

Attacking Bladder Cancer for a Better Tomorrow™

Disclaimer and Forward-Looking Statements We caution you that this presentation contains forward-looking statements about us and our industry. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, research and development plans, the anticipated timing, costs, design and conduct of our ongoing and planned clinical trials and preclinical studies for cretostimogene and any future product candidates, the timing and likelihood of regulatory filings and approvals for cretostimogene and any future product candidates, our ability to commercialize cretostimogene and any future product candidates, if approved, the pricing and reimbursement of cretostimogene and any future product candidates, if approved, the potential to develop future product candidates, the potential benefits of strategic collaborations and potential to enter into any future strategic arrangements, the timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated product development efforts, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: we currently depend entirely on the success of cretostimogene, which is our only product candidate and is based on a novel approach to the treatment of cancer; potential delays in the commencement, enrollment, and completion of clinical trials and preclinical studies; results from earlier clinical trials and preclinical studies not necessarily being predictive of future results; unfavorable results from clinical trials; unexpected adverse side effects or inadequate efficacy of cretostimogene that may limit its development, regulatory approval, and/or commercialization; preliminary or interim data results are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data and more patient data become available; our dependence on third parties in connection with manufacturing, shipping and clinical and preclinical testing; regulatory developments in the United States and foreign countries; our ability to obtain, maintain and enforce intellectual property protection for cretostimogene; we may use our capital resources sooner than we expect; we face significant competition; and other risks described in our filings with the SEC, including under the heading “Risk Factors” in our annual report on Form 10-K, our quarterly Form 10-Q and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation, and except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. Cretostimogene grenadenorepvec is an investigational engineered oncolytic immunotherapy (OIT). It is an investigational drug and is not approved by any regulatory agency. Its safety and efficacy has not been established. In BCG-unresponsive, Non-Muscle Invasive Bladder Cancer (NMIBC), cretostimogene has shown clinical benefit and has been generally well-tolerated as both a monotherapy and in combination with other therapies in clinical trials. Trade names, trademarks and service marks of other companies appearing in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names referred to in this presentation appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or that the applicable owner will not assert its rights to these trademarks and tradenames.
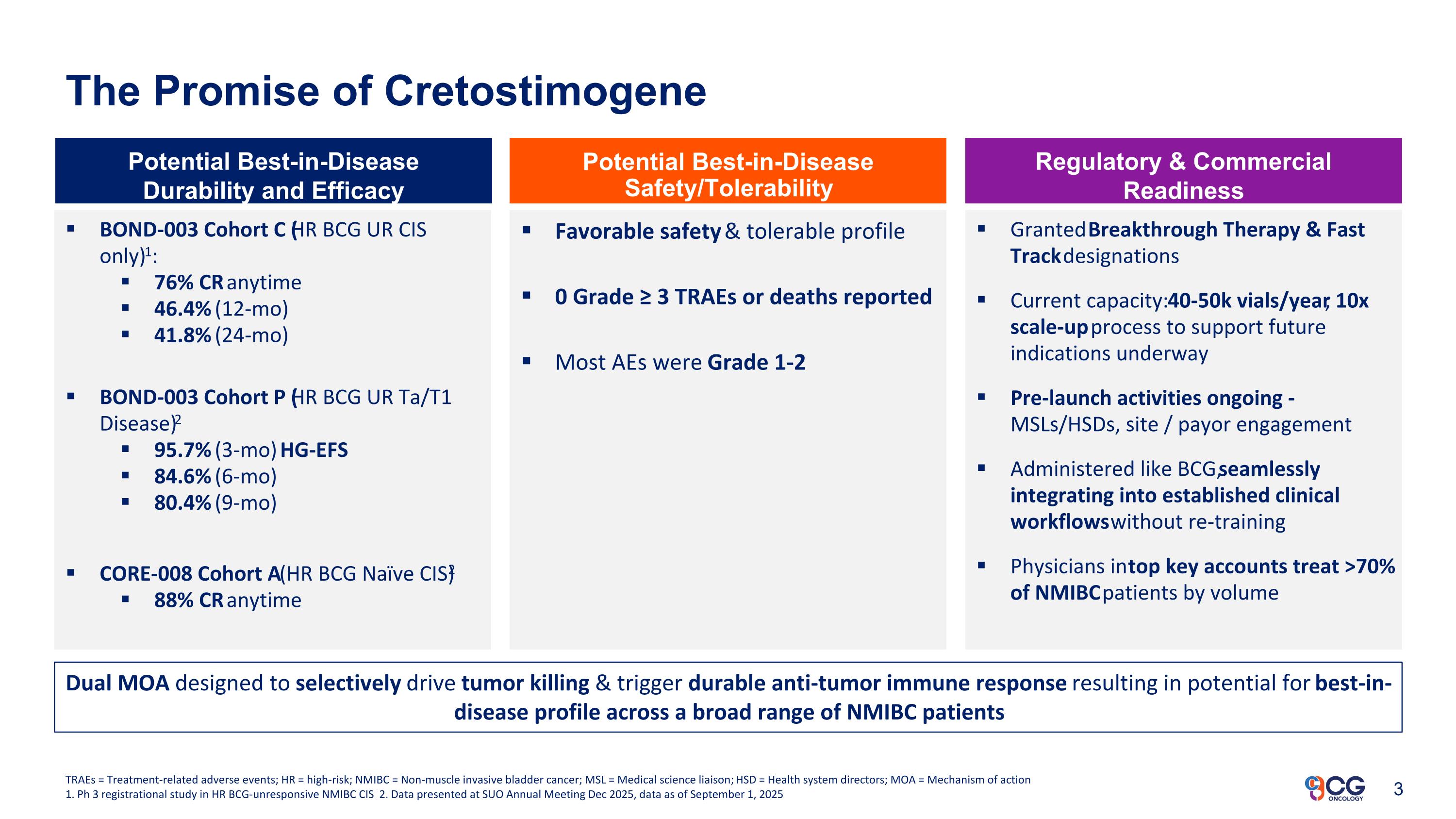
The Promise of Cretostimogene BOND-003 Cohort C (HR BCG UR CIS only)1: 76% CR anytime 46.4% (12-mo) 41.8% (24-mo) BOND-003 Cohort P (HR BCG UR Ta/T1 Disease)2 95.7% (3-mo) HG-EFS 84.6% (6-mo) 80.4% (9-mo) CORE-008 Cohort A (HR BCG Naïve CIS)2 88% CR anytime Potential Best-in-Disease Durability and Efficacy Potential Best-in-Disease Safety/Tolerability Regulatory & Commercial Readiness Favorable safety & tolerable profile 0 Grade ≥ 3 TRAEs or deaths reported Most AEs were Grade 1-2 Granted Breakthrough Therapy & Fast Track designations Current capacity: 40-50k vials/year; 10x scale-up process to support future indications underway Pre-launch activities ongoing - MSLs/HSDs, site / payor engagement Administered like BCG, seamlessly integrating into established clinical workflows without re-training Physicians in top key accounts treat >70% of NMIBC patients by volume TRAEs = Treatment-related adverse events; HR = high-risk; NMIBC = Non-muscle invasive bladder cancer; MSL = Medical science liaison; HSD = Health system directors; MOA = Mechanism of action 1. Ph 3 registrational study in HR BCG-unresponsive NMIBC CIS 2. Data presented at SUO Annual Meeting Dec 2025, data as of September 1, 2025 Dual MOA designed to selectively drive tumor killing & trigger durable anti-tumor immune response resulting in potential for best-in-disease profile across a broad range of NMIBC patients
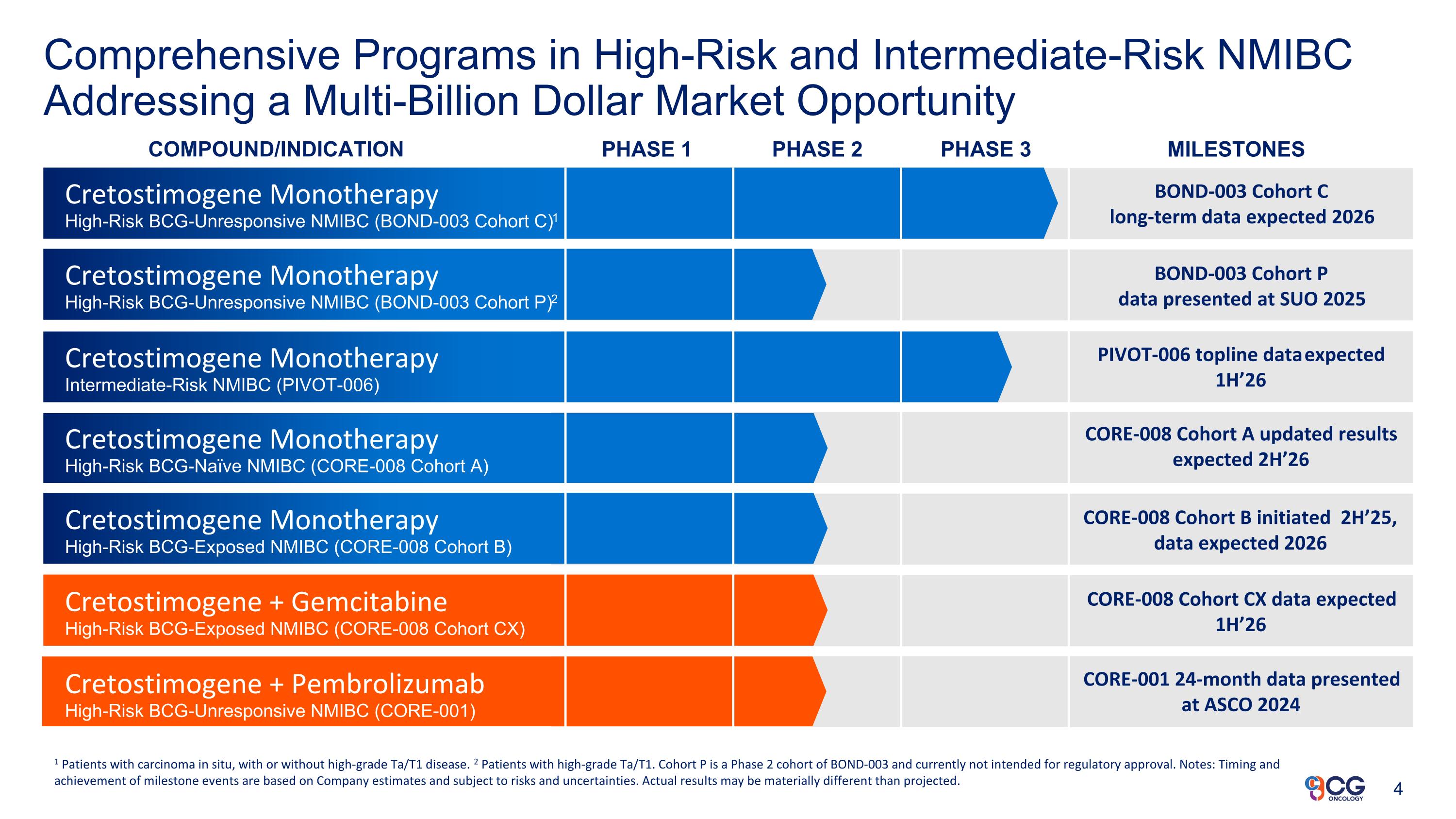
Comprehensive Programs in High-Risk and Intermediate-Risk NMIBC Addressing a Multi-Billion Dollar Market Opportunity COMPOUND/INDICATION PHASE 1 PHASE 2 PHASE 3 MILESTONES Cretostimogene Monotherapy High-Risk BCG-Unresponsive NMIBC (BOND-003 Cohort C)1 Cretostimogene Monotherapy Intermediate-Risk NMIBC (PIVOT-006) Cretostimogene Monotherapy High-Risk BCG-Naïve NMIBC (CORE-008 Cohort A) Cretostimogene Monotherapy High-Risk BCG-Exposed NMIBC (CORE-008 Cohort B) 1 Patients with carcinoma in situ, with or without high-grade Ta/T1 disease. 2 Patients with high-grade Ta/T1. Cohort P is a Phase 2 cohort of BOND-003 and currently not intended for regulatory approval. Notes: Timing and achievement of milestone events are based on Company estimates and subject to risks and uncertainties. Actual results may be materially different than projected. BOND-003 Cohort C long-term data expected 2026 PIVOT-006 topline data expected 1H’26 Cretostimogene + Pembrolizumab High-Risk BCG-Unresponsive NMIBC (CORE-001) BOND-003 Cohort P data presented at SUO 2025 Cretostimogene Monotherapy High-Risk BCG-Unresponsive NMIBC (BOND-003 Cohort P)2 CORE-008 Cohort A updated results expected 2H’26 CORE-008 Cohort B initiated 2H’25, data expected 2026 CORE-008 Cohort CX data expected 1H’26 CORE-001 24-month data presented at ASCO 2024 Cretostimogene + Gemcitabine High-Risk BCG-Exposed NMIBC (CORE-008 Cohort CX)
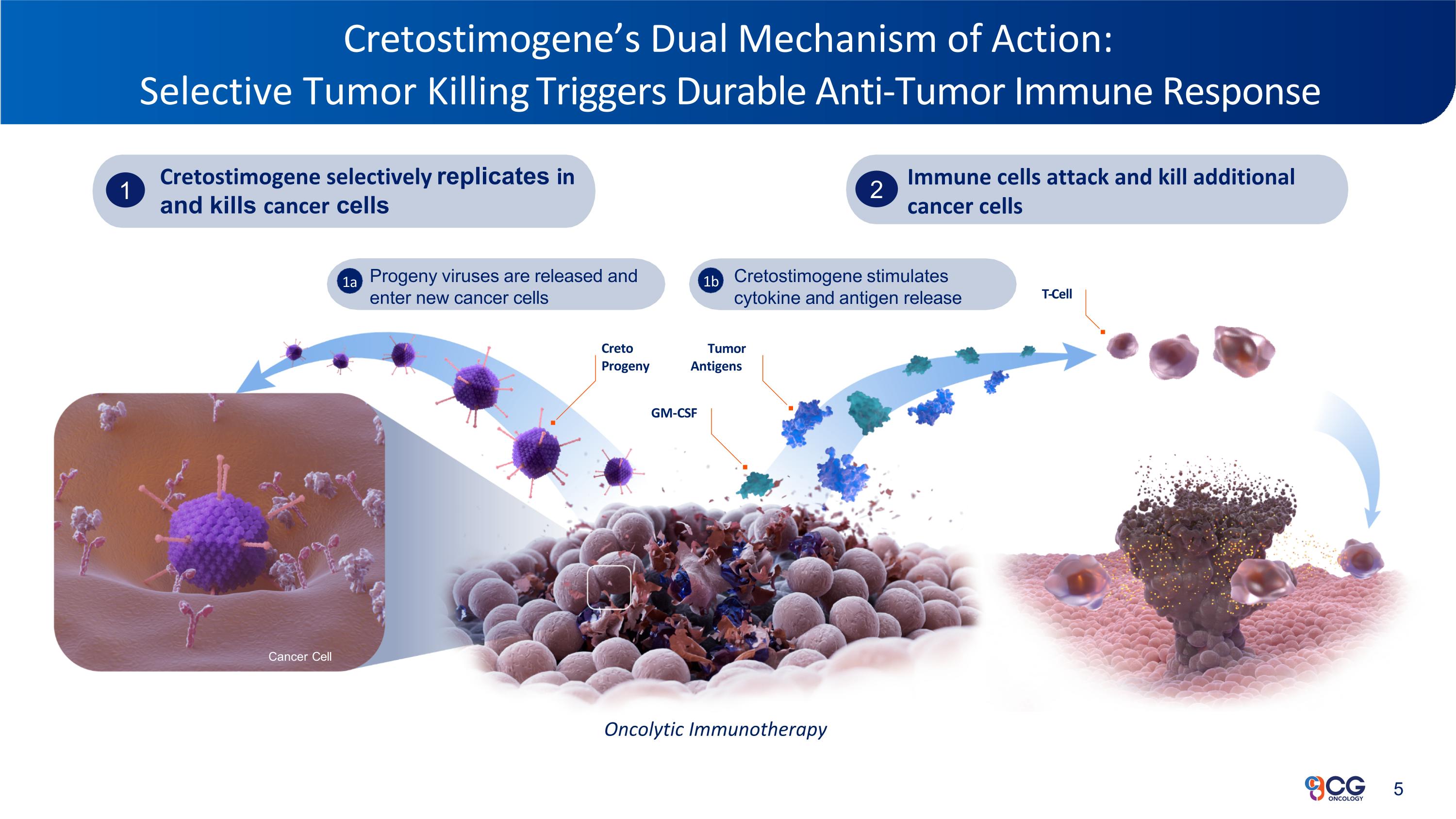
Cretostimogene’s Dual Mechanism of Action: Selective Tumor Killing Triggers Durable Anti-Tumor Immune Response Progeny viruses are released and enter new cancer cells Cretostimogene stimulates cytokine and antigen release 1a 1b Attacking Bladder Cancer for a Better Tomorrow™ Cancer Cell Creto Progeny Tumor Antigens GM-CSF Cretostimogene selectively replicates in and kills cancer cells 1 T-Cell Immune cells attack and kill additional cancer cells 2 Oncolytic Immunotherapy
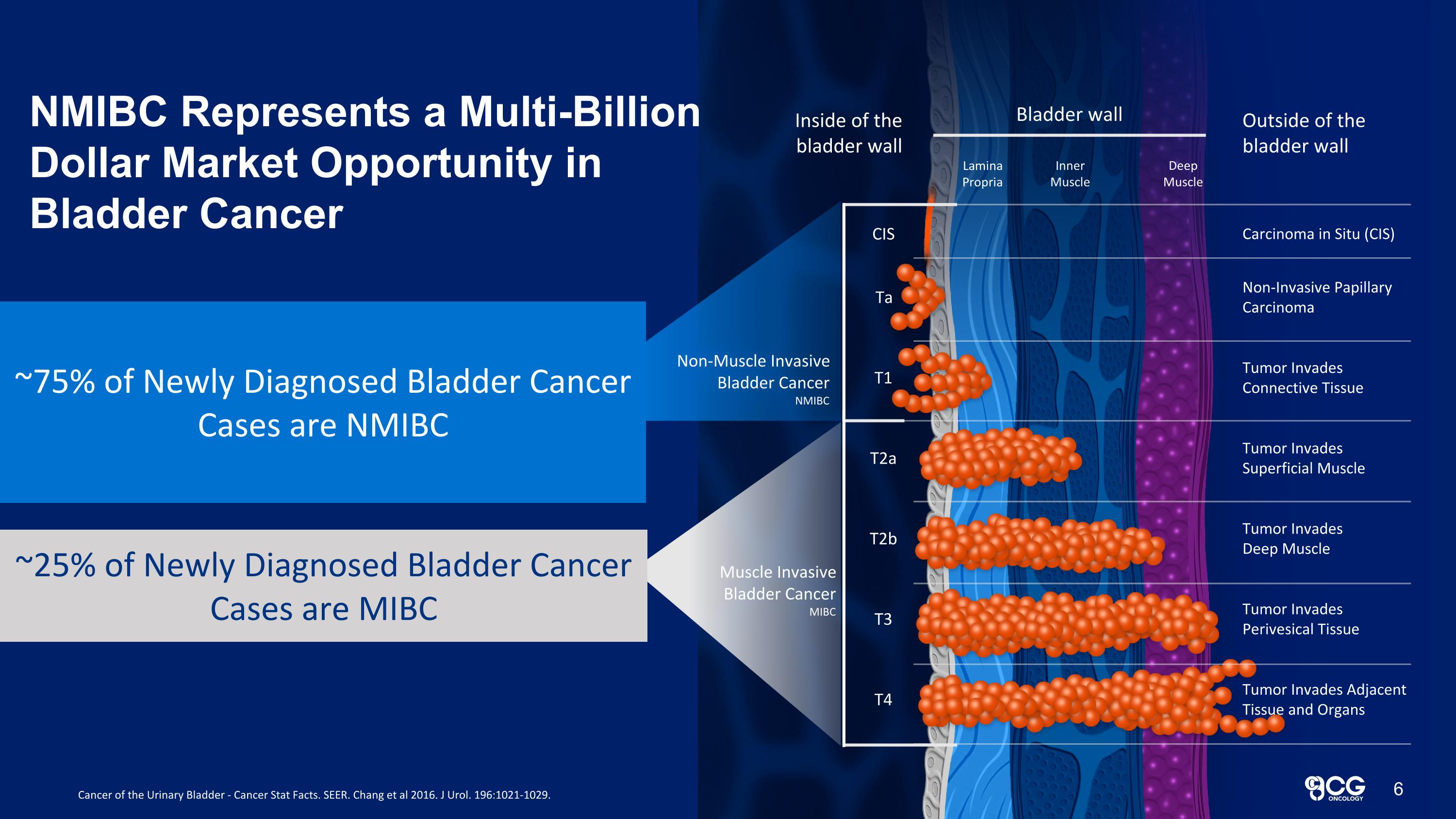
Bladder wall Lamina Propria Inner Muscle Deep Muscle Inside of the bladder wall Outside of the bladder wall Carcinoma in Situ (CIS) Non-Invasive Papillary Carcinoma Tumor Invades Connective Tissue Tumor Invades Deep Muscle Tumor Invades Perivesical Tissue Tumor Invades Adjacent Tissue and Organs Tumor Invades Superficial Muscle CIS Ta T1 T2b T3 T4 T2a Muscle Invasive Bladder Cancer MIBC New bladder cancer cases for 60,892 new patients 20,297 new patients ~75% of Newly Diagnosed Bladder Cancer Cases are NMIBC ~25% of Newly Diagnosed Bladder Cancer Cases are MIBC Cancer of the Urinary Bladder - Cancer Stat Facts. SEER. Chang et al 2016. J Urol. 196:1021-1029. NMIBC Represents a Multi-Billion Dollar Market Opportunity in Bladder Cancer 6 Non-Muscle Invasive Bladder Cancer NMIBC
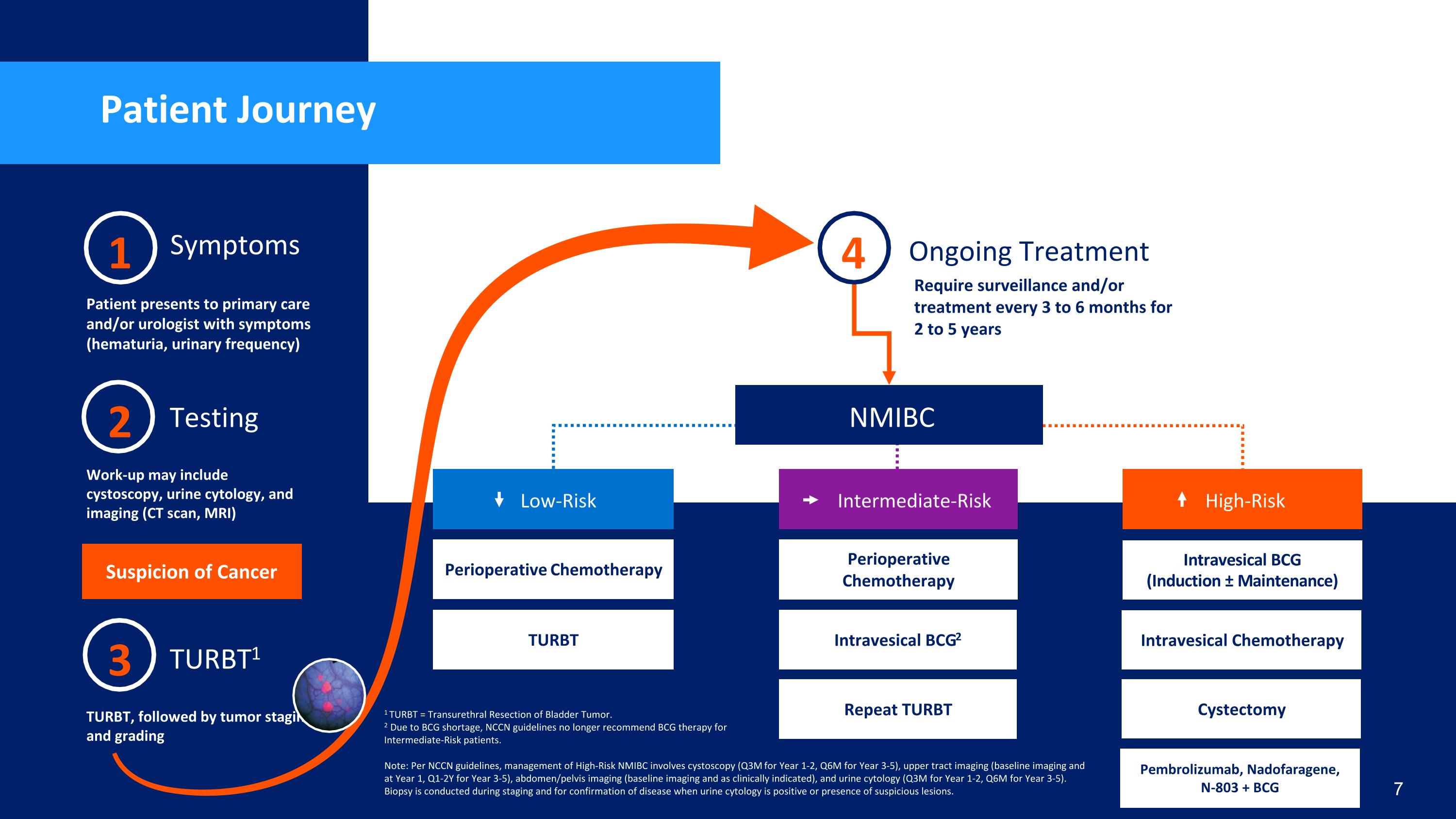
Patient Journey 2 Testing Work-up may include cystoscopy, urine cytology, and imaging (CT scan, MRI) 1 Symptoms Patient presents to primary care and/or urologist with symptoms (hematuria, urinary frequency) 3 TURBT1 TURBT, followed by tumor staging and grading Ongoing Treatment Require surveillance and/or treatment every 3 to 6 months for 2 to 5 years NMIBC Suspicion of Cancer 4 Perioperative Chemotherapy Low-Risk TURBT Note: Per NCCN guidelines, management of High-Risk NMIBC involves cystoscopy (Q3M for Year 1-2, Q6M for Year 3-5), upper tract imaging (baseline imaging and at Year 1, Q1-2Y for Year 3-5), abdomen/pelvis imaging (baseline imaging and as clinically indicated), and urine cytology (Q3M for Year 1-2, Q6M for Year 3-5). Biopsy is conducted during staging and for confirmation of disease when urine cytology is positive or presence of suspicious lesions. Intravesical BCG (Induction ± Maintenance) High-Risk Cystectomy *Quality of life implications Intravesical Chemotherapy Cystectomy Pembrolizumab, Nadofaragene, N-803 + BCG Perioperative Chemotherapy Intermediate-Risk Intravesical BCG2 Repeat TURBT 7 1 TURBT = Transurethral Resection of Bladder Tumor. 2 Due to BCG shortage, NCCN guidelines no longer recommend BCG therapy for Intermediate-Risk patients.
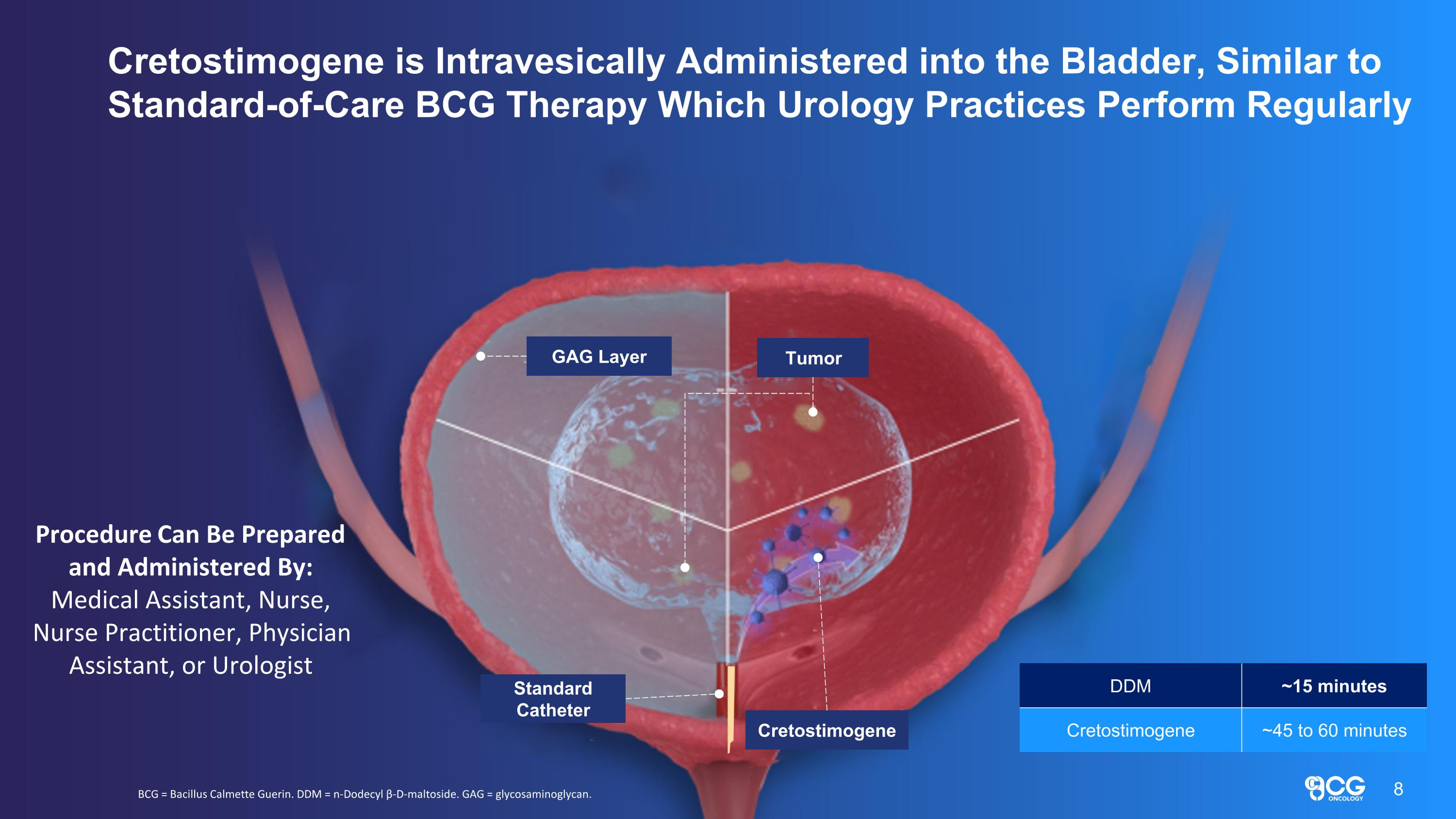
GAG Layer Tumor Standard Catheter Cretostimogene Cretostimogene is Intravesically Administered into the Bladder, Similar to Standard-of-Care BCG Therapy Which Urology Practices Perform Regularly BCG = Bacillus Calmette Guerin. DDM = n-Dodecyl β-D-maltoside. GAG = glycosaminoglycan. Procedure Can Be Prepared and Administered By: Medical Assistant, Nurse, Nurse Practitioner, Physician Assistant, or Urologist 8 DDM ~15 minutes Cretostimogene ~45 to 60 minutes
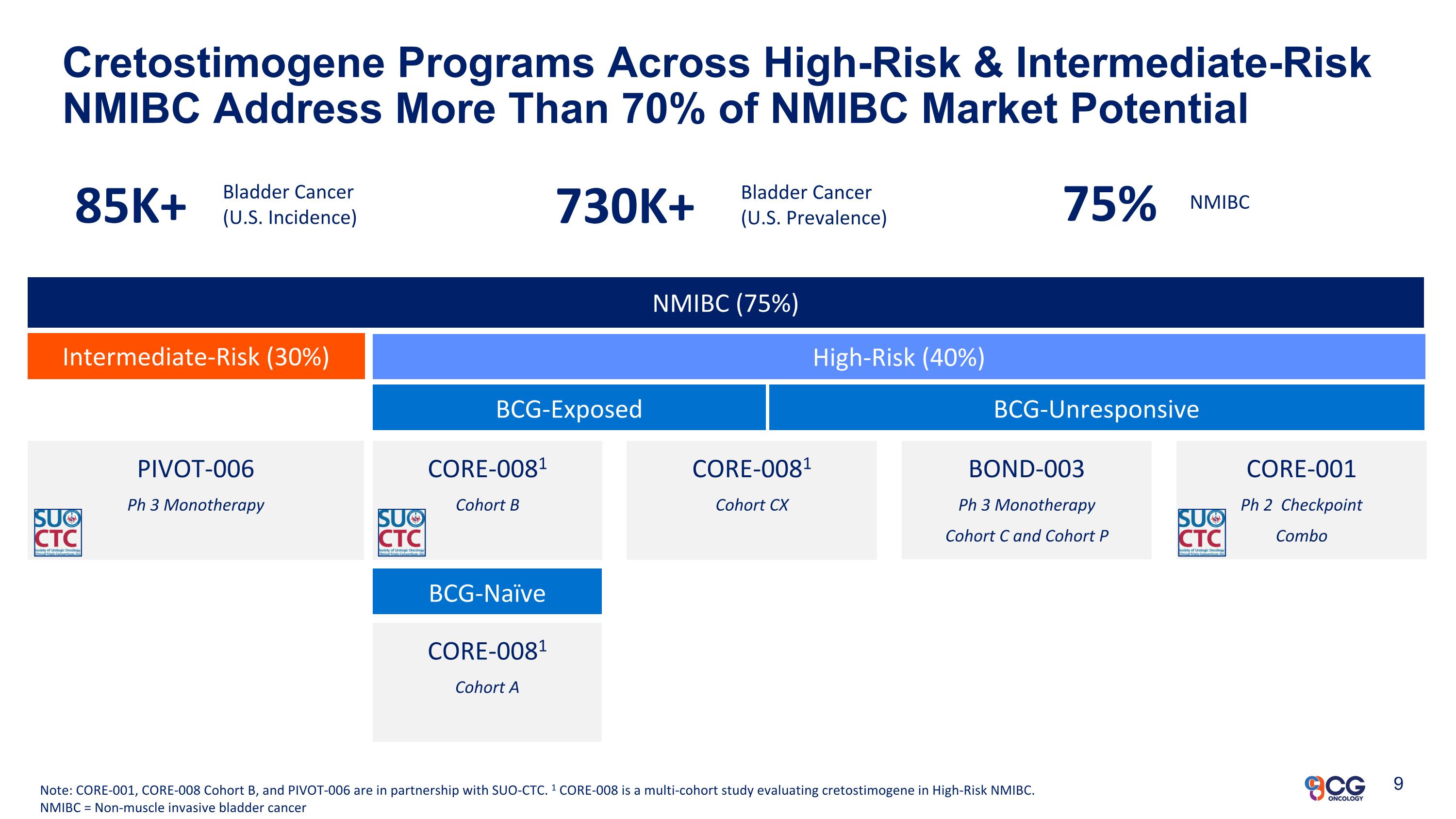
NMIBC (75%) Intermediate-Risk (30%) High-Risk (40%) BCG-Exposed BCG-Unresponsive BOND-003 Ph 3 Monotherapy Cohort C and Cohort P Cretostimogene Programs Across High-Risk & Intermediate-Risk NMIBC Address More Than 70% of NMIBC Market Potential Note: CORE-001, CORE-008 Cohort B, and PIVOT-006 are in partnership with SUO-CTC. 1 CORE-008 is a multi-cohort study evaluating cretostimogene in High-Risk NMIBC. NMIBC = Non-muscle invasive bladder cancer CORE-0081 Cohort B PIVOT-006 Ph 3 Monotherapy CORE-0081 Cohort A CORE-0081 Cohort CX BCG-Naïve CORE-001 Ph 2 Checkpoint Combo 85K+ Bladder Cancer (U.S. Incidence) 730K+ Bladder Cancer (U.S. Prevalence) 75% NMIBC 9
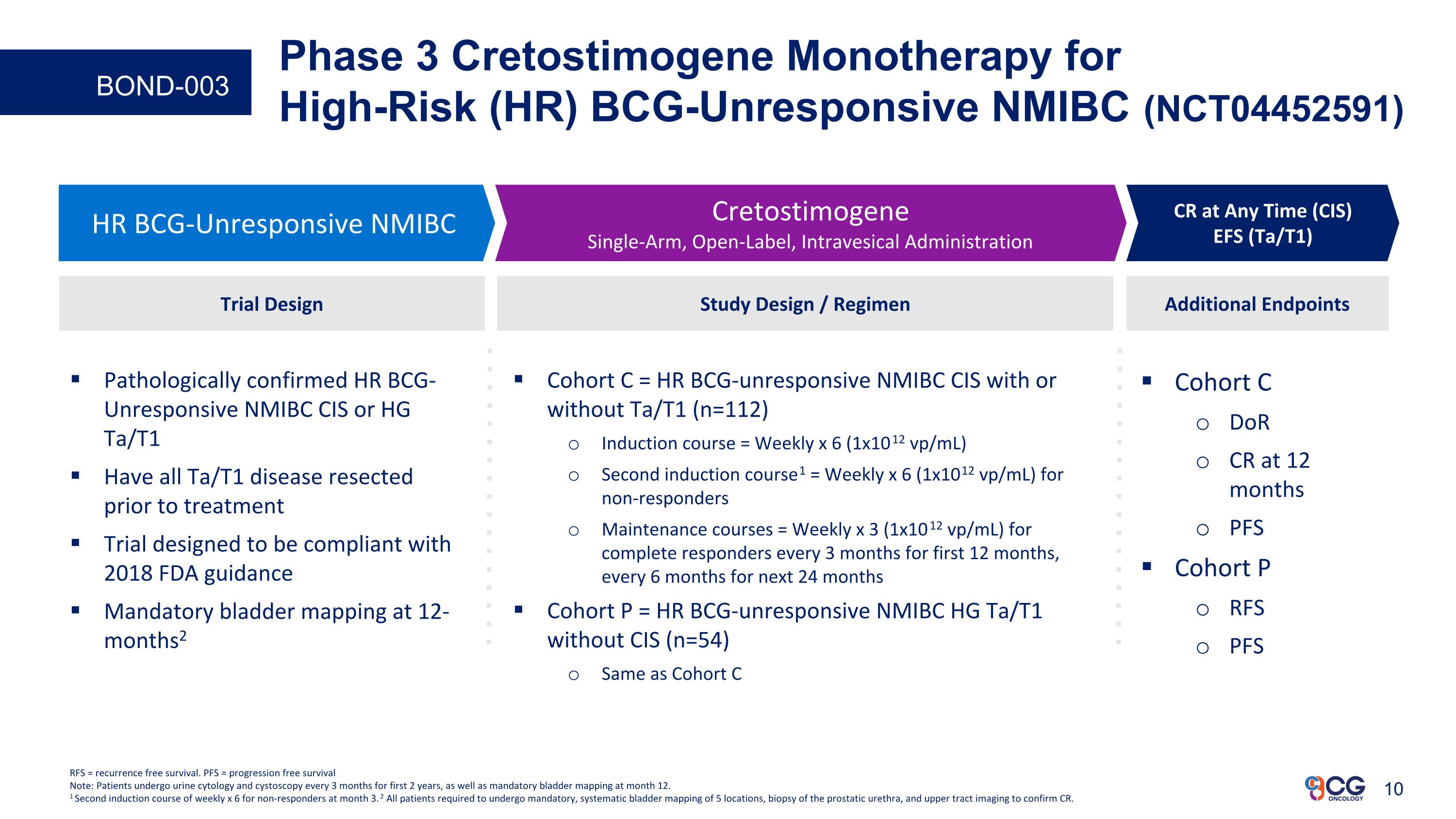
Phase 3 Cretostimogene Monotherapy for High-Risk (HR) BCG-Unresponsive NMIBC (NCT04452591) Cohort C DoR CR at 12 months PFS Cohort P RFS PFS Additional Endpoints HR BCG-Unresponsive NMIBC Cretostimogene Single-Arm, Open-Label, Intravesical Administration CR at Any Time (CIS) EFS (Ta/T1) Study Design / Regimen Cohort C = HR BCG-unresponsive NMIBC CIS with or without Ta/T1 (n=112) Induction course = Weekly x 6 (1x1012 vp/mL) Second induction course1 = Weekly x 6 (1x1012 vp/mL) for non-responders Maintenance courses = Weekly x 3 (1x1012 vp/mL) for complete responders every 3 months for first 12 months, every 6 months for next 24 months Cohort P = HR BCG-unresponsive NMIBC HG Ta/T1 without CIS (n=54) Same as Cohort C Trial Design Pathologically confirmed HR BCG-Unresponsive NMIBC CIS or HG Ta/T1 Have all Ta/T1 disease resected prior to treatment Trial designed to be compliant with 2018 FDA guidance Mandatory bladder mapping at 12-months2 RFS = recurrence free survival. PFS = progression free survival Note: Patients undergo urine cytology and cystoscopy every 3 months for first 2 years, as well as mandatory bladder mapping at month 12. 1 Second induction course of weekly x 6 for non-responders at month 3. 2 All patients required to undergo mandatory, systematic bladder mapping of 5 locations, biopsy of the prostatic urethra, and upper tract imaging to confirm CR. BOND-003
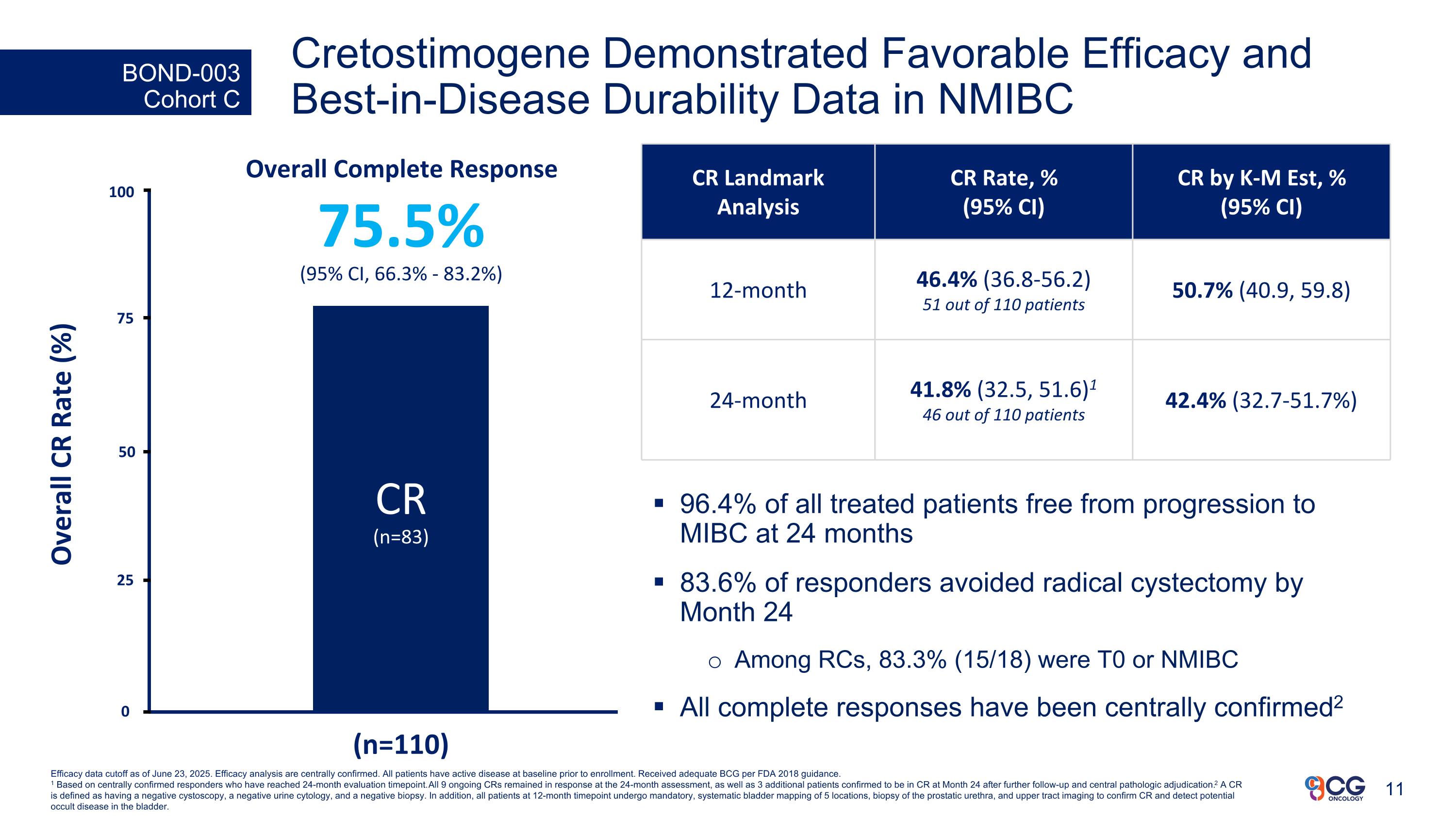
BOND-003 Cohort C Overall Complete Response 75.5% (95% CI, 66.3% - 83.2%) CR (n=83) 25 Overall CR Rate (%) (n=110) 50 75 100 0 Efficacy data cutoff as of June 23, 2025. Efficacy analysis are centrally confirmed. All patients have active disease at baseline prior to enrollment. Received adequate BCG per FDA 2018 guidance. 1 Based on centrally confirmed responders who have reached 24-month evaluation timepoint. All 9 ongoing CRs remained in response at the 24-month assessment, as well as 3 additional patients confirmed to be in CR at Month 24 after further follow-up and central pathologic adjudication. 2 A CR is defined as having a negative cystoscopy, a negative urine cytology, and a negative biopsy. In addition, all patients at 12-month timepoint undergo mandatory, systematic bladder mapping of 5 locations, biopsy of the prostatic urethra, and upper tract imaging to confirm CR and detect potential occult disease in the bladder. 96.4% of all treated patients free from progression to MIBC at 24 months 83.6% of responders avoided radical cystectomy by Month 24 Among RCs, 83.3% (15/18) were T0 or NMIBC All complete responses have been centrally confirmed2 Cretostimogene Demonstrated Favorable Efficacy and Best-in-Disease Durability Data in NMIBC CR Landmark Analysis CR Rate, % (95% CI) CR by K-M Est, % (95% CI) 12-month 46.4% (36.8-56.2) 51 out of 110 patients 50.7% (40.9, 59.8) 24-month 41.8% (32.5, 51.6)1 46 out of 110 patients 42.4% (32.7-51.7%)
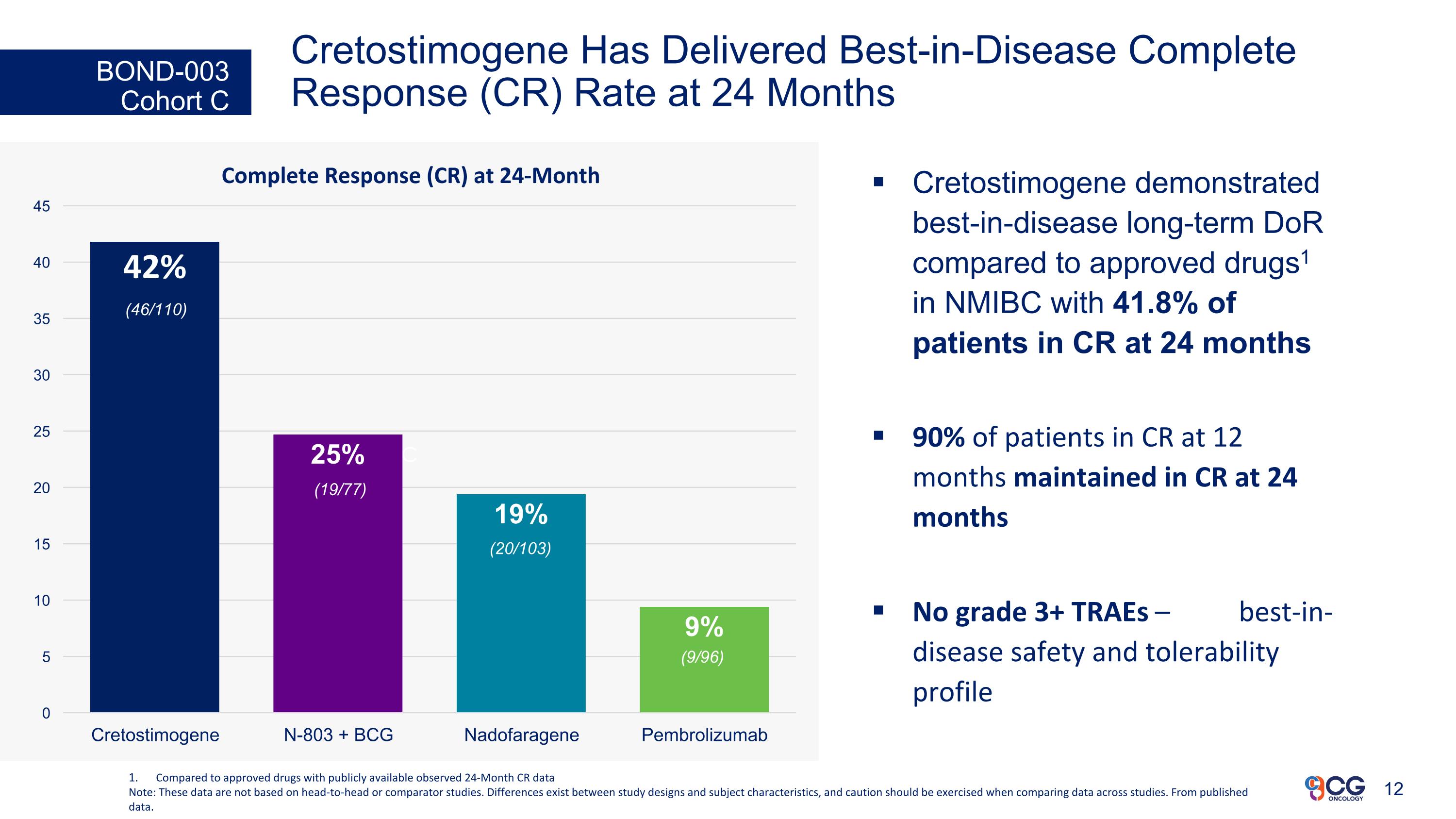
Cretostimogene Has Delivered Best-in-Disease Complete Response (CR) Rate at 24 Months Cretostimogene demonstrated best-in-disease long-term DoR compared to approved drugs1 in NMIBC with 41.8% of patients in CR at 24 months 90% of patients in CR at 12 months maintained in CR at 24 months No grade 3+ TRAEs – best-in-disease safety and tolerability profile BOND-003 Cohort C c Complete Response (CR) at 24-Month Compared to approved drugs with publicly available observed 24-Month CR data Note: These data are not based on head-to-head or comparator studies. Differences exist between study designs and subject characteristics, and caution should be exercised when comparing data across studies. From published data. (46/110) (19/77) (20/103) (9/96)
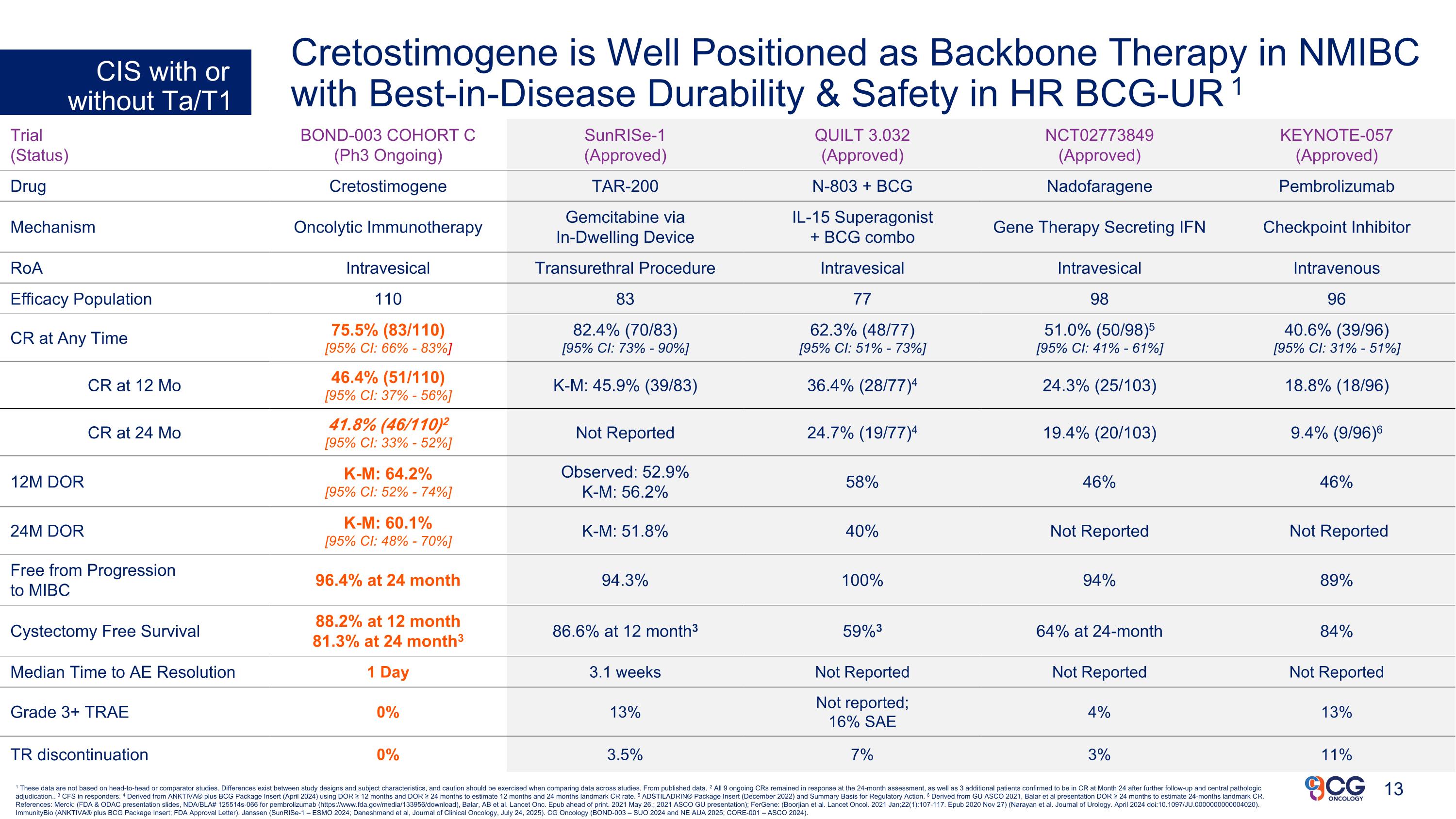
1 These data are not based on head-to-head or comparator studies. Differences exist between study designs and subject characteristics, and caution should be exercised when comparing data across studies. From published data. 2 All 9 ongoing CRs remained in response at the 24-month assessment, as well as 3 additional patients confirmed to be in CR at Month 24 after further follow-up and central pathologic adjudication.. 3 CFS in responders. 4 Derived from ANKTIVA® plus BCG Package Insert (April 2024) using DOR ≥ 12 months and DOR ≥ 24 months to estimate 12 months and 24 months landmark CR rate. 5 ADSTILADRIN® Package Insert (December 2022) and Summary Basis for Regulatory Action. 6 Derived from GU ASCO 2021, Balar et al presentation DOR ≥ 24 months to estimate 24-months landmark CR. References: Merck: (FDA & ODAC presentation slides, NDA/BLA# 125514s-066 for pembrolizumab (https://www.fda.gov/media/133956/download), Balar, AB et al. Lancet Onc. Epub ahead of print. 2021 May 26.; 2021 ASCO GU presentation); FerGene: (Boorjian et al. Lancet Oncol. 2021 Jan;22(1):107-117. Epub 2020 Nov 27) (Narayan et al. Journal of Urology. April 2024 doi:10.1097/JU.0000000000004020). ImmunityBio (ANKTIVA® plus BCG Package Insert; FDA Approval Letter). Janssen (SunRISe-1 – ESMO 2024; Daneshmand et al, Journal of Clinical Oncology, July 24, 2025). CG Oncology (BOND-003 – SUO 2024 and NE AUA 2025; CORE-001 – ASCO 2024). Trial (Status) BOND-003 COHORT C (Ph3 Ongoing) SunRISe-1 (Approved) QUILT 3.032 (Approved) NCT02773849 (Approved) KEYNOTE-057 (Approved) Drug Cretostimogene TAR-200 N-803 + BCG Nadofaragene Pembrolizumab Mechanism Oncolytic Immunotherapy Gemcitabine via In-Dwelling Device IL-15 Superagonist + BCG combo Gene Therapy Secreting IFN Checkpoint Inhibitor RoA Intravesical Transurethral Procedure Intravesical Intravesical Intravenous Efficacy Population 110 83 77 98 96 CR at Any Time 75.5% (83/110) [95% CI: 66% - 83%] 82.4% (70/83) [95% CI: 73% - 90%] 62.3% (48/77) [95% CI: 51% - 73%] 51.0% (50/98)5 [95% CI: 41% - 61%] 40.6% (39/96) [95% CI: 31% - 51%] CR at 12 Mo 46.4% (51/110) [95% CI: 37% - 56%] K-M: 45.9% (39/83) 36.4% (28/77)4 24.3% (25/103) 18.8% (18/96) CR at 24 Mo 41.8% (46/110)2 [95% CI: 33% - 52%] Not Reported 24.7% (19/77)4 19.4% (20/103) 9.4% (9/96)6 12M DOR K-M: 64.2% [95% CI: 52% - 74%] Observed: 52.9% K-M: 56.2% 58% 46% 46% 24M DOR K-M: 60.1% [95% CI: 48% - 70%] K-M: 51.8% 40% Not Reported Not Reported Free from Progression to MIBC 96.4% at 24 month 94.3% 100% 94% 89% Cystectomy Free Survival 88.2% at 12 month 81.3% at 24 month3 86.6% at 12 month3 59%3 64% at 24-month 84% Median Time to AE Resolution 1 Day 3.1 weeks Not Reported Not Reported Not Reported Grade 3+ TRAE 0% 13% Not reported; 16% SAE 4% 13% TR discontinuation 0% 3.5% 7% 3% 11% CIS with or without Ta/T1 Cretostimogene is Well Positioned as Backbone Therapy in NMIBC with Best-in-Disease Durability & Safety in HR BCG-UR 1
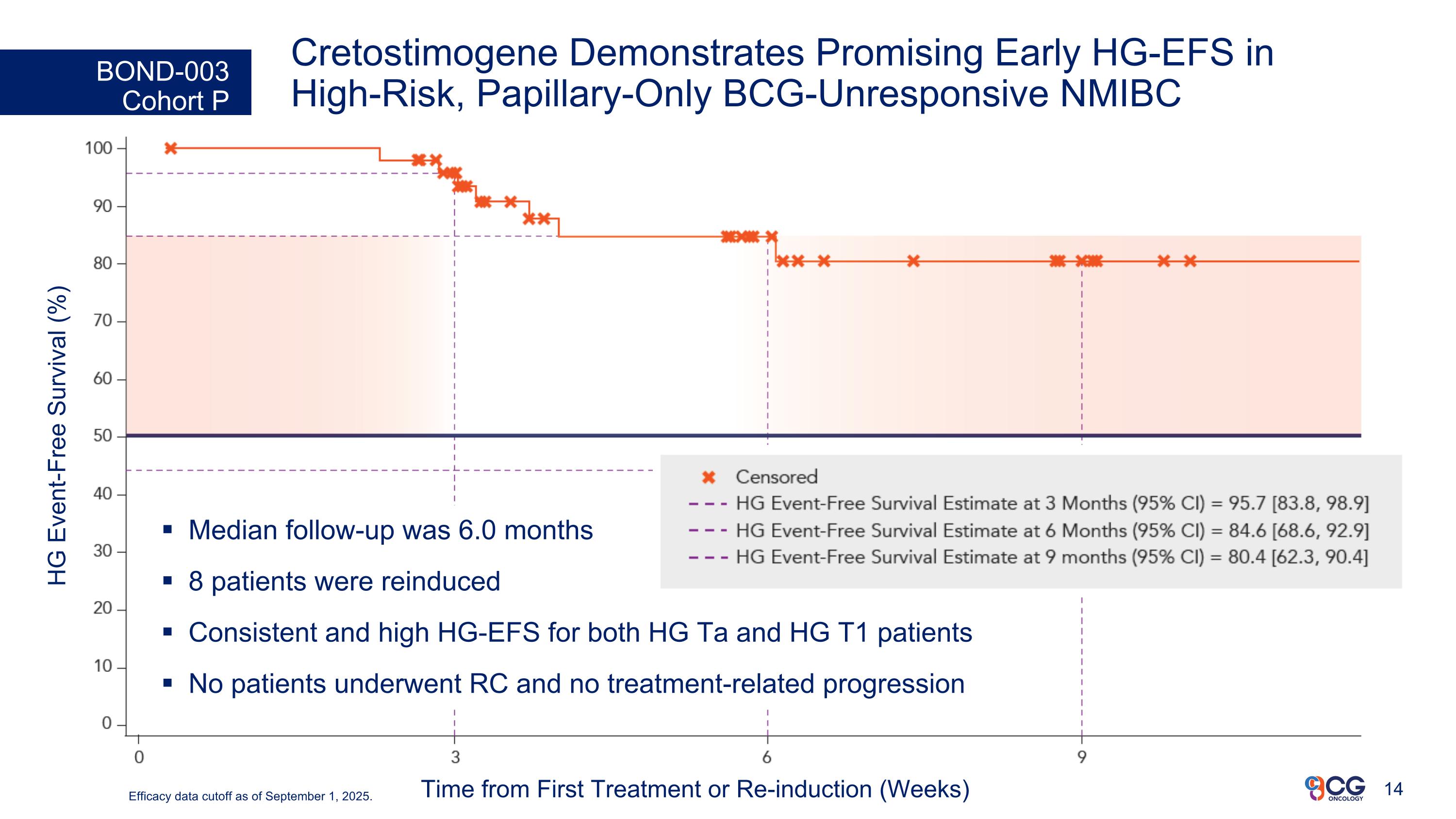
Cretostimogene Demonstrates Promising Early HG-EFS in High-Risk, Papillary-Only BCG-Unresponsive NMIBC BOND-003 Cohort P HG Event-Free Survival (%) Time from First Treatment or Re-induction (Weeks) Median follow-up was 6.0 months 8 patients were reinduced Consistent and high HG-EFS for both HG Ta and HG T1 patients No patients underwent RC and no treatment-related progression Efficacy data cutoff as of September 1, 2025.
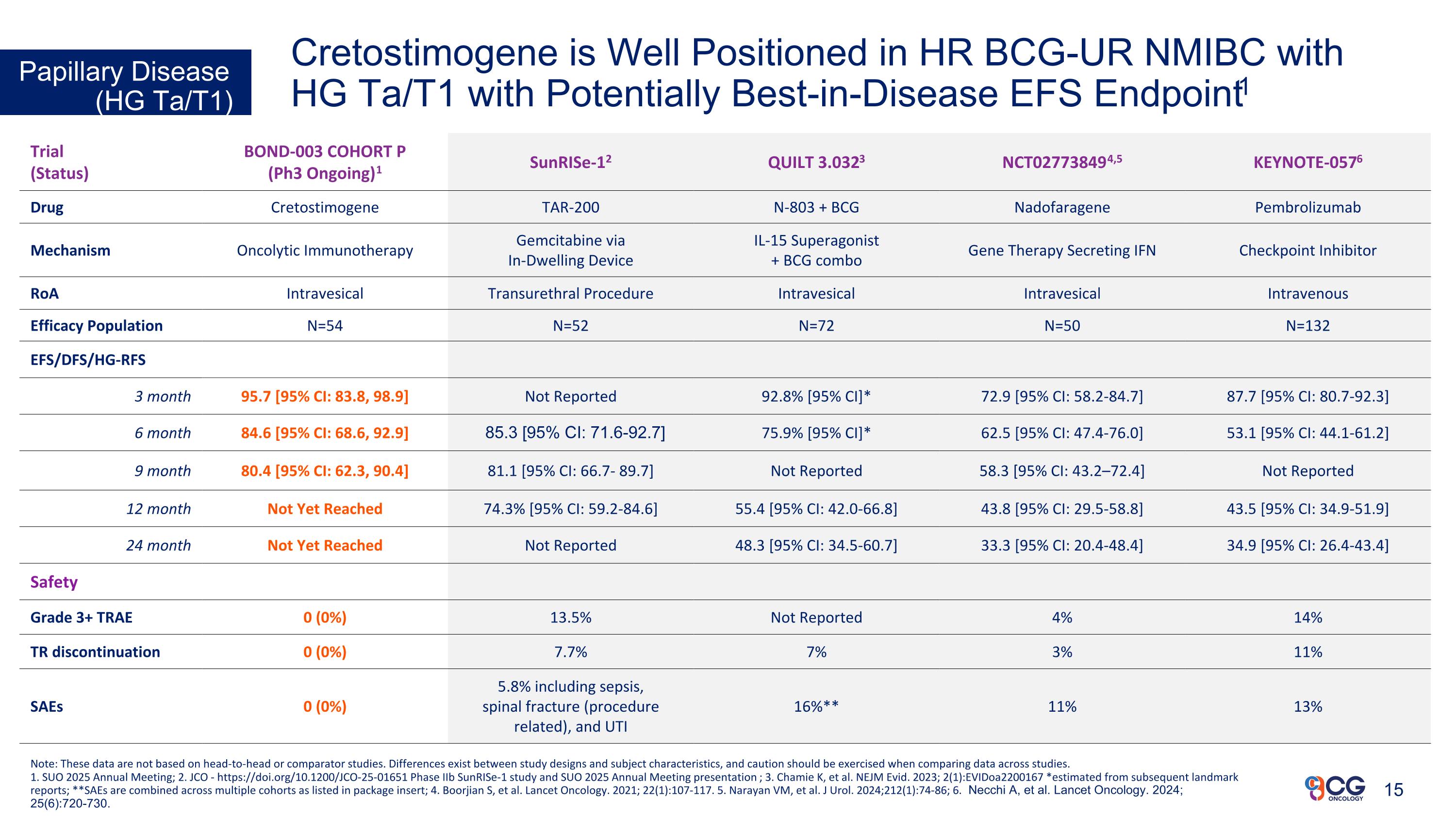
Cretostimogene is Well Positioned in HR BCG-UR NMIBC with HG Ta/T1 with Potentially Best-in-Disease EFS Endpoint1 Papillary Disease (HG Ta/T1) Trial (Status) BOND-003 COHORT P (Ph3 Ongoing)1 SunRISe-12 QUILT 3.0323 NCT027738494,5 KEYNOTE-0576 Drug Cretostimogene TAR-200 N-803 + BCG Nadofaragene Pembrolizumab Mechanism Oncolytic Immunotherapy Gemcitabine via In-Dwelling Device IL-15 Superagonist + BCG combo Gene Therapy Secreting IFN Checkpoint Inhibitor RoA Intravesical Transurethral Procedure Intravesical Intravesical Intravenous Efficacy Population N=54 N=52 N=72 N=50 N=132 EFS/DFS/HG-RFS 3 month 95.7 [95% CI: 83.8, 98.9] Not Reported 92.8% [95% CI]* 72.9 [95% CI: 58.2-84.7] 87.7 [95% CI: 80.7-92.3] 6 month 84.6 [95% CI: 68.6, 92.9] 85.3 [95% CI: 71.6-92.7] 75.9% [95% CI]* 62.5 [95% CI: 47.4-76.0] 53.1 [95% CI: 44.1-61.2] 9 month 80.4 [95% CI: 62.3, 90.4] 81.1 [95% CI: 66.7- 89.7] Not Reported 58.3 [95% CI: 43.2–72.4] Not Reported 12 month Not Yet Reached 74.3% [95% CI: 59.2-84.6] 55.4 [95% CI: 42.0-66.8] 43.8 [95% CI: 29.5-58.8] 43.5 [95% CI: 34.9-51.9] 24 month Not Yet Reached Not Reported 48.3 [95% CI: 34.5-60.7] 33.3 [95% CI: 20.4-48.4] 34.9 [95% CI: 26.4-43.4] Safety Grade 3+ TRAE 0 (0%) 13.5% Not Reported 4% 14% TR discontinuation 0 (0%) 7.7% 7% 3% 11% SAEs 0 (0%) 5.8% including sepsis, spinal fracture (procedure related), and UTI 16%** 11% 13% Note: These data are not based on head-to-head or comparator studies. Differences exist between study designs and subject characteristics, and caution should be exercised when comparing data across studies. 1. SUO 2025 Annual Meeting; 2. JCO - https://doi.org/10.1200/JCO-25-01651 Phase IIb SunRISe-1 study and SUO 2025 Annual Meeting presentation; 3. Chamie K, et al. NEJM Evid. 2023; 2(1):EVIDoa2200167 *estimated from subsequent landmark reports; **SAEs are combined across multiple cohorts as listed in package insert; 4. Boorjian S, et al. Lancet Oncology. 2021; 22(1):107-117. 5. Narayan VM, et al. J Urol. 2024;212(1):74-86; 6. Necchi A, et al. Lancet Oncology. 2024; 25(6):720-730. 15
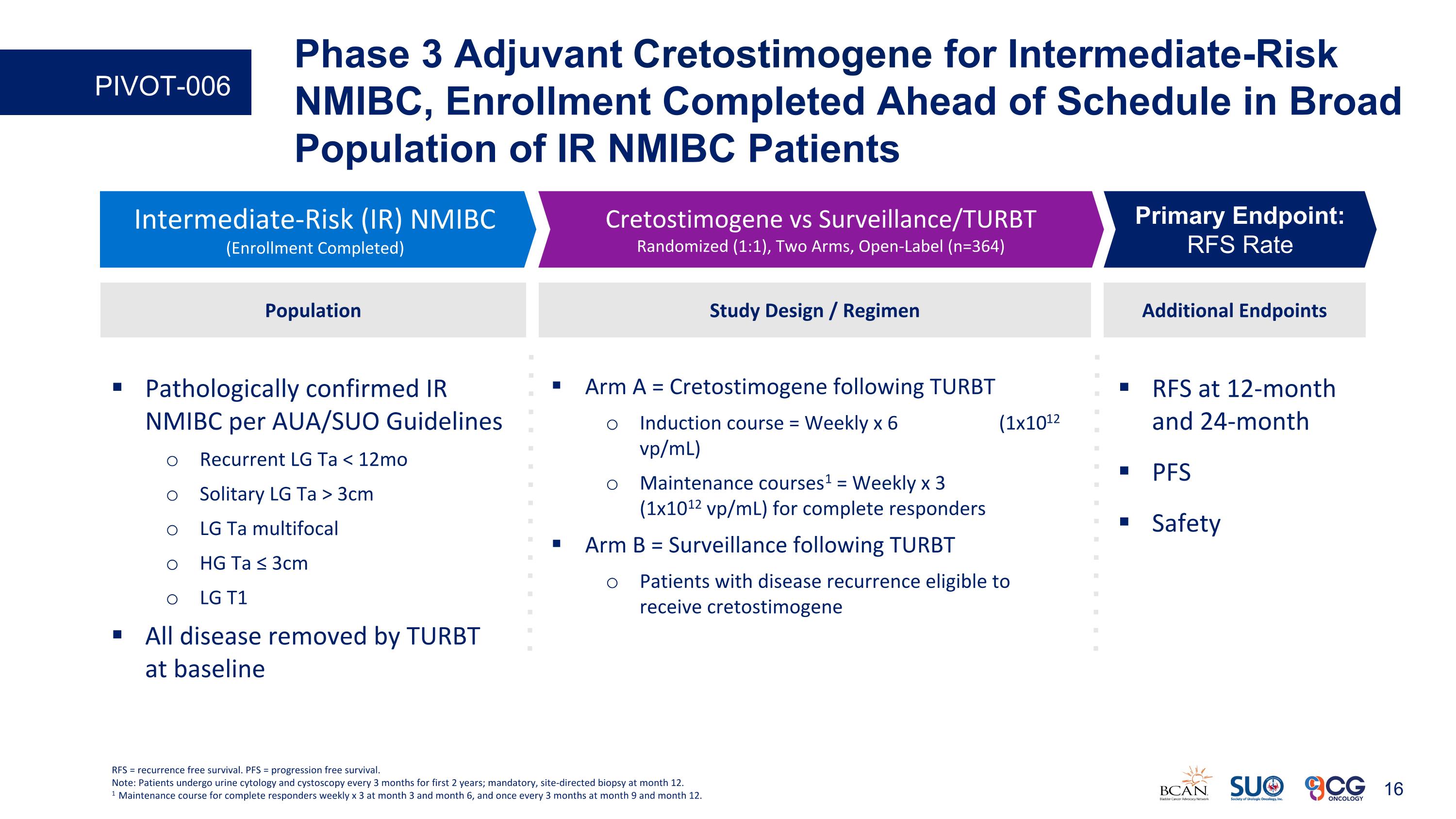
RFS at 12-month and 24-month PFS Safety Additional Endpoints Intermediate-Risk (IR) NMIBC (Enrollment Completed) Cretostimogene vs Surveillance/TURBT Randomized (1:1), Two Arms, Open-Label (n=364) Primary Endpoint: RFS Rate Study Design / Regimen Arm A = Cretostimogene following TURBT Induction course = Weekly x 6 (1x1012 vp/mL) Maintenance courses1 = Weekly x 3 (1x1012 vp/mL) for complete responders Arm B = Surveillance following TURBT Patients with disease recurrence eligible to receive cretostimogene Population Pathologically confirmed IR NMIBC per AUA/SUO Guidelines Recurrent LG Ta < 12mo Solitary LG Ta > 3cm LG Ta multifocal HG Ta ≤ 3cm LG T1 All disease removed by TURBT at baseline RFS = recurrence free survival. PFS = progression free survival. Note: Patients undergo urine cytology and cystoscopy every 3 months for first 2 years; mandatory, site-directed biopsy at month 12. 1 Maintenance course for complete responders weekly x 3 at month 3 and month 6, and once every 3 months at month 9 and month 12. Phase 3 Adjuvant Cretostimogene for Intermediate-Risk NMIBC, Enrollment Completed Ahead of Schedule in Broad Population of IR NMIBC Patients PIVOT-006
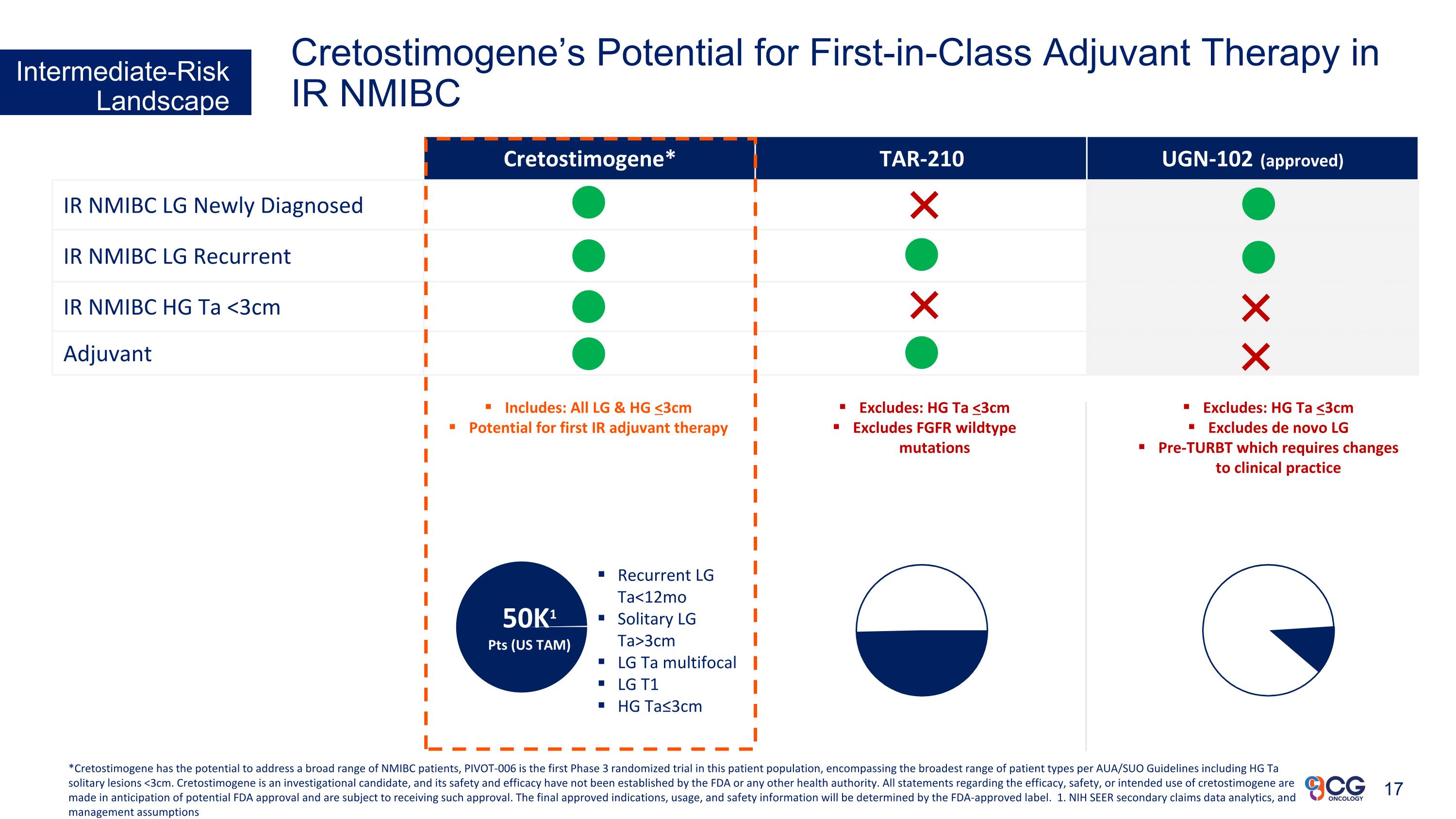
Cretostimogene* TAR-210 UGN-102 (approved) IR NMIBC LG Newly Diagnosed IR NMIBC LG Recurrent IR NMIBC HG Ta <3cm Adjuvant Excludes: HG Ta <3cm Excludes FGFR wildtype mutations Includes: All LG & HG <3cm Potential for first IR adjuvant therapy Excludes: HG Ta <3cm Excludes de novo LG Pre-TURBT which requires changes to clinical practice Recurrent LG Ta<12mo Solitary LG Ta>3cm LG Ta multifocal LG T1 HG Ta≤3cm 50K1 Pts (US TAM) Intermediate-Risk Landscape Cretostimogene’s Potential for First-in-Class Adjuvant Therapy in IR NMIBC *Cretostimogene has the potential to address a broad range of NMIBC patients, PIVOT-006 is the first Phase 3 randomized trial in this patient population, encompassing the broadest range of patient types per AUA/SUO Guidelines including HG Ta solitary lesions <3cm. Cretostimogene is an investigational candidate, and its safety and efficacy have not been established by the FDA or any other health authority. All statements regarding the efficacy, safety, or intended use of cretostimogene are made in anticipation of potential FDA approval and are subject to receiving such approval. The final approved indications, usage, and safety information will be determined by the FDA-approved label. 1. NIH SEER secondary claims data analytics, and management assumptions
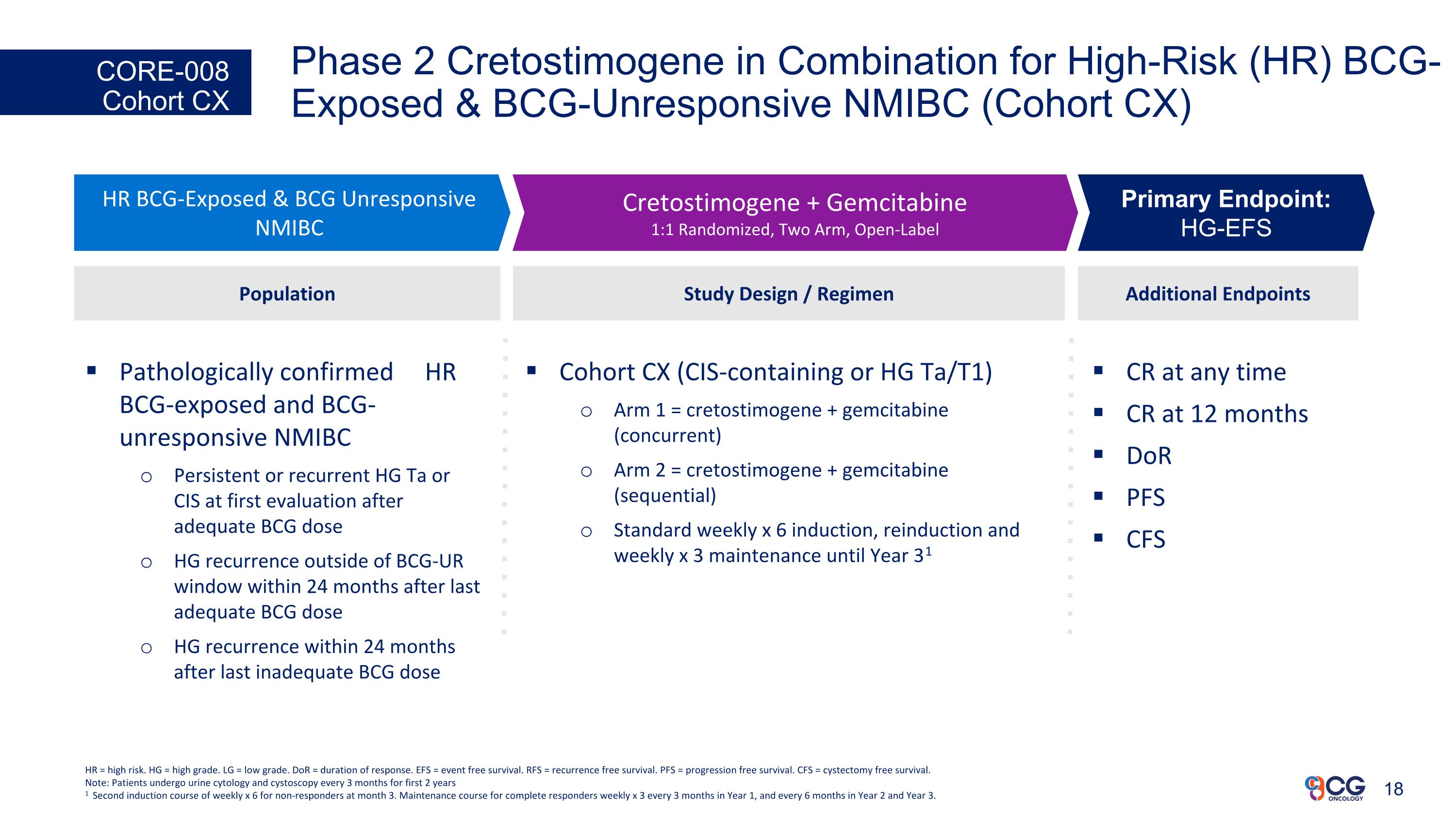
Phase 2 Cretostimogene in Combination for High-Risk (HR) BCG-Exposed & BCG-Unresponsive NMIBC (Cohort CX) CORE-008 Cohort CX CR at any time CR at 12 months DoR PFS CFS Additional Endpoints HR BCG-Exposed & BCG Unresponsive NMIBC Cretostimogene + Gemcitabine 1:1 Randomized, Two Arm, Open-Label Primary Endpoint: HG-EFS Study Design / Regimen Cohort CX (CIS-containing or HG Ta/T1) Arm 1 = cretostimogene + gemcitabine (concurrent) Arm 2 = cretostimogene + gemcitabine (sequential) Standard weekly x 6 induction, reinduction and weekly x 3 maintenance until Year 31 Population Pathologically confirmed HR BCG-exposed and BCG-unresponsive NMIBC Persistent or recurrent HG Ta or CIS at first evaluation after adequate BCG dose HG recurrence outside of BCG-UR window within 24 months after last adequate BCG dose HG recurrence within 24 months after last inadequate BCG dose HR = high risk. HG = high grade. LG = low grade. DoR = duration of response. EFS = event free survival. RFS = recurrence free survival. PFS = progression free survival. CFS = cystectomy free survival. Note: Patients undergo urine cytology and cystoscopy every 3 months for first 2 years 1 Second induction course of weekly x 6 for non-responders at month 3. Maintenance course for complete responders weekly x 3 every 3 months in Year 1, and every 6 months in Year 2 and Year 3.
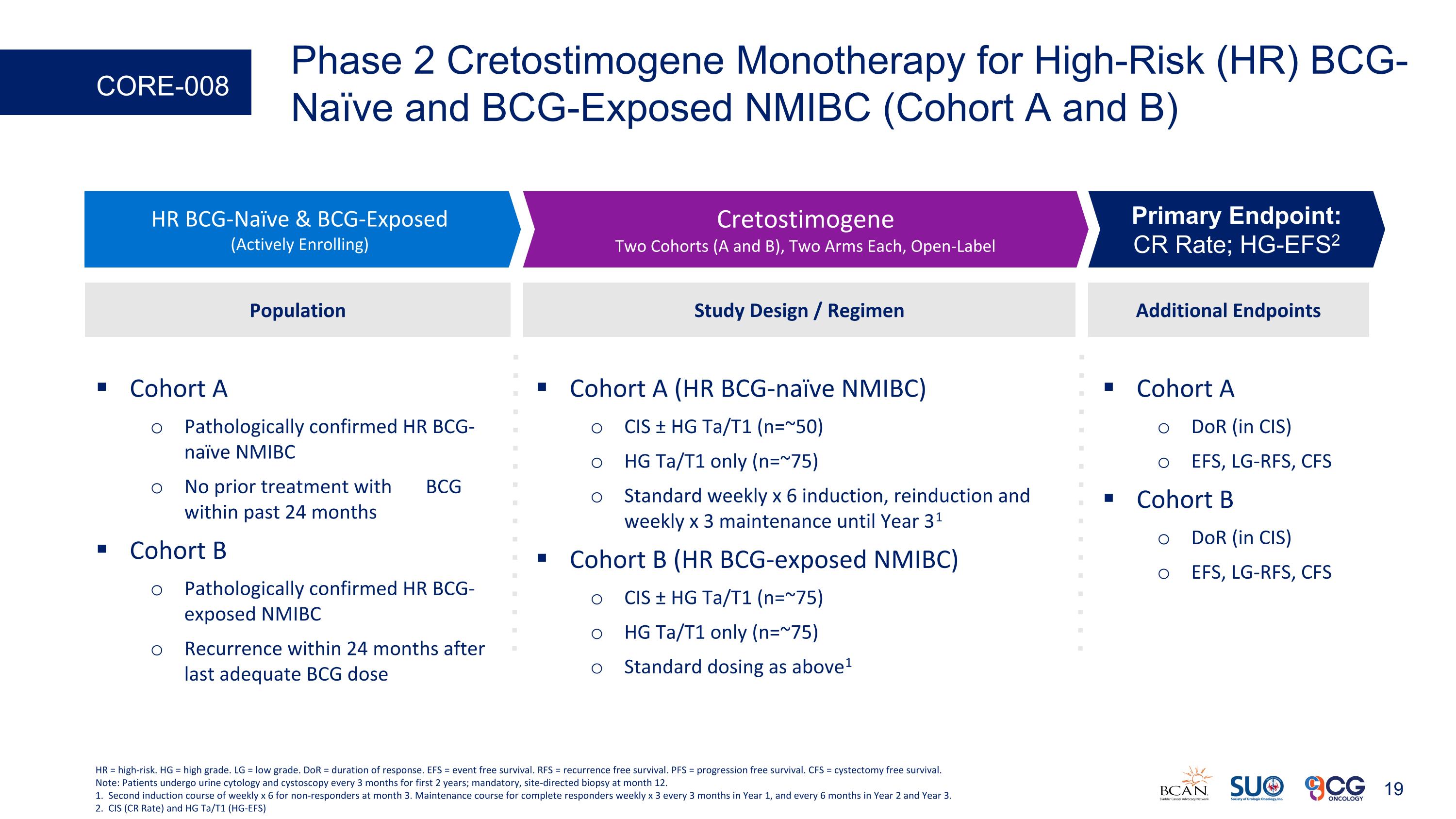
Phase 2 Cretostimogene Monotherapy for High-Risk (HR) BCG-Naïve and BCG-Exposed NMIBC (Cohort A and B) CORE-008 Cohort A DoR (in CIS) EFS, LG-RFS, CFS Cohort B DoR (in CIS) EFS, LG-RFS, CFS Additional Endpoints HR BCG-Naïve & BCG-Exposed (Actively Enrolling) Cretostimogene Two Cohorts (A and B), Two Arms Each, Open-Label Primary Endpoint: CR Rate; HG-EFS2 Study Design / Regimen Cohort A (HR BCG-naïve NMIBC) CIS ± HG Ta/T1 (n=~50) HG Ta/T1 only (n=~75) Standard weekly x 6 induction, reinduction and weekly x 3 maintenance until Year 31 Cohort B (HR BCG-exposed NMIBC) CIS ± HG Ta/T1 (n=~75) HG Ta/T1 only (n=~75) Standard dosing as above1 Population Cohort A Pathologically confirmed HR BCG-naïve NMIBC No prior treatment with BCG within past 24 months Cohort B Pathologically confirmed HR BCG-exposed NMIBC Recurrence within 24 months after last adequate BCG dose HR = high-risk. HG = high grade. LG = low grade. DoR = duration of response. EFS = event free survival. RFS = recurrence free survival. PFS = progression free survival. CFS = cystectomy free survival. Note: Patients undergo urine cytology and cystoscopy every 3 months for first 2 years; mandatory, site-directed biopsy at month 12. 1. Second induction course of weekly x 6 for non-responders at month 3. Maintenance course for complete responders weekly x 3 every 3 months in Year 1, and every 6 months in Year 2 and Year 3. 2. CIS (CR Rate) and HG Ta/T1 (HG-EFS)
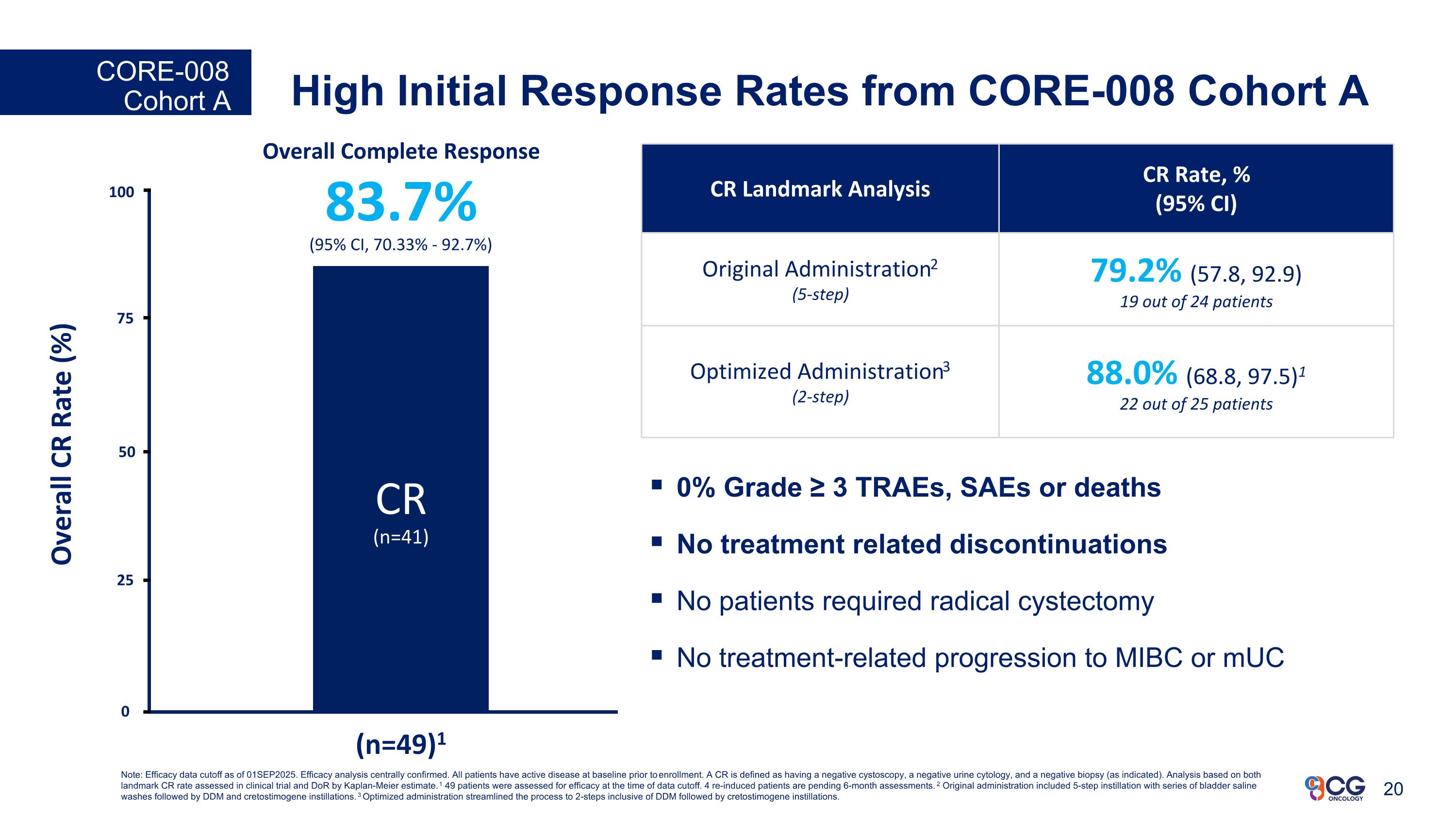
High Initial Response Rates from CORE-008 Cohort A CORE-008 Cohort A (n=49*) Overall Complete Response 83.7% (95% CI, 70.33% - 92.7%) CR (n=41) 25 Overall CR Rate (%) (n=49)1 50 75 100 0 CR Landmark Analysis CR Rate, % (95% CI) Original Administration2 (5-step) 79.2% (57.8, 92.9) 19 out of 24 patients Optimized Administration3 (2-step) 88.0% (68.8, 97.5)1 22 out of 25 patients 0% Grade ≥ 3 TRAEs, SAEs or deaths No treatment related discontinuations No patients required radical cystectomy No treatment-related progression to MIBC or mUC Note: Efficacy data cutoff as of 01SEP2025. Efficacy analysis centrally confirmed. All patients have active disease at baseline prior to enrollment. A CR is defined as having a negative cystoscopy, a negative urine cytology, and a negative biopsy (as indicated). Analysis based on both landmark CR rate assessed in clinical trial and DoR by Kaplan-Meier estimate. 1 49 patients were assessed for efficacy at the time of data cutoff. 4 re-induced patients are pending 6-month assessments. 2 Original administration included 5-step instillation with series of bladder saline washes followed by DDM and cretostimogene instillations. 3 Optimized administration streamlined the process to 2-steps inclusive of DDM followed by cretostimogene instillations.
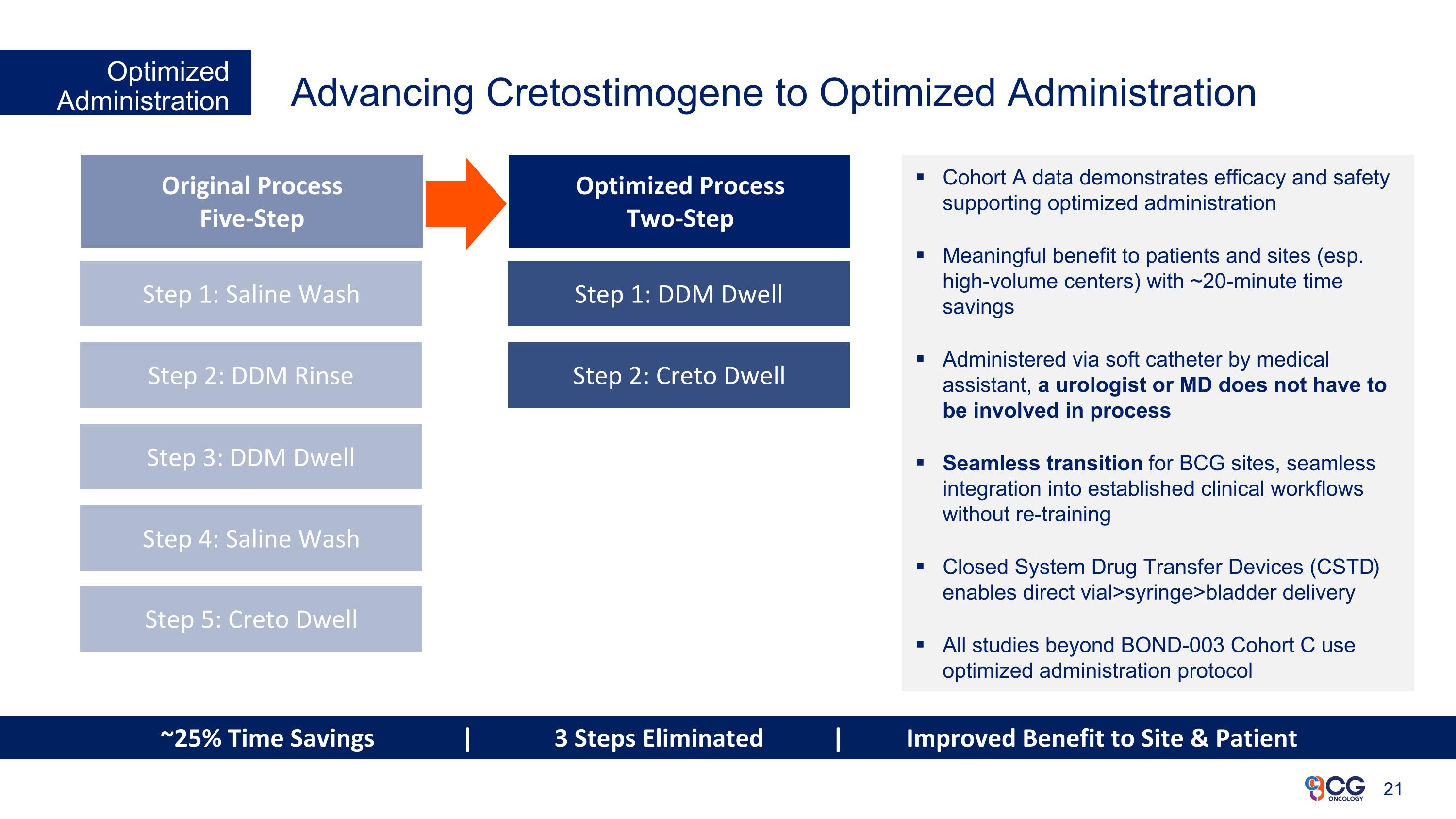
Advancing Cretostimogene to Optimized Administration Optimized Administration Cohort A data demonstrates efficacy and safety supporting optimized administration Meaningful benefit to patients and sites (esp. high-volume centers) with ~20-minute time savings Administered via soft catheter by medical assistant, a urologist or MD does not have to be involved in process Seamless transition for BCG sites, seamless integration into established clinical workflows without re-training Closed System Drug Transfer Devices (CSTD) enables direct vial>syringe>bladder delivery All studies beyond BOND-003 Cohort C use optimized administration protocol Original Process Five-Step Optimized Process Two-Step Step 1: Saline Wash Step 2: DDM Rinse Step 3: DDM Dwell Step 4: Saline Wash Step 1: DDM Dwell Step 2: Creto Dwell Step 5: Creto Dwell ~25% Time Savings | 3 Steps Eliminated | Improved Benefit to Site & Patient
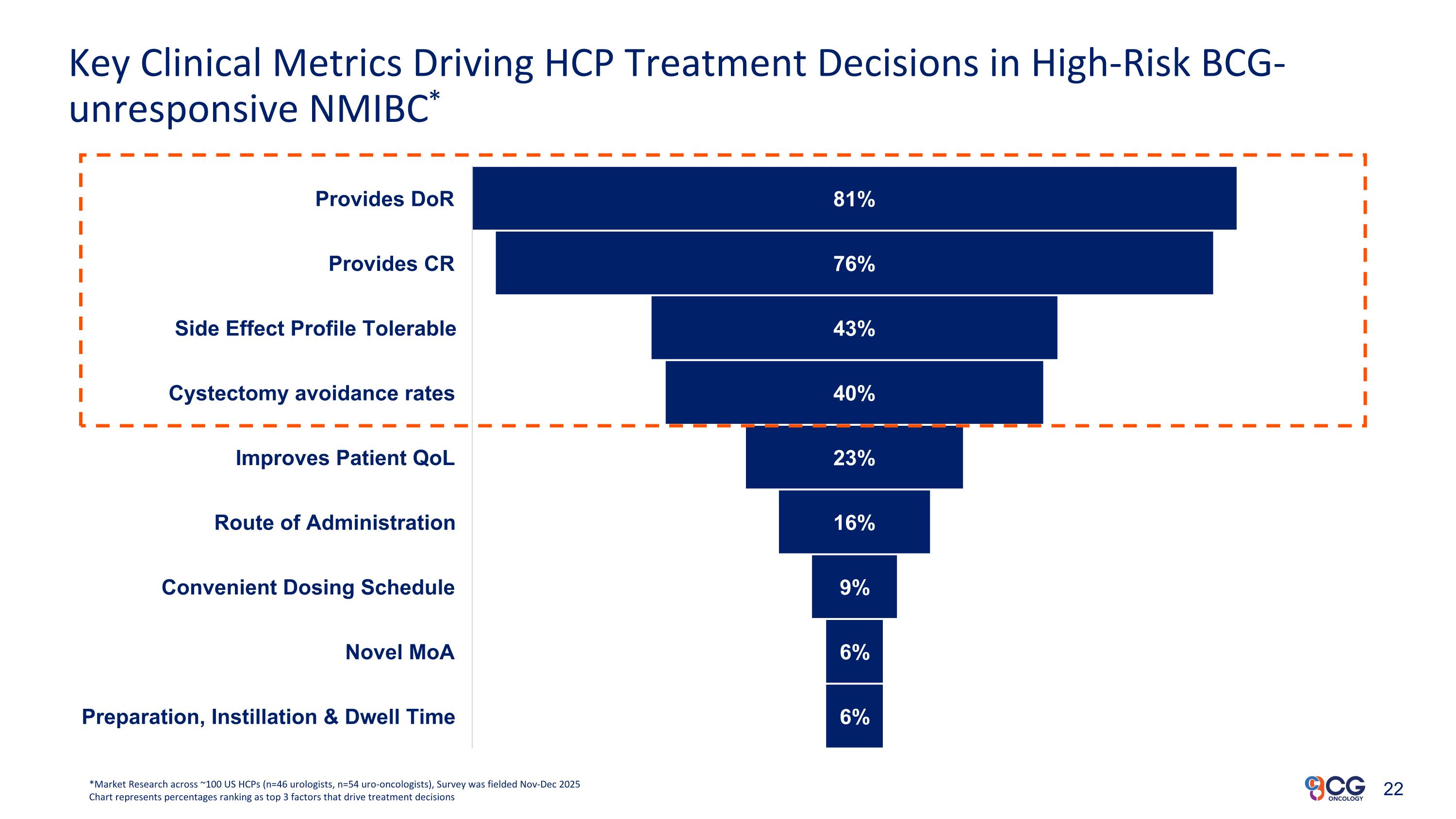
Key Clinical Metrics Driving HCP Treatment Decisions in High-Risk BCG-unresponsive NMIBC* *Market Research across ~100 US HCPs (n=46 urologists, n=54 uro-oncologists), Survey was fielded Nov-Dec 2025 Chart represents percentages ranking as top 3 factors that drive treatment decisions
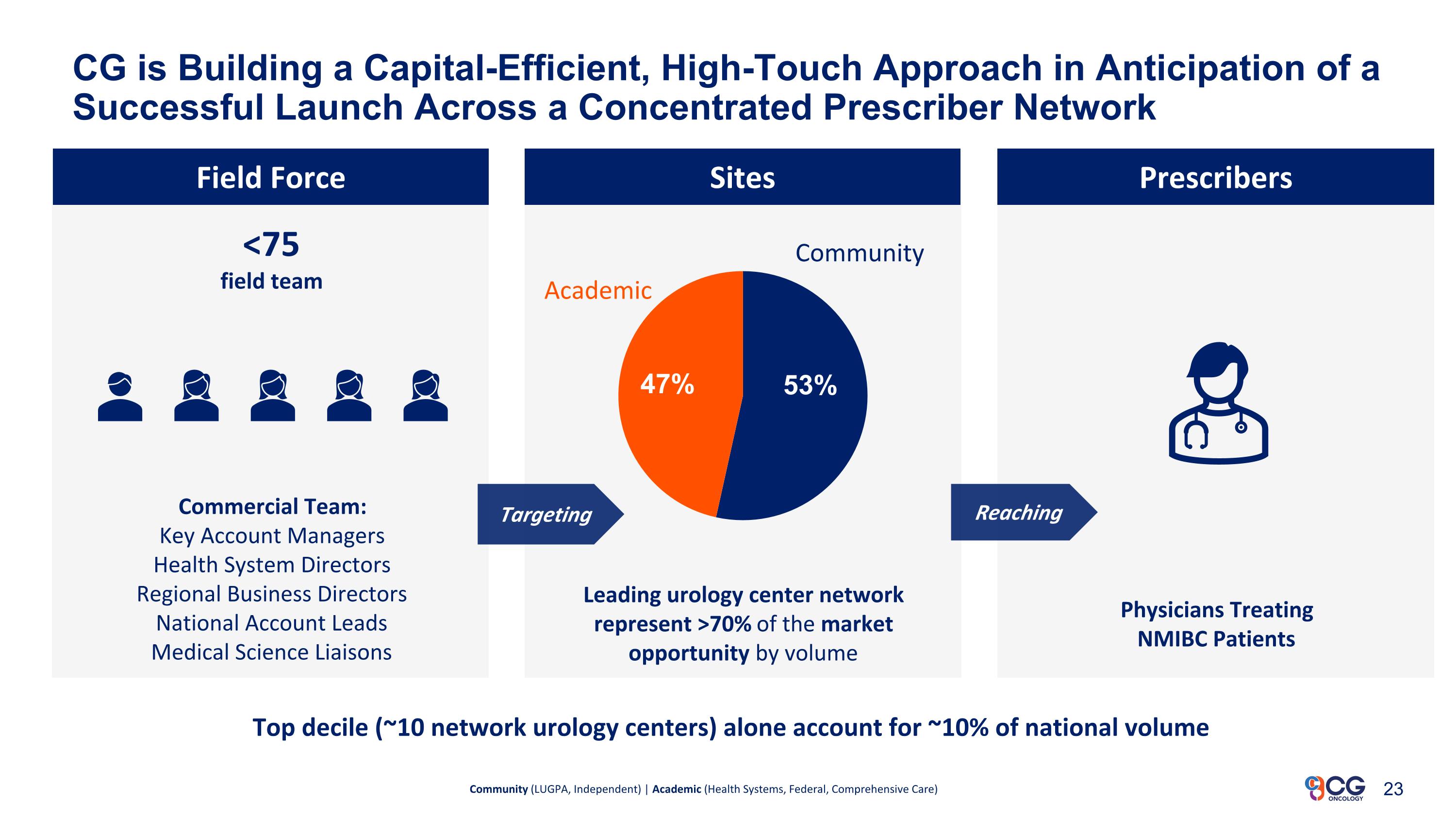
CG is Building a Capital-Efficient, High-Touch Approach in Anticipation of a Successful Launch Across a Concentrated Prescriber Network Field Force Commercial Team: Key Account Managers Health System Directors Regional Business Directors National Account Leads Medical Science Liaisons Physicians Treating NMIBC Patients Community (LUGPA, Independent) | Academic (Health Systems, Federal, Comprehensive Care) Top decile (~10 network urology centers) alone account for ~10% of national volume Targeting Reaching Sites Prescribers <75 field team Community Academic Leading urology center network represent >70% of the market opportunity by volume
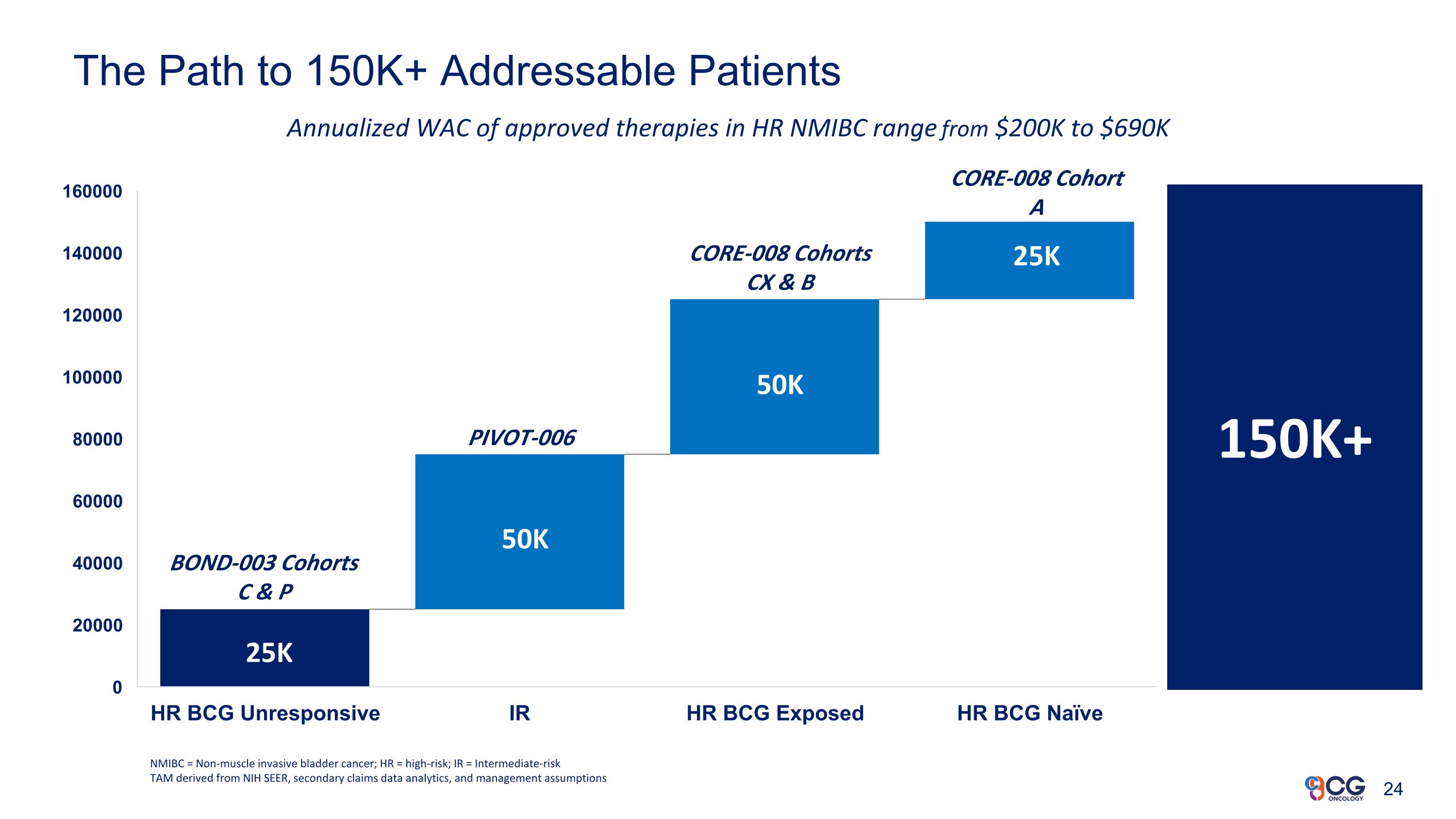
The Path to 150K+ Addressable Patients NMIBC = Non-muscle invasive bladder cancer; HR = high-risk; IR = Intermediate-risk TAM derived from NIH SEER, secondary claims data analytics, and management assumptions 150K+ Annualized WAC of approved therapies in HR NMIBC range from $200K to $690K 50K 50K 25K 25K BOND-003 Cohorts C & P PIVOT-006 CORE-008 Cohorts CX & B CORE-008 Cohort A
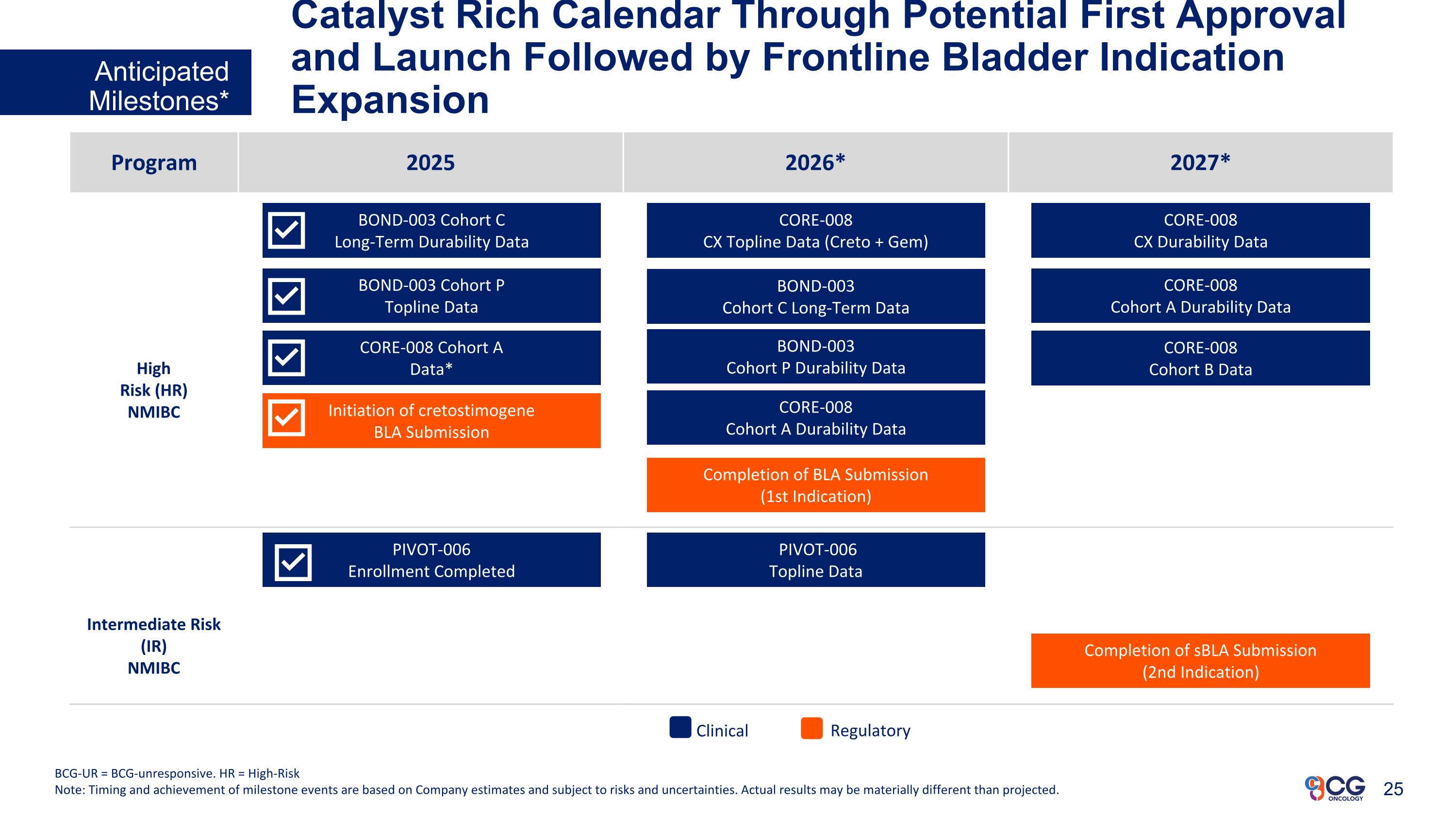
Catalyst Rich Calendar Through Potential First Approval and Launch Followed by Frontline Bladder Indication Expansion Anticipated Milestones* BCG-UR = BCG-unresponsive. HR = High-Risk Note: Timing and achievement of milestone events are based on Company estimates and subject to risks and uncertainties. Actual results may be materially different than projected. Program 2025 2026* 2027* High Risk (HR) NMIBC Intermediate Risk (IR) NMIBC Initiation of cretostimogene BLA Submission BOND-003 Cohort C Long-Term Durability Data PIVOT-006 Enrollment Completed BOND-003 Cohort P Topline Data CORE-008 Cohort A Data* Completion of BLA Submission (1st Indication) BOND-003 Cohort C Long-Term Data CORE-008 CX Topline Data (Creto + Gem) BOND-003 Cohort P Durability Data CORE-008 Cohort A Durability Data PIVOT-006 Topline Data Completion of sBLA Submission (2nd Indication) Regulatory Clinical CORE-008 CX Durability Data CORE-008 Cohort A Durability Data CORE-008 Cohort B Data

www.CGOncology.com CONTACT US CG Oncology, Inc. 400 Spectrum Center Dr Suite #2040 Irvine, CA 92618 GENERAL INQUIRIES Information@cgoncology.com MEDIA MediaRelations@cgoncology.com

b b b Arthur Kuan Chairman & CEO Business Insider’s 30 People Under 40 Who Are Transforming Healthcare 2020 Forbes 30 Under 30 featured honoree in healthcare b Ambaw Bellete President & COO Vijay Kasturi, M.D. Chief Medical Officer 30+ Years in Biotech & Life Sciences with multiple BLA approvals & launch experience Chairman of the Board for OncoSTING Board member of Axiom Reach Foundation 25+ Years as GU Medical Oncologist Managed Launch Plan for BAVENCIO® Supported multiple INDs, BLAs, and modalities to the clinic and market (TIVDAK®, PADCEV®, and ADCETRIS®) Swapnil Bhargava, Ph.D. Chief Technical Officer b 25 Years as In-House Counsel in Biopharmaceutical Industry Over $2.25B in transaction experience Joshua Patterson, Esq. General Counsel & CCO Executive Leadership Team Deep Industry Experience with Track Record of Success in Drug Development and Commercialization 27

Our Vision We see a world where urologic cancer patients can benefit from our innovative immunotherapies to live with dignity and have an enhanced quality of life. Our Mission We are focused on developing bladder-sparing therapeutics for patients afflicted with bladder cancer. Attacking Bladder Cancer for a Better Tomorrow™