

Corporate Presentation November 2025 .4

Disclaimers Cautionary Statement Regarding Forward-Looking Statements This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”, “believe”, “continue”, “could”, “estimate”, “expect”, “goa”, “intends”, “may”, “might”, “plan”, “possible”, “project”, “should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding the potential benefits of the amended protocol for cohort 1 of the LEGEND study, including the potential for patients enrolled under the amended protocol to experience better long-term complete response (“CR”) rates, detalimogene’s potential safety and ease of use profile, the development of detalimogene, the potential benefits of detalimogene, including its ability to become a first line therapy in BCG-unresponsive NMIBC, plans regarding regulatory interactions and a potential biologics license application (“BLA”) submission for detalimogene, estimates and forecasts of financial and performance metrics, projections of market opportunity and market share, the anticipated market acceptance of detalimogene, expectations and timing related to regulatory submissions and commercial product launches and the prospects for regulatory approval of detalimogene. These forward-looking statements are based on various estimates and assumptions, whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ("enGene"), are not predictions of annual performance, and are subject to risks and uncertainties. Such statements are subject to numerous important factors, risks and uncertainties, many of which are beyond enGene's control, that may cause actual events or results to differ materially from enGene's current expectations. For example, there can be no guarantee that detalimogene will successfully complete necessary clinical development phases, including achieving positive results in the pivotal cohort of the LEGEND study, or that those results or any feedback from regulatory authorities will ultimately lead to a BLA submission for, and the approval of, detalimogene. Management's expectations and, therefore, any forward-looking statements in this presentation could also be affected by risks, uncertainties and assumptions relating to a number of other factors, which could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the inability of preliminary clinical data to predict the final results of the trial, changes in the results from enGene’s clinical trials, including due to new data collected from the ongoing LEGEND study or future studies, subsequent analysis of existing data, and audit and verification procedures; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities; the Company’s ability to recruit and retain qualified scientific and management personnel, establish clinical trial sites and enroll patients in its clinical trials, execute on the Company’s clinical development plans; and ability to secure regulatory approval on anticipated timelines, and other risks and uncertainties detailed in filings with Canadian securities regulators on SEDAR+ and with the U.S. Securities and Exchange Commission (“SEC”) on EDGAR, including those described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the fiscal year ended October 31, 2024 (copies of which may be obtained at www.sedarplus.ca or www.sec.gov). You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report, as well as other documents if and when filed by enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements. There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans, or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward-looking statements should not be relied upon as representing enGene’s assessments as of any date subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein. Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Industry and Market Data This Presentation relies on and refers to certain information and statistics based on estimates by enGene’s management and/or obtained from third party sources which enGene believes to be reliable. enGene has not independently verified the accuracy or completeness of any such third party information, which involves elements of subjective judgment and analysis that may or may not prove to be accurate. None enGene, or its affiliates or any third parties that provide information to enGene or its affiliates, such as market research firms, guarantees the accuracy, completeness, timeliness, or availability of any information. None enGene, or its affiliates, or any third parties that provide information to enGene, and its affiliates, such as market research firms, is responsible for any errors or omissions (negligent or otherwise), regardless of the cause, or the results obtained from the use of such content. enGene may have supplemented such information where necessary, taking into account publicly available information about other industry participants and enGene management’s best view as to information that is not publicly available. Neither enGene nor its affiliates give any express or implied warranties with respect to the information included herein, including, but not limited to, any warranties regarding its accuracy or of merchantability or fitness for a particular purpose or use, and they expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special, or consequential damages, costs, expenses, legal fees, or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. Lead Program (detalimogene voraplasmid) The lead program described herein is an investigational drug therapy that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy or safety. Although the FDA has indicated that the Phase 2 portion of the current LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 clinical testing, which are not expected to be available until 2026.
Detalimogene Voraplasmid for Non-Muscle Invasive Bladder Cancer Designed for Seamless Integration into High-Volume Urology Practices Transformational Market Opportunity in Area of High Unmet Need Novel class of drug: non-viral gene therapy Powerful and coordinated immune activation across two major axes Clinical benefit and best-in-class ease-of-use Clinical activity, favorable safety, and a streamlined patient experience Near term planned catalysts Data Update: 2H 2026 BLA Submission: 2H 2026 Potential Launch: 2027
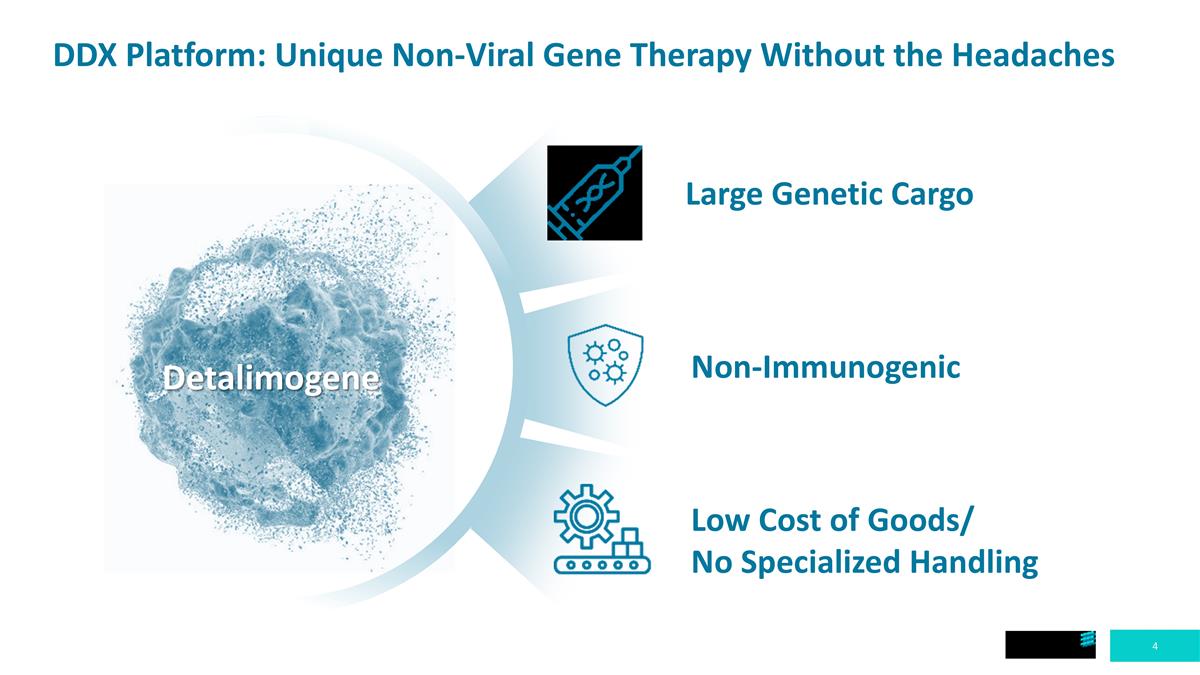
DDX Platform: Unique Non-Viral Gene Therapy Without the Headaches Large Genetic Cargo Non-Immunogenic Low Cost of Goods/ No Specialized Handling Detalimogene
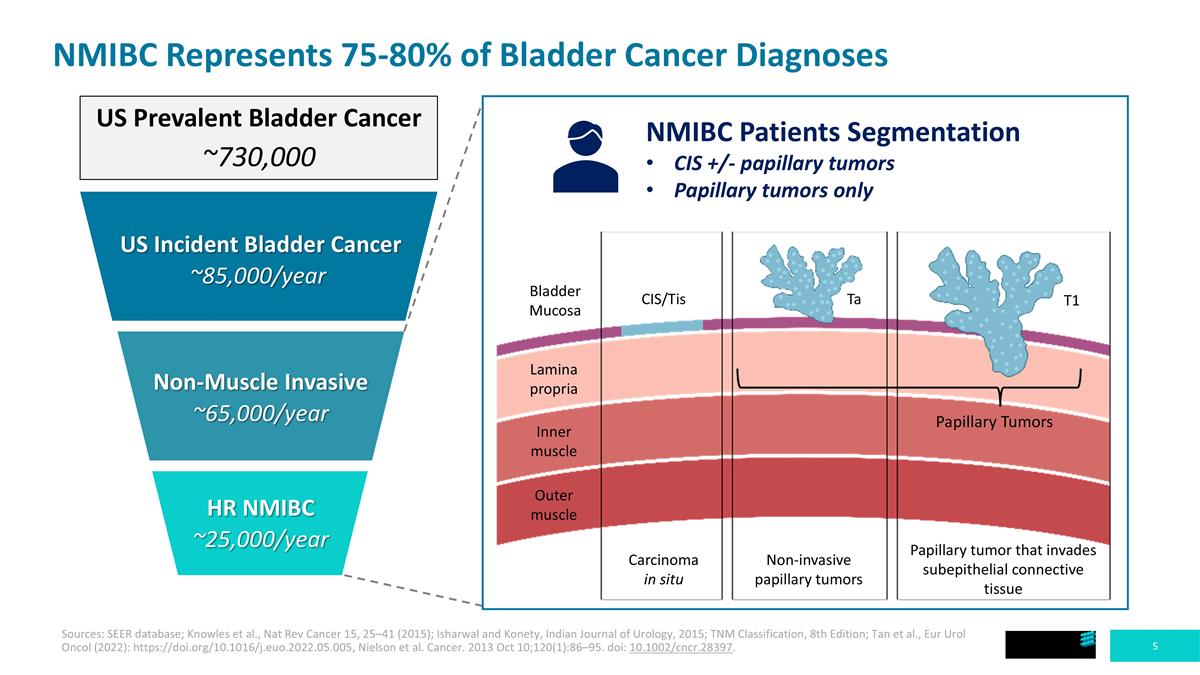
NMIBC Represents 75-80% of Bladder Cancer Diagnoses NMIBC Patients Segmentation CIS +/- papillary tumors Papillary tumors only US Incident Bladder Cancer ~85,000/year Non-Muscle Invasive ~65,000/year HR NMIBC ~25,000/year US Prevalent Bladder Cancer ~730,000 Carcinoma in situ Non-invasive papillary tumors Papillary tumor that invades subepithelial connective tissue Outer muscle Inner muscle Lamina propria Bladder Mucosa CIS/Tis Ta T1 Papillary Tumors Sources: SEER database; Knowles et al., Nat Rev Cancer 15, 25–41 (2015); Isharwal and Konety, Indian Journal of Urology, 2015; TNM Classification, 8th Edition; Tan et al., Eur Urol Oncol (2022): https://doi.org/10.1016/j.euo.2022.05.005, Nielson et al. Cancer. 2013 Oct 10;120(1):86–95. doi: 10.1002/cncr.28397.
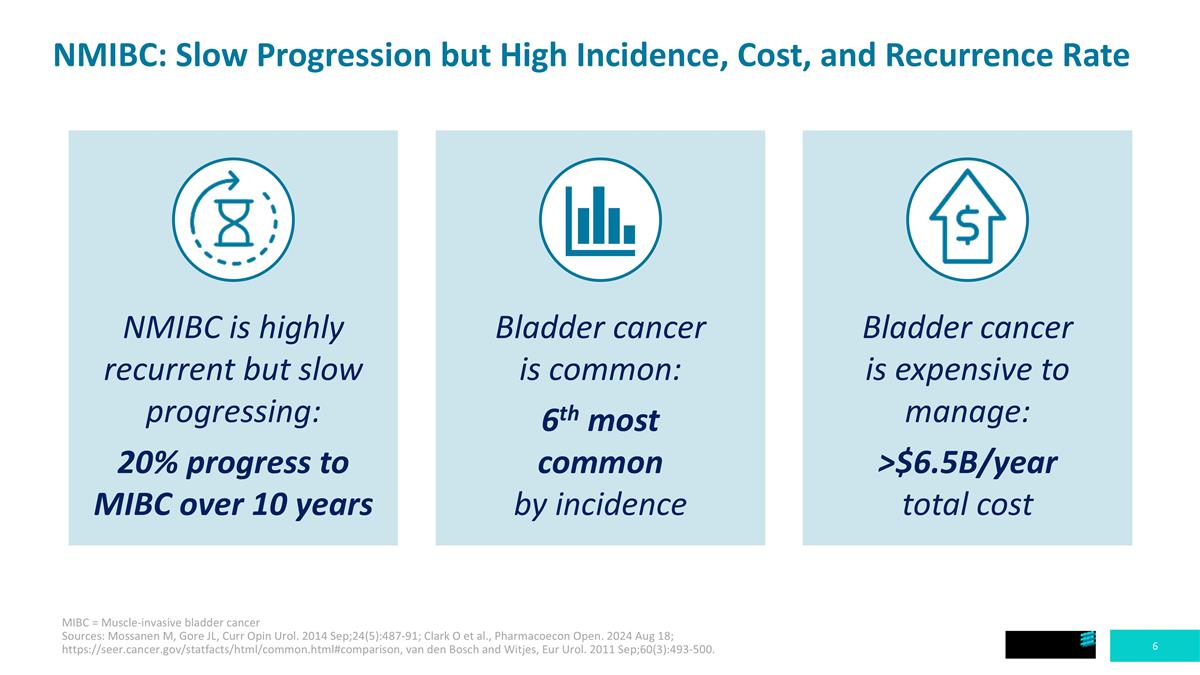
MIBC = Muscle-invasive bladder cancer Sources: Mossanen M, Gore JL, Curr Opin Urol. 2014 Sep;24(5):487-91; Clark O et al., Pharmacoecon Open. 2024 Aug 18; https://seer.cancer.gov/statfacts/html/common.html#comparison, van den Bosch and Witjes, Eur Urol. 2011 Sep;60(3):493-500. NMIBC: Slow Progression but High Incidence, Cost, and Recurrence Rate Bladder cancer is expensive to manage: >$6.5B/year total cost Bladder cancer is common: 6th most common by incidence NMIBC is highly recurrent but slow progressing: 20% progress to MIBC over 10 years
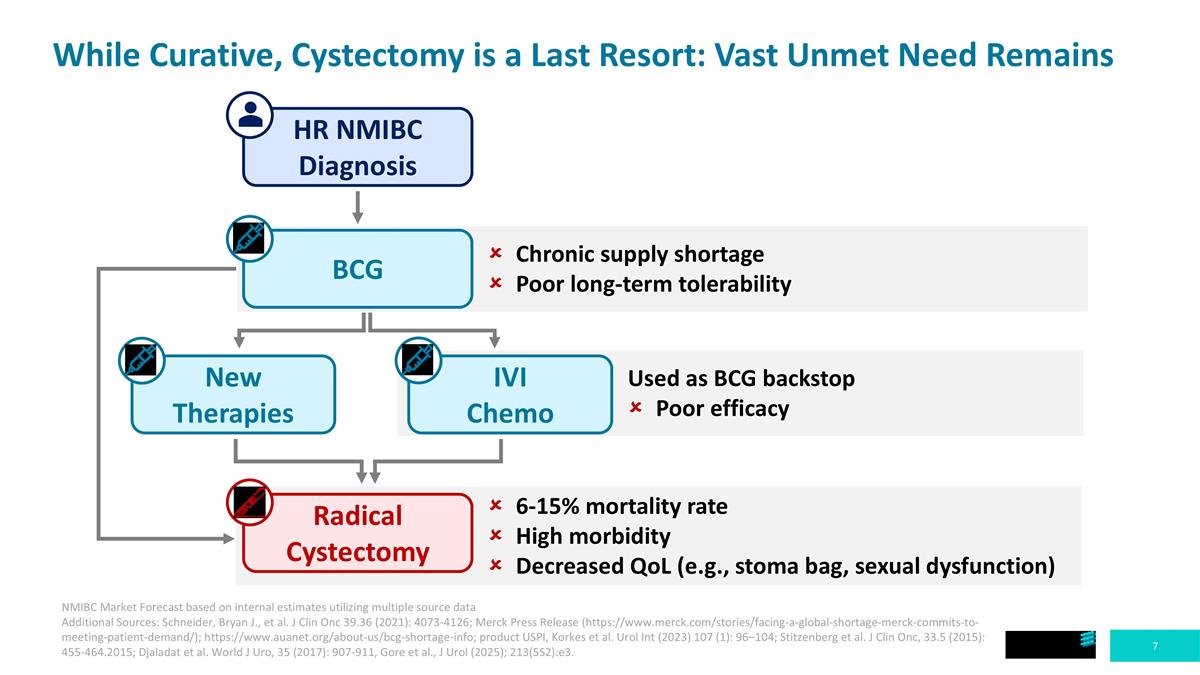
While Curative, Cystectomy is a Last Resort: Vast Unmet Need Remains HR NMIBC Diagnosis BCG New Therapies Radical Cystectomy IVI Chemo Chronic supply shortage Poor long-term tolerability Used as BCG backstop Poor efficacy 6-15% mortality rate High morbidity Decreased QoL (e.g., stoma bag, sexual dysfunction) NMIBC Market Forecast based on internal estimates utilizing multiple source data Additional Sources: Schneider, Bryan J., et al. J Clin Onc 39.36 (2021): 4073-4126; Merck Press Release (https://www.merck.com/stories/facing-a-global-shortage-merck-commits-to-meeting-patient-demand/); https://www.auanet.org/about-us/bcg-shortage-info; product USPI, Korkes et al. Urol Int (2023) 107 (1): 96–104; Stitzenberg et al. J Clin Onc, 33.5 (2015): 455-464.2015; Djaladat et al. World J Uro, 35 (2017): 907-911, Gore et al., J Urol (2025); 213(5S2):e3.
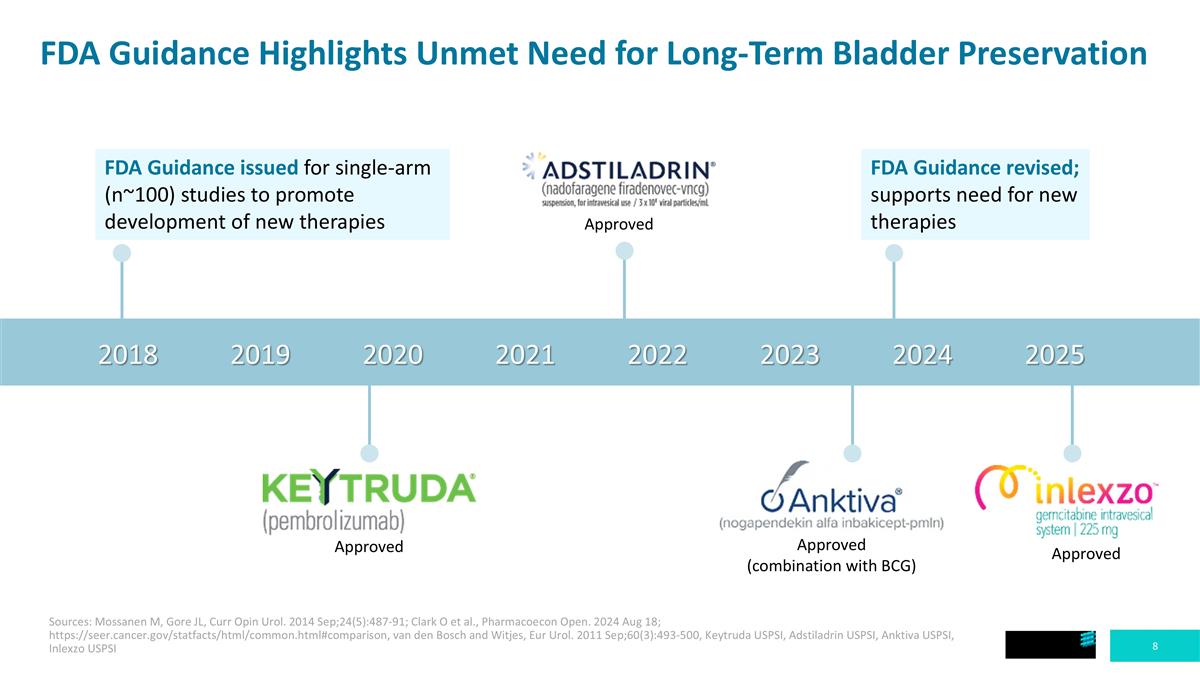
FDA Guidance Highlights Unmet Need for Long-Term Bladder Preservation 2018 2019 2020 2021 2022 2023 2024 2025 FDA Guidance issued for single-arm (n~100) studies to promote development of new therapies Approved FDA Guidance revised; supports need for new therapies Approved Approved (combination with BCG) Approved Sources: Mossanen M, Gore JL, Curr Opin Urol. 2014 Sep;24(5):487-91; Clark O et al., Pharmacoecon Open. 2024 Aug 18; https://seer.cancer.gov/statfacts/html/common.html#comparison, van den Bosch and Witjes, Eur Urol. 2011 Sep;60(3):493-500, Keytruda USPSI, Adstiladrin USPSI, Anktiva USPSI, Inlexzo USPSI
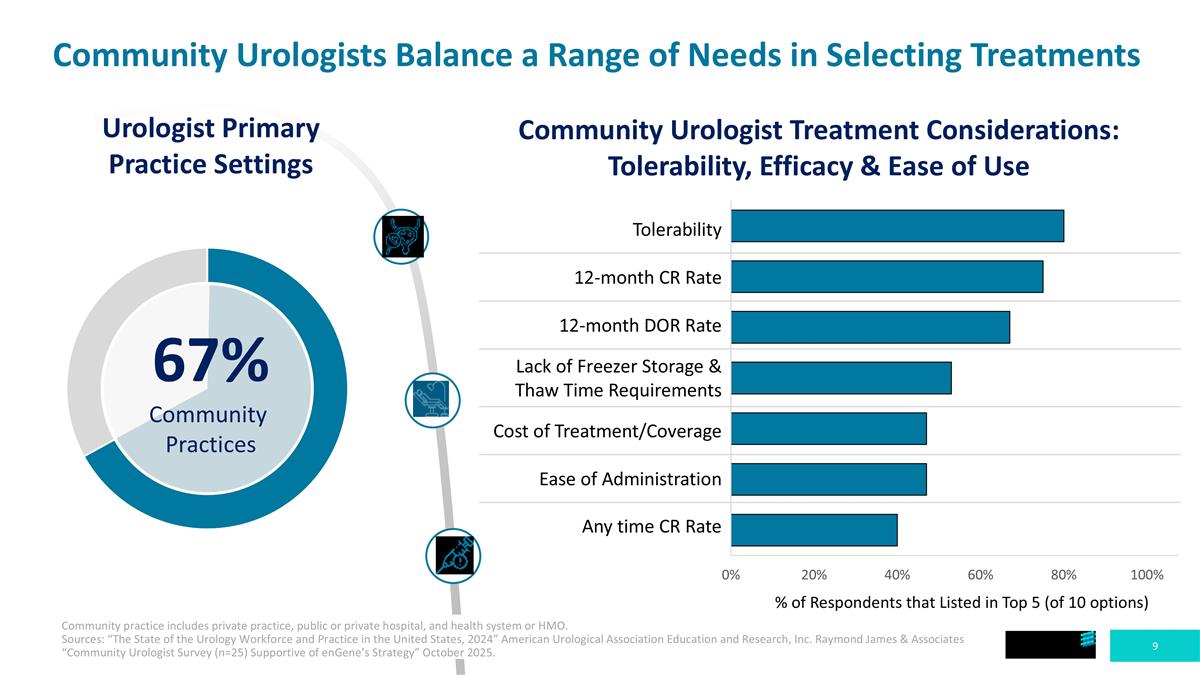
Tolerability 12-month CR Rate 12-month DOR Rate Lack of Freezer Storage & Thaw Time Requirements Cost of Treatment/Coverage Ease of Administration Any time CR Rate Community Urologists Balance a Range of Needs in Selecting Treatments Community Urologist Treatment Considerations: Tolerability, Efficacy & Ease of Use 67% Community Practices Urologist Primary Practice Settings % of Respondents that Listed in Top 5 (of 10 options) Community practice includes private practice, public or private hospital, and health system or HMO. Sources: “The State of the Urology Workforce and Practice in the United States, 2024” American Urological Association Education and Research, Inc. Raymond James & Associates “Community Urologist Survey (n=25) Supportive of enGene’s Strategy” October 2025.
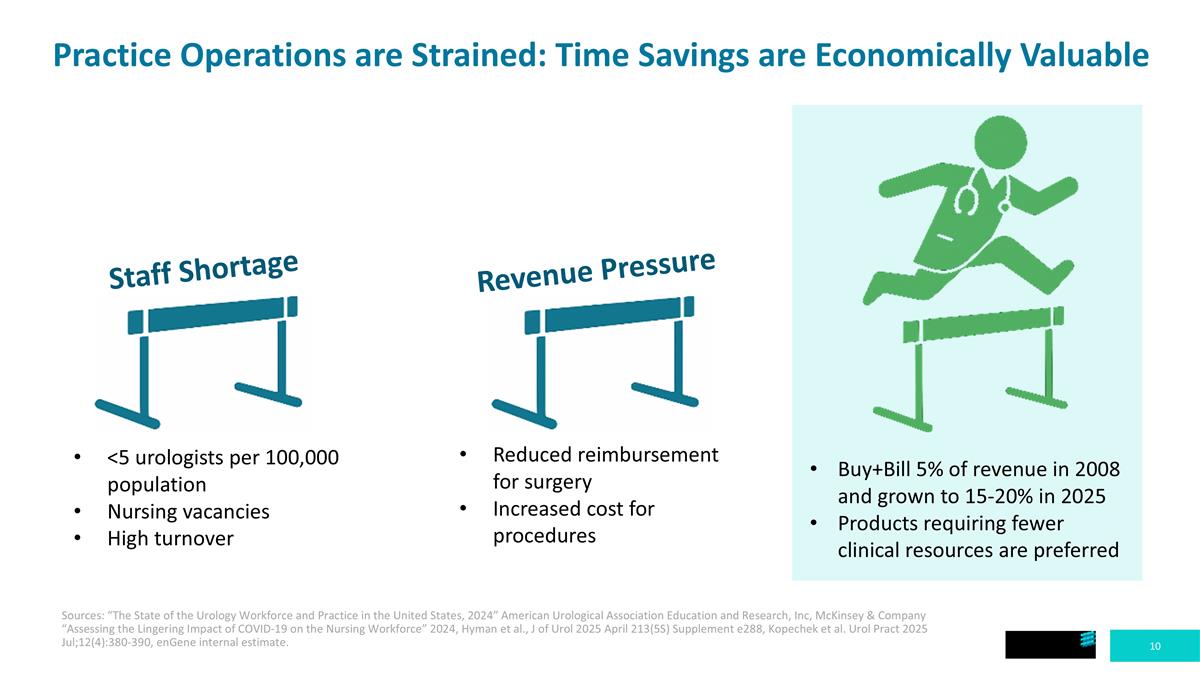
Buy+Bill 5% of revenue in 2008 and grown to 15-20% in 2025 Products requiring fewer clinical resources are preferred Practice Operations are Strained: Time Savings are Economically Valuable Sources: “The State of the Urology Workforce and Practice in the United States, 2024” American Urological Association Education and Research, Inc, McKinsey & Company “Assessing the Lingering Impact of COVID-19 on the Nursing Workforce” 2024, Hyman et al., J of Urol 2025 April 213(5S) Supplement e288, Kopechek et al. Urol Pract 2025 Jul;12(4):380-390, enGene internal estimate. <5 urologists per 100,000 population Nursing vacancies High turnover Staff Shortage Revenue Pressure Reduced reimbursement for surgery Increased cost for procedures
Detalimogene: Designed to Meet the Needs of Urologists and Patients Preliminary evidence of clinical activity* Non-viral gene therapy designed for streamlined clinical experience *LEGEND phase 1 reported data and September 2024 and November 2025 preliminary pivotal phase 2 reported data Efficacy Ease-of-Use Safety Generally mild treatment related adverse events*
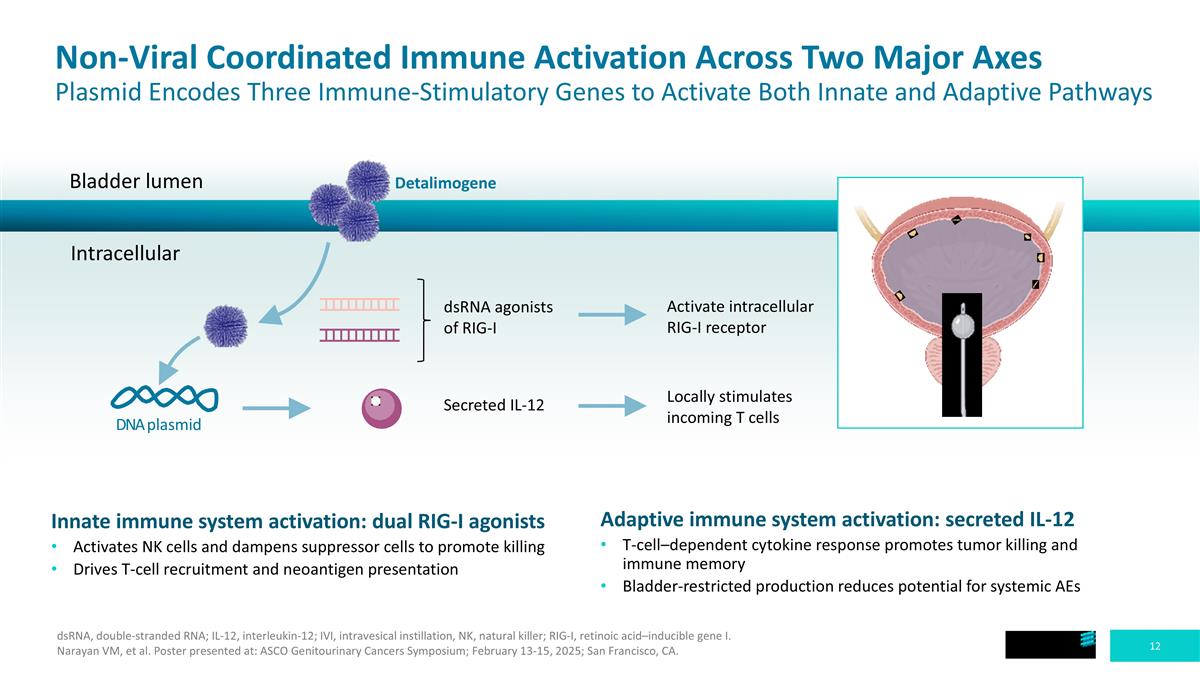
Non-Viral Coordinated Immune Activation Across Two Major Axes Plasmid Encodes Three Immune-Stimulatory Genes to Activate Both Innate and Adaptive Pathways Bladder lumen Intracellular Detalimogene DNA plasmid dsRNA agonists of RIG-I Secreted IL-12 Activate intracellular RIG-I receptor Locally stimulates incoming T cells Adaptive immune system activation: secreted IL-12 T-cell–dependent cytokine response promotes tumor killing and immune memory Bladder-restricted production reduces potential for systemic AEs Innate immune system activation: dual RIG-I agonists Activates NK cells and dampens suppressor cells to promote killing Drives T-cell recruitment and neoantigen presentation dsRNA, double-stranded RNA; IL-12, interleukin-12; IVI, intravesical instillation, NK, natural killer; RIG-I, retinoic acid–inducible gene I. Narayan VM, et al. Poster presented at: ASCO Genitourinary Cancers Symposium; February 13-15, 2025; San Francisco, CA.
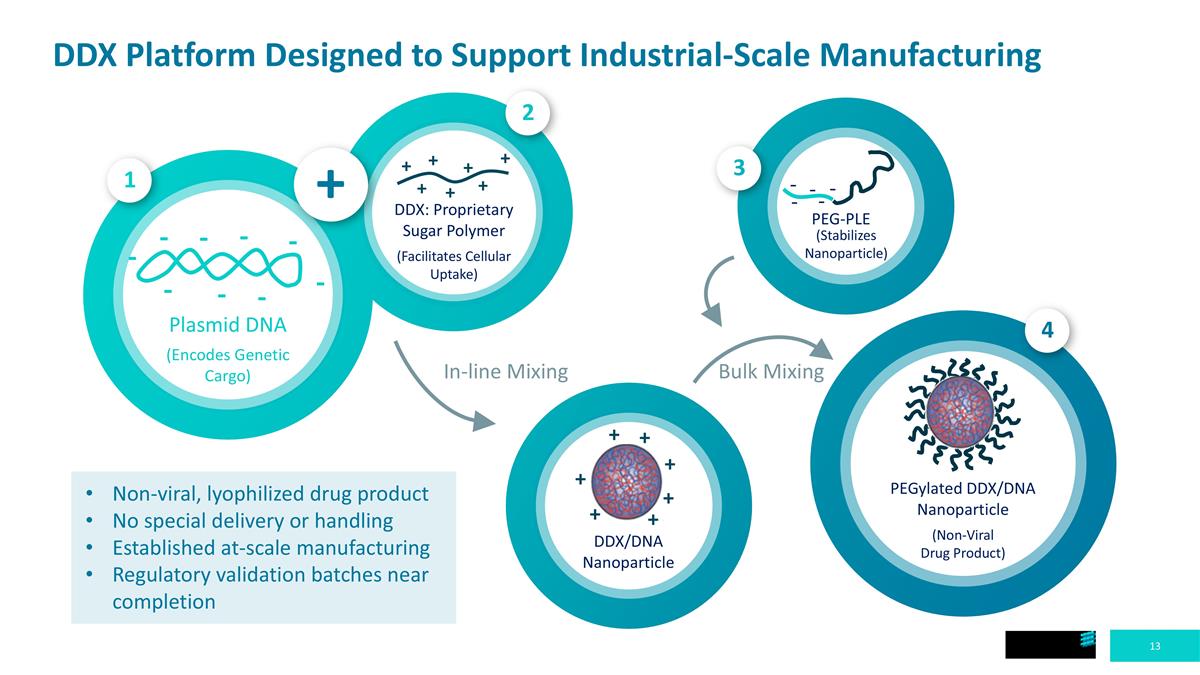
DDX Platform Designed to Support Industrial-Scale Manufacturing e e e e e - - - - - (Stabilizes Nanoparticle) PEG-PLE Bulk Mixing In-line Mixing PEGylated DDX/DNA Nanoparticle (Non-Viral Drug Product) DDX/DNA Nanoparticle + + + + + + + Non-viral, lyophilized drug product No special delivery or handling Established at-scale manufacturing Regulatory validation batches near completion + + + + + + + DDX: Proprietary Sugar Polymer (Facilitates Cellular Uptake) Plasmid DNA (Encodes Genetic Cargo) - - - - - - - - - + 2 4 3 1
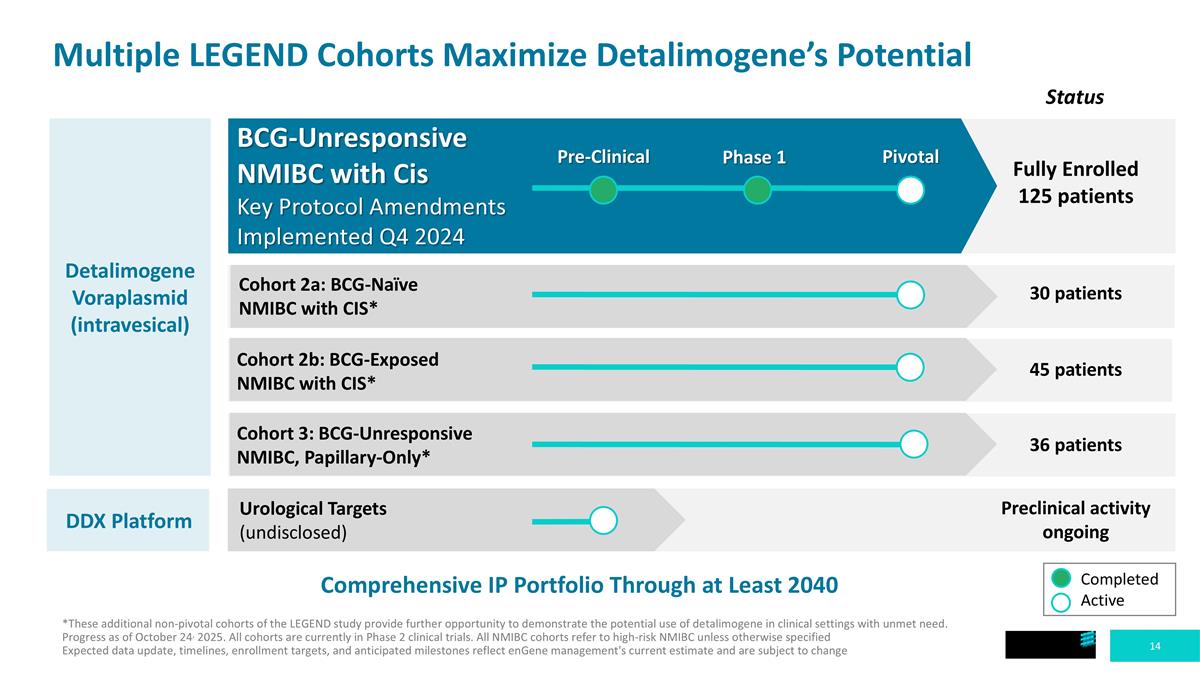
Multiple LEGEND Cohorts Maximize Detalimogene’s Potential Detalimogene Voraplasmid (intravesical) Comprehensive IP Portfolio Through at Least 2040 *These additional non-pivotal cohorts of the LEGEND study provide further opportunity to demonstrate the potential use of detalimogene in clinical settings with unmet need. Progress as of October 24, 2025. All cohorts are currently in Phase 2 clinical trials. All NMIBC cohorts refer to high-risk NMIBC unless otherwise specified Expected data update, timelines, enrollment targets, and anticipated milestones reflect enGene management's current estimate and are subject to change Fully Enrolled 125 patients 30 patients Preclinical activity ongoing 36 patients 45 patients BCG-Unresponsive NMIBC with Cis Key Protocol Amendments Implemented Q4 2024 Pre-Clinical Phase 1 Pivotal Cohort 2a: BCG-Naïve NMIBC with CIS* Cohort 2b: BCG-Exposed NMIBC with CIS* Cohort 3: BCG-Unresponsive NMIBC, Papillary-Only* Urological Targets (undisclosed) Status Completed Active DDX Platform

Pivotal Study Target Enrollment Achieved Patients: BCG-Unresponsive High-risk NMIBC with CIS Design: Global, single-arm, open label N = 125 (fully enrolled) Dosing2: Year 1: 800μg/ml IVI at weeks 1,2,5,6 Q3M Year 2-3 (Maintenance): 800μg/ml IVI at weeks 1,2 Q3M Primary Endpoint: Anytime CR rate Key Secondary Endpoint: Duration of Response 1: IVI = Intravesical. LEGEND allows patients from any cohort who are in complete response at 12 months to continue receiving detalimogene on a dose-reduced maintenance schedule throughout their second year of treatment. At the end of year two, patients may optionally elect to remain on maintenance therapy for another year, for a total of three years of therapy.
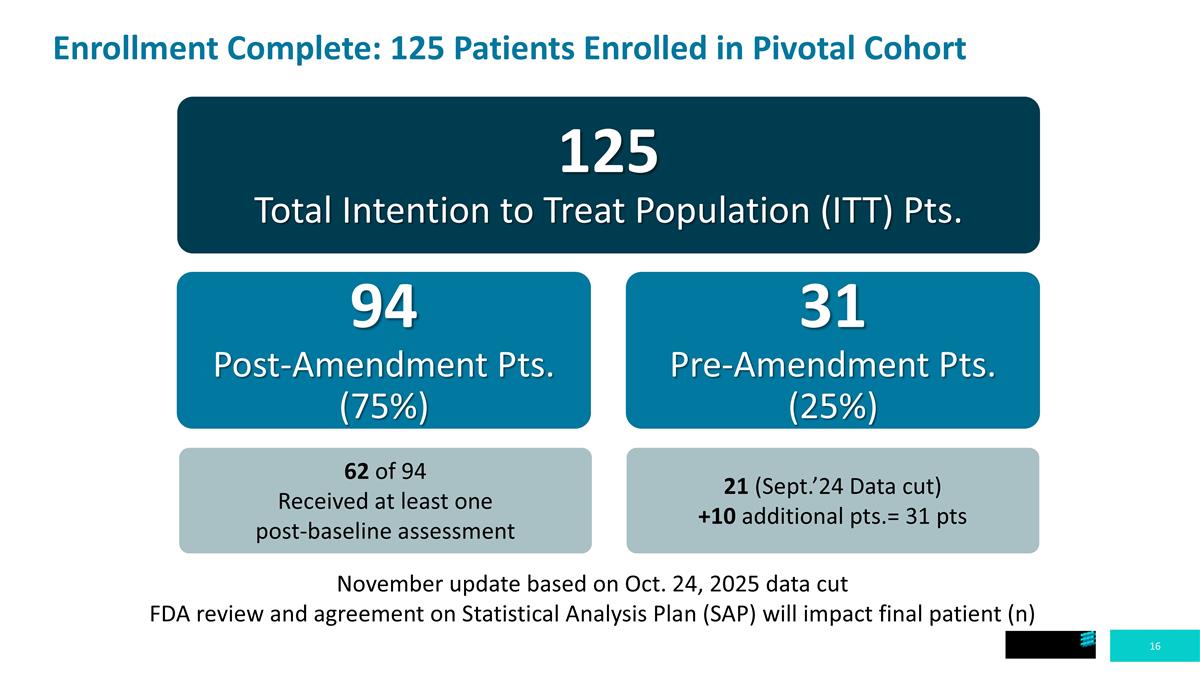
Enrollment Complete: 125 Patients Enrolled in Pivotal Cohort 125 Total Intention to Treat Population (ITT) Pts. 94 Post-Amendment Pts. (75%) 31 Pre-Amendment Pts. (25%) November update based on Oct. 24, 2025 data cut FDA review and agreement on Statistical Analysis Plan (SAP) will impact final patient (n) 62 of 94 Received at least one post-baseline assessment 21 (Sept.’24 Data cut) +10 additional pts.= 31 pts
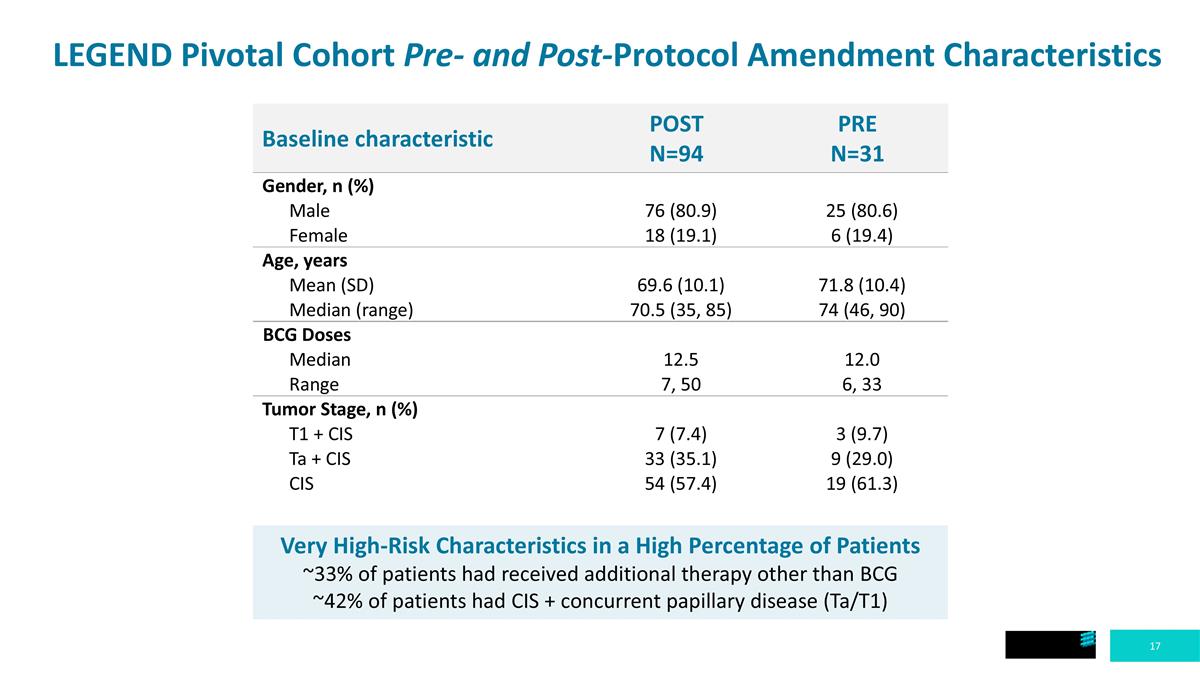
LEGEND Pivotal Cohort Pre- and Post-Protocol Amendment Characteristics Baseline characteristic POST N=94 PRE N=31 Gender, n (%) Male 76 (80.9) 25 (80.6) Female 18 (19.1) 6 (19.4) Age, years Mean (SD) 69.6 (10.1) 71.8 (10.4) Median (range) 70.5 (35, 85) 74 (46, 90) BCG Doses Median 12.5 12.0 Range 7, 50 6, 33 Tumor Stage, n (%) T1 + CIS 7 (7.4) 3 (9.7) Ta + CIS 33 (35.1) 9 (29.0) CIS 54 (57.4) 19 (61.3) Very High-Risk Characteristics in a High Percentage of Patients ~33% of patients had received additional therapy other than BCG ~42% of patients had CIS + concurrent papillary disease (Ta/T1)
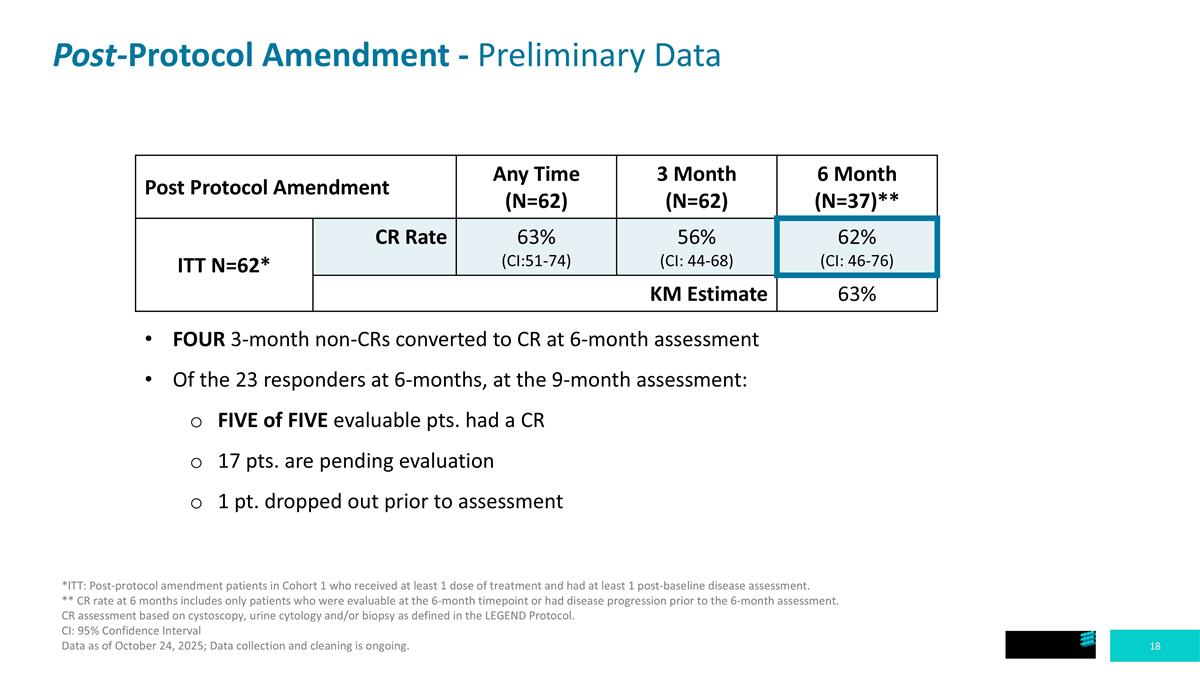
Post-Protocol Amendment - Preliminary Data Post Protocol Amendment Any Time (N=62) 3 Month (N=62) 6 Month (N=37)** ITT N=62* CR Rate 63% (CI:51-74) 56% (CI: 44-68) 62% (CI: 46-76) KM Estimate 63% *ITT: Post-protocol amendment patients in Cohort 1 who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing. FOUR 3-month non-CRs converted to CR at 6-month assessment Of the 23 responders at 6-months, at the 9-month assessment: FIVE of FIVE evaluable pts. had a CR 17 pts. are pending evaluation 1 pt. dropped out prior to assessment
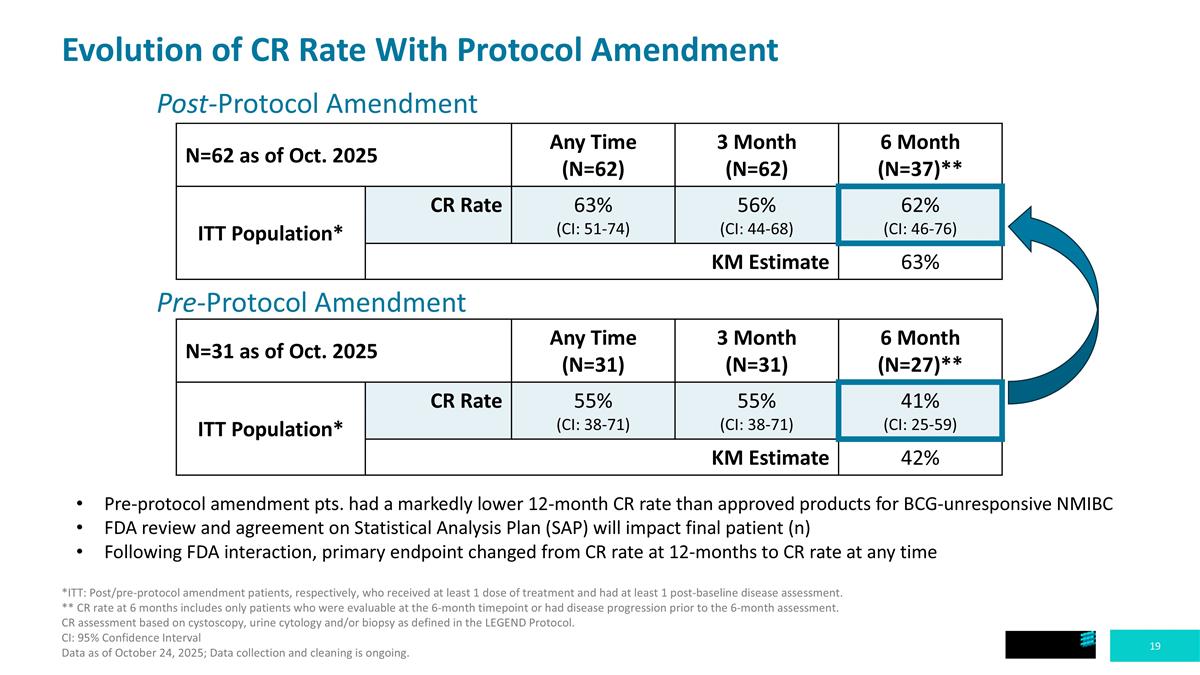
Post-Protocol Amendment N=31 as of Oct. 2025 Any Time (N=31) 3 Month (N=31) 6 Month (N=27)** ITT Population* CR Rate 55% (CI: 38-71) 55% (CI: 38-71) 41% (CI: 25-59) KM Estimate 42% Evolution of CR Rate With Protocol Amendment Pre-Protocol Amendment N=62 as of Oct. 2025 Any Time (N=62) 3 Month (N=62) 6 Month (N=37)** ITT Population* CR Rate 63% (CI: 51-74) 56% (CI: 44-68) 62% (CI: 46-76) KM Estimate 63% Pre-protocol amendment pts. had a markedly lower 12-month CR rate than approved products for BCG-unresponsive NMIBC FDA review and agreement on Statistical Analysis Plan (SAP) will impact final patient (n) Following FDA interaction, primary endpoint changed from CR rate at 12-months to CR rate at any time *ITT: Post/pre-protocol amendment patients, respectively, who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing.
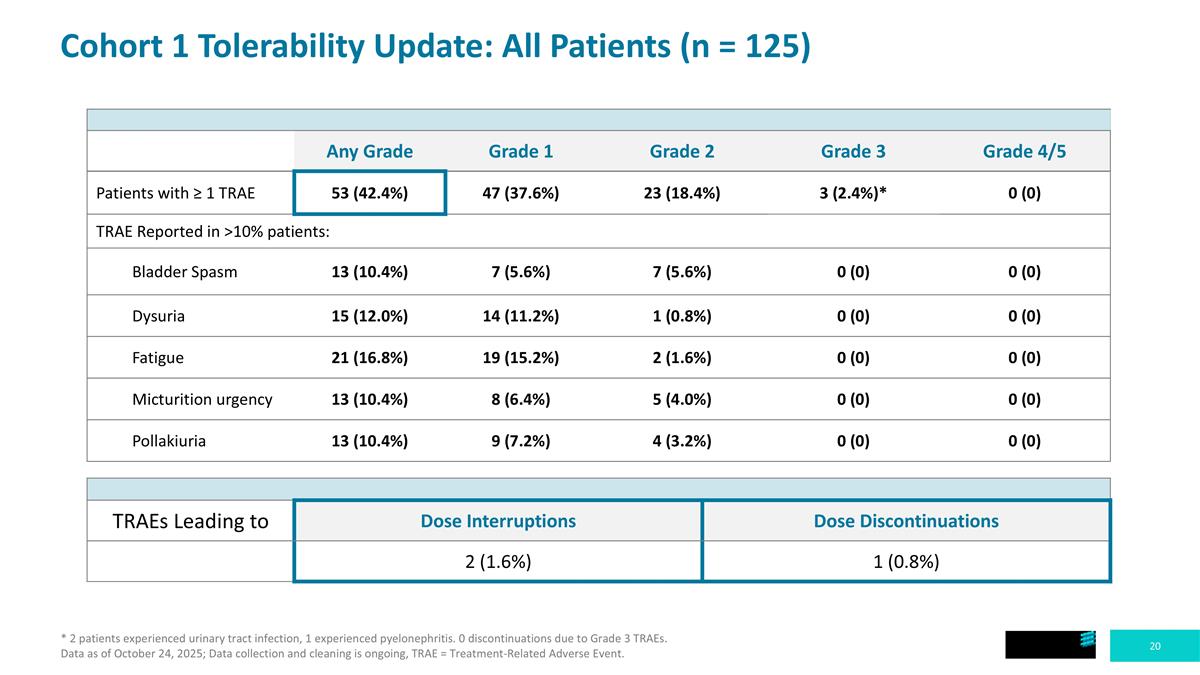
Cohort 1 Tolerability Update: All Patients (n = 125) * 2 patients experienced urinary tract infection, 1 experienced pyelonephritis. 0 discontinuations due to Grade 3 TRAEs. Data as of October 24, 2025; Data collection and cleaning is ongoing, TRAE = Treatment-Related Adverse Event. Any Grade Grade 1 Grade 2 Grade 3 Grade 4/5 Patients with ≥ 1 TRAE 53 (42.4%) 47 (37.6%) 23 (18.4%) 3 (2.4%)* 0 (0) TRAE Reported in >10% patients: Bladder Spasm 13 (10.4%) 7 (5.6%) 7 (5.6%) 0 (0) 0 (0) Dysuria 15 (12.0%) 14 (11.2%) 1 (0.8%) 0 (0) 0 (0) Fatigue 21 (16.8%) 19 (15.2%) 2 (1.6%) 0 (0) 0 (0) Micturition urgency 13 (10.4%) 8 (6.4%) 5 (4.0%) 0 (0) 0 (0) Pollakiuria 13 (10.4%) 9 (7.2%) 4 (3.2%) 0 (0) 0 (0) TRAEs Leading to Dose Interruptions Dose Discontinuations 2 (1.6%) 1 (0.8%)
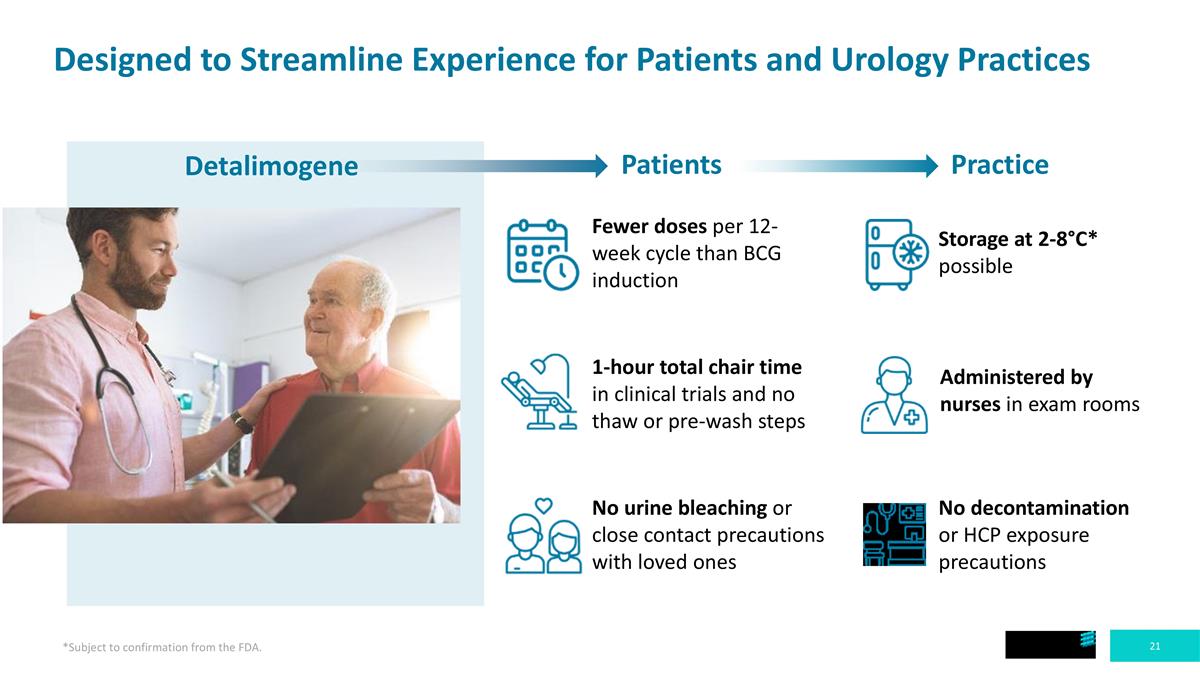
Patients Practice Designed to Streamline Experience for Patients and Urology Practices Fewer doses per 12-week cycle than BCG induction 1-hour total chair time in clinical trials and no thaw or pre-wash steps No urine bleaching or close contact precautions with loved ones Storage at 2-8°C* possible Administered by nurses in exam rooms No decontamination or HCP exposure precautions Detalimogene *Subject to confirmation from the FDA.
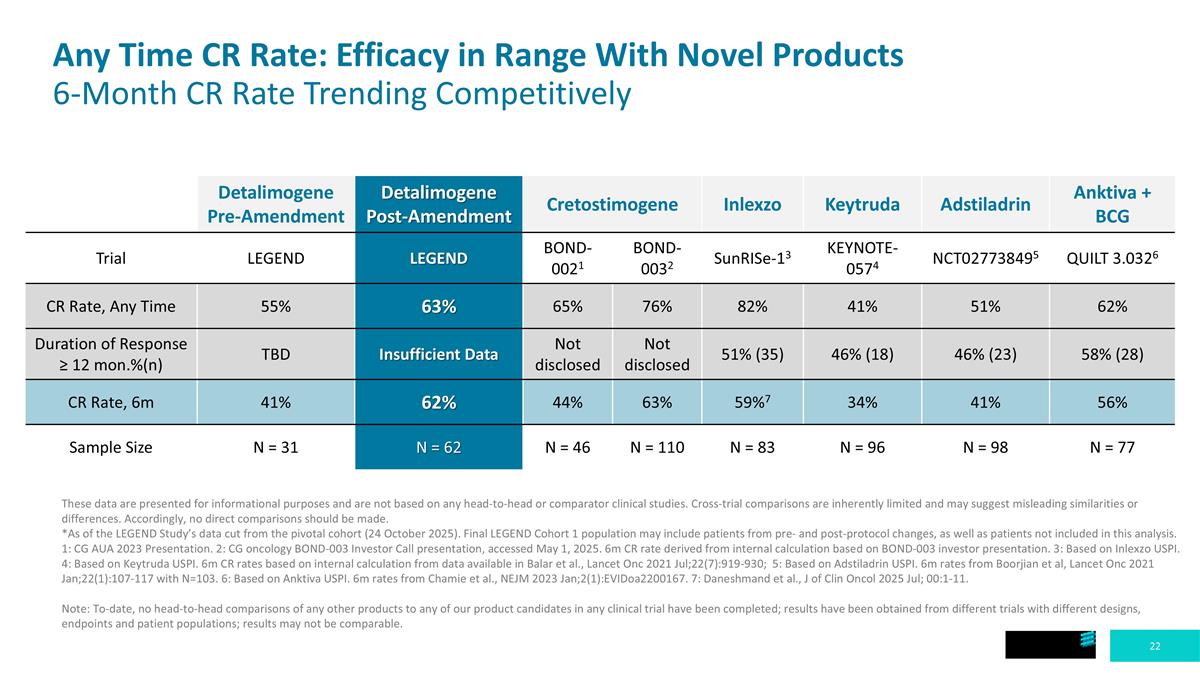
Any Time CR Rate: Efficacy in Range With Novel Products 6-Month CR Rate Trending Competitively These data are presented for informational purposes and are not based on any head-to-head or comparator clinical studies. Cross-trial comparisons are inherently limited and may suggest misleading similarities or differences. Accordingly, no direct comparisons should be made. *As of the LEGEND Study’s data cut from the pivotal cohort (24 October 2025). Final LEGEND Cohort 1 population may include patients from pre- and post-protocol changes, as well as patients not included in this analysis. 1: CG AUA 2023 Presentation. 2: CG oncology BOND-003 Investor Call presentation, accessed May 1, 2025. 6m CR rate derived from internal calculation based on BOND-003 investor presentation. 3: Based on Inlexzo USPI. 4: Based on Keytruda USPI. 6m CR rates based on internal calculation from data available in Balar et al., Lancet Onc 2021 Jul;22(7):919-930; 5: Based on Adstiladrin USPI. 6m rates from Boorjian et al, Lancet Onc 2021 Jan;22(1):107-117 with N=103. 6: Based on Anktiva USPI. 6m rates from Chamie et al., NEJM 2023 Jan;2(1):EVIDoa2200167. 7: Daneshmand et al., J of Clin Oncol 2025 Jul; 00:1-11. Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial have been completed; results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable. Detalimogene Pre-Amendment Detalimogene Post-Amendment Cretostimogene Inlexzo Keytruda Adstiladrin Anktiva + BCG Trial LEGEND LEGEND BOND-0021 BOND-0032 SunRISe-13 KEYNOTE-0574 NCT027738495 QUILT 3.0326 CR Rate, Any Time 55% 63% 65% 76% 82% 41% 51% 62% Duration of Response ≥ 12 mon.%(n) TBD Insufficient Data Not disclosed Not disclosed 51% (35) 46% (18) 46% (23) 58% (28) CR Rate, 6m 41% 62% 44% 63% 59%7 34% 41% 56% Sample Size N = 31 N = 62 N = 46 N = 110 N = 83 N = 96 N = 98 N = 77
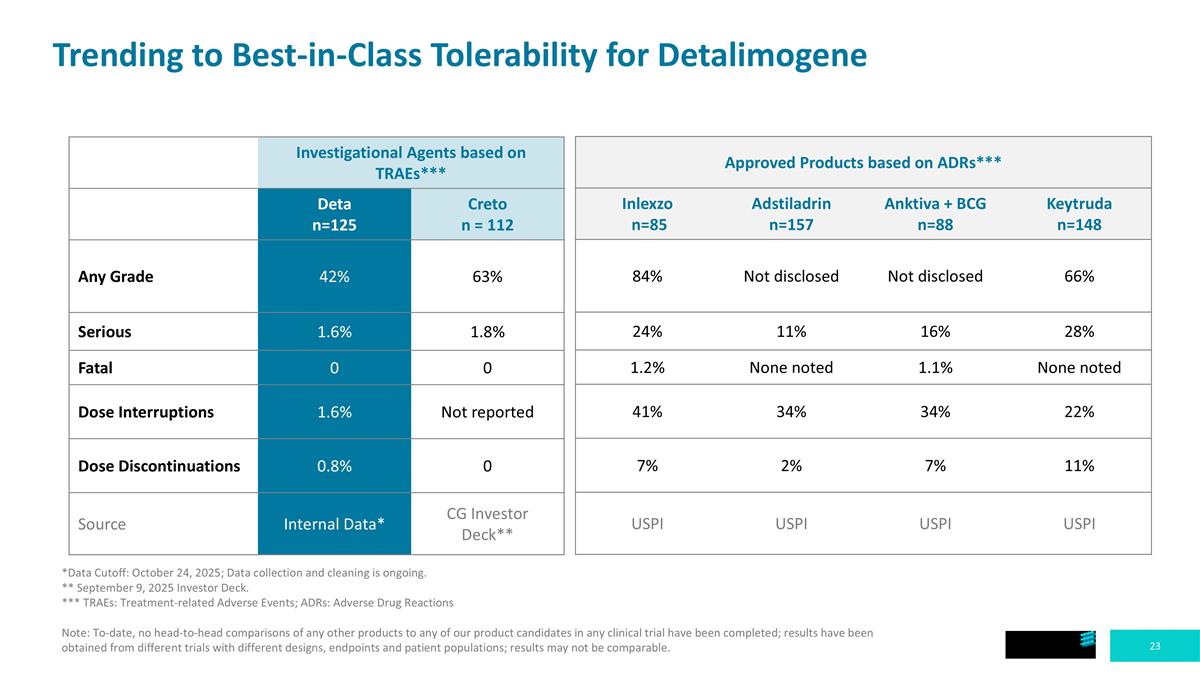
Trending to Best-in-Class Tolerability for Detalimogene Investigational Agents based on TRAEs*** Deta n=125 Creto n = 112 Any Grade 42% 63% Serious 1.6% 1.8% Fatal 0 0 Dose Interruptions 1.6% Not reported Dose Discontinuations 0.8% 0 Source Internal Data* CG Investor Deck** Approved Products based on ADRs*** Inlexzo n=85 Adstiladrin n=157 Anktiva + BCG n=88 Keytruda n=148 84% Not disclosed Not disclosed 66% 24% 11% 16% 28% 1.2% None noted 1.1% None noted 41% 34% 34% 22% 7% 2% 7% 11% USPI USPI USPI USPI *Data Cutoff: October 24, 2025; Data collection and cleaning is ongoing. ** September 9, 2025 Investor Deck. *** TRAEs: Treatment-related Adverse Events; ADRs: Adverse Drug Reactions Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial have been completed; results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable.
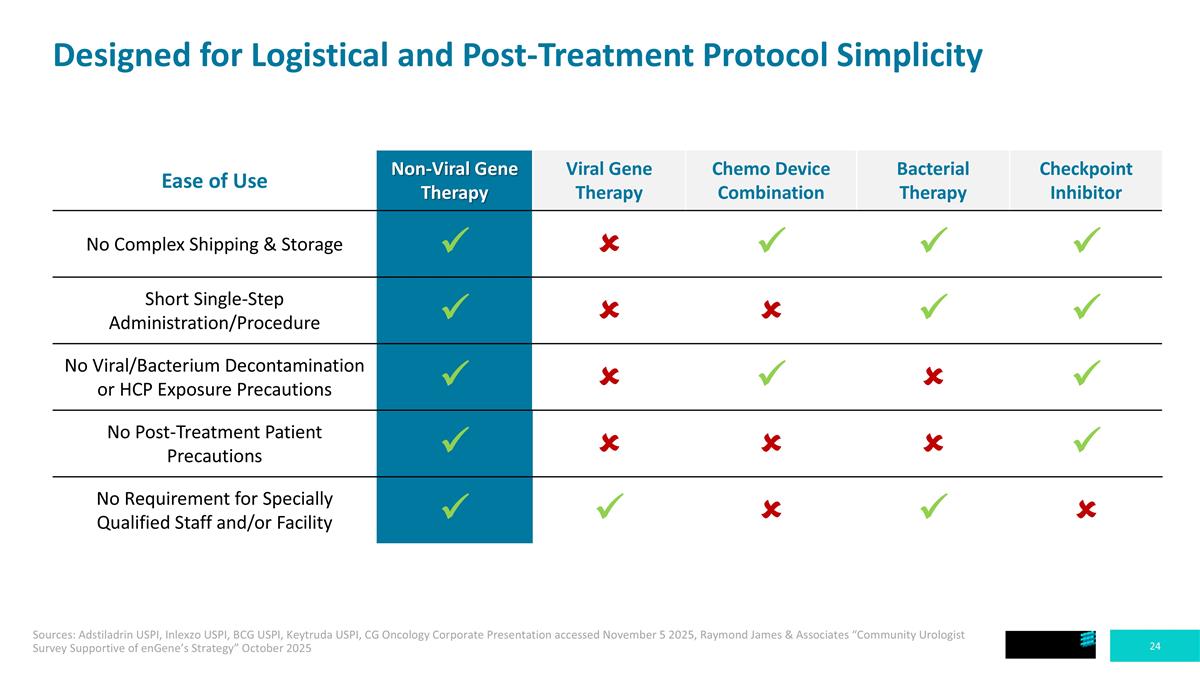
Designed for Logistical and Post-Treatment Protocol Simplicity Ease of Use Non-Viral Gene Therapy Viral Gene Therapy Chemo Device Combination Bacterial Therapy Checkpoint Inhibitor No Complex Shipping & Storage ü û ü ü ü Short Single-Step Administration/Procedure ü û û ü ü No Viral/Bacterium Decontamination or HCP Exposure Precautions ü û ü û ü No Post-Treatment Patient Precautions ü û û û ü No Requirement for Specially Qualified Staff and/or Facility ü ü û ü û Sources: Adstiladrin USPI, Inlexzo USPI, BCG USPI, Keytruda USPI, CG Oncology Corporate Presentation accessed November 5 2025, Raymond James & Associates “Community Urologist Survey Supportive of enGene’s Strategy” October 2025
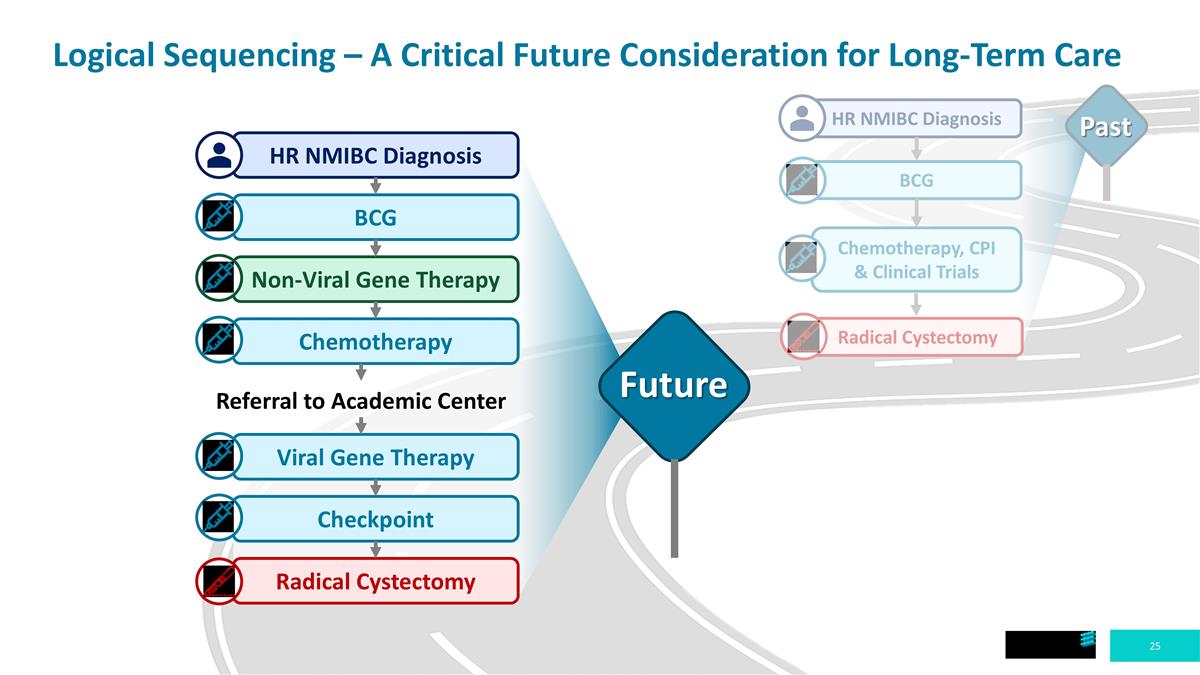
Logical Sequencing – A Critical Future Consideration for Long-Term Care HR NMIBC Diagnosis BCG Chemotherapy, CPI & Clinical Trials Radical Cystectomy HR NMIBC Diagnosis BCG Non-Viral Gene Therapy Chemotherapy Referral to Academic Center Viral Gene Therapy Checkpoint Radical Cystectomy Future Past

Experience Developing and Commercializing Highly Successful Medicines RON COOPER Chief Executive Officer Chief Executive Officer, Albireo Pharma President, Europe, Bristol Myers Squibb ANTHONY CHEUNG, Ph.D. Chief Scientific Officer Co-founder, enGene Co-inventor All Key enGene Patents ALEX NICHOLS, Ph.D. Chief Strategy & Operations Officer Co-Founder and CEO, Mythic Therapeutics Flagship Pioneering JOAN CONNOLLY Chief Technology Officer Chief Technology Officer, Albireo Pharma SVP Technical Operations, Stemline Therapeutics AMY POTT Chief Global Commercialization Officer Head, Rare Disease & Ophthalmology, Global Commercial, Astellas Head, Internal Medicine & Oncology, Commercial, Shire RYAN DAWS Chief Financial Officer CFO Roles: Concert Therapeutics, Obsidian Therapeutics Investment Banker Roles: Cowen, Stifel, Baird LEE GIGUERE Chief Legal Officer & Secretary CLO, Obsidian Therapeutics General Counsel, Chiasma Inc. Karyopharm Therapeutics, Boston Scientific, Goodwin Procter JILL BUCK Chief Development Officer Head, Clinical Development Operations, Rare Disease, Ipsen Albireo Pharma, Ziopharm Oncology, Synageva Biopharma HUSSEIN SWEITI, M.D. Chief Medical Officer Global Medical Head, Oncology Clinical Development, Johnson & Johnson MATTHEW BOYD Chief Regulatory Officer VP, Head of Regulatory Affairs, Zambon USA VP, Regulatory Affairs, Albireo Pharma
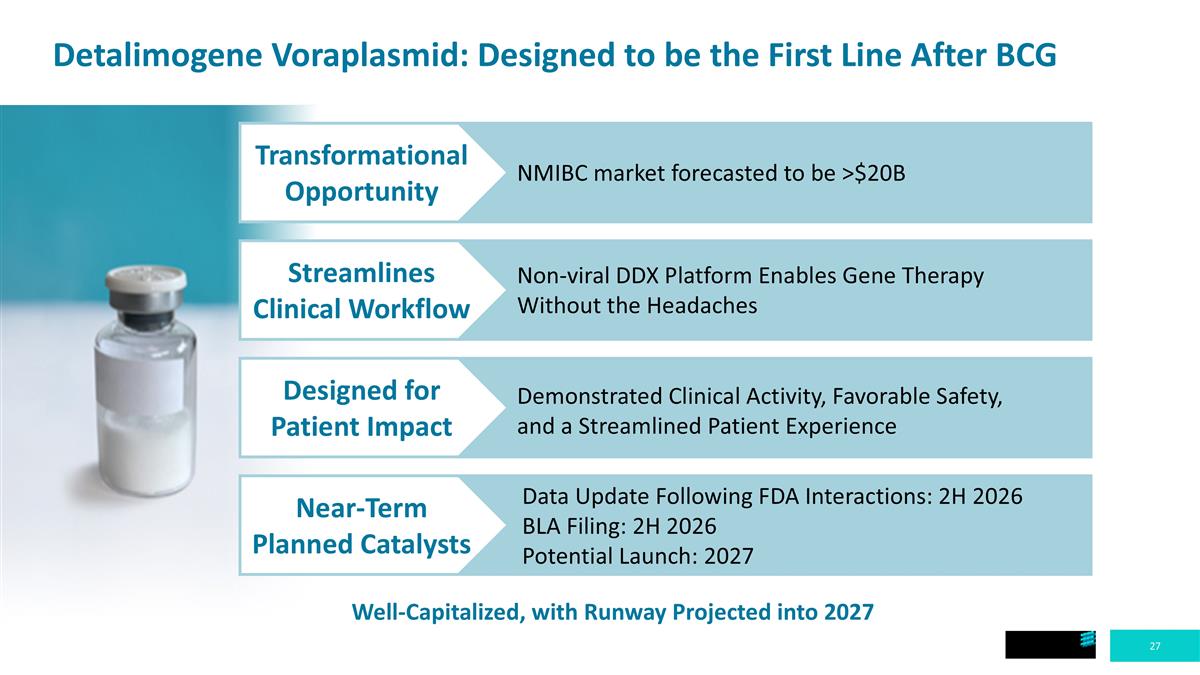
Detalimogene Voraplasmid: Designed to be the First Line After BCG Transformational Opportunity Streamlines Clinical Workflow Designed for Patient Impact Near-Term Planned Catalysts NMIBC market forecasted to be >$20B Non-viral DDX Platform Enables Gene Therapy Without the Headaches Demonstrated Clinical Activity, Favorable Safety, and a Streamlined Patient Experience Data Update Following FDA Interactions: 2H 2026 BLA Filing: 2H 2026 Potential Launch: 2027 Well-Capitalized, with Runway Projected into 2027

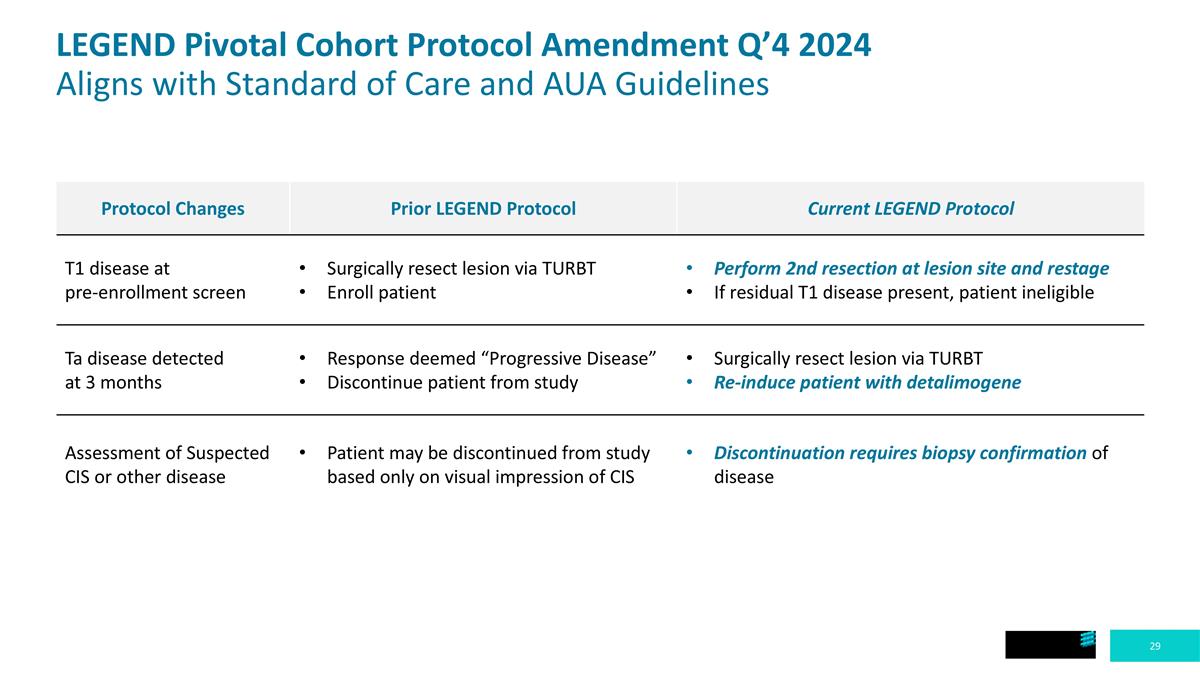
Protocol Changes Prior LEGEND Protocol Current LEGEND Protocol T1 disease at pre-enrollment screen Surgically resect lesion via TURBT Enroll patient Perform 2nd resection at lesion site and restage If residual T1 disease present, patient ineligible Ta disease detected at 3 months Response deemed “Progressive Disease” Discontinue patient from study Surgically resect lesion via TURBT Re-induce patient with detalimogene Assessment of Suspected CIS or other disease Patient may be discontinued from study based only on visual impression of CIS Discontinuation requires biopsy confirmation of disease LEGEND Pivotal Cohort Protocol Amendment Q’4 2024 Aligns with Standard of Care and AUA Guidelines
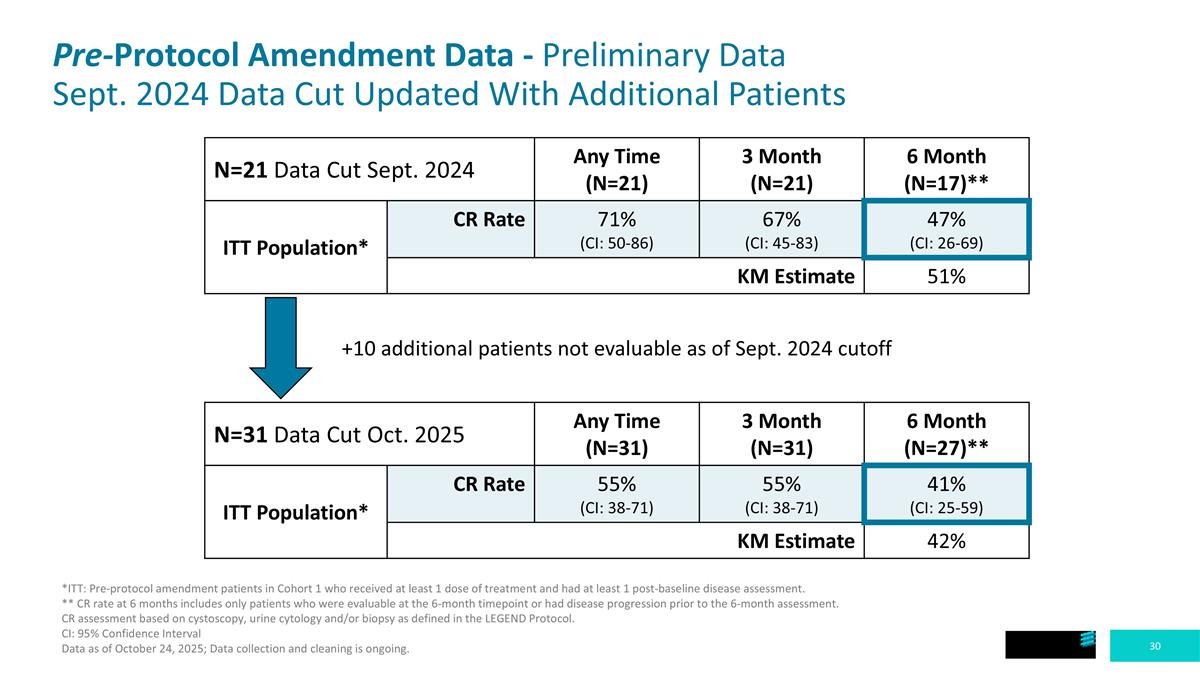
Pre-Protocol Amendment Data - Preliminary Data Sept. 2024 Data Cut Updated With Additional Patients N=31 Data Cut Oct. 2025 Any Time (N=31) 3 Month (N=31) 6 Month (N=27)** ITT Population* CR Rate 55% (CI: 38-71) 55% (CI: 38-71) 41% (CI: 25-59) KM Estimate 42% N=21 Data Cut Sept. 2024 Any Time (N=21) 3 Month (N=21) 6 Month (N=17)** ITT Population* CR Rate 71% (CI: 50-86) 67% (CI: 45-83) 47% (CI: 26-69) KM Estimate 51% +10 additional patients not evaluable as of Sept. 2024 cutoff *ITT: Pre-protocol amendment patients in Cohort 1 who received at least 1 dose of treatment and had at least 1 post-baseline disease assessment. ** CR rate at 6 months includes only patients who were evaluable at the 6-month timepoint or had disease progression prior to the 6-month assessment. CR assessment based on cystoscopy, urine cytology and/or biopsy as defined in the LEGEND Protocol. CI: 95% Confidence Interval Data as of October 24, 2025; Data collection and cleaning is ongoing.
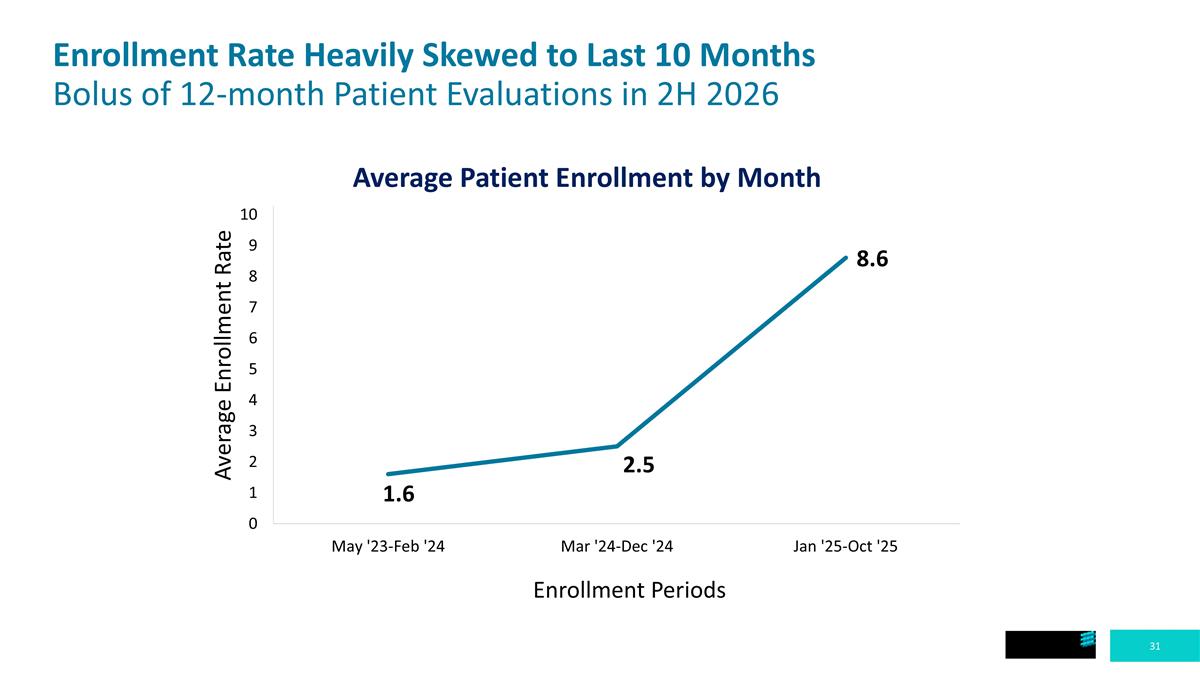
Enrollment Rate Heavily Skewed to Last 10 Months Bolus of 12-month Patient Evaluations in 2H 2026