

Pioneering a New Era of GPCR Drug Discovery J.P. Morgan Healthcare Conference 2026 January 2026 Nasdaq: SEPN

Forward-Looking Statements This presentation contains express or implied forward‐ looking statements of Septerna, Inc. (the “Company,” “we,” or “our”) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements other than statements of historical facts contained in this presentation, including statements regarding our business strategy, plans, estimated R&D program milestones and objectives of management are forward-looking statements. Such forward-looking statements include, but are not limited to, statements regarding: the continued advancement of our PTH1R agonist program, including the planned initiation of a Phase 1 clinical trial for SEP-479 in the first half of 2026 subject to successful completion of drug product manufacturing and regulatory submissions; the continued advancement of SEP-631, including the potential of SEP-631 to provide a convenient oral treatment option for patients with mast cell-driven diseases (e.g., CSU); the role of MRGPRX2 in mast cell-driven diseases; the completion of the Phase 1 clinical trial for SEP-631 and planned presentation of topline data at AAAAI Annual Meeting in March 2026; the timing, progress and results of conducting our research and development programs, including our plans to advance the TSHR program; the intended and potential benefits of the collaboration with Novo Nordisk, including our ability to jointly discover, develop and commercialize multiple potential oral small molecule therapies for obesity, type 2 diabetes, and other cardiometabolic diseases and the potential resulting milestones and royalties (if any); our ability to demonstrate, and the timing of, preclinical proof-of-concept in vivo and ex vivo for multiple programs; the potential of our proprietary Native Complex Platform ; the ability of preclinical observations to successfully translate into clinical outcomes; the size and growth potential of the markets for our current and future product candidates; our expectations regarding strategic plans for our business, product candidates, and technology; the scope of protection we are able to establish and maintain for intellectual property rights covering our Native Complex Platform and our product candidates; our ability to maintain existing collaborations and to identify and enter into future license agreements and collaborations; and the accuracy of our estimates regarding expenses and capital requirements, including our expected cash runway at least into 2029. Such forward-looking statements reflect the current views of the Company and are subject to known and unknown risks and other factors, which are, in some cases, beyond the Company’s control. Risks that contribute to the uncertain nature of the forward- looking statements include those risks and uncertainties set forth in the section titled Risk Factors in our most recent Annual Report on Form 10-K for the year ended December 31, 2024, as well as any subsequent filings with the Securities and Exchange Commission. Certain information in this presentation (including market data and statistical information) and statements made orally during this presentation are the good faith estimates of management and have been obtained from various sources (including third-party sources such as independent industry publications, governmental publications, and reports by market research firms), and we do not guarantee the accuracy or completeness of such information. No representations or warranties (expressed or implied) are made about the accuracy of such forward-looking statements, and there can be no assurance as to the reliability or correctness of such projections and actual results may vary materially from those projected. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. © 2026 SEPTERNA 2

Septerna: Pioneering a New Era Lead Programs of GPCR Drug Discovery with SEP-479 PTH1R Agonist: Potential first-in-class oral small molecule for hypoparathyroidism; Phase 1 Oral Small Molecules clinical trial initiation anticipated in 1H 2026 Native Complex Platform designed to SEP-631 MRGPRX2 NAM: Pipeline-in-a-product unlock the full potential of GPCR therapies opportunity for mast cell-driven diseases (e.g., CSU); Phase 1 SAD/MAD results anticipated at AAAAI Iterative structure-based drug design to rapidly Annual Meeting in March 2026 optimize and validate programs in animal models Portfolio strategy to drive value creation Discovery Stage Validated targets + early clinical readouts + multi-billion $ market opportunities TSHR NAM: Potential disease-modifying treatment for Graves’ disease and TED; progressing multiple lead Well-capitalized compounds toward development candidate selection Cash runway expected to support operating plans at Incretin Receptor Agonists: Potential multi-billion $ least into 2029 collaboration with Novo Nordisk for oral small molecules for metabolic diseases © 2026 SEPTERNA GPCR: G protein-coupled receptor; NAM: negative allosteric modulator; TED: thyroid eye disease; CSU: chronic spontaneous urticaria; AAAAI: American Academy of Allergy, Asthma & Immunology 3
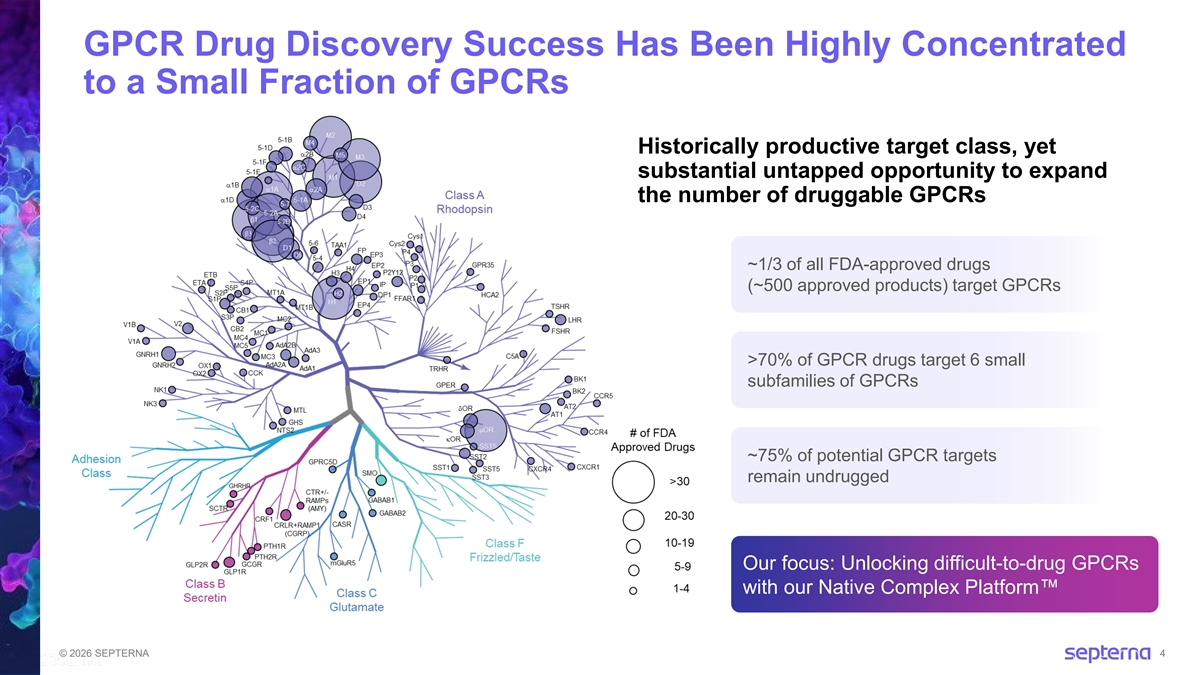
GPCR Drug Discovery Success Has Been Highly Concentrated to a Small Fraction of GPCRs Historically productive target class, yet substantial untapped opportunity to expand the number of druggable GPCRs ~1/3 of all FDA-approved drugs (~500 approved products) target GPCRs >70% of GPCR drugs target 6 small subfamilies of GPCRs ~75% of potential GPCR targets remain undrugged Our focus: Unlocking difficult-to-drug GPCRs with our Native Complex Platform © 2026 SEPTERNA 4
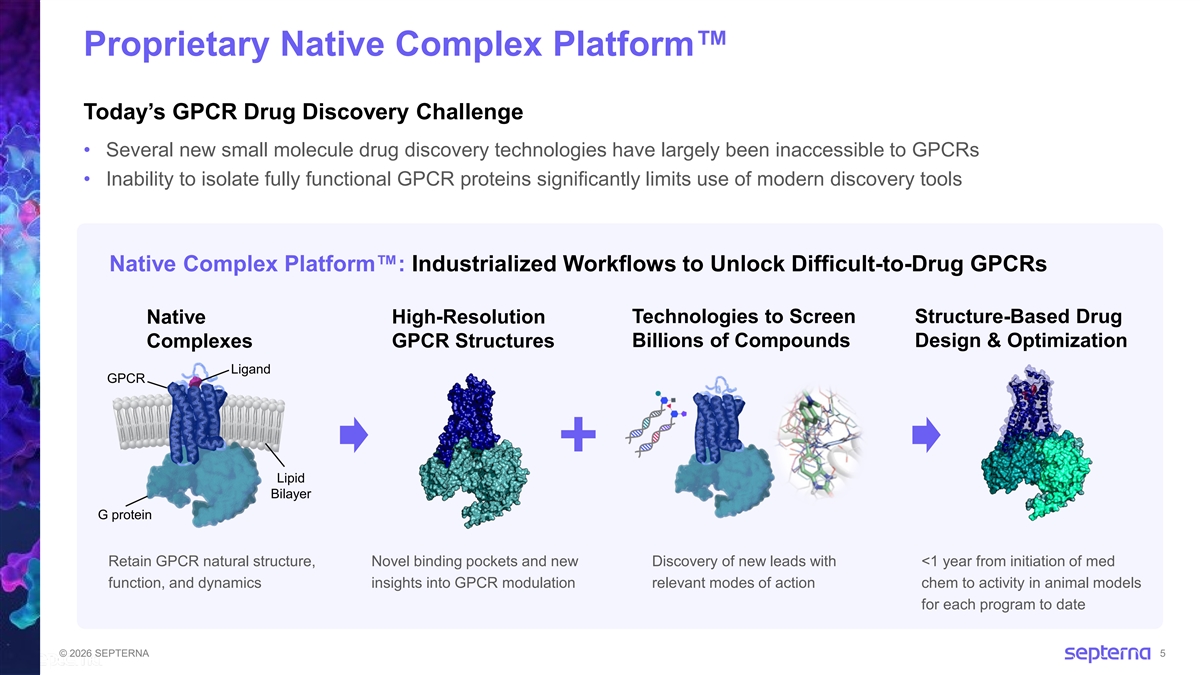
Proprietary Native Complex Platform Today’s GPCR Drug Discovery Challenge • Several new small molecule drug discovery technologies have largely been inaccessible to GPCRs • Inability to isolate fully functional GPCR proteins significantly limits use of modern discovery tools Native Complex Platform : Industrialized Workflows to Unlock Difficult-to-Drug GPCRs Native High-Resolution Technologies to Screen Structure-Based Drug Complexes GPCR Structures Billions of Compounds Design & Optimization Ligand GPCR Lipid Bilayer G protein Retain GPCR natural structure, Novel binding pockets and new Discovery of new leads with <1 year from initiation of med function, and dynamics insights into GPCR modulation relevant modes of action chem to activity in animal models for each program to date © 2026 SEPTERNA 5
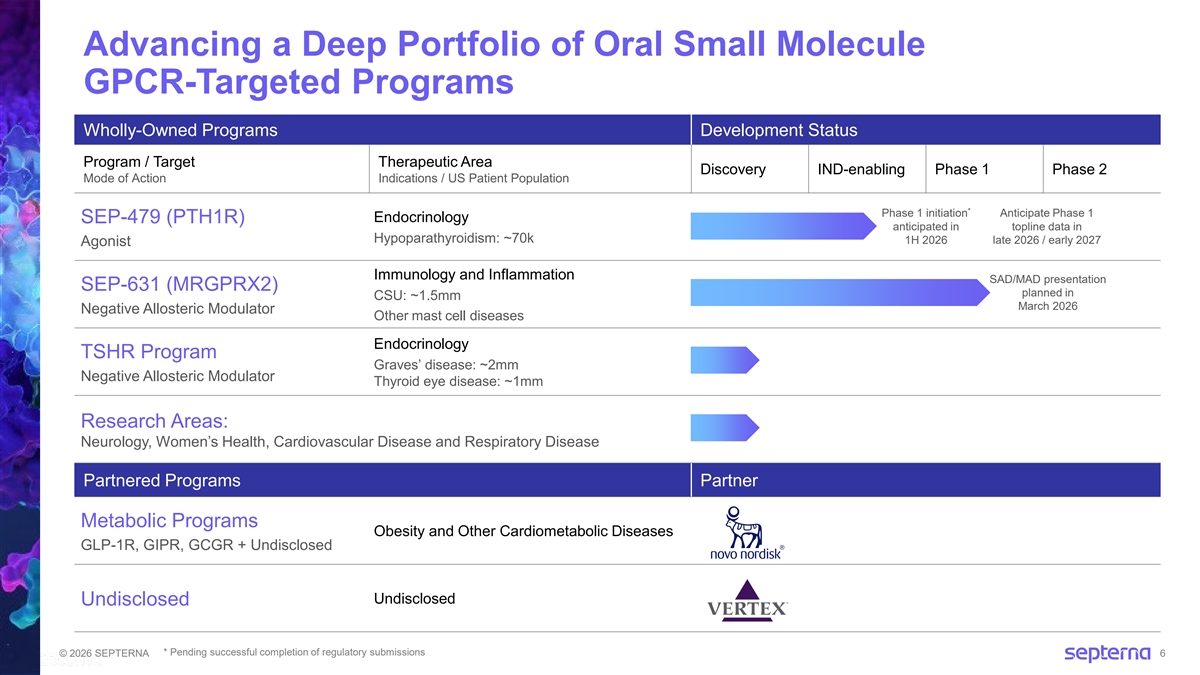
Advancing a Deep Portfolio of Oral Small Molecule GPCR-Targeted Programs Wholly-Owned Programs Development Status Program / Target Therapeutic Area Discovery IND-enabling Phase 1 Phase 2 Mode of Action Indications / US Patient Population * Phase 1 initiation Anticipate Phase 1 Endocrinology SEP-479 (PTH1R) anticipated in topline data in Hypoparathyroidism: ~70k 1H 2026 late 2026 / early 2027 Agonist Immunology and Inflammation SAD/MAD presentation SEP-631 (MRGPRX2) planned in CSU: ~1.5mm March 2026 Negative Allosteric Modulator Other mast cell diseases Endocrinology TSHR Program Graves’ disease: ~2mm Negative Allosteric Modulator Thyroid eye disease: ~1mm Research Areas: Neurology, Women’s Health, Cardiovascular Disease and Respiratory Disease Partnered Programs Partner Metabolic Programs Obesity and Other Cardiometabolic Diseases GLP-1R, GIPR, GCGR + Undisclosed Undisclosed Undisclosed * Pending successful completion of regulatory submissions © 2026 SEPTERNA 6

SEP-479: Oral Small Molecule PTH1R Agonist Targeting PTH1R for Hypoparathyroidism © 2026 SEPTERNA
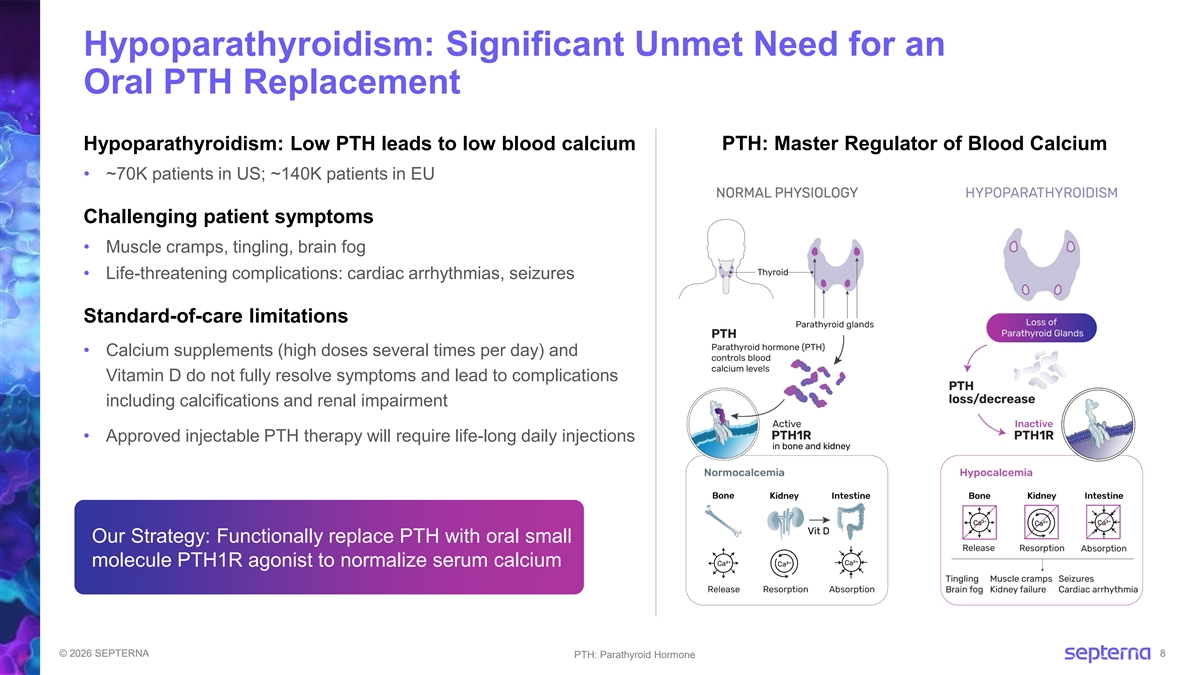
Hypoparathyroidism: Significant Unmet Need for an Oral PTH Replacement Hypoparathyroidism: Low PTH leads to low blood calcium PTH: Master Regulator of Blood Calcium • ~70K patients in US; ~140K patients in EU Challenging patient symptoms • Muscle cramps, tingling, brain fog • Life-threatening complications: cardiac arrhythmias, seizures Standard-of-care limitations • Calcium supplements (high doses several times per day) and Vitamin D do not fully resolve symptoms and lead to complications including calcifications and renal impairment • Approved injectable PTH therapy will require life-long daily injections Our Strategy: Functionally replace PTH with oral small molecule PTH1R agonist to normalize serum calcium © 2026 SEPTERNA 8 PTH: Parathyroid Hormone
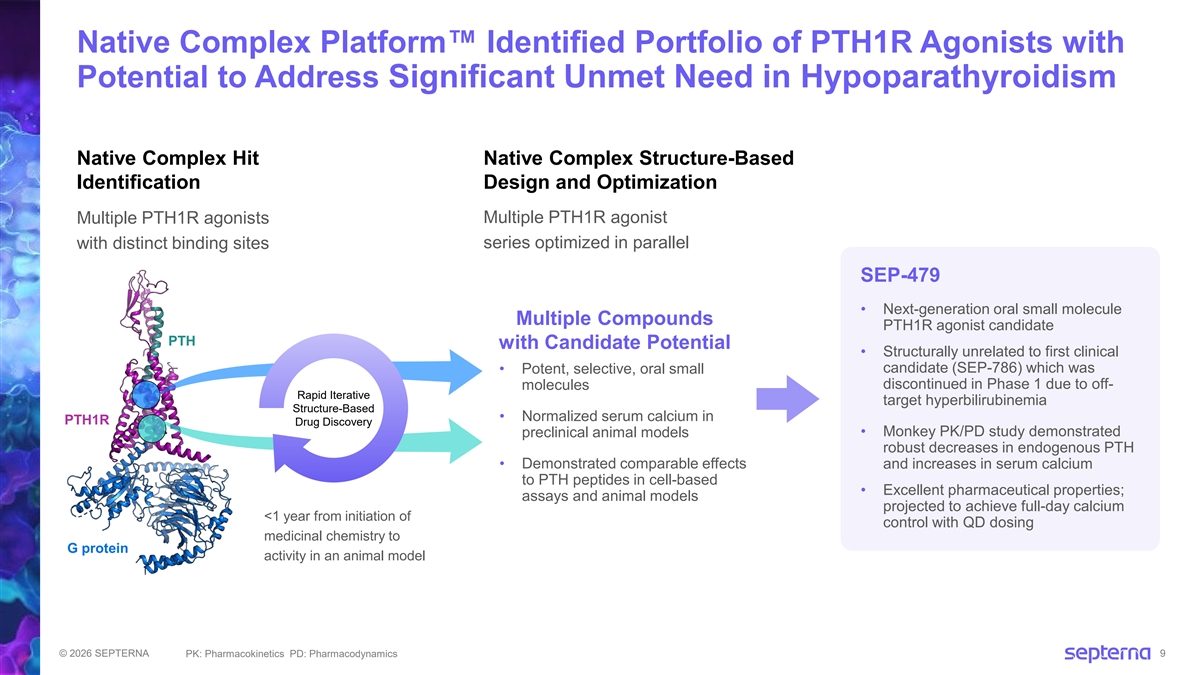
Native Complex Platform Identified Portfolio of PTH1R Agonists with Potential to Address Significant Unmet Need in Hypoparathyroidism Native Complex Hit Native Complex Structure-Based Identification Design and Optimization Multiple PTH1R agonists Multiple PTH1R agonist with distinct binding sites series optimized in parallel SEP-479 • Next-generation oral small molecule Multiple Compounds PTH1R agonist candidate PTH with Candidate Potential • Structurally unrelated to first clinical candidate (SEP-786) which was • Potent, selective, oral small discontinued in Phase 1 due to off- molecules Rapid Iterative target hyperbilirubinemia Structure-Based • Normalized serum calcium in PTH1R Drug Discovery • Monkey PK/PD study demonstrated preclinical animal models robust decreases in endogenous PTH • Demonstrated comparable effects and increases in serum calcium to PTH peptides in cell-based • Excellent pharmaceutical properties; assays and animal models projected to achieve full-day calcium <1 year from initiation of control with QD dosing medicinal chemistry to G protein activity in an animal model © 2026 SEPTERNA PK: Pharmacokinetics PD: Pharmacodynamics 9
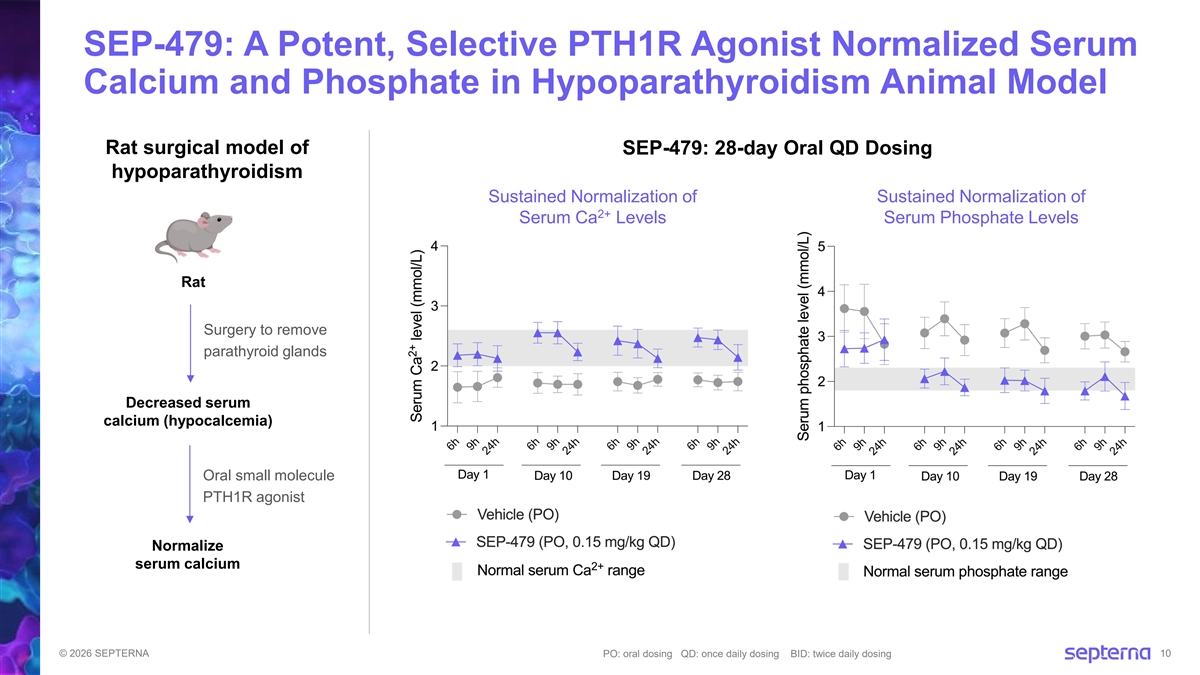
SEP-479: A Potent, Selective PTH1R Agonist Normalized Serum Calcium and Phosphate in Hypoparathyroidism Animal Model Rat surgical model of SEP-479: 28-day Oral QD Dosing hypoparathyroidism Sustained Normalization of Sustained Normalization of 2+ Serum Ca Levels Serum Phosphate Levels Rat Surgery to remove parathyroid glands Decreased serum calcium (hypocalcemia) Oral small molecule PTH1R agonist Normalize serum calcium © 2026 SEPTERNA PO: oral dosing QD: once daily dosing BID: twice daily dosing 10
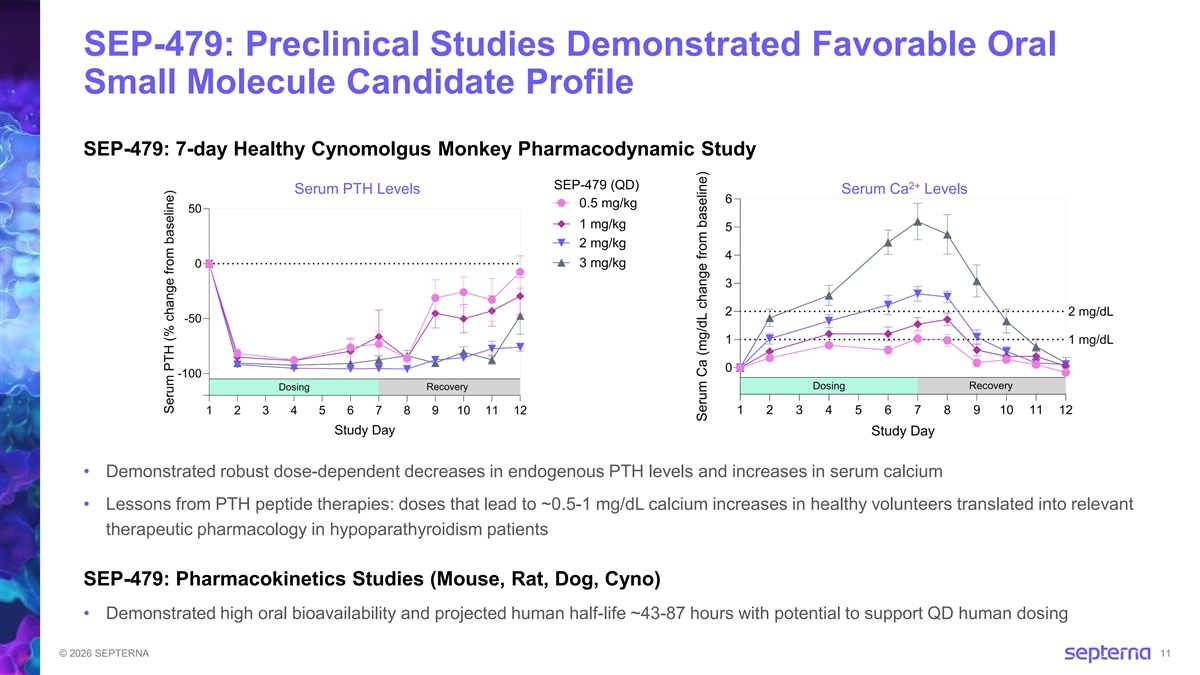
SEP-479: Preclinical Studies Demonstrated Favorable Oral Small Molecule Candidate Profile SEP-479: 7-day Healthy Cynomolgus Monkey Pharmacodynamic Study 2+ Serum PTH Levels Serum Ca Levels • Demonstrated robust dose-dependent decreases in endogenous PTH levels and increases in serum calcium • Lessons from PTH peptide therapies: doses that lead to ~0.5-1 mg/dL calcium increases in healthy volunteers translated into relevant therapeutic pharmacology in hypoparathyroidism patients SEP-479: Pharmacokinetics Studies (Mouse, Rat, Dog, Cyno) • Demonstrated high oral bioavailability and projected human half-life ~43-87 hours with potential to support QD human dosing © 2026 SEPTERNA 11

SEP-479: On Track to Initiate Phase 1 in 1H 2026 Non-clinical safety studies have completed • 28-day GLP toxicology studies completed in rats, dogs, and cynomolgus monkeys • SEP-479 was generally well tolerated in all non-clinical safety studies • Dose-limiting effect in each species was on-target hypercalcemia, as expected for a PTH1R agonist • No hyperbilirubinemia or inhibition of UGT1A1 was observed in any of the non-clinical studies, even at supratherapeutic doses Drug product manufacturing for Phase 1 trial is on track Phase 1 healthy volunteer SAD / MAD trial planned for initiation in Australia in 1H 2026 • Goal: Evaluate the safety, tolerability, PK and PD of oral SEP-479 in healthy adult volunteers • PD activity to be assessed by decreases in endogenous PTH levels and increases in serum calcium • Anticipate topline data in late 2026 / early 2027 © 2026 SEPTERNA 12

SEP-631: Oral Small Molecule MRGPRX2 NAM Targeting MRGPRX2 for Mast Cell-Driven Diseases, Including CSU © 2026 SEPTERNA
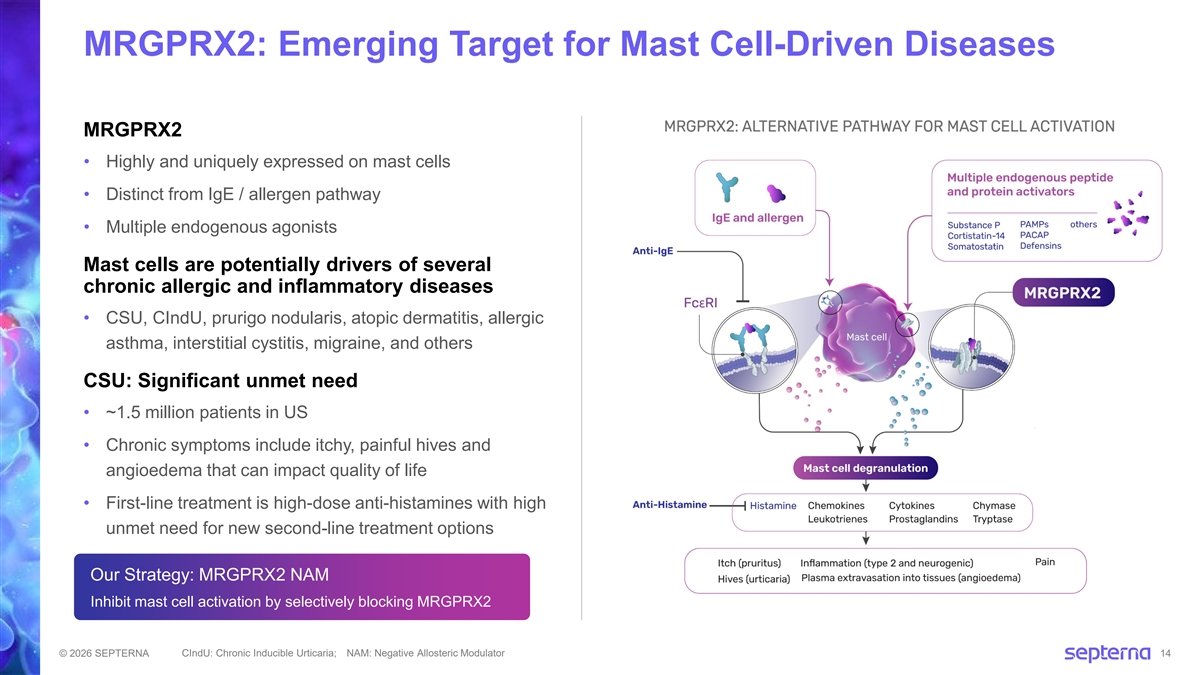
MRGPRX2: Emerging Target for Mast Cell-Driven Diseases MRGPRX2 • Highly and uniquely expressed on mast cells • Distinct from IgE / allergen pathway • Multiple endogenous agonists Mast cells are potentially drivers of several chronic allergic and inflammatory diseases • CSU, CIndU, prurigo nodularis, atopic dermatitis, allergic asthma, interstitial cystitis, migraine, and others CSU: Significant unmet need • ~1.5 million patients in US • Chronic symptoms include itchy, painful hives and angioedema that can impact quality of life • First-line treatment is high-dose anti-histamines with high unmet need for new second-line treatment options Our Strategy: MRGPRX2 NAM Inhibit mast cell activation by selectively blocking MRGPRX2 CIndU: Chronic Inducible Urticaria; NAM: Negative Allosteric Modulator © 2026 SEPTERNA 14
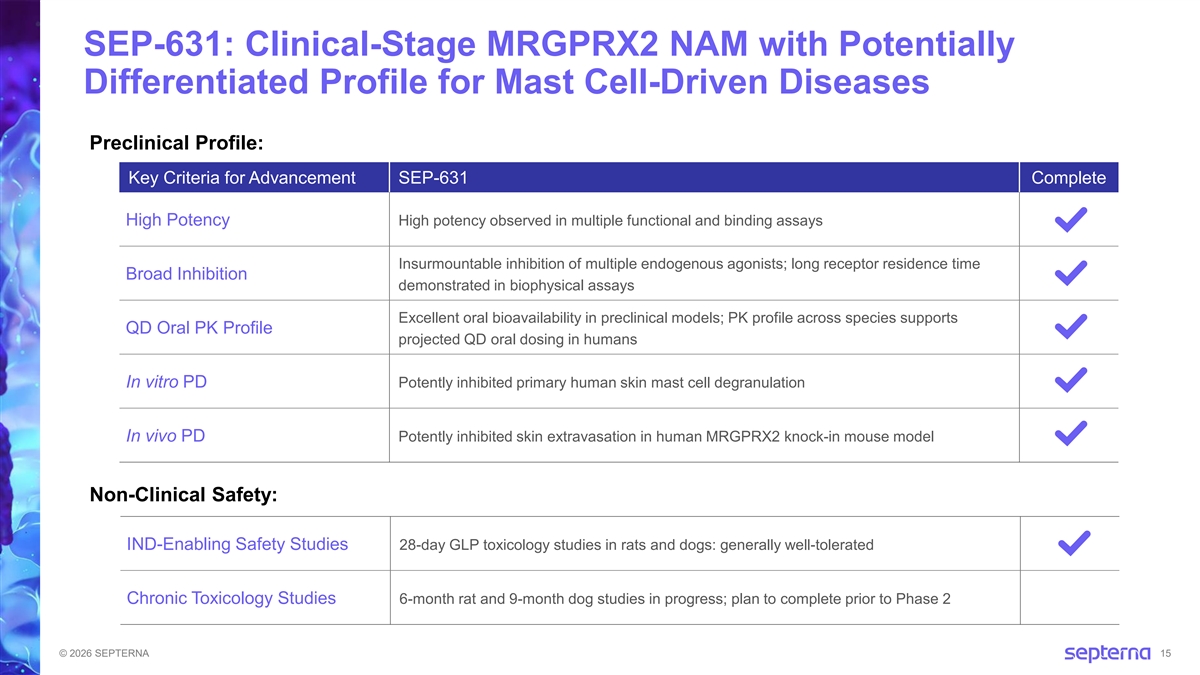
SEP-631: Clinical-Stage MRGPRX2 NAM with Potentially Differentiated Profile for Mast Cell-Driven Diseases Preclinical Profile: Key Criteria for Advancement SEP-631 Complete High Potency High potency observed in multiple functional and binding assays Insurmountable inhibition of multiple endogenous agonists; long receptor residence time Broad Inhibition demonstrated in biophysical assays Excellent oral bioavailability in preclinical models; PK profile across species supports QD Oral PK Profile projected QD oral dosing in humans In vitro PD Potently inhibited primary human skin mast cell degranulation In vivo PD Potently inhibited skin extravasation in human MRGPRX2 knock-in mouse model Non-Clinical Safety: IND-Enabling Safety Studies 28-day GLP toxicology studies in rats and dogs: generally well-tolerated Chronic Toxicology Studies 6-month rat and 9-month dog studies in progress; plan to complete prior to Phase 2 © 2026 SEPTERNA 15
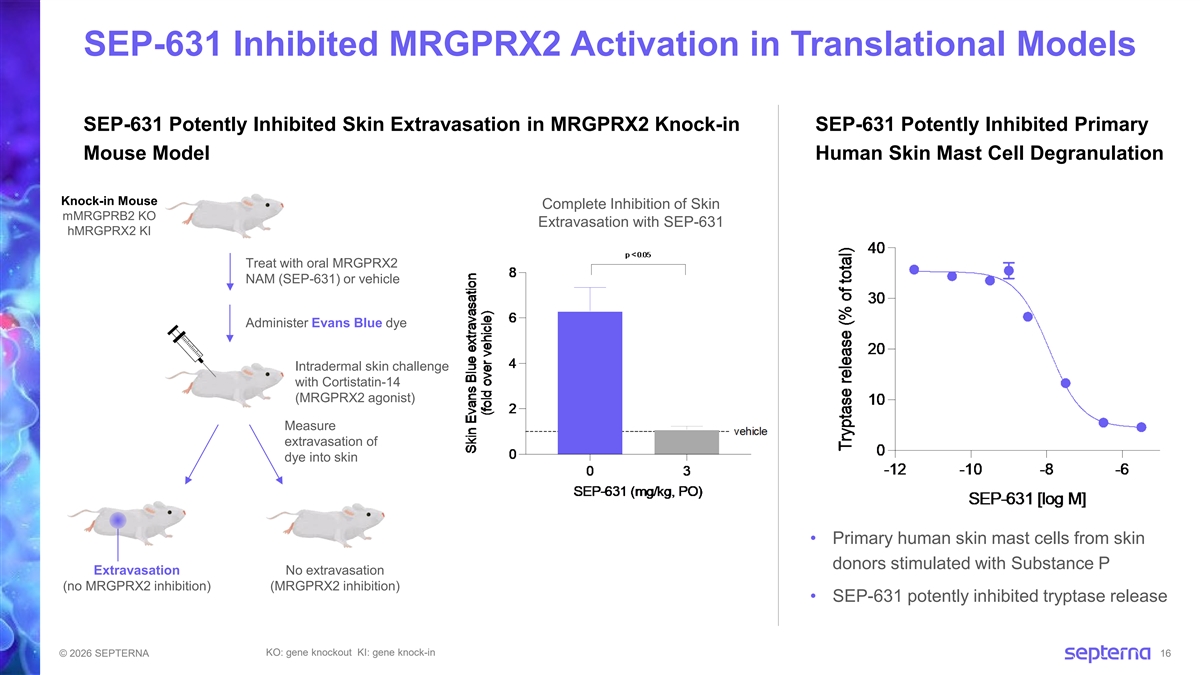
SEP-631 Inhibited MRGPRX2 Activation in Translational Models SEP-631 Potently Inhibited Skin Extravasation in MRGPRX2 Knock-in SEP-631 Potently Inhibited Primary Mouse Model Human Skin Mast Cell Degranulation Knock-in Mouse Complete Inhibition of Skin mMRGPRB2 KO Extravasation with SEP-631 hMRGPRX2 KI Treat with oral MRGPRX2 NAM (SEP-631) or vehicle Administer Evans Blue dye Intradermal skin challenge with Cortistatin-14 (MRGPRX2 agonist) Measure extravasation of dye into skin • Primary human skin mast cells from skin donors stimulated with Substance P Extravasation No extravasation (no MRGPRX2 inhibition) (MRGPRX2 inhibition) • SEP-631 potently inhibited tryptase release KO: gene knockout KI: gene knock-in © 2026 SEPTERNA 16

SEP-631 Phase 1 Trial is on Track – Presentation of SAD / MAD Results Planned for AAAAI Annual Meeting in March 2026 Phase 1 Goal: Evaluate the safety, tolerability, PK and PD of oral SEP-631 in healthy adult volunteers Study design: • Randomized, placebo-controlled, single ascending dose (SAD) and multiple ascending dose (MAD) trial • Oral tablets; QD dosing in MAD x 10 days Intradermal skin challenge to assess early pharmacological activity in all MAD cohorts • Icatibant induces wheal response • Wheal response measured both prior to and following SEP-631 dosing Skin Challenge (Pre-dose) Skin Challenge Objective (Post-dose) SEP-631 dosing L L H H N QD x 9 days N P P L = low-dose icatibant; H = high-dose icatibant; N = negative control (saline); P = positive control (histamine) AAAAI: American Academy of Allergy, Asthma & Immunology © 2026 SEPTERNA 17

TSHR NAM Program Oral Small Molecule Targeting TSHR for Graves’ Disease and TED © 2026 SEPTERNA
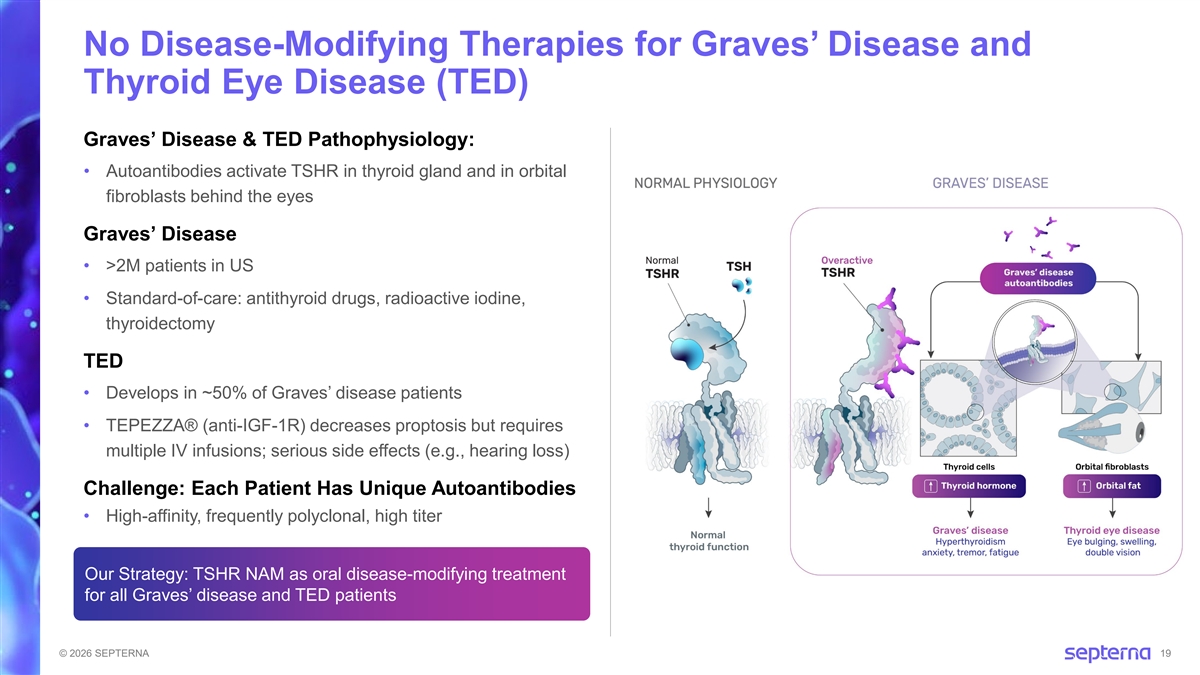
No Disease-Modifying Therapies for Graves’ Disease and Thyroid Eye Disease (TED) Graves’ Disease & TED Pathophysiology: • Autoantibodies activate TSHR in thyroid gland and in orbital fibroblasts behind the eyes Graves’ Disease • >2M patients in US • Standard-of-care: antithyroid drugs, radioactive iodine, thyroidectomy TED • Develops in ~50% of Graves’ disease patients • TEPEZZA® (anti-IGF-1R) decreases proptosis but requires multiple IV infusions; serious side effects (e.g., hearing loss) Challenge: Each Patient Has Unique Autoantibodies • High-affinity, frequently polyclonal, high titer Our Strategy: TSHR NAM as oral disease-modifying treatment for all Graves’ disease and TED patients © 2026 SEPTERNA 19
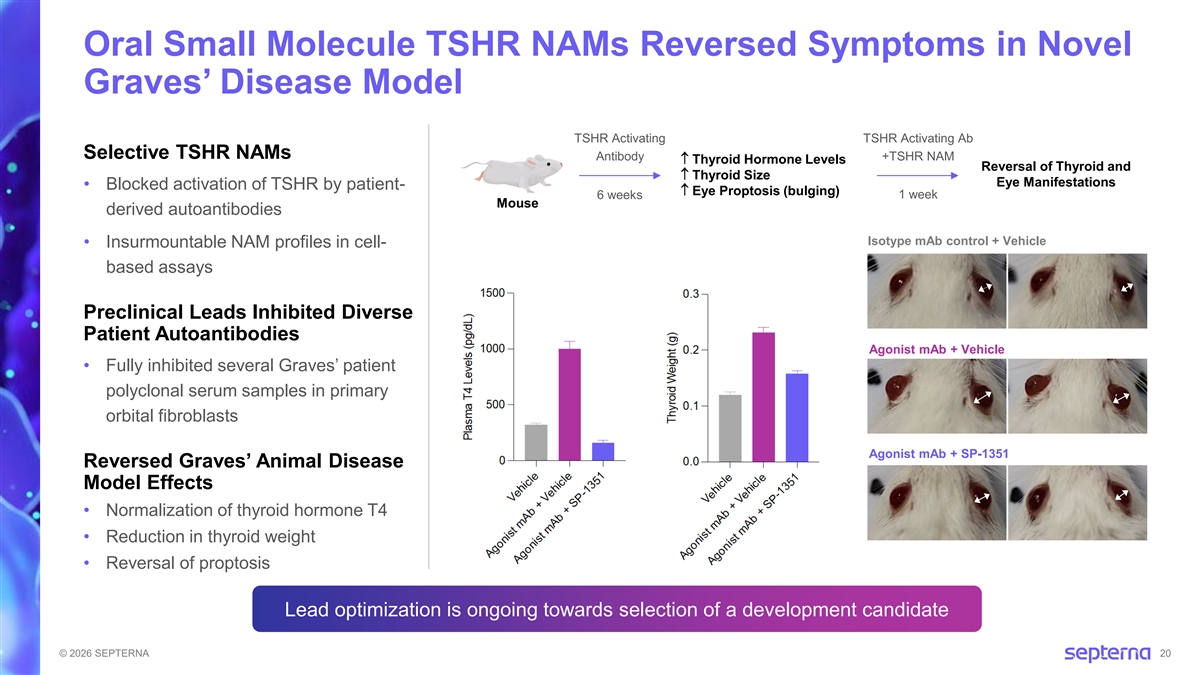
Oral Small Molecule TSHR NAMs Reversed Symptoms in Novel Graves’ Disease Model TSHR Activating TSHR Activating Ab Selective TSHR NAMs Antibody +TSHR NAM ↑ Thyroid Hormone Levels Reversal of Thyroid and ↑ Thyroid Size Eye Manifestations • Blocked activation of TSHR by patient- ↑ Eye Proptosis (bulging) 6 weeks 1 week Mouse derived autoantibodies Isotype mAb control + Vehicle • Insurmountable NAM profiles in cell- based assays Preclinical Leads Inhibited Diverse Patient Autoantibodies Agonist mAb + Vehicle • Fully inhibited several Graves’ patient polyclonal serum samples in primary orbital fibroblasts Agonist mAb + SP-1351 Reversed Graves’ Animal Disease Model Effects • Normalization of thyroid hormone T4 • Reduction in thyroid weight • Reversal of proptosis Lead optimization is ongoing towards selection of a development candidate © 2026 SEPTERNA 20

Metabolic Programs Oral Small Molecule Programs Targeting GLP-1R, GIPR, GCGR and Other Targets for Obesity, Diabetes, and Other Cardiometabolic Diseases © 2026 SEPTERNA
Collaboration with Novo Nordisk for Oral Small Molecules for Metabolic Diseases Septerna and Novo commenced four initial R&D programs targeting five GPCRs • Includes GLP-1, GIP and glucagon receptors • Collaboration includes Septerna’s preclinical, selective, oral, small molecule GIP receptor agonists Potential multi-billion $ opportunity • $195M upfront payment received in July ‘25 • ~$500M in R&D, regulatory and commercial milestones for each program Collaboration Objective: discover, develop • Mid-to-high single-digit tiered royalties based on global and commercialize multiple novel mono-, dual-, product sales or triple-acting oral small molecule drug • Opt-in right for worldwide profit-share for one program candidates directed to obesity, type 2 diabetes and other cardiometabolic diseases Novo responsible for coverage of all collaboration R&D expenses © 2026 SEPTERNA 22
Building a World-Class GPCR-Focused Biotechnology Company © 2026 SEPTERNA

Proven Leaders in GPCR Drug Development and Company Building Senior Leadership Dan Long, DPhil Jeff Finer, MD PhD Liz Bhatt, MS MBA Jae Kim, MD Gil Labrucherie, CFA JD Uwe Klein, PhD Samira Shaikhly Mark Wilson, JD CEO President & COO CMO CFO SVP Biological Sciences SVP Drug Discovery CPO CLO Board of Directors Academic Co-Founders • Jeff Finer, MD, PhD, CEO • Robert J. Lefkowitz, MD, Duke University Medical Center • Arthur Christopoulos, PhD, Monash University • Jeff Tong, PhD, Third Rock Ventures • Patrick Sexton, PhD, DSc, Monash University • Alan Ezekowitz, MD, DPhil, Third Rock Ventures • Abe Bassan, Samsara BioCapital Drug Discovery Advisory Board • Jake Simson, PhD, RA Capital • Ruth Wexler, PhD, formerly with BMS • Bernard Coulie, MD, PhD, MBA, Independent Director • John Lowe, PhD, formerly with Pfizer • Shalini Sharp, MBA, Independent Director • Craig Lindsley, PhD, Vanderbilt, formerly with Merck • Keith Gottesdiener, MD, Independent Director • Tom Baillie, PhD, DSc, formerly with Merck • David Lacey, MD, formerly with Amgen © 2026 SEPTERNA 24
Septerna: Pioneering a New Era of GPCR Drug Discovery Wholly-Owned Programs Development Status • Portfolio of oral small molecule Program / Target Discovery IND-enabling Phase 1 Phase 2 GPCR-targeted programs Mode of Action * Phase 1 initiation Anticipate Phase 1 SEP-479 (PTH1R) anticipated in topline data in • Multi-product pipeline, each with a 1H 2026 late 2026 / early 2027 Agonist multi-billion $ market opportunity SAD/MAD SEP-631 (MRGPRX2) presentation planned in Negative Allosteric Modulator March 2026 • Native Complex Platform drives TSHR Program rapid compound identification and Negative Allosteric Modulator portfolio expansion Partnered Programs Partner • Well-capitalized with cash runway Metabolic Programs Obesity and Other Cardiometabolic GLP-1R, GIPR, GCGR + expected to support operating plans Diseases Undisclosed at least into 2029 Undisclosed Undisclosed * Pending successful completion of regulatory submissions © 2026 SEPTERNA 25
Pioneering a New Era of GPCR Drug Discovery © 2026 SEPTERNA

Appendix © 2026 SEPTERNA
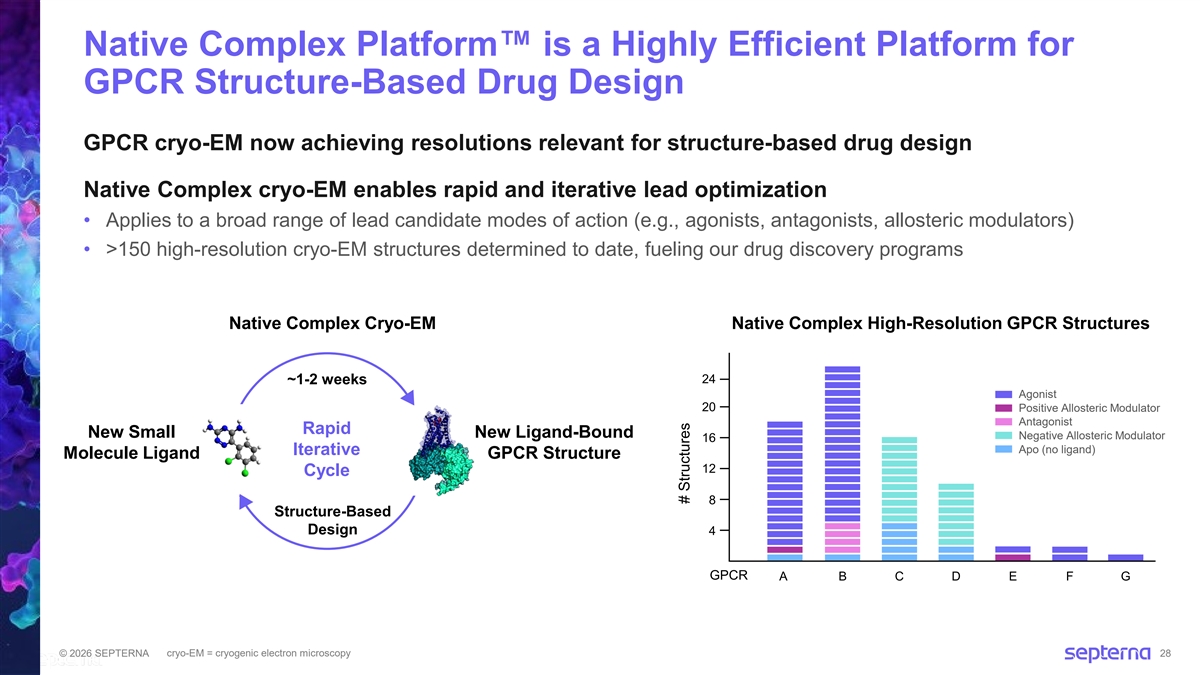
Native Complex Platform is a Highly Efficient Platform for GPCR Structure-Based Drug Design GPCR cryo-EM now achieving resolutions relevant for structure-based drug design Native Complex cryo-EM enables rapid and iterative lead optimization • Applies to a broad range of lead candidate modes of action (e.g., agonists, antagonists, allosteric modulators) • >150 high-resolution cryo-EM structures determined to date, fueling our drug discovery programs Native Complex Cryo-EM Native Complex High-Resolution GPCR Structures 24 ~1-2 weeks Agonist 20 Positive Allosteric Modulator Antagonist Rapid New Small New Ligand-Bound Negative Allosteric Modulator 16 Apo (no ligand) Iterative Molecule Ligand GPCR Structure 12 Cycle 8 Structure-Based Design 4 GPCR A B C D E F G cryo-EM = cryogenic electron microscopy © 2026 SEPTERNA 28 # Structures
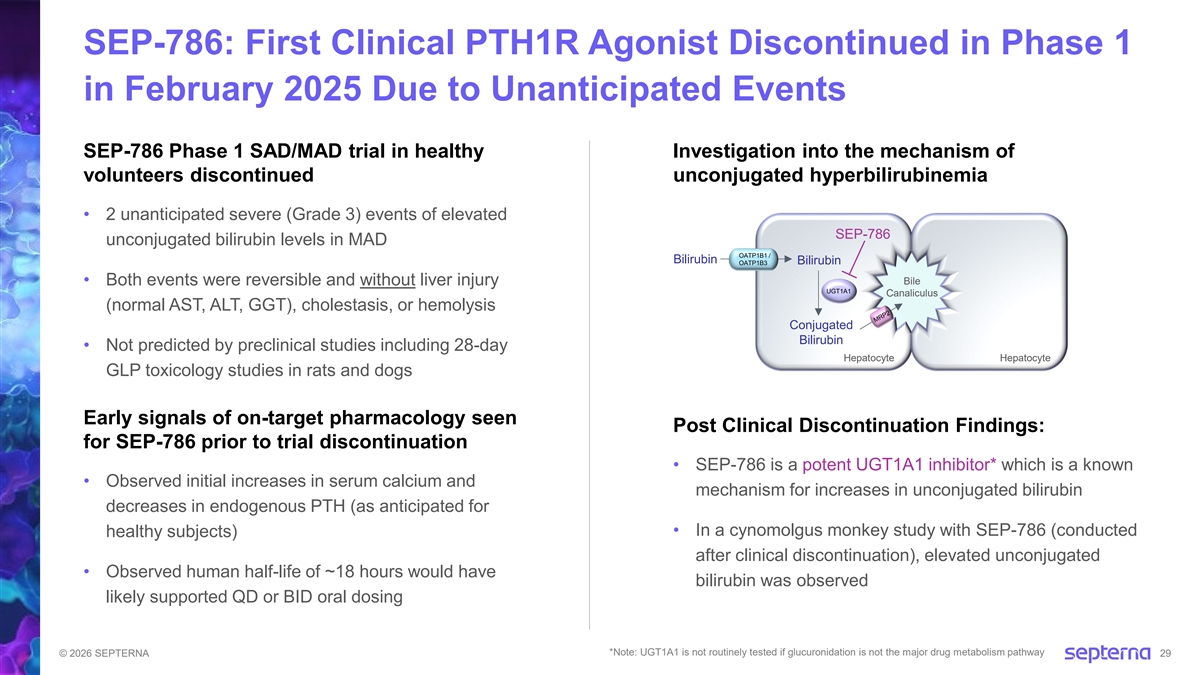
SEP-786: First Clinical PTH1R Agonist Discontinued in Phase 1 in February 2025 Due to Unanticipated Events SEP-786 Phase 1 SAD/MAD trial in healthy Investigation into the mechanism of volunteers discontinued unconjugated hyperbilirubinemia • 2 unanticipated severe (Grade 3) events of elevated SEP-786 unconjugated bilirubin levels in MAD OATP1B1 / Bilirubin OATP1B3 Bilirubin Bile • Both events were reversible and without liver injury UGT1A1 Canaliculus (normal AST, ALT, GGT), cholestasis, or hemolysis Conjugated Bilirubin • Not predicted by preclinical studies including 28-day Hepatocyte Hepatocyte GLP toxicology studies in rats and dogs Early signals of on-target pharmacology seen Post Clinical Discontinuation Findings: for SEP-786 prior to trial discontinuation • SEP-786 is a potent UGT1A1 inhibitor* which is a known • Observed initial increases in serum calcium and mechanism for increases in unconjugated bilirubin decreases in endogenous PTH (as anticipated for • In a cynomolgus monkey study with SEP-786 (conducted healthy subjects) after clinical discontinuation), elevated unconjugated • Observed human half-life of ~18 hours would have bilirubin was observed likely supported QD or BID oral dosing *Note: UGT1A1 is not routinely tested if glucuronidation is not the major drug metabolism pathway © 2026 SEPTERNA 29
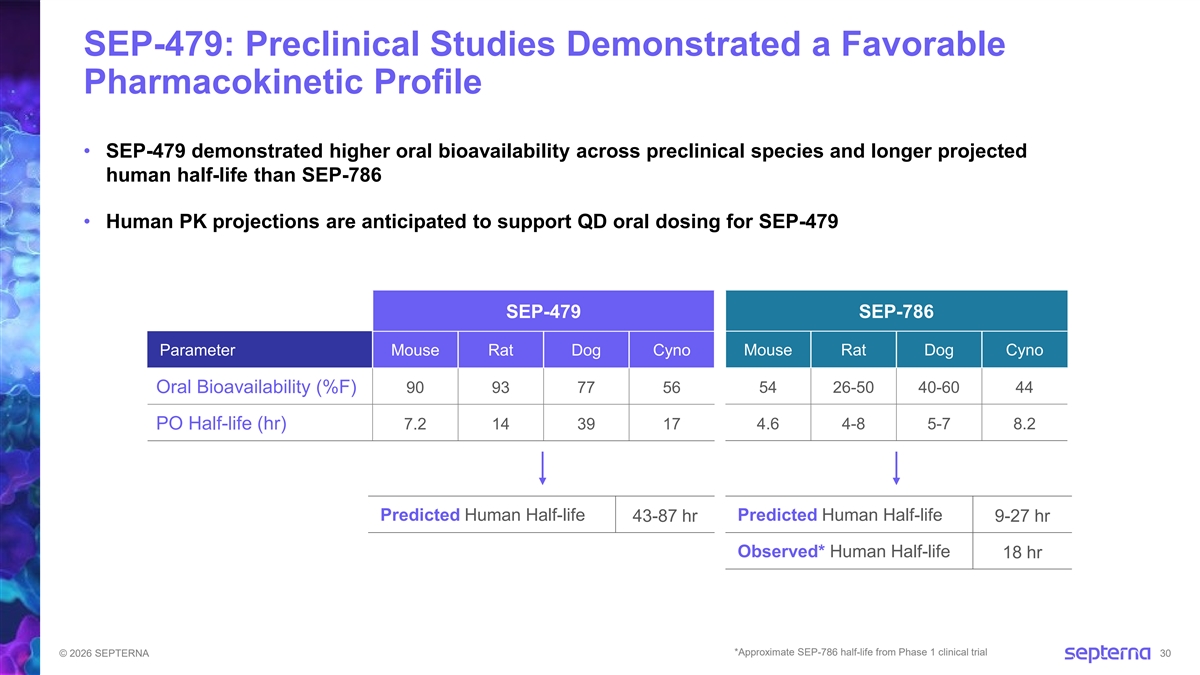
SEP-479: Preclinical Studies Demonstrated a Favorable Pharmacokinetic Profile • SEP-479 demonstrated higher oral bioavailability across preclinical species and longer projected human half-life than SEP-786 • Human PK projections are anticipated to support QD oral dosing for SEP-479 SEP-479 SEP-786 Parameter Mouse Rat Dog Cyno Mouse Rat Dog Cyno 54 26-50 40-60 44 Oral Bioavailability (%F) 90 93 77 56 PO Half-life (hr) 7.2 14 39 17 4.6 4-8 5-7 8.2 Predicted Human Half-life Predicted Human Half-life 43-87 hr 9-27 hr Observed* Human Half-life 18 hr *Approximate SEP-786 half-life from Phase 1 clinical trial © 2026 SEPTERNA 30