

Topline Data: KYSA-8 Registrational Trial of Miv-cel (KYV-101) in Stiff Person Syndrome (SPS) December 15, 2025 ©2025 Kyverna Therapeutics, Inc.

Disclaimer and Forward-Looking Statements This presentation contains forward-looking statements that are based on management’s beliefs and assumptions and information currently available to management of Kyverna Therapeutics, Inc. (“Kyverna”, “we”, “our,” or the “Company”). All statements other than statements of historical facts contained in this presentation are forward-looking statements. Forward looking statements include, but are not limited to, statements concerning: the Company’s future results of operations and financial position, business strategy, drug candidates, planned preclinical studies and clinical trials, results of preclinical studies, named-patient access data, ongoing clinical trials, research and development costs, plans for manufacturing, regulatory approvals, timing and likelihood of success, as well as plans and objectives of management for future operations. These forward-looking statements are subject to risks and uncertainties, including the factors described under the Risk Factors section of the Company’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q that the Company has filed or may subsequently file with the U.S. Securities and Exchange Commission. Actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. When evaluating Kyverna’s business and prospects, careful consideration should be given to these risks and uncertainties. These statements speak only as of the date of this presentation, and Kyverna undertakes no obligation to update or revise these statements. This presentation also contains estimates made by independent parties relating to industry market size and other data. These estimates involve a number of assumptions and limitations and you are cautioned not to give undue weight on such estimates. We have not independently verified the accuracy or completeness of such information, and we do not take any responsibility for the accuracy or completeness of such information. This presentation contains references to trademarks and marks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this presentation may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. The Company does not intend its use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of the Company by any other companies. This presentation includes results from named-patient basis access. Similar to expanded access or compassionate use in the United States, “IH” or “Individueller Heilversuch,” also known as “named-patient basis access,” is a regulatory scheme in Germany that allows for the supply of a treatment that has not received marketing authorization for an individual patient in response to a request by the treating physician on behalf of the named patient. This option can be pursued for the expected benefit of a patient who has exhausted all available treatment options, under the discretion of the treating physician, with the patient’s consent. The use of Miv-cel in the IH settings is not a substitute for, or intended to replace, our clinical trials. The goal is not to assess the effectiveness of a therapy, but rather to provide an individual patient with a possible efficacious approach when all other treatment options have failed, as determined by the patient’s physician. While we do not expect to be able to use the results from these activities as the basis for approval in our applications for marketing approval to the U.S. Food and Drug Administration (FDA) or other foreign regulatory agencies, we believe such activities may provide additional clinical insights beyond highly focused clinical trials in specific geographies. ©2025 Kyverna Therapeutics, Inc. 2

Today’s Agenda Speakers • Setting the Stage for Miv-cel’s Transformative Impact in SPS Warner Biddle Naji Gehchan, M.D., MSc, MBA Chief Executive Officer Chief Medical • Registrational Topline & Development Officer Results Q&A • Expanding Our Leadership in Autoimmune CAR T • Q&A Marc Grasso, M.D. Sham Dholakia, M.D., Ph.D. Amanda Piquet, M.D., FAAN Chief Financial Officer Chief Product Officer University of Colorado Anschutz Céline Dion Foundation Endowed Chair ©2025 Kyverna Therapeutics, Inc. 3 CAR, chimeric antigen receptor.

Kyverna’s Leadership in Autoimmune CAR T: Miv-cel’s Transformative Impact in SPS SPS is a debilitating, progressive autoimmune disease with no FDA-approved therapies and significant unmet medical need Miv-cel achieved statistically significant clinical benefit across all primary and secondary endpoints, reversing disability scores; patients remained free of immunotherapies as of last follow up; data supports BLA submission expected in 1H 2026 FIRST completed registration-enabling CAR T trial for autoimmune disease could pave the way for miv-cel to become the FIRST and ONLY approved therapy in SPS and CAR T-cell therapy for autoimmune disease Valuable commercial opportunity in SPS, supported by attractive market dynamics and focused commercialization strategy First-to-market opportunity in SPS lays foundation for expansion into broader indications ©2025 Kyverna Therapeutics, Inc. 4 CAR, chimeric antigen receptor.
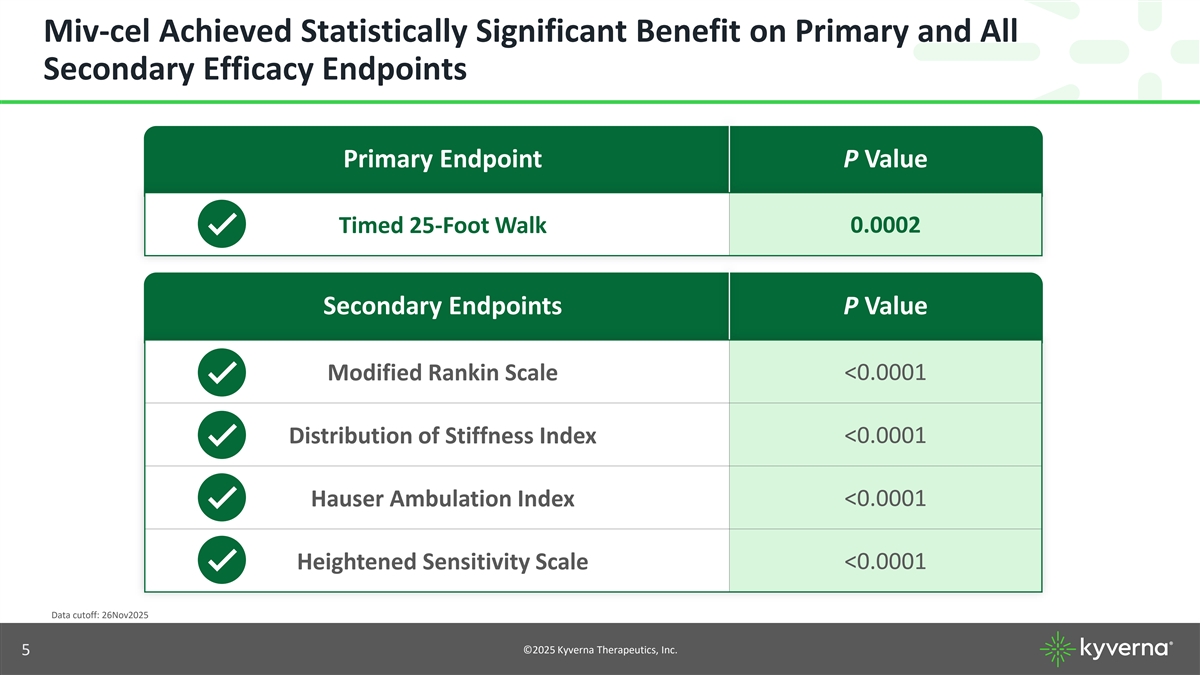
Miv-cel Achieved Statistically Significant Benefit on Primary and All Secondary Efficacy Endpoints Primary Endpoint P Value 0.0002 Timed 25-Foot Walk Secondary Endpoints P Value Modified Rankin Scale <0.0001 Distribution of Stiffness Index <0.0001 Hauser Ambulation Index <0.0001 <0.0001 Heightened Sensitivity Scale Data cutoff: 26Nov2025 ©2025 Kyverna Therapeutics, Inc. 5

Patient Video KYSA-8 Clinical Trial
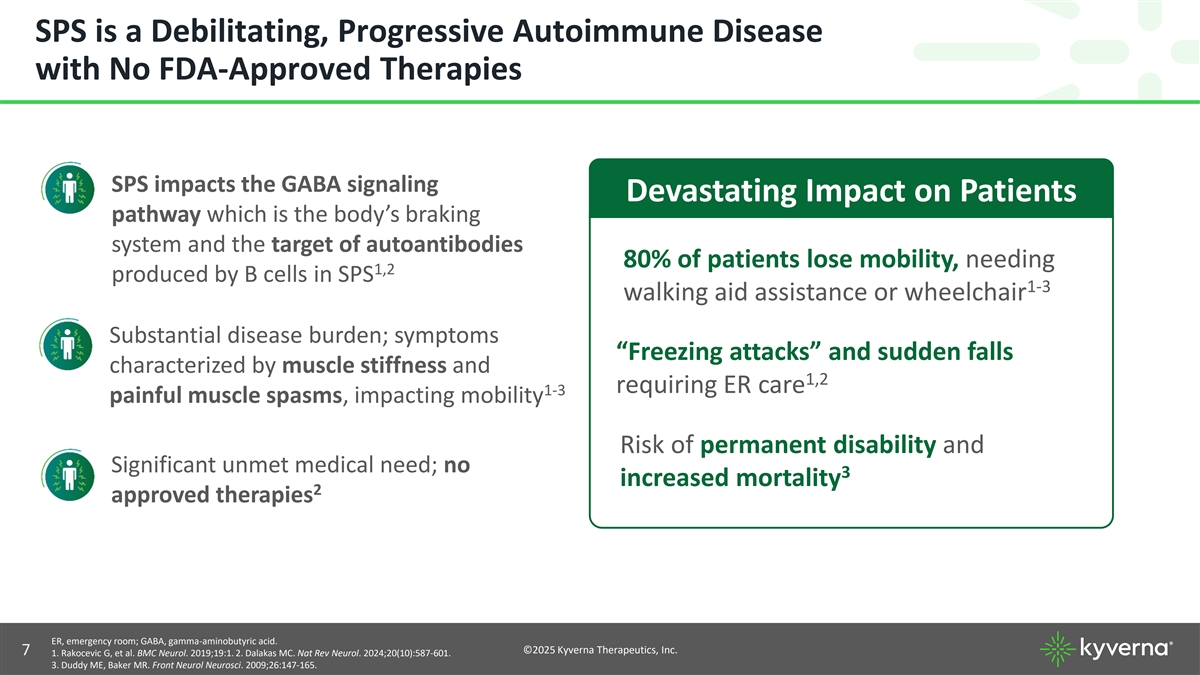
SPS is a Debilitating, Progressive Autoimmune Disease with No FDA-Approved Therapies SPS impacts the GABA signaling Devastating Impact on Patients pathway which is the body’s braking system and the target of autoantibodies 80% of patients lose mobility, needing 1,2 produced by B cells in SPS 1-3 walking aid assistance or wheelchair Substantial disease burden; symptoms “Freezing attacks” and sudden falls characterized by muscle stiffness and 1,2 requiring ER care 1-3 painful muscle spasms, impacting mobility Risk of permanent disability and Significant unmet medical need; no 3 increased mortality 2 approved therapies ER, emergency room; GABA, gamma-aminobutyric acid. ©2025 Kyverna Therapeutics, Inc. 7 1. Rakocevic G, et al. BMC Neurol. 2019;19:1. 2. Dalakas MC. Nat Rev Neurol. 2024;20(10):587-601. 3. Duddy ME, Baker MR. Front Neurol Neurosci. 2009;26:147-165.
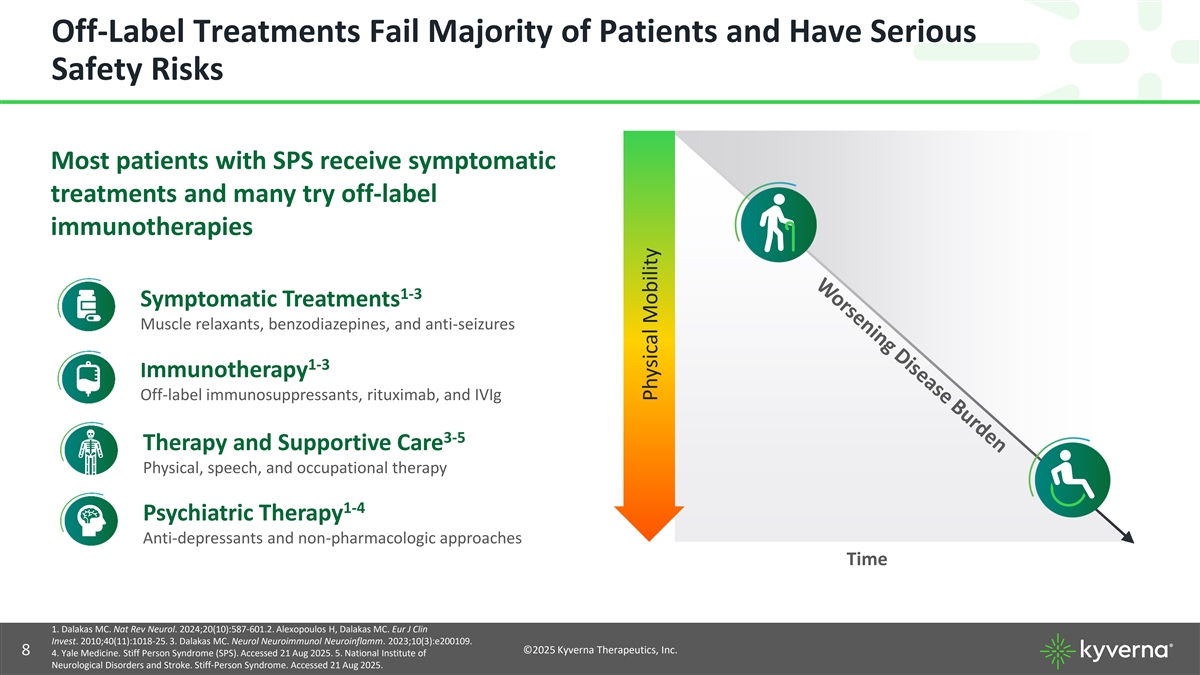
Off-Label Treatments Fail Majority of Patients and Have Serious Safety Risks Most patients with SPS receive symptomatic treatments and many try off-label immunotherapies 1-3 Symptomatic Treatments Muscle relaxants, benzodiazepines, and anti-seizures 1-3 Immunotherapy Off-label immunosuppressants, rituximab, and IVIg 3-5 Therapy and Supportive Care Physical, speech, and occupational therapy 1-4 Psychiatric Therapy Anti-depressants and non-pharmacologic approaches Time 1. Dalakas MC. Nat Rev Neurol. 2024;20(10):587-601.2. Alexopoulos H, Dalakas MC. Eur J Clin Invest. 2010;40(11):1018-25. 3. Dalakas MC. Neurol Neuroimmunol Neuroinflamm. 2023;10(3):e200109. ©2025 Kyverna Therapeutics, Inc. 8 4. Yale Medicine. Stiff Person Syndrome (SPS). Accessed 21 Aug 2025. 5. National Institute of Neurological Disorders and Stroke. Stiff-Person Syndrome. Accessed 21 Aug 2025. Physical Mobility
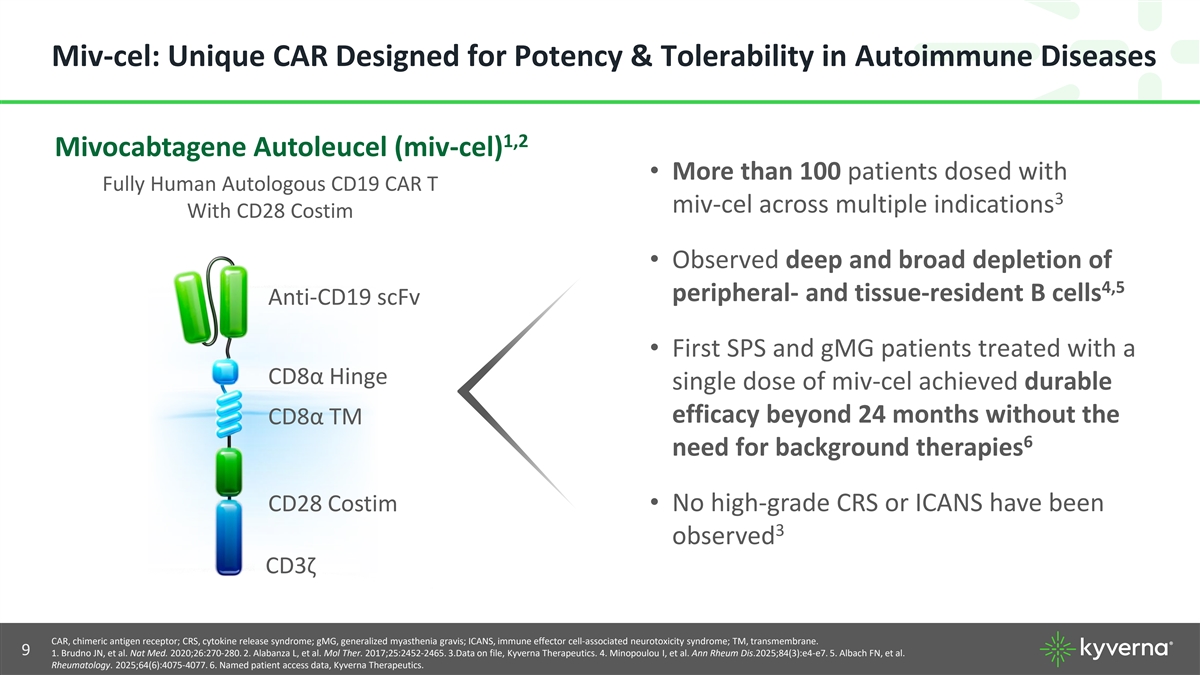
Miv-cel: Unique CAR Designed for Potency & Tolerability in Autoimmune Diseases 1,2 Mivocabtagene Autoleucel (miv-cel) • More than 100 patients dosed with Fully Human Autologous CD19 CAR T 3 miv-cel across multiple indications With CD28 Costim • Observed deep and broad depletion of 4,5 peripheral- and tissue-resident B cells Anti-CD19 scFv • First SPS and gMG patients treated with a CD8α Hinge single dose of miv-cel achieved durable efficacy beyond 24 months without the CD8α TM 6 need for background therapies CD28 Costim• No high-grade CRS or ICANS have been 3 observed CD3ζ CAR, chimeric antigen receptor; CRS, cytokine release syndrome; gMG, generalized myasthenia gravis; ICANS, immune effector cell-associated neurotoxicity syndrome; TM, transmembrane. ©2025 Kyverna Therapeutics, Inc. 9 1. Brudno JN, et al. Nat Med. 2020;26:270-280. 2. Alabanza L, et al. Mol Ther. 2017;25:2452-2465. 3.Data on file, Kyverna Therapeutics. 4. Minopoulou I, et al. Ann Rheum Dis.2025;84(3):e4-e7. 5. Albach FN, et al. Rheumatology. 2025;64(6):4075-4077. 6. Named patient access data, Kyverna Therapeutics.
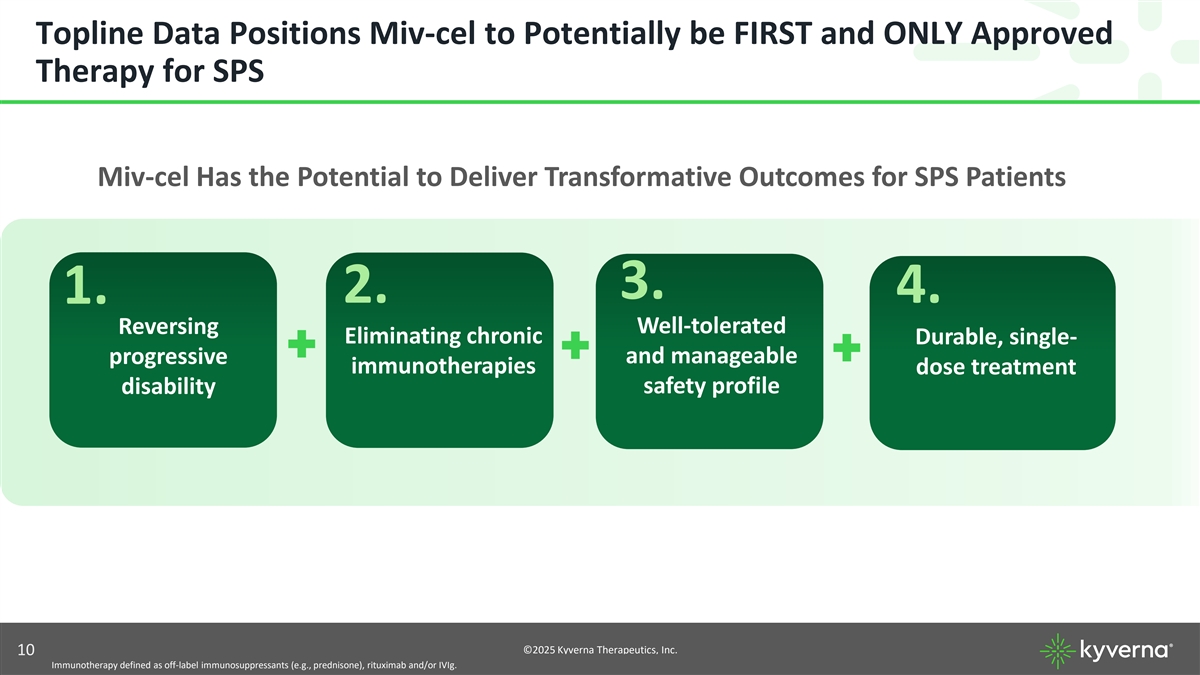
Topline Data Positions Miv-cel to Potentially be FIRST and ONLY Approved Therapy for SPS Miv-cel Has the Potential to Deliver Transformative Outcomes for SPS Patients 3. 2. 4. 1. Reversing Well-tolerated Eliminating chronic Durable, single- progressive and manageable immunotherapies dose treatment disability safety profile ©2025 Kyverna Therapeutics, Inc. 10 Immunotherapy defined as off-label immunosuppressants (e.g., prednisone), rituximab and/or IVIg.

Registrational Topline Results Naji Gehchan, M.D., MSc, MBA – Chief Medical and Development Officer
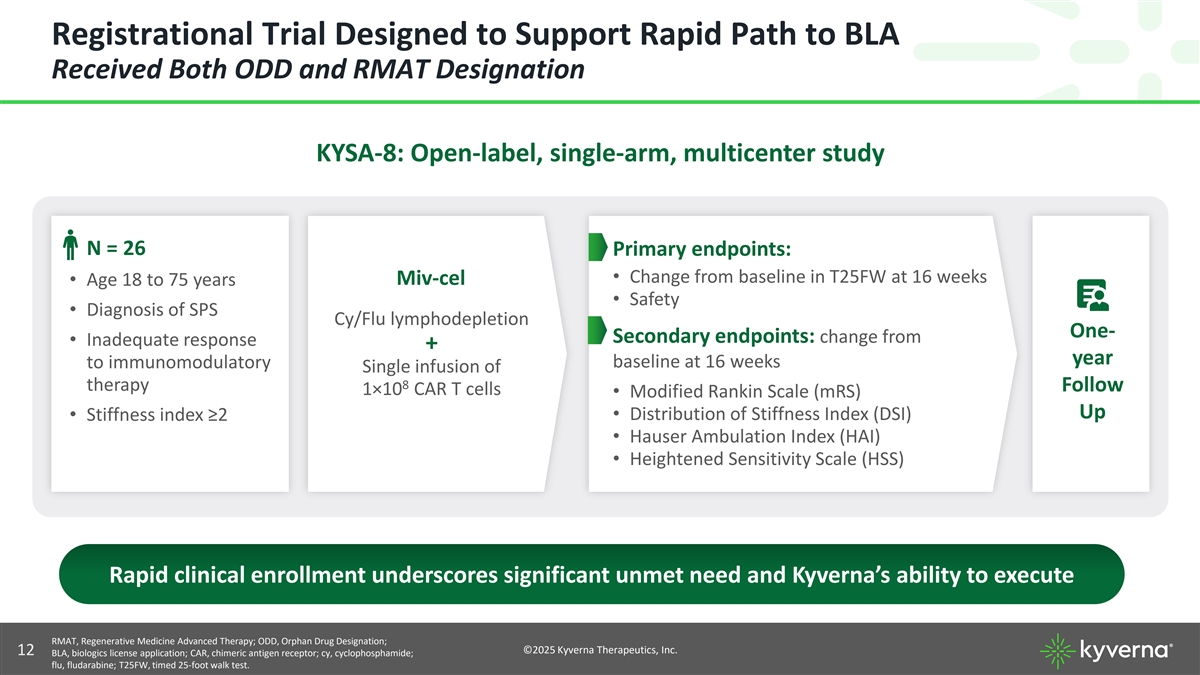
Registrational Trial Designed to Support Rapid Path to BLA Received Both ODD and RMAT Designation KYSA-8: Open-label, single-arm, multicenter study N = 26 Primary endpoints: • Change from baseline in T25FW at 16 weeks Miv-cel • Age 18 to 75 years • Safety • Diagnosis of SPS Cy/Flu lymphodepletion One- Secondary endpoints: change from • Inadequate response + year baseline at 16 weeks to immunomodulatory Single infusion of 8 therapy Follow 1×10 CAR T cells • Modified Rankin Scale (mRS) Up • Distribution of Stiffness Index (DSI) • Stiffness index ≥2 • Hauser Ambulation Index (HAI) • Heightened Sensitivity Scale (HSS) Rapid clinical enrollment underscores significant unmet need and Kyverna’s ability to execute RMAT, Regenerative Medicine Advanced Therapy; ODD, Orphan Drug Designation; ©2025 Kyverna Therapeutics, Inc. 12 BLA, biologics license application; CAR, chimeric antigen receptor; cy, cyclophosphamide; flu, fludarabine; T25FW, timed 25-foot walk test.
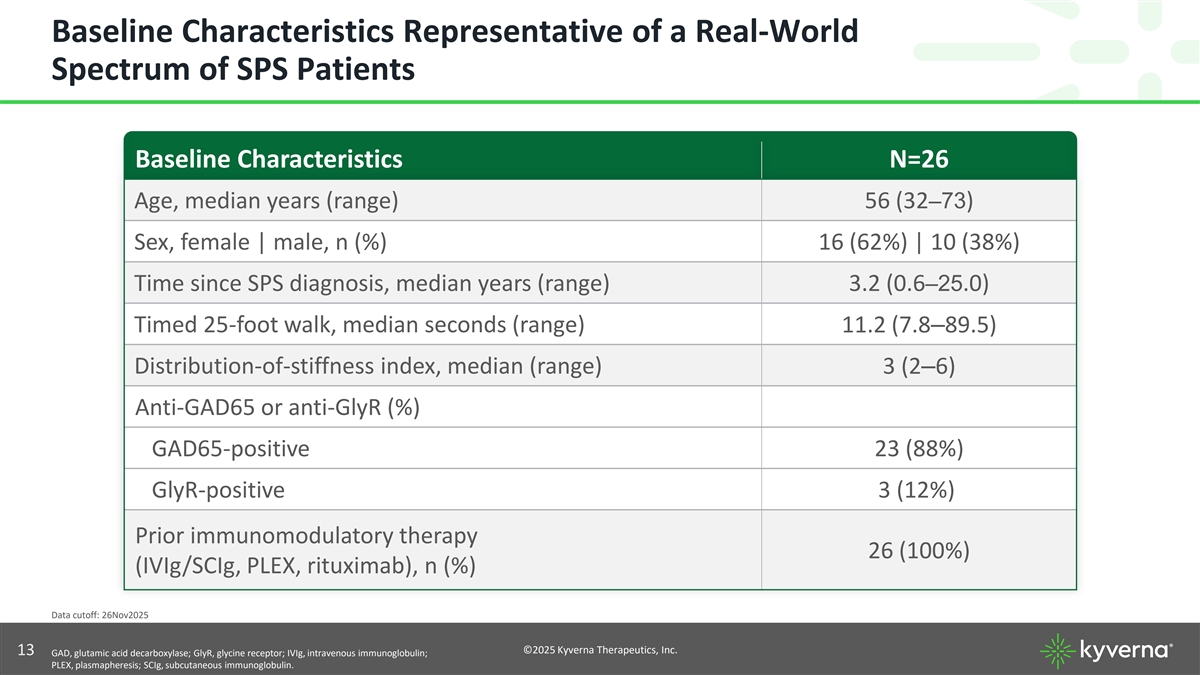
Baseline Characteristics Representative of a Real-World Spectrum of SPS Patients Baseline Characteristics N=26 Age, median years (range) 56 (32–73) Sex, female | male, n (%) 16 (62%) | 10 (38%) Time since SPS diagnosis, median years (range) 3.2 (0.6–25.0) Timed 25-foot walk, median seconds (range) 11.2 (7.8–89.5) Distribution-of-stiffness index, median (range) 3 (2–6) Anti-GAD65 or anti-GlyR (%) GAD65-positive 23 (88%) GlyR-positive 3 (12%) Prior immunomodulatory therapy 26 (100%) (IVIg/SCIg, PLEX, rituximab), n (%) Data cutoff: 26Nov2025 ©2025 Kyverna Therapeutics, Inc. 13 GAD, glutamic acid decarboxylase; GlyR, glycine receptor; IVIg, intravenous immunoglobulin; PLEX, plasmapheresis; SCIg, subcutaneous immunoglobulin.
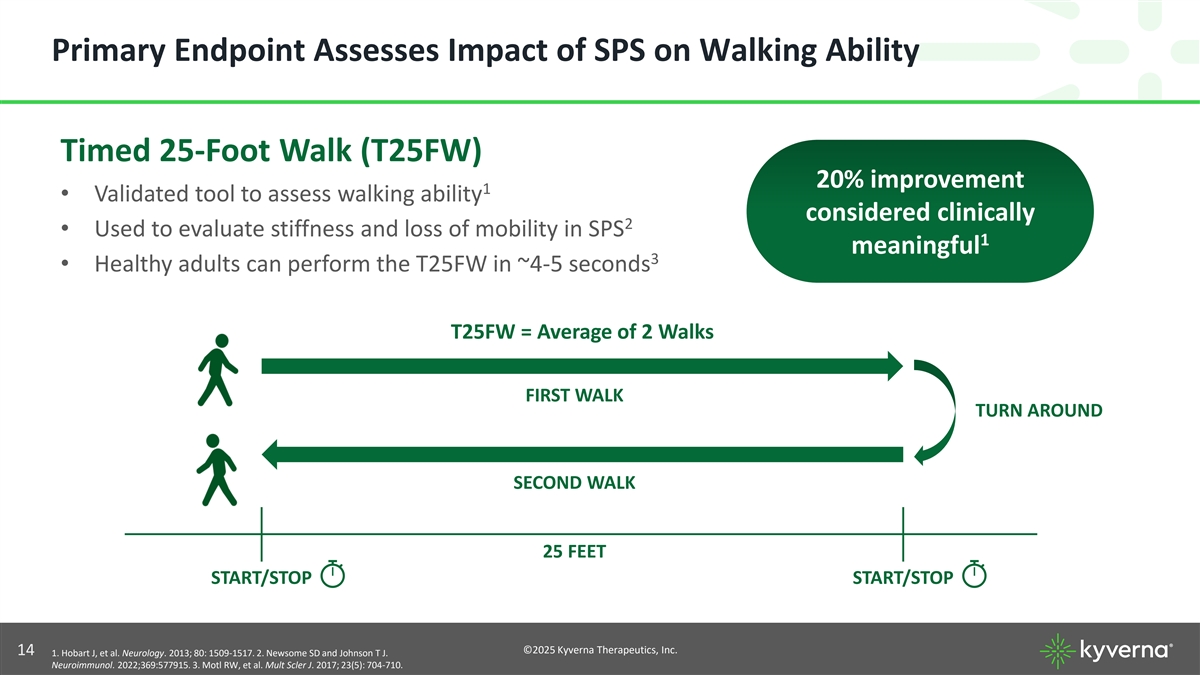
Primary Endpoint Assesses Impact of SPS on Walking Ability Timed 25-Foot Walk (T25FW) 20% improvement 1 • Validated tool to assess walking ability considered clinically 2 • Used to evaluate stiffness and loss of mobility in SPS 1 meaningful 3 • Healthy adults can perform the T25FW in ~4-5 seconds T25FW = Average of 2 Walks FIRST WALK TURN AROUND SECOND WALK 25 FEET START/STOP START/STOP ©2025 Kyverna Therapeutics, Inc. 14 1. Hobart J, et al. Neurology. 2013; 80: 1509-1517. 2. Newsome SD and Johnson T J. Neuroimmunol. 2022;369:577915. 3. Motl RW, et al. Mult Scler J. 2017; 23(5): 704-710.
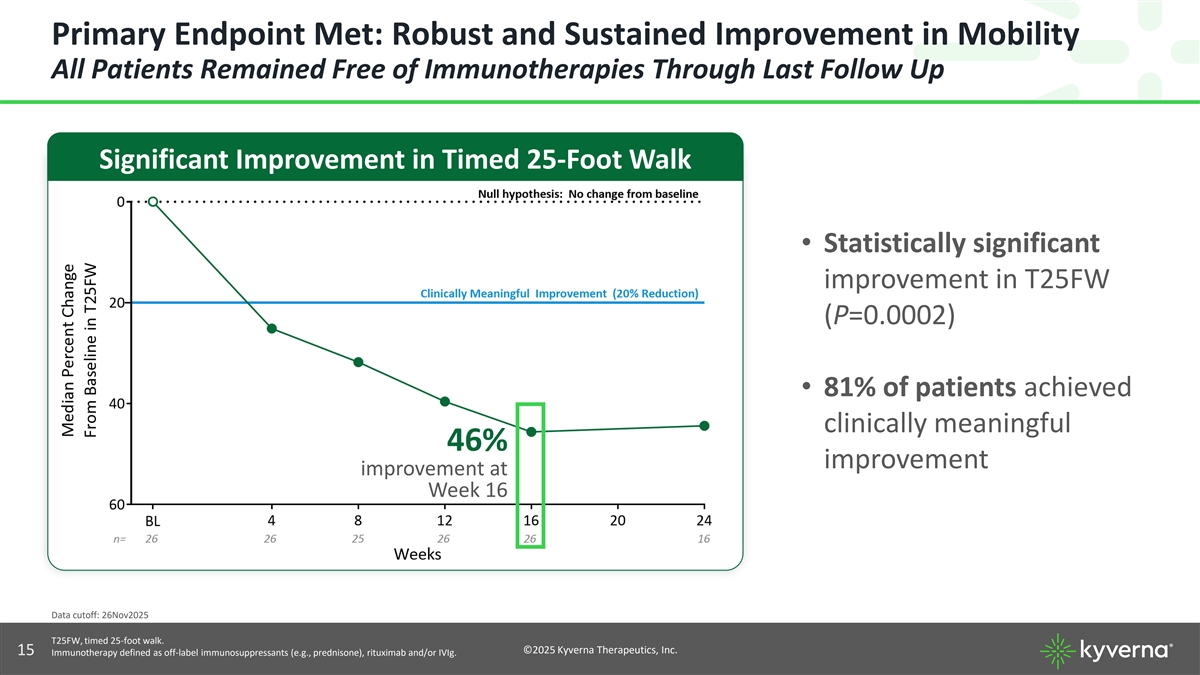
Primary Endpoint Met: Robust and Sustained Improvement in Mobility All Patients Remained Free of Immunotherapies Through Last Follow Up Significant Improvement in Timed 25-Foot Walk • Statistically significant improvement in T25FW (P=0.0002) • 81% of patients achieved clinically meaningful 46% improvement improvement at Week 16 Data cutoff: 26Nov2025 T25FW, timed 25-foot walk. ©2025 Kyverna Therapeutics, Inc. 15 Immunotherapy defined as off-label immunosuppressants (e.g., prednisone), rituximab and/or IVIg.
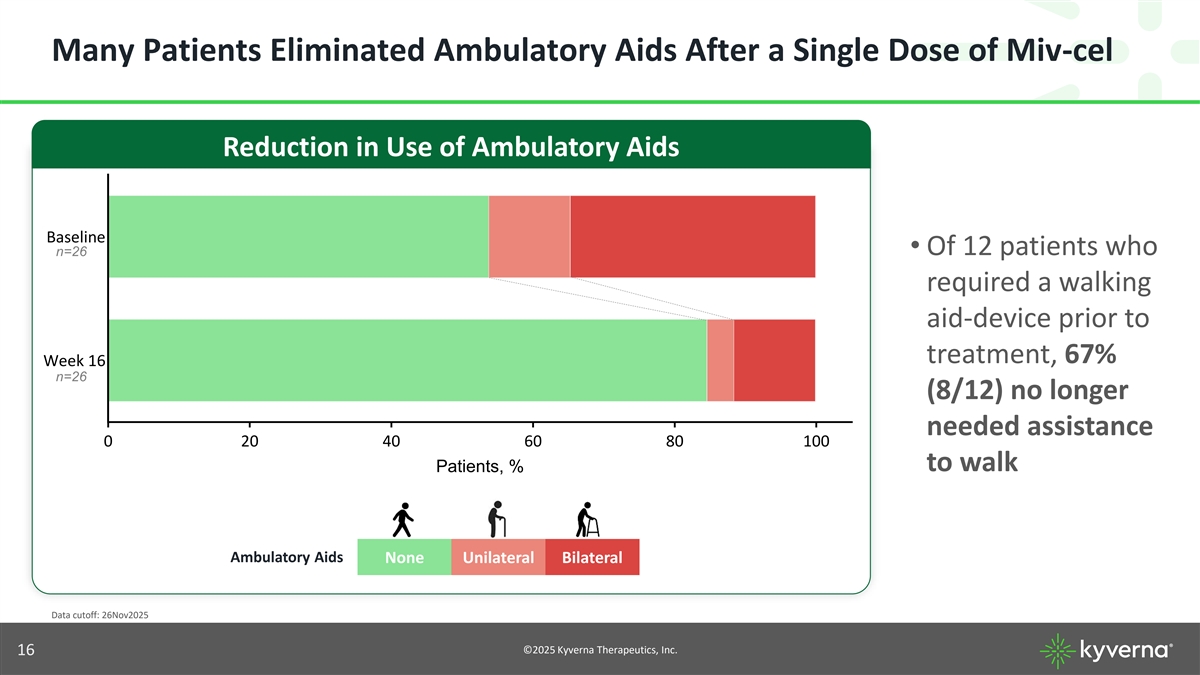
Many Patients Eliminated Ambulatory Aids After a Single Dose of Miv-cel Reduction in Use of Ambulatory Aids Baseline • Of 12 patients who n=26 required a walking aid-device prior to treatment, 67% Week 16 n=26 (8/12) no longer needed assistance 0 20 40 60 80 100 to walk Patients, % Ambulatory Aids None Unilateral Bilateral Data cutoff: 26Nov2025 ©2025 Kyverna Therapeutics, Inc. 16
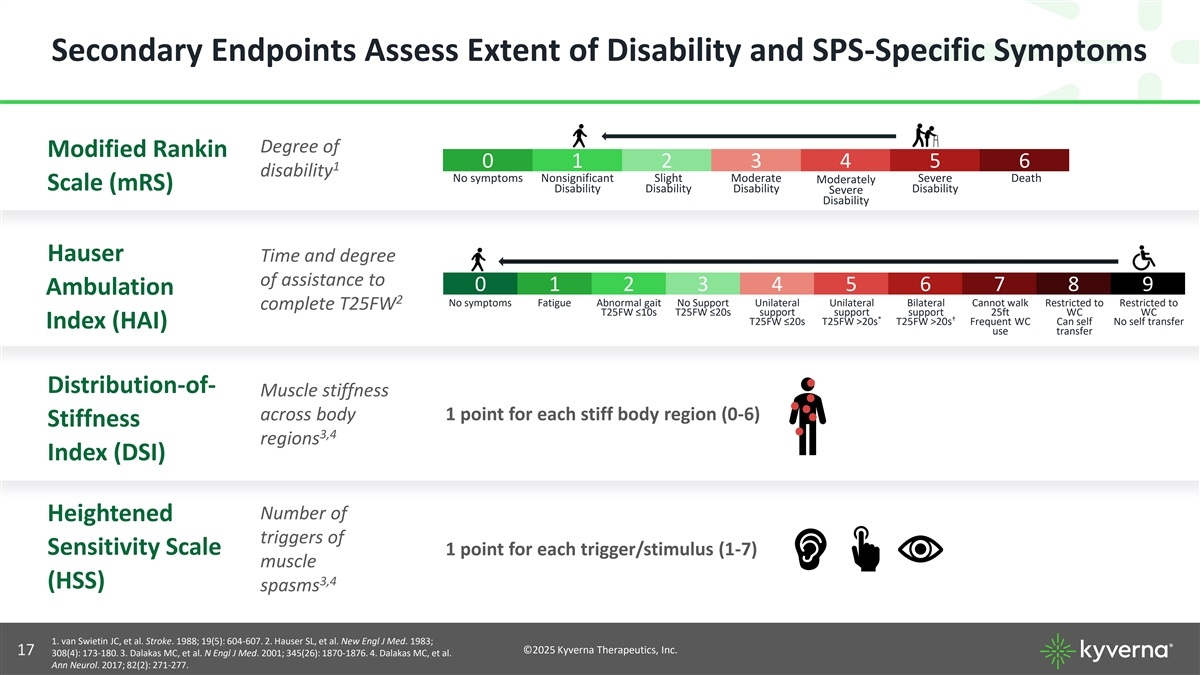
Secondary Endpoints Assess Extent of Disability and SPS-Specific Symptoms Degree of Modified Rankin 0 1 2 3 4 5 6 1 disability No symptoms Nonsignificant Slight Moderate Severe Death Moderately Scale (mRS) Disability Disability Disability Disability Severe Disability Hauser Time and degree of assistance to 0 1 2 3 4 5 6 7 8 9 Ambulation 2 No symptoms Fatigue Abnormal gait No Support Unilateral Unilateral Bilateral Cannot walk Restricted to Restricted to complete T25FW T25FW ≤10s T25FW ≤20s support support support 25ft WC WC * † T25FW ≤20s T25FW >20s T25FW >20s Frequent WC Can self No self transfer Index (HAI) use transfer Distribution-of- Muscle stiffness across body 1 point for each stiff body region (0-6) Stiffness 3,4 regions Index (DSI) Number of Heightened triggers of Sensitivity Scale 1 point for each trigger/stimulus (1-7) muscle 3,4 (HSS) spasms 1. van Swietin JC, et al. Stroke. 1988; 19(5): 604-607. 2. Hauser SL, et al. New Engl J Med. 1983; ©2025 Kyverna Therapeutics, Inc. 17 308(4): 173-180. 3. Dalakas MC, et al. N Engl J Med. 2001; 345(26): 1870-1876. 4. Dalakas MC, et al. Ann Neurol. 2017; 82(2): 271-277.
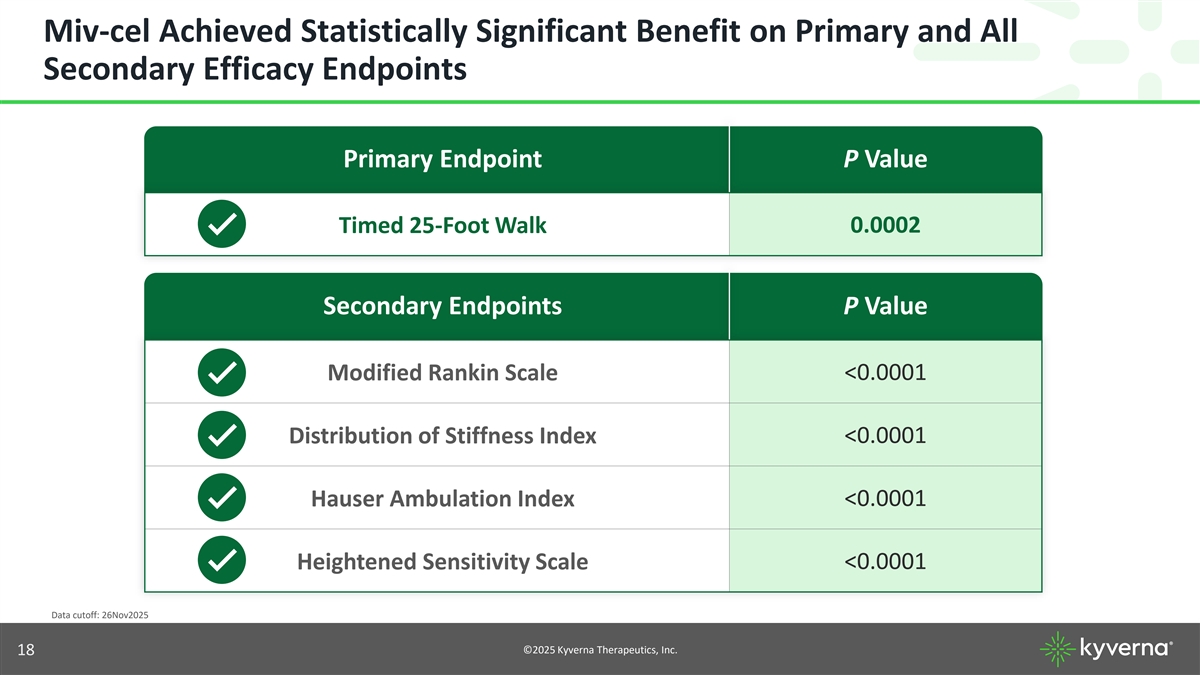
Miv-cel Achieved Statistically Significant Benefit on Primary and All Secondary Efficacy Endpoints Primary Endpoint P Value 0.0002 Timed 25-Foot Walk Secondary Endpoints P Value Modified Rankin Scale <0.0001 Distribution of Stiffness Index <0.0001 Hauser Ambulation Index <0.0001 <0.0001 Heightened Sensitivity Scale Data cutoff: 26Nov2025 ©2025 Kyverna Therapeutics, Inc. 18
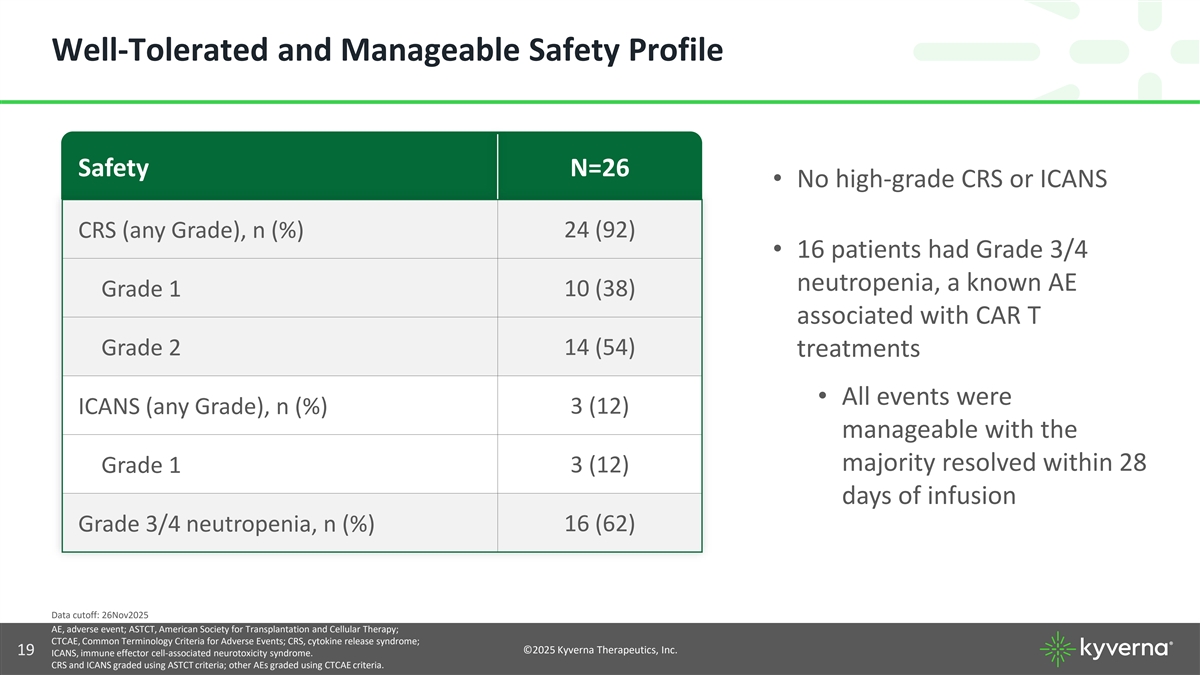
Well-Tolerated and Manageable Safety Profile Safety N=26 • No high-grade CRS or ICANS CRS (any Grade), n (%) 24 (92) • 16 patients had Grade 3/4 neutropenia, a known AE Grade 1 10 (38) associated with CAR T Grade 2 14 (54) treatments • All events were ICANS (any Grade), n (%) 3 (12) manageable with the majority resolved within 28 Grade 1 3 (12) days of infusion Grade 3/4 neutropenia, n (%) 16 (62) Data cutoff: 26Nov2025 AE, adverse event; ASTCT, American Society for Transplantation and Cellular Therapy; CTCAE, Common Terminology Criteria for Adverse Events; CRS, cytokine release syndrome; ©2025 Kyverna Therapeutics, Inc. 19 ICANS, immune effector cell-associated neurotoxicity syndrome. CRS and ICANS graded using ASTCT criteria; other AEs graded using CTCAE criteria.
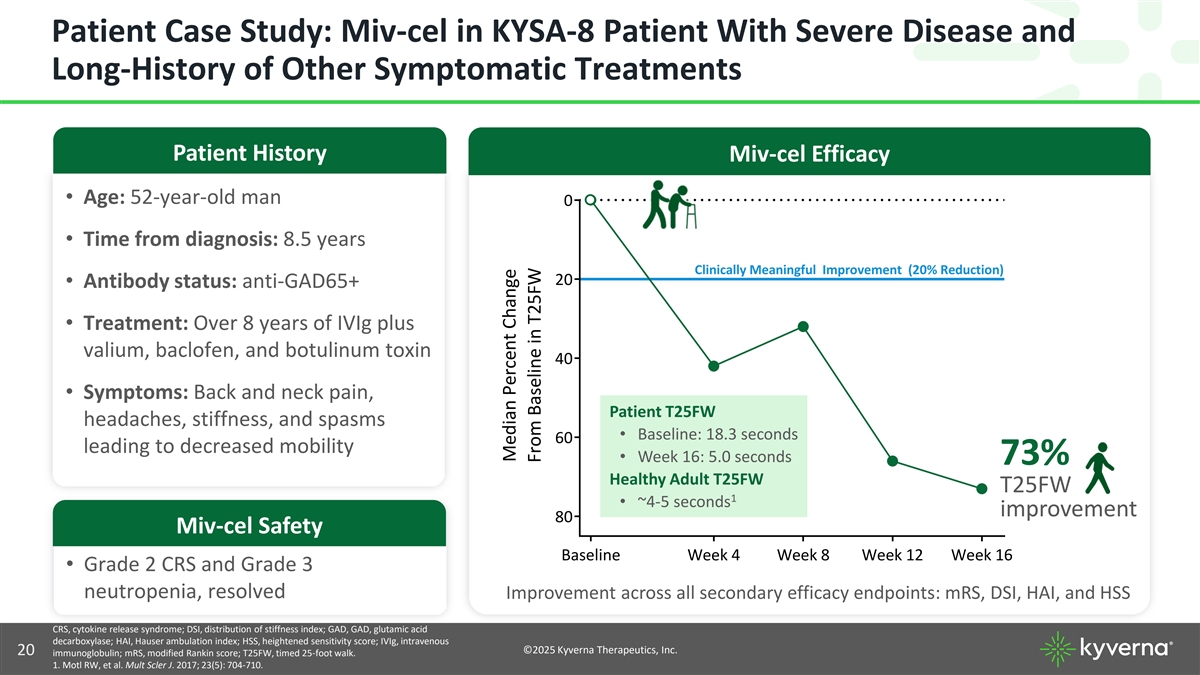
Patient Case Study: Miv-cel in KYSA-8 Patient With Severe Disease and Long-History of Other Symptomatic Treatments Patient History Miv-cel Efficacy • Age: 52-year-old man 0 • Time from diagnosis: 8.5 years Clinically Meaningful Improvement (20% Reduction) 20 • Antibody status: anti-GAD65+ • Treatment: Over 8 years of IVIg plus valium, baclofen, and botulinum toxin 40 • Symptoms: Back and neck pain, Patient T25FW headaches, stiffness, and spasms • Baseline: 18.3 seconds 60 leading to decreased mobility • Week 16: 5.0 seconds 73% Healthy Adult T25FW T25FW 1 • ~4-5 seconds improvement 80 Miv-cel Safety Baseline Week 4 Week 8 Week 12 Week 16 • Grade 2 CRS and Grade 3 neutropenia, resolved Improvement across all secondary efficacy endpoints: mRS, DSI, HAI, and HSS CRS, cytokine release syndrome; DSI, distribution of stiffness index; GAD, GAD, glutamic acid decarboxylase; HAI, Hauser ambulation index; HSS, heightened sensitivity score; IVIg, intravenous ©2025 Kyverna Therapeutics, Inc. 20 immunoglobulin; mRS, modified Rankin score; T25FW, timed 25-foot walk. 1. Motl RW, et al. Mult Scler J. 2017; 23(5): 704-710. Median Percent Change From Baseline in T25FW

Patient Video KYSA-8 Clinical Trial

Today’s Results Represent a Breakthrough in SPS Potentially the First and Only Approved Therapy in SPS and CAR T for Autoimmune First completed registration-enabling trial in autoimmune CAR T Miv-cel achieved highly statistically significant benefit on primary and all secondary efficacy endpoints, durable clinical improvement and reversed disability scores 100% elimination of off-label immunotherapies with a single dose Well-tolerated and manageable safety profile Data supports expected SPS BLA submission for miv-cel in 1H 2026 BLA, biologics license application; CAR, chimeric antigen receptor. ©2025 Kyverna Therapeutics, Inc. 22 Immunotherapy defined as off-label immunosuppressants (e.g., prednisone), rituximab and/or intravenous immunoglobulin.

Expanding Our Leadership in Autoimmune CAR T Warner Biddle – Chief Executive Officer
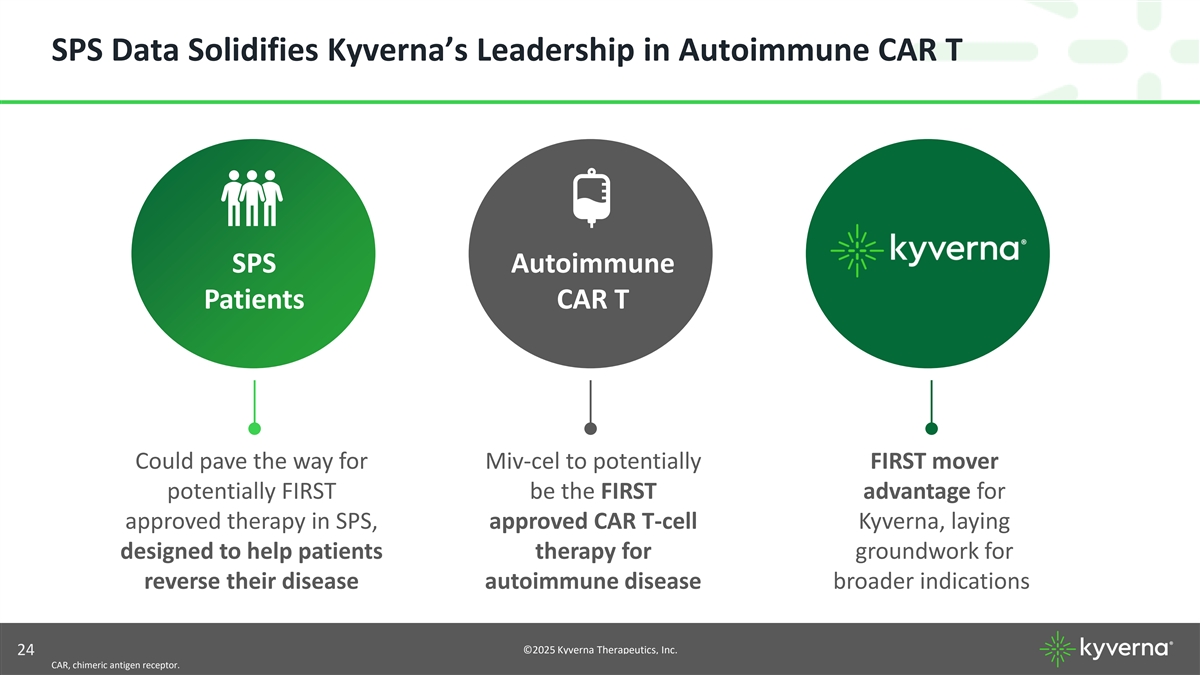
SPS Data Solidifies Kyverna’s Leadership in Autoimmune CAR T SPS Autoimmune Patients CAR T Could pave the way for Miv-cel to potentially FIRST mover potentially FIRST be the FIRST advantage for approved therapy in SPS, approved CAR T-cell Kyverna, laying designed to help patients therapy for groundwork for reverse their disease autoimmune disease broader indications ©2025 Kyverna Therapeutics, Inc. 24 CAR, chimeric antigen receptor.
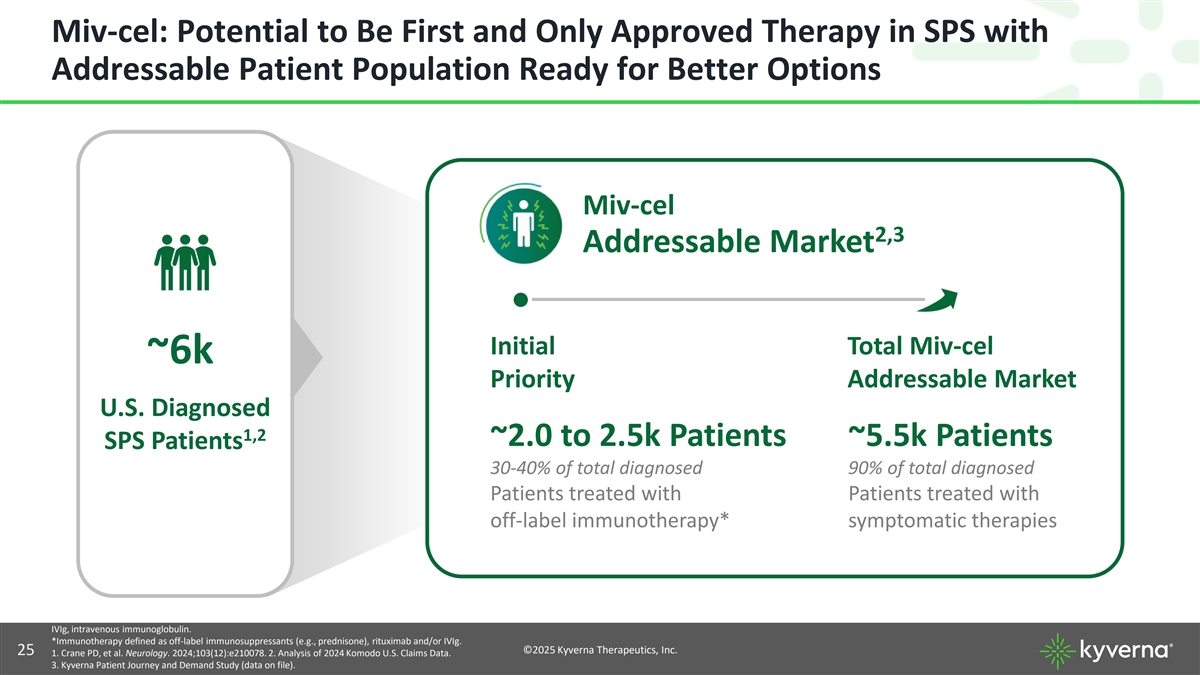
Miv-cel: Potential to Be First and Only Approved Therapy in SPS with Addressable Patient Population Ready for Better Options Miv-cel 2,3 Addressable Market Initial Total Miv-cel ~6k Priority Addressable Market U.S. Diagnosed 1,2 ~2.0 to 2.5k Patients ~5.5k Patients SPS Patients 30-40% of total diagnosed 90% of total diagnosed Patients treated with Patients treated with off-label immunotherapy* symptomatic therapies IVIg, intravenous immunoglobulin. *Immunotherapy defined as off-label immunosuppressants (e.g., prednisone), rituximab and/or IVIg. ©2025 Kyverna Therapeutics, Inc. 25 1. Crane PD, et al. Neurology. 2024;103(12):e210078. 2. Analysis of 2024 Komodo U.S. Claims Data. 3. Kyverna Patient Journey and Demand Study (data on file).
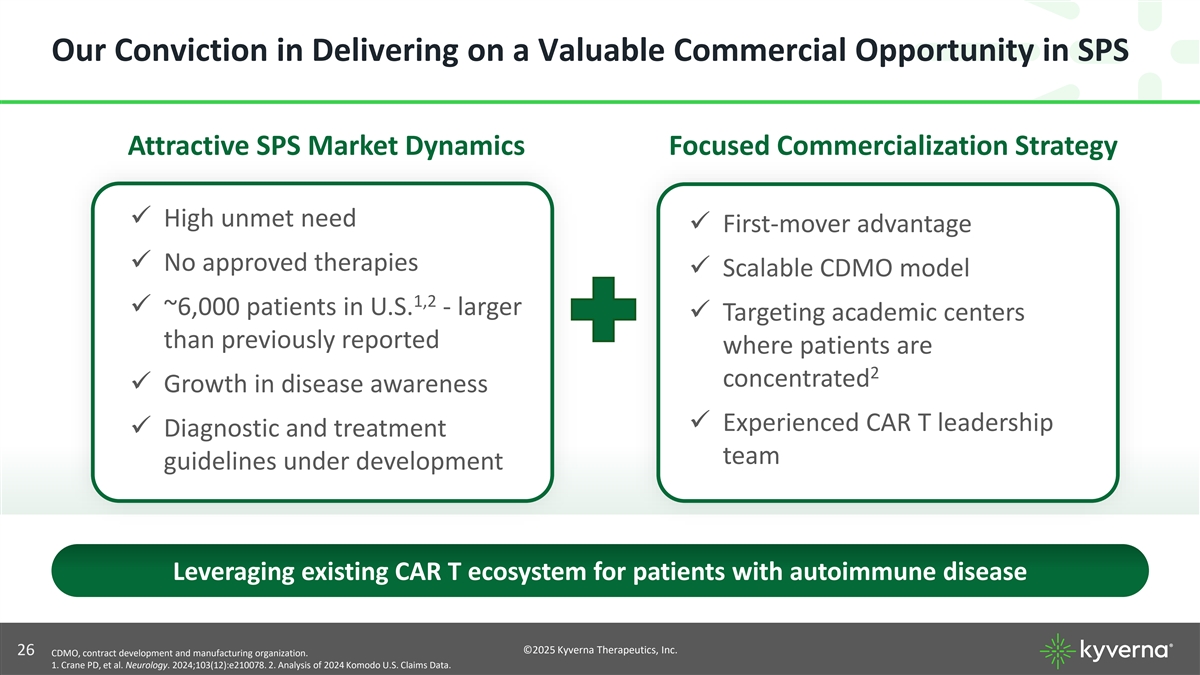
Our Conviction in Delivering on a Valuable Commercial Opportunity in SPS Attractive SPS Market Dynamics Focused Commercialization Strategy ✓ High unmet need ✓ First-mover advantage ✓ No approved therapies ✓ Scalable CDMO model 1,2 ✓ ~6,000 patients in U.S. - larger ✓ Targeting academic centers than previously reported where patients are 2 concentrated ✓ Growth in disease awareness ✓ Experienced CAR T leadership ✓ Diagnostic and treatment team guidelines under development Leveraging existing CAR T ecosystem for patients with autoimmune disease ©2025 Kyverna Therapeutics, Inc. 26 CDMO, contract development and manufacturing organization. 1. Crane PD, et al. Neurology. 2024;103(12):e210078. 2. Analysis of 2024 Komodo U.S. Claims Data.
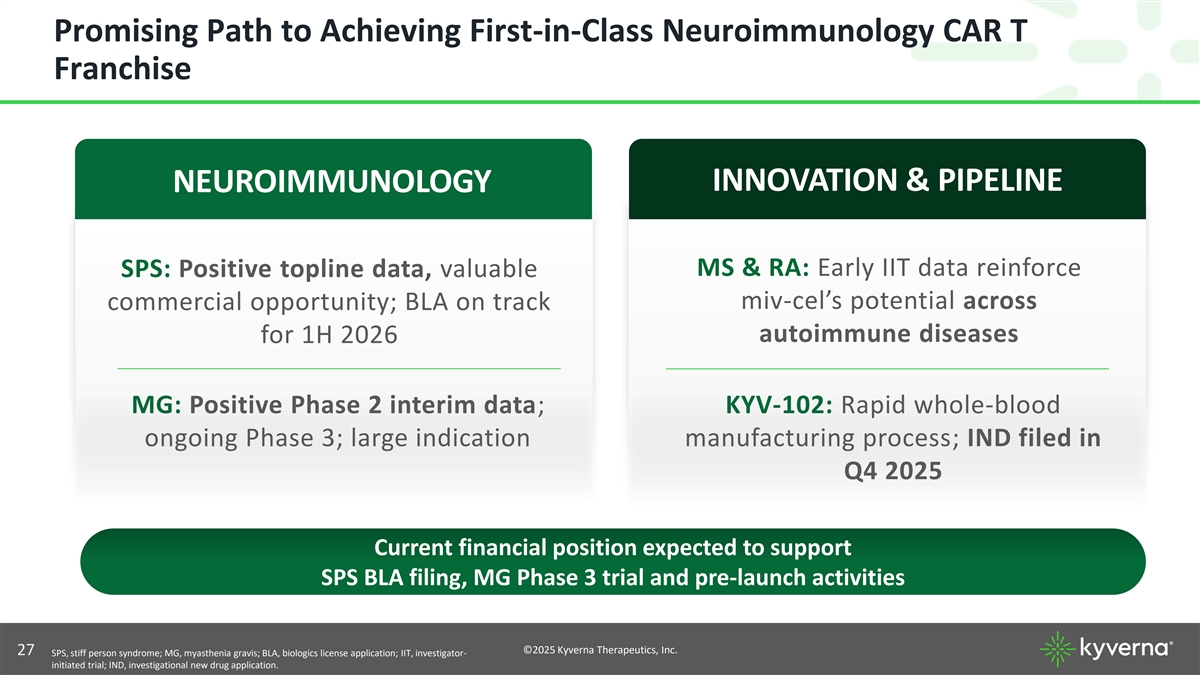
Promising Path to Achieving First-in-Class Neuroimmunology CAR T Franchise INNOVATION & PIPELINE NEUROIMMUNOLOGY MS & RA: Early IIT data reinforce SPS: Positive topline data, valuable miv-cel’s potential across commercial opportunity; BLA on track autoimmune diseases for 1H 2026 MG: Positive Phase 2 interim data; KYV-102: Rapid whole-blood ongoing Phase 3; large indication manufacturing process; IND filed in Q4 2025 Current financial position expected to support SPS BLA filing, MG Phase 3 trial and pre-launch activities ©2025 Kyverna Therapeutics, Inc. 27 SPS, stiff person syndrome; MG, myasthenia gravis; BLA, biologics license application; IIT, investigator- initiated trial; IND, investigational new drug application.

Patient Video KYSA-8 Clinical Trial

Warner Biddle Naji Gehchan, M.D., MSc, MBA Sham Dholakia, M.D., Ph.D. Chief Executive Officer Chief Medical Chief Product Officer & Development Officer Q&A Marc Grasso, M.D. Amanda Piquet, M.D., FAAN Chief Financial Officer University of Colorado Anschutz Céline Dione Foundation Endowed Chair

Kyverna’s Leadership in Autoimmune CAR T: Miv-cel’s Transformative Impact in SPS SPS is a debilitating, progressive autoimmune disease with no FDA-approved therapies and significant unmet medical need Miv-cel achieved statistically significant clinical benefit across all primary and secondary endpoints, reversing disability scores; patients remained free of immunotherapies as of last follow up; data supports BLA submission expected in 1H 2026 FIRST completed registration-enabling CAR T trial for autoimmune disease could pave the way for miv-cel to become the FIRST and ONLY approved therapy in SPS and CAR T-cell therapy for autoimmune disease Valuable commercial opportunity in SPS, supported by attractive market dynamics and focused commercialization strategy First-to-market opportunity in SPS lays foundation for expansion into broader indications ©2025 Kyverna Therapeutics, Inc. 30 CAR, chimeric antigen receptor.