

LEGEND Data Update November 11, 2025 .3

Disclaimers Cautionary Statement Regarding Forward-Looking Statements This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”, “believe”, “continue”, “could”, “estimate”, “expect”, “goa”, “intends”, “may”, “might”, “plan”, “possible”, “project”, “should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding the potential benefits of the amended protocol for cohort 1 of the LEGEND study, including the potential for patients enrolled under the amended protocol to experience better long-term complete response (“CR”) rates, detalimogene’s potential safety and ease of use profile, the development of detalimogene, the potential benefits of detalimogene, including its ability to become a first line therapy in BCG-unresponsive NMIBC, plans regarding regulatory interactions and a potential biologics license application (“BLA”) submission for detalimogene, estimates and forecasts of financial and performance metrics, projections of market opportunity and market share, the anticipated market acceptance of detalimogene, expectations and timing related to regulatory submissions and commercial product launches and the prospects for regulatory approval of detalimogene. These forward-looking statements are based on various estimates and assumptions, whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ("enGene"), are not predictions of annual performance, and are subject to risks and uncertainties. Such statements are subject to numerous important factors, risks and uncertainties, many of which are beyond enGene's control, that may cause actual events or results to differ materially from enGene's current expectations. For example, there can be no guarantee that detalimogene will successfully complete necessary clinical development phases, including achieving positive results in the pivotal cohort of the LEGEND study, or that those results or any feedback from regulatory authorities will ultimately lead to a BLA submission for, and the approval of, detalimogene. Management's expectations and, therefore, any forward-looking statements in this presentation could also be affected by risks, uncertainties and assumptions relating to a number of other factors, which could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the inability of preliminary clinical data to predict the final results of the trial, changes in the results from enGene’s clinical trials, including due to new data collected from the ongoing LEGEND study or future studies, subsequent analysis of existing data, and audit and verification procedures; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities; the Company’s ability to recruit and retain qualified scientific and management personnel, establish clinical trial sites and enroll patients in its clinical trials, execute on the Company’s clinical development plans; and ability to secure regulatory approval on anticipated timelines, and other risks and uncertainties detailed in filings with Canadian securities regulators on SEDAR+ and with the U.S. Securities and Exchange Commission (“SEC”) on EDGAR, including those described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the fiscal year ended October 31, 2024 (copies of which may be obtained at www.sedarplus.ca or www.sec.gov). You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report, as well as other documents if and when filed by enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements. There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans, or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward-looking statements should not be relied upon as representing enGene’s assessments as of any date subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein. Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Lead Program (detalimogene voraplasmid) The lead program described herein is an investigational drug therapy that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy or safety. Although the FDA has indicated that the Phase 2 portion of the current LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 clinical testing, which are not expected to be available until 2026.
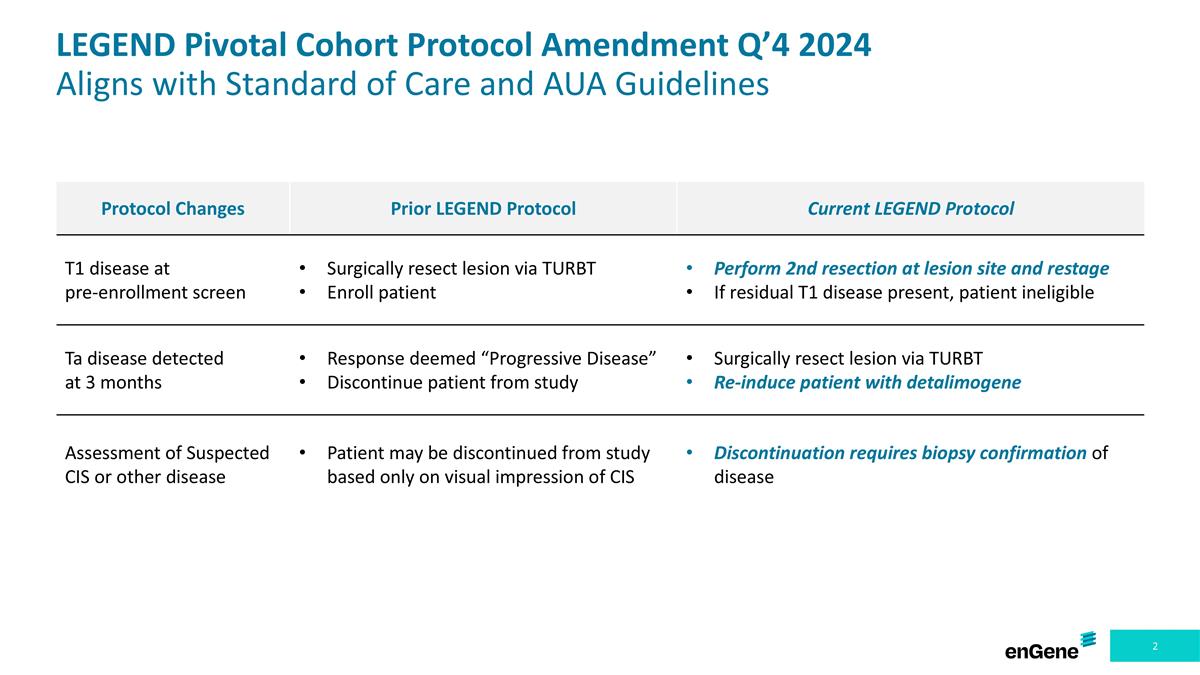
Protocol Changes Prior LEGEND Protocol Current LEGEND Protocol T1 disease at pre-enrollment screen Surgically resect lesion via TURBT Enroll patient Perform 2nd resection at lesion site and restage If residual T1 disease present, patient ineligible Ta disease detected at 3 months Response deemed “Progressive Disease” Discontinue patient from study Surgically resect lesion via TURBT Re-induce patient with detalimogene Assessment of Suspected CIS or other disease Patient may be discontinued from study based only on visual impression of CIS Discontinuation requires biopsy confirmation of disease LEGEND Pivotal Cohort Protocol Amendment Q’4 2024 Aligns with Standard of Care and AUA Guidelines
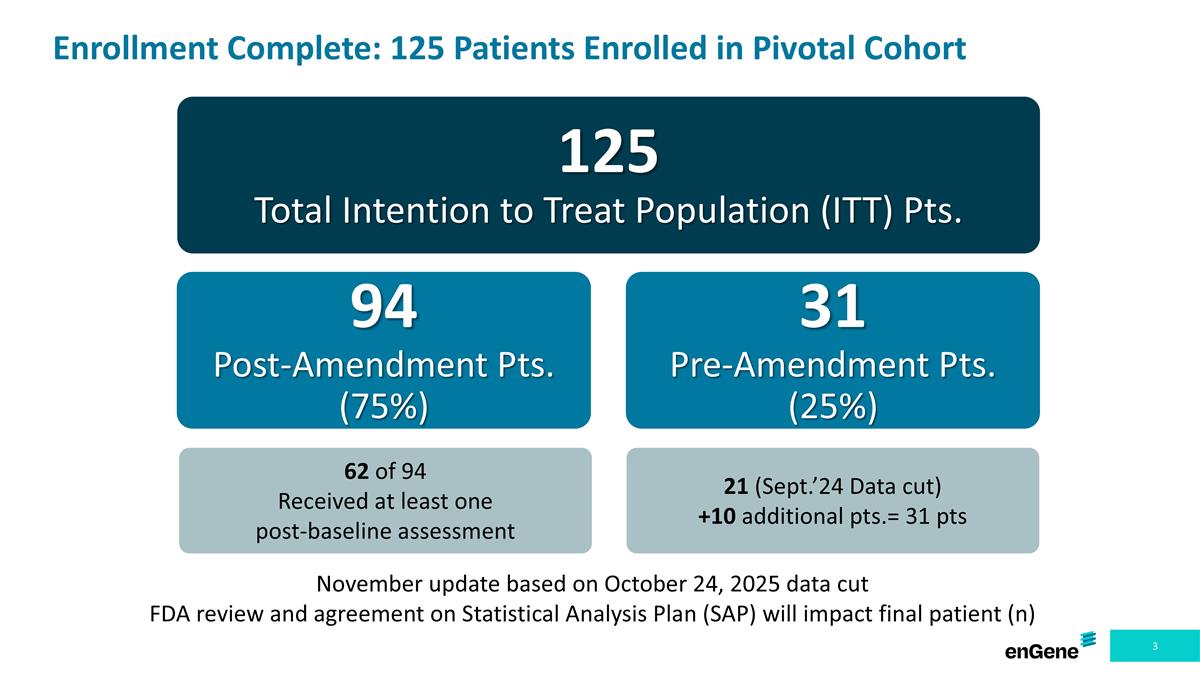
Enrollment Complete: 125 Patients Enrolled in Pivotal Cohort 125 Total Intention to Treat Population (ITT) Pts. 94 Post-Amendment Pts. (75%) 31 Pre-Amendment Pts. (25%) November update based on October 24, 2025 data cut FDA review and agreement on Statistical Analysis Plan (SAP) will impact final patient (n) 62 of 94 Received at least one post-baseline assessment 21 (Sept.’24 Data cut) +10 additional pts.= 31 pts
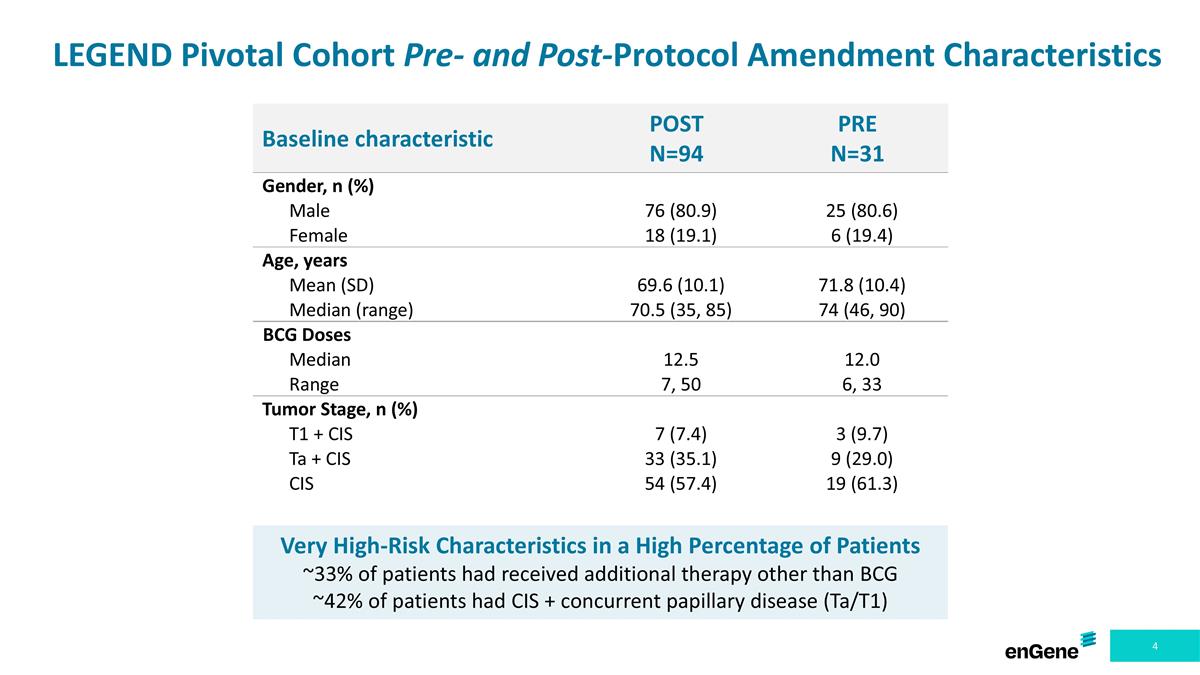
LEGEND Pivotal Cohort Pre- and Post-Protocol Amendment Characteristics Baseline characteristic POST N=94 PRE N=31 Gender, n (%) Male 76 (80.9) 25 (80.6) Female 18 (19.1) 6 (19.4) Age, years Mean (SD) 69.6 (10.1) 71.8 (10.4) Median (range) 70.5 (35, 85) 74 (46, 90) BCG Doses Median 12.5 12.0 Range 7, 50 6, 33 Tumor Stage, n (%) T1 + CIS 7 (7.4) 3 (9.7) Ta + CIS 33 (35.1) 9 (29.0) CIS 54 (57.4) 19 (61.3) Very High-Risk Characteristics in a High Percentage of Patients ~33% of patients had received additional therapy other than BCG ~42% of patients had CIS + concurrent papillary disease (Ta/T1)
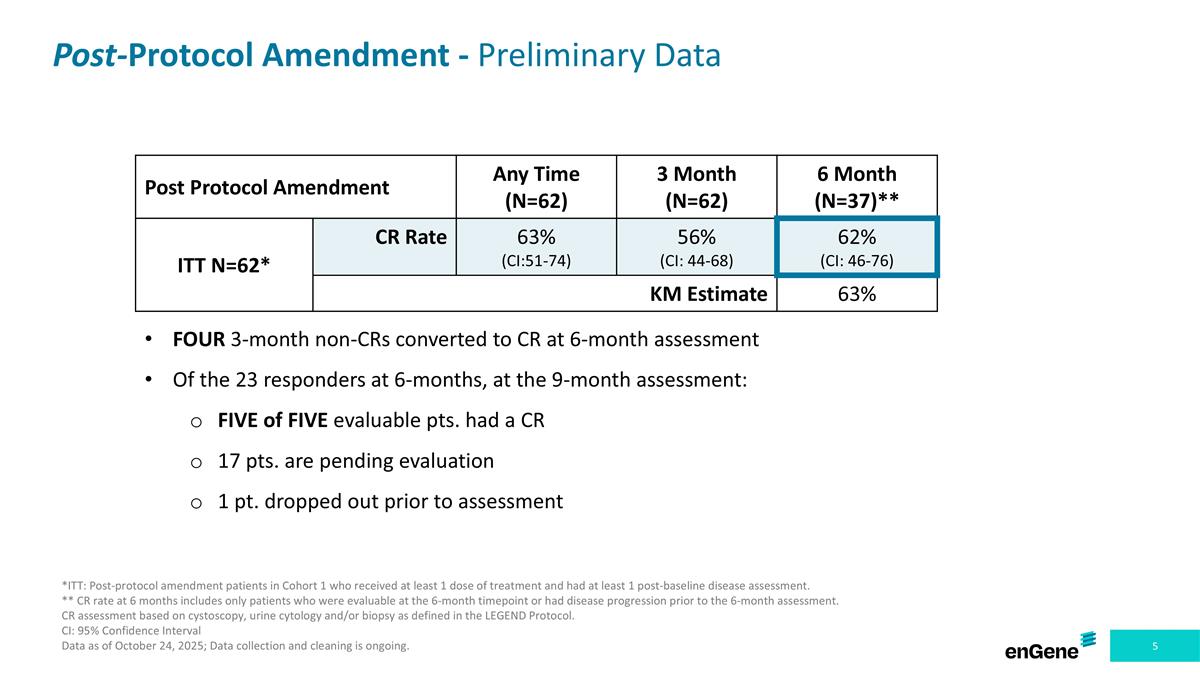
Post-Protocol Amendment - Preliminary Data Post Protocol Amendment Any Time (N=62) 3 Month (N=62) 6 Month (N=37)** ITT N=62* CR Rate 63% (CI:51-74) 56% (CI: 44-68) 62% (CI: 46-76) KM Estimate 63% *ITT: Post-protocol amendment patients in Cohort 1 who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing. FOUR 3-month non-CRs converted to CR at 6-month assessment Of the 23 responders at 6-months, at the 9-month assessment: FIVE of FIVE evaluable pts. had a CR 17 pts. are pending evaluation 1 pt. dropped out prior to assessment
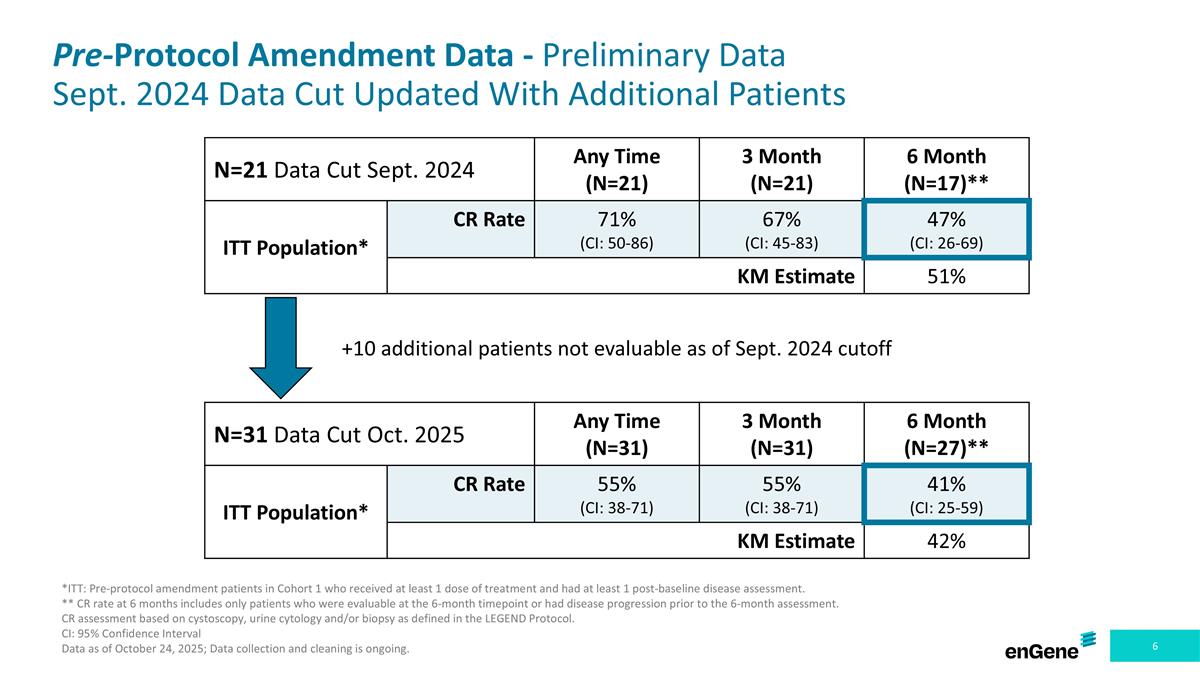
Pre-Protocol Amendment Data - Preliminary Data Sept. 2024 Data Cut Updated With Additional Patients N=31 Data Cut Oct. 2025 Any Time (N=31) 3 Month (N=31) 6 Month (N=27)** ITT Population* CR Rate 55% (CI: 38-71) 55% (CI: 38-71) 41% (CI: 25-59) KM Estimate 42% N=21 Data Cut Sept. 2024 Any Time (N=21) 3 Month (N=21) 6 Month (N=17)** ITT Population* CR Rate 71% (CI: 50-86) 67% (CI: 45-83) 47% (CI: 26-69) KM Estimate 51% +10 additional patients not evaluable as of Sept. 2024 cutoff *ITT: Pre-protocol amendment patients in Cohort 1 who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing.
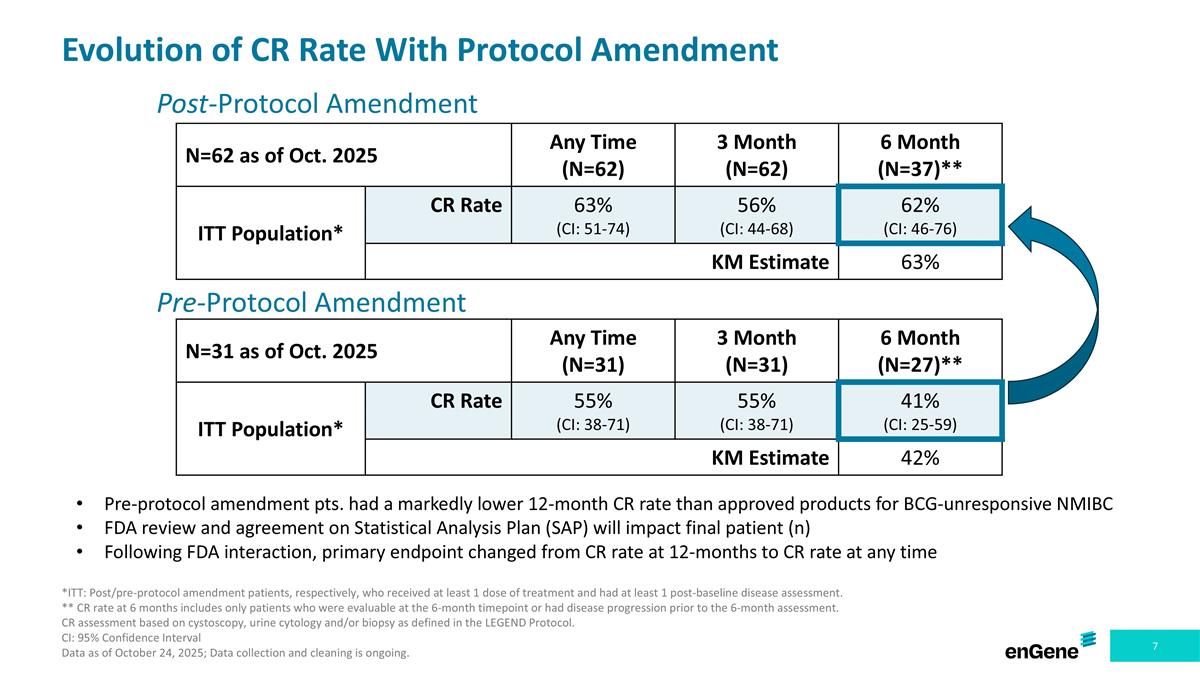
Post-Protocol Amendment N=31 as of Oct. 2025 Any Time (N=31) 3 Month (N=31) 6 Month (N=27)** ITT Population* CR Rate 55% (CI: 38-71) 55% (CI: 38-71) 41% (CI: 25-59) KM Estimate 42% Evolution of CR Rate With Protocol Amendment Pre-Protocol Amendment N=62 as of Oct. 2025 Any Time (N=62) 3 Month (N=62) 6 Month (N=37)** ITT Population* CR Rate 63% (CI: 51-74) 56% (CI: 44-68) 62% (CI: 46-76) KM Estimate 63% Pre-protocol amendment pts. had a markedly lower 12-month CR rate than approved products for BCG-unresponsive NMIBC FDA review and agreement on Statistical Analysis Plan (SAP) will impact final patient (n) Following FDA interaction, primary endpoint changed from CR rate at 12-months to CR rate at any time *ITT: Post/pre-protocol amendment patients, respectively, who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing.
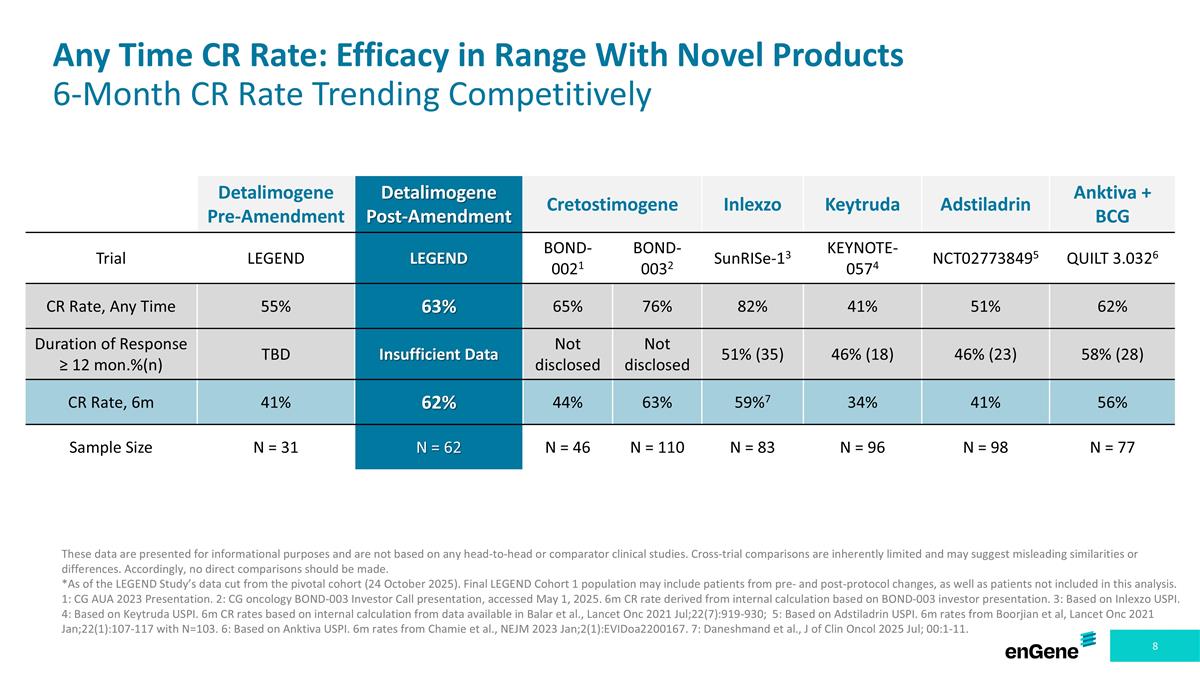
Any Time CR Rate: Efficacy in Range With Novel Products 6-Month CR Rate Trending Competitively These data are presented for informational purposes and are not based on any head-to-head or comparator clinical studies. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences. Accordingly, no direct comparisons should be made. *As of the LEGEND Study’s data cut from the pivotal cohort (24 October 2025). Final LEGEND Cohort 1 population may include patients from pre- and post-protocol changes, as well as patients not included in this analysis. 1: CG AUA 2023 Presentation. 2: CG oncology BOND-003 Investor Call presentation, accessed May 1, 2025. 6m CR rate derived from internal calculation based on BOND-003 investor presentation. 3: Based on Inlexzo USPI. 4: Based on Keytruda USPI. 6m CR rates based on internal calculation from data available in Balar et al., Lancet Onc 2021 Jul;22(7):919-930; 5: Based on Adstiladrin USPI. 6m rates from Boorjian et al, Lancet Onc 2021 Jan;22(1):107-117 with N=103. 6: Based on Anktiva USPI. 6m rates from Chamie et al., NEJM 2023 Jan;2(1):EVIDoa2200167. 7: Daneshmand et al., J of Clin Oncol 2025 Jul; 00:1-11. Detalimogene Pre-Amendment Detalimogene Post-Amendment Cretostimogene Inlexzo Keytruda Adstiladrin Anktiva + BCG Trial LEGEND LEGEND BOND-0021 BOND-0032 SunRISe-13 KEYNOTE-0574 NCT027738495 QUILT 3.0326 CR Rate, Any Time 55% 63% 65% 76% 82% 41% 51% 62% Duration of Response ≥ 12 mon.%(n) TBD Insufficient Data Not disclosed Not disclosed 51% (35) 46% (18) 46% (23) 58% (28) CR Rate, 6m 41% 62% 44% 63% 59%7 34% 41% 56% Sample Size N = 31 N = 62 N = 46 N = 110 N = 83 N = 96 N = 98 N = 77
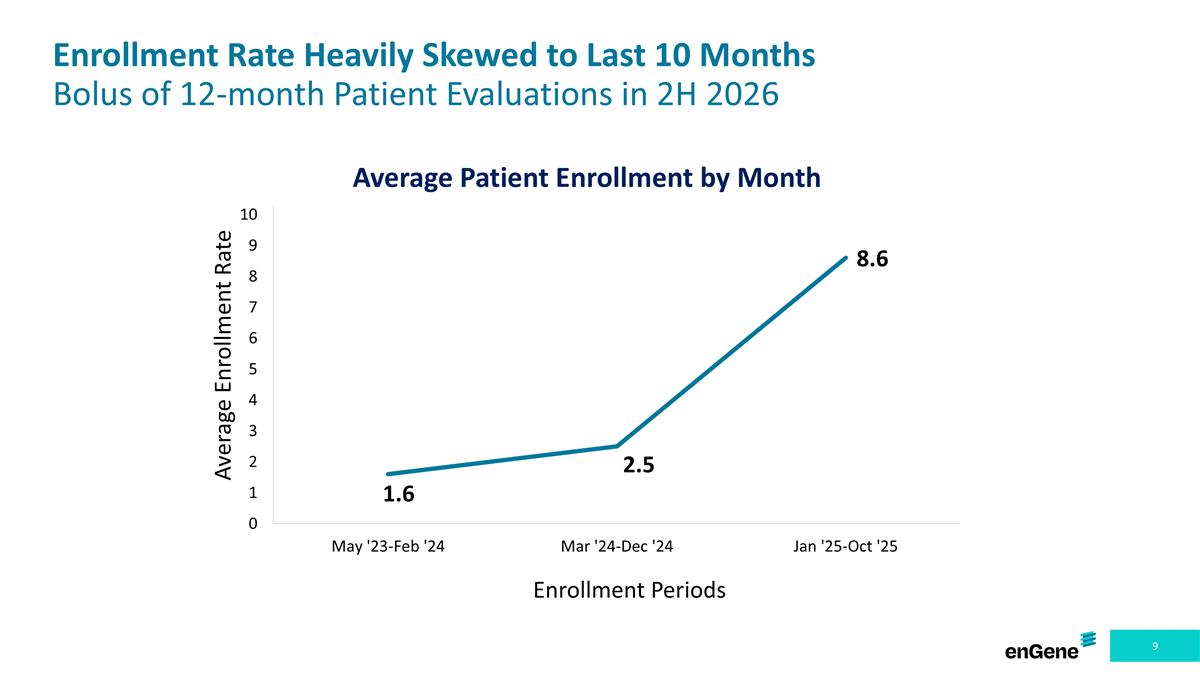
Enrollment Rate Heavily Skewed to Last 10 Months Bolus of 12-month Patient Evaluations in 2H 2026
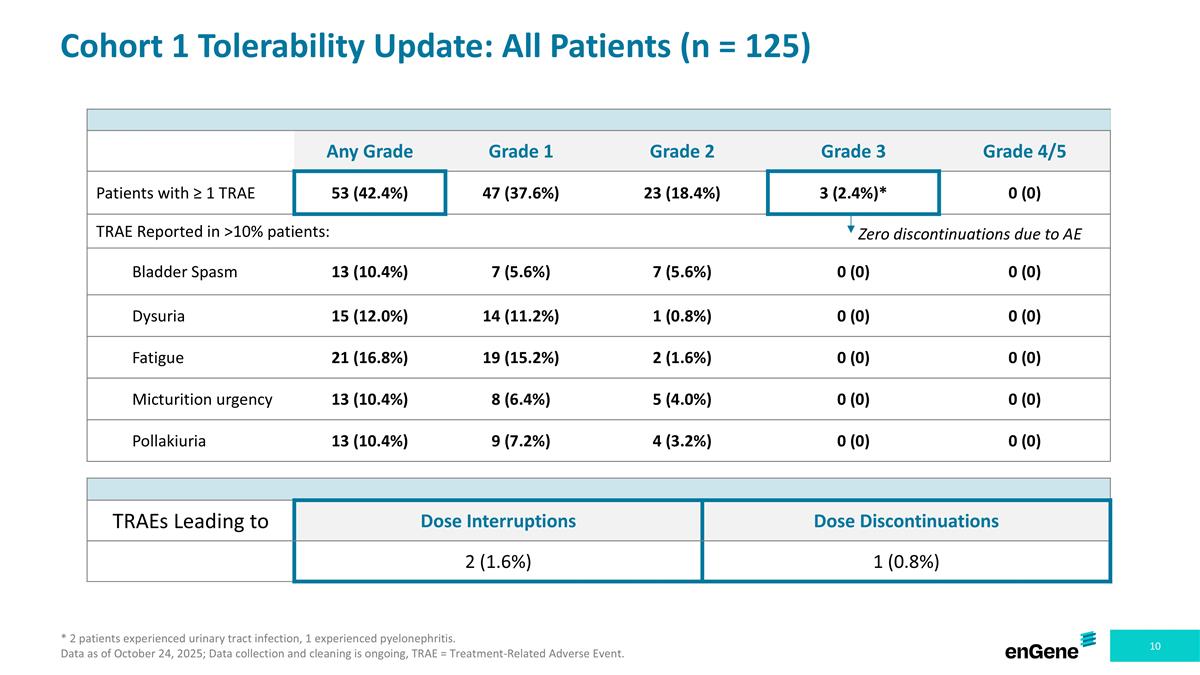
Cohort 1 Tolerability Update: All Patients (n = 125) * 2 patients experienced urinary tract infection, 1 experienced pyelonephritis. Data as of October 24, 2025; Data collection and cleaning is ongoing, TRAE = Treatment-Related Adverse Event. Any Grade Grade 1 Grade 2 Grade 3 Grade 4/5 Patients with ≥ 1 TRAE 53 (42.4%) 47 (37.6%) 23 (18.4%) 3 (2.4%)* 0 (0) TRAE Reported in >10% patients: Bladder Spasm 13 (10.4%) 7 (5.6%) 7 (5.6%) 0 (0) 0 (0) Dysuria 15 (12.0%) 14 (11.2%) 1 (0.8%) 0 (0) 0 (0) Fatigue 21 (16.8%) 19 (15.2%) 2 (1.6%) 0 (0) 0 (0) Micturition urgency 13 (10.4%) 8 (6.4%) 5 (4.0%) 0 (0) 0 (0) Pollakiuria 13 (10.4%) 9 (7.2%) 4 (3.2%) 0 (0) 0 (0) TRAEs Leading to Dose Interruptions Dose Discontinuations 2 (1.6%) 1 (0.8%) Zero discontinuations due to AE
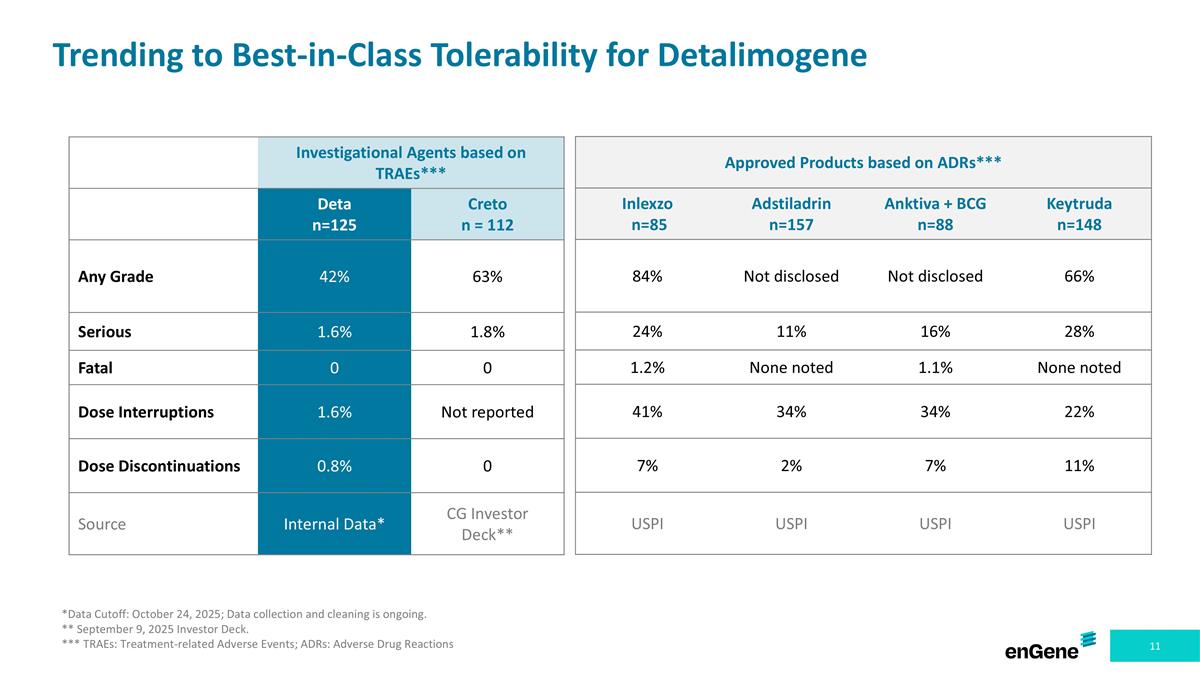
Trending to Best-in-Class Tolerability for Detalimogene Investigational Agents based on TRAEs*** Deta n=125 Creto n = 112 Any Grade 42% 63% Serious 1.6% 1.8% Fatal 0 0 Dose Interruptions 1.6% Not reported Dose Discontinuations 0.8% 0 Source Internal Data* CG Investor Deck** Approved Products based on ADRs*** Inlexzo n=85 Adstiladrin n=157 Anktiva + BCG n=88 Keytruda n=148 84% Not disclosed Not disclosed 66% 24% 11% 16% 28% 1.2% None noted 1.1% None noted 41% 34% 34% 22% 7% 2% 7% 11% USPI USPI USPI USPI *Data Cutoff: October 24, 2025; Data collection and cleaning is ongoing. ** September 9, 2025 Investor Deck. *** TRAEs: Treatment-related Adverse Events; ADRs: Adverse Drug Reactions
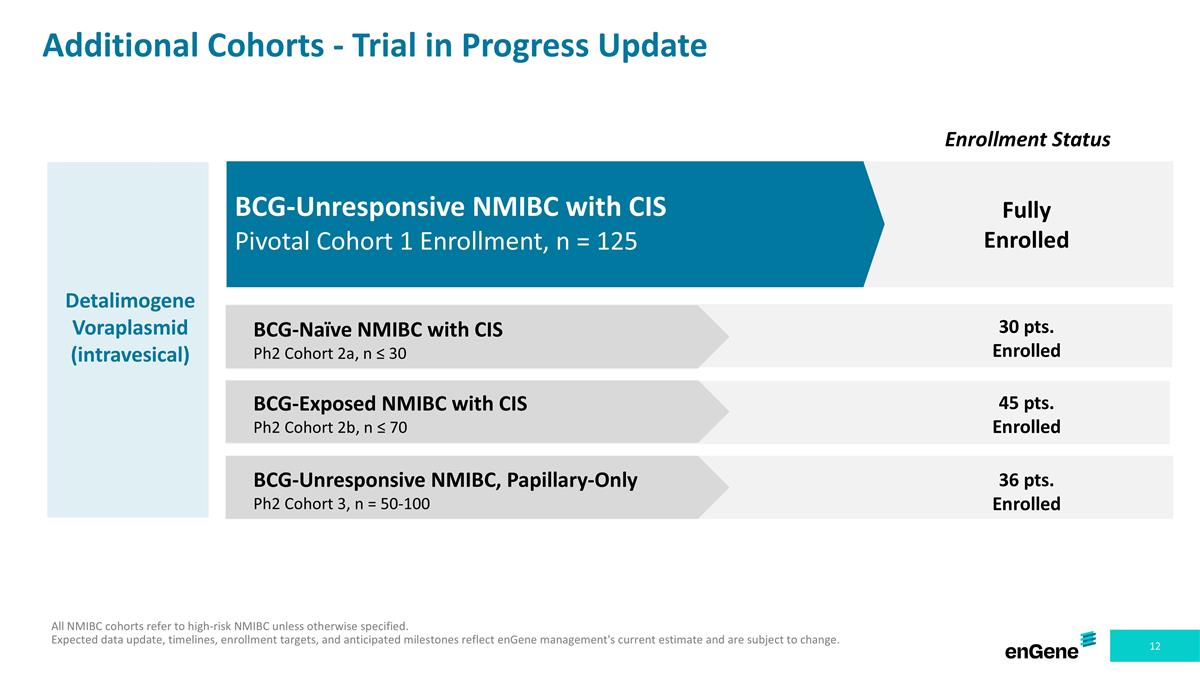
Additional Cohorts - Trial in Progress Update All NMIBC cohorts refer to high-risk NMIBC unless otherwise specified. Expected data update, timelines, enrollment targets, and anticipated milestones reflect enGene management's current estimate and are subject to change. Enrollment Status Detalimogene Voraplasmid (intravesical) BCG-Unresponsive NMIBC with CIS Pivotal Cohort 1 Enrollment, n = 125 BCG-Naïve NMIBC with CIS Ph2 Cohort 2a, n ≤ 30 BCG-Exposed NMIBC with CIS Ph2 Cohort 2b, n ≤ 70 BCG-Unresponsive NMIBC, Papillary-Only Ph2 Cohort 3, n = 50-100 30 pts. Enrolled Fully Enrolled 45 pts. Enrolled 36 pts. Enrolled

LEGEND Cohort 1 Update Summary Preliminary data suggest positive impact on efficacy post-protocol amendment Trending to best-in-class tolerability profile FDA review and agreement on Statistical Analysis Plan (SAP) will impact final patient (n) Heavily skewed enrollment curve delays insights into long-term efficacy Long-term durability data is limited to patients enrolled prior to protocol revision
Detalimogene: Designed to Meet the Needs of Urologists and Patients Preliminary evidence of efficacy* Non-viral gene therapy designed for streamlined clinical experience *LEGEND phase 1 reported data and September 2024 and November 2025 preliminary pivotal phase 2 reported data Efficacy Ease-of-Use Safety Generally mild treatment related adverse events*