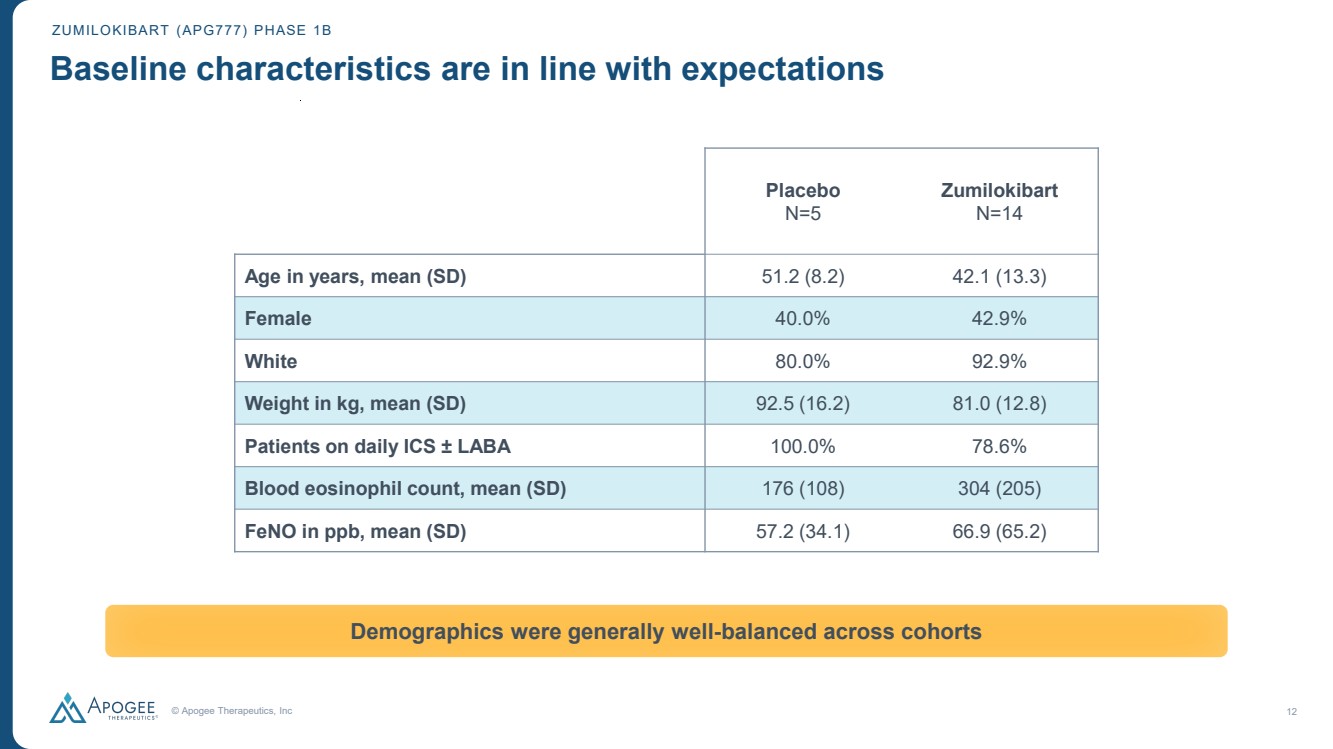
| © Apogee Therapeutics, Inc 12 ZUMILOKIBART (APG777) PHASE 1B Baseline characteristics are in line with expectations Demographics were generally well-balanced across cohorts Placebo N=5 Zumilokibart N=14 Age in years, mean (SD) 51.2 (8.2) 42.1 (13.3) Female 40.0% 42.9% White 80.0% 92.9% Weight in kg, mean (SD) 92.5 (16.2) 81.0 (12.8) Patients on daily ICS ± LABA 100.0% 78.6% Blood eosinophil count, mean (SD) 176 (108) 304 (205) FeNO in ppb, mean (SD) 57.2 (34.1) 66.9 (65.2) |
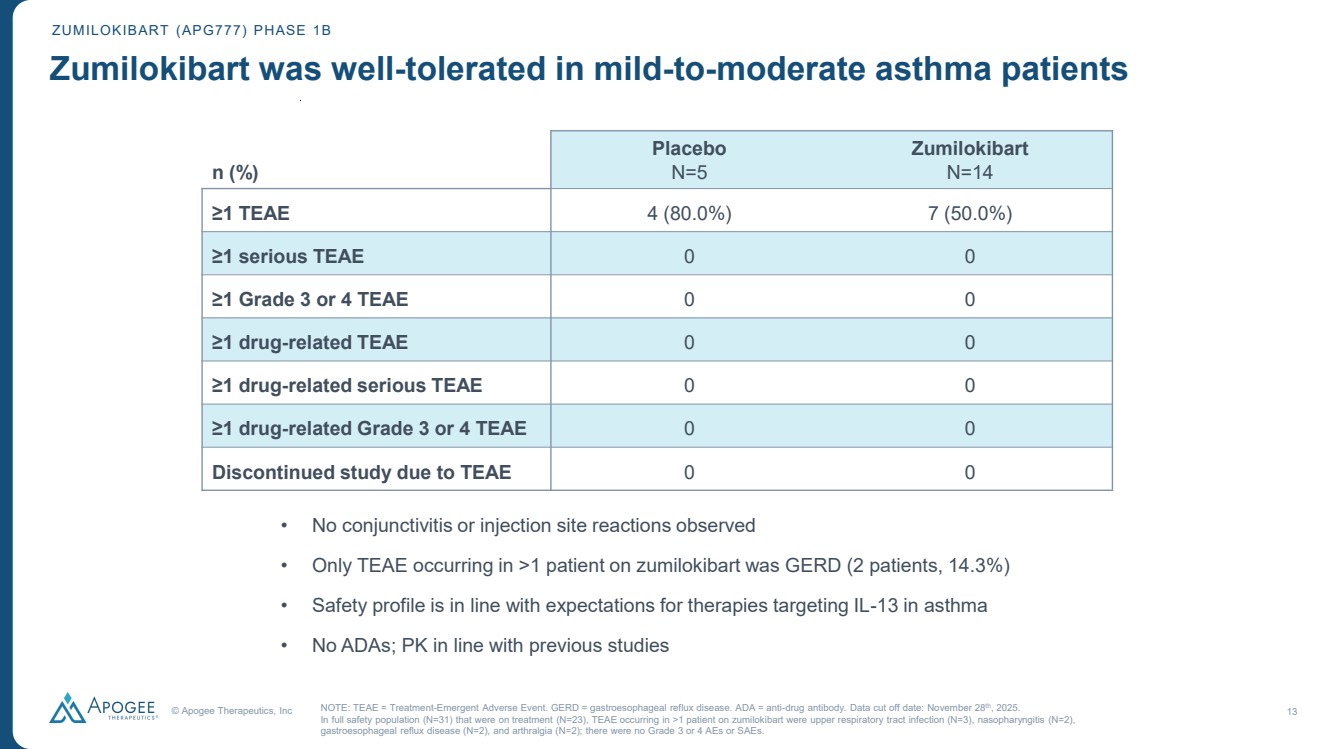
| © Apogee Therapeutics, Inc 13 n (%) Placebo N=5 Zumilokibart N=14 ≥1 TEAE 4 (80.0%) 7 (50.0%) ≥1 serious TEAE 0 0 ≥1 Grade 3 or 4 TEAE 0 0 ≥1 drug-related TEAE 0 0 ≥1 drug-related serious TEAE 0 0 ≥1 drug-related Grade 3 or 4 TEAE 0 0 Discontinued study due to TEAE 0 0 ZUMILOKIBART (APG777) PHASE 1B Zumilokibart was well-tolerated in mild-to-moderate asthma patients NOTE: TEAE = Treatment-Emergent Adverse Event. GERD = gastroesophageal reflux disease. ADA = anti-drug antibody. Data cut off date: November 28th, 2025. In full safety population (N=31) that were on treatment (N=23), TEAE occurring in >1 patient on zumilokibart were upper respiratory tract infection (N=3), nasopharyngitis (N=2), gastroesophageal reflux disease (N=2), and arthralgia (N=2); there were no Grade 3 or 4 AEs or SAEs. • No conjunctivitis or injection site reactions observed • Only TEAE occurring in >1 patient on zumilokibart was GERD (2 patients, 14.3%) • Safety profile is in line with expectations for therapies targeting IL-13 in asthma • No ADAs; PK in line with previous studies |
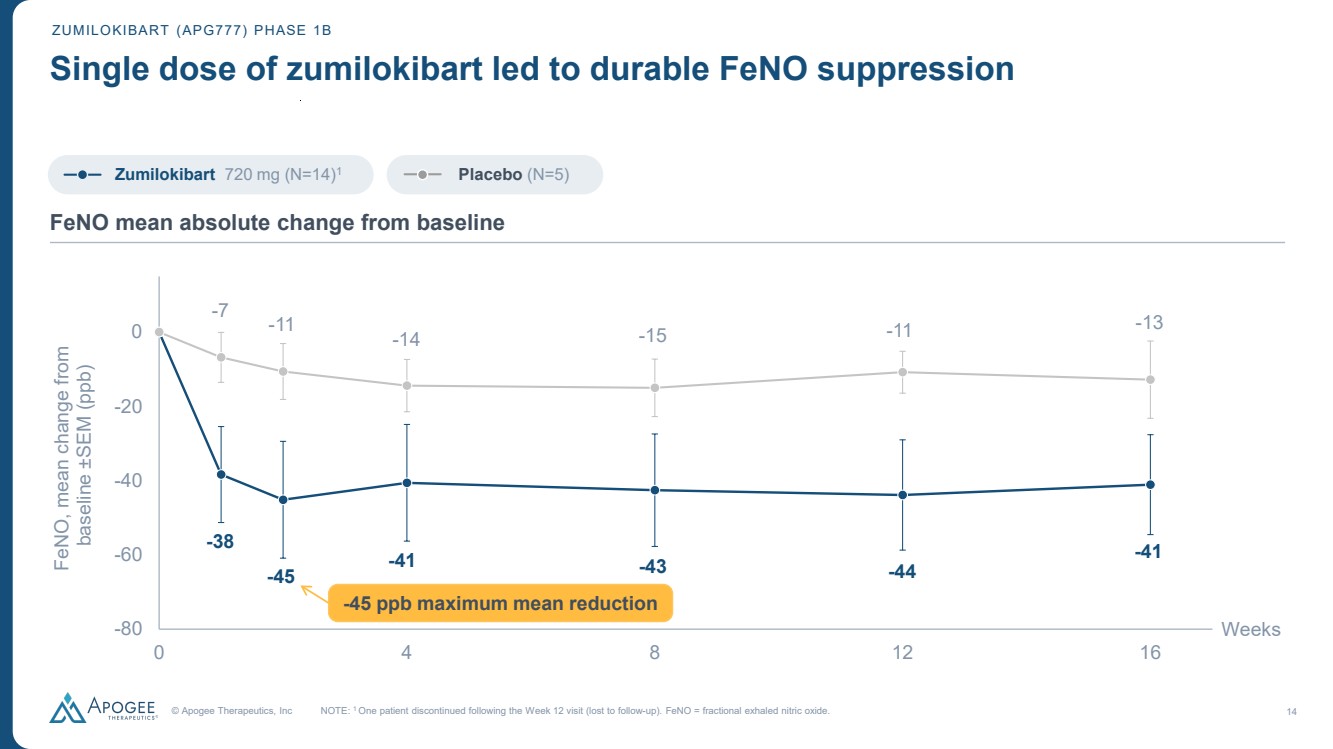
| © Apogee Therapeutics, Inc 14 ZUMILOKIBART (APG777) PHASE 1B Single dose of zumilokibart led to durable FeNO suppression -38 -45 -41 -43 -44 -41 -7 -11 -14 -15 -11 -13 -80 -60 -40 -20 0 0 4 8 12 16 FeNO, mean change from baseline ±SEM (ppb) Weeks NOTE: 1 One patient discontinued following the Week 12 visit (lost to follow-up). FeNO = fractional exhaled nitric oxide. FeNO mean absolute change from baseline Zumilokibart 720 mg (N=14)1 Placebo (N=5) -45 ppb maximum mean reduction |
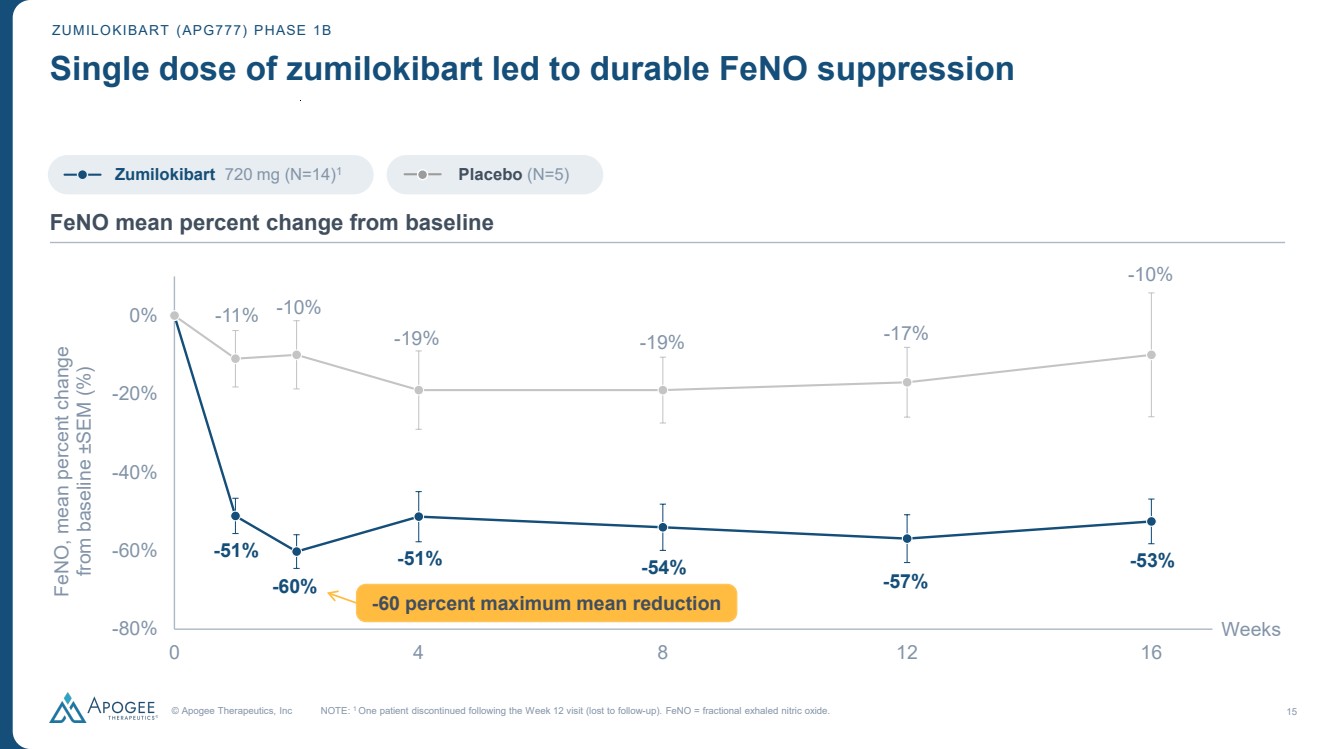
| © Apogee Therapeutics, Inc 15 ZUMILOKIBART (APG777) PHASE 1B Single dose of zumilokibart led to durable FeNO suppression -51% -60% -51% -54% -57% -53% -11% -10% -19% -19% -17% -10% -80% -60% -40% -20% 0% 0 4 8 12 16 FeNO, mean percent change from baseline ±SEM (%) Weeks NOTE: 1 One patient discontinued following the Week 12 visit (lost to follow-up). FeNO = fractional exhaled nitric oxide. FeNO mean percent change from baseline -60 percent maximum mean reduction Zumilokibart 720 mg (N=14)1 Placebo (N=5) |
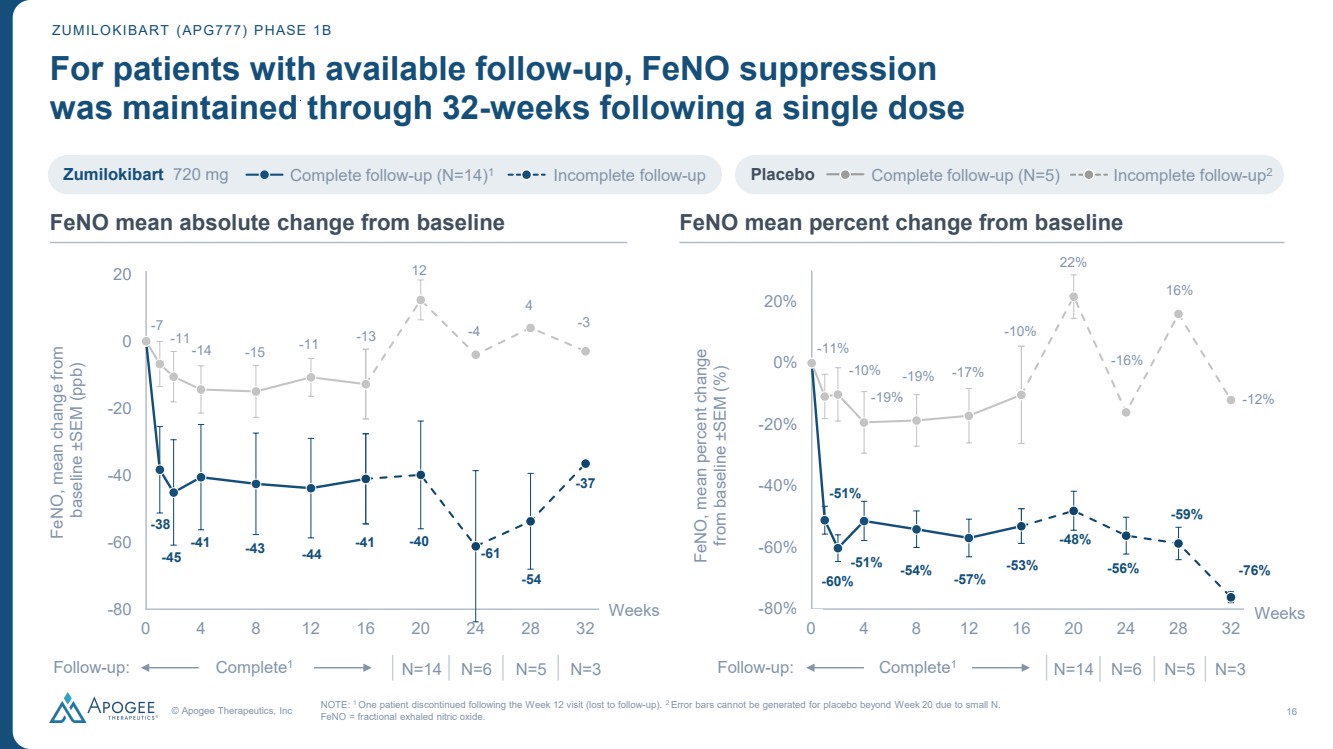
| © Apogee Therapeutics, Inc 16 -38 -45 -41 -43 -44 -41 -7 -11-14 -15 -11 -13 -40 -61 -54 -37 12 -4 4 -3 -100 -80 -60 -40 -20 0 20 0 4 8 12 16 20 24 28 32 FeNO, mean change from baseline ±SEM (ppb) Weeks -51% -60% -51% -54% -57% -53% -11% -10% -19% -19% -17% -10% -48% -56% -59% -76% 22% -16% 16% -12% -100% -80% -60% -40% -20% 0% 20% 0 4 8 12 16 20 24 28 32 FeNO, mean percent change from baseline ±SEM (%) Weeks ZUMILOKIBART (APG777) PHASE 1B For patients with available follow-up, FeNO suppression was maintained through 32-weeks following a single dose NOTE: 1 One patient discontinued following the Week 12 visit (lost to follow-up). 2 Error bars cannot be generated for placebo beyond Week 20 due to small N. FeNO = fractional exhaled nitric oxide. Zumilokibart 720 mg Complete follow-up (N=14) Incomplete follow-up 1 Placebo Complete follow-up (N=5) Incomplete follow-up2 Complete N=14 N=6 N=5 N=3 Follow 1 -up: Complete N=14 N=6 N=5 N=3 Follow 1 -up: 0 0 FeNO mean absolute change from baseline FeNO mean percent change from baseline |
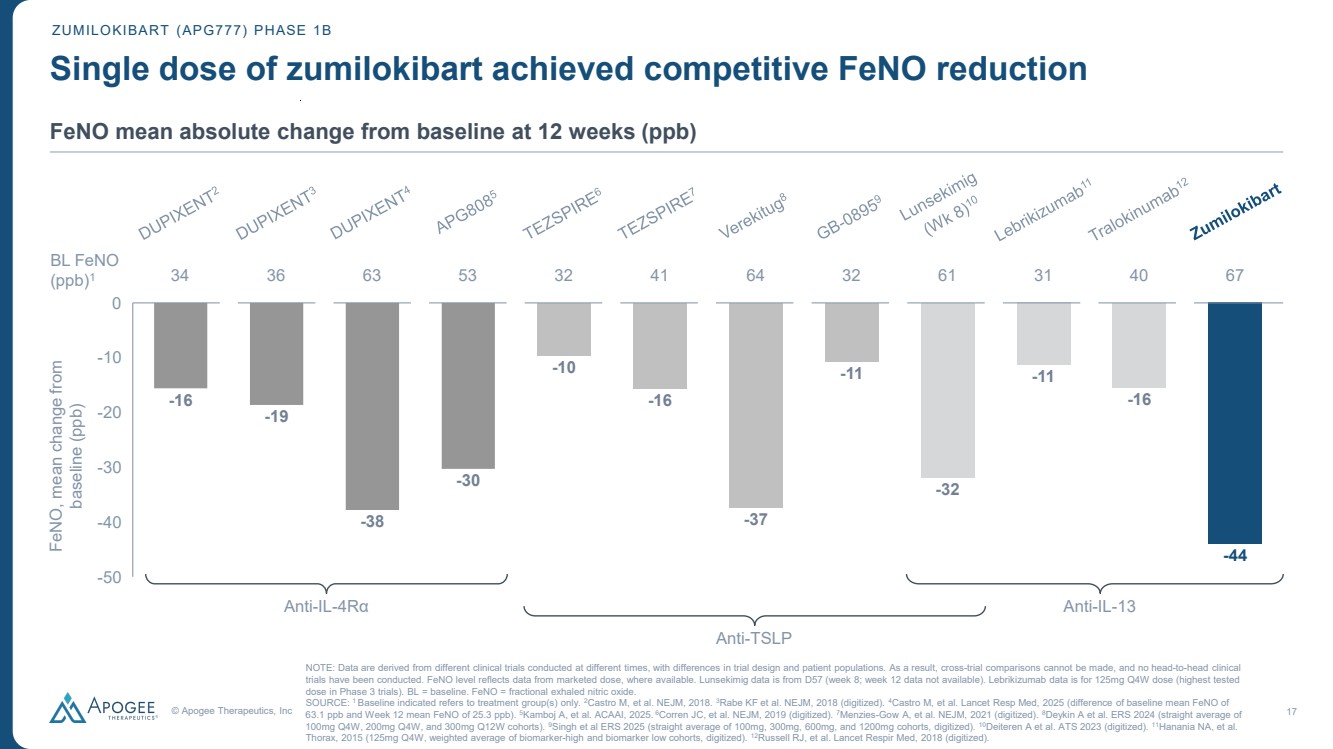
| © Apogee Therapeutics, Inc 17 Single dose of zumilokibart achieved competitive FeNO reduction -16 -19 -38 -30 -10 -16 -37 -11 -32 -11 -16 -44 -50 -40 -30 -20 -10 0 VESTIGE APG808 GB-0895 Zumi Anti-IL-4Rα Anti-TSLP Anti-IL-13 BL FeNO (ppb)1 34 36 63 53 32 41 64 32 61 31 40 67 NOTE: Data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. FeNO level reflects data from marketed dose, where available. Lunsekimig data is from D57 (week 8; week 12 data not available). Lebrikizumab data is for 125mg Q4W dose (highest tested dose in Phase 3 trials). BL = baseline. FeNO = fractional exhaled nitric oxide. SOURCE: 1 Baseline indicated refers to treatment group(s) only. 2Castro M, et al. NEJM, 2018. 3Rabe KF et al. NEJM, 2018 (digitized). 4Castro M, et al. Lancet Resp Med, 2025 (difference of baseline mean FeNO of 63.1 ppb and Week 12 mean FeNO of 25.3 ppb). 5Kamboj A, et al. ACAAI, 2025. 6Corren JC, et al. NEJM, 2019 (digitized). 7Menzies-Gow A, et al. NEJM, 2021 (digitized). 8Deykin A et al. ERS 2024 (straight average of 100mg Q4W, 200mg Q4W, and 300mg Q12W cohorts). 9Singh et al ERS 2025 (straight average of 100mg, 300mg, 600mg, and 1200mg cohorts, digitized). 10Deiteren A et al. ATS 2023 (digitized). 11Hanania NA, et al. Thorax, 2015 (125mg Q4W, weighted average of biomarker-high and biomarker low cohorts, digitized). 12Russell RJ, et al. Lancet Respir Med, 2018 (digitized). FeNO, mean change from baseline (ppb) ZUMILOKIBART (APG777) PHASE 1B FeNO mean absolute change from baseline at 12 weeks (ppb) |
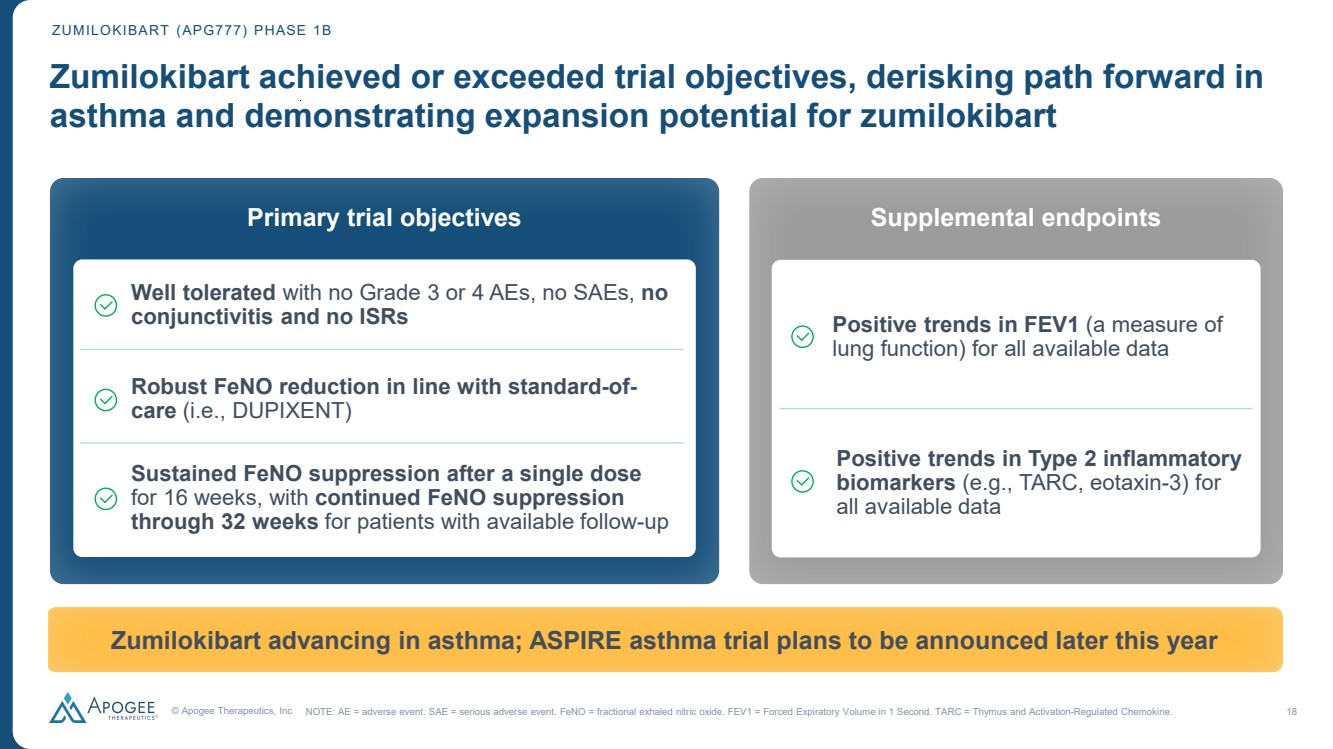
| © Apogee Therapeutics, Inc 18 Zumilokibart achieved or exceeded trial objectives, derisking path forward in asthma and demonstrating expansion potential for zumilokibart Primary trial objectives Well tolerated with no Grade 3 or 4 AEs, no SAEs, no conjunctivitis and no ISRs Robust FeNO reduction in line with standard-of-care (i.e., DUPIXENT) Zumilokibart advancing in asthma; ASPIRE asthma trial plans to be announced later this year Sustained FeNO suppression after a single dose for 16 weeks, with continued FeNO suppression through 32 weeks for patients with available follow-up ZUMILOKIBART (APG777) PHASE 1B NOTE: AE = adverse event. SAE = serious adverse event. FeNO = fractional exhaled nitric oxide. FEV1 = Forced Expiratory Volume in 1 Second. TARC = Thymus and Activation-Regulated Chemokine. Supplemental endpoints Positive trends in Type 2 inflammatory biomarkers (e.g., TARC, eotaxin-3) for all available data Positive trends in FEV1 (a measure of lung function) for all available data |
| IL-13 in asthma Mario Castro, MD, MPH University of Kansas School of Medicine |
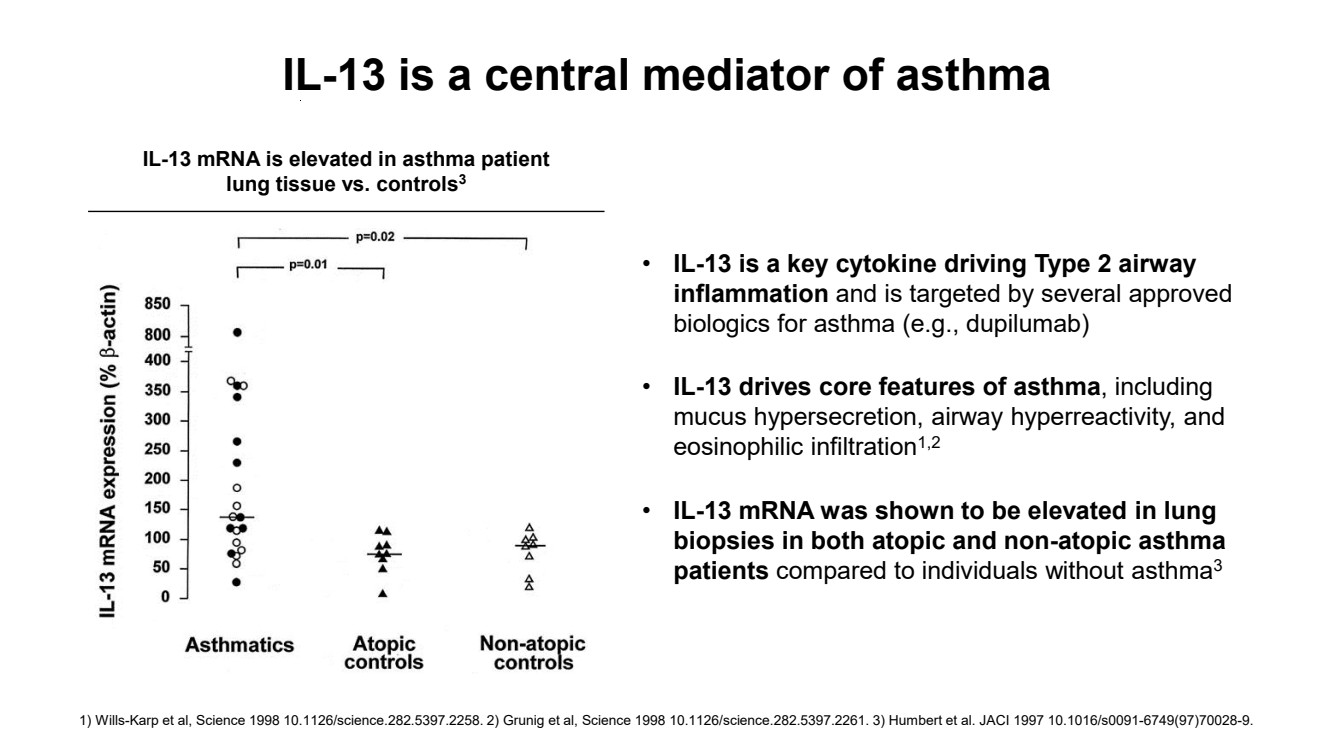
| IL-13 mRNA is elevated in asthma patient lung tissue vs. controls3 • IL-13 is a key cytokine driving Type 2 airway inflammation and is targeted by several approved biologics for asthma (e.g., dupilumab) • IL-13 drives core features of asthma, including mucus hypersecretion, airway hyperreactivity, and eosinophilic infiltration1,2 • IL-13 mRNA was shown to be elevated in lung biopsies in both atopic and non-atopic asthma patients compared to individuals without asthma3 IL-13 is a central mediator of asthma 1) Wills-Karp et al, Science 1998 10.1126/science.282.5397.2258. 2) Grunig et al, Science 1998 10.1126/science.282.5397.2261. 3) Humbert et al. JACI 1997 10.1016/s0091-6749(97)70028-9. |
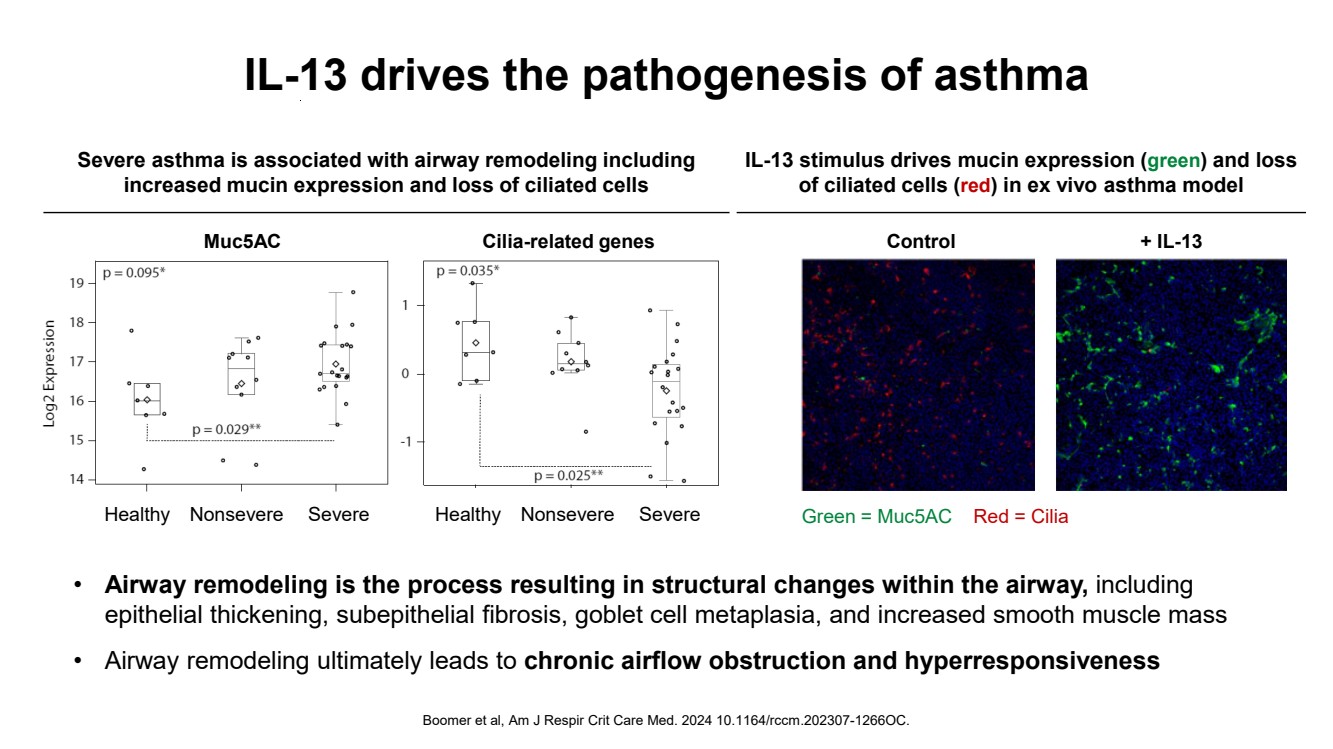
| IL-13 drives the pathogenesis of asthma Boomer et al, Am J Respir Crit Care Med. 2024 10.1164/rccm.202307-1266OC. Muc5AC Healthy Nonsevere Severe Cilia-related genes Healthy Nonsevere Severe Red = Cilia + IL-13 Green = Muc5AC Control Severe asthma is associated with airway remodeling including increased mucin expression and loss of ciliated cells IL-13 stimulus drives mucin expression (green) and loss of ciliated cells (red) in ex vivo asthma model • Airway remodeling is the process resulting in structural changes within the airway, including epithelial thickening, subepithelial fibrosis, goblet cell metaplasia, and increased smooth muscle mass • Airway remodeling ultimately leads to chronic airflow obstruction and hyperresponsiveness |
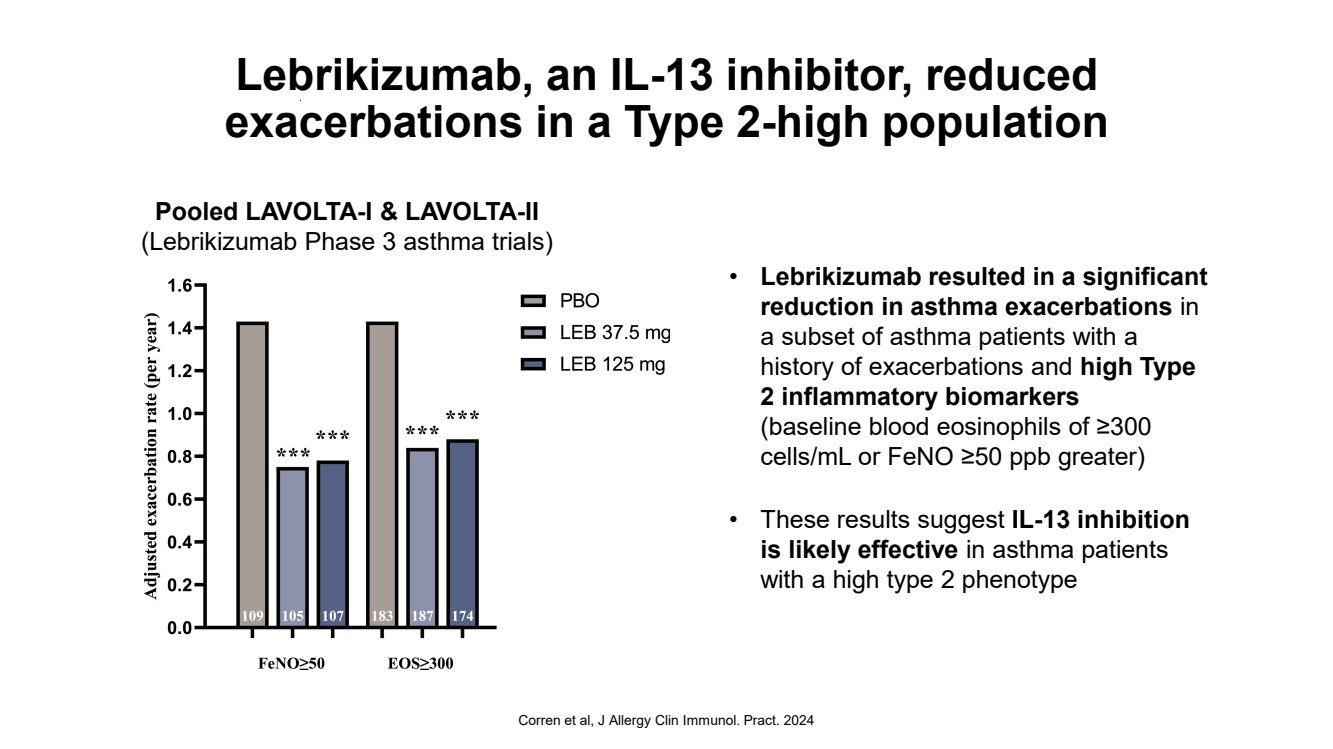
| Lebrikizumab, an IL-13 inhibitor, reduced exacerbations in a Type 2-high population • Lebrikizumab resulted in a significant reduction in asthma exacerbations in a subset of asthma patients with a history of exacerbations and high Type 2 inflammatory biomarkers (baseline blood eosinophils of ≥300 cells/mL or FeNO ≥50 ppb greater) • These results suggest IL-13 inhibition is likely effective in asthma patients with a high type 2 phenotype Corren et al, J Allergy Clin Immunol. Pract. 2024 Pooled LAVOLTA-I & LAVOLTA-II (Lebrikizumab Phase 3 asthma trials) |
| Summary • IL-13 is a central mediator of asthma and is consistently elevated in the lungs of asthma patients relative to healthy controls • IL-13 drives the pathogenesis of asthma (e.g., airway remodeling), directly contributing to the hallmark signs of asthma including chronic airflow obstruction and hyperresponsiveness • Post-hoc subgroup analyses of lebrikizumab (αIL-13 antibody) phase 3 studies demonstrated significant reduction in asthma exacerbations in patients with high Type-2 inflammatory biomarker levels and history of exacerbation • The phase I zumilokibart interim data demonstrates significant and durable improvement in lung function and suppression of FeNO with a single dose, with an acceptable safety profile, as one would expect from IL-13 inhibition in an appropriately selected population |
| Closing remarks Michael Henderson, MD Chief Executive Officer |
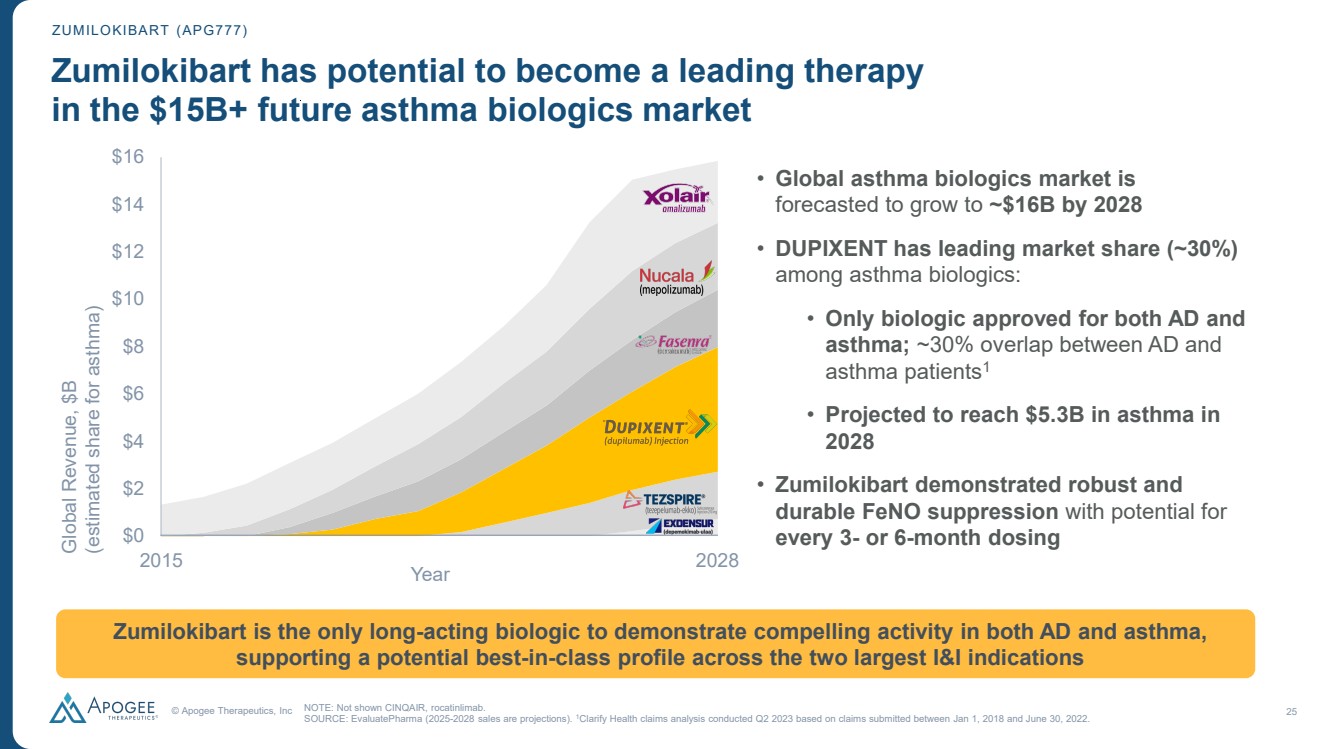
| © Apogee Therapeutics, Inc 25 ZUMILOKIBART (APG777) Zumilokibart has potential to become a leading therapy in the $15B+ future asthma biologics market $0 $2 $4 $6 $8 $10 $12 $14 $16 Global Revenue, $B (estimated share for asthma) NOTE: Not shown CINQAIR, rocatinlimab. SOURCE: EvaluatePharma (2025-2028 sales are projections). 1Clarify Health claims analysis conducted Q2 2023 based on claims submitted between Jan 1, 2018 and June 30, 2022. • Global asthma biologics market is forecasted to grow to ~$16B by 2028 • DUPIXENT has leading market share (~30%) among asthma biologics: • Only biologic approved for both AD and asthma; ~30% overlap between AD and asthma patients1 • Projected to reach $5.3B in asthma in 2028 • Zumilokibart demonstrated robust and durable FeNO suppression with potential for every 3- or 6-month dosing 2015 Zumilokibart is the only long-acting biologic to demonstrate compelling activity in both AD and asthma, supporting a potential best-in-class profile across the two largest I&I indications 2028 Year |
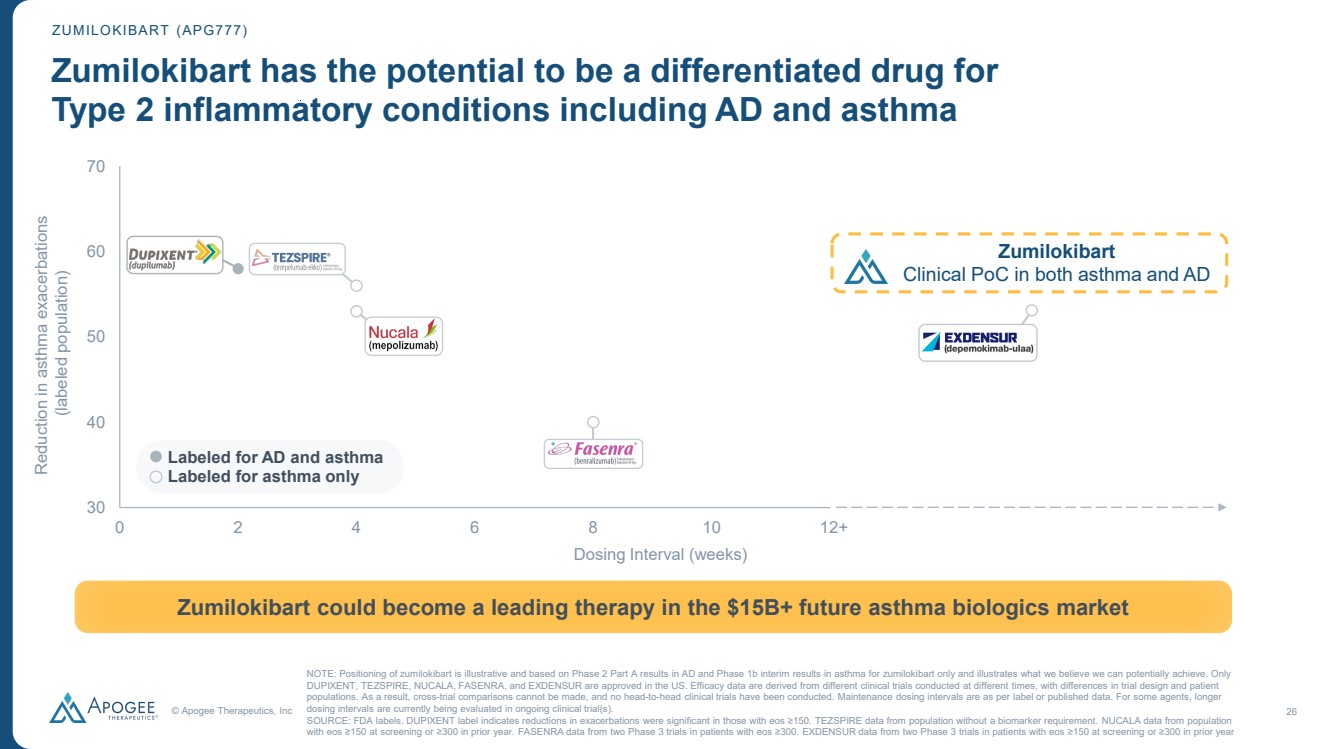
| © Apogee Therapeutics, Inc 26 30 40 50 60 70 0 2 4 6 8 10 12 ZUMILOKIBART (APG777) Zumilokibart has the potential to be a differentiated drug for Type 2 inflammatory conditions including AD and asthma NOTE: Positioning of zumilokibart is illustrative and based on Phase 2 Part A results in AD and Phase 1b interim results in asthma for zumilokibart only and illustrates what we believe we can potentially achieve. Only DUPIXENT, TEZSPIRE, NUCALA, FASENRA, and EXDENSUR are approved in the US. Efficacy data are derived from different clinical trials conducted at different times, with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. Maintenance dosing intervals are as per label or published data. For some agents, longer dosing intervals are currently being evaluated in ongoing clinical trial(s). SOURCE: FDA labels. DUPIXENT label indicates reductions in exacerbations were significant in those with eos ≥150. TEZSPIRE data from population without a biomarker requirement. NUCALA data from population with eos ≥150 at screening or ≥300 in prior year. FASENRA data from two Phase 3 trials in patients with eos ≥300. EXDENSUR data from two Phase 3 trials in patients with eos ≥150 at screening or ≥300 in prior year Zumilokibart could become a leading therapy in the $15B+ future asthma biologics market Dosing Interval (weeks) Labeled for AD and asthma Labeled for asthma only Reduction in asthma exacerbations (labeled population) 12+ Zumilokibart Clinical PoC in both asthma and AD |
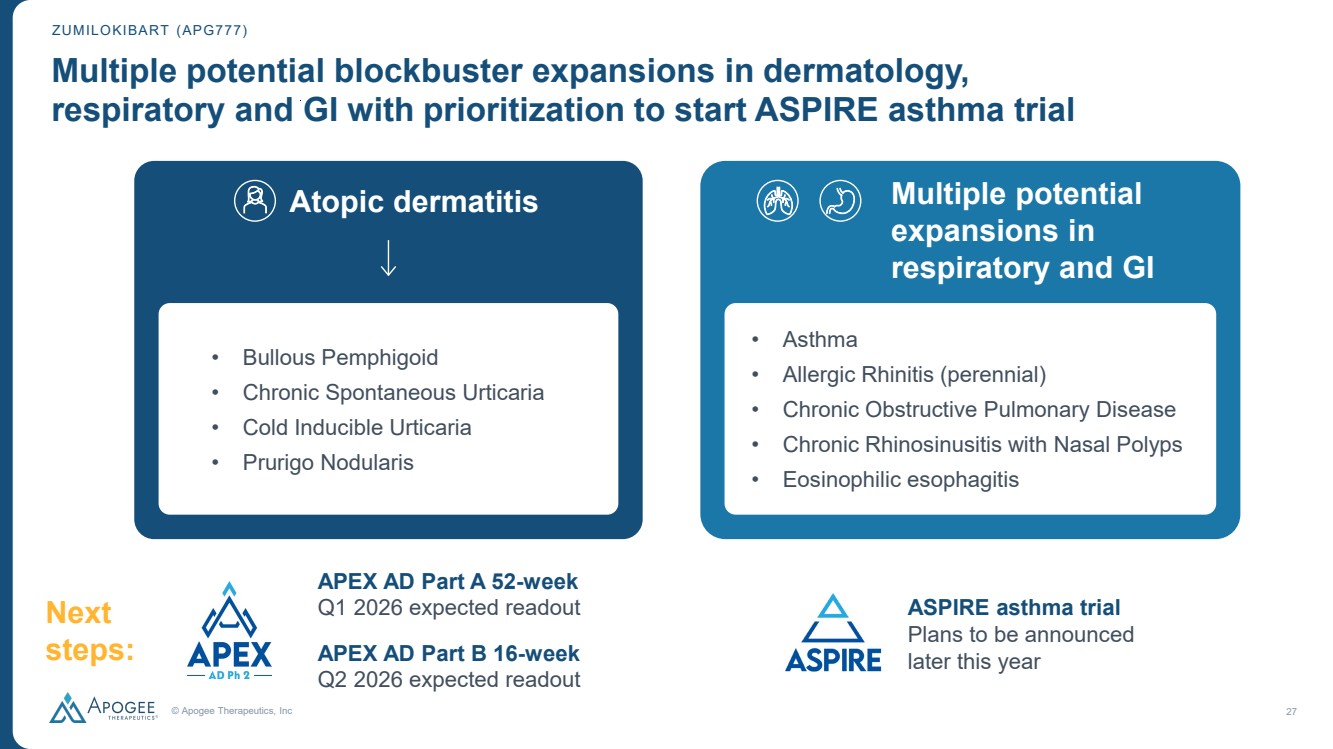
| © Apogee Therapeutics, Inc 27 Multiple potential blockbuster expansions in dermatology, respiratory and GI with prioritization to start ASPIRE asthma trial ZUMILOKIBART (APG777) • Bullous Pemphigoid • Chronic Spontaneous Urticaria • Cold Inducible Urticaria • Prurigo Nodularis APEX AD Part A 52-week Q1 2026 expected readout APEX AD Part B 16-week Q2 2026 expected readout Atopic dermatitis Next steps: • Asthma • Allergic Rhinitis (perennial) • Chronic Obstructive Pulmonary Disease • Chronic Rhinosinusitis with Nasal Polyps • Eosinophilic esophagitis ASPIRE asthma trial Plans to be announced later this year Multiple potential expansions in respiratory and GI |
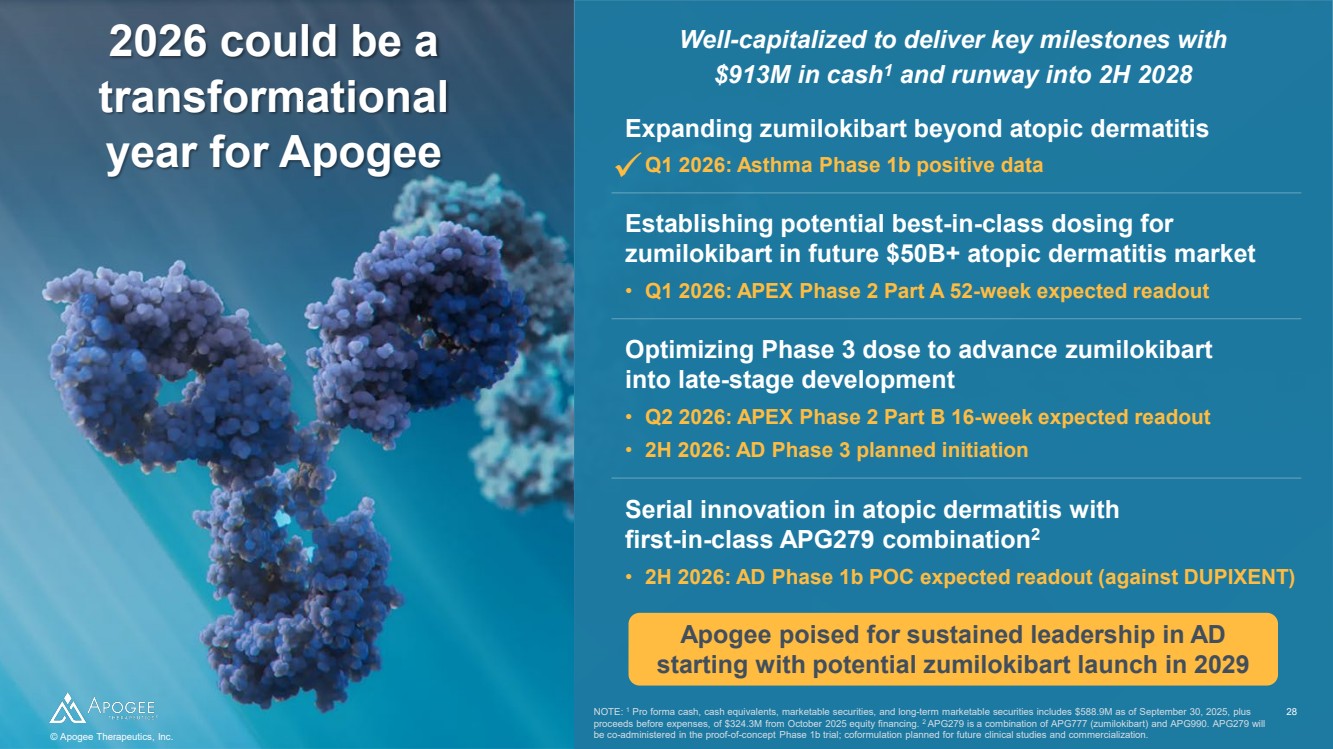
| 2026 could be a transformational year for Apogee Apogee poised for sustained leadership in AD starting with potential zumilokibart launch in 2029 NOTE: 1 Pro forma cash, cash equivalents, marketable securities, and long-term marketable securities includes $588.9M as of September 30, 2025, plus proceeds before expenses, of $324.3M from October 2025 equity financing. 2 APG279 is a combination of APG777 (zumilokibart) and APG990. APG279 will be co-administered in the proof-of-concept Phase 1b trial; coformulation planned for future clinical studies and commercialization. Well-capitalized to deliver key milestones with $913M in cash1 and runway into 2H 2028 © Apogee Therapeutics, Inc. 28 Establishing potential best-in-class dosing for zumilokibart in future $50B+ atopic dermatitis market • Q1 2026: APEX Phase 2 Part A 52-week expected readout • Q2 2026: APEX Phase 2 Part B 16-week expected readout • 2H 2026: AD Phase 3 planned initiation Optimizing Phase 3 dose to advance zumilokibart into late-stage development • 2H 2026: AD Phase 1b POC expected readout (against DUPIXENT) Serial innovation in atopic dermatitis with first-in-class APG279 combination2 • Q1 2026: Asthma Phase 1b positive data Expanding zumilokibart beyond atopic dermatitis |
| Apogee /ˈapəjē/ noun The highest point in the development of something; a climax or culmination |