UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER
THE SECURITIES EXCHANGE ACT OF 1934
October 20, 2025
Commission File Number: 001-39363
IMMATICS N.V.
Paul-Ehrlich-Straße 15
72076 Tübingen, Federal Republic of Germany
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
| Form 20-F |
☒ |
Form 40-F |
☐ |
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On October 20, 2025, Immatics N.V. (the “Company” or “Immatics”) provided updated data from 16 patients with metastatic uveal melanoma in the ongoing Phase 1b clinical trial evaluating anzu-cel PRAME cell therapy.
The data cutoff was September 24, 2025. As of the data cutoff, 16 patients with metastatic uveal melanoma were administered a one-time infusion of anzu-cel at the recommended Phase 2 dose (RP2D, 1 to 10 billion total TCR T cells) in the Phase 1b dose expansion. Patients received a median infused TCR T-cell dose of ~4 billion (range 1.62 - 8.43 billion TCR T cells) and had a median of 2 lines of prior systemic treatments. Patients had a median target lesion sum of a diameter of 103 mm (ranging from 31 to 210 mm), and 81% of patients had liver and extrahepatic metastasis.
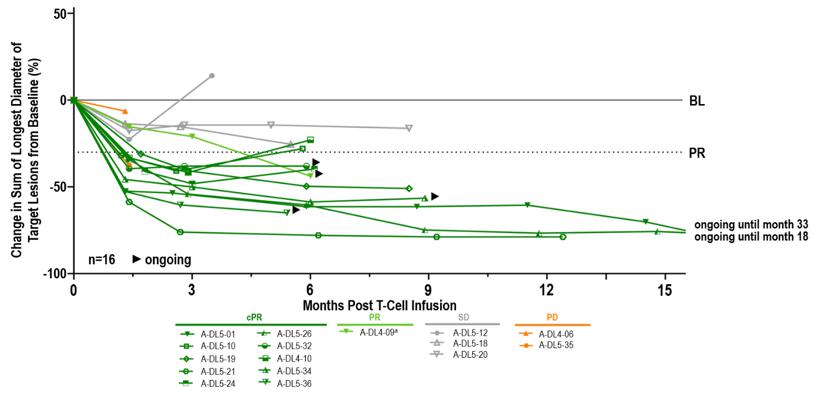
Anti-tumor Activity and Durability. Set forth below is the observed anti-tumor activity and durability of response of anzu-cel PRAME cell therapy:
| · | Confirmed objective response rate (cORR) of 67% (10/151) |
| · | Disease control rate (DCR) of 88% (14/16) |
| · | Median duration of response (mDOR) of 11 months (min 4.4, max 31.6 months) |
| · | Median progression-free survival (mPFS) of 8.5 months (min 1.4, max 32.9) at a mFU of 10.4 months. The PFS rate was 69% at six months and 39% at 12 months |
| · | Median overall survival (mOS) not reached (min 4.3+, max 34.2+ months) at a mFU of 14.3 months. The OS rate was 71% at 12 months |
Anti-tumor activity was observed in liver and extrahepatic metastases, including lung, lymph node, abdomen/peritoneum and others. 14/16 patients had target lesions in the liver and treatment with anzu-cel led to a median shrinkage in liver target lesion size of 49.6%.
Notably, 11 out of the 16 patients received a TCR bispecific (ten gp-100-targeting, one PRAME-targeting) as prior systemic treatment line, and thereof, six achieved a confirmed partial response, one a partial response and three stable disease. These results demonstrate anti-tumor activity of anzu-cel in patients who received prior TCR-based therapies.
1 Excluding one patient who withdrew consent with ongoing unconfirmed response.
Duration of Response

aPatient out of study at data-cut (withdrew consent); BL, baseline; (c)PR, (confirmed) partial response; PD, progressive disease; SD, stable disease.
Best Overall Response
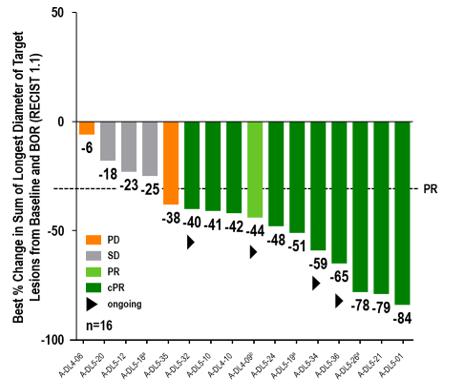
aMaximum change of target lesions and RECIST1.1 response at different timepoints. bPatient off study at data cutoff date (withdrew consent). 14/16 patients had liver target lesions with median best change of longest diameter of liver target lesions (range) of -49.6% (-100, 10). BOR, best overall response; (c)PR, (confirmed) partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; PD, progressive disease.
Safety Data. As shown in the table below, the most frequent treatment-emergent adverse events (TEAEs) were anticipated cytopenias associated with lymphodepletion. Expected and manageable cytokine release syndrome (CRS) was mostly Grade 1 or 2, which is consistent with the mechanism of action (Grade 1: 37.5%, Grade 2: 43.8%, Grade 3: 18.8%, Grade 4: 0%). No patients experienced long-term CRS, and most CRS was resolved by day 14. No anzu-cel-related Grade 5 events were observed. The tolerability profile in the uveal melanoma subset was generally consistent with the full anzu-cel tolerability profile in the Phase 1b.
TEAEs Occurring in ≥25% of Patients
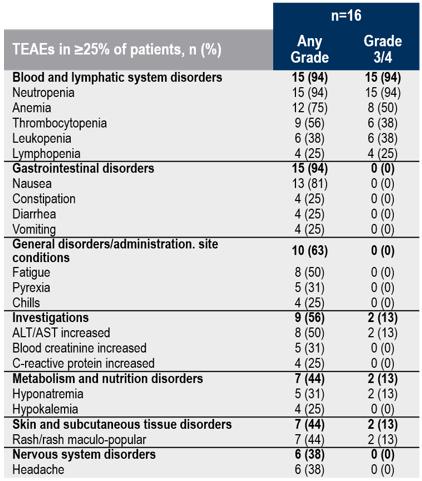
Patients are counted only once per adverse event and severity classification. ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAE, treatment-emergent adverse event.
Development Path. Based on the promising clinical data in patients with metastatic uveal melanoma, the Company has initiated a Phase 2 cohort with approximately 30 uveal melanoma patients planned. The cohort is being conducted at select centers in the United States and Germany with deep expertise in uveal melanoma. Given the high prevalence of PRAME expression in uveal melanoma, prospective PRAME testing is no longer required for inclusion in the clinical trial. The consistent tolerability, anti-tumor activity and pharmacokinetic profile of anzu-cel across both uveal and cutaneous melanoma provide a strong rationale for pursuing a parallel late-stage development strategy to serve both patient populations.
* * *
In connection with the foregoing, the Company issued a press release, a copy of which is attached hereto as Exhibit 99.1, and made available a presentation and an updated corporate presentation, copies of which are attached hereto as Exhibit 99.2 and Exhibit 99.3.
INCORPORATION BY REFERENCE
This Report on Form 6-K (other than Exhibit 99.1 hereto) shall be deemed to be incorporated by reference into the registration statements on Form S-8 (Registration Nos. 333-249408, 333-265820, 333-280935 and 333-288466) and the registration statements on Form F-3 (Registration Nos. 333-240260, 333-274218 and 333-286151) of Immatics N.V. and to be a part thereof from the date on which this report is filed, to the extent not superseded by documents or reports subsequently filed or furnished.
EXHIBIT INDEX
| Exhibit No. | Description |
| 99.1 | Press release dated October 20, 2025 |
| 99.2 | ESMO Data Presentation |
| 99.2 | Corporate presentation dated October 20, 2025 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IMMATICS N.V. | ||
| Date: October 20, 2025 | ||
| By: | /s/ Harpreet Singh | |
| Name: | Harpreet Singh | |
| Title: | Chief Executive Officer | |